Abstract
As a novel class of noncoding RNAs, microRNAs (miRNAs) can effectively silence their target genes at the posttranscriptional level. Various biological processes, such as cell proliferation, differentiation, and motility, are regulated by miRNAs. In different diseases and different stages of disease, miRNAs have various expression patterns, which makes them candidate prognostic markers and therapeutic targets. Abnormal miRNA expression has been detected in numerous neoplastic diseases in humans, which indicates the potential role of miRNAs in tumorigenesis. Previous studies have indicated that miRNAs are involved in nearly the entire process of tumor development. MicroRNA‐302a, miR‐302b, miR‐302c, miR‐302d, and miR‐367 are members of the miR‐302/367 cluster that plays various biological roles in diverse neoplastic diseases by targeting different genes. These miRNAs have been implicated in several unique characteristics of cancer, including the evasion of growth suppressors, the sustained activation of proliferative signaling, the evasion of cell death and senescence, and the regulation of angiogenesis, invasion, and metastasis. This review provides a critical overview of miR‐302/367 cluster dysregulation and the subsequent effects in cancer and highlights the vast potential of members of this cluster as therapeutic targets and novel biomarkers.
Keywords: biological phenomena, cell dedifferentiation, MIRN302, neoplasm, therapy
The microRNA (miR)‐302/367 cluster has been implicated in several peculiarities of cancer including evading growth suppressors, sustaining proliferative signaling, and evading cell death and senescence, angiogenesis, invasion and metastasis. This review provides a critical overview of miR‐302/367 cluster dysregulation and the subsequent effects in cancer and demonstrates the indispensable roles of this cluster as therapeutic targets and novel biomarkers at current state researches.

Abbreviations
- BAX
BCL2 associated X
- BC
breast cancer
- Bcl‐2
B cell leukemia/lymphoma 2 apoptosis regulator
- Bim
BCL2 like 11
- CircRASSF2
Circlular RNA RASSF2
- CCN
cyclin
- CCNA
cyclin A
- CCND1
cyclin D1
- CD49f
CD49 antigen‐like family member F
- CDK2
cyclin‐dependent kinase 2
- ceRNA
competing endogenous RNA
- circRNAs
circular RNAs
- COUPTFII
chicken ovalbumin upstream promoter transcription factor II
- CSC
cancer stem cell
- CXCR4
C‐X‐C chemokine receptor type 4
- DCUN1D1
cullin neddylation 1 domain containing 1
- DOCK4
dedicator of cytokinesis 4
- E2F1
E2F transcription factor 1
- E2F3
E2F transcription factor 3
- EGFR
epidermal growth factor receptor
- EMT
epithelial‐mesenchymal transition
- EndMT
endothelial‐mesenchymal transformation
- EOMES
eomesodermin
- ER
estrogen receptor
- ErbB4
Receptor tyrosine‐protein kinase erbB‐4
- Erk
extracellular regulated MAP kinase
- ESCC
esophageal squamous cell carcinoma
- GC
gastric cancer
- GCNF
germ cell nuclear factor
- GSK‐3β
glycogen synthase kinase‐3β
- HCC
hepatocellular carcinoma
- HECC
human endometrial carcinoma cell
- hESC
human embryonic stem cell
- IGF‐1R
insulin‐like growth factor 1 receptor
- iPSCs
induced pluripotent stem cells
- JNK
c‐Jun NH2‐terminal kinase
- KLF4
Kruppel Like Factor 4
- KPNA2
NF‐κB‐inducing kinase and karyopherin α2
- LATS2
large tumor suppressor kinase 2
- lncRNA
long noncoding RNA
- LSCC
laryngeal squamous cell carcinoma
- Mcl‐1
myeloid cell leukemia sequence 1
- MIAT
myocardial infarction‐associated transcript
- miRNA
microRNA
- mirPS
miRNA‐induced pluripotent stem
- MTDH
metadherin
- ncRNA
noncoding RNA
- NK
natural killer
- Notch4
notch receptor 4
- NR2F2
nuclear receptor subfamily 2 group F member 2
- OC
ovarian cancer
- OCT
organic cation/carnitine transporter
- PCa
prostate cancer
- P‐gp
P‐glycoprotein
- PRP‐1
proline‐rich polypeptide 1
- RACK1
receptor for activated C‐kinase 1
- S1pr1
sphingosine‐1‐phosphate receptor 1
- SDC1
syndecan 1
- SDF1
stromal cell‐derived factor 1
- SHH
sonic hedgehog
- SOX2
SRY‐box transcription factor 2
- Tcf3
transcription factor 3
- SSEA‐4
stage‐specific embryonic antigen‐4
- YAP
Yes‐associated transcriptional regulator
1. INTRODUCTION
MicroRNA, which was discovered in 1993, has become an emerging topic in biomedical research over the last few years. MicroRNAs are noncoding, single‐stranded small RNAs of approximately 21 nucleotides. Previous studies found that miRNAs are important components of cellular development processes, including the regulation of cell stemness and the maintenance and progression of cardiovascular diseases, neurological diseases, immunological diseases, and cancer.1 Specifically, miRNAs play a crucial role in tumor formation and are involved in nearly the entire process of tumor development. To regulate their target genes, miRNAs bind to complementary nucleotides within the 3′‐UTR or ORF of coding mRNAs.2 The genes are involved in a variety of biological processes, ranging from cell proliferation, apoptosis, growth, differentiation, and metabolism to vascularization and the immune response.
The has‐miR‐302/367 cluster, situated on chromosome band 4q25, is one of the most thoroughly studied groups of miRNAs. This cluster contains several members, including miR‐302a/b/c/d and miR‐367, which have various biological roles in diverse neoplastic diseases through different modes of gene regulation. This family is highly conserved and still evolving, and its potential targets and functions require further investigation.3 These targets regulate multiple biological behaviors in cells, including the cell cycle, EMT, epigenetic regulation, and vesicular transport.2 This cluster also plays a significant role in hESC renewal and pluripotency maintenance as key regulatory factors and are involved in cellular stress response mechanisms.4, 5 Cell stemness can be detected by markers such as Nanog and OCT4, which are core transcription factors that initiate their own pathways prior to regulation of the miR‐302/367 family.3 Nanog, OCT4, and Tcf3, as classical transcription factors, can bind and occupy the promoter of miR‐302 and regulate its expression.6 Researchers have found possible connections between the expression level of this cluster and tumor grade/state in many tissues. Significant changes in their expression levels were observed in tumor and adjacent tissue specimens. Most miRNAs in this cluster are tumor suppressors, but there are some exceptions.3, 7
Tumor initiation and progression are complex biological processes that occur in multiple stages and are influenced by several factors. Previous research showed that the miR302/367 cluster is involved in nearly the entire process of tumor development in various neoplasms. The miR‐302/367 family participates in numerous hallmarks of cancer, regulating to some degree on the evasion of growth suppression, sustained activation of proliferative signaling, evasion of cell death and senescence, and induction of angiogenesis, invasion, and metastasis.3, 7 This review highlights the status of miR‐302/367 cluster dysregulation in cancer and reveals the potential of miR302/367 family members as therapeutic targets and novel biomarkers.
2. REGULATORY NETWORK OF MIR‐302/367 CLUSTER EXPRESSION
Many molecules regulate miRNA expression levels and functions, which occur both inside and outside the cell, through effects on miRNA production and maturation. Cell viability and western blot assays showed that in glioblastoma, increased GSK‐3β activity promotes a positive feedback loop between DOCK4 and β‐catenin, which in turn upregulates miR‐302b production.8 Experimental results indicated that H3K9 activity is inhibited by the overexpression of PRP‐1 in metastasis‐derived chondrosarcoma, which leads to the downregulation of miR‐302c, thereby achieving antiproliferative effects.9 MicroRNA‐302/367, as well as OCT4, SOX2, KLF4, and c‐MYC, can be activated by engineered zinc‐finger transcription factors.10 OCT4, Nanog, SOX2, and EOMES clearly upregulate miR‐302 gene expression, and Tcf3 inhibits miR‐302 expression.10, 11 In GC, real‐time PCR analysis showed that the expression of the miR‐302 cluster is upregulated by RACK1, and this result was confirmed in cell lines and clinical cases.12 Electrophoretic mobility shift assays and chromatin ChIP assays confirmed that GCNF directly inhibits miR‐302 by binding its promoter.13 In addition to transcription factors, miRNA sponges or decoys, termed ceRNAs, can regulate the levels of functional miRNAs. Competing endogenous RNAs include both protein‐coding mRNAs and ncRNAs, such as long lncRNAs, pseudogenes, circRNAs, and viral RNAs, that release parental target mRNAs from miRNA control by competing with the miRNAs.14 Cross‐talk between ceRNAs adds a new dimension to the regulation of miRNA expression and function. A large number of miR‐302/367 family members can be encapsulated by exosomes and internalized by adjacent glioblastoma cells, thereby affecting cellular biological behavior.15 The regulation of miR‐302/367 cluster expression in the nucleus, cytoplasm, and extracellular space and the intercellular exchange of miR‐302/367 cluster members through exosomes contribute to the biological significance of the differential expression of this cluster (Figure 1).
Figure 1.

Regulatory network of microRNA (miR)‐302/367 cluster expression. Many molecules regulate miRNA function inside and outside the cell by modulating miRNA production and maturation. The glycogen synthase kinase‐3β (GSK‐3β)/ dedicator of cytokinesis 4 (DOCK4)/β‐catenin axis, zinc‐finger transcription factors (ZF‐TFs), Nanog, organic cation/carnitine transporter‐3/4 (OCT3/4), and receptor for activated C‐kinase 1 (RACK1) promote miR‐302 expression, whereas the proline‐rich polypeptide 1 (PRP‐1)/H3K9 axis, transcription factor‐3 (Tcf3), and germ cell nuclear factor (GCNF) inhibit miR‐302/367 expression. Competing endogenous RNAs, including noncoding RNAs, pseudogenes, mRNAs, circular RNAs, and viral RNAs, can release parental target mRNAs from miRNA control by competition with the miRNA through their function as miRNA sponges or decoys. miR‐302/367 can be encapsulated by exosomes and internalized by other cells, thereby affecting biological behavior. CASC11, Long noncoding RNA CASC11; CircRASSF2, circular RNA RASSF2; ES1,Long noncoding RNA ES1; Klf4, Kruppel like factor 4; lncRNA, long noncoding RNA; MIAT, myocardial infarction‐associated transcript; SOX2, SRY‐box transcription factor 2
3. REGULATORY ROLE AND MECHANISMS OF MIR‐302/367 CLUSTER REGARDING CELL STEMNESS
Stem cells can differentiate into other cell types and undergo self‐renewal to produce more daughter cells. MicroRNA‐302/367 can control the pluripotency and self‐renewal of hESCs, iPSCs, and CSCs.16 The special ability of this cluster to change cell fate from somatic to pluripotent underscores its participation in regulating cell stemness through various pathways (Figure 2).
Figure 2.

MicroRNA (miR)‐302/367 cluster participates in the regulation of cell stemness. The miR‐302/367 cluster controls the stemness of human embryonic stem cells, induced pluripotent stem cells, and cancer stem cells by regulating pluripotent differentiation, G1‐S phase transition, and self‐renewal. The 3 major regulatory pathways are connected by nodal proteins such as AKT and organic cation/carnitine transporter 4 (OCT4). MYC, P21, cyclin D (CCND), and β‐catenin are important in intracellular signaling networks in cancer. P21 and CCND are representative molecules involved in cytostasis and differentiation. Myc is a classical member of the proliferation and motility signaling networks. BMI, B cell‐specific Moloney murine leukemia virus integration site 1; CCNE, cyclin E; CDK, cyclin‐dependent kinase; E2F7, E2F transcription factor 7; Klf4, Kruppel like factor 4; NR2F2, nuclear receptor subfamily 2 group F member 2; SOX2, SRY‐box transcription factor 2
First, the miR‐302b/367 cluster acts as a specific and critical regulator of hESC pluripotency and differentiation.3, 17 Importantly, Oct3/4, Sox2, and Nanog, which are hESC‐specific transcription factors, can transcriptionally regulate the miR‐302/367 promoter.18 The downregulation of Notch4, a miR‐302a target gene, leads to the downregulation of the Nodal signaling pathway.19 In addition, GCNF efficiently regulates the expression of CCND1 during hESC differentiation through the inhibition of miR‐302a.13
Second, proper expression of this cluster is necessary for maintaining the stemness of iPSCs.20 By suppressing both the CDK2 and CCND‐CDK4/6 cell cycle pathways, this cluster can regulate iPSC tumorigenicity during the G1‐S phase transition.21 Moreover, some researchers have found that differentiation ability can be inhibited by elevated p53 activity in miRNA‐deficient iPSCs.22 Interestingly, miR‐302 can repress the expression of NR2F2, a target gene verified by reporter luciferase assays and RT‐PCR, and stimulate the expression of OCT4 to promote cell pluripotency.23
In addition to its function in hESCs and iPSCs, this cluster also plays a vital role in CSCs, also called tumor‐initiating cells.6 In the reproductive system, the miR‐302 cluster can reportedly regulate male germline stem cell self‐renewal through the posttranscriptional inhibition of gene expression. The miR‐302 cluster can promote CD49f and OCT4 expression and downregulate P21 expression.24 In addition, through an AKT1/OCT4‐dependent pathway, miR‐302 can regulate teratoma formation.25 Similarly, tumor cell differentiation is modulated by the apparent downregulation of miR‐302b in ESCC.26 One study on HCC showed that the E2F7/AKT/β‐catenin/CCND1 pathway is regulated by miR‐302a/d, which can inhibit the stemness of liver CSCs and the proliferation of tumor cells.27 Furthermore, some evidence from a glioblastoma mouse model showed that the cell‐to‐cell transfer of miR‐302/367 can result in the inhibition of CXCR4/SDF1, SHH, CCND, CCNA and E2F1, which are targets of miR‐302/367.15
4. FUNCTIONS AND MECHANISMS OF MIR‐302 CLUSTER IN TUMOR DEVELOPMENT AND PROGRESSION
Tumor development is a complex biological process regulated by several signals and factors. Tumor cells exert different biological behaviors, including proliferation, apoptosis, metastasis, and drug resistance. Previous results show that the miR‐302/367 cluster participates in nearly the entire process of tumor development in various neoplasms and exhibits different regulatory functions (Figure 3).3, 7
Figure 3.
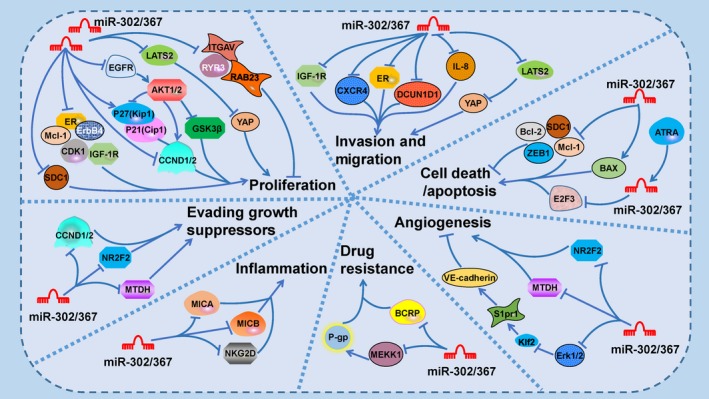
MicroRNA (miR)‐302/367 family participates in networks that regulate cancer hallmarks. Most members of the miR‐302/367 cluster inhibit the proliferation of tumor cells, but some contrary conclusions have been reached. The AKT signaling pathway plays an important role in regulating cell stemness and proliferation. In addition to stemness, cytostasis, and differentiation circuits, P21 and cyclin D (CCND) are involved in the regulation of cancer cell proliferation. Cancer cells show the classic hallmarks, including activation of invasion and metastasis, evasion of cell death and senescence, angiogenesis, the evasion of growth suppression and protumorigenic inflammation, as well as additional properties, such as chemotherapy/radiochemotherapy resistance. Some molecules, such as metadherin‐2 (MTDH2), nuclear receptor subfamily 2 group F member 2 (NR2F2), large tumor suppressor kinase 2 (LATS2), and Yes‐associated transcriptional regulator (YAP), contribute to multiple signaling interactions. Dual contributions are orchestrated and maintained by multiple signaling interactions, of which only a subset is illustrated. ATRA, All‐trans retinoic acid; BCRP, ATP binding cassette subfamily G member 2; CCND, cyclin D; CDK, cyclin‐dependent kinase; CXCR4, C‐X‐C chemokine receptor type 4; DCUN1D1, cullin neddylation 1 domain containing 1; E2F3, E2 transcription factor 3; EGFR, epidermal growth factor recptor; ER, estrogen receptor; ErbB4, Receptor tyrosine‐protein kinase erbB‐4; GSK‐3β, glycogen synthase kinase‐3β; IGF‐1R, insulin‐like growth factor 1 receptor; IL‐8, interleukin‐8; ITGAV, Integrin alpha‐V; Klf2, Kruppel like factor 2; Mcl‐1, myeloid cell leukemia sequence 1; MEKK, MAP/ERK kinase kinase 1; MICA/B, major histocompatibility complex class I chain‐related chain‐related proteins A and B; P‐gp, P‐glycoprotein; RAB23, Ras‐related protein 23; SDC1, syndecan 1; VE‐cadherin, vascular E‐cadherin; ZEB1, zinc finger E‐boxbinding 1
4.1. Role of miR‐302 cluster in sustaining proliferative signaling in cancer
The number of cells in normal tissue is essentially constant, and its regulation is guaranteed by a number of signaling pathways associated with cell proliferation. Increased cell proliferation is often observed in tumors, and tumor cells alter their biological behavior by promoting or inhibiting proliferative signals.28 That is, tumor cells have the characteristic of sustained proliferation. The miR‐302/367 family can affect the ability of tumor cells to proliferate continuously by regulating the cell cycle and other processes (Figure 3).
Members of this cluster exhibit tumor suppressor activity. In general, the growth ability of epithelial ovarian cancer, ESCC, HCC, BC, and glioma cells can be inhibited by miR‐302b.29, 30, 31, 32 According to previous studies, miR‐302b plays a tumor suppressor role in ESCC by repressing cell proliferation through the posttranscriptional downregulation of ErbB4.26 Similarly, miR‐302c functions as a tumor suppressor in BC by regulating ER to regulate cell proliferation and differentiation.32 The miR‐302/367 family inhibits melanoma and colorectal cancer cells by suppressing proliferation, and Ascl2 knockdown can arrest tumor growth by elevating miR‐302b, which significantly inhibits colon cancer progenitor cell function.33, 34 Evidence from in vivo and in vitro experiments indicates that DOCK4 can restrain glioblastoma progenitor cell proliferation by upregulating nuclear β‐catenin and subsequently increasing the expression of the miR‐302/367 family.8
Although miR‐302 was shown to inhibit clonal cell proliferation, tumor formation, and growth in some studies, other studies reached the opposite conclusion. The miR‐302d/LATS2 axis can promote cell proliferation by targeting the Hippo pathway in cardiomyocytes.35 In addition, upregulation of the miR‐302/367 cluster in PCa has protumorigenic effects in vivo and in vitro through enhanced cell proliferation, sphere formation, and migration through the miR‐302/367/LATS2/YAP pathway.36 However, in another PCa study, miR‐302a functioned as a tumor suppressor by influencing the AKT‐GSK‐3β‐CCND1 and AKT‐p27Kip1 pathways.37 The second PCa study did not verify the conclusions in animal models, and the clinical sample size was not large enough. Therefore, this conclusion needs further verification.
There are many studies on the mechanism of action of these family members in regard to the regulation of cell proliferation. miR‐302a can restrain the proliferation of colon cancer, cervical cancer, melanoma, and colorectal cancer cells.33, 37, 38, 39 Cyclins and CDKs are key regulatory factors that participate in DNA synthesis, chromosome separation, and cell division and are intimately involved in cell cycle regulation. Alterations in the AKT‐GSK‐3β‐CCND1 and AKT‐p27Kip1/p21Cip1 pathways lead to decreased AKT expression through miR‐302/367 family activity in ESCC and cervical cancer.37, 38, 39 MicroRNA‐302b can suppress HCC cell proliferation by targeting the EGFR/AKT2/CCND1 pathway.29, 30 As a novel direct target gene, SDC1 can be downregulated by miR‐302a, leading to the suppression of OC cell proliferation.39 Recent research found that the miR‐302/CDK1 axis can inhibit lung cancer cell proliferation, and this axis can be downregulated by the lncRNA CASC11.40 Another lncRNA, MIAT, can act as an upstream regulator of miR‐302b, promoting the proliferation of BC cells.41 Similarly, in LSCC, miR‐302b‐3p can downregulate IGF‐1R expression to inhibit tumor cell proliferation and migration, and these effects can be rescued by circRASSF2.42 Osteosarcoma cell proliferation can also be inhibited by miR‐302b through effects on cell cycle arrest.43 The miR‐302 cluster can suppress the CCND‐CDK4/6 and CCNE‐CDK2 pathways to regulate iPSC tumorigenicity. In contrast, this cluster can promote p16Ink4a and p14/p19Arf expression to silence BMI, a CSC marker.44 In addition, there is evidence for the tumorigenic activity of this cluster. MicroRNA‐367 can promote medulloblastoma cell proliferation and stem‐like traits by ryanodine receptor 3, integrin alpha‐V, and Ras‐related protein Rab‐23.16 In the abovementioned studies, different experiments reached different conclusions, possibly because the tumor types differed. Ultimately, miRNAs can target different genes in different diseases to exert their respective activities. Some experimental conclusions are derived from only in vitro studies and experimental animal models and have not been validated in large clinical cohorts. These contradictory conclusions need to be further validated by experimentation.
4.2. Role of miR‐302 cluster in activating tumor invasion and metastasis
Tumor cells can directly penetrate the neighboring space in a process called invasion. After a tumor progresses to a certain stage locally, the tumor cells can spread to distant locations through the circulatory system, which involve many signaling pathways.17 The miR‐302/367 family has the potential to alter cancer cell infiltration and metastasis (Figure 3).
MicroRNA‐302a/b/c showed inhibitory effects on the fitness of glioma, melanoma, osteosarcoma, colorectal cancer, BC, and ESCC cells.32, 45 In a clinical study, the expression of these miRNAs was downregulated in human GC, leading to more advanced tumor progression and a worse patient prognosis.46 Another study found that RACK1 downregulation can decrease miR‐302c expression, resulting in increased interleukin‐8 secretion and thereby promoting metastasis.12 As a critical regulator of metastasis, CXCR4 is downregulated at the expression level by miR‐302a, leading to decreased invasion and metastasis abilities of BC cells.45 The role of the miR‐302/367 cluster in BC is complex, and various studies have reached different conclusions. Estrogen receptor was shown by quantitative PCR and western blot analysis to be downregulated by miR‐302c, and luciferase reporter assays confirmed that CXCR4 and ER can be directly targeted by miR‐302c. In addition, the ER pathway can mediate invasion and migration and play an antitumor role.32 The downregulation of miR‐302b by its upstream regulator, MIAT, can also promote BC cell migration.41 High lncRNA ES1 transcript levels in high‐grade and P53‐mutated BC tissues can lead to miR‐302 upregulation and promote cell proliferation and migration.47 In specific BC cell lines, some scholars have found that vitamin C can reduce the reprogramming efficiency of the miR‐302/367 cluster by downregulating TET1 gene expression, which reverses the inhibition of cell invasion and proliferation by this cluster.48 Insulin‐like growth factor‐1R, can be directly targeted by miR‐302a, which plays a tumor suppressor role by inhibiting the invasion and migration of osteosarcoma cells.49 One recent study indicated that miR‐302‐3p can suppress cervical cancer metastasis through actions on its direct target, defective in DCUN1D1.50 In contrast, the circRASSF2/miR‐302b‐3p/IGF‐1R axis is protumorigenic in LSCC.42 Moreover, in ESCC, miR‐302b overexpression can attenuate lymph node metastasis by suppressing ErbB4.26
4.3. Role of miR‐302 cluster in resisting cancer cell death
Tumor cell apoptosis can occur at any stage of tumorigenesis as a mechanism by the host to prevent tumor progression, but abnormal cells can protect themselves from programmed cell death by apoptosis.26 Recent discoveries have shown that the miR‐302/367 cluster participates in signaling pathways that control apoptosis (Figure 3).
Among the increased miRNAs in mucoepidermoid carcinoma, miR‐302a shows the most pronounced change.51 In OC, miR‐302a downregulates SDC1 to increase tumor cell apoptosis.39 All‐trans retinoic acid can upregulate miR‐302b by way of a retinoic acid receptor‐α‐mediated pathway, whereas high miR‐302b expression promotes tumorigenic behaviors by directly targeting and downregulating E2F3, an important transcriptional regulator of glioblastoma proliferation and apoptosis.52 Correspondingly, Akt/pAkt, Bcl‐2, Bim, and caspase‐3 levels can be regulated by miR‐302b to enhance osteosarcoma cell apoptosis.43 One study of malignant pleural mesothelioma indicated that miR‐302b can inhibit tumor cell tumorsphere growth by targeting Mcl‐1 to inhibit proliferation and induce apoptosis.53 Additionally, in HECCs, miR‐302b/c/d was confirmed to promote apoptosis by downregulating zinc‐finger E‐box binding homeobox 1 and Bcl‐2 and upregulating BAX.54
4.4. Role of miR‐302 cluster in inducing tumor angiogenesis
Adequate angiogenesis and unstable vascular permeability provide excellent conditions for tumor survival and metastasis.33 Some studies have found that the miR‐302/367 family is closely related to tumor‐associated angiogenesis.55 (Figure 3).
The miR‐302/367 family could be a promising target for regulating oncogenesis by inhibiting angiogenesis in melanoma and colorectal cancer.33 Mechanistically, miR‐302c can inhibit angiogenesis by regulating EndMT and its direct functional target, MTDH in HCC.56 Moreover, miR‐302 represses the expression of the steroid/thyroid hormone receptor NR2F2, also known as COUPTFII, to inhibit angiogenesis.23 In‐depth research found that the miR‐302/367 cluster can limit tumor growth by restricting neovascularization and improving vascular stability through the Erk1/2‐Klf2‐S1pr1/VE‐cadherin pathway.55
4.5. Role of miR‐302 cluster in protumorigenic inflammation
Immune‐related molecules are important players in neoplastic disease. During tumor formation and progression, some miRNAs are linked to the immune response. Certain miRNAs are involved in the immune network controlled by estrogen.57 MicroRNA‐302c can regulate interferon expression induced by influenza A virus and downregulate innate immune responses induced by enterovirus 71 by targeting KPNA2, which is intracellularly associated with JNK1/JNK2 and p38. This finding indicates that miR‐302c has immunomodulatory function in cancer.58 According to some researchers, miR‐302c downregulation can enhance the sensitivity of cancer cells to NK cell‐mediated cytotoxicity; 1,25‐(OH)2D3 was confirmed to participate in this process, in which the NKG2D ligands MICA/B and ULBP2 are upregulated59 (Figure 3).
4.6. Role of miR‐302 cluster in evading growth suppressors in cancer
Cancer cells can induce and maintain positive growth‐stimulating signals and evade growth‐suppressive signals, in which the miR‐302 cluster is involved. Epithelial‐mesenchymal transition and cell reprogramming are effective means by which tumors evade growth inhibition. Several molecular mechanisms, including miRNA regulation, might directly and/or indirectly lead to phenotypic variations in tumor cell plasticity.19, 33, 56, 60 Metastatic melanoma cells can be epigenetically reprogrammed in an embryonic microenvironment through changes in the expression of miR‐302a and miR‐27b, suggesting that the metastatic phenotype and plasticity of tumor cells can be regulated by these 2 miRNAs.19 The upregulation of the miR‐302 family in A‐375 melanoma and HT‐29 colorectal cancer cells reverses EMT through a reprogramming process.33 Similarly, the EC‐specific miR‐302c inhibits HCC growth and angiogenesis by regulating EndMT through MTDH.56 The upregulation of miR‐302 by lncRNA ES1 promotes EMT in BC cells.47 Jhdm1a/1b, a vitamin C‐dependent H3K36 demethylase, can cooperate with Oct4 to activate the miR‐302/367 cluster, leading to cell reprogramming.61 In addition, a study found that miR‐302 can increase the efficiency of reprogramming through the repression of NR2F2,23 while some scholars found that vitamin C can reduce the reprogramming efficiency of the miR‐302/367 cluster by downregulating TET1 gene expression in specific BC cell lines.48 The miR‐302/367 cluster can promote somatic cell reprogramming and induce dedifferentiation into PSCs.2, 62, 63 In the initial stage of reprogramming to generate iPSCs, the miR‐302/367 cluster efficiently induces EMT and promotes cell morphological changes.2 The transformation of human adipose‐derived stem cells into iPSCs was more efficient in the presence of miR‐302.60 For example, ESC markers such as Nanog, OCT3/4, SOX2, SSEA‐3, and SSEA‐4 are detected in miR‐302‐transfected skin cancer cells, indicating that miR‐302 has the ability to gradually and continuously reprogram tumor cells into an ESC‐like pluripotent state.4 In addition, the miR‐302/367 cluster can effectively reprogram human fibroblasts into functional neurons.64
4.7. Other effects of miR‐302/367 cluster
In addition to the above regulatory effects, the miR‐302/367 family also affects cancer cell behavior by regulating cell resistance to chemotherapeutic drugs/radiotherapy and cellular energy metabolism (Figure 4).
Figure 4.
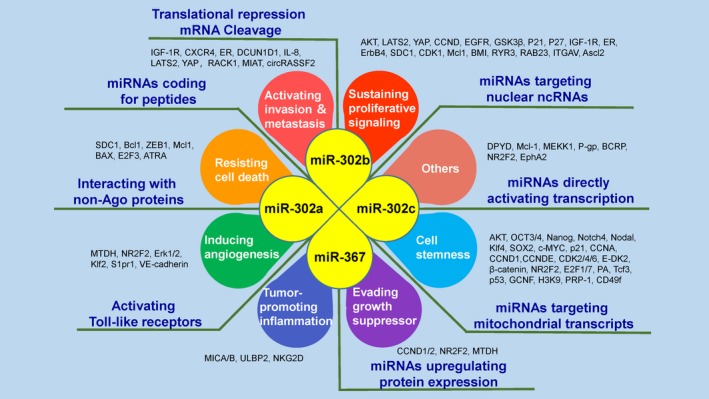
MicroRNA (miR‐302/367 cluster regulates tumorigenesis and development through multiple mechanisms. The miR‐302/367 cluster has been implicated in several of the hallmarks of cancer through interactions with various molecules and pathways, and the members of this cluster function by classical and noncanonical mechanisms of miRNA action. Through the networks that regulate the above characteristics, this cluster can affect the biological behaviors of tumor cells and induce different outcomes of cancer cells. As numerous hallmarks of cancer are regulated by the miR‐302/367 cluster, there are a variety of candidates for cancer treatment that involve different molecular targets and strategies. ATRA, All‐trans retinoic acid; BMI, B cell‐specific Moloney murine leukemia virus integration site 1; CCND, cyclin D; CDK, cyclin‐dependent kinase; CXCR4, C‐X‐C chemokine receptor type 4; DCUN1D1, cullin neddylation 1 domain containing 1; E2F3, E2 transcription factor 3; EGFR, epidermal growth factor recptor; ER, estrogen receptor; ErbB4, Receptor tyrosine‐protein kinase erbB‐4; GCNF, germ cell nuclear factor; GSK‐3β, glycogen synthase kinase‐3β; IGF‐1R, insulin‐like growth factor 1 receptor; IL‐8, interleukin‐8; ITGAV, Integrin alpha‐V; Klf2, Kruppel like factor 2; LATS2, large tumor suppressor kinase 2; Mcl‐1, myeloid cell leukemia sequence 1; MEKK, MAP/ERK kinase kinase 1; MIAT, myocardial infarction‐associated transcript; MICA/B, major histocompatibility complex class I chain‐related proteins A and B; MTDH, metadherin; NR2F2, nuclear receptor subfamily 2 group F member 2; PRP‐1, proline‐rich polypeptide 1; RAB23, Ras‐related protein 23; RACK1, receptor for activated C‐kinase 1; RYR3, Ryanodine receptor 3; SDC1, syndecan 1; VE‐cadherin, vascular E‐cadherin; YAP, Yes‐associated transcriptional regulator; ULBP2, UL16‐binding protein 2; ZEB1, zinc finger E‐boxbinding 1
First, miRNAs play important roles in modulating cell sensitivity to chemotherapeutic drugs. Studies have found that miR‐302a/b can enhance the drug susceptibility of testicular and OC cells.65 MicroRNA‐302b can promote HCC cell resistance to human adipose‐derived stem cells through 2 possible targets, Mcl‐1 and dihydropyrimidine dehydrogenase.66 Low miR‐302s expression is found in BC cells, especially in those resistant to chemotherapy.67, 68 The MAP/ERK kinase kinase 1 can be cooperatively inhibited by miR‐302s to suppress P‐gp, leading to an increased susceptibility of BC cells to adriamycin.67 Expression of BCRP can also be cooperatively inhibited by miR‐302s to increase the sensitivity of BC cells to mitoxantrone.69 Some reports stated that miR‐302 overexpression enhances the sensitivity of drug‐resistant hepatoma cells. Testicular germ cell tumors showed increased miR‐302a expression when treated with cisplatin, leading to increased drug sensitivity.65 The antiosteosarcoma effect of epirubicin can be enhanced by miR‐302b.43 Second, according to clinical results, different miRNAs show different responses to preoperative radiochemotherapy in locally advanced GC patients,70 indicating the potential application of microarray technology in candidates’ selection based on radio/chemotherapy sensitivity. Finally, NR2F2, which can regulate development, differentiation and metabolism, can be repressed by the miR‐302 clusters, which provides a mechanism for the deregulation of cellular energetics in cancer cells.23 An inverse correlation between miR‐302b and EphA2 expression was identified in GC, but the underlying mechanism is not clear.
5. MICRORNA‐302 CLUSTER IS AN EMERGING POTENTIAL BIOMARKER OR TARGET FOR CANCER THERAPY
The functions of miRNAs in various tumors have been widely recognized, and basic research on this topic has rapidly progressed. A miR‐34 mimic was made into a miRNA analogue drug called MRX34, which recently entered phase I clinical trials with the aim of treating cancer patients with liver involvement.71 This trial will provide reference data for the further clinical application of miR‐302/367‐based therapeutics. The miR‐302 cluster is involved in most biological processes and could provide many promising biomarkers or therapeutic targets for neoplastic disease. All of these different mechanisms provide directions for studying the biological significance of the miR‐302 family in specific tumors and strategies for clinical use (Figure 4).
The technology for extracting miRNA from serum is very well developed.72 The simultaneous downregulation of miRNA‐302a/d and overexpression of E2F7 showed a potential correlation with poor median overall survival and progression‐free survival in HCC patients.73 As highly sensitive and specific miRNAs, miR‐367‐3p and miR‐371 are good diagnostic factors for malignant germ cell tumors, including seminomas and embryonal carcinomas.74
Compared with traditional tumor markers, the serum levels of the miR‐302/367 family members, which include stem cell‐associated miRNAs, have significant advantages in sensitivity and specificity in diagnosing some patients with metastatic cancer.75 Exosomes are a novel research tool for studying the mechanisms of miRNAs in tumors, and exosomal miRNAs can predict the unique characteristics of some neoplasms.76 Therefore, exosomal miRNAs could potentially predict the disease stage, forecast the prognosis, and guide the treatment of advanced carcinomas. Given the tumor suppressor properties of the miR‐302/367 cluster, some researchers overexpressed miR‐302/367 in mice; these miRNAs were secreted in exosomes, which were then absorbed by tumor cells. This strategy effectively altered glioblastoma progression in the brains of mice.15
Many tumors show rapid progression and poor prognosis, and current methods have limited efficacy for aggressive carcinomas. As the miR‐302/367 family is involved in nearly the entire process of tumor development in various neoplasms and exerts different regulatory functions, these family members can be used to detect or treat cancer in numerous ways. MicroRNA‐302/367 levels could indicate tumor progression, and mimics or corresponding regulatory factors can be used to therapeutically regulate the various biological characteristics of tumor cells at different stages. Reprogramming tumor cells with miRNAs, for example, could provide a new way to treat cancer, and this approach may have additional advantages in more aggressive tumors.33 Chemically modified antisense oligonucleotides could efficiently and specifically inhibit miRNA function. Accordingly, miRNA‐targeted antisense oligonucleotides are novel therapies for several neoplastic diseases.77 The downregulation of miRNAs in some metastatic tumors indicates that miRNA mimics could be effective therapeutics for inhibiting cancer and preventing metastasis.45 Exogenous miR‐302a has shown potential therapeutic value in treating OC.39 Recent studies found that somatic pluripotency was more efficiently induced through the miR‐302/367 family without the need for exogenous transcription factors than through the standard approach involving OCT4/SOX2/KLF4/MYC. Basic research in an orthotopic xenograft mouse model of glioblastoma showed that cell‐based therapy with miR‐302/367‐expressing cells inhibits tumor growth.15 Currently, researchers can generate high‐quality iPSCs from most fibroblast sources by transfection with reprogramming‐modified mRNAs and mature ESC‐specific miR‐367/302 mimics.78 Cancer stem cells also represent a new approach to understanding the origin of cancer. Cancer cells transfected with miR‐302s, also called mirPS cells, offer new options for blocking cancer progression.4 Furthermore, this cluster offers new options for antiangiogenic therapy in cancer.56 As miRNA array technology can be used to screen miRNAs related to radiochemotherapy sensitivity in many cancers, it can provide biomarkers for miRNA‐targeted therapy and personalized radiochemotherapy.70 A synthetic RNA‐based CRISPR‐Cas9 system that is responsive to the miR‐Cas9 switch is under development.79
6. CONCLUSION AND PERSPECTIVE
The miR‐302 cluster shows multimodal tumor suppression in many cancers and is critically important in regulating cell stemness, multiple hallmarks of cancer cells, the cell response to chemotherapy drugs, and tumor immunity in classic and alternative ways, suggesting that this miRNA cluster has great value in the detection and treatment of neoplastic disease.
After the corresponding specificity is obtained, the circulating miR‐302/367 family will have considerable value in disease diagnosis and prognosis and in monitoring disease progression. In recent experiments, the malignant biological characteristics of some cancer cells have been successfully changed by regulating the miR302/367 cluster in vitro, which has laid a foundation for the clinical application of miRNAs. Viral vector transduction and miRNA synthesis (mimic or inhibitor) techniques can be used to overexpress or inhibit intracellular miRNA expression. Cell‐based therapy offers an alternative approach to miR‐302/367 cluster‐related treatment. MicroRNA mimics are promising for clinical use, but further study is needed to determine the mechanism of uptake by tumor cells in a patient’s body and how they function within tumor cells. As the miR‐302/367 family can increase the sensitivity of some tumor cells to radiotherapy or chemotherapy, the corresponding miRNA treatments could be applied before these therapies.
The miR‐302/367 family has been shown to efficiently reprogram human somatic cells to iPSCs; however, the safety and controllability of differentiation potential pose challenges in this kind of mirPS cell therapy for cancer. The new miR‐Cas9 switch technique has potential in the clinical application of this cluster. Further studies are necessary to verify the potential, feasibility, and safety of such treatments. Because the miR‐302/367 family has diverse expression patterns and specific tumor characteristics in different cancers at different stages, and the resulting biological behavior is dictated by interactions among complex target genes, it is of vital importance to identify the target genes and node proteins of this cluster. Therefore, a stable, safe, feasible, and convenient protocol is required for the application of the miR‐302/367 cluster as a promising biomarker and therapeutic.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENTS
This project was supported by grants from the National Natural Science Foundations of China (Nos. 81870775 and 81500855) and the Program for Innovation Team Building at Institutions of Higher Education in Chongqing in 2016.
Liu J, Wang Y, Ji P, Jin X. Application of the microRNA‐302/367 cluster in cancer therapy. Cancer Sci. 2020;111:1065–1075. 10.1111/cas.14317
REFERENCES
- 1. Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622‐638. [DOI] [PubMed] [Google Scholar]
- 2. Subramanyam D, Lamouille S, Judson RL, et al. Multiple targets of miR‐302 and miR‐372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29:443‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen L, Heikkinen L, Emily KK, Liang Y, Wong G. Evolutionary conservation and function of the human embryonic stem cell specific miR‐302/367 cluster. Comp Biochem Physiol Part D Genomics Proteomics. 2015;16:83‐98. [DOI] [PubMed] [Google Scholar]
- 4. Lin SL, Chang DC, Chang‐Lin S, et al. Mir‐302 reprograms human skin cancer cells into a pluripotent ES‐cell‐like state. RNA. 2008;14:2115‐2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pallocca G, Fabbri M, Sacco MG, et al. miRNA expression profiling in a human stem cell‐based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol. 2013;29:239‐257. [DOI] [PubMed] [Google Scholar]
- 6. Ali HRSM, Bavarsad MS, Arefian E, Jaseb K, Shahjahani M, Saki N. The Role of microRNAs in Stemness of Cancer Stem Cells. Oncol Rev. 2013;7:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jamshidi‐Adegani F, Langroudi L, Shafiee A, et al. Mir‐302 cluster exhibits tumor suppressor properties on human unrestricted somatic stem cells. Tumour Biol. 2014;35:6657‐6664. [DOI] [PubMed] [Google Scholar]
- 8. Debruyne DN, Turchi L, Burel‐Vandenbos F, et al. DOCK4 promotes loss of proliferation in glioblastoma progenitor cells through nuclear beta‐catenin accumulation and subsequent miR‐302‐367 cluster expression. Oncogene. 2018;37:241‐254. [DOI] [PubMed] [Google Scholar]
- 9. Galoian K, Qureshi A, D'Ippolito G, et al. Epigenetic regulation of embryonic stem cell marker miR302C in human chondrosarcoma as determinant of antiproliferative activity of proline‐rich polypeptide 1. Int J Oncol. 2015;47:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teo AK, Arnold SJ, Trotter MW, et al. Pluripotency factors regulate definitive endoderm specification through eomesodermin. Genes Dev. 2011;25:238‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bräutigam C, Raggioli A, Winter J. The Wnt/β‐catenin pathway regulates the expression of the miR‐302 cluster in mouse ESCs and P19 cells. PLoS ONE. 2013;8:e75315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen L, Min L, Wang X, et al. Loss of RACK1 Promotes Metastasis of Gastric Cancer by Inducing a miR‐302c/IL8 Signaling Loop. Cancer Res. 2015;75:3832‐3841. [DOI] [PubMed] [Google Scholar]
- 13. Wang H, Wang X, Archer TK, Zwaka TP, Cooney AJ. GCNF‐dependent activation of cyclin D1 expression via repression of Mir302a during ESC differentiation. Stem Cells. 2014;32:1527‐1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Oho AUID, Liu L, et al. miRspongeR: an R/Bioconductor package for the identification and analysis of miRNA sponge interaction networks and modules. BMC Bioinformatics. 2019;20:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fareh M, Almairac F, Turchi L, et al. Cell‐based therapy using miR‐302‐367 expressing cells represses glioblastoma growth. Cell Death Dis. 2017;8:e2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaid C, Silva PB, Cortez BA, Rodini CO, Semedo‐Kuriki P, Okamoto OK. miR‐367 promotes proliferation and stem‐like traits in medulloblastoma cells. Cancer Sci. 2015;106:1188‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fogel GB, Kai ZS, Zargar S, et al. MicroRNA dynamics during human embryonic stem cell differentiation to pancreatic endoderm. Gene. 2015;574:359‐370. [DOI] [PubMed] [Google Scholar]
- 18. Barroso‐del JA, Lucena‐Aguilar G, Menendez P. The miR‐302‐367 cluster as a potential stemness regulator in ESCs. Cell Cycle. 2009;8:394‐398. [DOI] [PubMed] [Google Scholar]
- 19. Costa FF, Seftor EA, Bischof JM, et al. Epigenetically reprogramming metastatic tumor cells with an embryonic microenvironment. Epigenomics. 2009;1:387‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeong HS, Lee JM, Suresh B, Cho KW, Jung HS, Kim KS. Temporal and spatial expression patterns of miR‐302 and miR‐367 during early embryonic chick development. Int J Stem Cells. 2014;7:162‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin SL, Ying SY. Mechanism and method for generating tumor‐free iPS cells using intronic MicroRNA miR‐302 induction. Methods Mol Biol. 2018;1733:265‐282. [DOI] [PubMed] [Google Scholar]
- 22. Liu Z, Zhang C, Skamagki M, et al. Elevated p53 Activities Restrict Differentiation Potential of MicroRNA‐Deficient Pluripotent Stem Cells. Stem Cell Reports. 2017;9:1604‐1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu S, Wilson KD, Ghosh Z, et al. MicroRNA‐302 increases reprogramming efficiency via repression of NR2F2. Stem Cells. 2013;31:259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu H, Zheng L, Wang L, Tang F, Hua J. MiR‐302 enhances the viability and stemness of male germline stem cells. Reprod Domest Anim. 2018;53(6):1580‐1588. [DOI] [PubMed] [Google Scholar]
- 25. Li HL, Wei JF, Fan LY, et al. miR‐302 regulates pluripotency, teratoma formation and differentiation in stem cells via an AKT1/OCT4‐dependent manner. Cell Death Dis. 2016;7:e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang M, Yang Q, Zhang L, et al. miR‐302b is a potential molecular marker of esophageal squamous cell carcinoma and functions as a tumor suppressor by targeting ErbB4. J Exp Clin Cancer Res. 2014;33:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma YS, Lv ZW, Yu F, et al. MicroRNA‐302a/d inhibits the self‐renewal capability and cell cycle entry of liver cancer stem cells by targeting the E2F7/AKT axis. J Exp Clin Cancer Res. 2018;37:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 29. Wang L, Yao J, Shi X, et al. MicroRNA‐302b suppresses cell proliferation by targeting EGFR in human hepatocellular carcinoma SMMC‐7721 cells. BMC Cancer. 2013;13:448. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Wang L, Yao J, Zhang X, et al. miRNA‐302b suppresses human hepatocellular carcinoma by targeting AKT2. Mol Cancer Res. 2014;12:190‐202. [DOI] [PubMed] [Google Scholar]
- 31. Ge T, Yin M, Yang M, Liu T, Lou G. MicroRNA‐302b suppresses human epithelial ovarian cancer cell growth by targeting RUNX1. Cell Physiol Biochem. 2014;34:2209‐2220. [DOI] [PubMed] [Google Scholar]
- 32. Leivonen SK, Mäkelä R, Ostling P, et al. Protein lysate microarray analysis to identify microRNAs regulating estrogen receptor signaling in breast cancer cell lines. Oncogene. 2009;28:3926‐3936. [DOI] [PubMed] [Google Scholar]
- 33. Maadi H, Moshtaghian A, Taha MF, et al. Multimodal tumor suppression by miR‐302 cluster in melanoma and colon cancer. Int J Biochem Cell Biol. 2016;81:121‐132. [DOI] [PubMed] [Google Scholar]
- 34. Zhu R, Yang Y, Tian Y, et al. Ascl2 knockdown results in tumor growth arrest by miRNA‐302b‐related inhibition of colon cancer progenitor cells. PLoS ONE. 2012;7:e32170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu F, Yang J, Shang J, et al. MicroRNA‐302d promotes the proliferation of human pluripotent stem cell‐derived cardiomyocytes by inhibiting LATS2 in the Hippo pathway. Clin Sci. 2019;133:1387‐1399. [DOI] [PubMed] [Google Scholar]
- 36. Guo Y, Cui J, Ji Z, et al. miR‐302/367/LATS2/YAP pathway is essential for prostate tumor‐propagating cells and promotes the development of castration resistance. Oncogene. 2017;36:6336‐6347. [DOI] [PubMed] [Google Scholar]
- 37. Zhang GM, Bao CY, Wan FN, et al. MicroRNA‐302a Suppresses Tumor Cell Proliferation by Inhibiting AKT in Prostate Cancer. PLoS ONE. 2015;10:e0124410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cai N, Wang YD, Zheng PS. The microRNA‐302‐367 cluster suppresses the proliferation of cervical carcinoma cells through the novel target AKT1. RNA. 2013;19:85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo T, Yu W, Lv S, Zhang C, Tian Y. MiR‐302a inhibits the tumorigenicity of ovarian cancer cells by suppression of SDC1. Int J Clin Exp Pathol. 2015;8:4869‐4880. [PMC free article] [PubMed] [Google Scholar]
- 40. Tong W, Han TC, Wang W, Zhao J. LncRNA CASC11 promotes the development of lung cancer through targeting microRNA‐302/CDK1 axis. Eur Rev Med Pharmacol Sci. 2019;23:6539‐6547. [DOI] [PubMed] [Google Scholar]
- 41. Alipoor FJ, Asadi MH, Torkzadeh‐Mahani M. MIAT lncRNA is overexpressed in breast cancer and its inhibition triggers senescence and G1 arrest in MCF7 cell line. J Cell Biochem. 2018;119:6470‐6481. [DOI] [PubMed] [Google Scholar]
- 42. Tian L, Cao J, Jiao H, et al. CircRASSF2 promotes laryngeal squamous cell carcinoma progression by regulating the miR‐302b‐3p/IGF‐1R axis. Clin Sci. 2019;133:1053‐1066. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Hu H, Song L, Cai L, Wei R, Jin W. Epirubicin‐mediated expression of miR‐302b is involved in osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett. 2013;222:1‐9. [DOI] [PubMed] [Google Scholar]
- 44. Ying S. MicroRNA Protocols 3rd ed. New York, NY: Humana Press; 2018: 265. [Google Scholar]
- 45. Liang Z, Bian X, Shim H. Inhibition of breast cancer metastasis with microRNA‐302a by downregulation of CXCR4 expression. Breast Cancer Res Treat. 2014;146:535‐542. [DOI] [PubMed] [Google Scholar]
- 46. Ma G, Li Q, Dai W, Yang X, Sang A. Prognostic implications of miR‐302a/b/c/d in human gastric cancer. Pathol Oncol Res. 2017;23:899‐905. [DOI] [PubMed] [Google Scholar]
- 47. Keshavarz M, Asadi MH. Long non‐coding RNA ES1 controls the proliferation of breast cancer cells by regulating the Oct4/Sox2/miR‐302 axis. FEBS J. 2019;286:2611‐2623. [DOI] [PubMed] [Google Scholar]
- 48. Ramezankhani B, Taha MF, Oho AUID, Javeri A, AUID‐ Oho. Vitamin C counteracts miR‐302/367‐induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J Cell Physiol. 2019;234:2672‐2682. [DOI] [PubMed] [Google Scholar]
- 49. Zhang C, Song G, Ye W, Xu B. MicroRNA‐302a inhibits osteosarcoma cell migration and invasion by directly targeting IGF‐1R. Oncol Lett. 2018;15:5577‐5583. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Jiang Y, Hou R, Li S, Li S, Dang G. MicroRNA‐302 inhibits cell migration and invasion in cervical cancer by targeting DCUN1D1. Exp Ther Med. 2018;16:1000‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Binmadi NO, Basile JR, Perez P, et al. miRNA expression profile of mucoepidermoid carcinoma. Oral Dis. 2018;24:537‐543. [DOI] [PubMed] [Google Scholar]
- 52. Chen PH, Shih CM, Chang WC, et al. MicroRNA‐302b‐inhibited E2F3 transcription factor is related to all trans retinoic acid‐induced glioma cell apoptosis. J Neurochem. 2014;131:731‐742. [DOI] [PubMed] [Google Scholar]
- 53. Khodayari N, Mohammed KA, Lee H, Kaye F, Nasreen N. MicroRNA‐302b targets Mcl‐1 and inhibits cell proliferation and induces apoptosis in malignant pleural mesothelioma cells. Am J Cancer Res. 2016;6:1996‐2009. [PMC free article] [PubMed] [Google Scholar]
- 54. Li Y, Huo J, Pan X, Wang C, Ma X. MicroRNA 302b–3p/302c‐3p/302d‐3p inhibits epithelial‐mesenchymal transition and promotes apoptosis in human endometrial carcinoma cells. Onco Targets Ther. 2018;11:1275‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pi J, Tao T, Zhuang T, et al. A MicroRNA302‐367‐Erk1/2‐Klf2‐S1pr1 pathway prevents tumor growth via restricting angiogenesis and improving vascular stability. Circ Res. 2017;120:85‐98. [DOI] [PubMed] [Google Scholar]
- 56. Zhu K, Pan Q, Jia LQ, et al. MiR‐302c inhibits tumor growth of hepatocellular carcinoma by suppressing the endothelial‐mesenchymal transition of endothelial cells. Sci Rep. 2014;4:5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burgos‐Aceves MA, Cohen A, Smith Y, Faggio C. A potential microRNA regulation of immune‐related genes in invertebrate haemocytes. Sci Total Environ. 2018;621:302‐307. [DOI] [PubMed] [Google Scholar]
- 58. Peng N, Yang X, Zhu C, et al. MicroRNA‐302 Cluster Downregulates Enterovirus 71‐Induced Innate Immune Response by Targeting KPNA2. J Immunol. 2018;201:145‐156. [DOI] [PubMed] [Google Scholar]
- 59. Min D, Lv XB, Wang X, et al. Downregulation of miR‐302c and miR‐520c by 1,25(OH)2D3 treatment enhances the susceptibility of tumour cells to natural killer cell‐mediated cytotoxicity. Br J Cancer. 2013;109:723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miyazaki S, Yamamoto H, Miyoshi N, et al. A Cancer Reprogramming Method Using MicroRNAs as a Novel Therapeutic Approach against Colon Cancer: Research for Reprogramming of Cancer Cells by MicroRNAs. Ann Surg Oncol. 2015;22(Suppl 3):S1394‐S1401. [DOI] [PubMed] [Google Scholar]
- 61. Wang T, Chen K, Zeng X, et al. The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin‐C‐dependent manner. Cell Stem Cell. 2011;9:575‐587. [DOI] [PubMed] [Google Scholar]
- 62. Subramanyam D, Blelloch R. From microRNAs to targets: pathway discovery in cell fate transitions. Curr Opin Genet Dev. 2011;21:498‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Onder TT, Daley GQ. microRNAs become macro players in somatic cell reprogramming. Genome Med. 2011;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhou C, Gu H, Fan R, Wang B, Lou J. MicroRNA 302/367 cluster effectively facilitates direct reprogramming from human fibroblasts into functional neurons. Stem Cells Dev. 2015;24:2746‐2755. [DOI] [PubMed] [Google Scholar]
- 65. Liu L, Lian J, Zhang H, et al. MicroRNA‐302a sensitizes testicular embryonal carcinoma cells to cisplatin‐induced cell death. J Cell Physiol. 2013;228:2294‐2304. [DOI] [PubMed] [Google Scholar]
- 66. Cai D, He K, Chang S, Tong D, Huang C. MicroRNA‐302b Enhances the Sensitivity of Hepatocellular Carcinoma Cell Lines to 5‐FU via Targeting Mcl‐1 and DPYD. Int J Mol Sci. 2015;16:23668‐23682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhao L, Wang Y, Jiang L, et al. MiR‐302a/b/c/d cooperatively sensitizes breast cancer cells to adriamycin via suppressing P‐glycoprotein(P‐gp) by targeting MAP/ERK kinase kinase 1 (MEKK1). J Exp Clin Cancer Res. 2016;35:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liang Z, Ahn J, Guo D, Votaw JR, Shim H. MicroRNA‐302 replacement therapy sensitizes breast cancer cells to ionizing radiation. Pharm Res. 2013;30:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang Y, Zhao L, Xiao Q, et al. miR‐302a/b/c/d cooperatively inhibit BCRP expression to increase drug sensitivity in breast cancer cells. Gynecol Oncol. 2016;141:592‐601. [DOI] [PubMed] [Google Scholar]
- 70. Liu X, Cai H, Sheng W, Huang H, Long Z, Wang Y. microRNAs expression profile related with response to preoperative radiochemotherapy in patients with locally advanced gastric cancer. BMC Cancer. 2018;18:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31:577. [DOI] [PubMed] [Google Scholar]
- 72. Gebremedhn S, Salilew‐Wondim D, Hoelker M, et al. Exploring maternal serum microRNAs during early pregnancy in cattle. Theriogenology. 2018;121:196‐203. [DOI] [PubMed] [Google Scholar]
- 73. Kaur S, Abu‐Shahba AG, Paananen RO, et al. Small non‐coding RNA landscape of extracellular vesicles from human stem cells. Sci Rep. 2018;8:15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13:715‐725. [DOI] [PubMed] [Google Scholar]
- 75. Terbuch A, Adiprasito JB, Stiegelbauer V, et al. MiR‐371a‐3p serum levels are increased in recurrence of testicular germ cell tumor patients. Int J Mol Sci. 2018; 19(10):3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu LX, Zhang BL, Yang Y, et al. Exosomal microRNAs as potential biomarkers for cancer cell migration and prognosis in hepatocellular carcinoma patient‐derived cell models. Oncol Rep. 2019;41:257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Balzano F, Cruciani S, Basoli V, et al. MiR200 and miR302: Two Big Families Influencing Stem Cell Behavior. Molecules. 2018;23(2):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McGrath PS, Diette N, Kogut I, Bilousova G. RNA‐based reprogramming of human primary fibroblasts into induced pluripotent stem cells. J Vis Exp. 2018;26:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hirosawa M, Fujita Y, Parr CJC, et al. Cell‐type‐specific genome editing with a microRNA‐responsive CRISPR‐Cas9 switch. Nucleic Acids Res. 2017;45:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]


