Abstract
Phoenixin-20 (PNX-20) is a bioactive peptide with hormone-like actions in vertebrates. In mammals, PNX stimulates hypothalamo-pituitary-gonadal hormones and regulate reproductive processes. Our immunohisto/cytochemical studies show PNX-like and the putative PNX receptor, SREB3-like immunoreactivity in the gonads of zebrafish, and in zebrafish liver (ZFL) cells. Intraperitoneal injection of zebrafish PNX-20 upregulates mRNAs encoding both salmon gonadotropin-releasing hormone (GnRH), and chicken GnRH-II and kisspeptin and its receptor in zebrafish hypothalamus. Similarly, luteinizing hormone receptor mRNA expression in the testis, follicle-stimulating hormone receptor in the ovary, and the kisspeptin system were upregulated in the gonads of PNX-20 injected fish. We also observed the upregulation of genes involved in the sex steroidogenic pathway (cyp11a1, cyp17a1, 17βhsd, cyp19a1a) in the gonads of PNX-20 administered fish. PNX-20 upregulates the expression of vitellogenin isoforms and estrogen receptor (esr2a and 2b) mRNAs in ZFL cells in vitro. Meanwhile, siRNA-mediated knockdown of PNX-20 resulted in the downregulation of all vitellogenin transcripts, further suggesting its possible role in vitellogenesis. PNX-20 treatment resulted in a significant increase in germinal vesicle breakdown in zebrafish follicles in vitro. Collectively, these results provide strong evidence for PNX-20 effects on the HPG axis and liver to promote reproduction in zebrafish.
Subject terms: Animal physiology, Zoology, Physiology, Reproductive biology
Introduction
Advances in computational biology, especially in the use of bioinformatics tools, helped in the discovery of many regulatory molecules with hormone-like actions. A recent example is phoenixin-20 (PNX-20), which was originally identified as a reproductive regulatory peptide1. Phoenixin was first isolated from the rat hypothalamus and bovine heart by two independent research groups1,2. The mature phoenixin peptide is produced from an uncharacterized protein called small integral membrane protein 20 (SMIM20) or chromosome 4 open reading frame 52 (C4orf52). Phoenixin is reported to be present in two active forms, a 20 amino acid peptide named PNX-20 and a 14 amino acid one called PNX-141,2. PNX-14 is identical in humans, rats, mice, pigs, and dogs. PNX-20 differs in one amino acid between the coding regions of human, canine and porcine sequences1. It was reported that PNX is expressed in various parts of the brain and peripheral tissues in mammals, with the highest expression in the hypothalamus. Treen et al., reported that a putative receptor of PNX is the G protein-coupled receptor 173 (GPR173), which is also called super conserved receptor expressed in brain 3 (SREB3)3.
PNX stimulates reproductive functions via acting on the HPG axis1. Later it was reported that PNX mediates its reproductive regulatory effects through GPR173, and cAMP-PKA dependent pathway, acting on both gonadotropin-releasing hormone (GnRH) and kisspeptin 1 (Kiss1) expressing neurons3. In mice, PNX positively influences Kiss1 transcription and GnRH mediated gonadotropin release1,4. Intracerebroventricular (ICV) injection of PNX-20 significantly increases plasma luteinizing hormone (LH) levels in a dose-related manner, and potentiate GnRH-induced LH secretion from cultured anterior pituitary cells in vitro5. Although PNX is originally identified and described as a regulator of reproductive functions, non-reproductive roles of PNX are also reported later on. The non-reproductive functions of PNX include the regulation of pain sensation2, memory retention6, and food intake7,8, cardiac functions9, vasopressin release10, and the proliferation and differentiation of white adipose (3T3-L1) cells11. Overall, PNX is now known to have multiple biological effects.
PNX in non-mammals is poorly understood. In fish (spotted scat; Scatophagus argus), short term fasting increases the expression of PNX-20 in the hypothalamus and refeeding attenuates it8. It was also reported that both in vitro and in vivo administration of PNX upregulates the expression of gnrh receptor (gnrhr), lh and fsh in the pituitary of spotted scat12. These results suggest PNX appears to have a regulatory role in reproduction in fish. This research aimed to determine whether PNX-20 has a reproductive regulatory role in zebrafish, a well-characterized model system in comparative endocrinology. We tested for possible effects of PNX-20 on the tissue-specific expression of mRNAs encoding reproductive regulatory peptides, and vitellogenin genes in the liver, and oocyte maturation in zebrafish. Our results indicate that PNX-20 is a positive modulator of the HPG axis and vitellogenesis, and promotes oocyte maturation in zebrafish.
Results
PNX-like and SERB3-like immunoreactivity (ir) was detected in the gonads of zebrafish
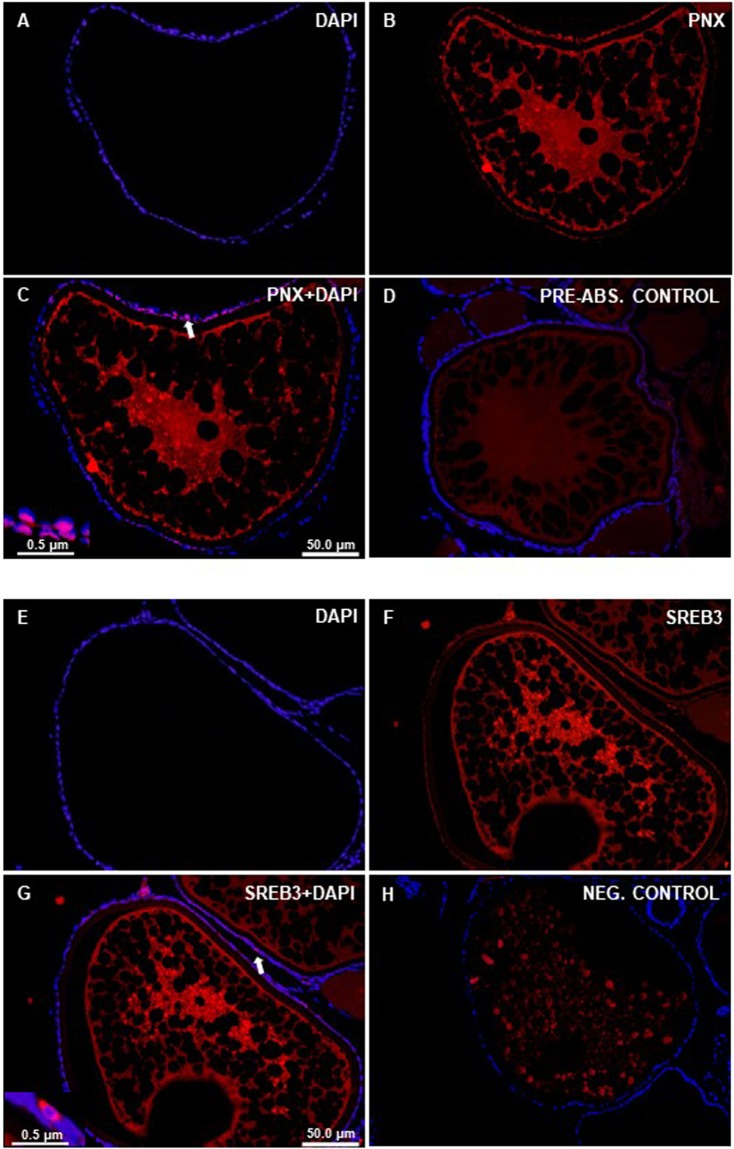
In the gonads of both male (Fig. 1) and female (Fig. 2) zebrafish, we found PNX-like and SREB3-like immunoreactivity in the supporting cell layers. No immunoreactive signals were found in the pre-absorption or secondary antibody alone negative controls for testis and ovary.
Figure 1.
Immunohistochemical localization of PNX-like and SREB3-like- ir in the testis of zebrafish. Figure shows representative sections of zebrafish testis (A–H) showing PNX-like (red; B,C) and SREB3-like (red; F,G) immunoreactivity. No PNX/SREB-3-like-ir was observed in both pre-absorption (D) and secondary antibody alone negative control (H). Nuclei shown in blue are stained with DAPI. Scale bars are indicated in each image.
Figure 2.
Immunohistochemical localization of PNX-like and SREB3-like- ir in the ovary of zebrafish. Figure shows representative sections of zebrafish ovary (A–H) showing PNX-like (red; B,C) and SREB3-like (red; F,G) immunoreactivity. No PNX/ SREB-3-like-ir was observed in both pre-absorption (D) and secondary antibody alone-negative controls (H). Nuclei shown in blue are stained with DAPI. Scale bars are indicated in each image.
PNX-like and SERB3-like immunoreactivity (ir) was detected in the zebrafish liver cell (ZFL) line
Both PNX-like and SREB3-like immunoreactivity was detected in the cytoplasm of ZFL cells (Fig. 3). No immunostaining was found in pre-absorption and negative controls.
Figure 3.
Immunohistochemical localization of PNX-like and SREB3-like- ir in the zebrafish liver cell line. Figure shows representative sections of zebrafish liver cell line (A–H) showing PNX-like (red; B,C) and SREB3-like (red; F,G) immunoreactivity. No PNX/SREB-3-like-ir was observed in both pre-absorption (D) and secondary antibody alone-negative controls (H). Nuclei are shown in blue are stained with DAPI. Scale bars are indicated in each image.
Intraperitoneally administered PNX-20 upregulates GnRH, kisspeptin and brain aromatase mRNA expression in the hypothalamus of zebrafish
A significant increase in salmon-type gonadotropin-releasing hormone (sgnrh or gnrh3) and chicken gonadotropin-releasing hormone (cgnrh-II or gnrh2) mRNA expression was noted in the hypothalamus of both male and female zebrafish post-PNX-20 administration (Fig. 4A–D). We observed a significant increase in the expression of kisspeptin 1 (kiss1, in both male and female treatment groups) (Fig. 4E,F) and its receptor (kiss1rb; only in the male fish group) (Fig. 4G) mRNAs in zebrafish hypothalamus. However, we did not observe any significant changes in the mRNA expression of kiss1rb in the hypothalamus of female fish (Fig. 4H). We also found a significant increase in the expression of kisspeptin 2 (kiss 2) mRNA in the hypothalamus of both male (1000 ng/g PNX-20) and female (both 100 and 1000 ng/g PNX-20) treatment groups (Fig. 4I,J). The brain aromatase (cytochrome P450, family 19, subfamily A, polypeptide 1b, cyp19a1b) mRNA expression was also significantly increased in the hypothalamus of female fish injected with 1000 ng/g PNX-20 (Fig. 4L), but no significant changes were found in the male fish group treated with PNX-20 (Fig. 4K).
Figure 4.

Expression levels of mRNAs encoding reproductive regulatory genes in the hypothalamus (A-L) of male (left panel) and female (right panel) zebrafish 1 h after intraperitoneal administration of 100 and 1000 ng/g bw of PNX-20. PNX administration significantly upregulates the mRNA expression of sgnrh (A,B), cgnrh-II (C,D), kiss-1 (E,F) and kiss-2 (I,J) in both male and female fish group. The kiss1rb (G) significantly upregulated in the male treatment group only. The brain aromatase (cyp19a1b) significantly upregulated in the female treatment group only (L). Data obtained by RT-qPCR are represented as mean + SEM (n = 6 fish/group). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
PNX-20 influence the expression of reproductive regulatory genes in the gonads of zebrafish
Sex-specific expression of lh receptor (lhr) and fsh receptor (fshr) mRNAs was observed in the gonads of zebrafish post-PNX-20 administration. lhr mRNA expression was significantly increased in the testis (100 ng/g PNX-20), and significantly decreased (1000 ng/g PNX-20) in the ovary of zebrafish (Fig. 5A,B). The fshr mRNA expression was significantly downregulated in the testis and significantly upregulated in the ovary of zebrafish injected with 1000 ng/g PNX-20 (Fig. 5C,D). A significant increase in kiss1 and kiss1rb mRNA expression in the testis and ovary of zebrafish treatment groups (Fig. 5E–H), and kiss2 mRNA expression in the testis of zebrafish injected with 100 ng/g PNX-20 (Fig. 5I) was detected. However, we didn’t observe any significant changes in the expression of kiss2 mRNA expression in the ovary of PNX 20 treated fish (Fig. 5J).
Figure 5.
Expression levels of mRNAs encoding gonadotropin receptors and kisspeptin system in the gonads (A-J) of male (left panel) and female (right panel) zebrafish post-PNX-20 administration. Upregulation of the mRNA expression of lhr in the testis (A) and downregulation in the ovary (B) of zebrafish was observed post-PNX-20 injection. However, fhsr mRNA expression downregulated in the testis (C) and upregulated in the ovary (D) post-PNX administration. The kiss-1 (E,F) and kiss1rb (G,H) mRNA expression significantly upregulated in both sexes. Significant increase in the kiss-2 mRNA expression observed only in the testis of the treatment group (I). Data obtained by RT-qPCR are represented as mean + SEM (n = 6 fish/group). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
PNX-20 influences genes involved in the gonadal sex steroidogenic pathway in zebrafish
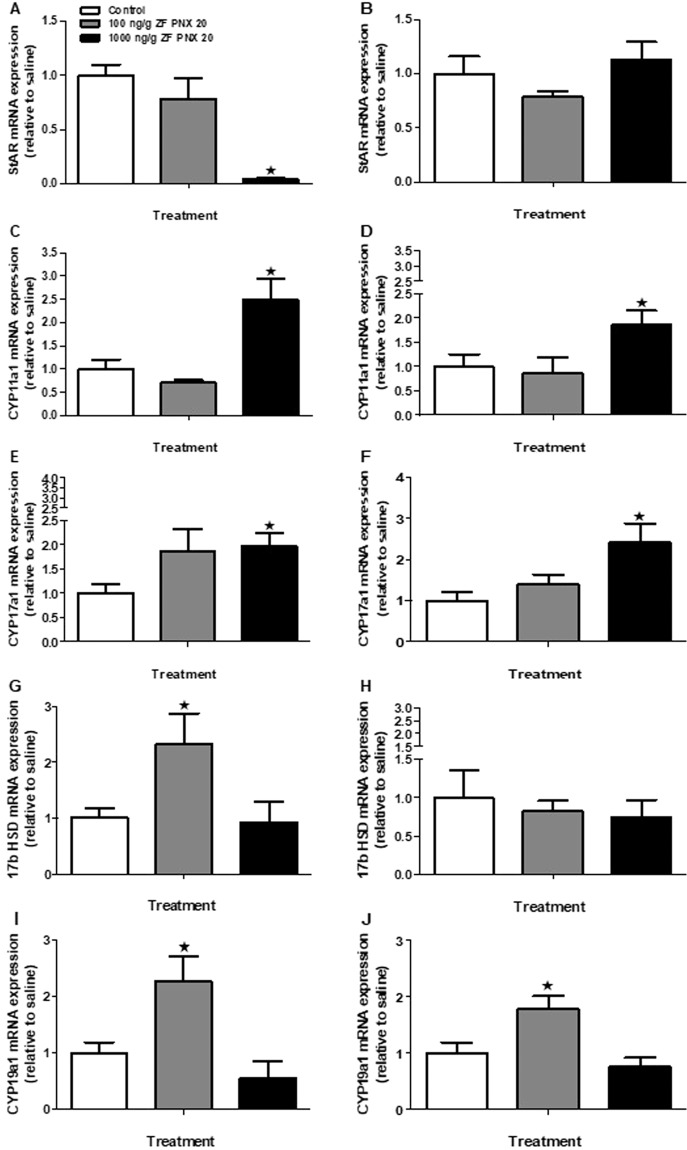
A significant decrease in steroidogenic acute regulatory protein (star) mRNA expression in the testis of 1000 ng/g PNX-20 treated fish group (Fig. 6A) was found. However, no significant changes in star mRNA expression were observed in the ovary of PNX treated fish (Fig. 6B). Cytochrome P450 family 11 subfamily A member 1 (cyp11a1, or the cholesterol side-chain cleavage enzyme) (Fig. 6C,D) and the cytochrome P450 family 17 subfamily A member 1 (cyp17a1, cytochrome P450 monooxygenase) (Fig. 6E,F) mRNA expression were significantly elevated in the gonads post-PNX-20 (1000 ng/g) administration. A significant increase in the expression of 17-beta hydroxysteroid dehydrogenase (17βhsd) was observed in the testis of zebrafish treated with 100 ng/g PNX-20 (Fig. 6G), but no significant changes were observed in the female fish (Fig. 6H). Similarly, the expression of cytochrome P450 family 19 subfamily A member 1 (cyp19a1a, gonadal aromatase) mRNA in the gonads of both male and female zebrafish was upregulated post 100 ng/g PNX-20 (Fig. 6I,J).
Figure 6.
Expression pattern of genes involved in the sex steroidogenic pathway in the gonads (A-J) of male (left panel) and female (right panel) zebrafish post-PNX-20 administration. A significant decrease in star mRNA expression was observed in the testis post-PNX-20 injection (A). PNX-20 upregulates the mRNA expression of cyp11a1 (C,D), cyp17a1 (E,F), cyp19a1a (I,J) in the gonads of male and female zebrafish. The 17βhsd upregulated only among the testis of treatment groups (G). Data obtained by RT-qPCR are represented as mean + SEM (n = 6 fish/group). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
Among gonadal genes that are important in zebrafish reproduction, anti-Müllerian hormone (amh) was significantly downregulated in the testis of zebrafish injected with 1000 ng/g PNX-20 (Fig. 7A). However, no significant changes in amh mRNA expression was observed in female fish injected with PNX-20 (Fig. 7B). Meanwhile, dmrt1 mRNA in the testis (100 ng/g) was increased (Fig. 7C) but was decreased (100 & 1000 ng/g) in the ovary (Fig. 7D). In the testis of 1000 ng/g PNX-20 treatment groups, both androgen receptor (ar) and estrogen receptor (er) mRNA expression was significantly downregulated (Fig. 7E,G). However, in the ovary of 1000 ng/g PNX-20 treated fish, both ar and er mRNA expression was significantly increased (Fig. 7F,H).
Figure 7.
mRNA expression pattern of amh, dmrt1 and sex-steroid hormone receptors in the gonads (A-H) of male (left panel) and female (right panel) zebrafish post-PNX-20 administration. The amh mRNA expression significantly decreased in the testis (A) of PNX injected fish group. However, dmrt1 mRNA significantly increased in the testis (C) and significantly decreased in the ovary (D). Both ar and er mRNAs downregulated in the testis (E,G). In the ovary of PNX injected fish ar and er mRNA’s upregulated (F,H). Data obtained by RT-qPCR are represented as mean + SEM (n = 6 fish/group). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
In vivo treatment of PNX-20 upregulates the expression of vitellogenin system mRNAs in ZFL cells
A significant increase in the vtg 1 mRNA expression was detected at 2, 6 and 24 hr post-PNX-20 treatment (Fig. 8A,B). As for vtg 2, we observed a significant increase in the expression at 6 and 24 hr post-PNX-20 treatment groups only (Fig. 8D), but not at 1 and 2 hr after PNX-20 treatment (Fig. 8C). However, similar to what we observed in case of vtg 1, the mRNA expression of vtg 3, 4, 5 and 7 were significantly upregulated at 2, 6 and 24 hr post-PNX-20 treatment (Fig. 8E–L).
Figure 8.

Vitellogenin mRNA expression pattern in the ZFL cells post-PNX-20 treatment (A-L). The left panel represents 1 and 2 hr and the right panel represents 6 and 24 hr treatment groups. No significant changes in any of the vitellogenin mRNA expression was observed in 1 hr treatment groups (1hr- A,C,E,G,I,K). Among 2 hr treatment groups, vtg 1, vtg 3, vtg 4, vtg 5 and vtg 7 mRNA expression was upregulated (2 hr- A,E,G,I,K). However, significant upregulation of all vtg’s was observed in the 6 and 24 hr treatment groups (B,D,F,H,J,L). Graphs represent pooled data from 2 independent study (‘n’ = 6 wells/treatment). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
PNX-20 upregulates the mRNA expression of estrogen receptor 2 (esr2) and sex hormone-binding globulin (shbg) in ZFL cells
PNX-20 treated caused a significant increase in esr-2a and esr-2b mRNA expression (Fig. 9C–F). However, we didn’t observe any significant changes in the expression of esr-1 mRNA expression in any treatment group (Fig. 9A,B). PNX-20 upregulated shbg mRNA expression at 2, 6 and 24 h (Fig. 9G,H).
Figure 9.
Expression pattern of estrogen receptor and shbg mRNA post-PNX-20 incubation in ZFL cells. The left panel represents 1 and 2 hr and the right panel represents 6 and 24 hr treatment groups. The mRNA expression of esr1a was not changed post-PNX- 20 treatment under any of the time points considered (A,B). Both esr2a, esr2b and shbg mRNA expression upregulated (C–H) in 2, 6 and 24 hr PNX-20 treatment groups. Graphs represent pooled data from 2 independent study (‘n’ = 6 wells/treatment). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
Significant reduction in smim20 mRNA expression was observed in the ZFL cells post siRNA treatments
The siRNA mediated knockdown resulted in a significant reduction in smim20 mRNA in ZFL cell line 72 hr post-incubation (Fig. 10B). However, no significant changes in smim20 mRNA expression was observed among 24 (data not included), 48 and 96 hr scrambled siRNA treatment groups (Fig. 10A,C). In addition, we didn’t observe any significant changes in smim20 mRNA expression in scrambled siRNA group post-72 hr treatment (Fig. 10D).
Figure 10.
mRNA expression of smim20 in the ZFL cells post siRNA and scrambled siRNA treatment. No significant changes in smim20 mRNA expression was observed in siRNA treated cells at 48 and 96 hr time points (A,C). A significant decrease in smim20 mRNA expression was observed at 72 hr post siRNA treatment (B) in all concentrations we tested compared to control. No significant changes in the mRNA expression of smim20 was observed among the scrambled siRNA treated groups (D). Graphs represent pooled data from 2 independent study (‘n’ = 6 wells/treatment). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
siRNA mediated PNX-20 knockdown downregulates vtg mRNAs in ZFL cells
All vtg (vtg 1, 2, 3, 4, 5 & 7) mRNAs were significantly downregulated in the siRNA treated group (Fig. 11A–F). We found no significant change in the expression of any of the vtg mRNAs in cells treated with the scrambled siRNA (data not shown).
Figure 11.
Effect of siRNA mediated gene knockdown of smim20 in vitellogenin mRNA expression profile in ZFL cells. Reduction in the mRNA expression of smim20 downregulates the expression of all vtg mRNA’s in the ZFL cells (A–F). Graphs represent pooled data from 2 independent study (‘n’ = 6 wells/treatment). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Asterisks denote significant differences between control and treated groups. p < 0.05 was considered statistical significance.
PNX-20 increased germinal vesicle breakdown and oocyte maturation in zebrafish
Incubation of zebrafish follicles with PNX-20 significantly increased germinal vesicle breakdown compared to control (Fig. 12). MIH (positive control) showed the highest response compared to both control and PNX-20 treated groups.
Figure 12.

PNX-20 promotes germinal vesicle breakdown and oocyte maturation in zebrafish. A significant increase in the maturation of oocytes were observed in ovarian follicles incubated with PNX-20 (A) compared to controls. However, follicles treated with MIH showed highest maturation rates compared to control and PNX treated group. Graphs represent pooled data from four independent study (n’ = 6 wells/treatment, 5–7 oocytes /well). One-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test was used for statistical analysis. Different letters (a, b, c) denote statistical differences between groups. p < 0.05 was considered statistical significance.
Discussion
PNX is a novel peptide with hormone-like actions to regulate reproduction in mammals. The research outlined here addressed whether PNX-20 has any role in the regulation of reproduction in zebrafish. Our research determined the effects of PNX-20 on all three tissues in the HPG axis, and liver, a major source of vitellogenin that is critical for ovarian follicle biology. Our immunohistochemical studies found PNX-like and SREB3-like immunoreactivity in the gonads, specifically in the supporting cell layers of developing ovarian follicles and spermatocytes. This provides new evidence for testis and ovary as a source of PNX-20 and sites of its action. The local expression suggests a possible role of PNX-20 in zebrafish reproduction. We then used 100 and 1000 ng/g BW PNX-20 to study whether PNX-20 has any role in zebrafish reproduction. A single I.P injection of PNX-20 significantly upregulated the expression of both sgnrh and cgnrh-II mRNAs in the hypothalamus of zebrafish. Both these GnRHs are expressed in zebrafish, with sGnRH has a pivotal role in the gonadotropin secretion from the pituitary13. Results suggest that PNX-20 directly acts on the GnRH system and upregulates the expression of GnRH in both male and female zebrafish. This is consistent with results obtained from mammals1,3–5 and non-mammals 12. Based on this, it is likely that the stimulatory effect of PNX-20 on the GnRH system is conserved across species. Similarly, we found an upregulation of kiss1, kiss2 (both male and female fish) and kiss1rb (only in males ) mRNA expression in the brain of zebrafish. The kisspeptin system is primarily involved in the regulation of reproduction in vertebrates14,15. Increased expression of kisspeptin system in PNX-20 treated fish suggests that in addition to the GnRH, PNX-20 influences another potent regulator of reproduction in the hypothalamus of zebrafish. It was reported that in the rat hypothalamus, PNX stimulates kisspeptin-1 transcription in both arcuate and anteroventral periventricular (AVPV) nucleus3. Kiss1 is abundantly expressed in the teleost habenula, a region involved in serotonin mediated behavior responses16. Studies in mammals reported that in addition to its reproductive roles, PNX-20 is a potent regulator of behavioral response as well6,17–19. Upregulation of kiss 1 post-PNX-20 administration suggests in addition to the reproductive regulatory role, PNX might be involved in the regulation of behavior in teleost. These results present a generally stimulatory role for PNX-20 on the hypothalamic reproductive hormones. Intracerebroventricular injection of PNX- 20 significantly increased the plasma LH levels in a dose-related manner to potentiate GnRH-induced LH secretion from cultured anterior pituitary cells of rats in vitro5. Due to the extremely small size of zebrafish pituitary gland, we were unable to collect this tissue and determine the effects of PNX-20 on pituitary hormones.
The effects of PNX-20 on zebrafish gonads were also positive. lhr mRNA expression in the testis increased, while its expression in the ovary decreased in response to PNX-20. Meanwhile, fshr expression was downregulated in the testis and upregulated in the ovary of the treatment group. This differential effect is likely due to the sex-specific variation in the effects of PNX-20. In agreement with the observations in the hypothalamus, an upregulation of kiss1, kiss1rb and kiss 2 (only in the male treatment group) mRNAs was identified in the gonads of zebrafish treated with PNX-20. Studies conducted in different fish species suggest that kisspeptin and gonadal development are positively correlated20. The upregulation of the kisspeptin system in the gonads of zebrafish is another possible mechanism by which PNX-20 influences zebrafish reproduction. However, we did not observe any significant changes in kiss1ra (kiss1r or kiss1 receptor a) mRNA expression in the hypothalamus and gonads of zebrafish injected with PNX-20 (data not shown).
Gonadal sex steroids are critical endocrine regulators of reproduction in vertebrates and are essential for the maturation of gametes and the development of secondary sexual characters21. Our gene quantification results indicate that PNX-20 upregulates the expression of critical genes involved in gonadal sex steroidogenesis in both male and female zebrafish. This includes the cyp11a1 (P450ssc), cyp17a1 (17α-hydroxylase) and cyp19a1a (gonadal aromatase) in which their expression was significantly increased in both male and female treatment groups. In addition, 17β hsd mRNA expression significantly increased in the testis of zebrafish injected with PNX-20. Our results suggest that PNX-20 has a role in the sex steroidogenesis in zebrafish. The only exception was the expression of star, which was significantly lower in the testis of the 1000 ng/g treatment group in our study. We also measured other reproduction-related genes. One among them is AMH, which has a role in sex determination, gonadal development and gametogenesis in fishes 22. Decreased expression of amh was observed in the testis of zebrafish treated with PNX-20. AMH is a steroidogenic suppressor in the gonads22. Decreased amh mRNA expression is additional evidence for the positive role of PNX-20 on sex steroidogenesis in male zebrafish. Another key regulator involved in vertebrate sex determination and gonadal development and gametogenesis is DMRT123. We observed a significant increase in the expression of dmrt1 mRNA in the testis, but it was decreased in the ovary of the PNX injected fish groups. This stands as another example for the sex-specific influence of PNX-20 on gonadal gene expression. It was reported that DMRT1 mutant zebrafish fail to develop into male fish or become sterile males, but ovary develops normally24. In addition, the loss of DMRT1 leads to conditions including gonadal dysgenesis, sex reversal syndromes, and reduced germ cells in the testis of vertebrates25–28. Upregulation of potent male reproductive promoter DMRT1 in the testis is additional evidence for the positive influence of PNX-20 in zebrafish gamete biology. Both ar and er mRNA expression was significantly higher in the ovary, but opposite effects were found in the case of testis. It was reported that in medaka, treatment with estradiol (E2) significantly increases the expression of both ER and AR, but treatment with 11-Ketotestosterone (11-KT) suppresses both AR and ER the brain29. It is possible that a similar kind of regulation happens in the gonads of the zebrafish, where the expression of steroidogenic genes and circulating steroid hormones were elevated, but the receptors (AR and ER) were decreased.
In addition to the HPG axis and related hormones, many other factors and biomolecules are important for reproductive success in zebrafish. One among them is vitellogenin, the principal yolk protein, synthesized and secreted predominantly by the liver. Vitellogenin has a critical role in the development and maturation of egg or ova in vertebrates30. We used ZFL cells to study the in vitro effects of PNX-20 on vitellogenin mRNA expression. We observed that all vitellogenin mRNAs (vtg 1, 2, 3, 4, 5 and 7) were significantly upregulated post-PNX-20 treatment in the ZFL cell line. Our results suggest that the upregulation of the vitellogenin mRNA expression may lead to elevated vitellogenin secretion, which leads to adequate availability of circulating vitellogenin, which then promotes the maturation of egg or developing ova. This is another possible mechanism by which PNX-20 influence (positively) zebrafish reproduction (in female fish). Liver vitellogenin synthesis is under the control of estrogen, which acts via estrogen receptors (esr’s). In our research, we observed that PNX-20 treatment leads to an increased expression of estrogen receptors (esr-2a and esr-2b), which is an additional (possible) mechanism by which PNX-20 influence vitellogenesis in zebrafish. We also found elevated expression of shbg, a liver-derived binding protein involved in the regulation of circulating sex steroid levels in the ZFL cell line post-PNX-20 treatment. In an alternate approach, we used siRNA mediated knockdown to study whether endogenous PNX-20 has any role in vitellogenin synthesis in the ZFL cell line. We found that the siRNA mediated gene knockdown of smim20 leads to decreased expression of all vitellogenin genes in the ZFL cell line. This suggests endogenous PNX-20 is a regulator of vitellogenin synthesis in zebrafish liver.
To study whether PNX-20 has any role in the maturation of gametes (ova), we used oocyte maturation assay, which is a well-established method to assess the direct action of ligand and hormones on oogenesis/oocyte development31–34. The incubation with zebrafish PNX-20 (both 100 and 100 ng/mL PNX-20) upregulated germinal vesicle breakdown and oocyte maturation in zebrafish follicles. This is in addition to the results we observed in both our in vivo and in vitro effect of PNX-20 on the overall increase in gene expression. This additional evidence for the direct action of PNX-20 on gamete maturation in zebrafish suggests extra means by which PNX-20 plays a positive modulatory role in fish reproduction.
The outcomes of this research provide several lines of novel information on exogenous PNX-20 in regulating reproduction. These include the regulation of vitellogenin, upregulation of steroidogenic mRNAs in the gonads, and stimulation of oocyte maturation in zebrafish. In addition, we discovered that endogenous PNX is also important for vitellogenin mRNA expression. Our results on the effects of PNX-20 on hypothalamic gnrh and kisspeptin mRNAs also concur with what has been reported in mammalian models, and at least in one fish species. These results help to conclude that the regulatory role (generally stimulatory) of PNX on reproduction is conserved across species. However, further studies are needed to confirm that the mRNA expression changes are also reflected at the protein level, both in tissues (enzymes) and in circulation (hormones). Similarly, pituitary expression and secretion of gonadotropins in response to PNX-20 need to be measured. While such limitations exist, this research and its outcomes have provided substantial new information to implicate PNX-20 in fish reproduction. Future studies on its role in other fish species, especially those cultured as a food source is strongly expected to benefit aquaculture.
Methods
Animals
Adult male and female zebrafish (Danio rerio; 12 months old; body weight: 1 g) were obtained from the Aquatic Toxicology Research Facility at the University of Saskatchewan. Fish were maintained in constantly aerated freshwater aquaria (10 L capacity), under a simulated 12 h light:12 h dark photoperiod (lights on at 07:00 AM). Fish were fed once daily (4% body weight ration) with slow sinking pellets (Aqueon, Franklin, WI, United States) at a scheduled feeding time. Tricaine methanesulfonate-222 (TMS-222, Syndel Laboratories, BC, Canada, 0.5%) was used for anesthetizing the fish (for intraperitoneal [I.P] injection) and euthanasia by spinal transection, followed by tissue collection. All animal studies were carried out according to the policies of the Canadian Council for Animal Care and were approved by the University of Saskatchewan Animal Research Ethics Board (Animal Use Protocol # 2012–0082).
Immunohistochemial and immunocytochemical localization of PNX and SREB3 in zebrafish gonads and zebrafish liver cell line
Identifying and localizing PNX and SREB3 immunoreactive cells in the gonads will assist us in predicting possible reproductive regulatory roles of PNX in zebrafish. Immunohistochemical studies (IHC) were conducted using methods described earlier35. The details of antibodies used in our research are rabbit anti PNX-14 primary antibody (Phoenix Pharmaceuticals, Burlingame, CA, United States, 1:1000 dilution) and rabbit anti-SREB3 antibody - N-terminal (Abcam, Cambridge, UK, 1:500 dilution). The secondary antibody used was Texas Red Goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, United States, 1:3000 dilution). All antibodies were diluted using antibody diluent (Abcam, UK). Antigen retrieval step was included for slides stained with SREB3 (1 mM EDTA, pH 8.0, and heat treatment, antigen retrieval protocol, Abcam). Preabsorption and secondary antibody alone controls were conducted as previously described36. Briefly, for the preabsorption control, zebrafish PNX-20 (custom synthesized, 10 μg) was preabsorbed with PNX-14 primary antibody overnight and this cocktail was used instead of the primary antibody for immunostaining. The negative controls were run only with the secondary antibody (no primary antibody incubation, used antibody diluent). Only the latter was used as a control for the SREB3 immunoreactivity. Vectashield medium containing DAPI dye (Vector Laboratories, Burlingame, CA, United States), which stain the nucleus of the cells blue, was used to mount the slides. The immunocytochemical (ICC) studies were conducted to localize PNX and SREB3 immunoreactivity in the zebrafish liver cell line (ZFL, Cat# ATCC CRL-2643, ATCC, Manassas, VA, United States). The ICC was conducted as previously described37 with slight modifications. Briefly, upon 60–70% confluency, cells grown in chamber slides (Corning, VWR, Canada) were fixed using ice-cold methanol (kept overnight at −20) for 2–5 minutes. The cells were then incubated with PBS-PF (5%), followed by cell permeabilization using PBS-Triton X-100 (0.2%) for 10 minutes. The slides were then incubated with blocking solution containing 5% protein block (Abcam) and BSA (5%) in PBS to prevent any non-specific antibody binding. The cells were then incubated with the primary antibody in antibody diluent solution (Abcam, UK) at 4 °C overnight. We used the same antibodies and dilutions as described above in the IHC section. No antigen retrieval steps were used for SREB3 stained slides (paraformaldehyde fixative is not used). Slides were mounted using Vectashield medium containing DAPI (Vector Laboratories). Both IHC and ICC slides were imaged using Olympus BX 51 microscope (Olympus Corporation, Tokyo, Japan) and analyzed using the DP controller software (Olympus Corporation, Japan). Since heterologous antibodies are used here, the immunostaining obtained is referred to as phoenixin-like and SREB3-like, to consider for the possible non-specific binding of antibodies.
Effect of exogenously administered PNX-20 on reproductive regulatory genes in the hypothalamus and gonads of zebrafish
Male and female zebrafish (age and weight-matched) were grouped (n = 6/group) in fish aquaria and were acclimated for 2 weeks. On the experiment day, fish were anesthetized (TMS-222, 0.5%) and intraperitoneally injected with 0.9% saline (control) or 100 or 1000 ng/g body weight (BW) custom synthesized zebrafish PNX-20 (AGVNQADIQPVGVKVWSDPY, Pacific Immunology, Ramona, CA, United States, Cat# 1711-PAC-21; ≥ 95% pure). The total injection volume used was 4 microliters (μl)/fish. This dose was decided based on our unpublished results from preliminary studies. Tissues (including the hypothalamus, and gonads) were collected 1 hour post-injection and stored at −80 °C until further analysis.
ZFL cell line maintenance and PNX-20 treatment
The liver is the primary site of synthesis of vitellogenin, the principal component of yolk protein critical for the development and maturation of egg or ova. To study whether PNX-20 has any role in vitellogenin system in zebrafish, we have incubated liver cells with zebrafish PNX-20. Zebrafish liver (ZFL) cell line was purchased from ATCC (ATCC CRL-2643, Manassas, VA, United States) and was cultured in a complete growth media [50 % L-15 (ATCC, VA, United States), 35 % DMEM HG (Gibco, Thermo Fisher Scientific, Sunnyvale, CA, United States), 15 % Ham's F12 (Gibco, Thermo Fisher Scientific, CA, United States), 0.15 g/L sodium bicarbonate (BioShop, Mainway, Burlington, ON, Canada), 15 mM HEPES (Millipore Sigma, Burlington, Massachusetts, United States), 0.01 mg/ml bovine insulin (Sigma-Aldrich, St. Louis, Missouri, United States), 50 ng/ml mouse EGF (Sigma-Aldrich, St. Louis, Missouri, United States), 5% heat-inactivated fetal bovine serum (Gibco, Thermo Fisher Scientific, CA, United States), 0.5% trout serum (Caisson Laboratories, Smithfield, Utah, United States) as suggested by ATCC. The cells were cultured in T25 ml flasks (Greiner Cellstar, VWR, PA, United States) and maintained at 28 degrees in an incubator. Cells were sub-cultured every 3 days, or when they reach 90% confluency. Passages 4–6 were used for all our experiments. For the in vitro peptide exposure studies, ZFL cells were cultured in 24 well plates (Greiner Cellstar, VWR, PA, United States) for all our experiments. Briefly, approximately 1 × 106 cells/well were allowed to grow in a 24 well plate for 2 days or until they reach 60–70% confluency. This was followed by replacing the complete growth media with media containing different doses of PNX-20 (0, 1, 10, 100 nM zebrafish PNX-20). All our experiments were run with ‘n’ = 6 wells/treatment, and all experiments were repeated twice. Control wells received complete growth media without any peptides (received an equal amount of sterile water instead) in it. The time points considered are 1, 2, 6, and 24 hr. At each time point, media from cells were removed and 500 μl of RiboZol RNA isolation reagent (aMReSCO, VWR, PA, United States) was added and incubated at room temperature for 5 minutes. This was followed by collecting the samples and stored them at −80 °C for total RNA extraction.
siRNA mediated knockdown of PNX-20
To study whether endogenous PNX-20 has any effect on the vitellogenin system in zebrafish liver, we used siRNA mediated gene knockdown approach using the ZFL cell line. Zebrafish PNX-20 siRNA and scrambled siRNA were custom synthesized (Dharmacon, Horizon Discovery, Waterbeach, United Kingdom). The scrambled siRNA represents the same composition of nucleotides; however, the arrangement of nucleotides in the sequence is highly dissimilar. Scrambled siRNA is to check or confirm whether the order of nucleotide or nucleotide integrity is critical for siRNA action. Both siRNAs and its scrambled controls were designed using online design tools (Genscript sequence scramble tool). Details of siRNAs are provided in Table 1.
Table 1.
The table is added with sequence information in siRNA format and correct nomenclature.
| Sense sequence | Antisense sequence |
|---|---|
| UACCUACUUUUCCUGCUUACGCUU | GCGUAAGCAGGAAAAGUAGGUAUU |
| Scrambled Sense sequence | Scrambled Antisense sequence |
| AGUACUUCGCUUCCUUCCUCUUU | AGAGGAAGGAAGCGAAGUACUUU |
The ZFL cells grown in 24 well plates (as described above) were treated with either different doses of siRNA (0, 1, 10, 100 nM) or scrambled siRNA. We used Lipofectamine RNAiMAX (Invitrogen, Thermo Fisher scientific CA, United States) mediated siRNA transfection method in our experiment. The Lipofectamine-siRNA mix was prepared in accordance with the manufacturer's instructions. Briefly, Lipofectamine was prepared using Opti-MEM serum-free media (Gibco, Thermo Fisher Scientific, CA, United States) and was mixed and incubated with siRNA, which devoid of any serum. The final concentrations for the treatments were prepared by adding the appropriate volume of the Lipofectamine-siRNA mix to complete growth media containing serum. This mixture was added to cells, which is about 50–60% confluent. The time points we used to check the level of knockdown were 24, 48, 72 and 96 hr post siRNA treatment. The total RNA extraction was carried out as described below. Cell viability and growth rate were closely monitored during the entire treatment period.
Total RNA extraction, cDNA synthesis, RT-PCR, and real-time quantitative PCR
Total RNA extraction from samples (tissues and cells), cDNA synthesis and RT-qPCR were conducted as described earlier38. RiboZol RNA isolation reagent (aMReSCO, VWR, Radnor, PA, United States) was used for total RNA was extraction, followed by DNase 1 (Thermo Fisher Scientific, CA, United States) treatment following the manufactures instructions. The purity and quantity of the total RNA extracted were determined by optical density (OD) absorption ratio (OD 260 nm/OD 280 nm) using a Nanodrop 2000 (Thermo Fisher Scientific, CA, United States). iScript cDNA synthesis kit (BioRad Laboratories, Hercules, CA, United States) was used for cDNA synthesis, following the manufacturer’s instructions. The CFX Connect Real-Time-quantitative PCR Detection System (Bio-Rad, CA, United States) and SensiFAST SYBR No-ROX Kit (Biolne, Froggabio, Toronto, ON, Canada,) were used for gene expression analysis. Livak method39 was used for the relative data analysis (2-ΔΔCt method). The quantitative gene expression of different genes and are normalized to the expression of the housekeeping genes, beta-actin and 18 s. Validation and optimization of the primers were carried out prior to qPCR to find the highest efficiency annealing temperatures. The qPCR conditions used were as follows: initial denaturation 95 °C (2 min), 35 cycles of denaturation: 95 °C (5 s), annealing: specific to each gene (25 s), elongation: 72 °C (20 s, when annealing temperature was less than 55 °C). A melting curve analysis was performed at 65 °C to 95 °C (5 s) was run to confirm the absence of any dimer formation or artifacts for each primer set. All samples were run in duplicates. Negative controls with no template DNA (used nuclease-free water instead) in the PCR mix were also run for each gene. The primer sequences and annealing temperatures used for each primer set for quantifying mRNA expression are listed in Table 2. Other than the primers used for vitellogenin genes (except vitellogenin 1), all primers were custom designed. Primer sequences for vitellogenin genes (vtg 2, 3, 4, 5,& 7) were previously reported40
Table 2.
List of primers used for PCR amplification of mRNAs.
| Gene | Accession no. | Primer sequence (5′−3′) | Annealing temperature (°C) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| 18 S rRNA | NM_173234.1 | GGCGAGGGTTCTGCATAATA | CATCCTTCGTGTCCTCAACA | 60 |
| β-actin | NM_131031.2 | TTCAAACGAACGACCAACCT | TTCCGCATCCTGAGTCAATG | 60 |
| sGnRH | NM_182887.2 | CCCGGTGGAAAAAGAAGCGT | CCCCGTCTGTCTGGAAATCTT | 60 |
| cGnRH-II | NM_181439.4 | AATGCAGTTACCTGAGACCG | AATCACGAATGAGGGCATCC | 60 |
| Kiss 1 | NM_001113489.1 | CTTCTCCATGGGTGCAGGTC | AATCGTGTGAGCATGTCCTGT | 60 |
| Kiss 1Rb | NM_001110531.1 | CCTTCTGTGCTGAAGACGTG | CTCGGTGCTCCTCCTTTTGA | 60 |
| Kiss 2 | NM_001142585.1 | ATGGAGCGAAGGCAGTTTGA | TGTCAGAGTCGCTGGTTGTC | 60 |
| FSH R | NM_001001812.1 | TTCCTGCTCAAACCCATTCC | GCATCCAAATCGGCTAGTCA | 60 |
| LH R | AY714133.1 | TGAAAGAGCAGCCAGGTACT | TGCTAAATTTCCTTTCGCCG | 60 |
| CYP11a1 | NM_152953.2 | ACCCTGCATAAATGAGCGTC | GACAGTGGAGTTTTGCGGTG | 60 |
| CYP17a1 | NM_212806.3 | AGACCCACCACAGACCTTTA | GCACAATCGGCCACTTAAAC | 60 |
| CYP19a1a | NM_131154.3 | ACCTCCACAAACTCTCACCT | TTGAGCGGGACTCCTAGAAA | 60 |
| CYP19a1b | NM_131642.2 | TTGGACGCATGCATAAGACA | ACCGAATGGCTGGAAGTAAC | 60 |
| StAR | NM_131663.1 | CTGTTTTCTGGCTGGGATGT | TCGCATGACAATACAGGTGG | 60 |
| 17βhsd | NM_205584.2 | TGTATCTGGAGGCGATGGAA | TGGAGGTGAGTTTCAGGCTA | 60 |
| DMRT1 | NM_205628.2 | CAGTCGCTCCATGTTGTCTT | AGTGGGCTGGTAAAGGTTGT | 60 |
| AMH | NM_001007779.1 | TTAAGGGACATCCTGCCTCA | AACACAAATACAGTCGGCGT | 60 |
| AR | NM_001083123.1 | CTCCAAAGCAAAGGACACCT | TTCGCCCATCTCGTTTTTCA | 60 |
| ER | NM_152959.1 | GATGTCCCTGCTCACCAACA | CAACACTTCCAGCCAAGAGC | 60 |
| PNX-20 | NM_001302624.1 | TTTGGAGGCTTCGTTGCAG | GGCTTGTAGGGATCAGACCA | 57 |
| SREB3 | NM_131498.1 | CCATTGCCCACCATCGTTTC | ATGAAGCCTAGCGTGTCGTT | 60 |
| VTG-1 | NM_001044897.3 | GAAGACTTTCTCGCCTGGAT | GCAGTACAGCAGTGGTCTAA | 60 |
| VTG-2 | NM_001044913.1 | GGTGACTGGAAGATCCAAG | TCATGCGGCATTGGCTGG | 57 |
| VTG-3 | NM_131265.1 | TTAGAACCAGCAAAGGATGC | CATCTCTTTTCTCCTTAAATAC | 57 |
| VTG-4 | NM_001045294.2 | GAGAGACTGCCAAAAATTGCCT | GGAGAGAAAATCCTTATCAATGGTG | 57 |
| VTG-5 | NM_001025189.2 | CCAAAAATTGTCACCACTTATGCT | CTTCATTCCTCCATGATATGCTTA | 60 |
| VTG-7 | NM_001102671.1 | CCATCTCAGAAGTCCTTTG | CAAACATTCTTGTGAAAATGAAGATA | 60 |
| Esr1 | NM_152959.1 | GACAGAAACCGTCGAAAGAG | CCGCGATCTTTACGAATACC | 58 |
| Esr2a | NM_180966.2 | GTCCGAGGTCTCAAGAGATA | AGGGCTTCTTCATGTCTTTC | 57 |
| Esr2b | NM_174862.3 | TCTCAGCACCTCTTTCCT | CATGCTAGCCTCAGTGTATG | 60 |
| SHBG | NM_001007151.3 | GAGCAGCAGGTGATCAAATA | CAAGGGTTAGGAACTCAA | 57 |
Oocyte maturation assay
Oocyte maturation assay was conducted as described earlier31,41. Briefly, 3–4 female zebrafish were euthanized as described above and ovaries were collected in sorting media containing 90% Leibovitz's L‐15 medium (Sigma, Cat# L1518) at pH 9.0 containing 10% bovine serum albumin (BSA). Individual oocytes or follicles in stage III were carefully separated from others under a dissection microscope. Follicle with size ranges from 0.55–0.62 mm in diameter were chosen for the study (oocytes at this stage respond to the maturation inducing hormone) as previously described32,33,42. Collected stage III follicles were either incubated in a 24 well plate with media (90% L-15 media pH 9 + 10% BSA) containing either 100 ng/mL maturation-inducing hormone (MIH; 4-Pregnen-17α,20β-diol-3-one) or 10 or 100 ng/mL ZF PNX-20. The control group was run without any hormones. To each well, 5–7 oocytes were added (n = 6 wells per treatment) and the experiment was repeated 4 times. The maturation rate was scored under a dissection microscope 24 hr post-incubation and the germinal vesicle break down, followed by changes in the appearance of the follicle from opaque to translucent is used to confirm maturation (fifth stage).
Statistical analysis
Quantitative qPCR gene expression data were analyzed using one-way ANOVA followed by Tukey’s multiple comparison test or Student–Newman–Keuls (SNK) test. p < 0.05 was considered statistical significance. PRISM version 5 (GraphPad Inc., United States) and IBM SPSS version 21 (IBM, United States) software were used for statistical analysis. PRISM version 5 (GraphPad Inc., United States) was used for generating graphs. Data are represented as mean + SEM.
Acknowledgements
We thank the animal order desk and the WCVM animal care unit staff for their assistance in the procurement of fish and their care. This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and the University of Saskatchewan Centennial Enhancement Chair in Comparative Endocrinology to SU. JR is a recipient of WCVM-CGPS PhD Scholarship.
Author contributions
J.J.R. planned and conducted the studies, analyzed and interpreted data, prepared the manuscript draft and revised it for submission. S.U. provided the original idea and funding, and helped in planning, designing experiments and assist in tissue sampling, data analysis and manuscript preparation and revisions.
Data availability
The data of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yosten GLC, et al. A Novel Reproductive Peptide, Phoenixin. J. Neuroendocrinol. 2013;25:206–215. doi: 10.1111/j.1365-2826.2012.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyu R-M, et al. Phoenixin: A novel peptide in rodent sensory ganglia. Neuroscience. 2013;250:622–631. doi: 10.1016/j.neuroscience.2013.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treen AK, Luo V, Belsham DD. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol. Endocrinol. Baltim. Md. 2016;30:872–888. doi: 10.1210/me.2016-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pałasz A, Rojczyk E, Bogus K, Worthington JJ, Wiaderkiewicz R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci. Lett. 2015;592:17–21. doi: 10.1016/j.neulet.2015.02.060. [DOI] [PubMed] [Google Scholar]
- 5.Stein LM, et al. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2016;311:R489–R496. doi: 10.1152/ajpregu.00191.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang JH, et al. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav. Brain Res. 2015;286:39–48. doi: 10.1016/j.bbr.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Schalla M, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides. 2017;96:53–60. doi: 10.1016/j.peptides.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, et al. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018;226:36–44. doi: 10.1016/j.cbpb.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Rocca C, et al. Phoenixin-14: detection and novel physiological implications in cardiac modulation and cardioprotection. Cell. Mol. Life Sci. CMLS. 2018;75:743–756. doi: 10.1007/s00018-017-2661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasparini S, et al. Novel regulator of vasopressin secretion: phoenixin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;314:R623–R628. doi: 10.1152/ajpregu.00426.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billert M, et al. Phoenixin-14 stimulates differentiation of 3T3-L1 preadipocytes via cAMP/Epac-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:1449–1457. doi: 10.1016/j.bbalip.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, et al. Phoenixin: Expression at different ovarian development stages and effects on genes ralated to reproduction in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019;228:17–25. doi: 10.1016/j.cbpb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Steven C, et al. Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen. Comp. Endocrinol. 2003;133:27–37. doi: 10.1016/S0016-6480(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa S, Parhar IS. Anatomy of the kisspeptin systems in teleosts. Gen. Comp. Endocrinol. 2013;181:169–174. doi: 10.1016/j.ygcen.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Tena-Sempere M, Felip A, Gómez A, Zanuy S, Carrillo M. Comparative insights of the kisspeptin/kisspeptin receptor system: lessons from non-mammalian vertebrates. Gen. Comp. Endocrinol. 2012;175:234–243. doi: 10.1016/j.ygcen.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa, S. & Parhar, I. S. Biological Significance of Kisspeptin–Kiss 1 Receptor Signaling in the Habenula of Teleost Species. Front. Endocrinol. 9, (2018). [DOI] [PMC free article] [PubMed]
- 17.Cowan A, et al. Phoenixin: A candidate pruritogen in the mouse. Neuroscience. 2015;310:541–548. doi: 10.1016/j.neuroscience.2015.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedrich T, et al. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019;1715:188–195. doi: 10.1016/j.brainres.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann T, et al. Phoenixin is negatively associated with anxiety in obese men. Peptides. 2017;88:32–36. doi: 10.1016/j.peptides.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Ohga, H., Selvaraj, S. & Matsuyama, M. The Roles of Kisspeptin System in the Reproductive Physiology of Fish With Special Reference to Chub Mackerel Studies as Main Axis. Front. Endocrinol. 9, (2018). [DOI] [PMC free article] [PubMed]
- 21.Yaron, Z. & Levavi-Sivan, B. Hormonal control of reproduction and growth | Endocrine Regulation of Fish Reproduction. In Encyclopedia of Fish Physiology (ed. Farrell, A. P.) 1500–1508 10.1016/B978-0-12-374553-8.00058-7. (Academic Press, 2011).
- 22.Pfennig F, Standke A, Gutzeit HO. The role of Amh signaling in teleost fish–Multiple functions not restricted to the gonads. Gen. Comp. Endocrinol. 2015;223:87–107. doi: 10.1016/j.ygcen.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webster KA, et al. Dmrt1 is necessary for male sexual development in zebrafish. Dev. Biol. 2017;422:33–46. doi: 10.1016/j.ydbio.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottolenghi C, et al. The region on 9p associated with 46,XY sex reversal contains several transcripts expressed in the urogenital system and a novel doublesex-related domain. Genomics. 2000;64:170–178. doi: 10.1006/geno.2000.6121. [DOI] [PubMed] [Google Scholar]
- 26.Õunap K, et al. Three patients with 9p deletions including DMRT1 and DMRT2: A girl with XY complement, bilateral ovotestes, and extreme growth retardation, and two XX females with normal pubertal development. Am. J. Med. Genet. A. 2004;130A:415–423. doi: 10.1002/ajmg.a.30269. [DOI] [PubMed] [Google Scholar]
- 27.Raymond CS, et al. A Region of Human Chromosome 9p Required for Testis Development Contains Two Genes Related to Known Sexual Regulators. Hum. Mol. Genet. 1999;8:989–996. doi: 10.1093/hmg/8.6.989. [DOI] [PubMed] [Google Scholar]
- 28.Wu, G.-C. et al. Testicular dmrt1 Is Involved in the Sexual Fate of the Ovotestis in the Protandrous Black Porgy. Biol. Reprod. 86, (2012). [DOI] [PubMed]
- 29.Towako Hiraki, et al. Female-specific target sites for both oestrogen and androgen in the teleost brain. Proceedings of the Royal Society B: Biological Sciences279(1749), 5014–5023 (2012). [DOI] [PMC free article] [PubMed]
- 30.Li H, Zhang S. Functions of Vitellogenin in Eggs. Results Probl. Cell Differ. 2017;63:389–401. doi: 10.1007/978-3-319-60855-6_17. [DOI] [PubMed] [Google Scholar]
- 31.Welch, E. L., Eno, C. C., Nair, S., Lindeman, R. E. & Pelegri, F. Functional Manipulation of Maternal Gene Products Using In Vitro Oocyte Maturation in Zebrafish. JoVE J. Vis. Exp. e55213 10.3791/55213 (2017). [DOI] [PMC free article] [PubMed]
- 32.Gonzalez, R. et al. Nesfatin-1 Regulates the Hypothalamo-Pituitary-Ovarian Axis of Fish. Biol. Reprod. 87, (2012). [DOI] [PubMed]
- 33.Shepperd E, Peng C, Unniappan S. Ghrelinergic system in fish ovaries and ghrelin inhibition of germinal vesicle breakdown in zebrafish oocytes. Gen. Comp. Endocrinol. 2012;176:426–431. doi: 10.1016/j.ygcen.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Clelland ES, Tan Q, Balofsky A, Lacivita R, Peng C. Inhibition of Premature Oocyte Maturation: A Role for Bone Morphogenetic Protein 15 in Zebrafish Ovarian Follicles. Endocrinology. 2007;148:5451–5458. doi: 10.1210/en.2007-0674. [DOI] [PubMed] [Google Scholar]
- 35.Hatef A, Yufa R, Unniappan S. Ghrelin O-Acyl Transferase in Zebrafish Is an Evolutionarily Conserved Peptide Upregulated During Calorie Restriction. Zebrafish. 2015;12:327–338. doi: 10.1089/zeb.2014.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundarrajan L, Yeung C, Hahn L, Weber LP, Unniappan S. Irisin regulates cardiac physiology in zebrafish. POS ONE. 2017;12:e0181461. doi: 10.1371/journal.pone.0181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatef A, Unniappan S. Gonadotropin-releasing hormone, kisspeptin, and gonadal steroids directly modulate nucleobindin-2/nesfatin-1 in murine hypothalamic gonadotropin-releasing hormone neurons and gonadotropes. Biol. Reprod. 2017;96:635–651. doi: 10.1095/biolreprod.116.146621. [DOI] [PubMed] [Google Scholar]
- 38.Rajeswari JJ, Hatef A, Golshan M, Alavi SMH, Unniappan S. Metabolic stress leads to divergent changes in the ghrelinergic system in goldfish (Carassius auratus) gonads. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2019;235:112–120. doi: 10.1016/j.cbpa.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Eide M, Rusten M, Male R, Jensen KHM, Goksøyr A. A characterization of the ZFL cell line and primary hepatocytes as in vitro liver cell models for the zebrafish (Danio rerio) Aquat. Toxicol. Amst. Neth. 2014;147:7–17. doi: 10.1016/j.aquatox.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Nair S, Lindeman RE, Pelegri F. In vitro oocyte culture-based manipulation of zebrafish maternal genes. Dev. Dyn. 2013;242:44–52. doi: 10.1002/dvdy.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clelland E, Peng C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009;312:42–52. doi: 10.1016/j.mce.2009.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available from the corresponding author upon reasonable request.