Abstract
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2006 and previously updated in 2009.
Tinnitus is described as the perception of sound or noise in the absence of real acoustic stimulation. It has been compared with chronic pain, and may be associated with depression or depressive symptoms which can affect quality of life and the ability to work. Antidepressant drugs have been used to treat tinnitus in patients with and without depressive symptoms.
Objectives
To assess the effectiveness of antidepressants in the treatment of tinnitus and to ascertain whether any benefit is due to a direct tinnitus effect or a secondary effect due to treatment of concomitant depressive states.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; PsycINFO; CINAHL; Web of Science; BIOSIS; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 5 January 2012.
Selection criteria
Randomised controlled clinical studies of antidepressant drugs versus placebo in patients with tinnitus.
Data collection and analysis
Two authors critically appraised the retrieved studies and extracted data independently. Where necessary we contacted study authors for further information.
Main results
Six trials involving 610 patients were included. Trial quality was generally low. Four of the trials looked at the effect of tricyclic antidepressants on tinnitus, investigating 405 patients. One trial investigated the effect of a selective serotonin reuptake inhibitor (SSRI) in a group of 120 patients. One study investigated trazodone, an atypical antidepressant, versus placebo. Only the trial using the SSRI drug reached the highest quality standard. None of the other included trials met the highest quality standard, due to use of inadequate outcome measures, large drop‐out rates or failure to separate the effects on tinnitus from the effects on symptoms of anxiety and depression. All the trials assessing tricyclic antidepressants suggested that there was a slight improvement in tinnitus but these effects may have been attributable to methodological bias. The trial that investigated the SSRI drug found no overall improvement in any of the validated outcome measures that were used in the study although there was possible benefit for a subgroup that received higher doses of the drug. This observation merits further investigation. In the trial investigating trazodone, the results showed an improvement in tinnitus intensity and in quality of life after treatment, but in neither case reached statistical significance. Reports of side effects including sedation, sexual dysfunction and dry mouth were common.
Authors' conclusions
There is as yet insufficient evidence to say that antidepressant drug therapy improves tinnitus.
Keywords: Humans, Amitriptyline, Amitriptyline/therapeutic use, Antidepressive Agents, Antidepressive Agents/therapeutic use, Depression, Depression/drug therapy, Nortriptyline, Nortriptyline/therapeutic use, Paroxetine, Paroxetine/therapeutic use, Randomized Controlled Trials as Topic, Selective Serotonin Reuptake Inhibitors, Selective Serotonin Reuptake Inhibitors/therapeutic use, Tinnitus, Tinnitus/drug therapy, Tinnitus/psychology, Trazodone, Trazodone/therapeutic use, Trimipramine, Trimipramine/therapeutic use
Plain language summary
Antidepressants for patients with tinnitus
Tinnitus is described as the perception of sound or noise in the absence of real acoustic stimulation, and it is frequently associated with depression or depressive symptoms. Six studies involving a total of 610 patients matched the inclusion criteria for this review. Four evaluated three tricyclic antidepressant agents (amitriptyline, nortriptyline and trimipramine) for the treatment of tinnitus. These studies did not find enough evidence to prove the efficacy of these agents in the management of tinnitus. One study evaluated paroxetine, a selective serotonin reuptake inhibitor antidepressant, and one evaluated trazodone, an atypical antidepressant. Neither of these studies showed benefit of paroxetine or trazodone in the treatment of tinnitus. Side effects, though relatively minor, were common in all groups of antidepressants. Further research is required.
Background
This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2006 and previously updated in 2009.
Tinnitus can be described as the perception of sound in the absence of external acoustic stimulation. For the patient it may be trivial or it may be a debilitating condition (Luxon 1993). The quality of the perceived sound can vary enormously from simple sounds such as whistling or humming to complex sounds such as music. The patient may hear a single sound or multiple sounds. Tinnitus may be perceived in one or both ears, within the head or outside the body. The symptom may be continuous or intermittent. The need to stratify subgroups of tinnitus patients in future clinical trials according to the type of tinnitus experienced by individual patients has been increasingly recognised (Elgoyhen 2010 ). Tinnitus is described in most cases as 'subjective', meaning that it cannot be heard by anyone other than the patient. While, for the patient, this perception of noise is very real, because there is no corresponding external sound it can be considered an 'hallucination', 'phantom' or false perception. 'Objective' tinnitus is a form of tinnitus which can be detected by an examiner, either unaided or using a listening aid such as a stethoscope or microphone in the ear canal. This form of tinnitus is much less common and usually has a definable cause such as sound generated by blood flow in or around the ear or unusual activity of the tiny muscles within the middle ear. Tinnitus may be associated with normal hearing or any degree of hearing loss and can occur at any age.
It is important to distinguish between clinically significant and non‐significant tinnitus (Davis 2000) and several different classifications have been proposed (Dauman 1992; McCombe 2001; Stephens 1991). Dauman, for example, makes a distinction between 'normal' (lasting less than five minutes, occurring less than once a week and experienced by most people) and 'pathological' tinnitus (lasting more than five minutes, occurring more than once a week and usually experienced by people with hearing loss).
Aetiology
Various theories for the pathophysiological cause of tinnitus have been proposed, but none are universally accepted and increasingly it seems likely that there is no single underlying pathological process. Also, there is evidence that a majority of people have the ability to detect tinnitus‐like activity in the right circumstances. Eighty volunteers who did not complain of tinnitus entered a sound‐proofed room and within five minutes 94% reported that they could hear noises, describing the same type of sound experiences that people with tinnitus describe (Heller 1953). In a more recent experiment 53 young adults were put in an anechoic chamber, firstly on their own and then with a non‐functioning loudspeaker (Del Bo 2008). Within four minutes 83% were aware of one or more sounds when they were alone and this rose to 92% when the loudspeaker was present. The researchers concluded that tinnitus‐like perceptions emerged in a non‐clinical population in a silent environment and indicated that suggestive mechanisms played only a minor role in their generation.
Almost any form of disorder involving the outer, middle or inner ear or the auditory nerve may be associated with tinnitus (Brummett 1980; Shea 1981). However, concentrating on the peripheral part of the auditory system has its limitations. If tinnitus was a consequence of hearing loss the degree of damage to the ear should correlate with the severity of the tinnitus. This is not the case and indeed it is possible to have severe tinnitus with no evidence of any aural pathology (Gabr 2011; Seidman 2010). Conversely, tinnitus can even exist without a peripheral auditory system: unilateral tinnitus is a common presenting symptom of vestibular schwannomas (acoustic neuromas) which are benign tumours of the vestibulo‐cochlear nerve. These are the most common tumours of the cerebellopontine angle, with an annual incidence estimated at between 1.4 and 2.02 per 100,000 of the population (Dawes 2000; Moffat 1995), although these figures are probably underestimates (Anderson 2000). One form of treatment of these lesions is surgical excision using a translabyrinthine route. In most cases this not only removes the tumour but also severs the cochlear nerve and destroys the inner ear. Despite the effective removal of their peripheral auditory mechanisms, 60% of these patients retain their tinnitus postoperatively (Baguley 1992). These observations have been used to produce a model of tinnitus that suggests that the trigger or ignition site for tinnitus may be at any point in the auditory system or even at a point outside the classical auditory system (Baguley 2006; Eggermont 2006). The process that maintains or promotes the tinnitus, however, is within the central auditory system. Further support for the involvement of central pathways is the observation that tinnitus is often reported as starting after a shock or emotional upset rather than any change in the auditory system.
Recent studies have increasingly focused on the interpretation of tinnitus as a central nervous system (CNS) disorder (Elgoyhen 2012). It has been suggested that tinnitus can be caused by excessive or abnormal spontaneous activity in the auditory system and in related cerebral areas (Jastreboff 1994; Kaltenbach 2000; Lockwood 1999; Moller 1997). There is experimental evidence supporting this theory (Lockwood 1999; Lockwood 2002): the flow of blood through the brain can be detected and imaged using positron emission tomography (PET), which is a method of computed tomography that detects energy emitted by radio nucleotides. In these studies, pure tones presented to patients with tinnitus activated more portions of the brain than in control (non‐tinnitus) patients, suggesting that 'abnormal connections' in the central auditory system may play a role in tinnitus perception. These initial observations of abnormal central auditory activity in tinnitus patients have been supported by subsequent functional imaging studies using both PET (Eichhammer 2007) and functional magnetic resonance imaging (fMRI) (Lanting 2008). Taking the role of central neural pathways one step further, tinnitus may be compared to chronic pain of central origin ‐ a kind of 'auditory pain' (Briner 1995; Sullivan 1994).
In 1990 existing facts about tinnitus were drawn together to create the neurophysiological model of tinnitus (Jastreboff 1990). In this model it was suggested that tinnitus is generated when a signal within the auditory system, which is normally suppressed by the subconscious brain, becomes noticed. If this perception is subjected to a form of negative emotional reinforcement a positive feedback loop is formed that results in maintenance of the sensation: perception of the original signal renders the awareness of tinnitus more annoying and intrusive which in turn heightens awareness of the signal and can ultimately lead to it becoming persistent.
In all these models of tinnitus generation and perception, the relationship between the symptom of tinnitus and central auditory processing has been emphasised. Advances in medical imaging and sophisticated investigations of brain signalling systems (i.e. magnetic resonance imaging (MRI), magnetoencephalography (MEG), quantitative‐electroencephalography (QEEG)) are contributing to highlight the role of different brain areas in processing sounds or signals coming from the ears. All these imaging techniques provide electrophysiologic and metabolic measures of activity in the brain. In particular, some studies have focused on several regions of hyperactivity in the auditory pathway of tinnitus patients: the cortex, prefrontal and temporo‐parietal areas, as well as non‐auditory brain structures, such as the limbic areas, hippocampus and amygdala. The hypothesis of 'plastic reorganisation' within certain brain areas, following an initial cochlear lesion with loss of hair cells (due to various causes, for example acoustic trauma, noise exposure, age‐related cell degeneration) is a suggestive one and it is gaining increasing attention (Leaver 2011; Rauschecker 2010) (Figure 1). However, this is not the only vision coming from research; other studies have focused on the characteristics of the brain's 'tonotopic map' (Langers 2012) or on the connectivity in brain networks associated with tinnitus (Burton 2012; Vanneste 2011).
1.
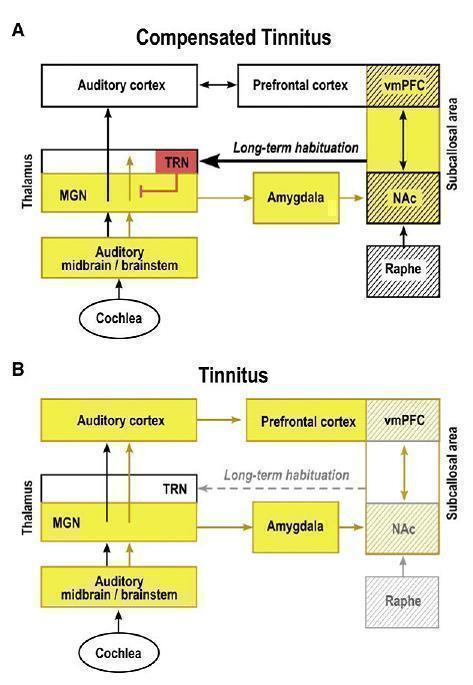
Tinnitus compensation: a proposed 'noise cancellation' model in tinnitus. Initially, a tinnitus signal results from peripheral deafferentiation (loss of hair cells from injury, age, noise exposure) and subsequent lesion‐induced reorganisation of central auditory structures. The nucleus accumbens and the associated paralimbic networks in the prefrontal cortex play an important role in long‐term habituation to continuous unpleasant sounds. As long as the NAc‐system is intact (A: "Compensated tinnitus"), the tinnitus signal is filtered out and will not be relayed to the auditory cortex. If the NAc‐system becomes compromised (B: Tinnitus), cancellation of the tinnitus signal at the thalamic level is no longer possible, long‐term reorganisation of auditory cortex sets in and tinnitus perception becomes potentially chronic. Structures with serotoninergic innervation are shown by hatching; inhibitory structures are shown in red: yellow indicates presence of tinnitus signal. NAc = nucleus accumbens; vmPFC = ventromedial prefrontal cortex; MGN = thalamus; TRN = talamic reticular nucleus.
Reprinted from Rauschecker JP, Leaver AN, Muhlau M. Turning out the noise: limbic‐auditory interactions in tinnitus. Neuron 2010;66:819‐25, with permission from Elsevier (Rauschecker 2010).
The limbic system mediates emotions and is of fundamental relevance in understanding why the sensation of tinnitus is in many cases so distressing for the patient. Involvement of these areas of the brain also suggests why, when symptoms are severe, tinnitus can be associated with major depression, anxiety and other psychosomatic and/or psychological disturbances, leading to a progressive deterioration of quality of life (Lockwood 1999; Sullivan 1989; Sullivan 1992; Sullivan 1993).
Prevalence
Epidemiological data reports are few. The largest single study was undertaken in the UK by the Medical Research Council Institute of Hearing Research and was published in 2000 (Davis 2000). This longitudinal study of hearing questioned 48,313 people: 10.1% described tinnitus arising spontaneously and lasting for five or more minutes at a time and 5% described it as moderately or severely annoying. However, only 0.5% reported tinnitus having a severe effect on their life. This is another of the paradoxes of tinnitus: the symptom is very common but the majority of people who experience it are not particularly concerned by it. These figures from the UK are broadly consistent with data collected by the American Tinnitus Association (ATA) which suggest that tinnitus may be experienced by around 50 million Americans, or 17% of the US population (ATA 2004). A recent report on a Chinese population showed an overall prevalence of 14.5% in the general population (Xu 2011) and in Japan, overall, 11.9% of 14,423 questionnaire respondents reported having tinnitus; the percentage was somewhat higher among men (13.2%) than women (10.8%) and the prevalence increased with age in both sexes (Fujii 2011). Another recent report from the USA also showed the prevalence of frequent tinnitus to increase with age, peaking at 14.3% between 60 and 69 years of age (Shargorodsky 2010).
Data also exist for Europe and Australia (Sindhusake 2003) and estimates suggest that tinnitus affects a similar percentage of these populations, with 1% to 2% experiencing debilitating tinnitus (Seidman 1998). The Oregon Tinnitus Data Archive (Meikle 1995) contains data on the characteristics of tinnitus drawn from a sample of 1630 tinnitus patients. The age groups with the greater prevalence are those between 40 and 49 years (23.9%) and between 50 and 59 years (25.6%). A useful graph displaying tinnitus and hearing impairment prevalence data has been presented by Lockwood (Figure 2) (Lockwood 2002).
2.
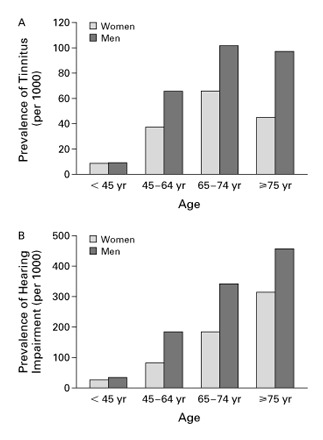
The Prevalence of Tinnitus (Panel A) and Hearing Impairment (Panel B). From: Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. New England Journal of Medicine 2002; 347(12):904‐10. Values are based on responses to the question "Do you have tinnitus (or) ringing in the ears or deafness (or) other trouble hearing?" Included in the National Center for Health Statistics Survey of noninstitutionalized Americans, 1999.
Diagnosis
By definition, subjective tinnitus is not detectable by anyone other than the patient. This means that diagnosis is entirely dependent on the patient's report. There are no objective measurements that can be made, which hampers both treatment and research. Initially, a patient complaining of tinnitus will undergo a basic clinical assessment including the relevant otological, general and family histories with specific questions regarding sleep disturbance and symptoms of psychological disease. Examination will focus on the ears, cranial nerve function, temporomandibular joints and neck. Investigations will be directed by the medical history and clinical examination: most patients with tinnitus require just a simple audiometric assessment with a pure‐tone audiogram and acoustic impedance test. Some patients require more detailed audiometric tests such as evoked response audiometry or otoacoustic emission testing. Certain clinical scenarios do require more detailed investigation: persistent, unilateral tinnitus may be due to a specific disorder of the auditory pathway and imaging of the cerebellopontine angle with magnetic resonance imaging (MRI) or computed tomography (CT) is important to exclude lesions such as vestibular schwannomas. Other more unusual lesions, such as glomus tumours, meningiomas, adenomas, vascular lesions or neuro‐vascular conflicts may also be detected by imaging (Marx 1999; Weissman 2000). Other diagnostic procedures such as haematological testing may occasionally be required. If these procedures exclude any specific pathologic cause, the tinnitus can be considered to be idiopathic.
Treatment
At present no specific therapy for tinnitus is acknowledged to be satisfactory in all patients. Many patients who complain of tinnitus and also have a significant hearing impairment will benefit from hearing aids or other sound‐masking techniques, although the evidence for real benefits for tinnitus patients is still inconclusive (Hobson 2010). However, these devices can be helpful in balancing the negative impact of hearing disability in respect of social life.
A wide range of pharmacological agents, including vasodilators, calcium antagonists, anti‐spasmodic drugs, local anaesthetics, anticonvulsants and benzodiazepines, have been suggested for tinnitus patients. Small effects have been demonstrated after administration of these treatments in patients with tinnitus, however evidence about efficacy still appears to be inconclusive (Hoare 2011). Furthermore, significant risk of bias is reported in most trials (Hoekstra 2011). However, it is important to note that an increasing number of pharmaceutical producers are developing compounds for tinnitus (Langguth 2009). It is important to note that all the medicines are used as 'off‐label' therapies, in that neither the US Food and Drug Administration (FDA) nor the European Medicines Agency (EMA) have a single medicine specifically approved for tinnitus treatment.
Mainstream psychological techniques such as cognitive behavioural therapy (CBT) can be used in the management of tinnitus (Andersson 1999). The neurophysiological model (Jastreboff 1990) gave rise to a treatment protocol called tinnitus retraining therapy (TRT) (Jastreboff 1993; Jastreboff 2004), a technique that uses a combination of low‐level, wide‐band sound and counselling to promote habituation. Complementary therapies such as acupuncture, aromatherapy, homeopathy and reflexology have also been utilised in the management of tinnitus. The use of Ginkgo biloba, cognitive behavioural therapy and hyperbaric oxygen therapy have been the subject of other Cochrane reviews (Bennett 2007; Hilton 2004; Martinez 2010). Improved understanding of the role of the central auditory system in tinnitus has led to the development of therapeutic modalities directed at the auditory pathways of the brain. Most notably there has been considerable interest in the use of repetitive transcranial magnetic stimulation (rTMS), although this is still an experimental technique (Kleinjung 2007; Meng 2011). Based on the current state of knowledge and clinical research, optimal management of tinnitus may involve multiple strategies.
Antidepressant therapy
There is a high co‐morbidity of tinnitus and psychiatric illness (McKenna 1991). However, there is argument as to whether tinnitus is more likely to occur in psychologically disturbed people or whether tinnitus causes the psychological disturbance. There is also debate about whether psychoactive drugs act on the central auditory system and reduce tinnitus directly, whether they act by treating concomitant psychological illness or whether they have a simultaneous effect on both the psychological disturbance and the tinnitus (McFerran 2008). There are suggestions that antidepressants act by treating patients' underlying psychological problems rather than directly affecting the tinnitus itself (Parnes 1997). However, many of the receptors that psychoactive drugs act upon are also present within central auditory pathways and it has been hypothesised that some drugs may have direct action against tinnitus. Some antidepressants have proved useful in treating some forms of chronic pain, such as trigeminal neuralgia, atypical facial pain and headache (McQuay 1997; Tomkins 2001). Tinnitus and chronic pain have certain similarities therefore it seems reasonable to ask whether antidepressants might help to reduce tinnitus.
The types of antidepressants used in treating tinnitus‐related symptoms are most commonly tricyclic antidepressants (including amitriptyline, imipramine and nortriptyline). Newer drugs such as selective serotonin reuptake inhibitors (SSRIs) (e.g. fluoxetine, paroxetine or other heterocyclic compounds) can also be used. It has been reported that with regard to their antidepressant effects tricyclic, heterocyclic and SSRI compounds are comparable in efficacy and SSRIs seem to be better tolerated (Kasper 1992; Parker 2001). In very few cases different types of antidepressants, such as trazodone, have been used (Dib 2007). Several medicines which are not currently classified as antidepressants are prescribed and administered for the treatment of depression (e.g. alprazolam, sulpiride and melatonin).
Objectives
To assess whether antidepressant drug therapy is effective in the management of patients suffering from tinnitus and whether any beneficial effect is due to reduction of tinnitus alone, reduction of concomitant depression or reduction of both.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials of antidepressant drugs versus placebo. Cross‐over trials were only included if data from before the cross‐over could be extracted.
Types of participants
Patients diagnosed with tinnitus in whom underlying pathological conditions such as Ménière's disease, vestibular schwannomas, cervical spine lesions or otosclerosis have been excluded or not detected (Gersdorff 2000; Jozefowicz 2004; Levo 2000).
Types of interventions
We investigated the treatment of tinnitus with tricyclic antidepressant agents, SSRI antidepressant agents and trazodone, an atypical antidepressant drug (chemically unrelated to tricyclic antidepressants). All the studies included in the review compared administration of the relevant drug (where data were extractable) versus placebo. We decided to investigate antidepressant treatment against placebo treatment as there is no 'gold standard' treatment for tinnitus. No studies comparing drug therapy with no treatment were retrieved by our search strategy.
Types of outcome measures
Primary outcomes
Improvement in tinnitus severity and disability, including the global negative impact and sense of discomfort associated with tinnitus ‐ described as 'positive change in tinnitus disability'
Secondary outcomes
Improvement in tinnitus perception, loudness or intensity (specific auditory disease evaluation)
Improvement/change in depressive symptoms or in depression scores
Improvement/change in global well‐being
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 5 January 2012, following a previous update search in 2008 and searches in 2006.
Electronic searches
We searched the following databases from their inception for published, unpublished and ongoing trials: the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2011, Issue 4); PubMed; EMBASE; CINAHL; PsycINFO; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; ISRCTN; ClinicalTrials.gov; ICTRP and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We also searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials.
Data collection and analysis
Selection of studies
One review author (PB) assessed every report identified by the search strategy described above for relevance to this review. Two review authors (PB, CD for the original review; PB, DM for the update), blinded to the decisions made by the other, assessed the trials according to the inclusion criteria and graded the methodological quality of the included studies. Any disagreement was resolved by discussion between the review authors. We contacted study authors if necessary for clarification.
Data extraction and management
Two review authors independently collected and extracted data onto a standard form developed by the Cochrane Ear, Nose and Throat Disorders Group.
Assessment of risk of bias in included studies
'Risk of bias' assessment was based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions 4.2.2 (Handbook 2004).
Adequacy of the randomisation process.
Adequacy of allocation concealment.
The possibility of attrition bias after allocation.
Detection bias: blinding of those assessing outcomes was particularly important in this review owing to the subjective basis of many of the outcome measures in the included studies (e.g. severity of tinnitus and/or associated depression).
Quality of the outcome measures: for the purpose of this review, it was also important to evaluate the adequacy, development and standardisation of the questionnaires used in the trials.
We then gave studies a single grade (A, B or C) for their overall methodological quality.
Data synthesis
Although the initial intention was to perform a meta‐analysis of the results it became apparent that this might prove unsatisfactory owing to methodological flaws and heterogeneity of the studies. We sought advice and after due consideration we felt that further analysis should not be undertaken at this time. If further data become available it may be possible to utilise data extracted from some of the current studies in a statistical analysis.
Results
Description of studies
Results of the search
The original searches (2006) retrieved 63 possibly relevant articles, two of which (Dobie 1992; Dobie 1993) were immediately excluded as reports of another paper (Sullivan 1993). Five studies were excluded because it was impossible to extract sufficient data (attempts were made to contact the authors concerned, but no replies were received) and we rejected a further 45 studies, after careful analysis of the full texts, as not relevant to this review. Most of these articles considered tinnitus as an adverse effect of other drug treatments, or as one of the symptoms within the context of other, mainly age‐related, neurological or psychiatric diseases. Of the remaining 11 studies, five adhered to the criteria given in the original protocol and were therefore included in this review. The remaining six trials, which we initially considered relevant for possible inclusion, were rejected for a variety of reasons that are listed in the table Characteristics of excluded studies.
In 2008 the update searches retrieved 45 potentially relevant references. Following independent assessment by PB and DM one was included in the review (Dib 2007) and two were added as excluded studies (Lopez‐Gonzalez 2007a; Lopez‐Gonzalez 2007b) (see Characteristics of excluded studies).
The searches conducted in January 2012 retrieved a total of 45 records, of which we assessed 39 for eligibility following removal of duplicates and studies already evaluated in the previous version of the review. Following the removal of a further 17 non‐clinical trials, we assessed 22 articles in full text. Three were relevant but excluded for the reasons shown in Characteristics of excluded studies (Holgers 2011; Jalali 2009; Roberts 2011). See Figure 3 for a flow chart showing the search process for the 2012 update.
3.
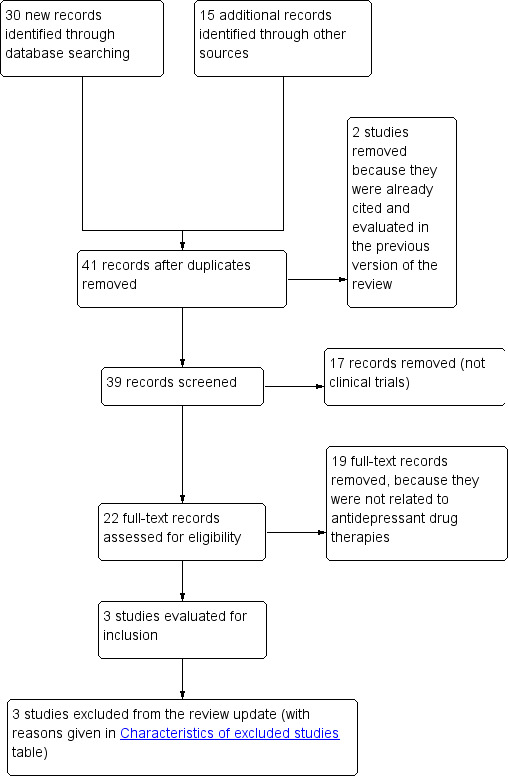
Study flow diagram: detail of literature review for the 2012 update.
Included studies
The six included studies were extremely varied in design, with validated diagnostic methods used only for the audiological tests, i.e. for the subjective evaluation of auditory disease or hearing impairment associated with tinnitus. However, for the evaluation of subjective tinnitus disease perception, of depressive symptoms, and of the global negative impact of tinnitus, there was a general lack of homogeneity, with the tests, scores, scales and questionnaires used differing significantly across studies.
Robinson 2005 evaluated the efficacy of paroxetine, a selective serotonin reuptake inhibitor (SSRI) agent in the relief of tinnitus. It was a double‐blind study with 120 participants who were randomised to treatment with paroxetine or placebo. An initial dose of 10 mg paroxetine per day was given, increasing in 10 mg increments every two weeks, to a maximal dose of 50 mg daily. The total duration was 100 days. The outcomes were based on audiometric measures and on the evaluation of improvement in tinnitus disability and subjective health‐related quality of life. All the evaluations were performed at baseline and at each follow‐up visit. The authors defined response on primary outcome as a 5 dB or 10 dB drop in perceived tinnitus volume, while tinnitus disability was assessed using the Tinnitus Handicap Questionnaire (Kuk 1990). The response on psychological and quality of life outcomes was assessed using a series of validated scales and questionnaires, together with a sequence of questions such as: "How bothered are you by your tinnitus?" or "How severe is your tinnitus?" Only one patient reported major depressive symptoms (included in the analysis).
Sullivan 1993 was a 12‐week, double‐blind, randomised trial of nortriptyline, a tricyclic antidepressant, versus placebo. One hundred and seventeen adult patients (aged between 50 and 80 years) with severe chronic tinnitus (i.e. of more than six months' duration and of sufficient severity to disrupt daily activities (defined by the investigators as producing a score of more than 600 on the 'disability' sub‐scale of the Iowa Tinnitus Handicap Questionnaire)) were recruited by a variety of methods (newspaper advertisements, American Tinnitus Association membership lists and letters to community otolaryngologists). Participants were required to have discontinued all psychotropic medications two weeks prior to the start of treatment (four weeks for fluoxetine). All subjects were assessed at baseline using otological and audiological assessments and a series of (mainly validated) measures, including sub‐sections of the National Institute of Mental Health (NIMH)‐Diagnostic Interview Schedule, the Hamilton Anxiety and Depression Rating Scales, Sheehan's 10‐point Disability Scales and the six‐point Multidimensional Pain Inventory. Following baseline evaluation, participants were randomly allocated to either a treatment (n = 63) or placebo group (n = 54), and were also subdivided by depression score into a major depression group (those with scores > 18 on the Hamilton Depression Scale) and a depression‐NOS ('not otherwise specified') group (those with scores < 18). Despite randomisation, the nortriptyline group had significantly more baseline depressive severity than the placebo group. Otherwise the groups were comparable at baseline. For the treatment group, the dosage of nortriptyline began at 25 mg at night and was titrated upwards by 25 mg per week until an adjusted blood level of nortriptyline between 50 and 150 ng/mL was reached (median number of capsules = 4; median nortriptyline level = 81 ng/mL). The placebo used consisted of lactose in identical capsules to the active drug. At six weeks, on trial completion, all participants re‐completed baseline questionnaires and had repeated audiometric and audiological assessments in order to assess the effect of treatment on i) levels of depression, ii) the disability/disruption caused by tinnitus, iii) the severity of auditory disease and iv) the global satisfaction with the treatment received. Twenty‐five participants dropped out during the trial (21.3%); 14 from the treatment group (the majority because of "anticholinergic side effects and sedation") and 11 from the placebo group (the majority because of "unsatisfactory therapeutic response and scheduling conflicts"). Results are presented in the original article for the 92 participants who completed the study; 38 of these had current major depression, while of the remaining 54 with depression‐NOS, 28% had a history of major depression.
Mihail 1988 was a double‐blind, cross‐over trial of the tricyclic antidepressant trimipramine versus placebo, with patients acting as their own controls. Twenty‐six consecutive male patients (mean age 52) presenting with subjective tinnitus at an army medical centre were enrolled and randomised to receive either a daily therapeutic dose of trimipramine (150 mg) or placebo (not specified) for six weeks, after which followed a rest period of four weeks followed by another six‐week test period, with all participants crossed over to the other treatment. All participants underwent baseline testing which comprised pure‐tone audiometry, auditory brain stem evoked responses, tinnitus frequency and intensity matching, and tinnitus masking levels. Participants were asked to grade their tinnitus on a seven‐point scale on which 1 represented very mild up to 7 which was very severe. Patients completed the Zung Depression Inventory (Zung 1973) and the Millon Behavioral Health Inventory (Millon 1982). The tests were performed at the beginning and end of each six‐week test period.
Podoshin 1995 was a randomised controlled trial of amitriptyline, a tricyclic antidepressant, versus placebo, and of biofeedback versus placebo biofeedback. Two hundred and twenty‐five adults with idiopathic subjective tinnitus were enrolled from a hospital ENT department and were randomly allocated to one of four treatment groups: Group A ‐ biofeedback (n = 62); Group B ‐ amitriptyline (10 mg three times daily for 10 weeks: n = 83; mean age 44); Group C ‐ placebo biofeedback (n = 40); and Group D ‐ placebo tablets (n = 40; mean age 52). The four groups were evaluated independently, with only Groups B and Group D (amitriptyline versus placebo) of interest to this review. Baseline audiometric testing was performed. The treatment period was 10 weeks, and tinnitus severity was measured at rest and during activity by a five‐point scale questionnaire and subsequently at weekly intervals.
Bayar 2001 was a randomised, parallel, single‐blind study comparing the effectiveness of a six‐week course of the tricyclic antidepressant amitriptyline (50 mg daily for the first week, then 100 mg daily for the following five weeks) with placebo (one lactose‐starch tablet daily for six weeks) for the treatment of subjective tinnitus. Thirty‐seven adult patients (no ages given) with a primary complaint of subjective tinnitus and no history of depression were enrolled in the trial and randomly allocated to either the treatment group (n = 20) or the placebo group (n = 17). Baseline tests included standard audiometry, audiometry using an extended range up to 18 kHz, acoustic impedance tests, auditory brainstem evoked response, tinnitus frequency and intensity matching. The severity of tinnitus in all participants was evaluated using a questionnaire based on a model devised by the American Tinnitus Association (ATA), in which patients were asked to grade the severity of their tinnitus using a 10‐point scale, with 1 mild and 10 severe. Factors such as the duration, severity and localisation of the tinnitus at baseline were generally comparable across the two groups.
Dib 2007 was a prospective, double‐blind, randomised controlled trial comparing trazodone and an unspecified placebo. Trazodone is a triazolopyridine derivative classified and currently used as an antidepressive agent. It is chemically unrelated to tricyclics and other known antidepressant agents. It acts as a serotonin uptake inhibitor, and has been shown to be effective in patients with major depressive disorders and other subsets, such as bipolar disorder. It is also useful in depressive disorders associated with insomnia and anxiety. In this study 85 participants aged between 45 and 80 years were recruited. Forty‐three received trazodone as 50 mg tablets once daily and 42 received placebo tablets at an identical schedule, for 60 days. Baseline audiometric testing was performed and the patients were placed into groups of normal hearing and mild or moderate sensorineural hearing loss, though the audiological criteria for that allocation is not explained. No pre‐trial assessment of psychological status was performed. Dispensing of the drug was described as double‐blind as only the pharmacist knew if drug or placebo was administered to patients. Main outcome measures were analogue scale scores of tinnitus intensity, the level of discomfort caused by tinnitus and the impact on the patient's quality of life. Measures were taken before and at the end of treatment.
Risk of bias in included studies
Two review authors subjected all six included trials to a critical review of their methodology and graded them for their overall methodological quality according to the stated criteria. Trial quality was generally low, as only one trial described adequate methods of randomisation, allocation concealment and blinding (Mihail 1988), and two of the trials had large losses to follow‐up (Mihail 1988; Sullivan 1993). The questionnaires used were also of generally low quality, with the exception of Sullivan 1993 and Robinson 2005.
Robinson 2005: overall grade A
This was a well‐designed study, with a good adherence to its methodological protocol. The randomisation process was not clear in the report but the lead author was contacted and confirmed that randomisation had been performed by computer in an external office (the hospital pharmacy). The characteristics of the paroxetine and placebo arms were well‐balanced. The patients were almost all non‐depressed, with only one who had typical major symptoms of depression. This patient did not have any other psychotic disorder and was therefore included in the trial. The audiometric measures were administrated in an appropriate manner, although measuring the tinnitus sensation level is a highly subjective test, and is not considered as a relevant primary outcome by many audiologists. Tinnitus disability was measured using the Tinnitus Handicap Questionnaire which is a well‐constructed, validated tool (Kuk 1990). The psychological questionnaires and scales were also well known and validated, as were the measures of general disability and well‐being. The drop‐out number was relatively high compared to the total randomised (26/120), although only 5/26 had complete lack of data necessitating exclusion from the analysis. The other 21 did not complete the trial protocol, mainly due to treatment‐related adverse effects, but were entered into the intention‐to‐treat analysis which was therefore based on a total of 115 patients. The authors replied to the review authors' questions about the nature of the placebo (dibasic calcium phosphate, per clinical supply) and supplied specific response data about the subgroup that reached the highest paroxetine dose (50 mg daily) (Robinson 2005).
Sullivan 1993: overall grade B
Although no method of randomisation or allocation concealment was described, the main author confirmed by e‐mail that participants, providers and investigators were blinded. However, blood samples were taken from the active treatment group to allow titration of the serum drug level. It is not clear whether the control group also had blood samples taken. If they did not this would bring the blinding of the study into question. There was a large drop‐out rate (21%), but the main questionnaires used were both relevant and standardised (Sullivan 1993).
Mihail 1988: overall grade C
In this cross‐over, double‐blind trial 26 participants acted as their own control. As no method of randomisation or allocation concealment was given in the report, the first author was contacted and completed a form in which randomisation by number table was confirmed, as was adequate allocation concealment (by office manager) and blinding (of participants, investigators and outcome assessors). However, study quality was reduced by the large number of participants who dropped out (seven patients = 27%) owing to lack of compliance. Also, despite having stated that an appropriate standardised questionnaire was to be used (based on Meikle 1984), a far less appropriate seven‐point scale questionnaire was actually used (based on Miller 1956) (Mihail 1988).
Podoshin 1995: overall grade C
This was a randomised controlled trial, but neither the method of randomisation nor allocation concealment were described, and there is no mention of blinding. The methodological quality was further reduced by the fact that the questionnaire used was not a standardised or recognised tinnitus questionnaire. The drop‐out rate was, however, reasonably low (seven patients in the amitriptyline group (9.2%) owing to adverse events). Attempts to contact the authors for more information were unsuccessful (Podoshin 1995).
Bayar 2001: overall grade C
This single‐blind, randomised trial fails to report a method either of randomisation or allocation concealment. The authors also fail to state if any patients dropped out of the trial, and an attempt to contact them was unsuccessful. This, combined with the non‐blinding of the trial investigators, greatly reduced trial quality. A questionnaire based on one devised by the American Tinnitus Association (ATA) was used to assess tinnitus disability (Bayar 2001).
Dib 2007: overall grade C
Although the authors stated that the trial was randomised and only the pharmacist knew what preparation was being given to the patients until their final clinical evaluation, neither the specific method of randomisation nor the allocation concealment technique were described. The placebo and active drug tablets were similar rather than identical as they had numerical markings: '24' for placebo and '23' for active drug. A potential weakness of this trial is the use of single analogue scale measurements at the start and end of the study as primary outcome measures. Repeated analogue scale measurements or use of standardised tinnitus and mental health questionnaires would have been preferable. No mention is made of whether any patients dropped out of the trial. Attempts to contact the lead author for further information were unsuccessful.
Effects of interventions
Evaluation of paroxetine
Robinson 2005
The outcome measures in this trial were audiometric, psychological and relative to general disability and quality of life. Ninety‐four out of 120 originally randomised patients completed the trial. Twenty‐six dropped out, mainly because of adverse effects. For this reason 115 were included in the intention‐to‐treat analysis, 57 from the paroxetine group and 58 from the placebo group. There were no significant differences in baseline characteristics and scores of patients in the two groups, either in the demographic or tinnitus variables.
Primary outcome measure: positive change in tinnitus disability
The authors found that paroxetine was not statistically superior to placebo in their primary and almost all of the secondary outcomes. Based on the data available from this trial, measures of tinnitus sensation level, psychological distress and general well‐being showed no significant variation between paroxetine and placebo groups. Hence they found no evidence to recommend paroxetine as a routine treatment for all non‐depressed tinnitus patients. However, they performed a post hoc analysis and found that in the subgroup that reached the maximal paroxetine dose (50 mg daily), some results were statistically superior for paroxetine compared to placebo. These results were: in the Tinnitus Handicap Questionnaire (THQ), Subscale 2, (F(1.76) = 8.36, P = 0.05); in two self reported questions: "How severe is your tinnitus?" (F(1.39) = 5.46, P = 0.025) and "How bothered are you from tinnitus?" (F(1.40) = 4.82, P = 0.034), and in one question reported to the audiologist: "How aggravated are you by your tinnitus?" (F(1.55) = 8.01, P = 0.006). For this reason, further studies are needed to investigate both the optimal paroxetine dose and the patient characteristics that are likely to achieve valid benefits by paroxetine therapy.
In the original paper, "perceived" tinnitus sensation level, evaluated by matching reported tinnitus to externally presented sounds, was chosen as one of the primary outcome measures. "Response" was defined in the original trial protocol as a 10 dB drop in perceived tinnitus volume. However, given the practical difficulty of accurately performing this test, and the lack of correlation between tinnitus level and tinnitus distress (Fowler 1942), it is questionable whether tinnitus sensation level should have been used as a primary outcome measure. For the purposes of this review, the more robust measure to be considered is probably the Tinnitus Handicap Questionnaire (Kuk 1990); this showed no overall benefit of paroxetine over placebo.
Secondary outcome measures
Improvement in tinnitus perception, loudness or intensity. Using audiological measures, the pure‐tone average was calculated pre‐ and post‐treatment for both ears in the placebo and paroxetine‐treated groups. The differences in hearing thresholds in the two groups of patients were not clinically significant. A statistically significant result was obtained in the 10 dB drop‐out measure by participants who reached the highest dose of 50 mg paroxetine (34.8% in the paroxetine group versus 12.0% in the placebo group, χ2 (1, n = 73) = 5.28, P = 0.027). Additionally, there was no significant worsening of tinnitus in those who received 50 mg of paroxetine versus those who did not reach the highest dose.
Improvement/change in depressive symptoms or in depression scores: depressive symptoms were assessed using the Hamilton Rating Scale of Depression (Hamilton 1960), 17‐item version, and the Beck Anxiety Inventory (Beck 1961). Changes in depressive symptoms showed unexpected results, inversely correlated with changes in tinnitus severity, but this was not estimated to be clinically significant, in consideration of the very low depressive or anxiety symptomatology of patients at baseline evaluation (only one patient met the criteria for major depressive episode). So there was, in practice, very little room for improvement (Robinson 2005).
Evaluation of tricyclic antidepressants
The authors of each of the trials that used tricyclic antidepressants used their results to suggest that there is a slight improvement in tinnitus with the use of these drugs. However, the quality of the studies was generally low, with heterogeneity in the presentation of data and results, and with short periods of follow‐up.
Sullivan 1993
Data were available for the 92 participants (49 in the treatment group; 43 in the placebo group) who completed the study (21% loss to follow‐up). Data were presented in one instance as percentages, and otherwise graphically or as adjusted group means (plus standard deviations).
Primary outcome measure: positive change in tinnitus disability
On trial completion, participants' overall satisfaction with the treatment that they had received was assessed via four ad hoc questions rather than by using the six standardised questionnaires (adapted for tinnitus) described in the study's methods section. To the first of these ad hoc questions, "Has the medication helped you?", 33% (16/49) of participants in the amitriptyline group stated that they had experienced no overall improvement in their tinnitus compared with 61% (26/43) in the placebo group. The authors fail to present the results for the next two questions ("Has your tinnitus improved?" and "Do you wish to continue the medication you are taking?"), stating only that the results "revealed no significant drug versus placebo differences for the sample considered as a whole" (p. 2256). (Results for the final question, "Do you wish further palliative treatment for your tinnitus?" are given as 36% of the treatment group and 57% of the placebo group requiring additional tinnitus treatment, however the question does not address any of this review's outcome measures).
Tinnitus disability at trial completion was also measured by the six (adapted) standardised questionnaires with results presented as before and after scores ("adjusted for baseline levels, gender, and lifetime history of mean group scores (plus standard deviations)"). From these results, the authors again reported "a significant drug treatment effect", but stated that this was "due primarily to differences on the self‐reported Multidimensional Pain Inventory (MPI) (Kerns 1985) tinnitus interference scale (F(1.54) = 9.42, P < 0.003), and the internal VAS [visual analogue scale] (F(1.54) = 4.25, P < 0.04). However, on all scales . . . the nortriptyline group had nonsignificantly lower disability scores at follow‐up than the placebo group." (p. 2255). In conclusion, the authors state that nortriptyline was demonstrated to decrease tinnitus disability significantly more than placebo for participants considered as a whole, but only the MPI tinnitus interference and internal disability VAS results were statistically significant.
Secondary outcome measures
Improvement in tinnitus perception, loudness or intensity: audiological evaluations included pure‐tone hearing thresholds, tinnitus matching, determination of the most comfortable and just uncomfortable loudness levels, and dynamic range. Of these, a significant treatment effect was shown in the matching of tinnitus loudness, with the treatment group matching their tinnitus to an external source of the same frequency at a level > 5 dB quieter than the placebo group. However, according to Figure 4 of the original paper, when this result is broken down by depression status (i.e. into major depression and depression‐NOS groups), tinnitus loudness only decreased in the major depression treatment group, and actually increased in the depression‐NOS treatment group. Participants with dynamic range greater than 76 dB were more likely to report that their tinnitus improved (χ2 = 4.32, P < 0.04).
Improvement/change in depressive symptoms or in depression scores: follow‐up depression severity was measured using the Hamilton Depression Scale, with mean group scores (adjusted for baseline depression assessment) as follows: nortriptyline group 10.6 ± 9.0 and placebo group 14.3 ± 10.0. Following an analysis of covariance, the authors concluded that the results indicated that participants receiving nortriptyline were significantly less depressed at follow‐up than those receiving placebo (F(1.84) = 5.27, P < 0.02). However, a separate analysis by depression status (i.e. by the major depression or depression‐NOS groups) showed no significant depression status effect.
From their assessment of the data, Sullivan et al concluded that the antidepressant nortriptyline decreases depression, functional disability and tinnitus loudness associated with severe chronic tinnitus and is superior to placebo. However, they also state that, "The drug effect was significant for the subjects considered as a whole but not significant in either the major depression or the depression‐NOS groups considered separately", explaining this by the fact that "despite randomisation to treatment, both nortriptyline groups were significantly more depressed [than both placebo groups] . . . [and that] the depression‐NOS group had substantial depressive symptoms . . . . [which] increased the likelihood of showing a drug effect in the combined group" (pp. 2256‐7) (Sullivan 1993).
Mihail 1988
Primary outcome measure: positive change in tinnitus disability
After 16 weeks one participant reported complete disappearance of tinnitus in the treatment group, eight reported an improvement, seven felt subjectively worse and three reported no change (in total 10/19 not improved), while in the placebo group, eight felt their symptoms improved, four felt they had worsened and seven reported no change (in total 11/19 not improved). It was concluded that there was no statistically significant benefit from treatment with trimipramine. In addition there was evidence of a strong placebo effect.
Secondary outcomes measures
Improvement in tinnitus perception, loudness or intensity: results were found to be statistically significant for the mean frequency of tinnitus matching at six weeks (4300 Hz versus 4800 Hz in the treatment group, 5800 Hz versus 6000 Hz in the placebo group). Other results reported in the original text are not of sufficient quality and/or clarity to be presented here.
Improvement/change in depressive symptoms or in depression scores: results from these assessments were not reported sufficiently clearly to be presented here (Mihail 1988).
Podoshin 1995
Results were presented graphically and by percentages, which made it impossible to obtain accurate data. Adverse effects (excessive sedation) led seven participants in the amitriptyline group to discontinue treatment.
Primary outcome measure: positive change in tinnitus disability
At the end of the 10‐week study period, 64 of the remaining 76 participants (84%) in the amitriptyline group reported no improvement in tinnitus during activity, and 55 (73%) reported no improvement at rest (86% and 75% respectively by intention‐to‐treat). In the corresponding placebo group, approximately 38 out of the 40 participants (95%) reported no improvement during both activity and rest.
Secondary outcome measures
Improvement in tinnitus perception, loudness or intensity: tinnitus was evaluated by comparing its pitch and intensity to tonal stimuli of audiometers, through a matching technique. However, no results from these tests are presented in the text.
Improvement/change in depressive symptoms or in depression scores: this outcome was not addressed by the trial (Podoshin 1995).
Bayar 2001
Primary outcome measure: positive change in tinnitus disability
Data were available on the percentage of participants with a reduction in subjective tinnitus at six weeks. Data are given for right and left ears of both treatment and control groups, with the authors finding a statistically significant improvement in both ears in the treatment group. No losses to follow‐up were declared, although it would appear that there was at least one drop‐out from the treatment group.
Secondary outcome measures
Improvement in tinnitus perception, loudness or intensity: the authors found that the conventional and high‐frequency audiometric tests, the acoustic impedance tests and the auditory brainstem response (ABR) results did not demonstrate any significant change, but that improvement in tinnitus intensity level did occur. This change was statistically significant in both right and left ears in the amitriptyline group, and in the left ear in the placebo group (P < 0.05).
Improvement/change in depressive symptoms or in depression scores: no evaluation of depressive symptoms was made in this trial either prior to treatment or after treatment. The authors asserted that participants were free from depression at enrolment but this statement is unsubstantiated.
The authors concluded that although amitriptyline did not significantly modify the audiological results, the treatment was effective in improving the overall global negative impact of tinnitus (Bayar 2001).
Evaluation of trazodone
Dib 2007
This study analysed tinnitus intensity, level of discomfort and life quality impact caused by tinnitus, by means of analogue scales scored from 0 to 10, administered to the patients before and after the treatment with trazodone or placebo. There were no significant differences in baseline characteristics of patients in the two groups, either in the demographic or tinnitus variables. Statistical methods used for each outcome appeared valid.
Primary outcome measures
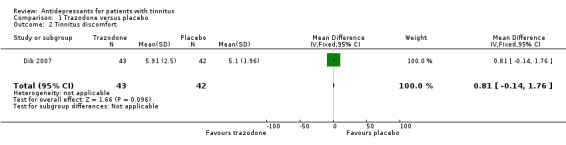
Results were presented in tabular form and both active and placebo groups showed modest improvement in tinnitus intensity, the level of discomfort caused by tinnitus and the impact on the patient's quality of life during the course of the trial. There was no statistical difference between the group receiving the active drug and those in the placebo arm. Interestingly, however, although statistical significance was not attained, those receiving placebo consistently showed greater improvement than those receiving trazodone. For example, those receiving trazodone reduced their level of discomfort score from a mean of 6.56 to 5.91 which is a 9.9% improvement: those receiving placebo showed a drop from 6.02 to 5.10, equating to an improvement of 15.3%.
Secondary outcome measures
The data analysis was repeated using hearing level, gender and age as variables. Outcome was not affected by whether the participants had normal hearing or mild or moderate hearing losses. Similarly the outcome was unaffected by the patient's gender. Looking at the age of the participants showed that patients aged 60 or more demonstrated greater improvement than those aged less than 60 years. This divergence, however, was almost entirely due to differences in the placebo arm: those on trazodone showed negligible changes related to their age.
Patients were also asked about other improvements that they had noted while receiving treatment. Those receiving trazodone described improvement in sleep (14%) and irritability reduction (4.7%). Those in the placebo arm reported more modest improvement in sleep (4.8%).
Side effects were relatively common but generally mild: 16.3% of the trazodone group and 7.1% of the placebo group reported a side effect. The most commonly reported side effect was sleepiness. Dib et al concluded that both trazodone and placebo groups showed slight improvement in tinnitus intensity and in the quality of life after treatment, but there was no statistical difference between the groups.
Discussion
There is no high‐quality evidence on the effect of tricyclic antidepressant drugs in the treatment of tinnitus. The trial evaluating the selective serotonin reuptake inhibitor (SSRI) paroxetine was considered to have grade A overall quality (Robinson 2005). Only one study evaluating the tricyclics was scored overall quality grade B (Sullivan 1993). We graded the other four trials C (Bayar 2001; Dib 2007; Mihail 1988; Podoshin 1995). Consequently different trials could not be combined for a summary of effect. The single trial that investigated a SSRI drug combined the highest level of outcome measures and a low risk of methodological bias (Robinson 2005).
The quality of the studies included in this review was generally low and they presented methodological heterogeneity, including the use of very different strategies to evaluate tinnitus and in the presentation of data and results. All the studies used several different measures for both audiological and psychological severity of tinnitus disturbance. Some studies reported partial baseline (beginning of trial) and end of follow‐up period data. All of the studies used different types of scales, questionnaires and scores. A major flaw was the use of non‐validated tinnitus handicap or distress measures in the three grade C trials.
There were several difficulties in assessing these studies as a set, including the use of different antidepressant drugs, the use of very different strategies to evaluate tinnitus characteristics, the relatively high number of drop‐outs and the very short‐term follow‐up periods. The protocol for treatment of acute depression with tricyclic drugs is that at least four weeks treatment is required and maintenance therapy can take from four to 12 months after remission of acute symptoms: the follow‐up period of the included studies was as low as four weeks and did not exceed 12 weeks. Only one of the trials (Robinson 2005) explicitly described an intention‐to‐treat analysis in their methods. The other trials run the consequent risk of overestimation of effects. Placebos used in five trials were either inert or not defined; in one trial (Dib 2007) placebo was determined to be in tablets, equal in shape and colour. Side effects of tricyclic and SSRI antidepressants are prominent and therefore the blinding of all the trials must be questioned.
One of the major questions regarding the use of antidepressants in the treatment of tinnitus patients is whether any beneficial effect is a primary effect on the tinnitus or a secondary effect due to control of concomitant depression. Among the studies included in this review only one (Sullivan 1993) analysed patients in different groups on the basis of depression status. In this study, a subgroup analysis for the major depression group (n = 38) and depression‐NOS group (n = 54) was performed by the authors, who state: "When major depression and depressive symptoms groups were considered separately, nortriptyline was superior to placebo on these same [tinnitus‐related disability and tinnitus loudness] measures, but differences did not achieve statistical significance" (p. 2251). Another study (Robinson 2005) was designed to measure differences between patients with and without depression. However, only one patient met the criteria for major depressive episode (as defined in DSM‐IV 1994) and it was therefore impossible to create meaningful subgroups. This study did examine whether any improvement in tinnitus was associated with improvement in depression. A negative correlation was discovered which is at variance with previous studies. It was suggested that these findings might have been attributed to the fact that depression/anxiety levels were very low in the patients who participated in the study and therefore there was no possibility of further improvement.
Authors' conclusions
Implications for practice.
There is no evidence that tricyclic antidepressants are effective or ineffective in the management of tinnitus. A solitary well‐constructed trial of a selective serotonin reuptake inhibitor (SSRI) showed no benefit for most outcome measures. The large drop‐out rate seen in some of the trials reflects the significant side effects produced by antidepressant drugs.
Implications for research.
A beneficial effect was seen in Robinson 2005 in the subgroup that received the maximal dose of the SSRI drug, which merits further investigation. Prospective, well‐designed, randomised trials of tricyclic antidepressant drugs and further trials of SSRIs are needed, in which general improvement in tinnitus disability is the primary outcome. The length of follow‐up must also be longer than that reported in the studies included in this review (preferably more than 12 months), with results monitored at regular intervals, to indicate the optimum duration of drug treatment. Future trials should have adequate sample sizes and use validated and standardised questionnaires to measure physical/auditory symptoms and psychological/depression status at baseline and at each follow‐up period. Participants recruited to trials should not have used antidepressants previously. The use of active placebos should be considered.
What's new
| Date | Event | Description |
|---|---|---|
| 13 June 2012 | New search has been performed | New searches run January 2012. |
| 13 June 2012 | New citation required but conclusions have not changed | We identified no new studies which met the inclusion criteria for the review following updated searches in January 2012. We excluded three further studies (Holgers 2011; Jalali 2009; Roberts 2011). There are therefore no changes to the conclusions of the review. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 10 August 2009 | New search has been performed | New searches were run in October 2008. One new study was included in the review (Dib 2007) and two further studies were excluded. There were no changes to the conclusions of the review. |
| 17 August 2006 | Amended | Converted to new review format. |
Acknowledgements
Many thanks to Jenny Bellorini for assisting the review author PB since his first approach to The Cochrane Collaboration.
Appendices
Appendix 1. Search strategies
| CENTRAL | PubMed | EMBASE (Dialog DataStar) | CINAHL (EBSCO) |
| 1. TINNITUS (MeSH term ‐ explode all trees) 2. tinnit* 3. (ear* near buzz*) or (ear* near ring*) or (ear* near roar*) or (ear* near click*) or (ear* near puls*) 4. 1 OR 2 OR 3 5. DEPRESSION (MeSH term) 6. depress* 7. 5 OR 6 8. DRUG THERAPY (MeSH term ‐ explode all trees) 9. PHARMACEUTICAL PREPARATIONS (MeSH term ‐ explode all trees) 10. PSYCHOPHARMACOLOGY (MeSH term ‐ explode all trees) 11. drug* 12. 8 OR 9 OR 10 OR 11 13. 7 AND 12 14. ANTIDEPRESSIVE AGENTS (MeSH term) 15. antidepress* 16. anti‐depress* OR anti depress* 17. ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN 18. DOXEPIN OR DULOXETINE OR ESCITALOPRAM OR FEMOXETINE OR FLESINOXAN OR FLUOXETINE OR FLUOXETIN* OR FLUVOXAMINE OR FLUVOXAMIN* OR GEPIRONE OR IMIPRAMINE OR IPSAPIRONE OR ISOCARBOXAZID* 19. LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE 20. REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE 21. 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 22. 13 OR 21 23. 4 AND 22 | #1 "Tinnitus"[Mesh] OR tinnit* #2 ((ear OR ears) AND (buzz* OR ring* OR roar* OR puls* OR click*)) #3 #1 OR #2 #4 "Depression" [Mesh] #5 depress* #6 #4 OR #5 #7 "Drug Therapy"[Mesh] #8 "PHARMACEUTICAL PREPARATIONS" [Mesh] #9 "PSYCHOPHARMACOLOGY" [Mesh] #10 drug* #11 #7 OR #8 OR #9 OR #10 #12 #6 AND #11 #13 "ANTIDEPRESSIVE AGENTS" [Mesh] #14 (ANTIDEPRESS* OR (SELECTIVE AND SEROTONIN AND REUPTAKE AND INHIBITOR*)) #15 (anti AND depress*) #16 ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN #17 LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE #18 REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE #19 #13 OR #14 OR #15 OR #16 OR #17 OR #18 #20 #12 OR #19 #21 #3 AND #20 | 1. TINNITUS.DE. 2. (TINNITUS OR TINNITIS).TI,AB. 3. (EAR$1 NEAR (BUZZ$4 OR RING$4 OR ROAR$4 OR CLICK$4 OR PULSE$1 OR PULSAT$3)).TI,AB. 4. 1 OR 2 OR 3 5. ANTIDEPRESSANT‐AGENT#.DE. 6. (ANTIDEPRESS$5 OR ANTI ADJ DEPRESS$5).TI,AB. 7. DEPRESSION‐DT.DE. 8. CLINICA‐PHARMACOLOGY#.DE. 9. DRUG‐THERAPY#.DE. 10. DRUG‐COMPARISON#.DE. 11. DEPRESS$4 AND DRUG$1.TI,AB. 12. ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN 13. LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN$1 OR PHENELZINE OR PROTRIPTYLINE 14. REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN$1 OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE 15. 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 16. 4 AND 15 | S1 (MH "Tinnitus") S2 TX tinnit* S3 S1 or S2 S4 (MH "Antidepressive Agents+") S5 TX ((ANTIDEPRESS* OR (SELECTIVE AND SEROTONIN AND REUPTAKE AND INHIBITOR*))) S6 TX ((anti AND depress*)) S7 TX (ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN) S8 TX (LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE) S9 TX (REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE) S10 S4 or S5 or S6 or S7 or S8 or S9 S11 S3 and S10 |
| Cochrane ENT Disorders Group Trials Register (ProCite database) | ISI Web of Science/BIOSIS Previews (Web of Knowledge) | CAB Abstracts (Ovid) | ICTRP |
| (tinnit* AND (antidepress* OR depress* OR serotonin OR ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN OR LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE OR REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE)) | #1 TS=tinnit* #2 TS=(ear AND (BUZZ* OR RING* OR ROAR* OR CLICK* OR PULSE* OR PULSAT*) #3 #2 OR #1 #4 TS=(ANTIDEPRESS* OR (SELECTIVE AND SEROTONIN AND REUPTAKE AND INHIBITOR*)) #5 TS=(anti AND depress*) #6 TS=(REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE OR LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE OR ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN #7 #6 OR #5 OR #4 #8 #7 AND #3 | #1 tinnit*.tw. #2 (antidepress* OR depress* OR serotonin OR ADINAZOLAM OR AMINEPTINE OR AMISULPIRIDE OR AMITRIPTYLINE OR AMOXAPINE OR ATOMOXETINE OR BUPROPION OR BUSPIRONE OR CITALOPRAM OR CLOMIPRAMINE OR CLORGYLINE OR CLOVOXAMINE OR DESIPRAMINE OR DIBENZEPIN OR DOTHIEPIN OR LEVOSULPIRIDE OR LITHIUM OR LOFEPRAMINE OR MAPROTILINE OR MELITRACEN OR MIANSERIN OR MILNACIPRAN OR MIRTAZAPINE OR MOCLOBEMIDE OR NEFAZODONE OR NOMIFENSINE OR NORTRIPTYLINE OR OPIPRAMOL OR PAROXETINE OR PAROXETIN* OR PHENELZINE OR PROTRIPTYLINE OR REBOXETINE OR RITANSERIN OR ROLIPRAM OR SELEGILINE OR SERTRALINE OR SERTRALIN* OR SIBUTRAMINE OR SULPIRIDE OR TANDOSPIRONE OR TIANEPTINE OR TRANYLCYPROMINE OR TRAZODONE OR TRIMIPRAMINE OR TRYPTOPHAN OR TRICYCLIC OR VENLAFAXINE OR VILOXAZINE OR ZIMELDINE)).tw. #3 1 and 2 |
tinnitus |
Data and analyses
Comparison 1. Trazodone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tinnitus intensity | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.70, 1.18] |
| 2 Tinnitus discomfort | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐0.14, 1.76] |
1.1. Analysis.
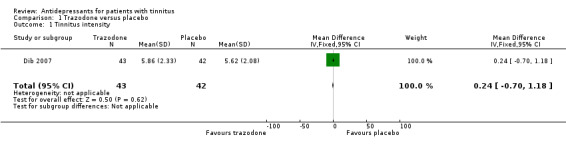
Comparison 1 Trazodone versus placebo, Outcome 1 Tinnitus intensity.
1.2. Analysis.
Comparison 1 Trazodone versus placebo, Outcome 2 Tinnitus discomfort.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayar 2001.
| Methods | Randomised, parallel, single‐blind trial | |
| Participants | 37 adults with subjective tinnitus (20 in treatment group, 17 in placebo group) | |
| Interventions | Administration of amitriptyline at scalar doses (50 mg to 100 mg, at night); placebo, 1 tablet at night Duration: 6 weeks |
|
| Outcomes | Severity and intensity of tinnitus (by audiologic and audiometric measures); tinnitus severity (by ATA questionnaire) ‐ at 6 weeks | |
| Notes | The randomisation method was not described Overall methodological quality: C |
|
Dib 2007.
| Methods | Randomised, parallel, double‐blind trial | |
| Participants | 85 patients presenting with tinnitus, aged between 45 and 80 years | |
| Interventions | Administration of either trazodone (50 mg daily) or placebo in tablets similar in shape, colour and size | |
| Outcomes | Tinnitus intensity level, tinnitus level of discomfort, tinnitus impact on quality of life; all by using analogue scales scoring between 0 to 10 | |
| Notes | Method of randomisation not described, although the authors declare that blinding of the medications was guaranteed by the hospital pharmacist until the end of the study | |
Mihail 1988.
| Methods | Randomised, double‐blind, cross‐over trial with patients as their own control | |
| Participants | 26 participants presenting with subjective tinnitus | |
| Interventions | Administration of either trimipramine (150 mg daily) or placebo Duration: 6‐week treatment, followed by 4‐week rest period, followed by a further 6‐week test period |
|
| Outcomes | Tinnitus severity (Meikle questionnaire) Tinnitus frequency and intensity (by audiometry) Tinnitus masking levels At 16 weeks |
|
| Notes | Overall methodological quality: C | |
Podoshin 1995.
| Methods | 4‐arm, randomised controlled trial No detail on randomisation method is given |
|
| Participants | 225 adults (in total) with idiopathic subjective tinnitus (83 in the amitriptyline group, 40 in control (placebo) group) | |
| Interventions | Administration of amitriptyline (10 mg 3 times daily) or placebo (1 tablet 3 times daily) for 10 weeks | |
| Outcomes | Improvement in degree of tinnitus at rest and during activity (by questionnaire and audiometry) at 10 weeks | |
| Notes | Only 2 of the 4 treatment arms (amitriptyline and placebo) were relevant to this review. The 2 other treatment arms were biofeedback (62 participants) versus control (40 participants) Results were mainly presented graphically Overall methodological quality: C |
|
Robinson 2005.
| Methods | Randomised, double‐blind, controlled trial | |
| Participants | 120 adult patients with chronic tinnitus of more than 6 months duration | |
| Interventions | Administration of paroxetine, 10 mg daily, increased to maximum of 50 mg daily Duration 100 days |
|
| Outcomes | Severity of tinnitus (audiometric measures) Evaluation of tinnitus disability (Tinnitus Handicap Questionnaire) Evaluation of general well‐being or disability (various questionnaires) Level of depression (various scales) |
|
| Notes | 26 patients dropped out of the study but only 5 of these were excluded from the analysis Overall methodological quality: A |
|
Sullivan 1993.
| Methods | Randomised, double‐blind, controlled trial No other information on method of randomisation or allocation concealment is given |
|
| Participants | 117 patients with chronic severe tinnitus of more than 6 months duration | |
| Interventions | Administration of nortriptyline (scalar doses) versus placebo (same number of capsules, average 4) Duration: 6 weeks |
|
| Outcomes | Severity of tinnitus (audiometric measures) Level of depression (Hamilton Depression Scale) Evaluation of tinnitus disability (various scales) at 6 weeks |
|
| Notes | Results are presented only for the 92 patients who completed the trial The same data are also reported in Dobie 1992 and Dobie 1993 (see table Characteristics of excluded studies) Overall methodological quality: B |
|
ATA = American Tinnitus Association
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dobie 1992 | ALLOCATION:
Randomised PARTICIPANTS: Same setting and number of patients (N = 92) as in a series of papers where the most definitive trial is the one included in this review (Sullivan 1993). This originates an explicit replication bias. |
| Dobie 1993 | ALLOCATION:
Randomised PARTICIPANTS: Same setting and number of patients (N = 92) as in a series of papers where the most definitive trial is the one included in this review (Sullivan 1993). This originates an explicit replication bias. |
| Holgers 2011 | ALLOCATION: Unclear (not declared) PARTICIPANTS: 75 patients meeting risk criteria for developing severe refractory tinnitus, without socially disabling hearing loss INTERVENTION: This study evaluated the same patient groups as the Zöger 2006 trial, which was already evaluated and excluded in the previous 2009 update of the present review. In the paper published in 2011 the follow‐up time was extended to 28 weeks and the authors focus specifically on evaluation of quality of life. Although this trial aimed to study sertraline, a benzodiazepine drug (oxazepam) was also administered to 9 out of the total 75 patients. Oxazepam was administered in the first 2 weeks to alleviate expected worsening of distress and to improve tolerability of the adverse side effects of sertraline (cited in the paper as "well known side effects of sertraline"). Being a benzodiazepine (a drug acting in the central nervous system) this may have an effect on tinnitus and represents a potential source of bias. OUTCOMES: High drop‐out rate with a relevant difference between the active drug group (40.5% drop‐outs after 28 weeks) and placebo group (10.5% in total after 28 weeks) |
| Jalali 2009 | ALLOCATION:
Cross‐over, randomised, triple‐blind, placebo‐controlled trial PARTICIPANTS: 36 patients between ages 21 and 65, with a complaint of non‐pulsatile tinnitus of more than 1 year duration. INTERVENTION: The study evaluated the effects of alprazolam on tinnitus. Although alprazolam is sometimes prescribed to treat depression, it is not actually classified as an antidepressive agent. Furthermore, patients in this trial were treated with an "active placebo" (chlorpheniramine maleate) to simulate the side effect of the active treatment (drowsiness). Although the authors say that it is "popularly believed that mild induced sedation (like that expected from chlorfeniramine) does not relieve tinnitus", this can originate strongly biased results (sleep disorders are one of most serious problems in tinnitus patients). |
| Katon 1993 | ALLOCATION:
Randomised PARTICIPANTS: Same setting and number of patients (N = 92) as in a series of papers where the most definitive trial is the one included in this review (Sullivan 1993). This originates an explicit replication bias. |
| Lopez‐Gonzalez 2007a | ALLOCATION:
Prospective, randomised, single‐blind, placebo‐controlled study PARTICIPANTS: 150 patients with subjective tinnitus INTERVENTION: The interventions included a combination of drugs: although sulpiride is sometimes used for its antidepressive efficacy, hydroxyzine is not actually classified as an antidepressive agent |
| Lopez‐Gonzalez 2007b | ALLOCATION:
Not randomised ("Un total de 100 pacientes diagnosticados de acùfenos se dividieron aleatoriamente a su llegada a la consulta en dos grupos de 50") PARTICIPANTS: 100 patients with tinnitus ("acufenos") INTERVENTION: The interventions included a combination of drugs: although sulpiride is sometimes used for its antidepressive efficacy, melatonin is not actually classified as an antidepressive agent |
| Roberts 2011 | ALLOCATION:
Randomised controlled trial PARTICIPANTS: 24 adult patients with tinnitus INTERVENTION: This trial is not placebo‐controlled, therefore it is excluded from the review. The trial compared vestipitant versus paroxetine in the treatment of tinnitus. There is no established evidence that the substance P antagonists/NK‐1 receptor antagonists, to which the drug vestipitant belongs, are effective antidepressants. Most of the drugs in the same class are (or have been tested as) antiemetics. The report also lacks a description of the dose (dosage) of the drugs investigated in the trial. There is only a general reference to "predose" or "postdose". This is an important criticism, because we cannot know the precise treatment and the trial cannot be 'replicated'. |
| Sullivan 1989 | ALLOCATION: Not randomised |
| Sullivan 1994 | ALLOCATION:
Randomisation not explicit. There is no mention of randomisation or allocation method, although the context and the recruited patients were the same as Sullivan 1993. PARTICIPANTS: Same setting and number of patients (N = 92) as in a series of papers where the most definitive trial is the one included in this review (Sullivan 1993). This originates an explicit replication bias. |
| Zöger 2006 | ALLOCATION:
Randomised PARTICIPANTS: 76 patients with tinnitus were investigated with measures of tinnitus severity, depression and anxiety INTERVENTION: Although this trial aimed to study sertraline, a benzodiazepine drug (oxazepam) was also administered to 9 out of the total 76 patients OUTCOMES: High drop‐out rate and if oxazepam‐treated patients are excluded from the analysis, the percentage of patients lost from analysis becomes unacceptably high (31% in treatment group, 26% in placebo group) |
Differences between protocol and review
In the protocol we planned to include tricyclic and SSRI antidepressant agents. At the update of the review in 2008, we also included a new trial which had studied trazodone, an atypical antidepressant drug chemically unrelated to tricyclic antidepressants (Dib 2007).
Contributions of authors
Paolo Baldo: lead author, planning and writing of the protocol, study selection, data extraction and analysis, writing of the review.
Don McFerran: study selection, analysis and interpretation of data, quality assessment, clinical ENT overview, writing of the review.
Carolyn Doree: planning and executing the search strategy, advice about the possibility of performing a meta‐analysis of included studies.
Paola Molin: interpretation of data relative to psychological profiles, quality of life and depression in patients with tinnitus, evaluation of questionnaires and tests performed in the studies.
Sara Cecco: data extraction (review update).
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bayar 2001 {published data only}
- Bayar N, Boke B, Turan E, Belgin E. Efficacy of amitriptyline in the treatment of subjective tinnitus. Journal of Otolaryngology 2001;30(5):300‐3. [DOI] [PubMed] [Google Scholar]
Dib 2007 {published data only}
- Dib GC, Akemi Kasse C, Alives de Andrade T, Gugel Testa JR, Cruz OLM. Tinnitus treatment with trazodone. Brazilian Journal of Otolaryngology 2007;73(3):390‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mihail 1988 {published data only}
- Mihail RC, Crowley JM, Walden BE, Fishburne JF, Reinwall JE, Zajtchuk JT. The tricyclic trimipramine in the treatment of subjective tinnitus. Annals of Otology, Rhinology and Laryngology 1988;97(2 Pt 1):120‐3. [DOI] [PubMed] [Google Scholar]
Podoshin 1995 {published data only}
- Podoshin L, Ben‐David Y, Fradis M, Malatskey S, Hafner H. Idiopathic subjective tinnitus treated by amitriptyline hydrochloride/biofeedback. International Tinnitus Journal 1995;1(1):54‐60. [PubMed] [Google Scholar]
Robinson 2005 {published data only}
- Robinson SK, Viirre ES, Bailey KA, Gerke MA, Harris JP, Stein MB. Randomized placebo‐controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosomatic Medicine 2005;67(6):981‐8. [DOI] [PubMed] [Google Scholar]
Sullivan 1993 {published data only}
- Sullivan M, Katon W, Russo J, Dobie R, Sakai C. A randomized trial of nortriptyline for severe chronic tinnitus. Archives of Internal Medicine 1993;153:2251‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Dobie 1992 {published data only}
- Dobie RA, Sullivan MD, Katon WJ, Sakai CS, Russo J. Antidepressant treatment for tinnitus patients. Acta Otolaryngologica 1992;112:242‐7. [DOI] [PubMed] [Google Scholar]
Dobie 1993 {published data only}
- Dobie RA, Sakai CS, Sullivan MD, Katon WJ, Russo J. Antidepressant treatment of tinnitus patients: report of a randomized clinical trial and clinical prediction of benefit. American Journal of Otology 1993;14(1):18‐23. [PubMed] [Google Scholar]
Holgers 2011 {published data only}
- Holgers K, Zöger S, Svedlund J. The impact of sertraline on health‐related quality of life in severe refractory tinnitus: a double‐blind, randomized, placebo‐controlled study. Audiological Medicine 2011;9:67‐72. [Google Scholar]
Jalali 2009 {published data only}
- Jalali MM, Kousha A, Naghavi SE, Solemani R, Banan R. The effects of alprazolam on tinnitus: a cross‐over randomized clinical trial. Medical Science Monitor 2009;15(11):155‐60. [PubMed] [Google Scholar]
Katon 1993 {published data only}
- Katon W, Sullivan M, Russo J, Dobie R, Sakai C. Depressive symptoms and measures of disability: a prospective study. Journal of Affective Disorders 1993;27:245‐54. [DOI] [PubMed] [Google Scholar]
Lopez‐Gonzalez 2007a {published data only}
- Lopez‐Gonzalez MA, Moliner‐Peiro F, Alfaro‐Garcia J, Esteban‐Ortega F. Sulpiride plus hydroxyzine decrease tinnitus perception. Auris Nasus Larynx 2007;34:23‐7. [DOI] [PubMed] [Google Scholar]
Lopez‐Gonzalez 2007b {published data only}
- Lopez‐Gonzalez MA, Santiago AM, Esteban‐Ortega F. Sulpiride and melatonin decrease tinnitus perception modulating the audiolimbic dopaminergic pathway. Journal of Otolaryngology 2007;36(4):213‐9. [DOI] [PubMed] [Google Scholar]
Roberts 2011 {published data only}
- Roberts C, Inamadar A, Koch A, Kitchiner P, Dewit O, Merlo‐Pich E, et al. A randomized, controlled study comparing the effects of vestipitant or vestipitant and paroxetine combination in subjects with tinnitus. Otology & Neurology 2011;32:721‐7. [DOI] [PubMed] [Google Scholar]
Sullivan 1989 {published data only}
- Sullivan MD, Dobie RA, Sakai CS, Katon WJ. Treatment of depressed tinnitus patients with nortriptyline. Annals of Otology, Rhinology and Laryngology 1989;98(11):867‐72. [DOI] [PubMed] [Google Scholar]
Sullivan 1994 {published data only}
- Sullivan M, Katon W, Russo J, Dobie R, Sakai C. Coping and marital support as correlates of tinnitus disability. General Hospital Psychiatry 1994;16:259‐66. [DOI] [PubMed] [Google Scholar]
Zöger 2006 {published data only}
- Zöger S, Svedlund J, Holgers KM. The effects of sertraline on severe tinnitus suffering ‐ a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Psychopharmacology 2006;26(1):32‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2000
- Anderson TD, Loevner LA, Bigelow DC, Mirza N. Prevalence of unsuspected acoustic neuroma found by magnetic resonance imaging. Otolaryngology ‐ Head and Neck Surgery 2000;122(5):643‐6. [DOI] [PubMed] [Google Scholar]
Andersson 1999
- Andersson G, Lyttkens L. A meta‐analytic review of psychological treatments for tinnitus. British Journal of Audiology 1999;33(4):201‐10. [DOI] [PubMed] [Google Scholar]
ATA 2004
- American Tinnitus Association. http://www.ata.org 2004.
Baguley 1992
- Baguley DM, Moffat DA, Hardy DG. What is the effect of translabyrinthine acoustic schwannoma removal upon tinnitus?. Journal of Laryngology and Otology 1992;106:329‐31. [DOI] [PubMed] [Google Scholar]
Baguley 2006
- Baguley DM. What progress have we made with tinnitus? The Tonndorf Lecture 2005. Acta Oto‐Laryngologica. Supplement 2006;556:4‐8. [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbauch J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Bennett 2007
- Bennett MH, Kertesz T, Yeung P. Hyperbaric oxygen for idiopathic sudden sensorineural hearing loss and tinnitus. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004739.pub3] [DOI] [PubMed] [Google Scholar]
Briner 1995
- Briner W. A behavioral nosology for tinnitus. Psychological Reports 1995;77(1):27‐34. [DOI] [PubMed] [Google Scholar]
Brummett 1980
- Brummett RE. Drug‐induced ototoxicity. Drugs 1980;19(6):412‐28. [DOI] [PubMed] [Google Scholar]
Burton 2012
- Burton H, Wineland A, Bhattacharya M, Nicklaus J, Garcia KS, Piccirillo JF. Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neuroscience 2012; Vol. 13, issue 3:1‐15. [http://www.biomedcentral.com/1471‐2202/13/3] [DOI] [PMC free article] [PubMed]
Dauman 1992
- Dauman R, Tyler RS. Some considerations on the classification of tinnitus. Proceedings of the Fourth International Tinnitus Seminar. Bordeaux, 1992:225‐9.
Davis 2000
- Davis A, Refaie A. Epidemiology of tinnitus. In: Richard Tyler editor(s). Tinnitus Handbook. San Diego: Singular Publishing Group, 2000. [Google Scholar]
Dawes 2000
- Dawes PJ, Metha D, Arullendran P. Screening for vestibular schwannoma: magnetic resonance imaging findings and management. Journal of Laryngology and Otology 2000;114(8):584‐8. [DOI] [PubMed] [Google Scholar]
Del Bo 2008
- Bo L, Forti S, Ambrosetti U, Costanzo S, Mauro D, Ugazio G, et al. Tinnitus aurium in persons with normal hearing: 55 years later. Otolaryngology ‐ Head and Neck Surgery 2008;139(3):391‐4. [DOI] [PubMed] [Google Scholar]
DSM‐IV 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
Eggermont 2006
- Eggermont JJ. Cortical tonotopic map reorganization and its implications for treatment of tinnitus. Acta Oto‐Laryngologica. Supplement 2006;556:9‐12. [DOI] [PubMed] [Google Scholar]
Eichhammer 2007
- Eichhammer P, Hajak G, Kleinjung T, Landgrebe M, Langguth B. Functional imaging of chronic tinnitus: the use of positron emission tomography. Progress in Brain Research 2007;166:83‐8. [DOI] [PubMed] [Google Scholar]
Elgoyhen 2010
- Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discovery Today 2010;15(7‐8):300‐5. [DOI] [PubMed] [Google Scholar]
Elgoyhen 2012
- Elgoyhen AB, Langguth B, Vanneste S, Ridder D. Tinnitus: network pathophysiology‐network pharmacology. Frontiers in Systems Neuroscience 2012;6:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowler 1942
- Fowler EP. The "illusion of loudness" of tinnitus ‐ its etiology and treatment. Laryngoscope 1942;52:275‐85. [Google Scholar]
Fujii 2011
- Fujii K, Nagata C, Nakamura K, Kawachi T, Takatsuka N, Oba S, et al. Prevalence of tinnitus in community‐dwelling Japanese adults. Journal of Epidemiology 2011;21(4):299‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabr 2011
- Gabr TA, El‐Hay MA, Badawy A. Electrophysiological and psychological studies in tinnitus. Auris Nasus Larynx 2011;38(6):678‐83. [DOI] [PubMed] [Google Scholar]
Gersdorff 2000
- Gersdorff M, Nouwen J, Gilain C, Decat M, Betsch C. Tinnitus and otosclerosis. European Archives of Oto‐rhino‐laryngology 2000;257(6):314‐6. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2004
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.2 [updated March 2004]. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Handbook 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Heller 1953
- Heller MF, Bergman M. Tinnitus aurium in normally hearing persons. Annals of Otology, Rhinology and Laryngology 1953;62:73‐83. [DOI] [PubMed] [Google Scholar]
Hilton 2004
- Hilton M, Stuart E. Ginkgo biloba for tinnitus. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003852.pub2] [DOI] [PubMed] [Google Scholar]
Hoare 2011
- Hoare DJ, Kowalkowski BA, Kang S, Hall DA. Systematic review and meta‐analyses of randomized controlled trials examining tinnitus management. Laryngoscope 2011;121:1555‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hobson 2010
- Hobson J, Chisholm E, Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD006371.pub2] [DOI] [PubMed] [Google Scholar]
Hoekstra 2011
- Hoekstra CEL, Rynja SP, Zanten GA, Rovers MM. Anticonvulsants for tinnitus. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007960.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jastreboff 1990
- Jastreboff PJ. Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neuroscience Research 1990;8(4):221‐54. [DOI] [PubMed] [Google Scholar]
Jastreboff 1993
- Jastreboff PJ, Hazell JW. Neurophysiological approach to tinnitus: clinical implications. British Journal of Audiology 1993;27:7‐17. [DOI] [PubMed] [Google Scholar]
Jastreboff 1994
- Jastreboff PJ, Hazell JW, Graham RL. Neurophysiological model of tinnitus: dependence of the minimal masking level on treatment outcome. Hearing Research 1994;80(2):216‐32. [DOI] [PubMed] [Google Scholar]
Jastreboff 2004
- Jastreboff PJ, Hazell JWP. Tinnitus Retraining Therapy. Implementing the Neurophysiological Model. Cambridge: Cambridge University Press, 2004. [Google Scholar]
Jozefowicz 2004
- Jozefowicz‐Korczynska M, Lukomski M, Pajor A. Electronystagmographic evaluation of vestibular system in tinnitus patients with degenerative cervical spine lesions. Otolaryngologia Polska 2004;58(2):349‐53. [PubMed] [Google Scholar]
Kaltenbach 2000
- Kaltenbach JA. Neurophysiologic mechanisms of tinnitus. Journal of the American Academy of Audiology 2000;11(3):125‐37. [PubMed] [Google Scholar]
Kasper 1992
- Kasper S, Fuger J, Moller HJ. Comparative efficacy of antidepressants. Drugs 1992;43(Suppl 2):11‐22 (discussion 22‐3). [DOI] [PubMed] [Google Scholar]
Kerns 1985
- Kerns RD, Turk DC, Rudy TE. The West Haven‐Yale Multidimensional Pain Inventory. Pain 1985;23(4):345‐56. [DOI] [PubMed] [Google Scholar]
Kleinjung 2007
- Kleinjung T, Steffens T, Londero A, Langguth B. Transcranial magnetic stimulation (TMS) for treatment of chronic tinnitus: clinical effects. Progress in Brain Research 2007;166:359‐67. [DOI] [PubMed] [Google Scholar]
Kuk 1990
- Kuk FK, Tyler RS, Russell D, Jordan H. The psychometric properties of a tinnitus handicap questionnaire. Ear and Hearing 1990;11:434‐45. [DOI] [PubMed] [Google Scholar]
Langers 2012
- Langers DRM, Kleine E, Dijk P. Tinnitus does not require macroscopic tonotopic map reorganization. Frontiers in Systems Neuroscience 2012;6:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langguth 2009
- Langguth B, Salvi R, Elgoyhen AB. Emerging pharmacotherapy of tinnitus. Expert Opinion in Emerging Drugs 2009;14(4):687‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanting 2008
- Lanting CP, Kleine E, Bartels H, Dijk P. Functional imaging of unilateral tinnitus using fMRI. Acta Otolaryngologica 2008;128:415‐21. [DOI] [PubMed] [Google Scholar]
Leaver 2011
- Leaver AM, Renier L, Chevillet MA, Morgan S, Kim HJ, Rauschecker JP. Dysregulation of limbic and auditory networks in tinnitus. Neuron 2011;69:33‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levo 2000
- Levo H, Blomstedt G, Pyykko I. Tinnitus and vestibular schwannoma surgery. Acta Oto‐Laryngologica. Supplement 2000;543:28‐9. [DOI] [PubMed] [Google Scholar]
Lockwood 1999
- Lockwood AH, Salvi RJ, Burkard RF, Galantowicz PJ, Coad ML, Wack DS. Neuroanatomy of tinnitus. Scandinavian Audiology. Supplementum 1999;51:47‐52. [PubMed] [Google Scholar]
Lockwood 2002
- Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. New England Journal of Medicine 2002;347(12):904‐10. [DOI] [PubMed] [Google Scholar]
Luxon 1993
- Luxon LM. Tinnitus: its causes, diagnosis and treatment. BMJ 1993;306:1490‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez 2010
- Martinez Devesa P, Waddell A, Perera R, Theodoulou M. Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD005233.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marx 1999
- Marx SV, Langman AW, Crane RC. Accuracy of fast spin echo magnetic resonance imaging in the diagnosis of vestibular schwannoma. American Journal of Otolaryngology 1999;20(4):211‐6. [DOI] [PubMed] [Google Scholar]
McCombe 2001
- McCombe A, Baguley D, Coles R, McKenna L, McKinney C, Windle‐Taylor P. Guidelines for the grading of tinnitus severity: the results of a working group commissioned by the British Association of Otolaryngologists ‐ Head and Neck Surgeons. Clinical Otolaryngology 2001;26:388‐93. [DOI] [PubMed] [Google Scholar]
McFerran 2008
- McFerran DJ, Baguley DM. The efficacy of treatments for depression used in the management of tinnitus. Audiological Medicine 2008;6(1):40‐7. [Google Scholar]
McKenna 1991
- McKenna L, Hallam RS, Hinchcliffe R. The prevalence of psychological disturbance in neuro‐otology outpatients. Clinical Otolaryngology and Allied Sciences 1991;16:452‐6. [DOI] [PubMed] [Google Scholar]
McQuay 1997
- McQuay HJ, Moore RA. Antidepressants and chronic pain. BMJ 1997;314(7083):763‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meikle 1984
- Meikle MA, Vernon J, Johnson RM. The perceived severity of tinnitus: some observations concerning a large population of tinnitus clinic patients. Otolaryngology ‐ Head and Neck Surgery 1984;92:689‐96. [DOI] [PubMed] [Google Scholar]
Meikle 1995
- Meikle MB, Johnson RM, Griest SE, Press LS, Charnell MG. Oregon Tinnitus Data Archive 95‐01. http://www.tinnitusarchive.org/ 1995.
Meng 2011
- Meng Z, Liu S, Zheng Y, Philips JS. Repetitive transcranial magnetic stimulation for tinnitus. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007946.pub2] [DOI] [PubMed] [Google Scholar]
Miller 1956
- Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychological Review 1956;63:81‐7. [PubMed] [Google Scholar]
Millon 1982
- Millon T, Green C, Meagher R. Millon Behavioral Health Inventory Manual. 3rd Edition. Minneapolis, MN: National Computer Systems Inc, 1982. [Google Scholar]
Moffat 1995
- Moffat DA, Hardy DG, Irving RM, Viani L, Beynon GJ, Baguley DM. Referral patterns in vestibular schwannomas. Clinical Otolaryngology 1995;20:80‐3. [DOI] [PubMed] [Google Scholar]
Moller 1997
- Moller AR. Similarities between chronic pain and tinnitus. American Journal of Otology 1997;18(5):577‐85. [PubMed] [Google Scholar]
Parker 2001
- Parker G, Roy K, Wilhelm K, Mitchell P. Assessing the comparative effectiveness of antidepressant therapies: a prospective clinical practice study. Journal of Clinical Psychiatry 2001;62(2):117‐25. [DOI] [PubMed] [Google Scholar]
Parnes 1997
- Parnes SM. Current concepts in the clinical management of patients with tinnitus. European Archives of Otorhinolaryngology 1997;254:406‐9. [DOI] [PubMed] [Google Scholar]
Rauschecker 2010
- Rauschecker JP, Leaver AN, Muhlau M. Turning out the noise: limbic‐auditory interactions in tinnitus. Neuron 2010;66:819‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seidman 1998
- Seidman MD. Glutamate antagonists, steroids and antioxidants as therapeutic options for hearing loss and tinnitus and the use of an inner ear drug delivery system. International Tinnitus Journal 1998;4(2):148‐54. [PubMed] [Google Scholar]
Seidman 2010
- Seidman MD, Standring RT, Dornhoffer JL. Tinnitus: current understanding and contemporary management. Current Opinion in Otolaryngology & Head and Neck Surgery 2010;18:363‐8. [DOI] [PubMed] [Google Scholar]
Shargorodsky 2010
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. American Journal of Medicine 2010;123(8):711‐8. [DOI] [PubMed] [Google Scholar]
Shea 1981
- Shea A. Otosclerosis and tinnitus. Journal of Laryngology and Otology Supplement 1981;4:149‐50. [PubMed] [Google Scholar]
Sindhusake 2003
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. International Journal of Audiology 2003;42(5):289‐94. [DOI] [PubMed] [Google Scholar]
Stephens 1991
- Stephens D, Hetu R. Impairment, disability and handicap in audiology: towards a consensus. Audiology 1991;30:185‐200. [DOI] [PubMed] [Google Scholar]
Sullivan 1992
- Sullivan M, Katon WJ, Russo J, Dobie R, Sakai C. Somatization, co‐morbidity, and the quality of life: measuring the effect of depression upon chronic medical illness. Psychiatric Medicine 1992;10(3):61‐76. [PubMed] [Google Scholar]
Tomkins 2001
- Tomkins GE, Jackson JL, O'Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta‐analysis. American Journal of Medicine 2001;111(1):54‐63. [DOI] [PubMed] [Google Scholar]
Vanneste 2011
- Vanneste S, Heyning P, Ridder D. The neural network of phantom sound changes over time: a comparison between recent‐onset and chronic tinnitus patients. European Journal of Neuroscience 2011;34:718‐31. [DOI] [PubMed] [Google Scholar]
Weissman 2000
- Weissman JL, Hirsch BE. Imaging of tinnitus: a review. Radiology 2000;216(2):342‐9. [DOI] [PubMed] [Google Scholar]
Xu 2011
- Xu X, Bu X, Zhou L, Xing G, Liu C, Wang D. An epidemiologic study of tinnitus in a population in Jangsu Province, China. Journal of the American Academy of Audiology 2011;22(9):578‐85. [DOI] [PubMed] [Google Scholar]
Zung 1973
- Zung WW. From art to science. The diagnosis and treatment of depression. Archives of General Psychiatry 1973;29:328‐37. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Baldo 2006
- Baldo P, Doree C, Lazzarini R, Molin P, McFerran D. Antidepressants for patients with tinnitus. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003853.pub2] [DOI] [PubMed] [Google Scholar]


