Abstract
Background
Allergies to cats are the most common animal‐origin allergy, and affect approximately 1 in 5 adults worldwide. The prevalence of allergy to furry animals has been increasing, and allergy to cats is a major risk factor for the development of asthma and rhinitis. The diagnosis of cat allergy is now well established. The exact significance of component‐resolved diagnosis in the diagnosis of cat allergy remains to be fully understood. Allergen avoidance is effective but often has a psychologic impact. Allergen immunotherapy is not well demonstrated. There is a need for innovative approaches to better manage cat allergens. Next‐generation care pathways for asthma and rhinitis will define the place of cat allergen avoidance.
Methods and Results
This manuscript, based on content presented at the European Academy of Allergy and Clinical Immunology Congress 2019, provides information on the prevalence and impact of cat allergies and the molecular biology of Fel d 1, the major cat allergen.
Discussion
The authors present the scientific basis of a novel care pathway that utilizes anti‐Fel d 1 IgY antibodies to safely and effectively neutralize Fel d 1 after its production by the cat but before human exposure.
Conclusion
Efficacy of a feline diet with an egg product ingredient containing anti‐Fel d 1 IgY antibodies was demonstrated in vitro, ex vivo, and in vivo, and further validated by a pilot exposure study involving cat‐allergic human participants.
Keywords: allergens, anti‐Fel d1 IgY, blocking antibodies, cat allergies, Fel d 1
Abbreviations
- AIT
allergen immunotherapy
- AR
allergic rhinitis
- ARIA
Allergic Rhinitis and its Impact on Asthma
- BAMSE
Swedish abbreviation for Children, Allergy, Milieu, Stockholm, Epidemiology
- ICP
Integrated care pathway
- POLLAR
Impact of air POLLution on Asthma and Rhinitis
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
1. INTRODUCTION
Allergies to cats are the most common mammalian‐origin allergy in humans1, 2, 3 and affect approximately 1 in 5 adults worldwide.4, 5 Fel d 1 is the major cat allergen, accounting for up to 96% of human allergic sensitization to cats and 60%‐90% of the overall antigenicity of cats and cat dander.2, 5, 6, 7, 8, 9
Traditional care pathways for cat allergies focus on treating patients who are exposed to cat allergens. The following review expands on information presented during a sponsored symposium at the European Academy of Allergy and Clinical Immunology (EAACI) Annual Congress on June 4, 2019, in Lisbon, Portugal. The symposium, titled “Keep the cat, changes the care pathway: a transformational approach to managing cat allergy,” presented a research breakthrough that reduces cat allergens while maintaining normal production of Fel d 1 by the cat and without impacting the cat's overall physiology. This new approach provides an opportunity for a novel clinical care pathway that allows the cat to remain in the home.
2. FEL D1, THE MAJOR CAT ALLERGEN
To date, eight cat‐origin allergens have been identified and registered through the World Health Organization/International Union of Immunological Societies (WHO/IUIS) Allergen Nomenclature Sub‐Committee.2, 10, 11, 12 Fel d 1, a secretoglobin, is the major cat allergen.2, 5, 6, 7, 8, 9, 13 (Table 1) Although monosensitization to Fel d 1 is common, individuals sensitized to the cat allergens Fel d 2 (an albumin allergen) and Fel d 4 (a lipocalin allergen) are usually also sensitized to Fel d 1.3 Fel d 1 is a sufficiently dominant allergen that IgE sensitization to Fel d 1 is equivalent to cat extract in predicting cat allergy.3, 13, 14
Table 1.
Human sensitization rates to feline‐origin allergens. (Excerpted from EAACI Molecular Allergology User's Guide, 2016.)
| Allergen | Biochemical name | Human sensitization rate |
|---|---|---|
| Fel d1 | Uteroglobin | 60%‐100% |
| Fel d2 | Serum albumin | 14%‐54% |
| Fel d3 | Cystatin | 10% |
| Fel d4 | Lipocalin | 63% |
| Fel d5 | Immunoglobulin A | 38% |
| Fel d6 | Immunoglobulin M | unknown |
| Fel d7 | Lipocalin | 38% |
| Fel d8 | Latherin‐like protein | 19% |
All cats produce Fel d 1 regardless of breed, age, hair length, sex, housing (indoors vs outdoors), or body weight; there are no allergen‐free or hypoallergenic cats.2, 9, 15, 16, 17, 18, 19 Fel d 1 production varies widely among individual cats and may vary widely throughout the year in the same cat.15, 16, 20 Bastien et al16 observed an 80‐fold difference in salivary Fel d 1 levels between the lowest‐producing and highest‐producing cats in a 64‐cat group and up to a 76‐fold difference between the lowest and highest salivary Fel d 1 levels in individual cats. Studies have shown that male cats produce 3‐5 times less Fel d 1 after neutering; these findings, combined with observations that Fel d 1 production could be restored to preneutering levels with the administration of exogenous testosterone, suggest an influential role of testosterone on Fel d 1 production.1, 2, 21, 22
Fel d 1 is produced primarily in the salivary and sebaceous glands and in lesser amounts in the lacrimal and anal glands.2, 23 It is spread throughout the cat's hair during grooming and shed into the environment with hair and dander.2, 17 Fel d 1's biological function for the cat is as yet unknown, but a pheromone/chemical signaling role has been proposed.2, 5, 22, 24
Fel d 1 easily becomes and remains airborne in dander and dust particles; up to 60% of Fel d 1 is carried by particles <5 microns in diameter.2 9 It is passively transferred on clothing2, 5, 19; as a result, the allergen is ubiquitous and has been documented in homes without cats, private vehicles, and public transportation and buildings at levels (≥8 µg Fel d 1 per gram of dust) that exceed threshold value associated with sensitization.2, 5, 7, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Although Fel d 1 levels in schools are detectable, they are often low and may not induce symptoms,35 but a Swedish study found that indirect exposure to cat may be associated with worsening of asthma in cat‐allergic students.26
2.1. Fel d 1‐induced sensitization and IgE‐mediated allergy
Upon initial exposure to an allergen (such as Fel d 1), antigen‐presenting cells (eg, dendritic cells and macrophages) capture and process the allergen, then present antigenic peptides to T helper cells. Stimulated by the presence of specific cytokines (eg, interleukin‐4, interleukin‐13), the T helper cells acquire a type 2 phenotype (Th2) and recruit B lymphocytes to produce and secrete IgE.36, 37, 38 The Th2 phenotype is thought to have evolved as a response to helminth parasites, but also plays a beneficial role in a number of physiologic functions.37 Excessive or mis‐targeted Th2 cell responses may result in allergic responses, including atopy, allergic rhinitis, and allergic asthma.37
The allergen‐specific IgE binds to high‐affinity immunoglobulin receptors (FcϵRI; considered high‐affinity because their affinity for IgE is 100 times greater for IgE than for IgG) on the surface of mast cells and basophils to create allergen‐specific receptors.38, 39 Very little IgE is found in the circulation as soluble IgE.40
Mast cells, located in mucosal and epithelial tissues near potential points of allergen entry into the body (such as the respiratory tract mucosa), play a critical role in the allergic response.40 With subsequent exposure to the allergen following sensitization, the allergen binds to and crosslinks two or more IgE:FcϵRI complexes on mast cells, triggering degranulation, and the release of inflammatory mediators (eg, histamine; heparin; interleukins 3, 4, and 5; leukotrienes, prostaglandins, thromboxanes).38, 39, 40, 41, 42 Inhaled allergens, such as Fel d 1, induce mast cell activation in the respiratory tract, resulting in symptoms of airway constriction, increased mucous production, and coughing.40 These mediators also attract additional cells that amplify the inflammatory response, creating a potentially self‐propagating inflammatory cycle.40
3. CAT ALLERGIES: A COMMON PROBLEM WITH CONSEQUENCES FOR HUMANS AND CATS
The prevalence of allergy to furry animals has been increasing, and allergy to cats is a major risk factor for the development of asthma and rhinitis.43 The diagnosis of cat allergy is now well established.9 Allergen avoidance is effective, but often has a psychologic impact. Allergen immunotherapy (AIT) is not well demonstrated.44 There is a need for innovative approaches to better manage cat allergy.
3.1. Prevalence
Most people in Western societies spend over 90% of their lives indoor, and indoor allergens play an important role in allergic sensitization and symptoms. The percentage of homes with a pet ranges from around 5% in Spain, 20% in Sweden, 25% in the United States, and up to 65% in New Zealand.9, 43, 45
Allergies to cat affect 7%‐25% of the population and represent a growing public health concern as these rates increase.3, 7, 9 The prevalence of cat allergy varies between countries, timing of exposure, and allergic predisposition.9 Cat allergy is found both in developed and developing countries,46, 47 starts early in infancy and progresses up to young adulthood,13, 48 and can be observed in children who live in a house where cats are not present.49
3.2. Mono‐ and polysensitization
Cross‐reactivity or co‐sensitization exists between some animal allergens, and many patients are allergic to cats and dogs, but also to other furry animals. However, since the major cat allergen Fel d 1 does not cross‐reacts with other animals, co‐sensitization is more likely.
In the BAMSE cohort,13 sensitizations to Fel d 1 in childhood were significantly associated with symptoms to cat at age 16 years. Polysensitization to 3 or more allergen molecules from cat was a better longitudinal predictor of cat symptoms than results of IgE tests with cat allergen extract. Cross‐sectionally, cat‐polysensitized children had higher IgE levels and more frequent symptoms to cat than monosensitized children.
In the West Sweden Asthma Study, the characterization of sensitization to furry animal allergen components was assessed in an adult population.3 Fel d 1 was the most prevalent cat component in monosensitized individuals, whereas all cat allergens including Fel d 1 were detected in polysensitized individuals. This study may be of relevance for the stratification of patients who would be exposed to low‐allergencity cats.
Sensitization to furry animal allergen components is an important predictor of asthma, rhinitis, and markers of asthma severity.50 Current asthma and asthma symptoms following contact with cats were associated with co‐sensitization to Fel d 1 and Fel d 4. This association was seen already at moderate‐level sensitization (1‐15 ISU) to Fel d 4, at which level most children were sensitized to Fel d 1, as well.51 There is a wide heterogeneity among cat‐allergic individuals with multiple clusters of sensitization. Each sensitization cluster (nonsensitized, Fel d 1‐driven sensitized, and multisensitized clusters) was associated with substantial increased risk of asthma, rhinitis, concomitant asthma, and rhinitis.50 There was, however, no association with asthma exacerbations, FEV1 predicted values, emergency visits, or regular oral steroid use. In another study, children with severe allergic asthma had higher serum IgE levels to cat, dog, and horse. Molecular‐based allergy diagnostics revealed a more complex molecular spreading of allergen components (polysensitization) in children with the most severe disease.52
3.3. The allergic burden
Classical symptoms of cat allergy include rhinitis, asthma, and/or conjunctivitis. Quality of life is impaired in patients with allergic rhinitis (AR) or asthma, who may suffer from poor quality sleep, fatigue, reduced alertness, lower work productivity and concentration, and mood changes.53, 54, 55 Learning may be impaired in children with allergic rhinitis.53 The direct costs to the allergy sufferer are compounded by the indirect costs to society of impaired productivity and performance.56 The severity of AR symptoms is the most common factor associated with impairment of productivity, but AR‐related sleep disturbances may contribute to the issue.56 According to a digitally based mobile health (mHealth) initiative, 90% of app users with uncontrolled AR experienced some work productivity impairment and over 50% experienced severe work impairment.56 These impairments may result not only from the allergic condition itself, but also from undesired effects of medications taken for symptom prevention and relief.53, 57
In addition to their role in AR, allergies to cats and dogs are a major risk factor in the development of asthma.7, 19, 47, 58 It is estimated that pets are the third‐leading cause of IgE‐mediated allergic asthma. Cat‐allergic children experience twice as many days with asthma symptoms and require more frequent dosing of β‐agonists and steroids during the second week of the school term in classrooms with moderate‐to‐high prevalence of home cat ownership.26 More than 500 000 emergency visits were attributed to cat allergen‐induced asthma attacks in a cat‐sensitive and exposed population of patients with asthma.58
The psychologic impact of cat allergy is of great concern. Many patients or parents of allergic children fail to comply with their physician's recommendations to avoid the animal; this can be understood especially when there are emotional consequences to making the asked for lifestyle changes.
3.4. Allergy's impact on cat welfare and the human‐animal bond
Pet ownership confers numerous health benefits, including positive influences on blood pressure and cardiovascular health, loneliness, depression and mental health, and weight management.59 Many owners consider their cats part of the family.45, 59, 60, 61, 62, 63 For these reasons, allergists' guidelines‐based recommendations to remove the cat from the home are often met with resistance.8, 9, 19, 64
Allergies can limit the interactions between the allergic person and their cat, interfering with the human‐animal bond. According to the American Veterinary Medical Association, the human‐animal bond is described as “a mutually beneficial and dynamic relationship between people and animals that is influenced by behaviors essential to both. This includes, among other things, emotional, psychological, and physical interactions of people, animals and the environment.”65 Physical contact plays an important role in the strength and longevity of the human‐cat bond.66, 67 Many cat owners, and particularly female owners, also value their cat's cleanliness60; understanding that grooming is an important aspect of cleanliness as well as a primary method for dispersion of cat allergens throughout the cat's hair and subsequently into the environment, this highly desired behavioral feature facilitates allergen exposure for allergic individuals.
Allergy to cats directly impacts cat welfare because allergy is a commonly provided reason for relinquishment of cats to shelters,68, 69, 70, 71, 72 as well as a barrier to cat adoption and ownership.72, 73
3.5. Diagnosis of cat allergy
The diagnosis of cat allergy is based on symptoms occurring during exposure and the demonstration of an IgE‐mediated sensitization to cat allergens.9 Skin prick tests with standardized extracts are reliable. Serum‐specific IgE with a crude extract is equally important. However, the results of these tests should be confronted to symptoms as they only indicate a cat sensitization.
Most recently, molecular‐based (component‐resolved) diagnosis has become available. However, its exact significance in the diagnosis of cat allergy remains to be fully understood.74 On the other hand, for the prediction of asthma severity50, 51 or persistence to symptoms,13 molecular‐based diagnosis may be relevant. One major advantage of the current molecular‐based diagnosis is certainly the determination of the primary sensitization source, which is not feasible by using extracts. This implies, of course, a better management of allergic patients.
Exposure tests are not recommended in routine diagnosis of cat allergy.9
4. CURRENT MANAGEMENT
Several recommendations have been proposed to reduce cat allergenicity, but to date, none of them present convincing evidence.
4.1. Allergen load reduction
Polysensitized individuals will often show more severe symptoms than monosensitized individuals when exposed to environmental allergens, indicating that allergens have an additive effect.40, 75 This reinforces the concept of total allergen load, which represents the sum of the individual allergens in the environment at that time. If the total allergen load exceeds an individual's allergic threshold, that individual develops allergic symptoms.
The total allergen load and allergic threshold represent the clinical manifestation of the underlying molecular process: similar to the allergic threshold associated with an individual, mast cells and basophils bearing the IgE receptors have a threshold at which degranulation and mediator release are triggered. The cellular threshold has been estimated to required crosslinking of 2000 out of an estimated 500 000‐1 000 000 high‐affinity IgE receptor complexes on the cell surface.40, 76 High exposure levels of one allergen may be sufficient to trigger degranulation and mediator release, while lower levels do not; however, multiple allergens present at subthreshold levels may have a cumulative effect that exceeds the threshold and triggers the chain of events leading to allergic symptoms.40 The number of IgE receptors bound, not the identity of the allergen(s), determine if the process is initiated.
If the allergen load can be reduced by avoiding or reducing the level of exposure to one or more of the contributing allergens, the cumulative level of allergen exposure may fall below an individual's threshold and improve or prevent allergy symptoms.40, 75
4.1.1. Avoidance
Removing the cat from the home is the most commonly recommended measure, although it is not supported by evidence.8, 9, 19, 77 Fel d 1 is a “sticky” allergen, and it may take months for symptoms to improve following removal of the cat from the household, particularly if the household is carpeted.78, 79 Wood et al79 observed that up to 20 weeks were required following removal of the cat for Fel d 1 levels to reduce to those found in homes without cats.
On the other hand, it is suggested that symptomatic patients may become tolerant to the cat after some months of continuous exposure and a specific IgG4 response is associated with tolerance.80 Tolerance duration is, however, unknown, and many adults who had cats in childhood may develop severe asthmatic reactions when re‐exposed to cats many years later.
So‐called “hypoallergenic pets” have been marketed to allergy sufferers, but their clinical relevance has never been demonstrated.9, 18 Although patents have been filed for gene editing to produce allergen‐free cats and the topic has received intermittent media attention for more than a decade, the truly allergen‐free cat has been elusive.81
4.1.2. Environmental control
Environmental control measures are also recommended in order to reduce environmental allergen levels, and measures implemented in cat‐sensitized households may include the following:
Keeping the cat outdoors8
Use of HEPA filters in vacuums and HVAC systems8, 9, 19, 83, 84
Wet mopping floors and surfaces82
Washing walls82
Ventilation to increase air change rate per hour85
Changing clothes before moving from an area of high allergen levels to one with lower levels8
Allergists' recommendations may include washing the cat to physically remove allergens from its hair.2, 9, 86, 87 This recommendation has poor compliance,82 due largely to the feline species' aversion to bathing. In addition, although immersion bathing is effective for lowering allergen levels on the cat's hair, the effects of bathing are transient; allergen levels return to baseline within 24 hours of bathing.2, 86, 87
Although these measures may reduce the allergen load,82 they are effort‐intensive, costly, and may be difficult to sustain long term.77 In addition, the effects may be transient.8, 19 Multifaceted interventions are recommended for best results.8, 9 Because Fel d 1 is such a ubiquitous allergen, sensitized individuals that successfully reduce the allergen load in their own homes will still be exposed to potentially high levels of cat allergens at work, in homes of cat‐owning family members or friends, and in public places.2, 5, 7, 25, 26, 27, 28, 29, 30, 31, 32
Perinasal mechanical barriers are intended to entrap airborne allergens and prevent them from contacting the respiratory mucosa. A hydroxypropyl methylcellulose barrier had positive effects on nasal symptom score, ocular symptom score, total symptom score, and quality of life score in patients with perennial and seasonal AR.88
4.2. Pharmaceutical interventions
There is no specificity for the treatment of symptoms induced by cats. However, patients with a known cat allergy may prevent the onset of symptoms by using medications before cat exposure. Rapidly acting medications are favored for both asthma and rhinitis.89
4.3. Immunotherapy
Davila et al9 recommended that immunotherapy with cat epithelium would be indicated in patients with allergic respiratory disease under circumstances in which there is exposure, assessing the viability and efficacy of environmental control measures, drug therapy, and patient preferences. However, there is a limited body of high‐quality evidence on the effectiveness and safety of cat AIT from large randomized controlled trials either for subcutaneous (SCIT) or for sublingual immunotherapy (SLIT) and no high‐quality data on its cost‐effectiveness.44 Around 200 patients have been enrolled in double‐blind trials for SCIT, and results are inconsistent. The available evidence on effectiveness is mixed based on studying a limited array of immunological, physiological, and patient‐reported outcome measures. Based on this evidence and extrapolating on the wider evidence base in AIT, it is likely that some patients may benefit from this modality of treatment, particularly those with moderate‐to‐severe disease who are inadequately controlled on allergen avoidance measures and pharmacotherapy and those who are monosensitized to Fel d 1. Further evidence is, however, required from larger trials before more definitive advice can be offered.44
Many trials with T‐cell peptides have shown limited efficacy and/or led to nonimmediate allergic reactions during treatment90, 91, 92 although immunologic effects were demonstrated.93, 94
4.4. Primary Prevention
The role of pet keeping in early life to prevent cat allergy is still a matter of debate. Early exposure to pets before 1 year of age may have a protective effect in preventing allergic sensitizations, but studies to date have produced conflicting results. However, a meta‐analysis from 11 pooled European birth cohorts concluded that there was no evidence for a protective or “harmful” effect of cat ownership on sensitization.95 To date, there is no consensus regarding animal exposure and preventing later onset of asthma or other allergic diseases.
5. NEXT‐GENERATION CARE PATHWAYS OF CAT ALLERGY
5.1. Care pathways in the digital transformation of health
Integrated care pathways (ICPs) are structured multi‐disciplinary care plans detailing the key steps of patient care.96 They promote the translation of guideline recommendations into local protocols and their application to clinical practice. An ICP forms all or part of the clinical record, documents the care given, and facilitates the evaluation of outcomes for continuous quality improvement.97 They empower patients as well as their health and social carers.
ICPs differ from practice guidelines as they are utilized by a multi‐disciplinary team and focus on the quality and co‐ordination of care. ICPs need to record variations from planned care.98 An ICP is intended to inform and encourage thought and adaptation. Clinicians are free to exercise their own professional judgments as appropriate. However, any alteration to the practice identified within this ICP must be noted as a variance.99 Variance analysis may be used to optimize the ICPs linked with pay‐for‐performance (P4P),100, 101, 102, 103 audit and feedback, and integration of recommendations with electronic medical records.
ICPs are already the standard of care in different areas of medicine such as oncology104 or palliative care.105 Some have already been proposed for asthma or COPD.
There is a need to support the digital transformation of health and care with ICPs. ICPs have been proposed with a focus on mHealth technologies that should enhance self‐management and adherence to guidelines and ICPs. An innovative patient‐centered approach for ICPs has been proposed by the ARIA expert group for rhinitis and asthma multi‐morbidity.56, 106, 107, 108, 109
5.2. Multisectoral care pathways for rhinitis and asthma
A large number of AR patients do not consult physicians because they think their AR symptoms are “normal” and/or trivial. However, AR negatively impacts social life, school, and work productivity.110 Many AR patients use over the counter (OTC) drugs and only a fraction have had a medical consultation.111 The vast majority of patients who visit general practitioners or specialists have moderate/severe rhinitis.112, 113, 114, 115, 116 Thus, ICPs should consider a multi‐disciplinary approach as proposed by AIRWAYS ICPs (Figure 1).
Figure 1.
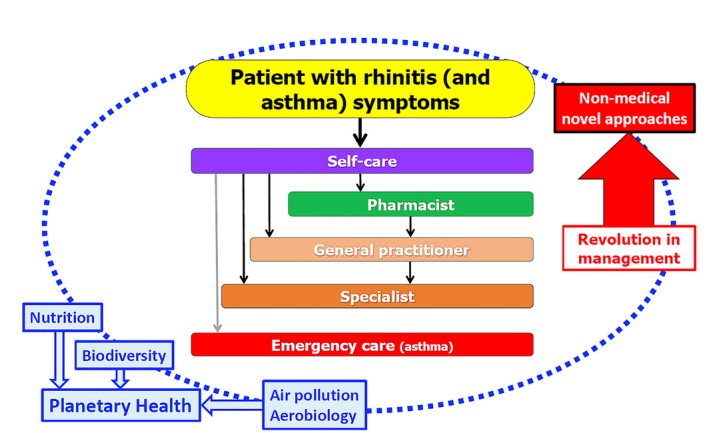
Multi‐disciplinary approach as proposed by AIRWAYS ICPs
5.3. Next‐generation care pathways for rhinitis and asthma using ICP
Although environmental factors play a major role in allergic diseases, no guideline or recommendation has included them. Based on the results of POLLAR (Impact of air pollution on asthma and rhinitis,55 ARIA next‐generation ICPs will embed exposure to environmental factors like pollen and air pollution as well as novel approaches like artificial intelligence that have not been considered up to now. As there is increasing evidence that patients' choices and behaviors have an impact on the planet, this will be the background of ARIA Planetary Health.117
Another interesting approach is to propose nonmedical approaches to control allergic symptoms. In all allergy textbooks, allergen avoidance is always required but most studies failed to prove efficacy for house dust mites. However, new data in stratified severe asthmatic patients are encouraging.118 In cat allergy, a reduction in cat allergenicity can represent a revolution in the management of cat‐allergic patients and next‐generation care pathways should recognize the results of the studies when they will be completed. Whether a patient stratification is needed should be further established.
6. NEUTRALIZING FEL D 1 AT ITS SOURCE
Anecdotal evidence has suggested that altering a cat's diet may affect allergen production, but specific nutritional interventions have not been evaluated using the scientific method. Pezzali et al119 postulated that dietary modification to reduce sebum production may lower Fel d 1 production by cats: possible modifications included isoflavones and phytoestrogens to reduce androgen production; reducing the omega‐6/omega‐3 fatty acid ratio in the diet; and reducing the glycemic index of the cat's diet by lowering carbohydrate levels.119 However, the clinical efficacy of their proposed modifications has not, to the authors' knowledge, been investigated.
Novel strategies have been developed to treat Fel d 1‐induced allergy in human subjects by immunizing cats against their own major allergen.120 A conjugate vaccine consisting of recombinant Fel d 1 and a virus‐like particle derived from the cucumber mosaic virus containing the tetanus toxin‐derived universal T‐cell epitope tt830‐843 (CuMVTT) was used to immunize cats. All cats induced a strong and sustained specific IgG antibody response. The induced anti‐Fel d 1 antibodies were of high affinity and exhibited a strong neutralization ability tested both in vitro and in vivo. A reduction in the endogenous allergen level and a reduced allergenicity of tear samples were observed.120 However, clinical studies are needed to confirm the approach.
Because the biological function of Fel d 1 for the cat is currently unknown, the potential health and welfare effects of ceasing its production are also unknown; for this reason, we sought to develop an approach that does not alter the cat's production of Fel d 1. It is understood that patients receiving immunotherapy develop “blocking antibodies” (usually IgG) which help to prevent the allergic cascade38; therefore, our research hypothesized that it could be possible to use a similar process to neutralize the Fel d 1 allergen after its production by the cat but before its activation of allergy effector cells. This approach attempts to reduce allergenic (active) Fel d 1 by binding the allergen with anti‐Fel d 1 polyclonal egg IgY antibodies.
6.1. Effective neutralization of Fel d1 requires binding at multiple epitopes
Fel d 1 is a four‐subunit (tetrameric) protein composed of two covalently linked heterodimers, each of which contains two distinct chains (Chain 1, a polypeptide, and Chain 2, a glycopeptide with N‐linked oligosaccharides) that are encoded by separate genes and linked with disulfide bridges.2, 10, 11, 24, 121, 122, 123, 124, 125, 126, 127 (Figure 2) Despite variation in Fel d 1 due to differential gene expression for the two chains, core fragments are preserved and the structural variation in Fel d 1 has a low impact on its allergenicity.125
Figure 2.
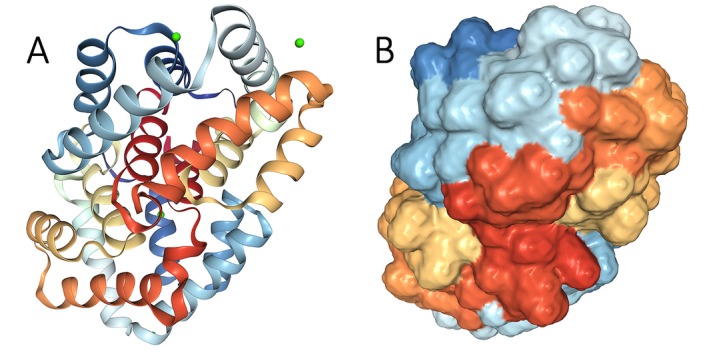
Fel d 1 crystalline (A) and three‐dimensional (B) structures, showing four subunits composed of two covalently linked heterodimers containing two distinct chains. From https://www.rcsb.org/structure/2EJN, open source image
The three‐dimensional structure of Fel d 1 is unique and complex, with an internal cavity and two external calcium‐binding sites.2 The heterodimer structure is important in allergenicity of Fel d 1 because IgE reactivity directed against the individual chains is far less than the level of reactivity against the heterodimer, suggesting the importance of protein conformation in Fel d 1‐IgE binding.2, 7, 128 Reduction and alkylation eliminate Fel d 1's allergenic activity, further suggesting conformation‐dependent IgE binding sites.7, 129 Three epitopes were identified on Fel d 1: two epitopes on Chain 1 and one epitope on Chain 2.130, 131 More recently, Tasaniyananda et al132 identified spatially juxtaposed residues on Chain 1 that indicate an additional epitope on that chain.
Monoclonal antibodies against Fel d 1 do not effectively neutralize the allergen's ability to induce an immune response. A commercially produced rabbit‐origin anti‐Fel d 1 monoclonal antibody (Fel d 1 major allergen 1 polypeptide chain 1 antibody, product no. FELD1‐121AP, FabGennix) (FGI) did not prevent binding of Fel d 1 to Fel d 1‐specific IgE in a modified chimeric ELISA.133 In contrast, a commercially produced rabbit antiserum containing polyclonal antibodies to Fel d 1 (Rabbit anti‐Fel d 1 (PA‐FD1), Indoor Biotechnologies) (Indoor Poly) was able to bind several epitopes on the Fel d 1 molecule and successfully blocked Fel d 1 binding to IgE in vitro in a dose‐dependent manner.133
Monoclonal and polyclonal anti‐Fel d 1 antibodies were pre‐incubated with cat saliva and evaluated utilizing a humanized rat basophil cell line and a beta‐hexosaminidase assay to indicate degranulation and mediator release. The polyclonal antibody reduced mediator release in a dose‐dependent fashion but the monoclonal antibody had no blocking action on mediator release.133
These findings are consistent with those of Orengo et al,38 who observed that monoclonal antibodies against Fel d 1 did not effectively block IgE and mast cell degranulation in basophil activation assay, but a combination of monoclonal antibodies directed at different epitopes—resulting in simulated polyclonal binding to Fel d 1—was effective. The simulated polyclonal antibody combination also blocked cutaneous anaphylaxis in a mouse model, confirming that multiple epitopes must be bound to prevent Fel d 1‐induced IgE crosslinking and cellular activation.38
6.2. Anti‐Fel d 1 IgY effectively neutralizes active Fel d 1 in vitro and ex vivo
Based on the findings that polyclonal binding is necessary to neutralize Fel d 1's allergenicity, Satyaraj et al133 evaluated the efficacy of avian egg yolk‐derived immunoglobulin Y (IgY) directed against Fel d 1. IgY is an avian equivalent to mammalian IgG and is found in chicken serum and egg yolks. Chickens naturally produce IgY against environmental antigens and transfer the IgY into their eggs to provide passive immunity to their offspring.134, 135 Anti‐Fel d 1 IgY can be induced by exposing hens to Fel d 1. Based on this principle, anti‐Fel d 1 IgY were produced using well‐established immunization methods.133 Anti‐Fel d 1 IgY blocked the binding of salivary Fel d 1 to Fel d 1‐specific IgE in vitro in a dose‐dependent manner similar to the Indoor Poly polyclonal antibody in both the modified chimeric ELISA and basophil activation assay.133 (Figure 3).
Figure 3.
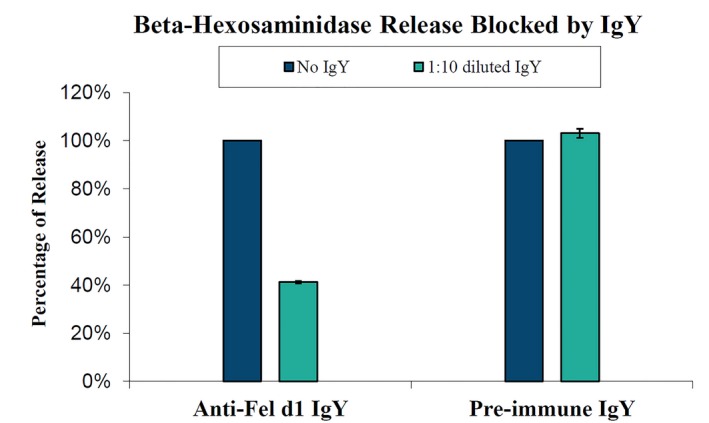
Beta‐hexosaminidase assay results from eggs from blocking experiments using eggs from chickens immunized to Fel d 1 to produce anti‐Fel d 1 IgY (immune) and eggs from the same chickens prior to Fel d 1 exposure (pre‐immune). β‐hexosaminidase levels are expressed as percentages of baseline levels from control samples incubated without antibodies
6.3. Anti‐Fel d 1 IgY effectively neutralizes active Fel d 1 in vivo
Building on the previous in vitro and ex vivo studies, the next step in the investigative process was to determine whether the anti‐Fel d 1 IgY produced effective Fel d 1 blocking in cats in vivo.
6.4. Feline test diet with added anti‐Fel d 1 IgY reduces active Fel d 1 in saliva
Based on the in vitro and ex vivo study results, it was hypothesized that feeding cats anti‐Fel d 1 IgY would reduce immunologically active (allergenic) Fel d 1 (active Fel d 1; aFel d 1) in cat saliva. In a pilot study, saliva was collected (Salivette®) from six healthy, adult domestic shorthair cats before their morning feeding and at 1, 3, and 5 hours postfeeding. All of the cats received a control diet (without anti‐Fel d 1 IgY) for a 2‐week baseline period, followed by 6 weeks on the test diet (control diet with added anti‐Fel d 1 IgY). A significant decrease in salivary aFel d 1 was detected within 2 weeks of starting the test food, and the average decrease over the 6‐week treatment period was 29.57%.136
In the second trial in the study, saliva was collected from twenty healthy, adult domestic shorthair cats 5 hours after their morning feeding, 5 days a week for the duration of the 5‐week study. Cats were fed a control diet for a 1‐week baseline period, followed by either the control diet (control group) or the control diet with an egg product containing anti‐Fel d 1 IgY (test group) for 4 weeks. Salivary aFel d 1 was significantly reduced by Week 3 in the cats receiving the anti‐Fel d 1 IgY in their diet, with a mean reduction of 24%, while the control group did not show any significant reduction in active Fel d 1 with a mean reduction of only 4%.136 (Figure 4).
Figure 4.
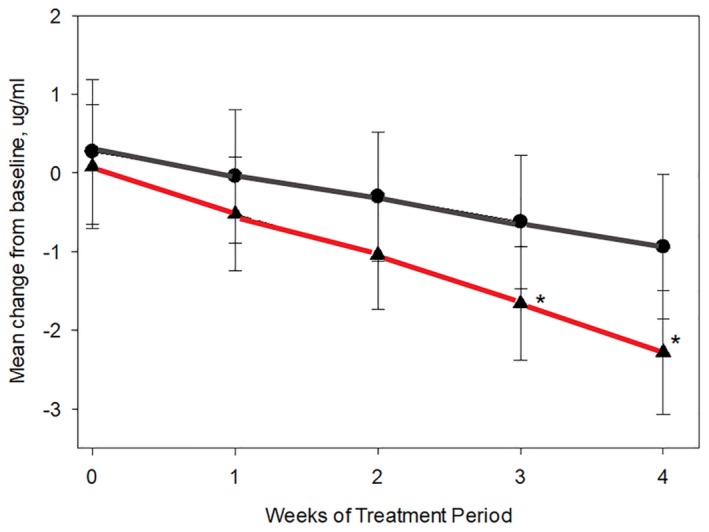
Reduction in salivary active Fel d 1 levels (µg/mL) in response to a diet with an egg product ingredient containing anti‐Fel d 1 IgY. The line with circle data points depicts data from the control diet group and the line with triangle data points depicts data from the test diet group. Asterisks denote statistical significance (P < .05) compared with baseline based on linear mixed model analysis (Source: CC‐BY‐NC Satyaraj et al, 2019136)
This study demonstrated that a diet with egg product ingredient containing anti‐Fel d 1 IgY effectively reduced salivary aFel d 1 levels of cats.
6.5. Feline test diet with added anti‐Fel d 1 IgY reduces Fel d 1 in hair and dander
Previous studies demonstrated that anti‐Fel d 1 IgY blocked IgE‐mediated degranulation in vitro and ex vivo and significantly reduced salivary aFel d 1 levels in cats in vivo. Fel d 1 enters the environment through shed hair and dander; therefore, the next step in the validation process was to determine the effects of anti‐Fel d 1 IgY on aFel d 1 levels in the cat's hair and dander.
Hair was collected (by brushing) from 105 healthy domestic shorthair cats four times over a 2‐week baseline period, then weekly during a 10‐week treatment period during which the cats received a food with an egg product ingredient containing anti‐Fel d 1 IgY. Active Fel d 1 levels in the hair and dander collected by brushing were significantly reduced starting in Week 3 of the treatment period and remained at reduced levels for the remainder of the treatment period. The aFel d 1 reduction ranged from 31% to 77%, with an average aFel d 1 reduction of 47%.137 (Figure 5) Cats with the highest baseline aFel d 1 levels showed the greatest decrease in aFel d 1 during the treatment period. (Figure 6).
Figure 5.
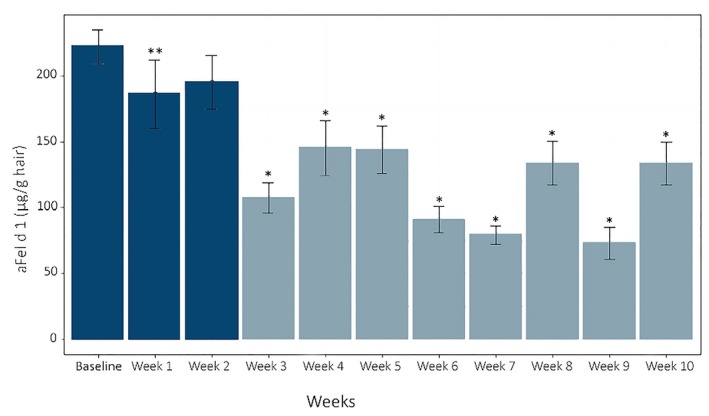
Active Fel d 1 levels (µg/g hair) means and SE across weeks. Means were significantly reduced from baseline at week 1 (P < .05) and weeks 3 through 10 (P < .001) using linear mixed effect models and P‐value adjustments using the single‐step method Columns with the lighter color denote values that were statistically different from baseline (Source: CC BY 3.0, Satyaraj et al, 2019137)
Figure 6.
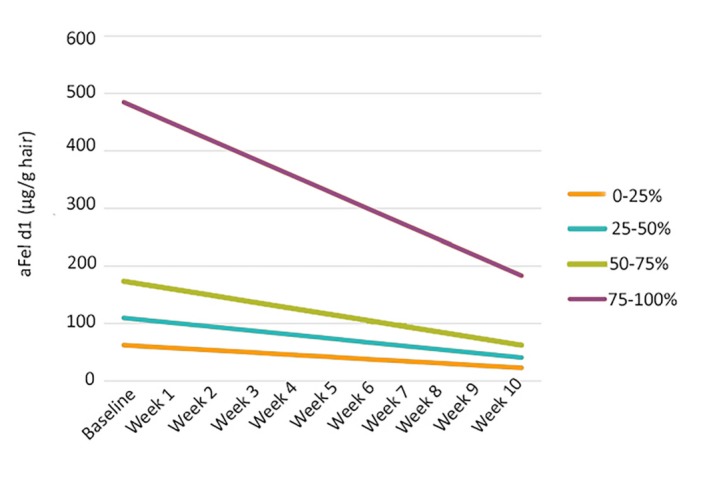
Change in active Fel d 1 (µg/g hair) means over time based on initial concentrations. Cats were divided into quartiles based on their baseline aFel d 1 levels and linear regression was used to estimate the initial level (intercept) and the change in aFel d 1 over time (slope). The graph represents a total of 1470 samples obtained during the 12‐week study. The slope of decline in aFel d 1 levels was significantly steeper for those cats in the highest quartile (P < .001) but did not differ among the three lower quartiles (P > .1) based on ANOVA with Tukey Post hoc Tests. Cats with the highest Fel d 1 production showed the greatest response to the intervention (Source: CC BY 3.0, Satyaraj et al, 2019137)
This study demonstrated that a diet with egg product ingredient containing anti‐Fel d 1 IgY effectively reduced aFel d 1 levels in the hair and dander of cats.
6.6. Anti‐Fel d 1 IgY is safe for cats
Many cat owners view their cats as part of the family45, 60, 61, 62 and will often go to great lengths to keep their cat in the home despite allergies.64 However, although many allergic owners will compromise their own health to keep their cat, they are unlikely to accept approaches that they feel may put their cat's health and well‐being at risk.
All egg products with egg yolk contain IgY. Egg products containing specifically targeted IgY have been used safely in human and veterinary medicine.138, 139, 140, 141 The anti‐Fel d 1 IgY is safe for cats, based on a comprehensive safety study that fed an egg product ingredient with multiple levels of anti‐Fel d 1 IgY, including levels many times higher than those used in efficacy studies.142
Based on the principle of allergen load reduction, complete elimination of Fel d 1 production is not necessary. Our approach does not neutralize 100% of the cat's Fel d 1; in essence, it converts moderate and high Fel d 1‐producing cats to the equivalent of low or moderate producers without altering the cat's production of the allergen. Cats produce varying levels of Fel d 1 depending on neuter status, sex, and genetics and can be healthy regardless of their Fel d 1 levels15, 121; our approach preserves some biologically available Fel d 1 while reducing the active allergen.
6.7. Clinical impact of anti‐Fel d 1 IgY
Reducing the levels of active Fel d 1 in a cat's saliva and hair can reduce the amount of cat allergens shed into the environment on hair and dander, thereby reducing the total allergen load in the environment. If the allergen load is reduced to a level below the individual's allergic threshold, clinical allergy symptoms may be prevented. In order to determine the effects of feeding cats a diet with an egg product containing anti‐Fel d 1 IgY on clinical symptoms in human allergy sufferers, a controlled exposure study was conducted.143
Volunteer participants (n = 114) were screened for the study. Subjects with asthma were excluded. Subjects were screened and selected for participation if they met the following criteria: history of strong cat sensitivity; positive skin prick test to standardized cat allergen extract; and documented variable response to high vs low levels of Fel d 1 on preliminary testing. Eleven subjects met all criteria and completed the study.
Eight healthy, domestic shorthair cats were fed either a control diet (n = 4) or a test diet (n = 4) composed of the control diet with added egg product ingredient containing anti‐Fel d 1 IgY for 8 weeks. During the last 4 weeks of the study, blankets used by the cats as bedding were collected and used to load the chambers described below.
Portable greenhouse chambers (SpringHouse Clear Growth, FlowerHouse, Inc.) were used as individual exposure chambers to provide a controlled environment for the study. Each chamber was loaded with a blanket (as previously described) from either a control diet‐fed cat (control exposure) or a test diet‐fed cat (test exposure); a fan to circulate air within the chamber; a chair for the participant; and a Petri dish to collect settled dust to determine Fel d1 levels within the chamber.
Participants underwent a priming exposure with high Fel d 1 levels to prime the immune response to the allergen and establish a benchmark for comparison, followed by random assignment to either the control or the test exposure the following week and the opposite exposure 2 weeks later. Exposures lasted 3 hours or until symptoms became intolerable, and patients recorded their symptoms and severity on a Total Nasal Symptom Score (TNSS) and Total Ocular Symptom Score (TOSS) sheet every 15 minutes during the exposure.
TNSS values were significantly reduced in test exposures compared with the priming exposure, but the scores were not statistically different between the priming and control exposures. Mixed model analysis demonstrated that nasal congestion subscores were significantly improved, and other subscores of the TNSS showed nonsignificant trends toward improvement. Although the TOSS score was not significantly reduced, the mixed model analysis showed that the subscores for itchy eyes and scratchy eyes were significantly improved for the test exposures, but not the control exposures, when compared to priming exposures.143
This pilot study demonstrated that feeding cats a diet with an egg product ingredient containing anti‐Fel d 1 IgY decreases the environmental Fel d 1 levels in a controlled environment and produces a significant improvement in Total Nasal Symptom Score and some ocular symptoms in cat‐allergic human subjects. Further research is indicated to determine the efficacy of anti‐Fel d 1 IgY in a home setting.
7. CONCLUSION
When presented with a cat‐allergic, cat‐owning patient, allergists are often compelled to recommend removal of the cat from the home in order to reduce the environmental allergen load and relieve clinical symptoms of allergy. However, this recommendation is often met with resistance because cat owners consider their cats to be members of the family and are not willing to re‐home or relinquish their cat. A new approach, using anti‐Fel d 1 IgY incorporated into the cat's food, reduces immunologically active Fel d 1 in the cat's saliva and on their shed hair and dander, ultimately reducing active Fel d 1 in the environment and improving clinical symptoms in cat‐sensitized individuals. This approach offers healthcare providers an opportunity to reframe their conversations with cat‐allergic patients, allowing a focus on proactive measures without the emotional toll associated with recommending the removal of a beloved cat from the home.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the work.
Satyaraj E, Wedner HJ, Bousquet J. Keep the cat, change the care pathway: A transformational approach to managing Fel d 1, the major cat allergen. Allergy. 2019;74(Suppl. 107):5–17. 10.1111/all.14013
REFERENCES
- 1. Morris DO. Human allergy to environmental pet danders: a public health perspective. Vet Derm. 2010;21:441‐449. [DOI] [PubMed] [Google Scholar]
- 2. Bonnet B, Messaoudi K, Jacomet F, et al. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Clin Immunol. 2018;14(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki S, Nwaru BI, Ekerljung L, et al. Characterization of sensitization to furry animal allergen components in an adult population. Clin Exp Allergy. 2019;49:495‐505. [DOI] [PubMed] [Google Scholar]
- 4. Bousquet PJ, Chinn S, Janson C, Kogevinas M, Burney P, Jarvis D. Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy. 2007;62:301‐309. [DOI] [PubMed] [Google Scholar]
- 5. Zahradnik E, Raulf M. Respiratory allergens from furred mammals: environmental and occupational exposure. Vet Sci. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsolakis N, Malinovschi A, Nordvall L, et al. Sensitization to minor cat allergen components is associated with type‐2 biomarkers in young asthmatics. Clin Exper Allergy. 2017;48:1186‐1194. [DOI] [PubMed] [Google Scholar]
- 7. Chan SK, Leung D. Dog and cat allergies: current state of diagnostic approaches and challenges. Allergy Asthma Immunol Res. 2018;10:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosme‐Blanco W, Arce‐Ayala Y, Malinow I, et al. Primary and secondary environmental control measure for allergic diseases In: Mahmoudi M, Ledford CT, eds. Allergy and Asthma. Warren, MI: Springer International Publishing; 2018:1‐36. [Google Scholar]
- 9. Dávila I, Domínguez‐Ortega J, Navarro‐Pulido A, et al. Consensus document on dog and cat allergy. Allergy. 2018;73:1206‐1222. [DOI] [PubMed] [Google Scholar]
- 10. Tasaniyananda N, Tungtrongchitr A, Seesuay W, et al. Quantification of Fel d 1 in house dust samples of cat allergic patients by using monoclonal antibody specific to a novel IgE‐binding epitope. Asian Pac J Allergy Immunol. 2018;36:8‐15. [DOI] [PubMed] [Google Scholar]
- 11. Pomés A, Chapman MD, Wünschmann S. Indoor allergens and allergic respiratory disease. Curr Allergy Asthma Rep. 2018;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. WHO/IUIS Allergen Nomenclature Sub‐Committee . Allergen Nomenclature: Fel d 1. http://www.allergen.org/viewallergen.php?aid=319. Accessed June 3, 2019.
- 13. Asarnoj A, Hamstein C, Waden K, et al. Sensitization to cat and dog allergen molecules in childhood and prediction of symptoms of cat and dog allergy in adolescence: a BAMSE/MeDALL study. J Allergy Clin Immunol. 2016;137:813‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Academy of Allergy and Clinical Immunology . Molecular Allergology User's Guide. 2016. https://www.eaaci.org/resources/3873-eaaci-molecular-allergology-user-s-guide.html. Accessed June 7, 2019.
- 15. Nicholas C, Wegienka G, Havstad S, Ownby D, Johnson CC. Influence of cat characteristics on Fel d 1 levels in the home. Ann Allergy Asthma Immunol. 2008;101:47‐50. [DOI] [PubMed] [Google Scholar]
- 16. Bastien BC, Gardner C, Satyaraj E. Influence of time and phenotype on salivary Fel d 1 in domestic shorthair cats. J Feline Med Surg. 2019. 10.1177/1098612X19850973. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly SM, Karsh J, Marcelo J, et al. Fel d 1 and Fel d 4 levels in cat fur, saliva and urine. J Allergy Clin Immunol. 2018;142:1990‐1992. [DOI] [PubMed] [Google Scholar]
- 18. Butt A, Rashid D, Lockey RF. Do hypoallergenic cats and dogs exist? Ann Allergy Asthma Immunol. 2012;108:74‐76. [DOI] [PubMed] [Google Scholar]
- 19. Salo PM, Arbes SJ, Jaramillo R, et al. Prevalence of allergic sensitization in the U.S.: Results from the National Health and Nutrition Examination Survey (NHANES) 2005‐2006. J Allergy Clin Immunol. 2018;134:350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wentz PE, Swanson MC, Reed CE. Variability of cat‐allergen shedding. J Allergy Clin Immunol. 1990;85:94‐98. [DOI] [PubMed] [Google Scholar]
- 21. Zielonka TM, Charpin D, Berbis P, et al. Effects of castration and testosterone on Fel d 1 production by sebaceous glands of male cats: 1 – immunological assessment. Clin Exper Allergy. 1994;24:1169‐1173. [DOI] [PubMed] [Google Scholar]
- 22. Jalilcolome J, Deandrade A, Birnbaum J, et al. Sex difference in Fel d 1 allergen production. J Allergy Clin Immunol. 1996;98:165‐168. [DOI] [PubMed] [Google Scholar]
- 23. Plattsmills T, Vervloet D, Thomas W, Aalberse R, Chapman M. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100:S2‐S24. [DOI] [PubMed] [Google Scholar]
- 24. Durairaj R, Pageat P, Bienboire‐Frosini C. Another cat and mouse game: deciphering the evolution of the SCGB superfamily and exploring the molecular similarity of major cat allergen Fel d 1 and mouse ABP using computational approaches. PLoS ONE. 2018;13:e0197618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Almqvist C, Larsson PH, Egmar AC, Hedrén M, Malmberg P, Wickman M. School as a risk environment for children allergic to cats and a site for transfer of cat allergen to homes. J Allergy Clin Immunol. 1999;103:1012‐1017. [DOI] [PubMed] [Google Scholar]
- 26. Almqvist C, Wickman M, Perfetti L, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163:694‐698. [DOI] [PubMed] [Google Scholar]
- 27. Gulbahar O, Sin A, Mete N, Kokuludag A, Kirmaz C, Sebik F. Sensitization to cat allergens in non‐cat owner patients with respiratory allergy. Ann Allergy Asthma Immunol. 2003;90:635‐639. [DOI] [PubMed] [Google Scholar]
- 28. Martin IR, Wickens K, Patchett K, et al. Cat allergen levels in public places in New Zealand. NZ Med J. 1998;111:356‐358. [PubMed] [Google Scholar]
- 29. Niesler A, Ścigala G, Ludzeń‐Izbińska B. Cat (Fel d 1) and dog (Can f 1) allergen levels in cars, dwellings and schools. Aerobiologia. 2016;32:571‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liccardi G, Calzetta L, Baldi G, et al. Allergic sensitization to common pets (cats/dogs) according to different possible modalities of exposure: an Italian multicenter study. Clin Mol Allergy. 2018;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siebers R, Jones B, Bailey L, Aldridge D, Draper J, Ingham T. Indoor allergen exposure in primary school classrooms in New Zealand. NZ Med J. 2019;132:1495. [PubMed] [Google Scholar]
- 32. Grant T, Rule AM, Koehler K, Wood RA, Matsui EC. Sampling devices for indoor allergen exposure: pros and cons. Curr Allergy Asthma Rep. 2019;19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sander I, Lotz A, Neumann HD, et al. Indoor allergen levels in settled airborne dust are higher in day‐care centers than at home. Allergy. 2018;73(6):1263‐1275. [DOI] [PubMed] [Google Scholar]
- 34. Esty B, Phipatanakul W. School exposure and asthma. Ann Allergy Asthma Immunol. 2018;120(5):482‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Permaul P, Hoffman E, Fu C, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol. 2012;23(6):543‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hales BJ, Chai LY, Hazell L, et al. IgE and IgG binding patterns and T‐cell recognition of Fel d 1 and non‐Fel d 1 cat allergens. J Allergy Clin Immunol. 2013;1:656.e5–665.e5. [DOI] [PubMed] [Google Scholar]
- 37. Matthias J, Zielinski CE. Shaping the diversity of Th2 cell responses in epithelial tissues and its potential for allergy treatment. Eur J Immunol. 2019. 10.1002/eji.201848011. [DOI] [PubMed] [Google Scholar]
- 38. Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nature Comm. 2018;9:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinet JP. The high‐affinity IgE receptor (FcεRI): from physiology to pathology. Annu Rev Immunol. 1999;17:931‐972. [DOI] [PubMed] [Google Scholar]
- 40. Krystel‐Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi‐functional master cell. Front Immunol. 2016;6:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murphy K, Weaver C. Janeway's Immunbiology. New York, NY: Garland Science; 2017:601‐637. [Google Scholar]
- 42. Nopp A, Johansson S, Lundberg M, Oman H. Simultaneous exposure of several allergens has an additive effect on multisensitized individuals. Allergy. 2006;61:1366‐1368. [DOI] [PubMed] [Google Scholar]
- 43. Konradsen JR, Fujisawa T, van Hage M, et al. Allergy to furry animals: New insights, diagnostic approaches, and challenges. J Allergy Clin Immunol. 2015;135(3):616‐625. [DOI] [PubMed] [Google Scholar]
- 44. Dhami S, Agarwal A. Does evidence support the use of cat allergen immunotherapy? Curr Opin Allergy Clin Immunol. 2018;18(4):350‐355. [DOI] [PubMed] [Google Scholar]
- 45. Burns K. Pet ownership stable, veterinary care variable. https://www.avma.org/News/JAVMANews/Pages/190115a.aspx. Accessed June 3, 2019.
- 46. Oluwole O, Arinola GO, Ana GR, et al. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant‐antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Glob J Health Sci. 2013;5(4):28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kabengele BO, Kayembe J‐M, Kayembe PK, Kashongue ZM, Kaba DK, Akilimali PZ. Factors associated with uncontrolled asthma in adult asthmatics in Kinshasa, Democratic Republic of Congo. PLoS ONE. 2019;14:e0215530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nissen SP, Kjaer HF, Host A, Nielsen J, Halken S. The natural course of sensitization and allergic diseases from childhood to adulthood. Pediatr Allergy Immunol. 2013;24(6):549‐555. [DOI] [PubMed] [Google Scholar]
- 49. Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29(5):611‐617. [DOI] [PubMed] [Google Scholar]
- 50. Nwaru BI, Suzuki S, Ekerljung L, et al. Furry animal allergen component sensitization and clinical outcomes in adult asthma and rhinitis. J Allergy Clin Immunol Pract. 2019;7(4):1230.e4‐1238.e4. [DOI] [PubMed] [Google Scholar]
- 51. Bjerg A, Winberg A, Berthold M, Mattsson L, Borres MP, Ronmark E. A population‐based study of animal component sensitization, asthma, and rhinitis in schoolchildren. Pediatr Allergy Immunol. 2015;26(6):557‐563. [DOI] [PubMed] [Google Scholar]
- 52. Konradsen JR, Nordlund B, Onell A, Borres MP, Gronlund H, Hedlin G. Severe childhood asthma and allergy to furry animals: refined assessment using molecular‐based allergy diagnostics. Pediatr Allergy Immunol. 2014;25(2):187‐192. [DOI] [PubMed] [Google Scholar]
- 53. Leynaert B, Neukirch C, Liard R, Bousquet J, Neukirch F. Quality of life in allergic rhinitis and asthma. Am J Respir Crit Care Med. 2000;162:1391‐1396. [DOI] [PubMed] [Google Scholar]
- 54. Canonica GW, Mullol J, Pradalier A, Didier A. Patient perceptions of allergic rhinitis and quality of life: findings from a survey conducted in Europe and the United States. World Allergy Organ J. 2008;1:138‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bousquet J, Anto JM, Annesi‐Maesano I, et al. POLLAR: impact of air POLLution on asthma and rhinitis; a European Institute of Innovation and Technology Health (EIT) Project. Clin Transl Allergy. 2018;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bousquet J, Arnavielhe S, Bedbrook A, et al. MASK 2017: ARIA digitally‐enabled, integrated, person‐centred care for rhinitis and asthma multimorbidity using real‐world‐evidence. Clin Trans Allergy. 2018;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brown LC, Slavin RG. Improving quality of life in patients with allergic rhinitis: The pharmacist's role. Pharmacy Times. 2005: available at https://www.pharmacytimes.com/publications/issue/2005/2005-06/2005-06-9606. Accessed March 1, 2019.
- 58. Gergen PJ, Mitchell HE, Calatroni A, et al. Sensitization and exposure to pets: the effect on asthma morbidity in the US population. J Allergy Clin Immunol Pract. 2018;6:101‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Human‐Animal Bond Research Initiative . The Pet Effect 2018. available at https://habri.org/the-pet-effect. Accessed June 7, 2019.
- 60. Turner DC. A review of over three decades of research on cat‐human and human‐cat interactions and relationships. Behav Processes. 2017;141:297‐304. [DOI] [PubMed] [Google Scholar]
- 61. Arahori M, Kuroshima H, Hori Y, Takagi S, Chijiiwa H, Fujita K. Owners' view of their pets' emotions, intellect, and mutual relationship: Cats and dogs compared. Behav Processes. 2017;141:316‐321. [DOI] [PubMed] [Google Scholar]
- 62. Pongrácz P, Szapu JS. The socio‐cognitive relationship between cats and humans – Companion cats (Felis catus) as their owners see them. Appl Anim Behav Sci. 2018;207:57‐66. [Google Scholar]
- 63. Finka LR, Ward J, Farnworth MJ, Mills DS. Owner personality and the well‐being of their cats share parallels with the parent‐child relationship. PLoS ONE. 2019;14:e0211862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Safdar K. My cat allergy is killing me, but Cupcake stays. Wall Street Journal. https://www.wsj.com/articles/my-cat-allergy-is-killing-me-but-cupcake-stays-11553784452. Accessed June 3, 2019.
- 65. American Veterinary Medical Association . Human‐Animal Bond. https://www.avma.org/KB/Resources/Reference/human-animal-bond/Pages/Human-Animal-Bond-AVMA.aspx. Accessed June 7, 2019.
- 66. Adamelli S, Marinelli L, Normando S, Bono G. Owner and cat features influence the quality of life of the cat. Appl Anim Behav Sci. 2005;94:89098. [Google Scholar]
- 67. Vitale Shreve KR, Mehrkam LR, Udell M. Social interaction, food, scent or toys? A formal assessment of domestic pet and shelter cat (Felis silvestris catus) preferences. Behav Processes. 2017;141:322‐328. [DOI] [PubMed] [Google Scholar]
- 68. Weiss E, Gramann S, Drain N, Dolan E, Slater M. Modification of the feline‐ality assessment and the ability to predict adopted cats' behaviors in their new homes. Animals. 2015;5:71‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zito S, Morton J, Vankan D, et al. Reasons people surrender unowned and owned cats to Australian animal shelters and barriers to assuming ownership of unowned cats. J Appl Anim Welf Sci. 2016;19:303‐319. [DOI] [PubMed] [Google Scholar]
- 70. Coe JB, Young I, Lambert K, Dysart L, Nogueira Borden L, Rajić A. A scoping review of published research on the relinquishment of companion animals. J Appl Anim Welf Sci. 2014;17:253‐273. [DOI] [PubMed] [Google Scholar]
- 71. Eriksson P, Loberg J, Andersson M. A survey of cat shelters in Sweden. Anim Welf. 2009;18:283‐288. [Google Scholar]
- 72. American Humane Association . Keeping pets (dogs and cats) in homes: a three‐phase retention study. Phase I: reasons for not owning a dog or cat. 2012. https://www.americanhumane.org/app/uploads/2016/08/aha-petsmart-retention-study-phase-1.pdf. Accessed June 3, 2019.
- 73. Svanes C, Zock J, Anto J, et al. Do asthma and allergy influence subsequent pet keeping? An analysis of childhood and adulthood. J Allergy Clin Immunol. 2018;118:691‐698. [DOI] [PubMed] [Google Scholar]
- 74. Uriarte SA, Sastre J. Clinical relevance of molecular diagnosis in pet allergy. Allergy. 2016;71(7):1066‐1068. [DOI] [PubMed] [Google Scholar]
- 75. Wickman M, Egmar A‐C, Emenius G, et al. When allergies complicate allergies. Allergy. 2005;60(S79):14‐18. [DOI] [PubMed] [Google Scholar]
- 76. MacGlashan D. IgE and FcεRI regulation. Ann NY Acad Sci. 2005;1050:73‐88. [DOI] [PubMed] [Google Scholar]
- 77. Brożek JL, Bousquet J, Baena‐Cagnani CE, et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466‐476. [DOI] [PubMed] [Google Scholar]
- 78. Warner JO. Use of temperature‐controlled laminar airflow in the management of atopic asthma: clinical evidence and experience. Ther Adv Respir Dis. 2017;11(4):181‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wood RA, Chapman MD, Adkinson NF, Eggleston PA. The effect of cat removal on allergen content in household‐dust samples. J Allergy Clin Immunol. 1989;83:730‐734. [DOI] [PubMed] [Google Scholar]
- 80. Kelly LA, Erwin EA, Platts‐Mills TA. The indoor air and asthma: the role of cat allergens. Curr Opin Pulm Med. 2012;18(1):29‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sheridan K. Don't hold your breath for allergy‐free cats. MIT Biotechnology Review. 2018. https://www.technologyreview.com/s/611671/dont-hold-your-breath-for-allergy-free-cats/. Accessed July 12, 2019.
- 82. Steina Björnsdottir U, Jakobinudottir S, Runarsdottir V, Juliusson S. The effect of reducing levels of cat allergen (Fel d 1) on clinical symptoms in patients with cat allergy. Ann Allergy Asthma Immunol. 2003;91:189‐194. [DOI] [PubMed] [Google Scholar]
- 83. Wood R, Johnson E, Van natta M, Chen P, Eggleston P. A placebo‐controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158:115‐120. [DOI] [PubMed] [Google Scholar]
- 84. Gore RB, Bishop S, Durrell B, Curbishley L, Woodcock A, Custovic A. Air filtration units in homes with cats: can they reduce personal exposure to cat allergen? Clin Exp Allergy. 2003;33:765‐769. [DOI] [PubMed] [Google Scholar]
- 85. Wickman M, Egmar AC, Emenius G, et al. Fel d 1 and Can f 1 in settled dust and airborne Fel d 1 in allergen avoidance day‐care centers for atopic children in relation to number of pet‐owners, ventilation and general cleaning. Clin Exp Allergy. 1999;29:626‐632. [DOI] [PubMed] [Google Scholar]
- 86. Avner DB, Perzanowski MS, Platts‐Mills TAE, Woodfolk JA. Evaluation of different techniques for washing cats: Quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J Allergy Clin Immunol. 1997;100:307‐312. [DOI] [PubMed] [Google Scholar]
- 87. Nageotte C, Park M, Havstad S, Zoratti E, Ownby D. Duration of airborne Fel d 1 reduction after cat washing. J Allergy Clin Immunol. 2006;118:521‐522. [DOI] [PubMed] [Google Scholar]
- 88. Sirin Kose S, Atakul G, Asilsoy S, Karaman O, Uzuner N, Anal O. Efficacy of allergen‐blocker mechanical barrier gel on symptoms and quality of life in patients with allergic rhinitis. Eur Arch Otorhinolaryngol. 2018;276:729‐734. [DOI] [PubMed] [Google Scholar]
- 89. Bachert C, Bousquet J, Hellings P. Rapid onset of action and reduced nasal hyperreactivity: new targets in allergic rhinitis management. Clin Transl Allergy. 2018;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Norman PS, Ohman JL, Long AA, et al. Treatment of cat allergy with T‐cell reactive peptides. Am J Respir Crit Care Med. 1996;154(6 Pt 1):1623‐1628. [DOI] [PubMed] [Google Scholar]
- 91. Couroux P, Patel D, Armstrong K, Larche M, Hafner RP. Fel d 1‐derived synthetic peptide immuno‐regulatory epitopes show a long‐term treatment effect in cat allergic subjects. Clin Exp Allergy. 2015;45(5):974‐981. [DOI] [PubMed] [Google Scholar]
- 92. Neighbour H, Soliman M, Steacy LM, et al. The allergic rhinitis clinical investigator collaborative (AR‐CIC): verification of nasal allergen challenge procedures in a study utilizing an investigational immunotherapy for cat allergy. Clin Transl Allergy. 2018;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pene J, Desroches A, Paradis L, et al. Immunotherapy with Fel d 1 peptides decreases IL‐4 release by peripheral blood T cells of patients allergic to cats. J Allergy Clin Immunol. 1998;102(4 Pt 1):571‐578. [DOI] [PubMed] [Google Scholar]
- 94. Moldaver DM, Bharhani MS, Rudulier CD, Wattie J, Inman MD, Larche M. Induction of bystander tolerance and immune deviation after Fel d 1 peptide immunotherapy. J Allergy Clin Immunol. 2019;143(3): 1087.e4‐1099.e4. [DOI] [PubMed] [Google Scholar]
- 95. Lødrup Carlsen KC, Roll S, Carlsen K‐H, et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS ONE. 2012;7(8):e43214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bousquet J, Addis A, Adcock I, et al. Integrated care pathways for airway diseases (AIRWAYS‐ICPs). Eur Resp J. 2014;44:304‐323. [DOI] [PubMed] [Google Scholar]
- 97. Overill S. A practical guide to care pathways. J Integr Care. 1998;2:93‐98. [Google Scholar]
- 98. How to produce and evaluate an integrated care pathway (ICP): information for staff. Great Ormond Street Hospital for Children. http://wwwgoshnhsuk. 2010.
- 99. Integrated Care Pathways Users in Scotland (ICPUS) . A workbook for people starting to develop integrated care pathways. 2007: 23‐54.
- 100. Campbell SM, Reeves D, Kontopantelis E, Sibbald B, Roland M. Effects of pay for performance on the quality of primary care in England. N Engl J Med. 2009;361(4):368‐378. [DOI] [PubMed] [Google Scholar]
- 101. Kirschner K, Braspenning J, Akkermans RP, Annelies Jacobs JE, Grol R. Assessment of a pay‐for‐performance program in primary care designed by target users. Fam Pract. 2012;30(2):161‐171. [DOI] [PubMed] [Google Scholar]
- 102. Rodriguez HP, Perry L, Conrad DA, Maynard C, Martin DP, Grembowski DE. The reliability of medical group performance measurement in a single insurer's pay for performance program. Med Care. 2012;50(2):117‐123. [DOI] [PubMed] [Google Scholar]
- 103. Pinnock H, Burton C, Campbell S, et al. Clinical implications of the Royal College of Physicians three questions in routine asthma care: a real‐life validation study. Prim Care Respir J. 2012;21(3):288‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ouwens M, Hulscher M, Hermens R, et al. Implementation of integrated care for patients with cancer: a systematic review of interventions and effects. Int J Qual Health Care. 2009;21(2):137‐144. [DOI] [PubMed] [Google Scholar]
- 105. Chan R, Webster J. End‐of‐life care pathways for improving outcomes in caring for the dying. Cochrane Database Syst Rev. 2010;1:CD008006. [DOI] [PubMed] [Google Scholar]
- 106. Bousquet J, Devillier P, Arnavielhe S, et al. Treatment of allergic rhinitis using mobile technology with real‐world data: the MASK observational pilot study. Allergy. 2018;73(9):1763‐1774. [DOI] [PubMed] [Google Scholar]
- 107. Bousquet J, Bedbrook A, Czarlewski W, et al. Guidance to 2018 good practice: ARIA digitally‐enabled, integrated, person‐centred care for rhinitis and asthma. Clin Transl Allergy. 2019;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bousquet J, Hellings PW, Agache I, et al. Allergic rhinitis and its impact on asthma (ARIA) Phase 4 (2018): change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. 2019;143(3):864‐879. [DOI] [PubMed] [Google Scholar]
- 109. Bousquet J, Onorato GL, Bachert C, et al. CHRODIS criteria applied to the MASK (MACVIA‐ARIA Sentinel NetworK) good practice in allergic rhinitis: a SUNFRAIL report. Clin Transl Allergy. 2017;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 111. Bousquet J, van Cauwenberge P, Khaltaev N; Members of the Workshops . ARIA in the pharmacy: management of allergic rhinitis symptoms in the pharmacy. Allergic Rhinitis and its impact on asthma. Allergy. 2004;59(4):373‐387. [DOI] [PubMed] [Google Scholar]
- 112. Bousquet J, Neukirch F, Bousquet P, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care. J Allergy Clin Immunol. 2006;117(1):158‐162. [DOI] [PubMed] [Google Scholar]
- 113. Bousquet J, Annesi‐Maesano I, Carat F, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. 2005;35(6):728‐732. [DOI] [PubMed] [Google Scholar]
- 114. Bousquet PJ, Devillier P, Tadmouri A, Mesbah K, Demoly P, Bousquet J. Clinical relevance of cluster analysis in phenotyping allergic rhinitis in a real‐life study. Int Arch Allergy Immunol. 2015;166(3):231‐240. [DOI] [PubMed] [Google Scholar]
- 115. del Cuvillo A, Montoro J, Bartra J, et al. Validation of ARIA duration and severity classifications in Spanish allergic rhinitis patients ‐ The ADRIAL cohort study. Rhinology. 2010;48(2):201‐205. [DOI] [PubMed] [Google Scholar]
- 116. Jáuregui I, Dávila I, Sastre J, et al. Validation of ARIA (Allergic Rhinitis and its Impact on Asthma) classification in a pediatric population: the PEDRIAL study. Pediatr Allergy Immunol. 2011;22(4):388‐392. [DOI] [PubMed] [Google Scholar]
- 117. Whitmee S, Haines A, Beyrer C, et al. Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation‐Lancet Commission on planetary health. Lancet. 2015;386(10007):1973‐2028. [DOI] [PubMed] [Google Scholar]
- 118. Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: a randomised trial of mite impermeable bedcovers. Am J Respir Crit Care Med. 2017;196(2):150‐158. [DOI] [PubMed] [Google Scholar]
- 119. Pezzali JG, Smith SC, Aldrich CG. An overview of the effect of diet on the allergenicity of cats to susceptible humans. SOJ Vet Sci. 2018;4:1‐9. [Google Scholar]
- 120. Thoms F, Jennings GT, Maudrich M, et al. Immunization of cats to induce neutralizing antibodies against Fel d1, the major feline allergen in human subjects. J Allergy Clin Immunol. 2019;144(1):193‐203. [DOI] [PubMed] [Google Scholar]
- 121. Morgenstern JP, Griffith IJ, Brauer AW, et al. Amino acid sequence of Fel d 1, the major allergen of the domestic cat: Protein sequence analysis and cDNA cloning. Proc Natl Acad Sci. 1991;88:9690‐9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kaiser L, Grönlund H, Sandalova T, et al. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J Biol Chem. 2003;278:37730‐37735. [DOI] [PubMed] [Google Scholar]
- 123. Kaiser L, Grönlund H, Sandalova T, et al. Three‐dimensional structure of Fel d 1, the major allergen in cat. Int Arch Allergy Immunol. 2003;132:25‐26. [DOI] [PubMed] [Google Scholar]
- 124. Griffith IJ, Craig S, Pollock J, et al. Expression and genomic structure of the genes encoding Fel d 1, the major cat allergen from the domestic cat. Gene. 1992;113:263‐268. [DOI] [PubMed] [Google Scholar]
- 125. Bienboire‐Frosini C, Lebrun R, Vervloet D, Pageat P, Ronin C. Distribution of core fragments from the major cat allergen Fel d 1 is maintained among the main anatomical sites of production. Int Arch Allergy Immunol. 2010;152:197‐206. [DOI] [PubMed] [Google Scholar]
- 126. Rogers BL, Morgenstern JP, Garman RD, et al. Recombinant Fel d 1: expression, purification, IgE binding and reaction with cat‐allergic human T cells. Molec Immunol. 1993;30:559‐568. [DOI] [PubMed] [Google Scholar]
- 127. Duffort OA, Carreira J, Nitti G, et al. Studies on the biochemical structure of the major cat allergen Felis Domesticus 1. Molec Immunol. 1991;28:301‐309. [DOI] [PubMed] [Google Scholar]
- 128. Bond JF, Brauer AW, Segal DB, Nault AK, Rogers BL, Kuo MC. Native and recombinant Fel d 1 as probes into the relationship of allergen structure to human IgE immunoreactivity. Molec Immunol. 1993;30:1529‐1541. [DOI] [PubMed] [Google Scholar]
- 129. Ring PC, Wan H, Schou C, Kroll Kristensen A, Roepstorff P, Robinson C. The 18‐kDa form of cat allergen Felis domesticus (Fel d 1) is associated with gelatin‐ and fibronectin‐degrading activity. Clin Exper Allergy. 2000;30:1085‐1906. [DOI] [PubMed] [Google Scholar]
- 130. Emara M, Royer PJ, Abbas Z, et al. Recognition of the major cat allergen Fel d 1 through the cysteine‐rich domain of the mannose receptor determines its allergenicity. J Biol Chem. 2011;286:13033‐13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. van Milligen FJ, van't Hof W,van den Berg M,Aalberse RC. IgE epitopes on the cat (Felis domesticus) major allergen Fel d 1: a study with overlapping synthetic peptides. J Allergy Clin Immunol. 1994;93:34‐43. [DOI] [PubMed] [Google Scholar]
- 132. Tasaniyananda N, Tungtrongchitr A, Seesuay W, et al. A novel IgE‐binding epitope of the major cat allergen, Fel d 1. Biochem Biophys Res Comm. 2016;470:593‐598. [DOI] [PubMed] [Google Scholar]
- 133. Satyaraj E, Sun P, Sherrill S. Fel d1 blocking antibodies against the major cat allergen Fel d 1 Poster presented at: European Academy of Allergy and Clinical Immunology; June 1‐5, 2019; Lisbon, Portugal. [Google Scholar]
- 134. Hamal KR, Burgess SC, Pevzner IY, Erf GF. Maternal antibody transfer from dams to their egg yolks, egg whites, and chicks in meat lines of chickens. Poultry Sci. 2006;85:1364‐1372. [DOI] [PubMed] [Google Scholar]
- 135. Bedrani L, Helloin E, Guyot N, Réhault‐Godbert S, Nys Y. Passive maternal exposure to environmental microbes selectively modulates the innate defences of chicken egg white by increasing some of its antibacterial activities. BMC Microbiol. 2013;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Satyaraj E, Li Q, Sun P, et al. Anti‐Fel d 1 immunoglobulin Y antibody‐containing egg ingredient lowers allergen levels in cat saliva. J Fel Med Surg. 2019. 10.1177/1098612X19861218. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Satyaraj E, Gardner C, Filipi I, Cramer K, Sherrill S. Reduction of active Fel d 1 from cats using an antiFel d 1 egg IgY antibody. Immun Inflamm Dis. 2019;7:68‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Karlsson M, Kollberg H, Larsson A. Chicken IgY: utilizing the evolutionary difference. Worlds Poult Sci J. 2004;60:341‐348. [Google Scholar]
- 139. Van Nguyen S, Umeda K, Yokoyama H, Tohya Y, Kodama Y. Passive protection of dogs against clinical disease due to Canine parvovirus‐2 by specific antibody from chicken egg yolk. Can J Vet Res. 2006;70:62‐64. [PMC free article] [PubMed] [Google Scholar]
- 140. Rahman S, Van Nguyen SA, Icatlo FC Jr, Umeda K, Kodama Y. Oral passive IgY‐based immunotherapeutics. Human Vacc Immunother. 2013;9:1039‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz‐Asplund J, Terzolo HR. Chicken egg yolk antibodies (IgY‐technology): a review of progress in production and use in research and human and veterinary medicine. ATLA, Alt Lab Anim. 2005;33:129‐154. [DOI] [PubMed] [Google Scholar]
- 142. Matulka RA, Thompson L, Corley D. Multi‐level safety studies of anti‐Fel d 1 IgY ingredient in cat food. Frontiers in Veterinary Pharmacology and Toxicology (in review). [DOI] [PMC free article] [PubMed]
- 143. Wedner HJ, Satyaraj E, Gardner C, et al. Pilot study to determine the effect of feeding cat food made with egg product containing anti‐Fel d 1 antibodies to cats on human allergy symptoms Poster presented at: European Academy of Allergy and Clinical Immunology; June 1–5, 2019; Lisbon, Portugal. [Google Scholar]


