Abstract
The use of specific care bundles to control infection has been demonstrated to be effective, including ventilator-associated pneumonia (VAP). However, the proposal of VAP rates as a quality indicator has generated debate due a lack of a gold standard definition. In 2013, the Centers for Disease Control and Prevention (CDC) replaced surveillance definitions from VAP to other preventable conditions related to mechanical ventilation (ventilator-associated events: VAE), which may predict outcomes. This represents a new opportunity to overcome many of the weaknesses of traditional VAP surveillance and broadens the focus of surveillance encompassing other preventable conditions related to mechanical ventilation.
Keywords: Children, Infection-related ventilator-associated complication (IVAC), Pediatric ventilator-associated event (PedVAE), Possible ventilator-associated pneumonia (PVAP), Quality improvement, Respiratory infection, Ventilator-associated condition (VAC), Ventilator-associated event (VAE), Ventilator-associated pneumonia (VAP), Ventilator-associated tracheobronchitis (VAT)
Introduction
In recent years, the use of specific care bundles to control infection in the intensive care unit has been demonstrated to be effective, including ventilator-associated pneumonia (VAP) (Heck, 2012; López-Pueyo et al., 2013). Thus, zero VAP rates have been proposed as an indicator of quality in the Intensive Care Unit (ICU). This has generated debate regarding a lack of a gold standard definition and the recognition of ventilator-associated tracheobronchitis (VAT) not only as an intermediate status preceding VAP, but as an independent source of morbidity by itself worthy of antibiotic treatment (Craven et al., 2016; Nseir et al., 2014; Klompas, 2012). The difficulties with the interpretation of chest X-ray opacities in any critical patient, the sole determinant between the two of them, makes a challenge to differentiate consistently between VAT and VAP. Moreover, this difficulty on the classification of ventilator-associated infections may explain the poor correlation between the sustained decrease of VAP rates, antimicrobial use, and clinical outcomes reported in the last years (Graat et al., 2006; Nora and Póvoa, 2017).
In view of these circumstances, in 2013, the Centres for Disease Control and Prevention (CDC) replaced surveillance definitions from VAP to other preventable conditions related to mechanical ventilation (ventilator-associated events: VAE), which may predict outcomes, focusing on respiratory worsening and removing the need for the Chest X-ray interpretation (Klompas, 2013). In these new definitions, the respiratory worsening becomes the key new point, advocating for identifying and monitoring all those processes that can cause respiratory deterioration (infectious or not) in the ventilated patient. This respiratory worsening is specified as the presence of hypoxemia and easily objectified by the changes recorded in two ventilator settings: fraction of inspired oxygen (FiO2) and positive end-expiratory pressure (PEEP).
2013-Ventilator-Associated Events (VAE) Definition
There are three definition tiers within the 2013-VAE algorithm (Fig. 1A):
-
(1)
Ventilator-associated condition (VAC), when the respiratory worsening fulfills some criteria for detecting hypoxemia defined as an increase in daily minimum PEEP ≥ 3 cm H2O or FiO2 ≥ 0.20 sustained for at least 2 calendar days following a baseline period (2 calendar days) of stability or improvement.
-
(2)
Infection-related ventilator-associated complication (IVAC), if in view of the above, and the presence of general evidence of infection/inflammation, defined as altered leukocyte count (≥ 12,000 cells/mm3 or ≤ 4000 cells/mm3) and/or temperature (> 38 °C or < 36 °C), a new antimicrobial prescription has been started and sustained for at least 4 calendar days by the attending physician, and
-
(3)
Possible or probable pneumonia (PsVAP/PrVAP), if in addition to the above, there is a microbiological confirmation of a lower respiratory tract infection, defined as: purulent respiratory secretions or positive culture (qualitative, semi-quantitative or quantitative) in case of possible VAP or more strict microbiological criteria in case of probable VAP, where purulent secretions plus quantitative criteria are mandatory except for positive lung histopathology, positive pleural fluid culture and other tests as Legionella spp.
Fig. 1.

2013 CDC classification and 2015 update. §Suspicion of infection: leucocytosis (≥ 12,000 cells/mL)/leucopenia (≤ 4000 cells/mL) or fever (≥ 38°C)/hypothermia (≤ 36°C).
This algorithm is not a clinical definition and it was not designed for use in the management of patients, but for hospital surveillance. The aim was to establish a new surveillance system to improve reliability on detecting quickly and easily VAP and other mechanical ventilation complications related to an increased intensive care unit length of stay and mortality. The final propose use was to be implemented for public reporting, inter-facility comparisons, and pay-for-performance programs in the United States. However, its clinical correlation is currently being reviewed and actively studied area.
Tiers 1 and 2 (VAC and IVAC) were definitions suitable for potential use in public reporting and comparing among facilities. Tier 3 (Ps/PrVAP), incorporating laboratory evidence, depends on microbiological techniques, some of them unavailable in certain institutions, so it was thought for internal use definitions and internal quality improvement. These three subtiers are mutually exclusive and VAE is referred as the sum of them.
VAE Surveillance Updates and Clinical Correlation
Several modifications to the VAE definitions have been made since January 2013 in the light of the issues raised by the CDC’s National Healthcare Safety Network (NHSN) users (Magill et al., 2014):
-
•
PEEP values between 0 and 5 cm H2O were considered equivalents for the purposes of VAE surveillance. Thus, an increase in the daily minimum PEEP to at least 8 cm H2O sustained for at least 2 calendar days is mandatory to meet the VAC definition.
-
•
Daily minimum PEEP and FiO2 were defined as those maintained for at least 1 h.
-
•
Purulent respiratory secretions definition was adapted and a list of antimicrobial agents eligible for IVAC was refined by removing selected antimicrobial agents that would not be used, or would be unlikely to be used, in treating a lower respiratory infection in critically ill patient (Table 1 ).
Table 1.
Antimicrobial agents eligible for the diagnosis of IVACa.
| Antibiotics | ||
| Amikacin Ampicillin Ampicillin/sulbactam Azithromycin Aztreonam Cefazolin Cefepime Cefotaxime Cefotetan Cefoxitin Ceftaroline Ceftazidime Ceftazidime/avibactam Ceftizoxime Ceftolozane/tazobactam Ceftriaxone Cefuroxime Ciprofloxacin Clarithromycin |
Clindamycin Colistimethate Dalbavancin Doripenem Doxycycline Ertapenem Fosfomycin Gemifloxacin Gentamicin Imipenem/cilastatin Levofloxacin Linezolid Meropenem Metronidazole Minocycline Moxifloxacin Nafcillin Oritavancin Oxacillin |
Penicillin G Piperacillin Piperacillin/tazobactam Polymyxin B Quinupristin/dalfopristin Rifampin Sulfamethoxazole/trimethoprim Sulfisoxazole Tedizolid Telavancin Telithromycin Tetracycline Ticarcillin/clavulanate Tigecycline Tobramycin Vancomycin |
| Antifungals | ||
| Amphotericin B Amphotericin B liposomal Anidulafungin Caspofungin |
Fluconazole Isavuconazonium Itraconazole Micafungin |
Posaconazole Voriconazole |
| Antivirals | ||
| Oseltamivir | Zanamivir | Peramivir |
IVAC, infection-related ventilator-associated complication.
In the 2015 update, probable and possible pneumonia were classified together as possible ventilator associated pneumonia (PVAP), and a new category (IVAC-plus) was created, comprising overall events meeting at least IVAC definition: the sum of IVAC + PVAP events (Fig. 1B). Moreover, episodes of mechanical ventilation were advised to be monitored and used as a new denominator for surveillance.
As for ventilator monitoring settings, if no value is maintained for at least 1 h, the lowest value is considered the daily minimum value. Upgrades regarding microbiological issues include community associated fungal pathogens exclusion, addition of six new antimicrobial agents and the use of non-culture based diagnostic test methods (e.g., PCR: polymerase chain reaction), being the current criteria to meet the PVAP definition resumed in Table 2 .
Table 2.
Criteria that can be used to meet the PVAPa definition.
|
|
|
Purulent respiratory secretions: ≥ 25 neutrophils and ≤ 10 squamous epithelial cells per low power field |
|
|
PVAP, possible ventilator-associated pneumonia.
Pathogenic virus in respiratory samples: influenza, respiratory syncytial, adenovirus, parainfluenza, rhinovirus, human metapneumovirus, coronavirus.
Some changes are aimed to avoid misinterpretations of infection occurring during mechanical ventilation not attributable to a ventilation complication per se. For instance, the removal of antimicrobial agents that would be unlikely indicated in treating lower respiratory infection in a critically ill patient (e.g. oral chloramphenicol, nitrofurantoin, enteral vancomycin, daptomycin). However, meeting Infection-related Ventilator-Associated Complication (IVAC) definition does not mean that the “infection related” event is necessarily respiratory in origin. IVAC refers to any infectious event associated with a deterioration in respiratory function. In the context of the critical patient, the new empiric antimicrobial agent may have dual purposes and not aimed, at least exclusively, to a respiratory infection. What is more, central line associated blood stream infections (CLABSI) or secondary blood stream infections can trigger an IVAC (e.g. by inducing acute respiratory distress syndrome, hydric overload). Otherwise, it could be IVAC due to a ventilator-associated respiratory infection that not meet PVAP criteria and even though VAP or VAT episodes that not meet any VAE criteria as it has been reported (Fan et al., 2016; Ramírez-Estrada et al., 2018; Peña-López et al., 2017; Sim et al., 2016). Thus, infectious ventilator-associated complications and non-infectious associated complications could be correlated with IVAC-plus and VAC events, respectively, IVAC-plus encompassing IVAC and PVAP with the purpose of avoiding missing some ventilator-associated infections but not referring exclusively to them. Nevertheless, some VAP and VAT still are being omitted, as it has been pointed out above.
Considerations When Reviewing VAE Research in the Literature
There has been a lack of understanding of the VAE algorithm that have generated confusion in the literature due to an inappropriate application of the terms designed by the CDC.
First, VAE has not to be simplified as the new ventilator-associated pneumonia/ventilator-associated respiratory infections surveillance, since it broadens the spectrum of surveillance to other mechanical ventilation complications. Thus, it seems logical that multiple studies confirm a poor correlation between VAE and VAP or VARI. What it do not make sense that this fact impels some authors against the use of the VAE algorithm by arguing that they are not the same (Pugh et al., 2016; Nair and Niederman, 2017). Actually, the aim of the VAE algorithm is to be a good tool for surveillance and preventability purposes with impact on outcomes (Klompas and Berra, 2016; Klompas et al., 2015). Since there is not a good clinical correlation between them, and above all, VAE not including some VARI, they has to be interpreted as those events occurring during mechanical ventilation, which are worthy of appropriate efforts to preventability purposes because of their clear impact on outcomes. Among them but not limited, the infectious ones, which represents only those ventilator-associated respiratory infections with a great impact on patient outcomes.
Second, VAE frequently are referred as VAC in the literature (Boyer et al., 2015; Damas et al., 2015; Stoeppel et al., 2014; Bouadma et al., 2015) when VAC only includes those events with respiratory worsening exclusively, excluding those events that also fulfill IVAC or PVAP criteria. Thus, all VAC are VAE but not vice versa. Some studies about incidence and prevalence referring to VAC as VAE may be misleading and they have to be interpreted adequately to avoid errors when comparing taxes.
On another hand, the three tiers of VAE (VAC, IVAC, and PVAP) are excluding among them and not one including another, as it can be misinterpreted by some figures using Venn diagrams (Spalding et al., 2017). In these diagrams, the groups are depicted in a series of different size circles, frequently VAC covering IVAC and PVAP. While it is true that the three of them share the first requirement to be defined as VAE, the respiratory worsening, and IVAC shares with PVAP the changes in temperature/white cell count and the antimicrobial prescription, all three tiers only are encompassed by the term VAE. The last update of the new algorithm solved this problem adding a new concept in the algorithm: the IVAC-plus events, that encompasses the other two tiers of the algorithm, IVAC + PVAP. Lately, some authors emphasize the first two tiers as “VAC only/alone” or “IVAC only/alone” when talking about VAEs subtypes to avoid misinterpretations (Klompas et al., 2014; Nakahashi et al., 2018) but other authors can still lead to misunderstanding using the term “IVAC only” meaning IVAC but again using the term IVAC meaning IVAC-plus (Chao et al., 2018).
In this scenario can be cautious when reading about incidence, clinical correlation and outcomes of both VAE and the three different tiers of VAE (VAC, IVAC, PVAP) apart from the updates of each definition e.g. studies corresponding to years previous to the 2015 update refer to possible pneumonia as a different concept as subsequent studies following the new update. Automated surveillance for ventilator-associated events through an updated electronic algorithm can help to solve some of these misunderstanding problems in addition to save hours of staff time spent in chart review (Hebert et al., 2018; Shenoy et al., 2018) but again, the terms could not to be properly used (Mann et al., 2015).
Pediatric VAE Definitions and VAE Surveillance in Children
The VAE surveillance definition algorithm implemented by the NHSN in January 2013 was initially available for use in adult locations only. Later, a Pediatric VAE definition using changes in mean airway pressure (MAP) instead of PEEP setting, in addition of changes in the fraction of inspired oxygen was proposed (Cocoros et al., 2017) (Fig. 2A) and the CDC decided move forward with Pediatric VAE (PedVAE) development and implementation in NHSN. From 2019, inpatient location eligible to participate in pediatric VAE surveillance are those neonatal and pediatric locations in acute care hospitals, long term acute care hospitals, and inpatient facilities in the United States. As adults, chronic care units in acute care facilities are not eligible to participate in pediatric VAE surveillance.
Fig. 2.
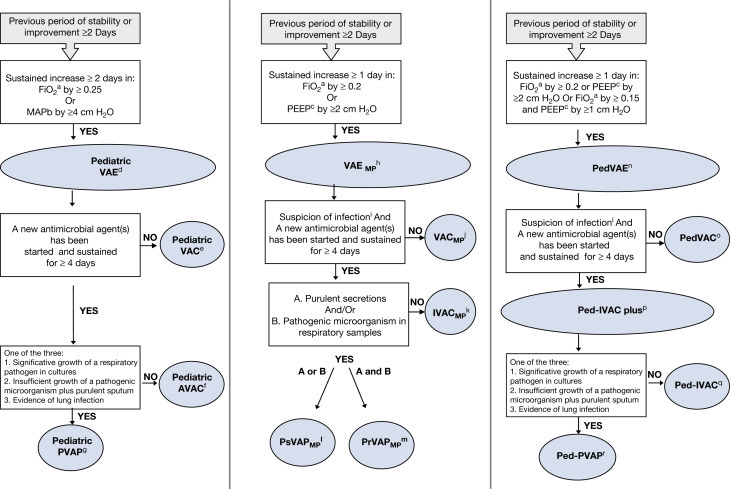
Pediatric VAE CDC definition and other pediatric modified proposals. aFraction of inspired oxygen; bMean airway pressure; cPositive end expiratory pressure; dPediatric VAE (PedVAE): pediatric ventilator-associated event; ePediatric VAC (PedVAC): pediatric ventilator-associated condition; fPediatric AVAC: pediatric VAC with antimicrobial use; gPediatric PVAP: pediatric possible ventilator-associated pneumonia; hVAEMP: Ventilator-Associated Event modified pediatric criteria; iSuspicion of infection: fever (≥ 38 °C)/hypothermia (≤ 36 °C) or leukocytosis (≥ 12,000 cells/mL)/leukopenia (≤ 4000 cells/mL); jVAC MP: Ventilator associated condition modified pediatric criteria; kIVACMP: Infection-related Ventilator-Associated complication modified pediatric criteria; lPsVAPMP: Possible Ventilator-Associated Pneumonia modified pediatric criteria; mPrVAPMP: Probable Ventilator-Associated Pneumonia modified pediatric criteria; nPedVAE: Pediatric Ventilator-Associated Event; oPedVAC: Pediatric Ventilator-Associated VAC; pPedIVAC-plus: Pediatric Infection-related Ventilator-Associated complication-plus; qPed-IVAC: Pediatric Infection-related Ventilator-Associated complication; rPedPVAP: Pediatric Possible ventilator-associated pneumonia.
Pediatric VAE CDC definition differs from adult VAE apart from the use of MAP instead of PEEP (a sustained increase of the daily minimum MAP ≥ 4 cm H2O versus PEEP increase ≥ 3 cm H2O) also in the changes in the fraction of inspired oxygen (increase in daily minimum FiO2 of ≥ 0.25 instead of 0.20). In both cases, the respiratory worsening has to be sustained for at least 2 calendar days for meeting VAE criteria.
Likewise PEEP values considered equivalents for the purposes of surveillance in adult VAE definitions, for in patients < 30 days-old, MAP values of 0–8 cm H2O are considered equivalent and the same for MAP values 0–10 cm H2O when referring to patients ≥ 30 days-old; Therefore, an increase in the daily minimum MAP to at least 12 cm H2O and 14 cm H2O respectively, sustained for 2 calendar days, would be needed to meet the pediatric VAE definition.
Patients on high-frequency oscillatory ventilation are included in pediatric VAE surveillance and also patients who are receiving a conventional mode of mechanical ventilation or high-frequency ventilation in treatment with surfactant, corticosteroids, nitric oxide therapy, heliox or epoprotenol therapy. As in adults, pediatric VAE are defined by a 14-day period, starting on the day of onset of worsening oxygenation (the event day) within a new pediatric VAE cannot be identified or reported until this 14-day period has elapsed.
The pediatric VAE definition adopted by the CDC is based on a multicentre retrospective cohort study involving 8862 patients in which several daily minimum MAP and FiO2 values were tested (4, 5, 6, 7, and 0.20, 0.25, 0.30, respectively). The final decision of the new definition threshold to identify VAC in pediatric patients (FiO2 0.25 and MAP of 4 cm H2O) was reached searching for a “reasonable” event rate associated with higher morbidity and mortality. Although officially recommended for use in USA pediatric facilities from 2019, it has not been clinically correlated or compared with the 2008-CDC definitions to date. From then on, other pediatric definitions have been proposed. The first VAE variation (Beardsley, 2016) applied a daily minimum PEEP value of at least 2 cm H2O instead of 3 cm H2O used in the adult VAE surveillance definition and they found that this pediatric VAE variation was consistent with PNU1/VAP surveillance definition in terms of incidence. However, only one mechanical ventilation episode met both surveillance definitions. In the same year, there was used another modification of VAC/VAE criteria (VAC/VAEMP) (Cirulis et al., 2016) for also a minimum daily PEEP value greater or equal to 2 cm H2O instead of 3 cm H2O but sustained for at least 1 day instead the 2 calendar days considered in other pediatric and adult VAE definitions (Fig. 2B). This study, conducted in pediatric traumatic brain injury patients also showed low sensitivity to identify all PNU1/VAP using the new proposal. Another VAE variation (Peña-López et al., 2017) supported the use of an even more less restrictive new pediatric VAE definition (Ped-VAE) by expanding on Cirulis et al. proposal with an additional modification: increase in FiO2 of 0.15 plus an increase in PEEP of ≥ 1 cm H2O sustained for ≥ 1 day, apart from the increase of the daily minimum PEEP value of at least 2 cm H2O or 0.20 FiO2 sustained for ≥ 1 day (Fig. 2C). This is the only pediatric VAE definition prospectively validated in a cohort of children, which was resulted to have greater prediction accuracy for outcomes than 2008-CDC definitions and the adult VAE algorithm without effect on mortality.
All modified pediatric VAE proposals apart from CDC focus on changing the thresholds to meet the first tier of VAE (VAC) and retain the parameters for IVAC and PVAP used in adult definitions. The reasons argued by the authors for this less restrictive algorithm for pediatric VAE definitions were that most children present good pulmonary baseline conditions and can withstand mechanical complications better than children suffering from chronic conditions or adults, and that childhood is needed a shorter recovery time than in adulthood. Indeed, when the VAE adult criteria were applied by Peña-López et al. to children from a Pediatric Intensive Care Unit, incidence of VAE was fewer than in adults and their repercussion on mortality higher (Peña-López et al., 2017). By contrast, they obtained a fourfold increase in VAEs and the double of PVAP when using their less restrictive criteria for a respiratory worsening in their Ped-VAE proposal, and keeping the repercussion on outcomes. As in the adult population, the agreement between the new 2013-VAE and old 2008-CDC PNU/VAP definitions in children is poor (Mohd Ali et al., 2019) and the same for the new proposed pediatric definitions tested (Peña-López et al., 2017). Once again, a misinterpretation of these definitions may lead to reject their use but, on the contrary, an in-depth review can generate great interest not only for surveillance but also for their clinical use. Until now, the importance of monitoring ventilator settings as a surrogate for oxygenation had not been previously recognized even though hypoxemia had been suggested to be a main variable in assessing pneumonia resolution. Short courses of antibiotics have been suggested in suspected or confirmed infectious episodes non fulfilling VAE criteria (Klompas et al., 2017). Oxygenation variations have been proposed as the cornerstone in the assessment of the ventilated patient when suspecting and treating a ventilator-associated infection, going one step beyond Chest X-ray opacities and inflammatory parameters (Rello et al., 2018). More research is needed to confirm this new approach.
Conclusions
In summary, the ventilator-associated event definition implemented by the CDC and proposed as the new National Healthcare Safety Network surveillance definition in the United States to replace their former definition of VAP represents a new opportunity to overcome many of the weaknesses of traditional VAP surveillance and broadens the focus of surveillance encompassing other preventable conditions related to mechanical ventilation. The use of these new criteria in children requires an adaptation of the VAE definition in accordance with the peculiarities of the pediatric population. On another hand, neither VAE definition nor VAE tiers are neither sensitive nor specific for VAP or VAT, making difficult to create a clinical correlation; moreover, some VAE subtype terms (VAC and IVAC) are frequently misused in the literature leading to confusion. More research will be needed in the next years to establish the usefulness of the VAE algorithm and its applicability as a quality indicator.
Biographies

Yolanda Peña-López earned her Medical Degree (2000) From the University of Barcelona graduating at the top of her class and receiving the Extraordinary Degree Award and the Professor Romero Prize for the highest score on the national medical examination test. Following residency in Pediatrics at the Vall d’Hebron University Hospital, she completed additional training in Pediatric Critical Care at the same hospital becoming a staff member. She is a Neonatal, Pediatric Advanced and Trauma Life Support instructor with Aeromedical Retrieval and Transport Certification.
Peña is a dedicated clinician, lecturer and researcher. She provides advance care for patients admitted to the Pediatric Intensive Care Department as well to children at Burn Service at Vall d’Hebron Hospital Campus. Over these years, she has participated in nurse and medical training in Basic and Advanced Life Support and renal replacement therapy. She is also has lectured Postgraduate and Master’s Degrees, among others, Master Degree in Emergency Care and technical proceedings of the critically ill patient (University of Barcelona) and Master Degree in Biomedical and Translational Research from the Vall d’Hebron Institute of Research. She has textbook contributions in pediatric critical transport and renal replacement therapy. Peña is also an active member of the Spanish Pediatric Critical Care Society Infectious Diseases Workgroup and she is part of the team running the Health-Care Infection Control and Prevention in critically ill children in Catalonia (VINCat). She joined the Clinical Research/Innovation in Pneumonia and Sepsis Group at Vall d’Hebron Institute of Research and she completed her PhD about respiratory infections in mechanically ventilated children and ventilator-associated events. Currently, she is coordinating a national multi-centre study assessing the impact on outcomes of the ventilator-associated events in children. Other research interests include acute kidney injury and continuous renal replacement, burn and immunocompromised critically ill children. Some of her work has been awarded from the Spanish Pediatric Cardiology Society and the Argentine Society of Intensive Therapy.

Sergio Ramírez-Estrada was born in Medellin, Colombia, in 1982. He graduated in medicine in 2005 from Fundación Universitaria San Martín, Colombia and completed his intensive care medicine residency at Vall d’Hebron Hospital in Barcelona, Spain. He is currently the chief of the critical Care Department at the Corachan Clinic in Barcelona, Spain and collaborates with the Clinical Research and Innovation in Pneumonia and Sepsis (CRIPS) Group at Vall d’Hebron Research Institut.
Ramírez-Estrada has texbook contributions in ventilator associated pneumonia, his research include studies on prevention and control of hospital-acquired infections and severe community acquired pneumonia, epidemiology of ventilator-associated events, and treatment of infections in critically ill patients.

Jordi Rello earned in 1985 his medical degree at University of Barcelona, Spain and completed his residence/fellowship in Critical Care (1990) in Hospital de Sant Pau in Barcelona. Dr. Rello completed additional training in infection control and ID epidemiology at Harvard Medical School, in Boston, as part of his PhD in Infectious Diseases.
He has conducted clinical practice as consultant, leaded research and trained fellows in critical care from 1990, with special focus on severe pneumonia and precision medicine in sepsis. After serving as critical care consultant in Hospital Clinic in Barcelona and Hospital Tauli in Sabadell, Dr Rello become Head of the ICU Department in 1999 in the University Hospital de Tarragona and in 2010 at the University Hospital Vall d’Hebron, Barcelona. He has directed 20 doctoral thesis, being part of his academic duties as Professor of Medicine at the Universitat Autònoma de Barcelona and Rovira and Virgili University in Tarragona.
Currently, he is Director of the Clinical Research and Innovation in Pneumonia and Sepsis (CRIPS) Group at Vall d’Hebron Research Institute and CIBERES: http://www.vhir.org/gr/crips, ubicated in Vall d’Hebron Barcelona Hospital Campus.
Rello has > 500 indexed manuscripts with 37,464 citations (overall 2850 per year from 2013), an H-index of 103 (65 from 2014) and i-10 index of 365 (281 from 2014)—Google Scholar accessed January 2019. He has been awarded in 2018 as international honorary professor of CSCCM—Chinese Medical Association.
References
- Beardsley A.L. Ventilator-associated infections need a new approach. Pediatric Critical Care Medicine. 2016;17:587. doi: 10.1097/PCC.0000000000000730. [DOI] [PubMed] [Google Scholar]
- Bouadma L., Sonneville R., Garrouste-Orgeas M., Darmon M., Souweine B., Voiriot G., Kallel H., Schwebel C., Goldgran-Toledano D., Dumenil A.-S., Argaud L., Ruckly S., Jamali S., Planquette B., Adrie C., Lucet J.-C., Azoulay E., Timsit J.-F., OUTCOMEREA Study Group Ventilator-associated events: Prevalence, outcome, and relationship with ventilator-associated pneumonia. Critical Care Medicine. 2015;43:1798–1806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]
- Boyer A.F., Schoenberg N., Babcock H., McMullen K.M., Micek S.T., Kollef M.H. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015;147:68–81. doi: 10.1378/chest.14-0544. [DOI] [PubMed] [Google Scholar]
- Chao W.-C., Chang W.-L., Wu C.-L., Chan M.-C. Using objective fluid balance data to identify pulmonary edema in subjects with ventilator-associated events. Respiratory Care. 2018;63:1413–1420. doi: 10.4187/respcare.06221. [DOI] [PubMed] [Google Scholar]
- Cirulis M.M., Hamele M.T., Stockmann C.R., Bennett T.D., Bratton S.L. Comparison of the new adult ventilator-associated event criteria to the Centers for Disease Control and Prevention Pediatric Ventilator-Associated Pneumonia Definition (PNU2) in a population of pediatric traumatic brain injury patients. Pediatric Critical Care Medicine. 2016;17:157–164. doi: 10.1097/PCC.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocoros N.M., Priebe G.P., Logan L.K., Coffin S., Larsen G., Toltzis P., Sandora T.J., Harper M., Sammons J.S., Gray J.E., Goldmann D., Horan K., Burton M., Checchia P.A., Lakoma M., Sims S., Klompas M., Lee G.M. A pediatric approach to ventilator-associated events surveillance. Infection Control and Hospital Epidemiology. 2017;38:327–333. doi: 10.1017/ice.2016.277. [DOI] [PubMed] [Google Scholar]
- Craven D.E., Hudcova J., Lei Y., Craven K.A., Waqas A. Pre-emptive antibiotic therapy to reduce ventilator-associated pneumonia: “Thinking outside the box”. Critical Care (London, England) 2016;20:300. doi: 10.1186/s13054-016-1472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas P., Frippiat F., Ancion A., Canivet J.-L., Lambermont B., Layios N., Massion P., Morimont P., Nys M., Piret S., Lancellotti P., Wiesen P., D’orio V., Samalea N., Ledoux D. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: A randomized controlled trial with subglottic secretion suctioning. Critical Care Medicine. 2015;43:22–30. doi: 10.1097/CCM.0000000000000674. [DOI] [PubMed] [Google Scholar]
- Fan Y., Gao F., Wu Y., Zhang J., Zhu M., Xiong L. Does ventilator-associated event surveillance detect ventilator-associated pneumonia in intensive care units? A systematic review and meta-analysis. Critical Care (London, England) 2016;20:338. doi: 10.1186/s13054-016-1506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graat M.E., Choi G., Wolthuis E.K., Korevaar J.C., Spronk P.E., Stoker J., Vroom M.B., Schultz M.J. The clinical value of daily routine chest radiographs in a mixed medical-surgical intensive care unit is low. Critical Care (London, England) 2006;10:R11. doi: 10.1186/cc3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert C., Flaherty J., Smyer J., Ding J., Mangino J.E. Development and validation of an automated ventilator-associated event electronic surveillance system: A report of a successful implementation. American Journal of Infection Control. 2018;46:316–321. doi: 10.1016/j.ajic.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Heck K. Decreasing ventilator-associated pneumonia in the intensive care unit: A sustainable comprehensive quality improvement program. American Journal of Infection Control. 2012;40:877–879. doi: 10.1016/j.ajic.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Klompas M. Is a ventilator-associated pneumonia rate of zero really possible? Current Opinion in Infectious Diseases. 2012;25:176–182. doi: 10.1097/QCO.0b013e3283502437. [DOI] [PubMed] [Google Scholar]
- Klompas M. Complications of mechanical ventilation--the CDC’s new surveillance paradigm. The New England Journal of Medicine. 2013;368:1472–1475. doi: 10.1056/NEJMp1300633. [DOI] [PubMed] [Google Scholar]
- Klompas M., Berra L. Should ventilator-associated events become a quality Indicator for ICUs? Respiratory Care. 2016;61:723–736. doi: 10.4187/respcare.04548. [DOI] [PubMed] [Google Scholar]
- Klompas M., Kleinman K., Murphy M.V. Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infection Control and Hospital Epidemiology. 2014;35:502–510. doi: 10.1086/675834. [DOI] [PubMed] [Google Scholar]
- Klompas M., Anderson D., Trick W., Babcock H., Kerlin M.P., Li L., Sinkowitz-Cochran R., Ely E.W., Jernigan J., Magill S., Lyles R., O’Neil C., Kitch B.T., Arrington E., Balas M.C., Kleinman K., Bruce C., Lankiewicz J., Murphy M.V., E Cox C., Lautenbach E., Sexton D., Fraser V., Weinstein R.A., Platt R., Prevention Epicenters C.D.C. The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. American Journal of Respiratory and Critical Care Medicine. 2015;191:292–301. doi: 10.1164/rccm.201407-1394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klompas M., Li L., Menchaca J.T., Gruber S., Centers for Disease Control and Prevention Epicenters Program Ultra-short-course antibiotics for patients with suspected ventilator-associated pneumonia but minimal and stable ventilator settings. Clinical Infectious Diseases. 2017;64:870–876. doi: 10.1093/cid/ciw870. [DOI] [PubMed] [Google Scholar]
- López-Pueyo M.J., Olaechea-Astigarraga P., Palomar-Martínez M., Insausti-Ordeñana J., Alvarez-Lerma F., ENVIN–HELICS Study Group Quality control of the surveillance programme of ICU-acquired infection (ENVIN-HELICS registry) in Spain. The Journal of Hospital Infection. 2013;84:126–131. doi: 10.1016/j.jhin.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Magill S.S., Rhodes B., Klompas M. Improving ventilator-associated event surveillance in the National Healthcare Safety Network and addressing knowledge gaps: Update and review. Current Opinion in Infectious Diseases. 2014;27:394–400. doi: 10.1097/QCO.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann T., Ellsworth J., Huda N., Neelakanta A., Chevalier T., Sims K.L., Dhar S., Robinson M.E., Kaye K.S. Building and Validating a Computerized Algorithm for Surveillance of Ventilator-Associated Events. Infection Control and Hospital Epidemiology. 2015;36:999–1003. doi: 10.1017/ice.2015.127. [DOI] [PubMed] [Google Scholar]
- Mohd Ali N.A., Jauncey-Cooke J., Bogossian F. Ventilator-associated events in children: A review of literature. Australian Critical Care. 2019;32:55–62. doi: 10.1016/j.aucc.2018.11.063. [DOI] [PubMed] [Google Scholar]
- Nair G.B., Niederman M.S. Using ventilator-associated pneumonia rates as a health care quality indicator: A contentious concept. Seminars in Respiratory and Critical Care Medicine. 2017;38:237–244. doi: 10.1055/s-0037-1602580. [DOI] [PubMed] [Google Scholar]
- Nakahashi S., Imai H., Imanaka H., Ohshimo S., Satou T., Shima M., Yanagisawa M., Yamashita C., Ogura T., Yamada T., Shime N. Ventilator-associated events: Prevalence and mortality in Japan. Journal of Thoracic Disease. 2018;10:6942–6949. doi: 10.21037/jtd.2018.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora D., Póvoa P. Antibiotic consumption and ventilator-associated pneumonia rates, some parallelism but some discrepancies. Annals of Translational Medicine. 2017;5:450. doi: 10.21037/atm.2017.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir S., Martin-Loeches I., Makris D., Jaillette E., Karvouniaris M., Valles J., Zakynthinos E., Artigas A. Impact of appropriate antimicrobial treatment on transition from ventilator-associated tracheobronchitis to ventilator-associated pneumonia. Critical Care (London, England) 2014;18:R129. doi: 10.1186/cc13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-López Y., Pujol M., Campins M., Lagunes L., Balcells J., Rello J. Assessing prediction accuracy for outcomes of ventilator-associated events and infections in critically ill children: A prospective cohort study. Clinical Microbiology and Infection. 2017 doi: 10.1016/j.cmi.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Pugh R., Harrison W., Harris S., Roberts H., Scholey G., Szakmany T., WICSARG Investigator sand WHAIP Is HELICS the right way? Lack of chest radiography limits ventilator-associated pneumonia surveillance in Wales. Frontiers in Microbiology. 2016;7:1271. doi: 10.3389/fmicb.2016.01271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Estrada S., Lagunes L., Peña-López Y., Vahedian-Azimi A., Nseir S., Arvaniti K., Bastug A., Totorika I., Oztoprak N., Bouadma L., Koulenti D., Rello J., The EU-VAE Study Investigators Group Assessing predictive accuracy for outcomes of ventilator-associated events in an international cohort: The EUVAE study. Intensive Care Medicine. 2018 doi: 10.1007/s00134-018-5269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rello J., Peña-López Y., Solé-Violan J. Implications of oxygenation variations in ventilated patients with respiratory infections. Minerva Anestesiologica. 2018;84:423–425. doi: 10.23736/S0375-9393.18.12560-0. [DOI] [PubMed] [Google Scholar]
- Shenoy E.S., Rosenthal E.S., Shao Y.-P., Biswal S., Ghanta M., Ryan E.E., Suslak D., Swanson N., Junior V.M., Hooper D.C., Westover M.B. Real-time, automated detection of ventilator-associated events: Avoiding missed detections, misclassifications, and false detections due to human error. Infection Control and Hospital Epidemiology. 2018;39:826–833. doi: 10.1017/ice.2018.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim J.K., Oh J.Y., Min K.H., Hur G.Y., Lee S.H., Lee S.Y., Kim J.H., Shin C., Shim J.J., Kang K.H. Clinical significance of ventilator-associated event. Journal of Critical Care. 2016;35:19–23. doi: 10.1016/j.jcrc.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Spalding M.C., Cripps M.W., Minshall C.T. Ventilator-associated pneumonia: New definitions. Critical Care Clinics. 2017;33:277–292. doi: 10.1016/j.ccc.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeppel C.M., Eriksson E.A., Hawkins K., Eastman A., Wolf S., Minei J., Minshall C.T. Applicability of the National Healthcare Safety Network’s surveillance definition of ventilator-associated events in the surgical intensive care unit: A 1-year review. Journal of Trauma and Acute Care Surgery. 2014;77:934–937. doi: 10.1097/TA.0000000000000425. [DOI] [PubMed] [Google Scholar]
Relevant Websites
- https://www.cdc.gov/nhsn/PDFs/pscManual/10-VAE_FINAL.pdf—CDC Device-associated Module. Ventilator-associated event (VAE), January 2019.
- https://www.cdc.gov/nhsn/pdfs/training/2018/vae-508.pdf—CDC VAE training.


