Abstract
Study Objectives
Sleep problems are a core feature of post-traumatic stress disorder (PTSD). The aim of this study was to find a robust objective measure for the sleep disturbance in patients having PTSD.
Methods
The current study assessed EEG power across a wide frequency range and multiple scalp locations, in matched trauma-exposed individuals with and without PTSD, during rapid eye movement (REM) and non-REM (NREM) sleep. In addition, a full polysomnographical evaluation was performed, including sleep staging and assessment of respiratory function, limb movements, and heart rate. The occurrence of sleep disorders was also assessed.
Results
In patients having PTSD, NREM sleep shows a substantial loss of slow oscillation power and increased higher frequency activity compared with controls. The change is most pronounced over right-frontal sensors and correlates with insomnia. PTSD REM sleep shows a large power shift in the opposite direction, with increased slow oscillation power over occipital areas, which is strongly related to nightmare activity and to a lesser extent with insomnia. These pronounced spectral changes occur in the context of severe subjective sleep problems, increased occurrence of various sleep disorders and modest changes in sleep macrostructure.
Conclusions
This is the first study to show pronounced changes in EEG spectral topologies during both NREM and REM sleep in PTSD. Importantly, the observed power changes reflect the hallmarks of PTSD sleep problems: insomnia and nightmares and may thus be specific for PTSD. A spectral index derived from these data distinguishes patients from controls with high effect size, bearing promise as a candidate biomarker.
Keywords: post-traumatic stress disorder, sleep, polysomnography, quantitative electroencephalography, spectral analysis
Statement of Significance.
Here, we investigate sleep disturbances in post-traumatic stress disorder (PTSD) and present evidence for an objective neural marker for PTSD sleep problems. Sleep EEG spectral power across the brain and polysomnographical results are compared in matched trauma-exposed individuals with and without PTSD. During non-REM sleep, PTSD patients show a substantial loss of slow oscillation power, yet increased power for higher frequencies. These effects appear over frontal areas and correlate with insomnia. PTSD REM sleep shows increased slow oscillation power over occipital areas. The latter effects positively correlate to nightmare activity and insomnia. A spectral index derived from these data distinguishes patients from controls with high effect size, bearing promise as a candidate biomarker for PTSD.
Introduction
Post-traumatic stress disorder (PTSD) is a highly debilitating disorder with a lifetime prevalence of 7%–8% [1, 2]. Sleep problems are the most prevalent symptoms of PTSD with roughly 70% of patients experiencing co-occurring sleep disorders [3, 4]. The sleep problems typically include nightmares, distressed awakenings, nocturnal panic attacks, sleep terrors, and insomnia [5].
Sleep abnormalities following trauma are a strong predictor for future development of PTSD [6–9]. However, sleep abnormalities may also pre-date trauma [10] and then also predict subsequent PTSD [9]. These and other findings suggest that sleep disturbances play an important role in the development and maintenance of PTSD [11]. This role may be related to sleep’s crucial involvement in memory consolidation [12, 13], reduction of memories’ emotional tone [14–17] and emotional regulation in general [14, 18, 19].
While the subjective sleep impairments in PTSD are well established, the underlying physiological abnormalities are less clear. Several studies have focused on the macrostructure of sleep, measuring variables such as the amount of time slept, periods of wakefulness after sleep-onset and the amount of time spent in the different sleep stages. A meta-analysis of such studies reports an increase in stage 1 sleep (the lightest sleep stage), decreased slow-wave sleep (SWS) or deep sleep, and increased density of rapid eye movements (REMs) in PTSD patients [20]. The reported average effect sizes are modest, however, with small effects for stage 1 (d = 0.24) and SWS (d = −0.28), and a small to medium effect for REM density (d = 0.43).
While sleep stages are informative about the temporal organization of sleep, they have limited value for quantifying the spectral content of the sleep electroencephalography (EEG). Sleep staging is by definition a categorical analysis in which a large amount of variance with regard to the frequency content of the signal goes undetected. Moreover, sleep staging regards neuronal population dynamics as a whole-brain state, resulting in a spatially globalized analysis. However, EEG-recorded activity varies substantially across different regions of the brain, during sleeping and waking alike. This spatial variance is largely disregarded in standard polysomnography.
A more precise way to quantify the frequency content of the EEG entails the analysis of spectral power. A few controlled studies of PTSD sleep report on such analyses, albeit only for recordings on single, often central, derivations [21–26]. The most consistent observation in these studies is reduced non-REM (NREM) delta (0.5–4 Hz) activity in patients [23–25]. Activity in this frequency range indexes sleep depth, suggesting reduced sleep depth in PTSD, in line with the findings of reduced SWS. However, as with the macroscopic findings, reduced delta activity is not consistently observed in all studies [22, 27]. Thus, none of the physiological sleep measures assessed thus far provides a reliable correlate of the severe subjective sleep problems in PTSD.
The primary aim of the current study was to obtain a better understanding of the neural underpinnings of PTSD sleep disturbances, through the assessment of spatially distributed brain activity. Secondly, we hoped to find a robust neural correlate of PTSD sleep problems, leading the way toward the development of a biomarker. Such a biomarker would importantly facilitate further research on PTSD, including the objective evaluation of (sleep-oriented) therapeutic strategies.
With these goals in mind, we performed a comprehensive investigation of sleep in patients with PTSD and matched, traumatized participants without PTSD. Central to the investigation was a spectral analysis of the sleep EEG captured over four, widely spaced, electrode locations (as compared to the most common method in sleep EEG, which captures only a single location). We considered a broad frequency range (0.5–50 Hz) and adopted a division into frequency bands in line with state-of-the-art knowledge on the neural underpinnings of oscillatory population dynamics. In particular, we separated the 0.5–4 Hz delta band considered in previous studies, into slow oscillations (SOs: 0.5–1.5 Hz), which have a cortical origin [28–30], and higher delta frequencies (1.5–4 Hz), which have a thalamic origin [31]. The range of the SO band was based on the maxima in EEG power spectra of young adult humans during normal nocturnal sleep, which show a broad peak centered around 0.7–0.8 Hz [32].
As an a priori hypothesis, we considered that SO dynamics might be disrupted in PTSD. Indeed, SOs [33] orchestrate sleep-related cortical communication [34] and have been shown to play an important role in (emotional) memory consolidation [35, 36] and emotional resilience [14]. As such, their disruption might lead to abnormal trauma memory consolidation and impaired recovery from emotional trauma. Furthermore, SO dynamics are most pronounced in EEG recordings over frontal cortical areas. The moderate reductions of 0.5–4 Hz power, observed over central locations previously, might actually reflect a more frontally centered SO power deficit, picked up in the diluted form.
While the above hypothesis concerns NREM sleep, the subjective sleep complaints associated with PTSD are likely associated with both NREM and REM sleep. Insomnia, commonly found in PTSD patients, has been associated with disruptions in NREM sleep and sometimes REM sleep (although the changes in polysomnography derived variables tend to be less pronounced than expected based on subjective reports [37]). Nightmares primarily occur during REM sleep [3, 4], although they have also been described to occur during N2 in patients with PTSD [38].
In addition to the EEG spectral analysis, a full sleep physiological evaluation (polysomnography) was performed, including visual sleep staging, assessments of limb movements, respiratory, and cardiac function. Furthermore, we evaluated subjective sleep quality and the presence of sleep disorders by means of validated questionnaires. These measures allowed us to relate any abnormalities in brain activity to sleep symptomatology.
Method
Participants
Participants in the experiment were traumatized police officers and military veterans, with (N = 16) or without (N = 14) PTSD. Participants with PTSD were recruited at ARQ Centrum‘45, the Dutch national center for diagnostics and treatment of PTSD, part of ARQ National Psychotrauma Centre. Participants all met the criteria for chronic PTSD and five participants met the criteria for delayed-onset PTSD. Participants in the PTSD and control group were matched on age, gender, education level, and professional background (Table 1). No differences in alcohol-related disorders and use were found between groups (Table 1). Participants were asked to refrain from medication use prior to the experiment. However, in seven PTSD patients medication (SSRIs, antipsychotics, anti-depressants, sedatives or hypnotics) could, for medical ethical reasons, not be interrupted. All participants gave written informed consent. For additional details see Supplementary materials.
Table 1.
Sociodemographic and clinical characteristics of participants with PTSD and trauma-exposed controls
| Characteristics | PTSD group | Control group |
|---|---|---|
| Professional background (n, %) | ||
| Police | 12 (75%) | 10 (71%) |
| Veteran | 4 (25%) | 4 (29%) |
| Mean age (SD) | 45.6 (7.9) | 44.4 (8.7) |
| Gender (n, %) | ||
| Male | 15 (94%) | 13 (93%) |
| Female | 1 (6%) | 1 (7%) |
| Educational level (n, %) | ||
| Lower vocational education | 3 (19%) | 0 (0%) |
| Middle vocational education | 10 (63%) | 11 (79%) |
| Higher vocational education | 3 (19%) | 3 (21%) |
| Clinical characteristics | ||
| CAPS score (mean, SD) | 82.8 (11.6) | 5.3 (4.7) |
| Alcohol dependence (n, %) | 0 (0%) | 0 (0%) |
| Alcohol abuse (n, %) | 0 (0%) | 1 (7%) |
| Number of drinks per week (mean, SD) | 2.4 (3.3) | 4.6 (4.5) |
CAPS, clinical-administered PTSD scale.
Clinical assessments and sleep-related questionnaires
The presence or absence of PTSD according to DSM-IV criteria was diagnosed with the Clinician Administered PTSD Scale (CAPS), following assessment of trauma history with the Life Events Checklist [39]. We used the CAPS score on item B2 as an indicator of nightmare severity (combined score of frequency and intensity). Past and present comorbid psychiatric disorders according to DSM-IV criteria were assessed with the MINI-PLUS clinical interview [40].
The presence of sleep disorders that were not physiologically evaluated (see next section) was assessed with the SLEEP-50 [41]. The subjective sleep quality on the night of the polysomnography was assessed the morning after, with the Dutch Sleep Quality Scale [42].
Polysomnography and general procedure
All experimentation, including sleep recording, was performed at the in-patient facility of ARQ Centrum’45. The current study is part of a larger registered clinical project ([43]; for details see Supplementary Materials). Participants slept at the department twice, in the context of a broad diagnostic assessment before treatment onset. Data for the current study was recorded on one of the two nights, with the order of the nights counterbalanced over participants and disease status (PTSD, trauma-control) through semi-randomization. Subjective sleep quality did not differ significantly between the two nights, either in participants with PTSD or in trauma-controls (p’s > 0.1). All reported clinical and sleep diagnostics were obtained within six to eight weeks of the sleep recording.
Polysomnographic data was recorded successfully for 13 participants with and 14 without PTSD. Participants were given the opportunity to sleep undisturbed for 9 hours during a lights-off period starting between 11 and 12 pm, depending on habitual sleep times. Polysomnography, using ambulatory 16-channel Porti amplifiers (TMS-i) and Galaxy sleep analysis software (PHI-international), consisted of EEG recording (F3, F4, C4 and O2, referenced to average mastoids), two EOG electrodes monitoring eye-movements, and two for submental EMG. Further sensors were ECG monitoring heart rate, plethysmography monitoring blood oxygenation, piezo leg sensors to detect leg movements, probes measuring nasal airflow, and a piezo respiratory band for the thoracic respiratory effort to monitor breathing and sleep apnea. The sample rate for all signals was 512 Hz.
Data analysis
Sleep stages were scored visually according to the American Academy of Sleep Medicine (AASM) criteria [44]. For each recording, we calculated total sleep time, sleep latency, REM latency, wake after sleep onset, and sleep efficiency. We also determined the amounts of light sleep (N1+N2), SWS (N3) and REM sleep, in minutes and as a percentage of total sleep time.
The frequency content of the EEG was analyzed using fast Fourier transform-based spectral analysis (4s time windows with 50% overlap, 0.25Hz bin size; Hamming window), on each electrode (F3, F4, C4 and O2) for NREM sleep and REM sleep separately. Through visual inspection of the data, EEG epochs containing artifacts were removed. Next, for each frequency bin, the power per 30s epoch was computed and summated over all epochs in the same sleep stage. Normalized power for each sleep stage was calculated dividing power per frequency bin by total power in the 0.5-50Hz range. Finally, normalized power bins were merged across frequencies in each of the following bands: SOs (0.5–1.5Hz), delta (1.5–4Hz), theta (4–8Hz), alpha (8–12Hz), sigma (12–16Hz), beta (16–30Hz) and gamma (30–50Hz).
Apneas and hypopneas, oxygen desaturations, leg movements and R-peaks in the ECG were automatically scored (Galaxy, PHI-international) and manually checked. From these measures an apnea/hypopnea index, oxygen saturation index, periodic leg movement index, leg movement index, and heart rate were calculated (details in Supplementary materials).
Sleep macrostructure and non-EEG physiological variables were statistically analyzed using independent samples t-tests (two-tailed) or Mann–Whitney U tests. Relative spectral power was log-transformed to achieve a Gaussian distribution and analyzed taking into account unequal variances between power variables for different frequency bands. The full data set was analyzed through mixed design MANOVA with planned comparisons. Factors in the design were Diagnosis (PTSD, trauma-control), Sleep State (NREM, REM), and Electrode (F3, F4, C4, O2), entering power in each frequency band as a separate dependent variable (thus avoiding sphericity violations) and using Roy’s largest root as test statistic. This first-level analysis was followed by ANOVA on the individual dependent variables and analyses of planned contrasts for significant ANOVA effects. The model contained all main and interaction effects of the factor Diagnosis. Correlation analyses were performed using Spearman’s rho. Finally, effect sizes were calculated as Glass’ delta [45].
Results
Sleep quality and sleep disorders
Means and standard deviations of subjectively assessed sleep quality and sleep disorders are given in Table 2. As expected, PTSD patients rated their sleep quality as extremely poor (t = −4.9, p < 0.0005) and scored very high on insomnia (insomnia: t = 9.3, p < 0.0005) and nightmares (W = 142.5, p < 0.0005) compared to trauma-controls. Furthermore, symptoms of periodic limb movement disorder were increased in patients (t = 2.4, p = 0.026). Considering diagnostic threshold criteria, out of 16 PTSD patients 13 met criteria for insomnia, 11 for nightmare disorder, 8 for periodic limb movement disorder, and 1 for a circadian rhythm sleep disorder. In the control sample, the number of participants crossing a diagnostic threshold ranged between 0 and 3 across all scales. Finally, PTSD patients’ daily functioning complaints associated with sleep problems were much higher than trauma-controls’ (W = 105, p < 0.0005).
Table 2.
Questionnaire-based sleep variables
| Variable | Group | Mean | STD | P |
|---|---|---|---|---|
| Sleep quality | PTSD | 5.9 | 3.8 | *** |
| CTRL | 11.6 | 2.6 | ||
| Insomnia | PTSD | 23.3 | 4.9 | *** |
| CTRL | 10.9 | 1.7 | ||
| Nightmares | PTSD | 3.8 | 2.8 | *** |
| CTRL | 0.1 | 0.5 | ||
| PLMD | PTSD | 7.1 | 2.9 | * |
| CTRL | 5.1 | 1.3 | ||
| CRSD | PTSD | 5.2 | 2.3 | |
| CTRL | 5.2 | 2.0 | ||
| Sleepwalking | PTSD | 3.3 | 0.8 | |
| CTRL | 3.5 | 1.7 | ||
| Daily functioning | PTSD | 22.0 | 4.1 | *** |
| CTRL | 9.9 | 2.5 |
CRSD, circadian rhythm sleep disorder; PLMD, periodic limb movement disorder.
*p < 0.05.
***p < 0.0005.
Non-EEG physiological measures to assess movement and breathing-related sleep disorders (Table 3) showed an increased Limb movement index in PTSD patients compared to trauma-controls (W = 158.0, p = 0.039). Heart rate and respiratory variables did not differ significantly between groups (p’s > 0.1), but one PTSD participant was diagnosed with sleep apnea.
Table 3.
Non-EEG physiological variables
| Variable | Group | Mean | STD | P |
|---|---|---|---|---|
| Limb movement index | PTSD | 58.1 | 42.9 | * |
| CTRL | 33.8 | 13.6 | ||
| Respiratory disturbance index | PTSD | 1.4 | 2.2 | |
| CTRL | 1.6 | 1.4 | ||
| SpO2 desaturation 3% index | PTSD | 3.3 | 2.5 | |
| CTRL | 4.3 | 3.5 | ||
| SpO2 desaturation 4% index | PTSD | 1.4 | 1.1 | |
| CTRL | 1.8 | 2.0 | ||
| Mean SpO2 | PTSD | 94.0 | 1.6 | |
| CTRL | 94.6 | 1.2 |
SpO2, blood oxygen saturation level.
*p < 0.05.
Sleep macrostructure
Sleep macrostructural variables (Table 4) also differed between groups. Participants with PTSD displayed significantly more awakenings during sleep, both in terms of the absolute number (t = 2.4, p = 0.025) and the frequency of awakenings (awakenings/TST: t = 3.0, p = 0.006), increased wake after sleep onset (t = 2.3, p = 0.037), a tendency toward longer sleep latency (W = 164.5, p = 0.077) and, consequently, reduced sleep efficiency (t = −2.5, p = 0.025). Furthermore, the PTSD group showed trend-level changes in sleep stage composition compared to trauma-controls: N1 percentage was somewhat increased (t = 2.0, p = 0.056), while there was a decrease in N3 percentage (t = −1.9, p = 0.067) and time spent in N3 (t = −2.0, p = 0.057). Finally, REM latency in the PTSD group was significantly increased (t = 2.2, p = 0.043). For other variables no significant differences were found (p’s > 0.1).
Table 4.
Sleep macrostructure
| Variable | Group | Mean | STD | P |
|---|---|---|---|---|
| Total sleep time (min) | PTSD | 393.0 | 82.6 | |
| CTRL | 429.6 | 45.9 | ||
| Sleep latency (min) | PTSD | 21.1 | 24.1 | |
| CTRL | 10.0 | 5.3 | ||
| REM latency (min) | PTSD | 130.8 | 81.0 | * |
| CTRL | 79.3 | 36.3 | ||
| Sleep efficiency | PTSD | 82.9 | 11.5 | * |
| CTRL | 90.9 | 3.6 | ||
| Wake after sleep onset (min) | PTSD | 56.5 | 34.9 | * |
| CTRL | 33.3 | 16.6 | ||
| WASO/TST | PTSD | 0.2 | 0.1 | * |
| CTRL | 0.1 | 0.0 | ||
| Awakenings (#) | PTSD | 31.3 | 8.5 | * |
| CTRL | 24.1 | 7.5 | ||
| Awakenings/TST (#/min) | PTSD | 4.9 | 1.7 | ** |
| CTRL | 3.4 | 1.0 | ||
| Arousal index (#/min) | PTSD | 23.8 | 6.8 | |
| CTRL | 21.4 | 7.2 | ||
| N1 (min) | PTSD | 67.3 | 26.2 | |
| CTRL | 57.8 | 22.1 | ||
| N1 (%) | PTSD | 17.3 | 5.5 | |
| CTRL | 13.4 | 4.8 | ||
| N2 (min) | PTSD | 238.0 | 45.6 | |
| CTRL | 248.7 | 21.3 | ||
| N2 (%) | PTSD | 61.1 | 6.6 | |
| CTRL | 58.3 | 5.7 | ||
| N3 (min) | PTSD | 18.2 | 19.2 | |
| CTRL | 35.5 | 26.1 | ||
| N3 (%) | PTSD | 4.4 | 4.2 | |
| CTRL | 8.3 | 6.3 | ||
| REM (min) | PTSD | 68.6 | 36.7 | |
| CTRL | 87.6 | 29.3 | ||
| REM (%) | PTSD | 17.1 | 7.3 | |
| CTRL | 20.0 | 4.6 |
Arousal index, number of arousals/total sleep time; TST, total sleep time; WASO, wake after sleep onset.
*p < 0.05, **p < 0.01.
Spectral analysis
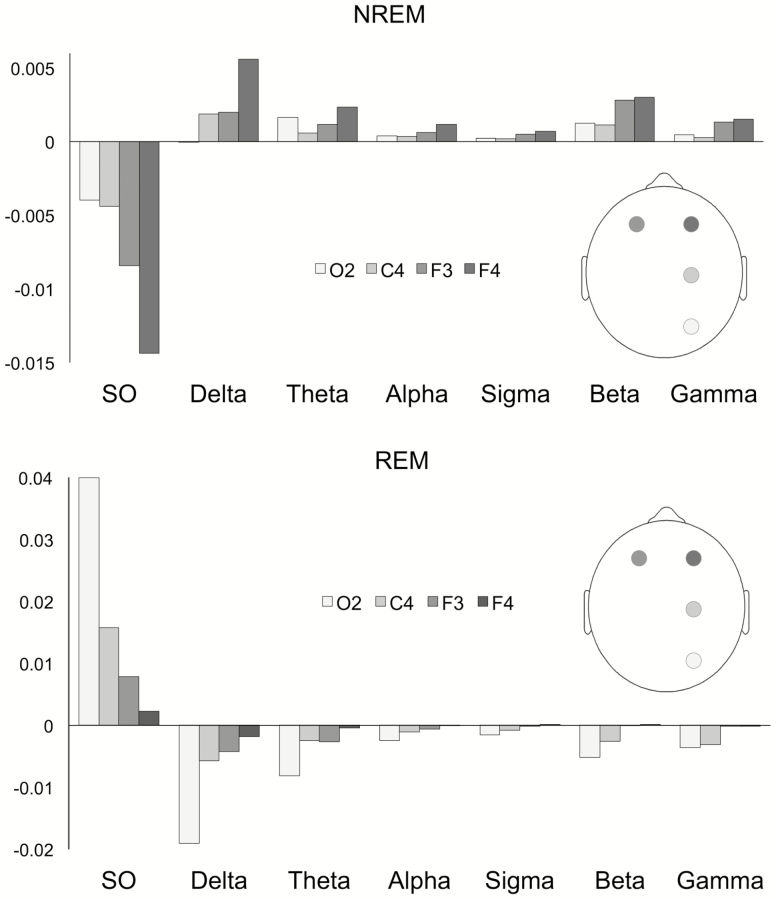
The sleep EEG spectral analysis revealed a striking pattern of differences between PTSD patients and controls. An overview of these differences, across frequencies and spatial positions, is shown in Figure 1 in terms of the power deviations in the PTSD group from control group values (individual group means and SDs are given in Supplementary Table S1). As can be seen, there is a selective loss of SO power in PTSD NREM sleep and a power increase across higher frequency bands (Figure 1A). The pattern is apparent across all derivations, but is the most pronounced over frontal electrodes, especially in the right hemisphere. PTSD REM sleep (Figure 1B) shows a more or less opposite pattern, with increased SO power and power loss in higher frequency bands. This pattern is the most pronounced on the occipital electrode.
Figure 1.
Mean differences in normalized power between patients with PTSD and trauma-controls in NREM (upper panel) and REM sleep (lower panel), across derivations and EEG frequency bands. Difference values were calculated as [Mean power value PTSD group − Mean power value control group]. SO, slow oscillations.
MANOVA with factors Diagnosis (PTSD, trauma-control), Sleep State (NREM, REM), and Electrode (F3, F4, C4, O2) showed a non-significant effect of Diagnosis (Θ = 0.64, F = 2.0, df 7.20, p = 0.11), a significant interaction effect of Diagnosis and Sleep State (Θ = 0.92, F = 2.6, df 7.20, p = 0.04), reflecting that group differences are different for NREM and REM sleep, and a significant Diagnosis by Electrode interaction (Θ = 0.32, F = 3.4, df 7.74, p = 0.003), which indicates that group differences differ across electrodes. The 3-way interaction (Diagnosis*Sleep State*Electrode) was not significant (Θ = 0.15, F = 1.6, df 7.74, p = 0.14).
In the follow-up ANOVAs, the Diagnosis by Sleep State interaction was highly significant for all frequency bands, with p-values ranging between p = 0.001 (in the SO band: F = 15.2, df 1.25, p = 0.001) and p = 0.008 (gamma band: F = 8.2, df 1.25, p = 0.008). Further analyses were thus conducted for the two sleep states separately, through repeated measures ANOVA with factors Diagnosis (PTSD, trauma-control) and Electrode (F3, F4, C4, O2) (Table 5).
Table 5.
Results of repeated measures ANOVA’s per sleep state and frequency band
| Frequency band | Effect | NREM | REM | ||||
|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | ||
| Slow waves 0.5–1.5 Hz | Group | 9.3 | 1.25 | 0.005* | 7.4 | 1.25 | 0.01* |
| Group * electrode | 1.4 | 1.75 | 0.25 | 3.3 | 1.75 | 0.025* | |
| Delta 1.5–4 Hz | Group | 6.2 | 1.25 | 0.019* | 6.8 | 1.25 | 0.015* |
| Group * electrode | 2.2 | 1.75 | 0.01* | 0.5 | 1.75 | 0.66 | |
| Theta 4–8 Hz | Group | 4.7 | 1.25 | 0.040* | 5.1 | 1.25 | 0.033* |
| Group * electrode | 1.8 | 1.75 | 0.16 | 0.6 | 1.75 | 0.63 | |
| Alpha 8–12 Hz | Group | 6.7 | 1.25 | 0.016* | 3.8 | 1.25 | 0.061 |
| Group * electrode | 2.5 | 1.75 | 0.088 | 0.5 | 1.75 | 0.65 | |
| Sigma 12–16 Hz | Group | 7.0 | 1.25 | 0.014* | 3.7 | 1.25 | 0.065 |
| Group * electrode | 2.4 | 1.75 | 0.08 | 0.6 | 1.75 | 0.62 | |
| Beta 16–30 Hz | Group | 7.5 | 1.25 | 0.011* | 2.9 | 1.25 | 0.10 |
| Group * electrode | 2.0 | 1.75 | 0.12 | 0.6 | 1.75 | 0.65 | |
| Gamma 30–50 Hz | Group | 5.3 | 1.25 | 0.030* | 3.7 | 1.25 | 0.066 |
| Group * electrode | 1.5 | 1.75 | 0.23 | 0.5 | 1.75 | 0.70 |
*p < 0.05.
For NREM sleep, a significant effect of Diagnosis was found for all frequency bands (p’s < 0.05), supporting that in PTSD power is consistently decreased in the SO range and significantly increased in all other bands, with respect to control. The anterior–posterior gradient in the power change was tested through the Diagnosis*Electrode interaction, assessing the contrast between anterior F4 and posterior O2. The contrast was statistically significant for the delta, alpha, sigma, and beta bands (p < 0.05) and reached trend-level significance (p < 0.1) for all other bands (SO, theta, beta, and gamma), confirming larger changes in anterior than posterior region.
For REM sleep, the main effect of Diagnosis was significant for the SO, delta, and theta bands and reached trend-level significance in all remaining bands (alpha, sigma, beta, gamma). These results reflect the power increase in the SO band and decrease for higher frequencies in PTSD compared to control. The posterior–anterior gradient in this effect was again assessed through the Diagnosis*Electrode O2 to F4 contrast. The contrast was only significant for the SO band (p < 0.05), suggesting that only the SO power increase is significantly localized over posterior brain areas.
To assess possible effects of medication on these results, we compared patients with and without medication on SO power, which showed the largest abnormalities across both NREM and REM sleep. Given the small numbers, this comparison has very low power to detect effects. Having said this, no differences were found either during NREM (t12 = 0.53, p = 0.61) or REM sleep (t12 = 0.84, p = 0.42). More importantly, the differences in SO power between patients and trauma-controls were still statistically significant if the patients on medication were removed from the analysis (NREM: t20 = −2.3, p = 0.03; REM: t14.6 = 2.7, p = 0.01), suggesting medication effects did not notably drive the abnormalities in patients.
Correlation of power changes in PTSD with experienced sleep problems
To investigate the relation of abnormalities in oscillatory sleep dynamics with experienced sleep problems in PTSD, we focused on the largest power changes in the investigated space-frequency domain. That is, SO power, the most strongly affected band across the combined sleep states, on right-frontal F4 for NREM sleep and on occipital O2 for REM sleep. Each variable was correlated with the two hallmark sleep problems in PTSD: insomnia and nightmares (Supplementary Figure S1).
Reduced right-frontal SO power in NREM sleep was related to increased insomnia (r = −0.46, p = 0.017), but was not related at all to nightmare severity (p > 0.1). Conversely, occipital SO power in REM sleep showed a large positive correlation with nightmare severity (r = 0.64, p = 0.048), as well as a significant correlation with insomnia (r = 0.50, p = 0.007).
A candidate biomarker for PTSD-sleep problems
We explored the extent to which a single variable, reflecting both the NREM and REM sleep spectral abnormalities, might be used as a biomarker. To this purpose, we calculated a “PTSD spectral sleep index” (PSSI) as the ratio between right-frontal NREM SO power and occipital REM sleep SO power. The clinical relevance of this index was assessed through the effect size of disease status (PTSD vs trauma-control). We observed an effect size of 3.4, which is considered very large [46, 47].
Importantly, a biomarker should correlate with diagnostic measures obtained with standardized diagnostic instruments. Accordingly, the PSSI shows a highly significant correlation with participants’ CAPS scores (r = 0.60, p = 0.001; Supplementary Figure S2).
Discussion
In the present study, we investigated EEG power in PTSD over a large frequency domain at multiple positions across the scalp, in both REM and NREM sleep. Our findings reveal substantial power differences between patients with PTSD and traumatized controls. Specifically, patients show a strong shift away from the lowest frequency band (SO band) toward the higher frequencies during NREM sleep, in particular over the right-frontal cortex. On the other hand, during REM sleep SO power is increased at the expense of higher frequency power, over the occipital part of the brain. The latter abnormality is strongly related to nightmare activity, while both REM and NREM abnormalities show a robust relation to insomnia. Abnormalities in PTSD sleep macrostructure were also observed but were much less pronounced than the power abnormalities. These changes in sleep macro- and microstructure co-occur with severe sleep pathology, apparent from subjective as well as physiological assessments.
The findings in NREM sleep support the hypothesis of deregulated SO dynamics in PTSD, showing a preferential reduction of SO power in patients over frontal areas, where SOs are most frequently generated. Interestingly, a recent study recording from a forehead location produced a similar observation, suggesting a reduced amount of NREM sleep with dominant power in the slow oscillation (0.1–1 Hz) range in participants with PTSD compared to healthy subjects ([26; note limitations with regard to age and gender matching of participant groups).
Notably, slow oscillations have been implicated in orchestrating sleep-related information processing [34], including memory reactivation and consolidation [48–53]. Activity in the spindle and beta/gamma bands, which is increased in our patient group, has been shown to be more directly associated with memory reprocessing [54], with local spindles reflecting the reactivation of specific memory content. Our findings, therefore, suggest a possible deregulation of these processes in PTSD, perhaps involving exaggerated reprocessing and consolidation of trauma memories. This, in turn, could lead to the overgeneralized, intrusive trauma memories that constitute a key symptom of PTSD [55, 56]. Further research into this putative relation is currently ongoing in our lab.
Importantly, the frontal loss of SO power also entails a reduction in sleep depth. Sleep depth is traditionally indexed by low-frequency power over a central electrode, but recent studies have shown that SO power, as well as other oscillatory brain dynamics, are also regulated locally, in relation with brain regions’ wake-time activity [57]. Deep sleep is essential for sleep’s homeostatic recovery function and the resolution of sleep pressure [58]. It is, moreover, crucial for a myriad of anabolic and restorative processes, including build-up of energy molecules and the immune system [59–61]. Thus, chronic deficiencies in deep sleep have important health consequences [62–64].
Turning to REM sleep, we surprisingly found a power shift toward the SO band, which was most prominent on the occipital channel. The abnormality is highly correlated with nightmare severity. Previous studies have reported increased REM sleep delta (0.5–4 Hz) in nightmare disorder [65], and in association with sleep onset hypnagogic imagery [66]. Furthermore, delta power is higher in phasic compared to tonic REM sleep [67], the former being the part of REM sleep marked by REMs and hosting the most vivid dreaming [68]. The combined findings suggest that increased REM sleep SO power in PTSD patients may be related to the visual imagery and/or REMs associated with nightmares. Accordingly, the posterior focus of the power increase might reflect a neural source in the visual cortex, or in posterior areas controlling eye movements (e.g. posterior temporoparietal areas or cerebellum [69]). It would be interesting to assess these possibilities through high-density EEG, which allows a more precise estimation of EEG activity’s neural sources.
Note that the neural mechanisms underlying SO power abnormalities in PTSD REM and NREM sleep may not be related. NREM slow oscillations in the EEG reflect synchronized transitions of bistable cortical neurons between so-called up-states and down-states. This dynamic and the resulting EEG slow oscillations are not compatible with the REM sleep state in the same network. However, given local components to sleep–wake regulation [57] and considering recent findings in rodents [70], such slow oscillations might occur locally, while other parts of the cortical mantle display REM sleep-like activity. Still, given the posterior location and the correlation with nightmare activity, it is perhaps more likely that REM sleep SO power reflects neural activity unrelated to the typical NREM slow oscillation dynamic.
REM sleep has also been related to memory reprocessing, in particular regarding emotional memories [14, 19, 71, 72]. A comprehensive account of how observed physiological changes during REM and NREM sleep in PTSD might lead to erratic memory consolidation is given in a recent review [73].
Our findings regarding sleep macrostructure in PTSD are generally in line with previous findings [20]; we observed a notable decrease in sleep efficiency, with increased awakenings and wake-time after sleep onset and a tendency toward longer sleep latency. Patients also showed trend-level changes in sleep stage composition, involving decreased N3 and increased N1, as well as increased REM latency, which was not reported previously. Of interest is a comparison of these results with those of spectral analysis. In fact, the pronounced spectral abnormalities in patients’ sleep EEG are only marginally (for NREM sleep) or not at all (for REM sleep) captured by the analysis of sleep stage composition. Given the nature and limitations of the method, this should not be surprising. Nevertheless, many clinical studies assessing brain activity during sleep still revert to sleep staging as the method of choice. We would like to advocate that spectral analysis across multiple brain locations presents a more sensitive and more generic method to assess oscillatory brain activity during sleep.
The pronounced spectral abnormalities in PTSD sleep, described above, occur in the context of pronounced sleep pathology. As expected, the large majority of PTSD participants experienced severe insomnia (81%) and nightmare pathology (69%). More interestingly, we observed excessive limb movements during sleep in a large percentage of patients (50% reached the diagnostic threshold for periodic limb movement disorder). These findings might, in part, be related to the reduced NREM sleep depth in patients, as movement normally diminishes with sleep depth. However, the polysomnographical recordings showed that limb movements also often occurred during both phasic and tonic REM sleep. This is highly abnormal, as REM-associated hypotonia normally prevents such movements. Indeed, this observation points in the direction of REM sleep behavior disorder, symptoms of which, such as acting out dreams, have clinically been noted in patients with PTSD [74]. Interestingly, enhanced leg movements in both REM and NREM sleep have also been observed in nightmare sufferers with and without PTSD [75]. Therefore, the REM sleep movements might be related to the intense negative dreaming and related high arousal, which is experienced by PTSD patients and nightmare sufferers alike.
Besides contributing to our understanding of sleep disturbance in PTSD, our findings may have practical implications. Indeed, the spectral fingerprint of PTSD sleep presents a pattern of abnormalities that has not been observed before. These abnormalities, moreover, appear to reflect the main proponents of PTSD sleep pathology, namely insomnia and nightmares, the combination of which has some specificity for PTSD. This fosters the exciting notion that a spectral biomarker of PTSD sleep problems might be obtained. A reliable biomarker would be highly useful in PTSD diagnostic practice and would importantly facilitate further research. Our first steps toward exploring this idea are encouraging, showing that a combined NREM-REM spectral index distinguishes PSTD patients from trauma-controls with strikingly high effect size. However, further research should investigate whether our findings can be replicated in a larger and more diverse sample of patients and, subsequently, whether the candidate biomarker has specificity versus other sleep and affective disorders.
As a final consideration, and limiting generalization of our results, the patients in this study have severe and chronic PTSD. As such, physiological abnormalities might be particularly pronounced. Also, as the sample consisted of treatment-seeking police officers and veterans, some caution is warranted in extrapolating to other PTSD populations.
In conclusion, our findings reveal substantial, abnormalities in the microarchitecture of PTSD sleep, including altered SO dynamics in both sleep states. These changes are likely to affect sleep’s homeostatic recovery function and memory reprocessing during sleep. The right frontal hotspot of abnormalities during NREM sleep may be related to reprocessing of negative memories, while the occipital REM sleep abnormalities are related to nightmare activity. Studies involving emotional memory measures, high-density EEG sleep recordings and larger samples, needed to confirm and extend these initial findings, are ongoing in our lab.
These findings provide new insights into the neural basis of PTSD-related sleep disorder and its role in PTSD etiology. Furthermore, given their robustness and potential specificity, the sleep microstructural deficits bear the promise of delivering a biomarker. Neuroscientifically informed treatment interventions aimed at targeting specific PTSD symptoms will be essential to future research agendas [76]. The objective disease marker for typical sleep problems in PTSD, found in the present study, could importantly enhance such interventions. In particular, those aimed at the debilitating sleep problems associated with PTSD.
Supplementary Material
Acknowledgments
We thank the staff of ARQ Centrum‘45 for their involvement in patient recruitment and varied practical support that has been crucial to the realization of this study.
Funding
We thank Amsterdam Brain and Cognition (ABC), University of Amsterdam, for financially supporting this work and Marnus Witte for assisting in part of the analyses.
Conflictof interest statement: none clared.
References
- 1. de Vries GJ, et al. The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. J Trauma Stress. 2009;22(4):259–267. [DOI] [PubMed] [Google Scholar]
- 2. Kessler RC, et al. Posttraumatic stress disorder in the national comorbidity survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. [DOI] [PubMed] [Google Scholar]
- 3. Ohayon MM, et al. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–478. [DOI] [PubMed] [Google Scholar]
- 4. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spoormaker VI, et al. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12(3):169–184. [DOI] [PubMed] [Google Scholar]
- 6. Mellman TA, et al. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159(10):1696–1701. [DOI] [PubMed] [Google Scholar]
- 7. Koren D, et al. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. [DOI] [PubMed] [Google Scholar]
- 8. Harvey AG, et al. The relationship between acute stress disorder and posttraumatic stress disorder: a prospective evaluation of motor vehicle accident survivors. J Consult Clin Psychol. 1998;66(3):507–512. [DOI] [PubMed] [Google Scholar]
- 9. Bryant RA, et al. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep. 2010;33(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Babson KA, et al. Temporal relations between sleep problems and both traumatic event exposure and PTSD: a critical review of the empirical literature. J Anxiety Disord. 2010;24(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pace-Schott EF, et al. Effects of sleep on memory for conditioned fear and fear extinction. Psychol Bull. 2015;141(4):835–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Talamini LM, et al. Sleep directly following learning benefits consolidation of spatial associative memory. Learn Mem. 2008;15(4):233–237. [DOI] [PubMed] [Google Scholar]
- 13. Axmacher N, et al. Cognitive Neuroscience of Memory Consolidation. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 14. Talamini LM, et al. Sleeping worries away or worrying away sleep? Physiological evidence on sleep-emotion interactions. PLoS One. 2013;8(5):e62480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pace-Schott EF, et al. Napping promotes inter-session habituation to emotional stimuli. Neurobiol Learn Mem. 2011;95(1):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoo SS, et al. The human emotional brain without sleep—a prefrontal amygdala disconnect. Curr Biol. 2007;17(20):R877–R878. [DOI] [PubMed] [Google Scholar]
- 17. Franzen PL, et al. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biol Psychol. 2009;80(3):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wassing R, et al. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci USA. 2016;113(9):2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deliens G, et al. Sleep and the processing of emotions. Exp Brain Res. 2014;232(5):1403–1414. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi I, et al. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. [DOI] [PubMed] [Google Scholar]
- 21. Cowdin N, et al. Theta frequency activity during rapid eye movement (REM) sleep is greater in people with resilience versus PTSD. Exp Brain Res. 2014;232(5):1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen DJ, et al. Quantitative electroencephalography during rapid eye movement (REM) and non-REM sleep in combat-exposed veterans with and without post-traumatic stress disorder. J Sleep Res. 2013;22(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richards A, et al. Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. J Sleep Res. 2013;22(6):679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neylan TC, et al. Delta sleep response to metyrapone in post-traumatic stress disorder. Neuropsychopharmacology. 2003;28(9):1666–1676. [DOI] [PubMed] [Google Scholar]
- 25. Woodward SH, et al. PTSD-related hyperarousal assessed during sleep. Physiol Behav. 2000;70(1-2):197–203. [DOI] [PubMed] [Google Scholar]
- 26. Onton JA, et al. In-home sleep recordings in military veterans with posttraumatic stress disorder reveal less REM and deep sleep <1 Hz. Front Hum Neurosci. 2018;12:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Otte C, et al. Effects of metyrapone on hypothalamic-pituitary-adrenal axis and sleep in women with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):952–956. [DOI] [PubMed] [Google Scholar]
- 28. Steriade M, et al. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13(8):3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timofeev I, et al. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J Neurophysiol. 1996;76(6):4152–4168. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez-Vives MV, et al. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3(10):1027–1034. [DOI] [PubMed] [Google Scholar]
- 31. Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–d899. [DOI] [PubMed] [Google Scholar]
- 32. Mölle M, et al. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002;22(24):10941–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steriade M, et al. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13(8):3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cox R, et al. Slow oscillations during sleep coordinate interregional communication in cortical networks. J Neurosci. 2014;34(50):16890–16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cairney SA, et al. Complimentary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation. Cerebral Cortex.bht349. 2015;25(6):1565–1575. [DOI] [PubMed] [Google Scholar]
- 36. Cairney SA, et al. Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep. 2014;37(4):701–7, 707A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riemann D, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. [DOI] [PubMed] [Google Scholar]
- 38. Phelps AJ, et al. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41(1). doi:10.1093/sleep/zsx188. [DOI] [PubMed] [Google Scholar]
- 39. Blake DD, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 40. Sheehan D, et al. Diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- 41. Spoormaker VI, et al. Initial validation of the SLEEP-50 questionnaire. Behav Sleep Med. 2005;3(4):227–246. [DOI] [PubMed] [Google Scholar]
- 42. Visser P, et al. Sleep and mood: measuring the sleep quality. In: Priest RG, Pletscher A, Ward J, eds. Sleep research. Lancaster, PA: MTP Press; 1979: 135–145. [Google Scholar]
- 43. de Boer M, et al. The role of sleep in emotional memory processing and neurocognitive functioning in patients with posttraumatic stress disorder. CCMO-register. 2012. [Google Scholar]
- 44.C, Iber, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 45. Hedges L, et al. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 46. Lane DM. Hyperstat Online Textbook; 2003. [Google Scholar]
- 47. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 48. Binder S, et al. Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul. 2014;7(4):508–515. [DOI] [PubMed] [Google Scholar]
- 49. Binder S, et al. Transcranial slow oscillation stimulation during NREM sleep enhances acquisition of the radial maze task and modulates cortical network activity in rats. Front Behav Neurosci. 2013;7:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heib DP, et al. Slow oscillation amplitudes and up-state lengths relate to memory improvement. PLoS One. 2013;8(12):e82049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ngo HV, et al. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78(3):545–553. [DOI] [PubMed] [Google Scholar]
- 52. Marshall L, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–613. [DOI] [PubMed] [Google Scholar]
- 53. Chauvette S, et al. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75(6):1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cox R, et al. Local sleep spindle modulations in relation to specific memory cues. Neuroimage. 2014;99:103–110. [DOI] [PubMed] [Google Scholar]
- 55. Kheirbek MA, et al. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nat Neurosci. 2012;15(12):1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shelton DJ, et al. A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res. 2013;256:20–26. [DOI] [PubMed] [Google Scholar]
- 57. Siclari F, et al. Local aspects of sleep and wakefulness. Curr Opin Neurobiol. 2017;44:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borbély AA, et al. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:559–568. [DOI] [PubMed] [Google Scholar]
- 59. Scheen AJ, et al. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol Endocrinol Metab. 1996;271:E261–E270. [DOI] [PubMed] [Google Scholar]
- 60. Obal F Jr, et al. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. [DOI] [PubMed] [Google Scholar]
- 61. Sassin JF, et al. Human growth hormone release: relation to slow-wave sleep and sleep-walking cycles. Science. 1969;165(3892):513–515. [DOI] [PubMed] [Google Scholar]
- 62. Tasali E, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105(3):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spiegel K, et al. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. [DOI] [PubMed] [Google Scholar]
- 64. Renegar KB, et al. Effects of short-term sleep deprivation on murine immunity to influenza virus in young adult and senescent mice. Sleep. 1998;21(3):241–248. [PubMed] [Google Scholar]
- 65. Simor P. Nightmare Disorder in Light of Neuropsychological and Polysomnographic Investigations [PhD thesis]. Budapest, Hungary: Budapest University of Technology and Economics, PhD school in Psychology, Department of Cognitive Sciences; 2013.
- 66. Germain A, et al. EEG power associated with early sleep onset images differing in sensory content. Sleep Res Online. 2001;4:83–90. [Google Scholar]
- 67. Simor P, et al. EEG spectral power in phasic and tonic REM sleep: different patterns in young adults and children. J Sleep Res. 2016;25(3):269–277. [DOI] [PubMed] [Google Scholar]
- 68. De Carli F, et al. Activation of the motor cortex during phasic rapid eye movement sleep. Ann Neurol. 2016;79(2):326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pouget P. The cortex is in overall control of ‘voluntary’ eye movement. Eye (Lond). 2015;29(2):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Funk CM, et al. Local slow waves in superficial layers of primary cortical areas during REM sleep. Curr Biol. 2016;26(3):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Poe GR, et al. Cognitive neuroscience of sleep. Prog Brain Res. 2010;185:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cunningham TJ, et al. Emotional memory consolidation during sleep. In: Axmacher N, Rasch B, eds. Cognitive Neuroscience of Memory Consolidation. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 73. Vanderheyden WM, et al. Trauma exposure and sleep: using a rodent model to understand sleep function in PTSD. Exp Brain Res. 2014;232(5):1575–1584. [DOI] [PubMed] [Google Scholar]
- 74. Roepke S, et al. Nightmares that mislead to diagnosis of reactivation of PTSD. Eur J Psychotraumatol. 2013;4(1). doi:10.3402/ejpt.v4i0.18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Germain A, et al. Sleep pathophysiology in posttraumatic stress disorder and idiopathic nightmare sufferers. Biol Psychiatry. 2003;54(10):1092–1098. [DOI] [PubMed] [Google Scholar]
- 76. Lanius RA, et al. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. Eur J Psychotraumatol. 2015;6:27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.