Abstract
Background
The quantity and distribution of adipose tissue may be prognostic measures of mortality in colorectal cancer patients, and such associations may vary by patient sex.
Methods
This cohort included 3262 stage I–III colorectal cancer patients. Visceral and subcutaneous adipose tissues were quantified using computed tomography. The primary endpoint was all-cause mortality. Restricted cubic splines estimated statistical associations with two-sided P values.
Results
Visceral adipose tissue was prognostic of mortality in a reverse L-shaped pattern (nonlinear P = .02); risk was flat to a threshold (∼260 cm2) then increased linearly. Subcutaneous adipose tissue was prognostic of mortality in a J-shaped pattern (nonlinear P < .001); risk was higher at extreme (<50 cm2) but lower at intermediate values (>50 to ≤560 cm2). Patient sex modified the prognostic associations between visceral adipose tissue (Pinteraction = .049) and subcutaneous adipose tissue (Pinteraction = .04) with mortality. Among men, visceral adiposity was associated with mortality in a J-shaped pattern (nonlinear P = .003), whereas among women, visceral adiposity was associated with mortality in a linear pattern (linear P = .008). Among men, subcutaneous adiposity was associated with mortality in an L-shaped pattern (nonlinear P = .01), whereas among women, subcutaneous adiposity was associated with mortality in a J-shaped pattern (nonlinear P < .001).
Conclusions
Visceral and subcutaneous adipose tissue were prognostic of mortality in patients with colorectal cancer; the shape of these associations were often nonlinear and varied by patient sex. These results offer insight into the potential biological mechanisms that link obesity with clinical outcomes in patients with cancer, suggesting that the dysregulated deposition of excess adiposity is prognostic of mortality.
Body mass index (BMI) is a surrogate measure for total body adiposity (1). In a pooled analysis of 25 291 patients with early-stage colon cancer, men with class II or III obesity (BMI ≥35.0 kg/m2) were 16% more likely to die compared with men of normal weight (BMI 18.5−24.9 kg/m2); however, no such association was observed in women (2). BMI does not distinguish muscle from adiposity, nor does it differentiate visceral and subcutaneous adipose tissue regions (3). Men preferentially store adiposity viscerally, whereas women store adiposity subcutaneously (4). Visceral adiposity secretes a variety of protumorigenic metabolites relative to subcutaneous adiposity (5–7), which may explain, in part, why BMI is prognostic in men but not in women (8).
The quantity and distribution of adipose tissue may be powerful prognostic measures of mortality in patients with colorectal cancer (9,10). However, studies to date have offered conflicting insight (11), concluding that excess visceral adiposity increases (12), decreases (13), or has no influence (14,15) on the risk of poor clinical outcomes. Emerging evidence also suggests that excess subcutaneous adiposity decreases the risk of poor clinical outcome (16,17). Adiposity is correlated with muscle, and it is not known if the prognostic effects of adipose tissue quantity and distribution are independent of muscle (18). The current study tested the hypothesis that visceral and subcutaneous adipose tissue, measured using abdominal computed tomography (CT) imaging, are prognostic of mortality in 3262 patients with stage I–III colorectal cancer and that such prognostic associations are modified by patient sex.
Methods
Study Population and Design
The cohort—Colorectal, Sarcopenia, Cancer and Near-term Survival—was derived from the Kaiser Permanente Northern California (KPNC) cancer registry, with ascertainment of all patients diagnosed with stage I–III invasive colorectal cancer between the years 2006 and 2011, age 18−80 years, who underwent surgical resection for colorectal cancer (n = 4465). We excluded 693 patients without abdominal or pelvic CT images, 411 patients without valid measures of body mass, and 99 patients whose CT images were unreadable because of poor image quality. The final analytic sample included 3262 patients (Supplementary Figure 1, available online). A waiver of written informed consent was obtained by the study investigators. This study was approved by the KPNC and University of Alberta institutional review boards.
Measures of Body Composition
Height (meters) and weight (kilograms) were measured at the time of diagnosis by medical assistants per KPNC clinic standards. BMI was calculated as kilograms per square meter of height (kg/m2). Body composition was measured using CT images originally collected for clinical purposes with sliceOmatic software (V5.0, TomoVision, Montreal, Canada). A single-slice transverse image at the third lumbar vertebra was used, because tissue cross-sectional areas at this lumbar region correlate with whole-body (19) and visceral and subcutaneous tissue volumes both in men and women (20). Tissues were demarcated with a semiautomated procedure using Hounsfield Unit thresholds of −29 to 150 for muscle tissue, −150 to −50 for visceral adipose tissue, and −190 to −30 for subcutaneous adipose tissue. A random subsample of 50 CT images was analyzed by two investigators blinded to outcome, and the remaining images were analyzed by a single trained investigator blinded to outcome. The coefficients of variation for visceral adipose tissue area and subcutaneous adipose tissue area were 1.1% and 2.7%, respectively (21).
Covariates
The KPNC electronic medical record was used to obtain baseline information on age, sex, race and ethnicity, and smoking history. The KPNC cancer registry was used to obtain information on the anatomical site of cancer, cancer stage, and the administration of chemotherapy and radiation. Conditions from the Charlson comorbidity index were obtained from the electronic medical record with a 36-month lookback interval from the time of cancer diagnosis (22). The above-described covariate data were 99.9% complete. The measurement of physical activity was implemented across KPNC beginning in October 2009; consequently, only 447 (13.7%) of our cohort had physical activity measures available from 36 months before colorectal cancer diagnosis and up to 12 months after diagnosis. Sensitivity analyses were conducted including physical activity measures as a covariate. Additional sensitivity excluded patients who had a BMI classified as underweight at colorectal cancer diagnosis (<18.5 kg/m2).
Study Outcomes
The primary study outcome was all-cause mortality, defined as the time from CT image acquisition to death from any cause. The secondary study outcome was colorectal cancer-specific mortality, defined as the time from CT image acquisition to death attributable to colorectal cancer. Deaths were identified from the California state death registry, National Death Index using Social Security Administration data, and KPNC electronic mortality files through December 31, 2016. Deaths were classified as cancer specific if colorectal cancer was documented as an underlying or contributing cause of death on the death certificate through January 31, 2015.
Statistical Analysis
Investigators often model visceral and subcutaneous adipose tissue using categorical variables. However, categorization induces discontinuities in mortality risk between exposure categories that reduce statistical power and are often difficult to justify biologically and interpret clinically. In contrast to prior studies, we modeled visceral and subcutaneous adipose tissue as continuous variables using restricted cubic splines. Cubic splines accommodate nonlinearity and provide statistically efficient and visually intuitive descriptions of prognostic associations (23). Model parsimony and the Akaike information criterion were used to select the optimal number of knots for visceral and subcutaneous adipose tissue. In the absence of available literature, knot locations could not be biologically prespecified and were based on default locations using data-derived quantities (23).
Multivariable-adjusted Cox proportional hazards models were used to evaluate associations between baseline visceral and subcutaneous adipose tissue and mortality risk. Models were adjusted for age, race and ethnicity, cancer site, cancer stage, chemotherapy, radiation therapy, smoking history, Charlson comorbidity index, and height. In addition to muscle area, we simultaneously adjusted statistical models for visceral adipose tissue and subcutaneous adipose tissue to evaluate their independent effects. Linearity and nonlinearity were inspected visually using spline plots and examined statistically using likelihood ratio tests (23). The assumption of proportional hazards was examined by visual inspection of graphical log-log plots and tested statistically in a generalized linear regression of the scaled Schoenfeld residuals on time (24). Sensitivity analyses that included physical activity as a covariate used the missing-indicator method (25). The Pearson correlation coefficient was used to quantify the strength of the association between visceral and subcutaneous adipose tissues.
Effect modification was examined by including the multiplicative interaction terms in the regression models and examined using the likelihood ratio test. Based on prior studies that identified sex-by-BMI interactions (2,26), sex was prespecified as the primary effect modifier of interest. Baseline characteristics were compared between men and women using the χ2 test for categorical variables and the t test for continuous variables. In post hoc analyses, we also examined the following subgroups: age, cancer site, cancer stage, smoking history, and muscle area. Because of known limitations in statistical power, the threshold for statistical significance for interactions was prespecified at P less than .10 (27). To conclude the presence of effect modification, a statistically significant likelihood ratio test P value and clinically meaningful visual differences in effect size estimates from the spline plots were required (28). All statistical tests were two-sided.
Results
Characteristics of the Study Cohort
The average (SD) age of the 3262 participants was 62.6 (11.4) years, and 49.9% were women (Table 1). Baseline CT images were obtained a median of 6 days (interquartile range = 0−12 days) after biopsy-proven diagnosis of colorectal cancer. During a median follow-up of 6.9 years (interquartile range = 5.3−8.4 years), 879 (26.9% of the cohort) deaths occurred, with 451 (51.3% of all deaths) attributable to colorectal cancer (Supplementary Table 1, available online).
Table 1.
Baseline demographic, disease, behavioral, and anthropometric characteristics overall and stratified by sex
| Characteristic | Overall cohort (N = 3262) | Sex stratified |
P * | |
|---|---|---|---|---|
| Men (N = 1634) | Women (N = 1628) | |||
| Mean age (SD), y | 62.6 ± 11.4 | 62.0 ± 11.3 | 63.2 ± 11.5 | .002 |
| Race, no. (%) | .59 | |||
| White | 2118 (65.0) | 1063 (65.1) | 1055 (64.8) | |
| Asian | 520 (16.0) | 261 (16.0) | 259 (15.9) | |
| Hispanic | 365 (11.2) | 193 (11.8) | 172 (10.6) | |
| Black | 234 (7.2) | 105 (6.4) | 129 (7.9) | |
| Other or unknown | 25 (0.6) | 12 (0.7) | 13 (0.8) | |
| Site, no. (%) | <.001 | |||
| Colon | 2315 (71.0) | 1082 (66.2) | 1233 (75.7) | |
| Rectum | 947 (29.0) | 552 (33.8) | 395 (24.3) | |
| Stage, no. (%) | .18 | |||
| I | 979 (30.0) | 501 (30.7) | 478 (29.4) | |
| II | 1030 (31.6) | 531 (32.5) | 499 (30.6) | |
| III | 1253 (38.4) | 602 (36.8) | 651 (40.0) | |
| Chemotherapy, no. (%) | 1703 (52.2) | 866 (53.0) | 837 (51.4) | .36 |
| Radiation, no. (%) | 506 (15.5) | 312 (19.1) | 194 (11.9) | <.001 |
| Smoking history, no. (%) | <.001 | |||
| Never | 1516 (46.5) | 634 (38.8) | 882 (54.2) | |
| Former | 1347 (41.3) | 771 (47.2) | 576 (35.4) | |
| Current | 396 (12.2) | 226 (13.8) | 170 (10.4) | |
| Unknown | 3 (0.1) | 3 (0.2) | 0 (0.0) | |
| Charlson comorbidity index, no. (%) | .32 | |||
| 0 | 1995 (61.2) | 981 (60.0) | 1014 (62.3) | |
| 1 | 946 (29.0) | 482 (29.5) | 464 (28.5) | |
| ≥2 | 321 (9.8) | 171 (10.5) | 150 (9.2) | |
| Mean height (SD), m | 1.69 ± 0.10 | 1.76 ± 0.08 | 1.62 ± 0.07 | <.001 |
| Mean body mass (SD), kg | 80.9 ± 20.6 | 88.7 ± 19.2 | 73.4 ± 19.2 | <.001 |
| Mean body mass index (SD), kg/m2 | 28.1 ± 6.0 | 28.3 ± 5.2 | 27.9 ± 6.7 | .09 |
| Mean muscle area (SD), cm2 | 140.7 ± 38.2 | 168.6 ± 30.6 | 112.7 ± 20.3 | <.001 |
| Mean visceral adipose tissue area (SD), cm2 | 155.7 ± 110.0 | 201.3 ± 116.2 | 110.0 ± 80.6 | <.001 |
| Mean subcutaneous adipose tissue area (SD), cm2 | 212.6 ± 120.3 | 187.4 ± 105.3 | 237.8 ± 128.9 | <.001 |
The χ2 test was used to compare distributions of categorical variables and a t test for distributions of continuous variables. All statistical tests were two-sided.
The median BMI was 27.2 kg/m2 (range = 14.0−59.6 kg/m2), muscle area was 135.9 cm2 (range = 60.3−319.6 cm2), visceral adipose tissue was 138.6 cm2 (range = 0.1−676.7 cm2), and subcutaneous adipose tissue was 184.9 cm2 (range = 0.0−931.1 cm2). Visceral and subcutaneous adipose tissue were moderately and positively correlated (r = 0.37, 95% CI = 0.34 to 0.40, P < .001). Demographic, clinical, and behavioral characteristics were associated with visceral and subcutaneous adipose tissue area (Supplementary Table 2, available online).
Prognostic Effects of Adipose Tissue Distribution on Mortality
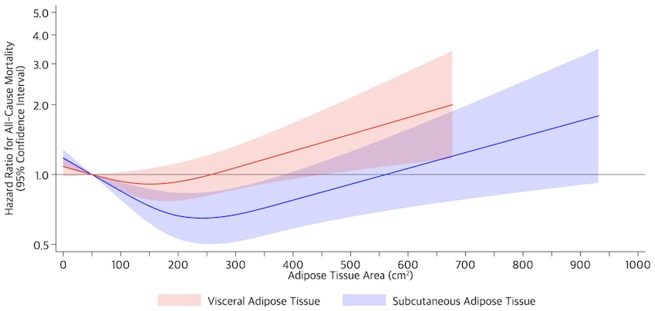
Visceral adipose tissue was prognostic of all-cause mortality in a reverse L-shaped pattern (nonlinear P = .02); risk was flat to a threshold (∼260 cm2), then increased linearly (Figure 1; Supplementary Table 3, available online). Subcutaneous adipose tissue was prognostic of all-cause mortality in a J-shaped pattern (nonlinear P < .001); risk was higher at the extreme (<50 cm2) but lower at intermediate values (>50 to ≤560 cm2; Supplementary Table 4, available online). Subcutaneous adipose tissue (nonlinear P = .02) but not visceral adipose tissue (linear P = .24, nonlinear P = .32) was prognostic of colorectal cancer-specific mortality (Supplementary Figure 2, available online). Sensitivity analysis that adjusted for physical activity did not substantively alter the shape or magnitude of the above-described effect estimates (Supplementary Figure 3, available online). The exclusion of 61 (1.9%) patients with underweight BMI (<18.5 kg/m2) did not substantively alter the shape or magnitude of the above-described effect estimates.
Figure 1.
Risk of all-cause mortality on the relative hazard scale in 3262 patients with colorectal cancer. Shaded regions indicate 95% confidence bands for risk of mortality as a function of visceral (red) and subcutaneous (blue) adipose tissue area. Estimates are adjusted for age, sex, race and ethnicity, cancer site, cancer stage, chemotherapy, radiation therapy, smoking history, Charlson comorbidity index, height, muscle area, subcutaneous adipose tissue area (for visceral adipose tissue area models), and visceral adipose tissue area (for subcutaneous adipose tissue area models).
Patient Sex Modified the Prognostic Effects of Adipose Tissue Distribution on Mortality
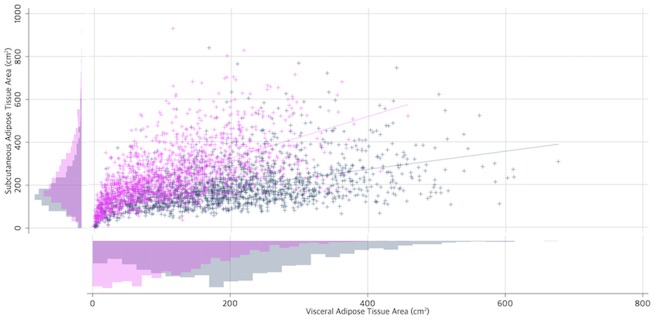
Analyses stratified by patient sex demonstrated that men had more visceral adipose tissue (+91.3 cm2, 95% CI = 84.4 to 98.2 cm2, P < .001) and less subcutaneous adipose tissue (−50.4 cm2, 95% CI = −58.5 to −42.3 cm2, P < .001) than women. The correlation between visceral and subcutaneous adipose tissue was moderate among men (r = 0.47, 95% CI = 0.43 to 0.50, P < .001) and women (r = 0.61, 95% CI = 0.57 to 0.63, P < .001; Figure 2).
Figure 2.
Distribution and correlation between visceral adipose tissue area and subcutaneous adipose tissue area among 3262 men (navy blue) and women (magenta) with colorectal cancer.
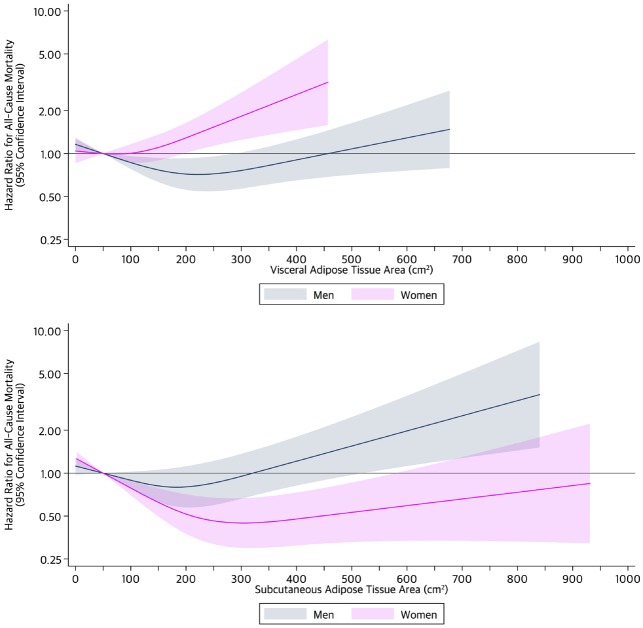
Patient sex modified the prognostic association of visceral adipose tissue and all-cause mortality (Pinteraction = .049). Among men, visceral adiposity was associated with mortality in a J-shaped pattern (nonlinear P = .003), whereas among women, visceral adiposity was associated with mortality in a linear pattern (linear P = .008; Figure 3). Patient sex modified the prognostic association of subcutaneous adipose tissue and all-cause mortality (Pinteraction = .04). Among men, subcutaneous adiposity was associated with mortality in an L-shaped pattern (nonlinear P = .01), whereas among women, subcutaneous adiposity was associated with mortality in a J-shaped pattern (nonlinear P < .001). Patient sex did not modify the prognostic association between visceral adipose tissue (Pinteraction = .44) or subcutaneous adipose tissue (Pinteraction = .39) with colorectal cancer-specific mortality (Supplementary Figure 4, available online). Sensitivity analysis that adjusted for physical activity did not substantively alter the shape or magnitude of the above-described effect estimates (Supplementary Figure 5, available online). The exclusion of 61 (1.9%) patients with underweight BMI (<18.5 kg/m2) at colorectal cancer diagnosis did not substantively alter the shape or magnitude of the above-described effect estimates.
Figure 3.
Risk of all-cause mortality by sex and adipose tissue compartment on the relative hazard scale in 3262 patients with colorectal cancer. Shaded regions indicate 95% confidence bands for risk of mortality as a function of visceral (top) and subcutaneous (bottom) adipose tissue area among men (navy blue) and women (magenta). Estimates are adjusted for age, race and ethnicity, cancer site, cancer stage, chemotherapy, radiation therapy, smoking history, Charlson comorbidity index, height, muscle area, subcutaneous adipose tissue area (for visceral adipose tissue area models), and visceral adipose tissue area (for subcutaneous adipose tissue area models).
Age, cancer site, cancer stage, smoking history, and muscle area did not modify the prognostic associations of visceral and subcutaneous adipose tissue with mortality (results not shown).
Discussion
In this population-based cohort study of 3262 patients with stage I–III colorectal cancer, abdominal adipose tissue quantity and distribution were prognostic of all-cause mortality. The shapes of these associations were generally nonlinear and modified by patient sex. Among men, larger quantities of abdominal subcutaneous adipose tissue were associated with a higher risk of mortality, whereas among women, larger quantities of abdominal visceral adipose tissue were associated with an increased risk of mortality. Conversely, among men, moderate quantities of visceral adipose tissue were associated with a lower risk of mortality, whereas among women, moderate quantities of subcutaneous adipose tissue were associated with a lower risk of mortality.
Previous studies have modeled adipose tissue using categories, which has led to inconsistent findings. In a retrospective cohort study of 62 patients with stage I–III colorectal cancer, a visceral adipose tissue area greater than 130 cm2 was associated with a statistically significantly higher risk of all-cause mortality (hazard ratio [HR] = 7.0, 95% CI = 2.0 to 24.6) (12). In another study of 219 patients with stage I–III colorectal cancer, visceral adipose tissue in the higher 50th percentile was associated with statistically significantly higher risk of disease recurrence and mortality in patients with stage II disease (HR = 2.72, 95% CI = 1.21 to 6.10) and a pattern of lower risk of disease recurrence and mortality in patients with stage III disease, but this did not reach statistical significance (HR = 0.50, 95% CI = 0.23 to 1.06) (13). The large sample size of the current study, combined with the application of restricted cubic splines, afforded us greater statistical power and model flexibility to characterize the associations of abdominal adipose tissue with mortality. Unlike the prior study (13), no effect modification by cancer stage was identified in the current study. The prognostic associations of adiposity were independent of muscle area, which has been previously reported as a predictor of mortality in cancer patients (18). Notably, moderate amounts of visceral adiposity among men and moderate amounts of subcutaneous adiposity among women were not associated with mortality; the nadir risks of mortality for visceral adiposity in men were 200−250 cm2 and for subcutaneous adiposity in women were 275−325 cm2.
Prior studies that investigated the association between BMI and mortality reported effect modification by patient sex for early-stage colon cancer (Pinteraction = .034) (2) and metastatic colorectal cancer (Pinteraction < .001) (26). The underlying biological mechanism of effect modification by patient sex is hypothesized to relate to adipose tissue distribution and resultant metabolic perturbations (9,10). Biopsy studies of patients with colorectal cancer demonstrated that visceral adipose tissue exhibits altered inflammatory, lymphocytic, and fatty acid secretory properties compared with subcutaneous adipose tissue (5–7). In the current study, correlational analyses between these tissues demonstrated that patients with higher areas of subcutaneous adipose tissue did not necessarily also have higher areas of visceral adipose tissue. This counters the hypothesis that patients who have poor outcomes are those who have both excess visceral adipose tissue and excess subcutaneous adipose tissue when measured at the third lumbar vertebra.
Our data are consistent with the hypothesis that dysregulated deposition of adiposity is prognostic of mortality: Men who preferentially store excess adiposity subcutaneously and women who preferentially store excess adiposity viscerally are at a statistically significantly higher risk of mortality than their counterparts who store adiposity in the regions expected for their sex. Sex steroid hormones are the principal factor that influences adipose tissue deposition (29). Testosterone suppression in healthy young men increases subcutaneous adipose tissue deposition (30), whereas ovarian hormone suppression in healthy premenopausal women increases visceral adipose tissue deposition (31). Similarly, the decline in estrogen that naturally occurs during menopause in women is associated with increases in visceral adipose tissue deposition (32). Sex steroid hormones that regulate adipose tissue deposition, including estradiol and testosterone, are associated with cancer risk and prognosis (33,34).
Study limitations include the observational design and the potential for residual confounding. Because this study relied on routine clinically acquired data, measures of socioeconomic status, diet, or menopausal status were not available. The inclusion of these variables may modestly influence our effect size estimates, but it is unlikely that the overall shape of these prognostic associations would substantively change. Body composition was measured at the third lumbar vertebra at a solitary time point. Although the third lumbar vertebra is strongly correlated with visceral and subcutaneous adipose tissue volumes (20), anatomic differences in the distribution of adiposity between men and women may have an influence on our findings (35); nevertheless our data demonstrate that the third lumbar vertebra is prognostic in both sexes. We did not have complete measures of body mass or composition before the diagnosis of cancer; therefore, we cannot rule out the possibility that some patients may have experienced tumor-induced changes in adiposity. However, we did not identify any interactions with clinical cancer stage.
There are several strengths of this study. The main strengths are the large sample size and population-based, racially and ethnically diverse sample. The large sample size offered sufficient statistical power to evaluate associations by sex-specific subgroups. The use of clinically acquired CT images, coupled with recently developed semi- and fully automated radiologic techniques to quantify adiposity using clinical imaging, make the integration of body composition measures into clinical practice cost effective and offers the opportunity to personalize oncology care.
In conclusion, abdominal visceral and subcutaneous adipose tissue quantity was prognostic of all-cause mortality in patients with stage I–III colorectal cancer, and the strength and shape of these prognostic associations varied by patient sex. Measurements of body composition using CT can be seamlessly integrated into clinical care and used to identify those at risk for poor outcome.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under award numbers K99-CA218603, K01-CA226155, R01-CA175011, and R25-CA203650, and the National Institute of General Medicine Sciences of the National Institutes of Health under award number U54-GM104940.
Notes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or decision to submit the manuscript for publication.
Dr Brown reports receiving grants from the National Cancer Institute during the conduct of the study. Dr Prado reports grants from various sources during the conduct of the study. Drs Cespedes Feliciano, Xiao, and Kroenke report receiving grants from the National Cancer Institute during the conduct of the study. Dr Meyerhardt reports receiving consulting fees from Ignyta, Cota, and Taiho during the 36 months before publication (all fees <$5000).
Supplementary Material
References
- 1. Brown JC, Cespedes Feliciano EM, Caan BJ.. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9(7):1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JC, Meyerhardt JA.. Obesity and energy balance in GI cancer. J Clin Oncol. 2016;34(35):4217–4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bredella MA. Sex differences in body composition In: Mauvais-Jarvis F, ed. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity. Basel, Switzerland: Springer; 2017:9–27. [Google Scholar]
- 5. Del Corno M, D’Archivio M, Conti L, et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and omega6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget. 2016;7(39):63093–63105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donninelli G, Del Corno M, Pierdominici M, et al. Distinct blood and visceral adipose tissue regulatory T cell and innate lymphocyte profiles characterize obesity and colorectal cancer. Front Immunol. 2017;8:643.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liesenfeld DB, Grapov D, Fahrmann JF, et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: the ColoCare study. Am J Clin Nutr. 2015;102(2):433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O’Sullivan J, Lysaght J, Donohoe CL, et al. Obesity and gastrointestinal cancer: the interrelationship of adipose and tumour microenvironments. Nat Rev Gastroenterol Hepatol. 2018;15(11):699–714. [DOI] [PubMed] [Google Scholar]
- 9. Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ulrich CM, Himbert C, Holowatyj AN, et al. Energy balance and gastrointestinal cancer: risk, interventions, outcomes and mechanisms. Nat Rev Gastroenterol Hepatol. 2018;15(11):683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao J, Mazurak VC, Olobatuyi TA, et al. Visceral adiposity and cancer survival: a review of imaging studies. Eur J Cancer Care (Engl). 2018;27(2):e12611.. [DOI] [PubMed] [Google Scholar]
- 12. Lee CS, Murphy DJ, McMahon C, et al. Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. J Gastrointest Canc. 2015;46(3):243–250. [DOI] [PubMed] [Google Scholar]
- 13. Rickles AS, Iannuzzi JC, Mironov O, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg. 2013;17(1):133–143. [DOI] [PubMed] [Google Scholar]
- 14. Kang J, Baek SE, Kim T, et al. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis. 2012;27(4):497–505. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto N, Fujii S, Sato T, et al. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol. 2012;8(4):337–345. [DOI] [PubMed] [Google Scholar]
- 16. Black D, Mackay C, Ramsay G, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017;24(8):2241–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ebadi M, Martin L, Ghosh S, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117(1):148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 19. Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338. [DOI] [PubMed] [Google Scholar]
- 20. Schweitzer L, Geisler C, Pourhassan M, et al. What is the best reference site for a single MRI slice to assess whole-body skeletal muscle and adipose tissue volumes in healthy adults? Am J Clin Nutr. 2015;102(1):58–65. [DOI] [PubMed] [Google Scholar]
- 21. Brown JC, Caan BJ, Prado CM, et al. Body composition and cardiovascular events in patients with colorectal cancer: a population-based retrospective cohort study. JAMA Oncol. 2019;5(7):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 23. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York City, NY: Springer; 2015. [Google Scholar]
- 24. Grambsch PM, Therneau TM.. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 25. Groenwold RH, White IR, Donders AR, et al. Missing covariate data in clinical research: when and when not to use the missing-indicator method for analysis. CMAJ. 2012;184(11):1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med. 1983;2(2):243–251. [DOI] [PubMed] [Google Scholar]
- 28. Matthews JN, Altman DG.. Statistics notes: interaction 2: compare effect sizes not P values. BMJ. 1996;313(7060):808.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power ML, Schulkin J.. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99(5):931–940. [DOI] [PubMed] [Google Scholar]
- 30. Woodhouse LJ, Gupta N, Bhasin M, et al. Dose-dependent effects of testosterone on regional adipose tissue distribution in healthy young men. J Clin Endocrinol Metab. 2004;89(2):718–726. [DOI] [PubMed] [Google Scholar]
- 31. Shea KL, Gavin KM, Melanson EL, et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015;22(10):1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tchernof A, Desmeules A, Richard C, et al. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89(7):3425–3430. [DOI] [PubMed] [Google Scholar]
- 33. Folkerd EJ, Dowsett M.. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28(26):4038–4044. [DOI] [PubMed] [Google Scholar]
- 34. Lin JH, Zhang SM, Rexrode KM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11(4):419–424.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.