Abstract
Investigation of the microbial community in the female reproductive tract using sequencing techniques has revealed that endometrial samples obtained through a transvaginal catheter are dominated by Lactobacillus species. Dysbiotic changes in the endometrial microbiota may be associated with implantation failure or early spontaneous abortion in patients undergoing assisted reproductive technology (ART) treatment. Whether or not there is an endometrial microbiota in early pregnancy is unknown.
Herein we describe, the human endometrial microbiota in a patient who subsequently had an 8th week spontaneous clinical miscarriage with euploid embryos in the next cycle and, for the first time, during a successful pregnancy in which the endometrial fluid was sampled at 4 weeks of gestation. The microbial profile found on the endometrial sample prior to the spontaneous abortion had higher bacterial diversity and lower Lactobacillus abundance than the endometrial fluid from the healthy pregnancy. Functional metagenomics detected different Lactobacillus species between the two samples. Lactobacillus crispatus was present in the endometrium prior to the spontaneous abortion, as were other bacteria involved in dysbiosis, which had an unstable functional pattern characterized by transposases and insertion elements.
Lactobacillus iners was the most prevalent microbe found in the endometrium during early pregnancy, associating its presence with defense mechanisms and basal functions. These novel observations prompt future investigations to understand the potential implications of microbiology on healthy and pathologic human pregnancy.
Keywords: 16S rRNA, Assisted Reproductive Treatments, Endometrial microbiota, Lactobacillus crispatus, Lactobacillus iners, Pregnancy, Reproductive tract microbiome, Spontaneous abortion, Whole Metagenomic Sequencing
Condensation
The endometrial microbiota in the same woman who subsequently had a spontaneous abortion with euploid embryos had a different profile than that of an early successful pregnancy.
INTRODUCTION
The efforts of the Human Microbiome Project (HMP) has highlighted the importance of microorganisms and their genomes in several human niches and has emphasized the importance in human health and disease.1 The female reproductive tract contributes up to 9% of the human microbiota.2 Until recently, the main research focus has been on the vaginal microbiota.3 However, accumulating evidence suggests the existence of a different bacterial ecosystem in the endometrium,4–8 challenging the traditional dogma of the sterility of the human uterus.9,10
The vaginal microbiota has been investigated for years using microbial culture, microscopy, and culture-independent techniques, showing that the predominant bacteria are Lactobacilli.3 The endometrial cavity has been traditionally considered sterile, and the isolation of Enterobacteriaceae, Streptococcus, Staphylococcus, and Escherichia coli from the tip of the embryo transfer catheter has been linked with poor reproductive outcomes in patients undergoing in vitro fertilization (IVF).11 The development of culture-independent techniques, – especially 16S ribosomal RNA (16S rRNA) gene sequencing – allows interrogation of low-biomass sites. Shotgun Metagenomics Sequencing/Whole Metagenome Sequencing (SMS/WMS) allows investigation of species diversity and certain functional properties.12,13
Using 16S rRNA sequencing in specimens obtained through a transcervical catheter, the microbiota profile in the human endometrial fluid can be classified as Lactobacillus-dominated (LD) or non-LD (NLD), established by a cut-off of 90% Lactobacilli. Dysbiotic profiles (i.e., imbalanced bacterial composition for a given niche) characterized by an NLD microbiota together with specific pathogens have been associated with lower implantation, pregnancy, ongoing pregnancy, and live birth rates, as well as an increase in clinical spontaneous abortions.5,14
During pregnancy, the presence of pathogenic bacteria in the reproductive tract has been associated with obstetric complications such as spontaneous preterm birth and fetal death.15,16 The vaginal microbiota is significantly different between pregnant and non-pregnant women. These differences can be observed in terms of structure and stability; during pregnancy it is more stable and less diverse than that in nonpregnant women due to domination by Lactobacillus spp. and a lower frequency of bacteria associated with bacterial vaginosis.17–20 The higher stability of the vaginal microbiota during pregnancy can be attributed to high hormonal concentration of estrogen, the absence of menses, or changes in cervical and vaginal fluid.18 The dominance of vaginal Lactobacillus in pregnancy may have a protective role against pathogenic bacteria ascending to the maternal-fetal interface, where they can confer risk for the ongoing pregnancy.21,22 Here, we report the first incidental case characterizing the endometrial microbiota taxonomically and functionally – using 16S rRNA sequencing and WMS – prior to an embryo transfer that resulted in spontaneous abortion and during a 4th week gestation in the same woman who subsequently had a successful pregnancy (Figure 1).
Figure 1.
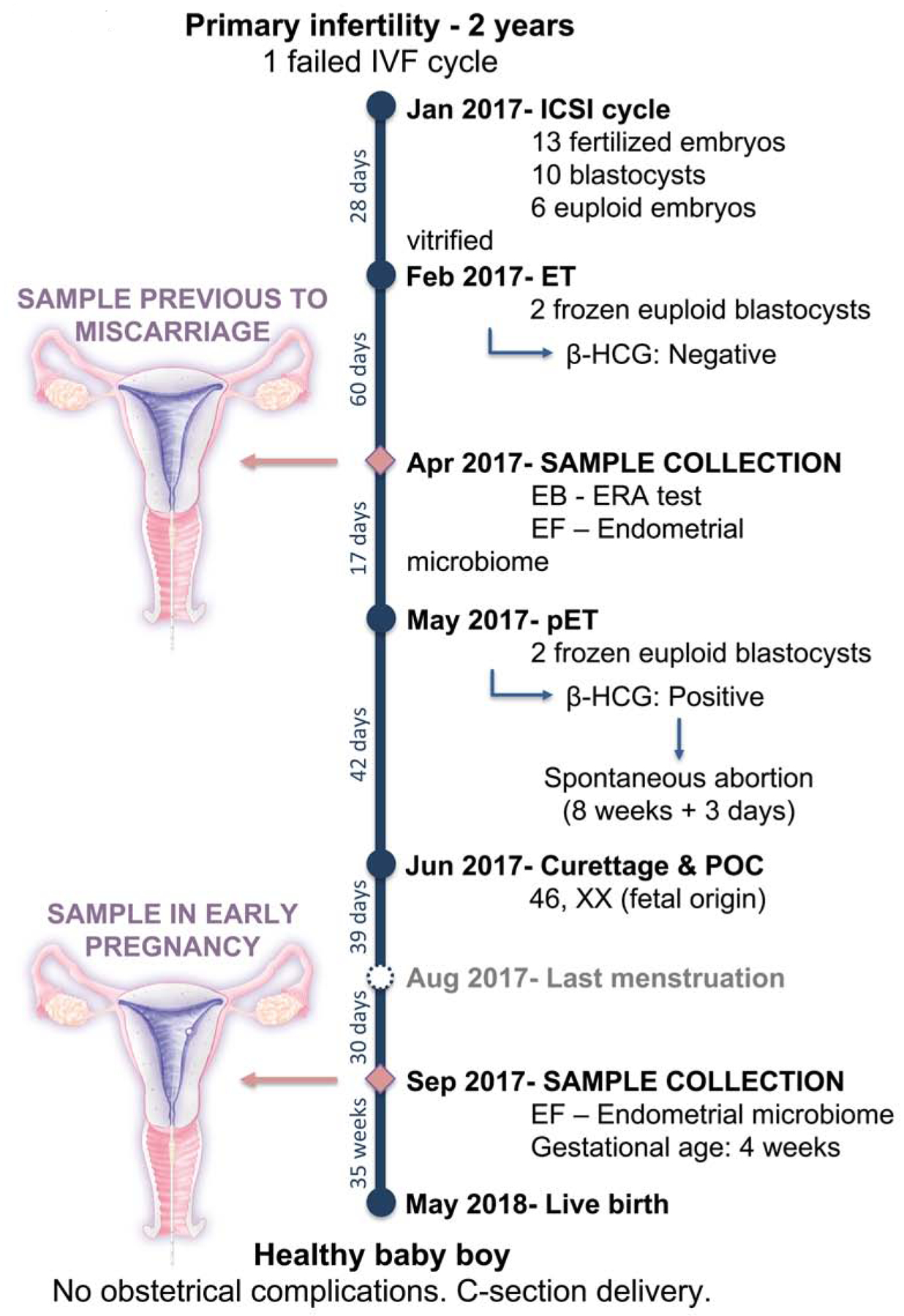
Flow chart of the clinical evolution of the patient during the spontaneous abortion and successful pregnancy. EB: Endometrial biopsy; EF: Endometrial fluid; ERA: Endometrial Receptivity Analysis; β-HCG: beta human chorionic gonadotropin; ET: embryo transfer; ICSI: intracytoplasmic sperm injection; IVF: in vitro fertilization; LD: Lactobacillus dominated; NLD: Non-Lactobacillus dominated; P: Progesterone; pET: personalized embryo transfer following the recommendation of ERA test; POC: Product of Conception.
PATIENT AND METHODS
A 28-year old woman with primary infertility for two years had undergone one unsuccessful IVF cycle (Figure 1). The patient did not have medical or surgical complications, had a body mass index (BMI) of 22, and a negative serological test for human immunodeficiency virus, hepatitis B virus, hepatitis C virus and syphilis. Her husband had normal semen analysis results, and neither had chromosomal abnormalities.
As a result of her first intracytoplasmic sperm injection (ICSI) cycle, 14 metaphase-II oocytes were retrieved resulting in 13 zygotes after ICSI. Of these, 10 embryos reached the blastocyst stage, resulting in 6 euploid embryos identified by pre-implantation genetic testing for aneuploidies (PGT-A), that were vitrified.
After the first embryo transfer (ET) of two euploid blastocysts, the pregnancy test was negative. Two months later, a sample of endometrial fluid was collected and stored for microbiota analysis prior to the endometrial biopsy used for the endometrial receptivity analysis (ERA) to guide personalized embryo transfer (pET). Subsequently, two euploid blastocysts were transferred in April 2017. Pregnancy was achieved, and the β-HCG concentration was 278.9 mIU/mL. One gestational sac 8 mm in diameter was visualized using transvaginal ultrasound during the 5th week of pregnancy. A spontaneous clinical miscarriage occurred at the 8th week of gestation, and dilation and curettage (D&C) was performed. The patient received azithromycin, 500 mg per day for 3 days. The analysis of the products of conception confirmed that the embryo was chromosomally normal with a profile 46, XX of fetal origin. Two months after the D&C, the patient was seen at the time of the expected menstruation to start a new embryo transfer cycle. In this visit, endometrial fluid was collected and stored to investigate changes in the microbiota. Subsequently, it became evident that the patient had conceived spontaneously, and was 4 weeks pregnant when the sample of endometrial fluid was obtained. The pregnancy continued uneventfully, and the patient delivered a healthy male infant weighing 3,700 g by cesarean section at 40 weeks of gestation.
Endometrial fluid had been collected under a protocol approved by the local Ethics Committee at the Instituto Valenciano de Infertilidad (Federal Wide Assurance number: FWA00027749; protocol number 1606-IGX-044-CS). The patient provided written informed consent for the aspiration of the endometrial fluid and the subsequent publication of her case.
Sample collection
Endometrial fluid samples were obtained by transcervical aspiration with a double lumen embryo transfer catheter as previously described.23 The specimens were collected in sterile tubes containing 50 μL of RNAlater solution (Thermo Fisher Scientific, Waltham, MA) following manufacturer’s instructions and stored at −80°C until use.
DNA extraction
Total DNA was isolated performing a pre-digestion step with lysozyme, lysostaphin and mutanolysin in order to degrade the cell wall of bacteria, followed by extraction with QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The genomic DNA was quantified using Tape Station (Agilent technologies, Waldbronn, Germany) and subjected to preamplification and sequencing for the identification of microbiota represented in the endometrial fluid.
16S ribosomal RNA sequencing
16S rRNA gene microbiota profiles were obtained using the Ion 16S metagenomics kit (ThermoFisher Scientific, Waltham, MA). This kit includes two primer sets (V2-4-8 and V3-6, 7-9) that selectively amplify the corresponding hypervariable regions of the 16S ribosomal subunit. The amplified fragments were sequenced on the Ion S5 XL system (ThermoFisher Scientific) and the results were analyzed using the QIIME 2.0 package (https://qiime2.org/) and RDP classifier 2.2 for taxonomic assignment. QIIME was used to calculate the alpha diversity and rarefaction curves before filtering. Positive controls of Escherichia coli DNA along with blank controls were included in the assays to detect any potential contamination from reagents.
Whole metagenome sequencing
The endometrial microbiome functional composition was assessed by WMS with the Illumina platform, using the Nextera DNA Flex Library Preparation kit (Illumina, San Diego, CA) following the manufacturer’s instructions. The sample collected during early successful pregnancy yielded sufficient DNA to analyze in two technical replicates starting from the same preparation of genomic DNA but sequencing the sample twice with independent amplifications and library preparations. Because both technical replicates yielded equivalent results, the results presented herein are representative of both aliquots. The libraries were sequenced on the NextSeq 500 system (Illumina, San Diego, CA). The reads generated by the Illumina sequencing platform were quality trimmed and length filtered using PRINSEQ.24 Paired-end reads were merged using Fast Length Adjustment of Short reads (FLASh) software tool25 and, finally, host-reads were removed using Burrows-Wheeler Aligner (BWA) mapper against human genome reference.26
Functional and taxonomical joint profiling was performed using the HMP Unified Metabolic Analysis Network (HUMAnN2) pipeline.27 This method combines taxonomic profiling of samples using MetaPhlAn2,28 which provides a panmicrobial annotation, using a combination of clade-specific markers and functional annotation inferred by the pangenomic database resulting from MetaPhlAn2 taxonomical classification. Another annotation to assess taxonomical classification robustness was obtained using the KRAKEN software with complete bacterial, archaeal and viral NCBI Reference Sequence (RefSeq) genomes database MiniKraken DB_4GB.29 The presence of biomedical interest protein families, such as G protein-coupled receptors (GPCRs) ligands producers, was assessed with InterProScan 5 and PFAM reference protein database.30,31 Finally, the pipeline outputs were processed using the R statistical software32 for statistical description and graphical representation of the sample’s taxonomical and functional profile.
Data availability
The Sequence data that support the findings of this study have been deposited as compressed fastq.gz files in the Sequence Read Archive (SRA) with the primary accession codes PRJNA514966 (http://www.ncbi.nlm.nih.gov/bioproject/514966).
RESULTS
The 16S rRNA sequencing of the endometrial fluid obtained in the cycle prior to the spontaneous miscarriage showed a non-Lactobacillus-dominant profile with 5% Actinobacteria, 19% Firmicutes, and 76% Proteobacteria. From these phyla, 15% of Lactobacilli was encountered together with several pathogenic bacterial genera previously reported to affect the reproductive tract such as Enterobacteriaceae (3%), Streptococcus (2%), Pseudomonas (2%), and Staphylococcus (0.8%). The microbiota during the successful 4-week pregnancy in the same patient revealed a Lactobacillus-dominated profile with 91% of Firmicutes and only 9% of Proteobacteria. Interestingly enough, Lactobacillus was the only bacteria present under the Firmicutes phylum accounting for 91% of the sample (Figure 2A).
Figure 2.
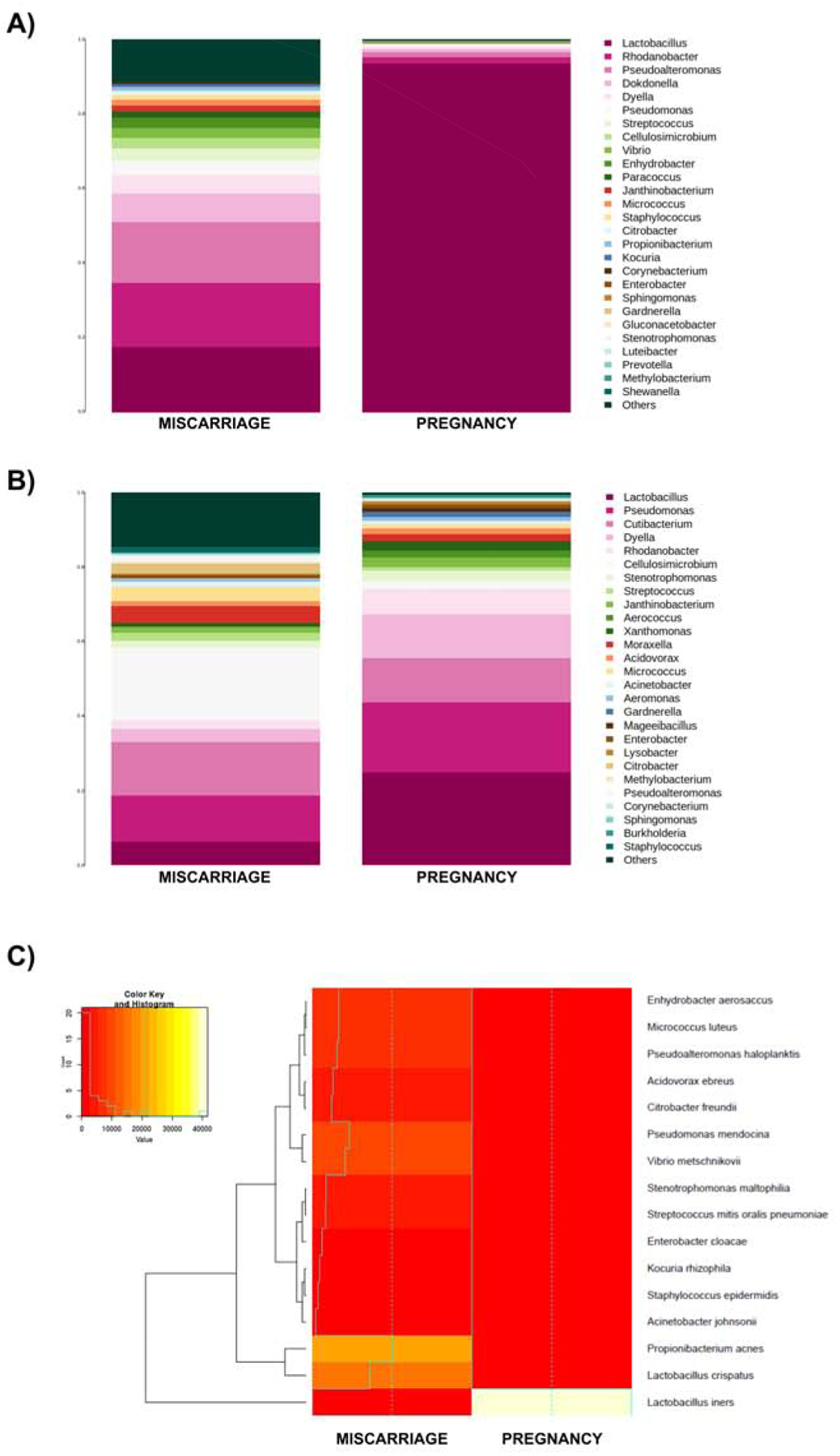
Endometrial microbiota profile assessed by 16S ribosomal RNA gene sequencing and whole-metagenome sequencing (WMS). (A) Microbiota composition profiles showing the most-abundant genera and their relative abundance in the sample preceding a spontaneous clinical miscarriage (MISCARRIAGE) or a successful pregnancy (PREGNANCY) in the same woman using 16S sequencing or (B) WMS. (C) Heatmap showing the bacterial composition with associated functional pattern analyzed by WMS.
Furthermore, the metagenomic analyses by WMS yielded a total of 238,778,133 reads. After quality control and filtering of human reads, only 0.1%–1% of reads corresponded to bacterial DNA while the vast majority of the sequences mapped to human DNA (Table 1). As in the 16S rRNA sequencing results, the taxonomic analysis by WMS showed a dysbiotic non-Lactobacillus-dominant profile in the endometrial fluid obtained prior to the spontaneous abortion, and alternatively, higher Lactobacillus abundance in the endometrial fluid sample collected in the presence of an embryo with successful implantation (Figure 2B). However, when analyzing the complexity of the microbial communities with the WMS technology in both samples, certain bacterial genera not represented in the 16S rRNA sequencing were detected such as Cutibacterium, Acidovorax, Xanthomonas, and Aerococcus (Figure 2B). Although the taxonomic assignment derived from WMS showed greater microbial diversity than 16S rRNA sequencing, when functional and taxonomic analyses were combined, the microbial diversity present in each sample was reduced. Due to this, the functional metagenomic analysis showed that the sample collected prior to the clinical spontaneous abortion contained Lactobacillus crispatus as the predominant Lactobacillus (15%) and a variety of bacterial genera, such as Propionibacterium (21%), Pseudomonas (10%), and Streptococcus (3.5%). In contrast, in the sample collected during the successful pregnancy, Lactobacillus iners was the only microbe found in the endometrium (Figure 2C).
Table 1.
Sequencing reads obtained after sequencing, quality control and elimination of human reads.
| Sample | Raw reads | Cleaned reads (%) | Joined reads (%) | Non-human reads (%) |
|---|---|---|---|---|
| MISCARRIAGE | 126,325,813 | 115,991,731 (91.8%) | 56,197,765 (44.5%) | 1,291,879 (1%) |
| PREGNANCY | 112,452,320 | 102,731,745 (91.4%) | 41,138,063 (36.6%) | 76,160 (0.1%) |
Functional metagenomics analysis also revealed different Lactobacillus species in the two samples (Figure 2C). L. iners was the only microbe present in the endometrium during successful early pregnancy, thus potentially associating its presence with defense mechanisms and basal functions – particularly, translation, energy production and cell division. In contrast, L. crispatus along with other non-Lactobacillus species were dominant in the endometrium prior to spontaneous abortion, and this community had a heterogeneous functional pattern characterized by transposases and insertion elements (Figure 3A).
Figure 3.
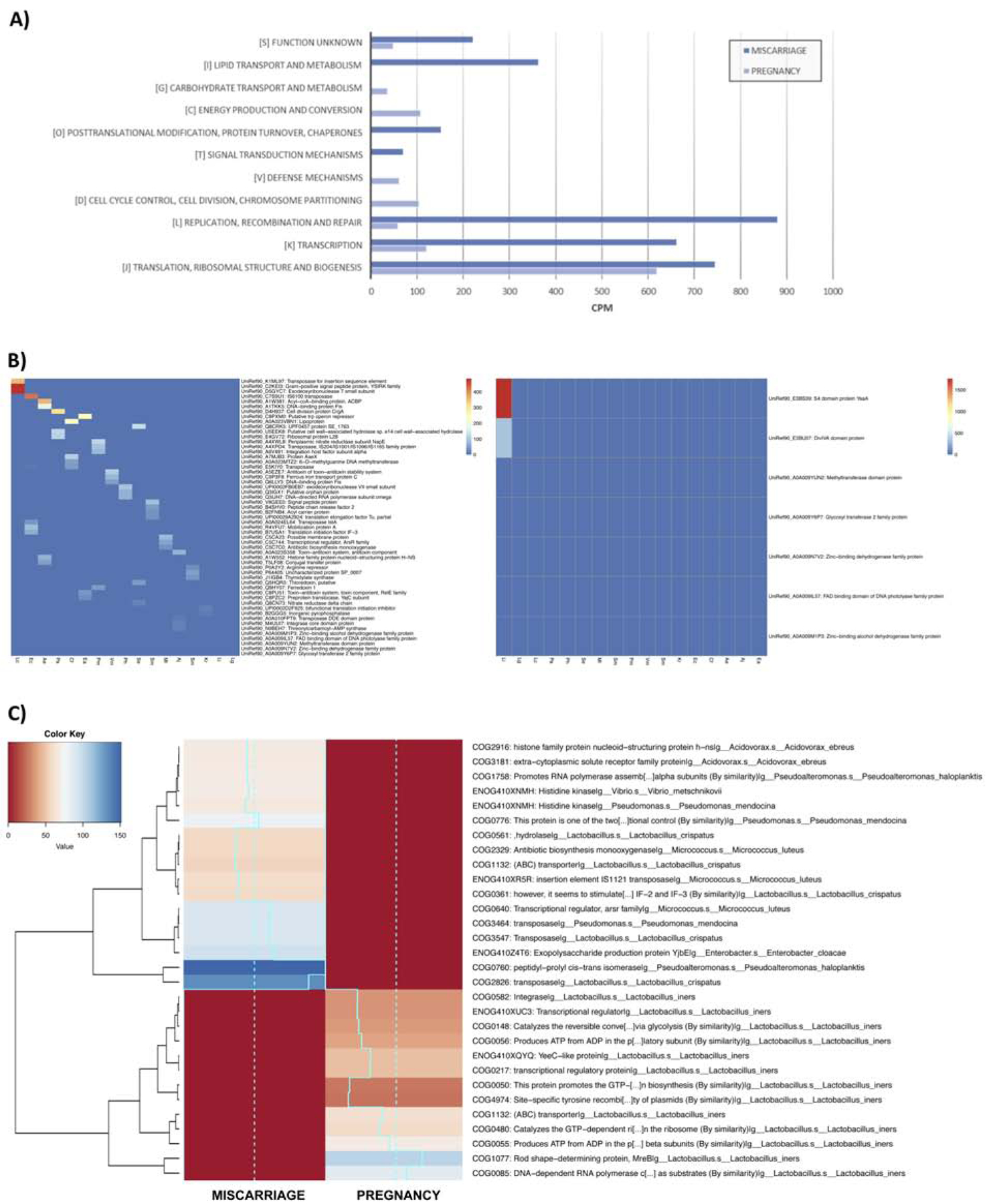
Functional pattern associated with taxonomy assessed by whole-metagenome sequencing. (A) Bar graph summarizing the 20 most detected functions obtained with the COGs results. (B) The functional metagenomic analysis was carried out in the sample preceding a miscarriage (left panel) and a successful pregnancy (right panel) using the information obtained from UniRef database and (C) Clusters of Orthologous Groups (COGs) associated with a specific taxonomy. Ae: Acidovorax ebreus; Aj: Acinetobacter johnsonii; Cf: Citrobacter freundii; Ea: Enhydrobacter aerosaccus; Ec: Enterobacter cloacae; Kr: Kocuria rhizophila; Lc: Lactobacillus crispatus; Lg: Lactobacillus gasseri; Li: Lactobacillus iners; Ml: Micrococcus luteus; Pa: Propionibacterium acnes; Ph: Pseudoalteromonas haloplanktis; Pm: Pseudomonas mendocina; Se: Staphylococcus epidermidis; Sm: Stenotrophomonas maltophilia; Sm: Streptococcus mitis; Vm: Vibrio metschnikovii.
The results of the metagenomic sequencing showed both taxonomic and functional differences in the two endometrial microbiomes from the same patient. The functional metagenomic analysis was performed using the information obtained from UniRef database and Clusters of Orthologous Groups (COGs) considering the proteins and functions associated with a specific taxonomy, respectively. After analyzing the most represented proteins in each sample, a greater functional annotation associated with several bacteria was observed in the sample preceding the spontaneous abortion, whereas in the sample obtained during the successful pregnancy, only proteins associated with L. iners were detected (Figure 3B). We also observed distinct functional profiles when comparing the main COG groups present in both samples (Figure 3C). “Information storage and processing” was the most represented functional category in both samples, with 2,285 and 798 counts per million in the sample associated with spontaneous abortion and successful pregnancy, respectively (ftp://ftp.ncbi.nlm.nih.gov/pub/COG/COG/fun.txt). Moreover, of the 25 COG subcategories established in the database, the endometrium prior to miscarriage showed an unstable functional pattern characterized by transposases and insertion elements belonging to the subcategory “[L] Replication, recombination and repair”. For instance, we found transposases and mobile elements, like Tra8, the only member of the superfamily cl28582, (COG2826), and a member of the superfamily cl27435 (COG3547) (Figure 3B). In contrast, the microbiome during early pregnancy subcategory “[J] translation, ribosomal structure and biogenesis” was the most represented. Notably, functions associated with defense mechanisms (subcategory [V]), carbohydrate metabolism and energy production (subcategories [C] [G]), and cell division (subcategory [D]) were only represented in the sample from the successful pregnancy, where the predominant bacterium was Lactobacillus (Figure 3A).
Microbes produce G protein-coupled receptor (GPCR) ligands to communicate with the human host and regulate their physiology.33 In both endometrial fluid samples, we sought sequences associated with the N-acyl synthase protein family PF13444, the consensus PFAM profile of the G protein-coupled receptor. In the endometrial microbiome prior to the spontaneous abortion, we identified 44 sequences corresponding to molecules of the Gcn5-related N-acetyltransferases (GNAT) domain, while in the microbiome of the early pregnancy, these sequences were not found.
COMMENTS
This case represents the first glimpse of the endometrial microbiome during a successful pregnancy. Moreover, we found an abnormal endometrial microbiome prior to spontaneous abortion in the same patient, with euploid embryos.
The microbiota of the reproductive tract is an important determinant of health and disease.34–37 Spontaneous abortion is a syndrome caused by multiple etiologies, reflecting the interaction of embryonic, maternal, and microbial factors.38 The role of the host-microbial relationship in determining pregnancy outcome is poorly understood.
Although it has been demonstrated that the reproductive tract of healthy women can be colonized by L. iners,39 it has been often identified in transitional communities between bacterial vaginosis and a normal microbiota.40 For example, L. iners was found to be dominant after treatment for bacterial vaginosis.41 In our study, transition to an L. iners-dominated microbiota after a period of instability – clinical miscarriage, followed by D&C and antibiotic treatment – was observed in the endometrial fluid present during early pregnancy when the embryo was already implanted. The genome of L. iners contains an iron-sulfur (Fe-S) cluster that limits the iron availability. This system may be used as a defense mechanism, providing a competitive advantage against other bacterial pathogens, or it may play a role in providing nutrients and surviving in adverse conditions such as menstruation.42 Correspondingly, it has been found that during menstruation the abundance of L. iners in the vaginal community increases while the number of L. crispatus decreases.40,43 The potential of L. iners to sequester iron could confer this microorganism with an advantage in respect to other bacteria in order to colonize the uterine cavity after D&C, where the environmental conditions are characterized by the presence of blood, similar to menstruation.
Mendes-Soares et al. characterized the genomes of several L. iners strains and found they lack several proteins related to the acetyltransferase GNAT family and various transcriptional regulators.44 Indeed, these results are in agreement with our findings. The GNAT domain is implicated in bacterial antibiotic resistance, chromatin remodeling, as well as anabolic and catabolic functions. Three putative ligands have been found in the ChEMBL database related to the GNAT domain: Luspatercept, Ecallantide, and Rilonacept, which correspond to inhibitors of activin receptor type-2B, plasma kallikrein, and interleukin-1β (IL-1β), respectively (Table 2). Ecallantide (KALBITOR) and Rilonacept (ARCALYST) are FDA-approved drugs with important effects on human health (www.accessdata.fda.gov). Rilonacept is an IL-1 blocker indicated for treatment of cryopyrin-associated periodic syndrome, associated with mutations in the cryopyrin gene, which produces an overactive inflammasome and excessive release of IL-1β that drives inflammation. Rilonacept blocks IL-1β signaling by acting as a soluble decoy receptor that binds IL-1β, preventing activation of IL-1 receptors. In both mice and humans, IL-1ra binds to IL-1R type 1 receptor, preventing signal transduction blocking its physiological responses in vivo such as hypoglycemia, induction of IL-6, and corticosterone production.45,46 Embryonic implantation in mice is blocked by IL-1 receptor antagonist.47 Our group demonstrated that blockade of maternal endometrial IL-1R t1 with IL-1ra prevents implantation in the mouse by interfering with embryonic attachment, without adverse effects on blastocyst formation, hatching, fibronectin attachment, outgrowth, and migration in vitro.47
Table 2.
Potential ligands of the GNAT sequences found in the sample obtained prior to spontaneous abortion. Source: ChEMBL database.
| Name | Compound ID | Drug Phase | Mechanism of Action | ChEMBL Target |
|---|---|---|---|---|
| LUSPATERCEPT | 3039545 | 3 | Activin receptor type-2B antagonist | Activin receptor type-2B |
| ECALLANTIDE (Kalbitor) | 1201837 | Approved | Plasma kallikrein inhibitor | Plasma kallikrein |
| RILONACEPT (Arcalyst) | 1201830 | Approved | lnterleukin-1 beta inhibitor | lnterleukin-1 beta |
L. crispatus and L. iners are common inhabitants of the healthy reproductive tract. These two species are closely related and are thought to perform similar ecological functions. Nevertheless, there is a wide range of activity within strains of all bacteria, including Lactobacillus spp., and differences in their genomes can explain their specificity for a given niche. Unlike other species studied, L. crispatus has the largest genome with a unique DNA polymerase, bacteriocin, and toxin-antitoxin genes that encode mobile genetic elements, especially transposases,48,49 consistent with the large number of functions related to mobile elements observed in the sample collected prior to spontaneous abortion. Also, other factors may influence the reproductive tract microbiota. Further studies are needed to determine the precise role of these interesting species in endometrial health and disease and whether these strains can serve as biomarkers of reproductive success or failure.
The main cause of clinical miscarriage in humans is embryo aneuploidy.50 The strength of the investigation of the endometrial microbiota is based on the fact that the chromosomal status of the embryos transferred was assessed prior to embryo transfer, confirmed in the products of conception after spontaneous abortion, and in the baby born after a successful pregnancy, ruling out embryo aneuploidy as a possible cause of miscarriage.
Predominantly, most of the high-throughput studies that characterize the endometrial microbiota have identified bacterial taxa to the genus, family, or order level, but have not been able to distinguish between bacterial species. For this reason, one of the main contributions of this study is to describe the distinct endometrial community in pregnancy and previous to miscarriage using WMS and bioinformatics tools that provide resolution at the species level.
However, some limitations must be acknowledged. First, there is some controversy about the existence of an indigenous intrauterine microbiome in the placenta or amniotic fluid in uncomplicated pregnancies51,52,53,54 or the endometrium of reproductive age women, although several studies analyzing endometrial samples from abdominal hysterectomies have pointed to it.4,6–8,55 The HMP has revealed that samples collected from the vagina contain a large amount of human DNA (~96%).1 Considering that the endometrial microbiota is a low-biomass ecosystem and its bacterial load is estimated to be between 100 and 10,000 times lower than the vaginal microbiota,4,8 the percentage of reads corresponding to bacteria found in our study was not unexpected. Despite the limited coverage, there were enough reads to perform the analysis with 1,291,879 and 76,160 reads in the first and in the second endometrial fluid, respectively.
Also, we have observed differences between the microbial profiles obtained by taxonomic-only or taxonomic coupled to functional analysis. A possible explanation for such differences could be the potential noise introduced in the sample by the DNA extraction kit, as it has been shown that DNA from bacterial genera such as Methylobacterium, Stenotrophomonas, Janthinobacterium, etc. could be contained in laboratory reagents, hence affecting microbiota analysis in low-biomass samples at the taxonomic-only level.56
Finally, the samples of endometrial fluid were collected using a transcervical catheter. We cannot exclude that some level of contamination with cervical and vaginal microorganisms may have occurred. However, there are no alternative non-invasive means to obtain endometrial samples, particularly in early gestation. The merit of studying the endometrial microbiota using endometrial fluid collected in this manner needs to be ascertained by clinical studies that examine reproductive success given a particular microbial profile. Our findings are consistent with reports by other investigators that isolation of bacterial pathogens from the embryo transfer catheter tip is associated with poor IVF outcomes.57–62 This raises the question of whether the microbial communities present in the reproductive tract exert their effects either inside or in close proximity to the uterine cavity, modifying physiological conditions in the uterine cavity and reproductive fitness.
Conclusions
Bacteria may facilitate or hamper human conception. Our results are the first observation of taxonomic and functional differences in the endometrial fluid microbiota between an early successful pregnancy and prior to spontaneous miscarriage with euploid embryos in the same patient. Functional metagenomic and 16S rRNA sequencing showed a bacterial community with lower richness and diversity and higher Lactobacillus abundance in early successful pregnancy compared to miscarriage. Ultimately, using WMS, we describe distinct functional profiles in which basal metabolism and transcription regulation are main functions in successful pregnancy. If confirmed, these findings would highlight the emerging relevance of commensal microbes in the endometrium. Our observations may also have implications to understand the causes of first trimester spontaneous abortion and facilitate development of diagnostic tools, which could be the basis for alternative and personalized therapeutic procedures with interventions to change the endometrial microbiota.
Supplementary Material
AJOG at a glance:
A. Why was the study conducted?
To address the question of whether there is a human endometrial microbiota in early pregnancy. This question became tractable because endometrial fluid was collected when pregnancy had not been diagnosed. Therefore, it was possible to characterize the endometrial microbiota in the cycle prior to a spontaneous abortion and during a successful pregnancy.
B. What are the key findings?
There were taxonomic and functional differences between the microbiota found in endometrial fluid collected during an early successful pregnancy and prior to a spontaneous abortion with euploid embryos in the same patient.
C. What does this study add to what is already known?
This study describes the differences in the microbial community of the endometrium in a successful pregnancy compared to that of a pregnancy failure. This observation suggests that an endometrial microbiota is present in normal pregnancy and that its composition can be different prior to a spontaneous abortion. These observations support that the endometrial microbiota may be associated with different reproductive outcomes.
ACKNOWLEDGEMENTS
The authors thank Dr. Daniel Ramon, PhD, vice president R&D, ADM Health & Wellness for critical reading of the manuscript and Maria Contini & Oscar Simon from American School of Valencia for editing this manuscript. No compensation was provided for their contribution.
Financial support
This work was partially supported by Igenomix Foundation and the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. IG-G is supported by a Formación de Profesorado Universitario grant (FPU15/01923) from the Spanish Ministry of Education, Madrid, Spain. DB is supported by Torres-Quevedo grant (PTQ-16-08454) from the Spanish Ministry of Economy and Competitiveness. FV is supported by Miguel Servet program type II (CPII18/00020) and FIS project (PI18/00957) from Instituto de Salud Carlos III, Spain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
IM, DB, DP-V, MG-M, and CS are partial or full-time employed by Igenomix S.L. IG-G, FV and RR report no conflict of interest.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: Dr. Roberto Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Contributor Information
Inmaculada MORENO, Valencia, Spain, Igenomix Foundation-Instituto de Investigación Sanitaria Hospital Clínico (INCLIVA) and Igenomix S.L..
Iolanda GARCIA-GRAU, Valencia, Spain, Igenomix Foundation-Instituto de Investigación Sanitaria Hospital Clínico (INCLIVA) and Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia..
Davide BAU, Valencia, Spain, Igenomix S.L..
David PEREZ-VILLAROYA, Valencia, Spain, Igenomix S.L..
Marta GONZALEZ-MONFORT, Valencia, Spain, Igenomix Foundation-Instituto de Investigación Sanitaria Hospital Clínico (INCLIVA) and Igenomix S.L..
Felipe VILELLA, Valencia, Spain, Igenomix Foundation-Instituto de Investigación Sanitaria Hospital Clínico (INCLIVA)..
Roberto ROMERO, Detroit, MI, USA, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), Bethesda, Maryland, and Detroit, Michigan, USA; Department of Obstetrics and Gynecology, University of Michigan, Ann Arbor, MI, USA; Department of Epidemiology and Biostatistics, Michigan State University, East Lansing, MI, USA; Center for Molecular Medicine and Genetics, Wayne State University, Detroit, MI, USA; Detroit Medical Center, Detroit, Michigan, USA; Department of Obstetrics and Gynecology, Florida International University, Miami, Florida, USA.
Carlos SIMON, Valencia, Spain, Igenomix Foundation-Instituto de Investigación Sanitaria Hospital Clínico (INCLIVA) and Igenomix S.L; Department of Pediatrics, Obstetrics and Gynecology, School of Medicine, University of Valencia, Valencia, Spain; Department of Obstetrics and Gynecology, Baylor College of Medicine, Houston, TX, USA; IVI Valencia, Valencia, Spain; BIDMC Harvard University, Boston, MA, USA..
REFERENCES
- 1.Human Microbiome Project Consortium. A framework for human microbiome research. Nature 2012; 486 (7402):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res 2009;19(12):2317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell CM, Haick A, Nkwopara E, et al. Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obstet Gynecol 2015;212(5):611.e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno I, Codoñer FM, Vilella F, et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am J Obstet Gynecol 2016;215(6):684–703. [DOI] [PubMed] [Google Scholar]
- 6.Walther-António MR, Chen J, Multinu F, et al. Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med 2016;8(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles SM, Hardy BL, Merrell DS. Investigation of the microbiota of the reproductive tract in women undergoing a total hysterectomy and bilateral salpingo-oopherectomy. Fertil Steril 2017;107(3):813–20.e1. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Song X, Wei W, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun 2017;8(1):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tissier H. Recherches sur la flore Intestinale des Nourrissons [Thesis no 529]. Paris: 1900. [Google Scholar]
- 10.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 2004;82(4):799–804. [DOI] [PubMed] [Google Scholar]
- 11.Moreno I, Simon C. Microbiological diagnosis: The human endometrial microbiome- Endometritis In Simon C & Giudice LC (Eds.). The endometrial factor: A reproductive precision medicine approach. CRC Press; 2017. 65–77. [Google Scholar]
- 12.Mor A, Driggers PH, Segars JH. Molecular characterization of the human microbiome from a reproductive perspective. Fertil Steril 2015; 104:1344–50. [DOI] [PubMed] [Google Scholar]
- 13.Quince C, Walker AW, Simpson JT, Loman NJ, Segata N. Shotgun metagenomics, from sampling to analysis. Nat Biotechnol. 2017;35(9):833–44. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Grau I, Perez-Villaroya D, Bau D, Gonzalez-Monfort M, Vilella F, Moreno I, Simon C. Taxonomical and functional assessment of the endometrial microbiota in a context of recurrent reproductive failure: a case report. Pathogens 2019;8(4). pii: E205. doi: 10.3390/pathogens8040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anahtar MN, Gootenberg DB, Mitchell CM, Kwon DS. Cervicovaginal microbiota and reproductive health: the virtue of simplicity. Cell Host Microbe 2018;23(2):159–68. [DOI] [PubMed] [Google Scholar]
- 16.Flenady V, Wojcieszek AM, Middleton P, et al. Stillbirths: recall to action in high-income countries. Lancet 2016;387(10019):691–702. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of nonpregnant women. Microbiome 2014;2(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walther-António MR, Jeraldo P, Berg Miller ME, et al. Pregnancy’s stronghold on the vaginal microbiome. PLoS One 2014;9(6):e98514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Sci Rep 2015;5:8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiGiulio DB, Callahan BJ, McMurdie PJ, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A 2015;112(35):11060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 2014b;71: 330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stout MJ, Zhou Y, Wylie KM, Tarr PI, Macones GA, Tuuli MG. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol 2017;217(3):356.e1–356.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilella F, Ramirez L, Berlanga O, et al. PGE2 and PGF2α concentrations in human endometrial fluid as biomarkers for embryonic implantation. J Clin Endocrinol Metab 2013;98(10):4123–32. [DOI] [PubMed] [Google Scholar]
- 24.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011;27(6):863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011;27(21):2957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010;26(5):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abubucker S, Segata N, Goll J, et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput Biol 2012;8(6):e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truong DT, Franzosa EA, Tickle TL, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods 2015;12(10):902–3. [DOI] [PubMed] [Google Scholar]
- 29.Wood DE, Salzberg SL. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 2014;15(3):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones P, Binns D, Chang HY, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics 2014;30(9):1236–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Punta M, Coggill PC, Eberhardt RY, et al. The Pfam protein families database. Nucleic Acids Res 2012;40(Database issue):D290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Development Core Team. 2008. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org. [Google Scholar]
- 33.Cohen LJ, Esterhazy D, Kim SH, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017;549(7670):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wylie KM, Wylie TN, Cahill AG, Macones GA, Tuuli MG, Stout MJ. The vaginal eukaryotic DNA virome and preterm birth. Am J Obstet Gynecol 2018;219(2):189.e1–189.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu DM, Seferovic M, Pace RM, Aagaard KM. The microbiome in preterm birth. Best Pract Res Clin Obstet Gynaecol 2018;52:103–13. [DOI] [PubMed] [Google Scholar]
- 36.Strauss JF 3rd, Romero R, Gomez-Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018;218(3):294–314.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayigga L, Kateete DP, Anderson DJ, Sekikubo M, Nakanjako D. Diversity of vaginal microbiota in sub-Saharan Africa and its effects on HIV transmission and prevention. Am J Obstet Gynecol 2019. February;220(2):155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update 2016. Jan-Feb;22(1):116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMillan A, Macklaim JM, Burton JP, Reid G. Adhesion of Lactobacillus iners AB-1 to human fibronectin: a key mediator for persistence in the vagina? Reprod Sci 2013;20(7):791–6. [DOI] [PubMed] [Google Scholar]
- 40.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012;4(132):132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: Friend or Foe? Trends Microbiol 2017;25(3):182–91. [DOI] [PubMed] [Google Scholar]
- 42.Macklaim JM, Gloor GB, Anukam KC, Cribby S, Reid G. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santiago GL, Tency I, Verstraelen H, et al. Longitudinal qPCR study of the dynamics of L. crispatus, L. iners, A. vaginae, (sialidase positive) G. vaginalis, and P. bivia in the vagina. PLoS One 2012;7(9):e45281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol 2014;196(7):1458–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mengozzi M, Bertini R, Sironi M, Ghezzi P. Inhibition by interleukin 1 receptor antagonist of in vivo activities of interleukin 1 in mice. Lymphokine Cytokine Res 1991:10:405–7. [PubMed] [Google Scholar]
- 46.Granowitz EV, Porat R, Mier JW, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 1992;4:353–60. [DOI] [PubMed] [Google Scholar]
- 47.Simón C, Frances A, Piquette GN, Zurawsky G, Deng W, Polan ML. The immune mediator interleukin-1 receptor antagonist (IL-1ra) prevents embryonic implantation. Endocrinology 1994;134:521–8. [DOI] [PubMed] [Google Scholar]
- 48.Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.France MT, Mendes-Soares H, Forney LJ. Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. Appl Environ Microbiol 2016;82(24):7063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am J Med Genet A 2016;170(10):2671–80. [DOI] [PubMed] [Google Scholar]
- 51.De Goffau MC, Lager S, Sovio U, et al. Human placenta has no microbiome but can contain potential pathogens. Nature 2019;572(7769):329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theis KR, Romero R, Winters AD, et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol. 2019;220(3):267.e1–267.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rehbinder EM, Lødrup Carlsen KC, Staff AC, et al. Is amniotic fluid of women with uncomplicated term pregnancies free of bacteria? Am J Obstet Gynecol. 2018;219(3):289.e1–289.e12. [DOI] [PubMed] [Google Scholar]
- 54.Seferovic MD, Pace RM, Carroll M, et al. Visualization of microbes by 16S in situ hybridization in term and preterm placentas without intraamniotic infection. Am J Obstet Gynecol. 2019;221(2):146.e1–146.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winters AD, Romero R, Gervasi MT, et al. Does the endometrial cavity have a molecular microbial signature? Sci Rep 2019;9(1):9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egbase PE, al-Sharhan M, al-Othman S, al-Mutawa M, Udo EE, Grudzinskas JG. Incidence of microbial growth from the tip of the embryo transfer catheter after embryo transfer in relation to clinical pregnancy rate following in-vitro fertilization and embryo transfer. Hum Reprod 1996;11(8):1687–9. [DOI] [PubMed] [Google Scholar]
- 58.Fanchin R, Harmas A, Benaoudia F, Lundkvist U, Olivennes F, Frydman R. Microbial flora of the cervix assessed at the time of embryo transfer adversely affects in vitro fertilization outcome. Fertil Steril 1998;70(5):866–70. [DOI] [PubMed] [Google Scholar]
- 59.Egbase PE, Udo EE, al-Sharhan M, Grudzinskas JG. Prophylactic antibiotics and endocervical microbial inoculation of the endometrium at embryo transfer. Lancet 1999;354(9179):651–2. [DOI] [PubMed] [Google Scholar]
- 60.Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril 2000;74(6):1118–24. [DOI] [PubMed] [Google Scholar]
- 61.Salim R, Ben-Shlomo I, Colodner R, Keness Y, Shalev E. Bacterial colonization of the uterine cervix and success rate in assisted reproduction: Results of a prospective survey. Hum Reprod 2002;17(2):337–40. [DOI] [PubMed] [Google Scholar]
- 62.Selman H, Mariani M, Barnocchi N, et al. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. J Assist Reprod Genet 2007;24(9):395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Sequence data that support the findings of this study have been deposited as compressed fastq.gz files in the Sequence Read Archive (SRA) with the primary accession codes PRJNA514966 (http://www.ncbi.nlm.nih.gov/bioproject/514966).


