Introduction
Ivabradine is a unique antiarrhythmic drug that was designed to affect chronotropy with no effect on inotropy or lusitropy. It was first approved by the European Medicines Agency in 2005 for stable angina treatment, and then in 2009 the indication was expanded to include uncontrolled angina with heart rates ≥60 beats per minute (bpm) on optimal beta-blocker therapy.1 Based on the SHIFT – HF trial in 2011, it got accepted for use in patients with systolic heart failure with heart rate greater than 70 bpm on maximally tolerated beta-blocker therapy or who have contraindication(s) to beta-blockers.2 It got approved by the Food and Drug Administration (FDA) in 2015 for use in heart failure.1 The FDA did not recommend its use for angina in the United States because the use of ivabradine in coronary disease and angina did not improve outcomes based on the SIGNIFY trial.3 Ivabradine has been on the market for less than a decade in the United States and longer than that in Europe. As its use is expanded worldwide, cases of ivabradine toxidromes are likely to be common. Thus far there are only 3 reported cases of overdoses worldwide4, 5, 6 based on literature search. We report on a 19-year-old woman who was prescribed ivabradine for inappropriate sinus tachycardia and had acute ivabradine toxicity. This case report will add to our repertoire of knowledge on ivabradine toxicity and its pathophysiology in human subjects.
Case report
A 19-year-old woman on ivabradine 7.5 mg twice daily for inappropriate sinus tachycardia presented to the emergency department after ingesting extra doses of ivabradine. She impulsively took about 20–30 tabs of 7.5 mg all at once, after a recent squabble with her boyfriend. She denied any co-ingestants except alcohol. She had instant regret and called her friend for help. She attempted to induce emesis, without success. She arrived at the emergency department within 1 hour after ingesting the excessive dose of ivabradine. She denied any chest pain but had intermittent dizziness, nausea, and vomiting and had looked pale.
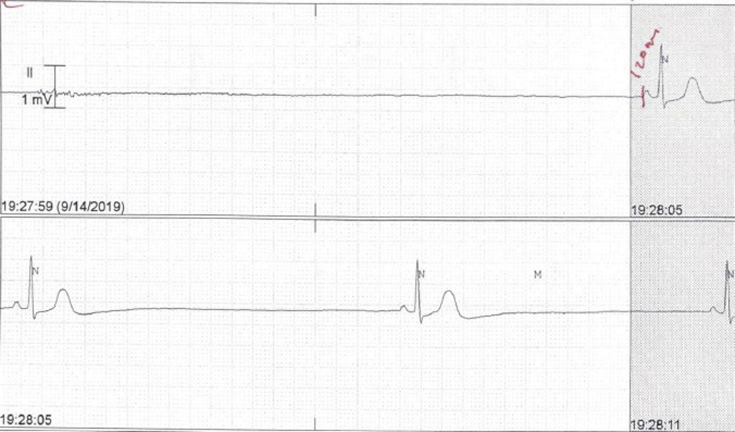
In the emergency department, she was found to be severely bradycardic with a heart rate of 20 bpm and multiple asystoles ranging from 6.6 to 11.72 seconds. She was in cardiogenic shock with blood pressure 75/51. Her presenting electrocardiogram showed marked sinus bradycardia with a heart rate of 35 bpm and QT/QTc of 470/358 ms. Her blood tests were unremarkable, with normal electrolytes and renal function. A urine drug screen was negative, and she had a blood ethanol level of 139.1 mg/dL. She received activated charcoal within 2 hours of ingestion. Two doses of 1 mg intravenous atropine were given, with no response (Figure 1).
Figure 1.
Severe sinus bradycardia and asystole showing no atropine.
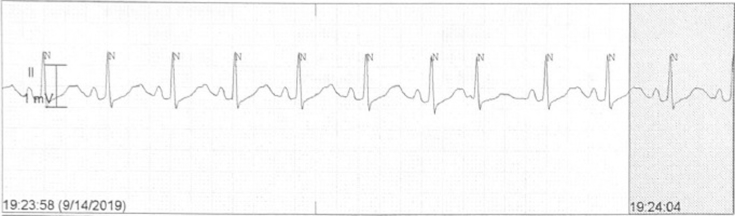
She was started on dopamine drip, titrated to a maximum dose of 15 mcg/kg/min, but could only achieve a maximum heart rate of 50 bpm. Isoproterenol 5 mcg/min was added and titrated to 10 mcg/min, and the heart rate improved to between 80 and 100 bpm. She continued to have prolonged episodes of sinus arrest. She also developed severe tremors from isoproterenol.
Figure 2 illustrates the rhythm strip, which shows improvement in the heart rate in response to dopamine and isoproterenol.
Figure 2.
Heart rate response to dopamine and isoproterenol.
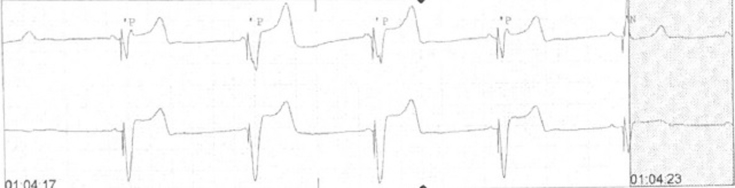
She was taken immediately to the cath lab for an externalized temporary pacemaker. A Medtronic 5076-52 device (Medtronic Inc, Minneapolis, MN) was inserted via the right internal jugular vein with ultrasound guidance. The lead was positioned in the right ventricle apical septum, and the pacemaker was set to VVI 60 bpm, with a capture threshold of 0.4 mA at 0.5 ms. Post threshold testing, she was noted to be entirely dependent on the device (Figure 3 shows pacemaker dependency), and she had no underlying escape rhythm or spontaneous electrical activity. Dopamine and isoproterenol were discontinued after the pacemaker was secured. Her tremors resolved with washout of isoproterenol. The next day, the pacemaker setting was changed to VVI 50 bpm to allow for the patient’s sinus rate to manifest. Thirty-six hours post-overdose, the temporary pacemaker was removed, and she had native sinus rhythm with a heart rate of 65 bpm consistently.
Figure 3.
Pacemaker dependency after weaning isoproterenol and dopamine.
The psychiatry team did a suicide assessment, and she was subsequently discharged home on sertraline.
Discussion
Ivabradine antagonizes the sinoatrial nodal tissue, thus inhibiting early depolarization of the cell membrane.4 It blocks the sodium (Na+) / potassium (K+) channel intracellularly, thereby inhibiting the mixed Na+/K+ currents called funny currents (If). This inhibition of cation movement during diastole decreases the gradient of depolarization, thus increasing the interval for the next depolarization current. Ivabradine is a class 0 antiarrhythmic drug that exhibits dose dependency and use dependency; ie, the degree of bradycardia increases as does dosage, and its effect is pronounced when tachycardic.1,7 The selective effect of ivabradine on If channels allows it to slow heart rate without affecting inotropy or total systemic vascular resistance.8
On average, heart rate reduction following ivabradine was 14 bpm after 1 hour; the PR and QRS intervals are unaffected.9 Electrophysiologic parameters of sinus node function after 0.2 mg/kg of slow bolus intravenous ivabradine resulted in a nonsignificant increase in the sinoatrial conduction time (SACT) by 20 ± 12 ms. In contrast, the sinus node recovery time (SNRT) increased significantly by 123 ± 51 ms after 1 hour.9 Thus, the resultant bradycardia is due to prolonged SNRT and SACT rather than a reduction in the sinus node automaticity.
Therapeutic dosing ranges from 2.5 to 7.5 mg twice daily. Peak serum concentration is achieved within 1 hour when taken on an empty stomach. Ivabradine is 70% protein bound and the volume of distribution is about 100 L. Ivabradine is metabolized by the cytochrome P450 enzyme CYP3A4 and undergoes first-pass metabolism; therefore, it is prone to multiple drug–drug interactions.1 Ivabradine has an active metabolite, which is an N-demethylated derivative. The effective half-life of ivabradine is approximately 6 hours. Four percent is excreted unchanged in urine and excretion of metabolites occurs via feces and urine.10, 11, 12
Its use for the treatment of inappropriate sinus tachycardia is off-label in the United States as the FDA’s only approved indication for ivabradine is in heart failure with reduced ejection fraction patients with NYHA II–IV on optimal medical therapy but unable to achieve heart rate ≤70 bpm despite maximal hemodynamically tolerable beta-blocker dose.
Our patient presented with profound symptoms of sinus bradycardia with runs of sinus arrest/asystole with a maximum duration of 11.72 s. The prolonged period of asystole could be due to the dose-dependent prolongation of SACT and SNRT. Atropine was ineffective, as she received 2 doses of 1 mg without any improvement in the bradycardia. Diminished response to atropine is a feature of ivabradine-induced bradycardia. This phenomenon was addressed in the European scientific discussions of ivabradine based on animal studies. Two out of the 3 case reports found atropine to be useful4,5 and were able to establish normal rate with repeated doses. Conversely, in our case and that of Gómez Casal and colleagues,6 the use of atropine was ineffective as a first-line agent. A possible explanation could be that our patient and that of Gómez Casal and colleagues exhibited profound toxicity vs Maskell and colleagues4 and Mathiaux and colleagues.5 The nadir of heart rate for our patient and that of Gómez Casal and colleagues were 15 bpm and 18 bpm, respectively, whereas in Maskell and colleagues and Mathiaux and colleagues, the nadir of heart rate was 31 and 60 bpm, respectively. We therefore hypothesize that the response to atropine may be influenced by the severity of bradycardia.
While our patient responded to a combination of dopamine and isoproterenol, Gómez Casal and colleagues6 reported that the use of isoproterenol alone was ineffective. The use of a temporary pacemaker in refractory ivabradine-induced bradycardia to chronotropic agents was reported first by Gómez Casal and colleagues.6 Our patient required temporary pacemaker placement owing to refractory episodes of asystole. We chose to insert an externalized pacemaker because of the advantages of increased stability, because it allows for early mobility, and because of the unknown duration of need. The sudden dependency on pacing was probably owing to the early withdrawal of dopamine and isoproterenol before sinus node recovery.
Table 1 summarizes the presentation and treatment strategies used in published case reports of ivabradine toxicity.
Table 1.
Varied presentations of patients who presented with ivabradine toxicity
| Case report | Age | Sex | Dose (mg) | HR nadir | Chronotropic agents | Temporary pacemaker | Co-ingestants | Gastric lavage/charcoal | Levels μg/dL | Time to recovery |
|---|---|---|---|---|---|---|---|---|---|---|
| Mathiaux et al 2014 | 47 | M | 280 | 50 | Atropine | No | Bromazepam | Yes | 375 | 48 |
| Gómez Casal et al 2015 | 27 | F | 235 | 18 | Isoproterenol | Yes | Spironolactone 625 mg | - | - | - |
| 6s pause | Losartan 350 mg | |||||||||
| Loperamide 20 mg | ||||||||||
| Maskell et al 2016 | 26 | F | 250 | 31 | Atropine | No | - | Yes | 525 | 36 |
| Osei et al 2020 | 19 | F | 225 | 15 | Dopamine and isoproterenol | Yes | Alcohol | Yes | - | 36 |
HR = heart rate.
Mathiaux and colleagues,5 in their conclusion, noted the absence of correlation between blood concentration and the severity of the effects of overdosage. Unfortunately, Gómez Casal and colleagues6 did not report serum levels of ivabradine. Since their patient had very severe symptoms from ivabradine compared to those in the Mathiaux and Maskell reports, it would have been interesting to know whether she achieved higher levels of serum ivabradine levels.
When managing suspected ivabradine toxicity, it is essential to elicit from the history of other potential other causes of drug-induced bradycardia like beta-blockers and calcium blocker toxicities.
Apart from bradycardia, other arrhythmogenic effects of ivabradine include atrial fibrillation and QT prolongation. A meta-analysis of 11 studies concluded that ivabradine use resulted in about a 15% increase in the risk of developing atrial fibrillation.13 The average QT interval was significantly prolonged by 29–38 ms at 30 minutes and 1 hour, respectively.9 The prolonged QT interval can be associated with torsades de pointes.14 This phenomenon is common when used together with other QT-prolonging drugs like macrolides.13,15
Conclusion
To the best of our knowledge, our case is the fourth case of reported ivabradine toxicity, and the second case reported in the United States. There seems to be a varied response to atropine and isoproterenol. Also, the severity of presentation varies based on the number of pills ingested or the serum levels of ivabradine. There is no recommended standardized treatment protocol for ivabradine toxicity.
Key Teaching Points.
-
•
Ivabradine is a pure negative chronotropic agent.
-
•
Ivabradine overdose results in severe sinus bradycardia with periods of asystole, which can be refractory to atropine.
-
•
Ivabradine significantly prolongs the sinus node recovery time and QT interval.
-
•
Dopamine and/or isoproterenol may be effective in the short term to improve heart rate.
-
•
A temporary pacemaker may be needed as a bridge to maintain the heart rate and thus allow elimination of ivabradine from the body.
References
- 1.Koruth J.S., Lala A., Pinney S., Reddy V.Y., Dukkipati S.R. The clinical use of ivabradine. J Am Coll Cardiol. 2017;70:1777–1784. doi: 10.1016/j.jacc.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Swedberg K., Komajda M., Böhm M. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 3.Fox K., Ford I., Steg P.G., Tardif J.-C., Tendera M., Ferrari R. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–1099. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 4.Maskell K., Tse A., Wolf C.E., Troendle M. Acute on chronic ivabradine overdose: a case report. J Med Toxicol. 2016;12:189–191. doi: 10.1007/s13181-016-0537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathiaux F., Dulaurent S., Julia F., Gaulier J.-M. Case report of Ivabradine intoxication. J Anal Toxicol. 2014;38:231–232. doi: 10.1093/jat/bku015. [DOI] [PubMed] [Google Scholar]
- 6.Gómez Casal V., Lage Cendon L., Lago Preciado G., Vara Adrio S. [Ivabradine poisoning with suicide intention] Med Intensiva. 2015;39:577–579. doi: 10.1016/j.medin.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Nawarskas J.J., Bowman B.N., Anderson J.R. Ivabradine: a unique and intriguing medication for treating cardiovascular disease. Cardiol Rev. 2015;23:201–211. doi: 10.1097/CRD.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 8.Vilaine J.-P., Bidouard J.-P., Lesage L., Reure H., Péglion J.-L. Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol. 2003;42:688. doi: 10.1097/00005344-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Camm A.J., Lau C.-P. Electrophysiological effects of a single intravenous administration of Ivabradine (S 16257) in adult patients with normal baseline electrophysiology. Drugs R D. 2003;4:83–89. doi: 10.2165/00126839-200304020-00001. [DOI] [PubMed] [Google Scholar]
- 10.Drug Monograph: Ivabradine (Corlanor) https://www.ebmconsult.com/articles/monograph-Ivabradine-corlanor [Internet]
- 11.Jiang J., Tian L., Huang Y., Li Y., Xu L. Pharmacokinetic and safety profile of Ivabradine in healthy Chinese men: a phase I, randomized, open-label, increasing single- and multiple-dose study. Clin Ther. 2013;35:1933–1945. doi: 10.1016/j.clinthera.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Rosa G.M., Ferrero S., Ghione P., Valbusa A., Brunelli C. An evaluation of the pharmacokinetics and pharmacodynamics of Ivabradine for the treatment of heart failure. Expert Opin Drug Metab Toxicol. 2014;10:279–291. doi: 10.1517/17425255.2014.876005. [DOI] [PubMed] [Google Scholar]
- 13.Lees-Miller J.P., Guo J., Wang Y., Perissinotti L.L., Noskov S.Y., Duff H.J. Ivabradine prolongs phase 3 of cardiac repolarization and blocks the hERG1 (KCNH2) current over a concentration-range overlapping with that required to block HCN4. J Mol Cell Cardiol. 2015;85:71–78. doi: 10.1016/j.yjmcc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Martin R.I.R., Pogoryelova O., Koref M.S., Bourke J.P., Teare M.D., Keavney B.D. Atrial fibrillation associated with Ivabradine treatment: meta-analysis of randomised controlled trials. Heart. 2014;100:1506–1510. doi: 10.1136/heartjnl-2014-305482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocco G., Jerie P. Torsades de pointes induced by the concomitant use of ivabradine and azithromycin: an unexpected dangerous interaction. Cardiovasc Toxicol. 2015;15:104–106. doi: 10.1007/s12012-014-9274-y. [DOI] [PubMed] [Google Scholar]