To the editor
The first available reports indicate that renal involvement is relatively frequent in patients with novel coronavirus disease 2019 (COVID-19) due to the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Up to 43% of patients present with proteinuria (including 10% with heavy proteinuria), 11% with hematuria, and 3.5% to 5% with acute kidney injury.1 , 2 Both proteinuria and acute kidney injury are associated with increased mortality.1 However, the exact mechanisms underlying renal injury in patients with COVID-19 are unclear as renal pathology data are lacking.
We report on a 63-year-old black male patient who developed acute kidney injury in the setting of COVID-19. He had a history of hypertension treated with atenolol, nifedipine, and olmesartan. He initially presented with intense fatigue, high-grade fever (39.7 °C), and respiratory distress (respiratory rate, 36 breaths/min; arterial blood O2 saturation, 86%) requiring O2 supplementation (4 l/min). On admission, his serum creatinine was 1.2 mg/dl. He was diagnosed with COVID-19 based on a positive reverse transcriptase–polymerase chain reaction test for SARS-CoV-2 in a nasopharyngeal swab sample.
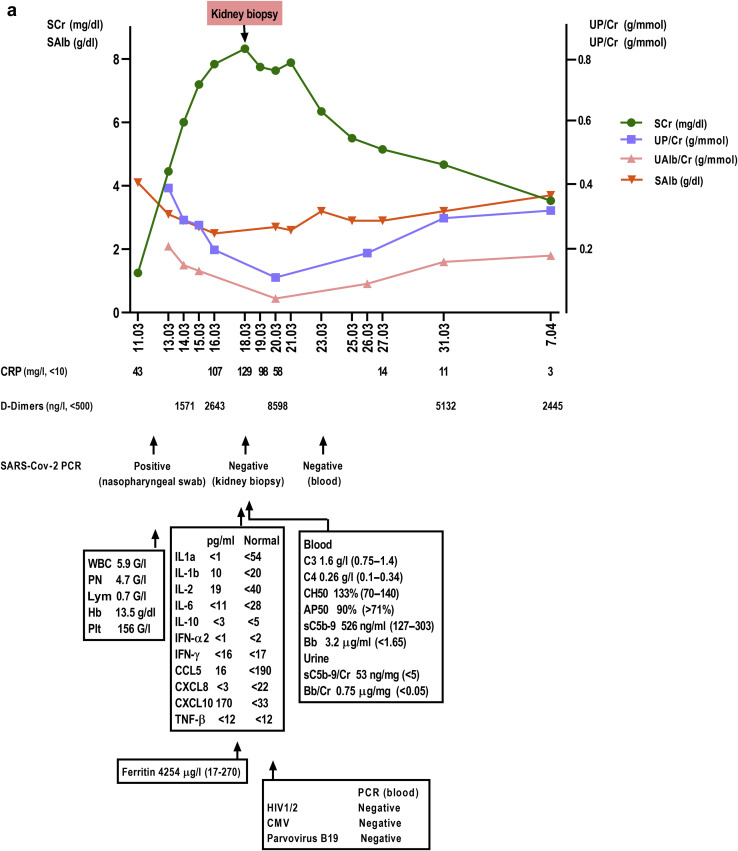
Shortly after his admission, he developed oliguria and rapidly progressive acute kidney injury (Kidney Disease: Improving Global Outcomes stage 3) with a serum creatinine of 4.4 mg/dl at day 4 (Figure 1 a). Laboratory tests showed an increase in the C-reactive protein level, lymphopenia, increased D-dimers serum level, hypoalbuminemia, massive proteinuria (5 g/l consisting of 50% of albumin), and reduced sodium urinary excretion (sodium excretion fraction: 0.4%). The patient had no episodes of hypotension and remained hypertensive for most of his hospital stay. His respiratory condition gradually improved and the O2 supplementation was decreased (0.5 l/min at day 8). Serum levels of a range of cytokines, including interleukin-6, were normal. However, the further increase of C-reactive protein serum levels was associated with systemic complement activation (soluble C5b-9, Bb fragment) and worsening of acute kidney injury with a serum creatinine peaking at 8.4 mg/dl at day 8 (Figure 1a). The patient did not receive SARS-CoV-2–specific experimental treatment (protease inhibitors, remdesivir, and hydroxychloroquine) or any nephrotoxic drug.
Figure 1.
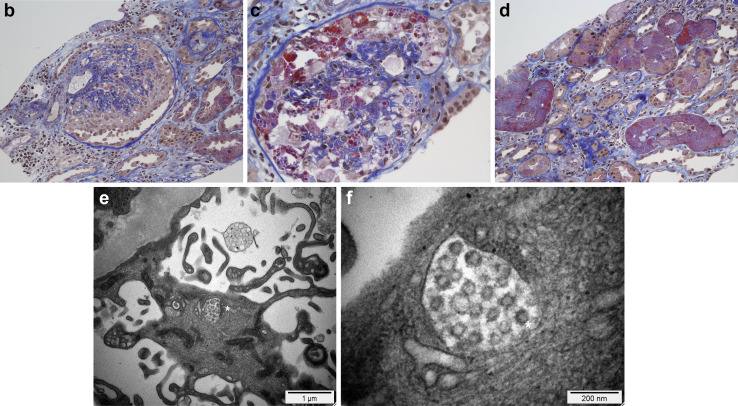
A 63-year-old black male patient was admitted for acute respiratory distress associated with novel coronavirus disease 2019. (a) The main laboratory results for this patient are shown. He rapidly developed acute kidney injury without hemodynamic compromise. His respiratory status improved but inflammatory syndrome persisted and renal function further deteriorated. (b–d) Illustrative images of his kidney biopsy are shown. Light microscopy study (Masson's trichrome stain, original magnification [b,d] ×200 and [c] ×400) showed the following: first, a severe collapsing glomerulopathy (focal segmental glomerulosclerosis) characterized by (b,c) the global collapse of shrinking capillary loops and the detachment from the basement membrane of (b) hypertrophic, proliferating podocytes (or “cobblestone pattern,” [asterisk]), which contained numerous (c) protein reabsorption vacuoles (asterisk). (d) Second, acute tubular lesions with focal tubular necrosis, dilatation, and the presence of intratubular reabsorption vacuoles (asterisks), reflecting the heavy proteinuria. Immunofluorescence study did not show any significant immune deposits. (e,f) Electron microscopy study (original magnification [e] ×15,000 and [f] ×73,000) disclosed within the podocytes cytoplasm vacuoles containing numerous (e) spherical particles (asterisk) measuring between 50 to 110 nm and surrounded by (f) spikes measuring 9 to 10 nm (“solar corona” [asterisk]). These particles may correspond to viral inclusion bodies reported with the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).3 AP50, alternative pathway activity 50%; Bb, Bb fragment; CCL, CC chemokine ligand; CH50, hemolytic complement activity 50%; CMV, cytomegalovirus; CXCL, CXC chemokine ligand; CRP, C-reactive protein; G, × 109; Hb, hemoglobin; IFN, interferon; IL, interleukin; Lym, lymphocytes; PCR, polymerase chain reaction; Plt, platelet count; PN, polynuclear neutrophils; SAlb, serum albumin; sC5b-9, soluble C5b-9; SCr, serum creatinine; TNF-β, tumor necrosis factor-β; UAlb/Cr, urinary albumin over creatinine ratio; UP/Cr, urinary protein over creatinine ratio; WBC, white blood cell count. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
A kidney biopsy was performed at day 8. Light microscopy examination disclosed 2 main features: severe collapsing focal segmental glomerulosclerosis (FSGS) (Figure 1b and c) and acute tubular necrosis (Figure 1d) without any significant interstitial inflammation. The immunofluorescence study revealed no significant immune deposits (including anti-C5b-9 staining). A reverse transcriptase–polymerase chain reaction for SARS-CoV-2 in RNA extracted from a frozen biopsy tissue sample was negative (reverse transcriptase–polymerase chain reaction was similarly negative in blood) (Figure 1a). Electron microscopy study (Figure 1e and f) disclosed, in the podocyte cytoplasm, vacuoles containing numerous spherical particles that may correspond to viral inclusion bodies reported with SARS-CoV-2.3
Further work-up showed that the patient was homozygous for the at-risk apolipoprotein A (APOL1) G1 variant (A342G and I348M). No specific treatment was implemented. The patient maintained a urinary output and did not require dialysis. Renal function subsequently improved and proteinuria decreased (Figure 1a). The patient was discharged on day 17 with a serum creatinine of 5.5 mg/dl and persistent proteinuria (1.8 g/l).
To the best of our knowledge, this is the first description of the pathological features of renal injury in the setting of COVID-19 outside autopsies series. The most striking finding in our patient was the collapsing FSGS. This finding suggests that FSGS could account for the heavy proteinuria reported in a significant proportion of patients with COVID-19.1 The collapsing FSGS is a known complication of other viral infections, in particular, HIV,4 as well as cytomegalovirus5 and parvovirus B19.6 For HIV a direct toxic viral effect on podocytes has been documented.7 The receptor for SARS-CoV-2, membrane-bound angiotensin-converting enzyme 2, is expressed on podocytes.8 , 9 However, polymerase chain reaction for SARS-CoV-2 was negative in kidney biopsy samples, but the technique has a notoriously low rate of detection in nonrespiratory samples (including blood and urine),10 and the quality of the extracted RNA material was poor. Besides, electron microscopy findings in our patient do not provide definite proof of the presence of SARS-CoV-2 in podocytes, and one cannot exclude that the detected vesicles in podocytes may correspond to nonviral particles. Collapsing FSGS, with or without acute tubular necrosis, can also complicate the course of hemophagocytic syndrome,11 a disorder characterized by an increased release of a wide range of cytokines. In our patient, normal levels of cytokines, in particular interleukin-6, while inflammation markers were still increased, plead against this hypothesis. However, a potential virus-driven intrarenal cytokines release cannot be excluded.
Besides, our patient is of African origin and is homozygous for the APOL1 at-risk G1 variant. This variant may have contributed to the pathogenesis of collapsing FSGS, because APOL1 is a recognized risk factor for the development collapsing FSGS in HIV and non-HIV patients.4
In our patient, acute tubular necrosis developed in the absence of hemodynamic compromise or severe pulmonary involvement. This suggests that tubular injury in COVID-19 patients, unlike that seen in coronavirus-associated SARS,12 is not predominantly ischemic. The possible underlying mechanisms are a direct viral toxicity on tubular cells that also harbor angiotensin-converting enzyme 2 or a cytokine-mediated tubular damage. In addition, the initial heavy proteinuria in our patient may have also contributed to tubular necrosis.
Overall, in contrast to lung injury, kidney injury in COVID-19 does not appear to include a predominant inflammatory component. This observation suggests that collapsing FSGS, potentially resulting from a direct viral effect on podocytes, probably belongs to the spectrum of COVID-19–associated renal involvement.
Acknowledgments
We would like to thank Dr. Heidi Fodstad, Department of Genetics, Lausanne University Hospital (CHUV), Lausanne, Switzerland, for the analysis of the APOL1 gene variants.
References
- 1.Cheng Y., Luo R., Wang K. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg A.Z., Naicker S., Winkler C.A., Kopp J.B. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11:150–160. doi: 10.1038/nrneph.2015.9. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson L., Boriskin Y., McPhee I. Acute cytomegalovirus infection complicated by collapsing glomerulopathy. Nephrol Dial Transplant. 2003;18:187–189. doi: 10.1093/ndt/18.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Waldman M., Kopp J.B. Parvovirus-B19-associated complications in renal transplant recipients. Nat Clin Pract Nephrol. 2007;3:540–550. doi: 10.1038/ncpneph0609. [DOI] [PubMed] [Google Scholar]
- 7.Lu T.C., He J.C., Wang Z.H. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–8182. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye M., Wysocki J., William J. Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: implications for albuminuria in diabetes. J Am Soc Nephrol. 2006;17:3067–3075. doi: 10.1681/ASN.2006050423. [DOI] [PubMed] [Google Scholar]
- 9.Perico L., Benigni A., Remuzzi G. Should COVID-19 concern nephrologists? Why and to what extent? The emerging impasse of angiotensin blockade. Nephron. 2020;144:213–221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaunat O., Delahousse M., Fakhouri F. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int. 2006;69:1892–1898. doi: 10.1038/sj.ki.5000352. [DOI] [PubMed] [Google Scholar]
- 12.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]