Abstract
Background
The Ontario Breast Screening Program recommends annual mammography to women age 50–74 years at increased risk because of family history of breast or ovarian cancer or personal history of ovarian cancer or mammographic density 75% or greater. Few studies have examined the diagnostic accuracy of recommendations based on risk factors and included screen film as well as digital mammography.
Methods
A retrospective design identified concurrent cohorts of women age 50–74 years screened annually or biennially with digital mammography only between 2011 and 2014 and followed until 2016 or breast cancer diagnosis. Diagnostic accuracy measures were compared between women screened annually because of first-degree relative of breast or ovarian cancer or personal history of ovarian cancer (n = 67 795 women), mammographic density 75% or greater (n = 51 956), or both (n = 3758) and those screened biennially (n = 526 815). The association between recommendation and sensitivity and specificity was assessed using generalized estimating equation models. All P values are two-sided.
Results
For annual screening because of family or personal history vs biennial, sensitivity was statistically significantly higher (81.7% vs 70.6%; OR = 1.86, 95% CI = 1.48 to 2.34), particularly for invasive cancers and postmenopausal women. Although there was no statistically significant difference in sensitivity for annual screening for mammographic density 75% or greater, specificity was statistically significantly lower (91.3%; OR = 0.87, 95% CI = 0.80 to 0.96) vs biennial (92.3%), particularly for women age 50–59 years.
Conclusion
Compared with biennial screening, annual screening improved detection for women with a family or personal history of breast and/or ovarian cancer, supporting screening that is more frequent. The benefit for annual screening for women with higher mammographic density must be weighed against possible harms of increased false positives.
Offering women at higher risk more tailored breast screening may help improve detection of earlier-stage disease and reduce the risk of having an interval cancer. Interval cancers detected between screening examinations are more likely than screen-detected cancers to have a poorer prognosis (1–3). Although guidelines recommend mammography screening every 2 to 3 years for women age 50–74 years (4,5), women at increased risk may benefit from more frequent screening. However, benefits must be weighed against possible harms, such as increased number of false-positive screens that can cause anxiety, distress, and unnecessary biopsies (6–8). Maintaining a high sensitivity and therefore a low rate of interval cancers is integral to the success of a screening test. A high specificity reflects the ability of a test to accurately identify cases that are not cancer, reducing the number of false positives.
The Breast Cancer Surveillance Consortium published several large, observational studies comparing the benefits and harms of different screening intervals (9–14). These studies suggested that annual mammography had minimal additional benefit over biennial mammography for women 50 years or older as well as those with dense breasts or using hormone therapy (9,10), or more closely adhering to screening guidelines (14). Women who underwent biennial mammography had a lower cumulative risk of false-positive results compared with those who underwent annual mammography (11). However, most women received screen-film mammography, and outcomes were examined by time since last screen rather than recommendation. In Breast Screen New South Wales. women with a first-degree relative with breast cancer offered annual compared with biennial screening were more likely to be diagnosed with smaller node–negative invasive cancers (15). Another Canadian study found that offering annual screening minimally improved the estimated survival rates for women age 50–74 years (16).
Few studies have examined diagnostic accuracy of screening recommendations based on risk factors and included screen-film and digital mammography alike. Digital mammography has received attention as an improved imaging modality for breast screening with higher detection rates among women more likely to have their cancer missed by screen-film mammography (17,18). Although women are screened every 2 years in the Ontario Breast Screening Program (OBSP), those at increased breast cancer risk are screened annually. This provides a unique opportunity to determine the effectiveness of these screening recommendations using digital mammography only. Diagnostic accuracy measures were compared between concurrent cohorts of women age 50–74 years screened annually because of first-degree relative of breast or ovarian cancer or a personal history of ovarian cancer, mammographic density 75% or greater or a first-degree relative of breast or ovarian cancer or personal history of ovarian cancer and mammographic density 75% or greater, and those at average risk screened biennially.
Methods
Study Population
The OBSP has operated since 1990 to deliver a population-based breast screening program to eligible women and has provided digital mammography since 2006 (19). Women are not eligible if they had a prior breast cancer or augmentation mammoplasty or if they currently have acute breast symptoms. At OBSP centers, quality assurance on equipment meets that specified by the Canadian Association of Radiologist’s Mammography Accreditation Program, and radiologists and technologists are accredited under the Canadian Association of Radiologist’s Mammography Accreditation Program. During this study, women were screened at 162 OBSP centers. The study was approved by the University of Toronto Research Ethics Board, and informed consent was not required.
This study employs a cohort design to identify concurrent groups of women age 50–74 years screened in the OBSP with a digital mammogram between January 1, 2011, and December 31, 2014, followed until diagnosis of breast cancer or December 31, 2016. Women screened annually could have two or more first-degree female relatives with breast cancer at any age, one first-degree female relative with breast cancer younger than 50 years, one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, one first-degree female relative with ovarian cancer at any age, and/or mammographic density 75% or greater. This cohort was grouped on an intention-to-treat basis, according to whether the woman was offered a biennial or annual screening recommendation rather than time since last screen. Only women whose screening recommendation remained the same over the study period were included. Rescreen mammograms were identified as the index screen and grouped by recommendation of previous screen. The final cohort included women screened annually because of a first-degree relative of breast or ovarian cancer or personal history of ovarian cancer (n = 67 795), mammographic density 75% or greater (n = 51 956), or both (n = 3758), and those screened biennially (n = 526 815).
Demographic and Breast Cancer Risk Factors
Information for all women screened within the OBSP was obtained from data routinely collected by the Integrated Client Management System (ICMS). Relevant risk factor information was obtained during the screening visit through a personal interview with the technologist. For family or personal history, data on female first-degree relatives with breast or ovarian cancer and age of diagnosis, male first-degree relatives with breast cancer, and personal history of ovarian cancer were collected. Age at menarche (≤11 years, >11 years) and menopausal status (premenopausal, postmenopausal) were also measured. Women were defined as current estrogen users if they reported taking estrogen at their last screening examination. Women’s postal code of residence at screening was linked to the 2011 Canadian Census (20) to determine community status and socioeconomic status. Community status included urban (population ≥10 000), rural (<10 000 and a strong metropolitan influenced zone [MIZ]), rural remote (<10 000 and a moderate MIZ), and rural very remote (<10 000 and a weak or no MIZ). Socioeconomic status was defined by five income quintiles (Q1 [lowest]–Q5 [highest]).
Screening and Assessment Characteristics
Information on screening visit and assessment was obtained through the ICMS. Digital mammograms were defined as rescreens for women who had more than one OBSP mammogram. Age and year at screening were based on the dates of all rescreen mammograms prior to diagnosis. Time interval (in months) was calculated between index rescreen date and prior mammogram date (range = 11 months to 5 years). Screening result and mammographic density (<75%; ≥75%) were recorded by the radiologist when recording findings from the mammogram. Radiologists are aware of all previous imaging and clinical history, including family history, prior to interpreting mammograms. Screening mammograms resulting in a call back for further work-up were considered abnormal. Assessment procedures from an abnormal screening mammogram included breast imaging and breast biopsy and known final outcomes for each procedure coded as benign or screen-detected breast cancer.
Selection of Breast Cancer Cases
Women were followed prospectively to December 31, 2016, to determine if there was a breast cancer diagnosis. Breast cancers detected within 12 months of the index abnormal rescreen mammogram date were classified as screen detected. Women with interval cancers were identified from record linkage using AutoMatch (21) with the Ontario Cancer Registry, estimated to be 98% complete for breast cancer (22). Intervals cancers included those diagnosed before the next screening examination after a normal or benign index rescreen episode (normal mammogram or abnormal mammogram that had benign assessment) within 1 year for annual and 2 years for biennial.
For women diagnosed with primary breast cancer, histological classification (invasive, ductal carcinoma in situ [DCIS]) was obtained from the ICMS and Ontario Cancer Registry. Morphology was coded using the International Classification of Diseases for Oncology, version 3.0 (23).
Performance Measures
The performance measure definitions used for this study are primarily those adopted by the Canadian Partnership Against Cancer, with slight modifications (24). Performance measures were examined only for rescreens because they would have been exposed to a previous screening recommendation. Abnormal recall rate was the percentage of mammograms referred for further testing because of an abnormal screening result. Nonmalignant biopsy rate was the number of nonmalignant open and core biopsies per 1000 screening examinations. Nonmalignant results included benign, indeterminate or equivocal, high-risk lesions, or nonprimary breast cancers. The positive predictive value was the proportion of abnormal mammograms with completed follow-up found to have breast cancer after diagnostic work-up. Cancer detection rate was defined as the number of screen-detected invasive or DCIS breast cancers per 1000 screening examinations. Sensitivity was defined as the percentage of all cancers (true positive + false negative) that were screen detected (true positive). Specificity was defined as the percentage of women without breast cancer (true negative + false positive) who had a true negative screening mammogram.
Statistical Analysis
All analyses used the screening mammogram as the unit of analysis; women may have had more than one mammogram during the study period. Logistic regression using generalized estimating equation (GEE) models evaluated the association between recommendation and risk of abnormal screen (abnormal or normal), nonmalignant biopsy among screens (with nonmalignant biopsy or without), cancer detection (screen detected or no cancer), cancer detection among abnormal screens (cancer or no cancer), sensitivity (screen detected or interval), and specificity (true negative or false positive) (25). The primary comparison is based on an intention-to-treat analysis according to screening recommendation. All models were adjusted for time (in days) between index and prior mammogram date, age and year of screen, estrogen therapy use, and community status, and controlled for clustering including a random effect by screening center. The inversed logits from the GEE model were used to estimate performance measures and approximate 95% confidence intervals (CI) based on the least-square means of fixed effects. Corresponding odds ratios (OR) and 95% confidence intervals were also calculated overall and in stratified analyses. Logistic regression analyses using GEE models were conducted using SAS version 9.4 (26), and a two-tailed 5% statistical significance level was used.
Results
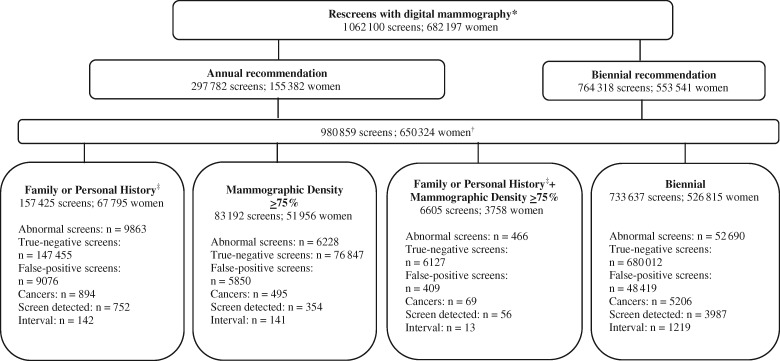
Of the 682 197 women screened between 2011 and 2014, there were 1 062 100 digital mammography rescreens with a final result and where time between index rescreen date and prior mammogram date was from 11 months to 5 years (Figure 1). This included 297 782 (28.0%) annual screens because of family history of breast or ovarian cancer or personal history of ovarian cancer and/or mammographic density 75% or greater and 764 318 (72.0%) biennial screens. After excluding screens (n = 81 241) among women whose screening recommendation changed over the study period, the cohort comprised 980 859 screens (n = 157 425 family or personal history; n = 83 192 mammographic density ≥75%; n = 6605 family or personal history plus density ≥75%; n = 733 637 biennial).
Figure 1.
Mammographic screening examinations among women age 50–74 years in the Ontario Breast Screening Program (OBSP) between January 1, 2011, and December 31, 2014, by screening recommendation. *Includes screens with final result and where index screen is between 11 months and 5 years from previous screen. †Excludes screens among women whose screening recommendation changed between their previous and index screen (family history n = 14 846; mammographic density n = 28 675; family history and mammographic density n = 7039; biennial n = 30 681). ‡Two or more first-degree female relatives with breast cancer at any age, one first-degree female relative with breast cancer younger than 50 years, one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, or one first-degree female relative with ovarian cancer at any age.
The majority of women screened biennially had their index rescreen within 19–36 months of their previous screen; the majority of women screened annually had their index rescreen within 11–18 months of their previous screen (Table 1). Most women screened biennially were age 60–74 years and postmenopausal, had menarche at older than 11 years, reported no use of estrogen therapy, lived in urban areas, and were in the three highest income quintiles. Women screened annually because of family or personal history were more likely to be age 60–74 years and postmenopausal and live in rural areas, but less likely to have had menarche at older than 11 years and be in higher income quintiles than women screened biennially. Conversely, women screened annually because of mammographic density 75% or greater with or without a family or personal history were less likely to be age 60–74 years and postmenopausal and live in rural areas, but more likely to have had menarche at older than 11 years, report current use of estrogen therapy, and be in higher income quintiles than women screened biennially.
Table 1.
Breast cancer risk factor and demographic characteristics and adjusted odds ratios (OR) and 95% confidence intervals (CI) among women age 50 to 74 years screened in the Ontario Breast Screening Program between 2011 and 2014 by screening recommendation (n = 980 859 screens)
| Characteristics | Biennial recommendation(n = 733 637) | Annual recommendation by reason |
|||||
|---|---|---|---|---|---|---|---|
| Family or personal history* (n = 157 425) | OR (95% CI) | Mammographic density ≥75% (n = 83 192) | OR (95% CI) | Family or personal history* + density ≥75% (n = 6605) | OR (95% CI) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | ||||
| Time between previous and index rescreen, mo | |||||||
| 11–18 | 39 019 | 144 399 | — | 72 688 (87.4) | — | 5870 | — |
| (5.3) | (91.7) | (88.9) | |||||
| 19–24 | 357 465 | 5285 | — | 3516 | — | 269 (4.1) | — |
| (48.7) | (3.4) | (4.2) | |||||
| 25–36 | 298 287 | 4956 | — | 4285 | — | 279 (4.2) | — |
| (40.7) | (3.2) | (5.2) | |||||
| 37–48 | 26 980 | 1841 | — | 1807 | — | 115 (1.7) | — |
| (3.7) | (1.2) | (2.2) | |||||
| 49–60 | 11 886 | 944 | — | 896 | — | 72 (1.1) | — |
| (1.6) | (0.6) | (1.1) | |||||
| Age at screening, y† | |||||||
| 50–59 | 290 830 | 53 796 | 1.00 | 50 715 | 1.00 | 3549 | 1.00 |
| (39.6) | (34.2) | (Referent) | (61.0) | (Referent) | (53.7) | (Referent) | |
| 60–74 | 442 807 | 103 629 | 1.27 | 32 477 | 0.42 | 3056 | 0.56 |
| (60.4) | (65.8) | (1.25 to 1.28)§ | (39.0) | (0.41 to 0.43)§ | (46.3) | (0.54 to 0.59)§ | |
| Year of screening† | |||||||
| 2011 | 117 835 | 28 439 | 1.00 | 10 718 | 1.00 | 840 | 1.00 |
| (16.1) | (18.1) | (Referent) | (12.9) | (Referent) | (12.7) | (Referent) | |
| 2012 | 153 130 | 33 585 | 0.91 | 13 796 | 0.99 | 1055 | 0.97 |
| (20.9) | (21.3) | (0.89 to 0.92)§ | (16.6) | (0.97 to 1.02) | (16.0) | (0.88 to 1.06) | |
| 2013 | 197 902 | 43 168 | 0.90 | 25 393 | 1.42 | 1776 | 1.27 |
| (27.0) | (27.4) | (0.89 to 0.92)§ | (30.5) | (1.39 to 1.46)§ | (26.9) | (1.17 to 1.38) | | |
| 2014 | 264 770 | 52 233 | 0.82 | 33 285 | 1.40 | 2934 | 1.57 |
| (36.1) | (33.2) | (0.80 to 0.83)§ | (40.0) | (1.37 to 1.43)§ | (44.4) | (1.45 to 1.69)§ | |
| Age at menarche, y† | |||||||
| ≤11 | 130 077 | 30 983 | 1.00 | 9737 | 1.00 | 836 | 1.00 |
| (18.3) | (20.1) | (Referent) | (12.1) | (Referent) | (13.0) | (Referent) | |
| >11 | 582 732 | 123 004 | 0.89 | 70 732 | 1.61 | 5575 | 1.48 |
| (81.8) | (79.9) | (0.88 to 0.90)§ | (87.9) | (1.57 to 1.64)§ | (87.0) | (1.37 to 1.59)§ | |
| Missing | 20 828 | 3438 | — | 2723 | — | 194 | — |
| Menopausal status† | |||||||
| Premenopausal | 35 650 | 4521 | 1.00 | 10 502 | 1.00 | 579 | 1.00 |
| (4.9) | (2.9) | (Referent) | (12.7) | (Referent) | (8.9) | (Referent) | |
| Postmenopausal | 689 743 | 151 737 | 1.44 | 72 025 | 0.67 | 5965 | 0.86 |
| (95.1) | (97.1) | (1.40 to 1.49)§ | (87.3) | (0.65 to 0.68)§ | (91.2) | (0.79 to 0.95)** | |
| Missing | 8244 | 1167 | — | 665 | — | 61 | — |
| Current use of estrogen† | |||||||
| No | 675 042 | 144 548 | 1.00 | 74 598 | 1.00 | 5919 | 1.00 |
| (92.9) | (92.9) | (Referent) | (90.4) | (Referent) | (90.6) | (Referent) | |
| Yes | 51 658 | 11 032 | 1.00 | 7881 | 1.37 | 617 | 1.37 |
| (7.1) | (7.1) | (0.98 to 1.02) | (9.6) | (1.33 to 1.40)§ | (9.4) | (1.26 to 1.48)§ | |
| Missing | 6937 | 1845 | — | 713 | — | 69 | — |
| Community status† | |||||||
| Urban | 624 468 | 129 683 | 1.00 | 75 456 | 1.00 | 5780 | 1.00 |
| (85.2) | (82.4) | (Referent) | (90.8) | (Referent) | (87.6) | (Referent) | |
| Rural | 41 532 | 10 268 | 1.19 | 3343 | 0.68 | 340 | 0.90 |
| (5.7) | (6.5) | (1.16 to 1.21)§ | (4.0) | (0.66 to 0.71)§ | (5.2) | (0.81 to 1.00) | |
| Rural remote | 47 993 | 12 551 | 1.24 | 3535 | 0.66 | 378 | 0.90 |
| (6.5) | (8.0) | (1.21 to 1.26)§ | (4.3) | (0.63 to 0.68)§ | (5.7) | (0.81 to 1.00) | |
| Rural very remote | 19 335 | 4868 | 1.19 | 796 | 0.36 | 100 | 0.59 |
| (2.6) | (3.1) | (1.16 to 1.23)§ | (1.0) | (0.33 to 0.39)§ | (1.5) | (0.48 to 0.72)§ | |
| Missing | 309 | 55 | — | 62 | — | 7 | — |
| Income quintile† | |||||||
| 1 = lowest | 106 493 | 24 325 | 1.00 | 9439 | 1.00 | 834 | 1.00 |
| (14.6) | (15.5) | (Referent) | (11.4) | (Referent) | (12.5) | (Referent) | |
| 2 | 135 892 | 29 866 | 0.97 | 13 935 | 1.14 | 1070 | 1.00 |
| (18.6) | (19.0) | (0.95 to 0.98)§ | (16.8) | (1.11 to 1.18)§ | (16.3) | (0.91 to 1.09) | |
| 3 | 151 248 | 32 260 | 0.94 | 16 064 | 1.17 | 1248 | 1.04 |
| (20.7) | (20.6) | (0.92 to 0.96)§ | (19.4) | (1.14 to 1.20)§ | (19.0) | (0.95 to 1.13) | |
| 4 | 163 817 | 34 436 | 0.93 | 19 401 | 1.29 | 1564 | 1.19 |
| (22.4) | (22.0) | (0.91 to 0.95)§ | (23.4) | (1.26 to 1.33)§ | (23.8) | (1.10 to 1.30)§ | |
| 5 = highest | 173 931 | 35 986 | 0.91 | 24 082 | 1.52 | 1859 | 1.35 |
| (23.8) | (22.9) | (0.90 to 0.93)§ | (29.0) | (1.49 to 1.56)§ | (28.3) | (1.24 to 1.46)§ | |
| Missing | 2256 | 552 | — | 271 | — | 30 | — |
Two or more first-degree female relatives with breast cancer at any age; one first-degree female relative with breast cancer younger than 50 years, one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, or one first-degree female relative with ovarian cancer at any age.
Adjusted for age at screen and/or year of screening.
Compared with biennial P < .001.
P = .03.
P = .002 . All P values (two-sided) were calculated using logistic regression with generalized estimating equation models.
Abnormal recall rate was statistically significantly higher for annual screening because of mammographic density 75% or greater (9.4%; 95% CI = 8.4 to 10.6) compared with biennial (8.3%; 95% CI = 7.6 to 9.1) (Table 2). Among all three annually screened groups, the nonmalignant biopsy rate was statistically significantly higher compared with biennial (6.4 per 1000; 95% CI = 5.4 to 7.7), particularly for mammographic density 75% or greater with (9.8 per 1000; 95% CI = 7.0 to 13.6) or without a family or personal history (9.7 per 1000; 95% CI = 7.9 to 11.9). Positive predictive value was statistically significantly higher for annual screening because of family or personal history plus density (15.5%; 95% CI = 11.5 to 20.4) compared with biennial (7.8%; 95% CI = 6.8 to 8.8). Similarly, cancer detection rate was statistically significantly higher for annual screening because of family or personal history plus density (14.1 per 1000; 95% CI = 10.4 to 19.0) compared with biennial (6.5 per 1000; 95% CI = 5.8 to 7.3), with the exception of premenopausal women. Annual screening because of mammographic density 75% or greater resulted in statistically significantly higher cancer detection rates for DCIS (1.6 per 1000; 95% CI = 1.1 to 2.2) compared with biennial (1.1 per 1000; 95% CI = 0.9 to 1.3).
Table 2.
Performance measures and adjusted odds ratios (OR) and 95% confidence intervals (CI) among women age 50–74 years screened within the Ontario Breast Screening Program between 2011 and 2014 by screening recommendation (n = 980 859 screens)
| Performance measure | Biennial recommendation (n = 733 637) (Referent) |
Annual recommendation by reason |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Family or personal history* (n = 157 425) |
Mammographic density ≥75% (n = 83 192) |
Family or personal history* + mammographic density ≥75% (n = 6605) |
|||||||||
| No. (screens) | Rate (95% CI) | No. (screens) | Rate (95% CI) | OR (95% CI) | No. (screens) | Rate (95% CI) | OR (95% CI) | No. (screens) | Rate (95% CI) | OR (95% CI) | |
| Abnormal recall rate, %† | 52 690 | 8.3 | 9863 | 8.4 | 1.01 | 6228 | 9.4 | 1.14 | 466 | 9.1 | 1.10 |
| (733 637) | (7.6 to 9.1) | (157 425) | (7.6 to 9.2) | (0.97 to 1.04) | (83 192) | (8.4 to 10.6) | (1.05 to 1.25)¶ | (6605) | (7.8 to 10.6) | (0.97 to 1.26) | |
| Nonmalignant biopsy rate per 1000† | 4017 | 6.4 | 759 | 7.1 | 1.10 | 579 | 9.7 | 1.51 | 44 | 9.8 | 1.52 |
| (733 637) | (5.4 to 7.7) | (157 425) | (5.9 to 8.5) | (1.01 to 1.21) | | (83 192) | (7.9 to 11.9) | (1.32 to 1.73)** | (6605) | (7.0 to 13.6) | (1.14 to 2.04)‡‡ | |
| Positive predictive value, %† | 3987 | 7.8 | 752 | 8.3 | 1.07 | 354 | 7.5 | 0.96 | 56 | 15.5 | 2.17 |
| (52 690) | (6.8 to 8.8) | (9863) | (7.1 to 9.6) | (0.98 to 1.17) | (6228) | (6.2 to 8.9) | (0.81 to 1.13) | (466) | (11.5 to 20.4) | (1.55 to 3.04)** | |
| Cancer detection rate per 1000† | 3987 | 6.5 | 752 | 7.0 | 1.07 | 354 | 7.0 | 1.08 | 56 | 14.1 | 2.17 |
| (733 637) | (5.8 to 7.3) | (157 425) | (6.1 to 8.0) | (0.98 to 1.17) | (83 192) | (6.0 to 8.2) | (0.93 to 1.24) | (6605) | (10.4 to 19.0) | (1.61 to 2.94)** | |
| Breast cancer type†,‡ | |||||||||||
| Invasive | 3280 | 5.4 | 617 | 5.6 | 1.05 | 262 | 5.2 | 0.98 | 39 | 9.7 | 1.83 |
| (732 927) | (4.7 to 6.1) | (157 290) | (4.9 to 6.5) | (0.95 to 1.16) | (83 100) | (4.4 to 6.2) | (0.84 to 1.14) | (6588) | (6.6 to 14.3) | (1.25 to 2.67)§§ | |
| DCIS | 681 | 1.1 | 131 | 1.2 | 1.17 | 87 | 1.6 | 1.49 | 17 | 4.2 | 3.93 |
| (730 328) | (0.9 to 1.3) | (156 804) | (0.9 to 1.7) | (0.96 to 1.42) | (82 925) | (1.1 to 2.2) | (1.11 to 1.99)†† | (6566) | (2.5 to 4.9) | (2.42 to 6.39)** | |
| Age at screen, y† | |||||||||||
| 50–59 | 1078 | 4.7 | 161 | 4.8 | 1.02 | 183 | 5.8 | 1.22 | 21 | 9.8 | 2.09 |
| (290 830) | (4.1 to 5.5) | (53 796) | (3.8 to 6.0) | (0.84 to 1.23) | (50 715) | (4.5 to 7.4) | (0.98 to 1.53) | (3549) | (6.4 to 15.0) | (1.38 to 3.14)** | |
| 60–74 | 2909 | 9.9 | 591 | 10.8 | 1.09 | 171 | 9.7 | 0.97 | 35 | 22.1 | 2.25 |
| (442 807) | (7.4 to 13.2) | (103 629) | (8.1 to 14.3) | (0.98 to 1.21) | (32 477) | (7.0 to 13.3) | (0.82 to 1.16) | (3056) | (14.8 to 32.9) | (1.53 to 3.32)** | |
| Menopausal status§ | |||||||||||
| Premenopausal | 175 | 4.9 | 27 | 6.7 | 1.27 | 59 | 6.9 | 1.30 | 4 | 7.9 | 1.49 |
| (35 650) | (3.6 to 7.8) | (4521) | (3.7 to 12.4) | (0.77 to 2.11) | (10 502) | (4.3 to 11.1) | (0.89 to 1.90) | (579) | (2.9 to 21.3) | (0.56 to 3.98) | |
| Postmenopausal | 3737 | 5.1 | 717 | 5.5 | 1.07 | 284 | 5.3 | 1.03 | 52 | 11.3 | 2.23 |
| (689 743) | (4.7 to 5.6) | (151 737) | (4.9 to 6.1) | (0.97 to 1.18) | (72 025) | (4.5 to 6.2) | (0.89 to 1.19) | (5965) | (8.3 to 15.5) | (1.63 to 3.06)** | |
Two or more first-degree female relatives with breast cancer at any age, one first-degree female relative with breast cancer younger than 50 years, one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, or one first-degree female relative with ovarian cancer at any age. DCIS = ductal carcinoma in situ.
Adjusted for age at screen, year of screen, menopausal status, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Thirty-eight cancers with missing morphology excluded (29 biennial; 4 family or personal history; 5 mammographic density ≥75%).
Adjusted for age at screen, year of screen, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Compared with biennial P = .04.
P = .003
P < .001
P = .007
P = .005
P = .002. All P values (two-sided) were calculated using logistic regression with generalized estimating equation models.
Sensitivity was statistically significantly higher for annual screening because of family or personal history compared with biennial (81.7% vs 70.6%; OR = 1.86, 95% CI = 1.48 to 2.34), particularly for invasive cancers (OR = 1.87, 95% CI = 1.46 to 2.39) (Table 3). Women age 50–59 years (OR = 1.69, 95% CI = 1.08 to 2.65), 60–74 years (OR = 1.92, 95% CI = 1.47 to 2.49), or postmenopausal (OR = 1.89, 95% CI = 1.49 to 2.39) also had statistically significantly higher sensitivity for annual screening because of family or personal history compared with biennial. There was no statistically significant difference in sensitivity for annual screening because of mammographic density 75% or greater compared with biennial overall or in stratified analyses. Among women screened annually because of family or personal history plus density, sensitivity was statistically significantly higher among postmenopausal women compared with biennial (OR = 2.39, 95% CI = 1.07 to 5.34).
Table 3.
Sensitivity and adjusted odds ratios (OR) and 95% confidence intervals (CI) among women age 50–74 years screened within the Ontario Breast Screening Program between 2011 and 2014 by screening recommendation (n = 6664 cancers)
| Characteristics | Biennial recommendation (n = 5206) (Referent) |
Annual recommendation by reason |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family or personal history* (n = 894) |
Mammographic density ≥75% (n = 495) |
Family or personal history* + mammographic density ≥75% (n = 69) |
||||||||||
| SD cancers (all cancers) | Sensitivity, % (95% CI) | SD cancers (all cancers) | Sensitivity, % (95% CI) | OR (95% CI) | SD cancers (all cancers) | Sensitivity, % (95% CI) | OR (95% CI) | SD cancers (all cancers) | Sensitivity, % (95% CI) | OR (95% CI) | ||
| Overall† | 3987 | 70.6 | 752 | 81.7 | 1.86 | 354 | 70.6 | 1.00 | 56 | 82.2 | 1.93 | |
| (5206) | (65.5 to 75.2) | (894) | (76.9 to 85.8) | (1.48 to 2.34) | | (495) | (65.0 to 75.7) | (0.79 to 1.27) | (69) | (68.7 to 90.7) | (0.94 to 3.99) | ||
| Breast cancer type†,‡ | ||||||||||||
| Invasive | 3280 | 68.2 | 617 | 80.0 | 1.87 | 262 | 66.9 | 0.94 | 39 | 77.8 | 1.64 | |
| (4386) | (62.8 to 73.1) | (749) | (74.6 to 84.5) | (1.46 to 2.39) | | (391) | (60.4 to 72.8) | (0.74 to 1.20) | (51) | (62.1 to 88.2) | (0.78 to 3.45) | ||
| DCIS | 681 | 82.7 | 131 | 90.0 | 1.89 | 87 | 84.7 | 1.16 | 17 | 92.4 | 2.53 | |
| (791) | (72.5 to 89.6) | (141) | (78.5 to 95.7) | (0.93 to 3.84) | (99) | (70.4 to 92.8) | (0.56 to 2.44) | (18) | (57.1 to 99.1) | (0.35 to 18.47) | ||
| Age, y† | ||||||||||||
| 50–59 | 1078 | 69.1 | 161 | 79.1 | 1.69 | 183 | 70.1 | 1.05 | 21 | 77.3 | 1.52 | |
| (1465) | (62.6 to 75.0) | (202) | (69.0 to 86.5) | (1.08 to 2.65)¶ | (269) | (61.5 to 77.5) | (0.74 to 1.49) | (27) | (55.0 to 90.4) | (0.58 to 3.98) | ||
| 60–74 | 2909 | 78.8 | 591 | 87.7 | 1.92 | 171 | 78.1 | 0.96 | 35 | 90.3 | 2.49 | |
| (3741) | (64.5 to 88.4) | (692) | (77.5 to 93.7) | (1.47 to 2.49) | | (226) | (61.7 to 88.7) | (0.70 to 1.31) | (42) | (73.1 to 97.0) | (0.85 to 7.32) | ||
| Menopausal status§ | ||||||||||||
| Premenopausal | 175 | 72.0 | 27 | 78.7 | 1.44 | 59 | 72.5 | 1.03 | 4 | 64.7 | 0.72 | |
| (248) | (58.3 to 82.5) | (37) | (58.2 to 90.8) | (0.62 to 3.34) | (91) | (56.4 to 84.3) | (0.54 to 1.95) | (7) | (23.8 to 91.5) | (0.13 to 3.82) | ||
| Postmenopausal | 3737 | 72.1 | 717 | 83.0 | 1.89 | 284 | 72.1 | 1.00 | 52 | 86.1 | 2.39 | |
| (4858) | (67.9 to 75.9) | (848) | (79.1 to 86.3) | (1.49 to 2.39) | | (389) | (66.9 to 76.8) | (0.79 to 1.27) | (61) | (73.6 to 93.2) | (1.07 to 5.34)** | ||
Two or more first-degree female relatives with breast cancer at any age, one first-degree female relative with breast cancer younger than 50 years, one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, or one first-degree female relative with ovarian cancer at any age. DCIS = ductal carcinoma in situ; SD = screen detected.
Adjusted for age at screen, year of screen, menopausal status, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Thirty-eight cancers with missing morphology excluded (29 biennial; 4 family or personal history; 5 mammographic density ≥75%).
Adjusted for age at screen, year of screen, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Compared with biennial P < .001.
P = .02.
P = .03. All P values (two-sided) were calculated using logistic regression with generalized estimating equation models.
Specificity was statistically significantly lower for annual screening because of mammographic density 75% or greater compared with biennial overall (91.3% vs 92.3%; OR = 0.87, 95% CI = 0.80 to 0.96) and among women age 50–59 years (OR = 0.85, 95% CI = 0.77 to 0.94) or postmenopausal (OR = 0.88, 95% CI = 0.80 to 0.96) (Table 4). Women age 50–59 years (OR = 0.84, 95% CI = 0.73 to 0.98) or premenopausal (OR = 0.71, 95% CI = 0.54 to 0.95) also had statistically significantly lower specificity for annual screening because of family or personal history plus density compared with biennial. Specificity was statistically significantly lower among premenopausal women screened annually because of family or personal history compared with biennial (OR = 0.81, 95% CI = 0.72 to 0.92).
Table 4.
Specificity and adjusted odds ratios (OR) and 95% confidence intervals (CI) among women age 50–74 years screened within the Ontario Breast Screening Program between 2011 and 2014 by screening recommendation (n = 974 195 screens)
| Annual recommendation by reason |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biennial recommendation(n = 72 431) (Referent) |
Family or personal history* (n = 156 531) |
Mammographic density ≥75% (n = 82 697) |
Family or personal history* + mammographic density ≥75% (n = 6536) |
||||||||||
| TN (TN + FP) | Specificity, % (95% CI) | TN (TN + FP) | Specificity, % (95% CI) | OR (95% CI) | TN (TN + FP) | Specificity, % (95% CI) | OR (95% CI) | TN (TN + FP) | Specificity, % (95% CI) | OR (95% CI) | |||
| Overall† | 680 012 | 92.3 | 147 455 | 92.3 | 1.00 | 76 847 | 91.3 | 0.87 | 6127 | 92.1 | 0.97 | ||
| (728 431) | (91.6 to 93.1) | (156 531) | (91.5 to 93.1) | (0.96 to 1.04) | (82 697) | (90.2 to 92.3) | (0.80 to 0.96) | | (6536) | (90.7 to 93.3) | (0.84 to 1.11) | |||
| Age, y† | |||||||||||||
| 50–59 | 268 840 | 92.2 | 50 188 | 91.9 | 0.97 | 46 615 | 90.9 | 0.85 | 3258 | 90.8 | 0.84 | ||
| (289 365) | (91.4 to 92.9) | (53 594) | (91.0 to 92.7) | (0.92 to 1.02) | (50 446) | (89.7 to 91.9) | (0.77 to 0.94)¶ | (3522) | (89.3 to 92.1) | (0.73 to 0.98)†† | |||
| 60–74 | 411 172 | 93.3 | 97 267 | 93.4 | 1.02 | 30 232 | 92.7 | 0.92 | 2869 | 94.2 | 1.18 | ||
| (439 066) | (92.2 to 94.2) | (102 937) | (92.3 to 94.4) | (0.97 to 1.07) | (32 251) | (91.3 to 93.9) | (0.83 to 1.02) | (3014) | (92.5 to 95.6) | (0.95 to 1.46) | |||
| Menopausal status‡ | |||||||||||||
| Premenopausal | 32 528 | 92.1 | 4068 | 90.4 | 0.81 | 9495 | 91.0 | 0.88 | 513 | 89.2 | 0.71 | ||
| (35 402) | (90.8 to 93.1) | (4484) | (88.7 to 91.9) | (0.72 to 0.92)§ | (10 411) | (89.4 to 92.4) | (0.77 to 1.00) | (572) | (85.5 to 92.1) | (0.54 to 0.95)‡‡ | |||
| Postmenopausal | 639 952 | 93.0 | 142 291 | 93.1 | 1.01 | 66 744 | 92.1 | 0.88 | 5559 | 93.1 | 1.01 | ||
| (684 885) | (92.3 to 93.7) | (150 889) | (92.3 to 93.8) | (0.97 to 1.05) | (71 636) | (91.1 to 93.1) | (0.80 to 0.96)** | (5904) | (91.8 to 94.2) | (0.87 to 1.17) | |||
Two or more first-degree female relatives with breast cancer at any age, one first-degree female relative with breast cancer younger than 50 years; one first-degree male relative with breast cancer at any age, a personal history of ovarian cancer, or one first-degree female relative with ovarian cancer at any age. FP = false positive; TN = true negative.
Adjusted for age at screen, year of screen, menopausal status, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Adjusted for age at screen, year of screen, use of estrogen therapy at last screen, community status, time (days) between previous and index rescreen date, and clustering by screening center attended.
Compared with biennial P < .001.
P = .004.
P = .001.
P = .006.
P = .03.
P = .02. All P values (two-sided) were calculated using logistic regression with generalized estimating equation models.
Discussion
Our study compared diagnostic accuracy of annual screening based on family history of breast or ovarian cancer or personal history of ovarian cancer and/or mammographic density 75% or greater for women age 50–74 years with biennial screening for average- risk women. Annual screening was effective for women with a family or personal history with statistically significantly higher sensitivity compared with biennial screening, irrespective of age. This association was greater for women diagnosed with invasive cancers and who were postmenopausal. Sensitivity was similar for annual screening because of high mammographic density compared with biennial. However, specificity was statistically significantly lower for annual screening because of mammographic density 75% or greater compared with biennial, particularly for women age 50–59 years.
Women with a family history of breast or ovarian cancer are at increased risk compared with the general population, with greater risk according to the number, closeness, and age of the affected relative(s) (27–30). For women age 50 years and older with a family history, several Canadian breast screening programs recommend more-frequent screening intervals (24). Our study found annual screening to be effective for women with a first-degree relative of breast and/or ovarian cancer or personal history of ovarian cancer. Although radiologists have access to the information collected on family history, this knowledge did not influence their referral pattern, as demonstrated by similar referral rates for these women and those at average risk. The statistically significantly greater detection of invasive cancers on rescreens was also an expected finding, reflecting the increased risk of breast cancer in these women.
The greater overall sensitivity for women screened annually for family or personal history compared with women screened biennially, irrespective of age, indicates a lower risk of interval cancers, which have poorer prognostic features (1–3). Although previous observational studies have reported higher sensitivity among women 40 years or older screened annually vs biennially (9,10,13,14), there was no evidence of a greater risk of adverse breast tumor characteristics for women screened less frequently, except for those age 40–49 years (10). However, these earlier studies did not examine indicators by family history of breast cancer, although some did adjust for it in their analyses (10,14). Another study found that offering women with a first-degree family history of breast cancer annual vs biennial screening resulted in more favorable prognostic outcomes of screen-detected cancers (15). In our study, the only associated harm of more frequent screening among women with a family or personal history was a slightly lower specificity in premenopausal women, which is expected because their breast density is typically higher (14).
Because our earlier study noted that breast cancer risk associated with extensive mammographic density is limited to the 12 months after a screening examination and most likely due to masking, screening with digital mammography could reduce the increased risk of interval cancers (31). Our result that sensitivity was similar for women screened annually with mammographic density 75% or greater as for women screened biennially may reflect that all women were screened with digital mammography. Digital mammography is more sensitive for women with higher mammographic density (32,33) and would be expected to identify more cancers hidden by dense tissue (34) because the contrast resolution is superior to screen-film mammography.
However, annual screening did result in an increased abnormal recall rate for women with higher mammographic density. The additional referrals may be harmful because these women were also more likely to have nonmalignant biopsies. Specificity was also slightly lower for annual screening because of high mammographic density compared with biennial, especially for women age 50–59 years whose mammographic density may have been greater. Another study also found that the cumulative risk of a false-positive mammography result was higher among women age 40–49 years undergoing annual mammography with extremely dense breasts but lower among those age 50–74 years having biennial mammography with scattered fibroglandular densities or fatty breasts (9).
Mammographic density may have affected screening outcomes differently in women with and without a family history. The higher positive predictive value and cancer detection rate among the small cohort of women screened annually for both risk factors reflects the increased risk of breast cancer in these women. However, similar to women screened annually for mammographic density only, these women had a higher nonmalignant biopsy rate and lower specificity among those age 50–59 years compared with biennial screening.
Strengths of our study include the comparison of concurrent cohorts, which minimizes the potential for differences between cohorts in radiologist experience or technology over time. Given our study included women screened in an organized breast screening program, all radiologists and equipment meet minimum quality standards and methods of follow-up are similar. We used a conservative approach by limiting our analyses to women whose screening recommendation remained the same over the study period. Our large sample size allowed us to adjust for multiple risk factors among women and clustering by screening center. In addition, our study included only digital mammography screens, which have been shown to be more sensitive for women with high mammographic density.
Our study had some limitations. Family or personal history was based on self-reported data, and misclassification might have occurred. However, the accuracy of reporting breast cancer in first-degree relatives has generally been found to be high (35) and data was collected through personal interviews. Based on our study design and similar to others (9,10,12–14), the follow-up period was not comparable among the cohorts, and therefore accuracy was measured differently between the annual and biennial screening groups. The accuracy values are limited by the screening interval of the woman and differ from calculations considering follow-up within 1 year of screening. Although we measured and adjusted for differences in patient populations, cohort designs are limited by nonrandomization of exposure, and therefore our results may be limited by inherent differences among the cohorts.
Uncertainties exist regarding effective screening recommendations for women at increased risk. Higher sensitivity in women with a family or personal history indicate that these women may have the potential to benefit from annual breast cancer screening. For women with mammographic density 75% or greater, annual screening with digital mammography was effective for detection of breast cancers (similar sensitivity) compared to women screened biennially. However, they had a slight increase in false-positive screens (lower specificity) and greater risk of nonmalignant biopsies. Women at increased risk should be advised of the benefits and harms of annual screening and supported to make an informed decision.
Funding
This work was supported by the Canadian Cancer Society (grant number 316078).
Notes
The funder had no involvement in the design, conduct, or reporting of the study; the writing of the manuscript; or the decision to publish the manuscript. The authors declare that they have no financial disclosures or conflicts of interest. We thank Cancer Care Ontario for use of its data.
References
- 1. Gilliland FD, Joste N, Stauber PM, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92(9):743–749. [DOI] [PubMed] [Google Scholar]
- 2. Chiarelli AM, Edwards SA, Sheppard AJ, et al. Favourable prognostic factors of subsequent screen-detected breast cancers among women aged 50–69. Eur J Cancer Prev. 2012;21(6):499–506. [DOI] [PubMed] [Google Scholar]
- 3. Cowan WK, Angus B, Gray JC, Lunt LG, Al-Tamimi SR.. A study of interval breast cancer within the NHS breast screening programme. J Clin Pathol. 2000;53(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Task Force on Preventive Health Care. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ. 2018;190(49):E1441–E1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siu AL. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2016;164(4):279–296. [DOI] [PubMed] [Google Scholar]
- 6. Brett J, Austoker J, Ong G.. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last breast screening appointment. J Public Health Med. 1998;20(4):396–403. [DOI] [PubMed] [Google Scholar]
- 7. Sutton S, Saidi G, Bickler G, Hunter J.. Does routine screening for breast cancer raise anxiety? Results from a three wave prospective study in England. J Epidemiol Community Health. 1995;49(4):413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brett J, Austoker J.. Women who are recalled for further investigation for breast screening: psychological consequences 3 years after recall and factors affecting re-attendance. J Public Health Med. 2001;23(4):292–300. [DOI] [PubMed] [Google Scholar]
- 9. Kerlikowske K, Zhu W, Hubbard RA, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal hormone therapy. JAMA Intern Med. 2013;173(9):807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White E, Miglioretti DL, Yankaskas BC, et al. Biennial versus annual mammography and the risk of late-stage breast cancer. J Natl Cancer Inst. 2004;96(24):1832–1839. [DOI] [PubMed] [Google Scholar]
- 11. Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL.. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography. Ann Intern Med. 2011;155(8):481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dittus K, Geller B, Weaver DL, et al. Impact of mammography screening interval on breast cancer diagnosis by menopausal status and BMI. J Gen Intern Med. 2013;28(11):1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Braithwaite D, Zhu W, Hubbard RA, et al. Screening outcomes in older US women undergoing multiple mammograms in community practice: does interval, age, or comorbidity score affect tumor characteristics or false positive rates? J Natl Cancer Inst. 2013;105(5):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miglioretti DL, Zhu W, Kerlikowske K, et al. Breast tumor prognostic characteristics and biennial vs annual mammography, age and menopausal status. JAMA Oncol. 2015;1(8):E1–E9. doi:10.1001/jamaoncol.2015.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Randall D, Morrell S, Taylor R, Hung WT.. Annual or biennial mammography screening for women at a higher risk with a family history of breast cancer: prognostic indicators of screen-detected cancers in New South Wales, Australia. Cancer Causes Control. 2009;20(5):559–566. [DOI] [PubMed] [Google Scholar]
- 16. Wai ES, D’Yachkova Y, Olivotto IA, et al. Comparison of 1- and 2-year screening intervals for women undergoing screening mammography. Br J Cancer. 2005;92(5):961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353(17):1773–1783. [DOI] [PubMed] [Google Scholar]
- 18. Kerlikowske K, Hubbard RA, Miglioretti DL, et al. ; Breast Cancer Surveillance Consortium. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155(8):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiarelli AM, Edwards SA, Prummel MV, et al. Digital compared with screen-film mammography: performance measures in concurrent cohorts within an organized breast screening program. Radiology. 2013;268(3):684–693. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Canada. Postal CodeOM Conversion File Plus (PCCF+) Version 6D, Reference Guide . July 2016 Postal Codes. Statistics Canada Catalogue no. 82-E0086-XDB. Ottawa, Canada: Minister of Industry; 2017. [Google Scholar]
- 21. Jaro MA. Probabilistic linkage of large public health data files. Stat Med. 1995;14(5–7):491–498. [DOI] [PubMed] [Google Scholar]
- 22. Holowaty E, Marrett L, Fehringer G.. Cancer Incidence in Ontario: Trends and Regional Variations in the 1980s. Toronto: Ontario Cancer Treatment and Research Foundation; 1995. [Google Scholar]
- 23.World Health Organization. International Classification of Diseases for Oncology: ICD-O. Geneva: World Health Organization; 2000. [Google Scholar]
- 24.Canadian Partnership Against Cancer. Breast Cancer Screening in Canada: Monitoring and Evaluation of Quality Indicators—Results Report, January 2011 to December 2012. Toronto: Canadian Partnership Against Cancer; 2016. [Google Scholar]
- 25. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. New York, USA: Oxford University Press; 2003:35–64. [Google Scholar]
- 26.SAS Institute Inc. Statistical Analysis Software (ed. 9.4) Cary, NC: SAS Institute; 2013.
- 27.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358(9291):1389–1399. [DOI] [PubMed] [Google Scholar]
- 28. Pharoah PD, Day NE, Duffy S, Easton DF, Ponder BA.. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800–809. [DOI] [PubMed] [Google Scholar]
- 29. Bevier M, Sundquist K, Hemminki K.. Risk of breast cancer in families of multiple affected women and men. Breast Cancer Res Treat. 2012;132(2):723–728. [DOI] [PubMed] [Google Scholar]
- 30. Ziogas A, Gildea M, Cohen P, Bringman D, Taylor TH, Seminara D.. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(1):103–111. [PubMed] [Google Scholar]
- 31. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. [DOI] [PubMed] [Google Scholar]
- 32. Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V.. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA. 1996;276(1):33–38. [PubMed] [Google Scholar]
- 33. Sala E, Warren R, McCann J, Duffy S, Day N, Luben R.. Mammographic parenchymal patterns and mode of detection: implications for the breast screening programme. J Med Screen. 1998;5(4):207–212. [DOI] [PubMed] [Google Scholar]
- 34. Dershaw DD. Status of mammography after the Digital Mammography Imaging Screening Trial: digital versus film. Breast J. 2006;12(2):99–102. [DOI] [PubMed] [Google Scholar]
- 35. Murff HJ, Spigel DR, Syngal S.. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. JAMA. 2004;292(12):1480–1489. [DOI] [PubMed] [Google Scholar]