Abstract
High-intensity exercises including tethered efforts are commonly used in training programs for athletes, active and even sedentary individuals. Despite this, the knowledge about the external and internal load during and after this effort is scarce. Our study aimed to characterize the kinetics of mechanical and physiological responses in all-out 30 seconds (AO30) tethered running and up to 18 minutes of passive recovery. Additionally, in an innovative way, we investigated the muscle oxygenation in more or less active muscles (vastus lateralis and biceps brachii, respectively) during and after high-intensity tethered running by near-infrared spectroscopy – NIRS. Twelve physically active young men were submitted to AO30 on a non-motorized treadmill to determine the running force, velocity and power. We used wearable technologies to monitor the muscle oxygenation and heart rate responses during rest, exercise and passive recovery. Blood lactate concentration and arterial oxygen saturation were also measured. In a synchronized analysis by high capture frequency of mechanical and physiological signals, we advance the understanding of AO30 tethered running. Muscle oxygenation responses showed rapid adjustments (both, during and after AO30) in a tissue-dependence manner, with very low tissue saturation index observed in biceps brachii during exercise when compared to vastus lateralis. Significant correlations between peak and mean blood lactate with biceps brachii oxygenation indicate an important participation of less active muscle during and after high-intensity AO30 tethered running.
Subject terms: Optical spectroscopy, Data acquisition, Metabolism, Respiration, Biomedical engineering
Introduction
Physical exercise performed at different intensities promotes distinct physiological responses during both activity and recovery process1,2. High-intensity and short-volume efforts are widely used in the sports context and have also been extensively adopted in non-athlete training programs, such as high-intensity interval training (HIIT) and sprint interval training3,4. In the same way, high-intensity tethered exercises (including resisted sled sprinting) performed maximally have been applied to improve physical and athletic performances5,6. Despite that, there is a lack of knowledge about the mechanical and physiological kinetics during all-out tethered exercise and recovery. This gap can compromise the training load interpretation when this type of effort is adopted.
Training load is described as external and internal, depending on which measurements of the athlete/participant are assessed7. External load is defined as the amount and quality of work performed (e.g. distance covered, velocity and exercise power). On the other hand, the internal load indicates the physiological and psychophysiological responses of the organism to the effort imposed from the external load. However, internal-load indicators, especially during exercise, and the integration of external and internal loads need to be improved7.
Most exercise and recovery studies investigate systemic responses to the observed internal load, such as heart rate and blood lactate, the latter being considered a reliable metabolite to indicate the exercise intensity1. Although very significant for training direction, so far this metabolite is still often obtained invasively. Furthermore, the blood lactate measurement commonly occurs only a few times during an effort protocol or rest (e.g. only a few points on the timeline), not allowing full monitoring. Lactate is a product of one metabolic pathway (glycolysis) and a substrate for mitochondrial respiration, being regarded as the link between glycolytic and aerobic pathways8. This ‘chief messenger’8 is more highly produced in more active muscles during high-intensity exercise and is released into the bloodstream; it can be removed during and after physical exercise with important participation of less active muscles in this task. The lactate efflux and influx in skeletal fiber are mediated by MCT4 and MCT1, respectively9,10 and there is a dependence on the supply and utilization of oxygen by higher and lower activity muscles during and after exercise, since the lactate removal process is provided by the oxidative pathway in this tissue. Thus, it is possible that the key to maximum physiological equilibrium (i.e., at maximal lactate steady state1,11) and even the best chronic adaptations promoted by high-intensity training, is precisely in the muscle responses of more or less active muscles. So, the peripheral respiratory dynamics in exercise and recovery integrated with the mechanical power responses need to be improved, especially in running exercise, due to the extensive use in sports and training programs.
Most investigations involving power efforts and muscle oxygenation analysis during high-intensity exercises were performed on a cycle ergometer12–15 and in repeated sprint13,16,17 but with measurements conducted in one muscle group16,18 or in independent exercise to compare arm vs leg oxygen responses13. Rissanen et al.19 performed simultaneous analysis in biceps brachii and vastus lateralis in incremental treadmill running, observing differences between less and more active muscle oxygenation, especially in severe-intensity exercise. To our knowledge, the monitoring of the external load by mechanical power in a high-intensity running effort or all-out running concurrently with oxygen saturation analysis in two muscles (more and less active) has not yet been investigated. We believe that, in part, this lack is due to the reduced number of protocols/ergometers capable of identifying the precise running power. Additionally, in contrast to more stationary exercise, such as that conducted on a cycle ergometer, in order to analyse the peripheral oxygenation in a running effort (with freedom of limb movement) the use of wearable equipment is desirable.
In the first case our research group developed an innovative ergometer capable of acquiring accurate values of mechanical parameters during running efforts20–22 based on the tethered running concept23,24. The non-motorized treadmill (NMT) is composed of velocity and force sensors to determine the individual performance in running exercise by the high capture frequency of these signals (1000 Hz)20,21. Similar to the classical Wingate test25, the all-out 30 seconds (AO30) have been used to identify the force, velocity and power (peak, mean, minimum and fatigue index) in a tethered system22,26. However, as it is widely used in training and evaluation programs, the characterization of external and internal load responses during AO30 tethered efforts and in post-exercise still needs to be improved.
The near-infrared spectroscopy (NIRS) technique, purposed at first in 197727,28 is based on the light absorption of oxygenated and deoxygenated hemoglobin and myoglobin in the near infrared tissue, using the interaction of light at different wavelengths29. NIRS is a non-invasive method that has been shown to be a significant tool capable of estimating the muscle oxygenation events, such as variations in oxyhemoglobin (O2Hb), deoxyhemoglobin (HHb), total hemoglobin (tHb) and tissue saturation index (TSI) in skeletal muscle30,31. This technique based on optical principles has been commonly used in clinical studies involving pathologies and exercise prescription32,33 and recently focused on inactive participants12, active subjects34,35 and athletes16,36–38 to improve the knowledge about physiological and performance responses. In a recent systematic review, Perrey and Ferrari39 suggested that the popularity of muscle oxygenation studies in exercise increased after the commercialization of portable wireless muscle oximeters. In this context, by allowing continuous and sensitive monitoring with high frequency of physiological signal capture, the use of NIRS − potentially and in the near future − may contribute to the improvement of the organization of exercise and training load monitoring aimed at improving health and performance.
Considering the significant application of high-intensity tethered exercises in training programs and the knowledge gap regarding the acute responses during and after this effort, our study aimed to characterize the kinetics of mechanical and physiological responses in all-out 30 s running effort and up to 18 minutes of passive recovery after this kind of exercise. Additionally, in an innovative way, we investigated the muscle oxygenation in more or less active muscles (vastus lateralis and biceps brachii, respectively) during and after high-intensity tethered running using wearable NIRS. Based on a previous study using an incremental running test19, we hypothesize, there will be a significant difference between arm and leg oxygenation during and after an all-out 30 second tethered running effort. Additionally there will be a significant relationship between muscle oxygenation and blood lactate responses.
Methods
Subjects
Twelve physically active young men were evaluated (22 ± 1 years, body mass 71.4 ± 2.7kg, height 178 ± 2 cm). Subjects answered the International Physical Activity Questionnaire, in which the minimum score to classify them as ‘physically active’ was used as inclusion criterion40. All subjects reported no metabolic, cardiovascular or orthopaedic disease and no use of medications or drugs. This study was conducted in agreement with the ethical recommendations of the Declaration of Helsinki and all experiments were approved by the Research Ethics Committee of The School of Medical Sciences (protocol number 99783318.4.0000.5404). After having received information about the experimental procedures and risks, all individual participants signed an informed consent form.
Experimental design
The experimental design consisted of three laboratory visits, separated by 24–48 h. Firstly, subjects received information about the experimental design and signed a consent form. Subjects answered the International Physical Activity Questionnaire (IPAQ) and a questionnaire for health characterization. Next, participants were submitted to anthropometric and body composition measurements41. On the second day, the tethered running familiarization was conducted on a non-motorized treadmill (NMT). Subsequently, during the third day, subjects were submitted to AO30s tethered running test to determine running force, velocity and power. The participants were also equipped with wearable technology (NIRS on the upper limb and lower limb and a heart rate monitor) to obtain muscle oxygenation and heart rate responses, respectively. Ergometer specifications and equipment are shown in Fig. 1. After the AO30 running test, participants remained at rest for 18 minutes to capture of physiological and muscle responses. Rest in the dorsal decubitus position was adopted to minimize the discomfort experienced by active individuals after all-out efforts.
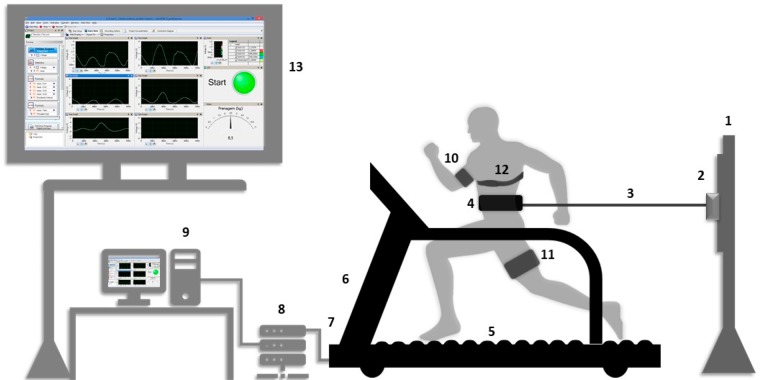
Figure 1.
(1) Height adjustment bar; (2) load cell; (3) steel cable; (4) belt attached to the steel cable and participant; (5) sensorized platform by four load cells; (6) non-motorized treadmill (NMT); (7) Hall-effect sensor positioned on the treadmill’s front cylinder to determine the velocity that results from the connection with the acquisition system; (8) acquisition system composed of portable amplifier (MKTC5-10, MK) and an acquisition module (NI USB-6009); (9) computer; (10) NIRS Portamon unit fixed to the upper limb (biceps brachii muscle); (11) NIRS Portamon unit fixed to the lower limb (vastus lateralis muscle); (12) heart rate monitor (Polar V-800), (13) monitor showing the force, velocity and power during tethered test (online signals).
Exercise test protocol (AO30) and post-exercise (recovery)
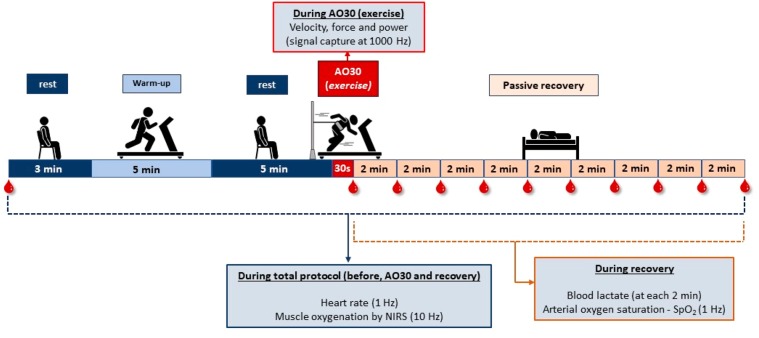
All procedures were conducted in a controlled laboratory (temperature = 22 °C ± 1 °C (SD); relative humidity = 50% ± 2%; luminosity = ~300 lx). To perform the AO30 test conducted in the third session, participants were equipped with wearable equipment (NIRS and heart rate monitor) and kept in rest (sitting position) during a 3 minute period aimed to establish baseline responses. After this, before the AO30 test, the subjects warmed up on a motorized treadmill running (Inbramed Super ATL, Inbrasport, Brazil) for 5 minutes at 7.0 km/h. After the warm-up, 5 minutes of recovery was taken to return the physiological responses to rest. Then the AO30 tethered running was carried out with mechanical and physiological responses recorded (muscle oxygenation and HR). The participants received constant verbal encouragement during the tethered test exercise.
Immediately after the exercise, a pulse oximeter was plugged on to the subject’s finger and blood capillary samples were extracted from the ear lobe by heparinized capillary tubes. With the aim of conducting the recovery investigations, HR, arterial oxygen saturation (SpO2) and muscle oxygenation were continuously monitored for up to 18 minutes after AO30, with blood capillary samples extracted (ear lobe) immediately and every two minutes during this recovery period. The timeline of the AO30 test procedures and recovery are explained in Fig. 2.
Figure 2.
Experimental high-intensity exercise session adopted to test the mechanical and physiological responses, and muscle oxygenation in upper and lower limb.
Mechanical measurements in AO30
The AO30 tests were performed on a standardized non-motorized treadmill20–22. Subjects ran with an inextensible steel cable in series with a load cell (CSL/ZL-500, MK Controle e Instrumentação Ltda, Brazil) attached to their waist for a directly horizontal force measurement22. Vertical force during the running test was also captured by four load cells positioned under a platform (NMT). Velocity was obtained as the first derivative of the treadmill displacement using a Hall-effect sensor. Thus, power was obtained by the product between force and velocity. The acquisition system consisted of a strain gauge (CSA/ZL-500 MK Control, Sao Paulo, Brazil), a portable amplifier (MKTC5–10, MK Control, Sao Paulo, Brazil) and an acquisition module (NI USB-6009, National Instruments, Austin, USA). Mechanical measures were captured via signals (LabView Signal Express 2009 National Instruments®) with 1000 Hz acquisition. The NMT system was calibrated daily21 modulated and subsequently transferred to MatLab (R2008a MatLab®, MathWorkstm). Peak, mean and minimum of the force and power were displayed in absolute and relative body mass values.
Muscle oxygenation measurements and analyses
Changes in the muscle oxygenation were assessed continually during the rest, exercise and recovery periods using a NIRS. For this, two PortaMon devices (Artinis Medical Systems BV, Zetten, Netherlands) including three light source transmitters (each one with two wavelengths between 750 and 850 nm) at 30, 35, and 40 mm distance to the receiver, were used to determine the tissue saturation index (TSI, %). These devices were also used to obtained the changes in the oxyhemoglobin ([O2Hb]), deoxyhemoglobin ([HHb]) and total hemoglobin ([tHb]) concentrations by analysis of the deeper trace (deepest optode, 40 mm).
These NIRS devices were positioned on two right muscles: the biceps brachii (BB), in medial biceps brachii portion14,42,43, an upper limb muscle considered as less active during running exercise, and the vastus lateralis belly (VL), 15 cm above the proximal edge of the patella and 5 cm towards the external side44,45 parallel to the long axis of the muscle19 considered more active during running effort16,46.The equipment was wrapped tightly in a transparent plastic to avoid humidity and which created a waterproof barrier13, and by a dark band, to secure the probe and protect it from environmental light.
The concentration values of muscle oxygen were analysed by entering the differential pathlength factor (DPF) values. According to the manufacturer’s instructions, the DPF for different tissues and participant characteristics must be selected according to the literature. In this sense, considering the PortaMon NIRS equipment, several studies analysing the vastus lateralis muscle applied DPF (range of 3.7 to 4.16 DPF) for participants and experimental designs with similar characteristics to our study39. We, therefore, adopted 3.83 to VL47,48. On the other hand, there is a lack of information about DPF for biceps brachii13. In the present study, we applied DPF 3.78 to BB, based on Fauss et al.49, which used similar values to triceps brachii, and also on Duncan50, which measured DPF in male adult arms.
The signal was captured at 10 Hz. After signal capture, the data were smoothed using the 10th order low- pass-zero phase Butterworth filter (cut-off frequency 0.1 Hz)16 provided by recording and analysis Artinis software (Oxysoft, Artinis Medical System, Netherlands). The change (Δ) in [O2Hb], [HHb], and [tHb], in micromolar units (µM), were obtained by subtracting these values from the baseline data12,51, considering the final 30 seconds of the baseline period17 of 3 minutes (Fig. 2).
Heart rate, arterial oxygen saturation and blood lactate concentration
Heart rate was continuously recorded every 1 second by a heart rate monitor (Polar V800, Finland). Blood samples (25 µl) were collected at rest and during post-exercise from the ear lobe with a heparinized capillary, and deposited into microtubes (Eppendorf, 1.5 ml) containing 50 µl of 1% sodium fluoride (NaF). The samples were frozen at –20 °C before being homogenized and determined by a lactate analyser (YSI-2300-STAT-Plus™, Yellow Springs, USA). Estimations of arterial oxygen saturation were accomplished immediately after AO30 and during all recovery phase with a pulse oximeter (OXIFAST Takaoka, SP, Brazil).
Statistical analysis
All results are expressed as mean ± error of the mean (SEM). Distribution of the normality and variance homogeneity were initially tested by the Shapiro−Wilk and Levene test, respectively. One-way repeated measures analysis of variance (ANOVA) was applied to study the effect of the time during the exercise (on mechanical and physiological responses) and during of the recovery phase (on physiological responses). A two-way analysis of variance (ANOVA) for repeated measures (effects of time and site device NIRS) investigated the difference between upper limb (BB) and lower limb (VL) muscle oxygenation (Δ[O2Hb], Δ[HHb], Δ[tHb], and TSI) in AO30 (at each 1 s) and post-exercise (immediately after exercise and at each 2 minutes until 18 minute). Newman−Keuls post hoc test was used to detect these differences. Paired Student’s t-test was applied to assess the difference between peak, mean and minimum responses of local tissue oxygenation. The relationship among mechanical, physiological and muscle oxygen responses in exercise and recovery were obtained by Pearson’s linear regression test. All statistical analyses were performed using STATISTICA software (7.0 version). Considering that this is the first methodological study to characterise the mechanical, physiological, and muscle oxygenation in AO30 in tethered running and in the line of exercise and physical training studies, statistical significance was set at P ≤ 0.05.
Results
The participants characterization is expressed in Table 1.
Table 1.
Participants’ characteristics (n = 12).
| Participants’ characteristics | |
|---|---|
| Mean ± SEM | |
| Age (year) | 22 ± 1 |
| Body mass (kg) | 71.4 ± 2.7 |
| Height (cm) | 178 ± 2 |
| Body fat percentage (%) | 8.7 ± 1 |
| Vastus laterais skinfold (mm) | 13.0 ± 1.4 |
| Biceps brachii skinfold (mm) | 3.7 ± 0.2 |
| HR rest (bpm) | 74 ± 3 |
| [Lac] rest (mM) | 0.9 ± 0.1 |
| SpO2 rest (%) | 98.1 ± 0.2 |
HR-heart rate; [Lac] - blood lactate concentration; SpO2- arterial oxygen saturation.
Exercise responses
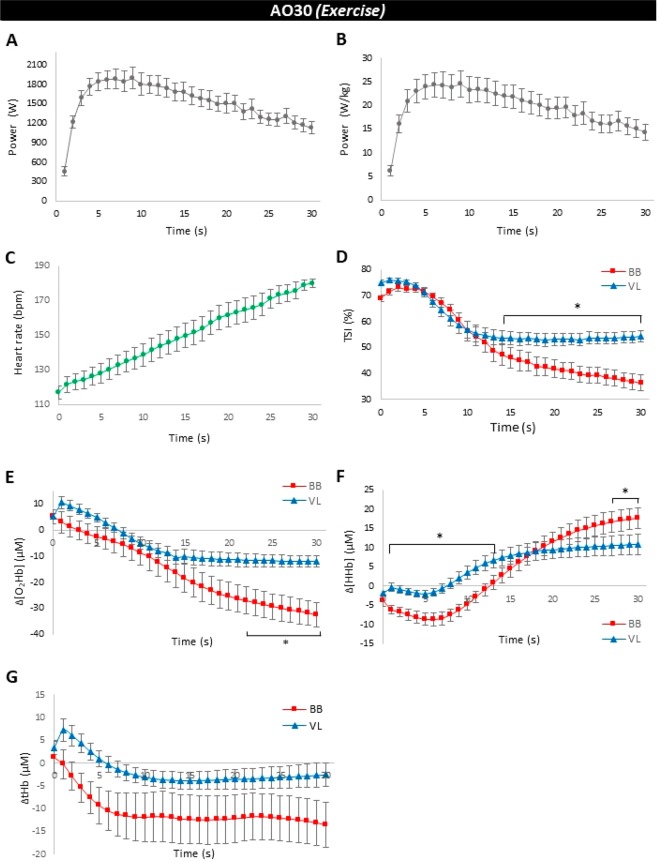
The main values (peak, mean and minimum results) of the mechanical parameter obtained by AO30 tethered running are summarized in Table 2. The curves of absolute and relative power output during AO30 test are shown in Fig. 3 (Panels A and B). We observed strong and significant relationships between absolute peak power and relative peak power (r = 0.87, P = 0.000). Peak power also showed a significant correlation between mean power (r = 0.81, P = 0.001) and relative mean power (r = 0.97, P = 0.000), absolute peak and mean force (r = 0.95, P = 0.000 and r = 0.92, P = 0.000, respectively) and peak and mean velocity (r = 0.83, P = 0.001 and r = 0.71, P = 0.001, respectively). Repeated measures ANOVA one-way showed an effect of time of test on absolute power (F = 5.95, P = 0.000) and relative power (F = 9.37, P = 0.000). Both, relative and absolute power increased during the first seconds of the test, and attained peak values around the 6th second of tethered running (Fig. 3, Panel A and B). After that time, there was a reduction in the power values, with significant difference among the last seconds of the test (29th and 30th s) compared to 6–9 seconds (P ≤ 0.05). Fatigue index (FI = (peak power − minimum power)/peak power *100) was 47.0 ± 2.7% (Table 2).
Table 2.
Peak, mean and minimum absolute and relative (to body mass) mechanical results obtained during tethered running exercise (AO30) on a non-motorized treadmill (NMT) (n = 12).
| Mechanical Parameters | |
|---|---|
| AO30s – Exercise | |
| Mean ± SEM | |
| Power | |
| Peak power (W) | 2017.8 ± 159.7 |
| Mean power (W) | 1514.9 ± 119.6 |
| Minimum power (W) | 460.8 ± 59.5 |
| Peak power (W/kg) | 28.2 ± 1.6 |
| Mean power (W/kg) | 21.2 ± 1.3 |
| Minimum power (W/kg) | 6.6 ± 0.9 |
| Force | |
| Peak Force (N) | 427.9 ± 23.1 |
| Mean Force (N) | 365.9 ± 20.9 |
| Minimum Force (N) | 251.8 ± 19.1 |
| Peak Force (N/kg) | 6.0 ± 0.3 |
| Mean Force (N/kg) | 5.2 ± 0.3 |
| Minimum Force (N/kg) | 3.6 ± 0.3 |
| Velocity | |
| Peak Velocity (m/s) | 5.0 ± 0.1 |
| Mean Veocity (m/s) | 4.1 ± 0.1 |
| Fatigue index (%) | 47.0 ± 2.7 |
Figure 3.
Results obtained during all-out 30 s (AO30 exercise) at tethered running in a non-motorized treadmill (mean ± SEM at each 1 s, n = 12). (A) Absolute running power (W), (B) Relative running power (W/kg), (C) Heart rate (bpm), (D) Tissue saturation index (TSI) (%) in biceps brachii (BB) and vastus lateralis (VL), (E) Changes (ΔµM) in oxyhemoglobin ([O2Hb]) occurred in BB and VL, (F) Changes (ΔµM) in deoxyhemoglobin ([HHb]) in BB and VL, and (G) Changes (ΔµM) in total hemoglobin ([tHb]) occurred in BB and VL, measured by near infrared-spectroscopy (NIRS). *Indicates the difference between responses obtained in BB and VL at the same moment (P ≤ 0.05).
Figure 3 shows the curves of heart rate (Panel C), TSI (Panel D), deltas of oxyhemoglobin (Δ[O2Hb]), deoxyhemoglobin (Δ[HHb]) and total hemoglobin (Δ[tHb]) (Panels E, F and G, respectively) during 30 s of tethered running. HR was affected by time (F = 9.26, P < 0.001), increasing throughout the AO30 test, with higher and significant values observed after 16th second compared to the first seconds of exercise. The last HR value (HR in 30th second) was higher than HR at the 1st to 15th s of test and the peak, mean and minimum HR values were 180 ± 2, 151 ± 6 and 121 ± 5 bpm, respectively.
A two-way repeated measures ANOVA presented the effect of time of test (F = 29.21, P < 0.001), site device NIRS (F = 109.78, P < 0.001) and interaction between time x site device NIRS (F = 2.99, P < 0.001) to TSI. In the upper limb (BB) the percentage of tissue saturation decreased during the initial 10 seconds of AO30 (significant difference compared to the 1st second exercise from the 9th test seconds), and then showed stabalisation in lower values. The same results were observed by TSI in VL, but a significant decrease occurred from the 7th second of the exercise. When comparing the TSI response in BB and VL at the same times of the test, a greater drop was observed in BB, with different results after the 14th second. Higher TSI values in more active muscle in our experimental conditions (VL) were observed during exercise.
With regard to oxygen availability (Δ[O2Hb]) and oxygen utilization (Δ[HHb]), an inverse curve behaviour was observed, as expected (Panels E and F). There is an effect of time (F = 12.17, P < 0.001 and F = 24.27, P < 0.001) and site of NIRS (F = 121.31, P < 0.001 and F = 7.96, P = 0.005) for Δ[O2Hb] and Δ[HHb], with significant interaction between time x site NIRS to Δ[HHb] (F = 3.61, P < 0.001), but not to Δ[O2Hb] (F = 1.08, P = 0.351). Delta BB Δ[O2Hb] response presented a drop during all exercise, but decreased significantly after 17 second of the test. In the leg muscle (VL), this delta was significantly reduced after 7th second, with stabilization of Δ[O2Hb] after the 14th second. The comparison between Δ[O2Hb] responses in BB and VL at the same test times revealed only significant differences in the last 8 seconds of the AO30 (22–30 seconds), with more oxygen available to leg muscle (VL) (P ≤ 0.05) (Fig. 3, panel E). Delta of [HHb] apparently reduced during the first exercise seconds for both, BB and VL muscles. In the arm (BB), higher values of delta [HHb] were observed after 14 second of the exercise, and a drop after 18 second compared to 1–7 seconds for the leg (VL). There was no significant difference of Δ[tHb] between BB and VL during the AO30 exercise, but greater interindividual variation was visualized in the upper limb (P < 0.05).
Recovery responses
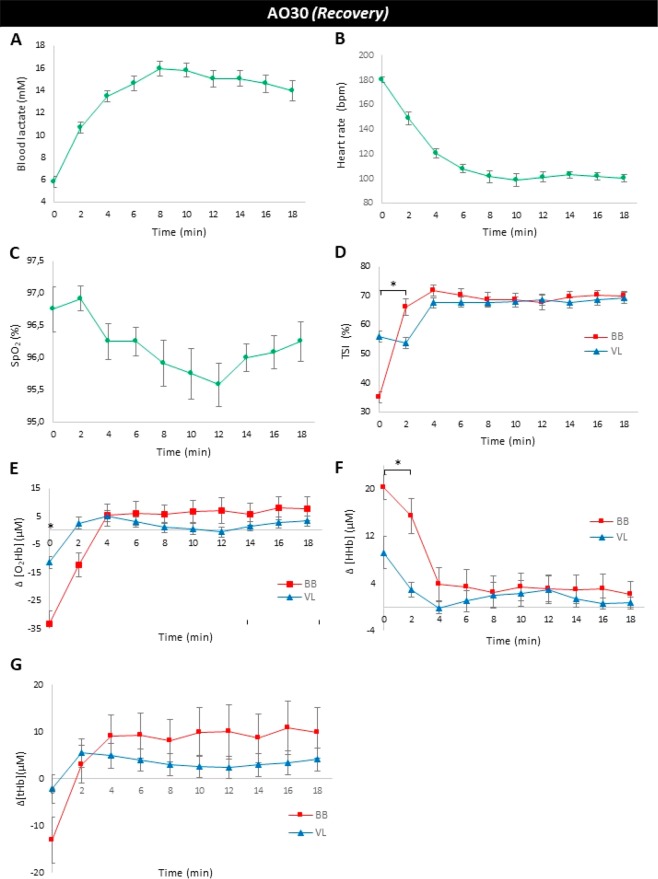
Table 3 and Fig. 4 summarize the main results of the physiological responses after AO30 in tethered running. Repeated one-way measures ANOVA showed effect of time on recovery of HR (F = 45.2, P = 0.000) and blood lactate (F = 20.49, P = 0.00), but not on SpO2 (F = 2.0, P = 0.055). The peak HR in the recovery phase occurred at 180 ± 2 bpm, which is equivalent to 91% of HRmax predicted by age (HRmax = 220-age). The HR curve showed a decrease in values after 2 minutes post-exercise. Despite lower results visualized during post-exercise, HR at 18th minute did not return to baseline value (100 ± 3 bpm vs 74 ± 3 bpm, respectively) (Fig. 4, Panel B). Immediately after AO30, the blood lactate started to increase (Fig. 4, Panel A) and the peak lactate (peak [Lac]) was attained individually at 8–10 minutes of the passive recovery, at 16.2 ± 0.7 mM. In addition, the rate of blood lactate recovery ((peak[Lac] – minimum [Lac])/peak [Lac]*100) was 64.4 ± 2.3%, which contributed to the partial removal of lactate observed at the end of AO30 (13.9 ± 0.9 mM) but not a return to the baseline concentration (0.94 ± 0.13 mM). SpO2 was not significantly modified during our protocol (Fig. 4, Panel C).
Table 3.
Peak, mean and minimum values of the heart rate (bpm), blood lactate concentration (mM), time to reach the peak of blood lactate concentration (min), rate of blood lactate recovery ((peak [Lac]–minimum [Lac])/peak [Lac] *100) (%) and arterial oxygen saturation (%) during post-exercise (AO30 recovery) (n = 12).
| Physiological Responses | |
|---|---|
| AO30s – Recovery | |
| Mean ± SEM | |
| Heart rate | |
| Peak HR (bpm) | 180 ± 2 |
| Mean HR (bpm) | 116 ± 3 |
| Minimum HR (bpm) | 95 ± 5 |
| Blood lactate | |
| Peak [Lac] (mM) | 16.2 ± 0.7 |
| Mean [Lac] (mM) | 13.5 ± 0.6 |
| Minimum [Lac] (mM) | 5.8 ± 0.5 |
| Time to peak [Lac] (min) | 9.2 ± 0.5 |
| [Lac] rate (%) | 64.4 ± 2.3 |
| SpO2 | |
| Peak SpO2 (%) | 97.1 ± 0,3 |
| Mean SpO2 (%) | 96.2 ± 0.2 |
| Minimum SpO2 (%) | 95.2 ± 0.3 |
HR-heart rate; [Lac] - blood lactate concentration; SpO2 – arterial oxygen saturation.
Figure 4.
Results obtained after all-out 30 s (AO30 recovery) at tethered running in a non-motorized treadmill (mean ± SEM at end and each 2 min during passive recovery, n = 12) (A) Blood lactate concentration (mM), (B) Heart rate (bpm), (C) arterial oxygen saturation – SpO2 (%), (D) Tissue saturation index (TSI) (%) in biceps brachii (BB) and vastus lateralis (VL), (E) Changes (ΔµM) in oxyhemoglobin ([O2Hb]) occurred in BB and VL, (F) Changes (ΔµM) in deoxyhemoglobin ([HHb]) in BB and VL, and (G) Changes (ΔµM) in total hemoglobin ([tHb]) occurred in BB and VL, measured by near infrared-spectroscopy (NIRS). *Indicates the difference between responses obtained in BB and VL at the same moment (P ≤ 0.05).
Muscle oxygenation curves in upper and lower limb are expressed in Fig. 4 (panels D–G). Two-way ANOVA for repeated measures presented the effect of time of test (F = 30.10, P < 0.001), site device NIRS (F = 108.99, P < 0.001) and interaction between time × site NIRS (F = 5.23, P < 0.001) to TSI. Differently from that observed with HR and blood lactate concentration, TSI in both muscles increased after the end of exercise, but in BB, this phenomenon was faster than VL. After the 4th minute of recovery, there were no differences between TSI in BB and VL, with values returning to close to those at rest.
In the recovery phase, oxy- and deoxyhemoglobin in both tissues (BB and VL) returned to initial values after 4 minutes of rest (Fig. 4, Panels E and F). When comparing the muscle oxygenation recovery curves to upper and lower limb tissues, we observed significant differences between BB and VL only at the first 4 minutes of recovery (to oxy- and deoxyhemoglobin), with BB showing re-oxygenation more quickly compared to VL. Delta of the total hemoglobin (Δ[tHb]) was not altered in BB and VL during the recovery phase, and the two-way ANOVA for repeated measures showed an effect of time (F = 2.46, P = 0.011) but did not indicate effects of site NIRS (F = 3.60, P = 0.590) and interaction between time × site NIRS (F = 1.11, P = 0.354) for this response.
In addition, the comparison and relationships between peak, mean and minimum values of the TSI and delta of oxyhemoglobin, deoxyhemoglobin and total hemoglobin in the biceps brachii and vastus lateralis (during exercise and recovery) are shown in Table 4. There were significant differences between TSI (mean and minimum values) in BB and VL during AO30, as well as minimum Δ[O2Hb] and peak and minimum Δ[HHb] between BB and VL muscles. After AO30 (recovery phase), we observed higher values of TSI in more active muscle (VL) compared to less active muscle (BB). Despite this, total hemoglobin was not significantly influenced by the recovery phase.
Table 4.
Peak, mean and minimum values of the muscle oxygenation (tissue saturation index and ΔµM of oxyhemoglobin [O2Hb], deoxyhemoglobin [HHb] and total hemoglobin [tHb]) in biceps brachii (BB) and vastus lateralis (VL) muscles in AO30 (exercise and recovery). Additionally, this table shows the differences and correlations between muscle oxygenation in BB and VL (n = 12).
| Muscle Oxygenation | ||||
|---|---|---|---|---|
| BB | VL | BB vs VL | ||
| Mean ± SEM | Mean ± SEM | P value | r value (P value) | |
| AO30s – Exercise | ||||
| TSI | ||||
| Peak (%) | 76 ± 2 | 79 ± 2 | 0.230 | 0.02 (0.939) |
| Mean (%) | 52 ± 2 | 60 ± 2 | 0.010 | 0.30 (0.331) |
| Minimum (%) | 31 ± 3 | 50 ± 4 | 0.000 | 0.55 (0.062) |
| Δ[O2Hb] | ||||
| Peak (µM) | 8 ± 3 | 8 ± 2 | 0.991 | −0,71 (0.825) |
| Mean(µM) | −16 ± 4 | −8 ± 2 | 0.160 | −0.56 (0.058) |
| Minimum (µM) | −33 ± 5 | −13 ± 2 | 0.007 | −0.36 (0.241) |
| Δ[HHb] | ||||
| Peak (µM) | 19 ± 2 | 10 ± 3 | 0.001 | 0.69 (0.013) |
| Mean (µM) | 5 ± 2 | 6 ± 2 | 0.613 | 0.48 (0.117) |
| Minimum (µM) | −10 ± 2 | −2 ± 1 | 0.003 | −0.16(0.629) |
| Δ[tHb] | ||||
| Peak (µM) | 3 ± 4 | 7 ± 3 | 0.406 | −0.47 (0.125) |
| Mean (µM) | −11 ± 5 | −2 ± 2 | 0.131 | −0.28 (0.383) |
| Minimum (µM) | −18 ± 5 | −5 ± 2 | 0.039 | −0.23 (0.478) |
| AO30s – Recovery | ||||
| TSI | ||||
| Peak (%) | 69 ± 2 | 75 ± 1 | 0.027 | −0.46 (0.134) |
| Mean (%) | 61 ± 2 | 71 ± 2 | 0.005 | −0.50 (0.096) |
| Minimum (%) | 35 ± 2 | 56 ± 2 | 0.000 | 0.37 (0.231) |
| Δ[O2Hb] | ||||
| Peak (µM) | 10 ± 5 | 6 ± 2 | 0.503 | −0,39 (0.213) |
| Mean(µM) | 1 ± 4 | 1 ± 2 | 0.988 | −0.82 (0.001) |
| Minimum (µM) | −33 ± 5 | −11 ± 2 | 0.003 | −0.36 (0.250) |
| Δ[HHb] | ||||
| Peak (µM) | 22 ± 2 | 9 ± 3 | 0.000 | 0.49 (0.109) |
| Mean (µM) | 6 ± 2 | 2 ± 2 | 0.207 | 0.16 (0.628) |
| Minimum (µM) | 0 ± 2 | −1 ± 1 | 0.927 | −0.09 (0.785) |
| Δ[tHb] | ||||
| Peak (µM) | 14 ± 6 | 7 ± 3 | 0.361 | −0.27 (0.388) |
| Mean (µM) | 7 ± 5 | 3 ± 3 | 0.574 | −0.35 (0.261) |
| Minimum (µM) | −14 ± 4 | −3 ± 3 | 0.052 | −0.07 (0.819) |
Results are expressed by mean ± standard error of the mean (SEM). Significance was pre-fixed at P ≤ 0.05.
In order to analyse the relationship between mechanical, physiological and muscle oxygenation in more or less active muscle, the Pearson linear regression test was applied. We did not observe a significant relationship among mechanical parameters (force, velocity and power) with physiological and muscle responses. On the other hands, significant correlations were observed between fatigue index with blood lactate and muscle oxygenation on BB. FI was significantly correlated with peak, mean and minimum [Lac] (r = 0.63, P = 0.027; r = 0.66, P = 0.020, and r = 0.68, P = 0.015, respectively), and it was inversely correlated with peak and mean Δ[tHb] in BB during AO30 exercise.
We chose to observe the correlations considering the points of the timeline close to the time of reaching the peak of lactate (i.e., in the 8th and 10th minutes of the recovery). In fact, although the BB and VL muscle oxygenation responses are similar in comparative analysis, the peak [Lac] showed only significant correlation with TSI and Δ[HHb] in BB at the 8th minute (r = 0.62, P = 0.028 and r = −0.75, P = 0.008, respectively) and at the 10th minute (r = 0.63, P = 0.028 and r = −0.77, P = 0.003, respectively), but not with the same responses in the VL. Adopting the minimum, mean and peak values to the muscle responses (as shown in Table 4), we observed an inverse relationship between peak [Lac] with minimum Δ[HHb] in biceps brachii during exercise (r = −0.61, P = 0.034), mean and minimum Δ[HHb] in recovery phase (r = −0.79, P = 0.002; and r = −0.86, P = 0.001, respectively) and mean and minimum TSI values in BB after exercise (r = 0.58, P = 0.046, and r = 0.66, P = 0.019). Still in this way, mean [Lac] showed an inverse and significant correlation with mean and minimum Δ[HHb] in BB at post-effort (r = −0.77, P = 0.03, and r = −0.82, P = 0.030), and with a mean of TSI in BB during recovery phase (r = 0.64, P = 0.025). Relationships between this metabolite and vastus lateralis oxygenation were only observed by peak [Lac] with mean Δ[O2Hb] (r = 0.58, P = 0.46) and mean [Lac] with mean and minimum Δ[tHb] values (r = 0.66, P = 0.019, and r = 66, P = 0.020, respectively), both during AO30 responses.
Discussion
The main highlight of the study was purposing a synchronized form to investigate the mechanical, physiological and oxygenation responses in more or less active muscles during and after high-intensity exercise (AO30) in tethered running conditions. To the best of our knowledge, this is the first study to evaluate muscle oxygen responses in this type of exercise. Here, we used robust tools to obtain power running in an anaerobic effort using high-frequency signal capture and muscle oxygenation measurements with NIRS of the upper and lower limb. The NIRS used here were characterized by wearable and wireless technology currently at the frontiers of knowledge in exercise physiology. We are certain that it is necessary to improve the understanding of the interaction among physiological signals observed during and after high-intensity effort, as in tethered exercises.
The choice to analyse running exercise was due to the importance of this motor skill in high-intensity efforts employed in the sports modality. Our choice to use the tethered system was based on the path to allow quantification of the running power, since tethered running training and sled training have been frequently used in sports evaluation52,53, and physical training programs aimed at improving velocity and power54,55.
Regarding the mechanical responses throughout the AO30, we measured both vertical and horizontal force components during the running exercise to calculate the power run (Fig. 1). The current findings of peak and mean power (Table 2 and Fig. 3, Panels A and B) were higher than those observed by other authors who used the same ergometer to evaluate recreational endurance runners26 and young soccer player athletes in this motorized treadmill56, but using only the horizontal force component. In this sense and to check this, when analysed at the same form of the cited authors, the peak and mean powers (peak power = 714.4 ± 35.0 W and 10.0 ± 0.3 W/kg) were similar to those observed by them. The peak power (Fig. 3, panels A and B) was obtained around 6 second at the AO30, and the curve fit behaviour in tethered running was similar to that observed in another 30 s all-out kind of exercise, such as power responses in cycle ergometer25 and force curve in swimming57 and kayak effort58. In contrast, the peak of running velocity in our experiment was obtained within a lower time than that recently observed by Morrison et al.17 who submitted amateur athletes to four series of AO4s sprints in a non-motorized treadmill (Woodway, Waukesha, Wisconsin). These differences can be attributed to the sample characteristics, time of effort and the use of the tethered run, in our experimental design.
Classically, all-out efforts like 30 s have been used to evaluate mechanical power and the anaerobic system efficiencies59,60. The physiological responses during and after AO30 tethered running confirm the high-intensity nature of this exercise to our active subjects, as it occurs, for example, in sprint interval exercise12,48. Within a single short duration bout (30 s), HR reached near maximum values (Fig. 3, Panel B) and the exercise showed a lactic anaerobic characteristic, with peak lactate concentration reaching high values (16.3 ± 0.7 mM). These physiological responses did not return to the rest values even after 18 minutes of recovery (Fig. 4, Panels A and B). In contrast, as observed by Morrison et al.17 in repeated treadmill sprints, the arterial saturation oxygenation (SpO2) was not altered by AO30 (Fig. 4, panel C).
In 2011, Ferarri, Muthalib and Quaresma30 conducted an interesting review aimed at understanding the skeletal muscle physiology using NIRS technology. Thus, years ago these authors suggested as a possible future direction the association of the muscle oxygenation measurement with other physiological responses monitored during tests and training (for example, HR and blood lactate concentration). Recently, this way has been accomplished and some studies aimed to adopt these recommendations16,18.
However, despite the significance of tissue oxygenation18 there are very few studies investigating the more and less active muscle responses during and after exercise14,42,61–63 especially in running effort12,19. According to Perrey and Ferrari in a recent review39, the majority of the NIRS studies examined the responses of the vastus lateralis muscle. On the other hand, we believe that knowledge about simultaneous muscle oxygenation in different tissues can provide the potential to improve comprehension of the internal load, especially in dynamics of exercise:rest ratio prescription in physical and sport training programs.
Based on our current knowledge, there is an important lack of investigations evaluating the Δ[HHb] or TSI in simultaneous analyses of arm and leg muscles during and after high-intensity running exercise19, and in all-out tethered running. Thus, our main results on muscle oxygenation during AO30 were the differences shown between tissue saturation (TSI) in biceps brachii (BB) and vastus lateralis (VL) (Fig. 3, panel D). Although the TSI decreased significantly for both muscles studied here, there was a greater drop in the biceps brachii (from ~70% to ~35%) compared to the VL responses (from ~75% to ~55%) suggesting an integrative physiological adjustment to maintain the more active musculature during high-intensity exercise, at least in short duration all-out tethered running. In biceps brachii, TSI at the end of all-out effort was very low (35 ± 2%) (Fig. 3 and Table 4), which is what strengthens this hypothesis regarding the difference between leg and arm muscles in AO30 tethered running.
Our results corroborated a previous study involving simultaneous biceps brachii and vastus lateralis during high-intensity incremental treadmill exercise19 Rissanen et al.19 investigated the BB, VL and alveolar gas exchange of healthy male volunteers submitted to an incremental running effort (started at 8 km/h, with a 1 km/h increase every 3 minutes until volitional exhaustion). In that study an initial moderate decrease in the oxygenation level in low-intensity exercise was observed followed by a rapid decrease in severe effort (greater for BB when compared to VL), suggesting that the O2 delivery to the less active muscle (BB) may be limited by the increase in ventilation in high-intensity running exercise. Although we investigated here the AO30 running effort unlike incremental treadmill running, it is possible that the acceleration of ventilatory responses in all-out exercise promoted a similar effect to that suggested by Rissanen19 on the TSI of the less active muscle.
Additionally, according to Secher and Volantis64 the greater TSI drop in the less active muscle as observed here in BB can be explained by the sympathetic flow induced by exercise, promoting vasoconstriction in this tissue and consequently a redirection of the blood flux to the more active muscle. A similar way was used by Shiroishi et al.65 to explain the decreased muscle oxygenation in the non-exercising limb during a graded leg cycling exercise, adopting NIRS and ultrasound measurements.
Kriel et al.12,66 suggested that Δ[HHb] is potentially unaffected by changes in perfusion, blood volume and arterial hemoglobin concentration in high-intensity exercise, in contrast to Δ[O2Hb]. So, due to these characteristics, we will focus our discussion on the delta of deoxyhemoglobin responses (Δ[HHb]). During AO30, we observed the drop in Δ[HHb] results in both BB and VL muscles. After this, Δ[HHb] presented stabilization to the vastus lateralis muscle oxygenation. At 28–30 seconds of exercise, the O2 utilization (signalized by Δ[HHb]) was higher in BB if compared to VL. We did not observe differences between Δ[tHb] in BB and VL during the A030 effort but a greater interindividual variation was shown in biceps brachii, which should be considered in future studies. Bhambhani67 based on the reports of Bae et al.68 suggested a significant contribution of the aerobic metabolism during high-intensity short duration exercise (such as the anaerobic Wingate test). Corroborating with these authors, our results showed an aerobic contribution during AO30 in tethered running, along with additional information about the significant participation of the less active muscle in this process.
Still comparing biceps brachii vs vastus lateralis oxygen responses, in a recent and interesting study, Willis et al.13 investigated the leg vs arm cycling repeated sprints with blood restriction and systemic hypoxia on peripheral and cerebral oxygenation. These authors observed greater changes in Δ[HHb] and Δ[tHb] in BB, suggesting that the arm is more responsible or sensitive to oxygen changes, especially induced by hypoxia, and it has a greater capacity to increase oxygen extraction in comparison to leg muscle. Although Willis et al.13 analysed the oxygenation of BB and VL, the purpose of those authors was not to investigate the responses of these muscles as more or less active in the same exercise, which makes comparison with our results difficult.
Another significant feature of our study was to monitor the metabolic and muscle oxygenation responses in a synchronized form up to 18 minutes after exercise by selecting investigation windows at every two minutes (Fig. 4). Thus, it was possible to observe which physiological responses return faster to the pre-exercise conditions, since recovery is an important component to improve physical training adaptations. Using these analyses, we observed that general responses (HR and blood lactate) did not return to baseline values after the recovery time chosen, at least in the active subjects studied here. However, the muscle oxygenation in the arm and leg are quickly adjusted in post-exercise, specially in BB, indicating that the use of NIRS technology showed it has higher sensitivity than classical exercise intensity markers, as well as can be used to improve the exercise monitoring and training prescription, as suggested by other research groups30,39.
As pertinently pointed out by Barstow69, the standardisation of protocols using NIRS is necessary. The attention to the instructions/limitations of each piece of equipment is important in order to minimise the possible intrinsic errors of the measurement; for example, the influence of the skin melanina and adipose tissue thickness69. In the present study, considerable differences were seen between BB and VL skinfold, which could account for some the of the differences seen in oxygenation responses. Aiming to minimise this methodological aspect, we use a NIRS devices that appears to be less sensitive to variations in adipose layer thickness70 applying the spatially resolved spectroscopy technique to analyse the TSI, and we choose the deeppest optode (40 mm) to investigated the [O2Hb], [HHb], and [tHb] in both muscles.
Focusing on muscle oxygenation responses after AO30, we observed differences between TSI and Δ[HHb] in the arm and leg muscles, especially in the initial minutes of exercise. BB showed lower TSI values immediately after AO30 than VL, but there was a quick and important reoxygenation in BB compared to VL (TSI in BB to from ~35% to ~70% within two minutes of recovery) (Fig. 4, Panel D). Our results are the opposite of Osawa et al.14, who investigated the recovery of BB after supramaximal cycling exercise with legs (140% of VO2peak for 30 s and then no-load cycle exercise for 4 minutes). They observed that recovery of tissue oxygenation in biceps brachii did not occur immediately after effort and the reoxygenation in the arm was slower than in the leg. However, the exercise type adopted by Osawa et al.14 (cycling efforts for leg) and recovery process (arm in rest and leg in movement) are different that applied by us. In the light of our study, for high-intensity running exercise, the biceps brachii seems to play an important role during process recovery, which implies in future proposals of aerobic physical training to potentiate this muscle response.
In high-performance sports and physical training programmes, blood lactate concentration is widely used as a tool to quantify exercise intensity and to monitor training effects, given that this metabolite has the highest muscle production, release into the bloodstream8, and accumulates in response to exercise intensity1. Therefore, we proposed studying the relationship between blood lactate with muscle oxygenation parameters. Here, even though there was significant correlation between blood lactate concentration and the fatigue index in the time of the recovery, around the time to attain the peak of blood lactate, we only observed a significant correlation between peak [Lac] value to biceps brachii TSI and Δ[HHb] (in the 8th and 10th minute of the post-exercise period). In addition, when we applied Pearson’s product-moment test to analyse the correlations between blood lactate and peak, mean, and minimum muscle oxygenation responses, the most significant results were obtained with BB in exercise and recovery, but not with VL (the greatest blood lactate producer in our experimental design). These results reinforce an important contribution of the less active muscles on blood lactate responses, suggesting that more attention should be paid to this factor in exercise and training prescription. Corroborating with Willis et al.13, we also suggest that coaches and practitioners plan different training protocols for arms and legs to increase performance and physical adaptations, particularly for intensity running exercise.
Finally, in addition to other current measurements, such as HR, blood lactate, and VO2, we believe that very shortly peripherical oxygenation (including more or less active muscles) will be used to measure the internal load of training and recovery sessions, such as proposed here. We still agree that, despite some limitations69, wearable NIRS technology is a significant tool for monitoring the effects of training programs.
Due to this being a characterisation study about the kinetics of mechanical and physiological responses in all-out 30 seconds (AO30) tethered running followed by 18 minutes of passive recovery, some limitations must be considered. We did not use a gas analyser to investigate the oxygen uptake, even knowing the relevance of the VO2, as a continuous measurement in the experimental design adopted here. We considered that the use of the mask in this methodology could compromise our main goals. Another limitation of this study was sample composition, comprising only active male subjects. Future investigations with a similar protocol could be conducted with athletes of different modalities and also female participants.
In summary, here we provide the characterization of the mechanical, physiological and muscle oxygenation kinetics during and after high-intensity exercise, in particular improving the understanding of all-out tethered running. In addition to the important mechanical loading imposed, this kind of exercise promotes a high internal load during effort observed by physiological measurements, which do not return to rest values although after 18 minutes of passive recovery (at least by blood lactate and HR of the active subjects). On the other hand, muscle oxygenation responses presented faster adjustments (both during and after AO30) in a tissue-dependence manner, with very low TSI values observed in biceps brachii during the all-out effort. In addition, the significant correlations between peak and mean blood lactate with biceps brachii oxygenation indicate an integrative response of less active muscle oxygenation and metabolic events during and after high-intensity AO30 tethered running.
Acknowledgements
The study was supported by São Paulo Research Foundation – FAPESP (2009/08535-5, 2012/06355-2, 2016/50250-1, and 2018/05821-6), National Council for Scientific and Technological Development – CNPq (307718/2018-2, 308117/2018-2), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. So, we would like to express our thanks for this support.
Author contributions
F.B.M.G. and C.A.G. contributed to the proposal of ideas, the conception, design of the work, interpretation of data, the writing of the main manuscript text, preparing figures and tables and contributed to funding acquisition. M.A.M. contributed to the proposal of ideas, conception and funding acquisition. A.B.M., F.M.R., C.C., J.P.C. contributed to acquisition and data analysis. All authors reviewed the manuscript and have approved the submitted version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Billat VL, Sirvent P, Py G, Kolarlsztein JP, Mercier J. The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med. 2003;20:407–426. doi: 10.2165/00007256-200333060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Jones AM, Burnley M, Black MI, Poole DC, Vanhatalo A. The maximal metabolic steady state: redefining the ‘gold standard’. Physiol. Rep. 2019;7:e14098. doi: 10.14814/phy2.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017;595:2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2014;39:409–412. doi: 10.1139/apnm-2013-0187. [DOI] [PubMed] [Google Scholar]
- 5.McMorrow BJ, Ditroilo M, Egan B. Effect of heavy resisted sled sprint training during the competitive season on sprint and change-of-direction performance in professional soccer players. Int. J. Sports Physiol. Perform. 2019;14:1066–1073. doi: 10.1123/ijspp.2018-0592. [DOI] [PubMed] [Google Scholar]
- 6.Petrakos G, Morin JB, Egan B. Resisted sled sprint training to improve sprint performance: A systematic review. Sports Med. 2016;46:381–400. doi: 10.1007/s40279-015-0422-8. [DOI] [PubMed] [Google Scholar]
- 7.Impellizzeri FM, Marcora SM, Coutts AJ. Internal and external training load:15 years on. Int. J. Sports Physiol. Perform. 2019;14:270–273. doi: 10.1123/ijspp.2018-0935. [DOI] [PubMed] [Google Scholar]
- 8.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflug. Arch. - Eur. J. Physiol. 2004;447:619–628. doi: 10.1007/s00424-003-1067-2. [DOI] [PubMed] [Google Scholar]
- 10.Bonen A, et al. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am. J. Physiol. Endocrinol. Metab. 2000;279:1131–1138. doi: 10.1152/ajpendo.2000.279.5.E1131. [DOI] [PubMed] [Google Scholar]
- 11.Faude O, Kindermann W, Meyer T. Lactate threshold concepts: how valid are they? Sports Med. 2009;39:469–490. doi: 10.2165/00007256-200939060-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kriel Y, Askew CD, Solomon C. Sprint interval exercise versus continuous moderate intensity exercise: acute effects on tissue oxygenation, blood pressure and enjoyment in 18-30 year old inactive men. PeerJ. 2019;7:e7077. doi: 10.7717/peerj.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis SJ, Borrani F, Millet GP. Leg-vs arm-cycling repeated sprints with blood flow restriction and systemic hypoxia. Eur. J. Appl. Physiol. 2019;119:1819–1828. doi: 10.1007/s00421-019-04171-0. [DOI] [PubMed] [Google Scholar]
- 14.Osawa T, Shiose K, Takahashi H. Delayed onset of reoxygenation in inactive muscles after high-intensity exercise. Adv. Exp. Med. Biol. 2017;977:255–260. doi: 10.1007/978-3-319-55231-6_35. [DOI] [PubMed] [Google Scholar]
- 15.Boone J, Koppo K, Barstow TJ, Bouckaert J. Effect of exercise protocol on deoxy[Hb + Mb]: incremental step versus ramp exercise. Med. Sci. Sports Exerc. 2010;42:935–942. doi: 10.1249/MSS.0b013e3181c0ecea. [DOI] [PubMed] [Google Scholar]
- 16.Woorons X, Dupuy O, Mucci P, Millet GP, Pichon A. Cerebral and muscle oxygenation during repeated shuttle run sprints with hypoventilation. Int. J. Sports Med. 2019;40:376–384. doi: 10.1055/a-0836-9011. [DOI] [PubMed] [Google Scholar]
- 17.Morrison JD, Quinn K, MacDonald LA, Billaut F, Minahan C. Repeated treadmill sprints impair cognitive performance in amateur team-sport athletes when performed in normobaric hypoxia. J. Sports Sci. Med. 2019;18:369–375. [PMC free article] [PubMed] [Google Scholar]
- 18.Bhambhani Y, Fan JL, Place N, Rodriguez-Falces J, Kayser B. Electromyographic, cerebral, and muscle hemodynamic responses during intermittent, isometric contractions of the biceps brachii at three submaximal intensities. Front. Physiol. 2014;5:190. doi: 10.3389/fphys.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rissanen AP, et al. Alveolar gas exchange and tissue oxygenation during incremental treadmill exercise, and their associations with blood O2 carrying capacity. Front. Physiol. 2012;3:1–11. doi: 10.3389/fphys.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira VH, et al. Complex network models reveal correlations among network metrics, exercise intensity and role of body changes in the fatigue process. Sci. Rep. 2015;5:10489. doi: 10.1038/srep10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gama MCT, Dos Reis IGM, Sousa FAB, Gobatto CA. The 3-min all-out test is valid for determining critical power but not anaerobic work capacity in tethered running. PLoS One. 2018;13:e0192552. doi: 10.1371/journal.pone.0192552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa FAB, Vasque RE, Gobatto CA. Anaerobic metabolism during short all-out efforts in tethered running: Comparison of energy expenditure and mechanical parameters between different sprint durations for testing. PLoS One. 2017;12:e0179378. doi: 10.1371/journal.pone.0179378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zemková E, Hamar D. “All-out” tethered running as an alternative to Wingate anaerobic test. Kinesiol. - Int. J. Fundamental Appl. Kinesiol. 2004;36:165–170. [Google Scholar]
- 24.Cheetham ME, Williams C, Lakomy HK. A laboratory running test: metabolic responses of sprint and endurance trained athletes. Br. J. Sports Med. 1985;19:81–84. doi: 10.1136/bjsm.19.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bar-Or O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987;4:381–394. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 26.Zagatto AM, Miyagi WE, Sousa FA, Gobatto CA. Relationship between anaerobic capacity estimated using a single effort and 30-s tethered running outcomes. PLoS One. 2017;12:e0172032. doi: 10.1371/journal.pone.0172032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jobsis, F. F. Non-invasive, infra-red monitoring of cerebral O2 sufficiency, blood volume, HbO2-Hb shifts and bloodflow. Acta Neurol Scand Suppl64, 452–453, PMID 268870 (1977). [PubMed]
- 28.Jobsis FF. What is a molecular oxygen sensor? What is a transduction process? Adv. Exp. Med. Biol. 1977;78:3–18. doi: 10.1007/978-1-4615-9035-4_1. [DOI] [PubMed] [Google Scholar]
- 29.Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J. Biomed. Opt. 2016;21:091313. doi: 10.1117/1.JBO.21.9.091313. [DOI] [PubMed] [Google Scholar]
- 30.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2011;369:4577–4590. doi: 10.1098/rsta.2011.0230. [DOI] [PubMed] [Google Scholar]
- 31.Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J. Biomed. Opt. 2007;12:062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 32.Lee BY, Ostrander E, Karmakar M, Frenkel L, Herz B. Noninvasive quantification of muscle oxygen in subjects with and without claudication. J. Rehabil. Res. Dev. 1997;34:44–51. [PubMed] [Google Scholar]
- 33.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. J. Gerontol. 49, B128-134. 1994 doi: 10.1093/geronj/49.3.b128. [DOI] [PubMed] [Google Scholar]
- 34.Lagerwaard B, Keijer J, McCully KK, de Boer VCJ, Nieuwenhuizen AG. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur. J. Appl. Physiol. 2019;119:1799–1808. doi: 10.1007/s00421-019-04169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oueslati F, Boone J, Ahmaidi S. Respiratory muscle endurance, oxygen saturation index in vastus lateralis and performance during heavy exercise. Respir. Physiol. Neurobiol. 2016;227:41–47. doi: 10.1016/j.resp.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Jones B, Cooper CE. Near infrared spectroscopy (NIRS) observation of vastus lateralis (muscle) and prefrontal cortex (brain) tissue oxygenation during synchronised swimming routines in elite athletes. Adv. Exp. Med. Biol. 2018;1072:111–117. doi: 10.1007/978-3-319-91287-5_18. [DOI] [PubMed] [Google Scholar]
- 37.Jones B, Hamilton DK, Cooper CE. Muscle oxygen changes following Sprint Interval Cycling training in elite field hockey players. PLoS One. 2015;10:e0120338. doi: 10.1371/journal.pone.0120338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesford CM, Laing S, Cooper CE. Using portable NIRS to compare arm and leg muscle oxygenation during roller skiing in biathletes: a case study. Adv. Exp. Med. Biol. 2013;789:179–184. doi: 10.1007/978-1-4614-7411-1_25. [DOI] [PubMed] [Google Scholar]
- 39.Perrey S, Ferrari M. Muscle Oximetry in Sports Science: A Systematic Review. Sports Med. 2018;48:597–616. doi: 10.1007/s40279-017-0820-1. [DOI] [PubMed] [Google Scholar]
- 40.Ainsworth BE, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 41.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br. J. Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, et al. Comparisons of muscle oxygenation changes between arm and leg muscles during incremental rowing exercise with near-infrared spectroscopy. J. Biomed. Opt. 2010;15:017007. doi: 10.1117/1.3309741. [DOI] [PubMed] [Google Scholar]
- 43.Ogata H, et al. Relationship between oxygenation in inactive biceps brachii muscle and hyperventilation during leg cycling. Physiol. Res. 2007;56:57–65. doi: 10.33549/physiolres.930888. [DOI] [PubMed] [Google Scholar]
- 44.Kitada T, Machida S, Naito H. Influence of muscle fibre composition on muscle oxygenation during maximal running. BMJ Open. Sport. Exerc. Med. 2015;1:e000062. doi: 10.1136/bmjsem-2015-000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner LA, et al. Inspiratory loading and limb locomotor and respiratory muscle deoxygenation during cycling exercise. Respir. Physiol. Neurobiol. 2013;185:506–514. doi: 10.1016/j.resp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 46.Guidetti L, Rivellini G, Figura F. EMG patterns during running intra-and inter-individual variability. J. Eletromyogr Kinesiol. 1996;6:37–48. doi: 10.1016/1050-6411(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 47.Buchheit M, Laursen PB, Ahmaidi S. Effect of prior exercise on pulmonary O2 uptake and estimated muscle capillary blood flow kinetics during moderate-intensity field running in men. J. Appl. Physiol. 2009;107:460–470. doi: 10.1152/japplphysiol.91625.2008. [DOI] [PubMed] [Google Scholar]
- 48.Buchheit M, Bishop D, Haydar B, Nakamura FY, Ahmaidi S. Physiological responses to shuttle repeated-sprint running. Int. J. Sports Med. 2010;31:402–409. doi: 10.1055/s-0030-1249620. [DOI] [PubMed] [Google Scholar]
- 49.Faiss, et al. Repeated double-poling training in hypoxia by competitive cross-country skiers. Med. Sci. Sports Exerc. 2015;47:809–17. doi: 10.1249/MSS.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 50.Duncan A, et al. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys. Med. Biol. 1995;40:295–304. doi: 10.1088/0031-9155/40/2/007. [DOI] [PubMed] [Google Scholar]
- 51.Jones B, Hesford CM, Cooper CE. The use of portable NIRS to measure muscle oxygenation and haemodynamics during a reapeted sprint running test. Adv. Exp. Med. Biol. 2013;789:185–191. doi: 10.1007/978-1-4614-7411-1_26.. [DOI] [PubMed] [Google Scholar]
- 52.Sousa F, Dos Reis I, Ribeiro L, Martins L, Gobatto C. Specific Measurement of Tethered Running Kinetics and its Relationship to Repeated Sprint Ability. J. Hum. Kinet. 2015;49:245–256. doi: 10.1515/hukin-2015-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira VH, et al. Computational and complex network modeling for analysis of sprinter athletes’ performance in track field tests. Front. Physiol. 2018;9:843. doi: 10.3389/fphys.2018.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawamori N, Newton RU, Hori N, Nosaka K. Effects of weighted sled towing with heavy versus light load on sprint acceleration ability. J. Strength. Cond. Res. 2014;28:2738–2745. doi: 10.1519/JSC.0b013e3182915ed4. [DOI] [PubMed] [Google Scholar]
- 55.Cronin J, Hansen K, Kawamori N, McNair P. Effects of weighted vests and sled towing on sprint kinematics. Sports Biomech. 2008;7:160–172. doi: 10.1080/14763140701841381. [DOI] [PubMed] [Google Scholar]
- 56.Andrade VL, et al. Running-based Anaerobic Sprint Test as a Procedure to Evaluate Anaerobic Power. Int. J. Sports Med. 2015;36:1156–1162. doi: 10.1055/s-0035-1555935. [DOI] [PubMed] [Google Scholar]
- 57.Papoti M, Martins LE, Cunha SA, Zagatto AM, Gobatto CA. Effects of taper on swimming force and swimmer performance after an experimental ten-week training program. J. Strength. Cond. Res. 2007;21:538–542. doi: 10.1519/R-14894.1. [DOI] [PubMed] [Google Scholar]
- 58.Messias LHD, et al. All-out test in tethered canoe system can determine anaerobic parameters of elite kayakers. Int. J. Sports Med. 2015;36:803–808. doi: 10.1055/s-0035-1548766. [DOI] [PubMed] [Google Scholar]
- 59.MacIntosh BR, MacEachern P. Paced effort and all-out 30-second power tests. Int. J. Sports Med. 1997;18:594–599. doi: 10.1055/s-2007-972687. [DOI] [PubMed] [Google Scholar]
- 60.Green S. Measurement of anaerobic work capacities in humans. Sports Med. 1995;19:32–42. doi: 10.2165/00007256-199519010-00003. [DOI] [PubMed] [Google Scholar]
- 61.Vogiatzis I, et al. Intercostal muscle blood flow limitation in athletes during maximal exercise. J. Physiol. 2009;587:3665–3677. doi: 10.1113/jphysiol.2009.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones B, Cooper CE. Underwater near-infrared spectroscopy: muscle oxygen changes in the upper and lower extremities in club level swimmers and triathletes. Adv. Exp. Med. Biol. 2016;876:35–40. doi: 10.1007/978-1-4939-3023-4_4. [DOI] [PubMed] [Google Scholar]
- 63.Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front. Physiol. 2014;5:142. doi: 10.3389/fphys.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Secher NH, Voliantis S. Are the arms and leg in competition for cardiac output? Med. Sci. Sports Exerc. 2006;38:1793–1803. doi: 10.1249/01.mss.0000230343.64000.ac. [DOI] [PubMed] [Google Scholar]
- 65.Shiroishi K, et al. Decreased muscle oxygenation and increased arterial blood flow in the non-exercising limb during leg exercise. Adv. Exp. Med. Biol. 2010;662:379–84. doi: 10.1007/978-1-4419-1241-1_552010. [DOI] [PubMed] [Google Scholar]
- 66.Kriel Y, Kerherve HA, Askew CD, Solomon C. The effect of active versus passive recovery periods during high intensity intermittent exercise on local tissue oxygenation in 18 - 30 year old sedentary men. PLoS One. 2016;11:e0163733. doi: 10.1371/journal.pone.0163733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhambhani YN. Muscle oxygenation trends during dynamic exercise measured by near infrared spectroscopy. Can. J. Appl. Physiol. 2004;29:504–23. doi: 10.1139/h04-033. [DOI] [PubMed] [Google Scholar]
- 68.Bae SY, et al. Comparison of muscle oxygen consumption measured by near infrared continuous wave spectroscopy during supramaximal and intermittent pedalling exercise. Int. J. Sports Med. 2000;21:168–174. doi: 10.1055/s-2000-8880. [DOI] [PubMed] [Google Scholar]
- 69.Barstow TJ. Understanding near infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2018;126:1360–1376. doi: 10.1152/japplphysiol.00166. [DOI] [PubMed] [Google Scholar]
- 70.McManus CJ, Collison J, Cooper CE. Performance comparision of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J. Biomed. Opt. 2018;23:1–14. doi: 10.1117/1.JBO.23.1.015007. [DOI] [PubMed] [Google Scholar]