Abstract
Cereblon (CRBN) is a target for immunomodulatory drugs. This study investigated the prognostic value of the expression of CRBN‐pathway genes on the clinical relevance of lenalidomide (Len) treatment and evaluated the levels of CRBN‐binding proteins and mutations in these genes after Len treatment. Forty‐eight primary multiple myeloma cells were collected prior to treatment with Len and dexamethasone (Ld) and 25 paired samples were obtained post‐Ld therapy. These tumor cells were used to determine the expression and mutated forms of the CRBN‐pathway genes. Following normalization with CRBN levels, there was a significantly reduced IKZF1/CRBN ratio in samples that responded poorly to Ld therapy. Moreover, patients with low ratios of IKZF1/CRBN showed a significantly shorter progression‐free survival (PFS) and overall survival (OS) than those with higher ratios. However, patients with high ratios of KPNA2/CRBN showed a significantly shorter PFS and OS than patients with lower ratios. Of the 25 paired samples analyzed, most samples showed a reduction in the expression of CRBN and an increase in IKZF1 gene expression. No mutations were observed in CRBN, IKZF1, or CUL4A genes in the post‐Ld samples. In conclusion, a decreased expression of IKZF1 and increased expression of KPNA2 compared to that of CRBN mRNA predicts poor outcomes of Ld therapy.
Keywords: CRBN, IKZF1, KPNA2, lenalidomide, multiple myeloma
This study investigated the prognostic value of the expression of cereblon (CRBN)‐pathway genes on the clinical relevance of lenalidomide treatment and evaluated the levels of CRBN‐binding proteins, mutations in these genes, and the methylation status of the CRBN promoter sequence. In conclusion, a decreased expression of IKZF1 and increased expression of KPNA2 compared to that of CRBN mRNA predicts poor outcomes of lenalidomide and dexamethasone therapy.

1. INTRODUCTION
Lenalidomide (Len) is an immunomodulatory drug (IMiD) that plays an essential role in the treatment of multiple myeloma (MM). It is usually used in combination with dexamethasone, known as Ld therapy. Due to its immunoreactivity,1 such as the costimulation of T cells2 and natural killer cells,3 combining it with the mAbs elotuzumab4 and daratumumab5, 6 is efficient for the treatment of relapsed/refractory (RR) cases of MM.
Cereblon (CRBN) is an adaptor of substrates in the CRL4CRBN E3 ubiquitin ligase complex and, following binding with Len, alters the substrate specificity of the complex.7, 8 This results in the ubiquitination and degradation of several factors, such as transcriptional factors IKZF1, IKZF3, and a nuclear transport protein, KPNA2.9, 10, 11 Degradation of these factors inhibit the survival and progression of MM cells.
Previous studies have shown that the protein and mRNA levels of CRBN‐binding factors like IKZF1 and IKZF3 are important for the efficacy of Len treatment9, 12, 13, 14 and could serve as predictive and prognostic markers for Len‐treated MM patients. Such studies9, 14 have reported the impact of low IKZF1 and/or IKZF3 levels on the poor outcome of Ld therapy. Using gene expression profiling, Zhu et al9 reported a correlation between low expression of IKZF1 and a poor response leading to reduced survival in Ld‐treated patients (n = 44), and Pourabdollah et al14 undertook immunohistochemistry of bone marrows from Ld‐treated MM patients (n = 50) to show that low levels of IKZF1 and IKZF3 lead to shorter progression‐free survival (PFS) and overall survival (OS). In contrast, Krönke et al reported higher IKZF1 mRNA levels as a poor prognostic factor of PFS in patients treated with Len and intensive chemotherapy (n = 60).13 However, Dimopoulos et al did not identify a correlation between the expression of IKZF1 and IKZF3, and patient response to Len12 from their immunohistochemical analysis (n = 23). These contradictory results could be attributed to the small populations, difference in experimental techniques in determining IKZF1 and IKZF3 expression, and Len‐containing regimens. Therefore, the link between the expression of CRBN‐associated genes and prognosis of MM remains unclear.
Apart from the prognostic value of CRBN‐pathway factors, their altered expression post‐Len treatment, including gain/loss of mutations, are yet to be understood. Although most Len‐treated patients develop resistance, the mechanism(s) underlying its acquired resistance remain unknown. Some studies have reported that MM cells from patients with reduced CRBN expression were resistant to Len15, 16; however, there is little known about the altered expression of the CRBN‐binding factors. Although several mutations in CRBN and related genes have been identified in the population with RR MM,17 their clinical relevance is poorly understood.
To investigate the prognostic value of Ld therapy, we determined the expression of CRBN mRNA and associated genes and correlated their levels with the efficacy and outcome of Ld therapy. We also evaluated the expression and mutation(s) in the CRBN‐pathway genes in post‐Ld samples.
2. MATERIALS AND METHODS
2.1. Patient samples
A total of 83 patients with RR MM were treated with Ld between July 2010 and May 2017 at Nagoya City University Hospital (Aichi, Japan). Patients who were treated with Ld as maintenance or induction therapy prior to high‐dose chemotherapy were excluded from the study.
Bone marrow (BM) specimens were collected from 48 patients just before Ld therapy. Among these patients, BM samples were obtained from 25 patients just after Ld therapy; of them, 22 patients showed acquired refractoriness to Ld. All the patients provided written informed consent prior to sampling, according to the requirements of the Declaration of Helsinki. The Ethical Committee of Nagoya City University Graduate School of Medical Sciences approved this study.
2.2. Sample preparation
Bone marrow mononuclear cells were isolated by density centrifugation (Ficoll‐Paque PLUS; GE Healthcare Bio‐Sciences). Primary MM cells were isolated from the BM mononuclear cell fraction using anti‐CD138 Ab‐conjugated magnetic beads and the AutoMACS pro‐separator (Miltenyi Biotec) as previously described.18, 19, 20 DNA and RNA were extracted using the QIAprep Miniprep kit (Qiagen). Using global RT‐PCR, the expression of 3 translocation‐related genes (CCND1, FGFR3, and c‐MAF) were analyzed in the primary MM cells as described previously.21
2.3. Quantification of CRBN and related genes
Total RNA was reverse transcribed to cDNA with an oligo(dT) primer and the Superscript III kit (Invitrogen Life Technologies). The cDNAs were diluted in double‐distilled H2O. mRNA levels of CRBN, IKZF1, IKZF3, and KPNA2 were determined using the RT‐PCR. Reverse transcription PCR was carried out in a 20‐µL reaction mixture containing 2 µL diluted cDNA, 10 µL THUNDERBIRD SYBR qPCR Mix (Toyobo), and 0.3 µmol/L of each primer using the StepOnePlus Real‐Time PCR System (Thermo Fisher Scientific). β‐Actin levels were used for normalization. The following primers were used that selected for the common isoforms of the genes: CRBN‐forward (F), 5′‐AGGCTTGCAACTTGAATCTGATAGG‐3′; CRBN‐reverse (R), 5′‐GCAACAGAGCAGATCGCGTTA‐3′; IKZF1‐F, 5′‐GCTGCAATACCACTTGGGAACA‐3′; IKZF1‐R, 5′‐CCACCCTCAAGACTGGGACTTAGA‐3′; IKZF3‐F, 5′‐TTGCATGCTCAGGTTCTCAGTCTA‐3′; IKZF3‐R, 5′‐CAATGGTCATCCACAGACCACA‐3′; KPNA2‐F, 5′‐AGTGAACAAGCTGTCTGGGCTCTA‐3′; and KPNA2‐R, 5′‐AGTGGGTCAACTGCACCGTACTTA‐3′.
2.4. Targeted DNA sequencing and analysis of mutations
Targeted amplicon sequencing was carried out in the genomic DNA isolated from the BMs of the 22 paired samples. Samples were analyzed for mutations in the complete coding exons of 5 genes: CRBN, IKZF1, IKZF3, CUL4A, and TP53 (the panel included 40 amplicons). DNA was quantified using the Quantus Fluorometer (Promega). Subsequently, a DNA library was prepared using the NEBNext Ultra II DNA Library Prep Kit (New England BioLabs), quantified by quantitative PCR using the QIAseq Library Quant Assay Kit (Qiagen), and sequenced using the MiSeq (Illumina) sequencer. All steps were carried out as per the instructions provided. The sequence data have been submitted to the DNA Data Bank of Japan under accession number JGAS00000000208.
Mutations in the exons were analyzed using the biomedical genomics workbench (CLC bio) with a cut‐off of 5% for somatic variants (variant allele frequency). The average depth of sequencing coverage was 903× and the limit for a variant was 200×. Common single nucleotide polymorphisms (SNPs) were excluded. The SNPs and somatic mutations were derived from the public databases Single Nucleotide Polymorphism Database (dbSNP), HapMap, 1000 Genomes Project, and the Catalogue of Somatic Mutations in Cancer (COSMIC; Wellcome Trust Sanger Institute).
2.5. Analysis of methylation by methylation‐specific PCR
Methylation in the CRBN promoter was determined in the BM‐derived genomic DNAs from the 22 post‐Ld samples using methylation‐specific PCR (MSP). DNA modifications were analyzed by bisulphite conversion using the MethylEasy Xceed Rapid DNA Bisulphite Modification Kit (TaKaRa). The MSP analysis was undertaken using the EpiScope MSP kit (TaKaRa). EpiScope Methylated HeLa gDNA (TaKaRa) was used as a positive control. The methylated (CRBN‐M) and unmethylated (CRBN‐UM) primers were designed to detect the CpG island in the CRBN promoter (from transcription starting site +62 to +227). The sequences of the CRBN‐M and CRBN‐UM primers were: 5′‐GCG TAT AAT ATG GGT AAT TAT TTG TC ‐3′ and 5′‐GAT ACG ACC CTA CTA AAC TAA CTC G ‐3′ for CRBN‐M, and 5′‐ TGT ATA ATA TGG GTA ATT ATT TGT TGT ‐3′ and 5′‐ AAT ACA ACC CTA CTA AAC TAA CTC A ‐3′ for CRBN‐UM. We used 6 ng bisulfite‐converted DNA as a template. Polymerase chain reaction was carried out according to the kit manual. Subsequently, agarose gel electrophoresis and ethidium bromide staining were undertaken.
2.6. Statistical analysis
Statistical analyses were undertaken using GraphPad Prism software. Patient characteristics and gene expression were compared using the Mann‐Whitney U test, Wilcoxon signed‐rank test (paired sample), and Fisher’s exact test. Survival was compared by the Kaplan‐Meier method using the log‐rank and Wilcoxon test; P < .05 indicated statistical significance.
3. RESULTS
3.1. Patient characteristics
All patients with RR MM who underwent Ld therapy at the Nagoya City University Hospital between July 2010 and May 2017 were eligible for this study. A total of 48 patients provided BM samples used for our investigation, whose characteristics have been described in Table 1. The patients underwent 1‐6 lines of therapies before this study, with the median value of 2. The majority of these patients (43; 90%) were previously treated with bortezomib‐containing therapies, 25 (50%) of whom showed refractoriness. Eleven patients (23%) were previously treated with thalidomide‐containing therapies, 10 (90%) of whom were resistant. Using RT‐PCR, 16 (33%), 11 (23%), and 7 (15%) patient samples showed overexpression of the translocation‐related genes CCND1, FGFR3, and c‐MAF, respectively.
Table 1.
Characteristics of patients with multiple myeloma treated with lenalidomide and dexamethasone
| Number of patients | 48 (100) |
| Age (y) | |
| Median (range) | 69 (45‐84) |
| Sex | |
| Male/female | 19/29 |
| M‐protein | |
| IgG | 21 (44) |
| IgA | 11 (23) |
| BJP | 11 (23) |
| IgD | 4 (8) |
| Nonsecretory | 1 (2) |
| Stage (ISS) | |
| I | 11 (23) |
| II | 20 (42) |
| III | 17 (35) |
| RT‐PCR and FISH | |
| CCND1 | 15 (33) |
| t(11;14)+ | 8 (53) |
| 11 polysomy | 3 (20) |
| NA | 2 (13) |
| ND | 2 (13) |
| FGFR3 | 11 (23) |
| t(4;14)+ | 10 (91) |
| NA | 1 (9) |
| c‐MAF | 7 (15) |
| t(14;16)+ | 5 (71) |
| NA | 2 (29) |
| Triple negative a | 11 (27) |
| ND | 4 (8) |
| ASCT | |
| +/− | 14/34 |
| Prior therapies | |
| Median (range) | 2 (1‐6) |
| Prior bortezomib therapy | |
| +/− | 43 (90)/5 |
| Prior thalidomide therapy | |
| +/− | 11 (23)/37 |
| Best response | |
| CR | 3 (6) |
| VGPR | 5 (10) |
| PR | 24 (50) |
| SD | 13 (27) |
| PD | 2 (4) |
| NE | 1 (2) |
Data are shown as n (%) unless otherwise indicated.
Abbreviations: ASCT, autologous stem cell transplantation; BJP, Bence Jones protein; CR, complete response; ISS, International Staging System; NA, no abnormality; ND, not done; NE, not evaluated; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
CCND1‐, FGFR3‐, c‐MAF‐negative in RT‐PCR.
3.2. Correlation between expression of CRBN‐related genes and efficacy of Ld therapy
3.2.1. Impact of expression of CRBN‐related genes on response and survival after Ld therapy
Primary MM cells from 48 samples were subjected to quantitative PCR analysis. Expression of CRBN and 3 related genes, IKZF1, IKZF3, and KPNA2, were measured and compared with the outcome of Ld therapy. Based on their responses to Ld therapy, the patients were classified as good responders (complete response [CR] + very good partial response [VGPR] + partial response [PR], n = 32) or poor responders (stable disease [SD] + progressive disease [PD], n = 15). One patient was not evaluated based on treatment response. There was no significant difference in the expression of the 4 genes between good and poor responders (Figure 1A). In addition, no significant differences were observed in the CRBN expression levels between each response category (Figure S1).
Figure 1.

Correlation of the expression of cereblon (CRBN)‐pathway genes with response to lenalidomide and dexamethasone (Ld) therapy and survival in treated multiple myeloma patients. Expression of 4 CRBN‐pathway genes, CRBN, IKZF1, IKZF3, and KPNA2, were measured from 48 primary MM cells from patients. A, Correlation of gene expression with its respective clinical response to Ld therapy. Based on their responses to Ld therapy, the patients were classified as good responders and poor responders. Good responders consisted of patients who achieved complete response (CR), very good partial response (VGPR), and partial response (PR). Poor responders consisted of patients achieved stable disease (SD) and progressive disease (PD). B, C, Comparison of progression‐free survival (B) and overall survival (C) between the groups with low or high expression of each gene
All the samples were divided into 2 groups with low or high expression of each gene based on their median values and the difference in survival was evaluated within the groups. As shown in Figure 1B and 1C, no significant differences were observed in the 4 genes tested between the groups. Furthermore, no significant differences were observed in response categories (Table S1).
3.2.2. Relevance of the ratio of CRBN‐related genes to CRBN in response to Ld therapy and survival of patients
Subsequently, we used CRBN expression to adjust the levels of the 3 genes determined as per previous reports.9, 22 Ratios of IKZF1, IKZF3, and KPNA2 to CRBN were calculated and analyzed for determining patient response to Ld therapy. As shown in Figure 2A, the ratio of IKZF1/CRBN was significantly lower in poor responders than in good responders (P = .0107). No significant difference was observed in the IKZF3/CRBN and KPNA2/CRBN ratios. We compared patient survival between the 2 groups based on gene expression (Figure 2B); PFS was significantly shorter in the group with a low ratio of IKZF1/CRBN (log‐rank, P = .168; Wilcoxon, P = .0397) and a high ratio of KPNA2/CRBN (log‐rank, P = .022; Wilcoxon, P = .17). Similarly, as shown in Figure 2C, OS was reduced in this group (low IKZF1/CRBN: log‐rank, P = .0342; Wilcoxon, P = .0205; high KPNA2/CRBN: log‐rank, P = .0201; Wilcoxon, P = .0698).
Figure 2.
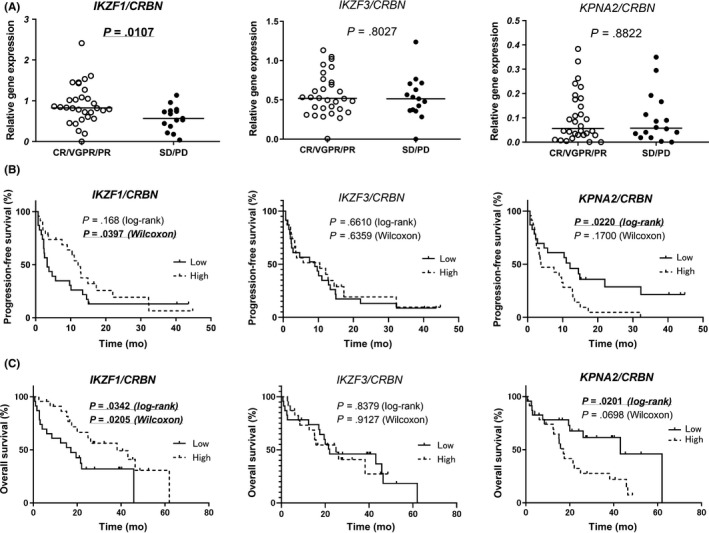
Clinical relevance of the levels of cereblon (CRBN)‐related genes in multiple myeloma patients. A, Correlation of expression of each CRBN‐related gene, normalized by the expression of CRBN, with the response to lenalidomide and dexamethasone (Ld) therapy. B, C, Comparison of progression‐free survival (B) and overall survival (C) between the groups with low or high expression of each CRBN‐related gene normalized by the expression of CRBN. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response
The samples were divided into 4 groups based on the expression of different pairs of genes, ie, CRBN and a related gene, and the duration of survival in each group was compared. The median values for the expression of each gene were used to represent low or high expression. Upon analysis of CRBN and IKZF1 expression, we observed a trend of difference in the duration of PFS (Figure 3A, P = .0668) and significant difference in OS (Figure 3A, P = .0032). The group with high CRBN and low IKZF1 showed a poor PFS (2.3 months) and OS (3.1 months) compared to the other groups (Figure 3A). No significant differences were observed in PFS and OS in the CRBN and IKZF3 groups (Figure 3B). There was a significant difference in the duration of OS (Figure 3C, P = .0495) in the CRBN and KPNA2 groups, wherein the group with low CRBN and low KPNA2 showed a prolonged OS (47.4 months) compared to the other groups (Figure 3C).
Figure 3.
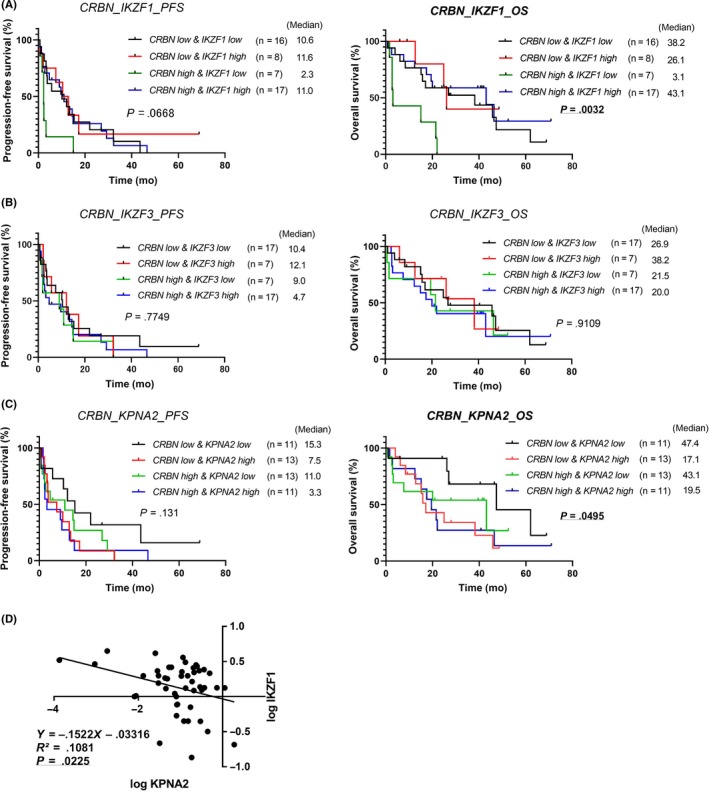
Correlation of the expression of 2 genes, cereblon (CRBN) and its related gene (IKZF1, IKZF3, or KPNA2) with the relevance to the duration of survival of multiple myeloma patients by lenalidomide plus dexamethasone therapy. All patient samples were divided into 4 groups according to their expression of the respective pair of genes, ie, CRBN and each related gene. A‐C, Comparison of progression‐free survival (PFS, left panels) and overall survival (OS, right panels) between 4 groups are shown in each graph. D, Linear regression model showing the relationship between low levels of IKZF1 and high levels of KPNA2
We then compared the gene expression of the 3 substrates of CRBN, ie, IKZF1, IKZF3, and KPNA2, reciprocally. Using a linear regression model, the relationship between low levels of IKZF1 and high levels of KPNA2 was shown (Figure 3D).
3.3. Alteration in expression of CRBN and related genes after Ld therapy
Of the 48 patients, 25 paired samples were collected just before and after Ld therapy; these samples were analyzed for the expression of CRBN, IKZF1, IKZF3, and KPNA2, and determined if there was an alteration in expression after Ld therapy.
A significant decrease in CRBN expression was observed after Ld therapy (Wilcoxon test, P = .0296; Figure 4A, left). Seventeen samples (17/25, 68%) showed reduced CRBN expression after Ld therapy, while 8 samples (8/25, 32%) showed an increased expression (Figure 4B, left). On testing for IKZF1 levels, 19 samples (19/25, 76%) showed an increased expression after Ld therapy (Wilcoxon test, P = .0034), while 6 samples (6/25, 24%) showed a reduction (Figure 4A,B, middle left). No significant difference was observed in the expression of IKZF3 (Figure 4A,B, middle right). The majority of samples (24/25, 96%) showed reduced KPNA2 gene expression after Ld therapy (Wilcoxon test, P < .0001; Figure 4A,B, right). In addition, there was no relationship between CRBN (or IKZF1) alteration and treatment response to Len (Table S2).
Figure 4.
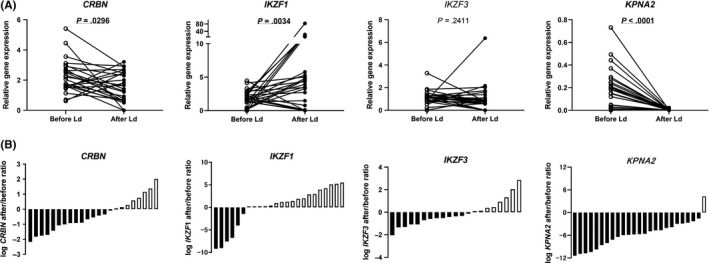
Alteration in gene expressions after lenalidomide plus dexamethasone (Ld) therapy for multiple myeloma. A, Comparison of the expression of cereblon (CRBN) and its related genes before and after Ld therapy. B, Ratio of the alteration of the expression of each gene in post‐Ld samples
3.4. Mutations in CRBN and related genes
Of the 25 paired samples, 22 were subjected to the targeted sequencing of 5 genes. As shown in Figures 4, 5, 4 mutations in 2 genes were detected (Figure 5A and Table 2). One mutation was detected in pre‐Ld samples and 3 mutations were detected in post‐Ld samples. No mutations were detected in CRBN, IKZF1, or CUL4A, whereas somatic mutations were found in TP53 and IKZF3 (3 samples and 1 sample, respectively). One patient harbored a mutation in TP53 with high frequencies in both pre‐ and post‐Ld samples, and 2 patients acquired mutations in TP53 and IKZF3.
Figure 5.
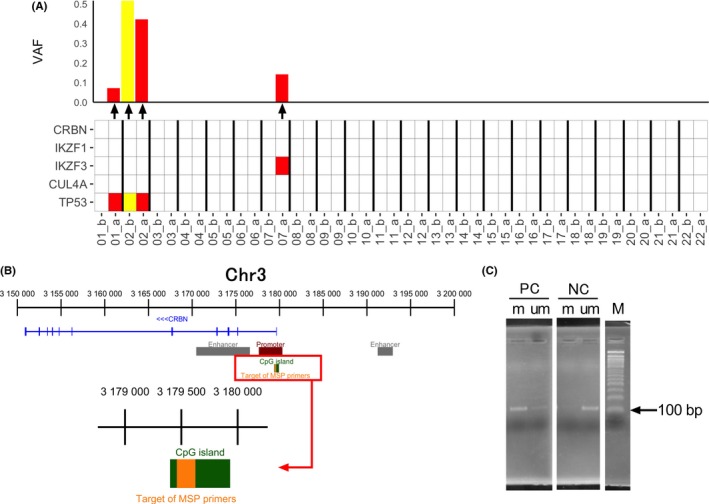
Mutations in cereblon CRBN and its related genes and methylation of the CRBN promoter in post‐lenalidomide plus dexamethasone (Ld) samples. A, Mutations detected in 5 genes (CRBN, IKZF1, IKZF3, CUL4A, and TP53) in primary multiple myeloma cells collected before (b) and after (a) Ld therapy. VAF, variant allele frequency. B, Target region of the methylation‐specific primers in the CpG island in the CRBN promoter. C, Agarose gel electrograms of the positive (methylated region) and negative controls (unmethylated region) observed by methylation‐specific PCR. m, methylated primer; M, marker; NC, negative control; PC, positive control; um, unmethylated primer
Table 2.
List of mutations that have been described in Figure 5A
| Patient# | Gene | Chromosome | Region | Coding | Type | VAF (%) | Transcript | Ensembl transcript ID | Location | Function | Protein | COSMIC | dbSNP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 01_a | TP53 | 17 | 7 578 212 | c.637C > T | SNV | 7.1 | NM_000546.5 | ENST00000420246 | protein_coding | Nonsense | p.Arg213* | COSM10654 | rs397516436 |
| 02_b | TP53 | 17 | 7 579 882 | c.31G > C | SNV | 51.8 | NM_000546.5 | ENST00000420246 | protein_coding | Missense | p.Glu11Gln | COSM11606 | rs201382018 |
| 02_a | TP53 | 17 | 7 579 882 | c.31G > C | SNV | 42.2 | NM_000546.5 | ENST00000420246 | protein_coding | Missense | p.Glu11Gln | COSM11606 | rs201382018 |
| 07_a | IKZF3 | 17 | 37 922 270 | c.1303C > T | SNV | 14.2 | NM_001257408 | ENST00000346872 | protein_coding | – | p.Pro435Ser | No | No |
3.5. Methylation analysis
As shown in Figure 5B, we examined CRBN promoter methylation in the 22 post‐Ld samples using the MSP designed to detect methylated site(s) within the CpG island (Figure 5C). None of the samples showed hypermethylation (data not shown).
4. DISCUSSION
In this study, we identified that the expression of IKZF1 and KPNA2 (normalized to CRBN levels) was associated with the efficacy of Ld therapy. We also explored the presence of mutation in CRBN‐related genes and methylation in the CRBN promoter in the clinical specimens obtained after Ld treatment.
Previous reports have shown that the expression of CRBN can be associated with the survival of MM patients treated with IMiDs.9, 16, 23 Therefore, the levels of substrates regulated by CRBN are expected to be promising markers for the efficacy of IMiDs. However, results from previous studies have been inconsistent, suggesting the need for adjusting the calculated expression of CRBN‐related factors, such as normalization by CRBN levels, to determine a correlation with Ld therapy. In our study, mRNA levels of CRBN, IKZF1, IKZF3, and KPNA2, were not significantly linked to Ld efficacy (PFS and OS). However, after normalizing these levels according to that of CRBN, we identified that a low ratio of IKZF1/CRBN and a high ratio of KPNA2/CRBN correlated with a poor response to Ld therapy that might serve as a biomarker for Ld treatment. Moreover, coevaluating the levels of CRBN and its related genes showed that a low IKZF1 and high CRBN corresponded to shortest survival after Ld therapy.
The relative quantitative relationship between IKZF1 and CRBN gene expression has not been reported. IKZF1/3 are transcription factors involved in lymphocyte differentiation and mutations in IKZF1 have been associated with B cell malignancies, such as acute lymphoblastic leukemia.24 Lenalidomide binds CRBN to alter the substrate specificity of the E3 ubiquitin ligase complex. This alteration induces downstream effects, such as degradation of IKZF1/3 and downregulation of interferon regulatory factor‐4. These effects cause MM cytotoxicity. A previous report9 discussed that myeloma cells surviving with low IKZF1 are less dependent on IKZF1‐associated signaling for proliferation and survival. The authors also reported that CRBN protein accumulated during Len exposure through the inhibition of its proteasomal degradation by Len treatment. The function of the accumulated CRBN was modulated during Len exposure, leading to the dramatic degradation of IKZF1 and IKZF3 expression. These findings support the speculation that those MM cells, in which the CRBN gene is highly expressed, might show an abundant production of CRBN protein before treatment, with the production of the protein being further enriched during Len exposure. The accumulated CRBN protein might dilute the action of Len treatment in MM cells, leading to the insufficient modulation of CRBN protein during Len treatment. In addition, MM cells with low levels of IKZF1 are less dependent on IKZF1‐associated signaling for proliferation and survival and, thus, are insensitive to the proapoptotic signaling induced by IKZF1 degradation. Based on these speculated events, MM cells showing high expression of CRBN and low expression of IKZF1 could display poor sensitivity to Len treatment due to insufficient CRBN modulation and low dependency on the IKZF1 signaling pathway. In our cohort, cases that had undergone prior thalidomide treatment or intensive lines of prior therapy, and which were considered as a high risk group, displayed high expression of the CRBN gene before Len treatment (data not shown). By contrast, in the group with low CRBN and high IKZF1, Len could have induced marked degradation of IKZF1, resulting in a favorable therapeutic effect. Further experiments using a large cohort of MM patients are needed to validate the association of CRBN‐related genes with the efficacy of Ld treatment and elucidate the cause for the poor response of Ld therapy in patients with low IKZF1 and high CRBN.
The nuclear transport protein KPNA2 is associated with B cell development25 and has recently been reported to be a potential cancer biomarker for a variety of cancers,26, 27 along with being associated with cancer progression and metastasis through translocation of cancer‐associated nuclear cargo proteins, such as TP53 and MYC.28 Zhu et al reported that KPNA2 is one of the substrates of CRBN and high KPNA2 levels correlate with shorter OS in MM patients.9 Similarly, our study revealed that MM cells with high expression of KPNA2 showed poor PFS and OS after Ld therapy (Figure 2B,C, left). The role of KPNA2 in the pathogenesis and prognosis of MM remains unclear. In this study, the expression of KPNA2 and IKZF1 showed an inverse correlation (Figure 3D); the relationship between IKZF1 and KPNA2 gene expression has not been reported. Therefore, we speculate that MM cells with highly expressed KPNA2 feature low expression of IKZF1 (Figure 3D) and that these MM cells have a specific and IKZF1‐independent proliferative signaling pathway. This specific proliferative signaling might not be affected by the alteration of KPNA2 expression during Len exposure. Continuing on this line of reasoning, MM cells featuring high expression of KPNA2 might have specific proliferative signaling and could maintain this signaling during Len exposure, even though both IKZF1 and KPNA2 are degraded. Thus, MM cells that highly express KPNA2 harbor an IKZF1‐independent signaling pathway that functions in proliferation and survival and could be insensitive to Len treatment. Precisely how these cells resist Len treatment remains unclear.
In a previous study, Zhu et al showed the loss of CRBN expression in 9 Len‐treated MM patients. Analysis of the MM cell lines in their preclinical study showed that reduced expression of CRBN was associated with IMiD resistance.16 However, this analysis included a small number of clinical samples; thus, altered expression of CRBN and its factors could not fully be examined in the post‐Ld samples. Within the 22 paired samples examined, although a fraction of patients showed increased CRBN expression, there was a significant reduction in CRBN expression after Ld therapy. A reduction of CRBN could facilitate the non‐CRBN‐dependent process of proliferation/survival in MM cells29 and contribute to the poor sensitivity to IMiDs.
Using a targeted sequence analysis of the clinical samples collected pre‐ and post‐Ld therapy, we found 3 patients with mutations in TP53 but no mutations in CRBN or IKZF1 in the 22 patients tested. This is inconsistent with previous reports that have identified mutations in CRBN in 6 (12%) out of the 50 RR patients.17 This inconsistency might be due to the difference in duration of Ld therapy. Our cohort was treated for shorter times (median, 194 days; range, 3‐952 days) than those in the previous study (median, 1146 days; range, 154‐1912 days). In our cohort, this short duration of Ld therapy seemed to explain the absence of mutations in CRBN. Unlike the previous report, our study mainly targeted the patients who received Ld therapy and did not involve intensive regimens, such as the combination of Len with proteasome inhibitor or mAb. Thus, PFS and treatment duration were shorter than that in the previous report testing the CRBN mutation. The differences in treatment duration and combination with other agents could affect the incidence of mutations of CRBN and related genes following Len treatment. Further experiments involving more samples are needed to ascertain the incidence and role of mutations in CRBN and related genes in RR MM patients. In this study, a nonsynonymous mutation was found in IKZF3 in 1 patient; however, this mutation was not recorded in any databases (eg, COSMIC) and the role of this mutation is unclear.
As there was significantly reduced CRBN expression in the post‐Ld samples, we explored the methylation status of the CRBN promoter; there was no hypermethylation in the promotor in the clinical samples. This is consistent with the observations by Dimopoulos et al,29 who showed no involvement of promoter methylation in the regulation of CRBN expression in Len‐resistant MM cell lines. However, the mechanism by which CRBN expression is downregulated remains to be understood. Further studies are needed to determine the role and mechanism of the reduction of CRBN expression and the concomitant sensitivity/resistance to IMiDs.
One limitation of this study was the small number of patients with various backgrounds. Treatment outcomes can be affected by a variety of variables, including conventional assessments for risk and tumor burden, history and clinical courses of previous treatments and subsequent treatments at relapse or progression after Ld, doses of Ld therapy, and comorbidities. Further analyses of a uniform group of patients are needed to clarify the role of CRBN and related markers in the sensitivity and/or resistance of Ld therapy.
Intrapatient genetic heterogeneity of MM cells was recently implicated as a critical factor for evaluating the prognosis of MM.30 In this study, we did not obtain samples from different sites of the tumor lesion in the same patients. Therefore, we did not evaluate the heterogeneity of the expression and mutation of CRBN and related genes among the tumor lesions in the same patient. Future studies evaluating the clonal heterogeneity of MM cells should address this point.
In conclusion, we have shown that a reduced expression of IKZF1 and increased expression of KPNA2, normalized by the levels of CRBN, might predict poor outcomes of Ld therapy. In the clinical samples, neither mutations in the CRBN‐pathway genes nor hypermethylation of the CRBN promoter was associated with the reduced expression of CRBN. Our results provide a better understanding of the sensitivity and resistance of MM cells to Len treatment and identify a patient population that would benefit from Len.
CONFLICT OF INTEREST
SI received research funding and honoraria from Ono Pharmaceutical, Janssen Pharmaceutical, Celgene, Bristol‐Myers Squibb, Takeda Yakuhin, and Novartis Pharma. SI also received research funding from Kyowa Hakko Kirin, Sanofi, MSD, and Daiichi Sankyo. MR received research funding from Celgene. MT has accepted researchers from Daiichi Sankyo, MSD, and Ono Pharmaceutical. The other authors have no conflict of interest.
Supporting information
FigS1
TableS1
TableS2
ACKNOWLEDGMENTS
This work was partly supported by a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (16K07179, 16K09855, and 19K07756), the National Cancer Center Research and Development Fund (26‐A‐4), the Accelerating Regulatory Science Initiative from the Ministry of Health, Labour and Welfare, and the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (16ck0106077h0002 and 19ck0106348h0002).
Tachita T, Kinoshita S, Ri M, et al. Expression, mutation, and methylation of cereblon‐pathway genes at pre‐ and post‐lenalidomide treatment in multiple myeloma. Cancer Sci. 2020;111:1333–1343. 10.1111/cas.14352
Takuto Tachita and Shiori Kinoshita contributed equally to this work.
REFERENCES
- 1. Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7‐CD28 pathway. Blood. 2004;103:1787‐1790. [DOI] [PubMed] [Google Scholar]
- 3. Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte‐mediated antibody‐dependent cellular cytotoxicity of rituximab‐treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650‐4657. [DOI] [PubMed] [Google Scholar]
- 4. Dimopoulos MA, Lonial S, Betts KA, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4‐year follow‐up and analysis of relative progression‐free survival from the randomized ELOQUENT‐2 trial. Cancer. 2018;124:4032‐4043. 10.1002/cncr.31680. [DOI] [PubMed] [Google Scholar]
- 5. Spencer A, Lentzsch S, Weisel K, et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica. 2018;103:2079‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dimopoulos MA, San‐Miguel J, Belch A, et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica. 2018;103:2088‐2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015;126:2366‐2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito T, Ando H, Suzuki T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345‐1350. [DOI] [PubMed] [Google Scholar]
- 9. Zhu YX, Braggio E, Shi C‐X, et al. Identification of cereblon‐binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014;124:536‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu G, Middleton RE, Sun H, et al. The myeloma drug Lenalidomide promotes the Cereblon‐dependent destruction of ikaros proteins. Science. 2014;343(6168):305‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krönke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dimopoulos K, Fibiger Munch‐Petersen H, Winther Eskelund C, et al. Expression of CRBN, IKZF1, and IKZF3 does not predict lenalidomide sensitivity and mutations in the cereblon pathway are infrequent in multiple myeloma. Leuk Lymphoma. 2019;60:180‐188. [DOI] [PubMed] [Google Scholar]
- 13. Krönke J, Kuchenbauer F, Kull M, et al. IKZF1 expression is a prognostic marker in newly diagnosed standard‐risk multiple myeloma treated with lenalidomide and intensive chemotherapy: a study of the German Myeloma Study Group (DSMM). Leukemia. 2017;31:1363‐1367. [DOI] [PubMed] [Google Scholar]
- 14. Pourabdollah M, Bahmanyar M, Atenafu EG, Reece D, Hou J, Chang H. High IKZF1/3 protein expression is a favorable prognostic factor for survival of relapsed/refractory multiple myeloma patients treated with lenalidomide. J Hematol Oncol. 2016;9(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopez‐Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326‐2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu YX, Braggio E, Shi C‐X, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118:4771‐4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kortum KM, Mai EK, Hanafiah NH, et al. Targeted sequencing of refractory myeloma reveals a high incidence of mutations in CRBN and Ras pathway genes. Blood. 2016;128:1226‐1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshida T, Ri M, Kinoshita S, et al. Low expression of neural cell adhesion molecule, CD56, is associated with low efficacy of bortezomib plus dexamethasone therapy in multiple myeloma. PLoS ONE. 2018;13:e0196780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Narita T, Ri M, Masaki A, et al. Lower expression of activating transcription factors 3 and 4 correlates with shorter progression‐free survival in multiple myeloma patients receiving bortezomib plus dexamethasone therapy. Blood. Cancer J. 2015;5(12):e373‐e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ri M, Tashiro E, Oikawa D, et al. Identification of Toyocamycin, an agent cytotoxic for multiple myeloma cells, as a potent inhibitor of ER stress‐induced XBP1 mRNA splicing. Blood Cancer J. 2012;2:e79‐e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoshida T, Ri M, Fujinami H, et al. Impact of chromosomal abnormalities on the efficacy of lenalidomide plus dexamethasone treatment in patients with relapsed/refractory multiple myeloma. Int J Hematol. 2019;110:228‐236. [DOI] [PubMed] [Google Scholar]
- 22. Mithraprabhu S, Morley R, Khong T, et al. Monitoring tumour burden and therapeutic response through analysis of circulating tumour DNA and extracellular RNA in multiple myeloma patients. Leukemia. 2019;33:2022‐2033. [DOI] [PubMed] [Google Scholar]
- 23. Broyl A, Kuiper R, Van Duin M, et al. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood. 2013;121:624‐627. [DOI] [PubMed] [Google Scholar]
- 24. Virely C, Moulin S, Cobaleda C, et al. Haploinsufficiency of the IKZF1 (IKAROS) tumor suppressor gene cooperates with BCR‐ABL in a transgenic model of acute lymphoblastic leukemia. Leukemia. 2010;24:1200‐1204. [DOI] [PubMed] [Google Scholar]
- 25. Kovac CR, Emelyanov A, Singh M, Ashouian N, Birshtein BK. BSAP (Pax5)‐importin alpha 1 (Rch1) interaction identifies a nuclear localization sequence. J Biol Chem. 2000;275:16752‐16757. [DOI] [PubMed] [Google Scholar]
- 26. Christiansen A, Dyrskjøt L. The functional role of the novel biomarker karyopherin α 2 (KPNA2) in cancer. Cancer Lett. 2013;331:18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Müller T, Tolkach Y, Stahl D, et al. Karyopherin Alpha 2 Is an Adverse Prognostic Factor in Clear‐Cell and Papillary Renal‐Cell Carcinoma. Clin Genitourin Cancer. 2019;17:e167‐e175. [DOI] [PubMed] [Google Scholar]
- 28. Ma A, Tang M, Zhang L, et al. USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene. 2019;38:2405‐2419. [DOI] [PubMed] [Google Scholar]
- 29. Dimopoulos K, Søgaard Helbo A, Fibiger Munch‐Petersen H, et al. Dual inhibition of DNMTs and EZH2 can overcome both intrinsic and acquired resistance of myeloma cells to IMiDs in a cereblon‐independent manner. Mol Oncol. 2018;12:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rasche L, Chavan SS, Stephens OW, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi‐region sequencing. Nat Commun. 2017;8(1):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigS1
TableS1
TableS2


