Abstract
With the deepening of research, proteomics has developed into a science covering the study of all the structural and functional characteristics of proteins and the dynamic change rules. The essence of various biological activities is revealed from the perspectives of the biological structure, functional activity and corresponding regulatory mechanism of proteins by proteomics. Among them, phospholipid-binding protein is one of the hotspots of proteomics, especially annexin A1, which is widely present in various tissues and cells of the body. It has the capability of binding to phospholipid membranes reversibly in a calcium ion dependent manner. In order to provide possible research ideas for researchers, who are interested in this protein, the biological effects of annexin A1, such as inflammatory regulation, cell signal transduction, cell proliferation, differentiation and apoptosis are described in this paper.
Keywords: Annexin A1, Inflammatory, Signal pathway, Cell proliferation, Apoptosis
Introduction
In 1977, Carl et al.1 isolated and purified a protein from the adrenal medulla of cattle, which can aggregate chromaffin cells. At the time, people called the protein “synexin” (Greek means meeting), now annexin A7.2 Later, the protein was found in tissue cells of many species.3 In 1990s, these proteins, relating to phospholipid binding in a calcium dependent manner, were named the annexin family officially.4 To date, more than one thousand annexin proteins have been identified in eukaryotic cells, which account for about 2% of total cellular proteins.5 According to their distribution in the biological world, annexin family was divided into 5 groups, with vertebrates as group A, invertebrates as group B, fungi and single-celled organisms as group C, plants as group D and protists as group E.6,7 Among them, group A has a total of 13 members, namely A1-A13, which are widely present in human tissues.8 Annexins normally have an unique N-terminal domain and a core domain with conserved structure, which is consisted of four (annexin A1-5, A7-13) or eight groups (annexin A6).9 Each group gets a repeat sequence consisting of 70 amino acid residues and extends to the C-terminal.8 In a calcium dependent manner, repeat sequence binds to the phospholipid bilayer structure on the cell membrane reversibly. Annexin's core domains have approximately 50% homology in amino acid sequence, whereas different patterns of gene expression and tissue specificity are derived from their unique N-terminal sequences.10 It has been found that annexins perform different biological functions. Once deregulation in gene expression or protein activity of annexins occurred, they can lead to “an annexin disease”. In annexin family, annexin A1 (ANXA1) was discovered at early stages. In 1979, ANXA1 was first recognized as a glucocorticoid regulated protein, which was believed to play an inhibitory role in synthesis of arachidonic acid (AA).11 In 1986, ANXA1 was successfully cloned for the first time. In the early days, it was also known as lipocotin I, calpactin II, phospholipase A2 (PLA2) inhibitory protein.12
ANXA1 gene and protein structure
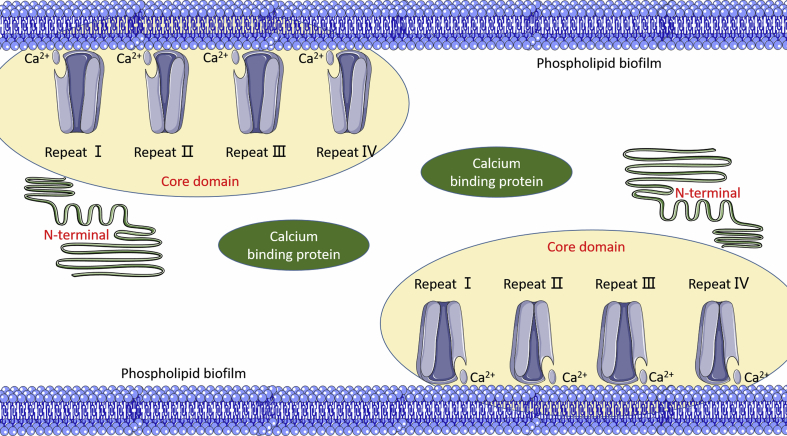
The ANXA1 gene, located on human chromosome 9q12-q21.2, is composed of 13 exons and 12 introns, and the ANXA1 protein is composed of 1041 bp coding 346 amino acid residues in its complementary deoxyribonucleic acid (cDNA).13 CAAT boxes and TATA boxes are included in promoters for genes, which both are required to maintain the minimum activity of the promoter.14 ANXA1 has a relative molecular weight of 38 kD and has a protein molecular structure shared by the annexin family: a core domain consisting of an N-terminus consisting of 44 amino acid residues and four homologous repeats (Repeat I, II, III, IV), each of which contains five α-helices and is arranged into a disc-like structure curvedly with a calcium (Ca) ion binding site thereon.15 As a result, it can be combined with the hydrophilic head of the phospholipid. The N-terminal, functional regulatory region of ANXA1, is also a Ca ion sensitive region.14 ANXA1 protein is inactive in the absence of Ca ions, and its N-terminus is present as a D-helix in the repeat III of the core domain. When the concentration of Ca ions increases, the N-terminus is released from repeat III. Then, the Ca ion binding site is unveiled. Once Ca ions combine the binding site to form a hydrophobic structure, it can interact with other molecules. Thus, the cell membranes aggregates with each other, exerting its biological function (Fig. 1).15
Fig. 1.
The structure of annexin A1 protein.
Secretion and cell source of ANXA1
ANXA1 protein is widely found in various cells of the body. In immune cells, the main source of ANXA1 is granulocytes, especially neutrophils.16 Among lymphocytes, T lymphocytes contain a small amount of ANXA1 protein, while B lymphocytes and platelets have no expression.16 In addition, ANXA1 can also be found in mature cells such as monocyte and macrophage, pulmonary epithelial cells, fibroblasts, and renal mesangial cells.17, 18, 19 Under the stimulation of inflammation, neutrophils and monocyte and are activated, and ANXA1 rapidly exerts its biological effect, as reach extracellular membranes.20 The exact mechanism of secretion varies. Adenosine triphosphate (ATP) binding transport is the main form of ANXA1 secretion in macrophages.20 In neutrophils, most of the intracellular ANXA1 are present in gelatinase granules. When neutrophils are buoyed by chemokines or adhere to the monolayer endothelial cells, ANXA1 moves quickly to the cell membrane from intracellular space, and then the chemotaxis of chemotactic factor is relieved in Ca ion concentration dependent manner. Then, chemotaxis of neutrophil and endothelium becomes low.
Biological function of ANXA1
ANXA1 and regulation of inflammation
ANXA1 and inflammation-related enzymes
ANXA1, also known as PLA2 inhibitory protein, prevents the formation of AA inflammatory precursors.21 There are two types of PLA2 in vivo: cytosolic PLA2 (cPLA2) and serotype (sPLA2). Among them, cPLA2 is a key enzyme for the synthesis and release of lipid icosane inflammatory mediators, and its inhibitors can prevent the expression of activated neutrophil surface integrin and the release of interleukin - 1β (IL-1β) from lipopolysaccharide-stimulated monocytes. The study data showed that both the endogenous full-length ANXA1 protein and its C-terminal fragment can bind to the phospholipid binding site of cPLA2 through hydrophobic interaction, which specifically inhibits the activity of PLA2 by interfering with the binding of cPLA2 to the membrane and inhibits the phosphorylation of cPLA2.22 While the extrinsic ANXA1 or its N-terminal fragments competes with the endogenous substrate in the Src homology domain of cPLA2, inhibiting the ras-MAPK signal transduction pathway to activate cPLA2.23 ANXA1 can also inhibit the synthesis of induced nitric oxide synthase in macrophages and induced cyclooxygenase 2 (COX-2) in activated microglia by inducing the release of IL-10, an anti-inflammatory factor.24
ANXA1 and inflammatory factors
Min et al.25 found that melanocyte stimulating hormone (α-MSH) inhibits tumor necrosis factor (TNF)-α-induced mature dendritic cells by up-regulating the expression of ANXA1, thereby exerting its anti-inflammatory effect. However, when ribonucleic acid (RNA) interference down-regulated the expression of ANXA1, the anti-inflammatory effect of α-MSH disappeared. Girol et al.26 found that the full-length ANXA1 and N-terminal active peptide Ac2-26 can alleviate ocular inflammation caused by endotoxin in mice ocular tissues and in vitro ARPE-19 cells. In the study of pleurisy mice model, ANXA1 and Ac2-26 can control inflammatory progression by inducing neutrophil apoptosis. Other studies have shown that Ac2-26 can inhibit the expression of inflammatory factor TNF-α in a mice model of intestinal ischemia reperfusion injury.27 In addition, some studies have shown that ANXA1 can inhibit the generation of IL-6 in pulmonary fibroblast cell lines stimulated by pro-inflammatory factor IL-1 through the p38-MAPK signaling pathway.28,29 Recent studies have demonstrated that in the lipopolysaccharide (LPS)-stimulated endotoxin animal model, serum IL-6 and TNF-α levels were higher in the ANXA1 knockout mice compared with normal mice.30 Similarly, in LPS-stimulated ANXA1-deficient macrophages, LPS can promote the production of IL-6 and TNF-α through the extracellular regulated protein kinases (ERK)-MAPK and c-Jun N-terminal kinase (JNK)-MAPK signaling pathways.30 The anti-inflammatory effects of ANXA1 may also be closely linked to the anti-inflammatory factor IL-10.31 Anti-inflammatory effects of lipoxin and ANXA1 disappeared in the rats whose IL-10 was knocked out, whereas in wild type rats, administration of lipoxin and ANXA1 protein through activating receptors (Lipoxin A4/ANXA1 receptor (ALXR) can promote the mass production of IL-10, thereby inhibiting inflammatory damage.31
ANXA1 and inflammatory cells
In the inflammatory response, ANXA1 and its N-terminal fragment showed strong inhibitory effects on migration of neutrophils and monocytes, which was related to abscission of L-selectin, carboxylation of N-glycans, the activation of ALXR and fingerprint recognition (FPR) and competitive binding of integrin α4β1.21,31, 32, 33 Under normal conditions, both granulocytes and monocytes in the blood are rich in ANXA1. Due to the lack of activation or adhesion, the anti-inflammatory activity of ANXA1 was not stimulated. In stationary leukocytes, especially polymorphonuclear (PMN), ANXA1 is mostly concentrated in cytoplasmic gelatinase particles.11 In the initial stage of inflammation, leukocytes are activated and penetrate the blood vessel wall to reach the reaction site due to the adhesion of chemokines. Under the action of inflammatory factors, the neutrophils in the post-capillary microveins of the infected site bind to endothelial cells, and neutrophils release enzyme particles, thereby a large amount of ANXA1 are stimulated to move to the cell surface, and specifically bind to the plasma membrane of the cell by means of Ca ion regulation.34 Externalized ANXA1 interacts with adhesion molecules that mediate the interaction between leukocyte endothelial cells, promoting the adherent neutrophils/monocytes to shed from the endothelial cells, thereby maintaining the dynamic balance of adhesion and desorption between PMN/monocytes and endothelial cells, and regulating the level of extravascular extravasation in inflammation to exert anti-inflammatory effects.35,36
FPR is a family of formyl-methionine-leucine-phenylalanine G protein-coupled receptors that are chemically driven and capable of being recognized by the peptide segments of bacteria, thus acting as a chemical driver to direct neutrophils movement to the site of bacterial infection.31 When an inflammatory response occurs, ANXA1 or its N-terminal peptide segments can bind to FPR in neutrophils, causing the receptor desensitization to lose its chemical drive effect and inhibiting the migration of neutrophils and monocytes, thereby the aggregation of leukocytes at the site of injury or infection is reduced.37 Studies have shown that, in the inflammatory model of FPR gene knockout, the effect of endogenous ANXA1 inhibits leukocyte aggregation and migration is significantly reduced, and the leukocyte aggregation is more in inflammatory response than that in normal rats.38 On the contrary, after the exogenous administration of ANXA1 or, it was observed that leukocytes adhesion and shedding increased, and the number of white blood cells entering the blood vessel was significantly reduced. Walther et al.39 showed that ANXA1 binds to its N-terminus and activates neutrophil FPR to inhibit its transendothelial process, and suggested that this effect may be related to the induction of L-selectin shedding by ANXA1 binds to FPR.
Other studies have shown that ANXA1 can activate ERK1/2 instantaneously after binding to ALXR, which leads to a rapid increase in the concentration of Ca ions in cells, thereby reducing the adhesion and migration of PMN.40 Solito et al.33 found that ANXA1 and integrin α4β1 co-localize on the surface of monocytes. A number of studies have shown that endogenous ANXA1 and exogenous ANXA1 exogenously compete with vascular cell adhesion molecule-1 (VCAM-1) for binding to integrin α4β1, inhibit adhesion of monocytes to vascular endothelial cells, and participate in the regulation of early inflammatory response.41,42 It has been proved that in a rat model of peritonitis, the use of a monoclonal antibody can inhibit the migration of neutrophils/monocytes by blocking the function of carboxylated N-glycans.43 In the inflammatory response induced by the ANXA1 knockout mice model, the expression of cyclooxygenase-2 (COX-2) and cPLA2 was up-regulated.44 Meanwhile, migration/phagocytosis ability of leukocyte and expression of adhesion molecules were abnormal. Yona et al43 showed that the deletion of ANXA1 gene affected the expression of macrophage surface adhesion molecules in peripheral tissues, thereby their phagocytic ability was changed selectively in the gene deficient mice. Meanwhile, expression of COX-2, release of prostaglandin E2 (PGE2), production of reactive oxygen species all increased.45 Recent studies have shown that the expression of ANXA1 in neutrophils is closely linked to serum cortisol concentration, and is also affected by serum levels of gonadotropin, luteinizing hormone and follicle stimulating hormone, while the expression of ANXA1 in lymphocytes gradually decreased with age.46
ANXA1 and signal transduction
ANXA1 protein has multiple phosphorylation sites and is a substrate for epidermal growth factor (EGF) receptor kinase, platelet derived growth factor (PDGF) receptor kinase, tyrosine protein kinase, serine, and threonine kinase.47, 48, 49 Studies have shown that ANXA1 tyrosine phosphorylation occurs during EGF receptor activation and internalization.47 Previous studies have revealed that the expression level of ANXA1 is coupled with the regulation of ERK pathway.50 ANXA1 can inhibit cell proliferation by inhibiting the expression of cyclin D1 through the ERK pathway. And the anti-inflammatory effect of ANXA1 is mainly regulated by the FPRs signaling pathway.26,38 In human tissues, FPRs include FPR1 and FPR2, which are the receptors for ANXA1 and Ac2-26.41 Perretti et al.51 found that neutrophils and macrophages release ANXA1 by autocrine or paracrine after induction by glucocorticoids. ANXA1 regulates the ERK-MAPK and T cell receptor (TCR) signaling pathways after binding with FPR1, and affects nuclear factor of activated T cells (NFAT), nuclear transcription factor NF-κB and the activity of activator protein-1 (AP-1), thereby regulating the activity, proliferation and differentiation of T cells and exerting their anti-inflammatory effects.26,28,42 These are all evidence that ANXA1 is involved in cell signaling.
ANXA1 and cell proliferation
Initial studies found that ANXA1 can bind to the EGF receptor, and subsequently found its effect on cell proliferation.47 It was believed that ANXA1 can inhibit the mitotic signal transduction pathway induced by hepatocyte growth factor (HGF), as well as the proliferation of T cells stimulated by monoclonal antibody CD3 monoclonal antibody (OKT3).52 ANXA1 also can affect the formation and activity of protein complex upstream of the ERK-MAPK pathway to reduce cell proliferation and inhibit the expression of cyclin D1. Studies have shown a definite relationship between ANXA1 with cell cycle. When the cells were in the G2/M phase, the expression of ANXA1 decreased by 40%, but the mechanism of decline was still unknown.53
ANXA1 and cell differentiation
ANXA1 is associated with the regulation of cell differentiation in bone and muscle tissues. Damazo et al.43 knocked out the mice ANXA1 gene to observe its effect on bone development. They noted delayed abnormal cranial suture structure, incomplete cranial suture fusion, and intracranial osteogenesis. It is believed that the normal expression of ANXA1 plays an important role in the normal development of skull. In vitro experiments by Bizzarro et al.37 found that ANXA1 expression was up-regulated when mice c2c12 myoblasts differentiated into myocytes. On the contrary, ANXA1 was down-regulated when c2c12 myoblasts were inhibited to differentiate into myocytes. After expression of ANXA1 was down-regulated by RNA interference, the differentiation of c2c12 myoblasts was decreased. It was concluded that the protein level of ANXA1 was positively correlated with the degree of differentiation of c2c12. At the same time, ANXA1 also promotes T cell differentiation. Huggins et al.54 demonstrated that ANXA1 has the effect of promoting the activation and differentiation of T cells stimulated by dendritic cells in mixed lymphocyte reaction. ANXA1 also regulates T cell differentiation via the FPRs signaling pathway. ANXA1 binds to FPR1 to activate ERK and Akt signaling pathways and enhance T cell receptor (TCR) activity. The downstream transcription factors include NFAT, nuclear transcription factor -κB and AP-1, etc., thus promoting differentiation of T cells into helper T cells.55
ANXA1 and apoptosis
Studies have shown that overexpression of ANXA1 in neutrophils, human histiocytic lymphoma cells (U-937) and bronchoalveolar epithelial cells promotes apoptosis.33 This effect is caused by the activation of Capase-3 and the transient intracellular Ca influx, which leads to the instantaneous increase of Ca ion concentration in cells, and thus leads to the dephosphorylation of the pro-apoptotic protein bad. And the phagocytosis of macrophages on apoptotic neutrophils can be further promoted by the endogenous ANXA1 from apoptotic neutrophils. Arur et al.56 showed that ANXA1 might be an endogenous ligand for phosphatidylserine (PS) that mediates phagocytosis of apoptotic cells. ANXA1 aggregates into a PS-rich domain on the outer layer of the apoptotic cytoplasmic membrane through Caspase-3 mediated mechanism, mediating phagocytic recognition, phagocytosis of apoptotic cells, and release of anti-inflammatory mediators, accompanied by release of intracellular Ca ions.26 It can be seen that ANXA1 plays a role in inhibiting inflammatory response and allowing safe clearance of apoptotic cells during phagocytic recognition and phagocytosis. The recognition and phagocytosis of apoptotic leukocytes is also important in the regression of inflammation. The phagocytosis of apoptotic leukocytes by phagocytic cells such as macrophages can inhibit the expression of pro-inflammatory factors (such as TNF-α), also release anti-inflammatory factors (such as IL-10, TGF-β, etc.). Further studies have confirmed that ANXA1 can change the small guanosine triphosphate (GTP) enzymes (such as Rho A, Rac and Cdc42) by binding to ALXR on the surface of macrophages, so as to recombine myosin and actin and participate in the cytoskeletal changes of macrophages, thus guiding the phagocytosis of macrophages on apoptotic cells.41,57
The relationship between ANXA1 and disease
ANXA1 and carotid atheromatous plaque formation
ANXA1 plays a positive endogenous defense by stimulating an inflammatory response associated with carotid plaque formation. In many trials, it has been shown to mimic the anti-inflammatory effects of corticosteroids. Cheuk et al.58 studied 34 patients with carotid atherosclerosis and found that increased expression of ANXA1 can increase the stability of carotid atherosclerotic plaque.
ANXA1 and rheumatoid arthritis
The early lesions of rheumatoid arthritis are in the synovial membrane, and many synovial cells proliferate in the synovial tissue, accompanied by infiltration of various inflammatory cells. The researchers found that ANXA1 and its amino-terminal active residues in the synovial tissue inhibit the adhesion and exudation of neutrophils by the formate carboxy tyrosine receptor that occupies the surface of inflammatory cells, thereby preventing the spread of inflammation.59,60 Some scholars have succeeded in improving the symptoms of rheumatoid arthritis in rats by increasing the expression of ANXA1.59,60
ANXA1 and acute idiopathic pulmonary fibrosis
In the neutrophils of patients with acute idiopathic pulmonary fibrosis, the expression of ANXA1 was significantly increased, and its expression was up-regulated in association with CD4+ T cells, confirming that ANXA1 may play an important immunopathological role in the development of acute idiopathic pulmonary fibrosis.
ANXA1 and liver fibrosis
Zagoura et al.61 found that ANXA1 can increase reactivity with the severity of liver fibrosis in the body. The more obvious the fibrosis, the more the expression of ANXA1 is up-regulated, and the anti-inflammatory and anti-fibrotic effects are maximized. At the same time, it begins to rise in the early stage of fibrosis, and peaks in the middle stage, indicating that its anti-inflammatory liver protection is concentrated in the early and middle stages.
ANXA1 and IgA nephropathy
Some scholars have found that the expression intensity of ANXA1 in renal tissues of human IgA nephropathy and animal models is negatively correlated with the degree of tubular damage, indicating inhibition in the development of nephropathy.62,63 It is speculated that ANXA1 may have an important defense effect against the production of glomerular inflammatory mediators, thereby inhibiting the development of IgA nephropathy.
ANXA1 and post-traumatic brain injury
Post-traumatic brain injury is a common traumatic disease in neurosurgery. It has the characteristics of high incidence, critical illness and poor prognosis. Studies have confirmed that blood-brain barrier destruction, inflammatory reaction, free radical damage after post-traumatic brain injury lead to secondary brain damage, which in turn impairs neurological function. In recent years, it has been found that the expression of serum ANXA1 is decreased after cerebral hemorrhage, and the increase of ANXA1 can improve the vascular endothelial cells and neuronal necrosis, maintain the integrity of the blood-brain barrier, and reduce brain edema after cerebral hemorrhage.
ANXA1 and skin wound healing
Jin et al.64 found that ANXA1 is widely expressed in neutrophils and monocytes in the inflammatory phase after skin injury, and is suitable for the inference of wound formation time in early trauma, especially the inference of wound injury time around 1 day. Therefore, detecting the expression of ANXA1 can further increase the accuracy of skin wound formation time inference.
Conclusion
Most of the membrane proteins are conserved in evolution, and most of them are intracellular proteins. ANXA1 is one of the early discovered and concerned protein molecules. Due to its ability to bind to Ca ions, ANXA1 is involved in a series of Ca-dependent membranous biological activities in tissue cells. Since its discovery, although many studies have been conducted on the biological functions of ANXA1, including biofilm formation, ion channel establishment, inflammation regulation, and cell signal transduction, its specific regulatory mechanisms have not been fully understood. Therefore, this paper attempts to systematically expound studies on its various biological functions, and provides a basis for the future studies on the complex regulatory network of ANXA1.
Funding
This study was supported by National Project of International Science and technology Cooperation program of China (2015DFA33050), National Natural Science Foundation of China for Youths (81601949).
Ethical Statement
Not applicable.
Acknowledgements
The authors are appreciated for the valuable comments and discussions with each member of our group.
Footnotes
Peer review under responsibility of Chinese Medical Association.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.Morris S.J., Hughes J.M., Whittaker V.P. Purification and mode of action of synexin: a protein enhancing calcium-induced membrane aggregation. J Neurochem. 1982;39:529–536. doi: 10.1111/j.1471-4159.1982.tb03977.x. [DOI] [PubMed] [Google Scholar]
- 2.Ye Weihua, Li Yong, Fan Liqiao. Effect of annexin A7 suppression on the apoptosis of gastric cancer cells. Mol Cell Biochem. 2017;429:33–43. doi: 10.1007/s11010-016-2934-4. [DOI] [PubMed] [Google Scholar]
- 3.Hong K., Düzgüneş N., Ekerdt R. Synexin facilitates fusion of specific phospholipid membranes at divalent cation concentrations found intracellularly. Proc Natl Acad Sci USA. 1982;79:4642–4644. doi: 10.1073/pnas.79.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geisow M.J., Walker J.H., Boustead C. Annexins–new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987;7:289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- 5.Aliyu Isah Abubakar, Ling King-Hwa, Hashim Nurfariesha Md. Annexin A2 extracellular translocation and virus interaction: a potential target for antivirus-drug discovery. Rev Med Virol. 2019;29:e2038. doi: 10.1002/rmv.2038. [DOI] [PubMed] [Google Scholar]
- 6.McCulloch Kathryn M., Yamakawa Izumi, Shifrin David A., Jr. An alternative N-terminal fold of the intestine-specific annexin A13a induces dimerization and regulates membrane-binding. J Biol Chem. 2019;294:3454–3463. doi: 10.1074/jbc.RA118.004571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piovezan Anna P., Batisti Ana P., Benevides Maria L. Hydroalcoholic crude extract of casearia sylvestris Sw. reduces chronic post-ischemic pain by activation of pro-resolving pathways. J Ethnopharmacol. 2017;204:179–188. doi: 10.1016/j.jep.2017.03.059. [DOI] [PubMed] [Google Scholar]
- 8.Weyd Heiko. More than just innate affairs - on the role of annexins in adaptive immunity. Biol Chem. 2016;397:1017–1029. doi: 10.1515/hsz-2016-0191. [DOI] [PubMed] [Google Scholar]
- 9.Grewal Thomas, Hoque Monira, Conway James R.W. Annexin A6-A multifunctional scaffold in cell motility. Cell Adhes Migrat. 2017;11:288–304. doi: 10.1080/19336918.2016.1268318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouzenzana Jamel, Pelosi Ludovic, Briolay Anne. Identification of the first Oomycete annexin as a (1-->3)-beta-D-glucan synthase activator. Mol Microbiol. 2006;62:552–565. doi: 10.1111/j.1365-2958.2006.05389.x. [DOI] [PubMed] [Google Scholar]
- 11.Park J.-C., Baik S.H., Han S.-H. ANXA1 restores Aβ1-42 -induced blood-brain barrier disruption through the inhibition of RhoA-ROCK signaling pathway. Aging Cell. 2017;16:149–161. doi: 10.1111/acel.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon Joon Hyun, Lee Jea Hwang, Kim Ki Soon. Regulation of cytosolic phospholipase A2 phosphorylation by proteolytic cleavage of annexin A1 in activated mast cells. J Immunol. 2012;188:5665–5673. doi: 10.4049/jimmunol.1102306. [DOI] [PubMed] [Google Scholar]
- 13.Liu Aifeng, Huang Weiguo, Zeng Guqing. Expression of the ANXA1 gene is associated with suppression of growth, invasion and metastasis of nasopharyngeal carcinoma. Mol Med Rep. 2014;10:3059–3067. doi: 10.3892/mmr.2014.2656. [DOI] [PubMed] [Google Scholar]
- 14.Smith P.D., Davies A., Crumpton M.J. Structure of the human annexin VI gene. Proc Natl Acad Sci USA. 1994;91:2713–2717. doi: 10.1073/pnas.91.7.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly S.R., Moss S.E. Functional analysis of the human annexin I and VI gene promoters. Biochem J. 1998;332:681–687. doi: 10.1042/bj3320681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Peng, Zhou Yuxiang, Liu Zan. Interaction between ANXA1 and GATA-3 in immunosuppression of CD4+ T cells. Mediat Inflamm. 2016;2016:1701059. doi: 10.1155/2016/1701059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto Michelle A., Ribeiro Ana Luíza C., Costa Bruno RC. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood. 2017;129:2896–2907. doi: 10.1182/blood-2016-09-742825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salles J.P., Gayral-Taminh M., Fauvel J. Sustained effect of angiotensin II on tyrosine phosphorylation of annexin I in glomerular mesangial cells. J Biol Chem. 1993;268:12805–12811. [PubMed] [Google Scholar]
- 19.Damazo Amílcar S., Sampaio André Lf, Nakata Cintia Mag. Endogenous annexin A1 counter-regulates bleomycin-induced lung fibrosis. BMC Immunol. 2011;12:59. doi: 10.1186/1471-2172-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancaleone Vincenzo, Mitidieri Emma, Flower Roderick J. ANXA1 mediates hydrogen sulfide properties in the control of inflammation. J Pharmacol Exp Therapeut. 2014;351:96–104. doi: 10.1124/jpet.114.217034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parente Luca, Solito Egle. Annexin 1: more than an anti-phospholipase protein. Inflamm Res. 2004;53:125–132. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi Masakiyo, Murata Hitoshi, Sonegawa Hiroyuki. Truncation of annexin A1 is a regulatory lever for linking epidermal growth factor signaling with cytosolic phospholipase A2 in normal and malignant squamous epithelial cells. J Biol Chem. 2007;282:35679–35686. doi: 10.1074/jbc.M707538200. [DOI] [PubMed] [Google Scholar]
- 23.John C.D., Christian H.C., Morris J.F. Kinase-dependent regulation of the secretion of thyrotrophin and luteinizing hormone by glucocorticoids and annexin 1 peptides. J Neuroendocrinol. 2003;15:946–957. doi: 10.1046/j.1365-2826.2003.01081.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferlazzo Viviana, D'Agostino Pietro, Milano Salvatore. Anti-inflammatory effects of annexin-1: stimulation of IL-10 release and inhibition of nitric oxide synthesis. Int Immunopharm. 2003;3:1363–1369. doi: 10.1016/S1567-5769(03)00133-4. [DOI] [PubMed] [Google Scholar]
- 25.Min Yan, Han Deping, Fu Zhanjiang. α-MSH inhibits TNF-α-induced maturation of human dendritic cells in vitro through the up-regulation of ANXA1. Acta Biochim Biophys Sin. 2011;43:61–68. doi: 10.1093/abbs/gmq109. [DOI] [PubMed] [Google Scholar]
- 26.Girol A.P., Mimura K.K., Drewes C.C. Anti-inflammatory mechanisms of the annexin A1 protein and its mimetic peptide Ac2-26 in models of ocular inflammation in vivo and in vitro. J Immunol. 2013;190:5689–5701. doi: 10.4049/jimmunol.1202030. [DOI] [PubMed] [Google Scholar]
- 27.Guido B.C., Zanatelli M., Tavares-de-Lima W. Annexin-A1 peptide down-regulates the leukocyte recruitment and up-regulates interleukin-10 release into lung after intestinal ischemia-reperfusion in mice. J Inflamm. 2013;10:10. doi: 10.1186/1476-9255-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia Y., Morand E.F., Song W. Regulation of lung fibroblast activation by annexin A1. J Cell Physiol. 2013;228:476–484. doi: 10.1002/jcp.24156. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y.H., Toh M.-L., Clyne C.D. Annexin 1 negatively regulates IL-6 expression via effects on p38 MAPK and MAPK phosphatase-1. J Immunol. 2006;177:8148–8153. doi: 10.4049/jimmunol.177.11.8148. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y.H., Aeberli D., Dacumos A. Annexin-1 regulates macrophage IL-6 and TNF via glucocorticoid-induced leucine zipper. J Immunol. 2009;183:1435–1445. doi: 10.4049/jimmunol.0804000. [DOI] [PubMed] [Google Scholar]
- 31.Volpato L.K., Horewicz V.V., Bobinski F. ANXA1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis. J Reprod Immunol. 2018;129:30–35. doi: 10.1016/j.jri.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 32.de Coupade C., Solito E., Levine J.D. Dexamethasone enhances interaction of endogenous annexin 1 with L-selectin and triggers shedding of L-selectin in the monocytic cell line U-937. Br J Pharmacol. 2003;140:133–145. doi: 10.1038/sj.bjp.0705413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solito E., Romero I.A., Marullo S. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the alpha 4 beta 1 integrin. J Immunol. 2000;165:1573–1581. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- 34.Brancaleone V., Dalli J., Bena S. Evidence for an anti-inflammatory loop centered on polymorphonuclear leukocyte formyl peptide receptor 2/lipoxin A4 receptor and operative in the inflamed microvasculature. J Immunol. 2011;186:4905–4914. doi: 10.4049/jimmunol.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliani S.M., Perretti M. Cell localization of the anti-inflammatory protein annexin 1 during experimental inflammatory response. Ital J Anat Embryol. 2001;106:69–77. [PubMed] [Google Scholar]
- 36.Hayhoe R.P.G., Kamal A.M., Solito E. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- 37.Belvedere R., Bizzarro V., Popolo A. Role of intracellular and extracellular annexin A1 in migration and invasion of human pancreatic carcinoma cells. BMC Canc. 2014;14:961. doi: 10.1186/1471-2407-14-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buss N.A., Gavins F.N., Cover P. Targeting the annexin 1-formyl peptide receptor 2/ALX pathway affords protection against bacterial LPS-induced pathologic changes in the murine adrenal cortex. Faseb J. 2015;29:2930–2942. doi: 10.1096/fj.14-268375. [DOI] [PubMed] [Google Scholar]
- 39.Walther A., Riehemann K., Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- 40.Pan B., Kong J., Jin J. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim Biophys Acta. 2016;1861:501–512. doi: 10.1016/j.bbalip.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Peshavariya H.M., Taylor C.J., Goh C. Annexin peptide Ac2-26 suppresses TNFalpha-induced inflammatory responses via inhibition of Rac1-dependent NADPH oxidase in human endothelial cells. PLoS One. 2013;8:e60790. doi: 10.1371/journal.pone.0060790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vago J.P., Tavares L.P., Garcia C.C. The role and effects of glucocorticoid-induced leucine zipper in the context of inflammation resolution. J Immunol. 2015;194:4940–4950. doi: 10.4049/jimmunol.1401722. [DOI] [PubMed] [Google Scholar]
- 43.Damazo A.S., Yona S., Flower R.J. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roviezzo F., Getting S.J., Paul-Clark M.J. The annexin-1 knockout mouse: what it tells us about the inflammatory response. J Physiol Pharmacol. 2002;53:541–553. [PubMed] [Google Scholar]
- 45.Santos J., Gonzalez-Sanchez L., Matabuena-Deyzaguirre M. A role for stroma-derived annexin A1 as mediator in the control of genetic susceptibility to T-cell lymphoblastic malignancies through prostaglandin E2 secretion. Canc Res. 2009;69:2577–2587. doi: 10.1158/0008-5472.CAN-08-1821. [DOI] [PubMed] [Google Scholar]
- 46.Mulla A., Leroux C., Solito E. Correlation between the antiinflammatory protein annexin 1 (lipocortin 1) and serum cortisol in subjects with normal and dysregulated adrenal function. J Clin Endocrinol Metab. 2005;90:557–562. doi: 10.1210/jc.2004-1230. [DOI] [PubMed] [Google Scholar]
- 47.Poeter M., Radke S., Koese M. Disruption of the annexin A1/S100A11 complex increases the migration and clonogenic growth by dysregulating epithelial growth factor (EGF) signaling. Biochim Biophys Acta. 2013;1833:1700–1711. doi: 10.1016/j.bbamcr.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Eltoweissy M., Dihazi G.H., Muller G.A. Protein DJ-1 and its anti-oxidative stress function play an important role in renal cell mediated response to profibrotic agents. Mol Biosyst. 2016;12:1842–1859. doi: 10.1039/c5mb00887e. [DOI] [PubMed] [Google Scholar]
- 49.Molas R.B., de Paula-Silva M., Masood R. Ac2-26 peptide and serine protease of Bothrops atrox similarly induces angiogenesis without triggering local and systemic inflammation in a murine model of dorsal skinfold chamber. Toxicon. 2017;137:7–14. doi: 10.1016/j.toxicon.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X., Li X., Li X. ANXA1 silencing increases the sensitivity of cancer cells to low-concentration arsenic trioxide treatment by inhibiting ERK MAPK activation. Tumori. 2015;101:360–367. doi: 10.5301/tj.5000315. [DOI] [PubMed] [Google Scholar]
- 51.Perretti M., Di Filippo C., D'Amico M. Characterizing the anti-inflammatory and tissue protective actions of a novel ANXA1 peptide. PLoS One. 2017;12:e0175786. doi: 10.1371/journal.pone.0175786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goulding N.J., Ogbourn S., Pipitone N. The inhibitory effect of dexamethasone on lymphocyte adhesion molecule expression and intercellular aggregation is not mediated by lipocortin 1. Clin Exp Immunol. 1999;118:376–383. doi: 10.1046/j.1365-2249.1999.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y.J., Lee S.H. Pro-oxidant activity of sulforaphane and cisplatin potentiates apoptosis and simultaneously promotes autophagy in malignant mesothelioma cells. Mol Med Rep. 2017;16:2133–2141. doi: 10.3892/mmr.2017.6789. [DOI] [PubMed] [Google Scholar]
- 54.Huggins A., Paschalidis N., Flower R.J. Annexin-1-deficient dendritic cells acquire a mature phenotype during differentiation. Faseb J. 2009;23:985–996. doi: 10.1096/fj.08-119040. [DOI] [PubMed] [Google Scholar]
- 55.D'Acquisto F., Merghani A., Lecona E. Annexin-1 modulates T-cell activation and differentiation. Blood. 2007;109:1095–1102. doi: 10.1182/blood-2006-05-022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arur S., Uche U.E., Rezaul K. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 57.Davenport N.R., Sonnemann K.J., Eliceiri K.W. Membrane dynamics during cellular wound repair. Mol Biol Cell. 2016;27:2272–2285. doi: 10.1091/mbc.E16-04-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheuk B.L., Cheng S.W. ANXA1 expression in atherosclerotic carotid plaques and its relationship with plaque characteristics. Eur J Vasc Endovasc Surg. 2011;41:364–371. doi: 10.1016/j.ejvs.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Haridas V., Shetty P., Sarathkumar E. Reciprocal regulation of pro-inflammatory Annexin A2 and anti-inflammatory ANXA1 in the pathogenesis of rheumatoid arthritis. Mol Biol Rep. 2019;46:83–95. doi: 10.1007/s11033-018-4448-5. [DOI] [PubMed] [Google Scholar]
- 60.Mei Y.F., Dai S.M., Jia H.N. Expression of annexin A1 in peripheral blood cells of Naive rheumatoid arthritis patients and its influencing factors. Zhonghua Yixue Zazhi. 2017;97:1937–1941. doi: 10.3760/cma.j.issn.0376-2491.2017.25.004. [DOI] [PubMed] [Google Scholar]
- 61.Zagoura D., Trohatou O., Makridakis M. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine. 2019;45:542–552. doi: 10.1016/j.ebiom.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purvis G.S.D., Collino M., Loiola R.A. Identification of AnnexinA1 as an endogenous regulator of RhoA, and its role in the pathophysiology and experimental therapy of type-2 diabetes. Front Immunol. 2019;10:571. doi: 10.3389/fimmu.2019.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pietrani N.T., Ferreira C.N., Rodrigues K.F. ANXA1 concentrations is decreased in patients with diabetes type 2 and nephropathy. Clin Chim Acta. 2014;436:181–182. doi: 10.1016/j.cca.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 64.Jin X., Zhao J.X., Yao L. Expression of annexin A1 during skin incised wound healing in mice. Fa Yi Xue Za Zhi. 2019;35:5–10. doi: 10.12116/j.issn.1004-5619.2019.01.002. [DOI] [PubMed] [Google Scholar]