Abstract
Background
Non-Hispanic black (NHB) adults with cancer may have longer time-to-treatment than non-Hispanic whites (NHW) in the United States. Unequal access to medical care may partially account for this racial disparity. This study aimed to investigate whether there were racial differences in time-to-treatment and in treatment delays for patients diagnosed with colon cancer in the equal-access Military Health System (MHS).
Methods
Patients age 18–79 years diagnosed with colon adenocarcinoma between January 1, 1998, and December 31, 2014, were identified in the Department of Defense Central Cancer Registry and the MHS Data Repository–linked databases. Median time-to-treatment (surgery and chemotherapy) and 95% confidence intervals were compared between NHBs and NHWs in multivariable quantile regression models. Odds ratios and 95% confidence intervals of receiving delayed treatment defined by guidelines for NHBs relative to NHWs were estimated using multivariable logistic regression.
Results
Patients (n = 3067) had a mean age at diagnosis of 58.4 (12.2) years and the racial distribution was 76.7% NHW and 23.3% NHB. Median adjusted time-to-treatment was similar for NHB compared to NHW patients. The likelihood of receiving delayed treatment was similar between NHB and NHW patients.
Conclusions
In the MHS, there was no evidence of treatment delays for NHBs compared to NHWs, suggesting the role of equal access to medical care and insurance coverage in reducing racial disparities in colon cancer treatment.
Although overall incidence and mortality rates of colon cancer are declining in the United States. (1–3), non-Hispanic blacks (NHBs) continue to have higher mortality rates than non-Hispanic whites (NHWs) (1,3,4). Later tumor stage (1,4,5), more comorbidity (5), and suboptimal treatment (1,5), which may be related to lower access to care (6–9) among NHBs, have been described as major contributors to racial disparities in survival. Regarding treatment, it has been shown that appropriate and timely treatment is associated with better survival in colon cancer (10–20) and delayed treatment can negatively affect clinical outcomes (11,12,14–16, 21). Thus, it is important to study adherence to treatment guidelines and treatment timing for racial disparities research.
Disparities in the timing and delivery of colon cancer treatment between NHBs and NHWs have been observed (5,22–28). These studies reported that NHBs are less likely to receive recommended adjuvant chemotherapy for stage II or III disease. There is also evidence of longer time-to-treatment for NHBs than NHWs, with studies reporting NHB patients being more likely to have treatment delays of more than 30 or 90 days after diagnosis (22,29). Reasons for treatment delays and nonadherence are likely multifactorial and may be related to access to care (19,22,30–33). Cancer treatment delays and nonadherence to treatments may also be related to social and cultural factors, health education, or patient–clinician communication about treatment (23,34–36). In the general US population, minorities have less access to care (37–39), making it hard to assess potential effects of the latter independent of access to care. Research in an equal-access health system can provide clues as to whether racial groups differ in time-to-treatment and treatment delays independent of access to care.
The Military Health System (MHS) of the US Department of Defense (DoD) provides universal access to care to more than 9.6 million beneficiaries (40,41). The DoD beneficiaries have access to direct care at military treatment facilities (MTFs) and purchased care through a network of civilian hospitals and clinics. The DoD provides benefit plans for eligible beneficiaries to cover direct and purchased care with little to no out-of-pocket costs regardless of race (41). The main benefit plans managed through TRICARE include Prime, Select, and TRICARE for Life, which is supplemental insurance for those with Medicare coverage (42). The objective of this study was to investigate whether there were racial differences in time-to-treatment and in treatment delays among DoD beneficiaries with colon cancer.
Methods
Data Sources
This study used the DoD Central Cancer Registry (CCR) and the MHS Data Repository (MDR)-linked data, which were described previously (43,44). In brief, the CCR contains information on demographics, cancer diagnosis, treatment, and vital status for patients diagnosed or treated at MTFs beginning in 1998 (45) using North American Association of Central Cancer Registries reporting standards (46). The MDR includes administrative and claims data for inpatient, outpatient, and ancillary services provided at MTFs (direct care) and from civilian providers (purchased care) (47). The CCR and MDR data linkage was approved by the institutional review boards of the Walter Reed National Military Medical Center and the Defense Health Agency.
Study Population
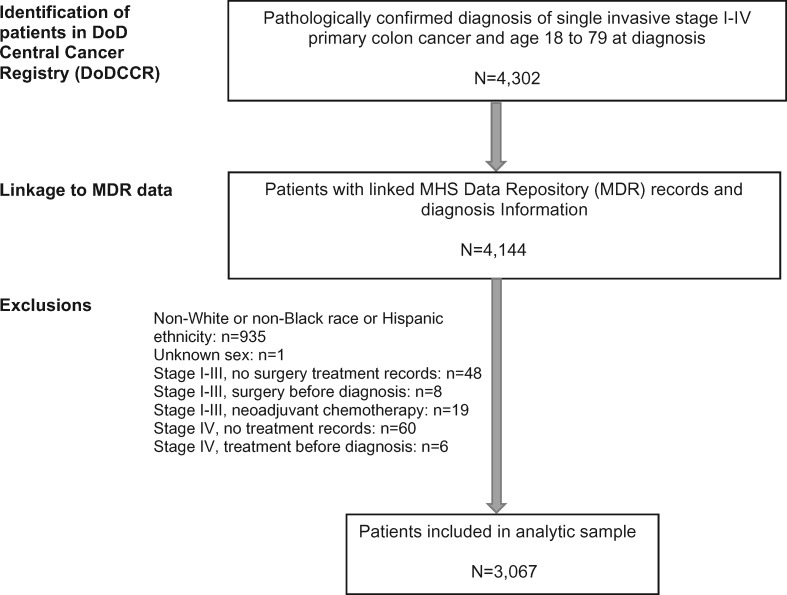
Patients with a pathologically diagnosed primary colon adenocarcinoma (International Classification of Diseases—Oncology, Third Edition [ICD-O-3] topography codes C18.0 and C18.2–C18.9) between January 1, 1998, and December 31, 2014, were identified in the CCR database (Figure 1). Patients with cancer in the rectosigmoid junction (C19.9) were excluded because of debate as to whether it should be classified as “colon” or “rectal” for treatment and surveillance purposes (48). Eligible patients were NHW and NHB men and women with stages I–IV invasive colon cancer diagnosed between the ages of 18 to 79 years who had MDR records. Patients age 80 years or older were excluded because of insufficient evidence to support treatment guidelines in older patients (49). Patients with multiple primary cancers were excluded to minimize potential effects of other cancers on results. Exclusions were also made according to treatment information. We excluded patients with stages I–III tumors who did not have surgery, patients with stage IV tumors who did not have any treatment because of the inability to assess treatment and its timing, and patients with a surgery date prior to diagnosis because this may represent emergent surgical procedures related to symptomatic presentation and advanced disease. Patients with stage I–III tumors who received neoadjuvant chemotherapy were also excluded because neoadjuvant treatment is not a standard procedure for colon cancer (50–52) and can markedly increase time to surgery. Figure 1 shows the selection and exclusion of patients.
Figure 1.
Selection of patients diagnosed with invasive primary colon cancer in the Department of Defense (DoD) Central Cancer Registry (CCR) and Military Health System Data Repository (MDR) linked data, 1998–2014.
Study Variables
The CCR database contained ICD-O-3 primary tumor (T), lymph node (N), and metastasis (M) to define TNM pathologic and clinical stage; tumor grade; tumor location; and diagnosis date. Tumor stage was consolidated to stage I, II, III, or IV, and tumor grade was defined as well-differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), nondifferentiated (G4), or undetermined or unknown (GX) using American Joint Committee on Cancer criteria (53,54). Tumor location was grouped as right or ascending, traverse, left or descending, and overlapping or unknown. The date of diagnostic confirmation in CCR was consolidated with the date of the first MDR diagnosis record (ICD-9-CM: 153.x) using a systematic process to determine diagnosis date.
Surgery is recommended as the primary treatment for stages I–III colon cancer (51,55–57). Adjuvant chemotherapy is recommended for patients with stage II colon cancer who are at high risk for recurrence or progression of the disease and for patients with stage III colon cancer (50,55,57). Because treatment recommendations differ between high-risk and low-risk stage II tumors, we subdivided stage II into high risk and low risk. High-risk features include tumor grades 3 and 4, bowel obstruction, localized perforation, fewer than 12 lymph nodes examined, lymphatic or vascular invasion, or close, indeterminate, or positive surgical margins (49,51,55). Bowel obstruction, localized perforation, and lymphatic or vascular invasion were identified in MDR by presence of ICD-9 diagnosis codes in the 30 days before cancer diagnosis through 30 days after surgery. The time interval was applied to minimize the likelihood of including diagnoses related to other gastrointestinal conditions or to recurrence or progression of cancer. Other high-risk features were obtained from CCR. Patients were considered high-risk stage II if they exhibited at least one of the high-risk features at time of diagnosis or surgery.
The first dates of surgery and chemotherapy treatment were determined from consolidated CCR and MDR records. The CCR database contains the first date of surgery and chemotherapy (if administered). The first MDR record(s) for surgery and chemotherapy were identified using ICD-9 procedure and Common Procedural Terminology codes (58). Adjuvant chemotherapy was defined as the first chemotherapy administration occurring within 6 months of surgery. This period was selected to appropriately capture adjuvant treatment that may have been delayed beyond the clinical recommendations (49,52) while reducing the likelihood of including treatment for disease recurrence or progression.
Several international organizations have established guidelines for the timing of colon cancer treatment. A treatment time target of less than 6 weeks from diagnosis to surgery has been acknowledged as an appropriate quality measure in nonmetastatic disease (59–61). In 2012, the European Society for Medical Oncology released a consensus report suggesting adjuvant chemotherapy be started no later than 8 to 12 weeks after surgery when indicated (52). Patients with stage IV colon cancer are recommended systemic therapy with or without surgery based on tumor and clinical characteristics (50,55). For this cancer stage, it is recommended that treatment be initiated as soon as medically able to improve prognosis (50).
The study outcomes were time-to-treatment and delayed treatment. Time-to-treatment was measured as the time from diagnosis to surgery (stages I–III), time from surgery to adjuvant chemotherapy (high-risk stage II and stage III), or time from diagnosis to any treatment (stage IV). To determine whether a patient had delayed treatment, we used the existing stage-specific guidelines to establish definitions for timely colon cancer treatment as 1) receipt of cancer surgery within 6 weeks of diagnosis (stages I–III), 2) receipt of adjuvant chemotherapy within 8 weeks of surgery (high-risk stage II and stage III), and 3) receipt of initial treatment within 4 weeks of diagnosis (stage IV). Patients were classified as having delayed treatment if the intervals between diagnosis and treatment(s) exceeded these definitions. Patients were classified as having no stage-specific adjuvant treatment if they had high-risk stage II or stage III tumors and did not receive the recommended adjuvant chemotherapy.
Comorbid conditions were identified in MDR records using ICD-9-CM codes for medical conditions included in the Charlson Comorbidity Index (62), excluding cancer because it was the disease of interest. We also included acute and chronic gastrointestinal comorbidities because they may affect time-to-treatment and treatment delays (63). To reduce the possibility of false or suspected diagnoses, comorbid conditions were included if there were at least one inpatient or three outpatient records with a diagnosis code occurring more than 30 days before cancer diagnosis. The number of conditions was categorized as 0, 1, or 2 or more both for the Charlson index and gastrointestinal comorbidity.
Other variables included age at diagnosis, marital status, active-duty status, military service or sponsor branch, TRICARE service region, benefit type, and care source. Benefit types included Prime, Select (formerly Standard or Extra), and TRICARE for Life. Care sources included direct care, purchased care, or both. Benefit type and care source were defined based on records in the 3 months before diagnosis and 3 months following diagnosis.
Statistical Analysis
First, we examined distributions of patient demographic, tumor, and treatment characteristics by race using two-sided χ2 tests for categorical variables with statistical significance at P less than .05. Then, we estimated the stage-specific median time-to-treatment intervals and associated 95% confidence intervals using quantile regression with the bootstrap method for standard errors (64,65). Regression parameter estimates for treatment intervals and 95% confidence intervals were compared between NHBs and NHWs both in univariable and multivariable models. Next, the odds ratios (OR) and 95% confidence intervals for the likelihood of having delayed treatment were estimated and compared between NHBs and NHWs (referent) in univariable and multivariable logistic regression models. We also conducted these analyses comparing NHBs and NHWs by sex or age at diagnosis (<50 years and ≥50 years). The age categories were selected based on the recommended age for colorectal cancer screening (66) because this may influence the mode of detection, stage at diagnosis, and treatment timing. To assess the potential effects of excluding patients on racial comparisons, we compared distributions of race and age between eligible patients and those excluded from the study because of missing sex or unavailable treatment information (Figure 1). The excluded patients (n = 142) had a similar racial distribution but tended to be older than those included in the analysis. Analyses were conducted in SAS 9.4 (SAS Institute Inc, Cary, NC) using two-sided statistical tests. Statistical significance was determined by assessing the point estimates and 95% confidence intervals generated from quantile and logistic regression models. If the 95% confidence intervals around the point estimate excluded the no-effect value (median difference of 0 days in quantile regression or OR of 1 in logistic regression), the effect was considered to be statistically significant.
Results
The 3067 patients in the study had a mean age at diagnosis of 58.4 (12.2) years and mean follow-up time of 6.7 (5.2) years. The patients were 76.7% NHW and 23.3% NHB race. Table 1 shows patient demographic, tumor, and treatment characteristics by race. A higher proportion of NHBs were age 18–49 years at diagnosis, unmarried, and active-duty, had Prime benefit, used direct care, and lived in the north region compared with NHWs. Regarding cancer diagnosis, NHBs were more likely to be diagnosed with stage III or stage IV tumors than NHWs.
Table 1.
Demographic, tumor, and treatment characteristics of non-Hispanic black and white patients with colon cancer in the US Military Health System, 1998–2014
| Characteristics | Non-Hispanic white | Non-Hispanic black | P * |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Sex | .36 | ||
| Men | 1457 (62.0) | 430 (60.1) | |
| Women | 894 (38.0) | 286 (39.9) | |
| Age at diagnosis, y | <.001 | ||
| 18–49 | 454 (19.3) | 210 (29.3) | |
| 50–64 | 1083 (46.1) | 334 (46.6) | |
| 65–79 | 814 (34.6) | 172 (24.0) | |
| Marital status | <.001 | ||
| Married | 1859 (79.1) | 525 (73.3) | |
| Single | 80 (3.4) | 53 (7.4) | |
| Divorced, separated, or widowed | 340 (14.5) | 114 (15.9) | |
| Unknown | 72 (3.1) | 24 (3.4) | |
| Military branch | <.001 | ||
| Army | 727 (30.9) | 322 (45.0) | |
| Navy | 505 (21.5) | 106 (14.8) | |
| Air Force | 728 (31.0) | 181 (25.3) | |
| Marine Corps | 98 (4.2) | 22 (3.1) | |
| Other | 159 (6.8) | 38 (5.3) | |
| Unknown | 134 (5.7) | 47 (6.6) | |
| Active-duty status | <.001 | ||
| No | 2124 (90.3) | 602 (84.1) | |
| Yes | 227 (9.7) | 114 (15.9) | |
| Benefit type | <.001 | ||
| TRICARE Prime | 1202 (51.1) | 396 (55.3) | |
| TRICARE Select | 511 (21.7) | 128 (17.9) | |
| TFL/Medicare | 362 (15.4) | 69 (9.6) | |
| Unknown | 276 (11.7) | 123 (17.2) | |
| Care source | .32 | ||
| Direct (MTFs) | 1448 (61.6) | 467 (65.2) | |
| Purchased (private) | 144 (6.1) | 36 (5.0) | |
| Both | 727 (30.9) | 203 (28.3) | |
| Unknown | 32 (1.4) | 10 (1.4) | |
| Geographic region | <.001 | ||
| North | 661 (28.1) | 277 (38.7) | |
| South | 811 (34.5) | 272 (38.0) | |
| West | 800 (34.0) | 134 (18.7) | |
| Overseas | 79 (3.4) | 33 (4.6) | |
| Diagnosis year | <.001 | ||
| 1998–2001 | 831 (35.3) | 195 (27.2) | |
| 2002–2005 | 613 (26.1) | 186 (26.0) | |
| 2006–2009 | 486 (20.7) | 163 (22.8) | |
| 2010–2014 | 421 (17.9) | 172 (24.0) | |
| Tumor stage (AJCC) | <.001 | ||
| I | 628 (26.7) | 149 (20.8) | |
| Low-risk II | 294 (12.5) | 87 (12.1) | |
| High-risk II | 280 (11.9) | 53 (7.4) | |
| III | 675 (28.7) | 224 (31.3) | |
| IV | 474 (20.2) | 203 (28.3) | |
| Tumor grade (AJCC) | .55 | ||
| Well differentiated (G1) | 405 (17.2) | 110 (15.4) | |
| Moderately differentiated (G2) | 1392 (59.2) | 445 (62.1) | |
| Poorly differentiated (G3) | 341 (14.5) | 100 (14.0) | |
| Undifferentiated (G4) | 18 (0.8) | 3 (0.4) | |
| Unknown (GX) | 195 (8.3) | 58 (8.1) | |
| Tumor location | .03 | ||
| Right/ascending | 980 (41.7) | 342 (47.8) | |
| Transverse | 181 (7.7) | 54 (7.5) | |
| Left/descending | 1002 (42.6) | 268 (37.4) | |
| Overlapping/unknown | 188 (8.0) | 52 (7.3) | |
| Surgery type† | .43 | ||
| Local excision | 66 (3.5) | 12 (2.3) | |
| Subtotal/partial colectomy | 1691 (90.1) | 474 (92.4) | |
| Total colectomy | 22 (1.2) | 5 (1.0) | |
| Other | 98 (5.2) | 22 (4.3) | |
| Charlson Comorbidity Index conditions | .07 | ||
| 0 | 1644 (69.9) | 486 (67.9) | |
| 1 | 376 (16.0) | 140 (19.5) | |
| 2 or more | 331 (14.1) | 90 (12.6) | |
| Gastrointestinal comorbid conditions | .44 | ||
| 0 | 2057 (87.9) | 635 (88.7) | |
| 1 | 254 (10.8) | 76 (10.6) | |
| 2 or more | 30 (1.3) | 5 (0.7) | |
P value for two-sided χ2 tests of distribution for categorical variables. AJCC = American Joint Committee on Cancer, 6th edition (1998–2009) and 7th edition (2010–2014); MTF = military treatment facility; TFL = TRICARE for Life.
Reported for stages I–III only.
Table 2 shows the median time-to-treatment for NHWs and NHBs. There were no statistically significant racial differences in time-to-treatment within each stage and treatment group. When all stages were combined, there was also no statistically significant difference in overall time-to-treatment between NHBs and NHWs (difference = −0.4 days, 95% CI = −2.2 to 1.3 days) (data not shown in table).
Table 2.
Quantile regression estimated median time-to-treatment (95% confidence intervals [CI]) and difference in median time-to-treatment between non-Hispanic white and non-Hispanic black adults with colon cancer
| Stage group and treatment | Treatment interval | Non-Hispanic white |
Non-Hispanic black |
Model-estimated difference* |
|||
|---|---|---|---|---|---|---|---|
| No. | Time-to-treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | Unadjusted | Adjusted† | ||
| Stage I and stage II (low risk) | |||||||
| Surgery | Diagnosis–surgery | 922 | 7.0 (5.0 to 9.0) | 236 | 9.0 (3.7 to 14.2) | 2.0 (−3.4 to 7.4) | 1.4 (−1.9 to 4.7) |
| Stage II (high risk) and stage III | |||||||
| Surgery only | Diagnosis–surgery | 262 | 4.0 (1.2 to 6.8) | 66 | 7.0 (1.5 to 12.5) | 3.0 (−2.6 to 8.6) | 3.2 (−1.3 to 7.8) |
| Surgery and adjuvant chemotherapy | Diagnosis–surgery | 693 | 7.0 (5.4 to 8.5) | 211 | 7.0 (4.9 to 9.1) | 0.0 (−2.6 to 2.6) | −1.9 (−4.3 to 0.5) |
| Surgery–chemo | 42.0 (40.7 to 43.3) | 44.0 (41.0 to 47.0) | 2.0 (−1.8 to 5.8) | 1.2 (−2.8 to 4.1) | |||
| Stage IV | |||||||
| Surgery or chemotherapy | Diagnosis–treatment | 474 | 9.0 (6.4 to 11.6) | 203 | 7.0 (3.9 to 10.1) | −2.0 (−5.5 to 1.5) | −2.3 (−5.3 to 0.6) |
Difference modeled is non-Hispanic black compared with non-Hispanic white as the reference group.
Regression model adjusted for sex, age at diagnosis, marital status, military service branch, active-duty status, benefit type, care source, geographic region, year of diagnosis, tumor stage (except model including stage IV only), and Charlson and gastrointestinal comorbid conditions.
Table 3 shows the likelihood of delayed treatment by race. There were no statistically significant racial differences in likelihood of delayed surgery within the stage and treatment groups. Among patients with high-risk stage II and stage III tumors, 23.8% of NHBs and 27.4% of NHWs did not receive guideline-recommended adjuvant chemotherapy with an adjusted odds ratio (AOR) of 0.83 (95% CI = 0.61 to 1.13; NHB compared with NHW) (data not shown). Of patients receiving adjuvant chemotherapy, the likelihood of delayed chemotherapy was similar comparing NHB to NHW patients (AOR = 1.13, 95% CI = 0.77 to 1.67). For stage IV disease, there were no racial differences in the likelihood of delayed treatment (AOR = 1.11, 95% CI = 0.72 to 1.71). The overall likelihood of receiving delayed treatment or no stage-specific adjuvant treatment when stages I–IV were combined was similar between NHBs and NHWs (AOR = 1.12, 95% CI = 0.90 to 1.40) (data not shown in table).
Table 3.
Logistic regression estimated odds ratios (OR) and 95% confidence intervals (CI) associated with race-ethnicity for delayed treatment among patients with colon cancer, US Military Health System, 1998–2014
| Stage group and treatment | Non-Hispanic white |
Non-Hispanic black |
OR (95% CI)* | Adjusted OR (95% CI)*,† | ||
|---|---|---|---|---|---|---|
| No. | Delayed‡ No. (%) | No. | Delayed‡ No. (%) | |||
| Stage I and stage II (low risk) | ||||||
| Surgery | 922 | 80 (8.7) | 236 | 21 (8.9) | 1.03 (0.62 to 1.70) | 0.96 (0.56 to 1.63) |
| Stage II (high risk) and stage III | ||||||
| Surgery only | 262 | 18 (6.9) | 66 | 9 (13.6) | 2.14 (0.91 to 5.01) | 2.66 (0.98 to 7.21) |
| Surgery and adjuvant chemotherapy | 693 | — | 211 | — | — | — |
| Surgery | — | 26 (3.7) | — | 9 (4.3) | 1.14 (0.53 to 2.48) | 1.09 (0.47 to 2.53) |
| Chemotherapy | — | 165 (23.8) | — | 53 (25.1) | 1.07 (0.75 to 1.53) | 1.13 (0.77 to 1.67) |
| Stage IV | ||||||
| Surgery or chemotherapy | 474 | 92 (19.4) | 203 | 45 (22.2) | 1.18 (0.79 to 1.77) | 1.11 (0.72 to 1.71) |
Odds ratios presented are for non-Hispanic black compared with non-Hispanic white as reference group. OR = odds ratio.
Regression model adjusted for sex, age at diagnosis, marital status, military service branch, active-duty status, benefit type, care source, geographic region, year of diagnosis, tumor stage (except model including stage IV only), and Charlson and gastrointestinal comorbid conditions.
Delayed treatment defined as surgery more than 6 weeks after diagnosis (stages I–III); or adjuvant chemotherapy more than 8 weeks after surgery (stage II high risk or stage III); or first treatment more than 4 weeks after diagnosis (stage IV).
The median time-to-treatment by sex is shown in Table 4. For high-risk stage II or stage III cancer, NHB women receiving surgery and chemotherapy had statistically significant shorter adjusted median time-to-surgery than NHW women (difference = −4.3 days, 95% CI = −7.6 to −0.9). Among patients with stage IV tumors, NHB women had statistically significant shorter adjusted median time-to-treatment (d = −6.9 days, 95% CI = −11.7 to −2.0) than NHW women. However, the shorter time-to-treatment among NHB women did not extend to differences in delayed treatment, whereas the numbers with delayed treatment were small when stratified both by sex and tumor stage (data not shown). On the contrary, NHB women with high-risk stage II or stage III cancer who received only surgery were more likely to have delayed surgery than NHW women (AOR = 12.56, 95% CI = 1.48 to 106.52). However, there were only five NHB and seven NHW women with delayed surgery in this subgroup. There were no statistically significant differences in treatment outcomes among men. There were no differences in median time-to-treatment between races when patients were stratified by age at diagnosis (Table 5). No racial differences were observed by age in stage-specific delayed treatment, although the numbers with delayed treatment were small (data not shown).
Table 4.
Quantile regression estimated median time-to-treatment (95% confidence intervals [CI]) and difference in median time-to-treatment between non-Hispanic white and non-Hispanic black adults with colon cancer, stratified by sex
| Stage group, treatment, and treatment interval | Male |
Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Hispanic white |
Non-Hispanic black |
Model-estimated difference* | Non-Hispanic white |
Non-Hispanic black |
Model-estimated difference* | |||||
| No. | Time-to-treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | |||
| Stages I and stage II (low-risk) | ||||||||||
| Surgery | ||||||||||
| Diagnosis–surgery | 579 | 7.0 (3.9 to 10.1) | 149 | 8.0 (1.2 to 14.7) | −0.4 (−4.1 to 3.3) | 343 | 8.0 (5.4 to 10.6) | 87 | 13.0 (5.9 to 20.1) | 2.7 (−3.1 to 8.6) |
| Stage II (high risk) and stage III | ||||||||||
| Surgery only | ||||||||||
| Diagnosis–surgery | 159 | 4.0 (0.4 to 7.6) | 40 | 6.0 (0.3 to 11.7) | 0.1 (−5.1 to 5.4) | 103 | 4.0 (−0.5 to 8.5) | 26 | 11.0 (1.5 to 20.5) | 8.2 (−1.5 to 17.9) |
| Surgery and adjuvant chemotherapy | 415 | 124 | 278 | 87 | ||||||
| Diagnosis–surgery | 8.0 (5.8 to 10.2) | 8.0 (4.5 to 11.5) | −0.2 (−3.8 to 3.3) | 6.0 (4.0 to 8.0) | 3.0 (−1.5 to 7.5) | −4.3 (−7.6 to -0.9) | ||||
| Surgery–chemo | 42.0 (40.1 to 43.9) | 45.0 (40.9 to 49.1) | 2.0 (−2.1 to 6.0) | 42.0 (39.8 to 44.2) | 43.0 (38.6 to 47.4) | 2.0 (−2.3 to 6.2) | ||||
| Stage IV | ||||||||||
| Surgery or chemotherapy | ||||||||||
| Diagnosis–treatment | 304 | 8.0 (4.8 to 11.2) | 117 | 8.0 (4.3 to 11.7) | 1.0 (−2.7 to 4.7) | 170 | 11.0 (7.8 to 14.2) | 86 | 6.0 (1.1 to 10.9) | −6.9 (−11.7 to -2.0) |
Difference modeled is Non-Hispanic black compared with Non-Hispanic white as the reference group. Regression model adjusted for age, marital status, military service branch, active-duty status, benefit type, care source, geographic region, year of diagnosis, tumor stage (except model including stage IV only), and Charlson and gastrointestinal comorbid conditions.
Table 5.
Quantile regression estimated median time-to-treatment (95% confidence intervals [CI]) and difference in median time-to-treatment between non-Hispanic white and non-Hispanic black adults with colon cancer, stratified by age at diagnosis
| Stage group, treatment, and treatment interval | Age at diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age <50 y |
Age ≥50 y |
|||||||||
| Non-Hispanic white |
Non-Hispanic black |
Model-estimated difference* | Non-Hispanic white |
Non-Hispanic black |
Model-estimated difference* | |||||
| No. | Time-to-treatment, d (95% CI) | No. | Time-to- treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | No. | Time-to-treatment, d (95% CI) | |||
| Stages I and stage II (low risk) | ||||||||||
| Surgery | ||||||||||
| Diagnosis–surgery | 134 | 6.0 (1.4 to 10.6) | 51 | 7.0 (0.4 to 13.6) | 0.0 (−4.6 to 4.6) | 788 | 7.0 (4.9 to 9.1) | 185 | 11.0 (5.7 to 16.3) | 1.1 (−2.7 to 4.9) |
| Stage II (high risk) and stage III | ||||||||||
| Surgery only | ||||||||||
| Diagnosis–surgery | 21 | 4.0 (−5.2 to 13.2) | 9 | 13.0 (−2.3 to 28.3) | —† | 241 | 4.0 (1.0 to 7.0) | 57 | 7.0 (1.7 to 12.2) | 3.0 (−1.5 to 7.5) |
| Surgery and adjuvant chemotherapy | 178 | 83 | 515 | 128 | ||||||
| Diagnosis-surgery | 6.0 (3.0 to 9.0) | 6.0 (1.9 to 10.1) | −2.2 (−6.0 to 1.7) | 7.0 (4.9 to 9.1) | 7.0 (3.9 to 10.1) | −2.0 (−5.6 to 1.5) | ||||
| Surgery–chemo | 41.0 (38.7 to 43.3) | 46.0 (42.5 to 49.5) | 5.1 (−0.6 to 10.8) | 43.0 (41.3 to 44.7) | 42.0 (38.5 to 45.5) | 0.2 (−4.3 to 4.6) | ||||
| Stage IV | ||||||||||
| Surgery or chemotherapy | ||||||||||
| Diagnosis–treatment | 121 | 8.0 (3.9 to 12.1) | 67 | 7.0 (0.9 to 13.1) | 0.9 (−5.4 to 7.3) | 353 | 10.0 (7.0 to 13.0) | 136 | 7.0 (3.3 to 10.7) | −1.7 (−5.8 to 2.4) |
Difference modeled is non-Hispanic black compared with non-Hispanic white as the reference group. Regression model adjusted for sex, marital status, military service branch, active-duty status, benefit type, care source, geographic region, year of diagnosis, tumor stage (except model including stage IV only), and Charlson and gastrointestinal comorbid conditions. CI = confidence interval.
Small size (n = 30) precluded reliable adjusted estimate.
Discussion
Overall, patients in the MHS received stage-appropriate colon cancer treatments in line with clinical recommendations. NHB patients had similar time-to-treatment as NHW patients both overall and within each stage group. In stratified analysis, NHB women had shorter time-to-surgery than NHW women among those with high-risk stage II or stage III tumors receiving surgery and adjuvant chemotherapy and shorter time-to-treatment among patients with stage IV disease. Overall, there were no statistical differences in the likelihood of receiving delayed treatment beyond guidelines for surgery or stage-indicated adjuvant chemotherapy between NHBs and NHWs.
Potential racial differences in time-to-treatment are a concern because treatment delays may be associated with poorer outcomes (11,13). A National Cancer Database study found that black patients with colon cancer were more likely to have treatment delays greater than 30 days than white patients (29). In another study, 14% more NHB patients experienced prolonged delays greater than 90 days from symptom or screen-detected lesion to initiation of treatment than NHW patients (22). In contrast to these studies, we found that NHB and NHW patients with colon cancer had similar time-to-treatment in the MHS, where patients have equal access to care. In fact, we found shorter time-to-treatments for NHB women with high-risk stage II or III and stage IV disease than NHW women. Nevertheless, there was no evidence of racial differences in treatment delays overall.
Our findings build on previous evaluations of colon cancer treatment within the MHS (44,67). One study found no statistically significant differences in receipt of surgery and chemotherapy comparing NHB to NHW patients overall or among subgroups (44). Another study reported similar rates of adjuvant chemotherapy for NHB and NHW patients with stage III tumors (67). In a study of patients with stage III colon cancer treated within Veterans Affairs Medical Centers (VAMCs), also an equal-access system, investigators did not observe any racial disparities in receipt of chemotherapy (68). However, another VAMC study reported a higher likelihood of surgery delays of 45 days or more for black patients with stages I–III colon cancer than white patients (69). The inconsistency between the VAMC studies might be related to differences in study design and outcomes and point to the need for further evaluation of other factors that may influence treatment aside from access to care. Nevertheless, the consistent findings of no racial differences in the MHS may suggest the possible role of universal access to cancer care in minimizing the racial disparities in colon cancer treatment observed in the general US population (23–25). Our study further supports the effects of universal access by showing the similar rates of adherence to treatment guidelines among NHB and NHW patients. This is important for clinical care because receipt of timely treatment may have implications for reducing risk of recurrence and mortality (18,19) and possible racial disparities in these outcomes.
We included multiple metrics in our assessment of whether there are racial disparities in colon cancer treatment in the MHS. However, our study had limitations. First, although we used guideline-based definitions for defining treatment delays, recommendations vary among organizations. Nevertheless, we found similar results defining treatment delays by using the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommendation of adjuvant chemotherapy within 4 months of diagnosis when the treatment is indicated (49). Second, we considered only the first surgery and/or initiation of adjuvant therapy and did not include the full course of treatment. Thus, our findings related to racial differences in colon cancer treatment may not translate to the full course of therapy. Third, our study included patients diagnosed or treated at MTFs who were captured in the CCR data. Patients who received cancer care exclusively at civilian facilities were not included. Thus, our findings may not be generalizable to the entire beneficiary population. Lastly, subgroup analysis was limited by the relatively few patients who received treatment beyond the recommendations. Although this is good clinically, it limited our ability to detect racial differences in receiving delayed treatment.
In general, there was no evidence of longer time-to-treatment for NHB compared to NHW patients with colon cancer in the MHS. Further, the overall likelihood of receiving treatment adherent to recommended guidelines was similar between races. Our results support the role of universal access to care in reducing racial disparities in colon cancer treatment.
Funding
This project was supported by the Murtha Cancer Center Research Program of the Uniformed Services University of the Health Sciences and Walter Reed National Military Medical Center under the auspices of the Henry M. Jackson Foundation for the Advancement of Military Medicine.
Notes
The study sponsors did not have a direct role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The contents of this publication are the sole responsibility of the authors and do not reflect the views, assertions, opinions or policies of the Uniformed Services University of the Health Sciences, the DoD, or the Departments of the Army, Navy, or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the US government.
The authors have no conflicts of interest to disclose.
The authors thank the following institutes for their contributions to the original data linkage project: Division of Cancer Epidemiology and Genetics, National Cancer Institute; ICF Macro, Kennell and Associates, Inc, the Defense Health Agency, the Joint Pathology Center, and former Armed Forces Institute of Pathology.
References
- 1. White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C.. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5014–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures, 2019. Atlanta, GA: American Cancer Society; 2019. https://www.cancer.org/research/cancer-facts-statistics.html. Accessed January 11, 2019. [Google Scholar]
- 4. Alexander DD, Waterbor J, Hughes T, Funkhouser E, Grizzle W, Manne U.. African-American and Caucasian disparities in colorectal cancer mortality and survival by data source: an epidemiologic review. Cancer Biomark. 2007;3(6):301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai Y, Wang C, Civan JM, et al. Effects of cancer stage and treatment differences on racial disparities in survival from colon cancer: a United States population-based study. Gastroenterology. 2016;150(5):1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gorey KM, Kanjeekal SM, Wright FC, et al. Colon cancer care and survival: income and insurance are more predictive in the USA, community primary care physician supply more so in Canada. Int J Equity Health. 2015;14(1):109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tammana VS, Laiyemo AO.. Colorectal cancer disparities: issues, controversies and solutions. World J Gastroenterol. 2014;20(4):869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sineshaw HM, Ng K, Flanders WD, Brawley OW, Jemal A.. Factors that contribute to differences in survival of black vs white patients with colorectal cancer. Gastroenterology. 2018;154(4):906–915.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pulte D, Jansen L, Brenner H.. Social disparities in survival after diagnosis with colorectal cancer: contribution of race and insurance status. Cancer Epidemiol 2017;48:41–47. [DOI] [PubMed] [Google Scholar]
- 10. Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112(suppl 1):S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM.. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011;305(22):2335–2342. [DOI] [PubMed] [Google Scholar]
- 12. Des Guetz G, Nicolas P, Perret G-Y, Morere J-F, Uzzan B.. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46(6):1049–1055. [DOI] [PubMed] [Google Scholar]
- 13. Amri R, Bordeianou LG, Sylla P, Berger DL.. Treatment delay in surgically-treated colon cancer: Does it affect outcomes? Ann Surg Oncol. 2014;21(12):3909–3916. [DOI] [PubMed] [Google Scholar]
- 14. Czaykowski PM, Gill S, Kennecke HF, Gordon VL, Turner D.. Adjuvant chemotherapy for stage III colon cancer: Does timing matter? Dis Colon Rectum. 2011;54(9):1082–1089. [DOI] [PubMed] [Google Scholar]
- 15. Nachiappan S, Askari A, Mamidanna R, et al. The impact of adjuvant chemotherapy timing on overall survival following colorectal cancer resection. Eur J Surg Oncol. 2015;41(12):1636–1644. [DOI] [PubMed] [Google Scholar]
- 16. Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer. 2015;51(17):2553–2561. [DOI] [PubMed] [Google Scholar]
- 17. Roland CL, Schwarz RE, Tong L, et al. Is timing to delivery of treatment a reliable measure of quality of care for patients with colorectal adenocarcinoma? Surgery. 2013;154(3):421–428. [DOI] [PubMed] [Google Scholar]
- 18. Cronin DP, Harlan LC, Potosky AL, Clegg LX, Stevens JL, Mooney MM.. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterology. 2006;101(10):2308–2318. [DOI] [PubMed] [Google Scholar]
- 19. Hines RB, Barrett A, Twumasi-Ankrah P, et al. Predictors of guideline treatment nonadherence and the impact on survival in patients with colorectal cancer. J Natl Compr Canc Netw. 2015;13(1):51–60. [DOI] [PubMed] [Google Scholar]
- 20. Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107(11):2581–2588. [DOI] [PubMed] [Google Scholar]
- 22. Jones LA, Ferrans CE, Polite BN, et al. Examining racial disparities in colon cancer clinical delay in the colon cancer patterns of care in Chicago study. Ann Epidemiol. 2017;27(11):731–738.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baldwin LM, Dobie SA, Billingsley K, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005;97(16):1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dimou A, Syrigos KN, Saif MW.. Disparities in colorectal cancer in African-Americans vs whites: before and after diagnosis. World J Gastroenterol. 2009;15(30):3734–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demissie K, Oluwole OO, Balasubramanian BA, Osinubi OO, August D, Rhoads GG.. Racial differences in the treatment of colorectal cancer: a comparison of surgical and radiation therapy between whites and blacks. Ann Epidemiol. 2004;14(3):215–221. [DOI] [PubMed] [Google Scholar]
- 26. Potosky AL, Harlan LC, Kaplan RS, Johnson KA, Lynch CF.. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002;20(5):1192–1202. [DOI] [PubMed] [Google Scholar]
- 27. Shavers VL, Brown ML.. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. [DOI] [PubMed] [Google Scholar]
- 28. Berry J, Bumpers K, Ogunlade V, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27(1):59–83. [DOI] [PubMed] [Google Scholar]
- 29. Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253(4):779–785. [DOI] [PubMed] [Google Scholar]
- 30. Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM.. Association of delays in surgery for melanoma with insurance type. JAMA Dermatol. 2017;153(11):1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bestvina CM, Zullig LL, Yousuf Zafar S.. The implications of out-of-pocket cost of cancer treatment in the USA: a critical appraisal of the literature. Future Oncol. 2014;10(14):2189–2199. [DOI] [PubMed] [Google Scholar]
- 32. Abdelsattar ZM, Hendren S, Wong SL.. The impact of health insurance on cancer care in disadvantaged communities. Cancer. 2017;123(7):1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30(9):972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bouwman F, Smits A, Lopes A, et al. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery–an institutional study and systematic review of the literature. Gynecol Oncol. 2015;139(2):369–376. [DOI] [PubMed] [Google Scholar]
- 35. Antonio M, Carmona-Bayonas A, Saldana J, et al. Factors predicting adherence to a tailored-dose adjuvant treatment on the basis of geriatric assessment in elderly people with colorectal cancer: a prospective study. Clin Colorectal Cancer. 2018;17(1):e59–e68. [DOI] [PubMed] [Google Scholar]
- 36. Lin C, Clark R, Tu P, Bosworth HB, Zullig LL.. Breast cancer oral anti-cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat. 2017;165(2):247–260. [DOI] [PubMed] [Google Scholar]
- 37. Grant SR, Walker GV, Guadagnolo BA, Koshy M, Allen PK, Mahmood U.. Variation in insurance status by patient demographics and tumor site among nonelderly adult patients with cancer. Cancer. 2015;121(12):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayberry RM, Mili F, Ofili E.. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(suppl 1):108–145. [DOI] [PubMed] [Google Scholar]
- 39. Wheeler SM, Bryant AS.. Racial and ethnic disparities in health and health care. Obstet Gynecol Clin North Am. 2017;44(1):1–11. [DOI] [PubMed] [Google Scholar]
- 40.Health.mil. Falls Church, VA: Defense Health Agency. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency. Accessed January 31, 2019.
- 41.Defense Health Agency Decision Support Division. Evaluation of the TRICARE Program: Fiscal year 2018 report to Congress. 2018. https://health.mil/Military-Health-Topics/Access-Cost-Quality-and-Safety/Health-Care-Program-Evaluation/Annual-Evaluation-of-the-TRICARE-Program. Accessed April 25, 2019.
- 42.Defense Health Agency. TRICARE. www.tricare.mil. Accessed April 5, 2019.
- 43. Enewold L, Zhou J, McGlynn KA, et al. Racial variation in breast cancer treatment among Department of Defense beneficiaries. Cancer. 2012;118(3):812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gill AA, Enewold L, Zahm SH, et al. Colon cancer treatment: are there racial disparities in an equal-access healthcare system? Dis Colon Rectum. 2014;57(9):1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The Department of Defense Joint Pathology Center. DoD Cancer Registry Program. 2014. https://www.jpc.capmed.mil/education/dodccrs/index.asp. Accessed April 5, 2019.
- 46.North American Association of Central Cancer Registries. Central Registry Standards. 2018. https://www.naaccr.org. Accessed April 25, 2019.
- 47.Defense Health Agency. Military Health System Data Repository. https://www.health.mil/Military-Health-Topics/Technology/Clinical-Support/Military-Health-System-Data-Repository. Accessed October 26, 2017.
- 48. D’Souza N, de Neree Tot Babberich MPM, Lord A, et al. The rectosigmoid problem. Surg Oncol. 2018;27(3):521–525. [DOI] [PubMed] [Google Scholar]
- 49. Desch CE, McNiff KK, Schneider EC, et al. American Society of Clinical Oncology/National Comprehensive Cancer Network quality measures. J Clin Oncol. 2008;26(21):3631–3637. [DOI] [PubMed] [Google Scholar]
- 50.National Comprehensive Cancer Network. Colon Cancer, version 1.2018. Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2018. https://www.NCCN.org. Accessed March 1, 2018.
- 51. van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50(1):1.e1–1.e34. [DOI] [PubMed] [Google Scholar]
- 52. Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479–2516. [DOI] [PubMed] [Google Scholar]
- 53. Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed Chicago, IL: Springer; 2002. [Google Scholar]
- 54. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene GL, Triotti A.. AJCC Cancer Staging Manual. 7th ed Chicago, IL: Springer; 2010. [Google Scholar]
- 55. Engstrom PF, Arnoletti JP, Benson AB III, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw. 2009;7(8):778–831. [DOI] [PubMed] [Google Scholar]
- 56. Benson AB III, Bekaii-Saab T, Chan E, et al. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11(5):519–528. [DOI] [PubMed] [Google Scholar]
- 57. Benson AB III, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw. 2014;12(7):1028–1059. [DOI] [PubMed] [Google Scholar]
- 58. Eaglehouse YL, Georg MW, Richard P, Shriver CD, Zhu K.. Costs for colon cancer treatment comparing benefit types and care sources in the U.S. Military Health System. Mil Med. 2019:1–9; doi: 10.1093/milmed/usz065. [DOI] [PubMed] [Google Scholar]
- 59.Cancer Care Ontario. Target wait times for cancer surgery in Ontario. https://www.cancercareontario.ca/en/content/target-wait-times-cancer-surgery-ontario. Accessed March 1, 2018.
- 60.National Health Service England. Delivering Cancer Waiting Times: A Good Practice Guide 2014. https://www.england.nhs.uk/wp-content/uploads/2015/03/delivering-cancer-wait-times.pdf. Accessed March 5, 2018.
- 61. Beets G, Sebag-Montefiore D, Andritsch E, et al. ECCO essential requirements for quality cancer care: colorectal cancer. A critical review. Critic Rev Oncol Hematol. 2017;110:81–93. [DOI] [PubMed] [Google Scholar]
- 62. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 63. Pruitt SL, Harzke AJ, Davidson NO, Schootman M.. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control. 2013;24(5):961–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Le Cook B, Manning WG.. Thinking beyond the mean: a practical guide for using quantile regression methods for health services research. Shanghai Arch Psychiatry. 2013;25(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Choi D, Hoffman KA, Kim M-O, McCarty D.. A high-resolution analysis of process improvement: use of quantile regression for wait time. Health Serv Res. 2013;48(1):333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.U.S. Preventive Services Task Force. Final Update Summary: Colorectal Cancer Screening. 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening. Accessed May 11, 2018.
- 67. Hofmann LJ, Lee S, Waddell B, Davis KG.. Effect of race on colon cancer treatment and outcomes in the Department of Defense healthcare system. Dis Colon Rectum. 2010;53(1):9–15. [DOI] [PubMed] [Google Scholar]
- 68. Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ.. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118(13):3345–3355. [DOI] [PubMed] [Google Scholar]
- 69. Merkow RP, Bilimoria KY, Sherman KL, McCarter MD, Gordon HS, Bentrem DJ.. Efficiency of colorectal cancer care among veterans: analysis of treatment wait times at Veterans Affairs Medical Centers. J Oncol Pract. 2013;9(4):e154–e163. [DOI] [PMC free article] [PubMed] [Google Scholar]