Introduction
Postoperative arrhythmias commonly occur after pediatric cardiac surgery and can result in significant hemodynamic deterioration.1 The combination of electrolyte imbalance, increased catecholamines, and postoperative myocardial injury and ischemia create a substrate for an array of arrhythmias. While patients with reentry tachycardias can usually be managed by vagal maneuvers, adenosine administration, rapid atrial pacing, or electrical cardioversion, patients with automatic arrhythmias are often managed by decreasing intrinsic catecholamines, antiarrhythmic therapy, and correction of electrolyte imbalances.1,2 In infants with congenital heart disease or ventricular dysfunction, the ability to tolerate these arrhythmias is often limited. As early as 1976, Waldo and colleagues3 described a technique utilizing “a pair of temporarily implanted atrial epicardial wire electrodes to pace the heart” to suppress the “supraventricular tachycardia and maintain the ventricular response rate in a therapeutically desirable range.”3 There is little data in the pediatric population on how one can leverage pacing techniques to manage automatic arrhythmias. We describe 2 cases of rapid atrial pacing utilizing the properties of the atrioventricular (AV) node to control the ventricular rate, thereby providing time for antiarrhythmic therapy to be fully realized.
Case report
Case 1
Case 1 involved a 5-month-old boy with hypoplastic left heart syndrome who following stage 1 Sano had long VA tachycardia managed on propranolol. He had had no clinical arrhythmias on therapy prior to the second stage of his palliation. He subsequently underwent a bidirectional Glenn with resultant trivial tricuspid regurgitation and mildly depressed right ventricular function. He returned to the cardiac intensive care unit postoperatively on esmolol and nitroprusside. On postoperative day (POD) 1, he developed frequent atrial ectopy and bursts of rapid narrow complex tachycardia at rates of 280 beats per minute (bpm) with variable VA intervals that could not be terminated with traditional rapid atrial pacing. His arrhythmia mechanism seemed consistent with an automatic focus. In addition to esmolol, he was loaded with 15 mg/kg procainamide followed by an infusion titrated to 40 mcg/kg/min, without apparent effect. During periods of this narrow complex tachycardia, his hemodynamics were significantly compromised, with mean arterial pressures between 15 and 20 by arterial line tracing. Given that his tachycardia was worsening, the decision was made to convert him to amiodarone. While being loaded with 5 mg/kg of amiodarone, he persistently had salvos of tachycardia with almost no periods of sinus rhythm. As the surgical team was called to discuss cardiopulmonary support, the decision was made to attempt to perform ultra-rapid atrial pacing to induce rate-dependent AV block and thereby control the ventricular rate and hopefully restore effective cardiac output in this fragile postoperative single-ventricle physiology. An external defibrillator was present in the event atrial fibrillation was induced. Using a Medtronic 8392 Dual Chamber (Medtronic, Minneapolis, MN) temporary external pacemaker and performing nearly continuous manual depression of the high-rate atrial pacing button, it was noted that pacing at 400 bpm was able to suppress the ectopic atrial tachycardia (EAT) but resulted in an irregular ventricular rate of 200 bpm on average, with poor hemodynamics. Pacing was increased steadily to 700 bpm (85 ms) with a resultant ventricular rate of 178 bpm and improvement in blood pressure (Figure 1). While an additional bolus of amiodarone was being given, the atrial pacing rate was decreased slowly to 600 bpm (100 ms) with resultant 4:1 AV conduction and a ventricular rate of 150 bpm with stable hemodynamics. Pacing at 600 bpm was continued for 2 hours by electrophysiology (EP) staff at the bedside. For the ensuing 8 hours, this rate was decreased to 300 bpm using a Medtronic 5328 Programmable Stimulator (Medtronic, Minneapolis, MN), which can continuously atrial pace at these rates. By POD 2, on an amiodarone infusion, he had no recurrence of his narrow complex tachycardia and was transitioned to oral amiodarone by POD 8. He was discharged to home 2 weeks later without complications. Follow-up 1 year later noted no recurrence of his supraventricular tachycardia (SVT) (by clinical status and screening Holters) while on amiodarone, with plans to continue on amiodarone until his Fontan operation.
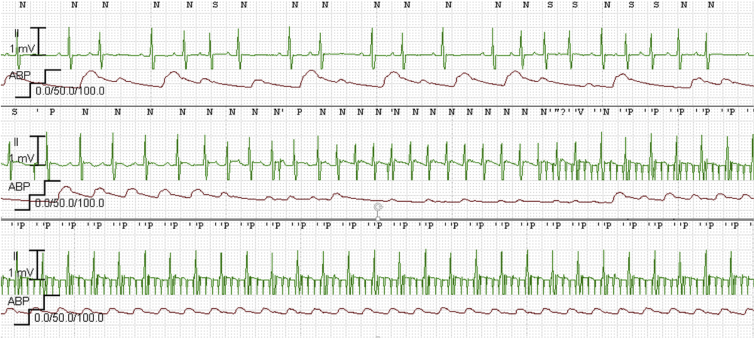
Figure 1.
Telemetry demonstrating sinus rhythm with blocked and conducted atrial premature beats followed by a burst of narrow complex tachycardia at ∼270 beats per minute (bpm), with flattening of arterial line. The initiation of rapid atrial pacing at 700 bpm (85 ms) and resultant ventricular rate of 178 bpm resulted in an improvement in mean arterial pressures from 20s to 40s.
Case 2
Case 2 involves a 6-month-old boy with long segment left coronary artery ostial atresia with associated severe left ventricular (LV) dysfunction and mitral regurgitation (MR) who underwent left main coronary artery ostial resection with pulmonary homograft patch plasty and mitral valve repair with residual severe MR and moderate LV dysfunction. On POD 4, he developed atrial tachycardia (AT). His AT demonstrated an unusual pattern of automaticity with variable gears and morphology most consistent with a multifocal process, with associated systolic blood pressures in the 40s and with salvos occurring in grouped patterns. The diagnosis was confirmed by atrial wire tracing in tachycardia. He was initially started on esmolol 50 mcg/kg/min given his ventricular dysfunction, with minimal improvement. He was subsequently loaded with procainamide at 10 mg/kg followed by an infusion at 30 mcg/kg/min. He continued to have salvos of AT on esmolol and procainamide. Esmolol was discontinued and amiodarone started with a load of 10 mg/kg, followed by an infusion of 10 mg/kg/day. Despite this therapy, he continued to have AT, with multiple gears including at rates of 150 bpm, a predominant rate of 200 bpm, which conducted 1:1, and occasionally 220 bpm, which conducted mostly 2:1. By POD 10, his resting electrocardiogram had a corrected QT interval of 490 ms and still with recurrent AT, his procainamide was discontinued and esmolol restarted in addition to amiodarone. Overnight on POD 10, his AT became hemodynamically unstable, with evidence of poor perfusion despite increases in his esmolol and amiodarone. He was paralyzed, cooled, and sedated. Using a Medtronic 8392 Dual Chamber temporary external pacemaker, rapid atrial pacing was performed to 800 bpm, with resultant 6:1 conduction and a ventricular rate of 134 bpm, suppressing his AT and stabilizing his hemodynamics while amiodarone was increased (Figures 2 and 3) over about 2–3 hours. This required EP staff to remain at the bedside to manually depress the rapid atrial pacing button, taking intermittent breaks to check the underlying rhythm. Once the patient was stabilized, esmolol was discontinued and a Medtronic 5328 Programmable Stimulator was used to steady rate atrial pace at 200 ms. This resulted in 2:1 AV conduction and a ventricular rate of 150 bpm with stable hemodynamics for approximately 6 hours. Over the next 24 hours, the pacing rate was steadily decreased, and on an amiodarone infusion at 20 mg/kg/day he remained in normal sinus rhythm off temporary pacing. On POD 12, oral flecainide was added to his amiodarone and only short residual nonsustained bursts of AT with slower rates (150–180 bpm) occurred. Unfortunately, given persistent MR, he returned to the operating room for mitral valve repair, left atrial cryomaze, and oversewing of left atrial appendage, following which he had no significant atrial arrhythmias. His amiodarone was discontinued 3 days after his repeat surgery. He was discharged home 37 days after his initial surgery on no antiarrhythmics and without complications.
Figure 2.
Telemetry demonstrating narrow complex, irregular tachycardia with variable P-wave morphologies followed by initiation of rapid atrial pacing at 800 beats per minute (bpm) (75 ms) with 6:1 conduction and a resultant ventricular rate of around 133 bpm (450 ms).
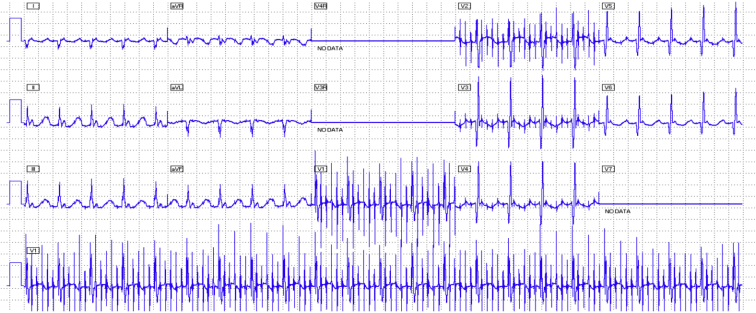
Figure 3.
Twelve-lead electrocardiogram during rapid atrial pacing at 800 beats per minute (75 ms) with resultant 6:1 conduction, resulting in stabilization of the ventricular rate and hemodynamics.
Discussion
In this case series we present 2 cases of hemodynamically unstable AT, both occurring in infants with congenital heart disease following surgical repair. Both patients were ultimately converted to sinus rhythm with intravenous amiodarone therapy, but their hemodynamics were stabilized during the course of medical therapy using ultra-rapid atrial pacing and the decremental properties of the AV node to allow for ventricular rate control.
The use of rapid atrial pacing to terminate reentrant SVT through the use of esophageal pacing and temporary wires has been well described.4, 5, 6, 7, 8, 9 These manuscripts also highlighted the possibility of inducing atrial fibrillation—although this often led to subsequent termination of the tachycardia and conversion to sinus rhythm, it also stresses the importance of having an external defibrillator present in case rapid ventricular conduction occurs with the more chaotic atrial fibrillation. Although in infants and children the risk of inducing atrial fibrillation is lower than in the adult population, caution and preemptive measures (external defibrillator and pads) during ultra-rapid pacing were employed in both these cases. Interestingly, aggressive atrial pacing, to the point of purposely causing atrial fibrillation, was documented in a study by Waldo and colleagues3 in 1976. Much like the 2 patients in our case series, ultra-rapid atrial pacing was used not to terminate the AT, but to provide improved rate control. Waldo and colleagues studied 2 groups: in 1 group comprising patients with postoperative atrial arrhythmias (paroxysmal AT, ectopic atrial tachycardia, and frequent premature atrial contractions), these patients were managed acutely with continuous atrial pacing at rates sufficient to achieve 2:1 AV block for short periods of time in the postoperative period.3
The use of rapid atrial pacing has been extended to those with permanent pacemakers. The risks and benefits of this therapy were well described in the study by Rhodes and colleagues10 in 1995. This study showed the benefit of antitachycardia pacing on patients with recurrent intra-atrial reentrant tachycardia, but also warned of the potential to worsen the clinical scenario by pacing a patient into a more rapidly conducted AT. As such, when performing rapid pacing for automatic tachycardias, the operator must ensure their pacing rate provides a slower ventricular rate response than the underlying clinical arrhythmia.
The technology to deliver this therapy is limited. Temporary bedside pacers (Medtronic 5392 and 53041; Medtronic, Minneapolis, MN) have maximum standard pacing rates of 200 bpm. For infants, atrial refractoriness can be <180 ms and 1:1 AV conduction can be seen at 300 bpm. Bedside pacemakers allow ultra-rapid pacing using manual pacing modes in which a button must be held down constantly by the operator. This, rightly so, keeps the operator at the bedside monitoring this intervention closely, while also monitoring the effect of antiarrhythmics being administered concurrently. Once the AV node has slowed, less rapid pacing can be performed in a sustained fashion using a portable EP stimulator (Medtronic model 5328), which can be programmed down to cycle lengths of 200 ms.
Pharmacological therapy will almost always be needed when ultra-rapid atrial pacing is performed. In our case series, ultra-rapid atrial pacing was a temporizing measure, providing hemodynamic stability while antiarrhythmics were slowly loaded into our patients. The pharmacological strategy for AV node control in these cases was beta blockers; neither digoxin nor calcium channel blockers were used. Although amiodarone was the ultimate pharmacological therapy of choice—with some effect on AV nodal conduction—this was primarily used for the strategy of rhythm rather than rate control. The concomitant use of medications, whether for rate control or rhythm control, has been described,11 making atrial pacing more effective in converting a patient back to sinus rhythm. An armamentarium of pacing strategies and antiarrhythmics is often needed in postoperative pediatric and congenital patients. Our case series provides an encouraging example of using ultra-rapid atrial pacing on a case-by-case basis as a bridge to achieve hemodynamic stability and improved cardiac output in infants with automatic AT.
Conclusions
Traditional rapid atrial pacing is an effective tool in treating reentrant SVTs. Combined with antiarrhythmic therapy, sinus rhythm can be restored in the majority of pediatric and congenital patients in the postoperative setting. Sustained ultra-rapid atrial pacing for automatic tachycardias has not been as well described but, as shown in our case series, has a role to play in select cases. Caution must be used in this pacing strategy so as to not worsen the clinical situation. Slowly increasing the atrial pacing rate above the underlying AT can help restore hemodynamic stability while allowing more cautious loading of potent antiarrhythmic therapy. The technology to deliver sustained pacing rates >300 bpm as therapy requires additional expertise to safely operate.
Key Teaching Points.
-
•
In 2 infants with congenital heart disease with hemodynamically unstable postoperative automatic atrial tachycardias, ultra-rapid atrial pacing was successfully utilized to control and slow the ventricular rate for short periods of time.
-
•
Acute pharmacological therapy should be utilized in conjunction with this pacing therapy.
-
•
This combination of therapies may help avoid the need for extracorporeal membrane oxygenation support in those hemodynamically unstable patients.
-
•
Caution should be employed when utilizing this pacing strategy so as not to worsen the clinical situation by causing more rapid conduction.
Footnotes
Dr Chandler is currently at the Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, Illinois.
Dr Whitehill is currently at Children’s Healthcare of Atlanta, Atlanta, Georgia.
M.E.A. contributes to Uptodate and receives some financial remuneration for those contributions. S.F.C., R.D.W., E.S.D., F.F.T., and D.Y.M. have no conflicts to disclose.
References
- 1.Bar-Cohen Y., Silka M.J. Management of postoperative arrhythmias in pediatric patients. Curr Treat Options Cardiovasc Med. 2012;14:443–454. doi: 10.1007/s11936-012-0195-4. [DOI] [PubMed] [Google Scholar]
- 2.Wang J.N., Wu J.M., Tsai Y.C. Ectopic atrial tachycardia in children. J Formos Med Assoc. 2000;99:766–770. [PubMed] [Google Scholar]
- 3.Waldo A.L., MacLean W.A., Karp R.B. Continuous rapid atrial pacing to control recurrent or sustained supraventricular tachycardias following open heart surgery. Circulation. 1976;54:245–250. doi: 10.1161/01.cir.54.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Gallagher J.J., Smith W.M., Kerr C.R. Esophageal pacing: a diagnostic and therapeutic tool. Circulation. 1982;65:336–341. doi: 10.1161/01.cir.65.2.336. [DOI] [PubMed] [Google Scholar]
- 5.Volkmann H., Dannberg G., Heinke M. Termination of tachycardias by transesophageal electrical pacing. Pacing Clin Electrophysiol. 1992;15:1962–1966. doi: 10.1111/j.1540-8159.1992.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 6.Lister J.W., Cohen L.S., Bernstein W.H. Treatment of supraventricular tachycardias by rapid atrial stimulation. Circulation. 1968;38:1044–1059. doi: 10.1161/01.cir.38.6.1044. [DOI] [PubMed] [Google Scholar]
- 7.Langendorf R., Pick A. Ventricular response in atrial fibrillation. Role of concealed conduction in the AV junction. Circulation. 1965;32:69–75. doi: 10.1161/01.cir.32.1.69. [DOI] [PubMed] [Google Scholar]
- 8.Haft J.I., Kosowsky B.D., Lau S.H. Termination of atrial flutter by rapid electrical pacing of the atrium. Am J Cardiol. 1967;20:239–244. doi: 10.1016/0002-9149(67)90084-7. [DOI] [PubMed] [Google Scholar]
- 9.Barold S.S. Newer concepts in electrical pacing of the heart. Med J Aust. 1969;2:103–106. doi: 10.5694/j.1326-5377.1969.tb105633.x. [DOI] [PubMed] [Google Scholar]
- 10.Rhodes L.A., Walsh E.P., Gamble W.J. Benefits and potential risks of atrial antitachycardia pacing after repair of congenital heart disease. Pacing Clin Electrophysiol. 1995;18:1005–1016. doi: 10.1111/j.1540-8159.1995.tb04741.x. [DOI] [PubMed] [Google Scholar]
- 11.Heldal M., Orning O.M. Effects of flecainide on termination of atrial flutter by rapid atrial pacing. Eur Heart J. 1993;14:421–424. doi: 10.1093/eurheartj/14.3.421. [DOI] [PubMed] [Google Scholar]