Abstract
Pericyte, a kind of pluripotent cell, may regulate the irrigation flow and permeability of microcirculation. Pericytes are similar to the smooth muscle cells, which express several kinds of contractile proteins and have contractility. The dysfunction of pericytes is related to many microvascular diseases, including hypoxia, hypertension, diabetic retinopathy, fibrosis, inflammation, Alzheimer's disease, multiple sclerosis, and tumor formation. For a long time, their existence and function have been neglected. The distribution, structure, biomarker, related signaling pathways as well as the roles of pericytes on vascular diseases will be introduced in this review.
Keywords: Pericytes, Vascular diseases, Microcirculation
Introduction
Pericytes, embedded in the vascular basement, have recently come into focus as regulators of vascular permeability, development, and maturation. Pericytes are implicated in the development of diabetic retinopathy, microvascular dysfunction and inflammation. In this review, we will summarize the currently available data describing the distribution, ontogeny, and identity of pericytes and its function in normal physiology and the pathogenesis of vascular diseases.
Distribution and structure of pericyte
Capillary wall consists of two types of cells, endothelial cells (ECs) and pericytes. Pericytes are encapsulated within the basement membrane and ensheathe the capillary endothelium throughout the body. The pericytes envelop the endothelial wall of the microcirculation including pre-capillaries, capillaries, post-capillary venules, venules, and arterioles.1,2 Moreover, the coverage of pericytes is more extensive in post-capillary venules than in capillaries. Expressed by EC/pericyte ratio, pericyte density is different in different tissues, which might indicate specialized functions in different organs. Pericyte density and coverage appear to correlate with endothelial barrier properties (brain > lungs > muscle). Interestingly, pericyte density is the highest in the central nervous system (CNS) and retina (1:1 EC/pericytes ratio3, 4, 5) compared to the vasculature of other organs and tissues (2.5:1 EC/pericyte ratio in the kidney, 7:1-9:1 EC/pericyte ratio in the lung, 10:1 EC/pericyte ratio in the skin and liver, 100:1 EC/pericyte ratio in skeletal muscle6, 7, 8). The density of pericytes appears to correlate positively with endothelial barrier selectivity and stringency,7 suggesting that pericytes may have a certain functional role just in the CNS and retina. Nees et al9 also found that the pericyte density of the cardiac microvasculature was thought to be closer to that of the cerebral vasculature with endothelial-pericyte ratios of 2:1-3:1. It is estimated that there are approximately 3.6 × 107 pericytes/cm3 in left ventricular tissue and the number of pericytes is more than the number of myocytes in unit volume of left ventricular tissue.
Transmission electronic microscopy (TEM) observation found that pericytes have a large, round nucleus, less cytoplasm, and much rough endoplasmic reticulum. They embrace endothelial wall of the vessel and may extend to the neighboring vessels. The cytoplasm of a pericyte forms cytoplasmic processes, which contains many myofilament, actin, myosin and tropomyosin, no Ⅷ factor. The mature capillary pericytes have discoid nucleus consisted of heterochromatin and electron density around the nucleus is surrounded by a small amount of cytoplasm containing protein-producing organelles, glycogen particles, liposome, dictyosome, endoplasmic reticulum and mitochondria. Depending on the vascular bed and their differentiation state, pericytes exhibit various morphologies ranging from typical flat and stellate shape in the CNS to a more round shape in kidneys. So the morphological characteristics of pericytes are different in different microcirculation and even different regions in the same microcirculation.10 For example, the mid-capillary pericytes are sparse with slender and spindle-shaped cytoplasmic processes, and do not express alpha-smooth muscle actin (α-SMA), whereas the pre- and post-capillary pericytes were large with thick and star-shaped processes, and are rich in α-SMA expression.
The majority of the pericyte-endothelial interface is separated by a basement membrane (BM), a feature that makes it possible to identify pericyte by TEM.11 Pericytes have numerous finger-like cytoplasmic processes that extend longitudinally spanning the abluminal surface of several endothelial tube and occasionally bridge neighboring capillary branches. At capillary branch points, which commonly harbor a pericyte soma, primary processes are often found to extend along each branch. These primary processes extending along the length of the capillary give rise to secondary processes that run perpendicular to the primary processes, partially encircling the capillary with tips of secondary processes making connections with endothelial cells.12 So far as know, pericytes are connected to the endothelial cells by 3 major types of intercellular junctions: peg-socket type, gap junctions and adhesion plaques.13 In the areas lacking a BM, pericyte cytoplasmatic elongations (the so-called pegs) are embeded in endothelial invaginations of the endothelial membrane (the sockets), including N-cadherin, the adherens junction protein and gap junctions (connexin-43/30) that allow direct chemical communication between the neighboring cells through the diffusion of nutrients, metabolites, secondary messengers, ions and various molecules.14 At some points of contact, the spot-like adherence junctions called adhesion plaques consist of predominately of fibronectin mediating the connection of the basement membrane to the plasma membrane via integrins, which attach cells to each other and to the extracellular matrix and underlie actin cytoskeletal networks of pericytes and endothelium (Fig. 1).15
Fig. 1.
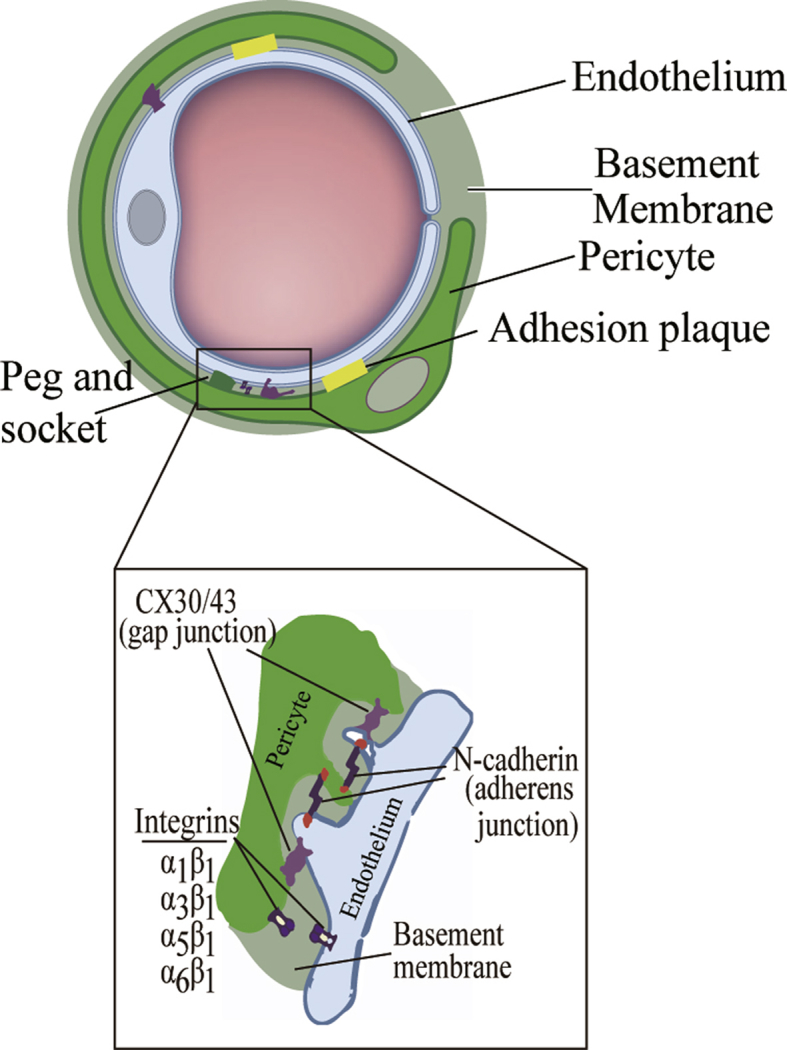
Ultrastructural characteristics of pericytes and pericyte-endothelial interactions.
Origin of pericytes
Pericytes were first characterized in the 19th century by Rouget as a mural cell population.16 Due to their perivascular position, Rouget cells were renamed as pericytes by Zimmermann.17 The source of pericytes has long been controversial. Initially, pericytes expressed myogenic phenotypes are believed to be similar to smooth muscle cells. Pericytes contain contractile filaments composed of vimentin and α-SMA, and are capable of vasomotion function.18 Probably, the majority of pericytes in the CNS are both mesoderm-derived mesenchymal stem cells and neuroectoderm-derived neural crest cells, as demonstrated in chick-quail chimeras.19 Recent studies on thymus, retina, choroid and aortic outflow tract showed that pericytes are also derived from differentiated neural crest-derived cells.20 On the other hand, the origins of pericytes from coelomic organs, such as the gut, lung, and liver, have been mapped to the mesothelium derived. Coronary vessel pericytes in the heart appear to have a similar development.21 Here, the cardiac pericytes are derived from the epicardial mesothelium.22 However, coronary pericytes developed from endocardial cells undergoing endothelial-to-mesenchymal transition. Recent publications showed that B cells derived from bone marrow may be the progenitor cells pools of pericytes, and endothelial cells and fibroblasts could be induced to pericytes. However, most scholars believed that pericytes capable of differentiation are similar to mesenchymal stem cells.2 It is clear that there is still a lack of understanding regarding pericyte ontogeny and development. More work to identify the origin of a type of pericyte could help identify their potential functions for particular pathological processes.
Identification markers of pericyte
Pericytes express a range of antigenic markers, which is similar to their morphological and structure diversity and helps their identification. No single pericyte specific marker is known, so their identification is based on the combination of multiple markers. Membrane bound markers for pericytes include platelet-derived growth factor receptor-beta (PDGFR-β),23, 24, 25 neural/glial antigen 2 (NG-2),23,26, 27, 28 CD146,23,24 alanine aminopeptidase (CD13),14,23,24,29 and endoglin, together with mesenchymal stem cell markers, such as CD90 and CD105.23,26, 27, 28 Commonly, cytosolic markers for pericyte identification include α-SMA, non-muscle myosin, desmin, nestin and vimentin, etc.14,23,24,26,30,31
A new cytosolic marker that promises for specific pericyte identification is the regulator of G protein signaling 5 (RGS5).14,32 RGS5 acts as a GTPase activating protein, which plays a role in the proliferation and recruitment of pericytes during vascular development and wound healing. Other cell surface antigens expressed by pericytes are SUR2 (ATP-binding cassette transporter subfamily C member 9 or ABCC9),33 Kir 6.1 (potassium inwardly rectifying channel subfamily J member 8),33 delta-like non-canonical Notch ligand 1 (DLK1),33 and alkaline phosphatase (ALP).34, 35, 36
It is important to emphasize that pericytes represent a heterogeneous population with identifiable subsets when characterized beyond a population level. For instance, pericytes on venules express desmin and α-SMA, whereas those on capillaries express desmin but are negative for α-SMA.24,31,37 Chen et al.38 showed that adventitial pericytes on the cardiac express CD34+/CD31-/CD146-, whereas microvascular cardiac pericytes express CD146+/CD34-/GATA-4/CD56-/CD117-. Park et al.39 found that cerebral pericytes express CD13+/CD73+/CD90+/CD105+/CD45-/CD31-.
It is important to clear that marker expression for pericytes however may be labile. For instance, pericyte α-SMA may be hardly expressed in both normal skin and brain, but may be increased obviously after tumorigenesis. Knowledge about pericyte subsets and their roles remains limited, more cell surface markers are identified and more pericyte subsets with diversity function can be expected to be found.
Pericyte function
Contribution to microvascular barrier function
Recent studies have shown that pericytes play an important role in endothelial barrier development and maintenance of integrity. The most stringent endothelial barriers are found in the vasculature of CNS and retina. In CNS, pericytes contact with microglia cells, neurons, ECs, and astrocyte end-feet that together form the so-called functional neurovascular unit (NVU).40 The role of astrocytes in blood brain barrier (BBB) regulation in NVU is well demonstrated and the importance of NVU pericytes has also been reported. Daneman et al.41 showed that pericyte recruitment was important to BBB formation during embryogenesis a week before astrocytes were recruited. They also showed that pericyte coverage was a decisive role in vascular permeability.
Pericytes are further shown in vitro to regulate the BBB at the level of endothelial junctions. In pericyte co-cultures with ECs and astrocyte, the presence of pericytes increases the trans-endothelial electrical resistance in comparison with single cultured ECs. Researchers found that pericytes and astrocyte could all increase the endothelial barrier integrity; however, pericytes had better protective effect on endothelial barrier than astrocyte after hypoxia.42 Studies further showed that loss of pericytes could damage the BBB in vivo. In PDGFRβ ± mice with hypomorphic alleles of PDGFRβ that renders these animals pericyte deficient, tight junction (TJ) protein like claudin-5, occludin, and zonula occludens-1 and adherent junction protein like cadherin were reduced in the microvasculature of the CNS.4,41 This coincided with BBB breakdown and vascular leakage. Further studies found that paracrine signaling between pericytes and ECs mediated by transforming growth factor-β (TGF-β) and angiopoietin-1 (Ang-1) contributed to endothelial barrier maintenance.43 Pericyte could secrete TGF-β, a multifunctional cytokine, to support BBB integrity through stabilizing the structure of actin filaments in ECs. Pericytes derived Ang-1 could activate Tie-2 receptor to promote the expression of occludin in ECs.44 Taken together, these studies point out an essential role for pericytes in endothelial barrier function regulation.
Contractile function
Subcellular structural pericyte marker proteins such as α-SMA, desmin, vimentin and cyclic guanosine monophosphate (C-GMP) associated with constriction contribute to the actin filament bundles located near the EC side, and regulate cell contractility.13 Pericytes were detected in vitro to response to the vasoconstrictors like angiotensin-Ⅱ(Ang-Ⅱ), serotonin and vasodilators like nitric oxide, cholinergic agonists, adenosine by measuring the surface area of the collagen lattice.45 Previous studies have shown that the contraction of pericytes participated in the developments and advances of many diseases, such as cardiopathy and CNS diseases. Peppiatt et al.46 found that retinal pericytes given by electrical stimulation could activate the microvascular constriction. Researches showed that some factors-induced contraction of pericytes had biphasic effects. Hyperoxic condition has been shown to induce pericyte contraction, whereas hypoxia condition can trigger pericyte relaxation.47 Studies found that some signaling pathways such as Rho guanosine triphosphatase (GTPase) and calpain pathways played an important role in regulation of the contraction of pericytes. Because of the staggering complexity of the environment in vivo, the contraction of pericytes in vitro cannot be equated with the contraction in vivo. So research on the contraction of pericytes has long been difficult. Recent studies confirmed that pericytes were capable of constringency function by some new techniques such as diaminofluorescein-2 diacetate (DAF-2DA) and two-photon microscopy.48,49 These data are related to the concept that pericyte contractility plays a role in the regulation of the blood flow in the microvasculature.
Angiogenesis function
Pericytes play an important role in forming and stabilizing the angiogenesis. Recruited pericytes settle into the new vessels to release paracrine factors, such as PDGFR-β, to promote vascular maturation.50 Ramsauer et al.51 found that pericytes stabilized the formation of capillary-like structures by co-cultured with endothelial cells and astrocytes. Persidsky et al.52 have shown that pericytes could regulate endothelial cell migration, proliferation, and differentiation. Studies reported that pericytes participated in new blood vessels formation and sprouting by some signaling molecules such as PDGFR-β, TGF-β, vascular endothelial growth factor (VEGF), Ang-1 and S1P.50 Pericytes release VEGF to promote endothelial cell maturation.53 TGF-β derived by pericytes can induce mesenchymal cell around blood vessels to differentiate into pericytes and smooth muscle cells.54 PDGF-β derived by endothelial cell can recruit pericytes to stabilize the blood vessels and promote pericytes migration and proliferation.55 Ang-1 released by pericytes binds to Tie-2 in endothelial cells, which increases the expression of heparin binding epidermal growth factor-like growth factor (HB-EGF) and plays an important role in vascular stability.
Stem cell potential
Research has shown that pericytes separated from different tissues are capable of differentiating into mesenchymal cells, such as smooth muscle cells, fibroblasts, adipose cell and so on. Crisan et al.31 found that pericytes expressed some stem cells markers, including CD44, CD73, CD90, CD105. In fact, the characteristics of the brain pericytes are similar with mesenchymal stem cells (MSCs). For example, brain pericytes and MSCs derived from mesoderm have common immunogenicity and differentiation potential.56 Further studies showed that brain pericyte can regenerate itself and be able to differentiate into neurons, astrocytes, and oligodendrocytes. Present studies have shown that pericytes transplanted into the severe combined immunodeficient (SCID) mice could generate skeletal muscle fiber.57 In short, pericytes have the characteristics of mesenchymal stem cells, which may participate in various diseases such as nervous system and leads to new drug targets for therapies.58
Immune regulation function
The high expression of acid phosphatase of lysosome that increased with age and injury in pericytes has the function of phagocytosis. Pericytes can intake the small molecules or soluble substances in blood or brain parenchyma by phagocytosis, pinocytosis and endocytosis.59 Once BBB breakdown, pericytes can phagocytose the exudation of the red cells.60 Monteiro et al.61 found that pericytes separated from basement membrance could differentiate into perivascular microglial cells to play phagocytosis in brain injury conditions. In the physiological condition, brain pericytes express adhesion molecules at a lower level, including intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and major histocompatibility complex (MHC) class I. However, in pathologic conditions, pericytes influenced by inflammatory cytolines such as TNF-α, IFN-γ generate MHC class II and presents them to T lymphocytes.62 In addition, even after induction of class I and II MHC molecules, pericytes are unable to stimulate allogeneic CD4 T cell proliferation and in fact rendered the T cells unresponsive to endothelial cells of the same donor.63 This behavior of pericytes indicates the possibility of pericytes in allogenic stem cell therapy.
Pericytes in diseases
BBB maintenance
Pericytes wrapped in the tightly bound endothelial cells on capillaries are an important constituent of neurovascular unit and BBB. Studies showed that pericytes participated in the regulation of cerebral blood flow, neurovascular growth, steady neural network and blood-brain barrier integrity. Several studies have already demonstrated that loss of pericyte coverage resulted in the breakdown of the BBB and endothelial barrier function. Pericytes maintained the function of BBB by releasing the Ang-1 and TGF-β1. Ang-1 secreted by pericytes increased the expression of the occludin in ECs; however, occludin at a lower level induces the breakdown of the tight junction and causes vascular permeability.44 Many studies also identified that the loss of pericytes was associated with the breakdown of the endothelial tight junction in the pathogenesis of stroke, multiple sclerosis, cerebral infection, epilepsy, and Parkinson's disease.64 Dohgu et al.43 found that TGF-β1 derived by pericytes could directly regulate BBB function. Transwell co-cultures of brain capillary ECs and brain derived pericytes, the presence of pericytes increased the trans-endothelial electrical resistance in comparison with single cultured ECs.65 TGF-β1 activated mitogen-activated protein kinase (MAPK) signaling increases the expression of TJ proteins and P-gp efflux in ECs to improve the BBB function. However, Shen et al.66 found that TGF-β1 increases the tyrosine phosphorylation of VE-cadherin and claudin-5 in ECs and aggravates the paracellular permeability. Thus, the effect of TGF-β1 derived from pericytes remains to be further studied in health and disease. Yemischi et al.67 showed that pericytes remained contraction despite the no-reflow phenomenon in brain ischemia–reperfusion rat model. This is mediated by the excessive production of the reactive oxygen species, which activates intracellular Ca2+ levels. Furthermore, Dore-Duffy also found that the upregulation of ET-1, which stimulated the expression of α-SMA pericytes, caused the inappropriate vasoconstriction of the pericytes and reduced the vessel diameter.68
Loss of pericyte in diabetic retinopathy
With the diabetes mellitus course being longer, the diabetic retinopathy (DR) is one of the serious complications. The marked characteristic of this course is severe microangiopathy, which eventually results in blindness. Loss of pericyte coverage of the retinal capillaries results in endothelial cell proliferation, thickened basal membrane and retinal barrier breakdown, which develop into the stricture of the vascular lumen, the changes of blood flow dynamics, the occlusion of the retinal capillaries and severe hemorrhage and retinal detachment. So pericytes play an important role in the regulation of the pathological angiogenesis. The early changes of diabetic retinopathy are related with the loss of retinal capillary pericytes stimulated by chronic hyperglycemia, which indicates that pericyte apoptosis is an important cause of pathological angiogenesis in diabetes.69,70 Shin et al.71 found that the increased expression of phosphorylated signal transducer and activat of transcription 1 (STAT 1) upregulated the expression of Bim on the retinal pericytes of rat with diabetes mellitus. Current study findings suggest that oxidative stress is one of the main causes of pericyte apoptosis.72 Ang-1/Tie-2 and PDGFβ/PDGFRβ signallings appeared to play an important role in the pericyte-mediated pathogenesis of DR. For example, Ang-2 related with higher levels of pro-fibrotic factors was 30-fold upregulated in the retina of diabetic rats.73 While treatment with Ang-1 in a diabetic retinopathy rat model could reduce endothelial injury and endothelial barrier breakdown in the retina.74 Studies in PDGFR-β knockout mice reported that PDGFR-β deficiency caused severe pericyte dissipation in the retinal capillaries, which resulted in the vascular malformation during the processing of the vascular formation.75 Many studies on pericytes were still restricted on experiments in vitro. The exact mechanisms about the retinal pericyte lesion and the development of DR are still remained to be clarified.
Pericytes in inflammation
Recent studies have proved that pericytes play an important role in inflammatory response.76,77 Proebstl et al.78 found that once neutrophils derived from venule migrated to the endothelial cells or endothelial cells wall of the inflamed tissues, the junctional intercellular gap between pericytes and endothelial cells enlarged. They also found that pericytes had high sensitivity in the inflammatory signaling, and as precursor of immune cell, pericytes drived leukocytes to the inflammatory site. Researches in vitro showed that pericytes stimulated by TNF-α could generate a series of immune responses. Pericytes could enhance the endothelial cell interactions by increasing the macrophage recruitment.79 The animal experimental results showed that the desquamation of pericytes could lead to the damage of blood-brain barrier in LPS-induced sepsis of rats.80 Ziegler et al.81 also found that Ang-2 antagonist improved the apoptosis of pericytes, the permeability of blood vessel and the changes of hemodynamics in septic rats. Additionally, studies found that cytokines derived by pericytes, such as sphingosine-1-phosphate (S1P), VEGF, BDNF, upregulated the adhesion molecules and the tight junction proteins on EC to enhance the vascular endothelial barrier function.82 Recent researches showed that pericytes, when stimulated with LPS or TNF-α, secreted large amounts of pro-inflammatory molecules, anti-inflammatory molecules, cytokines and chemokines. LPS-stimulated pericytes displayed IL-12, IL-13, IL-9 and increased protein expression of chemokines and adhesion molecules such as CCL-3/4, ICAM-1, VCAM-1 and E-selectin.83
Pericytes in cardiovascular diseases
The function of pericytes in the myocardial vasculature has been attracted an increasing attention. Nees et al.84 identified that larger myocardial arterioles and coronary arteries contained significant quantities of pericytes, while larger venules and veins only existed smaller amounts of pericytes. Others also demonstrated that pericytes played a significant role in the formation of the BM. High levels of type IV collagen expressed by pericytes contributed to the myocardial basement membrane formation.85 These studies indicated that pericytes play an important role in maintaining vascular homeostasis in heart.
Research showed that loss of pericytes exposed to tyrosine kinase inhibitors (sunitinib) in the cardiac microvasculature lead to coronary flow reserve impairing, interstitial permeability increase and cardiac microvascular dysfunction.86 Dysregulation of pericytes control of myocardial tissue resulted in ischemic heart disease. Juchem et al.87 demonstrated that abnormal pericyte contractions were the major causes of coronary no-reflow. Cardiac microvasculature stimulated by the inflammation released the tissue factor and activated the coagulation cascade, which lead to fibrin crosslinking, thrombus formation and lumen occlusion.88 It has been implicated that the increased level of endothelin-1 is the main reason for the phenomenon of coronary no-reflow in patients with acute myocardial infarction, which is related with an increase in a-SMA positive pericytes and a reduction of the vessel diameter.68 A great number of studies have showed that pericytes participated in the development of cardiovascular diseases, such as aortic valve sclerosis, ischemic cardiomyopathy. Meanwhile, pericytes also have the potential for treatment of cardiovascular disease. Chen et al.89 showed that pericytes derived from human skeletal muscle significantly improved the left ventricular myocardial contractility, reduced myocardial fibrosis and inflammatory infiltrate in the infarct area. Avolio et al.90 found that pericytes on the great saphenous vein improved the infraction area and fibrosis with myocardial infarction. In addition to the cardiovascular disease, other researchers in the field have already used pericytes as a source of therapeutic cells to treat multiple diseases.
Perspective
An iterated concern in pericyte biology is the identification of the cell type. Investigators have started to appreciate the impact and characteristic pericytes of various regions in different organs. So, further efforts must be made to characterize the specific markers for pericyte subpopulations so as to better establish their roles in health and diseases.
In regard to clinical applications, the mesenchymal stem cells are currently the most widely explored. Although pericytes have similar properties to MSC. The use of pericytes for regenerative medicine is still a relatively new area of research and novel therapeutic approaches are continually emerging. One such approach is that delivery of exogenous pericytes for regenerative therapy. The secretory capacities of different cell types are now explored as such possible therapeutic regenerative agents in a variety of diseases. More considerations should be addressed about the secretome of pericytes and its potential implications for tissue regeneration. Pericytes represent an obvious candidate product for improving vascular growth and perfusion of ischemic tissues.
Funding
This study was supported by the Key Projects and innovation group of National Natural Science Foundation of China (81830065, 81721001). These funding agencies had no role in study design, collection/analyses of data, decision to publish, or manuscript preparation.
Ethical Statement
Not applicable.
Declaration of Competing Interest
No conflict of interest exists in the submission of this manuscript.
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.DeRuiter M., Poelmann R., VanMunsteren J. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 2.Rajantie I., Ilmonen M., Alminaite A. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armulik A., Genove G., Mae M. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 4.Bell R.D., Winkler E.A., Sagare A.P. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathiisen T.M., Lehre K.P., Danbolt N.C. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 6.Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflügers Archiv. 2013;465:775–778. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Flores L., Gutierrez R., Madrid J.F. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 8.Rudziak P., Ellis C.G., Kowalewska P.M. Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues. Mediat Inflamm. 2019;2019:4123605. doi: 10.1155/2019/4123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nees S., Weiss D.R., Senftl A. Isolation, bulk cultivation, and characterization of coronary microvascular pericytes: the second most frequent myocardial cell type in vitro. Am J Physiol Heart Circ Physiol. 2012;302:H69–H84. doi: 10.1152/ajpheart.00359.2011. [DOI] [PubMed] [Google Scholar]
- 10.Hashitani H., Mitsui R. Role of pericytes in the initiation and propagation of spontaneous activity in the microvasculature. Adv Exp Med Biol. 2019;1124:329–356. doi: 10.1007/978-981-13-5895-1_14. [DOI] [PubMed] [Google Scholar]
- 11.Bababeygy S.R., Cheshier S.H., Hou L.C. Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cell Dev. 2008;17:11–18. doi: 10.1089/scd.2007.0117. [DOI] [PubMed] [Google Scholar]
- 12.Petrova T.V., Karpanen T., Norrmen C. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 13.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K., Kamouchi M., Arimura K. Extracellular acidification activates cAMP responsive element binding protein via Na+/H+ exchanger isoform 1-mediated Ca(2)(+) oscillation in central nervous system pericytes. Arterioscler Thromb Vasc Biol. 2012;32:2670–2677. doi: 10.1161/ATVBAHA.112.254946. [DOI] [PubMed] [Google Scholar]
- 16.Sims D.E. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- 17.Majesky M.W. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 18.Creazzo T.L., Godt R.E., Leatherbury L. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- 19.Korn J., Christ B., Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol. 2002;442:78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- 20.Karow M., Sanchez R., Schichor C. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Asahina K., Zhou B., Pu W.T. Septum transversum-derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology. 2011;53:983–995. doi: 10.1002/hep.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphreys B.D., Lin S.L., Kobayashi A. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisan M., Yap S., Casteilla L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Crisan M., Corselli M., Chen W.C. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozerdem U., Stallcup W.B. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall A.P. Review of the pericyte during angiogenesis and its role in cancer and diabetic retinopathy. Toxicol Pathol. 2006;34:763–775. doi: 10.1080/01926230600936290. [DOI] [PubMed] [Google Scholar]
- 27.Ozerdem U., Grako K.A., Dahlin-Huppe K. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dynam. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 28.Smith S.W., Chand S., Savage C.O. Biology of the renal pericyte. Nephrol Dial Transplant. 2012;27:2149–2155. doi: 10.1093/ndt/gfs134. [DOI] [PubMed] [Google Scholar]
- 29.Xueyong L., Shaozong C., Wangzhou L. Differentiation of the pericyte in wound healing: the precursor, the process, and the role of the vascular endothelial cell. Wound Repair Regen. 2008;16:346–355. doi: 10.1111/j.1524-475X.2008.00374.x. [DOI] [PubMed] [Google Scholar]
- 30.Ribatti D., Nico B., Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 31.Crisan M., Corselli M., Chen C.W. Multilineage stem cells in the adult: a perivascular legacy? Organogenesis. 2011;7:101–104. doi: 10.4161/org.7.2.16150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bondjers C., Kalén M., Hellström M. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol. 2003;162:721–729. doi: 10.1016/S0002-9440(10)63868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bondjers C., He L., Takemoto M. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J. 2006;20:1703–1705. doi: 10.1096/fj.05-4944fje. [DOI] [PubMed] [Google Scholar]
- 34.Vono R., Fuoco C., Testa S. Activation of the pro-oxidant PKCβII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes. 2016;65:3691–3704. doi: 10.2337/db16-0248. [DOI] [PubMed] [Google Scholar]
- 35.Farup J., De Lisio M., Rahbek S.K. Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle. J Appl Physiol. 2015;119:1053–1063. doi: 10.1152/japplphysiol.01108.2014. [DOI] [PubMed] [Google Scholar]
- 36.Sharma U., Pal D., Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem. 2014;29:269–278. doi: 10.1007/s12291-013-0408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes S., Gardiner T., Hu P. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Chen W.C., Baily J.E., Corselli M. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cell. 2015;33:557–573. doi: 10.1002/stem.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park T.I., Feisst V., Brooks A.E. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci Rep. 2016;6:26587. doi: 10.1038/srep26587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sa-Pereira I., Brites D., Brito M.A. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- 41.Daneman R., Zhou L., Kebede A.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Ahmad A., Taboada C.B., Gassmann M. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cerebr Blood Flow Metabol. 2011;31:693–705. doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dohgu S., Takata F., Yamauchi A. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Hori S., Ohtsuki S., Hosoya K. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 45.Rucker H.K., Wynder H.J., Thomas W.E. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51:363–369. doi: 10.1016/s0361-9230(99)00260-9. [DOI] [PubMed] [Google Scholar]
- 46.Peppiatt C.M., Howarth C., Mobbs P. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerkar S., Speyer C., Tyburski J. Reactive oxygen metabolites induce a biphasic contractile response in microvascular lung pericytes. J Trauma. 2001;51:440–445. doi: 10.1097/00005373-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Dai M., Nuttall A., Yang Y. Visualization and contractile activity of cochlear pericytes in the capillaries of the spiral ligament. Hear Res. 2009;254:100–107. doi: 10.1016/j.heares.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Klett F., Offenhauser N., Dirnagl U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dore-Duffy P., LaManna J.C. Physiologic angiodynamics in the brain. Antioxidants Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 51.Ramsauer M., Krause D., Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 52.Persidsky Y., Ramirez S.H., Haorah J. Blood–brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 53.Hagedorn M., Balke M., Schmidt A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dynam. 2004;230:23–33. doi: 10.1002/dvdy.20020. [DOI] [PubMed] [Google Scholar]
- 54.Sinha S., Hoofnagle M.H., Kingston P.A. Transforming growth factor-beta1 signaling contributes to development of smooth muscle cells from embryonic stem cells. Am J Physiol Cell Physiol. 2004;287:C1560–C1568. doi: 10.1152/ajpcell.00221.2004. [DOI] [PubMed] [Google Scholar]
- 55.Stenzel D., Nye E., Nisancioglu M. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–924. doi: 10.1182/blood-2008-10-186239. [DOI] [PubMed] [Google Scholar]
- 56.Lamagna C., Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J Leukoc Biol. 2006;80:677–681. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- 57.Dellavalle A., Sampaolesi M., Tonlorenzi R. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 58.Lange S., Trost A., Tempfer H. Brain pericyte plasticity as a potential drug target in CNS repair. Drug Discov Today. 2013;18:456–463. doi: 10.1016/j.drudis.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 59.Thomas W.E. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 60.Shulman S., Katzenstein H., Abramowsky C. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30:442–447. doi: 10.3109/15513815.2011.618873. [DOI] [PubMed] [Google Scholar]
- 61.Monteiro R.A., Rocha E., Marini-Abreu M.M. Do microglia arise from pericytes? An ultrastructural and distribution study in the rat cerebellar cortex. J Submicr Cytol Pathol. 1996;28:457–469. [PubMed] [Google Scholar]
- 62.Navarro R., Compte M., Alvarez-Vallina L. Immune regulation by pericytes: modulating innate and adaptive immunity. Front Immunol. 2016;7:480. doi: 10.3389/fimmu.2016.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maier C.L., Pober J.S. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arterioscler Thromb Vasc Biol. 2011;31:183–189. doi: 10.1161/ATVBAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luissint A.C., Artus C., Glacial F. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Shen W., Li S., Chung S.H. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90:323–332. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Yemisci M., Gursoy-Ozdemir Y., Vural A. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 68.Dore-Duffy P., Wang S., Mehedi A. Pericyte-mediated vasoconstriction underlies TBI-induced hypoperfusion. Neurol Res. 2011;33:176–186. doi: 10.1179/016164111X12881719352372. [DOI] [PubMed] [Google Scholar]
- 69.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 70.Hammes H.P., Feng Y., Pfister F. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin E.S., Huang Q., Gurel Z. STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell Death Dis. 2014;5:e986. doi: 10.1038/cddis.2013.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Assero G., Lupo G., Anfuso C.D. High glucose and advanced glycation end products induce phospholipid hydrolysis and phospholipid enzyme inhibition in bovine retinal pericytes. Biochim Biophys Acta. 2001;1533:128–140. doi: 10.1016/s1388-1981(01)00151-2. [DOI] [PubMed] [Google Scholar]
- 73.Pfister F., Wang Y., Schreiter K. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- 74.Cai J., Kehoe O., Smith G.M. The angiopoietin/Tie-2 system regulates pericyte survival and recruitment in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:2163–2171. doi: 10.1167/iovs.07-1206. [DOI] [PubMed] [Google Scholar]
- 75.Lindahl P., Johansson B.R., Leveen P. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 76.Stark K., Eckart A., Haidari S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 77.Alon R., Nourshargh S. Learning in motion: pericytes instruct migrating innate leukocytes. Nat Immunol. 2013;14:14–15. doi: 10.1038/ni.2489. [DOI] [PubMed] [Google Scholar]
- 78.Proebstl D., Voisin M.B., Woodfin A. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yotsumoto F., You W.K., Cejudo-Martin P. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. OncoImmunology. 2015;4 doi: 10.1080/2162402X.2014.1001204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nishioku T., Dohgu S., Takata F. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29:309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ziegler T., Horstkotte J., Schwab C. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013 doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimizu F., Sano Y., Saito K. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37:401–409. doi: 10.1007/s11064-011-0626-8. [DOI] [PubMed] [Google Scholar]
- 83.Smyth L.C.D., Rustenhoven J., Park T.I. Unique and shared inflammatory profiles of human brain endothelia and pericytes. J Neuroinflammation. 2018;15:138. doi: 10.1186/s12974-018-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nees S., Juchem G., Eberhorn N. Wall structures of myocardial precapillary arterioles and postcapillary venules reexamined and reconstructed in vitro for studies on barrier functions. Am J Physiol Heart Circ Physiol. 2012;302:H51–H68. doi: 10.1152/ajpheart.00358.2011. [DOI] [PubMed] [Google Scholar]
- 85.He Q., Spiro M.J. Isolation of rat heart endothelial cells and pericytes: evaluation of their role in the formation of extracellular matrix components. J Mol Cell Cardiol. 1995;27:1173–1183. doi: 10.1016/0022-2828(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 86.Chintalgattu V., Rees M.L., Culver J.C. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5:169–187. doi: 10.1126/scitranslmed.3005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Juchem G., Weiss D.R., Gansera B. Pericytes in the macrovascular intima: possible physiological and pathogenetic impact. Am J Physiol Heart Circ Physiol. 2010;298:H754–H770. doi: 10.1152/ajpheart.00343.2009. [DOI] [PubMed] [Google Scholar]
- 88.O'Farrell F.M., Attwell D. A role for pericytes in coronary no-reflow. Nat Rev Cardiol. 2014;11:427–432. doi: 10.1038/nrcardio.2014.58. [DOI] [PubMed] [Google Scholar]
- 89.Chen C.W., Okada M., Proto J.D. Human pericytes for ischemic heart repair. Stem Cell. 2013;31:305–316. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Avolio E., Meloni M., Spencer H.L. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81–e94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]


