Introduction
Arrhythmia-induced cardiomyopathy is a reversible form of left ventricular (LV) systolic dysfunction caused by a high burden of atrial or ventricular arrhythmias. It most frequently occurs in patients with atrial fibrillation or premature ventricular complexes (PVCs).1, 2, 3 Impairment of LV systolic function secondary to frequent premature atrial complexes (PACs) has been rarely described.
We present the case of a young man with frequent PACs and decrease in LV ejection fraction (LVEF). Catheter ablation successfully eliminated the PACs and LVEF subsequently normalized. Three similar cases reported in the literature are reviewed. In conjunction, these cases support the existence of reversible PAC-induced cardiomyopathy.
Case report
A 44-year-old man presented with several months of nearly constant palpitations and “skipped beats.” His past medical history was notable for hypertension and obesity, treated with gastric bypass surgery 9 years prior. His family history was significant for a father and brother with nonischemic cardiomyopathy. He denied alcohol and illicit drug use. One year prior to presentation, 12-lead electrocardiogram documented normal sinus rhythm. Echocardiogram demonstrated LVEF 65%, mild concentric LV hypertrophy, diastolic dysfunction, and mild biatrial dilation.
Electrocardiogram performed to assess his current symptoms of palpitations was notable for sinus rhythm with frequent PACs (Figure 1). Holter monitoring documented 19% burden of unifocal PACs. Echocardiogram was now notable for LVEF 40%, without LV dilation or regional wall motion abnormalities. Coronary computed tomography angiogram showed normal coronary arteries. Given symptoms as well as concern for PAC-induced cardiomyopathy, catheter ablation was performed.
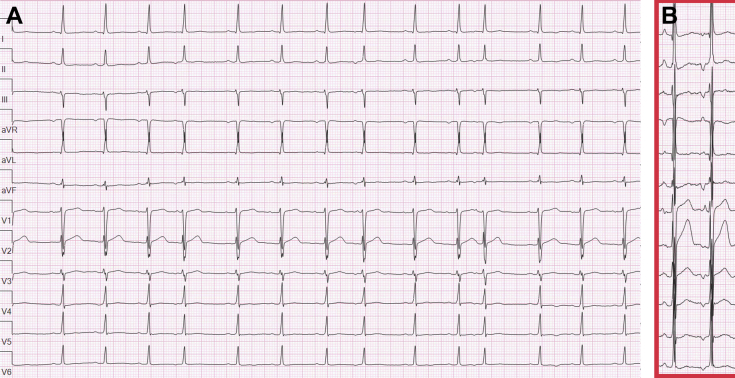
Figure 1.
Burden and morphology of premature atrial complexes (PAC). A: Twelve-lead electrocardiogram (ECG) shows sinus rhythm with frequent unifocal PACs. B: Twelve-lead ECG with increased gain. In contrast to the sinus P wave (first), the PAC (second) has an earlier precordial transition and is superiorly directed, consistent with a site of origin along the inferior tricuspid annulus.
The patient presented to the electrophysiology lab in sinus rhythm with frequent unifocal PACs. Activation mapping of the right and left atria was performed (Carto; Biosense Webster, Diamond Bar, CA) with earliest activation along the inferoseptal tricuspid annulus (Figure 2). At this site, the local electrogram was fractionated and preceded the P-wave onset by 33 ms. Ablation here eliminated the PAC. One month later, repeat Holter monitoring documented only 2 PACs during 24 hours. Echocardiogram performed 2 months after ablation was notable for LVEF 56%.
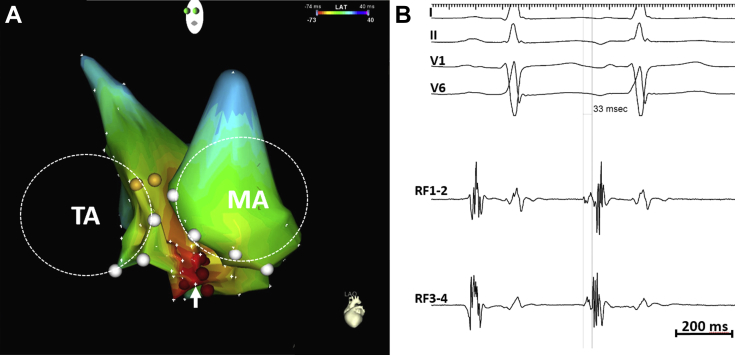
Figure 2.
Premature atrial complex ablation. A: Activation mapping of the right atrium (RA) and left atrium (LA) is notable for earliest site of activation along the inferoseptal tricuspid annulus, with radial spread. The RA and LA are projected in a left anterior oblique view. The dashed circles indicate the location of the tricuspid annulus (TA) and mitral annulus (MA). B: The bipolar electrogram at the site of earliest activation is fractionated and precedes the onset of the P wave by 33 ms.
Discussion
We present the case of a patient whose LVEF decreased from 65% to 40% in the setting of frequent PACs. Following PAC elimination, without any change in medications, his LVEF increased to 56%. This is among a small number of similar cases reported in the literature.
Literature review
Arrhythmia-induced cardiomyopathies are well known to occur in patients with atrial fibrillation,1,4 atrial flutter,5 other supraventricular tachycardias,6 PVCs2,3 and incessant ventricular tachycardia.7 Cardiomyopathy induced by frequent PACs is, however, less common. Hasdemir and colleagues8 described a patient with frequent PACs (20.9% burden) and LVEF 48%. Ten months following successful ablation of the PAC from the junction of the superior vena cava and right atrium, his LVEF normalized. A similar case was reported by Vervueren and colleagues.9 This patient presented with 35%–40% burden of PACs and LVEF 28%. Mapping demonstrated several PAC sites of origin within the pulmonary veins. Following pulmonary vein isolation, PACs were eliminated and LVEF improved to 50% within months, and to 65% 2 years following ablation. Recently, Mazzella and colleagues described a patient with 19.9% PACs and LVEF 40%.10 Three months after successful ablation of the PAC from the arcuate ridge, the LVEF improved to 50%. These cases, in conjunction with ours, support the existence of PAC-induced cardiomyopathy, which can be reversed with elimination of PACs.
Mechanism of PAC-induced cardiomyopathy
Arrhythmia-induced cardiomyopathies can be caused by a number of mechanisms, including tachycardia, irregularity, and dyssynchrony.4 As opposed to PVC-induced cardiomyopathy, where both irregularity and dyssynchrony occur, the predominant mechanism underlying PAC-induced cardiomyopathy is irregularity. This is analogous to atrial fibrillation with controlled ventricular rate, which can also cause reversible cardiomyopathy.11 Current knowledge of the pathophysiologic changes that lead to arrhythmia-induced cardiomyopathy derives largely from animal models of heart failure induced by prolonged periods of rapid right ventricular pacing. A constellation of hemodynamic, structural, electrical, and neurohormonal changes have been observed in these experiments,12 as well as others mimicking frequent PVCs with bigeminy right ventricular pacing.13 In a dog model of atrial pacing simulating atrial bigeminy, the LVEF decreased from 69% at baseline to 32% after 4 weeks.14 In a pig model of frequent PACs, LVEF also decreased, though to a lesser extent than with frequent PVCs.13 Interestingly, despite conducting with narrow QRS complexes, these PACs induced a mild degree of ventricular dyssynchrony as measured by pressure-volume loop catheters.
Gunda and colleagues15 studied 846 patients with PAC burden quantified by 14-day Holter monitors. While there were slightly more patients with LVEF <50% in the group with highest PAC burden (>5%), the association was far weaker than for PVCs. It should be noted, however, that even in the group with PACs >5%, the majority of patients had a burden <12% (median 8.5% with interquartile range 6.1–12.1). Similar to PVCs, it is probable that for PAC-induced cardiomyopathy to occur, PACs must be of sufficient burden. In our case, as well as the 3 previously reported cases, the PAC burden was ≥19%. In addition to a certain PAC threshold, it is also possible that an underlying susceptibility or predisposition must be present for PAC-induced cardiomyopathy to develop.
Conclusion
We report a case of PAC-induced cardiomyopathy. While it is less common than other arrhythmia-induced cardiomyopathies, providers should be aware that PAC-induced cardiomyopathy exists and can be reversed with elimination of PACs.
Key Teaching Points.
-
•
Arrhythmia-induced cardiomyopathies are well known to occur in patients with atrial fibrillation, atrial flutter, other supraventricular tachycardias, premature ventricular complexes, and incessant ventricular tachycardia.
-
•
We present a case of premature atrial complex–induced cardiomyopathy, now the fourth such report.
-
•
Elimination of premature atrial complexes can normalize left ventricular systolic function in patients with premature atrial complex–induced cardiomyopathy.
Footnotes
This work was supported by the Pennsylvania Steel Company EP Research Fund.
References
- 1.Gentlesk P.J., Sauer W.H., Gerstenfeld E.P. Reversal of left ventricular dysfunction following ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:9–14. doi: 10.1111/j.1540-8167.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 2.Hyman M.C., Mustin D., Supple G. Class IC antiarrhythmic drugs for suspected premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2018;15:159–163. doi: 10.1016/j.hrthm.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Mountantonakis S.E., Frankel D.S., Gerstenfeld E.P. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–1614. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Gopinathannair R., Etheridge S.P., Marchlinski F.E., Spinale F.G., Lakkireddy D., Olshansky B. Arrhythmia-induced cardiomyopathies: mechanisms, recognition, and management. J Am Coll Cardiol. 2015;66:1714–1728. doi: 10.1016/j.jacc.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luchsinger J.A., Steinberg J.S. Resolution of cardiomyopathy after ablation of atrial flutter. J Am Coll Cardiol. 1998;32:205–210. doi: 10.1016/s0735-1097(98)00183-1. [DOI] [PubMed] [Google Scholar]
- 6.Packer D.L., Bardy G.H., Worley S.J. Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol. 1986;57:563–570. doi: 10.1016/0002-9149(86)90836-2. [DOI] [PubMed] [Google Scholar]
- 7.Sosa E., Scanavacca M. Incessant ventricular tachycardia due to spontaneous automaticity in the Purkinje network inducing reversible left ventricular dysfunction. Europace. 2011;13:292–294. doi: 10.1093/europace/euq305. [DOI] [PubMed] [Google Scholar]
- 8.Hasdemir C., Simsek E., Yuksel A. Premature atrial contraction-induced cardiomyopathy. Europace. 2013;15:1790. doi: 10.1093/europace/eut141. [DOI] [PubMed] [Google Scholar]
- 9.Vervueren P.L., Delmas C., Berry M. Reversal of dilated cardiomyopathy after successful radio-frequency ablation of frequent atrial premature beats, a new cause for arrhythmia-induced cardiomyopathy. J Atr Fibrillation. 2012;5:627. doi: 10.4022/jafib.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzella A.J., Kouri A., O'Quinn M.P., Royal S.H., Syed F.F. Improvement in left ventricular ejection fraction after radiofrequency catheter ablation of premature atrial contractions in a 23-year-old man. HeartRhythm Case Rep. 2019;5:524–527. doi: 10.1016/j.hrcr.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu L.F., Jais P., Sanders P. Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med. 2004;351:2373–2383. doi: 10.1056/NEJMoa041018. [DOI] [PubMed] [Google Scholar]
- 12.Shinbane J.S., Wood M.A., Jensen D.N., Ellenbogen K.A., Fitzpatrick A.P., Scheinman M.M. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 13.Walters T.E., Rahmutula D., Szilagyi J. Left ventricular dyssynchrony predicts the cardiomyopathy associated with premature ventricular contractions. J Am Coll Cardiol. 2018;72:2870–2882. doi: 10.1016/j.jacc.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 14.Pacchia C.F., Akoum N.W., Wasmund S., Hamdan M.H. Atrial bigeminy results in decreased left ventricular function: an insight into the mechanism of PVC-induced cardiomyopathy. Pacing Clin Electrophysiol. 2012;35:1232–1235. doi: 10.1111/j.1540-8159.2012.03466.x. [DOI] [PubMed] [Google Scholar]
- 15.Gunda S., Akyeampong D., Gomez-Arroyo J. Consequences of chronic frequent premature atrial contractions: association with cardiac arrhythmias and cardiac structural changes. J Cardiovasc Electrophysiol. 2019;30:1952–1959. doi: 10.1111/jce.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]