Abstract
Hepatitis E virus (HEV) is an emerging zoonotic pathogen that has received an increasing amount of attention from virologists, clinicians, veterinarians, and epidemiologists over the past decade. The host range and animal reservoirs of HEV are rapidly expanding and a plethora of emerging HEV variants have been recently identified, some of which have the potential for interspecies infection. In this review, the detection of genetically diverse HEV variants, classified into and presumably associated with the species Orthohepevirus C, currently comprising HEV genotypes C1 and C2, by either serological or molecular approach is summarized. The distribution, genomic variability, and evolution of Orthohepevirus C are analyzed. Moreover, the potential risk of cross-species infection and zoonotic transmission of Orthohepevirus C are discussed.
Keywords: Orthohepevirus C, hepatitis E virus, genetic variability, molecular evolution, host, zoonosis
1. Introduction
Hepatitis E Virus (HEV) is the causative viral pathogen of hepatitis E, originally considered a self-limiting disease occurring only in developing countries with poor sanitary conditions [1]. Mortality rates in infected pregnant women can reach up to 25% during hepatitis E outbreaks in these countries [2]. In the late 1990s, hepatitis E was recognized to be a zoonotic disease and swine was found to be the natural host of HEV in the USA. Soon afterwards, a large number of autochthonous cases were reported in different Europe countries associated with the consumption of undercooked meat [3]. Hepatitis E poses a significant health risk for immunocompromised individuals, such as solid organ transplant recipients and patients with human immunodeficiency virus infection, in whom chronic infections can occur. Ribavirin is commonly administered as an off-label treatment for chronic HEV infection, but significant side-effects often limit its use [4]. Furthermore, HEV may also cause extrahepatic manifestations, including renal and neurological injuries, pancreatitis, cryoglobulinemia, and hematological disorders [5]. In light of this, hepatitis E is being increasingly recognized as a serious public health burden worldwide.
HEV was first isolated in 1983 and the viral genome was subsequently cloned and sequenced [6,7]. HEV is approximately 27–34 nm in diameter. Viral genomes are 6.4–7.2 kb long capped positive-sense single-stranded RNA containing three open readings frames (ORF1, ORF2, and ORF3), 5′- and 3′- untranslated regions, a 7-methylguanylate cap at the 5′ end and a polyadenine tract at the 3′-end. ORF1 encodes a non-structural polyprotein containing a methyltransferase, papain-like cysteine protease, macro domain, RNA helicase, and RNA-dependent RNA polymerase. ORF2 encodes the capsid protein. ORF3 overlaps partially with ORF2 and encodes a multifunctional phosphoprotein associated with viral egress [8]. Recent studies have reported that HEV particles are non-enveloped and infectious in bile and feces, while quasi-enveloped and less infectious/non-infectious in sera [9].
HEV differs from all other human hepatitis viruses in that it is closely related to non-human viruses [10]. HEV is the prototype of the family Hepeviridae, which includes the genera Orthohepevirus and Piscihepevirus. The genus Orthohepevirus is further divided into four species Orthohepevirus A to D. Orthohepevirus A contains HEV variants isolated from human, pig, wild boar, deer, mongoose, rabbit, and camel; Orthohepevirus B from chicken; Orthohepevirus C from rat, greater bandicoot, Asian musk shrew, ferret, and mink; and Orthohepevirus D from bat. The genus Piscihepevirus occurs in cutthroat trout and possibly other fish species [11]. Based on the host range of viruses and phylogeny of viral genomic sequences, proposals are also made for the designation of HEV genotypes and subgenotypes [12]. Nevertheless, numerous novel HEV strains remain unclassified due to the large degree of divergence or the lack of complete genomic sequences.
The species Orthohepevirus C is separated into two genotypes: HEV-C1 and HEV-C2. HEV-C1 contains variants derived from hosts in the orders Rodentia and Soricomorpha, and HEV-C2 from the order Carnivora [8,11]. By means of the application and development of new molecular techniques such as metagenomic sequencing, a growing number of distinct HEV variants have been discovered in a variety of animal species globally and the species Orthohepevirus C has been rapidly extended in the last years [13]. Although the zoonotic potential of Orthohepevirus C is still under debate, two recent clinical cases describing persistent hepatitis in a liver transplant patient in Hong Kong and severe acute hepatitis in an immunocompetent patient in Canada, respectively, have been attributed to infection with rat HEV [14,15]. Furthermore, seven additional rat HEV infections have been confirmed lately in Hong Kong [16]. In this review, we aim to compile and discuss the recent discoveries of HEV variants within the species Orthohepevirus C and their potential risk of cross-species infection and zoonotic transmission to humans.
2. Detection and Distribution of Orthohepevirus C
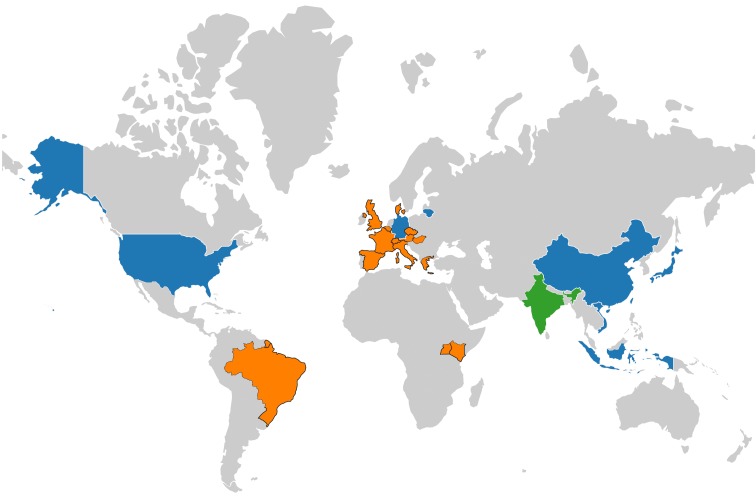
The first study concerning the prevalence of anti-HEV antibodies in wild rats was conducted in the USA in 1999 [17]. In 2000, another study from the USA reported on the HEV seroprevalence among 13 rodent species, providing evidence for widespread infection of Orthohepevirus C in rodents and suggesting these animals might be the natural reservoirs for this species of HEV [18]. Subsequently, Orthohepevirus C RNA was detected in feces of Norway rats in Germany in 2010 using a nested broad-spectrum reverse transcription polymerase chain reaction (RT-PCR) and the complete genomes were sequenced and found to be highly divergent from human HEV strains [19,20]. Thus far, anti-HEV or anti-rat HEV antibodies have been detected in 23 animal species, including 12 and 10 species within the families Muridae and Cricetidae of the order Rodentia, respectively, and one species within the family Soricidae of the order Soricomorpha, from eight countries (the USA, Japan, Germany, China, Vietnam, Lithuania, Indonesia, and India). Additionally, Orthohepevirus C sequences have been detected in 22 countries (the USA, Germany, France, Denmark, China, Lithuania, Hungary, Austria, Switzerland, Italy, Spain, Greece, Belgium, Czech Republic, England, Indonesia, Vietnam, Uganda, the Netherlands, Japan, Brazil, and Kenya) and 25 animal species, containing eight species within the family Muridae and 10 species within the family Cricetidae of the order Rodentia, one species within the family Ursidae and two species within the family Mustelidae of the order Carnivore, two species within the family Falconidae of the order Falconiformes, one species within the family Soricidae of the order Soricomorpha, and one species within family Hominidae of the order Primates. Worldwide detection and distribution of Orthohepevirus C is displayed in Figure 1. Although HEV serology and nucleic acid detection remains negative in several of these animal species, this may be linked to limitations of current detection assays, such as poor specificity and low sensitivity.
Figure 1.
Worldwide detection and distribution of Orthohepevirus C. Countries with both Orthohepevirus C RNA and antibodies detection are in blue; countries with only antibodies detection are in green; countries with only RNA detection are in orange. World map was created by using a free and open source geographic information system Quantum Geographic Information System version 3.10 (http://qgis.osgeo.org) and free vector and raster map data from Natural Earth (http://www.naturalearthdata.com).
2.1. Seroprevalence of Anti-HEV Antibodies
To date, 11 seroprevalence studies have been carried out on Rattus norvegicus (Brown rat/Norway rat). In the USA, the seroprevalences of anti-HEV antibodies were 76.9% (83/108 samples tested positive) between 1986 and 1997, 68.5% (135/197) between 1994 and 1998, and 73.5% (144/196) between 2005 and 2006, considerably higher than in other countries [17,18,21]. In Japan, the rates were 31.5% (114/362) from 2000 through 2002, while another study with unreported sample collection dates resulted in 28.6% seroprevalence (16/56) [22,23]; in Germany, the seroprevalence was 24.5% (36/147) between 2007 and 2010 [24], comparable to China with 27.8% (64/230) between 2011 and 2012 [25]. In Vietnam, two separate studies revealed similar seroprevalences of 20.3% (25/123) and 22.3% (21/94) in 2011 and an additional study between 2012 and 2013 demonstrated a lower seroprevalence of 12.3% (48/389) [26,27,28]. In a recent study in Lithuania, the seroprevalence was 31.2% (34/109) between 2014 and 2017; however, data were derived from both Norway rats (27) and Black rats (82) and individual species rates were not indicated [29].
Six studies have been carried out in Rattus rattus (Black rat) with results varying considerably. In the USA, one study reported a seroprevalence of 90.2% (102/113) between 1986 and 1997, yet only 38.3% (31/81) between 1994 and 1998 in another [17,18]. In Indonesia, three studies have been conducted with positive rates of 18.1% (21/116) between 2011 and 2012, 37.1% (137/369) in 2012, and 4.1% (10/242) between 2014 and 2016 [30,31,32]. In Japan, seroprevalence was 13.3% (12/90) from 2000 through 2002 [22].
Three studies in Vietnam found anti-HEV seroprevalences in Rattus tanezumi (Oriental house rat) of 25.0% (4/16) and 33.3% (2/6) in 2011 and 19.6% (9/46) between 2012 and 2013 [26,27,28]. In a large-scale investigation of sera of wild rats in China between 2011 and 2012, anti-HEV IgG rates in the species Rattus rattoides losea (Losea rat), Rattus flavipectus (Yellow-breasted rat), Rattus rattus hainanus, and Bandicota indica (Greater bandicoot rat), were 21.5% (26/121), 19.9% (34/171), 11.8% (2/17), and 23.0% (40/174), respectively [25]. In India, prevalence of anti-HEV Immunoglobulin G (IgG) was 15.8% (9/57) in 1985 in Rattus rattus rufescens (Indian black rat), 3.6% (2/55) in 1990 in Rattus rattus andamanesis (Sikkim rat), and 54.5% (12/22) in 1985 in Bandicota bengalensis (Lesser bandicoot rat) [33]. In the USA, two species within the family Muridae, Rattus exulans (Polynesian rat) and Mus musculus (House rat), had seroprevalences of 83.3% (15/18) between 1986 and 1997 and 14.3% (2/14) between 1994 and 1998, respectively [17,18]. Lastly, a study conducted in Brazil in 2005 found two of four wild rats (species unknown) were positive for anti-HEV IgG [34].
Antibodies against HEV were also found in multiple rodent species within the family Cricetidae. Seroprevalence rates in Clethrionomys gapperi (Southern red-backed vole), Neotoma albigula (White-throated woodrat), Neotoma mexicana (Mexican woodrat), Neotoma micropus (Southern plains woodrat), Oryzomys palustris (Marsh rice rat), Peromyscus boylei (Brush mice), Peromyscus eremicus (Cactus mouse), Peromyscus leucopus (White-footed mouse), Peromyscus maniculatus (North American deermouse), and Sigmodon hispidus (Hispid cotton rat) were 66.7 (4/6), 59.1 (13/22), 57.1 (48/84), 12.5 (1/8), 24.4 (10/41), 8.3 (2/24), 42.9 (3/7), 10.0 (5/50), 11.0 (11/91), and 32.7 (37/113), respectively. Rodent sera were collected during 1994–1998 throughout the USA and assessed by two in-house established serological tests including antigens based on a mosaic protein composed of recombinant HEV ORF2 and ORF3 and a 55-kDa HEV ORF2 protein [18].
Additionally, seroprevalence in Suncus murinus (House shrew) within the family Soricidae of the order Soricomorpha was 10.4% (27/260) for anti-HEV IgG and 4.6% (12/260) for Immunoglobulin M (IgM), respectively, with samples collected between 2011 and 2012 in China [35]. The studies in terms of seroprevalence of anti-HEV antibodies are summarized in Table 1.
Table 1.
Seroprevalence of anti-HEV antibodies.
| Order | Family | Species (Scientific Name) | Common Name 1 | No. Positive/No. Tested (%) 3 | Sampling Site (s) (Year) | Assay | Reference |
|---|---|---|---|---|---|---|---|
| Rodentia | Muridae | Rattus norvegicus | Brown rat/Norway rat | 83/108 (76.9) | The USA (1986–1997) | In-house anti-HEV ELISA | [17] |
| 135/197 (68.5) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||||
| 144/196 (73.5) | The USA (2005–2006) | In-house anti-HEV ELISA | [21] | ||||
| 114/362 (31.5) | Japan (2000–2002) | In-house anti-HEV ELISA | [22] | ||||
| 16/56 (28.6) | Japan (N.A.) | Viragent HEV-Ab kit | [23] | ||||
| 36/147 (24.5) | Germany (2007–2010) | In-house anti-rat HEV ELISA | [24] | ||||
| 64/230 (27.8) | China (2011–2012) | In-house anti-rat HEV ELISA | [25] | ||||
| 25/123 (20.3) | Vietnam (2011) | In-house anti-rat HEV ELISA | [26] | ||||
| 21/94 (22.3) | Vietnam (2011) | In-house anti-rat HEV ELISA | [27] | ||||
| 48/389 (12.3) | Vietnam (2012–2013) | In-house anti-rat HEV ELISA | [28] | ||||
| 34/109 (31.2) 4 | Lithuania (2014–2017) | In-house anti-rat HEV ELISA | [29] | ||||
| Rattus rattus | Black rat | 102/113 (90.2) | The USA (1986–1997) | In-house anti-HEV ELISA | [17] | ||
| 31/81 (38.3) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||||
| 21/116 (18.1) | Indonesia (2011–2012) | In-house anti-HEV ELISA | [30] | ||||
| 137/369 (37.1) | Indonesia (2012) | In-house anti-rat HEV ELISA | [31] | ||||
| 10/242 (4.1) | Indonesia (2014–2016) | In-house anti-rat HEV ELISA | [32] | ||||
| 12/90 (13.3) | Japan (2000–2002) | In-house anti-HEV ELISA | [22] | ||||
| Rattus tanezumi | Oriental house rat | 4/16 (25.0) | Vietnam (2011) | In-house anti-rat HEV ELISA | [26] | ||
| 2/6 (33.3) | Vietnam (2011) | In-house anti-rat HEV ELISA | [27] | ||||
| 9/46 (19.6) | Vietnam (2012–2013) | In-house anti-rat HEV ELISA | [28] | ||||
| Rattus rattoides losea | Losea rat | 26/121 (21.5) | China (2011–2012) | In-house anti-rat HEV ELISA | [25] | ||
| Rattus flavipectus | Yellow-breasted rat | 34/171 (19.9) | China (2011–2012) | In-house anti-rat HEV ELISA | [25] | ||
| Rattus rattus hainanus | N.A. 2 | 2/17 (11.8) | China (2011–2012) | In-house anti-rat HEV ELISA | [25] | ||
| Bandicota indica | Greater bandicoot rat | 40/174 (23.0) | China (2011–2012) | In-house anti-rat HEV ELISA | [25] | ||
| Rattus rattus rufescens | Indian black rat | 9/57 (15.8) | India (1985) | In-house anti-HEV ELISA | [33] | ||
| Rattus rattus andamanesis | Sikkim rat | 2/55 (3.6) | India (1990) | In-house anti-HEV ELISA | [33] | ||
| Bandicota bengalensis | Lesser bandicoot rat | 12/22 (54.5) | India (1985) | In-house anti-HEV ELISA | [33] | ||
| Rattus exulans | Polynesian rat | 15/18 (83.3) | The USA (1986–1997) | In-house anti-HEV ELISA | [17] | ||
| Mus musculus | House rat | 2/14 (14.3) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Cricetidae | Clethrionomys gapperi | Southern red-backed vole | 4/6 (66.7) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | |
| Neotoma albigula | White-throated woodrat | 13/22 (59.1) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Neotoma mexicana | Mexican woodrat | 48/84 (57.1) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Neotoma micropus | Southern plains woodrat | 1/8 (12.5) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Oryzomys palustris | Marsh rice rat | 10/41 (24.4) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Peromyscus boylei | Brush mice | 2/24 (8.3) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Peromyscus eremicus | Cactus mouse | 3/7 (42.9) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Peromyscus leucopus | White-footed mouse | 5/50 (10.0) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Peromyscus maniculatus | North American deermouse | 11/91 (11.0) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Sigmodon hispidus | Hispid cotton rat | 37/113 (32.7) | The USA (1994–1998) | In-house anti-HEV ELISAs | [18] | ||
| Soricomorpha | Soricidae | Suncus murinus | House shrew | 27/260 (10.4) | China (2011–2012) | In-house anti-rat HEV ELISA | [35] |
1 Taxonomy and common names refer to https://www.iucnredlist.org/. 2 N.A. stands for not available. 3 Seroprevalence indicates presence of anti-HEV IgG. 4 Positive rate derived from both Rattus norvegicus and Rattus rattus, no individual species rates reported. HEV, hepatitis E virus; ELISA, enzyme-linked immunosorbent assay.
Differing seroprevalence of anti-HEV antibodies may be due to different serological tests, which have been carried out using commercial anti-HEV enzyme-linked immunosorbent assay (ELISA) kits or conducted with in-house established assays based on diverse antigens (truncated or complete human and rat HEV ORF2 protein) in numerous labs independently. Furthermore, although there is only a single serotype of HEV, the cross-reaction of other known or unknown viral pathogens cannot be excluded and may impact the results. Finally, no HEV seroprevalence studies have been performed on species of the orders Carnivora and Falconiformes.
2.2. Detection of Orthohepevirus C Genomes
Molecular biology methods, including RT-PCR amplification, Sanger sequencing and metagenomic analysis with next-generation sequencing, have facilitated the detection of Orthohepevirus C (previously designated rat HEV) RNA from liver, feces, and blood specimens of various animal species. Furthermore, comprehensive sequence and phylogenetic analyses enable genotyping of viral strains. Consequently, an increasing number of novel Orthohepevirus C variants have been discovered around the world whose assignment remain to be determined.
Orthohepevirus C sequences were initially detected in Germany in the widespread rat species Rattus norvegicus (Brown rat/Norway rat) [19,20]. To date, the vast majority of Orthohepevirus C genomes have been identified in Norway rats, from countries including the USA, China, Germany, France, Denmark, Lithuania, England, Hungary, Austria, Switzerland, Italy, Spain, Greece, Belgium, the Czech Republic, and Vietnam, with all viral strains belonging to HEV-C1 [19,20,24,25,29,36,37,38,39,40,41,42,43,44,45,46]. Orthohepevirus C genomes have been detected in numerous other members of the family Muridae: HEV-C1 from Rattus rattus (Black Rat) has been detected in Indonesia, China, Kenya, and 12 other European countries comprising Lithuania, Germany, Hungary, Denmark, Austria, Switzerland, France, Italy, Spain, Greece, Belgium, and the Czech Republic [29,30,32,41,47,48]; Rattus tanezumi (Oriental house rat) in Vietnam and China have also been reported to harbor the virus [26,36,44,49]; in China, HEV-C1 RNA has been detected in Rattus losea (Losea rat), Rattus flavipectus (Yellow-breasted rat), and Bandicota indica (Greater bandicoot rat) [25,43,44]; Apodemus chevrieri (Chevrier’s field mouse) and Apodemus agrarius (Striped field mouse) were positive for Orthohepevirus C detection; however, these variants are phylogenetically divergent from known HEV-C1 or HEV-C2, and thus, cannot yet be classified [36,50].
Consistent with serological studies, Orthohepevirus C RNA was found in several species within other rodent families, including Cricetidae, containing Eothenomys melanogaster (Père David’s vole), Eothenomys Inez (Inez’s red-backed vole), Myodes rufocanus (Grey red-backed vole), Microtus gregalis (Narrow-headed vole), Cricetulus migratorius (Gray dwarf hamster), and Cricetulus barabensis (Striped dwarf hamster) from China, Microtus arvalis (Common vole) from Hungary and Germany, Myodes glareolus (Bank vole) from Germany, and Necromys Lasiurus (Hairy-tailed bolo mouse) and Calomys tener (Delicate vesper) from Brazil [36,50,51,52,53]. The assignment of these newly identified HEV variants remains unclear.
Additionally, Orthohepevirus C sequences have been detected in other animal species of mammals. Within the family Soricidae of the order of Soricomorpha, the species Suncus murinus (House shrew/Asian musk shrew) harbor HEV-C1 in China and Asian musk shrew have been implied as a reservoir for HEV-C1 from wild rats, while the virus in the species Crocidura olivieri (Olivier’s shrew) from Kenya cannot be assigned due to increased genetic divergence [35,44,48,50].
In the order Carnivora, HEV-C1 has been identified in a Syrian brown bear (species Ursus arctos syriacus, family Ursidae) in a German zoo, most likely the result of a spillover infection from free-roaming Norway rats [42]. In 2012, HEV-C2 was firstly reported in Western polecats (species Mustela putorius) from the Netherlands [54]. Soon afterwards, HEV-C2 was detected in ferrets from the USA, Japan, and China and in minks from Denmark and China [54,55,56,57]. Both species Mustela putorius and Neovison vison belong to the carnivore family Mustelidae. Even though a short (362 nucleotide) partial ORF1 sequence was identified in a red fox (species Vulpes vulpes, family Canidae), classification into Orthohepevirus C requires comparisons based upon complete genome sequences, as recommended by the International Committee on the Taxonomy of Viruses (ICTV) Hepeviridae Study group [58].
Recently, novel HEV variants have been reported from common kestrel (species Falco tinnunculus) and red-footed falcon (species Falco vespertinus) within the family Falconidae of the order Falconiformes in Hungary, notably the viral strain from kestrel showed higher sequence similarity to members of Orthohepevirus C than to Orthohepevirus B and clustered together with aforementioned rodent HEV variants from China and Brazil; therefore, the kestrel-derived HEV strain is included in this review [59].
Finally, nine HEV-C1 strains have been reported in humans, eight being typical rat HEV sequences from Hong Kong and the other divergent rat HEV sequence derived possibly from Uganda [14,15,16]. However, the precise source and transmission pattern of these nine HEV-C1 strains remain unclear. The studies with regards to the detection of Orthohepevirus C genomes are listed in Table 2.
Table 2.
Detection of Orthohepevirus C genomes.
| Order | Family | Species (Scientific Name) | Common Name 1 | Sampling Site (s) (Year) | Virus Genotype | Genomic Sequence | Reference |
|---|---|---|---|---|---|---|---|
| Rodentia | Muridae | Rattus norvegicus | Brown rat/Norway rat | The USA (2003), Germany (2007–2010), Germany (2009–2016), France (2011–2012), Denmark (2012), China (2011–2012; 2013–2016; 2014–2017), Lithuania (2014–2017), 11 European countries (2005–2016) 2, England (2014–2016), Vietnam (2011; 2012–2013), Museum collections 3 | HEV-C1 | Partial, Nearly complete 5, Complete | [19,20,24,25,28,29,36,37,38,39,40,41,42,43,44,45,46] |
| Rattus rattus | Black rat | Indonesia (2011–2012; 2014–2016), Lithuania (2014–2017), Italy (2015), 11 European countries (2005–2016), Kenya (2016); Museum collections | HEV-C1 | Partial, Complete | [29,30,32,41,47,48] | ||
| Rattus tanezumi | Oriental house rat | Vietnam (2011; 2012–2013), China (2013–2016; 2014–2017) | HEV-C1 | Partial, Complete | [26,28,36,44,49] | ||
| Rattus losea | Losea rat | China (2011–2012; 2014–2017) | HEV-C1 | Partial, Nearly complete | [25,44] | ||
| Rattus flavipectus | Yellow-breasted rat | China (2011–2012) | HEV-C1 | Partial | [25,43] | ||
| Bandicota indica | Greater bandicoot rat | China (2011–2012) | HEV-C1 | Partial | [25] | ||
| Apodemus chevrieri | Chevrier’s field mouse | China (2013–2015) | N.A. 4 | Partial, Complete | [50] | ||
| Apodemus agrarius | Striped field mouse | China (2013–2016) | N.A. | Complete | [36] | ||
| Cricetidae | Eothenomys melanogaster | Père David’s vole | China (2013–2015) | N.A. | Partial, Complete | [36,50] | |
| Eothenomys inez | Inez’s red-backed vole | China (2013–2016) | N.A. | Complete | [36] | ||
| Myodes rufocanus | Grey red-backed vole | China (2013–2016) | N.A. | Nearly complete | [36] | ||
| Microtus gregalis | Narrow-headed vole | China (2013–2016) | N.A. | Complete | [36] | ||
| Cricetulus migratorius | Gray dwarf Hamster | China (2013–2016) | N.A. | Complete | [36] | ||
| Cricetulus barabensis | Striped dwarf hamster | China (2013–2016) | N.A. | Complete | [36] | ||
| Microtus arvalis | Common vole | Hungary (2016), Germany and the Czech Republic (2009–2013) | N.A. | Partial, Complete | [52,53] | ||
| Myodes glareolus | Bank vole | Germany (2009–2013) | N.A. | Partial | [53] | ||
| Necromys lasiurus | Hairy-tailed bolo mouse | Brazil (2008–2013) | N.A. | Nearly complete | [51] | ||
| Calomys tener | Delicate vesper mouse | Brazil (2008–2013) | N.A. | Partial | [51] | ||
| Soricomorpha | Soricidae | Suncus murinus | House shrew/Asian musk shrew | China (2011–2012; 2013–2016; 2014–2017) | HEV-C1 | Partial | [35,43,44] |
| Crocidura olivieri | Olivier’s shrew | Kenya (2016) | N.A. | Partial | [48] | ||
| Carnivora | Ursidae | Ursus arctos syriacus | Syrian brown bear | Germany (2011–2016) | HEV-C1 | Partial | [42] |
| Mustelidae | Mustela putorius | Western polecat/ferret | The Netherlands (2010), the USA (2013), Japan (2009–2013), China (2016), | HEV-C2 | Partial, Complete | [54,55,56] | |
| Neovison vison | American mink | Denmark (2008–2011), China (2016), | HEV-C2 | Partial | [56,57] | ||
| Falconiformes | Falconidae | Falco tinnunculus | Common kestrel | Hungary (2014) | N.A. | Partial, Complete | [59] |
| Falco vespertinus | Red-footed falcon | Hungary (2014) | N.A. | Partial, | [59] | ||
| Primates | Hominidae | Homo sapiens | Human | Hong Kong (2017–2019), Uganda (2017) | HEV-C1 | Complete | [14,15,16] |
1 Taxonomy and common names refer to https://www.iucnredlist.org/. 2 Sampling sites include Germany, Hungary, Denmark, Austria, Switzerland, France, Italy, Spain, Greece, Belgium, and the Czech Republic. 3 Liver tissue samples from Rattus norvegicus and Rattus rattus rats from museum collections covering localities primarily in the USA plus additional samples from China, Honduras, Madagascar, Mexico, Nicaragua, Peru, Russia, and Vietnam. 4 N.A. stands for not assigned. 5 Nearly complete indicates viral genome lacking 5′ end.
3. Genomic Characterization of Orthohepevirus C
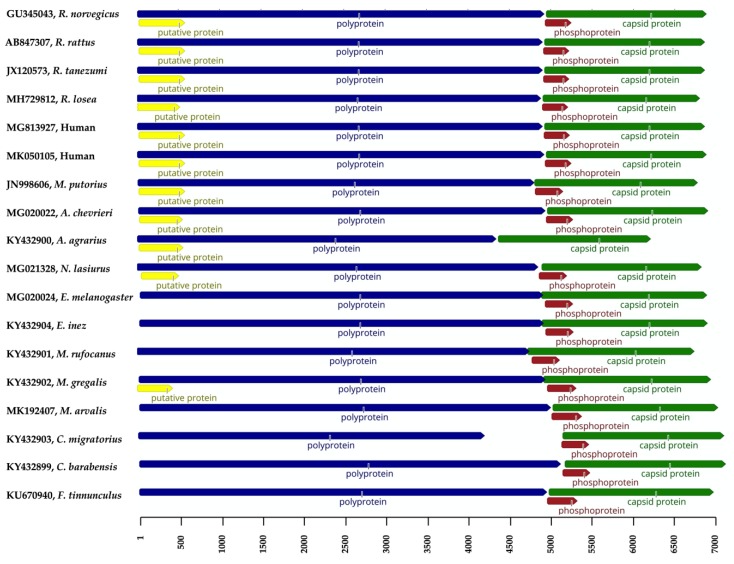
Despite the detection of Orthohepevirus C RNA in numerous species, the vast majority of deposited sequences consist of partial HEV ORF1 or ORF2 genes. Complete genomic sequences of Orthohepevirus C have been obtained from various mammal species, including Rattus norvegicus, Rattus rattus, Rattus tanezumi, Rattus losea, Apodemus chevrieri, Apodemus agrarius, Eothenomys melanogaster, Eothenomys inez, Myodes rufocanus, Microtus gregalis, Microtus arvalis, Necromys lasiurus, Cricetulus migratorius, Cricetulus barabensis, Mustela putorius, Falco tinnunculus, and human (Table 1). For a better comparative analysis and genomic characterization of Orthohepevirus C, the schematic description of genomic organization of representative Orthohepevirus C variants from individual animal species is depicted in Figure 2.
Figure 2.
Schematic description of genomic organization of Orthohepevirus C variants. Virus designations including GenBank accession numbers and host information are indicated on the left. HEV open reading frames (ORFs) 1, 2, 3, and the putative ORF4 are represented in blue, green, brown, and yellow, respectively. The scale is in bases.
Overall, the genomic length of Orthohepevirus C sequences ranges from 6.8 to 7.2 kb, excluding those without sequenced 5′ or 3′ ends. However, the distinct strain RtAa-HEV/JL2014 (GenBank accession no. KY432900) from Apodemus agrarius, which consists of only 6286 nucleotides and has a significant shorter ORF1 with 4334 nucleotides; moreover, RtAa-HEV/JL2014 lacks ORF3. Since it has been recently reported that the ORF3 is essential for the release of membrane-associated rat HEV particles, the absence of ORF3 in Apodemus agrarius needs further verification [60]. In addition to RtAa-HEV/JL2014, the ORF1 of the strain RtCm-HEV/XJ2016 (GenBank accession no. KY432903) from Cricetulus migratorius is only 4182 nucleotides in length. However, the short viral genome and ORF1, as well as the absence of ORF3 may be associated with errors in assembling reads to contigs and mapping assembled contigs to references in the metagenomic study [36].
A unique ORF (tentatively named ORF4), located within the 5′ region of ORF1 has been proposed in certain rat and ferret HEV strains [32,55]. As shown in Figure 2, all the HEV-C1 strains from the species including Rattus norvegicus, Rattus rattus, Rattus tanezumi, and Rattus losea within the family Muridae, two HEV-C1 analogues identified from humans, HEV-C2 strains from the family Mustelidae, and the HEV variants from the species Necromys lasiurus and Microtus gregalis within the family Cricetidae harbor ORF4. In contrast, this putative ORF is not found in other species. A recent study has shown that ORF4 is not necessary for the active replication of rat HEV [60]. Therefore, the functional role of the putative ORF4 remains poorly understood. Furthermore, due to the significant genetic variability within Orthohepevirus C, the molecular evolution of ORF4 is as of yet unclear.
Typically, HEV ORF2 overlaps partially with ORF3 but not ORF1. However, three HEV variants in the species Eothenomys melanogaster, identified from two separate studies, possess ORF2 genes overlapping with ORF1. This is also the case for HEV strains in the species Eothenomys inez, Myodes rufocanus, and Microtus gregalis within the family Cricetidae [8,36,50]. Additionally, the unique motif at the 5‘-untranslated regions containing the 10 nucleotides GCAACCCCG is exclusively present in HEV variants of the family Muridae [31]. Finally, Orthohepevirus C variants utilize distinct translational frames, as described in our recent study [43].
Although many novel HEV variants within the Orthohepevirus C species have been discovered in diverse wild rodents in China in a study based on metagenomic analysis, each species has only a single viral complete genomic sequence [36]. The genomic features and genetic heterogeneity of these HEV variants in individual animal species awaits the availability of more complete genome sequences.
4. Genetic Variability of Orthohepevirus C
As mentioned, the majority of described Orthohepevirus C sequences are partial genomic fragments (<500 bp). In order to determine the genetic variability of HEV variants within the species Orthohepevirus C, we have included 48 currently available complete or nearly complete genomic sequences of Orthohepevirus C from GenBank. The pairwise comparisons of complete genome and deduced ORFs of Orthohepevirus C is illustrated in Table 3.
Table 3.
Pairwise comparisons of complete genome and deduced ORFs of Orthohepevirus C variants.
| Orthohepevirus C (No. of Variants) 1 | % Identity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEV-C1 (20) 2 | HEV-C2 (10) | RdAcHEV (2) | RdAaHEV (1) | RdEmHEV (3) | RdEiHEV (1) | RdMrHEV (1) 3 | RdMgHEV (1) | RdMaHEV (6) 4 | RdNlHEV (1) | RdCmHEV (1) | RdCbHEV (1) | |
| Complete genome (nt) | ||||||||||||
| HEV-C1 | 65.3–67.7 | 62.6–65.0 | 56.5–59.1 | 53.2–55.4 | 54.3–55.4 | 53.1–55.2 | 53.8–55.5 | 52.9–55.3 | 54.8–56.0 | 49.9–51.3 | 52.6–54.1 | |
| HEV-C2 | 63.5–65.9/69.5–74.0 | 58.5–58.6 | 62.1–62.8 | 54.6–55.6 | 54.7–54.9 | 53.3–53.7 | 54.8–55.0 | 53.1–54.4 | 54.9–55.1 | 53.4–53.6 | 51.1–51.5 | |
| RdAcHEV | 61.0–63.9/64.4–67.8 | 61.0–61.6/67.8–68.5 | 66.2–66.4 | 54.3–55.0 | 54.5–54.7 | 53.6–53.8 | 54.5–54.9 | 53.0–54.1 | 54.5–54.6 | 52.7 | 50.4–51.0 | |
| RdAaHEV | 54.8–56.5/57.1–61.1 | 56.3–56.6/61.9–62.1 | 63.2–63.5/72.9–73.6 | 51.8–51.9 | 52.3 | 51.0 | 51.6 | 49.7–50.8 | 52.0 | 50.5 | 48.2 | |
| RdEmHEV | 51.4–53.4/49.6–53.5 | 53.1–53.7/52.5–54.3 | 51.9–52.9/51.3–53.2 | 49.1–49.5/47.6–48.6 | 72.2–72.6 | 62.1–63.0 | 66.9–67.3 | 65.7–67.2 | 59.5–59.9 | 54.5–54.6 | 52.2–52.5 | |
| RdEiHEV | 52.7–53.7/50.0–52.7 | 53.1–53.2/53.3–53.6 | 52.6–52.7/51.8–52.1 | 49.6/48.4 | 70.3–70.9/80.2–82.4 | 62.6 | 67.1 | 64.9–65.9 | 60.1 | 55.0 | 52.7 | |
| RdMrHEV | 51.7–53.3/51.6–54.2 | 51.4–52.9/52.8–53.1 | 51.8–52.0/52.3–52.7 | 48.3/47.9 | 60.6–62.0/67.8–69.1 | 61.0/66.8 | 61.4 | 62.0–62.4 | 58.3 | 54.0 | 52.2 | |
| RdMgHEV | 52.3–53.8/50.4–52.9 | 53.0–53.1/52.9–53.8 | 52.5–53.0/51.8–52.1 | 49.3/48.2 | 66.2–67.0/73.0–74.4 | 66.4/73.1 | 60.1/66.0 | 76.0–77.3 | 59.3 | 54.0 | 52.8 | |
| RdMaHEV | 50.9–53.9/49.6–54.0 | 51.1–52.5/51.7–54.0 | 50.8–52.0/51.2–52.7 | 47.4–48.7/49.4–50.6 | 65.1–67.1/70.9–74.5 | 64.0–65.5/69.9–72.4 | 60.5–61.4/64.6–62.2 | 74.0–75.9/86.6–89.6 | 58.9–59.5 | 53.4–54.2 | 51.3–52.1 | |
| RdNlHEV | 53.5–54.9/53.4–55.8 | 54.6/55.4–55.5 | 53.5–53.7/53.7–53.9 | 50.4/50.5 | 59.4–59.8/60.9–62.3 | 59.9/61.4 | 57.5/61.5 | 58.5/60.9 | 57.4–58.7/59.6–61.0 | 53.4 | 51.4 | |
| RdCmHEV | 48.0–49.5/47.5–50.8 | 48.1–48.9/49.7–50.4 | 47.7/49.2–49.4 | 44.6/45.0 | 49.3–49.9/50.1–51.8 | 49.6/50.7 | 49.7/49.9 | 49.6/50.0 | 47.6–49.1/48.4–50.3 | 49.6/50.1 | 51.1 | |
| RdCbHEV | 48.3–49.7/46.5–48.8 | 49.6–49.9/49.4–50.2 | 48.6–49.1/48.6–48.8 | 45.7/45.2 | 50.6–50.8/48.4–49.6 | 49.9/48.9 | 50.5/48.3 | 51.5/49.1 | 49.4–50.5/46.7–48.0 | 49.9/48.6 | 46.1/45.4 | |
| ORF1 (nt/aa) | ||||||||||||
| ORF2 (nt/aa) | ||||||||||||
| HEV-C1 | 66.5–72.8/76.4–81.6 | 63.2–69.7/70.7–74.9 | 59.6–66.1/68.2–73.3 | 57.0–61.9/60.7–63.0 | 56.8–61.3/59.7–62.0 | 56.5–61.1/60.2–63.6 | 57.2–61.7/61.2–64.3 | 56.6–61.0/58.7–63.2 | 56.6–60.6/63.0–65.7 | 56.6–60.4/60.1–61.8 | 53.1–57.1/56.4–59.4 | |
| HEV-C2 | 59.0–63.6/37.0–45.4 | 64.5–65.4/70.4–71.0 | 62.9–63.4/69.8–70.2 | 58.5–60.4/58.9–59.6 | 58.1–58.6/58.0–59.3 | 57.7/60.9–61.8 | 59.6–60.9/60.6–61.5 | 57.7–59.2/57.7–59.9 | 58.9–59.4/61.3–62.5 | 57.9–58.3/59.3–59.8 | 56.7/56.8–58.1 | |
| RdAcHEV | 59.2–62.6/39.6–50.0 | 54.6–56.4/32.1–35.7 | 70.1–70.4/85.6–85.8 | 60.4–61.5/62.3–62.9 | 59.6–60.3/61.6–61.8 | 59.1–59.6/61.2–61.8 | 60.3–60.8/63.2–63.5 | 58.1–60.0/60.1–61.6 | 59.4–59.5/61.8–62.3 | 59.3/59.2 | 55.1–56.3/57.4–58.0 | |
| RdAaHEV | N.A. 5 | N.A. | N.A. | 58.0–58.4/60.4–60.7 | 58.7/60.4 | 57.4/59.0 | 57.4/60.2 | 55.1–56.4/57.9–58.7 | 57.6/60.4 | 57.1/57.8 | 53.8/55.6 | |
| RdEmHEV | 40.1–44.8/20.0–27.8 | 41.3–46.7/21.0–26.9 | 40.9–44.3/22.2–28.2 | N.A. | 76.5–77.5/89.0–89.2 | 65.4–66.2/70.6–70.9 | 68.9–70.0/76.3–76.6 | 66.6–68.3/72.1–73.6 | 62.6–63.8/68.4–69.0 | 58.0–59.1/60.3–60.6 | 57.3–58.4/60.2–61.0 | |
| RdEiHEV | 42.2–47.5/20.7–29.3 | 40.7–43.6/20.3–26.3 | 42.6–42.9/23.7–25.4 | N.A. | 80.4–81.8/62.8–67.3 | 66.7/70.9 | 69.6/77.1 | 67.8–68.6/72.7–73.9 | 63.9/69.3 | 60.7/61.4 | 59.5/61.7 | |
| RdMrHEV | 37.1–39.4/19.5–24.6 | 37.8–38.1/19.7–20.5 | 35.4–37.1/22.7 | N.A. | 52.0–53.4/30.8–32.5 | 56.2/39.0 | 66.1/69.7 | 65.1–66.2/67.4–68.6 | 63.2/68.5 | 59.0/60.6 | 57.8/60.9 | |
| RdMgHEV | 38.6–41.6/19.5–24.4 | 39.1–41.0/19.7–22.8 | 36.9–38.2/20.8–21.6 | N.A. | 59.4–60.3/43.7–44.5 | 61.1/44.2 | 57.1/36.3 | 78.9–80.2/90.8–92.1 | 64.1/67.1 | 59.9/62 | 57.3/61.4 | |
| RdMaHEV | 36.3–40.2/10.2–18.9 | 36.5–38.9/10.9–17.8 | 37.8–39.5/10.1–15.5 | N.A. | 55.6–59.7/25.8–31.5 | 56.9–61.1/26.2–31.7 | 58.0–61.8/21.1–26.8 | 81.9–82.2/36.3–41.9 | 61.9–62.9/67.3–67.7 | 57.1–58.4/59.7–60.3 | 56.3–57.0/58.7–59.6 | |
| RdNlHEV | 32.9–35.0/14.9–24.0 | 31.7–32.0/14.4–15.2 | 32.9–34.0/17.2–18.0 | N.A. | 40.8–41.9/24.6–27.9 | 44.6/27.6 | 37.9/24.2 | 41.4/30.3 | 43.5–45.9/20.3–23.4 | 59.8/64.1 | 57.5/61.2 | |
| RdCmHEV | 38.2–43.1/17.8–27.1 | 37.6–38.1/17.7–22.6 | 40.3–41.7/23.3–25.0 | N.A. | 40.5–41.3/20.2–21.0 | 44.5/22.6 | 39.6/22.2 | 43.3/21.7 | 40.2–42.3/14.9–19.5 | 34.2/18.9 | 58.5/63.5 | |
| RdCbHEV | 36.6–39.4/20.5–25.0 | 36.9–38.0/17.2–21.6 | 37.6–37.9/21.7–23.5 | N.A. | 33.9–35.0/16.0–20.2 | 35.8/21.8 | 33.2/15.6 | 32.3/19.7 | 31.2–32.2/15.9–21.4 | 33.0/15.9 | 39.9/18.9 | |
| ORF3 (nt/aa) | ||||||||||||
1 RdAcHEV, RdAaHEV, RdEmHEV, RdEiHEV, RdMrHEV, RdMgHEV, RdMaHEV, RdNlHEV, RdCmHEV, and RdCbHEV stand for HEV variants derived from the species Apodemus chevrieri, Apodemus agrarius, Eothenomys melanogaster, Eothenomys inez, Myodes rufocanus, Microtus gregalis, Microtus arvalis, Necromys lasiurus, Cricetulus migratorius, and Cricetulus barabensis, respectively. 2 Due to the sequence identity and phylogeny, two variants from humans (GenBank accession numbers MG813927 and MK050105) are included in the HEV-C1. 3 Nearly complete genome. 4 Due to the sequence identity and phylogeny, a variant from Falco tinnunculus (GenBank accession number KU670940) is included in the RdMaHEV. 5 N.A. stands for not available. Nucleotide (nt) and amino acid (aa) sequences were aligned using the MAFFT algorithm. Pairwise distances were computed in MEGA version 7. The GenBank Accession numbers are as follows: MH729810, MH729811, MH729812, MG813927, AB847307, JN167538, JX120573, JN167537, KM516906, MK050105, GU345043, AB847308, GU345042, AB847305, AB847306, AB847309, LC225388, LC225389, KY432898, and KY432906 for HEV-C1; AB890001, JN998606, JN998607, AB890374, LC057247, LC057248, LC177788, LC177789, LC177790, LC177791, and LC177792 for HEV-C2; MG020022 and MG020023 for RdAcHEV; KY432900 for RdAaHEV; KY432905, MG020024 and MG020025 for RdEmHEV; KY432904 for RdEiHEV; KY432901 for RdMrHEV; KY432902 for RdMgHEV; MK192405, MK192406, MK192407, MK192408, MK1924059, and KU670940 for RdMaHEV; MG021328 for RdNlHEV; KY432903 for RdCmHEV; and KY432899 for RdCbHEV. Lightface type indicates nt identity, and boldface type indicates aa identity. Viral strains from rodent families Muridae and Cricetidae and carnivore family Mustelidae are highlighted with grey, blue, and green background, respectively.
Unexpectedly, the genomic comparisons between HEV-C1 and other newly identified HEV strains derived from the rodent species Apodemus chevrieri, Apodemus agrarius, Eothenomys melanogaster, Eothenomys inez, Myodes rufocanus, Microtus gregalis, Microtus arvalis, Necromys lasiurus, Cricetulus migratorius, and Cricetulus barabensis exhibit comparable or even higher divergence than is present between HEV-C1 and HEV-C2, even though HEV-C2 strains have only been found in the carnivore species Mustela putorius. Nucleotide identities in Orthohepevirus C variants from individual rodent species vary between 48.2–67.3%. Higher identities are observed between Eothenomys melanogaster and Eothenomys Inez, ranging from 72.2 to 72.6%, and from 76.0 to 77.3% between Microtus gregalis and Microtus arvalis. Similar results have been observed for the nucleotides and amino acids of the 3 coding regions. Notably, for the comparison of ORF3, most of the HEV variants in their respective group are highly divergent and exhibit low identities, especially at amino acid level (<20%). These data highlight the fact that substantial genetic variability of HEV is present within the order Rodentia, raising a need for increased efforts to characterize the variants circulating in the respective habitats. Only through this will we be able to better assess the risk potential of these viruses to animal and human health.
Due to the high identity (> 75%) to HEV-C1 strains, two HEV variants detected in humans in Hong Kong and Uganda can be considered as HEV-C1 [14,15]. Furthermore, the kestrel HEV strain identified in Hungary shares relatively high similarity (> 83%) to the HEV strains from the species Microtus arvalis from Hungary, Germany, and the Czech Republic, which is comparable to sequence identities within common vole variants (between 81.5% and 91.9%). Therefore, it is assumed that the detection of HEV variants in birds of prey (Common kestrel and Red-footed falcon) may be linked to the consumption of wild rodents, in particular common voles [52,53,59].
5. Molecular Evolution of Orthohepevirus C
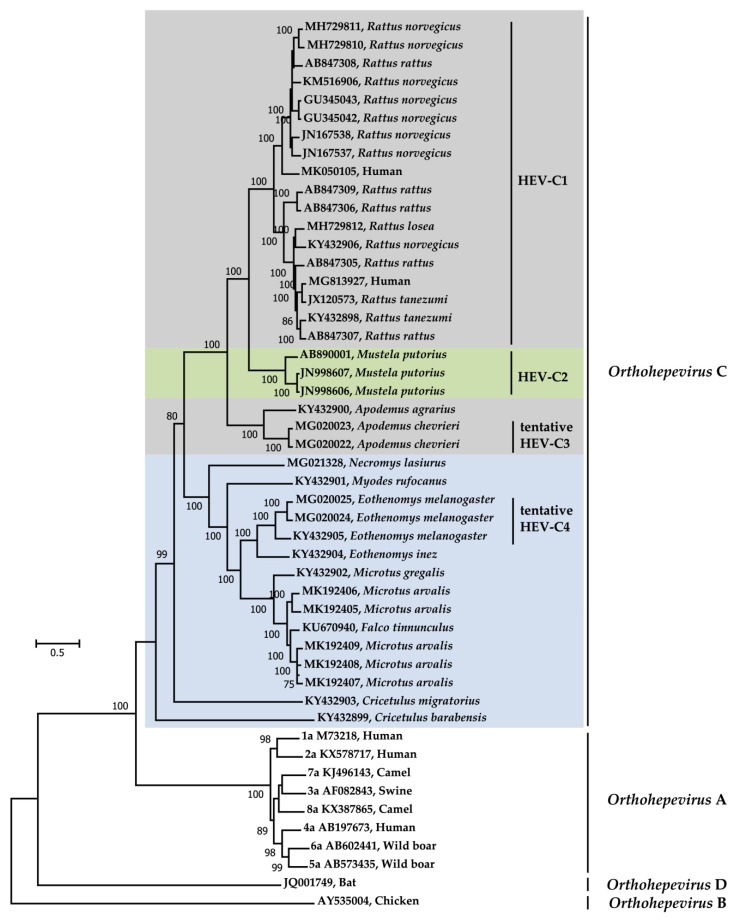
In order to investigate the dynamics of the molecular evolution of Orthohepevirus C, phylogenetic analysis was conducted using representative complete genomic sequences within the genus Orthohepevirus of the Hepeviridae family (Figure 3). The discrimination of genotypes within the species Orthohepevirus A and C is in accordance with consensus proposals for the classification from ICTV Hepeviridae study group [11]. Recently, we have reported novel HEV variants in Chevrier’s field mouse (species Apodemus chevrieri, family Muridae) and Père David’s vole (species Eothenomys melanogaster, family Cricetidae) in China and two viral genomes of each animal species were amplified and characterized. Due to the significant divergence from HEV-C1 and HEV-C2, we have proposed these variants be classified as putative HEV-C3 and putative HEV-C4, which has been included and discussed in several recent research articles [13,50,53]. Afterwards, highly diverse HEV variants have been discovered in other wild rodent species around the world [36,51,53]. Collectively, phylogenetic tree shows that Orthohepevirus C variants form a separate branch within the genus Orthohepevirus. Predictably, HEV variants detected in the rodent genus Rattus and the carnivore species Mustela putorius cluster into two independent clades within the species Orthohepevirus C, which have already been defined as HEV-C1 and HEV-C2, respectively. Even though newly identified HEV variants are phylogenetically separate from HEV-C1 and HEV-C2, HEV variants from the rodent genus Apodemus (including species Apodemus agrarius and Apodemus chevrieri), Eothenomys (including species Eothenomys melanogaster and Eothenomys Inez), and Microtus (including the species Microtus gregalis and Microtus gregalis) clustered into three distinct clusters, which are associated with rodent taxonomy and host specificity. Taken together, it is hypothesized that co-evolutionary model, and not the host-switch pattern, might be applied regarding the species Orthohepevirus C [10]. However, the HEV variants derived from the rodent family Cricetidae, particularly RtCm-HEV/XJ2016 (GenBank accession no. KY432903) from the species Cricetulus migratorius) and RtCb-HEV/HeB2014 (GenBank accession no. KY432899) from Cricetulus barabensis, demonstrate a high degree of divergence within the Orthohepevirus C clade. With no doubt that the evolutionary history of Orthohepevirus C will be better elucidated when diversified HEV strains are discovered in insectivores and carnivores in the future.
Figure 3.
Maximum-likelihood phylogeny of Orthohepevirus C variants within the genus Orthohepevirus. Representatives of Orthohepevirus A, B, and D variants are reference strains according to the International Committee on Taxonomy of Viruses (ICTV) consensus proposals [11]. Virus designations include GenBank accession numbers and host information. Species and genotypes are indicated on the right. Viral strains from rodent families Muridae and Cricetidae and carnivore family Mustelidae are highlighted with grey, blue, and green background, respectively. Complete genome sequences were aligned using the MAFFT algorithm. Phylogenetic reconstructions were performed using MEGA version 7 with 1000 bootstrap replicates. General Time Reversible (GTR) + Gamma Distributed (G) + Invariable Sites (I) nucleotide substitution model was selected based on Find Best-Fit Substitution Model (ML) in MEGA version 7. Bootstrap values (>75%) are indicated at specific nodes. Scale bars indicate the number of nucleotide substitutions per site.
The two HEV variants derived from a Chinese and Canadian patient, respectively, cluster within the HEV-C1 branch, providing further evidence of a potential zoonotic origin [14,15]. Concordant with pairwise comparisons of complete viral genomes and coding regions, the HEV strain (GenBank accession no. KU670940) identified in a common kestrel (Falco tinnunculus) from Hungary clusters together with the five reported HEV variants from European common voles (Microtus arvalis). Whether Orthohepevirus C variants from common voles indeed can infect common kestrel remains to be determined [52,53,59].
Thus far, all HEV variants detected in rodents have been isolated from the families Muridae and Cricetidae. Considering that the order Rodentia comprises 33 families and 2277 species (>40% of all mammal species), therefore forming the largest mammalian order, we believe that these HEV variants are only the tip of the iceberg. Future studies will undoubtably reveal further novel variants within Orthohepevirus C with new Orthohepevirus C genotypes being assigned in the coming years. Although it is evident from our analyses that HEV variants within each genotype of Orthohepevirus C (including HEV-C1, HEV-C2 and other unassigned groups) are highly divergent from one another, subtype differentiation has not yet been proposed by ICTV Hepeviridae study group [11,31,53].
It is a well-known fact that evolution of HEV within the species Orthohepevirus A undergoes different patterns between different genotypes (HEV-1 to HEV-8), which is associated with at least two distinct epidemiological profiles of the species [61]. Increasingly genetically diversified viruses within Orthohepevirus C have been identified; to what extent of viral evolutionary discrepancy of the species is largely unclear.
6. Cross-Species Transmission of Orthohepevirus C
Human hepatitis E cases in industrialized countries are mainly caused by zoonotic transmission of HEV-3 and HEV-4 within the species Orthohepevirus A; domestic swine, wild boar, and sika deer have been confirmed to be the reservoirs. Typically, HEV zoonotic transmission occurs via three routes. Firstly, population groups coming into direct contact with infected animals, such as veterinarians and workers on pig farms or slaughterhouses, who were proven to have significantly higher anti-HEV IgG seroprevalence rates than the general population and other veterinarians in some European countries; secondly, foodborne transmission via consumption of undercooked infectious meat products (mostly pork), such as sausages and liver, which is common in European countries; and thirdly, environmental contamination by animal feces, since the use of pig manure in agriculture may lead to contamination of water sources and agricultural products [3]. Furthermore, consumption of food products (milk and meat) from camels and subsequent infection with a camelid HEV-7 strain was implicated in a case of chronic hepatitis E after transplantation in a patient from the United Arab Emirates [62]. Using reverse genetic systems, the potential of zoonotic infection of HEV-7 as well as HEV-5 from wild boar have been confirmed by experimentally infecting primates in Japan [63,64]. Recently, one study has demonstrated that HEV-8 from Bactrian camels in multiple regions in China was able to cause chronic hepatitis in cynomolgus macaques (human surrogate), indicating a high risk of zoonosis [65]. Lastly, rabbit HEV-3ra might also pose a potential threat to humans, based on described cross-species infection of pigs and cynomolgus macaques [66,67].
Rodents are extraordinarily abundant and diversified in multiple continental habitats and live in close proximity to humans, domestic animals, and wild animals. This interface has resulted in the rodent origin of a plethora of zoonotic viruses, such as Lassa virus, Lymphocytic choriomeningitis virus, Sin Nombre virus, Hantaan virus, and Seoul virus, all of which have caused severe diseases in humans in the past decades [68]. Increasing amounts of evidence suggest that rodents may also play a role in the spread and epidemiology of HEV. Numerous studies from different countries have reported that rodent sera tested positive for anti-HEV antibodies were able to cross-react with human-derived HEV antigens [17,18,21,22,23]. Initially, a 1996 study from Thailand reported experimental infection of three Wistar laboratory rats with a human pathogenic HEV strain derived from patient feces acquired during a hepatitis E outbreak in Nepal in 1992 [69]. Later, a Chinese study demonstrated the successfully infection of Balb/c nude mice with swine HEV and active infection of Sprague-Dawley after intrahepatic inoculation with infectious HEV-4 RNA [70]. A German study reported detectable HEV RNA and anti-HEV antibodies in Wistar rats inoculated with a wild boar-derived HEV-3 strain [71]. In addition, multiple rodent cell lines have been successfully infected with HEV-3 [72]. Finally, zoonotic HEV-3 was reported in wild rats in the USA, England, and Japan, and a rabbit HEV-3ra sequence was detected in a Norway rat from Belgium. However, viral RNA was mostly detected in intestines and feces and seldom in the liver in these cases [23,41,46]. In contrast, the results from a series of studies demonstrated that rats are susceptible to rat HEV but not HEV-1, HEV-2, or HEV-4 [37,73]. Therefore, the question whether rodents are truly natural reservoirs of human HEV or act as only intermediate hosts is not yet conclusively answered.
Although rodents have been confirmed to be the primary natural reservoirs of HEV variants within the species Orthohepevirus C, the potential risk of these viruses concerning cross-species infection and zoonotic transmission to humans remains a controversial topic [13]. In the past, Orthohepevirus C variants were understudied and neglected due to their genetic divergence (identities <52%) to the principle human pathogenic HEV strains (HEV-1 to HEV-4). It has been reported that pigs or non-human primates (rhesus monkeys) inoculated with rat HEV showed no evidence of infection, indicating that it is not a source of human infection [37,66]. In contrast, after screening with ELISAs based on the capsid protein of Norway rat HEV, several sera from forestry works of eastern Germany were shown to have strong reactivity [74]. In a recent study from Vietnam, IgG antibody titers against HEV-C1 antigen in three of the 99 sera of hospitalized febrile patients were more than eight-fold higher than those against HEV-1 antigen, while IgM antibodies against HEV-C1 antigen were detected in acute sera from two of the three patients using both ELISA and Western blotting and one patient developed illness with mild liver dysfunction [75]. Furthermore, in Japan, a rat HEV reverse genetics system was demonstrated to be replication competent in a human cell line, while another study reported that rat HEV from Rattus rattus could propagate efficiently in human hepatoma cell lines including PLC/PRF/5, HuH-7, and HepG2 [76]. Intriguingly, it was reported that a typical rat HEV caused persistent hepatitis in a liver transplant patient in Hong Kong and that the virus was cleared after ribavirin treatment. Additionally, a divergent rat HEV from Uganda induced severe acute hepatitis in an immunocompetent patient from Canada. These two case reports provide first evidence of a possible zoonotic potential of rat HEV [14,15]. Furthermore, based on comprehensive clinical and epidemiological analyses, seven rat HEV infections have been further identified in Hong Kong, and these strains are extremely close to an isolate from a rat captured near the residences of patients. Remarkably similar to Orthohepevirus A, rat HEV have caused chronic viral infection in immunosuppressed individuals as well as extrahepatic manifestations [16]. Jointly, it is highly presumable that rat HEV may be a cause of human hepatitis. Nevertheless, there are still many open questions regarding zoonotic transmission of Orthohepevirus C. What is the viral determinant for rat HEV cross-species infection? What is the effectivity of antiviral therapy, especially regarding ribavirin, against rat HEV infection? Will other rat HEV analogues (e.g., newly identified HEV strains from wild rodents, insectivores, and carnivores) be capable of infecting humans?
Interspecies transmission of Orthohepevirus C variants has been characterized in several studies as well. House shrews (Suncus murinus) have been reported as a reservoir of rat HEV worldwide; however, a novel divergent HEV strain has been detected in a Olivier’s shrew (Crocidura olivieri) from Kenya and formed a separate monophyletic branch from HEV-C1, indicating the relationship between the viral species Orthohepevirus C and the animal order Soricomorpha should be more complex than previously considered [35,43,44,48]. Although ferret HEV (HEV-C2) can cause both acute and persistent infection in ferrets, researchers failed to infect monkeys and rats with ferret HEV [77].
7. Conclusions and Outlook
An increasing number of HEV variants are being detected in diverse animal species, including mammals, avian, and fish, with some of these species having been confirmed as natural reservoirs for HEV and sources of zoonotic infection. With the advancement of techniques such as high-throughput sequencing in metagenomics studies, no doubt more novel HEV variants will be discovered in the near future. The detection of numerous emerging HEV variants within the species Orthohepevirus C has expanded the known host range of HEV dramatically. This raises critical concerns about the potential risk of cross-species infection and zoonotic transmission to humans, as the biology, ecology, natural history, and pathogenesis of these viruses are largely unknown. After three recent reports identified totally nine human hepatitis E cases linked to rat HEV, the necessity to broaden our knowledge concerning the molecular epidemiology of circulating Orthohepevirus C variants is clear, and the potential risks of these viruses to public health should be reassessed. To elucidate the zoonotic potential of Orthohepevirus C variants, the patterns of transmission, mechanisms of replication, and functional roles of proteins merit further investigation.
Author Contributions
B.W. wrote the manuscript; D.H. edited the manuscript; B.W. and D.H. analyzed the data and prepared the tables and figures; and X.-L.Y. and C.-T.B. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by a grant from the German Federal Ministry of Health with regard to a decision of the German Bundestag by the Federal Government (CHED-project grant No: ZMVI1-2516-AUK-701/BMG: 321-4471-02/157). D.H. is supported by the Claussen-Simon-Stiftung (Claussen-Simon Foundation) “Dissertation Plus” program, Germany, and the Fazit-Stiftung Promotionsstipendium. B.W. is supported by the China Scholarship Council (CSC), Beijing, China. The content is responsibility only of the authors and does not represent the views of the Claussen-Simon-Stiftung or the CSC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rein D.B., Stevens G.A., Theaker J., Wittenborn J.S., Wiersma S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 2.Bose P.D., Das B.C., Kumar A., Gondal R., Kumar D., Kar P. High viral load and deregulation of the progesterone receptor signaling pathway: Association with hepatitis E-related poor pregnancy outcome. J. Hepatol. 2011;54:1107–1113. doi: 10.1016/j.jhep.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 3.Pavio N., Meng X.J., Doceul V. Zoonotic origin of hepatitis E. Curr. Opin. Virol. 2015;10:34–41. doi: 10.1016/j.coviro.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Kamar N., Izopet J., Tripon S., Bismuth M., Hillaire S., Dumortier J., Radenne S., Coilly A., Garrigue V., D’Alteroche L., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N. Engl. J. Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 5.Pischke S., Hartl J., Pas S.D., Lohse A.W., Jacobs B.C., Van der Eijk A.A. Hepatitis E virus: Infection beyond the liver? J. Hepatol. 2017;66:1082–1095. doi: 10.1016/j.jhep.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Balayan M.S., Andjaparidze A.G., Savinskaya S.S., Ketiladze E.S., Braginsky D.M., Savinov A.P., Poleschuk V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983;20:23–31. doi: 10.1159/000149370. [DOI] [PubMed] [Google Scholar]
- 7.Tam A.W., Smith M.M., Guerra M.E., Huang C.C., Bradley D.W., Fry K.E., Reyes G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology. 1991;185:120–131. doi: 10.1016/0042-6822(91)90760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Purdy M.A., Harrison T.J., Jameel S., Meng X.J., Okamoto H., Van der Poel W.H.M., Smith D.B., Ictv Report C. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017;98:2645–2646. doi: 10.1099/jgv.0.000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montpellier C., Wychowski C., Sayed I.M., Meunier J.C., Saliou J.M., Ankavay M., Bull A., Pillez A., Abravanel F., Helle F., et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology. 2018;154:e218. doi: 10.1053/j.gastro.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Rasche A., Sander A.L., Corman V.M., Drexler J.F. Evolutionary biology of human hepatitis viruses. J. Hepatol. 2019;70:501–520. doi: 10.1016/j.jhep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith D.B., Simmonds P., International Committee on Taxonomy of Viruses Hepeviridae Study Group. Jameel S., Emerson S.U., Harrison T.J., Meng X.J., Okamoto H., Van der Poel W.H., Purdy M.A. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014;95:2223–2232. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith D.B., Simmonds P., Izopet J., Oliveira-Filho E.F., Ulrich R.G., Johne R., Koenig M., Jameel S., Harrison T.J., Meng X.J., et al. Proposed reference sequences for hepatitis E virus subtypes. J. Gen. Virol. 2016;97:537–542. doi: 10.1099/jgv.0.000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney S.P. The Current Host Range of Hepatitis E Viruses. Viruses. 2019;11:452. doi: 10.3390/v11050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sridhar S., Yip C.C.Y., Wu S., Cai J., Zhang A.J., Leung K.H., Chung T.W.H., Chan J.F.W., Chan W.M., Teng J.L.L., et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018;24:2241–2250. doi: 10.3201/eid2412.180937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andonov A., Robbins M., Borlang J., Cao J., Hatchette T., Stueck A., Deschambault Y., Murnaghan K., Varga J., Johnston L. Rat Hepatitis E Virus Linked to Severe Acute Hepatitis in an Immunocompetent Patient. J. Infect. Dis. 2019;220:951–955. doi: 10.1093/infdis/jiz025. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar S., Yip C.C., Wu S., Chew N.F., Leung K.H., Chan J.F., Zhao P.S., Chan W.M., Poon R.W., Tsoi H.W., et al. Transmission of rat hepatitis E virus infection to humans in Hong Kong: A clinical and epidemiological analysis. Hepatology. 2020 doi: 10.1002/hep.31138. [DOI] [PubMed] [Google Scholar]
- 17.Kabrane-Lazizi Y., Fine J.B., Elm J., Glass G.E., Higa H., Diwan A., Gibbs C.J., Jr., Meng X.J., Emerson S.U., Purcell R.H. Evidence for widespread infection of wild rats with hepatitis E virus in the United States. Am. J. Trop. Med. Hyg. 1999;61:331–335. doi: 10.4269/ajtmh.1999.61.331. [DOI] [PubMed] [Google Scholar]
- 18.Favorov M.O., Kosoy M.Y., Tsarev S.A., Childs J.E., Margolis H.S. Prevalence of antibody to hepatitis E virus among rodents in the United States. J. Infect. Dis. 2000;181:449–455. doi: 10.1086/315273. [DOI] [PubMed] [Google Scholar]
- 19.Johne R., Heckel G., Plenge-Bonig A., Kindler E., Maresch C., Reetz J., Schielke A., Ulrich R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010;16:1452–1455. doi: 10.3201/eid1609.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johne R., Plenge-Bonig A., Hess M., Ulrich R.G., Reetz J., Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J. Gen. Virol. 2010;91:750–758. doi: 10.1099/vir.0.016584-0. [DOI] [PubMed] [Google Scholar]
- 21.Easterbrook J.D., Kaplan J.B., Vanasco N.B., Reeves W.K., Purcell R.H., Kosoy M.Y., Glass G.E., Watson J., Klein S.L. A survey of zoonotic pathogens carried by Norway rats in Baltimore, Maryland, USA. Epidemiol. Infect. 2007;135:1192–1199. doi: 10.1017/S0950268806007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano M., Ding X., Li T.C., Takeda N., Kawabata H., Koizumi N., Kadosaka T., Goto I., Masuzawa T., Nakamura M., et al. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol. Res. 2003;27:1–5. doi: 10.1016/S1386-6346(03)00192-X. [DOI] [PubMed] [Google Scholar]
- 23.Kanai Y., Miyasaka S., Uyama S., Kawami S., Kato-Mori Y., Tsujikawa M., Yunoki M., Nishiyama S., Ikuta K., Hagiwara K. Hepatitis E virus in Norway rats (Rattus norvegicus) captured around a pig farm. BMC Res. Notes. 2012;5:4. doi: 10.1186/1756-0500-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johne R., Dremsek P., Kindler E., Schielke A., Plenge-Bonig A., Gregersen H., Wessels U., Schmidt K., Rietschel W., Groschup M.H., et al. Rat hepatitis E virus: Geographical clustering within Germany and serological detection in wild Norway rats (Rattus norvegicus) Infect. Genet. Evol. 2012;12:947–956. doi: 10.1016/j.meegid.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Li W., Guan D., Su J., Takeda N., Wakita T., Li T.C., Ke C.W. High prevalence of rat hepatitis E virus in wild rats in China. Vet. Microbiol. 2013;165:275–280. doi: 10.1016/j.vetmic.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Li T.C., Yoshimatsu K., Yasuda S.P., Arikawa J., Koma T., Kataoka M., Ami Y., Suzaki Y., Mai le T.Q., Hoa N.T., et al. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2011;92:2830–2837. doi: 10.1099/vir.0.034835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koma T., Yoshimatsu K., Yasuda S.P., Li T., Amada T., Shimizu K., Isozumi R., Mai L.T., Hoa N.T., Nguyen V., et al. A survey of rodent-borne pathogens carried by wild Rattus spp. in Northern Vietnam. Epidemiol. Infect. 2013;141:1876–1884. doi: 10.1017/S0950268812002385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obana S., Shimizu K., Yoshimatsu K., Hasebe F., Hotta K., Isozumi R., Nguyen H.T., Le M.Q., Yamashiro T., Tsuda Y., et al. Epizootiological study of rodent-borne hepatitis E virus HEV-C1 in small mammals in Hanoi, Vietnam. J. Vet. Med. Sci. 2017;79:76–81. doi: 10.1292/jvms.16-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simanavicius M., Juskaite K., Verbickaite A., Jasiulionis M., Tamosiunas P.L., Petraityte-Burneikiene R., Zvirbliene A., Ulrich R.G., Kucinskaite-Kodze I. Detection of rat hepatitis E virus, but not human pathogenic hepatitis E virus genotype 1-4 infections in wild rats from Lithuania. Vet. Microbiol. 2018;221:129–133. doi: 10.1016/j.vetmic.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Mulyanto, Depamede S.N., Sriasih M., Takahashi M., Nagashima S., Jirintai S., Nishizawa T., Okamoto H. Frequent detection and characterization of hepatitis E virus variants in wild rats (Rattus rattus) in Indonesia. Arch. Virol. 2013;158:87–96. doi: 10.1007/s00705-012-1462-0. [DOI] [PubMed] [Google Scholar]
- 31.Mulyanto, Suparyatmo J.B., Andayani I.G., Khalid, Takahashi M., Ohnishi H., Jirintai S., Nagashima S., Nishizawa T., Okamoto H. Marked genomic heterogeneity of rat hepatitis E virus strains in Indonesia demonstrated on a full-length genome analysis. Virus Res. 2014;179:102–112. doi: 10.1016/j.virusres.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Primadharsini P.P., Mulyanto, Wibawa I.D.N., Anggoro J., Nishizawa T., Takahashi M., Jirintai S., Okamoto H. The identification and characterization of novel rat hepatitis E virus strains in Bali and Sumbawa, Indonesia. Arch. Virol. 2018;163:1345–1349. doi: 10.1007/s00705-018-3736-7. [DOI] [PubMed] [Google Scholar]
- 33.Arankalle V.A., Joshi M.V., Kulkarni A.M., Gandhe S.S., Chobe L.P., Rautmare S.S., Mishra A.C., Padbidri V.S. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. J. Viral. Hepat. 2001;8:223–227. doi: 10.1046/j.1365-2893.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 34.Vitral C.L., Pinto M.A., Lewis-Ximenez L.L., Khudyakov Y.E., dos Santos D.R., Gaspar A.M. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Memorias do Instituto Oswaldo Cruz. 2005;100:117–122. doi: 10.1590/S0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- 35.Guan D., Li W., Su J., Fang L., Takeda N., Wakita T., Li T.C., Ke C. Asian musk shrew as a reservoir of rat hepatitis E virus, China. Emerg. Infect. Dis. 2013;19:1341–1343. doi: 10.3201/eid1908.130069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z., Lu L., Du J., Yang L., Ren X., Liu B., Jiang J., Yang J., Dong J., Sun L., et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome. 2018;6:178. doi: 10.1186/s40168-018-0554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purcell R.H., Engle R.E., Rood M.P., Kabrane-Lazizi Y., Nguyen H.T., Govindarajan S., St Claire M., Emerson S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011;17:2216–2222. doi: 10.3201/eid1712.110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf S., Reetz J., Johne R., Heiberg A.C., Petri S., Kanig H., Ulrich R.G. The simultaneous occurrence of human norovirus and hepatitis E virus in a Norway rat (Rattus norvegicus) Arch. Virol. 2013;158:1575–1578. doi: 10.1007/s00705-013-1646-2. [DOI] [PubMed] [Google Scholar]
- 39.Widen F., Ayral F., Artois M., Olofson A.S., Lin J. PCR detection and analysis of potentially zoonotic Hepatitis E virus in French rats. Virol. J. 2014;11:90. doi: 10.1186/1743-422X-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayral F., Artois J., Zilber A.L., Widen F., Pounder K.C., Aubert D., Bicout D.J., Artois M. The relationship between socioeconomic indices and potentially zoonotic pathogens carried by wild Norway rats: A survey in Rhone, France (2010–2012) Epidemiol. Infect. 2015;143:586–599. doi: 10.1017/S0950268814001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryll R., Bernstein S., Heuser E., Schlegel M., Dremsek P., Zumpe M., Wolf S., Pepin M., Bajomi D., Muller G., et al. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet. Microbiol. 2017;208:58–68. doi: 10.1016/j.vetmic.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Spahr C., Ryll R., Knauf-Witzens T., Vahlenkamp T.W., Ulrich R.G., Johne R. Serological evidence of hepatitis E virus infection in zoo animals and identification of a rodent-borne strain in a Syrian brown bear. Vet. Microbiol. 2017;212:87–92. doi: 10.1016/j.vetmic.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Wang B., Cai C.L., Li B., Zhang W., Zhu Y., Chen W.H., Zhuo F., Shi Z.L., Yang X.L. Detection and characterization of three zoonotic viruses in wild rodents and shrews from Shenzhen city, China. Virol. Sin. 2017;32:290–297. doi: 10.1007/s12250-017-3973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He W., Wen Y., Xiong Y., Zhang M., Cheng M., Chen Q. The prevalence and genomic characteristics of hepatitis E virus in murine rodents and house shrews from several regions in China. BMC Vet. Res. 2018;14:414. doi: 10.1186/s12917-018-1746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy E.G., Williams N.J., Jennings D., Chantrey J., Verin R., Grierson S., McElhinney L.M., Bennett M. First detection of Hepatitis E virus (Orthohepevirus C) in wild brown rats (Rattus norvegicus) from Great Britain. Zoonoses Public Health. 2019;66:686–694. doi: 10.1111/zph.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lack J.B., Volk K., Van Den Bussche R.A. Hepatitis E virus genotype 3 in wild rats, United States. Emerg. Infect. Dis. 2012;18:1268–1273. doi: 10.3201/eid1808.120070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Sabato L., Ianiro G., Monini M., De Lucia A., Ostanello F., Di Bartolo I. Detection of hepatitis E virus RNA in rats caught in pig farms from Northern Italy. Zoonoses Public Health. 2020;67:62–69. doi: 10.1111/zph.12655. [DOI] [PubMed] [Google Scholar]
- 48.Onyuok S.O., Hu B., Li B., Fan Y., Kering K., Ochola G.O., Zheng X.S., Obanda V., Ommeh S., Yang X.L., et al. Molecular Detection and Genetic Characterization of Novel RNA Viruses in Wild and Synanthropic Rodents and Shrews in Kenya. Front. Microbiol. 2019;10:2696. doi: 10.3389/fmicb.2019.02696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T.C., Ami Y., Suzaki Y., Yasuda S.P., Yoshimatsu K., Arikawa J., Takeda N., Takaji W. Characterization of full genome of rat hepatitis E virus strain from Vietnam. Emerg. Infect. Dis. 2013;19:115–118. doi: 10.3201/eid1901.121007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B., Li W., Zhou J.H., Li B., Zhang W., Yang W.H., Pan H., Wang L.X., Bock C.T., Shi Z.L., et al. Chevrier’s Field Mouse (Apodemus chevrieri) and Pere David’s Vole (Eothenomys melanogaster) in China Carry Orthohepeviruses that form Two Putative Novel Genotypes Within the Species Orthohepevirus C. Virol. Sin. 2018;33:44–58. doi: 10.1007/s12250-018-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Souza W.M., Romeiro M.F., Sabino-Santos G., Jr., Maia F.G.M., Fumagalli M.J., Modha S., Nunes M.R.T., Murcia P.R., Figueiredo L.T.M. Novel orthohepeviruses in wild rodents from Sao Paulo State, Brazil. Virology. 2018;519:12–16. doi: 10.1016/j.virol.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kurucz K., Hederics D., Bali D., Kemenesi G., Horvath G., Jakab F. Hepatitis E virus in Common voles (Microtus arvalis) from an urban environment, Hungary: Discovery of a Cricetidae-specific genotype of Orthohepevirus C. Zoonoses Public Health. 2019;66:259–263. doi: 10.1111/zph.12543. [DOI] [PubMed] [Google Scholar]
- 53.Ryll R., Heckel G., Corman V.M., Drexler J.F., Ulrich R.G. Genomic and spatial variability of a European common vole hepevirus. Arch. Virol. 2019;164:2671–2682. doi: 10.1007/s00705-019-04347-1. [DOI] [PubMed] [Google Scholar]
- 54.Raj V.S., Smits S.L., Pas S.D., Provacia L.B., Moorman-Roest H., Osterhaus A.D., Haagmans B.L. Novel hepatitis E virus in ferrets, the Netherlands. Emerg. Infect. Dis. 2012;18:1369–1370. doi: 10.3201/eid1808.111659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T.C., Yang T., Ami Y., Suzaki Y., Shirakura M., Kishida N., Asanuma H., Takeda N., Takaji W. Complete genome of hepatitis E virus from laboratory ferrets. Emerg. Infect. Dis. 2014;20:709–712. doi: 10.3201/eid2004.131815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L., Gong W., Fu H., Li M., Zhang Y., Luo Z., Xu Q., Wang L. Hepatitis E virus detected from Chinese laboratory ferrets and farmed mink. Transbound. Emerg. Dis. 2018;65:e219–e223. doi: 10.1111/tbed.12720. [DOI] [PubMed] [Google Scholar]
- 57.Krog J.S., Breum S.O., Jensen T.H., Larsen L.E. Hepatitis E virus variant in farmed mink, Denmark. Emerg. Infect. Dis. 2013;19:2028–2030. doi: 10.3201/eid1912.130614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bodewes R., van der Giessen J., Haagmans B.L., Osterhaus A.D., Smits S.L. Identification of multiple novel viruses, including a parvovirus and a hepevirus, in feces of red foxes. J. Virol. 2013;87:7758–7764. doi: 10.1128/JVI.00568-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reuter G., Boros A., Matics R., Kapusinszky B., Delwart E., Pankovics P. Divergent hepatitis E virus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus), Hungary. Infect. Genet. Evol. 2016;43:343–346. doi: 10.1016/j.meegid.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi T., Takahashi M., Jirintai S., Nishizawa T., Nagashima S., Nishiyama T., Kunita S., Hayama E., Tanaka T., Okamoto H., et al. An analysis of two open reading frames (ORF3 and ORF4) of rat hepatitis E virus genome using its infectious cDNA clones with mutations in ORF3 or ORF4. Virus Res. 2018;249:16–30. doi: 10.1016/j.virusres.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Sridhar S., Teng J.L.L., Chiu T.H., Lau S.K.P., Woo P.C.Y. Hepatitis E Virus Genotypes and Evolution: Emergence of Camel Hepatitis E Variants. Int. J. Mol. Sci. 2017;18:869. doi: 10.3390/ijms18040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee G.H., Tan B.H., Teo E.C., Lim S.G., Dan Y.Y., Wee A., Aw P.P., Zhu Y., Hibberd M.L., Tan C.K., et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:e353. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 63.Li T.C., Zhou X., Yoshizaki S., Ami Y., Suzaki Y., Nakamura T., Takeda N., Wakita T. Production of infectious dromedary camel hepatitis E virus by a reverse genetic system: Potential for zoonotic infection. J. Hepatol. 2016;65:1104–1111. doi: 10.1016/j.jhep.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Li T.C., Bai H., Yoshizaki S., Ami Y., Suzaki Y., Doan Y.H., Takahashi K., Mishiro S., Takeda N., Wakita T. Genotype 5 Hepatitis E Virus Produced by a Reverse Genetics System Has the Potential for Zoonotic Infection. Hepatol. Commun. 2019;3:160–172. doi: 10.1002/hep4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Teng J.L.L., Lau S.K.P., Sridhar S., Fu H., Gong W., Li M., Xu Q., He Y., Zhuang H., et al. Transmission of a Novel Genotype of Hepatitis E Virus from Bactrian Camels to Cynomolgus Macaques. J. Virol. 2019;93 doi: 10.1128/JVI.02014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cossaboom C.M., Cordoba L., Sanford B.J., Pineyro P., Kenney S.P., Dryman B.A., Wang Y., Meng X.J. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J. Gen. Virol. 2012;93:1687–1695. doi: 10.1099/vir.0.041509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu P., Bu Q.N., Wang L., Han J., Du R.J., Lei Y.X., Ouyang Y.Q., Li J., Zhu Y.H., Lu F.M., et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg. Infect. Dis. 2013;19:559–565. doi: 10.3201/eid1904.120827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meerburg B.G., Singleton G.R., Kijlstra A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 69.Maneerat Y., Clayson E.T., Myint K.S., Young G.D., Innis B.L. Experimental infection of the laboratory rat with the hepatitis E virus. J. Med. Virol. 1996;48:121–128. doi: 10.1002/(SICI)1096-9071(199602)48:2<121::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 70.Huang F., Zhang W., Gong G., Yuan C., Yan Y., Yang S., Cui L., Zhu J., Yang Z., Hua X. Experimental infection of Balb/c nude mice with Hepatitis E virus. BMC Infect. Dis. 2009;9:93. doi: 10.1186/1471-2334-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schlosser J., Dahnert L., Dremsek P., Tauscher K., Fast C., Ziegler U., Groner A., Ulrich R.G., Groschup M.H., Eiden M. Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits. Viruses. 2018;11:1. doi: 10.3390/v11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shukla P., Nguyen H.T., Torian U., Engle R.E., Faulk K., Dalton H.R., Bendall R.P., Keane F.E., Purcell R.H., Emerson S.U. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc. Natl. Acad. Sci. USA. 2011;108:2438–2443. doi: 10.1073/pnas.1018878108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li T.C., Yoshizaki S., Ami Y., Suzaki Y., Yasuda S.P., Yoshimatsu K., Arikawa J., Takeda N., Wakita T. Susceptibility of laboratory rats against genotypes 1, 3, 4, and rat hepatitis E viruses. Vet. Microbiol. 2013;163:54–61. doi: 10.1016/j.vetmic.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Dremsek P., Wenzel J.J., Johne R., Ziller M., Hofmann J., Groschup M.H., Werdermann S., Mohn U., Dorn S., Motz M., et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012;201:189–200. doi: 10.1007/s00430-011-0221-2. [DOI] [PubMed] [Google Scholar]
- 75.Shimizu K., Hamaguchi S., Ngo C.C., Li T.C., Ando S., Yoshimatsu K., Yasuda S.P., Koma T., Isozumi R., Tsuda Y., et al. Serological evidence of infection with rodent-borne hepatitis E virus HEV-C1 or antigenically related virus in humans. J. Vet. Med. Sci. 2016;78:1677–1681. doi: 10.1292/jvms.16-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jirintai S., Suparyatmo J.B., Takahashi M., Kobayashi T., Nagashima S., Nishizawa T., Okamoto H. Rat hepatitis E virus derived from wild rats (Rattus rattus) propagates efficiently in human hepatoma cell lines. Virus Res. 2014;185:92–102. doi: 10.1016/j.virusres.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Li T.C., Yang T., Yoshizaki S., Ami Y., Suzaki Y., Ishii K., Kishida N., Shirakura M., Asanuma H., Takeda N., et al. Ferret hepatitis E virus infection induces acute hepatitis and persistent infection in ferrets. Vet. Microbiol. 2016;183:30–36. doi: 10.1016/j.vetmic.2015.11.014. [DOI] [PubMed] [Google Scholar]