OVERVIEW
The dystonias are defined by excessive muscular contraction producing involuntary abnormal movements.1 The clinical appearance of abnormal movements in the dystonias varies widely.2 Many movements tend to be slow and twisting, often with sustained abnormal postures. Sometimes dystonic movements are quick or tremor-like. Whether slow or fast, dystonic movements are not random. They tend to recur in the same pattern. They also tend to be worsened by voluntary effort.
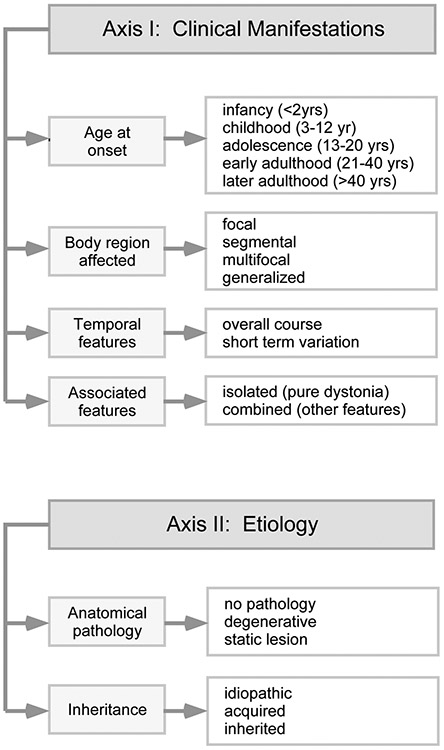
The dystonias may arise at any age, from infancy to late adulthood. They may affect muscles in almost any region of the body. Most dystonias are chronic. Some are static, some are progressive, and some have characteristic fluctuations over time. In some cases, dystonia is the only problem. For others, dystonia is one part of a more complex syndrome. Because of the remarkable variability in the clinical expression of dystonia, it is helpful to have a strategy for lumping and splitting them into related groups. Currently, the clinical features important for the classification of dystonia include age at onset, body region affected, variation over time, and whether or not dystonia is combined with other relevant features (Figure 1).1,3
Figure 1. Classification of the dystonias.
The many types of dystonia are classified according to two axes. The first axis relates to clinical features. The main factors important for clinical classification include age at onset, body distribution, temporal features, and whether or not dystonia is combined with other neurological or medical problems. The main factors important for etiological classification include whether the disorder is associated with relevant brain pathology, and whether the disorder is acquired or inherited. However, a large proportion of cases remain idiopathic.
Etiologies for dystonia are also quite varied.4 Most are idiopathic, and a cause cannot be found, even after extensive diagnostic evaluation. Although a cause can only be found in the minority of cases, there are many potential causes, both acquired and inherited. Acquired causes of dystonia include medications or toxins, structural defects such as vascular events or space-occupying lesions, and infectious or other inflammatory processes. There also are many inherited causes for the dystonias. There are only a few genes associated with isolated dystonia, but more than 100 genes associated with combined dystonias.5 Because there are so many etiologies for dystonia and most of them are quite rare, obtaining a definitive etiological diagnosis can be challenging. However, an etiological diagnosis is important because some of them have very specific treatments.
This article focusses on treatments for dystonia, both medical and surgical. Treatments are available for all types of dystonia. Most can benefit from symptomatic therapies, which include oral medications, local injections of botulinum toxin, and surgical interventions. For some, there are specific treatments that target underlying disease mechanisms.
PATIENT EVALUATION
History and examination.
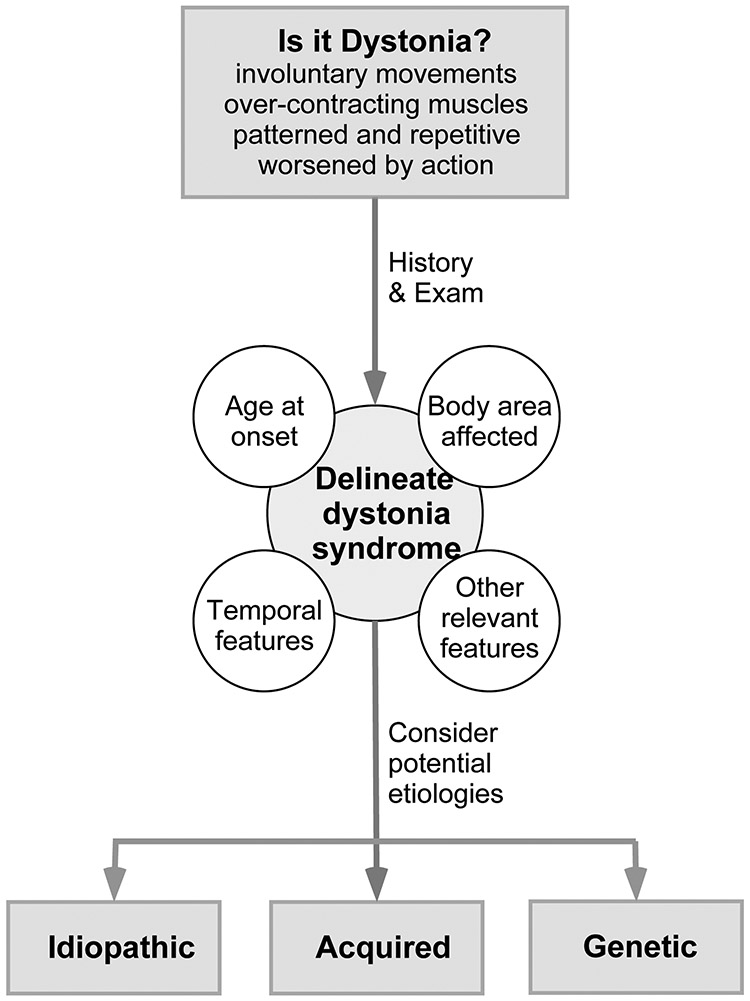
A thorough clinical evaluation is important for guiding therapeutic strategies. The basic overall strategy is described here. A more detailed approach is available in prior reviews.4,6 The clinical evaluation begins with a careful history and examination, focusing on the four clinical features used to classify the dystonias into specific subgroups (Figure 2). The age when dystonia first began provides a guide for potential etiologies. Those that begin in adults are more often idiopathic, while those that begin in children often have an identifiable cause.
Figure 2. Diagnostic approach.
This algorithm provides a basic strategy for the diagnostic approach to most patients with dystonia. The initial step is to determine whether the disorder being considered is a type of dystonia, or something that may mimic dystonia. The next step is to use the history and examination to delineate the dystonia syndrome, using the main clinical features used for classification. The third step is to consider potential etiologies.
The evolution of symptoms over time provides a further guide for potential etiologies. The adult-onset focal dystonias tend to evolve over a period of a few weeks to a few years, and subsequent evolution is slower or hard to detect. 7-13 More rapid evolution over hours or days can point to a functional (psychogenic) cause, a drug effect such as a neuroleptic, a vascular or immunological event, or an inherited disorder such as the syndrome of rapid-onset dystonia-parkinsonism syndrome. Diurnal fluctuations are characteristic of dopa-responsive dystonia, while sudden attacks are more characteristic of the paroxysmal dyskinesias.
The history and exam should also address if dystonia is the only problem, or if there are other relevant problems that are part of a recognizable syndrome.4 The term isolated dystonia (previously called primary dystonia) refers to a disorder where dystonia is the only problem.1,3 Since tremor occurs in approximately half of all patients with dystonia, it is allowed in the current definition of isolated tremor. The term combined dystonia is used when dystonia is one part of a more complex syndrome that includes other relevant neurological or medical problems. For example, dystonia combined with myoclonus can point to one group of etiologies, while dystonia combined with liver disease might point to a different group of etiologies.4
Finally, the body region affected is important to define because it provides an important guide to the therapeutic approach. For example, botulinum toxin (BoNT) often is the treatment of first choice for focal dystonias, while oral medications or surgery are more often used for generalized dystonias.
Laboratory investigations.
For most adult-onset dystonias, laboratory investigations depend on body area affected, temporal features, and any relevant accompanying problems. For most adult-onset focal or segmental dystonias, diagnostic testing is often unfruitful. Electromyography often shows excessive muscle activity, but it is not usually conducted because the findings are not specific to dystonia. Brain imaging is not required, although laryngoscopy is useful to rule out structural defects of the vocal cords in the laryngeal dystonias.14 Brain imaging can be useful to exclude a structural process for hemidystonia and generalized dystonia. Brain imaging can also be helpful for in adult-onset patients when there are additional neurological features, or unusually rapid progression. Genetic testing is not required in idiopathic adult-onset cases, but should be considered if there are other affected family members or the dystonia is part of a syndrome that points to a specific inherited disorder.
For most early-onset dystonias (children and young adults), laboratory testing is almost always conducted, because there is a greater likelihood of finding a cause. Brain imaging is valuable, because it can point to specific structural defects, or specific gray or white matter diseases. The accompanying clinical features and temporal evolution may point to specific syndromes, which can be evaluated by specific tests of blood, urine or cerebrospinal fluid. Genetic testing should be considered when the clinical evaluation points to a specific syndrome that is inherited, or when there are multiple affected family members. When there is a clear correlation between the clinical syndrome and a specific genetic disorder, it is reasonable to conduct focused genetic tests. If the clinical syndrome can be explained by multiple different genes, a dystonia gene panel or clinical exome is more efficient. Serial genetic testing of individual genes should be avoided, because the cost of 2-3 individual genes is often more expensive than a panel that includes nearly 100 genes. It is important to know that commercially available clinical gene panels vary dramatically. Some include all genes known to cause any type of dystonia (~100 genes), while others are selective for only the most common ones (10-12 genes). Recent studies have shown that the larger panels can achieve a molecular diagnosis for 10-30% of patients with dystonia, especially in early-onset cases.15
If a definitive diagnosis is not obtained after appropriate testing, it is useful to remember the “test of time”. Patients should be re-evaluated in follow-up, and the diagnosis reconsidered if and when the initial clinical phenotype evolves.
NON-PHARMACOLOGICAL TREATMENT OPTIONS
Counseling.
Counseling is important for three reasons. First, many patients with dystonia experience a diagnostic odyssey in which they have described their symptoms to multiple doctors over several years without getting a clear diagnosis.16-19 They often try multiple treatments that produce little or no benefit. This process creates the impression that they have some mysterious and incurable disorder. Some counseling is helpful to address this incorrect impression. Patients may be directed to several online resources for more information (Table 1).
Table 1.
Online resources for dystonia
| Type of dystonia | Source | Address |
|---|---|---|
| Blepharospasm and oromandibular syndrome | Benign Essential Blepharospasm Research Foundation | blepharospasm.org |
| All types of dystonia | Dystonia Coalition | rarediseasenetwork.org/cms/dystonia |
| All types of dystonia | Dystonia Medical Research Foundation | dystonia-foundation.org |
| All types dystonia | National Institute of Neurological Disorders and Stroke | ninds.nih.gov/disorders/dystonias |
| Laryngeal dystonia | National Spasmodic Dysphonia Association | dysphonia.org |
| Cervical dystonia | National Spasmodic Torticollis Association | spasmodictorticollis.org |
The second important reason for counseling is that there is a high risk of depression and anxiety in many types of dystonia.20 There also is significant social withdrawal due to embarrassment from overtly stigmatizing symptoms. These problems should be discussed openly and addressed with appropriate referrals if necessary.
The third reason that counseling is important is that finding the ideal treatment regimen often is a trial and error process. Oral medications have unpredictable efficacy and frequent side effects, sometimes requiring serial testing with multiple options, each requiring dose titration. The botulinum toxins require customization of the dose pattern over several treatment cycles, which can take several months. Deep brain stimulation (DBS) requires fine-tuning of stimulator settings, sometimes for 6-12 months. The trial and error approach and the time required to optimize therapies can be frustrating for patients. An open discussion regarding this process is important so that patients have realistic expectations and do not abandon treatment efforts too early.
Physical therapy.
Patients with dystonia often ask for physiotherapy. These procedures seem very intuitive in view of the spasms and pain that result from excessive involuntary muscle contractions. Options frequently requested include strengthening of non-dystonic muscles, “retaining” exercises, and stretching of tight muscles. Many small open trials of various physiotherapy methods have reported positive results for different types of dystonia, but the most rigorous studies have failed to demonstrate any lasting benefits.21,22 Despite the absence of evidence, it is reasonable to offer physiotherapy according to specific patient needs, especially those with severe generalized dystonias who may develop contractures.
Complementary and alternative medicine.
Patients also frequently ask about related procedures such as chiropractic therapy, yoga, meditation, other relaxation or stress reduction programs, acupuncture and dry needling, and others.23 Here again, there is little evidence for long-term benefit, although some patients enjoy these options. Usually there is no harm in these procedures, apart from chiropractic methods which occasionally can be overly aggressive or painful.
PHARMACOLOGICAL TREATMENT OPTIONS
Symptom-based drugs.
Patients with dystonia are offered a wide variety of medications.24-29 Evidence-based reviews have been published,30,31 but none of the available agents has been tested in rigorously controlled clinical trials. Their use is driven largely by anecdotal experience and expert opinion. The available therapeutic options fall into two broad categories. These include symptomatic drugs that are useful for many types of dystonia (Table 2), and drugs that are useful only in special circumstances (Table 3).
Table 2.
Oral medications for symptomatic treatment of dystonia
| Group | Examples | Notes |
|---|---|---|
| Benzodiazepines | alprazolam, chlordiazepoxide, clonazepam, diazepam | typical side effects include impaired concentration, sedation, fatigue, impaired coordination, withdrawal reactions |
| Muscle relaxants | carisoprodol, chlorzoxazone, cyclobenzeprine, metaxolone, methocarbamol, orphenadrine | typical side effects vary by class, but most centrally-acting muscle relaxants can cause some degree of sedation and fatigue |
| Anti-spasticity agents | baclofen, tizanidine | typical side effects include impaired concentration, sedation, fatigue, impaired coordination, dizziness, weakness, withdrawal reactions |
| Anti-cholinergics | benztropine, biperidin, ethopropazine, ophenadrine, procyclidine, trihexyphenidyl | typical side effects include impaired concentration, memory loss, fatigue, dry mouth and eyes, constipation, urinary retention, blurry vision |
| Levodopa | carbidopa/levodopa, benserazide/levodopa | typical side effects include nausea, orthostatic hypotension |
| Others | cannabidiol, cyproheptidine, deutetrabenazine, gabapentin, lithium, mexilitine, nabilone, riluzole, tetrabenazine, valbenazine, zolpidem | these drugs have been reported to be useful in anecdotal reports, so they are not widely used |
Table 3.
Inherited dystonias with specific treatments
| Treatment strategy | Disorders |
|---|---|
| Removal of a toxic substance | cerebrotendinous xanthomatosis manganese transporter deficiencies Niemann-Pick type C Wilson’s disease |
| Vitamins and/or cofactors | abetalipoproteinemia ataxia with vitamin E deficiency biotin-thiamin responsive basal ganglia disease cerebral folate deficiency cobalamin deficiency CoQ10 deficiency Homocystinuria Pyruvate dehydrogenase deficiency |
| Avoidance of triggers | Alternating hemiplegia of childhood biotin-thiamin responsive basal ganglia disease GLUT1 deficiency glutaric aciduria methylmalonic aciduria Propionic acidemia rapid-onset dystonia-parkinsonism |
| Specific medications | AADC deficiency dopa-responsive dystonia episodic ataxia type 2 GLUT1 deficiency MOCS1 deficiency paroxysmal kinesigenic dyskinesia |
| Dietary restrictions | abetalipoproteinemia GLUT1 deficiency homocystinuria maple syrup urine disease methylmalonic aciduria propionic acidemia |
Further details regarding these disorders and their specific treatments can be found in a prior comprehensive review.50
A large international survey of medication use across the adult-onset dystonias revealed the benzodiazepines to be the most commonly prescribed, followed by muscle relaxants, anti-spasticity drugs, anticholinergics, and dopamine-related drugs.25 Among children with dystonia, the most commonly used medications included baclofen and trihexyphenidyl, followed by levodopa and diazepam.29 The benzodiazepines are used by 10-30% of all cases, depending on the type of dystonia.25,29 They include alprazolam, chlordiazepoxide, clonazepam, diazepam and others. They seem to provide at least partial benefits for many patients. Common side effects are sedation and impaired concentration. Other side effects include confusion, depression, and dizziness. The benzodiazepines are known to be habit-forming, and sudden discontinuation can lead to withdrawal effects. As a result, their use must be carefully monitored.
Anti-spasticity agents include baclofen and tizanidine (in addition to the benzodiazepines noted above). They are used by 5-10% of adult dystonia patients,25 and 40-50% of children with dystonia.29 Baclofen is the most common. It is particularly popular in childhood, when there is co-existing spasticity, such as in cerebral palsy.32 For both adults and children, the most common side effects are sedation and impaired concentration. Baclofen can also cause confusion or dizziness. At high doses, sudden discontinuation can lead to serious withdrawal effects. Baclofen also can be administered intrathecally, via indwelling pumps.33 This approach minimizes non-specific side effects. However, the pumps must be refilled from time to time, and occasionally fail or become infected. Unrecognized pump failures, for example caused by a blockage in the tubing, can lead to serious withdrawal reactions.
Drugs that block acetylcholine receptors (muscarinic subtype) are at least partly effective for many subtypes of dystonia.28,29 They include benztropine, biperidin, ethopropazine, orphenadrine, procyclidine and trihexyphenidyl. Trihexyphenidyl is the most commonly used, and high doses are typically required. Although the anticholinergics were among the first to be recognized as useful across many types of dystonia, they are used by only 2-5% of adults with dystonia,25 and 30-40% of children with dystonia.29 A major limitation is that high doses are often required, and side effects become limiting as the dose is titrated. The most concerning side effects in adults involve problems with cognition such as impaired concentration, memory loss, confusion, and sedation. Other frequent side effects include dry mouth or dry eyes, urinary retention, constipation, depression, blurry vision and worsening of narrow-angle glaucoma. There are now multiple studies linking long-term anticholinergic use with dementia, raising concern for chronic use in adults with dystonia.34 It is often claimed that children tolerate high doses better than adults, although impaired performance in school can be difficult to identify in young children,35,36 and the risk of late life dementia following chronic early exposure is unknown. In one study, more than 50% of children taking trihexyphenidyl experienced dose-limiting side effects.29
Muscle relaxant drugs also intuitively seem attractive, because dystonia involves excessive muscle contractions leading to soreness. They are often prescribed by primary care providers and the are used by 5-10% of adult patients, rarely in children. The drugs included in this category include carisoprodol, chlorzoxazone, cyclobenzeprine, metaxalone, methocarbamol, and others.37 Side effects vary among these options, but all are centrally acting. As a result, some degree of sedation or fatigue is common, except perhaps for metaxolone. Carisoprodol is a controlled substance (schedule IV) and therefore requires special attention. Many patients seem to benefit at least partially, especially when there is pain and soreness from muscle spasms. However, patients should be counseled that most muscle relaxants have been approved for only short-term use (2-3 weeks), and the potential long-term effects of chronic use are not well known.
Drugs for special circumstances.
This category includes drugs that target a mechanism known to cause a specific type of dystonia. The most well-known in this class is levodopa, which is highly effective for dopa-responsive dystonia.38-40. Because of its remarkable efficacy, a levodopa trial is mandatory for all children and young adults with unexplained dystonia, including those with a diagnosis of cerebral palsy. Children may respond to doses as low as half of a 25/100 mg tablet of carbidopa/levodopa two times daily. Larger doses are sometimes required, so an adequate trial must titrate the dose to 20 mg/kg daily divided across 3-4 daily doses for at least a month. It is not uncommon for adult neurologists to assume the care of children with cerebral palsy when they reach adulthood, and a repeat trial of levodopa is often required because of inadequate documentation of a levodopa trial, or inadequate dose titration. In addition to dopa-responsive dystonia, levodopa can sometimes be helpful for dystonia in other disorders. They ataxia telangiectasia, spinocerebellar ataxia type 3, Parkinson’s disease, and others.41,42 Levodopa is not typically used for the more common adult-onset focal dystonias,43 although occasional patients do respond.44
Another well-known treatment strategy involving drugs that target a specific mechanism involves copper-lowering agents for Wilson’s disease, which results from an inherited defect in a copper transporter leading to toxic copper accumulation.45 Penecillamine and tetrathiomolybdenate are copper chelators that leech copper from the body, and zinc is a naturally occurring heaving metal that reduces copper absorption from the gastrointestinal tract. Treatment strategies depend on both severity and features of disease, and there remains significant controversy regarding the optimal approach.46 A series of related disorders recently have been described with toxic manganese accumulation due to manganese transporter defects. These disorders have a presentation very similar to Wilson’s disease, and they respond to manganese chelators.47-49
Although many reviews addressing special treatments for specific forms dystonia focus on dopa-responsive dystonia and Wilson’s disease, the list of options is much longer. A recent review described more than 30 inherited disorders with disease-specific treatments, and more than half of them included dystonia, either in isolation or as part of a multi-system disorder.50 Only a few examples are highlighted here, with a more complete list of the disorders and their treatment approach is provided in Table 3. For example, several disorders that usually present in early childhood dystonia as one part of a multisystem syndrome can be treated by supplementation with vitamins or essential cofactors. Examples include biotin-thiamine responsive basal ganglia disease, biotinidase deficiency, and ataxia with vitamin E deficiency. Other disorders can be managed with dietary changes. Examples include GLUT1 deficiency syndromes, maple syrup urine disease, and abetalipoproteinemia. Dystonic spasms in paroxysmal kinesigenic dyskinesia respond extraordinarily well to very low doses of carbamazepine and related anticonvulsants. Although all of these disorders are quite rare, early diagnosis is critical because treatments can be life-altering. For many, late diagnosis can lead to irreversible neurological defects.
Another category of treatable dystonias include autoimmune disorders, some of which have identifiable antibodies (Table 4).51,52 One of the most well-known examples is Sydenham’s chorea, where chorea usually predominates. However, many children with Sydenham’s chorea also have dystonic posturing, and dystonia may be the dominant problem. Antibacterial therapy is important. For most of the other disorders, typically dystonia is part of a multisystem disorder, sometimes combined with encephalopathy. Once again, early diagnosis is valuable because many are associated with underlying malignancy. Treatment of the malignancy is essential, and may sometimes be combined with immunomodulatory agents.
Table 4.
Dystonia associated with antibodies
| Anbody | Typical age at onset |
Clinical features | Association with malignancy |
|---|---|---|---|
| AQP4 | children or adults | tonic spasms may resemble paroxysmal dystonia; often combined with optic neuritis and/or myelitis | sometimes |
| CV2 or CRMP5 | children or adults | dystonia may be combined with chorea, parkinsonism, optic neuritis, myelitis and encephalopathy | frequent |
| D2R | children | dystonia may be combined with chorea or parkinsonism, in setting of encephalopathy that includes prominent behavioral and sleep disturbances | not usually |
| GAD or amphiphysin | adults | stiff-person syndrome may present with a syndrome that is easily mistaken for dystonia | occasional |
| GABAAR | children or adults | dystonia may be combined with chorea ataxia, sometimes opsoclonus-myoclonus in setting of broader encephalopathy | frequent |
| LGI1 | adults | faciobrachial dystonic seizures may resemble paroxysmal segmental dystonia, often with encephalopathy and sleep disorder | occasional |
| Ma2 | children or adults | dystonia may be combined with parkinsonism and sleep disorder in setting of broader encephalopathy | frequent |
| NMDAR | children or adults | dystonia may paroxysmal or combined with chorea, ataxia, myoclonus, parkinsonism, oculogyric crises in setting of broader encephalopathy | frequent |
| Ri or ANNA-2 | adults | dystonia may be combined with ataxia, myoclonus, rigidity, opsoclonus and cranial nerve signs of brainstem encephalitis | frequent |
LOCAL TREATMENT OPTIONS
Overview of botulinum toxins.
The most common forms of dystonia emerge in adults and affect a limited number of muscles. The treatment of choice for many of these focal dystonias is local injection of botulinum neurotoxin (BoNT). These treatments can also be applied to the most troublesome areas among patients with broader muscle involvement in generalized dystonias, such as the abnormal head/neck movements or tight leg muscles often seen dyskinetic cerebral palsy.
The BoNTs are synthetic derivatives of naturally-occurring toxins made by the bacterium Clostridium botulinum, the cause of botulism. Seven serotypes are known, and two have been developed as therapeutics, serotypes A and B (Table 5). The most commonly available formulations of serotype A include abobotulinumtoxinA (Dysport™), incobotulinumtoxinA (Xeomin™), and onabotulinumtoxinA (Botox™). Serotype B is available as rimabotulinumtoxinB (Myobloc™). Several less widely available formulations are available in specific parts of the world. While doses vary among the BoNT products, their overall effects are similar.
Table 5.
Most commonly used botulinum toxin preparations
| Onabotulinum toxinA |
Abobotulinum toxinA |
Incobotulinum toxinA |
Rimabotulinu toxinB |
|
|---|---|---|---|---|
| Trade name | Botox™ | Dysport™ | Xeomin™ | Myobloc™ |
| Vial supplied | vacuum dried | freeze dried | powder | liquid |
| Doses available (units) | 100, 200 | 300, 500 | 50, 100 | 1000, 2500, 5000 |
| Storage temperature | refrigerate | refrigerate | room temperature | refrigerate |
| Dose equivalents* | 1 | 2.5 – 3 | 1 | 40 |
As the first FDA-approved BoNT, onabotulinumtoxinA was defined as having a standard strength of 1 unit. The approximate dose-equivalents for the others are compared against this value.
For many types of dystonia, the BoNTs reduce abnormal movements, pain and spasms, and disability. The doses and muscles injected must be customized for each patient. Benefits emerge within a week, and they last for approximately 12 weeks.53 The utility of BoNT has been summarized in several systematic and evidence-based reviews.54-57 There are also many practical guides describing muscle selection and dosing.58,59 Only the most common applications are described below.
Cervical dystonia (torticollis).
Numerous studies have demonstrated the long-term efficacy of BoNT for cervical dystonia.55,56,60 The BoNTs attenuate abnormal head movements, they reduce neck pain, and they improve overall quality of life. While good responses can be achieved in the vast majority of patients with cervical dystonia, there are a few subtypes of cervical dystonia where it can be very difficult to get satisfactory results, such as those with prominent anterocollis, tremor-dominant cervical dystonia, or longstanding abnormal postures.61,62
Side effects are usually minor and transient. Dysphagia can result when large doses applied to anterior neck muscles (sternocleidomastoids and scalenes) spread to nearby muscles of swallowing in the pharynx. Neck extensor weakness (head drop) can occur when large doses are applied to posterior neck muscles (semispinalis capitus, splenius cervicus) that hold up the head. A systemic flu-like syndrome may also occur in the minority of patients.63
Craniofacial dystonia (blepharospasm and Meige syndrome).
Many studies also have demonstrated the long-term efficacy of BoNT for blepharospasm.55,60 The BoNTs reduce spasms of eye closure and excessive blinking, and they improve overall quality of life. The vast majority of patients see good effects, with only minor or transient side effects. Ptosis or diplopia can occur from spread of high doses applied to the upper middle eye region, or from inadvertent injections that are too deep and fall behind the fascial plane that separates anterior eyelid muscles (orbicularis oculi) from posterior muscles (levator palpebra and muscles controlling eye movements).
Patients with blepharospasm sometimes develop apraxia of eyelid opening, a phenomenon that refers to inability to open the eyes without apparent spasm. Eyelid opening apraxia may also be the sole manifestation of blepharospasm. This phenomenon may be caused by spasms of the eyelid muscles, which are not overtly visible on exam. Good responses are more difficult to achieve in these patients, unless the eyelid muscles are treated too.64-66
Approximately half of patients with blepharospasm experience spread of muscle spasms to the lower face, jaw, and/or tongue.9,12,13 The combination of dystonia in the upper face and oromandibular regions is sometimes called “Meige syndrome”. Occasionally, the oromandibular regions are affected in isolation.67,68 Although many neurologists are reluctant to treat the lower face and jaw, good outcomes can be achieved with proper dosing and muscle patterns.68-71
Limb dystonias (writer’s cramp).
The most common limb dystonias in adults involve the hand and/or arm and present as task-specific disorders such as writer’s cramp and muscian’s dystonias. In children, the lower limb is more often involved, and most often result from cerebral palsy or an inherited disorder. Achieving satisfactory results for limb dystonias is possible, but not as straightforward as treatment of the craniofacial dystonias.72 In the limbs, special attention must be directed towards distinguishing dystonic muscles from compensatory ones. The most common side effects from treating limb dystonias is weakness, which results from doses that are too high or treatment of compensatory muscles.
Laryngeal dystonias (spasmodic dysphonia).
BoNTs are widely used for the treatment of the laryngeal dystonias.73-75 Reliably good responses can be expected for the adductor spasmodic dysphonia, with reduction in voice breaks, reduction in speaking effort, and increased quality of life. A very common side effect is a hoarse or weak voice following treatment. Dysphagia is less common, but can be severe. Satisfactory responses are more challenging to obtain for abductor spasmodic dysphonia, or when involvement spreads to the pharyngeal or respiratory muscles.
SURGICAL TREATMENT OPTIONS
Overview.
Surgical interventions can be offered when satisfactory responses cannot be achieved with BoNT and/or oral medications. The surgical options are grouped here into two main categories; deep brain stimulation (DBS) and ablative procedures. In both cases, access to a multidisciplinary team of experts is required for patient selection and management of potential complications.
Deep brain stimulation (DBS).
A thorough presurgical evaluation is valuable, because the outcomes of DBS depend on careful patient selection.76-78 Etiology plays an important role in predicting outcomes, which are reliably good for some causes and reliably poor for others. It is often claimed that isolated dystonias respond more reliably to DBS than dystonias combined with other neurological features. This view is oversimplified. For example, good outcomes are can be reliably anticipated for isolated dystonia caused by defects in the TOR1A gene (DYT1), but outcomes for isolated dystonia caused by defects in THAP1 gene (DYT6) are less predictable.79 Good outcomes also can be expected for some combined dystonia syndromes such as myoclonus-dystonia (SCGE gene) and X-linked dystonia-parkinsonism (TAF1 gene), but not for rapid-onset dystonia-parkinsonism (ATP1A3 gene).80
Etiology also influences outcomes of DBS in acquired forms of dystonia. For example, reliably good outcomes can be anticipated for tardive dystonia due to neuroleptics. On the other hand, outcomes for dystonic cerebral palsy are less reliable.35 Knowing etiologies, both genetic and acquired, is therefore critical for proper counseling regarding potential risks and benefits. Patients who have etiologies for their dystonia that are known to be associated with unpredictable outcomes should be counseled so they can make a fully informed decision.
Even when etiology cannot be determined, certain clinical characteristics predict outcomes from DBS. In general, patients with mobile dystonia fare better than those with fixed postures associated with underlying contractures. Patients with short disease durations fare better than those with longstanding dystonia. Patients who are younger fare better than those who are older, although very young patients (<12 years old) experience higher rates of complications such as migration of leads or infections of the equipment. Other characteristics associated with poorer outcomes from DBS include significant medical comorbidities that increase risk for any surgery, significant psychiatric comorbidities that increase risk for suicide or non-compliance, advanced or progressive dementia, patients who live far from a DBS center and may have difficulty with frequent travel, and dystonia syndromes that are combined with significant spasticity or ataxia.
With proper patient selection, long-term outcomes for DBS are good, with benefits lasting for many years.81-83 Long-term complications are not uncommon, so close monitoring by the DBS team is important. For example, one study of 47 cases with dystonia due to defects in the TOR1A gene (DYT1) followed for more than 10 years reported an 8.5% incidence of delayed equipment infections requiring antibiotic treatment or re-operation, an 8.5% incidence of equipment failure requiring re-operation, and a 4.3% incidence of re-operations to reposition electrodes.84
Ablation procedures.
Currently, DBS is the most commonly used surgical method for all subtypes of dystonia, because it is reversible and tunable. However, prior to DBS, specific brain regions were commonly ablated. Ablative procedures are experiencing a renaissance, because of steady improvements in delineating the subtypes of dystonia and brain regions where reliably good outcomes can be expected.77,78 In fact, ablative procedures may be preferable to DBS in some circumstances.
Small lesions of the thalamus appear to be safe and reliable for certain task-specific dystonias of the upper limb.85,86 With a permanent result that has few apparent side effects, such lesions may be preferable over DBS. There is less risk of infection with ablation, both immediately and in the long term. Ablation also may be preferred by particularly small or young patients who do not want the hassle of permanent indwelling DBS hardware. Permanent ablation may also be a reasonable option for patients who cannot make frequent return visits for DBS management, patients who cannot afford DBS surgery, or as a palliative procedure for progressive neurodegenerative disorders with severe disability.
The most common method for surgical ablation involves making an electrolytic lesion through a temporarily placed electrode. Focused ultrasound has recently been shown to be effective as an ablative tool for tremor, and some have advocated using it for dystonia too87. However, experience with focused ultrasound for is still limited, and outcomes seem to vary. Although focused ultrasound is sometimes claimed to be superior to traditional surgical methods because it is “non-invasive”, it is important to emphasize that it still produces a brain lesion, along with the usual potentially permanent side effects.
Ablative surgical procedures targeting peripheral nerves or nerve roots were commonly used for dystonia in the past and are still sometimes offered in some situations. Selective peripheral denervation can be done for patients with cervical dystonia for medically resistant patients who do not want DBS. Success rates for selective peripheral denervation are similar to those for DBS.88,89 Typical side effects include local scarring, local muscle atrophy and weakness, dysphagia, and dysesthesia or hypoesthesia relating to destruction of sensory nerves to the neck.
Several related surgical procedures may be offered to patients with blepharospasm. They include suspension of the frontalis, surgical shortening of the levator palpebrae, removal of excess eyelid skin, and stripping of orbicularis oculi muscles.90-92 Only a minority of patients with blepharospasm have these procedures, because they are offered only by a few centers. However, it is good to jnow about them because patients may ask. In general, experience is limited, and there are no methodical studies that address their long-term safety and efficacy.
Several surgical procedures are sometimes offered to patients with laryngeal dystonia as well.93,94 Most of these aim at re-organizing the nerves or muscles of the larynx. Once again, experience is limited, and there are no large-scale studies demonstrating the long-term efficacy and safety of these procedures.
EXPERIMENTAL THERAPEUTICS AND THE FUTURE OF TREATMENT
Unmet needs.
Oral agents currently available have limited efficacy for most dystonias, except for a few subtypes of dystonia where there are mechanism-specific drugs. BoNT can be very effective, but it is not suitable for all types of dystonia. In addition, there has increasing awareness that approximately a third of patients discontinue BoNT.95 Similarly, some patients with dystonia are not suitable surgical candidates, or they don’t want surgery. Because of the increasing awareness of a significant unmet need, and special FDA incentives for developing new treatments for rare disorders, there has been an increase in enthusiasm for developing new treatments for different types of dystonia.96
Clinicaltrials.gov listed 161 active or recently completed interventional trials for dystonia in October 2019 (clinicaltrials.gov). Included were trials of oral agents, trials of a novel BoNT and different ways of using existing BoNTs, trials involving DBS, and trials involving non-invasive brain stimulation. For example, there are several ongoing or recently completed trials including ampicillin for dystonia caused by the TOR1A gene, cannabis for pediatric dystonias, levetiracetam for cervical or oromandibular dystonia, perampanel for cervical dystonia, sodium oxybate for laryngeal dystonia, and zonegran for myoclonus-dystonia.
Oral medications.
The anticholinergics have broad efficacy across many types of dystonia, but their use is limited by non-specific side effects. Most of these drugs non-selectively target all four major subclasses of muscarinic receptors, and have off-target effects too. These observations have stimulated interest in developing anticholingerics that may have fewer side effects, by more specifically targeting the muscarinic receptor subtypes most likely to be relevant to dystonia.97 There also has been increasing interest in the relationship between dystonia and Parkinson disease. Some levodopa-induced dyskinesias have a dystonic quality, so drugs being developed to treat these dyskinesias may be repurposed for some types of dystonia.98
In addition to the development of potential new drugs with broad symptomatic effects, the remarkable advances in delineating genetic mechanisms for rare subtypes of dystonia has opened the door to new mechanism-specific interventions. For example, pantothenate-kinase associated neurodegeneration has been linked with defects in the synthesis of coenzymeA and toxic accumulation of brain iron.99 In a recent large double-blind, placebo-controlled trial, the iron chelator deferiprone significantly reduced brain iron stores in patients with this disorder.100 Clinical improvement in dystonia was not clinically significant, but the duration of the trial may have been insufficient to detect a disease-modifying effect. Another large randomized double-blinded, placebo-controlled clinical trial using a synthetic precursor of coenzymeA was recently completed (clinicaltrials.gov NCT03041116), and additional trials are anticipated. It therefore seems likely that new mechanism-specific therapeutics will be available for this devastating disorder. Similar advances are occurring in other disorders.
Botulinum toxins.
A new formulation of BoNT (daxibotulinumtoxinA) is being evaluated in clinical trials now (clinicaltrials.gov NCT03608397). In addition to this potential new BoNT, there have been advances in our understanding of the optimal strategies for application of existing BoNTs. For example, the increasing awareness that approximately a third of patients discontinue BoNT95 has stimulated interest in better understanding the reasons for discontinuation. One of the main reasons given by patients is apparently poor efficacy, which is sometimes attributed to BoNT resistance. Although measurable titers of antibodies to BoNT can be detected in many patients who are treated with BoNT,101 true immunologically-mediated resistance has become quite uncommon with current manufacturing processes.102 Instead, several studies have indicated that the more common reason for poor responses is due to inadequate dosing or suboptimal muscle selection.61,103,104 Increasing awareness of the reasons for apparently poor efficacy has pointed to the need for more rigorous training in the optimal use of the BoNTs.
Another reason for poor patient satisfaction can be related to the duration of benefit of BoNT. Because the average duration of benefit is approximately 12 weeks, most patients are traditionally scheduled for re-treatment at fixed intervals of 12 weeks.105 However, 12 weeks is an average, and the range varies from 8-16 weeks. Providing a fixed interval of 12 weeks for all patients leads some to experience significant fluctuations in symptom severity, a phenomenon that has been dubbed the “yoyo” effect.96 Thus there has been increasing appreciation that the dosing interval needs to be customized, along with the dose and muscle pattern.106
The need for EMG guidance during BoNT procedures has been controversial for a long time. Some experts advocate EMG for nearly all procedures, while others advocate reliance on the clinical examination. Available studies comparing outcomes with and without EMG are inconclusive.56 In our experience, EMG is almost never required for blepharospasm, because subcutaneous injections readily diffuse to the thin underlying muscles of the face. EMG often is required for dystonia involving the hand, because of the large number of small muscles that are grouped closely together in the hand and forearm. EMG is required only in a minority of cases of cervical dystonia, for example those with particularly thick necks, deep muscle involvement, or very complex movement patterns. More recently, ultrasound guidance has been gaining in popularity, because muscles can be more precisely visualized.107 The place of ultrasound guidance for the various types of dystonia is still being explored.
Deep brain stimulation.
Significant advances in DBS therapy are anticipated in the next few years. For example, the most common target for dystonia has been the internal segment of the globus pallidus. However, other targets are being explored now, such as the subthalamic nucleus.108 As our understanding of the neuroanatomical basis of dystonia has expanded, targets outside of the basal ganglia are being considered too.109,110 The exploration of other targets may provide new surgical options for subtypes of dystonia that don’t respond reliably to current strategies.
There have also been technological advances including development of batteries that have a longer lifespans or are re-chargeable, and smaller impulse generators suitable for small children or those who are particularly thin. These advances will minimize re-operations and surgical complications. There have also been advances in directional electrodes that can be aimed more precisely at their targets to limit side effects from non-specific stimulation. Adaptive stimulation that responds to specific physiological triggers may also provide greater efficacy and extend battery life by delivering stimulation only when it is needed.77,78 All of these advances are likely to improve long-term surgical outcomes.
In addition to DBS, there has been increasing interest in non-invasive brain stimulation techniques, including transcranial magnetic stimulation and transcranial direct current stimulation.111 Some positive results have been reported for small and unblinded trials, and larger trials are planned.
PUTTING IT ALL TOGETHER
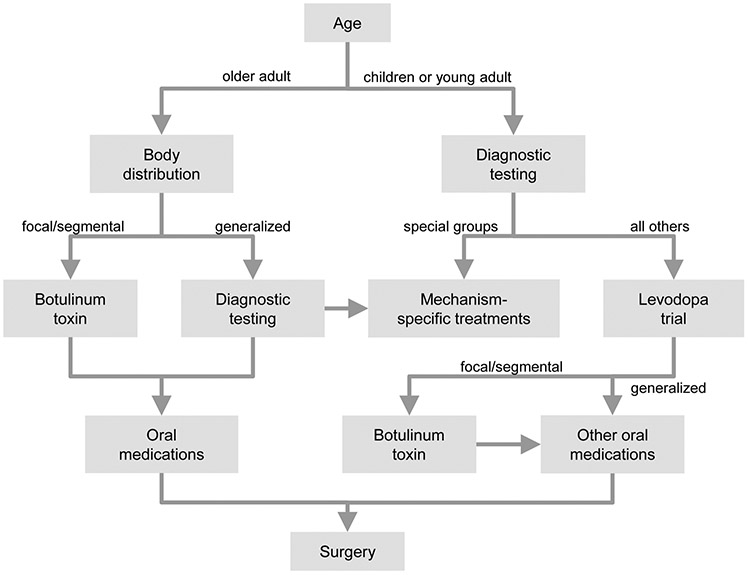
Simple algorithms that describe a universal approach for addressing all dystonias are challenging to construct, because of the remarkable heterogeneity of the dystonias. However, a basic algorithmic approach with some general principles can be provided (Figure 3), with the caveat that real-life management must be done on a case-by-case basis.
Figure 3. Treatment approach.
This algorithm provides a basic strategy for the therapeutic approach to most patients with dystonia. The clinical and etiological evaluations are important, because They influence optimal treatments strategies. For children, diagnostic testing is essential because there is a greater likelihood of discovering a treatable cause. When a cause cannot be found, a trial of oral levodopa is conducted to rule out dopa-responsive dystonia. For adults, dystonia is more often idiopathic, so extensive workup is often not needed. Instead, the approach depends on body distribution. For the focal dystonias, botulinum toxins provide a useful starting point. Oral medications can be used, but often are not well-tolerated. Those who have unsatisfactory responses can be offered surgery. Generalized dystonias or dystonia combined with other neurological problems in adults requires some additional diagnostic testing, to disclose a potentially treatable cause.
A thorough diagnostic evaluation is an important starting place, including attention to all 4 of the factors relevant for clinical classification. These factors include age at onset, body region affected, temporal features, and associated features. Age at onset plays an important starting role. For most adults, dystonia is idiopathic, so extensive diagnostic testing is often not conducted. Instead, the approach to treatment depends more on body distribution. For focal and segmental dystonias, the BoNTs are the treatment of first choice, sometimes with adjunctive oral agents, especially for the troughs in the cyclical responses to BoNT over time. When BoNT plus oral agents are insufficient, DBS should be considered. Generalized and hemidystonias are uncommon in adults, and some additional diagnostic testing with brain MRI may be required. BoNT is rarely adequate, so oral agents and DBS play a more important role.
For children, some diagnostic testing is valuable because the chances of finding an etiology are greater, and certain populations have specific treatments. Brain imaging is important to disclose particularly categories of disease, such as focal defects or grey or white matter diseases. Other diagnostic tests will depend on the nature of the dystonia syndrome. All children and young adults should be given a trial of levodopa to address dopa-responsive dystonia. If patients do not respond to levodopa, other oral agents and botulinum toxin can be considered. DBS can be considered when more conservative measures fail.
LONG-TERM OUTCOMES
Long-term prognoses vary among the different subtypes of dystonia. The most common subtypes include the adult-onset focal dystonias. For these patients, progression is most obvious in the first 3-6 months, although continued progression may occur for years.7-13 The 5-year risk of spread from the originally affected area is 10-20% for cervical dystonia, limb dystonias, and laryngeal dystonias. The 5 year risk of spread for blepharospasm is approximately 50%. Complete remission occurs in 5-10% of the adult-onset focal dystonias, but remission usually is temporary.112 Despite the chronic and sometimes progressive nature of the adult-onset focal dystonias, adequate symptom control is feasible in the majority with BoNT, oral medications, and rarely surgical intervention.
The childhood-onset dystonias are far less common. Progression to segmental and generalized dystonia is more frequent, and usually occurs over a period of several years. Further progression in adulthood is less common. For rare subtypes of the early-onset dystonias, there are specific mechanism-based oral medications that can have a dramatic effect, if started early.50,51 When mechanism-specific treatments are not available, symptom-based oral medications and BoNT can be helpful, and surgical intervention is more frequently required.
In summary, all dystonias are treatable. The majority benefit at least partly from symptom-based management, and several rare subtypes have specific treatments addressing the underlying disease mechanisms. For both adults and children, the list of treatment options is likely to grow in the near future, as ongoing research improves our ability to exploit existing therapies, and uncovers additional causes and biological mechanisms where new interventions can be designed.96
KEY POINTS.
The dystonias are defined by excessive muscle contractions leading to involuntary postures and/or repetitive abnormal movements.
The clinical manifestations of the dystonias are very varied. These manifestations are grouped according to four clinical characteristics which include age at onset, body region affected, changes over time, and whether or not there are relevant accompanying neurological or medical features.
The etiologies for the dystonias also are very varied. Most adult-onset cases are idiopathic, while most childhood-onset cases have a discoverable cause. There are many known causes, both inherited and acquired.
Treatments are tailored to the clinical manifestations and the etiology, if known. They include oral medications, botulinum toxin injections, and surgical procedures.
SYNOPSIS.
The dystonias are a large and heterogenous group of disorders characterized by excessive muscle contractions leading to abnormal postures and/or repetitive movements. Their clinical manifestations vary widely, and there are many potential causes. Despite the heterogeneity, helpful treatments are available for the vast majority of patients. Symptom-based therapies include oral medications, intramuscular botulinum toxins, and surgical interventions. For some subtypes of dystonia, specific mechanism-based treatments are available too. Advances in understanding the biological basis for many types of dystonia have led to numerous recent clinical trials, so additional treatments are likely to become available in the very near future.
Acknowledgments
DISCLOSURE STATEMENT
H. A. Jinnah has active or recent grant support from the US government (National Institutes of Health), private philanthropic organizations (the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now) and industry (Cavion Therapeutics, Ipsen Pharmaceuticals, Retrophin Inc., Revance). Dr. Jinnah has also served on advisory boards or as a consultant for Allergan Inc., CoA Therapeutics, Medtronic Inc., and Retrophin Inc. He has received honoraria or stipends for lectures or administrative work from the American Academy of Neurology, the American Neurological Association, the Dystonia Medical Research Foundation, the International Neurotoxin Society, the International Parkinson’s Disease and Movement Disorders Society, The Parkinson’s Disease Foundation, and Tyler’s Hope for a Cure. Dr. Jinnah serves on the Scientific Advisory Boards for several private foundations including the Benign Essential Blepharospasm Research Foundation, Cure Dystonia Now, the Dystonia Medical Research Foundation, the Tourette Association of America, and Tyler's Hope for a Cure. He also is principle investigator for the Dystonia Coalition, which has received the majority of its support through NIH grants NS116025 and NS065701 from the National Institutes of Neurological Disorders and Stroke and previously grant TR 001456 from the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences. The Dystonia Coalition has received additional material or administrative support from industry sponsors (Allergan Inc. and Merz Pharmaceuticals) as well as private foundations (The American Dystonia Society, Beat Dystonia, The Benign Essential Blepharospasm Foundation, Cure Dystonia Now, Dystonia Europe, Dystonia Inc., Dystonia Ireland, The Dystonia Medical Research Foundation, The Foundation for Dystonia Research, The National Spasmodic Dysphonia Association, and The National Spasmodic Torticollis Association).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: A consensus update. Mov Disord. 2013;28:863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balint B, Mencacci NE, Valente EM, et al. Dystonia. Nat Rev Dis Primers. 2018;4:25. [DOI] [PubMed] [Google Scholar]
- 3.Jinnah HA, Albanese A. The new classification for the dystonias: Why was it needed and how was it accomplished? Mov Disord Clin Pract. 2014;1:280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung VS, Jinnah HA, Bhatia K, Vidailhet M. Assessment of the patient with dystonia: An update on dystonia syndromes. Mov Disord. 2013;28:889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinnah HA, Sun YV. Dystonia genes and their biological pathways. Neurobiol Dis. 2019;in press. [DOI] [PubMed] [Google Scholar]
- 6.Jinnah HA. The dystonias. Continuum. 2019;in press. [DOI] [PubMed] [Google Scholar]
- 7.Esposito M, Fabbrini G, Ferrazzano G, et al. Spread of dystonia in patients with idiopathic adult-onset laryngeal dystonia. Eur J Neurol. 2018;25:1341–1344. [DOI] [PubMed] [Google Scholar]
- 8.Norris SA, Jinnah HA, Espay AJ, et al. Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov Disord. 2016;31:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svetel M, Pekmezovic T, Tomic A, Kresojevic N, Kostic VS. The spread of primary late-onset focal dystonia in a long-term follow up study. Clin Neurol Neurosurg. 2015;132:41–43. [DOI] [PubMed] [Google Scholar]
- 10.Martino D, Berardelli A, Abbruzzese G, et al. Age at onset and symptom spread in primary adult-onset blepharospasm and cervical dystonia. Mov Disord. 2012;27:1447–1450. [DOI] [PubMed] [Google Scholar]
- 11.Svetel M, Pekmezovic T, Jovic J, et al. Spread of primary dystonia in relation to initially affected region. J Neurol. 2007;254:879–883. [DOI] [PubMed] [Google Scholar]
- 12.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. [DOI] [PubMed] [Google Scholar]
- 13.Abbruzzese G, Berardelli A, Girlanda P, et al. Long-term assessment of the risk of spread in primary late-onset focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:392–396. [DOI] [PubMed] [Google Scholar]
- 14.Ludlow CL, Domangue R, Sharma D, et al. Consensus-based attributes for identifying patients with spasmodic dysphonia and other voice disorders. JAMA Otolaryngol Head Neck Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powis Z, Towne MC, Hagman KDF, et al. Clinical diagnostic exome sequencing in dystonia: Genetic testing challenges for complex conditions. Clin Genet. 2019;in press. [DOI] [PubMed] [Google Scholar]
- 16.Macerollo A, Superbo M, Gigante AF, Livrea P, Defazio G. Diagnostic delay in adult-onset dystonia: Data from an Italian movement disorder center. J Clin Neurosci. 2015;22:608–610. [DOI] [PubMed] [Google Scholar]
- 17.Creighton FXJ, Hapner ER, Klein AM, Rosen A, Jinnah HA, M.M. J Diagnostic delays in spasmodic dysphonia: A call for clinician education. J Voice. 2015;29:592–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertram KL, Williams DR. Delays to the diagnosis of cervical dystonia. J Clin Neurosci. 2015;25:62–64. [DOI] [PubMed] [Google Scholar]
- 19.Tiderington E, Goodman EM, Rosen AR, et al. How long does it take to diagnose cervical dystonia? J Neurol Sci. 2013;335:72–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zurowski M, Marsh L, McDonald W. Psychiatric comorbidities in dystonia: Emerging concepts. Mov Disord. 2013;28:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Pauw J, Van der Velden K, Meirte J, et al. The effectiveness of physiotherapy for cervical dystonia: a systematic literature review. J Neurol. 2014. [DOI] [PubMed] [Google Scholar]
- 22.Prudente CN, Zetterberg L, Bring A, Bradnam L, Kimberley TJ. Systematic review of rehabilitation in focal dystonias: Classification and recommendations. Mov Disord Clin Pract. 2018;5:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming BM, Schwab EL, Nouer SS, Wan JY, LeDoux MS. Prevalence, predictors, and perceived effectiveness of complementary, alternative and integrative medicine in adult-onset primary dystonia. Parkinsonism Relat Disord. 2012;18:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jankovic J Medical treatment of dystonia. Mov Disord. 2013;28:1001–1012. [DOI] [PubMed] [Google Scholar]
- 25.Pirio Richardson S, Wegele AR, Skipper B, Deligtisch A, Jinnah HA. Dystonia treatment: Patterns of medication use in an international cohort. Neurology. 2017;88:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jinnah HA, Factor S. Diagnosis and treatment of dystonia In: Jankovic J, ed. Neurologic Clinics. Vol 33: Elsevier; 2015:77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thenganatt MA, Jankovic J. Treatment of dystonia. Neurotherapeutics. 2014;11:139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene P, Shale H, Fahn S. Experience with high dosages of anticholinergic and other drugs in the treatment of torsion dystonia. Adv Neurol. 1988;50:547–556. [PubMed] [Google Scholar]
- 29.Lumsden DE, Kaminska M, Tomlin S, Lin JP. Medication use in childhood dystonia. Eur J Paediatr Neurol. 2016;20:625–629. [DOI] [PubMed] [Google Scholar]
- 30.Albanese A, Barnes MP, Bhatia KP, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: repor of an EFNS/MDS-ES task force. Eur J Neurol. 2006;13:433–444. [DOI] [PubMed] [Google Scholar]
- 31.Balash Y, Giladi N. Efficacy of pharmacological treatment of dystonia: evidence-based review including meta-anaylsis of the effect of botulinum toxin and other cure options. Eur J Neurol. 2004;11:361–370. [DOI] [PubMed] [Google Scholar]
- 32.Greene P Baclofen in the treatment of dystonia. Clin Neuropharmacol. 1992;15:276–288. [DOI] [PubMed] [Google Scholar]
- 33.Bonouvrie LA, Becher JG, Vles JSH, Vermeulen RJ, Buizer AI, Group IS. The effect of intrathecal baclofen in dyskinetic cerebral palsy: The IDYS trial. Ann Neurol. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andre L, Gallini A, Montastruc F, et al. Association between anticholinergic (atropinic) drug exposure and cognitive function in longitudinal studies among individuals over 50 years old: a systematic review. Eur J Clin Pharmacol. 2019;in press. [DOI] [PubMed] [Google Scholar]
- 35.Fehlings D, Brown L, Harvey A, et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60:356–366. [DOI] [PubMed] [Google Scholar]
- 36.Harvey AR, Baker LB, Reddihough DS, Scheinberg A, Williams K. Trihexyphenidyl for dystonia in cerebral palsy. Cochrane Database Syst Rev. 2018;5:CD012430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.See S, Ginzburg R. Choosing a skeletal muscle relaxant. Am Fam Physician. 2008;78:365–370. [PubMed] [Google Scholar]
- 38.Wijemanne S, Jankovic J. Dopa-responsive dystonia--clinical and genetic heterogeneity. Nat Rev Neurol. 2015;11:414–424. [DOI] [PubMed] [Google Scholar]
- 39.Lee WW, Jeon BS. Clinical spectrum of dopa-responsive dystonia and related disorders. Curr Neurol Neurosci Rep. 2014;14:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurian MA, Gissen P, Smith M, Heales S Jr., Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–733. [DOI] [PubMed] [Google Scholar]
- 41.Charlesworth G, Mohire MD, Schneider SA, Stamelou M, Wood NW, Bhatia KP. Ataxia telangiectasia presenting as dopa-responsive cervical dystonia. Neurology. 2013;81:1148–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilder-Smith E, Tan EK, Law HY, Zhao Y, Ng I, Wong MC. Spinocerebellar ataxia type 3 presenting as an L-DOPA responsive dystonia phenotype in a Chinese family. J Neurol Sci. 2003;213:25–28. [DOI] [PubMed] [Google Scholar]
- 43.Lang AE. Dopamine agonists and antagonists in the treatment of idiopathic dystonia. Adv Neurol. 1988;50:561–570. [PubMed] [Google Scholar]
- 44.Fan X, Donsante Y, Jinnah HA, Hess EJ. Dopamine receptor agonist treatment of idiopathic dystonia: A reappraisal in humans and mice. J Pharmacol Exp Ther. 2018;365:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sturm E, Piersma FE, Tanner MS, Socha P, Roberts EA, Shneider BL. Controversies and variation in diagnosing and treating children with Wilson disease: Results of an international survey. J Pediatr Gastroenterol Nutr. 2016;63:82–87. [DOI] [PubMed] [Google Scholar]
- 47.Tuschl K, Meyer E, Valdivia LE, et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat Commun. 2016;7:11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuschl K, Clayton PT, Gospe SM Jr., et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90:457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quadri M, Federico A, Zhao T, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jinnah HA, Albanese A, Bhatia KP, et al. Treatable inherited rare movement disorders. Mov Disord. 2018;33:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balint B, Vincent A, Meinck HM, Irani SR, Bhatia KP. Movement disorders with neuronal antibodies: syndromic approach, genetic parallels and pathophysiology. Brain. 2018;141:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baizabal-Carvallo JF, Jankovic J. Movement disorders in autoimmune diseases. Mov Disord. 2012;27:935–946. [DOI] [PubMed] [Google Scholar]
- 53.Marsh WA, Monroe DM, Brin MF, Gallagher CJ. Systematic review and meta-analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia. BMC Neurol. 2014;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson DM, Blitzer A, Brashear A, et al. Assessment: Botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70:1699–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86:1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castelao M, Marques RE, Duarte GS, et al. Botulinum toxin type A therapy for cervical dystonia. Cochrane Database Syst Rev. 2017;12:CD003633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallett M, Albanese A, Dressler D, et al. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon. 2013;67:94–114. [DOI] [PubMed] [Google Scholar]
- 58.Truong D, Dressier D, Hallet M, Zachary C. Manual of Botulinum Toxin. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- 59.Jost W, Valerius KP. Pictoral Atlas of Botulinum Toxin Injection: Dosage, Localization, Application. Berlin: Quintessence Publishing Company; 2008. [Google Scholar]
- 60.Jankovic J Botulinum toxin: State of the art. Mov Disord. 2017;in press. [DOI] [PubMed] [Google Scholar]
- 61.Jinnah HA, Goodmann E, Rosen AR, Evatt M, Freeman A, Factor S. Botulinum toxin treatment failures in cervical dystonia: causes, management, and outcomes. J Neurol. 2016;263:1188–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira JJ, Colosimo C, Bhidayasiri R, Marti MJ, Maisonobe P, Om S. Factors influencing secondary non-response to botulinum toxin type A injections in cervical dystonia. Parkinsonism Relat Disord. 2015;21:111–115. [DOI] [PubMed] [Google Scholar]
- 63.George EB, Cotton AC, Shneyder N, Jinnah HA. A strategy for managing flu-like symptoms after botulinum toxin injections. J Neurol. 2018;265:1932–1933. [DOI] [PubMed] [Google Scholar]
- 64.Esposito M, Fasano A, Crisci C, Dubbioso R, Iodice R, Santoro L. The combined treatment with orbital and pretarsal botulinum toxin injections in the management of poorly responsive blepharospasm. Neurol Sci. 2013;35:397–400. [DOI] [PubMed] [Google Scholar]
- 65.Rana AQ, Shah R. Combination of blepharospasm and apraxia of eyelid opening: a condition resistant to treatment. Acta Neurol Belg. 2012;112:95–96. [DOI] [PubMed] [Google Scholar]
- 66.Aramideh M, Ongerboer de Visser BW, Brans JW, Koelman JH, Speelman JD. Pretarsal application of botulinum toxin for treatment of blepharospasm. J Neurol Neurosurg Psychiatry. 1995;59:309–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Termsarasab P, Tanenbaum DR, Frucht SJ. The phenomenology and natural history of idiopathic lower cranial dystonia. J Clin Mov Disord. 2014;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Alegre P, Schneider RL, Hoffman H. Clinical, etiological, and therapeutic features of jaw-opening and jaw-closing oromandibular dystonias: A decade of experience at a single treatment center. Tremor Other Hyperkinet Mov (N Y). 2014;4:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scorr LM, Silver MR, Hanfelt J, et al. Pilot single-blind trial of abobotulinumtoxinA in oromandibular dystonia. Neurotherapeutics. 2018;15:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nastasi L, Mostile G, Nicoletti A, Zappia M, Reggio E, Catania S. Effect of botulinum toxin treatment on quality of life in patients with isolated lingual dystonia and oromandibular dystonia affecting the tongue. J Neurol. 2016;263:1702–1708. [DOI] [PubMed] [Google Scholar]
- 71.Moscovich M, Chen ZP, Rodriguez R. Successful treatment of open jaw and jaw deviation dystonia with botulinum toxin using a simple intraoral approach. J Clin Neurosci. 2015;22:594–596. [DOI] [PubMed] [Google Scholar]
- 72.Lungu C, Karp BI, Alter K, Zolbrod R, Hallett M. Long-term follow-up of botulinum toxin therapy for focal hand dystonia: outcome at 10 years or more. Mov Disord. 2011;26:750–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watts CC, Whurr R, Nye C. Botulinum toxin injections for the treatment of spasmodic dysphonia. Cochrane Database Syst Rev. 2004;3:CD004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel AB, Bansberg SF, Adler CH, Lott DG, Crujido L. The Mayo Clinic Arizona spasmodic dysphonia experience: A demographic analysis of 718 patients. Ann Otol Rhinol Laryngol. 2015;124:859–863. [DOI] [PubMed] [Google Scholar]
- 75.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 2015;125:1751–1757. [DOI] [PubMed] [Google Scholar]
- 76.Hale AT, Monsour MA, Rolston JD, Naftel RP, Englot DJ. Deep brain stimulation in pediatric dystonia: a systematic review. Neurosurg Rev. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cury RG, Kalia SK, Shah BB, Jimenez-Shahed J, Prashanth LK, Moro E. Surgical treatment of dystonia. Expert Rev Neurother. 2018;18:477–492. [DOI] [PubMed] [Google Scholar]
- 78.Krack P, Martinez-Fernandez R, Del Alamo M, Obeso JA. Current applications and limitations of surgical treatments for movement disorders. Mov Disord. 2017;32:36–52. [DOI] [PubMed] [Google Scholar]
- 79.Bruggemann N, Kuhn A, Schneider SA, et al. Short- and long-term outcome of chronic pallidal neurostimulation in monogenic isolated dystonia. Neurology. 2015;84:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jinnah HA, Alterman R, Klein C, et al. Deep brain stimulation for dystonia: a novel perspective on the value of genetic testing. J Neural Transm (Vienna). 2017;124:417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tagliati M, Krack P, Volkmann J, et al. Long-term management of DBS in dystonia: response to stimulation, adverse events, battery changes, and special considerations. Mov Disord. 2011;26 Suppl 1:S54–62. [DOI] [PubMed] [Google Scholar]
- 82.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029–1038. [DOI] [PubMed] [Google Scholar]
- 83.Reese R, Gruber D, Schoenecker T, et al. Long-term clinical outcome in Meige syndrome treated with internal pallidum deep brain stimulation. Mov Disord. 2011;26:691–698. [DOI] [PubMed] [Google Scholar]
- 84.Panov F, Gologorsky Y, Connors G, Tagliati M, Miravite J, Alterman RL. Deep brain stimulation in DYT1 dystonia: a 10-year experience. Neurosurgery. 2013;73:86–93. [DOI] [PubMed] [Google Scholar]
- 85.Horisawa S, Ochiai T, Goto S, et al. Safety and long-term efficacy of ventro-oral thalamotomy for focal hand dystonia: A retrospective study of 171 patients. Neurology. 2019;92:e371–e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horisawa S, Taira T, Goto S, Ochiai T, Nakajima T. Long-term improvement of musician's dystonia after stereotactic ventrooralthalamotomy. Ann Neurol. 2013;74:648–654. [DOI] [PubMed] [Google Scholar]
- 87.Krishna V, Sammartino F, Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: Advances in diagnosis and treatment. JAMA Neurol. 2018;75:246–254. [DOI] [PubMed] [Google Scholar]
- 88.Contarino MF, Van Den Munckhof P, Tijssen MA, et al. Selective peripheral denervation: comparison with pallidal stimulation and literature review. J Neurol. 2014;261:300–308. [DOI] [PubMed] [Google Scholar]
- 89.Ravindran K, Kumar N, Englot DJ, Wilson TJ, Zuckerman SL. Deep brain stimulation versus peripheral denervation for cervical dystonia: a systematic review and meta-analysis. World Neurosurg. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Georgescu D, Vagefi MR, McMullan TF, McCann JD, Anderson RL. Upper eyelid myectomy in blepharospasm with associated apraxia of lid opening. Am J Ophthalmol. 2008;145:541–547. [DOI] [PubMed] [Google Scholar]
- 91.Grivet D, Robert PY, Thuret G, et al. Assessment of blepharospasm surgery using an improved disability scale: study of 138 patients. Ophthal Plast Reconstr Surg. 2005;21:230–234. [DOI] [PubMed] [Google Scholar]
- 92.Pariseau B, Worley MW, Anderson RL. Myectomy for blepharospasm 2013. Curr Opin Ophthalmol. 2013;24:488–493. [DOI] [PubMed] [Google Scholar]
- 93.Ludlow CL. Treatment for spasmodic dysphonia: limitations of current approaches. Curr Opin Otolaryngol Head Neck Surg. 2009;17:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ludlow CL, Adler CH, Berke GS, et al. Research priorities in spasmodic dysphonia. Oto Head Neck Surg. 2008;139:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jinnah HA, Comella CL, Perlmutter J, Lungu C, Hallett M, Dystonia Coalition I. Longitudinal studies of botulinum toxin in cervical dystonia: Why do patients discontinue therapy? Toxicon. 2018;147:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pirio Richardson S, Jinnah HA. New approaches to discovering drugs that treat dystonia. Expert Opin Drug Discov. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 97.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. [DOI] [PubMed] [Google Scholar]
- 98.Calabresi P, Standaert DG. Dystonia and levodopa-induced dyskinesias in Parkinson's disease: Is there a connection? Neurobiol Dis. 2019;132:104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tello C, Darling A, Lupo V, Perez-Duenas B, Espinos C. On the complexity of clinical and molecular bases of neurodegeneration with brain iron accumulation. Clin Genet. 2018;93:731–740. [DOI] [PubMed] [Google Scholar]
- 100.Klopstock T, Tricta F, Neumayr L, et al. Safety and efficacy of deferiprone for pantothenate kinase-associated neurodegeneration: a randomised, double-blind, controlled trial and an open-label extension study. Lancet Neurol. 2019;18:631–642. [DOI] [PubMed] [Google Scholar]
- 101.Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92:e48–e54. [DOI] [PubMed] [Google Scholar]
- 102.Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and Botulinum toxin therapy: A systematic review and meta-analysis. Neurotox Res. 2015;25:105–117. [DOI] [PubMed] [Google Scholar]
- 103.Nijmeijer SW, Koelman JH, Standaar TS, Postma M, Tijssen MA. Cervical dystonia: improved treatment response to botulinum toxin after referral to a tertiary centre and the use of polymyography. Parkinsonism Relat Disord. 2013;19:533–538. [DOI] [PubMed] [Google Scholar]
- 104.Cordivari C, Misra VP, Vincent A, Catania S, Bhatia KP, Lees AJ. Secondary nonresponsiveness to botulinum toxin A in cervical dystonia: the role of electromyogram-guided injections, botulinum toxin A antibody assay, and the extensor digitorum brevis test. Mov Disord. 2006;21:1737–1741. [DOI] [PubMed] [Google Scholar]
- 105.Evidente VG, Pappert EJ. Botulinum toxin therapy for cervical dystonia: the science of dosing. Tremor Other Hyperkinet Mov. 2014;4:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ojo OO, Fernandez HH. Is it time for flexibility in botulinum inter-injection intervals? Toxicon. 2015;107:72–76. [DOI] [PubMed] [Google Scholar]
- 107.Castagna A, Albanese A. Management of cervical dystonia with botulinum neurotoxins and EMG/ultrasound guidance. Neurol Clin Pract. 2019;9:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wagle Shukla A, Ostrem JL, Vaillancourt DE, Chen R, Foote KD, Okun MS. Physiological effects of subthalamic nucleus deep brain stimulation surgery in cervical dystonia. J Neurol Neurosurg Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Franca C, de Andrade DC, Teixeira MJ, et al. Effects of cerebellar neuromodulation in movement disorders: A systematic review. Brain Stimul. 2018;11:249–260. [DOI] [PubMed] [Google Scholar]
- 110.Horisawa S, Arai T, Suzuki N, Kawamata T, Taira T. The striking effects of deep cerebellar stimulation on generalized fixed dystonia: case report. J Neurosurg. 2019:1–5. [DOI] [PubMed] [Google Scholar]
- 111.Quartarone A, Rizzo V, Terranova C, et al. Therapeutic use of non-invasive brain stimulation in dystonia. Front Neurosci. 2017;11:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mainka T, Erro R, Rothwell J, Kuhn AA, Bhatia KP, Ganos C. Remission in dystonia - Systematic review of the literature and meta-analysis. Parkinsonism Relat Disord. 2019;in press. [DOI] [PubMed] [Google Scholar]