Abstract
Synthetic progestogens (progestins) have been linked to increased breast cancer risk; however, the role of endogenous progesterone in breast physiology and carcinogenesis is less clearly defined. Mechanistic studies using cell culture, tissue culture, and preclinical models implicate progesterone in breast carcinogenesis. In contrast, limited epidemiologic data generally do not show an association of circulating progesterone levels with risk, and it is unclear whether this reflects methodologic limitations or a truly null relationship. Challenges related to defining the role of progesterone in breast physiology and neoplasia include: complex interactions with estrogens and other hormones (eg, androgens, prolactin, etc.), accounting for timing of blood collections for hormone measurements among cycling women, and limitations of assays to measure progesterone metabolites in blood and progesterone receptor isotypes (PRs) in tissues. Separating the individual effects of estrogens and progesterone is further complicated by the partial dependence of PR transcription on estrogen receptor (ER)α-mediated transcriptional events; indeed, interpreting the integrated interaction of the hormones may be more essential than isolating independent effects. Further, many of the actions of both estrogens and progesterone, particularly in “normal” breast tissues, are driven by paracrine mechanisms in which ligand binding to receptor-positive cells evokes secretion of factors that influence cell division of neighboring receptor-negative cells. Accordingly, blood and tissue levels may differ, and the latter are challenging to measure. Given conflicting data related to the potential role of progesterone in breast cancer etiology and interest in blocking progesterone action to prevent or treat breast cancer, we provide a review of the evidence that links progesterone to breast cancer risk and suggest future directions for filling current gaps in our knowledge.
Graphical Abstract
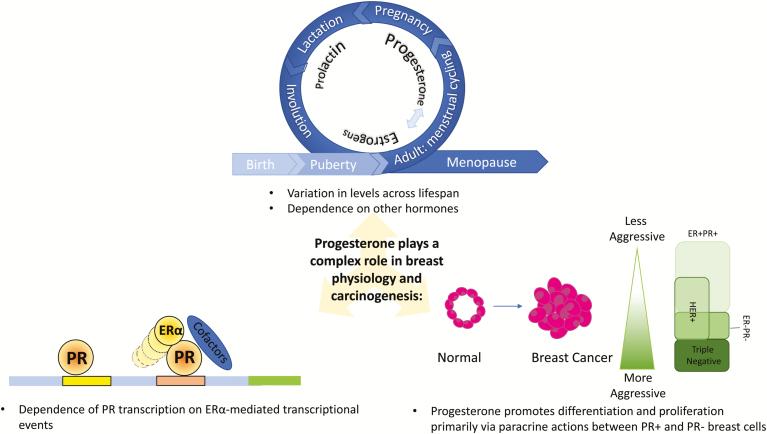
Graphical Abstract.
ESSENTIAL POINTS.
Estrogen and progesterone are sequentially involved in pubertal breast development primarily via a paracrine mechanism
Preclinical and clinical evidence support differentiation and proliferative roles of progesterone in the adult breast through primarily paracrine actions between PR+ and PR- breast cells
RANKL expression and high serum progesterone levels are highly correlated in the human breast; further, RANKL expression is required for progesterone-induced proliferation in the breast
To understand the role of progesterone in initiation and progression of breast cancer, it is prerequisite to understand how epithelial cells of the mammary gland control their fate.
Hormones are pivotal in controlling physiologic proliferation of normal breast epithelium, and therefore progesterone may influence early events in breast carcinogenesis.
In breast cancer, the proliferative effect of progesterone is mediated primarily by PR-B; extranuclear signaling actions of PR are also mediated predominantly by PR-B
Given recent improvements in assay sensitivity, research is needed to evaluate the association between endogenous progesterone/progesterone metabolites and breast cancer risk in the general population and among high-risk women (eg, BRCA 1 and BRCA 2 mutation carriers)
The critical importance of ovarian sex steroid hormones (estrogens and progesterone) in promotion and maintenance of the growth of breast cancers was suggested more than 100 years ago when George Beatson demonstrated that bilateral oophorectomy resulted in the remission of breast cancer in a premenopausal woman (1). Subsequently, recognition that many estrogen receptor-positive (ER+) breast cancers are estrogen dependent led to the development of highly effective adjuvant and chemopreventive agents for these tumors. Epidemiologic evidence has also demonstrated that some exogenous synthetic progestogens (progestins) administered with estrogen as menopausal hormone therapy or as contraception increase breast cancer risk. The finding that estrogen+progestin menopausal hormone therapy increases breast cancer risk led to a major decline in long-term use of these agents to alleviate postmenopausal symptoms (2). In contrast to the pharmacologic effects of progestins that have been clearly linked to breast cancer risk, the etiologic role of endogenous progesterone in the development of breast cancer is uncertain.
Mechanistic studies implicate progesterone in the development of breast cancer, whereas limited epidemiologic data have not provided strong support for a risk relationship with circulating levels. Data generated primarily by the Wiebe laboratory (3) suggest that progesterone metabolites have pro- and anti-carcinogenic effects, and that the balance of these factors may contribute to breast cancer risk, but this hypothesis has received limited attention in population-based research to date, primarily due to the lack of available assays. Finally, studies implicate progesterone signaling in the pathogenesis of breast cancers among BRCA1 mutation carriers (4), suggesting that chemoprevention to interrupt downstream signaling may be protective.
Complex factors contribute to the challenge of defining the role of progesterone in breast physiology and neoplasia, including: 1) dependence on and interaction with estrogens and other hormones (eg, androgens, prolactin, etc.), 2) variation in exposure levels, eg, during the menstrual cycle or pregnancy, 3) availability of sensitive assays, 4) limited data about the roles of progesterone metabolites, and 5) difficulties in assessing progesterone receptor (PR) isotypes in routinely prepared clinical specimens. Determining individual effects of estrogens and progesterone is complicated because transcription of PR is driven partly, but not exclusively, by estrogen receptor (ER)α-mediated transcriptional events (5, 6). Further, many of the actions of both estrogens and progesterone, particularly in “normal” breast tissues, are driven by paracrine mechanisms in which ligand binding to receptor-positive cells evokes secretion of factors that influence cell division of neighboring receptor-negative cells. Thus, levels in tissues and blood may differ and biological effects may be under local control.
Given these conflicting data and the interest in exploring whether blocking progesterone/PR signaling has utility in breast cancer prevention and treatment, we critically review the evidence that links progesterone to breast cancer risk and provide future directions for filling existing gaps in our knowledge. The aims of this review article are as follows: 1) provide background on the formation, transport, and metabolism of progesterone; 2) provide a summary of pharmacologic differences among exogenous progesterone and progestins; 3) describe what is known about physiologic levels of progesterone and breast development across the reproductive lifespan, including during pregnancy; 4) review the molecular mechanisms related to progesterone action; 5) summarize the current state of knowledge regarding progesterone and breast carcinogenesis; 6) summarize breast cancer risks associated with oral contraceptives and menopausal hormone therapy; and 7) provide a summary of unanswered questions, challenges, and future directions.
Progesterone Formation, Transport, and Metabolism
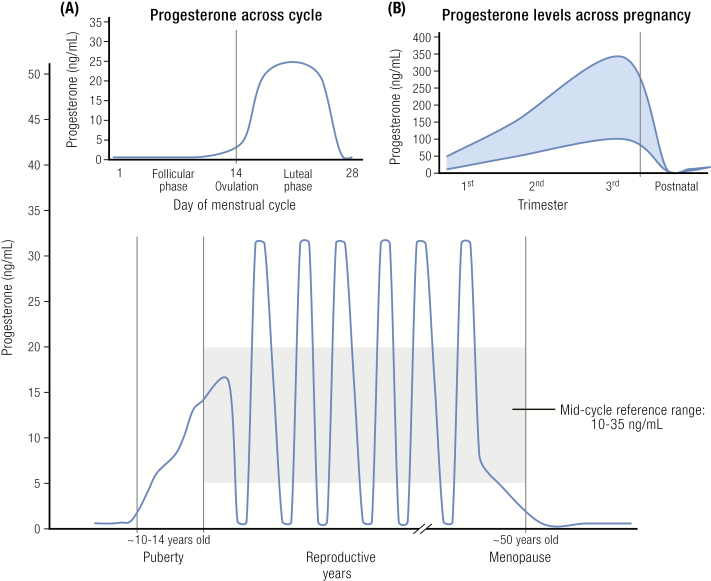
Progesterone is a 21-carbon steroid that exerts its primary physiological functions through binding to the progesterone receptors A and B (PR-A and PR-B), which initiate transcription of targeted genes resulting in conversion of proliferative endometrium to secretory endometrium in an estrogen-primed uterus. The physiological role of progesterone is primarily confined to the peri- and post-ovulatory phases of the menstrual cycle, and to pregnancy. In the menstrual cycle, progesterone is produced by the corpus luteum beginning in the early postovulatory phase. Its production rate increases from about 1 mg/day in the follicular phase to about 25 mg/day in the luteal phase (Fig. 1) (7). The biosynthesis of progesterone by the corpus luteum requires continued luteinizing hormone (LH) stimulation. During the follicular phase of the cycle serum progesterone levels are <1 ng/mL (8). The ovarian and adrenal contributions to the total progesterone production rate appear to be equal at that time. Subsequently, progesterone levels rise to about 1–2 ng/mL on the day of the LH surge and continue to rise, reaching a plateau of approximately 10–35 ng/mL during the mid-luteal phase; then levels decline until the end of the menstrual cycle (8). Most women have on average 35 years of predictable menstrual cycles with an average 14-day follicular phase and 14-day luteal phase.
Figure 1.
Progesterone levels across the lifespan: [Inset A) across the menstrual cycle, B) across pregnancy].
Legend: Progesterone levels increase from <1 ng/mL before age 10 to 10–12 ng/mL around puberty. Throughout the reproductive years levels range from 3 to 35 ng/mL in the luteal phase, decreasing in the late luteal phase, and are <1 ng/mL in the follicular phase (Inset A). In postmenopausal women, progesterone levels wane to <0.2 ng/mL. In pregnant women (Inset B), progesterone levels increase from 10–35 ng/mL in the 1st trimester to 100–300 ng/mL in the 3rd trimester; progesterone levels then drop to low levels postnatally as prolactin levels increase to facilitate lactation and return to average reproductive age ranges when ovulation returns.
In contrast to the menstrual cycle, progesterone production is considerably higher in pregnancy. During the first 10 weeks of pregnancy, maternal serum progesterone levels are in the luteal phase range of 10–35 ng/mL. The placenta then begins to secrete sizable amounts of progesterone, resulting in a steady rise throughout the remainder of pregnancy. The production rate of progesterone during the late stage of pregnancy is as high as 300 mg/day. At term, serum progesterone levels range from 100 to 300 ng/mL (8).
As women age, ovarian follicle loss increases exponentially with accelerated loss as a woman enters the menopausal transition. The menopausal transition is characterized by increasing menstrual cycle irregularity, including lengthy periods of anovulation with fluctuation in hormone levels, as women progress over a period of 2–6 years towards having their final menstrual periods (aged 51.4 years on average) (reviewed in (9, 10)). In brief, FSH levels begin to rise on average 6 years before the final menstrual period (11). Estrogen levels remain relatively constant through the early years of the menopausal transition, and then begin to fall about 2 years prior to the final menstrual period, reaching the low stable levels characteristic of postmenopausal women about 2 years after the final menstrual period (9, 12). In contrast to relatively consistent estrogen levels, urinary pregnanediol glucuronide levels in ovulatory cycles (representing progesterone excretion) slowly decline during the menopausal transition, about 7% annually, signaling progressive luteal dysfunction as a characteristic of the menopausal transition (12). Early follicular inhibin B levels, which reflect the diminishing quantity and quality of ovarian follicles, and anti-Müllerian hormone levels consistently decline prior to the menopausal transition, becoming nondetectable approximately 4–5 years before the final menstrual period (9).
In postmenopausal women, the major source of progesterone is the adrenals, which produce less than 1 mg/day resulting in serum progesterone levels that are generally <0.2 ng/mL.
In blood, progesterone is bound to corticosteroid-binding globulin (CBG) and to albumin (13). In cycling women only about 20% of progesterone is bound to CBG, and the rest predominantly to albumin; about 2–3% of progesterone is unbound (free). However, in pregnancy CBG increases considerably and about 40% of progesterone is CBG-bound. It is plausible that the difference in bound and unbound progesterone between pregnant and non-pregnant women could explain some of the breast cancer risk reduction associated with pregnancy, although to our knowledge this has not been evaluated in epidemiologic studies.
Progesterone is highly vulnerable to enzymatic action by hepatic reductases and hydroxysteroid dehydrogenases because its structure contains two ketone groups and a double bond (14). Thus, the molecule is transformed to two isomers of dihydroprogesterone, four pregnanolone isomers, and eight isomers of pregnanediol. In addition, progesterone can undergo hydroxylation by cytochrome p450 enzymes. Subsequently, all progesterone metabolites with a hydroxyl group can be sulfated and glucuronidated, and their conjugated products are then excreted primarily in urine, but also in feces. Theoretically, progesterone can have over 100 metabolites when the unconjugated, sulfated, and glucuronidated metabolites are combined (summarized in (15)).
In general, progesterone acts on the reproductive tract to prepare it for initiation and maintenance of pregnancy. The major physiologic roles of progesterone are mediated in the uterus and ovary. After ovulation the corpus luteum releases progesterone which stimulates maturation of the uterine lining to facilitate implantation; progesterone maintains pregnancy through stimulation of uterine growth and differentiation and suppression of myometrial contractility.
Exogenously Administered Progesterone and Progestins
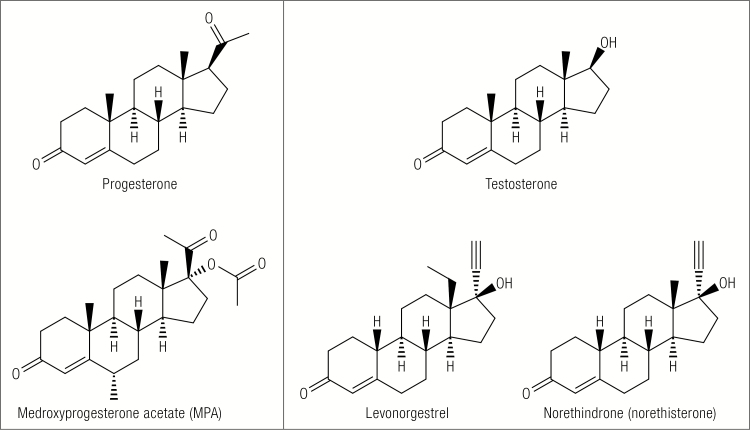
Orally administered progesterone has limited biological effects because it is poorly absorbed, even in micronized form, and is metabolized extensively during the hepatic first pass. For this reason and because more potent progestational compounds were needed for effective antifertility treatment, progestins were developed. A variety of progestins are now available not only for contraception, but also for menopausal hormone therapy to prevent endometrial hyperplasia and lessen the risk of endometrial cancer in estrogen-treated postmenopausal women. The progestins can be classified, based on their chemical structures, as those related to progesterone (C-21 progestins) and those structurally related to testosterone (C-19 progestins) (16). An example of a C-21 progestin is medroxyprogesterone acetate (MPA), whereas norethindrone (norethisterone) and levonorgestrel are C-19 related progestins (Fig. 2). Differences in pharmacologic properties of progesterone and some of the commonly used progestins are summarized in Table 1.
Figure 2.
Chemical structures of progesterone and testosterone and for comparison common C-21 and C-19 related progestins.
Table 1.
Pharmacologic difference among progestogens
| 1. Progesterone | • A natural progestogen (21 carbons) • Undergoes extensive metabolism during hepatic first pass • Approximately 20% is bound to CBG • Has low bioavailability (<5%) • Half-life is 16–18 hours after oral dosing • Binds with relatively high affinity to the PRs and MR • Typical daily dose used for endometrial protection is 200 mg |
| 2. Medroxyprogesterone acetate | • Structurally related to progesterone • Acetate group at the carbon 21 position limits metabolism (steric hinderance) • Does not bind to CBG or SHBG • Has high bioavailability (>90%) • Its half-life is ~22 hours • Binds with high affinity to the PRs • Has relatively high receptor/ligand binding affinity (RBA) for the AR but its androgenic activity is controversial • Has high RBA for the GR, displays higher binding affinity for the GR than cortisol • Common daily doses for endometrial protection are 2.5 and 5 mg |
| 3. Norethindrone (Norethisterone) | • Structurally related to testosterone • Ethinyl group at carbon 17 limits metabolism; can be converted to ethinyl estradiol • Binds to SHBG • Its bioavailability is ~64% and half-life is ~8 hours • Binds with relatively high affinity to the PRs and has some androgenic activity |
| 4. Levonorgestrel | • Structurally related to testosterone • Metabolism is very limited during hepatic first pass • Binds to SHBG • Its bioavailability is 89–99% and half-life 10–13 hours • It binds with high affinity to the PRs and AR, and has substantial androgenic activity |
| 5. Desogestrel | • Structurally related to testosterone; it is prodrug that is rapidly converted to etonogestrel during hepatic first pass • Etonogestrel binds to SHBG, has a bioavailability of 62–76% and half-life of 12–24 hours, and has a high affinity for the PRs |
| 6. Drospirenone | • Structurally related to spironolactone • Does not bind to SHBG or CBG • Has bioavailability of ~66% and half-life of ~32 hours • Has a relatively low RBA for the PRs but a very high RBA for the MR; exhibits both anti-androgenic and anti-mineralcorticoid properties |
Adapted with permission from Stanczyk, F.Z., et al., Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev, 2013. 34(2): p. 171–208.
Progesterone’s Role in Breast Development
Much of what is known about breast development has been derived from studies of rodents, which provides a useful, albeit imperfect, model for breast development in women. The breast develops at puberty with the establishment of menstrual cycling (17, 18). Estrogen and progesterone are sequentially involved in pubertal breast development primarily via a paracrine mechanism (19–21). Specifically, in vivo studies of hormonal ablation via ovariectomy and subsequent hormone replacement suggest that estrogens drive the first stage of pubertal development in the breast, whereas both estradiol and progesterone are responsible for cellular proliferation in the mammary gland (reviewed in (22); references to “mammary” gland throughout this manuscript refer to animal model data). Cell proliferation in the mammary epithelium ceases in ovariectomized mice; however, reintroduction of estradiol is sufficient to induce epithelial cell proliferation and restores ductal outgrowths consistent with intact pubertal animals. Estradiol alone is not sufficient to induce mammary gland cellular proliferation in pregnant animals (23). Similarly, progesterone is not sufficient to stimulate cellular proliferation in the absence of estrogen, as demonstrated by experimental studies conducted in ovariectomized mice showing that PR expression in mammary epithelium is dependent on interactions of estrogen with ERα to induce PR transcription (24–26). As is emphasized throughout this review, progesterone acts primarily through a paracrine mechanism, which makes cell culture experiments challenging. We are not aware of any co-culture systems for luminal progenitor cells that enable testing amplification of paracrine progesterone signaling. Further, progesterone’s paracrine signaling makes clinical translation a challenge as well, given that levels of other circulating hormones/growth factors and their cognate receptors likely influence the response to or effects of progesterone.
The dependence on hormones in mouse models is also relevant in humans, as the development of breast buds (thelarche) corresponds with increasing ovarian estrogen secretion preceding menarche and the introduction of cyclic ovarian progesterone secretion via menstrual cycling (27). In normal breast development in humans, it has been demonstrated that estrogen and progesterone are required for ductal elongation and side branching, respectively, across the reproductive years (28). However, the mouse and human differ substantially in several ways, including different levels of circulating hormones and hormone metabolites, processing by different liver enzymes, and different regulation of aromatase (29).
Androgens, on the other hand, inhibit breast development (reviewed in (30)). Higher absolute levels of androgens (regardless of estrogen level) suppress breast development, while low circulating testosterone relative to normal estradiol levels has been shown to stimulate breast development.
The mature breast is characterized by ducts and side branches (extralobular terminal ducts and lobular buds), but the highly branched architecture and terminal end buds characteristic of pregnancy are generally absent (18). Thus, pregnancy is another important developmental stage for the breast. During early pregnancy, the breast epithelium undergoes rapid and pronounced expansion and physiologic differentiation to form the highly branched architecture (lobular buds into intralobular ducts and ductules) of the breast. This expansion facilitates further differentiation of terminal duct lobular units (TDLUs) during late pregnancy to prepare for high levels of milk production resulting in lactation (31, 32). Similarly, studies of human breast tissues donated for research show that parous women have a greater density of breast lobules than nulliparous women and that this effect may be greatest within 10 years of a live birth (33).
Progesterone is critical for inducing ductal side-branching of the mammary gland which is essential for lobuloalveolar development during pregnancy (reviewed in (22)). PR null mouse mammary glands fail to undergo alveolar morphogenesis during pregnancy (28); in contrast, forced over-expression of PR results in increased ductal side-branching in adult virgin mice (34). It has further been demonstrated in selective knockout models in mice that PR-B is the primary mediator of progesterone’s proliferative effects during pregnancy (35, 36). Additional data indicate that progesterone mediates the secretion of Wnt4 from PR+ mammary cells, further supporting progesterone’s paracrine mode of action to regulate side-branching during early pregnancy (37). Additional data also suggest that progesterone acts directly on the ERα+/PR+ progenitor cell population by inducing receptor activator nuclear transcription factor kappa B ligand (RANKL) secretion from these mammary cells. This factor then directly binds to its receptor, RANK (also referred to as tumor necrosis factor superfamily 11A (TNFRSF11A)), to induce side branching and alveolar development (35, 38, 39). As mentioned previously, given that progesterone acts primarily through a paracrine mechanism, cell culture experiments are challenging. Co-culture systems for luminal progenitor cells are needed to allow testing amplification of paracrine progesterone signaling. Inevitably, as research evolves to test context-dependent signaling and transcriptional events involving multiple receptors and complex mixtures of ligands, our understanding of the role of hormones in breast development and cancer will improve. This information will be necessary to translate the existing laboratory and epidemiologic research into clinically actionable treatments and provide context on whether progesterone agonists or antagonists may be more useful.
Molecular Mechanisms Related to Progesterone Action
Progesterone Receptors
Steroid hormones, including progesterone, function by binding to cytoplasmic and nuclear proteins. Progesterone binds to two predominant PR isoforms, PR-A and PR-B. These isoforms are transcribed from the same gene (PGR) by two distinct promoters, which have different transcriptional and functional activities. PR-B has an additional stretch of amino acids located at the amino-terminus of the receptor. PR-B can encode a transactivation function that is specific to the PR-B protein, which plays an essential role in specifying target genes that can be activated by PR-B protein but not PR-A protein (6, 40, 41).
Reproductive tissue levels of PR-A and PR-B and their ratio varies based on developmental stage and hormonal status of the tissue (42, 43). When PR-A and PR-B are equivalent in cells, the receptors can dimerize and bind DNA as three species: A:A, B:B, and A:B (heterodimer) (44). PR-A and PR-B are capable of binding progesterone, dimerizing, and interacting with the progesterone response element and transcriptional machinery to regulate gene expression (41, 45). Mediation of the regulatory effects of progesterone depends on differential transactivation properties contributed to these complexes by the PR-B-specific domain (45–47). Further, the ratio of PR-A to PR-B in target cells as well as dimerization likely predict the overall cellular response to progesterone (45, 48).
Complicating research in this area, isoforms PR-A (94 kDa) and PR-B (114 kDa) can be readily distinguished by western blot due to their significant size difference of 20 kDa, but owing to similarities in structure, antibodies have failed to reliably distinguish them by immunohistochemical methods. Antibodies originally found to immunohistochemically detect the PR-A isoform alone have been found later to detect both PR-A and PR-B, and sample preparation methods appear to be important for PR-B epitope exposure. Recently, a monoclonal antibody Ab-6 (Thermo Fisher) has been described to uniquely detect PR-B due to its likely binding to the extra-stretch at the N-terminal (49) although this remains to be validated in larger studies including demonstrating consistency of the results across pathology laboratories.
Ligand-occupied PR binds to DNA and recruits pro-regulatory proteins (coactivators or corepressors) that interact with the transcription apparatus to modulate gene expression. When bound to ligand, PR controls the transcription of genes, which in the breast include genes for ER, insulin receptors, epidermal growth factor (EGF) and its receptors, and transforming growth factor (TGF)-alpha. Thus, progesterone is theorized to indirectly affect proliferation by modulating levels of growth factors and their cognate receptors (6). Progesterone may also modulate transcriptional activity via interaction with other transcription factors or by preventing access of transcriptional activators to DNA regulatory regions (squelching). Transcription of PR target genes can be affected by synthetic PR ligands through selective recruitment or blockade of regulatory cofactors. This action can result in differential regulation of gene expression in various progesterone target tissues (50). It has been shown in reproductive cancer cell lines that PR target genes, including CCND1, CDKN1A, DUSP1, EGFR, and PGR, are dependent on MAPK activity (51).
Progesterone Signaling via the PR in the Normal Breast
Role of PR-A and PR-B
As demonstrated in mouse models, estrogen and progesterone act sequentially on mammary epithelium to signal mammary gland development. With respect to hormone receptor signaling, estradiol and epithelial ERα signaling are necessary for ductal elongation during early puberty (52–54); further, it has been demonstrated that progesterone/PR are not necessary for this stage of early breast development (55). Increased estrogen levels induce PR expression; this is known as ‘estrogen priming’ and is common to most progesterone target tissues. PR signaling by progesterone is required in the epithelial compartment for side branching and alveologenesis (28, 55–57). Recent studies demonstrate different roles of PR-A and PR-B in estrogen signaling and ER chromatin binding (58–60). More specifically it has been demonstrated that PR-B is uniquely required for the latter stage of breast development (ie, side branching and alveologenesis) (35, 36). Thus, as the mouse reaches maturity, cyclic progesterone exposure results in ductal development and dichotomous branching that fills the mammary fat pad. During pregnancy, progesterone signaling via PR and prolactin signaling via the prolactin receptor (PrlR) in the epithelial compartment are required for branching and alveolar proliferation and differentiation (61, 62).
Paracrine mechanism
Specific progesterone effects that are distinct from estrogen and vice versa remain to be delineated; the co-expression and functional interdependence of these hormones poses challenges for distinguishing their respective functions. A paracrine mechanism is suggested to mediate estrogen and progesterone-induced proliferation of mammary epithelial cells. Studies supporting a paracrine mechanism for progesterone have demonstrated that approximately 40% of adult mouse mammary epithelial cells are PR+ and largely non-proliferative. These PR+ epithelial cells are located next to or near PR- cells that are proliferative (63, 64). Direct evidence derives from a study that demonstrated the return of proliferation in transplanted PR knockout mammary epithelial cells that were in close proximity to wild type PR+ mammary epithelial cells (28). The majority of PR+ epithelial cells are also ERα+ in the adult mouse mammary. It has been demonstrated in the mouse model and suggested in humans that PR upregulated target gene expression of ER and estradiol are required to maintain high expression of PR in mammary epithelium. Recently, it was also shown that PR-B dominantly activates more ER target gene expression than PR-A, whereas PR-A represses PR-B, ER, and other steroid receptors (reviewed in (41)). Further, excess expression of either PR-A or PR-B has been shown to disproportionately affect mammary gland development leading to increased lateral ductal branching or inappropriate lobulo-alveolar growth, respectively (34, 64).
Preclinical and clinical evidence support differentiation and proliferative roles of progesterone in the adult human breast through primarily paracrine actions between PR+ and PR- breast cells. Evidence from mouse models also suggests that expansion of the mammary stem cell population is driven by progesterone and PR signaling (20, 65). Consistent with this finding, research shows the expansion of stem cells, measured as an increase in the number of bipotent cells, after progesterone stimulation of normal human breast cells in matrix-embedded culture (66–68). However, it is not clear whether expression of PR isoforms differentially affects stem cell expansion in humans; in mouse models it is suggested that PR-A expression is restricted to luminal cells and PR-B expression is present in both luminal cells and a proportion of basal and mammary stem cells (20). Thus, the experimental data provide support that regulated expression of both PR-A and PR-B is critical for the mammary gland (and potentially human breast tissue) to respond appropriately to progesterone. This further highlights the need for accurate methods to quantify PR-A and PR-B expression in tissue that can be utilized in a clinical setting.
RANK pathway
The RANK pathway acts as the primary mediator of progesterone-driven proliferation in mammary epithelial tissues. Selective induction of high level RANKL expression in mammary epithelial tissues is associated with the high progesterone phase of the reproductive cycle and at this stage co-expression of PR is found in nearly 100% of RANKL+ mammary cells (69). RANKL was believed to work solely through RANK to provide developmental signaling that promotes mammary alveologenesis essential for lactation (70–72). Recently, leucine-rich repeat-containing G-protein coupled receptor 4 (LGR4), a receptor for R-spondins (RSPOs), has been shown to be a second RANKL receptor (73). LGR4 knockout mouse phenocopies RANK knockout, ie, mammary glands of LGR4 knockout mice have delayed ductal development, fewer terminal end buds, and decreased side-branching. It has been suggested that loss of LGR4 leads to decrease in Wnt signals important for alveologenesis (74). Both loss and overexpression of RANK in mice prevents development of pregnancy-induced milk-secreting structures, suggesting a possible dual role of RANK signaling in regulation of alveologenesis in pregnant mice (23, 70, 71, 75, 76). During pregnancy, RANKL expression is upregulated in mammary epithelial cells and is essential for development of the lobulo-alveolar mammary structures and the formation of a lactating mammary gland (35, 70, 75).
Hormones can regulate the expression of RANKL protein or mRNA in the human breast specifically; this regulation has been demonstrated for progesterone, estradiol, and prolactin (70, 77, 78). Increased RANKL mRNA expression and high serum progesterone levels are highly correlated in fine-needle aspirate samples from normal human breast; further RANKL expression is required for progesterone-induced proliferation in the breast (78). RANKL protein or mRNA expression in normal human breast tissue is higher in high progesterone conditions, ie, during pregnancy and during the luteal phase of menstrual cycle, as well as in women on combined hormone therapy. Expression of both RANKL mRNA and protein is induced by prolactin and parathyroid hormone protein-related peptide; RANKL expression is higher in luminal mammary cells of pregnant mice vs. virgin mice (19). However, estrogens, (either in the presence of a natural estrogen-rich environment (70) or via injection of estradiol and progesterone (20)) in conjunction with progesterone are required to induce RANKL mRNA and protein expression in mammary tissue (mouse). In normal human breast tissue, higher estradiol levels induced RANKL expression in the context of both high (luteal phase) and low (midcycle) progesterone levels (78), further supporting the complicated regulatory mechanisms of increased RANKL expression with respect to menstrual cycle hormonal fluctuations. Evaluating characteristics or patterns of altered RANKL expression may help clarify the divergent breast cancer risk across women with normal menstrual cycles. Osteoprotegerin is a soluble member of the TNF receptor superfamily that regulates osteoclastogenesis (79); it is secreted by osteoblasts and acts like a cytokine. Osteoprotegerin is a decoy receptor for RANKL, and has been shown to be produced by breast cancer tumors and to promote tumor growth and metastasis (reviewed in (80)). In vitro osteoprotegerin binds to TRAIL as a decoy receptor and prevents cell death. Higher circulating osteoprotegerin levels have been associated with increased risk of receptor negative breast cancer and a possible inverse relationship with receptor positive breast cancer (81), although limited to one study. It has been suggested that the inverse relationship with hormone receptor positive breast cancers is due to interference with RANKL signaling. This hypothesis is supported by in vitro data showing similar results in human breast tumors samples exposed to osteoprotegerin and RANKL (78). Further, lower circulating levels of osteoprotegerin has been observed in BRCA carriers in general, while a recent study reported an increased breast cancer risk with low osteoprotegerin levels in 206 BRCA carriers among whom 18 incident breast cancers were observed (82, 83).
Membrane-Bound Progesterone Receptors (“non-genomic” mechanisms of progesterone action)
The nuclear receptors described above (section IV.A.) are the key mediators of the biologic effects of progestogens. However, progesterone has also been shown to elicit its biologic effects via non-genomic mechanisms through activation of signal transduction pathways that are mediated by cell membrane associated PRs distinct from the classical PR (84, 85). The “non-classical” effects of progesterone can be elicited rapidly in various tissues, in contrast to the classical PR-mediated effects which require time to induce transcription and translation of genes into protein products. Among the rapid non-nuclear signaling pathways known to be activated by progesterone are the following: extracellular signal-regulated kinase (ERK) pathways, cyclic AMP/protein kinase A (PKA) pathway, cyclic GMP/protein kinase G (PKG) pathway, Ca++ influx/protein kinase C (PKC) activation pathway, and the phosphoinositide 3-kinase (PI3K)/Akt pathway (86).
Two types of distinct cell surface associated proteins unrelated to the classical PRs have been identified: membrane PRs (mPRs) and the progesterone receptor membrane component 1 (PGRMC1). The mPRs have a molecular mass of approximately 40 kDa and are comprised of different subtypes. In contrast to the mPRs, PGRMC1 is part of a multiprotein progesterone-binding complex that has multiple functions including activating cytochrome P450 enzymes that metabolize steroid hormones (87–89). PGRMC1 is expressed in breast tissue and several other tissues and is suggested to mediate the antiapoptotic effects of progesterone (88–92). PGRMC1 expression in blood cells does not vary across the menstrual cycle but was found to be downregulated in postmenopausal women, women with premature ovarian failure, and women with polycystic ovary syndrome (93).
PGRMC1 is overexpressed in breast cancer. It has also been reported that MPA, but not progesterone, increases cell viability and proliferation in the context of low estradiol levels, and that the proliferative effects are differential by PGRMC1 expression, with a more pronounced effect observed for PGRMC1 overexpressing cells compared with low PGRMC1 expressing cells (94, 95). However, it is unclear if this can entirely explain the absence of increased breast cancer risk with the menopausal hormone therapy formulations, estrogen-progesterone and estrogen-dydrogesterone (structurally similar to progesterone) compared to other menopausal estrogen-progestin formulations that are associated with transient increases in breast cancer risk among current and recent users (96).
Progesterone and Stem Cell Fate in the Breast
To understand the role of progesterone in the initiation and progression of breast cancer, it is prerequisite to understand how epithelial cells of the mammary gland control their fate. Mammary development starts from an embryonic mammary anlage that differentiates through coordinated stages to generate a diverse set of self-renewing, transitional, and terminal cell types (97, 98). By adulthood the normal adult female mammary gland has become a continuous, branching epithelial structure with a distinct outer basal/myoepithelial layer and an inner luminal epithelial layer. The ability to enzymatically dissociate these cells into a suspension of viable single cells and separate them by multicolor flow sorting into subpopulations has led to a major leap in our understanding of the biology of the mammary gland (99, 100).
In humans, the CD49f+/EpCAM-low basal cells contain all of the stem/bipotent/myoepithelial progenitor activities as well as differentiated myoepithelial cells (101). The CD49f+/EpCAM+ luminal progenitor cells contain luminal lineage-restricted progenitors at an approximately 30% purity with the other 70% of the cells in this fraction having undefined activities. The entire CD49f-/EpCAM+ luminal cell fraction is devoid of cells with clonogenic activity (102). A growing number of studies suggest that luminal progenitors are naturally at risk for acquiring cancer-predisposing mutation(s) due to the presence of a number of mechanisms that are contributors of genome instability, namely chronic oxidative stress (103), telomere dysfunctional state (102), and elevated R-loop structures (104).
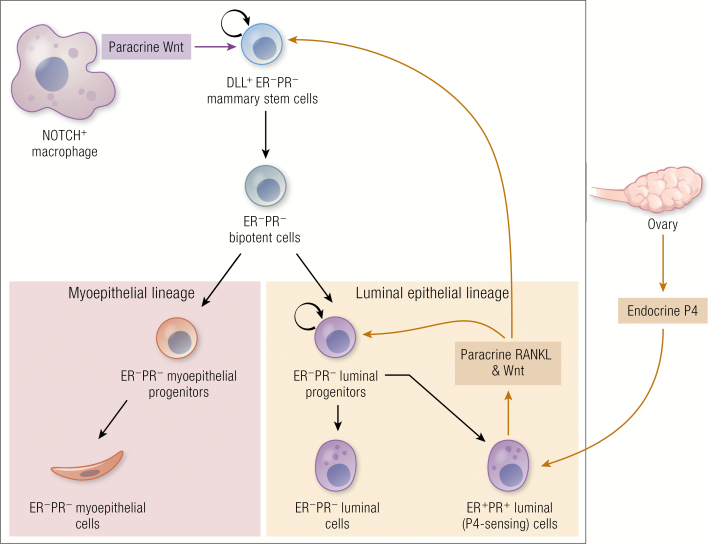
In humans, immunomagnetically purified in vitro transformed EpCAM+ luminal cells and CD10+ basal/myoepithelial cells were shown to generate ER+ and ER- subtypes and a metaplastic subtype, respectively, which resembled human breast cancers (105). With fluorescence-activated cell sorting (FACS) purified subsets of human breast epithelial cells, it was further shown that a single oncogene was sufficient to de novo transform basal cells and luminal progenitor subsets and not terminal luminal cells in highly immunodeficient mice providing new insight into the mechanism of breast cancer initiation (106). In mice, activation of the mutant PIK3CA gene in a cytokeratin-5 expressing basal/myoepithelial lineage generated only luminal ER+PR+ tumors, whereas its expression in cytokeratin-8 expressing luminal lineage cells generated both luminal ER+PR+ and basal-like ER-PR- tumors (107). Interestingly, neither stem cell-rich basal nor luminal progenitor-rich fractions express PR. The ER and PR are commonly found to be co-expressed within a developmentally mature subset of non-cycling cells in the luminal cell-fraction. According to the stem cell model, the ER+PR+ luminal cells act as a conduit for ovarian steroids to regulate more primitive mammary cells in the basal cells and luminal progenitor fraction and in turn control their own numbers (20). The ER+PR+ luminal cells, also referred to as P4 sensors, directly respond to progesterone and express mitogenic signals namely RANKL and Wnt, which activate RANK and Wnt pathways in more primitive mammary cells (19, 20, 108) (Fig. 3), while progesterone-mediated RANKL/Wnt signals are necessary but not sufficient to drive mammary development (109). A recent mouse study found that the NOTCH proteins in mammary-resident macrophages interact with their Delta-like ligand (DLL) expressed by mammary stem cells and produce several Wnt signals additionally required to reach the necessary threshold for mammary cell expansion during the menstrual cycle (109, 110). The highly complex multistep control of mammary cell expansion mediated by multiple cell types, using endocrine and paracrine signals, suggests that the risk mechanisms associated with breast cancers need to be investigated in humans to understand the full impact of progesterone signaling on cancer-susceptible primitive human breast cells. Owing to the recently acquired knowledge of the complexity of paracrine mechanisms through which progesterone controls mammary tissue turnover, the downstream gene targets of indirect effector NFκB specifically regulated by progesterone has not been resolved yet. Understanding these gene targets may be useful in identifying possible antibodies/treatments that could be tested in the context of either prevention or treatment/prognosis.
Figure 3.
Progesterone regulation of functionally-defined mammary epithelial cell hierarchy.
Recent studies examining the epithelial subpopulations in breast-cancer-susceptibility gene BRCA1 mutation carriers have found abnormally high numbers of functionally intact subsets of luminal progenitor cells with RANK expression and derailed progesterone regulation, and with high levels of persistent DNA damage. This abnormality, linked to breast cancer risk in BRCA1 carriers, is attributed to progesterone mediated RANKL expression by the terminal ER+PR+ luminal cell fraction (111, 112). Therefore, dismantling luminal cell/luminal progenitor communication via progesterone/RANKL with RANKL-neutralizing antibody denosumab (Genentech) is perceived as a strategy to control luminal progenitor numbers and possibly prevent breast cancer in BRCA1 mutation carriers (111, 113). An international trial to repurpose denosumab as a breast cancer precision prevention drug in a genetically pre-defined subset of high-risk patients (namely BRCA1/2) is currently underway. While this is a highly promising development, more caution is warranted since the knowledge of the extent of denosumab exposure and its response among the BRCA1/2 carriers with distinct mutations will determine the outcome of the first precision prevention trial for breast cancer.
Progesterone and Lobule Involution Among Women
Breast lobules are microscopic structures that represent the main source of breast cancer precursors and with aging these structures undergo simplification and obsolescence (33). Studies of women who have undergone benign breast biopsies demonstrate that reduced levels of TDLU involution are associated with increased breast cancer risk, independent of other breast cancer risk factors (32, 114, 115). Analyzing the association between serum hormone concentrations among premenopausal women and levels of TDLU involution of normal breast tissue donated for research demonstrated that higher progesterone levels in the luteal phase were associated with fewer lobules per unit area [odds ratio (OR) 0.80, 95% confidence interval (CI) 0.72–0.95; p<0.0001] (116). However, this analysis was based on 237 premenopausal women whose samples were evaluated with chemiluminescent immunometric assays, which may lack the sensitivity and specificity of newer LC-MS/MS assay methods (117). In the same study (116), analyses of postmenopausal women were limited by a smaller sample size (n=148) and low assay sensitivity (large number of samples below assay detection limit), which precluded quantitation.
Progesterone Signaling via the PR in Breast Cancer Development
Equal amounts of PR-A and PR-B are present in healthy adult breast tissue and the same is true in benign breast lesions, whereas this ratio is altered in breast cancer (reviewed in (118)). In the breast, it is suggested (and supported with mouse model data) that progesterone stimulates normal human breast epithelium through a paracrine mechanism (48, 118) and is a risk factor for breast cancer, because it promotes pre-neoplastic progression via stimulation of cyclical proliferation of mammary stem cell pools or occult tumor initiating cells in the mature breast epithelium (118). It is further suggested that cancer progression is a result of progesterone/PR signaling and a switch from paracrine to autocrine regulation of proliferation (57, 118, 119). In breast cancer, the proliferative effect of progesterone is mediated primarily by PR-B. Extranuclear signaling actions of PR are also mediated predominantly by PR-B (120). It is unclear if extranuclear signaling actions of PR occur in normal breast tissue, or if this represents a mechanism by which progesterone and PR in breast cancers overtake signaling pathways normally used by growth factors or other cell surface receptors (118). In contrast, PR-A does not efficiently mediate rapid activation of the protein kinase signaling pathway, making it ineffective at extranuclear signaling (120–122). In human breast cancer cell lines, PR-B was found to regulate gene expression of more genes than PR-A, with modest overlap in regulated genes by both receptors (123), further supporting the important role of PR-B in breast carcinogenesis. In breast cancer cell lines, PR-A is needed for appropriate responsiveness to progesterone, whereas PR-B-induced target gene expression has been shown to enhance the carcinogenicity of tamoxifen (124). Further, it has been suggested that antiprogestins may be more effective for tumors expressing PR-A (125).
Recent studies suggest that the PR’s role in breast cancer is more than just as a marker of ER activity (126, 127). Specifically, studies suggest PR acts as a binding partner as well as a modifier of ER activity that targets gene selection; however, findings on this topic have been inconsistent. Mohammed and colleagues (126) suggest that studies evaluating progesterone in addition to an antiestrogen may be informative, while Singhal and colleagues (127) supported the use of a selective PR modulator/antagonist to co-target both ER and PR in receptor positive breast cancers as a means for enhancing treatment, as compared with individual therapies. Even more complex interactions were suggested in a study from the Lange laboratory that supported not only targeting ER/PR in hormone receptor positive cancers, but also the insulin-like growth factor 1 receptor (IGF1R) (128). As such, progesterone and PR are not the only receptors/ligand combination that likely play a role in breast cancer. Steroid receptor crosstalk, including an overview of ER/PR crosstalk in the context of primarily breast cancer, was recently reviewed by Truong and Lange (129).
DNA Repair Enzyme Mutations and Hormonally-Related Breast Cancers
Pathogenic mutations in DNA repair genes such as BRCA1, BRCA2, CHEK2, PALB2, ATM, P53 and others are associated with elevated risk for breast cancers. While some gene mutations are linked to triple negative breast cancers (eg, BRCA1) others are linked to ER+ breast cancer (eg, CHEK2). The developmental and mechanistic origin of these tumors are poorly described in the literature. A recent study that investigated BRCA1 mutation carriers concluded that BRCA1 mutation could drive aberrant luminal progenitor expansion independent of progesterone (130). A more recent study from the same group has shown that BRCA1 mutation carriers have deregulated progesterone signaling leading to higher proliferation and DNA damage in a progesterone-sensitive RANK+ luminal progenitor subset (111). An independent study of BRCA1 mutation carriers observed that luminal progenitors displayed an aberrant differentiation program but failed to find expanded luminal progenitors in their samples (131). Furthermore, data from a mouse model provided evidence to suggest that aberrant expansion of BRCA1 null luminal progenitor is linked to the replication-stress associated DNA damage response, where proliferation of mammary progenitors is perpetuated by damage-induced, autologous NF-κB signaling (112). There are other functions of BRCA1 relevant to genomic stability that are compromised in patients carrying a deleterious mutant allele of this gene. Recent studies show that haploinsufficiency of BRCA1 leads to accumulation of R-loop, a DNA-RNA hybrid structure associated with transcriptional regulation and genomic instability in luminal progenitors (104). Chromosomal aberrations have been associated with mammary progenitors in BRCA1 insufficiency as well as experimental models of human mammary progenitors mimicking loss of BRCA1 function (132, 133). Together, human and murine studies provide credence to the notion that the origin of BRCA1-mutation associated triple negative breast cancers is in the ER- luminal progenitors. Such detailed examination of cancer risk mechanisms at the level of cellular differentiation is simply not available for other DNA repair genes.
There is some evidence to support higher circulating levels of luteal phase estrogen and progesterone in BRCA carriers compared with noncarriers (134). Specifically, it has been suggested that the increased hormone levels observed in carriers are due to a defect in ovarian hormone biosynthesis that leads to an increased risk of breast and ovarian cancer (134). However, the link between premenopausal luteal phase circulating hormones and ovarian cancer risk is unclear. Additional research is required to determine whether hormones influence the potential mutagenic effects of BRCA mutations (or possibly other DNA repair enzyme mutations) and thus provide data to support organ specific penetrance.
Progesterone and Breast Carcinogenesis
Progestins Versus Progesterone
Breast cancers arise from the epithelial cells of the breast. It is now well accepted that pharmacologic and physiologic concentrations of estradiol increase the mitogenic activity of epithelial cells, while the influence of progesterone continues to be debated. Inconsistent evidence suggests that progesterone can increase, decrease, or have no effect on mitotic activity and proliferation in breast epithelial cells (135–138). In contrast, a relative consensus has been reached that long-duration exposure to pharmacologic progestogen levels combined with estrogen, either through use of contraceptives (transient, albeit small elevation in risk with current use) or menopausal hormone therapy (primarily with estrogen+progestin formulations, but not estrogen+progesterone or dydrogesterone), increases breast cancer risk (139–143). In addition to possible progestational effects, the progestins used in menopausal hormone therapy and oral contraception can have antiandrogenic, proandrogenic, glucocorticoid, and antimineralocorticoid effects (14, 16) (summarized in Table 1). Thus, the potential effects of endogenous levels of progesterone in breast cancer cannot be inferred from studies of exogenous hormones, and epidemiologic studies evaluating circulating levels of progesterone are limited.
Progesterone and Proliferation
In vitro studies suggest that progesterone decreases mitotic activity of normal human breast epithelium and partially inhibits estrogen-induced proliferation in human cancer cell lines (135, 144). These data are limited and do not appear to be consistent with the in vivo effects of progesterone on mammary cells (145). Recent discoveries suggest that functionally distinct epithelial and non-epithelial mammary cells are involved in mediating progesterone/RANKL-induced paracrine signaling to promote expansion and differentiation of mammary stem and progenitor cells, and these are not adequately captured in the in vitro studies. It may therefore be challenging to reproduce the mammary-specific effects of progesterone in vitro, especially in 2-dimensional cell culture systems. The 3-dimensional primary tissue-organoid based short-term culture system has been shown to capture some proliferative effects of progesterone on mammary tissue ex vivo but more studies are required (111).
Maximum breast epithelial proliferation measured in surgical biopsies has been reported for early follicular (136), early luteal (137), and late luteal phases (138, 146), with only the latter suggesting potential mitogenic activity for progesterone. A new model has been proposed to explain the delay in response. According to this model, mammary stem cell numbers oscillate during the menstrual cycle, in response to oscillations of serum progesterone and macrophage numbers in the breast (109). Further investigation can shed light on the link between progesterone and macrophage coordination to increase local Wnt concentration in order to increase mammary stem cell expansion.
Contrarily, it has also been suggested that increased progesterone tissue concentrations are correlated with decreased mitotic activity in normal breast epithelium in women (145). Specifically, treatment with hydro-alcoholic gel provided very limited changes in plasma concentrations of estradiol and progesterone but produced markedly different levels of steroid accumulation in the breast tissue that was consistent with the treatment (eg, higher concentrations of progesterone in the progesterone and estradiol+progesterone groups, and higher concentrations of estradiol in the estradiol and estradiol+progesterone treated groups). Mitotic activity was highest in the estradiol-only groups, and lower in the progesterone-only and estradiol+progesterone group, providing evidence that adding 10–13 days of progesterone to estradiol reduced the proliferative effect of estradiol alone (145). However, it is unclear whether the change in progesterone concentrations reflect pharmacologic or physiologic levels during a relevant period of the menstrual cycle (147). One study showed that breast cell proliferation is higher in the luteal than follicular phase (characterized by higher serum progesterone relative to estradiol levels) (137). At physiologic levels, it is unclear whether this enhanced proliferation is due to progesterone exerting a mitotic effect or a weak antimitotic effect that is insufficient to block the mitotic effect of increased estradiol (147). As such, the association between progesterone and malignant transformation of breast epithelial cells may be dependent on the levels of estradiol, or other sex steroid hormones.
Hormones exert important effects on proliferation of normal breast epithelium, and therefore, progesterone is a plausible inducer of proliferation in the early phases of breast cancer development. In one model (148), risk of breast cancer was postulated to be determined by cumulative exposure of breast tissue to estrogen, but this same hypothesis could be relevant for the combined effects of estrogen and progesterone exposure over the menstrual cycle, and thus over a woman’s reproductive years. Indirect evidence supporting this model included increased risk of breast cancer with events that increase estrogen exposure, but also plausibly increase progesterone exposure (possibly relative to estrogen) over the menstrual life course, including early age at menarche, shorter menstrual cycle length, late first full-term pregnancy, and late menopause. Inverse associations have also been demonstrated for factors related to potentially reduced exposure to estrogen and/or progesterone including oophorectomy and early menopause. Cumulative effects of these exposures seem to be associated with more accurate risk assessment than individual factors. Improved accuracy could also be due to more precise characterization of total exposure to progesterone that reflects exposure of TDLU epithelium that has not undergone differentiation induced by pregnancy and lactation, with resulting post-lactational involution (and “reset” or turnover of damaged cells). The model described above by Pike and colleagues (148) treated breast cancer as a composite outcome. Given advances in the understanding of etiologic heterogeneity in age-specific incidence and risk factor associations for breast cancer subtypes, it is realistic that this model should be refined by major subtype and/or hormone receptor status (149). Further, the cell of origin of the breast cancer subtype (eg, basal or luminal) may be differentially sensitive to progesterone. For example, if the cell of origin of basal breast cancer is more stem-like and sensitive to progesterone, this could explain the epidemiologic risk factor of high parity (higher lifetime progesterone exposure) for basal-like tumors. In contrast, the cell of origin for luminal cancers is likely less sensitive to progesterone, and epidemiologic risk factors like parity are associated with reduced risk.
Factors Contributing to Variations in Progesterone
Individual variations in progesterone exposure may also be attributed to other factors, and specifically this could help explain the paradoxical association of obesity (ie, high body mass index (BMI)) with pre- and postmenopausal breast cancer risk. Obese BMI is inversely associated with premenopausal breast cancer risk overall, and positively associated with postmenopausal breast cancer risk (150). However, the association among premenopausal women is heterogeneous by hormone receptor status (151, 152). Premenopausal obesity is associated with increased risk of ER-, PR-, and triple negative breast cancers (ER-/PR-/HER2-) and decreased risk of hormone receptor positive tumors (151, 152). However, the increased breast cancer risk with obesity in postmenopausal women was not heterogeneous across hormone receptor status (151, 152). The association in postmenopausal women is likely a result of increased estradiol due to higher aromatase activity related to increased adipose tissue in overweight/obese women. However, the reduced risk of hormone receptor positive tumors in premenopausal women is more likely explained, in part, by reduced progesterone exposure due to dampening of progesterone peaks in premenopausal women with high BMI that can result in longer cycle length and increased frequency of anovulatory cycles, thereby reducing breast epithelial cell proliferation (153). Alternatively, or complementary, to the contribution of increased circulating estrogen concentrations with increased adiposity, insulin resistance could also be a mechanism by which obesity is associated with increased breast cancer risk. Insulin is a modest growth factor that promotes cell growth, division, and migration, and inhibits apoptosis. While insulin is a weaker mitogen than other prominent growth factors (eg, insulin-like growth factors, platelet-derived growth factor, vascular endothelial growth factors, etc.) its specific mitogenic action potentiates cellular responsiveness to other growth factors, in a sense amplifying its mitogenic capabilities (reviewed in (154)).
Plausible Mechanisms
In terms of potential mechanisms for progesterone’s ability to cause breast cancer, we have emphasized data throughout the review supporting that both estrogen and progesterone are required to elicit substantial cellular proliferation. Research supporting genotoxic effects of progesterone is very limited. Studies evaluating blood DNA damage via the comet assay, dominant lethal assays in mice, chromosomal aberrations in rats, and DNA double strand breaks in mice and rats were all null (155–157). Individual studies have demonstrated that treatment with progesterone induces an increase in chromosomal aberrations in embryonic human fibroblasts or aneuploidy by a non-disjunctional mechanism (158, 159). No direct DNA damage (via measurement of DNA adducts) was demonstrated in the liver after treatment with progesterone (160, 161). Global gene expression in human breast tissues (non-cancer) donated for research from 20 premenopausal women revealed that 255 genes were differentially expressed in a study comparing tissues collected during the follicular and luteal phase (162). Of the 255 genes, 221 were increased in the luteal phase with functions related to cell cycle, mitosis, and DNA damage and repair; 3 paracrine factors were also identified, including RANKL, WNT4, and epiregulin (162). Currently, there is no direct evidence to suggest that progesterone has the capability to initiate tumors via inducing enzymes and proteins involved in nucleic acid synthesis or through activation of oncogenes. The expansion of a cancer-susceptible luminal progenitor population is the proposed indirect developmental effect of progesterone that may contribute to tumor initiation. The discovery of widespread telomere dysfunction in ostensibly normal luminal progenitors in the cancer-free breast (102) and widespread telomere fusion observed in early breast cancer lesions (163) have provided strong support for the specific cell-of-origin theory for breast cancers.
Other Hormones and Breast Carcinogenesis
Elevated circulating androgen levels are consistently associated with increased breast cancer risk, but the underlying mechanism of action is unclear (164). Androgens may increase risk indirectly through aromatization to estrogens, whose role in breast cancer etiology is well established (165), or decrease risk by exerting antiestrogenic and antiproliferative effects via androgen receptor (AR) signaling (164, 166). Androgenic activity via the AR is responsible for healthy functioning of many organs in women (166) including the breast, where estrogens stimulate while androgens inhibit development, and the balance between these regulates breast development. In breast tumors, AR expression has been detected in up to 85% of cases (164), although this varies by breast cancer subtype; while almost all ER+ cancers express AR, only 10–35% of triple negative (ER-/PR-/HER2-) breast cancers express AR (167).
Elevated levels of circulating postmenopausal androgens (androstenedione and testosterone) and androgen precursors (dehydroepiandrosterone (DHEA) and its sulfated form (DHEAS)) increase breast cancer risk, with women in the top quartile of these hormones at 2–3 fold increased risk versus women in the lowest quartile (165, 168). Because these androgens are the obligate precursors for estrogens, it is not known whether their observed effect is independent of the estrogen association. In addition, epidemiologic studies have not examined the functional role of androgen metabolites synthesized within the breast. Indeed, the normal breast contains the steroidogenic enzymes needed to convert circulating precursors to biologically active forms (169), with the derived 5α-reduced androgens, particularly dihydrotestosterone (DHT), being the most potent. In women, however, levels of circulating DHT are often below assay detection, and do not reflect peripheral 5α-reductase activity (170). Rather, androsterone glucuronide (ADT-G), a distal metabolite of DHT, together with androstanediol glucuronide (found as 2 isomers: 5α-androstane-3α,17β diol-3-glucuronide (3α-diol-3G) and 5α-androstane-3α,17β diol-17-glucuronide (3α-diol-17G)), have been shown to reflect total tissue-level androgenic activity better than the proandrogens (eg, testosterone, androstenedione, etc.) (170, 171). Additional research is necessary to understand the interplay between progesterone, estrogen, and androgens in breast cancer risk with careful attention to potential interactive effects of the hormones and possible differences in associations by breast cancer subtypes.
Unlike female breast cancer, breast cancer in males is exceptionally rare (less than 1% of female breast cancer risk) and does not plateau after age 50. The slowed increase in incidence in women at the menopausal transition likely reflects in part the substantial decrease in circulating levels of a number of hormones including estrogen and progesterone. Further support for the importance of estrogen in both male and female breast cancer etiology is lent by the association with anthropometry, reproductive factors, and circulating hormones (172, 173). Obesity increases postmenopausal breast cancer risk and male breast cancer risk by similar magnitudes, likely as a result of higher levels of bioavailable estrogen via peripheral conversion of androgens to estrogens (172). Increased risk for male breast cancer with higher circulating levels of estradiol and a null association with ADT-G (173) suggests that the substantially lower rate of these cancers among men is likely due in part to much lower lifetime estrogen exposure than among women. Progesterone levels across the life course are also substantially lower in men than women, but they have not been evaluated in the context of male breast cancer risk to date.
Prolactin is another hormone (described above) that interacts with progesterone and their respective receptors to influence ductal and luminal epithelial cell proliferation during pregnancy (reviewed in (174)). High circulating prolactin is related to breast cancer risk factors, such as nulliparity and high mammographic density. Further, higher levels of circulating prolactin are associated with increased risk of predominantly receptor positive pre- and post-menopausal breast cancer risk (175). It has been suggested that both progesterone and prolactin may influence the epithelial cell hierarchy via RANKL feedback through mammary stem cells and luminal progenitors; however, more research is needed to understand the shared signaling pathways and whether they could serve as potential therapeutic targets (174).
Breast Cancer Risks Associated With Endogenous and Exogenous Progesterone/Progestin Exposure
Endogenous Progesterone Exposure
Few population-based studies have assessed circulating progesterone levels and breast cancer risk. One study of postmenopausal women including 322 breast cancer cases and 643 matched controls (176) showed no association between prediagnostic circulating progesterone levels and breast cancer risk. However, the study was limited because almost 30% of samples had undetectable levels using an RIA assay, with preceding organic solvent and Celite column chromatography steps, and a limit of detection of 3 ng/dL. Thus, the influence of endogenous progesterone on breast cancer risk among postmenopausal women has been infrequently studied primarily due to low levels of circulating progesterone, the large sample volume required for assays, and inadequate sensitivity of available assays.
Studies of circulating progesterone in premenopausal women and subsequent breast cancer risk include 6 published studies, with the majority measuring progesterone in luteal phase blood samples (177–182). Of these studies, 4 included a limited number of cases (n≤104) and generally reported null results (177, 178, 182) or a modest reduction in breast cancer risk with higher levels of circulating progesterone (179). The 2 largest studies evaluating progesterone concentrations in premenopausal women utilized either direct chemiluminescent immunoassays on automated platforms (180) or direct RIA using a commercial kit (181). Among 501 breast cancer cases and 1030 matched controls from the Nurses’ Health Study II, luteal phase progesterone was not associated with breast cancer risk (180). There was no difference in the association by menopausal status at cancer diagnosis (180). No association with progesterone among 801 premenopausal breast cancer cases and 1132 matched controls, irrespective of timing of the blood draw in the menstrual cycle, was seen in the European Prospective Investigation into Cancer and Nutrition (EPIC) (181). Analyses limited to progesterone levels from 232 cases and 323 controls measured during the luteal phase of the menstrual cycle also suggested no association (181).
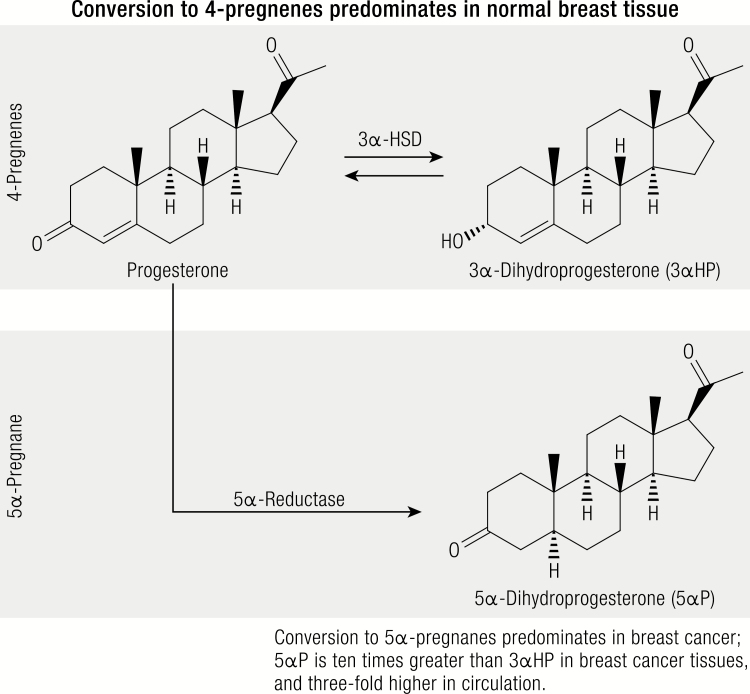
Circulating progesterone levels are significantly lower among postmenopausal women as compared to premenopausal women, posing a challenge for immunoassay techniques (117). Furthermore, available commercial kits measure only progesterone and do not measure relevant major metabolites which may also play a role in the carcinogenic process. A number of progesterone metabolites have been characterized in normal breast and cancerous breast cancer tissue and broadly fall into 2 groups, 1) 4-pregnenes: metabolites that retain their double bond, and 2) 5α-pregnanes: metabolites in which 5α-reductase has reduced the double bond (3). Different relative distributions in the 4-pregnenes and 5α-pregnanes have been demonstrated in normal and cancerous breast tissue (Fig. 4). In normal breast tissue the conversion of progesterone to pregnene metabolites exceeds or predominates the conversion of progesterone to 5α-pregnane metabolites, whereas the opposite is true in breast cancer tissue. Thus, it has been hypothesized, and laboratory studies have confirmed, that the promotion of breast cancer may be related to relative changes in concentrations of cancer-inhibiting 4-pregnenes (eg, 3α-dihydroprogesterone (3αHP)) and cancer-promoting 5α-pregnanes (eg, 5α-dihydroprogesterone (5αP)) (183). Further, 5αP and 3αHP have been shown to act with equal efficacy on all breast cell lines tested, regardless of their receptor status, estrogen sensitivity, and tumorigenicity (184). While the ovary is the primary source of progesterone in premenopausal women, smaller amounts are produced in the adrenals and adipose tissue, and local production of 3αHP and 5αP metabolites may occur in the breast (183). However, to date, no epidemiologic studies have evaluated the role of circulating progesterone metabolites and breast cancer risk in premenopausal or postmenopausal women.
Figure 4.
Primary pathways of progesterone metabolism in breast tissue.
Legend: In normal tissue, pregnenes (progesterone is a pregnene) are the predominant compounds. All of the 4 pregnenes (not shown) can be irreversibly converted to 5α-pregnane (respectively) via 5α-reductase. The 2 metabolites, 3αHP and 5αP, show the greatest differences between tumorous and nontumorous tissue; the ratio of 5αP-to-3αHP is more than 10-fold higher comparing breast tumors to normal breast tissue. Metabolic activities of the hormones are similar in pre- and postmenopausal women, by age, and by ER-status.
Exogenous Progesterone and Progestin Exposure
Information about the risk of breast cancer associated with isolated progesterone or progestin use is scant. Although oral and parenteral progestin-only formulations are used for contraception by premenopausal women, clinical breast cancer data on the progestin-only effect is limited because the reproductive age group of contraceptive users has a low baseline risk of breast cancer.
Oral contraceptives are widely used but the majority contain an estrogen combined with a progestin (combined oral contraceptives (COC)). Progestin-only pills (POP) are also available. Their primary use is for contraception in women in which estrogen is contraindicated. COCs act by inhibiting ovulation, whereas POPs primarily prevent conception by developing thick, hostile cervical mucus and atrophy of the endometrium. In postmenopausal women, progestogens are widely used in combination with an estrogen to protect the uterus. The result of adding a progestogen to estrogen in COCs or menopausal hormone therapy creates a complex and confusing situation because there is no consensus on the background effect of the estrogen itself and the effect of its dose, delivery system, and treatment time. Additionally, in premenopausal women the addition of a progestogen may prevent ovulation, thus both the direct effect of the progestogen on the breast and indirect effects associated with anovulation are inseparable.
Sparse data on progestin-only contraceptive usage and breast cancer risk
To date, a limited number of studies have been published on POPs and breast cancer risk (reviewed in (185–187)). The most recent, and largest, included a prospective population-based cohort in Denmark with contraceptive information on 1.8 million premenopausal women, of which 11,517 developed breast cancer during an average of 11 years of follow-up (187). Current or recent use of the levonorgestrel POP was associated with increased breast cancer risk compared to never use of hormonal contraception [levonorgestrel POP RR (95% CI): 1.93 (1.18–3.16)]. However, the number of women exposed to levonorgestrel POP in this analysis was relatively small. Other formulations of POPs (norethindrone or desogestrel) with larger proportions of users were not associated with breast cancer risk (187). In this same study, the levonorgestrel-releasing intrauterine system was also associated with increased breast cancer risk: 1.21 (1.11–1.33), whereas progestin-only implants and injections (DMPA) were not associated with risk (187). A recent review of the literature relating contraceptives to breast cancer risk estimated that progestin exposure at 6 months of levonorgestrel-releasing intrauterine system use would be roughly half of the progestin exposure with estrogen+progestin menopausal hormone therapy (ie, 2.5 mg of MPA), which is similarly associated with increased breast cancer risk and is thus a plausible association (188). They also observed that the current levonorgestrel-releasing intrauterine system use in the Danish cohort study did not take into account prior contraceptive use with its associated known increases in risk, which could have contributed to the increased breast cancer incidence (188). Further the Danish cohort study had too little exposure to DMPA, contraceptive implants, and most oral POPs to report precise risk estimates for those progestin-only methods, which are more widely used in the United States (188). A comparable increased breast cancer risk [Standardized Incidence Ratio (95% CI): 1.19 (1.13–1.25)] with the levonorgestrel-releasing intrauterine system was observed in a cancer registry linkage analysis using data from the prescription National Reimbursement Registry among 93,843 Finnish women; and in an updated analyses reported that the increased risk was apparent for both ductal [SIR (95% CI): 1.20 (1.14–1.25)] and lobular breast cancer [SIR (95% CI): 1.33 (1.20–1.46)] (189, 190). For the latter study, the same limitation of not being able to account for prior COC use is relevant since the prescription reimbursement registry does not capture lifetime exposure history, and thus the associations with current levonorgestrel-releasing intrauterine system use could be confounded by prior COC use.
In a large, US population-based case-control study in which POP use accounted for 0.5 percent of total oral contraceptive use, POP use was not associated with breast cancer [OR 0.9 (95% CI not reported)] (191). Progestin-only injections or implants were also not associated with breast cancer risk in the same study population (192). The Norwegian and Swedish Women’s Lifestyle and Health cohort study included 1008 primary breast cancers diagnosed among 103,027 women. Current use of POPs (which could have included former use of COCs) was associated with increased breast cancer risk [RR (95% CI): 1.6 (1.0–2.4)], while exclusive use of POPs (regardless of duration of use) was not associated with breast cancer risk [RR (95% CI): 1.1 (0.8–1.7) 29 cases out of 3435 vs. never, 261 cases out of 28,171] (193). In a study from the large French prospective cohort study (Etude Epidémiologique de Femmes de la Mutual Générale de l’Education Nationale, referred to as the E3N study or cohort), ever use of POPs after the age of 40 was not associated with breast cancer risk. Some elevations were reported in subgroup analyses, suggesting that prolonged use of oral progestin (more than 4.5 years of continuous use) was associated with increased breast cancer risk among older women (194). Earlier studies, specifically 8 case-control studies conducted between 1986 and 1996, reviewed in an IARC monograph, reported no risk of breast cancer among women using progestin-only contraceptives (pill or injectable DMPA) compared to non-users (186).
Combined oral contraceptives and breast cancer risk
COCs have been associated with a small magnitude increase in breast cancer risk. The Collaborative Group on Hormonal Factors in Breast Cancer was set up in 1992 to bring together and reanalyze data from 54 studies in 25 countries to assess the relationship between the risk of breast cancer and use of COCs (195). The study involved data from a total of 53,297 women with breast cancer and 100,239 women without breast cancer. The results provide strong evidence for the following conclusions. First, there is a small increase in the relative risk (RR) of having breast cancer diagnosed in current users (RR: 1.24) and in the 10 years following termination of COC use (RR: 1.16 after 1–4 years and RR: 1.07 after 5–9 years). Second, there is no significant excess risk of having breast cancer diagnosed 10 or more years after stopping use. In addition, the data show that the cancers diagnosed in women who had used COCs were less advanced clinically than in never-users. Increased risk among women currently taking COCs and diminishing risks after cessation of use, suggest a promotional, rather than an initiating, effect of progestin in these medications on breast cancer risk.
Menopausal hormone therapy and breast cancer risk
The relationship between risk of breast cancer and use of menopausal hormone therapy has been investigated in many epidemiologic studies. A 1997 review by the Collaborative Group on Hormonal Factors in Breast Cancer brought together and reanalyzed data from 51 epidemiologic studies that represented about 90% of the worldwide evidence on this topic (140). The main analyses of the relation between risk of breast cancer and use of hormone therapy included 53,865 postmenopausal women (17,949 cases and 35,916 controls) with known age at menopause and use of menopausal hormone therapies. The results show that the increase in the relative risk of breast cancer associated with each year of use in current and recent users is small. Therefore, inevitably some studies show significant associations and others do not. However, combination of the results across many studies has the advantage of reducing such fluctuations. The increased risk of breast cancer was not significant until 5 years of menopausal hormone therapy use. For women who used menopausal hormones for 5 years or longer, the relative risk was 1.35 (95% CI: 1.21–1.49). The risk was reduced after discontinuing menopausal hormone therapy use and largely, if not completely, disappeared after about 5 years. Information about the hormonal constituents of the menopausal hormone therapy preparations used the most was available for only 4,640 (39%) of eligible women. The women in the main analysis had their breast cancers diagnosed on average in 1985, when the type of hormone used was predominantly estrogen alone; only 12% of the women used estrogen and progestogen combinations. There was no significant variation in the relative risk of breast cancer according to the formulation or dose of the estrogen used by most women, and no evidence of marked differences between menopausal hormone preparations containing estrogen alone and those containing both estrogen and progestogen.
In a later review (196), data from 8 randomized controlled trials and 19 epidemiologic studies were used to determine whether there is an association between menopausal hormone therapy use and breast cancer. The review also addressed a number of other questions, including whether data exist to show a differential effect from use of menopausal hormone therapy products, doses, regimens, and routes of administration. The results show that the average risk of breast cancer with use of estrogen alone was 0.79 (95% CI: 0.61–1.02) in 4 randomized trials involving 12,643 women, whereas the risk with estrogen-progestin use was 1.24 (95% CI: 1.03–1.50) in 4 randomized trials involving 19,756 women. In contrast, the average relative risks reported in the epidemiologic studies were higher in current users: 1.18 (95% CI: 1.01–1.38) for estrogen alone and 1.70 (95% CI: 1.36–2.17) for estrogen-progestin; however, the risks were not as strong in ever users: 1.08 (95% CI: 0.97–1.20) and 1.31 (95% CI: 1.12–1.53), respectively (196).
For the analyses of the association of breast cancer risk by hormone formulation, dose, regimen and route of administration, the number of studies used in each group varies. For progestogen types, only 2 studies included risks with current exposure (139, 197). The analyses compared a C-21 related progestogen, namely MPA, with 2 C-19 related progestogens, norethindrone and levonorgestrel. Breast cancer risks with C-21 and C-19 related progestogens were virtually the same (2.14, 95% CI: 1.18–3.87 and 2.14, 95% CI: 1.68–2.72, respectively) (196). Only one study reported information on progestogen dose; the Women’s Health Study evaluated MPA dosages over the ranges of <5 to 10 mg/day; the trend from lowest to highest dose was not significant (198).
Although the 2 major reviews of data reanalysis just described include a substantial number of studies (51 epidemiologic studies in one review, and 8 randomized controlled studies and 19 epidemiologic studies in the other review), none of these studies include progesterone data. However, this changed with the publication of results from the E3N study to investigate risk factors for cancer in women, including exogenous hormones (142, 199). Although oral progestins were used most widely in the E3N study, there was also substantial use of exogenous progesterone. The progestins included dydrogesterone, medrogestone, chlormadinone acetate, cyproterone acetate, promegestone, nomegestrol acetate, norethindrone acetate, and MPA.
In an updated report on the E3N cohort evaluating 80,377 postmenopausal women 40–65 years of age at enrollment and followed for up to 12 years, use of estrogen alone was associated with a 29% increased breast cancer risk (95% CI: 1.02–1.65) (199). This increased breast cancer risk with use of estrogen alone (almost exclusively estradiol compounds and mostly administered transdermally) differs from that of the WHI estrogen-alone trial which found a decreased risk with oral conjugated equine estrogens (200).
The association of estrogen-progestogen combinations with breast cancer risk varied significantly according to the type of progestogen (199). The RR was 1.00 (95% CI: 0.83–1.22) for estrogen-progesterone, 1.16 (95% CI: 0.94–1.43) for estrogen-dydrogesterone, and 1.69 (95% CI: 1.50–1.91) for estrogen combined with other progestogens, which included medrogestone, chlormadinone acetate, promegestone, nomegestrol acetate norethindrone acetate, and MPA; the increased breast cancer risk was consistent across these latter estrogen-progestogen formulations. Estrogen alone, estrogen-progesterone and estrogen-dydrogesterone did not differ significantly from one another in breast cancer risk, but the risks were all significantly lower than that of estrogen combined with the other progestogens. It should be noted that the chemical structure of dydrogesterone differs from that of progesterone only in that the methyl group at the carbon 10 is in the alpha-orientation and there is a double bond between carbons 6 and 7. The E3N study was the first epidemiologic study to show that estrogen-progesterone and estrogen-dydrogesterone combinations may be the least harmful menopausal hormone therapies with respect to postmenopausal breast cancer risk. However, more evidence is required to make firm clinical recommendations for use of these formulations, compared to other formulations, in managing menopausal symptoms.
Challenges, Future Directions, Unanswered Questions
There are many gaps in our current knowledge of the cellular hierarchy model of mammary gland development and regulation of its cellular content and glandular function throughout life. Though it is assumed that the progesterone-sensor luminal cells constitute a developmentally terminal ‘post-mitotic’ state, the following observations raise doubts that cannot be ignored: 1) Lack of sufficient evidence in the literature that functionally and molecularly-defined ER+PR+ luminal cells are direct products of ER-PR- luminal progenitor cells; 2) In humans, the telomere length of the terminal luminal cell-fraction is longer than luminal progenitor cells in younger women and gradually declines with age (102); 3) A study looking at the synthetic nucleoside labeling cycling cells within defined mammary subsets in a mouse model has argued that luminal progenitor and luminal cells could represent 2 distinctly self-sustained lineages with shared luminal features (201); 4) It appears that the post-mitotic ER+ luminal cells which generally co-express PR can be induced to grow and expand in vitro (202). If ER+PR+ are indeed self-sustaining luminal cell lineages, then our understanding of how progesterone controls mammary cells may have to be revisited. Hence, the current mammary stem cell model requires further scrutiny. There is much to be learned about PR and its ligands with respect to downstream signaling and genomic and cellular targets. Better antibodies are needed to detect PR isoforms and cell systems to capture paracrine signaling events. Advances in these areas will provide a broad spectrum of exciting new discoveries that will improve not only the biologic understanding of progesterone in breast cancer, but also the clinical relevance.
There are many unanswered questions pertaining to progestogens and breast cancer risk. Epidemiologic evaluation of the role of endogenous progesterone, including newly identified progesterone metabolites, in the etiology of premenopausal or postmenopausal breast cancer risk is limited. The latter is primarily due to limited assay sensitivity while the former is due to limited annotation of the menstrual phase of blood draw or urine collection in prospective epidemiologic studies that include breast cancer outcomes. Studies have either included samples collected from women across the menstrual cycle or limited to luteal phase samples. It is not clear if this is the correct approach to determining risk, whether the maximum level from the luteal cycle is the appropriate measure, or whether the usual level during the early follicular phase could be more relevant to risk. Further, it could be the change in progesterone levels from follicular to luteal, or the concentration relative to estradiol that is most relevant to risk. Finally, cumulative exposure over a lifetime or levels during the final years of the menopausal transition could be important. At present, it is not clear what the appropriate comparison should be, and typical sample collections are limited to only one time point during the menstrual cycle, which would preclude the ability to evaluate the change in progesterone level over a menstrual cycle. Given recent advances in assay technology (LC-MS/MS assays can detect circulating progesterone concentrations ~0.1 ng/dL (117)), clarification of an association between endogenous progesterone and any relevant metabolites with breast cancer risks among premenopausal and postmenopausal women should be resolved in the near term. These studies should consider whether associations differ by tumor characteristics, most importantly molecular subtype and receptor status, as well as by race, BMI, and likely other genetic and non-genetic factors (eg, other circulating hormones). Studies are also needed to evaluate the association between progesterone/progesterone metabolites and breast cancer risk in higher risk groups, including women with ductal carcinoma in situ, a history of benign breast disease, or increased mammographic breast density, and specifically among BRCA1 mutation carriers, as antiprogestin therapy may be particularly relevant to this subpopulation.
Questions remain as to whether POPs or progestogen-only menopausal hormone therapy are related to breast cancer risks. In addition, breast cancer risks with low-dose and newer formulations of oral contraceptives and menopausal hormone therapy remain unresolved. Thus, continued monitoring of breast cancer associations with COCs, new generation progestin-containing contraceptives, and progestogen-containing menopausal hormone therapy use is needed. Further, it is unclear if progesterone and progestins relate to breast cancer risk through direct or indirect proliferative effects, as well as the potential for genotoxicity, DNA damage, tumor-initiating effects via induction of enzymes and proteins involved in nucleic acid synthesis, or through activation of oncogenes.
In this review we did not extensively summarize the expansive literature about the possible role of progesterone or PR in breast cancer prognosis and/or treatment. In section IV.F., we reference recent support for a more active role of PR in hormone receptor positive breast cancers. These studies individually made additional therapeutic recommendations beyond treatment with tamoxifen alone. As a result, multiple clinical trials have been developed to test combination hormone therapies targeting both PR and ER in breast cancer treatment. It is clear in this context that the clinical trials are outpacing the basic science in that there is more to learn regarding steroid receptor crosstalk in breast cancer (129), yet the clinical trials are actively testing combination therapies that include PR targets. At a minimum we will learn clinically relevant information from these trials. If the emerging laboratory and epidemiologic data support a role of progesterone in the etiology of breast cancer, it is plausible that similar trials (focused on prevention) could be planned to evaluate the use of combination therapies in women at high risk of breast cancer who would be given tamoxifen as current standard clinical practices.
Although many unanswered questions about the role of progesterone in breast physiology and carcinogenesis remain, there have been multiple advances over recent years. It is likely that continued progress in our understanding of the potential proliferative role of progesterone in the adult breast, including advances in stem cell technology, may clarify the role of progesterone in the initiation and progression of breast cancers. Finally, development of sensitive LC-MS/MS assay technology will likely facilitate evaluation of progesterone and progesterone metabolites in large epidemiologic studies of breast cancer that will ultimately address some of the data gaps outlined in this review.
Acknowledgments
Margaret Groves, M.Phil., E.L.S., for her help in preparation of the manuscript.
Financial Support: Funded in part by the intramural research program of the National Cancer Institute, NIH, DHHS; Mayo Clinic Breast Cancer SPORE (CA116201-12CEP).
Disclosure summary: All authors have nothing to disclose.
References
- 1. Beatson G. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment, with illustrative cases. Lancet Infect Dis. 1896;104–105. [PMC free article] [PubMed] [Google Scholar]
- 2. Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health. 2012;66(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, Seachrist JL. The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000;60(4):936–943. [PubMed] [Google Scholar]
- 4. Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314(5804):1467–1470. [DOI] [PubMed] [Google Scholar]
- 5. Hewitt SC, Korach KS. Progesterone action and responses in the alphaERKO mouse. Steroids. 2000;65(10–11):551–557. [DOI] [PubMed] [Google Scholar]
- 6. Horwitz KB, The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992;13(2):146–163. [DOI] [PubMed] [Google Scholar]
- 7. Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation. In: De Groot LJ, et al. , ed. Endotext. South Dartmouth (MA); 2000. [Google Scholar]
- 8. Tal R, Taylor HS, Burney RO, Mooney SB, Giudice LC. Endocrinology of pregnancy. In: De Groot LJ, et al. , eds. Endotext. South Dartmouth (MA); 2000. [Google Scholar]
- 9. Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perlman B, Kulack D, Goldsmith LT, Weiss G. The etiology of menopause: not just ovairan dysfunction but also a role for the central nervous system. Global Reproductive Health. 2018;3(2):e8. [Google Scholar]
- 11. Randolph Jr JF, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93(5):1711–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westphal U. Steroid-protein interactions II. Monogr Endocrinol. 1986;27:1–603. [PubMed] [Google Scholar]
- 14. Stanczyk F. Pharmacokinetics of progesterone administered orally and parenterally. In: Sitruk-Ware R, Mishell DR, eds. Progestins and Antiprogestins in Clinical Practice. Marcel-Dekker: New York; 2000:393–400. [Google Scholar]
- 15. Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68(10–13):879–890. [DOI] [PubMed] [Google Scholar]
- 16. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR Jr. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34(2):171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5(2):119–137. [DOI] [PubMed] [Google Scholar]
- 18. Javed A, Lteif A. Development of the human breast. Semin Plast Surg. 2013;27(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asselin-Labat ML, Vaillant F, Sheridan JM. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. [DOI] [PubMed] [Google Scholar]
- 20. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 21. Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology. 2008;149(5):2098–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13(6):385–396. [DOI] [PubMed] [Google Scholar]
- 23. Beleut M, Rajaram RD, Caikovski M, et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci U S A. 2010;107(7):2989–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haslam SZ, Shyamala G. Effect of oestradiol on progesterone receptors in normal mammary glands and its relationship with lactation. Biochem J. 1979;182(1):127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang S, Counterman LJ, Haslam SZ. Progesterone action in normal mouse mammary gland. Endocrinology. 1990;127(5):2183–2189. [DOI] [PubMed] [Google Scholar]
- 26. Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A. 2007;104(37):14718–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolf RM, Long D. Pubertal development. Pediatr Rev. 2016;37(7):292–300. [DOI] [PubMed] [Google Scholar]
- 28. Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95(9):5076–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chow JD, Simpson ER, Boon WC. Alternative 5’-untranslated first exons of the mouse Cyp19A1 (aromatase) gene. J Steroid Biochem Mol Biol. 2009;115(3–5):115–125. [DOI] [PubMed] [Google Scholar]
- 30. Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11(5):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horseman ND, Hernandez LL. New concepts of breast cell communication to bone. Trends Endocrinol Metab. 2014;25(1):34–41. [DOI] [PubMed] [Google Scholar]
- 32. Radisky DC, Visscher DW, Frank RD, et al. Natural history of age-related lobular involution and impact on breast cancer risk. Breast Cancer Res Treat. 2016;155(3):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Figueroa JD, Pfeiffer RM, Patel DA, et al. Terminal duct lobular unit involution of the normal breast: implications for breast cancer etiology. J Natl Cancer Inst. 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95(2):696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100(17):9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289(5485):1751–1754. [DOI] [PubMed] [Google Scholar]
- 37. Brisken C, Heineman A, Chavarria T, et al. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14(6):650–654. [PMC free article] [PubMed] [Google Scholar]
- 38. Cao YX, Bonizzi G, Seagroves TN, et al. IKK alpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107(6):763–775. [DOI] [PubMed] [Google Scholar]
- 39. Mukherjee A, Soyal SM, Li J, et al. Targeting RANKL to a specific subset of murine mammary epithelial cells induces ordered branching morphogenesis and alveologenesis in the absence of progesterone receptor expression. FASEB J. 2010;24(11):4408–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDonnell DP, Unraveling the human progesterone receptor signal transduction pathway Insights into antiprogestin action. Trends Endocrinol Metab. 1995;6(4):133–138. [DOI] [PubMed] [Google Scholar]
- 41. Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357(1–2):18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21(2):155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lamb CA, Fabris VT, Jacobsen B, Molinolo AA, Lanari C. Biological and clinical impact of imbalanced progesterone receptor isoform ratios in breast cancer. Endocr Relat Cancer 2018. doi:10.1530/ERC-18-0179. [DOI] [PubMed] [Google Scholar]
- 44. Hill KK, Roemer SC, Churchill ME, Edwards DP. Structural and functional analysis of domains of the progesterone receptor. Mol Cell Endocrinol. 2012;348(2):418–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tung L, Abdel-Hafiz H, Shen T, et al. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol. 2006;20(11):2656–2670. [DOI] [PubMed] [Google Scholar]
- 46. Mulac-Jericevic B, Conneely OM. Reproductive tissue selective actions of progesterone receptors. Reproduction. 2004;128(2):139–146. [DOI] [PubMed] [Google Scholar]
- 47. Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol. 1993;7(10):1256–1265. [DOI] [PubMed] [Google Scholar]
- 48. Conneely OM, Lydon JP, De Mayo F, O’Malley BW. Reproductive functions of the progesterone receptor. J Soc Gynecol Investig. 2000;7(1 Suppl):S25–S32. [DOI] [PubMed] [Google Scholar]
- 49. Fabris V, Abascal MF, Giulianelli S, et al. Isoform specificity of progesterone receptor antibodies. J Pathol Clin Res. 2017;3(4):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diep CH, Ahrendt H, Lange CA. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids. 2016;114:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143(6):2357–2365. [DOI] [PubMed] [Google Scholar]
- 53. Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2(4):393–402. [DOI] [PubMed] [Google Scholar]
- 54. Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci U S A. 2006;103(7):2196–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 56. Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14(3):359–368. [DOI] [PubMed] [Google Scholar]
- 57. Sivaraman L, Hilsenbeck SG, Zhong L, et al. Early exposure of the rat mammary gland to estrogen and progesterone blocks co-localization of estrogen receptor expression and proliferation. J Endocrinol. 2001;171(1):75–83. [DOI] [PubMed] [Google Scholar]
- 58. Diep CH, Knutson TP, Lange CA. Active FOXO1 is a key determinant of isoform-specific progesterone receptor transactivation and senescence programming. Mol Cancer Res. 2016;14(2):141–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singhal H, Greene ME, Zarnke AL, et al. Progesterone receptor isoforms, agonists and antagonists differentially reprogram estrogen signaling. Oncotarget. 2018;9(4):4282–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abdel-Hafiz HA, Dudevoir ML, Perez D, Abdel-Hafiz M, Horwitz KB. SUMOylation regulates transcription by the progesterone receptor A isoform in a target gene selective manner. Diseases. 2018;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68(10–13):771–778. [DOI] [PubMed] [Google Scholar]
- 62. Ismail PM, Amato P, Soyal SM, et al. Progesterone involvement in breast development and tumorigenesis--as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids. 2003;68(10–13):779–787. [DOI] [PubMed] [Google Scholar]
- 63. Ismail PM, Li J, DeMayo FJ, O’Malley BW, Lydon JP. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol. 2002;16(11):2475–2489. [DOI] [PubMed] [Google Scholar]
- 64. Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80(2):137–148. [DOI] [PubMed] [Google Scholar]
- 65. Axlund SD, Sartorius CA. Progesterone regulation of stem and progenitor cells in normal and malignant breast. Mol Cell Endocrinol. 2012;357(1–2):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Graham JD, Mote PA, Salagame U, et al. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150(7):3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Finlay-Schultz J, Sartorius CA. Steroid hormones, steroid receptors, and breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2015;20(1–2):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alferez DG, Simoes BM, Howell SJ, Clarke RB. The role of steroid hormones in breast and effects on cancer stem cells. Curr Stem Cell Rep. 2018;4(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tanos T, Sflomos G, Echeverria PC, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5(182):182ra55. [DOI] [PubMed] [Google Scholar]
- 70. Fata JE, Kong YY, Li J, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103(1):41–50. [DOI] [PubMed] [Google Scholar]
- 71. Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC. RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol. 2007;27(4):1442–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sisay M, Mengistu G, Edessa D. The RANK/RANKL/OPG system in tumorigenesis and metastasis of cancer stem cell: potential targets for anticancer therapy. Onco Targets Ther. 2017;10:3801–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo J, Yang Z, Ma Y, et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med. 2016;22(5):539–546. [DOI] [PubMed] [Google Scholar]
- 74. Wang Y, Dong J, Li D, et al. Lgr4 regulates mammary gland development and stem cell activity through the pluripotency transcription factor Sox2. Stem Cells. 2013;31(9):1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fernandez-Valdivia R, Mukherjee A, Ying Y, et al. The RANKL signaling axis is sufficient to elicit ductal side-branching and alveologenesis in the mammary gland of the virgin mouse. Dev Biol. 2009;328(1):127–139. [DOI] [PubMed] [Google Scholar]
- 76. Cordero A, Pellegrini P, Sanz-Moreno A, et al. Rankl impairs lactogenic differentiation through inhibition of the Prolactin/Stat5 pathway at midgestation. Stem Cells. 2016;34(4):1027–1039. [DOI] [PubMed] [Google Scholar]
- 77. Wang J, Gupta A, Hu H, Chatterton RT, Clevenger CV, Khan SA. Comment on “Progesterone/RANKL Is a Major Regulatory Axis in the Human Breast”. Sci Transl Med. 2013;5(215):215le4. [DOI] [PubMed] [Google Scholar]
- 78. Tanos T, Sflomos G, Echeverria PC, et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci Transl Med. 2013;5(182):182ra55. [DOI] [PubMed] [Google Scholar]
- 79. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. [DOI] [PubMed] [Google Scholar]
- 80. Weichhaus M, Chung ST, Connelly L. Osteoprotegerin in breast cancer: beyond bone remodeling. Mol Cancer. 2015;14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fortner RT, Sarink D, Schock H, et al. Osteoprotegerin and breast cancer risk by hormone receptor subtype: a nested case-control study in the EPIC cohort. BMC Med. 2017;15(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Widschwendter M, Burnell M, Fraser L, et al. Osteoprotegerin (OPG), the endogenous inhibitor of receptor activator of NF-kappaB ligand (RANKL), is dysregulated in BRCA mutation carriers. EBioMedicine. 2015;2(10):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oden L, Akbari M, Zaman T, et al. Plasma osteoprotegerin and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Oncotarget. 2016;7(52):86687–86694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thomas P, Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76(1–2):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Singh M, Su C, Ng S. Non-genomic mechanisms of progesterone action in the brain. Front Neurosci. 2013;7:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Losel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1--many tasks for a versatile protein. Steroids. 2008;73(9–10):929–934. [DOI] [PubMed] [Google Scholar]
- 88. Peluso JJ, Romak J, Liu J. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone’s antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology. 2008;149(2):534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cahill MA, Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105(1–5):16–36. [DOI] [PubMed] [Google Scholar]
- 91. Neubauer H, Adam G, Seeger H, et al. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12(3):230–239. [DOI] [PubMed] [Google Scholar]
- 92. Neubauer H, Clare SE, Wozny W, et al. Breast cancer proteomics reveals correlation between estrogen receptor status and differential phosphorylation of PGRMC1. Breast Cancer Res. 2008;10(5):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schuster J, Karlsson T, Karlstrom PO, Poromaa IS, Dahl N. Down-regulation of progesterone receptor membrane component 1 (PGRMC1) in peripheral nucleated blood cells associated with premature ovarian failure (POF) and polycystic ovary syndrome (PCOS). Reprod Biol Endocrinol. 2010;8:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Neubauer H, Yang Y, Seeger H, et al. The presence of a membrane-bound progesterone receptor sensitizes the estradiol-induced effect on the proliferation of human breast cancer cells. Menopause. 2011;18(8):845–850. [DOI] [PubMed] [Google Scholar]
- 95. Neubauer H, Ruan X, Schneck H, et al. Overexpression of progesterone receptor membrane component 1: possible mechanism for increased breast cancer risk with norethisterone in hormone therapy. Menopause. 2013;20(5):504–510. [DOI] [PubMed] [Google Scholar]
- 96. Stanczyk FZ, Can the increase in breast cancer observed in the estrogen plus progestin arm of the Women’s Health Initiative trial be explained by progesterone receptor membrane component 1? Menopause. 2011;18(8):833–834. [DOI] [PubMed] [Google Scholar]
- 97. Makarem M, Spike BT, Dravis C, Kannan N, Wahl GM, Eaves CJ. Stem cells and the developing mammary gland. J Mammary Gland Biol Neoplasia. 2013;18(2):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev. 2014;28(11):1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stingl J, Emerman JT, Eaves CJ. Enzymatic dissociation and culture of normal human mammary tissue to detect progenitor activity. Methods Mol Biol. 2005;290:249–263. [DOI] [PubMed] [Google Scholar]
- 100. Stingl J, Raouf A, Eirew P, Eaves CJ. Deciphering the mammary epithelial cell hierarchy. Cell Cycle. 2006;5(14):1519–1522. [DOI] [PubMed] [Google Scholar]
- 101. Eirew P, Stingl J, Raouf A, et al. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14(12):1384–1389. [DOI] [PubMed] [Google Scholar]
- 102. Kannan N, Huda N, Tu L, et al. The luminal progenitor compartment of the normal human mammary gland constitutes a unique site of telomere dysfunction. Stem Cell Rep. 2013;1(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kannan N, Nguyen LV, Makarem M, et al. Glutathione-dependent and -independent oxidative stress-control mechanisms distinguish normal human mammary epithelial cell subsets. Proc Natl Acad Sci U S A. 2014;111(21):7789–7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhang X, Chiang HC, Wang Y, et al. Attenuation of RNA polymerase II pausing mitigates BRCA1-associated R-loop accumulation and tumorigenesis. Nat Commun. 2017;8:15908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Keller PJ, Arendt LM, Skibinski A, et al. Defining the cellular precursors to human breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2772–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Nguyen LV, Pellacani D, Lefort S, et al. Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature. 2015;528(7581):267–271. [DOI] [PubMed] [Google Scholar]
- 107. Van Keymeulen A, Lee MY, Ousset M, et al. Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature. 2015;525(7567):119–123. [DOI] [PubMed] [Google Scholar]
- 108. Rajaram RD, Buric D, Caikovski M, et al. Progesterone and Wnt4 control mammary stem cells via myoepithelial crosstalk. EMBO J. 2015;34(5):641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kannan N, Eaves CJ. Macrophages stimulate mammary stem cells. Science. 2018;360(6396):1401–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chakrabarti R, Celia-Terrassa T, Kumar S, et al. Notch ligand Dll1 mediates cross-talk between mammary stem cells and the macrophageal niche. Science. 2018;360(6396):eaan4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nolan E, Vaillant F, Branstetter D, et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22(8):933–939. [DOI] [PubMed] [Google Scholar]
- 112. Sau A, Lau R, Cabrita MA, et al. Persistent activation of NF-kappaB in BRCA1-deficient mammary progenitors drives aberrant proliferation and accumulation of DNA damage. Cell Stem Cell. 2016;19(1):52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sigl V, Owusu-Boaitey K, Joshi PA, et al. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Research. 2016;26(7):761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98(22):1600–1607. [DOI] [PubMed] [Google Scholar]
- 115. Figueroa JD, Pfeiffer RM, Brinton LA, et al. Standardized measures of lobular involution and subsequent breast cancer risk among women with benign breast disease: a nested case-control study. Breast Cancer Res Treat. 2016;159(1):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Khodr ZG, Sherman ME, Pfeiffer RM, et al. Circulating sex hormones and terminal duct lobular unit involution of the normal breast. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Trabert B, Falk RT, Stanczyk FZ, McGlynn KA, Brinton LA, Xu X. Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry. Horm Mol Biol Clin Investig. 2015;23(3):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol. 2012;357(1–2):4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57(22):4987–4991. [PubMed] [Google Scholar]
- 120. Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21(2):359–375. [DOI] [PubMed] [Google Scholar]
- 121. Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol. 2008;22(4):823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol. 2005;19(2):327–339. [DOI] [PubMed] [Google Scholar]
- 123. Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277(7):5209–5218. [DOI] [PubMed] [Google Scholar]
- 124. Miller MM, James RA, Richer JK, Gordon DF, Wood WM, Horwitz KB. Progesterone regulated expression of flavin-containing monooxygenase 5 by the B-isoform of progesterone receptors: implications for tamoxifen carcinogenicity. J Clin Endocrinol Metab. 1997;82(9):2956–2961. [DOI] [PubMed] [Google Scholar]
- 125. Lanari C, Wargon V, Rojas P, Molinolo AA. Antiprogestins in breast cancer treatment: are we ready? Endocr Relat Cancer. 2012;19(3):R35–R50. [DOI] [PubMed] [Google Scholar]
- 126. Mohammed H, Russell IA, Stark R, et al. Progesterone receptor modulates ERalpha action in breast cancer. Nature. 2015;523(7560):313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Singhal H, Greene ME, Tarulli G, et al. Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv. 2016;2(6):e1501924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Daniel AR, Gaviglio AL, Knutson TP, et al. Progesterone receptor-B enhances estrogen responsiveness of breast cancer cells via scaffolding PELP1- and estrogen receptor-containing transcription complexes. Oncogene. 2015;34(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Truong TH, Lange CA. Deciphering steroid receptor crosstalk in hormone-driven cancers. Endocrinology. 2018;159(12):3897–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Lim E, Vaillant F, Wu D, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15(8):907–913. [DOI] [PubMed] [Google Scholar]
- 131. Proia TA, Keller PJ, Gupta PB, et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell. 2011;8(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sedic M, Skibinski A, Brown N, et al. Haploinsufficiency for BRCA1 leads to cell-type-specific genomic instability and premature senescence. Nat Commun. 2015;6:7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. He Z, Kannan N, Nemirovsky O, BRCA1 controls the cell division axis and governs ploidy and phenotype in human mammary cells. Oncotarget. 2017;8(20):32461–32475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Widschwendter M, Rosenthal AN, Philpott S, et al. The sex hormone system in carriers of BRCA1/2 mutations: a case-control study. Lancet Oncol. 2013;14(12):1226–1232. [DOI] [PubMed] [Google Scholar]
- 135. Gompel A, Malet C, Spritzer P, Lalardrie JP. Kuttenn F, Mauvais-Jarvis P. Progestin effect on cell proliferation and 17 beta-hydroxysteroid dehydrogenase activity in normal human breast cells in culture. J Clin Endocrinol Metab. 1986;63(5):1174–1180. [DOI] [PubMed] [Google Scholar]
- 136. Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS Jr. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104(1):23–34. [PMC free article] [PubMed] [Google Scholar]
- 137. Potten CS, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Going JJ, Anderson TJ, Battersby S, MacIntyre CC. Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol. 1988;130(1):193–204. [PMC free article] [PubMed] [Google Scholar]
- 139. Beral V, Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. [DOI] [PubMed] [Google Scholar]
- 140. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet. 1997;350(9084):1047–1059. [PubMed] [Google Scholar]
- 141. Calle EE, Feigelson HS, Hildebrand JS, Teras LR, Thun MJ, Rodriguez C. Postmenopausal hormone use and breast cancer associations differ by hormone regimen and histologic subtype. Cancer. 2009;115(5):936–945. [DOI] [PubMed] [Google Scholar]
- 142. Fournier A, Berrino F, Riboli E, Avenel V, Clavel-Chapelon F. Breast cancer risk in relation to different types of hormone replacement therapy in the E3N-EPIC cohort. Int J Cancer. 2005;114(3):448–454. [DOI] [PubMed] [Google Scholar]
- 143. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11(2):266–301. [DOI] [PubMed] [Google Scholar]
- 145. Chang KJ, Lee TT, Linares-Cruz G, Fournier S, de Lignieres B. Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil Steril. 1995;63(4):785–791. [PubMed] [Google Scholar]
- 146. Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol. 1986;10(6):382–393. [DOI] [PubMed] [Google Scholar]
- 147. Spicer DV, Ursin G, Pike MC. Progesterone concentrations--physiologic or pharmacologic? Fertil Steril. 1996;65(5):1077–1078. [DOI] [PubMed] [Google Scholar]
- 148. Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. [DOI] [PubMed] [Google Scholar]
- 149. Anderson WF, Rosenberg PS, Prat A, Perou CM, Sherman ME. How many etiological subtypes of breast cancer: two, three, four, or more? J Natl Cancer Inst. 2014;106(8):dju165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(3):454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103(3):250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pierobon M, Frankenfeld CL. Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;137(1):307–314. [DOI] [PubMed] [Google Scholar]
- 153. Dowsett M, Folkerd E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat. 2015;149(1):1–4. [DOI] [PubMed] [Google Scholar]
- 154. Draznin B. Mechanism of the mitogenic influence of hyperinsulinemia. Diabetol Metab Syndr. 2011;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Braz MG, Salvadori DM. Lack of genotoxicity induced by endogenous and synthetic female sex hormones in peripheral blood cells detected by alkaline comet assay. Environ Mol Mutagen. 2007;48(5):414–420. [DOI] [PubMed] [Google Scholar]
- 156. Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS One. 2013;8(2):e56161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Siddique YH, Afzal M. A review on the genotoxic effects of some synthetic progestins. Int J Pharmacol. 2008;4:410–430. [Google Scholar]
- 158. Kayani MA, Parry JM. The detection and assessment of the aneugenic potential of selected oestrogens, progestins and androgens using the in vitro cytokinesis blocked micronucleus assay. Mutat Res Genet Toxicol Environ Mutagen. 2008;651(1–2):40–45. [DOI] [PubMed] [Google Scholar]
- 159. Palomba S, Santagni S, La Sala GB. Progesterone administration for luteal phase deficiency in human reproduction: an old or new issue? J Ovarian Res. 2015;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Feser W, Kerdar RS, Baumann A, Korber J, Blode H, Kuhnz W. DNA adduct formation of selected sex steroids in human liver slices in vitro. Toxicol in Vitro. 1998;12(4):353–364. [DOI] [PubMed] [Google Scholar]
- 161. Werner S, Kunz S, Beckurts T, Heidecke CD, Wolff T, Schwarz LR. Formation of DNA adducts by cyproterone acetate and some structural analogues in primary cultures of human hepatocytes. Mutat Res Genet Toxicol Environ Mutagen. 1997;395(2–3):179–187. [DOI] [PubMed] [Google Scholar]
- 162. Pardo I, Lillemoe HA, Blosser RJ, et al. Next-generation transcriptome sequencing of the premenopausal breast epithelium using specimens from a normal human breast tissue bank. Breast Cancer Res. 2014;16(2):R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tanaka H, Abe S, Huda N, et al. Telomere fusions in early human breast carcinoma. Proc Natl Acad Sci U S A. 2012;109(35):14098–14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. McNamara KM, Sasano H. The intracrinology of breast cancer. J Steroid Biochem Mol Biol. 2015;145:172–178. [DOI] [PubMed] [Google Scholar]
- 165. Key T, Appleby P, Barnes I, Reeves G; Endogenous Hormones and Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. [DOI] [PubMed] [Google Scholar]
- 166. Tarulli GA, Butler LM, Tilley WD, Hickey TE. Bringing androgens up a NOTCH in breast cancer. Endocr Relat Cancer. 2014;21(4):T183–T202. [DOI] [PubMed] [Google Scholar]
- 167. Hickey TE, Robinson JL, Carroll JS, Tilley WD. Minireview: The androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26(8):1252–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Eliassen AH, Hankinson SE. Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol. 2008;630:148–165. [PubMed] [Google Scholar]
- 169. Labrie F. Intracrinology in action: importance of extragonadal sex steroid biosynthesis and inactivation in peripheral tissues in both women and men. J Steroid Biochem Mol Biol. 2015;145:131–132. [DOI] [PubMed] [Google Scholar]
- 170. Labrie F, Belanger A, Belanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol. 2006;99(4–5):182–188. [DOI] [PubMed] [Google Scholar]
- 171. Stanczyk FZ, Azen CG, Pike MC. Effect of finasteride on serum levels of androstenedione, testosterone and their 5 alpha-reduced metabolites in men at risk for prostate cancer. J Steroid Biochem Mol Biol. 2013;138:10–16. [DOI] [PubMed] [Google Scholar]
- 172. Brinton LA, Cook MB, McCormack V, Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst. 2014;106(3):djt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Brinton LA, Key TJ, Kolonel LN, et al. Prediagnostic sex steroid hormones in relation to male breast cancer risk. J Clin Oncol. 2015;33(18):2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lee HJ, Ormandy CJ. Interplay between progesterone and prolactin in mammary development and implications for breast cancer. Mol Cell Endocrinol. 2012;357(1–2):101–107. [DOI] [PubMed] [Google Scholar]
- 175. Wang M, Wu X, Chai F, Zhang Y, Jiang J. Plasma prolactin and breast cancer risk: a meta- analysis. Sci Rep. 2016;6:25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. [DOI] [PubMed] [Google Scholar]
- 177. Thomas HV, Key TJ, Allen DS, et al. A prospective study of endogenous serum hormone concentrations and breast cancer risk in premenopausal women on the island of Guernsey. Br J Cancer. 1997;75(7):1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18(2):79–85. [PubMed] [Google Scholar]
- 179. Micheli A, Muti P, Secreto G, et al. Endogenous sex hormones and subsequent breast cancer in premenopausal women. Int J Cancer. 2004;112(2):312–318. [DOI] [PubMed] [Google Scholar]
- 180. Fortner RT, Eliassen AH, Spiegelman D, Willett WC, Barbieri RL, Hankinson SE. Premenopausal endogenous steroid hormones and breast cancer risk: results from the Nurses’ Health Study II. Breast Cancer Res. 2013;15(2):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kaaks R, Tikk K, Sookthai D, et al. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status - results from the EPIC cohort. Int J Cancer. 2014;134(8):1947–1957. [DOI] [PubMed] [Google Scholar]
- 182. Schernhammer ES, Sperati F, Razavi P, et al. Endogenous sex steroids in premenopausal women and risk of breast cancer: the ORDET cohort. Breast Cancer Res. 2013;15(3):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wiebe JP, Zhang G, Welch I, and Cadieux-Pitre HA. Progesterone metabolites regulate induction, growth, and suppression of estrogen- and progesterone receptor-negative human breast cell tumors. Breast Cancer Res. 2013;15(3):R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Wiebe JP, Progesterone metabolites in breast cancer. Endocr Relat Cancer. 2006;13(3):717–738. [DOI] [PubMed] [Google Scholar]
- 185. Samson M, Porter N, Orekoya O, et al. Progestin and breast cancer risk: a systematic review. Breast Cancer Res Treat. 2016;155(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hormonal contraceptives, progestogens only. IARC Monogr Eval Carcinog Risks Hum. 1999;72:339–397. [PMC free article] [PubMed] [Google Scholar]
- 187. Morch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard O. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239. [DOI] [PubMed] [Google Scholar]
- 188. Westhoff CL, Pike MC. Hormonal contraception and breast cancer. Contraception. 2018;98(3):171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Soini T, Hurskainen R, Grenman S, Maenpaa J, Paavonen J, Pukkala E. Cancer risk in women using the levonorgestrel-releasing intrauterine system in Finland. Obstet Gynecol. 2014;124(2 Pt 1):292–299. [DOI] [PubMed] [Google Scholar]
- 190. Soini T, Hurskainen R, Grenman S, et al. Levonorgestrel-releasing intrauterine system and the risk of breast cancer: A nationwide cohort study. Acta Oncol. 2016;55(2):188–192. [DOI] [PubMed] [Google Scholar]
- 191. Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346(26):2025–2032. [DOI] [PubMed] [Google Scholar]
- 192. Strom BL, Berlin JA, Weber AL, et al. Absence of an effect of injectable and implantable progestin-only contraceptives on subsequent risk of breast cancer. Contraception. 2004;69(5):353–360. [DOI] [PubMed] [Google Scholar]
- 193. Kumle M, Weiderpass E, Braaten T, Persson I, Adami HO, Lund E. Use of oral contraceptives and breast cancer risk: the Norwegian-Swedish Women’s Lifestyle and Health Cohort Study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1375–1381. [PubMed] [Google Scholar]
- 194. Fabre A, Fournier A, Mesrine S, et al. Oral progestagens before menopause and breast cancer risk. Br J Cancer. 2007;96(5):841–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Breast cancer and hormonal contraceptives: further results. Collaborative Group on Hormonal Factors in Breast Cancer. Contraception. 1996;54(3 Suppl):1S–106S. [DOI] [PubMed] [Google Scholar]
- 196. Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11(6):545–560. [DOI] [PubMed] [Google Scholar]
- 197. Stahlberg C, Pedersen AT, Lynge E, et al. Increased risk of breast cancer following different regimens of hormone replacement therapy frequently used in Europe. Int J Cancer. 2004;109(5):721–727. [DOI] [PubMed] [Google Scholar]
- 198. Porch JV, Lee IM, Cook NR, Rexrode KM, Burin JE. Estrogen-progestin replacement therapy and breast cancer risk: the Women’s Health Study (United States). Cancer Causes Control. 2002;13(9):847–854. [DOI] [PubMed] [Google Scholar]
- 199. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N cohort study. Breast Cancer Res Treat. 2008;107(1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Giraddi RR, Shehata M, Gallardo M, Blasco MA, Simons BD, Stingl J. Stem and progenitor cell division kinetics during postnatal mouse mammary gland development. Nat Commun. 2015;6:8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Fridriksdottir AJ, Kim J, Villadsen R, et al. Propagation of oestrogen receptor-positive and oestrogen-responsive normal human breast cells in culture. Nat Commun. 2015;6:8786. [DOI] [PMC free article] [PubMed] [Google Scholar]