Abstract
Background
The β-lactamase inhibitor relebactam can restore imipenem activity against imipenem-nonsusceptible gram-negative pathogens. We evaluated imipenem/relebactam for treating imipenem-nonsusceptible infections.
Methods
Randomized, controlled, double-blind, phase 3 trial. Hospitalized patients with hospital-acquired/ventilator-associated pneumonia, complicated intraabdominal infection, or complicated urinary tract infection caused by imipenem-nonsusceptible (but colistin- and imipenem/relebactam-susceptible) pathogens were randomized 2:1 to 5–21 days imipenem/relebactam or colistin+imipenem. Primary endpoint: favorable overall response (defined by relevant endpoints for each infection type) in the modified microbiologic intent-to-treat (mMITT) population (qualifying baseline pathogen and ≥1 dose study treatment). Secondary endpoints: clinical response, all-cause mortality, and treatment-emergent nephrotoxicity. Safety analyses included patients with ≥1 dose study treatment.
Results
Thirty-one patients received imipenem/relebactam and 16 colistin+imipenem. Among mITT patients (n = 21 imipenem/relebactam, n = 10 colistin+imipenem), 29% had Acute Physiology and Chronic Health Evaluation II scores >15, 23% had creatinine clearance <60 mL/min, and 35% were aged ≥65 years. Qualifying baseline pathogens: Pseudomonas aeruginosa (77%), Klebsiella spp. (16%), other Enterobacteriaceae (6%). Favorable overall response was observed in 71% imipenem/relebactam and 70% colistin+imipenem patients (90% confidence interval [CI] for difference, –27.5, 21.4), day 28 favorable clinical response in 71% and 40% (90% CI, 1.3, 51.5), and 28-day mortality in 10% and 30% (90% CI, –46.4, 6.7), respectively. Serious adverse events (AEs) occurred in 10% of imipenem/relebactam and 31% of colistin+imipenem patients, drug-related AEs in 16% and 31% (no drug-related deaths), and treatment-emergent nephrotoxicity in 10% and 56% (P = .002), respectively.
Conclusions
Imipenem/relebactam is an efficacious and well-tolerated treatment option for carbapenem-nonsusceptible infections.
Clinical Trials Registration
Keywords: carbapenem resistant, KPC, nosocomial pneumonia, cIAI, cUTI
Imipenem/relebactam (IMI/REL) is a suitable treatment option for serious gram-negative infections, including those caused by carbapenem-nonsusceptible pathogens in high-risk patients. IMI/REL may be preferable to colistin-based therapy, given comparable efficacy but IMI/REL’s significantly lower nephrotoxicity and improved overall tolerability.
Multidrug-resistant (MDR) gram-negative pathogens are endemic worldwide [1] and cause infections associated with high mortality and morbidity [1–4]. In patients at high risk for poor treatment outcomes (eg, immunocompromised or critically ill), appropriate antibacterial therapy must be initiated promptly to improve survival [5]. Carbapenems are a treatment mainstay in high-risk patients, and carbapenem-resistant pathogens are among the highest-level bacterial threats [2, 4]. To overcome resistance, carbapenems can be combined with suitable β-lactamase inhibitors (BLIs).
Relebactam is a novel non–β-lactam, small-molecule BLI that inhibits class A carbapenemases (eg, Klebsiella pneumoniae carbapenemase [KPC]) and class C cephalosporinases (eg, AmpC) [6], 2 β-lactamases that are frequently involved in carbapenem nonsusceptibility. Relebactam is suitable for combination with the well-established carbapenem imipenem/cilastatin (IMI): inhibiting AmpC frequently restores Pseudomonas aeruginosa susceptibility to imipenem but not other carbapenems, neither agent is subject to efflux in P. aeruginosa, and their pharmacokinetic/pharmacodynamic profiles complement each other [7, 8]. Combining relebactam with IMI (IMI/REL) can restore IMI activity against many imipenem-nonsusceptible gram-negative pathogens, including extended-spectrum β-lactamase (ESBL)-, AmpC-, and KPC-producing Enterobacteriaceae [9, 10]. Two phase 2 trials found IMI/REL safe and no less effective than IMI for complicated intraabdominal and urinary tract infections [11, 12]. Pharmacokinetic/pharmacodynamic analyses [11, 13] confirmed 500 mg IMI plus 250 mg REL as the optimum IMI/REL dose for further clinical evaluation.
We conducted a randomized, controlled, double-blind trial to compare IMI/REL with colistin-based therapy for imipenem-nonsusceptible, serious, gram-negative bacterial infections. This was a noninferential, descriptive study intended to generate clinical data in this medically important patient population as part of a streamlined drug development program.
METHODS
Study Design
RESTORE-IMI 1 (protocol MK-7655A-013) was a phase 3, randomized, double-blind study to evaluate the efficacy and safety of IMI/REL vs colistin+IMI for imipenem-nonsusceptible infections at 35 hospitals (17 countries). The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. The study protocol is available in the Supplementary Materials.
Patients
Eligible patients were aged ≥18 years, hospitalized, and required intravenous antibacterial treatment for hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP), complicated urinary tract infections (cUTIs), or complicated intraabdominal infections (cIAIs) caused by imipenem-nonsusceptible, imipenem/relebactam-susceptible, and colistin-susceptible pathogens and lacking clinical improvement on any prior therapy. Diagnosis of eligible infections was based on standard definitions (Supplementary Materials).
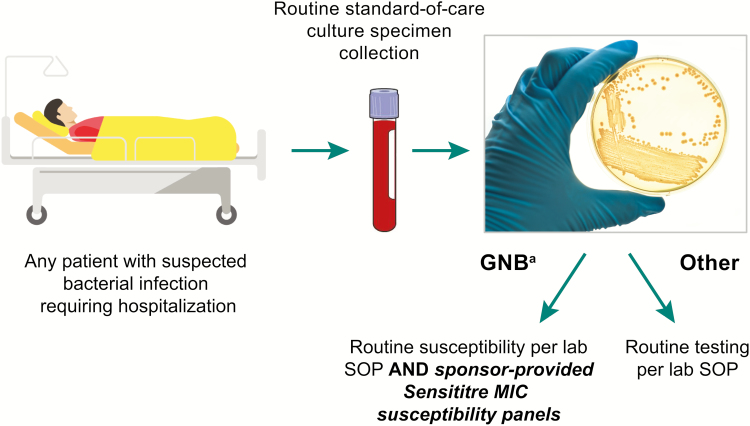
Patients were eligible based on local prescreening susceptibility testing: each site tested all gram-negative isolates from urine, intraabdominal, and lower respiratory tract samples for susceptibility to the 3 study drugs (Figure 1). Broth microdilution testing using Sensititre panels (ThermoFisherScientific, Waltham, MA) standardized across sites was used to test the samples. Susceptibility was interpreted according to each site’s applicable guidelines (either the Clinical and Laboratory Standards Institute or the European Committee on Antimicrobial Susceptibility Testing) and confirmed for each isolate at a central laboratory, using the respective site’s interpretative guideline [14, 15]. Intermediate-susceptible pathogens were regarded as nonsusceptible. Imipenem breakpoints were applied to imipenem/relebactam. Once patients with eligible pathogens were identified, investigators decided whether to enter these patients into the formal screening process in order to assess other eligibility criteria (Supplementary Materials).
Figure 1.
Prescreening for gram-negative isolates nonsusceptible to imipenem but susceptible to both imipenem/relebactam and colistin. aFrom specimen types of interest, including blood, urinary, intraabdominal, or lower respiratory sources. If information on infection-site specimen source was not available, all gram-negative bacteria were to be tested. Abbreviations: GNB, gram-negative bacteria; MIC, minimum inhibitory concentration; SOP, standard operating procedure.
Important exclusion criteria were Acute Physiology and Chronic Health Evaluation II (APACHE II) score >30; creatinine clearance (CrCL) <15 mL/min; requiring hemodialysis/peritoneal dialysis; concomitant systemic/inhaled agents active against Enterobacteriaceae (new taxonomy: Enterobacterales), Pseudomonas spp., and gram-negative anaerobic bacilli; prior colistin-based therapy; pulmonary obstructions (eg, lung cancer) in HAP/VAP; and complete obstruction of any portion of the urinary tract in cUTI (Supplementary Materials).
Randomization and Masking
Eligible patients were randomized (stratified by infection type) via a centralized, interactive, voice-integrated web response system 2:1 to intravenous IMI/REL (500 mg/250 mg every 6 hours) plus colistimethate sodium placebo or intravenous colistimethate sodium (loading dose to achieve 300 mg colistin base activity, followed by maintenance doses up to 150 mg colistin base activity, every 12 hours), hereafter referred to as colistin, plus IMI (500 mg every 6 hours). Patients and all investigational staff (except pharmacists who prepared infusions) remained blinded to treatment assignments throughout the study. The unblinded study pharmacist masked infusion bags with opaque sleeves.
Procedures
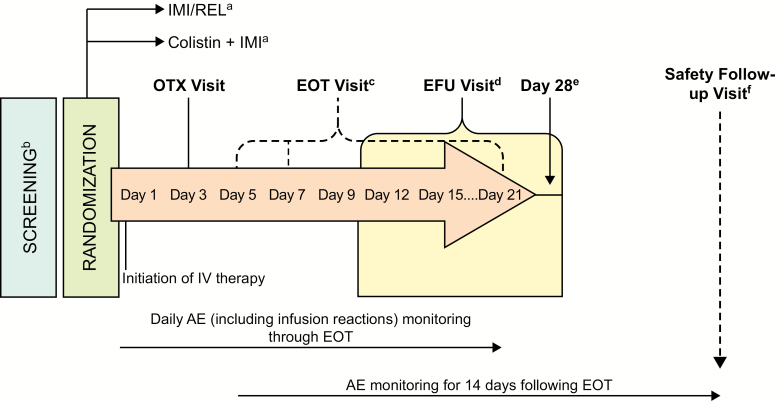
IMI/REL, IMI, and colistin doses were adjusted based on renal function (Supplementary Materials) and administered over 30 ± 5 minutes. Minimum treatment duration was 5 days (cIAI, cUTI) or 7 days (HAP/VAP), with a 21-day maximum. Patients were screened for eligibility ≤24 hours before randomization (Figure 2). Study visits were performed on day 1 (randomization), day 3 (on-therapy visit [OTX]), and at the end of therapy (EOT). Following study therapy completion, patients were evaluated at an early follow-up visit (EFU) 5–9 days post-EOT and on day 28 (could have occurred on the same day as EFU). Clinical signs and symptoms were assessed daily during therapy and at EOT, EFU, and day 28. In HAP/VAP, chest X rays were obtained on day 1 and at EOT.
Figure 2.
Assessment schedule. aPatients in these treatment groups had bacterial infections that were imipenem-nonsusceptible but susceptible to imipenem plus relebactam as well as to colistin. b≤24 hours prior to randomization. c≤24 hours after the last dose of IV study therapy. Minimum duration of IV therapy was 5 full days for complicated intraabdominal infections and complicated urinary tract infections and 7 full days for hospital-acquired pneumonia/ventilator-associated pneumonia. Maximum duration could not exceed 21 days without study sponsor approval. d5 to 9 days (up to an additional 2 days) following the end of therapy. e28 days (up to an additional 3 days) following randomization. fIf the day 28 visit occurred prior to 14 days after the end of therapy, an additional safety follow-up visit was required. Abbreviations: AE, adverse event; day 28, day 28 postrandomization; EFU, early follow-up; EOT, end of therapy; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; IV, intravenous; OTX, on therapy.
Infection site cultures (aerobic for all specimens plus anaerobic for intraabdominal specimens) for prescreening susceptibility testing at the local laboratory were collected ≤1 week before randomization. In cUTI, additional cultures were obtained at OTX, EOT, and EFU, and in HAP/VAP or cIAI whenever there was evidence of persistent/progressive infection or the patient underwent surgical/drainage procedures. Blood cultures were to be collected on day 1 and, if positive, repeated until 2 consecutive negative cultures were achieved.
Adverse events (AEs) were collected from the first dose through 14 days following completion of study therapy. A full assessment schedule, including laboratory evaluations, is provided in the Supplementary Materials.
Outcomes
The microbiologic modified intent-to-treat population (mMITT), which was the primary efficacy population, comprised all randomized patients with ≥1 dose of study treatment and cultures (collected within 1 week of enrollment) confirming ≥1 qualifying (according to central laboratory results) gram-negative pathogen from the primary infection site. The safety population comprised all randomized patients with ≥1 dose of study treatment according to the actual treatment received.
The primary efficacy endpoint was overall response in the mMITT population. Overall response was assessed centrally and defined differently for each infection type based on regulatory guidance as follows: HAP/VAP, 28-day all-cause mortality [16]; cIAI, day 28 clinical response [17]; and cUTI, composite clinical and microbiologic response at EFU [18]. Favorable clinical response was defined as resolution of baseline signs and symptoms and favorable microbiologic response was defined as eradication of baseline uropathogens; patients who died or had missing data were considered treatment failures. AE incidence in the safety population was another primary endpoint. Secondary endpoints were day 28 clinical response (mMITT population), 28-day all-cause mortality (mMITT population), and treatment-emergent nephrotoxicity (safety population). In patients with normal baseline renal function (serum creatinine <1.2 mg/dL), nephrotoxicity was defined as serum creatinine doubling (to >1.2 mg/dL) or ≥50% CrCL reduction. In patients with preexisting renal dysfunction (serum creatinine ≥1.2 mg/dL), nephrotoxicity was defined as serum creatinine increases ≥1 mg/dL, ≥20% CrCL reduction, or need for renal replacement therapy. All randomized, including mMITT, patients were treated and followed up identically.
Statistical Analyses
This was an estimation trial without formal statistical testing for efficacy endpoints. Within-group 95% confidence intervals (CIs) were calculated using the Agresti and Coull method. Between-group differences were calculated using the Miettinen and Nurminen method using 90% CIs and stratified by infection type where appropriate. Treatment-emergent nephrotoxicity rates were prospectively compared using the 2-sided P value from the Fisher exact test. Other safety data were analyzed using a tiered classification (Supplementary Materials). Sample size was based on logistical feasibility, not statistical considerations. Randomization of approximately 54 patients was planned, with the goal of obtaining approximately 45 participants (approximately 15 per infection type) who met mMITT inclusion criteria.
RESULTS
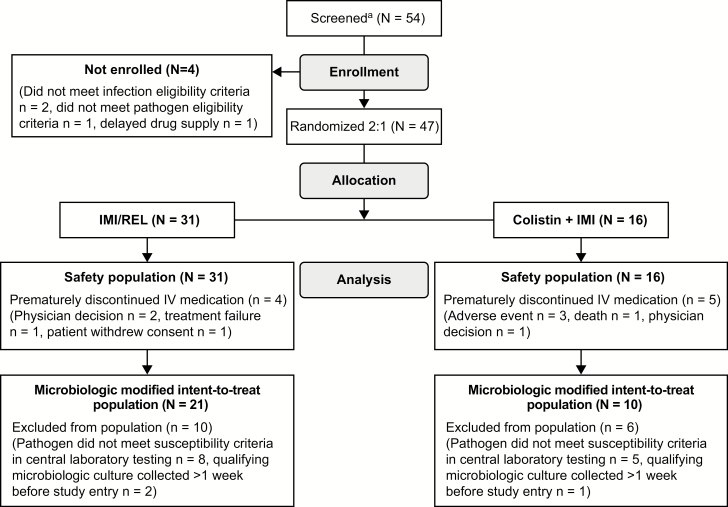
Sixteen sites from 11 countries (Supplementary Materials) enrolled patients between October 2015 and September 2017. All randomized patients (31 IMI/REL, 16 colistin+IMI) received ≥1 dose of assigned study treatment. The mMITT population comprised 31 patients (Figure 3): 11 HAP/VAP, 16 cUTI, and 4 cIAI. Baseline demographic, clinical, and microbiologic characteristics were similar between treatment arms (Table 1). Notably, 29% of mMITT patients had APACHE-II scores >15 (higher in HAP/VAP and cIAI than cUTI), 23% had CrCL <60 mL/min, and 35% were aged ≥65 years. Prior antibacterial therapies were largely comparable between arms, but prior meropenem was more frequent in IMI/REL patients (Supplementary Materials).
Figure 3.
Study analysis populations flow chart. aLocal diagnostic laboratories at each investigative site were asked to prescreen all incoming gram-negative isolates obtained from infection sites of interest against the sponsor-provided screening panels for resistance to IMI and susceptibility to IMI/REL and colistin. Investigators were notified of all isolates that met microbiologic eligibility criteria. Investigators then reviewed the patient’s general information to determine whether to proceed with actual screening, that is, based on protocol-specified procedures. Many patients with eligible isolates did not enter the formal screening process if, for example, the investigator was able to readily determine that a patient did not meet a major entry criterion. Screening was performed after obtaining patient consent. Abbreviations: IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; IV, intravenous.
Table 1.
Baseline Demographics and Clinical Characteristics in the Modified Microbiologic Intent-to-Treat Population
| Baseline characteristic | IMI/REL (n = 21) | Colistin + IMI (n = 10) | Total (n = 31) |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 13 (61.9) | 7 (70.0) | 20 (64.5) |
| Female, n (%) | 8 (38.1) | 3 (30.0) | 11 (35.5) |
| Age, y | |||
| <65, n (%) | 15 (71.4) | 5 (50.0) | 20 (64.5) |
| ≥65, n (%) | 6 (28.6) | 5 (50.0) | 11 (35.5) |
| Median (range) | 59 (19–75) | 61 (49–80) | 59 (19–80) |
| Weight, kg | |||
| Median (range) | 75 (53.0–132.3) | 75.6 (52.8–117.0) | 75 (52.8–132.3) |
| Creatinine clearance (mL/min) | |||
| ≥90, n (%) | 8 (38.1) | 3 (30.0) | 11 (35.5) |
| <90 to ≥60, n (%) | 8 (38.1) | 4 (40.0) | 12 (38.7) |
| <60 to ≥30, n (%) | 3 (14.3) | 2 (20.0) | 5 (16.1) |
| <30 to ≥15, n (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Not available, n (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Acute Physiology and Chronic Health Evaluation II score | |||
| ≤15, n (%) | 14 (66.7) | 8 (80.0) | 22 (71.0) |
| >15, n (%) | 7 (33.3) | 2 (20.0) | 9 (29.0) |
| Patients with HAP/VAP, median (range) | 16 (0, 26) | 22 (14, 23) | 18 (0, 26) |
| Patients with cIAI, median (range) | 18 (17, 19) | 14.5 (14, 15) | 16 (14, 19) |
| Patients with cUTI, median (range) | 5 (0, 17) | 7 (4, 15) | 5.5 (0, 17) |
| Primary diagnosis | |||
| HAP, n (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| VAP, n (%) | 7 (33.3) | 2 (20.0) | 9 (29.0) |
| cUTI (urinary tract abnormalities), n (%) | 5 (23.8) | 3 (30.0) | 8 (25.8) |
| cUTI (acute pyelonephritis), n (%) | 6 (28.6) | 2 (20.0) | 8 (25.8) |
| cIAI, n (%) | 2 (9.5)a | 2 (20.0)b | 4 (12.9) |
| Bacteremiac | |||
| Yes, n (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| No, n (%) | 5 (23.8) | 2 (20.0) | 7 (22.6) |
| Unknown, n (%)c | 15 (71.4) | 7 (70.0) | 22 (71.0) |
| Qualifying causative pathogens | |||
| Citrobacter freundii, n (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Enterobacter cloacae, n (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Klebsiella oxytoca, n (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Klebsiella pneumoniae, n (%) | 3 (14.3) | 1 (10.0) | 4 (12.9) |
| Pseudomonas aeruginosa, n (%) | 16 (76.2) | 8 (80.0) | 24 (77.4) |
| β-lactamasesd | |||
| Class A | |||
| Older spectrum β-lactamases | |||
| SHVe | 2 (9.5) | 1 (10.0) | 3 (9.7) |
| TEM | 7 (33.3) | 3 (30.0) | 10 (32.3) |
| Extended spectrum β-lactamases | |||
| CTX-M | 7 (33.3) | 4 (40.0) | 11 (35.5) |
| SHVe | 1 (4.8) | 0 | 1 (3.2) |
| VEB | 0 | 0 | 0 |
| Serine carbapenemases | |||
| KPC | 4 (19.0) | 1 (10.0) | 5 (16.1) |
| Class C | |||
| Chromosomal AmpC | |||
| PDC | 16 (76.2) | 8 (80.0) | 24 (77.4) |
| Plasmid-mediated AmpC | |||
| ACT | 0 | 0 | 0 |
| CMY | 1 (4.8) | 0 | 1 (3.2) |
| DHA | 1 (4.8) | 0 | 1 (3.2) |
| Class D | |||
| OXA-48 | 0 | 1 (10.0) | 1 (3.2) |
Abbreviations: cIAI, complicated intraabdominal infection; cUTI, complicated urinary tract infection; HAP, hospital-acquired bacterial pneumonia; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam; KPC, Klebsiella pneumoniae carbapenemase; VAP, ventilator-associated bacterial pneumonia.
aPerforated colon (n = 1), liver abscess (n = 1).
bPerforated jejunum/ileum and colon, postsurgical peritonitis (n = 1), intraabdominal abscess (n = 1). cBaseline blood cultures were available from only 9 patients in the overall modified microbiologic intent-to-treat population.
dQualifying baseline pathogens could have had multiple β-lactamases detected. Of isolates with class A β-lactamases, all but 3 isolates carried multiple class A β-lactamases. A detailed breakdown by pathogen is provided in the Supplementary Materials.
eOlder spectrum β-lactamases were SHV-1 and SHV-11. Extended spectrum β-lactamase was SHV-28.
Qualifying baseline pathogens in mMITT patients, mostly (65%) from cultures obtained ≤3 days before randomization (Supplementary Materials), were P. aeruginosa (77%), Klebsiella spp. (16%), and other Enterobacteriaceae (6%). All patients with HAP/VAP and cIAI had P. aeruginosa, except 1 with cIAI due to Citrobacter freundii. Patients with cUTI also mostly had P. aeruginosa, along with Klebsiella spp. and Enterobacter spp. (Supplementary Materials). Detected β-lactamases included AmpC (84% of mMITT patients), ESBLs (35%), KPC (16%), and OXA-48 (3%) (Supplementary Materials). Minimum inhibitory concentrations (MICs) for study drugs are listed in the Supplementary Materials. Resistance rates to most nonstudy antibacterial agents were high (Supplementary Materials). In the mMITT population, mean (range) treatment duration was 11.4 (2–18) days with IMI/REL and 10.8 (2–20) days with colistin+IMI; median treatment duration was 12.5 days and 9.8 days, respectively. Treatment compliance was 100%.
Most mMITT patients achieved a favorable overall response (IMI/REL, 71%; colistin+IMI, 70%; difference, –7.3%; 90% CI, –27.5%, 21.4%; Table 2). In both arms, most patients with HAP/VAP and cUTI, but none with cIAI, achieved favorable overall response (Table 2). Among cIAI patients, 1 out of 2 in both arms had unfavorable overall responses because of missing/indeterminate data.
Table 2.
Primary and Secondary Prospective Efficacy Endpoints (in the Modified Microbiologic Intent-to-Treat Population) and Secondary Prospective Safety Endpoints (in the Safety Population)
| IMI/REL (n = 21) | Colistin + IMI (n = 10) | Unadjusted Difference | Adjusted Differencea | ||||
|---|---|---|---|---|---|---|---|
| Endpoint | n | % (95% CI)b | n | % (95% CI)a | % | % | 90% CI |
| Primary endpoint | |||||||
| Favorable overall responsec | 15 | 71.4 (49.8, 86.4) | 7 | 70.0 (39.2, 89.7) | 1.4 | –7.3 | (–27.5, 21.4) |
| Hospital-acquired bacterial pneumonia/ ventilator-associated bacterial pneumonia | 7/8 | 87.5 (50.8, 99.9) | 2/3 | 66.7 | 20.8 | ||
| Complicated intraabdominal infection | 0/2d | 0.0 | 0/2e | 0.0 | 0.0 | ||
| Complicated urinary tract infection | 8/11 | 72.7 (42.9, 90.8) | 5/5 | 100.0 (51.1, 100.0) | –27.3 (–52.8, 12.8) | ||
| Secondary endpoints | |||||||
| Favorable clinical response (day 28) | 15f | 71.4 (49.8, 86.4) | 4g | 40.0 (16.7, 68.8) | 31.4 | 26.3 | (1.3, 51.5) |
| 28-day all-cause mortality | 2 | 9.5 (1.4, 30.1) | 3 | 30.0 (10.3, 60.8) | –20.5 | –17.3 | (–46.4, 6.7) |
| Treatment-emergent nephrotoxicityh | 3/29 | 10.3 (2.8, 27.2) | 9/16 | 56.3 (33.2, 76.9) | –45.9 (–69.1, –18.4) | ||
CIs are not presented if the number of patients with assessment was <4.
Abbreviations: CI, confidence interval; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam.
aBased on the Miettinen and Nurminen method stratified by infection site.
bBased on the Agresti and Coull method.
cOverall response defined as hospital-acquired bacterial pneumonia/ventilator-associated bacterial pneumonia (HAP/VAP), survival through day 28; complicated intraabdominal infection (cIAI), clinical response at day 28; complicated urinary tract infection (cUTI), composite clinical and microbiological response 5–9 days after the end of therapy (EOT).
dOne of these patients had cIAI due to Citrobacter freundii encoding Klebsiella pneumoniae carbapenemase KPC-2 and the plasmid-borne AmpC CMY-48; this patient died on day 3 due to pneumonia, with the cIAI not deemed contributory to this death (indeterminate overall response). The other patient had cIAI due to Pseudomonas. aeruginosa encoding the chromosomal AmpC PDC-30, with persistence at the on-therapy visit (OTX) and failure at EOT, the on-therapy visit (EFU), and day 28 (unfavorable overall response).
eOne of these patients had cIAI due to P. aeruginosa encoding the chromosomal AmpC PDC-1, with indeterminate response at OTX, response of “improved” at EOT, and indeterminate response at EFU and day 28 (indeterminate overall response). The other patient had cIAI due to P. aeruginosa isolate encoding the chromosomal AmpC PDC-39, with investigator-assessed response of “improved” at OTX, “cure” at EOT, and “sustained cure” at EFU and day 28; based on prospectively defined rules, prior to unblinding the patient was imputed as having had an unfavorable overall response due to having received confounding, protocol-prohibited antibacterial therapy.
fTwo HAP/VAP patients with favorable overall response (ie, alive at day 28) had an unfavorable clinical response at day 28, while 2 cUTI patients had favorable clinical response at day 28 but (due to lack of microbiologic response) did not have a favorable overall response.
gOf 3 patients with favorable overall response (defined differently for each infection type, see footnote “c”) but lack of day 28 favorable clinical response, 1 patient with HAP/VAP experienced confirmed clinical failure but was still alive by day 28, 1 patient with HAP/VAP due to P. aeruginosa and Delftia acidovorans had an indeterminate clinical response (ie, extenuating circumstances precluded response classification, but the patient still required mechanical ventilation and had hypoxemia) but was still alive by day 28, and 1 patient with cUTI was lost to follow-up by day 28 but had favorable composite clinical and microbiological response 5–9 days after EOT.
hAssessed in evaluable safety population patients (IMI/REL, n = 29; colistin + IMI, n = 16).
Favorable overall response against P. aeruginosa was observed in 13/16 (81%) of IMI/REL and 5/8 (63%) of colistin+IMI patients; against Enterobacteriaceae, response rates were 2/5 (40%) and 2/2 (100%), respectively (Supplementary Materials). Of the 3 patients with Enterobacteriaceae and unfavorable overall response, 1 had cIAI due to C. freundii and 2 cUTI due to K. pneumoniae. The patient with C. freundii (carrying KPC-2, a plasmid-borne AmpC, and TEM-1 β-lactamases) was a 59-year-old male with a complex medical history (recurrent rectal carcinoma, small bowel resection, pleural effusions, and emphysema) who died on day 3 due to pneumonia (cIAI not deemed contributory). Both patients with K. pneumoniae (1 carrying KPC-2, 3 different ESBLs, and TEM-1 and the other carrying KPC-3, SHV-11, and TEM-1) had favorable clinical and microbiologic responses at EOT but recurrence at EFU. Neither patient had clinical signs or symptoms associated with the recurring pathogen; these 2 were the only cUTI patients with chronic intermittent urinary catheterization, which likely contributed to recurrence. Of the 2 patients with confirmed baseline bacteremia (despite being protocol-required, baseline blood cultures were available for only 9 patients), 0/1 receiving IMI/REL and 1/1 receiving colistin+IMI had favorable overall response. Prior meropenem therapy did not impact overall response (Supplementary Materials). Patients with baseline qualifying cultures obtained >3 days before randomization did not have better outcomes than those with cultures collected ≤3 days before; favorable overall response rates were 5/11 (45%) and 17/20 (85%), respectively.
Favorable clinical response rates were 31% higher with IMI/REL than with colistin+IMI at day 28 and were also higher at EOT and EFU (Supplementary Materials). Day 28 all-cause mortality was 20% lower with IMI/REL (Table 2). All mMITT patients with cUTI achieved favorable microbiological response at all visits, except 3 of 11 IMI/REL patients at EFU due to missing urine cultures (n = 1) and baseline pathogen recurrence at EFU without changes in susceptibility (n = 2; see above). There were no instances of treatment-emergent IMI/REL nonsusceptibility.
Treatment-emergent nephrotoxicity was significantly less frequent (P = .002) with IMI/REL than with colistin+IMI: 3/29 (10%) vs 9/16 (56%), respectively (95% CI for difference, –69.1%, –18.4%). Significantly (P = .047) fewer patients receiving IMI/REL (0/31, 0%) than colistin+IMI (2/16, 13%) experienced clinically relevant elevations in hepatic transaminases, a predefined tier 1 safety parameter. Most patients (71% IMI/REL, 81% colistin+IMI) had ≥1 AE (Table 3). Drug-related AEs were reported in 16% of IMI/REL vs 31% of colistin+IMI patients, and serious AEs in 10% vs 31%, respectively. There were also fewer deaths with IMI/REL (6% vs 19%); no deaths were considered drug-related. Three patients (19%) in the colistin+IMI and none in the IMI/REL arm discontinued treatment due to AEs (drug-related blood creatinine increase, drug-related CrCL increase, and treatment-emergent infection; n = 1 each). The most common AEs were pyrexia (13%), increased aspartate aminotransferase (AST; 13%), increased alanine aminotransferase (ALT; 11%), and nausea (11%; Table 4, Supplementary Materials). Incidence of pyrexia was comparable in both groups; increased AST, increased ALT, and nausea were less frequent with IMI/REL (Table 4). The only IMI/REL drug-related AE in >1 patient was decreased CrCL (6%), also reported in 13% of colistin+IMI patients (Table 4).
Table 3.
Summary of Adverse Events During Intravenous Therapy and the 14-Day Follow-up Period in the Safety Population
| Patients With AEs | IMI/REL (n = 31) | Colistin + IMI (n = 16) | Unadjusted Difference (95% Confidence Interval)a |
|---|---|---|---|
| At least 1 AE, n (%) | 22 (71.0) | 13 (81.3) | –10.3 (–33.1, 18.0) |
| Drug-related AEs, n (%) | 5 (16.1) | 5 (31.3) | –15.1 (–42.3, 9.2) |
| Serious AEs, n (%) | 3 (9.7) | 5 (31.3) | –26.1 (–47.8,1.3) |
| Serious drug-related AEs, n (%) | 0 (0.0) | 0 (0.0) | 0.0 (–19.7, 11.2) |
| Deaths, n (%) | 2 (6.5) | 3 (18.8) | –12.3 (–37.8, 6.5) |
| Drug-related deaths, n (%) | 0 (0.0) | 0 (0.0) | 0.0 (–19.7, 11.2) |
| Discontinued drug due to AE, n (%) | 0 (0.0) | 3 (18.8) | –18.8 (–43.3, –6.2) |
| Discontinued drug due to drug-related AE, n (%) | 0 (0.0) | 2 (12.5) | –12.5 (–36.3, –0.3) |
Abbreviations: AE, adverse event; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam.
aBased on the Miettinen and Nurminen method.
Table 4.
Specific Treatment-emergent and Drug-related Adverse Events During Intravenous Therapy and the 14-Day Follow-up Period in the Safety Population, as Reported by the Investigator
| IMI/REL (n = 31) | Colistin + IMI (n = 16) | |
|---|---|---|
| Treatment-emergent AEsa (incidence ≥10% in either treatment arm), n (%) | ||
| Pyrexia | 4 (12.9) | 2 (12.5) |
| Increased aspartate aminotransferase | 3 (9.7) | 3 (18.8) |
| Increased alanine aminotransferase | 2 (6.5) | 3 (18.8) |
| Nausea | 2 (6.5) | 3 (18.8) |
| Decreased creatinine renal clearance | 2 (6.5) | 2 (12.5) |
| Increased γ-glutamyltransferase | 1 (3.2) | 2 (12.5) |
| Increased blood alkaline phosphatase | 1 (3.2) | 2 (12.5) |
| Infusion site phlebitis | 1 (3.2) | 2 (12.5) |
| Dizziness | 0 (0.0) | 2 (12.5) |
| Increased blood bilirubin | 0 (0.0) | 2 (12.5) |
| Increased blood creatinine | 0 (0.0) | 4 (25.0) |
| Oral hypoesthesia | 0 (0.0) | 2 (12.5) |
| Drug-related AEsb (any incidence), n (%) | ||
| Decreased creatinine renal clearance | 2 (6.5) | 2 (12.5) |
| Hyperglycemia | 1 (3.2) | 0 |
| Infusion site erythema | 1 (3.2) | 0 |
| Pyrexia | 1 (3.2) | 0 |
| Dizziness | 0 | 2 (12.5) |
| Increased alanine aminotransferase | 0 | 1 (6.3) |
| Increased aspartate aminotransferase | 0 | 1 (6.3) |
| Increased blood creatinine | 0 | 1 (6.3) |
| Oral hypoesthesia | 0 | 1 (6.3) |
| Leukopenia | 0 | 1 (6.3) |
Abbreviations: AE, adverse event; IMI, imipenem/cilastatin; IMI/REL, imipenem/cilastatin plus relebactam.
aTreatment-emergent AEs refers to all reported AEs, regardless of their causality.
bDrug-related AEs refers to those treatment-emergent events that were deemed as related to study treatment by the investigator.
DISCUSSION
This randomized, controlled, double-blind trial that compared IMI/REL with colistin-based therapy showed that IMI/REL was effective for treating carbapenem-nonsusceptible gram-negative infections without the nephrotoxicity associated with colistin. Primary efficacy outcomes were similar between treatment arms, with favorable overall response in about 70% of patients. IMI/REL-treated patients had higher clinical response rates and lower 28-day mortality than those who received colistin-based therapy, but the study was not powered for statistical inference. Furthermore, the incidence of treatment-emergent nephrotoxicity, serious AEs, and drug-related AEs was higher with colistin. These results are noteworthy given the rigorous study design, that is, a carefully blinded evaluation of IMI/REL vs a dose-optimized, single-comparator regimen.
The study population comprised patients at high risk for poor outcomes, including death. In addition to having confirmed MDR, often severe infections, many participants also had high APACHE II scores, renal insufficiency or other comorbidities, substantial clinical complexity, extensive pretrial antibacterial therapy, or were elderly. Treatment groups were well-balanced at baseline. The pathogen distribution aligned with previous reports that suggested that carbapenem resistance is more common in Pseudomonas than in Enterobacteriaceae [3, 9, 19, 20]. Prior treatments, except meropenem, also appeared comparable. There were no trends in outcomes with prior meropenem. Of note, eligible patients had to be failing any prior therapy and have imipenem-nonsusceptible pathogens, reflected in the high cross-resistance of baseline pathogens to commonly used first-line therapies. High-risk patients, especially those potentially (due to local/institutional epidemiology and/or presence of risk factors) or definitively affected by carbapenem-resistant pathogens, should rapidly receive appropriate therapy to improve survival and shorten hospital length of stay [5]. Colistin is widely included in guidelines for treating carbapenem-resistant pathogens [21–25], despite being associated with dose-dependent nephrotoxicity and neurotoxicity [26]. Poor clinical outcomes and resistance emergence, worsened by inadequate dosing strategies, are also frequent concerns with colistin [1]. Recent guidelines recommend colistin-based combination therapy, including with carbapenems, over colistin monotherapy when treating carbapenem-resistant Enterobacteriaceae (CRE) and P. aeruginosa infections [21, 25, 27].
Given colistin’s role in treating carbapenem-resistant infections, its use as a comparator in several similar clinical trials, and its potential synergy with carbapenems, we chose the combination of colistin with IMI as the active comparator [21, 25, 27]. This choice also enabled the double-blind study design and a straightforward comparison of REL and colistin, since all patients received identical β-lactam background therapy. IMI is an optimal partner carbapenem for REL since REL’s pharmacokinetic/pharmacodynamic profile, including renal elimination and interaction with various resistance mechanisms, complements that of IMI [7, 8]. In combination with 250 mg REL, standard-dose (500 mg) IMI appears effective even against highly resistant pathogens [11, 13].
The combination of IMI with REL can overcome many gram-negative resistance mechanisms (including β-lactamase production/overexpression, porin loss, and efflux) [19, 20, 28–31] and is active against most strains of KPC producers, ESBL producers, and MDR/carbapenem-resistant Pseudomonas but not metallo-β-lactamases (eg, NDM) and/or class D β-lactamases (eg, OXA-48). REL generally does not improve imipenem susceptibility in Acinetobacter baumannii [6]. IMI/REL is a potential monotherapy agent, with coverage of many gram-positive and anaerobic pathogens [7, 10].
The RESTORE-IMI 1 trial was unique for several reasons. Data were generated specifically in the target population of interest (patients with carbapenem-nonsusceptible infections). The double-blinded study design allowed for robust assessment of IMI/REL safety and efficacy, and limiting comparator treatment to a single regimen rather than “best available therapy” reduced variability and ensured that all patients received appropriate therapy (only patients with colistin-susceptible infections were enrolled). Colistin dosing was optimized based on contemporary pharmacokinetic/pharmacodynamic modeling [32, 33]. Finally, pathogen susceptibility was confirmed rapidly through study-agent–specific test panels concurrently with each site’s standard, first-line susceptibility testing, allowing timely therapy initiation at the same point for all patients (ie, once pathogens were confirmed to meet microbiologic eligibility criteria), thus ensuring a rigorous treatment comparison.
This study had several limitations, including the small sample size. Under certain circumstances, for example, when the parent drug of a BL/BLI combination has established safety and efficacy, regulatory agencies allow streamlined drug development programs for antibacterials that target unmet medical need. These programs aim to generate limited clinical data in the target population, supplemented with preclinical and pharmacokinetic/pharmacodynamic studies and the parent compound’s existing safety and efficacy data. Streamlined development programs acknowledge that large noninferiority trials in MDR infections are not feasible. Patients must meet regulatory definitions for the specific infection types included, be ill enough to require salvage therapy but not too ill to participate and contribute meaningful data, and be hospitalized at experienced clinical trial sites. The scarcity of patients with confirmed carbapenem-nonsusceptible infections who meet these requirements and consent for such trials was reflected in the small number of eligible, enrolled participants. Most exclusions from the primary efficacy population were due to differences in susceptibility testing between local and central laboratories. This was not unexpected since eligibility required susceptibility to 3 different agents and repeat test results frequently differ by 1 or 2 dilutions, which can affect susceptibility interpretation. The small sample size was mitigated by the well-established safety and efficacy profile of the IMI component of IMI/REL and the robust safety, tolerability, and efficacy data from the IMI/REL phase 2 program [11, 12]. Few cIAI patients were enrolled, largely because there were fewer prescreening isolates from intraabdominal than urinary and lower respiratory tract specimens. The lack of favorable response among cIAI patients could have been due to their medical complexity, evidenced by extensive pretrial antibacterial therapy and high APACHE II scores. Furthermore, 50% of cIAI patients had unfavorable outcomes due to indeterminate responses (data missing for any reason except cIAI-attributable death). Relatively few patients with CRE were enrolled, thus limiting interpretability among this subpopulation. Since in vitro susceptibility can be considered a reasonable predictor of favorable therapeutic response [34], IMI is recommended for treating infections caused by imipenem-susceptible pathogens (including Enterobacteriaceae) [22–24, 35]. Relebactam restores in vitro imipenem susceptibility against most CRE [6, 9, 10, 13], and IMI/REL can therefore be expected to have clinical efficacy against imipenem/relebactam-susceptible CRE strains. Another limitation is that while treatment-emergent nephrotoxicity was protocol-defined to apply a consistent, objective definition, alternative definitions of kidney injury exist [36].
Our results support IMI/REL as a suitable treatment option for serious gram-negative infections, including those caused by carbapenem-nonsusceptible pathogens in high-risk patients. IMI/REL may be preferable to colistin-based therapy for treating carbapenem-nonsusceptible infections, given that IMI/REL had comparable efficacy but significantly less nephrotoxicity and other AEs.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and their families and caregivers for participating in this study, along with all investigators and site personnel. A full list of primary investigators for protocol MK-7655A-013 (by country) who enrolled patients is provided in the Supplementary Materials (Supplementary Table 5). Medical writing assistance was provided by Dominik Wolf, MSc, and Dean Campbell, PhD, both of Merck & Co., Inc. Editorial assistance was provided by Michele McColgan, BA, of Merck & Co., Inc.
Financial support. Funding for this research was provided by Merck Sharp & Dohme (MSD) Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. MSD’s data sharing policy, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
Potential conflicts of interest. J. M.’s institution received research support from Merck & Co., Inc. C. M. d. O., V. S., and O. L. received institutional research funding from Merck & Co., Inc. I. Kö. has been an advisory board member and speaker for MSD, AbbVie, Gilead, and Pfizer. H. W. B. has been a (scientific advisory board) consultant to Merck & Co., Inc. K. S. K. received grant support from Merck & Co., Inc., and has been a consultant for Merck & Co., Inc., Melinta, Achaogen, Allergan, and Shinogi. T. M. F. has been a consultant to Merck & Co., Inc., bioMérieux, Curetis, Glaxo Smith Kline, Meiji, MotifBio, Nabriva, Paratek, Shionogi, and the Medicines Company. M. L. B., I. Kh., J. D., H.-K. J., R. W. T., A. A., K. Y., N. A. K., J. R. B., and A. P. are employees of MSD, a subsidiary of Merck & Co., Inc. and may own stock and/or hold stock options in the company. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 2013; 13:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/. Accessed 3 June 2019. [Google Scholar]
- 3. Cerceo E, Deitelzweig SB, Sherman BM, Amin AN. Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist 2016; 22:412–31. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. 2017. Available at: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ [Google Scholar]
- 5. Zilberberg MD, Shorr AF, Micek ST, Vazquez-Guillamet C, Kollef MH. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit Care 2014; 18:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:2286–90. [DOI] [PubMed] [Google Scholar]
- 7. Rizk ML, Rhee EG, Jumes PA, et al. Intrapulmonary pharmacokinetics of relebactam, a novel beta-lactamase inhibitor, dosed in combination with imipenem-cilastatin in healthy subjects. Antimicrob Agents Chemother 2018; 62.:e01411–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rizk ML, Jumes P, Lasseter K, et al. Pharmacokinetics of MK-7655, a novel b-lactamase inhibitor (BLI), in combination with imipenem/cilastatin (IPM/CIL) in subjects with impaired renal function. In: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC). San Francisco, CA, 2012; A-010.
- 9. Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem/relebactam against gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J Antimicrob Chemother 2018; 73:1872–9. [DOI] [PubMed] [Google Scholar]
- 10. Papp-Wallace KM, Barnes MD, Alsop J, et al. Relebactam is a potent inhibitor of the KPC-2 beta-lactamase and restores the susceptibility of imipenem against KPC-producing Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e00174–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother 2016; 60:6234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double-blind, phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother 2017; 72:2616–26. [DOI] [PubMed] [Google Scholar]
- 13. Wu J, Racine F, Wismer MK, et al. Exploring the pharmacokinetic/pharmacodynamic relationship of relebactam (MK-7655) in combination with imipenem in a hollow-fiber infection model. Antimicrob Agents Chemother 2018; 62:e02323–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute. M07-A10. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Wayne, PA: CLSI, 2015. [Google Scholar]
- 15. Clinical and Laboratory Standards Institute. M100-S27. Performance standards for antimicrobial susceptibility testing. 27 ed. Wayne, PA: CLSI, 2017. [Google Scholar]
- 16. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research. Community-acquired bacterial pneumonia: developing drugs for treatment, guidance for industry. Silver Spring, MD: Center for Drug Evaluation and Research, 2014. [Google Scholar]
- 17. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry. Complicated intra-abdominal infections: developing drugs for treatment. Silver Spring, MD: Center for Drug Evaluation and Research, 2018. [Google Scholar]
- 18. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry. Complicated urinary tract infections: developing drugs for treatment. Silver Spring, MD: Center for Drug Evaluation and Research, 2015. [Google Scholar]
- 19. Shortridge D, Pfaller MA, Castanheira M, Flamm RK. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. hospitals (2013–2016) as part of the surveillance program: program to assess ceftolozane-tazobactam susceptibility. Microb Drug Resist 2018; 24:563–77. [DOI] [PubMed] [Google Scholar]
- 20. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against gram-negative clinical isolates in New York City. Antimicrob Agents Chemother 2015; 59:4856–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother 2018; 73(suppl 3):iii2–78. [DOI] [PubMed] [Google Scholar]
- 22. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:133–64. [DOI] [PubMed] [Google Scholar]
- 23. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63:e61–e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 25. Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39:10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee YJ, Wi YM, Kwon YJ, Kim SR, Chang SH, Cho S. Association between colistin dose and development of nephrotoxicity. Crit Care Med 2015; 43:1187–93. [DOI] [PubMed] [Google Scholar]
- 27. Carrara E, Bragantini D, Tacconelli E. Combination versus monotherapy for the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 2018; 31:594–9. [DOI] [PubMed] [Google Scholar]
- 28. Chalhoub H, Sáenz Y, Rodriguez-Villalobos H, et al. High-level resistance to meropenem in clinical isolates of Pseudomonas aeruginosa in the absence of carbapenemases: role of active efflux and porin alterations. Int J Antimicrob Agents 2016; 48:740–3. [DOI] [PubMed] [Google Scholar]
- 29. Melinta Therapeutics Inc. Vabomere (meropenem and vaborbactam for injection. Prescribing information. Lincolnshire, IL: Melinta Therapeutics Inc, 2018. [Google Scholar]
- 30. European Centre for Disease Prevention and Control. Rapid risk assessment: emergence of resistance to ceftazidime-avibactam in carbapenem-resistant Enterobacteriaceae. Solna, Sweden: European Centre for Disease Prevention and Control, 2018. [Google Scholar]
- 31. Chalhoub H, Tulkens, PM, Van Bambeke F. Avibactam is a substrate for Mex-AB-OprM in Pseudomonas aeruginosa. In: 27th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID). Vienna, Austria, 2017.
- 32. Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nation RL, Garonzik SM, Li J, et al. Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rex JH, Pfaller MA. Has antifungal susceptibility testing come of age? Clin Infect Dis 2002; 35:982–9. [DOI] [PubMed] [Google Scholar]
- 35. Merck & Co Inc. PRIMAXIN® I.V. (imipenem and cilastatin for injection). Last revised October 2014 ed. Merck & Co., Inc., Whitehouse Station, NJ, 2014. [Google Scholar]
- 36. Brown ML, Motsch J, Kaye KS, et al. Nephrotoxicity associated with imipenem/cilastatin/relebactam (IMI/REL) versus imipenem/cilastatin plus colistin (IMI+CST) in patients with imipenem-nonsusceptible (NS) bacterial infections. In: IDWeek. San Francisco, CA, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.