Abstract
Toxoplasma gondii is a protozoan parasite, occurring worldwide, endangers human health and causes enormous economic losses to the Ministry of Agriculture. A safe and effective vaccination is needed to handle these problems. In addition, ideal vaccine production is a challenge in the future. In this study, we knocked out the adenylosuccinate lyase (ADSL) gene and found that the gene reduces the growth rate of T. gondii tachyzoites in vitro under standard growth conditions by plaque or replication experiments. Furthermore, mice that were immunized with tachyzoites of the ME49ΔADSL strain induced 100% protection efficacy against challenge with the type 1 strain RH, type 2 strain ME49 and type 3 strain VEG. All mice that were immunized with ME49ΔADSL had a survival rate of 100% when they were reinfected with wild-type strains, either 30 days or 70 days after immunization, and immunization was also protective against homologous infection with 50 T. gondii ME49 tissue cysts. In addition, the level of Toxoplasma-specific IgG was significantly elevated at 30 and 70 days after immunization. ME49ΔADSL induced high levels of Th1 cytokines (interferon gamma (IFN-γ), interleukin (IL)-12) at 4 weeks after immunization and spleen cell cultures from mice vaccinated for 150 days were able to produce robust INF-γ and IL-12 levels in the supernatant. The results of the present study showed that ΔADSL vaccination induced a T. gondii-specific cellular immune response against further infections. These results suggest that the ADSL-deficient vaccine can induce anti-Toxoplasma gondii humoral and cellular immune responses and has 100% immune protection against post-challenge by the type 1 strain RH, type 2 strain ME49 and type 3 strain VEG. It will be used as an excellent candidate for live vaccines and may contribute in a positive meaning to control human toxoplasmosis.
Keywords: Toxoplasma gondii, adenylosuccinate lyase (ADSL), attenuated live vaccine, protective immunity
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite that is distributed throughout the world and infects almost all warm-blooded animals [1]. The majority of T. gondii infection is asymptomatic in hosts with normal immune function, but systemic infections are more common in immunodeficient patients, such as those with AIDS, organ transplants, malignant tumors, etc. [2,3,4]. Congenital toxoplasmosis can also cause miscarriage, premature birth, teratogenic effects or stillbirth in pregnant women, especially in early pregnancy, and the incidence of teratogenesis is high for fetuses [5]. In animals, millions of lambs are still lost worldwide due to the miscarriage of ewe caused by toxoplasmosis, and huge economic losses are caused in agriculture [6,7]. Ethylamine, sulfonamide and other drugs can inhibit T. gondii folic acid metabolism but are ineffective for cysts, which will develop resistance over time [8]. Therefore, the development of a T. gondii vaccine is urgently needed for the prevention and control of toxoplasmosis and the reduction of economic losses. Currently, the live tachyzoites of strain S48 is a commercially available vaccine (Toxovax®), which has been used to reduce neonatal mortality in lambs [9,10]. Although the exact mechanism of the Toxovax® vaccine is not completely clear, the success of Toxovax® is a milestone in the development of a Toxoplasma vaccine. In the past ten years, research on T. gondii vaccines, such as protein, DNA vaccine and recombinant vaccines, has been carried out. Some soluble or secretory proteins are obtained from cultured tachyzoites as killed vaccines or native parasite antigens. However, these vaccines did not provide sufficient protection [11,12]. Then the recombinant DNA technology is an alternative strategy. A large number of Toxoplasma recombinant antigens have been widely tried. These include dense-granule proteins (GRAs), micronemes (MICs) and surface antigens (SAGs) [13,14,15]. Although these subunit vaccines were easier to produce and more stable than native antigens vaccines, they only induced partial immune protection against further parasite infection [16,17]. Thus, vaccine of safe and effective is urgently needed for T. gondii. In recent years, genetically modified strains as vaccines have become a major research focus. Many scientists have begun to study genetically modified parasites, and the virulence of T. gondii was weakened by deleting certain genes, as has been achieved in some studies [18,19,20,21,22]. In addition, early studies have shown that T. gondii is a purine auxotroph organism [23,24]. Hence, T. gondii possesses significant machinery for purine salvage, and this extensive machinery may enable this parasite to survive and replicate in an extensive range of mammalian cell types [25]. Adenylosuccinate lyase (ADSL) is present in the T. gondii salvage pathway to make IMP from AMP by a two-step reaction [25].
In this study, the ADSL gene was knocked out, and the protective immunity of the ME49ΔADSL strain was comprehensively evaluated to obtain an excellent vaccine to confront T. gondii infection in mice models .The results showed that the ADSL gene reduces the growth rate of T. gondii tachyzoites in vitro and induces anti-Toxoplasma humoral and cellular immune responses, and the strain induces a 100% efficient protective immune response against multiple Toxoplasma gondii strains, including the virulent strain RH, whether in the context of long-term or short-term. The modified strain will be used as an excellent potential toxoplasmosis vaccine that is able to protect animals from further infections of a variety of T. gondii strains.
2. Materials and Methods
2.1. Mice and Parasite Strains
Eight-week-old female ICR mice were obtained from the Huazhong Agricultural University Laboratory Animal Center, HuBei, China. All animals were housed in specific pathogen-free conditions. Mice were adapted to the environment for one week before use. All animal experiments were permitted by the ethics committee of Huazhong Agricultural University (HZAUMO-2019-009). The Toxoplasma gondii type I strain RHΔhxgprt, type 2 strain ME49, type 3 strain VEG, RFLP genotype ToxoDB #9 strain C7719, ToxoDB #3 strain TgPIG-WH1 and mutant strain ME49:ΔADSL used in this study were maintained in vitro in human foreskin fibroblast (HFF) cells (purchased from the ATCC, Rockefeller, MD, USA) and were propagated in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Inc., Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin. All strains and cells were culture using standard techniques [26].
2.2. Disruption of ADSL by the CRISPR-Cas9 System
ADSL (TGME49_277760) deletions in ME49 strains were performed by the CRISPR/Cas9 system, which utilized homologous recombination to replace the target gene, and the associated method was performed as described previously [27,28]. Briefly, the resistance cassette loxp-DHFR*-loxp was amplified from pDONR-G265-loxP-DHFR*-loxp, and 1-kbp 5′- and 3′-homology arms were obtained from the genomic DNA from ME49 strains. These fragments were concatenated into the pUC19 vector and then assembled into the pADSL:loxp-DHFR*-loxp plasmid, which can serve as a template to replace ADSL with loxp-DHFR*-loxp. All the primers used during plasmid construction are listed in Table 1, and all plasmids were proven to work by DNA sequencing before use. Furthermore, we modified pSAG1:CAS9-U6:sgUPRT to replace the UPRT targeting guide RNA (gRNA) with a single-guide RNA (sgRNA) that disrupted the ADSL gene by using Q5 site-directed mutagenesis. Toxoplasma gondii ME49 was transfected with the linearized pADSL:loxp-DHFR*-loxp homologous fragment and ADSL-specific CRISPR plasmid as previously described [27,28] and selected with 1 µM pyrimethamine (Life Technologies, Inc., Rockville, MD, USA).
Table 1.
Primers used in this study.
| Primer | Sequence | Use |
|---|---|---|
| gRNA-ADSL-Fw | 5′- GACACTGCTCAATTTCGTCGGTTTTAGAGCTAGAAATAGC-3′ | To construct the ADSL specific CRISPR plasmid |
| gRNA-R | 5′-AACTTGACAT CCCCA TTTAC-3′ | To construct the ADSL specific CRISPR plasmid |
| pUC19-Fw pUC19-Rv |
5′-GGCGTAATCATGGTCATAGC-3′ 5′-CTCGAATTCACTGGCCGTCG-3′ |
Amplification of pUC19 for Gibson assembly Amplification of pUC19 for Gibson assembly |
| loxp-DHFR*-loxp-Fw loxp-DHFR*-loxp-Rv 5′-GGACACGCTGAACTTGTGGC-3′ |
5′-CAACCCGCGCAGAAGACATC-3′ | Amplification of loxp-DHFR*-loxp for Gibson assembly Amplification of loxp-DHFR*-loxp for Gibson assembly |
| U5ADSL-Fw pADSL: loxp- DHFR*-loxp construction |
5′-GTTGTAAAACGACGGCCAGTCTAATTTTTGCCGGGTCTGG-3′ | Amplification of 5′-homology of ADSL for |
| U5ADSL-Rv pADSL: loxp- DHFR*-loxp construction |
5′-GATGTCTTCTGCGCGGGTTG CGAGACGAAGAAGAGAGC-3′ | Amplification of 5′-homology of ADSL for |
| U3ADSL-Fw pADSL: loxp -DHFR*-loxp construction |
5′-GCCACAAGTTCAGCGTGTCCACCTCAGTTGTCGGCACCGT-3′ | Amplification of 3′-homology of ADSL for |
| U3ADSL-Rv pADSL: loxp--DHFR*-loxp construction |
5′-GCTATGACCATGATTACGCCCGCAGACTCAAATCTATTC-3′ | Amplification of 3′-homology of ADSL for |
| 5′-UpU5 ADSL | 5′-GCTCTTGCTTTCGTCGCTGTC-3′ | PCR1 of ΔADSL:DHFR |
| 3′-InDHFR*-Fw | 5′-CGTGACCACGCCAAAGTAG-3′ | PCR1 of ΔADSL:DHFR |
| 5′-InDHFR*-Rv | 5′-GCACTTGCAGGATGAATTCC-3′ | PCR2 of ΔADSL:DHFR |
| 3′-DnU3ADSL | 5′-GTGTCTCGCACATGCGCGTT-3′ | PCR2 of ΔADSL:DHFR |
| 5′-UpgRNA ADSL | 5′- AACGCAATCAAGACGCTGGC-3′ | PCR3 of ΔADSL:DHFR |
| 3′-DngRNA ADSL | 5′- CGTCACTGTACCAGCGAGCAG-3′ | PCR3 of ΔADSL:DHFR |
2.3. Virulence Tests and Cyst Formation of Mutants Versus WT Strains in Mice
Freshly egressed tachyzoites of the ME49 strain and ME49ΔADSL strain were filtered with a 3.0 μm membrane filter and then harvested to intraperitoneally (ip) infect 8-week-old female ICR mice (10 mice per strain). Subsequently, the clinical symptoms of toxoplasmosis were observed, and mortality was recorded for 30 days. Blood samples were collected from the mouse tail vein at 30 days. After clot retraction, serum samples were collected and detected by enzyme-linked immunosorbent assay (ELISA) with T. gondii soluble antigens as coating antigen to confirm infection. Soluble Toxoplasma antigens were prepared by a previously described method [29]. Subsequently, brain cysts from seropositive mice were harvested, and DBA-FITC (Vector Laboratories, Burlingame, CA, USA) dyeing as previously described [30] was used to calculate the number of Toxoplasma cysts. The results of brain cyst number and cumulative mortality determination in the control and experimental group mice were graphed as Kaplan–Meier survival plots and then analyzed in Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
2.4. Toxoplasma Plaque Assay
HFF monolayer cells were seeded into 6-well plates and cultured at 37 °C with 5% CO2. Purified tachyzoites were quantified with a hemocytometer under a Nikon Eclipse TS100 phase contrast microscope (Nikon Instruments, Tokyo, Japan) and then inoculated onto host cells (200 parasites per well). Subsequently, the six-well plates containing parasites were placed at 37 °C with 5% CO2 for 13 days. After incubation, HFF cells were fixed, stained with 0.5% crystal violet solution, washed with PBS and imaged.
2.5. Assay for Parasite Replication In Vitro
The growth ability of wild-type andME49△ADSL strains was detected as previously described [31]. Briefly, tachyzoites that were grown in HFF cells for 3 days were purified by 3.0 μm membrane filtration and then inoculated in a 24-well plate that was seeded with HFF cells. After samples were incubated for 1 h at 37 °C with 5% CO2, the parasites that failed to invade cells were washed away, and the cultures were continued for 24 h at 37 °C. Subsequently, rabbit anti-TgALD and mouse anti-SAG1 were used as primary antibodies to detect extracellular parasites and total parasites, respectively. SAG1 has been reported to be anchored to the parasite membrane [32,33]. Alexa 488-conjugated goat anti-mouse and Alexa 594-conjugated goat anti-rabbit IgGs were used to stain as a secondary antibodies by diluting 1:1000. The number of parasites per vacuole was calculated under fluorescence microscopy. A minimum of 100 total parasitophorous vacuoles was examined for one sample. All strains were tested three times independently.
2.6. Protection of the ME49ΔADSL Strain Against Acute Infection, Toxoplasma-Specific lgG levels and Cytokine Detection
In this experiment, the immune protection of ME49ΔADSL tachyzoites against acute T. gondii infection was studied. Briefly, female ICR mice were immunized with 100 me49ΔADSL tachyzoites intraperitoneally. Then, 32 and 72 days after immunization, seropositive mice were challenged intraperitoneally with 500 tachyzoites of RHΔhxgprt or ME49 or 1 × 104 tachyzoites of C7719, VEG or TgPIG-WH1 or orally with 50 fresh cysts of ME49, as previously described [22]; natural mice that were not immunized were used as controls. In addition, serum samples were collected from ΔADSL-immunized mice 30 days and 70 days after immunization. Sera from mice that were not immunized were used as a control, and then these sera were used to assay the level of secreted interleukin-12p70 (IL-12p70), interferon gamma (INF-γ) and IL-4 by commercial ELISA kits following the manufacturer’s instructions (BioLegend, Inc., USA). T. gondii-specific IgG was measured by enzyme-linked immunosorbent assay (ELISA) as described previously [29].
2.7. Cytokine Production by Splenocytes After T. gondii Antigen Stimulation
One hundred fifty days after ICR mouse immunization, the spleens of seropositive mice were collected and ground gently in RPMI 1640 medium (Life Technologies, Inc., Rockville, MD, USA) with a 45 µm filter. Subsequently, the samples were centrifuged for 10 min at 1000× g and then resuspended in 10 mL of red cell lysis solution (Biosharp, Inc., Beijing, China) for 5 min at ambient temperature. The splenocyte suspensions were centrifuged as above and washed with RPMI 1640 medium. Trypan blue was used to assay the viable cells, and 3 × 105 viable splenocytes that were counted by a hemocytometer under a Nikon Eclipse TS100 phase contrast microscope (Nikon Instruments, Tokyo, Japan) were seeded into 96-well flat-bottom tissue culture plates in a final volume of 100 µL of RPMI 1640 supplemented with 20% FBS (Life Technologies, Inc., Rockville, MD, USA) and 1% penicillin–streptomycin mixture. After 1 day of incubation, TSA (final concentration of 50 µg/mL) was used to stimulate the splenocytes. Meanwhile, concanavalin A (final concentration of 5 µg/mL) and 20% FBS RPMI 1640 medium were added to stimulate the splenocytes as positive and negative controls, respectively. All supernatants were collected after stimulation to detect the level of cytokines, which was performed as above. In addition, non-immunized naïve mice were subjected to the same experiment as a control.
2.8. Statistical Analysis
All data were analyzed in Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) using Student’s t tests, Gehan–Breslow–Wilcoxon tests or one-way ANOVA with Bonferroni post-tests, as indicated in figure legends.
3. Results
3.1. Generation of ADSL-Deficient Type II T. gondii
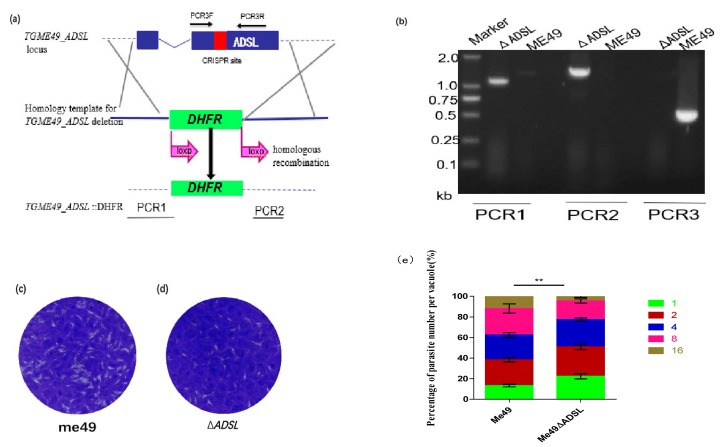
To study the biological function of the ADSL gene against Toxoplasma gondii, we generated a TgADSL mutant by using the CRISPR/Cas9-mediated genome editing system Figure 1a. A CRISPR plasmid containing the ADSL gene sgRNA and homology template ADSL:DHFR* was simultaneously transferred into the type II strain ME49. Subsequently, pyrimethamine was used to select stable single clones, which were confirmed successfully by diagnostic polymerase chain reactions (PCRs) Figure 1b. The correct integration of the DHFR marker at the ADSL locus was demonstrated by PCR1 and PCR2. The validation of PCR3 further proved the successful acquisition of the ADSL mutant strain Figure 1b. To measure the impact of TgADSL disruption on parasite growth, we performed plaque assays. Compared with the parental strain, the knockout strain showed obvious growth defects according to plaques and replication results Figure 1c–e, suggesting that the ADSL gene plays an important role in the in vitro growth of Toxoplasma gondii.
Figure 1.
Generation and analysis of an ADSL deletion mutant. (a) Schematic illustration of knocking out ADSL in ME49 to produce ΔADSL. (b) Diagnostic polymerase chain reaction (PCR) for a ΔADSL mutant clone. (c, d) Plaque assay comparing the growth of the ΔADSL mutant to that of the wild-type strain ME49. (e) Tachyzoite replication under standard tissue culture conditions. HFF cells were infected with purified tachyzoites, invading parasites were cultured for 24 h, and then fluorescently stained to determine the number of parasites in each parasitophorous vacuole (PV), ** p < 0.01, two-way analysis of variance.
3.2. Effects of ADSL Mutation on Virulence and Reduced Cyst Formation In Vivo
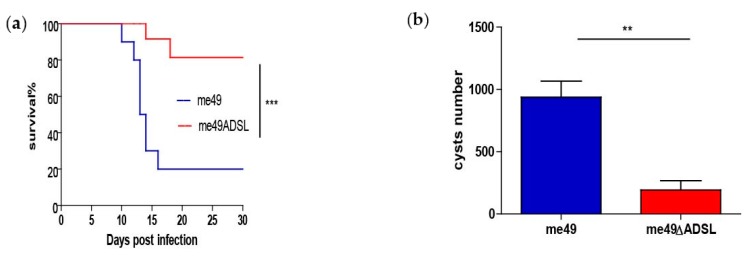
The consequences of ADSL deletion on the acute infection stage of the parasite were studied by performing a survival curve analysis. Briefly, 100 tachyzoites of both the parental strain ME49 and ADSL deletion mutants that were infected intraperitoneally into mice, and the survival rate results are shown in Figure 2a. That the survival rates at 30 days for the ME49 and ΔADSL mutants groups were 20% and 70% Figure 2a, respectively, indicated that ADSL deletion led to attenuated virulence of the parasite. Subsequently, brains were collected in survivor mice at 30 days post-infection, and brain cysts were quantified by DBA-FITC staining. The cyst number in ΔADSL mutants was significantly reduced and was compared with that of the ME49 strain under the same experimental conditions. The result is shown in Figure 2b.
Figure 2.
Virulence and cyst formation of the ΔADSL mutant in mice. (a) Virulence assessment of the indicated strains in ICR mice, as shown in Figure 2a. (b) Brain cyst counts for the mice in () that survived at day 30, as shown in Figure 2b. ** p < 0.01, *** p < 0.001, Gehan–Breslow–Wilcoxon tests.
3.3. Immune Responses of Immunized Mice to Acute Tachyzoites Infection
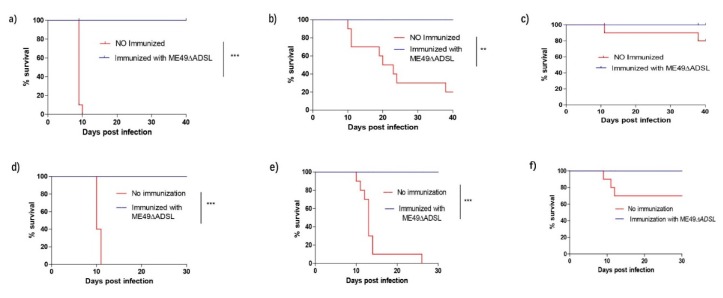
The results suggest that the virulence of the ADSL mutant was weakened, as described above. To examine whether mice after ADSL immunization were resistant to acute infection of Toxoplasma gondii, ICR mice were challenged intraperitoneally with 500 tachyzoites of RHΔhxgprt, 1 × 103 tachyzoites of ME49, or 1 × 104 tachyzoites of VEG 30 days after immunization with the ADSL mutants. Meanwhile, naïve mice were challenged with the same strain and the same corresponding dose as a control. The mortality rates for non-immunized mice challenged with RH were 100%. The mortality rates of ME49- and VEG-challenged naïve mice were 80% and 20%, respectively. By contrast, the survival rate of all immunized mice was 100%, with infection with any of the three strains, and no obvious clinical symptoms of toxoplasmosis were observed during 40 days of infection Figure 3a–c.
Figure 3.
ΔADSL parasite immunization protected mice from Toxoplasma gondii tachyzoite infection. ICR mice were pre-immunized with 100 tachyzoites of the ME49 ΔADSL mutant. (a–c) Thirty days post-immunization, the immunized mice were challenged with 500 RH tachyzoites (a), 103 ME49 tachyzoites (b) or 103 VEG tachyzoites ((c); 10 mice for each strain) by intraperitoneal injection, and their survival was monitored for another 40 days. (d–f) 75 days post-immunization, 500 tachyzoites of the RH Δhxgprt (d), ME49 (e) or VEG (f) strains were used to challenge the mice, and mouse survival was monitored for another 30 days. Non-immunized mice were included as controls. ** p < 0.01 *** p < 0.001, Gehan–Breslow–Wilcoxon tests.
Subsequently, the long-time protective immunity was studied for immunized ICR mice. Briefly, ICR mice at 72 days after immunization with ME49ΔADSL were challenged with 500 tachyzoites of RHΔhxgprt or ME49 or 1 × 104 tachyzoites of VEG. The survival of the mice was monitored for another 40 days. The survival of all immunized mice was 100% for the three strains, and no symptoms were observed post-infection. However, the mortality rate of naïve mice infected with the RH and type II ME49 strains was 100%, while VEG caused 30% mortality Figure 3d–f.
The above results not only indicate that short- and long-term protection were furnished by ME49ΔADSL vaccination against acute infection but also suggest that the ΔADSL strain provided protection against bradyzoite infection.
3.4. T. gondii-Specific IgG Antibodies and Cytokine Production After Vaccination
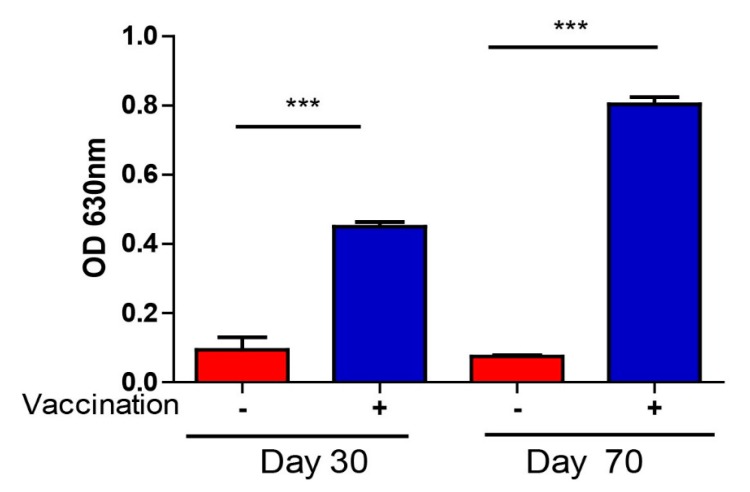
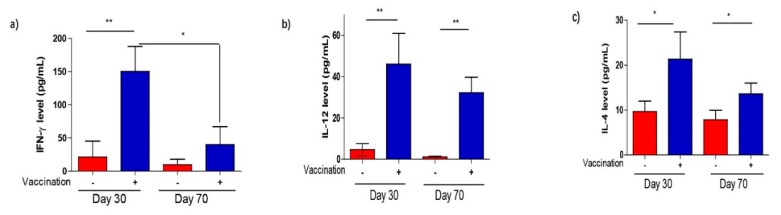
To further explore the immunogenicity of ΔADSL, the specific anti-T. gondii IgG antibody level was detected by ELISA in sera samples that were acquired from vaccinated mice at 30 and 70 days. The results showed that the IgG titer in immunized mice was significantly higher than that in non-immunized mice at 30 and 70 days Figure 4. However, IgG levels were relatively stable over time (Figure 4) for immunized mice. The results suggested that ΔADSL strain infection elicited a strong humoral response. Subsequently, we also collected vaccinated mouse sera at 30 and 70 days to measure the levels of Th1 (IFN-γ and IL-12) and Th2 (IL-4) cytokines by ELISA. The IFN-γ-IL-12 axis, serving as a representative of Th1-type cells, mediates the main cellular immune response to T. gondii. The levels of these cytokines were significantly elevated at 30 and 70 days compared to those in control mice (Figure 5a–c). Mice immunized for 70 days showed a significant decrease in IFN-γ levels compared to those immunized for 30 days (Figure 5a). In summary, these results suggested that vaccination with ΔADSL induced cellular and humoral immune responses against secondary infections of T. gondii.
Figure 4.
Relative levels of Toxoplasma-specific IgG, as determined by indirect ELISA. Sera from naïve mice were used as controls, and three mice from each group were analyzed. *** p < 0.001, NS: not significant, Student’s t-test.
Figure 5.
Levels of selected cytokines in the sera of mice 30 and 70 days after ΔADSL immunization. (a–c) Levels of the indicated cytokines measured by ELISA. Sera from naïve mice were used as controls, and three mice from each group were analyzed. * p < 0.05, ** p < 0.01, NS: not significant, Student’s t-test.
3.5. Cytokine Production After Vaccination in Response to Stimulation with T. gondii Antigen
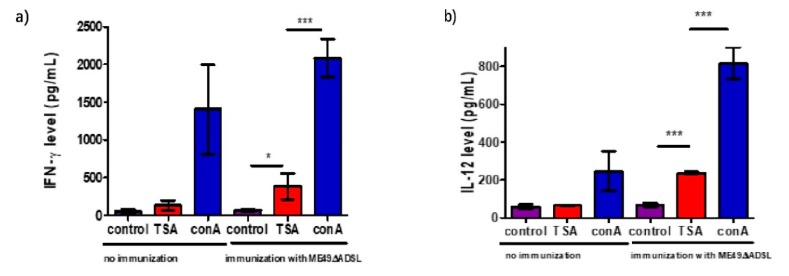
To verify the antigen recall response of immunized mice, we checked the cytokine levels of splenocytes that were infected with the ADSL mutant for 150 days and non-immunized mice that were used as a control. The splenocytes were stimulated with TSA prepared as described above, and culture supernatants were used to detect the IFN-γ and IL-12 levels by ELISA. The results showed that TSA stimulated high levels of the pro-inflammatory cytokines INF-γ and IL-12 (Figure 6a,b), while non-stimulated or nonvaccinated splenocytes served as a control. IFN-γ is a major regulator of cell-mediated immunity that activates hematopoietic and nonhematopoietic effector cells to control parasite replication, as previously reported [34,35,36]. In our study, we found that INF-γ and IL-12 were produced from vaccinated splenocytes upon TSA stimulation. During a second infection, effective cellular immunity is activated and makes a major contribution.
Figure 6.
Cytokine production by splenocytes of ME49 ΔADSL-vaccinated mice after Toxoplasma antigen stimulation. ICR mice were vaccinated with 100 tachyzoites of ME49 ΔADSL, and splenocytes were harvested 150 days post-vaccination. The in vitro cultured splenocytes were then treated with fetal bovine serum (control), total soluble Toxoplasma antigen (TSA) or concanavalin A (conA, positive control) for 72 h. Subsequently, the levels of IFN-γ (a) and IL-12 (b) in the culture supernatants were measured by ELISA. Splenocytes isolated from non-immunized naïve mice were included as controls. Three mice from each group were analyzed, and each treatment condition was repeated three times. * p < 0.05, *** p < 0.001, Student’s t-test.
4. Discussion
It is well known that the purine salvage pathways are essential for the growth and development of T. gondii [23,24,25,37]. T. gondii possesses many enzymes involved in interconversion and salvage of host purines, and adenylosuccinate lyase (ADSL) is one of them [25]. However, the role of the ADSL gene in the growth of T. gondii is unclear. In this study, ADSL was knocked out by using the gene editing system CRISPR/Cas9 in the ME49 strain. Subsequently, the results of plaque and replication assays showed that the gene reduces the growth rate of T. gondii tachyzoites in vitro under standard growth conditions. In addition, virulence experiments indicate that the replication of the ADSL mutant strain in vivo was slowed down and the ability to form cysts was weakened. The specific mechanism for these results is unclear, and we suspect that the reduction of AMP synthesis required for T. gondii due to the ADSL deficiency [23] thereby reduces the precursors of nucleotide synthesis and affects the growth of Toxoplasma gondii. Next, the ΔADSL mutant was investigated for potential immunization as a live-attenuated vaccine against reinfection of the T. gondii wild-type strain in a mouse model. ΔADSL-infected mice survived when the dose of infection was 100. Therefore, for all subsequent experiments, 100 ME49ΔADSL tachyzoites were used as the vaccination dose, which not only provided a balance between safety and high immunogenicity but also represented a promising attenuated live vaccine strain.
We then tested the antibody and cytokine levels induced in ΔADSL-immunized mice by ELISA. These results suggested a combination of humoral and cellular immune responses and their protective effects against T. gondii attack, but the cellular immune response was dominant. ΔADSL-immunized mice evoked a high level of anti-T. gondii IgG at 30 and 70 days after immunization, indicating that IgG antibodies play an important protective role in further infections with T. gondii tachyzoites. Initiation of IL-12 and IFN-γ production in host immune cells is essential for host resistance to parasites [38]. In our study, we found that ΔADSL-immunized mice produced higher levels of IL-12, IFN-γ, and IL-4 at 30 and 70 days post-infection than non-immunized mice. It is well known that the production of IFN-γ and IL-12 that is due to infection by T. gondii stimulates T cells, macrophages, dendritic cells, and neutrophils [38,39]. In addition, the Th2 cytokine IL-4 is critical for neutralizing the inflammation and immunopathologies caused by the Th1 response [40]. The balance of Th1 and Th2 cytokine levels was observed to control tachyzoite transmission in ADSL-immunized mice. ADSL-immunized mice produced immune protection against post-challenge of other various Toxoplasma gondii strains, such as the type I strain RHΔhxgprt, type 2 strain ME49 and type 3 strain VEG (i.e., 100% survival rate), whereas nonvaccinated mice died within 10 dpi. It is noteworthy that this immune protection can provide complete long-term or short-term protection against the virulent strain RH Δhxgprt. We detected cytokines from spleen cell culture supernatants at day 150 post-ΔADSL immunization and found a significant increase in the level of the IFN-γ and IL-12 cytokines after stimulation with TSA. The results showed that immunized mice could activate cellular immunity and clear secondary infections efficiently better than non-immunized mice. In addition, we found that ΔADSL immunization induced high levels of IL-12 and IFN-γ for a fairly long duration after infection, and this effect may be caused by the unique propagation dynamics of the ΔADSL mutants in vivo. Therefore, the continued IL-12 and IFN-γ induction might be a consequence of these parasite activities, and prolonged IL-4 may be to provide balance.
In conclusion, we demonstrated that a single ip vaccination of 100 ME49:ΔADSL tachyzoites provided immune protection against challenge with various T. gondii strains by stimulating cellular and humoral immune responses. In addition, ME49:ΔADSL immunization could improve the survival rate and reduce cyst production compared with those of unimmunized groups. Moreover, the ΔADSL mutant also generated high levels of Th1 (IFN-γ and IL-12) immunity and elevated Th2 (IL-4) protective immunity, as indicated at 30 and 70 days post-immunization, and protected animals from further infections with various T. gondii wild-type strains. Although our research from mice models demonstrated the ΔADSL mutant vaccine could be used as an excellent potential vaccine, further investigations are needed to check the safety of vaccination and efficiency of protection in other host species like swine and sheep.
Author Contributions
R.F. conceived and designed the study, L.W., C.Y., D.T. and J.Y. performed the experiments, R.F. and L.W. drafted the manuscript, and L.W. analyzed the data. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31572510), and Da Bei Nong Group Promoted Project for Young Scholar of HZAU (Grant No. 2017DBN001).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Dubey J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Luft B.J., Conley F., Remington J.S., Laverdiere M., Wagner K.F., Levine J.F., Craven P.C., Strandberg D.A., File T.M., Rice N., et al. Outbreak of central-nervous-system toxoplasmosis in western Europe and North America. Lancet. 1983;1:781–784. doi: 10.1016/S0140-6736(83)91847-0. [DOI] [PubMed] [Google Scholar]
- 3.Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 4.Remington J.S. Toxoplasmosis in the adult. Bull. N. Y. Acad. Med. 1974;50:211–227. [PMC free article] [PubMed] [Google Scholar]
- 5.Roux C., Desmont G., Mulliez N., Gaulier M., Tufferaud G., Marmor D., Herbillon A. Toxoplasmosis and pregnancy. Evaluation of 2 years of prevention of congenital toxoplasmosis in the maternity ward of Hopital Saint-Antoine (1973-1974) J. Gynecol. Obstet. Biol. Reprod. (Paris) 1976;5:249–264. [PubMed] [Google Scholar]
- 6.Dubey J.P., Miller S., Powell E.C., Anderson W.R. Epizootiologic investigations on a sheep farm with Toxoplasma gondii-induced abortions. J. Am. Vet. Med. Assoc. 1986;188:155–158. [PubMed] [Google Scholar]
- 7.Dubey J.P., Welcome F.L. Toxoplasma gondii-induced abortion in sheep. J. Am. Vet. Med. Assoc. 1988;193:697–700. [PubMed] [Google Scholar]
- 8.Alday P.H., Doggett J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017;11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxton D., Innes E.A. A commercial vaccine for ovine toxoplasmosis. Parasitology. 1995;110:S11–S16. doi: 10.1017/S003118200000144X. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins M.F., O’Connell E. Effect on lambing percentage of vaccinating ewes with Toxoplasma gondii. N. Z. Vet. J. 1983;31:181–182. doi: 10.1080/00480169.1983.35017. [DOI] [PubMed] [Google Scholar]
- 11.Daryani A., Hosseini A.Z., Dalimi A. Immune responses against excreted/secreted antigens of Toxoplasma gondii tachyzoites in the murine model. Vet. Parasitol. 2003;113:123–134. doi: 10.1016/S0304-4017(03)00044-X. [DOI] [PubMed] [Google Scholar]
- 12.Waldeland H., Frenkel J.K. Live and killed vaccines against toxoplasmosis in mice. J. Parasitol. 1983;69:60–65. doi: 10.2307/3281275. [DOI] [PubMed] [Google Scholar]
- 13.Cao A., Liu Y., Wang J., Li X., Wang S., Zhao Q., Cong H., He S., Zhou H. Toxoplasma gondii: Vaccination with a DNA vaccine encoding T- and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015;33:6757–6762. doi: 10.1016/j.vaccine.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 14.Dautu G., Munyaka B., Carmen G., Zhang G., Omata Y., Xuenan X., Igarashi M. Toxoplasma gondii: DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Exp. Parasitol. 2007;116:273–282. doi: 10.1016/j.exppara.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Yang C.D., Chang G.N., Chao D. Protective immunity against Toxoplasma gondii in mice induced by a chimeric protein rSAG1/2. Parasitol. Res. 2004;92:58–64. doi: 10.1007/s00436-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 16.Bastos L.M., Macedo A.G., Jr., Silva M.V., Santiago F.M., Ramos E.L., Santos F.A., Pirovani C.P., Goulart L.R., Mineo T.W., Mineo J.R. Toxoplasma gondii-Derived Synthetic Peptides Containing B- and T-Cell Epitopes from GRA2 Protein Are Able to Enhance Mice Survival in a Model of Experimental Toxoplasmosis. Front. Cell. Infect. Microbial. 2016;6:59. doi: 10.3389/fcimb.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajissa K., Zakaria R., Suppian R., Mohamed Z. Epitope-based vaccine as a universal vaccination strategy against Toxoplasma gondii infection: A mini-review. J. Adv. Vet. Anim. Res. 2019;6:174–182. doi: 10.5455/javar.2019.f329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foroutan M., Ghaffarifar F., Sharifi Z., Dalimi A., Jorjani O. Rhoptry antigens as Toxoplasma gondii vaccine target. Clin. Exp. Vaccine Res. 2019;8:4–26. doi: 10.7774/cevr.2019.8.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gigley J.P., Fox B.A., Bzik D.J. Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect. Immun. 2009;77:5380–5388. doi: 10.1128/IAI.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J.L., Elsheikha H.M., Zhu W.N., Chen K., Li T.T., Yue D.M., Zhang X.X., Huang S.Y., Zhu X.Q. Immunization with Toxoplasma gondii GRA17 Deletion Mutant Induces Partial Protection and Survival in Challenged Mice. Front. Immunol. 2017;8:730. doi: 10.3389/fimmu.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J.L., Li T.T., Elsheikha H.M., Chen K., Cong W., Yang W.B., Bai M.J., Huang S.Y., Zhu X.Q. Live Attenuated Pru: Deltacdpk2 Strain of Toxoplasma gondii Protects against Acute, Chronic, and Congenital Toxoplasmosis. J. Infect. Dis. 2018;218:768–777. doi: 10.1093/infdis/jiy211. [DOI] [PubMed] [Google Scholar]
- 22.Xia N., Zhou T., Liang X., Ye S., Zhao P., Yang J., Zhou Y., Zhao J., Shen B. A Lactate Fermentation Mutant of Toxoplasma Stimulates Protective Immunity Against Acute and Chronic Toxoplasmosis. Front. Immunol. 2018;9:1814. doi: 10.3389/fimmu.2018.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug E.C., Marr J.J., Berens R.L. Purine metabolism in Toxoplasma gondii. J. Biol. Chem. 1989;264:10601–10607. [PubMed] [Google Scholar]
- 24.Perrotto J., Keister D.B., Gelderman A.H. Incorporation of precursors into Toxoplasma DNA. J. Protozool. 1971;18:470–473. doi: 10.1111/j.1550-7408.1971.tb03356.x. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhary K., Darling J.A., Fohl L.M., Sullivan W.J., Jr., Donald R.G., Pfefferkorn E.R., Ullman B., Roos D.S. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 2004;279:31221–31227. doi: 10.1074/jbc.M404232200. [DOI] [PubMed] [Google Scholar]
- 26.Roos D.S., Donald R.G., Morrissette N.S., Moulton A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- 27.Shen B., Brown K., Long S., Sibley L.D. Development of CRISPR/Cas9 for Efficient Genome Editing in Toxoplasma gondii. Methods Mol. Biol. 2017;1498:79–103. doi: 10.1007/978-1-4939-6472-7_6. [DOI] [PubMed] [Google Scholar]
- 28.Shen B., Brown K.M., Lee T.D., Sibley L.D. Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. MBio. 2014;5:e01114. doi: 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pishkari S., Shojaee S., Keshavarz H., Salimi M., Mohebali M. Evaluation of Toxoplasma gondii soluble, whole and excretory/secretary antigens for diagnosis of toxoplasmosis by ELISA test. J. Parasit. Dis. 2017;41:289–291. doi: 10.1007/s12639-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melzer T.C., Cranston H.J., Weiss L.M., Halonen S.K. Host Cell Preference of Toxoplasma gondii Cysts in Murine Brain: A Confocal Study. J. Neuroparasitol. 2010;1 doi: 10.4303/jnp/N100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia N., Yang J., Ye S., Zhang L., Zhou Y., Zhao J., David Sibley L., Shen B. Functional analysis of Toxoplasma lactate dehydrogenases suggests critical roles of lactate fermentation for parasite growth in vivo. Cell. Microbiol. 2018;20 doi: 10.1111/cmi.12794. [DOI] [PubMed] [Google Scholar]
- 32.Burg J.L., Perelman D., Kasper L.H., Ware P.L., Boothroyd J.C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- 33.Kasper L.H. Isolation and characterization of a monoclonal anti-P30 antibody resistant mutant of Toxoplasma gondii. Parasite Immunol. 1987;9:433–445. doi: 10.1111/j.1365-3024.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- 34.Saraav I., Wang Q., Brown K.M., Sibley L.D. Secretory Microneme Proteins Induce T-Cell Recall Responses in Mice Chronically Infected with Toxoplasma gondii. mSphere. 2019;4 doi: 10.1128/mSphere.00711-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturge C.R., Yarovinsky F. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect. Immun. 2014;82:3090–3097. doi: 10.1128/IAI.01722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki Y., Orellana M.A., Schreiber R.D., Remington J.S. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 37.Schwartzman J.D., Pfefferkorn E.R. Toxoplasma gondii: Purine synthesis and salvage in mutant host cells and parasites. Exp. Parasitol. 1982;53:77–86. doi: 10.1016/0014-4894(82)90094-7. [DOI] [PubMed] [Google Scholar]
- 38.Yarovinsky F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014;14:109–121. doi: 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 39.Hunter C.A., Sibley L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazzinelli R.T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kuhn R., Muller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4 + T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]