Abstract
Background
To minimise the risk of implant failures after their placement, dental implants are kept load‐free for 3 to 8 months to establish osseointegration (conventional loading). It would be beneficial if the healing period could be shortened without jeopardising implant success. Nowadays implants are loaded early and even immediately and it would be useful to know whether there is a difference in success rates between immediately and early loaded implants compared with conventionally loaded implants.
Objectives
To evaluate the effects of (1) immediate (within 1 week), early (between 1 week and 2 months), and conventional (after 2 months) loading of osseointegrated implants; (2) immediate occlusal versus non‐occlusal loading and early occlusal versus non‐occlusal loading; (3) direct loading versus progressive loading immediately, early and conventionally.
Search methods
The following electronic databases were searched: the Cochrane Oral Health Group's Trials Register (to 8 June 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 4), MEDLINE via OVID (1946 to 8 June 2012) and EMBASE via OVID (1980 to 8 June 2012). Authors of identified trials were contacted to find unpublished randomised controlled trials (RCTs). There were no restrictions regarding language or date of publication.
Selection criteria
All RCTs of root‐form osseointegrated dental implants, having a follow‐up of 4 months to 1 year, comparing the same implant type immediately, early or conventionally loaded, occlusally or non‐occlusally loaded, or progressively loaded or not. Outcome measures were: prosthesis and implant failures and radiographic marginal bone level changes.
Data collection and analysis
Data were independently extracted, in duplicate, by at least two review authors. Trial authors were contacted for missing information. Risk of bias was assessed for each trial by at least two review authors, and data were extracted independently, and in duplicate. Results were combined using fixed‐effect models with mean differences (MD) for continuous outcomes and risk ratios (RR) for dichotomous outcomes with 95% confidence intervals (CI). Summary of findings tables of the main findings were constructed.
Main results
Forty‐five RCTs were identified and, from these, 26 trials including a total of 1217 participants and 2120 implants were included. Two trials were at low risk of bias, 12 were at high risk of bias and for the remaining 12 the risk of bias was unclear. In nine of the included studies there were no prosthetic failures within the first year, with no implant failures in seven studies and the mean rate of implant failure in all 26 trials was a low 2.5%. From 15 RCTs comparing immediate with conventional loading there was no evidence of a difference in either prosthesis failure (RR 1.90; 95% CI 0.67 to 5.34; 8 trials) or implant failure (RR 1.50; 95% CI 0.60 to 3.77; 10 trials) in the first year, but there is some evidence of a small reduction in bone loss favouring immediate loading (MD ‐0.10 mm; 95% CI ‐0.20 to ‐0.01; P = 0.03; 9 trials), with some heterogeneity (Tau² = 0.01; Chi² = 14.37, df = 8 (P = 0.07); I² = 44%). However, this very small difference may not be clinically important. From three RCTs which compared early loading with conventional loading, there is insufficient evidence to determine whether or not there is a clinically important difference in prosthesis failure, implant failure or bone loss. Six RCTs compared immediate and early loading and found insufficient evidence to determine whether or not there is a clinically important difference in prosthesis failure, implant failure or bone loss. From the two trials which compared occlusal loading with non‐occlusal loading there is insufficient evidence to determine whether there is a clinically important difference in the outcomes of prosthesis failure, implant failure or bone loss. We did not identify any trials which evaluated progressive loading of implants.
Authors' conclusions
Overall there was no convincing evidence of a clinically important difference in prosthesis failure, implant failure, or bone loss associated with different loading times of implants. The quality of the evidence is assessed as very low due to high and unclear risk of bias of primary studies and there is some evidence of reporting bias so clinicians should treat these findings with caution. A high value of insertion torque (at least 35 Ncm) seems to be one of the prerequisites for a successful immediate/early loading procedure. More well‐designed RCTs are needed and should be reported according to the CONSORT guidelines (www.consort‐statement.org/), and registered with a trials registry.
Plain language summary
Interventions for replacing missing teeth: different times for loading dental implants
When people have dental implants in their jaws, they usually wait several months for the bone around the implants to heal before artificial teeth are attached to the implant. During this period they use removable dentures. This review looked at the effects of attaching artificial teeth either the same day that the implant was placed, or early (after only 6 weeks) compared to the usual delay of at least 3 months. Some studies also compared the artifical tooth being attached so that it did not touch the opposite tooth (non‐occlusal loading). The search of studies was updated on 8th June 2012. The review found no evidence that attaching artificial teeth either immediately, after 6 weeks (early) or after at least 3 months (conventional) led to any important differences in the failure of the implant or the artifical tooth, or to the amount of bone which surrounded the implant (any bone loss would be an undesirable consequence). More research is needed in this area.
Summary of findings
for the main comparison.
| Conventional compared with immediate loading of dental implants | ||||||
|
Patient or population: patients requiring dental implants Settings: dental practice Intervention: immediate loading Comparison: conventional loading | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional | Immediate | |||||
|
Prosthesis failure at 1 year |
Low risk population | RR 1.90 (0.67 to 5.34) | 381 (8) | +OOO2, 3 very low | ||
| 10 per 10001 | 19 per 1000 (7 to 53) | |||||
| High risk population | ||||||
| 100 per 1000 | 190 per 1000 (70 to 534) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
1. The prosthetic failure rate in the conventional loading group is 1.2%
2. Eight studies: five at high and three at unclear risk of bias
3. There is some evidence of publication bias
CI = confidence interval RR = risk ratio GRADE Working Group grades of evidence: High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderatn quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
2.
| Conventional compared with early loading of dental implants | ||||||
|
Patient or population: patients requiring dental implants Settings: dental practice Intervention: early loading Comparison: conventional loading | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional | Early | |||||
|
Prosthesis failure at 1 year |
Low risk population | RR 5.00 (0.63 to 39.67) | 48 (1) | +OOO2, 3 very low | ||
| 10 per 10001 | 50 per 1000 (6 to 397) | |||||
| High risk population | ||||||
| 100 per 1000 | 241 per 1000 (63 to 1000) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
1. The prosthetic failure rate in the conventional loading group is 1.2%
2. Only one small study at high risk of bias
3. There is some evidence of publication bias
CI = confidence interval RR = risk ratio GRADE Working Group grades of evidence: High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
3.
| Early compared with immediate loading of dental implants | ||||||
|
Patient or population: patients requiring dental implants Settings: dental practice Intervention: immediate loading Comparison: early loading | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Early | Immediate | |||||
|
Prosthesis failure at 1 year |
Low risk population | RR 0.69 (0.25 to 1.87) | 408 (4) | +OOO2, 3 very low | ||
| 50 per 10001 | 35 per 1000 (13 to 94) | |||||
| High risk population | ||||||
| 240 per 1000 | 163 per 1000 (60 to 446) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
1. The prosthetic failure rate in the conventional loading group is 1.2%
2. Four trials: one trial high, one unclear and two at low risk of bias
3. There is some evidence of publication bias
CI = confidence interval RR = risk ratio GRADE Working Group grades of evidence: High quality (++++): Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality (+++O): Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality (++OO): Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality (+OOO): We are very uncertain about the estimate.
Background
Description of the condition
Missing teeth and supporting oral tissues have traditionally been replaced with dentures or bridges to restore the ability of patients to eat and speak and improve appearance. However, in several instances, patients are not satisfied with the function of removable dentures and it is not always possible to place a fixed bridge if the number of remaining abutment teeth is insufficient.
Description of the intervention
Since the 1970s, osseointegrated dental implants have offered an alternative (Brånemark 1977). They are surgically inserted into the jaw bones to support a dental prosthesis and are retained because of the intimacy of bone growth onto their surface (osseointegration). Dental implants have undoubtedly been one of the most significant scientific breakthroughs in dentistry over the past 30 years.
Primary implant stability and lack of micromovements are considered to be two of the main factors necessary for achieving predictable high success of osseointegrated oral implants (Albrektsson 1981). A successful osseointegrated oral implant is anchored directly to bone, however, in the presence of movement a soft tissue interface may encapsulate the implant causing its failure (Brunski 1979). To minimise the risk of soft tissue encapsulation, it has been recommended to keep the implants load‐free during the healing period: 3 to 4 months in mandibles (lower jaws) and 6 to 8 months in maxillae (upper jaws) (Brånemark 1977).
How the intervention might work
In general, during the healing period removable prostheses are used, however, many patients find these temporary prostheses rather uncomfortable and it would therefore be beneficial if the healing period could be shortened without jeopardising implant success. In 1990, the first longitudinal clinical trial was published suggesting that implants could be loaded immediately or early in the mandibles of selected patients (Schnitman 1990). Nowadays, immediately and early loaded implants are commonly used, particularly in mandibles of good bone quality (Brånemark 1999). Some authors also advocate that the use of some specific implant surface preparation is able to reduce the healing time (Roccuzzo 2001). To decrease the risk of immediately loaded implants failing early, various 'clinical tricks' have been suggested such as underpreparation of the implant site to achieve high primary stability (Cannizzaro 2003); the use of a non‐occluding temporary prosthesis during the first 2 months of healing (Testori 2003); or the progressive loading of the prostheses (Appleton 2005).
Why it is important to do this review
It would be useful to know whether there are differences in success rates between immediately or early loaded implants compared with conventionally loaded implants in different clinical indications such as in full and partial edentulism, in mandibles and maxillae, and if there are some surface modifications of the implants able to promote faster bone healing. The role of the surface characteristics is considered in another Cochrane review (Esposito 2007).
It is likely that the effect of loading at different times would become apparent during the first 4 months to 1 year of loading and therefore it was decided to make all comparisons at 4 months to 1 year after loading, preferably at 1 year when possible.
A few systematic reviews (Ioannidou 2005; Del Fabbro 2006, Sennerby 2008) have been published after the previous versions of the present review, however, they did not focus on the highest level of evidence (randomised controlled trials), therefore their results have to be interpreted with great caution.
Objectives
To determine the effects of osseointegrated dental implants loaded immediately, early or conventionally on clinical outcomes: prosthesis failure, implant failure and bone level.
To determine the effects of osseointegrated dental implants loaded occlusally or non‐occlusally immediately or early during the osseointegration period, on clinical outcomes: prosthesis failure, implant failure and bone level.
To determine the effects of osseointegrated dental implants directly or progressively loaded immediately, early and conventionally on clinical outcomes: prosthesis failure, implant failure and bone level.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of parallel group design and of split‐mouth design including root‐form osseointegrated dental implants having a follow‐up of 4 months to 1 year after loading (whenever possible the 1‐year data were used).
Types of participants
Patients who are having osseointegrated root‐form dental implants.
Types of interventions
Trials comparing the same osseointegrated root‐form dental implants loaded at different times. For the purpose of this review 'immediate' loading was defined as an implant put in function within 1 week after its placement; 'early' loading as those implants put in function between 1 week and 2 months; and 'conventional' (also termed 'delayed') loading as those implants loaded after 2 months. In particular the following comparisons were planned: (1) immediately versus conventionally loaded implants; (2) early versus conventionally loaded implants; (3) immediately versus early loaded implants. Both 'occlusally' and 'non‐occlusally' immediately loaded implants were considered as immediately loaded implants in this review. 'Non‐occlusally loaded' implants are those implants provisionally rehabilitated with restorations not in direct occlusion in static or dynamic lateral movements with the antagonistic dentition.
Trials comparing the same osseointegrated root‐form dental implants occlusally or non‐occlusally loaded during the osseointegration phase (immediately and early loading).
Trials comparing the same osseointegrated root‐form dental implants directly or progressively loaded, immediately, early or conventionally. Progressive loading is defined as the load of the implants obtained by gradual height increase of the occlusal table in increments from a state of infraocclusion to full occlusion.
Types of outcome measures
Prosthesis failure if secondary to implant failure.
Implant failures (implant mobility and removal of stable implants dictated by progressive marginal bone loss).
Radiographic marginal bone level changes on intraoral radiographs taken with a parallel technique.
Search methods for identification of studies
Electronic searches
For the identification of studies included or considered for this review, we developed detailed search strategies for each database to be searched. These were based on the search strategy developed for MEDLINE (OVID) but revised appropriately for each database. The MEDLINE search (Appendix 1) used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision), as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011).
We searched the following electronic databases.
The Cochrane Oral Health Group's Trials Register (to 8 June 2012) (Appendix 2)
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 4) (Appendix 3)
MEDLINE via OVID (1946 to 8 June 2012) (Appendix 1)
EMBASE via OVID (1980 to 8 June 2012) (Appendix 4).
The search of EMBASE was linked to the Cochrane Oral Health Group filter for identifying RCTs (Appendix 4). There were no restrictions on language or date of publication.
Searching other resources
Unpublished studies
We wrote to all the authors of the identified RCTs to identify any unpublished studies and we checked the bibliographies of all identified RCTs and relevant review articles. We used personal contacts in an attempt to identify unpublished or ongoing RCTs. In the first version of this review we also wrote to more than 55 oral implant manufacturers and we requested information on trials through an internet discussion group (implantology@yahoogroups.com), however, we discontinued this due to poor yield.
Handsearching
Handsearching was done as part of the Cochrane Worldwide Handsearching Programme, see the Cochrane Masterlist of the journals searched to date.
The following journals have been identified as being important to be handsearched for this review.
British Journal of Oral and Maxillofacial Surgery
Clinical Implant Dentistry and Related Research
Clinical Oral Implants Research
European Journal of Oral Implantology
Implant Dentistry
International Journal of Oral and Maxillofacial Implants
International Journal of Oral and Maxillofacial Surgery
International Journal of Periodontics and Restorative Dentistry
International Journal of Prosthodontics
Journal of Clinical Periodontology
Journal of Dental Research
Journal of Oral Implantology
Journal of Oral and Maxillofacial Surgery
Journal of Periodontology
Journal of Prosthetic Dentistry.
Where these have not already been searched as part of the Cochrane Worldwide Handsearching Programme, the journals were handsearched by one review author up to the month in which the last electronic search was undertaken.
Data collection and analysis
Selection of studies
The titles and abstracts (when available) of all reports identified through the electronic searches were scanned independently by two review authors. For studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, the full report was obtained. The full reports obtained from all the electronic and other methods of searching were assessed independently by two review authors to establish whether the studies did meet the inclusion criteria or not. Disagreements were resolved by discussion. Where resolution was not possible, a third review author was consulted. All studies meeting the inclusion criteria then underwent validity assessment and data extraction. Studies rejected at this or subsequent stages were recorded in the Characteristics of excluded studies table, and reasons for exclusion recorded.
Data extraction and management
Data were extracted by two review authors independently and in duplicate using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. Any disagreement was discussed and a third review author consulted where necessary. All study authors were contacted for clarification or missing information. Data were excluded until further clarification was available or if agreement could not be reached.
For each trial the following data were recorded.
Year of publication, country of origin and source of study funding.
Details of the participants including demographic characteristics and criteria for inclusion.
Details of the type of intervention.
Details of the outcomes reported, including method of assessment, and time intervals.
Assessment of risk of bias in included studies
The risk of bias assessment of the included trials was undertaken independently and in duplicate by two review authors as part of the data extraction process. In the case that the paper to be assessed had one or more review authors in the authors list, it was independently evaluated only by those review authors not involved in the trial.
This was conducted using the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011). It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and 'other issues'). Each domain includes one specific entry in a 'Risk of bias' table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering prespecified questions about the adequacy of the study in relation to the entry.
Summarising risk of bias for a study
After taking into account the additional information provided by the authors of the trials, studies were grouped into the following categories. We assumed that the risk of bias was the same for all outcomes and each study was assessed as follows.
| Risk of bias | Interpretation | Within a study | Across studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Further quality assessment was carried out to assess sample size calculations, definition of exclusion/inclusion criteria, and comparability of control and test groups at entry. The quality assessment criteria were pilot tested using several articles.
Measures of treatment effect
For dichotomous outcomes, the estimates of effects of interventions were expressed as risk ratios (RR) together with 95% confidence intervals (CI). For continuous outcomes, mean differences (MD) and standard deviations (SD) were used to summarise the data for each group using mean differences and 95% CIs.
Unit of analysis issues
The statistical unit was the participant and not the prosthesis or implant, unless the clustering of the implants within the participants had been taken into account in the analysis.
Dealing with missing data
All trial authors were contacted to retrieve missing data when necessary. Data were excluded until further clarification was available if agreement could not be reached. Methods in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions were used to estimate missing standard deviations (Higgins 2011).
Assessment of heterogeneity
The significance of any discrepancies in the estimates of the treatment effects from the different trials was to be assessed by means of Cochran's test for heterogeneity, and heterogeneity would have been considered significant if P < 0.1. The I² statistic, which describes the percentage total variation across studies that is due to heterogeneity rather than chance, was to be used to quantify heterogeneity with I² over 50% being considered substantial heterogeneity.
Assessment of reporting biases
If there had been sufficient numbers of trials (more than 10) in any meta‐analysis, publication bias would have been assessed according to the recommendations on testing for funnel plot asymmetry (Egger 1997), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If asymmetry was identified we would have examined possible causes.
Data synthesis
A meta‐analysis was only done when there were studies of similar comparisons reporting the same outcome measures. Risk ratios were combined for dichotomous data, and mean differences for continuous data, using fixed‐effect models. Random‐effects models were used when there were more than three studies in a meta‐analysis. Data from split‐mouth studies were to be combined with data from parallel group trials with the method outlined by Elbourne (Elbourne 2002), using the generic inverse variance method in RevMan. The techniques described by Follmann were used to estimate the standard error (SE) of the difference for split‐mouth studies, where the appropriate data were not presented and could not be obtained (Follmann 1992). Numbers needed to treat (NNT) were to be calculated for participants affected by implant failures. The Cochrane Handbook recommendations were followed for RCTs with parallel design with zero‐cell counts (Higgins 2011). The fixed value of 0.5 was automatically added to all cells with zero‐cell counts and risk ratios calculated with the RevMan software. If there were no events in both arms, no calculations were undertaken because in this situation the study does not provide any indication of the direction or magnitude of the relative treatment effect.
One study presented the mean difference (SE) for the mesial and distal radiographic scores separately (Lindeboom 2006). To calculate the total score, the mean differences were averaged and a conservative standard error was calculated assuming zero correlation. For rare events, odds ratios (OR) for split‐mouth trials were calculated using the Becker‐Balagtas methods outlined in Curtin 2002. As OR are similar to RR when the event rate is low we have simply used this value in place of RR for these studies. When using the generic inverse variance to combine studies of parallel design with studies of split‐mouth design, studies with both zero events could not be imputed in the meta‐analyses because RevMan 5 software does not allow it.
Subgroup analysis and investigation of heterogeneity
The following subgroup analyses were planned, however, there were insufficient studies in the meta‐analysis to undertake this.
Whether implants were placed in mandibles or maxillae.
Whether single or multiple splinted implants were used.
Sensitivity analysis
It was planned to undertake sensitivity analyses to examine the effect of the study quality on the overall estimates of effect. In addition, the effect of including unpublished literature on the review's findings was also to be examined. There were too few trials in the meta‐analyses to undertake these analyses to investigate to what extent the risk of bias might have influenced the results. This will be done in future updates as soon as sufficient numbers of trials having different risk of bias ratings are available.
Presentation of main results
Summary of findings tables were developed for the comparisons of different loading times for the outcome 'prosthesis failure', which were considered the main results. The quality of the body of evidence was assessed with reference to the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates, the risk of publication bias, and the magnitude of the effect. The quality of the body of evidence for each of the main results was categorised as high, moderate, low or very low.
Results
Description of studies
Characteristics of the trial settings and investigators
Of the 45 potentially eligible trials (Polson 2000; Chiapasco 2001; Roccuzzo 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Testori 2003; Fischer 2004; Salvi 2004; Appleton 2005; Ottoni 2005; Hall 2006; Lindeboom 2006; Oh 2006; Romanos 2006; Turkyilmaz 2006; Assad 2007; Göthberg 2007; Testori 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008c; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Merli 2008; Schincaglia 2008; Zöllner 2008; Degidi 2009; Degidi 2009; De Rouck 2009; Cannizzaro 2010; Enkling 2010; Shibly 2010; Van de Velde 2010; den Hartog 2011; Jokstad 2011, Kim 2011; Mackie 2011; Tealdo 2011; Barewal 2012; Meloni 2012), 19 trials had to be excluded. Eight trials were excluded because they were not randomised controlled trials (RCTs) (Ottoni 2005; Romanos 2006; Turkyilmaz 2006; Degidi 2009; Degidi 2010; Mackie 2011; Tealdo 2011; Barewal 2012), five trials because of various additional confounding factors (Roccuzzo 2001; Testori 2003; Göthberg 2007; Cannizzaro 2008c; Van de Velde 2010), three trials because they tested comparisons outside the scope of the present review (Salvi 2004; Jokstad 2011; Kim 2011), one trial was excluded due to insufficient data presented (Polson 2000), one trial because of conflicting data presented (Shibly 2010), and one trial because data were mixed by the parallel‐group and split‐mouth design and participants with problems were removed from the analyses (Appleton 2005).
Of the 26 included trials (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Fischer 2004; Hall 2006; Lindeboom 2006; Oh 2006; Assad 2007; Testori 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Merli 2008; Schincaglia 2008; Zöllner 2008; De Rouck 2009; Cannizzaro 2010; Enkling 2010; den Hartog 2011; Meloni 2012), 13 were conducted in Italy (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Crespi 2008; Donati 2008; Merli 2008; Schincaglia 2008; Cannizzaro 2010; Meloni 2012), three in New Zealand (Payne 2002; Tawse‐Smith 2002; Hall 2006), two in The Netherlands (Lindeboom 2006; den Hartog 2011), two in Turkey (Turkyilmaz 2007; Güncü 2008), one in Sweden (Fischer 2004), one in Belgium (De Rouck 2009), one in Switzerland (Enkling 2010), one in USA (Oh 2006), one in Egypt (Assad 2007), and one was run in several countries (Zöllner 2008). Twenty‐three trials had a parallel group study design and three a split‐mouth study design (Cannizzaro 2008d; Güncü 2008; Meloni 2012). One trial of parallel group design had 10 participants treated according to a split‐mouth design (Donati 2008); these 10 participants were excluded from the calculations in the present review.
Fifteen trials were conducted at university dental clinics (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Hall 2006; Lindeboom 2006; Oh 2006; Assad 2007; Turkyilmaz 2007; Crespi 2008; Güncü 2008; Schincaglia 2008; De Rouck 2009; Enkling 2010; den Hartog 2011), eight in private practices (Cannizzaro 2003; Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Donati 2008; Merli 2008; Cannizzaro 2010), one in a specialist public clinic (Fischer 2004), and two in both university clinics and private practices (Zöllner 2008; Meloni 2012).
Sixteen trials received support from industry (Payne 2002; Tawse‐Smith 2002; Fischer 2004; Hall 2006; Lindeboom 2006; Oh 2006; Testori 2007; Turkyilmaz 2007; Cannizzaro 2008d; Donati 2008; Merli 2008; Zöllner 2008; Cannizzaro 2010; Enkling 2010; den Hartog 2011; Meloni 2012). All studies included only adults.
Characteristics of participants
The mean age of the participants ranged from 35 to 63 with a minimum age of 18 and a maximum age of 80, however, some trials failed to report the mean age (five trials: Payne 2002; Tawse‐Smith 2002; Assad 2007; Enkling 2010; Meloni 2012), and some the age range (eight trials: Fischer 2004; Turkyilmaz 2007; Donati 2008; Zöllner 2008; De Rouck 2009; Enkling 2010; den Hartog 2011; Meloni 2012). The number of males and females was unclear in four trials (Tawse‐Smith 2002; Hall 2006; Enkling 2010; Meloni 2012). One trial only included male participants (Assad 2007). The remaining 21 trials included more women than men (or equal numbers).
The trials included between 10 and 266 participants, with a median of 30. Eleven trials placed multiple implants in participants: seven in the mandible (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Assad 2007; Turkyilmaz 2007; Enkling 2010), two in the maxilla (Fischer 2004; Cannizzaro 2008b) and two in both jaws (Testori 2007; Merli 2008), all participants being edentulous in the specific jaw. Nine trials placed single implants in each participant: seven in the maxilla (Hall 2006; Lindeboom 2006; Oh 2006; Crespi 2008; Donati 2008; De Rouck 2009; den Hartog 2011), one in the mandible (Schincaglia 2008), and one in either jaw (Cannizzaro 2010). All these participants were partially edentulous. Two trials placed both single and multiple implants in participants in either jaw (Cannizzaro 2003; Zöllner 2008). A further three split‐mouth trials placed one implant from each comparison group in each participant: two trials in the mandible (Güncü 2008; Meloni 2012), and one in either jaw (Cannizzaro 2008d).
Inclusion and exclusion criteria
The majority of trials, with seven exceptions (Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Donati 2008; Schincaglia 2008; Cannizzaro 2010), used quite strict inclusion criteria and included mainly ideal participants. This choice is understandable since it is common sense to load implants immediately or early only in selected cases, for instance when implants are placed with high insertion torques in good quality bone of adequate volume in participants not having parafunctional habits.
Main inclusion criteria
Completely edentulous mandible (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Assad 2007; Turkyilmaz 2007; Cannizzaro 2008a; Enkling 2010).
Completely edentulous maxilla able to harbour at least five implants (Fischer 2004; Cannizzaro 2008b).
Partially edentulous participants (both mandibles and maxillae) (Cannizzaro 2003; Testori 2007; Merli 2008).
Partially edentulous participants (both mandibles and maxillae) in the posterior jaws (premolar and molar areas) allowing the placement of at least 8 mm long implants, and the bone thickness at implant sites had to be of at least 6 mm (Zöllner 2008).
Bilaterally missing first mandibular molars (Güncü 2008; Meloni 2012).
Missing one first or second mandibular molar allowing the placement of one at least 8.5 mm long implant, and the bone thickness at implant site had to be of at least 7 mm (Schincaglia 2008).
Missing one single tooth in the anterior (premolar to premolar) maxilla, with adjacent teeth present, allowing the placement of at least 10 mm long implants with a 2.5 mm diameter (Hall 2006).
Missing one single tooth in the anterior (premolar to premolar) maxilla, allowing the placement of at least 10 mm long implants with a 3.7 mm diameter with a flapless procedure (Oh 2006).
Missing one single tooth in the anterior (first premolar to first premolar) maxilla with adjacent natural teeth, allowing the placement of at least 13 mm long implant with a diameter of 3.5 mm (den Hartog 2011).
Missing two teeth and enough bone to allow placement of two 7 mm long implants and the bone thickness at implant sites had to be of at least 5.5 mm (Cannizzaro 2008d).
Missing one or more single teeth in the anterior (premolar to premolar) maxilla allowing the placement of at least 8 mm long implants with a 3.4 mm diameter with no bone fenestration (Lindeboom 2006).
Missing one or more single teeth in the anterior (premolar to premolar) jaws allowing the placement of at least 8 mm long implants with a 4 mm diameter with no bone fenestration (Donati 2008).
Missing a single tooth with residual bone height of at least 10 mm and width of at least 5 mm (Cannizzaro 2010).
Single fresh extraction sockets in the anterior maxilla (15 to 25) in presence of four bone walls and at least 4 mm of bone beyond the root apex (Crespi 2008).
Single fresh extraction sockets in the anterior maxilla (15 to 25) in presence of an intact buccal wall, at least 5 mm of bone beyond the root apex and of both neighbouring teeth (De Rouck 2009).
13 to 15 mm of residual anterior mandibular bone or more (Chiapasco 2001; Payne 2002; Tawse‐Smith 2002).
10 mm of residual anterior mandibular bone or more (Romeo 2002).
Elderly participants (55 to 80 years) (Payne 2002; Tawse‐Smith 2002).
Sufficient bone to allow placement of two 15 mm long implants (Turkyilmaz 2007).
Sufficient bone to allow placement of two 9.5 mm long implants with a diameter of 4 mm (Enkling 2010).
Sufficient bone to allow placement of at least 13 mm long implants and with a diameter of 3.7 mm (Cannizzaro 2003; Assad 2007; Crespi 2008).
Sufficient bone to allow placement of 11.5 mm long implants with a diameter of 4 mm (Güncü 2008).
Sufficient bone to allow placement of at least 10 mm long implants and with a diameter of 3.7 mm (Cannizzaro 2008a; Cannizzaro 2008b).
Sufficient bone to allow placement of at least 9.5 mm long implants, and the bone thickness at implant sites had to be of at least 5.5 mm (Merli 2008).
Residual bone height of at least 10 mm and thickness of at least 6 mm (Meloni 2012).
Minimal insertion torque of 45/48 Ncm to be immediately loaded (Cannizzaro 2003; Cannizzaro 2008a; Cannizzaro 2008b; den Hartog 2011).
Minimal insertion torque of 40 Ncm to be immediately loaded (Cannizzaro 2008d; Merli 2008).
Minimal insertion torque of 35 Ncm to be immediately loaded (De Rouck 2009; Cannizzaro 2010; Enkling 2010; Meloni 2012).
Minimal insertion torque of 30 Ncm for single implants (Lindeboom 2006; Testori 2007), and 20 Ncm for splinted implants (Testori 2007).
Minimal insertion torque of 25 Ncm and primary implant stability ISQ > 60 to be immediately loaded (Crespi 2008).
Minimal primary implant stability of 20 Ncm to be immediately loaded (Donati 2008; Schincaglia 2008).
Main exclusion criteria
Any evidence of current or previous smoking (Payne 2002; Tawse‐Smith 2002).
Smoking (Lindeboom 2006; Güncü 2008; den Hartog 2011).
Smoking more than 10 cigarettes per day (Chiapasco 2001; Cannizzaro 2003; Fischer 2004; Crespi 2008; Zöllner 2008; De Rouck 2009; Meloni 2012).
Smoking more than 20 cigarettes per day (Romeo 2002; Hall 2006).
Any systemic disease likely to compromise implant surgery (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Fischer 2004; Lindeboom 2006; Assad 2007; Testori 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Merli 2008; Zöllner 2008; De Rouck 2009; Cannizzaro 2010; Meloni 2012).
Presence of severe systemic conditions (ASA III) (Schincaglia 2008; den Hartog 2011).
Previously bone grafted bone jaws (Payne 2002; Tawse‐Smith 2002; Fischer 2004; Hall 2006; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b).
In need of tissue augmentation procedures (Güncü 2008; Schincaglia 2008; Meloni 2012).
Previously irradiated jaws (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Testori 2007; Turkyilmaz 2007; Cannizzaro 2010; den Hartog 2011; Meloni 2012), or jaws irradiated less than 1 year before (Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008).
Bone quality type IV (very soft bone) according to the classification of Lekholm 1985 detected at the time of surgery (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Cannizzaro 2010), or on radiographs (Hall 2006).
History of bruxism (Payne 2002; Tawse‐Smith 2002).
Severe clenching or bruxism (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Hall 2006; Lindeboom 2006; Testori 2007; Cannizzaro 2008d; Crespi 2008; Güncü 2008; Merli 2008; Zöllner 2008; De Rouck 2009; Cannizzaro 2010; Meloni 2012).
Severe maxillo‐mandibular skeletal discrepancy (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Lindeboom 2006).
Extraction sockets with healing less than 3 months (Donati 2008; den Hartog 2011), 4 months (Schincaglia 2008; Zöllner 2008), and 6 months (Güncü 2008).
If primary implant stability could not be achieved (Hall 2006; Zöllner 2008).
Previous history of failed implants (Hall 2006).
Less than 4 mm (Merli 2008), or 5 mm (Meloni 2012), of keratinised mucosa.
Presence of dehiscence or fenestrations of the post‐extractive sites (Crespi 2008; De Rouck 2009).
Presence of peri‐apical lesions or any other abnormalities in the maxillary anterior region as determined on radiographs (den Hartog 2011).
Unknown exclusion criteria (Oh 2006; Assad 2007; Enkling 2010).
Sample size
A priori sample size calculation was performed in eight trials (Lindeboom 2006; Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008; Schincaglia 2008; den Hartog 2011). The sample size of one trial was calculated assuming that treatment modalities were equivalent (Lindeboom 2006): 21 implants were needed in each group to reject the null hypothesis that the treatments were not equivalent with a power of 80% and a type I error rate of 0.05. Non‐equivalence was defined as a difference in implant stability quotient (ISQ) values measured with Osstell of 10 or more. Twenty‐five implants (24 participants) were included in each group. Calculations of three trials (Testori 2007; Cannizzaro 2008d; Merli 2008) were based on the outcome (implant failure) of another RCT of similar design (Ottoni 2005), and it was calculated that 26 participants per group were needed to complete the trial. Unfortunately, because of an independent decision of the clinicians in violation of the research protocol, only 25 participants were included in the immediately loaded group in one trial (Testori 2007). The other two trials achieved the planned sample size (Cannizzaro 2008d; Merli 2008). The sample size calculation for the other two trials was based on a theoretical estimate of implant failures and 286 participants should have been included in each group (Cannizzaro 2008a; Cannizzaro 2008b). The sample size could not be achieved and the number of failures which actually occurred were much less than those estimated in the calculations, therefore the number of participants to be included to detect a difference should have been much greater. Another trial calculated the sample size on a peri‐implant marginal bone level change difference of 0.3 mm among immediately versus conventionally loaded implants based on an error of 5% and a power of 80% (Schincaglia 2008): 14 participants were needed in each group and 15 participants per group were enrolled. For another trial (De Rouck 2009), calculations were based on data from a previous cohort study on immediate single implants. A difference in soft tissue dimensions of 0.5 mm between the groups was defined as clinically relevant. Based on standard deviations (SD) of 0.7 mm for both groups, an a error level of 5% and statistical power of 80%, a sample size of 24 participants per group was calculated. In this study 26 participants per group were recruited. Cannizzaro 2010 calculated the sample size for the primary outcome measure (implant failure) based on the findings of another similar trial. A two group continuity corrected Chi² test with a 0.05 two‐sided significance level will have 90% power to detect the difference between a proportion of 0.999 and a proportion of 0.920 for participants experiencing at least one implant failure (odds ratio of 0.0012) when the sample size in each group is 154. It was originally decided to recruit 80 participants in each group, each centre recruiting 10 participants, however, only 40 participants were recruited, 20 per group. den Hartog 2011 used non‐inferiority analysis defined as 0.5 mm mean marginal bone loss. It was assumed that a mean marginal bone loss of 1 mm (SD 0.6) would occur from implant placement to 18 months thereafter for implants restored according to a conventional protocol. With a one‐sided significance level of 5% and a power of 90%, a minimum of 26 participants per group was required. The number of participants per group was set at 31 to deal with withdrawal.
Characteristics of interventions
(1) Immediate versus conventional loading
Immediate loading was compared with conventional loading in 15 trials (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Hall 2006; Oh 2006; Assad 2007; Turkyilmaz 2007; Crespi 2008; Donati 2008; Güncü 2008; Schincaglia 2008; De Rouck 2009; Enkling 2010; den Hartog 2011; Meloni 2012).
Chiapasco 2001; Romeo 2002; Assad 2007 and Turkyilmaz 2007 compared four implants in each edentulous mandible immediately loaded after insertion (2 to 7 days) with four implants conventionally loaded after 3 to 8 months.
Cannizzaro 2003 compared one or more implants in partially edentulous participants in both mandibles and maxillae loaded the same day with implants conventionally loaded (3.5 months for mandibles and 4.5 months for maxillae).
Hall 2006 compared one single implant loaded the same day with one single implant conventionally loaded at 6 months in the anterior maxilla (between premolars).
Oh 2006 compared one single implant loaded the same day with one single implant conventionally loaded at 4 months placed with a flapless procedure in the anterior maxilla (between premolars).
Crespi 2008 compared one single implant in fresh extraction sockets in the maxillary aesthetic zone immediately occlusally loaded the same day with one single implant conventionally loaded at 3 months.
Donati 2008 compared one single implant immediately loaded within 24 hours with one single implant conventionally loaded at 3 months in area 15 to 25 and 35 to 45. Immediately loaded sites were treated with two different preparation techniques (drills versus osteotomes). Ten participants were treated according to a split‐mouth design and were excluded.
Güncü 2008 and Meloni 2012, in split‐mouth design trials, compared one single mandibular implant in the first molar site loaded the same day with one single contralateral implant loaded after 3 months.
Schincaglia 2008 compared one single implant loaded within 24 hours with one implant loaded after 3 months in the first or second mandibular molar site.
Enkling 2010 compared two interforaminal immediately loaded implants with two implants loaded after 3 months supporting mandibular overdentures.
De Rouck 2009 compared one single immediate post‐extractive implant loaded the same day with one implant loaded after 3 months between second to second upper premolars.
den Hartog 2011 compared one single implant loaded within 24 hours with one implant loaded after 3 months between second to second upper premolars.
(2) Early versus conventional loading
Early loading was compared with conventional loading in three trials (Payne 2002; Tawse‐Smith 2002; Fischer 2004).
Payne 2002 and Tawse‐Smith 2002 compared two implants in fully edentulous mandibles early loaded at 6 weeks or conventionally loaded at 12 weeks.
Fischer 2004 compared five to six implants in fully edentulous maxillae early loaded (9 to 18 days) or conventionally loaded (2.5 to 5.1 months).
(3) Immediate versus early loading
Immediate loading was compared with early loading in six trials (Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008; Zöllner 2008).
Testori 2007 compared implants in both mandibles and maxillae of partially edentulous participants, immediately but non‐occlusally loaded (when possible) within 48 hours with implants early loaded at 2 months.
Cannizzaro 2008a compared two implants in fully edentulous mandibles loaded the same day or early loaded at 6 weeks.
Cannizzaro 2008b compared five to eight implants, placed flapless, in fully edentulous maxillae loaded the same day or early loaded at 2 months.
Cannizzaro 2008d, in a split‐mouth design, compared one single 7 mm long implant, placed flapless, occlusally loaded the same day with one implant early loaded at 6 weeks.
Merli 2008 compared implants, placed flapless, in both mandibles and maxillae of partially edentulous participants, immediately but non‐occlusally loaded (when possible) within 72 hours with implants early non‐occlusally loaded at 6 weeks.
Zöllner 2008 compared one to four implants in both posterior mandibles and maxillae of partially edentulous participants, immediately but non‐occlusally loaded the same day with implants early non‐occlusally loaded at 1 month.
(4) Occlusal versus non‐occlusal loading
Occlusal loading was compared with non‐occlusal loading in two trials (Lindeboom 2006; Cannizzaro 2010).
Lindeboom 2006 compared immediately occlusally loaded single implants with immediately non‐occlusally loaded implants within 1 day in the anterior and premolar region of the maxilla.
Cannizzaro 2010 compared immediately occlusally loaded single implants with immediately non‐occlusally loaded implants the day of placement.
(5) Progressive loading
No trial could be included.
Implant systems
Nineteen different implant systems were used in the trials in this review.
3i® Osseotite FNT (3i Biomet, Palm Beach, Florida, USA) titanium tapered screws (Testori 2007).
3i® Nanotite (3i Biomet, Palm Beach, Florida, USA) titanium grade 5 cylindrical screws (Cannizzaro 2008d).
Astra OsseoSpeed® (Astra Tech Dental, Mölndal, Sweden) titanium grade 1 screws (Donati 2008).
BioComp® (BioComp Industries BV, Vught, The Netherlands) tapered titanium plasma sprayed (TPS) screws (Lindeboom 2006).
Brånemark® (Nobel Biocare AB, Göteborg, Sweden) Mark II type turned titanium grade 1 screws (Chiapasco 2001).
Brånemark® (Nobel Biocare AB, Göteborg, Sweden) TiUnite Mark III type titanium grade 1 screws (Turkyilmaz 2007; Güncü 2008), wide body (Schincaglia 2008).
ITI® SLA (Institut Straumann AG, Waldenburg, Switzerland) solid sand‐blasted large‐grit acid‐etched titanium grade 4 screws (Payne 2002; Romeo 2002; Fischer 2004).
ITI® SLA active (Institut Straumann AG, Waldenburg, Switzerland) solid sand‐blasted large‐grit acid‐etched titanium grade 4 screws, three standard plus implants were also used (Zöllner 2008).
NobelReplace Tapered Groovy (Nobel Biocare AB, Göteborg, Sweden) TiUnite titanium grade 4 screws (De Rouck 2009; den Hartog 2011; Meloni 2012).
Outlink (Sweden & Martina, Padova, Italy) titanium plasma‐sprayed cylindrical screws (Crespi 2008).
SICace® (SIC invent AG, Basel, Switzerland) titanium screws (Enkling 2010).
Southern® (Southern Implants Irene, South Africa) sand‐blasted acid‐etched titanium grade 4 screws (Tawse‐Smith 2002; Hall 2006).
Steri‐Oss® (Steri‐Oss, Yorba Linda, California, USA) HL series, 3.8 mm in diameter acid‐etched titanium grade 4 screws (Tawse‐Smith 2002).
Thommen® (SPI®Element System; Thommen Medical AG, Waldenburg, Switzerland) sand‐blasted acid‐etched screws. In some of the post‐extraction sites SPI®Contact troncoconical screws were used (Merli 2008).
Zimmer® tapered SwissPlus (Zimmer Dental, Carlsbad, California, USA) implants (Cannizzaro 2008a; Cannizzaro 2008b).
Zimmer® Spline Twist MTX (Zimmer Dental, Carlsbad, California, USA) HA‐blasted and acid‐etched titanium screws (Cannizzaro 2003).
Zimmer® unknown type (Zimmer Dental, Carlsbad, California, USA) dental implants (Oh 2006).
Zimmer® Screw‐Vent® (but described as Paragon, Core‐Vent Corporation, Las Vegas, USA) titanium screws (Assad 2007).
Z‐Look3® (Z‐System, Oensingen, Switzerland) one‐piece zirconia sand‐blasted screws (Cannizzaro 2010).
Early and conventionally loaded implants were used according to a submerged (two‐stage) procedure, i.e. the implants were covered by the mucosa during the healing phase, thus a second surgical intervention was necessary to connect the abutments to the implants (Chiapasco 2001; Hall 2006; Assad 2007; Crespi 2008; Donati 2008; De Rouck 2009; Enkling 2010; den Hartog 2011), or according to a non‐submerged (one‐stage) protocol, i.e. the abutments were directly connected to the implants, thus a second operation was avoided (Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Fischer 2004; Oh 2006; Testori 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Güncü 2008; Merli 2008; Schincaglia 2008; Zöllner 2008; Cannizzaro 2010; Meloni 2012). More specifically the Z‐Look3 one‐piece zirconia implants used by Cannizzaro 2010 could only be inserted according a non‐submerged technique.
Removable overdentures were retained by clip attachments to a bar supported by four implants (Chiapasco 2001; Romeo 2002; Assad 2007), or two implants (Cannizzaro 2008a; Enkling 2010), or were retained by two unsplinted ball attachments (Payne 2002; Tawse‐Smith 2002; Turkyilmaz 2007).
Fixed maxillary full‐arch prostheses, without using provisional ones, were connected to the implant in one trial (Fischer 2004). In another trial provisional cemented metal reinforced acrylic full‐arch maxillary prostheses were replaced by metal ceramic or metal resin full‐arch prostheses after 2 to 3 months (Cannizzaro 2008b).
Temporary resin bridges/crowns were fabricated and then replaced by final restorations in 13 trials (Cannizzaro 2003; Hall 2006; Lindeboom 2006; Testori 2007; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Merli 2008; Zöllner 2008; De Rouck 2009; Cannizzaro 2010; den Hartog 2011). In nine of these studies, only single crowns were used (Hall 2006; Lindeboom 2006; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; De Rouck 2009; Cannizzaro 2010; den Hartog 2011).
Temporary resin crowns were fabricated and then replaced by definitive metal‐ceramic crowns in the immediately loaded group, whereas permanent metal‐ceramic crowns were delivered in the conventionally loaded group in three trials (Oh 2006; Schincaglia 2008; Meloni 2012).
Occlusal or non‐occlusal immediate loading
In 17 trials, the prostheses were put in full occlusion (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Fischer 2004; Oh 2006; Assad 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Schincaglia 2008; Enkling 2010).
In seven trials, the prostheses were not put in full static or dynamic occlusion for 2 months (Hall 2006; Testori 2007), 3 months (Meloni 2012), 5 months (Zöllner 2008), or 6 months (Merli 2008; De Rouck 2009; den Hartog 2011).
In two trials (Lindeboom 2006; Cannizzaro 2010), single crowns were randomised into full occlusion or not for 4 to 5 months (Cannizzaro 2010), or 6 months (Lindeboom 2006).
Characteristics of outcome measures
Prosthesis failures (all trials with the exception of Zöllner 2008, for which the number of prosthetic failures was assumed to be identical to the number of implant failures).
Implant failures (all trials).
Radiographic bone level changes were assessed in all trials with two exceptions (Oh 2006; Cannizzaro 2008a). However, the peri‐implant bone level measurements of eight trials were not included in the present analyses because they were performed on panoramic radiographs (Chiapasco 2001; Romeo 2002), because data were presented in a way we could not use (Fischer 2004; Assad 2007; Donati 2008; Zöllner 2008; De Rouck 2009), or because they just related to the 3‐year follow‐up (Merli 2008).
Risk of bias in included studies
Allocation
Sequence generation
Fifteen (65%) of the included studies described an adequate method of sequence generation and were assessed as being at low risk of bias for this domain (Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Lindeboom 2006; Testori 2007; Cannizzaro 2008d; Schincaglia 2008; Donati 2008; Zöllner 2008; Merli 2008; Cannizzaro 2008a; Cannizzaro 2008b; De Rouck 2009; Cannizzaro 2010; den Hartog 2011; Meloni 2012). One study described randomisation by drawing lots till one group was "full" and then simply putting all the rest into the other group, a process we assessed at high risk of selection bias (Chiapasco 2001). For the other eight studies, the risk of bias for this domain was unclear because insufficient detail on the method of sequence generation was available either from the report or from emails with the author.
Allocation concealment
Allocation concealment was reported as being done adequately in nine (35%) of the included studies (Lindeboom 2006; Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008; Cannizzaro 2010; den Hartog 2011; Meloni 2012). In nine studies, it was unclear from the report and communication with authors whether allocation had been adequately concealed, and these studies were assessed at unclear risk of bias for this domain (Payne 2002; Tawse‐Smith 2002; Hall 2006; Oh 2006; Assad 2007; Crespi 2008; Donati 2008; De Rouck 2009; Enkling 2010). In eight studies this was done prior to surgery and these trials were assessed at high risk of bias for this domain.
Overall less than half of the included studies (35%) are at low risk of selection bias (Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Cannizzaro 2010; den Hartog 2011; Lindeboom 2006; Meloni 2012; Merli 2008; Testori 2007).
Blinding
Blinding of operators and trial participants to the loading time is not possible in these trials. We acknowledge that this introduces a potential risk of performance bias in all of the included studies, but we have not assessed each included study for this domain.
However, blinding of outcome assessment is possible and we assessed this in the included studies. Blinded outcome assessment was reported in 13 studies (Chiapasco 2001; Payne 2002; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Lindeboom 2006; Hall 2006; Oh 2006; Cannizzaro 2008d; Güncü 2008; Cannizzaro 2008a; Cannizzaro 2008b; Enkling 2010), and was not done in three studies (Fischer 2004; Turkyilmaz 2007; den Hartog 2011). In the remaining 10 studies it was unclear whether outcome assessment was conducted by examiners blinded to allocated treatment and these studies were assessed at unclear risk of detection bias.
Incomplete outcome data
One study was assessed at high risk of attrition bias due to missing outcome data which was unequally missing from each group (Zöllner 2008). With the low event rate in this study missing outcome data is a likely source of bias. In the remaining 25 included studies all randomised participants were included in the outcome assessments and risk of attrition bias was low.
Selective reporting
We assessed all of the trials included in this review to be at low risk of selective reporting bias because all studies reported the main outcomes of this review.
Other potential sources of bias
An additional source of bias was identified in six included studies (Tawse‐Smith 2002; Lindeboom 2006; Testori 2007; Merli 2008; Cannizzaro 2008b; De Rouck 2009). Different radiographic assessment techniques were used in each group in the study by De Rouck 2009, which introduced a high risk of bias. A high rate of protocol violations occurred in the early loading group in the trial by Merli 2008, which introduced a high risk of bias. In the study by Tawse‐Smith 2002, there was a very different distribution of the length of the implants between the groups with different loading times together with different levels of operator skill associated with the placement of the two different types of implants used in this trial, factors which were assessed as introducing a high risk of bias to this study. Similarly in three other studies there were other differences between the groups besides loading time: Lindeboom 2006, in which more larger diameter implants were used in the immediately occlusally loaded group; Testori 2007, in which more early loaded implants were placed in maxillae; Cannizzaro 2008b, in which more immediately loaded implants were placed in fresh extraction sockets compared to the early loaded group. The clinical significance, if any, of these findings is difficult to interpret, so these three studies were assessed as being at unclear risk of other bias. For six trials, the baseline participant characteristics were not described in sufficient detail to enable an assessment to be made (Assad 2007; Turkyilmaz 2007; Crespi 2008; Donati 2008; Enkling 2010; Meloni 2012).
The overall risk of bias assessment after having incorporated the additional information, kindly provided by the authors of the included trials, is summarised in Figure 1 and Figure 2. Summarising the risk of bias for each study, two trials were judged to be at low risk of bias (Cannizzaro 2008a; Cannizzaro 2008d), 12 trials were judged to be at an unclear risk of bias (Payne 2002; Hall 2006; Lindeboom 2006; Oh 2006; Testori 2007; Assad 2007; Crespi 2008; Donati 2008; Cannizzaro 2008b; Cannizzaro 2010; Enkling 2010; Meloni 2012), whereas 12 trials were judged to be at high risk of bias (Chiapasco 2001; Romeo 2002; Tawse‐Smith 2002; Cannizzaro 2003; Fischer 2004; Turkyilmaz 2007; Güncü 2008; Schincaglia 2008; Zöllner 2008; Merli 2008; De Rouck 2009; den Hartog 2011).
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
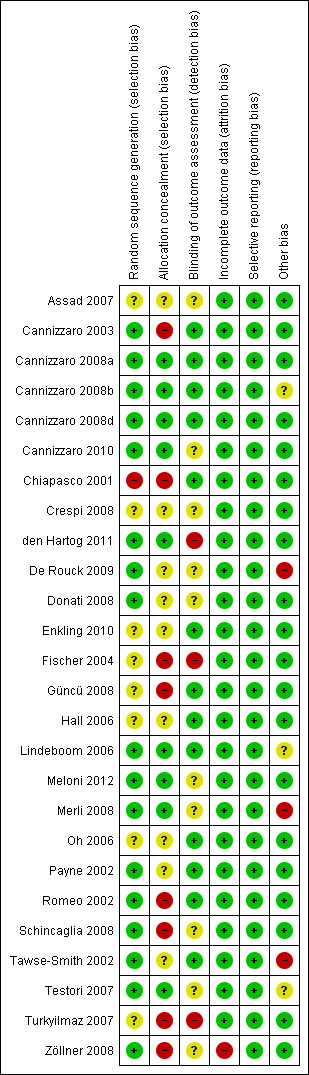
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Analysis of data
As the number of failures was very low (0 or 1) in the split‐mouth studies (one per meta‐analysis), there was no clustering, therefore we presented the data as parallel group data rather than generic inverse variance to enable the reader to view the data. This made no difference to the effect estimates.
Effects of interventions
See: Table 1; Table 2; Table 3
SeeTable 1; Table 2; and Table 3.
In total 2120 implants were originally placed in 1217 participants. The numbers of participants and implants for each comparison are shown below.
| Comparison |
Test Group 1 participants* (implants) |
Test Group 2 participants (implants) |
|
| Immediate versus conventional | All (15 trials) | 299 (432) | 236 (401) |
| Mandible (8 trials) | 98 (199) | 66 (199) | |
| Maxilla (6 trials) | 187 (187) | 156 (156) | |
| Both (1 trial) | 14 (46) | 14 (46) | |
| Early versus conventional | All (3 trials) | 52 (167) | 44 (119) |
| Mandible (2 trials) | 36 (72) | 36 (72) | |
| Maxilla (1 trial) | 16 (95) | 8 (47) | |
| Immediate versus early | All (6 trials) | 268 (464) | 230 (449) |
| Mandible (1 trials) | 30 (30) | (30) | |
| Maxilla (1 trials) | 15 (90) | 15 (87) | |
| Both (4 trial) | 223 (314) | 185 (302) | |
| Occlusal versus non‐occlusal | All (2 trials) | 44 (44) | 44 (44) |
| Maxilla (1 trials) | 24 (24) | 24 (24) | |
| Both (1 trial) | 20 (20) | 20 (20) |
* Participants from the split‐mouth trials are only included once in the 'Test Group 1' column.
During the follow‐up considered in this review (1 year of function for all trials with the exception of Oh 2006 and Cannizzaro 2008d, for which we could only use the 6 and 9 months data, respectively), 57 implants failed. Twenty‐nine of the failed implants were immediately loaded, 20 were early loaded and eight conventionally loaded. Of the 990 planned/placed restorations (unknown number of prostheses placed in Zöllner 2008, we assumed that for each implant failure corresponded one prosthesis failure), 45 (or 43 depending on the success criteria adopted) failed: 22 in the immediately loaded group, 14 (or 12 depending on the success criteria adopted) in the early loaded group, and 4 in the conventionally loaded group. The majority of prosthesis failures occurred in four trials: six prostheses failed in one trial (Tawse‐Smith 2002): five (42%) of those (or three (25%) depending on the success criteria adopted) were early loaded. Three (25%) immediately loaded prostheses failed in one trial (Oh 2006); five (10%) immediately loaded single crowns failed in one trial (Lindeboom 2006) and five (12.5%) immediately loaded single crowns failed in another trial (Cannizzaro 2010).
The meta‐analyses for prosthesis failures, implant failures and marginal bone level changes at 1 year, with the exception of Oh 2006 (6 months data used), Cannizzaro 2008d (6 months data used for radiographs and 9 months data for prosthesis and implant failures), Crespi 2008 (2 years data used for radiographs), and Lindeboom 2006 (18 months data used for radiographs), are presented in Data and analyses 'Comparisons 1 to 4'.
(1) Immediate versus conventional loading after 1 year of function (Comparison 1)
Fifteen trials were included (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Hall 2006; Oh 2006; Turkyilmaz 2007; Assad 2007; Crespi 2008; Güncü 2008; Schincaglia 2008; Donati 2008; De Rouck 2009; Enkling 2010; den Hartog 2011; Meloni 2012).
Data on the numbers of participants, and the number of prosthetic and implant failures are given in the table below.
| Design | Prosthetic failures (immediate loading first) | Implant failures (immediate loading first) | |
| Chiapasco 2001 | Parallel | No failures | 1/10, 1/10 |
| Romeo 2002 | Parallel | No failures | 0/10, 1/10 |
| Cannizzaro 2003 | Parallel | 0/14, 1/14 | 0/14, 1/14 |
| Hall 2006 | Parallel | 1/13, 0/12 | 1/13, 0/12 |
| Oh 2006 | Parallel | 3/12, 0/12 | 3/12, 0/12 |
| Assad 2007 | Parallel | No failures (n = 5) | No failures (n = 5) |
| Turkyilmaz 2007 | Parallel | No failures (n = 10) | No failures (n = 10) |
| Crespi 2008 | Parallel | No failures (n = 20) | No failures (n = 20) |
| Donati 2008 | Parallel | 3/86, 0/51 | 3/86, 0/51 |
| Güncü 2008 | Split‐mouth | 1/13, 0/13 | 1/13, 0/13 |
| Schincaglia 2008 | Parallel | 1/15, 0/15 | 1/15, 0/15 |
| De Rouck 2009 | Parallel | 1/25, 2/24 | 1/25, 2/24 |
| Enkling 2010 | Parallel | No failures (n = 15) | No failures (n = 15) |
| den Hartog 2011 | Parallel | 1/31, 0/31 | 1/31, 0/31 |
| Meloni 2012 | Split‐mouth | No failures (n = 20) | No failures (n = 20) |
Chiapasco 2001 (parallel group design) compared four immediately loaded (2 to 3 days) Brånemark implants with four conventionally loaded (4 to 8 months) implants supporting bar‐retained overdentures in totally edentulous mandibles of adequate shape and quality for 2 years. Ten participants were originally included in each group. No baseline differences were apparent for sex, age, and length of the implants used between the two groups. No withdrawals at 1 year. One implant failed in each group.
Romeo 2002 (parallel group design) compared four immediately loaded (2 days) ITI SLA implants with four conventionally loaded (3 to 4 months) implants supporting bar‐retained overdentures in totally edentulous mandibles of adequate shape and quality for 2 years. Ten participants were originally included in each group. It was unclear whether there were baseline differences between the two groups. No withdrawals at 1 year. One implant failed for peri‐implantitis in the conventionally loaded group.
Cannizzaro 2003 (parallel group design) compared single crowns/bridges immediately loaded (same day) Zimmer Spline twist implants with conventionally loaded implants (3.5 and 4.5 months in mandibles and maxillae respectively) in partially edentulous participants for 2 years. Fourteen participants were originally included in each group. There were no apparent baseline differences with respect to sex, age, bone quality, implant position and length between the two groups. No withdrawals at 1 year. One prosthesis/implant failed at abutment connection in the conventionally loaded group. There was no statistically significant difference in prosthesis failures, implant failures and marginal bone level changes between the different loading strategies (Analysis 1.1; Analysis 1.2; Analysis 1.3).
1.3. Analysis.

Comparison 1 Immediate versus conventional loading, Outcome 3 Marginal bone level changes.
Hall 2006 (parallel group design) compared single immediately non‐occlusally loaded (same day) Southern tapered implants with conventionally loaded implants (6 months) in the anterior maxilla (premolar to premolar) for 1 year. Fourteen participants were originally included in each group. There were no apparent baseline differences for sex, age, bone quality, bone quantity and implant length between the two groups. One participant emigrated from the immediately loaded group (the implant was in function) versus two participants who emigrated to Australia from the conventionally loaded group at 1 year. One prosthesis/implant failed at abutment connection in the immediately loaded group.
Oh 2006 (parallel group design) compared single immediately loaded (same day) Zimmer implants with conventionally loaded implants (4 months) in the anterior maxilla (premolar to premolar), placed with a flapless technique, for 6 months. Twelve participants were originally included in each group. There were no apparent baseline differences for sex, age, bone quality, soft tissue thickness, and implant position between the two groups. No withdrawals at 1 year. Three prostheses/implants failed in the immediately loaded group.
Assad 2007 (parallel group design) compared four immediately loaded (within 4 days) Screw‐Vent implants with four conventionally loaded (4 months) implants supporting bar‐retained overdentures in totally edentulous mandibles of adequate shape for 2 years. Ten participants were originally included five in each group. It was unclear whether there were baseline differences between the two groups. No withdrawals at 1 year. No implant failed.
Turkyilmaz 2007 (parallel group design) compared two unsplinted immediately loaded (1 week) Brånemark TiUnite implants with two unsplinted conventionally loaded (3 months) implants supporting overdentures in totally edentulous mandibles of adequate shape for 2 years. Ten participants were originally included in each group. It was unclear whether there were baseline differences between the two groups. No withdrawals at 1 year. No implant failed.
Crespi 2008 (parallel group design) compared single Outlink Sweden & Martina 13 mm long implants placed in fresh extraction sockets immediately loaded (same day) with identical implants conventionally loaded at 3 months in maxillae (premolar to premolar area) for 2 years. Twenty participants were originally included in each group. There were no baseline differences in implant diameter and position between the two groups. No withdrawals at 1 year. No implant failed.
Donati 2008 (parallel group design) compared one immediately loaded (within 1 day) Astra OsseoSpeed implant with one conventionally loaded (3 months) implant replacing a tooth in position 15 to 25 and 35 to 45 for 1 year. Three groups were formed: two groups had implants immediately loaded. The immediately loaded groups differed in the preparation of the implant site: a conventional preparation with drills (44 participants), and a preparation with osteotomes (42 participants). We considered these two groups as a single group. The control group consisted of 53 participants who had implant sites conventionally prepared and loaded. Ten participants who were treated according to a split‐mouth design had to be excluded from the analyses. It was unclear whether there were baseline differences between the three groups. There were two withdrawals at 1 year from the conventionally loaded group because of poor health conditions. Three crowns/implants failed from the immediately loaded groups: one from the conventionally prepared sites and two from the osteotomes prepared sites.
Güncü 2008 (split‐mouth design) compared one immediately loaded (same day) Brånemark TiUnite implant with one contralateral conventionally loaded (3 months) implant replacing first mandibular molars for 1 year. Thirteen participants were originally included. No baseline differences were apparent between the contralateral sites. No withdrawals at 1 year. One implant/crown failed in the immediately loaded group. There was no statistically significant difference in prosthesis, implant failures and marginal bone level changes between the different loading strategies (Analysis 1.1; Analysis 1.2; Analysis 1.3).
Schincaglia 2008 (parallel group design) compared one immediately loaded (within 1 day) Brånemark TiUnite implant with one conventionally loaded (3 to 4 months) implant replacing first or second mandibular molars for 1 year. Fifteen participants were originally included in each group. There were no apparent baseline differences in implant position and insertion torque between the two groups. However, the implants of the immediately loaded group were longer than those in the conventionally loaded group. No withdrawals at 1 year. One implant/crown failed in the immediately loaded group.
De Rouck 2009 (parallel group design) compared single NobelReplace Tapered Groovy TiUnite implants placed in fresh extraction sockets immediately loaded (same day) with identical implants conventionally loaded at 3 months in anterior maxillae (premolar to premolar area) for 1 year. Twenty‐six participants were originally included in each group, however, two participants from the conventional loading and one from the immediate loading groups were excluded because loss of buccal wall at extractions (conventional loading) and insufficient primary implant stability (20 Ncm; immediate loading). The implant‐bone gap was grafted with granules of anorganic bovine bone. There were no baseline differences in age, gender, implant length, diameter and position between the two groups. No further withdrawals at 1 year. One immediately loaded and two conventionally loaded implants failed.
Enkling 2010 (parallel group design) compared two immediately loaded interforaminal implants with two conventionally loaded (3 months) implants supporting bar‐retained overdentures in totally edentulous mandibles for 1 year. Sixteen participants were originally included in each group. It was unclear whether there were baseline differences between the two groups. The outcome of two participants (one from each group) was not provided. No implant failed.
den Hartog 2011 (parallel group design) compared one immediately loaded (within 1 day) NobelReplace Tapered Groovy TiUnite implant with one conventionally loaded (3 months) implant replacing a tooth in position 14 to 24 for 18 months post‐insertion. Thirty‐one participants were originally included in each group. There were no apparent baseline differences in reason for tooth loss, implant position, length, diameter and bone augmentation between the two groups. When needed sites where augmented with a mixture of autogenous bone and anorganic bovine bone and resorbable collagen barriers both before implant placement and at implant placement. No withdrawals at 18 months. One crown/implant failed from the immediately loaded group.
Meloni 2012 (split‐mouth design) compared one immediately loaded (within 1 day) NobelReplace Tapered Groovy TiUnite implant with one contralateral conventionally loaded (3 months) implant replacing first mandibular molars for 1 year. Twenty participants were originally included. It was unclear whether there were baseline differences between the two groups. No withdrawals at 1 year. No implant failed.
Summary of effects of interventions ‐ immediate versus conventional loading
For the outcome of prosthesis failures, the meta‐analysis of eight trials (381 participants) (Cannizzaro 2003; Hall 2006; Oh 2006; Donati 2008; Güncü 2008; Schincaglia 2008; De Rouck 2009; den Hartog 2011) found no evidence of a difference, risk ratio (RR) 1.90 (95% confidence interval (CI) 0.67 to 5.34) random‐effects, with no heterogeneity (Analysis 1.1). The meta‐analysis was based only on eight trials because there were no prosthesis failures in the other seven trials (110 participants) and therefore the risk ratios for these trials could not be calculated. Of the eight trials included in the meta‐analysis, five trials were at high risk of bias (Cannizzaro 2003; Güncü 2008; Schincaglia 2008; De Rouck 2009; den Hartog 2011), and three trials were at unclear risk of bias (Hall 2006; Oh 2006; Donati 2008).
1.1. Analysis.
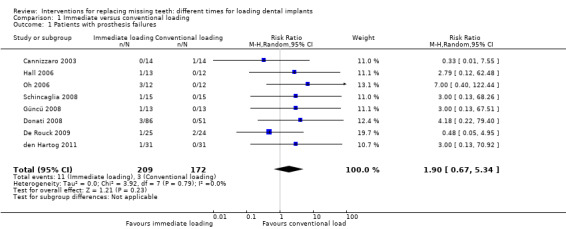
Comparison 1 Immediate versus conventional loading, Outcome 1 Patients with prosthesis failures.
For implant failures, the meta‐analysis of 10 trials (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Hall 2006; Oh 2006; Donati 2008; Güncü 2008; Schincaglia 2008; De Rouck 2009; den Hartog 2011) found no evidence of a difference, RR 1.50 (95% CI 0.60 to 3.77) random‐effects, with no heterogeneity (Analysis 1.2). In a further five trials (total 70 participants) there were no implant failures in either group, so risk ratios could not be calculated and they could not be included in the meta‐analysis. Of the 10 trials included in the meta‐analysis, seven trials were at high risk of bias (Chiapasco 2001; Romeo 2002; Cannizzaro 2003; Güncü 2008; Schincaglia 2008; De Rouck 2009; den Hartog 2011) and three trials were at unclear risk of bias (Hall 2006; Oh 2006; Donati 2008).
1.2. Analysis.
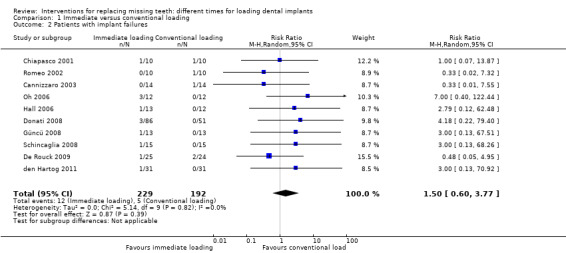
Comparison 1 Immediate versus conventional loading, Outcome 2 Patients with implant failures.
For marginal bone level changes, the meta‐analysis of nine trials (Cannizzaro 2003; Hall 2006; Turkyilmaz 2007; Crespi 2008; Güncü 2008; Schincaglia 2008; Enkling 2010; den Hartog 2011; Meloni 2012) found reduced bone loss associated with immediately loaded implants, MD ‐0.10 mm (95% CI ‐0.20 to ‐0.01; P = 0.03) random‐effects, with some heterogeneity (Tau² = 0.01; Chi² = 14.37, df = 8 (P = 0.07); I² = 44%) (Analysis 1.3). This is based on five trials at high risk of bias (Cannizzaro 2003; Turkyilmaz 2007; Güncü 2008; Schincaglia 2008; den Hartog 2011), and four at unclear risk of bias (Hall 2006; Crespi 2008; Enkling 2010; Meloni 2012).
(2) Early versus conventional loading after 1 year of function (Comparison 2)
Three trials were included (Payne 2002; Tawse‐Smith 2002; Fischer 2004).
Data on the numbers of participants experiencing at least one prosthetic and implant failure are given in the table below.
| Design | Prosthetic failures (early loading first) | Implant failures (early loading first) | |
| Payne 2002 | Parallel | No failures | No failures |
| Tawse‐Smith 2002 | Parallel | 5/24, 1/24 | 5/24, 1/24 |
| Fischer 2004 | Parallel | No failures | 1/16, 2/8 |
Payne 2002 (parallel group design) compared two unsplinted early loaded (6 weeks) ITI SLA implants with two unsplinted conventionally loaded (12 weeks) implants supporting overdentures in totally edentulous mandibles of adequate shape and quality for 2 years. Twelve participants were originally included in each group. There were no apparent baseline differences in gender, bone quality and quantity between the two groups. Two withdrawals occurred from the conventionally loaded group at 1 year. No implant failed.
Tawse‐Smith 2002 (parallel group design) compared two unsplinted early loaded (6 weeks) Southern or Steri‐Oss implants with two unsplinted conventionally loaded (12 weeks) implants supporting overdentures in totally edentulous mandibles of adequate shape and quality for 2 years. Twelve participants were originally included in each of the four groups (Southern early loaded, Steri‐Oss early loaded, Southern conventionally loaded, Steri‐Oss conventionally loaded). There were no apparent baseline differences in bone quality and quantity between the two groups. However, the implants of both the Steri‐Oss and Southern early loaded groups were shorter than those in the conventionally loaded groups, and we were unable to determine the reasons for this. In the article Steri‐Oss implants were described as having a turned surface, but after having analysed the surface of one implant, kindly provided by the authors, it was realised that the implant surface was chemically treated. No withdrawals at 1 year. Seven Steri‐Oss implants failed in five participants of the early loaded group versus one Steri‐Oss implant in the conventionally loaded group. No implants failed in the Southern groups. Most of the failed implants were placed by a surgeon who only placed some Steri‐Oss implants.
Fischer 2004 (parallel group design) compared five to six early loaded (9 to 18 days) ITI SLA implants with five to six conventionally loaded (2.5 to 5.1 months) ITI EstheticPlus implants supporting fixed maxillary cross‐arch bridges for 5 years. Sixteen participants were originally included in the early and eight in the conventionally loaded group. There were no apparent baseline differences in implant length and cantilever length between the two groups. No withdrawals at 1 year. No prosthesis failures. One implant failed in the early loaded group versus two implants in two participants in the conventionally loaded group.
Summary of effects of interventions ‐ early versus conventional loading
For the outcome of prosthesis failure, one trial (Tawse‐Smith 2002) at high risk of bias found no difference between early and conventional loading (Analysis 2.1), and two further trials (one at high risk of bias and the other at unclear risk of bias) had no prosthesis failures in either group in the first year. There is insufficient evidence from these three trials to determine whether there is a clinically important difference between early loading and conventional loading with regard to the outcome of prosthesis failure.
2.1. Analysis.

Comparison 2 Early versus conventional loading, Outcome 1 Patients with prosthesis failures.
For the outcome of implant failure, the meta‐analysis of two trials (Tawse‐Smith 2002; Fischer 2004), both at high risk of bias, found insufficient evidence to determine whether there was a difference between early and conventional loading with regard to implant failure in the first year, RR 1.55 (95% CI 0.46 to 5.18) fixed‐effect, with no evident heterogeneity (Analysis 2.2).
2.2. Analysis.
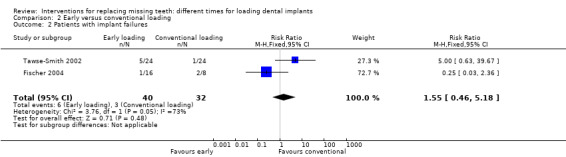
Comparison 2 Early versus conventional loading, Outcome 2 Patients with implant failures.
For marginal bone level changes, the meta‐analysis of two trials (Payne 2002; Tawse‐Smith 2002), one at high and the other at unclear risk of bias, found insufficient evidence to determine whether there was a difference in bone loss associated with early or conventional loading of implants, MD ‐0.04 (95% CI ‐0.15 to 0.07) fixed‐effect, with no evident heterogeneity (Analysis 2.3).
2.3. Analysis.
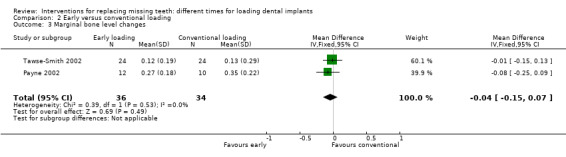
Comparison 2 Early versus conventional loading, Outcome 3 Marginal bone level changes.
(3) Immediate versus early loading after 1 year of function (Comparison 3)
Six trials were included (Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008; Zöllner 2008) in this comparison.
Data on the numbers of participants experiencing at least one prosthetic and implant failure are given in the table below.
| Design | Prosthetic failures (immediate loading first) | Implant failures (immediate loading first) | |
| Testori 2007 | Parallel | 1/25, 0/27 | 1/25, 0/27 |
| Cannizzaro 2008a | Parallel | 0/30, 2/30 | 0/30, 2/30 |
| Cannizzaro 2008b | Parallel | No failures | 1/15, 2/15 |
| Cannizzaro 2008d | Split‐mouth | 1/30, 1/30 | 1/30, 1/30 |
| Merli 2008 | Parallel | No failures | No failures |
| Zöllner 2008 | Parallel | 4/138, 6/128 | 4/138, 6/128 |
Testori 2007 (parallel group design) compared immediately non‐occlusally loaded 3i FNT implants (within 48 hours) with early loaded implants (2 months) supporting single crowns/partial bridges for 4 years. Twenty‐five participants were originally included in the immediately loaded group and 27 in the early loaded group. There were no baseline differences with respect to sex, age, bone quality, implant length and number between the two groups, however, more implants of the early loaded group were placed in maxillae than those of the immediately loaded group. No withdrawals at 1 year. One single implant and its related provisional crown failed after 2 months in the immediately loaded group.
Cannizzaro 2008a (parallel group design) compared two immediately loaded Zimmer SwissPlus implants (within 12 hours) with two early loaded implants (6 weeks), placed with a flapless technique, supporting mandibular bar‐retained overdentures for 1 year. Thirty participants were included in each group. There were no apparent baseline differences for sex, age, smoking habits, number of maxillary dentures, number of immediate post‐extractive implants, and implant length between the two groups. Two implants were immediately replaced with larger diameter ones to obtain the implant insertion torque (> 48 Ncm) required. No withdrawals at 1 year. Two implants failed in two participants of the early loaded group which determined the failure of the overdentures, however both implants were successfully replaced.
Cannizzaro 2008b (parallel group design) compared five to eight immediately loaded Zimmer SwissPlus implants (within 12 hours) with five to eight early loaded implants (2 months), placed with a flapless technique, supporting fixed maxillary cross‐arch bridges for 1 year. Fifteen participants were included in each group. There were no apparent baseline differences for sex, age, smoking habits, number of mandibular dentures, and implant length between the two groups, though more immediate post‐extractive implants were placed in the early loaded group. Four implants were immediately replaced with larger diameter ones to obtain the implant insertion torque (> 48 Ncm) required. No withdrawals at 1 year. No prosthesis failures. One implant did not achieve a sufficient primary stability and was immediately removed and not replaced. Four implants failed: one in the immediately loaded group and three in two participants of the early loaded group.
Cannizzaro 2008d (split‐mouth design) compared single Biomet 3i Nanotite 6.5 mm long cylindrical implants, placed with a flapless technique, immediately occlusally loaded (same day) with identical implants early loaded (6 weeks) for 5 years. Thirty participants were originally included. There were no baseline differences in the number of post‐extractive sites, bone quality, implant diameter and position between the two groups. Eight implants were immediately replaced with larger diameter ones to obtain the implant insertion torque (> 40 Ncm) required. The randomisation of one implant of the immediately loaded group was subverted: the implant was early loaded according to the research protocol since a sufficient implant insertion torque (> 40 Ncm) could not be obtained. No withdrawals at 1 year. One implant and its related crown failed from each group.
Merli 2008 (parallel group design) compared immediately non‐occlusally loaded Thommen implants (within 72 hours) with early non‐occlusally loaded implants (6 weeks), placed with a flapless technique, supporting single crowns/partial bridges for 1 year. Thirty participants were included in the immediately loaded group and 30 in the early loaded group. The randomisation of two participants in each group was subverted: two participants of the immediately loaded group were treated as early loaded participants according to the research protocol since a sufficient implant insertion torque (> 40 Ncm) could not be obtained, whereas two participants of the early loaded group were immediately loaded by mistake. Five additional participants of the early loaded group were actually conventionally loaded. There were no apparent baseline differences with respect to sex, age, bone quality, implant length and number between the two groups. No withdrawals at 1 year. No prosthesis or implant failures.
Zöllner 2008 (parallel group design) compared immediately non‐occlusally loaded implants (the same day) with early non‐occlusally loaded implants (1 month), placed in posterior jaws (premolar and molars areas) supporting single crowns/partial bridges for 1 year. One‐hundred‐and‐thirty‐eight participants were treated in the immediately loaded group and 128 in the early loaded group. There were no apparent baseline differences in bone quality, implant length, number and position between the two groups. Unclear whether five withdrawals occurred in the immediately loaded group and one in the early loaded group prior to implant placement or during a 5‐month follow‐up period and it is unclear how many drop‐outs occurred at 1 year. Four implants failed in the immediately loaded group versus six in the early loaded group. We assumed that an equal number of prostheses were lost, since we were not able to obtain this information from the authors.
Summary of effects of interventions ‐ immediate versus early loading
For the outcome of prosthesis failure, the meta‐analysis of four trials (Testori 2007; Cannizzaro 2008a; Cannizzaro 2008d; Zöllner 2008) found insufficient evidence to determine whether there is a difference between immediate and early loading, RR 0.69 (95% CI 0.25 to 1.87) random‐effects, with no evident heterogeneity (Analysis 3.1). Two of these trials were at low risk of bias (Cannizzaro 2008a; Cannizzaro 2008d), one at unclear risk (Testori 2007) and the other at high risk of bias (Zöllner 2008).
3.1. Analysis.
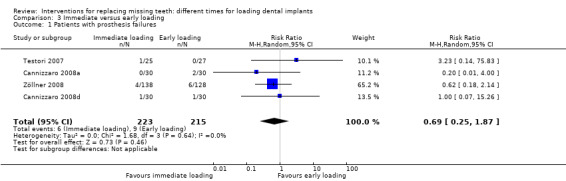
Comparison 3 Immediate versus early loading, Outcome 1 Patients with prosthesis failures.
For the outcome of implant failure, the meta‐analysis of five trials (Testori 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Zöllner 2008) (two low, two unclear and one at high risk of bias) found insufficient evidence to determine whether there is a difference between immediate and early loading, RR 0.65 (95% CI 0.26 to 1.64) random‐effects, with no evident heterogeneity (Analysis 3.2).
3.2. Analysis.
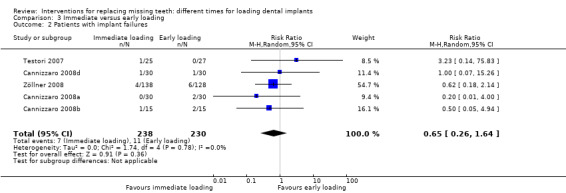
Comparison 3 Immediate versus early loading, Outcome 2 Patients with implant failures.
For the outcome of radiographic bone levels, the meta‐analysis of three trials (Testori 2007; Cannizzaro 2008b; Cannizzaro 2008d) (one low and two at unclear risk of bias) there was insufficient evidence to determine whether there is a difference in bone loss between immediate and early loading of implants, MD ‐0.06 (95% CI ‐0.16 to 0.03) random‐effects, with no evident heterogeneity (Analysis 3.3).
3.3. Analysis.
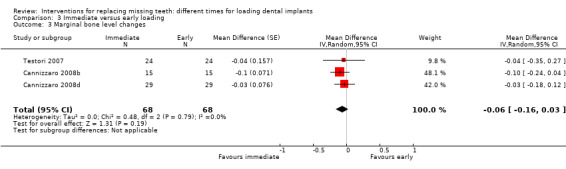
Comparison 3 Immediate versus early loading, Outcome 3 Marginal bone level changes.
(4) Occlusal versus non‐occlusal loading (Comparison 4)
Two trials were included (Lindeboom 2006; Cannizzaro 2010) in this comparison.
Data on the numbers of participants experiencing at least one prosthetic and implant failure are given in the table below.
| Design | Prosthetic failures (occlusal loading first) | Implant failures (occlusal loading first) | |
| Lindeboom 2006 | Parallel | 2/24, 3/24 | 2/24, 3/24 |
| Cannizzaro 2010 | Parallel | 3/20, 2/20 | 3/20, 2/20 |
Lindeboom 2006 (parallel group design) compared immediately occlusally loaded with immediately non‐occlusally loaded single BioComp implants for 1 year. Twenty‐four participants (25 implants) were included in each group. There were no apparent baseline differences in sex, age, previously grafted sites, and implant position between the two groups, though more larger diameter implants were used in the occlusally loaded group. No withdrawals at 1 year. Five crowns/implants failed in five participants: two in the occlusally loaded group and three in the non‐occlusally loaded group.
Cannizzaro 2010 (parallel group design) compared immediately occlusally loaded with immediately non‐occlusally loaded single one‐piece Z‐Look3 zirconia implants for 1 year. Twenty participants were included in each group. There were no apparent baseline differences in sex, age, bone quality, implant position and implant sizes between the two groups. No withdrawals at 1 year. Five crowns/implants failed in five participants: three in the occlusally loaded group and two in the non‐occlusally loaded group.
Summary of effects of interventions ‐ occlusal versus non occlusal loading
The meta‐analysis of two trials (Lindeboom 2006; Cannizzaro 2010), both at unclear risk of bias, found insufficient evidence to determine whether there is a difference between immediate occlusal and non‐occlusal loading, with regard to prosthesis failure, RR 1.00 (95% CI 0.31 to 3.22) fixed‐effect, with no evident heterogeneity (Analysis 4.1), implant failure, RR 1.00 (95% CI 0.31 to 3.22) fixed‐effect, with no evident heterogeneity (Analysis 4.2), or bone loss, MD 0.03 (95% CI ‐0.10 to 0.15) fixed‐effect, with no evident heterogeneity (Analysis 4.3).
4.1. Analysis.
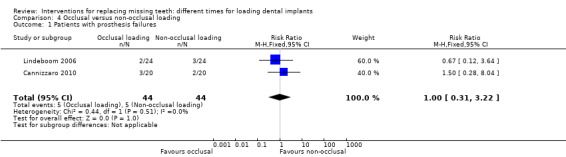
Comparison 4 Occlusal versus non‐occlusal loading, Outcome 1 Patients with prosthesis failures.
4.2. Analysis.

Comparison 4 Occlusal versus non‐occlusal loading, Outcome 2 Patients with implant failures.
4.3. Analysis.
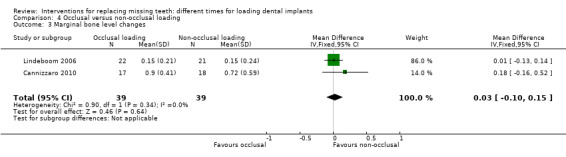
Comparison 4 Occlusal versus non‐occlusal loading, Outcome 3 Marginal bone level changes.
(5) Progressive loading
No trial could be included.
Subgroup analyses
No subgroup analysis was conducted as the maximum number of trials within any meta‐analysis was 10 (Analysis 1.2), and there was no evidence of heterogeneity.
Discussion
Summary of main results
The question of whether implants could be immediately or early loaded after their insertion has relevant clinical implications since the treatment period could be drastically reduced for the patients' benefit. The main outcome for this type of study is the success of the prosthesis since implant loss may not always jeopardise prosthesis success. It was decided to consider only a relatively short follow‐up (4 months to 1 year) since it was felt that such follow‐ups would be sufficient to understand the role of loading on the establishment of osseointegration.
The 26 trials included in this update of the review were divided into four groups based on the comparison being evaluated.
Comparison 1: Immediate versus conventional loading (after 3 months): From 15 randomised controlled trials (RCTs), there was no evidence of a difference in either prosthesis failure (risk ratio (RR) 1.90, 95% confidence interval (CI) 0.67 to 5.34) or implant failure (RR 1.50, 95% CI 0.60 to 3.77) in the first year, but there is some evidence of a small reduction in bone loss favouring immediate loading (MD ‐0.10 mm, 95% CI ‐0.20 to ‐0.01; P = 0.03), with some heterogeneity (Tau² = 0.01; Chi² = 14.37, df = 8 (P = 0.07); I² = 44%).
Comparison 2: Early (6 weeks) versus conventional loading: From three RCTs there is insufficient evidence to determine whether or not there is a clinically important difference in prosthesis failure, implant failure or bone loss.
Comparison 3: Immediate versus early loading: From the six RCTs in this group there is insufficient evidence to determine whether or not there is a clinically important difference in prosthesis failure, implant failure or bone loss between immediate and early loaded implants
Comparison 4: Occlusal versus non‐occlusal loading: From the two trials in this group there is insufficient evidence to determine whether there is a clinically important difference in the outcomes of prosthesis failure, implant failure or bone loss between occlusal and non‐occlusal loading.
Despite including 26 trials with over 1200 patients, the low failure rate of both prostheses and implants in all the included trials means there is still insufficient information to draw definitive conclusions. The only statistically significant difference was a very small reduction in bone loss associated with immediate loading compared to conventional loading, MD ‐0.10 mm (95% CI ‐0.20 to ‐0.01; P = 0.03) but this difference may be too small to be clinically important. There was insufficient evidence from the smaller number of trials in comparisons 2 to 4 to determine whether or not there was a difference in bone loss.
Overall completeness and applicability of evidence
Although this review has included 26 RCTs, the low failure rate of both implants and prostheses in these trials means that there is still insufficient evidence to support definitive conclusions. However, research suggests that thousands of participants would need to be included in randomised trials in order to produce conclusive evidence (Thorlund 2011), and this is unlikely to occur, at least in the short term.
The risk of implant failure can be substantially minimised by proper patient selection and well‐trained operators. It could make more clinical sense to load an implant immediately if the implant was inserted with a sufficient torque and if there are not other factors believed to negatively influence its prognosis. In the case of poor primary implant stability or other suspected negative prognostic variables, it might be preferable to wait for a conventional healing period.
It is possible that some specific factors might have played a determinant role in the final outcome in some of the trials. Factors such as the surgical skill of the operators, or the flapless placement of dental implants, which is technically demanding, might have contributed to the sub‐optimal success rates of immediately loaded implants. Insertion torque is another factor which may be associated with subsequent implant failure, but there is no conclusive evidence as to the minimum insertion torque required to prevent failure. In one trial (Ottoni 2005) an alternation method was used to allocate two single implants between premolars according to a split‐mouth design to immediate non‐occluding loading or conventional loading. Ten out of 23 (44%) immediately loaded implants failed versus only one out of 23 of the conventionally loaded implants. A strong correlation between implant failures and the initial insertion torque of the implants was found. In fact nine out of 10 implants (90%) inserted with a 20 Ncm torque failed, versus only one out of 13 (8%) placed with at least 32 Ncm torque in the immediately loaded group. More recently, an RCT compared success rates of immediately loaded single implants placed according to a split‐mouth design with insertion torques between 25 and 35 Ncm or superior to 80 Ncm in 50 participants (Cannizzaro 2012). Seven of the implants placed with 25 to 35 Ncm torque failed versus none of the implants placed with more than 80 Ncm. In addition, since RCTs reporting excellent success rates for immediately or early loaded implants used techniques to insert the implants with high insertion torques, it can be concluded that a high degree of primary stability at implant insertion is a key prerequisite for successful immediate or early loading procedures. What is the ideal insertion torque? A very accurate answer cannot be given yet, but for single implants insertion torques superior to 35 Ncm are mandatory in order to obtain predictable success rates.
Another important question is how difficult is it to obtain a sufficiently high insertion torque? In a few trials, the number of implants which were immediately replaced in order to obtain the required insertion torque was reported (Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Merli 2008). It is interesting to observe that in one study, eight out of 60 placed implants (13%) were immediately replaced by larger diameter ones to obtain an insertion torque > 40 Ncm (Cannizzaro 2008d). While this appears to be a costly procedure, the following observations should be made: (1) all implants were only 6.6 mm long and were placed according to a flapless procedure some even in fresh extraction sites (30%), therefore it is understandable that is not easy to achieve high insertion torques; (2) this was a clinical trial and it was decided at protocol level that the operator could replace the implants with larger diameter ones in order to conduct the study in the most appropriate way. In everyday clinical practice, the loading of implants which could not achieve the desired torque, could be delayed to allow osseointegration to take place.
It is debated whether immediate 'non‐occluding' loading (i.e. a provisional restoration is placed on the implants but not in contact with the opposite dentition, also called 'immediate provisionalisation'), as opposed to 'occlusal' loading (the restoration is in full occlusal contact with the opposite dentition), could be considered as a real immediate loading procedure. From a patient's point of view, this difference may not be very significant since patients do prefer to have their new teeth as soon as possible (Schropp 2004). In addition, non‐occluding restorations are actually functionally used when chewing. There are only two RCTs included in this review that investigated whether it is better to avoid static or dynamic occlusal contacts at implants immediately restored with single provisional crowns (Lindeboom 2006; Cannizzaro 2010). Their meta‐analyses did not find any statistically significant difference comparing immediate direct occlusal loading versus non‐occlusal loading, because the combined sample size was too small to reach definitive conclusions. Substantial implant failure rates (10% in Lindeboom 2006 and 12.5% in Cannizzaro 2010) were reported In both trials, however, it is difficult to find a reliable explanation for this observation.
In this review update we added the objective to determine whether loading implants progressively, when compared to directly, either immediately, early or conventionally could provide additional beneficial effects. We were unable to include any trial evaluating this technique. The only trial that was identified compared progressive versus direct loading after a healing period of 5 months and included only five participants (Appleton 2005). It was excluded also because it was judged not to be randomised. However, from the general results of the current review it is highly unlikely that progressive loading could provide any clinical benefit; on the contrary it would make loading procedures more time‐consuming and expensive.
The generalisation from the results of the included trials in this review to ordinary clinical practice should be made with extreme caution. In the majority of the included trials, the inclusion criteria were strict and only patients known to be ideal candidates for implant treatment were recruited. In general, operators were highly experienced, and it is important to observe that in those trials with less experienced operators, prosthetic failure rates were higher, ranging from 25% to 42% (depending on the success criteria adopted) (Tawse‐Smith 2002), and 44% (Ottoni 2005). On the other hand, it has been shown that in selected patients it is possible to load dental implants immediately with excellent success rates.
Quality of the evidence
The present review update unfortunately has not brought a great deal of new evidence. In fact, despite the updated search identifying 14 new RCTs, too many of these trials had to be excluded. The most common reason for study exclusion was that some of these trials were not actually randomised as described in the original articles. In addition, we have to say that some of the RCTs currently included in the present review may not be actually randomised. How to explain this recent explosion of 'fake' RCTs? Authors now are starting to be aware that the most reliable study design to evaluate effectiveness of therapy interventions is the RCT, but instead of conducting truly randomised controlled trials, in some cases, a simple controlled trial (sometimes even a retrospective study) is described as randomised, despite the fact that no robust prospective randomised allocation of participants to treatment ever took place. These 'RCTs' may even be published in peer‐reviewed journals, which means that the peer‐review process has failed, in some cases, to detect this issue. There is still a long way to go in order to determine the best interventions for our patients, and if too 'easy' or expedient shortcuts are taken in order to have articles more easily published or cited, there is a serious risk of providing misleading information to clinicians and patients, which is exactly the opposite of what we need.
Sometimes the evaluation of the included trials was complex and may be not completely reliable due to the insufficient information presented in the articles. For instance, there are two large multicentre sponsored trials which could only be included thanks to the kindness of the main authors who provided us with a lot of additional relevant information which was not included in the trial reports (Donati 2008; Zöllner 2008). While it is recognised that running large multicentre clinical trials is not an easy task, more efforts should be made at protocol level to decrease the risk of bias, for instance by randomising participants after implant placement, to have good allocation concealment, to select centres able to conduct clinical research, to clearly report drop‐outs and exclusions and their reasons, and moreover to report results according to international standards (www.consort‐statement.org). In particular, the power of one trial was decreased by having two groups testing different techniques for installing implants to be immediately loaded, and by having 10 participants treated according to a split‐mouth design, despite the study being designed with parallel arms (Donati 2008). There was no report of drop‐outs for the other trial (Zöllner 2008), meaning that it was unknown how many participants completed the trial after 1 year, and it was also unclear how many prostheses were delivered. Interestingly, it was acknowledged that the lack of allocation concealment possibly resulted in participants being treated differently (implants in the immediate loaded group were placed deeper probably as a consequence of higher insertion torques). While the authors attributed no clinical significance to this observation, it clearly underscores the importance of having a proper allocation concealment (Zöllner 2008).
Potential biases in the review process
We conducted a sensitive search of electronic databases and made extensive efforts to obtain information about both published and unpublished trials from researchers and manufacturers of implants. However, we have had to exclude some potentially relevant trials because we could not be sure that allocation was randomised and we were unable to obtain this information from the authors. We also suspect that there is some publication bias, underestimating failures of immediately loaded implants as suggested by a series of published abstracts (Polson 2000), and the information of a trial aborted in the UK due to excessive implant failures. Unfortunately, despite our request, the information was not made available to us. This high risk of publication bias has been considered in the overall assessment of the quality of the body of evidence in the Table 1; Table 2; and Table 3.
Agreements and disagreements with other studies or reviews
One of the problems associated with evaluating the outcomes following implant placement and loading is the lack of information on long‐term outcomes. There are many variables which are likely to affect the success of implants, including but not limited to bone quality and quantity, clinician skill and experience, diameter and length of implant and nature and time of loading. Many of these factors interact.
Our review has included all of the randomised trials available to date, but the low failure rate of implants in most of these trials means that statistically it is not possible to determine whether there is any important difference between different loading times. Other published reviews, less rigorous than Cochrane reviews, have reached similar conclusions (Alsabeeha 2010; Romanos 2010; Chung 2011). In these non‐Cochrane reviews, clinician judgement based on adequate alveolar bone heights and primary implant stability (Javed 2010), and patient preference are factors in the choice of loading time. Where clinical judgement is that immediate loading is appropriate, and patient desire to shorten the treatment period and avoid an extended period of edentulism is expressed, immediate loading of dental implants is an acceptable alternative to conventional protocols if a sufficient implant primary stability is obtained.
Authors' conclusions
Implications for practice.
The failure rate for all the interventions was low, suggesting that under ideal conditions surgeons are able to achieve a high rate of success in loading implants immediately, early or conventionally. Overall there was no convincing evidence of a clinically important difference in either implant failure, prosthesis failure, or bone loss associated with different loading times of implants. A high value of insertion torque (about 35 Ncm) seems to be one of the prerequisites for a successful procedure. The quality of evidence is assessed as very low due to high and unclear risk of bias of the majority of the primary studies and there is some evidence of reporting bias so clinicians should treat these findings with caution.
Implications for research.
More well‐designed randomised controlled trials (RCTs) are needed to understand how reliable the protocols for immediate and early loading are. Such trials should be correctly designed and reported according to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (www.consort‐statement.org). It is suggested that priority should be given to trials assessing the effectiveness of immediately versus early implant loading to improve patient satisfaction and decrease treatment time.
What's new
| Date | Event | Description |
|---|---|---|
| 30 April 2013 | Amended | Rewording of the conclusions of the review. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 20 February 2013 | New citation required and conclusions have changed | Methods updated. One previously included study now excluded. Five new included studies. Changes to the conclusions of the review. New co‐author. |
| 20 February 2013 | New search has been performed | Searches updates to 8 June 2012. |
| 31 October 2008 | New citation required and conclusions have changed | Change of authorship. We have added a secondary hypothesis, previously described in the subgroup analyses. We have updated the review adding 11 new included studies (Lindeboom 2006 (previously excluded); Assad 2007; Turkyilmaz 2007; Cannizzaro 2008a; Cannizzaro 2008b; Cannizzaro 2008d; Crespi 2008; Donati 2008; Güncü 2008; Schincaglia 2008; Zöllner 2008) and additional data from two previously included studies (Merli 2007; Testori 2007). Conclusions slightly changed. |
| 31 October 2008 | New search has been performed | The searches were updated in June 2008. |
| 10 June 2008 | Amended | Converted to new review format. |
| 15 February 2007 | New citation required and conclusions have changed | Change of authorship. We have updated the review adding seven new included studies (Cannizzaro 2003 (previously excluded); Fischer 2004; Hall 2006; Oh 2006; Romanos 2006; Merli 2007; Testori 2007), and excluding eight new trials. We also included studies with a minimum follow up of 6 months in function. Conclusions slightly changed. |
| 15 February 2007 | New search has been performed | The searches were updated in February 2007. |
| 15 March 2004 | New search has been performed | The searches were updated in March 2004. |
| 15 March 2004 | New citation required and conclusions have changed | Change of authorship. We have updated the review and added three new included studies (Payne 2002; Romeo 2002; Romanos in press). We have added to the methods of the review section the following possible subgroup analyses to be conducted in the future if appropriate data become available: (1) Whether implants were placed in mandibles or maxillae (2) Whether implants were placed in partially or fully edentulous jaws (3) Whether implants were placed in the anterior or posterior jaw (4) Different number of inserted implants (for instance overdentures supported by two versus overdentures supported by four implants) (5) Whether turned (machined) or implants with a roughened surface were used (6) Whether the trial was supported by implant manufacturer(s) or not. |
Acknowledgements
We wish to thank Anne Littlewood and Joanne Leese (Cochrane Oral Health Group) for their assistance with literature searching and obtaining copies of the studies respectively; Luisa Fernandez Mauleffinch and Philip Riley (Cochrane Oral Health Group) for their help with the preparation of this review; Peter Thomsen and Mark Willings, Hubert Achille, and Paul Coulthard for contributing as co‐authors to previous versions of this review; and Gioacchino Cannizzaro, Matteo Chiapasco, Mauro Donati, Norbert Enkling, Kerstin Fischer, Baris Güncü, Michael Hotze, Jerome Lindeboom, Silvio Meloni, Mauro Merli, Judith Maria Pinheiro Ottoni, Alan Payne, George Romanos, Eugenio Romeo, Andrew Tawse‐Smith, Ilser Turkyilmaz and Axel Zöllner for providing us with information on their trials. We would also like to thank the following referees: Matteo Chiapasco, Kerstin Fischer, Anne‐Marie Glenny, Lee Hooper, Jerome Lindeboom, David R Moles, Ian Needleman, Michele Nieri, Alan Payne, and Andrew Tawse‐Smith.
Appendices
Appendix 1. MEDLINE (OVID) search strategy
1. exp Dental Implants/ 2. exp Dental Implantation/ or dental implantation 3. exp Dental Prosthesis, Implant‐Supported/ 4. ((osseointegrated adj implant$) and (dental or oral)) 5. dental implant$ 6. (implant$ adj5 dent$) 7. (((overdenture$ or crown$ or bridge$ or prosthesis or restoration$) adj5 (Dental or oral)) and implant$) 8. "implant supported dental prosthesis" 9. ("blade implant$" and (dental or oral)) 10. ((endosseous adj5 implant$) and (dental or oral)) 11. ((dental or oral) adj5 implant$) 12. OR/1‐11
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. The Cochrane Oral Health Group's Trials Register search strategy
(dental‐implants OR "dental implant*" OR "oral implant*" OR dental‐implantation OR dental‐prosthesis‐implant‐supported OR "implant supported" OR "implant supported prosthesis" OR dental‐implantation‐endosseous‐endodontic OR "endosseous implant*" OR blade‐implantation OR "blade implant*" OR (implant* AND (oral OR dental)) or dental‐implantation‐subperiosteal OR "subperiosteal implant" OR (implant* AND overdenture*) OR ((overdenture* OR crown* OR bridge* OR prosthesis OR prostheses OR restoration*) AND ("dental implant*" OR "Oral implant" OR (zygoma* AND implant*))))
Appendix 3. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 DENTAL IMPLANTS explode all trees (MeSH) #2 DENTAL IMPLANTATION explode all trees (MeSH) #3 DENTAL PROSTHESIS IMPLANT‐SUPPORTED single term (MeSH) #4 ((osseointegrat* near implant*) and (dental* or oral*)) #5 (dental next implant*) #6 (implant* near dent*) #7 dental‐implant* #8 ((overdenture* near dental*) and implant*) #9 ((overdenture* near oral*) and implant*) #10 ((crown* near dental*) and implant*) #11 ((crown* near oral*) and implant*) #12 ((bridge* near dental*) and implant*) #13 ((bridge* near oral*) and implant*) #14 ((prosthesis near dental*) and implant*) #15 ((prosthesis near oral*) and implant*) #16 ((prostheses near dental*) and implant*) #17 ((prostheses near oral*) and implant*) #18 ((restoration* near dental*) and implant*) #19 ((restoration* near oral*) and implant*) #20 (implant next supported next dental next prosthesis) #21 (blade next implant*) #22 ((endosseous near implant*) and dental) #23 ((endosseous near implant*) and oral*) #24 ((dental* near implant*) or (oral* near implant*)) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24)
Appendix 4. EMBASE (OVID) search strategy
1. tooth implantation/ 2. ((implant‐supported or implant$) adj support$).mp. 3. ((osseointegrated adj implant$) and (dental or oral)).mp. 4. ((dental implant$ or dental‐implant or implant$) adj (dent$ or oral or tooth)).mp. 5. (((overdenture$ or crown$ or bridge$ or prosthesis or prostheses or restoration$) adj5 (dental or oral)) and implant$).mp. 6. "implant supported dental prosthesis".mp. 7. ("blade implant$" and (dental or oral or tooth or teeth)).mp. 8. ((endosseous adj5 implant$) and (dental or oral or tooth or teeth)).mp. 9. ((dental or oral or tooth or teeth) and implant$).mp. 10. or/1‐9
The above subject search was linked to the Cochrane Oral Health Group filter for EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Data and analyses
Comparison 1. Immediate versus conventional loading.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with prosthesis failures | 8 | 381 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.67, 5.34] |
| 2 Patients with implant failures | 10 | 421 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.60, 3.77] |
| 3 Marginal bone level changes | 9 | 293 | Mean Difference (Random, 95% CI) | ‐0.10 [‐0.20, ‐0.01] |
Comparison 2. Early versus conventional loading.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with prosthesis failures | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.63, 39.67] |
| 2 Patients with implant failures | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.46, 5.18] |
| 3 Marginal bone level changes | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.15, 0.07] |
Comparison 3. Immediate versus early loading.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with prosthesis failures | 4 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.25, 1.87] |
| 2 Patients with implant failures | 5 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.26, 1.64] |
| 3 Marginal bone level changes | 3 | 136 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.16, 0.03] |
Comparison 4. Occlusal versus non‐occlusal loading.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with prosthesis failures | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.22] |
| 2 Patients with implant failures | 2 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.22] |
| 3 Marginal bone level changes | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.10, 0.15] |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Chiapasco 2001.
| Methods | Trial design: randomised, parallel group trial Location: Milan, Italy Number of centres: one (University of Milan Dental clinic) Recruitment period: 1996 to 1997 Funding source: Z systems partially supported this trial |
|
| Participants | Inclusion criteria: patients that have been edentulous in the mandible for at least 3 months. Mandibles allowing the placement of 4 implants at least 13 mm long Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected at implant insertion (none), previously irradiated jaws, severe bruxism, smoking habits (more than 10 cigarettes a day) and any systemic diseases likely to compromise implant surgery Age at baseline: mean age 58.4 years, (44 to 73) Gender: M5/F15 Number randomised: 20 Number evaluated: 20 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 10) Immediate loading: 4 implants immediately loaded (within 3 days of insertion) Gp B (n = 10) Conventional loading: 4 implants supporting a bar and an overdenture conventionally loaded 4 to 8 months later Brånemark (Nobel Biocare AB, Göteborg, Sweden) submerged turned titanium MKII screws were used Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, Periotest, marginal bone level changes on panoramic radiographs, plaque accumulation, modified bleeding index, probing pocket depth 1‐year data used | |
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Reported in the article: "Patients were randomly assigned to 1 of 2 treatment groups: immediate loading (test group, n=10) or delayed loading (control group, n=10)" Author replied that "a drawing lot was used, however since there was a numeric imbalance between the 2 groups, once a group was completed the remaining patients were allocated to the other group" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Reviewer comments: with the methods of randomisation used by the author it is not possible to ensure a proper allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "measurements were made by a blinded post‐graduate training specialist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Tawse‐Smith 2002.
| Methods | Trial design: randomised, parallel group trial Location: Dunedin, New Zealand Number of centres: one (university dental clinic of the University of Otago, Dunedin, New Zealand) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients with edentulous mandibles having 13 to 15 mm of residual anterior bone height Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected at implant insertion (none), previously bone‐grafted or irradiated jaws, history of bruxism, any evidence of current or previous smoking and any systemic diseases likely to compromise implant surgery Age at baseline: range 55‐80 years Gender: not reported Number randomised: 48 (4 groups, 2 groups evaluated each implant system) Number evaluated: 48 (no withdrawals at 1 year) |
|
| Interventions |
Comparison: Early versus conventional loading Gp A (n = 12) Early loading: 2 unsplinted implants with ball attachments loaded at 6 weeks Gp B (n = 12) Conventional loading: 2 unsplinted implants with ball attachments supporting an overdenture loaded at 12 weeks Patients in groups A&B used Steri‐Oss® (Steri‐Oss, Yorba Linda, California, USA) non‐submerged acid‐etched titanium screws HL series, 3.8 mm in diameter Gp C (n = 12) Early loading: 2 unsplinted implants with ball attachments loaded at 6 weeks Gp D (n = 12) Conventional loading: 2 unsplinted implants with ball attachments supporting an overdenture loaded at 12 weeks Patients in groups C&D used Southern (Southern Implants Ltd, Irene, South Africa) non‐submerged sand‐blasted acid‐etched titanium screws Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, Periotest, marginal bone level changes on standardised intraoral radiographs, plaque accumulation, modified sulcus bleeding index, probing pocket depth, width of the keratinised mucosa 1‐year data used |
|
| Notes | Sample size calculation: not reported Most of the failed implants were placed by a surgeon who placed only Steri‐Oss implants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "Participants were randomly allocated to either the Sterioss system or the Southern Implant system on a one‐by‐one basis. For each system, participants were further allocated with maximum concealment into 2 subgroups (12 participants), in whom mandibular implant overdentures and their respective matrices were inserted following the standard 12‐week healing period, or the test group (12 participants), in whom a 6‐week healing period was followed by loading in a similar manner" Author replied that "Table of random numbers were used to randomly allocated participants" |
| Allocation concealment (selection bias) | Unclear risk | Reported in the article: "participants were randomly allocated to either the Sterioss system or the Southern Implant system on a one‐by‐one basis. For each system, participants were further allocated with maximum concealment into 2 subgroups (12 participants), in whom mandibular implant overdentures and their respective matrices were inserted following the standard 12‐week healing period, or the test group (12 participants), in whom a 6‐week healing period was followed by loading in a similar manner" No clarifying reply to letter |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "measurements were made by calibrated blinded outcome assessors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | High risk | It appears that implants were not placed in a balanced way by different operators but that one inexperienced operator, placing preferentially one type of implants, might have biased the success rates |
Romeo 2002.
| Methods | Trial design: randomised, parallel group trial Location: Milan, Italy Number of centres: one (dental clinic of the University of Milan, Italy) Recruitment period: 1997‐1999 Funding source: not stated |
|
| Participants | Inclusion criteria: patients that have been edentulous in the mandible for at least 3 months. Mandibles allowing the placement of 4 implants of at least 10 mm length Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected at implant insertion (none), previously irradiated jaws, severe bruxism, smoking habits (more than 20 cigarettes a day) and any systemic diseases likely to compromise implant surgery Age at baseline: mean 63.2 (range 42‐73 years) Gender: M8/F12 Number randomised: 20 Number evaluated: 20 (no withdrawals at 1 year) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 10) Immediate loading: 4 implants immediately loaded (within 2 days from insertion) Gp B (n = 10) Conventional loading: 4 implants supporting a bar and an overdenture conventionally loaded (3 to 4 months) ITI (Institut Straumann AG, Waldenburg, Switzerland) SLA non‐submerged solid titanium screws were used Duration of follow‐up: 2 years |
|
| Outcomes | ||
| Notes | Sample size calculation: not reported but trial authors note that small sample size was a limitation of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "Patients were randomly attributed to the test (immediate loading) or control (delayed loading), each one made up of 10 patients" Author replied that "a drawing lot was used" Reviewer comments: with the methods of randomisation used by the author it is unlikely to have 2 numerically balanced groups |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that "the operator knew patient allocation the day of intervention" Reviewer comments: with the methods of randomisation used by the author it is not possible to ensure a proper allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "measurements were made by a calibrated blinded outcome assessor" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Payne 2002.
| Methods | Trial design: randomised, parallel group trial Location: Dunedin, New Zealand Number of centres: one (University of Otago dental clinic, Dunedin, New Zealand) Recruitment period: not stated Funding source: study was supported by ITI Research Foundation (Research Grant #203/2000 RCL), Institut Straumann AG (Waldenburg, Switzerland, Ivovclar Vivadent (Auckland New Zealand), Radiographic Supplies (Christchurch New Zealand) & Colgate Oral Care, New Zealand |
|
| Participants | Inclusion criteria: elderly patients with edentulous mandibles having 13 to 15 mm of residual anterior bone height Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected at implant insertion (none), previously bone‐grafted or irradiated jaws, history of bruxism, any evidence of current or previous smoking and any systemic diseases likely to compromise implant surgery Age at baseline: range 55‐80 years Gender: M12/F12 Number randomised: 24 Number evaluated: 22 (2 withdrawals at 1 year from the conventionally loaded group for emigration) |
|
| Interventions |
Comparison: Early versus conventional loading Gp A (n = 12) Early loading: 2 unsplinted implants with ball attachments early loaded at 6 weeks Gp B (n = 12) Conventional loading: 2 unsplinted implants with ball attachments supporting an overdenture conventionally loaded at 12 weeks ITI (Institut Straumann AG, Waldenburg, Switzerland) SLA non‐submerged solid titanium screws were used Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, Periotest, Osstell, marginal bone level changes on standardised intraoral radiographs, plaque accumulation, modified sulcus bleeding index, probing pocket depth, width of the keratinised mucosa 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "Using a table of random numbers participants were randomly allocated, with maximum allocation concealment (Esposito et al. 2001), to two treatment groups, each with 12 participants" |
| Allocation concealment (selection bias) | Unclear risk | Reported in the article: "Using a table of random numbers participants were randomly allocated, with maximum allocation concealment (Esposito et al. 2001), to two treatment groups, each with 12 participants" No clarifying reply to letter |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "measurements were made by calibrated blinded outcome assessors" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Cannizzaro 2003.
| Methods | Trial design: randomised, parallel group trial Location: Pavia, Italy (private practice) Number of centres: one Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: partially edentulous patients (both mandibles and maxillae) allowing the placement of at least 13 mm long implants with a diameter of 3.7 mm. For implants to be immediately loaded, a primary implant stability of 45 Ncm had to be achieved at insertion Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected at implant insertion, less than 3 years irradiated jaws, severe bruxism, smoking habits (more than 10 cigarettes per day), substance abusers, pregnancy, uncontrolled diabetes, and any systemic diseases likely to compromise implant surgery Age at baseline: 18 to 72 years, Group 1 mean 37.1 years, Group 2 mean 38.6 years Gender: 14M/14F Number randomised: 28 Number evaluated: 28 (at 1 year) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 14) Immediate loading: One or more adjacent implants restored first with acrylic restorations in full occlusion and then with cemented metal‐ceramic prostheses, loaded the same day Gp B (n = 14) Conventional loading: One or more adjacent implants restored first with acrylic restorations in full occlusion and then with cemented metal‐ceramic prostheses,loaded after 3.4 months (mandibles) or 4.5 months (maxillae) Zimmer (Zimmer, Carlsbad, Ca, USA) Spline Twist MTX titanium screws were used Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, Periotest, Osstell, marginal bone level changes on standardised intraoral radiographs, plaque accumulation, probing pocket depth 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Nothing reported in the article Author replied that "random number tables were used" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that "just before the intervention the operator was informed of which therapy to deliver" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in the article: "A member of the treatment team recorded the following parameters 1 month after prosthesis seating (baseline) and every 6 months until conclusion of the study in June 2001" Author replied that: "3 blinded outcome assessors were used: Dr Fontana S. for radiographic evaluation, Dr Ignaccolo S. for Periotest evaluation, and Dr Leone M. for periodontal indexes evaluation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Fischer 2004.
| Methods | Trial design: randomised, parallel group trial Location: Falun, Sweden Number of centres: one (County Hospital, Falun, Sweden) Recruitment period: April 1999 to September 2000 Funding source: Institut Straumann supported this study |
|
| Participants | Inclusion criteria: patients with edentulous maxillae allowing the placement of 5 to 6 implants Exclusion criteria: smoking habits (more than 10 cigarettes a day), use of augmentation procedures at the implanted sites, and any systemic diseases likely to compromise implant surgery Age at baseline: mean 64 years Gender: immediate M6/F10; conventional M2/F6 Number randomised: 24 Number evaluated: 24 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 16) Immediate loading: 5‐6 implants per patient restored directly with definitive full‐arch titanium‐resin prosthesis with cantilevers (7‐11 mm long) at 9‐18 days. ITI SLA (Institut Straumann AG, Waldenburg, Switzerland) non‐submerged solid titanium screw type implants were used Gp B (n = 8) Conventional loading 5‐6 implants per patient restored directly with definitive full‐arch titanium‐resin prosthesis with cantilevers (7‐11 mm long) after 2.5‐5.1 months. Submerged ITI EstheticPlus implants in the conventionally loaded group (except for one patient) Duration of follow‐up: 5 years |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on standardised intraoral radiographs, plaque index, sulcus bleeding index, width of the keratinised mucosa 1‐year data used | |
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "Patients were randomized and consecutively enrolled in the study according to predefined inclusion and exclusion criteria" Reviewer comments: patients were randomised with a 2 to 1 ratio, i.e. 16 in the early loading group and 8 in the conventional loading group. There are no advantages by doing this and authors had further reduced their already small sample size |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that "When patient had signed the informed consent form a sealed treatment code envelope was opened through which patient was allocated to either the test or the control group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nothing reported in the article Author replied that "outcome assessors were not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Hall 2006.
| Methods | Trial design: randomised, parallel group trial Location: Dunedin, New Zealand Number of centres: one (University of Otago Dental Clinic, Dunedin, New Zealand) Recruitment period: not stated Funding source: Southern Implants (Irene, south Africa) and Radiographic Supplies (Christchurch, New Zealand, supported this trial |
|
| Participants | Inclusion criteria: patients missing a single tooth in anterior maxilla (premolar to premolar) with adjacent teeth present, allowing the placement of at least 10 mm long implant with a diameter of 2.5 mm Exclusion criteria: patients with type IV bone quality (very soft bone) according to the Lekholm and Zarb classification detected on radiographs, severe bruxism, smoking habits (more than 20 cigarettes per day), previous history of failed implants, and sites requiring augmentation surgery Age at baseline: mean 43.25 range 23 to 71 years Gender: not stated Number randomised: 28 Number evaluated: 25 (3 withdrawals at 1 year, 1 for the immediately loaded group and 2 from the conventionally loaded group for emigration) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 14) Immediate loading non‐occlusal ‐ single implants restored first with acrylic restorations (not in occlusion), then by screw‐connected metal‐ceramic crowns Gp B (n = 14) Conventional loading Single implants had screw‐retained provisional crowns placed after 6 months Definitive screw‐retained metal ceramic crowns were placed into occlusion for all participants 8 weeks after provisionalisation Southern (Southern Implants Ltd, Irene, South Africa) tapered sand‐blasted acid‐etched titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, Periotest, marginal bone level changes on standardised intraoral radiographs, plaque accumulation, sulcus bleeding index, unspecified peri‐implant soft tissues and prosthetic outcomes measures including the Papilla Index by Jemt 1997 1‐year data used |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "Participants.....were randomly allocated using sealed envelopes to the conventional loading...." The reply of the author failed to clarified the issue |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported in the article The reply of the author failed to clarified the issue |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "outcome assessors were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented. 3 withdrawals at 1 year, 1 for the immediately loaded group (included in outcome based on email responses) and 2 from the conventionally loaded group for emigration |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Lindeboom 2006.
| Methods | Trial design: randomised, parallel group trial Location: Amsterdam, the Netherlands Number of centres: one (Oral and Maxillofacial Surgery Department of the Academic Medical Center of the University of Amsterdam, The Netherlands) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: healthy (ASA I) patients missing single teeth in anterior maxilla (premolar to premolar), allowing the placement with no fenestration of a at least 8 mm long implant with a diameter of 3.4 mm placed with an insertion torque of at least 30 Ncm Exclusion criteria: patients with smoking habits, parafunctional habits, drug or alcohol abuse, lack of a stable occlusion, lack of adequate proper oral hygiene and compliance and any systemic diseases likely to compromise implant surgery Age at baseline: 42.3 years ± 13.1 (range 19‐78) Gender: M17/F31 Number randomised: 48 Number evaluated: 48 |
|
| Interventions |
Comparison: Occlusal versus non‐occlusal immediate loading Gp A (n = 24) Immediate loading occlusal: Single implants immediately restored within 1 day with acrylic single crowns in occlusion Gp B (n = 24) Immediate loading non occlusal: Single implants immediately restored within 1 day with acrylic single crowns not in occlusion Permanent ceramic crowns were provided to both groups after 6 months BioComp® (BioComp Industries BV, Vught, The Netherlands) tapered TPS screws were used Duration of follow‐up :1 year |
|
| Outcomes | Prosthesis/implant failures, Osstell, marginal bone level changes on intraoral radiographs, Papilla Index by Jemt 1997 and midbuccal gingival levels 1‐year data used |
|
| Notes | Sample size calculation:Not reported, but authors note that small sample size in this study was the reason that "no definite conclusions could be drawn" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "Patients were allocated to either immediate loading or immediate provisionalization groups by means of computer generated randomization" |
| Allocation concealment (selection bias) | Low risk | Nothing reported in the article Author replied that "The treatment allocation (after computer randomization) was presented at placement of the provisional. so that the patient was treated with an implant, and after that was moved to the next room with an impression post for provisional fabrication. At that point a call was received from the Clinical Epidemiology Unit (performing randomization)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "outcome assessors were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Unclear risk | The distribution of small and larger diameter implants is different in the two groups. There are more large diameter implants in the immediate loading group |
Oh 2006.
| Methods | Trial design: randomised, parallel group trial Location: Ann Arbor, USA Number of centres: one (University of Michigan dental clinic, Ann Arbor, USA) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: 2 single teeth missing in the anterior maxilla (premolar to premolar) allowing the placement of at least 10 mm long implants with a diameter of 3.8 mm Exclusion criteria: none specified Age at baseline: immediate 45.2 ±13.2; delayed 47.3 ±17.8 Gender: immediate M8/F4; delayed M2/F10 Number randomised: 24 Number evaluated: 24 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 12) Immediate loading: Single implants placed with a flapless technique and restored first with acrylic restorations and then 10‐14 days later replaced with cemented metal‐ceramic crowns Gp B (n = 12) Conventional loading: Single implants placed with a flapless technique and restored with cemented metal‐ceramic crowns after 4 months Zimmer (Zimmer, Carlsbad, Ca, USA) non‐submerged implants were used Duration of follow‐up: 6 months |
|
| Outcomes | Prosthesis/implant failures, probing pocket depths, plaque index, sulcus bleeding index, marginal level of soft tissues, soft tissue thickness, width of the keratinised mucosa, Papilla Index by Jemt 1997 6‐month data used |
|
| Notes | Sample size calculation: not reported but small sample size noted by the authors as a limitation of this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "The subjects were randomly assigned to one of two groups: IL (12 patients) or DL (12 patients)" No reply to letter |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported in the article No reply to letter |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in the article: "All of the clinical measurements were performed by one calibrated blind examiner at baseline (at the time of implant loading) and at 2, 4, and 6 months after implant loading" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Turkyilmaz 2007.
| Methods | Trial Design: randomised, parallel group trial Location: Ankara, Turkey Number of centres: one (Faculty of Dentistry, Hecettepe University, Ankara, Turkey) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients with edentulous mandibles allowing the placement of 2 x 15 mm long implants Exclusion criteria: patients with previously bone‐grafted or irradiated jaws, and any systemic diseases likely to compromise implant surgery Age at baseline: immediate 62.4 ± 8.6 years); delayed 62.3 ± 7.1 years Gender: M8/F12 Number randomised: 20 Number evaluated: 20 (no withdrawals at 1 year) |
|
| Interventions |
Comparison: Early versus conventional loading Gp A (n = 5) Immediate loading: 2 unsplinted implants with ball attachments supporting an overdenture immediately loaded at 1 week Gp B (n = 5) Conventional loading: 2 unsplinted implants with ball attachments conventionally loaded at 3 months Brånemark® (Nobel Biocare AB, Göteborg, Sweden) non‐submerged TiUnite Mark III type titanium screws were used Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, Osstell, marginal bone level changes on standardised intraoral radiographs, complications 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "The patients randomly allocated into two groups pre‐operatively" Author replied that "We actually toss a coin (heads or tails) and created groups randomly" Reviewer comment: "it is possible but highly unlikely to create 2 groups with identical number of patients by tossing a coin" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that "The surgeon did not know the groups before implant placement" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nothing reported in the article Author replied that "the outcome assessor was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Assad 2007.
| Methods | Trial design: parallel group RCT Location: Prosthodontic Department, Al Zahraa University Hospital, Egypt Number of centres: one Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients with edentulous mandibles allowing the placement of 4 12 mm long and 3.7 mm wide implants Exclusion criteria: any systemic or local disease that might contraindicate implant placement Age at baseline: 48 to 63 years Gender: all male Number randomised: 10 patients, 4 implants per patient Number evaluated: 10 patients, 4 implants per patient |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 5) Immediate loading: 4 implants immediately loaded (within 4 days of insertion) Gp B (n = 5) Conventional loading: 4 implants supporting a bar and an overdenture conventionally loaded (4 months after placement) Screw‐Vent (Paragon, Core‐Vent Corporation, Las Vegas, NV, USA) submerged titanium screws were used on all patients. "All patients received maxillary conventional complete dentures and mandibular bar retained dentures" Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, implant percussion, marginal bone level changes on standardised intraoral radiographs, gingival index, plaque index, probing pocket depth reported at baseline, 6,12,18 & 24 months | |
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "Patients participating in this study were randomly divided into 2 equal groups, each containing 5 edentulous patients" No reply to letter |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported in the article No reply to letter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing reported in the article No reply to letter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Testori 2007.
| Methods | Trial design: multicenter, randomised, parallel group trial Location: Italy Number of centres: five (private practices in Italy) Recruitment period: prior to May 2005 Funding source: not stated |
|
| Participants | Inclusion criteria: partially edentulous patients (both mandibles and maxillae) allowing the placement of at least 8.5 mm long implants with a diameter of 4 mm. For implants to be immediately loaded, a primary implant stability of 20 or 30 Ncm had to be achieved at insertion for implants which were going to be splinted and single implants, respectively Exclusion criteria: patients irradiated in the head and neck area, severe bruxism, substance abusers, pregnancy, uncontrolled diabetes, and any systemic diseases likely to compromise implant surgery, lack of opposing occluding dentition, need for augmentation procedures Age at baseline: immediate 51.6 (range 27‐74 years); delayed 51.3 (range 34‐73 years) Gender: immediate M13/F12; delayed M8/F17 Number randomised: 52 Number evaluated: 52 (no withdrawals at 1 year) |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 25) Immediate loading: 1 or more implants restored first with acrylic restorations (not in occlusion) and then with cemented metal‐ceramic crowns, within 48 hours Gp B (n = 27) Early loading: 1 or more implants restored first with acrylic restorations (not in occlusion) and then with cemented metal‐ceramic crowns, after 2 months 3i (3i Biomet , Palm Beach, FL, USA) Osseotite FNT tapered titanium screws were used; 1 of the centres used some other similar prototypes in both groups Duration of follow‐up :4 years |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on intraoral radiographs, buccal peri‐implant marginal soft tissue levels 1‐year data used |
|
| Notes | Sample size calculation: reported that 26 patients per group would be required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A manually generated restricted randomization list was used to create two groups with equal number of patients" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "Only one of the investigators, not involved in the selection and treatment of the patients, was aware of the randomization sequence and could have access to the randomization list stored in a pass‐word protected portable computer. The randomized codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially only after the implants to be included in the trial were inserted, therefore treatment allocation was concealed to the investigator in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the article: "Prosthesis and implant failures were assessed by the treating clinicians who were therefore not blinded....Radiographic bone level and height the clinical crown changes and outcome measures were assessed by 2 independent, blinded, calibrated outcome assessors. One measured all radiographs and the other the clinical crown height on plaster models. All radiographs were coded so that the outcome assessor was blinded to which group the implants belonged to, nor was the assessor informed of the aims of the study. Since the assessor did not know which crown(s) to measure on the plaster models, the implant‐supported crown(s) were marked with a black spot. The outcome assessors did not know the dates that either the radiographs or the plaster models were created" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented |
| Other bias | Unclear risk | There was a difference between the groups in the number of implants placed in the maxillae (14/52 in immediate loading group vs 31/52 in early loading group) |
Cannizzaro 2008d.
| Methods | Trial Design: randomised, split‐mouth group trial Location: Pavia, Italy (private practice) Number of centres: one Recruitment period: September to November 2007 Funding source: implants and prosthetic components were donated by Biomax and Biomet 3i |
|
| Participants | Inclusion criteria: partially edentulous patients needing 2 x 7 mm long single implants in bone at least 5.5 wide. For implants to be immediately loaded, a primary implant stability of 40 Ncm had to be achieved at insertion Exclusion criteria: irradiated in the head and/or neck less than 1 year previously, substance abusers, pregnancy or lactation, uncontrolled diabetes, severe bruxism or clenching, and any systemic diseases likely to compromise implant surgery, need for augmentation procedures with exception of Bio‐Oss in fresh extractions sockets, lack of opposite occluding dentition/prosthesis, psychiatric problems Age at baseline: 35 years (18‐57) Gender: M15/F15 Number randomised: 30 Number evaluated: 30 |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 15) Immediate loading: 2 single implants, planned to be placed with a flapless procedure, immediately (same day) loaded with acrylic crowns replaced by metal‐ceramic crowns after 9 weeks Gp B (n = 15) Early loading: 2 single implants, planned to be placed with a flapless procedure, early loaded after 6 weeks with acrylic crowns replaced by metal‐ceramic crowns after 3 weeks 7 mm* long Biomet 3i (Palm Beach, Florida, USA) Nanotite cylindrical titanium‐alloy implants non‐submerged were used Duration of follow‐up: 4 years |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on intraoral radiographs, patient satisfaction, complications 9‐month data used |
|
| Notes | Sample size calculation: reported that 26 patients were required to show a change in failure rate from 39% to 4%, so the trial recruited 30 participants to allow for drop‐outs * In the first paper (Cannizzaro 2008d) with follow‐up data 9 months after loading, these implants were described as 6.5 mm because the implant height without the external hexagon is 6.5 mm. In the subsequent paper(Cannizzaro 2012) the same implants are described as 7 mm ‐ a measure which included the external hexagon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A computer‐generated restricted randomisation list was used to create two groups with equal numbers of patients by Dr Marco Esposito, who was not involved in patient recruitment or treatment and had access to the randomisation list stored in a password‐protected portable computer" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes.Envelopes were opened sequentially only after the two implants were inserted, therefore treatment allocation was concealed to the investigator (GC) in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in the article: "Complications were assessed by the treating dentist (GC), who was not blinded, but implant stability and ISQ values were recorded by an independent dentist who was not aware of patient allocation (ML)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented |
| Other bias | Low risk | None detected |
Cannizzaro 2008a.
| Methods | Trial Design: randomised, parallel group trial Location: Pavia,Italy (private practice) Number of centres: one Recruitment period: November 2004 to December 2005 Funding source: "No commercial support was received by the investigators" |
|
| Participants | Inclusion criteria: patients with edentulous mandibles allowing the placement of 2 implants at least 10 mm long and with a diameter of 3.7 mm. For implants to be immediately loaded, a primary implant stability of 48 Ncm had to be achieved at insertion Exclusion criteria: general contraindications to implant surgery, poor oral hygiene and motivation, substance abuse, pregnancy or lactation, uncontrolled diabetes, and any systemic diseases likely to compromise implant surgery, need for augmentation procedures, lack of opposite occluding dentition/prosthesis, psychiatric problems Age at baseline: immediate 62 (44‐72); early 61 (36‐80) Gender: M25/F35 Number randomised: 60 Number evaluated: 60 (after 1 year) |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 30) Immediate loading: 2 implants, placed with a flapless procedure, supporting a bar and an overdenture immediately (same day) Gp B (n = 30) Early loading: 2 implants, placed with a flapless procedure, supporting a bar and an overdenture loaded early (6 weeks) Zimmer SwissPlus (Zimmer, Carlsbad, Ca, USA) non‐submerged solid titanium screws were used Duration of follow‐up: 1‐year |
|
| Outcomes | Prosthesis/implant failures, Osstell, patient satisfaction, complications 1‐year data used |
|
| Notes | Sample size calculation: authors suggest that a "sample size of 300 patients pre group may be needed to detect a difference in prosthesis or implant failures" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "The operator coded the selected implants sites on the forms as site number 1 and site number 2...... A computer generated restricted randomization list was created by one of the authors who was not involved in patient recruitment or treatment, and had access to the random list stored in a password‐protected portable computer (Dr Marco Esposito)" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The randomized codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially only after both implants were placed, therefore treatment allocation was concealed to the investigator in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in the article: "Complications were assessed by the treating dentist (Dr Gioacchino Cannizzaro) who was not blinded. Two independent dentists (Dr Michele Leone and Dr Cinzia Torchio) not aware of patient allocation evaluated implant stability and peri‐implant marginal bone levels changes on periapical radiographs, respectively" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented |
| Other bias | Low risk | None detected |
Crespi 2008.
| Methods | Trial Design: randomised, parallel group trial Location: Milan, Italy Number of centres: one ("Vita Salute" University, San Rafaele Hospital) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients rehabilitated with single implants in maxillary (premolar to premolar) fresh extraction sockets with 4 bony walls and at least 4 mm of bone beyond the root apex and the presence of adjacent teeth Exclusion criteria: presence of dehiscence or fenestration of the residual bony walls, signs of acute infection around the alveolar bone at the implant site, bruxism, patients smoking more than 10 cigarettes a day, uncontrolled diabetes, coagulation disorders, alcohol or drug abuse Age at baseline: mean 47.21 years (24‐68 years) Gender: M16/F24 Number randomised: 40 Number evaluated: 40 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 20) Immediate loading: 13 mm long single implants loaded immediately (the same day) Gp B (n = 20) Conventional loading: 13 mm long single implants loaded conventionally at 3 months Outlink (Sweden & Martina, Padova, Italy) titanium solid screws were used Duration of follow‐up: 2 years |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on periapical radiographs 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "All patients required a single‐tooth extraction for root fractures, caries or endodontic reasons, or periapical disease and were randomly assigned to the test or control group" No reply to letter |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported in the article No reply to letter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing reported in the article No reply to letter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Donati 2008.
| Methods | Trial design: multicenter, randomised, parallel group trial with 3 arms Location: Italy Number of centres: eight (private practices in Italy) Recruitment period: not stated Funding source: "This study was supported by grants from Astra Tech Dental" |
|
| Participants | Inclusion criteria: patients missing a single tooth in area 15‐25 and 35‐45 allowing the placement of 8 mm long and 4 mm large single implant Exclusion criteria: post‐extractive implants (at least 3 months healing was required), insertion torque < 20 Ncm, and any systemic diseases likely to compromise implant surgery Age at baseline: M mean age 46.7 (SD18.3); F Mean age 44.2 (SD 12.9) Gender: M70/F81 Number randomised: 139 (151 patients were randomised (57, 50, 54 tooth sites), however, 10 of these patients had 2 tooth sites treated according to a split mouth design and their data were excluded from this systematic review) Number evaluated: 137 patients (2 withdrawals from conventionally loaded group due to poor health) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 44) Immediate loading: Standard preparation procedure for implant placement and immediate functional loading Gp B (n = 42) Immediate loading: Modified preparation procedure with osteotomies, followed by immediate functional loading Gp C (n = 51) Conventional loading: Single implants restored after 3 months with occluding screw‐retained acrylic crowns, replaced after 6 months by cemented or screw‐retained metal‐ceramic crowns Astra OsseoSpeed® (Astra Tech Dental, Mölndal, Sweden) titanium grade 1 screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on standardised intraoral radiographs, plaque accumulation, mucositis, probing pocket depth, changes in papilla height and width of the keratinised mucosa 1‐year data used |
|
| Notes | Sample size calculation: not reported 10 patients who were treated according to a split‐mouth design, and their data were excluded from this systematic review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A randomization protocol was produced from a computer‐generated list for the distribution of subjects in the three treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Nothing reported in the article Author replied failed to clarify the matter |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the article: "The radiographs were analyzed by an experienced radiologist who was blinded with regard to treatment groups" Author replied that "the outcome assessor for the clinical outcomes was not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented after clarification by the author |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Schincaglia 2008.
| Methods | Trial Design: randomised, parallel group trial Location: Bologna, Italy Number of centres: one (School of Dentistry, University of Bologna, Italy) Recruitment period: 2002‐2004 Funding source: not stated |
|
| Participants | Inclusion criteria: patients missing 1 mandibular first or second molar allowing the placement of a single implant at least 8.5 mm long and 5 mm wide with a minimal insertion torque of 20 Ncm Exclusion criteria: severe systemic conditions (ASA III), in need of bone augmentation, and if the tooth was extracted less than 4 months before Age at baseline: immediate 51.87 (range 31‐75 years); delayed 49.2 (range 35‐68 years) Gender: immediate M4/F11; delayed M5/F10 Number randomised: 30 Number evaluated: 30 (no withdrawals at 1 year) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 15) Immediate loading: Single implants restored the same day with occluding screw‐retained acrylic crowns, replaced after 3 to 4 months by cemented or screw‐retained metal‐ceramic crowns Gp B (n = 15) Conventional loading: Single implants were initially connected to a healing abutment and directly restored after 3‐4 months with cemented or screw‐retained metal‐ceramic crowns Brånemark® (Nobel Biocare AB, Göteborg, Sweden) non‐submerged TiUnite Mark III Wide‐Platform type titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on intraoral radiographs, buccal peri‐implant marginal soft tissue levels 1‐year data used |
|
| Notes | Sample size calculation: reported that 14 implants in each group were required to give 80% power detect a difference of 0.3 mm (SD 0.4 mm) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "Patients were allocated to either the immediate loading or in the delayed loading group using a randomization table" Author replied that: "The patients were consecutively assigned to test and control groups according to a predetermined randomization table" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that: "the surgeon was not blinded in relation to the type of treatment before the implant placement" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the article: "A blinded examiner made the bone height measurements" The author reply to our request of clarification implied that the other clinical outcome measures were not assessed by a blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Zöllner 2008.
| Methods | Trial Design: multicentre randomised, parallel group trial Location: 10 countries Number of centres: 19 Recruitment period: not stated Funding source: sponsor |
|
| Participants | Inclusion criteria: patients missing teeth in premolar and molar areas allowing the placement of 8 mm long and 4.1 mm large single implant Exclusion criteria: post‐extractive implants (at least 4 months healing was required), lack of primary implant stability, opposing fixed dentition, smoking > 10 cigarettes per day, severe bruxism/clenching, and any systemic diseases likely to compromise implant surgery Age at baseline: 46.3 ± 12.8 years Gender: not stated Number randomised: 266 Number evaluated: unclear. Possibly 5 withdrawals from the immediately loaded group (3 subjects withdrew consent and 2 could not be located) and 1 from the early loaded group (withdrew consent) at 5 months (it is unclear whether patients were lost prior to or after implant placement) and unknown number of withdrawals at 1 year |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 138) Immediate loading: temporary acrylic restoration out of occlusal contact, placed on the day of surgery, with permanent occluding restoration (either single crown or 2‐4 unit fixed prosthesis cemented or screw‐retained restorations made of porcelain, acrylic resin or gold) placed 20‐23 weeks after surgery Gp B (n = 128) Early loading: temporary acrylic restoration out of occlusal contact, placed 28‐34 days after surgery, with permanent occluding restoration (either single crown or 2‐4 unit fixed prosthesis cemented or screw‐retained restorations made of porcelain, acrylic resin or gold) placed 20‐23 weeks after surgery ITI® SLA active (Institut Straumann AG, Waldenburg, Switzerland) solid sand‐blasted large‐grit acid‐etched titanium grade 4 screws, 3 standard plus implants were also used Duration of follow‐up: 1 year (interim analysis) |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on standardised intraoral radiographs 1‐year data used |
|
| Notes | Sample size calculation: reported that 35 patients per group would be required to detect a difference of 0.3 mm in bone loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the first article: "This is an ongoing prospective randomized study in which patients receive between one and four implants in the posterior maxilla or mandible.... Patients were randomized into immediate or early loading arms" Reported in the second article: "The randomization list was generated for each center by an independent statistician using block sizes of 10; randomization numbers were sequential, and each group (immediate or early) had an equal number in each block" Author replied that: "Patients were randomized into two loading arms according to a master randomization list to receive a temporary restoration (single crown or 2‐4 unit fixed partial denture) out of occlusal contact. A randomization list defined which patient is treated within the immediate or early loading arms. The randomization list was generated by an independent statistician for each of the 19 centers using 2 block sizes of 10 for each center, with each center having its own unique center number and sequential randomization number, and an equal number (i.e. 5 each) within each block randomized to either group immediate or early" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the first article Reported in the second article: "The sequential randomization was placed in sealed envelopes for each center, which were opened before surgery after obtaining signed informed consent from the patient" Author replied that: "The sequential randomization for each center was placed in a sealed envelope, which the investigator was allowed to open at least one to 5 days prior to the planned surgery and only after signed informed consent from the patient had been obtained. Patients were consecutively enrolled into the study and had the sealed treatment code envelope that corresponded to their time of entry, patient 1 received envelope 1, the 2nd patient received envelope 2 and so on for each center. Master copies of the randomization lists were held by the sponsor and the organization who performed the randomization" Comment: as the group allocation was known prior to surgery we have assessed this as high risk bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing reported in the article Author replied that: "The analysis of bone level change was performed by an independent expert who was blinded to the allocation of the treatment group. Secondary objective were to evaluate the effectiveness in terms of implant survival, implant success and patient satisfaction at month 5. Secondary parameters were assessed by each clinician. They were not blinded in respect of treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Possibly 5 withdrawals from the immediately loaded group (3 subjects withdrew consent and 2 could not be located) and 1 from the early loaded group (withdrew consent) at 5 months (it is unclear whether patients were lost prior to or after implant placement) and unknown number of withdrawals at 1 year |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Cannizzaro 2008b.
| Methods | Trial design: randomised, parallel group trial Location: Pavia, Italy (private practice) Number of centres: one Recruitment period: November 2004 to November 2005 Funding source: "No commercial support of any form has been received by the investigators" |
|
| Participants | Inclusion criteria: patients with edentulous maxillae allowing the placement of 5 to 8 implants at least 10 mm long and with a diameter of 3.7 mm. For implants to be immediately loaded, a primary implant stability of 48 Ncm had to be achieved at insertion Exclusion criteria: irradiated head & neck less than 1 year previously, substance abusers, pregnancy or lactation, uncontrolled diabetes, and any systemic diseases likely to compromise implant surgery, need for augmentation procedures, lack of opposite occluding dentition/prosthesis, psychiatric problems Age at baseline: immediate 62 (45‐75); early 56 (42‐69) Gender: M15/F15 Number randomised: 30 Number evaluated: 30 |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 15) Immediate loading: 5‐8 implants, placed with a flapless procedure, immediately (same day) restored with metal reinforced acrylic provisional prostheses replaced 2 to 3 months after by full‐arch metal ceramic or metal resin prostheses with short cantilevers Gp B (n = 15) Early loading: 5‐8 implants, placed with a flapless procedure, restored after 2 months with metal reinforced acrylic provisional prostheses replaced 2 to 3 months after by full‐arch metal ceramic or metal resin prostheses with short cantilevers Zimmer SwissPlus (Zimmer, Carlsbad, Ca, USA) non‐submerged solid titanium screws were used Duration of follow‐up: 1‐year |
|
| Outcomes | Prosthesis/implant failures, Osstell, marginal bone level changes on intraoral radiographs, patient satisfaction, complications 1‐year data used |
|
| Notes | Sample size calculation: not reported, but authors note that the actual sample size was insufficient to detect a statistically significant difference in prosthesis/implant failures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A computer generated restricted randomisation list was used to create two groups with equal numbers of patients by one of the authors who was not involved in patient recruitment or treatment and had access to the randomisation list stored in a password‐protected portable computer (Dr Marco Esposito)" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The randomised codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially only after all the implants were inserted, therefore treatment allocation was concealed to the investigator in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported in the article: "Complications were assessed by the treating dentist (Dr Gioacchino Cannizzaro), who was not blinded. Independent dentists who were not aware of patient allocation evaluated implant stability, including ISQ values (Dr Michele Leone) and marginal bone levels changes (Dr Cinzia Torchio)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented |
| Other bias | Unclear risk | More implants (n = 22) were placed in fresh extraction sockets in the early loaded group compared to the immediate loading group (n = 12 implants placed in fres extraction sockets) |
Merli 2008.
| Methods | Trial Design: randomised, parallel group trial Location: Rimini, Italy Number of centres: one (private practice) Recruitment period: not stated Funding source: partial support for this study was provided by Thommen Medical AG, Wldenburg, Switzerland |
|
| Participants | Inclusion criteria: partially edentulous patients (both mandibles and maxillae) allowing the placement of at least 9.5 mm long implants and the bone thickness at implant sites had to be of at least 5.5 mm. For implants to be immediately loaded, a primary implant stability of 40 Ncm had to be achieved at implant insertion Exclusion criteria: patients irradiated in the head and neck area within the previous year, severe bruxism, substance abusers, pregnancy, uncontrolled diabetes, and any systemic diseases likely to compromise implant surgery, lack of opposing occluding dentition, a need for bone‐augmentation procedures with exception of Bio‐Oss granules in post‐extractive sites, presence of less than 4 mm of keratinised mucosa Age at baseline: immediate 50.3 (28 to 72 years); early 48.7 (19 to 68 years) Gender: immediate M10/F20; early M12/F18 Number randomised: 60 Number evaluated: 60 |
|
| Interventions |
Comparison: Immediate versus early loading Gp A (n = 30 patients, 35 implants) Immediate loading: 1 or more implants placed with a flapless technique and restored with non‐occluding acrylic restorations, within 72 hours Gp B (n = 30 patients, 34 implants) Early loading: 1 or more implants placed with a flapless technique and restored with non‐occluding acrylic restorations after 6 weeks For all patients permanent restoration with occluding cemented metal‐ceramic crowns was performed after 6 months SPI®Element System (Thommen Medical AG, Waldenburg, Switzerland) sand‐blasted acid‐etched screws, and in some of the post‐extraction sites SPI®Contact troncoconical screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures and complications 1‐year data used |
|
| Notes | Sample size calculation: reported that 26 patients per group would be required to show a difference in proportion of failures from 39% to 4% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the first article: "A manually generated restricted randomization list was used to create two groups with equal numbers of patients. Only one of the investigators, not involved in the selection and treatment of the patients, was aware of the randomization sequence and had access to the randomization list stored in a password‐protected portable computer" |
| Allocation concealment (selection bias) | Low risk | Reported in the first article: "The randomized codes were enclosed in sequentially numbered, identical, opaque, sealed envelopes. Envelopes were opened sequentially only after the implants to be included in the trial were inserted, therefore treatment allocation was concealed to the investigator in charge of enrolling and treating the patients included in the trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the first article: "These outcome measures (prosthetic failures, implant failures and complications) were assessed by an independent assessor (FB), who was not blinded to the interventions" Reported in the third article: "Although patients and the surgeon were aware of the allocated arm, radiographic outcome assessor (G. Mariotti) was kept blinded to the allocation. Prosthesis failure, implant failure and complications were assessed by an independent assessor (M. Moscatelli), who was not blinded to the intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes were presented |
| Other bias | High risk | A substantial rate of protocol deviations were present, for instance of the 30 patients allocated to the early loading group, 5 were conventionally loaded and 2 immediately loaded |
Güncü 2008.
| Methods | Trial design: randomised, split‐mouth group trial Location: Ankara Turkey Number of centres: one (Faculty of Dentistry, Hecettepe University, Ankara, Turkey) Recruitment period: November 2004 to August 2005 Funding source: not reported |
|
| Participants | Inclusion criteria: patients missing both mandibular first molars allowing the placement of 11.5 mm long and 4 mm large single implants with an implant to crown length ratio 1/1 Exclusion criteria: smoking, osteoporosis, severe parafunctional habits, post‐extractive implants, untreated periodontal disease, poor oral hygiene, drug or alcohol abuse, need of augmentation procedures at the implanted sites, and any systemic diseases likely to compromise implant surgery Age at baseline: 41.09 years (± 8.46) range 30‐55 Gender: M4/F8 Number randomised: 12 Number evaluated: 12 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 12) Immediate loading: Single implants restored the same day with acrylic crown and after 1 week with occluding cemented metal‐ceramic crowns Gp B (n = 12) Conventional loading: Single implants restored after 3 months with occluding cemented metal‐ceramic crowns Brånemark® (Nobel Biocare AB, Göteborg, Sweden) non‐submerged TiUnite Mark III type titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, Osstell, marginal bone level changes on intraoral radiographs, plaque index, gingival index, probing depths, bleeding time index 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported in the article: "Randomization for IL and CL selection was performed by coin toss" |
| Allocation concealment (selection bias) | High risk | Nothing reported in the article Author replied that: "Coin toss was performed before implant placement during surgery for each patient. Group allocation was concealed to the investigators" Reviewers comment: if the coin was tossed before implant placement obviously allocation could not be concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nothing reported in the article Author replied that "the outcome assessor was blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented after clarification by the author |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
De Rouck 2009.
| Methods | Trial design: randomised, parallel group trial Location: Bruxelles, Belgium Number of centres: one (Dental Clinic of the Free University) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients requiring the immediate replacement of a single tooth in the anterior maxilla (15‐25) having both neighbouring teeth and at least 5 mm of bone height apical to the alveolus of the failing tooth to allow implant placement with a minimal insertion torque of 35 Ncm Exclusion criteria: systemic diseases, smoking more than 10 cigarettes a day, bruxism, lack of posterior occlusion, non‐treated periodontal diseases, presence of active infection (pus, fistula) around the hopeless tooth, loss of the labial crest after extraction of the failing tooth Age at baseline: immediate 55 years (SD 13); conventional 52 years (SD 12) Gender: immediate M11/F13; conventional M12/F13 Number randomised: 52 Number evaluated: 49 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 26) Immediate loading: Single immediate post‐extractive implants restored the same day with non‐occluding screw‐retained acrylic crowns, replaced after 6 months by permanent metal‐ceramic crowns Gp B (n = 26) Conventional loading: Implants were conventionally loaded and restored after 3 months with screw‐retained acrylic crowns, replaced after 3 months by permanent metal‐ceramic crowns. A collagen membrane (Bio‐Gide, Geistlich Biomaterials) covered the implant prior to crown placement The implant‐bone gap was grafted with granules of anorganic bovine bone Nobelreplace Tapered Groovy (Nobel Biocare AB, Göteborg, Sweden) TiUnite titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, marginal bone level changes on intraoral radiographs, plaque, bleeding on probing, probing pocket depths, papilla levels, midfacial mucosal levels, patient's esthetic satisfaction 1‐year data used |
|
| Notes | Sample size calculation: paper states that 24 patients per group were required to show a difference in soft tissue dimensions of 0.5 mm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "This was performed by means of a computer‐generated randomization scheme" |
| Allocation concealment (selection bias) | Unclear risk | Reported in the article: "After screening for recruitment, impressions were made to fabricate an acrylic stent for recording purposes. Thereupon, patients were randomly allocated to the ‘immediate restoration group’ (IRG) or the ‘delayed restoration group’ (DRG)". Allocation concealment not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nothing reported in the article No reply to letter |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported in the article: "From the 52 patients that had been recruited (actually randomised), 49 were included in the study [IRG: 24 patients, DRG: 25 patients]. In two patients the labial crest was partially lost following tooth removal. Another patient listed for immediate restoration was excluded as the insertion torque was only 20 Ncm" |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | High risk | The author used different baseline radiographic assessment making the 2 groups actually non‐comparable |
Cannizzaro 2010.
| Methods | Trial design: multicenter, randomised, parallel group trial Location: Italy Number of centres: four (private dental practices) Recruitment period: February to November 2008 Funding source: Z systems partially supported this trial |
|
| Participants | Inclusion criteria: patients missing a single tooth with residual bone height of at least 10 mm and width of at least 5 mm allowing implant placement with an insertion torque of at least 35 Ncm Exclusion criteria: general contraindications to implant surgery, lack of opposite occluding dentition in the area intended for implant placement, acute infection in the area intended for implant placement, immunosuppression or immunodepression, untreated periodontitis, poor oral hygiene and motivation, irradiation in the head or neck area, bruxism, under treatment or past treatment with intravenous amino‐bisphosphonates, uncontrolled diabetes, pregnant or lactating, substance abuse, psychiatric disorders or unrealistic expectations, participation in other clinical trials interfering with present protocol, having been referred only for implant placement or unable to be followed for at least one year, requiring the use of a membrane at implant placement, implant sites subjectively evaluated as being characterised by soft bone quality Age at baseline: occlusal 38 (918‐54); non‐occlusal 39 (26‐55) Gender: M17/F23 Number randomised: 40 Number evaluated: 40 |
|
| Interventions |
Comparison: Occlusal versus non‐occlusal immediate loading Gp A (n = 20) Immediate loading: (Occlusal) Single implants immediately restored the same day with acrylic single crowns in occlusion Gp B (n = 20) Immediate loading: (Non‐occlusal) Single implants immediately restored the same day with acrylic single crowns not in occlusion Permanent ceramic crowns were cemented after 4 to 5 months in both groups Z‐Look3® (Z‐System, Oensingen, Switzerland) one‐piece zirconia sand‐blasted screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, marginal bone levels, complications 1‐year data used |
|
| Notes | Sample size calculation: sample size calculation reported and states that the original intention was to recruit 80 patients in each group to detect a difference between a proportion of 0.999 and 0.920 (patients experiencing at least one implant failure) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A computer generated restricted randomisation list was created. Only one investigator (ME), who was not involved in the selection and treatment of the patients, knew the randomization sequence and had access to the randomization list stored in a pass‐word protected portable computer" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The randomised codes were enclosed in sequentially‐numbered, identical, opaque, sealed envelopes. Flap closure was obtained with vicryl 4.0 and the envelope containing the randomisation code was opened to disclose patient allocation........ Envelopes were opened sequentially after the implants were placed. Therefore, treatment allocation was concealed to the investigators in charge of enrolling and treating the patients" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the article: "One dentist (CT) who treated 10 patients performed all radiographic assessments without knowing group allocation for the patients of the other 3 centres, therefore the outcome assessor was blinded for 30 out of 40 patients........ " Author replied that: "implant success was assessed by blinded outcome assessors at each centers" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Enkling 2010.
| Methods | Trial design: randomised, parallel group trial Location: not reported Number of centres: not reported Recruitment period: not reported Funding source: not reported |
|
| Participants | Inclusion criteria: patients with edentulous mandibles allowing the placement of 2 interforaminal implants 9.5 mm long and wit 4 mm in diameter with an insertion torque of at least 35 Ncm. Patients were dissatisfied with the retention of their dentures after having wore them for at least 2 months Exclusion criteria: not reported Age at baseline: not reported Gender: not reported Number randomised: 32 Number evaluated: 30 (due to missing data) |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 16) Immediate loading: overdentures on 2 mandibular interforaminal implants using a prefabricated bar (SFI‐Bar®, C+M, Biel, Switzerland) Gp B (n = 16) Conventional loading: (3 months) loading of overdentures on 2 mandibular interforaminal implants using a prefabricated bar (SFI‐Bar®, C+M, Biel, Switzerland) SICace® (SIC invent AG, Basel, Switzerland) titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Prosthesis/implant failures, marginal bone levels, complications, oral‐health‐related‐quality‐of life (OHRQoL), plaque index, probing pocket depth, bleeding on probing, sounding depth 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided in the abstract Author replied that: "We recruited pairs of patients regarding the parameters age and gender. In each pair the allocation to the immediate or delayed loading group was randomized by a computer generated list" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in the abstract No reply to this question |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information provided in the abstract Author replied that: "The clinical assessors as well as the assessors for the x‐rays (post‐graduate students) were blinded and were independent of the surgical and prosthetic team" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information provided in the abstract Author replied that: "After the first year, we had two drop‐outs as two paired female patients died due to age reasons" The problem is that the data at 1 year for one patient per group is missing |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
den Hartog 2011.
| Methods | Trial design: randomised, parallel group trial Location: Groningen, The Netherlands Number of centres: one (University of Groningen, The Netherlands) Recruitment period: January 2005 to February 2008 Funding source: study was funded by Nobel Biocare AB, Goteborg, Sweden (grant # 2004‐288) |
|
| Participants | Inclusion criteria: patients missing a maxillary single tooth from first to first premolars allowing the placement of a single implant at least 13 mm long and 3.5 mm wide with a minimal insertion torque of 45 Ncm Exclusion criteria: severe systemic conditions (ASA III), presence of periodontal disease, previous irradiation, smoking, presence of peri‐apical lesions or any other abnormalities in the maxillary anterior region, and missing tooth extracted less than 3 months before study start Age at baseline: immediate 38.4 (± 14.0); conventional 40.1 (± 14.4) Gender: immediate M9/F22; conventional M17/F14 Number randomised: 62 Number evaluated: 62 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 31) Immediate loading: implants were restored within 24 hours with provisional non‐occluding acrylic crowns, replaced after 6 months by cemented or screw‐retained metal‐ceramic crowns Gp B (n = 31) Conventional loading implants were also restored after 3 months with provisional occluding acrylic crowns, replaced after 3 months by cemented or screw‐retained occluding metal‐ceramic crowns Nobel Replace Tapered Groovy (Nobel Biocare AB, Göteborg, Sweden) submerged TiUnite titanium screws were used Duration of follow‐up: 18 months |
|
| Outcomes | Crown and implant success, marginal bone levels, recession, various aesthetic indexes, patient satisfaction 1‐year data used for crown/implant success and 18 months form marginal bone level changes |
|
| Notes | Sample size calculation: paper states that "sample size was too small to demonstrate whether immediate loading was non‐inferior to conventional loading with respect to implant survival" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "A specifically designed locked computer software program was used to randomly assign patients to one of two study groups.... Randomization by minimization (Altman 1991) was used to balance possible prognostic variables between the treatment groups. Minimization was used for the variables age (<30 years, >31 <60 years, >60 years), location of the implant site (central or lateral incisor, canine or first premolar)and whether or not a pre‐implant augmentation procedure was indicated based on a clinical and diagnostic cast assessment" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The allocation result was kept in a locked computer file that was not accessible for the examiner and the practitioners. The surgeon who inserted the implants was informed about the allocation on the day of surgery, before implant surgery was started" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reported in the article: "The examiner was blinded to the photographs and the radiographs taken at T6m and T18m. The radiographic examination could not be blinded to the radiographs collected after implant placement (baseline, T0), as the study group could be deduced from these radiographs.... The examiner was blinded for the protocol that was applied for a particular patient (for clinical measurements).....The examiner was blinded to the group allocation (for the aesthetic outcome assessment" Reviewer comment: "it was possible to take baseline radiograph not showing group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected. |
Meloni 2012.
| Methods | Trial design: multicenter, randomised, split‐mouth trial Location: Sassari, Italy Number of centres: three (University of Sassari and in two private practices in Sassari, Italy) Recruitment period: not stated Funding source: not stated |
|
| Participants | Inclusion criteria: patients missing 2 bilateral mandibular first molars allowing the placement of a single implant at least 8 mm long and 4.3 mm wide with a minimal insertion torque of 35 Ncm Exclusion criteria: bone height < 10 mm, bone thickness < 6 mm, < 5 mm of keratinised gingiva, general contraindications to implant surgery; lack of occluding dentition in the area intended for immediate loading, periodontitis, bruxism, immunosuppression, previous history of irradiation of the head and neck area, uncontrolled diabetes, smoking >10 cigarettes/day, poor oral hygiene, current or past treatment with bisphosphonates, substance abuse, psychiatric disorder, need of bone augmentation, pregnancy or lactation Age at baseline: not stated Gender: not stated Number randomised: 20 Number evaluated: 20 |
|
| Interventions |
Comparison: Immediate versus conventional loading Gp A (n = 5) Immediate loading: implants restored within 24 hours with provisional non‐occluding acrylic crowns, replaced after 4 months by cemented definitive zirconia‐ceramic or metal‐ceramic crowns on individualised titanium‐zirconia abutments Gp B (n = 5) Conventional loading: implants restored after 4 months with cemented definitive zirconia‐ceramic or metal‐ceramic crowns on individualised titanium‐zirconia abutments NobelReplace Tapered Groovy (Nobel Biocare AB, Göteborg, Sweden) non‐submerged TiUnite titanium screws were used Duration of follow‐up: 1 year |
|
| Outcomes | Crown and implant success, complications, marginal bone levels, probing pocket depth (PPD) and bleeding on probing (BOP) 1‐year data used |
|
| Notes | Sample size calculation: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported in the article: "In each eligible patient, the mandibular right or left molar was randomly selected to receive either an immediate or delayed provisional crown; the randomisation code was assigned by an independent operator not involved in the trial, and was placed in envelopes with a code done by a dedicated computer programme combining a sequence of numbers with the two different procedures" |
| Allocation concealment (selection bias) | Low risk | Reported in the article: "The envelope containing a randomisation code to assign the immediate and the delayed site was opened only in this moment (after implant placement) by a blinded independent medical doctor and the immediate loading site was assigned" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported in the article: "A blinded radiologist (F.G.), unaffiliated with the study centre, interpreted all radiographs" Author replied that: "all outcomes were assessed by blinded assessors with the exceptions of implant stability that was assessed by the surgeon" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data presented |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to be presented |
| Other bias | Low risk | None detected |
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Polson 2000 | Trial comparing immediate versus conventional loading. Insufficient data presented. No reply to letter |
| Roccuzzo 2001 | Trial comparing early versus conventional loading of different implant types |
| Testori 2003 | Trial comparing immediate non‐occluding loading versus conventional loading but at the same time patients were also randomised to 2 different implant types |
| Salvi 2004 | Trial comparing 2 early loading procedures (2 versus 6 weeks). Such comparison does not fit within this review |
| Appleton 2005 | Trial comparing progressive versus direct loading after an healing period of 5 months. It was unclear whether the trial was actually randomised, there was a mixed study design parallel and split‐mouth and implants with problems not accounted for biasing the results. No replay to letter |
| Ottoni 2005 | Trial comparing immediate versus conventional loading. The author informed us that the study was not a randomised controlled trial |
| Turkyilmaz 2006 | Trial comparing early versus conventional loading. The author informed us that the study was not a randomised controlled trial |
| Romanos 2006 | Trial comparing immediate versus conventional loading. The author informed us that the study was not a randomised controlled trial |
| Göthberg 2007 | Ongoing trial comparing immediate versus early loading in partially edentulous patients. Patients are subsequently randomised again to 3 different abutment solutions (no abutment, abutment with a rough surface and abutment with a machined surface) |
| Cannizzaro 2008c | Trial comparing flapless surgery + modified implant installation technique + immediate loading versus conventional surgery and loading |
| Degidi 2009 | Trial comparing immediate versus conventional loading, judged not to be randomised. No reply to letter |
| Van de Velde 2010 | Trial comparing immediate versus early loading. Groups not comparable at baseline since implants were placed with flapless‐guide surgery only in the immediately loaded group |
| Shibly 2010 | Trial comparing immediate (the same day) loading with delayed loading (3 months) of single post‐extractive implants. Author stated in both publications that 1 immediately loaded implants and 2 delayed loading implants failed. However in the table the presented the opposite results. In addition one patient dropped out but it was not possible to understand from which group. No reply to letter |
| Degidi 2010 | Trial comparing immediate occlusal versus non‐occlusal loading, judged not to be randomised. No reply to letter |
| Tealdo 2011 | Trial comparing immediate versus conventional loading. It is not a randomised controlled trial |
| Jokstad 2011 | Trial comparing 2 early loading procedures (10 days versus 6‐8 weeks). Such comparison does not fit within this review |
| Kim 2011 | Trial comparing 2 conventional loading after 4 months versus conventional loading after 6 months. Such comparison does not fit within this review |
| Mackie 2011 | Trial comparing early loading at 2 weeks with early loading at 6 weeks with conventional loading at 12 weeks. A 'posthumous cocktail' of different RCTs, some of them already included in this review |
| Barewal 2012 | Trial comparing immediate versus early versus conventional loading. The 'stratified' randomisation method used by the authors inevitably generated 3 non‐comparable groups, in fact the trial is not randomised |
Differences between protocol and review
The new aim of evaluating progressive loading has been added, though no study could be included.
Contributions of authors
Conceiving, designing and co‐ordinating the review (Marco Esposito (ME)). Developing search strategy and undertaking searches (ME). Screening search results and retrieved papers against inclusion criteria (ME, Maria Gabriella Grusovin (GG), Hassan Maghaireh (HM). Appraising quality and extracting data from papers (ME, GG, HM). Writing to authors for additional information (ME, GG, Helen Worthington (HW)). Data management for the review and entering data into RevMan (HW, ME). Analysis and interpretation of data (ME, HW). Writing the review (ME). Providing general advice on the review (GG, HM). Performing previous work that was the foundation of current study (ME, HW).
Sources of support
Internal sources
School of Dentistry, The University of Manchester, UK.
-
MAHSC, UK.
The Cochrane Oral Health Group is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
External sources
-
Cochrane Oral Health Group Global Alliance, UK.
All reviews in the Cochrane Oral Health Group are supported by Global Alliance member organisations (British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; Canadian Dental Hygienists Association, Canada; National Center for Dental Hygiene Research & Practice, USA and New York University College of Dentistry, USA) providing funding for the editorial process (http://ohg.cochrane.org).
-
National Institute for Health Research (NIHR), UK.
CRG funding acknowledgement: The NIHR is the largest single funder of the Cochrane Oral Health Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Declarations of interest
Marco Esposito is among the authors of six of the included, and one of the excluded studies, however, he was not involved in the quality assessment of these trials. Marco Esposito is working as independent methodological consultant for various implant related projects for some of the companies whose implants were used both in the included and excluded trials, however, in this review, implant brands were not under evaluation.
Edited (no change to conclusions)
References
References to studies included in this review
Assad 2007 {published data only}
- Assad AS, Hassan SA, Shawky YM, Badawy MM. Clinical and radiographic evaluation of implant‐retained mandibular overdentures with immediate loading. Implant Dentistry 2007;16(2):212‐23. [DOI] [PubMed] [Google Scholar]
Cannizzaro 2003 {published data only}
- Cannizzaro G, Leone M. Restoration of partially edentulous patients using dental implants with a microtextured surface: a prospective comparison of delayed and immediate full occlusal loading. International Journal of Oral and Maxillofacial Implants 2003;18(4):512‐22. [PubMed] [Google Scholar]
Cannizzaro 2008a {published data only}
- Cannizzaro G, Leone M, Esposito M. Immediate versus early loading of two implants placed with a flapless technique supporting mandibular bar‐retained overdentures: a single‐blinded, randomised controlled clinical trial. European Journal of Oral Implantology 2008;1(1):33‐43. [PubMed] [Google Scholar]
Cannizzaro 2008b {published data only}
- Cannizzaro G, Torchio C, Leone M, Esposito M. Immediate versus early loading of flapless‐placed implants supporting maxillary full‐arch prostheses: a randomised controlled clinical trial. European Journal of Oral Implantology 2008;1(2):127‐39. [PubMed] [Google Scholar]
Cannizzaro 2008d {published data only}
- Cannizzaro G, Felice P, Leone M, Ferri V, Viola P, Esposito M. Immediate versus early loading of 6.5 mm‐long flapless‐placed single implants: a 4‐year after loading report of a split‐mouth randomised controlled trial. European Journal of Oral Implantology 2012;5(2):111‐21. [PubMed] [Google Scholar]
- Cannizzaro G, Leone M, Torchio C, Viola P, Esposito M. Immediate versus early loading of 7 mm long flapless‐placed single implants: a split‐mouth randomised controlled clinical trial. European Journal of Oral Implantology 2008;1(4):277‐92. [PubMed] [Google Scholar]
Cannizzaro 2010 {published data only}
- Cannizzaro G, Torchio C, Felice P, Leone M, Esposito M. Immediate occlusal versus non‐occlusal loading of single zirconia implants. A multicenter pragmatic randomised clinical trial. European Journal of Oral Implantology 2010;3(2):111‐20. [PubMed] [Google Scholar]
Chiapasco 2001 {published and unpublished data}
- Chiapasco M, Abati S, Romeo E, Vogel G. Implant‐retained mandibular overdentures with Branemark System MKII implants: a prospective comparative study between delayed and immediate loading. The International Journal of Oral and Maxillofacial Implants 2001;16(4):537‐46. [PubMed] [Google Scholar]
Crespi 2008 {published data only (unpublished sought but not used)}
- Crespi R, Capparé P, Gherlone E, Romanos GE. Immediate versus delayed loading of dental implants placed in fresh extraction sockets in the maxillary esthetic zone: a clinical comparative study. The International Journal of Oral and Maxillofacial Implants 2008;23(4):753‐8. [PubMed] [Google Scholar]
den Hartog 2011 {published data only}
- Hartog L, Raghoebar GM, Stellingsma K, Vissink A, Meijer HJ. Immediate non‐occlusal loading of single implants in the aesthetic zone: a randomized clinical trial. Journal of Clinical Periodontology 2011;38(2):186‐94. [DOI] [PubMed] [Google Scholar]
De Rouck 2009 {published data only}
- Rouck T, Collys K, Wyn I, Cosyn J. Instant provisionalization of immediate single‐tooth implants is essential to optimize esthetic treatment outcome. Clinical Oral Implants Research 2009;20(6):566‐70. [DOI] [PubMed] [Google Scholar]
Donati 2008 {published and unpublished data}
- Donati M, Scala V, Billi M, Dino B, Torrisi P, Berglundh T. Immediate functional loading of implants in single tooth replacement: a prospective clinical multicenter study. Clinical Oral Implants Research 2008;19(8):740‐8. [DOI] [PubMed] [Google Scholar]
Enkling 2010 {published and unpublished data}
- Albrecht D, Marki L, Fabiam M, Bayer S, Stark H, Mericske‐Stern R, Enkling N. Impact of implant‐retained‐overdentures on OHRQoL: immediate‐ vs. delayed‐loading (Abstract 076). Clinical Oral Implant Research. 2011; Vol. 22:913.
- Enkling N, Albrecht D, Bayer S, Stark H, Mericske‐Stern R. [Immediate loading of interforaminal implants using a chairside fabricated bur (oral presentation)]. Clinical Oral Implants Research. 2010; Vol. 21:1013.
Fischer 2004 {published and unpublished data}
- Fischer K, Stenberg T. Early loading of ITI implants supporting a maxillary full‐arch prosthesis: 1‐year data of a prospective, randomized study. The International Journal of Oral and Maxillofacial Implants 2004;19(3):374‐81. [PubMed] [Google Scholar]
- Fischer K, Stenberg T. Three‐year data from a randomized, controlled study of early loading of single‐stage dental implants supporting maxillary full‐arch prostheses. The International Journal of Oral and Maxillofacial Implants 2006;21(2):245‐52. [PubMed] [Google Scholar]
- Fischer K, Stenberg T, Hedin M, Sennerby L. Five‐year results from a randomized, controlled trial on early and delayed loading of implants supporting full‐arch prosthesis in the edentulous maxilla. Clinical Oral Implants Research 2008;19(5):433‐41. [DOI] [PubMed] [Google Scholar]
Güncü 2008 {published and unpublished data}
- Güncü GN, Tozum TF, Güncü MB, Yamalik N. Relationships between implant stability, image‐based measures and nitric oxide levels. Journal of Oral Rehabilitation 2008;35(10):745‐53. [DOI] [PubMed] [Google Scholar]
- Güncü GN, Tozum TF, Güncü MB, Yamalik N, Tumer C, Karabulut E, et al. Myeloperoxidase as a measure of polymorphonuclear leukocyte response in inflammatory status around immediately and delayed loaded dental implants: a randomized controlled clinical trial. Clinical Implant Dentistry & Related Research 2008;10(1):30‐9. [DOI] [PubMed] [Google Scholar]
- Güncü GN, Tözüm TF, Güncü MB, Yamalik N, Tumer C. A 12‐month evaluation of nitrite oxide metabolism around immediate and conventionally loaded dental implants. Implant Dentistry 2009;18(1):27‐37. [DOI] [PubMed] [Google Scholar]
- Güncü MB, Aslan Y, Tümer C, Güncü GN, Uysal S. In‐patient comparison of immediate and conventional loaded implants in mandibular molar sites within 12 months. Clinical Oral Implants Research 2008;19(4):335‐41. [DOI] [PubMed] [Google Scholar]
Hall 2006 {published and unpublished data}
- Hall J, Payne AG, Purton DG, Torr B, Duncan WJ, Silva RK. Immediately restored, single tapered implants in the anterior maxilla: prosthodontic and aesthetic outcome after one year. Clinical Implant Dentistry & Related Research 2007;9(1):34‐45. [DOI] [PubMed] [Google Scholar]
- Hall JA, Payne AG, Purton DG, Torr B. A randomized controlled clinical trial of conventional and immediately loaded tapered implants with screw‐retained crowns. International Journal of Prosthodontics 2006;19(1):17‐9. [PubMed] [Google Scholar]
- Tan D, Hall J, Duncan W, Thomson WH. Immediate‐loading of single‐tooth, narrow, tapered Southern implants. http://iadr.confex.com/iadr/anz05/techprogram/abstract_72085.htm 2005:(Abstract No 0099).
Lindeboom 2006 {published and unpublished data}
- Lindeboom JA, Frenken JW, Dubois L, Frank M, Abbink I, Kroon FH. Immediate loading versus immediate provisionalization of maxillary single‐tooth replacements: a prospective randomized study with BioComp implants. Journal of Oral and Maxillofacial Surgery 2006;64(6):936‐42. [DOI] [PubMed] [Google Scholar]
Meloni 2012 {published data only}
- Meloni SM, Riu G, Pisano M, Riu N, Tullio A. Immediate versus delayed loading of single lower molars. One year results from a randomised controlled trial. The European Journal of Oral Implantology 2012;5(4):345‐53. [PubMed] [Google Scholar]
Merli 2008 {published and unpublished data}
- Merli M, Bernardelli F, Esposito M. Immediate non‐occlusal versus early loading of dental implants placed flapless in partially edentulous patients. Preliminary results from a randomized controlled clinical trial. The International Journal of Periodontics and Restorative Dentistry 2008;28(5):453‐9. [PubMed] [Google Scholar]
- Merli M, Merli A, Bernardelli F, Lombardini F, Esposito M. Immediate versus early non‐occlusal loading of dental implants placed flapless in partially edentulous patients. One‐year results from a randomized controlled clinical trial. European Journal of Oral Implantology 2008;1(3):207‐20. [PubMed] [Google Scholar]
- Merli M, Moscatelli M, Mariotti G, Piemontese M, Nieri M. Immediate versus early non‐occlusal loading of dental implants placed flapless in partially edentulous patients: a 3‐year randomized clinical trial. Journal of Clinical Periodontology 2012;39(2):196‐202. [DOI] [PubMed] [Google Scholar]
Oh 2006 {published data only}
- Oh TJ, Shotwell JL, Billy EJ, Wang HL. Effect of flapless implant surgery on soft tissue profile: a randomized controlled clinical trial. Journal of Periodontololgy 2006;77(5):874‐82. [DOI] [PubMed] [Google Scholar]
Payne 2002 {published and unpublished data}
- Payne AG, Tawse‐Smith A, Duncan WD, Kumara R. Conventional and early loading of unsplinted ITI implants supporting mandibular overdentures. Clinical Oral Implants Research 2002;13(16):603‐9. [DOI] [PubMed] [Google Scholar]
Romeo 2002 {published and unpublished data}
- Romeo E, Chiapasco M, Lazza A, Casentini P, Ghisolfi M, Iorio M, et al. Implant‐retained mandibular overdentures with ITI implants: A comparison of 2‐year results between delayed and immediate loading. Clinical Oral Implants Research 2002;13(5):495‐501. [DOI] [PubMed] [Google Scholar]
Schincaglia 2008 {published and unpublished data}
- Schincaglia GP, Marzola R, Fazi G, Scapoli C, Scotti R. Replacement of mandibular molars with single‐unit restorations supported by wide‐body implants: immediate versus delayed loading. A randomized controlled study. International Journal of Oral and Maxillofacial Implants 2008;23(3):474‐80. [PubMed] [Google Scholar]
Tawse‐Smith 2002 {published and unpublished data}
- Tawse‐Smith A, Payne AG, Kumara R, Thomson WM. Early loading of unsplinted implants supporting mandibular overdentures using a one‐stage operative procedure with two different implant systems: a 2‐year report. Clinical Implant Dentistry & Related Research 2002;4(1):33‐42. [DOI] [PubMed] [Google Scholar]
Testori 2007 {published data only}
- Capelli M, Esposito M, Zuffetti F, Galli F, Fabbro M, Testori T. A 5‐year report from a multicentre randomised clinical trial: immediate non‐occlusal versus early loading of dental implants in partially edentulous patients. European Journal of Oral Implantology 2010;3(3):209‐19. [PubMed] [Google Scholar]
- Galli F, Capelli M, Zuffetti F, Esposito M, Testori T. Immediate non‐occlusal vs early loading of dental implants in partially edentulous patients: a multicentre randomized clinical trial. Peri‐implant bone and soft‐tissue levels. Clinical Oral implants Research 2008;19(6):546‐52. [DOI] [PubMed] [Google Scholar]
- Testori T, Galli F, Capelli M, Zuffetti F, Esposito M. Immediate non‐occlusal versus early loading of dental implants in partially edentulous patients: 1‐year results from a multicenter, randomized controlled clinical trial. The International Journal of Oral and Maxillofacial Implants 2007;22(5):815‐22. [PubMed] [Google Scholar]
Turkyilmaz 2007 {published and unpublished data}
- Turkyilmaz I, Tumer C. Early versus late loading of unsplinted TiUnite surface implants supporting mandibular overdentures: a 2‐year report from a prospective study. Journal of Oral Rehabilitation 2007;34(10):773‐80. [DOI] [PubMed] [Google Scholar]
Zöllner 2008 {published and unpublished data}
- Ganeles J, Zöllner A, Jackowski J, Bruggenkate C, Beagle J, Guerra F. Immediate and early loading of Straumann implants with a chemically modified surface (SLActive) in the posterior mandible and maxilla: 1‐year results from a prospective multicenter study. Clinical Oral Implants Research 2008;19(11):1119‐28. [DOI] [PubMed] [Google Scholar]
- Zöllner A, Ganeles J, Korostoff J, Guerra F, Krafft T, Brägger U. Immediate and early non‐occlusal loading of Straumann implants with a chemically modified surface (SLActive) in the posterior mandible and maxilla: interim results from a prospective multicenter randomized‐controlled study. Clinical Oral Implants Research 2008;19(5):442‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Appleton 2005 {published data only}
- Appleton RS, Nummikoski, PV, Pigno MA, Cronin RJ, Chung KH. A radiographic assessment of progressive loading on bone around single osseointegrated implants in the posterior maxilla. Clinical Oral Implants Research 2005;16(2):161‐7. [DOI] [PubMed] [Google Scholar]
Barewal 2012 {published data only}
- Barewal RM, Stanford C, Weesner TC. A randomized controlled clinical trial comparing the effects of three loading protocols on dental implant stability. The International Journal of Oral & Maxillofacial Implants 2012;27(4):945‐56. [PubMed] [Google Scholar]
Cannizzaro 2008c {published data only}
- Cannizzaro G, Leone M, Consolo U, Ferri V, Esposito M. Immediate functional loading of implants placed with flapless surgery versus conventional implants in partially edentulous patients. A 3‐year randomized controlled clinical trial. The International Journal of Oral and Maxillofacial Implants 2008;23(5):867‐75. [PubMed] [Google Scholar]
Degidi 2009 {published data only (unpublished sought but not used)}
- Degidi M, Nardi D, Piattelli A. Immediate versus one‐stage restoration of small‐diameter implants for a single missing maxillary lateral incisor: a 3‐year randomized clinical trial. Journal of Periodontology 2009;80(9):1393‐8. [DOI] [PubMed] [Google Scholar]
Degidi 2010 {published data only (unpublished sought but not used)}
- Degidi M, Nardi D, Piattelli A. A comparison between immediate loading and immediate restoration in cases of partial posterior mandibular edentulism: a 3‐year randomized clinical trial. Clinical Oral implants Research 2010;21(7):682‐7. [DOI] [PubMed] [Google Scholar]
Göthberg 2007 {unpublished data only}
- Göthberg C, Slotte C, Bergendal T, Thomsen P, Suska F, Zoric N, et al. Tissue reactions around implants in fixed partial dentures and evaluation of healing times. A prospective, randomized, controlled study. Ongoing trial.
Jokstad 2011 {published data only}
- Jokstad A, Ellner S, Gussgard A. Comparison of two early loading protocols in full arch reconstructions in the edentulous maxilla using the Cresco prosthetic system: a three‐arm parallel group randomized‐controlled trial. Clinical Oral Implants Research 2011;22(5):455‐63. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim YK, Kim SG, Park JY, Yi YJ, Bae JH. Comparison of clinical outcomes of sinus bone graft with simultaneous implant placement: 4‐month and 6‐month final prosthetic loading. Oral Surgery, Oral Medicine, Oral Pathotology, Oral Radiology, and Endodontics 2011;111(2):164‐9. [DOI] [PubMed] [Google Scholar]
Mackie 2011 {published data only}
- Mackie A, Lyons K, Thomson WM, Payne AG. Mandibular two‐implant overdentures: prosthodontic maintenance using different loading protocols and attachment systems. The International Journal of Prosthodontics 2011;24(5):405‐16. [PubMed] [Google Scholar]
Ottoni 2005 {published and unpublished data}
- Ottoni JM, Oliveira ZF, Mansini R, Cabral AM. Correlation between placement torque and survival of single‐tooth implants. International Journal of Oral and Maxillofacial Implants 2005;20(5):769‐76. [PubMed] [Google Scholar]
Polson 2000 {published data only}
- Lee H, Polson AM, Salama H, Rose L, Minsk L, Sharkey D, et al. Safety and effectiveness of mandibular implants used in immediate loading: 5‐year findings. Journal of Periodontology 2002;73(9, 2002 Annual Meeting Abstract):1091. [Google Scholar]
- Lee I, Polson AM, Sharkey D, Polson AP, Salama H, Minsk L, et al. Five‐year clinical status of dental implants after immediate loading. Journal of Dental Research 2003;Spec Issue A:(Abstract No 0850). [Google Scholar]
- Polson AM, Lee I, Salama H, Minsk L, Sharkey D, Rose L, et al. 3‐year evaluation of safety and effectiveness of implants used in an immediate loading situation for the edentulous mandible (abstract). Journal of Periodontology 2000;71(12, 2000 Annual Meeting Abstract):1914. [Google Scholar]
- Polson AM, Sharkey D, Lee I, Polson AP, Minsk L, Salama H, et al. Three year alveolar bone levels of dental implants after immediate loading. Journal of Dental Research 2001;80(AADR Abstracts):211 (Abstract No 1405). [Google Scholar]
- Polson AM, Sharkey D, Minsk L, Lee I, Polson AP, Salama H, et al. Two year alveolar bone levels of dental implants after immediate loading. Journal of Dental Research 2000;79(IADR Abstracts):337 (Abstract No 1550). [Google Scholar]
- Polson AM, Sharkey D, Polson AP, Salama H, Minsk L, Rose L, et al. Four year clinical status of dental implants after immediate loading. Journal of Dental Research 2002;81(Spec Issue A):A‐101 (Abstract No 0616). [Google Scholar]
Roccuzzo 2001 {published data only}
- Roccuzzo M, Aglietta M, Bunino M, Bonino L. Early loading of sandblasted and acid‐etched implants: a randomized‐controlled double‐blind split‐mouth study. Five‐year results. Clinical Oral Implants Research 2008;19(2):148‐52. [DOI] [PubMed] [Google Scholar]
- Roccuzzo M, Bunino M, Prioglio F, Bianchi SD. Early loading of sandblasted and acid‐etched (SLA) implants: a prospective split‐mouth comparative study. Clinical Oral Implants Research 2001;12(6):572‐8. [DOI] [PubMed] [Google Scholar]
Romanos 2006 {published and unpublished data}
- Romanos GE. Immediate loading in the posterior mandible. A clinical study. Clinical Oral Implants Research 2002;13(4):xxi (Abstract No 52). [DOI] [PubMed] [Google Scholar]
- Romanos GE, Nentwig G‐H. Immediate versus delayed functional loading of implants in the posterior mandible: a 2‐year prospective clinical study of 12 consecutive cases. The International Journal of Periodontics and Restorative Dentistry in press. [PubMed]
Salvi 2004 {published data only}
- Salvi GE, Gallini G, Lang NP. Early loading (2 or 6 weeks) of sandblasted and acid‐etched (SLA) ITI(R) implants in the posterior mandible. Clinical Oral Implants Research 2004;15(2):142‐9. [DOI] [PubMed] [Google Scholar]
Shibly 2010 {published data only (unpublished sought but not used)}
- Shibly O, Kutkut A, Patel N, Albandar JM. Immediate Implants with Immediate Loading vs. Conventional Loading: 1‐Year Randomized Clinical Trial. Clinical Implant Dentistry and Related Research 2010;14(5):663‐71. [DOI] [PubMed] [Google Scholar]
- Shibly O, Patel N, Albandar JM, Kutkut A. Bone regeneration around implants in periodontally compromised patients: a randomized clinical trial of the effect of immediate implant with immediate loading. Journal of Periodontology 2010;81(12):1743‐51. [DOI] [PubMed] [Google Scholar]
Tealdo 2011 {published data only}
- Tealdo T, Bevilacqua M, Menini M, Pera F, Ravera G, Drago C, Pera P. Immediate versus delayed loading of dental implants in edentulous maxillae: a 36‐month prospective study. International Journal of Prosthodontics 2011;24(4):294‐302. [PubMed] [Google Scholar]
Testori 2003 {published data only}
- Testori T, Bianchi F, Fabbro M, Szmukler‐Moncler S, Francetti L, Weinstein RL. Immediate non‐occlusal loading vs. early loading in partially edentulous patients. Practical Procedures and Aesthetic Dentistry 2003;15(10):787‐94. [PubMed] [Google Scholar]
Turkyilmaz 2006 {published and unpublished data}
- Turkyilmaz I. Clinical and radiological results of patients treated with two loading protocols for mandibular overdentures on Branemark implants. Journal of Clinical Periodontology 2006;33(3):233‐8. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Sennerby L, Tumer C, Yenigul M, Avci M. Stability and marginal bone level measurements of unsplinted implants used for mandibular overdentures: a 1‐year randomized prospective clinical study comparing early and conventional loading protocols. Clinical Oral Implants Research 2006;17(5):501‐5. [DOI] [PubMed] [Google Scholar]
- Turkyilmaz I, Tözüm TF, Tumer C, Ozbek EN. A 2‐year clinical report of patients treated with two loading protocols for mandibular overdentures: early versus conventional loading. Journal of Periodontology 2006;77(12):1998‐2004. [DOI] [PubMed] [Google Scholar]
Van de Velde 2010 {published data only}
- Velde T, Sennerby L, Bruyn H. The clinical and radiographic outcome of implants placed in the posterior maxilla with a guided flapless approach and immediately restored with a provisional rehabilitation: a randomized clinical trial. Clinical Oral Implants Research 2010;21(11):1223‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Albrektsson 1981
- Albrektsson T, Brånemark PI, Hansson HA, Lindström J. Osseointegrated titanium implants. Requirements for ensuring a long‐lasting, direct bone‐to‐implant anchorage in man. Acta Orthopaedica Scandinavica 1981;52(2):155‐70. [DOI] [PubMed] [Google Scholar]
Alsabeeha 2010
Brunski 1979
- Brunski JB, Moccia AF Jr, Pollack SR, Korostoff E, Trachtenberg DI. The influence of functional use of endosseous dental implants on the tissue‐implant interface. I. Histological aspects. Journal of Dental Research 1979;58(10):1953‐69. [DOI] [PubMed] [Google Scholar]
Brånemark 1977
- Brånemark P‐I, Hansson BO, Adell R, Breine U, Lindström J, Hallén O, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10‐year period. Stockholm: Almqvist & Wiksell International, 1977. [PubMed] [Google Scholar]
Brånemark 1999
- Brånemark PI, Engstrand P, Öhrnell LO, Gröndahl K, Nilsson P, Hagberg K, et al. Brånemark Novum: a new treatment concept for rehabilitation of the edentulous mandible. Preliminary results from a prospective clinical follow‐up study. Clinical Implant Dentistry & Related Research 1999;1(1):2‐16. [DOI] [PubMed] [Google Scholar]
Cannizzaro 2012
- Cannizzaro G, Leone M, Ferri V, Viola P, Federico G, Esposito M. Immediate loading of single implants inserted flapless with medium or high insertion torque: a 6‐month follow‐up of a split‐mouth randomised controlled trial. The European Journal of Oral Implantology 2012;5(4):333‐42. [PubMed] [Google Scholar]
Chung 2011
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Del Fabbro 2006
- Fabbro M, Testori T, Francetti L, Taschieri S, Weinstein R. Systematic review of survival rates for immediately loaded dental implants. The International Journal of Periodontics and Restorative Dentistry 2006;26(3):249‐63. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Esposito 2007
- Esposito M, Murray‐Curtis L, Grusovin MG, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different types of dental implants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003815.pub3] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ioannidou 2005
- Ioannidou E, Doufexi A. Does loading time affect implant survival? A meta‐analysis of 1,266 implants. Journal of Periodontology 2005;76(8):1252‐8. [DOI] [PubMed] [Google Scholar]
Javed 2010
Jemt 1997
- Jemt T. Regeneration of gingival papillae after single‐implant treatment. The International Journal of Periodontics and Restorative Dentistry 1997;17(4):326‐33. [PubMed] [Google Scholar]
Lekholm 1985
- Lekholm U, Zarb GA. Patient selection and preparation. In: Brånemark P‐I, Zarb GA, Albrektsson T editor(s). Tissue integrated prostheses ‐ osseointegration in clinical dentistry. Chicago: Quintessence Publishing Co, 1985:199‐209. [Google Scholar]
Romanos 2010
Schnitman 1990
- Schnitman PA, Wöhrle PS, Rubenstein JE. Immediate fixed interim prostheses supported by two‐stage threaded implants: methodology and results. Journal of Oral Implantology 1990;16(2):96‐105. [PubMed] [Google Scholar]
Schropp 2004
- Schropp L, Isidor F, Kostopoulos L, Wenzel A. Patient experience of, and satisfaction with, delayed‐immediate vs. delayed single‐tooth implant placement. Clinical Oral Implants Research 2004;15(4):498‐503. [DOI] [PubMed] [Google Scholar]
Sennerby 2008
- Sennerby L, Gottlow J. Clinical outcomes of immediate/early loading of dental implants. A literature review of recent controlled prospective clinical studies. Australian Dental Journal 2008;53 Suppl 1:S82‐88. [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PLoS One 2011; Vol. 6, issue 10:e25491. [DOI] [PMC free article] [PubMed]
References to other published versions of this review
Esposito 2003
- Esposito M, Worthington HV, Coulthard P. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003878] [DOI] [PubMed] [Google Scholar]
Esposito 2004
- Esposito M, Worthington HV, Thomsen P, Coulthard P. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003878.pub2] [DOI] [PubMed] [Google Scholar]
Esposito 2007b
- Esposito M, Grusovin MG, Willings M, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD003878.pub3] [DOI] [PubMed] [Google Scholar]
Esposito 2007c
- Esposito M, Grusovin MG, Willings M, Coulthard P, Worthington HV. The effectiveness of immediate, early, and conventional loading of dental implants. A Cochrane systematic review of randomized controlled clinical trials. The International Journal of Oral and Maxillofacial Implants 2007;22(6):893‐904. [PubMed] [Google Scholar]
Esposito 2009
- Esposito M, Grusovin MG, Achille H, Coulthard P, Worthington HV. Interventions for replacing missing teeth: different times for loading dental implants. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003878.pub4] [DOI] [PubMed] [Google Scholar]


