Abstract
We previously revealed that tumor cell‐derived angiopoietin‐like protein 2 (ANGPTL2) accelerates the metastatic capacity of tumors in an autocrine/paracrine manner by activating tumor cell motility and invasiveness and the epithelial‐mesenchymal transition. However, the effects of ANGPTL2 on cancer cell glycolytic metabolism, which is a hallmark of tumor cells, are unknown. Here we report evidence supporting a role for tumor cell‐derived ANGPTL2 in establishing a preference for glycolytic metabolism. We report that a highly metastatic lung cancer cell subline expressing abundant ANGPTL2 showed upregulated expression of the glucose transporter GLUT3 as well as enhanced glycolytic metabolism relative to a less metastatic parental line. Most notably, ANGPTL2 overexpression in the less metastatic line activated glycolytic metabolism by increasing GLUT3 expression. Moreover, ANGPTL2 signaling through integrin α5β1 increased GLUT3 expression by increasing transforming growth factor‐β (TGF‐β) signaling and expression of the downstream transcription factor zinc finger E‐box binding homeobox 1 (ZEB1). Conversely, ANGPTL2 knockdown in the highly metastatic subline decreased TGF‐β1, ZEB1, and GLUT3 expression and antagonized glycolytic metabolism. In primary tumor cells from patients with lung cancer, ANGPTL2 expression levels correlated with GLUT3 expression. Overall, this work suggests that tumor cell‐derived ANGPTL2 accelerates activities associated with glycolytic metabolism in lung cancer cells by activating TGF‐β‐ZEB1‐GLUT3 signaling.
Keywords: ANGPTL2, cancer metabolism, GLUT3, lung cancer, ZEB1
Tumor cell‐derived angiopoietin‐like protein 2 (ANGPTL2) accelerates metastatic capacity of tumors in an autocrine/paracrine manner by activating tumor cell motility and invasiveness and epithelial‐mesenchymal transition. Here we report evidence supporting a role for tumor cell‐derived ANGPTL2 in establishing a preference for glycolytic metabolism. Overall, this work suggests that tumor cell‐derived ANGPTL2 accelerates activities associated with glycolytic metabolism in lung cancer cells by activating TGF‐β‐ZEB1‐GLUT3 signaling.
1. INTRODUCTION
The reprogramming of energy metabolism represented by the Warburg effect is a hallmark of cancer.1 The common feature of such altered cellular metabolism is increased glucose consumption and a shift from oxidative phosphorylation to glycolysis.2 Increased rates of glycolysis provide cancer cells with growth and survival advantages and are associated with tumor aggressiveness and malignancy.3, 4 The GLUT (SLC2A) family of glucose transporter proteins is a key regulator of glucose uptake across cell membranes.5 Among its 14 members, GLUT1 is broadly expressed in various cancer cells and plays a pivotal role in glycolytic metabolism.6 Interestingly, a recent report indicates that GLUT3 is also expressed in glioblastoma,7 colon cancer,8 and lung cancer,9 suggesting that GLUT3 also functions in metabolic activity in cancer cells.
The epithelial‐mesenchymal transition (EMT) plays essential roles in development, tissue regeneration, wound healing, and organ fibrosis.10 Transforming growth factor‐β (TGF‐β) functions to induce EMT11, 12 by increasing expression of the transcription factors Snail and zinc finger E‐box binding homeobox 1 (ZEB1), which repress epithelial markers such as E‐cadherin, and by inducing mesenchymal markers, such as vimentin.13, 14 In tumor cells, the EMT is associated with increased tumor cell invasion and metastasis.15 Interestingly, recent studies indicate that the EMT promotes changes in cancer metabolism, antagonizes cancer cell apoptosis and senescence, and increases immunosuppression.10, 16
We have reported that angiopoietin‐like protein 2 (ANGPTL2), which functions as a proinflammatory mediator in various diseases, including cancer,17 promotes the EMT by activating the TGF‐β pathway in a chemically induced skin squamous cell carcinoma mouse model.18 We also showed that, in an autocrine/paracrine manner, tumor cell‐derived ANGPTL2 accelerates aggressive metastatic phenotypes in tumor cells by activating cell motility, invasiveness, and the EMT in human lung and breast cancers and osteosarcoma.19, 20, 21 However, ANGPTL2 function has not been studied in the context of cancer cell metabolism.
Here, we report that, in primary tumor cells from patients with lung cancer, ANGPTL2 expression levels positively correlate with those of GLUT3, but not GLUT1. Our in vitro analysis using lung cancer cell lines revealed that tumor cell‐derived ANGPTL2 signaling through integrin α5β1 increases GLUT3 expression by activating the TGF‐β‐ZEB1 pathway, thereby activating glycolytic metabolism in lung cancer cells.
2. MATERIALS AND METHODS
2.1. Human studies
Tissue samples were resected from 96 lung cancer patients at the Department of Thoracic Surgery of Kumamoto University Hospital. All specimens were diagnosed as lung cancer by a pathologist. All studies were approved by the Ethics Committee of Kumamoto University.
2.2. Immunohistological staining
Formalin‐fixed, paraffin‐embedded specimens were cut into 4‐μm sections and deparaffinized. Sections were autoclaved with citrate buffer (pH 6.0) for antigen retrieval. Sections were incubated with 3% H2O2 for 5 minutes to block endogenous peroxidase activity and then incubated with anti‐ANGPTL2 Ab and anti‐GLUT3 (1:100, HPA006539; Sigma‐Aldrich), diluted with Block Ace (KAC) at 4°C overnight. After washing with PBS, sections were incubated for 30 minutes with EnVision+ System‐HRP‐labeled Polymer Anti‐rabbit (Dako) for visualization with DAB (Dojindo). Slides were counterstained 20 seconds with hematoxylin.
2.3. Total RNA extraction and real‐time quantitative RT‐PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) and from human tissue samples using the Total RNA Extraction Miniprep System (Viogene). DNase‐treated RNA was reversed‐transcribed using a PrimeScript RT reagent kit (Takara Bio). The PCR products were analyzed using a Thermal Cycler Dice Real Time System (Takara Bio). The PCR primer sequences are shown in Table S1. Relative transcript abundance was normalized to that of RPS18 mRNA.
2.4. Cell culture
The human lung cancer lines NCI‐H460 (H460) and NCI‐H460‐LNM35 (LNM35) were previously described22 and provided by Dr T. Takahashi (Aichi Cancer Center, Japan). NCI‐H1975 (H1975) was purchased from ATCC. HCC15 (H15) was established at the Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center23 and generously donated by Dr Adi F. Gazdar (University of Texas Southwestern Medical Center). H460, LNM35, H1975, and H15 cells were cultured in RPMI‐1640 medium supplemented with 10% FCS at 37°C in a humidified 5% CO2 atmosphere. For some experiments, cells were treated with 10 μM MEK inhibitor U0126 (662005; Millipore) for 6 h in normal growth medium.
2.5. Plasmid transfection
For stable transfection, H460, H1975, and H15 cells were transfected with ANGPTL2 or empty vectors24 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol and selected in 400‐800 μg/mL G418.
2.6. Immunoblot analysis
Solubilized proteins were subjected to SDS‐PAGE, and proteins were electrotransferred to PVDF membranes. Immunoblotting was carried out with Abs against ANGPTL2 (1:2000, BAF1444; R&D Systems) and Hsc70 (1:2000, #sc7298; Santa Cruz Biotechnology). Immunodetection was carried out using an ECL kit (GE Healthcare) according to the manufacturer's protocol.
2.7. Flow cytometry
Cells were suspended in MACS buffer (Miltenyi Biotec) and stained with the following Abs: anti‐GLUT3 (ab15311; Abcam), anti‐integrin α5β1 (MAB1969; Millipore), anti‐integrin αvβ3 (MAB1976Z; Millipore), anti‐integrin αvβ5 (MAB1961; Millipore), and anti‐integrin α9β1 (Sc‐59969; Santa Cruz Biotechnology). Cells were incubated with appropriate secondary Abs. Viable cells were identified as unstained with 7‐AAD (Beckman Coulter). Stained cells were analyzed by BD FACSVerse (BD Biosciences). Data analysis was undertaken using FlowJo software (TreeStar).
2.8. Glucose uptake and lactate production assays
Glucose uptake was determined using a Glucose Uptake‐Glo Assay (Promega) and lactate production by using a Lactate Assay Kit‐WST (Dojindo), according to each manufacturer's protocols.
2.9. Immunofluorescence
For ZEB1 staining, cells were first fixed for 20 minutes in acetone and ethanol (1:1) and then blocked in 5% normal goat serum (Nichirei Biosciences). Cells were incubated with anti‐ZEB1 Abs (1:50, #sc515797; Santa Cruz Biotechnology) and then with Alexa 488‐conjugated anti‐mouse Abs. Nuclei were counterstained with DAPI.
2.10. Knockdown of ZEB1
H460 cells were reseeded in 12‐well plates and transfected with ZEB1 siRNA (SYK [ID SR304746] Trilencer‐27 human siRNA; OriGene). As a control, we used Trilencer‐27 Universal Scrambled Negative Control (OriGene). Total RNA was extracted for quantitative RT‐PCR (qRT‐PCR) analysis 24 hours later.
2.11. Knockdown of ANGPTL2
ANGPTL2‐specific knockdown LNM35 cells was described previously.19
2.12. Statistical analyses
Statistical analyses were undertaken using GraphPad Prism 8 software (GraphPad Software). Statistical parameters and methods are reported in figures and corresponding legends. Results with P values less than .05 were considered significant (*P < .05; **P < .01; ***P < .001). Comparisons between 2 groups were carried out using the unpaired 2‐tailed t test. Comparisons between 3 or more groups were undertaken using one‐way ANOVA with Tukey's multiple comparison test. Potential correlations of ANGPTL2, GLUT1 (SLC2A1), GLUT3 (SLC2A3), or ZEB1 expression in lung cancer specimens were evaluated by calculating Spearman's correlation coefficient.
3. RESULTS
3.1. Glucose transport 3 abundantly expressed in aggressive lung cancer cells
The NCI‐H460‐LNM35 (LNM35) line is a highly metastatic subline of the human large‐cell lung carcinoma cell line NCI‐H460 (H460).22 Because enhanced glycolytic metabolism is reportedly associated with tumor aggressiveness,4, 25 we asked whether LNM35 and H460 cells differed in terms of glycolytic metabolism by comparing glucose uptake and lactate production in both lines. Both glucose uptake and lactate production were significantly increased in LNM35 relative to H460 cells (Figure 1A,B), suggesting activation of glycolytic metabolism in the highly metastatic line.
Figure 1.
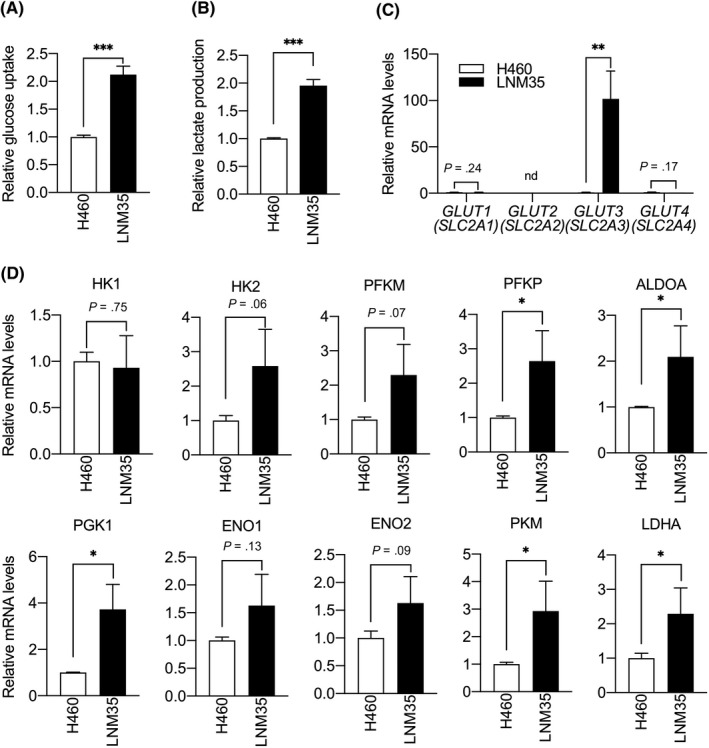
Aggressive lung cancer cells show increased glycolytic metabolism and glucose transporter 3 (GLUT3) expression. A, Relative glucose uptake in H460 and LNM35 cells. Levels in H460 cells were set to 1. Data are means ± SD; n = 3 per group. ***P < .001, unpaired t test. B, Relative lactate production in H460 and LNM35 cells. Levels in H460 cells were set to 1. Data are means ± SD; n = 4 per group. ***P < .001, unpaired t test. C, Comparison of levels of GLUT1‐4 (SLC2A1‐4) transcripts in H460 and LNM35 cells. Levels in H460 cells were set to 1. Data are means ± SD; n = 3 per group. nd, not detected. **P < .01, unpaired t test. D, Comparison of levels of indicated transcripts in H460 and LNM35 cells. Levels in H460 cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, unpaired t test. ALDOA, aldolase fructose‐bisphosphate A; ENO, enolase; HK, hexokinase; LDHA, lactate dehydrogenase A;PFKM, phosphofructokinase muscle; PFKP, phosphofructokinase platelets; PGK1, phosphoglycerate kinase 1; PKM, pyruvate kinase M
As GLUT family glucose transporters are critical for glucose transport and reportedly contribute to tumor progression in various cancers, including lung cancer,6, 26, 27 we compared expression levels of GLUT1 (SLC2A1), GLUT2 (SLC2A2), GLUT3 (SLC2A3), and GLUT4 (SLC2A4) in H460 and LNM35 cells. Interestingly, GLUT1, GLUT2, and GLUT4 expression was comparable in both lines, whereas expression levels of GLUT3 in LNM35 cells were significantly higher than in H460 cells (Figure 1C). To further investigate factors potentially accelerating glucose uptake and lactate production in LNM35 cells, we compared expression of transcripts encoding the glycolytic enzymes hexokinase 1 (HK1), hexokinase 2 (HK2), phosphofructokinase muscle (PFKM), phosphofructokinase platelets (PFKP), aldolase fructose‐bisphosphate a (ALDOA), phosphoglycerate kinase 1 (PGK1), enolase 1 (ENO1), enolase 2 (ENO2), pyruvate kinase M (PKM), and lactate dehydrogenase A (LDHA) in H460 and LNM35 cells (Figure 1D). Quantitative RT‐PCR analysis revealed that PFKP, ALDOA, PGK1, PKM, and LDHA mRNA levels were slightly higher in LNM35 cells than in H460 cells. These results suggest that enhanced glycolytic metabolism seen in LNM35 cells might be primarily attributable to increased GLUT3 expression in LNM35 relative to H460 cells.
3.2. Angiopoietin‐like protein 2 expression induces GLUT3 expression and enhances glycolytic metabolism in lung cancer cells
We previously reported that increased metastatic ability of LNM35 relative to H460 cells is due to significant activation of ANGPTL2‐dependent tumor cell motility and invasiveness in LNM35 cells.19 Accordingly, ANGPTL2 transcripts were more abundant in LNM35 than in H460 cells (Figure 2A). Here, we also found that LNM35 cells show increased GLUT3 expression and enhanced glycolytic metabolism compared to H460 cells, as shown in Figure 1. These findings suggest that ANGPTL2 activity is linked to metabolic preference in tumor cells. To assess this possibility, we established 2 independent H460 cell lines, 1 overexpressing ANGPTL2 (H460/ANGPTL2) and the other a control H460 line overexpressing empty vector (H460/vector) (Figure 2B), and compared glucose uptake and lactate production in both (Figure 2C,D). Glucose uptake and lactate production in H460/ANGPTL2 lines were significantly increased relative to corresponding activities in control H460/vector cells. Moreover, expression levels of GLUT3, but not GLUT1, in the H460/ANGPTL2 line was markedly increased relative to expression in control H460/vector cells (Figure 2E). Consistently, FACS analysis with an anti‐GLUT3 Ab revealed an increase in GLUT3 protein levels on the surface of H460/ANGPTL2 relative to control H460/vector cells (Figure 2F).
Figure 2.
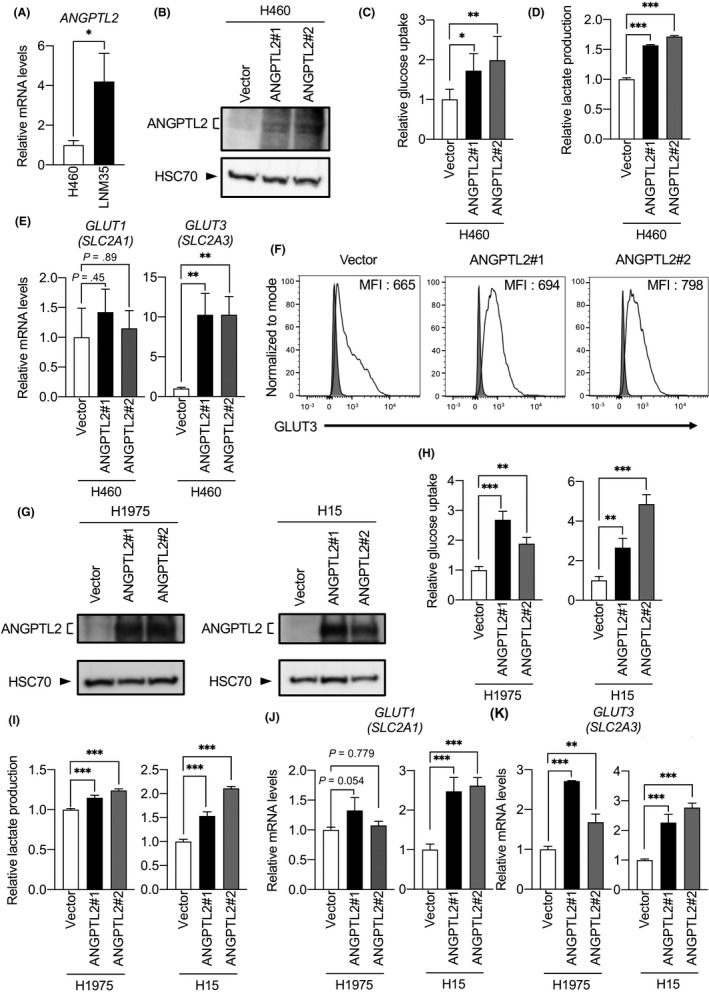
Angiopoietin‐like protein 2 (ANGPTL2) increases glucose transporter 3 (GLUT3) expression and glycolytic metabolism. A, Comparison of levels of ANGPTL2 transcripts in H460 and LNM35 cells. Levels in H460 cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, unpaired t test. B, Representative immunoblotting for ANGPTL2 in H460/ANGPTL2 and H460/vector cells. Heat shock cognate protein 70 (HSC70) served as a loading control. C, Relative glucose uptake in H460/ANGPTL2 and H460/vector cells. Levels in H460/vector cells were set to 1. Data are means ± SD; n = 6 per group. *P < .05, **P < .01, one‐way ANOVA test followed by Tukey's multiple comparison test. D, Relative lactate production in H460/ANGPTL2 and H460/vector cells. Levels in H460/vector cells were set to 1. Data are means ± SD; n = 3 per group. ***P < .001, one‐way ANOVA test followed by Tukey's multiple comparison test. E, Comparison of levels of GLUT1 (SLC2A1) and GLUT3 (SLC2A3) transcripts in H460/ANGPTL2 and H460/vector cells. Levels in H460/vector cells were set to 1. Data are means ± SD; n = 3 per group. **P < .01, one‐way ANOVA test followed by Tukey's multiple comparison test. F, Representative profiles showing cell surface expression of GLUT3 (black line traces) or isotype‐matched control (gray filled traces) in H460/ANGPTL2 and H460/vector cells, as assessed by flow cytometry analysis. Gating of living cells was carried out after background assessment. MFI, median fluorescence intensity. G, Representative immunoblotting for ANGPTL2 in H1975/ANGPTL2 and H1975/vector cells, and in H15/ANGPTL2 and H15/vector cells. HSC70 served as a loading control. H, I, Relative glucose uptake and lactate production in H1975/ANGPTL2 and control cells, and H15/ANGPTL2 and control cells. Levels in respective vector control cells were set to 1. Data are means ± SD; n = 3 per group. **P < .01, ***P < .001, one‐way ANOVA test followed by Tukey's multiple comparison test. J, K, Comparison of levels of GLUT1 (SLC2A1) and GLUT3 (SLC2A3) transcripts in H1975/ANGPTL2 and control cells, and H15/ANGPTL2 and control cells. Levels in respective vector control cells were set to 1. Data are means ± SD; n = 3 per group. **P < .01, ***P < .001, one‐way ANOVA test followed by Tukey's multiple comparison test
To assess whether ANGPTL2 activity alters GLUT3 expression in other lung cancer lines, we established 2 additional ANGPTL2‐overexpressing lines plus corresponding controls: the human lung adenocarcinoma line H1975 (H1975/ANGPTL2 and H1975/vector) and the human lung squamous cell carcinoma line H15 (H15/ANGPTL2 and H15/vector) (Figure 2G). As in H460 cells, glucose uptake and lactate production in H1975/ANGPTL2 and H15/ANGPTL2 lines were significantly increased relative to corresponding controls (Figure 2H,I). Quantitative RT‐PCR revealed that expression levels of GLUT3 in H1975/ANGPTL2 and H15/ANGPTL2 were higher than in control cells (Figure 2J,K). Taken together, these results suggest that ANGPTL2 enhances glycolytic metabolism in lung cancer cells by increasing GLUT3‐dependent glucose transport.
3.3. Angiopoietin‐like protein 2 induces GLUT3 expression through the TGF‐β–ZEB1 pathway
We next asked how ANGPTL2 increases GLUT3 expression in lung cancer cells. Glucose transporter 3 transcription is reportedly regulated by the DNA binding protein ZEB1, which is upregulated by TGF‐β signaling during the EMT in nonsmall‐cell lung cancer cells.26 Furthermore, we previously reported that ANGPTL2 promotes the EMT by activating the TGF‐β pathway.18 Based on these findings, we hypothesized that ANGPTL2 induces GLUT3 expression in lung cancer cells by activating TGF‐β‐induced ZEB1 expression. To assess this possibility, we first investigated the EMT status of H460/ANGPTL2 and H460/vector control cells (Figure 3A). Expression levels of CDH1 (E‐cadherin), an epithelial marker, in H460/ANGPTL2 cells were markedly lower than those seen in control H460/vector cells. By contrast, expression levels of VIM (vimentin), a mesenchymal marker, in H460/ANGPTL2 cells were significantly higher than those seen in control H460/vector cells. Consistently, we found significantly increased expression of TGFB1 (TGF‐β1) and the EMT‐associated transcription factors ZEB1 and SNAI1 in H460/ANGPTL2 relative to control H460/vector cells (Figure 3B). Furthermore, TGFB1 and ZEB1 transcript levels were higher in H1975/ANGPTL2 and H15/ANGPTL2 than in corresponding control cells (Figure 3C,D). We also observed increased nuclear accumulation of ZEB1 in H460/ANGPTL2 relative to control H460/vector cells (Figure 3E,F). These results suggest that tumor cell‐derived ANGPTL2 increases GLUT3 expression by activating both the TGF‐β1‐ZEB1 pathway and the EMT.
Figure 3.
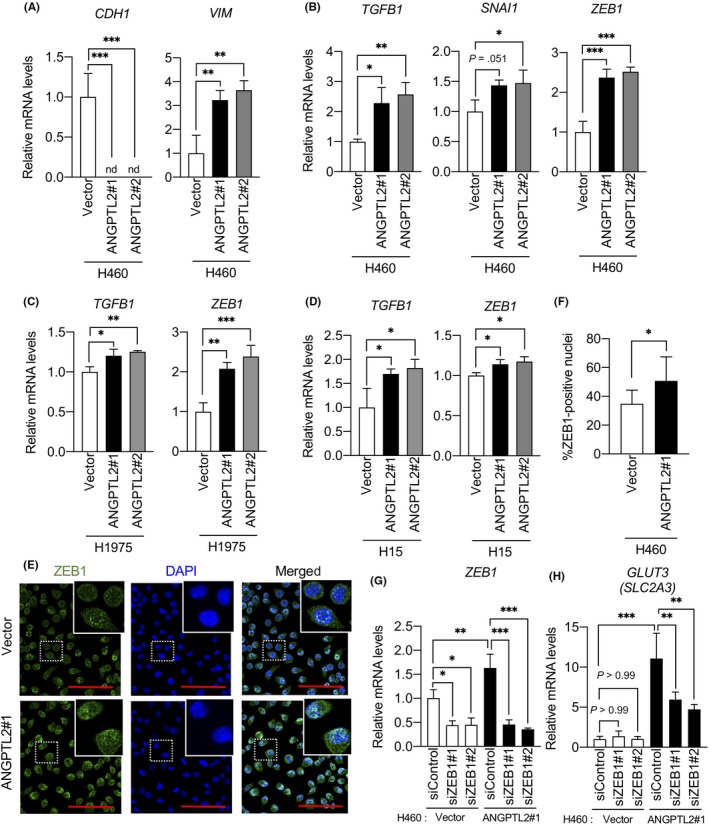
Angiopoietin‐like protein 2 (ANGPTL2) induces glucose transporter 3 GLUT3 expression through zinc finger E‐box binding homeobox 1 (ZEB1). A, B, Comparison of levels of indicated transcripts in H460/ANGPTL2 and H460/vector cells. Levels seen in H460/vector cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, **P < .01, ***P < .001, one‐way ANOVA test followed by Tukey's multiple comparison test. C, D, Comparison of levels of indicated transcripts in H1975/ANGPTL2 and control cells, and in H15/ANGPTL2 and control cells. Levels in respective vector control cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, **P < .01, ***P < .001, one‐way ANOVA test followed by Tukey's multiple comparison test. E, Immunofluorescent staining for ZEB1 (green) in H460/ANGPTL2 and H460/vector cells. Nuclei are counterstained with DAPI (blue). Scale bar = 100 μm. Insets show magnified images. F, Quantification of ZEB1‐positive nuclei. Data are means ± SD; n = 8 per group. *P < .05, unpaired t test. G, H, Comparison of levels of indicated transcripts in H460/ANGPTL2 and H460/vector cells transfected with ZEB1‐specific siRNAs (siZEB1#1 or siZEB1#2) or negative control siRNA (siControl). Levels in control siRNA H460/vector cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, **P < .01, ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test
We then asked whether GLUT3 expression in lung cancer cells changes after suppression of ZEB1 expression in H460/ANGPTL2 and H460/vector cells. To do so, we transfected both lines with either ZEB1‐specific siRNAs (siZEB1#1 and siZEB1#2) or control scramble siRNA (siControl) (Figure 3G,H). ZEB1 knockdown in H460/ANGPTL2 cells significantly decreased GLUT3 expression, whereas we observed no change in GLUT3 expression in siControl‐transfected H460/ANGPTL2 cells (Figure 3H). Interestingly, we observed no change in GLUT3 expression in H460/vector cells, even in ZEB1 knockdown cells (Figure 3G,H). Thus, we conclude that increased GLUT3 expression due to ANGPTL2 expression is due to ZEB1 upregulation, suggesting that ANGPTL2 activates the TGF‐β‐ZEB1 pathway and increases GLUT3 expression.
3.4. Angiopoietin‐like protein 2 signaling through integrin α5β1 increases ZEB1‐mediated GLUT3 expression
We next assessed molecular mechanisms underlying ANGPTL2‐mediated activation of the TGF‐β‐ZEB1 pathway. As ANGPTL2 signaling through integrin α5β1 enhances cancer cell metastasis, including EMT activation,20, 21 we evaluated integrins expressed on the H460 cell surface by flow cytometry. As shown in Figure 4A, integrin α5β1 is expressed on H460 cells, suggesting that ANGPTL2 activates TGF‐β‐ZEB1 signaling through this receptor. Accordingly, TGFB1 and ZEB1 expression significantly decreased in H460/ANGPTL2 cells treated by an anti‐integrin α5β1 blocking Ab (α5β1 Ab) as compared to a control IgG‐treated (cIgG) group (Figure 4B). Signaling through the ANGPTL2‐integrin α5β1 axis reportedly activates ERK signaling and subsequent TGF‐β expression.28 Indeed, qRT‐PCR analysis revealed that TGFB1 transcript levels were significantly decreased following treatment of H460/ANGPTL2 cells with the MEK inhibitor U0126 (Figure 4C), suggesting that ANGPTL2 could upregulate TGFB1 through integrin α5β1‐ERK signaling. Furthermore, GLUT3 induction in H460/ANGPTL2 cells was significantly suppressed after cells were treated with integrin α5β1 blocking Ab (Figure 4D). Collectively, these results suggest that tumor cell‐derived ANGPTL2 autocrine/paracrine signaling through integrin α5β1 increases GLUT3 expression by activating the TGF‐β‐ZEB1 pathway.
Figure 4.
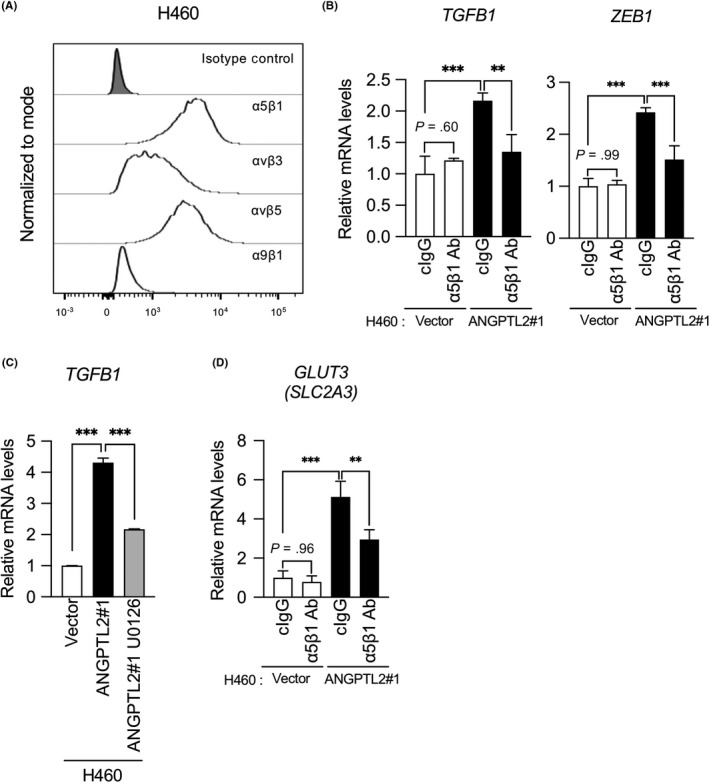
Angiopoietin‐like protein 2 (ANGPTL2) induces zinc finger E‐box binding homeobox 1 (ZEB1)‐dependent glucose transporter 3 (GLUT3) expression through integrin α5β1. A, Typical profiles of cell surface expression of integrins in H460 cells, as assessed by flow cytometry. Gating of living cells was carried out after background assessment. B, Comparison of levels of indicated transcripts in H460/ANGPTL2 and H460/vector cells treated with integrin α5β1‐blocking Ab or control IgG (cIgG). Levels in H460/vector cells treated with control IgG were set to 1. Data are means ± SD; n = 3 per group. **P < .01, ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test. C, Comparison of levels of TGFB1 transcripts in H460/ANGPTL2 and H460/vector control cells, either nontreated or treated with the MEK inhibitor U0126. Levels in vector control cells were set to 1. Data are means ± SD; n = 3 per group. ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test. D, Comparison of levels of GLUT3 (SLC2A3) transcripts in H460/ANGPTL2 and H460/vector cells treated with integrin α5β1‐blocking Abs or cIgG. Levels in H460/vector cells treated with control IgG were set to 1. Data are means ± SD; n = 3 per group. **P < .01, ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test
3.5. Angiopoietin‐like protein 2 knockdown reduces glycolytic metabolism in aggressive lung cancer cells
To confirm ANGPTL2 function in metabolic reprogramming of lung cancer cells, we generated 3 independent LNM35 cell sublines expressing a microRNA‐mediated RNA interference expression vector designed to knockdown ANGPTL2, namely, miANGPTL2#1, miANGPTL2#2, and LacZ (miLacZ) (Figure 5A). Expression of TGFB1, ZEB1, and GLUT3 in both LNM35/miANGPTL2#1 and LNM35/miANGPTL2#2 cells was significantly decreased relative to LNM35/miLacZ cells (Figure 5B,C). Moreover, glucose uptake and lactate production in both LNM35/miANGPTL2#1 and LNM35/miANGPTL2#2 cells were significantly reduced compared to levels seen in control LNM35/miLacZ cells (Figure 5D,E). We conclude that tumor cell‐derived ANGPTL2 autocrine/paracrine signaling drives glycolytic metabolism phenotypes in lung cancer cells.
Figure 5.
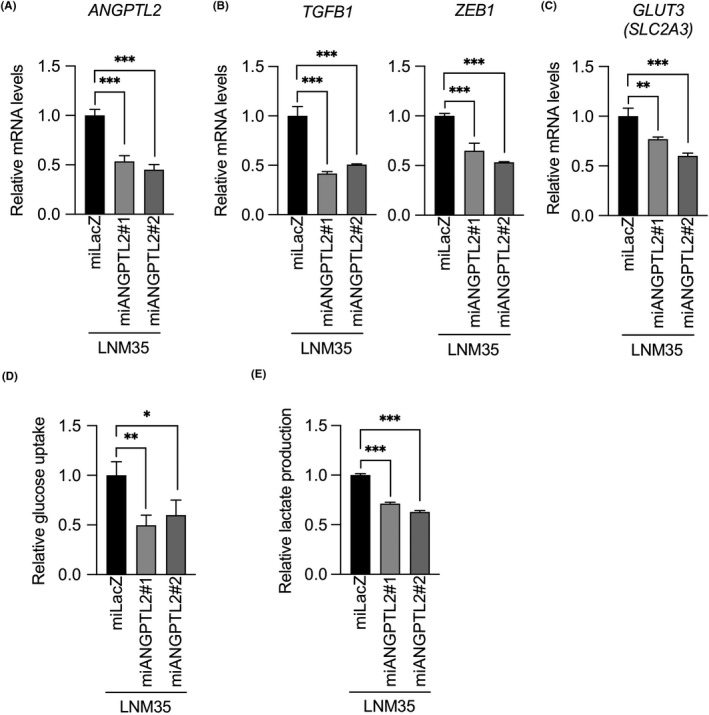
Angiopoietin‐like protein 2 (ANGPTL2) knockdown in lung cancer cells decreases glycolytic metabolism. A‐C, Comparison of levels of indicated transcripts in ANGPTL2 knockdown (miANGPTL2#1 and miANGPTL2#2) and control (miLacZ) LNM35 cells. Levels in control cells were set to 1. Data are means ± SD; n = 3 per group. **P < .01, ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test. D, Relative glucose uptake in ANGPTL2 knockdown and control LNM35 lines. Levels in control cells were set to 1. Data are means ± SD; n = 3 per group. *P < .05, **P < .01, one‐way ANOVA followed by Tukey's multiple comparison test. E, Relative lactate production in ANGPTL2 knockdown and control LNM35 lines. Levels in control cells were set to 1. Data are means ± SD; n = 3 per group. ***P < .001, one‐way ANOVA followed by Tukey's multiple comparison test
3.6. Angiopoietin‐like protein 2 expression in primary tumor tissues correlates with GLUT3 expression
Finally, we assessed a potential correlation in ANGPTL2, GLUT3, and ZEB1 mRNA expression levels in primary tumor tissues from 96 lung cancer patients (Figure 6A). ANGPTL2 expression was positively correlated with GLUT3 and ZEB1 expression in these specimens (r = .3536 and r = .5676), whereas we observed no correlation between ANGPTL2 and GLUT1 expression in these samples (r = .0716). Furthermore, we observed a positive correlation between ZEB1 and GLUT3 expression in these specimens (r = .6788). Immunohistochemical analysis of surgical specimens of primary tumors from patients with lung cancer using anti‐ANGPTL2 and anti‐GLUT3 Abs revealed relatively less GLUT3 expression in tumor tissues expressing low ANGPTL2 protein (Figure 6B, patients 1 and 2). By contrast, lung cancer cells expressing abundant ANGPTL2 protein showed high GLUT3 expression (Figure 6B, patients 3 and 4).
Figure 6.
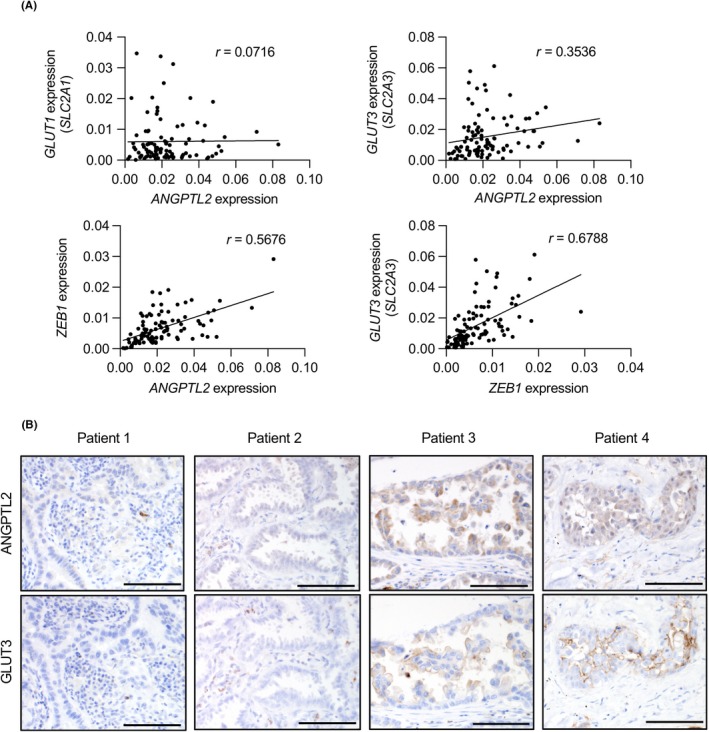
Angiopoietin‐like protein 2 (ANGPTL2) expression correlates with glucose transporter 3 (GLUT3) expression in human lung cancer. A, Correlation between (upper) ANGPTL2 and GLUT1 (SLC2A1) or ANGPTL2 and GLUT3 (SLC2A3) or (lower) ZEB1 and ANGPTL2 or ZEB1 and GLUT3 (SLC2A3) expression in primary tumor tissues from lung cancer patients (n = 96). Relative transcript abundance was normalized to that of RPS18 mRNA. Spearman's correlation coefficient (r) was calculated as 0.0716 (upper left), 0.3536 (upper right), 0.5676 (lower left), and 0.6788 (lower right). B, Immunostaining for ANGPTL2 and GLUT3 in surgical specimens of primary tumors from 4 indicated patients with lung cancer. Scale bar = 100 μm
4. DISCUSSION
Here, we provide evidence supporting a role for tumor cell‐derived ANGPTL2 in promoting glycolytic metabolism in lung cancer cells. We report that a highly metastatic subline of a human large‐cell lung carcinoma cell line showing high ANGPTL2 expression showed enhanced glycolytic metabolism and increased GLUT3 expression compared with the less metastatic parental line. Mechanistically, through autocrine/paracrine signaling, tumor cell‐derived ANGPTL2 signaling through integrin α5β1–ERK signaling increased expression of GLUT3 and glycolytic metabolism by activating the TGF‐β‐ZEB1 pathway in tumor cells. Conversely, ANGPTL2 suppression in tumor cells decreased GLUT3 expression and blocked glycolytic metabolism. Notably, we observed a positive correlation between ANGPTL2 and GLUT3 expression in primary tumors from patients with lung cancer. Overall, our findings show that tumor cell‐derived ANGPTL2 signaling increases GLUT3 expression through the TGF‐β‐ZEB1 pathway, enhancing glycolytic metabolism (Figure 7).
Figure 7.
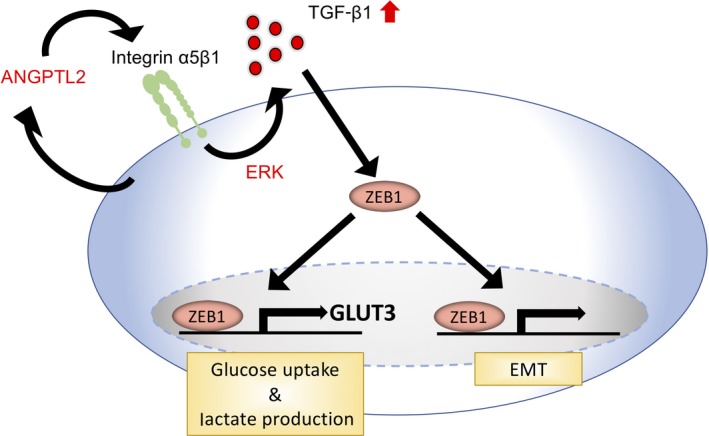
Model of proposed molecular mechanism underlying angiopoietin‐like protein 2 (ANGPTL2)‐mediated metabolic reprogramming. In lung cancer cells, tumor cell‐derived ANGPTL2 activates the transforming growth factor‐β (TGF‐β)‐zinc finger E‐box binding homeobox 1 (ZEB1) pathway through integrin α5β1‐ERK and induces not only the epithelial‐mesenchymal transition (EMT) but also glucose transporter 3 (GLUT3) expression, enhancing glycolytic metabolism
The tumor microenvironment, which is characterized by hypoxia and/or undernutrition, accelerates production of tumor cell‐derived humoral factors, such as cytokines and growth factors, leading to the EMT in tumor cells.29, 30 Epithelial‐mesenchymal transition‐dependent tumor invasion and metastasis is accompanied by increased cancer cell stemness and drug resistance.31 Furthermore, more recent studies report that the EMT is associated with altered metabolism in cancer cells32 and that EMT‐induced metabolic reprogramming in favor of glycolytic phenotypes promotes tumor aggressiveness.33 We previously showed that hypoxia and undernutrition increase ANGPTL2 expression in tumor cells and thereby enhance tumor cell metastatic capacity.19 The present study now shows that ANGPTL2 induces not only the EMT but also metabolic reprogramming in lung cancer cells through activating the TGF‐β pathway. These findings show that, in the tumor microenvironment, tumor cell‐derived ANGPTL2 serves as an important mediator of both EMT induction and metabolic reprogramming in tumor cells, enhancing tumor aggressiveness.
Mechanistically, we reveal here that activation of ANGPTL2‐dependent glycolytic metabolism is attributable to TGF‐β signaling activated in tumor cells. Accordingly, we previously reported that ANGPTL2 upregulates TGF‐β expression through integrin α5β1‐ERK signaling in renal tubular epithelial cells and macrophages.28 Moreover, we also reported that ANGPTL2 increases TGF‐β expression through the integrin α5β1‐p38‐MAPK pathway in osteosarcoma cells.21 Our findings strongly suggest that ANGPTL2 activity upregulates TGF‐β expression through integrin α5β1‐ERK signaling. Hence, we conclude that ANGPTL2‐activated ERK and/or p38 MAPK signaling contributes to activation of the TGF‐β pathway in lung cancer cells, thereby increasing ZEB1 and GLUT3 expression and activating glycolytic activity. Interestingly, others report that nuclear factor‐κB (NF‐κB) contributes to ZEB1 induction in cancer cells34 and that NF‐κB increases GLUT3 expression in cancer cells.35 Because ANGPTL2‐integrin α5β1 signaling activates the NF‐κB pathway in tumor cells,21 it is now of interest to investigate whether NF‐κB functions in ANGPTL2‐integrin α5β1 signaling‐mediated upregulation of GLUT3 expression in lung cancer cells.
To produce ATP as energy and to maintain cellular survival, nontumor epithelial cells rely primarily on mitochondrial oxidative phosphorylation. In contrast, cancer cells produce ATP primarily through glycolysis, regardless of whether cellular conditions are aerobic or not, a metabolic switch known as the Warburg effect.36, 37 Enhanced glycolytic metabolism seen in cancer cells is due to induction of glycolysis‐related genes, including GLUT factors.38 Glucose transporter 1 is reportedly expressed in various cancers, including brain, colon, and lung cancers, and contributes to glucose uptake in those tumor cells.39, 40, 41 Similar to GLUT1, GLUT3 is also expressed in some cancers, including lung cancer.27, 41 Both GLUT1 and GLUT3 expression in tumor cells reportedly correlates with poor prognosis of lung cancer patients.9 Conversely, several studies report that suppressing GLUT expression in tumor cells blocks tumor progression.42, 43 The present study indicates that ANGPTL2 expression is correlated with GLUT3 expression in primary tumors from patients with lung cancer. Moreover, our in vitro experiments using lung cancer lines reported here revealed that ANGPTL2 signaling increases GLUT3 expression and enhances glycolytic metabolism, whereas ANGPTL2 suppression decreased GLUT3 expression and antagonized glycolytic metabolism. These findings are consistent with our previous reports that ANGPTL2 expression in tumor cells correlates with metastatic ability and invasiveness of cancer cells and poor prognosis of cancer patients.19 They also suggest that ANGPTL2‐dependent GLUT3 expression in tumor cells might underlie poor prognosis of lung cancer patients both by activating glycolytic metabolism and increasing cancer malignancy.
In summary, we show that tumor cell‐derived ANGPTL2 enhances glycolytic metabolism in lung cancer cells by increasing GLUT3 expression through the integrin α5β1‐TGF‐β‐ZEB1 pathway. We also show that ANGPTL2 knockdown inactivates glycolytic metabolism in lung cancer cells. Based on these findings, we propose that tumor cell‐derived ANGPTL2 regulates the preference for glycolytic metabolism in cancer cells. Taken together with our previous reports that ANGPTL2 promotes aggressive metastatic phenotypes by activating tumor cell motility and invasiveness and the EMT, tumor cell‐derived ANGPTL2 could be a therapeutic target useful to antagonize cancer malignancy.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Kiyoka Tabu, Noriko Shirai, and Ayaka Yoshida for technical assistance. We also thank Dr Adi F. Gazdar (University of Texas Southwestern Medical Center) for the gift of the HCC15 cell line. This work was supported by the Scientific Research Fund of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (grant no. 17K08663 to M. Endo, grant no. 18K15246 to H. Horiguchi, and grant no. 18K07236 to T. Kadomatsu), the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST) (grant no. 13417915 to Y. Oike), the CREST program of the Japan Agency for Medical Research and Development (AMED) (grant no. JP19gm0610007 to Y. Oike), and the Takeda Science Foundation (to Y. Oike and to T. Kadomatsu).
Osumi H, Horiguchi H, Kadomatsu T, et al. Tumor cell‐derived angiopoietin‐like protein 2 establishes a preference for glycolytic metabolism in lung cancer cells. Cancer Sci. 2020;111:1241–1253. 10.1111/cas.14337
REFERENCES
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 2. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441‐464. [DOI] [PubMed] [Google Scholar]
- 3. DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 4. Icard P, Shulman S, Farhat D, Steyaert J‐M, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1‐11. [DOI] [PubMed] [Google Scholar]
- 5. Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298(2):E141‐E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24(6):650‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flavahan WA, Wu Q, Hitomi M, et al. Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake. Nat Neurosci. 2013;16(10):1373‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim E, Jung S, Park WS, et al. Upregulation of SLC2A3 gene and prognosis in colorectal carcinoma: analysis of TCGA data. BMC Cancer. 2019;19(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Younes M, Brown RW, Stephenson M, Gondo M, Cagle PT. Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer. 1997;80(6):1046‐1051. [DOI] [PubMed] [Google Scholar]
- 10. Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139(5):871‐890. [DOI] [PubMed] [Google Scholar]
- 11. Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF‐beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127(6 Pt 2):2021‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan E‐J, Olsson A‐K, Moustakas A. Reprogramming during epithelial to mesenchymal transition under the control of TGFbeta. Cell Adh Migr. 2015;9(3):233‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peinado H, Quintanilla M, Cano A. Transforming growth factor beta‐1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278(23):21113‐21123. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y, El‐Naggar S, Darling DS, Higashi Y, Dean DC. Zeb1 links epithelial‐mesenchymal transition and cellular senescence. Development. 2008;135(3):579‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212‐226. [DOI] [PubMed] [Google Scholar]
- 16. Sciacovelli M, Frezza C. Metabolic reprogramming and epithelial‐to‐mesenchymal transition in cancer. FEBS J. 2017;284(19):3132‐3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aoi J, Endo M, Kadomatsu T, et al. Angiopoietin‐like protein 2 accelerates carcinogenesis by activating chronic inflammation and oxidative stress. Mol Cancer Res. 2014;12(2):239‐249. [DOI] [PubMed] [Google Scholar]
- 18. Aoi J, Endo M, Kadomatsu T, et al. Angiopoietin‐like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71(24):7502‐7512. [DOI] [PubMed] [Google Scholar]
- 19. Endo M, Nakano M, Kadomatsu T, et al. Tumor cell‐derived angiopoietin‐like protein ANGPTL2 is a critical driver of metastasis. Cancer Res. 2012;72(7):1784‐1794. [DOI] [PubMed] [Google Scholar]
- 20. Masuda T, Endo M, Yamamoto Y, et al. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Odagiri H, Kadomatsu T, Endo M, et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7(309):ra7. [DOI] [PubMed] [Google Scholar]
- 22. Kozaki K, Miyaishi O, Tsukamoto T, et al. Establishment and characterization of a human lung cancer cell line NCI‐H460‐LNM35 with consistent lymphogenous metastasis via both subcutaneous and orthotopic propagation. Cancer Res. 2000;60(9):2535‐2540. [PubMed] [Google Scholar]
- 23. Girard L, Zochbauer‐Muller S, Virmani AK, Gazdar AF, Minna JD. Genome‐wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non‐small cell lung cancer, and loci clustering. Cancer Res. 2000;60(17):4894‐4906. [PubMed] [Google Scholar]
- 24. Kubota Y, Oike Y, Satoh S, et al. Cooperative interaction of Angiopoietin‐like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci USA. 2005;102(38):13502‐13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonatelli M, Silva ECA, Carcano FM, et al. The Warburg effect is associated with tumor aggressiveness in testicular germ cell tumors. Front Endocrinol (Lausanne). 2019;10:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masin M, Vazquez J, Rossi S, et al. GLUT3 is induced during epithelial‐mesenchymal transition and promotes tumor cell proliferation in non‐small cell lung cancer. Cancer Metab. 2014;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ancey PB, Contat C, Meylan E. Glucose transporters in cancer – from tumor cells to the tumor microenvironment. FEBS J. 2018;285(16):2926‐2943. [DOI] [PubMed] [Google Scholar]
- 28. Morinaga J, Kadomatsu T, Miyata K, et al. Angiopoietin‐like protein 2 increases renal fibrosis by accelerating transforming growth factor‐beta signaling in chronic kidney disease. Kidney Int. 2016;89(2):327‐341. [DOI] [PubMed] [Google Scholar]
- 29. Jiang J, Tang Y, Liang X. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11(8):714‐723. [DOI] [PubMed] [Google Scholar]
- 30. Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao D, He J. Epithelial mesenchymal transition and lung cancer. J Thorac Dis. 2010;2(3):154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu M, Quek L‐E, Sultani G, Turner N. Epithelial‐mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 2016;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kang H, Kim H, Lee S, Youn H, Youn B. Role of metabolic reprogramming in epithelial‐mesenchymal transition (EMT). Int J Mol Sci. 2019;20(8): E2042 10.3390/ijms20082042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajabi H, Alam M, Takahashi H, et al. MUC1‐C oncoprotein activates the ZEB1/miR‐200c regulatory loop and epithelial‐mesenchymal transition. Oncogene. 2014;33(13):1680‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zha X, Hu Z, Ji S, et al. NFkappaB up‐regulation of glucose transporter 3 is essential for hyperactive mammalian target of rapamycin‐induced aerobic glycolysis and tumor growth. Cancer Lett. 2015;359(1):97‐106. [DOI] [PubMed] [Google Scholar]
- 36. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309‐314. [DOI] [PubMed] [Google Scholar]
- 37. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;662:654‐662. [DOI] [PubMed] [Google Scholar]
- 39. Glucose transport: meeting the metabolic demands of cancer, and applications in glioblastoma treatment. Am J Cancer Res. 2016;6(8):1599‐1608. [PMC free article] [PubMed] [Google Scholar]
- 40. Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 41. Kurata T, Oguri T, Isobe T, Ishioka S, Yamakido M. Differential expression of facilitative glucose transporter (GLUT) genes in primary lung cancers and their liver metastases. Jpn J Cancer Res. 1999;90(11):1238‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Cao Y, Zhang W, et al. A small‐molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell‐cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11(8):1672‐1682. [DOI] [PubMed] [Google Scholar]
- 43. Chan DA, Sutphin PD, Nguyen P, et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3(94):94ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


