Abstract
Background
Placebo interventions are often claimed to substantially improve patient‐reported and observer‐reported outcomes in many clinical conditions, but most reports on effects of placebos are based on studies that have not randomised patients to placebo or no treatment. Two previous versions of this review from 2001 and 2004 found that placebo interventions in general did not have clinically important effects, but that there were possible beneficial effects on patient‐reported outcomes, especially pain. Since then several relevant trials have been published.
Objectives
Our primary aims were to assess the effect of placebo interventions in general across all clinical conditions, and to investigate the effects of placebo interventions on specific clinical conditions. Our secondary aims were to assess whether the effect of placebo treatments differed for patient‐reported and observer‐reported outcomes, and to explore other reasons for variations in effect.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 4, 2007), MEDLINE (1966 to March 2008), EMBASE (1980 to March 2008), PsycINFO (1887 to March 2008) and Biological Abstracts (1986 to March 2008). We contacted experts on placebo research, and read references in the included trials.
Selection criteria
We included randomised placebo trials with a no‐treatment control group investigating any health problem.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted study authors for additional information. Trials with binary data were summarised using relative risk (a value of less than 1 indicates a beneficial effect of placebo), and trials with continuous outcomes were summarised using standardised mean difference (a negative value indicates a beneficial effect of placebo).
Main results
Outcome data were available in 202 out of 234 included trials, investigating 60 clinical conditions. We regarded the risk of bias as low in only 16 trials (8%), five of which had binary outcomes.
In 44 studies with binary outcomes (6041 patients), there was moderate heterogeneity (P < 0.001; I2 45%) but no clear difference in effects between small and large trials (symmetrical funnel plot). The overall pooled effect of placebo was a relative risk of 0.93 (95% confidence interval (CI) 0.88 to 0.99). The pooled relative risk for patient‐reported outcomes was 0.93 (95% CI 0.86 to 1.00) and for observer‐reported outcomes 0.93 (95% CI 0.85 to 1.02). We found no statistically significant effect of placebo interventions in four clinical conditions that had been investigated in three trials or more: pain, nausea, smoking, and depression, but confidence intervals were wide. The effect on pain varied considerably, even among trials with low risk of bias.
In 158 trials with continuous outcomes (10,525 patients), there was moderate heterogeneity (P < 0.001; I2 42%), and considerable variation in effects between small and large trials (asymmetrical funnel plot). It is therefore a questionable procedure to pool all the trials, and we did so mainly as a basis for exploring causes for heterogeneity. We found an overall effect of placebo treatments, standardised mean difference (SMD) ‐0.23 (95% CI ‐0.28 to ‐0.17). The SMD for patient‐reported outcomes was ‐0.26 (95% CI ‐0.32 to ‐0.19), and for observer‐reported outcomes, SMD ‐0.13 (95% CI ‐0.24 to ‐0.02). We found an effect on pain, SMD ‐0.28 (95% CI ‐0.36 to ‐0.19)); nausea, SMD ‐0.25 (‐0.46 to ‐0.04)), asthma (‐0.35 (‐0.70 to ‐0.01)), and phobia (SMD ‐0.63 (95% CI ‐1.17 to ‐0.08)). The effect on pain was very variable, also among trials with low risk of bias. Four similarly‐designed acupuncture trials conducted by an overlapping group of authors reported large effects (SMD ‐0.68 (‐0.85 to ‐0.50)) whereas three other pain trials reported low or no effect (SMD ‐0.13 (‐0.28 to 0.03)). The pooled effect on nausea was small, but consistent. The effects on phobia and asthma were very uncertain due to high risk of bias. There was no statistically significant effect of placebo interventions in the seven other clinical conditions investigated in three trials or more: smoking, dementia, depression, obesity, hypertension, insomnia and anxiety, but confidence intervals were wide.
Meta‐regression analyses showed that larger effects of placebo interventions were associated with physical placebo interventions (e.g. sham acupuncture), patient‐involved outcomes (patient‐reported outcomes and observer‐reported outcomes involving patient cooperation), small trials, and trials with the explicit purpose of studying placebo. Larger effects of placebo were also found in trials that did not inform patients about the possible placebo intervention.
Authors' conclusions
We did not find that placebo interventions have important clinical effects in general. However, in certain settings placebo interventions can influence patient‐reported outcomes, especially pain and nausea, though it is difficult to distinguish patient‐reported effects of placebo from biased reporting. The effect on pain varied, even among trials with low risk of bias, from negligible to clinically important. Variations in the effect of placebo were partly explained by variations in how trials were conducted and how patients were informed.
Plain language summary
Placebo interventions for all clinical conditions
Placebo interventions are often claimed to substantially improve many clinical conditions. However, most reports on effects of placebos are based on unreliable studies that have not randomised patients to placebo or no treatment.
We studied the effect of placebo treatments by reviewing 202 trials comparing placebo treatment with no treatment covering 60 healthcare problems. In general, placebo treatments produced no major health benefits, although on average they had a modest effect on outcomes reported by patients, such as pain. However, the effect on pain varied from large to non‐existent, even in well‐conducted trials. Variations in the effect of placebo was partly explained by variations in how trials were conducted, the type of placebo used, and whether patients were informed that the trial involved placebo.
Summary of findings
Summary of findings for the main comparison. Effect of placebo interventions across all clinical conditions (main findings).
| Outcomes |
Effect [1] (95% CI) |
No. of participants (studies) | Quality of the evidence | Comments |
| All clinical conditions (binary outcomes) | RR 0.93 (0.88 to 0.99) | 6041 (44) | Moderate | Moderate heterogeneity. No statistically significant differences between patient‐reported, and observer‐reported binary outcomes. No statistically significant effect on: pain, nausea, smoking or depression [2]. Out of three pain trials with low risk of bias (1109 patients), one German acupuncture trial found a large effect, and two trials found no effect [3]. |
|
All clinical conditions (continuous outcomes) |
SMD ‐0.23 (‐0.28 to ‐0.17) | 10,525 (158) | Moderate | Moderate heterogeneity. Statistically significant differences between patient‐reported, and observer‐reported outcomes, SMD ‐0.26 (‐0.32 to ‐0.19) versus ‐0.13 (‐0.24 to ‐0.02). Meta‐regression explained 54% of the variation in effect [3]. |
|
Pain [2] (continuous outcomes) |
SMD ‐0.28 (‐0.36 to ‐0.19) | 4154 (60) | Moderate | Moderate heterogeneity. Seven trials (1198 patients) had low risk of bias, but heterogeneity was substantial: four German acupuncture pain trials found large effects, and three other pain trials found negligible effects [3]. |
|
Nausea [2] (continuous outcomes) |
SMD ‐0.25 (‐0.46 to ‐0.04) | 452 (7) | Moderate | Low heterogeneity. The pooled result for all nausea trials was similar to the pooled result of the two nausea trials with low risk of bias [3]. |
|
Depression [2] (continuous outcomes) |
SMD ‐0.25 (‐0.55 to 0.05) | 324 (8) | Moderate | Moderate heterogeneity. The pooled result for all depression trials was similar to the result of the single depression trial with low risk of bias [3]. |
|
Other outcomes [2] (continuous outcomes) Smoking, dementia, obesity, hypertension, insomnia, anxiety, asthma, phobia) |
Range of SMD: ‐0.63 (‐1.17 to ‐0.08) to ‐0.16 (‐0.48 to 0.16) |
1317 (41) | Low | There was a statistically significant, but unreliable, effect on asthma and phobia [3]. |
[1]. RR: relative risk; SMD: standardised mean difference.
[2]. Clinical conditions studied in three trials or more.
[3]. See Additional tables.
Background
There has been a widespread belief that placebo interventions have considerable and reliable effects. This view was influenced by the seminal paper 'The Powerful Placebo' (Beecher 1955), which was one of the first attempts to combine results from several randomised trials. Narrative reviews from the 1980s and 1990s similarly concluded that placebo interventions substantially improve both patient‐reported and observer‐reported outcomes in a large proportion of patients with a wide range of clinical conditions, such as pain, asthma, high blood pressure, and even myocardial infarction (Brown 1998; Lasagna 1986).
However, a careful analysis concluded that Beecher's paper is flawed (Kienle 1997). Most reports on placebo, including Beecher's and the reviews quoted above, have estimated the effect of placebo as the difference before and after treatment in a placebo arm of a randomised trial. Thus, though the information in a loose sense comes from randomised trials, the estimation of the effect is not based on a comparison between patients who have been randomly allocated to a placebo group and to a no‐treatment group. Without such a comparison, the effect of a placebo intervention cannot be distinguished from the natural course of the disease, and other factors, for example regression to the mean (the tendency for extreme measurements to be closer to the mean when repeated) (Gøtzsche 1994; Hróbjartsson 2002b). The reported large effect of placebo interventions could therefore, at least in part, be an artefact of inadequate research methods.
There is no formal definition of placebo that most clinicians and researchers agree upon (Gøtzsche 1994; Hróbjartsson 2002b). In clinical trials placebos are generally control treatments with a similar appearance to the study treatments, but without their essential components. It is generally assumed that any effect of a placebo intervention, for instance a sugar pill, is unrelated to its essential component, the sugar, but caused by the special interaction between patient and healthcare provider associated with the treatment ritual. However, the phrase 'placebo' is also sometimes used more broadly to describe, for example, any psychologically‐mediated factor that potentially influences health. In this review we evaluate the effect of placebo in its narrow sense, as an intervention, based on trials that randomise patients to a placebo intervention group and to a no‐treatment control group.
The two previous versions of this review were published in 2001 (Hróbjartsson 2001) and in 2004 (Hróbjartsson 2004a). Both reviews found that placebo interventions in general do not have clinically important effects, but that there were possible beneficial effects on patient‐reported outcomes, especially pain. Since then several relevant trials have been published.
Objectives
Our primary aims were to assess the effect of placebo interventions in general across all clinical conditions, and to investigate the effects of placebo interventions on specific clinical conditions.
Our secondary aims were to assess whether the effect of placebo treatments differed for patient‐reported and observer‐reported outcomes, and to explore other reasons for variations in effect
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials with a placebo group and a no‐treatment group were considered for inclusion. Both parallel and crossover trials, in any language, were included, as well as unpublished studies when methodology and results could be accessed in written form.
Trials were excluded if patients were allocated by a quasi‐random method, e.g. day of month or date of birth. Trials were also excluded if it was clear that the person who assessed objective outcomes was aware of group assignments, or if the dropout rate exceeded 50%.
Types of participants
Patients with a health problem, defined broadly as any somatic or psychiatric disease or symptom. We also included trials testing the prophylactic effect of placebo in a clinical setting on healthy participants. Trials were excluded if they involved healthy participants who had a condition inflicted upon them, e.g. pain, in a non‐clinical, experimental setting, or patients who were paid a fee.
Types of interventions
We pragmatically defined a placebo intervention as any intervention which was clearly labelled a placebo in a trial report (by using the term placebo or an analogous term, e.g. sham, fake, dummy, or non‐ or unspecific treatment).
Trials were excluded when it was very likely that the alleged placebo intervention had an effect which was not related to the treatment ritual alone (e.g. movement techniques for postoperative pain). The no‐treatment control groups consisted of patients who did not receive placebo interventions. We included trials in which both the placebo and no‐treatment control groups received the same basic treatment.
Types of outcome measures
One outcome per trial was extracted for the main analyses. We primarily chose the outcome indicated as the main outcome in a trial report (e.g. used for a power calculation). If a main outcome was not clearly indicated we chose the outcome measure we considered most relevant to patients. We preferred patient‐reported to observer‐reported outcomes, and binary to continuous outcomes because we find such outcomes are generally more relevant to patients. We preferred post‐treatment data, since follow‐up data may be more prone to bias because of patients leaving the trial and diminution of the effect. Outcomes were not selected based on effect size or statistical significance.
Search methods for identification of studies
The search strategy was based primarily on an electronic search of five databases. The references of all included articles and selected reviews and books on placebo were read systematically for citations of potentially eligible trials. Furthermore, we contacted 28 researchers who had made significant contributions to the field, and asked if they knew of relevant trials.
We searched the following databases:
The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4 2007);
MEDLINE (1966 to March 2008);
EMBASE (1980 to March 2008);
PsycINFO (1887 to March 2008); and
Biological Abstracts (1986 to March 2008).
The search strategies were developed iteratively based on synonyms of 'placebo', randomised clinical trials', and 'no‐treatment'. Our comments under each of the headings explain variations in the search strategy (see Appendix 1).
Data collection and analysis
Reports that described potentially eligible trials were read in full by one author (AH), who excluded all studies that clearly did not comply with the inclusion criteria. Both authors read all other potentially eligible trial reports in full and made a decision on study inclusion independently; any disagreement was resolved by discussion.
We extracted information from the trial reports using a pilot‐tested standardised data chart. The decision about which outcome to choose was made by both authors independently, and disagreements were resolved by discussion. All outcomes of each trial were listed. If outcome data were not available, we contacted the trial authors. All binary outcomes events were converted so they represent 'failures' or unsuccessful events. Similarly for continuous outcomes, all scales were converted so that higher scores indicate more intense symptoms.
For trials with binary outcomes we calculated the relative risk (RR) (if less than 1, it indicates a positive effect of the placebo intervention). For trials with continuous outcomes (and with data on ranking scales, for simplicity also called continuous in the following), we calculated the standardised mean difference (SMD) (a negative value indicates a positive effect of the placebo intervention). Trials reporting results measured on an ordinal scale were analysed as if they were continuous. For crossover trials we used data from the first period only. If that was not possible we used the summary data as if they had been derived from a parallel trial. We preferred final values, but used change from baseline if these were the only available data.
As we expected heterogeneity, we calculated the pooled results with random effect models (Mantel‐Haenszel method for RRs, and inverse variance method for SMDs). We estimated the degree of heterogeneity using the I2 test. The I2 statistic can be interpreted as the proportion of the observed discrepancy in the estimation of the effect, within a group of trials, which cannot be accounted for by random variation (Higgins 2003). All results are reported with 95% confidence intervals and all P values are two‐tailed.
We calculated the pooled effect of placebo overall for trials with binary outcomes and for trials with continuous outcomes. We also calculated the pooled effect on separate clinical conditions when they had been studied in three trials or more, and the pooled effect of trials with patient‐reported and observer‐reported outcomes. The threshold of three trials was chosen pragmatically, inspired by Linde (Linde 1997), in order to reduce the risk of spurious positive or negative findings in single trials. For each trial we plotted the effect by its standard error. The symmetry of such 'funnel plots' was assessed both visually, and formally with Egger's test (Egger 1997), to see if the effect decreased with increasing sample size.
We defined trials with low risk of bias as those fulfilling the following three criteria: adequate concealment of allocation, dropout rate no more than 15%, and inclusion of at least 50 patients. We pre‐specified these thresholds based on a pragmatic intention of providing a simple risk of bias assessment in a review with many trials. The role of trial size is debated, but was included because small trials are often more poorly conducted than larger trials.
To study whether specific subgroups of trials reported higher or lower effects of placebo we compared two or more subgroups, with tests of interaction, involving the following 14 factors:
Type of placebo: i) pharmacological placebo, e.g. a pill; versus. ii) physical placebo, e.g. a machine without current; versus iii) psychological placebo, e.g. a neutral conversation.
Type of outcome: i) patient‐reported outcomes essentially private to a patient, e.g. pain; versus ii) patient‐reported outcomes potentially observable by another person at the time they occurred, e.g. haematuria; versus iii) observer‐reported outcomes dependent on the cooperation of a patient, e.g. measurement of forced expiratory volume); versus iv) observer‐reported outcomes not dependent on patient cooperation, e.g. assessment of oedema); versus v) observer‐reported outcomes in the form of laboratory data, e.g. blood sugar.
Placebo as add‐on treatment: i) placebo treatment was the only intervention; versus ii) placebo treatment was an add‐on treatment to a basic care treatment, also given to the patients in the no‐treatment control group.
Dropout rate: i) the dropout rate exceeded 15% or was not reported; versus ii) the dropout rate was 15% or lower.
Blinding of observer: i) the trial report stated explicitly that the data collector of an observer‐reported outcome was blinded; versus ii) the trial report did not state this explicitly.
Blinding of patients and treatment providers: i) placebo and active experimental groups were compared in a ‘double‐blind’ design; versus ii) that was not the case, or not stated.
The trial’s objective: i) the trial report stated explicitly that the objective was to assess the effect of placebo treatment; versus ii) no such explicit objective was stated.
Concealment of allocation: i) the allocation of patients was clearly concealed; versus ii) the allocation of patients was not clearly concealed.
Type of distribution: i) clear signs of a non‐Gaussian distribution, or of a difference in variance between the placebo and the no‐treatment groups; versus ii) no such signs. We regarded it a clear sign of a non‐Gaussian distribution when 1.64 standard deviations exceeded the mean of naturally positive outcomes (CCC Stat Pol 1999). A difference in variance was assessed using F tests.
Reporting of a primary outcome: i) clear indication of a primary outcome in the trial report; versus ii) no clear indication of a primary outcome (in which case we decided which outcome to extract).
Sample size: i) the analysis involved at least 50 patients; versus ii) the analysis involved less than 50 patients.
Risk of bias: i) clearly concealed allocation of patients, and dropout rate of 15% or lower, and sample size of at least 50 patients; versus ii) those criteria not fulfilled.
Information to patients: i) patients were not informed that the trial involved a placebo intervention (instead they were informed that the trial compared two active interventions with a control group); versus ii) the trial report was unclear on this point, or stated that patients were aware that the trial involved a placebo intervention.
Format of outcome: Final values versus change from baseline.
Subgroup analyses 1‐12 were pre‐specified before we started searching for trials for the present update. Subgroup analyses 13 and 14 were post‐hoc (see Discussion).
We furthermore conducted supplementary meta‐regression analyses involving the trials with continuous outcomes. We specified 11 co‐variates: the factors involved in subgroup analysis 1‐4, 6‐10, and 13, as well as trial precision (1/SE). For the meta‐regression we modified our initial categorisations in two cases. Type of placebo (pharmacological, psychological or physical) was redefined as a binary co‐variate: physical placebo; versus not. Similarly, type of outcome was dichotomised so that we analysed patient‐involved outcomes (patient‐reported outcomes and observer‐reported outcomes involving patient cooperation); versus not. The meta‐regression analyses involved: a) multiple meta‐regression with all 11 covariates, and b) multiple meta‐regression with stepwise elimination of the co‐variate with the highest P value until the analysis only included co‐variates with P < 0.05.
Results
Description of studies
The search strategy (Appendix 1) identified 1215 potentially eligible trial reports. We excluded 620 non‐clinical or non‐randomised trials, 252 without a placebo or a no‐treatment group, 35 duplicate publications and 11 with clearly unblinded assessment of observer‐reported outcomes. A further 63 trials were excluded for other reasons, e.g. dropout rates over 50%.
Thus, we included 234 trials. In 29 trials we were unable to extract relevant outcome data, and three trials involved assessment of harm. The main meta‐analyses therefore included 202 trials.
There were 18 crossover trials of which 12 (330 patients) were handled as parallel trials. In 196 trials there was a third active treatment group in addition to the placebo and the no‐treatment groups. In 164 of these trials, the effect of placebo was not mentioned as an objective of the study. The trial reports were published in five languages between 1946 and 2008.
Outcomes were binary in 44 trials, and continuous in 158. Counting only patients in the placebo and no‐treatment groups, the trials with binary outcomes included 6041 patients, and had a median size of 54 patients (10 and 90 percentiles: 20 and 618); the trials with continuous outcomes included 10,525 patients and had a median size of 40 (10 and 90 percentiles: 18 and 149).
The typical pharmacological placebo intervention was a lactose tablet. The typical physical placebo implied a machine turned off, e.g. sham transcutaneous electrical nerve stimulation. The typical psychological placebo was a non‐directional, neutral discussion between patient and treatment provider, a so‐called 'attention placebo'. No‐treatment typically implied 'observation only' or 'standard therapy'. In the latter case all patients received standard therapy, and the placebo intervention was an additional treatment.
The trials investigated 60 clinical conditions: alcohol abuse, allergy, anaemia, anxiety, aphtous ulcers, asthma, attention‐deficit‐hyperactivity disorder, bacterial infections, benign prostatic hyperplasia, blood donation reactions, breathlessness, bulimia nervosa, carpal tunnel syndrome, compulsive nail biting, dementia, depression, dermatitis, difficulty of colonoscopy, diabetes, dry eye, enuresis, epilepsy, faecal soiling, fatigue, gag reflex, herpes simplex infection, irritable bowel syndrome, hypercholesterolaemia, hyperglycaemia, hypertension, ileus, infertility, insomnia, insufficient cervical dilatation, jet lag, labour, marital discord, menopause, mental handicap, orgasmic difficulties, overweight, procedural discomfort during bronchoscopy, upper respiratory infection, venous ulcers, vitiligo, pain, nausea, Parkinson's disease, patient involvement in adolescent diabetic care, phobia, physical activity, poor oral hygiene, Raynaud's disease, schizophrenia, seasickness, secondary erectile dysfunction, smoking, stress related to dental treatment, treatment adherence, or undiagnosed ailments.
Five trials call for special attention (Brinkhaus 2006; Linde 2005; Melchart 2005; Witt 2005; Scharf 2006).The trials all studied the effect of acupuncture on pain. They were conducted in Germany, published between 2005 and 2007, had a very similar design, and four of the five trials had overlapping authors. In the following they are called 'the German acupuncture trials'. They studied the effect of 6 to 8 weeks of acupuncture and placebo acupuncture on osteoarthritis pain, low back pain, migraine, and tension type headache. The trials were medium‐sized to large, their allocation concealment adequate and dropouts were below 15%. They reported substantial effects of placebo acupuncture, SMDs ranged from ‐0.56 to ‐0.82, and the single trial with a binary outcome reported an RR of 0.69. They differed from other trials in that they combined low risk of bias with large effects.
A more detailed description of the studies can be seen in the Characteristics of included studies table.
Risk of bias in included studies
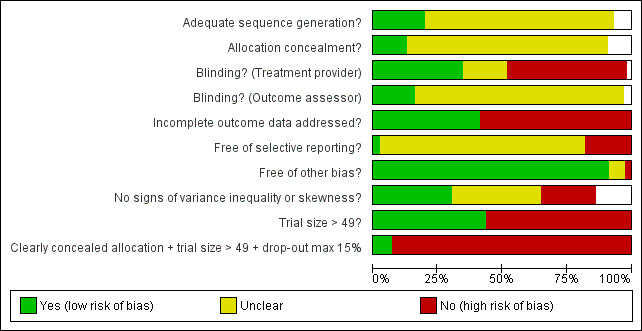
The methodological quality of the trials was generally mediocre, but quite variable (Figure 1; Figure 2).
1.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
2.
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
All included trials were randomised, but in only 28 trials (12%) was it clear that patient allocation had been adequately concealed. In 88 trials the dropout rate was 15% or lower, and in the remaining 114 trials it was above 15% (or not reported). In 86 trials the sample size was 50 or more. We regarded the risk of bias as low in 16 trials (8%), five of which had binary outcomes.
In 61 trials the comparison between placebo and an experimental active treatment was described as ‘double blind’, whereas in the remaining 141 such trials comparisons were not double blind (or not reported). Observer‐reported outcomes were clearly assessed by a blinded observer in 22 trials, but this was unclear in 41 trials.
Effects of interventions
See: Table 1
Binary outcomes (44 trials; 6041 patients)
The funnel plot was symmetrical around a single peak (Figure 3). There was no statistically significant difference between the results in small and large trials (Egger’s test, P = 0.49). Heterogeneity was moderate (P < 0.001, I2 45%).
3.
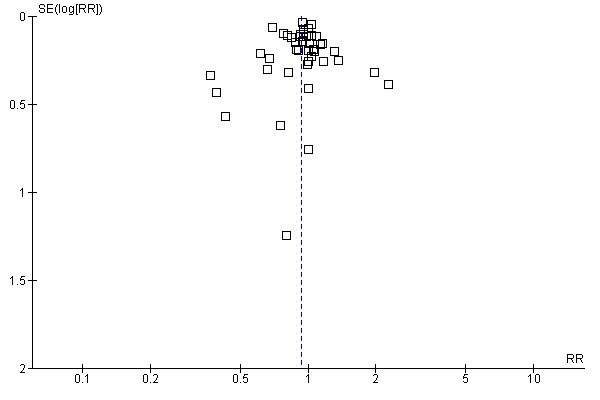
Funnel plot of comparison: 1 Main analysis: overall pooled analyses, outcome: 1.1 Binary outcomes.
The pooled effect was RR 0.93 (0.88 to 0.99) (Analysis 1.1) (Table 1). The effect for patient‐reported outcomes was RR 0.93 (0.86 to 1.00) and for observer‐reported outcomes RR 0.93 (0.85 to 1.02) (Table 20).
1.1. Analysis.
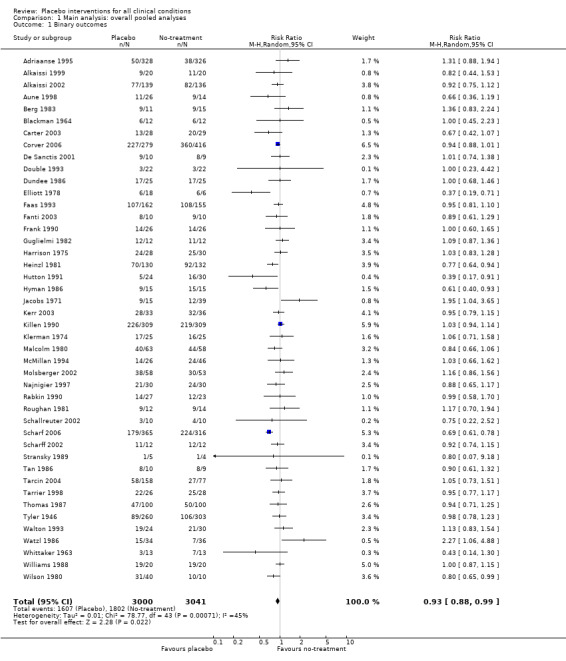
Comparison 1 Main analysis: overall pooled analyses, Outcome 1 Binary outcomes.
1. Effect of placebo interventions across all clinical conditions (binary outcomes).
| All trials | Trials with low risk of bias | Quality of the evidence | |||||
| Outcomes | Relative Risk (95% CI) | No. of participants (studies) | Comments | Relative Risk (95% CI) | No. of participants (studies) | Comments | |
| All clinical conditions | 0.93 (0.88 to 0.99) | 6041 (44) | Symmetrical funnel plot Moderate heterogeneity |
0.90 (0.76 to 1.08) | 1,438 (5) | Substantial heterogeneity. One German acupuncture trial found RR 0.69 (0.61 to 0.78) and the other four trials RR 0.96 (0.87 to 1.06) | Moderate |
| Trials with patient‐reported outcomes | 0.93 (0.86 to 1.00) | 4046 (31) | Symmetrical funnel plot Moderate heterogeneity |
0.89 (0.72 to 1.11) | 845 (4) | Substantial heterogeneity (see above). | Moderate |
| Trials with observer‐reported outcomes | 0.93 (0.85 to 1.02) | 1995 (13) | Symmetrical funnel plot Moderate heterogeneity |
0.95 (0.77 to 1.17) | 54 (1) | One small trial | Moderate |
We categorised five trials as having low risk of bias. The pooled effect of these trials was RR 0.90 (0.76 to1.08). The analysis involved considerable heterogeneity (P < 0.001; I2 78%) caused by one German acupuncture pain trial with a RR of 0.69 (0.61 to 0.78). The pooled effect of the other four trials was RR 0.96 (0.87 to 1.06) (P = 0.63; I2 0%).
Four clinical problems had been investigated in at least three trials with binary outcomes: nausea, pain, and relapse in prevention of smoking and depression. Placebo interventions had no statistically significant effect on these clinical conditions, but confidence intervals were wide (Table 21).
2. Effect of placebo interventions on specific clinical conditions (binary outcomes).
| All trials | Trials with low risk of bias | Quality of the evidence | |||||
| Condition [1] |
Relative risk (95% CI) |
No. of participants (studies) | Comments |
Relative risk (95% CI) |
No. of participants (studies) | Comments | |
| Pain | 0.92 (0.76 to 1.11) | 1207 (6) | Substantial heterogeneity | 0.89 (0.67 to 1.19) | 1109 (3) | No heterogeneity | Moderate |
|
0.98 (0.88 to 1.10) | 525 (5) | No heterogeneity | 1.00 (0.84 to 1.20) | 428 (2) | No heterogeneity | |
|
0.69 (0.61 to 0.78) | 681 (1) | NA | 0.69 (0.61 to 0.78) | 681 (1) | NA | |
| Nausea | 0.94 (0.82 to 1.07) | 732 (6) | No heterogeneity | 0.92 (0.75 to 1.12) | 275 (1) | NA | Moderate |
| Smoking | 0.89 (0.73 to 1.10) | 887 (6) | Substantial heterogeneity | NA | NA | NA | Low |
| Depression | 1.03 (0.78 to 1.34) | 152 (3) | No heterogeneity | NA | NA | NA | Low |
[1]. Clinical conditions studied in three trials or more.
[2]. German acupuncture trials.
NA: not applicable.
Continuous outcomes (158 trials; 10,525 patients)
The funnel plot was asymmetrical (Figure 4). Small trials tended to report higher effects of placebo than larger trial (Egger’s test, P = 0.03). There was moderate heterogeneity (P < 0.001, I2 42%).
4.
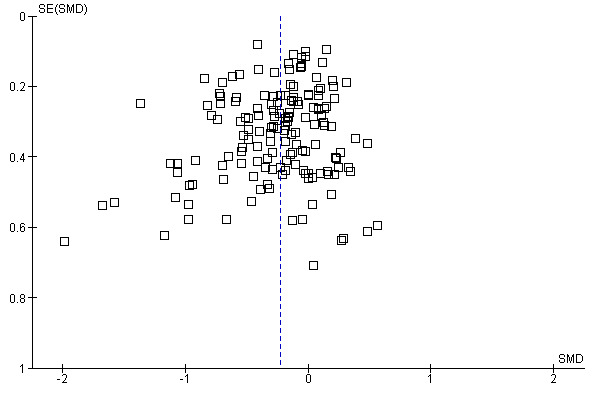
Funnel plot of comparison: 1 Main analysis: overall pooled analyses, outcome: 1.2 Continuous outcomes.
The effect estimates of the individual trials spanned roughly from SMD ‐2.0 to 0.5, and the effects of large trials varied considerably. Because of heterogeneity and funnel plot asymmetry it is a questionable procedure to pool all the trials, and we did so mainly as a basis for exploring causes for heterogeneity.
The pooled effect was SMD ‐0.23 (‐0.28 to ‐0.17) (Analysis 1.2) (Table 1). The effect for patient‐reported outcomes (SMD ‐0.26 (‐0.32 to ‐0.19)) was statistically significantly different from the effect for observer‐reported outcomes (SMD ‐0.13 (‐0.24 to ‐0.02), (test of interaction, P = 0.045)) (Table 22).
1.2. Analysis.
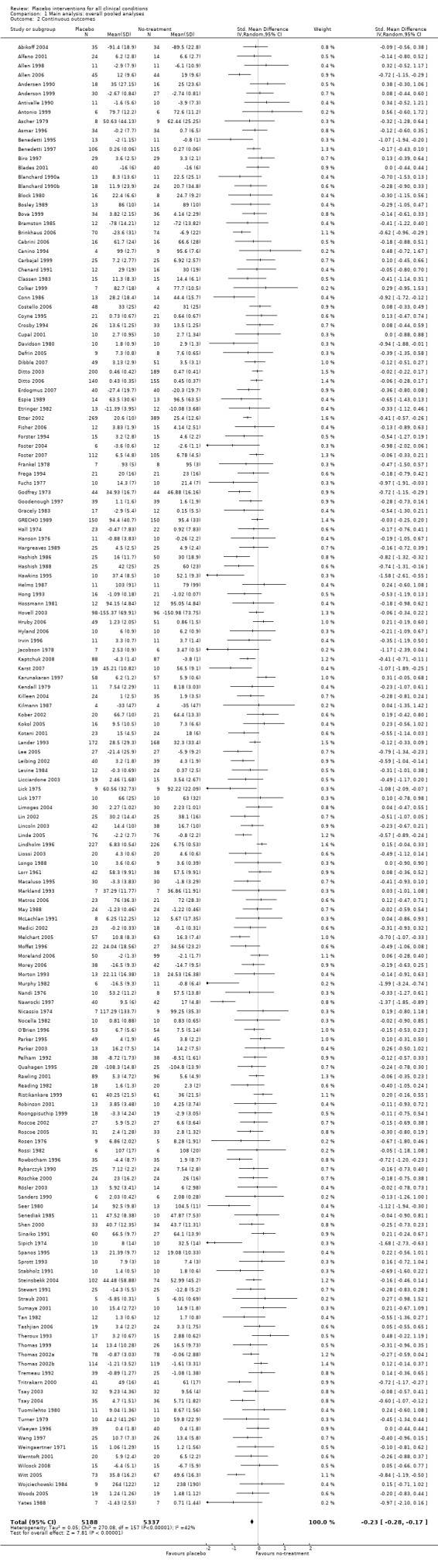
Comparison 1 Main analysis: overall pooled analyses, Outcome 2 Continuous outcomes.
3. Effect of placebo interventions across all clinical conditions (continuous outcomes).
| All trials | Trials with low risk of bias | Quality of the evidence | |||||
| Outcomes |
Standardised mean difference (95% CI) |
No. of participants (studies) | Comments |
Standardised mean difference (95% CI) |
No. of participants (studies) | Comments | |
| All clinical conditions | ‐0.23 (‐0.28 to ‐0.17) | 10,525 (158) | Asymmetrical funnel plot Moderate heterogeneity |
‐0.38 (‐0.55 to ‐0.22) | 1610 (11) | Substantial heterogeneity. Four German acupuncture trials had a pooled SMD ‐0.68 (‐0.85 to ‐0.50), whereas 7 other trials had a pooled SMD of ‐0.19 (‐0.31 to ‐0.07) | Moderate |
| Trials with patient‐reported outcomes | ‐0.26 (‐0.32 to ‐0.19) | 8000 (109) | Asymmetrical funnel plot Moderate heterogeneity |
‐0.39 (‐0.57 to ‐0.22) | 1543 (10) | Substantial heterogeneity (see above). | Moderate |
| Trials with observer‐reported outcomes | ‐0.13 (‐0.24 to ‐0.02) | 2513 (49) | Asymmetrical funnel plot Moderate heterogeneity |
‐0.25 (‐0.73 to 0.23) | 67 (1) | One small trial | Moderate |
We categorised 11 trials as having a low risk of bias. The pooled SMD for these trials was ‐0.38 (‐0.55 to ‐0.22), but heterogeneity was considerable (P < 0.001; I2 62%) and caused by four German acupuncture pain trials. The pooled effect of the other seven trials was ‐0.19 (‐0.31 to 0.07) with no heterogeneity (P = 0.67; I2 0%).
Eleven clinical problems had been investigated in at least three trials with continuous outcomes: anxiety, asthma, dementia, depression, hypertension, insomnia, nausea, overweight, pain, phobia, and smoking (Table 23). Confidence intervals were wide for most conditions. Placebo interventions had a statistically significant effect on pain, phobia, nausea, and asthma. Below we describe the results of these trials.
4. Effect of placebo interventions on specific clinical conditions (continuous outcomes).
| All trials | Trials with low risk of bias | Quality of the evidence | |||||
| Condition [1] | Standardised mean difference (95% CI) | No. of participants (studies) | Comments | Standardised mean difference (95% CI) | No. of participants (studies) | Comments | |
| Pain | ‐0.28 (‐0.36 to ‐0.19) | 4154 (60) | Moderate heterogeneity | ‐0.45 (‐0.69 to ‐0.21) | 1198 (7) | Substantial heterogeneity | Moderate |
|
‐0.22 (‐0.30 to ‐0.14) | 3534 (56) | Low heterogeneity | ‐0.13 (‐0.28 to 0.03) | 637 (3) | No heterogeneity | |
|
‐0.68 (‐0.85 to ‐0.50) | 544 (4) | No heterogeneity | ‐0.68 (‐0.85 to ‐0.50) | 544 (4) | No heterogeneity | |
| Nausea | ‐0.25 (‐0.46 to ‐0.04) | 452 (7) | Low heterogeneity | ‐0.19 (‐0.49 to 0.11) | 174 (2) | No heterogeneity | Moderate |
| Depression | ‐0.25 (‐0.55 to 0.05) | 324 (8) | Moderate heterogeneity | ‐0.23 (‐0.63 to 0.21) | 123 (1) | Moderate | |
| Hypertension | ‐0.17 (‐0.46 to 0.12) | 308 (10) | Low heterogeneity | NA | 0 (0) | Low | |
| Anxiety | ‐0.16 (‐0.48 to 0.16) | 286 (7) | Moderate heterogeneity | NA | 0 (0) | Low | |
| Asthma | ‐0.35 (‐0.70 to ‐0.01) | 203 (4) | Moderate heterogeneity | NA | 0 (0) | Low | |
| Obesity | ‐0.20 (‐0.57 to 0.17) | 188 (8) | Moderate heterogeneity | NA | 0 (0) | Low | |
| Insomnia | ‐0.19 (‐0.50 to 0.12) | 164 (6) | No heterogeneity | NA | 0 (0) | Low | |
| Dementia | ‐0.18 (‐0.55 to 0.20) | 111 (3) | No heterogeneity | NA | 0 (0) | Low | |
| Phobia | ‐0.63 (‐1.17 to ‐0.08) | 57 (3) | No heterogeneity | NA | 0 (0) | Low | |
[1]. Clinical conditions studied in three trials or more.
[2]. German acupuncture trials.
NA: not applicable.
Pain
There were 60 trials with 4154 patients that evaluated the effect on pain based on continuous outcomes, e.g. pain intensity measured on a 100 milimetre (mm) visual analogue scale. The funnel plot was asymmetrical, as larger trials tended to report lower effects than smaller trials (Figure not shown). This tendency was not statistically significant (Egger’s test, P = 0.20), but the intercept, which indicates the degree of asymmetry, was similar to the intercept for the analysis involving all trials with continuous outcomes. The statistically significant heterogeneity (P < 0.001) was moderate (I2 42%).
The effect estimates of the individual trials spanned roughly from SMD ‐1.0 to SMD 0.5, with a peak around SMD ‐0.15, and with several medium‐sized trials reporting effects between SMD ‐0.5 and ‐1.0. The pooled SMD was ‐0.28 (‐0.36 to ‐0.19).
We categorised seven pain trials as having low risk of bias. Their pooled SMD was ‐0.45 (‐0.69 to ‐0.21), but with substantial heterogeneity (I2 75%). Four German acupuncture trials had a pooled effect of ‐0.68 (‐0.85 to ‐0.50), whereas the other three pain trials had a pooled effect of SMD ‐0.13 (‐0.28 to 0.03) (Analysis 18.3). When grouped this way neither had any heterogeneity (I2 0%).
18.3. Analysis.
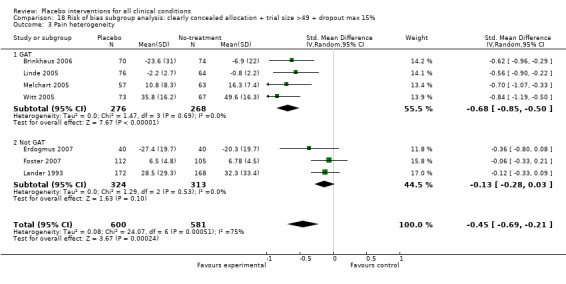
Comparison 18 Risk of bias subgroup analysis: clearly concealed allocation + trial size >49 + dropout max 15%, Outcome 3 Pain heterogeneity.
The mean standard deviation for the 16 trials with a 100 mm visual analogue pain scale was 24 mm. Thus, the effect on pain on a 100 mm scale based on the four German acupuncture trials was 16 mm, and 3 mm based on the other trials.
A similar pattern was seen among the six pain trials with binary outcomes, including 1207 patients. The pooled effect was RR 0.92 (0.77 to 1.11). There was substantial heterogeneity (P < 0.001; I2 76%). The single German acupuncture trial reported a marked effect of RR 0.69 (0.61 to 0.78), whereas the five other trials had a pooled RR of 0.98 (0.88 to 1.09). When so grouped there was no heterogeneity (I2 0%).
Nausea
Seven trials with 452 patients studied the effect of placebo on nausea based on continuous outcomes. No statistically significant heterogeneity (P = 0.30) was found (I2 17%). The pooled SMD was ‐0.25 (‐0.46 to ‐0.04). The mean standard deviation for trials using a 100 mm visual analogue scale (or similar) was 27 mm. The effect on reported nausea was thus 7 mm on a 100 mm scale. We categorised two nausea trials as having low risk of bias. They had a similar pooled effect, SMD = ‐0.19 (‐0.49 to 0.11).
Six trials with 732 patients evaluated the effect of nausea on binary outcomes. No statistically significant heterogeneity was found (P = 0.95; I2 0%). The pooled RR was 0.94 (0.82 to 1.07). We categorised one trial as having low risk of bias. This trial had a similar effect, RR 0.92 (0.75 to 1.12).
Phobia
Three trials with 57 patients evaluated the effect of placebo on phobia based on continuous outcomes, e.g. assessment of fear of snakes. No statistically significant heterogeneity (P = 0.52) was found (I2= 0%).The pooled SMD was ‐0.63 (95% CI ‐1.17 to ‐0.08). The trials were very small with sample sizes of 14, 18 and 25 patients, and the concealment of allocation was unclear in all three cases. No trials with binary outcomes investigated phobia.
Asthma
Four trials with 203 patients evaluated the effect of placebo on asthma. No statistically significant heterogeneity was found (P = 0.52; I2 0%). The pooled SMD was ‐0.35 (‐0.70 to ‐0.01). The marginally statistically significant pooled result is primarily driven by one trial published in 1976 reporting an effect on children. No trial reported adequate concealment of allocation, and no trial with binary outcomes investigated asthma. The risk of bias is considerable in this analysis and we find it uncertain whether placebo has an effect on asthma.
Trials not reporting data necessary for meta‐analyses
In 29 out of the 234 trials (12%), outcome data had not been reported in a way that was suited for meta‐analysis, and three trials reported harms. Based on a qualitative assessment, there was no clear tendency for the findings in the 29 trials without outcome data to be different from the findings in the 202 trials we meta‐analysed.
Trials studying harms
Three trials (1218 patients) studied harmful effects of placebo interventions. One trial with binary outcomes (1066 patients) found no statistically significant increase of nausea in patients treated with placebo (RR 1.36 (0.95 to 1.95)). The two trials with continuous outcomes (128 and 24 patients) also found no statistically significant harmful effect of placebo intervention (SMDs ‐0.19 (‐0.53 to 1.06) and 0.83 (‐0.01 to 1.67)).
Subgroup analyses and meta‐regression analyses
We found no statistically significant differences between the subgroups of trials with binary outcomes (data not shown).
For trials with continuous outcomes the effect of physical placebo interventions, SMD ‐0.31 (‐0.41 to ‐0.22) was higher than the effect of pharmacological placebo interventions, SMD ‐0.10 (‐0.20 to ‐0.01), (test of interaction, P = 0.002). Furthermore, the observed difference between patient‐reported outcomes and observer‐reported outcomes was primarily driven by a small negative effect on laboratory outcomes, SMD 0.16 (0.01 to 0.30), and a small effect on observer‐reported outcomes not involving the patients' cooperation, SMD ‐0.12 (‐0.29 to 0.05). The effect on observer‐reported outcomes involving the patients' cooperation, SMD ‐0.26 (‐0.41 to ‐0.12), was very similar to the effects on patient‐reported outcomes.
The pooled effect of the 23 trials that falsely informed the patients that they could receive two active treatments or no‐treatment (i.e. the possibility of a placebo intervention was not revealed) was SMD ‐0.39 (‐0.53 to ‐0.26). This effect was higher than in the trials that correctly informed patients that a placebo intervention was a possibility (or this aspects was not reported clearly) SMD ‐0.19 (‐0.25 to ‐0.13) (test of interaction, P = 0.008). We also found a statistically significantly higher effect in the 28 trials with an aim of studying the effect of placebo, SMD ‐0.34 (‐0.46 to ‐0.22) as compared with trials that did not state this aim, ‐0.20 (‐0.26 to ‐0.14) (test of interaction, P = 0.04).
We found no statistically significant impact on results of the following methodological factors: whether the placebo treatment provider and patients had been blinded, whether placebo was an add‐on treatment, whether observers had been blinded (when outcomes were observer‐reported), whether the data indicated non‐Normal distributions of continuous outcomes, or whether the trial report had defined a primary outcome. The effects of adequately concealed trials with continuous outcomes were somewhat larger than the effect of trials where concealment was unclear (test of interaction, P = 0.05). There was no statistically significant difference between the effect of placebo measured as final values, SMD ‐0.21 (‐0.28 to ‐0.14), and change from baseline, SMD ‐0.27 (‐0.37 to ‐0.16).
The results of the meta‐regression analyses are shown in Table 24. The meta‐regression model with stepwise elimination of co‐variates with the highest P values, identified four co‐variates with P values < 0.05: patient‐involved outcomes (patient‐reported outcomes and observer‐reported outcomes involving patient cooperation), physical placebos, information to patients, and the aim of the trial. The model explained 54% of the variation in the analysis of all trials with continuous outcomes. In this analysis, small sample size was close to being statistically significant (P = 0.09), and the analysis of the funnel plot (Egger's test) did find such an association (P = 0.03). Thus, we regard sample size and effect as associated.
5. Meta‐regression analyses.
| All trials (n = 158) | All trials excluding German acupuncture trials (n = 154) | |||||
| Model | Co‐variates [1] | Coefficient (SE) [2] | P value | Co‐variates | Coefficient (SE) | P value |
| Multiple meta‐regression of all co‐variates simultaneously | Pt‐involved outcome Study aim was placebo |
‐0.17 (0.084) ‐0.15 (0.072) |
0.047 0.043 |
Study aim was placebo | ‐0.18 (0.072) | 0.012 |
| Multiple meta‐regression by stepwise elimination | Pt‐involved outcome Physical placebos Placebo undisclosed Study aim was placebo |
‐0.18 (0.077) ‐0.13 (0.056) ‐0.17 (0.070) ‐0.14 (0.070) |
0.023 0.020 0.014 0.046 |
Pt‐involved outcome Study aim was placebo Precision |
‐0.19 (0.072) ‐0.18 (0.067) 0.025 (0.010) |
0.011 0.008 0.016 |
[1]. We studied 11 predefined co‐variates. A model based on stepwise elimination of the co‐variate with the highest P‐value resulted in four co‐variates with P < 0.05. The model had a tau2 = 0.0207, compared to the overall random effects meta‐analysis of tau2 = 0.0450. Thus, the model explains 54% of the initial variation. The model was sensitive to the exclusion of the four German acupuncture trials. The inclusion of these trials especially influenced the statistical significance of the importance of disclosing to patients that the trial involved a possible placebo treatment.
[2]. SE: Standard error
Discussion
We found a small and uncertain pooled effect of placebo interventions in 44 trials with binary outcomes and no difference between patient‐reported and observer‐reported outcomes. For 158 trials with continuous outcomes we found higher effects in small trials. The pooled effect of placebo on patient‐reported outcomes was modest, and on observer‐reported outcomes small and uncertain.
Out of 11 clinical conditions, investigated in three trials or more, there was a statistically significant effect of placebo on pain, nausea, phobia, and asthma. The pooled effect of placebo interventions on pain was very variable, also among trials with low risk of bias, spanning from clinically important to negligible. The pooled effect on nausea was modest, but consistent. The effect on phobia and asthma was very uncertain due to high risk of bias.
Larger effects of placebo interventions were associated with physical placebo interventions (e.g. sham acupuncture), patient‐involved outcomes (patient‐reported outcomes such as pain, and observer‐reported outcomes involving patient cooperation, such as depression rating scales), small trials, and trials with the explicit purpose of studying placebo. Larger effects of placebo were also found in the trials that falsely informed patients that the study compared two active treatments with no‐treatment.
Strengths and weaknesses
The two main strengths of our review are the randomised design of the included trials, and the large number of included trials. This enabled an comprehensive assessment of the clinical effect of placebo and provided a basis for analyses of the effect of placebo on specific clinical conditions, of the risk of bias, and of reasons for heterogeneity.
The main weakness of any review of the effect of placebo is that the comparison between placebo and no‐treatment cannot be conducted blindly. Patients will know whether they receive a treatment or not, and this may affect both their reporting of symptoms and their use of concomitant therapy. In trials with patient‐reported outcomes it is difficult to distinguish a true effect from biased reporting (response bias), as polite patients may tend to report what they think socially most acceptable. A review of signal detection analysis of experimental placebo studies on pain indicated that response bias was responsible for at least part of the patient‐reported effects (Allan 2002). This is in accord with our findings that the effect of placebo was twice as high for patient‐reported continuous outcomes as for observer‐reported ones.
The effect of placebo could be underestimated if the patients in the no‐treatment groups tended to seek treatment outside the trial more often than patients in the placebo groups. For example, the patients in the no‐treatment group of a long‐term pain trial could take more additional pain medication than the patients in the placebo group. Concomitant therapy was generally poorly reported, but in 13 three‐armed acupuncture trials, patients in the no‐treatment group reported taking more analgesic drugs than patients in the placebo group (Madsen 2009). The net direction of the two biases, response bias and co‐intervention bias, is difficult to predict, but it seems likely that they partly cancel each other out.
The funnel plot of trials with continuous outcomes was asymmetrical and lacked a clear peak, as the effect of large trials also varied considerably. This could indicate that some small trials with a neutral or negative result had not been included. However, the publication of such trials is not directly linked to the effect of the placebo intervention (but to the effect of the active intervention), so we find it less likely that unidentified trials could explain the higher effects of placebo reported in small trials. It seems more likely that the asymmetry is caused by a combination of true heterogeneity and poor methodological quality in small trials. Regardless, the overall pooled effect of trials with continuous outcomes should be interpreted cautiously.
We carried out several subgroup and meta‐regression analyses to explain the heterogeneity. Higher effects of placebo interventions were associated with patient‐involved outcomes (patient‐reported outcomes and observer‐reported outcomes involving patient cooperation), physical placebos, small trials, and trials with the explicit purpose of studying placebo. Ten of eleven co‐variates analysed were predefined before this update. The eleventh factor was whether patients had been falsely informed that they could receive two forms of active treatment or no‐treatment (and were not informed about the possibility of a placebo intervention). The German acupuncture trials informed their patients in this way, which prompted us to re‐read the other trial reports, extract relevant data, and include the factor in a post‐hoc analysis. The factor was statistically significant only when the German acupuncture trials were included in the analyses, implying some uncertainty as to its general importance. Furthermore, pooling of final values and change from baseline may be problematic when outcomes are presented as standardised mean differences. However, in a sensitivity analysis we found no statistically significant difference between the pooled effect of 40 trials that reported change from baseline as compared with the 118 trials that reported final values.
The meta‐regression model explained 54% of the initial variation found in the pooled analysis of trials with continuous outcomes. Subgroup analyses, and meta‐regression, are observational and there is a risk of confounding. We have found one randomised trial that studied a co‐variate involved in our meta‐regression analyses. Placebo acupuncture was found to have somewhat larger effects than pill placebo on pain (Kaptchuk 2006), supporting our observation that physical placebos are associated with larger effects than pharmacological ones.
Other reviews
One previous systematic review of randomised trials with placebo and no‐treatment groups identified 12 trials (Ernst 1995), which tended to report large effects of placebo.
Several laboratory studies indicate a neurobiological mechanism for the analgesic effect of placebo (Sauro 2005). These studies are often small, mostly based on healthy volunteers, and of short duration. The findings cannot easily be extrapolated to a clinical context, but they do elucidate the probable importance of, for example, endorphins in the analgesic response to placebo, and indicate that it is unlikely that response bias can account for all of the analgesic effect.
Other reviews have compared the effect of experimental treatments in trials that used placebo control groups, with similar trials that used no‐treatment control groups (Dush 1986; Grissom 1996; Kirsch 1998; Shapiro 1982; Smith 1980). Such comparisons are indirect, prone to confounding and therefore less reliable.
The previous versions of our review prompted several independent re‐analyses. Kamper and colleagues replicated our finding that the pooled effect of placebo on pain was low (Kamper 2008). Wampold and colleagues replicated our overall findings for both binary and continuous outcomes, despite modified inclusion criteria and some disagreement about how such estimates should be interpreted (Wampold 2005; Hróbjartsson 2007). Meissner and colleagues replicated our findings that effects of placebo on laboratory outcomes tended to be lower than on other observer‐reported outcomes (Meissner 2007). Vase and colleagues re‐analysed the clinical pain trials included in our review, and reported low effects in ordinary clinical trials and high effects in clinical and laboratory based 'mechanism studies' (Vase 2002). We pointed out several methodological errors, and suggested that the difference could be less pronounced (Hróbjartsson 2006). The German acupuncture trials, which were not 'mechanism studies', also indicated that clinical non‐mechanism trials can have quite substantial effects. Regardless, effects of placebo vary considerably, and the web of factors responsible for this variation is complex. Our regression analysis is one attempt to unfold the multifactorial background for effects of placebo.
Meaning of our review
This update confirms and modifies the findings of the previous versions of our review. Our approach can be seen as testing the hypothesis that placebo treatments have large effects across many clinical conditions and outcomes, and our results clearly indicate that this hypothesis is wrong.
However, our findings do not imply that placebo interventions have no effect. We found an effect on patient‐reported outcomes, especially on pain. Several trials of low risk of bias reported large effects of placebo on pain, but other similar trials reported negligible effect of placebo, indicating the importance of background factors. We identified three clinical factors that were associated with higher effects of placebo: physical placebos, patient‐involved outcomes (patient‐reported outcomes and observer‐reported outcomes involving patient cooperation), and falsely informing patients that the trial involved a comparison of two active treatments and no‐treatment. Furthermore, two methodological factors were also associated with higher effects: small sample size and the explicit aim of studying effect of placebo. So, despite a general picture of low effects, and the risk of response bias and small sample size bias, it is likely that large effects of placebo interventions may occur in certain situations.
Extrapolation of our findings to settings outside clinical trials rests on the premise that the nature of the treatment ritual in an experimental and a clinical setting is not fundamentally different. To analyse this empirically is challenging, however, as it seems impossible to study the effect of placebo treatments in clinical practice reliably without introducing an experimental setting (Hróbjartsson 1996).
It can be difficult to interpret whether a pooled standardised mean difference is large enough to be of clinical relevance. A consensus paper found that an analgesic effect of 10 mm on a 100 mm visual analogue scale represented a ‘minimal effect’ (Dworkin 2008). The pooled effect of placebo on pain based on the four German acupuncture trials corresponded to 16 mm on a 100 mm visual analogue scale, which amounts to approximately 75% of the effect of non‐steroidal anti‐inflammatory drugs on arthritis‐related pain (Gøtzsche 1990). However, the pooled effect of the three other pain trials with low risk of bias corresponded to 3 mm. Thus, the analgesic effect of placebo seems clinically relevant in some situations and not in others.
It is a question of definition whether our review evaluates the 'placebo effect'. This term does not only imply the effect of a placebo intervention as compared with a no‐treatment group, but is also used to describe various other aspects of the patient‐provider interaction, such as psychologically‐mediated effects in general, the effect of the patient‐provider interaction, the effect of suggestion, the effect of expectancies, and the effect of patients' experience of meaning (Hróbjartsson 2002b). As patients in the no‐treatment group also interact with treatment providers, a no‐treatment group is only untreated in the sense that they do not receive a placebo intervention (Hróbjartsson 1996). Our result is therefore neutral to many of the meanings of the term 'placebo effect' cited above, and we do not exclude the possibility of important effects of other aspects of the patient‐provider interaction, though the methodological problems of studying such effects reliably are demanding.
Despite ethical concerns of the deceit inherent in most placebo prescriptions (Rawlinson 1985), the clinical use of placebo interventions has been advocated in editorials and articles in leading journals (Bignal 1994; Brown 1998; Ho 1994) and by influential commentators (Cochrane 1989). Questionnaire surveys indicate that placebo interventions are sometimes used in clinical practice, such as vitamin B for fatigue, or antibiotics for presumed viral infections (Hróbjartsson 2003; Tilburt 2008). In our opinion a clinical placebo intervention is ethically acceptable only if it fulfils two criteria. First, patients must be informed about the nature of the intervention. Second, the effect of placebo must be reliably demonstrated in trials that disclose to patients that they receive placebo. None of the trials included in this review tested the effect of fully disclosed placebo interventions. The tendency was the opposite, for higher effects in trials where the possibility of a placebo intervention was obscured. Thefore, placebo prescription seems to lack both ethical and empirical justification.
The use of placebos in blinded randomised trials is a precaution directed against many forms of bias, and not only against effects of placebo. Unblinded patients may differ from blinded ones in their way of reporting beneficial and harmful effects of treatment, in their tendency to seek additional treatment outside the study, and in their risk of dropping out of the study. Furthermore, unblinded staff may differ in their use of alternative forms of care and in their assessment of outcomes. Thus, even if there were no true effect of placebo, one would expect to record differences between placebo and no‐treatment groups due to bias associated with lack of blinding.
Authors' conclusions
Implications for practice.
We did not find that placebo interventions have important clinical effects in general. However, in certain settings placebo interventions may influence patient‐reported outcomes, especially pain and nausea, though it is difficult to distinguish patient‐reported effects of placebo from response bias.
Most clinical placebo prescriptions involve deceit and the effect of placebo has not been tested in trials after full disclosure that the patients receive placebo. Therfore, we suggest that placebo interventions are not used outside clinical trials.
Implications for research.
The results of this review do not imply that no‐treatment control groups can replace placebo control groups in randomised clinical trials without a risk of bias.
Further research is needed to study the impact of bias (such as response bias and bias due to co‐intervention) on the estimated effect of placebo, to study the association between type of outcome and bias, to explore which factors in the clinical setting are associated with different effects of placebo, and to explore the duration of effects.
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2009 | New search has been performed | The update (published on issue 1 2010) includes 234 trials (52 trials added) and over 16,000 patients. The updated review includes more precise subgroup analysis, especially among trials with low risk of bias, and involves meta‐regression analyses to explain heterogeneity. |
| 11 November 2009 | New citation required and conclusions have changed | We have applied new methods and use Summary of Findings tables to assist in conveying the main findings. In contrast to the previous versions of the review (Hróbjartsson 2004a) we now find both a notable pooled effect of placebo in trials with low risk of bias, especially on pain, and a large variation in effects among trials with low risk of bias. Also new is the identification of five factors explaining roughly half of the variation. However, when all trials are pooled, disregarding the risk of bias, results are fairly similar to the previous versions. |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 12 June 2008 | Amended | Converted to new review format. |
| 18 April 2004 | New search has been performed | We identified 52 new trials and increased the number of included patients from 8525 to 11,737 (38%). Confidence intervals became narrower, more clinical conditions had been investigated by three trials or more. |
| 18 April 2004 | New citation required and conclusions have changed | We updated the review on issue 3 2004 of The Cochrane Library. The degree of heterogeneity between trials with continuous outcomes was more pronounced in this update, but the main findings were( identical to the previous version of the review (Hróbjartsson 2003a). |
Notes
This review was originally published with the title 'Placebo treatment versus no treatment' (Hróbjartsson 2003a). For the 2003‐4 update (Hróbjartsson 2004a), the title was changed to 'Placebo interventions for all clinical conditions'.
Acknowledgements
We thank Henrik R. Wulff, Jos Kleijnen and Iain Chalmers for valuable comments on previous versions of the text of the review. We are grateful for the lists of relevant trials provided by Gunvor Kienle, Andrew Vickers, Harald Walach, Clive Adams, and Iain Chalmers. We also thank Romana Klefter and Roberto Oliveri for translating trial reports from Polish and Italian, and we thank the numerous placebo‐trial researchers for access to additional data.
We are grateful for the assistance of Ulrik Felding in assisting us in assessing the risk of bias in the included trials and in converting the review to RevMan 5 format.
Appendices
Appendix 1. Electronic search strategies
Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2007)
(PLACEBO* or MOCK* or SHAM* or FAKE* or VEHICLE* or DUMM* or ATTENTION* CONTROL* or PSEUDO* TREAT* or UNSPECIFIC* or NON SPECIFIC*) and
(NO TREAT* or NON TREAT* or NOTREAT* or NONTREAT* or UNTREAT* or MINIMALTREAT* or MINIM* TREAT* or USUAL TREAT* or NO INTERV* or NON INTERV* or NOINTERV* or NONINTERV* or NO CONTACT* or NON CONTACT or NOCONTACT* or NONCONTACT or USUAL CONTACT* or USUAL CARE* or NO PILL* or NOPILL* or NONPILL* or NO TABLET* or NOTABLET* or NONTABLET* or NO MEDIC* or NON MEDIC* or NOMEDIC* or NONMEDIC* or UNMEDIC* or MINIM* MEDIC* or MINIMALMEDIC* or NO SURGER* or NON SURGER* or NOSURGER* or NONSURGER* or NO OPERAT* or NON OPERAT or NOOPERAT* or NONOPERAT* or WAITING LIST* or WAITINGLIST* or NO THERAP* or NON THERAP* or NOTHERAP* or NONTHERAP* or MINIM* THERAP* or MINIMALTHERAP* or USUAL* THERAP* or USUALTHERAP* or NATURAL COURSE or NATURAL DEVELOPMENT or NATURAL HISTORY or SPONTANEOUS COURSE or SPONTANEOUS DEVELOPMENT or SPONTANEOUS HISTORY or (TWO GROUPS) near CONTROL* or (THREE GROUPS) near CONTROL* or (FOUR GROUPS) near CONTROL* or (FIVE GROUPS) near CONTROL* or (SIX GROUPS) near CONTROL* or (SEVEN GROUPS) near CONTROL* or (TWO TREATMENT GROUPS) near CONTROL* or (THREE TREATMENT GROUPS) near CONTROL* or (FOUR TREATMENT GROUPS) near CONTROL* or (FIVE TREATMENT GROUPS) near CONTROL* or (SIX TREATMENT GROUPS) near CONTROL* or (SEVEN TREATMENT GROUPS) near CONTROL* ) and
(RANDOM* or DOUBLE* BLIND* or SINGLE* BLIND*)
Search strategy for MEDLINE 1966 to March 2008
(PLACEBO* or MOCK* or SHAM* or FAKE* or VEHICLE* or DUMM* or ATTENTION* CONTROL* or PSEUDO* TREAT* or UN?SPECIFIC* or NON?SPECIFIC*) and
(NO??TREAT* or NO TREAT* or NON TREAT* or UN?TREAT* or UN TREAT* or MINIM* TREAT* or USUAL?TREAT* or USUAL TREAT* or NO INTERV* or NON INTERV* or NO??INTERV* or NO CONTACT* or NON CONTACT* or NO??CONTACT?* or USUAL CONTACT* or USUAL CARE* or NO PILL* or NON PILL* or NO??PILL* or NO TABLET* or NON TABLET* or NO??TABLET* or NO MEDIC* or NON MEDIC* or NO??MEDIC* or UN MEDIC* or UN?MEDIC* or MINIM* MEDIC* or NO??SURGER* or NO OPERAT* or NON OPERAT* or NO??OPERAT* or NO SURGER* or NON SURGER* or NO??SURGER* or (NO THERAP* or NO??THERAP* or NON THERAP* or MINIM* THERAP* or USUAL* THERAP*) in AB or (NO THERAP* or NO??THERAP* or NON THERAP* or MINIM* THERAP* or USUAL* THERAP*) in TI or WAITING LIST* or WAITING?LIST* or ((NATURAL or SPONTANEOUS) NEAR1 (COURSE or DEVELOPMENT or HISTORY)) or ((TWO or "2" or THREE or "3" or FOUR or "4" or FIVE or "5" or SIX or "6" or SEVEN or "7") NEAR1 (GROUPS or TREATMENT GROUPS)) NEAR (CONTROL or CONTROLS)) and
(DOUBLE‐BLIND‐METHOD or SINGLE‐BLIND‐METHOD or RANDOM‐ALLOCATION or RANDOMIZED‐CONTROLLED‐TRIALS/ ALL SUBHEADINGS or CLINICAL‐TRIALS/ ALL SUBHEADINGS or (CLINICAL‐TRIAL or RANDOMIZED‐CONTROLLED‐TRIAL or CONTROLLED‐CLINICAL‐TRIAL) in PT or RANDOM* or (CLINICAL near TRIAL*) or DOUBLE* BLIND* or SINGLE* BLIND*) and HUMAN in TG
Search strategy for EMBASE 1980 to March 2008 (PLACEBO* or MOCK* or SHAM* or FAKE* or VEHICLE* or DUMM* or ATTENTION* CONTROL* or PSEUDO* TREAT* or UN?SPECIFIC* or NON?SPECIFIC*) and
(NO??TREAT* or NO TREAT* or NON TREAT* or UN?TREAT* or UN TREAT* or MINIM* TREAT* or USUAL?TREAT* or USUAL TREAT* or WITHOUT TREAT* or WITHOUT?TREAT* or NO INTERV* or NON INTERV* or NO??INTERV* or NO CONTACT* or NON CONTACT* or NO??CONTACT* or USUAL CONTACT* or USUAL CARE* or (NO THERAP* or NO??THERAP* or NON THERAP* or MINIM* THERAP* or USUAL* THERAP*) in AB or (NO THERAP* or NO??THERAP* or NON THERAP* or MINIM* THERAP* or USUAL* THERAP*) in TI or NO PILL* or NON PILL* or NO??PILL* or NO TABLET* or NON TABLET* or NO??TABLET* or WAITING LIST* or WAITING?LIST* or ((NATURAL or SPONTANEOUS) NEAR1 (COURSE or DEVELOPMENT or HISTORY)) or NO MEDIC* or NON MEDIC* or NO??MEDIC* or UN MEDIC* or UN?MEDIC* or MINIM* MEDIC* or NO OPERAT* or NON OPERAT* or NO??OPERAT* or NO SURGER* or NON SURGER* or NO??SURGER* or ((TWO or "2" or THREE or "3" or FOUR or "4" or FIVE or "5" or SIX or "6" or SEVEN or "7") NEAR1 (GROUPS or TREATMENT GROUPS)) NEAR (CONTROL or CONTROLS)) and
(CLINICAL‐TRIAL or RANDOMIZED‐CONTROLLED‐TRIAL or RANDOMIZATION or DOUBLE‐BLIND‐PROCEDURE or SINGLE‐BLIND‐PROCEDURE or CONTROLLED‐STUDY or MAJOR‐CLINICAL‐STUDY or CLINICAL‐ARTICLE or RANDOM* or (CLINICAL near TRIAL*) or DOUBLE* BLIND* or SINGLE* BLIND*) and HUMAN‐ in DE.
Search Strategy for PsycINFO 1887 to March 2008 Neither the indexation of clinical trials nor the reporting in abstracts in PsycINFO was helpful with respect to a reliable identification of randomised trials. With the purpose of minimising the number of missed randomised trials, any search terms aimed at identifying clinical trials were omitted. In a later manual filtering process abstracts were read in full.
(PLACEBO* or MOCK* or SHAM* or FAKE* or VEHICLE* or DUMM* or PSEUDO* TREAT* or ATTENTION* CONTROL* or UNSPECIFIC* or NON?SPECIFIC*) and
(NO??TREAT* or NO TREAT* or NON TREAT* or UN?TREAT* or UN TREAT* or MINIM* TREAT* or WITHOUT TREAT* or NO??INTERV* or NO INTERV* or NON INTERV* or UN?INTERV* or UN INTERV* or MINIM* INTERV* or WITHOUT INTERV* or NO??MEDIC* or NO MEDIC* or NON MEDIC* or UN?MEDIC* or UN MEDIC* or MINIM* MEDIC* or WITHOUT MEDIC* or NO??PILL* or NO PILL* or NON PILL* or NO??OPERAT* or NO OPERAT* or NON OPERAT* or UN?OPERAT* or UN OPERAT* or MINIM* OPERAT* or WITHOUT OPERAT* or NO??SURGER* or NO SURGER* or NON SURGER* or MINIM* SURGER* or WITHOUT SURGER* or WAITING?LIST* or WAITING LIST or VISITATION* or ((NATURAL or SPONTANEOUS) NEAR1 (COURSE* or DEVELOPMENT* or HISTORY*)) or ((TWO or "2" OR THREE OR "3" OR "4" OR FOUR OR FIVE OR "5" OR SIX "6" OR SEVEN OR "7") NEAR1 (GROUPS OR TREATMENT GROUOPS)) NEAR (CONTROL OR CONTROLS)) and not ANIMAL in (PO or DE).
Search strategy for Biological Abstracts 1986 to March 2008 (PLACEBO* or MOCK* or SHAM* or FAKE* or VEHICLE* or DUMM* or ATTENTION* CONTROL* or PSEUDO* CONTROL* or UN?SPECIFIC* or NON?SPECIFIC*) and (NO??TREAT* or NO TREAT* or NON TREAT* or UN?TREAT* or UN TREAT* or MINIM* TREAT* or USUAL?TREAT* or USUAL TREAT* or WITHOUT TREAT* or WITHOUT?TREAT* or NO INTERV* or NON INTERV* or NO??INTERV* or NO CONTACT* or NON CONTACT* or NO??CONTACT?* or NO CONTACT* or NON CONTACT* or NO??CONTACT* or USUAL CONTACT* or USUAL CARE* or NO PILL* or NON PILL* or NO??PILL* or NO TABLET* or NON TABLET* or NO??TABLET* or (NO THERAP* OR NO??THERAP* OR NON THERAP* OR MINIM* THERAP* OR USUAL* THERAP*) in TI or (NO THERAP* OR NO??THERAP* OR NON THERAP* OR MINIM* THERAP* OR USUAL* THERAP*) in AB or NO MEDIC* or NON MEDIC* or NO??MEDIC* or UN MEDIC* or UN?MEDIC* or MINIM* MEDIC* or NO OPERAT* OR NON OPERAT* OR NO??OPERAT* OR NO SURGER* OR NON SURGER* OR NO??SURGER* or WAITING LIST* OR WAITING?LIST* OR ((NATURAL OR SPONTANEOUS) NEAR1 (COURSE OR DEVELOPMENT OR HISTORY)) or ((TWO or "2" OR THREE OR "3" OR FOUR OR "4" OR FIVE OR "5" OR SIX OR "6" OR SEVEN OR "7") NEAR1 (GROUPS OR TREATMENT GROUPS)) NEAR (CONTROL OR CONTROLS)) and
(RANDOM* or (CLINICAL near TRIAL*) or DOUBLE* BLIND* or SINGLE* BLIND*) and (HUMAN‐ in OR or HUMAN in DE or HUMANS in ST).
Data and analyses
Comparison 1. Main analysis: overall pooled analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary outcomes | 44 | 6041 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.88, 0.99] |
| 2 Continuous outcomes | 158 | 10525 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.28, ‐0.17] |
Comparison 2. Main analysis: patient‐reported or observer‐reported outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported binary outcomes | 31 | 4046 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 2 Observer‐reported binary outcomes | 13 | 1995 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.85, 1.02] |
| 3 Patient‐reported continuous outcomes | 109 | 8000 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.32, ‐0.19] |
| 4 Observer‐reported continuous outcomes | 49 | 2513 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.02] |
2.1. Analysis.
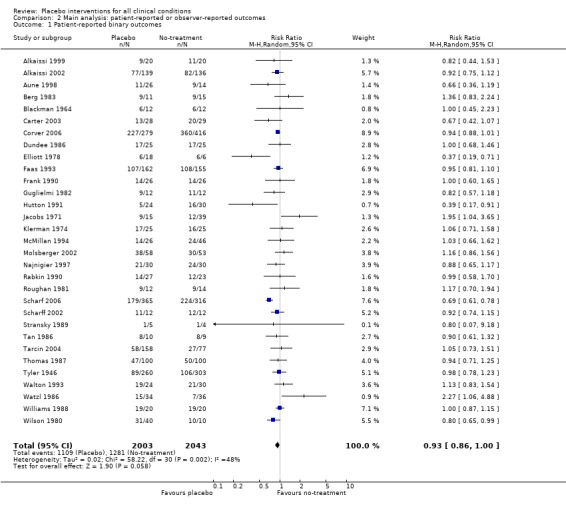
Comparison 2 Main analysis: patient‐reported or observer‐reported outcomes, Outcome 1 Patient‐reported binary outcomes.
2.2. Analysis.
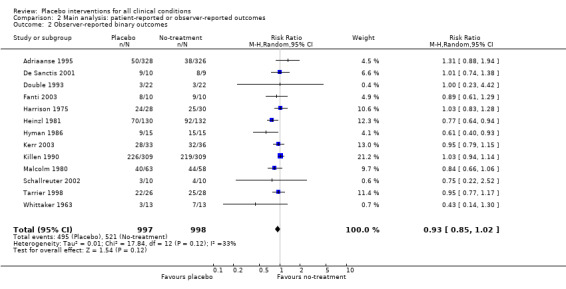
Comparison 2 Main analysis: patient‐reported or observer‐reported outcomes, Outcome 2 Observer‐reported binary outcomes.
2.3. Analysis.
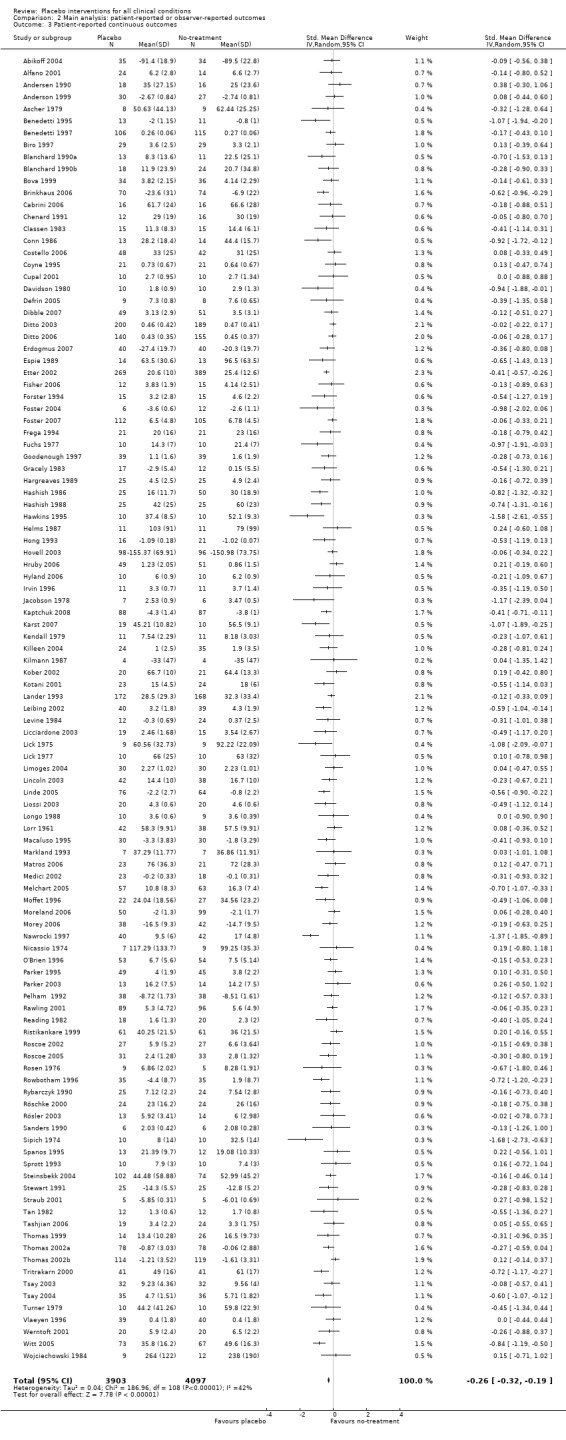
Comparison 2 Main analysis: patient‐reported or observer‐reported outcomes, Outcome 3 Patient‐reported continuous outcomes.
2.4. Analysis.
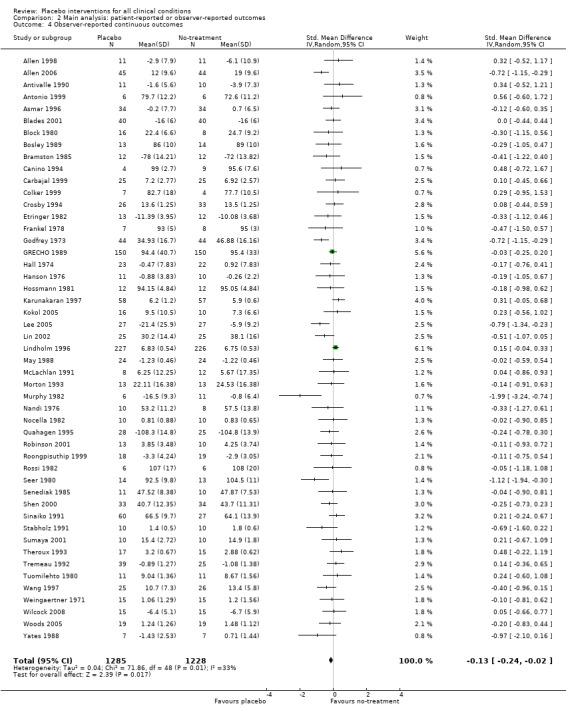
Comparison 2 Main analysis: patient‐reported or observer‐reported outcomes, Outcome 4 Observer‐reported continuous outcomes.
Comparison 3. Main analysis: clinical conditions investigated in three trials or more.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary outcomes | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Pain (incidence) | 6 | 1207 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.11] |
| 1.2 Depression (relapse prevention) | 3 | 152 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.34] |
| 1.3 Nausea | 6 | 732 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 1.4 Smoking (relapse, self report or biochemical measure) | 6 | 887 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.10] |
| 2 Continuous outcomes | 119 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Pain (VAS, ordinal scales, McGill score, escape medication, WOMAC index; absolute or improvement) | 60 | 4154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.36, ‐0.19] |
| 2.2 Insomnia (sleep onset latency in min, Pittsburgh sleep quality index change) | 6 | 164 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.50, 0.12] |
| 2.3 Hypertension (diastolic, mm Hg; absolute or improvement) | 10 | 308 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.46, 0.12] |
| 2.4 Nausea (VAS, Rhodes Inventory of Nausea and Vomiting, escape medication) | 7 | 452 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.46, ‐0.04] |
| 2.5 Smoking (cigarettes per day, self report) | 3 | 703 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.29, 0.23] |
| 2.6 Phobia (fear of snakes and spiders: snake slides test, behavioral avoidance test; absolute or improvement) | 3 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.17, ‐0.08] |
| 2.7 Asthma (bronchoconstriction: FEV1 or PEF; absolute or improvement) | 4 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.70, ‐0.01] |
| 2.8 Obesity (kg, pounds,%; absolute or improvement) | 8 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.57, 0.17] |
| 2.9 Depression (Hamilton's score, Beck Depression Inventory, Geriatric Depression Scale, Bf‐S scale) | 8 | 324 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.55, 0.05] |
| 2.10 Anxiety (modified versions of Spielberger's anxiety inventory, situational anxiety scale, cry score) | 7 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.48, 0.16] |
| 2.11 Dementia (various scales) | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.55, 0.20] |
3.1. Analysis.
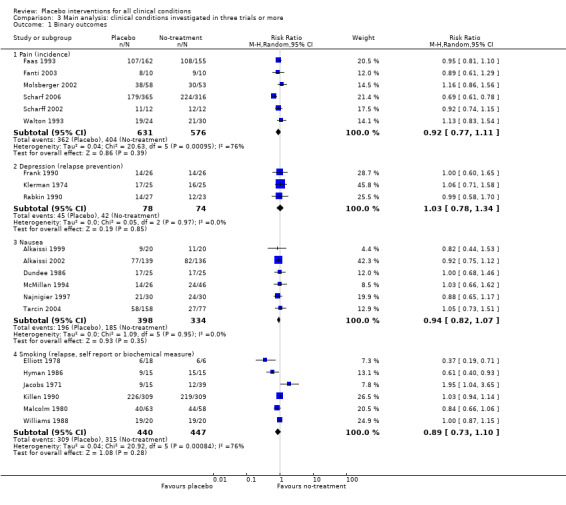
Comparison 3 Main analysis: clinical conditions investigated in three trials or more, Outcome 1 Binary outcomes.
3.2. Analysis.
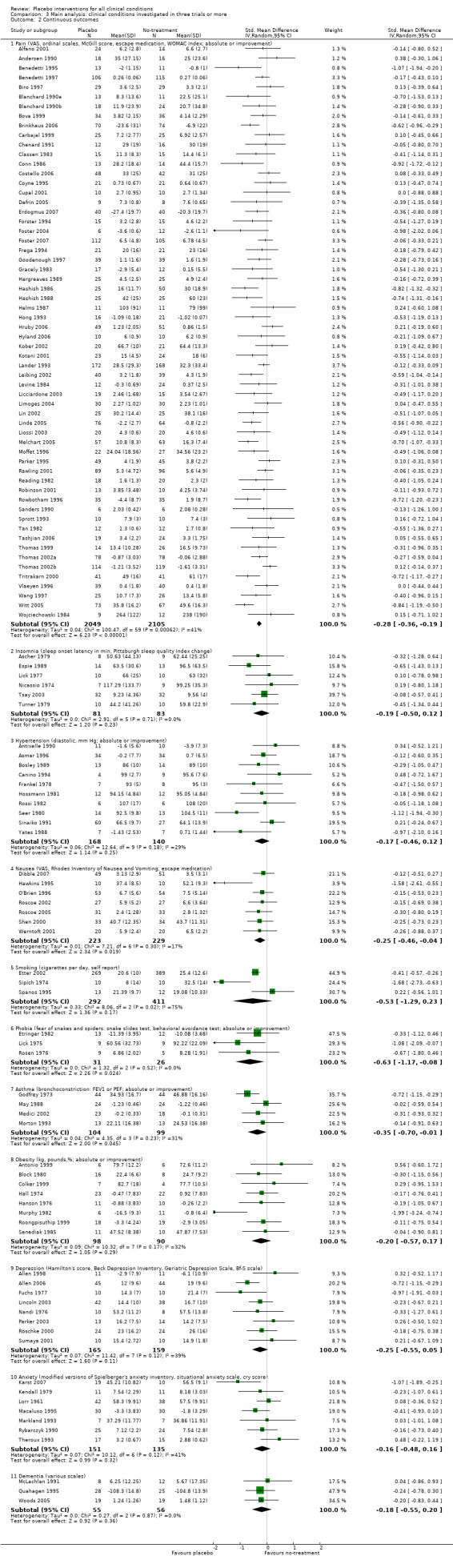
Comparison 3 Main analysis: clinical conditions investigated in three trials or more, Outcome 2 Continuous outcomes.
Comparison 4. Supplementary analysis: adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary outcomes | 1 | 1066 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.95, 1.95] |
| 1.1 Vomiting | 1 | 1066 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.95, 1.95] |
| 2 Continuous outcomes | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.74, 1.23] |
| 2.1 Cardiorespiratory safety during gastroscopy (heart rate per min) | 1 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.53, 0.16] |
| 2.2 Respiratory depressant response (liter per min) | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.01, 1.67] |
4.1. Analysis.
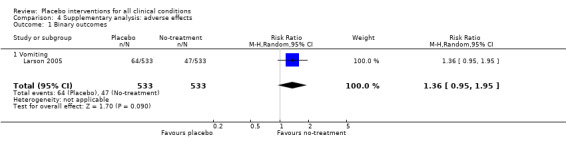
Comparison 4 Supplementary analysis: adverse effects, Outcome 1 Binary outcomes.
4.2. Analysis.
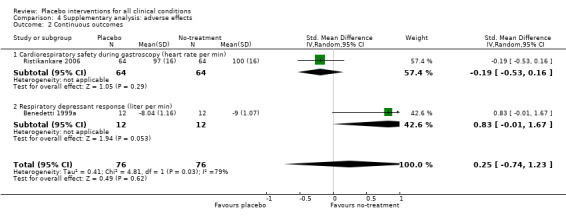
Comparison 4 Supplementary analysis: adverse effects, Outcome 2 Continuous outcomes.
Comparison 5. Effect modification subgroup analysis: type of outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient‐reported outcomes that are non‐observable | 12 | 2393 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.06] |
| 2 Patient‐reported outcomes that are observable | 19 | 1653 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.82, 1.02] |
| 3 Observer‐reported outcomes involving patient's cooperation | 4 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.09] |
| 4 Observer‐reported outcomes not involving patient's cooperation | 5 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.80, 1.06] |
| 5 Laboratory outcomes | 4 | 1423 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.74, 1.17] |
| 6 Patient‐reported outcomes that are non‐observable | 83 | 6004 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.36, ‐0.20] |
| 7 Patient‐reported outcomes that are observable | 26 | 1996 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.31, ‐0.11] |
| 8 Observer‐reported outcomes involving patient's cooperation | 22 | 878 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.41, ‐0.12] |
| 9 Observer‐reported outcomes not involving patient's cooperation | 22 | 906 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.05] |
| 10 Laboratory outcomes | 5 | 729 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.01, 0.30] |
5.1. Analysis.
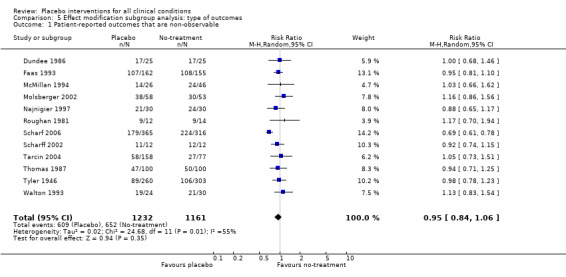
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 1 Patient‐reported outcomes that are non‐observable.
5.2. Analysis.
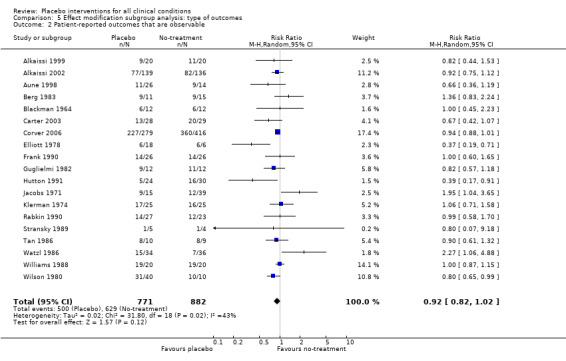
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 2 Patient‐reported outcomes that are observable.
5.3. Analysis.
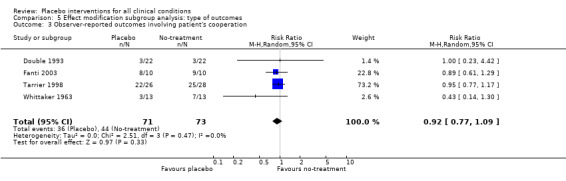
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 3 Observer‐reported outcomes involving patient's cooperation.
5.4. Analysis.
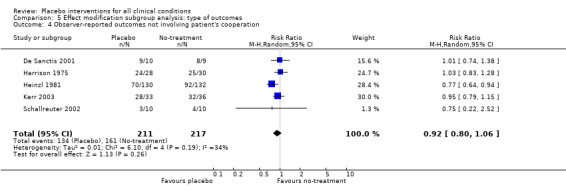
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 4 Observer‐reported outcomes not involving patient's cooperation.
5.5. Analysis.
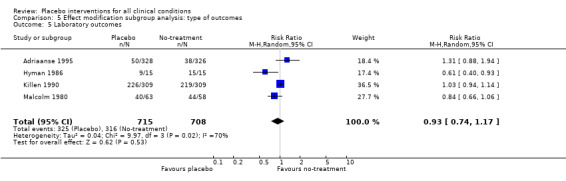
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 5 Laboratory outcomes.
5.6. Analysis.
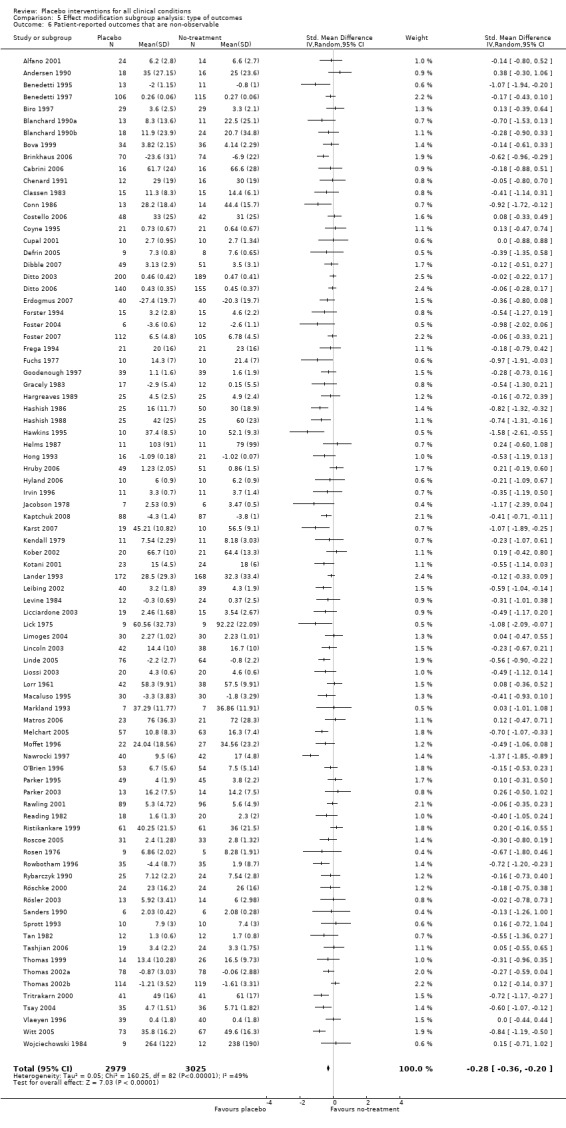
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 6 Patient‐reported outcomes that are non‐observable.
5.7. Analysis.
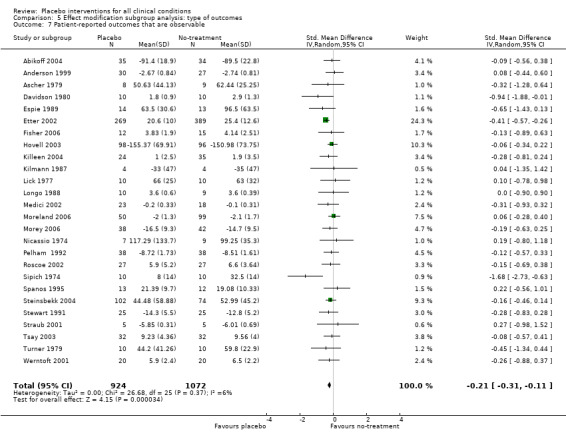
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 7 Patient‐reported outcomes that are observable.
5.8. Analysis.
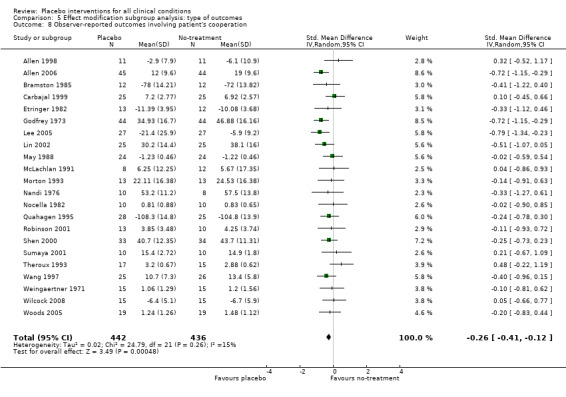
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 8 Observer‐reported outcomes involving patient's cooperation.
5.9. Analysis.
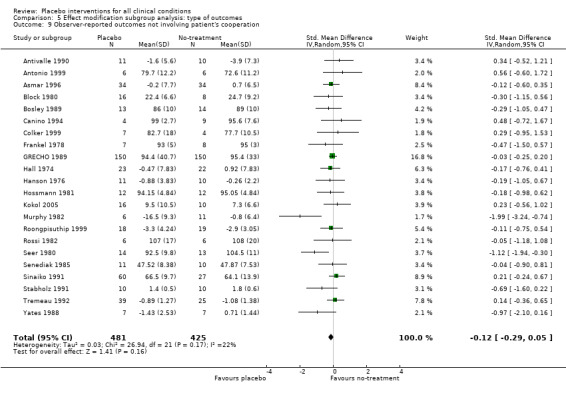
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 9 Observer‐reported outcomes not involving patient's cooperation.
5.10. Analysis.
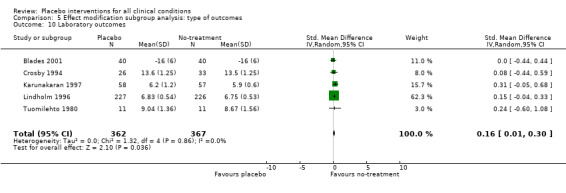
Comparison 5 Effect modification subgroup analysis: type of outcomes, Outcome 10 Laboratory outcomes.
Comparison 6. Effect modification subgroup analysis: the purpose of the trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 To study the effect of placebo was an explicit trial purpose | 11 | 1163 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 2 To study the effect of placebo was not an explicit trial purpose | 33 | 4878 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| 3 To study the effect of placebo was an explicit trial purpose | 28 | 2027 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.46, ‐0.22] |
| 4 To study the effect of placebo was not an explicit trial purpose | 130 | 8486 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.26, ‐0.14] |
6.1. Analysis.
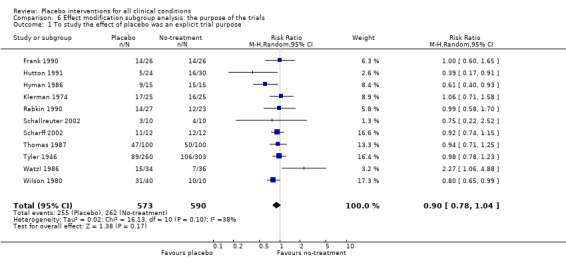
Comparison 6 Effect modification subgroup analysis: the purpose of the trials, Outcome 1 To study the effect of placebo was an explicit trial purpose.
6.2. Analysis.
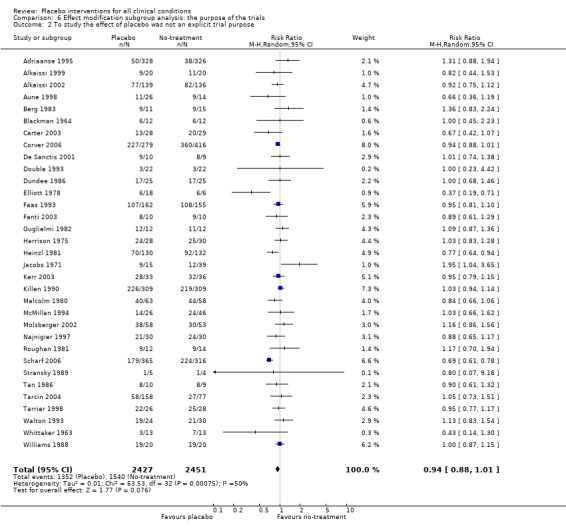
Comparison 6 Effect modification subgroup analysis: the purpose of the trials, Outcome 2 To study the effect of placebo was not an explicit trial purpose.
6.3. Analysis.
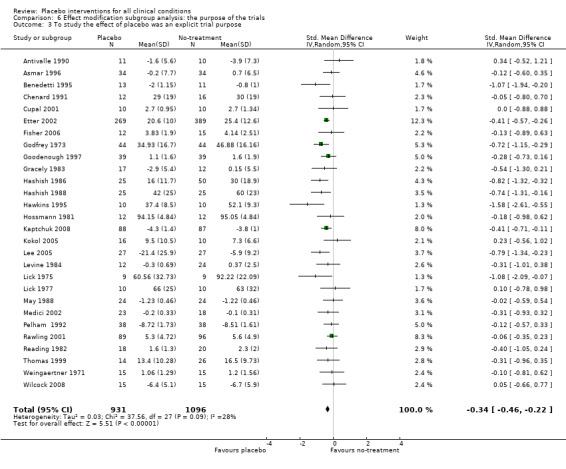
Comparison 6 Effect modification subgroup analysis: the purpose of the trials, Outcome 3 To study the effect of placebo was an explicit trial purpose.
6.4. Analysis.
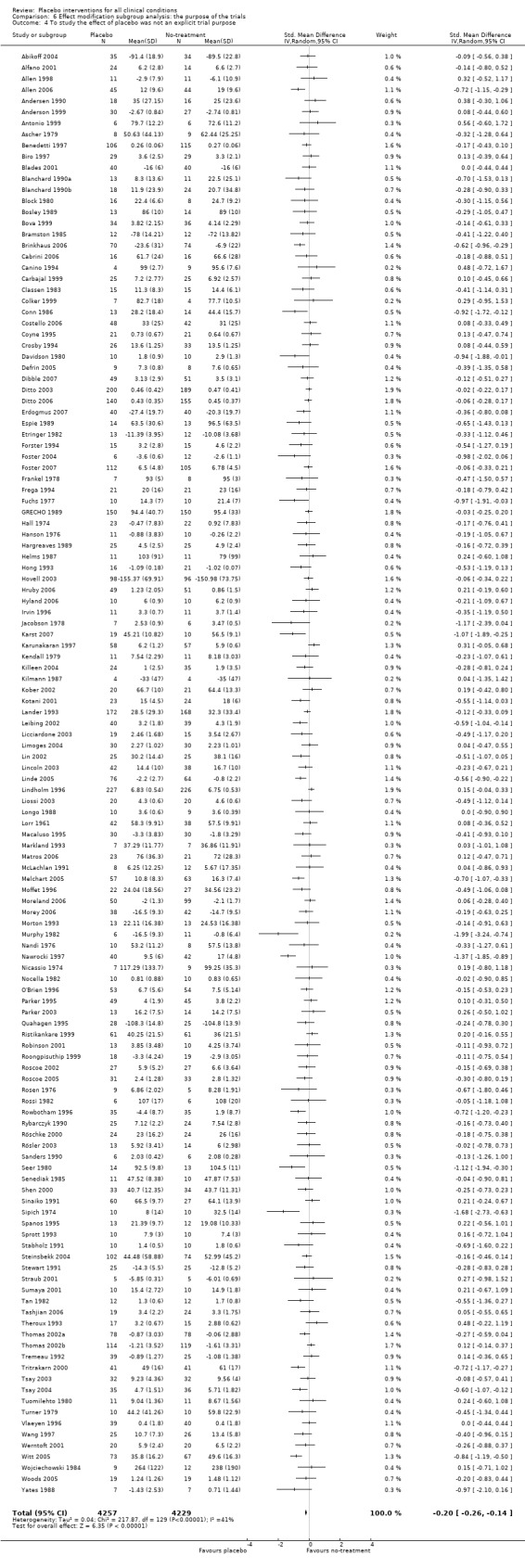
Comparison 6 Effect modification subgroup analysis: the purpose of the trials, Outcome 4 To study the effect of placebo was not an explicit trial purpose.
Comparison 7. Effect modification subgroup analysis: type of placebo interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Psychological placebos | 9 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.75, 1.04] |
| 2 Physical placebos | 11 | 2536 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.81, 1.01] |
| 3 Pharmacological placebos | 24 | 3207 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.89, 1.05] |
| 4 Psychological placebos | 53 | 2546 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.31, ‐0.12] |
| 5 Physical placebos | 61 | 3922 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.41, ‐0.22] |
| 6 Pharmacological placebos | 44 | 4045 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.20, ‐0.01] |
7.1. Analysis.
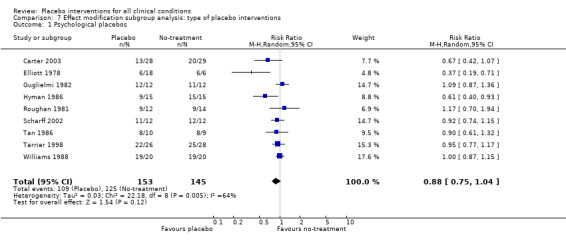
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 1 Psychological placebos.
7.2. Analysis.
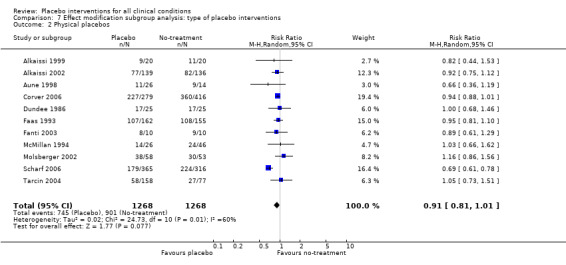
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 2 Physical placebos.
7.3. Analysis.
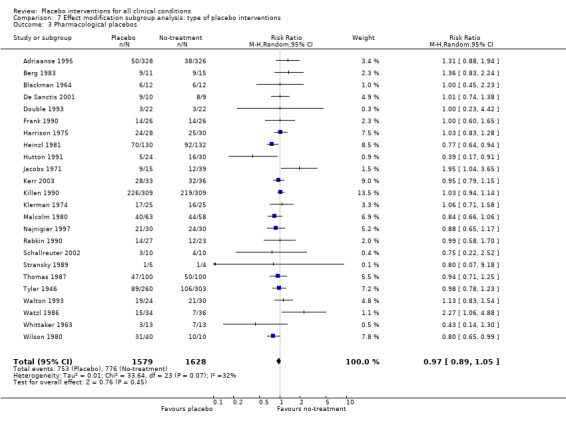
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 3 Pharmacological placebos.
7.4. Analysis.
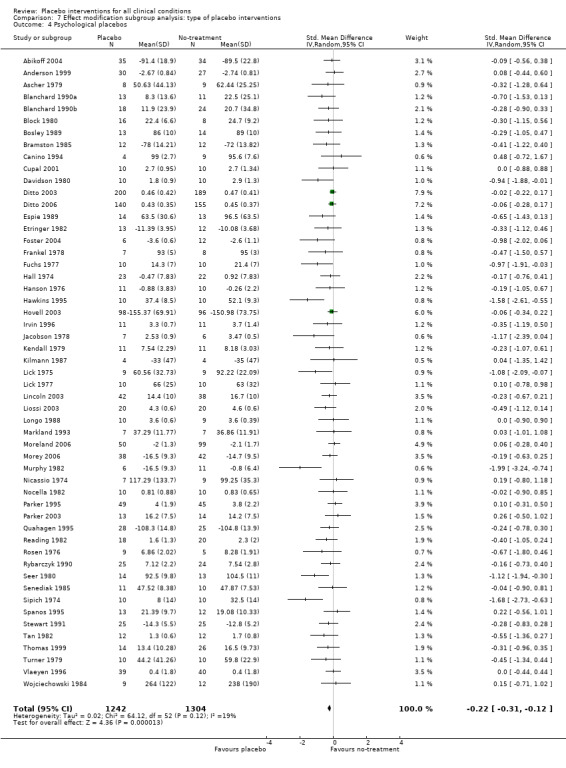
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 4 Psychological placebos.
7.5. Analysis.
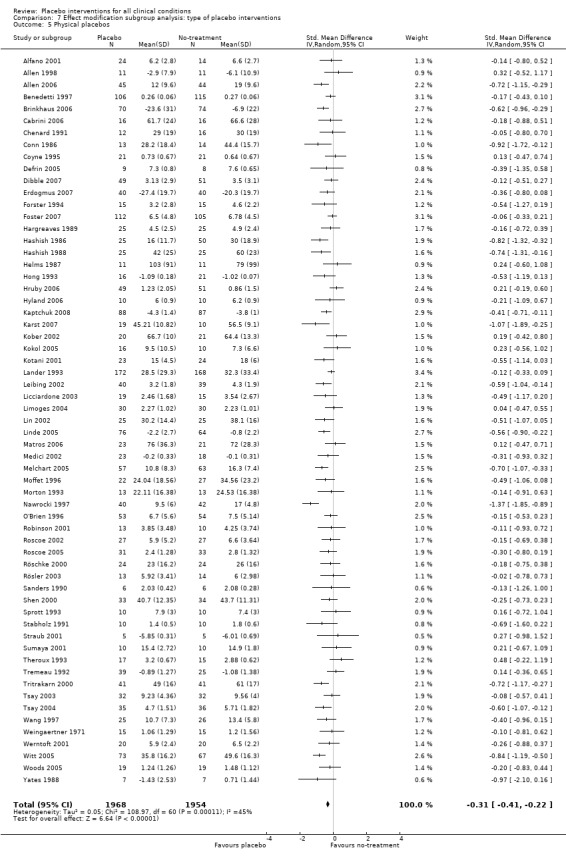
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 5 Physical placebos.
7.6. Analysis.
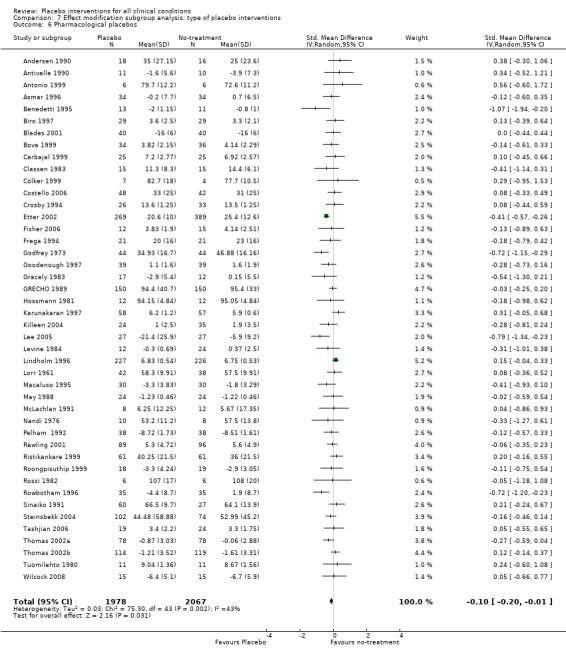
Comparison 7 Effect modification subgroup analysis: type of placebo interventions, Outcome 6 Pharmacological placebos.
Comparison 8. Effect modification subgroup analysis: patient information.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary outcomes: patients not informed that the trial involved placebo | 7 | 1085 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.12] |
| 2 Binary outcomes: patients informed that the trial involved placebo (or not stated) | 37 | 4956 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.87, 0.99] |
| 3 Continuous outcomes: patients not informed that the trial involved placebo | 23 | 1692 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.53, ‐0.26] |
| 4 Continuous outcomes: patients informed that the trial involved placebo (or not stated) | 135 | 8821 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.25, ‐0.13] |
8.1. Analysis.
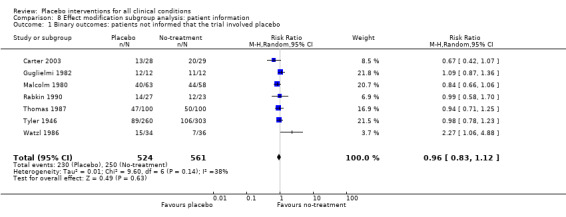
Comparison 8 Effect modification subgroup analysis: patient information, Outcome 1 Binary outcomes: patients not informed that the trial involved placebo.
8.2. Analysis.
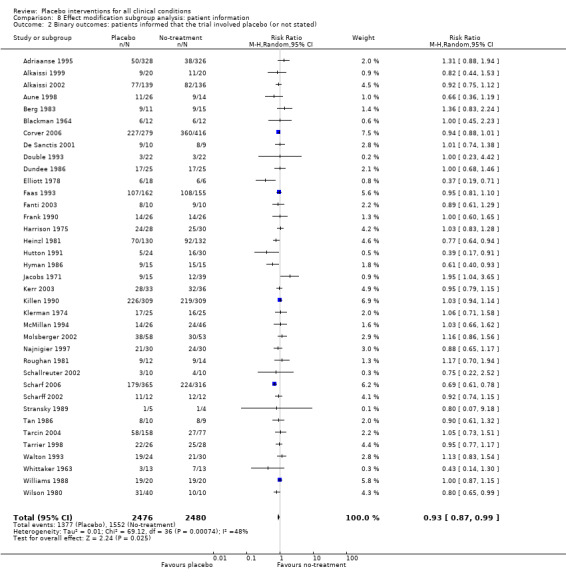
Comparison 8 Effect modification subgroup analysis: patient information, Outcome 2 Binary outcomes: patients informed that the trial involved placebo (or not stated).
8.3. Analysis.
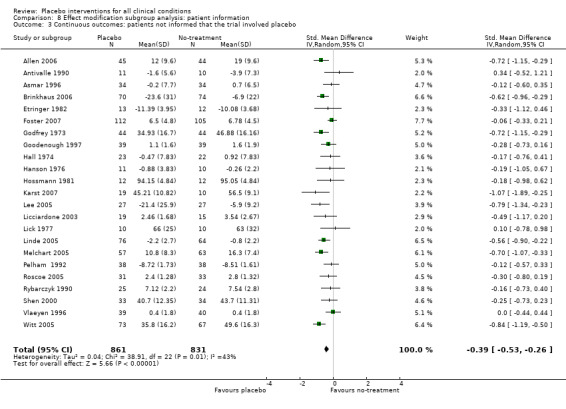
Comparison 8 Effect modification subgroup analysis: patient information, Outcome 3 Continuous outcomes: patients not informed that the trial involved placebo.
8.4. Analysis.
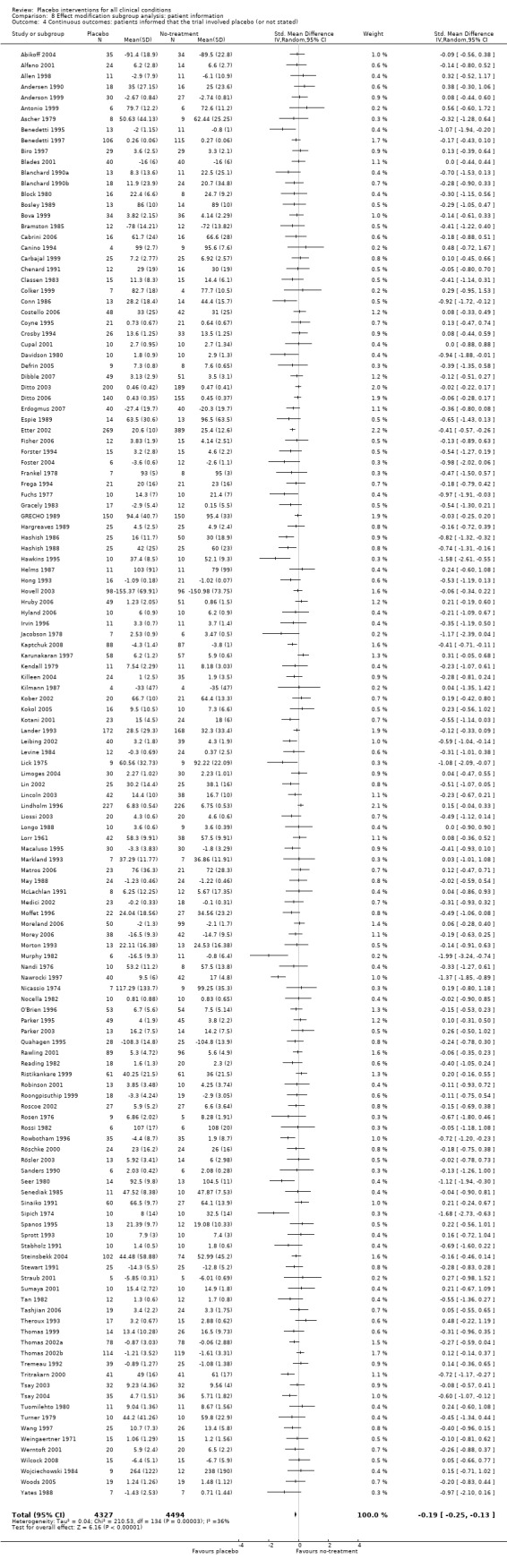
Comparison 8 Effect modification subgroup analysis: patient information, Outcome 4 Continuous outcomes: patients informed that the trial involved placebo (or not stated).
Comparison 9. Effect modification subgroup analysis: placebo as add‐on treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Add‐on treatment: yes | 17 | 1912 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.85, 1.07] |
| 2 Add‐on treatment: no or not stated | 27 | 4129 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.89, 1.00] |
| 3 Add‐on treatment: yes | 71 | 5423 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.31, ‐0.15] |
| 4 Add‐on treatment: no or not stated | 87 | 5090 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.30, ‐0.14] |
9.1. Analysis.
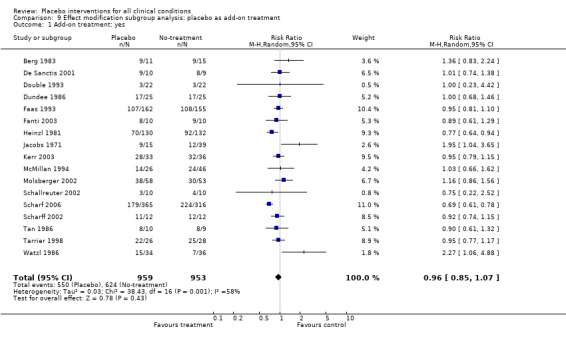
Comparison 9 Effect modification subgroup analysis: placebo as add‐on treatment, Outcome 1 Add‐on treatment: yes.
9.2. Analysis.
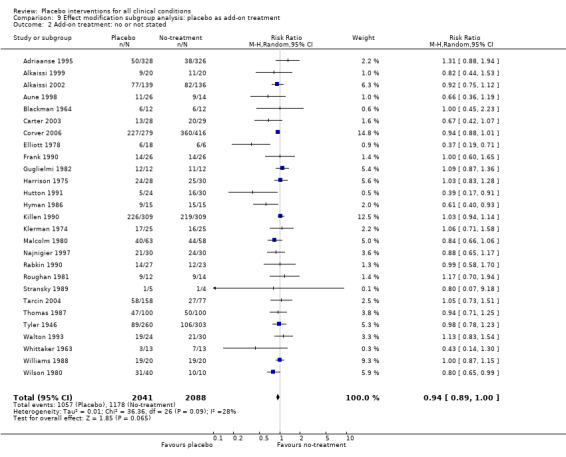
Comparison 9 Effect modification subgroup analysis: placebo as add‐on treatment, Outcome 2 Add‐on treatment: no or not stated.
9.3. Analysis.
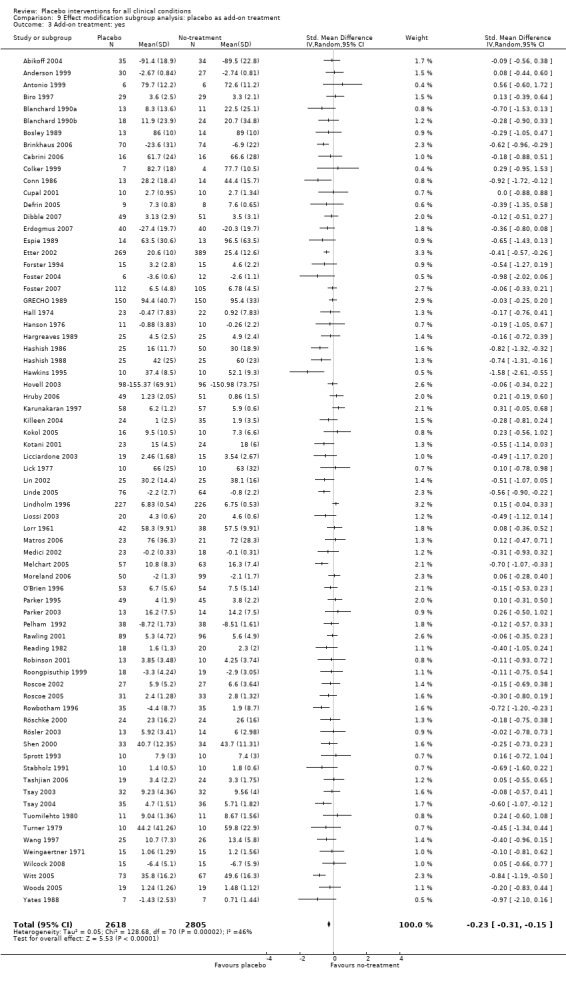
Comparison 9 Effect modification subgroup analysis: placebo as add‐on treatment, Outcome 3 Add‐on treatment: yes.
9.4. Analysis.
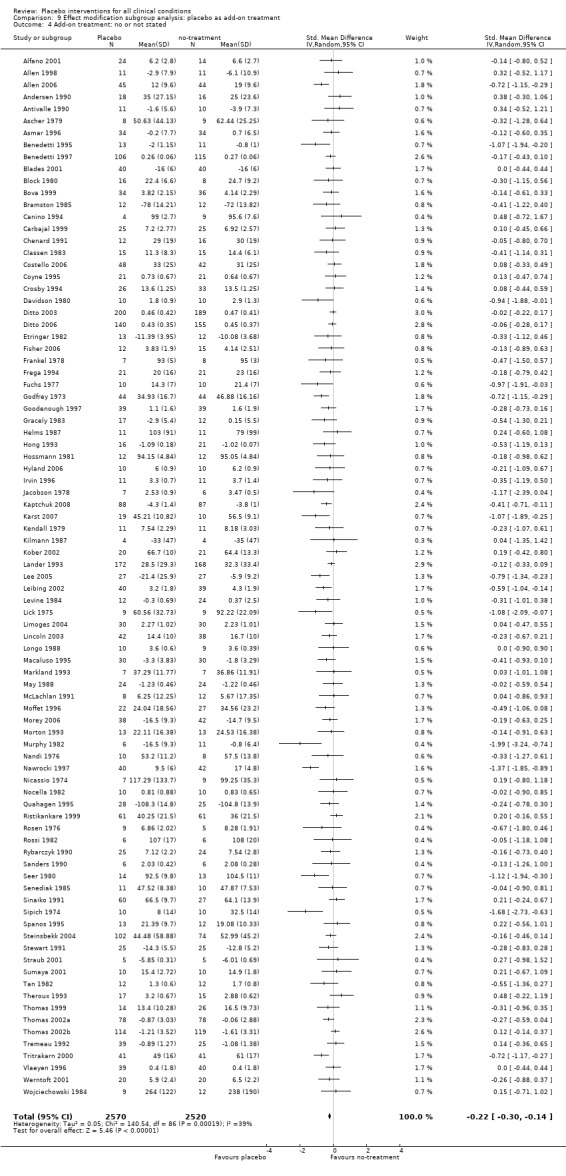
Comparison 9 Effect modification subgroup analysis: placebo as add‐on treatment, Outcome 4 Add‐on treatment: no or not stated.
Comparison 10. Risk of bias subgroup analysis: blinding of placebo treatment providers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Placebo intervention provider blinded: yes | 19 | 2707 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.05] |
| 2 Placebo intervention provider blinded: no or not stated | 25 | 3334 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.83, 0.99] |
| 3 Placebo intervention provider blinded: yes | 42 | 3069 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.27, ‐0.02] |
| 4 Placebo intervention provider blinded: no or not stated | 116 | 7444 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.32, ‐0.19] |
10.1. Analysis.
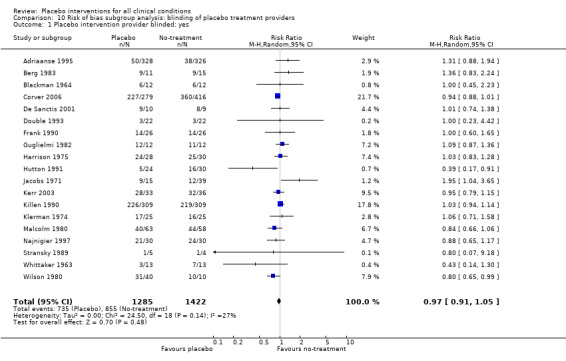
Comparison 10 Risk of bias subgroup analysis: blinding of placebo treatment providers, Outcome 1 Placebo intervention provider blinded: yes.
10.2. Analysis.
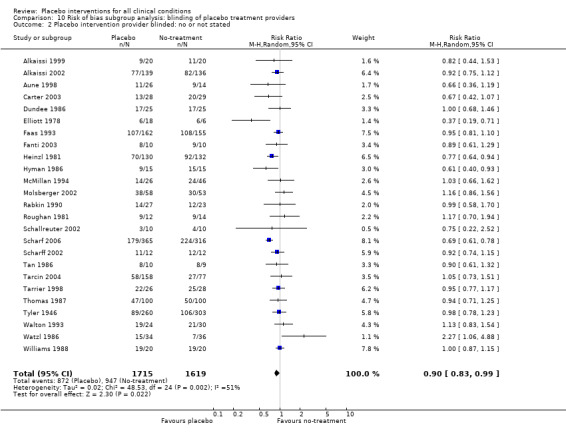
Comparison 10 Risk of bias subgroup analysis: blinding of placebo treatment providers, Outcome 2 Placebo intervention provider blinded: no or not stated.
10.3. Analysis.
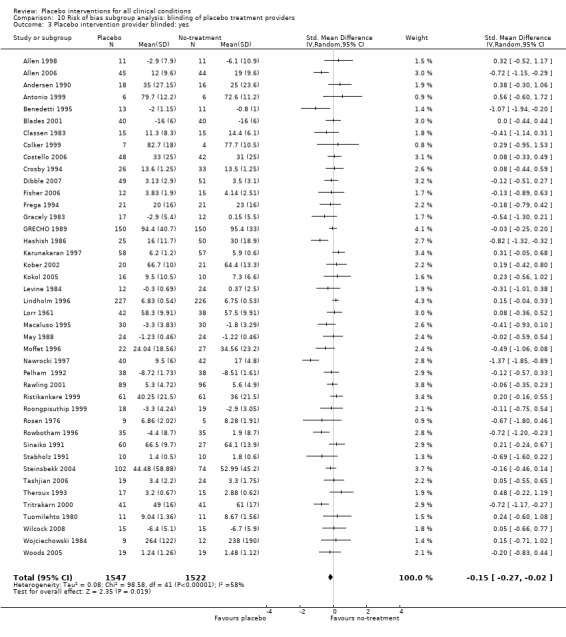
Comparison 10 Risk of bias subgroup analysis: blinding of placebo treatment providers, Outcome 3 Placebo intervention provider blinded: yes.
10.4. Analysis.
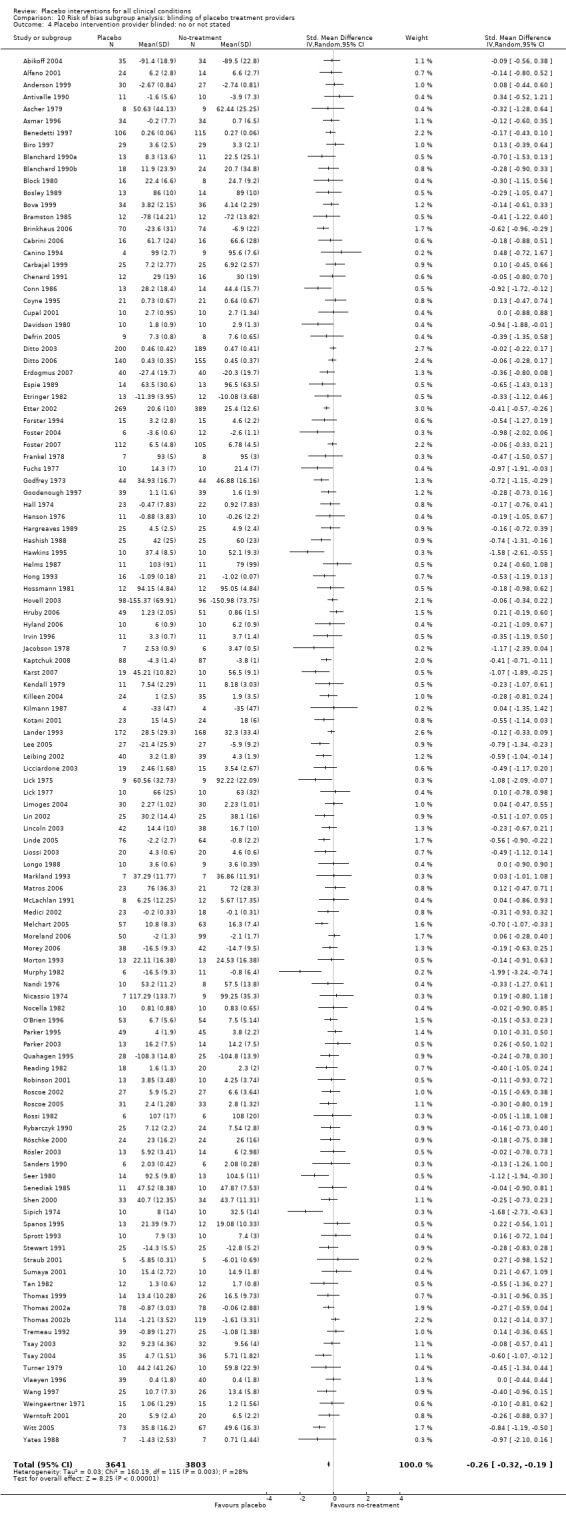
Comparison 10 Risk of bias subgroup analysis: blinding of placebo treatment providers, Outcome 4 Placebo intervention provider blinded: no or not stated.
Comparison 11. Risk of bias subgroup analysis: blinding of observer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blinding of observer: yes | 2 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.68, 1.07] |
| 2 Blinding of observer: not stated | 11 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3 Blinding of observer: yes | 20 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.42, ‐0.05] |
| 4 Blinding of observer: not stated | 29 | 1844 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.20, 0.06] |
11.1. Analysis.
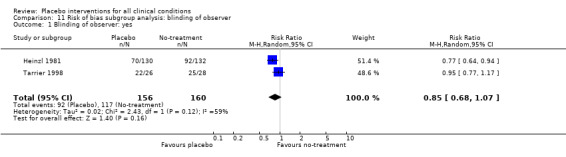
Comparison 11 Risk of bias subgroup analysis: blinding of observer, Outcome 1 Blinding of observer: yes.
11.2. Analysis.
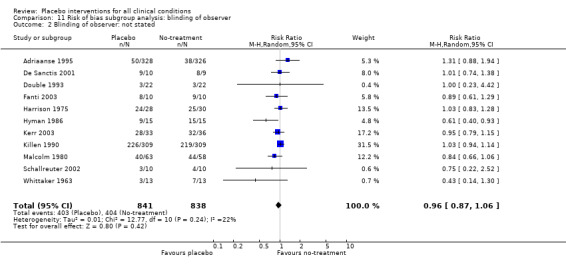
Comparison 11 Risk of bias subgroup analysis: blinding of observer, Outcome 2 Blinding of observer: not stated.
11.3. Analysis.
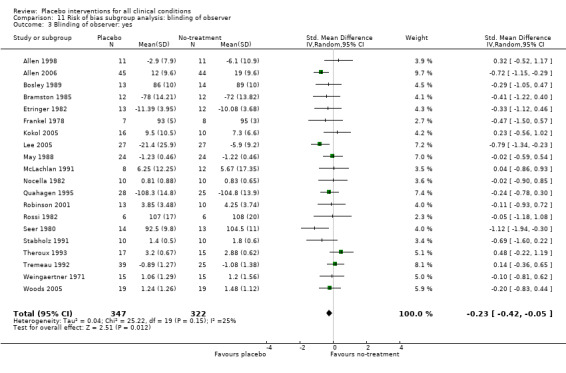
Comparison 11 Risk of bias subgroup analysis: blinding of observer, Outcome 3 Blinding of observer: yes.
11.4. Analysis.
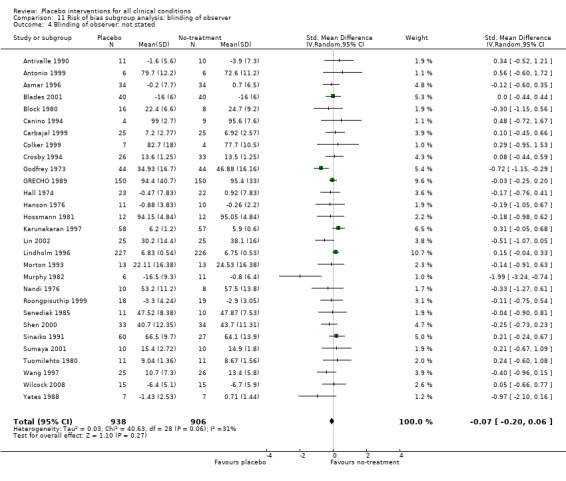
Comparison 11 Risk of bias subgroup analysis: blinding of observer, Outcome 4 Blinding of observer: not stated.
Comparison 12. Risk of bias subgroup analysis: variance inequality and skewness.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No signs of unequal variance or skewness | 72 | 4378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.33, ‐0.15] |
| 2 Signs of either unequal variance or skewness | 52 | 4108 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.30, ‐0.11] |
12.1. Analysis.
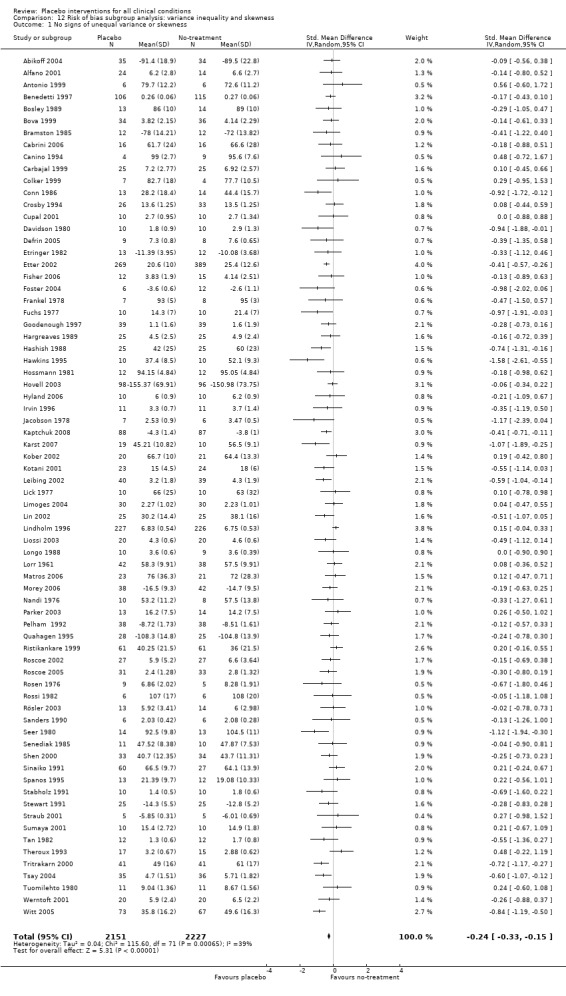
Comparison 12 Risk of bias subgroup analysis: variance inequality and skewness, Outcome 1 No signs of unequal variance or skewness.
12.2. Analysis.
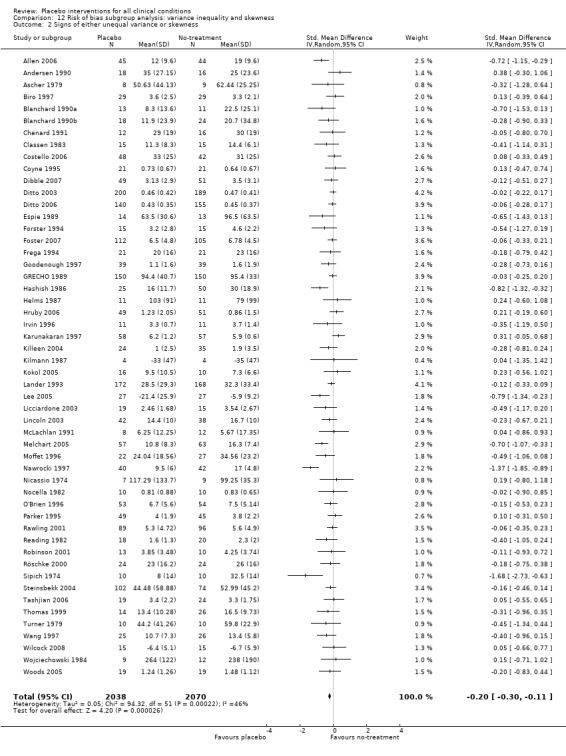
Comparison 12 Risk of bias subgroup analysis: variance inequality and skewness, Outcome 2 Signs of either unequal variance or skewness.
Comparison 13. Risk of bias subgroup analysis: selection of outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Selection of outcome: primary trial outcome clearly indicated | 31 | 4775 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 1.00] |
| 2 Selection of outcome: primary trial outcome not clearly indicated (or not selected) | 13 | 1265 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.89, 1.01] |
| 3 Selection of outcome: primary trial outcome clearly indicated | 85 | 6028 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.34, ‐0.19] |
| 4 Selection of outcome: primary trial outcome not clearly indicated (or not selected) | 73 | 4485 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.25, ‐0.09] |
13.1. Analysis.
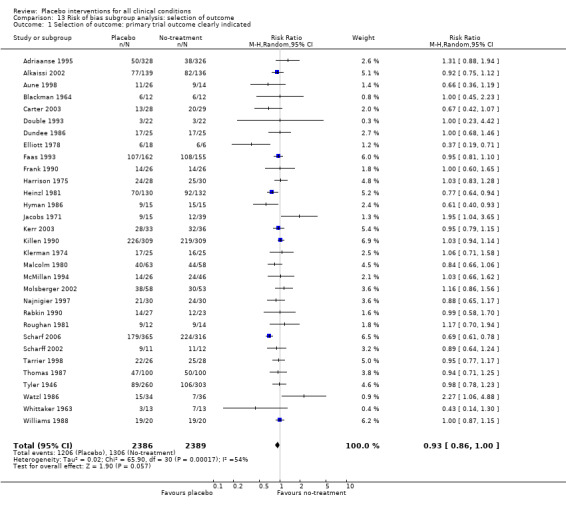
Comparison 13 Risk of bias subgroup analysis: selection of outcome, Outcome 1 Selection of outcome: primary trial outcome clearly indicated.
13.2. Analysis.
Comparison 13 Risk of bias subgroup analysis: selection of outcome, Outcome 2 Selection of outcome: primary trial outcome not clearly indicated (or not selected).
13.3. Analysis.
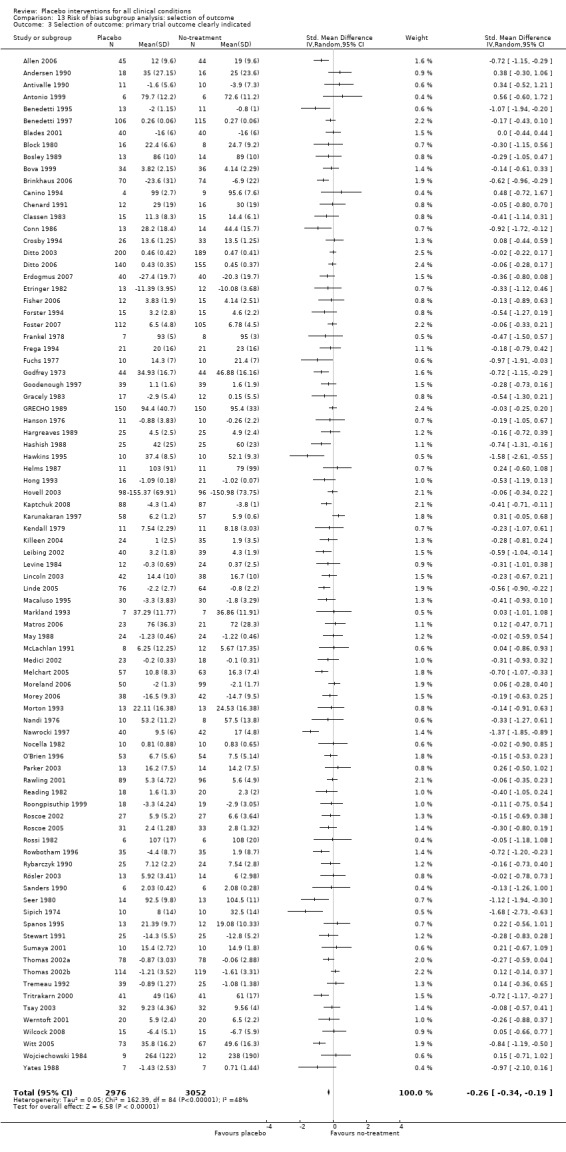
Comparison 13 Risk of bias subgroup analysis: selection of outcome, Outcome 3 Selection of outcome: primary trial outcome clearly indicated.
13.4. Analysis.
Comparison 13 Risk of bias subgroup analysis: selection of outcome, Outcome 4 Selection of outcome: primary trial outcome not clearly indicated (or not selected).
Comparison 14. Risk of bias subgroup analysis: format of outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome was final values | 118 | 7866 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.28, ‐0.14] |
| 2 Outcome was change from baseline | 40 | 2659 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.37, ‐0.16] |
14.1. Analysis.
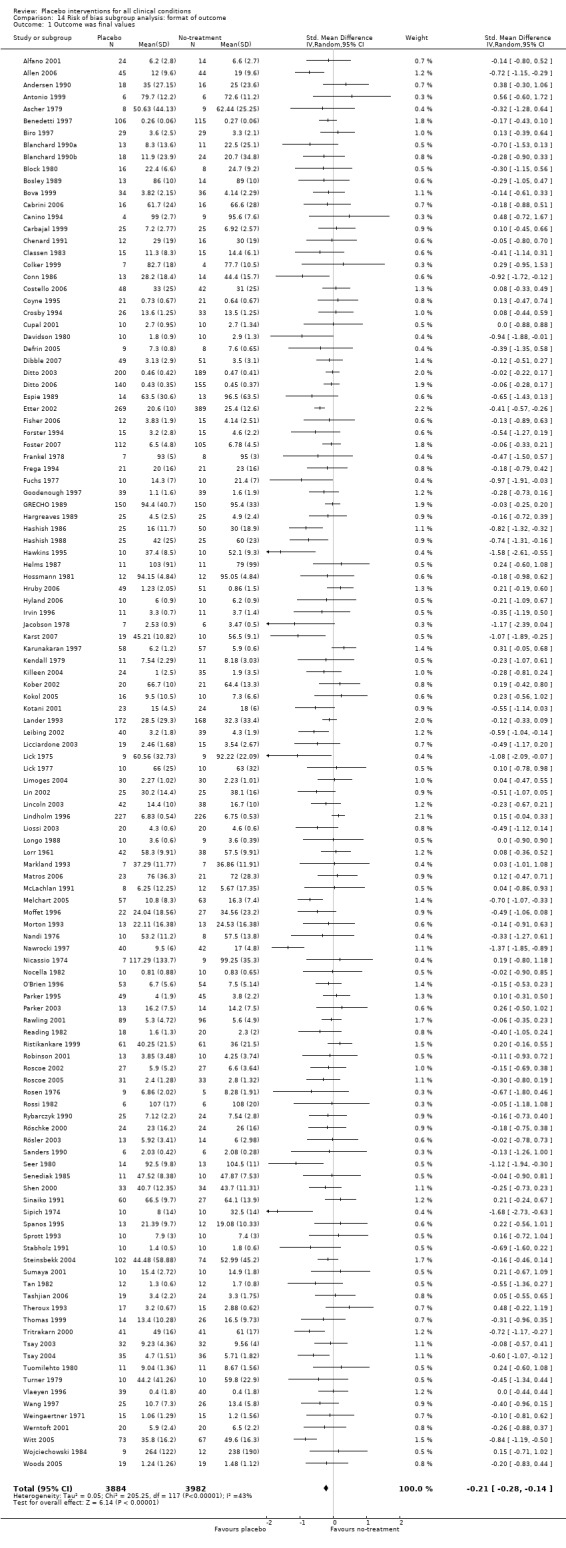
Comparison 14 Risk of bias subgroup analysis: format of outcome, Outcome 1 Outcome was final values.
14.2. Analysis.
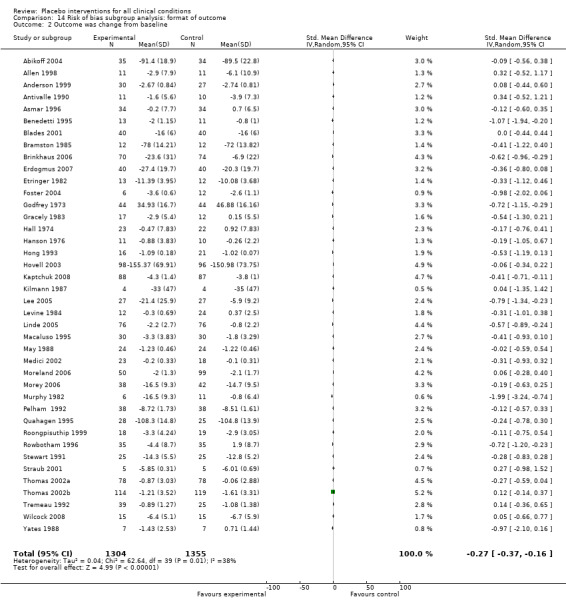
Comparison 14 Risk of bias subgroup analysis: format of outcome, Outcome 2 Outcome was change from baseline.
Comparison 15. Risk of bias subgroup analysis: concealed allocation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Concealed allocation adequate | 8 | 1554 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.02] |
| 2 Concealed allocation unclear | 36 | 4487 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.90, 1.01] |
| 3 Concealed allocation adequate | 20 | 2241 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.46, ‐0.22] |
| 4 Concealed allocation unclear | 138 | 8272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.26, ‐0.14] |
15.1. Analysis.
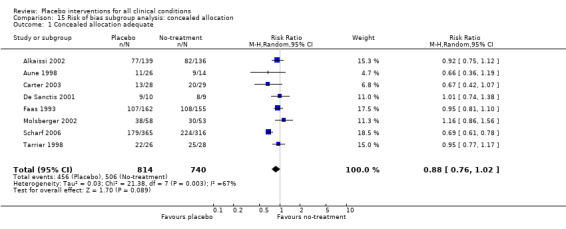
Comparison 15 Risk of bias subgroup analysis: concealed allocation, Outcome 1 Concealed allocation adequate.
15.2. Analysis.
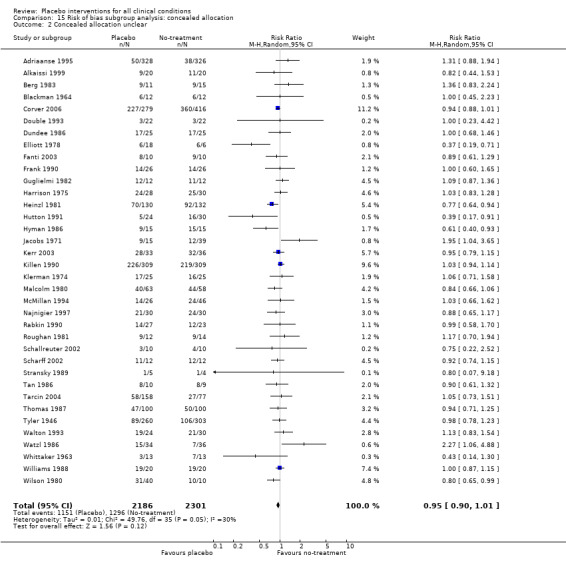
Comparison 15 Risk of bias subgroup analysis: concealed allocation, Outcome 2 Concealed allocation unclear.
15.3. Analysis.
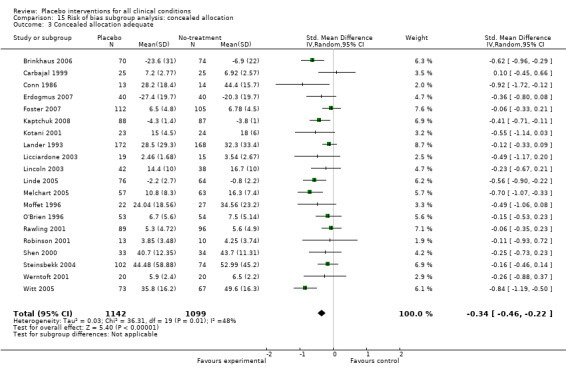
Comparison 15 Risk of bias subgroup analysis: concealed allocation, Outcome 3 Concealed allocation adequate.
15.4. Analysis.
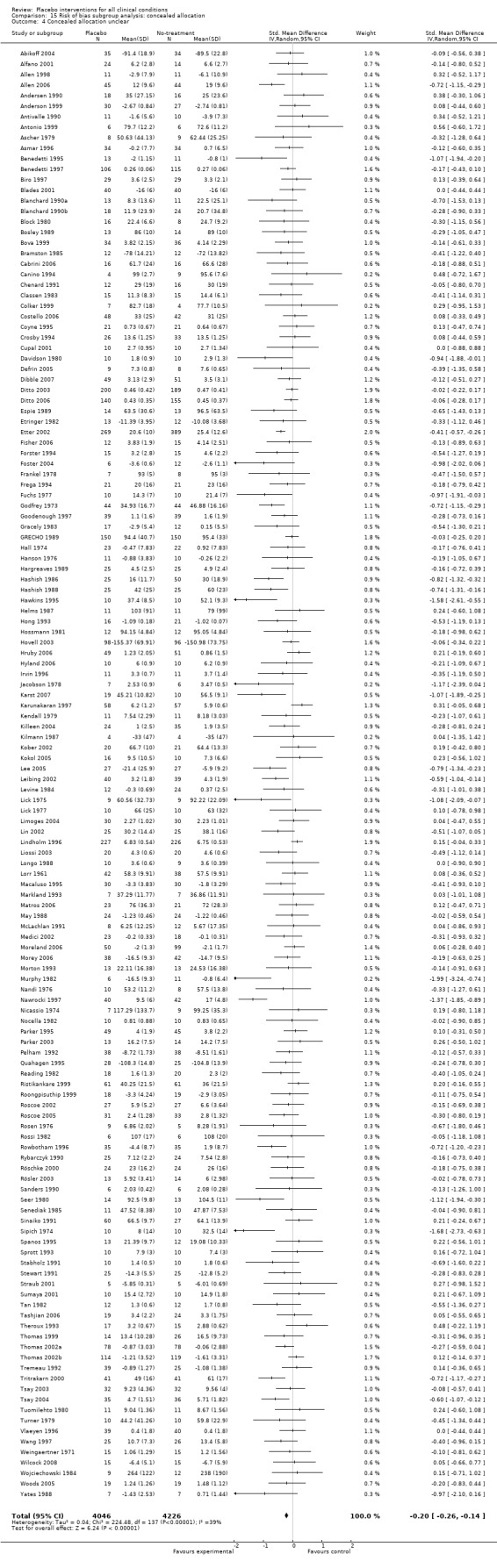
Comparison 15 Risk of bias subgroup analysis: concealed allocation, Outcome 4 Concealed allocation unclear.
Comparison 16. Risk of bias subgroup analysis: trial size.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Trial size >49 | 27 | 5586 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| 2 Trial size 49 or less | 17 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.80, 1.04] |
| 3 Trial size >49 | 65 | 8050 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.29, ‐0.14] |
| 4 Trial size 49 or less | 93 | 2463 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.32, ‐0.16] |
16.1. Analysis.
Comparison 16 Risk of bias subgroup analysis: trial size, Outcome 1 Trial size >49.
16.2. Analysis.
Comparison 16 Risk of bias subgroup analysis: trial size, Outcome 2 Trial size 49 or less.
16.3. Analysis.
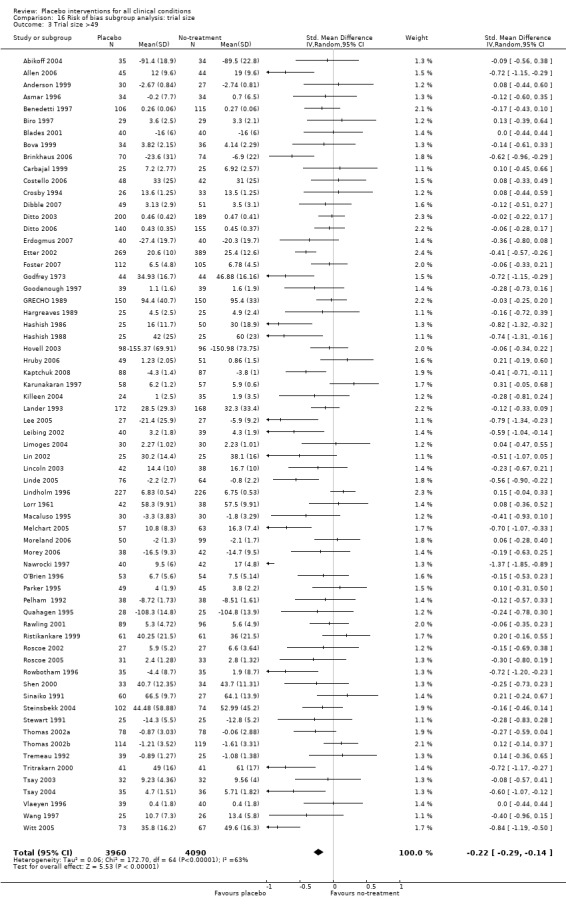
Comparison 16 Risk of bias subgroup analysis: trial size, Outcome 3 Trial size >49.
16.4. Analysis.
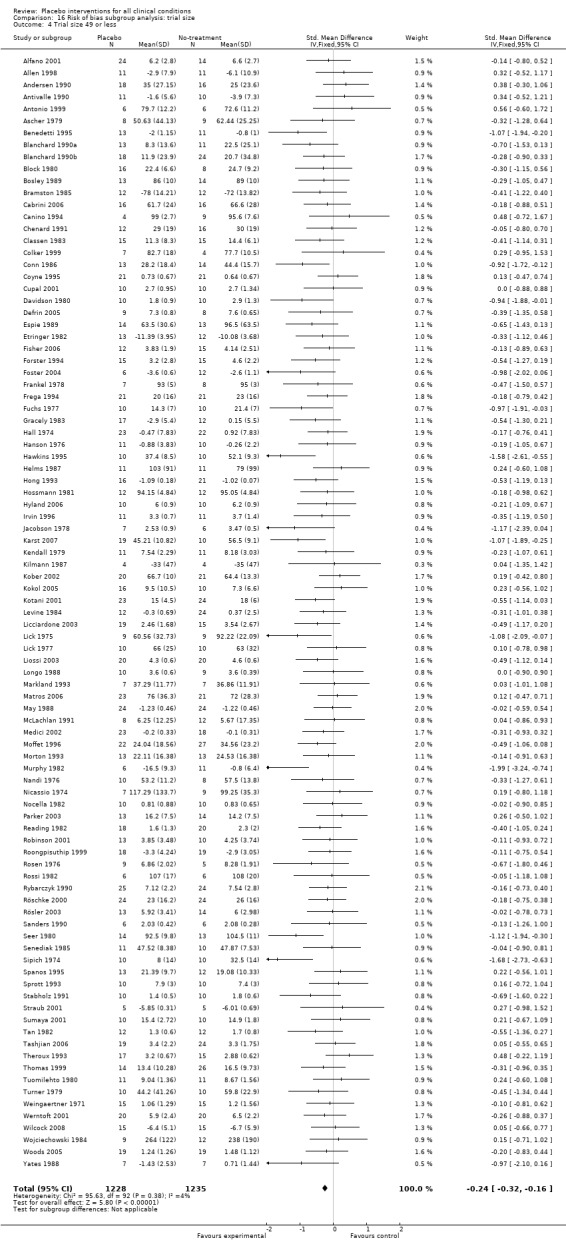
Comparison 16 Risk of bias subgroup analysis: trial size, Outcome 4 Trial size 49 or less.
Comparison 17. Risk of bias subgroup analysis: dropouts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropout rates no more than 15% | 24 | 3687 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.86, 0.99] |
| 2 Dropout rates >15%, or not stated | 20 | 2354 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.06] |
| 3 Dropout rates no more than 15% | 64 | 4973 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.34, ‐0.16] |
| 4 Dropout rates > 15%, or not stated | 94 | 5540 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.27, ‐0.13] |
17.1. Analysis.
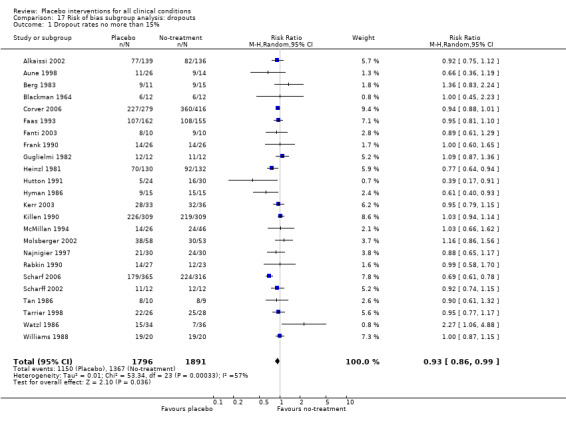
Comparison 17 Risk of bias subgroup analysis: dropouts, Outcome 1 Dropout rates no more than 15%.
17.2. Analysis.
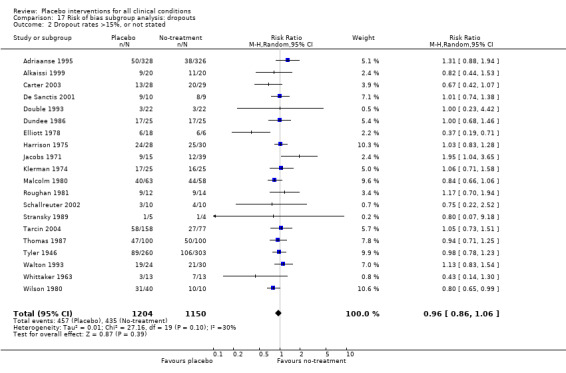
Comparison 17 Risk of bias subgroup analysis: dropouts, Outcome 2 Dropout rates >15%, or not stated.
17.3. Analysis.
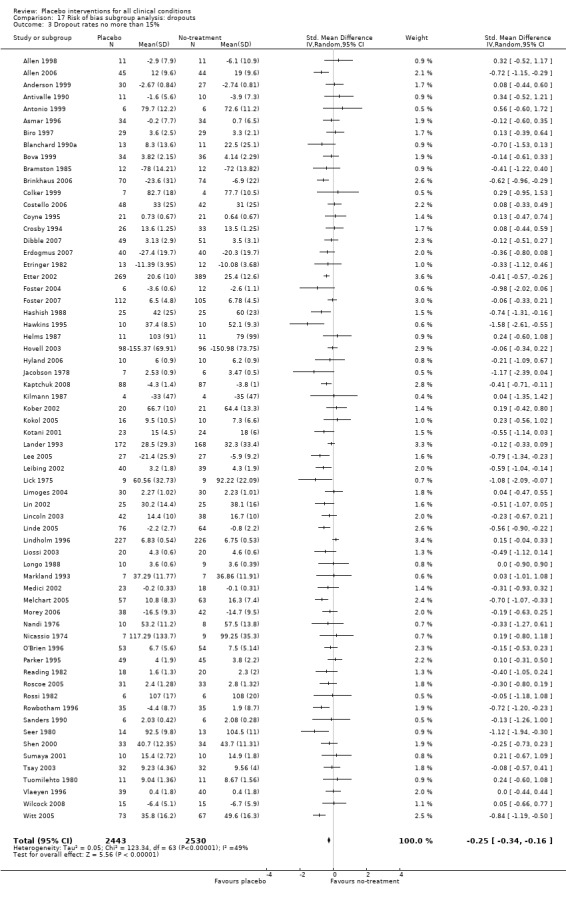
Comparison 17 Risk of bias subgroup analysis: dropouts, Outcome 3 Dropout rates no more than 15%.
17.4. Analysis.
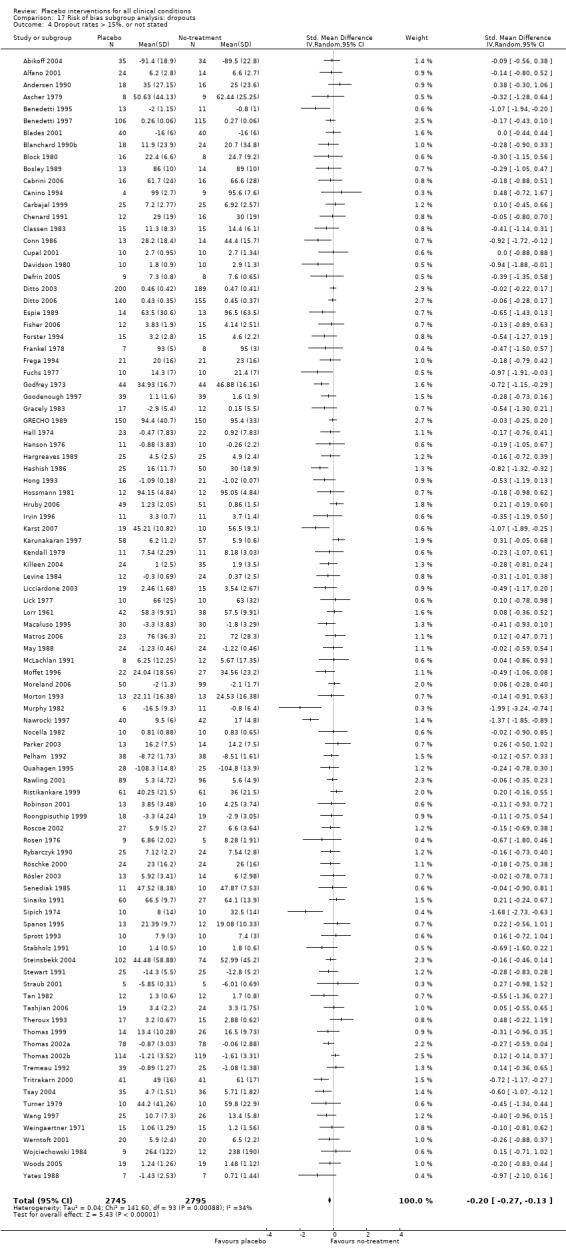
Comparison 17 Risk of bias subgroup analysis: dropouts, Outcome 4 Dropout rates > 15%, or not stated.
Comparison 18. Risk of bias subgroup analysis: clearly concealed allocation + trial size >49 + dropout max 15%.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Binary outcomes | 5 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.08] |
| 1.1 Pain (incidence) | 3 | 1109 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.67, 1.19] |
| 1.2 Schizophrenia (lack of 50% improvement) | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.17] |
| 1.3 Nausea | 1 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.12] |
| 2 Continuous outcomes | 11 | 1610 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.55, ‐0.22] |
| 2.1 Pain (various scales, see table of included studies for details) | 7 | 1181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.69, ‐0.21] |
| 2.2 Nausea (Rhodes inventory of nausea and vomiting, escape medication) | 2 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.49, 0.11] |
| 2.3 Irritable bowel syndrome (Global improvement scale) | 1 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.71, ‐0.11] |
| 2.4 Depression (Beck depression inventory) | 1 | 80 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.67, 0.21] |
| 3 Pain heterogeneity | 7 | 1181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.69, ‐0.21] |
| 3.1 GAT | 4 | 544 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐0.85, ‐0.50] |
| 3.2 Not GAT | 3 | 637 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.28, 0.03] |
18.1. Analysis.
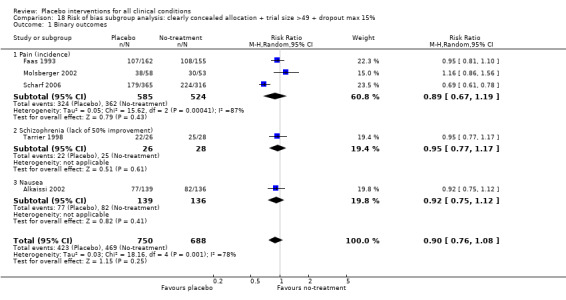
Comparison 18 Risk of bias subgroup analysis: clearly concealed allocation + trial size >49 + dropout max 15%, Outcome 1 Binary outcomes.
18.2. Analysis.
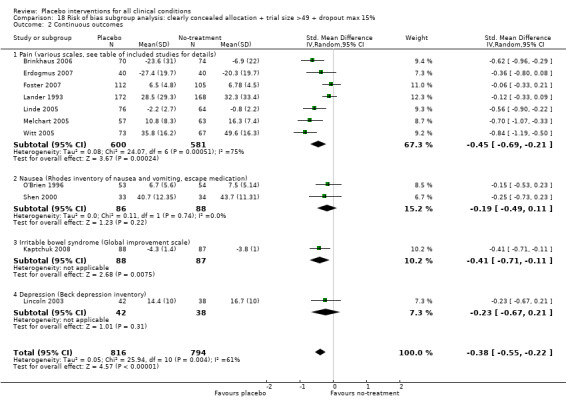
Comparison 18 Risk of bias subgroup analysis: clearly concealed allocation + trial size >49 + dropout max 15%, Outcome 2 Continuous outcomes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abikoff 2004.
| Methods | Design: three group parallel trial Purpose: study the effect of methylphenidate and multimodal psychological treatment on children with attention‐deficit/hyperactivity disorder | |
| Participants | Patients: children with attention‐deficit/hyperactivity disorder Baseline comparability: yes (except for socioeconomic status) | |
| Interventions | Placebo: sessions with attention control interventions with no social skills training Untreated: no sessions Experimental: sessions with social skills training (Co‐intervention: All patients received methylphenidate) | |
| Outcomes | Social skills rating scale (parents) Social skills rating scale (children) Taxonomy of problem situations (teachers) Direct school observations Parental practices Academic achievements Emotional status | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (attention control placebo/multimodal psychological intervention) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient (parents) reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 69 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Adams 1976.
| Methods | Design: three group parallel trial Purpose: examine the effect of adenine arabinoside on episodes of genital herpes | |
| Participants | Patients: out‐patients with episodes of genital herpes Baseline comparability: NS | |
| Interventions | Placebo: ointment or gel without adenine arabinoside Untreated: no ointment or gel Experimental: ointment or gel with adenine arabinoside (Co‐intervention: NS) | |
| Outcomes | Pain Mean duration of pain Mean duration of viral shedding Mean duration of lesions Mean duration any new lesion during treatment | |
| Notes | Relevant outcome data not reported in article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Thus ara‐A or placebo ointment or gel were given in a double‐blind fashion' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not reported in article |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 38 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Adriaanse 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of vaginal chlorhexidine disinfection on the transmission of group B streptococci from mother to child during labour | |
| Participants | Patients: women during labour Baseline comparability: yes | |
| Interventions | Placebo: vaginal gel without chlorhexidine Untreated: no vaginal gel Experimental: vaginal gel with chlorhexidine (Co‐intervention: NS) | |
| Outcomes | Bacterial transmission rate Infections | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Predefined block (10:10:10) allocation scheme' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Two groups were treated in a double‐blind manner with either a chlorhexidine or a placebo gel' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out >15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 654 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out >15% or NS |
Alfano 2001.
| Methods | Design: five group parallel trial Purpose: examine the effect of static magnetic fields for treatment of fibromyalgia | |
| Participants | Patients: subjects with fibromyalgia (American College of Rheumatology's diagnostic criteria) Baseline comparability: yes | |
| Interventions | Placebo: pads that have no magnetic property inserted into the beds of the patients for 6 months Untreated: no pads Experimental: two types of pads with static magnetic properties (Co‐intervention: NS) | |
| Outcomes | Pain (11 point numeric rating scale, 0 to 10) at six months Number of tender points Tender point pain intensity Functional status (fibromyalgia impact questionnaire) | |
| Notes | Results from two placebo groups reported as deriving from one group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer‐generated treatment list' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patients reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 38 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Alford 2003.
| Methods | Design: three group parallel trial Purpose: to evaluate the efficacy of a continuous postoperative bupivacaine infusion pump for pain management after anterior cruciate ligament reconstruction | |
| Participants | Patients: out‐patients requiring ACL reconstruction Baseline comparability: NS | |
| Interventions | Placebo: infusion catheter filled with saline Untreated: no catheter Experimental: infusion catheter filed with 0.25% bupivacaine solution. (Co‐intervention: ipsilateral femoral nerve block with 30mL 0.25% bupivacaine and 20 mL 0.25% bupivacaine intra‐articular injection. Postoperative pain management protocol: hydrocodone/acetaminophen, 5mg/500mg every 4 hours, and ibuprofen 800mg 3 times a day) | |
| Outcomes | Pain (11 point numeric rating scale, 0 to 10) Medication consumption Physical therapy performance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'random number draw conducted in the operating suite' |
| Allocation concealment? | Unclear risk | 'separate sealed notebook' |
| Blinding? Treatment provider | Low risk | 'Both patient and investigators were blinded to the catheter contents and group assignment' |
| Blinding? Outcome assessor | Low risk | 'The therapists were blinded to patient group assignment' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 16 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Alkaissi 1999.
| Methods | Design: three group parallel trial Purpose: examine the preventive effect of acupressure on nausea and vomiting after surgery | |
| Participants | Patients: women undergoing minor gynaecological surgery Baseline comparability: yes | |
| Interventions | Placebo: acupressure on a site which was not P6 during hospital stay Untreated: no acupressure Experimental: acupressure on P6 (Co‐intervention: metoclopramide and droperidol at request ) | |
| Outcomes | Proportion of patients with complete response (no nausea, vomiting, or rescue medication) after 24 hours Nausea (only) Vomiting Rescue medication Nausea after 24 hours | |
| Notes | In the no treatment group 6/20 had nausea at discharge, and 8/20 after 24 hours. The corresponding numbers for the placebo group were 7/20 and 1/20. According to protocol we extracted data at post‐treatment (discharge). 10 out of 60 patients dropped out ('evenly distributed between the groups') and were replaced by 10 new patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | 'The study was double‐blind...' but this referred to blinding of patient and outcome assessor. |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patients reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out >15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out >15% or NS |
Alkaissi 2002.
| Methods | Design: three group parallel trial Purpose: examine the preventive effect of acupressure on nausea and vomiting after surgery | |
| Participants | Patients: women undergoing minor gynaecological surgery Baseline comparability: yes | |
| Interventions | Placebo: acupressure bands not on P6 for 24 hours Untreated: no acupressure Experimental: acupressure on P6 (Co‐intervention: anaesthetic agents, rescue medication) | |
| Outcomes | Proportion of patients with complete response (no nausea, vomiting or rescue medication), time NS Apfel risk score Satisfaction with treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'Sealed envelope' |
| Blinding? Treatment provider | High risk | Described as double‐blind (Placebo/acupressure) but this referred to blinding of patient and outcome assessor. |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patients reported outcomes |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out <15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 275 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Allen 1998.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on depression | |
| Participants | Patients: out‐patients with depression Baseline comparability: no (depression scores) | |
| Interventions | Placebo: needling in acupuncture points not regarded having impact on depression Untreated: no needling Experimental: needling in acupuncture points regarded having impact on depression (Co‐intervention: NS) | |
| Outcomes | Hamilton Rating Scale for Depression (31‐item version, HAM‐D31) Proportion of patients with remission (50% reduction on HAM‐D31) Inventory of Depressive Symptomatology Beck Depression Inventory Beck Hopelessness Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The treating acupuncturists were blind to the experimental hypotheses...' (Placebo/acupuncture) |
| Blinding? Outcome assessor | Low risk | 'All patients were interviewed by trained raters blind to treatment condition...' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 22 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Allen 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on depression | |
| Participants | Patients: out‐patients with depression (score of 14 or more on the 17‐item Hamilton rating scale for depression) Baseline comparability: yes | |
| Interventions | Placebo: needling in acupuncture points not regarded having impact on depression Untreated: no needling Experimental: needling in acupuncture points regarded having impact on depression (Co‐intervention: NS) | |
| Outcomes | Hamilton Rating Scale for Depression (17 item) Beck Depression Inventory | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The treating acupuncturists were blind to the experimental hypotheses...' (Placebo/acupuncture) |
| Blinding? Outcome assessor | Low risk | 'Patients were blind to intervention condition, as were raters who assessed outcome' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 89 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Andersen 1990.
| Methods | Design: three group parallel trial Purpose: examine the effect of bromocriptine on breast pain and milk secretion after abortion | |
| Participants | Patients: women having undergone a second‐trimester abortion Baseline comparability: yes | |
| Interventions | Placebo: a tablet without bromocriptine Untreated: no tablet Experimental: a tablet containing bromocriptine (Co‐intervention: no) | |
| Outcomes | Breast pain (VAS) and serum prolactin (micro g/l) Breast tenderness Milk secretion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The part of the study that involved bromocriptine and placebo was carried out 'double‐blind'' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop out > 15 % or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 34 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Anderson 1999.
| Methods | Design: three group parallel trial Purpose: examine the effect of an office based intervention to maintain parent‐adolescent teamwork in diabetes management | |
| Participants | Patients: families with a young person with type 1 diabetes Baseline comparability: yes (age, gender, HbA1c, etc) | |
| Interventions | Placebo: over six months four 20 to 30 minute sessions with 'traditional' diabetic education with no focus on parental involvement Untreated: no sessions Experimental: sessions of parental‐adolescent teamwork therapy (Co‐intervention: standard diabetic care) | |
| Outcomes | Insulin routine score for parental involvement (4 point Likert scale) HbA1c (%) Blood glucose monitoring score Diabetes family conflict scale Diabetic family behavior checklist | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/teamwork intervention) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15 % |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 57 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Not clearly concealed allocation |
Antivalle 1990.
| Methods | Design: Two group, two period cross‐over trial Purpose: examine the effect of placebo intervention on blood pressure | |
| Participants | Patients: previously untreated out‐patients suffering from essential arterial hypertension Baseline comparability: yes (age, sex, blood pressure) | |
| Interventions | Placebo: 'tablet x 2 daily' Untreated: no tablet Experimental : no (Co‐intervention: no) | |
| Outcomes | Diastolic blood pressure reduction (mm Hg) | |
| Notes | The first period was considered a parallel trial. Results from the second period were disregarded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/ultrasound) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 21 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Antonio 1999.
| Methods | Design: three group parallel trial Purpose: examine the effect of 'guggulsterone phosphate compound' on the body composition and mood in obese adults | |
| Participants | Patients: out‐patients with body mass index >25 kg per square meter Baseline comparability: yes | |
| Interventions | Placebo: maltodextrin capsules daily for 6 weeks Untreated: no capsules Experimental: capsules with a compound of guggulsterone phosphate (Co‐intervention: dietary and exercise program) | |
| Outcomes | Weight (kg) Lean body mass Fat mass Percentage body fat Profile of Mood States questionnaire (POMS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'In this double‐masked, randomized study...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 12 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Ascher 1979.
| Methods | Design: three group parallel trial Purpose: examine the effect of paradoxical intention on insomnia | |
| Participants | Patients: out‐patients suffering from insomnia Baseline comparability: yes (sleep parameters) | |
| Interventions | Placebo: sessions of 'quasi‐desensitization' (neutral images paired with bedtime activity) Untreated: no sessions Experimental: paradoxical intention: (instructed to remain awake as long as possible and presented with the true theoretical background) (Co‐intervention: NS) | |
| Outcomes | Sleep latency (minutes) Awakenings Restedness rating Difficulty falling asleep | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/paradoxical intention) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 17 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Asmar 1996.
| Methods | Design: two period, two group, cross‐over trial Purpose: examine the effect of placebo on arterial hypertension | |
| Participants | Patients: out‐patients with untreated mild‐to‐moderate hypertension | |
| Interventions | Placebo: NS Untreated: no placebo (Co‐intervention: NS) | |
| Outcomes | Diastolic blood pressure (mm Hg) Systolic blood pressure (mm Hg) | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. The results from the trial were reported in two publications (without cross reference). In the original report there were 36 included patients, but in the subsequent report there appears only 26. The reported effect of placebo on diastolic blood pressure was higher in the second trial report. We decided to include the results from the original publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out < 15% |
| Free of selective reporting? | High risk | No protocol available. Authors were contacted. They shared data after request from the involved journal. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 68 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Not clearly concealed allocation |
Aune 1998.
| Methods | Design: three group parallel trial Purpose: examine the prophylactic effect of acupuncture on recurrent lower urinary tract infection (UTI) | |
| Participants | Patients: female out‐patients with recurrent UTI Baseline comparability: yes (age, number of UTI last 5 years) | |
| Interventions | Placebo: needling in areas that are not known acupuncture sites Untreated: no needling Experimental: needling in areas that are known acupuncture sites (Co‐intervention: NS) | |
| Outcomes | Number of patients who had infections during 6 months Number of infections during 6 months | |
| Notes | Patients were randomised to placebo and no treatment in a 2:1 ratio | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'computer based schedule' |
| Allocation concealment? | Low risk | 'sealed envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome. (Urine was tested when patients reported UTI symptoms) |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Banner 1983.
| Methods | Design: six group parallel trial Purpose: examine the effect of various techniques for reducing tension | |
| Participants | Patients: media recruited out‐patients who regularly experienced feelings of tension they wanted to reduce Baseline comparability: yes | |
| Interventions | Placebo: sessions where relaxation was enhanced by listening to soft music Untreated: no sessions Experimental: sessions with ‐visual and auditory feedback on the tension in the frontalis muscle ‐visual and auditory feedback on the finger temperature ‐combination of frontalis and temperature feedback procedures ‐relaxation enhanced by listening to a autogenic relaxation tape (Co‐intervention: NS) | |
| Outcomes | Tension rating scale EMG Finger temperature Frequency of problems | |
| Notes | Relevant outcome data not accessible: data lost (personal communication Banner CN) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/active) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | Relevant outcome data not accessible: data lost (personal communication Banner CN) |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 19 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Benedetti 1995.
| Methods | Design: eight group parallel trial Purpose: examine the effect of placebo and proglumide on postoperative pain | |
| Participants | Patients: postoperative in‐patients after thoracotomy Baseline comparability: yes (pain intensity) | |
| Interventions | Placebo: open infusion of saline Untreated: hidden infusion of saline Experimental: ‐open infusion of proglumide (0.05 mg, 0.5 mg, 5 mg) ‐hidden infusion of proglumide (0.05 mg, 0.5 mg, 5 mg) (Co‐intervention: NS) | |
| Outcomes | Pain (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'double‐blind randomized study' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out >15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Benedetti 1997.
| Methods | Design: three group parallel trial in five sub‐studies Purpose: examine the effect of transcutaneous electrical nerve stimulation (TENS) on acute postoperative pain | |
| Participants | Patients: postoperative in‐patients after thoracotomy Baseline comparability: yes (sex and age) | |
| Interventions | Placebo: TENS without batteries Untreated: no TENS Experimental: TENS with batteries (Co‐intervention: analgesics on demand, see outcome) | |
| Outcomes | Pain (overall analgesic medication within 12 hours) | |
| Notes | The results from five sub‐studies have been pooled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 221 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Benedetti 1999a.
| Methods | Design: five group parallel trial Purpose: examine the respiratory depressant response of placebo in patients newly treated with opioids | |
| Participants | Patients: lung cancer patients undergoing posterolateral thoracotomy, having repeatedly been treated with buprenorphine for three days, and were 'almost pain‐free' Baseline comparability: yes (age, gender, weight) | |
| Interventions | Placebo: saline injection (patients told it was continuation of analgesic medication) Untreated: no injection Experimental: naloxone injection (open and hidden) (Co‐intervention: additional doses of buprenorphine were administered and resulted in exclusion of the patient from the study, numbers NS) | |
| Outcomes | Respiratory depression (ventilation per minute) 73 hours after surgery Pain (11 point numerical scale, 0 to 10) | |
| Notes | The trial investigated the negative effect of placebo (respiratory depression). We included the trial in the review in a separate category (adverse effects), but not in the main analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The 60 patients... were investigated according to a randomized double‐blind design' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | High risk | See notes |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Berg 1983.
| Methods | Design: three group parallel trial Purpose: examine the effect of the additional treatment of an oral laxative 'Senokot' in patients with faecal soiling already treated with behavioural therapy | |
| Participants | Patients: children with severe and persistent faecal incontinence Baseline comparability: yes | |
| Interventions | Placebo: tablet without laxative Untreated: no tablet Experimental: tablet with laxative 'Senokot' (Co‐intervention: behavioural therapy) | |
| Outcomes | Number of children soiling more than once weekly | |
| Notes | Tablets delivered in packs marked A and B; selection bias may therefore have occurred, if the code was broken | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...the psychiatrist and psychologists did not know which tablets actually contained the laxative' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient (parents) reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | Tablets delivered in packs marked A and B; selection bias may therefore have occurred, if the code was broken |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 26 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Biro 1997.
| Methods | Design: five group parallel trial Purpose: examine the effect of topical anaesthesia methods for venous cannulation in adults | |
| Participants | Patients: patients in need of cannulation Baseline comparability: yes ('demographic data') | |
| Interventions | Placebo: cream without EMLA Untreated: no cream Experimental: ‐cream with EMLA ‐ethylchloride spray ‐lidocaine infiltration (Co‐intervention: yes, midazolam) | |
| Outcomes | Pain ratings (VAS) Number of patients with difficult punctures | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Unclear information |
| Allocation concealment? | Unclear risk | Unclear information |
| Blinding? Treatment provider | Low risk | 'The study was double‐blinded to the degree that the methodologies allowed' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcomes |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 58 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Unclear allocation concealment |
Blackman 1964.
| Methods | Design: three group parallel trial Purpose: examine the effect of imipramine on enuresis | |
| Participants | Patients: army recruits referred to a 'Mental Hygiene Consultation Service' with the complaint of enuresis Baseline comparability: yes (frequency of enuresis) | |
| Interventions | Placebo: tablet without imipramine Untreated: no tablet (observational group) Experimental: tablet with imipramine (Co‐intervention: NS) | |
| Outcomes | Number of patients with reduced frequency of enuresis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The subjects and experimenters were blind to which pills were the active medication' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Blades 2001.
| Methods | Design: three group, three period cross‐over trial Purpose: study the effect of oral antioxidant therapy on marginal dry eye | |
| Participants | Patients: out‐patients with marginal dry eyes | |
| Interventions | Placebo: capsules without antioxidants Untreated: no capsules Experimental: capsules with antioxidants (Co‐intervention: NS ) | |
| Outcomes | Glasgow Caledonian University threads phenol read thread test (G‐CUT) Tear thinning time McMonnies dry eye questionnaire Squamous cell metaplasia Goblet cell density | |
| Notes | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Latin squares' |
| Allocation concealment? | Unclear risk | No stated |
| Blinding? Treatment provider | Low risk | 'This trial was double‐masked...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 80 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Blanchard 1990a.
| Methods | Design: four group parallel trial Purpose: study the effect of abbreviated progressive muscle relaxation (PMR) and cognitive therapy on headache | |
| Participants | Patients: out‐patients with tension headache Baseline comparability: NS | |
| Interventions | Placebo: sessions of 'pseudomeditation' Untreated: no sessions Experimental: ‐sessions of abbreviated progressive muscle relaxation plus cognitive therapy ‐sessions of abbreviated progressive muscle relaxation (Co‐intervention: headache medication) | |
| Outcomes | Medication Index Headache Index Frequency of patients with headache reduction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/PMR) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Blanchard 1990b.
| Methods | Design: four group parallel trial Purpose: study the effect of thermal biofeedback and cognitive therapy on headache | |
| Participants | Patients: out‐patients with vascular headache Baseline comparability: NS | |
| Interventions | Placebo: sessions of 'pseudomeditation' Untreated: no sessions Experimental: ‐sessions of thermal biofeedback and cognitive therapy ‐sessions of thermal biofeedback (Co‐intervention: headache medication) | |
| Outcomes | Medication Index Headache Index Frequency of patients with headache reduction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/thermal biofeedback) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 42 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Block 1980.
| Methods | Design: three group parallel trial Purpose: examine the effect of rational emotive therapy on obese persons | |
| Participants | Patients: overweight adults Baseline comparability: yes (weight) | |
| Interventions | Placebo: sessions of deep muscle relaxation & discussions Untreated: no sessions Experimental: sessions with rational emotive therapy (Co‐intervention: information booklet on nutrition) | |
| Outcomes | Overweight (pounds) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/rational emotive therapy) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Bosley 1989.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive self‐management training on hypertension | |
| Participants | Patients: out‐patients with essential arterial hypertension Baseline comparability: NS | |
| Interventions | Placebo: general information on stress with no direct training suggestions Untreated: no training or information Experimental: cognitive self‐management training (Co‐intervention: antihypertensive medication, fixed ordination scheme: NS) | |
| Outcomes | Diastolic blood pressure (mm Hg) Psychological distress Coping style | |
| Notes | Standard deviations (SD) on diastolic blood pressure (mm Hg) not reported. SD estimated from another blood pressure study (Seer 1980: SD ˜ 10 mm Hg) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/training) |
| Blinding? Outcome assessor | Low risk | 'The nurses were blind to the treatment group to which subjects were assigned' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | Standard deviations (SD) on diastolic blood pressure (mm Hg) not reported. SD estimated from another blood pressure study (Seer 1980: SD ˜ 10 mm Hg) |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Bova 1999.
| Methods | Design: three group parallel trial Purpose: evaluate the usefulness of premedication with an oral anticholinergic for relief of pain associated with barium enema | |
| Participants | Patients: patients undergoing a pain inducing medical procedure (barium enema) Baseline comparability: NS | |
| Interventions | Placebo: tablet with no hyoscyamine 15 to 30 minutes before procedure Untreated: no tablet Experimental: tablet with hyoscyamine (Co‐intervention: NS) | |
| Outcomes | Pain (0 to 10 analogue scale) reported immediately after enema Side effects | |
| Notes | Patients with contraindications to hyoscyamine were 'moved to the placebo and no‐treatment group'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15 % |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 70 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation NOT clearly concealed |
Bramston 1985.
| Methods | Design: four group parallel trial Purpose: examine the effect of cognitive and behavioural social skills training with intellectually handicapped adults | |
| Participants | Patients: institutionalised intellectually handicapped adults Baseline comparability: yes (outcomes) | |
| Interventions | Placebo: unstructured training in 'money management' Untreated: no training Experimental: ‐cognitive social skills training ‐behavioural social skills training (Co‐intervention: standard care) | |
| Outcomes | Social skills assessment chart Staff questionnaire on social skills Preschool interpersonal problem solving | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/training) |
| Blinding? Outcome assessor | Low risk | 'None of the raters participated in the training programme or were aware of S‐group allocation' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Brill 1964.
| Methods | Design: six group parallel trial Purpose: to examine the effect of psycho‐ and pharmacotherapy on psychiatric out‐patients | |
| Participants | Patients: out‐patients with neuroses, borderline schizophrenia or personality disorders Baseline comparability: yes (rating scales) | |
| Interventions | Placebo: capsule without meprobamate, phenobarbital or prochlorperazine Untreated: no capsule (waiting list group) Experimental: capsule with ‐meprobamate ‐phenobarbital ‐prochlorperazine (Co‐intervention: NS) | |
| Outcomes | Patient's rating (anxiety, tension, irritability, concentration, alertness, mood, sleep, appetite, general feeling) Rating by relative and patient (getting along, nervousness, happiness, ability to handle personal problems, energy, physical health, presenting symptoms or problems, ability to work, ability to enjoy life, overall condition) MMPI (Minnesota Multi phasic Personality Inventory) Social Worker Interview Evaluation | |
| Notes | Number of patients assigned to untreated group was 34 contrasting the number in the other groups which had 50 to 54 patients. Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Used a double‐blind method of administration of drugs' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out >15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 89 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out >15% or NS |
Brinkhaus 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture in patients with chronic low back pain | |
| Participants | Patients: patients with chronic low back pain Baseline comparability: yes (age, gender, pain intensity) | |
| Interventions | Placebo: acupuncture on sites not regarded acupuncture sites Untreated: no acupuncture Experimental: acupuncture on sites regarded acupuncture sites (Co‐intervention: All patients were allowed to take non‐steroid anti‐inflammatory drugs if necessary) | |
| Outcomes | Pain (VAS) Back function (Funktionsfragebogen Hannover‐Rücken) Global assessment of effect Pain disability Index Emotional aspects of pain (Schmertzempfindungsskala) Depression (Allgemeine Depressionsskala) Quality of life (SF‐36) Number of days with pain Number of days with pain medication | |
| Notes | Patients in the no‐treatment group took medication on 6.3 days whereas the placebo group did so on 4.9 days (weeks 5 to 8) . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘a randomised list was generated using computer software [SAMPSIZE V2.0]’ |
| Allocation concealment? | Low risk | 'Centralised telephone randomization procedure' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupressure) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Low risk | Primary outcome specified in protocol |
| Free of other bias? | Unclear risk | Patients in the no‐treatment group took medication on 6.3 days whereas the placebo group did so on 4.9 days (weeks 5 to 8) . |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 144 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | |
Bullock 2002.
| Methods | Design: three group parallel trial Purpose: to study the effect of auricular acupuncture for alcohol dependence | |
| Participants | Patients: in‐patients with alcohol dependence Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: needling on sites not regarded acupuncture sites Untreated: no needling Experimental: needling on sites regarded acupuncture sites (Co‐intervention: conventional alcohol dependence treatment according to the 'Minnesota Model') | |
| Outcomes | Alcohol use (Timeline Follow‐back) Addiction severity index (ASI) Alcohol dependence scale Breathalyzer Alcohol desire (5‐point Likert scale) Health status (SF36 and Medical status composite score part of ASI) Beck depression inventory Self‐rating anxiety scale | |
| Notes | Outcome not reported so that meta‐analysis is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible. |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 267 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Cabrini 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture in reducing discomfort during fibreoptic bronchoscopy | |
| Participants | Patients undergoing diagnostic bronchoscopy Baseline comparability: yes |
|
| Interventions | Placebo: acupuncture on sites not regarded acupuncture sites Untreated: no acupuncture Experimental: acupuncture on sites regarded acupuncture sites (Co‐intervention: All patients were treated with airway topical anaesthesia) | |
| Outcomes | Discomfort (VAS) Anxiety Heart rate and pulse oximetry |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Acupuncturist aware of treatment group |
| Blinding? Outcome assessor | Unclear risk | Not relevant as outcome was patient‐reported |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐outs not described |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | SD x 1.64 < mean |
| Trial size > 49? | High risk | N = 32 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Camatte 1969.
| Methods | Design: ten group parallel trial Purpose: examine the effect of various forms of drugs and placebo on the pain associated with peptic ulcer disease | |
| Participants | Patients: patients with radiologically confirmed gastric ulcers Baseline comparability: NS | |
| Interventions | Placebo: patches without active substance Untreated: no patches, capsules or injections Experimental: patches, capsules, and injections with various drugs, e.g. bismuth (Co‐intervention: NS) | |
| Outcomes | Pain (number of days in pain) | |
| Notes | Outcome not reported so that meta‐analysis is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Nei Gruppi 2 e 10 il placebo veniva presentato sotto forma di compresse aventi il medesimo aspetto ed il medesimo colore di uno dei medicamenti impiegati nei Gruppi 3 e 9' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible. |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 72 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Camberg 1999.
| Methods | Design: three group three period cross‐over trial Purpose: examine the effect of 'Simulated Presence' (SimPres) on well‐being in nursing home residents with Alzheimer's disease | |
| Participants | Patients: nursing home residents with Alzheimer's disease | |
| Interventions | Placebo: audio tape of a person reading a text that is not personal nor interactive Untreated: no audio tape Experimental: personalised interactive audio tape that contains a telephone conversation with a family member or surrogate. (Co‐intervention: normal nursing home care) | |
| Outcomes | Mood (multidimensional observation scale for elderly) Interest (multidimensional observation scale for elderly) Prevalence of agitated behaviour Prevalence of withdrawn behaviour Agitation scale (Cohen‐Mansfield) Scale for observation of agitation in persons with dementia Positive affect rating scale Facial diagrams of mood (FACE) | |
| Notes | Outcome not reported so that meta‐analysis is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Latin squares' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Low risk | 'Observers were blinded to the study intervention' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible. |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 36 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Canino 1994.
| Methods | Design: three group parallel trial Purpose: examine the effect of behavioural treatment on hypertension | |
| Participants | Patients: out‐patients with primary hypertension Baseline comparability: yes (blood pressure) | |
| Interventions | Placebo: stressful life events were recorded and participants instructed to relax at home some time every day without any formal relaxation training Untreated: waiting list Experimental: 'Behavioural program' (deep muscle relaxation technique and anxiety management training) (Co‐intervention: no) | |
| Outcomes | Diastolic blood pressure (mm Hg) Urinary catecholamine concentration Anxiety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural program) |
| Blinding? Outcome assessor | Low risk | Automatic blood pressure measurement |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 13 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Carbajal 1999.
| Methods | Design: three group parallel trial Purpose: examine the analgesic effect of glucose, sucrose and pacifiers in term infants | |
| Participants | Patients: newborn infants in need of venipunctures Baseline comparability: yes | |
| Interventions | Placebo: 2 minutes before venipuncture sterile water was given to the infant orally with a syringe for 30 seconds Untreated: no sugar, pacifier or water Experimental: ‐glucose in syringe ‐sucrose in syringe ‐pacifier ‐sucrose in syringe and pacifier (Co‐intervention: no) | |
| Outcomes | Pain (Douleur Aiguë du Nouveau‐né (DAN) scale (0 to 10 points)) during venipuncture | |
| Notes | Outcome reported as medians (both placebo and no treatment: 7) and interquartile ranges (6‐10, and 5‐10)). Individual results were reported, and we recalculated the outcome as means and SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘random numbers table' |
| Allocation concealment? | Low risk | ‘treatment allocations inserted in opaque sealed envelopes numbered 1‐150. Investigators were blind to these allocations.' |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Carter 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of allergen avoidance for asthma among inner‐city children | |
| Participants | Patients: Children (5 to 16 years) with asthma Baseline comparability: yes | |
| Interventions | Placebo: mattresses permeable for allergens, ineffective roach traps Untreated: no mattresses Experimental: mattresses impermeable for allergens and effective roach bait (Co‐intervention: NS) | |
| Outcomes | Number of acute visits for asthma (hospitalisation, emergency department visits, unscheduled clinical visits) Allergen level (dust mite, cockroach, cat) Sensitization to common allergens | |
| Notes | Outcome not reported so that meta‐analysis is possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 55 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Carter 2003.
| Methods | Design: three group parallel trial Purpose: examine the effect of unguided self‐help for bulimia nervosa | |
| Participants | Patients: patients with bulimia nervosa Baseline comparability: yes (age, duration of bulimia nervosa) | |
| Interventions | Placebo: cognitive behaviour self‐help therapy Untreated: no self‐help therapy Experimental: non‐specific self‐help therapy (manual on 'self‐assertion for women' and hearing a plausible rationale) (Co‐intervention: NS) | |
| Outcomes | Frequency of binge eating Frequency of compensatory behaviours Eating disorder inventory scores Rosenberg self‐esteem score Beck depression inventory score Inventory of interpersonal problems score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Random numbers table' |
| Allocation concealment? | Low risk | 'numbered opaque sealed envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/self‐guide) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 57 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Cesarone 2001.
| Methods | Design: three group parallel trial Purpose: to evaluate the effect of local (foot) treatment with Essaven gel in subjects with diabetes mellitus and neuropathy without ulcers. | |
| Participants | Patients: out‐patients with diabetes mellitus and neuropathy without ulcers. Baseline comparability: NS | |
| Interventions | Placebo: Placebo‐gel Untreated: No‐treatment Experimental: 1g of Essaven gel applied to foot (Co‐intervention: Standard insulin management) | |
| Outcomes | Laser Doppler flowmetry measuring flux PO2/PCO2 (Kontron analyzer with a Combi sensor) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'the randomization process was controlled by an external statistical controller according to GCP rules' |
| Blinding? Treatment provider | Low risk | 'The trial was a double‐blind, placebo‐controlled study' |
| Blinding? Outcome assessor | Low risk | Objective measurements of flux and PO2/PCO2 |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 23 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Chenard 1991.
| Methods | Design: three group parallel trial Purpose: examine the effect of a back school treatment program and placebo intervention on chronic low back pain | |
| Participants | Patients: out‐patients with chronic low back pain Baseline comparability: yes | |
| Interventions | Placebo: sessions of transcutaneous electrical nerve stimulation (TENS) with the TENS machine off Untreated: no sessions Experimental: sessions of the 'Interactional Back School' Program (Co‐intervention: NS) | |
| Outcomes | Pain (VAS) | |
| Notes | Standard deviation of 10 cm visual analogue pain scales, pain means calculated from F‐test statistic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 28 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Classen 1983.
| Methods | Design: two group, four period cross‐over trial with a third untreated group assessed after the first period Purpose: examine the relationship between sensory suggestibility and treatment effect | |
| Participants | Patients: out‐patients suffering from chronic intermittent headaches Baseline comparability: pretreatment headache scores | |
| Interventions | Placebo: tablet without metamizole Untreated: no tablet Experimental: tablet with metamizole (Co‐intervention: NS) | |
| Outcomes | Pain (headache scores, 4‐item 6‐point scale) Sensitivity values (d') | |
| Notes | The first period was considered a parallel trial. Results from later periods were disregarded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 30 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Colker 1999.
| Methods | Design: three group parallel trial Purpose: examine the effect of a combination of Citrus aurantium extract, caffeine, and St. John's wort on obesity | |
| Participants | Patients: overweight individuals (body mass index > 25 kg per square meter) Baseline comparability: clinically relevant differences in age and body weight were not statistically significant | |
| Interventions | Placebo: maltodextrin capsules Untreated: no capsules Experimental: capsules with a compound of Citrus aurantium extract, caffeine, and St. John's wort (Co‐intervention: dietary and exercise program) | |
| Outcomes | Weight Percent body fat Fat mass Basal metabolic rate Profile of Mood States questionnaire (POMS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Using a double‐masked, randomized, placebo‐controlled protocol...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 11 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Conn 1986.
| Methods | Design: three group parallel trial Purpose: compare the efficacy of transcutaneous electrical nerve stimulation (TENS) with sham TENS on postoperative pain | |
| Participants | Patients: postoperative in‐patients (after appendicectomy) Baseline comparability: operative course not compared. | |
| Interventions | Placebo: TENS with machine off Untreated: no TENS Experimental: TENS with machine on (Co‐intervention: analgesics on demand) | |
| Outcomes | Pain (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sealed envelope' |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Corver 2006.
| Methods | Design: three group parallel trial Purpose: study the effect of house dust mite impermeable mattress covers on the development of respiratory symptoms, atopic eczema, and mite‐sensitization in children born to mothers with allergy | |
| Participants | Patients: pregnant women in third trimester with allergy Baseline comparability: yes (several factors), no for gender of children | |
| Interventions | Placebo: house dust mite permeable mattress (cotton) covers Untreated: no mattress covers Experimental: house dust mite impermeable mattress (polyester‐cotton) covers (Co‐intervention: NS) | |
| Outcomes | Wheezing at least once Recurrent wheezing Night cough without a cold Runny nose without a cold Atopic dermatitis House dust mite allergen level on bed Total IgE House dust mite specific IgE | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...participants were randomly allocated... in a double blind fashion' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 695 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Costello 2006.
| Methods | Design: three group parallel trial Purpose: study the effect of ethyl vinyl chloride spray on the pain associated with cannulation in children | |
| Participants | Patients: children (9 to18 years) undergoing cannulation Baseline comparability: yes (age, gender ratio) | |
| Interventions | Placebo: isopropyl alcohol spray Untreated: no spray Experimental: ethyl vinyl chloride spray (Co‐intervention: no) | |
| Outcomes | Pain intensity VAS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number allocation' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The investigators and nursing staff performin IV cannulation were blinded to the cannister's contents...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 90 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Not clearly concealed allocation |
Coyne 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of transcutaneous electrical nerve stimulation (TENS) on pain | |
| Participants | Patients: persons having a venepuncture to give blood Baseline comparability: yes | |
| Interventions | Placebo: placebo TENS Untreated: no TENS or placebo TENS Experimental: TENS (Co‐intervention: NS) | |
| Outcomes | Pain (VAS) | |
| Notes | Standard deviation of 10 cm visual analogue pain scale, means calculated from F‐test statistic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 42 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Crosby 1994.
| Methods | Design: four group parallel trial Purpose: examine the effect of iron supplement on acute blood loss anaemia after surgery | |
| Participants | Patients: postoperative out‐patients Baseline comparability: yes for age and sex. | |
| Interventions | Placebo: NS Untreated: no placebo or iron Experimental: iron supplement at two doses (Co‐intervention: no) | |
| Outcomes | Hb concentration (mg Hgl/dl) (59 days after surgery) Haematocrit Serum‐iron Serum‐ferritin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer‐generated table of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Consenting patients were randomized into four groups in a double‐blind fashion...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 59 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Cupal 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of relaxation + guided imagery (and attention‐placebo) on physical and psychological aspects of rehabilitation following orthopedic surgery | |
| Participants | Patients: patients having had performed anterior cruciate ligament reconstruction Baseline comparability: NS | |
| Interventions | Placebo: over a period of 6 months, 10 sessions of 30 to 40 minutes of encouragement, support and reminders to visualise a peaceful scene daily Untreated: no sessions Experimental: sessions of structured relaxation and guided imagery (Co‐intervention: physical therapy) | |
| Outcomes | Pain (11 point scale, 0 to 10) Re‐injury anxiety Knee strength (ratio of injured knee to the uninjured knee) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'random block' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/relaxation) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Davidson 1980.
| Methods | Design: five group parallel trial Purpose: examine the effect of psychological treatments on compulsive nail biting | |
| Participants | Patients: out‐patients regarding nail biting a serious problem and a source of personal shame Baseline comparability: yes | |
| Interventions | Placebo: factual information on nails, diseases of nails and of theories about pathological nail biting Untreated: waiting list Experimental: two types of psychological intervention separately, and one combined. (Co‐intervention: NS) | |
| Outcomes | Length of nails (mm) and estimated frequency of nail biting (per day) Estimated control over nail biting Cosmetic appearance rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/psychological intervention) |
| Blinding? Outcome assessor | Low risk | 'Post‐test and follow‐up sessions were conducted by an experimenter who had no knowledge of the groups to which subjects had been assigned during treatment' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
De Sanctis 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of Essaven gel in patients with venous microangiopathy and venous ulceration | |
| Participants | Patients: out‐patients with microangiopathy and venous ulcers Baseline comparability: yes (age, gender ratio) | |
| Interventions | Placebo: Gel not containing Essaven Untreated: no gel Experimental: Gel containing Essaven (Co‐intervention: elastic stockings) | |
| Outcomes | Ulcer healing rates Total symptom score (based on Pain, edema, alternation in social life and working handicaps, cost of care, deambulation) Microcirculatory parameters (flux, CO2, O2) | |
| Notes | No patient in either group had healed ulcers. For computing reasons we have entered data as one patient in each group had a healed ulcer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'The randomization process was controlled by an external statistical controller according to GCP rules' |
| Blinding? Treatment provider | Low risk | 'Operators were unaware of the contents of the tube, which was numbered' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 19 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Defrin 2005.
| Methods | Design: six group parallel trial Purpose: study the effect of segmental versus innocuous electrical stimulation for chronic pain relief | |
| Participants | Patients: out‐patients needing screening flexible endoscopy Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: stimulation with interferential current (IF) device off Untreated: no IF device treatment Experimental: stimulation with IF device on (Co‐intervention: analgesic medication. Patients were asked not to change regime during the study) | |
| Outcomes | Pain (VAS) Pain relief (%) Morning stiffness (VAS) Range of motion (Goniometry) Pain threshold | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/electrical stimulus device) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 17 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Dibble 2007.
| Methods | Design: three group parallel trial Purpose: study the effect of acupressure on chemo‐therapy induced nausea | |
| Participants | Patients: cancer patients receiving chemotherapy Baseline comparability: yes | |
| Interventions | Placebo: needling in S13 point theoretically inert for nausea Untreated: no needling Experimental: needling in P6 (Co‐intervention: antiemetic drugs) | |
| Outcomes | Nausea intensity (NRS) Rhodes Index of Nausea (3 items) Rhodes index of nausea and vomiting (1 item) Functional status (NRS) State‐Trait Anxiety Inventory | |
| Notes | Data provided from authors: mean nausea NRS (0 to 10) evening after getting chemotherapy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The researchers endeavoured to keep the research assistant masked as to the active point' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. Data provided from authors |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 100 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Ditto 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of applied muscle tension on blood donation reactions | |
| Participants | Patients: English speaking blood donors Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: applied muscle tension training for 2 minutes Untreated: no training Experimental: applied muscle tension training for 15 minutes (Co‐intervention: no) | |
| Outcomes | Blood donations reactions inventory score Proportion of cases in which donation chairs were reclined Proportion of full blood portion donated Doner's estimate of the probability of giving blood again Pain Anxiety Heart rate Blood pressure | |
| Notes | Trialists assumed that at least 5 minutes of applied muscle tension training was needed for an effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/applied muscle tension) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 389 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Ditto 2006.
| Methods | Same as Ditto 2003 | |
| Participants | Patients: french speaking blood donors Baseline comparability: yes (age, gender) | |
| Interventions | Same as Ditto 2003 | |
| Outcomes | Same as Ditto 2003 | |
| Notes | Same trial as Ditto 2003 but results for French speaking donors reported separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/applied muscle tension) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 295 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Doty 1975.
| Methods | Design: five group parallel trial Purpose: to examine the effect of social skills training on the interpersonal interaction of chronic psychiatric patients | |
| Participants | Patients: chronic psychiatric in‐patients Baseline comparability: probably (stratified by level of daily interaction) | |
| Interventions | Placebo: sessions with transactional game followed by lectures by the therapist Untreated: no sessions Experimental: ‐sessions with social skills training (role playing) ‐sessions with incentive condition (re‐enforcement by reward) ‐combination (Co‐intervention: yes, most patients received psychotropic drugs; type, dose and group distribution NS) | |
| Outcomes | % alone: the % of the time the individual was observed more than 4 feet away from another person. % silent: the % of the time the person was silent in a group discussion | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/social skills training) |
| Blinding? Outcome assessor | Low risk | 'The observers were blind to both the nature of the dependent variable to be extracted from their recordings and the group assignments...' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Double 1993.
| Methods | Design: three group, three period cross‐over trial Purpose: to examine the effect of discontinuation of antiparkinsonian medication in patients maintained on neuroleptics | |
| Participants | Patients: psychiatric in‐patients on concomitant antiparkinsonian and neuroleptic medication for over one year Baseline comparability: not relevant | |
| Interventions | Placebo: capsules with no antiparkinsonian medication Untreated: no capsules Experimental: capsules with antiparkinsonian medication (type and dose individual but fixed through trial) (Co‐intervention: yes, neuroleptics, fixed dose through trial except for three patients) | |
| Outcomes | Number of patients with relapse of parkinsonian symptoms (= need for escape medication) Extrapyramidal Symptom Rating Scale (ESRS) | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The medication periods were assigned blindly...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 44 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Dundee 1986.
| Methods | Design: three group parallel trial Purpose: examine the prophylactic effect of acupuncture on perioperative nausea | |
| Participants | Patients: surgical in‐patients undergoing minor gynaecological procedures Baseline comparability: 'broadly comparable', but no data presented | |
| Interventions | Placebo: needling on the lateral elbow crease, a point that is not on any recognized acupuncture line Untreated: no needling Experimental: needling at the P6 point (Neiguan) (Co‐intervention: 10 mg nalbuphene as routine premedication) | |
| Outcomes | Number of patients with nausea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Low risk | 'These assessments were performed by an observer who was unaware of which patients had undergone acupuncture' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Elliott 1978.
| Methods | Design: four group parallel trial Purpose: examine the effect of a multiple‐component treatment approach on smoking reduction | |
| Participants | Patients: smokers Baseline comparability: yes | |
| Interventions | Placebo: non‐directive discussions Untreated: no discussions Experimental: ‐rapid smoking (smoked every 6 seconds until unable to continue) ‐package treatment (rapid smoking, applied relaxation, covert sensitization, systematic desensitization, self‐reward and punishment etc) (Co‐intervention: NS) | |
| Outcomes | Number of abstinent smokers Mean number of cigarettes smoked / day | |
| Notes | The trial consisted of a primary intervention phase and a secondary booster phase. Only the allocation to the booster treatment was explicitly described as random. Contact with authors clarified that allocation in the primary phase was also random. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/package treatment) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Erdogmus 2007.
| Methods | Design: three group parallel trial Purpose: study the effect of physiotherapy | |
| Participants | Out‐patients having had a disc herniation operation Baseline comparability: yes (except for body mass index) | |
| Interventions | Placebo: sessions with neck massage Untreated: no sessions Experimental: sessions with physiotherapy‐based rehabilitation (Co‐intervention: yes) | |
| Outcomes | Low Back Pain Rating Scale Overall satisfaction Socioeconomic parameters State Trait Anxiety Inventory | |
| Notes | SDs were obtained from the reported CIs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘block randomization (SAS) was performed by the Department of Medical Statistics’ |
| Allocation concealment? | Low risk | 'sequentially numbered, sealed opaque envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/physiotherapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 80 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Espie 1989.
| Methods | Design: six group parallel trial Purpose: examine the effect of psychological treatments on chronic insomnia | |
| Participants | Patients: out‐patients suffering from chronic sleep‐onset insomnia Baseline comparability: yes (age, sex, duration of insomnia) | |
| Interventions | Placebo: imaginary belief treatment: neutral images paired with bed time activities. Untreated: waiting list Experimental: ‐relaxation therapy ‐stimulus control ‐paradoxical intervention ‐tailored therapy condition (reported in another publication) (Co‐intervention: hypnotics, fixed ordination and withdrawal scheme, compliance NS) | |
| Outcomes | Sleep latency (min) Sleep quality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'predetermined list of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/psychological intervention) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Etringer 1982.
| Methods | Design: three group parallel trial Purpose: examine the effect of 'participant modelling' (snake handling) therapy on snake phobia | |
| Participants | Patients: individuals who were unable to hold a snake for 10 sec Baseline comparability: yes | |
| Interventions | Placebo: sessions of 'graduated subliminal modelling'. Patients were exposed to blank slides and told they were subliminal pictures of snakes. Untreated: no sessions Experimental: sessions of 'participant modelling' (Co‐intervention: NS) | |
| Outcomes | Behavioral avoidance test (18 successive steps of snake interaction tasks). Fear arousal accompanying approach. Anticipatory fear. Self‐efficacy expectations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/participant modelling) |
| Blinding? Outcome assessor | Low risk | 'The assessors were kept blind as to each subject's particular treatment condition' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 25 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Etter 2002.
| Methods | Design: three group parallel trial Purpose: test the effect of nicotine replacement therapy, and placebo, in reducing cigarette consumption in smokers willing to intend to reduce number of smoked cigarettes but not to quit | |
| Participants | Patients: cigarette smokers unwilling to quit, but intending to reduce smoking by half Baseline comparability: not for gender (54% male in nicotine group and 44% in no treatment group); yes for age, cigarette consumption, intention to reduce consumption (and several other variables) | |
| Interventions | Placebo: transdermal patch, gum, or inhaler without nicotine (and information leaflet) mailed every other week at the choice of the patient for 6 months Untreated: no transdermal patch, gum, inhaler or leaflet Experimental: transdermal patch, gum, inhaler with nicotine (and information leaflet) (Co‐intervention: information booklet after three months) | |
| Outcomes | Mean number of cigarettes smoked per day after 6 months Score of smoking intensity (0 to 100) Score of total smoke inhalation per day (0 to 10) Mean number of reduction of smoked cigarettes per day Smoking cessation rate | |
| Notes | Not 1:1 randomisation. The combination of baseline inequality for gender, and lack of clear description of concealment of allocation indicate possible selection bias. In the no treatment group 7% did not provide information on smoking habits; in the placebo group it was 3%. For these individuals the baseline values were computed as the end result. Because the dropout rate was higher in the no‐treatment group this may have resulted in an inflated estimate of the mean number of smoked cigarettes, and thus a too optimistic estimate of the effect of placebo on smoking reduction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘computer generated list of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/drug) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | High risk | See notes |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 658 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Faas 1993.
| Methods | Design: three group parallel trial Purpose: examine the prophylactic effect of physical exercise and advice on daily living on recurrence of acute low back pain | |
| Participants | Patients: out‐patients visiting a GP with acute low back pain Baseline comparability: yes | |
| Interventions | Placebo: sessions with ultrasound at lowest possible frequency Untreated: no sessions (standard therapy) Experimental: sessions with exercise and advice on daily living (Co‐intervention: analgesics on demand, dose NS) | |
| Outcomes | Number of patients with recurrent low back pain episodes Duration of recurrent low back pain episodes Functional health status Mobility problems Influence on daily life In‐between consultation of the GP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sealed envelope' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/exercise) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 317 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Fanti 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of acupuncture on discomfort, pain and anxiety during colonoscopy | |
| Participants | Patients: patients scheduled to undergo colonoscopy Baseline comparability: yes (age, pre‐colonoscopy anxiety) | |
| Interventions | Placebo: needling and electrical stimulation on sites not regarded analgesic acupuncture sites Untreated: no needling Experimental: needling and electrical stimulation on sites regarded analgesic acupuncture sites (L14, S36, SP6, SP9) (Co‐intervention: midazolam 15 minutes before procedure and as required) | |
| Outcomes | Escape medication (midazolam) Pain at 4 times during the procedure (5‐point scale) Procedure acceptability (5‐point scale) Patient satisfaction Technical difficulty of the procedure (physician and nurse) Satisfaction with sedation (physician and nurse) Total procedural time | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘computer‐generated sequence of numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Fiorellini 2005.
| Methods | Design: three group parallel trial Purpose: evaluate the efficacy of bone induction for the placement of dental implants using recombinant human bone morphogenetic protein‐2. | |
| Participants | Patients: patients requiring local alveolar ridge preservation/augmentation of buccal wall defects following extraction of maxillary teeth. Baseline comparability: NS | |
| Interventions | Placebo: bioabsorbable collagen sponge (ACS) alone Untreated: No‐treatment Experimental: recombinant human bone morphogenetic protein‐2 delivered on a ACS. (Co‐intervention: preoperative antibiotics and 0.12% chlohexidine rinse (15ml)) | |
| Outcomes | Alveolar bone height and bone width (CT scan) Alveolar bone volume (CT scan) Bone density (CT scan) Bone biopsy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Two sequential cohorts of 40 patients each were randomized in a double‐masked manner...' |
| Blinding? Outcome assessor | Low risk | '... three independent masked CT scan reviewers...' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 37 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Fisher 2006.
| Methods | Design: three group parallel trial Purpose: study the feasibility of running a randomised trial aimed at evaluating specific and non‐specific effects in homeopathy | |
| Participants | Out‐patients with dermatitis Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: lactose pills Untreated: no pills Experimental: homeopathic pills (Co‐intervention: NS) | |
| Outcomes | Overall symptoms Skin symptoms Itching Sleep DLQI (dermatology life quality index) Use of steroids | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'randomisation list' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'double‐blind placebo' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Forster 1994.
| Methods | Design: three group parallel trial Purpose: examine the effect of transcutaneous nerve stimulation (TENS) on pain | |
| Participants | Patients: postoperative in‐patients (coronary artery bypass surgery) Baseline comparability: yes | |
| Interventions | Placebo: TENS with machine turned off Untreated: no TENS Experimental: TENS with machine turned on (Co‐intervention: analgesics as deemed appropriate by staff) | |
| Outcomes | Pain (verbal numerical scale) Lung function parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 30 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Foster 2004.
| Methods | Design: three group parallel trial Purpose: study the effect of the Trager approach on chronic headache | |
| Participants | Patients: outpatients with chronic headache Baseline comparability: yes | |
| Interventions | Placebo: attention treatment by physician including physical exam (15 to 20 minutes) Untreated: no treatment Experimental: Trager approach (movement based educational process to increase body awareness, learn relaxation skills, and practise pain‐free, balanced movement) (Co‐intervention: medication) | |
| Outcomes | The headache quality of life instrument Headache frequency, duration, and intensity Medication use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/Trager) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 18 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Foster 2007.
| Methods | Design: three group parallel trial Purpose: study the effect of acupuncture on osteoarthrosis of the knee | |
| Participants | Patients: older outpatients with osteoarthrosis of the knee Baseline comparability: yes | |
| Interventions | Placebo: needling with a non‐penetrating blunt needle Untreated: no needling Experimental: needling at with a proper acupuncture needle (Co‐intervention: exercise and advice) | |
| Outcomes | WOMAC pain sub‐scale WOMAC scale Function and general improvement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'a computed generated randomisation'. We assume 'a computer generated randomisation' |
| Allocation concealment? | Low risk | After inclusion of patients into the trials the 'physiotherapist telephoned an administrator at the research centre to ... receive ... a computed generated randomisation group.' |
| Blinding? Treatment provider | High risk | Acupuncturist knew type of acupuncture |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient‐reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop out <15% |
| Free of selective reporting? | Low risk | Protocol published. No sign of outcome selection bias for the primary outcome |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | SD x 1.67 >mean |
| Trial size > 49? | Low risk | N = 217 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three criteria fulfilled |
Frank 1990.
| Methods | Design: five group parallel trial Purpose: examine the effect of psychotherapy, imipramine and placebo on relapse of depression | |
| Participants | Patients: stable out‐patients with unipolar depression having improved markedly after imipramine medication and psychotherapy Baseline comparability: yes | |
| Interventions | Placebo: continuous treatment with ‐tablets containing no imipramine (content NS) plus psychotherapy sessions ‐tablets containing no imipramine (content NS) plus visits to a medication clinic Untreated: continuous treatment with psychotherapy but without any tablets Experimental: continuous treatment with ‐imipramine tablets and psychotherapy sessions ‐imipramine tablets and visits to a medication clinic ‐continuous treatment with imipramine tablets without psychotherapy sessions (Co‐intervention: NS) | |
| Outcomes | Number of patients with relapse of depression Time to recurrence of depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...both the patients and the members of their treatment team remained blind to whether they were receiving active medication or placebo' |
| Blinding? Outcome assessor | Low risk | Partly blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 52 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Frankel 1978.
| Methods | Design: three group parallel trial Purpose: examine the effect of biofeedback on arterial hypertension | |
| Participants | Patients: out‐patients with essential arterial hypertension Baseline comparability: yes | |
| Interventions | Placebo: sessions of discussions of past and present problems with no behavioural techniques taught Untreated: no sessions Experimental: training sessions for behavioural techniques (Co‐intervention: NS) | |
| Outcomes | Diastolic blood pressure (mm Hg) Multiple personality, depression and activity tests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number table' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/biofeedback) |
| Blinding? Outcome assessor | Low risk | '... nurses (who was blind to the patient's experimental status)...' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 15 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Frega 1994.
| Methods | Design: three group parallel trial Purpose: to evaluate the pain caused by laser vaporization of intraepithelial cervical neoplasia | |
| Participants | Patients: women with intraepithelial cervical neoplasia Baseline comparability: NS | |
| Interventions | Placebo: tablet without naproxen Untreated: no tablet Experimental: tablet with naproxen (Co‐intervention: NS) | |
| Outcomes | Pain (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 42 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Fuchs 1977.
| Methods | Design: three group parallel trial Purpose: examine the effect of self‐control behaviour therapy on depression | |
| Participants | Patients: female out‐patients with depression as defined by a multiple cut‐off procedure of the Minnesota Multi phasic Personality Inventory (D more than 69) Baseline comparability: yes (depression scores) | |
| Interventions | Placebo: sessions of discussion on past and present problems with no behavioural techniques taught Untreated: no sessions Experimental: sessions of behavioural techniques training (Co‐intervention: NS) | |
| Outcomes | Beck Depression inventory Minnesota Multi phasic Personality Inventory (depression scale and total evaluation) Group interaction activity and response elicitation Pleasant events activity and reinforcement potential Self‐evaluation Common associates test Concepts test | |
| Notes | The standard deviation (SD) on the improvement of the mean on Beck Depression Inventory was not reported. The SD was calculated from a F‐test statistic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behaviour therapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Gluckman 1980.
| Methods | Design: three group parallel trial Purpose: study the effect of a remedial program on visual‐motor perception in children with spina bifida | |
| Participants | Patients: Children with spina bifida who were able to respond to verbal and paper‐and‐pencil task Baseline comparability: yes | |
| Interventions | Placebo: sessions with stimulating environment (jig‐saw puzzles, drawing, colouring, reading etc) Untreated: no sessions Experimental: sessions with a program of development of visual perception (Co‐intervention: NS) | |
| Outcomes | Development test of visual perception (five sub‐tests) | |
| Notes | Outcome not reported so that meta‐analysis is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible. |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Godfrey 1973.
| Methods | Design: three group, three period cross‐over trial Purpose: to examine the effect of placebo on exercise induced asthma | |
| Participants | Patients: children with bronchial asthma Baseline comparability: not relevant | |
| Interventions | Placebo (according to active drug): ‐injections of saline ‐inhalations of saline ‐inhalations of lactose with sodium sulphate Untreated: no injections or inhalations Experimental: ‐inhalations of salbutamol ‐injections of atropine ‐inhalation of cromoglycate (Co‐intervention: NS) | |
| Outcomes | Fall in % peak expiratory flow (PEF) rate Number of patients with a drop in PEF rate of at least 15% | |
| Notes | Data originally came from three small cross‐over trials. The outcome data was not available from the first period only, and was calculated as deriving from one parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15 % or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | High risk | Data originally came from three small cross‐over trials. The outcome data was not available from the first period only, and was calculated as deriving from one parallel group trial. |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 88 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15 % or NS |
Goldstein 2000.
| Methods | Design: three group parallel trial Purpose: study the effect of eye movement desensitization and reprocessing (EMDR) on panic disorder with agoraphobia | |
| Participants | Patients: out‐patients with panic disorder with agoraphobia Baseline comparability: NS | |
| Interventions | Placebo: sessions with a credible attention‐placebo ('association and relaxation therapy') Untreated: no sessions Experimental: sessions with EMDR (Co‐intervention: anxiolytic drugs in moderate doses) | |
| Outcomes | Frequency of panic attacks Daily and weekly expectancy of panic attack Daily highest anxiety Daily average anxiety The agoraphobic cognitions questionnaire Body sensations questionnaire Brief body sensations interpretations Panic appraisal inventory The mobility inventory Beck depression inventory Beck anxiety inventory Brief symptom inventory Social adjustment scale (self‐report) Distress questionnaire | |
| Notes | After completion of waiting‐list period the no treatment group was randomised to placebo and active treatment. Results from the placebo group also included patients who originally were in the no‐treatment group. Contact with the authors made it clear that the data from the originally randomised patients had been lost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/EMDR) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. Contact to the authors made it clear that the data from the originally randomised patients had been lost. |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Goodenough 1997.
| Methods | Design: three group parallel trial Purpose: examine the effect of placebo medication and suggestion on acute pain associated with venepuncture | |
| Participants | Patients: children undergoing venepuncture in a hospital setting Baseline comparability: NS | |
| Interventions | Placebo: cream with no analgesic component and no suggestion of analgesic effect Untreated: no cream Experimental: cream with no analgesic component but with the suggestion of analgesic effect (Co‐intervention: NS) | |
| Outcomes | Pain (facial pain scale) Anxiety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/placebo+suggestion) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 78 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Gracely 1979.
| Methods | Design: three group parallel trial Purpose: examine the effect of placebo medication and naloxone on acute pain | |
| Participants | Patients: postoperative in‐patients after extraction of molars Baseline comparability: NS | |
| Interventions | Placebo: injection with naloxone vehicle Untreated: no injection Experimental: injection with naloxone (Co‐intervention: NS) | |
| Outcomes | Pain | |
| Notes | Data reported in a way not extractable for meta‐analysis. Original data lost (personal communication Gracely RH) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random table generated by a shuffle program' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Two groups of 12 subjects received double‐blind intravenous injections of either 10mg naloxone or naloxone vehicle (placebo)...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | No protocol available. Data reported in a way not extractable for meta‐analysis. Original data lost (personal communication Gracely RH) |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Gracely 1983.
| Methods | Design: three group parallel trial Purpose: examine the influence of hidden infusion of naloxone on the effect of fentanyl and placebo in acute pain | |
| Participants | Patients: postoperative in‐patients after extraction of molars Baseline comparability: NS | |
| Interventions | Placebo: injection with saline Untreated: no injection Experimental: injection with fentanyl (Co‐intervention: pre‐treatment hidden infusion of either naloxone or naloxone vehicle) | |
| Outcomes | Pain (McGill pain rating index) | |
| Notes | The trial report does not explicitly state that allocation was random, however personal communication with authors established that this is likely to have been the case. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random table generated by a shuffle program' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Hidden infusions of naloxone (10 mg) or naloxone vehicle were administered double‐blind...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 29 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Grammer 1984.
| Methods | Design: three group parallel trial Purpose: examine whether patients with allergic rhinitis would have lower or higher symptom scores depending upon the presence of asthma | |
| Participants | Patients: out‐patients with allergic, ragweed rhinitis Baseline comparability: NS | |
| Interventions | Placebo: injections without ragweed (caramelised glucose) Untreated: no injections Experimental: injections with polymerised ragweed (Co‐intervention: histamine, dosage part of composite outcome score) | |
| Outcomes | Rhinitis symptom‐medication scores (sneeze, nasal congestion, histamine medication) | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 31 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
GRECHO 1989.
| Methods | Design: four group parallel trial Purpose: examine the effect of two homoeopathic preparations on postoperative ileus | |
| Participants | Patients: postoperative in‐patients (after abdominal surgery) Baseline comparability: yes (sex, age, type and duration of operation) | |
| Interventions | Placebo: granule with no homoeopathic content Untreated: no granule Experimental: homoeopathic preparations 'opium' or 'raphanus' (Co‐intervention: use of laxatives higher in placebo than untreated) | |
| Outcomes | Time from abdominal closure to first stool and first flatus (hours) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... in a controlled, double‐blind therapeutic trial...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 300 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop out > 15% or NS |
Guglielmi 1982.
| Methods | Design: three group parallel trial Purpose: examine the effect of skin temperature biofeedback on Raynaud's disease | |
| Participants | Patients: out‐patients with primary Raynaud's disease Baseline comparability: yes (age and severity of attack) | |
| Interventions | Placebo: biofeedback training focused at relaxing forehead muscles Untreated: no training Experimental: biofeedback training focused at increasing finger temperature (Co‐intervention: no ) | |
| Outcomes | Number of patients with attacks Duration of attacks Severity of attacks Extent of hand involvement Number of symptoms experienced Pain Impairment Length of time spent in laboratory to relieve the attack Amount of relief obtained from laboratory training | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... a double‐blind design was adopted' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hall 1974.
| Methods | Design: four group parallel trial Purpose: examine the effect of two self‐management treatments on obese persons | |
| Participants | Patients: overweight adults Baseline comparability: NS | |
| Interventions | Placebo: sessions with relaxation training Untreated: no sessions Experimental: ‐sessions with combined self‐management technique: based on re‐enforcement principles ‐sessions with simple self‐management technique: based on re‐enforcement principles (Co‐intervention: information booklet on nutrition) | |
| Outcomes | Weight loss (percentage of initial body weight) | |
| Notes | 33 out of 84 patients were college students. The standard deviation (SD) of the mean % reduction of body weight was not reported. The SD was calculated from the reported F‐test statistic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/self‐management technique) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 45 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hallström 1988.
| Methods | Design: three group parallel trial Purpose: examine the effect of propranolol on tranquillizer dependence | |
| Participants | Patients: out‐patients who had not succeeded in stopping tranquilliser medication despite attempts Baseline comparability: yes for diazepam intake | |
| Interventions | Placebo: tablets without propranolol Untreated: no tablets Experimental: tablets of propranolol (Co‐intervention: NS) | |
| Outcomes | Abstinence 50% reduction of medication Time to abstinence Pulse rates | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Patients were also treated... under double blind conditions' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 18 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hanson 1976.
| Methods | Design: five group parallel trial Purpose: examine the effect of self‐management behavioural therapy on weight loss | |
| Participants | Patients: media‐recruited obese persons Baseline comparability: yes (weight) | |
| Interventions | Placebo: sessions of deep muscle relaxation therapy Untreated: no sessions (waiting list) Experimental: sessions with ‐behavioural training with standard therapist contact ‐behavioural therapy with low therapist contact ‐behavioural therapy with high therapist contact (Co‐intervention: antihypertensive medication, fixed ordination scheme: NS) | |
| Outcomes | Weight loss (percentage of initial body weight) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural therapy) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 21 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hargreaves 1989.
| Methods | Design: three group parallel trial Purpose: examine the effect of transcutaneous electrical nerve stimulation (TENS) on acute pain associated with wound dressing | |
| Participants | Patients: postoperative patients needing surgical wound dressing. Baseline comparability: NS | |
| Interventions | Placebo: TENS with no current passing to electrodes Untreated: no TENS Experimental: TENS with current passing to electrodes (Co‐intervention: analgesics administered at the same rate for all groups) | |
| Outcomes | Pain (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...the experimenter was unaware of the assigned intervention during the initial preparation of each subject for the study' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Harrison 1975.
| Methods | Design: three group parallel trial Purpose: examine the effect of doxycycline treatment on infertility | |
| Participants | Patients: couples suffering from infertility of unknown origin Baseline comparability: yes (age, duration of infertility) | |
| Interventions | Placebo: tablet without doxycycline Untreated: no tablet Experimental: tablet with doxycycline (Co‐intervention: NS ) | |
| Outcomes | Number of women who got pregnant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Double‐blind Trial with Doxycycline' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 58 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Hashish 1986.
| Methods | Design: five group parallel trial Purpose: examine the effect of ultrasound and placebo on pain and swelling | |
| Participants | Patients: postoperative in‐patients (after removal of impacted third molars) Baseline comparability: NS | |
| Interventions | Placebo: ultrasound with machine off Untreated: no ultrasound Experimental: ultrasound at three levels of intensity (Co‐intervention: analgesics, fixed ordination scheme) | |
| Outcomes | Pain (VAS) and facial swelling (cubic cm) Trismus C‐reactive protein | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... in a double‐blind controlled study...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 75 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Hashish 1988.
| Methods | Design: five group parallel trial Purpose: examine the mechanism of placebo effects in ultrasound treatment | |
| Participants | Patients: postoperative in‐patients (after removal of impacted third molars) Baseline comparability: NS | |
| Interventions | Placebo a: ultrasound without massage and machine off Placebo b: ultrasound with massage and machine off Untreated: no ultrasound or massage Experimental a: ultrasound without massage and machine on Experimental b: ultrasound with massage and machine on (Co‐intervention: analgesics on demand) | |
| Outcomes | Pain (VAS) and facial swelling (cubic cm) C‐reactive protein Trismus Plasma cortisol Anxiety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number tables' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'In a placebo‐controlled double‐blind clinical trial...' |
| Blinding? Outcome assessor | Low risk | 'All assessments and measurements were performed by an investigator (I.H.) who was unaware of the treatment group to which the patient has been allocated' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Hawkins 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of hypnosis and placebo on nausea and vomiting in children receiving chemotherapy | |
| Participants | Patients: in‐patients with cancer in need of nausea‐inducing chemotherapy Baseline comparability: chemotherapy type and dose NS | |
| Interventions | Placebo: sessions with psychologists who talked with the children about what they liked Untreated: no sessions Experimental: sessions with hypnosis (Co‐intervention: NS) | |
| Outcomes | Nausea (VAS) Vomiting | |
| Notes | The number of patients in each group was not reported. The number was estimated to n = 10 (total patient number 30 divided by 3 groups) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/hypnosis) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. The number of patients in each group was not reported. The number was estimated to n=10 (total patient number 30 divided by 3 groups) |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Heinzl 1981.
| Methods | Design: four group parallel trial Purpose: examine the effect of prostaglandin E2‐alpha on cervical dilatation | |
| Participants | Patients: in‐patients undergoing first trimester abortion Baseline comparability: yes | |
| Interventions | Placebo: intra‐cervically placed gel without prostaglandin Untreated: no gel or tablet Experimental: ‐intra‐cervically placed gel with prostaglandin E2‐alpha ‐tablet with prostaglandin E2‐alpha (Co‐intervention: Valium on fixed scheme and analgesic on demand, distribution NS) | |
| Outcomes | Successful cervical dilatation (number of patients with dilatation of cervical os > Hegar no. 6) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Low risk | 'The investigating doctor had no idea what treatment, if any, the patients had received' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 262 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Helms 1987.
| Methods | Design: four group parallel trial Purpose: examine the effect of acupuncture on dysmenorrhoea | |
| Participants | Patients: out‐patients with primary dysmenorrhoea Baseline comparability: yes, however somewhat lower pretreatment pain scores. | |
| Interventions | Placebo: needling at points not recognized as acupuncture points (lateral thighs and arms) Untreated a): no needling, minimum contact (our control group) b): no needling, visit by investigator Experimental: needling in correct acupuncture points (Co‐intervention: analgesics) | |
| Outcomes | Pain (scores) Use of analgesic medication Proportion of improved patients | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers table' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 22 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hong 1993.
| Methods | Design: six group parallel trial Purpose: examine the effect of different physical medicine modalities on pain threshold of a myofascial trigger point | |
| Participants | Patients: out‐patients with myofascial pain syndrome Baseline comparability: yes (sex, age, pain duration) | |
| Interventions | Placebo: sham ultrasound Untreated: no ultrasound Experimental: ‐spray and stretch ‐hydrocollator ‐ultrasound ‐massage (Co‐intervention: NS) | |
| Outcomes | Pain (Index of threshold change (ITS), based on measurements with a pressure algometer) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 37 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hossmann 1981.
| Methods | Design: four period, four group, cross‐over trial Purpose: examine the effect of hospitalisation (habituation to the medical environment and rest in hospital bed) and placebo on arterial hypertension | |
| Participants | Patients: patients with untreated essential hypertension | |
| Interventions | Placebo: oval pink tablet Untreated: no tablet Experimental: hospitalisation (Co‐intervention: NS) | |
| Outcomes | Diastolic arterial blood pressure (mm Hg) Systolic arterial blood pressure (mm Hg) Heart rate Plasma renin activity Plasma norepinephrine activity Anxiety scores Urinary catecholamines | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hovell 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of adherence coaching to treatment adherence for TB | |
| Participants | Patients: adolescents with TB Baseline comparability: yes | |
| Interventions | Placebo: sessions with self‐esteem counselling (12 sessions, 9 months) Untreated: no sessions Experimental: sessions with adherence counselling (Co‐intervention: TB medication) | |
| Outcomes | Cumulative number of pills Proportion of TB treatment completers (180 pills within 9 moths) Interaction of alcohol consumption and lack of treatment adherence | |
| Notes | We have multiplied the results by negative 1 to change the direction of the summary statistics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/coaching) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as outcome was patient reported (number of pills) |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 194 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Hruby 2006.
| Methods | Design: three group parallel trial Purpose: study the effect TENS for pain associated with cystoscopy | |
| Participants | Patients: outpatients needing flexible cystoscopy Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: sessions with TENS device not producing nerve stimulation Untreated: no sessions Experimental: sessions TENS device not producing nerve stimulation (Co‐intervention: lidocaine gel) | |
| Outcomes | Pain (VAS) Vital signs International prostate symptom score | |
| Notes | It is unclear wether the spread of the mean VAS pain score refer to standard errors or standard deviations. We assume that they are standard deviations as their size (1.50 and 2.05) are comparable to the standard deviations for other mean VAS pain scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | No protocol available. It is unclear wether the spread of the mean VAS pain score refer to standard errors or standard deviations. We assume that they are standard deviations as their size (1.50 and 2.05) are comparable to the standard deviations for other mean VAS pain scores. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 100 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Hutton 1991.
| Methods | Design: three group parallel trial Purpose: examine the effect of antihistamine decongestant and placebo on common cold symptoms in children | |
| Participants | Patients: paediatric primary care unit out‐patients (0.5 to 5 years) with common cold symptoms Baseline comparability: NS | |
| Interventions | Placebo: medication containing no antihistamines Untreated: no medication Experimental: medication containing antihistamines (brompheniramine, phenylephrine and phenylpropanolamine) (Co‐intervention: instructions to avoid, however 9/30 in untreated group took NSAID, 0/24 in placebo group) | |
| Outcomes | Number of children with improved rhinorrhoea (parental assessment) Individual cold symptoms (breathing problems, fever, cough, decreased appetite, crankiness, sleeping disturbance, vomiting) General assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Neither parents and guardians nor investigators were aware of the drug‐placebo assignment' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient (parents) reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Hyland 2006.
| Methods | Design: three group parallel trial Purpose: study the effect of calcaneal taping on plantar heel pain | |
| Participants | Patients: outpatients with plantar heel pain Baseline comparability: no (age, body mass) | |
| Interventions | Placebo: Cover‐Roll stretch bandage and leucotape that did not attempt to control the alignment/position of the calcaneus. The tape was 'simply being overlaid on the skin'. Untreated: no tape Experimental: calcaneal taping 'repositioning the calcaneal alignment closer to neutral'. (Co‐intervention: NS) | |
| Outcomes | Pain VAS (0 to 10) PSFS (patient‐specific function scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers table' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/taping) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Hyman 1986.
| Methods | Design: four group parallel trial Purpose: examine the effect of behaviour therapy, hypnosis and placebo on smoking cessation | |
| Participants | Patients: smokers wanting to quit Baseline comparability: yes | |
| Interventions | Placebo: sessions with general discussions of topics of concern to the participant Untreated: no sessions Experimental: ‐hypnosis sessions: negative aspects of smoking were repeated while patient was in trance ‐behavioural therapy sessions: focused smoking technique where participants were to concentrate on the aversive aspects of smoking (Co‐intervention: NS) | |
| Outcomes | Number of abstinent smokers (based on thiocyanate concentration) Number of abstinent smokers (based on self report) Mean smoking rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behaviour therapy) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 30 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Irjala 1993.
| Methods | Design: three group parallel trial Purpose: examine the relationship between quality of sleep the night before surgery and several biochemical cerebro‐spinal fluid indicators | |
| Participants | Patients: in‐patients in need of surgery Baseline comparability: yes (age, sex, weight, height) | |
| Interventions | Placebo: tablet with no midazolam or temazepam Untreated: no tablet Experimental: ‐tablet with midazolam ‐tablet with temazepam (Co‐intervention: no) | |
| Outcomes | Quality of sleep Concentrations of 3,4‐dihydroxyphenylglycol, 3‐methoxy‐4‐hydroxyphenylglycol, 3,4‐dihydroxyphenylacetic acid, homovanillic acid, tryptophan, 5‐hydroxyindoleacetic acid and cortisol | |
| Notes | Relevant outcome data not accessible: data lost (personal communication Irjala J) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... the anaesthetist (J.I.) was not aware of the groupings' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible: data lost (personal communication Irjala J) |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 35 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Irvin 1996.
| Methods | Design: three group parallel trial Purpose: examine the effect of relaxation therapy on postmenopausal symptoms | |
| Participants | Patients: postmenopausal women attending an out‐patient clinic Baseline comparability: yes | |
| Interventions | Placebo: sessions of leisure reading Untreated: no sessions Experimental: sessions of video instructed relaxation therapy (Co‐intervention: NS) | |
| Outcomes | Hot flashes: intensity (numerical rating scale) Hot flashes: frequency Profile of Mood State (anxiety, depression, anger, vigour, fatigue, confusion) Spielberger State‐Trait Anxiety Inventory | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers table' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/relaxation therapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 22 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Jacobs 1971.
| Methods | Design: five group parallel trial Purpose: examine the effect of different drugs, in addition to a supportive treatment program, on cessation of smoking | |
| Participants | Patients: male smokers wanting to quit Baseline comparability: yes (prognostic variables) | |
| Interventions | Placebo: tablet with no drug Untreated: no tablet Experimental: tablet with lobeline, dextroamphetamine or imipramine Untreated: no tablet (Co‐intervention: supportive treatment program, mostly group sessions) | |
| Outcomes | Number of abstinent smokers (cessation or reduction to < 10% of baseline value) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... administering five drug conditions in a random and double‐blind fashion...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Jacobson 1978.
| Methods | Design: four group parallel trial Purpose: examine the effect of behavioural therapy on marital discord | |
| Participants | Patients: couples with marital discord Baseline comparability: yes | |
| Interventions | Placebo: sessions of discussions with no behavioural elements or problem solving training Untreated: no sessions Experimental: ‐sessions of behavioural therapy + problem solving skills training based on good faith principles ‐sessions of behavioural therapy + problem solving skills training based on quid pro quo principles (Co‐intervention: NS) | |
| Outcomes | Marital happiness scale Marital adjustment scale Negative behaviour Positive behaviour | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/therapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 13 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Jakes 1992.
| Methods | Design: five group parallel trial Purpose: examine the effect of auditory masking and group cognitive therapy on tinnitus | |
| Participants | Patients: out‐patients suffering from tinnitus Baseline comparability: yes (outcome variables) | |
| Interventions | Placebo: auditory masking with noise at hearing threshold Untreated: no masking Experimental: ‐auditory masking with variable noise level ‐group cognitive therapy ‐group cognitive therapy and auditory masking with variable noise level (Co‐intervention: NS) | |
| Outcomes | Tinnitus Effects Questionnaire (insomnia, emotional distress, auditory perceptional difficulties) Interference with daily activities Crown Crisp Experimental Index | |
| Notes | Relevant outcome data not accessible: data lost (personal communication Jakes SC) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible: data lost (personal communication Jakes SC) |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 28 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Kaptchuk 2008.
| Methods | Design: three group parallel trial Purpose: study the effect of placebo treatment and patient‐practioner relationship | |
| Participants | Patients: outpatients with irritable bowel syndrome Baseline comparability: yes | |
| Interventions | Placebo: sessions of needling with a sham needle that does not penetrate the skin and limited patient‐provider interaction ('placebo acupuncture alone') Untreated: no session Experimental: sessions of needling with a sham needle that does not penetrate the skin and extensive patient‐provider interaction (placebo acupuncture augmented by warmth, attention and confidence') (Co‐intervention: NS) | |
| Outcomes | Global improvement scale scores (1 to 7 point ) Proportion of patients with adequate relief Symptom severity scale Quality of life scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sequentially numbered opaque sealed envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No primary outcomes mentioned in the protocol. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 175 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Karst 2007.
| Methods | Design: four group parallel trial Purpose: study the effect of auricular acupuncture on dental procedural anxiety | |
| Participants | Patients: outpatients undergoing dental extraction Baseline comparability: yes | |
| Interventions | Placebo: needling with non penetrative needle at points not regarded having effect on anxiety (finger and liver points)
Untreated: no needling
Experimental: ‐needling at ' ... relaxation, tranquillizer, and master cerebral points in the external ear ...'. ‐midazolam, intranasal (average dose 4mg) (Co‐intervention: NS) |
|
| Outcomes | Spielberger state‐trait anxiety inventory VAS Sedation score Quality of dental condition Physiological status | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'list of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 29 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Karunakaran 1997.
| Methods | Design: three group parallel trial Purpose: examine the effect of sulfonylurea therapy on patients with increased fasting plasma glucose | |
| Participants | Patients: out‐patients with increased but not diabetic fasting plasma glucose Baseline comparability: yes | |
| Interventions | Placebo: tablets containing no sulfonylurea Untreated: no tablets Experimental: tablets containing sulfonylurea (Co‐intervention: reinforced or basic health advice in a factorial design) | |
| Outcomes | Fasting plasma glucose (mmol/l) Haemoglobin A1c Plasma insulin Beta‐cell function Weight Blood lipids Arterial blood pressure | |
| Notes | Data from placebo and no‐treatment groups pooled in the original trial report. Contact with researchers provided unpooled data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... double‐blind placebo...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Data from placebo and no‐treatment groups pooled in the original trial report. Contact with researchers provided unpooled data. |
| Free of other bias? | High risk | See notes |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 115 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Kendall 1979.
| Methods | Design: four group parallel trial Purpose: examine the effect of cognitive‐behavioural and patient education interventions on the anxiety related to cardiac catheterization | |
| Participants | Patients: in‐patients undergoing cardiac catheterization Baseline comparability: yes | |
| Interventions | Placebo: pre‐catheterization session where patients' feelings were discussed, avoiding coping skills, procedures or factual information Untreated: no sessions (observational group) Experimental: sessions with: ‐cognitive‐behavioural training in anxiety coping strategies ‐patient education: factual information on heart diseases and the procedure of cardiac catherization (Co‐intervention: NS) | |
| Outcomes | Anxiety (Spielberger state trait anxiety inventory) Pain Anger | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/active) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 22 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Kerr 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of penicillin troches for recurrent aphthous ulcers | |
| Participants | Patients: outpatients with recurrent aphthous ulcers Baseline comparability: yes (age, gender, ulcer age) | |
| Interventions | Placebo: sessions with TENS device not producing nerve stimulation Untreated: no sessions Experimental: sessions TENS device producing nerve stimulation (Co‐intervention: installation of 4% lidocaine gel) | |
| Outcomes | Proportion of patients with healed ulcers Proportion of patients with no pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | urn randomisation '...the subject drawing a single envelope from a pool of sealed blank envelopes' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Both the investigators and treatment group subjects were blinded' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 69 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Killeen 2004.
| Methods | Design: three group parallel trial Purpose: study the effect of naltrexone for alcohol dependence | |
| Participants | Patients: outpatients with alcohol dependence Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: NS (12 weeks) Untreated: no placebo or naltrexone Experimental: naltrexone (Co‐intervention: various individual or group programs) | |
| Outcomes | Alcohol consumption (time line follow‐back: average drinks per day, percent days drinking, drinks per drinking days, heavy drinking days) Percentage with at least one heavy drinking episode Craving (obsessive compulsive drinking scale) Various explorative analyses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | urn randomisation |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 59 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Killen 1990.
| Methods | Design: 4 x 3 group parallel trial (see notes) Purpose: examine the effect of behavioural intervention (3 types) and pharmacological intervention (4 types) in smoking relapse prevention | |
| Participants | Patients: tobacco smokers abstinent for more than 48 hours and volunteering for a self‐help program Baseline comparability: yes | |
| Interventions | Placebo: gum with no nicotine Untreated: no gum Experimental: ‐nicotine gum ad libitum ‐nicotine gum with fixed regimen (Co‐intervention: NS) | |
| Outcomes | Number of abstinent smokers | |
| Notes | As the behavioural treatments showed no significant difference the 3 x 4 factorial design was collapsed into a four group parallel trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Assignment to gum condition was double‐blind...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 618 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Kilmann 1987.
| Methods | Design: five group parallel trial Purpose: study the effect of three different psychological interventions on secondary erectile dysfunction | |
| Participants | Patients: men with secondary erectile dysfunction Baseline comparability: yes | |
| Interventions | Placebo: sessions with factual information on sexual education and relationship enhancement Untreated: no sessions Experimental: ‐sessions with communication technique training ‐sessions with sexual technique training ‐sessions with combination treatment (Co‐intervention: NS) | |
| Outcomes | Coital success in % Marital adjustment test Sexual interaction inventory Sexual anxiety inventory Sexual pleasure rating | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/psychological interventions) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 8 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Klerman 1974.
| Methods | Design: 2 x 3 factorial design (high and low personal interaction versus amitriptyline, placebo, no treatment) Purpose: examine the effect of amitriptyline, placebo and psychotherapy treatment on relapse of depression | |
| Participants | Patients: out‐patients with neurotic depression having improved markedly after 1 to 2 months amitrityline medication Baseline comparability: yes | |
| Interventions | Placebo:
‐continuous treatment with tablets containing no amitriptyline (content NS)
Untreated:
‐tablet treatment discontinued (observational group)
Experimental:
‐continuous treatment with amitriptyline tablets (Co‐intervention: no) |
|
| Outcomes | Number of patients with relapse of depression Residual symptoms of depression Social adjustment | |
| Notes | As the high and low personal interaction treatments showed no significant difference the 2 x 3 factorial design was collapsed into a three group parallel trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... in a double‐blind controlled manner..' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Kober 2002.
| Methods | Design: three group parallel trial Purpose: study the analgesic effect of accupressure | |
| Participants | Patients: victims of minor trauma under transport to hospital Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: stimulation on sites not regarded analgesic acupressure sites Untreated: no stimulation Experimental: stimulation on sites regarded analgesic acupressure sites (Di4, KS9, KS6, BL60, LG20) (Co‐intervention: no) | |
| Outcomes | Pain (100 mm VAS) Anxiety (100 VAS) Heart frequency | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | 'opened an envelope' |
| Blinding? Treatment provider | Low risk | '... we conducted a prospective, randomized, double‐blinded study...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 41 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Kokol 2005.
| Methods | Design: three group parallel trial Purpose: study the effect of 685 nm laser and placebo on venous leg ulcers | |
| Participants | Patients: out‐patients with venous leg ulcers Baseline comparability: yes | |
| Interventions | Placebo: polychromatic red light Untreated: no light Experimental: 685 nm laser light (red) (Co‐intervention: standard venous leg ulcers treatments) | |
| Outcomes | Area of leg ulcers (photographs) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... weder vom Arzt noch vom Patienten unterschieden werden kann' |
| Blinding? Outcome assessor | Low risk | 'Erst nach Ende der Nachbeobachtungensphase (Tag 90) wurden die verwendeten als A und B be zeichneten Geräte decodiert (entblindet), um die Ergebnisse nicht zu beeinflussen' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 26 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Kotani 2001.
| Methods | Design: three group parallel trial Purpose: study the effect of insertion of intradermal needles into painful points on intractable scar pain | |
| Participants | Patients: out‐patients with intractable abdominal scar pain Baseline comparability: yes | |
| Interventions | Placebo: intradermal needling into nonpainful points Untreated: no needling Experimental: intradermal needling into painful points (Co‐intervention: diclofenac until 24 hours before pain evaluation) | |
| Outcomes | Number of analgesic tablets per week Proportion of patients with global pain relief (50% reduction or more) Pain, continuous (VAS) Pain, lancinating (VAS) Pain area (square cm) Threshold pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number table' |
| Allocation concealment? | Low risk | 'sequentially sealed opaque envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/needling into painful points) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 47 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Lamazza 1986.
| Methods | Design: three group parallel trial Purpose: examine the effect of pinaverium bromide (antispasmodic and analgesic drug) in the premedication of endoscopic retrograde cholangio‐pancreaticography (ERCP) and on the motor activity of the sphincter of Oddi | |
| Participants | Patients: with biliary and pancreatic disease and in need of a ERCP Baseline comparability: NS | |
| Interventions | Placebo: tablet with no pinaverium bromide Untreated: no tablet Experimental: tablet with pinaverium bromide (Co‐intervention: diazepam injection, 10 to 20 mg) | |
| Outcomes | Patient's tolerance of procedure Time for procedure Sphincter of Oddi pressure and wave pattern | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'A double‐blind study was carried out...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 12 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Lander 1993.
| Methods | Design: three group parallel trial Purpose: examine the effect of transcutaneous electrical nerve stimulation (TENS) for children's pain on blood sampling | |
| Participants | Patients: children attending out‐patient clinics undergoing venepuncture Baseline comparability: yes (age, sex, expected pain, anxiety) | |
| Interventions | Placebo: TENS with machine off Untreated: no TENS Experimental: TENS with machine on (Co‐intervention: NS) | |
| Outcomes | Pain (VAS, Faces Affective Pain Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sealed envelopes' |
| Blinding? Treatment provider | High risk | Not |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 340 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Larson 2005.
| Methods | Design: three group parallel trial Purpose: study the risk of vomiting and regurgitation in children with diarrhoea treated with Zink or placebo | |
| Participants | Patients: children with diarrhoea (short stay ward or outpatient clinic) Baseline comparability: yes | |
| Interventions | Placebo: dispersible tablet without Zink Untreated: no tablet Experimental: dispersible tablet with Zink (Co‐intervention: standard treatment for diarrhoea) | |
| Outcomes | Proportion of patients that vomit Proportion of patients that regurgitatee | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'numbered' and 'opaque envelopes' |
| Blinding? Treatment provider | Low risk | 'This randomized, double‐blind, placebo‐controlled clinical trial...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N =1066 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Lee 2005.
| Methods | Design: two group parallel trial Purpose: study the effect of placebo on cough | |
| Participants | Patients: out‐patients with cough and signs of upper respiratory infection Baseline comparability: NS | |
| Interventions | Placebo: capsule with vitamin E Untreated: no capsule (Co‐intervention: no) | |
| Outcomes | Changes in cough frequency per 15 minutes Cough suppression time | |
| Notes | Mean changes in cough frequency per 15 minutes calculated from individual patient data in Figure 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Low risk | 'microphone connected to a pen recorder' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Leibing 2002.
| Methods | Design: three group parallel trial Purpose: study the analgesic effect of acupuncture for low‐back pain | |
| Participants | Patients: out‐patients with non‐radiating low‐back pain of more than 6 months duration Baseline comparability: yes | |
| Interventions | Placebo: needling on sites not regarded analgesic acupuncture sites Untreated: no needling Experimental: needling on sites regarded analgesic acupressure sites (Co‐intervention: active physiotherapy) | |
| Outcomes | Pain (100 mm VAS) Pain disability (pain disability index) Psychological distress Spine flexion | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 79 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Levine 1984.
| Methods | Design: 8 group parallel trial (incomplete 3 x 3 factorial design) Purpose: examine the effect of open and hidden infusion of placebo on pain | |
| Participants | Patients: postoperative in‐patients (after removal of impacted third molars) Baseline comparability: NS | |
| Interventions | Placebo: open infusion of vehicle by bedside investigator Untreated: patient unaware of infusion of vehicle (either by machine or hidden investigator) Experimental: infusions of naloxone and morphine (8 and 12 mg) (Co‐intervention: NS) | |
| Outcomes | Pain (VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Each patient was randomly assigned to receive, after surgery, a double‐blind injection...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 36 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Levitt 1981.
| Methods | Design: three group parallel trial Purpose: examine the effect of investigating cardiovascular parameters in cancer patients receiving antiemetic cannabis treatment | |
| Participants | Patients: cancer patients receiving nausea inducing chemotherapy Baseline comparability: NS | |
| Interventions | Placebo: NS Untreated: no placebo or cannabis Experimental: ‐cannabis (three doses) ‐prochlorperazine (Co‐intervention: yes, chemotherapy) | |
| Outcomes | Emetic events Psychological events Heart rate Blood pressure Intraocular pressure | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 48 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Licciardone 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of osteopathic manipulative treatment techniques for chronic low‐back pain | |
| Participants | Patients: out‐patients with chronic low‐back pain Baseline comparability: yes | |
| Interventions | Placebo: simulated osteopathic manipulative treatment techniques Untreated: no simulated osteopathic manipulative treatment techniques Experimental: osteopathic manipulative treatment techniques (Co‐intervention: 'usual or other low back care') | |
| Outcomes | Back pain (VAS) SF‐36 health survey (physical functioning subscale) SF‐36 health survey (all subscales) Roland‐Morris disability questionnaire Lost work or school days Satisfaction with back care | |
| Notes | We selected back pain as the relevant outcome (and not the SF‐36 which was the basis for the power calculation), because also back pain was described as a 'main outcome', and because pain (and not quality of life) is mentioned in the aims section and the title. We extracted pain data (at six months) from Figure 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sequentially sealed envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/manipulation) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 34 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Lick 1975.
| Methods | Design: four group parallel trial Purpose: examine the effect of behavioural therapy and placebo on snake and spider phobia | |
| Participants | Patients: out‐patients suffering from phobia against snakes and spiders Baseline comparability: yes | |
| Interventions | Placebo: ‐with feedback: patients told that pictures with phobic stimulus would be flickered too rapidly for the conscious mind to register and phobic responses would be detected by a 'polygraph' that would deliver a mild but uncomfortable electrical shock. In fact only light was shown and no machine detected any phobias. The rate of shocks associated with lights was programmed to be reduced from 90% in the first session to 10% in the last session. The patients were shown outprints and explained that treatment was 'going well'. ‐ without feedback: similar procedure, but no outprints were shown and patients told that the machine reduced the number of shocks independently of their response. Patients were also 'assured throughout treatment that things were going well' Untreated: no behavioural or placebo procedure (waiting‐list) Experimental: systematic desensitization procedure (Co‐intervention: NS) | |
| Outcomes | Percentage of fear remaining (index) and pulse rate at confrontation with snake or spider Behavioural approach test Behavioural inhibition test Reaction to picture test Global behavioural improvement Therapy expectancy Warmth and competence of therapist | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural therapy) |
| Blinding? Outcome assessor | Low risk | '... experimenter who was blind to the conditions that the subjects had been assigned' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 18 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Lick 1977.
| Methods | Design: four group parallel trial Purpose: examine the effect of relaxation therapy and placebo on insomnia | |
| Participants | Patients: out‐patients suffering from chronic insomnia Baseline comparability: yes (time to fall asleep) | |
| Interventions | Placebo: 'T‐scope therapy', a sham procedure designed to induce expectancy Untreated: no relaxation or placebo therapy (waiting list) Experimental: relaxation therapy (Co‐intervention: sleep inducing drugs, no difference in % days in which patients took drugs in placebo and untreated groups) | |
| Outcomes | Sleep latency (min) Hours slept Quality of sleep Feelings on awakening Minnesota Multi phasic Personality Inventory Number of awakenings % days taking a sleeping pill | |
| Notes | 5 out of 40 participants were replaced by others approximately equivalent in age, sex and time to falling asleep. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/relaxation therapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | High risk | See notes |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Limoges 2004.
| Methods | Design: three group parallel trial Purpose: study the effect of TENS during sigmoidoscopy | |
| Participants | Patients: out‐patients needing screening endoscopy Baseline comparability: yes | |
| Interventions | Placebo: session with TENS machine off Untreated: no session Experimental: session with TENS machine turned on (Co‐intervention: NS) | |
| Outcomes | Pain intensity (1‐5) Bloating Nausea Pain or burning or tingling at electrode site Pain compared with previous sigmoidoscopy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | (but endoscopist and patients were blinded) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 60 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Unclear allocation concealment |
Lin 2002.
| Methods | Design: three group parallel trial Purpose: study the analgesic effect of electroacupuncture | |
| Participants | Patients: postoperative patients (hysterectomy) Baseline comparability: yes | |
| Interventions | Placebo: needling without electrical stimulation at ST‐36 Untreated: no needling Experiemental: needling with electrical stimulation at ST‐36 (Co‐intervention: pethidine and morphine at request) | |
| Outcomes | Use of morphine (mg at 8, 16 and 24 hours) Pain Time to first pethidine dose Heart rate Blood pressure Oxygen saturation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer‐generated' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Lincoln 2003.
| Methods | Design: three group parallel trial Purpose: study the effect of cognitive behavioral psychotherapy for post‐stroke depression | |
| Participants | Patients: out‐patients with a recent stroke and depression Baseline comparability: yes | |
| Interventions | Placebo: sessions with conversations focusing on daily events and physical effects of stroke and life changes Untreated: no sessions Experimental: sessions with cognitive behavioural psychotherapy (Co‐intervention: NS) | |
| Outcomes | Beck depression inventory Wakefield self‐assessment of depression inventory Extended activities of daily living scale London handicap scale Satisfaction with care rating | |
| Notes | Standard deviation for the mean scores on the Beck depression inventory were not reported. We took the standard deviation from another trial (Verduyn C 2003). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer generated' |
| Allocation concealment? | Low risk | 'sealed in opaque, consecutively numbered envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive behavioral psychotherapy) |
| Blinding? Outcome assessor | Low risk | 'Outcome assessments were administered by an assistant psychologist, who was blind to the group allocation...' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. Standard deviation for the mean scores on the Beck depression inventory were not reported. We took the standard deviation from another trial (Verduyn C 2003). |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 80 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Linde 2005.
| Methods | Design: three group parallel trial Purpose: study the effect of acupuncture on migraine | |
| Participants | Patients: out‐patients with migraine headache Baseline comparability: yes | |
| Interventions | Placebo: needling at places not regarded true acupuncture sites Untreated: no needling Experimental: needling at places regarded true acupuncture sites (Co‐intervention: standard headache treatment according to the guideline of the German Migraine and Headache Society) | |
| Outcomes | Number of headache days of moderate or severe intensity until week 9‐12 Number of migraine headaches Total number of headache days Proportion of treatment responders Number of days with medication Pain disability index Scale for assessing the emotional aspects of pain Algemeine Depressionskalla SF‐36 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random list generated with Sample Size 2.0' |
| Allocation concealment? | Low risk | 'centralized telephone randomization procedure' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Low risk | Primary outcome specified in protocol |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 157 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Lindholm 1996.
| Methods | Design: multi centre 2 x 3 factorial design (normal or intensive advice x pravastatin, placebo or no drug) Purpose: examine the effect of pharmacological and non‐pharmacological strategies on cardiovascular risk | |
| Participants | Patients: out‐patients with increased risk of cardiovascular disease and moderately increased serum‐cholesterol Baseline comparability: yes | |
| Interventions | Placebo: tablet with no pravastatin (with usual or intense advice) Untreated: no pharmacological intervention (with usual or intense advice) Experimental: pravastatin (with usual or intense advice) (Co‐intervention: usual health care advice on diet conducted by a GP or intense health care advice where usual advice is supplemented by group sessions) | |
| Outcomes | Serum‐cholesterol (mmol/l) Serum‐cholesterol: high and low density lipoprotein Serum‐triglycerides Blood‐glycose Blood pressure Framingham risk score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'A prospective, double‐blind, randomized, controlled trial...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 453 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly stated |
Liossi 2003.
| Methods | Design: four group parallel trial Purpose: study the effect of hypnosis on procedure‐related pain | |
| Participants | Patients: children (6 to 16 years) with cancer Baseline comparability: yes | |
| Interventions | Placebo: attention control treatment ('development of rapport, nonmedical play, nonmedical verbal interaction'. Therapist was 'supportive, warm, encouraged the child to express freely their interests, and formed a close relationship with the child.' Untreated: no hypnosis Experimental: ‐direct hypnosis (hypnotic indiction by reference to request for numbness and imagining 'numbing medicine', etc) ‐indirect hypnosis (hypnotic induction by reference to 'the setting sun metaphor' and the 'Mexican food metaphor') (Co‐intervention: standard medical care) | |
| Outcomes | Pain (6 point scale) Anxiety Observed distress | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Longo 1988.
| Methods | Design: three group parallel trial Purpose: examine the effect of psycho‐social intervention on recurrence of genital herpes simplex | |
| Participants | Patients: media and healthcare‐referred persons with recurrent genital herpes simplex with 4 or more attacks a year Baseline comparability: yes | |
| Interventions | Placebo: ‐sessions of social support consisting of discussions of interpersonal conflicts without any relaxation techniques or stress management. Untreated: no sessions (waiting‐list) Experimental: sessions of relaxation techniques and stress management + playing a relaxation tape during herpes attacks (Co‐intervention: NS) | |
| Outcomes | Herpes attack: severity (index) Herpes attack: frequency and duration Zung Depression Scale Profile of Mood States UCLA Loneliness Scale Multidimensional Health Locus of Control Scales | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/psycho‐social intervention) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 19 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Lorr 1961.
| Methods | Design: five group parallel trial Purpose: examine the effect of pharmacological treatment in addition to psychotherapy in patients with anxiety and hostility | |
| Participants | Patients: out‐patients with anxiety and hostility admitted to a veteran's Mental Hygiene Clinic for psychotherapy Baseline comparability: NS | |
| Interventions | Placebo: tablet containing lactose but not chlorpromazine, meprebomate or phenobarbital Untreated: no tablet (observational group) Experimental: ‐tablets with chlorpromazine ‐tablet with meprobamate ‐tablet with phenobarbital (Co‐intervention: sessions of individual psychotherapy) | |
| Outcomes | Anxiety (index) Hostility Discomfort | |
| Notes | 42% drop‐outs. The standard deviation (SD) of the anxiety score was not reported. The SD was calculated from the reported F‐test statistic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...12‐week double‐blind study' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 80 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Macaluso 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of fentanyl as premedication | |
| Participants | Patients: in‐patients undergoing same day surgery Baseline comparability: yes | |
| Interventions | Placebo: oralet without fentanyl Untreated: no oralet Experimental: oralet with fentanyl (Co‐intervention: NS) | |
| Outcomes | Anxiety (Spielberger state trait anxiety inventory) Heart rate Volume and pH of gastric contents Arterial pressure Respiratory frequency Oxygen saturation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'by computer program' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'All investigators were blinded to the type of oralet patients in Groups I and II consumed...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 60 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Malcolm 1980.
| Methods | Design: three group parallel trial Purpose: examine the effect of nicotine chewing gum treatment on smoking | |
| Participants | Patients: smokers Baseline comparability: yes | |
| Interventions | Placebo: chewing gum without nicotine Untreated: no chewing gum Experimental: chewing gum with nicotine (Co‐intervention: NS) | |
| Outcomes | Number of abstinent smokers (based on carboxyhaemoglobin levels) Number of abstinent smokers (based on self report) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The trial was double blind between the gum groups' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 121 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Markland 1993.
| Methods | Design: three group parallel trial Purpose: examine the effect of a relaxation tape session on preoperative anxiety | |
| Participants | Patients: in‐patients undergoing day‐case surgery Baseline comparability: yes, except for diastolic blood pressure | |
| Interventions | Placebo: session where patients listen to a recorded short story Untreated: no session Experimental: session where patients listen to a recorded relaxation procedure (Co‐intervention: NS) | |
| Outcomes | Anxiety (Spielberger state trait anxiety inventory) Heart rate Blood pressure Anaesthesia measures (dose of sedative, time to settle the patient, mean concentration per min of isoflurane, anaesthetist's score) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/relaxation procedure) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 14 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Matros 2006.
| Methods | Design: three group parallel trial Purpose: study the effect of gum chewing on postoperative ileus | |
| Participants | Patients: in‐patients having had performed abdominal surgery Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: Acupressure bracelet used on an inert location Untreated: no chewing gum or bracelet Experimental: chewing gum (Co‐intervention: standard postoperative care) | |
| Outcomes | Time to first flatus Time to first bowel movement Time to ready for discharge Time to actual discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer‐generated' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/chewing gum) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 44 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Mattarei 1985.
| Methods | Design: two period, two group, cross‐over trial Purpose: examine the effect of placebo on arterial hypertension |
|
| Participants | Patients: out‐patients with untreated mild essential hypertension Baseline comparability: NS | |
| Interventions | Placebo: capsule Untreated: no capsule (Co‐intervention: no) | |
| Outcomes | Diastolic blood pressure in mm Hg after 4 weeks Systolic blood pressure in mm Hg | |
| Notes | The outcome data was not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | The outcome data was not available. |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
May 1988.
| Methods | Design: four group, four period cross‐over trial Purpose: examine the effect of terbutaline, isotonic saline, ambient air and no‐treatment on chronic airway obstruction in asthmatics | |
| Participants | Patients: asthmatic out‐patients with reversible chronic airway obstruction Baseline comparability: no carry‐over effect | |
| Interventions | Placebo: ‐inhalation of air from a noisy nebulizer ‐inhalation of isotonic saline from a noisy nebulizer Untreated: inhalation of air through a mouthpiece disconnected from the nebulizer Experimental: inhalation of terbutaline from a noisy nebulizer (Co‐intervention: use of corticosteroids or bronchodilator drugs prohibited) | |
| Outcomes | Change in forced expiratory volume in 1 second (FEV1) Change in forced vital capacity (FVC) | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The treatment was given double‐blind...' |
| Blinding? Outcome assessor | Low risk | '... but the nurse in charge of spirometry did not know whether or not the patient was receiving treatment' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 48 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
McLachlan 1991.
| Methods | Design: three group parallel trial Purpose: examine the effect of the aluminium chelator desferrioxamine on progression of Alzheimer's disease | |
| Participants | Patients: out‐patients with probable Alzheimer's disease (memory problems, cerebral atrophy, no cerebral infarcts) Baseline comparability: NS | |
| Interventions | Placebo: tablets containing lecithin and no desferrioxamine Untreated: no tablets or injections (observational group) Experimental: injections with desferrioxamine (Co‐intervention: NS) | |
| Outcomes | Activities of daily living (ADL, rate of decline of video recorder home‐behavioural assessment) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'table of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/desferrioxamine) |
| Blinding? Outcome assessor | Low risk | '... trained raters who were not told about the nature of the study' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
McMillan 1994.
| Methods | Design: four group parallel trial Purpose: examine the prophylactic antiemetic effect of transcutaneous electrical stimulation (TENS) of the P6 (Neiguan) acupuncture point | |
| Participants | Patients: in‐patients in need of major surgery and at risk of opoid analgesic‐induced nausea Baseline comparability: yes (type of analgesic) | |
| Interventions | Placebo: TENS with machine turned off Untreated: no TENS Experimental: TENS with machine turned on (Co‐intervention: pharmacological analgesic and anti‐emetic medication) | |
| Outcomes | Number of patients with nausea Number of patients with vomiting | |
| Notes | Inconsistent data reporting in original trial report clarified by contact with author. Not 1:1 randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 72 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Medici 2002.
| Methods | Design: three group parallel trial Purpose: examine the effect of real and sham acupuncture on bronchial asthma | |
| Participants | Patients: out‐patients with chronic asthma Baseline comparability: yes | |
| Interventions | Placebo: needling at 11 sites not regarded true acupuncture sites Untreated: no needling Experimental: needling at 11 sites 'believed to have an anti‐asthmatic effect'. (Co‐intervention: standard pharmacological inhalation therapy) | |
| Outcomes | Proportion of patients scoring 1 to 4 on a VAS for nausea Proportion of patients scoring 1 to 4 on a VAS for difficulty of swallowing gastroscopy Proportion of patients scored 1 to 4 by gastrocopist on a VAS for nausea/retching Proportion of patient who accept re‐gastroscopy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 41 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Mehl‐Madrona 2007.
| Methods | Design: Five group parallel trial Purpose: To study the effect of acupuncture, craniosacral therapy, acupuncture and craniosacral therapy, attention control and waiting list control on asthma. | |
| Participants | Patients: out‐patients with chronic asthma Baseline comparability: NS | |
| Interventions | Placebo: Attention control Untreated: waiting list control Experimental: Acupuncture, craniosacral, acupuncture and craniosacral. (Co‐intervention: Standard medical management) | |
| Outcomes | Pulmonary function Asthma quality of life questionnaire. Profile of mood states. Beck depression inventory. Beck anxiety inventory. Medication form. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'random number generating program' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Low risk | 'The respiratory therapist performing pulmonary function testing was blind to the assignments of the subjects' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = ? |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Melchart 2005.
| Methods | Design: three group parallel trial Purpose: study the effect of acupuncture on tension type headache | |
| Participants | Patients: out‐patients with tension type headache Baseline comparability: yes for most aspects, however 'some differences in previous use of acupuncture and in parts of the pain questionnaire'. | |
| Interventions | Placebo: needling at places not regarded true acupuncture sites Untreated: no needling Experimental: needling at places regarded true acupuncture sites (Co‐intervention: standard headache treatment) | |
| Outcomes | Days with headache Hours with headache Headache scores Days with more than mild headache Days with analgesic drugs Number of days with medication Pain disability index SF‐36 Algemeine Depressionskala Scale for assessing the emotional aspects of pain Average pain (1 to 10 scale) | |
| Notes | Average days with analgesic drugs in placebo group was 2.6 (SD: 2.6), and in the no‐treatment group 4.4 (SD: 4.1) at week 12. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random list generated with sample size 2.0 by the statistician' |
| Allocation concealment? | Low risk | 'centralized telephone randomization procedure' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Low risk | Primary outcome specified in protocol |
| Free of other bias? | Unclear risk | Average days with analgesic drugs in placebo group was 2.6 (SD: 2.6), and in the no‐treatment group 4.4 (SD:4.1). |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 120 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Moffet 1996.
| Methods | Design: three group parallel trial Purpose: examine the effect of pulsed short wave therapy (PSW) on chronic pain | |
| Participants | Patients: out‐patients with chronic pain associated with osteoarthritis of hip or knee Baseline comparability: yes | |
| Interventions | Placebo: PSW with machine off Untreated: no PSW Experimental: PSW with machine on (Co‐intervention: NS) | |
| Outcomes | Pain (numerical/verbal rating scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'minimisation method' |
| Allocation concealment? | Low risk | 'minimisation method' |
| Blinding? Treatment provider | Low risk | '... double blind randomised controlled trial' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 49 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size is not 50 or more |
Molsberger 2002.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on chronic low back pain | |
| Participants | Patients: patients with low back pain of more than 6 weeks duration Baseline comparability: yes (age, pain) | |
| Interventions | Placebo: superficial needling at sites not regarded acupuncture sites (12 sessions over 4 weeks) Untreated: no needling Experimental: needling at acupuncture sites (Co‐intervention: conservative orthopaedic treatment (physiotherapy, exercise, back school, mud packs, infrared heat therapy, and diclofenac on demand)) | |
| Outcomes | Proportion of patients with 50% reduction of 100 mm pain VAS at one month Proportion of patients with 50% reduction of 100 mm pain VAS at follow‐up Proportion of patients with excellent or good ratings on a four‐point box scale | |
| Notes | According to protocol we extracted the post treatment outcome data at one month, overruling a secondary principle of extracting the primary outcome of a trial (follow up data at three months). The effect of placebo was neutral at post treatment (RR = 1.16, 0.86 to 1.56), but positive at follow‐up (RR = 0.64, 0.43 to 0.95). The drop out rate in the two groups was (3+7)/121 = 8% at one month, and (19+23)/121 = 35% at three months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'computer generated randomisation list' |
| Allocation concealment? | Low risk | 'central telephone randomisation' |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | High risk | See notes |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 111 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Moreland 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of a blood glucose monitoring manual in adults with diabetes | |
| Participants | Patients: out‐patients with diabetes Baseline comparability: yes | |
| Interventions | Placebo: session of diabetes education and a blood glucose meter ('attention control') Untreated: no session Experimental: session of diabetes education based on a blood glucose manual and a blood glucose meter (Co‐intervention: standard diabetes care) | |
| Outcomes | Frequency of blood glucose measurements Glycaemic control Knowledge of HbA1c Affect regarding blood glucoses results | |
| Notes | We have multiplied the results by negative 1 to change the direction of the effect in the analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/manual) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 149 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS and unclear allocation concealment |
Morey 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of exercise health counselling | |
| Participants | Patients: elderly out‐patients with chronic illnesses Baseline comparability: yes | |
| Interventions | Placebo: sessions of disease management or prevention unrelated to physical activity and with no efforts made to modify behaviour ('attention control') Untreated: no sessions Experimental: sessions aiming at providing patient centred motivational, behavioural, and cognitive techniques to increase physical activity (Co‐intervention: NS) | |
| Outcomes | Physical activity (CHAMPS questionnaire) ‐weekly frequency ‐caloric expenditure ‐estimated total minutes (estimated moderate minutes + estimated walk/bike minutes) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer‐generated' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (attention control placebo/exercise health counselling) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 80 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Morton 1993.
| Methods | Design: four group, four period cross‐over trial Purpose: examine the prophylactic effect of acupuncture on exercise induced asthma | |
| Participants | Patients: female out‐patients suffering from exercise induced asthma Baseline comparability: not relevant | |
| Interventions | Placebo: acupuncture with laser beam off Untreated: no acupuncture Experimental: ‐acupuncture with laser beam on ‐salbutamol inhalation (Co‐intervention: corticosteroids in 3 participants) | |
| Outcomes | Forced expiratory volume after 1 second (FEV1) as % of pretreatment value Proportion with bronchoconstriction (15% reduction in FEV1) | |
| Notes | The outcome data was treated as if coming from a parallel trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was treated as if coming from a parallel trial |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 26 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Murphy 1982.
| Methods | Design: 2 x 2 factorial design with two additional control groups Purpose: examine the effect of behavioural therapy with spouse involvement on weight loss | |
| Participants | Patients: self‐referred couples with at least one obese person Baseline comparability: yes (weight, sex, age) | |
| Interventions | Placebo: sessions with supportive group discussions about different weight loss programs Untreated: no sessions Experimental: sessions of behavioural therapy where the ‐person is alone and without spouse involvement by contract ‐person is alone in therapy and with spouse involvement by contract ‐couple is in therapy but without spouse involvement by contract ‐couple is in therapy and with spouse involvement by contract (Co‐intervention: corticosteroids in 3 subjects) | |
| Outcomes | Weight loss (pounds) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural therapy) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 17 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Mussell 1988.
| Methods | Design: five period cross‐over trial Purpose: examine the effect of trachea‐noise biofeedback in asthma | |
| Participants | Patients: 'asthmatics were recruited with informed consent' Baseline comparability: not relevant | |
| Interventions | Placebo: inhaled saline Type of untreated: no inhalation or biofeedback Type of experimental: ‐salbutamol inhalation ‐biofeedback wrong information ‐biofeedback correct information (Co‐intervention: no asthma medication) | |
| Outcomes | Forced expiratory volume after 1 second (FEV1) | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '...the active and placebo bronchodilator inhaler given double blind..' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 16 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Najnigier 1997.
| Methods | Design: three group parallel trial Purpose: examine the preventive effect of ondansetron on postoperative nausea | |
| Participants | Patients: in‐patients undergoing laparoscopic cholecystectomy Baseline comparability: NS | |
| Interventions | Placebo: tablets without ondansetron Untreated: no tablets Experimental: tablets with ondansetron (Co‐intervention: NS) | |
| Outcomes | Number of patients with nausea Number of patients with vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | Very likely a double‐blind study |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 60 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Nandi 1976.
| Methods | Design: three group parallel trial Purpose: examine the effect of imipramine on depression in patients not spontaneously seeking treatment | |
| Participants | Patients: out‐patients with clinical depression identified through a door to door survey in a rural community Baseline comparability: yes (score on Hamilton's depressive rating scale) | |
| Interventions | Placebo: tablets without imipramine (lactose) Untreated: no tablets (observational group) Experimental: tablets with imipramine (Co‐intervention: NS) | |
| Outcomes | Score on Hamilton's rating scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 18 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Naumann 1989.
| Methods | Design: three group parallel trial Purpose: examine the effect of transcutaneous electrical nerve stimulation (TENS) and placebo on postoperative pain | |
| Participants | Patients: postoperative patients Baseline comparability: NS | |
| Interventions | Placebo: TENS with no current Untreated: no TENS Experimental: TENS with current (Co‐intervention: standard pharmacological analgesic care) | |
| Outcomes | Number of analgesic injections | |
| Notes | Outcome not reported so that meta‐analysis is possible. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Der Versuchsaufbau entsprach Doppelblindbedingungen' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Outcome not reported so that meta‐analysis is possible. |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 117 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Nawrocki 1997.
| Methods | Design: three group parallel trial Purpose: examine the effect of transurethral microwave thermotherapy (TUMT) on benign prostatic hyperplasia | |
| Participants | Patients: out‐patients suffering from benign prostatic hyperplasia Baseline comparability: NS | |
| Interventions | Placebo: TUMT with no microwave emission Untreated: no TUMT Experimental: TUMT with machine on (Co‐intervention: NS) | |
| Outcomes | American Urologist Association's symptom score and Minimum urethral opening pressure (mm H20) Maximum urinary flow rate Post‐void residual urine volume Maximum detrusor pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'selecting ... numbered ... balls from a sealed bag' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The treatment of the first two groups was 'double‐blind'...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 82 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Nicassio 1974.
| Methods | Design: four group parallel trial Purpose: examine the effect of two different relaxation techniques on insomnia | |
| Participants | Patients: media recruited out‐patients suffering from insomnia Baseline comparability: yes (time to fall asleep) | |
| Interventions | Placebo: sessions of self relaxation without any technique being taught Untreated: no sessions Experimental: sessions of taught techniques of relaxation: ‐autogenic training (focusing on heaviness and warmth of legs and arms) ‐progressive relaxation (muscle tension‐release cycles) (Co‐intervention: no) | |
| Outcomes | Sleep latency (minutes) Hours slept Number of awakenings Overall quality of the night's sleep (fatigue, depression, ability to relax, feeling of anxiety, ability to function at work and irritability during day) Pupillography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/relaxation techniques) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 16 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Nocella 1982.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive‐behavior modification on coping with dental procedure stress | |
| Participants | Patients: children attending a dental clinic for a painful procedure Baseline comparability: yes (sex, procedure) | |
| Interventions | Placebo: one session where a child received the full attention of the experimenter without implementing strategies for stress coping Untreated: no session Experimental: one session where stress coping strategies of a cognitive‐behavioral nature were given (co‐intervention: NS) | |
| Outcomes | Frequency (per min) of behaviour expressing stress (facial grimaces, restlessness, moving legs and arms, sitting up, gripping chair and verbalizations) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive‐behavior intervention) |
| Blinding? Outcome assessor | Low risk | '... each child's behavior was categorized by a judge who was blind to treatment conditions' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
O'Brien 1996.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupressure on nausea associated with pregnancy | |
| Participants | Patients: pregnant women with nausea Baseline comparability: yes for baseline nausea | |
| Interventions | Placebo: acupressure on a neutral point (not P6) Untreated: no acupressure Experimental: acupressure on the point P6 (Co‐intervention: antiemetic medication, dietary and activity recommendations. The acupressure group used less antiemetic medication than the two other groups). | |
| Outcomes | Nausea (Rhodes inventory of nausea and vomiting) Vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'group assignments were computer generated' |
| Allocation concealment? | Low risk | 'numbered sealed envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupressure) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 107 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Parker 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of stress management on clinical outcomes of rheumatoid arthritis (RA) | |
| Participants | Patients: out‐patients with RA Baseline comparability: yes | |
| Interventions | Placebo: sessions where a education programme was discussed with each patient Untreated: no sessions Experimental: sessions with stress management (Co‐intervention: standard RA treatment, 74% of patients in placebo and 77% in untreated group continued on stable medication). | |
| Outcomes | Pain (VAS) McGill Pain Questionnaire Hassles scale Daily Stress Inventory Arthritis Helplessness Index Center for Epidemiologic Studies Depression Scale State‐Trait Anxiety inventory Arthritis Self‐Efficacy Scale Coping Strategy Questionnaire Arthritis impact Measurement Scale Disease Activity | |
| Notes | Relevant outcome data not accessible in trial report but retrieved by contact with authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/stress‐management) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out <15% |
| Free of selective reporting? | High risk | Relevant outcome data not accessible in trial report but retrieved by contact with authors. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 94 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Parker 2003.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive‐behavioural therapy on depression associated with rheumatoid arthritis | |
| Participants | Patients: in‐patients with rheumatoid arthritis Baseline comparability: no (age) | |
| Interventions | Placebo: general patient education program Untreated: no program Experimental: cognitive behavioural program (10 weekly visits) (Co‐intervention: standard medical care and anti‐depressive medication) | |
| Outcomes | Center for epidemiological studies‐depression scale (CES‐D) Hamilton rating scale for depression Geriatric depression scale Symptom checklist 90‐R Coping strategies questionnaire Daily stress inventory Hassles scale State‐trait anxiety inventory Arthritis helplessness index Arthritis self‐efficiency scale Arthritis impact measurement scale 2 multidimensional assessment of fatigue Pain (VAS) McGill pain questionnaire Rapid assessment of disease activity in rheumatology Erythrocyte sedimentation rate | |
| Notes | Standard deviation for mean CES‐D scores not reported. Standard deviation taken from another study (Kozora 2006): 7.5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive behavioural therapy) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | No protocol available. Standard deviation for mean CES‐D scores not reported. Standard deviation taken from another study (Kozora 2006): 7.5. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Pearl 1956.
| Methods | Design: three group parallel trial Purpose: examine the effect of reserpine on schizophrenic patients | |
| Participants | Patients: in‐patients with schizophrenia Baseline comparability: NS | |
| Interventions | Placebo: NS Untreated: no reserpine or placebo Experimental: reserpine (Co‐intervention: institutionalised patients, beside that NS) | |
| Outcomes | Multidimensional Scale for Rating Psychiatric Patients | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Except for the ward psychiatrist and nurse, no personnel involved were aware of patient's treatment. Persons dispensing the placebo were told it was a variant of reserpine' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 100 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Pelham 1992.
| Methods | Design: three group, multi‐period cross‐over trial (number of periods NS) Purpose: examine the perception of what influenced mood and behaviour (causal attribution) in boys treated for attention deficit hyperactivity disorder by methylphenidate or placebo | |
| Participants | Patients: boys with attention deficit hyperactivity disorder attending a summer camp Baseline comparability: not relevant | |
| Interventions | Placebo: tablet with no methylphenidate Untreated: no tablet Experimental: tablet with methylphenidate (Co‐intervention: ‐summer camp treatment program (behavioural therapy principles) ‐same boys also participated in trials with other drugs on days not included in the present study) | |
| Outcomes | Behaviour rating scales, including ratings of "Did you have a good day?" Attribution rating scales (what the children themselves thought influenced behaviour or mood) Mood/self‐esteem rating scales Forced‐Choice Questions | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... designed to examine ADHD boys' causal attributions in a double‐blind, within‐subject, placebo‐controlled medication trial' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 76 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Quahagen 1995.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive training program for patients with dementia | |
| Participants | Patients: out‐patients with possible or confirmed diagnosis of Alzheimer's disease Baseline comparability: yes | |
| Interventions | Placebo: passive cognitive stimulation where patients did not engage in training activities Untreated: no training Experimental: active cognitive stimulation where patients did engage in training activities (Co‐intervention: NS) | |
| Outcomes | Cognitive functioning (Mattis dementia rating scale) Behavioural functioning (Memory and behaviour problems checklist, part A) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive training program) |
| Blinding? Outcome assessor | Low risk | '... research assistants who, with rare exception, were blinded to the condition to which the family had been assigned' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 53 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Rabkin 1990.
| Methods | Design: two group parallel trial Purpose: examine the effect of continuous placebo treatment on relapse of chronic mild depression | |
| Participants | Patients: out‐patients with chronic, mild depression having improved markedly after a 10 day placebo medication Baseline comparability: yes | |
| Interventions | Placebo: continuous treatment with a tablet (content NS) Untreated: discontinued treatment with tablet (Co‐intervention: NS) | |
| Outcomes | Number of patients with relapse of depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Treatment provider was not blinded |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Rawling 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of fentanyl and placebo on the pain related to abortion | |
| Participants | Patients: women undergoing abortion Baseline comparability: yes | |
| Interventions | Placebo: saline injections Untreated: no injections Experimental: fentanyl injections (Co‐intervention: ibuprofen or acetaminophen, and lorazepam) | |
| Outcomes | Pain (11 point numerical pain scale, 0 to 10) after removal of speculum | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Low risk | 'sequentially numbered, opaque envelopes' |
| Blinding? Treatment provider | Low risk | 'Physicians, clinic staff, and women in the study did not know the contents of the syringes' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 185 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Reading 1982.
| Methods | Design: three group parallel trial Purpose: examine the effect of preoperative interview and placebo interview on postoperative pain and recovery | |
| Participants | Patients: women undergoing elective laparoscopy Baseline comparability: yes (age) | |
| Interventions | Placebo: preparation interview with neutral questions about hospitalisation in general Untreated: no preparation interview Experimental: preparation interview presenting 'information in a reassuring /supportive way' (Co‐intervention: analgesics on demand, type and dose NS, 7 from placebo and 9 from untreated group required pain medication) | |
| Outcomes | Pain (numerical/verbal rating scale) Time to return to health and work | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/interview) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 38 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Ristikankare 1999.
| Methods | Design: three group parallel trial Purpose: examine the tolerance and technical difficulty (and cardiorespiratory adverse effects) of sedative premedication during colonoscopy | |
| Participants | Patients: out‐patients undergoing colonoscopy Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: saline injections Untreated: no injections Experimental: midazolam injections (Co‐intervention: NS) | |
| Outcomes | Overall difficulty of colonoscopy, patient's report post procedure and after 2 weeks (100 mm VAS) Abdominal pain, patient's report (100 mm VAS) Discomfort, patient's report (100 mm VAS) Overall difficulty of colonoscopy, observer's report (100 mm VAS) Abdominal pain, observer's report (100 mm VAS) Discomfort, observer's report (100 mm VAS) Oxygen saturation in % Arterial blood pressure R‐R intervals on continuous ECG readings | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'An injection was administered for 30 to 60 seconds in a double‐blind manner...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 122 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Ristikankare 2006.
| Methods | Design: three group parallel group trial Purpose: study the effect of sedation and topical pharyngeal anaesthesia on cardiorespiratory safety during gastroscopy | |
| Participants | Patients: patients undergoing gastroscopy Baseline comparability: yes | |
| Interventions | Placebo: spray (NS) and injection (saline) Untreated: no spray or sedation injection Experimental: topical lidocaine spray or midazolam injection) (Co‐intervention: NS) | |
| Outcomes | Heart rate Blood pressure (diastolic and systolic) Saturation of Oxygen | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The patient, the endoscopist, and the endoscopy nurse were all blinded as to whether the patient received effective drug or placebo' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 128 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Robinson 2001.
| Methods | Design: three group parallel trial Purpose: examine the analgesic effect of transcutaneous electrical nerve stimulation (TENS) during colonoscopy | |
| Participants | Patients: out‐patients undergoing colonoscopy Baseline comparability: yes | |
| Interventions | Placebo: TENS without current Untreated: no TENS Experimental: TENS with current (Co‐intervention: midazoalam, and escape analgesic drugs) | |
| Outcomes | Breakthrough analgesia (mg nalbuphrine) Patient reported pain (100 point scale) Endoscopist rated pain (100 point scale) Post‐procedure evaluation questionnaire (physical discomfort, psychological distress, satisfaction) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'envelopes shuffled' |
| Allocation concealment? | Low risk | 'sealed envelopes' |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Low risk | 'Assesments were conducted by an assistant psychologist (CW) who did not attend the colonoscopy and was blind to study group' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 23 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Roongpisuthip 1999.
| Methods | Design: three group parallel trial Purpose: examine the effect of dexfenfluramine on weight loss and thermogenesis in obese individuals | |
| Participants | Patients: out‐patients with body mass index > 25 kilograms per square meter Baseline comparability: yes | |
| Interventions | Placebo: capsules for three months Untreated: no capsules Experimental: capsules with dexfenfluramine (Co‐intervention: 8 week behavioural weight loss program) | |
| Outcomes | Weight loss Other weight outcomes (waist/hip ratio, biceps fold, subscapular fold, arm circumference, etc) Daily activity and changes in behaviour Side effects | |
| Notes | 13 of 32 patients in the no treatment group dropped out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Patients in group I were randomly stratified into 2 subgroups... in a double‐blind, placebo‐controlled manner' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 37 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Roscoe 2002.
| Methods | Design: three period, Latin square, cross‐over trial Purpose: examine the effect of acustimulation on chemotherapy‐induced nausea | |
| Participants | Patients: cancer patients receiving chemotherapy who previously have experienced moderate or severe chemotherapy‐induced nausea | |
| Interventions | Placebo: acustimulation wrist‐band without stimulation of point PC‐6 Untreated: no acustimulation write band Experimental: acustimulation wrist band with stimulation on point PC‐6 (Co‐intervention: 'antiemetic pills') | |
| Outcomes | Antiemetic use (pills per day) Nausea Acute nausea Delayed nausea | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Roscoe 2005.
| Methods | Design: three group parallel trial Purpose: examine the effect of acustimulation on chemotherapy‐induced nausea in women with breast cancer | |
| Participants | Patients: patients about to receive their second chemotherapy treatment who experienced nausea or vomiting after the first treatment | |
| Interventions | Placebo: acustimulation wrist‐band without stimulation Untreated: no acustimulation wrist band Experimental: acustimulation wrist band with stimulation (Co‐intervention: standard clinical antiemetic prophylaxis, including a 5‐HT3 receptor antagonist) | |
| Outcomes | Acute nausea (7‐point scale) Delayed nausea Vomiting Quality of life Antiemetic medication | |
| Notes | SE values were provided in the original publication and these values were converted to SDs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 64 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Rosen 1976.
| Methods | Design: five group parallel trial Purpose: examine the effect of three types of behavioural therapy on snake phobia | |
| Participants | Patients: out‐patients suffering from snake phobia Baseline comparability: yes | |
| Interventions | Placebo: ‐posted instructions of factual information about snakes ('systematic re‐learning' ) Untreated: no behavioural or placebo procedure (waiting‐list) Experimental: ‐systematic desensitization procedure by posted instructions ‐systematic desensitization procedure by therapist ‐systematic desensitization procedure by minimal therapist contact through telephone (Co‐intervention: No) | |
| Outcomes | Anxiety score and heart rate and at slide provocation test Snake Attitude Questionnaire (SNAQ) Behavioural Approach test Fear Survey Schedule Rate of fear change | |
| Notes | 38% drop‐outs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '.... a self‐administered double‐blind placebo control...' |
| Blinding? Outcome assessor | Low risk | '... follow‐up assessments... were conducted by assistants blind to subjects' group assignment' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 14 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Rossi 1982.
| Methods | Design: three group, three period cross‐over trial Purpose: examine the effect of labetalol on hypertension | |
| Participants | Patients: in‐patients with essential hypertension Baseline comparability: yes | |
| Interventions | Placebo: tablet without labetalol Untreated: no tablet Experimental: tablet with labetalol (Co‐intervention: No) | |
| Outcomes | Diastolic blood pressure (mm Hg) | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 12 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Roughan 1981.
| Methods | Design: three group parallel trial Purpose: examine the effect of pelvic floor exercises on orgasmic potential | |
| Participants | Patients: female out‐patients not having had an orgasm for two years Baseline comparability: NS | |
| Interventions | Placebo: instruction to do relaxation exercises Untreated: no exercises (waiting‐list) Experimental: instructions to do pelvic floor exercises (Co‐intervention: no psychological counselling) | |
| Outcomes | Number of women having had orgasm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/exercise instructions) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 26 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Rowbotham 1996.
| Methods | Design: three group, four period cross‐over trial Purpose: examine the effect of lidocaine patches on post‐herpetic neuralgia | |
| Participants | Patients: out‐patients with post‐herpetic neuralgia Baseline comparability: not relevant | |
| Interventions | Placebo: patch with vehicle but no lidocaine Untreated: no patch (observational group) Experimental: patch with vehicle and lidocaine (two periods) (Co‐intervention: oral analgesics as prescribed before entering the trial, including escape medication, dose NS) | |
| Outcomes | Pain (VAS) Side effects Blood lidocaine | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... double‐blind controlled study...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 70 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Rupert 1978.
| Methods | Design: factorial design, 3 (biofeedback, placebo, no biofeedback) x 2 (instructions to increase or decrease heart rate) + 1 (no treatment) Purpose: examine the effect of biofeedback on anxiety and heart rate | |
| Participants | Patients: psychiatric in‐patients deemed to have a high degree of anxiety problems by their physician Baseline comparability: yes (anxiety scores, heart rate) | |
| Interventions | Placebo: biofeedback sessions with false positive feedback Untreated: no sessions (observational) Experimental: biofeedback sessions with correct feedback (Co‐intervention: anxiolytics, fixed dosage.) | |
| Outcomes | Anxiety Heart rate | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 16 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Rybarczyk 1990.
| Methods | Design: four group parallel trial Purpose: examine the effect of stress management interventions on preoperative anxiety | |
| Participants | Patients: older male in‐patients undergoing major surgery Baseline comparability: yes (anxiety scores) | |
| Interventions | Placebo: session of present focus interview prompting discussions on positive activities in the patient's present life Untreated: no session Experimental: ‐session of general reminiscence interview prompting patient to recall positive events from the first half of their life ‐session of challenge reminiscence interview prompting the patient to recall successfully met challenges (Co‐intervention: NS) | |
| Outcomes | Anxiety (Spielberger State‐trait Anxiety Inventory) Coping self‐efficacy inventory Physiological and postoperative adjustment measures | |
| Notes | Relevant outcome data not accessible in trial report but retrieved by contact with authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/stress management interventions) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15 % or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible in trial report but retrieved by contact with authors. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 49 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15 % or NS |
Röschke 2000.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on major depression | |
| Participants | Patients: inpatients with depression (score > 17 on 21 item Hamilton depression scale) Baseline comparability: yes for age and score on Hamilton depression scale; no for gender | |
| Interventions | Placebo: whole body needling in sites not regarded true acupuncture points for 30 minutes 3 times weekly for 4 weeks Untreated: no acupuncture Experimental: whole body acupuncture sessions (Co‐intervention: mianserin at fixed doses; diazepam 'if required' but actual medication the first four weeks was roughly comparable between groups) | |
| Outcomes | Self‐rating scale (Bf‐S) Global assessment scale (GAS) Bech‐Rafaelsen melancholia Scale (BRMS) Clinical global impressions scale (CGI) Need of diazepam medication | |
| Notes | SD for GAS and Self‐rating scale not reported. Authors were contacted and provided the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | SD for GAS and Self‐rating scale not reported. Authors were contacted and provided the data. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 48 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Rösler 2003.
| Methods | Design: three group parallel trial Purpose: examine the effect of single needle acupuncture in suppressing gag‐reflex during transesophageal echocardiography (TEE) | |
| Participants | Patients: acupuncture naive patients with presumed cardioembolic stroke or transient ischemic attack Baseline comparability: yes | |
| Interventions | Placebo: needling in the Chengjiang point (CV24) during TEE Untreated: no needling Experimental: needling 'at a sham point' (tip of the chin (Co‐intervention: 0.5% tetracaine spray) | |
| Outcomes | Gag‐reflex (10 point VAS) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'computer‐evoked' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Sanders 1990.
| Methods | Design: three group parallel trial Purpose: examine the effect of chiropractic spinal manipulation on acute low back pain | |
| Participants | Patients: out‐patients with acute low back pain Baseline comparability: no, stratified for sex | |
| Interventions | Placebo: light physical touch by investigator at L4/L5‐S1 region Untreated: no touch or manipulation Experimental: low amplitude high velocity manipulation of L4/L5‐S1 region (Co‐intervention: no) | |
| Outcomes | Pain (5‐point NRS) Plasma B‐endorphin concentration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'table of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/ manipulation) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 12 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Schallreuter 2002.
| Methods | Design: three group parallel trial Purpose: examine the effect of pseudocatalase cream and placebo on repigmentation in vitiligo | |
| Participants | Patients: out‐patients with vitiligo Baseline comparability: NS | |
| Interventions | Placebo: cream with no pseudocatalase Untreated: no cream Experimental: cream with pseudocatalase (Co‐intervention: climatotherapy) | |
| Outcomes | No or minimal repigmentation (photographs graded 0 to 3) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Scharf 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture in patients with osteoarthritis | |
| Participants | Patients: patients with symptomatic osteoarthritis of the knee Baseline comparability: yes (age, gender, pain duration) | |
| Interventions | Placebo: acupuncture on sites not regarded acupuncture sites Untreated: no acupuncture Experimental: acupuncture on sites regarded acupuncture sites (Co‐intervention: standard medical care (physiotherapy and NSAIDs)). | |
| Outcomes | Success rate (at least 36% change from baseline WOMAC scores) WOMAC (Western Ontario and McMaster Universities osteoarthritis) index Physical and mental health (SF‐36) Global patient assessment | |
| Notes | Patients in the no‐treatment group took more medication, and received more sessions of physiotherapy (median 10) than the other groups (median 6). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'block randomization with block size of 6, stratified by center, was computer‐generated by an independent statistician’ |
| Allocation concealment? | Low risk | 'Centralised telephone procedure' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupressure) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Low risk | Primary outcome specified in protocol |
| Free of other bias? | Unclear risk | See notes |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 681 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three criteria fulfilled |
Scharff 2002.
| Methods | Design: three group parallel trial Purpose: examine the effect of minimal‐contact thermal biofeedback and attention‐placebo on children's migraine | |
| Participants | Patients: children 7 to 17 years with migraine (minimum average 5 attacks a month) referred by neurologists Baseline comparability: yes (age, gender, headache index, days with headache, etc) | |
| Interventions | Placebo: four 1‐hour sessions within a period of 6 weeks with 'handcooling' sham biofeedback and general discussion about 'their lives and headache' Untreated: no sessions Experimental: sessions of thermal biofeedback ('hand warming') (Co‐intervention: ibuprofen and acetaminophen for headache. Instruction of 'not to change' medication habits) | |
| Outcomes | Incidence of patients with decrease in headache index of 50% or more Pain (Headache Index, 4‐point Likert scale) Temperature change Treatment credibility Child depression index (CDI) State‐trait anxiety inventory for children (STAIC) | |
| Notes | We have presented the outcome as no improvement for consistency of direction of outcomes. We excluded one patient from the numerators of both the placebo and no‐treatment groups to be able to compute the result, as relative risk cannot be calculated when all patients in a group have a negative outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'randomization table stratified by two age groups' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/biofeedback) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 23 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49, unclear allocation concealment |
Seer 1980.
| Methods | Design: three group parallel trial Purpose: examine the effect of meditation on hypertension | |
| Participants | Patients: out‐patients with essential arterial hypertension Baseline comparability: NS | |
| Interventions | Placebo: relaxation sessions Untreated: no relaxation or meditation sessions Experimental: meditation sessions (Co‐intervention: no) | |
| Outcomes | Diastolic blood pressure (mm Hg) Heart rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Low risk | '... trained psychologist who was blind to all experimental conditions' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 27 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Senediak 1985.
| Methods | Design: four group parallel trial Purpose: examine the effect of rapid versus gradual scheduling of behavioural weight reduction programme | |
| Participants | Patients: obese children Baseline comparability: yes | |
| Interventions | Placebo: discussion and relaxation sessions Untreated: no sessions (waiting list) Experimental: sessions with rapid versus gradual scheduling of a behavioural weight reduction programme (Co‐intervention: NS) | |
| Outcomes | Weight (kg) % overweight Subcapsular skin fold thickness Normal % skin fold thickness Caloric intake Activity output Expectancy and programme evaluation ratings | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural programme) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 21 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Shen 2000.
| Methods | Design: three group parallel trial Purpose: examine the effect of electroacupuncture for chemotherapy‐induced emesis | |
| Participants | Patients: female patients receiving chemotherapy Baseline comparability: yes for age, no for emesis with prior chemotherapy | |
| Interventions | Placebo: superficial needling at a location different from PC6 or ST36, no 'de Qi', or electrical stimulation ('minimal needling' ) Untreated: no needling Experimental: needling at PC6 or ST36, de Qi ‐sensation, and electrical stimulation (Co‐intervention: standard antiemetic regime and escape medication) | |
| Outcomes | Use of antiemetic medication Total number of emesis episodes Proportion of emesis free days | |
| Notes | Overall antiemetic escape medication was not reported. We report the use of prochlorperazine as outcome, which we consider the relevant drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number table' |
| Allocation concealment? | Low risk | 'serially numbered, sealed, opaque envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/electroacupuncture) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. Overall antiemetic escape medication was not reported. We report the use of prochlorperazine as outcome, which we consider the relevant drug. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 67 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Sibilio 1957.
| Methods | Design: three group parallel trial Purpose: examine the effect of promazine on the behaviour of chronic schizophrenics | |
| Participants | Patients: woman chronic schizophrenic in‐patients Baseline comparability: yes | |
| Interventions | Placebo: capsule with no promazine Untreated: no capsule Experimental: capsule with promazine (Co‐intervention: no) | |
| Outcomes | Behaviour change on Gardner Behavior Chart | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The double‐blind technique was employed throughout the study' |
| Blinding? Outcome assessor | Low risk | 'Those attendants who rated the patients' behavioral adjustment did not dispense medication and were unaware of the experimental group to which a patient was assigned' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 62 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Sinaiko 1991.
| Methods | Design: four group parallel trial Purpose: examine the effect of low sodium diet or potassium supplementation on adolescent blood pressure | |
| Participants | Patients: adolescents with systolic blood pressure above 109 mm Hg (boys) and 108 mm Hg (girls) Baseline comparability: yes | |
| Interventions | Placebo: capsules Untreated: no capsules Experimental: ‐ capsules with potassium ‐low sodium diet (Co‐intervention: NS) | |
| Outcomes | Diastolic blood pressure (mm Hg) at three years Systolic blood pressure | |
| Notes | Data from the no‐treatment group not published. Additional data received from the authors. Randomisation not 1:1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The capsule treatment is a double‐masked design...' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Data from the no‐treatment group not published. Additional data received from the authors. Randomisation not 1:1. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 87 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Sipich 1974.
| Methods | Design: five group parallel trial Purpose: examine the effect of 'covert sensitization' on smoking behaviour | |
| Participants | Patients: smokers Baseline comparability: yes (number of smoked cigarettes) | |
| Interventions | Placebo: sessions of listening to illusory subliminal messages Untreated: ‐no sessions with continuous monitoring of smoking rates ‐no sessions with pre‐post monitoring of smoking rates Experimental: ‐covert sensitization sessions (visualization of feelings of nausea and vomiting as imagining themselves smoking) ‐self control suggestion sessions (told to quit by their own effort) (Co‐intervention: NS) | |
| Outcomes | Mean number of cigarettes smoked per day | |
| Notes | Standard deviation of untreated and placebo means estimated from t‐test of baseline‐post intervention change in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/covert sensitization) |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | No protocol available. Standard deviation of untreated and placebo means estimated from t‐test of baseline‐post intervention change in the placebo group |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Sommerness 1955.
| Methods | Design: three group parallel trial Purpose: examine the effect of reserpine on the behaviour of chronic mentally ill patients | |
| Participants | Patients: chronic mentally ill men Baseline comparability: yes | |
| Interventions | Placebo: pill with no reserpine Untreated: no pill Experimental: pill with reserpine (Co‐intervention: yes, no group difference) | |
| Outcomes | Behaviour change Blood pressure Weight | |
| Notes | Relevant outcome data not accessible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers table' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The hospital pharmacist alone knew which group received reserpine or placebo' |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Relevant outcome data not accessible |
| Free of other bias? | Low risk | |
| Trial size > 49? | Low risk | N = 60 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Spanos 1995.
| Methods | Design: four group parallel trial Purpose: examine the effect of hypnosis on smoking reduction | |
| Participants | Patients: smokers Baseline comparability: yes (number of cigarettes smoked) | |
| Interventions | Placebo: sessions of listening to illusory subliminal messages Untreated: no sessions Experimental: ‐sessions of hypnosis ‐sessions called 'cognitive restructuring' with procedures identical to the hypnosis group but with no mention of hypnosis and no hypnotic induction (Co‐intervention: NS) | |
| Outcomes | Mean number of cigarettes smoked per day | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/hypnosis) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 25 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Sprott 1993.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on fibromyalgia | |
| Participants | Patients: in‐patients suffering from fibromyalgia Baseline comparability: NS | |
| Interventions | Placebo: laser acupuncture with laser off Untreated: no acupuncture Experimental: laser acupuncture with laser on (Co‐intervention: physio‐, thermo‐ and electrotherapy. Fixed scheme at start of treatment. Paracetamol on demand, intake NS) | |
| Outcomes | Pain (VAS and pain threshold) Number of positive tender points | |
| Notes | Standard deviations (SD) on 10 cm pain visual analogue scale data not reported. SD estimated from another pain RCT (Lander 1993: SD ˜ 3 cm) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | No protocol available. Standard deviations (SD) on 10 cm pain visual analogue scale data not reported. SD estimated from another pain RCT (Lander 1993: SD ˜ 3 cm) |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Stabholz 1991.
| Methods | Design: three group parallel trial Purpose: examine the effect of sustained release delivery system of chlorhexidine on oral hygiene of patients with Down's syndrome | |
| Participants | Patients: institutionalised with Down's syndrome Baseline comparability: NS | |
| Interventions | Placebo: teeth coating without chlorhexidine Untreated: no teeth coating Experimental: teeth coating with chlorhexidine (Co‐intervention: penicillin before scaling and polishing teeth, normal oral hygiene) | |
| Outcomes | Plaque index Gingival index Papillary bleeding Plaque bacterial counts | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'The study was double‐blind...' |
| Blinding? Outcome assessor | Low risk | '... the examiner was not aware which treatment was given' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Steinsbekk 2004.
| Methods | Design: four group parallel trial Purpose: examine the effect of homeopathy for upper respiratory infections (URTI) in children | |
| Participants | Patients: children with upper respiratory infection Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: lactose pill Untreated: no pill Experimental: ‐Self‐selected ultramolecular homeopathic pill ‐treatment by homeopath (Co‐intervention: no) | |
| Outcomes | Total URTI score Days with URTI Days with antibiotics Days with analgesic/antipyretic Visits to medical doctor Days with other illness, noises from the chest, or work absence due to ill child (all outcomes also as binary: proportion of children with days of ...) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'computer' |
| Allocation concealment? | Low risk | 'central' |
| Blinding? Treatment provider | Low risk | 'This trial was of double‐blind, randomized parallel group placebo controlled design' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 176 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Stewart 1991.
| Methods | Design: four group parallel trial Purpose: examine the effect of cognitive‐behavioural therapy on oral hygiene | |
| Participants | Patients: veteran out‐patients with normal oral hygiene Baseline comparability: yes | |
| Interventions | Placebo: sessions of lectures on non‐disease aspects of dentistry Untreated: no sessions Experimental: sessions of cognitive‐behavioural therapy (Co‐intervention: NS) | |
| Outcomes | Brushing frequency (per week) and Plaque Index Flossing frequency | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive‐behavioural therapy) |
| Blinding? Outcome assessor | Low risk | 'The dentist that rated plaque levels was blind to each subject's assigned experimental group' |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Drop‐out > 15% or NS |
Stransky 1989.
| Methods | Design: three group parallel trial Purpose: examine the effect of vitamin B6 on carpal tunnel syndrome | |
| Participants | Patients: out‐patients suffering from carpal tunnel syndrome Baseline comparability: NS | |
| Interventions | Placebo: tablet without vitamin B6 (content: dextrose) Untreated: no tablet Experimental: tablet with vitamin B6 (Co‐intervention: NS) | |
| Outcomes | Number of patients who improved in symptoms Improvement in median and ulnar nerve conduction latency | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'We undertook a randomized, double‐blind, placebo‐controlled study...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 9 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Straub 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of chiropractic care on jet lag | |
| Participants | Patients: junior elite athletes Baseline comparability: NS | |
| Interventions | Placebo: sham chiropractic manipulations for 19 days Untreated: no chiropractic sessions Experimental: chiropractic sessions (Co‐intervention: NS) | |
| Outcomes | Sleep disturbance (duration of sleep in hours, sleep onset in minutes, numbers of sleep bouts, movement and fragmentation index) Jet lag rating Mood (Profile of Mood States questionnaire) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'table of random numbers' |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (chiropractic manipulation/placebo manipulation) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 10 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Sumaya 2001.
| Methods | Design: three group, three period, cross‐over trial Purpose: examine the effect of bright light treatment on depression in institutionalised older adults | |
| Participants | Patients: institutionalised older adults with moderate to severe depression (baseline scores on the Geriatric Depression Scale 11 to 20) Baseline comparability: not relevant |
|
| Interventions | Placebo: sessions with light treatment with 300 Lux for 30 minutes per day for 1 week Untreated: no light treatment Experimental: light treatment with 10,000 Lux (Co‐intervention: NS) | |
| Outcomes | Geriatric Depression Scale at day 5 of each treatment period | |
| Notes | The outcome data was not available from the first period only, and was calculated as deriving from a parallel group trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | NS |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | The outcome data from this cross‐over trial was not available from the first period only, and was calculated as deriving from a parallel group trial. |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Tan 1982.
| Methods | Design: three group parallel trial Purpose: examine the effect of prophylactic behavioural‐cognitive training on procedural acute pain | |
| Participants | Patients: out‐patients having to undergo a painful diagnostic procedure (knee arthrogram) Baseline comparability: yes | |
| Interventions | Placebo: procedural information and pain experience discussion without any pain control skills training Untreated: no information or training Experimental: behavioural‐cognitive skills training for pain control (Co‐intervention: NS) | |
| Outcomes | Pain: McGill pain questionnaire Pain: radiologist's rating and videotaped pain behaviour Fear (self report and radiologist's rating) Discomfort (self report and radiologist's rating) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 24 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Tan 1986.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive‐behaviour therapy on psycho‐social problems and seizure control in epileptic patients | |
| Participants | Patients: epileptic out‐patients with psycho‐social problems (anxiety, depression) and inadequate seizure control Baseline comparability: yes | |
| Interventions | Placebo: sessions of supportive group counselling or discussion with no specific cognitive‐behaviour therapy Untreated: no sessions Experimental: sessions of behavioural‐cognitive therapy (Co‐intervention: ‐'professional counselling or psychiatric treatment' to all but 6 patients ‐anticonvulsant medication. The difference in serum‐level between the groups was not significant.) | |
| Outcomes | Number of patients with improvement in seizure frequency (frequency diary) Seizure rating by blinded observer Global rating of psycho‐social adjustment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural cognitive training) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 19 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trials size < 49 |
Tarcin 2004.
| Methods | Design: three group parallel trial Purpose: examine the effect of acustimulation in patients undergoing gastroscopy | |
| Participants | Patients: out‐patients undergoing gastroscopy Baseline comparability: yes (age, gender) | |
| Interventions | Placebo: ‐electrical device attached to electrodes and acustimulation performed on a site not regarded acupuncture site ‐electrical device attached to electrodes but no acustimulatiuon performed Untreated: no electrical device attached nor acustimulation performed Experimental: acustimulation on point P6 (Co‐intervention: no) | |
| Outcomes | Proportion of patients scoring 1 to 4 on a VAS for nausea Proportion of patients scoring 1 to 4 on a VAS for difficulty of swallowing gastroscopy Proportion of patients scored 1 to 4 by gastrocopist on a VAS for nausea/retching Proportion of patient who accept re‐gastroscopy | |
| Notes | The results from the two placebo groups were combined | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acustimulation) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 235 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Tarrier 1998.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive behaviour therapy on positive symptoms in patients with chronic schizophrenia | |
| Participants | Patients: people with chronic schizophrenia Baseline comparability: yes | |
| Interventions | Placebo: sessions of supportive counselling Untreated: no sessions Experimental: sessions of behavioural cognitive therapy (Co‐intervention: standard care including medication, fixed dose) | |
| Outcomes | Number of patients with improvement of positive symptoms by 50% or more Mean number and intensity of positive psychotic symptoms on Present state examination and Brief psychiatric rating scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'stratified block randomised procedure' |
| Allocation concealment? | Low risk | 'sealed envelopes' ... ' carried out by a third party' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/behavioural cognitive therapy) |
| Blinding? Outcome assessor | Low risk | 'Effort was made to blind the independent assessors...' ... '... suggesting that blinding was satisfactory' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Tashjian 2006.
| Methods | Design: three group parallel trial Purpose: examine the effect of zolpidem on postoperative pain | |
| Participants | Patients: out‐patients undergoing knee arthroscopy Baseline comparability: yes (age, gender, preoperative pain score) | |
| Interventions | Placebo: gelatin pills with no zolpidem Untreated: no pills Experimental: pills with zolpidem (Co‐intervention: yes (ibuprofen + hydrocodone/acetaminophen)) | |
| Outcomes | Pain (0 to 10 VAS; mean daily postoperative) Pain (mean morning and evening postoperative) Fatigue (0 to 10 VAS; mean daily postoperative) Fatigue (mean morning and evening postoperative) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | '... the surgeon was unaware if the patient was given zolpidem or placebo' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 43 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Theroux 1993.
| Methods | Design: three group parallel trial Purpose: examine the effect of intranasal midazolam in facilitating suturing of lacerations in children | |
| Participants | Patients: preschool children with lacerations visiting an emergency department Baseline comparability: yes | |
| Interventions | Placebo: nasal spray without midazolam Untreated: no nasal spray Experimental: nasal spray with midazolam (Co‐intervention: NS) | |
| Outcomes | Anxiety (cry score) Parent satisfaction Heart rate Blood pressure Respiratory rate Pulse oximetry Cry score Motion score Struggle score | |
| Notes | Data from placebo and no‐treatment groups pooled. Contact with researchers provided unpooled data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Allocation concealment? | Unclear risk | NS |
| Blinding? Treatment provider | Low risk | 'Blinding was maintained for the physician by having them leave the bedside for a short interval...' |
| Blinding? Outcome assessor | Low risk | 'Cry... was assessed by the physician...' (See above) |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15% or NS |
| Free of selective reporting? | High risk | Data from placebo and no‐treatment groups pooled. Contact with researchers provided unpooled data |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 32 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Thomas 1987.
| Methods | Design: 2 x 2 factorial design (positive or negative consultation x placebo tablet or no tablet) Purpose: examine the effect of positive consultation style and placebo tablet | |
| Participants | Patients: out‐patients attending a GP clinic with symptoms but without any physical signs and in whom no definite diagnosis could be made Baseline comparability: yes | |
| Interventions | Placebo: tablet containing thiamine Untreated: no tablet Experimental: ‐positive consultation: firm diagnosis + good prognosis ‐negative consultation: no diagnosis and uncertain prognosis (Co‐intervention: NS) | |
| Outcomes | Number of patients who improved Doctor‐patient contact Degree of communication Number of days before improvement Need for further treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | NS |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 200 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Thomas 1999.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive‐behavioural intervention, and placebo, on pain associated with sickle cell disease | |
| Participants | Patients: out‐patients with sickle cell disease (type HbSS) Baseline comparability: yes (age, gender, hospital admissions for painful crises, etc) | |
| Interventions | Placebo: one hourly session per week for two months of general discussions of the problems of living with sickle cell disease (attention‐placebo) Untreated: no sessions Experimental: sessions of cognitive‐behavioural therapy (Co‐intervention: NS) | |
| Outcomes | Pain (Short form of McGill pain questionnaire) General health questionnaire Coping strategies questionnaire Pain self‐efficacy questionnaire Beliefs about pain control questionnaire Number of hospital and emergency admissions Duration of hospital stay | |
| Notes | The result is probably unreliable because 38 of 97 patients dropped out (39%). In addition, 23 of 56 patients treated with placebo or active sessions were excluded because of failure to attend sessions or to complete assessments, no such exclusions described for no‐treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number table and was restricted to blocks of four ...' |
| Allocation concealment? | Unclear risk | 'a sequence of labeled cards contained in sealed numbered envelopes' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (cognitive‐behavioural intervention/placebo) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | Drop‐out > 15%. The result is probably unreliable because 38 of 97 patients dropped out (39%). In addition, 23 of 56 patients treated with placebo or active sessions were excluded because of failure to attend sessions or to complete assessments, no such exclusions described for no‐treatment group. |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Thomas 2002a.
| Methods | Design: 2x2 factorial design with an additional randomisation for one arm Purpose: examine the effect of a home based exercise programme on knee pain, and to determine the contribution of the contact with a therapist in explaining the outcome | |
| Participants | Patients: out‐patients with knee pain recruited through a postal questionnaire Baseline comparability: yes (age, gender, pain, weight, etc) | |
| Interventions | Placebo: tablet with dolomite (calcium and magnesium) twice weekly for two years Untreated: no tablets Experimental: ‐exercise (20‐30 minutes daily, initiated by four 30 minutes' instruction sessions within the first two months in the patients' home, and follow up visits every six months). ‐telephone (monthly telephone contact) (Co‐intervention: no information on use of analgesic drugs) | |
| Outcomes | Pain (WOMAC osteoarthritis index, 0 to 20) Knee stiffness Disability General physical function (SF‐36) Hospital anxiety and depression scale Isometric quadriceps muscle strength | |
| Notes | Not 1:1 randomisation. Dropout rate: 23.7%. Results not presented for placebo and no‐treatment, but authors provided additional data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'computer generated lists in permuted blocks of 10, stratified by sex and age' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (home based exercise/placebo) |
| Blinding? Outcome assessor | Unclear risk | Patient reported outcome (interviewer was blinded) |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | High risk | No protocol available. Results not presented for placebo and no‐treatment, but authors provided additional data. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 156 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Thomas 2002b.
| Methods | Same trial as Thomas 2002a, but results are presented as two subanalyses because there was one group of patients randomised to placebo or no treatment (see above), and another group randomised to telephone contact, exercise and placebo versus telephone contact and exercise. | |
| Participants | See Thomas 2002a | |
| Interventions | Placebo: dolomite (calcium and magnesium) twice weekly for two years Untreated: no tablets Experimental: see Thomas 2002a (Co‐intervention: exercise and telephone contact) | |
| Outcomes | See Thomas 2002a | |
| Notes | See Thomas 2002a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'computer generated lists in permuted blocks of 10, stratified by sex and age' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (home based exercise/placebo) |
| Blinding? Outcome assessor | Unclear risk | Patient reported outcome (interviewer was blinded) |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | High risk | No protocol available. Results not presented for placebo and no‐treatment, but authors provided additional data. |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 233 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Tremeau 1992.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture on cervical maturation | |
| Participants | Patients: women in 37th to 38th week of pregnancy and a Bishop score < 4 Baseline comparability: yes (Bishop score) | |
| Interventions | Placebo: acupuncture in relevant sites Untreated: no acupuncture (observational) Experimental: acupuncture 1 cm from relevant sites (Co‐intervention: standard care) | |
| Outcomes | Bishop score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Low risk | '... par un obstétricien ou une sage‐femme, ne connaissant ni l'un, ni l'autre, le groupe de tirage au sort de la patiente' |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 64 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Tritrakarn 2000.
| Methods | Design: five group parallel trial Purpose: examine the analgesic effect of EMLA cream (lidocaine and prilocaine) on pain associated with extracorporeal lithotripsy, and if effective, to examine which component (the cutaneous anaesthesia, the cream or the occlusive dressing) contributes to the analgesia | |
| Participants | Patients: out‐patients undergoing pain inducing extracorporeal lithotripsy because of renal stones Baseline comparability: yes | |
| Interventions | Placebo: no occlusive dressing ‐occlusive dressing without cream Untreated: no dressing or cream Experimental: 3 groups received occlusive dressing with and without cream (Co‐intervention: no) | |
| Outcomes | Pain (numerical verbal pain scale, 0 to 100) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | ' A randomized, double‐blind, crossover study...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 82 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Tsay 2003.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupressure on quality of sleep | |
| Participants | Patients: in‐patients with end stage renal disease Baseline comparability: yes | |
| Interventions | Placebo: acupressure on sites not regarded acupressure sites Untreated: no acupressure Experimental: acupressure on sites regarded acupressure sites (Co‐intervention: standard medical care) | |
| Outcomes | Pittsburgh sleep quality index Sleep log | |
| Notes | The trial report does not state the number of patients allocated to each group. We assumed that out of 98 patients in the three‐armed trial 32 patients entered the placebo group and 32 patients the no‐treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | High risk | No protocol available. The trial report does not state the number of patients allocated to each group. We assumed that out of 98 patients in the three‐armed trial 32 patients entered the placebo group and 32 patients the no‐treatment group |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 64 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Tsay 2004.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupressure on fatigue in patients with end‐stage renal disease | |
| Participants | Patients: patients with end‐stage renal disease Baseline comparability: yes | |
| Interventions | Placebo: acupressure on sites not regarded acupressure sites Untreated: no acupressure Experimental: acupressure on sites regarded acupressure sites (Co‐intervention: standard medical care) | |
| Outcomes | Revised Piper fatigue Scale Fatigue (100 mm VAS) Pittsburgh Sleep Quality Index Beck Depression Inventory | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 71 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Tuomilehto 1980.
| Methods | Design: three group parallel trial Purpose: examine the effect of high fibre intervention (guar gum) on serum lipoproteins and body weight | |
| Participants | Patients: out‐patients with hypercholesterolaemia Baseline comparability: NS | |
| Interventions | Placebo: granule with no guar gum (wheat flower) Untreated: no granule Experimental: granule with guar gum (Co‐intervention: diet instructions: decrease alcohol and fat, increase complex carbohydrates) | |
| Outcomes | Serum‐cholesterol: total (mmol/l) Serum‐cholesterol: high density lipoprotein Serum‐triglyceride Weight Blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | '... studied in a double‐blind controlled trial' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 22 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Turner 1979.
| Methods | Design: five group parallel trial Purpose: examine the effect of paradoxical intention therapy on insomnia | |
| Participants | Patients: out‐patients suffering from insomnia Baseline comparability: yes (sleep parameters) | |
| Interventions | Placebo: sessions of 'quasi‐desensitization' (neutral images paired with bedtime activity) Untreated: no sessions (waiting list) Experimental: sessions of ‐paradoxical intention therapy: instructions to remain awake as long as possible and presented with the true theoretical background ‐stimulus control: practical advice on bed time activities ‐progressive relaxation: training in relaxation techniques (Co‐intervention: hypnotics, comparable doses between groups) | |
| Outcomes | Sleep latency (min) Returning to sleep difficulty Restedness rating Falling asleep difficulty Hours of sleep Drug usage per week | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/paradoxical intention) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 20 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Tyler 1946.
| Methods | Design: three group parallel trial Purpose: examine the prophylactic effect of placebo and various drugs on seasickness | |
| Participants | Patients: soldiers undergoing amphibious training Baseline comparability: NS | |
| Interventions | Placebo: lactose capsules Untreated: no capsules Experimental: various belladonna alkaloid and barbiturate preparations (Co‐intervention: NS) | |
| Outcomes | Number of patients with seasickness | |
| Notes | The randomisation procedure was in principle open to selection bias, however, the allocation took place openly under full military discipline so we do not think selection bias was likely. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'cards from deck so stacked as to ensure a random distribution' |
| Allocation concealment? | Unclear risk | See notes |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 563 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Vlaeyen 1996.
| Methods | Design: three group parallel trial Purpose: examine the effect of cognitive therapy on fibromyalgia | |
| Participants | Patients: out‐patients with fibromyalgia Baseline comparability: yes | |
| Interventions | Placebo: sessions of group discussions on pain experience + educational programme on pain Untreated: no sessions Experimental: sessions of cognitive therapy + educational programme on pain (Co‐intervention: NS) | |
| Outcomes | Pain (McGill pain questionnaire) Pain: coping, control, behaviour % positive responders Relaxation Tension Catastrophing Activity Obsessive‐compulsive Fear Depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/cognitive therapy) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | Low risk | N = 79 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Walton 1993.
| Methods | Design: three group parallel trial Purpose: examine the prophylactic effect of penicillin on post treatment symptoms following root canal treatment for asymptomatic periapical pathosis | |
| Participants | Patients: out‐patients undergoing root canal treatment Baseline comparability: NS | |
| Interventions | Placebo: tablets without penicillin Untreated: no tablets Experimental: tablets with penicillin (Co‐intervention: NS) | |
| Outcomes | Number of patients in pain Swelling Side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 54 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Wang 1997.
| Methods | Design: four group parallel trial Purpose: examine the effect of transcutaneous acupoint electrical stimulation (TAES) on postoperative analgesic requirement | |
| Participants | Patients: patients having undergone lower abdominal surgical procedures Baseline comparability: yes | |
| Interventions | Placebo: TAES without electrical stimulation Untreated: no TAES Experimental: TAES with electrical stimulation (low and high) (Co‐intervention: standard operational procedures) | |
| Outcomes | Total opoid requirement (in equivalents of mg hydromorphone) in 24 hours Morphine (mg) delivered by PCA (patient controlled analgesia) device Number of times patients used PCA device (patient controlled analgesia) Supplemental opioid analgesics (i.m.) Supplemental oral analgesics Duration of PCA Duration of use of TAES Duration of stay in postanaesthesia care unit Duration of hospital stay Pain, 100 mm VAS Fatigue, 100 mm VAS Discomfort, 100 mm VAS Follow‐up questionnaire | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘computer‐generated randomization sequence' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/TAES) |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | Low risk | N = 51 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Watzl 1986.
| Methods | Design: 2 x 2 factorial design Purpose: examine the effect of strict control and placebo on relapse rate of abstinent alcoholics | |
| Participants | Patients: woman in‐patients attending an alcoholism clinic Baseline comparability: yes | |
| Interventions | Placebo: injections with saline Untreated: no injections Experimental: ‐strict external control of alcohol abstinence ‐no strict external control of alcohol abstinence (Co‐intervention: stay at a clinic with unspecified 'complex and extensive treatment program', e.g. in a few cases patients received 'liver preparation') | |
| Outcomes | Number of abstinent drinkers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/experimental) |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 70 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Allocation not clearly concealed |
Weingaertner 1971.
| Methods | Design: three group parallel trial Purpose: examine the effect of aversive stimulation (self‐administered electric shocks) on hallucinatious schizophrenic patients | |
| Participants | Patients: schizophrenic in‐patients (men) with auditory hallucinations Baseline comparability: yes | |
| Interventions | Placebo: patients equipped with a device that did not produce an electric shock and instructed to activate it when hallucinating (told that some people could not feel the shock) Untreated: not equipped with a device to induce electric shock Experimental: equipped with a device that did produce an electric shock and instructed to activate it when hallucinating (Co‐intervention: ‐medication (type and dose NS, four patients changed) ‐other experimental intervention (type NS, interference deemed implausible by authors) ‐group and milieu therapy (type, frequency NS)) | |
| Outcomes | Brief Psychiatric Rating Scale: hallucination scale Patient Data Sheet Symptom checklist Ward personnel comments | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/electric shock) |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 30 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Werntoft 2001.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupressure on nausea and vomiting during pregnancy | |
| Participants | Patients: pregnant women with nausea without treatment Baseline comparability: not for week of pregnancy (yes for age, week of pregnancy at start of nausea) | |
| Interventions | Placebo: acupressure waistband at the upper side of the wrist (not P6) for two weeks Untreated: no acupressure Experimental: acupressure waistband at P6 (Co‐intervention: no) | |
| Outcomes | Nausea (100 mm VAS) after 2 weeks Vomiting | |
| Notes | The drop‐out rate was 25% (20 out of 80). Trial probably stopped prematurely before inclusion of the planned 300 women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | each woman ‘drew an envelope from a box' |
| Allocation concealment? | Low risk | The drawn envelopes had 'the same appearance but different content', and 'The women were asked not to open the envelope until returning home' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupressure) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Whittaker 1963.
| Methods | Design: three group parallel trial Purpose: examine the effect of discontinuing medication with perphenazine in schizophrenia | |
| Participants | Patients: chronic schizophrenic in‐patients on perphenazine Baseline comparability: for age and length of hospital stay | |
| Interventions | Placebo: liquid solution with no perphenazine (content NS) Untreated: no liquid solution (observational group) Experimental: liquid solution with perphenazine (Co‐intervention: no other psychotropic drugs allowed) | |
| Outcomes | Number of patients with major relapse: need for known active medication Minor relapse: deterioration on symptom scales (psychiatric rating scale & Fergus Falls Behaviour Rating Scale) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | '...the trial was blind in that only the pharmacist knew which bottles were active' |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 26 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Wilcock 2008.
| Methods | Design: three group cross‐over trial Purpose: examine the effect of nebulised furosemide and placebo on breathlessness | |
| Participants | Patients: patients with cancer and breathlessness Baseline comparability: cross‐over trial | |
| Interventions | Placebo: inhalation of saline Untreated: no inhalation Experimental: inhalation of furosemide (Co‐intervention: standard cancer treatment | |
| Outcomes | Number reading test (number read per breath) Number reading test (total number) Arm exercise test Duration of arm test Borg Score at maximum equivalent work load Change in spirometric values | |
| Notes | Cross‐over trial. data from 1 period not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | 'The object of the current randomised, double‐blind, placebo‐controlled, cross‐over study...' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Unclear risk | Cross‐over trial. Data from 1 period not available. |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 30 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Williams 1988.
| Methods | Design: three group parallel trial Purpose: examine the effect of hypnosis on smoking cessation | |
| Participants | Patients: smokers that had attended one smoking program before Baseline comparability: NS | |
| Interventions | Placebo: one session in which the reason for smoking and attempts to stop were discussed Untreated: no session Experimental: single hypnosis session (Co‐intervention: NS) | |
| Outcomes | Number of abstinent smokers Mean number of cigarettes smoked per week | |
| Notes | All 20 patients in the placebo and 20 patients in the no‐treatment group smoked at post intervention. Data extracted as if one patient in each group did not smoke. This was done due to overcome software incapacity in computing data with no successes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/hypnosis) |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | High risk | N = 40 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Wilson 1980.
| Methods | Design: three group parallel trial Purpose: examine the effect of disulfiram and placebo implants on alcoholism | |
| Participants | Patients: alcoholics Baseline comparability: NS | |
| Interventions | Placebo: implants without disulfiram Untreated: no implants Experimental: implants with disulfiram (Co‐intervention: NS) | |
| Outcomes | Number of abstinent drinkers Mean time to first alcoholic consumption | |
| Notes | Patients were randomised to placebo and no treatment in a 4:1 ratio | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | 'Assignment to the disulfiram and placebo conditions was double‐blind' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (binary outcome) |
| Trial size > 49? | Low risk | N = 50 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | |
Witt 2005.
| Methods | Design: three group parallel trial Purpose: examine the effect of acupuncture for osteoarthritis of the knee | |
| Participants | Patients: patients with symptomatic osteoarthritis of the knee Baseline comparability: yes (WOMAC score) | |
| Interventions | Placebo: acupuncture on sites not regarded acupuncture sites Untreated: no acupuncture Experimental: acupuncture on sites regarded acupuncture sites (Co‐intervention: standard medical care. All patients were allowed to take non‐steroid anti‐inflammatory drugs if necessary) | |
| Outcomes | WOMAC (Western Ontario and McMaster Universities osteoarthritis) index Disability (Pain disability index) Physical and mental health (SF‐36) Pain (questionnaire for assessing the emotional aspects of pain) Depression (ADS depression scale) Days with limited function Days in pain (patient diary) Days with medication in weeks 5‐8 (patient diary) | |
| Notes | Patients in the no‐treatment group took medication on 5.8 days whereas the placebo group did so on 4.6 days (weeks 5 to 8). SE values were provided in the original publication and these values were converted to SD for analysis in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | ‘random list generated with Samp Size 2.0’ |
| Allocation concealment? | Low risk | 'centralised telephone randomisation procedure' |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/acupuncture) |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Low risk | Primary outcome specified in protocol |
| Free of other bias? | Unclear risk | Patients in the no‐treatment group took medication on 5.6 days whereas the placebo group did so on 4.6 days (weeks 5 to 8). |
| No signs of variance inequality or skewness? | Low risk | No variance inequality (F‐test not statistically significant) and no skewness (1.64 standard deviations does not exceed the mean) |
| Trial size > 49? | Low risk | N = 140 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | Low risk | All three categories fulfilled |
Wojciechowski 1984.
| Methods | Design: five group parallel trial Purpose: to demonstrate a) therapist bias and b) that a double blind design is feasible in psychotherapy | |
| Participants | Patients: women out‐patients with tension headache Baseline comparability: no (pre treatment headache index score) | |
| Interventions | Placebo: sessions with 'concentration therapy' and ‐positive therapist expectations ‐negative therapist expectations Untreated: no sessions Experimental: sessions with muscular relaxation therapy and ‐positive therapist expectations ‐negative therapist expectations (Co‐intervention: NS) | |
| Outcomes | Pain (Headache index score) Global therapist judgement of improvement Global patient judgement of improvement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | Low risk | '...the double blind design...' |
| Blinding? Outcome assessor | Unclear risk | Not relevant as patient reported outcome |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 21 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Woods 2005.
| Methods | Design: three group parallel trial Purpose: examine the effect of therapeutic touch on patients with dementia | |
| Participants | Patients: patients with Alzheimer's disease Baseline comparability: yes (age, gender, degree of dementia) | |
| Interventions | Placebo: mimic treatment that resembled therapeutic touch to the naive observer (no attempt to enter 'a quiet meditative state ... instead the practitioner did mental calculations). Untreated: no therapeutic touch Experimental: therapeutic touch (Co‐intervention: standard medical care). | |
| Outcomes | Modified Agitated Behavior Rating Scale (ABRS) Revised Memory and Behavior checklist | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random numbers table' |
| Allocation concealment? | Unclear risk | ‘Envelopes containing group assignments were opened just prior to the intervention to ensure blinding of all concerned during the pre‐test measurement’ |
| Blinding? Treatment provider | Low risk | 'Using a double‐blind (masked), three‐group experimental pre‐test/post‐test design...' |
| Blinding? Outcome assessor | Low risk | 'Six blind observers collected all of the data on a Behavior Monitoring Chart (BMC)' |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | High risk | Either variance inequality (F‐test statistically significant) or skewness (1.64 standard deviations exceeds the mean) |
| Trial size > 49? | High risk | N = 38 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Yan 2005.
| Methods | Design: three group parallel trial Purpose: study the effect of segmental vs. innocuous electrical stimulation for chronic pain relief | |
| Participants | Patients: patients with first acute stroke Baseline comparability: NS | |
| Interventions | Placebo: stimulation from electrical stimulation device with disconnected circuit and standard rehabilitation Untreated: standard rehabilitation only Experimental: functional electrical stimulation and standard rehabilitation (Co‐intervention: standard rehabilitation program) | |
| Outcomes | Composite spasticity scale (CSS) Maximum isometric voluntary contraction (MIVC) Walking ability (Up and Go (TUG) test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'random number produced by Jensen's computerized method of minimization' |
| Blinding? Treatment provider | High risk | Described as single‐blind (placebo/electrical stimulus device) |
| Blinding? Outcome assessor | Low risk | '... the assessor was blinded to the nature of intervention' |
| Incomplete outcome data addressed? All outcomes | Low risk | Drop‐out < 15% |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| Trial size > 49? | High risk | N = 28 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Yates 1988.
| Methods | Design: three group parallel trial Purpose: examine the effect of chiropractic treatment on blood pressure | |
| Participants | Patients: out‐patients with hypertension and thoracic subluxation Baseline comparability: NS | |
| Interventions | Placebo: session with a 'chiropractic adjusting device' without it performing the essential manipulative procedure Untreated: no session Experimental: session with a 'chiropractic adjusting device' performing the essential manipulative procedure (Co‐intervention: 5 patients in the placebo and untreated group received hypertensive medication, dose NS) | |
| Outcomes | Diastolic blood pressure reduction (mm Hg) Anxiety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? Treatment provider | High risk | Not described as double‐blind (placebo/chiropractic procedure) |
| Incomplete outcome data addressed? All outcomes | High risk | |
| Free of selective reporting? | Unclear risk | No protocol available |
| Free of other bias? | Low risk | |
| No signs of variance inequality or skewness? | Unclear risk | Not relevant (not naturally positive continuous outcomes e.g. change) |
| Trial size > 49? | High risk | N = 14 |
| Clearly concealed allocation + trial size > 49 + drop‐out max 15% | High risk | Trial size < 49 |
Outcomes: The first outcome listed is the one extracted for the review (all major outcomes for each trial are listed). NS: not stated. VAS: visual analogue scale. EMG: electromyography. GP: general practitioner. Hb: haemoglobin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbot 1995 | The 'placebo intervention' was 'compressed air with freon' which, sprayed on skin, lowers temperature: impure placebo. |
| Abikoff 1985 | All patients underwent a placebo run‐in period and only those not responding were included: not relevant participants. |
| Allen 1987 | Dropout was > 50%. |
| Allen 1996 | Randomisation was not described in the original paper. In a subsequent correspondence the authors described the method of randomisation: 'put three pieces of paper into a hat, each with the number 1, 2 or 3 and then drew a number each time a subject arrived and then assigned them accordingly '. There was no concealment of allocation. The number of patients in the placebo group was 105, in the no‐treatment group 75. |
| Amanzio 1999 | Pain was induced experimentally. |
| Amanzio 2001 | Not explicitly a randomised trial. |
| Archer 1992 | Not a randomised study. |
| Arnett 1990 | Inclusion of patients to no‐treatment groups started later than inclusion to active/placebo. |
| Avis 2008 | Patients received payment. |
| Babizhayev 2001 | No explicit randomisation between placebo and no‐treatment. |
| Barrett 1999 | Low self‐esteem is not regarded a clinical problem. |
| Beck 2002 | Male nursing home residents were not randomised, but 'assigned to the groups'. The proportion of males differed between the compared groups from 10% to 24%. |
| Benedetti 1998 | Allocation to placebo and no‐treatment was not explicitly random. |
| Benedetti 1999b | Pain was induced experimentally. |
| Benedict 1989 | The outcome in this study of chronic schizophrenia was reaction‐time which we regard not clinically relevant. |
| Bennet 2001 | The outcome was not clinical. |
| Benton 1988 | The trial studied the impact of vitamin supplements on the intelligence of normal schoolchildren, not regarded a clinical study. |
| Bergmann 1994 | No untreated group. |
| Beutler 1988 | The 'placebo' intervention consisted in 'paranormal healing at a distance', directed at a patient behind a screen, however the patients in the no treatment group also sat behind a screen, so the patient in both groups experienced the same: unacceptable no treatment group. |
| Bierman 1997 | The outcome in this alcohol addiction trial was 'sleep quality': regarded not clinically relevant. |
| Björkstén 1986 | Randomisation not mentioned. |
| Blackwell 1972 | Participants were 'medical students': not a clinical study. |
| Blanchard 1978 | 6/30 patients were reallocated after randomisation. |
| Borden 1989 | The clinically relevant outcomes (parents' and teachers' ratings) were not blinded. |
| Borkovec 1975 | The participants were college students screened by group test program and receiving research credit for participation: not a clinical setting. |
| Bornstein 1973 | The accumulated weight loss of the patients in the placebo group was 'positively reinforced': impure placebo. |
| Bouchet 1996 | The participants were normal subjects. |
| Brown 1999 | Pain was induced experimentally. |
| Buckalew 1972 | The participants were normal subjects. |
| Bullock 1999 | Drop out rate > 50%. |
| Bush 1985 | Allocation of participants to the different groups of the trial was not concealed. |
| Butler 1984 | The placebo intervention consisted in ordering difficult tasks for social phobics and practicing these tasks in ascending order: impure placebo. |
| Carlson 1993 | The outcomes were 1) a questionnaire assessing the boys' attributions and 2) performance at an experimental word puzzle test: regarded not clinically relevant. |
| Carpenter 1994 | Subjects were paid for participation. |
| Chambless 1984 | Drop‐out rate > 50%. |
| Chen 1999 | Unclear wether the trial was randomised. Authors contacted by e‐mail for clarification but did not respond. |
| Cole 1983 | Randomisation not stated. |
| Corletto 1999 | Placebo group got active treatment after 40 days. |
| Corson 1994 | The placebo intervention implied pain inducing needle sticks: not a pure placebo intervention. |
| Cottraux 1986 | Placebo group received not only a placebo intervention but also advice to reduce alcohol intake which is associated with smoking: impure placebo. |
| Cram 1980 | The no treatment group sessions ('chart headaches only' ) also included discussions of 'situational themes': unacceptable no treatment group. |
| Cristofalo 1999 | Normal subjects (athletes with no diagnosis of asthma). |
| Cullhed 1961 | Allocation by day of admission. |
| Dahlquist 1986 | Placebo group children were removed from parents. This was probably in itself anxiety inducing: impure placebo. |
| Daley 2007 | According to the protocol patients received money for entering the trial. |
| Diamond 1995 | The placebo used was saline. It is likely that saline has a physiological effect on congested nose, and is sold 'over the counter' in Denmark for this purpose: impure placebo. |
| Disney 1988 | Not explicitly a randomised trial. |
| Dobia 1985 | The 'placebo intervention' was relaxation therapy which was an integrated part of the experimental intervention: not a relevant placebo procedure. |
| Dundee 1988 | Patients allocated by day of admittance. |
| Egbert 1964 | The 'placebo' intervention in post‐operative patients having had intra‐abdominal surgery included instructions concerning pain modulating behaviour, e.g. how actively to relax abdominal muscles: impure placebo. |
| Eickholz 2002 | Split mouth design. Teeth were randomised, not patients or treatment periods. |
| Elkin 1985 | No untreated group. |
| Feather 1972 | A non‐clinical experimental setting where pain was induced by heat. |
| Fevery 1990 | The trial was designed to measure outcome after 6, 12 and 24 months. The placebo intervention was only comparable to the no treatment group the first 8 weeks: not a relevant comparison. |
| Fillmore 1992 | A non‐clinical experimental setting with normal subjects. |
| Fillmore 1994 | A non‐clinical experimental setting with normal subjects. |
| Fillmore 1994b | A non‐clinical experimental setting with normal subjects. |
| Flor 1983 | Untreated group received co‐intervention not given to the placebo group. |
| Fuller 1986 | No untreated group. |
| Gam 1998 | The placebo group received massage and exercise, the no treatment group did not: differential co‐intervention. |
| Gelfand 1963 | The subjects in this non‐clinical experimental pain trial were nursing students. |
| Goodale 1990 | No clear indication in the trial report that 'the reading group' constituted a placebo group. |
| Gowdey 1967 | 'Normal subjects': not a clinical problem. |
| Gregorio 1996 | The trial was designed to measure outcome after 6, 12 and 18 months. The placebo intervention was only comparable to the no treatment group the first 6 weeks: not a relevant comparison. |
| Gregory 1983 | The study was 'designed to investigate whether elderly hospitalized people can improve their performance if they are permitted a second attempt at the Set Test, a verbal fluency task': not a therapeutical clinical study. |
| Gryll 1978 | The allocation of participants was not explicitly stated to be random. |
| Haake 2007 | No no‐treatment group. The group receiving acupuncture was not treated with conventional treatment given to the no‐acupuncture group. |
| Hale 1986 | Allocation of participants was done in 'orderly sequence' following randomisation of the first patient. |
| Hall 1994 | 'Subjects reimbursed $20 at weeks 3 and 8 and $35 at week 12': participants paid. |
| Hargreaves 1983 | This laboratory study was an uncontrolled trial. |
| Hayden 1996 | The placebo used was saline. It is likely that saline has a physiological effect on congested nose, and is sold 'over the counter' in Denmark for this purpose: impure placebo. |
| Herth 2000 | Placebo not explicitly mentioned. |
| Hogarty 1973 | No untreated group. |
| Huber 1986 | The clinician who decided to remove the placenta knew which patients were in the untreated group: not blindly assessed. |
| Jensen 1991 | The trial was a 'laboratory study' with normal subjects: not a clinical study. |
| Kalman 1998 | The placebo group received dietary advice which was withheld from the no‐treatment group. |
| Kanner 1999 | The placebo treatment included 'intensive smoking‐cessation sessions' which was withheld from the the no‐treatment group. |
| Kelley 1976 | Subjects were normal children: not a clinical study. |
| Khandwala 1997 | According to personal communication with trial report authors, the vehicle was under suspicion of having an effect that was not only due to placebo and is actually sold 'over the counter' in the USA: impure placebo. |
| Klosko 1990 | Patients in the untreated group continued on anxiolytic medication. Patients in the placebo group discontinued their medication. The groups in this anxiety trial are not comparable. |
| Korner 1982 | No randomisation to placebo and untreated. |
| Lasagna 1954 | The study was not randomised. |
| Levine 1980 | The outcome was test anxiety which is not considered a clinically relevant outcome. |
| Liberman 1964 | Not a randomised study. |
| Lopez 1999 | Randomisation was conducted 'with due precaution to avoid differences among the subgroups in the children's mean ages and IQs'. |
| Lorr 1962 | Drop‐out rate > 50%. |
| Lujan 1992 | The headache was induced: an experimental setting. |
| Lynn 1983 | The placebo procedure is described as being of 'an active nature': impure placebo. |
| Manner 1987 | 7/20 placebo treated patients receive sedative anticholinergic premedication (glycopyrrolate) compared to 0/18 untreated patients. Also unclear whether the untreated patients were part of the randomization: differential co‐intervention. |
| Marchand 1993 | Patients were allocated through 'pseudo‐random assignment'. Contact to the authors clarified that this meant that randomisation was based on drawing pieces of paper with group assignments from a hat. There was no concealment of allocation. |
| McGrath 1988 | Suggestions to reduce impact of possible triggers in 'untreated group': unacceptable no treatment group. |
| Meehan 1985 | The no treatment group took prescribed and escape analgesics, the placebo group only escape analgesics. |
| Montgomery 1996 | A non‐clinical experimental study with normal subjects. |
| Nikolaou 1998 | Not a randomised trial. |
| Peart 1977 | No randomisation to untreated and placebo. |
| Penman 1956 | Post‐randomisation reallocations took place. |
| Pollo 2001 | Allocation to placebo and no‐treatment was not explicitly random. |
| Price 1999 | Healthy volunteers. Pain was induced experimentally. |
| Rampes 1997 | Drop‐out rate 33/58=56% [> 50%]. |
| Reich 1990 | The participants were normal older subjects: not a clinical study. |
| Robertson 1991 | 'All subjects were paid'. |
| Rodriguez 1997 | Post randomisation patient re‐allocation took place: 69 patients were randomised to the untreated group and but results were collected from 78 patients. |
| Roehrich 1993 | This alcohol study had neuropsychological and psychological test variables as outcomes: regarded not clinically relevant. |
| Roelofs 2000 | Paid healthy volunteers. Pain was induced experimentally. |
| Roos 1969 | The placebo intervention was designed to include active components: not a pure placebo. |
| Roth 1986 | Normal subjects. |
| Rustøen 1998 | Placebo not explicitly mentioned. |
| Sarles 1977 | Not a randomised trial. |
| Sartor 1980 | Not properly randomised. |
| Shaw 1974 | The placebo intervention consisted of listening to 'audiotapes designed to help persons cope with everyday fears and anxieties': not a pure placebo. |
| Sheikh 1986 | The participants were older 'normal' volunteers with 'age associated memory impairment' : not a clinical study. |
| Silvestri 1977 | Anxiety is the only outcome considered clinically relevant in this trial of the effect of implosive therapy for emotionally disturbed retardates, however, anxiety was not recorded in the no treatment group. |
| Skovlund 1991 | No untreated group. |
| Smith 2002 | The so‐called 'no treatment' group (but not the placebo group) received advice about changes in diet and the use of vitamin B6. |
| Spanos 1988 | 'All were paid $15 for their participation'. |
| Staats 1998 | Pain induced by 'hand exposure to ice water': not a clinical study. |
| Stanley 1989 | Not blindly assessed. |
| Suchman 1992 | Not blindly assessed. |
| Tashkin 1977 | Not a randomised trial. |
| Taylor 1977 | Anti‐hypertensive drugs were given at variable dose as co‐intervention. More patients in the placebo group than in the untreated group were increased in dose: differential co‐intervention. |
| Vacc 1980 | The investigation studied the effect of various interventions on maladaptive behaviour of otherwise normal children: not regarded a clinical study. |
| Van Damme 1998 | Healthy volunteers. |
| Volweider 1981 | Placebo procedure included physical exercise: not a pure placebo. |
| Weber 1975 | Averaged Electroencephalic audiometry (AEA) thresholds was the outcome in this study of CNS stimulant medication on children with minimal brain damage: outcome regarded not clinically relevant. |
| Weintraub 1992 | The placebo period (week 160 to 190) was followed by the untreated period (week 190 to 210), (no randomisation). |
| Windle 2001 | Allocation of patients by alternation. |
| Winnan 1982 | The Bernstein test is a diagnostic test. Pain was induced by infusion of acid: not a therapeutic clinical study. |
| Worner 1992 | Drop out rate > 50%. |
| Zeisset 1968 | The outcome was 'interview anxiety' which was not considered clinically relevant as a measure for general anxiety. |
Impure placebos: interventions with clearly specified contents or procedures that have an effect which, with considerable likelihood, goes beyond the effect of the treatment ritual. Such interventions are mostly 1) physical or pharmacological vehicles (see, e.g., Khandwala 1997), or 2) psychological 'placebos' with clear behavioural‐cognitive therapeutical elements (see, e.g., Bornstein 1973) or direct health related advice (see, e.g., Cottraux 1986).
Characteristics of studies awaiting assessment [ordered by study ID]
Shin 2005.
| Methods | Randomised trial |
| Participants | Women with hyperemesis gravidarum |
| Interventions | P6 acupuncture; placebo acupuncture; no acupuncture |
| Outcomes | Unclear |
| Notes | Reported in Korean |
Characteristics of ongoing studies [ordered by study ID]
Barret 2007.
| Trial name or title | PEP trial |
| Methods | Randomised trial |
| Participants | Out‐patients with common cold |
| Interventions | Placebo Standard and enhanced patient‐provider interaction Echinacea |
| Outcomes | Area under time severity curve based on the Wisconsin Upper Respiratory Symptom Survey |
| Starting date | NS |
| Contact information | bruce.barret@fammed.wisc.edu |
| Notes |
Differences between protocol and review
In the protocol we planned the following procedure for extraction of data: 'The primary outcome is that which is considered clinically most relevant to patients'. In the review we extracted data according to the following procedure: 'We primarily chose the outcome indicated as the main outcome in a trial report (e.g. through a power calculation). If a main outcome was not clearly indicated we chose the outcome measure we considered most relevant to patients'. The primary idea in the protocol was to minimise the risk of bias due to selective reporting of positive results in trials. We modified the procedure in an attempt to balance this risk against the risk of review author bias.
In the protocol for the first version of our review we specified a limited number of subgroup analyses (see Methods subgroup analyses 1‐8). Before we conducted this update we expanded the number of planned subgroup analyses (see Methods subgroup analyses 9‐12), and planned a number of meta‐regression analyses (see Methods). One subgroup‐analyses was conducted post‐hoc (see Methods subgroup analysis 13). Furthermore, in the protocol we planned to analyse trials that reported corresponding patient‐reported and observer‐reported outcomes. However, it became clear that the distinction between corresponding and not corresponding patient‐reported and observer‐reported outcomes was very subjective, and we decided to abort this comparison.
Contributions of authors
Asbjørn Hróbjartsson (AH) and Peter C. Gøtzsche (PCG) conceived the idea of the review. AH had the main responsibility for developing the search strategy, retrieving the trials, accessing additional data, and writing the first draft of the review. AH and PCG read all included trial reports. AH analysed the data.
Sources of support
Internal sources
No sources of support supplied
External sources
Faculty of Health Sciences, University of Copenhagen, Denmark.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abikoff 2004 {published data only}
- Abikoff H, Hechtman L, Klein RG, Gallagher R, Fleiss K, Etcovitch J, et al. Social functioning in children with ADHD treated with long‐term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43:820‐9. [DOI] [PubMed] [Google Scholar]
Adams 1976 {published data only}
- Adams HG, Benson EA, Alexander ER, Vontver LA, et al. Genital herpetic infection in men and women: clinical course and effect of topical application of adenine arabinoside. The Journal of Infectious Diseases 1976;133(supplement):A151‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adriaanse 1995 {published data only}
- Adriaanse AH, Kollée LAA, Muytjens HL, Nijhuis JG, Haan AFJ, Eskes TKAB. Randomized study of vaginal chlorhexidine disinfection during labor to prevent vertical transmission of group B streptococci. European Journal of Obstetrics, Gynecology and Reproductive Biology 1995;61:135‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alfano 2001 {published data only}
- Alfano AP, Taylor AG, Foresman PA, Dunkl PR, McConnell GG, Conaway MR, Gillies GT. Static magnetic fields for treatment of fibromyalgia: a randomised controlled trial. Journal of Alternative and Complementary Medicine 2001;7:53‐64. [DOI] [PubMed] [Google Scholar]
Alford 2003 {published data only}
- Alford JW, Fadale PD. Evaluation of postoperative bupivacaine infusion for pain management after anterior cruciate ligament reconstruction. Arthroscopy 2003;19(8):855‐861. [DOI] [PubMed] [Google Scholar]
Alkaissi 1999 {published data only}
- Alkaissi A, Stålnert M, Kalman S. Effect and placebo effect of acupressure (P6) on nausea and vomiting after outpatient gynaecological surgery. Acta anaesthesiologica Scandinavica 1999;43:270‐4. [DOI] [PubMed] [Google Scholar]
Alkaissi 2002 {published data only}
- Alkaissi A, Evartsson K, Johnsson VA, Ofenbartl L, Kalman S. P6 acupressure may relieve nausea and vomiting after gynecological surgery: an effectiveness study in 410 women. Canadian Journal of Anaesthesia 2002;49:1034‐9. [DOI] [PubMed] [Google Scholar]
Allen 1998 {published data only}
- Allen JJB, Schnyder RN, Hitt SK. The efficacy of acupuncture in the treatment of major depression in women. Psychological Science 1998;9:397‐401. [Google Scholar]
Allen 2006 {published data only}
- Allen JJB, Schnyer RN, Chambers AS, Hitt SK, Moreno FA, Manber R. Acupuncture for depression: a randomized controlled trial. Journal of Clinical Psychiatry 2006;67:1665‐73. [DOI] [PubMed] [Google Scholar]
Andersen 1990 {published data only}
- Andersen AN, Damm P, Tabor A, Pedersen IM, et al. Prevention of breast pain and milk secretion with bromocriptine after second‐trimester abortion. Acta Obstetricia et Gynecologica Scandinavica 1990;69:235‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Anderson 1999 {published data only}
- Anderson B, Brackett J, Ho J, Laffel LMB. An intervention to promote family teamwork in diabetes management tasks: relationships among parenteral involvement, adherence to blood glucose monitoring, and glycemic control in young adolescents with type 1 diabetes. In: Drotar D editor(s). Promoting adherence to medical treatment in chronic childhood illness: concepts, methods, and interventions. Mahwah, NJ,USA: Lawrence Erlbaum Associates, 2000:347‐65. [Google Scholar]
- Anderson B, Brackett J, Ho J, Laffel LMB. An office‐based intervention to maintain parent‐adolescent teamwork in diabetic management. Impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care 1999;22:713‐21. [DOI] [PubMed] [Google Scholar]
Antivalle 1990 {published data only}
- Antivalle M, Lattuada S, Salvaggio A, Paravicini M, et al. Placebo effect and adaptation to noninvasive monitoring of BP. Journal of Human Hypertension 1990;4:633‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Antonio 1999 {published data only}
- Antonio J, Colker CM, Torina G, Shi Q, Brink W, Kalman D. Effects of standardised guggulsterone phosphate supplement on body composition in overweight adults: a pilot study. Current Therapeutic Research 1999;60:220‐7. [Google Scholar]
Ascher 1979 {published data only}
- Ascher LM, Turner RM. Paradoxical intention and insomnia: an experimental investigation. Behaviour Research and Therapy. 1979;17:408‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Asmar 1996 {published data only}
- Asmar R, Boutelant S, Chaignon M, Guedon J, et al. Repeated measurment of non‐invasive ambulatory blood pressure: distinction between reproducibility and the proper effect of placebo. Blood Pressure Monitoring 1996;1:283‐8. [PubMed] [Google Scholar]
- Asmar R, Safar M, Queneau P. Evaluation of the placebo effect and reproducability of blood pressure measurement in hypertension. American Journal of Hypertension 2001;14:546‐52. [DOI] [PubMed] [Google Scholar]
- Queneau P, Asmar R, Safar M. [L'effect placebo sur les pressions artérielles systolique, diastolique et pulse]. Presse medicale 2002;31:1220‐3. [PubMed] [Google Scholar]
Aune 1998 {published data only}
- Aune A, Alraek T, Lihua H, Baerheim A. Acupuncture in the prophylaxis of recurrent lower urinary tract infection in adult women. Scandinavian Journal of Primary Health Care 1998;16:37‐9. [DOI] [PubMed] [Google Scholar]
- Aune A, Alræk T, Huo L, Baerheim A. Acupuncture in prevention of cystitis in women [Kan akupunktur forebygge blærekatarr hos kvinner?]. Tidsskrift for den Norske Laegeforening 1998;118(9):1370‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Banner 1983 {published data only}
- Banner CN, Meadows WM. Examination of the effectiveness of various treatment techniques for reducing tension. British Journal of Clinical Psychology 1983;22:183‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benedetti 1995 {published data only}
- Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet 1995;346:1231. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benedetti 1997 {published data only}
- Benedetti F, Amanzio M, Casadio C, Cavallo A, et al. Control of postoperative pain by transcutaneous electrical nerve stimulation after thoracic operations. Annals of Thoracic Surgery 1997;63:773‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benedetti 1999a {published data only}
- Benedetti F, Amanzio M, Baldi S, Casadio C, Maggi G. Inducing placebo respiratory depressant response in humans via opioid receptors. The European Journal of Neuroscience 1999;11:625‐31. [DOI] [PubMed] [Google Scholar]
Berg 1983 {published data only}
- Berg I, Forsythe I, Holt P, Watts J. A controlled trial of 'senokot' in faecal soiling treated by behavioural methods. Journal of Child Psychology and Psychiatry and Allied Disciplines 1983;24(4):543‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Biro 1997 {published data only}
- Biro P, Meier T, Cummins AS. Comparison of topical anaesthesia methods for venous cannulation in adults. European Journal of Pain 1997;1:37‐42. [DOI] [PubMed] [Google Scholar]
Blackman 1964 {published data only}
- Blackman S, Benton AJ, Cove LM. The effect of imipramine on enuresis. The American Journal of Psychiatry 1964;120:1194‐5. [DOI] [PubMed] [Google Scholar]
Blades 2001 {published data only}
- Blades KJ, Patel S, Aidoo KE. Oral antioxidant therapy for marginal dry eye. European Journal of Clinical Nutrition 2001;55(7):589‐97. [DOI] [PubMed] [Google Scholar]
Blanchard 1990a {published data only}
- Blanchard EB, Appelbaum KA, Radnitz CL, Michultka D, et al. Placebo‐controlled evaluation of abbreviated progressive muscle relaxation and of relaxation combined with cognitive therapy in the treatment of tension headache. Journal of Consulting and Clinical Psychology 1990;58:210‐5. [DOI] [PubMed] [Google Scholar]
Blanchard 1990b {published data only}
- Blanchard EB, Appelbaum KA, Radnitz CL, Morrill B, et al. A controlled evaluation of the thermal biofeedback and thermal biofeedback combined with cognitive therapy in the treatment of vascular headache. Journal of Consulting and Clinical Psychology 1990;58:216‐24. [DOI] [PubMed] [Google Scholar]
Block 1980 {published data only}
- Block J. Effects of rational emotive therapy on overweight adults. Psychotherapy: Theory, Research and Practice 1980;17(3):277‐80. [Google Scholar]
Bosley 1989 {published data only}
- Bosley F, Allen TW. Stress management training for hypertensives: cognitive and physiological effects. Journal of Behavioral Medicine 1989;12(1):77‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bova 1999 {published data only}
- Bova JG, Bhattacharjee N, Jurdi R, Bennet WF. Comparison of no medication, placebo and hyoscyamine for reducing pain during a barium enema. American Journal of Roentgenology 1999;172:1285‐7. [DOI] [PubMed] [Google Scholar]
Bramston 1985 {published data only}
- Bramston P, Spence SH. Behavioural versus cognitive social‐skills training with intellectually‐handicapped adults. Behaviour Research and Therapy 1985;23(3):239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brill 1964 {published data only}
- Brill NQ, Koegler RR, Epstein LJ, Forgy EW. Controlled study of psychiatric outpatient treatment. Archives of General Psychiatry 1964;10:581‐95. [DOI] [PubMed] [Google Scholar]
Brinkhaus 2006 {published data only}
- Brinkhaus B, Witt CM, Jena S, Linde K, Streng A, Wagenpfeil S, et al. Acupuncture in patients with chronic low back pain: a randomized controlled trial. Arch Intern Med 2006;166:450‐7. [DOI] [PubMed] [Google Scholar]
Bullock 2002 {published data only}
- Bullock ML, Kiresuk TJ, Sherman RE, Lenz SK, Culliton PD, Boucher TA, et al. A large randomized placebo controlled study of auricular acupuncture for alcohol dependence. Journal of Substance Abuse Treatment 2002;22:71‐7. [DOI] [PubMed] [Google Scholar]
Cabrini 2006 {published data only}
- Cabrini L, Gioia L, Gemma M, Melloni G, Caretta A, Ciriaco P, Puglisi A. Acupuncture for diagnostic bronchoscopy: a prospctive, randomized, placebo‐controlled study. American Journal of Chinese Medicine 2006;34:409‐15. [DOI] [PubMed] [Google Scholar]
Camatte 1969 {published data only}
- Camatte R, Gerolami A, Sarles H. Comparative study of the effect of various drugs and placebo on pain associated with gastro‐duodenal ulcers [Studio comparativo dell'azione di diversi trattamenti e dei placebo sulla crisi dolorosa dell'ulcera gastro‐duodenale]. La Clinica terapeutica 1969;49:411‐9. [PubMed] [Google Scholar]
Camberg 1999 {published data only}
- Camberg L, Woods P, Ooi WL, Hurley A, et al. Evaluation of simulated presence: a personalised approach to enhance well‐being in persons with Alzheimer's disease. JAGS 1999;47:446‐52. [DOI] [PubMed] [Google Scholar]
Canino 1994 {published data only}
- Canino E, Cardona R, Monsalve P, Acuna FP, et al. A behavioral treatment program as a therapy in the control of primary hypertension. Acta Cientifica Venezolana 1994;45:23‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Carbajal 1999 {published data only}
- Carbajal R, Chauvet X, Couderc S, Olivier‐Martin M. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ 1999;319:1393‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2001 {published data only}
- Carter MC, Perzanowski MS, Raymond A, Platts‐Mills TAE. Home intervention in the treatment of asthma among inner‐city children. The Journal of Allergy and Clinical Immunology 2001;108:732‐7. [DOI] [PubMed] [Google Scholar]
Carter 2003 {published data only}
- Carter JC, Olmsted MP, Kaplan AS, McCabe RE, Mills JS, Aime A. Self‐help for bulimia nervosa: a randomized controlled trial. American Journal of Psychiatry 2003;160:973‐8. [DOI] [PubMed] [Google Scholar]
Cesarone 2001 {published data only}
- Cesarone MR, Incandela L, Belcaro G, Sanctis MT, et al. Two‐week topical treatment with essaven gel patients with diabetic microangiopathy. A placebo‐controlled, randomized study. Angiology 2001;52(suppl.3):S43‐48. [DOI] [PubMed] [Google Scholar]
Chenard 1991 {published data only}
- Chenard JR, Marchand S, Charest J, Jinxue L, et al. Evaluation of a behavioral intervention for chronic low‐back pain: 'The interactional back school' [Évaluation d´un traitement comportmental de la lombalgie chronique: 'l´école interactionelle du dos']. Science et Comportement 1991;21(4):225‐39. [Google Scholar]
Classen 1983 {published data only}
- Classen W. Placebo application, personality, and headaches: a signal detection theory analysis of experimentally induced pain in comparison to clinical pain. Pharmacopsychiatry 1984;17:98‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Classen W, Feingold E, Netter P. Influence of sensory suggestibility on treatment outcome in headache patients. Neuropsychobiology 1983;10:44‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colker 1999 {published data only}
- Colker CM, Kalman DS, Torina GC, Perlis T, Street C. Effects of Citrus aurantium extract, caffeine, and St. John's wort on body fat loss, lipid levels, and mood states in overweight healthy adults. Current Therapeutic Research 1999;60:145‐53. [Google Scholar]
Conn 1986 {published data only}
- Conn IG, Marshall AH, Yadev SN, Daly J, Jaffer M. Transcutaneous electrical nerve stimulation following appendicectomy: the placebo effect. Annals of the Royal College of Surgeons of England 1986;68:191‐2. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Corver 2006 {published data only}
- Corver K, Kerkhof M, Brussee JE, Brunekreef B, Strien RT, Vos AP, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA‐study. Pediatric Allergy and Immunology 2006;17:329‐36. [DOI] [PubMed] [Google Scholar]
- Koopman LP, Strien RT, Kerkhof M, Wijga A, Smit HA, Jongste JC, et al. Placebo‐controlled trial of house dust mite‐impermeable mattress covers. American Journal of Respiratory and Critical Care Medicine 2002;166:307‐13. [DOI] [PubMed] [Google Scholar]
Costello 2006 {published data only}
- Costello M, Ramundo M, Christopher NC, Powel KR. Etyhyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clinical Pediatrics 2006;45:628‐32. [DOI] [PubMed] [Google Scholar]
Coyne 1995 {published data only}
- Coyne P, MacMurren M, Izzo T, Kramer T. Transcutaneous electrical nerve stimulator for procedural pain associated with intravenous needlesticks. Journal of Intravenous Nursing 1995;18(5):263‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Crosby 1994 {published data only}
- Crosby L, Palarski VA, Cottington E, Cmolik B. Iron supplementation for acute blood loss anemia after coronary artery bypass surgery: a randomized, placebo‐controlled study. Heart & Lung 1994;23:493‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Cupal 2001 {published data only}
- Cupal DD, Brewer BW. Effects of relaxation and guided imagery on knee strength, reinjury anxiety, and pain following anterior cruciate ligament reconstruction. Rehabilitation Psychology 2001;46:28‐43. [Google Scholar]
Davidson 1980 {published data only}
- Davidson A, Denney DR, Elliott CH. Suppression and substitution in the treatment of nailbiting. Behaviour Research and Therapy 1980;18:1‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Sanctis 2001 {published data only}
- Sanctis MT, Incandela L, Belcaro G, Cesarone MR. Topical treatment of venous microangiopathy in patients with venous ulceration with Essaven gel. A placebo controlled, randomized study. Angiology 2001;52 (suppl 3):S29‐34. [DOI] [PubMed] [Google Scholar]
Defrin 2005 {published data only}
- Defrin R, Ariel E, Peretz C. Segmental noxious versus innocuous electrical stimulation for chronic pain relief and the effect of fading sensation during treatment. Pain 2005;115:152‐60. [DOI] [PubMed] [Google Scholar]
Dibble 2007 {published data only}
- Dibble SL, Luce J, Cooper BA, Isreal J, Cohen M, Nussey B, Rugo H. Acupressure for chemotherapy‐induced nausea and vomiting: a randomized clinical trial. Oncology Nursing Forum 2007;34:813‐20. [DOI] [PubMed] [Google Scholar]
Ditto 2003 {published data only}
- Ditto B, France CR, Lavoie P, Roussos M, Adler PS. Reducing reactions to blood donation with applied muscle tension: a randomized controlled trial. Transfusion 2003;43:1269‐75. [DOI] [PubMed] [Google Scholar]
Ditto 2006 {published data only}
- Ditto B, France CR. The effects of applied tension on symptoms in French‐speaking blood donors: a randomized trial. Health Psychology 2006;25:433‐7. [DOI] [PubMed] [Google Scholar]
Doty 1975 {published data only}
- Doty DW. Role playing and incentives in the modification of the social interaction of chronic psychiatric patients. Journal of Consulting and Clinical Psychology 1975;43(5):676‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Double 1993 {published data only}
- Double DB, Warren GC, Evans M, Rowlands MP. Efficacy of maintenance use of anticholinergic agents. Acta Psychiatrica Scandinavica 1993;88:381‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dundee 1986 {published data only}
- Dundee JW. Belfast experience with P6 acupuncture antiemesis. The Ulster Medical Journal 1990;59(1):63‐70. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
- Dundee JW, Chestnutt WN, Ghaly RG, Lynas AGA. Traditional Chinese acupuncture: a potentially useful antiemetic?. British Medical Journal 1986;293:583‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundee JW, Ghaley RG, Bill KM, Chestnutt WN, Fitzpatrick KTJ, Lynas AGA. Effect of stimulation of the P6 antiemetic point on postoperative nausea and vomiting. British Journal of Anaesthesia 1989;63:612‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dundee JW, McMillan C. Clinical uses of P6 acupuncture antiemesis. Acupuncture & Electro‐therapeutics Research 1990;15:211‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lynas AGA, Ghaly RG, Dundee JW. Acupuncture reduced the emetic effects of nalbuphine premedication. British Journal of Anaesthesia 1986;58:1331P‐2P. [Google Scholar]
Elliott 1978 {published and unpublished data}
- Elliott CH, Denney DR. A multiple‐component treatment approach to smoking reduction. Journal of Consulting and Clinical Psychology 1978;46(6):1330‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Erdogmus 2007 {published data only}
- Erdogmus CB, Resch KL, Sabitzer R, Müller H, Nuhr M, Schöggl A, et al. Physiotherapy‐based rehabilitation following disc herniation operation. Spine 2007;32:2041‐9. [DOI] [PubMed] [Google Scholar]
Espie 1989 {published data only}
- Espie CA, Brooks DN, Lindsay WR. An evaluation of tailored psychological treatment of insomnia. Journal of Behavior Therapy and Experimental Psychiatry 1989;20(2):143‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Espie CA, Lindsay WR, Brooks DN, Hood EM, Turvey T. A controlled comparative investigation of psychological treatments for chronic sleep‐onset insomnia. Behaviour Research and Therapy 1989;27(1):79‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Etringer 1982 {published data only}
- Etringer BD, Cash TF, Rimm DC. Behavioral, affective and cognitive effects of participant modeling and an equally credible placebo. Behavior Therapy 1982;13:476‐85. [Google Scholar]
Etter 2002 {published data only}
- Etter J, Lazlo E, Zellweger J, Perrot C, Perneger TV. Nicotine replacement to reduce cigarette consumption in smokers who are unwilling to quit: a randomized trial. Journal of Clinical Psychopharmacology 2002;22:487‐95. [DOI] [PubMed] [Google Scholar]
Faas 1993 {published data only}
- Faas A, Chavannes AW, Eijk JTM, Gubbels JW. A randomized, placebo‐controlled trial of exercise therapy in patients with acute low back pain. Spine 1993;18(11):1388‐95. [MEDLINE: ] [PubMed] [Google Scholar]
- Faas A, Eijk JTM, Chavannes AW, Gubbels JW. A randomized trial of exercise therapy in patients with acute low back pain. Efficacy on sickness absence. Spine 1995;20(8):941‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fanti 2003 {published data only}
- Fanti L, Gemma M, Passaretti S, Guslandi M, Testoni PA, Casti A, Torri G. Electroacupuncture analgesia for colonoscopy: a prospective, randomised, placebo‐controlled study. The American Journal of Gastroenterology 2003;98:312‐6. [DOI] [PubMed] [Google Scholar]
Fiorellini 2005 {published data only}
- Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, et al. Randomized study evaluating recombinant human bone morphogenetic protein‐2 for extraction socket augmentation. Journal of Periodontology April 2005;76(4):605‐13. [DOI] [PubMed] [Google Scholar]
Fisher 2006 {published data only}
- Fisher P, McCarney R, Hasford C, Vickers A. Evaluation of specific and non‐specific effects in homeopathy: feasibility study for a randomised trial. Homeopath 2006;95:215‐22. [DOI] [PubMed] [Google Scholar]
Forster 1994 {published data only}
- Forster EL, Kramer JF, Lucy SD, Scudds RA, et al. Effects of TENS on pain, medications, and pulmonary function following coronary artery bypass graft surgery. Chest 1994;106(5):1343‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foster 2004 {published data only}
- Foster KA, Liskin J, Cen S, Abbot A, Armisen V, Globe D, et al. The Trager approach in the treatment of chronic headache: a pilot study. Alternative Therapies in Health and Medicine 2004;10:40‐6. [PubMed] [Google Scholar]
Foster 2007 {published data only}
- Foster NE, Thomas E, Barlas P, Hill JC, Young J, Mason E, Hay EM. Acupuncture as an adjunct to excercise based physiotherapy for osteoarthritis of the knee: randomised controlled trial. BMJ 2007;335:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 1990 {published data only}
- Frank E, Kupfer DJ. Does a placebo tablet affect psychotherapeutic treatment outcome? Results from the Pittsburgh study of maintenance therapies in recurrent depression. Psychotherapy Research 1992;2(2):102‐11. [Google Scholar]
- Frank E, Kupfer DJ, Buhari A, McEachran AB, Grochocinski VJ. Imipramine and weight gain during the long‐term treatment of recurrent depression. Journal of Affective Disorders 1992;26:65‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, et al. Three‐year outcomes for maintenance therapies in recurrent depression. Archives of General Psychiatry 1990;47:1093‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frankel 1978 {published data only}
- Frankel BL, Patel DJ, Horwitz D, Friedewald WT, et al. Treatment of hypertension with biofeedback and relaxation techniques. Psychosomatic Medicine 1978;40(4):276‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frega 1994 {published data only}
- Frega A, Stentella P, Renzi F, Gallo G, et al. Pain evaluation during carbon dioxide laser vaporization for cervical intraepithelial neoplasia: a randomized trial. Clinical and Experimental Obstetrics and Gynecology 1994;21(3):188‐91. [MEDLINE: ] [PubMed] [Google Scholar]
Fuchs 1977 {published data only}
- Fuchs CZ, Rehm LP. A self‐control behavior therapy program for depression. Journal of Consulting and Clinical Psychology 1977;45(2):206‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluckman 1980 {published data only}
- Gluckman S, Barling J. Effects of a remedial program on visual‐motor perception in spina bifida children. The Journal of Genetic Psychology 1980;136:195‐202. [DOI] [PubMed] [Google Scholar]
Godfrey 1973 {published data only}
- Godfrey S, Silverman M. Demonstration of a placebo response in asthma by means of exercise testing. Journal of Psychosomatic Research 1973;17:293‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldstein 2000 {published data only}
- Goldstein AJ, Beurs E, Chambless DL, Wilson KA. EMDR for panic disorder with agoraphobia: combination with waiting list and credible attention‐placebo control conditions. Journal of Consulting and Clinical Psychology 2000;68(6):947‐56. [PubMed] [Google Scholar]
Goodenough 1997 {published data only}
- Goodenough B, Kampel L, Champion GD, Laubreaux L, Nicholas K, Ziegler JB, et al. An investigation of the placebo effect and age‐related factors in the report of needle pain from venipuncture in children. Pain 1997;72:383‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gracely 1979 {published and unpublished data}
- Gracely RH, Deeter WR, Wolskee PJ, Wear BL, et al. The effect of naloxone on multidimensionel scales of postsurgical pain in nonsedated patients. Abstracts ‐ Society for Neuroscience 1979;5:609. [Google Scholar]
Gracely 1983 {published and unpublished data}
- Gracely RH, Dubner R, Wolskee PJ, Deeter W. Placebo and naloxone can alter post‐surgical pain by separate mechanisms. Nature 1983;306:264‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grammer 1984 {published data only}
- Grammer LC, Shaughnessy MA, Shaughnessy JJ, Patterson R. Asthma as a variable in a study of immunotherapy for allergic rhinitis. The Journal of Allergy and Clinical Immunology 1984;73(5, part 1):557‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRECHO 1989 {published data only}
- Fingerhut A. Use of homeopathy for the restoration of transit following abdominal surgery [Homéopathie dans la reprise du transit après chirurgie abdominale]. Chirurgie; Memoires de l'Academie de Chirurgie 1990;116:404‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- GRECHO, U292 inserm, ARC, GREPA. Evaluation of the effects of two homoeopathic preparations on the resumption of intestinal peristalsis after digestive tract surgery. A multicentre controlled trial [Evalution de deux produits homéopathiques sur la reprise du transit après chirurgie digestive. Un essai contrôlé multicentrique]. La Presse Médicale 1989;18(2):59‐62. [MEDLINE: ] [PubMed] [Google Scholar]
- Mayaux MJ, Guihard‐Moscato ML, Schwartz D, Benveniste J. Controlled clinical trial of homoepathy in postoperative ileus. Lancet 1988;i:528‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guglielmi 1982 {published data only}
- Guglielmi RS, Roberts AH, Patterson R. Skin temperature biofeedback for Raynaud's disease: a double‐blind study. Biofeedback and Self‐Regulation 1982;7(1):99‐120. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hall 1974 {published data only}
- Hall SM, Hall RG, Hanson RW, Borden BL. Permanence of two self‐managed treatments of overweight in university and community populations. Journal of Consulting and Clinical Psychology 1974;42(6):781‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hallström 1988 {published data only}
- Crouch G, Robson M, Hallstrom C. Benzodiazepine dependent patients and their psychological treatment. Progress in Neuro‐psychopharmacology & Biological Psychiatry 1988;12:503‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hallström C, Crouch G, Robsen M, Shine P. The treatment of tranquillizer dependence by propranolol. Postgraduate Medical Journal 1988;64(suppl 2):40‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Hanson 1976 {published data only}
- Hanson RW, Borden BL, Hall SM. Use of programmed instruction in teaching self‐management skills to overweight adults. Behavior Therapy 1976;7(3):366‐73. [Google Scholar]
Hargreaves 1989 {published data only}
- Hargreaves A, Lander J. Use of transcutaneous electrical nerve stimulation for postoperative pain. Nursing Research 1989;38(3):159‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Harrison 1975 {published data only}
- Harrison RF, Louvois J, Blades M, Hurley R. Doxycycline treatment and human infertility. Lancet 1975;i(7907):605‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hashish 1986 {published data only}
- Hashish I, Harvey W, Harris M. Anti‐inflammatory effects of ultrasound therapy: evidence for a major placebo effect. British Journal of Rheumatology 1986;25:77‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hashish 1988 {published data only}
- Hashish I, Hai HK, Harvey W, Feinmann C, Harris M. Reduction of postoperative pain and swelling by ultrasound treatment: a placebo effect. Pain 1988;33:303‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ho KH, Hashish I, Salmon P, Freeman R, Harvey W. Reduction of post‐operative swelling by a placebo effect. Journal of Psychosomatic Research 1988;32(2):197‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hawkins 1995 {published data only}
- Hawkins PJ, Liossi C, Ewart BW, Hatira P, Kosmidis VH, Varvutsi M. Hypnotherapy for control of anticipatory nausea and vomiting in children with cancer: preliminary findings. Psycho‐Oncology 1995;4:101‐6. [Google Scholar]
Heinzl 1981 {published data only}
- Heinzl S, Andor J. Preoperative administration of prostaglandin to avoid dilatation‐induced damage in first‐trimester pregnancy terminations. Gynecologic and Obstetric Investigation 1981;12:29‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Helms 1987 {published data only}
- Helms JM. Acupuncture for the management of primary dysmenorrhea. Obstetrics & Gynecology 1987;69(1):51‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Hong 1993 {published data only}
- Hong C, Chen Y, Pon CH, Yu J. Immediate effects of various physical medicine modalities on pain threshold of an active myofascial trigger point. Journal of Muscoloskeletal Pain 1993;1(2):37‐53. [Google Scholar]
Hossmann 1981 {published data only}
- Hossmann V, FitzGerald GA, Dollery CT. Influence of hospitalization and placebo therapy on blood pressure and sympathetic function in essential hypertension. Hypertension 1981;3:113‐8. [DOI] [PubMed] [Google Scholar]
Hovell 2003 {published data only}
- Hovell MF, Sipan CL, Blumberg EJ, Hofstetter CR, Slymen D, Friedman L, et al. Increasing Latino adolescents' adherence to treatment for latent tuberculosis infection: a controlled trial. American Journal of Public Health 2003;93:1871‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hruby 2006 {published data only}
- Hruby G, Ames C, Chen C, Yan Y, Sagar J, Baron P, Landman J. Assessment of efficacy of transcutaneous electrical nerve stimulation for pain management during office‐based flexible cystoscopy. Urology 2006;67:914‐7. [DOI] [PubMed] [Google Scholar]
Hutton 1991 {published data only}
- Hutton N, Wilson MH, Mellits D, Baumgardner R, et al. Effectiveness of an antihistamine‐decongestant combination for young children with the common cold: a randomized, controlled clinical trial. The Journal of Pediatrics 1991;118(1):125‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hyland 2006 {published data only}
- Hyland MR, Webber‐Gaffney A, Cohen L, Lichtman SW. Randomized controlled trial of calcaneal taping, sham taping, and plantar fascia stretching for the short‐term management of plantar heel pain. Journal of Orthopaedic and Sports Physical Therapy 2006;36:364‐71. [DOI] [PubMed] [Google Scholar]
Hyman 1986 {published data only}
- Hyman GJ, Stanley RO, Burrows GD, Horne DJ. Treatment effectiveness of hypnosis and behaviour therapy in smoking cessation: a methodological refinement. Addictive Behaviors 1986;11:355‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Irjala 1993 {published data only}
- Irjala J, Kanto J, Scheinin M. Monoamine metabolite and catecholamine measurements in cerebrospinal fluid in determining the quality of the pre‐operative night's sleep. European Journal of Anaesthesiology 1993;10:393‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Irvin 1996 {published data only}
- Irvin JH, Domar AD, Clark C, Zuttermeister PC. The effects of relaxation response training on menopausal symptoms. Journal of Psychosomatic Obstetrics and Gynaecology 1996;17:202‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacobs 1971 {published data only}
- Jacobs MA, Spilken AZ, Norman MM, Wohlberg GW, Knapp PH. Interaction of personality and treatment conditions associated with success in a smoking control program. Psychosomatic Medicine 1971;33(6):545‐56. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacobson 1978 {published data only}
- Jacobson NS. Specific and nonspecific factors in the effectiveness of a behavioral approach to the treatment of marital discord. Journal of Consulting and Clinical Psychology 1978;46(3):442‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jakes 1992 {published data only}
- Jakes SC, Hallam RS, McKenna L, Hinchcliffe R. Group cognitive therapy for medical patients: an application to tinnitus. Cognitive Therapy and Research 1992;16(1):67‐82. [Google Scholar]
Kaptchuk 2008 {published data only}
- Conboy LA, Wasserman RH, Jacobson EE, Davies RB, Legedza ATR, Park M, et al. Investigating placebo effects in irritable bowel syndrome: a novel research design. Contemporary Clinical Trials 2006;27(2):123‐34. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobsen EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;online:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karst 2007 {published data only}
- Karst M, Wintherhalter M, Münte S, Francki B, Hondronikos A, Eckardt A, et al. Auricular acupuncture for dental anxiety: a randomized controlled trial. Anesthesia & Analgesia 2007;104:295‐300. [DOI] [PubMed] [Google Scholar]
Karunakaran 1997 {published and unpublished data}
- Karunakaran S, Hammersley MS, Morris RC, Turner RC, et al. The fasting hyperglycaemia study: III. randomized controlled trial of sulfonylurea therapy in subjects with increased but not diabetic fasting plasma glucose. Metabolism 1997;46(12,suppl 1):56‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kendall 1979 {published data only}
- Kendall PC, Williams L, Pechacek TF, Graham LE, Shisslak C, Herzoff N. Cognitive‐behavioral and patient education interventions in cardiac catheterization procedures: the Palo Alto medical psychology project. Journal of Consulting and Clinical Psychology 1979;47(1):49‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kerr 2003 {published data only}
- Kerr AR, Drexel CA, Spielman AI. The efficacy and safety of 50 mg penicillin G potassium troches for recurrent aphthous ulcers. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics 2003;96:685‐94. [DOI] [PubMed] [Google Scholar]
Killeen 2004 {published data only}
- Killeen TK, Brady KT, Gold PB, Simpson KN, Faldowski RA, Tyson C, Anton RF. Effectiveness of naltrexone in a community treatment program. Alcoholism, Clinical and Experimental Research 2004;28:1710‐7. [DOI] [PubMed] [Google Scholar]
Killen 1990 {published data only}
- Fortmann SP, Killen JD, Telch MJ, Newman B. Minimal contact treatment for smoking cessation. A placebo controlled trial of nicotine polacrilex and self‐directed relapse prevention: initial results of the Stanford stop smoking project. JAMA 1988;260(11):1575‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Vardy A. Evaluation of a treatment approach combining nicotine gum with self‐guided behavioural treatments for smoking relapse prevention. Journal of Consulting and Clinical Psychology 1990;58(1):85‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kilmann 1987 {published data only}
- Kilman PR, Milan RJ, Boland JP, Nankin HR, Davidson E, West MO, et al. Group treatment of secondary erectile dysfunction. Journal of Sex & Marital Therapy 1987;13:168‐82. [DOI] [PubMed] [Google Scholar]
Klerman 1974 {published data only}
- Klerman GL, DiMascio A, Weisman M, Prusoff B. Treatment of depression by drugs and psychotherapy. The American Journal of Psychiatry 1974;131(2):186‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paykel ES, DiMascio A, Haskell D, Prusoff BA. Effects of maintenance amitriptyline and psychotherapy on symptoms of depression. Psychological Medicine 1975;5:67‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paykel ES, DiMascio A, Klerman GL, Prusoff BA, et al. Maintenance therapy of depression. Pharmakopsychiatrie, Neuro‐Psychopharmakologie 1976;9:127‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Paykel ES, Weissman MM, Prusoff BA. Social maladjustment and severity of depression. Comprehensive Psychiatry 1978;19(2):121‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weissman MM, Klerman GL, Paykel ES, Prusoff B. Treatment effects on the social adjustment of depressed patients. Archives of General Psychiatry 1974;30:771‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kober 2002 {published data only}
- Kober A, Scheck T, Greher M, Lieba F, Fleischhackl R, Fleischhackl S, et al. Prehospital analgesia with acupressure in victims of minor trauma: a prospective, randomized, double‐blinded trial. Anesthesia and Analgesia 2002;95:723‐7. [DOI] [PubMed] [Google Scholar]
Kokol 2005 {published data only}
- Kokol R, Berger C, Haas J, Kopera D. Venous leg ulcers: no improvement of wound healing with 685‐nm low level laser therapy. Randomised, placebo‐controlled, double‐blind study. Hautarzt 2005;56:570‐5. [DOI] [PubMed] [Google Scholar]
Kotani 2001 {published data only}
- Kotani N, Kushikata T, Suzuki A, Hashimoto H, Muraoka M, Matsuki A. Insertion of intradermal needles into painful points provides analgesia for intractable abdominal scar pain. Regional Anesthesia and Pain Medicine 2001;26:532‐8. [DOI] [PubMed] [Google Scholar]
Lamazza 1986 {published data only}
- Lamazza A, Tofi A, Bolognese A, Fontana B, Masi E, Frontespezi S. Effects of pinaverium bromide in the premedication of endoscopic retrograde cholangio‐pancreatography and on motor activity of the sphincter of Oddi. Current Medical Research and Opinion 1986;10(4):280‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lander 1993 {published data only}
- Lander J, Fowler‐Kerry S. TENS for children's procedural pain. Pain 1993;52:209‐16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larson 2005 {published data only}
- Larson CP, Hoque AB, Larson CP, Khan AM, Saha UR. Initiation of zinc treatment for acute childhood diarrhoea and risk for vomiting or regurgitation: a randomized, double‐blind, placebo‐controlled trial. Journal of Health, Population and Nutrition 2005;23:311‐9. [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee PC, Jawad MS, Hull JD, West WH, Shaw K, Eccles R. The antitussive effect of placebo treatment on cough associated with acute upper respiratory infection. Psychosomatic Medicine 2005;67:314‐7. [DOI] [PubMed] [Google Scholar]
Leibing 2002 {published data only}
- Leibing E, Leonhardt U, Köster G, Goerlitz A, Rosenfeldt JA, Hilgers R, Ramadori G. Acupuncture treatment of chronic low‐back pain: a randomized, blinded, placebo‐controlled trial with 9‐months follow‐up. Pain 2002;96:189‐96. [DOI] [PubMed] [Google Scholar]
Levine 1984 {published data only}
- Levine JD, Gordon NC. Influence of the method of drug administration of analgesic response. Nature 1984;312:755‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levitt 1981 {published data only}
- Levitt M, Wilson A, Bowman D, Kemel S, et al. Physiologic observations in a controlled clinical trial of the antiemetic effectiveness of 5, 10 and 15 mg of 9‐tetrahydrocannabinol in cancer chemotherapy. Ophtalmologic implications. Journal of Clinical Pharmacology 1981;21:103s‐9s. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Licciardone 2003 {published data only}
- Licciardone JC, Stoll ST, Fulda KG, Russo DP, Siu J, Winn W, Swift J Jr. Osteopathic manipulative treatment for chronic low back pain: a randomized controlled trial. Spine 2003;28:1355‐62. [DOI] [PubMed] [Google Scholar]
Lick 1975 {published data only}
- Lick J. Expectancy, false galvanic skin response feedback and systematic desensitization in the modification of phobic behavior. Journal of Consulting and Clinical Psychology 1975;43(4):557‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lick 1977 {published data only}
- Lick JR, Heffler D. Relaxation training and attention placebo in the treatment of severe insomnia. Journal of Consulting and Clinical Psychology 1977;45(2):153‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Limoges 2004 {published data only}
- Limoges MF, Rickabaugh B. Evaluation of TENS during screening flexible sigmoidoscopy. Gastroenterol Nurs 2004;27:61‐8. [DOI] [PubMed] [Google Scholar]
Lin 2002 {published data only}
- Lin JG, Lo MW, Wen YR, Hsieh CL, Tsai SK, Sun WZ. The effect of high and low frequency electroacupuncture in pain after lower abdominal surgery. Pain 2002;99:509‐14. [DOI] [PubMed] [Google Scholar]
Lincoln 2003 {published data only}
- Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: a randomized controlled trial. Stroke 2003;34:111‐5. [DOI] [PubMed] [Google Scholar]
Linde 2005 {published data only}
- Linde K, Streng A, Jurgens S, Hoppe A, Brinkhaus B, Witt C, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA 2005;293:2118‐25. [DOI] [PubMed] [Google Scholar]
Lindholm 1996 {published data only}
- Johanneson M, Borgquist L, Jönsson B, Lindholm LH. The cost effectiveness of lipid lowering in Swedish primary health care. Journal of Internal Medicine 1996;240:23‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lindholm LH, Ekbom T, Dash C, Isacsson Å, et al. Changes in cardiovascular risk factors by combined pharmacological and nonpharmacological strategies: the main result of the CELL study. Journal of Internal Medicine 1996;240:13‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liossi 2003 {published data only}
- Liossi C, Hatira P. Clinical hypnosis in the alleviation of procedure‐related pain in pediatric oncology patients. International Journal of Clinical and Experimental Hypnosis 2003;51:4‐28. [DOI] [PubMed] [Google Scholar]
Longo 1988 {published data only}
- Longo DJ, Clum GA, Yaeger NJ. Psychosocial treatment for recurrent genital herpes. Journal of Consulting and Clinical Psychology 1988;56(1):61‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lorr 1961 {published data only}
- Lorr M, McNair DM, Weinstein GJ, Michaux WW, et al. Mepromate and chlorpromazine in psychotherapy. Archives of General Psychiatry 1961;4:381‐9. [DOI] [PubMed] [Google Scholar]
Macaluso 1995 {published data only}
- Macaluso AD, Conelly AM, Hayes WB, Houb MC, et al. Oral transmucosal fentanyl citrate for premedication in adults. Anesthesia and Analgesia 1996;82:158‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malcolm 1980 {published data only}
- Malcolm RE, Sillet RW, Turner JAM, Ball KP. The use of nicotine chewing gum as an aid to stopping smoking. Psychopharmacology 1980;70:295‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Markland 1993 {published data only}
- Markland D, Hardy L. Anxiety, relaxation and anaesthesia for day‐case surgery. British Journal of Clinical Psychology 1993;32:493‐504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matros 2006 {published data only}
- Matros E, Rocha F, Zinner M, Wang J, Ashley S, Breen E, et al. Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized, placebo‐controlled trial. Journal of the American College of Surgeons 2006;202:773‐8. [DOI] [PubMed] [Google Scholar]
Mattarei 1985 {published data only}
- Matterei M, Venuto G, Dal Follo M, Miori R. Is placebo active in essential arterial hypertension? [Agisce il placebo nell'ipertensione arteriosa essenziale?]. Minerva Cardioangiologica 1985;33:725‐9. [PubMed] [Google Scholar]
May 1988 {published data only}
- May O, Hansen NCG. Comparison of terbutaline, isotonic saline, ambient air and non‐treatment in patients with reversible chronic airway obstruction. The European Respiratory Journal: Official Journal of the European Society for Clinical Respiratory Physiology 1988;1:527‐30. [MEDLINE: ] [PubMed] [Google Scholar]
McLachlan 1991 {published data only}
- McLachlan DRC, Dalton AJ, Kruck TPA, Bell MY, et al. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 1991;337:1304‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McLachlan DRC, Smith WL, Kruck TP. Desferrioxamine and Alzheimer's disease: video home behavior assessment of clinical course and measures of brain aluminum. Therapeutic Drug Monitoring 1993;15(6):602‐7. [MEDLINE: ] [PubMed] [Google Scholar]
McMillan 1994 {published and unpublished data}
- McMillan CM. Transcutaneous electrical stimulation of Neiguan anti‐emetic acupuncture point in controlling sickness following opioid analgesia in major orthopaedic surgery. Physiotherapy 1994;80(1):5‐9. [Google Scholar]
Medici 2002 {published data only}
- Medici TC, Grebski E, Wu J, Hinz G, Wuthrich B. Acupuncture and bronchial asthma: a long‐term randomized study of the effects of real versus sham acupuncture compared with controls in patients with bronchial asthma. Journal of Alternative and Complementary Medicine 2002;8:737‐50. [DOI] [PubMed] [Google Scholar]
Mehl‐Madrona 2007 {published data only}
- Mehl‐Medrona L, Kligler B, Silverman S, Lynton H, Merrell W. The impact of acupuncture and craniosacral therapy interventions on clinical outcomes in adults with asthma. Explore 2007;3(1):28‐36. [DOI] [PubMed] [Google Scholar]
Melchart 2005 {published data only}
- Melchart D, Streng A, Hoppe A, Brinkhaus B, Witt C, Wagenpfeil S, et al. Acupuncture in patients with tension‐type headache: randomised controlled trial. BMJ 2005;331:376‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moffet 1996 {published data only}
- Moffett JAK, Richardson PH, Frost H, Osborn A. A placebo controlled double blind trial to evaluate the effectiveness of pulsed short wave therapy for osteoarthritic hip and knee pain. Pain 1996;67:121‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Molsberger 2002 {published data only}
- Molsberger AF, Mau J, Pawelec DB, Winkler J. Does acupuncture improve the orthopedic management of chronic low back pain: a randomized, blinded, controlled trial with 3 months follow up. Pain 2002;99:579‐87. [DOI] [PubMed] [Google Scholar]
Moreland 2006 {published data only}
- Moreland EC, Volkening LK, Lawlor MT, Chalmers KA, Anderson BJ, Laffel LM. Use of a blood glucose monitoring manual to enhance monitoring adherence in adults with diabetes: a randomized controlled trial. Archives of Internal Medicine 2006;166:689‐95. [DOI] [PubMed] [Google Scholar]
Morey 2006 {published data only}
- Morey MC, Ekelund C, Pearson M, Crowley G, Peterson M, et al. Project LIFE: a partnership to increase physical activity in elders with multiple chronic illnesses. J Aging Physical Act 2006;14:324‐43. [DOI] [PubMed] [Google Scholar]
Morton 1993 {published data only}
- Morton AR, Fazio SM, Miller D. Efficacy of laser‐acupuncture in the prevention of exercise‐induced asthma. Annals of Allergy 1993;70:295‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Murphy 1982 {published data only}
- Murphy JK, Williamson DA, Buxton AE, Moody SC, et al. The long‐term effects of spouse involvement upon weight loss and maintenance. Behavior Therapy 1982;13:681‐93. [Google Scholar]
Mussell 1988 {published data only}
- Mussell MJ, Hartley JPR. Thrachea‐noise biofeedback in asthma: a comparison of the effect of thrachea‐noise biofeedback, a bronchodilator, and no treatment on the rate of recovery from excercise‐ and eucapnic hyperventilation‐induced asthma. Biofeedback and Self‐regulation 1988;13(3):219‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Najnigier 1997 {published data only}
- Najnigier B, Patkowski W, Zieniewicz K, Nyckowski P, et al. Zofran (ondansetron) in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy [Zofran w zapobieganiu nudnosciom i wymiotom po cholecystektomii laparoskopowej]. Acta Endoscopica Polona 1997;7(3):125‐8. [Google Scholar]
Nandi 1976 {published data only}
- Nandi DN, Ajmany S, Ganguli H, Banerjee G, et al. A clinical evaluation of depressives found in a rural survey in India. The British Journal of Psychiatry; the Journal of Mental Science 1976;128:523‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naumann 1989 {published data only}
- Naumann VC, Lange A. The use of transcutaneous electrical nerve stimulation for analgesia in the postoperative phase [Die Anwendung der transkutanen elektrischen Nervenstimulation zur Analgesie in der postoperativen Phase]. Z Physiother 1989;41:9‐13. [Google Scholar]
Nawrocki 1997 {published data only}
- Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. British Journal of Urology 1997;79:389‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicassio 1974 {published data only}
- Nicassio P, Bootzin R. A comparison of progressive relaxation and autogenic training as treatments for insomnia. Journal of Abnormal Psychology 1974;83(3):253‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nocella 1982 {published data only}
- Nocella J, Kaplan RB. Training children to cope with dental treatment. Journal of Pediatric Psychology 1982;7(2):175‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Brien 1996 {published data only}
- O'Brien B, Relyea MJ, Taerum T. Efficacy of P6 acupressure in the treatment of nausea and vomiting during pregnancy. American Journal of Obstetrics and Gynecology 1996;174(2):708‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parker 1995 {published and unpublished data}
- Parker JC, Smarr KL, Buckelew SP, Stucky‐Ropp RC, et al. Effects of stress management on clinical outcomes in rheumatoid arthritis. Arthritis and Rheumatism 1995;38(12):1807‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Parker 2003 {published data only}
- Parker JC, Smarr KL, Slaughter JR, Johnston SK, Priesmeyer ML, Hanson KD, et al. Management of depression in rheumatoid arthritis: a combined pharmacologic and cognitive‐behavioral approach. Arthritis and Rheumatism 2003;49:766‐77. [DOI] [PubMed] [Google Scholar]
Pearl 1956 {published data only}
- Pearl D, Vander Kamp H, Olsen AL, Greenberg PD, et al. The effects of reserpine on schizophrenic patients. The American Journal of Psychiatry 1956;112:936. [DOI] [PubMed] [Google Scholar]
Pelham 1992 {published data only}
- Pelham WE, Murphy DA, Vannatta K, Milich R, et al. Methylphenidate and attributions in boys with attention‐deficit hyperactivity disorder. Journal of Consulting and Clinical Psychology 1992;60(2):282‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quahagen 1995 {published data only}
- Quayhagen MP, Quayhagen M, Corbeil RR, Roth P, et al. A dyadic remediation program for care recipients with dementia. Nursing Research 1995;44(3):153‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Rabkin 1990 {published data only}
- Rabkin JG, McGrath PJ, Quitken FM, Tricamo E, et al. Effects of pill‐giving on maintenance of placebo response in patients with chronic mild depression. The American Journal of Psychiatry 1990;147:1622‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rawling 2001 {published data only}
- Rawling MJ, Wiebe ER. A randomized controlled trial of fentanyl for abortion pain. American Journal of Obstetrics and Gynecology 2001;185:103‐7. [DOI] [PubMed] [Google Scholar]
Reading 1982 {published data only}
- Reading AE. The effects of psychological preparation on pain and recovery after minor gynaecological surgery: a preliminary report. Journal of Clinical Psychology 1982;38(3):504‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ristikankare 1999 {published data only}
- Ristikankare M, Hartikainen, Heikkinen M, Janatuinen E, Julkunen R. Is routinely given conscious sedation of benefit during colonoscopy?. Gastroenterology Endoscopy 1999;49:566‐72. [DOI] [PubMed] [Google Scholar]
- Ristikankare M, Julkunen R, Mattila M, Laitinen T, et al. Conscious sedation and cardiorespiratory safety during colonoscopy. Gastrointestinal Endoscopy 2000;52:48‐54. [DOI] [PubMed] [Google Scholar]
Ristikankare 2006 {published data only}
- Ristikankare M, Julkunen R, Heikkinen M, Mattila M, Laitinen T, Wang SX, et al. Sedation, topical pharyngeal anesthesia and cardiorespiratory safety during gastroscopy. Journal of Clinical Gastroenterology 2006;40:899‐905. [DOI] [PubMed] [Google Scholar]
Robinson 2001 {published data only}
- Robinson R, Darlow S, Wright SJ, Watters C, Carr I, Gadsby G, Mayberry J. Is transcutaneous electrical nerve stimulation an effective analgesia during colonoscopy. Postgraduate Medical Journal 2001;77:445‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roongpisuthip 1999 {published data only}
- Roongpisuthipong C, Panpakdee O, Boontawee A, Kulapongse S, Tanphaichitr V. Possible thermogenesis with dexfenfluramine. Chotmaihet thangphaet [Journal of the Medical Association of Thailand] 1999;82:150‐9. [PubMed] [Google Scholar]
Roscoe 2002 {published data only}
- Roscoe JA, Morrow GR, Bushunow P, Tian L, Matteson S. Acustimulation wristbands for the relief of chemotherapy‐induced nausea. Alternative Therapies 2002;8:56‐62. [PubMed] [Google Scholar]
Roscoe 2005 {published data only}
- Roscoe JA, Matteson SE, Morrow GR, Hickok JT, Bushunow P, Griggs J, et al. Acustimulation wrist bands are not effective for the control of chemotherapy‐induced nausea in women with breast cancer. Journal of Pain and Symptom Management 2005;29:376‐84. [DOI] [PubMed] [Google Scholar]
Rosen 1976 {published data only}
- Rosen GM, Glasgow RE, Barrera M. A controlled study to assess the clinical efficacy of totally self‐administrated systematic desensitization. Journal of Consulting and Clinical Psychology 1976;44(2):208‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rossi 1982 {published data only}
- Rossi A, Ziacchi V, Lomanto B. The hypotensive effect of a single daily dose of labetalol: a preliminary study. International Journal of Clinical Pharmacology, Therapy and Toxicology 1982;20(9):438‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Roughan 1981 {published data only}
- Roughan PA, Kunst L. Do pelvic floor excercises really improve orgasmic potential?. Journal of Sex and Marital Therapy 1981;7(3):223‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rowbotham 1996 {published data only}
- Rowbotham MC, Davies PS, Verkempinck C, Galer BS. Lidocaine patch: double‐blind controlled study of a new treatment method for post‐herpetic neuralgia. Pain 1996;65:39‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rupert 1978 {published data only}
- Rupert PA, Holmes DS. Effects of multiple sessions of true and placebo heart rate biofeedback training on the heart rates and anxiety levels of anxious patients during and following treatment. Psychophysiology 1978;15(6):582‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rybarczyk 1990 {published data only}
- Rybarczyk BD, Auerbach SM. Reminiscence interviews as stress management interventions for older patients undergoing surgery. The Gerontologist 1990;30(4):522‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Röschke 2000 {published data only}
- Roschke J, Wolf C, Müller MJ, Wagner P, Mann K, Grozinger M, Bach S. The benefit from whole body acupuncture in major depression. Journal of Affective Disorders 2000;57:73‐81. [DOI] [PubMed] [Google Scholar]
- Rösche J. Wolf C, Kögel P, Wagner P, Bech S. Adjuvant whole body acupuncture for major depression. A placebo‐controlled study of patients in standardised mianserintreatment [Adjuvante Ganzkörper‐akupunktur bei Depression. Eine placebokontrollierte Studie unter standardisierter Mianserintherapie]. Nervenartz 1998;69:961‐7. [DOI] [PubMed] [Google Scholar]
Rösler 2003 {published data only}
- Rösler A, Otto B, Schreiber‐Dietrich D, Steinmetz H, Kessler KR. Single‐needle acupuncture alleviates gag reflex during transesophageal eccocardiography: a blinded, randomised, controlled pilot trial. The Journal of Alternative and Complementary Medicine 2003;9:847‐9. [DOI] [PubMed] [Google Scholar]
Sanders 1990 {published data only}
- Sanders G, Tepe R, Maloney P, Reinert O. The effect of spinal manipulation on subjects with acute low back pain: a comparison of visual analog pain scores and serum beta endorphin levels. Journal of Manipulative and Physiological Therapeutics 1990;13(1):58. [PubMed] [Google Scholar]
- Sanders GE, Reinert O, Tepe R, Maloney P. Chiropractic adjustive manipulation on subjects with acute low back pain: visual analog pain scores and plasma beta‐endorphin levels. Journal of Manipulative and Physiological Therapeutics 1990;13(7):391‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Schallreuter 2002 {published data only}
- Shallreuter KU, Moore J, Behrens‐Williams S, Panske A, Harari M. Rapid initiation of repigmentation in vitiligo with dead sea climatotherapy in combination with pseudocatalase (PC‐KUS). International Journal of Dermatology 2002;41:482‐7. [DOI] [PubMed] [Google Scholar]
Scharf 2006 {published data only}
- Scharf HP, Mansmann U, Streitberger K, Witte S, Krämer J, Maier C, et al. Acupuncture and knee osteoarthritis: a three‐armed randomized trial. Annals of Internal Medicine 2006;145:12‐20. [DOI] [PubMed] [Google Scholar]
Scharff 2002 {published data only}
- Scharff L, Marcus DA, Masek BJ. A controlled study of minimal‐contact thermal biofeedback treatment in children with migraine. Journal of Pediatric Psychology 2002;27:109‐19. [DOI] [PubMed] [Google Scholar]
Seer 1980 {published data only}
- Seer P, Raeburn JM. Meditation training and essential hypertension: a methodological study. Journal of Behavioral Medicine 1980;3(1):59‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Senediak 1985 {published data only}
- Senediak C, Spence SH. Rapid versus gradual scheduling of therapeutic contact in a family based behavioural weight control programme for children. Behavioural Psychotherapy 1985;13:265‐87. [Google Scholar]
Shen 2000 {published data only}
- Shen J, Wenger N, Glaspy J, Hays RD, Albert PS, Choi C, Shekelle PG. Electroacupuncture for control of myeloablative chemotherapy‐induced emesis. JAMA 2000;284:2755‐61. [DOI] [PubMed] [Google Scholar]
Sibilio 1957 {published data only}
- Sibilio JP, Andrew G, Dart D, Moore KB, et al. Treatment of chronic schizophrenia with promazine hydrochloride. AMA Archives of Neurology and Psychiatry 1957;78:419‐24. [DOI] [PubMed] [Google Scholar]
Sinaiko 1991 {published and unpublished data}
- Gómez‐Marín O, Prineas RJ, Sinaiko AR. The sodium‐potassium blood pressure trial in children. Design, recruitment, and randomization: the children and adolescent blood pressure program. Controlled Clinical Trials 1991;12:408‐23. [DOI] [PubMed] [Google Scholar]
- Sinaiko AR, Gómez‐Marín O, Prineas RJ. Effect of low sodium diet or potassium supplementation on adolescent blood pressure. Hypertension 1993; Vol. 21:989‐994. [DOI] [PubMed]
Sipich 1974 {published data only}
- Sipich JF, Russell RK, Tobias LL. A comparison of covert sensitization and 'nonspecific' treatment in the modification of smoking behavior. Journal of Behavior Therapy and Experimental Psychiatry 1974;5:201‐3. [Google Scholar]
Sommerness 1955 {published data only}
- Sommerness MD, Lucero RJ, Hamlon JS, Erickson JL, et al. A controlled study of reserpine on chronically disturbed patients. AMA Archives of Neurology and Psychiatry 1955;74:316‐9. [DOI] [PubMed] [Google Scholar]
Spanos 1995 {published data only}
- Spanos NP, Mondoux TJ, Burgess CA. Comparison of multi‐component hypnotic and non‐hypnotic treatments for smoking. Contemporary Hypnosis 1995;12(1):12‐19. [Google Scholar]
Sprott 1993 {published data only}
- Sprott H, Mennet P, Stratz T, Müller W. Efficacy of acupuncture in patients with generalised tendomyopathy (fibromyalgia) [Wirksamkeit der Acupunktur bei Patienten mit generaliserter Tendomyopathie (Fibromyalgie)]. Aktuelle Rheumatologie 1993;18:132‐5. [Google Scholar]
- Sprott H, Müller W. Efficacy of acupuncture in patients with fibromyalgia. Reumatologia 1994;32(4):414‐21. [Google Scholar]
Stabholz 1991 {published data only}
- Stabholz A, Shapira J, Shur D, Friedman M, et al. Local application of sustained‐release delivery system of chlorhexidine in Down's syndrome population. Clinical Preventive Dentistry 1991;13(5):9‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Steinsbekk 2004 {published data only}
- Steinsbekk A, Bentzen N, Fønnebø V, Lewith G. Self treatment with one of three self selected, ultramelecular homeopathic medicines for the prevention of upper respiratory tract infections in children. A double‐blind randomized placebo controlled trial. British Journal of Clinical Pharmacology 2005;59:447‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinsbekk A, Bentzen N, Fønnebø V, Lewith GT. Homeopathic care for the prevention of upper respiratory tract infections in children: A double‐blind randomized placebo controlled trial comparing individualised homeopathic care and waiting‐list controls. Complemantary Therapies in Medicine 2005;13:231‐8. [DOI] [PubMed] [Google Scholar]
- Steinsbekk A, Bentzen N, Fønnebø V, Lewith GT. Randomzed controlled trial on treatment by homeopaths and self‐treatment with homeopathic medicines: design and protocol. Journal of Alternative and Complementary Medecine 2004;10:1027‐32. [DOI] [PubMed] [Google Scholar]
Stewart 1991 {published data only}
- Stewart JE, Jacobs‐Schoen M, Padilla MR, Maeder LA, et al. The effect of cognitive behavioral intervention on oral hygiene. Journal of Clinical Periodontology 1991;18:219‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stransky 1989 {published data only}
- Stransky M, Rubin A, Lava NS, Lazaro RP. Treatment of carpal tunnel syndrome with vitamin B6: a double‐blind study. Southern Medical Journal 1989;89(7):841‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Straub 2001 {published data only}
- Straub WF, Spino MP, Alattar MM, Pfleger B, et al. The effect of chiropractic care on jet lag of Finnish junior elite athletes. Journal of Manipulative and Physiological Therapeutics 2001;24:191‐8. [PubMed] [Google Scholar]
Sumaya 2001 {published data only}
- Sumaya IC, Rienzi BM, Deegan JF, Moss DE. Bright light treatment decreases depression in institutionalized older adults: a placebo‐controlled crossover study. The Journals of Gerontology. Series A. Biological Sciences and Medical Sciences 2001;56A:M356‐60. [DOI] [PubMed] [Google Scholar]
Tan 1982 {published data only}
- Tan SY, Poser EG. Acute pain in a clinical setting: effects of cognitive‐behavioural skills training. Behaviour Research and Therapy 1982;20:535‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 1986 {published data only}
- Tan SY, Bruni J. Cognitive‐behavior therapy with adult patients with epilepsy: a controlled outcome study. Epilepsia 1986;27(3):225‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tarcin 2004 {published data only}
- Tarcin O, Gurbuz AK, Pocan S, Keskin O, Demirturk L. Acustimulation of the Neiguan point during gastroscopy: its effects on nausea and retching. Turkish Journal of Gastroenterology 2004;15:258‐62. [PubMed] [Google Scholar]
Tarrier 1998 {published data only}
- Tarrier N, Yusupoff L, Kinney C, McCarthy E. Randomised controlled trial of intensive cognitive behaviour therapy for patients with chronic schizophrenia. BMJ 1998;317:303‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tashjian 2006 {published data only}
- Tashjian RZ, Banerjee R, Bradley MP, Alford W, Fadale PD. Zolpidem reduces postoperative pain, fatigue, and narcotic consumption following knee arthroscopy: a prospective randomized placebo‐controlled double‐blinded study. Journal of Knee Surgery 2006;19:105‐11. [DOI] [PubMed] [Google Scholar]
Theroux 1993 {published data only}
- Theroux MC, West DW, Corddry DH, Hyde PM, et al. Efficacy of intranasal midazolam in facilitating suturing of lacerations in preschool children in the emergency department. Pediatrics 1993;91(3):624‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Thomas 1987 {published data only}
- Thomas KB. General practice consultations: is there any point in being positive?. British Medical Journal 1987;294:1200‐2. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 1999 {published data only}
- Thomas VJ, Dixon AL, Milligan P. Cognitive‐behaviour therapy for the management of sickle cell disease pain: an evaluation of a community based intervention. British Journal of Health Psychology 1999;4:209‐29. [Google Scholar]
Thomas 2002a {published data only}
- Thomas KS, Muir KR, Doherty M, Jones AC, O'Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ 2002;325:752‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2002b {published data only}
- Thomas KS, Muir KR, Doherty M, Jones AC, O'Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ 2002;325:752‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tremeau 1992 {published data only}
- Tremeau ML, Fontanie‐Ravier P, Teurnier F, Demouzon J. Protocol of cervical maturation by acupuncture [Protocole de maturation cervicale par acupuncture]. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 1992;21:375‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Tritrakarn 2000 {published data only}
- Tritrakarn T, Lertakyamanee J, Koompong P, Soontrapa S, Somprakit P, Tantiwong A, Jittapapai S. Both EMLA and placebo cream reduced pain during extracorporeal piezoelectric scock wave lithotripsy with the piezolith 2300. Anaesthesiology 2000;92:1049‐54. [DOI] [PubMed] [Google Scholar]
Tsay 2003 {published data only}
- Tsay SL, Chen ML. Acupressure and quality of sleep in patients with end‐stage renal disease: a randomised controlled trial. International Journal of Nursing Studies 2003;40(1):1‐7. [DOI] [PubMed] [Google Scholar]
Tsay 2004 {published data only}
- Tsay SL. Acupressure and fatigue in patients with end‐stage renal disease‐a randomized controlled trial. International Journal of Nursing Studies 2004;41:99‐106. [DOI] [PubMed] [Google Scholar]
Tuomilehto 1980 {published data only}
- Tuomilehto J, Voutilainen E, Huttunen J, Vinni S, et al. Effect of guar gum on body weight and serum lipids in hypercholesterolemic females. Acta Medica Scandinavica 1980;208:45‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turner 1979 {published data only}
- Ascher ML, Turner RM. A comparison of two methods for the administration of paradoxical intention. Behaviour Research and Therapy 1980;18:121‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turner RM, Ascher LM. Controlled comparison of progressive relaxation, stimulus control, and paradoxical intention therapies for insomnia. Journal of Consulting and Clinical Psychology 1979;47(3):500‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tyler 1946 {published data only}
- Tyler DB. The influence of a placebo, body position and medication on motion sickness. The American Journal of Physiology 1946;146:458‐66. [DOI] [PubMed] [Google Scholar]
Vlaeyen 1996 {published data only}
- Vlaeyen JWS, Teeken‐Gruben NJG, Goossens MEJB, Rutten‐van Mölken MPMH, et al. Cognitive‐educational treatment of fibromyalgia: a randomized clinical trial. I. Clinical effects. The Journal of Rheumatology 1996;23(7):1237‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Walton 1993 {published data only}
- Walton RE, Chiappinelli J. Prophylactic penicillin: effect on posttreatment symptoms following root canal treatment of asymptomatic periapical pathosis. Journal of Endodontics 1993;19(9):466‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang B, Tang J, White PF, Naruse R, et al. Effect of the intensity of transcutaneous acupoint electrical stimulation on the postoperative analgesic requirement. Anesthesia and Analgesia 1997;85:406‐13. [DOI] [PubMed] [Google Scholar]
Watzl 1986 {published data only}
- Watzl H, Olbrich R, Rist F, Cohen R. Placebo injections and alcohol surveillance in inpatient treatment of alcoholic women: An experimental study of two treatment characteristics [Placebo‐injektionen und Alkoholkontrollen in der stationären Behandlung alkoholkranker Frauen ‐ eine experimentelle Untersuchung zweier Behandlungsmerkmale]. Zeitschrift für Klinische Psychologie 1986;15(4):333‐45. [Google Scholar]
Weingaertner 1971 {published data only}
- Weingaertner AH. Self‐administered aversive stimulation with hallucinating hospitalized schizophrenics. Journal of Consulting and Clinical Psychology 1971;36(3):422‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Werntoft 2001 {published data only}
- Werntoft E, Dykes A. Effect of acupressure on nausea and vomiting during pregnancy. A randomized, placebo‐controlled, pilot study. The Journal of Reproductive Medicine 2001;46:835‐9. [PubMed] [Google Scholar]
Whittaker 1963 {published data only}
- Whittaker CB, Hoy RM. Withdrawal of perphenazine in chronic schizophrenia. The British Journal of Psychiatry; the Journal of Mental Science 1963;109:422‐7. [DOI] [PubMed] [Google Scholar]
Wilcock 2008 {published data only}
- Wilcock A, Walton A, Manderson C, Feathers L, Khoury B, Lewis M, et al. Randomised, placebo‐controlled trial of nebulised furosamide for breathlessness in patients with cancer. Thorax 2008;63:872‐5. [DOI: 10.1136/thx.2007.091538] [DOI] [PubMed] [Google Scholar]
Williams 1988 {published data only}
- Williams JM, Hall DW. Use of single session hypnosis for smoking cessation. Addictive Behaviors 1988;13:205‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wilson 1980 {published data only}
- Wilson A, Davidson WJ, Blanchard R. Disulfiram implantation. A trial using placebo implants and two types of controls. Journal of Studies on Alcohol 1980;41(5):429‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Witt 2005 {published data only}
- Witt C, Brinkhaus B, Jena S, Linde K, Streng A, Wagenpfeil S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 2005;366:136‐43. [DOI] [PubMed] [Google Scholar]
Wojciechowski 1984 {published data only}
- Wojciechowski FL. Behavioral treatment of tension headache: a contribution to controlled outcome research methodology. Gedrag ‐ Tijdschrift voor Psychologie 1984;12(5):16‐30. [Google Scholar]
Woods 2005 {published data only}
- Woods DL, Craven RF, Whitney J. The effect of therapeutic touch on behavioral symptoms of persons with dementia. Alternative Therapies in Health and Medicine 2005;11:66‐74. [PubMed] [Google Scholar]
Yan 2005 {published data only}
- Yan T, Hui‐chan CW, Li LS. Effects of functional electrical stimulation on the improvement of motor function of patients with acute stroke: a randomized controlled trial. National Medical Journal of China 2006;86(37):2627‐31. [PubMed] [Google Scholar]
- Yan T, Hui‐chan CWY, Li LSW. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke. Stroke 2005;36:80‐85. [DOI] [PubMed] [Google Scholar]
Yates 1988 {published data only}
- Yates RG, Lamping DL, Abram NL, Wright C. Effects of chiropractic treatment on blood pressure and anxiety: a randomized, controlled trial. Journal of Manipulative and Physiological Therapeutics 1988;11(6):484‐8. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbot 1995 {published data only}
- Abbot K, Fowler‐Kerry S. The use of topical refrigerent anesthetic to reduce injection pain in children. Journal of Pain and Symptom Management 1995;10(8):584‐90. [DOI] [PubMed] [Google Scholar]
Abikoff 1985 {published data only}
- Abikoff H, Gittelman R. Hyperactive children treated with stimulants. Archives of General Psychiatry 1985;42:953‐61. [DOI] [PubMed] [Google Scholar]
Allen 1987 {published data only}
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized trial. Surgery 1987;101:720‐30. [PubMed] [Google Scholar]
Allen 1996 {published and unpublished data}
- Allen KD, White DD, Walburn JN. Sucrose as an analgesic agent for infants during immunization injections. Archives of Pediatrics and Adolescent Medicine 1996;150:270‐4. [DOI] [PubMed] [Google Scholar]
Amanzio 1999 {published data only}
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation‐activated opioid systems versus conditioning‐activated specific subsystems. The Journal of Neuroscience 1999;19:484‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amanzio 2001 {published data only}
- Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non‐specific activation of endogenous opioids. Pain 2001;90:205‐15. [DOI] [PubMed] [Google Scholar]
Archer 1992 {published data only}
- Archer TP, Leier CV. Placebo treatment in congestive heart failure. Cardiology 1992;81:125‐33. [DOI] [PubMed] [Google Scholar]
Arnett 1990 {published data only}
- Arnett RM, Jones JS, Horger III EO. Effectiveness of 1% lidocaine dorsal penile nerve block in infant circumcision. American Journal of Obstetrics and Gynecology 1990;163:1074‐80. [DOI] [PubMed] [Google Scholar]
Avis 2008 {published data only}
- Avis NE, Legault C, Coeytaux RR, Pian‐Smith M, et al. A randomized, controlled pilot study of acupuncture treatment for menopausal hot flashes. Menopause 2008;15(6):1‐9. [DOI] [PubMed] [Google Scholar]
Babizhayev 2001 {published data only}
- Babizhayev MA, Deyev AI, Yermakova VN, Semiletov YA, et al. N‐acetylcarnosine, a natural histidine‐containing dipeptide, as a potent ophtalmic drug in treatment of human cataract. Peptides 2001;22:979‐94. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA, Yermakova VN, Deyev AI, Seguin MC. Imidazole‐containing peptidomimetic NACA as a potent drug for the medical treatment of age related cataract in humans. Journal of Anti‐Aging Medicine 2000;3:43‐62. [Google Scholar]
Barrett 1999 {published data only}
- Barrett PM, Webster HM, Wallis JR. Adolescent self‐esteem and cognitive skills training: a school‐based intervention. Journal of Child and Family Studies 1999;8(2):217‐27. [Google Scholar]
Beck 2002 {published data only}
- Beck CK, Vogelpohl TS, Rasin JH, Uriri JT, et al. Effects of behavioral interventions on disruptive behavior and effect in demented nursing home residents. Nursing Research 2002;51:219‐28. [DOI] [PubMed] [Google Scholar]
Benedetti 1998 {published data only}
- Benedetti F, Amanzio M, Baldi S, Casadio C, et al. The specific effects of prior opioid exposure on placebo analgesia and placebo respiratory depression. Pain 1998;75:313‐9. [DOI] [PubMed] [Google Scholar]
Benedetti 1999b {published data only}
- Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid system by target‐directed expectations of analgesia. The Journal of Neuroscience 1999;19:3639‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedict 1989 {published data only}
- Benedict RHB, Harris AE. Remediation of attention deficits in chronic schizophrenic patients: a preliminary study. British Journal of Clinical Psychology 1989;28:187‐8. [DOI] [PubMed] [Google Scholar]
Bennet 2001 {published data only}
- Bennet WD, Zeman KL, Foy C, Shaffer CL, et el. Effect of aerosolized uridine 5'‐triphosphate on mucociliary clearance in mild chronic bronchitis. American Journal of Respiratory and Critical Care Medicine 2001;164:302‐6. [DOI] [PubMed] [Google Scholar]
Benton 1988 {published data only}
- Benton D, Roberts G. Effect of vitamin and mineral supplementation on intelligence of a sample of schoolchildren. Lancet 1988;i:140‐3. [DOI] [PubMed] [Google Scholar]
Bergmann 1994 {published data only}
- Bergmann J, Chassany O, Gandiol J, Deblois P, et al. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer patients. Clinical Trials and Meta‐Analysis 1994;29:41‐7. [PubMed] [Google Scholar]
Beutler 1988 {published data only}
- Beutler JJ, Attevelt JTM, Schouten SA, Faber JA, et al. Paranormal healing and hypertension. BMJ 1988;296:1491‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bierman 1997 {published data only}
- Bierman DJ, Julien ECGJ. An explorative study on the effect of alpha brain wave training by means of photic stimulation on withdrawal reactions of alcoholics in a clinical setting [Een exploratief onderzoek naar het effect van alfa‐hersengolftraining door middel van photic stimulation op onthoudingsverschijnselen van alcoholafhankelijken in een klinische setting]. Tijdschrift voor Alcohol, Drugs en Andere Psychotrope Stoffen 1997;22(2):65‐73. [Google Scholar]
Björkstén 1986 {published data only}
- Björkstén B, Möller C, Broberger U, Ahlstedt S, et al. Clinical and immunological effects of oral immunotherapy with a standardized birch pollen extract. Allergy 1986;41:290‐5. [DOI] [PubMed] [Google Scholar]
Blackwell 1972 {published data only}
- Blackwell B, Bloomfield SS, Bumcher CR. Demonstration to medical students of placebo responders and non‐drug factors. Lancet 1972;i:1279‐2. [DOI] [PubMed] [Google Scholar]
Blanchard 1978 {published data only}
- Blanchard EB, Theobald DE, Williamson DA, Silver BV, et al. Temperature biofeedback in the treatment of migraine headaches. A controlled investigation. Archives of General Psychiatry 1978;35:581‐8. [DOI] [PubMed] [Google Scholar]
Borden 1989 {published data only}
- Bordon KA. Attributional outcomes: the subtle messages of treatment for attention deficit disorder. PhD‐Thesis, University of Illinois 1986.
- Bordon KA, Brown RT. Attributional outcomes: the subtle messages of treatments for attention deficit disorder. Cognitive Therapy and Research 1989;13(2):147‐60. [Google Scholar]
Borkovec 1975 {published data only}
- Borkovec TD, Kaloupek DG, Slame KM. The facilitative effect of muscle tension‐release in the relaxation treatment of sleep disturbance. Behavior Therapy 1975;6(3):301‐9. [Google Scholar]
Bornstein 1973 {published data only}
- Bornstein PH, Sipprelle CN. Group treatment of obesity by induced anxiety. Behaviour Research and Therapy 1973;11:339‐41. [DOI] [PubMed] [Google Scholar]
Bouchet 1996 {published data only}
- Bouchet C, Guilemin F, Briancon S. Nonspecific effects in longitudinal studies: impact on quality of life measures. Journal of Clinical Epidemiology 1996;49(1):15‐20. [DOI] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown FF, Robinson ME, Riley JL, Gremillion HA, McSoley J, Meyers G. Better palpation of pain: reliability and validity of a new pressure pain protocol in TMD. CRANIO: The Journal of Craniomandibular Practice 2000;18:58‐65. [DOI] [PubMed] [Google Scholar]
Buckalew 1972 {published data only}
- Buckalew LW. An analysis of experimental components in a placebo effect. The Psychological Record 1972;22:113‐9. [Google Scholar]
Bullock 1999 {published data only}
- Bullock ML, Kiresuk TJ, Pheley AM, Culliton PD, Lenz SK. Auricular acupuncture in the treatment of cocaine abuse. Journal of Substance Abuse Treatment 1999;16:31‐8. [DOI] [PubMed] [Google Scholar]
Bush 1985 {published data only}
- Bush C, Ditto B. A controlled evaluation of paraspinal EMG biofeedback in the treatment of chronic low back pain. Health Psychology 1985;4(4):307‐21. [DOI] [PubMed] [Google Scholar]
Butler 1984 {published data only}
- Butler G, Cullington A, Munby M, Amies P, et al. Exposure and anxiety management in the treatment of social phobia. Journal of Consulting and Clinical Psychology 1984;52(4):642‐50. [DOI] [PubMed] [Google Scholar]
Carlson 1993 {published data only}
- Carlson CL, Pelham WE, Milich R, Hoza B. ADHD boys' performance and attributions following success and failure: drug effects and individual differences. Cognitive Therapy & Research 1993;17(3):269‐87. [Google Scholar]
Carpenter 1994 {published data only}
- Carpenter DJ, Gatchel RJ, Hasegawa T. Effectiveness of a videotaped behavioral intervention for dental anxiety: the role of gender and the need for information. Behavioral Medicine 1994;20:123‐32. [DOI] [PubMed] [Google Scholar]
Chambless 1984 {published data only}
- Chambles DL, Sultan FE, Stern TE, O'Neill C, et al. Effect of pubococcygeal exercise on coital orgasm in women. Journal of Counseling and Clinical Psychology 1984;52(1):114‐8. [DOI] [PubMed] [Google Scholar]
Chen 1999 {published data only}
- Chen ML, Lin LC, Wu SC, Lin JG. The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 1999;54:M389‐94. [DOI] [PubMed] [Google Scholar]
Cole 1983 {published data only}
- Cole AD, Bond NW. Olfactory aversion conditioning and overeating: a review and some data. Perceptual and Motor Skills 1983;57:667‐78. [DOI] [PubMed] [Google Scholar]
Corletto 1999 {published data only}
- Corletto F. [Terapia dell'osteoporosi climaterica con estratto titolato di equiseto piu calcio (osteosil calcium). Studio in doppio cieco, randomizzato]. Minerva Ortopedica e Traumatologica 1999;50(5):201‐6. [Google Scholar]
Corson 1994 {published data only}
- Corson SL, Batzer FR, Gocial B, Kelly M. Is paracervical block anesthesia for oocyte retrieval effective?. Fertility and Sterility 1994;62(1):133‐6. [DOI] [PubMed] [Google Scholar]
Cottraux 1986 {published data only}
- Cottraux JA, Harf R, Boissel J, Schbath J, et al. Smoking cessation with behaviour therapy or acupuncture ‐ a controlled study. Behaviour Research and Therapy 1983;21(4):417‐24. [DOI] [PubMed] [Google Scholar]
Cram 1980 {published data only}
- Cram JR. EMG biofeedback and the treatment of tension headaches: a systematic analysis of treatment components. Behavior Therapy 1980;11:699‐710. [Google Scholar]
Cristofalo 1999 {published data only}
- Cristofalo MG, Savojardo M, Pecorello S, Galiano S, et al. The end of antioxidizing treatment of pulmonary illness: our experience [Ruolo degli antiossidanti nel danno polmonare. Nostre esperienze]. Medicino Dello Sport 1999;52(3):165‐75. [Google Scholar]
Cullhed 1961 {published data only}
- Cullhed S, Löfström B. Obstetric analgesia with pethidine and scopalamine. Lancet 1961;i:75‐7. [DOI] [PubMed] [Google Scholar]
Dahlquist 1986 {published data only}
- Dahlquist LM, Gil KM, Armstrong FD, DeLawyer DD, et al. Preparing children for medical examinations: the importance of previous medical experience. Health Psychology 1986;5(3):249‐59. [DOI] [PubMed] [Google Scholar]
Daley 2007 {published data only}
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of excercise therapy in women treated for breast cancer. Journal of Clinical Oncology 2007;25:1713‐21. [DOI] [PubMed] [Google Scholar]
Diamond 1995 {published data only}
- Diamond L, Dockhorn RJ, Grossman J, Kisicki JC, et al. A dose‐response study of the efficacy and safety of ipratropium bromide nasal spray in the treatment of the common cold. The Journal of Allergy and Clinical Immunology 1995;95(5, part 2):1139‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Disney 1988 {published data only}
- Disney JA, Graves RC, Cancro L, Payonk G, et al. An evaluation of 6 dentrifice formulations for supragingival anticalculus and antiplaque activity. Journal of Clinical Periodontology 1989;6:525‐8. [DOI] [PubMed] [Google Scholar]
Dobia 1985 {published data only}
- Dobia B, McMurray NE. Applicability of learned helplessness to depressed women undergoing assertion training. Australian Journal of Psychology 1985;37:71‐80. [Google Scholar]
Dundee 1988 {published data only}
- Dundee JW, Sourial FBR, Ghaly RG, Bell PF, et al. P6 acupressure reduces morning sickness. Journal of the Royal Society of Medicine 1988;81:456‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egbert 1964 {published data only}
- Egbert LD, Battit GE, Welch CE, Bartlett MK. Reduction of postoperative pain by encouragement and instruction of patients. The New England Journal of Medicine 1964;270(16):825‐7. [DOI] [PubMed] [Google Scholar]
Eickholz 2002 {published data only}
- Eickholz P, Kim TS, Bürklin T, Schacher B, et al. Non‐surgical periodontal therapy with adjunctive topical doxycycline: a double‐blind randomized controlled multicentre study (1). Study design and clinical results. Journal of Clinical Periodontology 2002;29:108‐17. [DOI] [PubMed] [Google Scholar]
Elkin 1985 {published data only}
- Elkin I, Parloff MB, Headly SW, Autry JH. NIMH treatment of depression collaborative research program. Archives of General Psychiatry 1985;42:305‐16. [DOI] [PubMed] [Google Scholar]
Feather 1972 {published data only}
- Feather BW, Chapman CR, Fisher SB. The effect of a placebo on the perception of painful radient heat stimuli. Psychosomatic Medicine 1972;34(4):290‐4. [DOI] [PubMed] [Google Scholar]
Fevery 1990 {published data only}
- Fevery J, Elewaut A, Michielsen P, Nevens F, et al. Efficiacy of interferon alfa‐2b with or without prednisone withdrawal in the treatment of chronic viral hepatitis B. A prospective double‐blind Belgian‐Dutch study. Journal of Hepatology 1990;11:s108‐12. [DOI] [PubMed] [Google Scholar]
Fillmore 1992 {published data only}
- Fillmore M, Vogel‐Sprott M. Expected effect of caffeine on motor performance predicts the type of response to placebo. Psychopharmacology 1992;106:209‐14. [DOI] [PubMed] [Google Scholar]
Fillmore 1994 {published data only}
- Fillmore MT, Mulvihill LE, Vogel‐Sprott M. The expected drug and its expected effect interact to determine placebo responses to alcohol and caffeine. Psychopharmacology 1994;115:383‐8. [DOI] [PubMed] [Google Scholar]
Fillmore 1994b {published data only}
- Fillmore MT, Vogel‐Sprott M. Psychomotor performance under alcohol and under caffeine: expectancy and pharmacological effects. Experimental and Clinical Psychology 1994;2:319‐27. [Google Scholar]
Flor 1983 {published data only}
- Flor H, Haag G, Turk DC, Koehler H. Efficacy of EMG biofeedback, pseudotherapy, and conventional medical treatment for chronic rheumatic back pain. Pain 1983;17:21‐31. [DOI] [PubMed] [Google Scholar]
Fuller 1986 {published data only}
- Fuller RK, Branchey L, Brightwell DR, Derman RM, et al. Disulfiram treatment of alcoholism. A Veterans Administration cooperative study. JAMA 1986;256(11):1449‐55. [PubMed] [Google Scholar]
Gam 1998 {published data only}
- Gam AN, Warming S, Larsen LH, Jensen B. Treatment of myofascial trigger‐points with ultrasound combined with massage and exercise ‐ a randomised controlled trial. Pain 1998;77:73‐9. [DOI] [PubMed] [Google Scholar]
Gelfand 1963 {published data only}
- Gelfand S, Ullmann LP, Krasner L. The placebo response: an experimental approach. Journal of Nervous and Mental Disease 1963;136:379‐87. [DOI] [PubMed] [Google Scholar]
Goodale 1990 {published data only}
- Goodale IL, Domar AD, Benson H. Allivation of premenstrual syndrome symptoms with the relaxation response. Obstetrics & Gynecology 1990;75(4):649‐55. [PubMed] [Google Scholar]
Gowdey 1967 {published data only}
- Gowdey CW, Hamilton JT, Philip RB. A controlled clinical trial using placebos in normal subjects. Canadian Medical Association Journal 1967;96:1317‐22. [PMC free article] [PubMed] [Google Scholar]
Gregorio 1996 {published data only}
- Gregorio GV, Jara P, Hierro L, Diaz C, et al. Lymphoblastoid interferon alfa with or without steroid pretreatment in children with chronic hepatitis B: a multicenter controlled trial. Hepatology 1996;23:700‐7. [DOI] [PubMed] [Google Scholar]
Gregory 1983 {published data only}
- Gregory SJ, Davies ADM, Binks MG. The improvement of verbal fluency in the elderly: the effects of practice on the set test and an alternative form. Educational Gerontology 1983;9(2‐3):139‐46. [Google Scholar]
Gryll 1978 {published data only}
- Gryll SL, Katahn M. Situational factors contributing to the placebo effect. Psychopharmacology 1978;57:253‐61. [DOI] [PubMed] [Google Scholar]
Haake 2007 {published data only}
- Haake M, Müller HH, Schade‐Brittinger C, Basler HD, Schäfer H, Maier C, et al. German acupuncture trials for chronic low back pain. Randomized, multicenter, blinded, parallel‐group trial with 3 groups. Archives of Internal Medicine 2007;167(17):1892‐8. [DOI] [PubMed] [Google Scholar]
Hale 1986 {published data only}
- Hale EH. A study of the relationship between therapeutic touch and the anxiety levels of hospitalized adults. PhD‐thesis, Texas Woman's University 1986.
Hall 1994 {published data only}
- Hall SM, Tunis S, Triffleman E, Banys P, et al. Continuity of care and desipramine in primary cocaine abusers. The Journal of Nervous and Mental Diseases 1994;182(10):570‐5. [PubMed] [Google Scholar]
Hargreaves 1983 {published data only}
- Hargreaves KM, Dionne RA, Mueller GP. Plasma beta‐endorphin‐like immunoreactivity, pain and anxiety following administration of placebo in oral surgery patients. Journal of Dental Research 1983;62:1170‐3. [DOI] [PubMed] [Google Scholar]
Hayden 1996 {published data only}
- Hayden FG, Diamond L, Wood PB, Korts DC, et al. Effectiveness and safety of intranasal ipratropium bromide in common colds. Annals of Internal Medicine 1996;125:89‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Herth 2000 {published data only}
- Herth K. Enhancing hope in people with a first recurrence of cancer. Journal of Advanced Nursing 2000;32:1431‐41. [DOI] [PubMed] [Google Scholar]
Hogarty 1973 {published data only}
- Hogarty GE, Goldberg SC. Drug and sociotherapy in the aftercare of schizophrenic patients. Archives of General Psychiatry 1973;28:54‐64. [DOI] [PubMed] [Google Scholar]
Huber 1986 {published data only}
- Huber MGP, Wildschut HIJ, Boer K, Kleiverda G, et al. Umbilical vein administration of oxytocin for the management of retained placenta: is it effective?. American Journal of Obstetrics and Gynecology 1991;164(5 part 1):1216‐9. [DOI] [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen MP, Karoly P. Motivation and expectancy factors in symptom perception: a laboratory study of the placebo effect. Psychosomatic Medicine 1991;53:144‐52. [DOI] [PubMed] [Google Scholar]
Kalman 1998 {published data only}
- Kalman D, Colker CM, Stark R, Minsch A, Wilets I, Antonio J. Effect of pyruvate supplemantation on body composition and mood. Current Therapeutic Research 1998;59:793‐802. [Google Scholar]
Kanner 1999 {published data only}
- Kanner RE, Connet JE, Williams DE, Buist AS. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disaese: the lung health study. The American Journal of Medicine 1999;106:410‐6. [DOI] [PubMed] [Google Scholar]
Kelley 1976 {published data only}
- Kelley CK. Play desensitization of fear of darkness in preschool children. Behaviour Research and Therapy 1976;14:79‐81. [DOI] [PubMed] [Google Scholar]
Khandwala 1997 {published and unpublished data}
- Binnie WH, Curro FA, Khandwala A, Inwegen RG. Amolexanox oral paste: a novel treatment that accelerates the healing of aphthous ulcers. Compendium 1997;18(7):1116‐26. [PubMed] [Google Scholar]
- Khandwala A, Inwegen RG, Alfano MC. 5% amlexanox oral paste, a new treatment for recurrent minor aphtous ulcers. I. Clinical demonstration of acceleration of healing and resolution of pain. Oral Surgery Oral Medicine Oral Pathology 1997;83(2):222‐30. [DOI] [PubMed] [Google Scholar]
Klosko 1990 {published data only}
- Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavior therapy in treatment of panic disorder. Journal of Consulting and Clinical Psychology 1990;58(1):77‐84. [DOI] [PubMed] [Google Scholar]
- Klosko JS, Barlow DH, Tassinari R, Cerny JA. A comparison of alprazolam and behavioural therapy in treatment of panic disorder. Journal of Psychotherapy Practice and Research 1994;3(2):166‐78. [PMC free article] [PubMed] [Google Scholar]
- Klosko JS, Barlow DH, Tassinari RB, Cerny JA. Comparison of alprazolam and cognitive behavior therapy in the treatment of panic disorder: a preliminary report. In: Hand I, Wittchen H editor(s). Panic and Phobias 2 ‐ Treatments and Variables affecting Course and Outcome. Berlin: Springer Verlag, 1988. [Google Scholar]
Korner 1982 {published data only}
- Korner PI, Bauer GE, Doyle AE, Edmondson KW, et al. Untreated mild hypertension. A report of the management committee of the Australian Therapeutic Trial in Mild Hypertension. Lancet 1982;i:185‐91. [PubMed] [Google Scholar]
Lasagna 1954 {published data only}
- Lasagna L, Mosteller F, Felsinger JM, Beecher HK. A study of the placebo response. American Journal of Medicine 1954;June:770‐9. [DOI] [PubMed] [Google Scholar]
Levine 1980 {published data only}
- Levine RA, O´Brian RM. Treatment of anxiety about college tests with negative practice and systematic desensitization: some negative findings. Psychological Reports 1980;46:823‐9. [DOI] [PubMed] [Google Scholar]
Liberman 1964 {published data only}
- Liberman R. An experimental study of the placebo response under three different situations of pain. Journal of Psychiatric Research 1964;2:233‐46. [DOI] [PubMed] [Google Scholar]
Lopez 1999 {published data only}
- Lopez‐Justicia MD, Martos FJ. The effectiveness of two programs to develop visual perception in Spanish schoolchildren with low vision. Journal of Visual Impairment and Blindness 1999;93:96‐103. [Google Scholar]
Lorr 1962 {published data only}
- Lorr M, McNair DM, Weinstein GJ. Early effects of chlordiazepoxide (librium) used with psychotherapy. Journal of Psychiatric Research 1962;1:257‐70. [DOI] [PubMed] [Google Scholar]
Lujan 1992 {published data only}
- Lujan M, Lopez‐Fiesco A, Martinez EL, Lopez GZ, et al. Experimental tension headache in humans: a double blind comparison of the analgesic effect of dipyrone, naproxen plus paracetamol or placebo. Proceedings of the Western Pharmacology Society 1992;35:201‐5. [PubMed] [Google Scholar]
Lynn 1983 {published and unpublished data}
- Lynn FE. Acupressure for the treatment of menstrual distress. PhD‐thesis, University of California 1983.
Manner 1987 {published data only}
- Manner T, Kanto J, Iisalo E, Lindberg, R, et al. Reduction of pain at venous cannulation in children with a eutectic mixture of lidocaine and prilocaine (EMLA cream): comparison with placebo cream and no local premedication. Acta Anaesthesiologica Scandinavica 1987;31:735‐9. [DOI] [PubMed] [Google Scholar]
Marchand 1993 {published data only}
- Marchand S, Charest J, Li J, Chenard J, Lavignolle B, Laurencelle L. Is TENS purely a placebo effect? A controlled study on chronic low back pain. Pain 1993;54:99‐106. [DOI] [PubMed] [Google Scholar]
McGrath 1988 {published data only}
- McGrath PJ, Humphreys P, Goodman JT, Keene D, et al. Relaxation prophylaxixs for childhood migraine: a randomized placebo‐controlled trial. Developmental Medicine and Child Neurology 1988;30:626‐31. [DOI] [PubMed] [Google Scholar]
Meehan 1985 {published data only}
- Meehan MC. The effect of therapeutic touch on the experience of acute pain in postoperative patients. PhD‐thesis, New York University 1985.
Montgomery 1996 {published data only}
- Montgomery G, Kirsch I. Mechanisms of placebo pain reduction: an empirical investigation. Psychological Science 1996;7(3):174‐6. [Google Scholar]
Nikolaou 1998 {published data only}
- Nikolaou C, Michalopoulou M, Segdista J, Kilidireas C, Rombos A, Kabouri K, Papageorgiou K. Cellular immunological parameters and antiviral antibodies in the motorneuron disease. Acta Microbiol Hellenica 1998;43:362‐9. [Google Scholar]
Peart 1977 {published data only}
- Peart WS, Cochrane AL, Dollery CT, Green KG, et al. Randomised controlled trial of treatment for mild hypertension: design and pilot trial. Report of Medical Research Council working party on mild to moderate hypertension. British Medical Journal 1977;1:1437‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penman 1956 {published data only}
- Penman AS, Dredge TE. Effect of reserpine and open‐ward‐privileges on chronic schizophrenics. AMA Archives of Neurology and Psychiatry 1956;76:42‐9. [DOI] [PubMed] [Google Scholar]
Pollo 2001 {published data only}
- Pollo A, Amanzio M, Arslantian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain 2001;93:77‐84. [DOI] [PubMed] [Google Scholar]
Price 1999 {published data only}
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 1999;83:147‐56. [DOI] [PubMed] [Google Scholar]
Rampes 1997 {published data only}
- Rampes H, Pereira S, Mortimer A, Manoharan S, Knowles M. Does electroacupuncture reduce craving for alcohol? A randomized controlled study. Complementary therapies in medicine 1997;5:19‐26. [Google Scholar]
Reich 1990 {published data only}
- Reich JW, Zautra AJ. Dispositional control beliefs and the consequences of control‐enhancing intervention. Journal of Gerontology 1990;45(2):46‐51. [DOI] [PubMed] [Google Scholar]
Robertson 1991 {published data only}
- Robertson C, Gatchel RJ, Fowler C. Effectiveness of a videotaped behavioral intervention in reducing anxiety in emergency oral surgery patients. Behavioral Medicine 1991;17(2):77‐85. [DOI] [PubMed] [Google Scholar]
Rodriguez 1997 {published data only}
- Rodriguez AP, Menesas FD, Quiroga RM, Perz L, et al. New experiences of finger pressure in prevention of acute respiratory infections [Nuevas experiencias de la digitopuntura en la prevencion de las infecciones resporatorias agudas]. Geriatrika 1997;13(8):23‐9. [Google Scholar]
Roehrich 1993 {published data only}
- Roehrich L, Goldman MS. Experience‐dependent neurophysiological recovery and the treatment of alcoholism. Journal of Consulting and Clinical Psychology 1993;61(5):812‐21. [DOI] [PubMed] [Google Scholar]
Roelofs 2000 {published data only}
- Roelofs J, ter Riet G, Peters ML, Kessels AGH, Reulen JPH, Menheere PPCA. Expectations of analgesia do not affect spinal nociceptive R‐III reflex activity: an experimental study into the mechanism of placebo‐induced analgesia. Pain 2000;89:75‐80. [DOI] [PubMed] [Google Scholar]
Roos 1969 {published data only}
- Roos P, Oliver M. Evaluation of operant conditioning with institutionalized retarded children. American Journal of Mental Deficiency 1969;74(3):325‐30. [PubMed] [Google Scholar]
Roth 1986 {published data only}
- Roth PM, Thrash WJ. Effect of transcutaneous electrical nerve stimulation for controlling pain associated with orthodontic tooth movement. American Journal of Orthodontics and Dentofacial Orthopedics 1986;90(2):132‐8. [DOI] [PubMed] [Google Scholar]
Rustøen 1998 {published data only}
- Rustøen T, Wiklund I, Hanestad BR, Moum T. Nursing intervention to increase hope and quality of life in newly diagnosed cancer patients. Cancer Nursing 1998;21:235‐45. [DOI] [PubMed] [Google Scholar]
Sarles 1977 {published data only}
- Sarles H, Camatte R, Sahel J. A study of the variation in the response regarding duodenal ulcers when treated with placebo by different investigators. Digestion 1977;16:289‐92. [DOI] [PubMed] [Google Scholar]
Sartor 1980 {published data only}
- Sartor, G, Schersten B, Carlström S, Melander A, Norden, Persson G. Ten‐year follow‐up of subjects with impaired glucose tolerance. Prevention of diabetes by tolbutamide and diet regulation. Diabetes 1980;29:41‐9. [DOI] [PubMed] [Google Scholar]
Shaw 1974 {published data only}
- Shaw DW, Thoresen CE. Effect of modeling and desensitation in reducing dentist phobia. Journal of Counseling Psychology 1974;21(5):415‐20. [Google Scholar]
Sheikh 1986 {published data only}
- Sheikh JI, Hill RD, Yesavage JA. Long‐term efficacy of cognitive training for age‐associated memory impairment: a six‐month follow up study. Developmental Neuropsychology 1986;2(4):413‐21. [Google Scholar]
Silvestri 1977 {published data only}
- Silvestri R. Implosive therapy treatment of emotionally disturbed retardates. Journal of Consulting and Clinical Psychology 1977;45(1):14‐22. [DOI] [PubMed] [Google Scholar]
Skovlund 1991 {published data only}
- Skovlund E. Should we tell trial participants that they might receive placebo?. Lancet 1991;337:1041. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Smith C, Crowther C. The placebo response and effect of time in a trial of acupuncture to treat nausea and vomiting in early pregnancy. Complementary Therapies in Medicine 2002;10:210‐6. [DOI] [PubMed] [Google Scholar]
- Smith C, Crowther C, Beilby J. Acupuncture to treat nausea and vomiting in early pregnancy: a randomized controlled trial. Birth 2002;29:1‐9. [DOI] [PubMed] [Google Scholar]
Spanos 1988 {published data only}
- Spanos NP, Stenstrom RJ, Johnston JC. Hypnosis, placebo, and suggestion in the treatment of warts. Psychosomatic Medicine 1988;50:245‐60. [DOI] [PubMed] [Google Scholar]
Staats 1998 {published data only}
- Staats P, Hekmat H, Staats A. Suggestion / placebo effects on pain: negative as well as positive. Journal of Pain and Symptom Management 1998;15(4):235‐43. [DOI] [PubMed] [Google Scholar]
Stanley 1989 {published data only}
- Stanley TH, Leiman BC, Rawal N, Marcus MA, et al. The effects of oral transmucosal fentanyl citrate premedication on preoperative behavioral responses and gastric volume and acidity in children. Anesthesia and Analgesia 1989;69:328‐35. [PubMed] [Google Scholar]
Suchman 1992 {published data only}
- Suchman AL, Ader R. Classic conditioning and placebo effects in crossover studies. Clinical Pharmacology and Therapeutics 1992;52(4):372‐7. [DOI] [PubMed] [Google Scholar]
Tashkin 1977 {published data only}
- Tashkin DP, Bresler DE, Kroening RJ, Kerschner H, et al. Comparison of real and simulated acupuncture and isoproterenol in methacholine‐induced asthma. Annals of Allergy 1977;39:379‐86. [PubMed] [Google Scholar]
- Tashkin DP, Katz RM, Kerschner H, Rachelefsky GS, et al. Comparison of aerosolized atropine, isoproterenol, atropine plus isoproterenol, disodium cromoglycate and placebo in the prevention of excercise‐induced asthma. Annals of Allergy 1977;39:311‐8. [PubMed] [Google Scholar]
Taylor 1977 {published data only}
- Taylor CB, Farquhar JW, Nelson E, Agras S. Relaxation therapy and high blood pressure. Archives of General Psychiatry 1977;34:399‐442. [DOI] [PubMed] [Google Scholar]
Vacc 1980 {published data only}
- Vacc NA, Greenleaf SM, Gerler ER Jr. Relaxing training and covert positive reinforcement with elementary school children. Elementary School Guidance & Counseling 1980;14(3):232‐5. [Google Scholar]
Van Damme 1998 {published data only}
- Damme L, Niruthisard S, Atisook R, Boer K, et al. Safety evaluation of nonoxynol‐9 gel in women at low risk of HIV infection. AIDS 1998;12:433‐7. [DOI] [PubMed] [Google Scholar]
Volweider 1981 {published and unpublished data}
- Volweider FH. A comparison of short‐term yoga and buddy‐orientated groups with chronic psychiatric patients. PhD‐thesis, University of Southern Missisippi 1981.
Weber 1975 {published data only}
- Weber BA, Sulzbacher SI. Use of CNS stimulant medication in averaged electroencephalic audiometry with children with MBD. Journal of Learning Disabilities 1975;8(5):300‐3. [Google Scholar]
Weintraub 1992 {published data only}
- Weintraub M, Sundaresan PR, Schuster B, Averbuch M, et al. Long term weight control study V (weeks 190 to 210). Clinical Pharmacology and Therapeutics 1992;51(5):614‐8. [DOI] [PubMed] [Google Scholar]
Windle 2001 {published data only}
- Windle PE, Borromeo A, Robles H, Ilacio‐Uy V. The effects of acupressure on the incidence of postoperative nausea and vomiting in postsurgical patients. Journal of Perianesthesia Nursing 2001;16:158‐62. [DOI] [PubMed] [Google Scholar]
Winnan 1982 {published data only}
- Winnan GR, Meyer CT, McCallum RW. Interpretation of the Bernstein Test: a reappraisal of criteria. Annals of Internal Medicine 1982;96:320‐2. [DOI] [PubMed] [Google Scholar]
Worner 1992 {published data only}
- Worner TM, Zeller B, Schwarz H, Zwas F, et al. Acupuncture fails to improve treatment outcome in alcoholics. Drug and Alcohol Dependence 1992;30:169‐73. [DOI] [PubMed] [Google Scholar]
Zeisset 1968 {published data only}
- Zeisset RM. Desensitization and relaxation in the modification of psychiatric patients' interview behavior. Journal of Abnormal Psychiatry 1968;73(1):18‐24. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Shin 2005 {published data only}
References to ongoing studies
Barret 2007 {published data only}
- Barrett B, Rakel D, Chewning B, Merchand L, Rabago D, Brown R, et al. Rationale and methods for a trial assessing placebo echinacea and doctor‐patient interaction in the common cold. Explore 2007;3:561‐72. [DOI] [PubMed] [Google Scholar]
Additional references
Allan 2002
- Allan LG, Siegel S. A signal detection theory analysis of the placebo effect. Eval Health Prof 2002;25:410‐20. [DOI] [PubMed] [Google Scholar]
Beecher 1955
- Beecher HK. The powerful placebo. JAMA 1955;159:1602‐6. [DOI] [PubMed] [Google Scholar]
Bignal 1994
- Bignal J. Science of placebos. Lancet 1994;344:904. [Google Scholar]
Brown 1998
- Brown WA. The placebo effect. Scientific American 1998;278:68‐3. [DOI] [PubMed] [Google Scholar]
CCC Stat Pol 1999
- Cochrane Consumers and Communication Review Group. Statistical policy. The Cochrane Library; Oxford: Update Software. Issue 1, 1999.
Cochrane 1989
- Cochrane AL. Effectiveness and efficiency. Random reflections on health services. Cambridge University Press for British Medical Journal & The Nuffield Provincial Hospitals Trust, 1989:31. [ISBN 0 7279 0282 2] [Google Scholar]
Dush 1986
- Dush DM. The placebo in psychosocial outcome evaluations. Evaluation & the Health Professions 1986;9:421‐38. [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, et al. Consensus statement. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M. Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 1995
- Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ 1995;311:551‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grissom 1996
- Grissom RJ. The magical number .7 +/‐ .2: meta‐meta‐analysis of the probability of superior outcome in comparisons involving therapy, placebo, and control. Journal of Consulting and Clinical Psychology 1996;64:973‐82. [DOI] [PubMed] [Google Scholar]
Gøtzsche 1990
- Gøtzsche PC. Sensitivity of effect variables in rheumatoid arthritis: a meta‐analysis of 130 placebo controlled NSAID trials. Journal of Clinical Epidemiology 1990;43:1313‐8. [DOI] [PubMed] [Google Scholar]
Gøtzsche 1994
- Gøtzsche PC. Is there logic in the placebo?. Lancet 1994;344:925‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 1994
- Ho VMS. The placebo effect: can we use it better?. BMJ 1994;309:69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 1996
- Hróbjartsson A. The uncontrollable placebo effect. European Journal of Clinical Pharmacology 1996;55:345‐8. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2002b
- Hróbjartsson A. What are the main methodological problems in the estimation of placebo effects?. Journal of Clinical Epidemiology 2002;55:430‐5. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2003
- Hróbjartsson A, Norup M. The use of placebo interventions in medical practice ‐ a national questionnaire survey of Danish clinicians. Evaluation and the Health Professions 2003;26:153‐65. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2006
- Hróbjartsson A, Gøtzsche PC. Unsubstantiated claims of large effects of placebo on pain: serious errors in meta‐analysis of placebo analgesia mechanism studies. Journal of Clinical Epidemiology 2006;59:336‐8. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2007
- Hróbjartsson A, Gøtzsche PC. Powerful spin in the conclusion of Wampold et al.'s re‐analysis of placebo versus no‐treatment trials despite similar results as in original review. Journal of Clinical Psychology 2007;63:373‐7. [DOI] [PubMed] [Google Scholar]
Kamper 2008
- Kamper SJ, Machado LA, Herbert RD, Maher CG, McAuley JH. Trial methodology and patient characteristics did not influence the size of placebo effects on pain. Journal of Clinical Epidemiology 2008;61(3):256‐60. [DOI] [PubMed] [Google Scholar]
Kaptchuk 2006
- Kaptchuk TJ, Stason WB, Davis RB, Legedza AR, Schnyer RN, Kerr CE, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ 2006;18:391‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kienle 1997
- Kienle GS, Kiene H. The powerful placebo effect: fact or fiction?. Journal of Clinical Epidemiology 1997;50:1311‐8. [DOI] [PubMed] [Google Scholar]
Kirsch 1998
- Kirsch I, Sappirstein G. Listening to prozac but hearing placebo: a meta‐analysis of antidepressant medication. Prevention and Treatment 1998;1:1‐17. [Google Scholar]
Lasagna 1986
- Lasagna L. The placebo effect. The Journal of Allergy and Clinical Immunology 1986;78 (1pt 2):161‐5. [DOI] [PubMed] [Google Scholar]
Linde 1997
- Linde K, Clausius N, Ramirez G, Melchart D, et al. Are the clinical effects of homoeopathy placebo effects? A meta‐analysis of placebo‐controlled trials. Lancet 1997;350:834‐43. [DOI] [PubMed] [Google Scholar]
Madsen 2009
- Madsen MV, Gøtzsche PC, Hróbjartsson A. Acupuncture treatment for pain. Systematic review of randomized clinical trials with acupuncture, placebo acupuncture and no‐acupuncture groups. BMJ 2009;338:a3115. [DOI: 10.1136/bmj.a3115] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meissner 2007
- Meissner K, Distel H, Mitzdorf. Evidence for placebo effects on physical but not on biochemical outcome parameters: a review of clinical trials. BMC Medicine 2007;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rawlinson 1985
- Rawlinson MC. Truth‐telling and paternalism in the clinic: philosophical reflections on the use of placebos in medical practice. In: White L, Tursky B, Schwartz GE editor(s). Placebo. Theory, Research, Mechanisms. New York: Guilford Press, 1985:403‐18. [Google Scholar]
Sauro 2005
- Sauro MD, Greenberg RP. Endogenous opiates and the placebo effect. A meta‐analytical review. Journal of Psychosomatic Research 2005;58:115‐20. [DOI] [PubMed] [Google Scholar]
Shapiro 1982
- Shapiro DA, Shapiro D. Meta‐analysis of comparative therapy outcome studies: a replication and refinement. Psychological Bulletin 1982;92:581‐604. [PubMed] [Google Scholar]
Smith 1980
- Smith ML, Glass GV, Miller TI. The Benefits of Psychotherapy. Baltimore: John Hopkins University Press, 1980. [Google Scholar]
Tilburt 2008
- Tilburt JC, Emanuel EJ, Kaptchuk TJ, Curlin FA, Miller FG. Prescribing 'placebo treatments: results of national survey of US internists and rheumatologists. BMJ 2008;337:a1938. [DOI: 10.1136/bmj.a1938] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vase 2002
- Vase L, Riley III JL, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain 2002;99:443‐52. [DOI] [PubMed] [Google Scholar]
Wampold 2005
- Wampold BE, Minami T, Tierney SC, Baskin TW, Bhati KS. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. Journal of Clinical Psychology 2005;61:835‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hróbjartsson 2001
- Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. The New England Journal of Medicine 2001;344(21):1594‐1602. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2002a
- Hróbjartsson A, Gøtzsche PC. What is the effect of placebo intervention? [Hvad er effekten af placebobehandling?]. Ugeskrift for Læger 2002;164:329‐33. [PubMed] [Google Scholar]
Hróbjartsson 2003a
- Hrobjartsson A, Gøtzsche PC. Placebo treatment versus no treatment. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003974] [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2004a
- Hróbjartsson A, Gøtzsche PC. Placebo treatment for all clinical conditions. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003974.pub2] [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2004b
- Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomised trials comparing placebo with no treatment. Journal of Internal Medicine 2004;256:91‐100. [DOI] [PubMed] [Google Scholar]


