Abstract
Background
Keloid and hypertrophic scars are common and are caused by a proliferation of dermal tissue following skin injury. They cause functional and psychological problems for patients, and their management can be difficult. The use of silicone gel sheeting to prevent and treat hypertrophic scarring is still relatively new and started in 1981 with treatment of burn scars.
Objectives
To determine the effectiveness of silicone gel sheeting for: (1) prevention of hypertrophic or keloid scarring in people with newly healed wounds (e.g. post surgery); (2) treatment of established scarring in people with existing keloid or hypertrophic scars.
Search methods
In May 2013 we searched the Cochrane Wounds Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations); Ovid EMBASE; and EBSCO CINAHL for this second update.
Selection criteria
Any randomised or quasi‐randomised controlled trials, or controlled clinical trials, comparing silicone gel sheeting for prevention or treatment of hypertrophic or keloid scars with any other non surgical treatment, no treatment or placebo.
Data collection and analysis
We assessed all relevant trials for methodological quality. Three review authors extracted data independently using a standardised form and cross‐checked the results. We assessed all trials meeting the selection criteria for methodological quality.
Main results
We included 20 trials involving 873 people, ranging in age from 1.5 to 81 years. The trials compared adhesive silicone gel sheeting with no treatment; non silicone dressing; other silicone products; laser therapy; triamcinolone acetonide injection; topical onion extract and pressure therapy. In the prevention studies, when compared with a no treatment option, whilst silicone gel sheeting reduced the incidence of hypertrophic scarring in people prone to scarring (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.21 to 0.98) these studies were highly susceptible to bias. In treatment studies, silicone gel sheeting produced a statistically significant reduction in scar thickness (mean difference (MD) ‐2.00, 95% CI ‐2.14 to ‐1.85) and colour amelioration (RR 3.49, 95% CI 1.97 to 6.15) but again these studies were highly susceptible to bias.
Authors' conclusions
There is weak evidence of a benefit of silicone gel sheeting as a prevention for abnormal scarring in high‐risk individuals but the poor quality of research means a great deal of uncertainty prevails. Trials evaluating silicone gel sheeting as a treatment for hypertrophic and keloid scarring showed improvements in scar thickness and scar colour but are of poor quality and highly susceptible to bias.
Plain language summary
Silicone gel sheeting for preventing the development of hypertrophic and keloid scars and for treating existing hypertrophic and keloid scars
As a wound heals, a scar can develop. Sometimes scars can develop abnormally, forming hypertrophic or keloid scars which are raised, unsightly and can cause both emotional problems and issues with movement for the people in which they develop. These types of scar are difficult to treat.
Keloid scarring is more common in darker skin and occurs after minor injuries such as insect bites, ear piercing and vaccinations. Keloid scars can also spread to the skin surrounding the injured area. Hypertrophic scarring is more common in lighter skin and is usually confined to the area injured. Hypertrophic scarring tends to follow surgery or burns. Hypertrophic and keloid scars are more likely to develop if the injury is on certain sites of the body, for example the lower face, neck and upper arms.
Silicone gel sheeting is a soft, self‐adhesive sheet that is applied to intact skin. It is thought to prevent the development of new abnormal scars and also to treat existing scars. This review aimed to assess the evidence on whether silicone gel sheeting prevents the development of abnormal scars in people with newly healed wounds or if it is an effective way to treat existing abnormal scars. Most of the studies identified were of poor quality and it is unclear whether silicone gel sheeting helps prevent abnormal scarring, or is effective in treating existing abnormal scars.
Background
Wounds, such as burns, surgical incisions and ulcers, are repaired through the deposition of components that form new skin. These components include blood vessels, nerves, elastin fibres (which give the skin some elasticity) and collagen fibres (for tensile strength), as well as glycosaminoglycans (GAGS) which form the gel‐like ground substance (or matrix) in which the structural fibres, nerves and blood vessels are embedded. In the early stages of healing, a cicatrix is formed. The cicatrix consists of a thin layer of skin (the pellicle) that covers the wound and subsequently contracts and becomes paler in colour, forming the scar.
Some scars develop abnormally, giving rise to keloid and hypertrophic scars. The scars arise from an excessive proliferation of dermal tissue following skin injury, with keloid scars developing in 5% to 15% of wounds (Wittenberg 1999). This proliferation of dermal tissue is due to both the production of fibrous tissue (fibroplasias) and the accumulation of abundant and randomly organised new collagen bundles.
O'Sullivan 1996 observed that although the terms 'keloid' and 'hypertrophic' have often been used synonymously, the two sorts of scarring are, in fact, significantly different. The principle clinical feature that distinguishes them is that in keloid scars the scar tissue progressively encroaches upon the normal skin surrounding it, producing a scar that appears irregular and pendulous in areas. Conversely, the hypertrophic scar is confined to the tissue damaged by the original injury. This type of scar increases in dimension by pushing out its margins, rather than invading surrounding tissue. Clinicians usually base diagnosis of keloid scarring on the overgrown boundaries and delayed onset of the scar (hypertrophic scars develop soon after injury) (Shaffer 2002).
Keloid scarring is reported to be more common in darker skin (Beers 1999; Niessen 1998), while hypertrophic scarring is more common in fair skin (Beers 1999). Examination of scars with an electron microscope shows keloid collagen to be thin and irregular with cross‐striations, suggesting immaturity, while keloid scars are deficient in lymphatics and their associated elastic fibres, and have a higher content of both water and soluble collagen than normal skin. Although hypertrophic scars have similar qualities in the early stages, after seven months the two become distinct as the water and collagen content of hypertrophic scars normalises (Raney 1993).
Hypertrophic scars tend to follow surgery and thermal injuries such as severe burns (Carney 1993; Eisenbeiss 1998; Shakespeare 1993), whereas keloid scars often originate after trivial injury such as ear piercing, insect bites and vaccination. The amount of scar tissue in a keloid scar exhibits little relation to the extent of the injury that caused it (O'Sullivan 1996).
Both types of scarring can cause functional and psychological problems for people, and their management can be difficult. Treatment options have included surgery, radiation therapy, steroid injections, pressure therapy, cryotherapy (treatment with liquid nitrogen) and laser therapy (Shaffer 2002). Many surgical techniques have been applied to remove keloids, either alone, or in combination with other treatments. Surgery alone has shown a high recurrence rate (Raney 1993).
Scars in specific sites of the body, including the lower face, presternum, pectoral area of the chest, upper back, ears, neck and outer (deltoid) area of the upper arms are more likely to develop abnormally (O'Sullivan 1996). People with scars in these high‐risk anatomical areas, or with a history of forming keloid scars, aim to prevent further scarring by observing certain principles that include: avoiding non essential cosmetic surgery, closing all wounds with minimal tension, and using pressure garments for four to six months after injury or surgery (O'Sullivan 1996).
The use of topical silicone for prevention and treatment of hypertrophic scarring is still relatively new. Silicone was first used, in gel form, for the treatment of burn scars at Australia's Adelaide Children's Hospital in 1981 (Perkins 1982). Silicone has since been produced in various forms, including: silicone cream compounds (Sawada 1992); silicone oil or gel with additives such as vitamin E (Palmieri 1995); in combination with other dressing media (Davey 1991); and as custom‐made silicone applications. This particular review is solely concerned with commercially produced adhesive silicone gel sheeting.
Silicone gel sheeting is a soft, self adhesive and semi‐occlusive sheet used for the treatment and prevention of both old and new hypertrophic and keloid scars. It is made from medical‐grade silicone (cross‐linked polydimethylsiloxane polymer) and reinforced with a silicone membrane backing (Katz 1992; Thomas 1997) thought to give it increased durability and make handling easier (Williams 1996).
Silicone gel sheeting is designed to be used on intact skin. It should not be used on open wounds and, according to the product information sheet supplied by the manufacturers (Smith & Nephew 2000), is contraindicated in people with dermatological conditions that disrupt the integrity of the skin (for example, severe acne or psoriasis).
The mode of action of silicone‐based products on scar tissue is unknown. Some researchers suggested that silicone may penetrate the skin, but studies by Ahn 1989 and Swanson 1974 found no evidence of silicone in the scar or stratum corneum. Quinn 1985 found that there was no significant difference in pressures obtained at the scar surface beneath the gel, and also concluded that there was no difference in scar surface temperature and oxygen tension, or water vapour transmissivity of the gel.
The cost of silicone gel sheeting (AUD 139 (Australian Dollars) recommended retail price for a 12 x 15 cm sheet, AUD 74 for a 12 x 6 cm sheet) may be moderated by the fact that, after rinsing, it can be reused by the patient or their carer. However, the fact remains that clinicians and funders of care will require clear evidence of its clinical effectiveness before recommending its use.
Objectives
The aim of this systematic review was to determine the effects of silicone gel sheeting in the:
prevention of hypertrophic or keloid scarring in people with newly healed wounds (e.g. post surgery); and
treatment of established scarring in people with keloid or hypertrophic scars after any type of wound.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled trials (RCTs) or quasi‐randomised controlled trials (QRCTs) (method for allocating participants to a treatment that is not strictly random, e.g. by date of birth, hospital record number, alternation) or controlled clinical trials (CCTs) (where an intervention group is compared to a comparison or control group) of interventions.
Types of participants
People with healed full‐thickness wounding (from any cause) where the skin was intact, with or without scarring at baseline.
Types of interventions
All comparisons of silicone gel sheeting with other conservative techniques (e.g. hydrocolloid dressings, non silicone gel sheeting, laser therapy or no intervention) were eligible.
We excluded comparisons of silicone gel sheeting with surgery. We excluded trials that reported only the absorption of silicone by the skin, but did not measure the effect on scar appearance.
Types of outcome measures
Primary outcomes
Prevention studies
The primary outcome measure was the number of people who developed keloid or hypertrophic scarring as determined by blood flow, hyperpigmentation, erythema (redness), scar thickness and regularity of scar.
Treatment studies
The primary measure was change in scar size (measured by area, length, volume, height, or width ‐ usually by ruler, taking an impression, or ultrasound).
Secondary outcomes
Prevention studies
Other measures of clinical outcome:
scar size (measured by area, length, volume, height, or width ‐ usually by ruler, taking an impression, or ultrasound);
scar colour (measured against standard colour charts), blood flow (measured using laser‐Doppler flowmetry) and scar appearance (measured on a three or five‐point scale with appropriate definitions);
skin elasticity (measured serially with the use of an elastometer);
development of complications (e.g. rashes, skin breakdown, measured on a numbered scale);
cosmetic appearance (cosmesis) as defined by patient opinion (using assessment scales) and physician observations;
patient tolerance, measured by reported side effects and adverse reactions;
preference for different modes of treatment, measured by patient choice after receiving at least two different types of treatment;
compliance, measured by physician and patient report.
Treatment studies
Other measures of clinical outcome:
scar colour (measured against standard colour charts), blood flow (measured using laser‐Doppler flowmetry) and scar appearance (measured on a three or five‐point scale with appropriate definitions);
skin elasticity (measured serially with the use of an elastometer);
development of complications (e.g. rashes, skin breakdown, measured on a numbered scale);
cosmesis as defined by patient opinion (using assessment scales) and physician observations;
patient tolerance, measured by reported side effects and adverse reactions;
preference for different modes of treatment measured by patient choice after receiving at least two different types of treatment;
compliance, measured by physician and patient report.
Search methods for identification of studies
For the search strategy for the first update of this review see Appendix 1.
Electronic searches
For this second update we searched the following databases in May 2013:
The Cochrane Wounds Group Specialised Register (searched 8 May 2013);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 4);
Ovid MEDLINE (2007 to April Week 4 2013);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations, May 07 2013);
Ovid EMBASE (2007 to 2013 Week 18);
EBSCO CINAHL (2007 to 3 May 2013)
We used the following search strategy to search CENTRAL:
#1 MeSH descriptor: [Keloid] explode all trees 61 #2 MeSH descriptor: [Cicatrix, Hypertrophic] explode all trees 84 #3 MeSH descriptor: [Hypertrophy] explode all trees 1125 #4 keloid* or hypertrophic or cicatrix 1030 #5 scar or scars or scarred or scarring 2163 #6 #1 or #2 or #3 or #4 or #5 3712 #7 MeSH descriptor: [Silicone Gels] explode all trees 34 #8 silicone next gel* 89 #9 silicone next sheet* 17 #10 silicone next dressing* 18 #11 #7 or #8 or #9 or #10 114 #12 #6 and #11 59
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2012). There were no restrictions with respect to language, date of publication or study setting.
Searching other resources
We examined the reference lists of relevant review articles and all included studies to identify further studies. We approached the major supplier of silicone gel sheeting (Smith and Nephew) for details of unpublished, ongoing and recently published trials. The search was not limited by language or publication status.
Data collection and analysis
Selection of studies
Two review authors (LOB, DJ) assessed the title and abstracts of potentially eligible trials independently. The review authors obtained papers that were potentially relevant and, using eligibility criteria, assessed their full text for inclusion independently. We resolved disagreements by discussion.
Data extraction and management
One review author extracted data and a second review author checked for accuracy. We used a standard data form to capture the following information:
characteristics of the study (design, method of randomisation, withdrawals/dropouts, funding source);
study participants (age, wound location, wound characteristics, scar type);
intervention (silicone gel, non silicone gel);
comparison intervention (e.g. laser therapy, compression, occlusive dressing);
duration of treatment;
outcome measures (type of scoring, timing of assessment, complications);
duration of follow‐up; and
results.
We requested additional unpublished data from primary authors and included these when available.
Assessment of risk of bias in included studies
Only RCTs, QRCTs or CCTs were included in this review because of the increased risk of bias with other types of study. Two review authors independently assessed the methodological quality of the included studies using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011) and any disagreement was discussed amongst all review authors to achieve a consensus. The 'Risk of bias' tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 3 for details of criteria on which the judgement was based). We assessed blinding and completeness of outcome data for each outcome separately, and completed a 'Risk of bias' table for each eligible study.
We have presented our assessment of risk of bias using a 'Risk of bias' summary figure (Figure 1), which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
1.
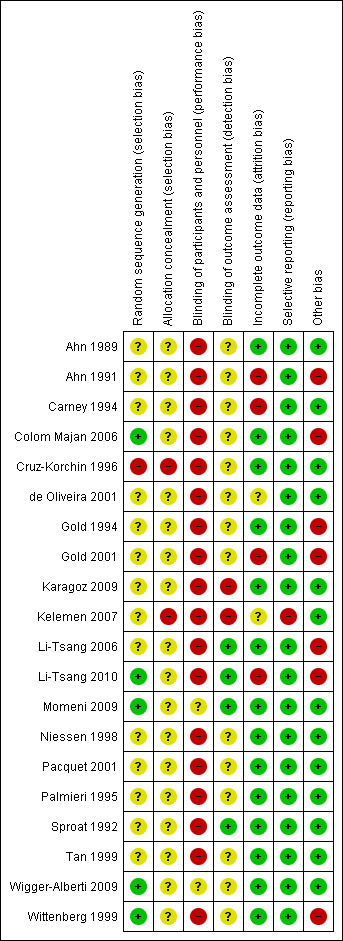
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Assessment of heterogeneity
We explored clinical heterogeneity by examining potentially influential factors such as age of people, cause of scar (e.g. if from recent surgery) and age of scar before treatment commenced. When statistical pooling was done, we tested for statistical heterogeneity using the Chi² test. If clinical heterogeneity was suspected, we combined the studies by narrative summary only. In the presence of statistical heterogeneity (i.e. when the Chi² was greater than degrees of freedom) but where other factors suggest pooling was appropriate, we used a random‐effects model. Otherwise we used a fixed‐effect model.
Data synthesis
The comparisons are as follows.
silicone gel sheeting compared with no treatment.
silicone gel sheeting compared with non silicone dressing.
silicone gel sheeting compared with other silicone products.
silicone gel sheeting compared with laser therapy.
silicone gel sheeting compared with triamcinolone acetonide injection treatment.
silicone gel sheeting compared with topical onion extract.
silicone gel sheeting compared with pressure therapy.
Data for prevention (i.e. for newly healed scars) and treatment (i.e. for existing keloid or hypertrophic scars) have been dealt with separately.
The analysis tables contain quantitative data from individual trial reports for prespecified outcomes and subgroups (e.g. those with a high risk of abnormal scarring versus normal population) for both dichotomous and continuous outcomes.
A narrative summary of results is presented. Results of dichotomous variables are presented as risk ratio (RR) with 95% confidence intervals (CI). We have used risk ratio rather than odds ratio, as event rates are high in these trials and odds ratios would give an inflated impression of the magnitude of effect. In addition, we have carried out statistical pooling on groups of studies which we considered to be sufficiently similar.
Results
Description of studies
Searches for this second update identified 13 potentially relevant articles. Independent scrutiny of the titles and abstracts by both review authors identified five new studies that met the inclusion criteria, bringing the total number of included studies to 20. Reasons for excluding the other studies can be found in the Characteristics of excluded studies.
We contacted 11 authors for additional trial data and five (de Oliveira 2001; Niessen 1998; Li‐Tsang 2006; Li‐Tsang 2010; Niessen 1998; Wigger‐Alberti 2009) kindly supplied these. We contacted the manufacturers of silicone gel sheeting (Smith & Nephew) and they supplied a categorised table of clinical trials conducted for key scar therapies. We checked this against the studies already sourced through the search strategy and ordered any papers not already considered, then subjected them to the same eligibility criteria as the other trials to determine whether they should be included. No further trials were identified from this source.
All 20 included trials compared silicone gel sheeting with either a control or another treatment. The studies were mainly single‐centre studies, although one included data from four hospitals (Niessen 1998). The studies were conducted in eight countries, with most being conducted in either North America (seven studies) or in Europe (eight studies). Where there were multiple trials for the same first author, we inspected for independence of study populations and found that that all were separate groups of participants. Prospective trial registration with unique trial numbering would help avoid duplication in systematic reviews.
The 20 included studies involved a total of 849 people aged between 1.5 to 81 years. The 'Characteristics of included studies' table provides details of individual studies. No age limits were explicitly applied, however where information was provided, most participants were adult.
In three studies (de Oliveira 2001; Gold 1994; Niessen 1998) a distinction was made between keloid and hypertrophic scarring and the results were discussed separately.
The trials made the following comparisons based on the objectives (i.e. to determine the effectiveness of silicone gel sheeting in preventing and treating hypertrophic and keloid scars):
(1) Silicone gel sheeting compared with no treatment
There were three prevention studies (Cruz‐Korchin 1996; Gold 2001; Niessen 1998) involving 245 people. There were eight treatment studies (Ahn 1989; Carney 1994; Colom Majan 2006; de Oliveira 2001; Li‐Tsang 2006; Li‐Tsang 2010; Tan 1999; Wittenberg 1999) involving 219 people. There were two studies that evaluated both prevention and treatment (Ahn 1991; Gold 1994) involving 82 people.
(2) Silicone gel sheeting compared with non silicone dressing
There were three treatment studies (de Oliveira 2001; Momeni 2009; Wigger‐Alberti 2009) involving 124 people.
(3) Silicone gel sheeting compared with other silicone products
There was one prevention study (Niessen 1998) involving 129 people. There were three treatment studies (Carney 1994; Karagoz 2009; Palmieri 1995) involving 152 people.
(4) Silicone gel sheeting compared with laser therapy
There were two treatment studies (Pacquet 2001; Wittenberg 1999) involving 40 people.
(5) Silicone gel sheeting compared with triamcinolone acetonide injection treatment
There were three treatment studies (Kelemen 2007; Sproat 1992; Tan 1999) involving 58 people.
(6) Silicone gel sheeting compared with topical onion extract
There was one treatment study (Karagoz 2009) involving 30 people.
(7) Silicone gel sheeting compared with pressure therapy
There was one treatment study (Li‐Tsang 2010) involving 54 people.
There were many different measurement techniques and tools used, which made pooling of results difficult. The comparability of people at baseline was generally good, although one study (Pacquet 2001) provided no information on the control group, making it impossible to judge whether those groups were comparable. Most studies were also explicit about their inclusion and exclusion criteria, which allowed a clearer definition of the study population.
In most trials silicone gel sheeting was applied for at least 12 hours per day, with five studies (de Oliveira 2001; Li‐Tsang 2006; Li‐Tsang 2010; Momeni 2009; Niessen 1998) specifying 24 hours per day, another (Carney 1994) stating "as many hours per day as possible", and a third (Palmieri 1995) specifying 10 hours per day. However, two studies (Pacquet 2001; Wigger‐Alberti 2009) did not indicate the number of hours that the silicone gel sheeting was worn by participants. One study (Niessen 1998) changed the type of silicone gel sheeting used (from Sil‐K to Epiderm which is more adhesive) when the initial results from the first group of people (n = 80) were described by the authors as "disappointing". Another study (Carney 1994) also used two different types of gel (Silastic Gel Sheeting and Cica‐Care) and analysed the treatment subgroups separately.
Descriptions and definitions of the type of scar (hypertrophic versus keloid) were adequate in 13 out of the 20 studies. Despite not giving a full description of the distinction between hypertrophic and keloid scars, de Oliveira 2001 classified their participants' scars as either one or the other, and separated the scar types in their analysis. Gold 2001 compared high‐risk (i.e. those with a history of abnormal scarring) and low‐risk participant groups in their results. Most other studies combined hypertrophic and keloid scars in their analyses, raising questions about the appropriateness of the study design (Shaffer 2002).
Given the long‐term process of remodeling and scarring, it is recommended that follow‐up continues for at least one year (Shaffer 2002). Only three studies (Carney 1994; Colom Majan 2006; Niessen 1998) had follow up of 12 months. Six studies (Ahn 1989; Gold 1994; Palmieri 1995; Sproat 1992; Tan 1999; Wigger‐Alberti 2009) followed people for three months or less, which is clearly inadequate.
Risk of bias in included studies
The quality of trial methodology varied widely. The results for individual trials are presented in the 'Characteristics of included studies' table. Overall, the quality of the trial methodology in the included studies was poor. We judged only two studies (Momeni 2009; Wigger‐Alberti 2009) to be at overall low risk of bias.
Allocation
Random sequence generation
Five studies (Colom Majan 2006; Li‐Tsang 2010; Momeni 2009; Wigger‐Alberti 2009; Wittenberg 1999) explicitly reported their method of generating the randomisation sequence and we judged them to be at low risk of selection bias. Colom Majan 2006, , Wigger‐Alberti 2009 and Wittenberg 1999 used a computer‐generated randomisation list; Sproat 1992 used a prescribed randomised sequence; Li‐Tsang 2010 used the drawing of lots for randomisation; Momeni 2009 used a random number table. One study (Cruz‐Korchin 1996) reported an inadequate method of randomisation and we judged this to be at high risk of selection bias. Cruz‐Korchin 1996 allocated treatment based on the patient's dominant hand. The remainder did not describe their methods and we judged them at unclear risk of selection bias.
Allocation concealment
One study (Cruz‐Korchin 1996) did not conceal allocation (they used the dominant or non dominant hand of the participant to decide where the material was placed) and we judged this to be at high risk of bias. The remaining studies did not report allocation concealment and were judged to be at unclear risk of bias.
Blinding
Blinding of participants and personnel
We judged two studies (Momeni 2009; Wigger‐Alberti 2009) to have unclear risk of performance bias. One study (Momeni 2009) attempted to blind participants only by using placebo gel sheets on one‐ half of the scar but did not attempt to blind research personnel. The other study (Wigger‐Alberti 2009) blinded research personnel by using an independent nurse to apply and remove all dressings in the investigators' absence. We judged the remaining studies to be at high risk of performance bias as they were unable to blind either participants or personnel due to the nature of the intervention.
Blinding of outcome assessor
We judged four studies (Li‐Tsang 2006; Li‐Tsang 2010; Momeni 2009; Sproat 1992) to have adequately blinded outcome assessors. Two studies (Li‐Tsang 2006; Li‐Tsang 2010) used an independent research assistant to judge the outcome; Momeni 2009 used an independent plastic surgeon; Sproat 1992 used photographs showed to five blinded observers and trained a 'blindfolded observer' to undertake measurements. We judged these four studies to be at low risk of detection bias. One study (Karagoz 2009) used the same outcome assessor at the beginning and end of the treatment and was not blinded. We judged this study was judged to be at high risk of detection bias. The remaining studies did not comment on blinding of outcome assessment and were judged to be at unclear risk of detection bias.
Incomplete outcome data
We judged fifteen15 studies (Ahn 1989; Colom Majan 2006; Cruz‐Korchin 1996; Gold 1994; Karagoz 2009; Kelemen 2007; Li‐Tsang 2006; Momeni 2009; Niessen 1998; Pacquet 2001; Palmieri 1995; Sproat 1992; Tan 1999; Wigger‐Alberti 2009; Wittenberg 1999) to be at low risk of attrition bias. In each of these studies the numbers lost to follow up were low and adequate reasons were given for these losses. We judged the remaining studies to be at high risk of attrition bias. Three studies (Ahn 1991; Carney 1994; Gold 2001) had a loss to follow up greater than 20%. One study (Li‐Tsang 2010) reported moderately high losses but was judged to be at high risk of bias because two thirds of drop outs came from the control group. One study (de Oliveira 2001) did not comment on attrition bias nor can it be ascertained from the data. We judged this study to be at unclear risk of attrition bias.
Clear statements of evidence of intention‐to‐treat (ITT) analysis were rarely presented in trial reports, and only two studies (Wigger‐Alberti 2009; Wittenberg 1999) performed an ITT analysis.
Selective reporting
We judged one study (Kelemen 2007) to be at high risk of reporting bias, as only one "interesting" case from each group was reported, and patient ratings were not reported at all. We assessed all other studies in the review were assessed as having low risk of reporting bias. Although study protocols were not sought, all outcomes mentioned in the methods were reported in the results and clinically meaningful outcomes presented.
Other potential sources of bias
We judged seven studies (Ahn 1991; Colom Majan 2006; Gold 1994; Gold 2001; Li‐Tsang 2006; Li‐Tsang 2010; Wittenberg 1999) to be at high risk of bias due to the influence of companies supplying the silicone gel sheeting. Two studies (Ahn 1991; Gold 1994) reported receiving grant money from the companies supplying the silicone gel sheeting. Two studies (Colom Majan 2006; Gold 2001) stated that the research was supported by the company supplying the silicone gel but gave no further information. In the remaining three studies the company donated the silicone gel sheeting (Li‐Tsang 2006; Li‐Tsang 2010; Wittenberg 1999). The remaining studies appear free from other sources of bias.
Effects of interventions
Where available quantitative data are presented in the analysis tables.
How the results are presented and what the terms mean
Results of dichotomous variables are presented as risk ratio (RR) with 95% confidence intervals (CI). Risk ratio has been used rather than odds ratio as event rates are high in these trials and odd ratios would give an inflated impression of the magnitude of effect. Where statistically significant heterogeneity existed (i.e. the Chi² was greater than degrees of freedom) we used a random‐effects model.
The types of outcomes measured in the studies are listed in the 'Characteristics of included studies' table. The primary outcome measure for prevention studies was the proportion of people who developed abnormal scarring in postoperative cases (measured in terms of blood flow, hyperpigmentation, erythema, thickness and regularity of scar). There were many different measurement techniques and tools used, making pooling of results difficult.
Comparison: silicone gel compared with no treatment
There were 13 studies (Ahn 1989; Ahn 1991; Carney 1994; Colom Majan 2006; Cruz‐Korchin 1996; de Oliveira 2001; Gold 1994; Gold 2001; Li‐Tsang 2006; Li‐Tsang 2010; Niessen 1998; Tan 1999; Wittenberg 1999) in this category. Three of the studies (Cruz‐Korchin 1996; Gold 2001; Niessen 1998) studied the prevention of scars for people undergoing surgery, eight studied the effect of silicone gel sheeting on existing hypertrophic or keloid scars (Ahn 1989; Carney 1994; Colom Majan 2006; de Oliveira 2001; Li‐Tsang 2006; Li‐Tsang 2010; Tan 1999; Wittenberg 1999) and two studies (Ahn 1991; Gold 1994) included both prevention and treatment.
I: Prevention studies
Of the five trials that compared silicone gel sheet with no treatment for prevention of scarring, four (Ahn 1991; Cruz‐Korchin 1996; Gold 2001; Niessen 1998) included people with healed surgical wounds, and one (Gold 1994) included people who had had keloid scars removed with CO2 laser. Two of the trials described people according to their risk of developing abnormal scarring ‐ Gold 1994 only recruited 'high‐risk' people, while Gold 2001 recruited 'low' and 'high' risk people and presented the results of these two groups separately.
Primary outcome: development of keloid or hypertrophic scarring
Cruz‐Korchin 1996 reported that fewer incisions treated with silicone gel sheeting became hypertrophic, though this difference was not significant(risk ratio (RR) 0.45, 95% confidence interval (CI) 0.19 to 1.07). Individually, two small trials (Gold 1994; Gold 2001) found no significant difference between the silicone gel sheeting and the control groups in terms of abnormal scarring in high‐risk individuals only (people who were prone to scarring), but when pooled (random‐effects) we found that silicone gel sheeting was associated with significantly fewer abnormal scars(RR 0.46, 95% CI 0.21 to 0.98). Ahn 1991 found significantly fewer abnormal scars in people treated with silicone gel sheeting (RR 0.05, 95% CI 0 to 0.76), whilst Niessen 1998 found a significant difference in favour of the control group(RR 2.71, 95% CI 1.19 to 6.22). When all five trials were pooled (random‐effects, I² = 69%) there was no significant difference in the number of people developing abnormal scars (RR 0.55, 95% CI 0.21 to 1.45) (Analysis 1.1). All these trials are susceptible to bias as they did not describe allocation concealment, blinding of outcome assessors or an intention‐to‐treat (ITT) analysis.
1.1. Analysis.
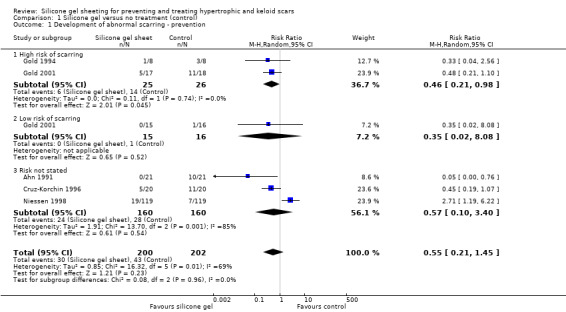
Comparison 1 Silicone gel versus no treatment (control), Outcome 1 Development of abnormal scarring ‐ prevention.
Secondary outcomes
Cruz‐Korchin 1996 reported transient rash and minor skin maceration as complications, but there was no statistically significant difference between the groups. Niessen 1998 reported transient rash, which resolved on removal of the silicone gel sheeting. Pooling these studies (fixed‐effect, I² = 0%) demonstrated a statistically significant difference in favour of the control groups. This means that more complications developed in the groups treated with silicone gel (RR 8.00, 95% CI 1.02 to 62.83) (Analysis 1.2).
1.2. Analysis.
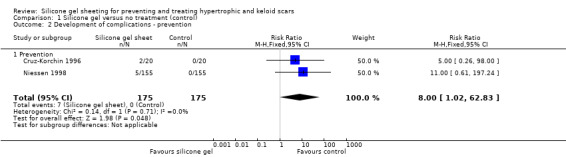
Comparison 1 Silicone gel versus no treatment (control), Outcome 2 Development of complications ‐ prevention.
II: Treatment studies
Ten trials compared silicone gel sheeting with control for treating abnormal scarring (Ahn 1989; Ahn 1991; Carney 1994; Colom Majan 2006; de Oliveira 2001; Gold 1994; Li‐Tsang 2006; Li‐Tsang 2010; Tan 1999; Wittenberg 1999). The majority of control groups were untreated. In two studies the control group received lanolin and massage. Six trials included people with hypertrophic scars resulting from thermal burns (Carney 1994; Gold 1994; Li‐Tsang 2006; Li‐Tsang 2010) or surgery (Colom Majan 2006; Wittenberg 1999). Three (Ahn 1991; Ahn 1989; de Oliveira 2001) included people with hypertrophic and keloid scarring, and one (Tan 1999) only included people with keloid scarring.
Primary outcome
As the studies used different outcome measures it was impossible to pool results. We examined outcomes of reduction of scar length and width (de Oliveira 2001), scar thickness (Li‐Tsang 2006; Li‐Tsang 2010) and reduction in scar size by 50% (Tan 1999). The studies found no significant difference between silicone gel sheeting and control for reduction in scar length, width and reduction of size by 50% (Analysis 1.3; Analysis 1.4; Analysis 1.7) but significant results for scar thickness favouring silicone gel (RR ‐2.00, 95% CI ‐2.14 to ‐1.85) (Analysis 1.5) although this was only two studies (Li‐Tsang 2006; Li‐Tsang 2010) with relatively small numbers (N = 77).
1.3. Analysis.
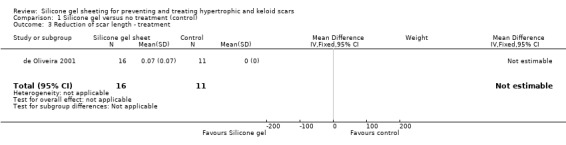
Comparison 1 Silicone gel versus no treatment (control), Outcome 3 Reduction of scar length ‐ treatment.
1.4. Analysis.
Comparison 1 Silicone gel versus no treatment (control), Outcome 4 Reduction in scar width ‐ treatment.
1.7. Analysis.
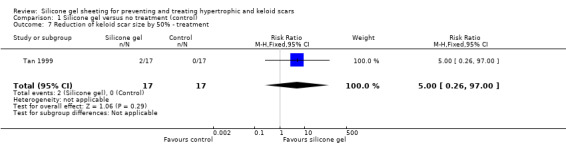
Comparison 1 Silicone gel versus no treatment (control), Outcome 7 Reduction of keloid scar size by 50% ‐ treatment.
1.5. Analysis.
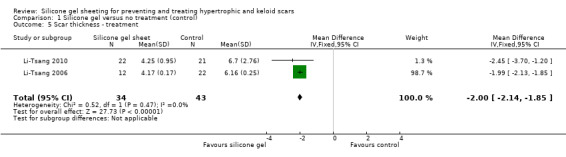
Comparison 1 Silicone gel versus no treatment (control), Outcome 5 Scar thickness ‐ treatment.
Secondary outcomes
All studies except Wittenberg reported secondary outcomes. There were no statistically significant differences between the treatment or control groups for improvements in scar appearance, scar colour and the relief of itching and pain.
Five studies (Colom Majan 2006; de Oliveira 2001; Li‐Tsang 2006; Tan 1999de Oliveira 2001Li‐Tsang 2010; Tan 1999) showed a statistically significant amelioration of scar colour (defined as a significant improvement in erythema) with silicone gel (pooled RR 3.49, 95% CI 1.97 to 6.15, fixed‐effect, I² = 53%) (Analysis 1.8). When a random‐effects model is applied this result is still statistically significant. It should be noted, however, that with the exception of Li‐Tsang 2010 who used spectrocolorimetry, this is a subjective outcome and only two studies (de Oliveira 2001; Li‐Tsang 2010) masked the outcome assessor. Also, only Li‐Tsang 2010 reported the method of randomisation and no studies had adequate allocation concealment.
1.8. Analysis.
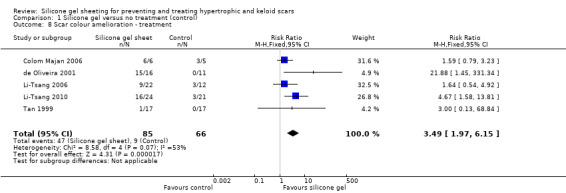
Comparison 1 Silicone gel versus no treatment (control), Outcome 8 Scar colour amelioration ‐ treatment.
Four studies (Ahn 1989; Ahn 1991; Carney 1994; Li‐Tsang 2006) reported a statistically significant improvement in scar elasticity in those people treated with silicone gel sheeting. Data were presented graphically (mean percentage of stretch and standard error of mean in Ahn 1989 and Ahn 1991; percentage of extensibility of scar in Carney 1994; mean only in Li‐Tsang 2006) with P values, but actual measurement data were not reported. We requested further information from trial authors, with two replies (Li‐Tsang 2006; Li‐Tsang 2010) resulting in new data. We treated reported data as dichotomous (i.e. improvement in elasticity compared with no improvement) and due to the high heterogeneity likely caused by the different measurement methods (I² = 55%), pooled using a random‐effects model resulting in a statistically significant improvement in scar elasticity (RR 3.03, 95% CI 1.02 to 8.99) (Analysis 1.9).
1.9. Analysis.
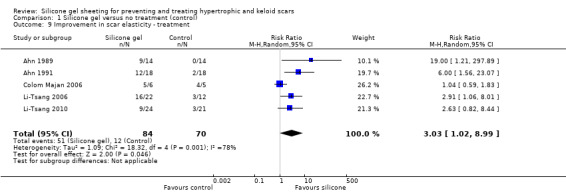
Comparison 1 Silicone gel versus no treatment (control), Outcome 9 Improvement in scar elasticity ‐ treatment.
Results for relief of pain and itch (Li‐Tsang 2006; Li‐Tsang 2010; Tan 1999) showed no statistically significant difference between the groups (Analysis 1.10).
1.10. Analysis.
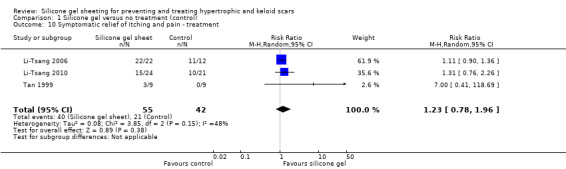
Comparison 1 Silicone gel versus no treatment (control), Outcome 10 Symptomatic relief of itching and pain ‐ treatment.
Three studies (Ahn 1989; Carney 1994; Colom Majan 2006) reported complications such as transient skin rashes, pruritis, itching or superficial maceration. Authors reported that these resolved promptly when the silicone gel sheeting was withdrawn, or when correct hygiene was practised. Combining results from Ahn 1989 and Colom Majan 2006 we found statistically significantly more complications reported for silicone gel sheeting than in the control group (RR 9.52, 95% CI 1.35 to 67.10, fixed‐effect, I² = 0%) (Analysis 1.11). No raw data were reported by Carney and email communication with the author did not produce further data.
1.11. Analysis.
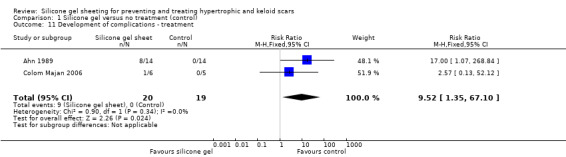
Comparison 1 Silicone gel versus no treatment (control), Outcome 11 Development of complications ‐ treatment.
Comparison: silicone gel compared with non silicone dressing
I: Prevention studies
No prevention studies were identified.
II: Treatment studies
Three studies (de Oliveira 2001; Momeni 2009; Wigger‐Alberti 2009) including 124 people compared silicone gel sheeting with non silicone gel sheeting. One study (de Oliveira 2001) classified scars as either hypertrophic or keloid, and the other two only included hypertrophic scars.
Primary outcome
There was no statistically significant difference between the two groups for reduction of scar width or scar length (Analysis 2.1; Analysis 2.2).
2.1. Analysis.
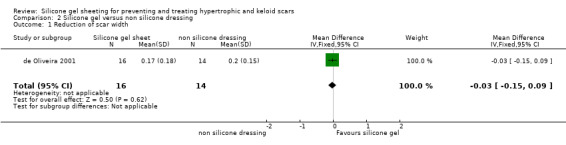
Comparison 2 Silicone gel versus non silicone dressing, Outcome 1 Reduction of scar width.
2.2. Analysis.
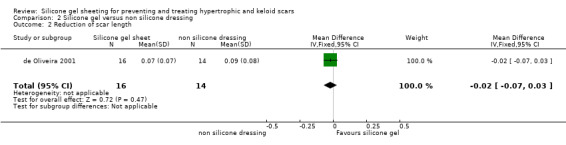
Comparison 2 Silicone gel versus non silicone dressing, Outcome 2 Reduction of scar length.
Secondary outcomes
There was no statistically significant difference between the two groups for amelioration of scar colour (RR 1.01, 95% CI 0.87 to 1.17) (Analysis 2.3). Two studies reported complications. de Oliveira 2001 reported irritative contact dermatitis which was resolved by washing the skin and removing the silicone gel sheeting for five hours. Wigger‐Alberti 2009 reported two adverse events described as local dermatitis which occurred in the silicone gel group. The final study (Momeni 2009) reported no adverse events in either group.
2.3. Analysis.
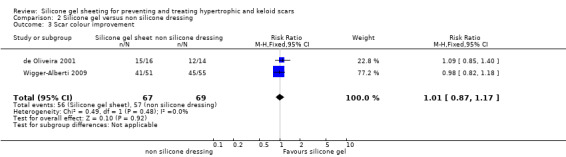
Comparison 2 Silicone gel versus non silicone dressing, Outcome 3 Scar colour improvement.
Comparison: silicone gel sheeting compared with silicone gel sheeting with different contact layers
I: Prevention studies
One study (Niessen 1998) involved 155 women undergoing bilateral breast reduction. This trial had three arms and scars were either treated with adhesive silicone gel sheeting (Epiderm adhesive), non adhesive silicone gel sheeting (Sil‐K) or covered with Micropore alone.
Primary outcome
The authors reported that 12 months after surgery no difference in hypertrophic scar development was found between the adhesive and non adhesive silicone gel sheets. However, they did not present separate data for the two intervention groups, but reported combined data for the silicone gel sheeting groups (adhesive plus non adhesive) compared with the control group. This trial was poorly reported and the method of allocating treatment to scar site was unclear. There was no blinded outcome assessment and no ITT analysis.
Secondary outcomes
Results obtained from the trial author (Niessen) by email on 238 scars (114 adhesive silicone gel group, 124 non adhesive silicone gel group) showed no statistically significant difference in results for scar width, height, colour and perfusion at 12 months post surgery (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4).
6.1. Analysis.
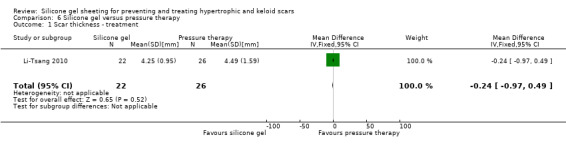
Comparison 6 Silicone gel versus pressure therapy, Outcome 1 Scar thickness ‐ treatment.
6.2. Analysis.
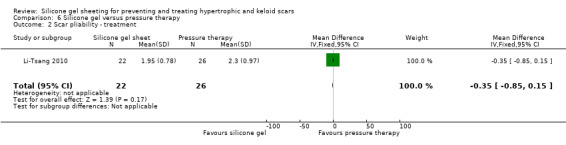
Comparison 6 Silicone gel versus pressure therapy, Outcome 2 Scar pliability ‐ treatment.
6.3. Analysis.
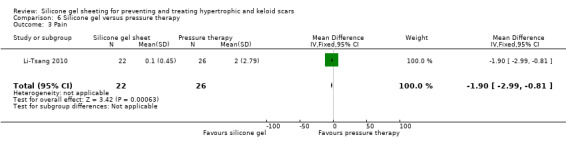
Comparison 6 Silicone gel versus pressure therapy, Outcome 3 Pain.
6.4. Analysis.
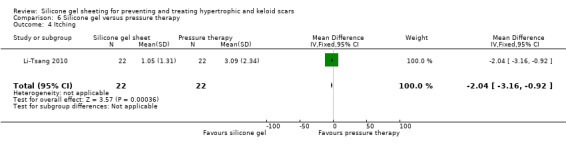
Comparison 6 Silicone gel versus pressure therapy, Outcome 4 Itching.
II: Treatment studies
Three studies were included in this category. One (Palmieri 1995) compared silicone gel sheets to silicone gel sheets with added vitamin E. This study involved 80 people with established hypertrophic and keloid scars resulting from either surgery or thermal burns. One (Karagoz 2009) compared silicone gel sheets to a paint‐on silicone gel and included a total of 30 people with hypertrophic scarring post‐thermal burns in this part of the study. The third study (Carney 1994) compared adhesive (Cica‐Care) with non adhesive (Silastic) silicone gel sheeting, and included 42 people with 47 hypertrophic scars.
Primary outcome
Palmieri 1995 reports that photographs of scar size, colour and cosmesis were objectively scored on a scale of zero to five, however these results appear to have been combined with patient self ratings of itching and pain on a Scott‐Huskisson scale. We contacted the authors for clarification, but they did not reply, therefore these data could not be used and it was impossible to draw conclusions about the effectiveness of either treatment for change in scar size, or determine whether the assessors were blinded to treatment allocation. Size of scar was not measured by Carney 1994 or Karagoz 2009.
Secondary outcomes
Carney 1994 did not provide a statistical analysis of the comparison between the two silicone gel sheets, but stated that after six months of treatment, 88.9% of scars in the non adhesive gel group, and 100% of the scars in the adhesive group were improved for colour, and 100% of both groups were improved for scar softness. We contacted the lead author and asked them for data, however the author replied that the actual data had not been retained.
Palmieri 1995 reported a combined subjective and objective score, which showed that 75% of people treated with silicone gel sheeting had improvements in cosmesis, pain and itching of at least 50%, compared with 90% of those treated with silicone gel plates with added vitamin E. There was a statistically significant improvement in favour of silicone gel sheet with added vitamin E (RR 0.79, 95% CI 0.65 to 0.96) (Analysis 3.5). No complications were reported.
3.5. Analysis.
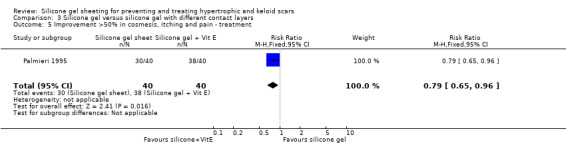
Comparison 3 Silicone gel versus silicone gel with different contact layers, Outcome 5 Improvement >50% in cosmesis, itching and pain ‐ treatment.
Karagoz 2009 used the Vancouver Scar Scale, reporting mean total scores and sub‐scale scores before and after treatment. They concluded that there was no significant difference between the silicone gel sheets and the paint‐on silicone group. Patient ratings of improvement on a four‐point scale were presented, but no statistical analysis was undertaken.
Given the presentation of results in these studies, it is impossible to draw a conclusion regarding the effectiveness of either treatment for the primary outcome (change in scar size).
Comparison: silicone gel sheeting compared with laser therapy
I: Prevention studies
No prevention studies were identified.
II: Treatment studies
Two studies (Pacquet 2001; Wittenberg 1999) involving 40 people compared the use of silicone gel sheeting with 585 nm pulsed dye laser therapy.
Primary outcome
Although scar size was measured in both studies, Pacquet 2001 did not report results at all and Wittenberg 1999 presented results for volume in graphical form only. We contacted both, but no responses were received.
Secondary outcomes
Pacquet 2001 found no statistically significant difference in scar erythema between people receiving silicone gel sheeting compared with those receiving 585 nm pulsed dye laser therapy. Similarly, Wittenberg 1999 also found no statistically significant difference in pain or burning, scar elasticity or fibrosis in people receiving these treatments. Since the published results in both papers were presented graphically and specific numerical data were not provided, no analyses tables or graphs are available in this review. No complications were reported by Pacquet 2001, although Wittenberg 1999 reported that one patient withdrew because of pain on laser treatment, and one patient was unable to use the silicone gel sheeting because of skin irritation.
Comparison: silicone gel sheeting compared with triamcinolone acetonide injection treatment
I: Prevention studies
No prevention studies were identified.
II: Treatment studies
Three studies involving 58 people were identified (Kelemen 2007; Sproat 1992; Tan 1999). Triamcinolone acetonide injections are an existing treatment for hypertrophic scars but can be painful.
Primary outcome
Tan 1999 reported that two out of the 17 people (12%) treated with silicone gel sheeting had a statistically significant reduction (defined as at least 50%) in the size of keloid scars, in contrast to the 16 out of 17 people (94%) who had a significant reduction when treated with intralesional injections of triamcinolone acetonide (40 mg/ml). The RR was 0.13 (95% CI 0.03 to 0.46) (Analysis 4.1). Sproat 1992 reported changes in scar height and width graphically. We contacted the researcher, but they had not kept specific numerical data, so no analyses tables or graphs are available in this review for these measures. Sproat 1992 reported that scar height decreased for both treatment groups, but that scar width increased in both (more so with triamcinolone acetonide injection). This trial report was not supported by any data analysis and therefore must be viewed with caution. Kelemen 2007 concluded that the intralesional steroid injection group had greater improvement of Vancouver scale scores after eight weeks than the silicone group, however they also did not provide any data analysis, so again these conclusions must be viewed with caution.
4.1. Analysis.
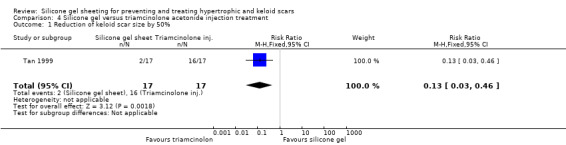
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 1 Reduction of keloid scar size by 50%.
Secondary outcomes
Tan 1999 reported that people treated with the injections showed a statistically significant improvement in erythema compared to those treated with silicone gel sheeting; the RR was 0.10 (95% CI 0.01 to 0.70) (Analysis 4.2). There was no statistically significant difference for symptomatic relief of itching and pain (Analysis 4.3). Sproat 1992 reported a statistically significant difference in mean time to symptomatic improvement (mean difference ‐2.90 days, 95% CI ‐3.93 to ‐1.87) (Analysis 4.4) and patient preference in favour of the silicone gel sheeting (RR 5.50, 95% CI 1.48 to 20.42) (Analysis 4.5). Kelemen 2007 reported a faster reduction in patient‐reported symptoms (measured on a Likert scale), but provided no data to support this claim.
4.2. Analysis.
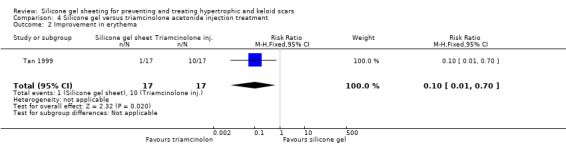
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 2 Improvement in erythema.
4.3. Analysis.
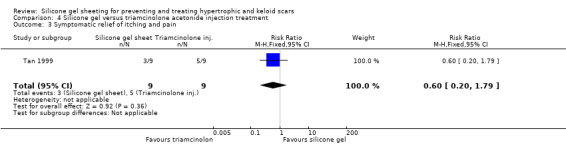
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 3 Symptomatic relief of itching and pain.
4.4. Analysis.
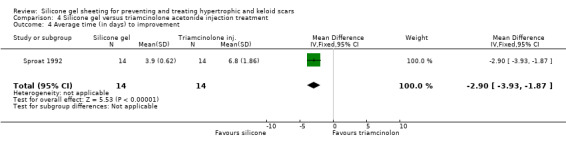
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 4 Average time (in days) to improvement.
4.5. Analysis.
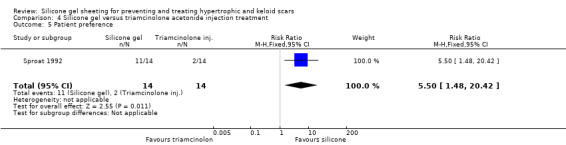
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 5 Patient preference.
Sproat 1992 reported that statistically significantly more participants in the triamcinolone injection group experienced complications (including severe pain (71% of people), skin atrophy, pigmentary changes and white bead‐like skin deposits (64% of people)) compared with one instance of superficial rash in the silicone gel sheeting group (which resolved on discontinuation of the sheeting for two days) (RR 0.10, 95% CI 0.01 to 0.68) (Analysis 4.6). Tan 1999 and Kelemen 2007 both reported that no adverse reactions occurred with either treatment.
4.6. Analysis.
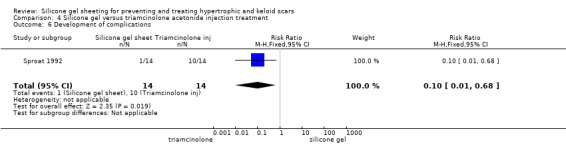
Comparison 4 Silicone gel versus triamcinolone acetonide injection treatment, Outcome 6 Development of complications.
Comparison: silicone gel sheeting compared with topical onion extract
I: Prevention studies
No prevention studies were identified.
II: Treatment studies
One study involving 30 people was identified (Karagoz 2009).
Primary outcome
This study used spectrocolorimeter and Tissue Ultrasound Palpation System (TUPS) to measure scar colour and thickness respectively. The Vancouver Scar Scale was used to measure scar pliability. All scores were reported as mean/standard deviation (SD) of total scores for each group. After six months of intervention, there was a significant difference between the total score for the silicone gel sheeting and topical onion extract groups in favour of the silicone gel sheeting group (MD 1.90, 95%CI 0.62 to 3.18 Analysis 5.1). Patient ratings of improvement on a four‐point scale were presented, but no statistical analysis was undertaken.
5.1. Analysis.
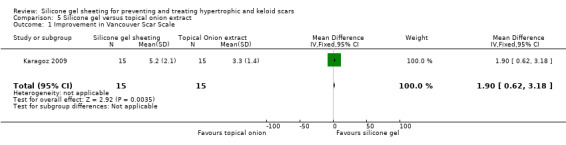
Comparison 5 Silicone gel versus topical onion extract, Outcome 1 Improvement in Vancouver Scar Scale.
Two participants in the silicone gel sheeting group developed skin maceration and pruritis, but this resolved after interrupting treatment for a week.
Comparison: silicone gel sheeting compared with pressure therapy
I: Prevention studies
No prevention studies were identified.
II: Treatment studies
One study involving 54 people was identified (Li‐Tsang 2010).
Primary outcome
This study used a spectrocolorimeter to measure colour (lightness, redness and yellowness), Tissue Ultrasound Palpation System (TUPS) to measure thickness and the Vancouver Scar Scale to measure pliability. Pain and itch were measured using a 10‐point visual analogue scale. Means and SDs were reported for all measures up to six months post commencement of treatment. No significant differences were found between the pressure therapy and the silicone gel sheeting groups on measures of scar thickness, scar colour lightness and yellowness, or pliability (Analysis 6.1; Analysis 6.2). Pain and itch were both significantly lower in the silicone gel sheeting group (Analysis 6.3; Analysis 6.4).
No complications were reported in either group.
Discussion
The introduction of silicone gel sheeting as both a prevention and treatment intervention in the early 1980s has led to a number of research trials of varied quality.
Whilst there is some weak evidence from two small trials (total 51 participants ‐ see comparison 1.1.1 in Analysis 1.1) that silicone gel‐treated incisions are less likely to become hypertrophic in high‐risk people, these trials had a high potential for selection and detection bias (method of randomisation unclear; no blinding of outcome assessors) and therefore must be viewed with a great deal of caution.
Similarly the findings that silicone gel sheeting improves the elasticity and colour of keloid scars came from low‐quality studies. Whilst triamcinolone appeared more effective at improving keloid scarring than silicone gel sheeting this finding too was from a single study with high susceptibility to bias (unclear randomisation; lack of blinding of outcome assessors).
Complications of silicone gel sheeting use including itch and skin rashes were reported in three trials and were more common than in control groups.
In this review, only randomised controlled trials (RCTs), quasi‐RCTs (QRCTs) and controlled clinical trials (CCTs) were considered, leading to a relatively small number of studies (20) and people (873) for evaluation. Few studies compared similar interventions or measured similar criteria. Several trials had methodological problems, and reported inadequately on their randomisation protocols and/or allocation concealment, or failed to undertake an intention‐to‐treat analysis. Blinding of outcome assessors, which would not have been difficult to achieve, was poorly reported. None of the included trials addressed health‐related quality of life, the minimum meaningful difference in scar characteristics such as size or colour, or the cost of treatments. In addition there is the potential for unit of analysis errors, some trials used the person as the unit of analysis, others the scar and in some cases the person was used as their own control, multiple scars on one person treated as being independent in the analysis would inflate precision of any pooled estimates, in general the reporting in this area was poor.
There was also some inconsistency in instruments of measurement, for example, different patient rating scales for pain/irritation/itch were used, making it difficult to compare results.
All but three of the studies had short duration of follow‐up (i.e. less than 12 months), which is inadequate given that scar remodeling and collagen synthesis continues for over a year.
It is interesting to note the difference between the results from the Niessen 1998 and Cruz‐Korchin 1996 trials when their clinical experiments were so similar (both treated women who had recently undergone breast reductions). Both researchers defined the difference in their trials, via letters published in Annals of Plastic Surgery (1997), in an attempt to explain their results. Niessen stated that the most important difference was the application of Micropore (3M) which provided support around the control (untreated) scars, thus demonstrating that "...it is not the silicone material itself that prevents the development of hypertrophic scar tissue". In her response, Cruz‐Korchin 1997 agreed that support would tend to reduce scar width, but silicone sheets would reduce width and flatten the hypertrophic scar. She also observed that her study population was composed mainly of Hispanics who are more prone to forming hypertrophic scars, and compared this to Niessen's study population of "fair‐skinned Caucasians, in whom hypertrophic scarring seldom occurs". At present this trial is the only one to have compared Micropore against silicone gel sheeting and, therefore, more research is needed to investigate whether the physical support of the scar is as effective as silicone gel sheeting.
In summary the effects of silicone gel sheeting on hypertrophic and keloid scarring are unclear and warrant rigorous evaluation.
Authors' conclusions
Implications for practice.
The main aims for practitioners dealing with wound healing and scar minimisation are good skin closure, elasticity, maintenance of functioning of underlying structures and good cosmetic appearance. There are many treatments available to prevent or minimise scarring (including, but not limited to, pressure therapy, topical moisturisers, surgical excision, intralesional corticosteroids, laser therapy, cryotherapy, silicone or non silicone gel sheeting) but these vary in how well they are tolerated, as some people find them painful, uncomfortable and/or expensive. Practitioners need to match treatments to the needs and wishes of their patients.
In this review, the evidence for the effects of silicone gel sheeting on scarring are obscured by the poor quality of the research. Thus, whilst there appeared to be fewer abnormal scars in people at high risk of developing hypertrophic or keloid scars, and improved scar colour and softness in existing scars treated with silicone gel sheeting, these findings are highly susceptible to bias. The increased incidence of adverse effects with silicone gel sheeting must also be taken in to account.
Implications for research.
Given the functional and psychological impact of hypertrophic and keloid scarring, it is surprising that there are so few high‐quality research trials investigating the preventative and treating qualities of silicone gel sheeting. Such information would be welcomed by practitioners, together with estimates of benefit and complication rates.
Robust research to clarify the issues discussed in this review would consist of a trial that incorporated the following criteria:
blinded allocation and outcome assessment;
standardised, objective, validated and repeatable outcome measurement;
adequate duration of follow‐up (at least 12 months, but preferably 18 months);
collection and reporting of recurrence data;
distinction between type of scar (hypertrophic versus keloid) and separation of results by scar type in the analysis.
A detailed list of suggestions for future research in keloid scar treatment is included in Shaffer 2002.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2013 | New citation required but conclusions have not changed | Five new studies were included (Karagoz 2009; Kelemen 2007; Li‐Tsang 2010; Momeni 2009; Wigger‐Alberti 2009). Seven studies were excluded and we requested additional data from three authors (responses from two were received at the time of writing). The review authors’ conclusions remain unchanged. |
| 8 May 2013 | New search has been performed | Second update, new searches. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 23 May 2008 | Amended | Converted to new review format. |
| 11 February 2008 | New search has been performed | For this first update, new searches were carried out in January and November 2007. Two new studies were included (Colom Majan 2006; Li‐Tsang 2006). Seven studies were excluded and we requested additional data from a further two (this has not been received at the time of writing). The review authors' conclusions remain unchanged. |
| 15 November 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank Anita Kainth, Michael Bigby and the Cochrane Wounds Group Editors (Nicky Cullum, David Margolis and Andrea Nelson) who refereed the protocol and review, June Poston who initiated the review, Jesus Lopez Alcalde, Arturo Marti‐Carvajal and Emese Mayhew, who provided translation assistance and Jenny Bellorini for copy editing the review. In addition, the authors would like to thank the staff at the Australasian Cochrane Centre who have been of enormous assistance, Dr Bess Fowler (formerly of Curtin University, School of Occupational Therapy) for support, encouragement and guidance and the staff of the Cochrane Wounds Group. Abhay Pandit performed previous work that was the foundation of the current review but did not contribute to the updating process, similarly Sally Stapley contributed to the first update but not to the original review nor the current update, we acknowledge their contributions.
Appendices
Appendix 1. Search strategy for the first review update 2008
Electronic searches
For this first update the following databases were searched:
The Cochrane Wounds Group Specialised Register (searched 21/11/07);
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library Issue 4, 2007
Ovid MEDLINE (2005 to November Week 1 2007)
Ovid EMBASE (2005 to 2007 Week 46)
Ovid CINAHL (2005 to November Week 3 2007)
The following search strategy was used to search CENTRAL: 1 MeSH descriptor Keloid explode all trees 2 MeSH descriptor Cicatrix, Hypertrophic explode all trees 3 MeSH descriptor Hypertrophy explode all trees 4 keloid* or hypertrophic or cicatrix 5 scar or scars or scarred or scarring 6 (#1 OR #2 OR #3 OR #4 OR #5) 7 MeSH descriptor Silicone Gels explode all trees 8 silicone NEXT gel* 9 silicone NEXT sheet* 10 silicone NEXT dressing* 11 (#7 OR #8 OR #9 OR #10) 12 (#6 AND #11)
The following search strategy was used in MEDLINE and was modified as necessary for EMBASE and CINAHL (available upon request). 1 exp Keloid/ 2 exp Cicatrix, Hypertrophic/ 3 exp Hypertrophy/ 4 (keloid$ or hypertrophic or cicatrix).mp. 5 (scar or scars or scarred or scarring).mp. 6 or/1‐5 7 exp Silicone Gels/ 8 (silicone adj gel$).mp. 9 (silicone adj sheet$).mp. 10 (silicone adj dressing$).mp. 11 or/7‐10 12 6 and 11
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying reports of randomised controlled trials. The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (Highly sensitive search strategies for identifying reports of randomised controlled trials in MEDLINE.
Searching other resources
The reference lists of relevant review articles and all included studies were examined to identify further studies. The major supplier of silicon gel sheeting (Smith and Nephew) was approached for details of unpublished, ongoing and recently published trials. The search was not limited by language or publication status.
Appendix 2. Medline, Embase and CINAHL search strategies
Medline
1 exp Keloid/ (1408) 2 exp Cicatrix, Hypertrophic/ (1151) 3 exp Hypertrophy/ (25586) 4 (keloid* or hypertrophic or cicatrix).tw. (17938) 5 (scar or scars or scarred or scarring).tw. (29856) 6 or/1‐5 (67405) 7 exp Silicone Gels/ (728) 8 (silicon* adj gel*).tw. (576) 9 (silicon* adj sheet*).tw. (165) 10 (silicon* adj dressing*).tw. (33) 11 or/7‐10 (1212) 12 6 and 11 (210) 13 randomized controlled trial.pt. (247106) 14 controlled clinical trial.pt. (40090) 15 randomized.ab. (201489) 16 placebo.ab. (93457) 17 clinical trials as topic.sh. (80864) 18 randomly.ab. (138656) 19 trial.ti. (75110) 20 or/13‐19 (557914) 21 (animals not (humans and animals)).sh. (1647357) 22 20 not 21 (507505) 23 12 and 22 (43)
Embase
1 exp Scar/ (32741) 2 (keloid* or hypertrophic or cicatrix).tw. (25878) 3 (scar or scars or scarred or scarring).tw. (45069) 4 or/1‐3 (76936) 5 exp Silicone Gel/ (793) 6 (silicon* adj gel*).tw. (776) 7 (silicon* adj sheet*).tw. (229) 8 (silicon* adj dressing*).tw. (50) 9 or/5‐8 (1475) 10 4 and 9 (389) 11 exp Clinical trial/ (802169) 12 Randomized controlled trial/ (290844) 13 Randomization/ (51197) 14 Single blind procedure/ (15897) 15 Double blind procedure/ (87219) 16 Crossover procedure/ (32445) 17 Placebo/ (169756) 18 Randomi?ed controlled trial$.tw. (82914) 19 RCT.tw. (10982) 20 Random allocation.tw. (931) 21 Randomly allocated.tw. (14603) 22 Allocated randomly.tw. (1227) 23 (allocated adj2 random).tw. (266) 24 Single blind$.tw. (9897) 25 Double blind$.tw. (92147) 26 ((treble or triple) adj blind$).tw. (248) 27 Placebo$.tw. (140349) 28 Prospective study/ (206934) 29 or/11‐28 (1107742) 30 Case study/ (16788) 31 Case report.tw. (170882) 32 Abstract report/ or letter/ (519805) 33 or/30‐32 (703087) 34 29 not 33 (1079210) 35 animal/ (730814) 36 human/ (8821758) 37 35 not 36 (489053) 38 34 not 37 (1056645) 39 10 and 38 (96)
CINAHL
S9 S5 and S8 S8 S6 or S7 S7 TI ( silicon* gel* or silicon* sheet* or silicon* dressing* ) or AB ( silicon* gel* or silicon* sheet* or silicon* dressing* ) S6 (MH "Silicones") S5 S1 or S2 or S3 or S4 S4 TI ( scar or scars or scarred or scarring ) or AB ( scar or scars or scarred or scarring ) S3 TI ( keloid* or hypertrophic or cicatrix ) or AB ( keloid* or hypertrophic or cicatrix ) S2 (MH "Cicatrix+") S1 (MH "Keloid")
Appendix 3. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)
High risk of bias
Any one of the following.
Not all of the study’s pre‐specified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Silicone gel versus no treatment (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Development of abnormal scarring ‐ prevention | 5 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.21, 1.45] |
| 1.1 High risk of scarring | 2 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.21, 0.98] |
| 1.2 Low risk of scarring | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.02, 8.08] |
| 1.3 Risk not stated | 3 | 320 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.10, 3.40] |
| 2 Development of complications ‐ prevention | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.02, 62.83] |
| 2.1 Prevention | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.02, 62.83] |
| 3 Reduction of scar length ‐ treatment | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Reduction in scar width ‐ treatment | 1 | 27 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Scar thickness ‐ treatment | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐2.14, ‐1.85] |
| 6 Scar pliability ‐ treatment | 2 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.83, ‐0.64] |
| 7 Reduction of keloid scar size by 50% ‐ treatment | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 97.00] |
| 8 Scar colour amelioration ‐ treatment | 5 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [1.97, 6.15] |
| 9 Improvement in scar elasticity ‐ treatment | 5 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 3.03 [1.02, 8.99] |
| 10 Symptomatic relief of itching and pain ‐ treatment | 3 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.78, 1.96] |
| 11 Development of complications ‐ treatment | 2 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.52 [1.35, 67.10] |
1.6. Analysis.
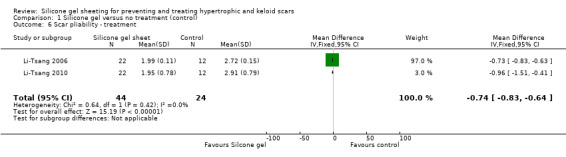
Comparison 1 Silicone gel versus no treatment (control), Outcome 6 Scar pliability ‐ treatment.
Comparison 2. Silicone gel versus non silicone dressing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction of scar width | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.15, 0.09] |
| 2 Reduction of scar length | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.07, 0.03] |
| 3 Scar colour improvement | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.87, 1.17] |
Comparison 3. Silicone gel versus silicone gel with different contact layers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Scar width ‐ prevention | 1 | 238 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.01, 1.61] |
| 2 Scar height ‐ prevention | 1 | 238 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 3 Scar colour ‐ prevention | 1 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.78, 0.38] |
| 4 Scar perfusion ‐ prevention | 1 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.25, 1.45] |
| 5 Improvement >50% in cosmesis, itching and pain ‐ treatment | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.96] |
3.1. Analysis.
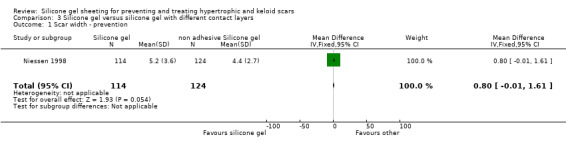
Comparison 3 Silicone gel versus silicone gel with different contact layers, Outcome 1 Scar width ‐ prevention.
3.2. Analysis.
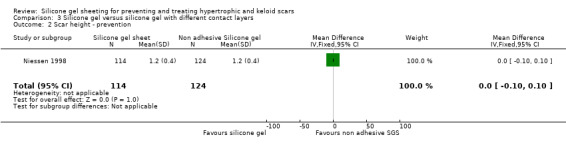
Comparison 3 Silicone gel versus silicone gel with different contact layers, Outcome 2 Scar height ‐ prevention.
3.3. Analysis.
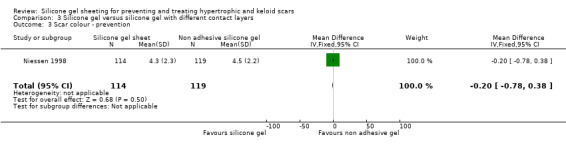
Comparison 3 Silicone gel versus silicone gel with different contact layers, Outcome 3 Scar colour ‐ prevention.
3.4. Analysis.
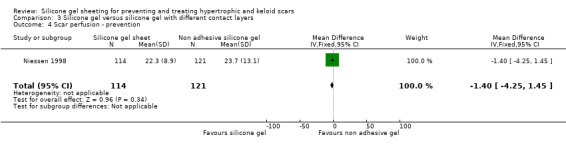
Comparison 3 Silicone gel versus silicone gel with different contact layers, Outcome 4 Scar perfusion ‐ prevention.
Comparison 4. Silicone gel versus triamcinolone acetonide injection treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction of keloid scar size by 50% | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.46] |
| 2 Improvement in erythema | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 0.70] |
| 3 Symptomatic relief of itching and pain | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.20, 1.79] |
| 4 Average time (in days) to improvement | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.9 [‐3.93, ‐1.87] |
| 5 Patient preference | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.5 [1.48, 20.42] |
| 6 Development of complications | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.1 [0.01, 0.68] |
Comparison 5. Silicone gel versus topical onion extract.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in Vancouver Scar Scale | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [0.62, 3.18] |
Comparison 6. Silicone gel versus pressure therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Scar thickness ‐ treatment | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.97, 0.49] |
| 2 Scar pliability ‐ treatment | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.85, 0.15] |
| 3 Pain | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐2.99, ‐0.81] |
| 4 Itching | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐2.04 [‐3.16, ‐0.92] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 1989.
| Methods | RCT | |
| Participants | Setting unclear ‐ the authors have affiliations with academic institutions in USA and Korea 10 patients (14 scars) Inclusion criteria: hypertrophic scars Exclusion criteria: not stated Sex: not stated Age: 19 to 78 years | |
| Interventions | silicone gel sheeting applied to 14 scars for at least 12 hours per day over 8 weeks (untreated adjacent or mirror image scars on same patients used as control) | |
| Outcomes | Length of follow‐up: measurements at 4, 8 and 12 weeks Clinical: scar elasticity, scar appearance, foreign body reaction Complications: occasional transient rashes or superficial maceration, both of which resolved promptly when treatment withdrawn | |
| Notes | Scars were photographed and biopsy specimens taken Elasticity quantitated serially with the use of an elastometer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information is provided on random sequence generation which we judged to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided on the allocation concealment which we judged to be at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "a 6 x 7cm test area of scar was selected so that an untreated control area was available". Comment: due to the nature of the intervention it was judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information is provided on the blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "silicone gel sheets were applied to 14 hypertrophic scars in 10 adults". Comment: results for all 14 scars are presented in table 1. There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to free of other sources of bias. Scars (not people) were the unit of analysis. |
Ahn 1991.
| Methods | RCT | |
| Participants | Teaching Hospital, USA 48 patients Inclusion criteria: 29 patients with fresh surgical incisions (32 pairs of scars) 19 patients with established hypertrophic scars (23 scars) Exclusion criteria: not stated Sex: not stated Age: 12 to 44 years | |
| Interventions | silicone gel bandage worn for at least 12 hours/day (untreated adjacent or mirror image scar on same patient used as control) | |
| Outcomes | Length of follow‐up: measurements at 1, 2 and 6 months Clinical: scar elasticity, scar volume Complications: rash (5), ulcer (3), pruritus (1), discomfort (1) | |
| Notes | Elasticity quantitated serially with the use of an elastometer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A test area of scar that was either 2 or 3 cm in length was arbitrarily selected so that an untreated control area of the same length and similar appearance was available from either the same or a mirror image anatomical site". Comment: no further information was given on how control and treatment areas were selected |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Patients were instructed to wear a small silicone gel sheet on the test area scar for at least 12 hours a day. No treatment or dressing was used on the control scars” Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Test and control scars were rated clinically by both the patient and the investigator at the completion of treatment.” Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Thirty‐two scar pairs were originally entered into the study. . . . Ten patients (11 scar pairs did not complete one month of treatment and thus were nonassessable; most of these patients were men who were minimally concerned about the appearance of their truncal scars” Comment: the loss to follow‐up was greater than 20% therefore the study was judged to be at high risk of attrition bias |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “The research was funded in part by a $10000 grant from Dow Corning Wright, Arlington, Tenn. The senior authors have received funds to conduct additional studies on silicone gel subsequent to this study from Dow Corning Wright. They do no own stock in Dow Corning Wright or its parent company. The authors certify that they have no affiliation with or financial involvement in any organisation with a direct financial interest in the subject matter or materials discussed in this article.” Comment: Dow Corning Wright was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
Carney 1994.
| Methods | RCT | |
| Participants | Teaching Hospital, UK 42 patients Inclusion criteria: hypertrophic scars Exclusion criteria: no other scar‐reducing treatment in previous month Sex: not stated Age: 2 to 65 | |
| Interventions | Half assigned to receive Silastic Gel Sheeting and half Cica‐Care (untreated scar on same patient used as control) | |
| Outcomes | Length of follow‐up: measurements made at monthly intervals for 6 months, then follow up at 3 and 6 months after ceasing treatment Clinical: scar elasticity, appearance, colour Complications: mild irritation, pruritis | |
| Notes | Extensometric measurements made. Irritation rated by patient on scale of 0 to 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Forty‐two patients were randomly assigned to SGS and CC groups and their hypertrophic scars were divided into treated and control areas”. Comment: no further information was given on how control and treatment areas were selected |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which was judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “The study took the form of an open, controlled trial.” Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Patients were required to attend a review clinic . . . at each visit the scar was photographed and the appearance of the treated and untreated area was assessed for state and colour . . . the extensibility of each scar was measured”. Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Forty‐two patients were enrolled in the trial, with a total of 47 scars”. The authors go on to state “the results specifically relating the colour and texture of the scars after 2 months (28 patients) and 6 months (21 patients)”. Comment: no specific mention of loss to follow‐up in made in the study. Due to the quote above we judged the study to be at high risk of attrition bias.Absence of reported data in trial. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Colom Majan 2006.
| Methods | RCT | |
| Participants | Special Sciences Institute, Spain 11 patients Inclusion criteria: adults > 18 years with postoperative scars Exclusion criteria: underlying relevant disease, known hypersensitivity to product used; inability to comply/attend follow‐up; keloid scar Sex: all female Age: 20 to 43 | |
| Interventions | silicone gel sheeting applied to 6 scars for 23 hours per day for a maximum of 1 week; 5 controls had no treatment | |
| Outcomes | Length of follow‐up: measurements made at monthly intervals for 6 months, then at 12 months Clinical: Vancouver scale for appearance; patient's rating of pain and itch Complications: local skin reaction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were randomly allocated to one of the two treatment options by a predetermined computer generated randomisation list”. Comment: participants were judged to be adequately randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which was judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Blinding was not possible as this was a dressing versus no dressing study”. Comment: the study was described as “an open randomised controlled clinical investigation”. Due to the nature of the treatment, we judged that participants and personnel could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Eleven female patients were randomised and enrolled . . . ten participants completed the 12‐month investigation; one in the treatment group discontinued for personal reasons.” Comment: we judged attrition bias to be low |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “This study was supported by Molnlycke Healthcare AB”. Comment: Molnlycke Healthcare AB was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
Cruz‐Korchin 1996.
| Methods | CCT | |
| Participants | Teaching Hospital, USA 20 patients (40 scars) Inclusion criteria: bilateral McKissock reduction mammaplasties Exclusion criteria: not stated Sex: all female Age: not stated | |
| Interventions | Pre‐cut silicone elastomer sheet worn for 12 hours/day for 2 months (untreated adjacent scar on opposite breast of same patient used as control) | |
| Outcomes | Length of follow‐up: measurements made at 2 months; follow‐up at 6 months Clinical: scar hypertrophy Complications: transient rash in 1 patient, minor skin maceration in 1 patient | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Half the patients used the sheet on the breast that corresponded to her dominant hand and the other half used the sheet on their non dominant side”. Comment: no method of randomisation was used in the study |
| Allocation concealment (selection bias) | High risk | Comment: no method of allocation concealment was used in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no further information is provided on blinding. Due to the nature of the treatment, we judged that participants and personnel could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 20 patients were enrolled in the study and table 2 in the results section gives outcomes for all 20 patients at 6 months follow up. Attrition bias was judged to be low risk. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
de Oliveira 2001.
| Methods | RCT | |
| Participants | Teaching Hospital, Brazil 26 patients (41 scars ‐ classified as either hypertrophic or keloid) Inclusion criteria: hypertrophic or keloid scars Exclusion criteria: radiation or corticosteroid therapy in last 12 months Sex: 5 male, 21 female Age: 15 to 53 years | |
| Interventions | Patients with 2 scars: one scar received silicone gel sheet, the other non silicone gel sheet (both worn 24 hours/day) Patients with 3 scars: as above with one "control" scar with no treatment | |
| Outcomes | Length of follow‐up: measurements made at 0, 30, 60, 90, 120 and 135 days Clinical: symptomatic relief of pain and itching, induration (hardness), length, width, colour of scar Intracicatrical pressure Complications: irritative contact dermatitis, which resolved with skin washing and removal of the gel for 5 hours | |
| Notes | Intracicatrical pressure defined as "the necessary pressure to inject a 0.5 ml of triamcinolone solution into the scar tissue" Colour measured by 1000 colour paint chart | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A clinical study was designed in which 26 patients with 41 hypertrophic or keloid scars were randomly chosen to receive silicone gel sheeting, non silicone gel sheeting or nothing”. Comment: no further information was given on how treatment areas were selected |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Every 2 days the [silicone gel] sheeting was removed, washed with water then reapplied to the underlying skin area . . . non silicone gel sheeting was removed after one week and replaced with fresh sheeting”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “On days 0, 30, 60, 90, 120 and 135 the following parameters were evaluated by the same research, except for intracicatrical pressure which was measured blindly by two researchers on day 135." Comment: it is unclear whether the parameters other than intracicatrical pressure were measured by a blinded assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information is given in the study on loss to follow‐up. Attrition bias was judged to be unclear. The scar was the unit of analysis and 27 scars were reported on, it is likely therefore that since 41 were randomised there were losses even though this was not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias. |
Gold 1994.
| Methods | CCT | |
| Participants | Gold Skin Care Centre, USA 34 patients Inclusion criteria: Phase 1: hypertrophic or keloid scar Phase 2: 2 distinct keloids on same body part removed by CO2 laser Phase 3: scars from thermal burns Exclusion criteria: not stated Sex: not stated Age: not stated | |
| Interventions | Phase 1 & 3: scar divided in half ‐ random allocation for each half to receive either silicone gel sheeting (minimum of 12 hours/day for 12 weeks); other half no treatment (control) Phase 2: one scar covered with silicone gel sheeting (as above) other scar untreated | |
| Outcomes | Length of follow‐up: 12 weeks Clinical: Phase 1: patient and physician evaluation of overall improvement and colour Phase 2: recurrence of keloid Phase 3: scar thickness and colour Complications: none reported | |
| Notes | Change rated on a 4‐point scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “lesions were divided in to two equal halves . . . sheeting was placed on half the scar for a minimum of 12 hours a day”. Comment: no information on randomisation was given in the study which we judged to be at unclear risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “lesions were divided in to two equal halves . . . sheeting was placed on half the scar for a minimum of 12 hours a day”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Both patient and physician evaluated the following features: change in thickness and colour in the treated half of the scar and overall effectiveness of the product”. Comment: no further information was given on blinding of outcome assessor which we judged to be at high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there was no loss to follow‐up in this study |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “Supported by a research grant from Dow Corning Wright. The author has no financial interest in Dow Corning Wright or its parent company”. Comment: Dow Corning Wright was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
Gold 2001.
| Methods | RCT | |
| Participants | Gold Skin Care Centre, USA 96 patients Inclusion criteria: dermatologic surgery patients, 2 groups: Low‐risk (no history of abnormal scarring); high‐risk (significant history of abnormal scarring) Exclusion criteria: not stated Sex: low‐risk group 30 male, 20 female; high‐ risk group 9 male, 37 female Age: 36.7 years (mean) | |
| Interventions | Random allocation to receive either silicone gel sheeting (minimum of 12 hours/day for 6 months) applied at 48 hours post surgery, or routine postoperative care (control group) | |
| Outcomes | Length of follow‐up: measurements made at 2, 4, 8, 12, 16, 20 and 24 weeks Clinical: patient's opinion, physician observations, scaled photographic analysis Complications: none reported | |
| Notes | Patient's opinion of the site was assessed in terms of discomfort, embarrassment, colour, height, texture and function and was recorded on a 4‐point scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the patients were randomised to receive either silicone gel sheet postoperatively or routine postoperative care”. Comment: no further information was given on how patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “the patients were randomised to receive either silicone gel sheet postoperatively or routine postoperative care” Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Assessments were made by physician observations of the site, patient opinions and scaled photographic analysis". Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “Ninety‐six patients entered the trial during the enrolment period. During the course of the study 19 patients from the low‐risk group and 11 from the high‐risk group were lost to follow up or withdrew before the 2‐month inspection could be made. That left 66 patients or 69% of the original group available for analysis”. Comment: we judged attrition bias to be high as loss to follow‐up was greater than 20% |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “This research was supported by Smith & Nephew, Largo, FL”. Comment: Smith & Nephew was the brand of silicone gel used in the trial. We judged this to cause a potentially high risk of bias. |
Karagoz 2009.
| Methods | RCT | |
| Participants | 32 patients (45 hypertrophic post‐burn scars): 12 men, 20 women; mean age 24 years Scar locations: head and neck (4 scars); upper limb (29 scars); lower limb (3 scars) and trunk (9 scars) Inclusion criteria: hypertrophic post‐burn scar; age of scar < 6 months Exclusion criteria: hypertrophic post‐burn scar due to chemical burns; age of scar > 6 months |
|
| Interventions | Random allocation to one of 3 groups (15 scars in each): Group 1; silicone gel (Scarfade) applied twice daily Group 2: silicone gel sheet (Epiderm) applied 24 hours (except during bathing) Group 3: topical onion extract including heparin and allantoin (Contractubex) applied twice daily (Also using Tubigrip, an elasticated tubular bandage, was recommended to all patients in the study) Duration of treatments: 6 months Study location: Haydarpasa Training Hospital, Istanbul, Turkey |
|
| Outcomes | Scar assessment using the Vancouver scar scale (assessing response to treatment on a 4‐point Likert scale): 'excellent' if scar change of 7 or more points; 'good' if scar change of 4 to 6 points; minimal response if scar change of one to 3 points; and 'no response' for no scar change Outcomes assessed on Vancouver scar scale: scar pigmentation; vascularity; liability; height before and after treatment Follow‐up: monthly when scars photographed |
|
| Notes | 2 patients in silicone gel sheet group (Group II) disrupted treatment for 1 week due to skin maceration and pruritis; all patients in Group II tolerated silicone gel sheet for at least 12 hours daily. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients with scars less than 6 months of age were assigned at random to three groups each containing 15 scars, and their treatment were continued for 6 months". Comment: no further information was given on how patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Scars were treated with silicone gel in group I, silicone gel sheet in group II, and topical onion extract including heparin and allantoin in group III". Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Scar assessments were performed at the beginning of the treatment, and at the end of the sixth month when the treatment was completed by the same physician" Comment: we judged the study to be at high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the CONSORT diagram in table 3 shows no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears free from other sources of bias |
Kelemen 2007.
| Methods | RCT | |
| Participants | 24 participants were drawn on the basis of pre‐determined criteria from a pool of 200 patients with linear hypertrophic wounds.8 men, 16 women; mean age 43 years (range 17 to 67). Inclusion criteria: active hypertrophic scar on a smooth anatomic site (for easy application of silicone sheet) Exclusion criteria: diabetes, immune and autoimmune diseases, any local or systemic steroid or non steroid treatment Study location: Baranya Megyei Korhaz (Hospital) – Medical University of Pecs, Hungary |
|
| Interventions | 24 patients were then randomly allocated to a treatment (n = 12) and a control (n = 12) group Treatment 1 (intralesional steroid group): injection of 10% solution of triamcinolone acetate (producer: Krka, Slovenia) using 1 ml/cm² with a ‘linear’ technique. The vial contains 40 mg active ingredient per 1 ml solution. For dilution 2% lidocaine (producer: Egis, Hungary) was used. Local anaesthetic was applied to ease the discomfort caused by the injection. The injection needle size varied between 12 and 19G according to the hardness of the scar. Treatment 2 (silicone sheet group): patients wore an appropriate size (exceeding the periphery of the scar by 2 cm in all directions) silicone sheet for 12 hours per day intermittently. They patients received training about how to use the sheets and used sheets were replaced. There were 2‐weekly check‐ups to measure progress. |
|
| Outcomes | Change in total score using Vancouver scar scale, subjective patient experience according to a 5‐point Likert scale | |
| Notes | Digital photographs used for scoring scar. The intralesional injection group was treated by health professionals whilst the silicone gel sheeting group was instructed how to apply the product themselves. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 24 participants were drawn on the basis of pre‐determined criteria from a pool of 200 patients with linear hypertrophic wounds who were treated between April 2001 to March 2004. These 24 patients were then randomly allocated to treatment 1 (n = 12) or treatment 2 (n = 12), but method unclear. |
| Allocation concealment (selection bias) | High risk | Comment: no information on allocation concealment was given in the study which was judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding took place |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes not well presented, only a couple of examples shown |
| Selective reporting (reporting bias) | High risk | Yes. Only 1 ‘interesting’/’unusual’ case from each group reported. There is not a lot of information about outcomes comparing the 2 groups. The authors only state that the intralesional steroid injection was more effective, worked faster and reduced subjective symptoms a lot quicker than the silicone gel. There are no statistical tables to show the progression and difference between the 2 groups week by week. There is one chart showing how Vancouver points change across the two groups for every 2 weeks. |
| Other bias | Low risk | Comment: the study appears free from other sources of bias |
Li‐Tsang 2006.
| Methods | RCT | |
| Participants | 45 Chinese patients Inclusion criteria: history of burns, scald or severe skin trauma resulting in hypertrophic scar Exclusion criteria: age > 50 years, scar > 20 cm² or < 3 mm thickness Sex: 29 male, 16 female Age: 29.65 years (mean) | |
| Interventions | Random allocation to receive either silicone gel sheeting (24 hours/day for 6 months) + 15‐minute lanolin deep massage twice a day versus lanolin massage only | |
| Outcomes | Length of follow‐up: measurements made at 1, 2, 4 and 6 months Clinical: scar pigmentation, thickness, Vancouver scale for appearance, patient's rating of pain and itch | |
| Notes | Colour and thickness measured with spectrocolorimeter and Tissue Ultrasound Palpation System (TUPS) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Forty‐five subjects were randomly allocated into the silicone gel sheeting group (SGS group) and the control group (MT group)”. Comment: no further information was given on how patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Twenty‐two subjects were placed in the experimental group with silicone gel sheeting (SGS) applied 24 h per day for 6 months while all subjects were taught to massage the scar daily for 15 min serving as the control intervention”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “the assessment was conducted by a research assistant who was blind to the subject grouping and was trained to administer all the assessments in standardized methods”. Comment: we judged the outcome assessor to be adequately blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There were 24 subjects allocated to the silicone gel sheeting (SGS) group and 21 subjects in the control (MT) group. Three subjects from the control group have dropped out due to long travelling incurred for reassessment". Comment: we judged attrition bias to be low risk |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “We would also like to thank Smith and Nephew (HK) Company Limited for their generous support to provide all the silicone gel sheeting (Cica‐Care) for our study". Comment: Smith & Nephew was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
Li‐Tsang 2010.
| Methods | RCT | |
| Participants | 104 patients with hypertrophic scars: 63 men, 41 women; mean age 21.8 years (SD of 18.7 years) Inclusion criteria: 'active' hypertrophic scars (more than 5 on the Vancouver scar scale); hypertrophic scars over limbs or body due to burns, scalds or trauma; scar surface area no greater than 16 cm² Exclusion criteria: patients with comorbid conditions (e.g. diabetes) Scar locations: upper limbs (44.2%); lower limbs (28.8%); other areas (26.9%) Study location: Jiangsu People's First Affiliated Hospital, China |
|
| Interventions | Random allocation of patients to one of 4 groups: 1) Pressure therapy (30 patients): "tailor‐made pressure garment with padding" 2) Silicon gel sheeting (Cica‐Care) (24 patients): worn for 24 hours/day (except bathing); secured with Micropore tape, if needed 3) Combined pressure therapy and silicone gel sheeting (29 patients); silicone gel sheeting worn under pressure garment 4) Control group (21 patients) Patients in all 4 groups instructed to perform lanolin massage on scar for 15 minutes daily Duration of intervention/control regimens: 6 months |
|
| Outcomes | Assessment at: baseline, 2, 4 and 6 months Scar colour: assessed using the spectrocolorimeter Scar thickness: assessed using the Tissue Ultrasound Palpation System (TUPS) Scar pliability: assessed using the Vancouver Scar Scale Pain and itchiness: assessed using a visual analogue scale (10‐point scale) |
|
| Notes | Smaller scar area chosen for study "in order to minimize the occurrence of confounding factors from scars that were too big and extensive" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The successfully recruited 104 subjects were randomly assigned into four experimental groups using the draw lots method". Comment: randomisation was judged to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "all the subjects chosen for the study were informed of their own intervention regime" Comment: participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The assessments were then conducted by the research assistant who did not know the intervention given to each subject but only their group number" Comment: we judged the study to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Because of the practical reasons such as living far away from the city, only 84 participants completed all assessments with a high final drop‐out rate (19.23%)". Comment: no intention‐to‐treat analysis was performed and most attrition (from 21 to 12 patients) was in the control group. We judged the study to be at high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: "We also thank Smith and Nephew (HK) Company Limited for providing all the silicone gel sheeting (Cica‐Care) for our study". Comment: Smith & Nephew was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
Momeni 2009.
| Methods | RCT | |
| Participants | 38 patients with hypertrophic burn scars: 16 male, 22 female; median age 22 years (1.5 to 60 years) Inclusion criteria: no history of keloid formation; healed, homogeneous burn scar of at least 5 cm by 5 cm in area Exclusion criteria: wound infection; open wound; sensitivity to silicone gel Study location: Iran University of Medical Sciences |
|
| Interventions | silicone gel sheeting (Cica‐Care) or a placebo (self adhesive propylene glycol and hydroxyethyl cellulose sheeting): both applied for 4 hours/day with a 4‐hourly daily increment to 24 hours/day (overlay taping also used as required) Each treatment applied to either half of one scar Treatment commenced 2 to 4 months after injury; exact duration of silicone sheet application not stated (recommendations of 12 to 24 hours/day are discussed only) |
|
| Outcomes | Pigmentation; vascularity; pliability; pain; itchiness (according to a modified version of the Vancouver scar scale excluding height) Assessment at 1 and 4 months (4 patients lost to follow‐up) |
|
| Notes | Front and profile views of wound changes recorded using a digital camera by a 'blind' evaluator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was used for the coding and randomisation of the gel and placebo samples". Comment: randomisation was judged to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: patients were blinded using placebo sheets; blinding of personnel not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "each participant was sent to another plastic surgeon for the wound to be evaluated blindly." Comment: assessors likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Four participants were lost to follow‐up (two because of distance and two because of failure respond)." Comment: loss to follow‐up is low and reasons given seem valid. We judged attrition bias to be low. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears free from other sources of bias |
Niessen 1998.
| Methods | RCT | |
| Participants | 4 University Hospitals, Netherlands 155 patients Inclusion criteria: bilateral breast reduction surgery Exclusion criteria: not stated Sex: all female Age: 14 to 69 years | |
| Interventions | Scars covered with silicone sheet held in place with Micropore tape, either left lateral and right medial sides, or right lateral and left medial side of scars Untreated "control" part of scar supported with Micropore tape silicone was worn for 24 hours/day for 3 months | |
| Outcomes | Length of follow‐up: measurements made at 2 weeks, 3 months, 6 months and 12 months Clinical: width, height, blood flow and colour of scar, patient complaints about itching and pain Complications: skin irritation | |
| Notes | Width measured by ruler; height judged as either 1 normal, 2 hypertrophic, 3 keloid Patient complaints assessed on a 10‐point scale (1 = no complaints, 10 = very severe itching or pain) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A total of 155 healthy women with a mean age of 31 (14 to 69) years participated in a prospective, randomised multicenter study”. Comment: no further information was given on how patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “...the silicone materials were applied with a little stretch tension and fixated with Micropore to obtain a proper contact with the skin. For an equal support to both parts of the scar, the untreated side was applicated with Micropore alone”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “To reduce observer variation, the first author examined all of the patients at follow‐up”. Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “From the 155 treated patients, 36 did not complete the study: 18 in the group treated by Sil‐K and 18 in the group treated by Epiderm”. Comment: the reasons for loss to follow‐up are clearly documented in table 1. The numbers are the same in each group and reasons given seem valid. As a result we judged the study to be at low risk of bias despite the overall loss to follow‐up being greater than 20%. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Pacquet 2001.
| Methods | CCT | |
| Participants | University Medical Centre, Belgium 20 patients Inclusion criteria: adults with post‐surgical or post‐traumatic keloid scars Exclusion criteria: no previous scar treatment Sex: 2 male, 9 female in laser group; not stated for silicone gel group Age: 17 to 63 years for treatment group (no information available on controls) | |
| Interventions | 11 patients treated with 585 nm pulsed dye laser (1 to 3 treatments at 6 to 8‐week intervals) 9 patients (controls) with application of silicone gel sheeting | |
| Outcomes | Length of follow‐up: measurements made on 5 occasions at 3‐week intervals Clinical: erythema and melanin of scar Complications: none reported | |
| Notes | Size measured by ruler Erythema and melanin measured by spectrophotometer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information is provided on random sequence generation which we judged to be at unclear risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is provided on the allocation concealment which we judged to be at unclear risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: this study compared silicone gel sheeting with a flash lamp pumped pulse dye laser. Due to the nature of the intervention it was judged that participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Clinical assessment of treatment efficacy was supported by comparisons of photographs". Comment: no further information is given on the blinding of participants which we judged to be at unclear risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Eleven adults presented with postsurgical or posttraumatic keloids". Comment: Figure 2 presents the results of all 11 patients therefore there was no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Palmieri 1995.
| Methods | RCT | |
| Participants | University trial, Italy 80 patients Inclusion criteria: adults with hypertrophic and keloid scars Exclusion criteria: psychological disturbance Sex: both; numbers not stated Age: 18 to 63 years | |
| Interventions | Random allocation to 2 groups: Scars covered with silicone plates with added vitamin E (5 g) Or Scars covered with silicone gel sheet Both worn for 10 hours/day (overnight) and fixed with tape | |
| Outcomes | Length of follow‐up: measurements made at 4 and 8 weeks Clinical: Scott‐Huskisson Scale (for pain and itching) Photography of front and side of scar ‐ evaluated on colour, size, cosmetic appearance Complications: none reported | |
| Notes | Scoring of scar appearance on a scale of 0 to 5; itching and pain recorded by patients on Scott‐Huskisson scale | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized in a simple blind study, into two groups”. Comment: no further information was given on how patients were randomised |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the study is described as simple blind. The intervention in this case was silicone gel sheeting with vitamin E versus silicone gel sheeting therefore it is likely that the participants were blind but the research personnel were not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Photographs were taken with identical frontal side views of the scar at the beginning and end of the trial and compared in terms of colour, size and cosmetic appearance of the scar and scored between 0 and 5". Comment: no further information was given on blinding of outcome assessor which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “No drop‐out was observed due to intolerance of the wearing overnight of the silicone plate”. Comment: no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Sproat 1992.
| Methods | RCT | |
| Participants | Teaching Hospital, Canada 14 patients Inclusion criteria: adults with symptomatic hypertrophic sternal scars after cardiac surgery Exclusion criteria: not stated Sex: 7 females, 7 males Age: 33 to 81 years | |
| Interventions | Matched design ‐ scar divided into halves (upper and lower) each receiving a different treatment: Half injected with Kenalog silicone gel sheet applied to other half for 12 hours/day for 12 weeks | |
| Outcomes | Length of follow‐up: measurements made weekly for 12 weeks Clinical: scar length, width, height measured by a blinded observer Photographs taken before and after Patient symptoms and rating of pain of injection Patient treatment preference Complications: Kenalog: skin atrophy, white bead‐like skin deposits, pigmentary changes; silicone: rash | |
| Notes | Patient treatment preference elicited at the end of the trial Photographs evaluated by 5 independent observers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fourteen poststernotomy cardiac patients were randomised to treatment in one half of the scar with Kenalog injection. Simultaneously the other half of the scar received the silicone gel sheet”. Comment: no further information was given on how control and treatment areas were selected |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Fourteen poststernotomy cardiac patients were randomised to treatment in one half of the scar with Kenalog injection. Simultaneously the other half of the scar received the silicone gel sheet”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Photographs of the scars were taken before and after the treatments and were evaluated by five independent observers at 12 weeks. Measurements of the scar were taken by a blindfolded observer”. Comment: as measurements of the scar were taken by a blindfolded observer we judged the outcome assessor to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “this trial was stopped when 11 patients had completed the treatment”. Comment: as a result of the above quote it was judged that no patients were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Tan 1999.
| Methods | CCT | |
| Participants | National Skin Centre, Singapore 20 patients (60 keloid scars) Inclusion criteria: adults with multiple keloid scars (acquired at least 2 years ago) located on the same anatomic site Exclusion criteria: not stated Sex: 18 male, 2 female Age: 19 to 40 | |
| Interventions | 3 scars on each subject: 1 scar as control (no treatment), 1 received silicone gel sheet, 1 injected with triamcinolone acetonide (40 mg/ml) at intervals of 4 weeks | |
| Outcomes | Length of follow‐up: measurements made at 4, 8 and 12 weeks Clinical: scar length, width, height; change in colour and texture; improvement in the symptoms of pain and/or pruritis Complications: none | |
| Notes | Clinical photographs were taken at baseline and at week 12 Patients rated pain/pruritis using a 5‐point scale 2 physicians recorded changes at each visit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “In each patient, three keloids of similar size were selected. One was assigned to no treatment (control) and one to each active treatment”. Comment: no further information is given on the method of randomisation used in the study |
| Allocation concealment (selection bias) | Unclear risk | Comment: no method of allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “In each patient, three keloids of similar size were selected. One was assigned to no treatment (control) and one to each active treatment. The first acted as control and was not given any treatment, the second was treated with occlusive silicone gel sheeting and the third was treated with intralesional injections”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “Clinical assessment was carried out by both the physician and patient”. Comment: no further information was given regarding outcome assessment which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Of the 20 patients, 17 completed the 12 weeks of treatment. Three patients were dropped from the trial (one defaulted from follow up and two defaulted from the treatment plan)”. Comment: overall loss to follow‐up was less than 20% and therefore judged to be a low risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Wigger‐Alberti 2009.
| Methods | RCT | |
| Participants | 60 outpatients with hypertrophic scars: 16 men, 44 women; mean age 38.2 years; mean scar duration 49.6 months (range 3 to 35.5 months) Inclusion criteria: scars of > 6 weeks duration; scars 5 to 10 mm wide and 60 mm long (or greater) Exclusion criteria: keloid scars; "clinically significant illness"; history of hypersensitivity or adverse reactions to adhesive dressings; breastfeeding or pregnant women or women "trying to become pregnant"; scars having undergone any of the following treatments: radiotherapy; intralesional glucocorticosteroids (6 months prior to study); surgical treatments including laser therapy (2 months prior to the study); topical glucocorticosteroids (2 months prior to the study); or any other topical product (1 month prior to the study) Study location: 3 dermatological units in Germany (University Department of Dermatology, Leipzig; proDERM Institute for Applied Dermatological Research, Schenefeld/Hamburg; University Department of Dermatology, Kiel) |
|
| Interventions | silicone sheet or polyurethane dressing: silicone sheet changed once a week; polyurethane dressing changed daily; removal of dressings permitted up to 1 hour daily (recording of times/dates of treatment interruptions and reapplication) Each treatment applied to either half of one scar by random allocation Duration of treatments: 12 weeks |
|
| Outcomes | Percentage change in overall scar index (SI) from baseline to week 12 Percentage change in overall SI between baseline and weeks 4 and 8 Differences in overall SI (absolute change) between baseline, weeks 4, 8 and 12 Changes in skin redness (measured by chromametry) at weeks 4, 8 and 12: photographs on days 1 and 85 also Patient questionnaire (on day 85 of study only): modifications to test area (assessed on a 5‐point Likert scale: ‐1 as 'worsened'; 0 as 'unaltered'; 1 as 'improved'; to 3 as 'complete improvement' Assessments at: baseline; weeks 4, 8 and 12 ("every measurement was made in triplicate, and the average value was used") |
|
| Notes | 67 participants recruited; 7 withdrew within first week of study "As all participants were outpatients, the study nurses also instructed them how to perform the remaining dressing changes at home. Patients were instructed how to apply the dressings properly and to replace them if they became loose" Participants instructed not to use adhesive tape with the polyurethane dressing "unless necessary" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The test product and the reference product were randomly allocated to one half of each treatment site by means of a randomisation list generated by the trial statistician" Comment: randomisation was judged to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on allocation concealment was given in the study which we judged to be at unclear risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "to partially blind the study, the dressings were applied and removed in each test centre by a study nurse in the investigator's absence." Comment: participants not blinded due to "the difference in dressing appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: percentage change in overall scar index (SI) from baseline to week 12: "performed observer blind". Unclear from study report if other assessments were 'blinded': i.e. percentage change of overall SI between baseline, weeks 4 and 8; overall SI (absolute change) across all time points; changes in skin redness |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients withdrew from the study on day 1 (one withdrew consent and one was lost to follow up).Five of the remaining 65 patients withdrew consent before the first assessment in week 4 (withdrawal of consent). The remaining 60 patients comprised the ITT population. However, five of these 60 patients terminated the study prematurely between days 29 and 85: three for personal reasons and two due to an allergic reaction, which may have been related to the silicone dressing." Comment: 12 participants lost in total; ITT performed. We judged attrition bias to be low. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | Low risk | Comment: the study appears to be free from other sources of bias |
Wittenberg 1999.
| Methods | RCT | |
| Participants | Teaching Hospital, USA 20 patients Inclusion criteria: adults with uniform, linear hypertrophic scars secondary to surgical wounds Exclusion criteria: treatment of the scar within the preceding 2 months, keloidal scarring, scars less than 8 cm long Sex: 5 male, 15 female Age: 24 to 81 | |
| Interventions | Each scar was divided into 3 sections, and each section was randomly assigned to 1 of 2 treatments (585 nm pulsed laser or silicone gel sheet) or designated as a control | |
| Outcomes | Length of follow‐up: measurements at 0, 8, 16, 24 and 40 weeks Clinical: hypertrophic scar blood flow, elasticity and volume Histological assessment of fibrosis, number of telangiectasias, number of mast cells Patient' subjective complaints of pruritis, pain and burning Consenting patients (n = 5) underwent punch biopsies at 0 and 40 weeks Complications: 1 patient unable to use silicone gel sheet due to skin irritation, 1 patient withdrew because of pain during laser treatment | |
| Notes | Elasticity measured by elastometer Blood flow measured with a laser Doppler Patients rated pain and burning on a quartile scale (1 = no/minimal pain, 4 = severe) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Each scar was divided into three sections and each section was either randomly assigned to 1 of 2 treatments (SGS or FLPDL) or designated as control using a computer‐generated randomization list”. Comment: we judged randomisation to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information is given on allocation concealment which we judged to be at unclear risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Patients were instructed to wear silicone gel sheeting on the designated site for at least 12 continuous hours per day . . . one section of the patients scar underwent flash lamp‐pumped pulsed‐dye laser . . . one section was randomised to control and left untreated for the study duration”. Comment: due to the nature of the treatment, we judged that participants and personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information was given regarding outcome assessment which we judged to be at unclear risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “20 [patients] were enrolled in the study. Patient 10 did not use SGS due to skin irritation. Patient 6 dropped out of the study at week 24 because of pain during laser treatment". Comment: loss to follow‐up was less than 20% and we therefore judged it to be at low risk of bias |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was not sought, however all measurements discussed in the methods are reported in the results and clinically meaningful outcomes presented. |
| Other bias | High risk | Quote: “We thank Smith and Nephew for supplying silicone gel sheeting” Comment: Smith & Nephew was the brand of silicone gel used in the trial. We judged this to cause a high risk of bias. |
CCT: controlled clinical trial; ITT: intention‐to‐treat; RCT: randomised controlled trial; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Mandeel 1998 | No control group |
| Berman 1999 | No control group |
| Berman 2008 | HSE gel (hydrocortisone, silicone and vitamin E) compared with onion extract, i.e. not silicone gel sheeting |
| Chan 2005 | Used paint‐on silicone (not silicone gel sheet) |
| Chernoff 2007 | No differentiation in outcome reporting between hypertrophic and keloid scars and post‐laser exfoliation erythema |
| Chuangsuwanich 2000 | No control group |
| Clugston 1995 | Non human subjects |
| D'Andrea 2002 | Silicone not the only intervention (combined with surgery) |
| de Giorgi 2009 | Patients with surgical wounds; zinc oxide cream compared with silicone gel, i.e. not silicone gel sheeting |
| Dockery 1994 | No control group |
| Donati 1991 | No control group |
| Fulton 1995 | No control group |
| Gold 1993 | No control group |
| Harte 2009 | Patients with hypertrophic burn scars; pressure garments versus pressure garments and silicone gel sheeting, therefore silicone gel sheeting not used on own |
| Hirshowitz 1993 | No control group |
| Hollands 1999 | Concentrates on local biochemical changes in skin (i.e. does not look at clinical outcomes such as scar size, colour, volume) |
| Hosnuter 2007 | No control group |
| Jenwitheesuk 2012 | Not silicone gel sheeting alone (was combined with onion extract) |
| Katz 1995 | No control group |
| Klopp 2000 | Silicone gel sheeting was applied in combination with pressure garment (not on own) |
| Lee 1996 | No control group |
| Loeding 1993 | silicone gel (not silicone gel sheeting) for hand injuries |
| Mercer 1989 | No control group |
| Musgrave 2002 | Outcome measured was effects on blood flow and perfusion |
| Muti E 1994 | No control group |
| Nikkonen 2001 | No control group |
| Reish 2008 | Discussion paper on scar treatments only, i.e. not a clinical trial |
| Ricketts 1996 | Non randomised pairs study that concentrates on local biochemical changes in skin (i.e. does not look at clinical outcomes such as scar size, colour, volume) |
| Sawada 1990 | Used silicone cream (not gel sheet) |
| Shigeki 1999 | Concentrates on local biochemical changes in skin (i.e. does not look at clinical outcomes such as scar size, colour, volume) |
| Signorini 2007 | Used paint‐on silicone (not silicone gel sheet) |
| So 2003 | Intervention patient education; both groups received silicone gel |
| Steinstraesser 2011 | Silicone gel sheeting was applied in combination with pressure garment (not on own) |
| Stoffels 2010 | Patients with hypertrophic scars; placebo compared with topical silicone spray (not silicone gel sheeting) |
| Suetake 2000 | Concentrates on local biochemical changes in skin (i.e. does not look at clinical outcomes such as scar size, colour, volume) |
| Van den K 2001 | Silicone gel sheeting was applied in combination with pressure garment (not on own) |
| van der Wal 2010 | Topical silicone gel not sheeting |
| Widgerow 2000 | No control group. Combines silicone gel sheet with surgery and steroids (injection and cream), i.e. no patients received silicone gel only |
Characteristics of studies awaiting assessment [ordered by study ID]
Fonseca Capdevila 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting translation |
Quddus‐ur‐Rehman 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | awaiting full text retrieval |
Contributions of authors
Lisa O'Brien co‐ordinated the review update, extracted data and checked the quality of data extraction, analysed or interpreted data, checked quality assessment, made an intellectual contribution to the review and wrote to study authors/experts/companies. Danny Jones extracted data, undertook quality assessment, analysed or interpreted data, completed the first draft of the review update, performed part of the writing or editing of the review update, made an intellectual contribution to the review update, approved the final review update prior to submission.
Contributions of editorial base:
Nicky Cullum: edited the review, advised on methodology, interpretation and review content. Approved the final review and first review update prior to submission. Andrea Nelson, Editor: edited the second review update, advised on methodology and approved the second review update prior to submission. Sally Bell‐Syer: coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review and the updates of the review. Ruth Foxlee: designed the search strategy, ran the searches and edited the search methods section for the updates.
Sources of support
Internal sources
No sources of support supplied
External sources
Australasian Cochrane Centre, Australia.
School of Occupational Therapy, Curtin University, Australia.
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahn 1989 {published data only}
- Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel: a new treatment for hypertrophic scars. Surgery 1989;106(4):781‐6; discussion 786‐7. [PubMed] [Google Scholar]
Ahn 1991 {published data only}
- Ahn ST, Monafo WW, Mustoe TA. Topical silicone gel for the prevention and treatment of hypertrophic scar. Archives of Surgery 1991;126(4):499‐504. [DOI] [PubMed] [Google Scholar]
Carney 1994 {published data only}
- Carney S, Cason CG, Gowar JP, Stevenson JH, McNee J, Groves AR, et al. Cica‐Care gel sheeting in the management of hypertrophic scarring. Burns 1994;20(2):163‐7. [DOI] [PubMed] [Google Scholar]
Colom Majan 2006 {published data only}
- Colom Majan JI. Evaluation of a self‐adherent soft silicone dressing for the treatment of hypertrophic postoperative scars: negative pressure wound therapy to treat hematomas and surgical incisions following high‐energy trauma. Journal of Wound Care 2006;15(5):193‐6. [DOI] [PubMed] [Google Scholar]
- Colom Maján JI. Treatment with a self‐adherent soft silicone gel dressing versus no treatment on postoperative scars. 12th Conference of the European Wound Management Association; 2002, 23‐25 May; Granada, Spain. 2002:336.
Cruz‐Korchin 1996 {published data only}
- Cruz‐Korchin NI. Effectiveness of silicone sheets in the prevention of hypertrophic breast scars. Annals of Plastic Surgery 1996;37(4):345‐8. [DOI] [PubMed] [Google Scholar]
de Oliveira 2001 {published data only}
- Oliveira GV, Nunes TA, Magna LA, Cintra ML, Kitten GT, Zarpellon S, et al. Silicone versus nonsilicone gel dressings: a controlled trial. Dermatologic Surgery 2001;27(8):721‐6. [DOI] [PubMed] [Google Scholar]
Gold 1994 {published data only}
- Gold M. Topical silicone gel sheeting ‐ 1999 update. 9th European Conference on Advances in Wound Management; 1999, 9‐11 November; Harrogate UK. 1999:21, Poster No.1.
- Gold MH. A controlled clinical trial of topical silicone gel sheeting in the treatment of hypertrophic scars and keloids. Journal of the American Academy of Dermatology 1994;30(3):506‐7. [DOI] [PubMed] [Google Scholar]
Gold 2001 {published data only}
- Gold M. The prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure. 20th World Congress of Dermatology; 2002, 1‐5 July; Paris. 2002:IC1743. [DOI] [PubMed]
- Gold MH, Foster TD, Adair MA, Burlinson K, Lewis T. Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatologic Surgery 2001;27(7):641‐4. [DOI] [PubMed] [Google Scholar]
Karagoz 2009 {published data only}
- Karagoz H, Yuksel F, Ulkur E, Evinc R. Comparison of efficacy of silicone gel, silicone gel sheeting, and topical onion extract including heparin and allantoin for the treatment of postburn hypertrophic scars. Burns 2009;35(8):1097‐103. [DOI] [PubMed] [Google Scholar]
Kelemen 2007 {published data only}
- Kelemen O, Kollar L, Menyhei G. A comparative clinical study of the treatment of hypertrophic scars with either intralaesional steroid or silicone gel sheeting [Hungarian]. Magyar Sebeszet 2007;60(6):297‐300. [DOI] [PubMed] [Google Scholar]
Li‐Tsang 2006 {published and unpublished data}
- Li‐Tsang CW, Lau JC, Choi J, Chan CC, Jianan L. A prospective randomized clinical trial to investigate the effect of silicone gel sheeting (Cica‐Care) on post‐traumatic hypertrophic scar among the Chinese population. Burns 2006;32(6):678‐83. [DOI] [PubMed] [Google Scholar]
Li‐Tsang 2010 {published and unpublished data}
- Li‐Tsang CWP, Zheng YP, Lau JCM. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. Journal of Burn Care and Research 2010;31(3):448‐57. [DOI] [PubMed] [Google Scholar]
Momeni 2009 {published data only}
- Momeni M, Hafezi F, Rahbar H, Karimi H. Effects of silicone gel on burn scars. Burns 2009;35(1):70‐4. [DOI] [PubMed] [Google Scholar]
Niessen 1998 {published and unpublished data}
- Niessen FB, Spauwen PH, Robinson PH, Fidler V, Kon M. The use of silicone occlusive sheeting (Sil‐K) and silicone occlusive gel (Epiderm) in the prevention of hypertrophic scar formation. Plastic and Reconstructive Surgery 1998;102(6):1962‐72. [DOI] [PubMed] [Google Scholar]
Pacquet 2001 {published data only}
- Pacquet P, Hermanns JF, Pierard GE. Effect of the 585 nm Flashlamp‐pumped pulsed dye laser for the treatment of keloids. Dermatologic Surgery 2001;27(2):171‐4. [DOI] [PubMed] [Google Scholar]
Palmieri 1995 {published data only}
- Palmieri B, Gozzi G, Palmieri G. Vitamin E added silicone gel sheets for treatment of hypertrophic scars and keloids. International Journal of Dermatology 1995;34(7):506‐9. [DOI] [PubMed] [Google Scholar]
Sproat 1992 {published data only}
- Sproat JE, Dalcin A, Weitauer N, Roberts RS. Hypertrophic sternal scars: silicone gel sheet versus Kenalog injection treatment. Plastic and Reconstructive Surgery 1992;90(6):988‐92. [PubMed] [Google Scholar]
Tan 1999 {published data only}
- Tan E, Chua SH, Lim JTE. Topical silicone gel sheet versus intralesional injections of triamcinolone acetonide in the treatment of keloids ‐ a patient‐controlled comparative clinical trial. Journal of Dermatological Treatment 1999;10(4):251‐4. [Google Scholar]
Wigger‐Alberti 2009 {published data only}
- Wigger‐Alberti W, Kuhlmann M, Wilhelm D, Mrowietz U, Eichhorn K, Ortega J, et al. Efficacy of a polyurethane dressing versus a soft silicone sheet on hypertrophic scars. Journal of Wound Care 2009;18(5):208‐14. [DOI] [PubMed] [Google Scholar]
Wittenberg 1999 {published data only}
- Wittenberg GP, Fabian BG, Bogomilsky JL, Schultz LR, Rudner EJ, Chaffins ML, et al. Prospective, single‐blind, randomised, controlled study to assess the efficacy of the 585‐nm flashlamp‐pumped pulsed‐dye laser and silicon gel sheeting in hypertrophic scar treatment. Archives of Dermatology 1999;135(9):1049‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Mandeel 1998 {published data only}
- Al‐Mandeel MS, Bang RL, Ebrahim MK. Re‐appraisal of Cica‐Care (silicone gel sheet) in the treatment of hypertrophic and keloid scars. Saudi Medical Journal 1998;19(6):741‐5. [Google Scholar]
Berman 1999 {published data only}
- Berman B, Flores F, Gold MH. Comparison of a silicone gel‐filled cushion and silicon gel sheeting for the treatment of hypertrophic or keloid scars. Dermatologic Surgery 99;25(6):484‐6. [DOI] [PubMed] [Google Scholar]
Berman 2008 {published data only}
- Berman B, Patel J, Perez O, Viera M. A prospective, randomized, investigator‐blinded, placebo‐controlled, comparative study evaluating the tolerability and efficacy of two topical medications versus placebo for the treatment of keloids and hypertrophic scars. Journal of the American Academy of Dermatology 2008;58(2):45. [Google Scholar]
Chan 2005 {published data only}
- Chan KY, Lau CL, Adeeb SM, Somasundaram S, Nasir‐Zahari M. A randomized, placebo‐controlled, double‐blind, prospective clinical trial of silicone gel in prevention of hypertrophic scar development in median sternotomy wound. Plastic and Reconstructive Surgery 2005;116(4):1013‐20. [DOI] [PubMed] [Google Scholar]
Chernoff 2007 {published data only}
- Chernoff WG, Cramer H, Su‐Huang S. The efficacy of topical silicone gel elastomers in the treatment of hypertrophic scars, keloid scars, and post–laser exfoliation erythema. Aesthetic Plastic Surgery 2007;31(5):495‐500. [DOI] [PubMed] [Google Scholar]
Chuangsuwanich 2000 {published data only}
- Chuangsuwanich A, Osathalert V, Muangsombut S. Self‐adhesive silicone gel sheet: a treatment for hypertrophic scars. Journal of Medical Association of Thailand 2000;83(4):439‐44. [PubMed] [Google Scholar]
Clugston 1995 {published data only}
- Clugston PA, Vistnes MD, Perry LC, Maxwell GP, Fisher J. Evaluation of silicone‐gel sheeting on early wound healing of linear incisions. Annals of Plastic Surgery 1995;34(1):12‐5. [DOI] [PubMed] [Google Scholar]
D'Andrea 2002 {published data only}
- D'Andrea F, Brongo S, Ferraro G, Baroni A. Prevention and treatment of keloids with intralesional verapamil. Dermatology 2002;204(1):60‐2. [DOI] [PubMed] [Google Scholar]
de Giorgi 2009 {published data only}
- Giorgi V, Sestini S, Mannone F, Papi F, Alfaioli B, Gori A, et al. The use of silicone gel in the treatment of fresh surgical scars: a randomized study. Clinical and Experimental Dermatology 2009;34(6):688‐93. [DOI] [PubMed] [Google Scholar]
Dockery 1994 {published data only}
- Dockery GL, Nilson RZ. Treatment of hypertrophic and keloid scars with SILASTIC gel sheeting. Journal of Foot and Ankle Surgery 1994;33(2):110‐9. [PubMed] [Google Scholar]
Donati 1991 {published data only}
- Donati L, Blandini D, Buraruffaldi Preis FW, Campiglio GL, Capretti A. Silicone gel in the treatment of hypertrophic scars following thermal injury. Rivista Italiana di Chirugia Plastica 1991;23(4):357‐65. [Google Scholar]
Fulton 1995 {published data only}
- Fulton JE Jr. Silicone gel sheeting for the prevention and management of evolving hypertrophic and keloid scars. Dermatological Surgery 1995;21(11):403‐4. [DOI] [PubMed] [Google Scholar]
Gold 1993 {published data only}
- Gold MH. Topical silicone gel sheeting in the treatment of hypertrophic scars and keloids: a dermatological experience. Journal of Dermatologic Surgery and Oncology 1993;19(10):912‐6. [DOI] [PubMed] [Google Scholar]
Harte 2009 {published data only}
- Harte D, Gordon J, Shaw M, Stinson M, Porter‐Armstrong A. The use of pressure and silicone in hypertrophic scar management in burns patients: a pilot randomized controlled trial. Journal of Burn Care and Research 2009;30(4):632‐42. [DOI] [PubMed] [Google Scholar]
Hirshowitz 1993 {published data only}
- Hirshowitz B, Ullman Y, Har‐Shai Y, Vilenski A, Peled IJ. Silicone occlusive sheeting (SOS) in the management of hypertrophic and keloid scarring, including the possible mode of action of silicone by static electricity. European Journal of Plastic Surgery 1993;16(1):5‐9. [Google Scholar]
Hollands 1999 {published data only}
- Hollands R, Spyrou NM. Elemental composition changes between breast tissue with and without silicone gel sheeting and hypertrophic scar tissue. Biological Trace Element Research 1999;Winter(71‐2):575‐83. [DOI] [PubMed] [Google Scholar]
Hosnuter 2007 {published data only}
- Hosnuter M, Payasli C, Isikdemir A, Tekerekoglu B. The effects of onion extract on hypertrophic and keloid scars. Journal of Wound Care 2007;16(6):251‐4. [DOI] [PubMed] [Google Scholar]
Jenwitheesuk 2012 {published data only}
- Jenwitheesuk K, Surakunprapha P, Jenwitheesuk K, Kuptarnond C, Prathanee S, Intanoo W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. International Wound Journal 2012;9(4):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 1995 {published data only}
- Katz BE. Silicone gel sheeting in scar therapy. Cutis 1995;56(1):65‐7. [PubMed] [Google Scholar]
Klopp 2000 {published data only}
- Klopp R, Niemer W, Fraenkel M, Weth A. Effect of four treatment variants on the functional and cosmetic state of matures scars. Journal of Wound Care 2000;9(7):319‐24. [DOI] [PubMed] [Google Scholar]
Lee 1996 {published data only}
- Lee SM, Ngim CK, Chan YY, Ho MJ. A comparison of Sil‐K and Epiderm in scar management. Burns 1996;22(6):483‐7. [DOI] [PubMed] [Google Scholar]
Loeding 1993 {published data only}
- Loeding LA, Guccione JM, Mustoe TA, Monafo WW, Edwards DF, Khouri RK. Effects of silicone gel on scar tissue in hand injuries. Journal of Hand Therapy 1993;6(1):59. [Google Scholar]
Mercer 1989 {published data only}
- Mercer NSG. Silicone gel in the treatment of keloid scars. British Journal of Plastic Surgery 1989;42(1):83‐7. [DOI] [PubMed] [Google Scholar]
Musgrave 2002 {published data only}
- Musgrave MA, Umraw N, Fish JS, Gomez M, Cartotto RC. The effect of silicone gel sheets on perfusion of hypertrophic burn scars. Journal of Burn Care and Rehabilitation 2002;23(3):208‐14. [DOI] [PubMed] [Google Scholar]
Muti E 1994 {published data only}
- Muti E, Bovetto G, Bogetti P, Balocco P, Bonesi A, Bocchiotti G, et al. The use of silicone gel in hypertrophic scars and keloids. Rivista Italiana di Chirugia Plastica 1994;26(3):277‐82. [Google Scholar]
Nikkonen 2001 {published data only}
- Nikkonen NM, Pitkanen JM, Al‐Qattan MM. Problems associated with the use of silicone gel sheeting for hypertrophic scars in the hot climate of Saudi Arabia. Burns 2001;27(5):498‐501. [DOI] [PubMed] [Google Scholar]
Reish 2008 {published data only}
- Reish RG, Eriksson E. Scar treatments: preclinical and clinical studies. Journal of the American College of Surgeons 2008;206(4):719‐30. [DOI] [PubMed] [Google Scholar]
Ricketts 1996 {published data only}
- Ricketts T, Saed GM, Fivenson DP. Cytokine mRNA changes during the treatment of hypertrophic scars with silicone and nonsilicone gel dressings. Dermatological Surgery 1996;22(11):955‐9. [DOI] [PubMed] [Google Scholar]
Sawada 1990 {published data only}
- Sawada Y, Sone K. Treatment of scars and keloids with a cream containing silicone oil. [see comment]. British Journal of Plastic Surgery 90;43(6):683‐8. [DOI] [PubMed] [Google Scholar]
Shigeki 1999 {published data only}
- Shigeki S, Nobuoka N, Murakami T, Ikuta Y. Release and skin distribution of silicone‐related compound(s) from a silicone gel sheet in vitro. Skin Pharmacology and Applied Skin Physiology 1999;12(5):284‐8. [DOI] [PubMed] [Google Scholar]
Signorini 2007 {published data only}
- Signorini M, Clementoni MT. Clinical evaluation of a new self‐drying silicone gel in the treatment of scars: a preliminary report. Aesthetic Plastic Surgery 2007;31(2):183‐7. [DOI] [PubMed] [Google Scholar]
So 2003 {published data only}
- So K, Umraw N, Scott J, Campbell K, Musgrave M, Cartotto R. Effects of enhanced patient education on compliance with silicone gel sheeting and burn scar outcome: a randomized prospective study. Journal of Burn Care and Rehabilitation 2003;24(6):411‐7; discussion 410. [DOI] [PubMed] [Google Scholar]
Steinstraesser 2011 {published data only}
- Steinstraesser L, Flak E, Witte B, Ring A, Tilkorn D, Hauser J, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plastic and Reconstructive Surgery 2011;128(4):306e‐13e. [DOI] [PubMed] [Google Scholar]
Stoffels 2010 {published data only}
- Stoffels I, Wolter TP, Sailer AM, Pallua N. The impact of silicone spray on scar formation. A single‐center placebo‐controlled double‐blind trial. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und verwandte Gebiete 2010;61(4):332‐8. [DOI] [PubMed] [Google Scholar]
Suetake 2000 {published data only}
- Suetake T, Sasai S, Zhen Y‐X, Tagami H. Effects of silicone gel sheet on the stratum corneum hydration. British Journal of Plastic Surgery 2000;53(6):503‐7. [DOI] [PubMed] [Google Scholar]
Van den K 2001 {published data only}
- Kerchove E, Stappaerts KHM, Boeckx WD, Staes F, Hof B, Monstrey S, et al. The influence of different occlusive plates on the erythema of hypertrophic burn scars. European Journal of Plastic Surgery 2001;24(4):182‐5. [Google Scholar]
van der Wal 2010 {published data only}
- Wal MB, Zuijlen PPM, Ven P, Middelkoop, E. Topical silicone gel versus placebo in promoting the maturation of burn scars; A randomized controlled trial. Burns 2011;37:S4. [DOI] [PubMed] [Google Scholar]
- Wal MBA, Zuijlen PP, Ven P, Middelkoop E. Topical silicone gel versus placebo in promoting the maturation of burn scars: a randomized controlled trial. Plastic and Reconstructive Surgery 2010;126(2):524‐31. [DOI] [PubMed] [Google Scholar]
Widgerow 2000 {published data only}
- Widgerow AD, Chait LA, Stals PJ. Triple keloid therapy: a combination of steroids, surgery and silicone gel strip/sheet for keloid treatment. European Journal of Plastic Surgery 2000;23(3):150‐1. [Google Scholar]
References to studies awaiting assessment
Fonseca Capdevila 2007 {published data only}
- Fonseca Capdevila E, López Bran E, Fernández Vozmediano JM, Torre Fraga, Nasarre IQ, Moreno Giménez JC. Prevention of scar sequels after excision of benign cutaneous lesions: multicentre, prospective, open label, controlled study comparing silicone gel versus silicone sheets in 131 patients with melanoctyic nevi [Prevención de secuelas cicatrizales de la extirpación de lesiones cutáneas benignas: estudio multicéntrico, prospectivo, abierto y controlado que compara un gel de silicona y láminas de silicona en 131 pacientes con nevos melanocíticos]. Piel 2007;22(9):421‐6. [Google Scholar]
Quddus‐ur‐Rehman 2012 {published data only}
- Quddus‐ur‐Rehman Latif U, Saqib AS, Elahi A. Comparison of different modalities of treatment of hypertrophic scars and keloids. Medical Forum Monthly 2012;23(3):7‐11. [Google Scholar]
Additional references
Beers 1999
- Beers MH, Berkow R. The Merck Manual of Diagnosis and Therapy. http://www.merck.com/pubs/mmanual/ 1999 (accessed 6 December 2007).
Carney 1993
- Carney SA. Hypertrophic scar formation after skin injury. Journal of Wound Care 1993;2(5):299‐302. [DOI] [PubMed] [Google Scholar]
Cruz‐Korchin 1997
- Cruz‐Corchin NI. Reply to letter by F Niessen, MD. Annals of Plastic Surgery 1997;38(5):547. [DOI] [PubMed] [Google Scholar]
Davey 1991
- Davey RB, Wallis KA, Bowering K. Adhesive contact media ‐ an update on graft fixation and burn scar management. Burns 1991;17(4):313‐9. [DOI] [PubMed] [Google Scholar]
Eisenbeiss 1998
- Eisenbeiss W, Peter FW, Bakhtiari C, Frenz C. Hypertrophic scars and keloids. Journal of Wound Care 1998;7(5):255‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Katz 1992
- Katz B. Silastic gel sheeting is found to be effective in scar therapy. Cosmetic Dermatology 1992;6:1‐3. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
O'Sullivan 1996
- O'Sullivan ST, O'Shaughnessy M, O'Connor TPF. Aetiology and management of hypertrophic scars and keloids. Annals of the Royal College of Surgeons of England 1996;78(3 Pt 1):168‐75. [PMC free article] [PubMed] [Google Scholar]
Perkins 1982
- Perkins K, Davey RB, Wallis KA. Silicone gel: a new treatment for burn scars and contractures. Burns, Including Thermal Injury 1982;9(3):201‐4. [DOI] [PubMed] [Google Scholar]
Quinn 1985
- Quinn KJ, Evans JH, Courtney JM, Gaylor JDS. Non‐pressure treatment of hypertrophic scars. Burns, Including Thermal Injury 1985;12(2):102‐8. [DOI] [PubMed] [Google Scholar]
Raney 1993
- Raney RW. Keloid Pathophysiology and Management. Grand Rounds Archive. The Baylor College of Medicine, Houston 1993.
Sawada 1992
- Sawada Y, Sone K. Hydration and occlusion treatment for hypertrophic scars and keloids. British Journal of Plastic Surgery 1992;45:599‐603. [DOI] [PubMed] [Google Scholar]
Shaffer 2002
- Shaffer JJ, Taylor SC, Cook‐Bolden F. Keloidal scars: a review with a critical look at therapeutic options. Journal of the American Academy of Dermatology 2002;46(2):S63‐S97. [DOI] [PubMed] [Google Scholar]
Shakespeare 1993
- Shakespeare P. Burn wound healing. Journal of Tissue Viability 1993;3(1):16‐24. [Google Scholar]
SIGN 2012
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 30 August 2012).
Smith & Nephew 2000
- Smith, Nephew. Cica‐Care: Scar Treatment Gel Sheet. Product instruction leaflet 2000.
Swanson 1974
- Swanson JW, LeBeau JW. The effect of implantation on the physical properties of silicone rubber. Journal of Biomedical Materials Research 1974;8(6):357‐67. [DOI] [PubMed] [Google Scholar]
Thomas 1997
- Thomas S. SMTL Dressings Datacard (Cica‐Care). http://www.dressings.org/Dressings/cica‐care.html 1997 (accessed 9 May 2013).
Williams 1996
- Williams C. Cica‐care: adhesive gel sheet. British Journal of Nursing 1996;5(14):875‐6. [DOI] [PubMed] [Google Scholar]


