Abstract
Background
As estrogens have been shown to have several potentially beneficial effects on the central nervous system, it is biologically plausible that maintaining high levels of estrogens in postmenopausal women by means of estrogen replacement therapy (ERT) could be protective against cognitive decline in women with Alzheimer's disease (AD) or other dementia syndromes.
Objectives
To investigate the effects of ERT (estrogens only) or HRT (estrogens combined with a progestagen) compared with placebo in randomized controlled trials (RCTs) on cognitive function of postmenopausal women with dementia.
Search methods
The Cochrane Dementia and Cognitive Improvement Group Specialized Register, which contains records from many medical databases, The Cochrane Library, EMBASE, MEDLINE, CINAHL, PsycINFO and LILACS were searched on 7 November 2007 using the terms ORT, PORT, ERT, HRT, estrogen*, oestrogen* and progesterone*.
Selection criteria
All double‐blind randomized controlled trials (RCTs) into the effect of ERT or HRT for cognitive function with a treatment period of at least two weeks in postmenopausal women with AD or other types of dementia.
Data collection and analysis
Abstracts of the references retrieved by the searches were read by two reviewers (EH and KY) independently in order to discard those that were clearly not eligible for inclusion. The two reviewers studied the full text of the remaining references and independently selected studies for inclusion. Any disparity in the ensuing lists was resolved by discussion with all reviewers in order to arrive at the final list of included studies. The selection criteria ensured that the blinding and randomization of the included studies was adequate. The two reviewers also assessed the quality of other aspects of the included trials. One reviewer (EH) extracted the data from the studies, but was aided and checked by JB from Cochrane.
Main results
A total of seven trials including 351 women with AD were analysed. Because different drugs were used at different studies it was not possible to combine more than two studies in any analysis.
On a clinical global rating, clinicians scored patients taking CEE as significantly worse compared with the placebo group on the Clinical Dementia Rating scale after 12 months (overall WMD = 0.35, 95% CI = 0.01 to 0.69, z = 1.99, P < 0.05).
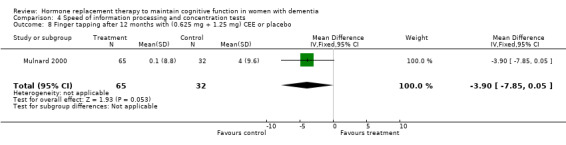
Patients taking CEE had a worse performance on the delayed recall of the Paragraph Test (overall WMD = ‐0.45, 95% CI = ‐0.79 to ‐0.11, z = 2.60, P < 0.01) after one month than those taking placebo. They had a worse performance on Finger Tapping after 12 months (WMD = ‐3.90, 95% CI = ‐7.85 to 0.05, z = 1.93, P < 0.05).
Limited positive effects were found for the lower dosage of CEE (0.625 mg/day) which showed a significant improvement in MMSE score only when assessed at two months, and disappeared after correction for multiple testing. No significant effects for MMSE were found at longer end points (3, 6 and 12 months of treatment). With a dosage of 1.25 mg/d CEE, short‐term significant effects were found for Trial‐Making test B at one month and Digit Span backward at four months. After two months of transdermal diestradiol (E2) treatment, a highly significant effect was observed for the word recall test (WMD = 6.50, 95% CI = 4.04 to 8.96, z = 5.19, P < 0.0001). No other significant effects were found for other outcomes measured.
Authors' conclusions
Currently, HRT or ERT for cognitive improvement or maintenance is not indicated for women with AD.
Plain language summary
There is no evidence of a positive effect that estrogen replacement therapy can maintain cognitive function for a longer period of time (> five months) in women with Alzheimer's disease
After the menopause, in women levels of estrogens decline. Estrogen replacement therapy (ERT) or replacement therapy with both estrogens and progestagens (hormone replacement therapy or HRT) might theoretically help to maintain cognitive function in postmenopausal women with dementia. We therefore investigated the results of randomized controlled trials of the effects of ERT and HRT on cognitive function in postmenopausal women with AD.
Overall, however, there was no evidence for positive effects of ERT or HRT which was sustained after two months of treatment. This is similar to results of studies of ERT and HRT in women without dementia, which additionally found that HRT increases the rate of dementia in women over 65 years.
Background
Nearly all cognitive functions decline, on average, with age, but there is a large variability which ranges from "successful" aging to dementia (Huppert 1997). The determinants of this variability are uncertain but elderly women seem to have a higher risk of developing Alzheimer's disease (AD) than elderly men (Launer 1999). While this may be because they reach an older age, and aging is a risk factor for AD, the age‐specific incidence of AD is also higher in women than in men (Fratiglioni 2000). It has been suggested that sex steroid hormone deficiencies in elderly women may play a role in this difference.
Estrogens (UK spelling oestrogens) are steroid hormones produced in women by the ovaries. The estrogen‐producing cells become depleted at menopause, and postmenopausal women have much lower estrogen levels than men. In men, and in women after the menopause, the main source of estrogens is from conversion of circulating androgen steroid hormone precursors. In women, the main source of androgen steroids are the ovaries (theca cells) and the adrenal cortex, whereas in men it is the testes. Estrogens have an important role in the female reproductive cycle, but animal and in vivo cell studies have suggested that estrogens can have beneficial effects on brain structures including those related to memory, such as the hippocampus and basal cholinergic forebrain (McEwen 1997). There appear to be a variety of mechanisms involved in this process, including anti‐amyloidgenic effects, antioxidant effects, dendritic sprouting and effects on various neurotransmitters involved in cognitive function (Silva 2001; McEwen 1997).
It is possible that maintaining high levels of estrogens in postmenopausal women by means of estrogen replacement therapy (ERT) or combined therapy with estrogens and progestagens (hormone replacement therapy ‐ HRT) could be protective against cognitive decline and the development of AD or other dementia syndromes. Post‐menopausal ERT or HRT is usually prescribed to treat menopausal symptoms, such as hot flashes (UK hot flushes) and night sweats. For hysterectomized postmenopausal women, replacement therapy is usually given as ERT but HRT is prescribed for postmenopausal women with a uterus to reduce the risk of endometrial hypertrophy and cancer. ERT use has been associated with an increased risk of breast as well as uterine cancer (but see Col 2001) and after the results of WHIMS were published (Shumaker 2003; Shumaker 2004), paradoxically now also with a doubled risk of dementia in women > 65 years (see discussion).
Most observational studies, however, suggested that the use of ERT and HRT is associated with a decreased risk of AD (Hogervorst 2000; Yaffe 1998a), but observational studies are subject to bias (Barrett‐Connor 1991). For instance, women who choose to use ERT or HRT after the menopause in general have a higher education, healthier life‐styles and are also healthier before using ERT or HRT than women who do not chose to use ERT or HRT (Matthews 1996). Taking ERT or HRT is thus associated with a healthier life style, which in turn can decrease the risk for dementia. In addition, despite estrogen's biologically plausible mechanisms for protecting the aging brain, two earlier reviews concluded that the human studies had substantial methodological problems and had produced conflicting results (Hogervorst 2000; Yaffe 1998a).
The present review assesses the evidence for effectiveness of ERT or HRT in treating the cognitive impairments of postmenopausal women with dementia. The evidence for effects of ERT or HRT on cognitive function in healthy postmenopausal women has been the subject of another review (Lethaby 2008).
Refer to Appendix 1 for a full list of abbreviations and their definitions.
Objectives
To assess the effects of hormone replacement therapy consisting of estrogens alone (ERT) or in combination with a progestagen (HRT) on cognitive function in women with dementia.
Methods
Criteria for considering studies for this review
Types of studies
All randomized placebo‐controlled trials (RCTs) were included in which treatment with ERT or HRT was administered to women with dementia to maintain cognitive function for at least two weeks. Trials in which the allocation to treatment or control was not randomized, or in which treatment allocation was not concealed, were excluded. This is because prior knowledge of treatment allocation may lead to biased patient allocation (Schulz 1995).
Types of participants
Postmenopausal women who had been diagnosed as having Alzheimer's disease or other dementia syndromes by standard consensus‐based criteria, such as ICD‐10, DSM (APA 1999) or NINCDS/ADRDA (McKhann 1984). Postmenopausal status was defined as established six months after the last menstrual period.
Types of interventions
Interventions containing estrogens alone (ERT) or when combined with a progestagen (HRT). All doses and dosing schedules and any mode of administration ‐ oral, subdermal, transdermal or intravenous ‐ were considered. Most commonly used ERTs are: CEE = conjugated equine estrogens: given orally in dosages of 0.625 or 1.25 mg per day (apparently also in 0.3 mg/day) which contains E1‐S = estrone sulphate; E2 = estradiol: usually given transdermally (0.1 to 0.05 mg e.g. Asthana 1999) or intramuscularly (2 mg/week, e.g. McDonald Caldwell 1952); Sometimes estrogens are given in combination with a progestagen (HRT) which is usually MPA = medroxyprogesteroneacetate or P = progesterone (McDonald Caldwell 1952; Honjo 1995; Birge 1997) to protect women with a uterus against endometrial hyperplasia and malignancies. This treatment regimen could be sequential or continuous and all dosages were considered.
Types of outcome measures
The primary outcome of interest was cognitive function, split into the more specific following categories:
General cognitive function tests
The Mini‐Mental Status Examination (MMSE), the Blessed Information, Memory and Concentration Test (BIMC), and the Alzheimer's Disease Assessment Scale‐cognitive subscale (ADAS‐Cog). In two studies (Honjo 1995; Zhang 2006) the Hasegawa Dementia Scale (HSD) was used as a test of general cognitive function.
Verbal memory tests
Paragraph recall or Logical Memory from the Wechsler Memory Scale (WMS); Paired Associate Learning (WMS); word lists: Buschke Selective Reminding Test (BSRT); CERAD 10 word list recall; verbal category Fluency tests for semantic memory; Digit Span forward for short term memory storage
Visual memory tests
Visual Retention tests (VRT from WMS): immediate and delayed recall; visual span; face recognition; the modified Rey‐Osterich Visual Memory Test
Language tests
Boston Naming Test, Token test
Speed and efficiency of information processing and concentration tests
Trail Making Test, part A (TMT‐A), Digit Symbol Substitution Test (DSST), letter cancellation test; and executive function or controlled information processing tests: Stroop interference test, Trail Making Test part B (TMT‐B), Digit Span backward. On most of the speed tests, a stronger drop in the time needed to respond indicates a positive result.
As secondary outcome measures, subjective scales of clinical change (a positive results indicates a worsening) and mood or depression were included:
Clinical impression of change scales
Clinician Interview‐Based Impression of Change (CIBIC), Clinical Dementia Rating scale (CDR), Clinician's Global Impression of Change (CGIC)
Depression scales
Hamiliton Depression Rating Scale (HDRS).
In this respect, the FDA standard tests for AD trials are the ADAS‐Cog and CIBIC and these should perhaps be used in future trials for comparability.
Search methods for identification of studies
See Cochrane Dementia and Cognitive Improvement Group methods used in reviews.
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) was searched on 7 November 2007 for all years up to December 2005. This register contains records from the major healthcare databases, The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing trial databases and other grey literature sources. The following search terms were used: ORT, PORT, ERT, HRT, estrogen*, oestrogen* and progesterone*.
The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS were searched separately on 7 November 2007 for records added to these databases after December 2005 to November 2007. The search terms used to identify relevant controlled trials on Alzheimer's disease and mild cognitive impairment for the Group's Specialized Register can be found in the Group's module on The Cochrane Library. These search terms were combined with the following search terms and adapted for each database, where appropriate: ORT, PORT, ERT, HRT, estrogen*, oestrogen* and progesterone*.
On 7 November 2007, the CDCIG Specialized Register consisted of records from the following databases:
Healthcare databases
CENTRAL: (The Cochrane Library 2006, Issue 1);
MEDLINE (1966 to 2006/07, week 5);
EMBASE (1980 to 2006/07);
PsycINFO (1887 to 2006/08, week 1);
CINAHL (1982 to 2006/06);
SIGLE (Grey Literature in Europe) (1980 to 2005/03);
LILACS: Latin American and Caribbean Health Science Literature (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) (last searched 29 August 2006);
Conference proceedings
ISTP (http://portal.isiknowledge.com/portal.cgi) (Index to Scientific and Technical Proceedings) (to 29 August 2006);
INSIDE (BL database of Conference Proceedings and Journals) (to June 2000);
Theses
Index to Theses (formerly ASLIB) (http://www.theses.com/) (UK and Ireland theses) (1716 to 11 August 2006);
Australian Digital Theses Program (http://adt.caul.edu.au/): (last update 24 March 2006);
Canadian Theses and Dissertations (http://www.collectionscanada.ca/thesescanada/index‐e.html): 1989 to 28 August 2006);
DATAD ‐ Database of African Theses and Dissertations (http://www.aau.org/datad/backgrd.htm);
Dissertation Abstract Online (USA) (http://wwwlib.umi.com/dissertations/gateway) (1861 to 28 August 2006);
Ongoing trials
UK
National Research Register (http://www.update‐software.com/projects/nrr/) (last searched issue 3/2006);
ReFeR (http://www.refer.nhs.uk/ViewWebPage.asp?Page=Home) (last searched 30 August 2006);
Current Controlled trials: Meta Register of Controlled trials (mRCT) (http://www.controlled‐trials.com/) (last searched 30 August 2006)
ISRCTN Register ‐ trials registered with a unique identifier
Action medical research
Kings College London
Laxdale Ltd
Medical Research Council (UK)
NHS Trusts Clinical Trials Register
National Health Service Research and Development Health Technology Assessment Programme (HTA)
National Health Service Research and Development Programme 'Time‐Limited' National Programmes
National Health Service Research and Development Regional Programmes
The Wellcome Trust
Stroke Trials Registry (http://www.strokecenter.org/trials/index.aspx) (last searched 31 August 2006);
Netherlands
Nederlands Trial Register (http://www.trialregister.nl/trialreg/index.asp) (last searched 31 August 2006);
USA/International
ClinicalTrials.gov (http://www.ClinicalTrials.gov) (last searched 31 August 2006) (contains all records from http://clinicalstudies.info.nih.gov/);
IPFMA Clinical trials Register: www.ifpma.org/clinicaltrials.html. The Ongoing Trials database within this Register searches http://www.controlled‐trials.com/isrctn, http://www.ClinicalTrials.gov and http://www.centerwatch.com/. The ISRCTN register and Clinicaltrials.gov are searched separately. Centerwatch is very difficult to search for our purposes and no update searches have been done since 2003.
The IFPMA Trial Results databases searches a wide variety of sources among which are:
http://www.astrazenecaclinicaltrials.com (seroquel, statins)
http://www.centerwatch.com
http://www.clinicalstudyresults.org
http://clinicaltrials.gov
http://www.controlled‐trials.com
http://ctr.gsk.co.uk
http://www.lillytrials.com (zyprexa)
http://www.roche‐trials.com (anti‐abeta antibody)
http://www.organon.com
http://www.novartisclinicaltrials.com (rivastigmine)
http://www.bayerhealthcare.com
http://trials.boehringer‐ingelheim.com
http://www.cmrinteract.com
http://www.esteve.es
http://www.clinicaltrials.jp
This part of the IPFMA database is searched and was last updated on 4 September 2006;
Lundbeck Clinical Trial Registry (http://www.lundbecktrials.com) (last searched 15 August 2006);
Forest Clinical trial Registry (http://www.forestclinicaltrials.com/) (last searched 15 August 2006).
The search strategies used to identify relevant records from MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module on The Cochrane Library.
In April 2008 one of the authors (EH) did another MEDLINE search update and also asked experts in the field whether they knew of any other ongoing trials. No new information could be added on the basis of this search.
Data collection and analysis
Selection of studies
Abstracts of the references retrieved by the search were read by two reviewers (EH and KY) in order to discard those that were clearly not eligible for inclusion. The two reviewers studied the full text of the remaining references and independently selected studies for inclusion. Any disparity in the ensuing lists was resolved by discussion with all reviewers in order to arrive at the final list of included studies. One reviewer (EH) extracted the data from the studies.
Quality assessment
Two reviewers assessed the quality of the studies according to the Cochrane Collaboration guidelines which focus on the allocation of treatment.
Category A (adequate) is where the report describes allocation of treatment by: (i) some form of centralized randomized scheme, such as having to provide details of an enrolled participant to an office by phone to receive the treatment group allocation; (ii) some form of randomization scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administrated sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, and opaque; (vi) other combinations of described elements of the process that provides assurance of adequate concealment.
Category B (intermediate) is where the report describes allocation of treatment by: (i) use of a "list" or "table" to allocate assignments; (ii) use of "envelopes" or "sealed envelopes"; (iii) stating the study as "randomized" without further detail.
Category C (inadequate) is where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of week, or any other such approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments.
Empirical research has shown that lack of adequate allocation concealment is associated with bias. Trials with unclear concealment measures have been shown liable to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but the bias is less pronounced than in inadequately concealed trials (Chalmers 1983; Schulz 1995).
Other aspects of the trial quality (methodology, statistics) were noted for the discussion.
Data collection
Data for the meta‐analyses were based on reported summary statistics for each study.
To test cognitive change after treatment, the main outcome of interest was the change from baseline to final assessment (mean difference in performance and SD of the mean difference in performance). The baseline assessment was defined as the last available assessment prior to randomization.
Data analysis
Meta‐analyses were performed on the mean difference of the continuous psychometric test scores. The mean difference was calculated as the difference between post‐treatment and baseline performance. The standard deviation (SD) of this difference was calculated as the square root of the variance of the baseline plus the variance of the final assessment (assuming the covariance between baseline and post‐treatment values is 0) as advised in Cochrane Collaboration guidelines unless data could be extracted. For studies that used the same treatment and the test outcome measure, the weighted mean difference (WMD) was employed in the meta‐analyses. In these analyses a fixed‐effect model was used if significant heterogeneity was not detected. If significant heterogeneity was detected (using chi‐square statistics), a possible explanation was sought and both fixed‐ and random‐effects models were reported. When I2 >50%, a sensitivity analysis will be performed. For studies that had employed different types of treatment or had used different tests but measured the same construct (e.g. visual memory), the standardized mean difference (SMD) was used with either fixed‐effect (when using the same test) or random‐effects models (when using a different test but measuring the same construct).
The null hypothesis tested was that, for any of the above outcomes, treatment had no effect in comparison with placebo.
Results
Description of studies
Nine double‐blind placebo‐controlled trials of postmenopausal women with dementia were identified (see Characteristics of included studies). However, in two (McDonald Caldwell 1952; Honjo 1995) there was insufficient information about the randomization procedure. In accordance with the Cochrane guidelines, these studies were excluded from analysis.
Subjects ‐ screening and selection
The total number of participants randomized in the trials varied from 14 to 120. In total, 351 women with dementia were included (266 had completed the studies) with an average of 38 participants per study. Drop‐outs were described in four studies (but not in Birge 1997 and Zhang 2006: although data provided by the investigator suggested no drop‐outs): 21/176 of treated women (11%) dropped out compared with 13/131 of placebo users (10%) for a wide variety of reasons, mostly unrelated to the medication. Most studies required very rigorous health screening (Mulnard 2000; Wang 2000; Henderson 2000; Asthana 1999; Asthana 2001). One study (Birge 1997) had less rigorous criteria (age < 70, depression, non‐AD dementia syndromes) and was a preliminary analysis on 20 subjects which was published in a general article on estrogen and hormonal replacement therapy. No follow‐up of these data (or a more detailed description of the study) has been reported. Power analyses had been carried out by two studies (Henderson 2000; Mulnard 2000) but were not mentioned in the smaller studies (Asthana 1999; Asthana 2001, Birge 1997) while Wang 2000 also failed to mention power analysis but would (on the basis of the calculations of the first two studies) have had sufficient numbers (n = 50).
Subjects ‐ dementia assessment
Most studies reported inclusion of people with dementia of the Alzheimer's type (DAT) or Alzheimer's disease (AD), except in Birge 1997 where patients had non‐AD dementia syndromes. Most studies employed the NINCDS/ADRDA criteria for probable AD (but see Birge 1997, where no criteria were given) and participants were in general considered to have mild to moderate dementia (MMSE between 10 and 28).
Subjects ‐ age and other confounding factors
The mean age of the women with AD was 75 years old, but some studies had a large age‐range (Mulnard 2000: range 56 to 91) and had thus included early and late onset AD. In one study only early age‐onset AD patients (< 63 years of age) were included with mild AD (Zhang 2006). Age, education and depression were usually not controlled for in the analyses. Some studies included only women who had undergone natural menopause (Asthana 1999) while others included mixed groups of surgically and naturally menopausal women (e.g. Henderson 2000; Mulnard 2000; Asthana 2001) or did not provide data on this.
Design
All studies used a parallel‐group design. Duration of treatment varied from eight weeks to 12 months, with an average of 4.4 months. We did not include the five week treatment time point of Asthana 1999 as the authors pointed out that data may not have been reliable, since two (of 12) participants had been tested elsewhere.
Cognitive assessments
Not all studies used similar cognitive tests which made comparison difficult. One of the problems in the otherwise well designed studies by Mulnard 2000 and Wang 2000 was, for instance, that no common test of verbal memory was used. Verbal memory has been thought likely to be most sensitive cognitive test to the effects of estrogen (Hogervorst 2000).
Different types of treatment and estradiol levels
Four RCTs prescribed Premarin (CEE produced by Wyeth). Of these, three used the 1.25 mg/day dosage (Henderson 2000; Wang 2000; Mulnard 2000) and two employed the lower dosage of 0.625 mg/day (Birge 1997; Mulnard 2000). Birge 1997 also added a progestagen to the estrogen to prevent endometrial hyperplasia. In one study (Zhang 2006) an Chinese estrogenic compound was used (Beimeili) containing conjugated oestrogen, which was also manufactured by Wyeth‐Ayerst in the USA and which was thought to probably be similar to CEE. Unfortunately little information could be found about this product which seems to be marketed mainly in China. Two studies used transdermal estradiol (Asthana 1999; Asthana 2001). Compliance checks were done using pill counts (Henderson 2000) or serum estrogen checks (Asthana 1999; Asthana 2001; Wang 2000; Mulnard 2000). Birge 1997 and Zhang 2006 gave no data on compliance checks.
Statistics
Some studies reported separate within‐group comparisons for participants in treatment and placebo groups (e.g. Birge 1997, Zhang 2006) which can result in chance accumulation and a risk of the type I error. Five studies had performed 'completers' analyses (Birge 1997; Asthana 1999; Henderson 2000; Zhang 2006; Mulnard 2000 but data not shown) and three had performed 'intention‐to‐treat' analyses (Mulnard 2000; Wang 2000; Asthana 2001).
Risk of bias in included studies
Three studies described their randomization procedures in detail (Henderson 2000; Mulnard 2000Asthana 2001) and received a Cochrane quality rating of A. In these studies an external person had performed the allocation. The other studies reported having 'randomly assigned treatments' but did not describe the randomization procedures in detail, and in accordance with Cochrane Collaboration standards received a quality rating of B. For two studies no randomization procedure was reported and these were not included in the analyses (Honjo 1989; McDonald Caldwell 1952).
Effects of interventions
Seven studies met inclusion criteria and had performed adequate or intermediate allocation procedures. These studies had all been published in peer‐reviewed journals.
Global cognitive functioning
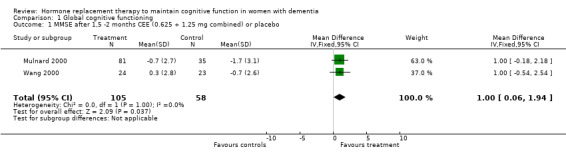
There was an overall positive effect for treatment on the MMSE score after 1 to 2 months with the combined low and high dosages (0.625 and 1.25 mg) CEE (WMD = 1.00, 95% CI = 0.06 to 1.94, z = 2.09, P < 0.05, test for heterogeneity, P = 1.00). However, this was the only test that showed a dosage effect: after two months the low dosage, but not the 1.25 mg dosage, was significantly better than placebo. The positive effect of the low dose on MMSE performance did not persist after six and 12 months. In addition, this effect is clinically irrelevant as there was only a one‐point difference on the MMSE between placebo and treatment. There was also no evidence for an overall short‐term effect of treatment on the MMSE score (P = 0.15), when the E2 transdermal treatment was included in the analyses. The test for heterogeneity was not significant (P = 0.27) and there was no difference using SMD with a fixed‐ or random‐effects model, suggesting that combining treatments did not violate prior assumptions of the analyses. The effect of combined CEE treatment (low and high dosage) on the MMSE score was not significant after 3, 6 and 12 months (P > 0.50). There was no evidence of an effect of treatment on the ADAS‐Cog after 1, 2, 4, 6 and 12 months of combined (0.625 mg + 1.25 mg) CEE treatment (P > 0.10). There was also no evidence of an effect of treatment (E2 and 0.625 mg CEE) on the BIMC after two and nine months (P > 0.30) and the tests for heterogeneity were not significant (P = 0.35). There was an overall effect of treatment on the HSD‐R after four months of treatment (WMD = 6.09, 95% CI = 0.98 to 11.20, z = 2.34, P < 0.05) in the early onset AD cases (Zhang 2006) when conjugated estrogen was compared to vitamin B1 treatment.
Memory tests
There was no significant effect of E2 transdermal or 1.25 mg CEE treatment on the immediate Paragraph Recall test (P > 0.15). However, controls had a slightly better performance after one month than 1.25 mg CEE users on the delayed Paragraph Recall test (WMD = ‐0.45, 95% CI = ‐0.79 to 10.11, z = 2.60, P < 0.01). There was a trend for a reversal of this effect after four months (P = 0.07). There was no evidence of a treatment effect on the BSRT immediate recall after two months of E2 transdermal treatment (P = 0.80). When these results were combined with that of another study of this research group using a combined measure of the immediate and delayed recall (derived from graphs), also no overall effect of two months treatment with transdermal E2 was detected (SMD random effects = 0.42, 95% CI = ‐0.29 to 1.12, z = 1.16, P = 0.25). However, the BSRT cued delayed recall was significantly better after two months of E2 transdermal than after placebo (WMD = 6.50, 95% CI = 4.04 to 8.96, z = 5.19, P < 0.0001). Performance on the CERAD word list (P = 0.60) and on the Paired Associate learning test (P = 0.16) were unchanged after nine months of 0.625 mg CEE + MPA compared to placebo.
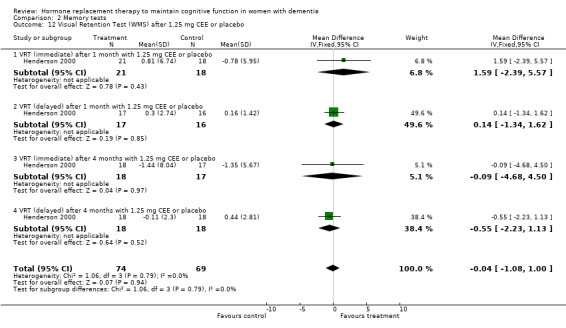
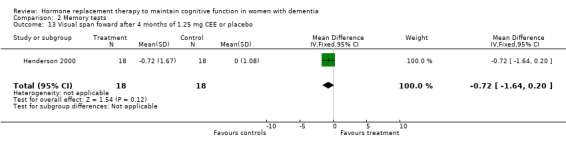
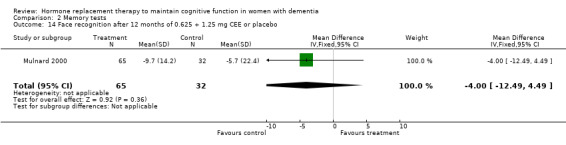
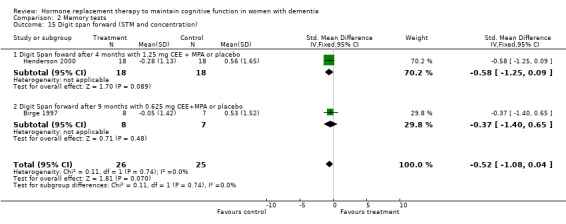
There was a trend (P = 0.07) for controls to have a better performance on Digit Span forward after four to nine months of CEE treatment (P = 0.09 for the four months treatment with 1.25 mg CEE, and P = 0.50 for the nine months treatment with 0.625 mg CEE + MPA). Differences in Category Fluency performance were in the same direction, but were also not significantly different (P = 0.11) for controls compared with CEE treatment after combined (0.625 + 1.25 mg) analyses after four (P = 0.82) and 12 months (P = 0.08) of treatment. Heterogeneity tests were not significant (Digit Span: P = 0.74 Fluency: P = 0.44) for these analyses. No evidence for a treatment effect was detected on visual memory tests, e.g. on the VRT after one and four months of 1.25 mg CEE (P = 0.90); or on the VRT after two months of E2 transdermal (P = 0.70); on the Rey Osterich visual memory test after two months of transdermal E2 (P = 0.29) or on the Visual Span after four months of 1.25 mg CEE (P = 0.12). Also, performance on the facial recognition tests did not differ significantly between placebo and combined (0.625 mg + 1.25 mg) CEE treatment after 12 months (P = 0.40). The CASI long term memory test showed no evidence (P > 0.30) of an effect with 1.25 mg CEE after two months. No difference was detected in SMD analyses using random‐ or fixed‐effects models, and heterogeneity tests were also non‐significant in these analyses.
Language tests
There was no evidence of a treatment effect on the Boston Naming test (P > 0.70) after four months of 1.25 mg CEE, or on the Token test after one month of 1.25 mg CEE (P = 0.80), or after two months of E2 transdermal (P = 0.50), or after four months of 1.25 mg CEE (P = 0.80).
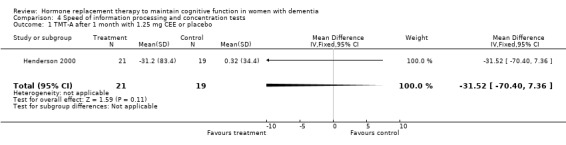
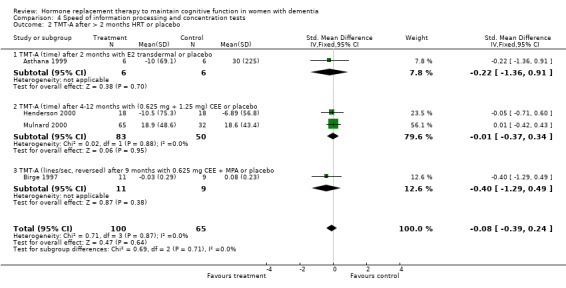
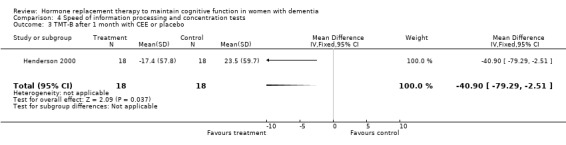
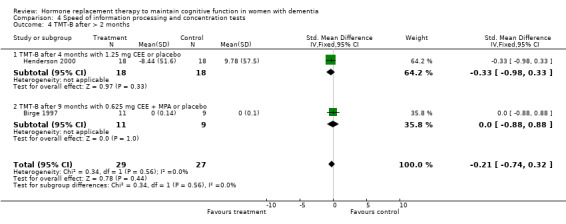
Speed of information processing and concentration tests
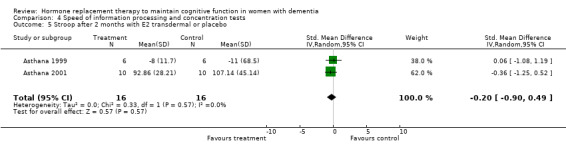
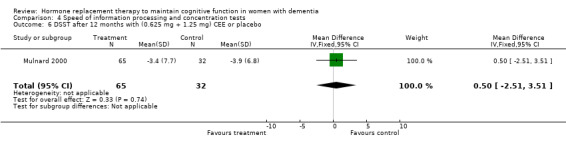
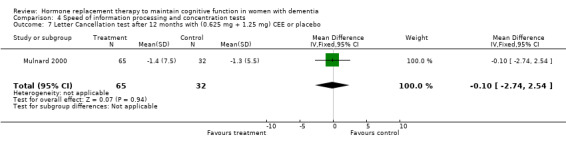
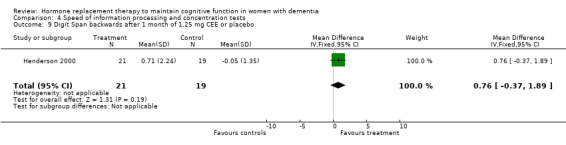
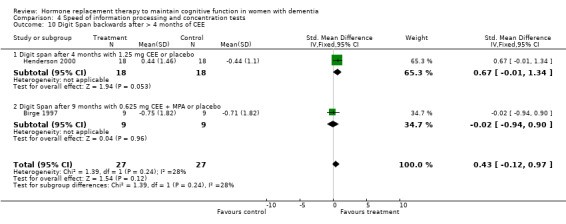
There was no evidence for an overall effect of placebo or treatment on TMT‐A performance. However, performance on the TMT‐B was better after one month of CEE (WMD = ‐40.90, 95% CI = ‐79.29 to ‐2.51, z = 2.09, P < 0.05), which was no longer significant after four and nine months of treatment (P > 0.40). There was no evidence of a treatment or control effect on the Stroop Interference test (P = 0.90), the DSST (P = 0.70), or the Letter Cancellation test (P = 0.90). Only Finger Tapping was significantly faster after 12 months in controls (WMD = ‐3.90, 95% CI = ‐7.85 to 0.05, z = 1.93, P < 0.05). Digit Span backward, as a measure of concentration and controlled information processing, was significantly better after four months of CEE (WMD = 0.67, 95% CI = ‐0.01 to 1.34, z = 1.94, P < 0.05) but not after one month of CEE (P = 0.12) or after nine months of CEE + MPA (P = 1.00).
Clinical impression of change, dementia severity and depression scales
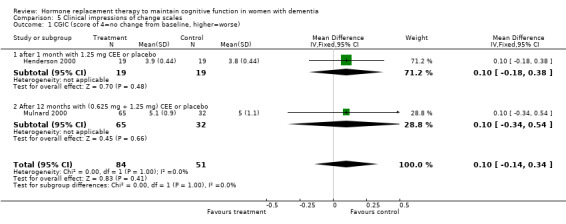
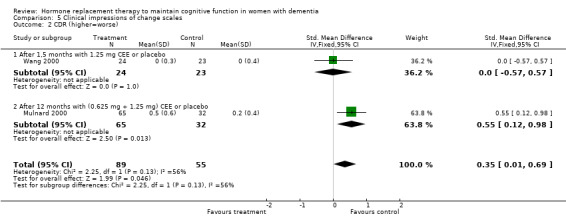
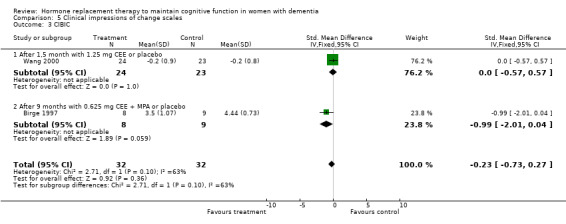
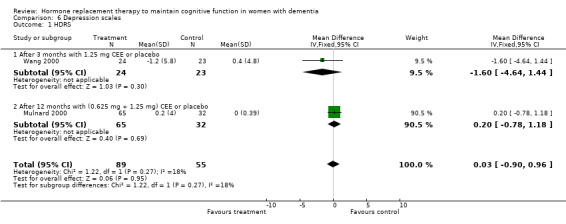
Both the CGIC and CIBIC showed no evidence of a treatment effect (P = 0.40). However, the CDR scores revealed that clinicians in general rated dementia severity to be less in participants taking placebo than in those on active treatment after 1.5 to 12 months (overall WMD = 0.35, 95% CI = 0.01 to 0.69, z = 1.99, P < 0.05). The Hamilton Depression rating scale did not show any evidence of a positive or negative effect of treatment (P = 1.00) after 3 or 12 months.
The heterogeneity test was not significant in any of the analyses, which could indicate that the results from studies were comparable and that the pooling of the data was valid. Using SMD with random‐effects models when a significant effect was found also did not alter results.
In sum, the following effects were seen: (i) There was a significant positive effect of a low dose of CEE up to two months when compared to placebo (Mulnard 2000). The higher dose of CEE and E2 did not show positive effects of treatment (Mulnard 2000, Wang 2000; Asthana 1999; Asthana 2001) (ii) After two months positive effects of low dose E2 on the delayed recall of the BSRT (Asthana 1999) was shown (but the reverse was found after one month of CEE on the delayed paragraph recall, which was better for controls). However, there was a trend for this to reverse again at four months within the same study by Henderson 2000. No effects were found on immediate recall or on other word learning lists, visual memory tests or language tests. (iii) After four months positive effects were seen after CEE (high dose) on Digit Span backward and the TMT‐B test, but a reverse trend was seen in the same study (Henderson 2000) after four months for the Digit Span forward test. No effects were seen on other complex speeded tests. (iv) After four months also a positive effect was seen of high dose CEE on the HDS‐R test (Zhang 2006) but this had not been significant between groups using the same test in another (not included) study (Honjo 1995) after a similar treatment duration with CEE and MPA. (v) After 12 months, controls performed better than CEE on the Finger Tapping and the CDR, with a similar trend for the verbal Fluency test.
Using the inverse* Bonferroni rule for multiple comparisons of the separate analyses involving the same test used in different studies (including those which had been performed, but for which we had no data to include in the analyses), most HRT and ERT effects disappeared: ‐ The effects of CEE on the MMSE (P = 0.04 x 4) and TMT‐B (P = 0.04 x 5) within one month of treatment, and on the Digit Span backward (P = 0.04 x 2) after four months of treatment did not remain. ‐ The effect of E2 transdermal treatment on the BSRT cued delayed recall after two months did remain (P = 0.0001 x 2) and that of the HDS‐R after four months of CEE in participants with early onset dementia (P = 0.02 x1). ‐ The findings of controls having a better performance than ERT users also remained, even after inverse Bonferroni corrections (Paragraph Delayed Recall after 1 month, P = 0.009 x 3; Finger Tapping after 12 months, P = 0.05 and CDR after 12 months, P = 0.01 x 2).
*the P value for a test becomes kP. For example, an uncorrected significance value of 0.02, where there are five tests, would become 5(0.02) or 0.1
Discussion
This review found no overall positive effect of HRT or ERT in maintaining cognitive function in AD. There was a limited positive effect (in time and effect size) on a test of global cognitive functioning, the MMSE. This effect was only significant after one to two months (but not after 3, 6, and 12 months) of a low dosage (0.625 mg) CEE treatment and disappeared after correction for multiple testing. In addition, this effect was small and clinically irrelevant (difference of 1 point on average). Others also reported not finding effects of two months transdermal estradiol (E2) treatment on the MMSE (Asthana 2001) but as we had no data available, these results could not be included in the meta‐analyses. Similarly, the positive time‐limited effect of CEE on two tests which measured concentration and executive function (the TMT‐B after one month CEE and Digit Span backward after four months CEE) disappeared after statistical correction for multiple testing. Only the effect of two months of a transdermal E2 treatment on cued delayed recall of a word list (BSRT Asthana 1999) remained after correction. However, a combined score (derived from graphs in the article) showed no effect in another study using the same treatment for the same period of time, but with a higher dose. No effects of HRT or ERT were seen on the delayed paragraph recall or on immediate verbal recall in general (of a paragraph or of a word list), on visual memory, language or on most speeded tests. There were no positive effects on the clinical rating scales or on mood. In several instances, placebo gave better performance than treatment, for instance on the Paragraph Delayed Recall after one month of CEE (although there was a trend for this to reverse after four months) and on Finger Tapping after 12 months. While it could be suggested that cognitive tests may not reflect clinical change, dementia severity was also judged to be less severe in controls than in cases who had been treated with CEE for 12 months. One study (Zhang 2006) reported an effect of a conjugated estrogen on a global test (HSD‐R) after four months against vitamin B1 (thiamine). Another Cochrane review could not report positive effects in RCTs investigating the effects of thiamine on cognitive function in AD (Rodríguez‐Martín 2001), but it is not entirely clear how to interpret these results without a perhaps more appropriate placebo.
The following aspects need perhaps to be taken into account when reviewing the results of our meta‐analyses.
‐ Size of studies and error introduced through recalculation of the SD of the mean difference The initial study (Asthana 1999) that maintained its significant effect of ERT was small (n = 12) and treatment with transdermal E2 was conducted for only a short period of time (two months). The later study (Asthana 2001) with a higher dose of the same treatment regimen (which also claimed similar effects) could not be replicated when data were extracted from graphs. A significant limitation of our analyses is that data needed to be extracted from graphs which may not have validly reflected the actual effect found (e.g. incorrect SE or introduction of error when extracting data using rulers). In addition, calculating the SD of the mean difference from extracted data was in our own analyses (using data of other treatment studies to compare actual and calculated SD of the mean difference) shown to sometimes overestimate the SD by a factor of two, especially in the smaller studies (n = 10‐15). Most of the data used in the present review were judged by the original authors in peer‐reviewed papers to show significant effects, which could, however, often not be replicated in our analyses of the individual studies. Instead many trends emerged which suggested a possible time‐dependent effect, with positive effects seen after 1‐2 months of treatments on some tests and negative effects seen after 12 months of estrogen treatment (see also Yesufu 2007)
‐ Type of cognitive test Our earlier meta‐analyses of the effects of estrogen treatment in healthy women (Yesufu 2007) found effects of E2 (intramuscular injections, but not of the transdermal E2 treatment) on verbal memory (in particular on one test, the Paired Associate learning test) and on some executive functions which had all been carried out by one research group. In the current review, the Paired Associate test had only been used in one study (Birge 1997). While effects seemed in favour of HRT, significance was not reached. As this was also a small study, it is difficult to assess whether the lack of effects could be attributed to the lack of power. Alternatively, these types of tests could be too complicated for AD cases. In this regard, cued recall, which is a much easier memory test, did show an effect of E2 treatment in the small AD study (Asthana 1999) for a limited duration of time. Other more complex cognitive tests (executive function) were shown to be affected by ERT both in women with and those without dementia, but again only for a limited period of time (< five months). It should be noted that several studies using similar tests in our analyses failed to replicate the significant short duration positive effects of ERT that had been reported by the authors.
‐ Type of treatment (estrogen, duration and dosage) While at present it is unclear whether the type of estrogen could account for differences in the results found, we can conclude that CEE does not have positive effects in women with AD after a longer period of treatment (> five months). This is in in line with the Chinese study (Zhang 2006) using a conjugated estrogen (developed by the same company who produces the CEE, Wyeth) which reported positive effects of ERT after four months when compared to vitamin B1.
It is unclear what the longer‐term effects of E2 treatment would be. A single‐blind study for 18 months in institutionalized women by McDonald Caldwell 1952 (in Hogervorst 2000, which could not be included in this analyses) suggested that the effects of intramuscular injections of 2 mg of E2/week also (similar to CEE) declined after 12 months on several cognitive tests.
Another important question has been whether adding a progestagen could alter effects (Honjo 1995). This has often been assumed, as animal studies have shown that progestagens can counteract several of the actions of estrogens on monoaminergic neurotransmitter systems and possibly on the vascular system (Hogervorst 2000). The one study (Birge 1997) of a longer duration, which added a progestagen to CEE, showed a slightly better performance on the Digit Span forward and the CIBIC compared with the studies that only used CEE (in a higher dosage).
Matters are complicated as the study which used the progestagen (Birge 1997) also used a lower dosage of CEE than the other studies (Wang 2000; Henderson 2000). While in another study the low dosage of CEE was seen to have positive effects on the MMSE for a short period, after 12 months the 0.625 mg dosage of CEE actually tended to lead to worse performance than placebo, while the higher 1.25 mg regimen was associated with better intermediate performance (Mulnard 2000). It is at present thus unclear (but also unlikely) whether the dosage of CEE or the addition of a progestagen could alter treatment effects.
It has been suggested by Toran‐Allerand 2000 that continuous longer‐term treatment with estrogens could result in a down‐regulation of estrogen receptors in the brain. On some tests a positive effect was indeed seen for a limited duration of time. These positive effects usually disappeared after five months and in some cases even reversed. Adding a progestagen could potentially reverse down‐regulation of receptors. However, the study by Birge 1997 did not seem to show a overall larger effect in comparison with the study by Mulnard where no progestagen was added.
‐ Participants (age at onset of AD and at testing, education, menopausal status and other potential confounds) The largest and longest study had a wide age range (Mulnard 2000: range 56 to 91). Age usually explains much of the variance in cognitive tests and could have over‐ridden the small effects of treatment. The age range in the Mulnard 2000 study also indicated that both participants with early‐ and late‐onset AD had been enrolled. This was also probably the case for the study by Wang 2000. Early‐ and late‐onset AD are thought to differ in pathogenesis, and this could possibly have interacted with ERT or HRT. Whether HRT or ERT could have an effect in women with early‐onset AD was investigated in the study by Zhang 2006 which intrigingly reported overall positive effects of treatment. This finding could support the 'window of opportunity' theory which states that hormones need to be given close to the natural age of menopause (around 50 years of age) to have a positive effect on the brain and which is substantiated by animal and human observational studies (Henderson 2008; Gibbs 2008).
Education and depression were often not controlled for, and an earlier review (Hogervorst 2000) suggested that women with low levels of education (who are additionally at risk for AD) may profit most from HRT or ERT. The MMSE in general had a wide range (10 to 28) indicating a wide variety of dementia severity in participants. Women with very mild dementia or mild cognitive impairment would be an important group to study as they may have more potential for benefit. Lastly, the current review did not include studies which selectively investigated women resident in nursing homes, and conclusions are therefore restricted to a largely community‐dwelling population.
In sum, from our meta‐analyses it has become clear that in the longer term (> two months) that any (if at all) potential positive effects of estrogens on the brain seem to reverse. Overall data do not suggest that treatment with HRT or ERT for longer than a few months is recommended to maintain cognitive function in postmenopausal women with or without dementia.
The large prospective randomized placebo‐controlled studies in the USA (Women's Health Initiative, WHI; PREPARE) (Schumaker 1998) and the UK (Women's International Study of long Duration estrogen after Menopause, WISDOM) were abruptly stopped after the WHI found increased risks for breast cancer and cardiovascular disease. In addition, a doubled risk for dementia was reported in women who had been randomized to Premarin (CEE) in combination with MPA treatment (Shumaker 2003) and a non significant effect for CEE alone was found in the same direction (Shumaker 2004). This led to a substantial drop in HRT and ERT use, although data suggested that many women resumed taking (different) estrogens after a couple of months (Wegienka 2006). The majority of observational studies suggested that the risk for AD is decreased with the use of HRT or ERT (Yaffe 1998a; Hogervorst 2000). Since Premarin is the most widely prescribed drug, it was believed that it had protective effects against the development of AD. Part of that effect may be due to the 'healthy user bias' (see introduction), but this does not take into account the overwhelming evidence for estrogen's protective effect on the brain as is still reported in animal and cell‐culture studies. Interestingly, also before WHI results were published, many women did not seem to use HRT for a long time (the majority < one year) to treat menopausal symptoms (Wegienka 2006).
Current evidence indicates that only short‐term treatment with HRT is advisable; longer‐term treatments should be avoided. Genotypes associated with sex steroid metabolism and AD should be investigated to see whether some women are more at risk for this than other women (Hogervorst 2007). Intermittent treatments could be pursued as an alternative treatment option in conjunction with this (Al‐Azzawi 2008).
Authors' conclusions
Implications for practice.
Currently, the long‐term use of HRT or ERT for cognitive improvement or maintenance in women with Alzheimer's disease is not indicated.
Implications for research.
Novel treatment strategies should be investigated for longer term treatment of cognitive dysfunction in AD.
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
| 9 April 2008 | New citation required and conclusions have changed | An update search of the literature review was done using Cochrane methods on 7 November 2007. The main author (EH) ran additional searches in MEDLINE using the keywords hrt, ert, estr* and Alzheimer* or dementia in April 2008. In addition experts in the field were asked whether they had any knowledge of trials that had been published or were ongoing. Professor Asthana kindly sent us his own papers but knew of no other studies. We included two studies by Asthana et al. (2001) and Zhang et al. (2006), which was translated from Chinese. All studies which included SERMS and non‐estrogenic compounds (GH, DHEA, corticosteroids, etc.) were excluded |
Acknowledgements
Marc Budge of OPTIMA Clare Bateman, Consumer Editor.
We also wish to acknowledge Xin‐Hui Chan for translating and extracting data from the Zhang 2006 study.
Appendices
Appendix 1. List of abbreviations and their definitions
AD, Alzheimer's disease Blessed or BIMC, Blessed Information Memory and Concentration test BSRT, Buschke's Selective Reminding Test BSO, Bilateral Salpingo‐Oopherectomy (removal of the ovaries) BVRT, Benton Visual Retention Test CEE, Conjugated Equine Estrogens or Premarin CVLT, Californian Verbal Learning Test DAT, Dementia of the Alzheimer's Type Dep, depressed DSM, Diagnostic Statistical Manual DSST, Digit Symbol Substitution Test E1, Estrone E2, Estradiol E3, Estriol ERT, Estrogen Replacement Therapy HRT, Hormone Replacement Therapy (estrogen plus progestagen) I.M., Intramuscular MANOVA, Multivariate Analysis of Variance MMSE, Mini‐Mental Status Examination MPA, Medroxyprogesterone Acetate NINCDS/ADRDA, National Institute of Neurological and Communicative Disorders and the Alzheimer's Disease and Related Disorders Association RCT, Randomized Controlled Trial SD, Standard Deviation SEM, Standard Error of the Mean SQRT, Square Root TMT, Trail Making Test VaD, Vascular Dementia Var, Variance VRT WMS, Visual Retention Test of the WMS (Visuospatial Memory) WHI, the Women's Health Initiative study WISDOM, Women's International Study of long Duration Oestrogen after the Menopause WMD, Weighted Mean Difference WMS, Wechsler Memory Scale
Data and analyses
Comparison 1. Global cognitive functioning.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MMSE after 1,5 ‐2 months CEE (0.625 + 1.25 mg combined) or placebo | 2 | 163 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.06, 1.94] |
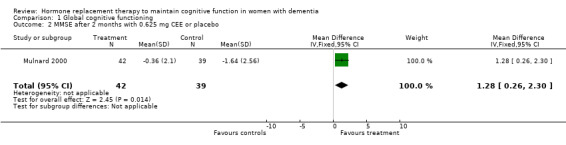
| 2 MMSE after 2 months with 0.625 mg CEE or placebo | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 1.28 [0.26, 2.30] |
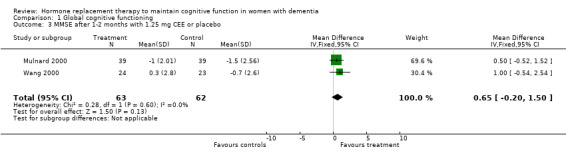
| 3 MMSE after 1‐2 months with 1.25 mg CEE or placebo | 2 | 125 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [‐0.20, 1.50] |
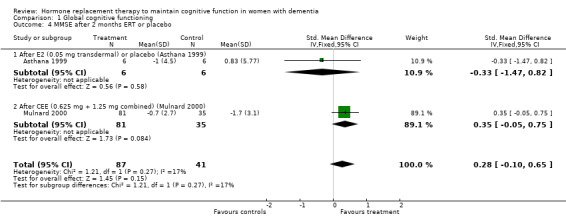
| 4 MMSE after 2 months ERT or placebo | 2 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.10, 0.65] |
| 4.1 After E2 (0.05 mg transdermal) or placebo (Asthana 1999) | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.47, 0.82] |
| 4.2 After CEE (0.625 mg + 1.25 mg combined) (Mulnard 2000) | 1 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.05, 0.75] |
| 5 MMSE after 3‐6 months CEE (0.625 + 1.25 mg) or placebo | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.75, 1.57] |
| 6 MMSE after 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.28, 2.08] |
| 7 ADAS‐Cog after 1‐2 months with (0.625 mg + 1.25 mg) CEE or placebo | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐1.20, 1.96] |
| 8 ADAS‐Cog after 4‐6 months with (0.625 mg + 1.25 mg) CEE or placebo | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.52, 1.73] |
| 9 ADAS‐Cog after 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐4.41, 0.41] |
| 10 ADAS‐Cog after 12 months with 0.625 mg CEE or placebo | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐6.01, 0.61] |
| 11 ADAS‐Cog after 12 months with 1.25 mg CEE or placebo | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐3.73, 1.33] |
| 12 Blessed (BIMC) after 2‐9 months ERT or placebo | 2 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.55 [‐9.71, 2.60] |
| 12.1 after 2 months E2 (0.05 mg transdermal) or placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐9.34, 9.74] |
| 12.2 after 9 months 0.625 mg CEE +MPA or placebo | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐6.24 [‐14.31, 1.83] |
| 13 HDS‐R after 16 weeks (estrogen) or vitamin B1 as placebo | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 6.09 [0.98, 11.20] |
1.1. Analysis.
Comparison 1 Global cognitive functioning, Outcome 1 MMSE after 1,5 ‐2 months CEE (0.625 + 1.25 mg combined) or placebo.
1.2. Analysis.
Comparison 1 Global cognitive functioning, Outcome 2 MMSE after 2 months with 0.625 mg CEE or placebo.
1.3. Analysis.
Comparison 1 Global cognitive functioning, Outcome 3 MMSE after 1‐2 months with 1.25 mg CEE or placebo.
1.4. Analysis.
Comparison 1 Global cognitive functioning, Outcome 4 MMSE after 2 months ERT or placebo.
1.5. Analysis.
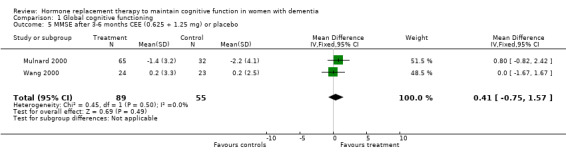
Comparison 1 Global cognitive functioning, Outcome 5 MMSE after 3‐6 months CEE (0.625 + 1.25 mg) or placebo.
1.6. Analysis.
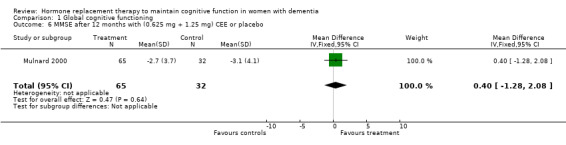
Comparison 1 Global cognitive functioning, Outcome 6 MMSE after 12 months with (0.625 mg + 1.25 mg) CEE or placebo.
1.7. Analysis.
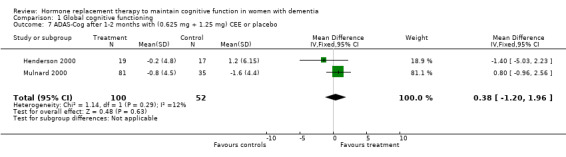
Comparison 1 Global cognitive functioning, Outcome 7 ADAS‐Cog after 1‐2 months with (0.625 mg + 1.25 mg) CEE or placebo.
1.8. Analysis.
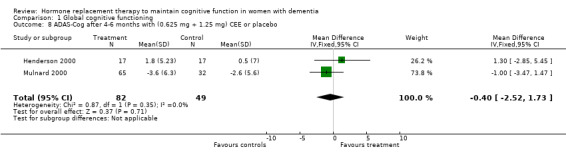
Comparison 1 Global cognitive functioning, Outcome 8 ADAS‐Cog after 4‐6 months with (0.625 mg + 1.25 mg) CEE or placebo.
1.9. Analysis.
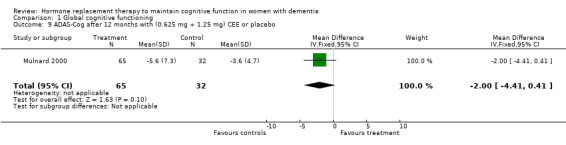
Comparison 1 Global cognitive functioning, Outcome 9 ADAS‐Cog after 12 months with (0.625 mg + 1.25 mg) CEE or placebo.
1.10. Analysis.
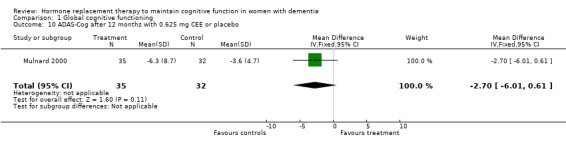
Comparison 1 Global cognitive functioning, Outcome 10 ADAS‐Cog after 12 months with 0.625 mg CEE or placebo.
1.11. Analysis.
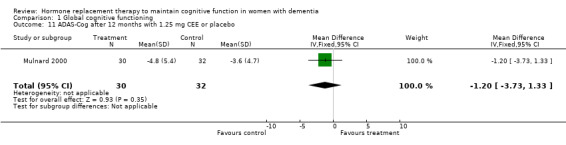
Comparison 1 Global cognitive functioning, Outcome 11 ADAS‐Cog after 12 months with 1.25 mg CEE or placebo.
1.12. Analysis.
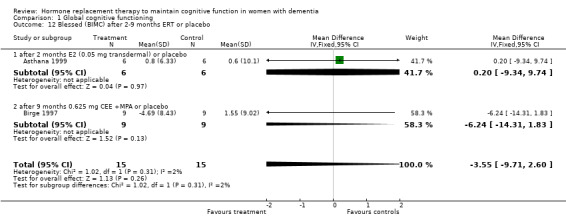
Comparison 1 Global cognitive functioning, Outcome 12 Blessed (BIMC) after 2‐9 months ERT or placebo.
1.13. Analysis.
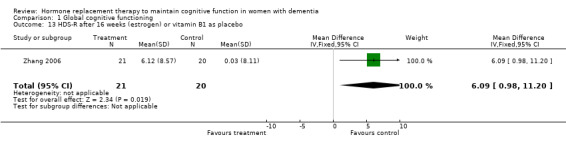
Comparison 1 Global cognitive functioning, Outcome 13 HDS‐R after 16 weeks (estrogen) or vitamin B1 as placebo.
Comparison 2. Memory tests.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Paragraph recall (immediate + delayed) after 2 months with E2 transdermal or placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.20 [‐6.98, 4.58] |
| 2 Paragraph recall (immediate) after 1 month with 1.25 mg CEE or placebo | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.99, 2.03] |
| 3 Paragraph recall (delayed) after 1 month with 1.25 mg CEE or placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.79, ‐0.11] |
| 4 Paragraph recall immediate after 4 months with 1.25 mg CEE or placebo | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [‐0.57, 3.73] |
| 5 Paragraph recall (delayed) after 4 months with 1.25 mg CEE or placebo | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐0.06, 1.72] |
| 6 Busche Selective Reminding (delayed recall) after 2 months with E2 transdermal or place | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [4.04, 8.96] |
| 6.1 BSRT delayed recall score (1999) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 6.5 [4.04, 8.96] |
| 7 Buschke Selective Reminding (Ir, IR+DR) after 2 months with E2 transdermal or placebo | 2 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.29, 1.12] |
| 7.1 Sub‐category | 2 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.29, 1.12] |
| 8 CERAD word list after 9 months with 0.625 mg CEE + MPA or placebo | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐2.87, 1.53] |
| 9 Paired Associate word learning after 9 months with 0.625 mg CEE + MPA or placebo | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 2.43 [‐0.94, 5.80] |
| 10 Verbal Fluency tests (semantic memory) | 2 | 133 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.65, 0.07] |
| 10.1 Category fluency after 4 months with 1.25 mg CEE or placebo | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.73, 0.58] |
| 10.2 Category Fluency after 12 months with 0.625 + 1.25 mg CEE or placebo | 1 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.81, 0.05] |
| 11 Visual Retention Test (WMS) after 2 months of E2 transdermal or placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐17.59, 27.59] |
| 11.1 VRT (immediate + delayed) after 2 months with E2 transdermal and placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐17.59, 27.59] |
| 12 Visual Retention Test (WMS) after 1.25 mg CEE or placebo | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐1.08, 1.00] |
| 12.1 VRT (immediate) after 1 month with 1.25 mg CEE or placebo | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.59 [‐2.39, 5.57] |
| 12.2 VRT (delayed) after 1 month with 1.25 mg CEE or placebo | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.34, 1.62] |
| 12.3 VRT (immediate) after 4 months with 1.25 mg CEE or placebo | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐4.68, 4.50] |
| 12.4 VRT (delayed) after 4 months with 1.25 mg CEE or placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.23, 1.13] |
| 13 Visual span foward after 4 months of 1.25 mg CEE or placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.64, 0.20] |
| 14 Face recognition after 12 months of 0.625 + 1.25 mg CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐12.49, 4.49] |
| 15 Digit span forward (STM and concentration) | 2 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.08, 0.04] |
| 15.1 Digit Span foward after 4 months with 1.25 mg CEE + MPA or placebo | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.25, 0.09] |
| 15.2 Digit Span forward after 9 months with 0.625 mg CEE+MPA or placebo | 1 | 15 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.40, 0.65] |
| 16 Other memory tests | 2 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [‐0.12, 0.85] |
| 16.1 CASI LTM after 1,5 week with 1.25 mg CEE or placebo | 1 | 47 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.26, 0.89] |
| 16.2 Visual memory adaptation Rey test Immediate recall after 2 months of transdemral E2 or placebo | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.48 [‐0.41, 1.37] |
2.1. Analysis.
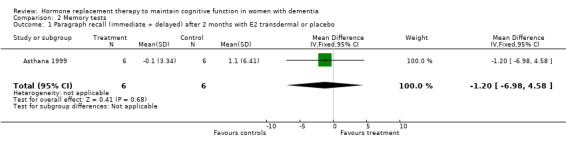
Comparison 2 Memory tests, Outcome 1 Paragraph recall (immediate + delayed) after 2 months with E2 transdermal or placebo.
2.2. Analysis.
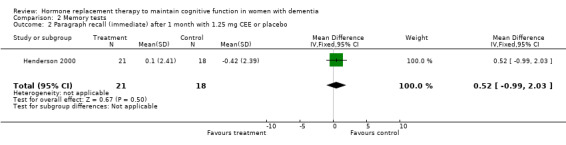
Comparison 2 Memory tests, Outcome 2 Paragraph recall (immediate) after 1 month with 1.25 mg CEE or placebo.
2.3. Analysis.
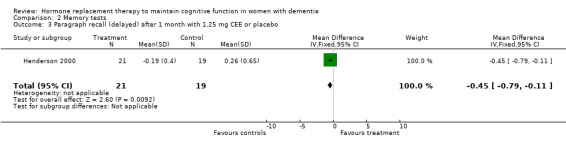
Comparison 2 Memory tests, Outcome 3 Paragraph recall (delayed) after 1 month with 1.25 mg CEE or placebo.
2.4. Analysis.
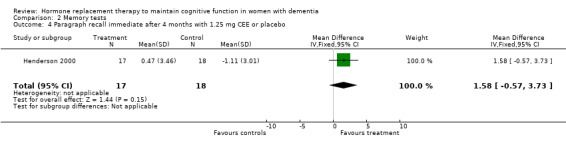
Comparison 2 Memory tests, Outcome 4 Paragraph recall immediate after 4 months with 1.25 mg CEE or placebo.
2.5. Analysis.
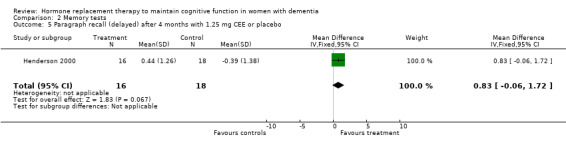
Comparison 2 Memory tests, Outcome 5 Paragraph recall (delayed) after 4 months with 1.25 mg CEE or placebo.
2.6. Analysis.
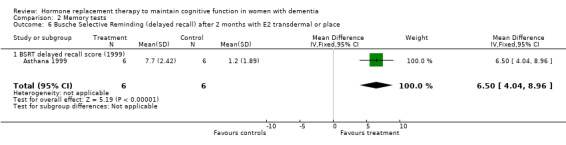
Comparison 2 Memory tests, Outcome 6 Busche Selective Reminding (delayed recall) after 2 months with E2 transdermal or place.
2.7. Analysis.
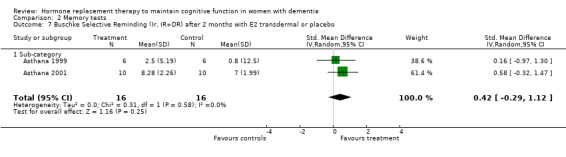
Comparison 2 Memory tests, Outcome 7 Buschke Selective Reminding (Ir, IR+DR) after 2 months with E2 transdermal or placebo.
2.8. Analysis.
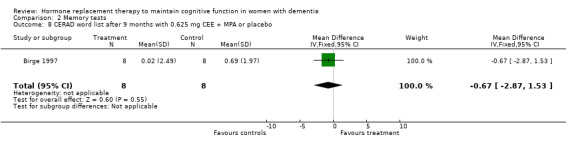
Comparison 2 Memory tests, Outcome 8 CERAD word list after 9 months with 0.625 mg CEE + MPA or placebo.
2.9. Analysis.
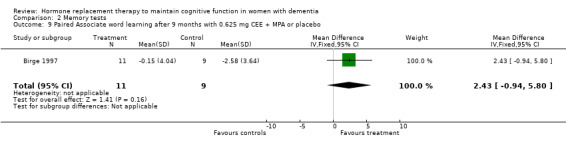
Comparison 2 Memory tests, Outcome 9 Paired Associate word learning after 9 months with 0.625 mg CEE + MPA or placebo.
2.10. Analysis.
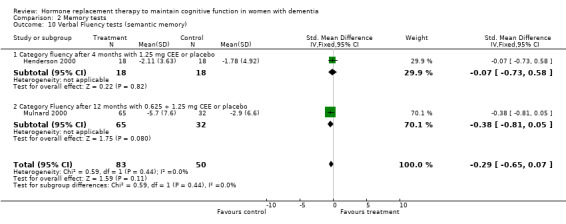
Comparison 2 Memory tests, Outcome 10 Verbal Fluency tests (semantic memory).
2.11. Analysis.
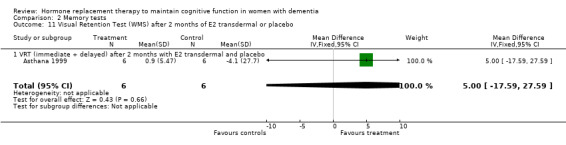
Comparison 2 Memory tests, Outcome 11 Visual Retention Test (WMS) after 2 months of E2 transdermal or placebo.
2.12. Analysis.
Comparison 2 Memory tests, Outcome 12 Visual Retention Test (WMS) after 1.25 mg CEE or placebo.
2.13. Analysis.
Comparison 2 Memory tests, Outcome 13 Visual span foward after 4 months of 1.25 mg CEE or placebo.
2.14. Analysis.
Comparison 2 Memory tests, Outcome 14 Face recognition after 12 months of 0.625 + 1.25 mg CEE or placebo.
2.15. Analysis.
Comparison 2 Memory tests, Outcome 15 Digit span forward (STM and concentration).
2.16. Analysis.
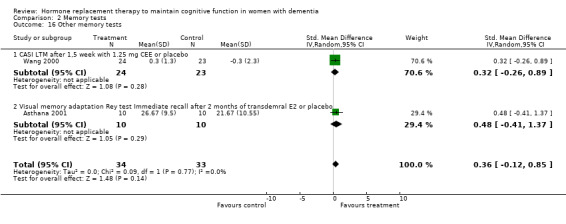
Comparison 2 Memory tests, Outcome 16 Other memory tests.
Comparison 3. Language tests.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Token test after 1‐2 months of HRT or placebo | 2 | 45 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.54, 0.63] |
| 1.1 Token test after 1 months with 1.25 mg CEE and placebo | 1 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.77, 0.60] |
| 1.2 Token test after 2 months with E2 transdermal or placebo | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.74, 1.56] |
| 2 Token test after 4 months with 1.25 mg CEE or placebo | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐3.39, 4.27] |
| 3 Boston Naming Test after 4 months with 1.25 mg CEE or placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐2.93, 4.49] |
3.1. Analysis.
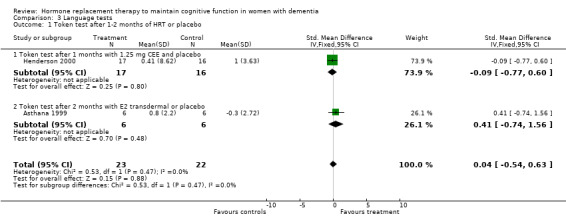
Comparison 3 Language tests, Outcome 1 Token test after 1‐2 months of HRT or placebo.
3.2. Analysis.
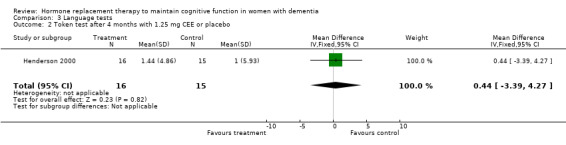
Comparison 3 Language tests, Outcome 2 Token test after 4 months with 1.25 mg CEE or placebo.
3.3. Analysis.
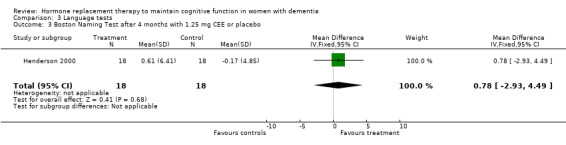
Comparison 3 Language tests, Outcome 3 Boston Naming Test after 4 months with 1.25 mg CEE or placebo.
Comparison 4. Speed of information processing and concentration tests.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TMT‐A after 1 month with 1.25 mg CEE or placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐31.52 [‐70.40, 7.36] |
| 2 TMT‐A after > 2 months HRT or placebo | 4 | 165 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.39, 0.24] |
| 2.1 TMT‐A (time) after 2 months with E2 transdermal or placebo | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐1.36, 0.91] |
| 2.2 TMT‐A (time) after 4‐12 months with (0.625 mg + 1.25 mg) CEE or placebo | 2 | 133 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.37, 0.34] |
| 2.3 TMT‐A (lines/sec, reversed) after 9 months with 0.625 mg CEE + MPA or placebo | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.29, 0.49] |
| 3 TMT‐B after 1 month with CEE or placebo | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐40.9 [‐79.29, ‐2.51] |
| 4 TMT‐B after > 2 months | 2 | 56 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.74, 0.32] |
| 4.1 TMT‐B after 4 months with 1.25 mg CEE or placebo | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.98, 0.33] |
| 4.2 TMT‐B after 9 months with 0.625 mg CEE + MPA or placebo | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.88, 0.88] |
| 5 Stroop after 2 months with E2 transdermal or placebo | 2 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.90, 0.49] |
| 6 DSST after 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐2.51, 3.51] |
| 7 Letter Cancellation test after 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐2.74, 2.54] |
| 8 Finger tapping after 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐3.9 [‐7.85, 0.05] |
| 9 Digit Span backwards after 1 month of 1.25 mg CEE or placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐0.37, 1.89] |
| 10 Digit Span backwards after > 4 months of CEE | 2 | 54 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.12, 0.97] |
| 10.1 Digit span after 4 months with 1.25 mg CEE or placebo | 1 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.67 [‐0.01, 1.34] |
| 10.2 Digit Span after 9 months with 0.625 mg CEE + MPA or placebo | 1 | 18 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.94, 0.90] |
4.1. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 1 TMT‐A after 1 month with 1.25 mg CEE or placebo.
4.2. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 2 TMT‐A after > 2 months HRT or placebo.
4.3. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 3 TMT‐B after 1 month with CEE or placebo.
4.4. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 4 TMT‐B after > 2 months.
4.5. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 5 Stroop after 2 months with E2 transdermal or placebo.
4.6. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 6 DSST after 12 months with (0.625 mg + 1.25 mg) CEE or placebo.
4.7. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 7 Letter Cancellation test after 12 months with (0.625 mg + 1.25 mg) CEE or placebo.
4.8. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 8 Finger tapping after 12 months with (0.625 mg + 1.25 mg) CEE or placebo.
4.9. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 9 Digit Span backwards after 1 month of 1.25 mg CEE or placebo.
4.10. Analysis.
Comparison 4 Speed of information processing and concentration tests, Outcome 10 Digit Span backwards after > 4 months of CEE.
Comparison 5. Clinical impressions of change scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CGIC (score of 4=no change from baseline, higher=worse) | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.14, 0.34] |
| 1.1 after 1 month with 1.25 mg CEE or placebo | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.18, 0.38] |
| 1.2 After 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.34, 0.54] |
| 2 CDR (higher=worse) | 2 | 144 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.01, 0.69] |
| 2.1 After 1,5 months with 1.25 mg CEE or placebo | 1 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 2.2 After 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [0.12, 0.98] |
| 3 CIBIC | 2 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.73, 0.27] |
| 3.1 After 1,5 month with 1.25 mg CEE or placebo | 1 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 3.2 After 9 months with 0.625 mg CEE + MPA or placebo | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.99 [‐2.01, 0.04] |
5.1. Analysis.
Comparison 5 Clinical impressions of change scales, Outcome 1 CGIC (score of 4=no change from baseline, higher=worse).
5.2. Analysis.
Comparison 5 Clinical impressions of change scales, Outcome 2 CDR (higher=worse).
5.3. Analysis.
Comparison 5 Clinical impressions of change scales, Outcome 3 CIBIC.
Comparison 6. Depression scales.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HDRS | 2 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.90, 0.96] |
| 1.1 After 3 months with 1.25 mg CEE or placebo | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐4.64, 1.44] |
| 1.2 After 12 months with (0.625 mg + 1.25 mg) CEE or placebo | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.2 [‐0.78, 1.18] |
6.1. Analysis.
Comparison 6 Depression scales, Outcome 1 HDRS.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asthana 1999.
| Methods | Design: randomized, placebo‐ controlled, double blind parallel groups 8 weeks | |
| Participants | Country: U.S.A. n=14 (12 completers) probable AD NINCDS/ADRDA, mild‐moderate dementia (MMSE: 17‐25), not institutionalised. Aged 79 (SD 8, 77‐85 yrs). Exclusion criteria: medical, neurological or psychiatric disease. HRT or cognition enhancer use last for the 2 months. Natural menopause ? (all underwent pap smears) | |
| Interventions | 1. E2 transdermal 0.05 mg 2. Placebo | |
| Outcomes | General (MMSE, BIMC) Memory: (BSRT, VRT, Paragraph recall) Speed (Stroop, TMT), Language: Fluency (letter), Token test | |
| Notes | HRT > placebo: on BSRT, Stroop (trends for VRT & Token test) 4/9 test Adverse events: 1 drop‐out due to skin irritations | |
Asthana 2001.
| Methods | Design: randomized, placebo‐ controlled, double blind parallel groups 8 weeks | |
| Participants | Country: U.S.A. n=20 (20 completers) probable AD NINCDS/ADRDA, mild‐moderate dementia (MMSE: 10‐29), Aged 80 (SD 7, 61‐90 yrs). Exclusion criteria: medical, neurological or psychiatric disease. Psychoactive medication, cholinesterase inhibitors or HRT use last for the 2 months. Mixed menopause (all underwent pap smears) | |
| Interventions | 1. E2 (17‐beta transdermal 0.10 mg 2. Placebo | |
| Outcomes | General (MMSE, BIMC) Memory: (BSRT, Paragraph recall, Rey Osterich Visual memory test, Visual Paired Associates, Oculomotor Delayed response), Boston Namin Test (semantic) Speed (Stroop, TMT), Treisman Visual Search Other: CIBIC, IADL, BPRS, PSMS | |
| Notes | HRT>placebo: attention: Stroop, memory: BSRT, Rey Visual Memory, BNT) Adverse events: breast tenderness (2 ERT), skin irritation patch (3 on ERT). No bleeding or spotting, no deep vein thrombosis, pulmonary embolism | |
Birge 1997.
| Methods | Design: randomized,placebo‐ controlled, double‐blind parallel groups 9 months | |
| Participants | Country: U.S.A. n=20 DAT (DSM?), mild dementia (CDRS <2) Aged 77 (SD 6, 67‐86). Exclusion criteria: Depression (GDS >5), age < 70, Other types of dementia syndromes | |
| Interventions | 1. CEE oral 0.625 mg/day + MPA 5 mg for 15 days every 3rd mth 2. Placebo | |
| Outcomes | General (BIMC) Memory (CERAD word list, Paired Associates, Digit Span), Speed (TMT), Other (Clock drawing) | |
| Notes | HRT >placebo: on Paired associate, Placebo > HRT: on BMIC, Digit Span 1/6 tests | |
Henderson 2000.
| Methods | Design: randomised, placebo‐ controlled, double‐blind parallel groups 4 months | |
| Participants | Country: U.S.A. n=42 (36 completers) probable AD (NINCDS), mild dementia (MMSE 10‐26). Aged 78 (SD 1). Mix natural and surgical menopause. Exclusion criteria: Contra‐ indications for HRT use, no use HRT or cognition enhancers last 3 months. | |
| Interventions | 1. CEE oral 1.25 mg/day 2. Placebo | |
| Outcomes | General (ADAS‐Cog, BIMC) Memory: (Paragraph recall, VRT, Digit Span), Speed: (TMT) Language (Naming, Token test) | |
| Notes | HRT > placebo on TMT‐B (wk 4), Placebo > HRT on VRT (wk 16), Paragraph recall + Digit span (wk 4), trend for reverse for both at wk 16 1/8 tests Adverse events: 3 vaginal spotting | |
Honjo 1995.
| Methods | Design: placebo‐ controll ed, double‐blind parallel groups, 7 weeks | |
| Participants | Country: Japan n=14 AD (criteria?) (13 completers), mild dementia (MMSE: 18 SD 6). Aged 84 (SD 5). Natural menopause (bleeding). No exclusion criteria ? | |
| Interventions | 1. CEE oral 1.25 mg /day for 3 wks + MPA 2.5 mg/day for last 3 weeks 2. Placebo | |
| Outcomes | General (MMSE and Japanese dementia scales: NSD HDS) | |
| Notes | HRT: all improved > baseline, 3/3 test. No between groups effect. Adverse events: 11 vaginal bleeding | |
McDonald Caldwell 1952.
| Methods | Design: placebo‐ controlled double‐blind parallel groups 6 months | |
| Participants | Country: U.S.A. n=30 nursing home residents, probably with dementia. Aged 75 years (54‐88). Natural menopause. Exclusion criteria: inability to communicate, evidence of neoplasm | |
| Interventions | 1. E2 i.m., 1 mg 3x/wk for 6 wks, then 2 mg/ wk +P 5‐10 mg for 1‐3 days/mth 2. Placebo | |
| Outcomes | General (IQ) Memory (WMS: Paragraph recall, Paired Associates,VRT, Digit Span) Speed (DSST, Stroop) | |
| Notes | HRT> placebo: on total memory (Paragraph recall, Paired Associates). No effect Digit Span, DSST, Stroop 2/5 | |
Mulnard 2000.
| Methods | Design: randomized, placebo‐ controlled, double‐blind parallel groups, 12 months | |
| Participants | Country: U.S.A., multi‐centre trial n= 120 probable AD NINCDS/ADRDA, (97 completers), mild‐ moderate dementia (MMSE: 12‐28). Aged 56‐91. Hysterectomized, mix natural and surgical menopause. Exclusion criteria: age < 60, depression, CVD, types of medication (no use HRT last 3 months, stabile use donazepil allowed). | |
| Interventions | 1. CEE oral 0.625 mg/day 2. CEE 1.25 oral mg/day 2. Placebo | |
| Outcomes | General (MMSE, ADAS‐Cog) Memory (new dot test, face recognition) Speed: (TMT, letter cancellation, finger tapping, DSST) Language (Fluency) | |
| Notes | HRT> placebo MMSE after 2 months, after 12 month worse (Fluency, finger tapping) 1/9 tests Adverse events: 4 had deep vein thrombosis, 2 vaginal bleeding | |
Wang 2000.
| Methods | Design: randomised, placebo‐ controlled, double‐blind parallel groups, 3 months | |
| Participants | Country: Taiwan n=50 prob AD NINCDS/ADRDA, (47completers) mild‐ moderate dementia (MMSE: 10‐26). Aged 72 (SD 9). Natural menopause ? (all had pap smears). Exclusion criteria: diabetes, cancer, hypertension, active disease, depression, use cognition enhancers last 3 months, use HRT last month. | |
| Interventions | 1. CEE oral 1.25 mg/day 2. Placebo | |
| Outcomes | General: (MMSE‐CE, CASI) | |
| Notes | no effect HRT over placebo 0/2 tests. Adverse events: 11 had vaginal bleeding | |
Zhang 2006.
| Methods | Design:
randomised, double‐blind, parallel groups 4 months |
|
| Participants | Country: China n=41 mild AD (DSM‐IV) Aged 47‐62 (55 +/‐0.4) years All menopause before age 65 Exclusion:dementia due to 'vessel, infection, toxication, metabolism, liver/kidney dysfunction or depression. All gave informed consent and had CT/MRI | |
| Interventions | 1. Beimeili (conjugated estrogen, Wyeth, USA) oral/1.25 mg /day 2. vit B1 20 mg 3x/day as Placebo | |
| Outcomes | General Hasegawa Dementia Scale‐R (HDS‐R) ADL | |
| Notes | HRT > vitamin B1 after 4 months on HDS‐R and ADL | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fillit 1986 | not RCT |
| Fillit 1994 | not RCT |
| Honjo 1989 | Not RCT |
| Kantor 1973 | Only used hospital adjustment scores and no cognitive tests |
| Ohkura 1994a | Not RCT |
| Ohkura 1994b | Not RCT |
| Ohkura 1995 | Not RCT |
| Rigaud 2003 | Comparison between HRT + rivastigmine vs. placebo + rivastigmine: HRT did not give further improvement over rivastigmine alone |
| Yoon 2003 | Open label |
Contributions of authors
‐EH: main reviewer, correspondence, all aspects of review. ‐KY: search for trials, extraction of data, interpretation data analyses, updating review. ‐MR: search for trials, extraction of data, interpretation data analyses, updating review. ‐FAH: drafting review versions, selection of trials, interpretation data analyses.
‐Contact editor for this review is Leon Flicker. ‐Consumer editor for this review is Clare Bateman. ‐This review has been peer reviewed by two external experts.
Sources of support
Internal sources
OPTIMA, Department of Pharmacology, University of Oxford, UK.
Loughborough University, UK.
External sources
Bristol‐Myers Squibb, USA.
Declarations of interest
None known.
Edited (conclusions changed)
References
References to studies included in this review
Asthana 1999 {published data only}
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen C, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with AD: results of a placebo‐controlled, double‐blind pilot study. Psychoneuroendocrinology 1999;24:657‐677. [DOI] [PubMed] [Google Scholar]
Asthana 2001 {published data only}
Birge 1997 {published data only}
- Birge SJ. The role of estrogen in the treatment of Alzheimer's disease. Neurology 1997;48:S36‐S41. [DOI] [PubMed] [Google Scholar]
Henderson 2000 {published data only}
- Henderson VW, Paganini‐Hill A, Miller BL, et al. Estrogen for Alzheimer's disease in women. Neurology 2000;54(2):295‐301. [DOI] [PubMed] [Google Scholar]
Honjo 1995 {published data only}
- Honjo H, Tanaka T, Urabe M, Okada H, Hayashi M, Hayashi K. Senile dementia‐Alzheimer's type and estrogen. Horm. Metab. Res. 1995;27:204‐207. [DOI] [PubMed] [Google Scholar]
McDonald Caldwell 1952 {published data only}
- Caldwell BM, Watson RI. Evaluation of psychologic effects of sex hormone administration in aged women: results of therapy after 6 months. Journal of Gerontology 1952;7:228‐44. [DOI] [PubMed] [Google Scholar]
Mulnard 2000 {published data only}
- Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer's disease. JAMA 2000;283:1007‐15. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang PN, Liao SQ, Liu CY, et al. Effects of estrogen on cognition, mood, and cerebral blood flow in AD. Neurology 2000;54:2061‐2066. [DOI] [PubMed] [Google Scholar]
Zhang 2006 {published data only}
- Zhang, YX, Luo, G, Guo ZJ, Cui, RY, Wang, LQ, Zhou CL. Quantitative evaluation of the intervention al effect of estrogen on Alzheimer's disease. Chinese Journal of Clinical Rehabilitation 2006;10(30):37‐91. [Google Scholar]
References to studies excluded from this review
Fillit 1986 {published data only}
- Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for senile dementia‐Alzheimer's type. Psychoneuroendocrinology 1986;11:337‐345. [DOI] [PubMed] [Google Scholar]
Fillit 1994 {published data only}
- Fillit H. Estrogens in the Pathogenesis and Treatment of Alzheimer's Disease in Postmenopausal Women. Annals of the New York Academy of Sciences 1994;743:233‐9. [DOI] [PubMed] [Google Scholar]
Honjo 1989 {published data only}
- Honjo H, Ogino Y, Naitoh K, et al. In vivo effects by estrone sulfate on the central nervous system‐senile dementia (Alzheimer's type). Journal of Steroid Biochemistry 1989;34:521‐5. [DOI] [PubMed] [Google Scholar]
Kantor 1973 {published data only}
- Kantor HI, Michael CM, Shore E. Estrogen for older women. American Journal of Obstetrics and Gynecology 1973;4:31‐47. [DOI] [PubMed] [Google Scholar]
Ohkura 1994a {published data only}
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Evaluation of estrogen treatment in female patients with dementia of the Alzheimer type. Endocrine Journal 1994;41:361‐71. [DOI] [PubMed] [Google Scholar]
Ohkura 1994b {published data only}
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Low‐dose estrogen replacement therapy for Alzheimer disease in women. Menopause 1994b;1:125‐30. [Google Scholar]
Ohkura 1995 {published data only}
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long‐term estrogen replacement therapy in female patients with dementia of the Alzheimer type 7 case reports. Dementia 1995;6:99‐107. [DOI] [PubMed] [Google Scholar]
Rigaud 2003 {published data only}
- Rigaud AS, Andre G, Vellas B, Touchon J, Pere MD. No additional benefit of HRT on response to rivastigmine in menopausal women with AD. Neurology 2003;60:148‐9. [DOI] [PubMed] [Google Scholar]
Yoon 2003 {published data only}
- Yoon B‐K, Kim DH, Kan Y, Kim J‐W, Shin M‐H, Na DL. Hormone replacement therapy in women with Alzheimer's disease: a randomised prospective study. Fertility and Sterility 2003;79(2):274‐80. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Azzawi 2008
- Al‐Azzawi F. Pulsatile nasal administration of estradiol for postmenopausal complaints. In: Hogervorst E, Henderson V, Gibbs B, Brinton‐Diaz R editor(s). Hormones, Cognition and Dementia. Cambridge: Cambridge University Press, 2008. [Google Scholar]
APA 1999
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association, 1999. [Google Scholar]
Barrett‐Connor 1991
- Barrett‐Connor E. Postmenopausal estrogen and prevention bias. Postmenopausal estrogen and prevention bias. Annals of Internal Medicine 1991;115:455‐6. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H, Junior. Bias in treatment assignment in controlled clinical trials. New England Journal of Medicine 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Christie 1990
- Christie, et al. Reduced plasma oestrogen stimulated neurophysin and delayed response to oestrogen challenge in AD. Psych Med 1990;20:773‐777. [DOI] [PubMed] [Google Scholar]
Col 2001
- Col NF, Hirota LK, Orr RK, Erban JK, Wong JB, Lau J. Hormone replacement therapy after breast cancer: a systematic review and quantitative assessment of risk. Journal of Clinical Oncology 2001;19(8):2357‐63. [DOI] [PubMed] [Google Scholar]
Fratiglioni 2000
- Fratiglioni L, Launer LJ, Andersen K, Breteler MM, Copeland JR, Dartigues JF, et al. Incidence of dementia and major subtypes in Europe: A collaborative study of population‐based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000;54(11):S10‐15. [PubMed] [Google Scholar]
Gibbs 2008
- Gibbs RB. Animal studies that support estrogen effects on cognitive performance and the cholinergic basis of the critical period hypothesis. In: Hogervorst E, Henderson V, Gibbs B, Brinton‐Diaz R editor(s). Hormones, Cognition and Dementia. Cambridge: Cambridge University Press, 2008. [Google Scholar]
Henderson 2008
- Henderson VH. The critical window hypothesis: hormone exposure and cognitive outcomes after menopause. In: Hogervorst E, Henderson V, Gibbs B, Brinton‐Diaz R editor(s). Hormones, Cognition and Dementia. Cambridge: Cambridge University Press, 2008. [Google Scholar]
Hogervorst 2000
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post‐menopausal women: a meta‐analysis. Neuroscience 2000;101(3):485‐512. [DOI] [PubMed] [Google Scholar]
Hogervorst 2007
- Hogervorst E, Bandelow S. Should surgically menopausal women be treated with estrogens to decrease the risk for dementia?. Neurology, Invited Editorial 2007;69(11):1070‐1. [DOI] [PubMed] [Google Scholar]
Huppert 1997
- Huppert F, Wilcock G. Ageing, cognition and dementia. Age and Ageing 1997;Dec 26(Suppl 4):20‐3. [DOI] [PubMed] [Google Scholar]
Launer 1999
- Launer LJ, Andersen K, Dewy ME, et al. Rates and risk factors for dementia and Alzheimer's disease. Neurology 1999;1:78‐84. [DOI] [PubMed] [Google Scholar]
LeBlanc 2001
- LeBlanc ES, Janowsky J, Chan BKS, Nelson HD. Hormone replacement therapy and cognition. JAMA 2001;285(11):1489‐99. [DOI] [PubMed] [Google Scholar]
Lethaby 2008
- Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD003122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 1996
- Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy: are users healthier than nonusers ?. American Journal of Epidemiology 1996;143(10):971‐8. [DOI] [PubMed] [Google Scholar]
McEwen 1997
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain implications for cognition and aging. Neurology 1997;48:S14‐S18. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;4:939‐44. [DOI] [PubMed] [Google Scholar]
Nappi 1999
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecologic and Obstetric Investigation 1999;47:29‐36. [DOI] [PubMed] [Google Scholar]
Paganini‐Hill 2001
- Paganini‐Hill A. Hormone replacement therapy and stroke: risk, protection or no effect? Hormone replacement therapy and stroke: risk, protection or no effect?. Maturitas 2001;38(3):243‐61. [DOI] [PubMed] [Google Scholar]
Rodríguez‐Martín 2001
- Rodríguez JL, Qizilbash N, López‐Arrieta J. Thiamine for Alzheimer's disease. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001498] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Schumaker 1998
- Schumaker, et al. The WHIMS: a trial of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials 1998;19:604‐621. [DOI] [PubMed] [Google Scholar]
Shumaker 2003
- Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, et al. and WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289(20):2651‐62. [DOI] [PubMed] [Google Scholar]
Shumaker 2004
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 2004;291(24):2947‐58. [DOI] [PubMed] [Google Scholar]
Silva 2001
- Silva I, Mor G, Naftolin F. Estrogen and the aging brain. Maturitas 2001;38(1):100‐1. [DOI] [PubMed] [Google Scholar]
Toran‐Allerand 2000
- Toran‐Allerand CD. Estrogen as a treatment for AD: commentary. JAMA 2000;284:307‐8. [PubMed] [Google Scholar]
Wegienka 2006
- Wegienka G, Havstad S, Kelsey JL. Menopausal hormone therapy in a health maintenance organization before and after women's health initiative hormone trials termination. Journal of Women's Health 2006;15(4):369‐78. [DOI] [PubMed] [Google Scholar]
Yaffe 1998a
- Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women. Journal of the American Medical Association 1998;279:688‐695. [DOI] [PubMed] [Google Scholar]
Yaffe 1998b
- Yaffe K, Grady D, Pressman A, Cummings S. Serum estrogen levels, cognitive performance and risk of cognitive decline in older community women. Journal of the American Geriatrics Sociaty 1998;46:816‐21. [DOI] [PubMed] [Google Scholar]
Yaffe 2000
- Yaffe K, et al. Cognitive decline in women with relation to non‐protein bound estradiol concentrations. Lancet 2000;356:708‐12. [DOI] [PubMed] [Google Scholar]
Yesufu 2007
- Yesufu A, Bandelow S, Hogervorst E. Meta‐analyses of the effect of hormone treatment on cognitive function in postmenopausal women. Women’s Health invited review 2007;3(2):173‐95. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hogervorst 2002
- Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003799] [DOI] [PubMed] [Google Scholar]


