Abstract
BACKGROUND
Drug shortages are common yet their impact on patient care and their commercial ramifications has not been adequately researched. In Australia a shortage of balsalazide (2012-2013) necessitated substitution with alternative 5-aminosalicylate (5-ASA) formulations for ulcerative colitis (UC).
AIM
To assess and compare the clinical and commercial sequelae of non-medical switching from balsalazide to another 5-ASA and/or return to balsalazide once supply resumed.
METHODS
A prospective cohort study of patients on balsalazide for mild-moderate UC was conducted where, strictly due to the national shortage (November 2012- January 2013), were switched to alternative 5-ASA and/or then returned to balsalazide once supply resumed. Clinical (Partial Mayo), endoscopic (Mayo score) activity, adverse effects (to alternative 5-ASA) and percentage market share (of continuous 5-ASA users) from baseline (i.e., time of switching due to shortage) through to five years were assessed.
RESULTS
Of 31 patients switched due to the shortage, 12 (38.7%) resumed balsalazide immediately once supply resumed, 8 (25.8%) prompted by adverse effects to the alternative 5-ASA used. Three patients (9.7%) had documented symptomatic improvement, 15 (48.4%) were unchanged and 13 (41.9%) had symptomatic worsening vs baseline (P < 0.01), after switching to an alternative 5-ASA. At 3 and 5y post switch, overall 26/31 (83.9%) and 23/31 (74.2%) had remained continuously on any 5-ASA therapy respectively. Twelve (38.7%) and 11 (35.5%) patients remained on balsalazide continuously at three and five years respectively after drug supply returned, equating to a loss of market share (within 5-ASA class) of 45.2% and 38.7% respectively.
CONCLUSION
This study of a balsalazide shortage in UC patients exemplifies the detrimental impact of a drug shortage on long term patient, disease and commercial outcomes.
Keywords: Inflammatory bowel disease, Ulcerative colitis, Drug supply, Drug shortage, Patient outcomes, Market share
Core tip: As a chronic disease, this study of a drug shortage in ulcerative colitis provides an excellent, novel insight into the short and long term effects of an sudden, unexpected nationwide drug shortage (in this case balsalazide) in patients previously in stable remission. The study highlights the importance of maintaining a seamless drug supply for both patients (given significant rates of disease worsening occurred, directly attributable to shortage), and drug manufacturers given the loss of market share engendered by even a short term drug shortage.
INTRODUCTION
Drug supply shortages are an insidious yet growing threat to the optimal medical management of chronic diseases, including ulcerative colitis (UC). Across many countries, healthcare settings and diseases, such shortages have been linked with patient deaths and significant morbidity[1,2]. Drug shortages may be defined by “a supply issue that affects the ability of preparation and/or dispensing of a drug such that it influences patient care and/or prescribers must use an alternative agent[3]”. They appear to be increasing for multiple reasons, including growing trends worldwide to operate drug supply chains on a just-in-time basis to maximise cost reduction and avoid excess inventory, brought about by lower profit margins due to the arrival of generics and other market competitive forces. Moreover, in most countries, there is no requirement for suppliers to report drug supply issues, often leading to clinicians and patients facing shortages with little or no warning. High cost drugs for relatively narrow indications in smaller markets appear more at risk of shortages, exemplified by the balsalazide shortage in Australia as studied here[4-6].
Over a three month period from November 2012 to January 2013 inclusive, a nationwide shortage in the supply of balsalazide (Colazal, Colazide®) in Australia necessitated a blanket, non-medical substitution with alternative 5-ASA formulations in patients with UC. Balsalazide is a prodrug of the active metabolite, mesalazine, which is linked to a carrier molecule via an azo bond and released in the colon by bacterial azo-reduction[7]. The 5-aminosalicylic acid (5-ASA) agents as a class (including balsalazide) are standard and effective therapies for the induction and maintenance of remission in UC, particularly in mild to moderate disease. In the setting of few randomized head-to-head trials or real-world comparative studies of different oral 5-ASA preparations, the formulations are, pragmatically, considered to be similar in efficacy when adjusting for dose for both induction and maintenance of UC[8]. For instance, 6.75 g daily of balsalazide (equivalent to 2.34 g of mesalazine) was compared with a pH-dependent acrylic resin coated mesalazine formulation (Asacol®) in three randomized-controlled, double-blind studies[9-11], with no significant differences in the primary endpoints between these formulations at 8 wk.
Therefore, this balsalazide shortage presented a unique opportunity to evaluate short and long term outcomes of a semi-controlled, unselected cohort of patients with UC in whom a non-medical switch in 5-ASA formulation was undertaken contemporaneously. Moreover, there are scant data regarding the short and long term implications of a drug shortage in patients with chronic diseases, including IBD. Hence, the aims of this prospective study were to assess the short and long term ramifications of a drug shortage imposed substitution of balsalazide to alternative 5-ASA formulations in patients with UC, in terms of: (1) Efficacy (including symptom-based, endoscopic activity and long-term outcomes); (2) Safety (including immediate adverse effects to alternative 5-ASA upon switching); and (3) The proportion of patients returning to the original product once supply resumed as a measure of loss of market share in a competitive drug category such as 5-ASA agents.
MATERIALS AND METHODS
A prospective, long term cohort study was undertaken of patients with a confirmed diagnosis of mild-moderate UC as per standard criteria, treated for a minimum of six months with balsalazide at specialist inflammatory bowel disease (IBD) Clinics at two Melbourne metropolitan hospitals, in whom switching to an alternative 5-ASA formulation (at the treating doctor’s discretion) was mandated by drug unavailability between November 1st 2012 to January 31st 2013. Patients who were switched from balsalazide to an alternative 5-ASA due to medical reasons, including intolerance, tablet burden or active disease were excluded from this study. After supply of balsalazide resumed, the occurrence of switching back to balsalazide was solely at the discretion and agreement of the patient and treating clinician.
Patient demographics, disease characteristics (including Montreal Classification extent/severity of IBD[12]), biomarkers including C-reactive protein (CRP) and faecal calprotectin (where available), medication changes (including adverse effects and concomitant medications) and symptom-based disease activity (via partial Mayo score) were collected immediately at baseline pre-switch and then at multiple timepoints (3-6 mo at post-switch subsequent clinic review, then at 3 years and 5 years) after 5-ASA substitution. A balsalazide dose of 6-6.75 g was assumed to be equivalent to sulfasalazine or mesalazine 2-2.4 g as per the manufacturer’s product information documentation.
Endoscopic data (via Mayo endoscopy score) were also collected at baseline (data included if endoscopy had occurred no more than 12 mo pre-switch), then post-switch (no less than 3 mo and no more than 12 mo from date of switch) and subsequently at three and five years (included if within six months in each case). A Mayo endoscopy score of 0 or 1 was deemed to represent endoscopic remission. Clinical remission was defined as a partial Mayo score of ≤ 2, where each subscore was ≤ 1. Patients with newly diagnosed ulcerative colitis in the preceding 12 mo prior November 1st 2012 were excluded from the analysis.
The impact on market share of balsalazide affected by the drug shortage was calculated as the proportion derived by the number of patients who were switched temporarily to an alternative 5-ASA before returning to balsalazide through to the three and five-year timepoints, divided by the total number of patients who, upon switching from balsalazide to an alternative 5-ASA, then remained on this or any alternative 5-ASA agent continuously to the respective timepoints.
Data were analyzed with GraphPad Prism version 7 (GraphPad software, La Jolla, United States 2018). Given the data were non-normally distributed and of relatively small sample size, non-parametric statistics were used. For continuous data, medians are presented and compared with Kruskal-Wallis (unpaired) or Wilcoxon (paired) tests. For categorical data, proportions were compared with Fisher exact tests. Patients without complete data at both baseline and five year follow-up timepoints were excluded from the study. A P < 0.05 was considered to be clinically significant. The statistical methods of this study were reviewed by Dr Danny Con, Biostatistician, Eastern Health. Ethics approval was obtained from the Eastern Health Office of Research and Ethics (LR 61/2015).
RESULTS
Thirty-one patients with UC were switched from balsalazide to an alternative 5-ASA formulation specifically due to the balsalazide drug shortage. The majority of patients were switched from balsalazide to multi-matrix (MMX) mesalazine (28, 90.3%). Further characteristics of this cohort are shown in Table 1.
Table 1.
Characteristics of the patient cohort (n = 31) who were switched from balsalazide (due to shortage) to an alternative aminosalicylate formulation
| Variable | Pre-switch (baseline) | Post-switch (at subsequent review)1 |
| Age (yr) (median, range) | 54 (20-79) | |
| Male sex (%) | 16 (51.6) | |
| Disease duration (yr) (median, range) | 10 (3-48) | |
| Montreal Classification, n (%) | ||
| Disease extent | ||
| Proctitis (E1) | 4 (12.9) | |
| Left sided colitis (E2) | 21 (67.7) | |
| Extensive colitis (E3) | 6 (19.4) | |
| Disease severity | ||
| Clinical remission (S0) | 14 (45.2) | 10 (32.2) |
| Mild (S1) | 16 (51.6) | 15 (48.4) |
| Moderate (S2) | 1 (3.2) | 6 (19.4) |
| Severe (S3) | 0 (0.0) | 0 (0.0) |
| Endoscopic (Mayo) subscore, n (%) | ||
| Mayo 0 | 6 (19.4) | 13 (41.9) |
| Mayo 1 | 9 (29.0) | 9 (29.0) |
| Mayo 2 | 13 (41.9) | 5 (16.1) |
| Mayo 3 | 3 (9.7) | 3 (9.7) |
| Endoscopic remission (Mayo 0/1) | 15 (48.4) | 22 (71.0) |
| Alternative 5-ASA formulation switched to | ||
| MMX mesalazine | 28 (90.3) | |
| Time-dependent, ethylcellulose coated2 | 2 (6.5) | |
| Sulfasalazine | 1 (3.2) | |
| Median balsalazide dose (g, range) | 5.3 (3.0-9.0) | - |
| Median equivalent mesalazine dose (g, range)3 | 2.1 (1.1-3.2) | 3.6 (2.0-4.8) |
| Concurrent Medical therapy, n (%) | ||
| Nil other | 7 (22.6) | |
| Topical aminosalicylate | 10 (32.2) | |
| Oral corticosteroid | 1 (3.2) | |
| Azathioprine/mercaptopurine | 14 (45.2) | |
| Methotrexate | 3 (9.7) | |
| Anti-TNF biologic | 0 (0.0) | |
| Other biologic | 0 (0.0) |
Median 3 mo after baseline–overall cohort data reported here (i.e., either on alternative aminosalicylate or had resumed balsalazide).
Marketed as Mezavant® (Shire Pty Ltd) and Pentasa® (Ferring Pty Ltd) in Australia respectively.
Based on Balsalazide Product Information[7].
Short term outcomes post switch compared to baseline (pre-switch)
Compared to baseline clinical (partial Mayo score) activity, three patients (9.7%) had documented symptomatic improvement (≥ 1 point reduction in partial Mayo score), 15 (48.4%) were unchanged and 13 (41.9%) had symptomatic worsening (≥ 1 point increase in partial Mayo score) (improvement vs worsening, P < 0.01, Fisher exact test) after switching from balsalazide to an alternative 5-ASA formulation.
Twenty-six (83.9%) of the cohort had endoscopic assessment both within 12 mo prior and then within 12 mo after the switch. Of these, compared to baseline endoscopic activity (Mayo endoscopy score), 13 (50%) patients had similar or improved endoscopic activity and 13 (50%) had worsening of their endoscopic activity post switch (P = 1.0, Fisher exact test). There were no significant differences (pre to post-switch) in serum CRP [median difference 0 mg/L (-6, 108)], serum ALT [1.0 (-15, 61)], serum GGT [0.5 (-8, 507)] or serum white cell count [-0.5 (-3.0, 3.3)] (all tests +/−3 mo of switch, each P > 0.2, Wilcoxon tests).
Based on the published dose equivalence of balsalazide compared to mesalazine[7], in all 31 patients there was an equal or increased effective mesalazine dose received after switching to the alternative 5-ASA formulation [median delta increase of 1.4 g (0, 3.2) mesalazine daily, P < 0.01 (Wilcoxon test)], Figure 1.
Figure 1.
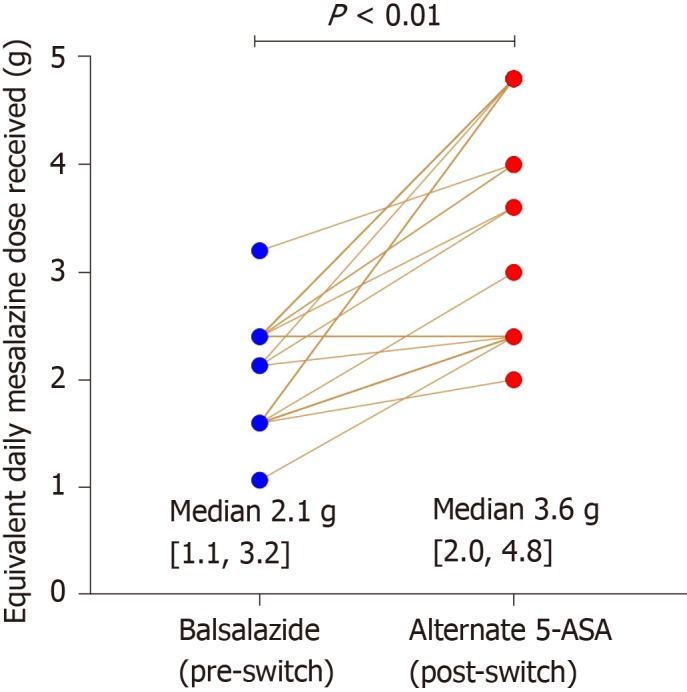
Change in equivalent mesalazine dose with switch from balsalazide to alternative aminosalicylate agent.
Adverse events with substitution of balsalazide to alternative 5-ASA agent during shortage
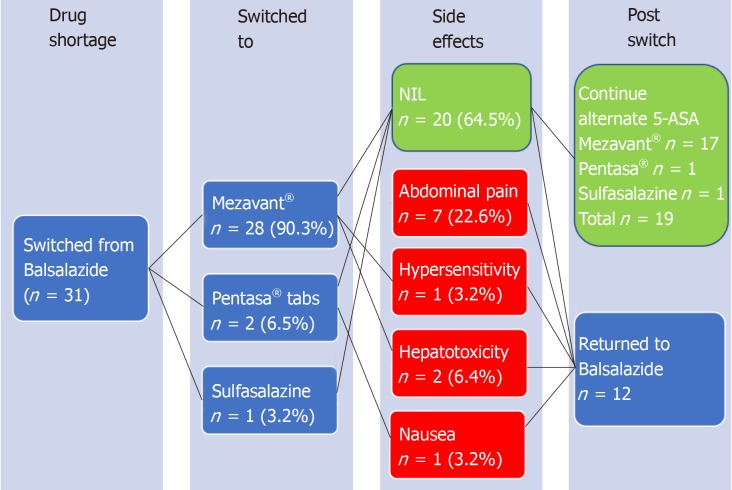
Adverse events were reported by 8/31 (16.2%) patients, all documented within 2 wk after switching, and attributable to, the alternative 5-ASA agent. These included one or more of hepatotoxicity (n = 2), abdominal pain (n = 6), nausea (n = 1) and/or hypersensitivity reaction (n = 1). Of these patients with adverse events, all 8 (100%) had prompt resolution of symptoms upon cessation of alternative 5-ASA therapy. Then upon switching back to balsalazide once supply returned, all 8 continued on balsalazide without further adverse event/s during the remainder of the follow-up period (Figure 2).
Figure 2.
Flow chart depicting the switch to alternative aminosalicylate resulting from the balsalazide shortage, with adverse effect rate of 35.5% occurring immediately post-switch, which in all cases resolved upon return to balsalazide.
Long term outcomes
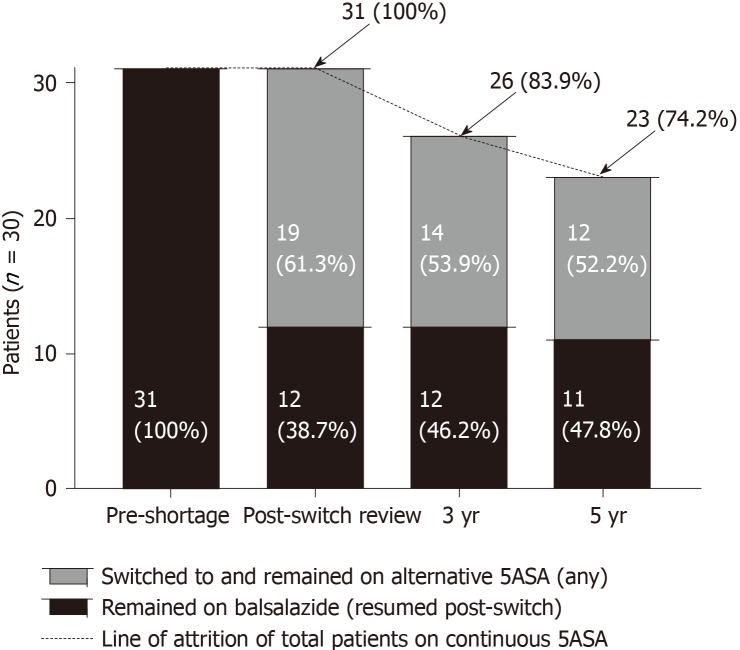
At three and five years following the switch, overall 26/31 (83.9%) and 23/31 (74.2%) patients had remained continuously on any 5-ASA therapy respectively. Twelve (38.7%) patients switched back to balsalazide as soon as supply returned (within three months). All twelve (38.7%) remained on balsalazide at 3 years, with 11 patients (35.5%) on balsalazide at 5 years.
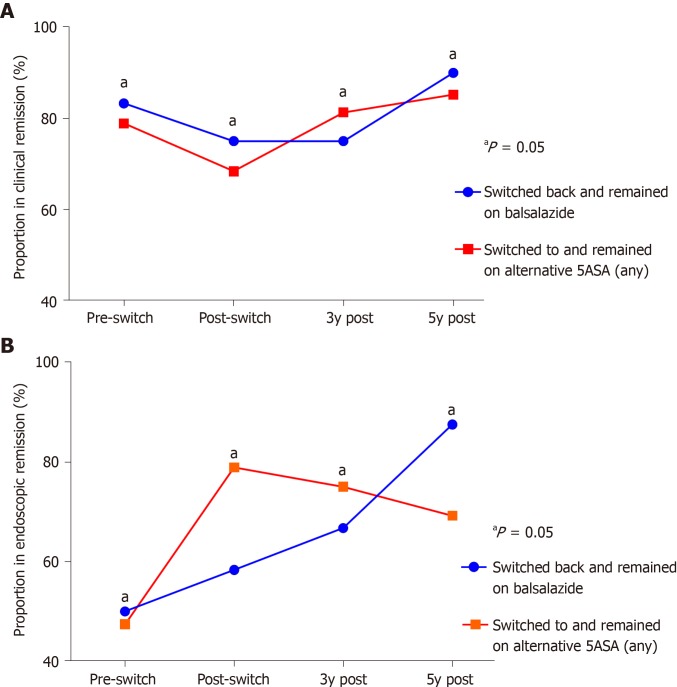
Compared to the total number and accounting for the resultant attrition of those on continuous 5-ASA therapy, there was a loss of long-term market share of 45.2% and 38.7% at three and five years respectively after, and as a direct result of, the balsalazide shortage (Figure 3). Also, at each subsequent timepoint following the switch through to five years, there were no significant differences in the rates of clinical or endoscopic remission between those who continued on alternative 5-ASA therapy vs those who had switched but then returned to balsalazide as soon as supply returned (Figure 4A-B).
Figure 3.
Long term outcome of balsalazide drug shortage on market share through to five years follow-up (compared to persistence on alternative aminosalicylate therapy).
Figure 4.
Comparison of rates of Clinical remission and Endoscopic remission over multiple timepoints in those who switched to and remained on alternative aminosalicylate vs those who switched back and remained on balsalazide. A: Clinical remission; B: Endoscopic remission.
Finally, there was no significant difference in rates of treatment escalation to immunomodulators or biologics, colectomy or mortality (all causes) between the two groups at both the 3 and 5-year follow-up timepoints. However, there was a higher rate of flares requiring hospitalization in those who switched from balsalazide then remained on an alternative 5-ASA (36.8 vs 0.0% at 5 years, P = 0.03, Fisher exact test), Table 2.
Table 2.
Long term cumulative outcomes including rates of treatment escalation, colectomy and mortality in those who continued on alternative aminosalicylate therapy (n = 19) vs those who switched but then returned to balsalazide as soon as supply returned (n = 12), n (%)
| Outcome (cumulative) | Post-switch review (baseline), n (%) | As of 3y follow-up1, n (%) | As of 5y follow-up1, n = (%) | |||
| Alternative 5-ASA2 | Resumed alsalazide | Alternative 5-ASA2 | Resumed balsalazide | Alternative 5-ASA2 | Resumed balsalazide | |
| Escalated to immunomodulator | 14 (73.7) | 5 (41.7) | 16 (84.2) | 5 (41.7) | 16 (84.2) | 5 (41.7) |
| Escalated to biologic | 0 (0) | 0 (0) | 3 (15.8) | 0 (0) | 6 (31.6) | 2 (16.7) |
| Hospitalised for flare3 | 0 (0) | 0 (0) | 3 (15.8) | 0 (0) | 7 (36.8)a | 0 (0)a |
| Colectomy | 0 (0) | 0 (0) | 1 (5.3) | 0 (0) | 1 (5.3) | 0 (0) |
| All-cause mortality4 | 0 (0) | 0 (0) | 2 (10.5) | 0 (0) | 2 (10.5) | 0 (0) |
Outcome occurring prior to or at timepoint.
5-ASA: Aminosalicylae.
First hospitalization counted for UC flare only.
Both deaths in cohort were unrelated to ulcerative colitis (one due to sarcoma and one acute myocardial infarction).
P < 0.05.
DISCUSSION
To our knowledge, this study is the first to demonstrate the long-term ramifications of a drug shortage in a cohort of patients with inflammatory bowel disease. Mild-moderate UC is an archetypal chronic disease in which to examine the effect of a drug shortage given it is a lifelong, relapsing-remitting disease in typically young, otherwise healthy patients, where remission is achieved and maintained in a significant proportion by daily administration of oral drugs such as mesalazine or balsalazide, with a highly favourable risk: benefit ratio. Long-term stability of disease control and outcomes depend primarily on adherence, and therefore continuous supply, of the drug. Consequently, a drug shortage has the potential to exert multiple deleterious flow-on effects including a flare or worsening of disease with possible hospitalization or colectomy, as well as significant anxiety, psychological distress, and loss of work productivity. Hence, such a shortage poses a risk for not only the patient but health payers and the drug manufacturer.
The most striking finding of this study is perhaps the significant loss of balsalazide’s market share resulting from the shortage to competitor 5-ASA formulations of approximately 40% which persisted even to 5 years in this cohort. Given the sudden, unexplained nature of the drug shortage for both clinicians and patients with no advanced notification of return of supply, all patients were switched immediately to alternative 5-ASA formulations in order to maintain treatment continuity. Once balsalazide was again available, it is perhaps unsurprising that given the loss of confidence in drug supply that most patients chose to remain on the alternative 5-ASA therapy. This study therefore presents a warning to drug manufacturers that despite a relatively short-duration, once-off drug shortage, the effects on patient and clinician confidence in a product may be far more lasting, especially where similarly effective, competing formulations are available. Indeed, a sustained loss of 40% of prior market share as depicted in this study highlights financial risks to pharmaceutical companies as a result of suboptimal manufacture and supply-chain processes, especially in chronic diseases.
In this study there was no significant impact elicited in either clinical or endoscopic assessed disease activity on a per-group basis between those who remained on an alternative 5-ASA formulation post-switch and those who returned to balsalazide after drug supply returned, at each of the assessment timepoints to five years. Although this might imply the bioequivalence of the 5-ASA formulations, given the small sample size in this study no further conclusions can be made. It is important to reiterate that all switches were performed for non-medical reasons (i.e., shortage) only and those switched for other reasons such as intolerance, active disease, or tablet burden were excluded from the study. However, on a per-patient basis, a greater proportion of patients suffered a symptomatic worsening than improvement of their colitis at initial review post-switch (P < 0.01) and there was a higher rate of flares requiring hospitalization through to five years (37% vs 0%, P = 0.03) despite no differences in rates of treatment escalation between the groups. One may therefore hypothesise that for a given individual, not all oral aminosalicylate preparations are equal and due to reasons including disparate tablet/granule composition, delivery system, pharmacodynamics and phenotypic differences, switching between agents within class may result in improved/worsened disease control and/or adverse effects. Regardless, these data exemplify the potential clinical sequelae of a drug shortage, which hitherto has not been well characterized in previous studies[13-15].
Notably, upon non-medical switching from balsalazide to an alternative 5-ASA formulation, approximately one-third of patients developed an adverse effect requiring drug cessation. This is a far higher proportion of adverse effects than typically seen in commencing 5-ASA agent/s in UC, and certainly higher than that reported in mesalazine registration trials[16,17]. Furthermore, many of the side effects appeared to be of idiosyncratic type (e.g., abdominal pain, Figure 2), occurred rapidly within 1-2 wk of commencement and all resolved upon cessation and recommencing balsalazide. It is plausible therefore that at least a proportion of these adverse effects might be explained by a nocebo effect– i.e., an effect occurring when negative expectations of the patient regarding a treatment cause the treatment to have a more negative effect than it otherwise would have, such as recently reported with the non-medical switching from originator to biosimilar infliximab in similar disease populations[18,19]. If so, this further illustrates the potential negative impact of a drug shortage on patients, especially in diseases like UC where psychological stress has been linked with flares and/or symptom provocation[20]. Another possible explanation is that, given the vast majority of patients were switched to MMX mesalazine, that this particular formulation might be the cause of adverse effects. Alternatively, the increase in side effects might have been explained by the almost universal increase in equivalent mesalazine dose received by patients switching to the alternative 5-ASA therapy (median increase of 1.6 g mesalazine), although multiple studies have demonstrated that adverse effects to mesalazine are not dose-dependent[21,22].
The authors acknowledge several limitations of this study, including the observational design and small sample size which limit the ability to make definitive conclusions given potential bias, or ascribe causality. However, this was an unselected, consecutive patient cohort who were all on the same treatment (balsalazide) prior to a non-medical therapeutic switch, were well phenotypically characterized (all mild-moderate UC), were all followed prospectively for five years and all treatments and disease (including endoscopic) assessments were recorded, thus alleviating much of the potential bias. Moreover, the vast majority (over 90%) were switched to MMX mesalazine upon shortage of balsalazide, further aiding uniformity. Other limitations of the study included that endoscopic and clinical assessment of disease activity prior to and post-switch was not performed at strictly uniform timepoints and faecal calprotectin testing was not routinely available for most patients during this study. Assessment by CRP was performed at the clinic visits by these patients, but CRP has known poor sensitivity in the assessment of disease activity in UC[23]. Finally, concomitant medications were not controlled in keeping with the “real world” nature of this cohort, however it should be noted that none of the patients were on biologic therapy at the time of drug shortage though a significant proportion were on concurrent immunomodulators.
In summary, this study has demonstrated the deleterious effects of a drug shortage in ulcerative colitis, with a higher than expected proportion of patients exhibiting a worsening of disease and/or significant side effects upon substitution of their maintenance agent balsalazide with an alternative in the same class. Furthermore, this study demonstrated the adverse commercial impact of a drug shortage for the manufacturer, with a 40% loss of market share persisting even to five years post-shortage. Despite enhanced globalization, supply chains and technological advances, drug shortages are increasingly common and relative low incidence chronic diseases such as IBD appear at higher risk. Hence, this study highlights the threat posed by drug shortages to patients, clinicians, healthcare payers and pharmaceutical companies alike, and the need to explore ways to minimise such occurrences in future.
ARTICLE HIGHLIGHTS
Research background
Drug shortages appear to be occurring more frequently, yet their clinical impact and sequelae are not yet well described. Here, a nationwide drug shortage of balsalazide occurred over several months in 2012-3, necessitating a sudden switch to alternative aminosalicyclate formulations.
Research motivation
In this study the impact of this balsalazide shortage was intensively assessed in a well characterized population of patients with ulcerative colitis over a five year period to assess short and long term effects of a drug shortage. We hypothesized that this and similar drug shortages can have significant detrimental impacts on disease course and patient outcomes.
Research objectives
This study aimed to elucidate the short and long term ramifications of a drug shortage in ulcerative colitis patients on (1) efficacy (including symptom-based, and objective disease assessments); (2) safety (including immediate adverse effects occurring after switching to alternative agents); and (3) the proportion of patients returning to the original product once supply resumed as a measure of loss of market share. This comprehensive, holistic assessment of drug shortage-related outcomes sets a benchmark for further quantitative research in this field.
Research methods
A prospective cohort study of patients on balsalazide for mild-moderate ulcerative colitis was conducted where, strictly due to the national shortage patients were switched to alternative 5-ASA and/or then returned to balsalazide once supply resumed. Clinical and disease activity assessments were performed at baseline pre-switch, then immediately and at 3 and 5 years after the drug shortage-imposed switch to assess short and long term sequelae.
Research results
Although in stable remission at the time of the drug shortage, almost half of the patients when switched from balsalazide had documented clinical worsening at their subsequent review, including several reporting side effects to the alternative formulation. Only a minority of patients returned to balsalazide after drug supply returned, equating to a loss of market share (within the same class) of approximately 40% even to five years post-shortage in this cohort. These data highlight the importance of maintaining a seamless drug supply for both patients (given significant rates of disease worsening occurred, directly attributable to shortage), and drug manufacturers given the loss of market share engendered by even a short term drug shortage.
Research conclusions
In one of the first published studies of its kind to date, this study of a balsalazide shortage in UC patients exemplifies the detrimental impact of a drug shortage on long term patient, disease and commercial outcomes. Hence, patients, clinicians and drug manufacturers should be more aware and explore ways to address and minimize this growing problem worldwide.
Research perspectives
Further prospective, larger scale studies are needed to document the impacts of drug shortages in patients across multiple chronic and/or life-threatening diseases. By documenting the scope of this problem in this manner, hopefully long term solutions can then be instituted accordingly.
Footnotes
Institutional review board statement: This study was approved as a low-risk application by the Eastern Health Office for Human Research & Ethics.
Informed consent statement: Patient consent was not deemed to be required for this non-interventional observational study using deidentified data.
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Invited Manuscript
Corresponding Author's Membership in Professional Societies: Gastroenterology and hepatology.
Peer-review started: November 12, 2019
First decision: December 23, 2019
Article in press: March 12, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: de Silva AP, Chiba T, Sikiric P S-Editor: Wang J L-Editor: A E-Editor: Qi LL
Contributor Information
Daniel R van Langenberg, Department of Gastroenterology, Eastern Health, Box Hill, Victoria 3128, Australia; Eastern Health Clinical School, Monash University, Box Hill, Victoria 3128, Australia. daniel.van-langenberg@monash.edu.
Richard Kai-Yuan Cheng, Department of Gastroenterology, Eastern Health, Box Hill, Victoria 3128, Australia; Department of Gastroenterology, Redcliffe Hospital, Redcliffe, Queensland 4020, Australia.
Mayur Garg, Department of Gastroenterology, Eastern Health, Box Hill, Victoria 3128, Australia; Eastern Health Clinical School, Monash University, Box Hill, Victoria 3128, Australia; Department of Gastroenterology, Royal Melbourne Hospital, Parkville, Victoria 3052, Australia.
Data sharing statement
Not applicable.
References
- 1.Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361–373. doi: 10.1016/j.mayocp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels K, Simoens S, Casteels M, Huys I. Insights into European drug shortages: a survey of hospital pharmacists. PLoS One. 2015;10:e0119322. doi: 10.1371/journal.pone.0119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742–1750. doi: 10.2146/ajhp180441. [DOI] [PubMed] [Google Scholar]
- 4.Pauwels K, Huys I, Casteels M, Simoens S. Drug shortages in European countries: a trade-off between market attractiveness and cost containment? BMC Health Serv Res. 2014;14:438. doi: 10.1186/1472-6963-14-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox ER, Tyler LS. Potential Association between Drug Shortages and High-Cost Medications. Pharmacotherapy. 2017;37:36–42. doi: 10.1002/phar.1861. [DOI] [PubMed] [Google Scholar]
- 6.Schweitzer SO. How the US Food and Drug Administration can solve the prescription drug shortage problem. Am J Public Health. 2013;103:e10–e14. doi: 10.2105/AJPH.2013.301239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colazide Product Information 2017. [updated 2017 March 31st] Available from: https://www.fresenius-kabi.com/au/documents/Colazide_PI.pdf.
- 8.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD000544. doi: 10.1002/14651858.CD000544.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Pruitt R, Hanson J, Safdi M, Wruble L, Hardi R, Johanson J, Koval G, Riff D, Winston B, Cross A, Doty P, Johnson LK. Balsalazide is superior to mesalamine in the time to improvement of signs and symptoms of acute mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:3078–3086. doi: 10.1111/j.1572-0241.2002.07103.x. [DOI] [PubMed] [Google Scholar]
- 10.Levine DS, Riff DS, Pruitt R, Wruble L, Koval G, Sales D, Bell JK, Johnson LK. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398–1407. doi: 10.1111/j.1572-0241.2002.05781.x. [DOI] [PubMed] [Google Scholar]
- 11.Green JR, Lobo AJ, Holdsworth CD, Leicester RJ, Gibson JA, Kerr GD, Hodgson HJ, Parkins KJ, Taylor MD. Balsalazide is more effective and better tolerated than mesalamine in the treatment of acute ulcerative colitis. The Abacus Investigator Group. Gastroenterology. 1998;114:15–22. doi: 10.1016/s0016-5085(98)70627-4. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Tao T, Grabarek Z, Gergely J, Collins JH. Cross-linking of residue 57 in the regulatory domain of a mutant rabbit skeletal muscle troponin C to the inhibitory region of troponin I. J Biol Chem. 1991;266:13746–13751. [PubMed] [Google Scholar]
- 13.Nonzee NJ, Luu TH. The Drug Shortage Crisis in the United States: Impact on Cancer Pharmaceutical Safety. Cancer Treat Res. 2019;171:75–92. doi: 10.1007/978-3-319-43896-2_6. [DOI] [PubMed] [Google Scholar]
- 14.Hedlund NG, Isgor Z, Zwanziger J, Rondelli D, Crawford SY, Hynes DM, Powell LM. Drug Shortage Impacts Patient Receipt of Induction Treatment. Health Serv Res. 2018;53:5078–5105. doi: 10.1111/1475-6773.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralls MW, Blackwood RA, Arnold MA, Partipilo ML, Dimond J, Teitelbaum DH. Drug shortage-associated increase in catheter-related blood stream infection in children. Pediatrics. 2012;130:e1369–e1373. doi: 10.1542/peds.2011-3894. [DOI] [PubMed] [Google Scholar]
- 16.Kruis W, Schreiber S, Theuer D, Brandes JW, Schütz E, Howaldt S, Krakamp B, Hämling J, Mönnikes H, Koop I, Stolte M, Pallant D, Ewald U. Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses. Gut. 2001;49:783–789. doi: 10.1136/gut.49.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Haens G, Hommes D, Engels L, Baert F, van der Waaij L, Connor P, Ramage J, Dewit O, Palmen M, Stephenson D, Joseph R. Once daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: a phase II, dose-ranging study. Aliment Pharmacol Ther. 2006;24:1087–1097. doi: 10.1111/j.1365-2036.2006.03082.x. [DOI] [PubMed] [Google Scholar]
- 18.Bakalos G, Zintzaras E. Drug Discontinuation in Studies Including a Switch From an Originator to a Biosimilar Monoclonal Antibody: A Systematic Literature Review. Clin Ther. 2019;41:155–173.e13. doi: 10.1016/j.clinthera.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Kristensen LE, Alten R, Puig L, Philipp S, Kvien TK, Mangues MA, van den Hoogen F, Pavelka K, Vulto AG. Non-pharmacological Effects in Switching Medication: The Nocebo Effect in Switching from Originator to Biosimilar Agent. BioDrugs. 2018;32:397–404. doi: 10.1007/s40259-018-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54:1481–1491. doi: 10.1136/gut.2005.064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata H, Yokoyama T, Mizushima S, Hagino A, Hibi T. Comparison of efficacy of once daily multimatrix mesalazine 2.4 g/day and 4.8 g/day with other 5-aminosalicylic acid preparation in active ulcerative colitis: a randomized, double-blind study. Intest Res. 2018;16:255–266. doi: 10.5217/ir.2018.16.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fockens P, Mulder CJ, Tytgat GN, Blok P, Ferwerda J, Meuwissen SG, Tuynman HA, Dekker W, Gasthuis K, van Hees PA. Comparison of the efficacy and safety of 1.5 compared with 3.0 g oral slow-release mesalazine (Pentasa) in the maintenance treatment of ulcerative colitis. Dutch Pentasa Study Group. Eur J Gastroenterol Hepatol. 1995;7:1025–1030. doi: 10.1097/00042737-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140:1817–1826.e2. doi: 10.1053/j.gastro.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.