Abstract
Background
Oral candidiasis (OC) associated with human immunodeficiency virus (HIV) infection occurs commonly and recurs frequently, often presenting as an initial manifestation of the disease. Left untreated, these lesions contribute considerably to the morbidity associated with HIV infection. Interventions aimed at preventing and treating HIV‐associated oral candidal lesions form an integral component of maintaining the quality of life for affected individuals.
Objectives
To determine the effects of any intervention in preventing or treating OC in children and adults with HIV infection.
Search methods
The search strategy was based on that of the Cochrane HIV/AIDS Review Group. The following electronic databases were searched for randomised controlled trials for the years 1982 to 2005: Medline, AIDSearch, EMBASE and CINAHL. The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, and the Cochrane Central Register of Controlled Trials (CENTRAL) were also searched through May 2005. The abstracts of relevant conferences, including the International Conferences on AIDS and the Conference on Retroviruses and Opportunistic Infections, as indexed by AIDSLINE, were also reviewed. The strategy was iterative, in that references of included studies were searched for additional references. All languages were included.
The updated database search was done for the period 2005 up to 2009. The following databases were searched: Medline, EMBASE, the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. AIDSearch was not searched for the updated search as it ceased publication during 2008.
Selection criteria
Randomised controlled trials (RCTs) of palliative, preventative or curative therapy were considered, irrespective of whether the control group received a placebo. Participants were HIV positive adults and children.
Data collection and analysis
Two authors independently assessed the methodological quality of the trials and extracted data. Study authors were contacted for additional data where necessary.
Main results
For the first publication of the review in 2006, forty studies were retrieved. Twenty eight trials (n=3225) met inclusion criteria. During the update search for the review a, further six studies were identified. Of these, five met the inclusion criteria and were included in the review. The review now includes 33 studies (n=3445): 22 assessing treatment and 11 assessing prevention of oropharyngeal candidiasis. Six studies were done in developing countries, 16 in the United States of America and the remainder in Europe.
Treatment Treatment was assessed in the majority of trials looking at both clinical and mycological cures. In the majority of comparisons there was only one trial. Compared to nystatin, fluconazole favoured clinical cure in adults (1 RCT; n=167; RR 1.69; 95% CI 1.27 to 2.23). There was no difference with regard to clinical cure between fluconazole compared to ketoconazole (2 RCTs; n=83; RR 1.27; 95% CI 0.97 to 1.66), itraconazole (2 RCTs; n=434; RR 1.05; 95% CI 0.94 to 1.16), clotrimazole (2 RCTs; n=358; RR 1.14; 95% CI 0.92 to 1.42) or posaconazole (1 RCT; n=366; RR1.32; 95% CI 0.36 to 4.83). Two trials compared different dosages of fluconazole with no difference in clinical cure. When compared with clotrimazole, both fluconazole (2 RCTs; n=358; RR 1.47; 95% CI 1.16 to 1.87) and itraconazole (1 RCT; n=123; RR 2.20; 95% CI 1.43 to3.39) proved to be better for mycological cure. Both gentian violet (1 RCT; n=96; RR 5.28; 95% CI 1.23 to 22.55) and ketoconazole (1 RCT; n=92; RR 5.22; 95% CI 1.21 to 22.53) were superior to nystatin in bringing about clinical cure. A single trial compared gentian violet with lemon juice and lemon grass with no significant difference in clinical cure between the groups. Prevention Successful prevention was defined as the prevention of a relapse while receiving prophylaxis. Fluconazole was compared with placebo in five studies (5 RCTs; n=599; RR 0.61; 95% CI 0.5 to 0.74) and with no treatment in another (1 RCT; n=65; RR 0.16; 95% CI 0.08 to 0.34). In both instances the prevention of clinical episodes was favoured by fluconazole. Comparing continuous fluconazole treatment with intermittent treatment (2 RCTs; n=891; RR 0.65; 95% CI 0.23 to 1.83), there was no significant difference between the two treatment arms. Chlorhexidine was compared with normal saline in a single study with no significant difference between the treatment arms.
Authors' conclusions
Five new studies were added to the review, but their results do not alter the final conclusion of the review.
Implications for practice Due to there being only one study in children, it is not possible to make recommendations for treatment or prevention of OC in children. Amongst adults, there were few studies per comparison. Due to insufficient evidence, no conclusion could be made about the effectiveness of clotrimazole, nystatin, amphotericin B, itraconazole or ketoconazole with regard to OC prophylaxis. In comparison to placebo, fluconazole is an effective preventative intervention. However, the potential for resistant Candida organisms to develop, as well as the cost of prophylaxis, might impact the feasibility of implementation. No studies were found comparing fluconazole with other interventions. The direction of findings suggests that ketoconazole, fluconazole, itraconazole and clotrimazole improved the treatment outcomes.
Implications for research It is encouraging that low‐cost alternatives are being tested, but more research needs to be on in this area and on interventions like gentian violet and other less expensive anti‐fungal drugs to treat OC. More well‐designed treatment trials with larger samples are needed to allow for sufficient power to detect differences in not only clinical, but also mycological, response and relapse rates. There is also a strong need for more research to be done on the treatment and prevention of OC in children as it is reported that OC is the most frequent fungal infection in children and adolescents who are HIV positive. More research on the effectiveness of less expensive interventions also needs to be done in resource‐poor settings. Currently few trials report outcomes related to quality of life, nutrition, or survival. Future researchers should consider measuring these when planning trials. Development of resistance remains under‐studied and more work must be done in this area. It is recommended that trials be more standardised and conform more closely to CONSORT.
Plain language summary
Interventions for the prevention and management of oral thrush associated with HIV infection in adults and children
Oral candidiasis (thrush) associated with human immunodeficiency virus (HIV) infection occurs commonly and recurs frequently, often presenting as an initial manifestation of the disease. Interventions aimed at preventing and treating HIV‐associated oral thrush form an integral component of maintaining the quality of life for affected individuals. This review evaluated the effects of interventions in preventing or treating oral thrush in children and adults with HIV infection. Thirty three trials (n=3445) were included. Twenty two trials investigated treatment and eleven trials investigate prevention. There was no difference with regard to clinical cure between fluconazole compared to ketoconazole, itraconazole, clotrimazole and posaconazole. Fluconazole, gentian violet and ketoconazole were superior to nystatin. Compared to placebo and no treatment, fluconazole was effective in preventing clinical episodes from occurring. Continuous fluconazole was better than intermittent treatment. Insufficient evidence was found to come to any conclusion about the effectiveness of clotrimazole, nystatin, amphotericin B, itraconazole, ketoconazole or chlorhexidine with regard to OC prophylaxis.
Background
Description of the condition
Oral candidiasis (OC) associated with human immunodeficiency virus (HIV) infection occurs commonly and recurs frequently (Arribas 2000; Greenspan 1992; Gennaro 2008; Reznik 2005), often presenting as an initial manifestation of the disease (Coogan 2005; Epstein 1998; Nittayananta 1997; Rachanis 2001). Though Candida albicans is most commonly implicated, other organisms have also been identified. If left untreated these lesions contribute considerably to the morbidity associated with HIV infection. Interventions aimed at preventing and treating HIV‐associated oral candidal lesions form an integral component of maintaining the quality of life for affected individuals. For brevity's sake, the term oral HIV lesions will be used for oropharyngeal infections associated with HIV/AIDS infection.
The spectrum of oral HIV lesions seen include those of fungal, viral, bacterial and neoplastic origin. This review deals with oral candidiasis, the most common oral HIV lesion seen in both children and adults (Ramos‐Gomez 1999; Williams 1993). Four forms occur (Williams 1993) ‐ pseudomembranous candidiasis, erythematous candidiasis, angular chelitis and mixed candidal lesions. OC has been found to occur more commonly in those with advancing HIV infection (Klein 1984), often requiring more aggressive forms of treatment. The presence of OC, which can be painful, may lead to a reduction in food intake (Oude Lashof 2004), or a reduction in the correct kinds of food, with subsequent malnutrition, which may compromise an already ill patient even more. Additionally, OC has been found to lead to the loss of taste and smell in HIV‐infected patients and can subsequently impair the intake of food and contribute to wasting (Heald 1997; Paillaud 2004). Xerostomia, a reduction in the flow of saliva, is a common occurrence (Arendorf 1998). It may occur as a result of the disease process or secondary to medications used. Not only does this condition render the normal protective components of saliva less effective, but it also interferes with the solubility of topical antifungals. It has also been found that concomitant drug therapy with antibiotics may influence the colonization and proliferation of the yeast within the oral cavity.
Description of the intervention
The treatment of mucosal fungal infections is dominated by the azole compounds, which can be used either topically or systemically. The antifungal agents for the treatment of OC in adults, together with their recommended dosages, are listed in Table 26. Similarly the antifungal agents for the treatment of OC in children are listed in Table 27, together with their dosages, as recommended by Ramos‐Gomez 1999. Mucosal diseases do however have the propensity for some patients to suffer from repeated relapses (Rex 2000). The suppression of OC is possible with the use of topical agents such as clotrimazole or nystatin, or with systemic agents such as ketoconazole or fluconazole (Gallant 1994; Pankhurst 2005). The routine use of prophylactic treatment in patients with OC may lead to the development of resistance, especially to the azole antifungal agents like fluconazole. Resistance has been found to develop in patients with advanced HIV disease or after repeated or long‐term therapy for OC (Epstein 1998). There is however an overall lack of data on resistance following antifungal usage (Patton 2001; Ioannidis 2005).
1. Antifungal Agents for use in Adults.
| Administration | Drug | Form | Dosage |
| Topical | Amphotericin‐B | Lozenge | 10 000 iu dissolved slowly in the mouth 3‐4 times a day for a minimum of 2 weeks |
| Topical | Nystatin | Cream | Apply to affected area twice a day |
| Topical | Nystatin | Oral suspension | 20ml 4 times a day; continue to use for several days post clinical resolution |
| Topical | Nystatin | Pastille | Dissolve tablet in mouth 5 times a day |
| Topical | Clotrimazole | Solution | 5ml 3‐4 times a day for 2 weeks minimum |
| Topical | Clotrimazole | Cream | apply to affected area 2‐3 times a day for 3‐4 weeks |
| Topical | Miconazole | Oral gel | apply to the affected area 3‐4 times a day |
| Topical | Miconazole | Cream | Apply twice a day and continue to use 10‐14 days after lesions heal |
| Systemic | Fluconazole | 150mg Capsules | 150mg stat or one 150mg capsule once a day for 2‐3 weeks |
| Systemic | Ketaconazole | 200mg Tablets | One to two tablets twice a day with food for 2 weeks |
| Systemic | Itraconazole | 100mg Capsules | one capsule per day taken immediately after meals for 2 weeks |
| Topical | Chlorhexidine gluconate (0,2%) | Mouthwash | 10ml to be swirled in the mouth for 1 timed minute and then spat out |
| Topical | Gentian Violet (0,5%) | Aqueous solution | Paint on affected area(s) of mouth three times daily |
2. Antifungal Agents for use in Children.
| Administration | Drug | Form | Dosage |
| Topical | Nystatin | Oral suspension | 1 to 5 ml suspension five times per day |
| Topical | Nystatin | 100 000 U/ml vaginal tablets | 1 in nipple TID |
| Topical | Clotrimazole | 10 mg troches | five times per day |
| Topical | Clotrimazole | 100 000 u/ml vaginal tablets | 1 in nipple TID |
| Systemic | Fluconazole | 2‐5 mg per kg | Once a day |
| Systemic | Ketoconazole | 4‐6 mg per kg | Once or twice a day |
How the intervention might work
While antifungals are available as either topical or systemic agents, the choice of treatment is influenced by many variables. Current criteria for prescription of treatment are either arbitrary or determined by availability and affordability within particular clinical settings, or based on specified hospital protocols. In resource‐poor settings the availability is dependent on the cost of treatment.
Whilst antiretroviral therapy may not cure OC, evidence suggests that individuals on Highly Active Antiretroviral Therapy (HAART) have less frequent and severe occurrences (Munro 2002; Schmidt‐West 2000). An observational cohort study, Arribas 2000 found that in patients with advanced HIV infection, antiretroviral therapy including a protease inhibitor, had a positive impact on OC. Yang 2006 investigated the effect of prolonged HAART on OC and found that it is highly effective in decreasing OC in association with a rise in CD4+ lymphocyte count. It has been reported that the risk of having OC can be halved in patients treated with HAART (Hodgson 2006).
Why it is important to do this review
This systematic review evaluated the current evidence about interventions for the prevention and management of oro‐pharyngeal candidiasis associated with HIV infection in both adults and children. The differences, as well as the similarities, between the developing and the developed world were taken into account where possible when evaluating the available evidence.
Objectives
The objective of this review was to determine the effects of any intervention in preventing and treating oropharyngeal candidiasis in children and adults with HIV infection, by reviewing randomised controlled clinical trials (RCTs) only.
Methods
Criteria for considering studies for this review
Types of studies
For the primary purpose of determining the effects of any given intervention, only randomised controlled clinical trials (RCTs) of palliative, preventive or curative therapy were considered, irrespective of whether the control group received a placebo. Quasi‐randomised trials were excluded.
Types of participants
HIV‐positive adults and children (We defined children according to the Centre for Disease Control and Prevention(CDC) as being ≤13 years and adolescents or adults as being > 13 years, Osmond 1998).
Participants were receiving one or more of the following:
Treatment for OC;
Prophylactic treatment for OC;
HAART.
Types of interventions
Any intervention aimed at preventing, treating or palliating HIV‐associated OC.
These included:
HAART;
Traditional medicines;
Scaling and Polishing, curettage;
A combination of the above.
A comparison of any of these interventions against placebo or no treatment or another drug or intervention.
Types of outcome measures
Primary outcomes
Presence or absence of clinical lesions (Williams 1993)
Severity of the lesions (as defined by the study)
Microbiological measures e.g. candidal counts.
Secondary outcomes
Secondary outcomes:
Quality of life indicators (as defined by study)
Any adverse events such as hypersensitivity and development of resistant strains were reported if recorded.
Search methods for identification of studies
Electronic searches
The search strategy was based on that of the HIV/AIDS Cochrane Review Group. For the first review publication, the following electronic databases were searched 13 May 2005 for RCTs for the years 1982 to 2005: Medline; AIDSearch; EMBASE and CINAHL. The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were also searched through May 2005.
The abstracts of relevant conferences, including the International Conferences on AIDS and the Conference on Retroviruses and Opportunistic Infections, as indexed by AIDSearch, were also searched. The reference lists of all review articles and primary articles identified were also searched. The abstracts of the International Conference on AIDS and STDs in Africa (ICASA) were not reviewed as we were unable to obtain access to the abstracts of the past conferences.
The search strategy was iterative, in that references of included studies were searched for additional references. All languages were included.
A search was undertaken using MeSH terms and the full strategy is listed in Appendix 1 and Appendix 2. These strategies was combined with the search strategy for RCTs as recommended by The Cochrane Collaboration (Alderson 2004).
The updated database search was done using the original search terms in July 2009 for the period 2005 up to 2009 and including the date the search was done. The following databases were searched: Medline, EMBASE, the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. AIDSearch was not searched for the updated search as it ceased publication during 2008.
Data collection and analysis
Selection of studies
Abstracts of all trials identified by electronic or bibliographic scanning were examined by two authors (EP and TY) working independently. Where necessary, the full text was obtained to determine the eligibility of studies for inclusion. Full studies were not examined in instances where both authors agreed that the study was not a RCT.
Data extraction and management
Data from eligible trials was extracted and coded by two independent authors (EP and TY) using a standardised data extraction form. Where there were differences they were resolved by the review mentor, Nandi Siegfried. The following information was collected for each trial: type and dose of intervention used; duration of treatment; patient characteristics, including number of patients, gender, age, and co‐morbid conditions; adverse events and length of trial follow‐up. Also noted were the various diagnostic criteria used for the identification of lesions, these included presumptive as well as definitive criteria (Williams 1993). Where necessary authors were contacted for additional information.
Assessment of risk of bias in included studies
EP and TY independently examined the components of each included trial for risk of bias using a standard form. This included information on the sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), and incomplete outcome data. We did not assess selective outcome reporting and other sources of bias. The methodological components of the trials were assessed and classified as adequate, inadequate or unclear as per Chapter 8 the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Where differences arose, these were resolved by discussions with the mentor for the review.
Sequence generation
Adequate: investigators described a random component in the sequence generation process such as the use of random number table, coin tossing, cards or envelops shuffling etc.
Inadequate: investigators described a non‐random component in the sequence generation process such as the use of odd or even date of birth, algorithm based on the day/date of birth, hospital or clinic record number.
Unclear: insufficient information to permit judgment of the sequence generation process.
Allocation concealment
Adequate: participants and the investigators enrolling participants cannot foresee assignment, e.g. central allocation; or sequentially numbered, opaque, sealed envelopes.
Inadequate: participants and investigators enrolling participants can foresee upcoming assignment, e.g. an open random allocation schedule (e.g. a list of random numbers); or envelopes were unsealed or nonopaque or not sequentially numbered.
Unclear: insufficient information to permit judgment of the allocation concealment or the method not.
Described.
Blinding
Adequate: blinding of the participants, key study personnel and outcome assessor, and unlikely that the blinding could have been broken. Or lack of blinding unlikely to introduce bias. No blinding in the situation where non‐blinding is not likely to introduce bias.
Inadequate: no blinding, incomplete blinding and the outcome is likely to be influenced by lack of blinding.
Unclear: insufficient information to permit judgment of adequacy or otherwise of the blinding.
Incomplete outcome data
Adequate: no missing outcome data, reasons for missing outcome data unlikely to be related to true outcome,or missing outcome data balanced in number across groups.
Inadequate: reason for missing outcome data likely to be related to true outcome, with either imbalance in number across groups or reasons for missing data.
Unclear: insufficient reporting of attrition or exclusions.
Unit of analysis issues
Data analysis was conducted using Review Manager (RevMan) version 5.0.24 (2010).
The weighted mean difference was calculated for continuous outcomes with 95% confidence intervals (CIs).
For dichotomous / binary outcomes, the relative risk was calculated with 95% CIs and for time to event data the median survival time, hazard ratios and 95% CI were included.
Where trials were similar enough, we conducted a meta‐analysis. Use was made of the random effects model to calculate the overall measure of effect, as significant heterogeneity was anticipated. When trials did not allow for meta‐analysis, we reported the results reported by the investigators.
Assessment of heterogeneity
Testing for between study heterogeneity was carried out using the Chi2 and I2 provided by the RevMan software. The Chi2 test for heterogeneity was computed with a P value of 0.10 to determine statistical significance. The I2 statistic was computed to quantify inconsistency across studies. A stratified analysis of children (< 13 years) and adults was carried out. We also planned to explore any significant heterogeneity by analysis of the following subgroups:
WHO and CDC clinical disease staging (CDC 1992; WHO 1993; Table 28);
CD4 cell counts: (> 200 cells/ml; 50‐200 cells/ml; < 50 cells/ml; and
Study who were taking antiretroviral therapy or those who were not.
3. Clinical disease staging systems.
| CDC | WHO |
| A | I |
| B | II, III |
| C | IV |
However, this was not possible due to insufficient data. Instead we have reported any possible reasons for clinical heterogeneity in narrative form.
Sensitivity analysis
We were also not able to conduct a sensitivity analysis to test the robustness of the results as most comparisons included only one trial.
Results
Description of studies
Results of the search
For the first publication of the review in 2006, 40 studies were retrieved. Twenty‐eight trials with a total of 3225 participants met the inclusion criteria. During the updated search for the review a further six studies were identified. Of these, five studies met the inclusion criteria and were included in the review. The review now includes 33 studies with a total of 3445 participants. The flow‐diagram in Figure 1 illustrates the retrieval and selection of studies included in the review.
1.
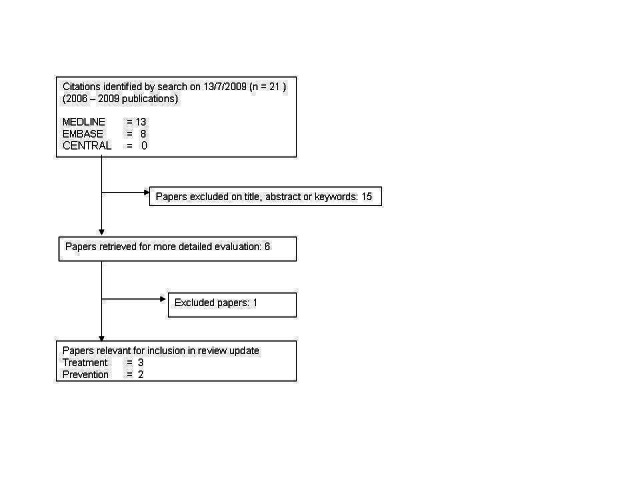
Flow diagram of study selection for review update
Included studies
As the review investigates both treatment and prevention of OC the review is structured to provide information for treatment and prevention separately
Treatment
Twenty‐two of the included trials looked at treatment (Arathoon 2002; Chavanet 1992; de Repentigny 1996; De Wit 1989; De Wit 1993; De Wit 1997; De Wit 1998; Graybill 1998a; Hamza 2008; Hernandez 1994; Linpiyawan 2000; Murray 1997; Nyst 1992; Phillips 1998a; Pons 1993; Pons 1997; Redding 1992; Smith 1991; Van Roey 2004; Vazquez 2002; Vazquez 2006; Wright 2009) of candidiasis. In one trial (Hernandez 1994) the participants were children aged between 7 weeks and 14 years. In three trials (Redding 1992; Smith 1991; Wright 2009) the age of the participants was not stated and in the remaining trials the participants were all adults.
Trials were conducted in different countries, in varying population groups and socioeconomic settings.
Eight of the included treatment trials were multicenter studies (de Repentigny 1996; De Wit 1997; Graybill 1998a; Hernandez 1994; Phillips 1998a; Pons 1993; Pons 1997; Vazquez 2006). Six trials were done in developing countries, namely in South Africa (Wright 2009), Tanzania (Hamza 2008), Thailand (Linpiyawan 2000; Nittayananta 2008), Uganda (Van Roey 2004), and Zaire (now the Democratic Republic of Congo) (Nyst 1992). Details of geographic location and whether studies are multi or single‐centre are described in Appendix 3.
Trials used different definitions for cure, ranging from subjective clinical assessment to the use of a formal scoring system. Mycological cure was based on culture and in some instances also on microscopy. One trial (Hernandez 1994) used a composite outcome consisting of clinical cure and eradication. Colony forming units are defined as the number of colony forming units per ml of oral rinse specimen (Samaranayake 1986). Treatment trials also followed patients who were cured post treatment to determine the relapse rate. Relapse was defined as either clinical recurrence of signs and/or symptoms, or mycological relapse.
Twelve of the trials reported CD4 cell counts Arathoon 2002; Chavanet 1992; de Repentigny 1996; De Wit 1997; De Wit 1998b; Graybill 1998a; Hamza 2008; Linpiyawan 2000; Phillips 1998a; Redding 1992; Smith 1991; Van Roey 2004). None of the trials investigating the treatment of oropharyngeal candidiasis recorded any information regarding antiretroviral treatment or HAART received by participants.
Prevention
Eleven of the included trials investigated the prevention of OC (Goldman 2005; Just‐Nubling 1991a; Leen 1990; MacPhail 1996; Marriott 1993; McKinsey 1999; Nittayananta 2008; Pagani 2002; Revankar 1998; Schuman 1997; Stevens 1991). In two trials (Just‐Nubling 1991a; Revankar 1998 ) the age of the participants was not stated, and in the remaining trials the participants were all adults.
Trials were conducted in different countries, in varying population groups and socioeconomic settings.
Two of the included prevention trials were multicenter studies (Goldman 2005; Schuman 1997). One trial was done in a developing country (Thailand Nittayananta 2008). Details of geographic location and whether studies are multi or single‐centre are described in Appendix 3
Eight trials reported CD4 cell counts (Goldman 2005; Just‐Nubling 1991a; MacPhail 1996; McKinsey 1999; Pagani 2002; Revankar 1998; Schuman 1997; Stevens 1991). Participants received antiretroviral treatment in two of the 11 included trials investigating prevention: in Marriott 1993 zidovudine was given to 25/44 patients in the intervention group and 18/40 in the placebo group; in Schuman 1997 antiretrovirals were given but no drugs were specified. They stated that 85% (138/162) of participants in the fluconazole group and 75% (121/161) in the placebo group received antiretrovirals.
Excluded studies
Sixteen of the identified studies (Barbaro 1995a; Barbaro 1995b; Blomgren 1998; Fichtenbaum 2000; Flynn 1995; Jandourek 1998; Lim 1991; Nebavi 1998; Moshi 1998Phillips 1996; Plettenberg 1994; Powderly 1995; Skiest 2007; Smith 2001; Soubry 1991; Uberti‐Foppa 1989) were excluded from the review. Three of these (Moshi 1998; Soubry 1991; Uberti‐Foppa 1989) were initially listed as still awaiting assessment as we tried to obtain additional information in order to assess their eligibility for inclusion. After several attempts to contact the authors failed it was decided to exclude them from the review. The reasons for exclusion of all studies are given in the table of Characteristics of excluded studies.
Risk of bias in included studies
When considering the risk of bias within the studies included in the review we only considered allocation, blinding and incomplete outcome data.
As with the description of the included studies the risk of bias for treatment and prevention studies are discussed separately.
The risk of bias assessment is summarised in Figure 2 and Figure 3
2.
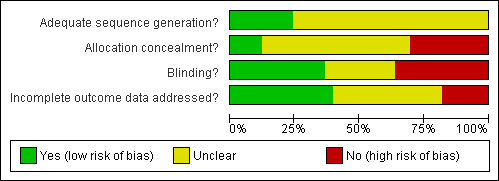
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
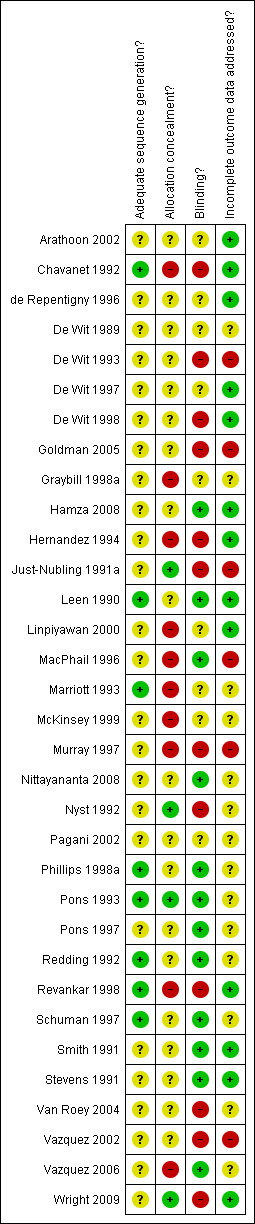
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Treatment
Allocation sequence generation
All trials stated that participants were randomised. One trial used a table of random numbers (Chavanet 1992) and three trials used computer generated randomisation sequences (de Repentigny 1996; Pons 1993; Redding 1992). One trial reported the use of block randomisation but did not describe how the allocation sequence was generated (Phillips 1998a ). The remainder did not describe how the allocation sequence was generated (Arathoon 2002; De Wit 1989; De Wit 1993; De Wit 1997; De Wit 1998b; Graybill 1998a; Hamza 2008; Hernandez 1994; Linpiyawan 2000; Murray 1997; Nyst 1992; Pons 1997; Smith 1991; Van Roey 2004; Vazquez 2002; Vazquez 2006; Wright 2009).
Four trials first stratified patients before randomisation into: oropharyngeal or oesophageal candidiasis (de Repentigny 1996); oropharyngeal candidiasis only or both oropharyngeal and esophageal candidiasis (Nyst 1992); HIV/AIDS or otherwise immunocompromised (Murray 1997); and AIDS‐related complex, or AIDS, or AIDS and esophageal candidiasis (Smith 1991).
Allocation concealment Allocation concealment was adequate in six trials (Chavanet 1992; De Wit 1997; Hamza 2008; Nyst 1992; Smith 1991; Wright 2009), inadequate in two (Graybill 1998a; Hernandez 1994) and unclear in the remaining trials.
Prevention
Allocation sequence generation
All trials stated that participants were randomised. Two trials used computer generated randomisation sequences Leen 1990; Marriott 1993). Two trials reported the use of block randomisation but did not describe how the allocation sequences were generated (Revankar 1998; Schuman 1997). The remainder did not describe how the allocation sequence was generated (Goldman 2005; Just‐Nubling 1991a; MacPhail 1996; McKinsey 1999; Nittayananta 2008; Pagani 2002; Stevens 1991).
Two trials first stratified patients before randomisation into: history of oropharyngeal candidiasis or no history of oropharyngeal candidiasis (MacPhail 1996) and CD4 count (≤ 50 vs > 50) and number of previous oropharyngeal episodes (< 2 vs ≥ 2) (Pagani 2002).
Allocation concealment Allocation concealment was adequate in one trial (Just‐Nubling 1991a), inadequate in one (Revankar 1998) and unclear in the remaining nine trials.
Blinding
Treatment
Six trials reported using double blinding (Arathoon 2002; de Repentigny 1996; De Wit 1989; De Wit 1997; Hamza 2008; Smith 1991). Placebos were used in all of these trials but did not state explicitly at which other point blinding occurred. Only providers were blinded in three trials (Pons 1993; Pons 1997; Redding 1992), and in four trials only the investigator party was blind (Graybill 1998a; Linpiyawan 2000; Murray 1997; Vazquez 2006). Both the participant and investigator were blinded in one trial(Phillips 1998a). In the rest no blinding was reportedly used (Chavanet 1992; De Wit 1993; De Wit 1998b; Hernandez 1994; Nyst 1992; Van Roey 2004; Vazquez 2002; Wright 2009).
Prevention
Patients, providers and investigators were blinded in one trial, Stevens 1991. Five trials reported using double blinding (Leen 1990; Marriott 1993; McKinsey 1999; Nittayananta 2008; Pagani 2002). Placebos were used in all of these trials but did not state explicitly at which other point blinding occurred. Both the participant and investigator were blinded in two trials (MacPhail 1996; Schuman 1997). In the rest of the trials no blinding was reportedly used (Goldman 2005; Just‐Nubling 1991a; Revankar 1998).
Incomplete outcome data
Treatment
Loss to follow‐up In 14 trials loss to follow‐up was less than 20%. In five trials (de Repentigny 1996; Graybill 1998a; Nyst 1992; Phillips 1998a; Smith 1991) loss to follow‐up was greater than 20%. Loss to follow‐up was unclear or not reported in three trials (Arathoon 2002; Murray 1997; Vazquez 2006).
Intention to Treat Analysis In six of the included studies it is indicated that intention‐to‐treat (ITT) analysis was done (Hamza 2008; Linpiyawan 2000; Pons 1993; Redding 1992; Vazquez 2006; Wright 2009). In two trials (Vazquez 2002; Vazquez 2006) the authors reported conducting a 'modified ITT' in that all randomised participants who received at least one dose of the study medication were included in the analysis. Fifteen trials did not include ITT (Arathoon 2002; Chavanet 1992; de Repentigny 1996; De Wit 1989; De Wit 1993; De Wit 1997; De Wit 1998; Graybill 1998a; Hernandez 1994; Murray 1997; Nyst 1992; Phillips 1998a; Pons 1997; Smith 1991; Smith 1991).
Prevention
Loss to follow‐up In five trials loss to follow‐up was less than 20%. In three trials (Goldman 2005; Marriott 1993; Stevens 1991) loss to follow‐up was greater than 20%. Loss to follow‐up was unclear or not reported in three trials (McKinsey 1999; Revankar 1998; Schuman 1997).
Intention to Treat Analysis In four of the included studies, it is indicated that intention‐to‐treat (ITT) analysis was done (Leen 1990; Marriott 1993; McKinsey 1999; Stevens 1991). Six trials did not include ITT (Goldman 2005; Just‐Nubling 1991a; MacPhail 1996; Nittayananta 2008; Pagani 2002;Revankar 1998). In one trial (Schuman 1997) it was not possible to determine whether or not they used ITT.
Effects of interventions
Treatment Twenty‐two of the included trials looked at treatment. Treatment success was assessed in the majority of trials by looking at both clinical and mycological cure. The number needed to treat (NNT) was calculated for those comparisons where the overall estimate of effect was statistically significant.
1) Fluconazole versus Ketoconazole Two trials, one in adults (De Wit 1989) (N = 37) and one in children (Hernandez 1994) (N = 46), compared oral fluconazole and ketoconazole. In the trial in adults, fluconazole was more effective than ketoconazole and favoured clinical cure (RR 1.50; 95% CI 1.04 to 2.15). This was, however, not the case in the study in children (RR 1.13; 95% CI 0.86 to 1.49) (Analysis 1.1). From the combined result for adults and children there was no significant difference between fluconazole and ketoconazole (RR 1.27; 95 %CI 0.97 to 1.66), and there was no significant heterogeneity (I2 32.4%; Chi2 1.48 with P = 0.22). Amongst adults (De Wit 1989) there was no significant differences in mycological cure. The trial in children did not give the results for mycological cure separately, but combined with clinical cure and that was also not significantly different between fluconazole and ketoconazole (Analysis 1.3).
1.1. Analysis.
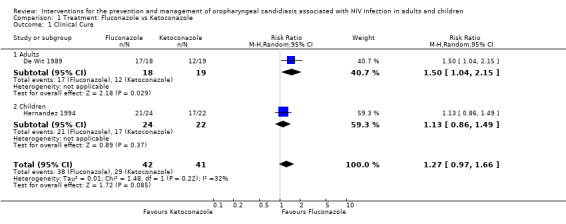
Comparison 1 Treatment: Fluconazole vs Ketoconazole, Outcome 1 Clinical Cure.
1.3. Analysis.
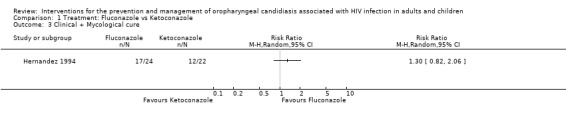
Comparison 1 Treatment: Fluconazole vs Ketoconazole, Outcome 3 Clinical + Mycological cure.
Mycologically confirmed relapses were more likely in patients receiving fluconazole. De Wit 1989 followed 13 of the 18 patients assigned to fluconazole, for one month post‐treatment during which time there were six relapses (46%) after a mean of 18 days (range 10 to 24 days) with fluconazole (18 randomised, and four lost to follow‐up) and, while there was one relapse in the ketoconazole group (19 randomised, 12 cured and three lost to follow‐up) 13 days after the end of treatment.
Gastrointestinal tract toxicity (GIT) was the main adverse effect. De Wit 1989 reported severe nausea in one fluconazole patient. Diarrhoea and abdominal pain occurred in one ketoconazole patient (Hernandez 1994), and an increase in the liver enzymes (ALT and AST) occurred more often in the ketoconazole group and is reported as being mild transitory laboratory abnormalities. One patient in the fluconazole group also had thrombocytopaenia (Hernandez 1994).
2) Fluconazole vs Itraconazole Three trials (N = 474) compared fluconazole with itraconazole (De Wit 1998; Graybill 1998a; Phillips 1998a). Graybill 1998a and Phillips 1998a both had three arms, one fluconazole and two itraconazole. In Graybill 1998a the itraconazole doses in the different arms were 200 mg per day for seven days and 200 mg per day for 14 days, in Phillips 1998a the doses in the two itraconazole arms were 100 mg per day for seven days and 14 days respectively. In order to include both itraconazole arms in the meta‐analysis the number of participants in the fluconazole arm was divided in two (Ramsay 2003). The combined RR for clinical cure was 1.12 (95% CI 0.92 to 1.36) with significant heterogeneity (I2 67.6%; Chi2 12.35 with P = 0.01) (Analysis 2.1).
2.1. Analysis.
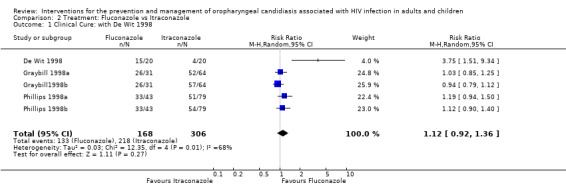
Comparison 2 Treatment: Fluconazole vs Itraconazole, Outcome 1 Clinical Cure: with De Wit 1998.
Because De Wit 1998 (RR 3.75; 95% CI 1.51 to 9.34) was an outlier, the meta‐analysis was repeated excluding this study. The revised analysis also did not indicate a benefit of one drug over the other, RR 1.05 (95% CI 0.94 to 1.16) with no significant heterogeneity (I2 4.3%; Chi2 3.14 with P = 0.37) (Analysis 2.2). Exploring sources of heterogeneity, mean CD4 cell counts were similar at baseline. Graybill 1998a, itraconazole (7 days) was 134 cells/mm3 (3 to 707), itraconazole (14 days) was 134 cells/mm3 and fluconazole 162 cells/mm3 (2 to 702). Phillips 1998a: fluconazole = 136 cells/ml; itraconazole (7 days) = 151 cells/ml and itraconazole (14 days) = 160 cells/mm3. De Wit 1998 reported 38 cells/mm3 for the fluconazole arm and 22 cells/mm3 for the itraconazole arm. None of the trials reported antiretroviral usage. De Wit 1998 however used itraconazole in tablet form as opposed to the oral solution used by both Graybill 1998a and Phillips 1998a and fluconazole as a single dose compared to a 2 week course in the other two studies.
2.2. Analysis.
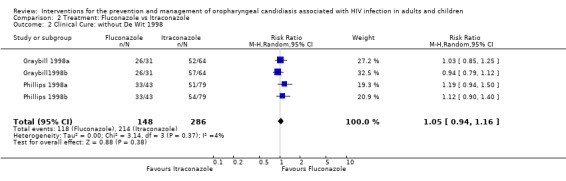
Comparison 2 Treatment: Fluconazole vs Itraconazole, Outcome 2 Clinical Cure: without De Wit 1998.
Use of fluconazole favoured mycological cure in one of the five trials. The combined RR indicated no benefit of one drug over the other (RR 1.14; 95% CI 0.90 to 1.46) with no significant heterogeneity (I2 43.0%; Chi2 7.02, P = 0.28) (Analysis 2.3). None of these showed a benefit in minimising post treatment relapse (RR 0.92; 95% CI 0.71 to 1.21) with no heterogeneity (Chi2 0.66, P = 0.96, I2 0%) (Analysis 2.4).
2.3. Analysis.
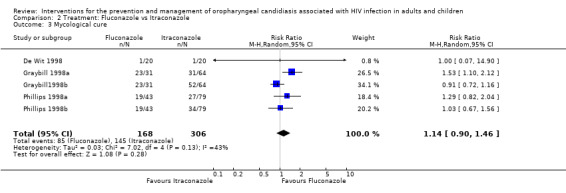
Comparison 2 Treatment: Fluconazole vs Itraconazole, Outcome 3 Mycological cure.
2.4. Analysis.
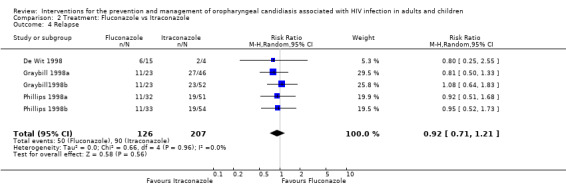
Comparison 2 Treatment: Fluconazole vs Itraconazole, Outcome 4 Relapse.
De Wit 1998 reported that no adverse events were experienced in either group. Graybill 1998a reported that 25% of participants in each arm experienced adverse events. Nausea, diarrhoea and abdominal pain were the most common events experienced. Respiratory side effects were experienced by 21% in the fluconazole arm and 12.5% in the itraconazole arm. Phillips 1998a reported no difference in the frequency of adverse events between the three treatment groups (33% in itraconazole twice a day and 48% in the itraconazole daily group and 43% in the fluconazole group). GIT symptoms were the most frequently reported adverse event. One participant in each of the treatment groups died of causes unrelated to the study drug.
3) Fluconazole vs Clotrimazole Two trials (Pons 1993; Redding 1992) (N = 358) compared fluconazole with clotrimazole. The combined RR for clinical cure (RR 1.14; 95% CI 0.92 to 1.42) using the random effects model indicated that no treatment was superior (Analysis 3.1). Fluconazole resulted in mycological cure in Pons 1993 but not in Redding 1992. The combined RR was 1.47; 95% CI 1.16 to 1.87 with no significant heterogeneity (I2 0%; Chi2 0.51; P=0.48) (Analysis 3.2). The NNT was calculated as 6 with 95% CI 4 to 15.
3.1. Analysis.
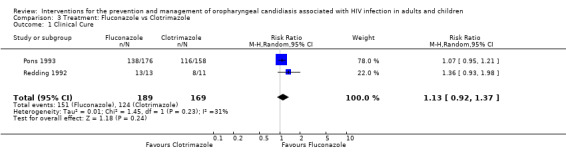
Comparison 3 Treatment: Fluconazole vs Clotrimazole, Outcome 1 Clinical Cure.
3.2. Analysis.
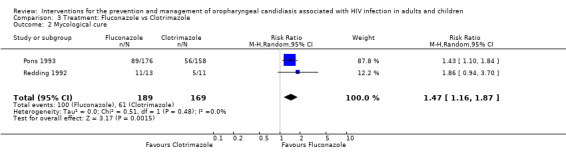
Comparison 3 Treatment: Fluconazole vs Clotrimazole, Outcome 2 Mycological cure.
By day 28 post treatment 1/13 (18%) fluconazole and 2/11 (18%) clotrimazole patients relapsed (Redding 1992) compared to 23/130 (18%) fluconazole and 48/96 (50%) clotrimazole (Pons 1993), combined RR 0.36; 95% CI 0.24 to 0.54 (no significant heterogeneity). While by day 42, four out of 13 (31%) fluconazole and five out of 11(45%) clotrimazole patients relapsed (Redding 1992) and 34/99 (34%) fluconazole patients and 23/58 (40%) clotrimazole patients relapsed (Pons 1993).
The reported adverse events were similar across both arms in Pons 1993 (18% in fluconazole vs 19% in clotrimazole) with GIT the most common. Less common events included headache, dizziness, pruritis, rash, sweating and dry mouth as well as liver function abnormalities. In the majority of cases the side‐effects were mild to moderate in severity. Two participants in the fluconazole and seven in the clotrimazole arms were withdrawn from the study because of non‐life threatening treatment adverse events. Redding 1992 reported that adverse events were infrequent in both treatment groups. No patients were withdrawn from therapy because of adverse events.
4) Fluconazole vs Fluconazole Two trials, De Wit 1993 (N = 56) and Hamza 2008 (n=220) compared different dosages of fluconazole. In De Wit 1993 one arm was 50 mg per day for 7 days and the other arm was 150 mg as a single dose. Hamza 2008 compared 150 mg per day for 14 days 750 mg as a single dose. The two studies were analysed as subgroups because of the difference in dosage as well as duration of treatment.
Clinical cure was not significantly different (Analysis 4.1). There was also no clear superiority between the dosages for mycological cure of OC (Analysis 4.2).
4.1. Analysis.
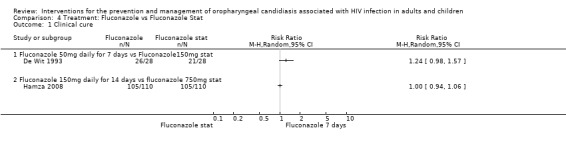
Comparison 4 Treatment: Fluconazole vs Fluconazole Stat, Outcome 1 Clinical cure.
4.2. Analysis.
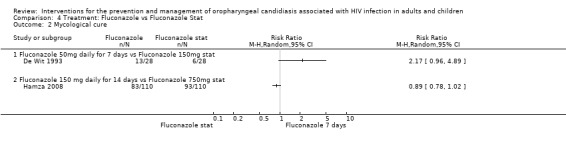
Comparison 4 Treatment: Fluconazole vs Fluconazole Stat, Outcome 2 Mycological cure.
In the De Wit 1993 study 26 patients were followed for two weeks after treatment (13 per treatment group). The relapse rates within the two treatment groups are compared in Analysis 4.3 and were not significantly different. Hamza 2008 followed 194 patients for 42 days after the commencement of treatment and reported 12 relapses out of 100 patients in the 150mg fluconazole arm and 12 out of 94 patients in the fluconazole stat arm.
4.3. Analysis.
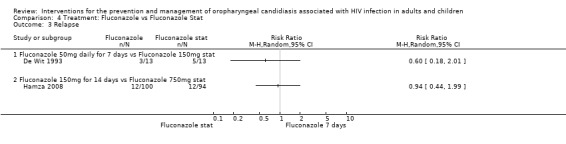
Comparison 4 Treatment: Fluconazole vs Fluconazole Stat, Outcome 3 Relapse.
Hamza 2008 reported the following adverse events: 14 day fluconazole: 6 patients reported gastrointestinal problems and for the single‐dose fluconazole: 6 nausea, vomiting, abdominal pain and/or diarrhoea; 1 headache; 1 heart palpitations and dizziness.
5) Fluconazole vs Nystatin One trial, Pons 1997 (N = 167) compared fluconazole with nystatin. Fluconazole favoured clinical cure, (RR 1.69; 95% CI 1.27 to 2.23) and mycological cure, (RR 10.37; 95% CI 3.89 to 27.66). For the outcome clinical cure, the NNT was calculated as 3 with 95%CI 2 to 7, and for mycological cure the NNT was 2 with 95%CI 2 to 3 (Analysis 5.1 and Analysis 5.2). GIT adverse events were the most common. One participant in each treatment group withdrew due to either nausea or vomiting. Liver enzymes were elevated in two participants in the fluconazole group. A sample of 13 participants per group was followed up for 2 weeks, 5/13 (38%) in the fluconazole stat group and 3/13 (23%) in the daily fluconazole group relapsed.
5.1. Analysis.
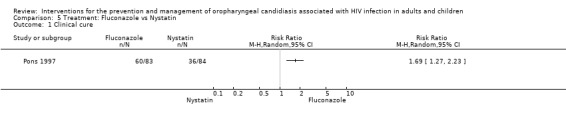
Comparison 5 Treatment: Fluconazole vs Nystatin, Outcome 1 Clinical cure.
5.2. Analysis.
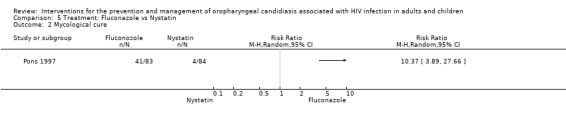
Comparison 5 Treatment: Fluconazole vs Nystatin, Outcome 2 Mycological cure.
6) D0870: 25 mg vs 10 mg One trial, De Wit 1997 (N = 27) compared different dosages of D0870, a new tri‐azole antifungal agent (Cartledge 1998, Yamada 1993). Neither dosage offered an advantages over the other in bringing about clinical cure, RR 0.97; 95% CI 0.59 to 1.58 (Analysis 6.1). Mean CD4 counts were different, however the reported ranges overlapped (100 mg D0870 = 48 cells/mm3; range 2 to 230 and 10 mg D0870 = 100 cells/mm3; range 2 to 355). By day 14 post treatment 2/13 (15%) of the 25 mg group and 3/14 (21%) of the other group relapsed (Analysis 6.2).
6.1. Analysis.
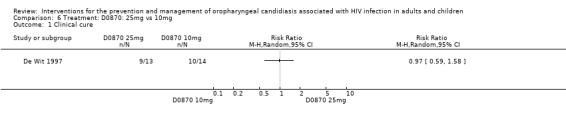
Comparison 6 Treatment: D0870: 25mg vs 10mg, Outcome 1 Clinical cure.
6.2. Analysis.
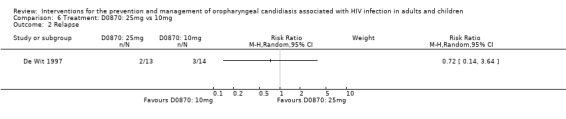
Comparison 6 Treatment: D0870: 25mg vs 10mg, Outcome 2 Relapse.
7) Itraconazole vs Clotrimazole Two trials (Linpiyawan 2000; Murray 1997) (N =152), compared itraconazole with clotrimazole. In Murray 1997 162 patients were enrolled. Of these, 123 were HIV+, 26 HIV‐ and the HIV status of the remaining 13 was unknown. For the purpose of this review, it was decided to use the 123 as the denominator. Itraconazole significantly favoured clinical cure in the Murray 1997 trial with the RR 2.03. Analysis 7.1: The combined RR 1.34; 95% CI 0.56 to 3.2, with significant heterogeneity (I2 84.9%; Chi2 6.60; P=0.01). The source of heterogeneity is unclear. Both trials used the same drug preparations and had similar participant profiles.
7.1. Analysis.
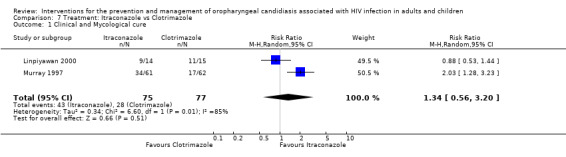
Comparison 7 Treatment: Itraconazole vs Clotrimazole, Outcome 1 Clinical and Mycological cure.
Only Murray 1997 reported on mycological cure as an outcome. Itraconazole favoured mycological cure (RR 2.20; 95% CI 1.43 to 3.39). Analysis 7.2: The NNT was calculated as 3 with 95% CI 2 to 5. One month post treatment, 46% of the itraconazole and 60% of the clotrimazole patients relapsed (Murray 1997). The median time to relapse was 31 days for itraconazole and 28 days for clotrimazole. In Linpiyawan 2000 3/9 (33%) patients in the itraconazole group and 5/5 (100%) patients in the clotrimazole group relapsed by week 4.
7.2. Analysis.
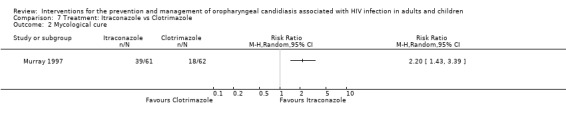
Comparison 7 Treatment: Itraconazole vs Clotrimazole, Outcome 2 Mycological cure.
In both studies, more participants receiving itraconazole developed GIT side effects. Two patients had transient elevation of liver enzymes (Linpiyawan 2000). Seven patients in the itraconazole group and three in the clotrimazole group had to discontinue participation prematurely as a result of adverse events (Murray 1997).
8) Melaleuca oral solution: alcohol free vs alcohol‐based One trial, Vazquez 2002 (N =27) compared an alcohol free melaleuca solution, also known as tea tree oil (Vazquez 2000), with an alcohol‐based solution of the same compound. Neither of the formulations offered any advantages in bringing about clinical cure (Analysis 8.1), mycological cure (Analysis 8.2) or preventing relapse (Analysis 8.3). By week four post treatment 0/2 (0%) alcohol‐free and 1/5 (20%) alcohol‐based patients had relapsed.
8.1. Analysis.

Comparison 8 Treatment: Melaleuca Oral Solution: Alcohol‐free vs Alcohol‐based, Outcome 1 Clinical cure.
8.2. Analysis.

Comparison 8 Treatment: Melaleuca Oral Solution: Alcohol‐free vs Alcohol‐based, Outcome 2 Mycological cure.
8.3. Analysis.

Comparison 8 Treatment: Melaleuca Oral Solution: Alcohol‐free vs Alcohol‐based, Outcome 3 Relapse.
Oral burning was experienced in eight participants receiving alcohol‐based solution and two receiving the alcohol‐free solution.
9) Amphotericin: Fat emulsion vs Glucose solution One trial, Chavanet 1992 (N =22) compared amphotericin in a fat emulsion with amphotericin glucose solution. The mean CD4 cell count for the glucose‐based solution group was 121 cells/mm3 and for the fat‐emulsion group it was 48 cells/mm3. Clinical cure was not significantly different between the two formulations (Analysis 9.1). Neither formulation provided any effect towards mycological cure (RR 1.0; 95%;CI 0.17 to 5.89) (Analysis 9.2).
9.1. Analysis.
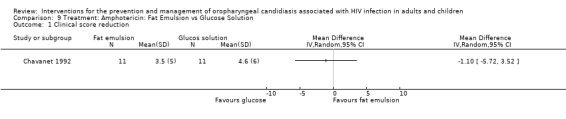
Comparison 9 Treatment: Amphotericin: Fat Emulsion vs Glucose Solution, Outcome 1 Clinical score reduction.
9.2. Analysis.
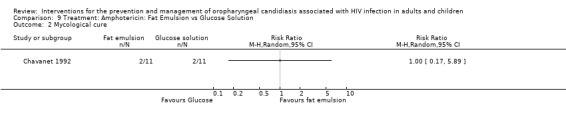
Comparison 9 Treatment: Amphotericin: Fat Emulsion vs Glucose Solution, Outcome 2 Mycological cure.
More frequent adverse events were experienced with the glucose preparation. Chills and fever were the most frequent side effects (66% vs 4%). Sweating and nausea were slightly less frequent in the fat emulsion group.
10) Itraconazole vs Ketoconazole Two trials (de Repentigny 1996, Smith 1991) (N =217) compared itraconazole with ketoconazole. The combined RR did not indicate the superiority of either treatment (Analysis 10.1). The mean days to clinical response was 32.4 ± 2.9 (95% CI 26.6 to 38.1) for itraconazole vs 28.9 ± 3.3 (95% CI 22.5 to 35.3) for ketoconazole (de Repentigny 1996). Mycological cure (de Repentigny 1996) was not favoured by either (RR 0.98; 95%CI 0.7 to 1.36) (Analysis 10.2). Within 21 days after treatment, 11/46 (24%) itraconazole and 15/52 (29%) ketoconazole patients relapsed (de Repentigny 1996). For Smith 1991 the relapse rate was > 80% in both arms within 3 months (Analysis 10.3).
10.1. Analysis.
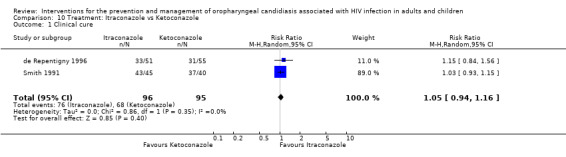
Comparison 10 Treatment: Itraconazole vs Ketoconazole, Outcome 1 Clinical cure.
10.2. Analysis.
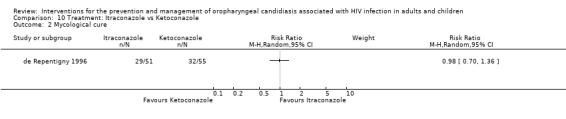
Comparison 10 Treatment: Itraconazole vs Ketoconazole, Outcome 2 Mycological cure.
10.3. Analysis.
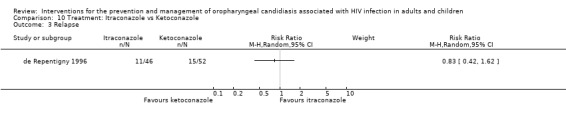
Comparison 10 Treatment: Itraconazole vs Ketoconazole, Outcome 3 Relapse.
While de Repentigny 1996 reported no significant differences in adverse event rate between the treatment groups, Smith 1991 reported that five patients had to stop ketoconazole due to serious toxic events (2 nausea, 2 hepatotoxicity and 1 generalised erythematous rash). One patient receiving itraconazole developed a maculopapular rash.
11)Ketoconazole vs Miconazole One trial, Van Roey 2004 (N = 357) compared ketoconazole with miconazole. The mean CD4 cell counts were similar with miconazole (102.3 ± 14.5 cells/mm3) and ketoconazole (109.5 ± 12.88 cells/mm3). Neither intervention clearly favoured clinical cure (RR 1.02; 95% CI 0.94 to 1.10). Of the ketoconazole patients 34/148 (23%) and 45/146 (31%) miconazole patients relapsed (RR 0.75; 95% CI 0.51 to 1.09). Fewer drug related adverse events were noted in the miconazole group.
Gentian Violet vs Ketoconazole vs Nystatin One trial, Nyst 1992, (N = 141), consisted of three intervention arms. Gentian violet (N = 49), ketoconazole (N = 45) and nystatin (N = 47). This trial was analysed in three separate comparisons as outlined below in comparisons 12 to 14. Two patients receiving gentian violet developed irritation and small superficial ulcers of the oral mucosa 24 hours after the start of therapy.
12) Gentian Violet vs Ketoconazole When comparing gentian violet with ketoconazole, clinical cure (Analysis 12.1) and mycological cure (Analysis 12.2) was not significantly different.
12.1. Analysis.
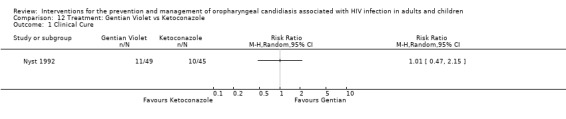
Comparison 12 Treatment: Gentian Violet vs Ketoconazole, Outcome 1 Clinical Cure.
12.2. Analysis.
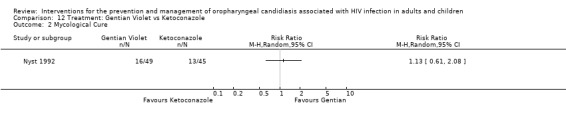
Comparison 12 Treatment: Gentian Violet vs Ketoconazole, Outcome 2 Mycological Cure.
13) Gentian Violet vs Nystatin When comparing gentian violet with nystatin, gentian violet favoured clinical cure (RR 5.28; 95% CI 1.23 to 22.55) (Analysis 13.1). Gentian violet favoured mycological cure (RR 5.12; 95% CI 1.59 to 16.42) (Analysis 13.2). The NNT was calculated as 6 with 95% CI 3 to 20 for clinical cure and as 4 with 95% CI 2 to 9 for mycological cure.
13.1. Analysis.
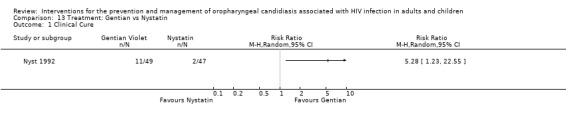
Comparison 13 Treatment: Gentian vs Nystatin, Outcome 1 Clinical Cure.
13.2. Analysis.
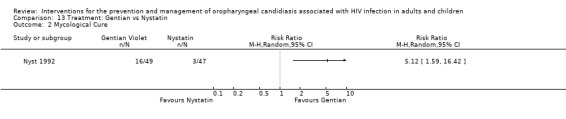
Comparison 13 Treatment: Gentian vs Nystatin, Outcome 2 Mycological Cure.
14) Ketoconazole vs Nystatin In the comparison of ketoconazole with nystatin, ketoconazole favoured clinical cure with RR 5.22 95% CI 1.21 to 22.53 (Analysis 14.1). Ketoconazole favoured mycological cure (RR 4.53; 95% CI 1.38 to 14.83) (Analysis 14.2). The NNT was calculated as 6 with 95% CI 3 to 23 for clinical cure and as 4 with 95% CI 3 to 13 for mycological cure.
14.1. Analysis.
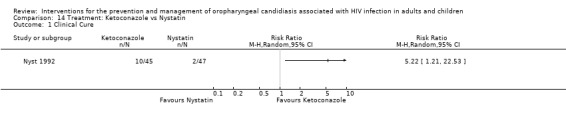
Comparison 14 Treatment: Ketoconazole vs Nystatin, Outcome 1 Clinical Cure.
14.2. Analysis.
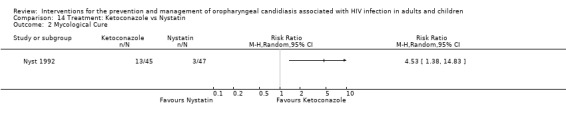
Comparison 14 Treatment: Ketoconazole vs Nystatin, Outcome 2 Mycological Cure.
15) Caspofungin vs Amphotericin B One trial, Arathoon 2002 (N = 52), compared intravenous caspofungin with intravenous amphotericin B. Compared to other treatment options for OC, these two interventions are very expensive (Klotz 2006). Neither treatment showed any superiority, see Analysis 15.1. Mycological cure was reported as more than 75% in each of the treatment arms. Relapse during the month following discontinuation of treatment was as high as 37% and was similar among the treatment groups. Significantly fewer patients receiving caspofungin developed drug‐related fever, chills, nausea or vomiting. The incidence of local reactions (infusion related) ranged from 6 ‐ 14% across treatment arms. Drug‐related laboratory abnormalities (raised ALT, AST, ALP, creatinine and decreased potassium) were more common in patients receiving amphotericin B (Analysis 15.1).
15.1. Analysis.
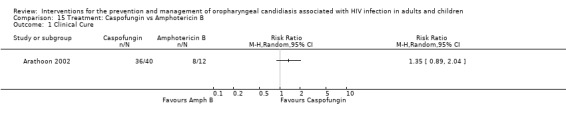
Comparison 15 Treatment: Caspofungin vs Amphotericin B, Outcome 1 Clinical Cure.
16) Posaconazole vs Fluconazole One trial, Vazquez 2006 (N=366), compared posaconazole with fluconazole. Clinical cure was not significantly different (RR 1.32; 95% CI 0.36 to 4.83). Mycological cure was significantly higher with posaconazole (RR 1.24; 95% CI 1.01 to 1.52), whereas the results for mycological eradication were not statistically significant (RR 1.18; 95% CI 0.98 to 1.42).
Analysis 16.1; Analysis 16.2; Analysis 16.3
16.1. Analysis.
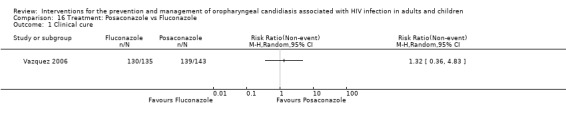
Comparison 16 Treatment: Posaconazole vs Fluconazole, Outcome 1 Clinical cure.
16.2. Analysis.
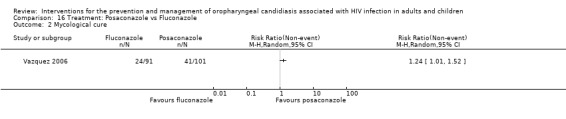
Comparison 16 Treatment: Posaconazole vs Fluconazole, Outcome 2 Mycological cure.
16.3. Analysis.
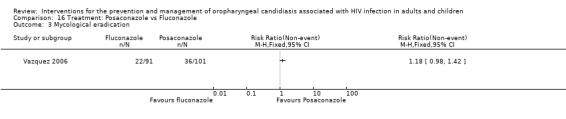
Comparison 16 Treatment: Posaconazole vs Fluconazole, Outcome 3 Mycological eradication.
Lemon juice vs Lemon Grass vs Gentian Violet One trial, Wright 2009 (N = 90), consisted of three intervention arms. Lemon juice (N = 30), lemon grass (N = 30) and gentian violet (N = 30). This trial was analysed in three separate comparisons as outlined below in comparisons 17 to 19. The adverse events reported for the gentian violet group were purple discolouration, cracked lips and dry mouth. In the lemon juice group, the events were reported as changed taste in the mouth, and for the lemon grass group only one adverse event was reported namely increased appetite.
17) Lemon Juice vs Gentian Violet When comparing lemon juice with gentian violet, clinical cure was not significantly different (RR 1.78; 95% CI 0.94 to 3.37) (Analysis 17.1). Clinical failure was more likely in the gentian violet group than the lemon juice group (RR 0.25; 95% CI 0.06 to 1.08) (Analysis 17.2) but not statistically significant. .
17.1. Analysis.
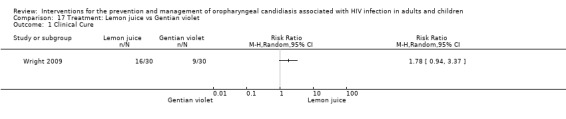
Comparison 17 Treatment: Lemon juice vs Gentian violet, Outcome 1 Clinical Cure.
17.2. Analysis.
Comparison 17 Treatment: Lemon juice vs Gentian violet, Outcome 2 Clinical failure.
18) Lemon Grass vs Gentian Violet When comparing lemon grass with gentian violet, clinical cure was not significantly different (RR 1.67; 95% CI 0.87 to 3.20) (Analysis 18.1). Clinical failure was more likely in the gentian violet group (RR 0.25; 95% CI 0.06 to 1.08) (Analysis 18.2) but not statistically significant.
18.1. Analysis.
Comparison 18 Treatment: Lemon grass vs gentian violet, Outcome 1 Clinical cure.
18.2. Analysis.
Comparison 18 Treatment: Lemon grass vs gentian violet, Outcome 2 Clinical Failure.
19) Lemon juice vs Lemon grass In the comparison of lemon juice with lemon grass, there was no difference between the two groups relating to clinical cure (Analysis 19.1). When looking at clinical failure there was also no difference between the two groups (Analysis 19.2).
19.1. Analysis.
Comparison 19 Treatment: Lemon juice vs Lemon grass, Outcome 1 Clinical cure.
19.2. Analysis.
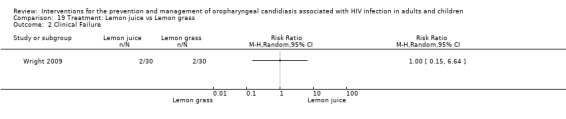
Comparison 19 Treatment: Lemon juice vs Lemon grass, Outcome 2 Clinical Failure.
Prevention
Eleven RCTs looked at prevention. Successful prevention was defined as the prevention of a relapse (a reduction in the number of clinical episodes) occurring while receiving prophylaxis.
1) Nystatin vs Placebo One trial, MacPhail 1996 (N = 128), compared different dosages of Nystatin (Two nystatin pastilles of 200 000 U and one Nystatin pastilles of 200 000 U) with placebo. The participants were stratified into a group with no previous history of OC (median CD4 330 cells/mm3 (range 3 to 752)) and those with a history of OC (median CD4 166 cells/ mm3 (range 2 to 888)) with each strata having three arms. Comparing Nystatin with placebo there was no significant difference (combined RR 0.85; 95% CI 0.69 to 1.05) (Analysis 20.1). When comparing the two different dosages, thus excluding the placebo arms (N = 43), the prevention of clinical episodes was favoured by 2 Nystatin pastilles of 200 000 U in both strata (combined RR 0.70; 95% CI 0.50 to 0.99) (Analysis 21.1).
20.1. Analysis.
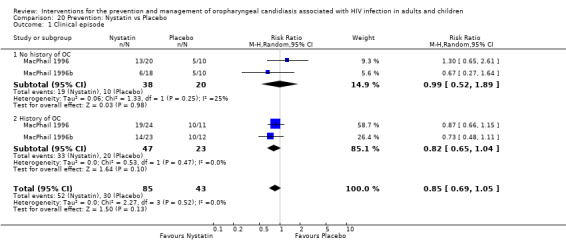
Comparison 20 Prevention: Nystatin vs Placebo, Outcome 1 Clinical episode.
21.1. Analysis.
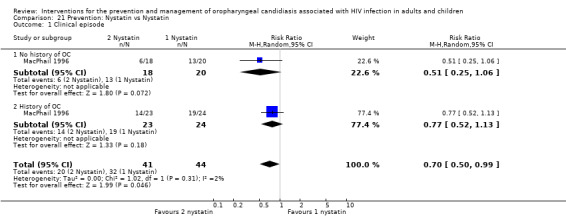
Comparison 21 Prevention: Nystatin vs Nystatin, Outcome 1 Clinical episode.
2) Fluconazole vs Placebo Five trials, Leen 1990; Stevens 1991; Marriott 1993; Pagani 2002; Schuman 1997, (N = 599) compared fluconazole with placebo. The prevention of clinical episodes was favoured by fluconazole (RR 0.61; 95% CI 0.50 to 0.74), with no significant heterogeneity (I2 11.5%; Chi2 4.52, P = 0.34) see Analysis 22.1. The NNT was calculated as 4 with 95% CI 3 to 6.
22.1. Analysis.
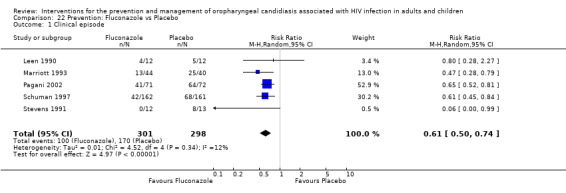
Comparison 22 Prevention: Fluconazole vs Placebo, Outcome 1 Clinical episode.
In Marriott 1993 mycologically confirmed relapse occurred in 12/25 (48%) fluconazole and 17/18 (94%) placebo patients. In Schuman 1997 clinical resistance developed in six fluconazole and seven placebo patients. Pagani 2002 reported the number of relapses per patient as well as the time to relapse (median time to first relapse was 175 days for fluconazole vs 35 days for placebo). Clinical resistance observed in five patients was associated with isolation of a C. albicans strain resistant to fluconazole. This was observed during the study in two patients in the fluconazole group and one in the placebo group, and also within one month of the study end in two patients in the fluconazole group. These patients had a cumulative dose of fluconazole before study entry of a mean value of 8.7 g compared with 2.9 g in patients without clinical failure.
In Marriott 1993 zidovudine was given to 25 out of 44 patients in the treatment group and 18 out of 40 in the placebo group. Schuman 1997 reported that antiretrovirals were given but does not indicate which drug was given. They state that 85% (138/162) participants in the fluconazole group and 75% (121/161) in the placebo group received antiretrovirals.
Leen 1990 reported that diarrhoea developed in one patient shortly after receiving fluconazole. Marriott 1993 reported that in the fluconazole group 40 intercurrent illnesses, nine adverse drug reactions and three deaths occurred, and in the placebo group there were five intercurrent illnesses, one adverse drug reaction and two deaths. Stevens 1991 reported no differences between the two groups with regard to adverse events.
Leen 1990 did not report on CD4 cell count, Stevens 1991 reported that 11 patients had a CD4 cell count of less than 200 cells/mm3 and in Marriott 1993 the median for the fluconazole group was 18 cells/mm3 with a range of 0 ‐ 299, and in the placebo group it was 38 cells/mm3 with a range of 0‐200. Schuman 1997 reported a median CD4 cell count of 172 cells/mm3 in the fluconazole group and 186 cells/mm3 in the placebo group. In Schuman 1997, 31% in the fluconazole group and 25 % in the placebo group had a CD4 cell count of less than 100 cells/mm3. Pagani 2002 reported no difference in CD4 cell count between the different groups within the study.
3) Fluconazole vs No treatment One trial, Just‐Nubling 1991a (N = 65) compared fluconazole with no treatment. In the fluconazole group there were two arms with different dosages, i.e. 50 mg (N = 20) and 100 mg (N = 22). In order to include both fluconazole arms in the meta‐analysis the number of participants in the no‐treatment arm was divided in two (Ramsay 2003). The prevention of clinical episodes was favoured by fluconazole in both dosage arms with RR 0.10 (95% CI 0.03 to 0.40) and 0.20 (95% CI 0.08 to 0.49) respectively. The meta‐analysis indicates that fluconazole was effective in the prevention of OC ( RR 0.16; 95%CI 0.08 to 0.34) (Analysis 23.1), with no significant heterogeneity (I2 0%; Chi2 0.65, P = 0.42). The NNT was calculated as 1 with 95%CI; 1 to 2. Just‐Nubling 1991a reported on the stage of patients disease as well as the number of relapses per patient. In the no‐treatment group 20/21 patients experienced 60 relapses, in the 50 mg fluconazole group 2/18 patients experienced 4 relapses and in the 100 mg fluconazole group 4/19 patients experienced 9 relapses. Just‐Nubling 1991a reported allergic exanthema as an adverse event in the fluconazole group. All patients had a CD4 cell count of less than 100 cells/mm3.
23.1. Analysis.
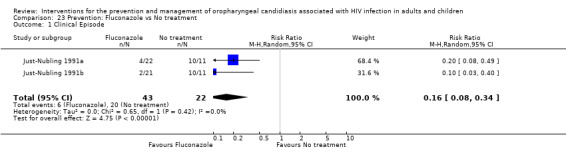
Comparison 23 Prevention: Fluconazole vs No treatment, Outcome 1 Clinical Episode.
4) Itraconazole vs Placebo One trial, McKinsey 1999 (N = 298), compared itraconazole with placebo. Only data from participants who met the eligibility criteria, were enrolled and received at least one dose of the test medication were reported. They enrolled 298 patients of whom three were ruled to be ineligible, two withdrew consent before receiving the study drug and one was taking disallowed medication. It is not clear from the text whether randomisation was done before or after the exclusion of these three participants.
Itraconazole was not superior to placebo (Analysis 24.1). Diarrhoea, nausea, vomiting, elevated liver enzyme levels, rash and Stevens‐Johnson syndrome were more common adverse events in the itraconazole arm of the study. The median CD4 cell count in the placebo group was 63 cells/mm3 and 57 cells/mm3 in the itraconazole group.
24.1. Analysis.
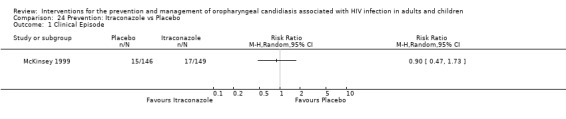
Comparison 24 Prevention: Itraconazole vs Placebo, Outcome 1 Clinical Episode.
5) Fluconazole: Intermittent vs Continuous
Two studies, Goldman 2005 and Revankar 1998 (N=891), compared the continuous use of fluconazole with intermittent use.
In Revankar 1998 the continuous use favoured the prevention of clinical episodes (RR 0.37; 95% CI 0.15 to 0.92). In the Goldman 2005 study there was no clear superiority between the two treatment arms (RR1.05; 95% CI 0.55 to 2.01).
The combined RR was 0.65 (95%CI 0.23 to 1.83) with significant heterogeneity (I2 71%; Chi2 3.40 with P=0.07) (Analysis 25.1). Possible sources of heterogeneity could be the difference in sample size between the studies, Goldman 2005( N=829) and Revankar 1998 (N=26), and also the difference in fluconazole dosage and length of treatment period.
25.1. Analysis.
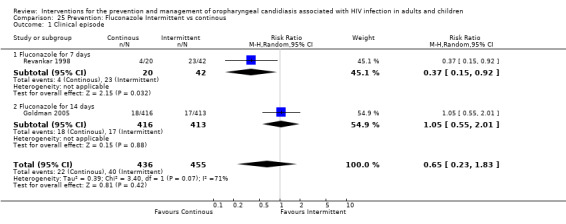
Comparison 25 Prevention: Fluconazole Intermittent vs continous, Outcome 1 Clinical episode.
Revankar 1998 reported resistance developing amongst 13/28 (46%) patients in the intermittent group and 9/16 (56%) patients in the continuous group. In the intermittent group 23/28 (82%) patients experienced relapses vs 4/16 (25%) in the continuous group. No information was given on adverse events. The mean CD4 cell count of patients on continuous therapy was 43 ± 37 cells/mm3 with a range of 4 to 116 compared with 44 ± 51 cells/mm3 with a range of 4 to 191 in patients receiving intermittent therapy. Goldman 2005 reported a total of 14 adverse events with four in the episodic arm (n=416) and 10 in the continuous arm (n=413). The median CD4 cell count of patients on continuous therapy was 52 cells/mm3 with the range of 0‐250 compared to 50 cells /mm3 with a range of 0‐209 in patients receiving intermittent therapy.
6) Chlorhexadine vs Normal Saline
One study, Nittayananta 2008 (N=75), compared the use of chlorhexidine with normal saline to prevent relapse of OC. The study reported the number of days from when patients started using the mouth‐rinse until relapse. Time to recurrence of OC was not statistically significant. The time to recurrence counted by number of visits ranged from 1‐ 15 (median 3) in the chlorhexidine group and 1‐8 (median 2) in the normal saline group (reported p>0.05).
Discussion
As the results of the review are divided into the treatment and prevention of oro‐pharyngeal candidiasis the discussion is structured in the same format.
Treatment A number of treatment options, both topical and systemic, are available for the treatment of oropharyngeal candidiasis. The main classes of antifungal agents used for the treatment of oral candidiasis are the polyenes (e.g. nystatin and amphotericin), imidazoles (e.g. clotrimazole) and the tri‐azoles such as fluconazole (Hunter 1998). Even though topical agents may be effective, they are very often unpalatable and their use can also be inconvenient (De Wit 1998b).
In this review, 22 trials investigating the treatment of OC met our inclusion criteria. For the outcome clinical cure, fluconazole was more effective than nystatin, had similar effects as posaconazole and had no difference in effect compared to ketoconazole, itraconazole and clotrimazole. The effect of itraconazole was the same as that of clotrimazole and ketoconazole. Gentian violet and ketoconazole had similar effects and were each better than nystatin. Ketoconazole also had the same effect as miconazole. In contrast to clinical cure, both fluconazole and itraconazole were better than clotrimazole for mycological cure, that is a negative Candida culture or negative KOH microscopic preparation.
One new study Wright 2009 compared gentian violet with lemon juice and lemon grass as treatment for the OC. There was no significant difference in effect between the three different interventions.
In a previously published review, Patton and colleagues (Patton 2001) found that the efficacy of fluconazole ranged from 87% to 100%, bringing about a complete clinical response, that is the absence of signs or symptoms of OC, or both. This is similar to what was found in this review, namely that the effectiveness of fluconazole in bringing about clinical cure of OC is the highest, followed by itraconazole. This is also similar to the findings of a trial comparing fluconazole with itraconazole for the treatment of OC in cancer patients (Oude Lashof 2004). The fact that itraconazole did not give significant results could be explained by drug interactions and also unpredictable absorption of itraconazole capsules (De Wit 1998b).
The sample size of the treatment trials ranged from 22 to 357 with ten trials having less than 100 participants. With such small sample sizes it is thus not possible to draw any real robust conclusions. Some trials had a very high loss to follow‐up creating underpowered studies. Due to the limited number of studies per comparison combined with the small sample size of the majority of studies the meta‐analysis did not assist in raising the power of the comparison to such an extent as to allow for meaningful results in most comparisons.
The trials did not always use the same outcome measures which also made it more difficult to combine the results. If trials were standardised and conformed to CONSORT (Altman 1996; Moher 1987) it would improve research, reporting and hence also clinical practice.
Five of the 22 studies investigating treatment of OC were conducted in developing countries (Hamza 2008; Linpiyawan 2000; Nyst 1992; Van Roey 2004; Wright 2009). Lack of availability and cost of some of the drugs in resource‐poor countries may limit the relevance to all settings.
In addition this review is not able to make definitive recommendations regarding the treatment of OC in HIV‐positive children as only one trial with children was found (Hernandez 1994). The sample size of the trial was also small (n=48).
Prevention Eleven trials investigated the prevention of OC in adults. As no trials investigating prevention in children was found this review is not able to make recommendations regarding OC prevention in HIV‐positive children. Compared to placebo and no treatment, fluconazole was effective in preventing clinical episodes from occurring. Revankar 1998 found that continuous fluconazole was more effective than intermittent treatment. In contrast, both nystatin and itraconazole were not effective in preventing OC. A review of studies of the use of nystatin in immunodepressed patients also found similar results (Gotzsche 2005). No trial was found that investigated the prevention of OC in children.
Only one study reported drug resistance testing for Candida species where there had been prophylactic failure (Pagani 2002). None of the studies reported on the impact of compliance with treatment on the results of studies. Compliance is important as failure of prophylaxis might be due to the patient's inability or unwillingness to adhere to the therapy rather than the true reflection of the treatment efficacy (Patton 2001).
Fluconazole, a bis‐tri‐azole antifungal agent, is not altered by gastric acidity and therefore has less risk of hepatotoxicity. There is however concern that prolonged use of fluconazole increases the risk of developing azole‐resistant Candida albicans (Martin 1999; Patton 2001). When the decision has to be made whether to provide prophylaxis for OC it is necessary to weigh the risks and cost against the benefits. In patients who are HIV positive, it is rare for OC to develop into possible fatal fungemia or even systemic candidiasis (Just‐Nubling 1991a) which makes waiting until the OC appears before starting treatment an alternative to prophylaxis.
Although the use of fluconazole reduces the risk of OC in patients with advanced HIV it is not recommended as primary prophylaxis because there is potential for resistant Candida organisms to develop as well as the cost of prophylaxis. For the same reasons, chronic prophylaxis is also not recommended (CDC 1999). In patients with low CD4 cell counts, the prolonged use of systemically absorbed azoles increases their risk of developing azole resistance.
The number of participants enrolled in the studies ranged from 13 to 323 with five of the nine included studies investigating prevention having less than 100 participants. Some trials had a very high loss of participants to follow‐up creating underpowered studies. Due to the limited number of studies per comparison combined with the small sample size of the majority of studies, the meta‐analysis did not assist in raising the power of the comparison to such an extent as to allow for meaningful results in most comparisons.
As in the case of the treatment trials, prevention trials did not always use the same outcome measures which made it more difficult to combine the results. Again standardisation and conforming closely to CONSORT (Altman 1996; Moher 1987) will improve research, reporting and clinical practice. None of the included studies investigating the prevention of OC were conducted in resource‐poor settings and this may limit the relevance of our results in some settings. As no trials investigating the prevention of OC in children were identified, this review is unable to come to any conclusion as to the prevention of OC in children.
HAART None of the included studies investigated the effects of HAART or any other form of antiretroviral treatment on OC treatment or prevention. The use of antiretroviral therapy in HIV infection may be associated oral lesions related to its side effects as well as reconstitution of the immune system (IRIS). While protease inhibitors have been shown to directly attenuate the adherence of Candida albicans to epithelial cells in vitro (Bektic 2001), (Cauda 1999; Cassone 1999). The impact of this intervention warrants further investigation with regard to clinical presentation and mycological effect.
Economics None of the trials provided any information on the cost‐effectiveness of either treatment or prophylaxis of OC. There is evidence that the cost‐effectiveness of prophylaxis of HIV‐related opportunistic infections varies widely, but no specifics were provided on OC (Freedberg 1998). In general, azoles are the more expensive compounds, with ketoconazole being cheaper, but with more side‐effects. One trial (Nyst 1992) reported the cost of gentian violet, nystatin and ketoconazole in Africa as this could have a major impact on the choice of treatment. Gentian violet is much cheaper at 0.5 US $/30 ml than ketoconazole (13‐17 US$/10 tablets) and nystatin oral suspension (of which 4 bottles of 2.4 million units are necessary per treatment course at 4‐5 US$/bottle).
Authors' conclusions
Implications for practice.
Four new studies were added to the review, but their results does not alter the final conclusion of the review.
Due to only one study in children it is not possible to make recommendations for treatment or prevention of OC in children. Amongst adults, there were few studies per comparison. Insufficient evidence was found to come to any conclusion about the effectiveness of clotrimazole, nystatin, amphotericin B, itraconazole or ketoconazole with regard to OC prophylaxis. The direction of findings suggests that ketoconazole, fluconazole, itraconazole and clotrimazole improved the treatment outcomes. In comparison to placebo, fluconazole is an effective preventative intervention. However, the potential for resistant Candida organisms to develop as well as the cost of prophylaxis might impact on the feasibility of implementation. No studies were found comparing fluconazole with other interventions.
Implications for research.
It is encouraging that low‐cost alternatives are being tested, but more research needs to be on in this area and interventions like gentian violet and other less expensive anti‐fungal drugs to treat OC to be evaluated in larger studies. More well designed treatment trials with larger sample size are needed to allow for sufficient power to detect differences in not only clinical, but also mycological response and relapse rates. There is also a strong need for more research to be done on the treatment and prevention of OC in children as it is reported that OC is the most frequent fungal infection in children and adolescents who are HIV positive. More research on the effectiveness of less expensive interventions also needs to be done in resource‐poor settings. Currently few trials report outcomes related to quality of life, impact on daily activities, nutrition, or survival. Future researchers should consider measuring these when planning trials. Development of resistance remains under‐studied and more work must be done in this area. Oral lesions associated with HIV form part of the clinical spectrum of immune reconstitution associated with ARV use. More stringent criteria needs to be applied to studies in order to elucidate the true effect of OC treatment medication in persons using ARV therapy. It is recommended that trials be more standardised and conform more closely to CONSORT.
What's new
| Date | Event | Description |
|---|---|---|
| 6 October 2010 | New search has been performed | New studies added, review updated. Conclusions unchanged. |
| 5 October 2010 | New citation required but conclusions have not changed | New studies added and review has been updated. Conclusions have not changed. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 2 February 2010 | New search has been performed | The literature search was updated to July 2009, Five new studies Goldman 2005, Hamza 2008, Nittayananta 2008, Vazquez 2006 and Wright 2009 met inclusion criteria and were included. |
| 21 April 2008 | New search has been performed | Converted to new review format. |
| 14 January 2008 | New search has been performed | We updated our search to January 2008. We identified three additional relevant trials of which two (Goldman 2005; Vazquez 2006) met our inclusion criteria and the third, Skiest 2007, was excluded. |
| 23 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Nandi Siegfried for her mentoring, help and advice. We also acknowledge the very valuable advice and assistance received from George Rutherford, Coordinating Editor of the Cochrane HIV/AIDS Review Group and Gail Kennedy, Review Group Coordinator of the Cochrane HIV/AIDS Review Group, and Peter Robinson, School of Clinical Dentistry, Sheffield, for extremely valuable input and advice on the final report of the review.
Appendices
Appendix 1. PubMed Search strategy
Database: PubMed 2005 ‐ 2009
Date: 13 July 2009
| Search | Most Recent Queries | Time | Result |
| #5 | Search ("2005"[Publication Date] : "2009"[Publication Date]) AND (#1 AND #2 AND #3) | 05:56:22 | 83 |
| #4 | Search #1 AND #2 AND #3 | 05:55:54 | 554 |
| #3 | Search ((CANDIDIASIS OR THRUSH OR CANDIDIOSIS OR CANDIDIASIS, ORAL OR "ORAL CANDIDIASIS" OR CANDIDA INFECT* OR CANDIDA) AND (ORAL DISEASES OR OROPHARYNX OR OROPHARYNGEAL)) | 05:55:29 | 3226 |
| #2 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immune deficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 05:55:04 | 253794 |
| #1 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 05:54:39 | 4096323 |
Appendix 2. EMBASE Search Strategy
Database: EMBASE 2008 ‐ 2009
Date: 13 July 2009
| Search | ||
| #7 | #1 AND #2 AND #5 AND #6 | 8 |
| #6 | ('oral disease'/exp OR 'oral disease') OR 'oral manifestation' OR ('oral manifestations'/exp OR 'oral manifestations') OR ('oropharynx'/exp OR 'oropharynx') OR oropharyngeal OR ('oral lesion'/exp OR 'oral lesion') OR 'oral lesions' AND [embase]/lim AND [2008‐2009]/py | 13,963 |
| #5 | #3 OR #4 | 2,306 |
| #4 | ('thrush'/exp OR 'thrush') OR candidiosis OR ('oral candidiasis'/exp OR 'oral candidiasis') OR 'candida infected' OR 'candida infections' OR ('candida infection'/exp OR 'candida infection') OR (('candida'/exp OR 'candida') AND infect*) [AND [embase]/lim AND [2008‐2009]/py | 2,306 |
| #3 | ('candidiasis'/exp OR 'candidiasis') AND [embase]/lim AND [2008‐2009]/py | 1,284 |
| #2 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR ('crossover procedure'/de) OR ('double‐blind procedure'/de) OR ('single‐blind procedure'/de) OR ('randomized controlled trial'/de)) AND (('human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus'/exp) OR ('human immunodeficiency virus vaccine'/de) OR ('b cell lymphoma'/de) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab)) AND [embase]/lim AND [2008‐2009]/py | 1,292 |
| #1 | (((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection'))) OR ((('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')))) OR ((((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus'))) OR ((('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus'))))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) AND [embase]/lim AND [2008‐2009]/py | 15,964 |
Database: EMBASE 2005 ‐ 2008
Date: 25 February 2008
| Search | ||
| #7 | #1 AND #2 AND #5 AND #6 | 30 |
| #6 | ('oral disease'/exp OR 'oral disease') OR 'oral manifestation' OR ('oral manifestations'/exp OR 'oral manifestations') OR ('oropharynx'/exp OR 'oropharynx') OR oropharyngeal OR ('oral lesion'/exp OR 'oral lesion') OR 'oral lesions' AND [2005‐2008]/py | 44,068 |
| #5 | #3 OR #4 | 6,298 |
| #4 | ('thrush'/exp OR 'thrush') OR candidiosis OR ('oral candidiasis'/exp OR 'oral candidiasis') OR 'candida infected' OR 'candida infections' OR ('candida infection'/exp OR 'candida infection') OR (('candida'/exp OR 'candida') AND infect*) AND [2005‐2008]/py | 5,939 |
| #3 | ('candidiasis'/exp OR 'candidiasis') AND [2005‐2008]/py | 3,751 |
| #2 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR ('crossover procedure'/de) OR ('double‐blind procedure'/de) OR ('single‐blind procedure'/de) OR ('randomized controlled trial'/de)) AND (('human immunodeficiency virus infection'/exp) OR ('human immunodeficiency virus'/exp) OR ('human immunodeficiency virus vaccine'/de) OR ('b cell lymphoma'/de) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab)) | 12,225 |
| #1 | (((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection'))) OR ((('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')))) OR ((((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus'))) OR ((('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus'))))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) | 271,716 |
Appendix 3. Geographic location of studies
| Study ID | Study Type | Country / Region | Multi‐centre | Multi‐country | Single‐centre | Single‐country |
| Arathoon 2002 | Treatment | South America | Yes | Yes | ||
| Chavanet 1992 | Treatment | France | Yes | Yes | ||
| de Repentigny 1996 | Treatment | Canada | Yes | Yes | ||
| De Wit 1989 | Treatment | Belgium | Yes | Yes | ||
| De Wit 1993 | Treatment | Belgium | Yes | Yes | ||
| De Wit 1997 | Treatment | Belgium; UK; France | Yes | Yes | ||
| De Wit 1998 | Treatment | Belgium | Yes | Yes | ||
| Graybill 1998a | Treatment | USA | Yes | Yes | ||
| Hamza 2008 | Treatment | Tanzania | Yes | Yes | ||
| Hernandez 1994 | Treatment | Spain | Yes | Yes | ||
| Linpiyawan 2000 | Treatment | Thailand | Yes | Yes | ||
| Murray 1997 | Treatment | USA | Yes | Yes | ||
| Nyst 1992 | Treatment | Zaire | Yes | Yes | ||
| Phillips 1998a | Treatment | Austria; Belgium; Canada; UK; Netherlands; Spain | Yes | Yes | ||
| Pons 1993 | Treatment | USA | Yes | Yes | ||
| Pons 1997 | Treatment | USA | Yes | Yes | ||
| Redding 1992 | Treatment | USA | Yes | Yes | ||
| Smith 1991 | Treatment | UK | Yes | Yes | ||
| Van Roey 2004 | Treatment | Uganda | Yes | Yes | ||
| Vazquez 2002 | Treatment | USA | Yes | Yes | ||
| Vazquez 2006 | Treatment | Europe; Latin America; Canada; Africa | Yes | Yes | ||
| Wright 2009 | Treatment | South Africa | Yes | Yes | ||
| Goldman 2005 | Prevention | USA | Yes | Yes | ||
| Just‐Nubling 1991a | Prevention | Germany | Yes | Yes | ||
| Leen 1990 | Prevention | UK | Yes | Yes | ||
| MacPhail 1996 | Prevention | USA | Yes | Yes | ||
| Marriott 1993 | Prevention | Australia | Yes | Yes | ||
| McKinsey 1999 | Prevention | USA | Yes | Yes | ||
| Nittayananta 2008 | Prevention | Thailand | Yes | Yes | ||
| Pagani 2002 | Prevention | Switzerland | Yes | Yes | ||
| Revankar 1998 | Prevention | USA | Yes | Yes | ||
| Schuman 1997 | Prevention | USA | Yes | Yes | ||
| Stevens 1991 | Prevention | USA | Yes | Yes |
Data and analyses
Comparison 1. Treatment: Fluconazole vs Ketoconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.97, 1.66] |
| 1.1 Adults | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.04, 2.15] |
| 1.2 Children | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.86, 1.49] |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Clinical + Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.2. Analysis.
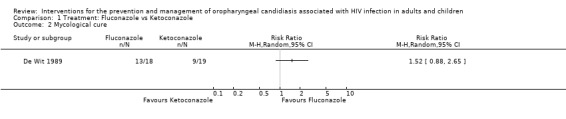
Comparison 1 Treatment: Fluconazole vs Ketoconazole, Outcome 2 Mycological cure.
Comparison 2. Treatment: Fluconazole vs Itraconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure: with De Wit 1998 | 5 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.36] |
| 2 Clinical Cure: without De Wit 1998 | 4 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.16] |
| 3 Mycological cure | 5 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.90, 1.46] |
| 4 Relapse | 5 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.21] |
Comparison 3. Treatment: Fluconazole vs Clotrimazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.37] |
| 2 Mycological cure | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.16, 1.87] |
| 3 Relapse | 2 | 250 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.54] |
3.3. Analysis.
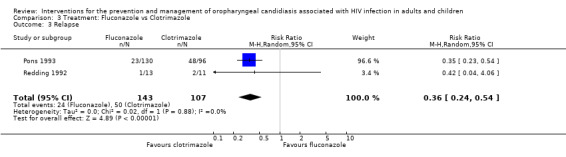
Comparison 3 Treatment: Fluconazole vs Clotrimazole, Outcome 3 Relapse.
Comparison 4. Treatment: Fluconazole vs Fluconazole Stat.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Fluconazole 50mg daily for 7 days vs Fluconazole150mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Fluconazole 150mg daily for 14 days vs fluconazole 750mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mycological cure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Fluconazole 50mg daily for 7 days vs Fluconazole 150mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fluconazole 150 mg daily for 14 days vs Fluconazole 750mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Relapse | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Fluconazole 50mg daily for 7 days vs Fluconazole 150mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Fluconazole 150mg for 14 days vs Fluconazole 750mg stat | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Treatment: Fluconazole vs Nystatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Treatment: D0870: 25mg vs 10mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 7. Treatment: Itraconazole vs Clotrimazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical and Mycological cure | 2 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.56, 3.20] |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Treatment: Melaleuca Oral Solution: Alcohol‐free vs Alcohol‐based.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 9. Treatment: Amphotericin: Fat Emulsion vs Glucose Solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical score reduction | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Treatment: Itraconazole vs Ketoconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.94, 1.16] |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 11. Treatment: Ketoconazole vs Miconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Relapse | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
11.1. Analysis.
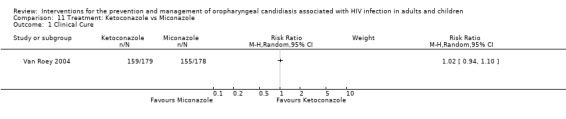
Comparison 11 Treatment: Ketoconazole vs Miconazole, Outcome 1 Clinical Cure.
11.2. Analysis.
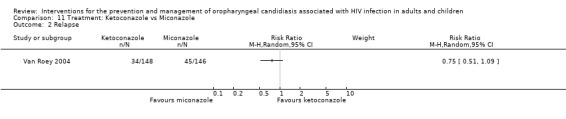
Comparison 11 Treatment: Ketoconazole vs Miconazole, Outcome 2 Relapse.
Comparison 12. Treatment: Gentian Violet vs Ketoconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Treatment: Gentian vs Nystatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 14. Treatment: Ketoconazole vs Nystatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Treatment: Caspofungin vs Amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Treatment: Posaconazole vs Fluconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Mycological cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Mycological eradication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 17. Treatment: Lemon juice vs Gentian violet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Clinical failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 18. Treatment: Lemon grass vs gentian violet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Clinical Failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 19. Treatment: Lemon juice vs Lemon grass.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical cure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Clinical Failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 20. Prevention: Nystatin vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical episode | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 1.1 No history of OC | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.52, 1.89] |
| 1.2 History of OC | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.65, 1.04] |
Comparison 21. Prevention: Nystatin vs Nystatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical episode | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.50, 0.99] |
| 1.1 No history of OC | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.25, 1.06] |
| 1.2 History of OC | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.52, 1.13] |
Comparison 22. Prevention: Fluconazole vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical episode | 5 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.50, 0.74] |
Comparison 23. Prevention: Fluconazole vs No treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Episode | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.08, 0.34] |
Comparison 24. Prevention: Itraconazole vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical Episode | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 25. Prevention: Fluconazole Intermittent vs continous.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical episode | 2 | 891 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.83] |
| 1.1 Fluconazole for 7 days | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.15, 0.92] |
| 1.2 Fluconazole for 14 days | 1 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.55, 2.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arathoon 2002.
| Methods | A Multi‐centre, single country study conducted in South America. Dates of enrolment and completion of study not reported. Analysis: no ITT |
|
| Participants | Eligibility criteria: patients who had oropharyngeal and/ oesophageal candidiasis Exclusion criteria: Pregnant, breast feeding, inadequate contraception, allergy or serious adverse event to glucan synthesis inhibitors or amphotericin B, previous failure with amphotericin B treatment, ongoing treatment with rifampicin or ritonavir, any underlying condition that deemed likely to confound interpretation of results or pose undue risk to the patient, abnormal laboratory results: hematocrit =< 27%; absolute neutrophil count < 1,000/ul; platelet count <= 75,000/ul; creatinine clearance < 50ml/min; prothrombin time > upper limit of normal and / or total bilirubin > 3 or more times upper limit of normal; ALT or AST > 5 or more times upper limit of normal Diagnosis confirmed by visualisation of Candida pseudohyphae in appropriate specimens. 140 patients enrolled C1=34 C2=34 C3=37 A1=35 |
|
| Interventions | C1 ‐ caspofungin acetate 35 mg
C2 ‐ caspofungin acetate 50 mg
C3 ‐ caspofungin acetate 70 mg
plus placebo
A1 ‐ amphotericin B (0,5mg/kg) or placebo
intravenously, once daily Patients were randomly allocated to one of four interventions and stratified according to presentation with either oropharyngeal infection alone or esophageal disease with or without oropharyngeal involvement. Patients were further subdivided according to previous refractory (S1) or responsive (S2) fluconazole therapy. Treatment duration: minimum 7 days, maximum of 14 days |
|
| Outcomes | Primary end point: Combined response of referable symptoms and visible lesions assessed 3‐4 days after discontinuation of study drug. Relapse: Recurrence of symptoms or signs of Candida infection during the month after discontinuation of therapy Microbiological eradication: Culture results 3‐4 days after discontinuation of therapy Adverse events: significantly fewer C recipients developed drug‐related fever, chills, nausea or vomiting. Incidence of local reactions (infusion related) ranged from 6‐14% across treatment arms. Drug related laboratory abnormalities (raised ALT, AST, ALP, Creatinine, and decreased K) were also more common in patients taking amphotericin B. |
|
| Notes | Ethics: IRB approved protocol and informed consent obtained 3 patients not HIV + Author contacted to clarify treatment allocation, allocation concealment, and blinding. No response to date 11/11/2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description given of how sequence generation was done |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Mention that double blinding was used but unclear who was blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐outs clearly described Loss to follow‐up: unclear how many patients completed treatment |
Chavanet 1992.
| Methods | A single‐centre trial with enrolment from June to December 1991. Location of study: France Loss to follow‐up: Amp Glucose 4/11 (36%) Amp Fat 0/11 (0%) Analysis: no ITT |
|
| Participants | Eligibility criteria: Patients who had oral candidiasis, 18 years and older, HIV positive Exclusion criteria: Esophagitis, oral Kaposi, hyperlipidaemia, known intolerance to amphotericin, pancreatitis, serum creatinine > 115 umol/l. Diagnosis confirmed using mycological analysis 22 patients enrolled Amphot‐glucose :11 Amphot‐fat : 11 |
|
| Interventions | Amphotericin deoxycholate dissolved in either:
5% glucose (amphotericin glucose) ‐ final concentration 1.6 g amphotericin / l or
parenteral fat emulsion (amphotericin fat emulsion) ‐ final concentration 2 g amphotericin / l Given as a 1 hour infusion of 1 mg/kg/day on four consecutive days. |
|
| Outcomes | Clinical scores of candidiasis ‐ 9 sites were inspected for the presence or absence of candidiasis which were examined for confluent, patchy or scattered lesions scored as 3,2 and 1 respectively. Evaluated the reduction in clinical score. Mycologic cure : culture from swab Clinical and biological tolerance Serum concentrations of amphotericin Adverse events: more frequent with glucose preparation. Chills and fever were most frequent side effect ‐ 66% vs 4%. Sweating and Nausea slightly less frequent in Fat emulsion group. |
|
| Notes | Ethics: Ethics approval obtained from local ethics committee, informed consent obtained from patients. Enrolment: June ‐ December 1991. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "..patients were assigned randomly to one of the two treatment groups. Group selection was determined by sequential assignment from a table of random numbers.." |
| Allocation concealment? | High risk | Sealed envelopes. Each envelope was opened immediately before the treatment was given. |
| Blinding? All outcomes | High risk | No blinding was used. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons given for discontinuation of intervention |
de Repentigny 1996.
| Methods | A multicentre study at ten centres in Montreal, Canada, including university and private practice settings. Date of enrolment: No information provided. Loss to follow‐up: Ictraconazole ‐ 8/51 (16%) Ketoconazole ‐ 6/55 (9%) Analysis: no ITT; patients considered evaluable if they received at least 5 days consecutive days of treatment |
|
| Participants | Eligibility criteria: symptoms and signs of oropharyngeal and/or esophageal candidiasis as confirmed by microscopy and culture; 16 years and older; HIV positive Exclusion criteria: < 16 years old; pregnant or lactating; no effective contraceptive method; history of allergy to imidazole; concomitant treatment with rifampicin or antimycotic; received antifungal treatment in last 2 weeks for esophageal candidiasis; stomatitis and/or esophagitis secondary to herpetic infection; life expectancy less that 3 months; liver enzyme elevation (ALT and AST) > 500 IU/ml; unable or willing to give informed consent 106 patients enrolled with oropharyngeal candidiasis 51 ‐ Ictraconazole 55 ‐ Ketoconazole |
|
| Interventions | Ictraconazole: two 100mg capsules plus one ketoconazole placebo tablet daily Ketoconazole: one 200mg tablet plus two itraconazole placebo capsules daily Treatment duration: 2 weeks After completion of treatment patients who cleared were followed up for a period of 6 weeks |
|
| Outcomes | Clinical cure: Signs and symptoms were categorised as none (0), mild (1), moderate (2) or severe (3). Clinical cure defined as successful if signs + symptoms = 0 or failed if signs + symptoms > 0. Mycologic cure: successful if microscopy and culture negative for Candida. Relapse: clinical evidence of buccal candidiasis with mycological confirmation Adverse events: no significant differences between treatment groups. Common reported adverse events were nausea, headache, rash, diarrhoea and taste perversion. |
|
| Notes | Ethics: IRB of each centre approved study and informed consent obtained Study reports clinical cure at day 21 and not day 14. Mycologic cure reported for end of treatment (day 14). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Randomly assign to treatment in 1:1 ratio according to a computer generated schedule. |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Report the use of double blinding, however did not specify who was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons given for withdrawal given. |
De Wit 1989.
| Methods | Location of study: Belgium From November 1986 ‐ February 1988 patients presenting with severe oropharyngeal candidiasis were evaluated for the study. Follow‐up: one month. Loss to follow up: During treatment ‐ Fluconazole 1/18 (6%) Ketoconazole 3/19 (16%) During follow‐up ‐ Fluconazole 3 Ketoconazole 3 Analysis: no ITT |
|
| Participants | Eligibility criteria: AIDS/ARC patients, 18 years and older, severe oropharyngeal candidiasis confirmed by culture. Exclusion criteria: esophageal Candidiasis, other systemic antifungal drugs, known sensitivity to imidazoles, previous abnormalities of blood or urine chemistry after ketoconazole, impaired renal function, moderate to severe liver disease, drugs capable of inducing more rapid clearance of ketoconazole or that reduce / neutralise gastric acid. Diagnosis confirmed by culture 37 patients fluconazole ‐ 18 ketoconazole ‐ 19 |
|
| Interventions | 50mg fluconazole once daily 200mg ketoconazole once daily Treatment duration: 28 days |
|
| Outcomes | Clinical cure ‐ complete disappearance of mucosal lesions and symptoms Mycological cure ‐ culture assessments Relapses ‐ weekly clinical and mycological assessments for 4 weeks after treatment completion Adverse events: severe nausea in 1 fluconazole patient, transient rise ( < 3 times baseline) ALT or AST in 1 fluconazole and 4 ketoconazole patient |
|
| Notes | Ethics: nil reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of allocation sequence generation not specified |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Reported double blinding. Specified that participants were blinded. Unclear who else was blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Reasons given for those lost to follow‐up during treatment, not those lost during follow‐up period. |
De Wit 1993.
| Methods | Pilot study conducted in Belgium Dates of enrolment not reported. Loss to follow up: Stat dose ‐ 4/28 (14%) Daily dose ‐ 1/28 (4%) Analysis: no ITT |
|
| Participants | Eligibility criteria: AIDS/ARC patients, 18 years and older, oropharyngeal candidiasis confirmed by culture Exclusion criteria: esophageal Candidiasis diagnosis confirmed by culture 56 patients fluconazole stat ‐ 28 fluconazole daily ‐ 28 |
|
| Interventions | 50mg fluconazole once daily for 7 days 150mg fluconazole as a single dose Treatment duration: 7 days , monitored 26 patients (13/group) for 2 weeks after treatment |
|
| Outcomes | Clinical cure Mycological cure Relapse Adverse events: Nil reported. |
|
| Notes | Ethics: nil reported Author contacted via email: response 29/10/2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of allocation sequence generation not specified. |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | High risk | No blinding used. |
| Incomplete outcome data addressed? All outcomes | High risk | No reasons given for loss to follow‐up |
De Wit 1997.
| Methods | Multicenter, multi‐country study ‐ 4 hospitals: Brussels; London; Manchester; Paris Dates of enrolment and/or randomisation not reported. Loss to follow‐up: D1 ‐ 3/13 (23%) D2 ‐ 0/14 (0%) Analysis: no ITT |
|
| Participants | Eligibility criteria: HIV positive, age 18‐62 years, patients who had oro‐pharyngeal with or without esophageal candidiasis Exclusion criteria: History of clinical failure of fluconazole, abnormal ECG, concomitant medication with agents known to induce cytochrome P450 or to interact with azoles, history of intolerance to azoles, acute or chronic liver disease. Diagnosis confirmed by colony forming units on culture 27 patients enrolled D1= 13 D2= 14 |
|
| Interventions | D1 ‐ D0870 100mg initial dose followed by 25 mg/day for 4 days D2 ‐ D0870 10mg once daily for 5 days | |
| Outcomes | Clinical response was recorded as cleared, improved, failure or not evaluable. Relapse: Patients were assessed 7 and 14 days after end of treatment for evaluation of relapses. Adverse events: 1 patients experienced dizziness (D1) and another had diarrhoea (D2). |
|
| Notes | Ethics: IRB of each centre approved protocol and informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of allocation sequence generation not specified. |
| Allocation concealment? | Unclear risk | "..sealed envelopes detailing the allocation randomised treatment" |
| Blinding? All outcomes | Unclear risk | Reported double blinding. Specified that participants were blinded. Unclear who else was blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Reasons for lost to follow‐up given |
De Wit 1998.
| Methods | A single‐centre study done at the reference hospital of Saint Pierre, Brussels, Belsgium Loss to follow‐up: Fluconazole ‐ 0/20 (0%) Ictraconazole ‐ 3/20 (15%) Analysis: no ITT No dates for start or end of study reported. |
|
| Participants | Inclusion criteria: 16‐65 years; AIDS / AIDS‐related complex, who are able to take oral medication; OPC suspected on clinical grounds Exclusion criteria: received antifungal treatment within 1 week before enrolment; or maintenance azole treatment for prevention of OPC during previous month; hepatic or renal disease; allergy to azole compounds or if they had had more than three OPC episodes during previous 2 months; end stage AIDS; fever of unknown origin; documented systemic fungal infections; any other acute concomitant infection; pregnancy and lactation. 40 enrolled Fluconazole =20 Ictraconazole = 20 diagnosis confirmed KOH and culture |
|
| Interventions | Fluconazole ‐ 150 mg stat Intraconazole ‐ 100 mg daily for 7 days | |
| Outcomes | Clinical Cure : Signs and symptoms of OPC were graded on scale from 0 (absent) to 3 (severe). Symptoms and signs included soreness, burning or pain, dysphagia, erythema, and presence of white plaques. Extent of lesions present was graded on a scale of 0 (no lesions) to 4 (lesions covering more than 70% of oral mucosa). These scores were combined to give an overall clinical score. Clinical cure was defined as disappearance of all symptoms (clinical score = 0), improvement defined as clinical score lower than baseline score and failure as no change in clinical score or worsening of clinical symptoms. Relapse: defined as initial improvement or cure followed by reappearance of symptoms within 30 days after baseline examination. Mycological evaluation: eradication was defined as recovery of less than 10 colony forming units (CFUs)/ml, improvement as a count below baseline count but above 10 CFUs/ml and failure as an increase or no change. Adverse events: none reported |
|
| Notes | Ethics: Written informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not specified |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | 3/20 lost to follow‐up in itraconazole group ‐ reasons provided. |
Goldman 2005.
| Methods | Multi‐centre prevention study conducted in the USA: May 1997 through April 2000 Loss to follow‐up: 184 from Episodic Fluconazole 205 from Continuous Fluconazole |
|
| Participants | Eligibility criteria:
Exclusion criteria:
Subjects who initiated treatment for opportunistic infection 14 days before trial commenced or medication in which coadministration of fluconazole is contraindicated. 829 Patients enrolled: 416 randomised to episodic or intermittent fluconazole 413 randomised to continuous fluconazole |
|
| Interventions | Fluconazole: Continuous: 200mg fluconazole orally 3 times weekly Episodic: Fluconazole only administered for OPC and EC episodes. |
|
| Outcomes | Primary: Time to development of fluconazole refractory mucosal Candida infection (FRI) Secondary:
|
|
| Notes | Written consent obtained from participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Sequence generation not reported |
| Allocation concealment? | Unclear risk | Concealment of allocation not reported |
| Blinding? All outcomes | High risk | Open‐label study ‐ patients, researchers and evaluators not blinded |
| Incomplete outcome data addressed? All outcomes | High risk | 53% loss to follow‐up |
Graybill 1998a.
| Methods | Location of study: USA
Multicentre: 12 centres Only patients randomised whose efficacy outcome was cured or improved was evaluable for follow up Loss to follow‐up: discrepancy between text (157) and tables (146) as to number who completed treatment According to table ‐ Ictraconazole 7d ‐ 10/64 (17%) Ictraconazole 14d ‐ 10/64 (17%) Fluconazole ‐ 13/62 (21%) Analysis: no ITT |
|
| Participants | Eligibility criteria: HIV positive adult patients randomised (> 13 years), clinical picture of oropharyngeal candidiasis with findings on direct microscope examination (KOH smear) consistent with Candida spp and subsequently confirmed by positive mycological culture. Exclusion criteria: presence of perioral lesions only, esophageal involvement, history of significant hepatic abnormalities / clinical evidence hepatic disease within two months of entering study, life expectancy < 1 month or clinical condition such that study completion could not be assured, hypersensitivity to imidazole or azole compounds, pregnant or breastfeeding, therapy with other antifungal agents, H2 receptor blockers, antacids, rifampicin, rifabutin, phenobarbital, phenytoin, carbamazepine, terfenadine, astemizole. Diagnosis confirmed using KOH and culture 190 enrolled and randomised: 64 Itraconazole 7 days 64 Itraconazole 14 days 62 Fluconazole 14 days |
|
| Interventions | Compared 2 regimens of itraconazole oral solutions
200mg/day for 7 days
200mg/kg for 14 days
with active control ‐ fluconazole tablets 200mg on day 1, 100mg daily for the remaining 13 days time period of interventions: 14 days |
|
| Outcomes | clinical response:
cured (clearance of all signs and symptoms) /
clinical improvement ( minimal signs and symptoms with no residual visible candida lesions) /
clinical deterioration (worsening or increasing signs and symptoms) secondary outcomes: changes in symptoms from baseline (soreness/ burning, erythema) ; extent of oral lesions; quantification of CFU's; mycological cure : yeast quantification of </= 20 cfu/ml; Culture Adverse events: 25% each treatment arm GIT adverse events. Nausea, diarrhoea, abdominal pain most common. Respiratory side effects 21% fluconazole, 12.5% itraconazole. |
|
| Notes | Ethics: IRB of each centre approved study, written informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of allocation sequence generation not specified |
| Allocation concealment? | High risk | "..open label.." |
| Blinding? All outcomes | Unclear risk | Third party blinded. |
Graybill1998b.
| Methods | The same as for Graybill 1998a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of allocation sequence generation not specified |
| Allocation concealment? | High risk | "..open label.." |
| Blinding? All outcomes | Unclear risk | Third party blinded. |
Hamza 2008.
| Methods | Participants recruited at HIV clinic of the Muhimbili National Hospital, Dar es Salaam, Tanzania from November 2006 through December 2007 Loss to follow‐up: 0% in both arms Analysis: ITT |
|
| Participants | Inclusion criteria: 18 years and older, documented HIV infection (determined by positive ELISA results and confirmed by Western‐blot analysis); clinical signs of OPC, visual lesions and microbiological confirmation. Exclusion criteria: Ongoing or previous topical or systemic antifungal therapy within 3 days before study enrolment. History of allergy to azole derivatives; abnormal liver function tests. Inability to tolerate oral dug administration. Pregnancy or breast‐feeding. Life expectancy of <4 weeks. Current participation in another clinical trial. Current treatment with drugs known to interact with fluconazole. documented systemic fungal infection. Symptoms suggestive of esophageal candidiasis. Fluconazole 14 days: 110 Fluconazole stat: 110 HAART: 41 in Stat group and 39 in 14 day group |
|
| Interventions | Fluconazole 14 days: 1 tablet of 150mg daily for two weeks plus 5 placebo tablets on first day of therapy. Fluconazole stat: Single dose of 750mg, i.e. 5 tablets of 150 mg on day one. One placebo tablet per day for two weeks. Treatment: 14 days Follow‐up: until 42 days from start of treatment |
|
| Outcomes | Primary outcomes:Clinical and mycological responses at end of treatment. Clinical cure = complete resolution of lesion, signs and symptoms of OPC. Improvement = reduction in number of lesions and symptoms, but persisting typical oropharyngeal lesions. Failure = no resolution of signs and symptoms. Mycological failure = any growth of Candida species on day 14 of culture. Secondary outcomes: relapse and safety Relapse = initial cure followed by reappearance of symptoms, signs and/or confirmation of positive yeast culture during a follow‐up period of four weeks at end of treatment. Adverse events reported in six patients in the 14‐day FCZ group, with all events being gastrointestinal. In the single‐dose group events were reported in 8 patients: 6 nausea, vomiting, abdominal pain, and/or diarrhea. One patient had a headache and 1 patient had heart palpitations. |
|
| Notes | Ethics: Ethics Committee of Muhimbili University of Health and Allied Sciences and Muhimbili National Hospital approved study protocol. Written informed consent from each participant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not specified. |
| Allocation concealment? | Unclear risk | Methods not specified |
| Blinding? All outcomes | Low risk | Double‐blind, double‐dummy. Both patients and evaluator blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | All participants included in final analysis |
Hernandez 1994.
| Methods | Open multicentre study in Spain with patients enrolled from January to July 1992. 4 week follow‐up Loss to follow‐up: Ketoconazole ‐ 1/22 (5%) Fluconazole ‐ 0/24 (0%) Analysis: no ITT |
|
| Participants | Eligibility: HIV patients randomised, able to swallow medication, age between 7 weeks to 14 years, had clinical signs and symptoms of oropharyngeal candidiasis and positive findings on microscopy pending confirmation by culture Exclusion criteria: Positive pregnancy test, Mycoses other than oropharyngeal candidiasis, life expectancy < 4 weeks Diagnosis confirmed using microscopy and culture 46 patients randomised enroled 24 fluconazole 22 ketoconazole |
|
| Interventions | Fluconazole oral suspension once daily in a dose of 3mg/kg body weight for 23/24 patients randomised
and 2mg/kg body weight in 1/24 patients randomised. Mean treatment duration 14 days (range 6‐33days) Ketaconazole oral suspension: once daily in a dose of 7mg/kg body weight in 19/22 patients randomised and 3,5mg/kg body weight in 3/22 patients. Mean treatment duration 16 days (range 5‐49days) |
|
| Outcomes | Clinical response:
Clinical cure ‐ resolution pretreatment signs and symptoms
Clinical improvement ‐ partial resolution pre‐treatment signs and symptoms
Clinical failure ‐ no change / worsening
Relapse ‐ initial improvement or resolution of signs and symptoms followed by worsening or reappearance. Mycological response: Cure ‐ complete eradication Candida + clinical cure Colonization ‐ positive culture and absence of clinical disease Failure ‐ positive culture and presence clinical disease Reinfection ‐ reappearance of Candida Adverse events : GIT toxicity ‐ 1 patient ketoconazole group (diarrhoea and abdominal pain). Two patients also had increased ALT and AST vs 1 in fluconazole. 1 in latter group also had thrombocytopaenia |
|
| Notes | Ethics: legal guardian / parents informed consent obtained. Patients in 2 groups were given different doses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not specified |
| Allocation concealment? | High risk | "open study" |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss to follow‐up less than 10% |
Just‐Nubling 1991a.
| Methods | Open prevention study in Germany. Patients randomised from May 1989 to January 1990. Loss to follow‐up: Group 1 ‐ 1/22 (5%) Group 2 ‐ 3/21 (14%) Group 3 ‐ 3/22 (14%) Analysis: no ITT |
|
| Participants | Inclusion criteria: Patients with advanced stages of HIV infection (stages 2b and 3 according to Frankfurt classification, stages 3‐6 according to Walter Reed); CD4 cells counts <100/mm3; at least 1 episode of candidiasis within previous 3 months Exclusion criteria: candida requiring Rx Diagnosis confirmed looking at culture (colony forming units). 58 participants Group 1 ‐22 Group 2 ‐ 21 Group 3 ‐ 22 |
|
| Interventions | Group1 ‐ no treatment Group 2 ‐ 50mg fluconazole daily Group 3 ‐ 100mg fluconazole daily | |
| Outcomes | Relapses of OC (lesions) Adverse events: Allergic exanthema in fluconazole group Of the 60 patients enrolled and randomised in the study, 7 were early dropouts. These 7 drop‐outs were replaced by recruiting and randomising 5 new patients into the study. |
|
| Notes | Ethics: no mention of ethics approval No demographics reported. Author contacted, no response to date 11/11/2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not specified |
| Allocation concealment? | Low risk | "Patients were randomised (sealed envelopes) and assigned.." |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | High risk | No ITT done |
Just‐Nubling 1991b.
| Methods | Same as for Just‐Nubling 1991a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not specified |
| Allocation concealment? | Low risk | "Patients were randomised (sealed envelopes) and assigned.." |
| Blinding? All outcomes | High risk | No blinding |
| Incomplete outcome data addressed? All outcomes | High risk | No ITT |
Leen 1990.
| Methods | Maintenance trial ‐ everyone received Fluconazole initially and only those in whom Rx was successful were included into the maintenance study Location of study: United Kingdom Loss to follow‐up: Fluconazole ‐ 2/9 (22%) Placebo ‐ 0/5 (0%) Analysis: ITT |
|
| Participants | Inclusion criteria: HIV+ males; AIDS/ARC; 18‐65 yrs; clinically and mycologically diagnosed severe oral mucosal candidiasis that was not immediately life‐threatening, but needed Rx Exclusion criteria: serum creatinine levels of 110 umol/l or >; patients with moderate to severe liver disease; patients using any other systemic antifungal drug; patients taking drugs that are cleared predominantly by metabolism and that have a low therapeutic ratio i.e. barbiturates, coumarin anticoagulants, oral hypoglycaemic agents; patients taking any other investigational drugs except certain drugs used for other opportunistic diseases Diagnosis confirmed microscopy and culture 24 enrolled, 14 randomised Fluconazole ‐ 9 Placebo ‐ 5 |
|
| Interventions | Fluconazole 150mg weekly Placebo Duration: 24 weeks |
|
| Outcomes | Relapse of candidiasis Adverse events: one patient developed diarrhoea shortly after receiving fluconazole. |
|
| Notes | Ethics: informed consent obtained; no mention of ethics approval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated randomisation |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Say double blind. Participants blind,but does not state who else is blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | ITT |
Linpiyawan 2000.
| Methods | Location of study: Thailand Loss to follow‐up: none Analysis: ITT |
|
| Participants | Inclusion criteria: oropharyngeal candidiasis, AIDS patients Exclusion criteria: treatment with any antifungal agent 2 weeks prior to study entry; pregnancy; breastfeeding; significant liver disease 29 enrolled Clotrimazole group = 15 Itraconazole group = 14 diagnosis confirmed using KOH and culture |
|
| Interventions | Clotrimazole troche 10mg five time daily Itraconazole oral soln 100mg/10ml twice daily Duration: 1 week |
|
| Outcomes | Clinical evaluation ‐ assessed by scoring method (0‐3, absent to severe) Mycological assessment ‐ KOH prep and fungal culture Global evaluation ‐ cure (all signs and symptoms resolved with no evidence of infection) ‐ improvement (decrease in clinical score without complete resolution) ‐ failure (lack of improvement or further deterioration) Adverse events: 2 patients had transient elevation of liver enzymes |
|
| Notes | Ethics: none mentioned No table with demographics. Mention in text that groups are comparable. Author contacted, no response to date 24/08/2004 Author contacted via email: 27/9/2004; 27/10/2004 ‐ no response by 08/11/2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation ‐ does not mention method used. |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Unclear risk | "...observer blinded..." |
| Incomplete outcome data addressed? All outcomes | Low risk | No Loss to follow‐up |
MacPhail 1996.
| Methods | Study was part of a large multi‐centre trial. Participants volunteers drawn from patients attending the Oral AIDS Clinic at the University of California, San Francisco, Date of enrolment: November 1987 and the multicenter study closed in March 1990. Loss to follow‐up at 20 weeks: Stratum 1 (No history of OC): 20/58 = 34% Stratum 2 (OC history): 14/70 =10% Loss to follow‐up not given separately for the different arms within each stratum. Analysis: no ITT |
|
| Participants | Inclusion criteria: HIV infected or AIDS patients; CD4 cells counts < 650 /ul Exclusion criteria: suspected or proven esophageal candidiasis; if required antifungal agent orally or intravenously within 72h of entry; known hypersensitivity to nystatin HIV infected patients stratified by history of oral candidiasis Stratum 1 (no history of oral candidiasis) Stratum 2 ( history of oral candidiasis i.e. presented to the centre with OC and was treated and cured/cleared clinically within 3‐7 days before randomisation) 128 participants Stratum 1 ‐ 58 Stratum 2 ‐ 70 diagnosis confirmed using KOH and culture |
|
| Interventions | Within each strata
Placebo ‐ 2 placebo pastilles daily
One nystatin (200,000 U) and 1 placebo pastille daily
Two nystatin pastilles daily Duration: 20 weeks |
|
| Outcomes | Delay in onset of oral candidiasis: defined as presence of removable white plaques, erythematous areas, angular cheilitis that were potassium hydroxide positive for fungal hyphae and culture positive for fungal spp Culture positive: defined as growth of one or more colony forming units. |
|
| Notes | Ethics: informed consent obtained; no mention of ethics approval | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation, does not mention method used to generate allocation sequence. |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Low risk | Double blind. Participant and investigators blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | 34% loss to follow‐up in stratum 1. No ITT, |
MacPhail 1996b.
| Methods | Same as MacPhail 1996 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation, does not mention method used to generate allocation sequence |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Low risk | Double blind. Participant and investigators blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | 34% loss to follow‐up in stratum 1. No ITT, |
Marriott 1993.
| Methods | Location of study: Australia Enrolment: Jan 1989 ‐ March 1990 Loss to follow‐up: Fluconazole: 13/44 = 29% Placebo: 14/40 = 35% Analysis: ITT |
|
| Participants | Inclusion criteria: male inpatients/outpatients, 18 years/older; with moderate to severe HIV infection who had been successfully treated with oropharyngeal candidiasis with fluconazole, 50mg daily for 14‐28 days. They were classified as clinically cured both at end of treatment and 7 days after the last dose of fluconazole Exclusion criteria: poor tolerance of fluconazole; taking drugs with low therapeutic ratio that are metabolized by the liver, such as barbiturates and coumarin anticoagulants; or if they were taking and other systemically administered antifungal drug; serum creatinine >= 220Umol/l; ALT, AST and ALP > 3 times upper limit of normal; total bilirubin >=350Umol/l and or prothrombin time > 5 seconds over control value 84 enrolled Fluconazole =44 Control group = 40 Diagnosis confirmed KOH and culture |
|
| Interventions | Weekly dose of 150mg fluconazole or placebo for 24 weeks (on the same day of each week) | |
| Outcomes | Clinical recurrence of oral Candidiasis
Mycological outcome Adverse events: Fluconazole group ‐ 40 intercurrent illnesses, 9 adverse drug reactions, 3 deaths with Placebo group ‐ 5 intercurrent illnesses, 1 adverse drug reaction and 2 deaths. |
|
| Notes | Ethics: approval obtained; signed informed consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random allocation. Sequence computer generated. |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Unclear risk | Double blind. Participants blinded, does not mention who else. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | loss to follow‐up > 20%. Analysis ITT |
McKinsey 1999.
| Methods | Location of study: USA, multicentre Enrolment: June 1993 ‐ April 1995 Loss to follow‐up: unclear Analysis: ITT |
|
| Participants | Inclusion criteria: >13 yrs; HIV +; confirmed by western blot; life expectancy of > 1 year; absolute CD4 count< 150/mm3 within 1 year prior to study; residence in a city with a higher incidence of histoplasmosis Exclusion criteria:use of investigational drug concurrently or within 1 month prior to initiation of study, pregnancy, lactation, failure to use medically approved and effective method of contraception; history of intolerance of imidazole or triazole compounds; inability to take oral medication; history of active histoplasmosis; active fungal infection; medication interact with itraconazole; elevated liver function test values 298 enrolled; 295 randomised Itraconazole group = 149 Control (placebo) group = 146 Diagnosis confirmed: yes (culture) |
|
| Interventions | Itraconazole 2 x 100mg capsules daily for 32 months Placebo |
|
| Outcomes | Primary: Safety and efficacy of itraconazole in preventing histoplasmosis Secondary: recurrence of other fungal infections Adverse events: diarrhoea, abdominal pain, nausea, vomiting, elevated liver enzymes, rash and Stevens‐Johnson syndrome. |
|
| Notes | Ethics: approval obtained from each study site IRB; written informed consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation; 1:1 ration, stratified by site with each site having an independent randomisation code. |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Unclear risk | Double blind; participants blinded, but not who else. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Not reported. |
Murray 1997.
| Methods | Multicentre, open‐label study. Location of study: USA Loss to follow‐up: unclear Analysis: no ITT |
|
| Participants | Inclusion criteria: immunocompromised ‐ HIV and other causes; >13 yrs; clinical and mycological confirmed OPC Exclusion criteria: presence of perioral lesions only, signs and symptoms suggestive of esophageal candidiasis, history of hepatic abnormalities, or clinical evidence of hepatic disease within 2 months of entering the study, life expectancy of < 1 month, history of hypersensitivity to imidazole or azole compounds, patients requiring therapy with histamine2‐receptor antagonists, antacids, rifampicin, rifabutin, phenobarbital, phenytoin, carbamazepine, terfenadine, astemizole. 162 enrolled 123 HIV/AIDS ‐ Itraconazole group = 61 ‐ Clotrimazole group = 62 Diagnosis confirmed: yes ‐ culture |
|
| Interventions | Itraconazole oral solution: once each day two 10mL aliquots of solution were to be swished vigorously in the mouth for several seconds and then swallowed Clotrimazole troches: 5 troches taken daily, each dissolved slowly in the mouth. Duration: 14 days. If clinical response to treatment at day 14 then observed for another month |
|
| Outcomes | Primary: Clinical cure judged on symptoms, extent of oral lesions and culture. Cured (clearance of symptoms), improved (minimal symptoms remaining with no residual visible lesions), unchanged or deteriorated. Secondary: recurrence of other fungal infections Adverse events: GIT symptoms mainly. 7 patients in itraconazole group and 3 in clotrimazole group had to discontinue study participation prematurely as a result of adverse events. |
|
| Notes | Ethics: approval obtained from each clinical centre IRB; written informed consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method how generated not stated |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | High risk | Investigator assessing the outcome was blinded. |
| Incomplete outcome data addressed? All outcomes | High risk | Loss to follow‐up unclear. No ITT |
Nittayananta 2008.
| Methods | Study conducted in Thailand with participants those who lived at Wiwekwanasom temple, or were outpatients at an internal medicine unit at Songklanagarind Hospital in Songkhla province in the south, of at Bamratnaradoon Institute in Nonthaburi, Thailand. Loss to follow‐up: Not reported Analysis: no ITT |
|
| Participants | Inclusion criteria: HIV‐infected heterosexual adults previously diagnosed as seropositive for antibodies to HIV. Presented with oral candidiasis. No current use or history of antifungal therapy last 3 months. Able to use mouth‐rinse properly. Able to come for follow‐up visits for at least a 3‐month period after complete treatment of OC. Willing to provide informed consent. Exclusion criteria: HIV‐seropositive without OC or with diabetes, history of organ transplantation, or any other immunosuppressive disease. Any current treatment of history of taking antifungals in last 3 months. 102 enrolled ‐ Chlorhexadine: 37 (aged 22 ‐ 52 years, mean 34 years) ‐ Normal saline: 38 (aged 22 ‐ 55 years, mean 38 years) Diagnosis confirmed: Yes ‐ oral rinse to determine CFU |
|
| Interventions | 0.12% chlorhexidine mouth‐rinse 0.9% normal saline |
|
| Outcomes | Primary: Prevention of relapse after antifungal therapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double‐blind, but unclear who was blinded |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Loss to follow up not reported. |
Nyst 1992.
| Methods | Single centre, single country study conducted in the medical wards of Mama Yemo Hospital, Kinshasa, Zaire. Patients enrolled into study from 9 May 1989 to 31 May 1990. Loss to follow‐up: Gentian violet ‐ 23/49 (47%) Ketoconazole ‐ 22/45 (49%) Nystatin ‐ 24/47 (51%) Analysis: no ITT |
|
| Participants | Inclusion criteria: adult patients; no anti mycotic treatment in previous 2 weeks; clinical diagnosis of oropharyngeal candidiasis; confirmed by microscopy. Exclusion criteria: none mentioned. 150 patients enrolled 9 excluded from analysis (3 HIV negative; 6 missing HIV status) 49 ‐ Gentian violet 45 ‐ Ketoconazole 47 ‐ Nystatin |
|
| Interventions | Gentian violet ‐ mouth washes with 0.5% aqueous solution 1.5ml bid; wash for 2 minutes then swallow Ketoconazole ‐ 200mg/day Nystatin ‐ mouth washes with oral suspension 200.000 U qid; wash for 2 minutes then swallow Duration: 10 days or longer until complete clearance of symptoms. |
|
| Outcomes | Primary: Clinical and mycological cure judged on clinical and microscopy assessment. Adverse events: 2 patients receiving gentian violet developed irritation and small superficial ulcers of the oral mucosa 24 hours of start of therapy. |
|
| Notes | Ethics: signed informed consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were stratified into patients with oropharyngeal candida only and patients with both oropharyngeal and esophageal candida. Within these strata consecutive patients received an incremental study number which has been randomised beforehand. Method of sequence generation not reported. |
| Allocation concealment? | Low risk | Listing of study numbers and treatments was kept by one of the authors who ws not involved in the initial assessment to the patients. Treatment to be given only disclosed after patient was enrolled in the study. |
| Blinding? All outcomes | High risk | No blinding due to characteristics of drugs ‐ one is a dye |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No ITT analysis done |
Pagani 2002.
| Methods | Recruited trial subjects from the Swiss HIV Cohort Study Patients were stratified according to CD4 count ( <=50 vs >50) and number of previous oropharyngeal episodes (<2 vs >=2) before randomisation Loss to follow‐up: Fluconazole ‐ 4/71 (6%) Placebo ‐ 1/72 (1%) Analysis: no ITT |
|
| Participants | Inclusion criteria: at least 16 years of age; HIV positive; documented oropharyngeal candidiasis who responded to a 7 day course of treatment with oral fluconazole 200mg daily. Exclusion criteria: < 16 years old; known hypersensitivity to azole compounds; documented Candida isolate resistant to fluconazole from baseline swab culture; ongoing systemic or topical secondary prevention for oropharyngeal candidiasis; ongoing fluconazole therapy for another reason; previous systemic antifungal drug within 15 days of planned study entry; creatinine > 150 micromol/L; ALT or ALP more than 5 times upper normal value. 143 patients enrolled 71 ‐ fluconazole therapy 72 ‐ placebo |
|
| Interventions | Fluconazole 150mg weekly
Placebo weekly Duration: planned follow‐up per patient = 18 months Duration: 7 ‐ 14 days |
|
| Outcomes | Primary outcome was the third relapse of oropharyngeal candidiasis, the occurrence of an adverse event requiring drug discontinuation, and the development of microbiological resistance to fluconazole in association with clinical resistance. Oropharyngeal candidiasis was considered to be clinically documented when examination showed raised confluent white patches on a hyperaemic base or erythema alone. In the latter case it was required that microscopic confirmation be done. Adverse events: no participant dropped out because of a fluconazole related adverse event. |
|
| Notes | Ethics: ethics approval obtained from Ethics committee of Centre Hospitalier Universitaire Vaudois; informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of sequence generation not described. |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Unclear risk | Says double‐blind, but does not report who was blinded. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No ITT analysis |
Phillips 1998a.
| Methods | Multicentre, multi‐country study conducted at 25 centres in seven countries, i.e. Austria, Belgium, Canada, Germany, the Netherlands, Spain and the United Kingdom, from June 1993 to August 1993. Loss to follow‐up: Inctraconacole BD ‐ 21/79 (27%) Ictraconazole daily ‐ 19/79 (24%) Fluconazole ‐ 18/86 (21%) Analysis: no ITT |
|
| Participants | Inclusion criteria: HIV positive adults, at least 19 yrs old, CD4 cell count < 400/mm3 previous month, had OPC Exclusion criteria: inability to take oral medication; systemic antifungal treatment within previous 2 weeks / intraoral topical antifungal treatment within 1 week before the trial; hypersensitivity to azoles; non‐responsive candidiasis to fluconazole or itraconazole, suspicion of candidal esophagitis, liver dysfunction or estimated creatinine clearance < 50ml/min, concurrent use of terfenadine, astemizole, phenytoin, carbamazepine, phenobarbital, rifampicin, oral anticoagulants or sulfonylureas not permitted during trial. Diagnosis confirmed: microscopy with KOH and culture Enrolled 244 79 Ictraconazole BD 79 Ictraconazole daily 86 Fluconazole |
|
| Interventions | Fluconazole capsules (100mg once daily for 14 days ) with placebo oral solution Itraconazole oral solution 100mg once daily for 14 days plus placebo capsules Itraconazole oral solution 100mg bd for 7 days plus placebo capsules |
|
| Outcomes | Clinical response ‐ based upon changes in investigator's rating of signs and symptoms. Severity of symptoms and signs were scored on 3 point scale. Extent of lesions was also scored. Responses classified as complete response or markedly improved, moderately improved, unchanged or deteriorated condition. Mycological efficacy ‐ based on presence or absence of fungal forms consistent with candidiasis Clinical relapse during follow up (at 1 and 2 weeks) Adverse events: GIT (nausea, vomiting, abdominal pain, anorexia and liver enzyme abnormalities), rash, fever, neurological (headache, coma, convulsions and hemiparesis) , hypotension and 1 death in the fluconazole arm. |
|
| Notes | Ethics: approval obtained from each participating centre and informed consent signed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block randomisation: blocks of 12 to one of three treatment groups, according to a predefined randomisation code. ensuring that an equal number of patients were allocated to each treatment group |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double‐blind: participant and investigator blinded. "Active and placebo forms provided by Jansen Research Foundation and were blinded so that neither the investigators or the patients were aware of their contents." |
Phillips 1998b.
| Methods | Same as for Phillips 1998a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Block randomisation: blocks of 12 to one of three treatment groups, according to a predefined randomisation code. ensuring that an equal number of patients were allocated to each treatment group |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double‐blind: participant and investigator blinded. "Active and placebo forms provided by Jansen Research Foundation and were blinded so that neither the investigators or the patients were aware of their contents." |
Pons 1993.
| Methods | Multicentre study conducted in the USA Loss to follow‐up: Fluconazole ‐ 10/176 (6%) Clotrimazole ‐ 17/158 (11%) Analysis: ITT |
|
| Participants | Inclusion criteria:> 17 years, with CDC criteria for AIDS, or serologic/virologic evidence of HIV infection, signs and symptoms of oropharyngeal candidiasis confirmed by KOH prep Exclusion criteria: patients with signs and symptoms of oesophagitis, pregnancy, using any antifungal treatment within 3 days preceding study entry, taking barbiturates, phenytoin, coumarin‐type anticoagulants, rifampicin, oral hypoglycaemics, cyclosporin, known history of intolerance or allergy to imidazoles or triazoles and patients unable to tolerate oral medication, moderate to severe liver disease, breast feeding, life expectancy < 4 weeks and patients unable or unwilling to be followed at the same centre for duration of study. Diagnosis confirmed using KOH and culture 334 enrolled and randomised Fluconazole ‐ 176 Clotrimazole ‐ 158 |
|
| Interventions | Fluconazole ‐ 100 mg once daily for 14 days Clotrimazole ‐ 10 mg five times daily for 14 days |
|
| Outcomes | Clinical cure ‐ resolution of signs and symptoms of oropharyngeal candidiasis Mycologic cure based on culture results Recurrence during follow‐up of cured patients Adverse events: GIT most common, less common included headache, dizziness, pruritus, rash, sweating and dry mouth as well as liver function abnormalities |
|
| Notes | Ethics: written informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated random number codes provided for each study centre. Patients assigned numbers in sequence. |
| Allocation concealment? | Low risk | Randomisation code held by the pharmacy |
| Blinding? All outcomes | High risk | Single‐blind, clinician assessing clinical response and who obtained culture specimens unaware of treatment regimen. Patients not blinded due to nature of treatment |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Analysis: ITT No reasons given for loss to follow‐up |
Pons 1997.
| Methods | Multicentre study in the USA Loss to follow‐up: Fluconazole ‐ 13/83 (16%) Nystatin ‐ 14 /84 (17%) Analysis: no ITT |
|
| Participants | Inclusion criteria: oropharyngeal candidiasis;CDC criteria for HIV/ AIDS; diagnosis confirmed by mycologic culture Exclusion criteria: patients taking other forms of antifungal therapy at/ within 3 days of enrolment; known intolerance to imidazoles, triazoles or polyene components of nystatin, inability to tolerate oral medications, moderate to severe liver disease, life expectancy < 4 weeks, inability to be followed at one centre for study duration. Clinical diagnosis confirmed with KOH and culture 167 patients enrolled and randomised 83 fluconazole 84 nystatin |
|
| Interventions | Fluconazole ‐ 200mg (20ml) loading dose once off on day 1 then 100mg (10ml) once daily for 14 days Nystatin ‐ 5ml (500,000 U) four times daily for 14 days Duration of intervention ‐ 14 days Follow up period ‐ after 28 and 48 days |
|
| Outcomes | Clinical cure ‐ defined as complete resolution of signs and symptoms of oropharyngeal candidiasis Mycologic cure: absence of candidal spp in cultures on day 14 Adverse events: GIT most common |
|
| Notes | Ethics: informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation, method not described |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | High risk | Single blind: clinical evaluator at each study point was unaware of treatment assignment. |
Redding 1992.
| Methods | Study conducted in USA, no dates provided. Loss to follow‐up: none during treatment Analysis: ITT |
|
| Participants | Eligibility criteria: HIV positive adults with thrush; subjective complaints of oral discomfort Exclusion criteria: Other anti‐fungals within 3 days before enrolment; pregnancy; breastfeeding; life expectancy < 4 weeks Diagnosis confirmed: yes ‐ KOH preparation/gram stain; positive culture 24 patients Fluconazole‐13 Clotrimazole‐11 |
|
| Interventions | Fluconazole 100mg tablets once per day
Clotrimazole 10mg troches five times per day Duration: 14 days Clinically cured patients followed‐up for relapse at d28 and d42 |
|
| Outcomes | Clinical cure: defined as absence of lesions
Colonisation: Positive culture of clinically cured
Relapse Adverse events: nausea; flatulence |
|
| Notes | Ethics: no mention No demographics reported Author contacted, no response to date 24/08/2004 Author contacted via email: 08/11/2004 (age groups) Location of study: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Single‐blind: clinician blinded |
| Incomplete outcome data addressed? All outcomes | Unclear risk | ITT analysis. |
Revankar 1998.
| Methods | Multicentre prevention trial. Patients followed up at Univ Texas Health Science Center in San Antonio and the Sout Texas Veterans Health Care System, Audie Murphy Division, San Antonio Texa, USA. Study period was 11 months. Loss to follow‐up: unclear Analysis: no ITT |
|
| Participants | Inclusion criteria: HIV positive with evidence of active oropharyngeal candidiasis by KOH and culture; CD4 cell count< 350x106/l; currently not taking any azole compound Exclusion criteria: known hypersensitivity to azole compounds; unable to take oral medications; pregnant; serum ALT /AST ratio > 10 x normal; serum ALP > 5x normal or bilirubin > 3 x normal. Diagnosis confirmed: yes ‐ KOH preparation and culture 62 patients enrolled 42 ‐ intermittent fluconazole therapy 20 ‐ continuous fluconazole therapy |
|
| Interventions | All patients treated with 200mg fluconazole on day 1 followed by 100mg/day for 4 days or until complete clinical response (resolution of symptoms and signs). Fluconazole ‐ intermittent : treated with fluconazole only during relapses of Candidiasis Fluconazole ‐ continuous : 200mg/day | |
| Outcomes | Primary outcomes ‐ clinical development of lesions
Microbiological counts Clinical response (defined as resolution of symptoms and clearance of lesions) Secondary outcomes ‐ development of yeast isolates with MIC > 16 ug/ml clinical failure to respond to 800mg/day of fluconazole |
|
| Notes | Ethics: approval obtained; informed consent taken Author contacted, no response to date 24/08/2004 Author contacted via email: 08/11/2004 (age groups) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 2:1 using permuted blocks of 6 |
| Allocation concealment? | High risk | Open label |
| Blinding? All outcomes | High risk | Open label study |
| Incomplete outcome data addressed? All outcomes | Low risk | No ITT. Those lost to follow‐up prior to 3 months of follow‐up not included in final analysis Reasons given why lost to follow‐up |
Schuman 1997.
| Methods | Multi‐centre study. Patients enrolled in the Women's Fungal Study (CPCRA 010) at 14 participating sites in the USA. Patients were enrolled between May 1992 and January 1994. The trial started ended on 30 November 1995, 22 months after the last patient was randomised. Median follow‐up was 29 months. Loss to follow‐up: unclear Analysis: unclear |
|
| Participants | Inclusion criteria: Female; HIV positive; >=13 years of age; CD4 count < 300 cells/mm3 or 20% total lymphocyte count Exclusion criteria: history of Candida esophagitis; receiving systemic antifungal agents; known intolerance of azoles; pregnant or breastfeeding Diagnosis confirmed: positive culture and >= 2 signs or symptoms Diagnosis probable: culture and 1 sign / symptom OR KOH positive and >= 2 signs / symptoms OR antifungal response and >= 2 signs / symptoms 323 participants enrolled 162 ‐ Fluconazole 161 ‐ placebo |
|
| Interventions | Fluconazole 200mg / week Placebo |
|
| Outcomes | First episode mucosal Candidiasis Prophylaxis failure ‐ second episode of confirmed oropharyngeal Candidiasis | |
| Notes | Ethics: ethics approval obtained; informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Centrally generated at CPCRA statistical centre; stratified by site and using permuted blocks of sizes 2 and 4 |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double‐blind: Patients and committee reviewing candidiasis |
Smith 1991.
| Methods | Patients were stratified at enrolment into one of three groups ‐ HIV constitutional disease (AIDS related complex), AIDS and AIDS plus esophageal candidiasis Study location: United Kingdom Dates for start and end of study not reported. Loss to follow‐up: Intraconazole ‐ 13/59 (22%) Ketoconazole ‐ 12/52 (23%) Analysis: no ITT. Patients placed on concomitant rifampicin, antacids, who were non‐compliant, or who died from other infections were excluded from the evaluation of efficacy. |
|
| Participants | Inclusion criteria: HIV positive; homosexual men; symptoms of oral disease or clinical appearance of candidiasis; mouth swab revealing numerous fungal hyphae on gram stain 111 participants enrolled 59 ‐ Intraconazole 52 ‐ Ketoconazole |
|
| Interventions | Intraconazole ‐ 200mg daily plus placebo ketoconazole
Ketoconazole ‐ 200mg twice a day plus placebo itraconazole Duration treatment: 28 days |
|
| Outcomes | Clinical evaluation was based on the degree of aphthae, erythema, angular stomatitis, mucosal ulceration and dysphagia and graded as absent (0), mild disease (1), moderate disease (2), or severe disease (3). A clinical response was defined by an improvement of 2 grades or to grade 0 in all signs when compared with pretreatment values. Mouth washings and swabs were cultured and graded according to the number of CFUs: 0‐10 (grade 0), 10‐100 (grade 1), 100‐1000 (grade 2) and > 1000 (grade 3). Mycological response was defined by either improvement of 2 grades or no growth. Relapse was defined as clinical evidence of buccal and /or esophageal candidiasis with mycological confirmation noted between or at monthly follow‐up visits after active treatment. Adverse events: five patients had to stop ketoconazole due to serious toxic events ‐ 2 nausea, 2 hepatotoxicity and 1 generalized erythematous rash. One patient on itraconazole developed a maculopapular rash. |
|
| Notes | Ethics: no mention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method not reported |
| Allocation concealment? | Low risk | Sealed envelopes |
| Blinding? All outcomes | Low risk | Report "double‐blind", unclear who apart from patient blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | No ITT. Those lost to follow‐up not included in evaluation of efficacy |
Stevens 1991.
| Methods | Prevention study in USA Loss to follow‐up: Fluconazole ‐ 3/12 (25%) Placebo ‐ 5/13 (38%) Analysis: ITT |
|
| Participants | Inclusion criteria: at least 18 years of age; previous history of at least one episode of thrush; AIDS or ARC Exclusion criteria: less than 18 years of age; pregnant or lactating; women not observing accepted birth control measures; history of allergy to azoles; inability to take oral drugs; serum creatinine > 221 micromol/L; receiving barbiturates, anticoagulants, hypoglycaemic drugs, immunotherapy, rifampicin or its derivatives, concurrent antifungal agents. Diagnosis confirmed: yes ‐ KOH preparation and culture 25 patients enrolled 12 ‐ fluconazole therapy 13 ‐ placebo |
|
| Interventions | Fluconazole 100mg tablets once per day
Placebo once daily Duration: 12 weeks |
|
| Outcomes | Relapse of thrush defined as the presence of white or rarely red oropharyngeal patches that when smears are examined produced the characteristic microscopic appearance of C Albicans or that on culture yielded C albicans or were positive in both assays. Adverse events: increased liver function tests, GIT symptoms. |
|
| Notes | Ethics: ethics approval obtained from IRb committee for protection of human subjects, Santa Clara Valley Medical Centre and California Institute for medical research, San Jose; informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of sequence generation not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | Low risk | Double‐blind: patients, investigators and caregivers. |
| Incomplete outcome data addressed? All outcomes | Low risk | ITT |
Van Roey 2004.
| Methods | Treatment study. Patients recruited by 6 investigators at 4 hospitals in Kampala, Uganda Loss to follow‐up: Ketoconazole ‐ 25/179 (14%) Miconazole ‐ 24/178 (13%) Analysis: no ITT |
|
| Participants | Inclusion criteria: at least 18 years of age; presumed HIV positive; Life expectancy > 6 months; living within 10 km from study site; presenting with clinical signs of oropharyngeal candidiasis Exclusion criteria: patients who received antifungal therapy within 2 weeks of entry (except local treatment for vaginal candidiasis), pregnant, known history of allergy or intolerance to trial drugs; concomitant use of rifampicin, rifabutin, isoniazid, phenobarbital, phenytoin, carbamazepine, methylprednisolone, terfenadine, astemizole or cisapride; significant hepatic abnormalities. Diagnosis was by clinical examination. Presence of mycelia as observed by microscopic evaluation was documented. 357 patients enrolled 179 ‐ ketoconazole therapy 178 ‐ miconazole nitrate |
|
| Interventions | Ketoconazole 400mg daily
Miconazole nitrate 10mg daily Duration: 7 ‐ 14 days |
|
| Outcomes | Primary outcome was clinical response after 1 week of treatment. Clinical cure was defined as an absence of signs and symptoms (score of 0) as confirmed by the investigator. Signs and symptoms were scored by the same physician at each visit. Symptoms defined as presence (2) or absence (0) of dysphagia, oral pain and loss of taste provided a maximum score of 6. Signs (extent of oral lesions [erythema or removable white plaques]) were scored as 0 = no lesions, 1 = covering < 1 cm2, 3 = moderate covering > 1 cm2 and involving both buccal mucosa and palatal or peritonsillar regions and 4 = severe lesions with extensive involvement of buccal mucosa and palatal peritonsillar regions and pharyngeal mucosa. A subgroup analysis on clinical response was performed based on baseline CD4 count, absence/presence of dysphagia at baseline and concurrent use of broad spectrum antibiotics during the treatment phase. Relapse, amongst those clinically cured, was defined as the recurrence of at least 1 sign or symptom 14 days after treatment stopped. Adverse events: fewer drug related adverse events in miconazole group. |
|
| Notes | Ethics: ethics approval obtained from AIDS research committee; informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation, method of sequence generation not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | High risk | |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No ITT |
Vazquez 2002.
| Methods | Treatment study. A single‐centre study conducted from July 1997 to December 1998 in the USA Loss to follow‐up: 5 of the 27 enrolled participants did not complete treatment. Does not give breakdown per study arm. Analysis: Modified intention to treat analysis done where all randomised participants who received at least one dose of study medication were included |
|
| Participants | Inclusion criteria: between 18 and 65 years of age; HIV positive; presenting with clinical signs of oropharyngeal candidiasis confirmed on KOH and subsequent culture; failed to respond to 14 days or more of 400mg fluconazole daily 27 patients enrolled 13 ‐ alcohol based melaleuca oral solution 14 ‐ alcohol‐free melaleuca oral solution |
|
| Interventions | alcohol based melaleuca oral solution ‐ 15 ml 4 times daily
alcohol‐free melaleuca oral solution ‐ 5 ml 4 times daily
Swish for 30‐60 seconds then expel; no rinsing afterwards for at least 30 minutes Duration: 2 weeks Add 2 weeks therapy if clinical improvement but nor complete clinical response at end of 2 weeks |
|
| Outcomes | Clinical and mycological response.
Clinical assessments were graded as cured (clearance of all symptoms and signs), improved (minimal signs or symptoms remaining), unchanged or deteriorated. An assessment at the end of therapy of cured or improved was considered clinical response. Mycological cure was defined as <20 CFU/ml. Mycological response was defined as a significant decrease in CFU/ml from the baseline yeast count. Overall response was then defined as a clinical plus a mycological response. Adverse events: oral burning experience in 8 receiving alcohol based solution and 2 receiving the alcohol free solution. |
|
| Notes | Ethics: ethics approval obtained; informed consent obtained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Method of sequence generation not reported |
| Allocation concealment? | Unclear risk | Not reported |
| Blinding? All outcomes | High risk | Reported as "open‐label" |
| Incomplete outcome data addressed? All outcomes | High risk | |
Vazquez 2006.
| Methods | Multicenter study conducted from 15 December 1998 to 27 October 1999 at 47 sites of which 19 were in USA and 28 located worldwide, primarily Europe, Latin America, Canada and South Africa. | |
| Participants | 366 patients enrolled and randomised to one of two treatment arms. Eligibility criteria:
Exclusion criteria:
|
|
| Interventions |
|
|
| Outcomes |
Primary: Clinical cure = proportion of patients who were clinically cured or showed improvement after 14 days of treatment Secondary:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No indication how sequence was generated |
| Allocation concealment? | High risk | Not reported |
| Blinding? All outcomes | Low risk | Evaluators were blinded/masked |
Wright 2009.
| Methods | Single Centre study conducted at the Moretele Hospice, South Africa Study period: 10 days Loss to follow‐up:
Analysis: ITT as well as analysis of only those who completed study. |
|
| Participants | Inclusion criteria: HIV+; positive diagnosis of oral thrush; currently not on any medication for oral thrush; willing to participate Exclusion criteria: Patients who did not have thrush at the time of the study. Patiens not expected to remain alive for the study period of 10 days. Grading and diagnosis of oral thrush according to Oral thrush scale. 90 patients enrolled and randomised.
Duration: 10 days. |
|
| Interventions | Gentian violet: 0.5% aqueous solution Lemon juice:20 ml lemon juice diluted with 10 ml water; 2‐3 drops 3 times per day Lemon grass: 12.5ml dried lemon grass in 500 ml boiling water. Drink 125 ml first day and thereafter 250 ml daily |
|
| Outcomes | Clinical success Clinical failure Adverse events reported:
|
|
| Notes | Ethics: Signed informed consent before participation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random allocation, but method of sequence generation not reported |
| Allocation concealment? | Low risk | Identical, sealed, opaque, sequentially numbered envelopes. |
| Blinding? All outcomes | High risk | Nothing reported regarding blinding. Difficult to blind gentian violet. |
| Incomplete outcome data addressed? All outcomes | Low risk | Analysis ITT as well as analysis of only those who completed study. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barbaro 1995a | Oesophageal candidiasis |
| Barbaro 1995b | Oesophageal candidiasis |
| Blomgren 1998 | Not all participants HIV positive. |
| Fichtenbaum 2000 | Patients not randomised |
| Flynn 1995 | Results not given according to stratified groups. |
| Jandourek 1998 | No Randomisation |
| Lim 1991 | Anti‐HIV positive haemophiliacs |
| Moshi 1998 | Authors contacted for additional information to facilitate status of study ‐ no response |
| Nebavi 1998 | Outcome measures |
| Phillips 1996 | No randomisation |
| Plettenberg 1994 | Not a controlled trial. No randomisation |
| Powderly 1995 | Primary endpoint: time to invasive fungal infection |
| Skiest 2007 | Not a randomised controlled trial. High risk of bias. Letter sent to authors to clarify study design. |
| Smith 2001 | Deep fungal infection. |
| Soubry 1991 | Authors contacted for additional information to facilitate status of study ‐ no response |
| Uberti‐Foppa 1989 | Authors contacted for additional information to facilitate status of study ‐ no response |
Characteristics of studies awaiting assessment [ordered by study ID]
Patel 2008.
| Methods | Patients, laboratory staff processing samples, clinic staff were blinded. "Each patient was allocated a mouth rinse randomly..." |
| Participants | 31 males and 119 females Mean age 38; range 21‐56 Not on antiretroviral therapy; treat for opportunistic infections as the need arose. |
| Interventions | 0.03% triclosan +0.025% sodium fluoride (Plax) 0.15% benzydamine hydrochloride (Andolex) 0.15% benzydamine hydrochloride + 0.12% chlorhexidine gluconate (Andolex C) 5% sodium bicarbonate Placebo |
| Outcomes | |
| Notes | Authors contacted to obtain more information on how the allocation sequence was generated. |
Characteristics of ongoing studies [ordered by study ID]
FDA 012M.
| Trial name or title | A randomised multicenter study of the efficacy, safety, and toleration of fluconazole or clotrimazole troches in the treatment of patients with oropharyngeal candidiasis in association with the acquired immunodeficiency syndrome |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Clincal Trials.gov: NCT00002282 |
FDA 236B.
| Trial name or title | An open study of the effect of itraconazole oral solution for the treatment of fluconazole refractory oropharyngeal candidiasis in HIV‐positive subjects |
| Methods | Patients receive itraconazole oral solution twice daily. Complete resolution of OC lesions on completion of treatment ‐ eligible for maintenance ‐ Decline maintenance are followed for six weeks. Relapse during follow‐up are retreated for 14 ‐ 28 days. If lesions clear, enter maintenance. Open label study. |
| Participants | Inclusion criteria: 1 to 65 yrs old; HIV antibody seropositivity or diagnosis of AIDS; Confirmed OC; Failed fluconazole treatment with past 14 days; Life expectancy of at least 3 months; NO symptoms of esophageal candidiasis; No prior disseminate candidiasis |
| Interventions | |
| Outcomes | Endpoint classification: Safety study Primary purpose: Treatment |
| Starting date | Not provided |
| Contact information | Not provided |
| Notes | No publications provided. Study completed. Clincal Trials.gov: NCT00002133 |
FDA 288A.
| Trial name or title | A multicenter, randomised, double‐blind, phase II study to evaluate the safety, tolerance and efficacy of multiple doses of SCH 56591 versus fluconazole in the treatment of oropharyngeal candidiasis (OPC) in HIV‐positive patients |
| Methods | Randomized, double‐blind, multi‐center study consisting of five arms |
| Participants |
Inclusion criteria: 18 to 65 yrs old; Documented HIV seropositivity; Pseudomembranous OC; Fungal stain or KOH consistent with Candida species, confirmed by a positive mycologic culture; Ability to swallow medicine Estimated enrolment: 500 |
| Interventions | 4 dose levels of SCH 56592 vs fluconazole |
| Outcomes | Endpoint classification: Safety study Primary purpose: Treatment |
| Starting date | Not available |
| Contact information | |
| Notes | Study completed. No publications found. Clincal Trials.gov: NCT00002399 |
FDA 305A.
| Trial name or title | Randomized, controlled trial of SCH 56592 oral suspension versus fluconazole suspension in the treatment of oropharyngeal candidiasis (OPC) in HIV positive patients |
| Methods | Patients receive SCH 56592 oral suspension or fluconazole suspension for 14 days. Patients remain on study for 44 days total and re monitored for safety and efficacy of study treatment. |
| Participants | Estimated enrolment: 300 Inclusion criteria: 18 yrs or older; HIV positive; Have thrush (OC); agree to sexual abstinence of use effective barrier methods of birth control; Able to take study medication and return to clinic |
| Interventions | |
| Outcomes | |
| Starting date | August 1998 |
| Contact information | |
| Notes | Study completed. No publications provided. Clincal Trials.gov: NCT00002446 |
Contributions of authors
Elizabeth Pienaar contributed to the protocol design, conducted the literature search, selected studies for inclusion, located copies of study reports, extracted data, and did the data entry and analysis.
Taryn Young selected studies for inclusion, extracted data and assisted with data entry and analysis.
Haly Holmes conceptualised the protocol and assisted with obtaining copies of study reports.
All authors contributed to the writing of the report.
Sources of support
Internal sources
South African Cochrane Centre, Medical Research Council, South Africa.
HIV/AIDS Mentoring Programme, South Africa.
External sources
Effective Health Care Alliance Programme, Department of International Development, UK.
Declarations of interest
We declare that we have no affiliation with or involvement in any organization or entity with a different financial interest in the subject matter of the review for example employment, consultancy, stock ownership, honoraria or expert testimony.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arathoon 2002 {published data only}
- Arathoon EG, Gotuzzo E, Noriega LM, Berman RS, DiNubile MJ, Sable CA. Randomized, double‐blind, multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiasis. Antimicrobial Agents & Chemotherapy 2002 Feb;46(2):451‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chavanet 1992 {published data only}
- Chavanet PY, Garry I, Charlier N, Caillot D, Kisterman J‐P, D'Athis M, et al. Trial of glucose versus fat emulsion in preparation of amphotericin for use in HIV‐infected patients with candidiasis. British Medical Journal 1992;305(17):921‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Repentigny 1996 {published data only}
- Repentigny L, Ratelle J. Comparison of itraconazole and ketoconazole in HIV‐positive patients with oropharyngeal or esophageal candidiasis. Human Immunodeficiency Virus Itraconazole Ketoconazole Project Group.. Chemotherapy 1996;42(5):374‐83. [DOI] [PubMed] [Google Scholar]
De Wit 1989 {published data only}
- Wit S, Goossens H, Weerts D, Clumeck N. Comparison of fluconazole and ketoconazole for oropharyngeal candidiasis in AIDS. Lancet 1989;1(8641):746‐8. [DOI] [PubMed] [Google Scholar]
De Wit 1993 {published data only}
De Wit 1997 {published data only}
- Wit S, Dupont B, Carteledge JD, Hawkins DA, Gazzard BG, Clumeck N, et al. A dose comparison study of a new triazole antifungal (D0870) in HIV‐positive patients with oral candidiasis.. AIDS 1997;11(6):759‐63. [DOI] [PubMed] [Google Scholar]
De Wit 1998 {published data only}
- Wit S, O'Doherty E, Vroey C, Clumeck N. Safety and efficacy of single‐dose fluconazole compared with a 7‐day regimen of itraconazole in the treatment of AIDS‐related oropharyngeal candidiasis. Journal of International Medical Research 1998;26(3):159‐70. [DOI] [PubMed] [Google Scholar]
- Wit S, Goossens H, Clumeck N. Single‐dose versus 7 days of fluconazole treatment for oral candidiasis in human immunodeficiency virus‐infected patients: A prospective, randomized study. Journal of Infectious Diseases 1993;168(5):1332‐3. [DOI] [PubMed] [Google Scholar]
Goldman 2005 {published data only}
- Goldman M, Cloud GA, Wade KD, Reboli AC, Fichtenbaum CJ, Hafner R, et al. A randomized study for the use of fluconazole in continuous versus episodic therapy in patients with advanced HIV infection and a history of oropharyngeal candidiasis: AIDS Clinical Trials Group STudy 323 / Mycoses Study Group Study 40. Clinical Infectious Diseases 2005;41:1473‐80. [DOI] [PubMed] [Google Scholar]
Graybill 1998a {published data only}
- Graybill JR, Vazquez J, Darouiche RO, Morhart R, Greenspan D, Tuazon C, et al. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. American Journal of Medicine 1998;104:33‐9. [DOI] [PubMed] [Google Scholar]
Graybill1998b {published data only}
- Graybill JR, Vazquez J, Darouiche RO, Morhart R, Greenspan D, Tuazon C, et al. Randomized trial of itraconazole oral solution for oropharyngeal candidiasis in HIV/AIDS patients. American Journal of Medicine 1998;104:33‐9. [DOI] [PubMed] [Google Scholar]
Hamza 2008 {published data only}
- Hamza OJM, Matee MIN, Bruggemann RJM, Moshi MJ, Simon ENM, Mugusi F, Mikx FHM, can der Lee HAL, Verweij PE, Ven AJAM. Single‐dose fluconazole versus 2‐week therapy for oropharyngeal Candidiasis in HIV‐infected Patiens: A randomized, double‐blind, double‐dummy trial. Clinical Infectious Diseases 2008;47:1270‐6. [DOI] [PubMed] [Google Scholar]
Hernandez 1994 {published data only}
- Hernandez‐Sampelayo T, Multicentre Study Group. Fluconazole versus Ketoconazole in the treatment of oropharyngeal candidiasis in HIV‐infected children. European Journal of Clinical Microbiology and Infectious Diseases 1994;13:340‐4. [DOI] [PubMed] [Google Scholar]
Just‐Nubling 1991a {published data only}
- Just‐Nubling G, Gentschew G, Meibner K, Odewald J, Staszewski S, Holm EB, et al. Fluconazole prophylaxis of recurrent oral candidiasis in HIV ‐positive patients. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(11):917‐21. [DOI] [PubMed] [Google Scholar]
Just‐Nubling 1991b {published data only}
- Just‐Nubling G, Gentschew G, Meibner K, Odewald J, Staszewski S, Holm EB, et al. Fluconazole prophylaxis of recurrent oral candidiasis in HIV ‐positive patients. European Journal of Clinical Microbiology and Infectious Diseases 1991;10(11):917‐21. [DOI] [PubMed] [Google Scholar]
Leen 1990 {published data only}
- Leen CL, Dunbar EM, Ellis ME, Mandal BK. Once‐weekly fluconazole to prevent recurrence of oropharyngeal candidiasis in patients with AIDS and AIDS‐related complex: a double‐blind placebo‐controlled study. [erratum appears in J Infect 1990 Sep;21(2):183.]. Journal of Infection 1990 Jul;21(1):55‐60. [DOI] [PubMed] [Google Scholar]
Linpiyawan 2000 {published data only}
- Linpiyawan R, Jittreprasert K, Sivayathorn A. Clinical trial: clotrimazole troche vs. itraconazole oral solution in the treatment of oral candidosis in AIDS patients. International Journal of Dermatology 2000 Nov;39(11):859‐61. [DOI] [PubMed] [Google Scholar]
MacPhail 1996 {published data only}
- MacPhail LA, Hilton JF, Dodd CL, Greenspan D. Prophylaxis with nystatin pastilles for HIV associated oral candidiasis. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1996;12(4):470‐6. [DOI] [PubMed] [Google Scholar]
MacPhail 1996b {published data only}
- MacPhail LA, Hilton JF, Dodd CL, Greenspan D. Prophylaxis with nystatin pastilles for HIV associated oral candidiasis. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1996;12(4):470‐6. [DOI] [PubMed] [Google Scholar]
Marriott 1993 {published data only}
- Marriott DJ, Jones PD, Hoy JF, Speed BR, Harkness JL. Fluconazole once a week as secondary prophylaxis against oropharyngeal candidiasis in HIV‐infected patients. A double‐blind placebo‐controlled study. Medical Journal of Australia 1993 Mar 1;158(5):312‐6. [DOI] [PubMed] [Google Scholar]
McKinsey 1999 {published data only}
- McKinsey DS, Wheat LJ, Cloud GA, Pierce M, Black JR, Bamberger DM, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo‐controlled, double‐blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clinical Infectious Diseases 1999 May;28(5):1049‐56. [DOI] [PubMed] [Google Scholar]
Murray 1997 {published data only}
- Murray PA, Koletar SL, Mallegol I, Wu J, Moskovitz BL. Itraconazole oral solution versus Clotrimazole troches for the treatment or oropharyngeal Candidiasis in immunocompromised patients.. Clinical Therapeutics 1997;19(3):471‐80. [DOI] [PubMed] [Google Scholar]
Nittayananta 2008 {published data only}
- Nittayananta W, DeRouen TA, Arirachakaran P, Laothumthut T, Pangsomboon K, Petsantad DS, et al. A randomized clinical trial of chlorhexidine in the maintenance of oral candidiasis‐free period in HIV infection. Oral Diseases 2008;14:665‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyst 1992 {published data only}
- Nyst MJ, Perriens JH, Kimputu L, Lumbila M, Nelson AM, Piot P. Gentian violet, ketoconazole and nystatin in oropharyngeal and esophageal candidiasis in Zairian AIDS patients.. Annales de la Societie Belge de Medecine Tropicale 1992;72(1):45‐52. [PubMed] [Google Scholar]
Pagani 2002 {published data only}
- Pagani JL, Chave JP, Casjka C, Galuser MP, Bille J. Efficacy, tolerability and development of resistance in HIV‐positive patients treated with fluconazole for secondary prevention of oropharyngeal candidiasis: a randomized, double‐blind, placebo‐controlled trial. Journal of Antimicrobial Chemotherapy 2002;50:231‐40. [DOI] [PubMed] [Google Scholar]
Phillips 1998a {published data only}
- Phillips P, Beule K, Frechette G, Tchamouroff S, Vandercam B, Weitner L, et al. A double‐blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clinical Infectious Diseases 1998;26:1368‐73. [DOI] [PubMed] [Google Scholar]
Phillips 1998b {published data only}
- Phillips P, Beule K, Frechette G, Tchamouroff S, Vandercam B, Weitner L, et al. A double‐blind comparison of itraconazole oral solution and fluconazole capsules for the treatment of oropharyngeal candidiasis in patients with AIDS. Clinical Infectious Diseases 1998;26:1368‐73. [DOI] [PubMed] [Google Scholar]
Pons 1993 {published data only}
- Barbacci M, Lichter S, Palenicek J, Chaisson RE, Yamaguchi E. Fluconazole or clotrimazole troche for oropharyngeal candidiasis in HIV‐1 positive patients. International Conference on AIDS. 1990; Vol. 6, issue 1:218 (abstract no. TH.B.385).
- Koletar SL, Russell JA, Fass RA, Plouffe JF. Comparison of oral fluconazole and clotrimazole troches as treatment for oral candidiasis in patients infected with human immunodeficiency virus. Antimicrobial Agents and Chemotherapy 1990;34(11):2267‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons V, Greenspan D, Debruin M. Therapy for oropharyngeal candidiasis in HIV‐infected patients: a randomized, prospective multicenter study of oral fluconazole versus clotrimazole troches. The Multicenter Study Group. [see comments.]. Journal of Acquired Immune Deficiency Syndromes 1993 Dec;6(12):1311‐6. [PubMed] [Google Scholar]
Pons 1997 {published data only}
- Pons V, Greenspan D, Lozada‐Nur F, McPhail L, Gallant JE, Tunkel A, et al. Oropharyngeal candidiasis in patients with AIDS: Randomized comparison of fluconazole versus nystatin oral suspensions. Clinical Infectious Diseases 1997;24(6):1204‐7. [DOI] [PubMed] [Google Scholar]
Redding 1992 {published data only}
- Redding SW, Farinacci GC, Smith JA, Fothergill AW, Rinaldi MG Fothergill AW, et al. A comparison between fluconazole tablets and clotrimazole troches for the treatment of thrush in HIV infection. Special Care in Dentistry 1992;12(1):24‐7. [DOI] [PubMed] [Google Scholar]
Revankar 1998 {published data only}
- Revankar SG, Kirkpatrick WR, McAtee RK, Dib OP, Fothergill AW, Redding SW, et al. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV‐infected patients: Clinical outcomes and development of fluconazole resistance. American Journal of Medicine 1998;105(1):7‐11. [DOI] [PubMed] [Google Scholar]
Schuman 1997 {published data only}
- Schuman P, Capps L, Peng G, Vasquez J, El‐Sadr W, Goldman AI, et al. Weekly fluconazole for the prevention of mucosal candidiasis in women with HIV infection. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1997;126(9):689‐96. [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith DE, Midgley J, Allan M, Connolly GM, Gazzard BG. Itraconazole versus ketaconazole in the treatment of oral oesophageal candidosis in patients infected with HIV.. AIDS 1991;5:1367‐71. [DOI] [PubMed] [Google Scholar]
Stevens 1991 {published data only}
- Lang OS, Stevens DA, Greene SI. Thrush(T) can be prevented in AIDS/ARC patients(pts): Randomized (R) double‐blind placebo (P)‐controlled study of 100mg fluconazole (F) daily.. International Conference on AIDS. 1990; Vol. 6, issue 2:395 (abstract no. 2165).
- Stevens DA, Greene SI, Lang OS. Thrush can be prevented in patients with acquired immunodeficiency syndrome and the acquired immunodeficiency syndrome‐related complex. Randomized, double‐blinded, placebo‐controlled study of 100mg oral Fluconazole daily.. Archives of Internal Medicine 1991;151(12):2458‐64. [PubMed] [Google Scholar]
Van Roey 2004 {published data only}
- Roey J, Haxaire M, Kamya M, Lwanga I, Katabira E. Comparative efficacy of topical therapy with a slow release mucoadhesive buccal tablet containing Miconazole nitrate versus systemic therapy with Ketoconazole in HIV‐positive patients with oropharyngeal Candidiasis.. Journal of Acquired Immune Deficiency Syndrome 2004;35(2):144‐50. [DOI] [PubMed] [Google Scholar]
Vazquez 2002 {published data only}
- Vazquez JA, Zawawi AA. Efficacy of alcohol‐based and alcohol‐free melaleuca oral solution for the treatment of fluconazole‐refractory oropharyngeal candidiasis in patients with AIDS. HIV Clinical Trials 2002;3(5):379‐85. [DOI] [PubMed] [Google Scholar]
Vazquez 2006 {published data only}
- Vazquez JA, Skiest DJ, Nieto L, Northland R, Sanne I, Gogate J, et al. A multicenter randomized trial evaluating posaconazole versus fluconazole for the treatment of oropharyngeal candidiasis in subjects with HIV/AIDS. Clinical Infectious Diseases 2006;42:1179‐86. [DOI] [PubMed] [Google Scholar]
Wright 2009 {published data only}
- Wright SC, Maree JE, Sibanyoni M. Treatment of oral thrush in HIV/AIDS patients with lemon juice and lemon grass (Cymbopogon citratus) and gentian violet. Phytomedicine 2009;16:118‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barbaro 1995a {published data only}
- Barbaro G, Barbarini G, Lorenzo G. Fluconazole vs. flucytosine in the treatment of esophageal candidiasis in AIDS patients: a double‐blind, placebo‐controlled study. Endoscopy 1995 Jun;27(5):377‐83. [DOI] [PubMed] [Google Scholar]
Barbaro 1995b {published data only}
- Barbaro G, Barbarini G, Lorenzo G. Fluconazole compared with itraconazole in the treatment of esophageal candidiasis in AIDS patients: a double‐blind, randomized, controlled clinical study. Scandinavian Journal of Infectious Diseases 1995;27(6):613‐7. [DOI] [PubMed] [Google Scholar]
Blomgren 1998 {published data only}
- Blomgren J, Berggren U, Jontall M. Fluconazole versus Nystatin in the treatment of oral candidosis.. Acta Odontologica Scandinavica 1998;56(44):202‐5. [DOI] [PubMed] [Google Scholar]
Fichtenbaum 2000 {published data only}
- Fichtenbaum CJ, Zackin R, Rajicic N, et al. Amphotericin B oral suspension for fluconazole‐refractory oral candidiasis in persons with HIV infection. AIDS 2000;14:845‐52. [DOI] [PubMed] [Google Scholar]
Flynn 1995 {published data only}
- Flynn PM, Cunningham CK, Kerkering T, San Jorge AR, Peters VB, Pitel PA, Harris J, Gilbert G, Castagnaro L, Robinson P. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. Journal of Pediatrics 1995 Aug;127(2):322‐8. [DOI] [PubMed] [Google Scholar]
Jandourek 1998 {published data only}
- Jandourek A, Vaishampayan JK, Vazquez JA. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS 1998;12(9):1033‐7. [PubMed] [Google Scholar]
Lim 1991 {published data only}
- Lim SG, Lee CA, Hales M, O'Doherty M, winter M, Kernoff PBA. Fluconazole for oropharyngeal candidiasis in anti‐HIV positive haemophiliacs.. Alimentary Pharmacology and Therapeutics 1991;5:199‐205. [DOI] [PubMed] [Google Scholar]
Moshi 1998 {published data only}
- Moshi AH, Jorgensen AF, Pallangyo K. Treatment of oral candidiasis: a study to determine the clinical response of sodium benzoate compared with nystatin suspension. AIDS 1998;12(16):2237‐8. [PubMed] [Google Scholar]
Nebavi 1998 {published data only}
- Nebavi F, Arnvielhe S, Guennec R, Menan E, Kacou A, Combe P, et al. Oropharyngeal candidiasis in AIDS patients from Abidjan (Ivory Coast): antifungal susceptibilities and multilocus enzyme electrophoresis analysis of Candida albican isolates. Pathologie Biologie 1998;46(5):307‐14. [PubMed] [Google Scholar]
Phillips 1996 {published data only}
- Phillips P, Zemcov J, Mahmood W, Montaner JSG, Craib K, Phillips P, Zemcov J, Mahmood W, Montaner JSG, Craib K, Clarke AM. Itraconazole cyclodextrin solution for fluconazole‐refractory oropharyngeal candidiasis in AIDS: correlation of clinical response with in vitro suceptability. AIDS 1996;10(12):1369‐76. [DOI] [PubMed] [Google Scholar]
Plettenberg 1994 {published data only}
- Plettenberg A, Stoehr A, Hoffken G, Bergs C, Tschechne B, Ruhnke M, Heise W, Dieckmann S, Meigel W. Fluconazole therapy of oral candidiasis in HIV‐infected patients: results of a multicentre study. Infection 1994 Mar‐Apr;22(2):118‐23. [DOI] [PubMed] [Google Scholar]
Powderly 1995 {published data only}
- Powderly WG, Finkelstein D, Feinberg J, Frame P, He W, Horst C, Koletar SL, Eyster ME, Carey J, Waskin H. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. [see comments.]. New England Journal of Medicine 1995 Mar 16;332(11):700‐5. [DOI] [PubMed] [Google Scholar]
Skiest 2007 {published data only}
- Skiest DJ, Vazquez JA, Anstead GM, Graybill JR, Reynes J, Ward D, et al. Posaconazole fot the treatment of azole‐refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clinical Infectious Diseases 2007;44:607‐14. [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith DE, Bell J, Johnson M, Youle M, Gazzard B, Tchamouroff S, Frechette G, Schlech W, Miller S, Spencer D, Seifert W, Peeters M, Beule K, The Itraconazole Prophylaxis Study Group. A randomized, double‐blind, placebo‐controlled study of itraconazole capsules for the prevention of deep fungal infections in immunodeficient patients with HIV infection. HIV Medicine 2001 Apr;2(2):78‐83. [DOI] [PubMed] [Google Scholar]
Soubry 1991 {published data only}
- Soubry R, Banyangiliki V, Clerinx J, Vandepere P, Taelman H, Bogaert J, Kagame A, Batungwamayo J. Comparison of itraconazole oral solution and fluconazole capsules in the treatment of oral and esophageal candidiasis in HIV‐infected patients. Preliminary results. Int Conf AIDS. 1991; Vol. 7, issue 1:232 (Abstract no. M.B.2201).
Uberti‐Foppa 1989 {published data only}
- Uberti Foppa C, Cernuschi M, Esposito R. Treatment of HIV+ patients with oropharyngeal and/or oesophageal candidiasis: The results of a D.B. study.. Int Conf AIDS. 1989; Vol. 5:474 (abstract no Th.B.P.348).
References to studies awaiting assessment
Patel 2008 {published data only}
- Patel M, Shackleton JA, Coogan MM, Galpin J. Antifungal effect of mouth rinses on oral Candida counts and salivary flow in treatment‐naïve HIV‐infected patients. AIDS Patient Care and STDs 2008;22(8):613‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
FDA 012M {published data only}
- A randomized multicenter study of the efficacy, safety, and toleration of Fluconazole or Clotrimazole troches in the treatment of patients with oropharyngeal candidiasis in association with the acquired immunodeficiency syndrome. http://ClinicalTrials.gov. [NCT00002282; Protocol ID FDA 012M]
FDA 236B {published data only}
- An open study of the effect of Itraconazole oral solution for the treatment of fluconazole refractory oropharyngeal candidiasis in HIV‐positive subjects.. http://ClinicalTrials.gov. [FDA 236B; NCT00002133]
FDA 288A {published data only}
- A multicenter, randomized, double‐blind, phase II study to evaluate the safety, tolerance and efficacy of multiple doses of SCH 56592 versus fluconazole in the treatment of oropharyngeal candidiasis (OPC) in HIV‐positive patients.. http://ClinicalTrials.gov. [FDA 288A; NCT00002399]
FDA 305A {published data only}
- Randomized controlled trial of SCH 56592 oral suspension versu fluconazole suspension in the treatment of orophryngeal candidiasis (OPC) in HIV‐positive patients. http://ClinicalTrials.gov. [FDA 305A; NCT00002446]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins J [editors]. Cochrane Reviewers' Handbook 4.2.2 (updated March 2004). The Cochrane Library, Issue 3, 2004. Oxford: Update Software. Updated quarterly, 2004. [Google Scholar]
Altman 1996
- Altman DG. Better reporting of randomised controlled trials: the CONSORT statement (editorial). BMJ 1996;313(7057):570‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arendorf 1998
- Arendorf TM, Bredekamp B, Cloete CA, Sauer G. Oral manifestations of HIV infection in 600 South African patients. J Oral Pathol Med 1998 Apr;27(4):176‐9. [DOI] [PubMed] [Google Scholar]
Arribas 2000
- Arribas JR, Hernandez‐Albujar S, Gonzalez‐Garcia JJ, Pena JM, Conzalez A, Canedo T, et al. Impact of protease inhibitor therapy on HIV‐related oropharyngeal candidiasis. AIDS 2000;14(8):979‐84. [DOI] [PubMed] [Google Scholar]
Bektic 2001
- Bektic J, Lell CP, Fuchs A, Stoiber H, Speth C, Lass‐Florl C, et al. HIV protease inhibitors attenuate adherence of Candida albicans to epithelial cells in vitro. FEMS Immunology and Medical Microbiology 2001;31:65‐71. [DOI] [PubMed] [Google Scholar]
Cartledge 1998
- Cartledge JD, Denning DW, Dupont B, Clumeck N, Wit S, Midgley J, Hawkins DA, Gazzard BG. Treatment of HIV‐related fluconazole‐resistant oral candidosis with D0870, a new triazole antifungal. AIDS 1998;12(4):411‐6. [DOI] [PubMed] [Google Scholar]
Cassone 1999
- Cassone A, Bernadis F, Torosantucci A, tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. Journal of Infectious Diseases 1999;180:448‐53. [DOI] [PubMed] [Google Scholar]
Cauda 1999
- Cauda R, Tacconelli E, Tumbarello M, Morace G, Bernadis F, Torosantucci A, Cassone A. Role of protease inhibitors in preventing recurrent oral Candidosis in patients with HIV infection: A prospective case‐control study. Journal of Acquired Immune Deficiency Syndromes 1999;21(1):20‐5. [DOI] [PubMed] [Google Scholar]
CDC 1992
- Centres for Disease Control. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS among Adolescents and Adults. Mortality and Morbidity Weekly Report 1992 Dec 18; Vol. 41, issue RR‐17:1‐19. [PubMed]
CDC 1999
- Centers for Disease Control and Prevention. 1999 USPHS/IDSA Guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus:U.S. Public Health Service (USPHS) and Infectious Diseases Society of America(IDSA). MMWR Morbidity and Mortality weekly Report 1999;48(RR‐10):1‐59; 61‐6. [PubMed] [Google Scholar]
Coogan 2005
- Coogan MM, Greenspan J, Challacombe SJ. Oral lesions in infection with human immunodeficiency virus. Bulletin of the World Health Organization 2005;83(9):700‐6. [PMC free article] [PubMed] [Google Scholar]
De Wit 1998b
- Wit S, O'Doherty E, Edwards J, Yates R, Smith RP, Clumeck AN. Pharmacokinetics of two multiple‐dosing regimens of D0870 in human immunodeficiency virsu‐positive patients: a Phase I study. Antimicrobial Agents and Chemotherapy 1998;42(4):903‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 1998
- Epstein JB, Polsky B. Oropharyngeal candidiasis: A review of its clinical spectrum and current therapies. Clinical Therapeutics 1998;20:40‐57. [DOI] [PubMed] [Google Scholar]
Freedberg 1998
- Freedberg K, Scharfstein JA, Seage 3rd GR, Losina E, Weinstein MC, Craven DE, Paltiel D. The cost‐effectiveness of preventing AIDS‐related opportunistic infections. JAMA 1998;279(2):130‐6. [DOI] [PubMed] [Google Scholar]
Gallant 1994
- Gallant JE, Moore RD, Chaisson RE. Prophylaxis for opportunistic infections in patients with HIV infection. Annals of Internal Medicine 1994;120(11):932‐44. [DOI] [PubMed] [Google Scholar]
Gennaro 2008
- Gennaro S, Naidoo S, Berthold P. Oral health & HIV/AIDS. MCN American Journal of Maternal and Child Nursing 2008;33(1):50‐7. [DOI] [PubMed] [Google Scholar]
Gotzsche 2005
- Gotzsche PC, Johansen HK. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002033] [Google Scholar]
Greenspan 1992
- Greenspan JS, Barr CE, Scuibba JJ, Winkler JR, US Oral Aids Collaborative Group. Oral manifestations of HIV Infection. Definitions, diagnostic criteria and principles of therapy.. Oral Surg Oral Med Oral Pathol 1992;73(2):142‐4. [DOI] [PubMed] [Google Scholar]
Heald 1997
- Healtd AE, Schiffman SS. Taste and smell. Neglected senses that contribute to the malnutrition of AIDS. North Carolina Medical Journal 1997 Mar‐Apr;58(2):100‐4. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Hodgson 2006
- Hodgson TA, Greenspan D, Greenspan JS. Oral lesion of HIV disease and HAART in industralized countries. Advances in Dental Research 2006;19:57‐62. [DOI] [PubMed] [Google Scholar]
Hunter 1998
- Hunter KD, Gibson J, Lockhart P, Pithie A, Bagg J. Fluconazole‐resistant Candida species in the oral flora of fluconazole‐exposed HIV‐positive patients.. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1998;85(5):558‐64. [DOI] [PubMed] [Google Scholar]
Ioannidis 2005
- Ioannidis J, Wilinson D. HIV: prevention of opportunistic infections. Clinical Evidence. Issue 14. London: BMJ Publishing Group, 2005:832‐51. [PubMed] [Google Scholar]
Klein 1984
- Klein RS, Harris CA, Small CB, et al. Oral candidiasis in high risk patients as the initial manifestation of the acquired immunodeficiency syndrome. New England Journal of Medicine 1984;311:354‐8. [DOI] [PubMed] [Google Scholar]
Klotz 2006
- Klotz SA. Oropharyngeal Candidiasis: A new treatment option. Clinical Infectious Diseases 2006;42:1187‐8. [DOI] [PubMed] [Google Scholar]
Martin 1999
- Martin MV. The use of fluconazole and itraconazole in the treatment of Candida albicans infections: a review. Journal of Antimicrobial Chemotherapy 1999;44:429‐37. [DOI] [PubMed] [Google Scholar]
Moher 1987
- Moher D, Schulz KF, Altman D, CONSORT Group. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 1987;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Munro 2002
- Munro C, Hube B. Antifungal therapy at the HAART of antiviral therapy.. Trends Microbiol 2002 Apr;10:173‐7. [DOI] [PubMed] [Google Scholar]
Nittayananta 1997
- Nittayananta W, Chingpanich S. Oral lesions in a group of Thai people with AIDS. Oral Diseases 1997;3(Suppl 1):41‐56. [DOI] [PubMed] [Google Scholar]
Osmond 1998
- Osmond DH. Classification, Staging, and Surveillance of HIV Disease. The AIDS knowledge base http://hivinsite.ucsf.edu/akb/1997/01class 1998.
Oude Lashof 2004
- Oude Lashof AML, Bock R, Herbrecht R. de pauw BE, Krcmery V, Aoun M, et al. An open multicentre comparatives study of the efficacy, safety and tolerance of fluconazole and itraconazole in the treatment of cancer patients with oropharyngeal candidiasis. European Journal of Cancer 2004;40(9):1314‐9. [DOI] [PubMed] [Google Scholar]
Paillaud 2004
- Paillaud E, Merlier I, Dupeyron C, Scherman E, Poupon J, Bories PN. Oral candidiasis and nutritional deficiencies in elderly hospitalised patients. British Journal of Nutrition 2004 Nov;92(5):861‐7. [DOI] [PubMed] [Google Scholar]
Pankhurst 2005
- Pankhurst CL. Candidiasis (oropharyngeal). Clinical Evidence. Issue 14. London: BMJ Publishing Group Ltd, 2005:1‐15. [Google Scholar]
Patton 2001
- Patton LL, Bonito AJ, Shugars DA. A systematic review of the effectiveness of antifungal drugs for the prevention and treatment of oropharyngeal candidiasis in HIV‐positive patients. Oral Surgery, Oral Medicine, Orla Pathology, Oral Radiology and Endodontics 2001;92:170‐9. [DOI] [PubMed] [Google Scholar]
Rachanis 2001
- Rachanis CC. Looking into the mouth ‐ oral manifestations of HIV infection. South African Journal of HIV Medicine 2001 Aug;5:27‐31. [Google Scholar]
Ramos‐Gomez 1999
- Ramos‐Gomez FJ, Flaitz C, Catapano P, Murray P, Milnes AR, Dorenbaum A. Classification, diagnostic criteria, and treatment recommendations for orofacial manifestations in HIV‐infected pediatric patients. Collaborative Workgroup on Oral Manifestations of Pediatric HIV Infection. [Murray P, Milnes AR, Dorenbaum A.]. Journal of Clinical Pediatric Dentistry 1999 Winter;23(2):85‐96. [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay C. How do you include trials with more than two groups into a single meta‐analysis?. http://www.epoc.uottawa.ca/FAQmultiplegroups2003.pdf 2003; Vol. [Accessed: 17/02/2005].
Rex 2000
- Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. Practice Guideline for the Treatment of Candidiasis. Clinical Infectious diseases 2000;30:662‐78. [DOI] [PubMed] [Google Scholar]
Reznik 2005
- Reznik DA. Oral manifestations of HIV disease. Topics in HIV Medicine 2005/2006;13(5):143‐8. [PubMed] [Google Scholar]
Samaranayake 1986
- Samaranayake L, MacFarlane T, Lo, et al. A comparison of oral rinse and imprint sampling techniques for the detection of yeast, coliform and Staphilococcus aureus damage in the oral cavity. Journal of Oral Pathology 1986;15:386‐8. [DOI] [PubMed] [Google Scholar]
Schmidt‐West 2000
- Schmidt‐Westhausen A, Priepke F, Bergman F, Reichardt P. Decline in the rate of oral opportunistic infections following introduction of highly active antiretroviral therapy. J Oral Pathol Med 2000;29:336‐41. [DOI] [PubMed] [Google Scholar]
Vazquez 2000
- Vazquez JA, Arganoza MT, Boikov D, Vaishampayan JK, Akins RA. In vitro susceptibilities of Candida and aspergillus species to Melaleuca alternifolia (tea tree) oil. Revista Iberoamericana de Micologia 2000;17:60‐3. [PubMed] [Google Scholar]
WHO 1993
- World Health Organisation. Interim proposal for a WHO staging system for HIV infection and diseases. Weekly Epidemiological Record 1993;65:221‐4. [Google Scholar]
Williams 1993
- Williams DM for EC‐Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. Classification and diagnostic criteria for oral lesions in HIV infection. Journal of Oral Pathology and Medicine 1993;22(7):289‐91. [PubMed] [Google Scholar]
Yamada 1993
- Yamada H, Tsuda T, Watanabe T, Ohashi M, Murakami K, Mochizuki H. In Vitro and In Vivo Antifungal Activities of D0870, a new triazole agent. Antimicrobial Agents and Chemotherapy 1993;37(11):2412‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2006
- Yang YL, Lo HJ, Hung CC, Li Y. Effect of prolonged HAART on oral colonization with Candida and candidiasis. BMC Infect Dis. 2006 Jan 20;6:8. 2006;20(6):8. [DOI] [PMC free article] [PubMed] [Google Scholar]


