Introduction
Approximately 20% of all ischemic strokes are cryptogenic—they have an uncertain cause despite thorough diagnostic evaluation.1 Many such patients undergo long-term cardiac monitoring for detection of atrial fibrillation (AF), but in the absence of clearly documented AF, there is controversy regarding the best approach to secondary prevention.2 While anticoagulation may seem reasonable for some patients, empiric anticoagulation has not been shown to prevent recurrent events and may not outweigh the risk of bleeding in patients with embolic stroke of unknown source (ie, cryptogenic strokes with embolic pattern on brain imaging but no documented embolic source).3,4
We recently reported on the application of artificial intelligence to the electrocardiogram (artificial intelligence–enabled electrocardiogram; AI-ECG) to identify patients who may have a particularly high likelihood of concomitant AF or atrial flutter, even though their presenting rhythm was sinus.5 We present a case of a patient with recurrent cryptogenic stroke in whom repeat ECGs and cardiac monitoring recorded sinus rhythm, but retrospective AI-ECG analysis demonstrated forewarning of AF risk 12 years prior to the first thromboembolic event.
Case report
A 92-year-old woman with hypertension, diabetes mellitus, and peripheral arterial disease presented with a left frontal stroke. Her workup demonstrated bilateral carotid atherosclerosis (right worse than left), left atrial enlargement but normal left atrial appendage (LAA) on transesophageal echocardiography, and sinus rhythm without evidence of AF on ECG and outpatient Holter monitoring (Figure 1). In the absence of documented AF, she was maintained on antiplatelet therapy with aspirin for secondary prevention.
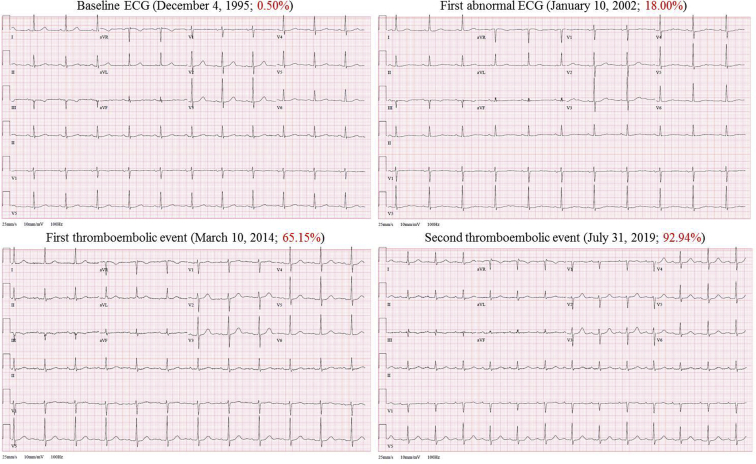
Figure 1.
Four of the patient’s standard 12-lead electrocardiograms (ECGs) recorded at different times: baseline ECG, first abnormal ECG identified by the artificial intelligence–enabled ECG (AI-ECG) algorithm, and ECGs performed at the time of the first and second thromboembolic events. Notice that all ECGs demonstrate sinus rhythm and are quite similar to each other and relatively unremarkable to the naked eye despite having very different predicted probabilities of concomitant atrial fibrillation (reported in red; abnormal is greater than 8.70%).
Five years later, the patient re-presented with a posterior circulation stroke and acute right leg ischemia. She underwent right femoral, iliac, superficial femoral, and profunda artery thrombectomy. An intraoperative transesophageal echocardiography revealed left atrial enlargement with a dilated LAA as well as an 11 × 5 mm left atrial thrombus near the os of the LAA. Again, ECG and cardiac monitoring consistently revealed sinus rhythm (Figure 1). The patient was initiated on therapeutic anticoagulation with warfarin. Thirty-day ambulatory cardiac monitoring continued to demonstrate only sinus rhythm.
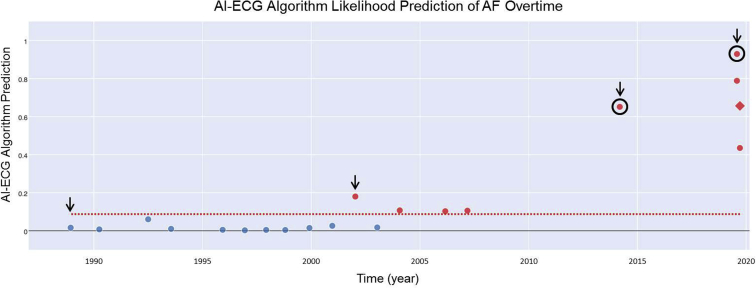
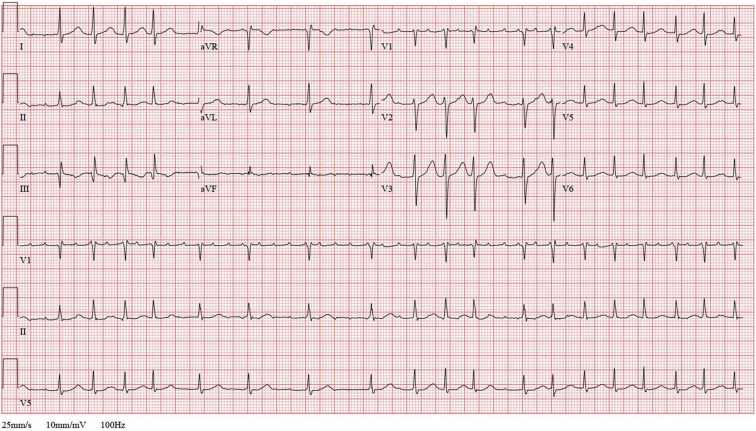
We then performed a retrospective AI-ECG analysis to assess the risk of concomitant (but undiagnosed) AF using all prior ECGs over the 19 years prior to her first thromboembolic event (Figure 2). The AI-ECG algorithm output demonstrated an increasing probability of AF over the observation period, with the greatest likelihood predictions of AF at the time of both thromboembolic events. Remarkably, within weeks of completing this initial analysis, the patient presented for follow-up and was found on ECG to have atrial flutter with variable atrioventricular block (Figure 3), thus confirming the AI-ECG-based suspicion that she was at increased risk of atrial arrhythmia.
Figure 2.
Retrospective artificial intelligence–enabled electrocardiogram (AI-ECG) analysis of the patient’s available ECGs in our electronic medical record over a 24-year period. Our current algorithm5 provides an alert to the provider for an abnormal ECG when a likelihood prediction value of greater than 8.70% (dotted red line) is projected. All abnormal ECGs are designated by a red marker in the figure. In this case, the first abnormal ECG would have been reported over 12 years prior to the patient’s first thromboembolic event. The first and second thromboembolic events are circled in black. The black arrows correspond to the ECGs depicted in Figure 1. The red diamond-shaped marker indicates when atrial flutter was recorded (Figure 3). AF = atrial fibrillation.
Figure 3.
Follow-up electrocardiogram demonstrating atrial flutter with variable atrioventricular block.
Discussion
As is often the case, thorough diagnostic workup failed to find an underlying cause for the patient’s recurrent strokes. Despite high CHA2DS2-VASc score (score = 8), the inability to identify AF or atrial flutter on standard 12-lead ECGs or cardiac rhythm monitoring precluded initial initiation of anticoagulation. It was not until years later when the patient presented with recurrent stroke and acute limb ischemia with evidence of left atrial thrombus on echocardiography that anticoagulation was commenced. Moreover, it was only after initial submission of this work that an atrial tachyarrhythmia was documented. In this case, the AI-ECG may have provided some additional information regarding the patient’s likelihood of undiagnosed AF or atrial flutter that, if further validated for this use, could have changed management and potentially prevented embolic events.
It is impossible to know which ECG features the deep neural network is utilizing in human terms to label a specific ECG owing to the absence of teleological meaning in intervening network layers. This is a subject of active research, as that understanding may provide additional insights into thromboembolic pathophysiology. The quality of the data input into the network might have an impact on the quality of the results; however, it is common to inject noise to provide networks with robustness in the setting of imperfect data, and with exposure to many ECGs, this factor is likely mitigated. Thus, while it is not certain what the AI-ECG algorithm is “seeing,” we have proposed that it may be detecting subclinical fibrosis, atrial myopathy, or repolarization changes that affect the surface ECG. Interestingly, by manual review, the ECGs demonstrate only minor abnormalities and are quite similar to each other despite having very different AI-ECG-based probabilities of concomitant AF (0.50% to 93%; Figure 1). The threshold of 8.7% was determined in previous work5 based on the point on the receiver operator characteristics curve that maximizes both sensitivity and specificity. The ideal cutoff point has been validated and tested in the original work and is now applied clinically.
Further study will be required to evaluate and validate the clinical utility of AI-ECG in patient care. These studies should include prospective validation of the algorithm for prediction of incident AF and stroke, determination of the optimal algorithm cut-points, and evaluation of a strategy of anticoagulation initiation guided by AI-ECG in a prospective clinical trial. If validated, the AI-ECG could even be considered as a strategy for identification of patients who may benefit from anticoagulation for primary stroke prevention as well as prevention of recurrent stroke.
Conclusions
This is the first case to demonstrate the potential utility of AI-ECG into clinical practice at the individual patient level. This case suggests that the AI-ECG may serve as a surrogate for AF and could influence management decisions in the setting of cryptogenic stroke.
Key Teaching Points.
-
•
Many patients with cryptogenic stroke are suspected to have underlying paroxysmal atrial fibrillation (AF). However, in the absence of proven AF, anticoagulation of these patients has not been shown to prevent recurrent ischemic strokes and may result in excess bleeding compared with aspirin.
-
•
The artificial intelligence–enabled electrocardiogram (AI-ECG) may identify patients with a particularly high likelihood of concomitant AF in the setting of sinus rhythm.
-
•
AI-ECG may serve as an AF/atrial myopathy risk marker and could influence management of patients with cryptogenic stroke. Further study will be required to evaluate and validate the clinical utility of AI-ECG in patient care.
References
- 1.Li L., Yiin G.S., Geraghty O.C. Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol. 2015;14:903–913. doi: 10.1016/S1474-4422(15)00132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saver J.L. Cryptogenic stroke. N Engl J Med. 2018;378:2191–2201. [Google Scholar]
- 3.Hart R.G., Sharma M., Mundl H. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2019;394:861–867. doi: 10.1056/NEJMc1809065. [DOI] [PubMed] [Google Scholar]
- 4.Diener H.C., Sacco R.L., Easton J.D. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906–1917. doi: 10.1056/NEJMoa1813959. [DOI] [PubMed] [Google Scholar]
- 5.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]