Abstract
Background
Antihypertensive drugs from the thiazide diuretic drug class have been shown to reduce mortality and cardiovascular morbidity. Loop diuretics are indicated and used to treat hypertension, but a systematic review of their blood pressure‐lowering efficacy or effectiveness in terms of reducing cardiovascular mortality or morbidity from randomized controlled trial (RCT) evidence has not been conducted.
Objectives
To determine the dose‐related decrease in systolic or diastolic blood pressure, or both, as well as adverse events leading to participant withdrawal and adverse biochemical effects (serum potassium, uric acid, creatinine, glucose and lipids profile) due to loop diuretics versus placebo control in the treatment of people with primary hypertension.
Search methods
We searched the Cochrane Hypertension Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 9), MEDLINE, MEDLINE In‐Process, EMBASE, and ClinicalTrials.gov to 27 October 2014.
Selection criteria
We included double‐blind randomized placebo‐controlled trials of at least three weeks duration comparing loop diuretic with a placebo in people with primary hypertension defined as blood pressure greater than 140/90 mmHg at baseline.
Data collection and analysis
Two review authors independently assessed the risk of bias and extracted data. We used weighted mean difference and a fixed effects model to combine continuous outcome data. We analysed the drop outs due to adverse effects using relative risk ratio.
Main results
Nine trials evaluated the dose‐related blood pressure‐lowering efficacy of five drugs within the loop diuretics class (furosemide 40 mg to 60 mg, cicletanine 100 mg to 150 mg, piretanide 3 mg to 6 mg, indacrinone enantiomer ‐2.5 mg to ‐10.0/+80 mg, and etozolin 200 mg) in 460 people with baseline blood pressure of 162/103 mmHg for a mean duration of 8.8 weeks. The best estimate of systolic/diastolic blood pressure‐lowering efficacy of loop diuretics was ‐7.9 (‐10.4 to ‐5.4) mmHg/ ‐4.4 (‐5.9 to ‐2.8) mmHg. Withdrawals due to adverse effects and serum biochemical changes did not show a significant difference.
We performed additional searches in 2012 and 2014, which found no additional trials meeting the minimum inclusion criteria.
Authors' conclusions
Based on the limited number of published RCTs, the systolic/diastolic blood pressure‐lowering effect of loop diuretics is ‐8/‐4 mmHg, which is likely an overestimate. We graded the quality of evidence for both systolic and diastolic blood pressure estimates as "low" due to the high risk of bias of included studies and the high likelihood of publication bias. We found no clinically meaningful blood pressure‐lowering differences between different drugs within the loop diuretic class. The dose‐ranging effects of loop diuretics could not be evaluated. The review did not provide a good estimate of the incidence of harms associated with loop diuretics because of the short duration of the trials and the lack of reporting of adverse effects in many of the trials.
Plain language summary
Loop diuretics cause modest blood pressure lowering
While more commonly used to reduce water retention, loop diuretics are are also indicated for lowering elevated blood pressure. We asked to what degree this drug class lowers blood pressure, whether individual drugs within the class produce different effects, and what the estimate of harms is associated with this class of drugs. We searched the available scientific literature to find all the trials that had addressed these questions. We found 9 trials studying the blood pressure‐lowering ability of 5 different loop diuretics (furosemide, cicletanine, piretanide, indacrinone and etozolin) in 460 participants. The blood pressure‐lowering effect was modest, with systolic pressure lowered by 8 mmHg and diastolic pressure by 4 mmHg. No loop diuretic drug appears to be any better or worse than others in terms of blood pressure‐lowering ability. Due to lack of reporting and the short duration of included trials, this review could not provide an estimate of the harms associated with loop diuretics.
Summary of findings
for the main comparison.
| Loop diuretics compared with placebo for primary hypertension | ||||
|
Patient or population: People with primary hypertension Settings: Outpatient Intervention: Loop diuretics at various doses Comparison: Placebo control | ||||
| Outcomes | MD with (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Decrease in SBP mmHg mean duration 8.8 weeks |
‐7.9(‐10.4 to ‐5.4) | 460 (9) |
⊕⊕⊝⊝ low1,2 | Not significantly different from thiazides, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or renin inhibitors. |
|
Decrease in DBP mmHg mean duration 8.8 weeks |
‐4.4(‐5.9 to ‐2.8) | 460 (9) |
⊕⊕⊝⊝ low1,2 | Not significantly different from thiazides, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or renin inhibitors. |
| CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1. Wide confidence intervals
2. High risk of bias including publication bias
Background
Loop diuretics are indicated as pharmacological agents for the treatment of hypertension. Of all known diuretics they are the most powerful, capable of causing the excretion of 15% to 25% of filtered sodium. Loop diuretics include furosemide (formerly frusemide), bumetanide, torsemide, piretanide, azosemide, ethacrynic acid, indacrinone, muzolimine, ozolinone, xipamide, and tienilic acid.
The first loop diuretics were mercurial agents, which are of historic interest only. In 1959 chemists synthesized furosemide, which was active orally and soon became the loop diuretic of choice. Additional loop diuretics have since been developed; the differences between them is in their pharmacokinetics.
Loop diuretics act from the lumen side of the nephron, hence urinary, rather than serum amounts, are the major determinants of response. Since they are extensively bound to serum protein albumin, they cannot enter the tubular lumen by glomerular filtration and reach this site by active secretion by the organic acid transport pump at the straight segment of the loop of Henle. These drugs are rapidly absorbed, with peak serum concentration attained within 0.5 to 2 hours. The onset of action of furosemide after oral dosing is slower than that of other loop diuretics because its absorption rate is slower than the rate of elimination (Seldin 1997).
The bioavailability of loop diuretics varies, with that of azosemide being 10%; bumetanide and torsemide 80% to 100%; and furosemide 40% to 60%, with a half‐life of 1 to 2 hours. Muzolimine, xipamide, and ozolinone (the active metabolite of etozolin) have longer half‐lives of 6 to 15 hours, and elimination is unchanged in people with renal insufficiency or congestive heart failure. The route of elimination varies. About half of intravenous furosemide is eliminated unchanged in the urine and most of the remainder is glucuronidated in the kidney itself. By contrast, nonrenal elimination of bumetanide and torsemide is in the liver.
Loop diuretics act primarily on the thick ascending limb of the loop of Henle, inhibiting the transport of sodium chloride out of the tubule into the interstitial tissue by inhibiting the Na+/K+/2Cl‐ cotransporter on the apical membrane. Furosemide and bumetanide have a direct inhibiting effect on the carrier, acting on the chloride binding site while ethacrynic acid forms a complex with cysteine, the complex being active form of the drug (Materson 1983). They lower blood pressure acutely because of their potent natriuretic effect and consequently fall in circulating blood volume. However, when used alone, loop diuretics may not have useful long‐term antihypertensive effect.
Another group of diuretics, the thiazides, inhibit Na+/Cl‐ reabsorption in the early part of distal tubule. Thiazide and thiazide‐related drugs include hydrochlorothiazide, bendroflumethiazide, chlorothiazide, methyclothiazide, trichlormethiazide, cyclothiazide, and chlorthalidone.
It is currently unknown whether the blood pressure‐lowering efficacy of either thiazide or loop diuretics is due solely to their diuretic effect. Diuretics were originally prescribed in starting doses that exceed the average prescribed dose that is used today.
Although the aim of antihypertensive drug therapy is to reduce systolic as well as diastolic blood pressure, the ultimate clinical goal is to lower the risk of cardiovascular‐related mortality and morbidity. A systematic review based on 19 RCTs in 39,713 participants has shown proven benefit in terms of reduced mortality (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.83 to 0.96), stroke (RR 0.63, 95% CI 0.57 to 0.71), coronary heart disease (RR 0.84, 95% CI 0.75 to 0.95), and cardiovascular morbidity (RR 0.70, 95% CI 0.66 to 0.76). Low‐dose thiazides reduced coronary heart disease (RR 0.72, 95% CI 0.61 to 0.84), but high‐dose thiazides (11 RCTs) did not (RR 1.01, 95% CI 0.85 to 1.20) (Wright 2009). In the same systematic review, first‐line low‐dose thiazide decreased blood pressure by 13 mmHg (99% CI 12 to 14)/5 (99% CI 4 to 6) as compared to placebo or no treatment, and first‐line high‐dose thiazide decreased blood pressure by 14 mmHg (99% CI 13 to 15)/7 (99% CI 6 to 8) (Wright 2009).
No systematic review of loop diuretics has been identified that measured either their blood pressure‐lowering efficacy or effectiveness in lowering cardiovascular mortality or morbidity in the treatment of primary hypertension. It is also important to establish whether loop diuretics lower blood pressure to the same degree as thiazide diuretics and other classes of antihypertensive drugs, and to know the blood pressure‐lowering dose response relationship to other effects of loop diuretics, such as the adverse metabolic effects. Individual drugs within the diuretic drug class might have differing dose‐related blood pressure‐lowering efficacy and adverse effects.
The aims of this systematic review were to:
determine the lowest dose with the maximum blood pressure‐lowering efficacy for each drug within the loop diuretic class.
establish dose equivalencies of different drugs within the loop diuretic family.
The information derived from this review should facilitate future reviews of head‐to‐head comparisons with other drug classes and assist clinicians in determining when to choose a loop diuretic and what dose to use.
Objectives
Primary objective
To determine the dose‐related decrease in systolic or diastolic blood pressure, or both, due to loop diuretics versus placebo control in the treatment of people with primary hypertension.
Secondary objectives
To determine the dose‐related adverse events leading to participant withdrawal and adverse biochemical effects including serum potassium, uric acid, creatinine, glucose, and lipids profile.
Methods
Criteria for considering studies for this review
Types of studies
Study design had to meet the following criteria: double‐blind placebo‐controlled trials; random allocation to loop diuretic group and parallel placebo group; duration of follow‐up of at least 3 weeks; blood pressure measurement at baseline (following washout) and at 1 or more time points between 3 to 12 weeks after starting treatment.
Types of participants
Participants had to have a baseline systolic blood pressure (SBP) of at least 140 mmHg and/or diastolic blood pressure (DBP) of at least 90 mmHg, measured in a standard way. We excluded from the analysis people with significant renal insufficiency and a documented serum creatinine level greater than 1.5 times the normal values. We did not restrict participants by age, gender, baseline risk, or any other comorbid conditions.
Types of interventions
Monotherapy with any loop diuretic, including: furosemide, bumetanide, piretanide, torsemide, azosemide, ethacrynic acid, tripamide, phenoxybenzoic acid, muzolimine, indacrinone, etozolin, ozolinone, cicletanine, tienilic acid (ticrynafen), and tizolemide.
Data from trials in which titration to a higher dose was based on blood pressure response were not eligible. Stepped‐up therapy given only to nonresponders would bias the results and therefore was not included in the analysis. Potassium supplementation was allowed in people with low serum potassium levels.
Types of outcome measures
Primary outcomes
Change in systolic and diastolic blood pressure compared to placebo. If blood pressure measurements were available at more than one time during the 24‐hour period, we used the trough measurement. Peak level was defined as blood pressure measurement within 12 hours of the dose, and trough level was defined as blood pressure measurements between 12 and 24 hours. If blood pressure measurements were available at more than 1 week within the 3‐ to 12‐week window, the weighted means of blood pressure measurement was calculated and used as the best estimate of the treatment effect.
Secondary outcomes
The number of participant withdrawals due to adverse events compared to placebo.
Change in the levels of serum potassium, uric acid, creatinine, glucose, and lipids compared to placebo. If measurements were available at more than one time within the acceptable window, then the weighted mean data was calculated and used as the best estimate of the treatment effect.
Search methods for identification of studies
We searched the following databases for primary studies: the Cochrane Hypertension Group Specialised Register (all years to 27 October 2014), the Cochrane Central Register of Controlled Trials (CENTRAL, 2014, Issue 9) via the Cochrane Register of Studies Online, Ovid MEDLINE (1946 to 27 October 2014), Ovid EMBASE (1974 to 27 October 2014), and ClinicalTrials.gov (all years to 27 October 2014). We searched the Database of Abstracts of Reviews of Effects (DARE, 2014 Issue 9) for related reviews.
The Cochrane Hypertension Group Specialised Register includes controlled trials from searches of AGRICOLA, Allied and Complementary Medicine (AMED), BIOSIS, CAB Abstracts, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, Food Science and Technology Abstracts (FSTA), Global Health, International Pharmaceutical Abstracts (IPA), LILACS, MEDLINE, ProQuest Dissertations & Theses Database, PsycINFO, Scirus, Web of Science, and the World Health Organization International Clinical Trials Registry Platform (ICTRP).
We searched electronic databases using a strategy combining the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms relating to loop diuretics and hypertension. We used no language restrictions. We translated the MEDLINE search strategy (Appendix 1) into EMBASE (Appendix 2), CENTRAL (Appendix 3), and the Cochrane Hypertension Group Specialised Register (Appendix 4) using the appropriate controlled vocabulary as applicable. Previous search strategies are in Appendix 5.
Searching other resources
We identified reference lists of all papers and relevant reviews.
We contacted authors of trials reporting incomplete information for the missing information.
Data collection and analysis
Selection of studies
Two review authors (VM, CJ) independently screened the titles and abstracts identified as a result of the search strategies prior to January 2009. Two review authors (VM, PR) independently screened the titles and the abstracts identified as a result of the search strategies from January 2009 to February 2012. Two review authors (PR, CJ) independently screened the titles and the abstracts identified as a result of the search strategies from February 2012 to October 2014. We rejected articles on initial screen if we were able to determine from the title or the abstract that the article was not a report of a randomized placebo‐controlled trial or did not meet the inclusion criteria. We then retrieved the full text of the remaining articles. We searched the bibliographies of pertinent articles, reviews, and texts for additional citations. Two review authors independently assessed the eligibility of the trials using a trial selection form. Any discrepancies were resolved by JMW and KB.
Data extraction and management
Two review authors (VM, CJ) abstracted data independently using a standard form and then cross‐checked. If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were preferred because of possible measurement error when estimating from graphs. A second review author confirmed all numeric calculations and extractions from graphs or figures. We resolved any discrepancies by consensus.
Assessment of risk of bias in included studies
Two review authors (VM, CJ) checked the methodological quality of the included studies according to The Cochrane Collaboration's recommended tool. We based the risk of bias within each included study on the six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting, with ratings of Yes (low risk of bias); No (high risk of bias); and Unclear (uncertain risk of bias). Refer to the ‘Risk of bias’ table for each study. Also see Figure 1 and Figure 2 for a graphical representation of our assessment of the risk of bias in the included studies.
1.
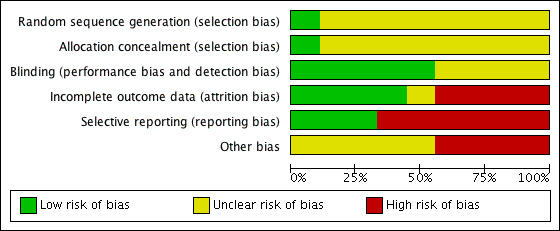
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
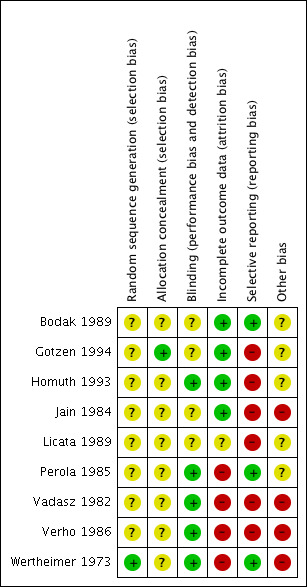
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
The position of the patient during blood pressure measurement can affect the blood pressure‐lowering effect. However, so as not to lose valuable data if only one position was reported, we included data from that position. When blood pressure measurement data in more than one position was available, sitting blood pressure was the first preference. If standing and supine blood pressure measurements were available, we used standing blood pressure.
Unit of analysis issues
Each trial is a unit in the systematic review analysis. For each trial, the placebo control group or the loop diuretic therapy group were compared to each other only within that trial and not with participants in any other trial.
Dealing with missing data
In case of missing information in the included studies, we contacted investigators through email, letter, and/or fax to obtain the missing information. Where missing information was not available, we included the best estimate based on information in the same trial or from other trials using the same dose.
In case of missing standard deviation (SD) of the change in blood pressure, we imputed the SD based on information in the same trial or from other trials using the same dose. We used the following hierarchy (listed from high to low preference) to impute SD values:
SD of change in blood pressure from a different position than that of the blood pressure data used
SD of blood pressure at the end of treatment
SD of blood pressure at the end of treatment measured from a different position than that of the blood pressure data used
SD of blood pressure at baseline, unless this measure was used for entry criteria (Musini 2009)
mean SD of change in blood pressure from other trials using the same drug and dose
mean weighted SD of change available from all other trials meeting the inclusion criteria
Most trials reported end‐of‐treatment SD, which we imputed as SD of change from baseline for SBP as well as DBP. For the two piretanide trials (Homuth 1993, Verho 1986), since the end‐of‐treatment SD was not reported, the baseline SD of the SBP or DBP could be used if it was not the entry criteria for inclusion in the study. Homuth 1993 did not report the criteria for inclusion (states as hypertensive patients), and Verho 1986 included patients based on DBP but did not report the baseline SD values for both SBP and DBP. We therefore calculated the mean weighted SD at the end of treatment across all trials and imputed it as SD of change. It was calculated as 13.7 mmHg for SBP in the treatment group and 15.9 mmHg for the placebo group, and 8.3 mmHg for DBP in the treatment group and 8.8 mmHg for the placebo group.
Data synthesis
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (2011 edition). We used Review Manager 5.3 (RevMan 2014) software to perform data synthesis and analyses. We expressed data for changes in blood pressure as well as serum levels of potassium, uric acid, creatinine, glucose, and lipids profile as the mean (±SD) change from baseline to follow‐up and combined using a mean difference method. We expressed withdrawals due to adverse effects (dichotomous outcome) for each comparison as risk ratios (RR) with 95% confidence intervals (CI). If there was a statistically significant RR difference, we also calculated the associated number needed to treat for an additional beneficial outcome/harmful outcome.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses according to age, gender, race, comorbid conditions, and baseline severity of hypertension (mild, moderate, or severe), but these were not possible due to insufficient data.
We performed test for heterogeneity of treatment effect between the trials using a standard Chi2 statistic for heterogeneity as mentioned in RevMan (RevMan 2014). We applied the fixed‐effect model to obtain summary statistics of pooled trials, unless significant between‐study heterogeneity was present, in which case we used the random‐effects model.
We used the funnel plot to examine publication bias.
Sensitivity analysis
We were to test the robustness of the results using several sensitivity analyses including:
trials of high quality versus poor quality
fixed‐effect versus random‐effects model
trials with blood pressure data measured in sitting position versus other measurements
trials with peak blood pressure measurements versus trials with trough blood pressure measurements
trials with published SDs of blood pressure change versus imputed SDs
trials that are industry sponsored versus non‐industry sponsored
However, due to lack of sufficient data it was not possible to perform any sensitivity analyses.
Results
Description of studies
The original search (Appendix 5) led to 380 citations, of which 264 (69.5%) were excluded after reading the abstract because they did not meet the minimum inclusion criteria. Of the remaining 116 citations, 74 (25.6%) were excluded after retrieving the trials and reading the detailed methodology. We considered 42 (11.0%) potentially appropriate studies for inclusion, of which 33 studies had to be excluded for various documented reasons (see Characteristics of excluded studies).
We ran the search again with an updated search strategy from January 2009 to February 2012 (Appendix 5). This led to 400 additional citations, of which no new studies met the minimum inclusion criteria.
We ran the search a third time with an updated search strategy on 27 October 2014 (Appendix 1; Appendix 2; Appendix 3; Appendix 4), which led to 721 additional citations. After removal of duplicates, we were left with 364 additional citations. 360 citations were not relevant to this review. The remaining four did not meet the minimum inclusion criteria for this review and were excluded.
Nine double‐blind RCTs out of the 1144 citations (0.8%) met the minimum inclusion criteria. These trials included 460 participants with mean age of 54.4 years (see Characteristics of included studies).
A complete account of the studies identified is presented in the study flow diagrams (Figure 3, Figure 4, Figure 5).
3.
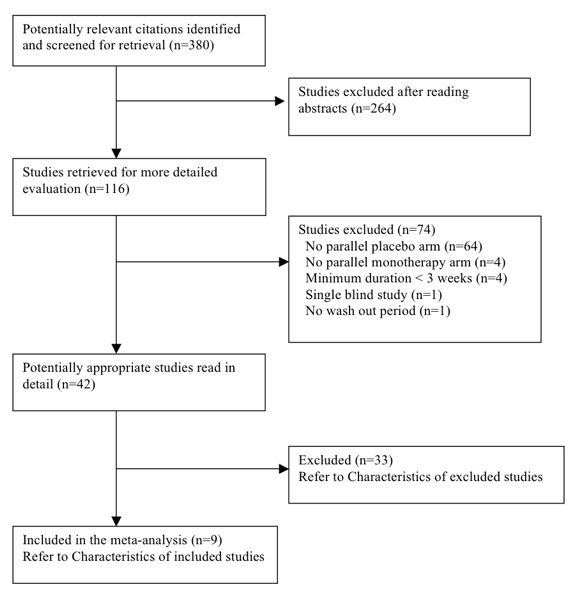
QUOROM Diagram ‐ Search: until January 2009
4.
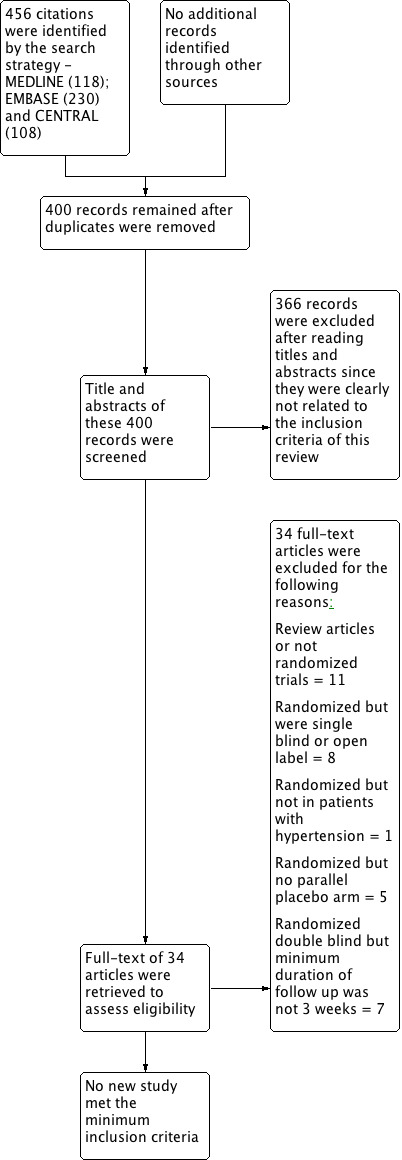
Updated PRISMA diagram ‐ Search updated from Jan 2009 to February 2012
5.
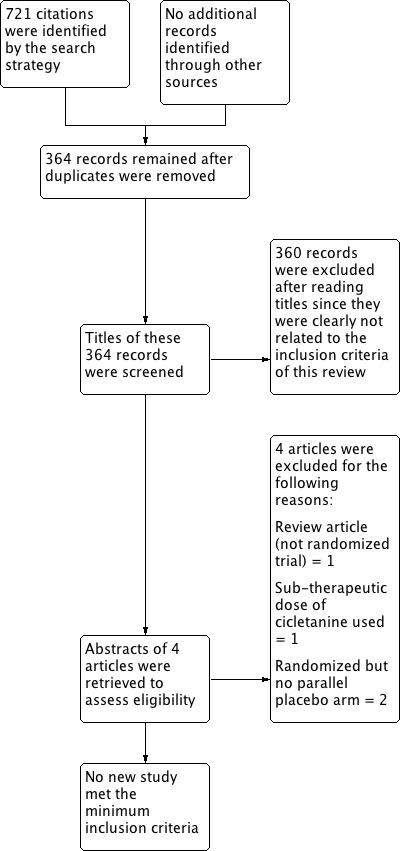
Updated PRISMA diagram ‐ Search updated on 27 October 2014
Placebo control was compared to cicletanine 100 mg in one trial (Gotzen 1994) and 150 mg in one trial (Bodak 1989); to furosemide 40 mg in two trials (Perola 1985, Wertheimer 1973) and to furosemide 60 mg in one trial (Vadasz 1982); to piretanide 3 mg in one trial (Homuth 1993) and to piretanide 6 mg in two trials (Homuth 1993, Verho 1986); to indacrinone at doses of ‐2.5/+80 mg, ‐5.0/+80 mg, or ‐10/+80 mg in one trial (Jain 1984); and to etozolin 200 mg in one trial (Licata 1989).
Refer to Characteristics of included studies for details on baseline characteristics.
The earliest study evaluating the antihypertensive efficacy of loop diuretic monotherapy using office blood pressure measurements was published in 1973. The other eight studies were published during the 1980s and early 1990s. Since 1994 no further studies have been published. Of the nine included studies, seven were published in English, one in German (Gotzen 1994), and one in French (Bodak 1989). One of the included studies was industry sponsored (Jain 1984); the remaining eight studies did not report the source of funding.
Since the number of trials meeting the inclusion criteria was very limited, to maximize data inclusion we included data in the Bodak 1989 trial at the end of 6 months, although the publication did not report data between 3 and 12 weeks. All other trials ranged from 4 to 12 weeks duration. The mean weighted duration of treatment across all trials was 8.8 weeks. Including or excluding this trial from the analysis showed no significant difference in SBP or DBP MD. The mean weighted baseline SBP/DBP across all nine trials was 162.3/103.4 mmHg.
Risk of bias in included studies
Refer to Figure 1 and Figure 2 for the overall 'Risk of bias' assessment.
Only Wertheimer 1973 and Gotzen 1994reported random sequence generation and allocation concealment, respectively. Five of the nine trials (55% of included studies) reported blinding adequately. Four of the nine trials (44%) reported incomplete outcome data. Only two of the nine trials (22%) reported all outcome data. Four of the nine trials (44%) had high risk of other bias, and in the remaining five trials it was unclear if other risk was present. In summary, the effect size reported in this review could be an overestimate due to the potential for high risk of bias.
Another source of bias likely to have a significant impact on this review is the selective publication of trials. Since it only included and appraised published trial evidence, this review was evaluated for the existence of publication bias. In the absence of bias, the funnel plot should resemble a symmetrical inverted funnel. The most common way to investigate whether or not a review is subject to publication bias is to examine for funnel plot asymmetry, as smaller studies with null results remained unpublished. Refer to Figure 6 and Figure 7, which show that publication bias was detected.
6.
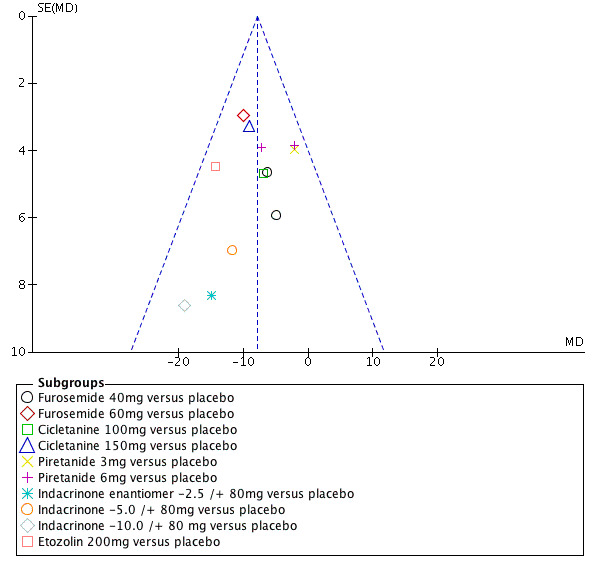
Funnel plot of comparison: 1 Loop diuretics vs placebo, outcome: 1.1 SBP.
7.
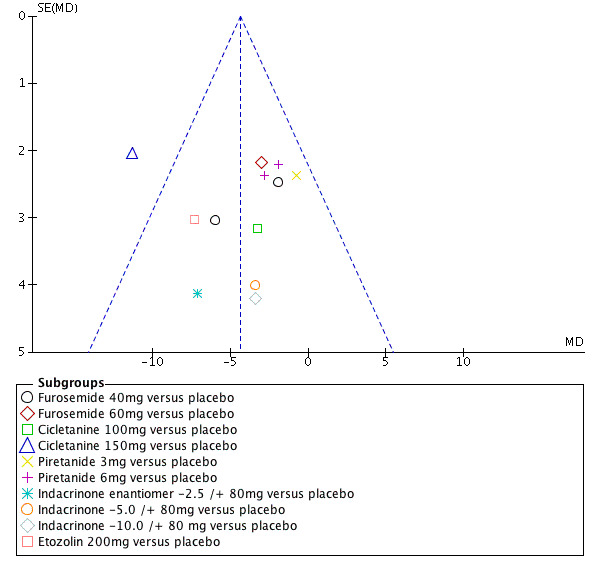
Funnel plot of comparison: 1 Loop diuretics vs placebo, outcome: 1.2 DBP.
Effects of interventions
See: Table 1
Systolic blood pressure
Furosemide 40 mg did not show a significant reduction in SBP (mean difference (MD) ‐5.80, 95% CI ‐13.0 to 1.4) mmHg. Furosemide 60 mg showed a significant reduction in SBP (MD ‐10.0, 95% CI ‐15.8 to ‐4.2), see Analysis 1.1. Combined doses of furosemide 40 mg and 60 mg showed a significant reduction in SBP (MD ‐8.4, 95% CI ‐12.8 to ‐3.9) mmHg.
Cicletanine 100 mg did not show a significant reduction in SBP (MD ‐7.00, 95% CI ‐16.2 to 2.2). Cicletanine 150 mg showed a significant reduction in SBP (MD ‐9.1, 95% CI ‐15.5 to ‐2.7) mmHg, see Analysis 1.1. Combined doses of cicletanine 100 mg and 150 mg showed a significant reduction of SBP (MD ‐8.4, 95% CI ‐13.7 to ‐3.1) mmHg.
Piretanide 3 mg as well as 6 mg did not show a significant reduction in SBP (MD ‐2.2, 95% CI ‐10.0 to 5.6) mmHg and (MD ‐4.7, 95% CI ‐10.0 to 0.7) mmHg, see Analysis 1.1. Combined doses of piretanide 3 mg and 6 mg did not achieve statistical significance (MD ‐3.9, 95% CI ‐8.3 to 0.6) mmHg.
Indacrinone enantiomers ‐2.5/+80 mg and ‐5.0/+80 mg did not show a significant reduction in SBP (MD ‐14.9, 95% CI ‐31.2 to 1.4) mmHg and (MD ‐11.7, 95% CI ‐25.4 to 2.0) mmHg, respectively. Indacrinone ‐10.0/+80 mg showed a significant reduction in SBP (MD ‐19.1, 95% CI ‐36.0 to ‐2.3) mmHg, see Analysis 1.1. Combined doses of the indacrinone enantiomers showed significant reduction in SBP (MD ‐14.7, 95% CI ‐23.1 to 5.8) mmHg.
Etozolin 200 mg reduced SBP (MD ‐14.3, 95% CI ‐23.1 to ‐5.6) mmHg, see Analysis 1.1.
1.1. Analysis.
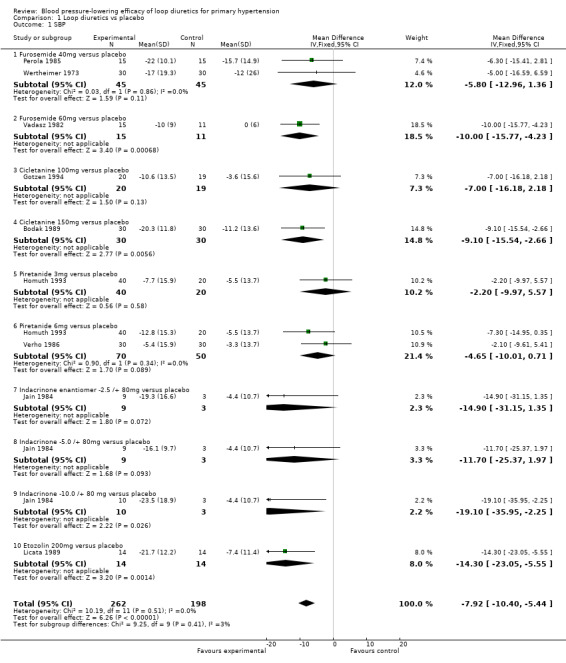
Comparison 1 Loop diuretics vs placebo, Outcome 1 SBP.
The 95% CI of SBP reduction across all individual drugs within the loop diuretics class as well as doses were wide and overlapped with each other. The heterogeneity was not significant P = 0.51 and I2 = 0%; test for subgroup differences was not significant P = 0.41 and I2 = 2.7%.
The best estimate of SBP‐lowering efficacy of all nine trials comparing loop diuretics to placebo control is (MD ‐7.9, 95% CI ‐10.4 to ‐5.4) mmHg for a mean duration of 8.8 weeks, see Analysis 1.1.
Diastolic blood pressure
Furosemide 40 mg as well as 60 mg did not show a significant reduction in DBP (MD ‐3.5, 95% CI ‐7.3 to 0.2) mmHg and (MD ‐3.0, 95% CI ‐7.3 to 1.3) mmHg, respectively, see Analysis 1.2. Combined doses of 40 mg and 60 mg showed a significant decrease in DBP (MD ‐3.3, 95% CI ‐6.1 to ‐0.5) mmHg.
Cicletanine 100 mg did not show a significant reduction in DBP (MD ‐3.3, 95% CI ‐9.5 to 2.9) mmHg. Cicletanine 150 mg showed a significant reduction in DBP (MD ‐11.3, 95% CI ‐15.3 to ‐7.3) mmHg, see Analysis 1.2. Combined doses of cicletanine 100 mg and 150 mg showed a significant reduction in DBP (MD ‐8.9, 95% CI ‐12.3 to ‐5.6) mmHg.
Piretanide 3 mg as well as 6 mg did not show a significant reduction in DBP (MD ‐0.8, 95% CI ‐5.4 to 3.8) mmHg and (MD ‐2.3, 95% CI ‐5.5 to 0.8) mmHg, see Analysis 1.2. Combined doses of piretanide 3 mg and 6 mg did not achieve statistical significance (MD ‐1.8, 95% CI ‐45.5 to 0.8) mmHg.
Indacrinone enantiomers ‐2.5/+80 mg, ‐5.0/+80 mg, and ‐10.0/+80 mg did not show a significant reduction in DBP (MD ‐7.1, 95% CI ‐15.2 to 0.9) mmHg, (MD ‐3.4, 95% CI ‐11.6 to 4.8) mmHg and (MD ‐3.4, 95% CI ‐11.6 to 4.8) mmHg, respectively, see Analysis 1.2. Combined doses of the indacrinone enantiomers also did not show a significant reduction in DBP (MD ‐4.6, 95% CI ‐9.3 to 0.01) mmHg.
Etozolin 200 mg showed a significant reduction in DBP (MD ‐7.3, 95% CI ‐13.2 to ‐1.4) mmHg, see Analysis 1.2.
1.2. Analysis.
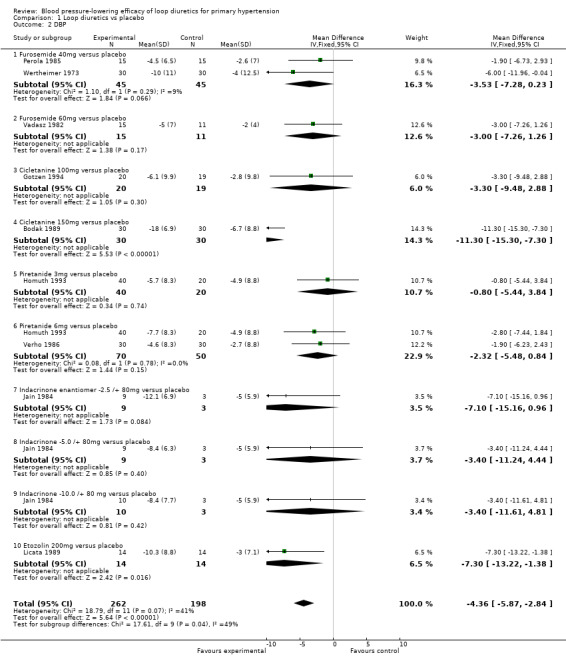
Comparison 1 Loop diuretics vs placebo, Outcome 2 DBP.
The 95% CI of DBP reduction across all individual drugs within the loop diuretics class were significant for cicletanine 150 mg and etozolin 200 mg. The heterogeneity was significant P = 0.07 and I2 = 41%; test for subgroup differences was significant P = 0.04 and I2 = 48.9%. However, excluding the cicletanine 150 mg DBP lowering effect in the Bodak 1989 trial from the overall estimate, both the heterogeneity and subgroup differences became nonsignificant with I2 = 0%. Refer to Figure 7 showing that the Bodak 1989 trial is an outlier with the DBP effect size outside the 95% CI of the overall effect size.
The best estimate of DBP‐lowering efficacy of all nine trials comparing loop diuretics to placebo control is (MD ‐4.4, 95% CI ‐5.9 to ‐2.8) mmHg for a mean duration of 8.8 weeks, see Analysis 1.2. Excluding the Bodak 1989 trial, the estimate of DBP lowering is decreased to (MD ‐3.2, 95% CI ‐4.8 to ‐1.6) mmHg.
Withdrawals due to adverse effects
Withdrawals due to adverse effects were reported in 6 of the 9 trials in 331 participants and were not statistically significant; risk ratio with 95% CI using fixed‐effect model was 1.9 (0.3 to 10.8), see Analysis 1.3.
1.3. Analysis.
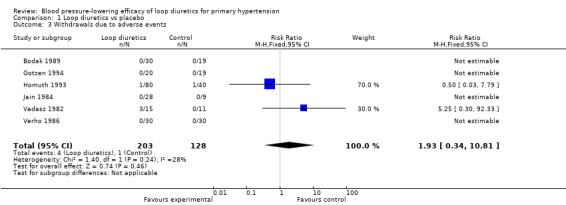
Comparison 1 Loop diuretics vs placebo, Outcome 3 Withdrawals due to adverse events.
Biochemical changes
Two of the nine trials reported data for changes in serum levels of potassium, uric acid, creatinine, blood glucose, serum cholesterol, and triglycerides as compared to placebo control (Bodak 1989, Perola 1985). Based on available data in 88 participants for a mean duration of 20 weeks, there was no statistically significant difference in any parameter, see Analysis 1.4, Analysis 1.5, Analysis 1.6, Analysis 1.7, Analysis 1.8 and Analysis 1.9.
1.4. Analysis.
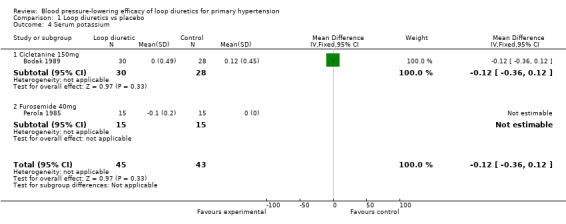
Comparison 1 Loop diuretics vs placebo, Outcome 4 Serum potassium.
1.5. Analysis.
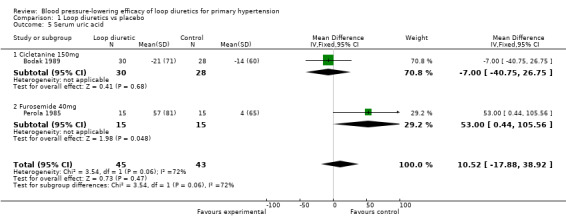
Comparison 1 Loop diuretics vs placebo, Outcome 5 Serum uric acid.
1.6. Analysis.
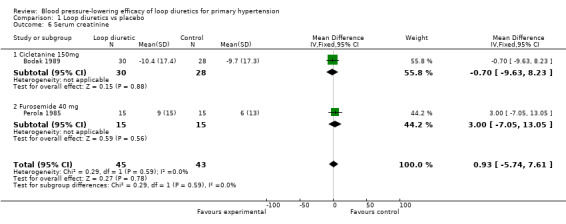
Comparison 1 Loop diuretics vs placebo, Outcome 6 Serum creatinine.
1.7. Analysis.
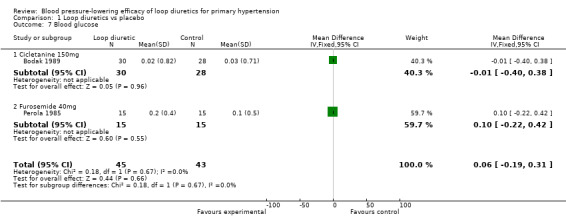
Comparison 1 Loop diuretics vs placebo, Outcome 7 Blood glucose.
1.8. Analysis.
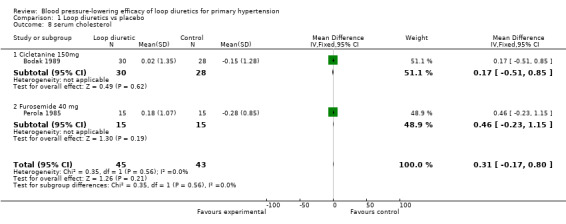
Comparison 1 Loop diuretics vs placebo, Outcome 8 serum cholesterol.
1.9. Analysis.
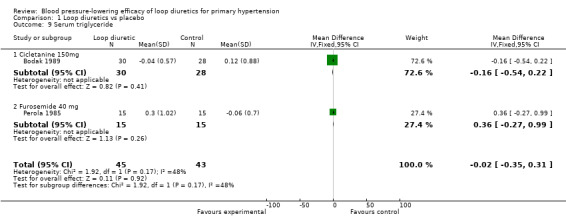
Comparison 1 Loop diuretics vs placebo, Outcome 9 Serum triglyceride.
Discussion
We found the paucity of data available in the public domain to evaluate the dose ranging effects of loop diuretics in the treatment of primary hypertension shocking and surprising, and suspect that there are trials meeting our inclusion criteria that have not been published. We hoped in 2009 that as a result of the publication of this systematic review trialists would contact us to provide us with more data. However, this has not happened. We continued to hope for this following the publication of this systematic review's 2012 update, but no additional data has been made available to date. The results of this systematic review emphasize the need for all studies, regardless of the findings, to be published and accessible for secondary analysis.
Only 9 trials with a mean duration of 8.8 weeks at a fixed dose of loop diuretics met the pre‐specified inclusion criteria. These trials reported data on 460 participants (242 treated with loop diuretics and 198 with placebo) with a weighted mean age of 54.4 years and a weighted mean baseline blood pressure of 162/103 mmHg. The best estimate at this time of the overall SBP‐ and DBP‐lowering efficacy of this drug class is 8/4 mmHg as compared to placebo control, based on double‐blind RCTs. However, we have graded this result as low‐quality evidence based on too few participants, high risk of bias of included studies, presence of publication bias, and the wide 95% confidence intervals, ranging from 5 mmHg to 11 mmHg systolic (Table 1). Considering the currently available data, the effect is not significantly different from thiazides, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or renin inhibitors. (Chen 2009, Heran 2008a, Heran 2008b, Musini 2008).
Due to the limited number of published studies, there is insufficient evidence for the various loop diuretics to generate dose‐response curves for systolic and diastolic blood pressure reduction. At any given dose of loop diuretics, there were only one or two studies contributing blood pressure data.
Given the limited data available, it is impossible with this analysis to be certain of any blood pressure‐lowering differences between one or more of the drugs. To assess whether or not there are differences between different drugs and evaluate the dose equivalence between drugs within the loop diuretic class would require head‐to‐head trials of different loop diuretics. However, at the present time, given that all the drugs within the loop diuretic class work by the same mechanism of action and the overlapping of the 95% CI in the blood pressure‐lowering effect, it is most likely that the blood pressure‐lowering effect of the different loop diuretics at equivalent doses is the same.
Authors' conclusions
Implications for practice.
Based on the limited number of published RCTs, the SBP/DBP‐lowering effect of loop diuretics is ‐8/‐4 mmHg, which is likely an overestimate due to the high risk of bias in the available studies and the high likelihood of publication bias. There are no clinically meaningful blood pressure‐lowering differences between different drugs within the loop diuretic class. We could draw no conclusions regarding the dose‐related decrease in systolic and diastolic blood pressure of loop diuretics in the treatment of primary hypertension. This review did not provide a good estimate of the incidence of harms associated with loop diuretics because of the short duration of the trials and the lack of reporting of adverse effects in many of the trials.
Implications for research.
Data from unpublished trials of the effect of loop diuretics on blood pressure needs to be made available. More RCTs are needed assessing the blood pressure‐lowering effect of loop diuretics as compared to placebo and as compared to other classes of drugs where the blood pressure‐lowering effect has already been established. In particular, the benefit of loop diuretics in the setting of renal insufficiency, the patient population where loop diuretics are often used for their antihypertensive effect, needs to be assessed.
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2015 | New citation required but conclusions have not changed | updated with a new search and new Summary of Findings table |
| 29 December 2014 | New search has been performed | Search updated, no new citations met the inclusion criteria. Summary of Findings table has been added. Minor copy editing performed to improve readability. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 10 July 2012 | New search has been performed | Search updated, no new citations met the minimum inclusion criteria. |
| 11 August 2008 | Amended | Search strategy includes Cochrane Central database. The search has been updated in Medline, Embase and Central until June 30th 2008. Converted to new review format. |
Acknowledgements
The review authors would like to acknowledge the help provided by the Cochrane Hypertension Group. We would like to thank Doug Salzwedel, the Trials Search Co‐ordinator of the Cochrane Hypertension Group, for conducting the updated searches; Stephen Adams for sorting search findings in Reference Manager and retrieving articles to complete this review; and Dr. Balraj Heran for extracting data from the graph of the Homuth 1993 trial.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 27 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp sodium potassium chloride symporter inhibitors/ 2 (sodium potassium chloride adj2 (cotransporter? or co‐transporter? or symporter)).tw. 3 (loop adj2 diuretic?).tw. 4 (azosemide or bemetanide or burinex or cicletanine or ethacrynic acid or etozolin).tw. 5 (frusemid? or furosemid? or fursemid? or indacrinone or muzolimine or ozolinone or phenoxybenzoic acid).tw. 6 (piretanide or ticrynafen or tienilic acid or tizolemid? or torasemid? or torsemid?).tw. 7 or/1‐6 8 hypertension/ 9 hypertens$.tw. 10 exp blood pressure/ 11 (blood pressure or bloodpressure).mp. 12 or/8‐11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomized.ab. 16 placebo.ab. 17 dt.fs. 18 randomly.ab. 19 trial.ab. 20 groups.ab. 21 or/13‐20 22 animals/ not (humans/ and animals/) 23 21 not 22 24 7 and 12 and 23
Appendix 2. EMBASE search strategy
Database: Embase <1974 to 2014 Week 43> Search Date: 27 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp loop diuretic agent/ 2 (loop adj2 diuretic?).tw. 3 (sodium potassium chloride adj2 (cotransporter? or co‐transporter? or symporter)).tw. 4 (azosemide or bemetanide or burinex or cicletanine or ethacrynic acid or etozolin).tw. 5 (frusemid? or furosemid? or fursemid? or indacrinone or muzolimine or ozolinone or phenoxybenzoic acid).tw. 6 (piretanide or ticrynafen or tienilic acid or tizolemid? or torasemid? or torsemid?).tw. 7 or/1‐6 8 or/1‐5 9 exp hypertension/ 10 hypertens$.tw. 11 exp blood pressure/ 12 (blood pressure or bloodpressure).tw. 13 or/9‐12 14 randomized controlled trial/ 15 crossover procedure/ 16 double‐blind procedure/ 17 (randomi?ed or randomly).tw. 18 (crossover$ or cross‐over$).tw. 19 placebo.ab. 20 (doubl$ adj blind$).tw. 21 assign$.ab. 22 allocat$.ab. 23 or/14‐22 24 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 25 23 not 24 26 7 and 13 and 25
Appendix 3. CENTRAL search strategy
Database: Cochrane Central Register of Controlled Trials <Issue 9 2014> via the Cochrane Register of Studies Online Search Date: 27 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 "sodium potassium chloride" near2 (cotransporter* or co‐transporter* or symporter) #2 loop near2 diuretic* #3 (azosemide or bemetanide or burinex or cicletanine or ethacrynic acid or etozolin) #4 (frusemid* or furosemid* or fursemid* or indacrinone or muzolimine or ozolinone or phenoxybenzoic acid) #5 (piretanide or ticrynafen or tienilic acid or tizolemid* or torasemid* or torsemid*) #6 #1 OR #2 OR #3 OR #4 OR #5 #7 antihypertens* OR hypertens* #8 ("blood pressure" or bloodpressure) #9 #7 OR #8 #10 #6 AND #9 #11 30/11/2013 TO 31/10/2014:DL #12 #10 AND #11
Appendix 4. Hypertension Group Specialised Register search strategy
Database: Hypertension Group Specialised Register Search Date: 27 October 2014 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 ("sodium potassium chloride" near2 (cotransporter* or co‐transporter* or symporter)) #2 (loop near2 diuretic*) #3 ((azosemide or bemetanide or burinex or cicletanine or ethacrynic acid or etozolin)) #4 ((frusemid* or furosemid* or fursemid* or indacrinone or muzolimine or ozolinone or phenoxybenzoic acid)) #5 ((piretenide or ticrynafen or tienilic acid or tizolemid* or torasemid* or torsemid*)) #6 #1 OR #2 OR #3 OR #4 OR #5 #7 #6 AND (RCT OR REVIEW OR Meta‐Analysis):DE
Appendix 5. Previous search strategies
2009 search strategy:
1. randomized controlled trial$.mp
2. randomized controlled trial.pt
3. controlled clinical trial.pt
4. controlled clinical trial$.mp
5. random allocation.mp
6. exp random allocation/
7. exp double‐blind method/
8. double‐blind.mp
9. exp single‐blind method/
10. single‐blind.mp
11. or/1‐10
12. (animals not human).sh
13. 11 not 12
14. clinical trial$.mp
15. clinical trial.pt
16. (clin$ adj25 trial$).mp
17. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp
18. random$.mp
19. exp research design/
20. research design.mp
21. or/14‐20
22. 21 not 12
23. 13 or 22
24. comparative stud$.mp
25. evaluation stud$.mp
26. follow up stud$.mp
27. prospective stud$.mp
28. (control$ or prospective$ or volunteer$).mp
29. or/24‐28
30. 29 not 12
31. 23 or 30
32. blood pressure.mp
33. exp hypertension/
34. hypertens$.mp
35. exp blood pressure/
36. or/32‐35
37. 31 and 36
38. loop diuretics.mp. or exp Loop Diuretic Agent/
39. Furosemide Plus Triamterene/ or exp Furosemide/ or furosemide.mp
40. Bumetanide.mp. or exp Bumetanide/
41. Ethacrynic acid.mp. or exp Etacrynic Acid/
42. muzolimine.mp. or exp Muzolimine/
43. torasemide.mp. or exp Torasemide/
44. Piretanide/ or Pirentanide.mp or exp Piretanide/
45. azosemide.mp. or exp Azosemide/
46. Ticrynafen.mp. or exp Tienilic Acid/
47. exp Tripamide/
48. Benzoic Acid Derivative/ or Phenoxybenzoic acid.mp.
49. Indacrinone.mp. or exp Indacrinone/
50. etozolin.mp. or exp Etozolin/
51. ozolinone.mp. or exp Ozolinone/
52. cicletanine.mp. or exp Cicletanine/
53. tienilic acid.mp. or exp Tienilic Acid/
54. tizolemide/ or tizolemide.mp.
55. or/38‐54
56. 37 and 55
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 29 February 2012 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp sodium potassium chloride symporter inhibitors/ 2 (azosemide or bemetanide or cicletanine or ethacrynic acid or etozolin or frusemid?or furosemide or indacrinone or muzolimine or ozolinone or phenoxybenzoic acid or piretanide or ticrynafen or tienilic acid or tizolemide or torsemide or tripamide).mp. (17271) 3 (sodium potassium chloride adj2 (cotransporter? or co‐transporter? or symporter)).tw. 4 symporter inhibitor?.tw. 5 ((loop or ceiling) adj2 diuretic?).tw. 6 or/1‐5 7 hypertension/ 8 hypertens$.tw. 9 exp blood pressure/ 10 (blood pressure or bloodpressure).mp. 11 or/7‐10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 dt.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 or/12‐19 21 animals/ not (humans/ and animals/) 22 20 not 21 23 6 and 11 and 22
Data and analyses
Comparison 1. Loop diuretics vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SBP | 9 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐7.92 [‐10.40, ‐5.44] |
| 1.1 Furosemide 40mg versus placebo | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐5.80 [‐12.96, 1.36] |
| 1.2 Furosemide 60mg versus placebo | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐15.77, ‐4.23] |
| 1.3 Cicletanine 100mg versus placebo | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐16.18, 2.18] |
| 1.4 Cicletanine 150mg versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐9.10 [‐15.54, ‐2.66] |
| 1.5 Piretanide 3mg versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐9.97, 5.57] |
| 1.6 Piretanide 6mg versus placebo | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐4.65 [‐10.01, 0.71] |
| 1.7 Indacrinone enantiomer ‐2.5 /+ 80mg versus placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐14.90 [‐31.15, 1.35] |
| 1.8 Indacrinone ‐5.0 /+ 80mg versus placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐11.70 [‐25.37, 1.97] |
| 1.9 Indacrinone ‐10.0 /+ 80 mg versus placebo | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐19.1 [‐35.95, ‐2.25] |
| 1.10 Etozolin 200mg versus placebo | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐14.30 [‐23.05, ‐5.55] |
| 2 DBP | 9 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐4.36 [‐5.87, ‐2.84] |
| 2.1 Furosemide 40mg versus placebo | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐3.53 [‐7.28, 0.23] |
| 2.2 Furosemide 60mg versus placebo | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐7.26, 1.26] |
| 2.3 Cicletanine 100mg versus placebo | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐9.48, 2.88] |
| 2.4 Cicletanine 150mg versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.3 [‐15.30, ‐7.30] |
| 2.5 Piretanide 3mg versus placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐5.44, 3.84] |
| 2.6 Piretanide 6mg versus placebo | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐2.32 [‐5.48, 0.84] |
| 2.7 Indacrinone enantiomer ‐2.5 /+ 80mg versus placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐7.1 [‐15.16, 0.96] |
| 2.8 Indacrinone ‐5.0 /+ 80mg versus placebo | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐11.24, 4.44] |
| 2.9 Indacrinone ‐10.0 /+ 80 mg versus placebo | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐11.61, 4.81] |
| 2.10 Etozolin 200mg versus placebo | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐7.30 [‐13.22, ‐1.38] |
| 3 Withdrawals due to adverse events | 6 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.34, 10.81] |
| 4 Serum potassium | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.12] |
| 4.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.12] |
| 4.2 Furosemide 40mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serum uric acid | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 10.52 [‐17.88, 38.92] |
| 5.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐40.75, 26.75] |
| 5.2 Furosemide 40mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 53.00 [0.44, 105.56] |
| 6 Serum creatinine | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐5.74, 7.61] |
| 6.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐9.63, 8.23] |
| 6.2 Furosemide 40 mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐7.05, 13.05] |
| 7 Blood glucose | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.19, 0.31] |
| 7.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.40, 0.38] |
| 7.2 Furosemide 40mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.22, 0.42] |
| 8 serum cholesterol | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.80] |
| 8.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.51, 0.85] |
| 8.2 Furosemide 40 mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.46 [‐0.23, 1.15] |
| 9 Serum triglyceride | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.35, 0.31] |
| 9.1 Cicletanine 150mg | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.54, 0.22] |
| 9.2 Furosemide 40 mg | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.27, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bodak 1989.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled study | |
| Participants | 132 participants aged more than 60 years with diastolic arterial pressure over 95mmHg and systolic over 160 mmHg. 62.9% participants were aged over 75 years. Mean age was 73.6 years; male 32/60 (53.3%) Baseline SBP/DBP with SD1 Placebo = 173.2 ± 14.7/104.7 ± 6.9 mmHg Cicletanine 150 mg = 176.3 ± 10.7/103.2 ± 7.6 mmHg Heart rate: placebo = 75.8 ± 6.7 and cicletanine = 76.9 ± 9 |
|
| Interventions | Study A: cicletanine 150 mg per day and placebo for a duration of 180 days cicletanine 150 mg = 30 and placebo = 30 Study B: cicletanine 50 mg and cicletanine 100 mg with no parallel placebo arm, so this study was excluded from the review. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure Normalization of blood pressure Serum sodium, potassium, glucose, uric acid, creatinine, cholesterol, and triglycerides |
|
| Notes | Article in French translated by Ciprian Jauca. End‐of‐treatment SBP/DBP ± SD were reported in table III page 104. Withdrawal due to adverse events reported in Study A: 2 participants left in placebo group due to serious adverse events. Biochemical parameters at baseline and end of treatment with SD were reported in table V page 105. Change from baseline to end point in SBP, DBP, and biochemical values was calculated and SD at end of treatment was imputed for meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "14 patients left the study early," 12 from the excluded Study B and 2 from the included Study A (both from the placebo group, both due to serious adverse events). |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported. |
| Other bias | Unclear risk | The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Gotzen 1994.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled study | |
| Participants | 39 participants with mild to moderate hypertension Mean age = 60 years; male (53.3%) Baseline SBP was 143.9 ± 10.3 mmHg and DBP was 85.5 ± 8.9 mmHg in placebo group Baseline SBP was 149.9 ± 15.5 mmHg and DBP was 91.1 ± 13.8 mmHg in cicletanine 100 mg group |
|
| Interventions | cicletanine 100 mg per day or placebo for 8 weeks duration cicletanine 100 mg = 20 and placebo = 19 |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure at end of treatment Also daytime (6.00am to 22.00pm) and nighttime (22.00pm to 6.00am) measurements |
|
| Notes | Article in German translated by Ciprian Jauca. Table II: End‐of‐treatment week 8 SBP in cicletanine 100 mg was 139.3 ± 13.5 mmHg and DBP was 85.0 ± 9.9 mmHg End‐of‐treatment week 8 SBP in placebo group was 140.3 ± 15.6 mmHg and DBP was 82.7 ± 9.8 mmHg All 39 participants completed the study (no withdrawals). Biochemical parameters were either not measured or not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Low risk | "optically identical placebo was administered double‐blind at 8:00 AM." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and their blood pressure measurements were reported. |
| Selective reporting (reporting bias) | High risk | Adverse events and biochemical markers were not reported. |
| Other bias | Unclear risk | The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Homuth 1993.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled study | |
| Participants | Participants were from 20 outpatient clinics in Germany and between 21 and 65 years of age (mean age = 52.5 years). 482 participants were recruited and 480 were randomized to 12 groups in a multifactorial design. (Table II page 668) Mean duration of high blood pressure 9 to 10 years; 25/120 (20.8%) participants were smokers; 5/120 (4.2%) with congestive heart failure; 6/120 (5%) with diabetes; 10/120 (8.3%) with uricemia; and 21/120 (17.5%) with hyperlipidemia. (Table III page 668) Baseline SBP was 161.0 ± 17.0 mmHg and DBP was 109 ± 5.0 mmHg in placebo group Baseline SBP was 160.0 ± 13.0 mmHg and DBP was 108.8 ± 6.0 mmHg in piretanide 3 mg group Baseline SBP was 165.0 ± 17.0 mmHg and DBP was 108.0 ± 7.0 mmHg in piretanide 6 mg group |
|
| Interventions | placebo; ramipril 2.5 mg, 5.0 mg, and 10 mg; and piretanide 3.0 mg or 6.0 mg; or combination of ramipril doses with the 2 piretanide doses for a treatment duration of 6 weeks given as a single daily morning dose The following are relevant treatment groups for this review (N = 120): piretanide 3 mg = 40; piretanide 6 mg = 40; placebo = 40 |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure in supine, sitting, and standing positions at baseline and weekly during week 1 to week 6. Specially trained personnel measured blood pressure with mercury column sphygmomanometers. The fifth Korotkoff sound was accepted as diastolic blood pressure after 3 minutes of rest. Thereafter patient assumed the sitting position, followed by standing position for ≥ 3 minutes, after which blood pressure was recorded again. Adverse events were assessed by means of questionnaire that was given at each participant visit. |
|
| Notes | Analysis was performed on an intention‐to‐treat basis. Data was abstracted from the response surface contour plot on page 669, which was based on a biquadratic regression model and was included in the meta‐analysis. The data abstracted is as follows: Change in SBP from baseline in placebo = ‐5.5 mmHg; piretanide 3 mg = ‐7.7 mmHg; and piretanide 6 mg = ‐12.8 mmHg Change in DBP from baseline in placebo = ‐4.9 mmHg; piretanide 3 mg = ‐5.7 mmHg; and piretanide 6 mg = ‐8.0 mmHg Withdrawal due to adverse events were reported placebo = 1 and piretanide = 1 (3 mg or 6 mg group was not reported). Biochemical parameters were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The regimens had identical appearance and the Physician, nurses and patient‐care personnel, as well as patients were unaware of the regimens." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total withdrawal in the 3 relevant treatment groups included in this review was 7/40 (17.5%) participants in placebo group and 2/80 (2.5%) participants in piretanide group. Analysis was an intention‐to‐treat analysis that included randomized patients, with any postrandomization data available during double‐blind phase included. |
| Selective reporting (reporting bias) | High risk | The study reported all outcome measures mentioned in the Methods section. However, the study did not report the absolute values of SBP, DBP at end of treatment as measured by the sphygmomanometer. Instead a graph of the response was presented as a surface contour plot, which was based on a biquadratic regression model. |
| Other bias | Unclear risk | The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Jain 1984.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled study | |
| Participants | 40 participants were recruited, of which 2 were lost to follow‐up early in the study and 1 did not receive double‐blind medication because of marginally increased SGOT and SGPT levels. 37 participants with sitting DBP between 90 and 104 mmHg after 4 weeks of participant‐blind placebo washout period were randomized to 4 treatment groups for a duration of 12 weeks. Throughout the study participants were instructed to follow a no‐salt‐added diet. Except for the occasional use of a laxative or a non‐narcotic analgesic, no concurrent medications were allowed. If potassium level dropped below 3.0 mEq/L on 2 consecutive visits, a potassium supplement was permitted. The population was mainly black females 27/37 (73%), except for the placebo group, which had 5 males and 4 females. Mean age ranged from 53 to 56 years; duration of hypertension ranged from 7.3 to 14.7 years. Baseline sitting SBP was 157.0 ± 18.0 mmHg and DBP was 96 ± 3.0 mmHg for ‐2.5/+80 mg group. Baseline sitting SBP was 149.0 ± 8.0 mmHg and DBP was 95 ± 4.0 mmHg for ‐5.0/+80 mg group. Baseline sitting SBP was 156.0 ± 20.0 mmHg and DBP was 95 ± 4.0 mmHg for ‐10.0/+80 mg group. Baseline sitting SBP was 150.0 ± 8.0 mmHg and DBP was 95 ± 4.0 mmHg for placebo group. |
|
| Interventions | 1 of the ratios of 1 enantiomers of indacrinone namely ‐2.5/+80 mg, ‐5.0/+80 mg, ‐10.0/+80 mg, or placebo for duration of 12 weeks once daily ‐2.5/+80 mg = 9, ‐5.0/+80 mg = 9, ‐10.0/+80 mg = 10, or placebo = 9 |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure (sitting and standing) Blood pressure was measured in duplicate after 5 minutes of sitting and 2 minutes of standing by a standard mercury sphygmomanometer by the same observer at approximately 24 hours after the previous day dosing. The fifth Korotkoff sound was accepted as diastolic blood pressure. Heart rate, ECG Laboratory data: serum uric acid, potassium, chloride, sodium, glucose, creatinine, BUN2 Body weight |
|
| Notes | Mean weighted SBP and DBP with mean weighted SD was calculated. There were no withdrawals due to adverse events. Biochemical parameters absolute data was not reported. No data comparing treatment groups to placebo was presented. Authors report: "No significant changes observed in mean serum sodium, chloride, glucose, creatinine, BUN2 and body weight during treatment with indacrinone enantiomers ‐2.5 and ‐5.0 and placebo when baseline data was compared to data at week 16. With indacrinone ‐10mg significant changes were seen in serum chloride from 104 to 99.5 mEq/L, serum glucose from 110 to 128mg/dl. Lipid changes were not monitored." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 40 participants, 2 were lost to follow‐up early in the study and 1 did not receive double‐blind medication because of marginal increase in SGOT and SGPT levels. Data was reported for the remaining 37 participants completing the trial. |
| Selective reporting (reporting bias) | High risk | "During the DB3 phase, if the DBP was greater than 104 mmHg on two consecutive visits, the patient was dropped as therapeutic failure." (page 280) Absolute values of serum biochemical changes were not reported. |
| Other bias | High risk | Industry‐funded study. |
Licata 1989.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 28 participants with essential hypertension (WHO4 stage I‐II) after 15 days washout period with placebo were randomized. Mean age 54 ± 10 years; men 16/28 (57%); 18/28 (64.3%) with mild hypertension and 10/28 (35.7%) with moderate hypertension Baseline sitting SBP was 171.4 ± 10.6 mmHg and DBP was 102.4 ± 5.4 mmHg for etozolin group. Baseline sitting SBP was 169.7 ± 10.2 mmHg and DBP was 103.7 ± 6.1 mmHg for placebo group. |
|
| Interventions | etozolin 200 mg once a day or placebo for 30 days 15‐day washout period on placebo All participants received 150 mEq to 200 mEq sodium daily. etozolin = 14 and placebo = 14 |
|
| Outcomes | Resting systolic blood pressure, diastolic blood pressure, mean blood pressure, heart rate (ECG tracing), and first‐pass radionuclide angiocardiography. Blood pressure was measured with a mercury sphygmomanometer after a 5‐minute rest at the first and fifth Korotkoff phase as the mean of 3 recordings 24 hours after the last dose. Daily urine volume, serum sodium, potassium, calcium, blood glucose, BUN2, cholesterol, and uric acid were measured. |
|
| Notes | Total withdrawals and withdrawal due to adverse effects were not reported. Change from baseline for biochemical parameters were reported in the publication for the etozolin group but not for the placebo group. Authors state: "No significant modifications emerged in hematological and metabolic picture." (page 265) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in the publication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total withdrawals were not reported. |
| Selective reporting (reporting bias) | High risk | Change from baseline for all outcomes was reported in the publication for the etozolin group, but biochemical parameters were not reported in the placebo group. |
| Other bias | Unclear risk | The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Perola 1985.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled, cross‐over study | |
| Participants | 15 mildly hypertensive people classified as WHO4 I (12 people) or WHO4 II (3 people). Ages ranged from 27 to 65 years (mean age is 48.3 years). Supine SBP 159.7 ± 17.0 and DBP 101.1 ± 8.1 mmHg in all participants Standing SBP 158.5 ± 17.9 and DBP 107.7 ± 7.2 mmHg in all participants |
|
| Interventions | furosemide 40 mg + triamterene 50 mg; furosemide 40 mg; triamterene 50 mg; or placebo for a treatment duration of 4 weeks each The following are relevant treatment groups for this review: furosemide 40 mg or placebo (N = 15 since cross‐over study) No mention of washout period between treatments. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, serum sodium, potassium, calcium, magnesium, creatinine, urate, transaminase, cholesterol, HDL‐cholesterol, triglyceride, glucose, plasma renin activity, plasma insulin, and C peptide. A nurse measured blood pressure on the right arm of each participant in the supine position as well as in the standing position (3 measurements) with accuracy of paired 2 mmHg. |
|
| Notes | The standing SBP and DBP were used in analysis. No data was reported during the parallel placebo arm period in the first 4‐week phase. Mean of 2‐ and 4‐weeks data was reported for each treatment group at end of 4 weeks. In order to maximize inclusion of data, since blood pressure was taken at end of 4 weeks of each treatment period, we assumed there was no carry‐over effect and included data from furosemide vs. placebo group at end of 4 weeks in all 15 randomized participants. Table 2 page 547 Withdrawals due to adverse events were not reported. Biochemical parameters are reported in table III and IV page 549 and 550. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Corresponding placebo tablets made the medication uniform for all patients, who received every morning a total of three tablets." (page 546) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The publication did not mention number of participant withdrawals. |
| Selective reporting (reporting bias) | Low risk | Table 1, 2, 3, and 4 in the publication provide data for all outcomes. |
| Other bias | Unclear risk | Since the study is a cross‐over trial, we would have preferred to use the parallel‐group data, which was not available. The authors have not stated whether order of treatment significantly affected results. The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Vadasz 1982.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled study | |
| Participants | 36 people with mild to moderate hypertension with DBP between 95 and 120 mmHg were enrolled. 26 completed the study. 7/18 participants in the placebo group and none in the frusemide group had to withdraw because of deterioration in their hypertension. 2 of the three dropouts in the frusemide group occurred in the second part of the trial when the dose was doubled after 4 weeks in some participants. The DBP at baseline was 108 ± 7 mmHg in the frusemide group and 98 ± 4 mmHg in the placebo group. "The same pattern was seen for standing DBP, and supine SBP and DBP." (page 201) Median age was 51 in frusemide group and 45 in placebo group. |
|
| Interventions | frusemide slow‐release formulation 60 mg or placebo given for 8 weeks The 1st weeks is fixed dose in all randomized patients, after which the dose was increased "in patients failing to achieve a clinically meaningful fall in blood pressure." (page 200) Therefore data at 4 weeks duration will be included for this review. frusemide = 15 and placebo = 11 Participants were advised to maintain a low‐salt diet. Potassium supplements were not prescribed. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, plasma electrolytes (sodium, potassium, chloride, and bicarbonate), blood glucose, BUN2, plasma uric acid, serum creatinine, hemoglobin, hematocrit, red‐cell count, white‐cell count, and blood film examination. Blood pressure was measured by a standardized technique using a random zero (Hawksley) sphygmomanometer. This was applied to the same arm generally by the same investigator at about the same time of the day. The patient rested for 10 to 15 minutes before supine pressure was recorded and stood for at least 2 minutes for the standing blood pressure reading. DBP was taken at the fifth Korotkoff phase. |
|
| Notes | "Two randomized groups were broadly comparable except for age since there were many older patients in the frusemide group." "The baseline supine diastolic was higher in frusemide group 108 mmHg ± 7 as compared to 98 ± 4 mmHg in placebo group. The same pattern was seen in erect diastolic, supine systolic and erect systolic blood pressure." Total withdrawals = 10/26 in the trial. Withdrawals due to adverse effects 3/15 in frusemide group and 0/11 in placebo group. Absolute value of biochemical changes were not reported. "None of the laboratory tests revealed any clinically meaningful changes. In particular no patients developed hypokalaemia, although there was one patient with relatively low serum potassium (< 3.2 mmol/L) at the end of treatment in the frusemide group." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated as double blind and "matching placebo" was administered. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is not known how the data for the 10 participants who were lost and did not complete the trial was accounted for. |
| Selective reporting (reporting bias) | High risk | SBP and DBP were reported, but other laboratory parameters were reported as: "None of the laboratory tests revealed any clinically meaningful changes." Absolute values at baseline or end of treatment were not provided. |
| Other bias | High risk | The two groups differed in their age pattern, baseline blood pressure level, and response to a low‐salt diet during washout run‐in period. The pattern of individual responses differed in the group. Despite the protocol, the 3 participants achieving normal blood pressure at week 4 in frusemide group dose was doubled in all 15 patients. The authors state that "despite the lack of comparability of the two groups firm clinical inferences could be drawn from the study." The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Verho 1986.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | 60 people with DBP between 95 and 120 mmHg were randomized. The study had no dropouts. Ages ranged from 37 to 65 years (mean age 52.5 years). Any existing hypertensive medication was discontinued, and after 2‐ to 3‐week placebo run‐in period, participants were randomized to piretanide 6 mg or placebo for 6 weeks. After 6 weeks, dose was doubled from 1 tablet daily to 1 tablet twice a day in participants who experienced an inadequate blood pressure‐lowering effect (supine DBP of > 95 mmHg). Therefore, we have included data at 6 weeks in this review. Baseline supine blood pressure: piretanide group = 163.8/101.4 mmHg and placebo group = 157.9/99.1 mmHg |
|
| Interventions | piretanide 6 mg = 30 or placebo = 30 for 12 weeks | |
| Outcomes | Systolic blood pressure, diastolic blood pressure, biochemical values Blood pressure was measured at rest after 10 minutes in recumbent position and 2 minutes after standing. The mean of 3 measurements was recorded each time. Standing SBP and DBP data could not be used since it was reported in a subset of randomized patients‐‐17 in piretanide group and 10 in the placebo group. Supine blood pressure measurements in all randomized participants were used for analysis. |
|
| Notes | There were no withdrawals due to adverse events. Since SD1 data at baseline, end of treatment, or change in blood pressure was not reported, the mean weighted SD1 was calculated from the other trials and imputed in the analysis. Changes in biochemical parameters were not reported at week 6 as required for this systematic review. Data at 12 weeks was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the publication. |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated as double blind: "The tablets were identical in appearance and each patient took the tablets as a single dose at breakfast." (page 386) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "No drop outs during the study." This was true only for supine blood pressure data in all randomized participants. However, the standing blood pressure data were reported in a small subset of participants 27/60 (45%) in figures. |
| Selective reporting (reporting bias) | High risk | Biochemical values at 6 weeks were not reported. |
| Other bias | High risk | Baseline supine blood pressure in piretanide group was significantly higher than in placebo group (163.8/101.4 mmHg vs. 157.9/99.1 mmHg, respectively). The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
Wertheimer 1973.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled, cross‐over study | |
| Participants | 60 participants from 3 different hospital clinics by investigators following the same protocol. People with DBP > 90 mmHg during 3‐ to 6‐week control period in which no antihypertensive medication was given. Men 28/60 (46.6%); black 26/60 (43.3%); and white 34/60 (56.6%); mean age 58.6 years. Baseline standing SBP 177 ± 21.9 mmHg and DBP 107 ± 10.4 mmHg (range 91 to 131 mmHg) for both groups Group 1: placebo followed by frusemide = 30 Group 2: frusemide followed by placebo = 30 |
|
| Interventions | furosemide 40 mg or placebo for 5 to 14 weeks, mean duration of 7.9 weeks Group 1: placebo followed by frusemide 40 mg = 30 Group 2: frusemide 40 mg followed by placebo = 30 No washout period and then cross‐over. Data after first parallel‐group phase could be used. |
|
| Outcomes | Systolic blood pressure, diastolic blood pressure, laboratory measures (hematocrit, complete blood cell count, urinanalysis, serum potassium, BUN2, and fasting blood glucose) Blood pressure was measured once or twice weekly with participants in standing, sitting, or supine position. |
|
| Notes | Blood pressure data in the first period between frusemide and parallel placebo group was useful for this systematic review. Authors report "the order in which treatment was administered influenced the results." (page 935) Blood pressure values were provided for group 1 vs. group 2 in first phase, which could be used at the end of 4 weeks. Also, difference between placebo and frusemide group was given in placebo and frusemide treatment arms at end of 4 weeks. (page 936) Biochemical parameter absolute values were not provided at the end of the first phase of the study. End‐of‐treatment data was provided. Withdrawal due to adverse effects during first phase was not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned treatment according to randomized scheme." (page 934) |
| Allocation concealment (selection bias) | Unclear risk | Not described in the publication. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Medication supplied as tablets in bottles with coded labels so that neither patient nor investigator knew which preparation was being given at any time. The placebo tablets were identical in appearance to those containing active ingredients." (page 934) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Separate data was not provided at end of first period for biochemical changes as well as total withdrawals. |
| Selective reporting (reporting bias) | Low risk | All outcomes at end of the study were reported in the publication. |
| Other bias | High risk | Authors report "the order in which treatment was administered influenced the results." (page 935) The authors did not declare sponsorship or funding of this study and conflicts of interest in the publication. |
1SD: standard deviation 2BUN: blood urea nitrogen 3DB: double‐blind 4WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonaduce 1981 | This double‐blind cross‐over study after placebo washout randomized participants to xipamide or chorthalidone. This study had no parallel placebo control arm. |
| Buckert 1984 | This double‐blind randomized study compared piretanide to two doses of hydrochlorothiazide and had no parallel placebo arm. |
| Campbell 1998 | This double‐blind, randomized, placebo‐controlled, cross‐over study was a single‐dose study and did not meet 3‐week minimum treatment duration for inclusion. |
| Charansonney 1997 | This double‐blind RCT after 3 weeks placebo washout randomized participants to piretanide, hydrochlorothiazide, or a combination of the two drugs. This study had no parallel placebo control arm. |
| Clerson 1989 | This double‐blind trial randomized 120 participants with essential hypertension uncontrolled on beta‐blocking therapy to placebo, cicletanine 50 mg/day, and cicletanine 100 mg/day in addition to beta‐blocker therapy. This trial had no monotherapy arm, so it did not meet our inclusion criteria. |
| Coca 2009 | This trial randomized 238 chronic heart failure patients to furosemide 40 mg/day and prolonged‐release torsemide 10 mg/day over 8 months. The study was not double‐blinded and had no placebo control arm. |
| Costello‐Boerrigter 2006 | This trial randomized 14 chronic heart failure patients to 30 mg tolvaptan or placebo on day 1, with cross‐over on day 3, and all patients received 80 mg furosemide on day 5. The study was not double blinded. |
| Di Somma 1990 | This study had no parallel placebo control arm. |
| Diez 2009 | This trial randomized 142 chronic heart failure patients to furosemide 40 to 160 mg/day and prolonged‐release torsemide 10 to 40 mg/day over 8 months. The study was not double blinded and had no placebo control arm. |
| Drolet 2010 | This double‐blind, placebo‐controlled trial randomized 28 healthy adults to placebo and sildenafil 20 mg 3 times per day over 5 days, followed by 150 mg and 300 mg of cicletanine on days 6 and 7, respectively, then crossed over. The trial did not study hypertensive patients over at least 3 weeks. |
| Dussol 2006 | This trial randomized 312 renal insufficiency patients to 1 of 4 treatment arms before a radiological procedure, including one that gave 3 mg/kg of body weight of furosemide intervenously after the procedure. The trial did not mention blinding and was not placebo controlled. |
| Fried 1979 | This double‐blind randomized trial compared tienilic acid to hydrochlorothiazide. This study had no parallel placebo arm. |
| Galleti 1991 | This study had no parallel placebo control arm. |
| Galloe 2006 | This double‐blind, double‐placebo‐controlled, cross‐over trial randomized 16 heart failure patients to 16 combinations of trandolapril and bumetanide over 7 days. The trial did not study hypertensive patients over at least 3 weeks. |
| Gupta 1981 | This double‐blind controlled study after 2 weeks placebo run‐in period randomized participants to 3 doses of piretanide. This study had no parallel placebo control arm. |
| Gupta 2010 | This double‐blind, placebo‐controlled, cross‐over trial randomized 30 heart failure patients to torasemide 5 mg or placebo for 3 months, with 2 months of washout between cross‐over. The trial did not report results for hypertensive patients. |
| Heijden 1998 | This double‐blind, placebo‐controlled randomized cross‐over trial in 27 people with essential hypertension was excluded because during the first 6‐week period, placebo was administered in single‐blind fashion, followed by second 6‐week period in which furosemide or bumetanide was administered in double‐blind fashion. In the third 6‐week period, placebo was again administered in single‐blind fashion, followed by fourth 6‐week period of double‐blind alternative active medication. There was no parallel placebo arm during active treatment in these randomized participants. |
| Holland 1979 | This study had no parallel placebo control arm. |
| Hua 2007 | This trial randomized 370 hypertensive patients to 15 antihypertensive drugs over 8 weeks. The trial did not contain a placebo control arm. |
| Iyalomhe 2007 | This trial randomized 40 mild‐to‐moderate hypertensive patients to hydrochlorothiazide 25 mg and furosemide 40 mg over 3 weeks. The trial was not double blinded and had no placebo control arm. |
| Jungers 1989 | This double‐blind RCT included 72 participants with mild‐to‐moderate hypertension, and after 2‐week single‐blind placebo run‐in period randomized participants to 50 mg/day or 100 mg/day cicletanine. This trial had no parallel placebo control arm. |
| Knoben 1982 | This trial had no parallel placebo control arm. |
| Kopp 1978 | This double‐blind randomized trial in 100 participants was carried out over 22 days with 5 days without medication, 12 days therapy, and 5 days follow‐up, and did not meet the criteria of a minimum of 3 weeks of treatment with etozolin or placebo. |
| Krogsgaard 1976 | This double‐blind randomized cross‐over study had no parallel placebo control arm. |
| Kumar 1984 | This double‐blind RCT included 17 participants who had 2 weeks placebo run‐in and were randomized to xipamid or hydrochlorothiazide. This study had no parallel placebo control arm. |
| Kundu 1986 | This 26‐week double‐blind trial randomized 49 hypertensive patients to 1 of 2 arms (each with 2 daily doses): single dose of furosemide and placebo, and 2 half doses of furosemide. The study did not directly compare a loop diuretic with placebo. |
| Kuramoto 1985 | This double‐blind comparative method study in 33 elderly people with essential hypertension compared those 60 years of age or older to those 59 years or younger, and had no parallel placebo control group. |
| Laffi 1991 | This double‐blind trial randomized 24 nonazotemic cirrhotic patients to torasemide 10 mg/day and furosemide 25 mg/day, both with potassium canrenoate 200 mg/day administration over 3 days. The trial did not study hypertensive patients for at least 3 weeks with a placebo control arm. |
| Leitch 1974 | This trial randomized 8 healthy doctors to furosemide 80 mg and bendrofluazide 10 mg over 4 days. Blinding information was not given. The trial did not study hypertensive patients for at least 3 weeks with a placebo control arm. |
| Magrini 1988 | Single‐blind cross‐over study. Short‐term administration of 25 mg of captopril. |
| Matthews 1997 | This double‐blind trial randomized 19 pre‐eclampsia patients to oral frusemide 40 mg or placebo daily for 7 days in the first postpartum week. The study participants were not hypertensives and the trial duration was not at least 3 weeks. |
| Mitrovic 2009 | This double‐blind trial randomized 111 heart failure patients to 1‐hour infusion of 5, 10, and 15 mg SLV320, an adenosine A1 receptor antagonist; placebo; or 40 mg furosemide over 8 days. The duration of the trial was not at least 3 weeks. |
| Miziara 1982 | This double‐blind trial randomized participants to chlorthalidone and furosemide after a 15‐day placebo period. There was no parallel placebo treatment arm. |
| Nami 1991 | This double‐blind, placebo‐controlled study randomized participants to single dose of etozolin (200 mg, 400 mg, and 600 mg), chlorthalidone (25 mg, 50 mg, and 75 mg), and placebo. This study therefore did not meet the criteria of a minimum of 3 weeks treatment. |
| Obel 1984 | This double‐blind, randomized, cross‐over study included 50 participants with hypertension and compared furosemide to bendrofluazide. The study had no parallel placebo arm. |
| Okun 1978 | Prospective, randomized, double‐blind, placebo‐controlled study. 30 male participants ranging in age from 28 to 60 years with mild‐to‐moderate hypertension were randomized to ticrynafen 250 mg, hydrochlorothiazide 50 mg, or placebo for 6 weeks. However, dosage could be increased from 1 to 2 tablets after 2 weeks of double‐blind treatment in any participant who had not had a 10 mmHg decrease in DBP and who had not experienced any serious adverse event. Dosage was increased in 8 of the 9 participants on placebo, 4 of the 9 participants on ticrynafen, and 5 of the 10 participants on hydrochlorothiazide. Since fixed‐dose monotherapy or dose increase in all randomized participants irrespective of response was not used, this trial was excluded. |
| Okun 1979 | This double‐blind study in 30 participants to a 6‐week regimen of either tienilic acid, hydrochlorothiazide, or placebo was excluded since after the first 2 weeks of double‐blind treatment, dosage was increased in any participant who had not had a 10 mmHg decrease in diastolic blood pressure. |
| Oli 1983 | This study in 22 non‐insulin Nigerian diabetics compared frusemide to placebo for a period of 9 weeks but was not a randomized trial. |
| Olshan 1981 | This trial in 12 white men with essential hypertension randomized participants to placebo or furosemide. Entry criteria was based on mean arterial pressure of 105 mmHg; end‐of‐treatment data was also reported as mean arterial pressure. |
| Pearson 1979 | Tienilic acid or ticrynafen (USAN) is a diuretic drug with uric acid‐lowering (uricosuric) action, formerly marketed for the treatment of hypertension. It was withdrawn in 1982, shortly after its introduction to the market, after case reports in the United States indicated a link between the use of tienilic acid and hepatitis. This double‐blind, placebo‐controlled, cross‐over trial was excluded as there was no washout period prior to randomization of participants. |
| Potter 1987 | This randomized double‐blind study compared atenolol with matching placebo in people already receiving fixed dose of captopril and frusemide. This study had no monotherapy arm. |
| Reyes 1990 | This study had no parallel placebo control arm. |
| Ronchi 1990 | This was a double‐blind RCT in which diuretic was added to nifedipine. The study had no diuretic monotherapy treatment arm. |
| Rush 1990 | This double‐blind trial randomized 11 oxygen‐dependent, spontaneously breathing infants with chronic bronchopulmonary dysplasia to furosemide 4 mg/kg in 2 divided doses on alternate days and placebo over 8 days. The study did not look at hypertensive patients for the minimum 3‐week duration. |
| Rutledge 1988 | This randomized double‐blind study compared intravenous enalapril to placebo in 42 moderate hypertensive patients and to intravenous furosemide in 23 severe hypertensive patients for 2 days. |
| Shah 2011 | This trial randomized 308 acute heart failure patients to 2x2 design of receiving low‐ and high‐dose furosemide (80 mg to 240 mg) and continuous and daily bolus dosing over 72 hours. The trial did not study hypertensive patients for at least 3‐months duration. |
| Udelson 2011 | This double‐blind trial randomized 83 people with heart failure and systolic dysfunction to daily furosemide 80 mg, tolvaptan 30 mg, the combination of furosemide 80 mg and tolvaptan 30 mg, and placebo over 8 days. The study was not at least 3‐weeks in duration. |
| Valmin 1980 | Double‐blind randomized cross‐over study in 26 hypertensive patients to 4 weeks of placebo followed by 6 weeks of different doses of frusemide and intervening placebo of 4 weeks. This study had no parallel placebo control arm. |
| van Kraaij 2003 | This double‐blind trial randomized furosemide‐taking participants to (1) continued furosemide and (2) halved furosemide dose for 1 week followed by placebo for 3 months. The trial did not contain a washout period. |
| Webster 1987 | This double‐blind randomized trial in 18 participants compared atenolol, propanolol, or placebo in patients not controlled on captopril 50 mg twice a day and frusemide 40 mg twice a day. There was no parallel loop diuretic monotherapy arm. |
| Witchitz 1974 | This double‐blind trial randomized 18 hypertensive patients to indapamide 2.5 mg and furosemide 40 mg over 48 hours. The study had no placebo control arm and was not at least 3 weeks in duration. |
| Yasky 1987 | This double‐blind randomized cross‐over study in 20 people with mild‐to‐moderate hypertension had a parallel placebo arm, but piretanide was not given as monotherapy. |
| Zamboli 2011 | This trial randomized 40 hypertensive chronic kidney disease patients to furosemide or a non‐diuretic antihypertensive treatment over 52 weeks. The study was not double blinded and had no placebo control arm. |
Differences between protocol and review
In the second update the search was run until February 2012. In order to maximize data inclusion, we also included trials that reported data after 12 weeks in the meta‐analysis. The second update in October 2014 followed the same methods as the 2012 update, but no new trials met the minimum inclusion criteria.
Contributions of authors
James Wright and Vijaya Musini formulated the idea for the review and developed the basis for the protocol.
Vijaya Musini designed the search strategy, undertook the initial and updated search, screened search results, collected data for the review, screened retrieved papers against eligibility criteria, appraised the risk of bias of papers, extracted data from papers, entered data into RevMan, analyzed and interpreted data, and wrote the review. Vijaya Musini also conducted the second search update until 17 December 2013 and screened all new titles and abstracts to determine if they met the minimum inclusion criteria.
Ciprian Jauca was the second author, identified trials meeting inclusion criteria, translated two non‐English studies (one in French and one in German), assessed risk of bias of all included studies, and performed data abstraction. For the 2012 and 2014 updates, Ciprian Jauca helped screen new titles and abstracts, retrieved the full text of potential articles, screened new citations to determine if they met the minimum inclusion criteria, and contributed minor edits to the final version of the updated review.
James Wright confirmed accuracy of data and was the third author to settle any discrepancies in inclusion criteria or data abstraction.
Ken Bassett confirmed accuracy of data and was the third author to settle any discrepancies in inclusion criteria or data abstraction.
Pouria Rezapour joined the review author team for the updates in 2012 and 2014, performed updated searches in 2012 and 2014, screened new titles and abstracts, retrieved the full text of potential articles, screened new citations to determine if they met the minimum inclusion criteria, and contributed minor edits to the final version of the updated review.
Sources of support
Internal sources
Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Canada.
External sources
-
Canadian Institutes of Health Research, Canada.
grant to the Hypertension Review Group.
Declarations of interest
Vijaya Musini: nothing to declare.
Pouria Rezapour: nothing to declare.
James Wright: nothing to declare.
Ken Bassett: nothing to declare.
Ciprian Jauca: nothing to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bodak 1989 {published data only}
- Bodak A, Gavardin Th, Salom M, Tarrade Th, Forette F, Jungers P. Efficacy and tolerance of cicletanine in arterial hypertension of the elderly [Efficacite et tolerance cicletanine chez le sujet age hypertendu]. Therapie 1989;44:101‐6. [PubMed] [Google Scholar]
Gotzen 1994 {published data only}
- Gotzen R, Schulte K‐L, Bonath Th. Duration of antihypertensive effectiveness during therapy with cicletanine in ambulatory 24‐h blood pressure measurement [Antihypertensive wirkdauer von cicletanin in der ambulanten 24‐h‐blutdruckmessung]. Herz/Kreisl 1994;26(11):377‐81. [Google Scholar]
Homuth 1993 {published data only}
- Homuth V, Faulhaber HD, Loose U, Loffler K, Luft FC. Usefulness of piretanide plus ramipril for systemic hypertension: a multicenter trial. The American Journal of Cardiology 1993;72:666‐71. [DOI] [PubMed] [Google Scholar]
Jain 1984 {published data only}
- Jain AK, Michael R, Ryan JR, McMahon FG. Antihypertensive and biochemical effects of indacrinone enantiomers. Pharmacotherapy 1984;4:278‐83. [DOI] [PubMed] [Google Scholar]
Licata 1989 {published data only}
- Licata G, Scaglione R, Parrinello G, Capuana G, Piovana U, Ganguzza A, et al. Clinical and hemodynamic effects of etozolin in essential hypertension: a double blind controlled trial. Current Therapeutic Research 1989;45(2):263‐7. [Google Scholar]
Perola 1985 {published data only}
- Perola P, Lehto MB H, Lammintausta R, Vikari J. Metabolic effects of furosemide and the combination of furosemide and triamterene. Current Therapeutic Research 1985;37(3):545‐53. [Google Scholar]
Vadasz 1982 {published data only}
- Vadasz TA, Chadha DR. Experience with once daily and twice daily slow release frusemide in hypertension. Journal of International Medical Research 1982;10:199‐203. [DOI] [PubMed] [Google Scholar]
Verho 1986 {published data only}
- Verho M, Rangoonwala B, Beloso M, Maass L, Bender R. Piretanide in the treatment of essential hypertension. A double blind comparison versus placebo. Drugs Under Experimental and Clinical Research 1986;XII(5):385‐91. [PubMed] [Google Scholar]
Wertheimer 1973 {published data only}
- Wertheimer L, Finnerty Jr FA, Bereu BA, Mich E, Hall RH. Furosemide in essential hypertension. A statistical analysis of three double blind studies. Archives Internal Medicine 1971;127:934‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonaduce 1981 {published data only}
- Bonaduce D, Ferrara N, Petretta M, Canonico V, Romango E, Rengo F. Comparison of antihypertensive activities of xipamid and chlorthalidone: a double blind randomized cross over trial. Current Medical Research and Opinion 1981;7(4):247‐52. [DOI] [PubMed] [Google Scholar]
Buckert 1984 {published data only}
- Buckert C, Muhlhausier W, Fratzer U, Verho M. A double blind multicentre study of piretanide and hydrochlorothiazide in patients with essential hypertension. Journal of International Medical Research 1984;12:81‐6. [DOI] [PubMed] [Google Scholar]
Campbell 1998 {published data only}
- Campbell N, Brant R, Stalts H, Stone J, Mahallati H. Fluctuations in blood lipid levels during furosemide therapy. Archives of Internal Medicine 1998;158(13):1461‐3. [DOI] [PubMed] [Google Scholar]
Charansonney 1997 {published data only}
- Charansonney SL, Lievre M, Laville M, Lion L, Derobert E, Visèle N, et al. The Eurieve Study: contrasting effect of piretanide and thiazides in mild to moderate hypertension. Therapie 1997;52:169‐177. [PubMed] [Google Scholar]
Clerson 1989 {published data only}
- Clerson P, Deltour L, Billaut P, Tarrade T, Berthet P, Pauchnat M. Efficacy and tolerance of cicletanine added to a previous beta blocking therapy in essential hypertensive patients [Evaluation de l'efficacite et de la tolerance du cicletanine chez des patients hypertendus traites par beta‐bloquants]. Archives des Maladies du Coeur et des Vaisseaux 1989;82:1293‐1297. [PubMed] [Google Scholar]
Coca 2009 {published data only}
- Coca A, Teresa E, Alfonso CB, Anguita M, Fernandez E, Dez J. Evaluation of the effects of prolonged release torasemide versus furosemide on myocardial fibrosis (Torafic Study): Design and baseline characteristics. Journal of Clinical Hypertension 2009;1:A97. [Google Scholar]
Costello‐Boerrigter 2006 {published data only}
- Costello‐Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, et al. Vasopressin‐2‐receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. American Journal of Physiology ‐ Renal Physiology 2006;290:F273‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diez 2009 {published data only}
- Diez J, Coca A, Teresa E, Anguita M, Castro‐Beiras A, Conthe P, et al. TORAFIC study protocol: torasemide prolonged release versus furosemide in patients with chronic heart failure. Expert Review of Cardiovascular Therapy 2009;7:897‐904. [DOI] [PubMed] [Google Scholar]
Di Somma 1990 {published data only}
- Somma S, Liguori V, Petitto M, Galletti P, Divitis O. The hemodynamic effect of etozolin in the treatment of hypertension. Current Therapeutic Research 1990;48(6):1044‐1052. [Google Scholar]
Drolet 2010 {published data only}
- Drolet DW, Coar W, Boinpally R, Walker G. Effect on peripheral blood pressure of a single oral dose of cicletanine HCl alone or in the presence of steady state sildenafil in healthy adult subjects. American Journal of Respiratory and Critical Care Medicine 2010:A3331.
Dussol 2006 {published data only}
- Dussol B, Morange S, Loundoun A, Auquier P, Berland Y. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrology Dialysis Transplantation 2006;21:2120‐6. [DOI] [PubMed] [Google Scholar]
Fried 1979 {published data only}
- Fried ED. Tienilic acid in the treatment of hypertension. Double blind assessment of tienilic acid in essential hypertension. Postgraduate Medical Journal 1979;55(Suppl 3):92‐97. [PubMed] [Google Scholar]
Galleti 1991 {published data only}
- Galleti F, Strazzullo P, Barba G, Cappuccio P, Iacone R, Mancici R. Diuretic therapy for mild hypertension: A comparison of the metabolic effects of etozoline and chlorthalidone during long term treatment. Current Therapeutic Research 1991;50(2):159‐166. [Google Scholar]
Galloe 2006 {published data only}
- Galloe AM, Skagen K, Christensen NJ, Nielsen SL, Frandsen EK, Bie P, et al. Dosage dependent hormonal counter regulation to combination therapy in patients with left ventricular dysfunction. Journal of Clinical Pharmacy and Therapeutics 2006;31:139‐47. [DOI] [PubMed] [Google Scholar]
Gupta 1981 {published data only}
- Gupta PS, Dave ML. Piretanide (HOE118): A new diuretic in essential hypertension. Current Therapeutic Research 1981;30(4):468‐476. [Google Scholar]
Gupta 2010 {published data only}
- Gupta S, Waywell C, Gandhi N, Clayton N, Keevil B, Clark AL, et al. The effects of adding torasemide to standard therapy on peak oxygen consumption, natriuretic peptides, and quality of life in patients with compensated left ventricular systolic dysfunction. European Journal of Heart Failure 2010;12:746‐52. [DOI] [PubMed] [Google Scholar]
Heijden 1998 {published data only}
- Heijden M, Donders SH, Cleophas TJ, Niemeyer MG, Meulen J, Bernink PJ, et al. A randomized placebo controlled study of loop diuretics in patients with essential hypertension: the bumetanide and furosemide on lipid profile (BUFUL) clinical study report. Journal of Clinical Pharmacology 1998;38:630‐635. [DOI] [PubMed] [Google Scholar]
Holland 1979 {published data only}
- Holland B, Gomez‐Sanchez C, Fairchild C, Kaplan NM. Role of renin classification for diuretic treatment of black hypertensive patients. Archives of Internal Medicine 1979;139:1365‐1370. [PubMed] [Google Scholar]
Hua 2007 {published data only}
- Hua CXi, Hua L, Li N, Wang L, Pang HM, Ming GH, et al. Efficacy of monotherapy with 15 antihypertensive agents in treating essential hypertension assessed by 24‐hour ambulatory blood pressure monitoring. Chung‐Kuo i Hsueh Ko Hsueh Yuan Hsueh Pao Acta Academiae Medicinae Sinicae 2007;29:792‐6. [PubMed] [Google Scholar]
Iyalomhe 2007 {published data only}
- Iyalomhe GBS, Omogbai EKI, Ozolua RI. Antihypertensive and some biochemical effects of hydrochlorothiazide and furosemide in some Nigerians. Journal of Medical Sciences 2007:977‐83.
Jungers 1989 {published data only}
- Jungers P, Gavardin T, Salom M, Tarrade T, Berthet P. Dosage study of cicletanine in elderly hypertensive patients: comparison of 50 vs 100mg daily dose [Etude comparative de deux posologies d' un nouvel antihypertenseur, le cicletanine chez le sujet age']. Archives des Maladies du Coeur et des Vaisseaux 1989;82(IV):125‐129. [PubMed] [Google Scholar]
Knoben 1982 {published data only}
- Knobem JM, Chada DR. Clinical experience with a fixed dose combination of penbutolol and furosemide in hypertension. Current Therapeutic Research 1982;31(3):281‐292. [Google Scholar]
Kopp 1978 {published data only}
- Kopp VH. The antihypertensive effect of etozolin [Zur antihypertensiven wirkung des diuretikums etozolin]. Fortschr. Med.96.jg. 1978;22:1194‐1198. [PubMed] [Google Scholar]
Krogsgaard 1976 {published data only}
- Krogsgaard AR, Trap‐Jensen J, Hartling O, Svendsen TL. Clinical and hemodynamic effects of high doses of slow release furosemide in arterial hypertension. A double blind cross over study with cyclopenthiazide. Acta Medica Scandinavica Supplementum 1976;602:110‐3. [PUBMED: PMID: 800315] [DOI] [PubMed] [Google Scholar]
Kumar 1984 {published data only}
- Kumar S, Pandi P, Wahi PL, Sharma PL. A randomized double blind clinical trial of xipamid and hydrochlorothiazide in essential hypertension. International Journal of Clinical Pharmacology 1984;22(10):349‐331. [PubMed] [Google Scholar]
Kundu 1986 {published data only}
- Kundu SC, Bhattacharya Td, Vakil HB. Diurnal pattern of blood pressure response to furosemide. Indian Practitioner 1986:757‐65.
Kuramoto 1985 {published data only}
- Kuramoto K, Masuyama Y. Comparison of clinical usefulness of diuretics in elderly and younger essential hypertension ‐ double blind trial using tripamide. Jpn J Geriat 1985;22:346‐353. [DOI] [PubMed] [Google Scholar]
Laffi 1991 {published data only}
- Laffi G, Marra F, Buzzelli G, Azzena G, Meacci E, Feo ML, et al. Comparison of the effects of torasemide and furosemide in nonazotemic cirrhotic patients with ascites: a randomized, double‐blind study. Hepatology 1991;13(6):1101‐5. [PubMed] [Google Scholar]
Leitch 1974 {published data only}
Magrini 1988 {published data only}
- Magrini F, Reggiani P, Roberts N, Meazza R, Ciulla M, Zanchetti A. Effects of angiotensin and angiotensin blockade on coronary circulation and coronary reserve. The American Journal of Medicine 1988;84(suppl 3A):55‐60. [DOI] [PubMed] [Google Scholar]
Matthews 1997 {published data only}
- Matthews G, Gornall R, Saunders NJ. A randomised placebo controlled trial of loop diuretics in moderate/severe pre‐eclampsia, following delivery. Journal of Obstetrics & Gynaecology 1997;17(1):30‐2. [DOI] [PubMed] [Google Scholar]
Mitrovic 2009 {published data only}
- Mitrovic V, Seferovic P, Dodic S, Krotin M, Neskovic A, Dickstein K, et al. Cardio‐renal effects of the A1 adenosine receptor antagonist SLV320 in patients with heart failure. Circulation: Heart Failure 2009;2:523‐31. [DOI] [PubMed] [Google Scholar]
Miziara 1982 {published data only}
- Miziara LJ, Goncalves JGF, Feres F. Comparative double blind trial with long acting furosemide and chlorthalidone in the treatment of essential hypertension [Ensaio comparativo duplo‐cego com furosemide de acao prolongada e chortalidona no tratamento da hipertensao essencial]. Farmacologica Clinica 1982;85(4):773‐778. [Google Scholar]
Nami 1991 {published data only}
- Nami R. Acute antihypertensive, diuretic and metabolic effect of etozolin and chlorthalidone. Panminerva Medica 1991;33:157‐163. [PubMed] [Google Scholar]
Obel 1984 {published data only}
- Obel A, Griffin L, Were J. Comparison of slow release frusemide and bendrofluazide in the treatment of moderate hypertension in Kenyan Negroes. Clinical Trials Journal 1984;21(6):443‐450. [Google Scholar]
Okun 1978 {published data only}
- Okun R, Beg MA. Tricynafen and hydrochlorothiazide in hypertension. Clinical Pharmacology & Therapeutics 1978;23(6):703‐711. [DOI] [PubMed] [Google Scholar]
Okun 1979 {published data only}
- Okun R, Beg MA. A double‐blind study of tienilic acid with two year follow‐up of patients with mild to moderate essential hypertension. Postgrad Med J 1979;55(Suppl 3):103‐9. [PUBMED: PMID: 388395] [PubMed] [Google Scholar]
Oli 1983 {published data only}
- Oli JM, Ikeakor IP. Sustained release frusemide and blood glucose levels in diabetics. Current Therapeutic Research 1983;34(3):537‐541. [Google Scholar]
Olshan 1981 {published data only}
- Olshan AR, O'Connor DT, Preston RA, Frigon RP, Stone RA. Involvement of kallikrein in the antihypertensive response to furosemide in essential hypertension. Journal of Cardiovascular Pharmacology 1981;3(1):161‐8. [DOI] [PubMed] [Google Scholar]
Pearson 1979 {published data only}
- Pearson RM, Bulpitt CJ, Havard CWH. Biochemical and haematological changes induced by tienilic acid combined with propranolol in essential hypertension. The Lancet 1979;1(8118):697‐9. [PUBMED: PMID: 85937] [DOI] [PubMed] [Google Scholar]
- Pearson RM, Bulpitt CJ, Havard CWH. Propranolol and tienilic acid in essential hypertension. Postgraduate Medical Journal 1979;55(3):115‐119. [PubMed] [Google Scholar]
Potter 1987 {published data only}
- Potter JF, Beevers DG. Atenolol improves blood pressure control in patients taking captopril and frusemide. Journal of Human Hypertension 1987;1:127‐130. [PubMed] [Google Scholar]
Reyes 1990 {published data only}
- Reyes AJ, Chiesa PD, Santucci MR, Batista LB, Olhaberry JV, Mosler AL, et al. Hydrochlorothiazide versus a non‐diuretic dose of torasemide as once daily antihypertensive monopharmacotherapy in elderly patients. A randomized double blind study.. Progress in Pharmacology and Clinical Pharmacology 1990;8(1):183‐209. [Google Scholar]
Ronchi 1990 {published data only}
- Ronchi E, Posca M, Sassi S, Agostara F, Palumbo G. Adding muzolimine or triamterene to nifedipine: effect on blood pressure. Current Therapeutic Research 1990;48(3):492‐498. [Google Scholar]
Rush 1990 {published data only}
- Rush MG, Engelhardt B, Parker RA, Hazinski TA. Double‐blind, placebo‐controlled trial of alternate‐day furosemide therapy in infants with chronic bronchopulmonary dysplasia. The Journal of Pediatrics 1990;117((1 Pt 1)):112‐8. [DOI] [PubMed] [Google Scholar]
Rutledge 1988 {published data only}
- Rutledge J, Ayers C, Davidson R, DiPette D, Guthrie G, Fisher M, et al. Effect of intravenous enalaprilat in moderate and severe systemic hypertension. The American Journal of Cardiology 1988;62:1062‐1067. [DOI] [PubMed] [Google Scholar]
Shah 2011 {published data only}
- Shah RV, McNulty S, Lee K, Michael Felker G, O'Connor CM, Givertz MM. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from DOSE‐AHF. Journal of the American College of Cardiology 2011;1:E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Udelson 2011 {published data only}
- Udelson JE, Bilsker M, Hauptman PJ, Sequeira R, Thomas I, O'Brien T, et al. A multicenter, randomized, double‐blind, placebo‐controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. Journal of Cardiac Failure 2011;17:973‐81. [DOI] [PubMed] [Google Scholar]
Valmin 1980 {published data only}
- Valmin K, Hansen T, Ronsted P. Treatment of benign essential hypertension with frusemide in different doses. Pharmacotherapeutica 1980;2:296‐304. [PubMed] [Google Scholar]
van Kraaij 2003 {published data only}
- Kraaij DJ, Jansen RW, Sweep FC, Hoefnagels WH. Neurohormonal effects of furosemide withdrawal in elderly heart failure patients with normal systolic function. European Journal of Heart Failure 2003;5(1):47‐53. [DOI] [PubMed] [Google Scholar]
Webster 1987 {published data only}
- Webster J, Petrie JC, Robb OJ, Witte K, Lovell HG. Atenolol or propanolol in hypertensive patients poorly controlled on captopril and frusemide. Journal of Human Hypertension 1987;1:121‐126. [PubMed] [Google Scholar]
Witchitz 1974 {published data only}
- Witchitz S, Giudicelli JF, El‐Guedri H, Kamoun A, Chiche P. Diuretic and anti‐hypertensive effects of indapalide. Comparison with furosemide. Therapie 1974;29(1):109‐18. [PubMed] [Google Scholar]
Yasky 1987 {published data only}
- Yasky J, Verho M, Rangoonwala B. Efficacy of a fixed dose combination of 40 mg penbutolol with 6 mg piretanide in the treatment of mild to moderate hypertension: a double blind study against placebo. Curr. Med. Res. Opi. 1987;10(6):397‐406. [DOI] [PubMed] [Google Scholar]
- Yasky J, Verho M, Rangoonwala B. Efficacy of a low dose fixed combination of penbutolol and piretanide in the treatment of mild to moderate hypertension: a double blind study against placebo. Pharmatherapeutica 1986;4(9):607‐16. [PUBMED: PMID: 3763655] [PubMed] [Google Scholar]
Zamboli 2011 {published data only}
- Zamboli P, Nicola L, Minutolo R, Chiodini P, Crivaro M, Tassinario S, et al. Effect of furosemide on left ventricular mass in non‐dialysis chronic kidney disease patients: a randomized controlled trial. Nephrology Dialysis Transplantation 2011;26:1575‐83. [DOI] [PubMed] [Google Scholar]
Additional references
Chen 2009
- Chen JMH, Heran BS, Wright JM. Blood pressure lowering efficacy of diuretics as second‐line therapy for primary hypertension. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007187.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008a
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heran 2008b
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003822.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Materson 1983
- Materson BJ. Insights into intrarenal sites and mechanism of action of diuretic agents. American Heart Journal 1983;106:188‐208. [DOI] [PubMed] [Google Scholar]
Musini 2008
- Musini VM, Fortin PM, Bassett K, Wright JM. Blood pressure lowering efficacy of renin inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007066.pub2] [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Wright JM. Factors affecting blood pressure variability: lessons learned from two systematic reviews of randomized controlled trials . PLoS ONE 2009;4(5):e5673. [DOI: 10.1371/journal.pone.0005673] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3.5. Copenhagen: The Cochrane Collaboration, 2014.
Seldin 1997
- Seldin D, Giebisch G. Diuretic Agents: Clinical Physiology and Pharmacology. San Diego (CA): Academic Press, 1997. [Google Scholar]
Wright 2009
- Wright JM, Musini VM. First‐line drugs for hypertension. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001841.pub2] [DOI] [PubMed] [Google Scholar]


