Abstract
Novel alternative antibacterial compounds have been persistently explored from plants as natural sources to overcome antibiotic resistance leading to serious foodborne bacterial illnesses. In this study, the ethanolic extracts from 239 traditional Chinese medicinal plants (TCMP)’ materials were screened to discover promising candidates that have strong antibacterial properties against multidrug-resistant Staphylococcus (S.) aureus and low cytotoxicity. The results revealed that 74 extracts exhibited good antibacterial activities (diameter of inhibition zone (DIZ) ≥ 15 mm). Furthermore, 18 extracts (DIZ ≥ 20 mm) were determined their minimum inhibitory concentrations (MIC) and minimum bactericide concentrations (MBC), ranging from 0.1 to 12.5 mg/mL and 0.78 to 25 mg/mL, respectively. In addition, most of the 18 extracts showed relatively low cytotoxicity (a median lethal concentration (LC50) >100 µg/mL). The 18 extracts were further determined to estimate possible correlation of their phenolic contents with antibacterial activity, and the results did not show any significant correlation. In conclusion, this study selected out some promising antibacterial TCMP extracts with low cytotoxicity, including Rhus chinensis Mill., Ilex rotunda Thunb., Leontice kiangnanensis P.L.Chiu, Oroxylum indicum Vent., Isatis tinctorial L., Terminalia chebula Retz., Acacia catechu (L.f.) Willd., Spatholobus suberectus Dunn, Rabdosia rubescens (Hemsl.) H.Hara, Salvia miltiorrhiza Bunge, Fraxinus fallax Lingelsh, Coptis chinensis Franch., Agrimonia Pilosa Ledeb., and Phellodendron chinense C.K.Schneid.
Keywords: medicinal plant, antimicrobial activity, drug resistance, foodborne pathogens, cytotoxicity, polyphenols
1. Introduction
Foodborne diseases are mainly transmitted by bacterial-contaminated food and pose serious public health problems that cause significant morbidity and mortality worldwide [1]. According to the World Health Organization (WHO) report, approximately 2.2 million people annually die from foodborne diseases caused by pathogens [2]. Staphylococcus (S.) aureus, one of the major foodborne pathogens, leads to a wide range of severe foodborne diseases due to its invasiveness, multidrug resistance, and virulence [3,4]. The indiscriminate use of antibiotics in animal husbandry and hospitals have accelerated the emergence of S. aureus to acquire resistance to various antibacterial agents [5,6,7,8]. The rapid development of multidrug resistance has led to a decrease in the effectiveness of commercial antibiotics and has resulted in untreatable infections [9]. Moreover, a challenging complication is presented by the slow pace of research and development of new antibiotics that may add to the existing arsenal of antimicrobials to circumvent the outbreak and transmission of drug resistant bacteria [10]. As such, to overcome the outbreak and proliferation of multidrug-resistant bacterial infections, novel antibacterial compounds of plant, animal, bacterial, algal, and fungal origin have been persistently explored as an alternative to conventional antibiotics. In recent years, plants have particularly played a pivotal role as viable sources for the discovery of strong antibacterial agents applied in the pharmaceutical, food, and animal feed industries.
Plants produce various secondary metabolites that have antimicrobial properties such as phenolics, alkaloids, flavonoids, tannins, terpenoids, polyacetylenes, and quinones [6,11,12,13]. These phytochemical compounds, namely phytoalexins, are naturally occurring antimicrobials produced by plant as active defense mechanisms against phytopathogens such as bacteria, fungi, and viruses in the environment [14]. In addition, these biomolecules possess a wide spectrum of chemical properties including unique chemical scaffolds and complex structures, providing potentiating inhibitory effects associated with possible distinctive mechanisms of action against microorganisms [15,16]. There have been several previous studies reporting a significant advantage of these phytochemicals to impede the development of drug-resistant bacteria [17,18]. Some of these phytochemicals and plant extracts are designated by the United States Food and Drug Administration (FDA) as Generally Recognized As Safe (GRAS), and have low toxicity and good acceptability to food and medical applications [18]. All these features in combination support that plants are a promising source of natural antimicrobials.
There has been a growing interest in discovering new and effective antimicrobial substances from traditional Chinese medicinal plants (TCMP). In China, 11,118 species of plants have been used as medicinal materials for centuries [19]. By virtue of diverse bioactive compounds synthesized from the plants, their therapeutic applications have been reported in many ethnopharmacological studies including anticancer, hepatoprotection, anti-inflammatory, antidiarrheal antioxidant, antiviral, and antimicrobial activities [20,21]. Numerous studies have been demonstrated on the antimicrobial activities of TCMP extracts against different types of microbes, but there still remain challenges to discover novel antimicrobial molecules from TCMP. Therefore, 239 TCMP materials (89 families, 206 genera, and 233 species) were selected from the Chinese literature that have antibacterial effects in this present study. It was aimed to take a large-scale screening of TCMP for the assessment of potentially strong antibacterial activity against multidrug-resistant S. aureus to evaluate the provision of promising baseline information for the potential use of TCMP as novel antimicrobial agents in pharmaceutical, food and animal feed industries.
2. Results and Discussion
2.1. Screening of Antibacterial TCMP Extracts against Antibiotic-Resistant S. aureus
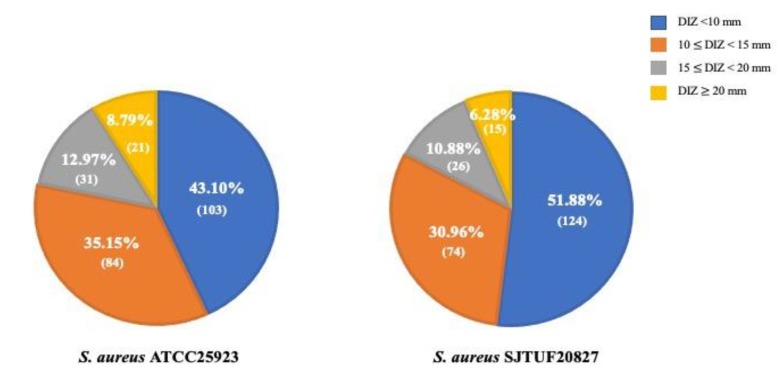
The 239 ethanolic TCMP extracts were used to screen for their potential antibacterial activity against S. aureus including one reference strain ATCC 25923 and one antibiotic-resistant strain SJTUF 20827 based on the agar diffusion method (Table S1 in Supplementary Material and Figure 1). Antibacterial activities of TCMP extracts, evaluated by the measurement of diameter of inhibition zone (DIZ), were classified as very strong (DIZ ≥ 20 mm), strong (20 > DIZ ≥ 15 mm), moderate (15 > DIZ ≥ 10 mm), and weak (DIZ < 10 mm) [22]. Fifty-two out of 239 extracts showed strong antibacterial activities against S. aureus ATCC 25923, whereas 41 extracts had strong antibacterial activities against S. aureus SJTUF 20827 (DIZ ≥ 15 mm) that had similar values of DIZ (12.9–34.6 mm) with conventional antibiotics such as oxacillin (32 µg/mL) and ampicillin (4 µg/mL). Of the extracts, Speranskia tuberculate Baill. exhibited the best inhibitory effects on the growth of both reference and antibiotic-resistant S. aureus strains with DIZ values 26.8 and 25.2 mm, respectively. In this study, antibacterial properties of TCMP against S. aureus may be attributed to the simplicity of its cell membrane structure and composition in Gram-positive bacteria, which can be easily accessible to permeation of hydrophobic active compounds in TCMP [13]. Therefore, antibacterial molecules can directly access the target sites both on the cell wall and in the cytoplasm, resulting in pore formation, intercellular constituent leakages, structural or functional abnormalities of the bacterial membrane phospholipid bilayer, and inhibition of its biosynthesis [23,24,25,26]. In addition, these active compounds are commonly targeted at multiple sites rather than one specific site of antibacterial action [27,28]. For example, Ebelle et al. [29] reported that the methanolic extract of Enantia chlorantha had several modes of antibacterial action including the extension of the bacterial latency period, the deactivation of H+ - ATPases activity in cell membrane, the loss of the salt tolerance of the S. aureus, and inhibition of the biofilm formation. Since TCMP extracts contain diverse chemical substances, they probably influence the bacteria cell constituents or molecular targets by various mechanisms.
Figure 1.
Screening of 239 TCMP extracts for antibacterial activities against S. aureus ATCC 25923 (reference strain) and SJTUF 20827 (antibiotic-resistant strain). Number in parentheses indicates the number of TCMP extracts, which exhibited DIZ value in four different ranges of DIZ value; DIZ < 10 mm, 10 ≤ DIZ < 15 mm, 15 ≤ DIZ < 20 mm and DIZ ≥ 20 mm.
The results also revealed that the reference S. aureus was more susceptible to TCMP extracts compared with the antibiotic-resistant strain. These differences may be attributed to the fact that the antibiotic-resistant bacteria have acquired complex resistance mechanisms to survive in the presence of the antimicrobial molecules, rendering the antibacterial agents inactive. Indeed, bacterial pathogens have developed strategies for antibacterial resistance by chemical alteration and destruction of antibacterial molecules, decrease in their penetration, extrusion of the antibacterial compound, and interference in their access to target sites [30,31]. In addition, they are able to synthesize a protective polysaccharide layer on their surface, known as a bacterial biofilm [32]. These resistant factors might influence the activity of TCMP extracts against antibiotic-resistant S. aureus in this study. Further studies are needed to determine the mechanisms of antibacterial action of the extracts and their specific targets in antibiotic-resistant S. aureus strains.
2.2. Selected TCMP Extracts with a Wide Range of Antibacterial Activities against Multidrug-Resistant Bacteria
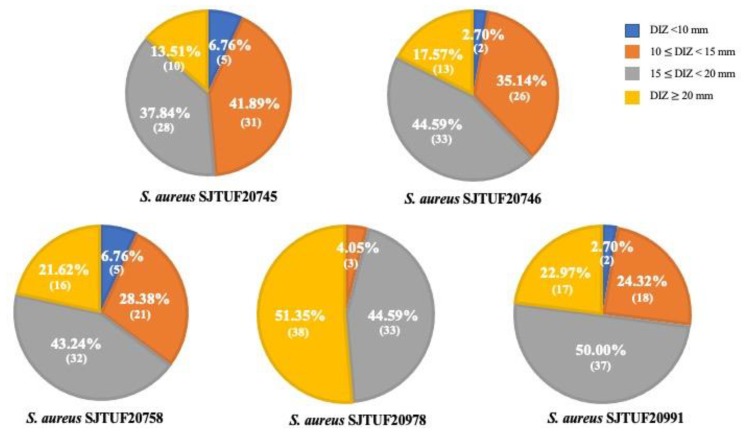
Based on initial screening results in which both the reference and antibiotic-resistant S. aureus model strains were found to be susceptible to TCMP extracts, we selected 74 strong TCMP extracts (DIZ ≥ 15 mm) and proceeded to the assessment of the wide range of antibacterial activities against multidrug-resistant S. aureus strains SJTUF 20745, 20746, 20758, 20978, and 20991 (Table 1). In this study, results showed that the selected TCMP extracts exhibited different antibacterial effects against multidrug-resistant pathogens; however, most extracts revealed strong and extensive antibacterial activities. As shown in Figure 2, 51.35%, 62.16%, 64.86%, 95.94%, and 77.97% of TCMP extracts possessed strong inhibitory effects (DIZ ≥ 15 mm) against S. aureus SJTUF 20745, 20746, 20758, 20978, and 20991, respectively.
Table 1.
Antibacterial activities of 74 selected TCMP extracts against multidrug-resistant S. aureus strains based on diameter of inhibition zone (DIZ).
| No. 1 | Family | Scientific Name | Common Name | Extracted Plant Part | DIZ (mm) against S. aureus | ||||
|---|---|---|---|---|---|---|---|---|---|
| SJTUF 20745 | SJTUF 20746 | SJTUF 20758 | SJTUF 20978 | SJTUF 20991 | |||||
| 3 | Anacardiaceae | Rhus chinensis Mill. | Nutgall tree | gall | 21 ± 1 | 21.5 ± 0.1 | 21.8 ± 0.4 | 25 ± 0.6 | 20.5 ± 0.3 |
| 8 | Apiaceae | Cnidium monnieri Cusson | Monnier’s snowparsley | fruit | 9 | 12.6 | NIZ | 17.6 ± 0.1 | 10.5 ± 0.2 |
| 22 | Aquifoliaceae | Ilex rotunda Thunb. | Kurogane holly | bark | 17.2 ± 0.5 | 17.6 ± 0.6 | 19.9 ± 0.8 | 17.4 ± 0.2 | 18.3 |
| 26 | Araceae | Spirodela polyrrhiza (L.) Schleid. | Common duckweed | aerial part | 9.1 | 10.4 ± 0.1 | 10.3 ± 0.1 | 17.5 ± 0.1 | 11.9 |
| 31 | Aristolochiaceae | Asarum heterotropoides F. Schmidt | Manchurian wildginge | rhizome & root | 14.3 ± 0.2 | 18 | 15.2 ± 0.2 | 29.8 ± 0.2 | 14.1 ± 0.1 |
| 36 | Asphodelaceae | Aloe ferox Mill. | Cape aloe | dried gel | 8.3 | 11.6 ± 0.4 | 8.7 | 14.6 ± 0.5 | 10.2 ± 0.3 |
| 46 | Asteraceae | Elephantopus scaber L. | Cucha cara | aerial part | 12.3 ± 0.5 | 13.2 ± 0.1 | 13.2 ± 0.2 | 15.1 | 13.8 ± 0.6 |
| 49 | Hemistepta lyrata Bunge | Lyre-shape hemistepta | aerial part | 12.4 | 12.3 ± 0.3 | 12.1 ± 0.4 | 16 | 14.3 | |
| 51 | Inula japonica Thunb. | Inula flower | flower | 16.4 ± 0.4 | 17 ± 1 | 15.7 | 20 ± 0.5 | 20.3 ± 0.1 | |
| 58 | Berberidaceae | Leontice kiangnanensis P. L. Chiu | Rhizoma corydalis | tuberous root | 19.1 ± 0.7 | 23.7 ± 0.3 | 22 ± 1 | 25.6 ± 0.1 | 22.4 ± 0.4 |
| 61 | Bignoniaceae | Oroxylum indicum Vent. | Indian trumpet flower | seed | 18.5 ± 0.3 | 25 ± 1 | 24.2 ± 0.2 | 30 ± 1 | 22.4 ± 0.2 |
| 62 | Boraginaceae | Lithospermum erythrorhizon Siebold & Zucc. | Purple gromwell | leaf | 11.8 ± 0.2 | 16.3 ± 0.2 | 13.5 ± 0.1 | 15.3 ± 0.1 | 13.4 |
| 65 | Brassicaceae | Isatis tinctoria L. | Dyer’s woad | leaf | 21 ± 1 | 22 ± 1 | 23 ± 1 | 24.8 ± 0.7 | 16.9 ± 0.3 |
| 81 | Combretaceae | Terminalia chebula Retz. | Myrobalan | fruit | 16.8 ± 0.3 | 18.1 | 20 ± 0.2 | 24.5 ± 0.1 | 20.2 ± 0.1 |
| 87 | Dioscoreaceae | Dioscorea bulbifera L. | aerial yam | tuberous root | 21 ± 1 | 21 ± 1 | 19.2 | 24.6 ± 0.5 | 20.8 ± 0.1 |
| 90 | Ebenaceae | Diospyros kaki Thunb. | Chinese persimmon | calyx | 16.7 ± 0.4 | 16.2 ± 0.3 | 16.7 | 19.5 ± 0.1 | 17.2 ± 0.2 |
| 92 | Ericaceae | Pyrola calliantha Andres | Chinese pyrola | aerial part | 13.7 ± 0.1 | 21.3 ± 0.5 | 13.3 | 18.2 ± 0.2 | 15.4 ± 0.1 |
| 95 | Euphorbiaceae | Euphorbia humifusa Willd. | Herba euphorbiae humifusae | aerial part | 13.8 ± 0.2 | 13 | 16.2 ± 0.6 | 18.1 ± 0.4 | 17.7 ± 0.2 |
| 96 | Speranskia tuberculata Baill. | Herba speranskiae tuberculatae | aerial part | 24.1 ± 0.4 | 25 ± 2 | 22.8 ± 0.3 | 27.7 ± 0.4 | 27.1 ± 0.5 | |
| 97 | Fabaceae | Acacia catechu (L.f.) Willd. | Catechu | branch | 23 ± 0.8 | 25 ± 2 | 27 ± 1 | 32 ± 2 | 25.1 ± 0.3 |
| 101 | Cassia occidentalis L. | Coffee senna | seed | 14.9 ± 0.1 | 14.1 ± 0.1 | 13.8 ± 0.4 | 14.8 ± 0.2 | 10.3 ± 0.1 | |
| 102 | Cassia tora L. | Sickle Senna | seed | 14 ± 0.4 | 14.2 ± 0.4 | 13.2 ± 0.8 | 16.6 ± 0.5 | 15.9 ± 0.1 | |
| 103 | Dalbergia odorifera T. C. Chen | Fragrant rosewood | trunk | 18.2 ± 0.5 | 20 ± 1 | 20 ± 1 | 28 ± 1 | 21.4 ± 0.5 | |
| 107 | Gleditsia sinensis Lam. | Chinese honey locust | branch | 14.6 ± 0.4 | 15.8 ± 0.2 | 17.4 | 20 ± 1 | 15.2 ± 0.4 | |
| 109 | Glycyrrhiza uralensis Fisch. | Licorice | rhizome & root | 12.8 ± 0.1 | 13.7 ± 0.4 | 16.1 ± 0.2 | 17.4 | 14.8 ± 0.1 | |
| 110 | Lablab purpureus (L.) Sweet | Lablab Bean | seed | 14.6 | 16.8 ± 0.3 | 19.2 | 22 ± 1 | 17.5 | |
| 112 | Quercus infectoria Oliv. | Aleppo oak | gall | 22.4 ± 0.5 | 22.3 ± 0.4 | 24.8 ± 0.5 | 26.6 ± 0.1 | 22.8 | |
| 114 | Sophora tonkinensis Gagnepain | Vietnamese sophora | rhizome & root | 14.3 ± 0.4 | 15.3 ± 0.2 | 13.5 | 20.2 ± 0.1 | 15.8 | |
| 115 | Spatholobus suberectus Dunn | Caulis spatholobi | stem | 17 ± 0.1 | 17.2 ± 0.2 | 19 ± 1 | 20.8 ± 0.2 | 19.4 ± 0.2 | |
| 122 | Hypericaceae | Hypericum japonicum Thunb. | Matted St. John’s-wort | aerial part | 17.8 ± 0.4 | 17.1 ± 0.1 | 14.7 | 22 ± 1 | 18.9 ± 0.2 |
| 126 | Lamiaceae | Isodon serra Kudo | Herba rabdosiae | aerial part | 15.8 ± 0.2 | 15.4 ± 0.2 | 18.7 ± 0.1 | 21.7 ± 0.1 | 17.3 ± 0.3 |
| 132 | Rabdosia rubescens (Hemsl.) H. Hara | Blushred rabdosia | aerial part | 18.8 ± 0.3 | 23 ± 1 | 19 ± 1 | 23.5 ± 0.1 | 22 ± 1 | |
| 133 | Salvia miltiorrhiza Bunge | Chinese salvia | rhizome & root | 17.3 ± 0.5 | 18.9 ± 0.5 | 22.3 ± 0.3 | 19.1 ± 0.4 | 19 ± 0.3 | |
| 139 | Lardizabalaceae | Sargentodoxa cuneata Rehder & E. H. Wilson | Sargentgloryvine | stem | 14.5 ± 0.1 | 13.8 ± 0.4 | 15.1 | 19.6 | 17.8 ± 0.3 |
| 140 | Lauraceae | Cinnamomum cassia (L.) Presl | Grey bollywood | branch | 15.2 | 14.6 ± 0.1 | 20 ± 0.2 | 18.1 ± 0.2 | 18.5 ± 0.6 |
| 148 | Lycopodiaceae | Diphasiastrum complanatum (L.) Holub | Groundcedar | aerial part | 20.8 ± 0.2 | 19.6 ± 0.1 | 21.7 ± 0.3 | 24.3 ± 0.3 | 22.6 |
| 151 | Magnoliaceae | Magnolia denudata Desr. | Lilytree | bud of flower | 15.2 | 16.1 ± 0.2 | 15.4 ± 0.2 | 17.2 ± 0.2 | 12.9 |
| 153 | Malvaceae | Bombax malabaricum DC. | Bombax | root bark | 10.3 ± 0.2 | 10.8 ± 0.2 | 10.4 ± 0.2 | 16.3 | 12.6 |
| 154 | Helicteres angustifolia L. | Narrowleaf screwtree | root | 17.7 ± 0.1 | 19.8 | 19.7 ± 0.5 | 26 ± 1 | 16.9 ± 0.1 | |
| 155 | Pterospermum heterophyllum Hance | Heterophyllous wingseedtree | root | 15.7 ± 0.2 | 18.2 ± 0.1 | 19.3 ± 0.2 | 21.4 ± 0.4 | 18.2 ± 0.1 | |
| 159 | Meliaceae | Melia azedarach L. | Chinaberry tree | bark & root bark | 18.4 ± 0.3 | 16.8 ± 0.3 | 22.2 ± 0.3 | 23.3 ± 0.6 | 19.8 |
| 168 | Oleaceae | Fraxinus fallax Lingelsh. | Largeleaf Chinese ash | bark | 13.4 ± 0.5 | 15.8 ± 0.3 | 20 ± 1 | 19.3 ± 0.7 | 19.5 |
| 169 | Jasminum nudiflorum Lindl. | Winter jasmine | bud of flower | 11.3 | 13.9 | 12.5 ± 0.1 | 15.9 ± 0.2 | 21.2 ± 0.8 | |
| 172 | Orchidaceae | Nervilia fordii Schltr. | Ford nervilla | rhizome & leaf | NIZ | 8.3 | NIZ | 17.3 | 9.9 ± 0.1 |
| 173 | Pholidota chinensis Lindl. | Chinese photinia herb | stem | 14.9 ± 0.3 | 17 ± 0.4 | 17.3 ± 0.2 | 22.4 ± 0.8 | 17.2 ± 0.3 | |
| 177 | Orobanchaceae | Striga asiatica (L.) Kuntze | Asiatic witchweed | aerial part | 11.9 | 11.1 | 8.8 | 19.4 ± 0.5 | 13.2 |
| 178 | Paeoniaceae | Paeonia lactiflora Pall. | Chinese peony | root | 15.6 ± 0.4 | 14.2 ± 0.1 | 17.4 | 16 ± 0.3 | 17.2 ± 0.2 |
| 179 | Paeonia suffruticosa Andrews | Moutan peony | root bark | 14.1 ± 0.1 | 18 ± 0.7 | 17 ± 0.3 | 18.2 ± 0.1 | 18.1 ± 0.2 | |
| 180 | Paeonia veitchii Lynch | Red Peony | root | 14.5 ± 0.1 | 15.7 ± 0.1 | 15.8 ± 0.3 | 16.7 | 15.5 ± 0.7 | |
| 182 | Phyllanthaceae | Phyllanthus emblica L. | Emblic | fruit | 16.6 ± 0.5 | 13.4 ± 0.3 | 19 ± 0.3 | 23.5 ± 0.2 | 18.6 ± 0.2 |
| 183 | Pinaceae | Pseudolarix amabilis Rehder | Chinese golden larch | root bark | 17.4 ± 0.5 | 17 ± 0.6 | 19.4 ± 0.4 | 17.4 | 19.7 |
| 185 | Poaceae | Bambusa tuldoides Munro | Puntingpole bamboo | stem | 9.4 | 9.3 | 8.1 ± 0.1 | 18.2 ± 0.3 | 9.7 ± 0.1 |
| 186 | Chrysopogon aciculatus Trin. | Mackie’s pest | aerial part | 11.4 ± 0.2 | 12.6 ± 0.4 | 12.3 ± 0.3 | 25 ± 1 | 14.2 | |
| 192 | Polygonaceae | Polygonum bistorta L. | Meadow bistort | rhizome | 15.3 ± 0.2 | 15.7 ± 0.2 | 14.4 ± 0.4 | 19.2 ± 0.4 | 15.1 ± 0.1 |
| 193 | Polygonum chinense L. | Chinese knotweed | aerial part | 14.2 ± 0.1 | 13.6 | 14.2 ± 0.1 | 19 ± 0.5 | 17.2 ± 0.1 | |
| 194 | Polygonum multiflorum Thunb. | Tuber fleeceflower | stem | 16.6 ± 0.3 | 15.2 ± 0.4 | 16.6 ± 0.3 | 21 ± 1 | 16.4 ± 0.1 | |
| 195 | Polygonum multiflorum Thunb. | Tuber fleeceflower | tuberous root | 14 ± 1 | 17.7 ± 0.4 | 14.7 ± 0.5 | 19.5 ± 0.3 | 14.9 | |
| 196 | Rumex obtusifolius L. | Bitter dock | root | 12.8 | 13 ± 0.2 | 18 | 22.9 ± 0.3 | 13.9 | |
| 197 | Primulaceae | Ardisia japonica Blume | Marlberry | aerial part | 16.5 ± 0.6 | 12.4 | 13.2 | 19.2 ± 0.1 | 19 ± 1 |
| 198 | Lysimachia christinae Hance | Herba lysimachiae | aerial part | 14.3 ± 0.3 | 14.3 ± 0.2 | 16.4 | 27 ± 2 | 16.2 ± 0.1 | |
| 200 | Ranunculaceae | Coptis chinensis Franch. | Chinese goldthread | rhizome | 23 ± 1 | 23.2 ± 0.4 | 22.5 ± 0.5 | 23 ± 1 | 22.3 ± 0.1 |
| 201 | Thalictrum aquilegifolium L. | French meadow-rue | rhizome & root | 14.4 | 15.4 ± 0.2 | 17.5 ± 0.6 | 17 ± 0.3 | 15.4 ± 0.4 | |
| 202 | Rosaceae | Agrimonia pilosa Ledeb. | Herba agrimoniae | aerial part | 16.5 ± 0.2 | 16 ± 0.4 | 16.9 ± 0.4 | 21.1 | 18.4 ± 0.4 |
| 203 | Duchesnea indica (Andr.) Focke | Indian strawberry | aerial part | 11.5 | 13.9 ± 0.4 | 13.8 ± 0.7 | 21.5 ± 0.2 | 15.4 ± 0.5 | |
| 205 | Geum aleppicum Jacq. | Aleppo avens | aerial part | 15.1 ± 0.5 | 14.7 ± 0.1 | 16.7 ± 0.3 | 22.7 ± 0.3 | 17.3 ± 0.3 | |
| 206 | Prunus mume Siebold & Zucc. | Japanese apricot | fruit | 12.1 ± 0.1 | 11.5 | 13.3 ± 0.1 | 16.3 ± 0.5 | 12.2 ± 0.9 | |
| 210 | Rubiaceae | Serissa serissoides (DC.) Druce | Snowrose | aerial part | 16.1 ± 0.3 | 18.9 | 21.5 ± 0.5 | 25.9 ± 0.1 | 15.9 ± 0.4 |
| 217 | Rutaceae | Phellodendron chinense C. K. Schneid. | Chinese corktree | bark | 20 ± 1 | 19.5 ± 0.3 | 18.8 ± 0.5 | 22.3 ± 0.3 | 21.8 |
| 218 | Zanthoxylum nitidum DC. | Shiny-leaf prickly-ash | root | 15.5 ± 0.4 | 17.7 ± 0.1 | 15.6 | 21 ± 1 | 14.5 ± 0.2 | |
| 223 | Saxifragaceae | Saxifraga stolonifera Meerb. | Creeping saxifrage | aerial part | 11.5 | 11.6 | 11.6 ± 0.4 | 14.6 ± 0.2 | 13.3 ± 0.2 |
| 227 | Solanaceae | Lycium chinense Mill. | Chinese boxthorn | root bark | 19 ± 0.2 | 19.6 ± 0.1 | 22.8 ± 0.1 | 24 ± 1 | 21.4 |
| 230 | Tamaricaceae | Tamarix chinensis Lour. | China tamarisk | branch & leaf | 13.6 ± 0.2 | 14.4 ± 0.1 | 13.7 ± 0.4 | 16.6 ± 0.2 | 19.2 |
| 231 | Thymelaeceae | Daphne genkwa Siebold & Zucc. | Chinese daphne | bud of flower | 22.1 ± 0.1 | 23 ± 1 | 11.4 ± 0.3 | 23.7 ± 0.1 | 25.8 ± 0.4 |
| 239 | Zingberaceae | Curcuma phaeocaulis Valeton | Rhizoma zedoariae | rhizome | 14.1 ± 0.4 | 17.8 ± 0.3 | 16.5 | 22.7 ± 0.4 | 16.8 ± 0.3 |
| Ampicillin | 21.7 ± 0.3 | 18.7 ± 0.5 | 18.5 | 20.2 ± 0.7 | 21.1 ± 0.5 | ||||
| Oxacillin | 15.5 ± 0.6 | 11.9 ± 0.7 | 13.3 ± 0.5 | 13.2 ± 0.4 | 18.2 ± 0.4 | ||||
| DMSO | NIZ | NIZ | NIZ | NIZ | NIZ | ||||
1 74 TCMP extracts (100 mg/mL) with strong inhibitory effects on the growth of reference S. aureus strain ATCC 25923 and antibiotic-resistant S. aureus strain SJTUF 20827 (DIZ ≥ 15 mm, Table S1 in Supplementary Material), were selected to investigate the wide range of antibacterial activities against multidrug-resistant S. aureus strains SJTUF 20745, 20746, 20758, 20978, and 20991. Diameter of inhibition zone (DIZ) was determined by the agar diffusion method in triplicate and value of DIZ was expressed as mean ± standard deviation (SD). DIZ values less than 8.0 mm was defined as “no inhibition zone (NIZ)”. Ampicillin (32 µg/mL) and oxacillin (4 µg/mL) were used as positive controls, while DMSO was used as a negative control.
Figure 2.
Selected TCMP extracts with the wide range of antibacterial activities against multidrug-resistant S. aureus. Number in parentheses indicates the number of TCMP extracts, which exhibited DIZ value in four different ranges of DIZ value; DIZ < 10 mm, 10 ≤ DIZ < 15 mm, 15 ≤ DIZ < 20 mm and DIZ ≥ 20 mm.
However, certain TCMP extracts such as Cnidium monnieri Cusson and Nervilia fordii Schltr. showed poor inhibitory effects against most multidrug-resistant S. aureus strains (Table 1). In total, TCMP extracts of S. tuberculate, Acacia catechu (L.f.) Willd., Coptis chinensis Franch., Quercus infectoria Oliv., Leontice kiangnanensis P.L. Chiu, Rhus chinensis Mill., Rabdosia rubescens (Hemsl.) H. Hara, and Dalbergia odorifera T.C. Chen had the best antibacterial activities against antibiotic-resistant bacteria with DIZ values ranging from 18.2 to 31.9 mm compared with tested conventional antibiotics (DIZ = 11.9–21.7 mm). The strong antibacterial activities of these TCMP have been reported in many studies, which are correlated well with the results obtained in this study. For example, previous study showed that the methanolic extract of A. catechu at a concentration of 100 mg/mL had a strong antibacterial activity against S. aureus, with DIZ value 20 mm [33]. Feng and Xu [34] demonstrated that R. rubescens extract had broad-spectrum inhibitory effects on S. aureus, Symphoricarpos albus and Bacillus subtilis strains with DIZ values in range of 17.5–22.8 mm, 18.2–22.3 mm, and 17.3–25.6 mm, respectively. The antibacterial effects of Q. infectoria gall extract against multidrug-resistant bacteria has also been studied [35]. The ethanolic extracts of Q. infectoria (1 mg/disc) exhibited inhibitory effects against methicillin-resistant S. aureus (MRSA, DIZ = 13.3–15.3 mm), methicillin-resistant coagulase-negative Staphylococcus (MRCoNS, DIZ = 19.3 mm), and multidrug-resistant Acinetobacter spp. (DIZ = 12.7 mm).
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericide Concentration (MBC) of TCMP Extracts against Multidrug-Resistant S. aureus
Next, 18 selected TCMP extracts with the highest DIZ values were subjected to investigations of their MIC and MBC against multidrug-resistant S. aureus strains (Table 2). The MIC values for the extracts ranged from 0.1 to 12.5 mg/mL and the MBC values ranged from 0.78 to 25 mg/mL. Of the TCMP extracts evaluated, multidrug-resistant S. aureus strains were impacted with a high degree of susceptibilities, expressed as MIC, to R. chinensis (0.1–0.195 mg/mL), followed by Q. infectoria (0.195 mg/mL), C. chinensis (0.195–0.39 mg/mL), D. odorifera ( 0.39 mg/mL), Agrimonia pilosa Ledeb. (0.1–0.78 mg/mL), A. catechu (0.195–0.78 mg/mL), Phellodendron chinense C.K. Schneid. (0.195–0.78 mg/mL), Terminallia chebula Retz. (0.39–0.78 mg/mL), Oroxylum indicum Vent. (0.39–1.56 mg/mL), and Spatholobus suberectus Dunn. (0.39–1.56 mg/mL). Conversely, Isatis tinctoria L. showed relatively poor antibacterial activity with the high MIC values (3.125–12.5 mg/mL) and MBC values (12.5–25 mg/mL). Accordant results were previously reported from other studies. In one example, Tian et al. [36] reported that gall extract of R. chinensis was more effective against S. aureus (MIC = 0.25 mg/mL) among tested microorganisms. Moirangthem et al. [37] found that the bark extract from O. indicum exhibited strong antibacterial effects against S. aureus (MIC = 62.5 µg/disc), and also showed a broad antibacterial spectrum against Gram-positive and Gram-negative bacteria with the MIC values ranging from 62.5 to 250 µg/disc. Similar to the results obtained in this study, Tayel et al. [38] reported that the extract of Q. infectoria exhibited strong inhibitory effects against S. aureus with the MIC value for 0.313 mg/mL. Wan et al. [35] also determined the antibacterial activities of Q. infectoria that MIC values of the ethanolic extract against multidrug-resistant bacteria were in the range of 0.03 to 0.63 mg/mL, indicating their strong antibacterial activities. Other TCMP extracts with good antibacterial activities against other bacterial pathogens reported in other studies [39,40,41], are in agreement with the results in Table 2. The observed slight differences in the susceptibility of test bacteria were due to different solvents, extraction methods, antibacterial test methods, harvesting time, and the variation in the proportion of bioactive compounds.
Table 2.
Minimal inhibitory concentration (MIC, mg/mL) and minimal bactericidal concentration (MBC, mg/mL) of selected TCMP extracts against multidrug-resistant S. aureus.
| No. | Scientific Name | S. aureus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATCC 25923 | SJTUF 20745 | SJTUF 20746 | SJTUF 20758 | SJTUF 20827 | SJTUF 20978 | SJTUF 20991 | |||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | (mg/mL) | |||||||||
| 3 | Rhus chinensis Mill. | 0.1 | 3.125 | 0.1 | 3.125 | 0.195 | 3.125 | 0.1 | 0.78 | 0.1 | 0.39 | 0.195 | 3.125 | 0.195 | 1.56 |
| 22 | Ilex rotunda Thunb. | 0.78 | 3.125 | 1.56 | 25 | 1.56 | > 25 | 1.56 | 6.25 | 1.56 | 12.5 | 0.78 | 12.5 | 0.78 | 12.5 |
| 51 | Inula japonica Thunb. | 3.125 | 6.25 | 1.56 | > 25 | 3.125 | 25 | 3.125 | > 25 | 3.125 | 6.25 | 1.56 | 6.25 | 3.125 | 6.25 |
| 58 | Leontice kiangnanensis P.L.Chiu | 0.78 | 1.56 | 0.78 | 25 | 0.78 | > 25 | 0.78 | 3.125 | 6.25 | 25 | 0.78 | 12.5 | 0.78 | 3.125 |
| 61 | Oroxylum indicum Vent. | 0.39 | 1.56 | 0.39 | 6.25 | 0.78 | 25 | 0.78 | 1.56 | 0.39 | 0.78 | 1.56 | 3.125 | 1.56 | 3.125 |
| 65 | Isatis tinctoria L. | 3.125 | 25 | 3.125 | > 25 | 6.25 | > 25 | 6.25 | 12.5 | 12.5 | 12.5 | 6.25 | > 25 | 12.5 | > 25 |
| 81 | Terminalia chebula Retz. | 0.195 | > 25 | 0.78 | > 25 | 0.39 | > 25 | 0.78 | 25 | 0.78 | 25 | 0.78 | 25 | 0.39 | 25 |
| 96 | Speranskia tuberculata Baill. | 0.78 | 25 | 0.78 | > 25 | 0.78 | 12.5 | 0.78 | 12.5 | 0.78 | 12.5 | 1.56 | 12.5 | 0.78 | 12.5 |
| 97 | Acacia catechu (L.f.) Willd. | 0.195 | 3.125 | 0.39 | 6.25 | 0.195 | 6.25 | 0.78 | 0.78 | 0.78 | 1.56 | 0.195 | 0.78 | 0.195 | 1.56 |
| 103 | Dalbergia odorifera T.C.Chen | 0.39 | 3.125 | 0.39 | 12.5 | 0.39 | 6.25 | 0.39 | 3.125 | 0.39 | 6.25 | 0.39 | 0.78 | 0.39 | 6.25 |
| 112 | Quercus infectoria Oliv. | 0.1 | 12.5 | 0.195 | 25 | 0.195 | 6.25 | 0.195 | 12.5 | 0.19 | 1.56 | 0.195 | 1.56 | 0.195 | 12.5 |
| 115 | Spatholobus suberectus Dunn | 0.195 | 3.125 | 1.56 | > 25 | 0.78 | 6.25 | 0.39 | 6.25 | 0.78 | 3.125 | 0.78 | 0.78 | 0.78 | 12.5 |
| 132 | Rabdosia rubescens (Hemsl.) H.Hara | 1.56 | 12.5 | 1.56 | > 25 | 1.56 | 12.5 | 1.56 | 6.25 | 1.56 | 3.125 | 0.78 | 1.56 | 1.56 | 12.5 |
| 133 | Salvia miltiorrhiza Bunge | 0.39 | 3.125 | 1.56 | 25 | 3.125 | 12.5 | 1.56 | 6.25 | 0.78 | 3.125 | 3.125 | 6.25 | 3.125 | 6.25 |
| 168 | Fraxinus fallax Lingelsh. | 1.56 | > 25 | 1.56 | > 25 | 1.56 | 25 | 3.125 | 12.5 | 1.56 | 12.5 | 3.125 | 12.5 | 3.125 | 25 |
| 200 | Coptis chinensis Franch. | 0.39 | 1.56 | 0.195 | 25 | 0.39 | 12.5 | 0.39 | 1.25 | 0.39 | 0.78 | 0.195 | 1.56 | 0.195 | 0.78 |
| 202 | Agrimonia pilosa Ledeb. | 0.1 | 1.56 | 0.1 | 6.25 | 0.195 | 1.56 | 0.78 | 3.125 | 0.39 | 1.56 | 0.195 | 6.25 | 0.1 | 1.56 |
| 217 | Phellodendron chinense C.K. Schneid. | 0.78 | 3.125 | 0.78 | 12.5 | 0.39 | 1.56 | 0.78 | 1.56 | 0.78 | 0.78 | 0.195 | 3.125 | 0.39 | 1.56 |
| Ampicillin (μg/mL) | 0.05 | >25 | 6.25 | > 25 | >25 | NA 1 | >25 | NA | >25 | NA | 3.125 | >25 | 0.1 | >25 | |
| Oxacillin (μg/mL) | 0.195 | > 25 | 0.39 | > 25 | 0.39 | > 25 | 0.39 | 25 | 0.195 | 12.5 | 0.195 | > 25 | 0.195 | 25 | |
18 selected TCMP extracts with the highest DIZ values (DIZ ≥ 20 mm) were subjected to investigations of their MIC and MBC against one reference (ATCC 25923), one erythromycin-resistant (SJTUF 20827) and five multidrug-resistant (SJTUF 20745, SJTUF 20746, SJTUF 20758, SJTUF 20978 and SJTUF 20991) S. aureus strains (n = 3). Ampicillin and Oxacillin were used as positive controls. 1 NA, not applicable.
In general, this study selected out some TCMP with strong and extensive growth inhibitory effects on multidrug-resistant S. aureus, including R. chinensis, Q. infectoria, C. chinensis, D. odorifera, A. pilosa, A. catechu, P. chinense, T. chebula, O. indicum, S. suberectus, S. tuberculate, Inula japonica Thunb., R. rubescens, and Salvia miltiorrhiza Bunge. Additionally, to the best of our knowledge, our study is the first to report the promising antimicrobials of plant extracts, including I. rotunda, I. japonica, L. kiangnanensis, S. tuberculate and F. fallax, all of which had strong and broad-range of antibacterial activities against multidurg- resistant S. aureus.
2.4. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) of TCMP Extracts
Plant species naturally produce various secondary metabolites leading to possession of valuable biological activities including antibacterial activity [20]. Selecting a potential antibacterial agent from plant-derived compounds might be an appropriate strategy since these substances are produced by the outcome of plant defense responses against biotic stress from bacterial, fungi, viruses, and abiotic stress, and commonly have antibacterial activities [18]. Major plant-derived compounds that are responsible for antibacterial activity include phenolics, phenolic acids, flavonoids, quinones, tannins, coumarins, terpenoids, and alkaloids [16]. The structural diversity and various chemical compositions of antibacterial compounds result in strong and broad-range of antibacterial activities with diverse mechanisms of antibacterial action [15].
Multiple studies have reported that phenolic compounds (phenolic acids, flavonoids, quinones, coumarins, lignans, stilbenes, and tannins) presented in TCMP contribute to good antibacterial properties against bacterial pathogens [42,43,44]. Polyphenolic compounds such as flavan-3-ols, flavonols, tannins, and phenolic acids are known to exhibit wide spectra and strong antimicrobial activity compared with other polyphenols [45]. The phenolic compounds are also able to suppress microbial virulence factors such as quorum sensing, bacterial biofilms, bacterial motility, bacterial toxins, and bacterial surfactant [16]. For example, Wang et al. [46] reported that vesticarpan derived from D. odorifera as the phenolic compound showed antibacterial activity against Ralstonia solanacearum. Four flavonoids isolated from D. odorifera, including sativanone, (3R)-vestitone, liquiritigenin and isoliquiritigenin, exhibited strong antibacterial activity against R. solanacearum with the DIZ values ranging from 11.2 to 16.6 mm [47]. Sithisarn et al. [48] demonstrated that flavones such as baicalein, baicalin, and chrysin in O. indicum are active compounds for antibacterial activities against Staphylococcus intermedius, Streptococcus suis, Pseudomonas aeruginosa and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli. Cho et al. [49] also found active flavonoids like 7-hydroxy-6-methoxy-flavanone and formononetin isolated from S. suberectus that inhibited S. aureus derived sortase A, which is responsible for anchoring surface protein virulence factors.
Therefore, we further determined the phenolic content in 18 TCMP extracts with strong antibacterial effect to demonstrate whether their major antibacterial compounds are attributed to polyphenols. Total phenolic content (TPC) and total flavonoid content (TFC) were determined by the Folin-Ciocalteu method and AlCl3-based colorimetric method, respectively. The TPC in the extracts were in the range of 31.4 to 646 mg gallic acid equivalent (GAE)/g dry weight (DW), whereas TFC were in the range of 5.27 to 377 mg catechin equivalent (CE)/g DW (Table 3). Consistent with previous studies, strong antibacterial TCMP extracts such as R. chinensis, Q. infectoria, A. Pilosa, A. catechu and S. suberectus contained high amounts of polyphenols, with TPC 632, 646, 371, 545, and 489 mg GAE/g DW, respectively, leading to a suspicion that polyphenols might be the actual active compounds responsible for antibacterial activity against S. aureus. A case in point being the gall extract of Q. infectoria which presented the highest polyphenol level (646 mg GAE/g DW), but it presented a relatively low flavonoids level (38 mg CE/g DW), indicating that major phenolic components in Q. infectoria might be consisted of non-flavonoids such as phenolic acids, stilbenes and lignans. Arina and Harisun [50] found that Q. infectoria gall mainly contained polyphenols with a high concentration of tannic acid (2233 mg/g). Tannins, well-known as antibacterial agents, have been shown to inhibit the growth of various pathogens by destroying their bacterial plasma membrane and forming hydrogen bonds between tannins and the proteins in bacterial cells, resulting in the protein denaturation and their coagulation [51]. Conversely, it was also found that some other strong antibacterial TCMP extracts like C. chinensis, S. tuberculate, and S. miltiorrhiza included relatively low TPC (95, 31.4 and 56 mg GAE/g DW, respectively) and TFC (23.1, 13.4 and 34.5 mg CE/g DW, respectively), suggesting that their major antibacterial substances might be nonphenolic antibacterial compounds.
Table 3.
Total phenolic content (TPC) and the total flavonoid content (TFC) in selected TCMP extracts.
| No. | Scientific Name | TPC (mg GAE/g DW) | TFC (mg CE/g DW) |
|---|---|---|---|
| 3 | Rhus chinensis Mill. | 632 ± 4 a | 36.8 ± 0.9 fg |
| 22 | Ilex rotunda Thunb. | 143 ± 3 fg | 16.9 ± 0.2 ijkl |
| 51 | Inula japonica Thunb. | 92 ± 6 i | 62 ± 3 e |
| 58 | Leontice kiangnanensis P. L. Chiu | 33 ± 1 k | 5.27 ± 0.08 l |
| 61 | Oroxylum indicum Vent. | 158 ± 3 f | 18.1 ± 0.6 ijk |
| 65 | Isatis tinctoria L. | 60 ± 3 j | 8.35 ± 0.2 kl |
| 81 | Terminalia chebula Retz. | 553 ± 4 b | 27 ± 0.8 ghi |
| 96 | Speranskia tuberculata Baill. | 31.4 ± 0.6 k | 13.4 ± 0.5 jkl |
| 97 | Acacia catechu (L.f.) Willd. | 545 ± 2 b | 377 ± 3 a |
| 103 | Dalbergia odorifera T. C. Chen | 215 ± 6 e | 62 ± 1 e |
| 112 | Quercus infectoria Oliv. | 646 ± 3 a | 38 ± 3 fg |
| 115 | Spatholobus suberectus Dunn | 489 ± 5 c | 214 ± 11 b |
| 132 | Rabdosia rubescens (Hemsl.) H. Hara | 135 ± 4 gh | 41 ± 1 f |
| 133 | Salvia miltiorrhiza Bunge | 56 ± 5 j | 34.5 ± 0.9 fgh |
| 168 | Fraxinus fallax Lingelsh. | 363 ± 15 d | 106 ± 5 d |
| 200 | Coptis chinensis Franch. | 95 ± 3 i | 23.1 ± 0.3 hij |
| 202 | Agrimonia pilosa Ledeb. | 371 ± 11 d | 154 ± 10 c |
| 217 | Phellodendron chinense C. K. Schneid. | 123 ± 2 h | 35 ± 2 fgh |
The experiments were performed in triplicate and the results were expressed as mean ± SD. One–way analysis of variance (ANOVA) plus post hoc Tukey test was performed and different superscript lowercase letters (a–l) indicated statistically significant difference (p < 0.05). GAE, gallic acid equivalent; CE, catechin equivalent; DW, dry weight.
Although the spectrophotometric methods such as Folin-Ciocalteu method and AlCl3-based colorimetric method are commonly used to quantify total phenolics in plant extracts [52,53,54,55,56,57], these analyses are not accurate measurements. The Folin-Ciocalteu assay can contribute to the overestimation of TPC due to the presence of reducing compounds such as ascorbic acid, reducing sugars, and aromatic amino acids (tyrosine and tryptophan), leading to the disruption of phenolic oxidation reaction [58,59,60,61]. Similar to the limitation for TPC, the AlCl3-based method for TFC has a constraint on the measurement of all classes of flavonoids in the extracts and the considerable contents of total flavonoid can be attributed to the presence of phenolic acids in the extract during the absorbance measurement at 510 nm [62]. Therefore, more accurate and precise analyses such as chromatographic methods would be necessary to conduct for qualification of total phenolic compounds in the TCMP extracts. In this present study, these spectrophotometric methods were used for simple comparison among the selected TCMP extracts. However, further research would be required using more accurate and reliable analytical methods such as chromatographic assays in order to investigate phytochemical constituents and to identify major antimicrobial compounds in each extract.
2.5. Cytotoxicity and Safety of the TCMP Extracts
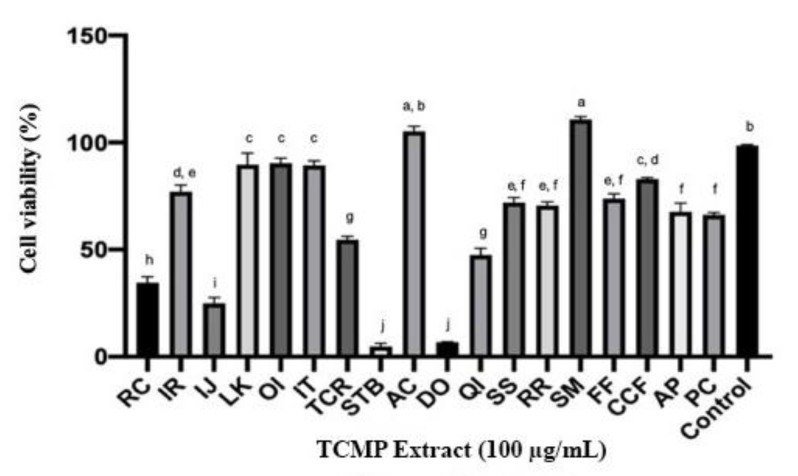
In order to utilize the crude TCMP extracts as antimicrobial agents in pharmaceuticals, food, and animal feed industries, it is essential to ensure their safety. The 18 TCMP extracts with good antibacterial effect were evaluated for their cytotoxicity in normal human foreskin fibroblast (HFF) cells. Cell viability varies with the extracts when the cells were exposed at the concentration of 100 μg/mL for 24 h (Figure 3). S. tuberculate extract had a considerably low level of cell viability with 4.83%, followed by D. odorifera (7.14%), I. japonica (24.9%), R. chinensis (34.5%), and Q. infectoria (47.5%). Additionally, in order to investigate the cytotoxic effects of the TCMP extracts, dose-response experiments were conducted (Figure S1 in Supplementary Material) and the median lethal concentration (LC50) values were calculated (Table 4). The cytotoxicity of plant extracts with LC50 value ≤ 20 μg/mL was regarded as a possible cytotoxic plant extract [63]. The results indicated that most plant extracts had low cytotoxicities with considerably high LC50 value ≥ 100 µg/mL, excluding S. tuberculate (LC50 = 25.9 µg/mL), D. odorifera (LC50 = 44.1 µg/mL), I. japonica (LC50 = 54.1 µg/mL), R. chinensis (LC50 = 77.6 µg/mL), and Q. infectoria (LC50 = 91.6 µg/mL), supporting by previous studies that also found a weak cytotoxicity of T. chebula [64,65], S. suberectus [66], Q. infectoria [67,68] and O. indicum [37]. However, it is necessary to test the toxicity by in vivo studies, since in vitro cellular toxicity might provoke different consequences in animals associated with gut interactions and bioavailability of the extracts [18].
Figure 3.
The viability of HFF cells exposed to selected TCMP extracts (100 µg/mL) for 24 h assessed by the colorimetric assay using MTT. RC = R. chinensis; IR = I. rotunda; IJ = I. japonica; LK = L. kiangnanensis; OI = O. indicum; IT = I. tinctorial; TCR = T. chebula; STB = S. tuberculate; AC = A. catechu; DO = D. odorifera; QI = Q. infectoria; SS = S. suberectus; RR = R. rubescens; SM = S. miltiorrhiza; FF = F. fallax; CCF = C. chinensis; AP = A. Pilosa; PC = P. chinense; Control = 0.1% DMSO. Results are expressed as mean ± standard deviation (SD) and the experiments were conducted in triplicate (p < 0.05).
Table 4.
Cytotoxicity (LC50) on the human foreskin fibroblast (HFF) cells and selectivity index (SI) of selected TCMP extracts. SI value greater than 1 indicate that the extract is more toxic to the pathogen than to human cells, suggesting possible safety.
| Scientific Name | Cytotoxicity (LC50, µg/mL) |
Selectivity Index (SI = LC50/MIC) | |
|---|---|---|---|
| 3 | Rhus chinensis Mill. | 77.6 | 0.77 |
| 22 | Ilex rotunda Thunb. | >100 | 554 |
| 51 | Inula japonica Thunb. | 54.1 | 0.02 |
| 58 | Leontice kiangnanensis P. L. Chiu | >100 | 2687 |
| 61 | Oroxylum indicum Vent. | >100 | NA |
| 65 | Isatis tinctoria L. | >100 | 76.0 |
| 81 | Terminalia chebula Retz. | >100 | 2.20 |
| 96 | Speranskia tuberculata Baill. | 25.9 | 0.03 |
| 97 | Acacia catechu (L.f.) Willd. | NA 1 | NA |
| 103 | Dalbergia odorifera T. C. Chen | 44.1 | 0.11 |
| 112 | Quercus infectoria Oliv. | 91.6 | 0.47 |
| 115 | Spatholobus suberectus Dunn | >100 | 138 |
| 132 | Rabdosia rubescens (Hemsl.) H. Hara | >100 | 158 |
| 133 | Salvia miltiorrhiza Bunge | NA | NA |
| 168 | Fraxinus fallax Lingelsh. | >100 | 5.55 |
| 200 | Coptis chinensis Franch. | >100 | 86.1 |
| 202 | Agrimonia pilosa Ledeb. | >100 | 204 |
| 217 | Phellodendron chinense C. K. Schneid. | >100 | 1.85 |
1 NA, not applicable.
To verify the safety of plant extracts, selectivity index (SI) of plant extracts was also determined by LC50 dividing by MIC value (Table 4). The SI value greater than 1 indicates that a plant extract is more toxic to the pathogen than the host cell [63]. In other words, the plant is safe and possible to be developed as antibacterial agents [13]. I. japonica extract had the lowest SI value of 0.02 against S. aureus, followed by S. tuberculate (0.03), D. odorifera (0.11), Q. infectoria (0.47), and R. chinensis (0.77). Besides the five extracts, most other plant extracts had strong inhibitory effects on S. aureus, but were less cytotoxic to human cells, suggesting that these plants could be developed as herbal medicine, food additive or preservative in the future.
2.6. Correlations Analysis among Polyphenolic Content, Antibacterial Effect, and Cytotoxicity of TCMP Extracts Cytotoxicity and Safety of the TCMP Extracts
The presence of polyphenolic compounds in the TCMP extracts can be related to strong antibacterial activity against multidrug-resistant S. aureus. In previous study, Shan et al. [42] demonstrated good linear correlations of phenolic content with the antibacterial activity of medicinal herbs against foodborne bacteria including S. aureus (r2 = 0.93). Pavić et al. [52] also reported that TPC in a medicinal plant, namely Ruta graveolen L., was strongly correlated to the MIC values of E. coli, B. subtilis, and S. aureus (r = 0.973, p < 0.050). These results suggest that polyphenols might contribute to antibacterial properties of plant extracts. The antibacterial activity of polyphenols can involve various mechanisms of action such as disrupting the cell integrity, destroying membrane proteins, increasing permeability of cell membrane, inhibiting biofilm formation, inactivating microbial enzymes, up/down-regulating proteins involved in DNA and RNA synthesis, and deprivation of metal iron by their chelating ability [69,70]. For instance, some phenolics such as chlorogenic acid, tannic acid, and caffeic acid inhibited on the bacterial growths due to hyperacidification at the plasma membrane interface, resulting in disrupting H+-ATPase pump and thereby causing cell death [53].
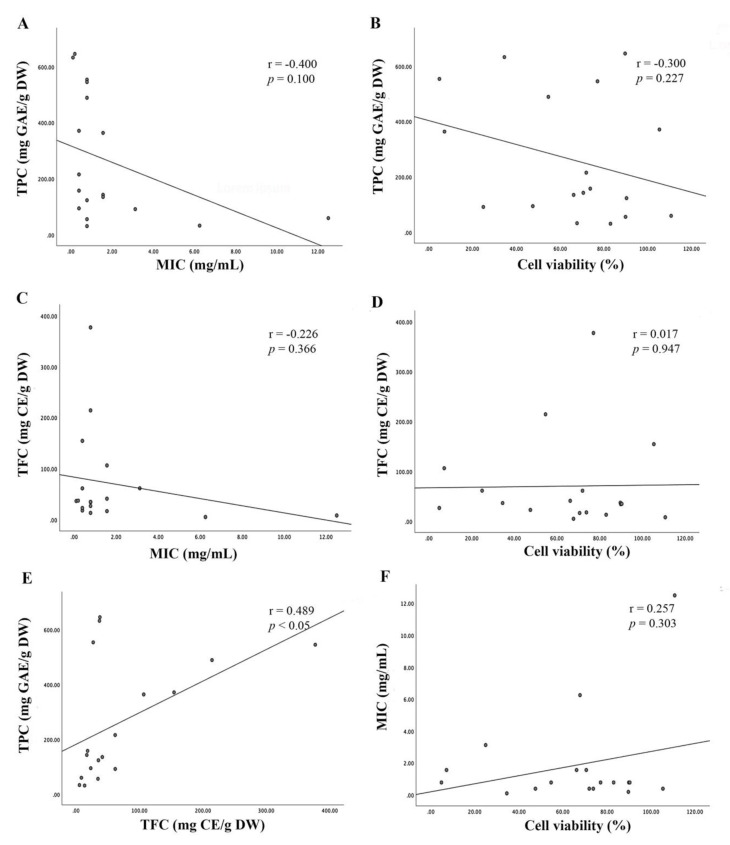
In order to understand the interrelationships between the antibacterial activity of TCMP extracts and polyphenolic contents, 18 TCMP extracts with good effects were used in an analysis of correlations among MIC, TPC, and TFC values (Figure 4). The results with high p values (p > 0.05) are interpreted as without statistical significance that might be confounded by the small sample size (n = 18) in this study because of p value’s dependence on the sample size [71,72]. Unlike p value, the effect size is independent of sample size [71]. In this study, Pearson’s correlation coefficient (r) was introduced as an effect size index to quantify difference among variables and to understand the correlations of antibacterial activity with phenolic contents. A moderate negative correlation was obtained between TPC and MIC with Pearson’s correlation coefficient r = −0.400 (p = 0.100), and a very weak correlation was also shown between TFC and MIC with r = −0.226 (p = 0.366), in agreement of the results obtained by a previous study [54], showing moderate correlations of antibacterial activity with phenolic contents in spice extracts (r = 0.541, p < 0.001).
Figure 4.
Pearson correlation analysis. (A) Correlation of total phenolic content (TPC) with minimum inhibitory concentration (MIC). (B) Correlation of TPC with cell viability (%) at 100 µg/mL. (C) Correlation of total flavonoid content (TFC) with MIC. (D) Correlation of TFC with cell viability (%). (E) Correlation of TPC with TFC. (F) Correlation of MIC with cell viability (%).
The r values were low and moderate based on the standards of effect size, which are classified as small (r = ± 0.2), medium (r = ± 0.3), and large (r = ± 0.5) [71]. The effect size can be influenced by factors such as amount of variability, linear relationship among two variables, presence of outlier and measurement errors [73]. The relationships between phenolic contents and antibacterial activities showed the inclination to nonlinearity, leading to the low values of Pearson’s correlation (Figure 4). In addition, the narrow range of MIC values (0.01–12.5 mg/mL) compared to TPC (31.4–646 mg GAE/g DW) and TFC (5.27–214 mg CE/g DW) might be attributed to low r values. This tendency for the weak correlations between phenolic contents and antibacterial activity might be suspected to the high levels of TPC, resulting from overestimated TPC values due to the presence of reducing compounds in the TCMP extracts [58,59,60,61]. Therefore, the phytochemical profiles of TCMP extracts need to be investigated by accurate and precise analyses in the future to reliably comprehend the correlations of antibacterial activity with phenolic compounds in the extracts. In other respects, the results may be also suggested that antibacterial activity of TCMP extracts is attributed to nonphenolic substances, especially the extracts of C. chinensis, S. tuberculate, and S. miltiorrhiza, which had relatively low polyphenols contents (Table 3). Indeed, Lee et al. [74] discovered that a terpenoid compound, namely dihydrotanshinone I, isolated from the roots of S. miltiorrhiza was the strong antibacterial compound against the broad range of gram-positive bacteria such as B. subtilis with a low MIC value of 3.1 µg/mL.
For the purpose of evaluating possible antibacterial compounds in TCMP extracts with their safety for use, we also analyzed the correlations of antibacterial activity with cell viability (%), showing the weak positive correlation with r = 0.257 (p = 0.303) (Figure 4). The results indicate that cytotoxic compounds might be discordant with antibacterial compounds [13,63]. Indeed, Dzoyem et al. [75] found that antibacterial compounds such as ursolic acid, quercitrin, and entadanin from Entada abyssinica were relatively low cytotoxic on Vero monkey kidney cells. Two flavonoids isolated from Pappea capensis, quercetin-3-O-rhamnoside and epicatechin, exhibited a wide range of antibacterial activity against B. subtilis, S. aureus, E. coli and Klebsiella pneumoniae and showed considerably low cytotoxic on Vero monkey kidney cells with LC50 values > 200 μg/mL [76]. Therefore, there still remain the possibilities to identify strong antibacterial compound(s) from S. tuberculate, I. japonica, D. odorifera and Q. infectoria that were relatively more toxic to the pathogen than the host cells (Table 4).
3. Materials and Methods
3.1. Chemicals and Reagents
Dimethyl sulfoxide (DMSO) and 3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Beyotime (Shanghai, China). Ethanol was purchased from Titan Chem. (Shanghai, China). Luria Bertani (LB) broth, agar bacteriological, and Mueller–Hinton broth were from Oxiod (Basingstoke, UK). Ampicillin and oxacillin were purchased from Meilune (Dalian, China). Sodium chloride, sodium nitrite, sodium hydroxide, potassium chloride, disodium phosphate and monopotassium phosphate were purchased from General-Reagent® (Shanghai, China). Aluminum chloride was purchased from Sinopharm Chemical Reagent (Shanghai, China). Sodium carbonate was from J&K Scientific (Beijing, China). Resazurin was purchased from Adamas (Shanghai, China). Gallic acid was from 87 Energy Chemical (Shanghai, China). Folin-Ciocalteu reagent was from Macklin (Shanghai, China). Deionized water was used in all experiments.
3.2. Collection of Plant Samples
The 239 dried TCMP (89 families, 206 genera, and 233 species) were collected from the markets in Shanghai, China. The TCMP samples presented in Table 1 and Table S1 in the Supplementary Material were sorted in term of family, scientific name, and common name identified from GBIF (http://www.gbif.org/) and Tropicos® (http://www.tropicos.org/).
3.3. Preparation of Plant Extracts
The dried TCMP samples were pulverized by a lab-scale miller (S025, IKA, Staufen, Germany). In this study, ethanol was used as an extraction solvent due to its safety and good extraction activity to obtain the desired antimicrobial components from the plant materials [77]. 4.0 g dried powder of each TCMP was extracted two times with 40 mL of 80% (v/v) ethanol using an ultrasound-assisted extraction method (1 h, 40 °C, and 480 W) to improve extraction efficiency and the yield of antibacterial compounds in the plants [55,78,79]. Each extract was centrifuged at room temperature (900× g, 15 min), and the supernatants were collected, combined, and concentrated using a rotary evaporator (RE-52AA, Shanghai Ya Rong Co., Ltd, Shanghai, China) at 40 °C under vacuum. The concentrated extract was dried by a vacuum freeze-dryer (SJIA-5FE, Ningbo Shuang Jia instrument Co., Ltd, Ningbo, China). The freeze-dried extract was dissolved in dimethyl sulfoxide (DMSO) at 100 mg/mL and was stored at −20 °C for further use.
3.4. Microorganisms and Culture Samples
One reference strain of S. aureus ATCC 25923 and six antibiotic-resistant S. aureus strains were used in this study. The list of six S. aureus consisted of five multidrug-resistant strains (SJTUF 20745, SJTUF 20746, SJTUF 20758, SJTUF 20978, and SJTUF 20991), and one erythromycin-resistant strain (SJTUF 20827), verified in our previos study [54], is shown in Table 5. S. aureus ATCC 25923 was used as a reference strain. Single colonies of the bacteria growing on Luria Bertani (LB) agar plate were inoculated in LB culture medium and grown overnight in a shaking incubator at 37 °C and 250 rpm. The bacterial suspension was used at a concentration of 1 × 106 colony-forming units (CFU)/mL for the following antibacterial experiments.
Table 5.
List of antibiotic-resistant S. aureus strains used in this study and their antibiotic resistance profiles [54].
| S. aureus Strain Name | Antibiotic Resistance Profile |
|---|---|
| SJTUF 20745 | Streptomycin, ciprofloxacin, clindamycin, erythromycin |
| SJTUF 20746 | Gentamicin, ciprofloxacin, clindamycin, erythromycin |
| SJTUF 20758 | Penicillin, streptomycin, clindamycin, erythromycin |
| SJTUF 20827 | Erythromycin |
| SJTUF 20978 | Ciprofloxacin, erythromycin, sulfisoxazole |
| SJTUF 20991 | Ciprofloxacin, clindamycin, erythromycin, tetracycline |
3.5. Determination of Antibacterial Activity
3.5.1. Measurement of Diameter of Inhibition Zone (DIZ)
The antibacterial activity of 239 TCMP extracts (100 mg/mL) against S. aureus ATCC 25923 and SJTUF 20827 was evaluated based on the DIZ determined by agar diffusion methods as described by Chan et al. [22] with some modifications. Ampicillin (32 μg/mL) and oxacillin (4 μg/mL) were used as the positive controls, and DMSO (60 μL/cup) were used as the negative controls. The DIZ values less than or equal to 8.0 mm was regarded as no antibacterial activity. In order to test the wide-spectrum antibacterial effects on multidrug-resistant S. aureus, 74 TCMP extracts with DIZ ≥ 15 mm from the screening results were further investigated their DIZ against another five multidrug-resistant S. aureus strains (SJTUF 20745, SJTUF 20746, SJTUF 20758, SJTUF 20978, and SJTUF 20991) as the procedure above. The assay was conducted in triplicate in two independent experiments.
3.5.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericide Concentration (MBC)
TCMP samples with DIZ ≥ 20 mm were used to determine their MIC and MBC against S. aureus strains in triplicate. The MIC and MBC were determined by a serial dilution microplate method and Mueller–Hinton (MH) agar counting, respectively [22,80]. Ampicillin and oxacillin were used as the positive controls. This assay was performed in triplicate in three independent experiments.
3.6. Determination of the Total Phenolic and Flavonoid Content
Total phenolic content (TPC) was determined using a Folin-Ciocalteu method, as previously described by Blainski et al. [81]. To quantify TPC, a linear calibration curve of gallic acid was established as a standard (). TPC was calculated using the linear equation and expressed as mg gallic acid equivalent (mg GAE)/g dry weight (DW) of the sample. Total flavonoids content (TFC) was quantified using an AlCl3-based colorimetric method [56]. Catechin was used as a standard to establish a linear calibration curve with function (). The assay was performed in triplicate in two independent experiments. TFC was calculated using the linear equation and expressed as mg catechin equivalent (mg CE)/g DW of sample.
3.7. In vitro Cytotoxicity Assay
The cytotoxicity of 18 TCMP ethanolic extracts on human foreskin fibroblast (HFF) cells, which are human normal cells, was determined by the colorimetric assay using 3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as described by Senthilraja and Kathiresan [82] with slight modification. Briefly, cells/mL were cultured with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum into each well of 96-well microplates and incubated at 37 °C in a humidified 5% CO2 incubator overnight. The cells were treated with various concentrations (ranging from 1.56 to 100 µg/mL) of TCMP extracts and incubated at 37 °C for 24 h, whereas the untreated cells were used as control. Next, cells in each well were treated with 100 µL MTT (5 mg/mL, in PBS) and were maintained for 3 h at 37 °C in the dark. After removal of MTT solution, 100 µL DMSO was added to dissolve insoluble formazan crystal. The absorbance was measured at 570 nm by a microplate reader (SpectraMax iD3, Molecular Devices, Silicon Valley, NC, USA). This assay was conducted in triplicate in four independent experiments. The percentage of cell viability was calculated based on the following equation:
| (1) |
The median lethal concentration (LC50) was calculated according to the log dose-response curve of cytotoxicity against concentration and was defined as a 50% reduction of cell viability compared with the control. The selectivity index (SI) value was calculated based on the equation below [63]:
| (2) |
3.8. Statistical Analysis
All assays were conducted in triplicate and all experimental results were expressed as mean ± standard deviation (SD). Statistical analysis was performed using Microsoft Excel 2018 (Microsoft, Seattle, WA, USA) and SPSS 22.0 (IBM SPSS Statistics, IBM Corp, Somers, NY, USA). The cytotoxicity data (cell viability and LC50) were analyzed by GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Statistical significance was defined at p-value less than 0.05.
4. Conclusions
This study conducted the large-scale screening of 239 TCMP extracts to discover strong antibacterial activities against multidrug-resistant S. aureus. This study selected out several TCMP extracts with promising antibacterial activity as well as low cytotoxicity, including R. chinensis, I. rotunda, L. kiangnanensis, O. indicum, I. tinctoria, T. chebula, A. catechu, S. suberectus, R. rubescens, S. miltiorrhiza, F. fallax, C. chinensis, A. pilosa, and P. chinense. The results of plant extracts, I. rotunda, I. japonica, L. kiangnanensis, S. tuberculate and F. fallax, were first reported about their antibacterial effects on S. aureus in the present study. Further study would be required to investigate phytochemical profiles in TCMP extracts by more sensitive analytical methods. It would be necessary to identify their antibacterial active compounds, potential mechanisms of antibacterial action, and in vivo toxicity prior to applying them as new antibacterial agents in pharmaceutical, food, and animal feed industries.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-0817/9/3/185/s1, Table S1: Screening 239 TCMP extracts for antibacterial activities against reference S. aureus ATCC 25923 and antibiotic-resistant S. aureus SJTUF 20827 based on diameter inhibition zone (DIZ, mm), Figure S1: Dose-response curves for the viability of HFF cells exposed to selected TCMP extracts.
Author Contributions
Conceptualization, R.-Y.G. and H.C.; methodology, G.K. and R.-Y.G.; validation, G.K.; formal analysis, G.K.; investigation, G.K., D.Z., and A.K.F.; data curation, G.K.; writing—original draft preparation, G.K. and R.-Y.G.; writing—review and editing, R.-Y.G., O.H., V.M., H.-B.L., X.-H.W., and H.C.; supervision, R.-Y.G. and H.C. All authors have read and agree to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2017YFC1600100), China Central Public-interest Scientific Institution Basal Research Fund (Y2020XK05), the Shanghai Pujiang Talent Plan (18PJ1404600), and the Agri-X Interdisciplinary Fund of Shanghai Jiao Tong University (Agri-X2017004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Akarca G. Composition and antibacterial effect on food borne pathogens of Hibiscus surrattensis L. calyces essential oil. Ind. Crops Prod. 2019;137:285–289. doi: 10.1016/j.indcrop.2019.05.043. [DOI] [Google Scholar]
- 2.Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Dopfer D., Fazil A., Fischer-Wallker C.L., Hald T., et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Tao X., Xia X., Yang B., Xi M., Meng J., Zhang J., Xu B. Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in retail raw chicken in China. Food Control. 2013;29:103–106. doi: 10.1016/j.foodcont.2012.06.002. [DOI] [Google Scholar]
- 4.Miao J., Liang Y., Chen L., Wang W., Wang J., Li B., Li L., Chen D., Xu Z. Formation and development of Staphylococcus biofilm: With focus on food safety. J. Food Saf. 2017;37:e12358. doi: 10.1111/jfs.12358. [DOI] [Google Scholar]
- 5.Fratianni F., Nazzaro F., Marandino A., Fusco M.D.R., Coppola R., De Feo V., De Martino L. Biochemical composition, antimicrobial activities, and anti–quorum-sensing activities of ethanol and ethyl acetate extracts from Hypericum connatum Lam. (Guttiferae) J. Med. Food. 2013;16:454–459. doi: 10.1089/jmf.2012.0197. [DOI] [PubMed] [Google Scholar]
- 6.Noumi E., Snoussi M., Merghni A., Nazzaro F., Quindós G., Akdamar G., Mastouri M., Al-Sieni A., Ceylan O. Phytochemical composition, anti-biofilm and anti-quorum sensing potential of fruit, stem and leaves of Salvadora persica L. methanolic extracts. Microb. Pathogen. 2017;109:169–176. doi: 10.1016/j.micpath.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Foster T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017;41:430–449. doi: 10.1093/femsre/fux007. [DOI] [PubMed] [Google Scholar]
- 8.Yadav A.K., Sirohi P., Saraswat S., Rani M., Singh M.P., Srivastava S., Singh N.K. Inhibitory mechanism on combination of phytic acid with methanolic seed extract of Syzygium cumini and sodium chloride over Bacillus subtilis. Curr. Microbiol. 2018;75:849–856. doi: 10.1007/s00284-018-1457-5. [DOI] [PubMed] [Google Scholar]
- 9.Frieri M., Kumar K., Boutin A. Antibiotic resistance. J. Infect. Public Health. 2017;10:369–378. doi: 10.1016/j.jiph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 11.Packiavathy I.A.S.V., Agilandeswari P., Musthafa K.S., Pandian S.K., Ravi A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012;45:85–92. doi: 10.1016/j.foodres.2011.10.022. [DOI] [Google Scholar]
- 12.Ahmed S., Liu H., Ahmad A., Akram W., Abdelrahman E.K., Ran F., Ou W., Dong S., Cai Q., Zhang Q., et al. Characterization of anti-bacterial compounds from the seed coat of Chinese windmill palm tree (Trachycarpus fortunei) Front. Microbiol. 2017;8:1894. doi: 10.3389/fmicb.2017.01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elisha I.L., Botha F.S., McGaw L.J., Eloff J.N. The antibacterial activity of extracts of nine plant species with good activity against Escherichia coli against five other bacteria and cytotoxicity of extracts. BMC Complement. Altern. Med. 2017;17:133. doi: 10.1186/s12906-017-1645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis K., Ausubel F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006;24:1504. doi: 10.1038/nbt1206-1504. [DOI] [PubMed] [Google Scholar]
- 15.Gyawali R., Ibrahim S.A. Natural products as antimicrobial agents. Food Control. 2014;46:412–429. doi: 10.1016/j.foodcont.2014.05.047. [DOI] [Google Scholar]
- 16.Silva L.N., Zimmer K.R., Macedo A.J., Trentin D.S. Plant natural products targeting bacterial virulence factors. ACS Chem. Rev. 2016;116:9162–9236. doi: 10.1021/acs.chemrev.6b00184. [DOI] [PubMed] [Google Scholar]
- 17.Carranza M.G., Sevigny M.B., Banerjee D., Fox-Cubley L. Antibacterial activity of native California medicinal plant extracts isolated from Rhamnus californica and Umbellularia californica. Ann. Clin. Microbiol. Antimicrob. 2015;14:29. doi: 10.1186/s12941-015-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma D.S., Tan L.T.H., Chan K.G., Yap W.H., Pusparajah P., Chuah L.H., Ming L.C., Khan T.M., Lee L.H., Goh B.H. Resveratrol—Potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018;9:102. doi: 10.3389/fphar.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R.S. In: Some Bioactive Natural Products from Chinese Medicinal Plants: Studies in Natural Products Chemistry. 1st ed. Atta-ur-Rahman, editor. Volume 21. Elsevier; Amsterdam, The Netherland: 2000. pp. 729–772. [DOI] [Google Scholar]
- 20.Yang L., Yang C., Li C., Zhao Q., Liu L., Fang X., Chen X.Y. Recent advances in biosynthesis of bioactive compounds in traditional Chinese medicinal plants. Sci. Bull. 2016;61:3–17. doi: 10.1007/s11434-015-0929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal Y., Liang Z., Zhao Z. Botanical drugs in Ayurveda and traditional Chinese medicine. J. Ethnopharmacol. 2016;194:245–259. doi: 10.1016/j.jep.2016.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Chan C.L., Gan R.Y., Shah N.P., Corke H. Polyphenols from selected dietary spices and medicinal herbs differentially affect common food-borne pathogenic bacteria and lactic acid bacteria. Food Control. 2018;92:437–443. doi: 10.1016/j.foodcont.2018.05.032. [DOI] [Google Scholar]
- 23.Dessen A., Di Guilmi A.M., Vernet T., Dideberg O. Molecular mechanisms of antibiotic resistance in Gram-positive pathogens. Curr. Drug Targets Infect. Disord. 2001;1:63–77. doi: 10.2174/1568005013343272. [DOI] [PubMed] [Google Scholar]
- 24.Hancock R.E. Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect. Dis. 2005;5:209–218. doi: 10.1016/S1473-3099(05)70051-7. [DOI] [PubMed] [Google Scholar]
- 25.Seow Y.X., Yeo C.R., Chung H.L., Yuk H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014;54:625–644. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira D.M., Melo F.G., Balogun S.O., Flach A., de Souza E.C.A., de Souza G.P., Ascêncio S.D. Antibacterial mode of action of the hydroethanolic extract of Leonotis nepetifolia (L.) R. Br. involves bacterial membrane perturbations. J. Ethnopharmacol. 2015;172:356–363. doi: 10.1016/j.jep.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Abreu A.C., McBain A.J., Simoes M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012;29:1007–1021. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 28.Borges A., Ferreira C., Saavedra M.J., Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 29.Ebelle Etame R., Mouokeu R.S., Pouaha C., Laurel C., Voukeng Kenfack I., Tchientcheu R., Assam Assam J.E., Monthe Poundeu F.S., Tiabou A.T., Etoa F.X., et al. Effect of fractioning on antibacterial activity of Enantia chlorantha Oliver (Annonaceae) methanol extract and mode of action. Evid. Based Complement. Alternat. Med. 2018;2018 doi: 10.1155/2018/4831593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliphant C.M., Eroschenko K. Antibiotic resistance, part 1: Gram-positive pathogens. J. Nurse Pract. 2015;11:70–78. doi: 10.1016/j.nurpra.2014.09.018. [DOI] [Google Scholar]
- 31.Munita J.M., Arias C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall C.W., Mah T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017;41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- 33.Negi B.S., Dave B.P. In vitro antimicrobial activity of Acacia catechu and its phytochemical analysis. Indian J. Microbiol. 2010;50:369–374. doi: 10.1007/s12088-011-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng S.S., Xu J.G. Profile of antioxidant and antibacterial activities of different solvent extracts from Rabdosia rubescens. Int. J. Food Sci. Technol. 2014;49:2506–2513. doi: 10.1111/ijfs.12576. [DOI] [Google Scholar]
- 35.Wan W.N.A., Masrah M., Hasmah A., Noor N.I. In vitro antibacterial activity of Quercus infectoria gall extracts against multidrug resistant bacteria. Trop. Biomed. 2014;31:680–688. [PubMed] [Google Scholar]
- 36.Tian F., Li B., Ji B., Yang J., Zhang G., Chen Y., Luo Y. Antioxidant and antimicrobial activities of consecutive extracts from Galla chinensis: The polarity affects the bioactivities. Food Chem. 2009;113:173. doi: 10.1016/j.foodchem.2008.07.062. [DOI] [Google Scholar]
- 37.Moirangthem D.S., Talukdar N.C., Bora U., Kasoju N., Das R.K. Differential effects of Oroxylum indicum bark extracts: Antioxidant, antimicrobial, cytotoxic and apoptotic study. Cytotechnology. 2013;65:83–95. doi: 10.1007/s10616-012-9463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tayel A.A., El-Sedfy M.A., Ibrahim A.I., Moussa S.H. Application of Quercus infectoria extract as a natural antimicrobial agent for chicken egg decontamination. Rev. Argent. Microbiol. 2018;50:391–397. doi: 10.1016/j.ram.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Mishra M.P., Rath S., Swain S.S., Ghosh G., Das D., Padhy R.N. In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria. J. King Saud Uni-Sci. 2017;29:84–95. doi: 10.1016/j.jksus.2015.05.007. [DOI] [Google Scholar]
- 40.Lou J., Mao Z., Shan T., Wang Q., Zhou L. Chemical composition, antibacterial and antioxidant properties of the essential oils from the roots and cultures of Salvia miltiorrhiza. J. Essent. Oil Bear. Plants. 2014;17:380–384. doi: 10.1080/0972060X.2014.895199. [DOI] [Google Scholar]
- 41.Miyasaki Y., Rabenstein J.D., Rhea J., Crouch M.L., Mocek U.M., Kittell P.E., Morgan M.A., Nichols W.S., Van Benschoten M.M., Hardy W.D., et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE. 2013;8:e61594. doi: 10.1371/journal.pone.0061594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan B., Cai Y.Z., Brooks J.D., Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007;117:112–119. doi: 10.1016/j.ijfoodmicro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Qu H., Zhang Y., Wang Y., Li B., Sun W. Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J. Pharm. Pharmacol. 2008;60:261–266. doi: 10.1211/jpp.60.2.0016. [DOI] [PubMed] [Google Scholar]
- 44.Tajkarimi M.M., Ibrahim S.A., Cliver D.O. Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199–1218. doi: 10.1016/j.foodcont.2010.02.003. [DOI] [Google Scholar]
- 45.Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang H., Mei W.L., Zeng Y.B., Zuo W.J., Guo Z.K., Chen L.L., Zhong H.M., Dai H.F. Phenolic compounds from Dalbergia odorifera. Phytochem. Lett. 2014;9:168–173. doi: 10.1016/j.phytol.2014.06.008. [DOI] [Google Scholar]
- 47.Zhao X., Mei W., Gong M., Zuo W., Bai H., Dai H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules. 2011;16:9775–9782. doi: 10.3390/molecules16129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sithisarn P., Rojsanga P., Sithisarn P. Inhibitory effects on clinical isolated bacteria and simultaneous HPLC quantitative analysis of flavone contents in extracts from Oroxylum indicum. Molecules. 2019;24:1937. doi: 10.3390/molecules24101937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho H., Chung B., Kim C.K., Oh D.C., Oh K.B., Shin J. Spatholobus suberectus Dunn. constituents inhibit sortase A and Staphylococcus aureus cell clumping to fibrinogen. Arch. Pharm. Res. 2017;40:518–523. doi: 10.1007/s12272-016-0884-8. [DOI] [PubMed] [Google Scholar]
- 50.Arina M.I., Harisun Y. Effect of extraction temperatures on tannin content and antioxidant activity of Quercus infectoria (Manjakani) Biocatal. Agric. Biotechnol. 2019;19:101104. doi: 10.1016/j.bcab.2019.101104. [DOI] [Google Scholar]
- 51.Mailoa M.N., Mahendradatta M., Djide N. Test of antimicrobial activity of tannins extract from guava leaves to pathogens microbial. Int. Asian Res. J. 2014;2:43–50. [Google Scholar]
- 52.Pavić V., Flaćer D., Jakovljević M., Molnar M., Jokić S. Assessment of total phenolic content, in vitro antioxidant and antibacterial activity of Ruta graveolens L. extracts obtained by choline chloride based natural deep eutectic solvents. Plants. 2019;8:69. doi: 10.3390/plants8030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paz M., Gúllon P., Barroso M.F., Carvalho A.P., Domingues V.F., Gomes A.M., Becker H., Longhinotti E., Delerue-Matos C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015;172:462–468. doi: 10.1016/j.foodchem.2014.09.102. [DOI] [PubMed] [Google Scholar]
- 54.Zhang D., Gan R.Y., Farha A.K., Kim G., Yang Q.Q., Shi X.M., Shi C.L., Luo Q.X., Xu X.B., Li H.B., et al. Discovery of antibacterial dietary spices that target antibiotic-resistant bacteria. Microorganisms. 2019;7:157. doi: 10.3390/microorganisms7060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figueiras Abdala A., Mendoza N., Valadez Bustos N., Escamillia Silva E.M. Antioxidant capacity analysis of blackberry extracts with different phytochemical compositions and optimization of their ultrasound assisted extraction. Plant Foods Hum. Nutr. 2017;72:258–265. doi: 10.1007/s11130-017-0616-3. [DOI] [PubMed] [Google Scholar]
- 56.Rebaya A., Belghith S.I., Baghdikian B., Leddet V.M., Mabrouki F., Olivier E., Cherif J.K., Ayadi M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae) J. Appl. Pharm. Sci. 2014;5:52–57. doi: 10.7324/JAPS.2015.50110. [DOI] [Google Scholar]
- 57.Sarker U., Oba S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegitable. Sci. Rep. 2019;9:18233. doi: 10.1038/s41598-019-52033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Del Hierro J.N., Herrera T., Garcia-Risco M.R., Fornari T., Reglero G., Martin D. Ultrasound-assisted extraction and bioaccesibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018;109:440–447. doi: 10.1016/j.foodres.2018.04.058. [DOI] [PubMed] [Google Scholar]
- 59.Medina-Medrano J.R., Mares-Quinones M.D., Valiente-Banuet J.I., Vazquez-Sanchez M., Alvarez-Bernal D., Villar-Luna E. Determination and quantification of phenolic compounds in methanolic extracts of Solanum ferrugineum (Solanaceae) fruits by HPLC-DAD and HPLC/ESI-MS/TOF. J. Liq. Chromatogr. Relat. Technol. 2017;40:900–906. doi: 10.1080/10826076.2017.1382376. [DOI] [Google Scholar]
- 60.Castro-Alves V.C., Cordenunsl B.R. Total soluble phenolic compounds quantification is not as simple as it seems. Food Anal. Methods. 2015;8:873–884. doi: 10.1007/s12161-014-9961-0. [DOI] [Google Scholar]
- 61.Medina M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011;59:1565–1571. doi: 10.1021/jf103711c. [DOI] [PubMed] [Google Scholar]
- 62.Pekal A., Pyrzynska K. Evaluation of aluminum complexation reaction for flavonoid content assay. Food Anal. Methods. 2014;7:1766–1782. doi: 10.1007/s12161-014-9814-x. [DOI] [Google Scholar]
- 63.Famuyide I.M., Aro A.O., Fasina F.O., Eloff J.N., McGaw L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019;19:141. doi: 10.1186/s12906-019-2547-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soumya K., Jesna J., Sudheesh S. Screening study of three medicinal plants for their antioxidant and cytotoxic activity. Int. J. Pharm. Sci. Res. 2018;9:3781–3787. doi: 10.13040/IJPSR.0975-8232.9(9).3781-87. [DOI] [Google Scholar]
- 65.Zia-Ul-Haq M., Raza Shah M., Qayum M., Ercisli S. Biological screening of selected flora of Pakistan. Biol. Res. 2012;45:375–379. doi: 10.4067/S0716-97602012000400008. [DOI] [PubMed] [Google Scholar]
- 66.Guo J.P., Pang J., Wang X.W., Shen Z.Q., Jin M., Li J.W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006;12:4078. doi: 10.3748/wjg.v12.i25.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Özbílgín A., Durmuşkahya C., Kílímcíoğlu A.A., Kayalar H., Kurt Ö., Ermíş V.Ö., Tabak T., Östan I. In vitro efficacy of Quercus infectoria Oliv. and Achillea millefolium L. extracts against Blastocystis spp. isolates. Kafkas Univ Vet Fak Derg. 2013;19:511–516. doi: 10.9775/kvfd.2012.8196. [DOI] [Google Scholar]
- 68.Kheirandish F., Delfan B., Mahmoudvand H., Moradi N., Ezatpour B., Ebrahimzadeh F., Rashidipour M. Antileishmanial, antioxidant, and cytotoxic activities of Quercus infectoria Olivier extract. Biomed. Pharmacother. 2016;82:208–215. doi: 10.1016/j.biopha.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 69.Papuc C., Goran G.V., Predescu C.N., Nicorescu V., Stefan G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017;16:1243–1268. doi: 10.1111/1541-4337.12298. [DOI] [PubMed] [Google Scholar]
- 70.Pires T.C., Dias M.I., Barros L., Calhelha R.C., Alves M.J., Santos-Buelga C., Ferreira I.C. Phenolic compounds profile, nutritional compounds and bioactive properties of Lycium barbarum L.: A comparative study with stems and fruits. Ind. Crops Prod. 2018;122:574–581. doi: 10.1016/j.indcrop.2018.06.046. [DOI] [Google Scholar]
- 71.Kim H.Y. Statistical notes for clinical researchers: Effect size. Restor. Dent. Endod. 2015;40:328. doi: 10.5395/rde.2015.40.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sullivan G.M., Feinn R. Using effect size- or why the p value is not enough. J. Grad. Med. Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goodwin L.D., Leech N.L. Understanding correlation: Factors that affect the size of r. J. Exp. Educ. 2006;74:251–266. doi: 10.3200/JEXE.74.3.249-266. [DOI] [Google Scholar]
- 74.Lee D.S., Lee S.H., Noh J.G., Hong S.D. Antibacterial activities of cryptotanshinone and dihydrotanshinone I from a medicinal herb, Salvia miltiorrhiza Bunge. Biosci. Biotechnol. Biochem. 1999;63:2236–2239. doi: 10.1271/bbb.63.2236. [DOI] [PubMed] [Google Scholar]
- 75.Dzoyem J.P., Melong R., Tsamo A.T., Tchinda A.T., Kapche D.G., Ngadjui B.T., McGaw L.J., Eloff J.N. Cytotoxicity, antimicrobial and antioxidant activity of eight compounds isolated from Entada abyssinica (Fabaceae) BMC Res. Notes. 2017;10:118. doi: 10.1186/s13104-017-2441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pendota S.C., Aderogba M.A., Moyo M., McGaw L.J., Mulaudzi R.B., Van Staden J. Antimicrobial, antioxidant and cytotoxicity of isolated compounds from leaves of Pappea capensis. S. Afr. J. Bot. 2017;108:272–277. doi: 10.1016/j.sajb.2016.10.021. [DOI] [Google Scholar]
- 77.Capello C., Fischer U., Hungerbühler K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007;9:927–934. doi: 10.1039/b617536h. [DOI] [Google Scholar]
- 78.Backes E., Pereira C., Barros L., Prieto M.A., Genena A.K., Barreiro M.F., Ferreira I. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound-based extraction techniques. Food Res. Int. 2018;113:197–209. doi: 10.1016/j.foodres.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Le A.V., Parks S.E., Nguyen M.H., Roach P.D. Effect of solvents and extraction methods on recovery of bioactive compounds from defatted gac (Momordica cochinchinensis Spreng.) seeds. Separations. 2018;5:39. doi: 10.3390/separations5030039. [DOI] [Google Scholar]
- 80.Elshikh M., Ahmed S., Funston S., Dunlop P., McGaw M., Marchant R., Banat I.M. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotech. Lett. 2016;38:1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blainski A., Lopes G., de Mello J. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Senthilraja P., Kathiresan K. In vitro cytotoxicity MTT assay in Vero, HepG2 and MCF-7 cell lines study of marine yeast. J. Appl. Pharm. Sci. 2015;5:80–84. doi: 10.7324/JAPS.2015.50313. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.