Abstract
As shown by the current COVID-19 pandemic, emergency departments (ED) are the front line for hospital-and-community-based care during viral respiratory disease outbreaks. As such, EDs must be able to reorganize and reformat operations to meet the changing needs and staggering patient volume. This paper addresses ways to adapt departmental operations to better manage in times of elevated disease burden, specifically identifying areas of intervention to help limit crowding and spread. Using experience from past outbreaks and the current COVID-19 pandemic, we advise strategies to increase surge capacity and limit patient inflow. Triage should identify and geographically cohort symptomatic patients within a designated unit to limit exposure early in an outbreak. Screening and PPE guidelines for both patient and staff should be followed closely, as determined by hospital administration and the CDC. Equipment needs are also greatly affected in an outbreak; we emphasis portable radiographic equipment to limit transport, and an upstocking of certain medications, respiratory supplies, and PPE.
Keywords: SARS-CoV-2, COVID-19, Emergency department, Operation, Staff
1. Introduction
The current pandemic caused by the rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) demands urgent guidance for clinicians faced with the novel illness. Emergency departments (ED) are the front lines for hospital-and-community-based care, serving as the main points for triaging patients as infected versus non-infected and sick versus not-sick. EDs across the nation are overwhelmed with patient numbers at baseline. A review by the National Emergency Department Survey showed annual ED visits increased from 89.6 million to 139 million from 2006 to 2017 (an increase of 55.13%) [1] [2]. This issue of overcrowding has the potential to increase exponentially during times of viral outbreak. For example, during the H1N1/2009 pandemic, one study estimated that the rate of ED visits attributable to influenza increased to 1000 per 100,000, doubling the average annual rate of 500 per 100,000 population for seasonal influenza [3]. It is essential that strategic measures be taken to anticipate surges in ED patient visits, particularly during times of infectious disease outbreak.
EDs maintain order through structured workflow and careful departmental geographic planning. However, the typical organization must be largely disrupted when anticipating how to limit infectious spread and care for enormous patient volume. The main concern during such times is to maintain high quality and high efficiency care, with emphasis on patient and provider safety, when demand far exceeds capacity. Though many departments throughout the country have disaster preparedness protocols in place, individual outbreaks differ by severity of illness, route of transmission, and level of contagion, which dramatically alters the number of patients presenting to hospital facilities for screening and/or care. Thus a more general guideline, as presented here, will help address the overarching issues that will occur with any viral epidemic/pandemic. This paper will address some of the ways to adapt daily routines in the ED to better manage times of elevated airborne disease burden, specifically identifying areas of intervention to help limit crowding and spread during viral respiratory disease outbreaks.
2. Managing increased patient volume and adding capacity
Interventions that limit unnecessary patient visits during a respiratory disease outbreak are essential to mitigate infectious exposure and maintain expeditious ED workflow. A panel of expert emergency physicians placed highest priority on ED interventions that would alleviate high patient volume, with their greatest concern being ED crowding [4]. Specific interventions included triaging patients to the most appropriate care setting through a website or call center, then standardizing ED admission criteria for patients with respiratory symptoms. This same panel felt disease severity was a lesser issue in the ED setting and more important for inpatient management [4].
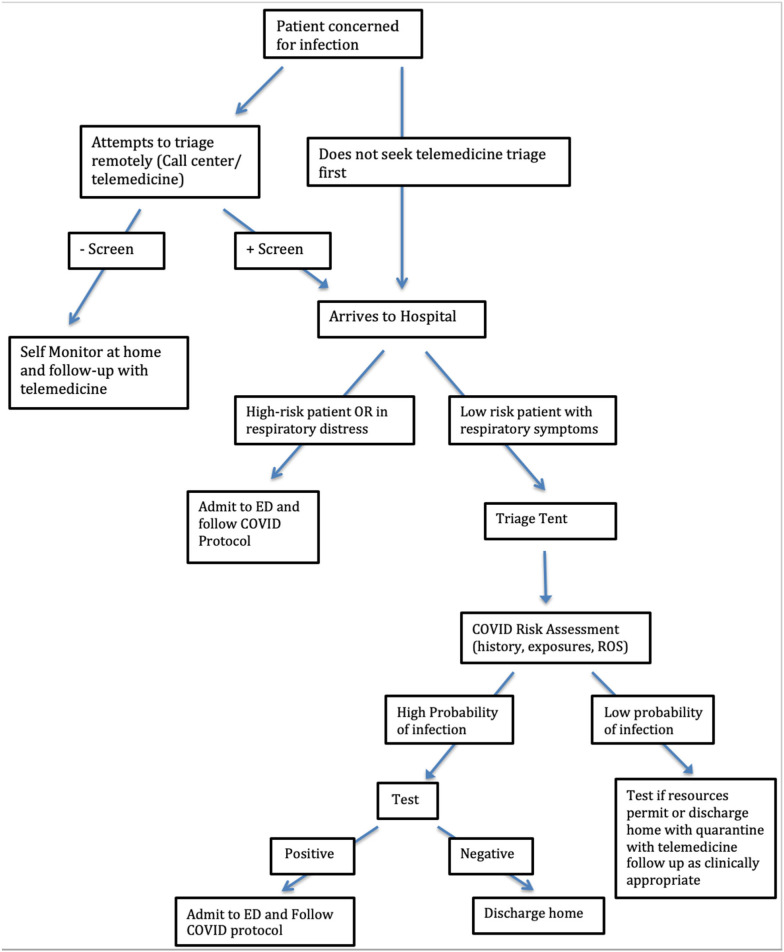
Limiting patient inflow can be accomplished by triage points before and upon ED arrival. Before presenting to the hospital, patients can be directed to a telemedicine visit for triage. While some portion of these patients will ultimately require hospital-based care, many can be counseled and/or tested in the outpatient setting. Once at the hospital, diverting low-risk patients with respiratory symptoms to an alternate site of care, such as a medical tent, is a strategy to protect ED bed capacity. At George Washington University Hospital during the COVID-19 pandemic, a tent was set up outside the hospital and adjacent to the outpatient clinic building. Patients who arrived to the ED with concern for COVID-19 were directed to the tent and seen by an advanced practice provider (APP) if they met criteria for age, heart rate, and temperature. Patients who did not meet these criteria were deemed higher risk and triaged to a designated ED treatment space (Fig. 1 ).
Fig. 1.
Clinical decision pathway.
ED directors should engage hospital leadership immediately to expand inpatient capacity, canceling elective surgeries and adding ICU and negative pressure rooms where feasible. With a reduced OR schedule, ED treatment spaces that typically would be occupied by ‘boarding’ patients can be recovered.
3. Identifying patients early
Triage is key to early recognition and rapid initiation of infection control precautions, which remain the most important strategies for controlling viral respiratory disease outbreaks [5]. However, as demonstrated with COVID-19, early identification can be difficult due to asymptomatic carriers and patients presenting with atypical symptoms, such as solely gastrointestinal complaints. Without immediate point-of-care tests, it is best to screen individuals through a balanced approach that incorporates clinical features and pertinent epidemiologic clues (including recent travel). This approach allows for the rapid identification of at-risk individuals while limiting the number of individuals who are tested or quarantined unnecessarily [5]. All patients who arrive in the ED with respiratory symptoms should be immediately masked with reinforcing signage posted in the waiting area.
4. Cohorting patients
With highly contagious viral disease that has both airborne and fomite transmission, a major problem is nosocomial transmission. Large healthcare systems may designate one hospital to serve as the primary hospital for infected patients. Within a single ED, there are no clear guidelines for the best organizational model, though it is clear that major adjustments must be made quickly when addressing highly contagious respiratory disease outbreaks. During the SARS outbreak, no decisions were made in the early stages about whether to cohort suspected and/or probable cases of SARS into a centralized section in EDs [6]. Thus, it is important to have collateral plans ready to put in place for when such viral outbreaks reoccur. One of the highest priority interventions should be to limit the number of staff and patients exposed by geographically cohorting patients with presumed or confirmed infection [4]. This operational approach must be implemented in the early stages of an outbreak as the efficacy declines significantly once an infection becomes widespread.
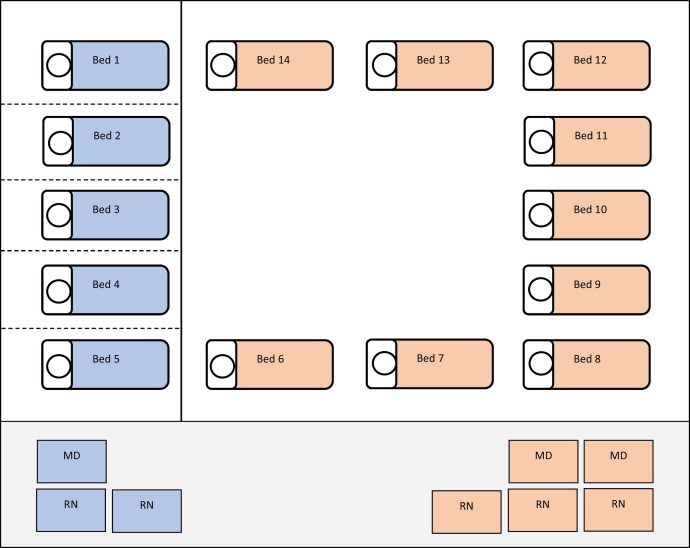
When cohorting patients it is most appropriate to designate a specific area of the department and establish a static geographic assignment model. In this model, providers and nurses are assigned specific rooms and automatically assigned patients who arrive in these rooms. When cohorting patients, the care team is assigned solely to the rooms in that cohort, thus limiting interaction with outside patients and staff. The providers and nurses may sit together at a workstation near the assigned rooms (Fig. 2 ) [7]. In terms of staffing these cohorts, it is most appropriate to use long shifts and overtime hours to limit the amount of staff turnover. Additionally, if possible, use staff who are immune (recovered) in these units and negative pressure rooms for these patients [8]. This operational plan must be flexible to change based on input from staff and the evolution of an individual outbreak situation. It is important to reinforce that cohorting is most effective in the early stages of an outbreak; there may come a point, depending on the level of contagion, where there are too many patients under investigation or confirmed positive to cohort them.
Fig. 2.
Cohort symptomatic patient to rooms in an isolated area of the department (blue). Using a static geographic model, designate providers and nurses to be assigned to these areas only. The non-cohort rooms (orange) can remain with the baseline department staffing model.
5. Infection control and environmental changes
Healthcare workers are particularly vulnerable to transmission of respiratory infections due to close proximity and department crowding, as highlighted during the 2003 SARS outbreak [9]. Currently, early data from Wuhan and Italy suggest a very high COVID-19 disease burden among healthcare providers [10]. Thus an important aspect of infection control is limiting patient-to-provider spread and provider-to-patient spread. A case-control study in five Hong Kong hospitals found that consistent practice of droplet and contact precaution significantly reduced the risk of infection in healthcare workers after exposures to patients with SARS [11]. Another study found that inadequate supply, and inconsistent use, of personal protection equipment (PPE) when in contact with SARS patients were independent risk factors for SARS infection [12]. A case-control study of 254 hospital staff with documented exposure to SARS patients found that consistent droplet and contact precaution was adequate to significantly reduce the number of infections from patient exposures [11]. PPE can be a highly controversial subject with conflicting recommendations from different sources. Current COVID-19 guidelines from the Surviving Sepsis Campaign and the CDC recommend that any healthcare provider (HCP) performing an aerosol-generating procedure on a suspected or confirmed COVID-19 patient (including endotracheal intubation, open suctioning, NIPPV, and administration of nebulized treatment) wear fitted respirator masks, like N95 or FFP2, along with contact precaution and eye protection [10] [13]. In order to best preserve respirator masks for high-risk procedures, providers working with non-ventilated patients are recommended to wear surgical/medical masks. ED leadership should plan for, and obtain, an appropriate supply of PPE and establish allocation procedures. For instance, in case of shortage, each staff member may be given one N95 respirator per shift that is ‘checked out’ rather than leaving boxes out in the open, with potential for anxious staff to use or take-home extra.
Additionally, although an initial face-to-face examination of the patient is typically required, subsequent interactions (updates, discharge instructions) may take place via phone or video-chat. Thus, EDs may wish to have smart phones or portable electronic devices (tablets or iPads) available for patients to communicate with staff who are outside the room.
New housekeeping protocols must be developed in conjunction with the Environmental Services director. Additional supplies will be needed. Additional staff may be added or diverted from other settings during times of peak room turnover. When the ED is seeing more than 100 persons under investigation (PUIs) per day, a 2-hour room clean time for contaminated rooms can devastate ED operations/flow and have real impact on patient care.
6. Staffing concerns and exposures
An essential aspect of infection control is testing and quarantining healthcare providers/staff as appropriate. Data from the COVID-19 pandemic in Italy suggests that EDs should be prepared to have at least 10% of staff become ill [8]. Thus, a conservative approach much be taken with strict guidelines for monitoring and testing HCPs.
Such exposures include prolonged close contact to COVID-19 patients without proper PPE or using a mask instead of a respirator during aerosol-generating procedures. Asymptomatic HCPs with low-risk exposures are able to work but should self-monitor with supervision from occupational health (or other hospital entity) for two weeks after last exposure. Low-risk exposures include most interactions with appropriate PPE. Self-monitoring includes making sure they are afebrile and asymptomatic before reporting to work [13]. This system collapses in communities with high rates of infection, where it is assumed that most HCPs have been exposed and must still work if asymptomatic and wearing appropriate PPE.
ED administration may consider implementing a system to evaluate staff for fevers and/or respiratory symptoms prior to starting work. Any HCP with fever or respiratory symptoms should immediately self-isolate. To mitigate impact on scheduling, EDs can have additional staff backup scheduled to cover in case a staff member calls out. Additionally, HCPs should be given priority for rapid-turnaround testing as this has enormous impact on available staff during a critical time.
7. Changes in medication stocking and supply
During a surge of a particular infectious disease, different formulations or quantities of medications may be required. Most importantly, metered-dose inhalers pose a smaller risk of infectious spread via respiratory droplet than nebulizers and are the preferred mode of delivery. As discussed above, the Surviving Sepsis Campaign classifies nebulized treatments as an aerosol-generating procedure that requires the administrating healthcare provider to wear fitted respirator masks [10]. Nebulizers generate aerosol particles 1–5 μm in size, with N95 respirators protecting against particles 0.3 μm or larger. These treatments pose the risk of generating a high volume of small particles and propelling them over a longer distance than the natural air dispersion pattern [14]. This is supported by evidence from the SARS outbreak, where a super-spreading event was largely related to the administration of salbutamol via jet nebulizer [15]. Though limited, existing evidence suggests that MDIs, when used correctly, are as effective as nebulized treatments [16]. Thus, EDs will need to increase stock of inhalers and spacers, in addition to instructing staff to preferentially order inhaler treatments. EMS personnel need to be included in this discussion, such that no patient should arrive in a stretcher with a nebulizer actively aerosolizing particles.
If noninvasive ventilation (NIV)-limiting strategies are chosen, more aggressive support medications for conditions that normally improve with NIV may be needed: magnesium and epinephrine for COPD, sublingual nitroglycerin and nitroglycerin infusion for CHF. In addition to upstocking, both pharmacy and ED leadership need to confirm Pyxis availability with emergent access to these medications readily available. If high volumes of patients requiring respiratory support are expected, additional stock of paralytics, induction agents, and medications for post-intubation sedation should be obtained and made easily accessible.
8. Radiology preparation
Radiographic personnel (including radiologists and technicians) are among the first-line healthcare workers at increased risk of exposure during a viral respiratory disease outbreak. Diagnostic imaging facilities should be prepared with guidelines to handle such an event. This includes using portable radiographic equipment whenever possible, which limit patient transport and can be easily cleaned afterwards [17]. The American College of Radiology has recommended against the use of CT as a first-line screening tool for viral respiratory infections, like COVID-19. CT should be reserved for patients with clinical indications that require CT and appropriate infection control measures should be in place before scanning subsequent patients [17]. According to the CDC and Spaulding Criteria, these equipment pieces are only in contact with intact skin and are therefore considered “noncritical items”. They can be cleaned via low-level disinfectants, which include isopropyl alcohol and ethyl alcohol, with single-use disposable disinfectant towels [18]. The use of satellite radiography centers and/or dedicated radiographic equipment can also decrease the risk of transmission. Additionally, if it is required that a patient be transported to the radiology department, it is essential that individual be wearing appropriate PPE throughout transport.
9. Respiratory support resources
Appropriate ventilatory support is a main concern when preparing for, and responding to, viral respiratory disease outbreaks. Looking at the current COVID-19 pandemic, the prevalence of severe to critical hypoxic respiratory failure is 19% [19]. Current guidelines recommend the use of high-flow nasal cannula over non-invasive positive pressure ventilation (NIPPV) for COVID-19 patients; this is due to the high failure rate and increased risk of intubation associated with NIPPV use in patients with non-cardiogenic acute hypoxemic respiratory failure [10]. High flow oxygen is also thought to pose lower risk for aerosolization than NIPPV.
The guidelines recommend early endotracheal intubation, which should be performed in a negative pressure room to prevent diffusion of the pathogen [10]. One consideration for limiting exposure to aerosolized pathogens is to use video-guided laryngoscopy over direct laryngoscopy as evidence suggests this may reduce the number of failed intubation attempts [20]. COVID-19 patients who require mechanical ventilation should be managed similarly to other patients with acute respiratory failure, with special attention to settings that limit ventilator-induced lung injury as ARDS is a primary cause of death in many patients. A stock of disposable tape measurers for patient height and a wall reference with ideal body weights may help hard-wire appropriate initial settings.
The concerns about ventilator shortages must also be addressed. Many states having strategies in place to allocate ventilators during large disease outbreaks. For example, the New York Ventilator Allocation Guidelines uses the Sequential Organ Failure Assessment (SOFA) to evaluate mortality risk; this, along with exclusion criteria and timed trials, determine a patient's priority level and length of access to ventilator therapy [21]. Hospitals anticipating a shortage may consider strategies to ventilate two patients using a single ventilator [22]. This concept remains highly complex and multifaceted with many ethical concerns (Table 1 ).
Table 1.
Amendments to ED operations during viral respiratory outbreaks.
| Operation | Response considerations |
|---|---|
| Patient volume/triage |
|
| Screening patients |
|
| Cohorting patients |
|
| Infection control and environmental changes |
|
| Screening/testing HCPs |
|
| Staffing concerns |
|
| ED stocking and supply |
|
| Radiology preparation |
|
| Respiratory support |
|
10. Limitations
Individual outbreak scenarios require unique responses. Strategies will vary largely based on hospital and department size, availability of staff, number of surrounding hospitals, community size, populations affected, and extent of disease spread. Additionally, the concerns addressed here are not exhaustive; they represent challenges to care in a viral respiratory disease outbreak and may not be applicable to other types of contagions. Finally, there is limited data with which to validate some of these interventions due to the recentness of the COVID-19 pandemic. There is a need for more formal evaluation of intervention quality and efficacy to further our understanding of the best response.
11. Conclusion
As the front-line for healthcare during a viral respiratory disease outbreak, EDs must be prepared to make expeditious operational adjustments to meet the expanding patient volume and limit infectious spread. The suggestions presented here limit unnecessary ED visits via establishment of a call-center, isolate patients through effective triage and geographic cohorting, mitigate viral spread with appropriate screening and PPE, and address equipment and medication stock concerns. While any viral outbreak requires an individualized response, sharing these suggestions for operational planning may assist in the development of emergency management protocol and better prepare departments during this pandemic and in the future.
Financial support
This is a non-funded study, with no compensation or honoraria for conducting the study.
CRediT authorship contribution statement
Tess Whiteside: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Erin Kane: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Bandar Aljohani: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Marya Alsamman: Data curation, Formal analysis, Writing - review & editing. Ali Pourmand: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors do not have a financial interest or relationship to disclose regarding this research project.
References
- 1.Lin M.P., Baker O., Richardson L.D., Schuur J.D. Trends in emergency department visits and admission rates among US acute care hospitals. JAMA Intern Med. 2018;178(12):1708–1710. doi: 10.1001/jamainternmed.2018.4725. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FastStats [Internet] 2020. https://www.cdc.gov/nchs/fastats/emergency-department.htm [cited 2020 Mar 29]. Available from.
- 3.Schanzer D.L., Schwartz B. Impact of seasonal and pandemic influenza on emergency department visits, 2003-2010, Ontario, Canada. Acad Emerg Med. 2013;20(4):388–397. doi: 10.1111/acem.12111. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugas A.F., Morton M., Beard R., Pines J.M., Bayram J.D., Hsieh Y.H. Interventions to mitigate emergencydepartment and hospital crowding during an infectious respiratory diseaseoutbreak: results from an expert panel. PLoS Curr. 2013;5 doi: 10.1371/currents.dis.1f277e0d2bf80f4b2bb1dd5f63a13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jernigan J.A., Low D.E., Hefland R.F. Combining clinical and epidemiologic features for early recognition of SARS. Emerging Infect Dis. 2004;10(2):327–333. doi: 10.3201/eid1002.030741. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W.-K., Wu H.-D.I., Lin C.-C., Cheng Y.-C. Emergency department response to SARS, Taiwan. Emerg Infect Dis. 2005;11(7):1067–1073. doi: 10.3201/eid1107.040917. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almulhim K.N., Shesser R., Pourmand A., Whiteside T., Kane E. The relationship between staff teaming models and emergency department efficiency. The American Journal of Emergency Medicine [Internet] 2020;0(0) doi: 10.1016/j.ajem.2020.03.015. https://www.ajemjournal.com/article/S0735-6757(20)30157-1/abstract Mar 10 [cited 2020 Mar 30]. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Novel coronavirus - SARS-CoV-2 - COVID-19- an update what emergency clinicians need to know.Pdf.Pdf [internet]. Google docs. https://drive.google.com/file/d/1c2sBW8SUBkNlwUaS68WMZ8ULxuXLRe8i/view?usp=drive_web&usp=embed_facebook [cited 2020 Mar 27]. Available from:
- 9.Wilder-Smith A., Low J.G.H. Risk of respiratory infections in health care workers: lessons on infection control emerge from the SARS outbreak. Southeast Asian J Trop Med Public Health. 2005 Mar;36(2):481–488. [PubMed] [Google Scholar]
- 10.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis campaign: guidelines on the management of critically ill Adults with coronavirus disease 2019 (COVID-19). 101. [DOI] [PMC free article] [PubMed]
- 11.Seto W.H., Tsang D., Yung R.W.H., Ching T.Y., Ng T.K., Ho M. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003 May 3;361(9368):1519–1520. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau J.T.F., Fung K.S., Wong T.W., Kim J.H., Wong E., Chung S. SARS transmission among hospital workers in Hong Kong. Emerging Infect Dis. 2004;10(2):280–286. doi: 10.3201/eid1002.030534. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC Coronavirus disease 2019 (COVID-19) [internet]. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/index.html [cited 2020 Apr 3]. Available from.
- 14.Amirav I., Newhouse M.T. RE: transmission of Corona virus by nebulizer- a serious, underappreciated risk! 2020 Mar 23. https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk [cited 2020 Mar 25]; Available from: [DOI] [PMC free article] [PubMed]
- 15.Hui D.S. Severe acute respiratory syndrome (SARS): lessons learnt in Hong Kong. J Thorac Dis. 2013;5(Suppl. 2):S122–S126. doi: 10.3978/j.issn.2072-1439.2013.06.18. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brocklebank D., Ram F., Wright J., Barry P., Cates C., Davies L. Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess. 2001;5(26):1–149. doi: 10.3310/hta5260. [DOI] [PubMed] [Google Scholar]
- 17.ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection | American College of Radiology [internet] https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection [cited 2020 Mar 23]. Available from.
- 18.Rational approach | disinfection & sterilization guidelines | guidelines library | infection control | CDC [internet] https://www.cdc.gov/infectioncontrol/guidelines/disinfection/rational-approach.html [cited 2020 Mar 23]. Available from.
- 19.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Lewis S.R., Butler A.R., Parker J., Cook T.M., Smith A.F. Videolaryngoscopy versus direct laryngoscopy for adult patients requiring tracheal intubation. Cochrane Database Syst Rev. 2016;(11) doi: 10.1002/14651858.CD011136.pub2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6472630/ [Internet]. 2016 Nov 15 [cited 2020 Mar 25]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zucker HA, Adler KP, Berens DP. Current members of the New York state task force on life and the law. 272.
- 22.Neyman G., Irvin C.B. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006;13(11):1246–1249. doi: 10.1197/j.aem.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]