Abstract
Background
Immune restoration on antiretroviral therapy (ART) can drive inflammation in people living with human immunodeficiency virus (HIV) who have pulmonary tuberculosis (TB), but its effects on the lungs have not been assessed. We evaluated associations between pulmonary inflammation, recovery of pathogen-specific CD4 T-cell function, and lung injury prior to and after ART initiation in adults with HIV and pulmonary TB.
Methods
This was a prospective cohort study in South Africa, following adults with HIV and pulmonary TB prior to and up to 48 weeks after ART initiation. Pulmonary-specific inflammation was defined as total glycolytic activity (TGA) on [18]F-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) at baseline and 4 weeks after ART initiation. Spirometry, respiratory symptom tests, and flow cytometry were performed at the same times to assess lung involvement and the frequency of mycobacteria-specific CD4 T-cells. In addition, we evaluated lung function longitudinally up to 48 weeks after ART initiation.
Results
Greater lung TGA on FDG PET-CT was associated with worse lung function and respiratory symptoms prior to ART initiation, and nearly half of subjects experienced worsening lung inflammation and lung function at Week 4 of ART. Worsening Week 4 lung inflammation and pulmonary function were both associated with greater increases in pathogen-specific functional CD4 T-cell responses on ART, and early decreases in lung function were independently associated with persistently lower lung function months after TB treatment completion.
Conclusions
Increases in pulmonary inflammation and decreases in lung function are common on ART, relate to greater ART-mediated CD4 T-cell restoration, and are associated with the persistent impairment of lung function in individuals with HIV/TB.
Keywords: HIV/tuberculosis, antiretroviral therapy, CD4 T-cell function, FDG positron emission tomography–computed tomography (PET-CT), pulmonary function
In this study from South Africa, approximately half of all adults with human immunodeficiency virus and pulmonary tuberculosis had increases in lung inflammation and decreases in lung functioning as their CD4 T-cell function recovered on antiretroviral therapy.
Antiretroviral therapy (ART) improves survival in adults living with human immunodeficiency virus (HIV) who have pulmonary tuberculosis (TB) [1–4], but ART may trigger the TB immune reconstitution inflammatory syndrome (TB-IRIS) [5–9]. Severe TB-IRIS, requiring hospitalization or medical procedures, occurred in approximately 40% of patients who started ART in the initial 2 weeks after TB treatment initiation in a trial in South Africa [10]. Furthermore, the effects of IRIS on involved organ function are largely unknown.
Nearly half of those with pulmonary TB have impaired lung function after cure, including either decreased forced expiratory volume in 1 second (FEV1), a measure of airflow obstruction; decreased functional vital capacity (FVC), a measure of restriction; or both [11–14]. These defects likely relate to pulmonary inflammation [12, 14–17]. Although inflammation may resolve quickly after starting TB treatment, individuals with pulmonary TB and HIV may experience incident lung involvement on ART, providing an opportunity to evaluate mediators of lung damage prospectively [7, 10, 18, 19]. This is important, as effects of IRIS on target organ function are unknown.
Scanning with [18]F-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) can characterize and quantify pulmonary inflammation in TB [20]. Furthermore, pulmonary enhancement on FDG PET-CT has been correlated with an improved probability of a microbiologic cure in adults with drug-resistant TB, indicating clinical relevance [21]. No studies, to our knowledge, have used this modality to study the relationship between lung inflammation and longer-term lung function in people with HIV/TB.
Animal models indicate an important role for CD4 T-cells in TB granuloma and cavity formation [22, 23]. We therefore tested the hypothesis that greater functional CD4 T-cell recovery on ART worsens pulmonary inflammation and impairs lung function in adults coinfected with HIV/TB. We also evaluated the relationship between pulmonary glycolytic activity, lung function, and respiratory symptoms prior to and early after ART initiation, and correlated early changes in lung function to longer-term pulmonary impairment on ART.
METHODS
Ethics
The Institutional Review Boards of the University of Pennsylvania, the University of Witwatersrand, and the Health Research Ethics Counsel in South Africa approved this study. All participants provided written informed consent.
Study Design
In the prospective Lung Function after TB-IRIS (LIFT-IRIS) study, participants are seen monthly until Week 24, then seen at Week 36 and Week 48 post-ART initiation. FEV1 and FVC, measured by spirometry and respiratory symptoms, as assessed by the chronic obstructive pulmonary disease assessment test (CAT) questionnaire [24], are determined at the pre-ART baseline visit and at 4, 12, 24, and 48 weeks after ART initiation. These measures are associated with survival and have had minimum clinically important differences estimated in other diseases (a 100–150 mL change for FEV1 and a 2-point score in the 40-point CAT) [24–27]. Within this parent study, we conducted an imaging substudy, enrolling individuals who agreed to undergo FDG PET-CT scanning prior to and 4 weeks after ART initiation. Relationships between pre-ART and early changes at Week 4 of ART in lung function, pathogen-specific CD4 T-cell function, and lung enhancement on FDG PET-CT is the primary focus of this report. Dramatic changes in cellular immune responses occur by Week 4 and are associated with clinical outcomes in individuals with HIV/TB [6, 28–31]. In addition, we used data from all subjects with available lung function data in longitudinal analyses to establish the relationship between changes in lung function from baseline to Week 4 on ART, and to longer-term lung function out to approximately 6 months after TB treatment completion.
Study Participants
Participants receiving care at 18 public sector clinics in Tembisa, South Africa, were screened and enrolled. People living with HIV who were ART-naive adults 18–70 years old with CD4 counts ≤200 cells/µl were initially enrolled, but the protocol was amended 11 June 2018 to include individuals with CD4 counts ≤500 cells/µl. Participants had a new diagnosis of pulmonary TB by sputum Xpert MTB/RIF assay, without evidence of rifampin resistance or TB meningitis, were not prisoners or taking corticosteroids, did not have diabetes, were not pregnant, and were willing to initiate standard TB therapy and ART (efavirenz plus tenofovir and emtricitabine). Participants could not have been on TB treatment for more than 2 months. Qualifying patients were asked to participate in the imaging substudy consecutively. Participants needed to complete at least the baseline FDG PET-CT scan to be included in imaging analyses. Those who had at least baseline, Week 4, and 1 subsequent lung function assessments at the time of data lock were included in an analysis relating baseline to Week 4 spirometric changes and longer-term lung function.
Clinical Data and Sample Collection
Symptoms were assessed every visit by study physicians with a standardized protocol. Outcomes were categorized using modified World Health Organization definitions (details in Supplementary Material). Paradoxical TB-IRIS was diagnosed using an internationally accepted definition [7]. A spirometer (EasyOne Pro, nDD Medical Technologies, Andover, MD) was used to assess lung function. The percentage of predicted FEV1 and FVC were calculated from appropriate reference ranges. The CAT questionnaire was administered by a study nurse and was not part of the IRIS assessment.
Flow Cytometry
Flow cytometry was conducted as previously described [28], and is detailed in the Supplementary Material.
[18]F-fluorodeoxyglucose Positron Emission Tomography–Computed Tomography
Details of the imaging methods are presented in the Supplementary Material. Briefly, we used an integrated 40-detector PET/CT scanner (Biograph 40 True Point PET/CT, Siemens Medical, IL) at the University of the Witwatersrand Nuclear Medicine Department. We (G. P. B. and M.-d.-T. V.) manually created a total pulmonary region of interest, then applied a threshold of >1 standardized uptake value (SUV) within this area to minimize the contribution of the normal lung. The activity for this 3-dimensional area was determined as the mean SUV (SUVmean) of the area, multiplied by the area’s volume. The aggregate activity in both lungs is referred to as the total glycolytic activity (TGA). We also recorded the lung SUVmax, presence of cavitation, the percentage of the lung affected (ie, volume with SUV > 1, divided by total lung volume), and the so-called “hard volume,” consisting of lung tissue between -100 and 200 Hounsfield units, which Chen et al [21] previously associated with microbiologic failure in drug-resistant TB.
Statistical Analysis
Details on our sample size are given in the Supplementary Material. Continuous variables were summarized using proportions, means, ranges, medians, and interquartile ranges (IQRs), as appropriate. In unadjusted analyses, continuous variables were evaluated using Spearman correlation coefficients and rank sum tests. Lung TGA was log10 transformed for graphical analyses. Adjusted analyses used linear regression (for baseline associations and for evaluations of changes in parameters from baseline to Week 4) and a generalized estimating equation (for repeated measures longitudinal analyses of lung function). Details of evaluation of the generalized estimating model, confounding, and corrections for multiple comparisons are given in the Supplementary Material.
RESULTS
Baseline Characteristics
Characteristics of the 48 participants in the imaging cohort are shown in Table 1. The median CD4 count was 87 cells/µl (IQR 48–191). Among the 48 completing 1 FDG PET-CT scan, 15 (31%) withdrew from the imaging study after the first scan, 2 (4%) were lost to follow-up, and the rest either completed TB treatment successfully (n = 18, 38%) or remained on study beyond Week 12 without evidence of TB treatment failure (n = 13, 27%). Baseline chest lesions were heterogeneous, as illustrated by examples in Figure 1A–C. In the imaging cohort, 10 subjects (21%) could not produce usable spirometry values, primarily due to coughing during the procedure, and were excluded from lung function analyses (characteristics shown in Supplementary Table 1). Among patients with baseline pulmonary function tests, FEV1 and FVC were highly correlated (ρ = 0.92, P < .001); therefore, FEV1/FVC ratios were generally preserved (Table 1). Furthermore, 16 of 48 (33%) subjects had a predicted FVC percent that was lower than 0.8, indicating restriction.
Table 1.
Pre–antiretroviral Therapy Characteristics of Adults With Human Immunodeficiency Virus and Pulmonary Tuberculosis
| Characteristic | Imaging Cohort (n = 48) |
Qualifying Combined Imaging and Parent Cohort (n = 63) |
|---|---|---|
| Female sex, n (%) | 26 (54%) | 23 (37%) |
| Age, mean (range) | 37 (22–54) | 37 (20–57) |
| Pre-ART CD4 T-cell count, median cells/µL (IQR) | 87 (48–191) | 118 (49–194)a |
| CD4 T-cell count >50 cells/µl, n (%) | 33 (69%) | 45 (74%)a |
| HIV viral load, median log10 copies/mL (IQR) | 5.1 (4.7–5.5) | 5.3 (4.8–5.8)a |
| Body mass index, median (IQR) | 18.8 (17.6–21.1) | 19.2 (18.4–21.4) |
| TB treatment to ART initiation interval, median days (IQR) | 21 (14–41) | 22 (15–38)a |
| Ever smoked, n (%) | 17 (35%) | 24 (38%) |
| Sputum culture positive, n (%) | 27 (56%) | 31 (49%) |
| Days to positivity among culture-positive subjects, median (IQR) | 14 (11–17) | 13 (8–15) |
| FEV1, L, median (IQR)b | 2.4 (1.9–2.8) | 2.4 (2.1–2.8) |
| FEV1, %, median (IQR)b | 79 (64–88) | 79 (64–90) |
| FVC, mL, median (IQR)b | 3.1 (2.5–3.6) | 3.2 (2.7–3.7) |
| FVC, %, median (IQR)b | 84 (70–97) | 86 (72–94) |
| FEV1/FVC ratio, median (IQR)b | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| Percent of lung involved, median (IQR) | 19% (6–31%) | NA |
| Cavitation on CT scan, n (%) | 18 (33%) | NA |
| Mean SUV, median (IQR) | 1.6 (1.3–2.2) | NA |
| Max SUV, median (IQR) | 8.7 (5.3–13.9) | NA |
| CT hard volume, median mL (IQR) | 37 (24–113) | NA |
| Region of interest volume, median mL (IQR) | 464 (148–718) | NA |
| Total lung glycolytic activity, median (IQR) | 768.8 (197.8–1585.1) | NA |
| Log10 total lung glycolytic activity, median (IQR) | 2.9 (2.3–3.2) | NA |
Abbreviations: ART, antiretroviral therapy; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; SUV, standard uptake value; TB, tuberculosis.
a n = 61.
b38 subjects in the imaging cohort completed usable spirometry at baseline.
Figure 1.
FDG PET-CT fusion images of adult subjects with HIV and pulmonary TB. A–C, Pre-ART scans in 3 subjects. D–F, Pre-ART (top) and Week 4 (bottom) scans in 3 different subjects who experienced increases in pulmonary glycolytic activity after ART initiation. Abbreviations: ART, antiretroviral therapy; FDG, [18]F-fluorodeoxyglucose; HIV, human immunodeficiency virus; PET-CT, positron emission tomography–computed tomography; TB, tuberculosis.
Pulmonary Total Glycolytic Activity Is Associated With Lung Function and Respiratory Symptoms Prior to Antiretroviral Therapy Initiation
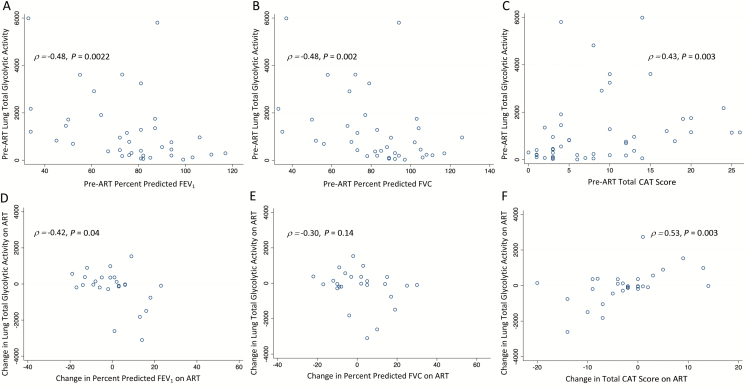
A higher pre-ART lung TGA was significantly associated with a lower percent predicted FEV1 (ρ = −0.48; P = .002), lower percent predicted FVC values (ρ = −0.48; P = .002), and higher CAT scores (ρ = 0.43; P = .003; Figure 2A–C and Table 2). Additional radiographic measures, including both individual components of TGA—the region of interest SUVmean and volume—were also associated with lung function and symptoms (Table 2). A higher CD4 count and lower HIV viral load were associated with a greater pre-ART lung TGA at baseline (Table 3), as were additional radiographic measures and cavitation (P ≤ .001).
Figure 2.
Relationships between lung total glycolytic activity and percent predicted FEV1, FVC, and total CAT score. Scatter plots of lung glycolytic activity and percent predicted (A) FEV1, (B) FVC, and (C) total symptom score pre-ART. Also shown are the correlations between changes from baseline to Week 4 post-ART in lung TGA and (D) FEV1, (E) FVC, and (F) CAT scores. Each dot represents an individual patient in the scatter plots. Abbreviations: ART, antiretroviral therapy; CAT, chronic obstructive pulmonary disease assessment test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Table 2.
Relationships Between Pre–Antiretroviral Therapy Clinical and Radiographic Characteristics in the Imaging Cohort
| Characteristic | FEV1, % | FVC, % | CAT Score | |||
|---|---|---|---|---|---|---|
| β (95% CI) | P Value | β(95% CI) | P Value | β (95% CI) | P Value | |
| Female sex, n (%) | −9.0 (−22.6 to 4.6) | .19 | −7.5 (−22.2 to 7.3) | .3 | 3.4 (−.6 to 7.5) | .09 |
| Age, per 10 years | −2.13 (−11.8 to 7.6) | .5 | −1.6 (−12.0 to 8.9) | >.5 | 0.8 (−1.9 to 3.5) | >.5 |
| CD4 count, cells/µl | −0.04 (−.0 to .01) | .10 | −0.05 (−.10 to .003) | .07 | 0.01 (−.002 to .02) | .09 |
| CD4 count >50 cells/µl | −11.5 (−25.9 to 3.0) | .12 | −10.3 (−26.1 to 5.4) | .19 | 4.2 (−.1 to 8.5) | .06 |
| HIV viral load, log10 copies/mL | 0.0 (−7.1 to 7.1) | >.5 | 1.0 (−6.7 to 8.6) | >.5 | 0.0 (−1.7 to 1.6) | >.5 |
| Body mass index | 1.3 (−.76 to 3.4) | .21 | 2.2 (.04–4.3) | .05 | −0.5 (−1.1 to .1) | .08 |
| Days between TB treatment initiation and ART initiation, per day | 0.03 (−.37 to .43) | >.5 | 0.08 (−.34 to .51) | >.5 | −0.05 (−.17 to .1) | .4 |
| Sputum culture positive | −10.0 (−23.6 to 3.8) | .15 | −10.5 (−25.3 to 4.2) | .16 | 5.2 (1.3–9.1) | .01 |
| Ever smoked | 3.7 (−10.7 to 18.1) | >.5 | 0.5 (−15.0 to 16.0) | >.5 | −0.7 (−5.0 to 3.7) | >.5 |
| Percent of lung involved, per 10 percentage point increase | −6.1 (−9.1 to −3.2) | <.001 | −6.7 (−9.9 to −3.5) | <.001 | 1.8 (1.0–2.8) | <.001 |
| Cavitation on CT scan | −5.3 (−20.0 to 9.6) | .5 | −7.6 (−23.5 to 8.3) | .3 | 5.6 (1.6–9.5) | .007 |
| Mean SUV, per 1 unit increase | −9.0 (−17.3 to −.8) | .03 | −8.8 (−17.8 to .2) | .06 | 1.2 (−1.3 to 3.7) | .3 |
| Max SUV, per 1 unit increase | −1.0 (−2.2 to .3) | .12 | −0.9 (−2.2 to .5) | .2 | 0.2 (−.2 to .6) | .2 |
| CT hard volume, per 10 mL higher | −0.8 (−1.3 to −.2) | .01 | −0.9 (−1.5 to −.3) | .007 | 0.1 (−.0 to .3) | .09 |
| Region of interest volume, per 100 mL higher | −1.8 (−3.1 to −.5) | .01 | −2.0 (−3.4 to −.6) | .007 | 0.6 (.2–1.0) | .004 |
| Total lung glycolytic activity, per 500 units higher | −2.8 (−5.0 to −.7) | .01 | −3.1 (−5.5 to −.8) | .01 | 0.6 (−.03 to 1.3) | .06 |
| Log10 total lung glycolytic activity, per 1 log10 higher | −18.0 (−28.8 to −7.1) | .002 | −18.1 (−30.0 to −6.2) | .004 | 4.3 (1.3–7.3) | .005 |
Data are from adults with HIV and pulmonary TB, for lung function (n = 38) and respiratory symptoms (n = 48). Shown are the β coefficient and 95% CI from a multivariable linear regression model. The β coefficient provides an estimate of the effect of a change of a certain magnitude in the parameter on the change in lung function or symptoms.
Abbreviations: ART, antiretroviral therapy; CAT, chronic obstructive pulmonary disease assessment test; CI, confidence interval; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HIV, human immunodeficiency virus; SUV, standard uptake value; TB, tuberculosis.
Table 3.
Relationships Between Pre–antiretroviral Therapy Clinical and Radiographic Characteristics in the Imaging Cohort
| Baseline Characteristic | Total Pulmonary Glycolytic Activity at Baseline, n = 48 | P Value | Change in Total Pulmonary Glycolytic Activity from Baseline to Week 4 on ART, n = 30 | P Value |
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | |||
| Female sex | −114.5 (−1015.3 to 786.3) | >.5 | 401.3 (−432.6 to 1235.1) | .3 |
| Age, per 10 years | 24.3 (−34.4 to 83.0) | .4 | −8.9 (−638.4 to 620.5) | >.5 |
| CD4 count, cells/µl | 6.6 (4.4–8.8) | <.001 | −2.1 (−4.4 to .3) | .08 |
| CD4 count>50 cells/µl | 1351.8 (469.7–2233.8) | .003 | −493.3 (−1399.0 to 412.2) | .3 |
| HIV viral load, log10 copies/mL | −704.1 (−1094.5 to −313.8) | .001 | 381.1 (−103.9 to 866.1) | .1 |
| Body mass index | −88.2 (−215.7 to 39.3) | .17 | 63.4 (−61.4 to 188.2) | .3 |
| Days between TB treatment initiation and ART initiation, per day | 10.5 (−15.5 to 36.4) | .4 | −26.3 (−48.1 to −4.6) | .02 |
| Sputum culture positive | 793.5 (−80.7 to 1667.7) | .07 | 457.2 (−404.9 to 1319.2) | .3 |
| Ever smoked | 412.1 (−519.0 to 1343.2) | .4 | −63.4 (−962.8 to 835.9) | >.5 |
| Percent of lung involved, per 10 percentage point increase | 580.3 (427.0–733.6) | <.001 | −217.2 (−416.0 to −18.4) | .03 |
| Cavitation on CT scan | 1347.9 (601.0–2094.9) | .001 | −494.7 (−1241.4 to 252.1) | .2 |
| Mean SUV, per 1 unit increase | 1577.1 (1289.7–1864.1) | <.001 | −561.9 (−993.0 to −130.8) | .01 |
| Max SUV, per 1 unit increase | 143.2 (69.6–216.8) | <.001 | −66.5 (−147.6 to 14.7) | .1 |
| CT hard volume, per 10 mL higher | 92.2 (75.6–108.8) | <.001 | −25.5 (−50.2 to −.7) | .04 |
| Region of interest volume, per 100 mL higher | 285.1 (243.2–327.1) | <.001 | −122.1 (−187.9 to −56.3) | .001 |
| Total lung glycolytic activity, per 500 units higher | NA | … | −188.1 (−291.6 to −84.6) | .001 |
| Log10 total lung glycolytic activity, per 1 log10 higher | NA | … | −994.7 (−1600.8 to −388.7) | .002 |
Data are from adults with HIV and pulmonary TB, for total pulmonary glycolytic activity at baseline (n = 48) and change in total pulmonary glycolytic activity from baseline to Week 4 of ART (n = 30). Shown are the β coefficient and 95% CI from a multivariable linear regression model.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CT, computed tomography; HIV, human immunodeficiency virus; SUV, standard uptake value; TB, tuberculosis.
Increases in Lung Total Glycolytic Activity on Antiretroviral Therapy Are Common and Are Associated With Worsening Lung Function and Respiratory Symptoms
Approximately 4 weeks after ART initiation, 30 subjects (63%) returned for a second FDG PET-CT. Those without a second scan had slightly lower baseline CD4 counts and higher CAT scores versus those with 2 scans (Supplementary Table 2). Immunologic and virologic responses to ART at Week 4 were robust (Supplementary Table 3).
There was no significant change in lung TGA from baseline to Week 4 on ART (Supplementary Table 3), but 12 of 30 subjects (40%) had increases in pulmonary glycolytic activity (Supplementary Figure 1A). Changes in lung TGA on ART were correlated with both changes in region of interest volumes (ρ = 0.9; P < .0001) and changes in SUVmean (ρ = 0.7, P < .0001).
Among the 30 patients who had 2 FDG PET-CT scans, only 3 (10%) met the definition of paradoxical TB-IRIS during 48 weeks of follow-up (described in Supplementary Material). Of the 3 subjects, 1 had a respiratory TB-IRIS presentation with increasing lung TGA, while the other 2 had nonrespiratory presentations with decreasing lung TGA. Examples of subjects developing increasing lung TGA on ART are shown in Figure 1D–F.
Of the 30 participants with a second FDG PET-CT scan, 25 had evaluable spirometry results at both visits. There was no statistically significant overall change in FEV1 or FVC values after 4 weeks of ART among these participants (Supplementary Table 3), but 12 of 25 (48%) experienced a decrease in FEV1 from baseline to Week 4 (Supplementary Figure 1B). Among those with an early FEV1 drop, the median percent and absolute decreases in FEV1 were −9% (IQR −14 to −4) and −265 mL (IQR −420 to −90), respectively. The median change in lung TGA was an increase of 250 (IQR −125 to 474) among those with an FEV1 decrease from baseline to Week 4, versus a decrease of 96 in those without an FEV1 decrease (IQR −1492 to −22; P = .06, rank-sum), and the change in lung TGA from baseline to Week 4 was inversely correlated with the change in FEV1 (ρ = −0.42; P = .04), but not with FVC (ρ = −0.30; P = .14; Figure 2D and 2E). Similarly, 8 subjects (28%) had increases in total CAT scores over 4 weeks of ART (median increase among those who increased = 4, IQR 2 to 11; Supplementary Figure 1C). The median change in lung TGA from baseline to Week 4 among those with worsening CAT scores was 730 (IQR −35 to 1258), versus −95 in others (IQR −449 to 123; rank-sum P = .005), and increasing lung TGA during 4 weeks of ART was positively correlated with increasing CAT scores (ρ = 0.53; P = .003; Figure 2F).
Among clinical factors, a lower CD4 count, higher viral load, and shorter interval between TB treatment initiation and ART initiation were all at least marginally (P < .10) associated with increasing lung TGA on ART (Table 3). Less radiographic involvement, including lower baseline lung TGA, was also associated with greater increases in lung TGA on ART (Table 3). In a multivariable model accounting for baseline lung TGA, baseline CD4 count, and viral load, each additional week in delay between TB treatment initiation and ART initiation was associated with a change in lung TGA at Week 4 of ART of −161.2 (95% CI −310.0 to −12.4; P = .04).
Purified Protein Derivative–specific CD4 T-Cell Recovery Is Associated With Increasing Pulmonary Total Glycolytic Activity and Declining Lung Function
Changes in lung TGA, FEV1, FVC, and symptom scores were not associated with changes in overall CD4 counts or changes in viral loads (data not shown, all P > .4). Participants with increasing lung TGA on ART had similar baseline frequencies (Figure 3A and 3D) but significantly greater increases in the frequency of purified protein derivative (PPD)-specific CD4 T-cells expressing tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ) from baseline to Week 4 post-ART (Figure 3B and 3E; P ≤ .01), as well as higher frequencies of these cells at Week 4 (Figure 3C and 3F; P ≤ .01). Similar relationships were seen in PPD-specific TNF-α + (total and TNF-α single-positive) CD4 T-cells and decreasing FEV1 on ART (Figure 4A–F). Associations between PPD-specific immune responses and FVC and symptoms, as well as HIV Gag-specific responses and measures of lung involvement, were not significant (Supplementary Figures 3 and 4; Supplementary Table 4). Associations between changes in lung TGA on ART and changes in TNF-α + and IFN-γ + CD4 T-cell responses (total TNF-α or IFN-γ; TNF-α +IFN-γ + double-positive; or TNF-α single-positive) to PPD remained significant in a multivariable model accounting for baseline lung TGA, baseline CD4 count, and viral load (Table 4). All associations were attenuated after adjusting for the time between TB treatment initiation and ART initiation (Table 4).
Figure 3.
Individuals with HIV who are coinfected with TB and have increases in lung inflammation, have rapid increases in PPD-specific CD4 T-cell responses following ART initiation. PBMCs isolated from patients at baseline and Week 4 post-ART initiation were stimulated with PPD to determine PPD-specific immune responses by flow cytometry. The gating strategy is shown in Supplementary Figure 2. Shown are the median frequencies and interquartile ranges of CD4 T-cells expressing total IFN-γ (G), IL-2 (2), or TNF-α (T) (A) at baseline, (B) change from baseline to Week 4 post-ART initiation, and (C) at Week 4 post-ART initiation in patients who had a decrease in lung TGA (black), compared to those who had an increase in lung TGA from baseline to Week 4 post-ART initiation (red). Also shown are the median frequencies and interquartile ranges of CD4 T-cells expressing any combination of the 3 cytokines in response to PPD stimulation determined by Boolean gating (D) at baseline, (E) change from baseline to Week 4 post-ART initiation, and (F) at Week 4 post-ART initiation. Data shown are following background subtraction (no stimulation condition). P values shown are from Wilcoxon rank-sum tests, followed by Benjamini–Hochberg correction for multiple comparisons. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IL, interleukin; IFN-γ,interferon gamma; PBMC, peripheral blood mononuclear cell; PPD, purified protein derivative; TB, tuberculosis; TNF-α, tumor necrosis factor-alpha; TGA, total glycolytic activity.
Figure 4.
Individuals with HIV who are coinfected with TB and have decreases in %FEV1, have increase in PPD-specific CD4 T-cell responses following ART initiation. PBMCs isolated from patients at baseline and Week 4 post-ART initiation were stimulated with PPD to determine PPD-specific immune responses by flow cytometry. The gating strategy is shown in Supplementary Figure 2. Shown are the median frequencies and interquartile ranges of CD4 T-cells expressing total IFN-γ (G), IL-2 (2), or TNF-α (T) (A) at baseline, (B) change from baseline to Week 4 post-ART initiation, and (C) at Week 4 post-ART initiation in patients who had an increase in %FEV1 (black), compared to those who had a decrease in %FEV1 (red) from baseline to Week 4 post-ART initiation. Also shown are the median frequencies and interquartile ranges of CD4 T-cells expressing any combination of the 3 cytokines in response to PPD stimulation, determined by Boolean gating (D) at baseline, (E) change from baseline to Week 4 post-ART initiation, and (F) at Week 4 post-ART initiation. Data shown are following background subtraction (no stimulation condition). P values shown are from Wilcoxon rank-sum tests, followed by Benjamini–Hochberg correction for multiple comparisons. Abbreviations: ART, antiretroviral therapy; %FEV1, percent forced expiratory volume in 1 second; HIV, human immunodeficiency virus; IFN-γ, interferon gamma; PBMC, peripheral blood mononuclear cell; PPD, purified protein derivative; TB, tuberculosis; TNF-α, tumor necrosis factor-alpha.
Table 4.
Relationships Between Changes in Purified Protein Derivative–specific CD4 T-Cell Type and Lung Total Glycolytic Activity in the Imaging Cohort
| PPD-specific CD4 T-cell Type, Change From Baseline to Week 4 of ART | Association With Change in Lung TGA on ART | Association With Change in Lung TGA on ART, Adjusted for Time to ART Initiation | ||
|---|---|---|---|---|
| β (95% CI) | P Value | β (95% CI) | P Value | |
| Total TNF-α + CD4 T-cells | 353.8 (20.3–687.3) | .04 | 239.7 (−153.7 to 633.1) | .22 |
| Total IFN-γ + CD4 T-cells | 981.4 (39.6–1923.3) | .04 | 701.6 (−308.7 to 1712.0) | .16 |
| TNF-α +IFN-γ + double-positive CD4 T-cells | 1834.6 (313.4–3355.8) | .02 | 1409.2 (−236.8 to 3055.2) | .09 |
| TNF-α + single-positive CD4 T-cells | 353.6 (−61.9 to 769.2) | .09 | 200.8 (−268.5 to 670.0 | .38 |
Data are from baseline to Week 4 post-ART changes in lung TGA among adults with HIV and pulmonary TB (n = 30). All estimates are adjusted for baseline lung TGA, baseline CD4 count and pre-ART HIV viral load. Each β coefficient estimate effect of 1% change in cell type on change in lung TGA from baseline to Week 4 of ART.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IFN-γ, interferon gamma; PPD, purified protein derivative; TB, tuberculosis; TGA, total glycolytic activity; TNF-α, tumor necrosis factor-alpha.
Early Decreases in Forced Expiratory Volume in 1 Second on Antiretroviral Therapy Are Associated With Decreasing Lung Function During and After TB Treatment
Of 63 subjects with baseline, Week 4, and at least 1 subsequent lung function assessment on ART, 23 were also in the imaging cohort and 40 were not (Supplementary Figure 6 and characteristics in Table 1). Raw data on FEV1 trajectories over time are shown in Supplementary Figure 5. These 63 subjects contributed 256 lung function assessments during up to 48 weeks after ART initiation. Approximately half (n = 32; 51%) had lung function data at Week 48 after ART initiation. A decrease in FEV1 from baseline to Week 4 was associated with, on average, an FEV1 that was 208 mL lower (95% CI −319 to −100 mL; P < .0002) throughout follow-up. This effect was independent of baseline FEV1, age, sex, height, and baseline CD4 count, and was also seen with FVC (261 mL lower, 95% CI −389 to −134 mL; P < .001). Parameter estimates are given in Supplementary Tables 5 and 6.
DISCUSSION
The primary findings of the present study are that (1) greater lung inflammation on FDG PET-CT is associated with worse lung function and symptoms prior to ART initiation; and (2) greater recovery of pathogen-specific CD4 T-cell function on ART is associated with increased pulmonary inflammation and decreased lung function in people living with HIV who also have pulmonary TB. Additional findings indicate that increases in pulmonary inflammation on ART appear to be common, appear to relate to the interval between TB treatment initiation and ART initiation, may not be clinically recognized as TB-IRIS, and may be associated with longer-term pulmonary impairments in individuals with HIV/TB.
The results are consistent with a previous study from Botswana demonstrating that a greater magnitude of CD4 increase from baseline to Week 4 of ART was associated with worse lung function months to years after TB cures in adults with pulmonary TB [31]. Greater increases in pathogen-specific, functional CD4 T-cells on ART are also associated with increased risks of paradoxical TB-IRIS [6, 28, 30, 32]. In this study, increases in circulating PPD-specific TNF-α + and IFN-γ + CD4 T-cells correlated significantly with early increases in lung TGA and decreases in FEV1 on ART. Furthermore, patients with lower CD4 counts, a shorter time to ART initiation, and less lung radiographic involvement at baseline were at higher risk of increased lung inflammation during virologic suppression. Taken together, these findings suggest ART-associated functional cellular immune recovery directed at the site of antigen may be contributing to incident lung inflammation in patients with pulmonary TB via an IRIS-like phenomenon. Also consistent with this are findings from Hammoud et al [33] that demonstrated correlations between changes in body glycolytic activity on ART and soluble inflammatory biomarkers associated with IRIS risk, including TNF-α.
In the present study, 40% of subjects experienced an increase in pulmonary TGA on ART, which appears higher than rates observed in those without HIV. Martinez et al [34] followed 21 HIV-uninfected adults being treated for TB with FDG PET-CTs at baseline and 1 month, and found that 19 of 21 patients had a decreasing lung SUVmax value at Week 4. Similarly, Malherbe et al [35] studied HIV-uninfected subjects and found that only 12% had an increase in lesion glycolytic activity at Week 4 (F. Malherbe, personal communication). These data are consistent with the concept that paradoxical reactions are observed in those without HIV, albeit less commonly than in those with HIV [36]. Although these previous reports in those without HIV did not measure lung function, our findings suggest that persistent inflammation could also relate to lung function in HIV-uninfected individuals with TB [34, 35]. Future studies should evaluate this possibility.
These findings may have clinical implications. The 200 mL lower FEV1 observed during up to 48 weeks of follow-up among those with a Week 4 decrease in FEV1 on ART is approximately double the FEV1 change considered clinically important in other pulmonary diseases [24–27] and exceeds the 175 mL chronic, persistent FEV1 deficit attributable to a second episode of TB in South African miners [12]. Furthermore, many with pulmonary inflammation in this study were not captured by standard definitions of TB-IRIS. Thus, there may be a role for routine assessments of lung function in at-risk subjects. The associations between pathogen-specific CD4 T-cell recovery, timing of ART initiation, and changes in lung TGA on ART are notable, and indicate that patients starting ART very soon after TB treatment initiation may require interventions. A recent trial by Meintjes et al [37] demonstrated that corticosteroids decrease the risk of IRIS in individuals with HIV/TB who are initiating ART, but effects on pulmonary function remain to be evaluated.
Incomplete data on spirometry or PET-CT could bias the study results. Furthermore, while this is a relatively large study of FDG PET-CT in TB, the power to detect various effects is limited. As such, further evaluations of the relationships presented here should be conducted in other cohorts. In addition, not all subjects were culture positive at the time of ART initiation, and the use of the Xpert MTB/RIF assay, which can miss up to one-third of patients [38], may limit generalizability.
Our findings should not support delays in ART initiation in individuals with HIV/TB, as ART initiation and cellular immune recovery on ART are associated with survival [1–4, 29]. Nonetheless, chronic lung damage appears to occur on ART, and this phenomenon might be understood with subsequent studies and lessened with targeted interventions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number R01AI120821 to G. P. B.) and the National Center for Advancing Translational Sciences (grant number KL2TR001879 to S. R.), the Penn Center for Acquired Immunodeficiency Syndrome (AIDS) Research (grant number P30AI045008 to S.R and G.P.B), the Center for AIDS Research at Emory University (grant number P30AI050409 to S.C.A), the National Institute of Allergy and Infectious Diseases (grant number K23AI134182 to S.C.A), and the Advancing Care & Treatment for Tuberculosis/Human Immunodeficiency Virus (to R. S. W. and G. C).
Potential conflicts of interest. The authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Ann Intern Med 2015; 163:32–9. [DOI] [PubMed] [Google Scholar]
- 2. Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365:1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blanc FX, Sok T, Laureillard D, et al. ; CAMELIA (ANRS 1295–CIPRA KH001) Study Team Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havlir DV, Kendall MA, Ive P, et al. ; Acquired Immunodeficiency Syndrome Clinical Trials Group Study A5221 Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andrade BB, Singh A, Narendran G, et al. Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLOS Pathog 2014; 10:e1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006; 20:F1–7. [DOI] [PubMed] [Google Scholar]
- 7. Meintjes G, Lawn SD, Scano F, et al. ; International Network for the Study of Human Immunodeficiency Virus–Associated Immune Reconstitution Inflammatory Syndrome Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect Dis 2015; 15:429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tadokera R, Meintjes G, Skolimowska KH, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J 2011; 37:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naidoo K, Yende-Zuma N, Padayatchi N, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med 2012; 157:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26:948–68. [DOI] [PubMed] [Google Scholar]
- 12. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000; 55:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ross J, Ehrlich RI, Hnizdo E, White N, Churchyard GJ. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010; 65:1010–5. [DOI] [PubMed] [Google Scholar]
- 14. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018; 27:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plit ML, Anderson R, Van Rensburg CE, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J 1998; 12:351–6. [DOI] [PubMed] [Google Scholar]
- 16. Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989; 83:195–8. [DOI] [PubMed] [Google Scholar]
- 17. Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015; 32:138–46. [DOI] [PubMed] [Google Scholar]
- 18. Laureillard D, Marcy O, Madec Y, et al. ; CAMELIA (ANRS 1295–CIPRA KH001) Study Team Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS 2013; 27:2577–86. [DOI] [PubMed] [Google Scholar]
- 19. Luetkemeyer AF, Kendall MA, Nyirenda M, et al. ; Adult Acquired Immunodeficiency Syndrome Clinical Trials Group A5221 Study Team Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr 2014; 65:423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lefebvre N, Argemi X, Meyer N, et al. Clinical usefulness of 18F-FDG PET/CT for initial staging and assessment of treatment efficacy in patients with lymph node tuberculosis. Nucl Med Biol 2017; 50:17–24. [DOI] [PubMed] [Google Scholar]
- 21. Chen RY, Dodd LE, Lee M, et al. PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med 2014; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heuts F, Gavier-Widén D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci USA 2013; 110:6482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flynn JL, Gideon HP, Mattila JT, Lin PL. Immunology studies in non-human primate models of tuberculosis. Immunol Rev 2015; 264:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta N, Pinto LM, Morogan A, Bourbeau J. The COPD assessment test: a systematic review. Eur Respir J 2014; 44:873–84. [DOI] [PubMed] [Google Scholar]
- 25. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014; 2:195–203. [DOI] [PubMed] [Google Scholar]
- 26. Cazzola M, MacNee W, Martinez FJ, et al. ; American Thoracic Society; European Respiratory Society Task Force on Outcomes of Chronic Obstructive Pulmonary Disease Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31:416–69. [DOI] [PubMed] [Google Scholar]
- 27. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med 2014; 189:250–5. [DOI] [PubMed] [Google Scholar]
- 28. Ravimohan S, Tamuhla N, Nfanyana K, et al. Robust reconstitution of TB-specific polyfunctional CD4+ T-cell responses and rising systemic IL-6 in paradoxical TB-IRIS. Clin Infect Dis 2016; 62:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravimohan S, Tamuhla N, Steenhoff AP, et al. Early immunologic failure is associated with early mortality among advanced HIV-infected adults initiating antiretroviral therapy with active tuberculosis. J Infect Dis 2013; 208:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahnke YD, Greenwald JH, DerSimonian R, et al. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood 2012; 119:3105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravimohan S, Tamuhla N, Kung SJ, et al. Matrix metalloproteinases in tuberculosis-immune reconstitution inflammatory syndrome and impaired lung function among advanced HIV/TB co-infected patients initiating antiretroviral therapy. EBioMedicine 2016; 3:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vignesh R, Kumarasamy N, Lim A, et al. TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr 2013; 64:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hammoud DA, Boulougoura A, Papadakis GZ, et al. Increased metabolic activity on 18F-fluorodeoxyglucose positron emission tomography-computed tomography in human immunodeficiency virus-associated immune reconstitution inflammatory syndrome. Clin Infect Dis 2019; 68:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinez V, Castilla-Lievre MA, Guillet-Caruba C, et al. (18)F-FDG PET/CT in tuberculosis: an early non-invasive marker of therapeutic response. Int J Tuberc Lung Dis 2012; 16:1180–5. [DOI] [PubMed] [Google Scholar]
- 35. Malherbe ST, Shenai S, Ronacher K, et al. ; Catalysis Tuberculosis–Biomarker Consortium Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 2016; 22:1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV-negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis 2007; 11:1290–5. [PubMed] [Google Scholar]
- 37. Meintjes G, Stek C, Blumenthal L, et al. ; PredART Trial Team Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med 2018; 379:1915–25. [DOI] [PubMed] [Google Scholar]
- 38. Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011; 184:132–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.