Abstract
Study Objectives
Recovery rates of systolic blood pressure (BP) and heart rate (HR) after exercise have been used to assess cardiovascular fitness, and slower recovery rates are predictors of coronary heart disease and cardiac mortality. The endogenous circadian system is known to modulate BP and HR at rest and during exercise. Here, we examined whether the post-exercise recovery rates of BP and HR are also under circadian control.
Methods
Twelve healthy adults (mean age = 26 ± 6 (SD) years; 6 female) participated in a 240 h forced desynchrony protocol in dim light where all behaviors, including 15 min cycle exercise tests at 60% maximal HR, were uniformly distributed across the circadian cycle. Circadian phases were assigned based on the rhythm of core body temperature. For each session, HR was measured continuously, and BP every 3–5 min throughout baseline, exercise, and recovery. Recovery was quantified as the proportional return to pre-exercise baseline levels following exercise ([peak exercise-recovery]/[peak exercise-baseline) × 100%], whereby 100% represents full recovery to baseline).
Results
There was a significant circadian rhythm in systolic BP recovery, with fastest recovery at the circadian phase corresponding to late afternoon (equivalent to ~5 pm) and slower recovery across the early morning (~8:30 am; p = 0.029, peak-to-trough: 9.2%). There were no significant circadian variations in post-exercise recovery rates of diastolic BP or HR.
Conclusions
The circadian system modulates the rate of recovery of systolic BP after exercise with fastest recovery in the biological afternoon. These data could have implications for exercise prescription and interpretation of clinical tests of stress recovery.
Keywords: circadian rhythms, exercise physiology, blood pressure
Statement of Significance.
Here we show, for the first time, a significant endogenous circadian rhythm in the recovery rate of systolic blood pressure (BP) after exercise in healthy young, lean adults. The fastest recovery of post-exercise systolic BP occurs in the late afternoon, while the slower recovery occurs across the night and into the early morning. Because recovery rates of systolic BP after exercise have been used to assess cardiovascular fitness and to detect risk for coronary artery disease, healthcare professionals may consider circadian variation when prescribing exercise for therapeutic intervention and when interpreting clinical tests of stress recovery.
Introduction
Electrocardiographic, heart rate (HR), and blood pressure (BP) responses to exercise are commonly monitored in athletes to assess cardiovascular adaptations to training, and in untrained and/or patient populations to detect potential cardiovascular disease [1]. The speeds of recovery of HR and BP immediately after stopping exercise are used to assess cardiovascular fitness and to predict an individual’s risk of cardiovascular diseases and cardiac mortality [2–5]. Moreover, the decrease of BP after acute exercise contributes to the hypotensive effects of chronic exercise training [6, 7] and HR recovery is used as a non-invasive indicator of cardiac autonomic control [8]. Thus, it is important to understand the mechanisms underlying the rate of post-exercise hemodynamic recovery.
The circadian system, consisting of the suprachiasmatic nucleus (SCN) in the hypothalamus and circadian oscillators in virtually all cells and tissues throughout the body, plays an important role in the regulation of cardiovascular function [9]. Animal studies have demonstrated that the SCN regulates the cardiovascular system through humoral factors and multi-synaptic neural projections to the heart and vasculature [10, 11] and that deletion or mutation of core clock genes (i.e., Bmal1, Clock, and tau) results in abnormal sympatho-adrenal function and vascular responses to stressors [12, 13]. It has been shown in humans that the circadian system regulates many autonomic functions (e.g., circulating catecholamines, markers of cardiac vagal modulation, BP, and HR) at rest, as well as their responses to behavioral stressors such as exercise and postural tilt [14, 15]. Indeed, we discovered that the greatest systolic BP increase in response to standardized exercise occurred in the biological evening [14]. Here, we analyzed data from the same study [14] to determine whether the post-exercise recovery rates of BP and HR are also under circadian control.
Methods
We note that other aspects of this study—which was designed to test separate hypotheses—have been previously published [14–20].
Participants
Twelve healthy participants [6 males; 6 females; age: mean ± SD (range) 25.8 ± 5.7 years (20–42 years); BMI: 23.6 ± 3.2 kg/m2 (19.9–29.6 kg/m2)] completed a 13 day inpatient protocol in the Clinical and Translational Research Center at Brigham and Women’s Hospital. All participants provided written informed consent prior to enrollment, and ethical approval was granted by the Partners Human Research Committee. Health status was determined by extensive medical history, physical, psychological, and laboratory examination. To ensure a stable circadian rhythmicity and minimize training and detraining effects, participants maintained a regular sleep–wake cycle of 8 h sleep per night and an exercise level equivalent to a 30 min brisk walk per day for 2–3 weeks immediately before admission to the laboratory (verified by sleep–wake diaries, call-in times to a time-stamped voice recorder and wrist actigraphy). Toxicology screens upon admission confirmed that participants were free of any drugs, including caffeine, alcohol, and nicotine.
Study protocol
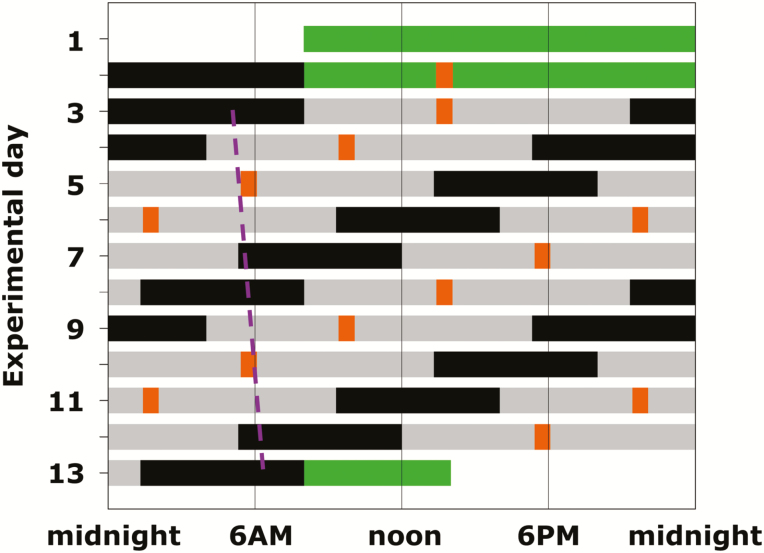
A forced desynchrony (FD) protocol was used to dissociate the separate effects of the circadian system and behavioral/environmental cycles upon variables of interest [21]. Participants lived in a private laboratory room, free of time cues, for 13 days and nights including two baseline days and nights (16 h wake/8 h scheduled sleep) followed by the FD portion of the protocol which consisted of 12 standardized 20 h “days” (13:20 h scheduled wakefulness; 6:40 h scheduled sleep; maintaining a 2:1 wake:sleep ratio) with controlled activity, posture, meals, sleep, room temperature, and light (<4 lx, Figure 1). Because the 20 h sleep–wake cycle in dim light is outside the range of entrainment, the circadian timing system cycles at its inherent rate of close to 24 h. Thus, the sleep and wake episodes, and the exercise tests, were uniformly distributed across the circadian cycle [22].
Figure 1.
Forced desynchrony protocol. Solid back bars, scheduled sleep (0 lux); gray bars, wakefulness in dim light [≈1.8 lux (≈0.0048 W/m2)]; green bars, baseline days and discharge day in normal room light (≈90 lux); orange bars, test batteries in dim light; dashed purple line, illustration of trajectory of circadian core body temperature minimum throughout the FD protocol as a circadian phase marker (average circadian period of 24.09 ± 0.06 h in these subjects).
Exercise stress test
On the day of admission, participants performed an initial exercise session on a cycle ergometer (Cybex Semirecumbent Cycle Ergometer) at 70 rpm to determine the personalized workload to achieve a target HR of approximately 60% of maximal HR (estimated as 220 beats/min minus age in years). Thereafter, each subsequent exercise stress test was performed at each participant’s personalized constant workload at 70 rpm. Exercise tests began at 5 h 25 min after each scheduled awakening, and consisted of 25 min rest for baseline, 15 min exercise, and 20 min recovery. During each of the three sections, participants maintained the same body posture (seated on the ergometer), did not drink or talk and were asked to limit any unscheduled movements.
Measurements
Blood pressure was measured using a automatic oscillometric cuff sphygmomanometer (Dinamap; Critikon) every 5 min during baseline and recovery and every 3 min during each exercise test. HR was assessed by three-lead ECG using a Vitaport (Temec Instruments) at 256 Hz throughout each baseline, exercise test, and recovery section.
Circadian phase estimation
Core body temperature derived from a rectal thermistor was continuously recorded and used to estimate circadian period and phase by non-orthogonal spectral analyses [21]. All data were assigned an endogenous circadian phase (0–359°) depending on the time from the fitted core body temperature minimum (set to 0°; equivalent to ~4:30 am in these participants) [14].
Post-exercise recovery measures
For systolic BP and diastolic BP, the rate of recovery after exercise was quantified as the proportional return to the pre-exercise average baseline levels following exercise cessation ([peak exercise-recovery]/[peak exercise-baseline) × 100%], whereby 100% represents full recovery to baseline). This is shown graphically in Supplementary Figure S1A. The decrease in HR after exercise usually follows a first order exponential decay [23, 24], so we quantified the HR recovery rate as the time constant of the beat-by-beat HR decay for the first 5 min after exercise (T300), whereby a shorter T300 corresponds to a quicker recovery to pre-exercise baseline levels. This decay function is shown graphically in Supplementary Figure S1B and described fully in the SI. In addition, because absolute differences in systolic BP from baseline to recovery and absolute differences in HR from peak exercise to 1 and 2 min into recovery have been associated with increased risk of coronary heart diseases or cardiac mortality [2, 25–27], we also assessed the circadian effects on these two outcomes.
Statistical analysis
Based on pre-hoc criteria to eliminate spurious outliers, values more than 3 SDs from the mean within each subject across all wake periods were excluded from analyses. The independent effects of the circadian cycle on the aforementioned recovery indices were assessed by cosinor analyses using mixed model analyses of variance with “participant” as a random factor. These models included a fundamental circadian component (~24 h; effect of “circadian phase”; where the exact period used was based on the individual’s personal circadian period derived from the core body temperature during the FD protocol), a harmonic component (~12 h), and a linear component (hours into FD protocol to account for any gradual changes across time into the protocol). To account for time-course within test sessions, elapsed time from start of exercise recovery (time since recovery: 1, 6, 11 min for BP; 1 and 2 min for HR) were included in the models. To account for the autocorrelation of the repeated measurements, we assumed first-order autoregressive or compound symmetry covariance structure as appropriate. The interaction between circadian phase and time into recovery was also explored and included if the effect was significant. Where appropriate based on data distributions, analyses were performed on log-transformed data and back-transformed to the original scales for reporting in order to aid interpretations. Estimates for log-normal distributed data were reported as geometric means (95% CI). Estimates for normally distributed data are reported as mean ± SEM. All statistical analyses were performed using statistical software (JMP Pro14; SAS Institute, Cary, NC). Statistical significance was accepted as p < 0.05.
Results
There were no significant interactions between circadian phase and time into recovery; therefore, all reported statistics are based on mixed model analyses without this interaction. The data for recovery rates of systolic BP and HR are shown in Figures 2 and 3, respectively (data in absolute units are presented in Supplementary Figure S2).
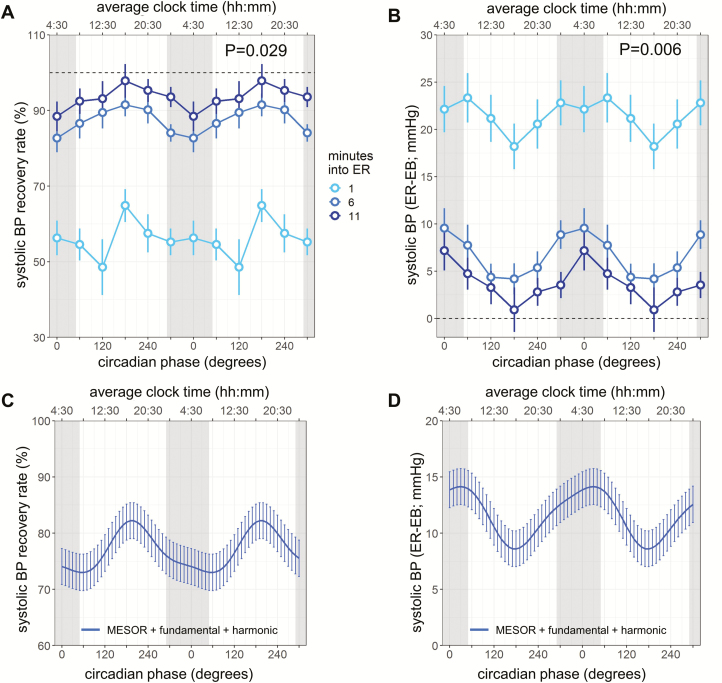
Figure 2.
Circadian rhythm in systolic BP recovery after exercise. Systolic BP recovery rates are expressed in two ways: (A, C) as the proportion of the return to the pre-exercise average baseline relative to the peak achieved during exercise ([peak exercise-recovery]/[peak exercise-baseline) × 100%]), (B, D) as the absolute difference between recovery and baseline (previously used for prognostic purpose). (A, B) Light, medium, dark blue circles, 1, 6, and 11 min into exercise recovery (ER), respectively; error bars, SEM; gray bars, group average habitual sleep episodes; horizontal dotted lines, baseline. (C, D) The cosinor fits to the SBP recovery indices. Each model reflects the summed MESOR, fundamental circadian and harmonic components, and averaged time since recovery effects. Cosinor analyses were performed on the 360° data sets, whereas data are double plotted (two identical circadian cycles) to aid visualization of rhythmicity. Lines, model fit; error bars, SEM; p values, significance of circadian effect from cosinor analyses.
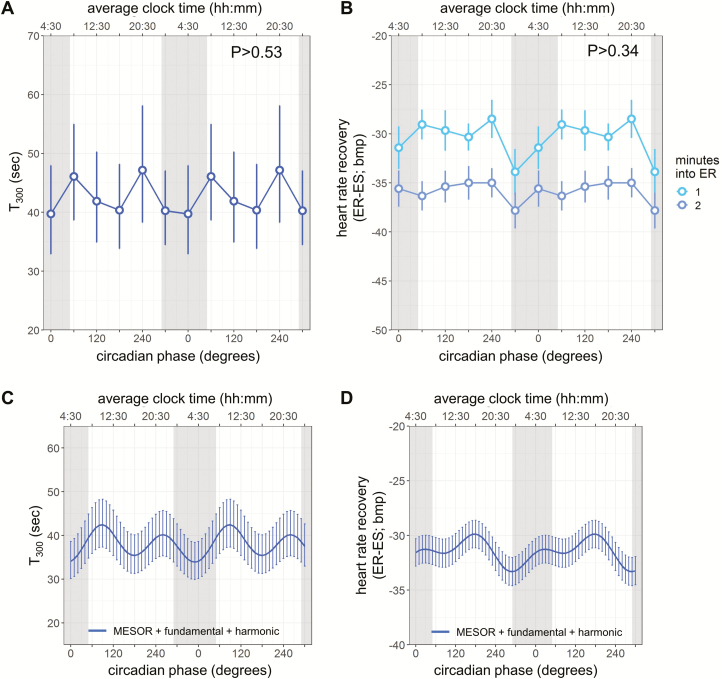
Figure 3.
Heart rate (HR) recovery after exercise across circadian cycles. HR recovery indices are expressed in two ways: (A, C) T300, time constant obtained by fitting a first order exponential decay curve to the HR responses for the first 5 min after exercise, and (B, D) as the difference between recovery and maximal HR during exercise (previously used for prognostic purpose). Light and medium blue circles, 1 and 2 min, into exercise recovery (for B only), respectively; gray bars, group average habitual sleep episodes. (C, D) The cosinor fits to the HR recovery indices. Each model reflects the summed MESOR, circadian fundamental and harmonic components, and averaged time since recovery effects (the latter for D only). Cosinor analyses were performed on the 360° data sets, whereas data are double plotted (two identical circadian cycles) to aid visualization of rhythmicity. Lines, model fit; error bars, 95% CI in (A, C) and SEM in (B, D); p values, significance of circadian effect from cosinor analyses.
The average systolic BP recovery rate across all circadian phases was 55.9 ± 2.1% [95% CI 51.9% to 60.0%], 87.5 ± 1.5% [95% CI 84.6% to 90.3%], and 93.4 ± 1.5% [95% CI 90.4% to 96.4%] at 1, 6, and 11 min into recovery, respectively (time since recovery: p < 0.0001). We first investigated whether the circadian system influences the systolic BP decline during the post-exercise recovery period (testing main effect of “circadian phase” on systolic BP recovery rate). There was a large amplitude endogenous circadian rhythm in systolic BP recovery rate (fundamental: p = 0.029, Figure 2, A and C). The fastest recovery (peak of the circadian rhythm) occurred in the biological late afternoon (circadian phase of 190°, corresponding to ~5:00 pm), while slowest recovery occurred in the biological morning (60°, ~8:30 am; peak-to-trough amplitude = 9.2%). Because we previously found circadian variation in systolic BP increase in response to exercise (i.e., reactivity), we attempted to determine whether the circadian system influences the systolic BP recovery beyond its effect on the reactivity. In a complementary analysis when including systolic BP reactivity (peak exercise-baseline) as a covariate, the circadian effect on the systolic BP recovery rate remained significant (fundamental: p = 0.03) without a significant effect of the systolic BP reactivity (p = 0.82).
We also investigated the circadian effects on the difference in systolic BP from baseline to recovery as it has been reported to predict the risk for acute myocardial infarction [27]. The average differences in systolic BP from baseline to recovery across all circadian phases at 1, 6, and 11 min into recovery were 21.5 ± 1.0 mmHg [95% CI 19.5 to 23.4], 6.7 ± 0.7 mmHg [95% CI 5.2 to 8.1], and 3.8 ± 0.8 mmHg [95% CI 2.3 to 5.2], respectively (time since recovery: p < 0.0001). This differences in systolic BP from baseline to recovery also showed a significant circadian rhythm (fundamental: p = 0.0061, Figure 2, B and D), with the smallest value (the fastest decline) similarly in the biological late afternoon (180°, ~4:30 pm; peak-to-trough amplitude = 5.5 mmHg). The significant circadian effect remained (fundamental: p = 0.0029) after adding systolic BP reactivity as a covariate (p < 0.0001). These results suggest that the circadian system influences the systolic BP recovery rate beyond its effect on the reactivity.
We did not find significant circadian influences on the recovery of diastolic BP (fundamental: all p > 0.077; Supplementary Figure S3).
We found no significant circadian rhythm in T300 (geometric mean 42.6 s, [95% CI 39.5 to 89.5]; fundamental: p > 0.53; Figure 3, A and C). Consistent with this result, there was no significant circadian variation in the HR decrease from exercise to 1 and 2 min into recovery (29.8 ± 0.8 bpm [95% CI 28.3 to 31.3], 34.9 ± 0.7 bpm [95% CI 33.6 to 36.3], respectively; fundamental: p > 0.34, time since recovery: p < 0.0001; Figure 3, B and D).
Discussion
To the best of our knowledge, this is the first report of the endogenous circadian effects on the recovery of BP and HR after exercise. We discovered that, independent of environmental and behavioral changes, the human endogenous circadian system influences the post-exercise recovery of systolic BP, with the fastest recovery in the biological late afternoon and slower recovery across the biological night and into the biological morning. However, we did not find significant circadian variations in diastolic BP or HR recovery following exercise. These data could have implications for exercise prescription or interpreting clinical tests of stress recovery.
Previous studies on diurnal or circadian variations in hemodynamic responses to exercise
There are clear day/night patterns in HR and BP in humans at rest and during exercise [28]. However, it is less clear whether such patterns persist during recovery from exercise, with studies showing either no diurnal patterns in HR recovery [29], less HR recovery in the afternoon [30, 31], or greater systolic BP declines following morning [32] or afternoon exercise [33, 34]. Since hemodynamic variables are sensitive to changes in body posture, fluid loss/intake, preceding activity levels and thermal stresses, it is possible that discrepancies in the results of these studies might be due to differences in these conditions between studies and between different times of day. Thus, to separate endogenous circadian effects from potential behavioral/environmental effects (e.g., induced by light–dark, rest–activity, fasting/feeding, sleep–wake cycles), we used a highly controlled FD protocol to standardize behavioral and environmental factors across the circadian cycle. Discrepancies between previous studies also might be due to differences in the definitions of post-exercise recovery which encompassed changes between peak exercise and recovery, changes between baseline and recovery, or the absolute values during recovery. Moreover, all of these indices would be influenced by underlying diurnal variations in HR and BP. Thus, to better compare the time-of-day effects on the magnitudes of recovery, in the current analyses we accounted for such diurnal variations. In our previous work, we demonstrated that there are endogenous circadian effects on the reactivity of systolic BP to exercise, with the greatest rise in the biological evening [14]. Interestingly, while this peak is close to the fastest recovery of systolic BP post-exercise presented here, our further analyses suggested that the circadian rhythm in the reactivity does not fully account for the circadian rhythm in the recovery rate. These results may suggest that different downstream pathways are involved in the circadian modulation of the hemodynamic reactivity and recovery to behavioral challenges.
Potential mechanisms underlying circadian variations in post-exercise hemodynamic recovery
Recovery of HR and BP following exercise involves multiple complex mechanisms [7]. For instance, HR deceleration is attributable to a coordinated interaction of parasympathetic re-activation and sympathetic withdrawal, with parasympathetic re-activation predominating in the early phase after exercise [8, 35]. Indeed, the time constant (T300) of the immediate HR drop following exercise has been used as marker of cardiac parasympathetic tone [8, 23, 36]. As we did not find a significant circadian rhythm in T300, we found no evidence of circadian regulation of post-exercise parasympathetic re-activation. The fall in systolic BP after exercise is attributed to a delayed increase in systemic vascular resistance to match the reduction in cardiac output. This is achieved by a combination of baroreflex resetting (results in reduced sympathetic outflow), reduced signal transduction from sympathetic activation into vasoconstriction, and local vasodilator mechanisms [37, 38]. Previous studies focusing on the daily pattern of exercise responses have shown that: (1) post-exercise forearm blood flow peaked between 3 and 7 pm [39], and (2) slower increase in systemic vascular resistance following afternoon exercise (4 pm) than morning exercise (8 am) [33]. These observations suggest faster post-exercise decline of systolic BP in the afternoon. Although the relative circadian contribution in these cases is not clear, these results are consistent with our observation of the fastest systolic BP recovery in the biological late afternoon. Moreover, both animal and human experimental work has found significant circadian regulation of vascular function through SCN projections and peripheral oscillators located in the vasculature [40, 41]. Taken together, it is possible that the observed circadian rhythm in systolic BP recovery is due to a circadian-governed slower return (increase) in systemic vascular resistance after exercise.
Potential implications
According to the Exercise Standards Statement from the American Heart Association (2013), continuous monitoring of HR and BP after the clinical exercise stress test is highly recommended, because some abnormal cardiac responses occur only in the recovery period [38]. Hemodynamic recovery after exercise is widely used to detect coronary artery disease, and predict cardiovascular events and all-cause death [2, 5, 26, 27, 42]. Thus, to ensure the reliability of the exercise test, it is important to account for any circadian variations in the measurements. We note that the sluggish recovery in the morning coincides with the highest frequency of adverse cardiovascular events in the general population [43], and it may be worth studying this phenomenon further in more vulnerable clinical populations. Moreover, it seems possible that the relatively sluggish recovery of systolic BP across the night could have relevance to night-shift workers. Our data also have possible implications for the timing of exercise when used therapeutically, especially considering that the circadian amplitude of systolic BP recovery (5.5 mmHg or 9.2%) is comparable to the order of magnitude achieved by antihypertensive medications [44]. A recent study recognized that the magnitude of BP decrease immediately following acute exercise predicts the BP lowering effect of chronic exercise [6]. Importantly, Brito and colleagues, using a randomized-control trial, found that treated hypertensive patients had more reduction in mean BP after a 10 week aerobic training performed in the evening than in the morning [45].
Strengths and limitations
Strengths of this study include the gold standard circadian protocol, the within-participant comparison, and the highly controlled in-laboratory monitoring which allowed us to separate circadian effects from any behavioral and environmental factors. Limitations of the study include the relatively small sample size. Thus, we may have been underpowered to statistically detect the circadian rhythm in some of the outcome variables. Nonetheless, this sample size is in keeping with similar prolonged, highly controlled, within-participant studies. Second, we used short submaximal exercise workload (15 min at 60%-HRmax) to minimize training effects of repeated testing. The circadian variations in hemodynamic recovery may be different with different forms of exercise. Third, we studied healthy young adults without a history of cardiovascular diseases. It is important to validate our findings in vulnerable populations who may have an altered circadian profile of cardiovascular function. It would also be of interest for future studies to examine whether the circadian rhythm in hemodynamic recovery may change in trained athletes, or in people who consistently perform exercise at a specific time of day. Fourth, we did not control the time of the study relative to the menstrual cycle, so we could not formally assess the effect of menstrual phase on any of the results in females. Furthermore, we only have hemodynamic measures for up to 20 min after exercise, and only measured BP every 5 min, such that we may have missed some resolution of the BP response dynamics. Last, it would be interesting to know whether the quicker drop of systolic BP in the first 10 min after exercise in the biological late afternoon can translate into a bigger magnitude of sustained post-exercise hypotension.
Supplementary Material
Acknowledgments
We thank the research volunteers, research staff, recruiters, and Brigham and Women’s Hospital’s Center for Clinical Investigation nursing and technical staff.
Ethics Approval
The study protocol was approved by the Partners Human Research Committee at Clinical and Translational Research Center of Brigham and Women’s Hospital. All participants provided written informed consent prior to enrollment and this consent could be revoked by the participants at any time.
Authors’ contributions
FAJLS and SAS designed research. FAJLS, KH, and SAS performed research and collected data. JQ and KH analyzed data. JQ, FAJLS, and SAS wrote the manuscript. All authors interpreted the data and revised the manuscript critically for important intellectual content.
Funding
This work was supported by the National Institutes of Health [R01HL76409 and NCRR-GCRC-M01-RR02635]. J.Q. was supported in part by American Diabetes Association grant #1-17-PDF-103 and R01DK102696. F.A.J.L.S. was supported in part by R01HL118601, R01DK099512, R01DK102696, R01DK105072, and R01HL140574. K.H. was in part supported by R01AG048108 and R01AG059867. S.A.S. was supported in part by R01HL125893, R01HL142064, R01HL125893-03S1, R01HL140577, and the Oregon Institute of Occupational Health Sciences via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Conflict of interest statement. FAJLS received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer. JQ, KH, and SAS declare no conflicts of financial interest. All authors declare no conflicts of non-financial interests.
References
- 1. Fletcher GF, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694–1740. [DOI] [PubMed] [Google Scholar]
- 2. Cole CR, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341(18): 1351–1357. [DOI] [PubMed] [Google Scholar]
- 3. Shetler K, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38(7):1980–1987. [DOI] [PubMed] [Google Scholar]
- 4. McHam SA, et al. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. J Am Coll Cardiol. 1999;34(3):754–759. [DOI] [PubMed] [Google Scholar]
- 5. Singh JP, et al. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99(14): 1831–1836. [DOI] [PubMed] [Google Scholar]
- 6. Liu S, et al. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med Sci Sports Exerc. 2012;44(9):1644–1652. [DOI] [PubMed] [Google Scholar]
- 7. Romero SA, Minson CT, Halliwill JR. The cardiovascular system after exercise. J Appl Physiol (1985). 2017;122 (4):925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borresen J, et al. Autonomic control of heart rate during and after exercise: measurements and implications for monitoring training status. Sports Med. 2008;38(8):633–646. [DOI] [PubMed] [Google Scholar]
- 9. Thosar SS, et al. Role of the circadian system in cardiovascular disease. J Clin Invest. 2018;128(6):2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheer FA, et al. Cardiovascular control by the suprachiasmatic nucleus: neural and neuroendocrine mechanisms in human and rat. Biol Chem. 2003;384(5):697–709. [DOI] [PubMed] [Google Scholar]
- 11. Schroeder A, et al. Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol. 2011;300(1):H241–H250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curtis AM, et al. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA. 2007;104(9):3450–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anea CB, et al. Vascular disease in mice with a dysfunctional circadian clock. Circulation. 2009;119(11):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheer FA, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA. 2010;107(47):20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu K, et al. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 2011;123(9):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheer FA, et al. Impact of mental stress, the circadian system and their interaction on human cardiovascular function. Psychoneuroendocrinology. 2019;103:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheer FA, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One. 2011;6(9):e24549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheer FA, et al. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring). 2013;21(3):421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scheer FA, et al. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood. 2014;123(4):590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shea SA, et al. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Czeisler CA, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–2181. [DOI] [PubMed] [Google Scholar]
- 22. Qian J, et al. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27(5):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imai K, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24(6):1529–1535. [DOI] [PubMed] [Google Scholar]
- 24. Perini R, et al. Plasma norepinephrine and heart rate dynamics during recovery from submaximal exercise in man. Eur J Appl Physiol Occup Physiol. 1989;58(8):879–883. [DOI] [PubMed] [Google Scholar]
- 25. Cole CR, et al. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132(7):552–555. [DOI] [PubMed] [Google Scholar]
- 26. Huang CL, et al. Usefulness of paradoxical systolic blood pressure increase after exercise as a predictor of cardiovascular mortality. Am J Cardiol. 2008;102(5):518–523. [DOI] [PubMed] [Google Scholar]
- 27. Laukkanen JA, et al. Systolic blood pressure during recovery from exercise and the risk of acute myocardial infarction in middle-aged men. Hypertension. 2004;44(6):820–825. [DOI] [PubMed] [Google Scholar]
- 28. Reilly T, et al. Some circulatory responses to exercise at different times of day. Med Sci Sports Exerc. 1984;16(5):477–482. [DOI] [PubMed] [Google Scholar]
- 29. Brito L, et al. Time of day affects heart rate recovery and variability after maximal exercise in pre-hypertensive men. Chronobiol Int. 2015;32(10):1385–1390. [DOI] [PubMed] [Google Scholar]
- 30. Prodel E, et al. Different times of day do not change heart rate variability recovery after light exercise in sedentary subjects: 24 hours Holter monitoring. Chronobiol Int. 2017;34(10):1354–1365. [DOI] [PubMed] [Google Scholar]
- 31. Cohen CJ, et al. Human circadian rhythms in resting and exercise pulse rates. Ergonomics. 1977;20(5):475–479. [DOI] [PubMed] [Google Scholar]
- 32. de Brito LC, et al. Post-exercise hypotension and its mechanisms differ after morning and evening exercise: a randomized crossover study. PLoS One. 2015;10(7):e0132458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones H, et al. The acute post-exercise response of blood pressure varies with time of day. Eur J Appl Physiol. 2008;104(3):481–489. [DOI] [PubMed] [Google Scholar]
- 34. Jones H, et al. Post-exercise blood pressure reduction is greater following intermittent than continuous exercise and is influenced less by diurnal variation. Chronobiol Int. 2009;26(2):293–306. [DOI] [PubMed] [Google Scholar]
- 35. Stanley J, et al. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med. 2013;43(12):1259–1277. [DOI] [PubMed] [Google Scholar]
- 36. Pierpont GL, Voth EJ. Assessing autonomic function by analysis of heart rate recovery from exercise in healthy subjects. Am J Cardiol. 2004;94(1):64–68. doi: 10.1016/j.amjcard.2004.03.032 [DOI] [PubMed] [Google Scholar]
- 37. Halliwill JR, et al. Postexercise hypotension and sustained postexercise vasodilatation: what happens after we exercise? Exp Physiol. 2013;98(1):7–18. [DOI] [PubMed] [Google Scholar]
- 38. Fletcher GF, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128(8):873–934. [DOI] [PubMed] [Google Scholar]
- 39. Kaneko M, et al. Circadian variation in human peripheral blood flow levels and exercise responses. J Appl Physiol. 1968;25(2):109–114. [DOI] [PubMed] [Google Scholar]
- 40. Thosar SS, et al. Circadian rhythm of vascular function in midlife adults. Arterioscler Thromb Vasc Biol. 2019;39(6):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paschos GK, et al. Circadian clocks and vascular function. Circ Res. 2010;106(5):833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nishime EO, et al. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284(11):1392–1398. [DOI] [PubMed] [Google Scholar]
- 43. Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12(2 Pt 2):35S–42S. [DOI] [PubMed] [Google Scholar]
- 44. Staessen JA, et al. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358(9290):1305–1315. [DOI] [PubMed] [Google Scholar]
- 45. Brito LC, et al. Morning versus evening aerobic training effects on blood pressure in treated hypertension. Med Sci Sports Exerc. 2019;51(4):653–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.