Abstract
Background
Several studies have demonstrated that the use of pancreatic duct stents following pancreaticoduodenectomy is associated with a lower risk of pancreatic fistula. However, to date there is a lack of accord in the literature on whether the use of stents is beneficial and, if so, whether internal or external stenting, with or without replacement, is preferable. This is an update of a systematic review.
Objectives
To determine the efficacy of pancreatic stents in preventing pancreatic fistula after pancreaticoduodenectomy.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science, and four major Chinese biomedical databases up to November 2015. We also searched several major trials registers.
Selection criteria
Randomized controlled trials (RCTs) comparing the use of stents (either internal or external) versus no stents, and comparing internal stents versus external stents, replacement versus no replacement following pancreaticoduodenectomy.
Data collection and analysis
Two review authors independently extracted the data. The outcomes studied were incidence of pancreatic fistula, need for reoperation, length of hospital stay, overall complications, and in‐hospital mortality. We showed the results as risk ratio (RR) or mean difference (MD), with 95% confidence interval (CI). We assessed the quality of evidence using GRADE (http://www.gradeworkinggroup.org/).
Main results
We included eight studies (1018 participants). The average age of the participants ranged from 56 to 68 years. Most of the studies were conducted in single centers in Japan (four studies), China (two studies), France (one study), and the USA (one study). The risk of bias was low or unclear for most domains across the studies.
Stents versus no stents
The effect of stents on reducing pancreatic fistula in people undergoing pancreaticoduodenectomy was uncertain due to the low quality of the evidence (RR 0.67, 95% CI 0.39 to 1.14; 605 participants; 4 studies). The risk of in‐hospital mortality was 3% in people who did receive stents compared with 2% (95% CI 1% to 6%) in people who had stents (RR 0.73, 0.28 to 1.94; 605 participants; 4 studies; moderate‐quality evidence). The effect of stents on reoperation was uncertain due to wide confidence intervals (RR 0.67, 0.36 to 1.22; 512 participants; 3 studies; moderate‐quality evidence). We found moderate‐quality evidence that using stents reduces total hospital stay by just under four days (mean difference (MD) ‐3.68, 95% CI ‐6.52 to ‐0.84; 605 participants; 4 studies). The risk of delayed gastric emptying, wound infection, and intra‐abdominal abscess was uncertain (gastric emptying: RR 0.75, 95% CI 0.24 to 2.35; moderate‐quality evidence) (wound infection: RR 0.73, 95% CI 0.40 to 1.32; moderate‐quality evidence) (abscess: RR 1.38, 0.49 to 3.85; low‐quality evidence). Subgroup analysis by type of stent provided limited evidence that external stents lead to lower risk of fistula compared with internal stents.
External versus internal stents
The effect of external stents on the risk of pancreatic fistula, reoperation, delayed gastric emptying, and intra‐abdominal abscess compared with internal stents was uncertain due to low‐quality evidence (fistula: RR 1.44, 0.94 to 2.21; 362 participants; 3 studies) (reoperation: RR 2.02, 95% CI 0.38 to 10.79; 319 participants; 3 studies) (gastric emptying: RR 1.65, 0.66 to 4.09; 362 participants; 3 studies) (abscess: RR 1.91, 95% CI 0.80 to 4.58; 362 participants; 3 studies). The rate of in‐hospital mortality was lower in studies comparing internal and external stents than in those comparing stents with no stents. One death occurred in the external‐stent group (RR 0.33, 0.01 to 7.99; low‐quality evidence). There were no cases of pancreatitis in participants who had internal stents compared with three in those who had external stents (RR 0.15, 0.01 to 2.73; low‐quality evidence). The difference between internal and external stents on total hospital stay was uncertain due to the wide confidence intervals around the average effect of 1.7 days fewer with internal stents (9.18 days fewer to 5.84 days longer; 262 participants; 2 studies; low‐quality evidence). The analysis of wound infection could not exclude a protective effect with either approach (RR 1.41, 0.44 to 4.48; 319 participants; 2 studies; moderate‐quality evidence).
Operative replacement of pancreatic juice versus not replacing pancreatic juice
There was insufficient evidence available from a small trial to ascertain the effect of replacing pancreatic juice.
Authors' conclusions
This systematic review has identified limited evidence on the effects of stents. We have not been able to identify convincing direct evidence of superiority of external over internal stents. We found a limited number of RCTs with small sample sizes. Further RCTs on the use of stents after pancreaticoduodenectomy are warranted.
Keywords: Humans, Hospital Mortality, Pancreatic Fistula, Pancreatic Fistula/prevention & control, Pancreaticoduodenectomy, Pancreaticoduodenectomy/adverse effects, Randomized Controlled Trials as Topic, Reoperation, Stents, Stents/classification, Time Factors, Treatment Outcome
Plain language summary
Stents for preventing pancreatic fistula after pancreaticoduodenectomy
Review question
Are pancreatic stents useful in preventing pancreatic fistula after pancreaticoduodenectomy?
Background
There is no accord in studies on whether the use of stents is beneficial and, if so, whether the use of internal or external stents, with or without replacement is preferable.
Study characteristics
The use of stents following pancreaticoduodenectomy was studied in 1018 participants from eight randomized controlled trials (RCTs).
Key results
We found no evidence that the use of stents leads to lower risk of fistula when compared with no stents. We also found no evidence of a difference between the use of internal and external stents. There was not enough evidence to determine the effects of replacement of pancreatic juice versus no replacement of pancreatic juice. Further RCTs on the use of stents after pancreaticoduodenectomy are needed.
Quality of the evidence
All eight included studies were reported as RCTs. The quality of the findings ranged from moderate to low across the different outcomes. The main limiting factor, which was the reason for a decrease in quality in some outcomes, was only one study included in internal stents versus no stents group. It is important to acknowledge the large potential impact if the average effect of one study differs in size or direction.
Summary of findings
Summary of findings for the main comparison. Stents versus no stents for the prevention of pancreatic fistula following pancreaticoduodenectomy.
| Stents versus no stents for the prevention of pancreatic fistula following pancreaticoduodenectomy | |||||
| Patient or population: people undergoing pancreaticoduodenectomy Settings: in hospital Intervention: stents versus no stents | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| No stents | Stents | ||||
| Pancreatic fistulas | 28 per 100 | 19 per 100 (11 to 32) | RR 0.67 (0.39 to 1.14) | 605 (4 studies) | ⊕⊕ОО LOW1,2 |
| External stents | |||||
| 32 per 100 | 18 per 100 (12 to 25) | RR 0.55 (0.38 to 0.79) | 371 (3 studies) | ⊕⊕⊕О MODERATE1 |
|
| Internal stents | |||||
| 22 per 100 | 27 per 100 (17 to 42) | RR 1.23 (0.78 to 1.94) | 234 (1 study) | ⊕⊕⊕О MODERATE1 |
|
| In‐hospital mortality | 3 per 100 | 2 per 100 (1 to 6) | RR 0.73 (0.28 to 1.94) | 605 (4 studies) | ⊕⊕⊕О MODERATE1 |
| Reoperation | 10 per 100 | 6 per 100 (3 to 12) | RR 0.67 (0.36 to 1.22) | 512 (3 studies) | ⊕⊕⊕О MODERATE1 |
| Total hospital stay | The mean total hospital stay in the intervention groups was 3.68 days lower (6.52 to 0.84 lower) | ‐ | 605 (4 studies) | ⊕⊕⊕О MODERATE1 |
|
| Delayed gastric emptying | 16 per 100 | 12 per 100 (4 to 38) | RR 0.75 (0.24 to 2.35) | 371 (3 studies) | ⊕⊕⊕О MODERATE1 |
| Intra‐abdominal abscess | 5 per 100 | 7 per 100 (2 to 19) | RR 1.38 (0.49 to 3.85) | 234 (1 study) | ⊕⊕ОО LOW3 |
| Wound infection | 15 per 100 | 11 per 100 (6 to 20) | RR 0.73 (0.40 to 1.32) | 605 (4 studies) | ⊕⊕⊕О MODERATE1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded one level due to imprecision. 2Downgraded one level due to inconsistency. 3Downgraded two levels due to imprecision (wide confidence intervals and low event rate).
Summary of findings 2. Internal stents versus external stents for the prevention of pancreatic fistula following pancreaticoduodenectomy.
| Internal stents versus external stents for the prevention of pancreatic fistula following pancreaticoduodenectomy | ||||||
| Patient or population: people undergoing pancreaticoduodenectomy Settings: in hospital Intervention: external stents versus internal stents | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| External stents | Internal stents | |||||
| Pancreatic fistulas | 18 per 100 | 26 per 100 (17 to 40) | RR 1.44 (0.94 to 2.21) | 362 (3 studies) | ⊕⊕ОО LOW1 |
Wide confidence intervals, which include very little difference and substantial increase in risk of fistula |
| In‐hospital mortality | 5 per 1000 | 2 per 1000 (0 to 44) | RR 0.33 (0.01 to 7.99) | 362 (3 studies) | ⊕⊕ОО LOW2 |
Only 1 event (in the external‐stent group) across all 3 studies |
| Reoperation | 1 per 100 | 3 per 100 (0 to 13) | RR 2.02 (0.38 to 10.79) | 319 (2 studies) | ⊕⊕ОО LOW2 |
‐ |
| Total hospital stay | The mean total hospital stay in the intervention groups was 1.67 days lower (9.18 lower to 5.84 higher) | 262 (2 studies) | ⊕⊕ОО LOW1,3 |
‐ | ||
| Delayed gastric emptying | 9 per 100 | 15 per 100 (6 to 38) | RR 1.65 (0.66 to 4.09) | 362 (3 studies) | ⊕⊕ОО LOW1,3 |
‐ |
| Pancreatitis | 23 per 1000 | 3 per 1000 (0 to 62) | RR 0.15 (0.01 to 2.73) | 262 (2 studies) | ⊕⊕ОО LOW1,3 |
‐ |
| Intra‐abdominal abscess | 7 per 100 | 13 per 100 (5 to 30) | RR 1.91 (0.8 to 4.58) | 362 (3 studies) | ⊕⊕ОО LOW1,3 |
‐ |
| Wound infection | 6 per 100 | 8 per 100 (2 to 25) | RR 1.41 (0.44 to 4.48) | 319 (2 studies) | ⊕⊕⊕О MODERATE3 |
‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to risk of bias. 2Downgraded two levels due to very serious imprecision. 3Downgraded one level due to imprecision.
Summary of findings 3. No replacement versus replacement for the prevention of pancreatic fistula following pancreaticoduodenectomy.
| No replacement versus replacement for the prevention of pancreatic fistula following pancreaticoduodenectomy | ||||||
| Patient or population: people undergoing pancreaticoduodenectomy Settings: in hospital Intervention: no replacement versus replacement | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Replacement | No replacement | |||||
| Pancreatic fistulas | 36 per 100 | 58 per 100 (31 to 100) | RR 1.6 (0.84 to 3.07) | 46 (1 study) | ⊕⊕ОО LOW1 |
‐ |
| In‐hospital mortality | See comment | See comment | Not estimable | 46 (1 study) | See comment | No events/data |
| Total hospital stay | The mean total hospital stay in the intervention groups was 16.3 days higher (13.24 to 19.36 higher) | ‐ | 46 (1 study) | ⊕⊕ОО LOW1 |
‐ | |
| Delayed gastric emptying | 32 per 100 | 33 per 100 (15 to 77) | RR 1.05 (0.46 to 2.41) | 46 (1 study) | ⊕⊕ОО LOW1 |
‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded two levels due to very serious imprecision. Single study with a small sample size and very wide confidence intervals.
Background
Description of the condition
Pancreatic fistula (PF) is one of the most common postoperative complications after pancreaticoduodenectomy (PD), the operation of choice for resectable tumors of the pancreatic head as well as the distal common bile duct and ampulla of Vater (Schmidt 2004; Bassi 2005; Diener 2008; Iqbal 2008; Diener 2011). PF was first described by Smith in 1953, and the concept was further developed in 1976 by Cameron et al that PF can result from pancreatic disease, trauma, or surgery. PF is defined as an abnormal communication between the pancreas and other organs due to leakage of pancreatic secretions from damaged pancreatic ducts. An external PF, also known as a pancreaticocutaneous fistula, occurs when the pancreatic duct communicates with the abdominal wall, whereas an internal PF communicates with the peritoneal cavity, the mediastinum, or other spaces. The pancreatic juice can lead to ascites, pleural effusions, and enzymatic mediastinitis. This can be followed by the loss of bicarbonate‐rich pancreatic fluid via the pancreatic fistula that can result in a hyperchloremic or normal anion gap metabolic acidosis. If the volume of pancreatic juice lost from the body is large, acidosis can occur, which is a serious problem (Smith 1953; Cameron 1976). The International Study Group on Pancreatic Fistula (ISGPF) defines PF as "output via an operatively placed drain or a subsequently placed percutaneous drain of any measurable volume of drain fluid on or after postoperative day three, with an amylase content greater than three times the upper normal serum value" (Bassi 2005). PF occurs in up to 56% of patients undergoing pancreaticoduodenectomy, and is regarded as the major factor leading to morbidity and mortality in people undergoing pancreaticoduodenectomy (Cullen 1994; Neoptolemos 1997; Yeo 1997; Boettger 1999; Gouma 2000; Satoi 2006; Kollmar 2007; Pratt 2008; Satoi 2008; Brown 2014). Various techniques have been suggested to prevent PF (reconstruction with pancreaticogastrostomy, modification of the pancreaticojejunal anastomosis technique, duct‐to‐mucosa anastomosis, mucosa‐to‐mucosa anastomosis, use of adhesive sealants, use of somatostatin and its analogues), but the optimal strategy is still unknown (Peng 2003; Wente 2007; Zeng 2008; Fingerhut 2009; Li 2009; You 2009). There have also been reports of laparoscopic PD, total laparoscopic PD, and robot‐assisted PD techniques, which were associated with a significant learning curve effect, expedited postoperative recovery, feasibility, and long‐term survival, but without an increase in the incidence of pancreatic fistula (Chen 2015; Dokmak 2015; Paniccia 2015; Piedimonte 2015; Wang 2015).
Description of the intervention
One of the possible preventive strategies for PF is placement of pancreatic duct stents, either internally or externally, as well as external drainage of pancreatic juice, with or without replacement (Roder 1999; Imaizumi 2006; Winter 2006; Poon 2007; Kamoda 2008; Berger 2009; Kimura 2009; Pessaux 2011; Motoi 2012; Wang 2014; Yokoyama 2014). The use of an internal stent implies placement of a plastic catheter across the pancreaticojejunostomy anastomosis for drainage into the jejunum (Roder 1999). The use of an external stent implies placement of a plastic catheter into the main pancreatic duct for external drainage of pancreatic juice (Poon 2007; Pessaux 2011; Yokoyama 2014) (Figure 1; Figure 2).
1.
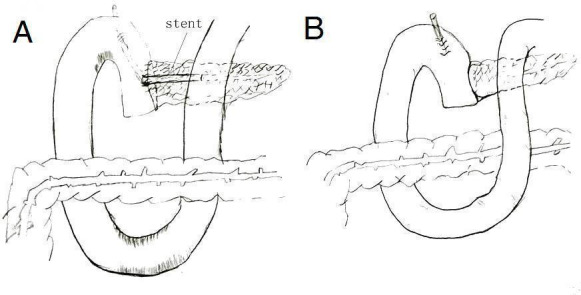
Stent versus no stent.
2.

Internal drainage versus external drainage (This picture redrawn with the permission of Ronnie Poon).
How the intervention might work
Given that PF is mainly caused by anastomotic leakage from the pancreaticojejunostomy anastomosis, the placement of an internal stent may be beneficial as it may direct pancreatic juice into the jejunum and thus protect the pancreaticojejunostomy anastomosis. The placement of external drainage may be beneficial as it may protect the pancreaticojejunostomy anastomosis by diverting pancreatic juice away from the anastomosis. The enteral replacement of externally drained pancreatic juice may reduce the incidence of postoperative PF by external pancreatic juice drainage from a stent applied by pancreaticojejunostomy (Roder 1999; Imaizumi 2006; Howard 2007; Poon 2007; Kimura 2009; Yokoyama 2014).
Why it is important to do this review
PF is one of the most common complications after pancreaticoduodenectomy. The quest for a preventive strategy is therefore of paramount importance. Several studies have demonstrated that the use of pancreatic duct stents is associated with a lower risk of PF (Winter 2006; Kamoda 2008; Berger 2009). The four published meta‐analyses have yielded conflicting results, and some are now out of date as several new RCTs have been published on this topic since 2011 (Motoi 2012; Wang 2014; Yokoyama 2014). Those meta‐analyses also had several methodological limitations. First, no subgroup analysis based on study design was conducted. Second, the only comparisons studied were external stent versus no stent or external stent versus internal stent. Third, some RCTs were not considered (Zhou 2011; Xiong 2012; Hong 2013; Ke 2015). The Cochrane review therefore needed updating. To date, there is no accord in the literature on whether the use of stents is beneficial and, if so, whether the use of internal or external stents, with or without replacement is preferable.
Objectives
To determine the efficacy of pancreatic stents in preventing pancreatic fistula after pancreaticoduodenectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs), irrespective of blinding, publication status, language, or publication date, and excluded all observational studies.
Types of participants
Inclusion criteria
People (regardless of age, sex, or ethnic group) who underwent pancreaticoduodenectomy for benign or malignant pathologies of the pancreas or periampullary region. We included studies employing the definition of pancreatic fistula according to the International Study Group for Pancreatic Fistula (ISGPF) and the Johns Hopkins definition (Bassi 2005). The operation of pancreaticoduodenectomy included both pylorus‐preserving pancreaticoduodenectomy (PPPD) and the classical Whipple resection.
Exclusion criteria
People who underwent emergency pancreaticoduodenectomy for trauma. People who were recruited before surgery but were found to have unresectable disease after laparoscopy or laparotomy.
Types of interventions
RCTs comparing the use of stents versus no stents after pancreaticoduodenectomy.
RCTs comparing the use of internal stents versus external stents after pancreaticoduodenectomy.
RCTs comparing the use of replacement versus no replacement of externally drained pancreatic juice after pancreaticoduodenectomy.
Any type of stents was allowed, depending on the preference of surgeons in the primary studies.
Types of outcome measures
An RCT had to report on our primary outcome and at least one of our secondary outcomes to be included in the review.
Primary outcomes
Incidence of pancreatic fistula
Secondary outcomes
In‐hospital mortality
Need for reoperation
Length of hospital stay
Overall complications
Search methods for identification of studies
Trials Search Co‐ordinator Yuhong Yuan developed a new search strategy and updated the search results. We applied no language restrictions during the search. We sought all relevant published and unpublished RCTs, irrespective of blinding, publication status, or publication date.
Electronic searches
We conducted a computerized literature search in:
CENTRAL (Cochrane Central Register of Controlled Trials) (Appendix 1);
MEDLINE (PubMed) (Appendix 2);
EMBASE (Appendix 3);
Web of Science (Appendix 4);
CBM (China Biological Medicine Database) (Appendix 5);
CNKI (Chinese National Knowledge Infrastructure Database) (Appendix 6);
Wangfang (Database of Chinese Ministry of Science & Technology) (Appendix 7);
VIP (Database of Chinese Science and Technology Periodicals) (Appendix 8).
We searched the above databases up to November 2015. We limited the search to studies in humans.
Searching other resources
We also searched the following trials registers and the references of included and excluded studies:
WHO ICTRP (World Health Organization International Clinical Trials Registry Platform) (http://www.who.int/trialsearch/);
ANZCTR (Australian New Zealand Clinical Trials Registry) (http://www.anzctr.org.au/);
ISRCTN (http://www.isrctn.org/) OR (http://www.controlled‐trials.com/);
ClinicalTrials.gov (http://www.clinicaltrials.gov/);
Chinese Clinical Trial Register (http://www.chictr.org.cn/);
Trials Central (www.trialscentral.org/);
Trials (http://www.trialsjournal.com/).
Data collection and analysis
The protocol was based on the guidelines of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group. We performed and updated the systematic review according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We also used the GRADE Working Group grades of evidence in this Cochrane review (www.gradeworkinggroup.org). We used the GRADE profiler to import data from Review Manager (RevMan) 5.3 and to create 'Summary of findings' tables (RevMan 2014). Downgrading of the quality of the evidence was due to risk of bias, inconsistency, indirectness, imprecision, and publication bias. We assessed the study limitations as none for low bias, one level for serious bias, or two levels for very serious bias.
For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Selection of studies
Two review authors (ZY Dong, Z Wang) independently screened titles and abstracts of all citations. We retrieved the full texts of eligible articles for assessment. Two review authors independently applied the inclusion criteria to all of these potentially eligible studies. The review authors had planned to resolve discrepancies in trial selection through discussion, asking a third review author (J Xu) for an opinion in order to reach consensus, but in the event the views of the two review authors were consistent.
A summary of the results of the search and included studies is shown in Figure 3.
3.

Study flow diagram.
Data extraction and management
Two review authors (ZY Dong, Z Wang) independently extracted data regarding participants' characteristics, study methods, interventions, and outcomes using a prespecified data extraction form. We resolved any disagreements by consensus. We obtained the major data of included studies, so there was no need to contact the authors of the original articles for details regarding methods and missing data as we had planned in this systematic review update, the first systematic review, and protocol.
Assessment of risk of bias in included studies
Two review authors (ZY Dong, Z Wang) independently assessed the methodological quality of the considered studies. We used the quality checklist recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The quality checklist for assessing the risk of bias covered six headings (sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting bias, other potential sources of bias), where a response of 'Yes' indicated a low risk of bias, 'Unclear' indicated an uncertain risk of bias, and 'No' indicated a high risk of bias (Higgins 2011). We resolved any discrepancies by discussion among all review authors. We recorded the final results in RevMan 5.3 (RevMan 2014), and generated two figures to illustrate the proportion of studies with each of the judgements (Figure 4; Figure 5).
4.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Adequate sequence generation
Low risk. Adequate sequence generation is reported using one of following approaches: random number tables or generated random numbers from statisticians, computer‐generated random numbers, central randomization (central voice systems or voice response systems). Coin tossing or shuffling used to generate the allocation sequence before the trials commenced was also considered eligible. We considered this to have a low risk of selection bias.
Unclear risk. The report does not specify one of the adequate methods outlined above but only mentions "random". We considered this to have a moderate risk of selection bias.
High risk. Other methods of allocation, such as quasi‐randomization, with a high risk of selection bias. We excluded these trials.
Allocation concealment
Low risk. Clearly indicated that the staff involved in recruitment of participants and participants themselves were not aware of the allocation.
Unclear risk. We considered concealed trials in which the authors did not report the approach taken to conceal allocation to have a moderate risk of selection bias.
High risk. We considered trials where allocation to a treatment option did not fall into one of the above categories or where there was no allocation concealment to have a high risk of selection bias.
Blinding
Low risk. We considered blinding to be done by a good method, double blinding was assumed when participants and results assessor were masked. We considered trials as having a low risk of both performance and detection bias.
Unclear risk. We considered trials where there was single blinding for the results assessor as having a moderate risk of both performance and detection bias. If single blinding was performed for participants but not the results assessor, we considered the trial to have a moderate risk of both performance and detection bias.
High risk. Open‐label study, non‐blinding was considered to result in a high risk of both performance and detection bias.
Incomplete outcome data
Low risk. According to the sample size, we considered trials where the number of lost participants was less than 5% was to have a low risk of incomplete data bias.
Unclear risk. According to the sample size, we considered trials where the number of lost participants was between 5% and 10% as having a moderate risk of incomplete data bias.
High risk. According to the sample size, we considered trials where the number of lost participants was greater than 15% as having a high risk of incomplete data bias.
Selective reporting bias
Where only favorable outcomes were reported and not any unfavorable ones.
Other potential sources of bias
Where a non‐inferiority trial was used, conflicts of interest.
Measures of treatment effect
We considered the data from each study according to the intention‐to‐treat principle. For dichotomous variables, we determined risk ratio with 95% confidence interval (CI) to interpret the results. We estimated continuous variables as the difference in means along with the corresponding 95% CI. For the same outcomes measured with different scales in different studies we used standardized mean differences with 95% CI for analysis. Two review authors entered all data into RevMan 5.3 (RevMan 2014).
Unit of analysis issues
We found eight RCTs suitable for inclusion in this review. We anticipated no unit of analysis issues.
Dealing with missing data
All necessary data were available from the included trials. We contacted the authors of the primary studies to request missing data. When the authors of primary studies did not reply, we used the available data for analysis.
Assessment of heterogeneity
We assessed two types of heterogeneity: statistical, and clinical and methodological heterogeneity.
For statistical heterogeneity, we evaluated the I² statistic and Chi² test for each outcome analyzed. We considered a P value less than 0.1 and an I² value more than 75% as high heterogeneity, and an I² value less than 25% as low heterogeneity. When we found heterogeneity we searched for the cause.
We assessed through clinical knowledge whether heterogeneity was clinical or methodological. We assessed clinical heterogeneity by comparing characteristics of participants, study designs, and interventions. We then divided the studies into five different groups according to the different interventions.
Assessment of reporting biases
We did not use funnel plots as the number of included studies was less than 10.
Data synthesis
We performed the data analysis using the meta‐analysis software RevMan 5.3 (RevMan 2014). We used a random‐effects model model in all analyses. The extracted data were combined by calculating a pooled estimate of the risk ratio and 95% CI for dichotomous data using a random‐effects model. Where continuous data outcomes were measured, the weighted mean difference (and 95% CI) were calculated, using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We intended to perform subgroup analysis as follows, if possible.
Studies with low risk of bias versus studies with high risk of bias.
Adult participants versus older participants.
Participants with mild disease versus those with severe disease.
Plastic stents versus metal stents.
Different drainage times.
Different duration of disease or hospital stay.
We considered these six items when analysing the results. We performed subgroup analysis on stents versus no stents (internal stents versus no stents; external stents versus no stents; replacement versus no replacement of externally drained pancreatic juice).
Sensitivity analysis
In the protocol we stated that we might perform sensitivity analyses to explore the stability and reliability of the evidence in order to enhance the strength of the evidence. However, as there were only eight included studies across the comparison groups, a sensitivity analysis was not undertaken.
Summary of findings tables
We employed the GRADE approach to interpret findings (Langendam 2013), and the GRADE profiler allowed us to import data from RevMan 5.3 to create 'Summary of findings' tables (GRADE 2008; RevMan 2014). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered.
We assessed the quality of the evidence for the following key outcomes:
Pancreatic fistula
In‐hospital mortality
Reoperation
Total hospital stay
Pancreatitis
Delayed gastric emptying
Intra‐abdominal abscess
Wound infection
Results
Description of studies
Results of the search
Figure 3 provides the details of the search process. We identified a total of 69 studies through electronic searches of CENTRAL in the Cochrane Library (n = 163), MEDLINE (n = 406), EMBASE (n = 1543), Web of Science search strategy (n = 961), and the four Chinese databases (CBM, VIP, CNKI, Wangfang) (n = 559), in addition, other sources and five relevant ongoing trials were found (n= 116).
Included studies
Eight RCTs including a total of 1018 participants undergoing pancreaticoduodenectomy met all of the inclusion criteria (Winter 2006; Poon 2007; Kamoda 2008; Tani 2010; Pessaux 2011; Motoi 2012; Wang 2014; Yokoyama 2014). The characteristics of the included trials are presented in Table 4 and Characteristics of included studies.
1. Study characteristics for included studies.
| Study ID | Country (number of centers) | Intervention group | Control group | Total number of participants | Age | Male: female | |||
| Intervention group | Control group | Intervention group | Control group | Intervention group | Control group | ||||
| Kamoda 2008 | Japan (1) | 22 | 21 | 22 | 22 | NS | NS | 8:14 | 7:14 |
| Motoi 2012 | Japan (1) | 47 | 46 | 47 | 46 | 60.0 (33 to 79) | 65.5 (32 to 80) |
26:21 | 29:17 |
| Pessaux 2011 | France (8) | 77 | 81 | 77 | 81 | 60.8 ± 11.8 | 60.6 ± 11.8 | 39:39 | 47:34 |
| Poon 2007 | Hong Kong, China (1) |
60 | 60 | 60 | 60 | 61 ± 12 | 62 ± 13 | 31:29 | 41:19 |
| Tani 2010 | Japan (1) | 49 | 50 | 50 | 50 | 70 (44 to 87) |
68 (35 to 84) |
28:22 | 27:23 |
| Wang 2014 | China (1) | 110 | 109 | 110 | 109 | 52 (53.6) | 56 (51.4) |
59:51 | 54:55 |
| Winter 2006 | USA (1) | 115 | 119 | 116 | 122 | 63 (27 to 89) | 67 (33 to 88) | 67:48 | 65:54 |
| Yokoyama 2014 | Japan (1) | 22 | 24 | 22 | 24 | 63.1 (44 to 79) | 63.3 (43 to 85) | 13:9 | 15:9 |
NS: Not stated
We also identified five ongoing studies (NCT00501176; NCT00855985; NCT01023594; NCT01634971; ChiCTR‐TRC‐14004830). NCT00501176 is still recruiting participants and is ongoing. NCT00855985 and NCT01023594 are still ongoing but are not recruiting participants. See Characteristics of ongoing studies.
Excluded studies
We excluded 17 references for the reasons listed in the Characteristics of excluded studies table.
Risk of bias in included studies
One of the limitations of this systematic review is the limited number of RCTs available. Only one study compared internal pancreatic duct stents with no stents following pancreaticoduodenectomy; one study compared external pancreatic duct stents with no stents; one study compared replacement of externally drained pancreatic juice with no replacement; and two studies compared internal pancreatic duct stents with external stents. Nevertheless, the trials included in this systematic review were all randomized, and two of eight studies were of low methodological quality. The methodological quality of the trials is shown in Figure 4 and Figure 5. We have presented the quality of the evidence (GRADE) in Table 1, Table 2, and Table 3.
Allocation
The majority of studies reported the use of randomly generated number patterns or random number tables (Winter 2006; Poon 2007; Tani 2010; Motoi 2012; Wang 2014). One trial mentioned that the process was "randomised," but provided no further details (Kamoda 2008). We considered the allocation concealment in four trials that used sealed envelopes and Consolidated Standards of Reporting Trials (CONSORT) flow diagram to be of high quality (Poon 2007; Tani 2010; Motoi 2012; Wang 2014). We considered the allocation concealment in two trials that specified a "generated number pattern" and "study design partially followed the method of a randomized controlled study" to be adequate (Winter 2006; Yokoyama 2014).
Blinding
There was no mention of participant or assessor blinding in any of the included trials. We have thus assigned them all an unclear risk of bias, as this is difficult to perform in operation.
Incomplete outcome data
Participants were adequately matched in all the included trials, which were all free from baseline imbalance bias. All trials had adequate follow‐up. Four trials reported postrandomization withdrawal of participants (Winter 2006; Kamoda 2008; Motoi 2012; Yokoyama 2014). These participants were excluded from the analysis, but as the rate of loss to follow‐up was less than 10%, we considered these trials to have a low risk of incomplete outcome data bias.
Selective reporting
All the trials reported on the primary outcome and were considered to be at low risk of selective outcome reporting bias. Except for Wang 2014, all the trials reported on sample size calculations.
Other potential sources of bias
The source of funding was described in Wang 2014, but not in any other of the trials.
Effects of interventions
See: Table 1; Table 2; Table 3
Stents versus no stents
Four RCTs including a total of 605 participants who underwent pancreaticoduodenectomy were analyzed (Winter 2006; Poon 2007; Pessaux 2011; Motoi 2012). The risk of pancreatic fistula (PF) did not differ significantly between the groups (risk ratio (RR) 0.67, 95% CI 0.39 to 1.14; 605 participants; 4 studies) (Analysis 1.1). The use of stents did not result in a statistically significant change in the risk of in‐hospital mortality (RR 0.73, 95% CI 0.28 to 1.94; 605 participants; 4 studies) (Analysis 1.2). There was no statistically significant difference in need for reoperation between the two groups (RR 0.67, 95% CI 0.36 to 1.22; P = 0.19) (Analysis 1.3). The total hospital stay was shorter in the external‐stents group in comparison with the no‐stents group (mean difference (MD) ‐3.68, 95% CI ‐6.52 to ‐0.84; 371 participants; 3 studies) (Analysis 1.4). The use of stents did not result in a statistically significant difference in the risk of delayed gastric emptying (RR 0.75, 95% CI 0.24 to 2.35; 371 participants; 3 studies) (Analysis 1.5). The use of stents did not result in a statistically significant difference in the risk of intra‐abdominal abscess (RR 1.38, 95% CI 0.49 to 3.85; 234 participants; 1 study) (Analysis 1.6). The use of stents did not result in a statistically significant difference in the risk of wound infection (RR 0.73, 95% CI 0.40 to 1.32; 605 participants; 4 studies) (Analysis 1.7). The incidence of PF was: for Poon 2007, 7% (4/60) in the stented group and 20% (12/60) in the non‐stented group; for Winter 2006, 27% (31/115) in the stented group and 22% (26/119) in the non‐stented group; for Pessaux 2011, 26% (20/77) in the stented group and 49% (34/81) in the non‐stented group; for Motoi 2012, 15% (7/47) in the stented group and 30% (14/46) in the non‐stented group.
1.1. Analysis.
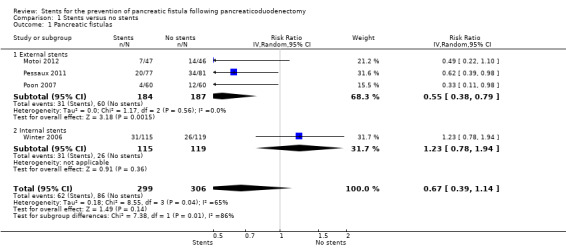
Comparison 1 Stents versus no stents, Outcome 1 Pancreatic fistulas.
1.2. Analysis.
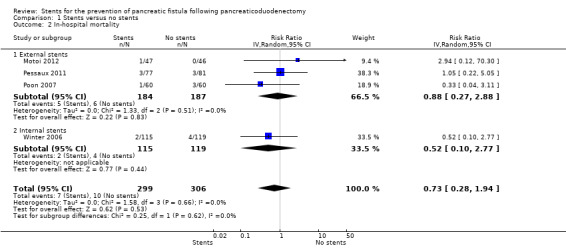
Comparison 1 Stents versus no stents, Outcome 2 In‐hospital mortality.
1.3. Analysis.

Comparison 1 Stents versus no stents, Outcome 3 Reoperation.
1.4. Analysis.
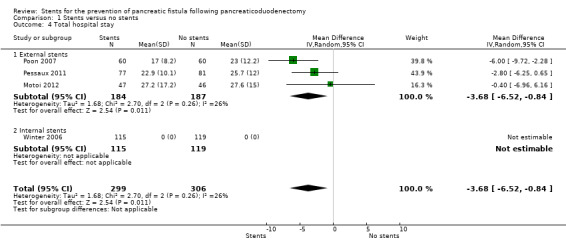
Comparison 1 Stents versus no stents, Outcome 4 Total hospital stay.
1.5. Analysis.

Comparison 1 Stents versus no stents, Outcome 5 Delayed gastric emptying.
1.6. Analysis.

Comparison 1 Stents versus no stents, Outcome 6 Intra‐abdominal abscess.
1.7. Analysis.

Comparison 1 Stents versus no stents, Outcome 7 Wound infection.
Subgroup analysis: external‐stents versus no‐stents group
Three RCTs including a total of 371 participants who underwent pancreaticoduodenectomy were analyzed (Poon 2007; Pessaux 2011; Motoi 2012). The incidence of PF was significantly lower in the external‐stents group compared to the no‐stents group (RR 0.55, 95% CI 0.38 to 0.79; P = 0.01) (Analysis 1.1). The use of external stents did not result in a statistically significant difference in in‐hospital mortality (RR 0.88, 95% CI 0.27 to 2.88; P = 0.83) (Analysis 1.2). Two RCTs reported on reoperation (Poon 2007; Pessaux 2011); there was no statistically significant difference in reoperation between the two groups (RR 0.76, 95% CI 0.36 to 1.60; P = 0.47) (Analysis 1.3). The total hospital stay was shorter in the external‐stents group than in the no‐stents group; there was a statistically significant difference between the two groups (MD ‐3.68, 95% CI ‐6.52 to ‐0.84; P = 0.01) (Analysis 1.4). The incidence of major complications was significantly lower in the external‐stents group compared to the no‐stents group for delayed gastric emptying (RR 0.75, 95% CI 0.24 to 2.35) and wound infection (RR 0.65, 95% CI 0.22 to 1.95) (Analysis 1.5; Analysis 1.7).
Subgroup analysis: internal‐stents versus no‐stents group
One RCT including a total of 234 participants who underwent pancreaticoduodenectomy was analyzed (Winter 2006). The incidence of PF did not differ significantly between the no‐stents group and the internal‐stents group (RR 1.23, 95% CI 0.78 to 1.94; P = 0.36) (Analysis 1.1). This difference remained statistically insignificant when we adopted the fixed‐effect model. The use of internal stents did not result in a statistically significant difference in in‐hospital mortality (RR 0.52, 95% CI 0.10 to 2.77; P = 0.44) (Analysis 1.2). There was no statistically significant difference for reoperation between the two groups (RR 0.52, 95% CI 0.18 to 1.47; P = 0.22) (Analysis 1.3). The total hospital stay was shorter in the no‐stents group (median of seven days) than in the internal‐stents group (median of eight days). The use of internal stents did not result in a statistically significant difference in major complications of intra‐abdominal abscess (RR 1.38, 95% CI 0.49 to 3.85) (Analysis 1.6) or wound infection (RR 0.71, 95% CI 0.39 to 1.29) (Analysis 1.7).
Internal stents versus external stents
Three RCTs including a total of 362 participants undergoing pancreaticoduodenectomy were analyzed (Kamoda 2008; Tani 2010; Wang 2014). The incidence of PF did not differ significantly between the groups (RR 1.44, 95% CI 0.94 to 2.21; P = 0.10) (Analysis 2.1). The use of internal stents did not result in a statistically significant in‐hospital mortality (RR 0.33, 95% CI 0.01 to 7.99; P = 0.50) (Analysis 2.2). Two RCTs reported on reoperation (Tani 2010; Wang 2014); there was no statistically significant difference in reoperation between the two groups (RR 2.02, 95% CI 0.38 to 10.79) (Analysis 2.3). Two RCTs reported total hospital stay (Kamoda 2008; Wang 2014); the total hospital stay was not statistically significantly different in the internal‐stents group (43.1 ± 19.9 days; 49 ± 45 days) compared with the external‐stents group (43.4 ± 9.9 days; 53 ± 48.2 days) (MD ‐1.67, 95% CI ‐9.18 to 5.84; P = 0.66) (Analysis 2.4). The use of internal stents did not result in a statistically significant difference in major complications of delayed gastric emptying (RR 1.65, 95% CI 0.66 to 4.09), events of pancreatitis (RR 0.15, 95% CI 0.01 to 2.73), intra‐abdominal abscess (RR 1.91, 95% CI 0.80 to 4.58), or wound infection (RR 1.41, 95% CI 0.44 to 4.48) (Analysis 2.5). The incidence of PF was: for Kamoda 2008, 36% (8/22) in the external‐stented group and 33% (7/21) in the internal‐stented group; for Tani 2010, 20% (10/50) in the external‐stented group and 26% (13/50) in the internal‐stented group; for Wang 2014, 27% (29/109) in the internal‐stented group and 14% (15/110) in the external‐stented group.
2.1. Analysis.
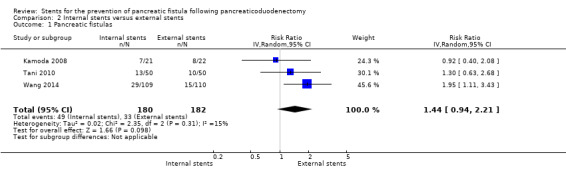
Comparison 2 Internal stents versus external stents, Outcome 1 Pancreatic fistulas.
2.2. Analysis.

Comparison 2 Internal stents versus external stents, Outcome 2 In‐hospital mortality.
2.3. Analysis.
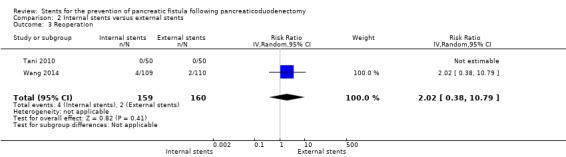
Comparison 2 Internal stents versus external stents, Outcome 3 Reoperation.
2.4. Analysis.

Comparison 2 Internal stents versus external stents, Outcome 4 Total hospital stay.
2.5. Analysis.

Comparison 2 Internal stents versus external stents, Outcome 5 Overall complications.
No replacement versus replacement of pancreatic juice
One RCT including a total of 46 participants undergoing panceaticoduodenectomy was analyzed (Yokoyama 2014). The incidence of PF did not differ significantly between the two groups (RR 1.60, 95% CI 0.84 to 3.07; P = 0.15) (Analysis 3.1). None of the participants in either group died. The use of replacement resulted in a statistically significant difference in total length of hospital stay (MD 16.30, 95% CI 13.24 to 19.36; P = 0.0001) (Analysis 3.3). The use of replacement did not result in delayed gastric emptying (RR 1.05, 95% CI 0.46 to 2.41) (Analysis 3.4). The incidence of PF was 58% (14/24) in the no‐replacement group and 36% (8/22) in the replacement group.
3.1. Analysis.

Comparison 3 No replacement versus replacement, Outcome 1 Pancreatic fistulas.
3.3. Analysis.
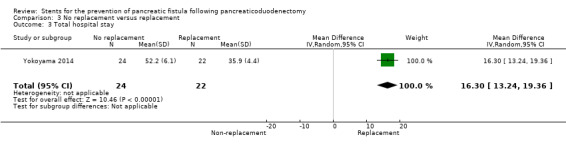
Comparison 3 No replacement versus replacement, Outcome 3 Total hospital stay.
3.4. Analysis.

Comparison 3 No replacement versus replacement, Outcome 4 Overall complications.
Ongoing trials
We found a total of five unpublished trials on the use of stents for the prevention of PF following pancreaticoduodenectomy, including one study that has been completed but not published yet. We emailed the author for possible data (see Characteristics of ongoing studies). We will include these new RCTs in future updates.
Discussion
Summary of main results
This is an updated systematic review incorporating a total of eight trials with 1018 participants undergoing pancreaticoduodenectomy with or without pancreatic duct stenting. We found no significant difference in terms of clinically meaningful outcomes between participants who had stents and those who did not have stents during pancreaticoduodenectomy. We found no statistically significant difference in terms of any of the studied outcomes between the use of internal and external stents (P > 0.05).
Overall completeness and applicability of evidence
The use of stents in people undergoing pancreaticojejunostomy (PJ) was first reported in 1986 (Manabe 1986), followed by several published reports that further advocated this technique (Hiraoka 1993; Hamanaka 1994; Roder 1999). Theoretically, the stent may provide some protection of the PJ anastomosis against activated pancreatic enzymes by directing the exocrine secretions into the jejunal lumen, and it may help to facilitate a precise placement of sutures through the pancreatic parenchyma or duct when performing the PJ anastomosis (Roder 1999; Winter 2006). Several factors were reported to influence the success of stenting in preventing a pancreatic fistula. These included the stent's material, size, and length; the timing of stent removal; the replacement of externally drained pancreatic juice; and the quality of the pancreatic remnant (Ohwada 2002; Winter 2006; Kamoda 2008; Tani 2010; Yokoyama 2014).
The data for the comparison internal versus external stent (Analysis 2.4) appears to indicate that the mean length of hospital stay in both arms of the two studies was twice as long as length of stay in the intervention arms of the three studies included in the external‐ versus no‐stent comparison (Analysis 1.4). However, this finding must be interpreted with caution, as the studies in the internal‐ versus external‐stent comparison, Kamoda 2008 and Wang 2014, had a more severe disease course than the studies in the external‐ versus no‐stent comparison (Poon 2007; Pessaux 2011; Motoi 2012).
Unfortunately, we were not able to assess the effect of the size of stent used in the meta‐analysis, as not all the included studies reported the size of the stents used. The typical description was that "the largest sized that could be easily passed into the pancreatic duct" was used (Winter 2006).
Quality of the evidence
A total of eight studies met our inclusion criteria. All eight included studies were reported as RCTs, but two trials just mentioned "randomized controlled study," and none reported on the use of concealed allocation. There was no mention of participant or assessor blinding in any of the included trials. Four trials used intention‐to‐treat analysis; the others used a per‐protocol analysis. Only one study did not describe whether informed consent was obtained. Five of the eight trials performed sample size calculations. Two of the eight trials were of low quality, while the other trails were of moderate quality according to the GRADE (GRADE 2008) and the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Table 1: Among the four studies of 605 participants, the quality of evidence varied between the outcomes from moderate to low. The main reason for downgrading the quality of the evidence was imprecision arising from wide confidence intervals around the effect estimates or low event rates. With only three studies included, it is important to acknowledge the large potential impact if the average effect of one study differs in size or direction. The individual outcomes we examined were downgraded one or two levels to reflect the high risk of bias due to imprecision or inconsistency. Since the imprecision of the results also lowers the quality of the evidence, we downgraded a further evidence level on that basis. Overall we believe the evidence is of low quality, which means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Table 2: Among the three studies of 362 participants, the quality of evidence was low in the pancreatic fistulas and in‐hospital mortality analyses. It was downgraded two levels due to risk of bias, wide confidence intervals, which include very little difference and substantial increase in risk of fistula, and only one event (in the external‐stent group) across all three studies. The quality of evidence was low in the reoperation analysis, downgraded one level due to very serious imprecision. The quality of evidence was low in the total hospital stay, delayed gastric emptying, development of pancreatitis, and intra‐abdominal abscess analyses, downgraded two levels due to risk of bias and one level for imprecision. The quality of evidence was moderate in the wound infection analysis, downgraded one level due to imprecision.
Table 3: In one study of 46 participants, the quality of evidence was low in the pancreatic fistulas, total hospital stay, and delayed gastric emptying analyses, downgraded two levels due to very serious imprecision, small sample size, and very wide confidence intervals.
Potential biases in the review process
The operation included two modifications, pylorus preserving pancreaticoduodenectomy (PPPD) and classical Whipple, which might introduce clinical heterogeneity. The length of the external stents varied between the trials and was mainly determined by practical considerations in individual participants. It is also worth noting that the stents were manufactured by different companies, and so the quality of the stents might be different, which in turn could be a source of bias. Another possible bias in the included trials is that the timing of stent removal varied greatly between the studies, which might influence the incidence of pancreatic fistula.
We searched four English and four Chinese databases; we may have missed studies in other languages. The included trails were from 2006 to 2014; the criteria for design and evaluation may have changed. Another limitation is that the economic benefit of the stents was not analyzed in the primary studies. This review did not analyze soft versus hard pancreatic remnant. While it was reported that soft pancreatic remnant is associated with a significantly higher risk of pancreatic fistula, no data were available in the primary studies to analyze this aspect (Tani 2010). Five of eight trials defined pancreatic fistula using International Study Group on Pancreatic Fistula (ISGPF), one trial using ISGPF or the Johns Hopkins (Kamoda 2008), one trial using the local definition and ISGPF (Winter 2006), and one trial using ISGPF and DeOliveira 2006 (Yokoyama 2014). In order to reduce selection bias, pancreatic fistula must be consistently defined.
Agreements and disagreements with other studies or reviews
This updated systematic review confirms that the use of external stents has the possible advantages of reducing the incidence of pancreatic fistulas and length of hospital stay as reported in a prospective random study (Poon 2007). And the use of replacement of externally drained pancreatic juice has the possible advantage of reducing length of hospital stay as reported in an RCT (Yokoyama 2014). Four published meta‐analyses have reported on this topic (Zhou 2011; Xiong 2012; Hong 2013; Ke 2015). The conclusion of one study was similar to our study (Xiong 2012), but some RCTs were not considered. The other meta‐analyses had several methodological limitations (Zhou 2011; Hong 2013; Ke 2015), so the conclusions were uncertain, and all four meta‐analyses did not compare replacement of externally drained pancreatic juice versus no replacement; only external stent versus no stent or external stent versus internal stent was analyzed.
Authors' conclusions
Implications for practice.
We have not been able to ascertain the effects of pancreatic duct stenting on the risk of pancreatic fistulas, in‐hospital mortality, and length of hospital stay after pancreaticoduodenectomy. The results of subgroup analyses that indicated superiority of external stents to internal stents may perform better than internal stents after pancreaticoduodenectomy may have little benefit in terms of incidence of pancreatic fistulas, reoperation, delayed gastric emptying, intra‐abdominal abscess, and wound infection when compared with internal stents were not confirmed by evidence from studies directly comparing these approaches to pancreatic duct stenting.
Implications for research.
Further randomized clinical trials are needed to evaluate the use of pancreatic duct stents. These trials need to have a sufficient sample size and should adopt blinded outcome assessment by researchers. Pancreatic fistula must be consistently defined. These trials should be rigorously reported according to the recommendations of the updated Consolidated Standards of Reporting Trials (CONSORT) Statement (http://www.consort‐statement.org/) (Schulz 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 12 May 2016 | Amended | Affiliations of Zhiyong Dong amended |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 14 December 2015 | New citation required but conclusions have not changed | Three new studies were identified for inclusion. The review conclusions remain unchanged. |
| 14 December 2015 | New search has been performed | Literature searches rerun in November 2015. |
Acknowledgements
We thank Toby Lasserson and Karin Dearness for modifying and editing our protocol, initial review, and updated review; Racquel Simpson, Trials Search Co‐ordinator, for developing the search strategy and identifying resources; Yuhong Yuan, Trials Search Co‐ordinator, for developing a new search strategy and updating the search results; and the other team members of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group for help during the development of this review. Fuyuhiko Motoi, for providing original data. Ronnie T Poon, for providing additional data.
Appendices
Appendix 1. CENTRAL search strategy (up to November 2015)
MeSH descriptor: [Pancreaticoduodenectomy] explode all trees
MeSH descriptor: [Pancreaticojejunostomy] explode all trees
MeSH descriptor: [Pancreatectomy] explode all trees
pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or (pancrea* near/3 duodenectom*):ti,ab,kw (Word variations have been searched)
pancreatojejunostom* or pancreaticojejunostom* or (pancrea* near/3 jejunostom*):ti,ab,kw (Word variations have been searched)
anastomosis near/3 (pancreatojejunal or jejunopancreatic):ti,ab,kw (Word variations have been searched)
pancreatogastrostom* or (pancrea* near/3 gastrostom*):ti,ab,kw (Word variations have been searched)
pancreatectom* or hemipancreatectom* or (whipple and pancrea*) or (PPD and pancrea*):ti,ab,kw (Word variations have been searched)
pancrea* near/5 (surger* or operation* or operated or operative or resect*):ti,ab,kw (Word variations have been searched)
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
MeSH descriptor: [Stents] explode all trees
stent* (Word variations have been searched)
MeSH descriptor: [Catheters] explode all trees
MeSH descriptor: [Catheterization] explode all trees
catheter* or cannula* or tube* or pipe* or "SEMS":ti,ab,kw (Word variations have been searched)
pancreatic duct* near/5 holder*:ti,ab,kw (Word variations have been searched) (0)
MeSH descriptor: [Drainage] explode all trees
drainag*:ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Suction] explode all trees
suction* or aspirate* or aspiration*:ti,ab,kw (Word variations have been searched)
#11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#10 and #21
MeSH descriptor: [Fistula] explode all trees
fistula* (Word variations have been searched)
leak*:ti,ab,kw (Word variations have been searched)
MeSH descriptor: [Postoperative Complications] explode all trees
(postoperat* or postsurgical or surgical or ((post or after) near/1 (operat* or surger*))) near/5 complication*:ti,ab,kw (Word variations have been searched)
#23 or #24 or #25 or #26 or #27
#22 and #28 (163)
Appendix 2. Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) search strategy (searched from 1946 to November 2015)
exp Pancreaticoduodenectomy/
exp Pancreaticojejunostomy/
exp Pancreatectomy/
(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or (pancrea* adj3 duodenectom*)).tw,kw.
(pancreatojejunostom* or pancreaticojejunostom* or (pancrea* adj3 jejunostom*)).tw,kw.
(anastomosis adj3 (pancreatojejunal or jejunopancreatic)).tw,kw.
(pancreatogastrostom* or (pancrea* adj3 gastrostom*)).tw,kw.
(pancreatectom* or hemipancreatectom*).tw,kw.
(Whipple and pancrea*).tw,kw.
("PPD" and pancrea*).tw,kw.
(pancrea* adj5 (surger* or operation* or operated or operative or resect*)).tw,kw.
or/1‐11
exp Stents/
stent*.mp.
exp Catheters/
exp Catheterization/
(catheter* or cannula* or tube* or pipe* or "SEMS").tw,kw.
(pancreatic duct* adj5 holder*).tw,kw.
exp Drainage/
drainag*.tw,kw.
exp Suction/
(suction* or aspirate* or aspiration*).tw,kw.
or/13‐22
12 and 23
exp Fistula/
fistula*.af.
leak*.tw,kw.
exp Postoperative Complications/
((postoperat* or postsurgical or surgical or ((post or after) adj (operat* or surger*))) adj5 complication*).tw,kw.
or/25‐29
24 and 30
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
randomly.ab.
trial.ab.
groups.ab.
or/32‐38
exp animals/ not humans.sh.
39 not 40
31 and 41 (406)
Appendix 3. EMBASE search strategy (searched from 1974 to November 2015)
exp pancreaticoduodenectomy/
exp pancreaticojejunostomy/
exp pancreas resection/ or exp pancreas surgery/
(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or (pancrea* adj3 duodenectom*)).tw,kw.
(pancreatojejunostom* or pancreaticojejunostom* or (pancrea* adj3 jejunostom*)).tw,kw.
(anastomosis adj3 (pancreatojejunal or jejunopancreatic)).tw,kw.
(pancreatogastrostom* or (pancrea* adj3 gastrostom*)).tw,kw.
(pancreatectom* or hemipancreatectom*).tw,kw.
(Whipple and pancrea*).tw,kw.
("PPD" and pancrea*).tw,kw.
(pancrea* adj5 (surger* or operation* or operated or operative or resect*)).tw,kw.
or/1‐11
exp stent/
exp catheter/
exp catheterization/
exp tube/
exp suction/
exp aspiration/
stent*.mp.
(catheter* or cannula* or tube* or pipe* or "SEMS").tw,kw.
(pancreatic duct* adj5 holder*).tw,kw.
drainag*.tw,kw.
(suction* or aspirate* or aspiration*).tw,kw.
or/13‐23
12 and 24
exp fistula/
fistula*.af.
leak*.tw,kw.
exp postoperative complication/
((postoperat* or postsurgical or surgical or ((post or after) adj (operat* or surger*))) adj5 complication*).tw,kw.
or/26‐30
25 and 31
random*.mp.
clinical trial:.mp.
exp health care quality/
double‐blind*.mp.
blind*.tw.
placebo:.mp.
or/33‐38
exp animal/ not human.sh.
39 not 40
32 and 41 (1543)
Appendix 4. Web of Science search strategy (up to November 2015)
#1) TS=(pancreaticoduodenectom* or pancreatoduodenectom* or duodenopancreatectom* or (pancrea* and duodenectom*) or pancreatojejunostom* or pancreaticojejunostom* or (pancrea* and jejunostom*) or (anastomosis and (pancreatojejunal or jejunopancreatic)) or pancreatogastrostom* or (pancrea* and gastrostom*) or pancreatectom* or hemipancreatectom* or (whipple and pancrea*) or (PPD and pancrea*) or (pancrea* and (surger* or operation* or operated or operative or resect*)))
#2) TS=(stent* or catheter* or cannula* or tube* or pipe* or "SEMS" or (duct* holder*) or drainag* or suction or aspirat*)
#3) #2 AND #1
#4) TS= (fistula* or leak* or ((postoperat* or postsurgical or surgical or ((post or after) and (operat* or surger*))) and complication*) )
#5) #4 AND #3
#6) TS=(random* or controlled or trial)
#7) #6 AND #5 (961)
Appendix 5. China Biological Medicine Database (CBM) search strategy
Searched from 1970 to November 2015
#1 壶腹癌 OR 壶腹部癌 OR 壶腹周围癌 OR 壶腹周围恶性肿瘤 OR Vater 壶腹癌OR 壶腹部良性肿瘤
#2 胆管下段癌 OR 胆总菅下端癌 OR 下段胆管癌 OR 胆总菅癌 OR 胆管肿瘤OR 胆囊癌
#3十二指肠癌 OR 十二指肠乳头腺癌 OR 十二指肠肉瘤 OR 胰头癌 OR 慢性胰腺炎 OR 胰腺癌
#4 #1 OR #2 OR#3
#5 胰十二指肠吻合术 OR 胰十二指肠切除术 OR 胰管胰头十二指肠切除术OR 胰头十二指肠切除术 OR 保留幽门的胰十二指肠切除术
#6 消化道重建 OR Child 术式 OR WhippIe 术式 OR 经典术式
#7 支架置入 OR 支架管 OR 金属支架 OR 塑料支架 OR 胰管内支撑管 OR 引流菅 OR 内引流 OR 外引流
#8 #5 OR #6 OR #7
#9 随机对照 OR 随机OR 双盲 OR 盲法
#10 病例对照 OR 对照组 OR 对照试验 OR 安慰剂
#11 #9 OR #10
#12 #4 AND #8 AND #11
Appendix 6. Chinese National Knowledge Infrastructure Database (CNKI)
Searched from 1979 to November 2015
# 1 胰十二指肠吻合术 OR 胰十二指肠切除术 OR 保留幽门胰十二指肠切除术 OR 消化道重建 OR Child 术式 OR WhippIe术式 OR 捆绑式胰肠吻合术
#2 支架置入 OR 金属支架 OR 塑料支架 OR 胰菅内支撑管 OR 引流管 OR 引流术 OR 外引流术
#3 #1 AND #2
Appendix 7. Chinese Wangfang database search strategy
Searched from 1998 to November 2015
#1 胰十二指肠吻合术 OR 胰十二指肠切除术 OR 保留幽门胰十二指肠切除术OR Child 术式 OR Whipple术式.
#2 支架 OR 胰菅内支撑菅 OR 引流菅 OR 引流术 OR 胰瘘
#3 #1 AND #2
Appendix 8. Database of Chinese Science and Technology Periodicals search strategy
Searched from 1994 to November 2015
#1 胰十二指肠吻合术 OR 胰十二指肠切除术 OR 胰管胰头十二指肠切除术 OR 十二指肠胰切除术 OR 胰头十二指肠切除术 OR 保留幽门的胰十二指肠切除术 OR 消化道重建 OR Child 术式 OR WhippIe术式.
#2 支架置入 OR 支架管 OR 金属支架 OR 塑料支架 OR 胰菅内支撑菅 OR 引流管 OR 内引流 OR 外引流
#3 #1 AND #2
Data and analyses
Comparison 1. Stents versus no stents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pancreatic fistulas | 4 | 605 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.39, 1.14] |
| 1.1 External stents | 3 | 371 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.38, 0.79] |
| 1.2 Internal stents | 1 | 234 | Risk Ratio (IV, Random, 95% CI) | 1.23 [0.78, 1.94] |
| 2 In‐hospital mortality | 4 | 605 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.28, 1.94] |
| 2.1 External stents | 3 | 371 | Risk Ratio (IV, Random, 95% CI) | 0.88 [0.27, 2.88] |
| 2.2 Internal stents | 1 | 234 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.10, 2.77] |
| 3 Reoperation | 3 | 512 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.36, 1.22] |
| 3.1 External stents | 2 | 278 | Risk Ratio (IV, Random, 95% CI) | 0.76 [0.36, 1.60] |
| 3.2 Internal stents | 1 | 234 | Risk Ratio (IV, Random, 95% CI) | 0.52 [0.18, 1.47] |
| 4 Total hospital stay | 4 | 605 | Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐6.52, ‐0.84] |
| 4.1 External stents | 3 | 371 | Mean Difference (IV, Random, 95% CI) | ‐3.68 [‐6.52, ‐0.84] |
| 4.2 Internal stents | 1 | 234 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Delayed gastric emptying | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 External stents | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.24, 2.35] |
| 5.2 Internal stents | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Intra‐abdominal abscess | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 External stents | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Internal stents | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Wound infection | 4 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.40, 1.32] |
| 7.1 External stents | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.22, 1.95] |
| 7.2 Internal stents | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.39, 1.29] |
Comparison 2. Internal stents versus external stents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pancreatic fistulas | 3 | 362 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.94, 2.21] |
| 2 In‐hospital mortality | 3 | 362 | Risk Ratio (IV, Random, 95% CI) | 0.33 [0.01, 7.99] |
| 3 Reoperation | 2 | 319 | Risk Ratio (IV, Random, 95% CI) | 2.02 [0.38, 10.79] |
| 4 Total hospital stay | 2 | 262 | Mean Difference (IV, Random, 95% CI) | ‐1.67 [‐9.18, 5.84] |
| 5 Overall complications | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Delayed gastric emptying | 3 | 362 | Risk Ratio (IV, Random, 95% CI) | 1.65 [0.66, 4.09] |
| 5.2 Events of pancreatitis | 2 | 262 | Risk Ratio (IV, Random, 95% CI) | 0.15 [0.01, 2.73] |
| 5.3 Intra‐abdominal abscess | 3 | 362 | Risk Ratio (IV, Random, 95% CI) | 1.91 [0.80, 4.58] |
| 5.4 Wound infection | 2 | 319 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.44, 4.48] |
Comparison 3. No replacement versus replacement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pancreatic fistulas | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 1.60 [0.84, 3.07] |
| 2 In‐hospital mortality | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Total hospital stay | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 16.30 [13.24, 19.36] |
| 4 Overall complications | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Delayed gastric emptying | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.2. Analysis.

Comparison 3 No replacement versus replacement, Outcome 2 In‐hospital mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kamoda 2008.
| Methods | Randomized clinical trial
Allocation sequence: unclear, only mentioned "allocated randomly"
Allocation concealment: unclear, not mentioned
Blinding: unclear, no mention Follow‐up: adequate, dropouts: n = 1 (2.27%) Intention‐to‐treat analysis: no, used "per protocol analysis" Informed consent: reported Sample size calculation: performed |
|
| Participants | Country: Japan. Single center: Kobe University Hospital, from January 2003 to January 2007. No. randomized: 44 Age: 23 participants ≧ 65 years, 20 participants ≨ 65 years Sex: Male/Female: 15/28 Inclusion criteria: Participants underwent a PD including pylorus‐preserving PD for pancreas, periampullary, and bile duct tumours. Exclusion criteria: The study was stopped when three unanticipated events occurred. Definition of pancreatic fistula: According to the ISGPF or the Johns Hopkins Conflicts of interest: no details provided |
|
| Interventions | 44 participants were randomly assigned to 1 of 2 groups: Group 1: Pancreaticojejunostomy with an external stent (n = 22) Group 2: Pancreaticojejunostomy with an internal stent (n = 22) | |
| Outcomes | The main outcome measures were: Pancreas fistula Events of pancreatitis Pancreatic duct dilatation Mortality Postoperative hospital stay |
|
| Notes | One participant in group 1 was excluded from study due to secondary fatty replacement of the remnant pancreas. 43 participants were analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only mentioned "allocated randomly" |
| Allocation concealment (selection bias) | Unclear risk | Only mentioned "allocated randomly" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information, and dropouts: n = 1 (2.27%) |
| Selective reporting (reporting bias) | Low risk | According to the outcomes reported |
| Other bias | Unclear risk | Not mentioned |
Motoi 2012.
| Methods | Randomized controlled trial (www.umin.ac.jp/ctr/index‐j.htm, clinical trial, registration no. UMIN000000952) Allocation sequence: random permuted blocks Allocation concealment: sealed envelope method. We considered both allocation sequence generation and allocation concealment to be adequate. Blinding: not mentioned Follow‐up: adequate. All participants were reviewed after operation to complete the follow‐up. Dropouts: n = 0 Informed consent: reported Intention‐to‐treat analysis: used Sample size calculation: not performed Informed consent: reported |
|
| Participants | Country: Sendai, Japan. Single center, elective between December 2007 and April 2010 No. randomized: 93 Age: No‐stent group: 65.5 (32 to 80) years; Stent group: 60.0 (33 to 79) years Gender: No‐stent group: Male/Female: 29/17; Stent group: Male/Female: 26/21 Inclusion criteria: Participants underwent PD Exclusion criteria: Unresectable disease and underwent conversion to a different surgical procedure after laparotomy Definition of pancreatic fistula: ISGPF Conflicts of interest: No potential conflicts of interest |
|
| Interventions | 93 people who underwent PD were randomized intraoperatively to either stented (47) or non‐stented (n = 46). Among participants with a dilated duct: 26 were randomized to the stented and non‐stented groups. Among participants with a non‐dilated duct: 21 were randomized to the stented group and 20 to the non‐stented group | |
| Outcomes | The main outcome measures were: Postoperative pancreatic fistula Overall morbidity Hospital mortality Duration of postoperative hospital stay |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Detailed information |
| Selective reporting (reporting bias) | Low risk | According to the detailed outcome |
| Other bias | Unclear risk | None |
Pessaux 2011.
| Methods | Randomized clinical trial (registered at www.clinicaltrials.gov, ID# NCT01068886)
Allocation sequence: central random numbers
Allocation concealment: sealed envelopes. We considered both allocation sequence generation and allocation concealment to be adequate.
Blinding: not mentioned Follow‐up: adequate. All participants were reviewed 6 weeks after operation to complete the follow‐up. Dropouts: n = 0 Informed consent: reported Intention‐to‐treat analysis: used Sample size calculation: not performed |
|
| Participants | Country: Strasbourg, France Multicenter, between January 2006 and March 2009 No. randomized: 158 Age: No‐stent group: 60.6 ± 11.8 years; Stent group: 60.8 ± 11.8 years Gender: Male/Female: 86/72 Inclusion criteria: Participants underwent conventional or pylorus‐preserving PD Exclusion criteria: Age less than 18 years, emergency surgery Definition of pancreatic fistula: ISGPF Conflicts of interest: No potential conflicts of interest |
|
| Interventions | 158 participants who underwent PD were randomized intraoperatively to either receive an external stent inserted across the anastomosis to drain the pancreatic duct (n = 77) or no stent (n = 81) | |
| Outcomes | The main outcome measures were: Pancreatic fistula Overall morbidity rate Mortality rate Length of hospitalization |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported |
| Selective reporting (reporting bias) | Low risk | According to the detailed outcomes |
| Other bias | Unclear risk | None detailed |
Poon 2007.
| Methods | Randomized clinical trial (registered in a clinical trial website of Hong Kong www.hkclinicaltrials.com, no. HKCTR‐12)
Allocation sequence: random numbers
Allocation concealment: sealed envelopes. We considered both allocation sequence generation and allocation concealment to be adequate.
Blinding: not mentioned Follow‐up: adequate. Lost to follow up: n = 0 Dropouts: n = 0 Informed consent: reported Intention‐to‐treat analysis: used, but no detailed information Sample size calculation: performed |
|
| Participants | Country: Hong Kong, China Single center: Department of Surgery, The University of Hong Kong, Queen Mary Hospital, between June 2000 and October 2006. No. randomized: 120 Age: 67 participants ≦ 65 years, 53 participants ≩ 65 years Gender: Male/Female: 72/48 Inclusion criteria: Underwent elective PD for benign or malignant pathologies of pancreas or periampullary region. Exclusion criteria: Underwent emergency PD for trauma. Ongoing acute pancreatitis at the time of operation. Recruited before surgery but were found to have unresectable disease after laparoscopy or laparotomy Definition of pancreatic fistula: amylase‐rich fluid (amylase concentration 3 times the upper limit of normal serum amylase level) collected from the peripancreatic drains after postoperative day 3 with a drainage volume of 10 mL per day Conflicts of interest: none |
|
| Interventions | 120 participants who underwent PD with end‐to‐side pancreaticojejunal anastomosis were randomly assigned to 1 of 2 groups; duct‐to‐mucosa anastomosis was performed in all participants. Group 1: An external stent inserted across the PJ anastomosis into the pancreatic duct and brought out externally via the jejunal loop and abdominal wall (n = 60) Group 2: No stent in the PJ anastomosis (n = 60) | |
| Outcomes | The main outcome measures were: Pancreatic fistula or leakage Overall morbidity rate Hospital mortality rate Postoperative hospital stay No. of days to resume oral diet No. of days on total parenteral nutrition |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information, and dropouts: n = 0 |
| Selective reporting (reporting bias) | Low risk | According to the outcomes reported |
| Other bias | Unclear risk | Not mentioned |
Tani 2010.
| Methods | A prospective randomized clinical trial (registered at www.clinicaltrials.gov, ID# NCT00628186) Allocation sequence: adequate Allocation concealment: adequate, used CONSORT flow diagram. We considered both allocation sequence generation and allocation concealment to be adequate. Blinding: no mention Follow‐up: adequate Dropouts: n = 0 Informed consent: reported Intention‐to‐treat analysis: used Sample size calculation: performed |
|
| Participants | Country: Japan Single center: Wakayama Medical University Hospital, between April 2005 and August 2007. No. randomized: 100 Age: 35 to 87 years Gender: Male/Female: 55/45 Inclusion criteria: Underwent PD for pancreatic head and periampullary disease. Exclusion criteria: Severe associated diseases that might prolong hospital stay. Condition inadequately diagnosed for this trial by a physician. Could not have a pancreatic stent placed Definition of pancreatic fistula: ISGPF Conflicts of interest: none |
|
| Interventions | 100 participants who underwent PD were randomly assigned to 1 of 2 groups: Group 1: External drainage (n = 50) Group 2: Internal drainage (n = 50) | |
| Outcomes | The main outcome measures were: Postoperative hospital stay Mortality and morbidity Pancreatic fistula Delayed gastric emptying Intra‐abdominal hemorrhage Intra‐abdominal abscess |
|
| Notes | One participant in group 1 died in the hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used CONSORT flow diagram |
| Allocation concealment (selection bias) | Low risk | Used CONSORT flow diagram |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information |
| Selective reporting (reporting bias) | Low risk | According to the outcomes reported |
| Other bias | Unclear risk | Not mentioned |
Wang 2014.
| Methods | A prospective randomized controlled trial (registration no. ChiCTR‐TRC‐14004830)
Allocation sequence: adequate, reported computer‐generated random numbers Allocation concealment: adequate. We considered both allocation sequence generation and allocation concealment to be adequate. Blinding: no mention Follow‐up: adequate, withdrawn: n = 0 Dropouts: n = 0 Intention‐to‐treat analysis: no mention Sample size calculation: no mention Informed consent: reported Ethics committee: approval |
|
| Participants | Country: China Single center: Department of Hepatobiliary and Pancreatic Surgery, the First Affiliated Hospital, Harbin Medical University, 219 participants were accrued from 1 January 2010 to 1 March 2013 No. randomized: 219 Age: 24 to 77 years Gender: Male/Female: 113/106 Inclusion criteria: Underwent PD Definition of pancreatic fistula: ISGPF (2005) definition Conflicts of interest: none |
|
| Interventions | All participants underwent PD. 219 participants were randomly assigned to 1 of 2 groups: Group 1: External drainage (n = 110) Group 2: Internal drainage (n = 109) |
|
| Outcomes | The main outcome measures were: Mortality, morbidity of pancreatic fistula Delayed gastric emptying Abdominal infection Intestinal obstruction Overall complications Intraoperative blood loss Operative duration Postoperative hospital stay |
|
| Notes | All participants underwent PD, no participants were withdrawn, and no participants dropped out | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clearly reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information |
| Selective reporting (reporting bias) | Low risk | Reported detailed outcomes |
| Other bias | Unclear risk | No mention |
Winter 2006.
| Methods | Randomized clinical trial
Allocation sequence: adequate, reported using a randomly generated number pattern.
Allocation concealment: adequate. We considered both allocation sequence generation and allocation concealment to be adequate.
Blinding: no mention Follow‐up: adequate, withdrawn: n = 4 Dropouts: n = 0 Intention‐to‐treat analysis: no, used per‐protocol analysis Sample size calculation: performed |
|
| Participants | Country: USA Single center: Johns Hopkins Medicine IRB, 238 participants were accrued between 8 March 2004 and 21 November 2005. No. randomized: 234 Age: 27 to 89 years Gender: Male/Female: 132/102 Inclusion criteria: Underwent a pylorus‐preserving PD Definition of pancreatic fistula: local definition and ISGPF Conflicts of interest: none |
|
| Interventions | In participants with a soft pancreas: 113 participants were randomly assigned to 1 of 2 groups: Stent (n = 57), No stent (n = 56) In participants with a hard pancreas: 121 participants were randomly assigned to 1 of 2 groups: Stent (n = 58), No stents (n = 63) |
|
| Outcomes | The main outcome measures were: Mortality Complications Reoperation Specific complications Postoperative length of stay |
|
| Notes | All participants underwent PD, but 4 participants were withdrawn from the study after enrollment and randomization. 234 participants who completed the study were analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated number pattern |
| Allocation concealment (selection bias) | Unclear risk | Randomly generated number pattern |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information, and withdrawn: n = 4 |
| Selective reporting (reporting bias) | Low risk | According to the outcomes reported |
| Other bias | Unclear risk | Not mentioned |
Yokoyama 2014.
| Methods | Randomized controlled study
Allocation sequence: not reported
Allocation concealment: not reported
Blinding: no mention Follow‐up: adequate, withdrawn: n = 18 Dropouts: n = 0 Intention‐to‐treat analysis: no, used per‐protocol analysis Informed consent: described Sample size calculation: performed |
|
| Participants | Country: Nagoya, Japan Single center: Nagoya University Hospital, 64 participants were accrued between April 2006 and August 2008. 18 participants were excluded (4 due to local or liver metastasis, 2 due to dislocation of pancreatic tube, 1 due to re‐operation, 11 due to lass than 100ml discharge from pancreatic tube) Non‐replacement of pancreatic duct drainage tube: Age: 63.3 (43 to 85) years; Gender: Male/Female: 15/9 Replacement of pancreatic duct drainage tube: Age: 63.1 (44 to 79) years; Gender: Male/Female: 13/9 Inclusion criteria: Underwent pancreaticoduodenectomies Definition of pancreatic fistula: ISGPF and Classification of surgical complication after pancreatic surgery by DeOliveira 2006 Conflicts of interest: none |
|
| Interventions | 64 participants were randomly assigned to 1 of 2 groups, excluded 18 participants: Non‐replacement of pancreatic juice (n = 24) Replacement of pancreatic juice (n = 22) |
|
| Outcomes | The main outcome measures were: Mortality Pancreatic fistula Delayed gastric emptying DeOliveira's score Postoperative length of stay |
|
| Notes | All participants underwent PD, but 18 participants were withdrawn from the study before enrollment and randomization. 46 participants who completed the study were analyzed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No mention, but "followed the method of a randomized controlled study" |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed information, and withdrawn: n = 18 |
| Selective reporting (reporting bias) | Low risk | No, according to results |
| Other bias | Unclear risk | None |
CONSORT: Consolidated Standards of Reporting Trials ISGPF: International Study Group on Pancreatic Fistula PD: pancreaticoduodenectomy PJ: pancreaticojejunal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bree 2004 | Case report |
| Chen 2004 | A randomized clinical trial, but the control and observation group did not meet the inclusion criteria |
| He 2004 | A randomized clinical trial, but the control and observation group did not meet the inclusion criteria |
| Imaizumi 2006 | Clinical controlled study, did not use random assignment |
| Ito 2012 | A retrospective clinical study, another meta‐analysis, Ke 2015, included this study |
| Kimura 2009 | Case control report |
| Kuroki 2011 | A randomized clinical trial, but reported about a prospective study limited to a normal pancreas without fibrosis sorted by using dynamic magnetic resonance imaging in pancreaticojejunostomy, and did not meet the inclusion criteria. Another meta‐analysis, Hong 2013, included this study |
| Lee 2009 | A randomized clinical trial, but did not meet the inclusion criteria. ClinicalTrials.gov identifier: NCT00679952 |
| Meng 2014 | A retrospective clinical study, another meta‐analysis, Ke 2015, included this study |
| Mullen 2005 | Controlled study, did not use random assignment |
| Ohwada 2002 | Non‐randomized controlled study, another meta‐analysis, Ke 2015, included this study |
| Roder 1999 | A prospective non‐randomized observation study |
| Schulick 2009 | A review |
| Shukla 2010 | A review |
| Smyrniotis 2010 | Clinical controlled study |
| Tani 2005 | Clinical controlled study |
| Yoo 2014 | A retrospective clinical study evaluating only degree of pancreatic parenchymal atrophy, another meta‐analysis, Ke 2015, included this study |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐14004830.
| Trial name or title | The effect of different pancreatic duct stent drainage modes on the early curative effect after pancreaticoduodenectomy, a prospective randomized controlled trial |
| Methods | Randomized parallel controlled trial |
| Participants | People ready to undergo pancreaticoduodenectomy due to benign and malignant diseases of pancreas and pars ampullaris aged 18 to 80 years |
| Interventions | Pancreatic duct stent is placed outside the body, pancreatic duct stent is placed 7 to 8 cm from the pancreaticojejunal anastomotic stoma in the intestine |
| Outcomes | Incidence of postoperative pancreatic fistula Postoperative pancreatic fistula healing time Incidence of postoperative‐related complications Incidence of global postoperative complications Intraoperative blood loss Surgical duration Duration of postoperative hospitalization Perioperative mortality Reoperation rate |
| Starting date | July 2014 |
| Contact information | Sun Bei Pro. Email: sunbei70@tom.com Tel: +86 13603656935 |
| Notes | Registration number: ChiCTR‐TRC‐14004830. http://www.chictr.org.cn/searchproj.aspx This group has published another relevant study in Chinese, which is included (Wang 2014) |
NCT00501176.
| Trial name or title | Randomized study comparing the effect of plastic stents to that of expandable metal stents as preoperative |
| Methods | Allocation: Randomized Control: Active control Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Open label Primary purpose: Treatment |
| Participants | The primary inclusion criteria are people with operable periampullary cancer and jaundice who have not previously undergone bile flow drainage |
| Interventions | Procedure: primary ERCP and secondary Whipple |
| Outcomes | Intraoperative measurement of the inflammatory reaction in the liver, hepatoduodenal ligament, and around the bile ducts with biopsies and culture from the bile. Stent dysfunction and cholangitis after ERCP and preoperative bile flow relief. Postoperative complications. Time in the hospital. Cost. Quality of life analysis |
| Starting date | July 2007 |
| Contact information | Farshad Frozanpor Email: farshad.frozanpor@sodersjukhuset.se |
| Notes | ClinicalTrials.gov identifier: NCT00501176 http://www.clinicaltrial.gov/ct2/show/NCT00501176?term=pancreaticod This trial is still recruiting. We have emailed for details but have not received a reply. Last updated: 11 June 2014 |
NCT00855985.
| Trial name or title | Anastomotic techniques in pancreaticoduodenectomy |
| Methods | Allocation: Randomized Control: Active control Intervention model: Parallel assignment Masking: Single blind (participant) Primary purpose: Treatment |
| Participants | Pancreaticoduodenectomy for adenocarcinoma of the pancreatic head resectable tumor |
| Interventions | Procedure: type of anastomosis after pancreaticoduodenectomy |
| Outcomes | Mortality 90 days Yes Hospital stay 90 days No Need for postoperative intervention 90 days Yes Major complication 90 days Yes |
| Starting date | July 2004 |
| Contact information | Hariharan Ramesh, MS MCh FRCS FACS Tel: 2701992 ext.: 2036 hramesh@vsnl.com |
| Notes | ClinicalTrials.gov identifier: NCT00855985 Website: http://www.clinicaltrials.gov/ The recruitment status of this study is unknown because the information has not been recently verified. Last updated: 24 September 2011 |
NCT01023594.
| Trial name or title | Comparison of feasibility between internal and external pancreatic drainage in pancreaticoduodenectomy |
| Methods | Allocation: Randomized Endpoint classification: Safety/efficacy study Intervention model: Parallel assignment Masking: Open label Primary purpose: Prevention |
| Participants | People who have malignant or benign disease that needs pancreaticoduodenectomy Participant age: ≥ 20 and ≤ 85 years |
| Interventions | External stent: Active comparator feeding tube insert at pancreaticojejunostomy site as a stent. And stent tube is brought out through jejunal loop below the hepaticojejunostomy site and abdominal wall. Intervention: Procedure: Pancreatic stent Internal stent: Active comparator short (5 cm) internal stent insertion at pancreaticojejunostomy site. Intervention: Procedure: Pancreatic stent |
| Outcomes | Evidence of pancreatic fistula confirmed by serum and drain amylase Pancreatic endocrine and exocrine function by blood test, stool exam |
| Starting date | January 2010 |
| Contact information | Contact: Sun‐Whe Kim, MD 82‐2‐2072‐2310 sunkim@plaza.snu.ac.kr Contact: Jin‐Young Jang, MD 82‐2‐2072‐2194 jangjy4@gmail.com |
| Notes | ClinicalTrials.gov identifier: NCT01023594 Website: http://www.clinicaltrials.gov/ This study is ongoing, but not recruiting participants. Last updated: 14 September 2015 |
NCT01634971.
| Trial name or title | External drainage versus internal drainage of pancreatic duct with a stent after pancreaticoduodenectomy (EDIDPD) |
| Methods | Allocation: Randomized Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Double blind (participant, caregiver, investigator) Primary purpose: Treatment |
| Participants | Inclusion criteria: Age more than 18 years Received pancreaticoduodenectomy Informed consent has been signed Exclusion criteria: Age less than 18 years Emergency surgery Past history of pancreatic surgery |
| Interventions | Intervention: Internal drainage of pancreatic duct after pancreatectomy, a stent was placed in the pancreatic duct Control: External drainage of the stent (the stent will be tans‐abdomen and as a drainage of pancreatic fluid) and internal drainage of the stent is defined as interventional (or experimental) group, with the stent very short (2 cm) and placed in jejunum |
| Outcomes | Postoperative pancreatic fistula Rate of postoperative surgical complications Rate of postpancreatectomy hemorrhage Delayed gastric emptying Bile leakage after pancreatic surgery Anastomotic leakage |
| Starting date | May 2012 |
| Contact information | Contact: Qiang Wu, MD PhD Tel.: 18622221080 Email: wuqiangtianjin@hotmail.com Tianjin Medical University Cancer Institute and Hospital, Tianjin, China 300060 |
| Notes | ClinicalTrials.gov identifier: NCT01634971. Website: http://www.clinicaltrials.gov/ This study is currently recruiting participants. Last updated: 22 January 2013 |
ERCP: endoscopic retrograde cholangiopancreatography
Differences between protocol and review
The first version of this systematic review was published in Issue 6, 2013, and needed to be updated. We designed a new search strategy, searching until the end of November 2015. We found three new RCTs, which included studies reporting that enteral replacement of externally drained pancreatic juice may reduce the rate of pancreatic fistula, and a significant decrease (30%) in levels of water, protein, bicarbonate, and enzyme output compared with those participants that did not undergo replacement after pancreaticoduodenectomy (Yasui 1988; Yokoyama 2014). We have therefore expanded the inclusion criteria to include RCTs comparing the use of replacement versus no replacement of externally drained pancreatic juice after pancreaticoduodenectomy, and we have added this as a third comparison of interest.
Contributions of authors
Zhiyong Dong drafted the protocol and updated the review, designed the new search strategy, performed the literature searches, and drafted the final updated review.
Jing Xu provided advice on the protocol and revised the review/updated review and provided advice on the clinical content of the review.
Maxim S Petrov revised the protocol, developed the methodology section of the protocol, and revised the final review and the updated review.
Zhiyong Dong and Zhen Wang independently performed data extraction, data management, and assessed studies for inclusion.
Sources of support
Internal sources
The First Affiliated Hospital of Guangxi Medical University, China.
External sources
The University of Auckland, New Zealand.
Declarations of interest
ZD: none known.
JX: none known.
ZW: none known.
MSP: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Kamoda 2008 {published data only}
- Kamoda Y, Fujino Y, Matsumoto I, Shinzeki M, Sakai T, Kuroda Y. Usefulness of performing a pancreaticojejunostomy with an internal stent after a pancreatoduodenectomy. Surgery Today 2008;38(6):524‐8. [DOI] [PubMed] [Google Scholar]
Motoi 2012 {published data only}
- Motoi F, Egawa S, Rikiyama T, Katayose Y, Unno M. Randomized clinical trial of external stent drainage of the pancreatic duct to reduce postoperative pancreatic fistula after pancreaticojejunostomy. British Journal of Surgery 2012;99(4):524‐31. [DOI] [PubMed] [Google Scholar]
Pessaux 2011 {published data only}
- Pessaux P, Sauvanet A, Mariette C, Paye F, Muscari F, Cunha ASA, et al. External pancreatic duct stent decreases pancreatic fistula rate after pancreaticoduodenectomy: prospective multicenter randomized trial. Annals of Surgery 2011;253(5):879‐85. [DOI] [PubMed] [Google Scholar]
Poon 2007 {published data only}
- Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Annals of Surgery 2007;246(3):425‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tani 2010 {published data only}
- Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, Shimizu A, et al. A prospective randomized controlled trial of internal versus external drainage with pancreaticojejunostomy for pancreaticoduodenectomy. American Journal of Surgery 2010;199(6):759‐64. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang G, Sun B, Jiang H, Li L, Ma Y, Wu L, et al. A prospective randomized controlled trial of pancreatic duct stent internal versus external drainage with pancreaticojejunostomy for the early curative effect after pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi 2014;52(5):333‐7. [PubMed] [Google Scholar]
Winter 2006 {published data only}
- Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. Journal of Gastrointestinal Surgery 2006;10(9):1280‐90. [DOI] [PubMed] [Google Scholar]
Yokoyama 2014 {published data only}
- Yokoyama Y, Ebata T, Igami T, Sugawara G, Nagino M. Is the enteral replacement of externally drained pancreatic juice valuable after pancreatoduodenectomy?. Surgery Today 2014;44:252‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bree 2004 {published data only}
- Bree ED, Melissas J, Schoretsanitis G, Sanidas E, Tsiftsis DD. Pylorus‐preserving pancreaticoduodenectomy with external pancreatic remnant drainage. Acta Chirurgica Belgica 2004;104:668‐72. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen CL, Chen CZ, He L, Lin H, Xie JQ. Application of outside of suction of bile and pancreatic juice by double T tubes in pancreato‐duodenectomy. Chinese Journal of Primary Medicine and Pharmacy 2004;11(7):839‐40. [Google Scholar]
He 2004 {published data only}
- He L, Chen CL, Chen CZ, Lin H, Xie JQ. Application of external drainage of bile and pancreatic juice in pancreatoduodenectomy. Journal of Postgraduates of Medicine (Surgery Edition) 2004;27(3):20‐1. [Google Scholar]
Imaizumi 2006 {published data only}
- Imaizumi T, Hatori T, Tobita K, Fukuda A, Takasaki K, Makuuchi H. Pancreaticojejunostomy using duct‐to‐mucosa anastomosis without a stenting tube. Journal of Hepato‐Biliary‐Pancreatic Surgery 2006;13(3):194‐201. [DOI] [PubMed] [Google Scholar]
Ito 2012 {published data only}
- Ito Y, Kenmochi T, Irino T, Egawa T, Hayashi S, Nagashima A, et al. Strategies to prevent pancreatic fistula after pancreaticoduodenectomy. Hepatogastroenterology 2012;59(120):2609‐13. [DOI] [PubMed] [Google Scholar]
Kimura 2009 {published data only}
- Kimura W. Pancreaticojejunal anastomosis, using a stent tube, in pancreaticoduodenectomy. Journal of Hepato‐Biliary‐Pancreatic Surgery 2009;16(3):305‐9. [DOI] [PubMed] [Google Scholar]
Kuroki 2011 {published data only}
- Kuroki T, Tajima Y, Kitasato A, Adachi T, Kanematsu T. Stenting versus non‐stenting in pancreaticojejunostomy: a prospective study limited to a normal pancreas without fibrosis sorted by using dynamic MRI. Pancreas 2011;40(1):25‐9. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee SE, Ahn YJ, Jang JY, Kim SW. Prospective randomized pilot trial comparing closed suction drainage and gravity drainage of the pancreatic duct in pancreaticojejunostomy. Journal of Hepato‐Biliary‐Pancreatic Surgery 2009;16(6):837‐43. [DOI] [PubMed] [Google Scholar]
Meng 2014 {published data only}
- Meng G, Xing Q, Yuan Q, Du Z, Wang Y, Meng H. Internal compared with external drainage of pancreatic duct during pancreaticoduodenectomy: a retrospective study. Chinese Journal of Cancer Research 2014;26(3):277‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullen 2005 {published data only}
- Mullen JT, Lee JH, Gomez HF, Ross WA, Fukami N, Wolff RA, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. Journal of Gastrointestinal Surgery 2005;9(8):1094‐105. [DOI] [PubMed] [Google Scholar]
Ohwada 2002 {published data only}
- Ohwada S, Tanahashi Y, Ogawa T, Kawate S, Hamada K, Tago KI, et al. In situ vs ex situ pancreatic duct stents of duct‐to‐mucosa pancreaticojejunostomy after pancreaticoduodenectomy with billroth I–type reconstruction. Archives of Surgery 2002;137:1289‐93. [DOI] [PubMed] [Google Scholar]
Roder 1999 {published data only}
- Roder JD, Stein HJ, Böttcher KA, Busch R, Heidecke CD, Siewert JR. Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy: a prospective study. Annals of Surgery 1999;299(1):41‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulick 2009 {published data only}
- Schulick RD, Yoshimura K. Stents, glue, etc.: Is anything proven to help prevent pancreatic leaks/fistulae?. Journal of Gastrointestinal Surgery 2009;13:1184–6. [DOI] [PubMed] [Google Scholar]
Shukla 2010 {published data only}
- Shukla PJ, Barreto SG, Fingerhut A. Do transanastomotic pancreatic ductal stents after pancreatic resections improve outcomes?. Pancreas 2010;39(5):561‐6. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2010 {published data only}
- Smyrniotis V, Arkadopoulos N, Kyriazi MA, Derpapas M, Theodosopoulos T, Gennatas C, et al. Does internal stenting of the pancreaticojejunostomy improve outcomes after pancreatoduodenectomy? A prospective study. Langenbeck's Archives of Surgery 2010;395(3):195‐200. [DOI] [PubMed] [Google Scholar]
Tani 2005 {published data only}
- Tani M, Onishi H, Kinoshita H, Kawai M, Ueno M, Hama T, et al. The evaluation of duct‐to‐mucosal pancreaticojejunostomy in pancreaticoduodenectomy. World Journal of Surgery 2005;29(1):76‐9. [DOI] [PubMed] [Google Scholar]
Yoo 2014 {published data only}
- Yoo D, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, et al. Pancreatic atrophy relative to external versus internal drainage of the pancreatic duct after pylorus‐preserving pancreaticoduodenectomy. Journal of Gastrointestinal Surgery 2014;18(9):1604‐9. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐14004830 {published data only}
- ChiCTR‐TRC‐14004830. The effect of different pancreatic duct stent drainage modes on the early curative effect after pancreaticoduodenectomy, a prospective randomized controlled trial. http://www.chictr.org.cn/showprojen.aspx?proj=4743 (accessed 28 April 2016).
NCT00501176 {published data only}
- NCT00501176. Randomized study comparing the effect of plastic stents to that of expandable metal stents as pre‐operative. http://www.clinicaltrials.gov/ct2/show/NCT00501176?term=pancreaticod (accessed 28 April 2016).
NCT00855985 {published data only}
- NCT00855985. Anastomotic techniques in pancreaticoduodenectomy. http://www.clinicaltrials.gov/show/NCT00855985 (accessed 28 April 2016).
NCT01023594 {published data only}
- NCT01023594. Comparison of feasibility between internal and external pancreatic drainage in pancreaticoduodenectomy. http://www.clinicaltrials.gov/show/NCT01023594 (accessed 28 April 2016).
NCT01634971 {published data only}
- NCT01634971. External drainage versus internal drainage of pancreatic duct with a stent after pancreaticoduodenectomy (EDIDPD). http://clinicaltrials.gov/show/NCT01634971 (accessed 28 April 2016).
Additional references
Bassi 2005
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8‐13. [DOI] [PubMed] [Google Scholar]
Berger 2009
- Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower‐Cherry M, Dutkevitch S, et al. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual‐institution trial. Journal of the American College of Surgeons 2009;208:738‐49. [DOI] [PubMed] [Google Scholar]
Boettger 1999
- Boettger TC, Junginger T. Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World Journal of Surgery 1999;23:164‐72. [DOI] [PubMed] [Google Scholar]
Brown 2014
- Brown EG, Yang A, Canter RJ, Bold RJ. Outcomes of pancreaticoduodenectomy: where should we focus our efforts on improving outcomes?. JAMA Surgery 2014;147(7):694‐9. [DOI] [PubMed] [Google Scholar]
Cameron 1976
- Cameron JL, Kieffer RS, Anderson WJ, Zuidema GD. Internal pancreatic fistulas: pancreatic ascites and pleural effusions. Annals of Surgery 1976;184(5):587‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015
- Chen S, Chen JZ, Zhan Q, Deng XX, Shen BY, Peng CH, et al. Robot‐assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid‐term follow‐up study. Surgical Endoscopy 2015;29(12):3698‐711. [DOI] [PubMed] [Google Scholar]
Cullen 1994
- Cullen JJ, Sarr MG, Ilstrup DM. Pancreatic anastomotic leak after pancreaticoduodenectomy: incidence, significance, and management. American Journal of Surgery 1994;168:295‐8. [DOI] [PubMed] [Google Scholar]
DeOliveira 2006
- DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Annals of Surgery 2006;244(6):931‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diener 2008
- Diener MK, Rahbari NN, Fischer L, Antes G, Büchler MW, Seiler CM. Duodenum‐preserving pancreatic head resection versus pancreatoduodenectomy for surgical treatment of chronic pancreatitis: A systematic review and meta‐analysis. Annals of Surgery 2008;247(6):950‐61. [DOI] [PubMed] [Google Scholar]
Diener 2011
- Diener M, Heukaeufer C, Schwarzer G, Seiler CM, Antes G, Knaebel HP, et al. Pancreaticoduodenectomy (classic Whipple) versus pylorus‐preserving pancreaticoduodenectomy (pp Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD006053.pub3] [DOI] [PubMed] [Google Scholar]
Dokmak 2015
- Dokmak S, Aussilhou B, Ftériche FS, Chaumont A, Malgras B, Belghiti J, et al. Laparoscopic pancreaticoduodenectomy: How I do it? (with video). Journal of Visceral Surgery 2015;152(6):393‐4. [DOI] [PubMed] [Google Scholar]
Fingerhut 2009
- Fingerhut A, Veyrie N, Ata T, Alexakis N, Leandros E. Use of sealants in pancreatic surgery: Critical appraisal of the literature. Digestive Surgery 2009;26(1):7‐14. [DOI] [PubMed] [Google Scholar]
Gouma 2000
- Gouma DJ, Geenen RCI, Gulik TM, Haan RJ, Wit LT, Busch OR, et al. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Annals of Surgery 2000;232(6):786‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Brozek J, Oxman A, Schunemann H. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Hamanaka 1994
- Hamanaka Y, Suzuki T. Total pancreatic duct drainage for leak proof pancreatojejunostomy. Surgery 1994;115(1):22‐6. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hiraoka 1993
- Hiraoka T, Kanemitsu K, Tsuji T, Saitoh N, Takamori H, Akamine T, et al. A method for safe pancreaticojejunostomy. American Journal of Surgery 1993;165(2):270‐2. [DOI] [PubMed] [Google Scholar]
Hong 2013
- Hong S, Wang H, Yang S, Yang K. External stent versus no stent for pancreaticojejunostomy: a meta‐analysis of randomized controlled trials. Journal of Gastrointestinal Surgery 2013;17(8):1516‐25. [DOI] [PubMed] [Google Scholar]
Howard 2007
- Howard JM. History of pancreatic head resection ‐‐ the evaluation of surgical technique. American Journal of Surgery 2007;194(4):S6‐S10. [Google Scholar]
Iqbal 2008
- Iqbal N, Lovegrove RE, Tilney HS, Abraham AT, Bhattacharya S, Tekkis PP, et al. A comparison of pancreaticoduodenectomy with pylorus preserving pancreaticoduodenectomy: A meta‐analysis of 2822 patients. European Journal of Surgical Oncology 2008;34(11):1237‐45. [DOI] [PubMed] [Google Scholar]
Ke 2015
- Ke FY, Wu XS, Zhang Y, Zhang HC, Weng MZ, Liu YB, et al. Comparison of postoperative complications between internal and external pancreatic duct stenting during pancreaticoduodenectomy: a meta‐analysis. Chinese Journal of Cancer Research 2015;27(4):397‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kollmar 2007
- Kollmar O, Moussavian MR, Bolli M, Richter S, Schilling MK. Pancreatojejunal leakage after pancreas head resection: anatomic and surgeon related factors. Journal of Gastrointestinal Surgery 2007;11:1699‐703. [DOI] [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schünemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2009
- Li HX. Controversies and appraisals about gastrointestinal reconstruction in pancreatoduodenectomy. Shijie Huaren Xiaohua Zazhi 2009;17(5):476‐81. [Google Scholar]
Manabe 1986
- Manabe T, Suzuki T, Tobe T. A secured technique for pancreatojejunal anastomosis in pancreaticoduodenectomy. Surgery, Gynecology and Obstetrics 1986;163(4):378‐80. [PubMed] [Google Scholar]
Neoptolemos 1997
- Neoptolemos JP, Russel RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and peri‐ampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. British Journal of Surgery 1997;84(10):1370‐6. [PubMed] [Google Scholar]
Paniccia 2015
- Paniccia A, Schulick RD, Edil BH. Total laparoscopic pancreaticoduodenectomy: A single‐institutional experience. Annals of Surgical Oncology 2015;22(13):4380‐1. [DOI] [PubMed] [Google Scholar]
Peng 2003
- Peng S, Mou Y, Su Y, Peng C, Cai X, Wu Y, et al. Binding pancreaticojejunostomy: 150 consecutive cases without leakage. Gastrointestinal Surgery 2003;7(2):297. [DOI] [PubMed] [Google Scholar]
Piedimonte 2015
- Piedimonte S, Wang Y, Bergman S, Vanounou T. Early experience with robotic pancreatic surgery in a Canadian institution. Canadian Journal of Surgery 2015;58(6):394‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pratt 2008
- Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World Journal of Surgery 2008;32(3):419‐28. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Satoi 2006
- Satoi S, Takai S, Matsui Y, Terakawa N, Iwaki R, Fukui J, et al. Less morbidity after pancreaticoduodenectomy of patients with pancreatic cancer. Pancreas 2006;33(1):45‐52. [DOI] [PubMed] [Google Scholar]
Satoi 2008
- Satoi S, Toyokawa H, Yanagimoto H, Yamamoto J, Kim S, Matsui Y, et al. A new guideline to reduce postoperative morbidity after pancreaticoduodenectomy. Pancreas 2008;37(2):128‐33. [DOI] [PubMed] [Google Scholar]
Schmidt 2004
- Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20‐year experience in 516 patients. Archives of Surgery 2004;139(7):718‐27. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1953
- Smith EB. Hemorrhagic ascites and hemothorax associated with benign pancreatic disease. Archives of Surgery 1953;67(1):52‐6. [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang M, Zhang H, Wu Z, Zhang Z, Peng B. Laparoscopic pancreaticoduodenectomy: single‐surgeon experience. Surgical Endoscopy 2015;29(12):3783‐94. [DOI] [PubMed] [Google Scholar]
Wente 2007
- Wente MN, Shrikhande SV, Müller MW, Diener MK, Seiler CM, Friess H, et al. Pancreaticojejunostomy versus pancreaticogastrostomy: systematic review and meta‐analysis. American Journal of Surgery 2007;193(2):171‐83. [DOI] [PubMed] [Google Scholar]
Xiong 2012
- Xiong JJ, Altaf K, Mukherjee R, Huang W, Hu WM, Li A, et al. Systematic review and meta‐analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. British Journal of Surgery 2012;99(8):1050‐61. [DOI] [PubMed] [Google Scholar]
Yasui 1988
- Yasui A, Nimura Y, Hayakawa N, Hayakawa T, Shibata T, Kondo T, et al. Feedback regulation of basal pancreatic secretion in humans. Pancreas 1988;3(6):681‐7. [DOI] [PubMed] [Google Scholar]
Yeo 1997
- Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s. Annals of Surgery 1997;226(3):248‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
You 2009
- You D, Jung K, Lee H, Heo J, Choi S, Choi D. Comparison of different pancreatic anastomosis techniques using the definitions of the International Study Group of Pancreatic Surgery: a single surgeon’s experience. Pancreas 2009;38:896‐902. [DOI] [PubMed] [Google Scholar]
Zeng 2008
- Zeng Q, Zhang Q, Han S, Yu Z, Zheng M, Zhou M, et al. Efficacy of somatostatin and its analogues in prevention of postoperative complications after pancreaticoduodenectomy: A meta‐analysis of randomized controlled trials. Pancreas 2008;36(1):18‐25. [DOI] [PubMed] [Google Scholar]
Zhou 2011
- Zhou Y, Yang C, Wang S, Chen J, Li B. Does external pancreatic duct stent decrease pancreatic fistula rate after pancreatic resection?: a meta‐analysis. Pancreatology 2011;11(3):362‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dong 2013
- Dong Z, Xu J, Wang Z, Petrov MS. Stents for the prevention of pancreatic fistula following pancreaticoduodenectomy. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008914.pub2] [DOI] [PubMed] [Google Scholar]


