Abstract
Anthrax toxin receptor 1 (ANTXR1), a type I transmembrane protein, is one of the receptors that facilitates the entrance of anthrax toxin into cells. Previous studies have confirmed the pivotal role of ANTXR1 in progression and tumorigenesis of diverse cancer types. However, the biological function of ANTXR1 in gastric cancer (GC) is still unknown. The present study aimed to investigate the role of ANTXR1 in GC and illuminate the potential molecular mechanisms. Bioinformatics analysis found that ANTXR1 expression was significantly upregulated in GC tissue and its overexpression was associated with poor prognosis of GC patients. Moreover, we confirmed the upregulation of ANTXR1 in GC cell lines and GC tissue by quantitative PCR, western blot analysis, and immunohistochemical analysis. Additionally, high protein expression level of ANTXR1 was positively associated with several clinicopathological parameters in GC patients. In our study, a series of in vitro and in vivo assays were undertaken through strategies of loss/gain‐of‐function and rescue assays. Consequently, our results indicated that ANTXR1 induced proliferation, cell cycle progression, invasion and migration, and tumorigenicity and induced suppressed apoptosis in GC. Mechanistic investigation indicated that ANTXR1 exerted its promoting effects on GC through activation of the PI3K/AKT/mTOR signaling pathway. In conclusion, our findings suggested that ANTXR1 plays a crucial role in the development and progression of GC and could serve as a novel prognostic biomarker and potential therapeutic target for GC.
Keywords: ANTXR1, EMT, gastric cancer, metastasis, proliferation
The authors observed that anthrax toxin receptor 1 (ANTXR1) expression was significantly upregulated in gastric cancer (GC) tissue and its overexpression was associated with poor prognosis of GC patients. A series of in vitro and in vivo assays were carried out through strategies of loss‐ or gain‐of‐function and rescue assays to find that ANTXR1 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway.
Abbreviations
- ANTXR1
anthrax toxin receptor 1
- EC
endothelial cell
- EMT
epithelial‐mesenchymal transition
- GC
gastric cancer
- IHC
immunohistochemistry
- p‐
phosphorylated
- PI
propidium iodide
- qPCR
quantitative PCR
- SVV
Seneca Valley virus
- TEM8
tumor endothelial marker 8
1. INTRODUCTION
Gastric cancer is one of the most common malignancies of the digestive system, the fifth most frequently diagnosed cancer, and the third leading cause of cancer death.1 The incidence rate of GC is markedly high in eastern Asia. The age‐standardized 5‐year net survival rate of patients with GC has been reported to be generally in the range 20%‐40%.2, 3 A majority of patients with GC are diagnosed with advanced stage or distant metastasis.4 Despite the development of techniques for the diagnosis and treatment of GC, prognosis for patients with advanced‐stage GC is still discouraging.5, 6, 7 Therefore, there is an urgent need to clarify the mechanism involved in the process and metastasis of GC, which will help elucidate specific molecular targets and improve diagnosis and prognosis.
Anthrax toxin receptor 1, also known as ATR, GAPO, or TEM8, is a type I transmembrane protein, is encoded by the highly conserved TEM8 gene.8 Tumor endothelial marker 8 is a highly conserved cell‐surface glycoprotein that was originally identified by its overexpression in ECs that line the tumor vasculature of colorectal cancer.8 Several studies have shown that TEM8 binds to the C5 domain of collagen type VI and promotes migration of ECs in vitro.9, 10 Furthermore, TEM8 plays a significant role in cell attachment and migration, and interacts with ECM proteins and the actin cytoskeleton. It also mediates adhesion of cells to type 1 collagen and gelatin, reorganization of the actin cytoskeleton, and promotes cell spreading.11 Previous studies found that TEM8 is involved in the angiogenic response of cultured umbilical vein ECs by regulating cell–matrix interactions on collagen.12 Originally, TEM8 was identified as one a cell surface receptor of anthrax toxin, so it was alternatively named ANTXR1.13 Recent studies identified ANTXR1 as the high‐affinity cellular receptor for SVV.14 Seneca Valley virus has shown encouraging results and a favorable safety profile as an oncolytic virus in clinical trials, and this finding offers a promising biomarker for selecting patient response to treatment.11, 15, 16, 17 The extracellular domains of ANTXR1 share homology with integrins, and interactions with collagen IV, collagen VI, and laminin suggest a possible function in basement membrane assembly and angiogenesis.18, 19 In comparison with the wide distribution in normal tissue of ANTXR2, ANTXR1 is overexpressed in tumor cells and the vasculature of developing carcinoma.9, 12 Previous studies reported that approximately 63% of cell lines surpass the expression cut‐off line of ANTXR1 among 1037 cell lines in the Cancer Cell Line Encyclopedia.14
In the present study, we found that ANTXR1 plays a critical role in promoting GC progression. A series of in vitro and in vivo assays revealed that knockdown of ANTXR1 in GC cells dramatically suppressed cell proliferation, cell cycle progression, invasion and migration, and tumorigenicity and induced apoptosis, whereas overexpression of ANTXR1 had the opposite effect. Furthermore, our mechanistic investigations revealed that ANTXR1 induced GC cell proliferation and aggressiveness by activating the PI3K/AKT/mTOR signaling pathway. Our findings indicated that ANTXR1 plays a role as a novel oncogene in GC and could be a potential diagnostic and therapeutic target.
2. MATERIALS AND METHODS
2.1. Tissue specimens
Human GC tissue and adjacent nonmalignant tissue were obtained from the Department of General Surgery in Xinhua Hospital affiliated with Shanghai Jiao Tong University (Shanghai, China). None of the patients received radiotherapy or chemotherapy before surgery. All diagnostic information was gathered based on the American Joint Committee on Cancer (8th edition) guidelines. We obtained informed consent from all patients and the study was approved by the Research Ethics Committee of Xinhua Hospital, School of Medicine, Shanghai Jiao Tong University (approval no. XHEC‐F‐2019‐029).
2.2. Cell lines and reagents
The 4 human GC cell lines, BGC823, MGC803, HGC27, and SGC7901, and human gastric mucosal epithelial cell line (GES‐1) were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China). All cells were authenticated by short tandem repeat profiling and cultured in RPMI‐1640 (Hyclone) containing 10% FBS (Gibco). These cell lines were incubated in a humidified incubator containing 5% CO2 at 37°C.
LY294002 (Cat. No. 154447‐36‐6) was purchased from MedChemExpress and dissolved in DMSO. Both HGC27/ANTXR1 and SGC7901/ANTXR1 cells were treated with LY294002 for 48 hours at the recommended concentration of 20 μM.
2.3. Total RNA extraction and qRT‐PCR
Total RNA was extracted from the cells or tissue samples using TRIzol reagent (Invitrogen). The cDNA was synthesized using the PrimeScript RT reagent kit with gDNA Eraser (TaKaRa) according to the manufacturer's instructions. Quantitative real‐time PCR was undertaken using SYBR Green (TaKaRa) according to the manufacturer's instructions.
Target genes were measured by using an Applied Biosystems StepOnePlus real‐time thermocycler (Applied Biosystems). The specific primer sequences used to amplify ANTXR1 were: 5′‐ACAGTTGGCTCACAAATTCATCA‐3′ (forward) and 5′‐ TCACTGGCCCTTTCAAATCCT‐3′ (reverse). GAPDH was used as an endogenous control in PCR analysis. Relative gene expression levels were quantified by the comparative 2−Ct method and cycle thresholds were normalized to GAPDH levels.
2.4. Western blot analysis
The protein was extracted by RIPA lysis buffer (Beyotime) containing cocktail protease inhibitors and cocktail phosphatase inhibitors. Protein quantification was undertaken using BCA assays (Beyotime). Proteins from cell and tissue samples were separated by SDS‐PAGE and electrotransferred onto PVDF membranes (Millipore). The PVDF membranes were blocked with 5% skimmed milk for 1 hour at room temperature. Subsequently, the bands were incubated with the following primary Abs including anti‐ANTXR1 (1:1000, #A6525; ABclonal), anti‐P‐PI3K (1:1000, #4228; Cell Signaling Technology), anti‐PI3K (1:1000, #4249; Cell Signaling Technology), anti‐AKT (1:1000, #4691; Cell Signaling Technology), anti‐P‐AKT (1:1000, #4060; Cell Signaling Technology), anti‐mTOR (1:1000, #2983; Cell Signaling Technology), anti‐P‐mTOR (1:1000, #5536; Cell Signaling Technology), anti‐E‐cadherin (1:1000, #3195; Cell Signaling Technology), anti‐vimentin (1:1000, #5741; Cell Signaling Technology), anti‐snail (1:1000, #3879; Cell Signaling Technology), anti‐cleaved caspase 3 (1:1000, #9662; Cell Signaling Technology), anti‐Bcl‐2 (1:1000, #4223; Cell Signaling Technology), anti‐cytochrome C (1:1000, #11940; Cell Signaling Technology), anti‐cyclin D1 (1:1000, #2978; Cell Signaling Technology), anti‐p21Cip1 (1:1000, #2947; Cell Signaling Technology), anti‐p27Kip1 (1:1000, #3686; Cell Signaling Technology), and anti‐β‐tubulin (1:3000, #AB0039; Abways) at 4°C overnight. The next day, the membranes were incubated with a goat anti‐rabbit HRP‐conjugated secondary Ab (1:1000, #A0208; Beyotime). Western blot analyses were repeated 3 times independently.
2.5. Cell transfection
Anthrax toxin receptor 1 was silenced in BGC823 and MGC803 cells with siRNA (Biotend) using Rfect transfection reagent (BAIDAI) according to the manufacturer's instructions using siRNA‐ANTXR1‐1 (sense, GGUGUGGUAAGAAACUCAAdTdT; antisense, UUGAGUUUCUUACCACACCdTdT) and siRNA‐ANTXR1‐2 (sense, GCAACUACAGUCAGAUUUdTdT; antisense, UAAAUCUGACUGUAGUUGCdTdT).
Overexpression of ANTXR1 in HGC27 and SGC7901 cells was achieved by lentiviral infection. For ANTXR1 overexpression in GC cells, the ANTXR1 gene was synthesized according to the human ANTXR1 mRNA sequence and was constructed into a lentiviral expression vector; empty vector was used as the control (Shanghai Genechem). Control cells were transfected with empty vectors. Overexpression plasmids lentiviral vector carrying GFP was synthesized by GeneChem. Lentiviral infection was carried out according to the manufacturer's instructions. Stably transfected cell lines were selected for 2 weeks using puromycin (1 μg/mL). The transfection efficiency in the infected cells was validated by western blotting, qRT‐ PCR, and fluorescence microscopy. The stable ANTXR1‐overexpressing cell lines were named HGC27/ANTXR1 and SGC7901/ANTXR1.
2.6. Cell proliferation assay
The CCK‐8 assay (Yeasen) is used to evaluate the level of cell proliferation. Gastric cancer cells were seeded into 96‐well plates at the density of 1000 cells per well with 100 μL complete medium. At each time point (6, 24, 48, 72, 96, and 120 hours), 10 μL CCK‐8 reagent was added to each well and incubated at 37°C. After 2 hours of incubation, we measured the optical density at 450 nm with a SpectraMax 190 Microplate Reader. Three independent experiments were carried out for the final results.
2.7. Colony formation assay
Gastric cancer cells were seeded into 6‐well plates at the density of 500 cells per well with 2.5 mL complete medium and cultured for 2 weeks. Two weeks later, colonies were fixed with 4% paraformaldehyde for 30 minutes and then stained with 0.5% crystal violet stain solution (Yeasen) for 30 minutes. The number of colonies containing more than 50 cells was counted.
2.8. Invasion and migration assays
Gastric cancer cells were resuspended in 100 μL serum‐free medium and then seeded at a density of 6 × 104 HGC27 or BGC823 cells (8 × 104 SGC7901 and 3 × 104 MGC803 cells) into the upper chamber of an 8‐μm pore size (Corning) or BioCoat Matrigel Invasion Chamber (Corning). The lower compartments were put into a 24‐well plate filled with 600 μL medium containing 10% FBS. The plate was incubated for 24 hours in a humidified tissue culture incubator at 37°C in a 5% CO2 atmosphere. After incubation, cells were fixed in 4% paraformaldehyde for 30 minutes and stained with 0.5% crystal violet stain solution (Yeasen) for 30 minutes. Cell numbers were counted in 5 random fields, and images were captured under a microscope at a magnification of 100×.
2.9. Apoptosis assay
An annexin V‐Alexa Fluor 647/PI Apoptosis Detection Kit (Cat No. 40304; Yeasen) was used to detect the apoptosis rate of GC cells according to the manufacturer's instructions. Briefly, GC cells were washed twice with cold PBS and then resuspended in 1× binding buffer. Next, 5 μL annexin V‐Alexa Fluor 647 and 10 μL PI staining solution were added into the binding buffer. After incubation for 15 minutes at room temperature in the dark, samples were analyzed by flow cytometry (BD Biosciences).
2.10. Cell cycle assay
Cell Cycle Analysis Kit (Beyotime) was used to analyze the cell cycle distribution. Gastric cancer cells were harvested from 6‐well plates and washed twice with cold PBS. Subsequently, the cells were fixed with 70% ethanol at 4°C overnight. The next day, the cells were treated with RNase A solution and stained with PI for 30 minutes at 37°C. The cell cycle distribution (G0/G1, S and G2/M) were measured by flow cytometry (BD Biosciences) and analyzed by ModFit software (BD Biosciences).
2.11. Subcutaneous xenograft models
Male BALB/c nude mice (4 weeks old) were purchased from Shanghai SLRC Laboratory Animal Co. and maintained under specific pathogen‐free conditions. One group contained 5 mice and each mouse was s.c. inoculated with 5 × 106 cells of SGC7901 with stable overexpression of ANTXR1 (SGC7901/ANTXR1) that had been resuspended in Matrigel (BD Biosciences). Tumor size was measured with calipers every 4 days, and the tumor volume was calculated using the following formula: π/6 × length × width2. Four weeks after injection, the mice were killed, and the xenograft tumors were dissected for IHC assays. All animal studies were carried out with the approval of the Ethics Committee of Xinhua Hospital of the School of Medicine at Shanghai Jiaotong University (Approval No. XHEC‐F‐2019‐029).
2.12. Statistical analysis
All experiments were independently repeated 3 times, and quantitative data were expressed as mean ± SD. Prism 7 (GraphPad Software) and SPSS 24 (SPSS Inc.) were used for statistical analyses. The 2‐tailed Student's t test was used to compare quantitative variables. The correlation between ANTXR1 expression and clinicopathological variables was calculated by Pearson's χ2 test. Kaplan‐Meier methods and log‐rank tests were used for the survival analysis. P values less than .05 were considered statistically significant. Significant differences are indicated by asterisks referring to the following P values: *P < .05, **P < .01, and ***P < .001.
3. RESULTS
3.1. Expression of ANTXR1 is upregulated in human GC tissue and GC cell lines
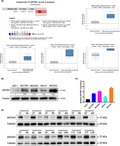
Based on the Oncomine database, we discovered that ANTXR1 expression is significantly upregulated in GC tissue compared with normal tissue. As shown in Figure 1A, 4 cases of datasets found that ANTXR1 mRNA levels were dramatically higher in cancer tissue. To further confirm the results of the Oncomine database, we tested expression levels of ANTXR1 in GC cells by qRT‐PCR and western blotting. The results showed that expression level of ANTXR1 in GC cell lines (BGC823, MGC803, HGC27, and SGC7901) was significantly higher than in human gastric mucosal epithelial cell line GES‐1 (Figure 1B,C). In order to further determine the expression level of ANTXR1 in clinical samples of human GC tissue, western blotting was used in 12 pairs of randomly selected human GC tissue and paired adjacent normal tissue. As described in Figure 1D, the expression of ANTXR1 in GC tissue was significantly higher than that in matched adjacent normal tissue. In addition, IHC was applied to examine the protein expression level of ANTXR1 in 103 GC tissues. The result showed 64.08% (66/103) in GC tissue, indicating a relatively higher level of ANTXR1 compared with 31.07% (32/103) in the matched adjacent normal tissue. In addition, we found that ANTXR1 protein was mainly located in the cytoplasm of benign and malignant epithelial cells (Figure 2A).
Figure 1.
Anthrax toxin receptor 1 (ANTXR1) expression was increased in gastric cancer (GC) tissue and GC cell lines. A, 4 datasets showed statistically significant ANTXR1 mRNA overexpression (red) based on the Oncomine database. B, C, Relative expression of ANTXR1 protein and mRNA in human gastric mucosal epithelial cell line GES‐1 and 4 GC cell lines (BGC823, MGC803, HGC27, and SGC7901) by western blotting and quantitative PCR. D, Expression of ANTXR1 protein in 12 pairs of randomly selected human GC tissue and paired adjacent normal tissue by western blotting
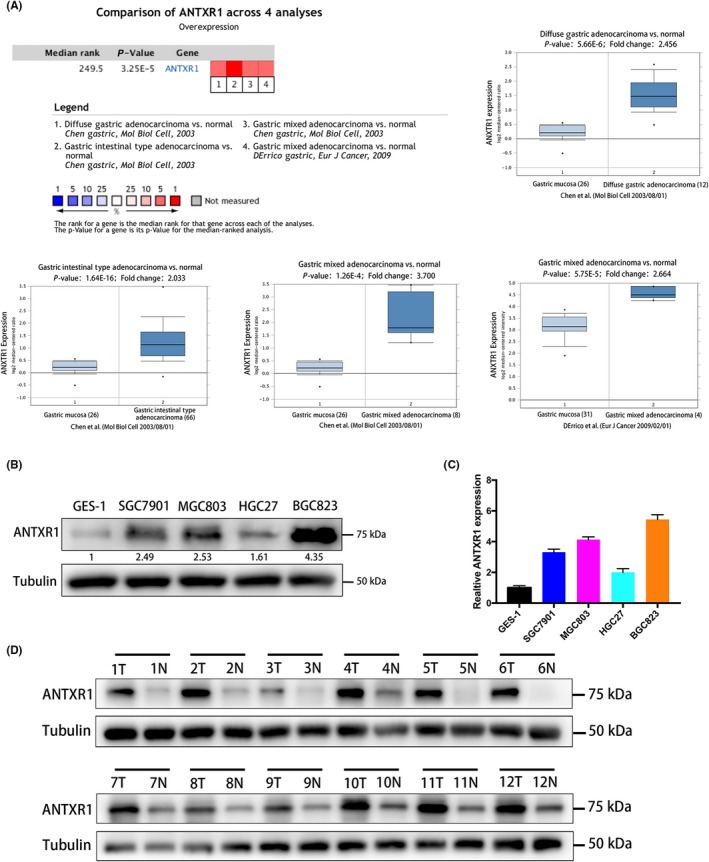
Figure 2.
Anthrax toxin receptor 1 (ANTXR1) overexpression is correlated with poor prognosis in gastric cancer (GC). A, Representative images of ANTXR1 expression in GC tissue and paired adjacent normal tissue by immunohistochemistry. The 6 images on the left are different staining intensities in GC tissue. Far right images show adjacent normal tissue. B, Expression level of ANTXR1 based on individual GC stages in the UALCAN database. C, Expression level of ANTXR1 based on GC tumor grade in the UALCAN database. D, E, Kaplan‐Meier curves of overall survival in GC patients stratified by ANTXR1 expression. Patients with higher ANTXR1 expression had poorer prognosis. *P < .05, **P < .01, ***P < .001. ns, non‐significant
Taken together, these results suggest that ANTXR1 is remarkably elevated in GC tissue and GC cell lines.
3.2. High ANTXR1 expression is associated with several clinicopathologic parameters and poor prognosis of GC patients
As summarized in Table 1, high protein expression level of ANTXR1 was positively associated with T stage (P = .001), lymph node metastasis (P = .002), and clinical stage (P < .001) in GC patients. However, there was no significant correlation between ANTXR1 expression and other clinicopathological parameters (P > .05).
Table 1.
Association of anthrax toxin receptor 1 (ANTXR1) expression with clinicopathologic parameters of gastric cancer (GC) patients
| Characteristics | Total (n = 103) | ANTXR1 protein expression | P value | |
|---|---|---|---|---|
| Low (n = 37) | High (n = 66) | |||
| Age (y) | ||||
| ≤60 | 46 | 15 | 31 | .529 |
| >60 | 57 | 22 | 35 | |
| Gender | ||||
| Male | 63 | 20 | 43 | .268 |
| Female | 40 | 17 | 23 | |
| Differentiation | ||||
| Well + moderate | 41 | 18 | 23 | .170 |
| Poor | 62 | 19 | 43 | |
| T classification | ||||
| T1 + T2 | 32 | 19 | 13 | .001 |
| T3 + T4 | 71 | 18 | 53 | |
| Lymph node metastasis | ||||
| N0 | 38 | 21 | 17 | .002 |
| N1‐3 | 65 | 16 | 49 | |
| Distant metastasis | ||||
| M0 | 82 | 33 | 49 | .071 |
| M1 | 21 | 4 | 17 | |
| AJCC stage | ||||
| I + II | 35 | 23 | 12 | <.001 |
| III + IV | 68 | 14 | 54 | |
Results showed 64.08% (66/103) in GC tissue indicated a relatively higher level of ANTXR1 compared with 31.07% (32/103) in the matched adjacent normal tissue. AJCC, American Joint Committee on Cancer.
We detected whether the mRNA expression of ANTXR1 was related to cancer stage in GC patients using the UALCAN database.20 As shown in Figure 2B, the results indicated that patients with a more advanced stage of GC tended to have higher ANTXR1 expression levels of their GC tissue, except the stage III group. Patients in stage IV expressed the highest mRNA levels of ANTXR1 (P < .05). Moreover, the data shown in Figure 2C indicate that patients with higher pathological grade tumors expressed higher levels of ANTXR1 mRNA (P < .05). However, no statistical significance was found between the grade I group and the normal group; we assumed the limited sample size in the grade I group caused the result.
Simultaneously, we used Kaplan‐Meier plotter (http://kmplot.com/) and OncoLnc (http://www.oncolnc.org/) to further detect the prognostic values of the mRNA expression of ANTXR1 in GC patients. The result by Kaplan‐Meier plotter revealed that high ANTXR1 expression was significantly associated with a shorter OS for all patients with GC (hazard ratio = 1.7; 95% confidence interval, 1.43‐2.02; P = 8e‐10; Figure 2D). The results by OncoLnc also shows the same tendency (P = .0174, Figure 2E).
3.3. Anthrax toxin receptor 1 promotes proliferation of GC cells
We undertook CCK‐8 and colony formation assays to evaluate the role of ANTXR1 in GC progression. First, we investigated the mRNA and protein endogenous ANTXR1 levels in GC cell lines (BGC823, MGC803, HGC27, and SGC7901) and human gastric mucosal epithelial cells (GES‐1). The ANTXR1 expression levels were higher in BGC823 and MGC803 cells than in HGC27 and SGC7901 cells (Figure 1B,C). Subsequently, we knocked down ANTXR1 in BGC823 and MGC803 cells and overexpressed ANTXR1 in HGC27 and SGC7901 cells to undertake loss‐of‐function and gain‐of‐function assays. To avoid off‐target effects by siRNA, we constructed ANTXR1‐knockdown cells using 2 ANTXR1‐specific siRNAs. Western blotting and qPCR analysis confirmed a significant decrease of ANTXR1 expression in BGC823 and MGC803 cells compared with the expression level of ANTXR1 in respective control cells (Figure 3A,B). The CCK8 and colony formation assays showed that GC cell proliferation abilities were significantly inhibited after downregulation of ANTXR1 (Figure 3C,D). Simultaneously, 2 stable ANTXR1‐overexpressed GC cell lines, HGC27/ANTXR1 and SGC7901/ANTXR1, were established (Figure 4A,B). As expected, the CCK‐8 and colony formation assays showed that GC cell proliferation abilities were remarkably enhanced after upregulation of ANTXR1 (Figure 4C,D).
Figure 3.
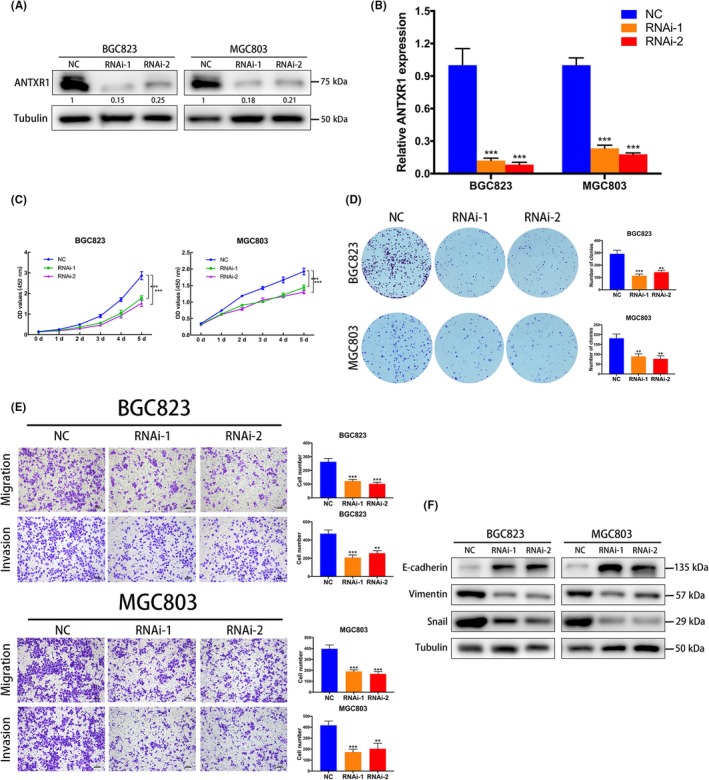
Downregulation of anthrax toxin receptor 1 (ANTXR1) inhibits proliferation, migration, and invasion of gastric cancer (GC) cells. A, B, Knockdown of ANTXR1 was confirmed at the protein and mRNA level in BGC823 and MGC803 cells by western blotting and quantitative PCR. C, D, ANTXR1 knockdown inhibited proliferation of BGC823 and MGC803 cells as determined by CCK‐8 and colony formation assays. E, Transwell assays were undertaken to measure the effects of ANTXR1 downregulation on migration and invasion abilities of BGC823 and MGC803 cells. F, Western blot analysis of E‐cadherin, Vimentin, and snail levels in GC cells transfected with ANTXR1‐specific siRNA or control siRNA. *P < .05, **P < .01, ***P < .001. NC, negative control
Figure 4.
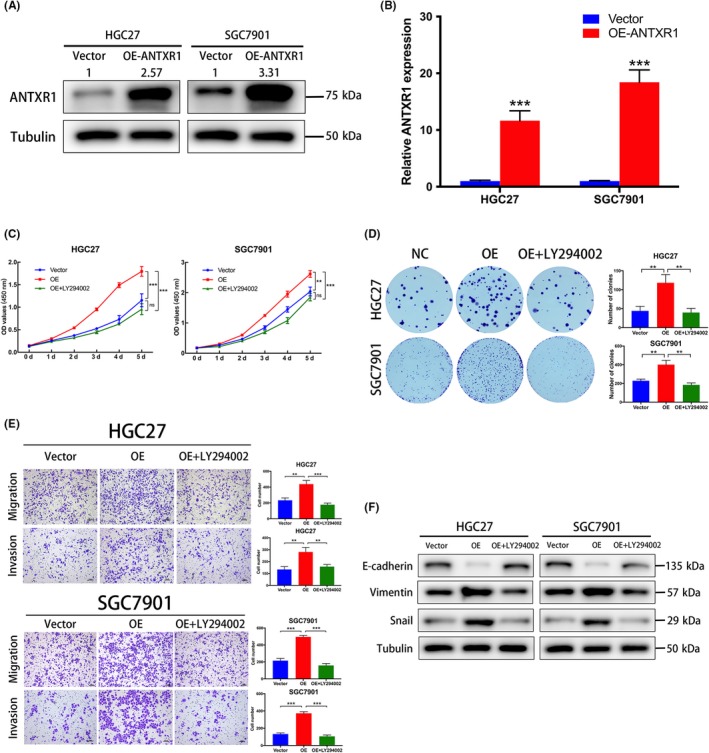
Overexpression of anthrax toxin receptor 1 (ANTXR1) promotes proliferation, migration, and invasion of gastric cancer (GC) cells. A, B, Overexpression (OE) of ANTXR1 was confirmed at the protein and mRNA level in HGC27 and SGC7901 cells by western blotting and quantitative PCR. C, D, Proliferation ability of ANTXR1 overexpression GC cells (HGC27/ANTXR1 and SGC7901/ANTXR1) without or with the PI3K inhibitor LY294002 (20 μM, 48 h) was determined by CCK‐8 and colony formation assays. E, Transwell assays were undertaken to measure the effects of ANTXR1 overexpression and LY294002 treatment on migration and invasion abilities of HGC27 and SGC7901 cells. F, Western blot analyses were used to measure the effects of ANTXR1 overexpression and LY294002 treatment on E‐cadherin, Vimentin, and snail levels. *P < .05, **P < .01, ***P < .001
3.4. Anthrax toxin receptor 1 promotes GC cell migration and invasion in vitro through EMT
To explore whether ANTXR1 expression levels affect GC metastasis, we undertook Transwell migration and Matrigel cell invasion assays. As shown in Figure 3E, downregulation of ANTXR1 remarkably repressed the migratory and invasive abilities of BGC823 and MGC803 cells. In contrast, overexpression of ANTXR1 remarkably enhanced the migratory and invasive abilities of HGC27 and SGC7901 cells (Figure 4E). Collectively, these results showed that ANTXR1 affects GC cell migration and invasion in vitro.
Furthermore, we assuming that ANTXR1 might be involved in EMT of GC cells, a pivotal process characterized by tumor cell migration and invasion. We detected the expression of EMT‐associated markers by western blotting and found that downregulation of ANTXR1 in BGC823 and MGC803 cells upregulated epithelial marker E‐cadherin, whereas mesenchymal markers Vimentin and EMT‐associated transcription factor Snail were downregulated (Figure 3F). By contrast, E‐cadherin was significantly decreased in ANTXR1‐overexpressed cells (HGC27/ANTXR1 and SGC7901/ANTXR1) cells, whereas Vimentin and Snail were increased (Figure 4F). Therefore, we concluded that ANTXR1 could promote GC cell migration and invasion in vitro through EMT.
3.5. Anthrax toxin receptor 1 accelerates cell‐cycle G1‐S transition in GC cells
To investigate the possible mechanism of ANTXR1 in GC progression, we undertook flow cytometry analysis. The result suggested that silencing ANTXR1 increased the percentage of G0/G1 phase cells and reduced S phase cells, whereas overexpressing ANTXR1 significantly decreased the percentage of G0/G1 phase cells and increased the proportion of S phase cells (Figure 5A,B). In summary, our data indicated that ANTXR1 accelerates the GC cell cycle and furthers promote the proliferation of GC cells.
Figure 5.
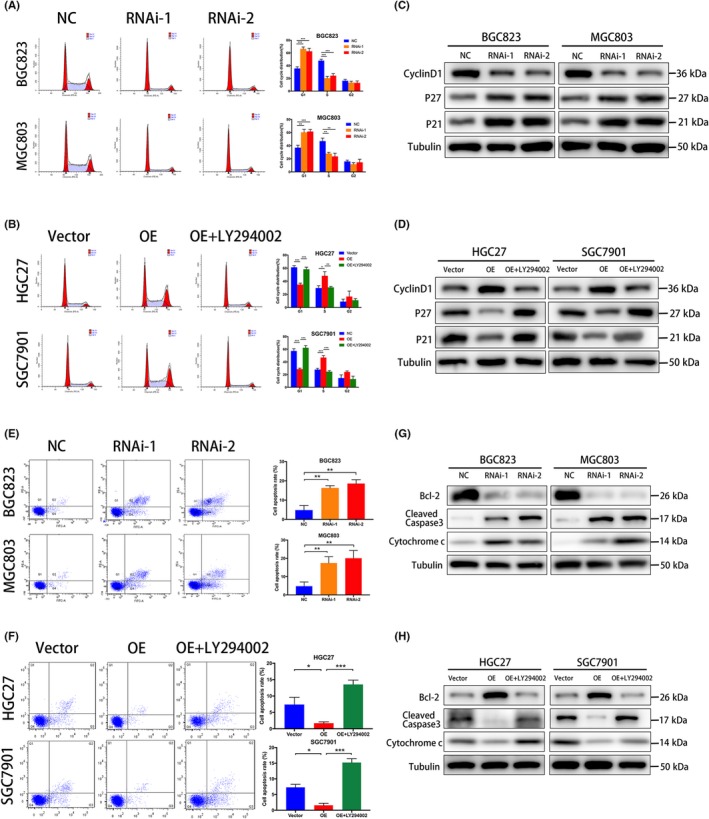
Anthrax toxin receptor 1 (ANTXR1) inhibits apoptosis of gastric cancer (GC) cells and accelerated cell cycle G1‐S transition in GC cells. A, B, Cell cycle distribution in different phases was analyzed by flow cytometry. C, D, Western blot analysis of cyclin D1, p21Cip1, and p27Kip1 in ANTXR1‐silenced cells, ANTXR1‐overexpressed (OE) cells, and ANTXR1‐overexpressed cells treated with LY294002. E, F, Cells were stained with annexin V‐Alexa Fluor 647/propidium iodide then apoptosis rates were assessed by flow cytometry. G, H, Western blot analysis of Bcl‐2, cleaved caspase 3, and Cytochrome C in ANTXR1‐silenced cells, ANTXR1‐overexpressed cells, and ANTXR1‐overexpressed cells treated with LY294002. *P < .05, **P < .01, ***P < .001
We further used western blotting analysis to explore whether ANTXR1 could adjust expressions of cell cycle‐related proteins, including cyclin D1, p21Cip1, and p27Kip1. As shown in Figure 5C,D, a significant decline of cyclin D1 was observed in ANTXR1‐silenced GC cells compared to control groups, whereas p21Cip1 and p27Kip1 increased. On the contrary, a significant downregulation of p21Cip1 and p27Kip1 was observed in ANTXR1‐overexpressed GC cells compared to control groups, whereas cyclin D1 was remarkably upregulated. Taken together, our results show that ANTXR1 accelerated cell‐cycle G1‐S transition through regulating expression of cyclin D1, p21Cip1, and p27Kip1.
3.6. Anthrax toxin receptor 1 inhibits apoptosis of gastric cancer cells
We used the annexin V‐Alexa Fluor 647/PI Apoptosis Detection Kit to evaluate the effects of ANTXR1 on GC cell apoptosis by flow cytometry. The results showed that the proportions of apoptotic cells in the ANTXR1‐overexpressed HGC27 and SGC7901 cells were lower than those in the control groups, whereas the ANTXR1‐silenced BGC823 and MGC803 cells have more apoptotic cells than the control groups (Figure 5E,F). To investigate the molecular mechanism underlying the cell apoptosis caused by ANTXR1, we evaluated the expression of apoptosis markers cleaved caspase‐3 and cytochrome C, and survival‐associated protein BCL‐2. The protein expression levels of cleaved caspase‐3 and cytochrome C were upregulated in ANTXR1‐silenced BGC823 and MGC803 cells but downregulated in both HGC27/ANTXR1 and SGC7901/ANTXR1 cells. In addition, the protein expression levels of BCL‐2 showed the opposite tendency (Figure 5G,H). Collectively, our data indicate that ANTXR1 could inhibit apoptosis of GC cells.
3.7. Anthrax toxin receptor 1 promotes tumorigenesis of GC cells
To investigate the biological function of ANTXR1 on GC tumor growth in vivo, we undertook xenograft growth assays in nude mice by s.c. injection of SGC7901/ANTXR1 cells or negative control cells. As shown in Figure 6A,B, SGC7901/ANTXR1 cells had a significantly enhanced ability to form s.c. tumor nodules in nude mice compared to negative control cells, as indicated by the xenograft tumor volume, weight, and tumor growth curves (Figure 6C,D). The tumor sizes in 2 groups reached 100 mm3 at the first week, when we started to measure and record. Moreover, IHC staining showed that the proliferation index Ki‐67 was higher in ANTXR1‐overexpressed cells derived from tumor nodules than those derived from negative control cells. In addition, expression of EMT markers, including E‐cadherin, N‐cadherin, and vinculin, were significantly changed between the 2 groups. Expression of E‐cadherin and vinculin were lower in the ANTXR1‐overexpressed group; conversely, expression of N‐cadherin was higher in the ANTXR1‐overexpressed group (Figure 6E). Taken together, these results indicate that ANTXR1 plays a crucial role in the tumorigenesis of GC cells in vivo.
Figure 6.
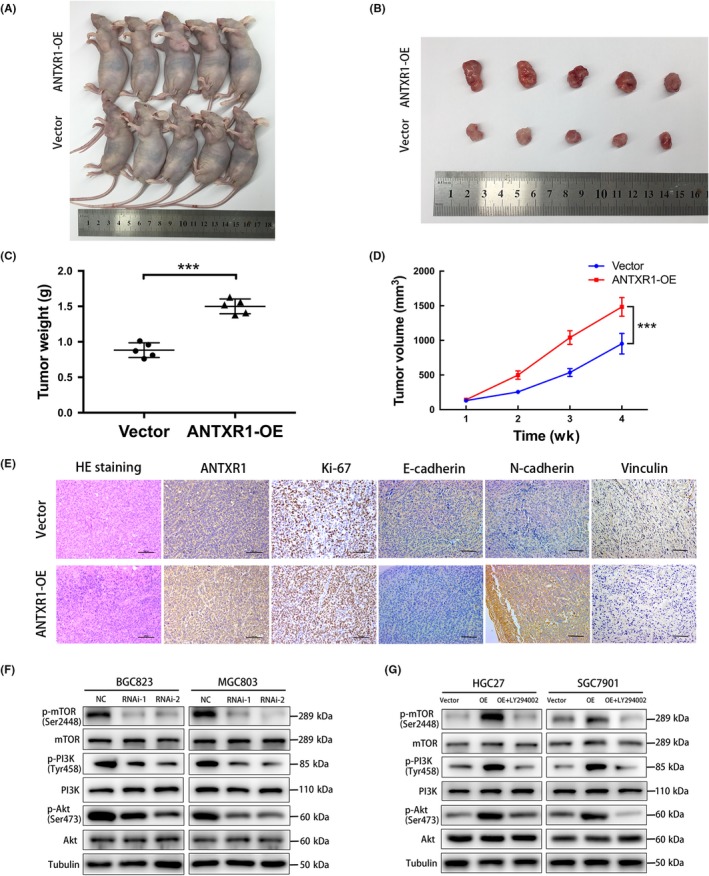
Anthrax toxin receptor 1 (ANTXR1) promotes the tumorigenesis of gastric cancer (GC) cells and promotes GC progression through activation of the PI3K/AKT/mTOR signaling pathway. A, B, Overexpression (OE) of ANTXR1 promoted tumor growth in the nude mouse model by xenograft growth assay. C, D, Tumor weights and tumor volumes in the empty vector control group and the ANTXR1‐overexpressed group. E, H&E staining and immunohistochemistry for ANTXR1, Ki67, E‐cadherin, N‐cadherin, and Vinculin in the empty vector control group and the ANTXR1‐overexpressed group. F, G, Western blot analysis of phosphorylated (p‐)PI3K, PI3K, p‐AKT, AKT, p‐mTOR, and mTOR expression in ANTXR1‐silenced cells, ANTXR1‐overexpressed cells, and ANTXR1‐overexpressed cells treated with LY294002. *P < .05, **P < .01, ***P < .001
3.8. Anthrax toxin receptor 1 promoted GC progression through the PI3K/AKT/mTOR signaling pathway
The PI3K/AKT/mTOR pathway plays a vital role in cancer cell transcription, translation, proliferation, survival and metastasis.22 A previous study suggested that ANTXR1 might have correlation with the PI3K/AKT pathway, by bioinformatics analysis.23 Therefore, we further explored whether the oncogenic effects of ANTXR1 were dependent on the PI3K/AKT/mTOR signaling pathway. As shown in Figure 6F,G, p‐PI3K, p‐AKT, and p‐mTOR were increased in ANTXR1‐overexpressed groups but decreased in ANTXR1‐silenced groups. To confirm these results, we used the PI3K inhibitor LY294002 to treat ANTXR1‐overexpressed GC cells (HGC27/ANTXR1 and SGC7901/ANTXR1). We found that LY294002 treatment significantly downregulated the promotional effects on the expression levels of p‐PI3K, p‐AKT, and p‐mTOR caused by ANTXR1 overexpression (Figure 6F,G). We also observed that the expression levels of the total PI3K, AKT, and mTOR showed no significant changes. Furthermore, we undertook rescue experiments to confirm that ANTXR1 promoted GC progression through the PI3K/AKT/mTOR signaling pathway. The CCK‐8 and colony formation assays revealed that LY294002 can dramatically suppress the proliferation ability of these ANTXR1‐overexpressed cells (Figure 4C,D). We also investigated whether LY294002 could inhibit the promotional effects on migration and invasion, G1‐S transition, or promote the inhibitory effect on cell apoptosis caused by ANTXR1 overexpression. We found that LY294002 treatment induced suppression of cell migration and invasion and G1‐S transition, along with promotion of cell apoptosis in ANTXR1‐overexpressed GC cells (Figures 4E and 5C,G).
4. DISCUSSION
The results of the current study indicated that expression of ANTXR1 was extensively upregulated in GC tissue and GC cell lines. Immunohistochemistry indicated an association between ANTXR1 expression and several clinicopathological characteristics of 103 patients with GC. Kaplan‐Meier analysis showed that high expression of ANTXR1 was positively correlated with poor survival in GC patients. Thus, we hypothesize that ANTXR1 contributes to supporting growth of GC and driving tumor cell invasion and metastatic dissemination.
Subsequently, we undertook a series of in vitro and in vivo assays to investigate the effects of ANTXR1 on GC cell growth. Our results indicated that knockdown of ANTXR1 suppressed GC cell proliferation, colony formation, migration, invasion, and tumorigenicity, and induced cell apoptosis and cell cycle arrest, whereas overexpression of ANTXR1 induced the opposite phenotype. Therefore, our findings provide further support for the hypothesis that ANTXR1 plays a vital role in the progression of GC and could be a novel diagnostic and therapeutic target.
Anthrax toxin receptor 1, a 564 amino acid type I transmembrane protein, is known as one of the receptors that facilitate the entrance of anthrax toxin into cells.13 Also known as TEM8, ANTXR1 specifically interacts with the protective antigen component of anthrax toxin and was originally identified by its overexpression in the endothelial cells that line the tumor vasculature of colorectal cancer.8, 24 Moreover, ANTXR1 has previously been found to be specifically expressed in the tumor microenvironment.25 In the context of cancer, ANTXR1 is upregulated in diverse cancer types and plays an important role in tumor development, such as lung cancer, breast cancer, and gallbladder carcinoma.26, 27, 28, 29, 30, 31 In addition, Shukla et al observed that ANTXR1 was expressed in 10.4% of T2 tumors, 79.5% of T3 tumors, and 84.8% of T4 tumors.27 Additional studies were completed with breast tumors and normal tumor tissue and showed similar results.32, 33 In the light of these previous reports, patients with ANTXR1 overexpression showed poor differentiation, advanced TNM stage, and significantly poorer prognosis. Prior to this work, Tuupanen and colleagues identified ANTXR1 as 1 of 33 candidate oncogenes by screening for base‐specific mutations.34 In addition, they also found that ANTXR1 is the most frequent target for hotspot mutations in a validation set. In early 2018, researchers had obtained promising results when they applied ANTXR1 as a target of CAR T cell therapy in the preclinical phase of triple‐negative breast cancer.35 Subsequently, another study reported that ANTXR1 is the potent antigenic target for CAR T cell therapy of GC.36 Taken together, it is a logical hypothesis that ANTXR1 might be involved in GC progression and metastasis. To the best of our knowledge, this is the first study to determine the biological function of ANTXR1 in GC and the correlation between expression of ANTXR1 and clinicopathological features of GC.
The PI3K/AKT/mTOR pathway is a classical signaling pathway playing a vital role in regulating cell growth, apoptosis, cell cycle, metastasis, and other cell biology processes in various cancer types, including GC.37, 38, 39, 40 Once active, AKT regulates cell proliferation and tumor progression by phosphorylating a variety of downstream cell cycle‐related and antiapoptotic proteins as well as transcription factors.41 Additionally, relevant studies have shown that the PI3K/AKT/mTOR pathway is activated in GC and GC patients with simultaneous expression of PI3K/AKT/mTOR had worse prognosis.42, 43 A bioinformatics study23 found that ANTXR1, as a differentially expressed gene, was associated with relapse‐free survival and overall survival in GC and a Kyoto Encyclopedia of Genes and Genomes pathway analysis found that the PI3K‐AKT signaling pathway was a significantly represented enriched pathway. To explore the regulation of molecular mechanisms of ANTXR1 for GC cell proliferation, cell cycle progression, invasion, and migration, we focused on the expression levels of proteins within the PI3K/AKT/mTOR pathway. In the present study, western blot analysis indicated that the expression levels of p‐PI3K, p‐AKT, and p‐mTOR were significantly decreased by knockdown of ANTXR1 in both BGC823 and MGC803 cells. Likewise, western blot analysis showed that the expression levels of p‐PI3K, p‐AKT, and p‐mTOR in the ANTXR1 overexpression group were significantly elevated, but not total AKT or mTOR, indicating that the PI3K/AKT/mTOR pathway might be involved in the molecular mechanism of ANTXR1 for GC progression. Moreover, our results showed that PI3K inhibitor LY294002 inhibits the function induced by overexpressed ANXTR1 in GC cells and reduced expression levels of p‐PI3K, p‐AKT, and p‐mTOR in the ANTXR1‐overexpression group. The above results contribute to establish a mechanistic rationale for validating our initial hypothesis that ANTXR1 promotes GC progression through the PI3K/AKT/mTOR signaling pathway.
In conclusion, our study showed that expression of ANTXR1 in GC tissue is significantly upregulated and closely associated with poor prognosis of GC patients. Additionally, our results suggested that ANTXR1 plays a critical role in promoting GC cell progression and invasiveness by activating the PI3K/AKT/mTOR signaling pathway. Our findings indicated that ANTXR1 could be a potential diagnostic and therapeutic target for GC.
DISCLOSURE
The authors have declared that no competing interest exists.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81572819).
Cai C, Dang W, Liu S, et al. Anthrax toxin receptor 1/tumor endothelial marker 8 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway. Cancer Sci. 2020;111:1132–1145. 10.1111/cas.14326
Cai, Dang, and Liu contributed equally to this work.
Contributor Information
Yunping Hu, Email: kevintony@126.com.
Jun Gu, Email: gujun02@xinhuamed.com.cn.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet. 2018;391(10125):1023‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654‐26664. [DOI] [PubMed] [Google Scholar]
- 5. Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127‐164. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. [DOI] [PubMed] [Google Scholar]
- 7. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363‐385. [DOI] [PubMed] [Google Scholar]
- 8. St Croix B, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197‐1202. [DOI] [PubMed] [Google Scholar]
- 9. Nanda A, Carson‐Walter EB, Seaman S, et al. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI). Cancer Res. 2004;64(3):817‐820. [DOI] [PubMed] [Google Scholar]
- 10. Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281(32):23227‐23236. [DOI] [PubMed] [Google Scholar]
- 11. Burke MJ, Ahern C, Weigel BJ, et al. Phase I trial of Seneca Valley Virus (NTX‐010) in children with relapsed/refractory solid tumors: a report of the Children's Oncology Group. Pediatr Blood Cancer. 2015;62(5):743‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell‐matrix interactions on collagen. Exp Cell Res. 2005;305(1):133‐144. [DOI] [PubMed] [Google Scholar]
- 13. Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414(6860):225‐229. [DOI] [PubMed] [Google Scholar]
- 14. Miles LA, Burga LN, Gardner EE, Bostina M, Poirier JT, Rudin CM. Anthrax toxin receptor 1 is the cellular receptor for Seneca Valley virus. J Clin Invest. 2017;127(8):2957‐2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudin CM, Poirier JT, Senzer NN, et al. Phase I clinical study of Seneca Valley Virus (SVV‐001), a replication‐competent picornavirus, in advanced solid tumors with neuroendocrine features. Clin Cancer Res. 2011;17(4):888‐895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burke MJ. Oncolytic Seneca Valley Virus: past perspectives and future directions. Oncolytic Virother. 2016;5:81‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst. 2007;99(21):1623‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell SE, Mavila A, Salazar R, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G‐protein signaling. J Cell Sci. 2001;114(Pt 15):2755‐2773. [DOI] [PubMed] [Google Scholar]
- 19. Burgi J, Kunz B, Abrami L, et al. CMG2/ANTXR2 regulates extracellular collagen VI which accumulates in hyaline fibromatosis syndrome. Nat Commun. 2017;8:15861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagy A, Lanczky A, Menyhart O, Gyorffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. West KA, Castillo SS, Dennis PA. Activation of the PI3K/AKT pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234‐248. [DOI] [PubMed] [Google Scholar]
- 23. Liu JB, Jian T, Yue C, et al. Chemo‐resistant gastric cancer associated gene expression signature: bioinformatics analysis based on gene expression omnibus. Anticancer Res. 2019;39(4):1689‐1698. [DOI] [PubMed] [Google Scholar]
- 24. Martchenko M, Jeong SY, Cohen SN. Heterodimeric integrin complexes containing beta1‐integrin promote internalization and lethality of anthrax toxin. Proc Natl Acad Sci USA. 2010;107(35):15583‐15588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finak G, Bertos N, Pepin F, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518‐527. [DOI] [PubMed] [Google Scholar]
- 26. Chen D, Bhat‐Nakshatri P, Goswami C, Badve S, Nakshatri H. ANTXR1, a stem cell‐enriched functional biomarker, connects collagen signaling to cancer stem‐like cells and metastasis in breast cancer. Cancer Res. 2013;73(18):5821‐5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maurya SK, Tewari M, Kumar M, Thakur MK, Shukla HS. Expression pattern of tumor endothelial marker 8 protein in gallbladder carcinomas. Asian Pac J Cancer Prev. 2011;12(2):507‐512. [PubMed] [Google Scholar]
- 28. Gong Q, Liu C, Wang C, Zhuang L, Zhang L, Wang X. Effect of silencing TEM8 gene on proliferation, apoptosis, migration and invasion of XWLC05 lung cancer cells. Mol Med Rep. 2018;17(1):911‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao C, Wang Z, Huang L, et al. Down‐regulation of tumor endothelial marker 8 suppresses cell proliferation mediated by ERK1/2 activity. Sci Rep. 2016;6:23419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alcala S, Martinelli P, Hermann PC, Heeschen C, Sainz B Jr. The Anthrax Toxin Receptor 1 (ANTXR1) is enriched in pancreatic cancer stem cells derived from primary tumor cultures. Stem Cells Int. 2019;2019:1378639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cullen M, Seaman S, Chaudhary A, et al. Host‐derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69(15):6021‐6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davies G, Cunnick GH, Mansel RE, Mason MD, Jiang WG. Levels of expression of endothelial markers specific to tumour‐associated endothelial cells and their correlation with prognosis in patients with breast cancer. Clin Exp Metastasis. 2004;21(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 33. Davies G, Rmali KA, Watkins G, Mansel RE, Mason MD, Jiang WG. Elevated levels of tumour endothelial marker‐8 in human breast cancer and its clinical significance. Int J Oncol. 2006;29(5):1311‐1317. [PubMed] [Google Scholar]
- 34. Tuupanen S, Hanninen UA, Kondelin J, et al. Identification of 33 candidate oncogenes by screening for base‐specific mutations. Br J Cancer. 2014;111(8):1657‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Byrd TT, Fousek K, Pignata A, et al. TEM8/ANTXR1‐specific CAR T cells as a targeted therapy for triple‐negative breast cancer. Cancer Res. 2018;78(2):489‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M. MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. J Cell Biochem. 2019;120(4):5010‐5017. [DOI] [PubMed] [Google Scholar]
- 37. Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin Cancer Biol. 2019;59:147‐160. [DOI] [PubMed] [Google Scholar]
- 38. Corti F, Nichetti F, Raimondi A, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: a review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45‐55. [DOI] [PubMed] [Google Scholar]
- 39. Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335‐350. [DOI] [PubMed] [Google Scholar]
- 40. Xing X, Zhang L, Wen X, et al. PP242 suppresses cell proliferation, metastasis, and angiogenesis of gastric cancer through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs. 2014;25(10):1129‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ying J, Xu Q, Liu B, Zhang G, Chen L, Pan H. The expression of the PI3K/AKT/mTOR pathway in gastric cancer and its role in gastric cancer prognosis. Onco Targets Ther. 2015;8:2427‐2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tapia O, Riquelme I, Leal P, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465(1):25‐33. [DOI] [PubMed] [Google Scholar]


