Test your knowledge!
Take an interactive quiz related to this article and view the A Case for Education quiz archive: https://www.heartrhythmcasereports.com/content/quiz_archive.
Introduction
Pulmonary vein isolation (PVI) forms the cornerstone of catheter ablation for atrial fibrillation (AF); however, the approach to PVI varies across centers. Over time, the technique of PVI has evolved from focal “trigger” ablation to ostial isolation and then diverged to wide circumferential isolation.1 Although the STAR AF II showed absence of incremental benefit of additional linear ablation and substrate modification over PVI in persistent AF, posterior wall isolation was not systematically studied.2 The posterior left atrium shares a common embryologic origin with the pulmonary veins, which are isolated en bloc during single ring isolation.3 A recent meta-analysis on single ring isolation technique showed a high acute procedural success rate of 92%–99%.4, 5, 6, 7 Here, we explore the physiological rationale of the single ring isolation technique and technical considerations for successful electrical isolation of the pulmonary vein and posterior wall to minimize risk of esophageal injury.
Rationale for single ring isolation to achieve pulmonary vein isolation
The left atrial wall between the pulmonary veins, referred to as left atrial dome, is derived embryologically from the solitary common pulmonary vein.3 During the development, the solitary common pulmonary vein is “cannibalized” to form the venous component consisting of the pulmonary veins and left atrial dome. The left atrial dome, although anatomically superior, has been termed as “posterior left atrial wall” in previous clinical studies, and the same terminology will be used for this manuscript. This posterior left atrium shares a unique electrophysiological characteristic and arrhythmogenic potential with the pulmonary veins owing to the common embryological origin.8,9 Single ring isolation is based on the physiological principle to electrically isolate the venous part of the left atrium. It not only results in debulking of the left atrium but also electrically isolates the posterior wall, with anticipated benefits by inclusion of proximal triggers and sites maintaining AF. Furthermore, this approach limits the amount of ablation in the posterior left atrium, thereby having a potential to reduce collateral damage to the esophagus and resultant atrial esophageal fistula. We utilize single ring isolation as the initial approach for patients with paroxysmal or persistent AF.
Anatomic consideration for single ring isolation
Anatomically, the left atrium consists of (1) the venous part receiving the pulmonary veins, (2) the smooth-walled vestibule conducting blood to the mitral valve, (3) the left atrial appendage, and (4) the atrial septum, which is shared with the right atrium.3 The left atrial dome represents the venous component between the 4 pulmonary veins.
The left atrial wall is composed of 1–3 overlapping layers of differently aligned myocardial fibers with varying wall thickness.10,11 The thickness of the left atrial wall varies, from 3.3 ± 1.2 mm anteriorly to 4.5 ± 0.6 mm on the roof to 3.9 ± 0.7 mm laterally.11 The part of the anterior wall immediately inferior to the Bachmann bundle and adjacent to the aorta can be very thin (1–2 mm) and is a common site for left atrial diverticulum. The posterior wall of the left atrium is a complex structure with thickness of 2.2 ± 0.3 mm at the pulmonary vein junction, which gradually decreases to 1.5 mm at 10 mm from the venoatrial junction. The left atrial wall is thick adjacent to the coronary sinus and measures 6.5 ± 2.5 mm with maximum thickness at the mitral isthmus ranging from 6 to 15 mm.
Anatomic dissections have shown that despite the individual variation, the left atrium has a distinctive pattern of arrangement of myocardial fiber (Figure 1). The vestibule of the left atrium consists of circumferential fibers parallel to the atrioventricular groove. The anterior wall has myocardial fibers arranged into a bundle oriented parallel to the atrioventricular groove. The Bachmann bundle inserts subepicardially into this bundle, which extends and bifurcates at the base of the left atrial appendage. The septopulmonary bundle of Papez arises from the anterosuperior septal raphe and fans to pass around the left and right pulmonary veins and pass longitudinally over the dome of the left atrium before bifurcating and merging into circumferential fibers of the vestibule on the left and posterior septal raphe on the right.10 However, in 10 of 26 specimens, the fibers on the dome ran laterally rather than longitudinally or obliquely. In these cases, the fibers around the inferior veins were less conspicuous.12 On the subendocardial aspect, most specimens demonstrate a septoatrial bundle originating from the anterior interatrial raphe, ascending the roof and left atrial dome prior to blending with the fibers of the septopulmonary bundle.10 The characteristic fiber pattern results in change in fiber orientation at the margin of the septopulmonary bundle and posterior-inferiorly where the septopulmonary bundle merges with the circumferential fibers of the vestibule. The fiber orientation of the left atrium has been shown to dictate preferential conduction in the posterior left atrial wall. Markides and colleagues8 have demonstrated that, irrespective of site of earliest activation of the left atrium in sinus rhythm, the left atrial activation pattern was determined by a line of conduction block. The line of block correlated anatomically with the margin of the septopulmonary bundle. The line of block, although present in sinus rhythm, was variable under paced conditions and therefore functional.8
Figure 1.
Myocardial fiber arrangement of roof and posterior wall of left atrium. Top image is a cranial view showing the roof and posterior wall (anatomical left atrial dome) of the left atrium with transillumination to demonstrate the thinner sections. The epicardium has been removed to show the longitudinal arrangement of the septopulmonary bundle (broken arrows posteriorly) and Bachmann bundle on the roof. In some specimens, septopulmonary fibers can have a lateral rather than the typical longitudinal or oblique arrangement (see text). Note the areas with discontinuity of fibers near left inferior pulmonary vein. Bottom image shows abrupt changes in orientation of the myocardial strands (broken arrows) in the posterior-inferior wall. Anatomically, this represents the transition between the venous left atrium and the vestibule and may be responsible for functional blocks. An interatrial muscle bundle (double-headed arrow) is present in this heart. IVC = inferior vena cava; LAA = left atrial appendage; LI = left inferior pulmonary vein; LS = left superior pulmonary vein; RI = right inferior pulmonary vein; RS = right superior pulmonary vein. Figure modified from Ho and colleagues.12
Technical aspects for single ring isolation
Lesion set
The single ring isolation technique is designed to electrically isolate the pulmonary veins and left atrial posterior wall en bloc, thereby isolating the embryologically derived venous left atrium. Single ring isolation differs from both box isolation and debulking ablation. In box isolation, the wide antral PVI is followed by roof and inferior lines connecting the superior and inferior aspects of the contralateral rings. In debulking ablation, the PVI is followed by sequential ablation of the earliest electrograms recorded along the posterior wall to achieve isolation of the posterior wall.
The technique and sequence of ablation for single ring isolation varies across centers. The approach in our institution for single ring isolation is to commence linear ablation anterior to the right pulmonary veins, followed by the roof and then the ridge between the left pulmonary veins and the left atrial appendage. The inferior line is completed last, as the double potentials are better appreciated once the remainder of the ring is completed. This minimizes energy delivery on the posterior wall and therefore should minimize the risk of collateral damage to the esophagus. Figure 2 shows single ring isolation in a patient with persistent AF. In this case, the patient reverted to sinus rhythm once the single ring was completed; however, the venous atrium within the ring continued in AF. Electrical isolation of pulmonary veins and posterior wall can be achieved en bloc in a small but significant proportion without completion of inferior line (Figure 3). We utilize steerable sheaths to ensure good contact and catheter stability to achieve transmural lesions.
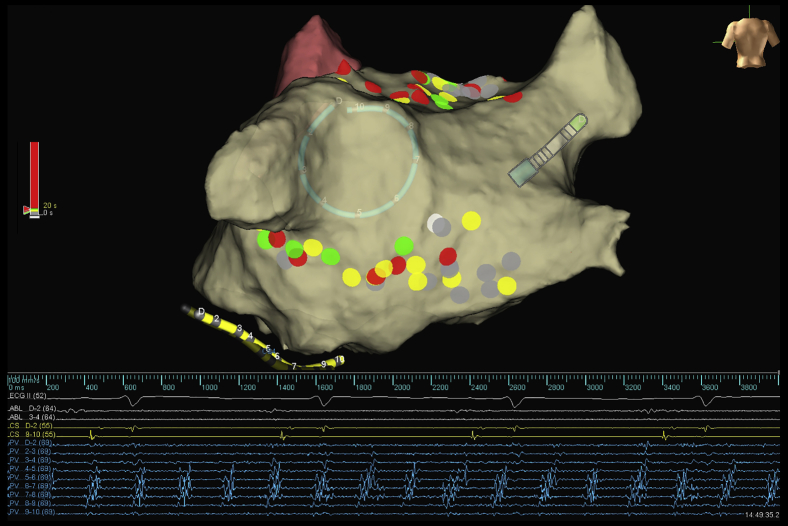
Figure 2.
Posterior view demonstrating single ring isolation in a patient with persistent atrial fibrillation (AF). Completion of ring resulted in sinus rhythm (coronary sinus electrogram and surface electrocardiogram) with ongoing AF within the ring. The lasso catheter is placed on the posterior wall adjacent to the left common pulmonary vein. The color of the lesions represents the ablation duration of the individual lesion (gray: 0–5 seconds; yellow: 6–10 seconds; green: 11–20 seconds; red: >20 seconds).
Figure 3.
“Esophageal-sparing” single ring isolation. Top images show the lesion set. The ablation was performed as follows: anterior to right pulmonary veins, roof, left pulmonary vein ridge, and then under the pulmonary veins. High-density activation map of posterior left atrium (bottom image) was then performed during coronary sinus pacing to confirm robustness of ablation anteriorly. The phenomenon of esophageal sparing will not be observed if there are gaps anteriorly. Note the rapid conduction along the posterior wall with slow conduction into the veins on either side. The slowing of conduction into pulmonary vein may represent the change in fiber orientation. Electrical isolation was achieved at the spot with the brown-colored lesion (bottom image) without completion of the inferior line. There was recovery during the wait period and additional burns were applied to achieve isolation (top images).
Roof line
There are anatomical considerations that determine successful single ring isolation without additional segmental lesions within the ring.
Left atrial diverticulum
The anterior left atrium, in particular adjacent to the right superior pulmonary vein, is a common site for left atrial diverticulum. The ablation line along the roof is undertaken posterior to an incidental left atrial diverticulum in this region. The left atrial diverticulum may have insertions of epicardial fibers from the right atrium, resulting in difficulty in achieving single ring isolation if the roof line is anterior to the diverticulum.
Bundle of Bachmann
A roof line that is too anterior may result in epicardial-to-endocardial activation through the Bachmann bundle into the ring, creating difficulties in achieving single ring isolation without segmental ablation within the ring. Furthermore, the sinus node artery (SNA) may arise from the left circumflex in one-third of patients and usually has a course along the anterior left atrium. An anterior roof line close to the left SNA may occasionally predispose to coronary injury and acute sinus node dysfunction. The left SNA may act as a heat sink when the roof line is anterior and may pose difficulty in achieving transmural lesions.13 Finally, a roof line transversely across the Bachmann bundle may predispose to interatrial conduction delay.14
Inferior line
The inferior ablation is performed last. As mentioned above, in a small but significant proportion of cases, the pulmonary veins and posterior wall are isolated en bloc with minimal ablation along the inferior line (Figure 3, top images). This phenomenon is recognized only if the ablation anterior to the veins and roof is robust and without any gaps. Hence, it is critical to confirm absence of gaps in the roof and anterior to pulmonary veins with a high-density activation map of the posterior wall prior to proceeding to the inferior line (Figure 3, bottom image). We propose the following reasons for “posterior wall–sparing” ring isolation: (1) abrupt change in fiber orientation, which may result in conduction blocks8,11; (2) muscle fiber discontinuity in this region11,15; (3) ablation at a distance: myocardial fibers conducting posteriorly are cut off by ablation inferior to pulmonary veins; and (4) left atrial scar in region of inferior line.
However, in some patients, additional segmental ablation in the posterior wall within the ring is required to achieve isolation of the pulmonary veins and posterior wall. The reasons for additional ablation within the ring are (1) inability to achieve transmural lesions owing to esophageal temperature rise posteriorly or instability anteriorly, (2) roof line anterior to Bachmann bundle or left atrial diverticulum with epicardial connection into the ring, and (3) less common epicardial connection from right atrium into the ring.
Avoiding esophageal injury
Left atrial to esophageal fistula is a rare but often fatal complication of catheter ablation for AF. The incidence varies from 0.01% to 0.2% with an estimated mortality of 40%–80%.16 Lower body mass index, rise in esophageal temperature beyond 38.5°C–39.5°C, Sensitherm temperature probe (St. Jude Medical, Inc., St. Paul, MN), gastroesophageal reflux, power >25 W on posterior left atrium, and general anesthesia are associated with esophageal injury after ablation. Reduced power on the posterior wall, luminal esophageal temperature monitoring, mechanical displacement of esophagus, and postprocedure proton pump inhibitors have been deployed as strategies to avoid esophageal injury. More recently, in vitro studies have shown that high-power, short-duration lesions may minimize collateral damage by forming wider lesions without increasing depth.17,18 The safety of this approach is supported by recent clinical studies.19
In our institution, luminal esophageal temperature monitoring is performed, and ablation is ceased if temperature rise >0.5°C is noted. The ablation is performed with 35–40 W for 5–10 seconds and contact force is limited to 5–10g to avoid tenting. The patients are routinely prescribed proton pump inhibitors for 4 weeks after ablation. The technique of single ring isolation, especially when the posterior wall is spared from ablation across the entire inferior line, may minimize delivery of radiofrequency energy adjacent to the esophagus and minimize risk of collateral injury.
Feasibility and long-term outcome of single ring isolation
Three studies, including 1 randomized study, have reported on the feasibility and outcomes of single ring isolation.5, 6, 7 A recent meta-analysis on posterior wall isolation reported an acute procedural success rate of 92%–99% for achieving posterior wall isolation with single ring isolation.4, 5, 6, 7 A rise in esophageal temperature during posterior wall ablation was reported to hinder electrical isolation. One study reported that single ring isolation could be achieved without additional ablation within the ring in 59% of patients.5 Lim and colleagues6 have compared single ring isolation with wide antral isolation in a randomized controlled trial and demonstrated reduced recurrence of AF and similar incidence of organized atrial arrhythmias after single ring isolation. They showed that AF-free survival at 2 years was better after single ring isolation (74% [95% CI, 65%–82%]) than wide antral isolation (61% [51%–70%]; P = .031). Survival free of organized atrial tachyarrhythmia was similar after both single ring and wide antral isolation (67% [57%–75%] vs 64% [54%–72%], respectively, at 2 years; P = .988).
Conclusion
The single ring isolation technique for PVI has a sound physiological principle for electrical isolation of the venous left atrium. This technique minimizes ablation adjacent to the esophagus and thereby may reduce risk of the rare but life-threatening complication of atrial esophageal fistula.
Footnotes
Financial Disclosures: Dr Mahajan is supported by the Health Professional Fellowship funded by the National Health and Medical Research Council of Australia and National Heart Foundation of Australia. Dr Thiyagarajah is supported by an Australian Postgraduate Award Scholarship from the University of Adelaide. Dr Lau is supported by the Robert J. Craig Lectureship from the University of Adelaide and by a fellowship from the Hospital Research Foundation. Dr Sanders is supported by the Practitioner Fellowship by the National Health and Medical Research Council of Australia. Dr Sanders is also supported by the National Heart Foundation of Australia.
Conflict of Interest Disclosures: Dr Mahajan reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott, Pfizer, and Bayer. Dr Mahajan reports having served on the advisory board of Abbott. Dr Mahajan reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott, and Bayer. Dr Lau reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Abbott, Biotronik, Bayer, Boehringer Ingelheim, and Pfizer. Dr Sanders reports having served on the advisory board of Medtronic, Abbott, Boston Scientific, CathRx, and Pacemate. Dr Sanders reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Medtronic, Abbott, and Boston Scientific. Dr Sanders reports that the University of Adelaide has received on his behalf research funding from Medtronic, Abbott, Boston Scientific, and Microport.
References
- 1.Mahajan R., Twomey D., Lau D.L., Sanders P. Atrial fibrillation ablation: Pulmonary vein isolation techniques, strategies and principles. In: Bhargava K., Asirvatham S.J., editors. Practical Cardiac Electrophysiology. 1st ed. New Delhi: Jaypee Brothers Medical Pub; 2016. pp. 375–386. [Google Scholar]
- 2.Verma A., Jiang C.Y., Betts T.R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 3.Anderson R.H., Brown N.A., Moorman A.F. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2–9. doi: 10.1002/dvdy.20578. [DOI] [PubMed] [Google Scholar]
- 4.Thiyagarajah A., Kadhim K., Lau D.H. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2019;12:e007005. doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 5.Thomas S.P., Lim T.W., McCall R., Seow S.C., Ross D.L. Electrical isolation of the posterior left atrial wall and pulmonary veins for atrial fibrillation: feasibility of and rationale for a single-ring approach. Heart Rhythm. 2007;4:722–730. doi: 10.1016/j.hrthm.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Lim T.W., Koay C.H., See V.A. Single-ring posterior left atrial (box) isolation results in a different mode of recurrence compared with wide antral pulmonary vein isolation on long-term follow-up: longer atrial fibrillation-free survival time but similar survival time free of any atrial arrhythmia. Circ Arrhythm Electrophysiol. 2012;5:968–977. doi: 10.1161/CIRCEP.111.970293. [DOI] [PubMed] [Google Scholar]
- 7.Kumagai K., Nakashima H. Noncontact mapping-guided catheter ablation of atrial fibrillation. Circ J. 2009;73:233–241. doi: 10.1253/circj.cj-08-0700. [DOI] [PubMed] [Google Scholar]
- 8.Markides V., Schilling R.J., Ho S.Y., Chow A.W., Davies D.W., Peters N.S. Characterization of left atrial activation in the intact human heart. Circulation. 2003;107:733–739. doi: 10.1161/01.cir.0000048140.31785.02. [DOI] [PubMed] [Google Scholar]
- 9.Mandapati R., Skanes A., Chen J., Berenfeld O., Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Papez J.W. Heart musculature of the atria. Am J Anat. 1920;27:255–285. [Google Scholar]
- 11.Ho S.Y., Sanchez-Quintana D., Cabrera J.A., Anderson R.H. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999;10:1525–1533. doi: 10.1111/j.1540-8167.1999.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 12.Ho S.Y., Cabrera J.A., Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythm Electrophysiol. 2012;5:220–228. doi: 10.1161/CIRCEP.111.962720. [DOI] [PubMed] [Google Scholar]
- 13.Yokokawa M., Sundaram B., Oral H., Morady F., Chugh A. The course of the sinus node artery and its impact on achieving linear block at the left atrial roof in patients with persistent atrial fibrillation. Heart Rhythm. 2012;9:1395–1402. doi: 10.1016/j.hrthm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Teuwen C.P., Yaksh A., Lanters E.A. Relevance of conduction disorders in Bachmann's bundle during sinus rhythm in humans. Circ Arrhythm Electrophysiol. 2016;9:e003972. doi: 10.1161/CIRCEP.115.003972. [DOI] [PubMed] [Google Scholar]
- 15.Douglas Y.L., Jongbloed M.R., Gittenberger-de Groot A.C. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97:662–670. doi: 10.1016/j.amjcard.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Han H.C., Ha F.J., Sanders P. Atrioesophageal fistula: clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol. 2017;10:e005579. doi: 10.1161/CIRCEP.117.005579. [DOI] [PubMed] [Google Scholar]
- 17.Ali-Ahmed F., Goyal V., Patel M., Orelaru F., Haines D.E., Wong W.S. High-power, low-flow, short-ablation duration-the key to avoid collateral injury? J Interv Card Electrophysiol. 2019;55:9–16. doi: 10.1007/s10840-018-0473-5. [DOI] [PubMed] [Google Scholar]
- 18.Leshem E., Zilberman I., Tschabrunn C.M. High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol. 2018;4:467–479. doi: 10.1016/j.jacep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Winkle R.A., Moskovitz R., Hardwin Mead R. Atrial fibrillation ablation using very short duration 50 W ablations and contact force sensing catheters. J Interv Card Electrophysiol. 2018;52:1–8. doi: 10.1007/s10840-018-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]