Abstract
Objectives
To evaluate the human abuse potential of pitolisant, a selective histamine 3 (H3)-receptor antagonist/inverse agonist recently approved by the US Food and Drug Administration for the treatment of excessive daytime sleepiness in adult patients with narcolepsy.
Methods
Nondependent, recreational stimulant users able to distinguish phentermine HCl 60 mg from placebo in a drug discrimination test were randomized in a four-period, double-blind, crossover design to receive single doses of pitolisant 35.6 mg (therapeutic dose), pitolisant 213.6 mg (supratherapeutic dose), phentermine HCl 60 mg, and placebo. The primary endpoint was maximum effect (Emax) on the 100-point Drug Liking (“at this moment”) visual analog scale.
Results
In 38 study completers (73.7% male; 65.8% white; mean age, 33.3 years), mean Drug Liking Emax was significantly greater for phentermine versus pitolisant 35.6 mg (mean difference, 21.4; p < 0.0001) and pitolisant 213.6 mg (mean difference, 19.7; p < 0.0001). Drug Liking Emax was similar for pitolisant (both doses) and placebo. Similarly, for key secondary measures of Overall Drug Liking and willingness to Take Drug Again, mean Emax scores were significantly greater for phentermine versus pitolisant (both doses) and similar for pitolisant (both doses) versus placebo. The incidence of adverse events was 82.1% after phentermine HCl 60 mg, 72.5% after pitolisant 213.6 mg, 47.5% after pitolisant 35.6 mg, and 48.8% after placebo administration.
Conclusions
In this study, pitolisant demonstrated significantly lower potential for abuse compared with phentermine and an overall profile similar to placebo; this suggests a low risk of abuse for pitolisant.
Clinical Trial Registration
ClinicalTrials.gov NCT03152123. Determination of the abuse potential of pitolisant in healthy, nondependent recreational stimulant users. https://clinicaltrials.gov/ct2/show/NCT03152123.
Keywords: drug abuse, narcolepsy, pitolisant
Statement of Significance.
Except for pitolisant, all medications approved in the United States for the treatment of narcolepsy are controlled substances due to their potential for abuse. Pitolisant, a selective histamine 3 (H3)-receptor antagonist/inverse agonist that enhances the activity of histaminergic neurons in the brain, was recently approved by the US Food and Drug Administration (FDA) for the treatment of excessive daytime sleepiness in adult patients with narcolepsy. This study shows that pitolisant has an abuse potential profile similar to that of placebo and significantly lower than that of phentermine (a mild stimulant), with no findings suggestive of a risk of abuse. Based on these findings, along with preclinical data, pitolisant was approved by the FDA without being scheduled as a controlled substance.
Introduction
Narcolepsy is a chronic, debilitating, rare neurological disorder of sleep–wake state instability that is characterized by excessive daytime sleepiness (EDS), cataplexy, and other manifestations of rapid eye movement (REM) sleep dysregulation [1, 2]. Narcolepsy imposes substantial psychosocial and economic burdens that include reductions in work productivity and quality of life, disruptions to interpersonal relationships, and increases in healthcare resource usage and associated costs [3–6]. For most patients, the management of narcolepsy requires lifelong pharmacological treatment [7, 8].
Stimulants and wake-promoting agents, approved for the treatment of EDS, act primarily via dopaminergic mechanisms [9–12]; these agents are prone to abuse and thus are classified as controlled substances. Stimulant abuse continues to be a public health concern. In the United States (2017), an estimated 1.8 million persons aged 12 or older (including 715,000 young adults aged 18 to 25 years) reported misusing prescription stimulants in the previous month [13]. Abuse of stimulants has been linked to multiple health concerns including cardiovascular disease and psychiatric symptoms, with methamphetamine abuse leading to particularly severe consequences (e.g. increased risk of stroke) [14–18]. Sodium oxybate, a central nervous system depressant approved for the treatment of EDS and cataplexy, works through an unknown mechanism of action, but the therapeutic effect is thought to be mediated by activity at gamma-aminobutyric acid B receptors [19, 20]. Sodium oxybate has a boxed warning regarding its risk of abuse (Schedule III controlled substance) and is available only via a restricted access Risk Evaluation and Mitigation Strategy (REMS) program [19]. Hence, there is a need for narcolepsy medications with novel mechanisms of action that are associated with minimal or no abuse potential.
Pitolisant is a first-in-class medication for the treatment of narcolepsy, with a novel mechanism of action [21, 22]. As a potent, highly selective histamine 3 (H3) receptor antagonist/inverse agonist, pitolisant enhances the activity of histaminergic neurons in the brain and activates the release of other wake-promoting neurotransmitters [21, 23, 24]. In contrast to known drugs of abuse (e.g. amphetamines, opioids, ethanol, nicotine, and cocaine) [25, 26] and wake-promoting agents (i.e. modafinil and armodafinil) [11], pitolisant does not work primarily through dopamine and does not cause an increase in dopamine levels in the nucleus accumbens (the brain’s main reward center) [27]. No findings suggestive of abuse potential have been observed in preclinical or clinical studies of pitolisant [27, 28].
The efficacy of pitolisant in the treatment of EDS and cataplexy in patients with narcolepsy has been demonstrated in randomized, placebo-controlled trials [28, 29]. Pitolisant is approved by the European Medicines Agency for the treatment of narcolepsy with or without cataplexy in adults [22, 30], and is not considered a controlled substance in the European Union. Pitolisant was recently approved by the US Food and Drug Administration (FDA) for the treatment of EDS in adult patients with narcolepsy [31]. As pitolisant is the first agent in a new pharmacological class that has central nervous system activity, it was necessary in the United States to evaluate the abuse potential of the drug in accordance with FDA requirements [32]. For example, assessment of the abuse potential of an investigational medication requires comparison with both an active drug with known abuse potential and placebo [32]. In the case of pitolisant, although it is not a psychostimulant, it has demonstrated wake-promoting effects [28, 29]; therefore, a moderately active psychostimulant (i.e. phentermine HCl; Schedule IV) would serve as an appropriate active comparator.
The current study was conducted in accordance with the 2017 FDA guidance on performing human abuse potential studies (Supplementary Table S1) [32]. The primary objective was to assess the abuse potential of pitolisant relative to phentermine and placebo after single-dose administration in nondependent, recreational stimulant users. The secondary objectives were to evaluate the pharmacokinetic profile of pitolisant and assess the safety and tolerability of a single dose of the drug.
Methods
Study design
This was a single-dose, randomized, double-blind, active- and placebo-controlled, four-sequence, four-period crossover study conducted at one clinical site in Toronto, Canada, between March 2017 and October 2017. The study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki (1964); the guidelines for Good Clinical Practice, as outlined by the International Conference on Harmonisation; and FDA guidance [32] on the assessment of the abuse potential of drugs. The study protocol was submitted to the FDA (Controlled Substance Staff) before conduct of the study (with no comments received) and was approved by the institutional review board IRB Services. All participants provided written informed consent before study-related procedures began.
Participants
This study enrolled healthy males and females aged 18 to 55 years (inclusive) with body mass index (BMI) in the range of 18.0 to 33.0 kg/m2 (inclusive). All participants were recreational stimulant users, defined as users of stimulants for nontherapeutic purposes (i.e. psychoactive effects) at least 10 times in the past year and at least 1 time in the previous 8 weeks.
Potential participants were excluded if they had a history of alcohol or other drug dependence (excluding caffeine and nicotine) within the previous 2 years, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria [33], and/or a history of attendance or a plan to participate in a rehabilitation program to treat alcohol or other substance dependence. Other exclusion criteria included clinically significant medical conditions, any history of suicidal ideation or behavior, and heavy use of tobacco products (>20 cigarettes per day and/or inability to abstain from nicotine-containing products for ≥6 hr per day). Use of concomitant medications or natural health products was prohibited, except for nicotine-containing substances, acetaminophen, vitamin or mineral supplements, selected contraceptives, and hormone replacement therapy. Negative alcohol breath test and negative urine drug screen results were required before admission for each study visit, although positive results for tetrahydrocannabinol (THC) were permitted at the discretion of the investigator, provided that residual levels were not indicative of recent use.
Procedure
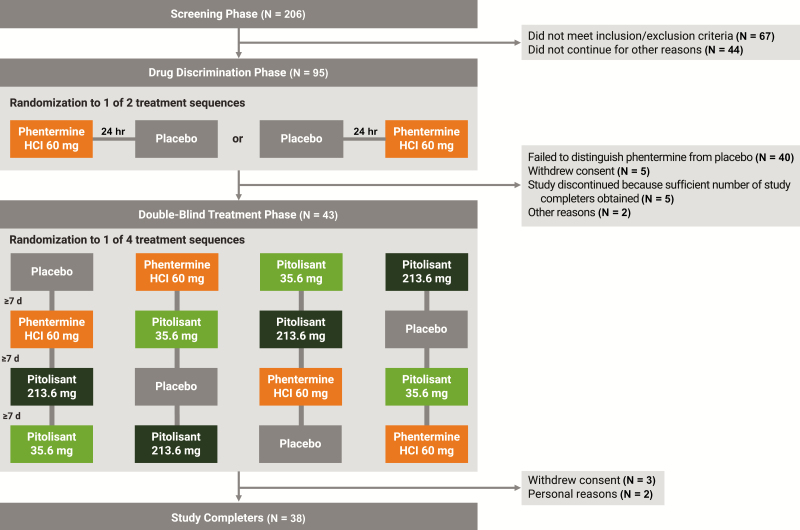
Following a screening visit, a drug discrimination test was first conducted to ensure that enrolled participants could discern the effects of the active comparator (phentermine HCl 60 mg) compared with placebo (Figure 1). After an overnight fast, participants received, in randomized order, phentermine or placebo, administered in a double-blind crossover manner, with each dose separated by approximately 24 hr. Ability to discriminate phentermine from placebo was based on the 100-point, bipolar Drug Liking visual analog scale (VAS), anchored with “strong disliking” at 0, “neither like nor dislike” at 50, and “strong liking” at 100, and defined by all of the following: peak score for phentermine of at least 65 points, peak score for placebo between 40 and 60 points (inclusive), and score difference of at least +15 points for phentermine relative to placebo. Consistent with the FDA guidance [32], only those participants who “report drug liking in response to the positive control and demonstrate a meaningfully different response from that produced by placebo…should participate in the Treatment Phase.” This methodology was used because a study population that is able to discriminate between the positive control (in the class of drug being tested) and placebo is necessary to assess for drug liking of the investigational agent. Participants were eligible to continue into the double-blind treatment phase if they met the discrimination criteria and were able to tolerate phentermine HCl 60 mg (e.g. no emesis within 4 hr postdose).
Figure 1.
Study design and participant disposition. HCl = hydrochloride.
During the double-blind treatment phase, each participant was to receive all four treatments: pitolisant 35.6 mg (optimal therapeutic dose), pitolisant 213.6 mg (supratherapeutic dose), phentermine HCl 60 mg (active comparator), and placebo. Participants were assigned to receive one of four treatment sequences via computer-generated randomization according to a 4 x 4 Williams square randomization design. Study medication was administered in a randomized, double-blind, crossover manner, with a minimum 7 day washout period between treatments. To maintain the integrity of the double-blind administration, participants received four matching capsules containing active drug and/or placebo at each treatment visit. Study medication was administered in the morning after a fasting period of at least 8 hr, and participants were required to fast for at least 4 hr postdose. Participants were admitted to the clinical research unit the day before each treatment was administered and remained until approximately 24 hr after dosing or until discharge was deemed safe by the investigator.
Pharmacodynamic measures
Pharmacodynamic measures used to assess subjective drug effects were consistent with FDA guidance for abuse potential studies (Table 1) [32]. The primary endpoint was the maximum effect (Emax) for Drug Liking (“at this moment”) assessed on a bipolar (0 to 100) VAS. Key secondary endpoints included Emax for global drug effects (i.e. Overall Drug Liking, Take Drug Again) and positive drug effects (i.e. Good Drug Effects, High). Additional VAS scores assessed other drug effects (e.g. Any Drug Effects, Bad Drug Effects, Relaxation/Agitation Effects, Drug Similarity). A modified version of the Addiction Research Center Inventory (ARCI) [34] consisted of items from four subscales: the Morphine-Benzedrine Group (MBG) scale, a measure of euphoria; the Amphetamine and Benzedrine Group scales, to assess stimulant effects; and the Lysergic Acid Diethylamide (LSD) scale, a measure of dysphoria.
Table 1.
Pharmacodynamic measures used to assess drug liking and other drug effects
| Measure | Description | Time(s) administered |
|---|---|---|
| Drug Effects VAS |
|
Predosea and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 24 hr postdose |
| Overall Drug Liking VAS |
|
8, 12, and 24 hr postdose |
| Take Drug Again VAS |
|
8, 12, and 24 hr postdose |
| Drug Similarity VAS |
|
24 hr postdose |
| Addiction Research Center Inventory [34] |
|
Predose and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 24 hr postdose |
aHigh and Relaxation/Agitation scales only.
BG = Benzedrine Group; LSD = lysergic acid diethylamide; MBG = Morphine–Benzedrine Group; THC = tetrahydrocannabinol; VAS = visual analog scale.
Pharmacokinetic evaluation
During each treatment period, a 6-mL blood sample was collected for pharmacokinetic analysis at each of the following time points: predose and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 16, and 24 hr postdose. The pharmacokinetic parameters calculated were maximum observed serum concentration (Cmax), time to reach maximum serum concentration (tmax), and area under the concentration-time curve from time 0 to the last measurable concentration (AUC0-last).
Safety evaluation
The incidence and severity of adverse events were assessed during the drug discrimination and treatment phases, from the time of study drug administration until approximately 24 hr postdose. Other safety assessments included vital signs measurements, physical examination findings, clinical laboratory test results, electrocardiogram parameters, and Columbia-Suicide Severity Rating Scale (C-SSRS) score [35].
Statistical analyses
The study was planned to randomize 44 participants into the treatment phase to ensure that at least 36 participants completed the study. As determined by a published algorithm [36], adjusting for four periods and four sequences, with a 1-sided significance level of 0.05 and a phentermine-placebo difference of 5 points, a sample size of 36 participants was estimated to have at least 90% power to detect a difference in Drug Liking VAS Emax, assuming within-group standard deviations of 17.5 for phentermine and 10.2 for placebo (based on unpublished data collected at the study site).
The analysis population for the pharmacodynamic endpoints consisted of participants who completed all four treatment periods and had at least one assessment on the Drug Liking VAS within 2 hr of tmax for each treatment (completers population). The pharmacokinetic analysis population included participants who received at least one dose of pitolisant in the treatment phase and had at least one evaluable serum pitolisant concentration postdose. The safety population included participants who received at least one dose of any study medication in the treatment phase.
Pharmacodynamic endpoints were analyzed using a mixed-effects model for a crossover study. The model included treatment, period, treatment sequence, and first-order carryover effect (if applicable) as fixed effects; baseline (predose) measurement as a covariate (if applicable); and participant nested within treatment sequence as a random effect. The residuals from the mixed-effects model were investigated for normality, and parametric (e.g. paired t-test) or nonparametric (e.g. sign test) analyses were conducted, as appropriate. Comparisons were 1-tailed for the primary and key secondary endpoints and 2-tailed for all other secondary endpoints; the significance level was set at 0.05 without adjustment for multiplicity.
For the primary endpoint (Drug Liking Emax), statistical analyses evaluated three discrete questions: (1) did phentermine produce reliable abuse-related responses compared with placebo (to assess study validity); (2) did pitolisant produce abuse-related responses that were smaller than those of phentermine (to assess the relative abuse potential of pitolisant); and (3) did pitolisant produce abuse-related responses that were similar to placebo (to assess the absolute abuse potential of pitolisant). For the first question, study validity was evaluated by comparing Drug Liking Emax between phentermine (the active control) and placebo; statistical testing evaluated the hypothesis that the mean score for phentermine was at least 5 points higher than the mean score for placebo. A statistically significant result would indicate that phentermine differed from placebo. Second, for comparisons of pitolisant (35.6 and 213.6 mg) to phentermine on Drug Liking Emax, statistical testing evaluated the hypothesis that the mean score for phentermine was higher (by any amount) than the mean score for pitolisant. A statistically significant result would indicate that phentermine differed from pitolisant. Third, for comparisons of pitolisant (35.6 and 213.6 mg) to placebo on Drug Liking Emax, statistical testing evaluated the hypothesis that the mean score for pitolisant was less than 11 points greater than the mean score for placebo. The margin of 11 points was based on recommendations from a meta-analysis of eight abuse potential studies [37]. For the third hypothesis, a statistically significant result would mean that pitolisant was similar to placebo; this hypothesis testing is in contrast to the statistical evaluation used in efficacy studies but is appropriate for measures of safety (such as abuse potential). Evaluation of key secondary endpoints was based on the same hypothesis testing conducted for Drug Liking Emax.
For other pharmacodynamic endpoints, statistical testing evaluated the hypothesis that the difference between treatments was not equal to 0, with the absence of a statistically significant between-group difference indicating that mean Emax values were not different.
Pharmacokinetic parameters for pitolisant (Cmax, tmax, AUC0-last) were calculated from serum concentration data using standard noncompartmental analysis and were summarized using descriptive statistics. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
Results
Participants
A total of 95 participants were enrolled in the drug discrimination phase (Figure 1). Of 43 randomized participants, 38 completed the study and constitute the primary analysis (completers) population. Participants in the completers population were predominantly male (73.7%) and white (65.8%), with a mean (range) age of 33.3 (21 to 54) years and a mean (range) BMI of 24.6 (18.9 to 32.5) kg/m2. In addition to recreational stimulant use (required for study participation, per protocol), almost all randomized participants (95.3%) reported a history of alcohol use. Recreational drug use also included cannabinoids (95.3%), morphine or other opioids (58.1%), central nervous system depressants (55.8%), hallucinogens (39.5%), and dissociative anesthetics (25.6%).
Primary pharmacodynamic outcome measure
Study validity and sensitivity were confirmed by the statistically significant difference in mean Drug Liking Emax for phentermine (78.7) compared with placebo (56.1; p < 0.0001; Table 2). Phentermine also differed significantly from placebo in the maximum effect (Emax) on all other VAS and ARCI scales, except for Bad Drug Effects (Table 3).
Table 2.
Analysis results for Drug Liking (“at this moment”) VAS Emax (primary endpoint) in the completers population (N = 38)
| Comparison | Mean (SE) or median (Q1-Q3) of the paired difference | P |
|---|---|---|
| Phentermine HCl 60 mg vs placeboa | 22.7 (2.86) | < 0.0001 |
| Phentermine HCl 60 mg vs pitolisant 35.6 mga | 21.4 (3.16) | < 0.0001 |
| Phentermine HCl 60 mg vs pitolisant 213.6 mga | 19.7 (3.52) | < 0.0001 |
| Pitolisant 35.6 mg vs placebob | 0.0 (0.0 to 6.0) | < 0.0001 |
| Pitolisant 213.6 mg vs placebob | 0.0 (0.0 to 11.0) | 0.0013 |
aPaired t-test was used to assess the difference between the two treatments. For these comparisons, the null hypothesis was that the mean difference for phentermine vs placebo was ≤5 and that the mean difference for phentermine vs pitolisant was ≤0.
bSign test (which evaluated statistical significance based on the proportion of patients for whom the pitolisant–placebo difference in Drug Liking Emax exceeded the prespecified threshold of 11) was used to assess the difference between the two treatments, because the paired differences were not normally distributed or quite symmetric. For these comparisons, the null hypothesis was that the difference between medians for pitolisant vs placebo was ≥11. Therefore, the significant results indicate that the null hypothesis was rejected and the Drug Liking Emax scores were similar for pitolisant and placebo.
Emax = peak maximum effect; Q1 = 25th percentile; Q3 = 75th percentile; SE = standard error; VAS = visual analog scale.
Table 3.
Summary of pharmacodynamic measures: mean (SE) Emax scores in the completers population (N = 38)a
| Measure | Placebo | Pitolisant 35.6 mg | Pitolisant 213.6 mg | Phentermine HCl 60 mg |
|---|---|---|---|---|
| Primary endpointb | ||||
| Drug Liking (“at this moment”) | 56.1 (2.1) | 57.3 (2.1)**** ,††††,c,d | 59.0 (2.1)**** ,††,c,d | 78.7 (2.8)†††† |
| Key secondary endpointsb | ||||
| Overall Drug Liking | 54.4 (2.2) | 52.7 (2.1)**** ,††††,c,d | 49.2 (4.3)** ,†††,c,d | 77.4 (3.8)†††† |
| Take Drug Again | 51.0 (2.9) | 49.4 (3.4)**** ,††††,c,d | 44.5 (4.9)**** ,†††,c,d | 78.7 (4.3)†††† |
| Good Drug Effects | 15.3 (4.4) | 15.7 (4.0)**** ,†,c,d | 26.3 (4.8)****c | 62.9 (5.2)†††† |
| High | 12.3 (3.9) | 15.7 (4.1)**** ,c | 35.3 (5.6)*** ,c | 58.6 (5.0)†††† |
| Other secondary endpointse | ||||
| Bad Drug Effects | 7.4 (3.2) | 6.7 (2.9) | 28.2 (5.7)* ,†† | 14.7 (3.5) |
| Relaxation/Agitation | 52.4 (2.1) | 53.2 (2.8) | 60.8 (2.9)†† | 62.9 (3.2)†† |
| Any Drug Effects | 17.2 (4.9) | 19.3 (4.5)**** | 41.6 (6.1)** ,††† | 63.6 (5.0)†††† |
| ARCI/MBG score | 3.4 (0.7) | 3.3 (0.7)**** | 3.9 (0.7)**** | 9.6 (0.9)†††† |
| ARCI/Amphetamine score | 2.7 (0.4) | 2.3 (0.4)**** | 3.1 (0.5)**** | 6.4 (0.5)†††† |
| ARCI/BG score | 6.0 (0.3) | 5.9 (0.3)**** | 6.1 (0.3)**** | 8.2 (0.4)†††† |
| ARCI/LSD score | 4.4 (0.3) | 4.5 (0.3)**** | 5.8 (0.4)†† | 6.1 (0.4)†††† |
a p values are based on a mixed-effects model for a crossover study and included treatment, period, treatment sequence, baseline, and carryover effects.
bBecause of the structure of the statistical hypothesis testing for the primary and key secondary endpoints, significant results for pitolisant compared with placebo indicate that mean Emax values were similar for pitolisant and placebo.
cStatistically significant comparison with phentermine indicates difference.
dStatistically significant comparison with placebo indicates similarity.
eFor other secondary endpoint comparisons, statistical significance indicates difference.
*p < 0.05 versus phentermine; **p < 0.01 versus phentermine; ***p < 0.001 versus phentermine; ****p < 0.0001 versus phentermine; †p < 0.05 versus placebo; ††p < 0.01 versus placebo; †††p < 0.001 versus placebo; ††††p < 0.0001 versus placebo.
ARCI = Addiction Research Center Inventory; BG = Benzedrine Group; Emax = peak maximum effect; LSD = lysergic acid diethylamine; MBG = Morphine-Benzedrine Group; SE = standard error.
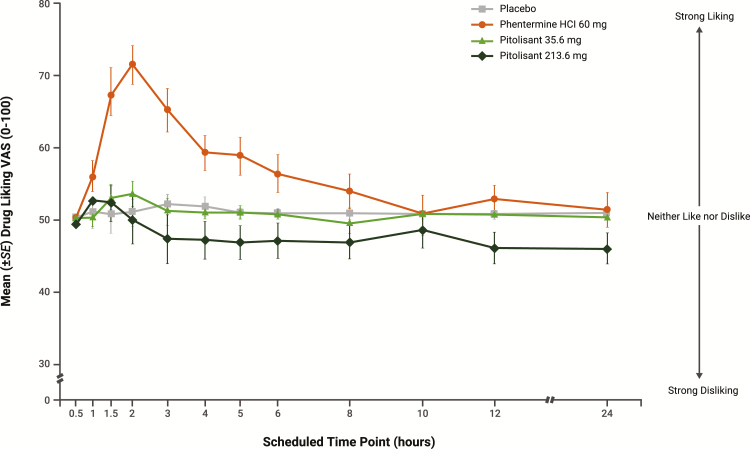
At early time points and continuing for up to 10 hr, mean scores for the primary outcome, Drug Liking (“at this moment”) VAS, were greater for phentermine compared with the other treatments; from 3 to 24 hr postdose, scores were similar for pitolisant 35.6 mg and placebo and consistently lower for pitolisant 213.6 mg relative to placebo (Figure 2). Mean Drug Liking Emax was significantly greater for phentermine (78.7) compared with pitolisant 35.6 mg (57.3) and pitolisant 213.6 mg (59.0; both p < 0.0001) and similar for pitolisant (both doses) compared with placebo (56.1). Because of the structure of the statistical hypothesis testing (difference between pitolisant and placebo < 11), the significant results for pitolisant 35.6 and 213.6 mg compared with placebo (Table 2) indicate that Drug Liking Emax scores were similar for pitolisant and placebo.
Figure 2.
Primary pharmacodynamic measure, Drug Liking VAS, over time (completers population, N = 38). Error bars represent ±1 SE. SE = standard error; VAS = visual analog scale.
Key secondary pharmacodynamic outcome measures
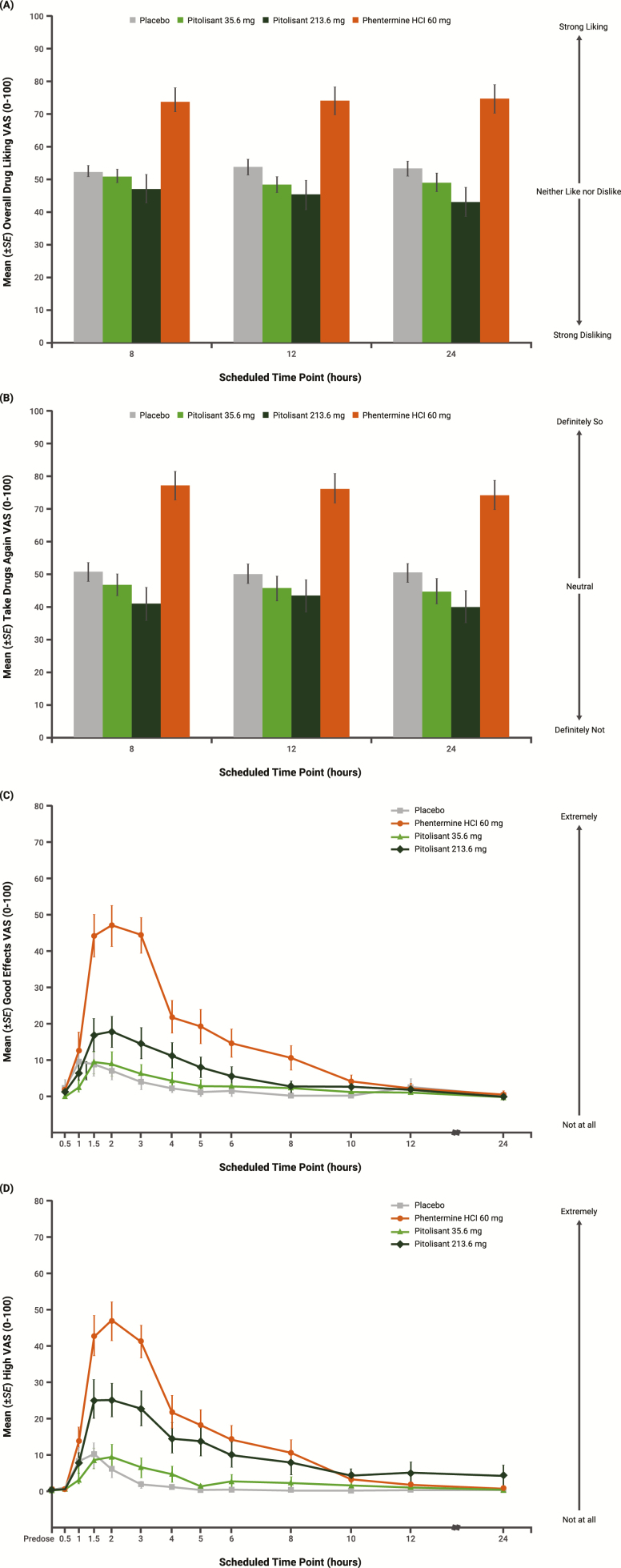
For the Overall Drug Liking VAS and Take Drug Again VAS, mean scores were greater for phentermine compared with both pitolisant treatments at all assessments (8, 12, and 24 hr postdose; Figure 3A and B); Emax scores were significantly greater for phentermine relative to pitolisant (both doses) and similar for pitolisant (both doses) compared with placebo (Table 3). Mean scores for the Good Drug Effects VAS were markedly greater for phentermine compared with pitolisant (both doses) between 1.5 and 3 hr postdose and remained higher until approximately 10 hr postdose (Figure 3C); mean Emax scores were significantly greater for phentermine relative to pitolisant (both doses) (Table 3). Pitolisant 35.6 mg was similar to placebo on Good Drug Effects Emax, but pitolisant 213.6 mg did not meet statistical criteria for similarity (<11-point difference from placebo). Mean scores for the High VAS were greatest for phentermine, intermediate for pitolisant 213.6 mg, and lowest for pitolisant 35.6 mg and placebo (Figure 3D). Mean Emax scores for High VAS were significantly greater for phentermine relative to pitolisant (both doses); neither dose of pitolisant met the statistical criteria for similarity (<11-point difference) to placebo (Table 3).
Figure 3.
Key secondary pharmacodynamic measures over time (completers population, N = 38) for (A) Overall Drug Liking, (B) Take Drug Again, (C) Good Drug Effects, and (D) High VAS. Error bars represent ±1 SE. SE = standard error; VAS = visual analog scale.
Other secondary pharmacodynamic outcome measures
On the other secondary VAS measures (Bad Drug Effects, Relaxation/Agitation, Any Drug Effects), mean Emax for pitolisant 35.6 mg was not different from placebo (Table 3). Emax on the Bad Drug Effects VAS was significantly greater for pitolisant 213.6 mg compared with both phentermine and placebo. On the Relaxation/Agitation VAS, Emax for pitolisant 213.6 mg was significantly different from placebo (in the direction of agitation) but not significantly different from phentermine. On the Drug Similarity VAS, pitolisant was rated as dissimilar (median score near 0) to all drug classes including stimulants, central nervous system (CNS) depressants, opioids, THC, hallucinogens, and dissociative drugs. For the stimulant and hallucinogen drug classes, median Drug Similarity VAS scores for phentermine were markedly greater than those for pitolisant or placebo. Median scores on the Overall Familiarity item were comparable (and near the neutral point) for pitolisant 35.6 mg (52.5) and placebo (52.0); median scores were in the direction of more familiar for phentermine (78.5) and less familiar for pitolisant 213.6 mg (19.0). Peak scores on the ARCI scales were significantly different for pitolisant (both doses) compared with phentermine and not different for pitolisant relative to placebo (Table 3), with the exception of the dysphoria (LSD) scale, on which mean Emax for pitolisant 213.6 mg was significantly greater than that for placebo and not different from phentermine.
Pharmacokinetic parameters
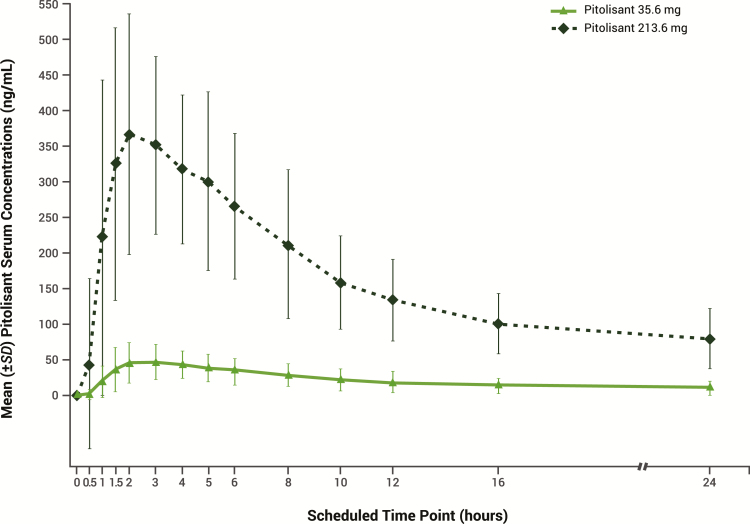
Mean (standard deviation [SD]) Cmax for pitolisant 35.6 mg was 54.5 (27.7) ng/mL and mean (SD) AUC0-last was 501.2 (308.4) ng·hr/mL (Figure 4); these parameters are consistent with the established pharmacokinetic profile of pitolisant. For pitolisant 213.6 mg (supratherapeutic dose), mean (SD) Cmax (425.0 [198.4] ng/mL) and AUC0-last (4001.5 [1523.9] ng·hr/mL) were approximately 7.8 times higher and 8.0 times higher, respectively, compared with pitolisant 35.6 mg. Median tmax was 3.0 hr for pitolisant 35.6 mg and 2.0 hr for pitolisant 213.6 mg. No pharmacokinetic and pharmacodynamic correlations or trends were identified between Cmax and the maximum pharmacodynamic effects (Emax) of pitolisant; in fact, higher plasma concentrations were associated with lower scores on the Drug Liking scale.
Figure 4.
Serum concentration of pitolisant over time (pharmacokinetic population, N = 40). SD = standard deviation.
Safety and tolerability
The overall rate of adverse events was similar for placebo and pitolisant 35.6 mg and somewhat higher for pitolisant 213.6 mg (supratherapeutic dose) and phentermine (Table 4). Headache was the most common adverse event for pitolisant, and the incidence appeared to be dose-related. The majority of adverse events were rated as mild in intensity by the investigator; adverse events rated as moderate were headache (3 participants after receiving placebo and 2 after receiving pitolisant 213.6 mg), inability to concentrate (1 participant after receiving phentermine), and vomiting (1 participant after receiving pitolisant 35.6 mg). There were no deaths, serious adverse events, or severe adverse events during the study, and no participants discontinued from the study because of adverse events.
Table 4.
Adverse events (safety population)a
| Adverse event, n (%)b | Placebo (N = 41) | Pitolisant 35.6 mg (N = 40) | Pitolisant 213.6 mg (N = 40) | Phentermine HCl 60 mg (N = 39) |
|---|---|---|---|---|
| Any adverse event | 20 (48.8) | 19 (47.5) | 29 (72.5) | 32 (82.1) |
| Headache | 5 (12.2) | 6 (15.0) | 10 (25.0) | 4 (10.3) |
| Euphoric mood | 4 (9.8) | 4 (10.0) | 7 (17.5) | 16 (41.0) |
| Somnolence | 6 (14.6) | 4 (10.0) | 3 (7.5) | 3 (7.7) |
| Hypervigilance | 1 (2.4) | 3 (7.5) | 5 (12.5) | 13 (33.3) |
| Nausea | 1 (2.4) | 2 (5.0) | 4 (10.0) | 0 (0.0) |
| Dizziness | 0 (0.0) | 1 (2.5) | 4 (10.0) | 1 (2.6) |
| Feeling hot | 0 (0.0) | 0 (0.0) | 4 (10.0) | 2 (5.1) |
aOccurring in >5% of participants for either pitolisant dose.
bCoded using the Medical Dictionary for Regulatory Activities version 20.0.
Six participants experienced clinically significant vital sign values that were recorded as adverse events (i.e. hypertension, blood pressure increased, and tachycardia), all after administration of phentermine. No participants exhibited any suicidal ideation or behavior during the study, as assessed by the C-SSRS.
Discussion
In this randomized, double-blind study involving recreational stimulant users, maximal scores on the primary endpoint of maximum Drug Liking (Emax) were significantly greater for phentermine HCl (60 mg) compared with pitolisant (single doses, 35.6 and 213.6 mg) and similar for pitolisant relative to placebo, with no apparent dose–response effect for pitolisant on Drug Liking. Results on the key secondary endpoints of Overall Drug Liking and willingness to Take Drug Again were supportive of the primary endpoint (Drug Liking “at this moment”). Across outcome measures, the profile for pitolisant 35.6 mg (the maximum therapeutic dose for the treatment of narcolepsy) was consistent with that for placebo, except for the High VAS, on which the maximal rating for pitolisant did not meet the statistical criteria for similarity with placebo.
Differences between phentermine and the supratherapeutic dose of pitolisant (213.6 mg) were evident on some pharmacodynamic measures. Maximal responses on the Good Drug Effects VAS and High VAS were significantly greater for phentermine compared with pitolisant 213.6 mg; peak scores for the supratherapeutic dose of pitolisant were greater than those for placebo. In addition, pitolisant 213.6 mg was associated with negative subjective effects, including significantly higher maximal scores than phentermine or placebo on the Bad Drug Effects VAS and significantly greater dysphoric effects than placebo on the ARCI-LSD scale, indicating that the higher dose of pitolisant produces aversive drug effects. Neither dose of pitolisant was associated with increased scores on the ARCI Amphetamine, Benzedrine Group, or MBG scales. By contrast, phentermine produced increased scores on all ARCI scales, indicative of significantly greater stimulant and euphoric effects compared with pitolisant (both doses) and placebo. Pitolisant was perceived as dissimilar to all drug classes including stimulants, CNS depressants, opioids, THC, hallucinogens, and dissociative agents. The clinical dose of pitolisant (35.6 mg) was rated as more similar to placebo than any other drug class. Study participants, all recreational stimulant abusers, were relatively familiar with the effects of phentermine but unfamiliar with the effects of high-dose pitolisant (213.6 mg), which supports the lack of similarity between pitolisant and stimulant medications. Overall, results from this study showed that pitolisant has significantly less abuse potential compared with phentermine (a known stimulant) and an overall profile that is similar to placebo, with no signal suggestive of abuse even at the supratherapeutic dose.
Study design and methodology were consistent with the latest FDA guidance for assessing the abuse potential of drugs [32]. Because recreational drug users often exceed medically recommended doses to enhance the subjective experience produced by a drug, FDA guidance recommends evaluating a supratherapeutic dose 2 to 3 times greater than the therapeutic dose [32]. In this study, a larger supratherapeutic dose was selected (pitolisant 213.6 mg, 6 times the therapeutic dose) because it exceeded the FDA recommendation yet was well tolerated by healthy volunteers in previous studies (data on file). Phentermine was chosen as the active comparator for pitolisant because it has wake-promoting effects, has some common adverse events (e.g. insomnia), and is a scheduled drug (Schedule IV controlled substance) with demonstrated abuse potential. Using a Schedule IV psychostimulant (e.g. phentermine) as the active comparator provides a more sensitive evaluation of abuse potential than using a Schedule II psychostimulant (e.g. methylphenidate and amphetamine), because it is more difficult to demonstrate significantly lower drug liking compared with a Schedule IV drug than to a Schedule II drug. The dose of phentermine used in this study (60 mg), although larger than the generally prescribed clinical doses (15 or 30 mg), was selected because it can be expected to reliably produce psychoactive effects, is in a range that can be safely administered, and is within the range (45–90 mg) used in previous abuse potential studies [38–40]. The sensitivity of the methodology and validity of the study were established by the statistically significant difference between phentermine and placebo on the primary endpoint of Drug Liking Emax (and also on secondary pharmacodynamic measures). These findings were consistent with results for phentermine reported in previous studies [38–40].
Human abuse potential studies utilize validated measures of subjective drug effects that evaluate the propensity of drug liking, positive and reinforcing effects, willingness to take drug again, perceived drug value, and pharmacological effects that are similar in nature to drugs with known abuse potential [41]. These measures have been routinely used by regulatory agencies (i.e. FDA, the US Drug Enforcement Administration [DEA], Health Canada) to make drug controlling and scheduling decisions based on a drug’s abuse potential profile. Furthermore, these measures are recognized surrogates to approximate potential real-world abuse of a drug and are recommended in the FDA’s 2017 guidance for the assessment of abuse potential [32]. Approximately 10-point difference between drugs was reported to be clinically meaningful for Drug Liking [42] and High Emax scores [43]. In a study of opioid medications, reductions in Overall Drug Liking Emax were significantly associated with lower rates of nonmedical use [44].
With the exception of pitolisant, all medications currently approved by the FDA for the treatment of narcolepsy (i.e. stimulants, wake-promoting agents, and sodium oxybate) are controlled substances [7]; thus, there has been a need for effective pharmacologic options to treat narcolepsy that have reduced potential for abuse. Methylphenidate [45], dextroamphetamine [46], and mixed amphetamine salts [47] are Schedule II controlled substances (high potential for abuse) and have long been recognized as drugs of abuse [34]. Modafinil [48] and armodafinil [49] are Schedule IV controlled substances (lower potential for abuse). Modafinil was shown to induce behavioral sensitization upon repeated administration in a preclinical animal study [27]. In addition, a positron emission tomography study in healthy volunteers showed modafinil to increase dopamine release in the human nucleus accumbens, a brain region associated with rewarding effects and abuse [50]. In an abuse potential study that included men with a history of polysubstance abuse (including cocaine), drug liking was significantly greater for modafinil (200, 400, or 800 mg) compared with placebo, but the overall pharmacologic profile (including responses on the ARCI Amphetamine scale) indicated lower abuse potential for modafinil compared with methylphenidate [51]. In a clinical trial in which modafinil was compared with pitolisant, 10% of patients in the modafinil group (but none in the pitolisant group) displayed amphetamine-like withdrawal symptoms [28].
Another dopaminergic agent—solriamfetol, a dopamine, and norepinephrine reuptake inhibitor—was recently approved by the FDA for the treatment of EDS in patients with narcolepsy or obstructive sleep apnea [52]. In an abuse potential study of adults with a history of recreational polydrug use (including a stimulant), drug liking was significantly greater for solriamfetol (300, 600, and 1200 mg) compared with placebo [40]. Overall, the abuse potential of solriamfetol appeared to be similar to or lower than that of phentermine [40]. Solriamfetol is classified as a Schedule IV controlled substance [52].
The sodium oxybate oral solution approved for the treatment of narcolepsy is a Schedule III controlled substance (abuse potential less than Schedule I or II but more than Schedule IV); it is the sodium salt of gamma-hydroxybutyrate (GHB), a Schedule I controlled substance (high or very high potential for abuse) [19]. In a study of men and women with a history of sedative/hypnotic abuse, drug liking was significantly greater for sodium oxybate (at doses up to 18 g/70 kg) compared with placebo; overall, the abuse potential of sodium oxybate was found to be greater than that of triazolam but less than that of pentobarbital [53]. In a study of nondependent, recreational users of both ethanol and other sedative-hypnotics, drug liking was significantly greater for sodium oxybate (at doses up to 10 g/70 kg) compared with placebo; drug liking for sodium oxybate was similar to that for alcohol, whereas willingness to take the drug again was significantly greater for sodium oxybate [54].
By contrast, the results of this study (which found that drug liking was similar for pitolisant and placebo) indicate that pitolisant was not associated with a profile suggesting a potential for recreational drug use or abuse. Based on these findings, along with preclinical data, pitolisant was approved by the FDA without being scheduled as a controlled substance by the DEA.
The minimal abuse potential observed for pitolisant, showing similarity to placebo, is consistent with its mechanism of action, which differs from that of other products used in the treatment of narcolepsy [55]. Pitolisant functions as an antagonist/inverse agonist at H3 autoreceptors, which increases the synthesis and release of histamine and enhances histaminergic signaling in the brain [56]. Studies in animals have shown that histaminergic signaling in the brain promotes wakefulness and suppresses non-REM and REM sleep [57]. Histamine activates wake-promoting neurons including norepinephrine neurons in the locus coeruleus, acetylcholine neurons in the pons and forebrain, and serotonin neurons in the dorsal raphe nucleus [58]; however, as noted above, histamine does not increase dopamine release in the nucleus accumbens [24, 27]. No cases of drug withdrawal syndrome were reported in the clinical development program for pitolisant in narcolepsy (8 studies), including during the 1 week placebo washout phase at the end of the pivotal trials [28, 29] or in patients who discontinued from the 1 year, open-label, long-term extension study (data on file). Furthermore, pitolisant partially counteracts the stimulant properties of cocaine, as demonstrated in an animal study [27]. Considering the preclinical and clinical evidence, pitolisant has a different profile than psychostimulants.
The pharmacokinetic characteristics of pitolisant observed in this study of recreational stimulant users were consistent with those reported in previous studies [59]. Pitolisant is rapidly absorbed after oral administration and reaches peak serum concentration in approximately 3 hr [59]; median tmax was 3.0 hr for pitolisant 35.6 mg and 2.0 hr for pitolisant 213.6 mg in this study. Pitolisant exposure increases in a dose-dependent fashion [30] and was approximately 8 times greater for the 213.6 mg dose relative to the 35.6 mg dose in this study. Consistent with the absence of any correlation or trend between the pharmacokinetic concentrations (i.e. Cmax) and pharmacodynamic effects (e.g. Drug Liking Emax) of pitolisant, no concentration-dependent abuse potential was observed in this study.
Pitolisant was well-tolerated in the study population of recreational stimulant users. The overall incidence of adverse events was comparable for pitolisant 35.6 mg and placebo, and there were no study discontinuations due to adverse events. Headache was the most common adverse event reported after administration of pitolisant in this study; in studies of patients with narcolepsy, the most common adverse events associated with pitolisant were headache, insomnia, and nausea [22, 31]. Unlike phentermine, pitolisant did not produce any clinically significant increases in blood pressure or heart rate.
Limitations of this study primarily concern the generalizability of the results. This study involved single-dose administration of pitolisant in a highly controlled setting to a relatively small population of nondependent stimulant users. Although this design is consistent with FDA guidance and results are considered an accurate predictor of abuse potential, generalizability to other populations or settings may be limited.
Conclusions
Pitolisant, a first-in-class medication with a novel mechanism of action, increases histaminergic signaling in the brain and has demonstrated efficacy in the treatment of adult patients with narcolepsy, with or without cataplexy. In this study of recreational stimulant users, pitolisant (at the recommended therapeutic dose of 35.6 mg and a supratherapeutic dose of 213.6 mg) produced pharmacodynamic responses that demonstrated significantly lower abuse potential compared with phentermine. In addition, the response profile of pitolisant for maximum Drug Liking (“at this moment”), Overall Drug Liking, and willingness to Take Drug Again were similar to placebo, demonstrating a lack of reinforcing drug effects. Pitolisant, especially at the therapeutic dose of 35.6 mg, showed no signals suggestive of abuse, which contrasts with the other classes of drugs currently available to treat patients with narcolepsy. Given the public health crisis related to abuse, misuse, and diversion of prescription drugs (which includes both opioids and stimulants), a new treatment with minimal risk of abuse is an important therapeutic option for healthcare professionals to treat patients with narcolepsy.
Supplementary Material
Acknowledgments
Technical editorial and medical writing assistance was provided under the direction of the authors by Nancy Holland, PhD, Synchrony Medical Communications, LLC, West Chester, PA, and funded by Harmony Biosciences, LLC, Plymouth Meeting, PA.
Work Performed: INC Research Toronto, Inc., Toronto, Ontario, Canada (now part of Syneos Health)
Funding
This study was funded by Bioprojet Pharma, Paris, France.
Conflict of interest statement. Dr Setnik was an employee of INC Research/inVentiv Health (now Syneos Health) at the time this study was conducted. Dr McDonnell and Ms Mills are employees of Syneos Health. Dr Scart-Grès is an employee of Bioprojet Pharma. Dr Schwartz is a cofounder of Bioprojet Pharma. Dr Robert is an employee of Bioprojet Biotech. Dr Dayno is an employee of Harmony Biosciences, LLC.
References
- 1. American Academy of Sleep Medicine. The International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2. Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27): 2654–2662. [DOI] [PubMed] [Google Scholar]
- 3. Flores NM, et al. The humanistic and economic burden of narcolepsy. J Clin Sleep Med. 2016;12(3):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maski K, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13(3):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration. The voice of the patient: narcolepsy.https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM402907.pdf. Published June 2014. Accessed August 21, 2019.
- 6. Black J, et al. The Burden of Narcolepsy Disease (BOND) study: health-care utilization and cost findings. Sleep Med. 2014;15(5):522–529. [DOI] [PubMed] [Google Scholar]
- 7. Thorpy MJ, et al. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16(1):9–18. [DOI] [PubMed] [Google Scholar]
- 8. Kallweit U, et al. Pharmacological management of narcolepsy with and without cataplexy. Expert Opin Pharmacother. 2017;18(8):809–817. [DOI] [PubMed] [Google Scholar]
- 9. Challman TD, et al. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75(7):711–721. [DOI] [PubMed] [Google Scholar]
- 10. Hodgkins P, et al. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur Child Adolesc Psychiatry. 2012;21(9):477–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wisor J. Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol. 2013;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loland CJ, et al. R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biol Psychiatry. 2012;72(5): 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Department of Health & Human Services, Substance Abuse and Mental Health Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. Rockville, MD: US Department of Health & Human Services; 2018. HHS Publication No. SMA 18–5068, NSDUH Series H-53. [Google Scholar]
- 14. Darke S, et al. Cardiovascular disease risk factors and symptoms among regular psychostimulant users. Drug Alcohol Rev. 2010;29(4):371–377. [DOI] [PubMed] [Google Scholar]
- 15. Frishman WH, et al. Cardiovascular manifestations of substance abuse: part 2: alcohol, amphetamines, heroin, cannabis, and caffeine. Heart Dis. 2003;5(4):253–271. [DOI] [PubMed] [Google Scholar]
- 16. Morton WA, et al. Methylphenidate abuse and psychiatric side effects. Prim Care Companion J Clin Psychiatry. 2000;2(5):159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winslow BT, et al. Methamphetamine abuse. Am Fam Physician. 2007;76(8):1169–1174. [PubMed] [Google Scholar]
- 18. Lappin JM, et al. Stroke and methamphetamine use in young adults: a review. J Neurol Neurosurg Psychiatry. 2017;88(12):1079–1091. [DOI] [PubMed] [Google Scholar]
- 19. Xyrem [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2018. [Google Scholar]
- 20. Pardi D, et al. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20(12):993–1018. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol. 2011;163(4):713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kollb-Sielecka M, et al. The European Medicines Agency review of pitolisant for treatment of narcolepsy: summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2017;33:125–129. [DOI] [PubMed] [Google Scholar]
- 23. Lin JS, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol Dis. 2008;30(1):74–83. [DOI] [PubMed] [Google Scholar]
- 24. Ligneau X, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy]propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. J Pharmacol Exp Ther. 2007;320(1):365–375. [DOI] [PubMed] [Google Scholar]
- 25. Volkow ND, et al. The brain on drugs: from reward to addiction. Cell. 2015;162(4):712–725. [DOI] [PubMed] [Google Scholar]
- 26. Di Chiara G, et al. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uguen M, et al. Preclinical evaluation of the abuse potential of Pitolisant, a histamine H3 receptor inverse agonist/antagonist compared with Modafinil. Br J Pharmacol. 2013;169(3):632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dauvilliers Y, et al. ; HARMONY I study group . Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. [DOI] [PubMed] [Google Scholar]
- 29. Szakacs Z, et al. ; HARMONY-CTP study group . Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(3):200–207. [DOI] [PubMed] [Google Scholar]
- 30. Syed YY. Pitolisant: first global approval. Drugs. 2016;76(13):1313–1318. [DOI] [PubMed] [Google Scholar]
- 31. Wakix [package insert]. Plymouth Meeting, PA: Harmony Biosciences, LLC; 2019. [Google Scholar]
- 32. US Department of Health & Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research. Assessment of Abuse Potential of Drugs. Guidance for Industry. Silver Spring, MD: US Department of Health & Human Services; 2017. [Google Scholar]
- 33. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th Edition, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 34. Martin WR, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258. [DOI] [PubMed] [Google Scholar]
- 35. Posner K, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun P. Using SAS PROC POWER to Perform Model-Based Power Analysis for Clinical Pharmacology Studies. Paper presented at: PharmaSUG 2010; May 23–26, 2010; Orlando, FL: http://www.pharmasug.org/cd/papers/SP/SP05.pdf. Accessed September 18, 2019. [Google Scholar]
- 37. Chen L, et al. An equivalence test for the comparison between a test drug and placebo in human abuse potential studies. J Biopharm Stat. 2013;23(2):294–306. [DOI] [PubMed] [Google Scholar]
- 38. Jasinski DR, et al. Abuse liability assessment of atomoxetine in a drug-abusing population. Drug Alcohol Depend. 2008;95(1-2):140–146. [DOI] [PubMed] [Google Scholar]
- 39. Schoedel KA, et al. Human abuse potential and cognitive effects of taranabant, a cannabinoid 1 receptor inverse agonist: a randomized, double-blind, placebo- and active-controlled, crossover study in recreational polydrug users. J Clin Psychopharmacol. 2012;32(4):492–502. [DOI] [PubMed] [Google Scholar]
- 40. Carter LP, et al. A randomized, double-blind, placebo-controlled, crossover study to evaluate the human abuse liability of solriamfetol, a selective dopamine and norepinephrine reuptake inhibitor. J Psychopharmacol. 2018;32(12):1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McColl S, et al. Research design strategies to evaluate the impact of formulations on abuse liability. Drug Alcohol Depend. 2006;83 (Suppl 1):S52–S62. [DOI] [PubMed] [Google Scholar]
- 42. Schoedel KA, et al. Defining clinically important differences in subjective abuse potential measures. Palm Springs, CA: College on Problems of Drug Dependence; June 9–14, 2012. Abstract 593. https://cpdd.org/wp-content/uploads/2016/07/CPDD12AbstractBook.pdf. Accessed June 25, 2019. [Google Scholar]
- 43. Eaton TA, et al. Determining the clinically important difference in visual analog scale scores in abuse liability studies evaluating novel opioid formulations. Qual Life Res. 2012;21(6):975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. White AG, et al. Positive subjective measures in abuse liability studies and real-world nonmedical use: potential impact of abuse-deterrent opioids on rates of nonmedical use and associated healthcare costs. J Opioid Manag. 2015;11(3):199–210. [DOI] [PubMed] [Google Scholar]
- 45. Ritalin [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2013. [Google Scholar]
- 46. Dexedrine [package insert]. Horsham, PA: Amedra Pharmaceuticals LLC; 2017. [Google Scholar]
- 47. Adderall [package insert]. Horsham, PA: Teva Pharmaceuticals; 2017. [Google Scholar]
- 48. Provigil [package insert]. North Wales, PA: Teva Pharmaceuticals; 2015. [Google Scholar]
- 49. Nuvigil [package insert]. North Wales, PA: Teva Pharmaceuticals; 2017. [Google Scholar]
- 50. Volkow ND, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301(11):1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14(1):53–60. [DOI] [PubMed] [Google Scholar]
- 52. Sunosi [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc; 2019. [Google Scholar]
- 53. Carter LP, et al. Relative abuse liability of GHB in humans: a comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology. 2006;31(11):2537–2551. [DOI] [PubMed] [Google Scholar]
- 54. Johnson MW, et al. Comparative abuse liability of GHB and ethanol in humans. Exp Clin Psychopharmacol. 2013;21(2): 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calik MW. Update on the treatment of narcolepsy: clinical efficacy of pitolisant. Nat Sci Sleep. 2017;9:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lin JS, et al. Histamine H3 receptors and sleep-wake regulation. J Pharmacol Exp Ther. 2011;336(1):17–23. [DOI] [PubMed] [Google Scholar]
- 57. Scammell TE, et al. Histamine: neural circuits and new medications. Sleep. 2019;42(1). doi: 10.1093/sleep/zsy183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haas HL, et al. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183–1241. [DOI] [PubMed] [Google Scholar]
- 59. Wakix Summary of Product Characteristics [SPC]. https://www.ema.europa.eu/documents/product-information/wakix-epar-product-information_en.pdf. 2016. Accessed August 21, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.