Abstract
Background
Fructus aurantii is a flavonoid derived from Citrus aurantium (bitter orange) that is used in traditional Chinese medicine (TCM) to treat gastric motility disorders. This study aimed to investigate the effects of low-dose and high-dose decoctions of Fructus aurantii in a rat model of functional dyspepsia (FD).
Material/Methods
Sprague-Dawley rats (n=90) were divided into nine study groups: the control group, the FD model group, the domperidone-treated (Domp) group, the low-dose raw Fructus aurantii (FA-L) group, the high-dose raw Fructus aurantii (FA-H) group, the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group, the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group, the low-dose Fructus aurantii with stir-fried wheat bran and honey (Honey-L) group, and the high-dose Fructus aurantii with stir-fried wheat bran and honey (Honey-H) group. The FD rat model was established by semi-starvation, followed by tail damping, stimulation, and forced exercise with fatigue. Change in weight, rate of gastric emptying and intestinal propulsion, and serum levels of leptin, motilin, vasoactive intestinal peptide (VIP), gastrin, calcitonin gene-related peptide (CGRP), ghrelin, and cholecystokinin were compared between the groups.
Results
In the FD model group, weight, rate of gastric emptying and intestinal propulsion significantly decreased, the expression of leptin, VIP and CGRP increased, and expression of motilin, gastrin, ghrelin, and cholecystokinin significantly decreased. Treatment with low-dose Fructus aurantii with stir-fried wheat bran significantly reversed these effects.
Conclusions
In the rat model of FD, low-dose Fructus aurantii with stir-fried wheat bran increased gastrointestinal motility and gastrointestinal hormone levels.
MeSH Keywords: Dyspepsia, Gastrointestinal Diseases, Gastrointestinal Hormones
Background
Functional dyspepsia (FD) is the term used to describe a syndrome of impaired motility of the upper gastrointestinal tract that affects the quality of life and health of patients [1]. The clinical symptoms of FD include chronic upper abdominal pain and discomfort without organic disease [2]. The pathogenesis and etiology of FD are complex and include impaired functional motility of the stomach and duodenum with associated psychological effects and effects on the quality of life [3]. The pathogenesis of FD remains poorly understood, and the treatment is mainly symptomatic [4,5].
Fructus aurantii is a flavonoid derived from Citrus aurantium (bitter orange) that is used in traditional Chinese medicine (TCM) to treat gastric motility disorders [6]. The taste of Fructus aurantii is bitter, and the compound has pharmacological effects that relive abdominal distention, according to TCM theory [7]. Also, Fructus aurantii is a prokinetic herb that relieves indigestion and gastrointestinal dysfunction, as well as chest pain [8]. The efficacy of herbal TCM is significantly associated with the chemical components, and different production methods may affect the content of the effective components of the processed products or decoctions used [9,10]. However, have been few studies on the effects of Fructus aurantii on FD and the mechanisms involved. A previously published study showed that the Weichang’an (WCA) tablet, which is used to treat FD, contains 12 active components including naringin, hesperidin, and neohesperidin derived from Fructus aurantii [11]. A further study showed that meranzin hydrate, a compound isolated from Fructus aurantii, increased gastric emptying and intestinal transit in patients with FD [12]. Therefore, it is possible that some active components from Fructus aurantii, such as naringin, hesperidin, neohesperidin, and meranzin hydrate may have roles in the effects Fructus aurantii in experimental models of FD.
Therefore, this study aimed to investigate the effects of low-dose and high-dose processed products, or decoctions, of Fructus aurantii in a rat model of FD. The rat model of FD was established by semi-starvation followed by tail damping, stimulation, and forced exercise with fatigue, as previously described [13].
Material and Methods
Preparation of the processed products, or decoctions, of Fructus aurantii and the study groups
Fructus aurantii was obtained from Tianqitang Pharmacy (Jiangxi, China), which was the dried immature fruit of Citrus aurantium. Bran from dried wheat, Triticun aestivum was obtained from Shanghai Xiangxu Agricultural Products Trading Co. Ltd. (Shanghai, China). Ninety specific pathogen-free (SPF), 7-week-old Sprague–Dawley rats (45 male and 45 female), weighing between 180–220 g, were obtained from the Guangdong Medical Laboratory Animal Center (Guangdong, China). The rats were housed in a room with a temperature of 21±2°C, relative humidity of 30–70% and a 12-hourly light and dark cycle, and fed with water and normal food. All animal studies were approved by the Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine.
Nine study groups included: the control group; the functional dyspepsia (FD) model group; the domperidone-treated (Domp) group; the low-dose raw Fructus aurantii (FA-L) group; the high-dose raw Fructus aurantii (FA-H) group; the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group; the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group; the low-dose Fructus aurantii with stir-fried wheat bran and honey (Honey-L) group; and the high-dose Fructus aurantii with stir-fried wheat bran and honey (Honey-H) group.
Fructus aurantii was stir-fried with wheat bran until the mixture became pale yellow. The wheat bran was sieved and cooled the Fructus aurantii. The Fructus aurantii to wheat bran ratio was 10: 1. Fructus aurantii was stir-fried with honey and bran with a wheat bran to honey ratio of 10: 3. After stir-frying on medium heat, until the Fructus aurantii became yellow, the wheat bran was sieved and cooled.
Concoction solutions were obtained by dilution in water. The high-dose concoction was diluted ten times in water for 30 min and then strained with gauze. The filtrates were combined, and the final concentrated obtained was 1 g/mL, which was stored at −4°C. The low-dose concoction was diluted to a concentration: 0.1 g/mL.
The domperidone aqueous solution was obtained by grinding domperidone tablets (10.0 mg) (certification No: H10910003; Xian Janssen Pharmaceutical Ltd, Beijing, China), which were dissolved in water at a concentration of 0.2 g/L. Carboxymethyl cellulose sodium (20 g) (Sigma-Aldrich, St. Louis, MO, USA) was diluted with 500 mL of double-distilled water and heated to dissolve the mixture. Then, 16 g of dried skimmed milk powder, 8 g of starch, 8 g of sucrose, and 2 g of activated charcoal were added and mixed into a paste and stored at −4°C.
Development of the rat model of functional dyspepsia (FD)
Ten rats were normally fed in the control group. The remaining 80 rats were randomly divided into eight groups of ten in the FD model group, the Domp group, the AF-L group, the AF-H group, the Bran-L group, the Bran-H group, the Honey-L group, and the Honey-H group. The rat model of FD was established by semi-starvation, followed by tail damping, stimulation, and forced exercise with fatigue, as previously described [13].
Briefly, the FD rat model was developed using semi-starvation in the rats by tail damping, provocation, and forced exercise fatigue with exercise four times a day for ten days. Tail damping involved using the head of hemostatic forceps wrapped with gauze, and the distal one-third of the rat tail was clamped by hemostatic forceps, without damaging the skin. The hemostatic clamp was released when the rat struggled to escape, and this was performed twice per day for 30 min each time with a 12 h interval. This stimulation was performed for 14 days with feeding on alternate days. Any skin injuries to the rats were swabbed with iodine to prevent infection. All rats were fed a normal diet after the model was developed.
Dosing of the Fructus aurantii decoctions and dosing of domperidone
The dosing of the rats was calculated according to the drug doses in humans, and the conversion between humans and experimental animals [14,15]. The routine dosage for Fructus aurantii of rats was 1.0 g/kg in the low-dose groups, and 10.0 g/kg dose in the high-dose groups. Also, the dose of domperidone was calculated in the same way. After the model was constructed, the rats were treated every morning since the 15th day.
The rats in the control group and the model group were given normal saline (10.0 mL/kg) by gavage. The rats in the Domp group were given domperidone (10.0 mL/kg) by gavage. The rats in the AF-L group, the Bran-L group, and the Honey-L group were given low doses of raw Fructus aurantii (1.0 g/kg), Fructus aurantii with stir-fried with wheat bran (1.0 g/kg), and Fructus aurantii stir-fried with honey and bran (1 g/kg) by gavage. The rats in the AF-H group, the Bran-H group, and the Honey-H group were given high doses of raw Fructus aurantii (10.0 g/kg), Fructus aurantii with stir-fried wheat bran (10.0 g/kg) and Fructus aurantii with stir-fried honey and bran (10.0 g/kg), by gavage. The rats in each study group were continuously monitored for 14 days. Before modeling (week 0), two weeks after modeling (week 2), and after treatment (week 4), the weight change of rats in each group were measured and recorded using an electronic balance (R200D; Sartorius, Göttingen, Germany).
Blood sampling from the abdominal aorta
During the development of the rat model of FD, attention was given to the changes in the behavior, hair, diet, drinking water, and the weight of the rats in each group. The endpoint evaluations at the end of the study included the appearance, the hair, activity, response to external stimuli, vigilance, and resistance to handling, weight, and the nature of the stool. On the 28th day of the study, the animals fasted for 24 h after the last treatment. On the 29th day of the study, all rats were fed with nutritious semisolid paste (2 mL) by gavage. After 30 min, the rats were anesthetized with 10% chloral hydrate (350 mg/kg) by intraperitoneal injection). Blood samples (3 mL) were taken from the abdominal aorta, the serum was separated and then frozen at −80°C. The anesthetized rats were sacrificed by cervical dislocation.
Investigation of gastric emptying and intestinal propulsion
After the rats were euthanized, the abdominal cavity was immediately opened, and if no intra-abdominal abnormalities were seen, the gastric cardia and pyloric orifice were ligated, and the entire stomach and small intestine were removed. The mucosal surfaces of the stomach and small intestine were observed for changes, included flushing, erosion, and ulcer. After the stomach was dried with filter paper, the entire stomach was weighed using an electronic balance (R200D; Sartorius, Göttingen, Germany). Then, the stomach was immersed in a 0.9% saline solution to clean out the gastric contents. Filter paper was used to dry the stomach, which was weighed.
The gastric remnant rate was calculated as follows:
The whole small intestine, from the pylorus to the ileocecal junction, and the distance of movement of ingested of graphite powder from the pylorus were measured to calculate the propulsive as follows:
One senior investigator was responsible for the study and for conducting the experiments.
Histology
The stomach and duodenal tissue from the rats were fixed in a 4% paraformaldehyde solution for 48 h. The tissue was dehydrated and paraffin wax-embedded. Tissue sections were cut at 4 μm and routinely stained with hematoxylin and eosin (H&E) (Beyotime Biotechnology, Shanghai, China) for light microscopy, using a BH-2 light microscope (Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay (ELISA)
Serum levels of leptin, motilin, vasoactive intestinal peptide (VIP), gastrin, and calcitonin gene-related peptide (CGRP) were measured by ELISA using MSK ELISA kits (LifeSpan BioSciences, Wuhan, China), according to the manufacturer’s instructions. The samples were incubated in 96-well plates at 37°C for 30 min. The washing solution was added into each well and incubated for 30 s, then discarded. Washing was repeated five times. The enzyme standard reagent was added into the well and incubated at 37°C for 30 min, except for the blank well. After washing, the chromogenic reagent was added into each well, in the dark, for 15 min at 37°C, and the reaction was terminated. The optical density (OD) of each sample was detected using a microplate 680 reader (Bio-Rad, Hercules, CA, USA) at a wavelength of 450 nm.
Immunohistochemistry
The gastric antral tissue and duodenal tissue sections were incubated in 0.01 mol/L of citric acid buffer solution for 5 min. The tissue sections were blocked in 5% normal goat serum (Origene, Beijing, China) for 30 min. The tissue sections were incubated in the primary rabbit antibody to ghrelin (1: 2000) (ab209790; Abcam, Cambridge, MA, USA) at 4°C overnight. After washing, the sections were incubated for 1 h at 37°C with the secondary goat anti-rabbit IgG (1: 2000) (ab150077; Abcam, Cambridge, MA, USA). The 3,3-diaminobenzidine (DAB) detection kit (Beyotime Biotechnology, China) was used. Histology was performed using a light microscope (Olympus, Tokyo, Japan). The images were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Western blot
The gastric antral mucous tissue and duodenal tissues of the rats were cut into small fragments, and placed in RIPA lysate buffer containing phenylmethyl sulfonyl fluoride (PMSF), and homogenized. A bicinchoninic acid (BCA) protein assay kit (Thermofisher Scientific, Waltham, MA, USA) was used to measure the protein concentration. Then, 4 μL of protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The PVDF membranes were incubated for 2 h in 10% dried skimmed milk powder. The PVDF membrane was incubated with the primary antibodies at 4°C overnight. The primary antibodies included a rabbit antibody to cholecystokinin (1: 1000) (ab83180; Abcam, Cambridge, MA, USA) and a mouse antibody to GAPDH (1: 1000) (ab8245; Abcam, Cambridge, MA, USA). Then, horseradish peroxidase (HRP)-conjugated secondary antibodies were incubated with the PVDF membrane at room temperature for 2 h, and included goat anti-rabbit IgG (1: 2000) (ab205718; Abcam, Cambridge, MA, USA), and goat anti-mouse IgG (1: 2000) (ab205719; Abcam, Cambridge, MA, USA). GAPDH was used as an internal standard. The membranes were stained using the BeyoECL Plus kit (Beyotime, Shanghai, China) and analyzed using BandScan version 5.0 (Bio-Rad, Hercules, CA, USA).
Statistical analysis
Each experiment was performed in triplicate. Study data were presented as the mean±standard deviation (SD). Data were analyzed using SPSS version 21.0 software (IBM Corp., Armonk, NY, USA). Statistical comparisons between groups were determined by Student’s t-test or one-way analysis of variance (ANOVA). A P-value <0.05 was considered to be statistically significant.
Results
The weight of the rats from the different groups
In this study, a rat model of FD was established by semi-starvation followed by tail damping, stimulation, and forced exercise with fatigue, as previously described [13]. The rats were divided into nine study groups: the control group, the FD model group, the domperidone-treated (Domp) group, the low-dose raw Fructus aurantii (FA-L) group, the high-dose raw Fructus aurantii (FA-H) group, the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group, the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group, the low-dose Fructus aurantii with stir-fried bran and honey (Honey-L) group, and the high-dose Fructus aurantii with stir-fried bran and honey (Honey-H) group. The findings showed that a low-dose of Fructus aurantii stir-fried with wheat bran had the most significant therapeutic effect on rat model of FD.
The rats in all treatment groups were treated by gavage, and the rats in the model group and the control group were given normal saline by gavage. The rats in each group were weighed before and after the study. At 2 weeks, the weight of the rats in the model group and all treatment groups were significantly lower than in the control group (Table 1) (P<0.05). At 4 weeks, the weight of the rats in all the treatment groups was significantly greater than in the model group. There was no difference in the weight between the control group and the Bran-L group (Table 1) (P>0.05). The weight of the rats in the model of FD was lower than that in the control group, while the weight of the rats in the Bran-L group recovered the most after the study.
Table 1.
Comparison of the weight of the rats before and after the development of the model of functional dyspepsia (FD) and the study groups.
| Study group | n | Before modeling (0 weeks) | After modeling (at 2 weeks) | After treatment (at 4 weeks) |
|---|---|---|---|---|
| Control | 10 | 186.48±10.62 | 225.75±10.23 | 257.92±10.25 |
| Model | 10 | 186.81±8.65 | 200.89±10.25** | 220.18±10.32** |
| Domp | 10 | 187.05±9.63 | 200.12±8.65** | 243.25±10.28*## |
| FA-L | 10 | 187.11±8.96 | 202.96±9.64** | 244.68±11.65*## |
| FA-H | 10 | 188.02±10.32 | 200.99±8.56** | 237.58±8.52**## |
| Bran-L | 10 | 186.56±8.95 | 201.75±9.92** | 249.97±7.64## |
| Bran-H | 10 | 187.25±9.95 | 200.68±10.12** | 237.88±8.25*## |
| Honey-L | 10 | 187.58±8.85 | 201.72±10.55** | 245.25±9.96*## |
| Honey-H | 10 | 188.02±9.32 | 200.67±9.65** | 237.62±10.11*## |
Data are expressed as the mean±standard deviation (SD). Sprague-Dawley rats (n=90) were divided into nine study groups: the control group, the FD model group, the domperidone-treated (Domp) group, the low-dose raw Fructus aurantii (FA-L) group, the high-dose raw Fructus aurantii (FA-H) group, the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group, the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group, the low-dose Fructus aurantii with stir-fried bran and honey (Honey-L) group, and the high-dose Fructus aurantii with stir-fried bran and honey (Honey-H) group.
P<0.05 vs. the control;
P<0.01 vs. the control;
P<0.01 vs. the model.
Comparison of gastrointestinal function in rats from the different groups
Compared with the control group, the gastric remnant rate in the model group was significantly increased, and the propulsive intestinal rate was significantly reduced (Table 2) (P<0.05). This finding indicated that the gastrointestinal motility of the rats was reduced, and the FD model was successfully established. Compared with the model group, the gastric remnant rate was significantly reduced and the intestinal propulsion rate was significantly increased in rats in the Domp group, the AF-L group, the AF-H group, the Bran-L group, the Bran-H group, and the Honey-L group (Table 2) (P<0.05). At the same dosage, the difference in the gastric remnant rate and the propulsive intestinal rate between the Bran group and the control group was the least, indicating that the effect of Fructus aurantii stir-fried with wheat bran on the treatment of the rat model of FD was greater than that of raw Fructus aurantii and of Fructus aurantii that was stir-fried with honey and bran. Compared with low-dose group, high-dose treatment increased the gastric remnant rate and reduced the intestinal propulsion rate in the rat model of FD, which indicated high-dose treatment reduced the therapeutic effect.
Table 2.
Comparison of the gastric remnant rate and propulsive intestinal rate in the model of functional dyspepsia (FD) and the study groups.
| Study group | n | Gastric remnant rate | Propulsive intestinal rate |
|---|---|---|---|
| Control | 10 | 51.25±5.52 | 47.25±5.65 |
| Model | 10 | 65.55±4.65** | 33.89±4.65** |
| Domp | 10 | 52.56±7.58## | 48.02±6.52## |
| FA-L | 10 | 56.48±5.35*## | 42.68±3.23*## |
| FA-H | 10 | 61.55±3.23**# | 39.55±3.12*# |
| Bran-L | 10 | 51.18±6.52## | 42.35±3.65*## |
| Bran-H | 10 | 56.85±5.26*# | 39.02±3.01*# |
| Honey-L | 10 | 61.06±4.23**# | 41.03±3.21*## |
| Honey-H | 10 | 69.58±3.25**## | 37.68±3.02**# |
Data are expressed as the mean±standard deviation (SD). Sprague-Dawley rats (n=90) were divided into nine study groups: the control group, the FD model group, the domperidone-treated (Domp) group, the low-dose raw Fructus aurantii (FA-L) group, the high-dose raw Fructus aurantii (FA-H) group, the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group, the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group, the low-dose Fructus aurantii with stir-fried bran and honey (Honey-L) group, and the high-dose Fructus aurantii with stir-fried bran and honey (Honey-H) group.
P<0.05 vs. the control;
P<0.01 vs. the control;
P<0.05 vs. the model;
P<0.01 vs. the model.
Histology of the stomach and duodenum in rats from the different groups
The effects of treatment on the gastric and duodenal mucosa were studied histologically. The appearance and morphology of the gastric and duodenal mucosa of rats in all treatment groups were normal without visible ulcer, erosion, or hemorrhage. The histology showed that the gastric mucosa (Figure 1A) and duodenal mucosa (Figure 1B) of the rats in all the treatment groups was intact, and there was no infiltration of inflammatory cells, which indicated that different doses of Fructus aurantii products did not damage the gastric and intestinal mucosa of the rats studied.
Figure 1.
Histology of the gastric mucosa and duodenal mucosa in different groups of rats and in the rat model of functional dyspepsia (FD) treated with low-dose and high-dose decoctions of Fructus aurantii. (A) Photomicrograph of the histology of the gastric mucosa in rats in each study group. Hematoxylin and eosin (H&E). (B) Photomicrograph of the histology of the duodenal mucosa in rats in each study group. Hematoxylin and eosin (H&E).
Comparison of serum levels of leptin, motilin, vasoactive intestinal peptide (VIP), gastrin, and calcitonin gene-related peptide (CGRP) in rats from the different groups
The serum levels of leptin, motilin, VIP, gastrin, and CGRP in each group were detected by enzyme-linked immunosorbent assay (ELISA). Compared with the control group, the leptin, VIP, and CGRP levels in the model group were significantly upregulated, while the motilin and gastrin levels were significantly down-regulated (Figure 2A–2E) (P<0.05). Compared with the model group, the leptin, VIP, and CGRP levels in all treatment groups were significantly down-regulated, while the motilin and gastrin levels were significantly upregulated (Figure 2A–2E) (P<0.05). No significant differences were observed in the levels of leptin, motilin, VIP, gastrin, and CGRP between the control and the Bran-L groups, suggesting that the levels of these gut hormones were restored to a normal level after Bran-L treatment.
Figure 2.
The serum levels of leptin, motilin, vasoactive intestinal peptide (VIP), gastrin, and calcitonin gene-related peptide (CGRP) were measured by enzyme-linked immunosorbent assay (ELISA). (A) Serum levels of leptin in each group. (B) Serum levels of motilin in each group. (C) Serum levels of VIP in each group. (D) Serum levels of gastrin in each group. (E) Serum levels of calcitonin gene-related peptide (CGRP) in each group. * P<0.05 vs. the control group; ** P<0.01 vs. the control group; # P<0.05 vs. the model group; ## P<0.01 vs. the model group.
Comparison of ghrelin expression in rats from the different groups
Immunohistochemistry was performed to investigate the level and distribution of ghrelin expression in the gastric antral and duodenal mucosa of the rats, as previously described [16,17]. Ghrelin expression was detected in cells in both the gastric and duodenal tissues (Figures 3, 4). Also, compared with the control group, ghrelin expression in the model group was significantly reduced in the gastric and duodenal tissues. However, ghrelin expression in all treatment groups was increased compared with the model group (Figures 3, 4) (P<0.05). Immunohistochemistry showed that the level of ghrelin in the duodenum and gastric antral mucosa of the rat model of FD was increased by treatment. Also, ghrelin expression was not significantly different between the control group and the Bran-L group, which indicated that Bran-L had the greatest effect on the expression of ghrelin in the rat model of FD, and could restore it to the level of normal.
Figure 3.
The expression of ghrelin in the gastric antral mucosal tissue of rats detected by immunohistochemistry. Magnification, ×100. Scale bar=100 μm. Magnification, ×200. Scale bar=50 μm. * P<0.05 vs. the control group; ** P<0.01 vs. the control group; ## P<0.01 vs. the model group.
Figure 4.
The expression of ghrelin in the duodenal mucosal tissue of rats detected by immunohistochemistry. Magnification, ×100. Scale bar=100 μm. Magnification, ×200. Scale bar=50 μm. * P<0.05 vs. the control group; ** P<0.01 vs. the control group; # P<0.05 vs. the model group; ## P<0.01 vs. the model group.
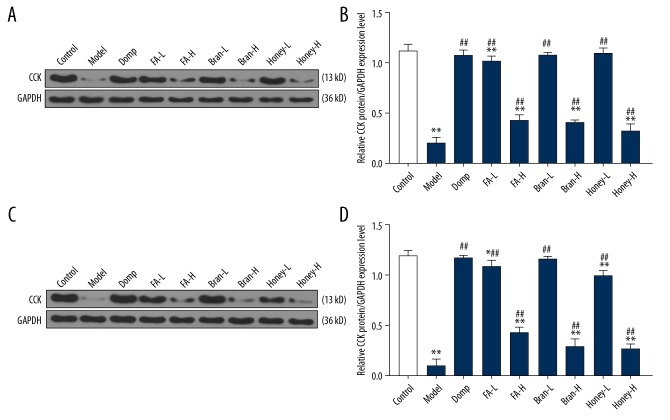
Comparison of cholecystokinin expression in rats from the different groups
Western blot was used to detect the protein expression levels of cholecystokinin in the rat duodenum and gastric antrum mucosa. Compared with the control group, the cholecystokinin expression in the duodenum (Figure 5A, 5B) and the gastric antral mucosa (Figure 5C, 5D) in the model group were significantly reduced (P<0.05). Compared with the model group, cholecystokinin expression in all treatment groups was significantly increased (P<0.05). No significant difference in cholecystokinin expression was found between the Bran-L group and the control group (P>0.05). These results showed that the cholecystokinin protein expression in the rat model of FD was increased after the treatment with Fructus aurantii decoction.
Figure 5.
The expression of cholecystokinin in the gastric antral mucosal tissue and duodenal mucosal tissue of rats measured by Western blot. (A) Cholecystokinin expression in the duodenal tissue of rats in each group was detected by Western blot. (B) The protein level of cholecystokinin in the duodenal tissue. (C) Cholecystokinin expression in the gastric antral mucosa of rats in each group was detected by Western blot. (D) The protein level of cholecystokinin in the gastric antral mucous. * P<0.05 vs. the control group; ** P<0.01 vs. the control group; ## P<0.01 vs. the model group.
Discussion
The commonly used treatments for functional dyspepsia (FD) in clinical practice include drugs that inhibit the production of gastric acid, drugs that increase gastrointestinal motility, and treatments for Helicobacter pylori [18,19. Domperidone is a peripheral dopamine receptor blocker that can improve gastrointestinal function and reduce the clinical symptoms of FD [20]. Fructus aurantii is derived from Citrus aurantium (bitter orange) that is used in traditional Chinese medicine (TCM) to treat gastric motility disorder [21]. The main effective bioactive constituents of Fructus aurantii are alkaloids, volatile oils, and flavonoids, which have anti-inflammatory and antioxidant effects in FD [21]. In this study, a rat model of FD was established by semi-starvation followed by tail damping, stimulation, and forced exercise with fatigue, as previously described [13]. The rats were divided into nine study groups: the control group, the FD model group, the domperidone-treated (Domp) group, the low-dose raw Fructus aurantii (FA-L) group, the high-dose raw Fructus aurantii (FA-H) group, the low-dose Fructus aurantii with stir-fried wheat bran (Bran-L) group, the high-dose Fructus aurantii with stir-fried wheat bran (Bran-H) group, the low-dose Fructus aurantii with stir-fried bran and honey (Honey-L) group, and the high-dose Fructus aurantii with stir-fried bran and honey (Honey-H) group. The findings showed that a low-dose of Fructus aurantii stir-fried with wheat bran had the most significant therapeutic effect on the rat model of FD.
In this study, after the FD rat model was established, the weight of the rats was measured and recorded before and after the study, as weight loss is a clinical feature of FD [22]. The animal experiments showed that the weight of the rats in the model group was significantly reduced, which indicated that the rat model of FD was successfully developed. Following treatment, the weight of the rats in the rat model of FD increased in all treatment groups, and the recovery of the weight of the domperidone-treated rats in the model of FD in Bran-L group showed the greatest outcome. Also, in this study, no ulcers, hemorrhage, or inflammation were observed in the gastric and duodenal mucosa of rats in each treatment group. The findings from this preliminary study indicated that Fructus aurantii processed products, or decoctions, were effective in treating FD, without gastrointestinal mucosal injury in rats, and the beneficial effect of Bran-L showed the greatest effect.
Gastric emptying is the process of emptying food from the stomach into the duodenum through the propulsive effects of the stomach and duodenum [23,24]. Most patients with FD have gastrointestinal motility disorder, which is associated with delayed gastric emptying [25]. There is a close association between slow intestinal motility and impaired gastric emptying [26]. In the present study, the investigation of the gastric remnant and small intestine propulsion in rats showed delayed gastric emptying and slow intestinal propulsion in the model group. Following treatment, the gastrointestinal motility in the rat model of FD was improved, and the effect of low-dose Fructus aurantii stir-fried with wheat bran on gastrointestinal motility was the best, which was consistent with the findings from domperidone treatment. Also, high-dose treatment reduced the therapeutic effects, which further supported the efficacy of low-dose bran stir-fried with Fructus aurantii.
Gastrointestinal hormones, secreted by endocrine cells on the gastrointestinal mucosa, is closely associated with gastrointestinal motility disorders [27]. Leptin is a neuroendocrine factor secreted by the hypothalamus and gastric mucosal cells, which can inhibit gastric emptying and enhance satiety [28]. Vasoactive intestinal peptide (VIP) is widely distributed in neural tissue and the gastrointestinal tract, inhibits gastrointestinal motility, delays gastric emptying, and slows small intestine motility [29]. Calcitonin gene-related peptide (CGRP) is widely distributed in the gastrointestinal tract neural plexus. CGRP has a role in visceral hypersensitivity, inhibits gastric acid secretion, slows gastrointestinal movement, and regulates gastrointestinal hormone secretion [30]. Jiang et al. [31] showed that Fructus aurantii water decoction could reduce the expression of VIP. The present study showed that serum levels of leptin, VIP, and CGRP in the rat model group were upregulated. After 14 days of treatment, the serum levels of leptin, VIP, and CGRP in the rat model of FD in each treatment group were reduced. These findings showed that the different treatments based on Fructus aurantii reduced the serum levels of leptin, VIP, and CGRP, to regulate the visceral sensory function of the gastrointestinal tract and improve gastrointestinal movement. Also, low-dose Fructus aurantii stir-fried with wheat bran was the most efficacious decoction.
Motilin is a gastrointestinal peptide hormone secreted cells in the small intestinal mucosa and can promote gastrointestinal motility and accelerate gastric emptying [32]. Gastrin promotes gastrointestinal motility and the secretion of gastric acid and pepsin [33]. Ghrelin is an appetite-stimulating factor secreted by gastric oxyntic cells, which can increase appetite, accelerate gastric emptying, and protect the gastrointestinal mucosa [34–36]. Cholecystokinin is widely distributed in gastrointestinal neurons and is involved in the regulation of gastrointestinal function [37]. Liang et al. [38] showedthat cholecystokinin expression was down-regulated in the duodenum and antrum of the rat model of FD. The findings from the present study showed down-regulation of motilin, gastrin, ghrelin, and cholecystokinin in the model group, and upregulation of motilin, gastrin, ghrelin, and cholecystokinin in the treated groups. These results showed that the processed products of Fructus aurantii could promote gastrointestinal movement and improve gastric motility disorders by regulating the secretion of gastrointestinal hormones in the rat model of FD. Also, low-dose Fructus aurantii stir-fried with wheat bran had the greatest efficacy in the rat model.
This study had several limitations. Because lesions in the duodenal bulb are more common than in other segments of the duodenum, as in celiac disease in children [39], it is necessary to subdivide and investigate different segments of the duodenum, which was not done in this study. This study also included only ten rats in each group, and future animal studies on gastric emptying and intestinal motility should be undertaken with larger study groups. Also, the mechanisms of action of Fructus aurantii and its decoctions in FD remain unknown.
Conclusions
This study aimed to investigate the effects of low-dose and high-dose decoctions of Fructus aurantii in a rat model of functional dyspepsia (FD). The findings showed that low-dose Fructus aurantii with stir-fried wheat bran increased gastrointestinal motility and gastrointestinal hormone levels, including leptin, vasoactive intestinal peptide (VIP), and calcitonin gene-related peptide (CGRP).
Footnotes
Source of support: This study was funded by the National Key R&D Program of China (Grant No. 2018YFC1707206), the Public Welfare Industry Research Project of State Administration of Traditional Chinese Medicine (Grant No. 201507002-2), the Natural Science Foundation of Jiangxi Province (Grant No.20192BAB215055), the Science and Technology Program of Jiangxi Provincial Health Committee (Grant No. 20195646), and the First-Class Subjects Foundation of Traditional Chinese Medicine of Jiangxi Province (Grant No. JXSYLXK-ZHYAO041), the Scientific Research Foundation for the Doctoral Program of Jiangxi University of Traditional Chinese Medicine (Grant No.2019WBZR007)
References
- 1.Jin Y, Zhao Q, Zhou K, et al. Acupuncture for functional dyspepsia: A single blinded, randomized, controlled trial. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/904926. 904926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ. Functional dyspepsia: New insights into pathogenesis and therapy. Korean J Intern Med. 2016;31(3):444–56. doi: 10.3904/kjim.2016.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng WP, Wang ZQ, Deng JQ, et al. The role of H. pylori CagA in regulating hormones of functional dyspepsia patients. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/7150959. 7150959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talley NJ. Functional dyspepsia: Advances in diagnosis and therapy. Gut Liver. 2017;11(3):349–57. doi: 10.5009/gnl16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamawaki H, Futagami S, Wakabayashi M, et al. Management of functional dyspepsia: State of the art and emerging therapies. Ther Adv Chronic Dis. 2018;9(1):23–32. doi: 10.1177/2040622317725479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HF, Zhang WG, Yuan JB, et al. Simultaneous quantification of polymethoxylated flavones and coumarins in Fructus aurantii and Fructus aurantii immaturus using HPLC-ESI-MS/MS. J Pharm Biomed Anal. 2012;59:90–95. doi: 10.1016/j.jpba.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Wagner H, Püls S, Barghouti T, et al. Fructus immaturus auranti i – Zhishi/fructus aurantii – Zhiqiao. In: Wagner H, Püls S, Barghouti T, et al., editors. Chromatographic Fingerprint Analysis of Herbal Medicines. V. Springer International Publishing AG; Cham Switzerland: 2017. pp. 31–34. [Google Scholar]
- 8.Li P, Zeng S-L, Duan L, et al. Comparison of Aurantii fructus immaturus and Aurantii fructus based on multiple chromatographic analysis and chemometrics methods. J Chromatogr A. 2016;1469:96–107. doi: 10.1016/j.chroma.2016.09.061. [DOI] [PubMed] [Google Scholar]
- 9.Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5) doi: 10.3390/molecules21050559. pii: E559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An K, Zhao D, Wang Z, et al. Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): Changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem. 2016;197(Pt B):1292–300. doi: 10.1016/j.foodchem.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Gao W, Liu Z, Zhang Z. Identification and simultaneous determination of twelve active components in the methanol extract of traditional medicine Weichang’an Pill by HPLC-DAD-ESI-MS/MS. Iran J Pharm Res. 2013;12(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu XJ, Huang X, Chen ZQ, et al. Pharmacokinetic study of the prokinetic compounds meranzin hydrate and ferulic acid following oral administration of Chaihu-Shugan-San to patients with functional dyspepsia. J Ethnopharmacol. 2011;137(1):205–13. doi: 10.1016/j.jep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Liang Q, Yan Y, Mao L, et al. Evaluation of a modified rat model for functional dyspepsia. Saudi J Gastroenterol. 2018;24(4):228–35. doi: 10.4103/sjg.SJG_505_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.China Pharmacopoeia Committee. The pharmacopoeia of the People’s Republic of China. Beijing, China: China Medical Science Press; 2019. [Google Scholar]
- 15.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 16.Kraus D, Reckenbeil J, Wenghoefer M, et al. Ghrelin promotes oral tumor cell proliferation by modifying GLUT1 expression. Cell Mol Life Sci. 2016;73(6):1287–99. doi: 10.1007/s00018-015-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka-Shintani M, Watanabe M. Distribution of ghrelin-immunoreactive cells in human gastric mucosa: Comparison with that of parietal cells. J Gastroengterol. 2005;40(4):345–49. doi: 10.1007/s00535-004-1550-3. [DOI] [PubMed] [Google Scholar]
- 18.Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroengterol. 2015;50(2):125–39. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa T, Masaoka T, Suzuki H. Functional dyspepsia: Pathogenesis, diagnosis, and treatment. J Gen Fam Med. 2016;17(3):204–10. [Google Scholar]
- 20.Jain C, Kumar M, Advani U, et al. Comparison of efficacy and safety of levosulpiride and domperidone in functional dyspepsia. Int Res J Pharmaceut Biosci. 2015;2(5):20–30. [Google Scholar]
- 21.Shu Z, Yang Y, Xing N, et al. Structural characterization and immunomodulatory activity of a pectic polysaccharide (CALB-4) from Fructus aurantii. Int J Biol Macromol. 2018;116:831–39. doi: 10.1016/j.ijbiomac.2018.01.165. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Ly HG, Carbone F, et al. Efficacy of mirtazapine in patients with functional dyspepsia and weight loss. Clin Gastroenterol Hepatol. 2016;14(3):385–92.e4. doi: 10.1016/j.cgh.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis. 2014;32(3):222–29. doi: 10.1159/000357854. [DOI] [PubMed] [Google Scholar]
- 24.Kusano M, Hosaka H, Kawada A, et al. Gastrointestinal motility and functional gastrointestinal diseases. Curr Pharm Des. 2014;20(16):2775–82. doi: 10.2174/13816128113199990572. [DOI] [PubMed] [Google Scholar]
- 25.Guo WJ, Yao SK, Zhang YL, et al. Impaired vagal activity to meal in patients with functional dyspepsia and delayed gastric emptying. J Int Med Res. 2018;46(2):792–801. doi: 10.1177/0300060517726442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon YJ, Lee JS, Cho YR, et al. Banha-sasim-tang improves gastrointestinal function in loperamide-induced functional dyspepsia mouse model. J Ethnopharmacol. 2019;238:111834. doi: 10.1016/j.jep.2019.111834. [DOI] [PubMed] [Google Scholar]
- 27.Tian H, Huang D, Li T, et al. The protective effects of total phenols in magnolia officinalix rehd. et wils on gastrointestinal tract dysmotility is mainly based on its influence on interstitial cells of Cajal. Int J Clin Exp Med. 2015;8(11):20279–86. [PMC free article] [PubMed] [Google Scholar]
- 28.Hammersjo R, Roth B, Hoglund P, Ohlsson B. Esophageal and gastric dysmotilities are associated with altered glucose homeostasis and plasma levels of incretins and leptin. Rev Diabet Stud. 2016;13(1):79–90. doi: 10.1900/RDS.2016.13.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins AB, Garnica-Siqueira MC, Zaia DAM, et al. Oxytocin participates on the effects of vasoactive intestinal peptide on food intake and plasma parameters. Mol Cell Biochem. 2018;437(1–2):177–83. doi: 10.1007/s11010-017-3106-x. [DOI] [PubMed] [Google Scholar]
- 30.Qiang L, Jiang Y. Electroacupuncture for functional dyspepsia and the influence on serum Ghrelin, CGRP and GLP-1 levels. World J Acupunct Moxibustion. 2018;28(2):86–90. [Google Scholar]
- 31.Jiang Y, Bai X, Zhu X, Li J. The effects of Fructus aurantii extract on the 5-hydroxytryptamine and vasoactive intestinal peptide contents of the rat gastrointestinal tract. Pharm Biol. 2014;52(5):581–85. doi: 10.3109/13880209.2013.854396. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Zhou X, Xiang X, et al. Effect of miRNA-19a on gastrointestinal motility in rats with functional dyspepsia. Exp Ther Med. 2018;15(6):4875–79. doi: 10.3892/etm.2018.6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao X, Suo HY, Qian Y, et al. Therapeutic effects of Lactobacillus casei Qian treatment in activated carbon-induced constipated mice. Mol Med Rep. 2015;12(2):3191–99. doi: 10.3892/mmr.2015.3737. [DOI] [PubMed] [Google Scholar]
- 34.Kazemi M, Eshraghian A, Hamidpour L, Taghavi S. Changes in serum ghrelin level in relation to meal-time in patients with functional dyspepsia. United European Gastroenterol J. 2015;3(1):11–16. doi: 10.1177/2050640614563373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goswami C, Shimada Y, Yoshimura M, et al. Motilin stimulates gastric acid secretion in coordination with ghrelin in Suncus murinus. PLoS One. 2015;10(6):e0131554. doi: 10.1371/journal.pone.0131554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi YJ, Kim N, Yoon H, et al. Increase in plasma acyl ghrelin levels is associated with abatement of dyspepsia following Helicobacter pylori eradication. J Gastroenterol. 2016;51(6):548–59. doi: 10.1007/s00535-015-1124-6. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Li F, Tang XD, et al. XiangshaLiujunzi decoction alleviates the symptoms of functional dyspepsia by regulating brain-gut axis and production of neuropeptides. BMC Complement Altern Med. 2015;15:387. doi: 10.1186/s12906-015-0913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiankun L, Lanfang M, Xiaojuan D, et al. Pingwei capsules improve gastrointestinal motility in rats with functional dyspepsia. J Tradit Chin Med. 2018;38(1):43–53. [PubMed] [Google Scholar]
- 39.De Leo L, Villanacci V, Ziberna F, et al. Immunohistologic analysis of the duodenal bulb: A new method for celiac disease diagnosis in children. Gastrointest Endosc. 2018;88(3):521–26. doi: 10.1016/j.gie.2018.05.014. [DOI] [PubMed] [Google Scholar]