Abstract
The survival benefit from docetaxel, cisplatin and 5‐fluorouracil (TPF) induction chemotherapy in oral squamous cell carcinoma (OSCC) patients is not satisfactory. Previously, we identified that stathmin, a microtubule‐destabilizing protein, is overexpressed in OSCC. Here, we further investigated its role as a biomarker that impacts on OSCC chemosensitivity. We analyzed the predictive value of stathmin on TPF induction chemotherapy and its impact on OSCC cell chemosensitivity. Then, we further investigated the therapeutic effects of the combination therapy of TPF chemotherapy and PI3K‐AKT‐mTOR inhibitors in vitro and in vivo. We found that OSCC patients with low stathmin expression benefited from TPF induction chemotherapy, while OSCC patients with high stathmin expression could not benefit from TPF induction chemotherapy. Stathmin overexpression promoted cellular proliferation and decreased OSCC cell sensitivity to TPF treatment. In addition, inhibition of the PI3K‐AKT‐mTOR signaling pathway decreased stathmin expression and phosphorylation. The combination therapy of TPF chemotherapy and PI3K‐AKT‐mTOR inhibitors exhibited a potent antitumor effect both in vitro and in vivo. Therefore, stathmin can be used as a predictive biomarker for TPF induction chemotherapy and a combination therapy regimen based on stathmin expression might improve the survival of OSCC patients.
Keywords: biomarker, induction chemotherapy, oral squamous cell carcinoma, personalized therapy, translational medicine
The evaluation of stathmin in biopsy tissues has potential as a clinical tool for predicting the outcomes of oral cancer patients undergoing docetaxel, cisplatin, and 5‐fluorouracil (TPF) induction chemotherapy. Combination of TPF chemotherapy and PI3K signaling pathway inhibitors showed potent inhibition of oral cancer cells and xenografts, in which stathmin is highly expressed. Therefore, we are exploring personalized strategies of stathmin expression‐based induction chemotherapy in oral cancer.

1. INTRODUCTION
Considerable efforts have been made to benefit patients with locally advanced oral squamous cell carcinoma (OSCC). Unfortunately, survival rates of these patients have not greatly improved.1, 2 Induction chemotherapy is effective in downgrading locally advanced cancers and in improving the probability of local tumor eradication with surgery and chemoradiotherapy. Docetaxel, cisplatin and 5‐fluorouracil (TPF) induction chemotherapy has been recommended in patients with head and neck squamous cell carcinoma. However, the survival benefit from this treatment in patients with OSCC has not been satisfactory.3, 4, 5, 6 Our previous trial also failed to demonstrate that the use of TPF induction chemotherapy in locally advanced OSCC patients could attain survival benefit.7 Therefore, personalized selection based on biomarkers for TPF induction chemotherapy is necessary.
Stathmin, an important microtubule‐destabilizing protein, is shown to be overexpressed in many human malignancies, including hepatocellular carcinoma and lung, gastric, breast, and head and neck cancers.8, 9, 10, 11 Our previous study suggested that stathmin acts as an oncogene and could be a potential antitumor therapeutic biomarker in OSCC.12 Several studies have shown that overexpression of stathmin influences the resistance of docetaxel and cisplatin.13, 14 Based on the above findings, a new therapy regimen targeting stathmin looks promising and needs to be researched.
PI3K‐AKT‐mTOR signaling pathway (hereafter referred to as the PI3K pathway) inhibitors have been effective in regulating the cytoskeletal protein machinery and modulating stathmin expression and phosphorylation.15, 16 Because the PI3K pathway is one of the most frequently dysregulated pathways in cancers, different types of inhibitors have been applied in the laboratory and clinic. The specific patient population in whom the potency and selectivity of PI3K pathway inhibitors can be further enhanced needs to be identified.17
In this study, which was based on a phase III trial of TPF induction chemotherapy in locally advanced OSCC, we hypothesized that stathmin can be used as a predictive biomarker for survival benefit from TPF induction chemotherapy. Moreover, we evaluated the antitumor efficiency of TPF chemotherapy combined with PI3K pathway inhibitors to explore a potential personalized treatment regime for stathmin‐overexpressed OSCC.
2. MATERIAL AND METHODS
2.1. Patients and immunohistochemical analyses
From March 2008 to December 2010, 256 patients with locally advanced OSCC from a prospective, randomized phase 3 trial (registration ID: NCT01542931) at the Ninth People’s Hospital Shanghai Jiao Tong University School of Medicine (Shanghai, China) were enrolled in the present study. This study was approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Approval Number: 201618, Shanghai, China), and written informed consent was obtained from each patient. Tumor samples were used for immunohistochemical staining against stathmin. Briefly, the sample sections were heated in a water bath at 100°C with a citrate buffer solution (pH 6.0) for 20 minutes to retrieve antigen. The primary antibodies included stathmin (#ab52630, 1:500 dilution; Abcam), Ki‐67 (#19972‐1‐AP, 1:50 dilution; Proteintech) and Bub1 (budding uninhibited by benzimidazoles 1, #13330‐1‐AP, 1:100 dilution; Proteintech). Stathmin immunohistochemical staining score was evaluated as previously described.12
2.2. Cell proliferation, cell cycle and apoptosis assays
For the cell proliferation assay, cells were seeded in 96‐well plates (2 × 103 cells per well, three replicates). After cell adherence, a CCK‐8 kit was used to calculate cell viability at various time points.
For the cell cycle and apoptotic assay, cells were treated with solvent, BKM‐120 (5 μmol L−1), docetaxel (1.5 ng/mL), cisplatin (2 μg/mL), 5‐fluorouracil (1 μg/mL), or a combination of BKM‐120 and docetaxel/cisplatin/5‐fluorouracil (dosage as above) for 48 hours. Cells were washed with cold PBS twice and then fixed in 70% ethanol for 4 hours. Then the staining was performed by using the PI/RNase staining kit (BD Pharmingen) according to the manufacturer’s protocol and analyzed by flow cytometry (FACSCalibur; BD Biosciences). Cell cycle distribution was analyzed by using FlowJo software (BD Biosciences).
Apoptotic assay was analyzed using the FITC‐Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturer’s protocol. Then the apoptosis was analyzed using flow cytometry and FlowJo software.
2.3. Other in vitro assays
Patient, tissue sample, cell line, cell culture and immunofluorescence analyses, stathmin RNA interference and gene transfection, real‐time PCR and western blotting were performed as previously described 7, 12 (Supplementary Methods).
2.4. In vivo studies
Thirty‐six male SPF BALB/c nude mice (nu/nu) were purchased from Shanghai Laboratory Animal Center (Shanghai, China) and housed under pathogen‐free conditions in the Animal Care Facilities at the Ninth People’s Hospital. All mice are 5 weeks old and weighing approximately 20 g. Animal body weight was measured every 3 days. Mice were killed using asphyxiation by CO2 when they showed a 15% reduction from initial body weight or the diameter of grafts exceeded 1.5 cm. All in vivo experiments in this study were approved by the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (Approval Number: 201618, Shanghai, China).
After lentivirus transfection with si‐stathmin and scramble control, 2 × 106 HN13 cells were injected subcutaneously to establish a xenograft model (n = 6 mice per group). When tumor volumes reached 50‐100 mm3, the mice were treated with TP‐based chemotherapy (docetaxel and cisplatin). The dosage of drugs (based on our preliminary experiment results): docetaxel (10 mg/kg intraperitoneally [i.p.] once a week), followed by cisplatin (2.5 mg/kg i.p. three times a week). Tumor size was measured every 7 days using the formula Volume = (Width2 × Length)/2. Mice were killed on day 35 and three tumor samples from each group were fixed in 10% formalin and embedded in paraffin. Routine paraffin sections were prepared for immunohistochemistry and immunofluorescence.
CAL27 cells showed relatively high stathmin expression,12 so this cell line was chosen to conduct follow‐up experiments. A total of 2 × 106 CAL27 cells expressing firefly luciferase were injected subcutaneously into the right flank. After the tumor volumes reached 50‐100 mm3, mice were randomized to the following four groups (n = 6 mice per group): control; BKM‐120; TPF; and combination (BKM‐120 and TPF). Mice from four groups were treated, respectively, with vehicle, BKM‐120 (1.5 mg/kg, oral administration every other day), two cycles of TPF chemotherapy drugs (i.p. first cycle: docetaxel, 7.5 mg/kg; cisplatin, 7.5 mg/kg; 5‐fluorouracil, 15 mg/kg on day 1, followed by 5‐fluorouracil, 15 mg/kg per day from days 2 to 5; the second cycle was administered from days 9 to 13), and BKM‐120 combined TPF chemotherapy drugs (dosage and administration method were as above). The dosage of drugs was determined from our preliminary experiment results. On the days of bioluminescence imaging, anesthesia was induced with inhalational 3%‐4% isoflurane in O2 (to avoid interference between anesthetic and luciferin if both were conducted through i.p.). Mice were then injected i.p. with 80 mg/kg D‐luciferin (Gold Biotechnology) dissolved in sterile saline. Bioluminescence imaging was obtained 15 minutes later using the IVIS Lumina III In Vivo Imaging System (PerkinElmer). All images of tumor‐bearing mice were obtained in identical imaging conditions. Imaging was performed every 6‐7 days after initiation of treatment. Mice were killed on day 25; three tumor samples from each group were fixed in 10% formalin and embedded in paraffin, then routine paraffin sections were prepared for immunohistochemistry. Another three tumor samples from each group were lysed for western blot.
2.5. Statistical analyses
Statistical analyses were performed using GraphPad Prism software, version 6 Demo (GraphPad Software) and SPSS 13.0 for Windows (SPSS). Post‐hoc power calculations were obtained using G*Power 3.1.9.4. Mean values were reported as the mean ± standard deviation. All hypothesis‐generating tests were two‐sided at a significance level of 0.05. The statistical significance of differences between two groups was analyzed using Student t tests. The statistical significance of differences among groups was tested using one‐way analysis of variance. The survival analysis was conducted using the Kaplan‐Meier method and the log‐rank test. The efficacy analysis was conducted using the intention‐to‐treat principle.
3. RESULTS
3.1. Stathmin overexpression correlates with higher clinical stages in oral squamous cell carcinoma patients
The TPF induction chemotherapy trial included 256 patients (age range: 26‐75 years; mean age: 55.4 years). Stathmin expression was evaluated by immunohistochemical staining, and this analysis included 170 patients (78 patients in the control group and 92 in the experimental group). According to quartile, 43 patients (25.3%) were grouped into the low stathmin expression group, 127 patients (74.7%) were grouped into the high stathmin expression group (Figure 1). No significant differences were detected in stathmin expression based on sex, age, primary tumor site, T stage, pathologic different grade, tobacco or alcohol use; however, patients with advanced lymph node metastasis and clinical stage IVA had higher stathmin expression than the patients with negative lymph node metastasis and at clinical stage III, respectively (Table 1).
Figure 1.
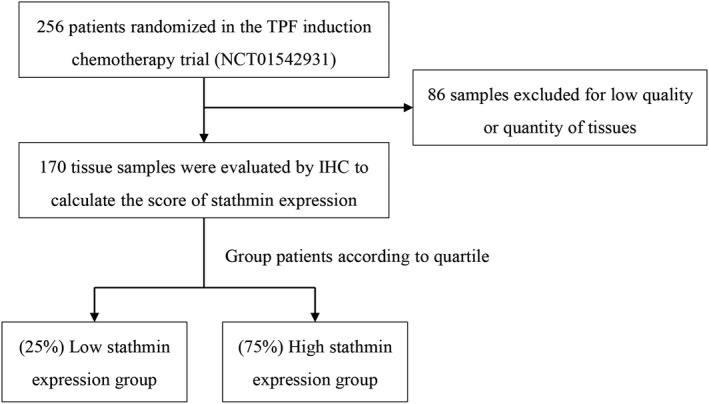
Flow diagram of patients and samples included in the analysis. IHC, immunohistochemistry; TPF, docetaxel, cisplatin and 5‐fluorouracil
Table 1.
Stathmin expression and baseline characteristics
| Characteristics |
Total patients N = 256 |
Stathmin expression | P‐valuea | |
|---|---|---|---|---|
|
Low N = 43 |
High N = 127 |
|||
| n (%) | n (%) | n (%) | ||
| Gender | ||||
| Male | 179 (69.9) | 25 (58.1) | 91 (71.7) | .129 |
| Female | 77 (30.1) | 18 (41.9) | 36 (28.3) | |
| Age (y) | ||||
| <60 | 168 (65.6) | 26 (60.5) | 87 (68.5) | .354 |
| ≥60 | 88 (34.4) | 17 (39.5) | 40 (31.5) | |
| Site | ||||
| Tongue | 113 (44.1) | 20 (46.5) | 52 (40.9) | .065 |
| Buccal | 45 (17.6) | 5 (11.6) | 25 (19.7) | |
| Gingiva | 40 (15.6) | 11 (25.6) | 19 (15.0) | |
| Floor of mouth | 30 (11.7) | 1 (2.3) | 18 (14.1) | |
| Palate | 18 (7.0) | 2 (4.7) | 9 (7.1) | |
| Retromolar trigone | 10 (3.9) | 4 (9.3) | 4 (3.1) | |
| Clinical T descriptor | ||||
| T1/T2 | 66 (25.8) | 12 (27.9) | 31 (24.4) | .885 |
| T3/T4 | 190 (74.2) | 31 (72.1) | 96 (75.6) | |
| Clinical N descriptor | ||||
| N0 | 110 (43.0) | 26 (60.5) | 48 (37.8) | .016 |
| N1 | 94 (36.7) | 14 (32.6) | 51 (40.2) | |
| N2 | 52 (20.3) | 3 (7.0) | 28 (22.0) | |
| Clinical stage | ||||
| III | 177 (69.1) | 39 (90.7) | 87 (68.5) | .004 |
| IVA | 79 (30.9) | 4 (9.3) | 40 (31.5) | |
| Pathologic differentiation | ||||
| Well | 80 (31.2) | 17 (39.5) | 32 (25.2) | .193 |
| Moderately | 165 (64.5) | 24 (55.8) | 86 (67.7) | |
| Poorly | 11 (4.3) | 2 (4.7) | 9 (7.1) | |
| Smoking statusb | ||||
| Current/former | 126 (49.2) | 28 (65.1) | 60 (47.2) | .052 |
| Never | 130 (50.8) | 15 (34.9) | 67 (52.8) | |
| Alcohol usec | ||||
| Positive | 98 (40.6) | 29 (67.4) | 73 (57.5) | .283 |
| Negative | 158 (59.4) | 14 (22.8) | 54 (42.5) | |
P‐value from the χ2 test was presented to compare the difference between low and high stathmin expression based on the different baseline factors.
Former/current smoker was defined as at least one pack‐year history of smoking.
Positive alcohol use was defined as current alcohol use of more than one drink per day for 1 year (12 ounces of beer with 5% alcohol, or five ounces of wine with 12%‐15% alcohol or one ounce of liquor with 45%‐60% alcohol).
3.2. Stathmin expression predicts the response to TPF induction chemotherapy in oral squamous cell carcinoma patients
The median follow‐up period of patients was 80 months. No significant differences were reported in overall survival (OS), distant metastasis‐free survival (DFS), locoregional recurrence‐free survival (LRFS) or distant metastasis‐free survival (DMFS) between patients with and without TPF induction chemotherapy or between low and high stathmin expression groups (Figure 2); however, patients with low stathmin expression benefited from TPF induction chemotherapy compared with patients treated without TPF induction chemotherapy with respect to OS (hazard ratio [HR] = 0.102, 95% confidence interval [CI] = 0.013 to 0.781, P = 0.028, power of test = 59.2%), DFS (HR = 0.070, 95% CI = 0.009 to 0.525, P = 0.010, power of test = 87.3%), LRFS (HR = 0.070, 95% CI = 0.009 to 0.524, P = 0.010, power of test = 86.5%) and DMFS (HR = 0.101, 95% CI = 0.013 to 0.767, P = 0.027, power of test = 60.9%; Figure 3A‐D). In contrast, among patients with high stathmin expression, the difference between the control and TPF induction chemotherapy groups was not significant (OS: HR = 0.692, 95% CI = 0.407 to 1.177, P = 0.174; DFS: HR = 0.888, 95% CI = 0.534 to 1.475, P = 0.646; LRFS: HR = 0.861, 95% CI = 0.517‐1.434, P = 0.565; and DMFS: HR = 0.698, 95% CI = 0.411 to 1.188, P = 0.185; Figure 3E‐H). Because both high stathmin expression and higher clinical stage (stage IVA) were found to be associated with a worse survival, we further performed exploratory prognostic analysis on the treatment of TPF induction chemotherapy in clinical stages III and IVA patients, respectively (Table S1, Figure S1). In the 126 patients at clinical stage III, the patients with low stathmin expression (31%) significantly benefited from TPF induction chemotherapy, while the patients with high stathmin expression (69%) did not benefit from TPF induction chemotherapy. Among the 44 patients at clinical stage IVA, there were only 4 patients (9%) with low stathmin expression; among the 4 patients, 3 patients received TPF induction chemotherapy and were still alive, and 1 patient did not receive TPF induction chemotherapy and died during the follow‐up period (Table S2). Among the other 40 patients with high stathmin expression (91%), the prognostic difference was not significant between the patients receiving and not receiving TPF induction chemotherapy.
Figure 2.
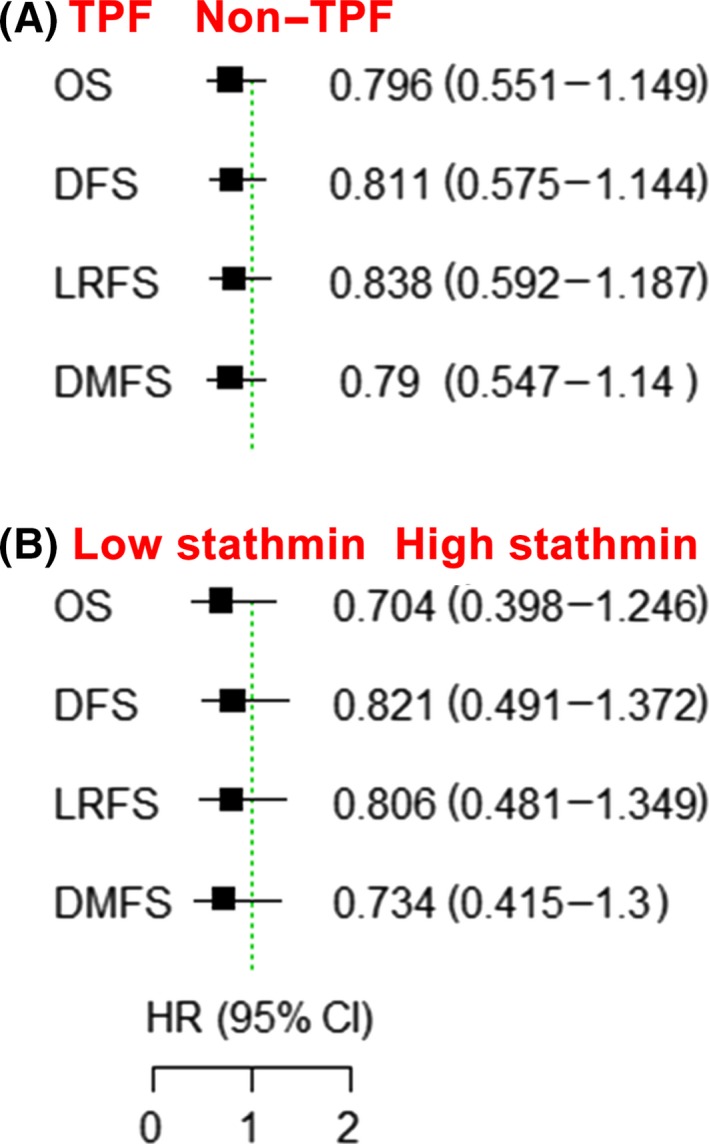
Stathmin expression is not a prognostic biomarker in oral squamous cell carcinoma (OSCC) patients as a whole. A, Clinical outcome between the docetaxel, cisplatin and 5‐fluorouracil (TPF) induction chemotherapy group and the non–TPF induction chemotherapy group with respect to OS, DFS, LRFS and DMFS. B, Clinical outcome between the low stathmin expression and high stathmin expression groups. CI, confidence interval; DFS, disease‐free survival; DMFS, distant metastasis‐free survival; HR, hazard ratio; LRFS, locoregional recurrence‐free survival; OS, overall survival
Figure 3.
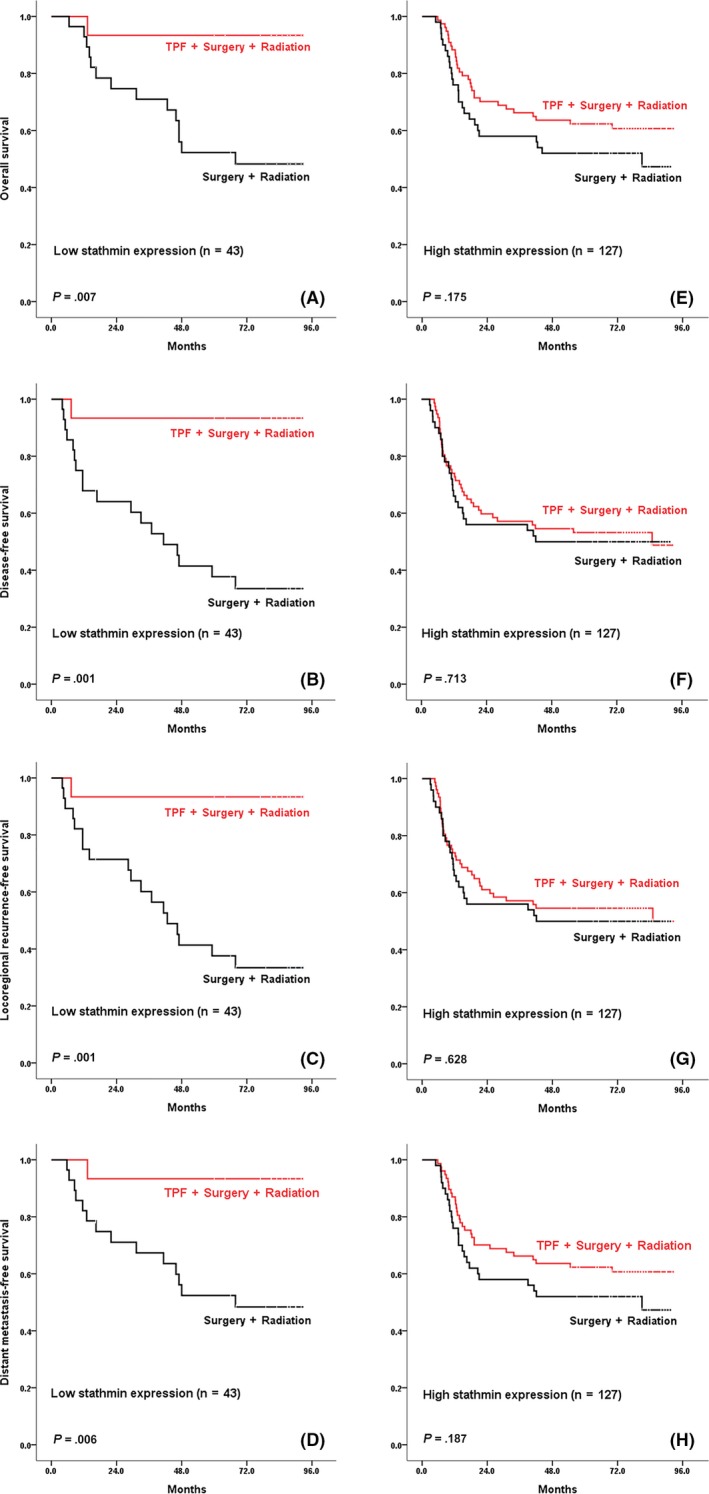
Patients in stathmin low expression subgroup benefit from docetaxel, cisplatin and 5‐fluorouracil (TPF) induction chemotherapy. OS, DFS, LRFS and DMFS were calculated from the date of randomization to death, recurrence, locoregional recurrence, and distant metastasis from any cause, respectively. A‐D, Patients with low stathmin expression. E‐H, Patients with high stathmin expression. P‐values are shown in the figure. DFS, disease‐free survival; DMFS, distant metastasis‐free survival; LRFS, locoregional recurrence‐free survival; OS, overall survival
3.3. Stathmin overexpression promotes cellular proliferation and decreases chemosensitivity in oral squamous cell carcinoma
We further investigated whether stathmin overexpression has an impact on the proliferation and the response to chemotherapy in OSCC cells. We transfected siRNA or pc‐DNA‐stathmin into OSCC cell lines and confirmed the silencing and overexpressing effect (Figure S2). Stathmin overexpression in the CAL27 and HN30 lines led to a significant increase in cellular proliferation (P < 0.05; Figure 4A), accompanied by decreased chemosensitivity to TPF chemotherapy drugs (Figure 4B). Correspondingly, CAL27 and HN4 cells were more sensitive to TPF chemotherapy drugs after silencing of stathmin expression (Figure S3).
Figure 4.
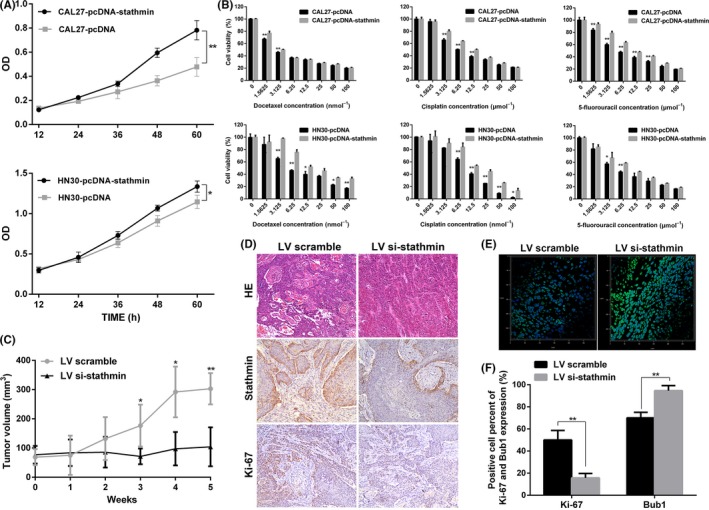
Stathmin overexpression promotes cell proliferation and decreases chemosensitivity to docetaxel, cisplatin and 5‐fluorouracil (TPF) chemotherapy in oral squamous cell carcinoma (OSCC). A, Cellular growth was measured with use of a CCK‐8 kit. B, Inhibition ratios after 72‐h treatment at various drug concentrations in CAL27 and HN30 cells with or without stathmin overexpression. C, OSCC xenograft models were established and treated with docetaxel and cisplatin chemotherapy; tumor growth in stathmin knockdown and control groups was presented and compared. D, HE staining and immunohistochemical staining against stathmin and Ki‐67 in the xenografts (original magnification × 200). E, Immunofluorescence for the percentage of Bub1‐positive cells in the xenografts. F, Differences in percentages of Ki‐67‐positive and Bub1‐positive cells. *P < 0.05, **P < 0.01. Data are expressed as mean ± SD
To test the effects of stathmin expression silencing on sensitivity to chemotherapy drugs in vivo, we injected HN13 lentivirus‐mediated siRNA‐stathmin and scramble cells subcutaneously into BALB/c nude mice and treated them with docetaxel and cisplatin. Tumor size in the siRNA‐stathmin group was significantly smaller than in the scramble group (P < 0.05; Figure 4C). Decreased levels of stathmin and Ki‐67 were detected in the siRNA‐stathmin group compared with the scramble group (Figure 4D). Bub1 is a spindle‐assembly checkpoint protein that controls proper chromosome segregation.18 The percentage of Bub1‐positive cells in the siRNA‐stathmin group was significantly higher than that in the scramble group (94.7 ± 4.5 vs 70.4 ± 5.0, P = 0.003; Figure 4E,F).
3.4. PI3K‐AKT‐mTOR inhibitors decrease stathmin expression and phosphorylation and enhance apoptosis induced by TPF chemotherapy in oral squamous cell carcinoma cells
In OSCC patients, the PI3K‐AKT‐mTOR signaling pathway was shown to be activated (Figure S4). We treated OSCC cell lines with various PI3K‐AKT‐mTOR signaling pathway inhibitors targeting different sites, and stathmin expression and phosphorylation were found to be significantly suppressed (Figures 5A and S5). In addition, A6730, an AKT inhibitor, induced potent inhibition of p‐AKT but slight inhibition of p‐S6, stathmin and p‐stathmin; KU0063794, a mTOR inhibitor, induced reliable inhibition of p‐S6 but slight inhibition of stathmin and p‐stathmin and no inhibition of p‐AKT; LY294002, a PI3K inhibitor, led to more potent stathmin and p‐stathmin inhibition compared with AKT and mTOR inhibitors, although rebound activation of p‐AKT was observed in LY294002‐treated cells. BKM‐120, a pan‐PI3K inhibitor, led to more potent and durable inhibition than did LY294002 and other downstream inhibitors (Figure 5B); therefore, BKM‐120 was selected for subsequent biological experiments.
Figure 5.
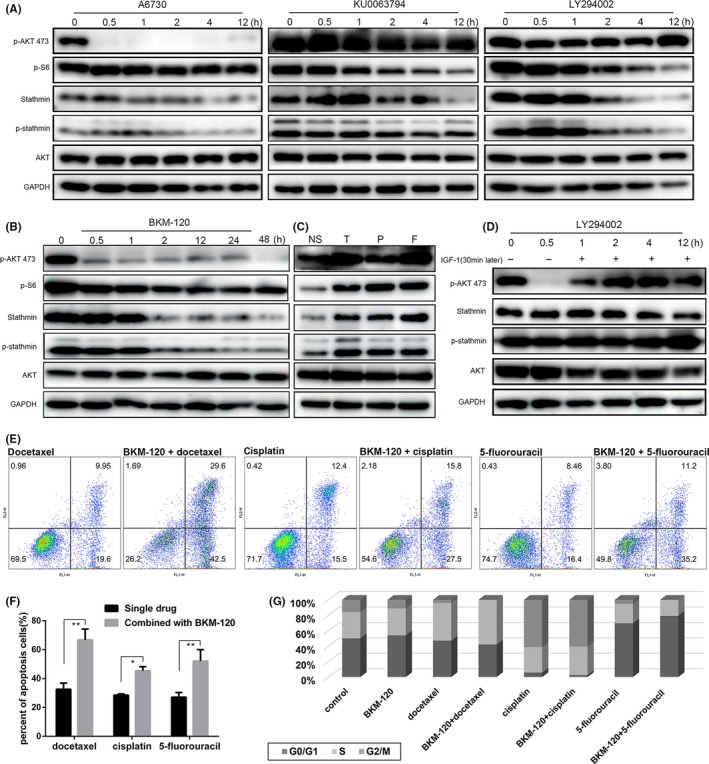
PI3K‐AKT‐mTOR pathway inhibitors decreased stathmin expression and phosphorylation. A‐C, CAL27 cells were treated with LY294002 (10 μmol L−1), A6730 (10 μmol L−1), KU0063794 (1 μmol L−1), BKM‐120 (5 μmol L−1) and TPF chemotherapy drugs (docetaxel: 1.5 ng/mL, cisplatin: 2 μg/mL, 5‐fluorouracil: 1 μg/mL), and then western blotting was performed. D, For the rescue experiment for stathmin inhibition, CAL27 cells were treated with LY294002 for 1 h, after which IGF‐1 (50 ng/mL) was added to the cell culture medium for an additional 11 h, then western blotting was performed. E, Cell apoptosis in groups treated with TPF chemotherapy drugs and in combined treatment groups. F, Statistical analysis of apoptosis ratio from two independent experiments. G, Percentages of different cell phases were visualized in the form of a histogram of CAL27 cells. *P < 0.05, **P < 0.01. Data are expressed as mean ± SD
We also treated cells with TPF chemotherapy drugs and found no significant change in stathmin expression and phosphorylation after treatment (Figure 5C). We used insulin‐like growth factor 1 (IGF‐1), which was considered an effective PI3K pathway agonist, to rescue the inhibition effect. CAL27 cells were treated with LY294002 for 1 hour, after which IGF‐1 (50 ng/mL) was added to the cell culture medium for an additional 11 hours; then the proteins from cell lysis were analyzed. The inhibition effect of stathmin expression and phosphorylation could be rescued by IGF‐1 (Figure 5D).
We then treated OSCC cells with BKM‐120 combined with chemotherapy drugs and detected cellular apoptosis and cell cycle arrest. The percentage of apoptotic cells in the combination groups was significantly higher than in the chemotherapy drug‐alone groups (Figure 5E,F). Docetaxel decreased the percentage of G1 and G2 phase cells and increased the percentage of S phase cells. Cisplatin arrested cells in the G2 phase, whereas 5‐fluorouracil arrested cells in the G1 phase. BKM‐120 heightened the effect in the cell cycle of these three drugs (Figure 5G and S6).
3.5. The combination of TPF chemotherapy and PI3K inhibition shows a potent antitumor effect in vivo
We used CAL27 cell line to establish xenograft tumor models. We divided tumor‐bearing mice into four groups and treated them, respectively, with solvent, BKM‐120, TPF drugs, and combination therapy consisting of BKM‐120 and TPF drugs. Tumors from the combination group grew significant more slowly than did those in the TPF group (Figure 6A,B). Animals from the combination group had greater weight loss than did those in the control group but had no greater weight loss than did those in the TPF group (Figure 6C). Tumors in the combination group had a more significant pathological response (Figure 6D,E). Western blot analysis of xenografts showed that BKM‐120 treatment significantly suppressed stathmin expression and phosphorylation in vivo (Figure 6F).
Figure 6.
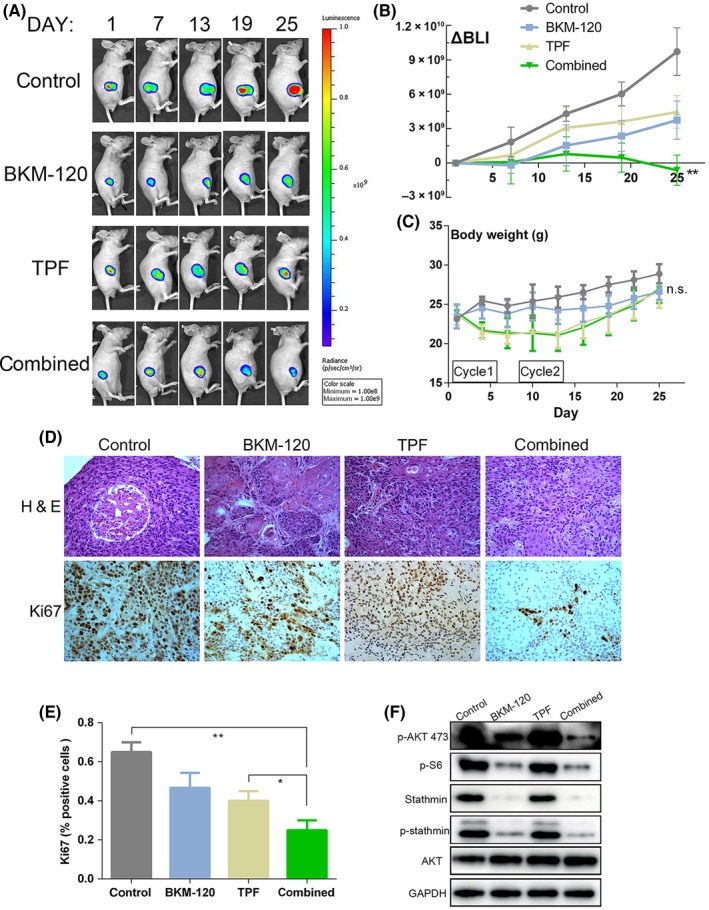
The combination of BKM‐120 and docetaxel, cisplatin and 5‐fluorouracil (TPF) chemotherapy potently inhibits the growth of oral squamous cell carcinoma (OSCC) xenografts. A, Bioluminescence imaging of tumor‐bearing mice. Representative images in four treatment groups are shown in the figure. B, Regions of interest from displayed images were revealed on the tumor sites and bioluminescence intensity (BLI) in tumors over time was measured, quantified as total radiance/s. C, Tumor‐bearing mice body weight in four treatment groups. D, Tumor samples were stained with H&E, and proliferating tumors cells were identified by immunohistochemistry for Ki67. Representative images from four groups are shown in the figure (original magnification × 200). E, Data shown represent mean ± SD of Ki67 index in of three random high‐power microscopic fields from tumors in each group. F, Tumor samples were harvested, lysed and analyzed by western blotting as indicated. *P < 0.05, **P < 0.01, n.s. = not significant. Data are expressed as mean ± SD
4. DISCUSSION
Our results showed that patients with low stathmin expression would benefit from TPF induction chemotherapy before radical surgery and postoperative radiotherapy. We further confirmed that stathmin overexpression led to improved cellular proliferation and decreased chemosensitivity to TPF chemotherapy drugs in OSCC cells and xenografts. In seeking to optimize the protocol for TPF induction chemotherapy in patients with high stathmin expression, a PI3K inhibitor was shown to be a good choice to improve the inductive effect of TPF chemotherapy.
Although the difference of smoking status between low and high stathmin expression group is not statistically significant in our study, we do find more non–smokers among low stathmin expression patients. The dosage of smoking is reported to be directly associated with the expression of serum markers of telomere dysfunction and DNA damage, including stathmin.19 However, no study has been published on the correlation between smoking and stathmin expression in tumor samples. It would be meaningful to investigate the real correlation between them in future research. Several previous studies showed stathmin could directly predict the prognosis of cancer patients,20, 21 but we did not obtain accordant results in this study. The possible reason is that patients in this study, following the inclusion criteria for our clinical trial, were all at clinical stages III and IVA. So, the prognosis analysis here should not be supposed to represent the prognostic value of stathmin in all OSCC patients. In spite of this, high stathmin expression is shown to be correlated with advanced lymph node metastasis and higher clinical stage in this study.
Furthermore, we demonstrated that stathmin could be used as a predictive biomarker for TPF chemotherapy. For OSCC patients with low stathmin expression, TPF induction chemotherapy should be recommended to improve clinical outcomes. This finding is consistent with previous studies.22, 23 In contrast, for OSCC patients with high stathmin expression, TPF induction chemotherapy failed to improve survival. Therefore, the standard treatment of surgery and postoperative radiotherapy without the addition of TPF induction chemotherapy is comparatively better in OSCC patients with high stathmin expression, considering the adverse effects of TPF chemotherapy drugs, the treatment period and the economic burden. Chemoresistance to docetaxel with stathmin overexpression may be caused by the microtubule‐destabilizing activity of stathmin, which may diminish binding to docetaxel and increase binding to vincristine‐like drugs.24 Chemoresistance to cisplatin and 5‐fluorouracil with stathmin overexpression may be caused by the participation of stathmin overexpression in the cellular response to DNA damage and caspase‐6‐mediated cellular apoptosis, as described in previous studies conducted in head and neck cancer or colorectal cancer, respectively.13, 25 To some extent, these reasons lead to the unsatisfactory clinical benefit of TPF induction chemotherapy in OSCC patients with high stathmin expression.
To identify a better regimen based on TPF induction chemotherapy for high stathmin expression patients, targeted therapy drugs were tried in this study. Because stathmin has been shown to correlate with the PI3K pathway activity in previous studies,15, 26 we chose PI3K inhibitors and confirmed that inhibition of the PI3K pathway could decrease stathmin expression and phosphorylation in OSCC, which is in line with previous research.16, 27, 28 We also found that knockdown or overexpression of stathmin did not significantly affect p‐AKT, suggesting that stathmin is at the downstream of AKT. Some studies in other types of cancer have shown that stathmin regulates the PI3K or mTOR pathway, but each study is focused only on stathmin expression or phosphorylation and few rescue experiments are conducted.29, 30 Another possible reason for this divergence is that stathmin may have some feedback effect on the upstream PI3K pathway.
The combination therapy of BKM‐120 and TPF chemotherapy that we used in the stathmin highly expressing OSCC xenograft model showed a potent therapeutic effect. This result is in line with a phase 2 trial that suggested that BKM‐120 in combination with paclitaxel could be an effective second‐line treatment for head and neck squamous cell carcinoma patients.31 However, two other trials failed to demonstrate improvement from the addition of BKM‐120 to chemotherapy in the full or in a PI3K pathway‐activated study population.32, 33 Here we find for the first time that PI3K inhibitors decrease stathmin expression and phosphorylation. Therefore, high stathmin expression is a potential selection criterion for adding PI3K pathway inhibitors to chemotherapy, especially when high stathmin expression predicts poor survival of TPF induction chemotherapy at the same time.
The predictive value of stathmin expression in OSCC patients for TPF induction chemotherapy was established by the retrospective analysis in this study. To further verify this predictive value, we have an ongoing prospective phase 2 trial of low stathmin expression as a predictive biomarker for OSCC patients receiving TPF induction chemotherapy followed by surgery and radiotherapy or chemoradiotherapy (NCT03326947). In the meantime, the detailed mechanism of the effects of PI3K pathway inhibitors on the regulation of stathmin expression and phosphorylation is still not clear. Moreover, the dosage of each component in the combination protocol of a PI3K inhibitor and TPF chemotherapy drugs has not been suggested from this study. More mechanical research concerning stathmin’s role in chemoresistance and the predictive value of targeted therapy is needed. Further clinical trials are warranted to verify this optimized personalized protocol based on stathmin expression in OSCC patients.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81672660, 81602370 and 81472519), the Shuguang Program of Shanghai Municipal Education Commission [17SG18], the Shanghai Municipal Commission of Health and Family Planning (SHDC12017X02 and 2018BR41), the Program of Shanghai Academic/Technology Research Leader (19XD1422300) and the Joint PhD Program of China Scholarship Council (201806230235). We thank the ATCC and the NIH for cell lines. We also thank the Department of Scientific Publications in the MD Anderson Cancer Center for the help with manuscript editing.
Ju W‐T, Ma H‐L, Zhao T‐C, et al. Stathmin guides personalized therapy in oral squamous cell carcinoma. Cancer Sci. 2020;111:1303–1313. 10.1111/cas.14323
Ju, Ma and Zhao are contributed equally to this work.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015;33:3269‐3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705‐1715. [DOI] [PubMed] [Google Scholar]
- 4. Kong L, Zhang Y, Hu C, Guo Y, Lu JJ. Effects of induction taxotere, platinum, and fluorouracil (TPF) chemotherapy in patients with stage III and IVA/B nasopharyngeal cancer treated with concurrent chemoradiation therapy: final results of two parallel phase 2 clinical trials. Cancer. 2017;123:2258‐2267. [DOI] [PubMed] [Google Scholar]
- 5. Haddad RI, Posner M, Hitt R, et al. Induction chemotherapy in locally advanced squamous cell carcinoma of the head and neck: role, controversy, and future directions. Ann Oncol. 2018;29:1130‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inhestern J, Schmalenberg H, Dietz A, et al. A two‐arm multicenter phase II trial of one cycle chemoselection split‐dose docetaxel, cisplatin and 5‐fluorouracil (TPF) induction chemotherapy before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC‐1). Ann Oncol. 2017;28:1917‐1922. [DOI] [PubMed] [Google Scholar]
- 7. Zhong L‐P, Zhang C‐P, Ren G‐X, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up‐front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niethammer P, Bastiaens P, Karsenti E. Stathmin‐tubulin interaction gradients in motile and mitotic cells. Science. 2004;303:1862‐1866. [DOI] [PubMed] [Google Scholar]
- 9. Baquero MT, Hanna JA, Neumeister V, et al. Stathmin expression and its relationship to microtubule‐associated protein tau and outcome in breast cancer. Cancer. 2012;118:4660‐4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nie W, Xu MD, Gan L, Huang H, Xiu Q, Li B. Overexpression of stathmin 1 is a poor prognostic biomarker in non–small cell lung cancer. Lab Invest. 2015;95:56‐64. [DOI] [PubMed] [Google Scholar]
- 11. Biaoxue R, Xiguang C, Hua L, Shuanying Y. Stathmin‐dependent molecular targeting therapy for malignant tumor: the latest 5 years’ discoveries and developments. J Transl Med. 2016;14:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma H‐L, Jin S‐F, Ju W‐T, et al. Stathmin is overexpressed and regulated by mutant p53 in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin X, Liao Y, Xie J, Liu S, Su L, Zou H. Op18/stathmin is involved in the resistance of taxol among different epithelial carcinoma cell lines. Cancer Biother Radiopharm. 2014;29:376‐386. [DOI] [PubMed] [Google Scholar]
- 14. Johnsson A, Zeelenberg I, Min Y, et al. Identification of genes differentially expressed in association with acquired cisplatin resistance. Br J Cancer. 2000;83:1047‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen JN, Sathyanarayanan S, Di Bacco A, et al. Pathway‐based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors. Sci Transl Med. 2000;2:43‐55. [DOI] [PubMed] [Google Scholar]
- 16. Wik E, Birkeland E, Trovik J, et al. High phospho‐Stathmin(Serine38) expression identifies aggressive endometrial cancer and suggests an association with PI3K inhibition. Clin Cancer Res. 2013;19:2331‐2341. [DOI] [PubMed] [Google Scholar]
- 17. Janku F, Yap TA, Meric‐Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15:273‐291. [DOI] [PubMed] [Google Scholar]
- 18. Marchetti F, Venkatachalam S. The multiple roles of Bub1 in chromosome segregation during mitosis and meiosis. Cell Cycle. 2010;9:58‐63. [DOI] [PubMed] [Google Scholar]
- 19. Song Z, Von Figura G, Liu Y, et al. Lifestyle impacts on the aging‐associated expression of biomarkers of DNA damage and telomere dysfunction in human blood. Aging Cell. 2010;9:607‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao C, Li H, Wang L, Sun W. An immunohistochemical study of Stathmin 1 expression in osteosarcoma shows an association with metastases and poor patient prognosis. Med Sci Monit. 2018;24:6070‐6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rong B, Nan Y, Liu H, Gao W. Increased stathmin correlates with advanced stage and poor survival of non–small cell lung cancer. Cancer Biomark. 2017;19:35‐43. [DOI] [PubMed] [Google Scholar]
- 22. Harada K, Ferdous T, Harada T, Ueyama Y. High expression of stathmin 1 is a strong prognostic marker in oral squamous cell carcinoma patients treated by docetaxel‐containing regimens. Clin Exp Med. 2017;17:45‐50. [DOI] [PubMed] [Google Scholar]
- 23. Kouzu Y, Uzawa K, Koike H, et al. Overexpression of stathmin in oral squamous‐cell carcinoma: correlation with tumour progression and poor prognosis. Br J Cancer. 2006;94:717‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alli E, Bash‐Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864‐6869. [PubMed] [Google Scholar]
- 25. Wu W, Tan XF, Tan HT, Lim TK, Chung MC. Unbiased proteomic and transcript analyses reveal that stathmin‐1 silencing inhibits colorectal cancer metastasis and sensitizes to 5‐fluorouracil treatment. Mol Cancer Res. 2014;12:1717‐1728. [DOI] [PubMed] [Google Scholar]
- 26. Bohnacker T, Prota AE, Beaufils F, et al. Deconvolution of Buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat Commun. 2017;8:14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci. 2007;104:7564‐7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang W, Huang S, Song L, Wang Z. STMN1, a prognostic predictor of esophageal squamous cell carcinoma, is a marker of the activation of the PI3K pathway. Oncol Rep. 2018;39:834‐842. [DOI] [PubMed] [Google Scholar]
- 29. Schimmack S, Taylor A, Lawrence B, et al. Stathmin in pancreatic neuroendocrine neoplasms: a marker of proliferation and PI3K signaling. Tumour Biol. 2015;36:399‐408. [DOI] [PubMed] [Google Scholar]
- 30. Wang Z, He R, Xia H, Wei Y, Wu S. Knockdown of STMN1 enhances osteosarcoma cell chemosensitivity through inhibition of autophagy. Oncol Lett. 2017;13:3465‐3470. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31. Soulières D, Faivre S, Mesía R, et al. Buparlisib and paclitaxel in patients with platinum‐pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL‐1): a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Oncol. 2017;18:323‐335. [DOI] [PubMed] [Google Scholar]
- 32. Martín M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan‐class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2‐ advanced breast cancer (BELLE‐4). Ann Oncol. 2017;28:313‐320. [DOI] [PubMed] [Google Scholar]
- 33. Loibl S, de la Pena L, Nekljudova V, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double‐blind, placebo‐controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


