Abstract
Colorectal cancer (CRC) is the second leading cause of cancer death worldwide. Therefore, it is important to establish useful methods for preventing CRC. One prevention strategy involves the use of cancer chemopreventive agents, including functional foods. We focused on the well‐known cancer chemopreventive agent curcumin, which is derived from turmeric. However, curcumin has the disadvantage of being poorly soluble in water due to its high hydrophobicity. To overcome this problem, the formation of submicron particles with surface controlled technology has been applied to curcumin to give it remarkably improved water solubility, and this derived compound is named Theracurmin. To date, the preventive effects of Theracurmin on hereditary intestinal carcinogenesis have not been elucidated. Thus, we used Apc‐mutant mice, a model of familial adenomatous polyposis, to evaluate the effects of Theracurmin. First, we showed that treatment with 10‐20 µM Theracurmin for 24 hours reduced nuclear factor‐κB (NF‐κB) transcriptional activity in human colon cancer DLD‐1 and HCT116 cells. However, treatment with curcumin mixed in water did not change the NF‐κB promoter transcriptional activity. As NF‐κB is a regulator of inflammation‐related factors, we next investigated the downstream targets of NF‐κB: monocyte chemoattractant protein‐1 (MCP‐1) and interleukin (IL)‐6. We found that treatment with 500 ppm Theracurmin for 8 weeks inhibited intestinal polyp development and suppressed MCP‐1 and IL‐6 mRNA expression levels in the parts of the intestine with polyps. This report provides a proof of concept for the ongoing Theracurmin human trial (J‐CAP‐C study).
Keywords: colorectal cancer chemoprevention, curcumin, min mice, nuclear factor‐κB, Theracurmin
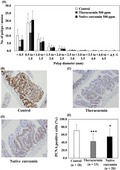
Min mice were fed a basal diet (open box), a diet containing 500 ppm Theracurmin (gray‐filled box), and a diet containing 500 ppm curcumin (black‐filled box) for 8 weeks.
Abbreviations
- AP‐1
activator protein‐1
- CRC
colorectal cancer
- DSS
dextran sodium sulfate
- IL
interleukin
- MCP‐1
monocyte chemoattractant protein‐1
- NF‐κB
nuclear factor‐κB
- NQO
NADP(H):quinone oxidoreductase
- PCNA
proliferating cell nuclear antigen
1. INTRODUCTION
Colorectal cancer is the second leading cause of cancer death (881 000 deaths worldwide), with an estimated incidence of 1.8 million in 2018.1 This number indicates that 1 in 10 patients with CRC will die from the disease. In addition, the incidence of CRC is high in developed countries.1 Thus, it is important to establish useful methods for preventing CRC. Popular methods of preventing CRC are lifestyle modifications, ie, regular physical activity, smoking abstinence, and healthy nutritional intake, and population screening methods for finding CRC during check‐ups, ie, fecal occult blood testing and colorectal endoscopy. However, a change in lifestyle depends on personal intention, and the efficacy of surveillance strategies is suboptimal and limits true effectiveness. Thus, we need to consider alternative strategies, such as the use of cancer chemoprevention, which includes the consumption of functional foods.
In this study, we focused on the well‐known cancer chemopreventive agent curcumin. Curcumin is a characteristic yellow‐colored compound derived from turmeric that possesses biological functions. Turmeric is produced from the root of the perennial herb Curcuma longa, a group in the ginger family. Numerous studies have reported that curcumin possesses antiinflammatory, anticancer and antioxidant properties by regulating various cell signaling pathways, such as the NF‐κB, signal transducer and activator of transcription 3, and NF‐E2 p45‐related factor‐2 pathways.2 In CRC research, curcumin has suppressed DSS‐induced carcinogenesis by decreasing inflammation‐related gene expression and epigenetic modifications in vivo.3 Furthermore, its safety has been confirmed in human clinical trials.4, 5
However, curcumin is problematic for direct use in human clinical trials because it is poorly soluble in water due to its high hydrophobicity, making the rate of absorption in the intestine poor.6 Thus, the majority of human trials cannot determine the efficacy curcumin. To overcome this problem, submicron particles with surface controlled technology has been applied to curcumin to remarkably improve its water solubility. This water‐soluble compound is named Theracurmin (Theravalues).6 Theracurmin shows antioxidant activity through the regulation of reactive oxygen species and NQO1 inhibition in esophageal squamous cell carcinoma cells.7 It also induces apoptosis in human prostate and bladder cancer cells.8 In CRC research, Theracurmin suppressed DSS‐induced carcinogenesis by decreasing inflammation‐related gene expression in vivo.9 However, the preventive effects of Theracurmin on hereditary intestinal carcinogenesis have not yet been elucidated. Therefore, we used Apc‐mutant mice, a model of familial adenomatous polyposis, in which we induced the development of many intestinal polyps through activation of β‐catenin signaling to evaluate the effects of Theracurmin and curcumin.
In this study, we found that Theracurmin significantly suppressed NF‐κB transcriptional activity and decreased intestinal tumorigenesis in Min mice, partly by modulating proinflammatory cytokine expression. A Theracurmin human trial (J‐CAP‐C study) is now ongoing (UMIN000018817)). This report provides proof of concept for the J‐CAP‐C study.
2. MATERIALS AND METHODS
2.1. Chemicals
Theracurmin and curcumin used for animal experiments were kindly gifted by Theravalues Corporation. In this study, Theracurmin, which contains curcumin (30%) within excipient (70%) as submicron form, was dissolved in water, and curcumin was dissolved in DMSO or water.
2.2. Cell culture
DLD‐1 and HCT116 cells, human colon adenocarcinoma cell lines, were purchased from ATCC. The DLD‐1 and HCT116 cells were maintained in DMEM supplemented with 10% FBS and antibiotics (100 µg/mL streptomycin and 100 U/mL penicillin) at 37°C with 5% CO2.
2.3. Luciferase reporter gene assay
To measure NF‐κB transcriptional activities, DLD‐1 and HCT116 colon cancer cells were transfected with NF‐κB (Promega) reporter plasmids using Polyethylenimine MAX MW 40 000 (PolyScience). Cells stably expressing NF‐κB‐Luc were treated with hygromycin and cloned. These cells are referred to as DLD1‐NF‐κB‐Luc and HCT116‐ NF‐κB‐Luc cells. For the Theracurmin and curcumin experiments, the DLD1‐NF‐κB‐Luc and HCT116‐ NF‐κB‐Luc cells were seeded in 96‐well plates (2 × 104 cells/well). After 24 hours of preincubation, the cells were treated with Theracurmin or curcumin for 24 hours. Firefly luciferase activity levels were determined using the Bright GLO Luciferase assay system (Promega). Basal NF‐κB luciferase activity in the control was set as 1.0. Data are expressed as the mean ± SD (n = 3).
2.4. Luciferase assays for AP‐1 transcriptional activity
To measure AP‐1 transcriptional activity, DLD‐1 colon cancer cells were seeded in 12‐well plates (4.0 × 105 cells/well). After 24 hours of incubation, the cells were transiently transfected with 1 μg/well of the pAP1‐Luc (Signosis) reporter plasmid and 100 ng/well pGL4.73 (hRluc/SV40) control plasmid (Promega) using Polyethylenimine Max MW 40 000 (PolyScience); transfected cells were cultured for an additional 24 hours, treated with the test agents for 24 hours, and firefly and Renilla luciferase activities were determined using the Bright GLO and Renilla GLO Systems (Promega), respectively. The basal luciferase activity of AP‐1 in the control was set as 1.0. The percentage of luciferase activity with each treatment was calculated from the data of triplicate wells, with values normalized to those of the Renilla luciferase activity. The data are expressed as the means ± SD (n = 4).
2.5. Quantitative real‐time PCR analyses
The DLD‐1 cells were seeded in 12‐well plates (1.5 × 105 cells/well). The cells were cultured in the presence of 5, 10, and 20 μM Theracurmin or curcumin for 24 hours. After 24 hours, the mRNA expression levels of the oxidative stress‐related factors were evaluated by quantitative real‐time PCR. Total RNA was isolated using RNAiso Plus (TaKaRa). Aliquots of 100 ng in a final volume of 20 µL were used for the synthesis of cDNA with a high capacity cDNA reverse transcription kit (Applied Biosystems) and oligo (dT) primers. Real‐time PCR was carried out using a CFX96/384 PCR detection system (Bio‐Rad) and Fast Start Universal SYBR Green Mix (Roche Diagnostics) according to the manufacturers’ instructions. The following primers were used to evaluate human mRNA levels: human MCP‐1 (5′‐CAG CCA GAT GCA ATC AAT GCC and 5′‐TGG AAT CCT GAA CCC ACT TCT), human IL‐6 (5′‐ ACT CAC CTC TTC AGA ACG AAT TG and 5′‐GTC GAG GAT GTA CCG AAT TTG T) and 18S ribosome (5′‐ GGC GCC CCC TCG ATG CTC TTA and 5′‐GCT CGG GCC TGC TTT GAA CAC TCT). In addition, to assess the effects of Theracurmin or curcumin on the mRNA expression levels of inflammation‐related factors in the Min mice, we used the following primer sequences: mouse MCP‐1 (5′‐TAA AAA CCT GGA AGT TGA CC and 5′‐GCA TAG CTT CAG ATT TAC GGG T), mouse IL‐6 (5′‐CTG CAA GAG ACT TCC ATC CAG TT and 5′‐GAA GTA GGG AAG GCC GTG G), and 18S ribosome (5′‐ GGC GCC CCC TCG ATG CTC TTA and 5′‐GCT CGG GCC TGC TTT GAA CAC TCT).
Each well was filled with a 12 µL sample mixture that included 6 µL of 2 × SYBR Green Master Mix, 6.4 µL distilled water, 2 µL DNA sample (10 ng/µL), and 4 µL primer set (1 µM each). The plates were run for 10 minutes at 95°C, for 40 cycles of 15 minutes at 95°C and for 1 minutes at 60°C. To assess the specificity of each primer set, the melting curves of the amplicons generated by the PCRs were analyzed.
2.6. Animals
Male C57BL/6‐Apc Min/+ mice (Min mice) were purchased from Jackson Laboratory. The mice (n = 3 or n = 4) were housed in plastic cages with sterilized softwood chips as bedding in a barrier‐sustained animal room maintained at 24 ± 2°C and 55% humidity under a 12/12‐hour light / dark cycle. The mice were fed an AIN‐76A powdered basal control diet mixed with Theracurmin or curcumin at concentrations of 0 and 500 ppm. Ten male 5‐week‐old Min mice were given 0‐500 ppm Theracurmin or curcumin for 8 weeks. All animals housed in the same cage were in the same treatment group (10 mice/group). Food and water were available ad libitum. The animals were observed daily for clinical symptoms and mortality. Body weight and food consumption were measured weekly. At the time they were killed, the mice were anesthetized, and blood samples were collected from their abdominal veins. Their intestinal tracts were removed and separated into the small intestine, cecum, and colorectum. The small intestine was divided into proximal segments (4 cm in length), and the remaining segments contained middle and distal halves. The number of polyps in the proximal segments was counted and collected under a stereoscopic microscope at the time of death. The remaining intestinal mucosa (the part without polyps) was removed by scraping, and the specimens were stored at –80°C until quantitative real‐time PCR analysis was carried out. The other regions were opened longitudinally and fixed flat between sheets of filter paper in 10% buffered formalin. Later, the number, size, and intestinal distribution of the polyps were assessed with a stereoscopic microscope. All experiments were carried out according to the “Guidelines for Animal Experiments in the National Cancer Center” and were approved by the Institutional Ethics Review Committee for Animal Experimentation of the National Cancer Center. The animal protocol was designed to minimize pain and discomfort to the animals. All animals were euthanized for tissue collection by isoflurane overdose.
2.7. Immunohistochemical staining of PCNA to evaluate proliferation of intestinal polyps in Min mice
The small intestines were fixed, embedded, and sectioned as Swiss rolls for further immunohistochemical examination using the avidin‐biotin complex immunoperoxidase technique after heating with 10 mM citrate buffer (pH 6.0). The primary Ab was monoclonal mouse anti‐PCNA Ab (Calbiochem) at a 100 × dilution. As the secondary Ab, biotinylated horse anti‐mouse IgG (Vector Laboratories) was used at a 200 × dilution. Staining was undertaken using avidin‐biotin reagents (Vectastain ABC reagents; Vector Laboratories), 3,3′‐diaminobenzidine, and hydrogen peroxide, and the sections were counterstained with hematoxylin to facilitate orientation. As a negative control, consecutive sections were immunostained without exposure to the primary Ab. The ratio of PCNA‐positive cells was calculated by the formula % = number of PCNA‐positive cells per polyp / total number of cells in the polyp (100× magnification).
2.8. Statistical analyses
The results are expressed as the mean ± SD according to the statistical analyses undertaken using Dunnett’s test. Differences were considered to be statistically significant at *P < .05, **P < .01, and ***P < .01.
3. RESULTS
3.1. Suppression of NF‐κB promoter transcriptional activity by Theracurmin treatment
To examine the effects of Theracurmin on NF‐κB promoter transcriptional activity, the stably transfected DLD1‐NF‐κB‐Luc cells and HCT116‐NF‐κB‐Luc cells were treated with 5, 10, and 20 µM Theracurmin for 24 hours (Figure 1). In DLD1‐NF‐κB‐Luc cells, 10 and 20 µM Theracurmin treatment decreased NF‐κB promoter transcriptional activity by 23% (P < .05) and 34% (P < .01), respectively, compared with the untreated control value (Figure 1A). In the other colorectal cancer cells, HCT116‐NF‐κB‐Luc cells, only 20 µM Theracurmin treatment decreased NF‐κB promoter transcriptional activity by 39.4% (P < .01) compared with the untreated control value (Figure 1B). Curcumin is known to remain mostly undissolved in water but completely dissolved in DMSO. However, Theracurmin is the compound that is derived from finely crushed curcumin, which makes it more soluble, and it is assumed to show more potent activity in the cells than curcumin mixed in water. Thus, NF‐κB promoter transcriptional activity was also examined using curcumin dissolved in DMSO or water treatment for 24 hours. Similar to the results of Theracurmin, treatment with 20 µM curcumin dissolved in DMSO decreased NF‐κB promoter transcriptional activity by 31.9% (P < .01) in HCT116‐NF‐κB‐Luc cells, but not in the DLD‐NF‐κB‐Luc cells, compared with the untreated control value (Figure 1C,D). As expected, treatment with curcumin dissolved in water did not change the NF‐κB promoter transcriptional activity in DLD‐NF‐κB‐Luc or HCT116‐NF‐κB‐Luc cells (Figure 1E,F). We also confirmed the effects of Theracurmin and curcumin on AP‐1 promoter transcriptional activity in DLD‐1 cells, and found that Theracurmin weakly inhibited AP‐1 promoter transcriptional activity from 5 μM (Figure 1G,H).
Figure 1.
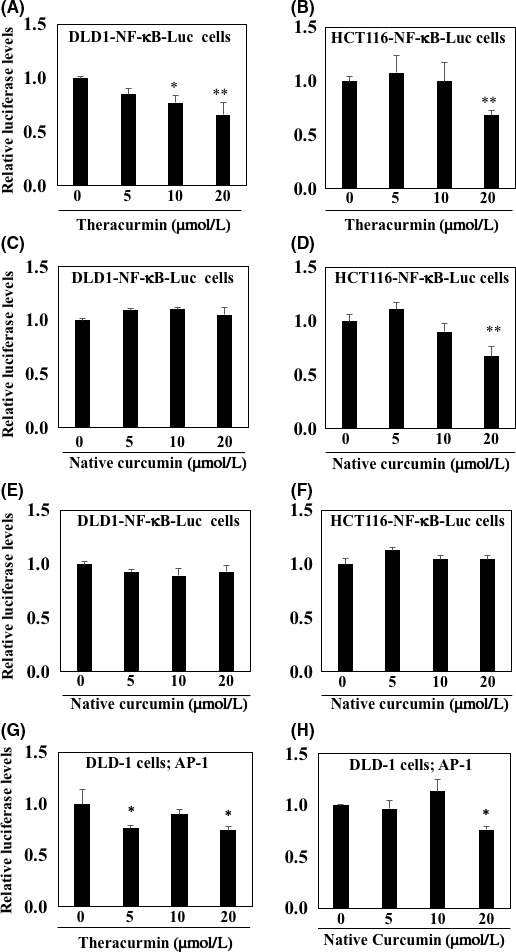
Effect of Theracurmin on nuclear factor (NF)‐κB and activator protein‐1 (AP‐1) promoter transcriptional activity in DLD‐1 and HCT116 colorectal cancer cells. A, B, DLD1‐NF‐κB‐Luc cells (A) and HCT116‐NF‐κB‐Luc cells (B) were treated with 5, 10, and 20 µM Theracurmin for 24 hours. C, D, DLD1‐NF‐κB‐Luc cells (C) and HCT116‐NF‐κB‐Luc cells (D) were treated with 5, 10, and 20 µM curcumin dissolved in DMSO for 24 hours. E, F, DLD1‐NF‐κB‐Luc cells (E) and HCT116‐NF‐κB‐Luc cells (F) were treated with 5, 10, and 20 µM curcumin dissolved in water for 24 hours. G, H, DLD‐1 cells were transiently transfected with pAP1‐Luc reporter plasmid and pGL4.73 (hRluc/SV40) control plasmid. After 24 hours, DLDL‐1 cells were treated with Theracurmin (G) or curcumin (H) dissolved in DMSO for 24 hours. Luciferase activity values with each treatment were normalized by those of Renilla luciferase activity. Basal luciferase activity level of the control was set as 1.0. Data are presented as the mean ± SD (n = 3). *P < .05, **P < .01 vs 0 for control
3.2. Suppression of inflammation‐related factor mRNA expression by Theracurmin treatment
To clarify the effects of Theracurmin on the downstream targets of NF‐κB, the mRNA expression levels of inflammation‐related factors were evaluated in the DLD‐1 cells after a 24 hours of treatment with 5, 10, and 20 μM Theracurmin. The MCP‐1 mRNA expression levels in cells treated with 10 or 20 μM Theracurmin decreased by 38.4% (P < .05) and 63% (P < .01), respectively, compared with the untreated control value (Figure 2A). In addition, treatments with 5, 10, and 20 μM Theracurmin decreased IL‐6 mRNA expression levels by 52.8% (P < .001), 62.2% (P < .001), and 41.5% (P < .001), respectively, compared with the level of the untreated control (Figure 2B). Moreover, the mRNA expression levels of inflammation‐related factors were also examined using a treatment of curcumin dissolved in DMSO for 24 hours. Similar to the results from the Theracurmin treatment, the results from the treatment with 20 μM curcumin indicated a decreased MCP‐1 mRNA expression level of 26.1% (P < .05) compared with the untreated control value (Figure 2C). In addition, 10 and 20 μM curcumin treatment decreased IL‐6 mRNA expression levels by 38.0% (P < .01) and 38.7% (P < .01), respectively, compared with the untreated control value (Figure 2D).
Figure 2.
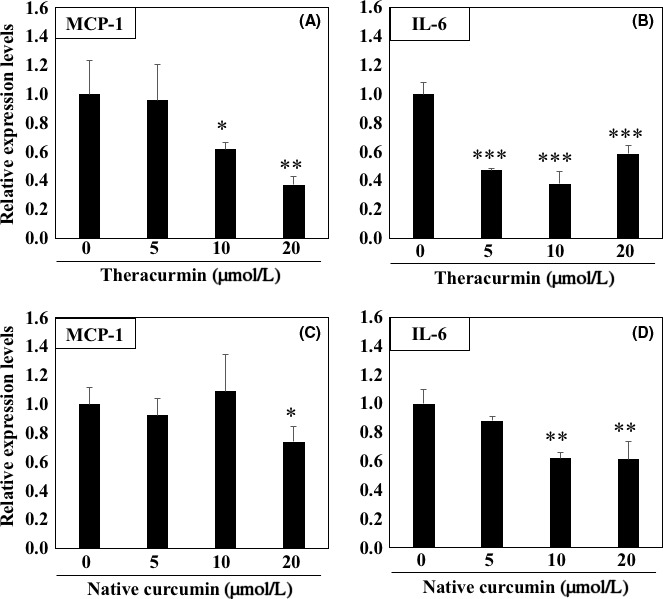
Expression levels of monocyte chemoattractant protein‐1 (MCP‐1) and interleukin‐6 (IL‐6) mRNA in DLD1 cells treated with Theracurmin. The DLD‐1 cells were seeded in 12‐well plates (1.5 × 105 cells/well) and cultured in medium containing Theracurmin (5, 10, and 20 μM) for 24 hours. A, B, After 24 hours, mRNA expression levels of MCP‐1 (A) and IL‐6 (B) were evaluated by quantitative real‐time PCR analysis. C, D, Similarly, curcumin (5, 10, and 20 μM) dissolved in DMSO was used for treatments, and the MCP‐1 (C) and IL‐6 (D) mRNA expression levels were evaluated. Basal mRNA expression levels of the control were set as 1.0. Data were normalized to those of the 18S ribosome. Data are presented the mean ± SD, n = 4. *P < .05, **P < .01, ***P < .001, vs 0 for control
3.3. Theracurmin suppressed intestinal polyp development in Min mice
Treatment of Min mice with Theracurmin or curcumin for 8 weeks did not affect their body weight, food intake, or clinical symptoms throughout the experimental period. There was no difference in average daily food intake among Min mice in the basal diet control group (n = 10 mice), Theracurmin‐treated group (n = 10 mice), or curcumin‐treated group (n = 10 mice). In addition, no changes in organ weight, which was measured as an indicator of toxicity, were observed. Table 1 summarizes the data regarding the number and distribution of the intestinal polyps in the basal diet control, Theracurmin‐treated, and curcumin‐treated groups. The number of polyps was mostly observed in the small intestine, and some mice did not have polyps in the colon. Treatment with 500 ppm Theracurmin decreased the total number of polyps to 65.5% of the untreated control value (P < .05 vs control group). In particular, a reduction in the number of polyps was observed in the distal segment of the small intestine (P < .05 vs control group). No significant differences in the number of polyps were observed in the other segments of the small intestine or the colon.
Table 1.
Number of intestinal polyps/mouse in Min mice treated with native curcumin or Theracurmin
| Treatment | Small intestinal | Colon | Total | ||
|---|---|---|---|---|---|
| Proximal | Middle | Distal | |||
| Control (n = 10) | 6.2 ± 7.5 | 8.8 ± 4.8 | 25.5 ± 3.8 | 0.4 ± 0.7 | 40.9 ± 10.2 |
| Theracurmin 500 ppm (n = 10) | 4.0 ± 2.0 | 6.0 ± 3.8 | 16.3 ± 6.1* | 0.5 ± 0.5 | 26.8 ± 10.3* |
| Native curcumin 500 ppm (n = 10) | 5.5 ± 2.8 | 9.2 ± 1.5 | 22.7 ± 6.4 | 0.6 ± 0.7 | 38.0 ± 7.8 |
Data are presented as the mean ± SD.
*P < .05, significantly different from untreated control group.
Figure 3 shows the size distribution of the intestinal polyps for the basal diet and Theracurmin‐treated groups. The majority of polyps were approximately 3.0 mm in diameter. Theracurmin treatment significantly reduced the number of polyps < 0.5 mm or 0.5 < 0.9 mm in diameter (P < .05 vs control group). However, treatment with 500 ppm curcumin failed to significantly decrease the total number of polyps.
Figure 3.
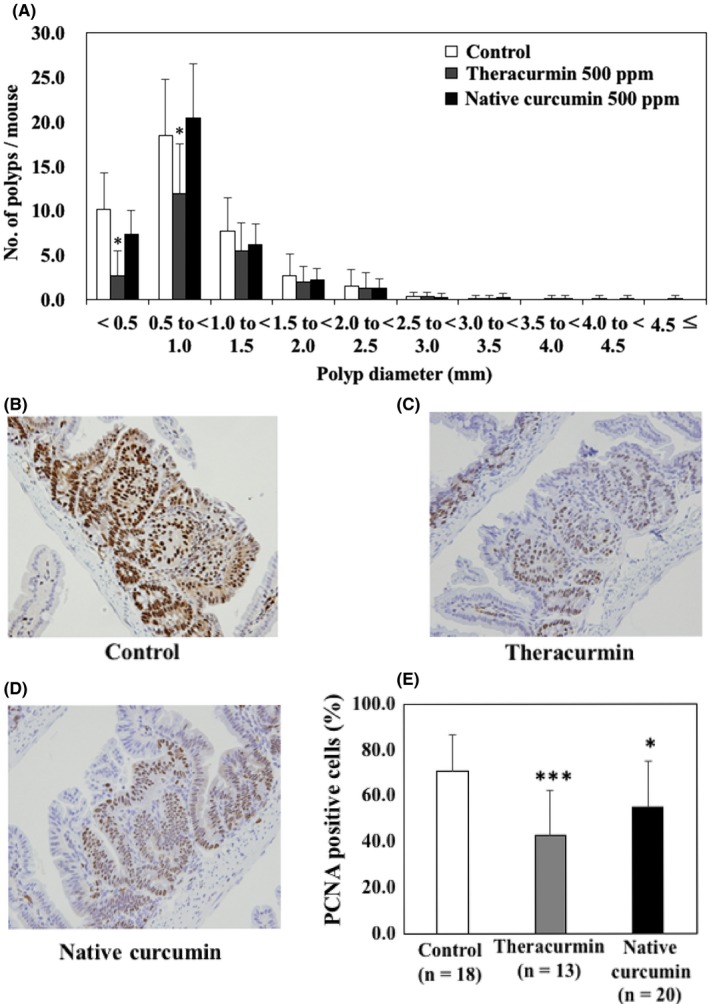
Effects of Theracurmin on the size distribution and proliferating cell nuclear antigen (PCNA)‐positive cells of intestinal polyps. A, Min mice were fed a basal diet (open column), a diet containing 500 ppm Theracurmin (gray column), or a diet containing 500 ppm curcumin (black column) for 8 weeks. The number of polyps per mouse in each size class is given as the mean ± SD. *P < .05 vs the control group. B‐D, Representative data of PCNA immunohistochemical staining from each group (B, basal diet group; C, Theracurmin group; D, curcumin group) are shown. Percentages of PCNA‐positive cells were calculated and are shown. Number of examined polyps is shown in parentheses. Data are presented as mean ± SD. *P < .05, ***P < .001, vs control group
Immunohistochemical staining of PCNA, which represents cell growth, supported the effect of Theracurmin on the growth of tumor epithelial cells. Proliferating cell nuclear antigen‐positive cells occupied 70.9% of the tumor epithelial cells in the polyp of the basal diet group (Figure 3B,E), 42.8% in the Theracurmin‐treated group (Figure 3C,E), and 55.1% in the curcumin‐treated group (Figure 3D,E).
3.4. Theracurmin suppressed inflammation‐related gene expression in intestinal polyps of Min mice
To clarify the mechanisms underlying Theracurmin‐mediated suppression of intestinal polyp formation, the expression of inflammation‐related genes in cells from parts of the intestine with polyps was investigated (Figure 4). Real‐time PCR revealed that treatment with 500 ppm Theracurmin for 8 weeks significantly suppressed MCP‐1 and IL‐6 mRNA expression in the parts of the intestine with polyps by 36.3% (P < .05) and 31.3% (P < .05), respectively, compared with the untreated control values (Figure 4). In the case of curcumin treatment, MCP‐1 and IL‐6 mRNA expression tended to be reduced.
Figure 4.
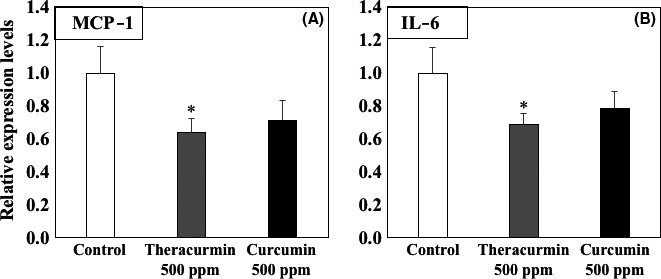
mRNA expression levels of inflammation‐related factors in the intestinal polyps of Min mice fed a basal diet (open column), a diet containing 500 ppm Theracurmin (gray column), or a diet containing 500 ppm curcumin (black column) for 8 weeks. Quantitative real‐time PCR analysis was undertaken to determine MCP‐1 (A) and IL‐6 (B) mRNA expression levels in parts of the Min mice intestine that had polyps. Data were normalized to those of the 18S ribosome. Basal mRNA expression levels of the control group were set as 1.0. Data are presented as the mean ± SD, n = 4. *P < .05 vs control group. IL‐6, interleukin‐6; MCP‐1, monocyte chemoattractant protein‐1
4. DISCUSSION
In the present study, Theracurmin was shown to suppress the transcriptional activity of the NF‐κB promoter in human colon cancer cells. Nuclear factor‐κB is an inflammation‐related transcription factor. Moreover, Theracurmin suppressed the mRNA expression levels of inflammation‐related factors. Finally, Theracurmin was found to inhibit intestinal polyp development in Min mice, which was assumed to be by suppressing the mRNA expression of MCP‐1 and IL‐6 mRNA in cells from parts of the intestine with polyps.
Theracurmin is expected to have similar biological functions to those identified in curcumin. Theracurmin is a highly absorbable and bioavailable preparation of curcumin. Curcumin is a well‐known and potent inhibitor of NF‐κB transcription. One plausible mechanism for this inhibition involves blocking IκBα degradation, which results in the suppression of the nuclear translocation of the functionally active NF‐κB subunit, p65.10 In colorectal cancer, curcumin activates 5′‐AMP‐activated protein kinase (AMPK) and suppresses p65 NF‐κB phosphorylation.11 Thus, Theracurmin is expected to suppress NF‐κB transcriptional activity. Indeed, our study indicated that Theracurmin inhibited NF‐κB transcriptional activity in DLD‐1 and HCT116 cells, which are human colon cancer cells. The findings from another study showing that Theracurmin inhibited NF‐κB transcriptional activity in HT‐29 cells and human colon cancer cells support our results.4 In addition, curcumin is known to inhibit AP‐1 transcriptional activity. Another function of curcumin is that of an antioxidant, scavenging nitric oxide, inhibiting nitric oxide synthase, and counteracting oxidative stress by inducing antioxidant enzymes that use glutathione.12 These expected activities of Theracurmin were not examined in this study. Further study is desired to clarify the function of Theracurmin.
In our study using DLD‐1 cells in vitro, Theracurmin treatment significantly suppressed MCP‐1 and IL‐6 mRNA expression. Suppression of these factors is considered to be mediated by NF‐κB transcriptional activity.13, 14 Proinflammatory cytokines, such as IL‐6, and selected chemokines, such as MCP‐1/CCL2, play important roles in the inflammation that is connected to colorectal cancer.15 To explain why Theracurmin suppressed the IL‐6 expression level in concentrations lower than those that inhibit NF‐κB transcriptional activity, we additionally evaluated the AP‐1 transcriptional activity. As a result, AP‐1 is suppressed by a dose of Theracurmin that suppresses the IL‐6 expression level. These data could explain the discrepancy in our data (Figures 1 and 2), in part.
The dysregulation of NF‐κB has been suggested to promote carcinogenesis. Thus, inhibition of NF‐κB transcriptional activity by Theracurmin might be related to anticarcinogenesis. In a subsequent experiment, we examined the effects of Theracurmin on intestinal carcinogenesis. To the best of our knowledge, this is the first report showing that Theracurmin can suppress intestinal polyp formation in mice. Several studies have reported that the number of intestinal polyps is decreased by curcumin. Among them, 1 paper reported findings similar to those from our research.16 In that paper, Min mice were given a placebo or a 2% curcumin mixed diet for 4‐18 weeks (n = 10/group). The curcumin treatment decreased the total number of intestinal polyps by 75% (P < .05). The authors concluded that the benefits of curcumin in colon cancer could be attributed, at least in part, to its mediation of antiinflammatory activity. Similar to their study, our study indicated that Theracurmin at low concentrations effectively suppressed the number of intestinal polyps. Our results in vivo suggest that Theracurmin has clinical benefits in terms of dose setting in humans not offered by curcumin.
In our previous report using Min mice, cancer chemopreventive agents mainly suppressed intestinal polyp development less than 1.5 mm in a diameter.17, 18, 19, 20, 21 Thus, our data are consistent with our previous data. In these Min mice, large polyps tended to be observed in the proximal part of the small intestine and became smaller as one approached the anal side. Our previous data indicated that antiinflammatory agents suppress intestinal polyp development mainly in the middle to distal parts of the small intestine.19, 20, 21 Thus, it is speculated that an antiinflammatory function of Theracurmin suppressed intestinal polyp development in this study.
We did not provide direct evidence of suppression of NF‐κB by Theracurmin in vivo. Thus, we cannot determine whether reduced expression of IL‐6 or MCP1 causes tumor suppression or tumor suppression causes a decrease in the IL‐6 and MCP‐1 levels. However, our working hypothesis is that inhibition of NF‐κB transcriptional activity led to suppression of its downstream expression of IL‐6 and MCP‐1, and resulted in the inhibition of intestinal polyp development. Direct evidence of suppression of NF‐κB by Theracurmin in vivo is desired in the near future.
The reason that Theracurmin is more potent than curcumin is that Theracurmin is made from curcumin with a submicron colloidal dispersion technique, which increases water solubility. As a result, the bioavailability of Theracurmin has been reported to be 27‐fold higher than curcumin in plasma.6 Yamaguchi et al showed that Theracurmin had an approximately 50‐fold stronger preventive effect than curcumin on noise‐induced hearing loss.22 Our study also supports the results indicating that the effective dose of Theracurmin is lower than that of curcumin. We are starting a Theracurmin human trial (J‐CAP‐C study; UMIN000018817). This study in vitro and in vivo provides a proof of concept for the J‐CAP‐C study.
In conclusion, we showed that Theracurmin significantly suppressed NF‐κB transcriptional activity and decreased intestinal tumorigenesis in Min mice, partly by modulating proinflammatory cytokine expression. Theracurmin could be used for cancer chemoprevention as a functional food source.
CONFLICT OF INTEREST
The authors have no potential conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors thank Ms Ruri Nakanishi and Mr Naoaki Uchiya, members of the National Cancer Center Research Core Facility, for their expert technical assistance in the animal experiments. This work was supported by Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development, AMED (18ck0106271h0002), and the Ministry of Agriculture, Fishery and Forestry, Japan (MAFF‐CPS‐2016‐1‐1).
Adachi S, Hamoya T, Fujii G, et al. Theracurmin inhibits intestinal polyp development in Apc‐mutant mice by inhibiting inflammation‐related factors. Cancer Sci. 2020;111:1367–1374. 10.1111/cas.14329
Saeko Adachi and Takahiro Hamoya contributed equally.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Kunnumakkara AB, Bordoloi D, Padmavathi G, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2017;174:1325‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo Y, Wu R, Gaspar JM, et al. DNA methylome and transcriptome alterations and cancer prevention by curcumin in colitis‐accelerated colon cancer in mice. Carcinogenesis. 2018;39:669‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanai H, Iida T, Takeuchi K, et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double‐blind, placebo‐controlled trial. Clin Gastroenterol Hepatol. 2006;4:1502‐1506. [DOI] [PubMed] [Google Scholar]
- 6. Sasaki H, Sunagawa Y, Takahashi K, et al. Innovative preparation of curcumin for improved oral bioavailability. Biol Pharm Bull. 2011;34:660‐665. [DOI] [PubMed] [Google Scholar]
- 7. Mizumoto A, Ohashi S, Kamada M, et al. Combination treatment with highly bioavailable curcumin and NQO1 inhibitor exhibits potent antitumor effects on esophageal squamous cell carcinoma. J Gastroenterol. 2019;54:687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang M, Ho JN, Kook HR, et al. Theracurmin® efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol Rep. 2016;35:1463‐1472. [DOI] [PubMed] [Google Scholar]
- 9. Ohno M, Nishida A, Sugitani Y, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE. 2017;12:e0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase‐2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30:45‐51. [DOI] [PubMed] [Google Scholar]
- 11. Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK‐induced inhibition of NF‐κB, uPA activator and MMP9. Oncol Lett. 2016;12:4139‐4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443‐E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laos S, Baeckström D, Hansson GC. Inhibition of NF‐kappaB activation and chemokine expression by the leukocyte glycoprotein, CD43, in colon cancer cells. Int J Oncol. 2006;28:695‐704. [PubMed] [Google Scholar]
- 14. Zhang YH, Lin JX, Vilcek J. Interleukin‐6 induction by tumor necrosis factor and interleukin‐1 in human fibroblasts involves activation of a nuclear factor binding to a kappa B‐like sequence. Mol Cell Biol. 1990;10:3818‐3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danese S, Mantovani A. Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin‐Yang interplay between inflammation and cancer. Oncogene. 2010;29:3313‐3323. [DOI] [PubMed] [Google Scholar]
- 16. Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin′s effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J Interferon Cytokine Res. 2011;31:219‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Niho N, Mutoh M, Takahashi M, Tsutsumi K, Sugimura T, Wakabayashi K. Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO‐1886, increasing lipoprotein lipase activity in Min mice. Proc Natl Acad Sci U S A. 2005;102:2970‐2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niho N, Takahashi M, Shoji Y, et al. Dose‐dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR gamma ligand. Cancer Sci. 2003;94:960‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niho N, Mutoh M, Komiya M, Ohta T, Sugimura T, Wakabayashi K. Improvement of hyperlipidemia by indomethacin in Min mice. Int J Cancer. 2007;121:1665‐1669. [DOI] [PubMed] [Google Scholar]
- 20. Nakatsugi S, Fukutake M, Takahashi M, et al. Suppression of intestinal polyp development by nimesulide, a selective cyclooxygenase‐2 inhibitor, in Min mice. Jpn J Cancer Res. 1997;88:1117‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimizu S, Fujii G, Takahashi M, et al. Sesamol suppresses cyclooxygenase‐2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in Apc Min/+ mice. J Clin Biochem Nutr. 2014;54:95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaguchi T, Yoneyama M, Onaka Y, Imaizumi A, Ogita K. Preventive effect of curcumin and its highly bioavailable preparation on hearing loss induced by single or repeated exposure to noise: A comparative and mechanistic study. J Pharmacol Sci. 2017;134:225‐233. [DOI] [PubMed] [Google Scholar]


