Abstract
Attention deficit/hyperactivity disorder (ADHD) is a highly prevalent neurodevelopmental disorder that emerges in childhood and persists into adulthood in a sizeable portion of afflicted individuals. The persistence of ADHD symptoms elevates the risk of adverse outcomes that result in substantial individual and societal burden. The objective of this study was to delineate neuroanatomical substrates associated with the diversity of adult outcomes of childhood ADHD, which may have considerable value for development of novel interventions that target mechanisms associated with recovery. Structural MRI and diffusion tensor imaging data from 32 young adults who were diagnosed with ADHD combined-type during childhood and 35 group-matched controls were analyzed. Adults with childhood ADHD were divided into 16 remitters and 16 persisters based on DSM-IV criteria. Compared to the controls, ADHD probands showed significantly reduced gray matter (GM) volume in right putamen and white matter (WM) volume in left parieto-insular fiber tracts. Within the ADHD probands, the remitters, as compared to persisters, showed significantly greater volume of right hippocampo-frontal and right parieto-insular WM fiber tracts, and those connecting caudate with the frontal, parietal, occipital, temporal, and insular cortices. Among ADHD probands, increased fractional anisotropy value of left caudate-parietal tract was significantly correlated with reduced hyperactive/impulsive symptoms. These findings suggest that optimal structural development in the WM tracts that connect caudate with cortical areas, especially in the caudate-parietal path, may play an important role in symptom remission in young adults with childhood ADHD.
Keywords: ADHD, Adult Outcome, Remission, Persistence, Magnetic Resonance Imaging (MRI), Diffusion Tensor Imaging (DTI)
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most commonly diagnosed neurodevelopmental disorders with a prevalence of approximately 9.5% in school-age children in the United States (Pastor, et al., 2015; Visser, et al., 2014). It is characterized by pervasive symptoms of inattentiveness, hyperactivity, and impulsivity, and a wide-range of behavioral and cognitive impairments in working memory, inhibitory control, and motivation (Sonuga-Barke, 2002). Approximately 65% of the children with ADHD have persistent impairing symptoms into adulthood, which elevates the risk of adverse outcomes linked to substantial individual and societal burden (Faraone, et al., 2006). Neural determinants of the diverse adult outcomes of childhood ADHD remain unknown. Elucidation of these mechanisms could set the stage for the development of novel interventions that yield enduring benefits and improve long-term outcomes.
A large number of existing studies suggest that ADHD symptoms in children are associated with widespread neuroanatomical and functional alterations of brain. Structural neuroimaging studies have found ADHD symptoms in childhood to be associated with decreased regional gray matter (GM) volume in frontal cortex, striatum and cerebellum (Bledsoe, et al., 2011; Ellison-Wright, et al., 2008; Mahone, et al., 2011). Reduced regional cortical GM thickness in frontal and parietal cortices have also been linked with ADHD symptoms (Almeida Montes, et al., 2013; Batty, et al., 2010). White matter (WM) structural deficits, especially reduced WM volume and/or fractional anisotropy (FA) in the fronto-parietal, fronto-limbic, corona radiate, cerebellar- and temporo-occipital, and internal capsule fiber tracts have been consistently demonstrated in children with ADHD (Durston, et al., 2004; Nagel, et al., 2011; Peterson, et al., 2011; Qiu, et al., 2011; Xia, et al., 2012). Additionally, a number of task-based functional MRI (fMRI) studies have reported significantly reduced task-responsive activation in frontal and parietal areas, dorsal anterior cingulate cortex (dACC), thalamus, and striatum in children with ADHD relative to the group-matched controls, when performing behavioral tasks that assess attentional and inhibitory control functions (such as the go/no-go task, stop signal task, continuous performance task, stroop task, etc) (Booth, et al., 2005; Durston, et al., 2007; Durston, et al., 2006; Durston, et al., 2003; Li, et al., 2013; Li, et al., 2012; Pliszka, et al., 2006; Smith, et al., 2006; Suskauer, et al., 2008). Significantly reduced activation in these cortical and subcortical regions have also been consistently reported in children with ADHD relative to controls, when performing tasks assessing working memory, decision making, reward processing, and interference control functions (Cao, et al., 2008; Konrad, et al., 2006; Vaidya, et al., 2005; Vance, et al., 2007). A meta-analysis of 55 task-based fMRI studies reported significantly decreased activation in frontoparietal and ventral attentional networks in children with ADHD, compared to the group-matched controls (Cortese, et al., 2012).
The majority of existing clinical and neuroimaging studies in ADHD have focused on understanding the neural correlates of symptoms in cross-sectional samples of children or young adults. Far fewer studies have examined neural substrates associated with the diverse adult outcomes of childhood ADHD. Compared to group-matched controls, Schneider et al. reported that adults with childhood ADHD showed significantly reduced activation in caudate, ACC, parietal regions, and increased activation in insular during cognitive control processing, with these functional anomalies positively associated with increased levels of inattentive and hyperactive/impulsive symptoms (Schneider, et al., 2010). In a sample of children with ADHD followed into adulthood, Schulz et al. found lower orbitofrontal, inferior frontal, anterior cingulate and parietal activation in probands with persistent ADHD relative to both probands with remitted ADHD and comparison subjects, with no differences between remitters and comparison subjects (Schulz, et al., 2017). In the same longitudinal sample of adults with childhood ADHD, Clerkin et al. found lower thalamo-frontal functional connectivity in the ADHD persisters relative to remitters during a cued-reaction time task (Clerkin, et al., 2013); and Luo et al. further depicted decreased nodal efficiency in middle frontal gyri (MFG) in the functional brain network for cue-evoked attention processing in the ADHD persisters relative to the remitters (Luo, et al., 2018). Finally, resting-state fMRI studies found that higher fronto-ACC connectivity in the executive control network may contribute to symptom reduction in adults with childhood ADHD (Francx, et al., 2015; Mattfeld, et al., 2014).
Neuroanatomical studies showed diverse results in adults with childhood ADHD. With structural MRI, Proal et al. found that compared to matched controls, ADHD probands had significantly decreased GM volume in prefrontal lobe, cerebellum, thalamus, and caudate, regardless of ADHD symptom remission or persistence (Proal, et al., 2011); while Shaw et al. showed that significantly reduced cortical thickness was linked with symptom persistence (Shaw, et al., 2013). A diffusion tensor imaging (DTI) study suggested that ADHD probands had WM disruptions in the superior longitudinal fasciculus (SLF) and cortico-limbic areas regardless of symptom remission or persistence (Gehricke, et al., 2017); another study found that greater adult inattentiveness, but not hyperactivity/impulsivity, was associated with lower FA in inferior occipito-frontal fasciculus and uncinated fasciculus (Shaw, et al., 2015); while Cortese et al. indicated no significant WM differences between the ADHD-remitters and -persisters (Cortese, et al., 2013). The inconsistent findings from these neuroimaging studies in adults with childhood ADHD may be partially explained by differences in imaging modalities, analytic methods, and study cohorts. These existing studies have demonstrated neuroanatomical alterations in adults with childhood ADHD. However, most of them applied only single imaging modality (either structural MRI or DTI) to investigate GM morphometrical or WM integrity properties, without reporting both the GM and WM patterns in the same study cohort, and their potential impact on the adult outcome of childhood ADHD. This study aimed to fill this gap by applying both structural MRI and DTI in the same study sample to identify the structural markers in GM and WM, that are associated with symptom persistence and remission in young adults with childhood ADHD. Based on findings of previous studies from our group and others, we hypothesized that more optimal structural development associated with the frontal and parietal lobes, such as greater regional GM thickness, higher FA of the WM tracts that connect subcortical structures (i.e. thalamus, caudate) and frontal/parietal cortices, may play an important role in symptom remission in young adults with childhood ADHD.
METHOD
Participants
The initial sample consisted of 106 young adults who had been clinically followed since childhood, including 60 probands who were diagnosed with ADHD combined-type (ADHD-C) when they were 7 – 11 years of age and 46 group-matched comparison subjects with no history of ADHD. Among the 60 ADHD probands, 16 were classified as ADHD persisters (ADHD-P) and 16 as ADHD remitters (ADHD-R), and were able to provide usable T1-weighted and DTI data. Those adults with ADHD-P endorsed at least five inattentive and/or hyperactive/impulsive symptoms and had a minimum of 3 symptoms in each domain. Those classified as ADHD-R endorsed no more than 3 inattentive or 3 hyperactive/impulsive symptoms in adulthood and had no more than 5 symptoms in total, to allow separation from the ADHD-P group.
Among the 46 young adults who were previously classified as non-ADHD, 35 had no more than three inattentive or hyperactive/impulsive symptoms and provided usable clinical and neuroimaging data. Therefore, 67 subjects (32 ADHD proband and 35 controls) were included in group-level clinical and neuroimaging data analyses.
Childhood diagnoses were based upon teacher ratings using the IOWA Conners’ Teachers Rating Scale (Loney and Milich, 1982) and parent interview using the Diagnostic Interview Schedule for Children version 2 (Shaffer, et al., 1989). The exclusion criteria in childhood were chronic medical illness; neurological disorder; diagnosis of schizophrenia, autism spectrum disorder, or chronic tic disorder; Full Scale IQ < 70; and not speaking English. The social economic status (SES) of each child was assessed using the Nakao-Treas Socioeconomic Prestige Index (Nakao and Treas, 1994).
Adult psychiatric status was assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First, et al., 2002), supplemented by a semi-structured interview for ADHD that was adapted from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K- SADS) (Kaufman, et al., 1997) and the Conners’ Adult ADHD Diagnostic Interview for DSM-IV (Epstein, et al., 2006). Exclusion criteria in adulthood were psychotropic medication that could not be discontinued and conditions that would preclude MRI (e.g., metal in body, pregnancy, too obese to fit in scanner). Clinical and demographic information are listed in Table 1.
Table 1:
Demographical, neurocognitive and clinical characteristics in groups of controls and ADHD probands (including ADHD remitters and persisters).
| Controls (N = 35) |
ADHD (N = 32) |
Remitters (N=16) |
Persisters (N=16) |
|||
|---|---|---|---|---|---|---|
| Mean (SD | Mean (SD) | P | Mean (SD) | Mean (SD) | p | |
| Age | 24.24 (2.3) | 24.60 (2.1) | 0.51 | 24.81 (2.3) | 24.39 (1.9) | 0.57 |
| Full-scale IQ | 104.21 (15) | 96.81 (14.3) | 0.07 | 99.58 (14.2) | 94.11 (11.5) | 0.24 |
| Socioeconomic status | 51.59 (14.8) | 43.73 (17.2) | 0.06 | 43.28 (14.9) | 40.56 (19.8) | 0.57 |
| CAARS (raw score) | ||||||
| Inattentive | 4.03 (3.5) | 8.09 (5.1) | <0.001 | 5.75 (4.9) | 10.44 (4.3) | 0.007 |
| Hyperacitive/impuls | 4.58 (2.6) | 8.66 (4.9) | <0.001 | 5.88 (3.8) | 11.44 (4.3) | 0.001 |
| ADHD Total | 8.82 (5.2) | 16.59 (9.2) | <0.001 | 11.94 (8.2) | 21.25 (7.9) | 0.003 |
| N (%) | N (%) | P | N (%) | N (%) | P | |
| Male | 30 (85.7) | 27 (84.4) | 0.88 | 14 (87.5) | 13 (81.3) | 0.63 |
| Right-handed | 31 (88.6) | 28 (87.5) | 0.89 | 14 (87.5) | 14 (87./5) | 1 |
| Race | 0.41 | 0.70 | ||||
| Caucasian | 14 (40.0) | 17 (53.1) | 8 (50.0) | 9 (56.3) | ||
| African American | 13 (37.1) | 7 (21.9) | 4 (25.0) | 3 (18.8) | ||
| More than one race | 6 (17.1) | 8 (25) | 4 (25.0) | 4 (25.0) | ||
| Asian | 2 (5.7) | 0 (0) | 0 (0) | 0 (0) | ||
| Ethnicity | 0.21 | 0.72 | ||||
| Hispanic/Latino | 12 (34.3) | 15 (46.9) | 7 (43.8) | 8 (50.0) | ||
CAARS: Conners’ Adult ADHD Rating Scale
All the ADHD probands had a history of treatment with short-acting psychostimulants. Mean duration of treatment was 2.03 years (SD = 3.21) for the subgroup of ADHD-R and 4.18 years (SD = 4.12) for the subgroup of ADHD-P (t = −1.604, p = 0.12). There were two subjects in the ADHD-P subgroup who were taking psychostimulants at the time of this study, and had a 48-hour medication wash-out period before MRI acquisition.
The study received Institutional Review Board Approval at the participating institutions. Participants provided signed informed consent and were reimbursed for their time and travel expenses.
MRI acquisition protocol
High resolution 3-dimensional T1-weighted structural MRI and DTI data were acquired using the same 3T Siemens Allegra (Siemens, Erlangen, Germany) head-dedicated MRI scanner. T1-weighted data was acquired using magnetization prepared rapid gradient echo (MPRAGE) pulse sequence with the following parameters: repetition time (TR) = 2.5 s, echo time (TE) = 4.38 ms, inversion time (TI) = 1.1 s, flip angle = 8°, voxel size = 0.94 mm × 0.94 mm × 1 mm, field of view (FOV) = 256 mm × 256 mm × 256 mm. DTI data was acquired using an echo planar imaging (EPI) pulse sequence with a b-value = 1250 s/mm2 along 12 independent, as well as a reference volume without diffusion-weighting b = 0 s/ mm2, non-collinear orientations with the following parameters: TR = 5.2 s, TE = 80 ms, flip angle = 90°, voxel size = 1.875 mm × 1.875 mm × 4 mm, FOV = 128 mm × 128 mm, imaging matrix = 128 × 96, number of slices = 63.
Individual-level structural MRI data analyses
T1-weighted data were reconstructed into a 3-dimensional cortical model for thickness and area estimations using FreeSurfer v.5.3.0 (https://surfer.nmr.mgh.harvard.edu). Each data point was first registered with the Talairach atlas to compute the transformation matrix using an affine registration method, which was developed and distributed by the Montreal Neurological Institute (MNI). Then intensity variations caused by magnetic field inhomogeneities were corrected using Voronoi partitioning algorithm. The skull was stripped using a deformable template model. Cutting planes were defined to separate the left and right hemispheres and to remove the cerebellum and brainstem. Two mess surfaces (mess of grids created using surface tessellation technique) were then generated between WM and GM (white matter surface), as well as between GM and cerebrospinal fluid (pial surface). The distance between the two closest vertices of the white matter and pial surfaces presented the cortical thickness at that specific location, validated using training data (Rosas, et al., 2002). Regional cortical thickness and area in 68 bilateral cortical regions were estimated based on the Desikan atlas (Desikan, et al., 2006).
Each of 37 subcortical structures/nuclei was first labelled after the initial registration with the Talairach atlas, and then refined based on a manually labelled model constructed according to prior knowledge of spatial relationships acquired with a training data set (Fischl, et al., 2002). Volume of each subcortical structure was then calculated.
To adjust head-size variation related influence on these cortical and subcortical GM measures, the head-size scaling factor of each subject was calculated by normalizing the T1-weighted data with the template provided in FSL/SIENA (Smith, et al., 2002). The normalized thickness and area of each cortical region and volume of each subcortical structure were finally estimated by multiplying the original value with the scaling factor of that subject.
Individual-level DTI data analyses
DTI data from each subject was first processed using the Diffusion Toolbox (FDT Version 3.0) from FSL (Behrens, et al., 2007). After eddy current and head motion corrections, the diffusion-weight images were registered to the additionally acquired non-diffusion-weighted reference image (b0 image) using an affine, 12 degrees of freedom registration. The FA value and principle diffusion direction at each brain voxel were calculated. WM probabilistic tractography between each pair of 18 regions-of- interest (ROIs) was constructed using the FSL/BEDPOSTX toolbox (Behrens, et al., 2007). These 18 ROIs (including thalamus, putamen and caudate nuclei from striatum, hippocampus, and frontal, parietal, occipital, temporal, and insular cortices in both hemispheres) were created based on the Harvard-Oxford Cortical Atlases and the Julich Histological Atlas from the MNI standard space, and mapped to the DTI data. We used the multi-fiber probabilistic connectivity-based method to determine the number of pathways between each seed and target ROIs. The default setting of parameters for Markov Chain Monte Carlo estimation of the probabilistic tractography was utilized: 5000 individual pathways were drawn on the principle fiber direction of each voxel within the seed ROI; curvature threshold of 80° to exclude implausible pathways; a maximum number of 2000 travel steps of each sample pathway and a 0.2 mm step length. The number of pathways that existed through each voxel from the remainder of the brain was labeled. The non-zero labeling voxels were taken as the initial elements of the tracts between the seed and target ROIs. The brain voxels with low probability of connection were removed from the tract, if one had a number of pathways that was less than the average of the pathway numbers from all the non-zero labeling voxels. A total of 20 cortico-cortical (including bilateral fronto-parietal, fronto-occipital, fronto-temporal, fronto-insular, parieto-occipital, parieto-temporal, parieto-insular, occipito-temporal, occipito-insular, temporo-insular) and 40 subcortico-cortical (including bilateral thalamo-frontal, thalamo-parietal, thalamo-occipital, thalamo-temporal, thalamo-insular, putamen-frontal, putamen-parietal, putamen-occipital, putamen-temporal, putamen-insular, caudate-frontal, caudate-parietal, caudate-occipital, caudate-temporal, caudate-insular, hippocampo-frontal, hippocampo-parietal, hippocampo-occipital, hippocampo-temporal, hippocampo-insular) WM fiber tracts were generated. Average FA and volume (number of voxels times voxel size) of each identified WM tract were estimated.
Group statistical analyses
The clinical, neurocognitive and demographic measures were compared using chi-square tests for discrete variables and unpaired two-sample t-tests for continuous variables, between groups of controls and ADHD probands, and further between the two ADHD subgroups (ADHD-R and ADHD-P) using SPSS18 (SPSS Inc, Somers, NY).
The structural MRI- and DTI-based neuroimaging measures (including regional cortical thickness, surface area, volume of each subcortical structure, FA and volume of each WM fiber tract) were compared between the groups of controls and ADHD probands, as well as between the subgroups of ADHD-R and ADHD-P, using analysis of covariance (ANCOVA) with gender, age, IQ and SES as covariates. Bonferroni correction for multiple comparisons (at a corrected α = 0.05) was applied to control potential false positive results of these group comparisons. For group comparisons in the structural MRI-based measures, we controlled alpha for 105 ROIs (i.e., 68 bilateral cortical regions and 37 subcortical structures). For the DTI-based measures, we controlled alpha for the 60 WM tracts analyzed. The brain-behavior association analyses controlled for the 18 partial correlation procedures conducted.
Partial correlation analysis was utilized to assess associations between the GM and WM brain measures that showed between-group differences (measures listed in Tables 2 and 3) and the clinical symptom measures (the raw scores for inattentive and hyperactive-impulsive symptoms derived from the CAARS collected during the visit of MRI scan) in the group of ADHD probands. Age, gender, IQ and SES were added as covariates. Bonferroni correction was used to correct the number of partial correlation procedures (a total of 16) at a corrected α = 0.05.
Table 2:
Gray matter neuroimaging measures that show significant between-group differences with age, gender, IQ and social economic status as covariates.
| Group | Anatomical location | Measure | F-value | p-value after Bonferroni correction |
|---|---|---|---|---|
| CON > PRO | R. Putamen | Volume | 8.892 | 0.045 |
| ADHD-R > ADHD-P | L./R. Parahippocampal gyrus | Regional Cortical Surface Area | 7.921/12.947 | 0.05/0.008 |
| L. Paracentral gyrus | 12.283 | 0.012 | ||
| R. Transverse temporal gyrus | 8.494 | 0.037 |
CON: group of controls; PRO: group of ADHD probands; ADHD-R: subgroup of ADHD remitters; ADHD-P: subgroup of ADHD persisters. L.: left hemisphere; R.: right hemisphere; p values were corrected using Bonferroni correction.
Table 3:
White matter neuroimaging measures that show significant between-group differences with age, gender, IQ and social economic status as covariates.
| Group | White matter fiber tract | Measure | F-value | p-value after Bonferroni correction |
|---|---|---|---|---|
| CON > PRO | L. parieto-insular tract | Volume | 9.928 | 0.041 |
| ADHD-R > ADHD-P | L./R. caudate-frontal tracts | Volume | 42.755/32.576 | <0.001/<0.001 |
| L./R. caudate-parietal tracts | 51.553/31.62 | <0.001/<0.001 | ||
| L./R. caudate-occipital tracts | 55.169/31.593 | <0.001/<0.001 | ||
| L./R. caudate-temporal tracts | 55.088/31.564 | <0.001/<0.001 | ||
| L./R. caudate-insular tracts | 55.155/31.527 | <0.001/<0.001 | ||
| R. hippocampo-frontal tract | 13.228 | 0.037 | ||
| R. parieto-insular | 12.785 | 0.038 |
CON: group of controls; PRO: group of ADHD probands; ADHD-R: subgroup of ADHD remitters; ADHD-P: subgroup of ADHD persisters. L.: left hemisphere; R.: right hemisphere; p values were corrected using Bonferroni correction.
RESULTS
As shown in Table 1, there were no significant demographic differences between the groups although relative to controls, ADHD probands tended to have lower IQ and SES.
Significantly decreased volume in right putamen was observed in ADHD probands when compared to controls (p = 0.045). Compared to the ADHD-P group, those with ADHD-R showed significantly increased cortical surface area in bilateral parahippocampal gyri (Left: p = 0.05; Right: p = 0.008), left paracentral gyrus (p = 0.012), and right transverse temporal gyrus (p = 0.037) (see Table 2). Group comparisons of the WM measures showed significantly decreased volume of the left parieto-insular fiber tract (p = 0.041) in ADHD probands relative to controls. Compared to ADHD-R, the subgroup of ADHD-P showed significantly decreased volume in two cortico-cortical fiber tracts (right hippocampo-frontal (p = 0.037) and right parieto-insular (p = 0.038)), and in the WM tracts connecting bilateral caudate nuclei of the striatum with all the five cortical ROIs of the same hemispheres (p < 0.001) (see Table 3).
Dimensional analyses between the GM and WM measures (listed in Tables 2 and 3) and the clinical symptom measures indicated that among the ADHD probands, greater FA of the left caudate-parietal WM fiber tract was significantly associated with reduced hyperactive/impulsive symptoms (Figure 1, r = −0.402, p = 0.031).
Figure 1:
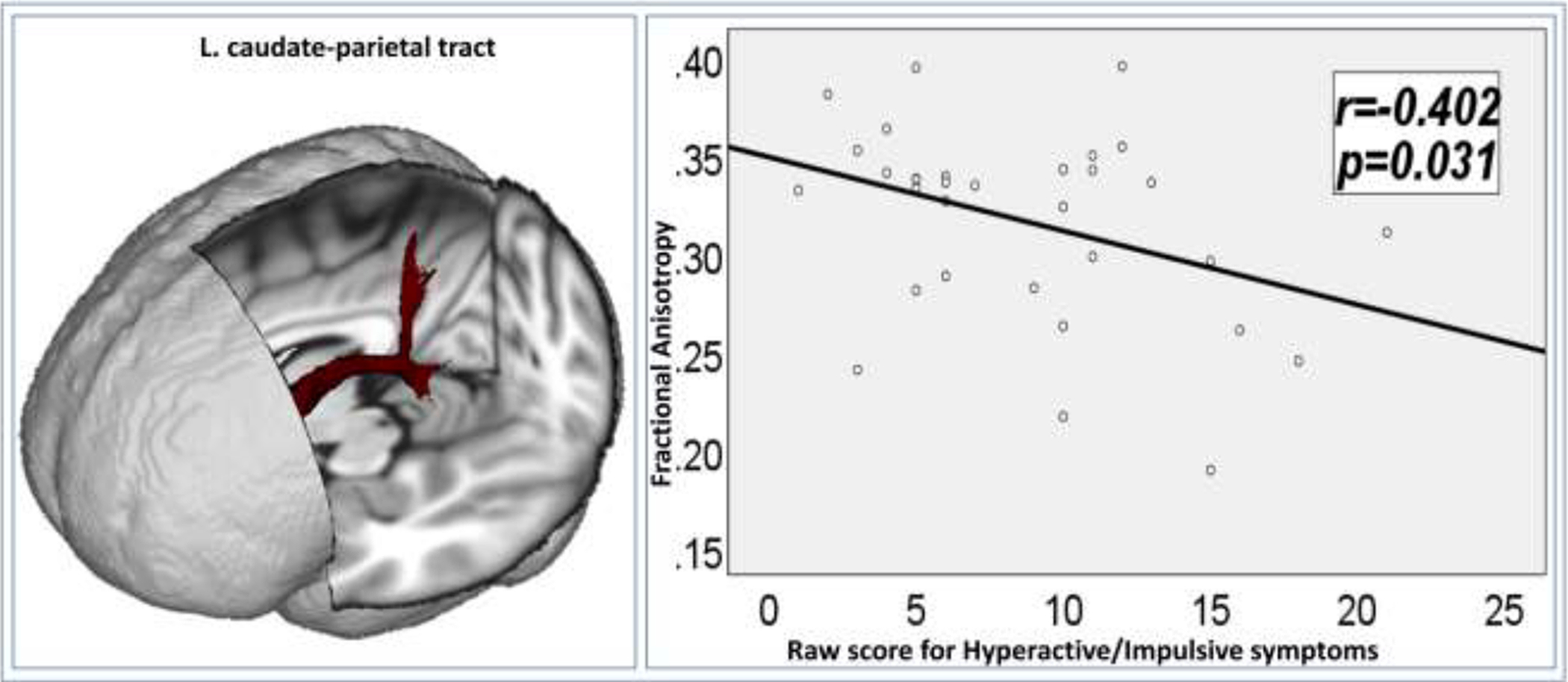
In the group of ADHD probands, greater fractional anisotropy of the left caudate-parietal white matter fiber tract was significantly associated with reduced hyperactive-impulsive symptoms.
DISCUSSION
The present study investigated GM and WM structural differences between young adults with childhood ADHD and group-matched controls, and between the subgroups of remitters and persisters within the ADHD probands. Compared to controls, significantly reduced GM volume of the putamen in right hemisphere was observed in the ADHD probands. The putamen and caudate nucleus together form the dorsal striatum, and play a key role in the cortico-thalamo-striatal-cortical (CTSC) loops for attention and higher order cognitive processes (Alexander, et al., 1986; Ring and Serra-Mestres, 2002). A large number of structural MRI and fMRI studies have reported the linkage of putamen-related anatomical and functional abnormalities and onset of ADHD in children (Ellison-Wright, et al., 2008; Frodl and Skokauskas, 2012; Max, et al., 2002; Nakao, et al., 2011). Putamen-related structural alterations have also been tested in neuroimaging studies focusing on adults with ADHD which yielded inconsistent results, with some reports of reduced putamen volume in adults with ADHD (Onnink, et al., 2014; Seidman, et al., 2011), and others reporting increased putamen volume (Greven, et al., 2015) or no significant differences (Seidman, et al., 2006) when compared to group-matched controls. The inconsistency of these existing studies may have been caused by technical differences for putamen extractions, and sample-related biases such as the very wide age ranges involved in these studies (Greven, et al., 2015). Adding to the literature, our result of significantly reduced putamen GM volume in young adults with childhood ADHD (regardless of their clinical outcomes) suggests its significant linkage with the emergence of ADHD during their childhood.
Compared to controls, we also found that the ADHD probands had significantly reduced volume of the left hemisphere parieto-insular WM tract; while relative to the ADHD remitters, the persisters had significantly smaller volume of the right hemisphere parieto-insular WM tract. The parieto-insular WM fiber tract is an important structural component of the vestibular system, and has been suggested to link with static and dynamic balance control (Frank and Greenlee, 2018; Perennou, et al., 2000; Shum and Pang, 2009; Ustinova, et al., 2001). Vestibular system deficiency, which can cause inappropriate postural condition or impaired balance function, has been found to be associated with cognitive deficits and behavioral symptoms in ADHD patients (Clark, et al., 2008; Haghshenas, et al., 2014; Shum and Pang, 2009). Merging with the results of existing studies, our findings of the underdeveloped parieto-insular WM fiber tracts in adults with childhood ADHD, especially in those with persistent ADHD symptoms, suggest that parieto-insular WM structural alterations may interact with the vestibular system functional alterations, and together contribute to the onset and symptom persistence of ADHD.
Within the probands, we further found that the ADHD remitters had significantly larger surface area in bilateral parahippocampal, left paracentral, and right transverse temporal gyri, as well as significantly greater volume of WM fiber tracts connecting caudate with the frontal, parietal, occipital, temporal, and insular cortices when compared to the persisters. Existing studies have reported that ADHD remitters had increased parahippocampal cortical thickness compared to ADHD persisters (Proal, et al., 2011). Further studies have implicated that parahippocampal gyrus interacts with the ventralateral prefrontal cortex (VLPFC), both significantly contribute to appropriate inhibitory control (Deacon, et al., 1983; Schulz, et al., 2005). Parahippocampal cortical volume reduction has been observed in both children and adolescents with ADHD, compared to group-matched controls (Carmona, et al., 2005; Noordermeer, et al., 2017).
Caudate plays a critically important role in cognitive control (Chiu, et al., 2017; Grahn, et al., 2008). Structural and functional deficits associated with caudate have been widely observed in children and adults with ADHD (Frodl and Skokauskas, 2012; Onnink, et al., 2014; Szekely, et al., 2017). Substantial structural MRI studies have revealed that children with ADHD had smaller caudate volume relative to controls (Castellanos, et al., 2002). Task-based fMRI studies showed significantly decreased caudate activation in children with ADHD (Vaidya, et al., 2005) and adults with childhood ADHD (Szekely, et al., 2017), during attention and inhibitory control processes. Our findings of significantly smaller volume of the WM fiber tracts connecting caudate with all five cortices bilaterally in the ADHD persisters suggest that caudate-associated widespread WM underdevelopment may play important roles in symptom persistence of ADHD. This hypothesis can also be supported by multiple existing DTI studies that showed immature WM organizations involving caudate and cortical structures in children and adults with ADHD (Ashtari, et al., 2005; Casey, et al., 1997; Castellanos, et al., 2002; Shang, et al., 2013).
In addition, we found that the FA of left caudate-parietal tracts was significantly negatively correlated with the CAARS raw score for hyperactive/impulsive symptoms in ADHD probands. The caudate-parietal WM tract is one of the most important structural component of the CTSC loops, which subserves maintaining the modifications of spatial attention via reinforcement learning, and supports the integration of reward, attention, and executive control (Jarbo and Verstynen, 2015). Reduced parietal activation during cognitive control has been linked to the persistence of ADHD symptoms in adults with childhood ADHD (Schulz, et al., 2017; Szekely, et al., 2017). Reduced caudate and parietal lobe activation during inhibitory control processing were found to be associated with increased inattentive and impulsive symptoms in adults with ADHD diagnosed in childhood (Schneider, et al., 2010). Together with these existing findings, we suggest that optimal structural development in the caudate-parietal WM tract may partially modulate the functional integrity of caudate and parietal cortex, and together contribute to symptom remission in adults with childhood ADHD.
In summary, together with existing findings, results of this study suggest that WM structural development in tracts that connect caudate with cortical areas, especially in the caudate-parietal path, is a critical determining factor of outcomes in adults with childhood ADHD. The current study has some limitations. First, our cohort consisted of both male and female subjects, but many more males. It is still unclear whether the neuropathological underpinnings of ADHD differ between males and females. To partially remove gender-related effects, sex was added as a fixed effect covariate in the group-level analyses. Second, the sample size of this study is relatively small. Therefore, the findings must be considered preliminary. Future work will need a much larger cohort from a longitudinal study consisting of multi-scan neuroimaging data, to determine the neural underpinnings of longitudinal trajectories of childhood ADHD.
Acknowledgement
This study was partially supported by research grants from the National Institute of Mental Health (R03MH109791, R15MH117368, and R01MH060698), the New Jersey Commission on Brain Injury Research (CBIR17PIL012), and the New Jersey Institute of Technology Start-up Award.
Role of funding source
Funding for this study was provided by NIMH (Grant/Award Numbers: R03MH109791, R15MH117368, and R01MH060698), NJ Department of Health (CBIR17PIL012), and the NJIT Faculty Seed Award. The funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare that they have no conflicts of interest.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci, 9:357–81. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Prado Alcantara H, Martinez Garcia RB, De La Torre LB, Avila Acosta D, Duarte MG (2013) Brain cortical thickness in ADHD: age, sex, and clinical correlations. J Atten Disord, 17:641–54. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA (2005) Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry, 57:448–55. [DOI] [PubMed] [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, Liotti M, Liddle PF, Paus T, Hollis C (2010) Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry, 49:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 34:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe JC, Semrud-Clikeman M, Pliszka SR (2011) Neuroanatomical and neuropsychological correlates of the cerebellum in children with attention-deficit/hyperactivity disorder--combined type. J Am Acad Child Adolesc Psychiatry, 50:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM (2005) Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD). J Child Psychol Psychiatry, 46:94–111. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Zhu C, Cao X, Sun L, Zhou X, Wang Y (2008) Alerting deficits in children with attention deficit/hyperactivity disorder: event-related fMRI evidence. Brain Res, 1219:159–68. [DOI] [PubMed] [Google Scholar]
- Carmona S, Vilarroya O, Bielsa A, Tremols V, Soliva JC, Rovira M, Tomas J, Raheb C, Gispert JD, Batlle S, Bulbena A (2005) Global and regional gray matter reductions in ADHD: a voxel-based morphometric study. Neurosci Lett, 389:88–93. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL (1997) Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 36:374–83. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL (2002) Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA, 288:1740–8. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Jiang J, Egner T (2017) The Caudate Nucleus Mediates Learning of Stimulus-Control State Associations. J Neurosci, 37:1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Arnold LE, Crowl L, Bozzolo H, Peruggia M, Ramadan Y, Bornstein R, Hollway JA, Thompson S, Malone K, Hall KL, Shelton SB, Bozzolo DR, Cook A (2008) Vestibular Stimulation for ADHD: randomized controlled trial of Comprehensive Motion Apparatus. J Atten Disord, 11:599–611. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM (2013) Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. Am J Psychiatry, 170:1011–9. [DOI] [PubMed] [Google Scholar]
- Cortese S, Imperati D, Zhou J, Proal E, Klein RG, Mannuzza S, Ramos-Olazagasti MA, Milham MP, Kelly C, Castellanos FX (2013) White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biol Psychiatry, 74:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX (2012) Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry, 169:1038–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW (1983) Afferent connections of the perirhinal cortex in the rat. J Comp Neurol, 220:168–90. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31:968–80. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, Scheres A, Castellanos FX, van Engeland H, Casey BJ (2007) Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry, 48:881–9. [DOI] [PubMed] [Google Scholar]
- Durston S, Hulshoff Pol HE, Schnack HG, Buitelaar JK, Steenhuis MP, Minderaa RB, Kahn RS, van Engeland H (2004) Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry, 43:332–40. [DOI] [PubMed] [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H (2006) Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry, 60:1062–70. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ (2003) Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry, 53:871–8. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Ellison-Wright Z, Bullmore E (2008) Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry, 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Johnson D, Conners CK (2006) Conners’ Adult ADHD Diagnostic Interview for DSM-IV.
- Faraone SV, Biederman J, Mick E (2006) The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med, 36:159–65. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J (2002) Structured clinical interview for DSM-IV-TR Axis I disorders, research version Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33:341–55. [DOI] [PubMed] [Google Scholar]
- Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, Franke B, Beckmann CF, Buitelaar JK, Mennes M (2015) The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex, 73:62–72. [DOI] [PubMed] [Google Scholar]
- Frank SM, Greenlee MW (2018) The parieto-insular vestibular cortex in humans: more than a single area? J Neurophysiol, 120:1438–1450. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N (2012) Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand, 125:114–26. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Kruggel F, Thampipop T, Alejo SD, Tatos E, Fallon J, Muftuler LT (2017) The brain anatomy of attention-deficit/hyperactivity disorder in young adults - a magnetic resonance imaging study. PLoS One, 12:e0175433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol, 86:141–55. [DOI] [PubMed] [Google Scholar]
- Greven CU, Bralten J, Mennes M, O’Dwyer L, van Hulzen KJ, Rommelse N, Schweren LJ, Hoekstra PJ, Hartman CA, Heslenfeld D, Oosterlaan J, Faraone SV, Franke B, Zwiers MP, Arias-Vasquez A, Buitelaar JK (2015) Developmentally stable whole-brain volume reductions and developmentally sensitive caudate and putamen volume alterations in those with attention-deficit/hyperactivity disorder and their unaffected siblings. JAMA Psychiatry, 72:490–9. [DOI] [PubMed] [Google Scholar]
- Haghshenas S, Hosseini MS, Aminjan AS (2014) A possible correlation between vestibular stimulation and auditory comprehension in children with attention-deficit/hyperactivity disorder. Psychology & Neuroscience, 7:159–162. [Google Scholar]
- Jarbo K, Verstynen TD (2015) Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci, 35:3865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36:980–8. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B (2006) Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry, 59:643–51. [DOI] [PubMed] [Google Scholar]
- Li X, Branch C, De La Fuente A, Xia S (2013) Role of pulvinar-cortical functional brain pathways in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 52:756–8. [DOI] [PubMed] [Google Scholar]
- Li X, Sroubek A, Kelly MS, Lesser I, Sussman E, He Y, Branch C, Foxe JJ (2012) Atypical pulvinar-cortical pathways during sustained attention performance in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 51:1197–1207 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney J, Milich R (1982) Hyperactivity, inattention and aggression in clinical practice,. In Wolraich M & R. D (Eds.). Advances in Developmental and Behavioral pediatrics, 2:113–147. [Google Scholar]
- Luo Y, Schulz KP, Alvarez TL, Halperin JM, Li X (2018) Distinct topological properties of cue-evoked attention processing network in persisters and remitters of childhood ADHD. Cortex, 109:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Ranta ME, Crocetti D, O’Brien J, Kaufmann WE, Denckla MB, Mostofsky SH (2011) Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc, 17:1047–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld AT, Gabrieli JD, Biederman J, Spencer T, Brown A, Kotte A, Kagan E, Whitfield-Gabrieli S (2014) Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain, 137:2423–8. [DOI] [PubMed] [Google Scholar]
- Max JE, Fox PT, Lancaster JL, Kochunov P, Mathews K, Manes FF, Robertson BA, Arndt S, Robin DA, Lansing AE (2002) Putamen lesions and the development of attention-deficit/hyperactivity symptomatology. J Am Acad Child Adolesc Psychiatry, 41:563–71. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, Nigg JT (2011) Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 50:283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Treas J (1994) Updating Occupational Prestige and Socioeconomic Scores: How the New Measures Measure up. Sociological Methodology, 24:1–72. [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D (2011) Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry, 168:1154–63. [DOI] [PubMed] [Google Scholar]
- Noordermeer SDS, Luman M, Greven CU, Veroude K, Faraone SV, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Heslenfeld DJ, Oosterlaan J (2017) Structural Brain Abnormalities of Attention-Deficit/Hyperactivity Disorder With Oppositional Defiant Disorder. Biol Psychiatry, 82:642–650. [DOI] [PubMed] [Google Scholar]
- Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Kan CC, Buitelaar J, Franke B (2014) Brain alterations in adult ADHD: effects of gender, treatment and comorbid depression. Eur Neuropsychopharmacol, 24:397–409. [DOI] [PubMed] [Google Scholar]
- Pastor P, Reuben C, Duran C, Hawkins L (2015) Association between diagnosed ADHD and selected characteristics among children aged 4–17 years: United States, 2011–2013. NCHS Data Brief:201. [PubMed] [Google Scholar]
- Perennou DA, Leblond C, Amblard B, Micallef JP, Rouget E, Pelissier J (2000) The polymodal sensory cortex is crucial for controlling lateral postural stability: evidence from stroke patients. Brain Res Bull, 53:359–65. [DOI] [PubMed] [Google Scholar]
- Peterson DJ, Ryan M, Rimrodt SL, Cutting LE, Denckla MB, Kaufmann WE, Mahone EM (2011) Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD). J Child Neurol, 26:1296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R 3rd, Xiong J, Liotti M (2006) Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry, 163:1052–60. [DOI] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, Lerch JP, He Y, Zijdenbos A, Kelly C, Milham MP, Castellanos FX (2011) Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Arch Gen Psychiatry, 68:1122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MG, Ye Z, Li QY, Liu GJ, Xie B, Wang J (2011) Changes of brain structure and function in ADHD children. Brain Topogr, 24:243–52. [DOI] [PubMed] [Google Scholar]
- Ring HA, Serra-Mestres J (2002) Neuropsychiatry of the basal ganglia. J Neurol Neurosurg Psychiatry, 72:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B (2002) Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology, 58:695–701. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Krick CM, Retz W, Hengesch G, Retz-Junginger P, Reith W, Rosler M (2010) Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults - a functional magnetic resonance imaging (fMRI) study. Psychiatry Res, 183:75–84. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Li X, Clerkin SM, Fan J, Berwid OG, Newcorn JH, Halperin JM (2017) Prefrontal and parietal correlates of cognitive control related to the adult outcome of attention-deficit/hyperactivity disorder diagnosed in childhood. Cortex, 90:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM (2005) Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry, 44:47–54. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Biederman J, Liang L, Valera EM, Monuteaux MC, Brown A, Kaiser J, Spencer T, Faraone SV, Makris N (2011) Gray matter alterations in adults with attention-deficit/hyperactivity disorder identified by voxel based morphometry. Biol Psychiatry, 69:857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J (2006) Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry, 60:1071–80. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Piacentini J, Schwab-Stone M, Wicks J (1989) Diagnostic Interview Schedule for Children-Parent Version (DISC-2.1P)
- Shang CY, Wu YH, Gau SS, Tseng WY (2013) Disturbed microstructural integrity of the frontostriatal fiber pathways and executive dysfunction in children with attention deficit hyperactivity disorder. Psychol Med, 43:1093–107. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W (2013) Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry, 74:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sudre G, Wharton A, Weingart D, Sharp W, Sarlls J (2015) White matter microstructure and the variable adult outcome of childhood attention deficit hyperactivity disorder. Neuropsychopharmacology, 40:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum SB, Pang MY (2009) Children with attention deficit hyperactivity disorder have impaired balance function: involvement of somatosensory, visual, and vestibular systems. J Pediatr, 155:245–9. [DOI] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K (2006) Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry, 163:1044–51. [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage, 17:479–89. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ (2002) Psychological heterogeneity in AD/HD--a dual pathway model of behaviour and cognition. Behav Brain Res, 130:29–36. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, Mostofsky SH (2008) Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci, 20:478–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely E, Sudre GP, Sharp W, Leibenluft E, Shaw P (2017) Defining the Neural Substrate of the Adult Outcome of Childhood ADHD: A Multimodal Neuroimaging Study of Response Inhibition. Am J Psychiatry, 174:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova KI, Chernikova LA, Ioffe ME, Sliva SS (2001) Impairment of learning the voluntary control of posture in patients with cortical lesions of different locations: the cortical mechanisms of pose regulation. Neurosci Behav Physiol, 31:259–67. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD (2005) Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry, 162:1605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, Cunnington R (2007) Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry, 12:826–32, 793. [DOI] [PubMed] [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ (2014) Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry, 53:34–46 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Li X, Kimball AE, Kelly MS, Lesser I, Branch C (2012) Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res, 204:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


