Summary
Background
The Food and Drug Administration has approved several pharmacotherapies for the treatment of obesity. This study assesses the cost‐effectiveness of six pharmacotherapies and lifestyle intervention for people with mild obesity (body mass indices [BMIs] 30 to 35).
Methods
A microsimulation model was constructed to compare seven weight loss strategies plus no treatment: intensive lifestyle intervention, orlistat, phentermine, phentermine/topiramate, lorcaserin, liraglutide, and semaglutide. Weight loss, quality‐of‐life scores, and costs were estimated using clinical trials and other published literature. Endpoints included costs, quality‐adjusted life years (QALYs), and incremental cost‐effectiveness ratios (ICERs) with a willingness‐to‐pay (WTP) threshold of $100 000/QALY. Results were analysed at 1‐, 3‐, and 5‐year time horizons.
Results
At each of the three follow‐up periods, phentermine was the cost‐effective strategy, with ICERs of $46 258/QALY, $20 157/QALY, and $17 880/QALY after 1, 3, and 5 years, respectively. Semaglutide was the most effective strategy in the 3‐ and 5‐year time horizons, with total QALYs of 2.224 and 3.711, respectively. However, the ICERs were prohibitively high at $1 437 340/QALY after 3 years and $576 931/QALY after 5 years. Deterministic and probabilistic sensitivity analyses indicated these results were robust.
Conclusions
Phentermine is the cost‐effective pharmacologic weight‐loss strategy. Although semaglutide is the most effective, it is not cost‐effective because of its high price.
Keywords: cost‐effectiveness analysis, obesity, pharmacotherapy, weight loss
1. INTRODUCTION
An estimated 70% of the population in the United States have overweight or obesity, a threefold increase over the last 40 years.1 Worldwide, an estimated 650 million adults live with overweight or obesity.2 With the large population of affected individuals, the economic costs of obesity are substantial. The cost of medical care related to obesity in adults is estimated at $85.7 billion in the United States3 but could be as much as $209.7 billion.4 On a global level, obesity‐related complications are estimated to cost $1.2 trillion by 2025, almost half of which will be spent in the United States alone.5 Weight reduction of as little as 5% in individuals with obesity is associated with improved health outcomes and reduced incidence of obesity‐related comorbidities, including cardiovascular disease and diabetes.6 Effective treatments and interventions are crucial but remain elusive.
Lifestyle intervention, with diet, physical activity, and behaviour modification, is the standard first‐line therapy for overweight and obesity. However, adaptive physiologic responses, such as increased appetite and decreased resting metabolic rate, make it difficult to maintain weight loss through lifestyle intervention alone.7 After initial weight loss in the first year, weight regain occurs at an average rate of 1 to 2 kg y−1.6, 8 For this reason, individuals with a body mass index (BMI) of at least 30 kg m−2 or a BMI of at least 27 kg m−2 with weight‐related comorbidities are eligible for pharmacotherapy.7, 9
The Food and Drug Administration (FDA) has approved several pharmacotherapies to treat overweight and obesity. As more agents enter the market, comparative efficacy and economic burdens are difficult to discern. Randomized, placebo‐controlled trials for orlistat, lorcaserin, liraglutide, and phentermine/topiramate have shown promising results for individuals with obesity, leading to FDA approval. In each trial, the majority of participants lost 5% to 10% of their baseline weight within the first year.10, 11, 12, 13
Phentermine and semaglutide are not FDA approved for long‐term treatment of obesity. However, both medications demonstrate efficacy and increasing use within the medical community.5, 14, 15 Phentermine is the most commonly prescribed pharmacotherapy for weight‐loss in the United States, despite FDA approval for only short‐term use (90 days) and a lack of large, long‐term clinical trials.15, 16, 17 In a randomized, placebo‐controlled trial, individuals with overweight or obesity lost an average of 9.3% of baseline body weight after 14 weeks.18 Once‐weekly semaglutide is FDA approved only as treatment for type 2 diabetes. However, a recent phase 2, randomized, double‐blind, placebo and active controlled trial with nondiabetic individuals found promising results for daily semaglutide as obesity treatment (n = 957). Results indicated that semaglutide doses of 0.2 mg d−1 or more significantly increased weight loss compared with both liraglutide (3.0 mg d−1) and placebo.5 In addition, a recent randomized, placebo‐controlled trial demonstrated that diabetic patients on semaglutide can sustain weight loss for at least 2 years.19
A clinical trial comparing all available pharmacotherapies for obesity is unlikely to occur in the future for various reasons, including the high costs and large sample sizes that would be required. The purpose of this study is to compare and analyse the cost‐effectiveness of six pharmacotherapies and intensive lifestyle intervention in patients with mild obesity (BMI between 30 and 35). It builds on previous cost‐effectiveness analyses20, 21 by focusing exclusively on pharmacotherapy treatment for a specific population and including phentermine and semaglutide. In addition, this analysis follows patients on long‐term treatment (over 1 year), in accordance with the increasing recognition of obesity as a chronic, relapsing medical disease.22
2. METHODS
2.1. Model overview
A microsimulation model was developed using Python 3.6.5 to assess the cost‐effectiveness of seven strategies plus no treatment: intensive lifestyle intervention (ILI), phentermine/topiramate (7.5/46 mg daily), liraglutide (3.0 mg daily), semaglutide (0.4 mg daily), orlistat (120 mg TID), lorcaserin (10 mg BID), and phentermine (37.5 mg daily). In addition, a scenario analysis that excludes phentermine was performed since phentermine is not FDA approved for long‐term treatment. Liraglutide and semaglutide are subcutaneous injections, while all other pharmacotherapies are oral. For the base case, 100 000 patients were modelled, with 75% females and initial age of 40 based on patient populations in the clinical trials. Initial BMI was 32.5 kg m−2, representing the midpoint BMI within the mildly obese classification (BMIs 30 to 35 kg m−2). Initial quality of life (QOL) was 0.720, based on estimates for people with mild obesity in a previous study.23
The model extended to 1, 3, and 5 years in order to estimate weight loss maintenance. Modelled patients could remain on active treatment for the duration of the modelled time horizon. Alternatively, patients could drop out of treatment or die from all causes. Dropout rates were estimated from the proportion of patients who dropped out of the respective clinical trials (Table 1). The therapy adherence rate was assumed to be constant for years 2 to 5. Mortality rates were estimated using BMI‐specific life tables from a previous analysis on the impact of obesity on mortality (Table S1).39 The model cycle length, or time between state transitions, was 1 month. All model inputs are listed in Table 1.
Table 1.
Base case model inputs
| No Treatment | ILI | Phentermine | Phentermine/Topiramate | Liraglutide | Orlistat | Lorcaserin | Semaglutide | |
|---|---|---|---|---|---|---|---|---|
| Monthly ΔBMI, year 1 | 0.012724 | −0.2525, 26, 27 | −0.5214 | −0.3128 | −0.2529 | −0.2630 | −0.2210 | −0.455 |
| Monthly ΔBMI, years 2 to 5 | 0.012724 | 0.03325, 26, 27 | 0.1414 | 0.002928 | 0.03629 | 0.0430 | 0.06610 | 0.001219 |
| Annual dropout rate (year 1), % | ‐‐ | 2.825, 27 | 35.014 | 30.912, 28 | 26.411, 29, 31 | 31.430 | 44.610 | 18.05 |
| Annual dropout rate (years 2‐5), % | ‐‐ | 2.225, 27 | 44.014 | 17.628 | 19.029 | 28.830 | 27.4 | 3.255, 19 |
| Annual cost, $ | ‐‐ | 55732 | 62318, 20 | 1,64720 | 17,09033 | 1,23434 | 2,76535 | 8,27336 |
| ΔQALY/ΔBMI | −0.005637, 38 | |||||||
| Dropout ΔBMI, monthly | 0.13810 | |||||||
Abbreviations: BMI, body mass index; ILI, intensive lifestyle intervention; QALY, quality‐adjusted life year.
2.2. Strategies for weight management
Patients receiving no treatment experienced slight weight gain over time, based on published literature.24 This group served as a reference group composed of individuals not attempting any self‐directed weight loss. For patients in the treatment strategies, weight change was based on data from randomized, placebo‐controlled clinical trials (Table 2). In cases with more than one published clinical trial, the trial with the longest duration was selected. All pharmacotherapy clinical trials also included lifestyle modification counselling.
Table 2.
Clinical trial data
| Treatment | Baseline Weight (kg) /BMI, kg m−2 | Sample Size | Weight After 1 y on Treatment, kg | Weight After 2 y on Treatment, kg | Source |
|---|---|---|---|---|---|
| ILI | 100.5/35.89 | 2440 | 91.93 | 96.37 | Look AHEAD trial27 |
| Phentermine | 98.4/35.6 | 269 | 81.08 | 85.90 | Hendricks et al14 |
| Phentermine/Topiramate | 102.2/36.1 | 153 | 91.67 | 92.80 | SEQUEL trial28 |
| Liraglutide | 97.6/34.8 | 93 | 89.3 | 91.5 | Astrup et al29 |
| Orlistat | 100.7/36.2 | 153 | 91.84 | 93.05 | Davidson et al30 |
| Lorcaserin | 100.4/36.2 | 1538 | 92.9 | 95.1 | BLOOM trial10 |
| Semaglutide | 111.5/39.3 | 102 | 96.11 | 96.15 | O'Neil et al5 |
Abbreviations: BMI, body mass index; ILI, intensive lifestyle intervention.
Most clinical trials contained data on weight loss for at least 2 years. For the model input, weight change was converted to rate of BMI change using average baseline weight and BMI values in the trial cohort (Table 1). Weight loss in the ILI strategy was based on data from the Look AHEAD study, which reported percent reduction from baseline weight over 8 years among type 2 diabetes patients receiving either ILI or diabetes support and education.25 For the 3‐year and 5‐year time horizons of the pharmacotherapy strategies, weight was assumed to increase linearly after the first year of pharmacotherapy. This assumption was based on the observed trends in the SCALE, BLOOM, and SEQUEL trials for liraglutide, lorcaserin, and phentermine/topiramate, respectively.10, 28, 29 For semaglutide, there were no clinical data past 1 year for a daily dose of 0.4 mg.5 However, the SUSTAIN‐6 trial followed type 2 diabetes patients on a weekly dose of 1.0 mg for 104 weeks.19 Change in weight after the first year from this study was used to estimate weight change on daily semaglutide after the first year in the model. For patients who dropped out of a weight loss strategy, the rate of weight regain was based on data from patients in the BLOOM trial who received lorcaserin in the first year and placebo in the second year.10 If a patient returned to baseline weight, the rate of weight gain was equivalent to patients on no treatment.
2.3. QOL adjustments and costs
QOL was dependent on weight change. A QOL constant of 0.0056 quality‐adjusted life years (QALYs) gained per BMI unit lost was used based on prior literature.37, 38
The model assumes a health care system cost perspective. Cost of no treatment was assumed to be zero. Costs of treatments were estimated from published literature (Table 1). The cost of semaglutide was estimated from the cost of once‐weekly 1.0 mg injections.36 For all pharmacotherapy arms, the cost for two physician visits ($178) was added to the first year, to account for the two visits expected for patients beginning weight loss medication.20 Costs of comorbidities and adverse events were not included. All costs from prior years were adjusted to 2019‐year dollars using the Consumer Price Index. Both costs and utilities were discounted at a rate of 3%.
2.4. Outcomes
Study endpoints included QALYs, total costs (US $2019), and incremental cost‐effectiveness ratios (ICERs). ICERs are calculated as the ratio of differences in costs and QALYs between a strategy and the next best alternative. A commonly used willingness‐to‐pay (WTP) threshold of $100 000/QALY determined cost‐effectiveness.40
2.5. Sensitivity analyses
To assess the impact of model input uncertainty on cost‐effectiveness results, one‐way sensitivity analyses and a probabilistic sensitivity analyses (PSAs) were performed. Deterministic one‐way sensitivity analyses were performed by varying one parameter at a time within prescribed bounds and recording the change in ICERs. All parameters were varied by +/−20% of the base case values. Probabilistic sensitivity analyses were performed by sampling all parameters simultaneously from probability distributions. The mean values for these distributions were the base case values for each parameter, and the standard deviations were 20% of the means. Gamma distributions were used for costs, and beta distributions were used for all other parameters. One thousand Monte Carlo samples were run per strategy with cohorts of 10 000 patients. The percentage of times each strategy was cost‐effective at various WTP thresholds was recorded.
3. RESULTS
3.1. Base case results
The results of the base case analysis are given in Table 3 and depicted as efficiency frontiers in Figure 1. An efficiency frontier plots cost and effectiveness for each strategy. Optimal strategies lie on the efficiency frontier (dashed line), while suboptimal (dominated) strategies lie below the frontier. For all time horizons, no treatment was the reference strategy (lowest cost and lowest effectiveness). Phentermine was the only strategy on the efficiency frontier after 1 year, dominating all other strategies. After years 3 and 5, semaglutide was also on the efficiency frontier.
Table 3.
Base case results
| Cost, $ | QALY | ICER, $/QALY | ICER Excluding Phentermine | |
|---|---|---|---|---|
| Year 1 | ||||
| No Treatment | 0 | 0.720 | ‐‐ | ‐‐ |
| Lorcaserin | 2064.83 | 0.725 | Dominated | Dominated |
| Liraglutide | 13 533.25 | 0.727 | Dominated | Dominated |
| Orlistat | 1108.36 | 0.727 | Dominated | Dominated |
| ILI | 674.61 | 0.728 | Dominated | 82 733 |
| Phentermine/Topiramate | 1424.02 | 0.728 | Dominated | Dominated |
| Semaglutide | 6972.12 | 0.733 | Dominated | 1 267 325 |
| Phentermine | 636.02 | 0.734 | 46 258 | |
| Year 3 | ||||
| No Treatment | 0 | 2.157 | ‐‐ | ‐‐ |
| Lorcaserin | 4229.10 | 2.177 | Dominated | Dominated |
| Liraglutide | 33 206.39 | 2.185 | Dominated | Dominated |
| Orlistat | 2284.81 | 2.187 | Dominated | Dominated |
| Phentermine/Topiramate | 3219.30 | 2.194 | Dominated | Dominated |
| ILI | 1673.68 | 2.198 | Dominated | 41 265 |
| Phentermine | 1100.83 | 2.212 | 20 157 | |
| Semaglutide | 19 375.75 | 2.224 | 1 437 340 | 661 326 |
| Year 5 | ||||
| No Treatment | 0 | 3.591 | ‐‐ | ‐‐ |
| Lorcaserin | 5331.07 | 3.616 | Dominated | Dominated |
| Liraglutide | 45 744.97 | 3.627 | Dominated | Dominated |
| Orlistat | 2862.47 | 3.633 | Dominated | Dominated |
| Phentermine/Topiramate | 4402.22 | 3.657 | Dominated | Dominated |
| ILI | 2605.55 | 3.657 | Dominated | 39 219 |
| Phentermine | 1240.45 | 3.660 | 17 880 | |
| Semaglutide | 30 704.99 | 3.712 | 576 931 | 520 262 |
Abbreviations: ICER, incremental cost‐effectiveness ratio; ILI, intensive lifestyle intervention; QALY, quality‐adjusted life year.
Figure 1.
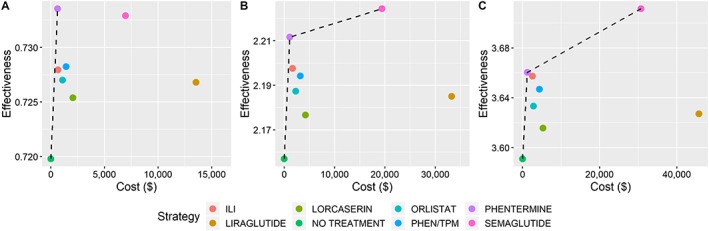
Base case results in cost‐effectiveness planes after (A) 1 year, (B) 3 years, and (C) 5 years. The dashed lines indicate the efficiency frontiers. PHEN/TPM, phentermine/topiramate
Phentermine was the cost‐effective strategy for each time horizon, with ICERs of $46 258/QALY, $20 157/QALY, and $17 880/QALY after 1, 3, and 5 years, respectively. Weight loss in the first year was the greatest on phentermine, making it the most effective treatment in the first year. However, this weight loss was not sustained, and patients returned to baseline weight by year 5 (Figure S1).
By contrast, patients on semaglutide maintained significant weight loss throughout the 5‐year time horizon. As a result, semaglutide was the most effective strategy in later years, with total QALYs of 2.224 and 3.711 in years 3 and 5, respectively. However, semaglutide was not cost‐effective. ICERs were prohibitively high at $1 437 340/QALY and $576 931/QALY in years 3 and 5, respectively.
When excluding phentermine from the analysis, ILI became the cost‐effective strategy. In this scenario, the ICERs for ILI were $82 733/QALY, $41 265/QALY, and $39 219/QALY in years 1, 3, and 5, respectively. Semaglutide remains cost‐ineffective but with lower ICER values: $661 326/QALY and $520 262/QALY in years 3 and 5, respectively.
3.2. One‐way sensitivity results
Figure 2 depicts one‐way sensitivity analyses over 3‐ and 5‐year time horizons, corresponding to years with multiple strategies on the efficiency frontier. Each time horizon compares phentermine and semaglutide, the two strategies on the efficiency frontier.
Figure 2.
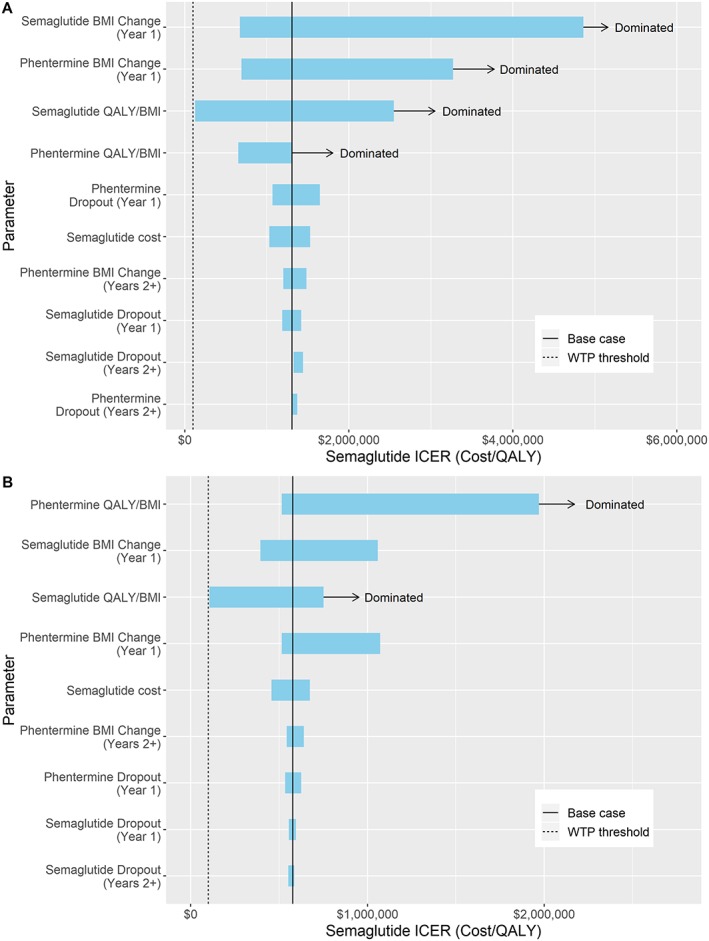
One‐way sensitivity analyses depicted as tornado diagrams. Range in semaglutide incremental cost‐effectiveness ratios (ICERs) when comparing phentermine vs semaglutide after (A) 3 years and (B) 5 years
For results over 3 years, changing the rate of BMI loss in year 1 and the QOL constant had substantial effects on resulting semaglutide ICERs. For each of these parameters, semaglutide was dominated under certain conditions. This was the case when the BMI lost in year 1 on phentermine was greater than 0.6 kg m−2, or the BMI lost in year 1 on semaglutide was less than 0.37 kg m−2. This was also the case when the QOL constant for phentermine was above 0.006 QALYs gained per BMI unit lost or the QOL constant for semaglutide was below 0.005 QALYs gained per BMI unit lost. Semaglutide approached the WTP threshold when its QOL constant was 0.017 QALYs gained per BMI unit lost, with an ICER of $127 062.
For results over 5 years, changing the rate of BMI loss in year 1 and the QOL constant also had the greatest effects on resulting semaglutide ICERs but not to the same extent as in the 3‐year time horizon. Semaglutide was dominated when the QOL constant for phentermine was above 0.009 QALYs gained per BMI unit lost or the QOL constant for semaglutide was below 0.004 QALYs gained per BMI unit lost. Semaglutide again approached the WTP threshold when its QOL constant was 0.017 QALYs gained per BMI unit lost, with an ICER of $106 873.
The results of these one‐way sensitivity analyses indicate that base case results were robust and phentermine remained the cost‐effective strategy under varying conditions. The semaglutide ICER did not fall below the WTP threshold in any test scenarios, although it became very close when the semaglutide QOL constant was much higher than the phentermine QOL constant.
3.3. PSA results
Probabilistic sensitivity analyses were performed on the model over the three time horizons. Acceptability curves are shown in Figure 3, and scatter plots of cost and effectiveness values are shown in Figure 4. The following results use a WTP threshold of $100 000/QALY. After 1 year, phentermine was the cost‐effective choice in 87.3% of runs, no treatment in 9.7% of runs, and ILI in 2.9% of runs. After 3 years, phentermine was the cost‐effective choice in 89.9% of runs and ILI in 9.2% of runs. After 5 years, phentermine was the cost‐effective choice in 77.4% of runs and ILI in 20.5% of runs.
Figure 3.
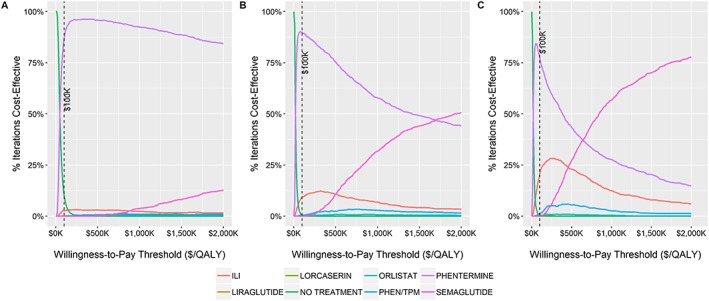
Probabilistic sensitivity analyses results depicted as acceptability curves after (A) 1 year, (B) 3 years, and (C) 5 years. The dashed line indicates the base case willingness‐to‐pay threshold. PHEN/TPM, phentermine/topiramate
Figure 4.
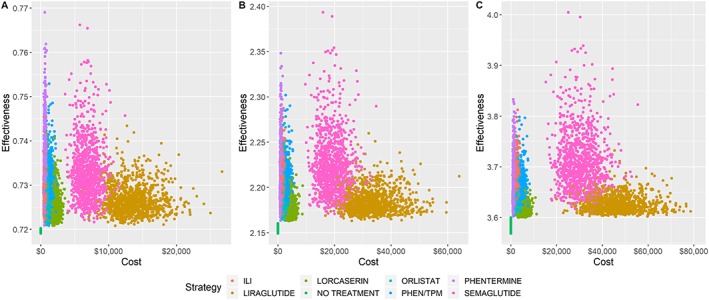
Probabilistic sensitivity analyses depicted in cost‐effectiveness planes after (A) 1 year, (B) 3 years, and (C) 5 years. PHEN/TPM, phentermine/topiramate
4. DISCUSSION
The aim of this analysis was to determine cost‐effectiveness among six pharmacotherapies and ILI used to treat obesity. A simulation model was used as a platform to incorporate weight loss and QOL data from clinical trials and other published literature. The modelling results found that phentermine was the cost‐effective strategy over 1‐, 3‐, and 5‐year time horizons. Phentermine resulted in the most weight loss in the first year and was the least expensive pharmacotherapy. Since weight loss on phentermine was not as well sustained after the first year compared with other therapies, semaglutide became the most effective strategy over 3‐ and 5‐year time horizons. Weight loss on semaglutide was second only to phentermine in the first year and was maintained over the 5‐year time horizon. Despite its effectiveness, semaglutide did not achieve cost‐effectiveness because of its high cost.
Treatment decisions are highly specific to individual values and preferences, and phentermine may not be the best choice for everyone. Phentermine is not recommended for patients with a history of cardiovascular disease because of the medication's side effect profile.15 This cost‐effectiveness analysis aimed to provide data to inform treatment decision making from a particular framework. Other considerations important to an individual patient could include previous attempts to lose weight, existing comorbid conditions, use of other medications, treatment side effects, and patient preference.7, 41 For this reason as well as a lack of FDA approval for long‐term use, a separate scenario analysis was performed that excluded phentermine. In this scenario, ILI was the cost‐effective treatment. While it did not lead to the most weight loss, ILI was much less costly than the more effective alternatives: semaglutide and liraglutide.
When excluding patients with a history of cardiovascular events, a large electronic health record study found significantly greater weight loss among “off‐label” phentermine users (those who take the drug longer than the FDA‐approved 90 days) without any increase in risk for cardiovascular events over 3 years.15 In addition, given the widespread off‐label use of phentermine for over 20 years without any evidence of serious side effects, the Endocrine Society includes phentermine in their Clinical Practice Guideline, assuming educated patient preference, response to treatment, and no history of cardiovascular disease or increase in blood pressure or pulse while on treatment.17
Two previous cost‐effectiveness analyses have examined pharmacotherapies, along with other commercial weight loss programmes.20, 21 However, this study is the first cost‐effectiveness analysis to directly compare six pharmacotherapies. This is also the first analysis to incorporate data from the recent clinical trial on the efficacy and safety of daily semaglutide for the treatment of obesity. In addition, other similar analyses did not include phentermine. A variety of treatments were included in the analysis in order to reflect decision making in clinical practice, primarily for patients who have unsuccessfully attempted weight loss with lifestyle intervention.
There were limitations to this analysis that should be acknowledged. This model did not directly incorporate adverse effects or side effects of treatment. Instead, QOL was dependent solely on weight loss. This was due to insufficient data regarding quality of life and the side effects of each drug. Treatment adherence was incorporated, however, which directly impacts therapy effectiveness and is largely dependent on adverse events and side effects. In addition, since most clinical trials included data for only 1 or 2 years, changes in weight were extrapolated past the first year for the extended time analyses with the assumption that weight linearly increased. This was based on the plateau or slight increase seen in many clinical trials after about 40 weeks. It was possible for patients to remain on treatment for all 5 years, which is uncommon and, in some cases, not recommended for some pharmacotherapies, particularly phentermine. In addition, many patients take medications intermittently, which was not considered in this modelling analysis. However, longer term continuous treatment regimens are becoming more common in accordance with the growing understanding of obesity as a chronic disease.15 Extensive sensitivity analyses were performed to evaluate the uncertainty resulting from model assumptions and indicated that the results were consistent despite changes in the parameters.
In summary, this modelling analysis found that phentermine is the cost‐effective pharmacotherapy currently on the market. This highlights the influence of drug cost and the need for further research into chronic therapy for patients with obesity. Longer‐term clinical trials that fully capture QOL, weight loss, comorbidities, and adverse events are needed to confirm these findings.
CONFLICT OF INTEREST STATEMENT
Dr Hur received consulting fees from Novo Nordisk outside the submitted work. Dr Corey received consulting fees from Bristol Myers Squibb, Novo Nordisk, and Gilead outside the submitted work and grant funding from Bristol Myers Squibb and Boehringer‐Ingelheim. Dr Kaplan is a consultant to Novo Nordisk. Novo Nordisk manufactures liraglutide and semaglutide.
Supporting information
Data S1. Supporting information
Lee M, Lauren BN, Zhan T, et al. The cost‐effectiveness of pharmacotherapy and lifestyle intervention in the treatment of obesity. Obes Sci Pract. 2020;6:162–170. 10.1002/osp4.390
Minyi Lee and Brianna Lauren contributed equally to the publication.
REFERENCES
- 1. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925‐1932. [DOI] [PubMed] [Google Scholar]
- 2. Pilitsi E, Farr OM, Polyzos SA, et al. Pharmacotherapy of obesity: available medications and drugs under investigation. Metabolism. 2019;92:170‐192. [DOI] [PubMed] [Google Scholar]
- 3. Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer‐and service‐specific estimates. Health Aff. 2009;28:w822‐w831. [DOI] [PubMed] [Google Scholar]
- 4. Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31:219‐230. [DOI] [PubMed] [Google Scholar]
- 5. O'Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double‐blind, placebo and active controlled, dose‐ranging, phase 2 trial. The Lancet. 2018;392:637‐649. [DOI] [PubMed] [Google Scholar]
- 6. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129:S102‐S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity pharmacotherapy. Med Clin North Am. 2018;102:135‐148. [DOI] [PubMed] [Google Scholar]
- 8. Dombrowski SU, Knittle K, Avenell A, Araújo‐Soares V, Sniehotta FF. Long term maintenance of weight loss with non‐surgical interventions in obese adults: systematic review and meta‐analyses of randomised controlled trials. BMJ: British Medical Journal. 2014;348:g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bray GA, Ryan DH. Medical therapy for the patient with obesity. Circulation. 2012;125:1695‐1703. [DOI] [PubMed] [Google Scholar]
- 10. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245‐256. [DOI] [PubMed] [Google Scholar]
- 11. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 12. Gadde KM, Allison DB, Ryan DH, et al. Effects of low‐dose, controlled‐release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo‐controlled, phase 3 trial. The Lancet. 2011;377:1341‐1352. [DOI] [PubMed] [Google Scholar]
- 13. Derosa G, Maffioli P, Salvadeo SAT, et al. Comparison of orlistat treatment and placebo in obese type 2 diabetic patients AU—Derosa, Giuseppe. Expert Opin Pharmacother. 2010;11:1971‐1982. [DOI] [PubMed] [Google Scholar]
- 14. Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long‐term phentermine pharmacotherapy for obesity. Obesity. 2011;19:2351‐2360. [DOI] [PubMed] [Google Scholar]
- 15. Lewis KH, Fischer H, Ard J, et al. Safety and effectiveness of longer‐term phentermine use: clinical outcomes from an electronic health record cohort. Obesity. 2019;27:591‐602. [DOI] [PubMed] [Google Scholar]
- 16. Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended‐release in obese adults. Obesity. 2013;21:2163‐2171. [DOI] [PubMed] [Google Scholar]
- 17. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 18. Kim KK, Cho H‐J, Kang H‐C, Youn B‐B, Lee K‐R. Effects on weight reduction and safety of short‐term phentermine administration in Korean obese people. Yonsei Med J. 2006;47:614‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 20. Finkelstein EA, Kruger E, Karnawat S. Cost‐effectiveness analysis of Qsymia for weight loss. Pharmacoeconomics. 2015;33:699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finkelstein EA, Verghese NR. Incremental cost‐effectiveness of evidence‐based non‐surgical weight loss strategies. Clinical Obesity. 2019;0:e12294. [DOI] [PubMed] [Google Scholar]
- 22. Velazquez A, Apovian CM. Updates on obesity pharmacotherapy. Ann N Y Acad Sci. 2018;1411:106‐119. [DOI] [PubMed] [Google Scholar]
- 23. Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, Avery AJ. The relationship between body mass index and health‐related quality of life: comparing the EQ‐5D, EuroQol VAS and SF‐6D. Int J Obes (Lond). 2007;31:189‐196. [DOI] [PubMed] [Google Scholar]
- 24. Malhotra R, Østbye T, Riley CM, Finkelstein EA. Young adult weight trajectories through midlife by body mass category. Obesity. 2013;21:1923‐1934. [DOI] [PubMed] [Google Scholar]
- 25. The Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity. 2014;22:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bray GA. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The Look AHEAD Research Group . Long‐term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four‐year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garvey WT, Ryan DH, Look M, et al. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr. 2011;95:297‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes (Lond). 2012;36:843‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235‐242. [DOI] [PubMed] [Google Scholar]
- 31. Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. The Lancet. 2009;374:1606‐1616. [DOI] [PubMed] [Google Scholar]
- 32. The Diabetes Prevention Program Research Group . The 10‐year cost‐effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent‐to‐treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah D, Risebrough NA, Perdrizet J, Iyer NN, Gamble C, Dang‐Tan T. Cost‐effectiveness and budget impact of liraglutide in type 2 diabetes patients with elevated cardiovascular risk: a US‐managed care perspective. Clinico Economics Outcomes Res: CEOR. 2018;10:791‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lacey LA, Wolf A, O'Shea D, Erny S, Ruof J. Cost‐effectiveness of orlistat for the treatment of overweight and obese patients in Ireland. Int J Obes (Lond). 2005;29:975‐982. [DOI] [PubMed] [Google Scholar]
- 35. Johnson G, Oliver NM. Lorcaserin (Belviq) for weight loss. Am Fam Physician. 2014;90:863‐866. [Google Scholar]
- 36. Wilkinson L, Hunt B, Johansen P, Iyer NN, Dang‐Tan T, Pollock RF. Cost of achieving HbA1c treatment targets and weight loss responses with once‐weekly semaglutide versus dulaglutide in the United States. Diabetes Therapy. 2018;9:951‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klebanoff MJ, Chhatwal J, Nudel JD, Corey KE, Kaplan LM, Hur C. Cost‐effectiveness of bariatric surgery in adolescents with obesity. JAMA Surg. 2017;152:136‐141. [DOI] [PubMed] [Google Scholar]
- 38. Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health. 2008;11:478‐486. [DOI] [PubMed] [Google Scholar]
- 39. Wang YC, Graubard BI, Rosenberg MA, et al. Derivation of background mortality by smoking and obesity in cancer simulation models. Med Decis Making. 2013;33:176‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness—The curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med. 2014;371:796‐797. [DOI] [PubMed] [Google Scholar]
- 41. Igel LI, Kumar RB, Saunders KH, Aronne LJ. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765‐1779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information


