Abstract
Background
Invasive fungal infection is an important cause of mortality and morbidity in very preterm and very low birth weight infants. Early diagnosis is difficult and treatment is often delayed. Systemically absorbed antifungal agents (usually azoles) are increasingly used as prophylaxis against invasive fungal infection in this population.
Objectives
To assess the effect of prophylactic systemic antifungal therapy on mortality and morbidity in very preterm or very low birth weight infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group. This included searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 8), MEDLINE, EMBASE, and CINAHL (to May 2015), conference proceedings, and previous reviews.
Selection criteria
Randomised controlled trials or quasi‐randomised controlled trials that compared the effect of prophylactic systemic antifungal therapy versus placebo or no drug or another antifungal agent or dose regimen in very low birth weight infants.
Data collection and analysis
We extracted data using the standard methods of the Cochrane Neonatal Review Group, with separate evaluation of trial quality and data extraction by two review authors.
Main results
We identified 15 eligible trials enrolling a total of 1690 infants. Ten trials (1371 infants) compared systemic antifungal prophylaxis versus placebo or no drug. These trials were generally of good methodological quality. Meta‐analysis found a statistically significant reduction in the incidence of invasive fungal infection (typical risk ratio (RR) 0.43, 95% confidence interval (CI) 0.31 to 0.59; risk difference (RD) −0.09, 95% CI −0.12 to −0.06). The average incidence of invasive fungal infection in the control groups of the trials (16%) was much higher than that generally reported from large cohort studies. Meta‐analysis did not find a statistically significant difference in the risk of death prior to hospital discharge (typical RR 0.79, 95% CI 0.61 to 1.02; typical RD −0.04, 95% CI −0.07 to 0.00). Very limited data on long‐term neurodevelopmental outcomes were available. Three trials that compared systemic versus oral or topical non‐absorbed antifungal prophylaxis did not detect any statistically significant effects on invasive fungal infection or mortality. Two trials that compared different dose regimens of prophylactic intravenous fluconazole did not detect any significant differences in infection rates or mortality.
Authors' conclusions
Prophylactic systemic antifungal therapy reduces the incidence of invasive fungal infection in very preterm or very low birth weight infants. This finding should be interpreted and applied cautiously since the incidence of invasive fungal infection was very high in the control groups of many of the included trials. Meta‐analysis does not demonstrate a statistically significant effect on mortality. There are currently only limited data on the long‐term neurodevelopmental consequences for infants exposed to this intervention. In addition, there is a need for further data on the effect of the intervention on the emergence of organisms with antifungal resistance.
Plain language summary
Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants
Review question: In very low birth weight infants, does the use of prophylactic systemic antifungal therapy decrease the risk of mortality and morbidity?
Background: Fungi such as candida (the organism that causes thrush) can cause severe infections in very low birth weight infants (birth weight less than 1.5 kg). These infections are often difficult to diagnose. It may be appropriate to attempt to prevent such infections by giving all very low birth weight infants antifungal drugs as a routine part of their care (systemic antifungal prophylaxis). This review assessed whether evidence exists that such a practice prevents severe fungal infection, death, and disability in very low birth weight infants.
Study characteristics: We identified 15 eligible trials enrolling a total of 1690 infants. These trials were generally of good quality.
Key findings: The overall analysis showed a reduction in the risk of severe fungal infection in infants who received systemic antifungal prophylaxis but did not show a difference in the risk of death. The trials did not assess the risk of long‐term problems, including disabilities.
Conclusions: There is evidence from some good‐quality trials that giving infants an antifungal drug regularly for the first four to six weeks after birth reduces the number of infants who develop severe infection. There is not yet any convincing evidence that death or disability rates are affected.
Background
Description of the condition
Invasive fungal infection, predominantly due to Candida species, is an increasingly common cause of mortality and morbidity in very preterm (less than 32 weeks) and very low birth weight (VLBW less than 1500 grams) infants (Kossoff 1998; Stoll 2003; Kaufman 2004; Benjamin 2006; Robinson 2009; Hornik 2012; Wynn 2012; Oeser 2013; Shane 2013). Invasive fungal infection accounts for about 10% of all cases of late‐onset invasive infection (diagnosed more than 72 hours after birth) in newborn infants. The risk of infection is inversely related to gestational age and birth weight. The reported incidence in very preterm or VLBW infants is about 1% to 5%. In extremely preterm (less than 28 weeks) or extremely low birth weight (ELBW less than 1000 grams) infants, incidences from 2% to 10% are reported and much higher incidences, up to 20%, have been reported for infants with birth weight less than 750 grams or gestational age at birth less than 26 weeks (Saiman 2000; Karlowicz 2002; Makhoul 2002; Stoll 2002; Clerihew 2006; Kaufman 2006; Vergnano 2011; Aliaga 2014).
Additional putative risk factors for invasive fungal infection in very preterm or VLBW infants include fungal colonisation at multiple sites, severe illness at birth, exposure to multiple courses of antibiotics, receipt of parenteral nutrition, the presence of a central venous catheter, preceding necrotising enterocolitis, and exposure to histamine receptor subtype 2 antagonists (Saiman 2000; Manzoni 2007; Barton 2014; Oeser 2014). Between‐centre differences in the incidence of invasive fungal infection may be due to all or some of these population characteristics and clinical practices.
In addition to fungaemia, infants may develop fungal pneumonia, meningitis, renal tract infection, ophthalmitis, osteomyelitis, endocarditis, liver abscesses and skin abscesses (Benjamin 2003; Clerihew 2006). The diagnosis of invasive fungal infection in very preterm or VLBW infants is often delayed because the clinical presentation can be similar to bacterial infections and because of difficulties in consistently recovering the infecting organisms from blood, cerebrospinal fluid, or urine (Camacho‐Gonzalez 2013). A high index of suspicion and the use of additional laboratory and clinical tests, including retinal examination, echocardiography, and renal ultrasonography, may be needed to confirm the suspected diagnosis (Benjamin 2003; Oeser 2014).
Mortality attributed to invasive fungal infection is more than 25%, higher than mortality attributed to late‐onset invasive bacterial infection in very preterm or VLBW infants (Stoll 1996; Saiman 2000; Makhoul 2002; Stoll 2002; Ascher 2012). Invasive fungal infection, particularly fungal meningitis, is also associated with long‐term morbidity, including adverse neurodevelopmental outcomes (Friedman 2000; Saiman 2000; Benjamin 2006; Wynn 2012; Adams‐Chapman 2013; Barton 2014).
Description of the intervention
Given the high mortality and morbidity associated with invasive fungal infection, and the difficulty in confirming a diagnosis, antifungal medications are frequently used as chemoprophylaxis against fungal colonisation and invasive fungal infection in very preterm or VLBW infants. Two broad chemoprophylactic strategies are employed in current clinical practice:
prophylaxis using oral ortopical non‐absorbed agents such as nystatin or miconazole. This intervention is assessed in another Cochrane Review (Austin 2009).
prophylaxis using systemically‐absorbed antifungal drugs that achieve fungicidal concentrations in tissue, blood, cerebrospinal fluid, and urine. Over the past 15 years, the prophylactic use of systemic antifungal agents, most commonly fluconazole or amphotericin B, has been adopted as routine practice in some neonatal centres (Burwell 2006; Clerihew 2008; O'Grady 2008; Kaufman 2010; Kaguelidou 2012; Oeser 2014,). This intervention is the subject of this Cochrane review.
Fluconazole
Fluconazole is a triazole antifungal which can be administered intravenously but is also well absorbed enterally. Fluconazole achieves good penetration into the cerebrospinal fluid and is excreted unchanged in the urine. Fluconazole is used commonly in neonatal practice and appears to be a safe treatment for newborn infants. The most frequently reported side effect is transient elevation of plasma levels of creatinine or hepatic enzymes described in about 5% of infants treated with fluconazole (Huttova 1998). There have also been adverse events including Stevens–Johnson syndrome, anaphylactic shock, and lengthening of the electrocardiogram QT interval reported in infants and other populations of patients (Gussenhoven 1991; Aydin 2012; Koklu 2014). Additionally, there is a potential risk of adverse effects as a result of drug interactions with medications that are prescribed for newborn infants, including cisapride, theophylline and thiazide diuretics (Neely 2001).
Amphotericin B
Amphotericin B, a polyene antifungal agent that reacts with sterols in cell membranes to cause cell lysis, is poorly absorbed via the enteral route and is given as an intravenous preparation. Drug toxicity, particularly nephrotoxicity, is a potential problem as amphotericin B also damages mammalian cell membranes. These adverse effects limit the total dose that may be given. Newer lipid complex formulations of amphotericin B deliver the active drug directly to the site of action on the fungal cell membrane. Because the lipid complex is more stable in mammalian cells, toxicity is reduced. Amphotericin B is highly protein‐bound and does not achieve good penetration into extracellular fluid spaces, including cerebrospinal fluid.
Why it is important to do this review
Given the difficulty in establishing an early diagnosis and the high level of associated morbidity and mortality, there is a need to assess the effect of strategies to prevent invasive fungal infection in very preterm or VLBW infants (Brecht 2009). This review aimed to evaluate the evidence from randomised controlled trials to determine that systemic antifungal prophylaxis prevents invasive fungal infection and reduces mortality and morbidity in very preterm or VLBW infants. A further major consideration is the potential for antimicrobial prophylaxis to drive the emergence of drug resistance (Brion 2007).
Objectives
To assess the effect of prophylactic systemic antifungal therapy on mortality and morbidity in very preterm or VLBW infants.
We examined the effects of these interventions:
systemic antifungal prophylaxis versus placebo or no drug;
systemic versus oral or topical non‐absorbed antifungal prophylaxis;
one systemic antifungal agent versus another agent or dose regimen.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials using random or quasi‐random patient allocation
Cluster randomised trials where the unit of randomisation was the neonatal nursery
Types of participants
Very preterm or VLBW infants with or without evidence of fungal colonisation but without evidence of invasive fungal infection at study entry.
Types of interventions
Trials comparing systemic antifungal prophylaxis with placebo or no drug, oral or topical antifungal prophylaxis, or another systemic antifungal agent or dose regimen. The drug may have been given by the intravenous or enteral route.
Types of outcome measures
Primary outcomes
1. Confirmed invasive fungal infection as determined by
culture of fungus from a normally sterile site e.g. cerebrospinal fluid, blood, urine, bone or joint, peritoneum, pleural space;
findings on autopsy examination consistent with invasive fungal infection;
findings on ophthalmological examination consistent with fungal ophthalmitis or retinitis;
pathognomonic findings on renal ultrasound examination such as 'renal fungal balls'.
2. Death prior to hospital discharge.
3. Development: (i) neurodevelopmental outcomes assessed using validated tools at 12 months or more corrected age, and classifications of disability including non‐ambulant cerebral palsy, developmental delay, auditory and visual impairment; (ii) cognitive and educational outcomes at 5 years or more e.g. intelligence quotient or indices of educational achievement measured using a validated tool (including school examination results).
Secondary outcomes
Bronchopulmonary dysplasia (oxygen supplementation at 36 weeks postmenstrual age).
Necrotising enterocolitis (Bell stage 2 or 3).
Retinopathy of prematurity: a) any stage; b) requiring treatment.
Duration of intensive care unit or hospital admission (days).
Emergence of organisms resistant to antifungal agents, as detected in individual infants enrolled in the study or, in the case of cluster randomised studies, on surveillance of other infants in the same unit in the study centre (including infants who were admitted to the unit following completion of the study).
Adverse drug reactions attributed to the antifungal agent, such as rash, gastrointestinal disturbance, abnormal hepatic or renal function, cardiac arrhythmias, thrombophlebitis, seizures, and anaphylaxis or toxicity sufficient to cease drug administration.
Search methods for identification of studies
We used the standard search strategy of the Cochrane Neonatal Review Group (http://neonatal.cochrane.org/).
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 8), MEDLINE (1966 to August 2015), EMBASE (1980 to August 2015), and CINAHL (1982 to August 2015) using a combination of the following text words and MeSH terms: [Infant, Newborn OR Infant, Premature OR Infant, Low Birth Weight OR infan* OR neonat*] AND [Mycoses/ OR fung* OR candid* OR Candida albicans OR Antifungal Agents/ OR Triazoles/ OR fluconazole OR azole OR Amphotericin B/]. We limited the search outputs with the relevant search filters for clinical trials as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not apply any language restriction [See appendix 1 for updated search strategy].
We searched ClinicalTrials.gov and Current Controlled Trials and the World Health Organization (WHO) International Clinical Trials Registry Platform for completed or ongoing trials.
Searching other resources
We examined reference lists in previous reviews and included studies. We searched the proceedings of the annual meetings of the Pediatric Academic Societies (1993 to 2015), the European Society for Paediatric Research (1995 to 2014), the Royal College of Paediatrics and Child Health (2000 to 2015), the Perinatal Society of Australia and New Zealand (2000 to 2015), the European Society for Paediatric Infectious Diseases (2005 to 2015), and the Infectious Diseases Society of America (2003 to 2015). We considered trials reported only as abstracts to be eligible if sufficient information was available from the reports, or from contact with the authors, to fulfil the inclusion criteria.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group.
Selection of studies
Two review authors screened the titles and abstracts of all studies identified by the above search strategy. We reassessed the full text of any potentially eligible reports and excluded those studies that did not meet all of the inclusion criteria. We discussed any disagreements until consensus was achieved.
Data extraction and management
We used a data collection form to aid extraction of relevant information from each included study. Two review authors extracted the data separately. We discussed any disagreements until consensus was achieved. We asked the investigators for further information if data from the trial reports were insufficient.
Assessment of risk of bias in included studies
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Group to assess the methodological quality of any included trials. We requested additional information from the trial authors to clarify methodology and results as necessary. We evaluated and reported the following issues in the 'Risk of bias' tables:
Sequence generation (the method used to generate the allocation sequence):
low risk: any truly random process, e.g. random number table; computer random number generator;
high risk: any non‐random process, e.g. odd or even date of birth; hospital or clinic record number;
unclear risk: no or unclear information provided.
Allocation concealment (the method used to conceal the allocation sequence):
low risk: e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes;
high risk: open random allocation, e.g. unsealed or non‐opaque envelopes, alternation; date of birth;
unclear: no or unclear information provided.
Blinding (the methods used to ensure blinding of participants, clinicians and caregivers, and outcome assessors):
low risk;
high risk;
unclear.
Incomplete outcome data (completeness of data including attrition and exclusions from the analysis for each outcome and any reasons for attrition or exclusion where reported): we will assess whether missing data are balanced across groups or are related to outcomes. Where sufficient information is reported or supplied by the trial authors, we will reinstate missing data in the analyses. We will categorise completeness as:
low risk: adequate (less than 10% missing data);
high risk: inadequate (more than 10% missing data);
unclear risk: no or unclear information provided.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs). We determined the number needed to treat for an additional beneficial outcome (NNTB) or an additional harmful outcome (NNTH) for analyses with a statistically significant difference in the RD.
Unit of analysis issues
The unit of analysis was the participating infant in individually randomised trials and the neonatal unit for cluster randomised trials.
Assessment of heterogeneity
We examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each RR analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than sampling error. If substantial heterogeneity (I² greater than 50%) was detected, we explored the possible causes (for example differences in study design, participants, interventions, or completeness of outcome assessments).
Assessment of reporting biases
We examined a funnel plot for asymmetry (if more than 10 trials).
Data synthesis
We used the fixed‐effect model in Review Manager 5.3 for meta‐analyses (as per Cochrane Neonatal Group recommendations). Where substantial heterogeneity existed, we planned to examine the potential causes in subgroup and sensitivity analyses.
Subgroup analysis and investigation of heterogeneity
We pre‐specified the following subgroup analyses:
ELBW infants (less than 1000 grams);
infants with fungal colonisation at trial entry.
Results
Description of studies
We identified 15 eligible trials: Kaufman 2001; Kicklighter 2001; Cabrera 2002; Kaufman 2005; Manzoni 2007a; Manzoni 2007b; Parikh 2007; Arrieta 2010; Kim 2010; Violaris 2010; Aydemir 2011a; Aydemir 2011b; Mersal 2013; Benjamin 2014; Kirpal 2015.
Participants
The trials were undertaken in tertiary perinatal centres in North America, Europe, Korea, or India within the past 15 years. In total, 1690 infants participated. The participants were VLBW infants (Kicklighter 2001; Cabrera 2002; Manzoni 2007a; Manzoni 2007b; Parikh 2007; Arrieta 2010; Kim 2010; Violaris 2010; Aydemir 2011a; Aydemir 2011b; Mersal 2013; Kirpal 2015); or ELBW infants (Kaufman 2001; Kaufman 2005); or infants of birth weight less than 750 grams (Benjamin 2014). Documented fungal colonisation was an eligibility criterion for Cabrera 2002 but not for any of the other trials.
Interventions
Ten trials compared systemic antifungal prophylaxis versus placebo or no drug. Nine trials used fluconazole (Kaufman 2001; Kicklighter 2001; Cabrera 2002; Manzoni 2007a; Parikh 2007; Kim 2010; Aydemir 2011a; Benjamin 2014; Kirpal 2015). One trial used amphotericin B (Arrieta 2010). Participating infants were enrolled within the first few days after birth and assigned to receive the intervention or placebo for four weeks (for VLBW infants) to six weeks (for ELBW infants). The study drug was given intravenously until the infants tolerated enteral intake and then either administered enterally (Cabrera 2002; Manzoni 2007a; Parikh 2007; Kim 2010; Aydemir 2011a; Benjamin 2014); or discontinued when intravenous access was no longer available (Kaufman 2001; Kaufman 2005; Arrieta 2010; Kirpal 2015).
Three trials compared systemic antifungal prophylaxis (fluconazole) with oral or topical antifungal prophylaxis (nystatin) (Violaris 2010; Aydemir 2011b; Mersal 2013).
-
Two trials compared different dose regimen
Kaufman 2005 compared two regimens of prophylaxis with fluconazole (regimen A: 3 mg/kg body weight every third day for the first two weeks, then every second day during the third and fourth weeks, then daily during the fifth and sixth weeks; regimen B: 3 mg/kg twice weekly for six weeks). Infants were assigned to intervention for six weeks or until intravenous access was discontinued.
Manzoni 2007b randomly allocated infants in the fluconazole group to either 3 mg/kg per 48 hours (regimen A) or 6 mg/kg per 48 hours (regimen B) for 30 days after birth (or for 45 days in ELBW infants).
Outcomes
The primary outcomes of the trials were fungal colonisation or invasive fungal infection. Data on deaths prior to hospital discharge were provided for 14 of the trials. Most trials monitored plasma levels of aspartate aminotransferase, alanine aminotransferase, total bilirubin, or alkaline phosphatase.
Investigators monitored the fluconazole minimal inhibitory concentrations of fungal isolates (from both surface colonisation and from invasive infection) during the surveillance period in five trials (Kaufman 2001; Kicklighter 2001; Kaufman 2005; Aydemir 2011a; Aydemir 2011b). Cabrera 2002 collected surveillance cultures from day seven, at weekly intervals until six weeks, and began prophylaxis once surveillance cultures were positive.
Only two trials have reported neurodevelopmental outcomes assessed beyond infancy (Kaufman 2001; Benjamin 2014).
Excluded studies
We excluded 16 studies (see table 'Characteristics of excluded studies'). These were all single centre retrospective observational studies that compared outcomes for cohorts of VLBW or ELBW infants cared for in an epoch immediately prior to the introduction of intravenous antifungal prophylaxis compared with infants cared for in the epoch after this intervention was adopted (Bertini 2005; Dutta 2005; Healy 2005; Aghai 2006; Manzoni 2006; Uko 2006; McCrossan 2007; Wadhawan 2007; Al Qurashi 2008; Healy 2008; Kim 2008; Manzoni 2008; Weitkamp 2008; Aziz 2010; Rueda 2010; Maede 2013).
Risk of bias in included studies
Quality assessments are described in the table 'Characteristics of included studies'.
The included trials were generally of good methodological quality. In most studies, allocation was concealed by separating the randomisation process from recruitment and enrolment. Caregivers, investigators, and assessors were all blind to the intervention in the systemic antifungal versus placebo trials, but not blinded in the systemic antifungal versus oral/topical antifungal trials. Follow‐up appeared to be complete for the outcomes reported in all of the included trials.
Effects of interventions
Systemic antifungal agent versus placebo or no drug (Comparison 1)
Ten trials compared systemic antifungal prophylaxis versus placebo or no drug. Nine trials used fluconazole (Kaufman 2001; Kicklighter 2001; Cabrera 2002; Manzoni 2007a; Parikh 2007; Kim 2010; Aydemir 2011a; Benjamin 2014; Kirpal 2015). One trial used amphotericin B (Arrieta 2010).
Primary outcomes
Confirmed invasive fungal infection (Outcome 1.1):
Only Kaufman 2001, Manzoni 2007a and Aydemir 2011a individually reported statistically significantly lower incidences in the intervention group. Meta‐analysis of data from all of the trials showed a statistically significant lower incidence of invasive fungal infection in the intervention group (typical RR 0.43, 95% CI 0.31 to 0.59; typical RD −0.09, 95% CI −0.12 to −0.06; NNTB 11, 95% CI 8 to 17 (Analysis 1.1; Figure 1). There was evidence of substantial statistical heterogeneity in this meta‐analysis (I² = 52%) but no evidence of funnel plot asymmetry (Figure 2).
1.1. Analysis.
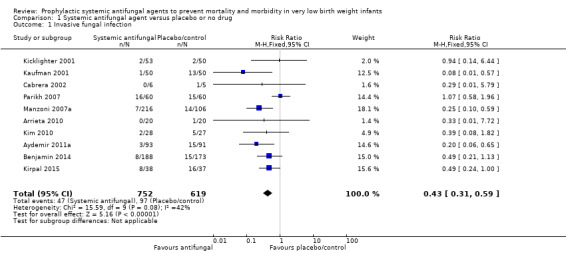
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 1 Invasive fungal infection.
1.
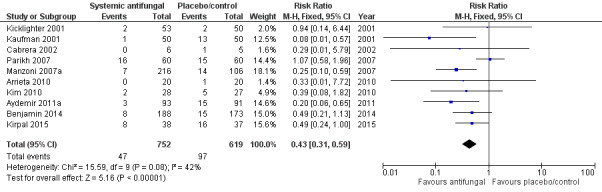
Forest plot of comparison: 1 Systemic antifungal agent versus placebo or no drug, outcome: 1.1 Invasive fungal infection.
2.
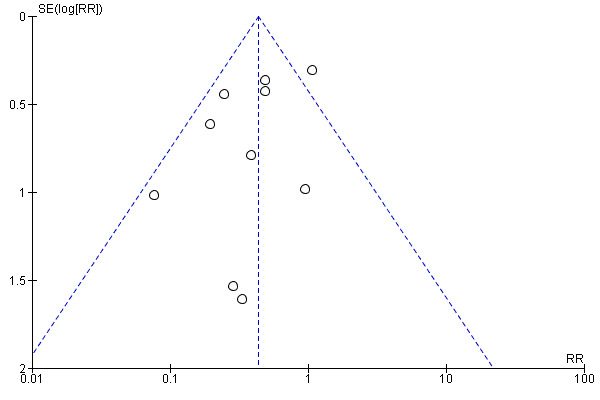
Funnel plot of comparison: 1 Systemic antifungal agent versus placebo or no drug, outcome: 1.1 Invasive fungal infection.
Death prior to hospital discharge (Outcome 1.2)
Data were reported by nine trials (Kaufman 2001; Kicklighter 2001; Manzoni 2007a; Parikh 2007; Arrieta 2010; Kim 2010; Aydemir 2011a; Benjamin 2014; Kirpal 2015). There were not any statistically significant differences in any of the individual trials or in a meta‐analysis of all data (typical RR 0.79, 95% CI 0.61 to 1.02; typical RD −0.04, 95% CI −0.07 to 0.00) (Analysis 1.2; Figure 3) There was no evidence of statistical heterogeneity (I² = 0%).
1.2. Analysis.
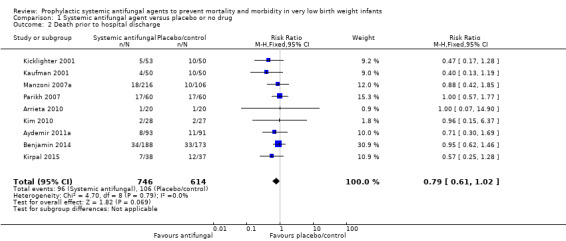
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 2 Death prior to hospital discharge.
3.
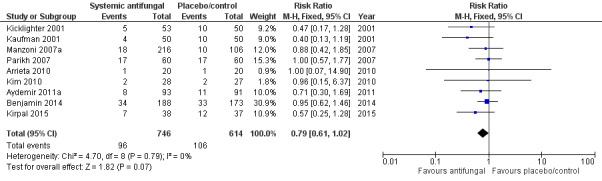
Forest plot of comparison: 1 Systemic antifungal agent versus placebo or no drug, outcome: 1.2 Death prior to hospital discharge.
Neurodevelopment (Outcomes 1.3 to 1.9)
Neurodevelopmental outcomes were reported by two trials (Kaufman 2001; Benjamin 2014).
Kaufman 2001 reported no significant difference in the incidence of developmental delay (modified Gesell test) or motor or sensory neurological impairment in infants assessed at a median age of 16 months. These findings were reported in abstract form only. Long‐term follow‐up assessments (at 8 to 10 years) conducted on 45% of surviving children did not find any statistically significant differences in Vineland Adaptive Behavior Scales‐II (Analysis 1.3) or self esteem scores assessed using the Child Health Questionnaire Parent‐Completed Form 28 (Analysis 1.4).
Benjamin 2014 reported no significant difference in a "neurodevelopmental impairment composite end‐point", defined as at least one of (i) Bayley‐III cognition composite score less than 70, (ii) cerebral palsy, (iii) deafness or, (iv) blindness at follow‐up at 18 to 22 months post term for trial participants (Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9).
1.3. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 3 VABS‐II Domain Scores.
1.4. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 4 Self esteem scores.
1.5. Analysis.
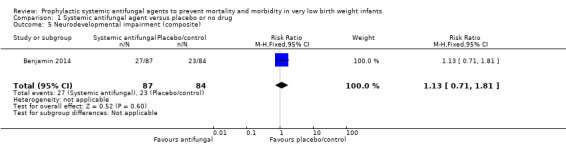
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 5 Neurodevelopmental impairment (composite).
1.6. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 6 Bayley‐III cognition composite score < 70.
1.7. Analysis.
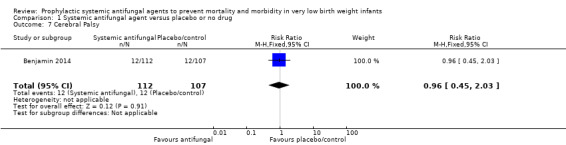
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 7 Cerebral Palsy.
1.8. Analysis.
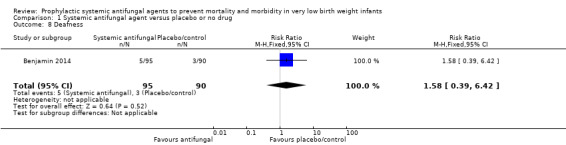
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 8 Deafness.
1.9. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 9 Blindness.
Secondary outcomes
Retinopathy of prematurity (Outcome 1.10)
Meta‐analysis did not show a statistically significant difference (typical RR 0.90, 95% CI 0.68 to 1.20; typical RD −0.02, 95% CI −0.06 to 0.03; 5 trials, 1022 infants) (Analysis 1.10).
1.10. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 10 Retinopathy of prematurity.
Necrotising enterocolitis (Outcome 1.11)
Meta‐analysis did not show a statistically significant difference (typical RR 0.90, 95% CI 0.62 to 1.29; typical RD −0.01, 95% CI −0.04 to 0.02; 7 trials, 1152 infants) (Analysis 1.11).
1.11. Analysis.

Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 11 Necrotising enterocolitis.
Chronic lung disease (Outcome 1.12)
Meta‐analysis did not show a statistically significant difference (typical RR 0.98, 95% CI 0.84 to 1.16; typical RD −0.01, 95% CI −0.06 to 0.05; 4 trials, 922 infants) (Analysis 1.12).
1.12. Analysis.
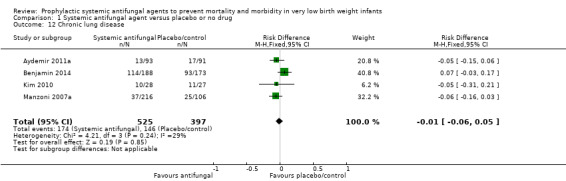
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 12 Chronic lung disease.
Length of hospital stay
Kim 2010 did not detect a statistically significant difference: MD −0.10 (95% CI −13.28 to 13.08) days (Analysis 1.13).
1.13. Analysis.
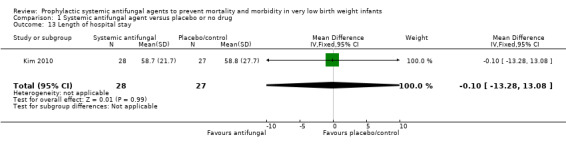
Comparison 1 Systemic antifungal agent versus placebo or no drug, Outcome 13 Length of hospital stay.
Parikh 2007 and Benjamin 2014 did not detect any statistically significant differences (but data for inclusion in meta‐analysis not reported).
Emergence of organisms resistant to antifungal agents
Four reports commented on this outcome but presented limited data.
Kaufman 2001 did not find any statistically significant changes in the minimal inhibitory concentration of fluconazole for fungal isolates during the 30 months study period.
Kicklighter 2001 did not find any statistically significant differences in the minimal inhibitory concentration of fluconazole for Candida albicans isolates between the study groups during the treatment period or for four weeks after discontinuation of the study drug.
Manzoni 2007a stated that "patterns of sensitivity to fluconazole remained the same".
Aydemir 2011a stated that "sensitivity to fluconazole did not vary during the study period" (no other data presented).
Adverse drug reactions attributed to the antifungal agent
There were no clinically significant adverse reactions attributed to antifungal agents in the included studies. No infants were withdrawn from the trials because of adverse effects.
Subgroup analyses
ELBW infants
Kaufman 2001 enrolled only ELBW infants. Benjamin 2014 enrolled only infants with birth weight less than 750 grams. Meta‐analysis of data from only these trials found a statistically significant effect on the incidence of invasive fungal infection (typical RR 0.30, 95% CI 0.14 to 0.63; typical RD −0.09, 95% CI −0.14 to −0.04), but no difference in mortality (typical RR 0.82, 95% CI 0.55 to 1.23; typical RD −0.03, 95% CI −0.10 to 0.04).
The other trials which recruited VLBW infants did not report subgroup data for ELBW infants. If these data become available, we will include them in an update of this review.
Infants with fungal colonisation at entry to study
Only the smallest trial restricted participation to infants with fungal colonisation (Cabrera 2002). Subgroup analysis of infants with fungal colonisation was not possible with the available data from the other trials.
Systemic antifungal agent versus oral or topical antifungal therapy (Comparison 2)
Three trials compared systemic antifungal prophylaxis (fluconazole) with oral or topical antifungal prophylaxis (nystatin) (Violaris 2010; Aydemir 2011b; Mersal 2013).
Primary outcomes
Confirmed invasive fungal infection (Outcome 2.1): Meta‐analysis did not find a statistically significant difference (typical RR 0.53, 95% CI 0.19 to 1.51; typical RD −0.03, 95% CI −0.07 to 0.02; 3 studies, 326 infants)) (Analysis 2.1).
2.1. Analysis.
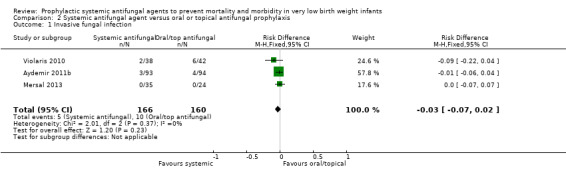
Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 1 Invasive fungal infection.
Death prior to hospital discharge (Outcome 2.2): Meta‐analysis did not find a statistically significant difference (typical RR 0.72, 95% CI 0.33 to 1.56; typical RD −0.02, 95% CI −0.08 to 0.03; 3 studies, 326 infants) (Analysis 2.2).
2.2. Analysis.

Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 2 Death prior to hospital discharge.
Neurodevelopmental outcomes: None of the trials reported neurodevelopmental outcomes.
Secondary outcomes
Incidence of bronchopulmonary dysplasia in surviving infants (Outcome 2.3):Aydemir 2011b did not find a statistically significant difference: RR 0.77 (95% CI 0.40 to 1.49), RD −0.04 (95% CI −0.16 to 0.07). Not reported by Violaris 2010 or Mersal 2013 .
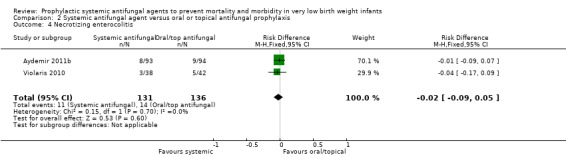
Incidence of necrotising enterocolitis (Outcome 2.4): Meta‐analysis of data from Violaris 2010 and Aydemir 2011b did not detect a statistically significant difference: typical RR 0.82 (95% CI 0.38 to 1.74), RD −0.02 (95% CI −0.09 to 0.05). Not reported by Mersal 2013 .
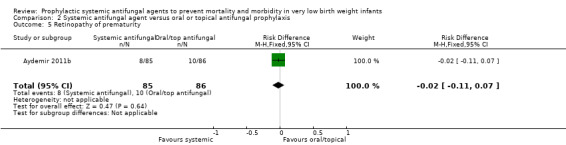
Incidence of retinopathy of prematurity (Outcome 2.5):Aydemir 2011b did not find a statistically significant difference in the incidence of retinopathy requiring surgery: RR 0.81 (95% CI 0.34 to 1.95), RD −0.02 (95% CI −0.11 to 0.07). Not reported by Violaris 2010 or Mersal 2013 .
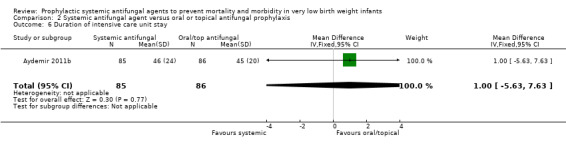
Duration of intensive care unit stay:Aydemir 2011b did not find a statistically significant difference: MD 1.00 (95% CI −5.63 to 7.63) days. Not reported by Violaris 2010 or Mersal 2013.
Emergence of organisms resistant to antifungal agents:Aydemir 2011b stated that "sensitivity to fluconazole did not vary during the study period" (no other data presented). Not reported by Violaris 2010 or Mersal 2013.
Adverse drug reactions attributed to the antifungal agent: There were no clinically significant adverse reactions attributed to antifungal agents in the included studies. No infants were withdrawn from the trials because of adverse effects.
Subgroup analyses
ELBW infants: The included trials enrolled VLBW infants and ELBW subgroup data were not available.
Infants with fungal colonisation at entry to study: None of the trials restricted participation to infants with fungal colonisation.
One systemic antifungal agent versus another agent or dose regimen (Comparison 3)
Kaufman 2005 and Manzoni 2007b compared two regimens of fluconazole prophylaxis.
Primary outcomes
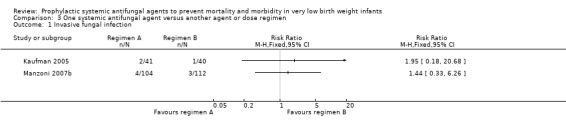
Confirmed invasive fungal infection (Outcome 3.1): Neither trial found a statistically significant difference:
Kaufman 2005: RR 1.95 (95% CI 0.18 to 20.7); RD 0.02 (95% CI −0.06 to 0.11)
Manzoni 2007b: RR 1.44 (95% CI 0.33 to 6.26); RD 0.01 (95% CI −0.04 to 0.11).
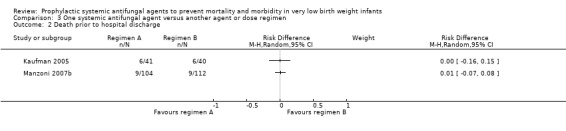
Death prior to hospital discharge (Outcome 3.2): Neither trial found a statistically significant difference:
Kaufman 2005: RR 0.98 (95% CI 0.34 to 2.77); RD 0.00 (95% CI −0.16 to 0.15);
Manzoni 2007b: RR 1.44 (95% CI 0.33 to 6.26); RD 0.01 (95% CI −0.04 to 0.06).
Neurodevelopmental outcome: Not reported by either trial.
Secondary outcomes
Emergence of organisms resistant to antifungal agents
Kaufman 2005 did not find any statistically significant difference in the mean minimal inhibitory concentration of fluconazole for fungi isolated from surveillance cultures from infants during the first 12 months versus the second 12 months of the study. Manzoni 2007b stated that "patterns of sensitivity to fluconazole remained the same".
Adverse drug reactions attributed to the antifungal agent
There were no clinically significant adverse reactions attributed to fluconazole and no infants were withdrawn from either study.
Subgroup analyses
ELBW infants: All participants in Kaufman 2005 were of ELBW. Manzoni 2007b did not provide ELBW subgroup data.
Infants with fungal colonisation at entry to study: Neither trial restricted participation to infants with fungal colonisation.
Discussion
Summary of main results
The available trial data indicate that prophylactic systemic antifungal therapy reduces the incidence of invasive fungal infection in VLBW infants. The pooled estimate suggests that treating 11 VLBW infants with prophylactic antifungal therapy would prevent one extra case of invasive fungal infection. Meta‐analysis did not find a statistically significant effect on all‐cause mortality and there were few data reported on long‐term neurodevelopmental outcomes.
We found limited trial data on outcomes for VLBW infants who received systemic versus oral or topical non‐absorbed antifungal prophylaxis. The three trials that examined this question did not find any statistically significant differences in the primary outcomes, but larger trials would be needed to exclude more modest yet important effect sizes. Similarly, the currently available trial data are insufficient to determine which dose regimens of antifungal prophylaxis are superior.
Overall completeness and applicability of evidence
High incidence of invasive fungal infection in controls
The main factor limiting generalisabilty of the findings of this review is the high incidence of invasive fungal infection in the placebo groups of some of the included trials. The average incidence of invasive fungal infection was 16% (range 4% to 43%) compared to incidences of 1% to 5% generally reported from other large cohort studies of VLBW infants (Kossoff 1998; Karlowicz 2002; Clerihew 2006; Vergnano 2011; Aliaga 2014). Consequently the effect size estimates from the meta‐analyses should be applied cautiously. In neonatal care centres where the incidence of invasive fungal infection is lower, a much larger number of infants than the number derived from the meta‐analysis would need to be exposed to prophylaxis to prevent a single extra case of invasive fungal infection. For example, 1% of VLBW infants and 2% of ELBW infants in a UK prospective national surveillance study developed invasive fungal infection (Clerihew 2006). In neonatal care centres where the incidence of invasive fungal infection matches this UK national estimate, 175 VLBW (or 88 ELBW) infants would need to be exposed to systemic antifungal prophylaxis in order to prevent a single extra case of invasive fungal infection.
Diagnostic sensitivity of microbiological culture affected by systemic antifungal prophylaxis
Another issue that may limit the validity of these trial data is that the diagnostic sensitivity of microbiological culture for invasive fungal infection may be lower in infants receiving systemic antifungal treatment (Schelonka 2003). This may have caused selective under‐diagnosis in the treatment group and over‐estimation of the effect size. Mortality was included as a primary outcome for this review since ascertaining this outcome is less likely to be affected by bias. Furthermore, as it is often difficult to precisely define the cause of death in VLBW infants, and since invasive fungal infection is not always diagnosed, all‐cause mortality rather than death attributed to fungal infection was the pre‐specified outcome. The mortality rates in the placebo cohorts were similar to rates in large cohort studies of VLBW infants cared for in similar settings (Horbar 2002). The review did not find a statistically significant effect of prophylactic systemic antifungal therapy on all‐cause mortality, with the 95% CI around this estimate of effect consistent with a 19% risk reduction to a 7% risk increase. When data from further trials are available, these may be included in this meta‐analysis to provide a more precise estimate of the effect on mortality.
Lack of data on antifungal resistance
There is a possibility that widespread use of systemic antifungal prophylaxis may lead to the emergence of antifungal resistance. A meta‐analysis of trials of fluconazole prophylaxis in immunosuppressed adults found evidence of an increased risk for colonisation, but not invasive infection, with fungi partially or completely resistant to fluconazole (Brion 2007). Although the data available from the trials identified in this review are reassuring in terms of the emergence of fluconazole resistance, the follow‐up periods (up to 30 months) of the trials are probably insufficient to detect clinically significant changes in the resistance profile of fungal isolates. Antifungal resistance may take many years following the introduction of fluconazole prophylaxis to become established in neonatal intensive care units (Sarvikivi 2008).
In the trial undertaken in an Indian neonatal care centre where fluconazole had been used routinely for treating infants with fungal infection during the preceding six years, the most common fungal isolates causing invasive infection were non‐albicans Candida species with relatively reduced azole susceptibility (Parikh 2007). This may partly explain why this trial did not detect a statistically significant effect of fluconazole prophylaxis on the incidence of invasive fungal infection. Continued mycological surveillance in those units where systemic antifungal prophylaxis is used is essential.
Regarding the potential adverse effects of prophylactic systemic antifungal therapy, there were no clinically significant drug‐related adverse events reported in these trials, nor was any infant withdrawn from any study because of unacceptable adverse reactions. To date, fluconazole has appeared to be a safe treatment for newborn infants with invasive fungal infection. Only a mild and transient elevation of plasma levels of hepatic enzymes has been described as a common side effect (Huttova 1998). However, there are rare but important side effects such as toxic epidermal necrolysis and Stevens–Johnson syndrome reported in other populations of patients. If fluconazole exposure becomes more widespread through use as prophylaxis then these side effects may be observed in newborn infants. Additionally, widespread use of prophylactic fluconazole may increase the risk of potential drug interactions with medications that are prescribed for VLBW infants including theophylline and thiazide diuretics (Neely 2001).
Quality of the evidence
The included trials, although small, were generally of good methodological quality with satisfactory allocation concealment and blinding using placebo in most cases. Assessment of in‐hospital outcomes was complete in all of the trials (Figure 4). Only very limited data on long‐term outcomes and on the emergence of antifungal resistance are available.
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Potential biases in the review process
The existence of substantial statistical heterogeneity in the meta‐analysis of the effect of antifungal prophylaxis on the incidence of invasive fungal infection raises concern that the estimate is not robust. The source of heterogeneity does not appear to be due to differences in either the participants or the intervention, or related to trial design or quality. One exception is the trial undertaken in India in which the most common fungal isolates causing invasive infection were non‐albicans Candida species with relatively reduced azole susceptibility (Parikh 2007). Removal of this trial from the meta‐analysis removed the statistical heterogeneity of the RR estimate and did not change the direction or size of the estimate.
Concern exists that widespread use of antifungal prophylaxis may drive the emergence of antifungal‐resistant species in the neonatal care centre. Limiting prophylaxis to infants at highest risk may help delay the emergence of antifungal resistance. Since invasive fungal infection is about twice as common in ELBW than VLBW infants, targeting prophylaxis to this population reduces the number of infants who need to be exposed to prophylaxis. Insufficient subgroup data were available to undertake the planned subgroup analysis of ELBW infants. If these data become available, they will be included in a future update of the review.
Similarly, a planned subgroup analysis of outcomes for infants who were colonised with fungi at trial entry was not possible. Colonisation, especially heavy gastrointestinal colonisation, has been suggested by some as a risk factor for invasive infection (Pappu‐Katikaneni 1990) but not other (Saiman 2000) observational studies. The subgroup data for only those infants colonised at trial entry were not available in the published reports of the largest studies (Kicklighter 2001; Kaufman 2005; Manzoni 2007a). As only about 10% of all of the participating infants were colonised at trial entry, it is unlikely that the analysis of these small numbers would provide clinically useful findings.
It is plausible that limiting the exposure of infants to systemic antifungal prophylaxis by using less intensive dose regimens may help in limiting the emergence of antifungal resistance. Two trials compared 'standard' dosing regimens to less intensive, lower dose regimens (Kaufman 2005; Manzoni 2007b). Neither found statistically significant differences on mortality before hospital discharge or the incidence of invasive fungal infection. However, the 95% confidence intervals were wide and further trials are needed to identify the most appropriate dosing regimen for this intervention.
Authors' conclusions
Implications for practice.
Systemic antifungal prophylaxis reduces the incidence of invasive fungal infection in VLBW and ELBW infants. The available trial data do not indicate a statistically significant effect on mortality and there are only limited data on long‐term neurodevelopmental outcomes. Lower dose regimens appear to be as effective at preventing invasive fungal infection as more frequently administered prophylaxis doses, but the 95% CI for these estimates are wide.
Implications for research.
Further randomised controlled trials of systemic antifungal prophylaxis could provide more precise estimates of the effect on mortality and neuro‐disability. Systemic antifungal prophylaxis may be compared with placebo or with topical or oral prophylaxis. Any trial should aim to assess long‐term outcomes, particularly disability‐free survival, as well as the effect on invasive fungal infection.
Because the burden of invasive fungal infection is confined mainly to the smallest and least mature infants, and because neonatologists who currently use systemic antifungal prophylaxis target infants thought to be at greatest risk, which are mainly ELBW or extremely preterm infants (or infants less than 26 weeks gestation or with birth weight less than 750 grams) with additional risk factors, a trial restricted to this population of infants or perhaps even smaller or lower gestation infants may be appropriate and acceptable (Burwell 2006; Parikh 2007; Clerihew 2008; Kaguelidou 2012).
Additionally, although randomised controlled trials may attempt to measure the effect of prophylaxis on antifungal resistance, there is also a need for on‐going local and national surveillance to detect the emergence of resistant organisms, particularly if prophylactic use of fluconazole becomes more widespread.
What's new
| Date | Event | Description |
|---|---|---|
| 2 September 2015 | New search has been performed | Our updated search identified four new trials for inclusion in this review update (Kim 2010; Mersal 2013; Benjamin 2014; Kirpal 2015). |
| 2 September 2015 | New citation required but conclusions have not changed | This updates the review "Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants" published in The Cochrane Database of Systematic Reviews, Issue 4, 2013 (Austin 2013). |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 31 August 2012 | New search has been performed | Our updated search in August 2012 identified 3 new trials for inclusion in this review update (Arrieta 2010; Aydemir 2011a; Violaris 2010). |
| 30 January 2009 | New search has been performed | This updates the review "Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants" published in The Cochrane Database of Systematic Reviews, Issue 4, 2007 (Clerihew 2007). Search updated January 2009. One new trial identified (Parikh 2007) and incorporated into review update. |
| 11 June 2008 | Amended | Converted to new review format. |
| 24 July 2007 | New search has been performed | This review updates the review "Prophylactic intravenous antifungal agents to prevent mortality and morbidity in very low birth weight infants" published in the Cochrane Database of Systematic Reviews, The Cochrane Library, Issue 1, 2004 (McGuire 2004). For this update, the title was changed to "Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants" since this better reflects the clinical context. Consequently, one small trial in which a systemic antifungal agent was administered enterally is now included (Violaris 1998). The electronic search was updated in May 2007. Two new trials that fulfilled eligibility criteria were identified. One of these is the largest trial of this intervention yet reported (Manzoni 2007a). Inclusion of this trial more than doubled the total number of participants in the review. Inclusion of the data in the meta‐analyses increased the precision of the estimates of effect size. The finding of a reduced incidence of invasive fungal infection in infants who received systemic antifungal prophylaxis was not altered. However, the previous finding of a statistically significantly lower mortality rate no longer holds. Six observational studies of the intervention were found and we have described these in the excluded studies section. |
Acknowledgements
We thank Rocio Rodriguez‐Lopez for updating the electronic search strategy.
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Appendices
Appendix 1. Update detailed electronic search strategy July 2014
Information Specialist: Rocio Rodriguez Lopez, CRD, UK
Databases:
MEDLINE (Ovid SP), 1946 – current;
EMBASE (Ovid SP), 1974 – current;
Cumulative Index to Nursing and Allied Health Literature Plus (CINAHL Plus) (EBSCO), 1937 – current;
Cochrane Central Register of Controlled Trials (CENTRAL)
The International Clinical Trials Registry Platform (ICTRP)
ClinicalTrials.gov
We applied a date limit July 2012 –onwards to the bibliographic databases.
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
exp infant, premature/ (41486)
exp infant, low birth weight/ (26342)
Infant, Premature, Diseases/ (17779)
(preterm* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW).ti,ab,hw. (189775)
or/1‐4 (192144)
exp Mycoses/ (104604)
exp Fungi/ (302448)
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s).ti,ab,hw. (459779)
or/6‐8 (604641)
and/5,9 (4150)
exp Antifungal Agents/ (134995)
exp azoles/ (523420)
(fungicid* or antifungal or azole*).ti,ab,rn. (66796)
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol).ti,ab,rn. (10206)
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine).ti,ab,rn. (4594)
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec).ti,ab,rn. (17395)
or/11‐16 (655015)
and/10,17 (820)
limit 18 to ed=20120601‐20140717 (100)
100 total results saved to Endnote library marked MEDLINE_17/07/2014 in Custom 4 field.
Database: EMBASE <1974 to 2014 May 21>
Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
prematurity/ (72156)
exp low birth weight/ (39615)
(preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW).ti,ab,hw. (232091)
or/1‐3 (235476)
exp mycosis/ (146509)
fungal colonization/ (2535)
exp fungus/ (386952)
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s).ti,ab,hw. (564479)
or/5‐8 (746245)
and/4,9 (5666)
exp antifungal agent/ (267508)
exp pyrrole derivative/ (57174)
(fungicid* or antifungal or azole*).ti,ab,rn. (51286)
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol).ti,ab,rn. (31540)
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine).ti,ab,rn. (12465)
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec).ti,ab,rn. (21126)
or/11‐16 (335058)
and/10,17 (1268)
limit 18 to em=201220‐201429 (225)
225 total results saved to Endnote library marked EMBASE_17/067/2014 in Custom 4 field.
CINAHL Plus Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
S18 S11 AND S17 34 S17 S12 OR S13 OR S14 OR S15 OR S16 5,975
S16 TX (Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec) 1,244
S15 TX (Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine) 195
S14 TX (Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol) 1,038
S13 TX (fungicid* or antifungal or azole*) 4,679
S12 (MH "Antifungal Agents+") 4,935
S11 S5 AND S10 535
S10 S6 OR S7 OR S8 OR S9 32,460
S9 TX (fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s) 27,232
S8 (MH "Fungi+") 7,672
S7 (MH "Mycosis Fungoides") 228
S6 (MH "Mycoses+") 10,176
S5 S1 OR S2 OR S3 OR S4 38,598
S4 TX (preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW) 37,954
S3 (MH "Infant, Premature, Diseases") 2,438
S2 (MH "Infant, Low Birth Weight+") 8,128
S1 (MH "Infant, Premature") 13,445
34 total results saved to Endnote library marked CINAHL_18/07/2014 in Custom 4 field.
Cochrane Library (CENTRAL) Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
ID Search Hits
MeSH descriptor: [Infant, Premature] explode all trees 2753
MeSH descriptor: [Infant, Low Birth Weight] explode all trees 1814
MeSH descriptor: [Infant, Premature, Diseases] explode all trees 2186
(preterm* or pre‐term* or pretermatur* or prematur* or (low and ("birth weight" or birthweight)) or ELBW or VLBW or LVW):ti,ab,kw (Word variations have been searched) 15350
#1 or #2 or #3 or #4 15681
MeSH descriptor: [Mycoses] explode all trees 2223
MeSH descriptor: [Fungi] explode all trees 1068
(fungus or fungi or fungal or fungemia or fungaemia or aspergillosis or candid* or mycos?s):ti,ab,kw (Word variations have been searched) 7137
#6 or #7 or #8 8166
#5 and #9 197
MeSH descriptor: [Antifungal Agents] explode all trees 1647
MeSH descriptor: [Azoles] explode all trees 29830
(fungicid* or antifungal or azole*):ti,ab,kw (Word variations have been searched) 2260
(Fluconazole or Fluconazol or Diflucan or Triflucan or Elazor or Biozolene or Flucostat or Pritenzol or Biocanol or Flucazol or Flunizol):ti,ab,kw (Word variations have been searched) 862
(Nystatin or Mycostatin or Nilstat or Nystop or Korostatin or Nystatinum or Biofanal or Nistatina or Nystaform or Nystatine):ti,ab,kw (Word variations have been searched) 336
(Amphotericin or Amphotericine or Fungizone or Ambisome or Amphocin or Abelcet or Amfotericina or Ampho‐Moronal or Amphotec):ti,ab,kw (Word variations have been searched) 813
#11 or #12 or #13 or #14 or #15 or #16 31769
#10 and #17 Online Publication Date from Jan 2012 to Jul 2014, in Trials 7
7 total results saved to Endnote library marked CENTRAL_17/07/2014 in Custom 4 field.
The International Clinical Trials Registry Platform (ICTRP) http://www.who.int/ictrp/en/ Searched online 17/07/14 Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(premature OR preterm OR "low birth weight") AND ( Nystatin OR Fluconazole OR Amphotericin)
5 total results saved to Endnote library marked ICTRP 17/07/2014 in Custom 4 field.
Clinical Trials.gov https://clinicaltrials.gov/ Searched online 17/07/14
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(premature OR preterm OR "low birth weight") AND ( Nystatin OR Fluconazole OR Amphotericin)
7 total results saved to Endnote library marked CLINICAL TRIALS.GOV 17/07/2014 in Custom 4 field.
Total Results
| Database | Results | After deduplication | Custom 4 field |
| MEDLINE and MEDLINE In‐Process | 100 | 90 | MEDLINE 17/07/2014 |
| EMBASE | 225 | 170 | EMBASE 17/07/2014 |
| CINAHL | 34 | 12 | CINAHL 17/07/2014 |
| CENTRAL | 7 | 3 | CENTRAL 17/07/2014 |
| The International Clinical Trials Registry Platform (ICTRP) | 5 | 5 | ICTRP 17/07/2014 |
| Clinical Trials.gov | 7 | 7 | CLINICAL TRIALS.GOV 17/07/2014 |
| Total | 378 | 287 |
Appendix 2. Update detailed electronic search strategy May 2015
Information Specialist: Colleen Ovelman, CNRG, US.
Search Date: May 18, 2015
Search Terms: (Mycoses OR fung* OR candid* OR Candida albicans OR Antifungal Agents OR Triazoles OR fluconazole OR azole OR Amphotericin B) AND
Plus the following database‐specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Total Studies Found: 367
Data and analyses
Comparison 1. Systemic antifungal agent versus placebo or no drug.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Invasive fungal infection | 10 | 1371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.59] |
| 2 Death prior to hospital discharge | 9 | 1360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.02] |
| 3 VABS‐II Domain Scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Communication | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐6.71, 10.71] |
| 3.2 Daily living skills | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐5.83, 6.83] |
| 3.3 Socialisation | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐2.64, 8.24] |
| 3.4 Motor skills | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐13.30, 7.30] |
| 4 Self esteem scores | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐10.74, 5.94] |
| 5 Neurodevelopmental impairment (composite) | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.81] |
| 6 Bayley‐III cognition composite score < 70 | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.67, 2.54] |
| 7 Cerebral Palsy | 1 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.45, 2.03] |
| 8 Deafness | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.39, 6.42] |
| 9 Blindness | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.06, 14.30] |
| 10 Retinopathy of prematurity | 5 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.20] |
| 11 Necrotising enterocolitis | 7 | 1152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.62, 1.29] |
| 12 Chronic lung disease | 4 | 922 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 13 Length of hospital stay | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐13.28, 13.08] |
Comparison 2. Systemic antifungal agent versus oral or topical antifungal prophylaxis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Invasive fungal infection | 3 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.07, 0.02] |
| 2 Death prior to hospital discharge | 3 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 3 Bronchopulmonary dysplasia | 1 | 171 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.16, 0.07] |
| 4 Necrotizing enterocolitis | 2 | 267 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 5 Retinopathy of prematurity | 1 | 171 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 6 Duration of intensive care unit stay | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐5.63, 7.63] |
2.3. Analysis.
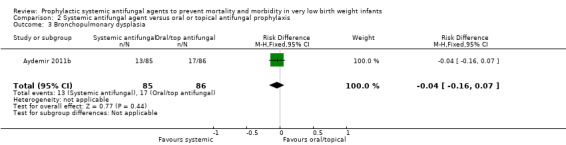
Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 3 Bronchopulmonary dysplasia.
2.4. Analysis.
Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 4 Necrotizing enterocolitis.
2.5. Analysis.
Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 5 Retinopathy of prematurity.
2.6. Analysis.
Comparison 2 Systemic antifungal agent versus oral or topical antifungal prophylaxis, Outcome 6 Duration of intensive care unit stay.
Comparison 3. One systemic antifungal agent versus another agent or dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Invasive fungal infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Death prior to hospital discharge | 2 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
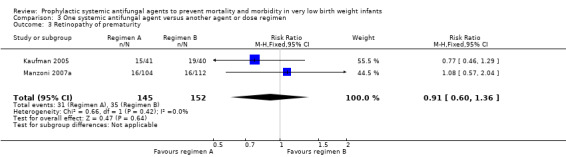
| 3 Retinopathy of prematurity | 2 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.36] |
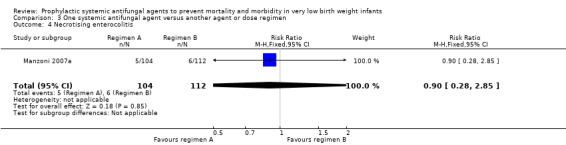
| 4 Necrotising enterocolitis | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.28, 2.85] |
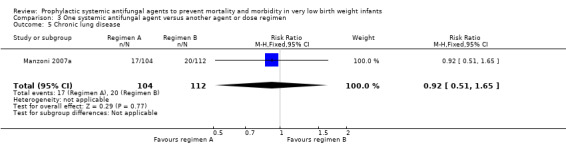
| 5 Chronic lung disease | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.51, 1.65] |
3.1. Analysis.
Comparison 3 One systemic antifungal agent versus another agent or dose regimen, Outcome 1 Invasive fungal infection.
3.2. Analysis.
Comparison 3 One systemic antifungal agent versus another agent or dose regimen, Outcome 2 Death prior to hospital discharge.
3.3. Analysis.
Comparison 3 One systemic antifungal agent versus another agent or dose regimen, Outcome 3 Retinopathy of prematurity.
3.4. Analysis.
Comparison 3 One systemic antifungal agent versus another agent or dose regimen, Outcome 4 Necrotising enterocolitis.
3.5. Analysis.
Comparison 3 One systemic antifungal agent versus another agent or dose regimen, Outcome 5 Chronic lung disease.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arrieta 2010.
| Methods | Randomised controlled trial | |
| Participants | 40 VLBW infants with central vascular catheter in situ | |
| Interventions | Intravenous liposomal amphotericin B 5 mg/kg (N = 20) versus dextrose water placebo (N = 20) once weekly until 6 weeks old | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Incidence of necrotising enterocolitis and severe intraventricular haemorrhage |
|
| Notes | Setting: Children's Hospital of Orange County, California, USA; 2004 to 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Pharmacy allocation from computer‐generated random sequence |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "open‐label, placebo‐controlled" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as "open‐label, placebo‐controlled" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "open‐label, placebo‐controlled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Aydemir 2011a.
| Methods | Randomised controlled trial | |
| Participants | 184 VLBW infants | |
| Interventions | Fluconazole 3 mg/kg (N = 93) every third day versus normal saline placebo (N = 91) until the 30th day after birth (or 45th day in ELBW infants) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fungi with native azole resistance Adverse drug reactions |
|
| Notes | Setting: Zekai Tahir Burak Maternity Hospital, Ankara, Turkey; 2008 to 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Aydemir 2011b.
| Methods | Randomised controlled trial | |
| Participants | 187 VLBW infants | |
| Interventions | Fluconazole 3 mg/kg (N = 93) every third day versus oral nystatin 100,000 U/ml 8 hourly (N = 94) until the 30th day after birth (or 45th day in ELBW infants) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fungi with native azole resistance Adverse drug reactions |
|
| Notes | Setting: Zekai Tahir Burak Maternity Hospital, Ankara, Turkey; 2008 to 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Report states placebo‐controlled but unclear how this was achieved |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Benjamin 2014.
| Methods | Randomised controlled trial | |
| Participants | 361 infants with BW < 750 grams and less than 120 hours old. Siblings were assigned to the same treatment group. Infants were excluded if they were receiving systemic antifungal therapy, were diagnosed with congenital or invasive candidiasis, or had liver or renal impairment. |
|
| Interventions | Fluconazole 6 mg/kg twice weekly (N = 188) versus normal saline placebo (N = 173) administered intravenously in infants with intravenous access, and enterally by orogastric tube to infants without intravenous access, for first six weeks of life. | |
| Outcomes | Death Definite or probable invasive candidiasis Neurodevelopmental impairment at 18 to 22 months corrected age Length of stay Chronic lung disease Retinopathy of prematurity Necrotising enterocolitis |
|
| Notes | Setting: 32 NICUs in United States. November 2008 to January 2011. Trial registration: http://clinicaltrials.gov/show/NCT00734539) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated. Interactive voice recognition system randomisation (Almac). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled. Blinding for the duration of the study, including at neurodevelopmental assessment at 19 to 22 months corrected age |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Cabrera 2002.
| Methods | Randomised controlled trial | |
| Participants | 11 VLBW infants with fungal colonisation detected on rectal, oro‐pharyngeal, or tracheal weekly surveillance cultures | |
| Interventions | Fluconazole 6 mg/kg (N = 6) versus placebo (N = 5) The dosage interval is not known. The study drug was given intravenously until intravenous access was no longer otherwise required, when oral study drug was given. The total duration of treatment with the study drug, or of follow‐up is not clear |
|
| Outcomes | Invasive fungal infection | |
| Notes | Published in abstract form only (some additional data obtained from authors) Setting: Medical School of Georgia, Augusta, USA; before 2002 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Allocation method not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Kaufman 2001.
| Methods | Randomised controlled trial | |
| Participants | 100 ELBW infants < 5 days old Infants with evidence of liver failure were not eligible for inclusion |
|
| Interventions | Fluconazole (N = 50) 3 mg/kg every third day for the first two weeks, then every second day during the third and fourth weeks, then daily during the fifth and sixth weeks versus normal saline placebo (N = 50). Assigned to intervention for six weeks, or until intravenous access discontinued | |
| Outcomes | Fungal colonisation and invasive infection Emergence of fluconazole resistance Adverse drug reactions Incidence of bacterial infections, necrotising enterocolitis, isolated intestinal perforation, ligation of patent ductus arteriosus, retinopathy of prematurity, abnormal findings on cranial ultrasonography Death prior to hospital discharge Neurodevelopmental status and quality of life of survivors at 8 to 10 years old assessed using the Vineland Adaptive Behavior Scales‐II (VABS‐II) and the Child Health Questionnaire Parent‐Completed Form 28 (CHQ‐PF28) respectively |
|
| Notes |
Kaufman 2001 reported that 13 of the 50 infants in the placebo group developed invasive fungal infection. Ten episodes were detected during the six‐weeks period when the intervention was administered, and three episodes occurred following discontinuation of the intervention. There were no episodes of invasive fungal infection in the fluconazole group during the six‐weeks intervention period. One case occurred following discontinuation of the intervention.
In the report of the outcomes in abstract form (published in Pediatric Research), the investigators state that invasive fungal infection occurred in nine, rather than 10, infants in the placebo group during the six‐weeks treatment period, and in two, rather than three, infants in the control group. These differences were related to less information being available at the time that the first (abstract) report was prepared (personal communication Dr Kaufman). Setting: University of Virginia School of Medicine, Charlottesville; 1998 to 2000 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, pharmacy randomly assigned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In‐hospital follow‐up complete. Long‐term follow‐up assessments (at 8 to 10 years) conducted on 46% and 43% of surviving children in the intervention and control groups, respectively |
Kaufman 2005.
| Methods | Randomised controlled trial | |
| Participants | 81 ELBW infants < 5 days old, and with either an endotracheal tube or central venous catheter in situ | |
| Interventions | Regimen A (N = 41): fluconazole 3 mg/kg every third day for the first two weeks, then every second day during the third and fourth weeks, then daily during the fifth and sixth weeks Regimen B (N = 40): fluconazole 3mg/kg twice weekly for 6 weeks Assigned to intervention for six weeks, or until intravenous access discontinued | |
| Outcomes | Fungal colonisation and invasive infection Mortality (all‐cause) was reported as a secondary outcome |
|
| Notes | Setting: University of Virginia School of Medicine, Charlottesville; before 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated, pharmacy randomly assigned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
Kicklighter 2001.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 103 VLBW infants < 3 days old Infants with evidence of liver failure, congenital heart disease, or congenital defects needing surgery were not eligible for inclusion |
|
| Interventions | Fluconazole 6 mg/kg (N = 53) or placebo (N = 50) every third day for one week than daily for three more weeks. Administered intravenously and then oro‐gastrically when tolerated | |
| Outcomes | Fungal colonisation and invasive infection Emergence of fluconazole resistance Adverse drug reactions Death prior to hospital discharge |
|
| Notes | Setting: Medical University of South Carolina; 1998 to 1999 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Random assignment by separate trials centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Kim 2010.
| Methods | Randomised controlled trial | |
| Participants | 55 VLBW infants with mechanical ventilation, central venous access and parenteral nutrition | |
| Interventions | Intravenous fluconazole commenced within first three days after birth for 4 to 6 weeks after birth at dose of 3 mg/kg (N = 28) versus placebo (N = 27) | |
| Outcomes | Invasive fungal infection Mortality Retinopathy of prematurity Necrotising enterocolitis Bronchopulmonary dysplasia Duration of hospital stay |
|
| Notes | Setting: Dongsan Medical Centre, Andong, Korea. Dates August 2008 to December 2009. Report is in Korean language: Dr Chun Soo Kim, principal investigator, kindly provided further information (July 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Kirpal 2015.
| Methods | Randomised controlled trial | |
| Participants | 80 VLBW infants receiving antibiotics for more than 3 days | |
| Interventions | Intravenous fluconazole (6 mg/kg) every other day for 7 days followed by every day till day 28 or discharge whichever was earlier (N = 40) versus placebo (N = 40). | |
| Outcomes | Invasive fungal infection Death |
|
| Notes | Setting: Departments of Paediatrics and Neonatology, Sharma PGIMS, Haryana and Fernandez Hospital, Hyderabad, India. Dates: May 2011 to November 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near complete follow‐up assessment |
Manzoni 2007a.
| Methods | Randomised controlled trial | |
| Participants | 322 VLBW infants | |
| Interventions | Fluconazole 3 mg/kg (N = 104) or 6 mg/kg (N = 112) versus placebo (N = 106) given every second day from birth for 30 days (or 45 days for ELBW infants (N = 216)) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fluconazole resistance |
|
| Notes | Setting: Eight level III neonatal units in Turin, Rome, Milan, or Pavia, Italy (2004 to 2006) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated and allocated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up assessment |
Manzoni 2007b.
| Methods | Randomised controlled trial | |
| Participants | 216 VLBW infants | |
| Interventions | Fluconazole 3 mg/kg (N = 104) versus 6 mg/kg (N = 112) given every second day from birth for 30 days (or 45 days for ELBW infants (N = 216)) | |
| Outcomes | Fungal colonisation and invasive infection Death prior to hospital discharge Emergence of fluconazole resistance |
|
| Notes | Setting: Eight level III neonatal units in Turin, Rome, Milan, or Pavia, Italy (2004 to 2006) Manzoni 2007b is the internal dose comparison of Manzoni 2007a intervention group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated and allocated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Mersal 2013.
| Methods | Randomised controlled trial | |
| Participants | 59 preterm infants < 30 weeks, birth weight < 1200 grams. Exclusion criteria: severe congenital anomalies, severe sepsis, intraventricular haemorrhage, persistent pulmonary hypertension, coagulopathy. |
|
| Interventions | Intravenous fluconazole 6 mg/kg every 72 hours until end of first week, then every 48 hours from second week to sixth week after birth (N = 35), or oral nystatin 1 ml (100,000 IU) every 8 h for six weeks (N = 24). Interventions commenced at one week of age. | |
| Outcomes | Invasive fungal infection Mortality |
|
| Notes | Location: Jeddah, Saudi Arabia. Participants enrolled February 2011 to February 2012. 60 infants were enrolled in the study. 3 were withdrawn from analysis (2 severe bacterial sepsis in fluconazole group, 1 Edwards syndrome). 57 were included in final analysis (24 in nystatin group, 33 in fluconazole group). The allocation group for the infant with Edwards syndrome is unknown. It is unclear as to whether the two infants with bacterial sepsis were withdrawn from analysis from the initial N = 35 (see above) or the N = 33 as the report quotes "2/33...in fluconazole group died because of bacterial sepsis...excluded from the study". The study author has been contacted and clarification if awaited. We have therefore assumed that there were a total of two deaths of N = 35 in the fluconazole group, and no deaths of N = 24 in the nystatin group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomisation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See notes above |
Parikh 2007.
| Methods | Randomised controlled trial | |
| Participants | 121 VLBW infants < 3 days old (one infant was withdrawn on day of randomisation and not included in any analyses) "Critically ill" infants and infants with biochemical evidence of hepatic insufficiency were not eligible for inclusion |
|
| Interventions | Fluconazole (N = 60) 6 mg/kg every third day for the first week after birth, then every day until four weeks versus "sugar solution" placebo (N = 60). Administered intravenously and then enterally when tolerated | |
| Outcomes | Fungal colonisation and invasive infection Emergence of fluconazole resistance Adverse drug reactions Death prior to hospital discharge |
|
| Notes | Most invasive fungal infection was due to non‐albicans Candida species (mainly C. glabrata) which were relatively less susceptible to fluconazole. Setting: KEM Hospital and Seth GS Medical College, Mumbai; 2003 to 2004 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Near‐complete follow‐up |
Violaris 2010.
| Methods | Randomised controlled trial | |
| Participants | 80 VLBW infants Haemodynamically unstable infants and infants with severe congenital anomalies or abnormal liver function tests were not eligible to participate |
|
| Interventions | Fluconazole (4 mg/kg) orally (N = 38) versus nystatin (100,000 units/kg/day) in each side of the mouth (N = 42), beginning on day five after birth. Medications were continued until full oral feedings were attained or systemic fungal infection was diagnosed | |
| Outcomes | Invasive fungal infection, invasive bacterial infection, biochemical indices related to liver function, mortality | |
| Notes | Setting: Brooklyn Hospital Center, New York; 1997 to 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computerised randomisation and allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unable to blind interventions |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind interventions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unable to blind interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghai 2006 | Observational (before‐after) study, not a randomised controlled trial |
| Al Qurashi 2008 | Observational (before‐after) study, not a randomised controlled trial |
| Aziz 2010 | Observational (before‐after) study, not a randomised controlled trial |
| Bertini 2005 | Observational (before‐after) study, not a randomised controlled trial |
| Dutta 2005 | Observational (before‐after) study, not a randomised controlled trial |
| Healy 2005 | Observational (before‐after) study, not a randomised controlled trial |
| Healy 2008 | Observational (before‐after) study, not a randomised controlled trial |
| Kim 2008 | Observational (before‐after) study, not a randomised controlled trial |
| Maede 2013 | Observational (before‐after) study, not a randomised controlled trial |
| Manzoni 2006 | Observational (before‐after) study, not a randomised controlled trial |
| Manzoni 2008 | Observational (before‐after) study, not a randomised controlled trial |
| McCrossan 2007 | Observational (before‐after) study, not a randomised controlled trial |
| Rueda 2010 | Observational (before‐after) study, not a randomised controlled trial |
| Uko 2006 | Observational (before‐after) study, not a randomised controlled trial |
| Wadhawan 2007 | Observational (before‐after) study, not a randomised controlled trial |
| Weitkamp 2008 | Observational (before‐after) study, not a randomised controlled trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Latif 2012.
| Methods | Randomised controlled trial |
| Participants | Infants and children in NICU and PICU |
| Interventions | Fluconazole versus placebo |
| Outcomes | Invasive fungal infection Mortality |
| Notes | Most participants were not of VLBW. The report does not provide subgroup data for VLBW infants. We have sought these data from the primary investigator (Sept 2015). |
Differences between protocol and review
None
Contributions of authors
Jemma Cleminson (JC) screened the titles and abstracts of all studies identified by the search strategy. JC and William McGuire (WM) screened the full text of the report of each study identified as of potential relevance. JC and WM extracted the data separately, compared data, and resolved differences by consensus. JC, Nicola Austin and WM completed the final review.
Sources of support
Internal sources
Christchurch Womens Hospital, New Zealand.
CRD, University of York, UK.
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
-
NIHR, UK.
This report is independent research funded by a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Arrieta 2010 {published data only}
- Arrieta AC, Shea K, Dhar V, Cleary JP, Kukreja S, Morris M, et al. Once‐weekly liposomal amphotericin B as Candida prophylaxis in very low birth weight premature infants: a prospective, randomized, open‐label, placebo‐controlled pilot study. Clinical Therapeutics 2010;32(2):265‐71. [PUBMED: 20206784] [DOI] [PubMed] [Google Scholar]
Aydemir 2011a {published data only}
- Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(3):F164‐8. [PUBMED: 20659937] [DOI] [PubMed] [Google Scholar]
Aydemir 2011b {published data only}
- Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Archives of Disease in Childhood 2011;96:F164‐8. [PUBMED: 20659937] [DOI] [PubMed] [Google Scholar]
Benjamin 2014 {published data only}
- Benjamin DK Jr, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, et al. Effect of Fluconazole Prophylaxis on Candidiasis and Mortality in Premature Infants. The Journal of the American Medical Association 2014;311(17):1742‐9. [PUBMED: 24794367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabrera 2002 {published data only}
- Cabrera C, Frank M, Carter D, Bhatia J. Fluconazole prophylaxis against systemic candidiasis after colonization: a randomized, double‐blinded study. Journal of Perinatology 2002;22(7):604. [Google Scholar]
Kaufman 2001 {published data only}
- Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. New England Journal of Medicine 2001;345(23):1660‐6. [PUBMED: 11759644] [DOI] [PubMed] [Google Scholar]
- Kaufman D, Boyle R, Hazen KC, Robinson M, Donowitz LG. Effectiveness of fluconazole prophylaxis in the prevention of colonization and invasive infection in preterm infants. Pediatric Research 2001;50:309A. [DOI] [PubMed] [Google Scholar]
- Kaufman D, Boyle R, Robinson M, Grossman LB. Long‐term safety of intravenous prophylactic fluconazole use in preterm infants less than 1000 grams. Pediatric Research 2003;53:2735. [Google Scholar]
- Kaufman DA, Cuff AL, Wamstad JB, Boyle R, Gurka MJ, Grossman LB, et al. Fluconazole prophylaxis in extremely low birth weight infants and neurodevelopmental outcomes and quality of life at 8 to 10 years of age. The Journal of Pediatrics 2011;158(5):759‐65.e1. [PUBMED: 21168853] [DOI] [PubMed] [Google Scholar]
Kaufman 2005 {published data only}
- Kaufman D, Boyle R, Hazen K, Patrie J, Robinson M, Grossman LB. Twice weekly fluconazole for prophylaxis for prevention of invasive Candida infection in high‐risk infants of <1000 grams birth weight. The Journal of Pediatrics 2005;147(2):172‐9. [PUBMED: 16126045] [DOI] [PubMed] [Google Scholar]
Kicklighter 2001 {published data only}
- Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics 2001;107(2):293‐8. [PUBMED: 11158461] [DOI] [PubMed] [Google Scholar]
- Kicklighter SD, Springer SC, Phaller MA, Messer S, Cox T, Hulsey TC, et al. Fluconazole for prophylaxis against fungal rectal colonization in the very low birth weight infant. Pediatric Research 2000;47:342A. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim CS. Prevention of invasive candida infections in the neonatal intensive care unit. Korean Journal of Pediatric Infectious Diseases 2011;18(1):15‐22. [Google Scholar]
- Kim CS, Hong SA, Lee SL, Kim HS. Effect of fluconazole prophylaxis to control candida infection in high‐risk preterm infants. Korean Journal of Perinatology 2010;21(4):378‐87. [Google Scholar]
Kirpal 2015 {published data only}
- Kirpal H, Gathwala G, Chaudhary U, Sharma D. Prophylactic fluconazole in very low birth weight infants admitted to neonatal intensive care unit: randomized controlled trial. The Journal of Maternal‐fetal & Neonatal Medicine 2015;Epub ahead of print:1‐5. [PUBMED: 25708488] [DOI] [PubMed] [Google Scholar]
Manzoni 2007a {published data only}
- Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. New England Journal of Medicine 2007;356(24):2483‐95. [PUBMED: 17568029] [DOI] [PubMed] [Google Scholar]
Manzoni 2007b {published data only}
- Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. New England Journal of Medicine 2007;356(24):2483‐95. [PUBMED: 17568029] [DOI] [PubMed] [Google Scholar]
Mersal 2013 {published data only}
- Mersal A, Alzahrani I, Azzouz M, Alsubhi A, Alsawaigh H, Albshri N, et al. Oral nystatin versus intravenous fluconazole as neonatal antifungal prophylaxis: non‐inferiority trial. Journal of Clinical Neonatology 2013;2(2):88‐92. [PUBMED: 24049751] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parikh 2007 {published data only}
- Parikh TB, Nanavati RN, Patankar CV, Rao S, Bisure K, Udani RH, et al. Fluconazole prophylaxis against fungal colonization and invasive fungal infection in very low birth weight infants. Indian Pediatrics 2007;44(11):830‐7. [PUBMED: 18057479] [PubMed] [Google Scholar]
Violaris 2010 {published data only}
- Violaris K, Carbone T, Bateman D, Olawepo O, Doraiswamy B, LaCorte M. Comparison of fluconazole and nystatin oral suspensions for prophylaxis of systemic fungal infection in very low birthweight infants. American Journal of Perinatology 2010;27(1):73‐8. [PUBMED: 19504425] [DOI] [PubMed] [Google Scholar]
- Violaris K, Doraiswamy B, Olawepo O, Gulrajani‐LaCorte M. Fluconazole versus nystatin prophylaxis for fungal infection in very low birth weight (VLBW) infants. Pediatric Research 1998;44:254A. [Google Scholar]
References to studies excluded from this review
Aghai 2006 {published data only}
- Aghai ZH, Mudduluru M, Nakhla TA, Amendolia B, Longo D, Kemble N, et al. Fluconazole prophylaxis in extremely low birth weight infants: association with cholestasis. Journal of Perinatology 2006;26(9):550‐5. [PUBMED: 16940972] [DOI] [PubMed] [Google Scholar]
Al Qurashi 2008 {published data only}
- Al Qurashi MA, Alallah J, Salma EB, et al. Fluconazole prophylaxis is effective in reducing systemic fungal infection in VLBW infants. A single center four years retrospective study. Pediatric Research. 2008; Vol. 63.
Aziz 2010 {published data only}
- Aziz M, Patel AL, Losavio J, Iyengar A, Berven M, Schloemer N, et al. Efficacy of fluconazole prophylaxis for prevention of invasive fungal infection in extremely low birth weight infants. The Pediatric Infectious Disease Journal 2010;29(4):352‐6. [PUBMED: 19934791] [DOI] [PubMed] [Google Scholar]
Bertini 2005 {published data only}
- Bertini G, Perugi S, Dani C, Filippi L, Pratesi S, Rubaltelli FF. Fluconazole prophylaxis prevents invasive fungal infection in high‐risk, very low birth weight infants. The Journal of Pediatrics 2005;147(2):162‐5. [PUBMED: 16126042] [DOI] [PubMed] [Google Scholar]
Dutta 2005 {published data only}
- Dutta S, Murki S, Varma S, Narang A, Chakrabarti A. Effects of cessation of a policy of neonatal fluconazole prophylaxis on fungal resurgence. Indian Pediatrics 2005;42(12):1226‐30. [PUBMED: 16424560] [PubMed] [Google Scholar]
Healy 2005 {published data only}
- Healy CM, Baker CJ, Zaccaria E, Campbell JR. Impact of fluconazole prophylaxis on incidence and outcome of invasive candidiasis in a neonatal intensive care unit. The Journal of Pediatrics 2005;147(2):166‐71. [PUBMED: 16126043] [DOI] [PubMed] [Google Scholar]
Healy 2008 {published data only}
- Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole‐resistant Candida species. Pediatrics 2008;121(4):703‐10. [PUBMED: 18381534] [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim MR, Chavda C, Jha V, Badugu S, Chung NY, Jean‐Baptiste D, et al. Prophylactic fluconazole therapy for very low birth weight infants colonised with Candida. Pediatric Research. 2008; Vol. 62.
Maede 2013 {published data only}
- Maede Y, Ibara S, Nagasaki H, Inoue T, Tokuhisa T, Torikai M, et al. Micafungin versus fluconazole for prophylaxis against fungal infections in premature infants. Pediatrics International 2013;55(6):727‐30. [PUBMED: 23773357] [DOI] [PubMed] [Google Scholar]
Manzoni 2006 {published data only}
- Manzoni P, Agrisio R, Mostert M, Leonessa M, Farina D, Latino MA, et al. Prophylactic fluconazole is effective in preventing fungal colonization and fungal systemic infections in preterm neonates: a single‐center, 6‐year retrospective cohort study. The Journal of Pediatrics 2006;117(1):e22‐32. [PUBMED: 16326690] [DOI] [PubMed] [Google Scholar]
Manzoni 2008 {published data only}
- Manzoni P, Leonessa M, Galletto P, Latino MA, Arisio R, Maule M, et al. Routine use of fluconazole prophylaxis in a neonatal intensive care unit does not select natively fluconazole‐resistant Candida subspecies. The Pediatric Infectious Disease Journal 2008;27(8):731‐7. [PUBMED: 18600191] [DOI] [PubMed] [Google Scholar]
McCrossan 2007 {published data only}
- McCrossan BA, McHenry E, O'Neill F, Ong G, Sweet DG. Selective fluconazole prophylaxis in high‐risk babies to reduce invasive fungal infection. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(6):F454‐8. [PUBMED: 17460023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rueda 2010 {published data only}
- Rueda K, Moreno MT, Espinosa M, Saez‐Llorens X. Impact of routine fluconazole prophylaxis for premature infants with birth weights of less than 1250 grams in a developing country. The Pediatric Infectious Disease Journal 2010;29(11):1050‐2. [PUBMED: 20571460] [DOI] [PubMed] [Google Scholar]
Uko 2006 {published data only}
- Uko S, Soghier LM, Vega M, Marsh J, Reinersman GT, Herring L, et al. Targeted short‐term fluconazole prophylaxis among very low birth weight and extremely low birth weight infants. Pediatrics 2006;117(4):1243‐52. [PUBMED: 16585321] [DOI] [PubMed] [Google Scholar]
Wadhawan 2007 {published data only}
- Wadhawan R, Dumois J, Escoto D, et al. Near elimination of Candida sepsis after fluconazole prophylaxis in ELBW infants‐experience from a tertiary level NICU. Pediatric Research. 2007; Vol. 62.
Weitkamp 2008 {published data only}
- Weitkamp JH, Ozdas A, LaFleur B, Potts AL. Fluconazole prophylaxis for prevention of invasive fungal infections in targeted highest risk preterm infants limits drug exposure. Journal of Perinatology 2008;28(6):405‐11. [PUBMED: 18185518] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Latif 2012 {published and unpublished data}
- Latif DA, Sultan MH, Mohamed HE. Efficacy of Prophylactic Fluconazole in Reducing Candidemia in High Risk NICU and PICU Patients. Life Science Journal 2012;9:817‐24. [Google Scholar]
Additional references
Adams‐Chapman 2013
- Adams‐Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al. Neurodevelopmental outcome of extremely low birth weight infants with candida infection. The Journal of Pediatrics 2013;163(4):961‐7. [PUBMED: 23726546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aliaga 2014
- Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics 2014;133(2):236‐42. [PUBMED: 24446441] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ascher 2012
- Ascher SB, Smith PB, Watt K, Benjamin DK, Cohen‐Wolkowiez M, Clark RH, et al. Antifungal therapy and outcomes in infants with invasive Candida infections. The Pediatric Infectious Disease Journal 2012;31(5):439‐43. [PUBMED: 22189522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 2009
- Austin N, Darlow BA, McGuire W. Prophylactic oral/topical non‐absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003478.pub3] [DOI] [PubMed] [Google Scholar]
Aydin 2012
- Aydin B, Dilli D, Zenciroglu A, Uzunalic N, Okumus N, Aydin M. Lengthening of QT interval caused by fluconazole in a newborn. Journal of Clinical Toxicology 2012;2:132. [Google Scholar]
Barton 2014
- Barton M, O'Brien K, Robinson JL, Davies DH, Simpson K, Asztalos E, et al. Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infectious Diseases 2014;14:327. [PUBMED: 24924877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2003
- Benjamin DK Jr, Poole C, Steibach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end‐organ damage: a critical appraisal of the literature using meta‐analytic techniques. Pediatrics 2003;112(3 Pt 1):634‐40. [PUBMED: 12949295] [DOI] [PubMed] [Google Scholar]
Benjamin 2006
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84‐92. [PUBMED: 16396864] [DOI] [PubMed] [Google Scholar]
Brecht 2009
- Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(1):F65‐9. [PUBMED: 18838467] [DOI] [PubMed] [Google Scholar]
Brion 2007
- Brion LP, Uko SE, Goldman DL. Risk of resistance associated with fluconazole prophylaxis: systematic review. Journal of Infection 2007;54(6):521‐9. [PUBMED: 17239952] [DOI] [PubMed] [Google Scholar]
Burwell 2006
- Burwell LA, Kaufman D, Blakely J, Stoll BJ, Fridkin SK. Antifungal prophylaxis to prevent neonatal candidiasis: a survey of perinatal physician practices. Pediatrics 2006;118(4):e1019‐26. [PUBMED: 16982807] [DOI] [PubMed] [Google Scholar]
Camacho‐Gonzalez 2013
- Camacho‐Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatric Clinics of North America 2013;60(2):367‐89. [PUBMED: 23481106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clerihew 2006
- Clerihew L, Lamagni TL, Brocklehurst P, McGuire W. Invasive fungal infection in very low birthweight infants: national prospective surveillance study. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91(3):F188‐92. [PUBMED: 16332924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clerihew 2008
- Clerihew L, McGuire W. Antifungal prophylaxis for very low birthweight infants: UK national survey. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(3):F238‐9. [PUBMED: 17768153] [DOI] [PubMed] [Google Scholar]
Friedman 2000
- Friedman S, Richardson SE, Jacobs SE, O'Brien K. Systemic candida infection in extremely low birth weight infants: short term morbidity and long term neurodevelopmental outcome. The Pediatric Infectious Disease Journal 2000;19(6):499‐504. [PUBMED: 10877162] [DOI] [PubMed] [Google Scholar]
Gussenhoven 1991
- Gussenhoven MJ, Haak A, Peereboom‐Wynia JD, van't Wout JW. Stevens‐Johnson syndrome after fluconazole. Lancet 1991;338(8759):120. [PUBMED: 1676446] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horbar 2002
- Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Members of the Vermont Oxford Network. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002;110(1 Pt 1):143‐51. [PUBMED: 12093960] [DOI] [PubMed] [Google Scholar]
Hornik 2012
- Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very‐low‐birth‐weight infants from a large group of neonatal intensive care units. Early Human Development 2012;88 Suppl 2:S69‐74. [PUBMED: 22633519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huttova 1998
- Huttova M, Hartmanova I, Kralinsky K, Filka J, Uher J, Kurak J, et al. Candida fungemia in neonates treated with fluconazole: report of forty cases, including eight with meningitis. The Pediatric Infectious Disease Journal 1998;17(11):1012‐5. [PUBMED: 9849984] [DOI] [PubMed] [Google Scholar]
Kaguelidou 2012
- Kaguelidou F, Pandolfini C, Manzoni P, Choonara I, Bonati M, Jacqz‐Aigrain E. European survey on the use of prophylactic fluconazole in neonatal intensive care units. European Journal of Pediatrics 2012;171(3):439‐45. [PUBMED: 21912893] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlowicz 2002
- Karlowicz MG, Rowen JL, Barnes‐Eley ML, Burke BL, Lawson ML, Bendel CM, et al. The role of birth weight and gestational age in distinguishing extremely low birth weight infants at high risk of developing candidemia from infants at low risk: a multicenter study. Pediatric Research 2002;51:301A. [Google Scholar]
Kaufman 2004
- Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very‐low‐birth‐weight infants. Clinical Microbiology Reviews 2004;17(3):638‐80. [PUBMED: 15258097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 2006
- Kaufman DA, Gurka MJ, Hazen KC, Boyle R, Robinson M, Grossman LB. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. The Pediatric Infectious Disease Journal 2006;25(8):733‐7. [PUBMED: 16874174] [DOI] [PubMed] [Google Scholar]
Kaufman 2010
- Kaufman DA, Manzoni P. Strategies to prevent invasive candidal infection in extremely preterm infants. Clinics in Perinatology 2010;37(3):611‐28. [PUBMED: 20813274] [DOI] [PubMed] [Google Scholar]
Koklu 2014
- Koklu E, Kalay S, Koklu S, Ariguloglu EA. Fluconazole administration leading to anaphylactic shock in a preterm newborn. Neonatal Network 2014;33(2):83‐5. [PUBMED: 24589899] [DOI] [PubMed] [Google Scholar]
Kossoff 1998
- Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: trends during fifteen years and clinical features of 111 cases. The Pediatric Infectious Disease Journal 1998;17(6):504‐8. [PUBMED: 9655543] [DOI] [PubMed] [Google Scholar]
Makhoul 2002
- Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late‐onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics 2002;109(1):34‐9. [PUBMED: 11773539] [DOI] [PubMed] [Google Scholar]
Manzoni 2007
- Manzoni P, Farina D, Galletto P, Leonessa M, Priolo C, Arisio R, et al. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. Journal of Perinatal Medicine 2007;35(3):220‐6. [PUBMED: 17378718] [DOI] [PubMed] [Google Scholar]
Neely 2001
- Neely MN, Schreiber JR. Fluconazole prophylaxis in the very low birth weight infant: not ready for prime time. Pediatrics 2001;107(2):404‐5. [PUBMED: 11158475] [DOI] [PubMed] [Google Scholar]
O'Grady 2008
- O'Grady MJ, Dempsey EM. Antifungal prophylaxis for the prevention of neonatal candidiasis?. Acta Paediatrica 2008;97(4):430‐3. [PUBMED: 18363952] [DOI] [PubMed] [Google Scholar]
Oeser 2013
- Oeser C, Lamagni T, Heath PT, Sharland M, Ladhani S. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000‐2009. The Pediatric Infectious Disease Journal 2013;32(1):23‐6. [PUBMED: 23241987] [DOI] [PubMed] [Google Scholar]
Oeser 2014
- Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal invasive fungal infection in England 2004‐2010. Clinical Microbiology and Infection 2014;20:936‐41. [PUBMED: 24479862] [DOI] [PubMed] [Google Scholar]
Pappu‐Katikaneni 1990
- Pappu‐Katikaneni LD, Rao KP, Banister E. Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses 1990;33(1):20‐3. [PUBMED: 2342516] [DOI] [PubMed] [Google Scholar]
Robinson 2009
- Robinson JL, Davies HD, Barton M, O'Brien K, Simpson K, Asztalos E, et al. Characteristics and outcome of infants with candiduria in neonatal intensive care ‐ a Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infectious Diseases 2009;9:183. [PUBMED: 19930662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saiman 2000
- Saiman L, Ludington E, Pfaller M, Rangel‐Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. The Pediatric Infectious Disease Journal 2000;19(4):319‐24. [PUBMED: 10783022] [DOI] [PubMed] [Google Scholar]
Sarvikivi 2008
- Sarvikivi E, Lyytikäinen O, Soll DR, Pujol C, Pfaller MA, Richardson M, et al. Emergence of fluconazole resistance in a Candida parapsilosis strain that caused infections in a neonatal intensive care unit. Journal of Clinical Microbiology 2005;43(6):2729‐35. [PUBMED: 15956390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schelonka 2003
- Schelonka RL, Moser SA. Time to positive culture results in neonatal Candida septicemia. The Journal of Pediatrics 2003;142(5):564‐5. [PUBMED: 12756391] [DOI] [PubMed] [Google Scholar]
Shane 2013
- Shane AL, Stoll BJ. Recent developments and current issues in the epidemiology, diagnosis, and management of bacterial and fungal neonatal sepsis. American Journal of Perinatology 2013;30(2):131‐41. [PUBMED: 23297182] [DOI] [PubMed] [Google Scholar]
Stoll 1996
- Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Late‐onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. The Journal of Pediatrics 1996;129(1):63‐71. [PUBMED: 8757564] [DOI] [PubMed] [Google Scholar]
Stoll 2002
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late‐onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110(2 Pt 1):285‐91. [PUBMED: 12165580] [DOI] [PubMed] [Google Scholar]
Stoll 2003
- Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Seminars in Perinatology 2003;27(4):293‐301. [PUBMED: 14510320] [DOI] [PubMed] [Google Scholar]
Vergnano 2011
- Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Archives of Disease in Childhood. Fetal and Neonatal Edition 2011;96(1):F9‐14. [PUBMED: 20876594] [DOI] [PubMed] [Google Scholar]
Wynn 2012
- Wynn JL, Tan S, Gantz MG, Das A, Goldberg RN, Adams‐Chapman I, et al. Outcomes following candiduria in extremely low birth weight infants. Clinical Infectious Diseases 2012;54(3):331‐9. [PUBMED: 22144537] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Austin 2013
- Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003850.pub4] [DOI] [PubMed] [Google Scholar]
Clerihew 2007
- Clerihew L, Austin N, McGuire W. Prophylactic systemic antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 4, Issue 2007 10.1002/14651858.CD003850.pub3. [DOI: 10.1002/14651858.CD003850.pub3] [DOI] [PubMed] [Google Scholar]
McGuire 2004
- McGuire W, Clerihew L, Austin N. Prophylactic intravenous antifungal agents to prevent mortality and morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003850.pub2] [DOI] [PubMed] [Google Scholar]


