Abstract
The modification of habitats in urban areas is thought to alter patterns of species interactions, by filtering specialist species and those at higher trophic levels. However, empirical studies addressing these hypotheses remain limited in scope and number. This work investigates (1) how main urban land uses affect predator-prey and mutualistic interactions, and (2) how specialist and generalist predators respond to size and availability of urban green spaces. In a large town in the UK, experimental colonies of ant-attended Black bean aphid Aphis fabae and non-ant-attended Pea aphid Acyrthosiphon pisum were monitored over two years. Ants were more frequently found in highly urbanised sites; however mutualistic ants were also more often encountered when the habitat was more plant diverse. Aphids were not affected by urban land uses, but A. fabae numbers were positively related to the presence of mutualists, and so indirectly affected by urbanisation. Predators were the only group negatively affected by increased urbanisation, and specialist species were positively related to increased proportion of urban green areas within the habitats. While this work supports the hypothesis that specialist predators are negatively affected by urbanisation, we also show that a fundamental ecological interaction, mutualism, is affected by urbanisation.
Subject terms: Ecology, Urban ecology
Introduction
Urbanisation is one of the defining environmental trends of recent times, almost completely modifying natural environments and significantly reducing local biodiversity1,2. Nevertheless, in most urban areas some native vegetation remains alongside introduced species within the urban matrix, mostly in parks and suburban gardens3, and their presence promotes biodiversity and provides ecosystem services (e.g. pollination, nutrient cycling). Urban areas can therefore be of value for biodiversity and conservation4. However, we have little understanding of how urbanisation modifies the patterns of species interactions.
At a simple level, urbanisation replaces natural systems with smaller sealed and impermeable areas, resulting in the reduction and fragmentation of habitat5. Beyond that, urbanisation changes key factors including local climate, nutrient availability, and disturbance levels6. Such factors change host plant quality, availability and accessibility (bottom-up factors), alter the abundance and diversity of natural enemies (top-down factors), and may modify the occurrence or intensity of mutualisms and competition (lateral factors)7,8. All such changes may alter how species interact, changing how ecological communities are structured in urban environments9.
Interactions between arthropod predators and their prey are particularly predisposed to being disrupted by urbanisation8,10, with specialist predators in particular likely to present a higher degree of sensitivity to the environmental disturbances that arises from habitat alteration10–12. It is not only consumptive interactions that may be affected by urbanisation. It is likely that mutualisms will also be affected13. The intensity and occurrence of mutualistic interactions can be strongly dependent on the physical and biological setting in which they occur14,15. Surprisingly, to our knowledge there are no studies that empirically evaluate how increased urbanisation might affect interactions between mutualists and other trophic groups (i.e. herbivores and enemies). Populations of insect herbivores, such as aphids and associated natural enemies and mutualists, can be used as a model system to address such questions.
Aphids are widespread and abundant in urban habitats16. Their populations are structured by host plant quality and availability, natural enemies, and for some species, interactions with ant mutualists17. Bottom-up effects are the consequence of variation in host plant diversity, quality and structure e.g.18. Top down effects are the result of the action of both generalist and specialist natural enemies, such as spiders, parasitoids, coccinellid beetles and hoverflies e.g.19. Lateral factors include the presence of mutualists; some aphid species are mymercophiles, tended and protected by ants in return for honeydew20. Each group of interactions may be affected by the biotic and abiotic changes typical of urban ecosystems, allowing us to tease apart how urbanisation may affect predator-prey and mutualistic interactions.
In this study, we are using a tri-trophic system of aphids and their associated predators and mutualistic ants to ask how the main land uses that compose cities (gardens, woodlands, roads and buildings), and the plant species richness of urban green areas affects aphid population numbers and the presence (or absence) of the mutualistic ants and predators associated with aphid colonies. Our study system was composed of two host plants (Vicia faba L.), one carrying a colony of Acyrthosiphon pisum Harris and the other a colony of Aphis fabae Scopoli, which were placed on a gradient of urbanisation in a large town in southern England. Both aphid species are known to suffer heavy predation20,21, but in contrast to A. pisum, A. fabae is regularly ant-attended. El‐Ziady and Kennedy22 demonstrated that the ant Lasius niger Linnaeus attending A. fabae accelerated the rate of multiplication and growth of the aphid colony and decreased the proportion of winged (dispersing) individuals among the adults. These ants showed “ownership behaviour”, acting aggressively against intruders such as ladybirds and hoverfly larvae. However, Pontin23 demonstrated that Lasius species regularly prey on aphids of non-myrmecophilus species. As such, our second question is how urban habitat features and plant richness can mediate interactions between aphids, predators and ants, given the behaviour of ants defending A. fabae against its predators, and also the potential competitive interspecific interactions of ants that prey on A. pisum with other predatory species. Lastly, we ask if specialist and generalist predators respond differently to the amount of green spaces present in the urban area.
Results
In total, we observed 18490 Acyrthosiphon pisum Harris (the pea aphid, hereafter PA) aphids and 46804 Aphis fabae Scopoli (the black bean aphid, hereafter BB) aphids, 377 PA predators and 374 BB predators, 244 ants preying on PA colonies and 1555 mutualistic ants on BB colonies. Two ant species, Myrmica rubra (L.) and Lasius niger (L.), were found on PA and BB colonies. Parasitized aphids were found in negligible numbers (not found on PA colonies and only found on period 3, 4 and 7 on BB colonies in few study sites). Analysis of the latter is reported in Rocha and Fellowes24. The proportion of habitat elements and their maximum and minimum values are shown in Table 1.
Table 1.
Mean proportion (±SE) and range values of habitat elements within 30 meters buffers of the study sites.
| Plant richness | Roads | Buildings | Woodland | Gardens | Green areas | |
|---|---|---|---|---|---|---|
| Mean (±SE) | 34.90 ± 1.24 | 0.287 ± 0.014 | 0.122 ± 0.008 | 0.191 ± 0.024 | 0.349 ± 0.016 | 0.545 ± 0.018 |
| Range | 14–100 | 0–0.774 | 0–0.463 | 0–1 | 0–0.719 | 0–0.848 |
Urban land use and interactions between aphids, ants and predators
Numbers of predators were positively correlated with aphid numbers (Table 2, models 3 and 4). The presence of predatory ants did not affect PA numbers, and the presence of mutualistic ants was associated with increased numbers of BB (model 2). Habitat features did not significantly affect aphid numbers (models 1 and 2). The presence of ants negatively affected the likelihood of finding predators on colonies of both aphid species (models 3 and 4). Fewer BB predators were found in areas with a higher proportion of roads, and an increased proportion of buildings in the habitat negatively affected the presence of PA predators, but positively affected the numbers of predatory ants, found on PA colonies (models 3, 4 and 5). Increased numbers of mutualistic ants on BB colonies were associated with increased BB colony size, higher local plant species richness and higher proportions of roads in the study sites (model 6).
Table 2.
Summary of models predicting abundance of Aphis fabae (BB) and Acyrthosiphon pisum (PA) and the occurrence of predators and ants found on colonies of each aphid species as response variables, and proportion of habitat types, plant richness and aphid species, predators and ants as explanatory variables.
| Model ID | AIC | Response variable | Explanatory variable | Coefficient value ± SE | P |
|---|---|---|---|---|---|
| 1 | 304.6 | PA aphid | Intercept | 1.103 ± 0.196 | 0.0000 |
| Presence of predators | 0.556 ± 0.090 | 0.0000 | |||
| Proportion of buildings | 0.549 ± 0.346 | 0.1152 | |||
| 2 | 279.4 | BB aphid | Intercept | 1.862 ± 0.185 | 0.0000 |
| Presence of predators | 0.188 ± 0.078 | 0.0165 | |||
| Presence of ants | 0.440 ± 0.078 | 0.0000 | |||
| Plant richness | −0.003 ± 0.002 | 0.1151 | |||
| Proportion of buildings | 0.557 ± 0.316 | 0.0796 | |||
| 3 | 182.9 | PA predators | Intercept | −1.101 ± 0.551 | 0.0457 |
| PA aphid | 1.664 ± 0.551 | 0.0000 | |||
| Presence of ants | −1.341 ± 0.502 | 0.0076 | |||
| Proportion of buildings | −3.320 ± 1.743 | 0.0568 | |||
| 4 | 212.2 | BB predators | Intercept | 0.342 ± 0.638 | 0.5920 |
| BB aphid | 0.790 ± 0.316 | 0.0124 | |||
| Presence of ants | −1.119 ± 0.407 | 0.0060 | |||
| Proportion of roads | −2.120 ± 0.926 | 0.0221 | |||
| 5 | 163.3 | PA ants | Intercept | −1.545 ± 0.420 | 0.0002 |
| Presence of predators | −1.147 ± 0.450 | 0.0108 | |||
| Proportion of buildings | 5.169 ± 1.682 | 0.0021 | |||
| 6 | 185.9 | BB ants | Intercept | −6.627 ± 1.229 | 0.0000 |
| BB aphid | 2.087 ± 0.419 | 0.0000 | |||
| Presence of predators | −0.888 ± 0.408 | 0.0294 | |||
| Plant richness | 0.036 ± 0.011 | 0.0015 | |||
| Proportion of roads | 2.042 ± 1.013 | 0.0438 |
AIC values for each model are given. Models with significant explanatory factors are shown in bold.
Relationship between generalist/specialist predators and urban green space
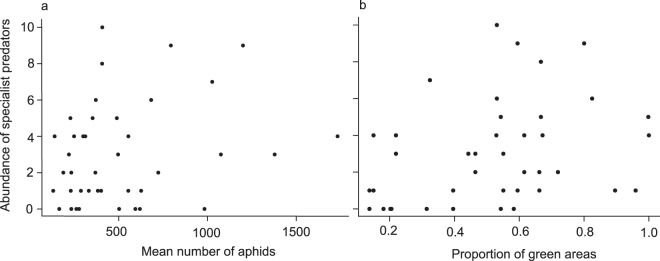
Generalist predators were more common than specialists (W = 538.5, Z = 2.38, P < 0.05; median values of generalists and specialists recorded per colony were 2 and 4 respectively). The best model in explaining the abundance of specialist predators had two positive and significant explanatory factors, the mean number of aphids and the proportion of green areas on the study sites (Table 3, model 1; Fig. 1). The best model in explaining the abundance of generalist predators had only one variable, proportion of green areas on the study sites; however, this factor was not statistically significant (Table 3, model 2).
Table 3.
Summary of models predicting the abundance of specialist predators (model 1) and generalist predators (model 2) found on both Aphis fabae and Acyrthosiphon pisum colonies.
| Model ID | AIC | Response variable | Explanatory variable | Coefficient value ± SE | P |
|---|---|---|---|---|---|
| 1 | 199.3 | Specialist predators | Intercept | −2.138 ± 0.915 | 0.019 |
| Mean number of aphids | 0.969 ± 0.319 | 0.002 | |||
| Proportion of green areas | 1.189 ± 0.391 | 0.002 | |||
| 2 | 41.8 | Generalist predators | Intercept | 0.499 ± 0.130 | 0.001 |
| Proportion of green areas | 0.383 ± 0.224 | 0.096 |
AIC values for each model are given. Models with significant explanatory factors are shown in bold.
Figure 1.
Abundance of specialist predators found on Aphis fabae and Acyrthosiphon pisum colonies according to (a) the abundance of aphids and (b) the proportion of green space in study sites.
Discussion
In this study we asked how habitat changes associated with urbanisation may alter patterns of interactions between insect herbivores, their predators and mutualistic ants. Additionally, we wanted to investigate if the amount of green space in urban environments is an important environmental factor selecting species of specialist or generalist aphid predators. We controlled for habitat associated variation in plant quality and initial aphid colony size, allowing us to disentangle effects resulting from changes in the presence of natural enemies and mutualist ant species. Local habitat factors (human-constructed surfaces, local plant species richness) did not affect aphid colony size, but the presence of ant mutualists was associated with an increase in numbers of black bean aphids. The presence of ants was associated with a reduction in insect predators, and both were more likely to be found on larger aphid colonies. Predator occurrence was reduced at study sites with higher proportions of roads (BB) and buildings (PA). However, ants showed the inverse pattern, where increased numbers of predatory ants (PA) were associated with an increased proportion of buildings, and attendance of BB colonies by mutualistic ants was positively associated with the proportion of roads and plant species richness. This suggests that anthropogenic changes associated with urbanisation may alter the structure of local ecological assemblages, with some taxa (predatory and mutualistic ants) benefiting more than others (specialist insect predators). A difference between numbers of specialist and generalist predator groups was also observed, as higher numbers of specialist predators are correlated to greater proportion of green areas in urban habitats, while generalist predators did not follow this trend. These findings are consistent with studies showing high sensitivity of monophagous and oligophagous species to urbanisation, habitat fragmentation and habitat loss11,25–29, but this is the first study to show that urbanisation changes the likelihood of such a mutualistic interaction.
We found that predators were reduced in areas with increased proportions of roads. Fundamentally, roads can act as barriers or filters to animal dispersal30, with studies finding that carabid beetles and wolf spiders are blocked by roads as narrow as 2.5 m wide31. Furthermore, the quantity and extent of impervious cover (paved surfaces structures such as buildings and roads) cause strong detrimental effects to arthropod diversity and abundance, including natural enemies such as parasitoids32–34. Specialist predators are linked to the presence of resources utilised by their prey, consequently, loss of prey habitat would also mean reduction of predator habitat35,36. Here we found that numbers of specialist predators were positively linked to the amount of local green space, but that numbers of generalists were not similarly affected. Overall, environmental changes are expected to be more disadvantageous to specialist species, as generalists are better able to adapt to varying habitat conditions and prey availability29,37,38.
Predatory ants found on PA colonies and mutualistic ants found on BB colonies were positively associated with the proportion of roads and buildings in the habitat, respectively. The presence of mutualistic ant-aphid interactions was positively associated with more plant diverse sites; aphid diversity in urban gardens is associated with plant diversity and abundance16. Urbanised areas may serve as habitat and corridors for dry-adapted and heat tolerant species such as ants39,40, and such habitats select for opportunistic, highly competitive ant species41,42. Lasius niger and Myrmica rubra live in colonies of several thousand individuals, showing aggressiveness and displacement against competitors43,44, both species are omnivores with varied diet which consists of honeydew, other invertebrates, pollen, seeds and human waste45 and are good candidates for benefitting from urban habitats46. Indirect interactions between ants and other natural enemies can be complex47. However, the negative effect found where both predatory and mutualistic ants displaced other predator species on our experimental aphid colonies was not surprising. In our study sites ants acted as predators on PA colonies, displaced predators of both aphid species, and acted as beneficial mutualists of BB aphids. This behaviour could be linked to the fact that ants not exclusively choose to just tend aphids or just prey on them, but as whether ants tend aphids for honeydew or eat them and their rate of attendance, depends upon food availability in the ants’ foraging areas20,48,49. According to Pontin23 ants would keep a balanced protein-carbohydrate food intake by initiating predation on attended aphids when other prey were in short supply, and though the study of Offenberg48 did not support Pontin’s hypothesis - who observed that when offered alternative sugar, the interaction moved from mutualism to exploitation due to decreased ant-tending and increased predation, and alternative prey had no significant effect – their work was not performed on “real life” habitats but in controlled laboratory conditions where only three different alternative prey were offered. The fact that ants with predatory behaviour against PA aphids were more likely to be found in highly urbanised habitats with increased proportions of buildings may also indicate an increased need for prey and protein rich food sources by ants in highly urbanised environments.
Little consideration has been given to the effects of habitat structure of cities in determining trophic dynamics and species interactions. Our data suggests that such variables play a major role for predatory and mutualistic interactions, with likely consequences for the structuring of urban insect communities. The reduction in specialist predator numbers, with a concurrent increase in ant presence, could also lead to a potential increase in herbivore populations of ant-attended species. This may affect the environmental services predators provide50,51. In our work we found that numbers of specialist predators were positively linked to the amount of local green space, but that numbers of generalists were not affected by the same variable. Some studies have found a constant number of generalist predators on gradients of human disturbance11,52, however others have found that generalists are even more abundant in cities than specialists28,53. There is a strong theoretical expectation that generalist and specialist predators will have distinctive responses associated with changes in habitat37. Overall, environmental changes are expected to be more disadvantageous to specialist species, as generalists are able to adapt more easily to varying habitat conditions29,38. Thus specialist aphid predators may particularly benefit from the increased amount of potential habitat promoted by the presence of urban green spaces.
Urbanisation is transforming the areas where most of the world’s people live. Understanding how patterns of species interactions change in such radically altered environments is of critical importance if we are to develop approaches to help maintain biodiversity in highly altered, novel environments. Here we show that for two abundant and widespread species of aphids, their specialist predators are lost from assemblages as urbanisation increases, but no such pattern is seen with generalists. We also observed that a facultative mutualism (ant attendance) increased with urbanisation, suggesting that urban areas might act as filter that benefits dominant ant species that thrive when tending aphid colonies. Together, these results suggest that insect communities in urban areas are not just simply depauperate, but how they are structured may differ. Urban ecosystems are indeed novel ecosystems, but just how novel they are remains to be fully explored.
Methods
Study area and habitat variables
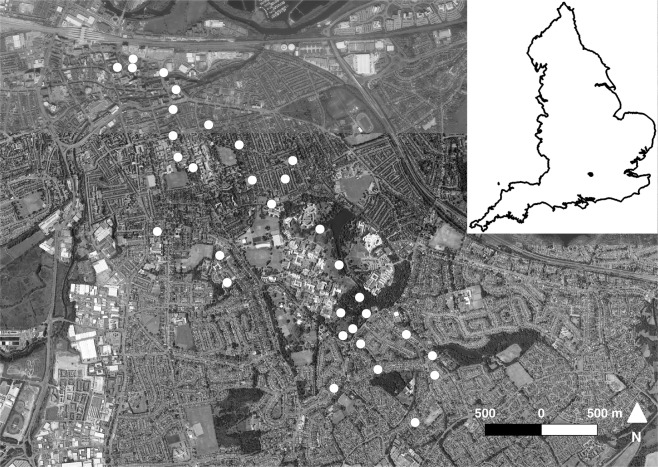
The study area is located in Greater Reading, Berkshire, UK (51°27′N, 0°58′W, Fig. 2). Reading is a large town with a population of 290 000, which covers an area of ca. 72 km254. For the first year of sampling, 27 experimental sites were studied and 32 sites in the second year. Site selection captured a gradient from highly urbanised sites on the town centre to suburban areas closer to rural areas located on the south24. Each study site was at least 110 meters apart. Habitat variables were obtained using GIS, utilising the topography layer from Digimap EDINA MasterMap, at a scale of 1:1250. Thirty meters radius buffers were delimited in each study site, and a reclassification of vectors was made to calculate proportions of area of the following habitat types within those buffers: green areas (gardens and parks), woodlands (shrubs and trees), and impervious surfaces, made up of buildings (any artificial structure made of concrete, brick or stone) and roads (roads, roadsides, tracks or paths made of surfaces such as asphalt), using QGIS 2.8.155. Additionally, plant species richness within the 30 meters radius buffers of each study site was estimated by counting plant morphospecies (defined as taxonomic groups which could be separated by eye in the field by a trained botanist). Thirty meters radius buffers were chosen as the optimal size to account for significant local habitat variation between study sites while solving practicalities regarding site access for plant diversity estimation.
Figure 2.
Study site location in Greater Reading, England (n = 32). Aerial image was obtained from Digimap EDINA Aerial. Figure created using QGIS 2.8.155.
Study systems and summer recording
Monoclonal cultures of Acyrthosiphon pisum Harris (PA) and Aphis fabae Scopoli (BB) were maintained in a laboratory using plastic and mesh cages. All cultures and experimental colonies were identically reared in a Controlled Temperature room at 20 ± 1 °C, 16:8 L:D h light regime and at ambient humidity on broad bean, Vicia faba L. (var. the Sutton dwarf). Plants were sown in pots with potting compost (Vitax Grower, Leicester, England) and watered as needed.
Three days before being allocated to the study sites, three adults from each aphid species were transferred from the monoclonal cultures and reared in cages containing 14 to 16-day-old broad bean plants (18–22 cm tall), to allow the colonies to become established. After three days, one colony of PA and one of BB on Vicia faba plants were placed at each study site (60–80 cm apart). Two days after the experimental colonies were placed in the field, aphid, ant and predator numbers were recorded for the first time, and recording subsequently occurred every four days, for five recording events in total. At the end of this sampling period, colonies were replaced. Sampling was repeated four times in 2015 (period one: May 16, 20, 24, 28 and June 1; period two: June 15, 19, 23, 27 and July 1; period 3: 16, 20, 24, 28 July and August 1; period four: August 14, 18, 22, 26 and 30), and three times in 2016 (period five: May 16, 20, 24, 28 and June 1; period six: June 16, 20, 24, 28 and July 2; period seven: July 29, and August 2, 6,10 and 14).
Data analysis
All statistical analyses were carried out using R 3.1.256.
Urban land use and interactions between aphids, ants and predators
Here, the dataset consisted of the cumulative numbers of aphids and the presence or absence of predators and ants in the five counting events on each of the seven sampling periods. Some colonies were lost during the experiment (due to poor plant health, herbivory, damage or theft by the public). This resulted in 183 observations of BB colonies and 177 observations for PA colonies. To analyse PA and BB aphid colony numbers we applied separate linear mixed models fitted by reduced maximum likelihood using package nlme57, and as explanatory variables we used presence or absence of ants and predators, proportion of gardens, buildings, roads, and plant species richness. Counts of aphids were log10-transformed to deal with extreme values and to standardize and homogenize model residuals. For these models we accounted for repeated sampling of the colonies through time by adding period as a random effect. We removed the proportion of woodland from the set of explanatory variables due to its correlation with garden and roads (−0.66 and −0.61, respectively).
To investigate which biotic and abiotic factors determined the occurrence of predators and ants on PA and BB colonies we performed separate logistic regression mixed models with a binomial error distribution (with canonical link logit) using the function glmer of package lme458, fitted by maximum likelihood59. When modelling predators we used as explanatory factors the proportion of gardens, buildings, roads and plant species richness, number of aphids and presence or absence of ants. When modelling ants we used the same habitat variables, as well as controlling for aphid numbers on the colonies and the presence or absence of predators.
Relationship between generalist/specialist predators and urban green space
Predators found attacking aphid species were summed together and classified according to Rotheray20 into specialists (obligate aphid predators) and generalists (opportunistic aphid predators). Ladybirds (Coccinellidae), lacewings (Chrysopidae), flower bugs (Anthocoridae), aphid midges (Cecidomyiidae) and hoverfly (Syrphidae) larvae were considered as specialist aphid predators; earwigs (Dermaptera), ground beetles (Carabidae), spiders (Araneae) and harvestmen (Opiliones) were considered as generalist aphid predators. In order to obtain a meaningful quantitative response and avoid an excess of zeroes, the dataset of the two sampling periods with higher predator numbers in 2015 (period one and two) and the other two periods with highest predator numbers of 2016 (sampling periods five and seven) were summed together. As some colonies of both aphid species were damaged across different sampling periods, they were discarded from the dataset, leaving 41 observations.
To address possible differences in abundance between numbers of specialist and generalist predators, we used a paired Wilcoxon signed-rank test59. In order to assess the effect of urban green spaces on numbers of specialist predators a generalised linear mixed method (GLMM) fitted by maximum likelihood (Laplace Approximation), with a Poisson error distribution and a log link function was performed60, relating numbers of specialist predators to the proportion of green spaces (i.e. sum of the proportions of gardens and woodlands within 30 m buffers), occurrence of ants and mean number of aphids, using package lme458. As numbers of generalist predators were over-dispersed, this variable was log10 transformed and then related to the proportion of green spaces, occurrence of ants and mean number of aphids using a linear mixed effect model fitted by reduced maximum likelihood on package nlme57. For these models, year of sampling was considered as a random factor, and mean numbers of aphids were log10 transformed in order to deal with extreme values and improve model convergence60.
For all analyses, model selection was done by model comparison using Akaike’s Information Criteria (AIC) by fitting the full model with the set of all explanatory variables and removing the least significant term on each step (refitting the model each time), until the optimal model is found59,60. We checked for collinearity between explanatory variables in all models through variance inflation factors (VIF), with VIF values higher than 3 indicating that covariation between predictors may impose a problem60. Our VIF values were in the range of 1.01–1.50. The response variables and model residuals were checked for spatial autocorrelation through spline correlograms on package ncf61, in which we did not find any significant spatial structure. We also confirmed the validity of models by checking normality, independence and homogeneity of model residuals.
Acknowledgements
We would like to thank the Reading Borough Council and all garden owners for permitting access to their properties. Science without Borders and CAPES-Brazil provided scholarship to the first author (BEX: 13531-13-1). Estevão N. F. Souza help us with the survey and counting of plant morphospecies.
Author contributions
Both E.A.R. and M.D.E.F. conceived and designed the study. E.A.R. obtained, compiled and analyzed the data. Both authors have written the manuscript. M.D.E.F. supervised the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Elise A. Rocha, Email: eliserocha1@gmail.com
Mark D. E. Fellowes, Email: m.fellowes@reading.ac.uk
References
- 1.MacGregor-Fors I, Morales-Pérez L, Schondube JE. Migrating to the city: responses of neotropical migrant bird communities to urbanization. Condor. 2010;112:711–717. doi: 10.1525/cond.2010.100062. [DOI] [Google Scholar]
- 2.Reis E, López-Iborra GM, Pinheiro RT. Changes in bird species richness through different levels of urbanization: implications for biodiversity conservation and garden design in Central Brazil. Landsc. Urban Plan. 2012;107:31–42. doi: 10.1016/j.landurbplan.2012.04.009. [DOI] [Google Scholar]
- 3.Parsons H, Major RE, French K. Species interactions and habitat associations of birds inhabiting urban areas of Sydney, Australia. Austral Ecol. 2006;31:217–227. doi: 10.1111/j.1442-9993.2006.01584.x. [DOI] [Google Scholar]
- 4.Pereira‐Peixoto MH, Pufal G, Staab M, Feitosa Martins C, Klein A. Diversity and specificity of host‐natural enemy interactions in an urban‐rural interface. Ecol. Entomol. 2016;41:241–252. doi: 10.1111/een.12291. [DOI] [Google Scholar]
- 5.McKinney ML. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 2008;11:161–176. doi: 10.1007/s11252-007-0045-4. [DOI] [Google Scholar]
- 6.Pickett STA, et al. Urban ecological systems: scientific foundations and a decade of progress. J. Environ. Manage. 2011;92:331–362. doi: 10.1016/j.jenvman.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Shrewsbury PM, Raupp MJ. Do top-down or bottom-up forces determine Stephanitis pyrioides abundance in urban landscapes? Ecol. Appl. 2006;16:262–272. doi: 10.1890/04-1347. [DOI] [PubMed] [Google Scholar]
- 8.Raupp MJ, Shrewsbury PM, Herms DA. Ecology of herbivorous arthropods in urban landscapes. Annu. Rev. Entomol. 2010;55:19–38. doi: 10.1146/annurev-ento-112408-085351. [DOI] [PubMed] [Google Scholar]
- 9.Faeth SH, Warren PS, Shochat E, Marussich WA. Trophic dynamics in urban communities. Bioscience. 2005;55:399–407. doi: 10.1641/0006-3568(2005)055[0399:TDIUC]2.0.CO;2. [DOI] [Google Scholar]
- 10.Burkman CE, Gardiner MM. Urban greenspace composition and landscape context influence natural enemy community composition and function. Biol. Control. 2014;75:58–67. doi: 10.1016/j.biocontrol.2014.02.015. [DOI] [Google Scholar]
- 11.Sorace A, Gustin M. Distribution of generalist and specialist predators along urban gradients. Landsc. Urban Plan. 2009;90:111–118. doi: 10.1016/j.landurbplan.2008.10.019. [DOI] [Google Scholar]
- 12.Turrini T, Sanders D, Knop E. Effects of urbanization on direct and indirect interactions in a tri-trophic system. Ecol. Appl. 2016;26:664–675. doi: 10.1890/14-1787. [DOI] [PubMed] [Google Scholar]
- 13.Philpott SM, et al. Local and landscape drivers of arthropod abundance, richness, and trophic composition in urban habitats. Urban Ecosyst. 2014;17:513–532. doi: 10.1007/s11252-013-0333-0. [DOI] [Google Scholar]
- 14.Chamberlain SA, Bronstein JL, Rudgers JA. How context dependent are species interactions? Ecol. Lett. 2014;17:881–890. doi: 10.1111/ele.12279. [DOI] [PubMed] [Google Scholar]
- 15.Mooney EH, et al. Abiotic mediation of a mutualism drives herbivore abundance. Ecol. Lett. 2016;19:37–44. doi: 10.1111/ele.12540. [DOI] [PubMed] [Google Scholar]
- 16.Rocha EA, et al. Influence of urbanisation and garden plants on the diversity and abundance of aphids and their ladybird and hoverfly predators. Eur. J. Entomol. 2018;115:140–149. doi: 10.14411/eje.2018.013. [DOI] [Google Scholar]
- 17.Wimp GM, Whitham TG. Biodiversity consequences of predation and host plant hybridization on an aphid-ant mutualism. Ecology. 2001;82:440–452. [Google Scholar]
- 18.Müller CB, Fellowes MDE, Godfray HCJ. Relative importance of fertiliser addition to plants and exclusion of predators for aphid growth in the field. Oecologia. 2005;143:419–427. doi: 10.1007/s00442-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 19.Hazell SP, Fellowes MDE. Intra‐specific variation affects the structure of the natural enemy assemblage attacking pea aphid colonies. Ecol. Entomol. 2009;34:34–42. doi: 10.1111/j.1365-2311.2008.01051.x. [DOI] [Google Scholar]
- 20.Rotheray, G. E. Aphid predators. (Richmond Publishing Co. Ltd., 1989).
- 21.Losey JE, Harmon J, Ballantyne F, Brown C. A polymorphism maintained by opposite patterns of parasitism and predation. Nature. 1997;388:269–272. doi: 10.1038/40849. [DOI] [Google Scholar]
- 22.El‐Ziady S, Kennedy JS. Beneficial effects of the common garden ant, Lasius niger L., on the black bean aphid, Aphis fabae Scopoli. Proc. R. Entomol. Soc. London. Ser. A, Gen. Entomol. 1956;31:61–65. [Google Scholar]
- 23.Pontin AJ. The numbers and distribution of subterranean aphids and their exploitation by the ant Lasius flavus (Fabr.) (Hym., Formicidae) Ecol. Entomol. 1978;3:203–207. doi: 10.1111/j.1365-2311.1978.tb00920.x. [DOI] [Google Scholar]
- 24.Rocha EA, Fellowes MDE. Does urbanization explain differences in interactions between an insect herbivore and its natural enemies and mutualists? Urban Ecosyst. 2018;21:405–417. doi: 10.1007/s11252-017-0727-5. [DOI] [Google Scholar]
- 25.Kitahara M, Fujii K. Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: an analysis based on the concept of generalist vs. specialist strategies. Res. Popul. Ecol. (Kyoto). 1994;36:187–199. doi: 10.1007/BF02514935. [DOI] [Google Scholar]
- 26.Posa MRC, Sodhi NS. Effects of anthropogenic land use on forest birds and butterflies in Subic Bay, Philippines. Biol. Conserv. 2006;129:256–270. doi: 10.1016/j.biocon.2005.10.041. [DOI] [Google Scholar]
- 27.Bergerot B, Fontaine B, Renard M, Cadi A, Julliard R. Preferences for exotic flowers do not promote urban life in butterflies. Landsc. Urban Plan. 2010;96:98–107. doi: 10.1016/j.landurbplan.2010.02.007. [DOI] [Google Scholar]
- 28.Lizée MH, Mauffrey JF, Tatoni T, Deschamps-Cottin M. Monitoring urban environments on the basis of biological traits. Ecol. Indic. 2011;11:353–361. doi: 10.1016/j.ecolind.2010.06.003. [DOI] [Google Scholar]
- 29.Deguines N, Julliard R, Flores M, Fontaine C. Functional homogenization of flower visitor communities with urbanization. Ecol. Evol. 2016;6:1967–1976. doi: 10.1002/ece3.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard TTF, Alexander LE. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998;29:207–231. doi: 10.1146/annurev.ecolsys.29.1.207. [DOI] [Google Scholar]
- 31.Mader HJ. Animal habitat isolation by roads and agricultural fields. Biol. Conserv. 1984;29:81–96. doi: 10.1016/0006-3207(84)90015-6. [DOI] [Google Scholar]
- 32.Magura T, Tóthmérész B, Molnár T. A species-level comparison of occurrence patterns in carabids along an urbanisation gradient. Landsc. Urban Plan. 2008;86:134–140. doi: 10.1016/j.landurbplan.2008.01.005. [DOI] [Google Scholar]
- 33.Bennett AB, Gratton C. Local and landscape scale variables impact parasitoid assemblages across an urbanization gradient. Landsc. Urban Plan. 2012;104:26–33. doi: 10.1016/j.landurbplan.2011.09.007. [DOI] [Google Scholar]
- 34.Bennett AB, Gratton C. Measuring natural pest suppression at different spatial scales affects the importance of local variables. Environ. Entomol. 2014;41:1077–1085. doi: 10.1603/EN11328. [DOI] [PubMed] [Google Scholar]
- 35.Swihart RK, Feng Z, Slade NA, Mason DM, Gehring TM. Effects of habitat destruction and resource supplementation in a predator–prey metapopulation model. J. Theor. Biol. 2001;210:287–303. doi: 10.1006/jtbi.2001.2304. [DOI] [PubMed] [Google Scholar]
- 36.Ryall KL, Fahrig L. Response of predators to loss and fragmentation of prey habitat: a review of theory. Ecology. 2006;87:1086–1093. doi: 10.1890/0012-9658(2006)87[1086:ROPTLA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Rand TA, Tscharntke T. Contrasting effects of natural habitat loss on generalist and specialist aphid natural enemies. Oikos. 2007;116:1353–1362. doi: 10.1111/j.0030-1299.2007.15871.x. [DOI] [Google Scholar]
- 38.Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 2011;9:222–228. doi: 10.1890/080216. [DOI] [Google Scholar]
- 39.Gibb H, Hochuli DF. Colonisation by a dominant ant facilitated by anthropogenic disturbance: effects on ant assemblage composition, biomass and resource use. Oikos. 2003;103:469–478. doi: 10.1034/j.1600-0706.2003.12652.x. [DOI] [Google Scholar]
- 40.Menke SB, et al. Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; an example from ants. Urban Ecosyst. 2011;14:135–163. doi: 10.1007/s11252-010-0150-7. [DOI] [Google Scholar]
- 41.Lessard JP, Buddle CM. The effects of urbanization on ant assemblages (Hymenoptera: Formicidae) associated with the Molson Nature Reserve, Quebec. Can. Entomol. 2005;137:215–225. doi: 10.4039/n04-055. [DOI] [Google Scholar]
- 42.Philpott, S. M., Perfecto, I., Armbrecht, I. & Parr, C. L. Ant diversity and function in disturbed and changing habitats In Ant Ecology (eds. Lach, lori, Parr, C. L. & Abbott, K.) 137–157 (Oxford University Press, 2010).
- 43.Novgorodova TA, Gavrilyuk AV. The degree of protection different ants (Hymenoptera: Formicidae) provide aphids (Hemiptera: Aphididae) against aphidophages. Eur. J. Entomol. 2012;109:187–196. doi: 10.14411/eje.2012.025. [DOI] [Google Scholar]
- 44.Garnas J, Groden E, Drummond FA. Mechanisms of competitive displacement of native ant fauna by invading Myrmica rubra (Hymenoptera: Formicidae) populations. Environ. Entomol. 2014;43:1496–1506. doi: 10.1603/EN14079. [DOI] [PubMed] [Google Scholar]
- 45.Czechowski W, Marko B, Radchenko A. Rubbish dumps reveal the diet of ant colonies: Myrmica schencki EM. and Myrmica rubra (L.) (Hymenoptera: Formicidae) as facultative pollen-eaters. Pol. J. Ecol. 2008;56:737–741. [Google Scholar]
- 46.Slipinski P, Zmihorski M, Czechowski W. Species diversity and nestedness of ant assemblages in an urban environment. Eur. J. Entomol. 2012;109:197–206. doi: 10.14411/eje.2012.026. [DOI] [Google Scholar]
- 47.Eubanks MD. Estimates of the direct and indirect effects of red imported fire ants on biological control in field crops. Biol. Control. 2001;21:35–43. doi: 10.1006/bcon.2001.0923. [DOI] [Google Scholar]
- 48.Offenberg J. Balancing between mutualism and exploitation: the symbiotic interaction between Lasius ants and aphids. Behav. Ecol. Sociobiol. 2001;49:304–310. doi: 10.1007/s002650000303. [DOI] [Google Scholar]
- 49.Nagy C, Cross JV, Markó V. Sugar feeding of the common black ant, Lasius niger (L.), as a possible indirect method for reducing aphid populations on apple by disturbing ant-aphid mutualism. Biol. Control. 2013;65:24–36. doi: 10.1016/j.biocontrol.2013.01.005. [DOI] [Google Scholar]
- 50.Olden JD, LeRoy Poff N, Douglas MR, Douglas ME, Fausch KD. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 2004;19:18–24. doi: 10.1016/j.tree.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Vincent D, Julliard R, Couvet D, Alexandre L, Jiguet F. Functional homogenization effect of urbanization on bird communities. Conserv. Biol. 2007;21:741–751. doi: 10.1111/j.1523-1739.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- 52.Gardiner MM, Prajzner SP, Burkman CE, Albro S, Grewal PS. Vacant land conversion to community gardens: influences on generalist arthropod predators and biocontrol services in urban greenspaces. Urban Ecosyst. 2014;17:101–122. doi: 10.1007/s11252-013-0303-6. [DOI] [Google Scholar]
- 53.Lowe EC, Wilder SM, Hochuli DF. Persistence and survival of the spider Nephila plumipes in cities: do increased prey resources drive the success of an urban exploiter? Urban Ecosyst. 2016;19:705–720. doi: 10.1007/s11252-015-0518-9. [DOI] [Google Scholar]
- 54.Office for National Statistics. Neighbourhood statistics. 2011 census data. https://www.ons.gov.uk/ (2013).
- 55.QGIS Development Team. QGIS Geographic Information System. https://qgis.org/en/site/ (2015).
- 56.R Development Core Team. R: A language and environment for statistical computing. https://www.r-project.org/ (2014).
- 57.Pinheiro, J. & Sarkar, D. Package nlme: linear and nonlinear mixed effects models. Version 3.1-127. https://cran.r-project.org/web/packages/nlme/index.html (2016).
- 58.Bates, D. et al. Package lme4: linear mixed-Effects models using ‘Eigen’ and S4. Version 1.1-10. https://cran.r-project.org/web/packages/lme4/index.html (2015).
- 59.Crawley, M. J. The R Book. (John Wiley & Sons, 2007).
- 60.Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. Mixed effects models and extensions in ecology with R (Gail, M., Krickeberg, K., Samet, J. M., Tsiatis, A. & Wong, W., eds). (Springer, 2009).
- 61.Bjornstad, O. N. Package ‘ncf’: spatial nonparametric covariance functions. Version 1.1-6. https://cran.r-project.org/web/packages/ncf/index.html (2015).