Abstract
Pancreaticoduodenectomy (PD) is the commonest procedure performed for pancreatic cancer. Pancreatic exocrine insufficiency (PEI) may be caused or exacerbated by surgery and remains underdiagnosed and undertreated. The aim of this review was to ascertain the incidence of PEI, its consequences and management in the setting of PD for indications other than chronic pancreatitis. A literature search of databases (MEDLINE, EMBASE, Cochrane and Scopus) was carried out with the MeSH terms “pancreatic exocrine insufficiency” and “Pancreaticoduodenectomy”. Studies that analysed PEI and its complications in the setting of PD for malignant and benign disease were included. Studies reporting PEI in the setting of PD for chronic pancreatitis, conference abstracts and reviews were excluded. The incidence of PEI approached 100% following PD in some series. The pre-operative incidence varied depending on the characteristics of the patient cohort and it was higher (46%-93%) in series where pancreatic cancer was the predominant indication for surgery. Variability was also recorded with regards to the method used for the diagnosis and evaluation of pancreatic function and malabsorption. Pancreatic enzyme replacement therapy is the mainstay of the management. PEI is common and remains undertreated after PD. Future studies are required for the identification of a well-tolerated, reliable and reproducible diagnostic test in this setting.
Keywords: Pancreatic exocrine insufficiency, Pancreaticoduodenectomy, Pancreatic enzyme replacement therapy, Pancreatic cancer, Malabsorption, Steatorrhoea
Core tip: Pancreatic exocrine insufficiency is highly prevalent after pancreaticoduodenectomy and has significant implications to patients’ quality of life, nutrition, post-operative survival and cancer related outcomes. The published literature reveals no uniform definition of pancreatic exocrine insufficiency after surgery and patients are often under diagnosed. Pancreatic enzyme replacement therapy is effective, well tolerated and is indicated routinely in this cohort of patients. Future studies need to focus on the identification of a well-tolerated, reliable and reproducible diagnostic test in this setting that will facilitate a uniform definition and management approach.
INTRODUCTION
Pancreatic enzymes are an essential component of normal digestion, without which severe malnutrition occurs. Nonetheless, pancreatic exocrine insufficiency remains widely under-diagnosed and undertreated. The physiological secretion of pancreatic enzymes is in response to nutritional intake in healthy individuals. The stimulation occurs through three phases: Cephalic, gastric and the most important intestinal phase[1]. The pancreatic enzyme secretion peaks at about 30 min after the exposure of the duodenum to nutrients and returns to baseline after about 2-4 h. The presence of undigested food, especially fat, in the terminal ileum exerts a robust negative feedback mechanism[2-7].
Pancreatic exocrine insufficiency (PEI) is a common and recognized outcome after pancreatic surgery. Multiple definitions have been used in the published literature based on various evaluation parameters. The “broadest” definition was presented in a systematic review by the Spanish pancreatic association and defined PEI as the inability of the pancreas to perform digestion in association with disturbed pancreatic function[8].
Pancreaticoduodenectomy (PD) is an operative procedure that involves resection of the pancreatic head in addition to the duodenum and bile duct. It is the most common pancreatic resection performed, especially in the setting of pancreatic malignancy. The effect of PD on pancreatic exocrine secretion is multifactorial. The degree of insufficiency is influenced by the pancreatic remnant[9], preservation or resection of the gastric antrum and duodenum[10], the use of a roux-en-Y loop with asynchrony of delivery of the pancreatic enzymes[11,12] and other factors, such as the peri-operative use of Octreotide[13]. In the context of pancreatic surgery, PEI has been associated with prolonged hospital stay[14], increased complication rates[15], reduced survival[16], worse quality of life[8] and nutritional deficiencies[17]. Furthermore, the presence of PEI may also impede the progression of patients to adjuvant chemotherapy in the setting of resections performed for malignancy.
The purpose of this paper is a comprehensive review of the current evidence on the incidence and management of PEI specifically in the setting of PD for indications other than chronic pancreatitis.
STUDY SELECTION
The intention was to proceed with a systematic review of the incidence and management of PEI in the setting of PD. Studies were selected in accordance with Preferred Reporting of Systematic Reviews and Meta-analyses (PRISMA) 2009 guidelines[18].
A literature search of databases (MEDLINE, EMBASE, Cochrane and Scopus) was carried out by two separate authors (AP and JA). The search was constructed by using the Medical Subject Heading (MeSH) terms “pancreatic exocrine insufficiency” and “Pancreaticoduodenectomy”. Studies that analysed the incidence of PEI in the setting of PD were included. Studies that focused on complications of PEI after PD were also included. Case reports, reviews, consensus statements, conference abstracts and articles in languages other than English were excluded. Studies on pancreatic resections for chronic pancreatitis were excluded, as well as studies that did not sub-classify patients according to the type of pancreatic resection and therefore data on PEI after PD could not be extracted.
The search led to a total of 746 hits. After removal of duplicates and articles in languages other than English, 556 articles remained. Further screening and full text review of articles resulted in a total of 34 articles eligible for inclusion in the review. The steps of the selection process are collated in Figure 1.
Figure 1.
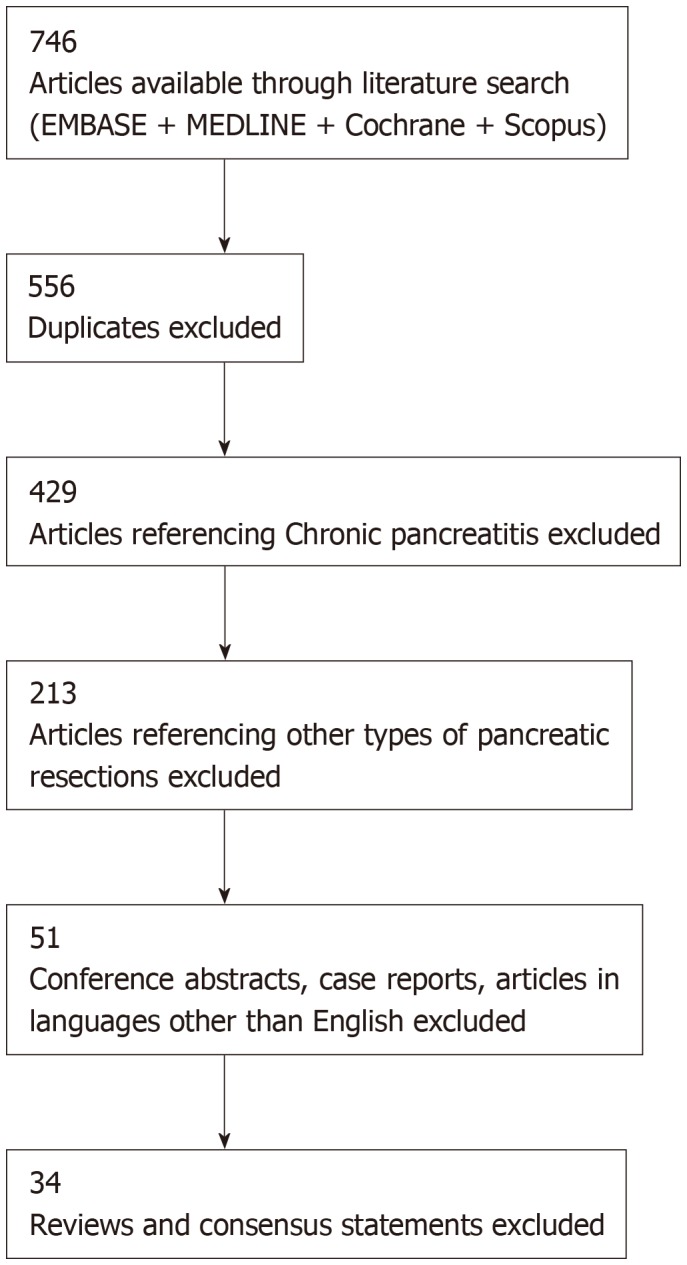
Literature review and selection process.
APPRAISAL OF LITERATURE
An attempt at data extraction revealed that studies used different parameters to define PEI as explained below in the results. This meant that a quantitative analysis of the results was not possible. Narrowing down studies further with stricter inclusion criteria meant that a large body of evidence would be left out of the analysis thereby subjecting the review to significant bias. A recent systematic review on the same subject, which included a total of only 9 studies, highlighted this aspect[19]. It was therefore decided to proceed with a qualitative narrative review of the subject.
DEFINITION OF PANCREATIC EXOCRINE INSUFFICIENCY
In chronic pancreatitis, PEI is defined by the presence of steatorrhoea and commonly assessed by the concentration of faecal elastase-1 (FE-1) in a random stool sample[20]. In this setting, FE-1 is known to reflect the level of pancreatic function and water reabsorption in the gastrointestinal tract[21,22]. It has been validated and correlates well with radiological findings and steatorrhoea in chronic pancreatitis[23-26]. FE-1 in the setting of chronic pancreatitis has also been used to grade the severity of PEI (Normal- > 200 µg/g stool; mildly impaired - 100-200 µg/g stool and severe - < 100 µg/g stool)[27].
Following pancreatic surgery, however, there is no consistent definition for PEI. Furthermore, various diagnostic tests have been used in this setting, while the accuracy of FE-1 is reduced making it an unreliable test. Table 1 highlights some of the most common parameters used to define PEI in patients undergoing pancreatic surgery and especially PD[28-34].
Table 1.
Definitions of Pancreatic Exocrine Insufficiency after pancreaticoduodenectomy
| Ref. | Definition of pancreatic exocrine insufficiency |
| Sabater et al[8] | Condition wherein the amount of pancreatic secretions is not enough to maintain normal digestion |
| Ghaneh et al[28] | Need for new pharmacological intervention for exocrine insufficiency i.e. PERT |
| Sikkens et al[11] | Faecal elastase-1 < 0.200 mg/g of faeces |
| Halloran et al[29] | Coefficient of fat absorption < 93% |
| Domínguez-Muñoz et al[30] | 13C-mixed triglyceride test (Percent cumulative dose of < 5% of 13CO2 at 7 h) |
| Yamaguchi et al[31] | BT-PABA excretion rate of < 70% |
| Kato et al[32] | Abnormal secretin stimulation test |
| Perez et al[33] | 72 h faecal fat estimation |
| Fang et al[34] | Faecal chymotrypsin estimation |
PERT: Pancreatic enzyme replacement therapy.
PARAMETERS FOR THE CLINICAL ASSESSMENT OF PANCREATIC EXOCRINE INSUFFICIENCY FOLLOWING PANCREATICODUODENECTOMY
The most characteristic clinical presentation of PEI is steatorrhoea, defined as the presence of more than 7 g of stool fat/day[35]. However, steatorrhoea is a late sign and associated with severe PEI (occurring after a loss of more than 90% of pancreatic function). Therefore, a methodical diagnostic approach is warranted, including complete medical and dietetic history, physical examination and serial anthropometric measurements, supplemented by biochemical tests and in some scenarios by relevant imaging investigations[36].
Due to the low diagnostic sensitivity of steatorrhoea, other PEI-related (but also not specific) symptomatology is important. A history of flatulence, bloating, urgency and abdominal discomfort or post-prandial abdominal pain may assist in the diagnosis of PEI. PEI is also associated with weight loss and reduction in muscle mass[10,37]. Other symptoms such as nausea, early satiety, vomiting, oral thrush and ulcers (secondary to concurrent chemotherapy) may adversely affect the dietary intake contributing to malnutrition in these patients. Dietary modifications (consciously or subconsciously by the patients), such as restriction of protein and/or fat intake, may result in masking the symptomatology, including steatorrhoea, and therefore lead to late or misdiagnosis[17,36].
A previous history of endocrine disorders (importantly diabetes mellitus), bowel conditions (such as coeliac disease, irritable bowel syndrome etc.), food intolerances or eating disorders is relevant. Previous surgery to the bowel (e.g. gastrectomy, small bowel resection, and colectomy) can also affect the gut function and alter microbiota causing symptoms that may aggravate or mimic PEI. Drugs like probiotics, antibiotics, laxatives, anti-diarrhoea agents also influence gut function, while others, such as steroids and insulin, can also have an additional impact on the patient’s weight in addition to affecting gut absorption[36]. Serial anthropometric measurements are invaluable to monitoring the nutritional status and important to assess the response to therapeutic interventions. Functional assessments, such as grip strength, mid arm circumference and triceps skin fold, together with weight changes, must be evaluated in the context of the patient’s symptoms and caloric intake.
BIOCHEMICAL PARAMETERS FOR THE ASSESSMENT OF PANCREATIC EXOCRINE INSUFFICIENCY
Relevant laboratory investigations fall into two main categories: (1) Evaluation of the nutritional status, and (2) Evaluation of the pancreatic function (Table 2). The first category includes tests such as the assessment of fat soluble vitamins, bone profile (calcium, parathyroid hormone), anaemia screen and glycaemic control. These can be used for the initial diagnosis, as well as for follow-up and evaluation of the treatment response. The second category includes tests that evaluate the pancreatic function and are further broadly sub-classified into those that evaluate the exocrine function of the pancreas and tests that measure the degree of malabsorption secondary to PEI. The latter ones focus mainly on fat malabsorption with the limitation that they cannot distinguish between pancreatic and extra-pancreatic causes. Currently there are no tests available to diagnose nitrogen malabsorption also known to occur in PEI[38], while colonic mechanisms exist to compensate for the malabsorption of carbohydrates[39,40].
Table 2.
Biochemical tests in the assessment of pancreatic exocrine insufficiency
| Nutritional assessment |
Evaluation of pancreatic function |
|
| Exocrine markers | Markers of malabsorption | |
| Fat soluble vitamins | Faecal elastase-1 | 72 h faecal fat estimation |
| Bone profile | Faecal chymotrypsin | BT-PABA absorption |
| Iron and Ferritin studies | Secretin stimulation test | 13C labelled trioctanoin breath test |
| Micronutrient status | ||
| Glycaemic status | ||
PABA: Para-aminobenzoic acid.
The 2018 ISGPS position statement considered 72 h faecal fat collection with a standard intake of fat as the gold standard test to diagnose fat malabsorption[17]. FE-1 measurement is one of the most commonly used methods to evaluate and subsequently define PEI. It is quick, non-invasive, and relatively easy to carry out in the clinical setting (on a spot faecal sample). Additionally, it is not influenced by the intake of pancreatic enzyme supplements. However, in the setting of PD, steatorrhoea occurs at a much higher FE-1 level (207 µg/g in patients post PD vs 15 µg/g in patients without a resection)[41], therefore its usefulness in this setting is questionable. Kato et al[32] detected PEI in 93% patients prior to PD (most of which with a diagnosis of pancreatic cancer) on the basis of the secretin stimulation test. The comparison of this test was with 13C- labelled trioctanoin breath assay and with parallel testing of para-aminobenzoic acid (PABA) and faecal chymotrypsin excretion. The sensitivities of both these tests were between 60% and 70% in the setting of obstructive jaundice and PD[32]. The current review thus, reveals a lack of consensus on the parameters used to evaluate PEI after PD.
INCIDENCE OF PANCREATIC EXOCRINE INSUFFICIENCYIN THE SETTING OF PANCREATICODUODENECTOMY
Due to the nature of this review, studies reporting outcomes among patients undergoing PD for chronic pancreatitis were excluded. The reported incidence of PEI after PD varied widely between 38% and 93% (Table 3)[42-55]. This is probably attributed to the heterogeneity of the patient cohorts and the diagnostic tests used.
Table 3.
Incidence of pancreatic exocrine insufficiency before and after pancreaticoduodenectomy
| Ref. | Pre-operative incidence of PEI | Post-operative incidence of PEI | Diagnostic test |
| Kato et al[32] | 93% | 80% | Secretin stimulation |
| Halloran et al[29] | - | 55% | Coefficient of fat absorption |
| Yuasa et al[42] | - | 64% | 13C- mixed triglyceride test |
| Nakamura et al[9] | - | 62.3% | |
| Hirono et al[43] | - | 51% | |
| Benini et al[41] | - | 87.5% | 72 h faecal fat estimation |
| Lemaire et al[44] | - | 94% | |
| Sato et al[45] | 46% | 33% | BT-PABA excretion |
| Fujino et al[46] | - | 75% | |
| Matsumoto et al[47] | 68% | 50% | Faecal elastase-1 |
| Van der Gaag et al[48] | - | 59% | |
| Tran et al[49] | - | 91% | |
| Pessaux et al[50] | - | 95% | |
| Jang et al[51] | - | 100% | |
| Falconi et al[52] | - | 24% | Faecal chymotrypsin |
| Fang et al[34] | - | 33% | |
| Bock et al[53] | - | 52.8% | Steatorrhoea |
| Rault et al[54] | - | 42% | |
| Van Berge Henegouwen et al[55] | - | 64.5% |
PEI: Pancreatic exocrine insufficiency; PABA: Para-aminobenzoic acid.
Halloran et al[29] showed an improvement in FE-1 after PD for pancreatic cancer, however, this was in the setting of a diminishing patient cohort (exclusion of patients with mortality)introducing the possibility of bias. Additionally, FE-1 did not compare accurately to the standard measure of PEI (Coefficient of Fat absorption). Other studies have consistently recorded improving pancreatic function in patients with ampullary cancer post-PD[56,57]. The proposed hypothesis in these studies was the relief of the obstruction by the ampullary tumour to the pancreatic duct draining a healthy pancreas.
The correlation of pre-operative PEI to post-operative PEI is difficult to assess as FE-1 is the most frequently used marker and has been shown to underestimate PEI after pancreatic resection[19]. Matsumoto et al[47] noted a significant post-operative drop in FE-1 levels in patients with normal pre-operative values, while FE-1 levels in those with pre-existing PEI remained relatively unchanged post-operatively. It is possible that these findings are limited not only by the use of FE-1 in post-operative assessment, but also by the short follow-up period. This is further supported by the diagnosis of PEI in all patients at a median post-operative time of 52 mo[58].
There are several studies that have investigated possible predictors of PEI after PD, such as the presence of a dilated pancreatic duct on computerized tomography (CT) scans or endoscopic ultrasound pre-operatively[59]. One study reported that a dilated pre-operative duct diameter (> 3 mm) was more likely to result in exocrine dysfunction at 2 mo after surgery measured by reduced PABA excretion[45]. This finding was however, not corroborated by Matsumoto et al[47] who suggested that the diminishing pancreatic parenchyma was the main reason for the reduced post-operative FE-1 levels. Furthermore, post-operative parenchymal thickness on CT was shown to be a predictor of PEI (based on the 13C-labelled mixed triglyceride test) with a sensitivity of 88.2% and specificity of 88.9% when the cut off was set at 13 mm[60]. Nonetheless, the use of imaging findings to clinically predict PEI remains in use predominantly in the setting of chronic pancreatitis[25,61,62].
TECHNICAL OPERATIVE FACTORS INFLUENCING PANCREATIC EXOCRINE INSUFFICIENCY
The pre- and post-operative incidence of PEI was studied with a BT-PABA test in patients undergoing classical PD versus Pylorus preserving PD (PPPD). The short term post-operative incidence was similar in both groups. The exocrine function recovered to pre-operative levels in the PPPD group, while this was not observed in the classical PD group. The study, however, was limited by the small patient cohort (10 classical PD vs 44 PPPD) and the potential for selection bias across the two groups, while the indications included both benign and malignant diagnoses[45].
The effect of the type of reconstruction, pancreatico-gastrostomy or pancreatico-jejunostomy, on PEI has also been studied (Table 4). Two retrospective studies reported that patients undergoing pancreatico-jejunostomy reconstruction for pancreatic head malignancy were significantly less likely to have PEI[54,63]. Others have also shown a similarly high incidence of PEI after pancreatico-gastrostomy in retrospective cohorts[9,44]. However, the retrospective comparative study by Jang et al[51] showed no significant difference between the two reconstruction methods (100% vs 95%). This conflicting evidence is most likely attributed to the use of different methods to measure and report the incidence of PEI, including 72 h faecal fat estimation, 13C-labelled mixed triglyceride breath test and measurement of FE-1.
Table 4.
Incidence of pancreatic exocrine insufficiency after pancreaticoduodenectomy–evidence on the role of the type of pancreatic reconstruction
| Ref. | Diagnostic test | Incidence of PEI–Pancreaticogastrostomy | Incidence of PEI–Pancreaticojejunostomy |
| Nakamura et al[9] | 13C Triglyceride breath test | 62.3% | - |
| Lemaire et al[44] | Faecal Fat excretion and faecal elastase-1 | 100% | - |
| Jang et al[51] | Faecal elastase-1 | 100% (severe) | 75% (severe); 20% (mild) |
| Roeyen et al[63] | Need for PERT +/- any abnormal pancreatic function test | 75% | 45.7% (P < 0.001) |
| Rault et al[54] | Steatorrhoea | 70% | 21.7% (P < 0.025) |
PEI: Pancreatic exocrine insufficiency; PERT: Pancreatic enzyme replacement therapy.
CONSEQUENCES OF PANCREATIC EXOCRINE INSUFFICIENCY AFTER PANCREATICODUODENECTOMY
In the perioperative setting, PEI can lead to malnutrition and this in turn to higher morbidity and mortality including a greater risk of a pancreatic leak[59,64,65]. Additionally, it may significantly affect quality of life and it has been shown to be an independent predictor of survival in advanced pancreatic cancer[66]. Similarly, cachexia, has shown to be associated with decreased survival with unresectable pancreatic cancer, with weight stabilization showing better prognosis[67-69].
There is increasing evidence that untreated PEI negatively affects survival following PD for cancer. Among consecutive patients undergoing PD for periampullary cancer those without treatment had significantly reduced survival; this was even more pronounced among the cohort with pancreatic duct dilation (≥ 3 mm)[70]. A further population based study used propensity matched analysis to adjust for key variables and in that study lack of treatment of PEI was associated with reduced survival and the survival benefit of pancreatic enzyme replacement therapy (PERT) was of a similar magnitude to surgery or chemotherapy[71].
The symptoms and consequences of PEI after PD are mainly related to the malabsorption of undigested food and nutrients, especially fat soluble vitamins (Vitamins A, D, E and K)[17] in the distal small bowel[72]. The classical symptoms of steatorrhoea, abdominal pain with bloating and cramping, flatulence, dyspepsia and nausea are however, not seen in patients with mild to moderate PEI[73]. Vitamin malabsorption may lead to symptoms such as xerophthalmia and night blindness (Vitamin A), neurological symptoms, ophthalmoplegia and ptosis (Vitamin E), abnormal bleeding (Vitamin K), osteomalacia and metabolic bone disease (Vitamin D). It is important to recognize these as potential complications of PEI early and start supplementation (parenterally if necessary) on a long term basis. Other complications such as weight loss, electrolyte imbalances and poor wound healing may also occur[17]. In cases where the indication for PD is cancer, malnutrition can delay the start of the recommended adjuvant chemotherapy or worse, render the patient unfit for the same. Finally, NAFLD is a rare and poorly recognized possible consequence of PEI after PD. It is believed to occur secondary to the malabsorption of essential amino acids leading to decreased plasma levels of apoprotein B[74], which, when combined with sub-optimal insulin secretion lead to peripheral lipolysis and greater hepatic fat deposition[75]. These changes have been shown to be reversible with the administration of PERT and subsequent improvements in body weight[76].
MANAGEMENT OF PANCREATIC EXOCRINE INSUFFICIENCY AFTER PANCREATICODUODENECTOMY
PERT is the mainstay of treatment of PEI. However, in the post-operative setting, there is a lack of consensus over the timing of initiation of PERT. While some authors recommend routine post-operative PERT[36,77], others advocate in favour of PERT only after clinical or biochemical diagnostic evidence of PEI[8,78]. In pancreatic cancer, due to the high incidence of PEI and obstructive jaundice, peri-operative use of PERT for all patients has been shown to be beneficial[36] and is recommended by the United Kingdom National Institute of Clinical Excellence guidelines[79].
Patient education is important for the correct use of PERT. Enzymes use is advisable with all meals, snacks and milky drinks, including various supplements. The conventional timing of administration is during or immediately after a meal in order to achieve optimal timing for mixing with the chyme. This hypothesis however, has not been studied in the setting of pancreatic surgery and the presence of pancreatico-biliary reconstruction and digestive asynchrony[16].
PERT is usually commenced at a dose of 50000-75000 units lipase with a meal and 25000-50000 units with each snack[10,80-82]. This may be titrated to the needs of the individual patient. In common clinical experience, patients over time learn to adjust the dose of PERT on the basis of their symptoms and diet. Nonetheless, close follow-up is required to ensure that management remains on track in the setting of changing (recovering or deteriorating) pancreatic function and/or patient diet (as some patients may compromise on the nutritional value of their diet rather than the PERT dose). The use and effectiveness of PEI should be monitored with serial anthropometric measurements and nutritional blood tests[36], including measurements for glycaemic control, as the use of effective PERT may result in manifestation of diabetes[83]. In addition to PERT, supplementation with vitamins and other micronutrients is recommended[84].
The gastrointestinal environment and acidity is important for the appropriate function of PERT. The lipase in PERT is inactivated by gastric acid activity. Consequently, commercially available PERT formulations are covered with pH sensitive, acid resistant microspheres that release the lipase at a pH of 5-6, similar to what is present in the native duodenum. Based on studies about the optimal sphere size required to produce the best dissociation in the duodenum, most commercial preparations have sphere size that varies from 1-2 mm[85,86]. In the post-operative PD setting, failure of the pancreas to produce bicarbonate is hypothesized to lead to an acidic environment in the duodenum and proximal jejunum, leading to inefficient activation of lipase[35,87]. The concurrent use of gastric acid suppression is therefore recommended. The use of a proton pump inhibitor is known to reduce faecal fat losses[88] and may also help reduce precipitation of bile salts[36].
PERT is generally well tolerated with minimal adverse effects. Rare reports on fibrosing colonopathy with the use of PERT are limited to paediatric patients, especially in the setting of cystic fibrosis[89-91]. There have been no such reports in the adult post-operative population. Many studies including open label PERT trials have not found significant adverse drug reactions[92-94].
Failure of PEI to improve after escalation of PERT dosage and gastric acid suppression must prompt further investigations for concurrent problems. The two commonest diagnoses in this setting are bile salt malabsorption and small bowel bacterial overgrowth[30,80,82]. Bile salt malabsorption occurs due to the change in the pH in the proximal small bowel secondary to deficiency of bicarbonate secretion from the pancreas. The cholecystectomy performed during PD may also contribute to the development of this condition[36,95]. The presence of a blind loop of bowel used for reconstruction is known to occur after PD and is documented in up to 65% of patients leading to small bowel bacterial overgrowth[36].
CONCLUSION
This literature review confirms that PEI is prevalent after PD even for indications other than chronic pancreatitis and may have severe implications with respect to patients’ survival, quality of life, nutrition and subsequent management. The lack of a uniform definition of PEI in this setting and the low diagnostic accuracy of the available tests introduce a wide variability in the reported results and suggested management. Pancreatic enzyme replacement therapy is effective, well tolerated and is indicated routinely in this cohort of patients. Future studies need to concentrate on the identification of a well-tolerated, reliable and reproducible diagnostic test that will facilitate a uniform definition and management approach.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: December 2, 2019
First decision: December 23, 2019
Article in press: March 22, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): D
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Löhr JM, Wang XB S-Editor: Zhang L L-Editor: A E-Editor: Qi LL
Contributor Information
Adithya M Pathanki, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Joseph A Attard, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Elizabeth Bradley, Department of Nutrition and Dietetics, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Sarah Powell-Brett, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Bobby V M Dasari, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
John R Isaac, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Keith J Roberts, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom.
Nikolaos A Chatzizacharias, Department of HPB and Liver Transplantation, Queen Elizabeth Hospital, University Hospitals Birmingham, Birmingham B15 2TH, United Kingdom. chatzizacharias@gmail.com.
References
- 1.Anagnostides A, Chadwick VS, Selden AC, Maton PN. Sham feeding and pancreatic secretion. Evidence for direct vagal stimulation of enzyme output. Gastroenterology. 1984;87:109–114. [PubMed] [Google Scholar]
- 2.Keller J, Rünzi M, Goebell H, Layer P. Duodenal and ileal nutrient deliveries regulate human intestinal motor and pancreatic responses to a meal. Am J Physiol. 1997;272:G632–G637. doi: 10.1152/ajpgi.1997.272.3.G632. [DOI] [PubMed] [Google Scholar]
- 3.Fried M, Mayer EA, Jansen JB, Lamers CB, Taylor IL, Bloom SR, Meyer JH. Temporal relationships of cholecystokinin release, pancreatobiliary secretion, and gastric emptying of a mixed meal. Gastroenterology. 1988;95:1344–1350. doi: 10.1016/0016-5085(88)90371-x. [DOI] [PubMed] [Google Scholar]
- 4.Beglinger C, Fried M, Whitehouse I, Jansen JB, Lamers CB, Gyr K. Pancreatic enzyme response to a liquid meal and to hormonal stimulation. Correlation with plasma secretin and cholecystokinin levels. J Clin Invest. 1985;75:1471–1476. doi: 10.1172/JCI111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LJ, Clain JE, Malagelada JR, Go VL. Control of human postprandial pancreatic exocrine secretion: a function of the gastroduodenal region. Dig Dis Sci. 1979;24:150–154. doi: 10.1007/BF01324743. [DOI] [PubMed] [Google Scholar]
- 6.Layer P, Schlesinger T, Gröger G, Goebell H. Modulation of human periodic interdigestive gastrointestinal motor and pancreatic function by the ileum. Pancreas. 1993;8:426–432. doi: 10.1097/00006676-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Keller J, Holst JJ, Layer P. Inhibition of human pancreatic and biliary output but not intestinal motility by physiological intraileal lipid loads. Am J Physiol Gastrointest Liver Physiol. 2006;290:G704–G709. doi: 10.1152/ajpgi.00411.2005. [DOI] [PubMed] [Google Scholar]
- 8.Sabater L, Ausania F, Bakker OJ, Boadas J, Domínguez-Muñoz JE, Falconi M, Fernández-Cruz L, Frulloni L, González-Sánchez V, Lariño-Noia J, Lindkvist B, Lluís F, Morera-Ocón F, Martín-Pérez E, Marra-López C, Moya-Herraiz Á, Neoptolemos JP, Pascual I, Pérez-Aisa Á, Pezzilli R, Ramia JM, Sánchez B, Molero X, Ruiz-Montesinos I, Vaquero EC, de-Madaria E. Evidence-based Guidelines for the Management of Exocrine Pancreatic Insufficiency After Pancreatic Surgery. Ann Surg. 2016;264:949–958. doi: 10.1097/SLA.0000000000001732. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Predictive factors for exocrine pancreatic insufficiency after pancreatoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2009;13:1321–1327. doi: 10.1007/s11605-009-0896-5. [DOI] [PubMed] [Google Scholar]
- 10.Tran TC, van Lanschot JJ, Bruno MJ, van Eijck CH. Functional changes after pancreatoduodenectomy: diagnosis and treatment. Pancreatology. 2009;9:729–737. doi: 10.1159/000264638. [DOI] [PubMed] [Google Scholar]
- 11.Sikkens EC, Cahen DL, de Wit J, Looman CW, van Eijck C, Bruno MJ. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014;48:e43–e46. doi: 10.1097/MCG.0b013e31829f56e7. [DOI] [PubMed] [Google Scholar]
- 12.Bruno MJ, Haverkort EB, Tytgat GN, van Leeuwen DJ. Maldigestion associated with exocrine pancreatic insufficiency: implications of gastrointestinal physiology and properties of enzyme preparations for a cause-related and patient-tailored treatment. Am J Gastroenterol. 1995;90:1383–1393. [PubMed] [Google Scholar]
- 13.Gullo L. Somatostatin analogues and exocrine pancreatic secretion. Digestion. 1996;57 Suppl 1:93. doi: 10.1159/000201408. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk SM, Heerkens HD, Tseng DSJ, Intven M, Molenaar IQ, van Santvoort HC. Systematic review on the impact of pancreatoduodenectomy on quality of life in patients with pancreatic cancer. HPB (Oxford) 2018;20:204–215. doi: 10.1016/j.hpb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Heerkens HD, van Berkel L, Tseng DSJ, Monninkhof EM, van Santvoort HC, Hagendoorn J, Borel Rinkes IHM, Lips IM, Intven M, Molenaar IQ. Long-term health-related quality of life after pancreatic resection for malignancy in patients with and without severe postoperative complications. HPB (Oxford) 2018;20:188–195. doi: 10.1016/j.hpb.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Bartel MJ, Asbun H, Stauffer J, Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: A review of the literature. Dig Liver Dis. 2015;47:1013–1020. doi: 10.1016/j.dld.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Gianotti L, Besselink MG, Sandini M, Hackert T, Conlon K, Gerritsen A, Griffin O, Fingerhut A, Probst P, Abu Hilal M, Marchegiani G, Nappo G, Zerbi A, Amodio A, Perinel J, Adham M, Raimondo M, Asbun HJ, Sato A, Takaori K, Shrikhande SV, Del Chiaro M, Bockhorn M, Izbicki JR, Dervenis C, Charnley RM, Martignoni ME, Friess H, de Pretis N, Radenkovic D, Montorsi M, Sarr MG, Vollmer CM, Frulloni L, Büchler MW, Bassi C. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2018;164:1035–1048. doi: 10.1016/j.surg.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Tseng DS, Molenaar IQ, Besselink MG, van Eijck CH, Borel Rinkes IH, van Santvoort HC. Pancreatic Exocrine Insufficiency in Patients with Pancreatic or Periampullary Cancer: A Systematic Review. Pancreas. 2016;45:325–330. doi: 10.1097/MPA.0000000000000473. [DOI] [PubMed] [Google Scholar]
- 20.Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem. 1996;42:222–226. [PubMed] [Google Scholar]
- 21.Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–586. doi: 10.1136/gut.39.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici R, Franzini C. Fecal elastase-1 as a test for pancreatic function: a review. Clin Chem Lab Med. 2002;40:325–332. doi: 10.1515/CCLM.2002.051. [DOI] [PubMed] [Google Scholar]
- 23.Usküdar O, Oğuz D, Akdoğan M, Altiparmak E, Sahin B. Comparison of endoscopic retrograde cholangiopancreatography, endoscopic ultrasonography, and fecal elastase 1 in chronic pancreatitis and clinical correlation. Pancreas. 2009;38:503–506. doi: 10.1097/MPA.0b013e31819f639f. [DOI] [PubMed] [Google Scholar]
- 24.Symersky T, van der Zon A, Biemond I, Masclee AA. Faecal elastase-I: helpful in analysing steatorrhoea? Neth J Med. 2004;62:286–289. [PubMed] [Google Scholar]
- 25.Gillams A, Pereira S, Webster G, Lees W. Correlation of MRCP quantification (MRCPQ) with conventional non-invasive pancreatic exocrine function tests. Abdom Imaging. 2008;33:469–473. doi: 10.1007/s00261-007-9286-1. [DOI] [PubMed] [Google Scholar]
- 26.Thomas PD, Forbes A, Green J, Howdle P, Long R, Playford R, Sheridan M, Stevens R, Valori R, Walters J, Addison GM, Hill P, Brydon G. Guidelines for the investigation of chronic diarrhoea, 2nd edition. Gut. 2003;52 Suppl 5:v1–15. doi: 10.1136/gut.52.suppl_5.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erickson JA, Aldeen WE, Grenache DG, Ashwood ER. Evaluation of a fecal pancreatic elastase-1 enzyme-linked immunosorbent assay: Assessment versus an established assay and implication in classifying pancreatic function. Clin Chim Acta. 2008;397:87–91. doi: 10.1016/j.cca.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Ghaneh P, Neoptolemos JP. Exocrine pancreatic function following pancreatectomy. Ann N Y Acad Sci. 1999;880:308–318. doi: 10.1111/j.1749-6632.1999.tb09534.x. [DOI] [PubMed] [Google Scholar]
- 29.Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11:535–545. doi: 10.1159/000333308. [DOI] [PubMed] [Google Scholar]
- 30.Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy: exocrine pancreatic insufficiency after gastrointestinal surgery. HPB (Oxford) 2009;11 Suppl 3:3–6. doi: 10.1111/j.1477-2574.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Yokohata K, Nakano K, Ohtani K, Ogawa Y, Chijiiwa K, Tanaka M. Which is a less invasive pancreatic head resection: PD, PPPD, or DPPHR? Dig Dis Sci. 2001;46:282–288. doi: 10.1023/a:1005644614104. [DOI] [PubMed] [Google Scholar]
- 32.Kato H, Nakao A, Kishimoto W, Nonami T, Harada A, Hayakawa T, Takagi H. 13C-labeled trioctanoin breath test for exocrine pancreatic function test in patients after pancreatoduodenectomy. Am J Gastroenterol. 1993;88:64–69. [PubMed] [Google Scholar]
- 33.Perez MM, Newcomer AD, Moertel CG, Go VL, Dimagno EP. Assessment of weight loss, food intake, fat metabolism, malabsorption, and treatment of pancreatic insufficiency in pancreatic cancer. Cancer. 1983;52:346–352. doi: 10.1002/1097-0142(19830715)52:2<346::aid-cncr2820520228>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Fang WL, Su CH, Shyr YM, Chen TH, Lee RC, Tai LC, Wu CW, Lui WY. Functional and morphological changes in pancreatic remnant after pancreaticoduodenectomy. Pancreas. 2007;35:361–365. doi: 10.1097/MPA.0b013e3180d0a8d5. [DOI] [PubMed] [Google Scholar]
- 35.Ghaneh P, Neoptolemos JP. Exocrine pancreatic function following pancreatectomy. Ann N Y Acad Sci. 1999;880:308–318. doi: 10.1111/j.1749-6632.1999.tb09534.x. [DOI] [PubMed] [Google Scholar]
- 36.Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology. 2015;15:449–455. doi: 10.1016/j.pan.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function - clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol. 2009;23:425–439. doi: 10.1016/j.bpg.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Caliari S, Benini L, Sembenini C, Gregori B, Carnielli V, Vantini I. Medium-chain triglyceride absorption in patients with pancreatic insufficiency. Scand J Gastroenterol. 1996;31:90–94. doi: 10.3109/00365529609031633. [DOI] [PubMed] [Google Scholar]
- 39.Owira PM, Winter TA. Colonic energy salvage in chronic pancreatic exocrine insufficiency. JPEN J Parenter Enteral Nutr. 2008;32:63–71. doi: 10.1177/014860710803200163. [DOI] [PubMed] [Google Scholar]
- 40.Temple SJ, Kim PT, Serrano PE, Kagedan D, Cleary SP, Moulton CA, McGilvray ID, Gallinger S, Greig PD, Wei AC. Combined pancreaticoduodenectomy and colon resection for locally advanced peri-ampullary tumours: analysis of peri-operative morbidity and mortality. HPB (Oxford) 2014;16:797–800. doi: 10.1111/hpb.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benini L, Amodio A, Campagnola P, Agugiaro F, Cristofori C, Micciolo R, Magro A, Gabbrielli A, Cabrini G, Moser L, Massella A, Vantini I, Frulloni L. Fecal elastase-1 is useful in the detection of steatorrhea in patients with pancreatic diseases but not after pancreatic resection. Pancreatology. 2013;13:38–42. doi: 10.1016/j.pan.2012.11.307. [DOI] [PubMed] [Google Scholar]
- 42.Yuasa Y, Murakami Y, Nakamura H, Uemura K, Ohge H, Sudo T, Hashimoto Y, Nakashima A, Hiyama E, Sueda T. Histological loss of pancreatic exocrine cells correlates with pancreatic exocrine function after pancreatic surgery. Pancreas. 2012;41:928–933. doi: 10.1097/MPA.0b013e31823d837d. [DOI] [PubMed] [Google Scholar]
- 43.Hirono S, Murakami Y, Tani M, Kawai M, Okada K, Uemura K, Sudo T, Hashimoto Y, Nakagawa N, Kondo N, Yamaue H. Identification of risk factors for pancreatic exocrine insufficiency after pancreaticoduodenectomy using a 13C-labeled mixed triglyceride breath test. World J Surg. 2015;39:516–525. doi: 10.1007/s00268-014-2832-4. [DOI] [PubMed] [Google Scholar]
- 44.Lemaire E, O'Toole D, Sauvanet A, Hammel P, Belghiti J, Ruszniewski P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br J Surg. 2000;87:434–438. doi: 10.1046/j.1365-2168.2000.01388.x. [DOI] [PubMed] [Google Scholar]
- 45.Sato N, Yamaguchi K, Yokohata K, Shimizu S, Morisaki T, Chijiiwa K, Tanaka M. Short-term and long-term pancreatic exocrine and endocrine functions after pancreatectomy. Dig Dis Sci. 1998;43:2616–2621. doi: 10.1023/a:1026686824173. [DOI] [PubMed] [Google Scholar]
- 46.Fujino Y, Suzuki Y, Matsumoto I, Sakai T, Ajiki T, Ueda T, Kuroda Y. Long-term assessments after pancreaticoduodenectomy with pancreatic duct invagination anastomosis. Surg Today. 2007;37:860–866. doi: 10.1007/s00595-007-3507-7. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto J, Traverso LW. Exocrine function following the whipple operation as assessed by stool elastase. J Gastrointest Surg. 2006;10:1225–1229. doi: 10.1016/j.gassur.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 48.van der Gaag NA, Berkhemer OA, Sprangers MA, Busch OR, Bruno MJ, de Castro SM, van Gulik TM, Gouma DJ. Quality of life and functional outcome after resection of pancreatic cystic neoplasm. Pancreas. 2014;43:755–761. doi: 10.1097/MPA.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 49.Tran TC, van 't Hof G, Kazemier G, Hop WC, Pek C, van Toorenenbergen AW, van Dekken H, van Eijck CH. Pancreatic fibrosis correlates with exocrine pancreatic insufficiency after pancreatoduodenectomy. Dig Surg. 2008;25:311–318. doi: 10.1159/000158596. [DOI] [PubMed] [Google Scholar]
- 50.Pessaux P, Aube C, Lebigot J, Tuech JJ, Regenet N, Kapel N, Caron C, Arnaud JP. Permeability and functionality of pancreaticogastrostomy after pancreaticoduodenectomy with dynamic magnetic resonance pancreatography after secretin stimulation. J Am Coll Surg. 2002;194:454–462. doi: 10.1016/s1072-7515(02)01126-2. [DOI] [PubMed] [Google Scholar]
- 51.Jang JY, Kim SW, Park SJ, Park YH. Comparison of the functional outcome after pylorus-preserving pancreatoduodenectomy: pancreatogastrostomy and pancreatojejunostomy. World J Surg. 2002;26:366–371. doi: 10.1007/s00268-001-0234-x. [DOI] [PubMed] [Google Scholar]
- 52.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 53.Bock EA, Hurtuk MG, Shoup M, Aranha GV. Late complications after pancreaticoduodenectomy with pancreaticogastrostomy. J Gastrointest Surg. 2012;16:914–919. doi: 10.1007/s11605-011-1805-2. [DOI] [PubMed] [Google Scholar]
- 54.Rault A, SaCunha A, Klopfenstein D, Larroudé D, Epoy FN, Collet D, Masson B. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg. 2005;201:239–244. doi: 10.1016/j.jamcollsurg.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 55.van Berge Henegouwen MI, Moojen TM, van Gulik TM, Rauws EA, Obertop H, Gouma DJ. Postoperative weight gain after standard Whipple's procedure versus pylorus-preserving pancreatoduodenectomy: the influence of tumour status. Br J Surg. 1998;85:922–926. doi: 10.1046/j.1365-2168.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- 56.Kodama M, Tanaka T. Residual function of exocrine pancreas after operation for chronic pancreatitis by N-benzoyl-L-tyrosyl-p-aminobenzoic acid test (NBT-PABA test) Digestion. 1984;30:41–46. doi: 10.1159/000199089. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Ichiba Y, Fujii Y, Kodama O, Dohi K. Clinical and experimental study of pancreatic exocrine function after pancreaticoduodenectomy for periampullary carcinoma. Surg Gynecol Obstet. 1988;166:200–205. [PubMed] [Google Scholar]
- 58.Nordback I, Parviainen M, Piironen A, Räty S, Sand J. Obstructed pancreaticojejunostomy partly explains exocrine insufficiency after pancreatic head resection. Scand J Gastroenterol. 2007;42:263–270. doi: 10.1080/00365520600849174. [DOI] [PubMed] [Google Scholar]
- 59.Domínguez-Muñoz JE, Alvarez-Castro A, Lariño-Noia J, Nieto L, Iglesias-García J. Endoscopic ultrasonography of the pancreas as an indirect method to predict pancreatic exocrine insufficiency in patients with chronic pancreatitis. Pancreas. 2012;41:724–728. doi: 10.1097/MPA.0b013e31823b5978. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura H, Murakami Y, Uemura K, Hayashidani Y, Sudo T, Ohge H, Sueda T. Reduced pancreatic parenchymal thickness indicates exocrine pancreatic insufficiency after pancreatoduodenectomy. J Surg Res. 2011;171:473–478. doi: 10.1016/j.jss.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 61.Manfredi R, Perandini S, Mantovani W, Frulloni L, Faccioli N, Pozzi Mucelli R. Quantitative MRCP assessment of pancreatic exocrine reserve and its correlation with faecal elastase-1 in patients with chronic pancreatitis. Radiol Med. 2012;117:282–292. doi: 10.1007/s11547-011-0774-6. [DOI] [PubMed] [Google Scholar]
- 62.Balci NC, Smith A, Momtahen AJ, Alkaade S, Fattahi R, Tariq S, Burton F. MRI and S-MRCP findings in patients with suspected chronic pancreatitis: correlation with endoscopic pancreatic function testing (ePFT) J Magn Reson Imaging. 2010;31:601–606. doi: 10.1002/jmri.22085. [DOI] [PubMed] [Google Scholar]
- 63.Roeyen G, Jansen M, Ruyssinck L, Chapelle T, Vanlander A, Bracke B, Hartman V, Ysebaert D, Berrevoet F. Pancreatic exocrine insufficiency after pancreaticoduodenectomy is more prevalent with pancreaticogastrostomy than with pancreaticojejunostomy. A retrospective multicentre observational cohort study. HPB (Oxford) 2016;18:1017–1022. doi: 10.1016/j.hpb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crucitti F, Doglietto GB, Viola G, Frontera D, De Cosmo G, Sgadari A, Vicari D, Rizzi A. Assessment of risk factors for pancreatic resection for cancer. World J Surg. 1998;22:241–247. doi: 10.1007/s002689900377. [DOI] [PubMed] [Google Scholar]
- 65.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 66.Partelli S, Frulloni L, Minniti C, Bassi C, Barugola G, D'Onofrio M, Crippa S, Falconi M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig Liver Dis. 2012;44:945–951. doi: 10.1016/j.dld.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 67.Davidson W, Ash S, Capra S, Bauer J Cancer Cachexia Study Group. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr. 2004;23:239–247. doi: 10.1016/j.clnu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Bachmann J, Ketterer K, Marsch C, Fechtner K, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer. 2009;9:255. doi: 10.1186/1471-2407-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heinrich S, Pestalozzi BC, Schäfer M, Weber A, Bauerfeind P, Knuth A, Clavien PA. Prospective phase II trial of neoadjuvant chemotherapy with gemcitabine and cisplatin for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:2526–2531. doi: 10.1200/JCO.2007.15.5556. [DOI] [PubMed] [Google Scholar]
- 70.Roberts KJ, Schrem H, Hodson J, Angelico R, Dasari BVM, Coldham CA, Marudanayagam R, Sutcliffe RP, Muiesan P, Isaac J, Mirza DF. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB (Oxford) 2017;19:859–867. doi: 10.1016/j.hpb.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology. 2019;19:114–121. doi: 10.1016/j.pan.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 72.Whitcomb DC, Lehman GA, Vasileva G, Malecka-Panas E, Gubergrits N, Shen Y, Sander-Struckmeier S, Caras S. Pancrelipase delayed-release capsules (CREON) for exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery: A double-blind randomized trial. Am J Gastroenterol. 2010;105:2276–2286. doi: 10.1038/ajg.2010.201. [DOI] [PubMed] [Google Scholar]
- 73.Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Gastroenterol Hepatol (N Y) 2011;7:401–403. [PMC free article] [PubMed] [Google Scholar]
- 74.Yao ZM, Vance DE. Reduction in VLDL, but not HDL, in plasma of rats deficient in choline. Biochem Cell Biol. 1990;68:552–558. doi: 10.1139/o90-079. [DOI] [PubMed] [Google Scholar]
- 75.Soliman AT, Alsalmi I, Asfour M. Hypoinsulinaemia has an important role in the development of oedema and hepatomegaly during malnutrition. J Trop Pediatr. 1996;42:297–299. doi: 10.1093/tropej/42.5.297. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S, Aoyama T, Tanaka E. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758–768. doi: 10.1007/s00535-011-0370-5. [DOI] [PubMed] [Google Scholar]
- 77.Working Party of the Australasian Pancreatic Club., Smith RC, Smith SF, Wilson J, Pearce C, Wray N, Vo R, Chen J, Ooi CY, Oliver M, Katz T, Turner R, Nikfarjam M, Rayner C, Horowitz M, Holtmann G, Talley N, Windsor J, Pirola R, Neale R. Summary and recommendations from the Australasian guidelines for the management of pancreatic exocrine insufficiency. Pancreatology. 2016;16:164–180. doi: 10.1016/j.pan.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Domínguez-Muñoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Gastroenterol Hepatol (N Y) 2011;7:401–403. [PMC free article] [PubMed] [Google Scholar]
- 79.National Institute for Health and Care Excellence. Pancreatic cancer in adults: diagnosis and management. NICE guideline [NG85], Recommendations. Available from: https://www.nice.org.uk/guidance/ng85/chapter/Recommendations#nutritional-management. [PubMed] [Google Scholar]
- 80.Imrie CW, Connett G, Hall RI, Charnley RM. Review article: enzyme supplementation in cystic fibrosis, chronic pancreatitis, pancreatic and periampullary cancer. Aliment Pharmacol Ther. 2010;32 Suppl 1:1–25. doi: 10.1111/j.1365-2036.2010.04437.x. [DOI] [PubMed] [Google Scholar]
- 81.Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap A, Varga M, Friess H. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharmacol Ther. 2013;37:691–702. doi: 10.1111/apt.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toouli J, Biankin AV, Oliver MR, Pearce CB, Wilson JS, Wray NH Australasian Pancreatic Club. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med J Aust. 2010;193:461–467. doi: 10.5694/j.1326-5377.2010.tb04000.x. [DOI] [PubMed] [Google Scholar]
- 83.O'Keefe SJ, Cariem AK, Levy M. The exacerbation of pancreatic endocrine dysfunction by potent pancreatic exocrine supplements in patients with chronic pancreatitis. J Clin Gastroenterol. 2001;32:319–323. doi: 10.1097/00004836-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 84.Armstrong T, Strommer L, Ruiz-Jasbon F, Shek FW, Harris SF, Permert J, Johnson CD. Pancreaticoduodenectomy for peri-ampullary neoplasia leads to specific micronutrient deficiencies. Pancreatology. 2007;7:37–44. doi: 10.1159/000101876. [DOI] [PubMed] [Google Scholar]
- 85.Meyer JH, Dressman J, Fink A, Amidon G. Effect of size and density on canine gastric emptying of nondigestible solids. Gastroenterology. 1985;89:805–813. doi: 10.1016/0016-5085(85)90576-1. [DOI] [PubMed] [Google Scholar]
- 86.Meyer JH, Elashoff J, Porter-Fink V, Dressman J, Amidon GL. Human postprandial gastric emptying of 1-3-millimeter spheres. Gastroenterology. 1988;94:1315–1325. doi: 10.1016/0016-5085(88)90669-5. [DOI] [PubMed] [Google Scholar]
- 87.Aloulou A, Puccinelli D, Sarles J, Laugier R, Leblond Y, Carrière F. In vitro comparative study of three pancreatic enzyme preparations: dissolution profiles, active enzyme release and acid stability. Aliment Pharmacol Ther. 2008;27:283–292. doi: 10.1111/j.1365-2036.2007.03563.x. [DOI] [PubMed] [Google Scholar]
- 88.Proesmans M, De Boeck K. Omeprazole, a proton pump inhibitor, improves residual steatorrhoea in cystic fibrosis patients treated with high dose pancreatic enzymes. Eur J Pediatr. 2003;162:760–763. doi: 10.1007/s00431-003-1309-5. [DOI] [PubMed] [Google Scholar]
- 89.FitzSimmons SC, Burkhart GA, Borowitz D, Grand RJ, Hammerstrom T, Durie PR, Lloyd-Still JD, Lowenfels AB. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–1289. doi: 10.1056/NEJM199705013361803. [DOI] [PubMed] [Google Scholar]
- 90.Smyth RL, van Velzen D, Smyth AR, Lloyd DA, Heaf DP. Strictures of ascending colon in cystic fibrosis and high-strength pancreatic enzymes. Lancet. 1994;343:85–86. doi: 10.1016/s0140-6736(94)90817-6. [DOI] [PubMed] [Google Scholar]
- 91.Nouisa-Arvanitakis S, Stapleton FB, Linshaw MA, Kennedy J. Therapeutic approach to pancreatic extract-induced hyperuricosuria in cystic fibrosis. J Pediatr. 1977;90:302–305. doi: 10.1016/s0022-3476(77)80657-4. [DOI] [PubMed] [Google Scholar]
- 92.Gullo L, Pezzilli R, Gaiani S. Tolerability and safety of the long-term administration of pancreatic extracts. Pancreas. 1997;14:210–212. doi: 10.1097/00006676-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 93.Ramesh H, Reddy N, Bhatia S, Rajkumar JS, Bapaye A, Kini D, Kalla M, Thorat V. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated minimicrospheres in patients with pancreatic exocrine insufficiency due to chronic pancreatitis. Pancreatology. 2013;13:133–139. doi: 10.1016/j.pan.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Gubergrits N, Malecka-Panas E, Lehman GA, Vasileva G, Shen Y, Sander-Struckmeier S, Caras S, Whitcomb DC. A 6-month, open-label clinical trial of pancrelipase delayed-release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther. 2011;33:1152–1161. doi: 10.1111/j.1365-2036.2011.04631.x. [DOI] [PubMed] [Google Scholar]
- 95.Bustillo I, Larson H, Saif MW. Small intestine bacterial overgrowth: an underdiagnosed cause of diarrhea in patients with pancreatic cancer. JOP. 2009;10:576–578. [PubMed] [Google Scholar]


