Any infection modifies the host immune status, potentially ameliorating or aggravating the pathophysiology of a simultaneous inflammatory condition. In the course of investigating how malaria infection modulates the severity of contemporaneous inflammatory diseases, we identified a nonpathogenic mouse virus in stabilates of two widely used rodent parasite lines: Plasmodium berghei K173 and Plasmodium yoelii 17X YM. We established that the protective effects of these Plasmodium lines on cerebral malaria and multiple sclerosis are exclusively due to this virus. The virus induces a massive type I interferon (IFN-I) response and causes quantitative and qualitative defects in the ability of dendritic cells to promote pathogenic T cell responses. Beyond revealing a possible confounding factor in rodent malaria models, our work uncovers some bases by which a seemingly innocuous viral (co)infection profoundly changes the immunopathophysiology of inflammatory diseases.
KEYWORDS: CD4 T cell, RNA virus, autoimmunity, coinfection, dendritic cells, inflammation, malaria
ABSTRACT
Coinfections shape immunity and influence the development of inflammatory diseases, resulting in detrimental or beneficial outcome. Coinfections with concurrent Plasmodium species can alter malaria clinical evolution, and malaria infection itself can modulate autoimmune reactions. Yet, the underlying mechanisms remain ill defined. Here, we demonstrate that the protective effects of some rodent malaria strains on T cell-mediated inflammatory pathologies are due to an RNA virus cohosted in malaria-parasitized blood. We show that live and extracts of blood parasitized by Plasmodium berghei K173 or Plasmodium yoelii 17X YM, protect against P. berghei ANKA-induced experimental cerebral malaria (ECM) and myelin oligodendrocyte glycoprotein (MOG)/complete Freund’s adjuvant (CFA)-induced experimental autoimmune encephalomyelitis (EAE), and that protection is associated with a strong type I interferon (IFN-I) signature. We detected the presence of the RNA virus lactate dehydrogenase-elevating virus (LDV) in the protective Plasmodium stabilates and we established that LDV infection alone was necessary and sufficient to recapitulate the protective effects on ECM and EAE. In ECM, protection resulted from an IFN-I-mediated reduction in the abundance of splenic conventional dendritic cell and impairment of their ability to produce interleukin (IL)-12p70, leading to a decrease in pathogenic CD4+ Th1 responses. In EAE, LDV infection induced IFN-I-mediated abrogation of IL-23, thereby preventing the differentiation of granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing encephalitogenic CD4+ T cells. Our work identifies a virus cohosted in several Plasmodium stabilates across the community and deciphers its major consequences on the host immune system. More generally, our data emphasize the importance of considering contemporaneous infections for the understanding of malaria-associated and autoimmune diseases.
INTRODUCTION
By extension of the “hygiene hypothesis” (1), the host immune system is “conditioned” by lifelong environmental encounters with micro- and macroorganisms (also known as the multibiome [2]), ranging from commensals to pathogens. In turn, this interplay is expected to influence, positively or negatively, the development and severity of inflammatory disorders, including allergy and autoimmune diseases (3).
With respect to multiple sclerosis (MS), an autoimmune demyelinating disease of the central nervous system (CNS), neurotropic virus infections were reported to confer an added risk of developing the disease in adolescents and young adults (4, 5). Although the mechanisms are still debated, it has been hypothesized that virus infections may lead to breakdown of self-tolerance in genetically predisposed individuals, in part by antigenic molecular mimicry and/or by bystander activation of autoreactive T cells via upregulation of major histocompatibility complex (MHC) and costimulatory molecules on dendritic cells (DC) (6). In experimental autoimmune encephalomyelitis (EAE), a mouse model of MS, virus-specific brain-resident T cells generated in early life following CNS virus infection are indeed able to promote the activity of autoreactive T cells, resulting in brain autoimmune attacks (7). In contrast to viruses, helminthic parasites are known to play a protective role in MS, most likely because (i) they promote tissue repair and (ii) they induce regulatory T and B cells as well as anti-inflammatory cytokines (e.g., interleukin 10 [IL-10] and transforming growth factor β [TGF-β]), which suppress pathogenic autoreactive granulocyte-macrophage colony-stimulating factor (GM-CSF)+ CD4+ T cell responses (reviewed in references 8, to ,10).
Beside extracellular helminths, intracellular parasites such as Plasmodium, the malaria-causing parasite, which affects almost one-half of the world’s population, can also elicit immune modulatory pathways, which could affect autoimmune reactions. Following an asymptomatic liver stage, the parasites reach the bloodstream and develop within red blood cells (RBC). Blood-stage malaria can be associated with mild to severe clinical symptoms, including anemia, acute respiratory distress syndrome, acidosis, renal failure, or cerebral malaria (CM), which is characterized by the sequestration of leukocytes and parasitized RBC (pRBC) in brain microcapillaries, leading to hypoxia and vascular damage. Interestingly, most of the reported modulatory effects of Plasmodium, which can affect DC and B and T cells, occur during blood stage. Examples of this modulation on DC include the increased apoptosis of blood-circulating DC (11, 12), an atypical/partial DC maturation profile (13), and a crippled ability to present antigens to CD4+ and CD8+ T cells (11, 14, 15). The impact of Plasmodium on DC may be direct, such as exposure to parasite effectors or by-products such as the heme crystal hemozoin (16), or indirect, such as the systemic activation by pattern recognition receptors such as Toll-like receptors (TLRs), which imprint a “refractory” state on DC (17), or by type I interferon (IFN-I), which impairs their Th1-promoting property (18). With regard to T cells, blood-stage malaria may cause T cell exhaustion, which can be restored by checkpoint inhibitor therapy (19). CD4+ T follicular helper (Tfh) cells normally play a critical role in parasite control during blood stage, as they enhance the activation of germinal center B cell responses and enable long-lasting more-efficient humoral immunity (20, 21). Yet during severe malaria, a strong Th1-polarizing environment promotes the development of dysfunctional T-bet+ “Th1-like” CD4+ Tfh cells (22, 23), which exhibit poor help activity on B cell responses and lead to B cell apoptosis or differentiation into short-lived plasma cells and atypical memory B cells (24).
While such immune modulatory processes are thought to partially underlie the poor naturally acquired immunity to malaria observed in areas of endemicity, they may also have a beneficial impact on the course of autoimmune disorders. More than half a century ago, the incidence of two autoimmune diseases, rheumatoid arthritis and systemic lupus erythematosus, was found to be up to 6 times less frequent in Nigerians than in Europeans, and it was proposed that parasitic infections, in particular, malaria, were responsible for alleviating the development of autoimmunity (25). In accordance, experimental infection with Plasmodium berghei suppressed the spontaneous development of renal disease in a mouse lupus model (26). Intriguingly, the prevalence and incidence of MS has increased following malaria eradication in Sardinia (27), and work using rodent-adapted Plasmodium strains has revealed an overall protective effect of malaria infection on EAE. Infection with Plasmodium chabaudi chabaudi AS (PccAS) reduced EAE severity, possibly due to the induction of regulatory CD4+ T cells (Treg) and the production of IL-10 and TGF-β (28). Moreover, transfer of DC incubated with extracts of P. berghei NK65 pRBC ameliorated EAE (29); although paradoxically, when induced in mice cured from that same parasite, EAE was aggravated (30). Currently, little is known about the molecular and cellular mechanisms by which Plasmodium infection influences CNS autoimmunity.
In addition, beside autoimmune contexts, the clinical evolution of malaria itself is influenced by coinfection with another Plasmodium species. In humans, the risk of developing symptomatic malaria seems to be lower in mixed P. falciparum/P. malariae or P. falciparum/P. vivax infections (31, 32). In mice, the development of experimental cerebral malaria (ECM), a deadly vascular pathology during which Th1 CD4+ T cells promote the sequestration of pathogenic CD8+ T cells in the brain vasculature (33), is inhibited by coinfection with Plasmodium yoelii yoelii 17X clone 1.1 (34) and by P. berghei K173 (35). In the former case, replication of the ECM-causing P. berghei ANKA parasites was found to be hampered by mixed infection, but in the latter case, there was no effect on parasite growth. Rather, protection was associated with an early production of IFN-γ and IL-10 cytokines at 24 h postinfection. Yet, the exact protective mechanisms remain ill defined.
Here, in order to elucidate the bases of Plasmodium-mediated protection against T cell-mediated inflammatory diseases, we have investigated rodent malaria strains that block ECM and EAE. We show that live blood, as well as blood extracts, parasitized by P. berghei K173 or P. yoelii 17X YM confers full protection against P. berghei ANKA-induced ECM and myelin oligodendrocyte glycoprotein (MOG)/complete Freund’s adjuvant (CFA)-induced EAE and that this is associated with a strong IFN-I signature. We report the identification of an RNA virus called lactate dehydrogenase-elevating virus (LDV) in the protective Plasmodium stabilates, and we find that LDV infection alone recapitulates all the protective effects. In brief, LDV infection triggers a massive IFN-I response, which leads to a decrease in the number and functional capacity of conventional dendritic cells (cDC), thereby preventing the provision of signal 3 cytokines that are responsible for the pathogenic polarization of CD4+ T cells in ECM and EAE.
(This article was submitted to an online preprint archive [36].)
RESULTS
P. berghei K173 infection does not cause ECM and elicits lower Th1 responses than P. berghei ANKA.
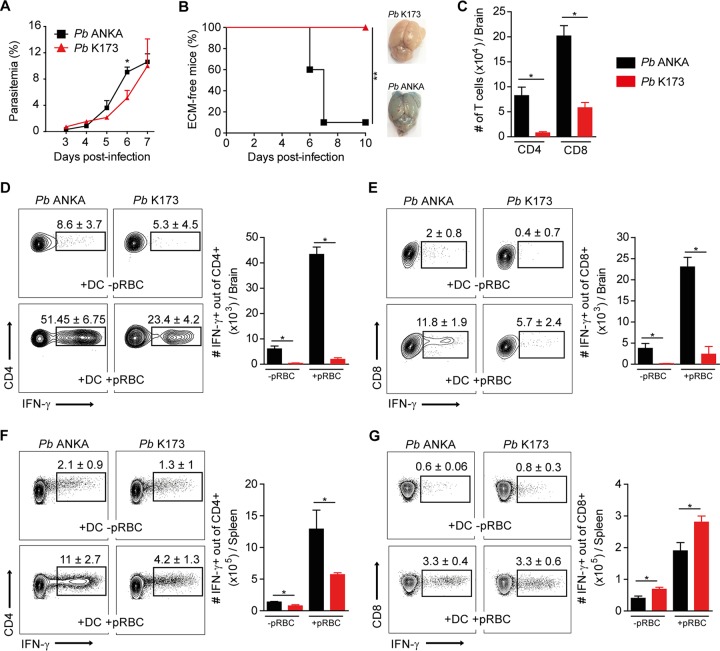
While the P. berghei ANKA isolate is widely used to induce ECM in C57BL/6 mice, the outcomes of P. berghei K173 infection vary. Some studies report its potential to induce ECM (37–39), while others suggest that it does not cause ECM (40) or that it even protects from this pathology (35). We first evaluated the immune responses and clinical outcomes following infections with our “in-house” P. berghei ANKA and P. berghei K173 stabilates. We infected mice intravenously (i.v.) with P. berghei ANKA- or P. berghei K173-infected pRBC and monitored the development of ECM and circulating parasitemia. Both parasites replicated in vivo (Fig. 1A), but only P. berghei ANKA led to ECM, characterized by brain edema and early death within 7 to 8 days of infection (Fig. 1B). At day 6 postinfection, the absence of ECM development upon P. berghei K173 infection was consistent with a lower number of brain-sequestered CD4+ and CD8+ T cells than with P. berghei ANKA (Fig. 1C). Moreover, the percentage of brain CD4+ (Fig. 1D) and CD8+ T cells (Fig. 1E) producing IFN-γ, an essential cytokine in ECM pathogenesis (33, 41, 42), was significantly decreased upon P. berghei K173 infection compared to that with P. berghei ANKA. In the spleen, the percentage of CD4+ T cells producing IFN-γ upon pRBC restimulation was reduced in P. berghei K173-infected mice (Fig. 1F), but no difference was observed in the percentage of IFN-γ+ CD8+ T cells (Fig. 1G). These data suggest that the inability of P. berghei K173 to elicit ECM is related to an impaired differentiation of CD4+ Th1 T cells in the spleen and a reduced sequestration of pathogenic CD8+ T cells in brain microcapillaries.
FIG 1.
Reduced Th1 responses and absence of ECM pathology following P. berghei K173 infection. C57BL/6 mice infected i.v. by injection of 106 P. berghei ANKA (Pb ANKA) or P. berghei K173 (Pb K173) pRBC. Blood circulating parasitemia (A) and ECM development (B) monitored following infection. Brain edema was visualized by Evans blue coloration. (C) Total numbers of CD4+ or CD8+ T cells collected from brain at day 6 after infection. Cells collected from brain (D and E) and spleen (F and G) at day 6 after infection were restimulated in vitro with MutuDC preloaded or not with pRBC. IFN-γ production by CD4+ T cells (D and F) or CD8+ T cells (E and G) detected by intracellular staining. Percentages on the representative dot plots show the median percentages of IFN-γ+ cells of total CD4+ or CD8+ T cells ± IQRs. Bar graphs show the medians ± IQRs of absolute numbers. Data are representative of 2 independent experiments. N = 5 mice per group.
P. berghei K173 infection triggers an early type I IFN response.
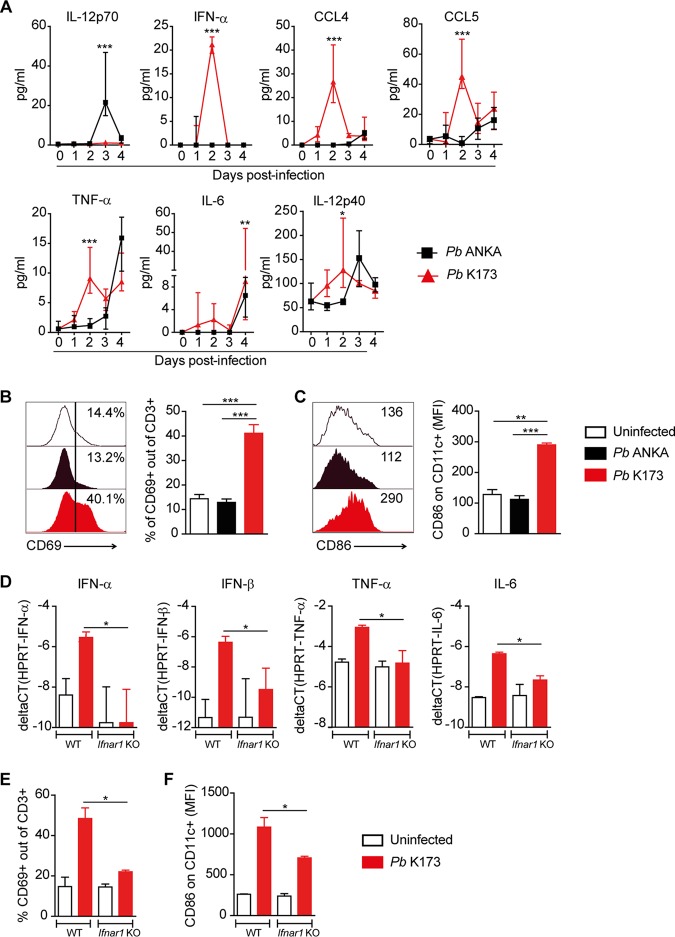
To investigate the molecular bases behind the lower Th1 responses in P. berghei K173-infected mice, we performed a kinetic analysis of several cytokines in the serum after infection. In accordance with the above findings, bioactive IL-12p70, a hallmark cytokine of Th1 responses, was induced exclusively by P. berghei ANKA but not by P. berghei K173 at day 3 postinfection (p.i.) (Fig. 2A). Instead, P. berghei K173 triggered an early production of IFN-α, CCL4, CCL5, tumor necrosis factor alpha (TNF-α), IL-6, and IL-12p40 at day 2 (Fig. 2A). In the spleen, P. berghei K173 infection induced a global activation of lymphocytes and DC, as exemplified by the upregulation of CD69 on T cells (Fig. 2B), B cells, and NK cells (not shown), and of CD86 on DC (Fig. 2C). These changes were suggestive of a systemic type I IFN (IFN-I) response (43). To confirm this hypothesis, we assessed whether they were dependent on IFN-I signaling. We analyzed the cytokine induction and T cell/DC activation in P. berghei K173-infected wild-type versus Ifnar1 knockout (KO) mice. Both the induction of IFN-α, IFN-β, TNF-α, and IL-6 genes and the systemic activation of splenic T cells and DC were abrogated in Ifnar1 KO mice (Fig. 2D to F). These data indicate that P. berghei K173- but not P. berghei ANKA-parasitized blood triggers a systemic IFN-I response, which correlates with defective Th1 differentiation and with ECM protection.
FIG 2.
P. berghei K173 but not P. berghei ANKA induces an early systemic type I IFN response. (A) Serum cytokines measured by Luminex assay at different time points after P. berghei (Pb) ANKA or P. berghei K173 infection. Proportions of CD69+ of CD3+ T cells (B) and geometric mean fluorescence intensities of CD86 expressed by CD11c+ cells (C) in the spleen at day 2 after infection. Bars show the medians ± IQRs. WT and Ifnar1 KO mice were infected with P. berghei K173 and analyzed at day 2 postinfection. (D) Cytokine gene expression in whole spleen analyzed by real-time qPCR. Proportions of CD69+ of CD3+ T cells (E) and geometric mean fluorescence intensities of CD86 on CD11c+ cells (F) in the spleen. Bars show the medians ± IQRs. (A, D, E, and F) Data from 1 experiment with 5 to 7 mice/group. (B and C) Data representative of 4 independent experiments with N = 4 mice/group.
Plasmodium-parasitized blood protects against ECM and EAE independently from live parasites.
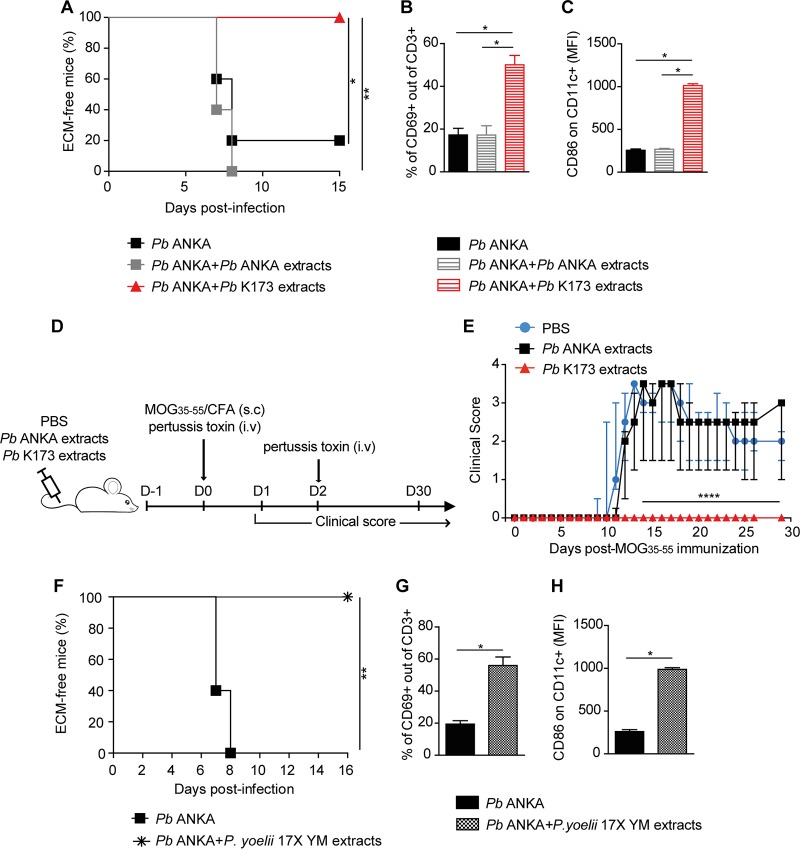
We next investigated if upon P. berghei K173/P. berghei ANKA coinfection, P. berghei K173 had a “dominant” protective effect on ECM. Coinfected mice were indeed protected from ECM (see Fig. S1A in the supplemental material) and, consistent with an impaired development of Th1 responses, blood CD4+ T cells displayed lower production of IFN-γ (Fig. S1B). To assess if live parasites were required for protection, we sonicated the P. berghei K173 stabilate, which precluded the parasites from replicating in vivo (not shown). Killed P. berghei K173 extracts still conferred full protection against ECM (Fig. 3A), and protection correlated with an early systemic activation of splenic T cells and DC (Fig. 3B and C). To examine if the protective potential of P. berghei K173 extracts could extend to other T cell-mediated inflammatory diseases, we evaluated their impact on experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis. EAE was induced by immunization with an MHC II peptide from the myelin oligodendrocyte self-antigen (MOG35–55) combined with CFA. Control mice injected with P. berghei ANKA blood extracts 1 day prior to MOG35–55/CFA immunization developed a classical EAE disease characterized by a progressive ascendant paralysis, but mice that received extracts of P. berghei K173 blood showed no sign of disease (Fig. 3D and E). This indicates that inhibition of ECM and EAE by P. berghei K173-parasitized blood operates independently from live parasites.
FIG 3.
Plasmodium-parasitized blood protects against ECM and EAE independently from live parasites. (A) ECM development following infection with 106 P. berghei (Pb) ANKA pRBC administered with or without sonicated extracts of P. berghei ANKA or P. berghei K173. (B, C) Proportions of CD69+ out of CD3+ T cells (B) and geometric mean fluorescence intensities of CD86 on CD11c+ cells (C) in the spleen at day 2 postinfection. Bars show the medians ± IQRs. (D) Experimental protocol. C57BL/6 mice were injected with P. berghei ANKA or P. berghei K173 pRBC sonicated extracts and immunized with MOG35–55/CFA to induce EAE. (E) Clinical scores monitored up day 30 postimmunization. Dots show the medians ± IQRs. (F) ECM development following infection with 106 P. berghei ANKA pRBC administered with or without sonicated extracts of 106 P. yoelii 17X YM pRBC. (G, H) Proportions of CD69+ out of CD3+ T cells (G) and geometric mean fluorescence intensities of CD86 on CD11c+ cells (H) in the spleen at day 2 postinfection. Bars show the medians ± IQRs. (A, B, and C) Data representative of 3 independent experiments with N = 5 mice/group. (E) Data representative of 2 experiments. N = 6 mice/group. (F, G, and H) Data from 2 experiments with N = 5 mice per group.
P. berghei K173 coinfection dampens P. berghei ANKA-induced IFN-γ production by CD4+ T cells. (A) ECM development in C57BL/6 mice infected with 106 P. berghei (Pb) ANKA pRBC or 106 P. berghei ANKA pRBC plus 106 P. berghei K173 pRBC. (B) IFN-γ expression by CD4+ T cells from peripheral blood measured from day 3 to day 7 after infection. Data from 1 experiment with N = 5 mice per group. Dots show the medians ± IQRs. Download FIG S1, PDF file, 0.3 MB (344.6KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
It was previously reported that coinfection with certain clones of P. yoelii parasites can inhibit ECM (34) and that the P. yoelii 17X YM strain triggers an IFN-I response (44, 45). Thus, we tested whether P. yoelii 17X YM, obtained here from the MR4/BEI distributor, would phenocopy P. berghei K173. Although P. yoelii 17X YM does not elicit ECM, it causes hyperparasitemia and is known to be lethal around day 7. To mitigate this confounding factor, we sonicated the P. yoelii 17X YM stabilate before coadministration with P. berghei ANKA. Akin to P. berghei K173 extracts, P. yoelii 17X YM extracts fully prevented the development of ECM (Fig. 3F) and induced T cell and DC activation at day 2 postinoculation (Fig. 3G and H). These data establish that at least two commonly used rodent malaria strains (P. berghei K173 and P. yoelii 17X YM) induce a systemic immune activation and protect from ECM.
P. berghei K173 and P. yoelii 17X YM stabilates contain LDV virus, which alone, disrupts Th1 responses and protects from ECM.
Intriguingly, we noticed that the protective activity of P. berghei K173 blood was contained in the plasma (see Fig. S2A) and was disrupted by UV treatment (Fig. S2B), two observations suggestive of the presence of a viral element. To verify this hypothesis, we subjected P. berghei ANKA and P. berghei K173 stabilates to comprehensive PCR rodent infectious agent (PRIA) testing. We found the presence of a virus, the lactate dehydrogenase-elevating virus (LDV), in P. berghei K173 (titer of 3,600 particles per 100 μl plasma) and P. yoelii 17X YM (titer of 14,600 particles per 100 μl plasma) stabilates, but not in P. berghei ANKA stabilates.
P. berghei K173 protects against ECM independently from the presence of the parasite. (A) ECM development in C57BL/6 mice infected with 106 P. berghei (Pb) ANKA pRBC and injected with plasma prepared from mice infected either with P. berghei ANKA or P. berghei K173 pRBC. (B) Same as in panel A except that the plasma from P. berghei K173-infected mice was subjected to ultraviolet (UV) radiation before coinjection with P. berghei ANKA pRBC. (A and B) Data from 1 experiment with N = 5 to 7 mice per group. Download FIG S2, PDF file, 0.3 MB (342.1KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
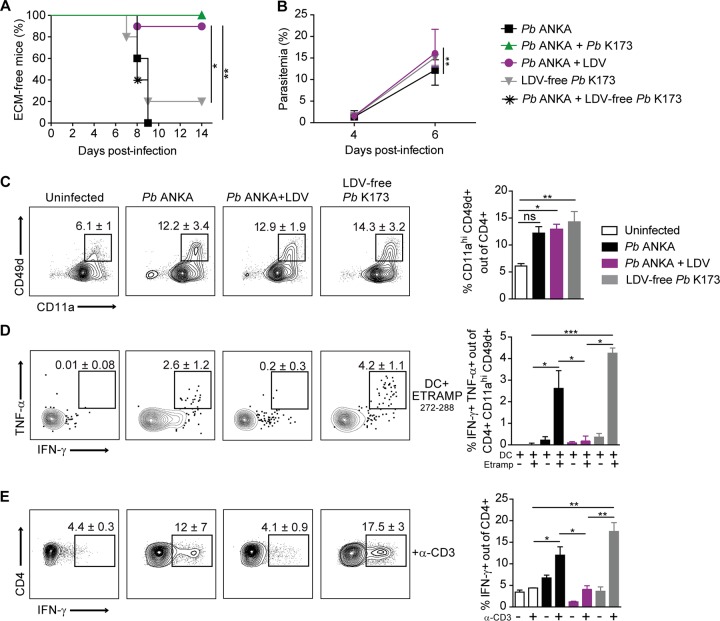
LDV is an enveloped, single-stranded positive RNA virus of the family Arteriviridae, order Nidovirales, known to cause persistent asymptomatic infection in mice, with long-lasting circulating viremia (46). This virus has been reported to modulate host responses, thereby either alleviating (47, 48) or exacerbating (49) immune-mediated diseases. To assess the direct implication of LDV in ECM protection, we decontaminated P. berghei K173 stabilates by fluorescence-activated cell sorting (FACS) of erythrocytes. We verified by PRIA that these new P. berghei K173 pRBC stocks were LDV free. We also prepared parasite-free LDV-containing plasma by collecting the plasma from mice injected with P. berghei K173 sonicated blood extracts. P. berghei ANKA-infected mice coinjected with LDV-containing plasma were protected from ECM, while those coinfected with LDV-free P. berghei K173 were no longer protected (Fig. 4A). Strikingly, the LDV-free P. berghei K173 stabilate was now causing ECM in the majority of mice (Fig. 4A), and these effects were not due to faster parasite growth (Fig. 4B). Since we had observed that ECM protection was linked to a reduced Th1 response, we analyzed the activation and production of Th1 cytokines by parasite-specific CD4+ T cells at day 6 postinfection. While there was no major difference in the percentages of activated (CD11a+ CD49d+) splenic CD4+ T cells between the groups (Fig. 4C), activated CD4+ T cells were unable to produce IFN-γ and TNF-α upon restimulation with the I-Ab-restricted ETRAMP272–288 antigenic peptide (Fig. 4D). This inhibition was also observed in the total CD4+ T cell population upon anti-CD3 stimulation (Fig. 4E). The results demonstrate that LDV is necessary and sufficient for ECM protection, most likely by abrogating the Th1 effector activity of CD4+ T cells.
FIG 4.
LDV alone, but not LDV-free P. berghei K173, impedes Th1 responses and prevents ECM development. ECM development (A) and blood circulating parasitemia (B) after infection or coinfection of C57BL/6 mice with the indicated inocula: P. berghei (Pb) ANKA pRBC, P. berghei ANKA plus P. berghei K173 pRBC, P. berghei ANKA pRBC plus LDV, LDV-free P. berghei K173 pRBC, P. berghei ANKA pRBC plus LDV-free P. berghei K173 pRBC. (C) Proportions of activated CD11a+ CD49d+ of spleen CD4+ T cells collected 6 days after infection. Cytokine production by spleen CD4+ T cells restimulated in vitro with MutuDC loaded with ETRAMP272–288 peptide (D) or with anti-CD3 (E). (D) Proportions of double IFN-γ+ TNF-α+ cells of activated CD4+ T cells upon ETRAMP272–288 restimulation. (E) Proportions of IFN-γ+ cells out of activated CD4+ T cells upon anti-CD3 restimulation. Numbers on the dot plots and bar graphs show median percentages ± IQRs. Data are representative of 2 independent experiments with N = 5 mice/group.
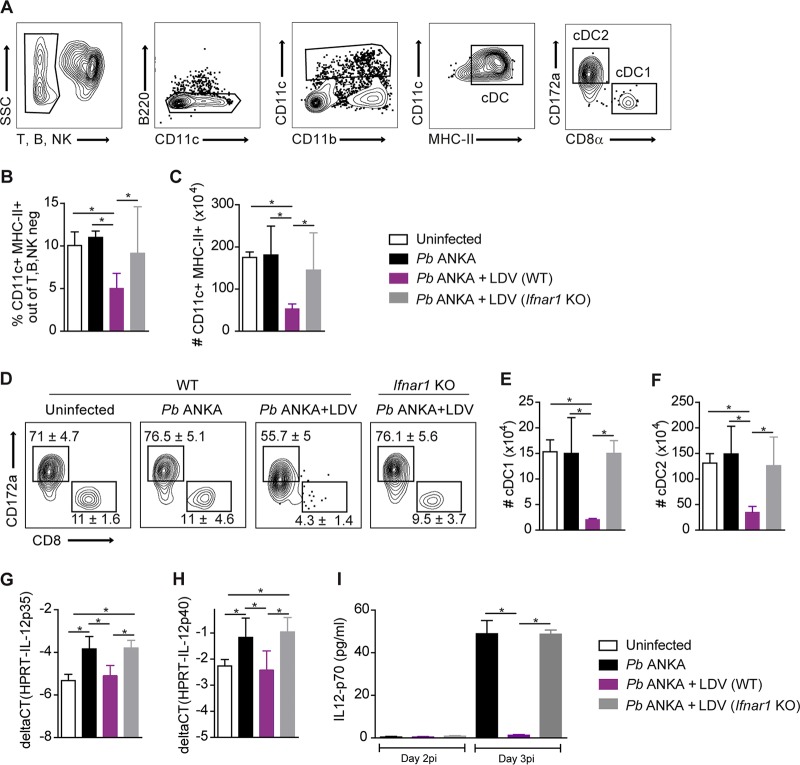
LDV induces Ifnar1-dependent depletion and functional impairment of splenic cDC.
To study how LDV impedes the Th1 CD4+ T cell response, we analyzed splenic conventional DC (cDC), which are primarily responsible for Th1 priming and polarization in this context (50–52). Two days following P. berghei ANKA/LDV coinfection, we observed a strong reduction in the proportion and numbers of total CD11c+ MHC II+ cDC, which was not observed in Ifnar1 KO mice (Fig. 5A to C). In proportion, the decrease was more pronounced for the CD8α+ cDC1 subset, but the CD172a+ cDC2 subset was also affected (Fig. 5D to F). In addition, we analyzed the capacity of the remaining cDC to make the pro-Th1 cytokine IL-12p70, which is composed of the IL-12p35 and IL-12p40 subunits. Upon day 2 of infection, P. berghei ANKA induced the upregulation of the IL-12p35 and IL-12p40 genes, which was blocked by LDV coinfection but rescued in Ifnar1 KO mice (Fig. 5G and H). Logically, this resulted in the lack of bioactive IL-12p70 in the serum at day 3 postinfection, an effect that was reversed in Ifnar1 KO mice (Fig. 5I). Taken together, our data establish that IFN-I signaling induced by LDV infection causes a quantitative reduction in cDC and a functional impairment in their ability to polarize Th1 CD4+ T cell responses.
FIG 5.
LDV causes an IFNAR-dependent decrease in number and IL-12p70 production of splenic conventional DC. C57BL/6 WT and Ifnar1 KO mice were infected i.v. with 106 P. berghei (Pb) ANKA pRBC or 106 P. berghei ANKA pRBC plus LDV. (A) Gating strategy used for cDC analysis 2 days after infection. Proportions of CD11c+ MHC II+ cDC of non-T, non-B, non-NK cells (B) and absolute numbers of cDC (C). (D) Representative dot plots showing the median percentages ± IQRs of cDC1 and cDC2 subsets. Absolute numbers of splenic cDC1 (E) and cDC2 (F). Real-time qPCR analysis of IL-12p35 (G) and IL-12p40 (H) gene expression by sorted CD11c+ cDC at day 2 after infection. (I) Plasmatic level of IL-12p70 measured by ELISA at day 2 and 3 after infection. (A to H) Data representative of 4 independent experiments with N = 5 mice/group. (I) Data representative of 2 independent experiments with N= 5 mice/group.
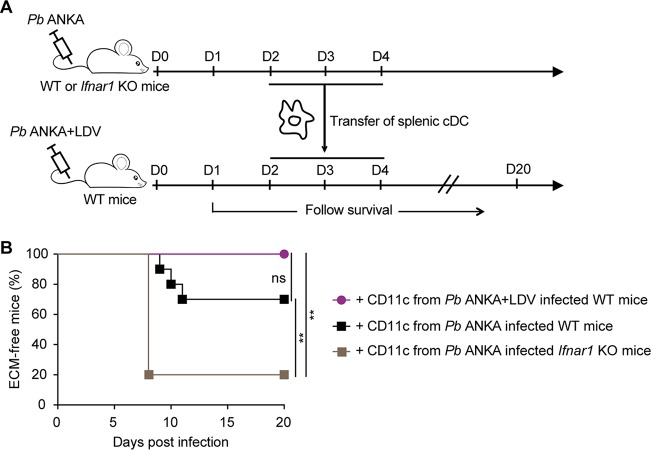
Type I IFNs can affect multiple cell types, including CD4+ T cells. Therefore, to determine to which extent the dysfunctional cDC compartment was responsible for LDV-mediated ECM protection, we tested if the transfer of functional cDC could restore disease in P. berghei ANKA/LDV coinfected mice. cDC were isolated from P. berghei ANKA-infected mice and transferred into LDV/P. berghei ANKA coinfected mice on days 3, 4, and 5 (Fig. 6A). This procedure restored ECM in only 3 of 10 transferred mice. We reasoned that even if fully functional at the time of injection, the transferred cDC may immediately be negatively conditioned by IFN-I in the LDV-infected environment. To overcome this effect, we isolated “IFN-I-insensitive” cDC from Ifnar1 KO P. berghei ANKA-infected mice and transferred them into LDV/P. berghei ANKA coinfected wild-type (WT) mice. This procedure restored ECM in 8 of 10 mice (Fig. 6B), indicating that crippling of cDC by IFN-I signaling is the predominant mechanism that underlies the LDV-mediated protection against ECM.
FIG 6.
cDC from P. berghei ANKA-infected Ifnar1 KO mice restore ECM upon transfer into P. berghei ANKA/LDV-coinfected mice. (A) Experimental protocol. WT or Ifnar1 KO donor mice were i.v. infected with 106 P. berghei (Pb) ANKA pRBC, with or without LDV. At days 2, 3, and 4 of infection, CD11c+ DC were magnetically sorted from spleen and transferred into P. berghei ANKA plus LDV coinfected WT mice. (B) ECM development. Data pooled from 2 independent experiments with N = 10 mice receiving WT DC and N = 5 mice receiving Ifnar1 KO DC.
LDV prevents EAE by disrupting the polarization of pathogenic autoreactive CD4+ T cell responses.
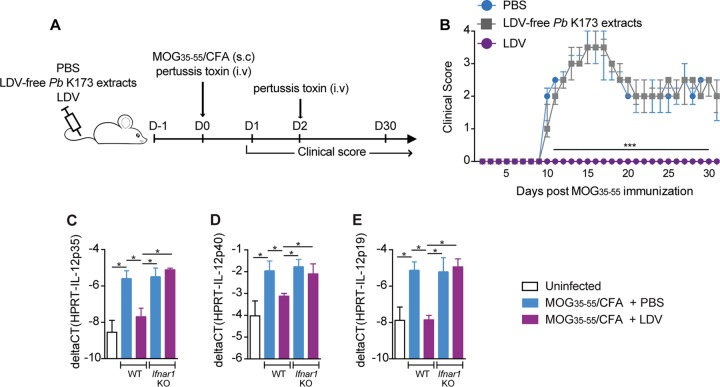
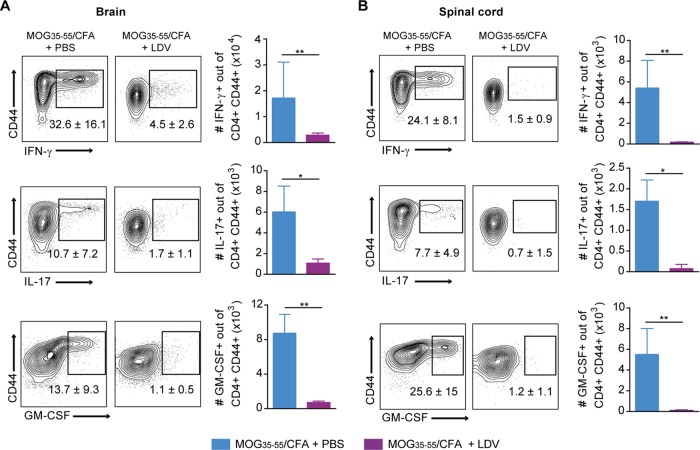
Finally, we investigated to which extent the protective mechanisms of P. berghei K173 extracts against EAE were analogous to those described in ECM. We first confirmed that LDV infection alone was protective against EAE. Mice injected with phosphate-buffered saline (PBS) or with sonicated extracts of LDV-free P. berghei K173 prior to MOG35–55/CFA immunization developed EAE, while mice that received LDV-containing plasma were fully protected (Fig. 7A and B). Upon day 2 of MOG35–55/CFA immunization, the IL-12p40, p35, and p19 subunit genes were induced in cDC isolated from draining lymph nodes (dLN), but this upregulation was blocked by LDV infection in an interferon α/β receptor (IFNAR)-dependent manner (Fig. 7C to E). These findings show that IFN-I signaling blocks the production by cDC of IL-23 (composed of IL-12p40 and p19 subunits), a key cytokine in the polarization of encephalitogenic CD4+ T cells (53). Accordingly, while the proportions of antigen-experienced (CD44+) CD4+ T cells were similar in the spleen and dLN 15 days after MOG35–55/CFA immunization (see Fig. S3A and C), the proportion of autoreactive CD44+ CD4+ T cells producing IFN-γ, IL-17, and GM-CSF in response to MOG35–55 stimulation was lower in LDV-infected mice (Fig. S3B and D). This decrease was confirmed by dosing the 3 cytokines in the supernatants of MOG35–55-stimulated spleen cells (Fig. S3E). In parallel to the impaired CD4+ T cell functional polarization in the spleen and dLN, we observed a major drop in the percentages and numbers of CD4+ T cells producing IFN-γ, IL-17, and GM-CSF in the brain (Fig. 8A) and spinal cord (Fig. 8B), in line with the absence of disease. In conclusion, our data show that LDV-mediated protection against EAE is mediated by an IFN-I signaling-dependent blockade of IL-23-dependent polarization of autoreactive GM-CSF-producing encephalitogenic CD4+ T cells.
FIG 7.
LDV alone, but not LDV-free P. berghei K173, prevents EAE development. (A) Experimental protocol. C57BL/6 mice were injected with PBS, LDV-free P. berghei (Pb) K173 pRBC sonicated extracts or LDV-containing plasma, and immunized with MOG35–55/CFA to induce EAE. (B) Clinical scores up to day 30 postimmunization. Dots show the medians ± IQRs. Real-time qPCR analysis of IL-12p35 (C), IL-12p40 (D), and IL-12p19 (E) gene expression on CD11c+ DC magnetically sorted from dLN of C57BL/6 WT or Ifnar1 KO mice, injected with PBS or infected with LDV 1 day prior to MOG35–55/CFA immunization. Analysis at day 1 postimmunization. (B) Data representative of 3 independent experiments with N = 5 mice/group. (C to E) Data representative of 2 independent experiments with N = 5 mice/group.
FIG 8.
LDV alone protects against EAE by blocking the encephalitogenic CD4+ T cell response. C57BL/6 mice were injected with PBS or LDV 1 day before MOG35–55/CFA immunization. Mononuclear cells from CNS and spinal cord harvested 15 days after MOG35–55/CFA immunization were restimulated with 100 μg MOG peptide for 24 h. Production of IFN-γ, IL-17, and GM-CSF visualized by intracellular staining in CD4+ T cells from brain (A) and spinal cord (B). Numbers on the representative dot plots show the median percentages of cytokine-positive cells of CD44+ CD4+ T cells ± IQRs. Histograms show the absolute numbers. Data representative of 2 independent experiments with N = 7 mice/group.
LDV dampens encephalitogenic CD4+ T cell differentiation in spleen and draining lymph node following MOG35–55/CFA immunization. C57BL/6 mice were injected with PBS or LDV one day before MOG35–55/CFA immunization. Mononuclear cells from spleen and dLN harvested 15 days after MOG35–55/CFA immunization were restimulated with 100 μg MOG peptide for 24 h. Proportions of activated CD44+ of CD4+ T cells in spleen (A) and dLN (C). Proportions of CD4+ T cells producing IFN-γ, IL-17, or GM-CSF, visualized by intracellular staining from spleen (B) and dLN (D). (E) Quantification of IFN-γ, IL-17, and GM-CSF cytokines measured by ELISA in the supernatant of MOG35–55-stimulated spleen cells. Data representative of 2 independent experiments with N = 7 mice/group. Download FIG S3, PDF file, 0.2 MB (218.4KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
The host immune status is shaped by continuous exposure to commensal as well as potentially pathogenic microorganisms. Depending on whether they exacerbate or alleviate immunopathology, the surrounding microbes may be harmful or beneficial. In this study, we initially sought to address why malaria infection influences the clinical outcome of a concurrent Plasmodium infection and autoimmune reactions. To investigate these mechanisms, we took advantage of rodent Plasmodium lines that prevent the development of ECM and EAE, two inflammatory diseases mediated by T cells. Unexpectedly, we found that the beneficial effects of the protective Plasmodium berghei K173 strain are entirely due to LDV, a nonpathogenic mouse virus, and that this virus is cohosted in stabilates of other parasite strains (e.g. P. yoelii 17X YM) commonly used by the community. We demonstrated that protection against ECM results from an IFN-I signaling-dependent depletion and functional impairment of splenic DC, which become incapable of producing IL-12p70 and polarizing pathogenic Th1 CD4+ T cell responses. Analogous to this mechanism, we found that the protection against EAE is linked to an IFN-I-dependent reduction in the production of encephalitogenic IL-23 and GM-CSF cytokines.
Based on the facts that (i) in our experiments, CD8+ T cell accumulation in the CNS was reduced (see Fig. 1C and E) despite a stronger induction of parasite-specific CD8+ T cell responses in the spleen (see Fig. 1G) and (ii) Th1 CD4+ T cells are known to enhance CD8+ T cell sequestration in the brain through the induction of CXCL9/10 (33), we hypothesize that the defective Th1 responses underlie ECM protection. However, because IL-12p70 may also heavily influence CD8+ T cells, we do not rule out the possibility that dysfunctional cDC also directly cripple the pathogenic CD8 T cell response.
This work reiterates the importance of screening products that are injected into animal models for the presence of unsuspected contaminants. LDV was initially discovered as a companion virus of transplantable tumor cell lines more than half a century ago (54). Our data establish that the virus is carried over in commonly used rodent Plasmodium stocks. Although LDV should not be an issue for Plasmodium strains that are regularly passaged through mosquitoes, the results of some studies on innate immune sensing and/or IFN-I responses performed with blood-passaged Plasmodium strains may need to be reinterpreted if these strains are LDV positive. So far, the timing of the contamination of certain Plasmodium stocks by LDV and the causes remain unclear. As LDV can be, though very poorly, transmitted to cage mates (46, 55), one may speculate that at times when the confinement of cages in animal facilities was less stringent, some Plasmodium-infected mice may have been exposed to LDV-contaminated bedding. Another possibility is that at some point, mice used to expand Plasmodium stocks have been injected with a biological product containing LDV. LDV is indeed a known contaminant of mouse-passaged ascitic fluids (56). In any case, we are confident that the contamination of strains used in this study occurred prior to their manipulation in our facility for various reasons: (i) for P. yoelii 17X YM, quantitative PCR (qPCR) detection of LDV was performed immediately on one-half of the obtained cryovials, before any mouse passage, (ii) all cages were kept isolated from each other on ventilated racks, (iii) for stock preparation, mice infected with different parasite strains were never mixed in the same cage, (iv) our “clean” Plasmodium stocks have remained LDV free in the course of this study, (v) the data on P. berghei K173 are fully consistent with earlier observations of ECM inhibition by this strain (35).
LDV is a mouse-specific virus that causes limited pathology but persists lifelong with detectable circulating viremia (46). Only one study from 1978 examined the outcome of LDV/malaria coinfection. It was observed that LDV exacerbates P. yoelii virulence by increasing parasitemia, leaving open the possibility that the phenotype of the lethal and nonlethal P. yoelii 17X substrains may be in part determined by the presence of LDV (57). Why, in this case, LDV aggravated the pathology remains unclear, but one could speculate that with malaria strains that do not cause cerebral malaria, the suppression of Th1 responses is deleterious for parasite control. Concerning autoimmunity, the beneficial role of LDV has been reported in various contexts such as lupus (58), diabetes (59), and EAE (47). In lupus, LDV-mediated protection was correlated with a decrease in IFN-γ production in the serum (60), but no mechanism was provided to explain the suppression of diabetes and EAE.
Beyond the importance of our findings for the community working on malaria models, our work has fundamental relevance to understand how viruses shape inflammatory diseases. We indeed reveal some of the molecular bases that underlie the protective effects of LDV in two settings involving T cell-mediated immunopathology. In the case of ECM, protection results from the functional paralysis and the strong depletion of splenic cDC, both of which are caused by IFN-I signaling. It was previously shown that LDV induces a rapid systemic IFN-I production by pDC in a TLR7-dependent manner (61). IFN-I signaling has been reported to play a dual role on the IL-12 pathway. On the one hand, IFN-I drives DC activation and maturation (62) and production of IL-12 (63), which may augment Th1 CD4 responses. On the other hand, IFN-I can selectively suppress IL-12p70 (64). It is likely that IFN-I dosage determines the outcome of IFN-I signaling on Th1 responses (65). While low levels of IFN-I signaling positively regulate Th1 responses, higher doses of IFN-I, as observed here upon massive viral replication in the first 2 days, rather impede the development of Th1. In addition to the functional alterations of cDC, we reported a major drop in the number of splenic cDC that is dependent on IFN-I signaling. It will be interesting to analyze whether the reduction in cDC is due to apoptosis induced by BH3-only proteins, such as the proapoptotic factor Bim (66).
In the case of EAE, LDV infection prior to MOG/CFA immunization blunted the production of IL-12 and IL-23 by cDC in the dLN, resulting in the absence of IFN-γ, IL-17, and GM-CSF production by MOG-specific CD4+ T cells, both in the periphery and in the CNS. It has now become clear that IL-12p35 (67), IFN-γ, and IL-17 (68) are dispensable for the development of MOG-induced EAE, while the key pathogenic cytokines are GM-CSF produced by CD4+ Th cells and IL-23, which may be produced by multiple sources (68, 69). Also, in contrast to ECM pathology in which cDC are required for the priming and polarization of Th1 CD4+ T cells (50–52), cDC are not necessary for the priming of encephalitogenic CD4+ Th cells in MOG protein-induced EAE (70), and mice lacking cDC even display an aggravated disease (71). Based on this, we propose that LDV-mediated protection against EAE is due to the global effect of IFN-I signaling, which inhibits IL-23 production by several different cell types of the dLN, including cDC, and that this prevents the differentiation of GM-CSF-secreting CD4+ T cells. In human DC, IFN-β has been shown to inhibit IL-23p19 production by DC, possibly through a STAT3-dependent induction of the negative regulator SOCS3 (72). The exact transcriptional and/or epigenetic reprogramming that cripples the IL-12 and IL-23 synthesis pathways in response to IFN-I remains to be fully unraveled in this context.
Type I IFNs are powerful signals that can modify the functionality of many cell types, including CD4+ T cells, which play a major role in the two diseases. For example, in EAE, IFN-I signaling in T cells can directly impair Th17 differentiation (73). During blood-stage malaria, CD4+ T cell-intrinsic IFN-I signaling induces T-bet and Blimp-1 expression, thereby promoting IL-10+ Tr1 responses in mice (74) as well as in humans (75). Therefore, it was possible that CD4+ T cells impacted by IFN-I may contribute to protection. Because ECM pathology in LDV-infected animals could be restored by transferring activated “IFN-I-insensitive” (Ifnar1 KO) DC from P. berghei ANKA-infected mice, we infer that the role, if any, of non-DC in the protection phenotype should be modest.
There have been other examples of viruses modifying the clinical course of a parasitic disease. In experimental malaria, coinfection with the chikungunya arbovirus also prevented ECM but through a distinct mechanism involving the IFN-γ-mediated retention of CXCR3-expressing pathogenic CD8+ T cells in the spleen (76). Another striking example is the discovery of a double-stranded RNA virus in Leishmania parasites (LRV), which is responsible for the exacerbation of the severity of mucocutaneous leishmaniasis via a TLR3/IFN-I-dependent proinflammatory response (77, 78). A notable difference is that LRV is an endogenous viral element of the parasite itself, while LDV is an exogenous mouse-specific virus that is cohosted in Plasmodium-parasitized blood. Nevertheless, it was observed that the aggravation and metastasis of Leishmania lesions were not exclusively associated with LRV and that they could also be enhanced by coinfection with exogenous viruses (79). Interestingly, a new RNA virus called Matryoshka RNA virus 1 (MaRNAV-1) was recently identified in P. vivax-infected blood samples (80). Like LRV, which infects the Leishmania parasite, MaRNAV-1 is thought to be a virus of the Plasmodium parasite. Future investigations should reveal whether this virus modulates the pathogenicity of P. vivax, as P. vivax malaria is associated with lower severity than P. falciparum malaria (81) and was suggested to be protective in cases of coinfection (32).
In conclusion, our data emphasize the notion that viral infections, even with viruses that are seemingly innocuous, can have dramatic consequences on a concurrent infectious or autoimmune disease. LDV is a mouse-specific virus, but the IFN-I-mediated modulatory mechanisms highlighted here may be fully relevant in humans. As constant exposure to an immense variety of commensal and noncommensal microbes profoundly shapes our health, it appears essential to actively and systematically characterize environmental microbial agents. From a fundamental standpoint, one could foresee the discovery of new languages in the host-pathogen cross talk. From a medical perspective, this research could suggest novel therapeutic immune modulatory molecules.
MATERIALS AND METHODS
Animals.
Animal care and use protocols were carried out under the control of the National Veterinary Services and in accordance with the European regulations (EEC Council Directive, 2010/63/EU, September 2010). Protocols inducing pain were approved under code APAFIS 4318-2016022913333485 v4, by the local ethical committee for animal experimentation (Comité d’Ethique sur l’Experimentation Animale, CEEA number [no.] 122 of US006/CREFRE) affiliated with the Comité National de Réflexion Ethique sur l’Expérimentation Animale. C57BL/6J (B6) mice were purchased from Envigo or Janvier (France) with similar results. Ifnar1 KO mice with at least 5 backcrosses on the C57BL/6 background were a gift from D. Hudrisier and E. Meunier (IPBS Toulouse). All mice were housed and bred under specific-pathogen-free conditions at the Centre Régional d’Exploration Fonctionnelle et de Ressources Expérimentales (CREFRE-Inserm UMS006). Mice used in experiments were male mice aged 8 to 10 weeks. The number of mice and experimental replicates are indicated in the respective figure legends.
Parasites and experimental infections.
Plasmodium berghei ANKA and Plasmodium berghei Kyberg 173 parasites were gifts from S. Picot (University of Lyon, France) and I. Landau (National Museum of Natural History, Paris, France), respectively. The identity of these parasites was confirmed by whole-genome sequencing. Plasmodium yoelii subs p. yoelii, strain YM (P. yoelii 17X YM, MRA-755) was obtained through BEI Resources, NIAID, NIH, in 2017, contributed by David Walliker. All Plasmodium strains were propagated in C57BL6/J mice. To prepare pRBC stocks, mice were bled with heparin, and the proportion of pRBC was evaluated by blood smear. The pRBC concentration was adjusted to 107 pRBC/ml in Alsever’s solution with 10% glycerin. One-milliliter aliquots were stored at −80°C. All infections were conducted by intravenous inoculation of 106 pRBC. For Evans blue staining, mice were injected i.v. with a solution of 1% Evans blue dissolved in 0.9% NaCl, and brain coloration was examined 1 h after dissection. To prepare the sonicated blood extracts, total blood was sonicated using an ultrasonic liquid processor (Branson, Sonifier) under the following parameters: 40 mA, pulse 2, 1 min. Blood was sonicated twice with a 1-min rest. For UV treatment, plasma was exposed for 40 min.
Parasitemia quantification.
Parasitemia was measured by blood smear or flow cytometry with similar results. For flow cytometry, 3 μl of tail blood was collected using an EDTA-coated Microvette (CB 300 K2E; Sarstedt) and diluted in 500 ml PBS. The diluted blood was labeled with antibodies directed against Ter119 (fluorescein isothiocyanate [FITC], TER-119, 1/30; Miltenyi Biotec), CD71 (phycoerythrin [PE], C2, 1/300; BD Pharmingen), and CD41‐PE‐Cy7 (MWReg30, 1/100; BioLegend), fixed, and permeabilized with 4% paraformaldehyde (PFA) with 0.6% saponin for 10 min, followed by 4′,6-diamidino-2-phenylindole (DAPI) staining.
LDV detection and dosage and decontamination of P. berghei K173 blood.
For the initial detection of LDV, P. berghei ANKA and P. berghei K173 stabilates were subjected to a mouse/rat comprehensive clear panel for PCR infectious agent testing (PRIA; Charles River). Further detection and quantitation of LDV particles were conducted with a simple PCR LDV test (Charles River) on mouse plasma.
To free P. berghei K173 blood from the virus, C57BL6/J mice were injected with 106 P. berghei K173 pRBC; 10 days after injection, intracardiac blood was collected with heparin and washed with PBS. RBC and platelets were stained with anti-Ter119 (allophycocyanin [APC], REA847, 1/50; Miltenyi Biotec) and CD41 (PE, MWReg30, 1/300; BD Biosciences), and then RBC were separated from platelets by magnetic sorting using anti-PE microbeads (Miltenyi Biotec). The Ter119+ CD41− fraction was stained again with the same anti-Ter119 and CD41 antibodies, and RBC were FACS sorted with an Aria cell sorter (BD Biosciences). New B6 mice were injected with 106 sorted pRBC. Five days later, a new stock of pRBC was prepared as indicated above. Confirmation of the absence of LDV was performed by PCR (Charles River).
Induction of EAE and clinical score.
The MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) peptide was purchased from Polypeptide Laboratories (San Diego, CA, USA). Mice were immunized subcutaneously with 50 μg of MOG35–55 peptide emulsified in complete Freund’s adjuvant (CFA; BD Difco, Franklin Lakes, NJ, USA) containing 500 μg of killed Mycobacterium tuberculosis (strain H37a; Difco). Mice were injected intravenously with 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA, USA) at days 0 and 2 postimmunization. Clinical scores were recorded daily by an experimenter blinded to the experimental groups. Scores were assigned as follows: 0, no sign of disease; 1, loss of tone in the tail; 2, hind limb paresis; 3, hind limb paralysis; 4, tetraplegia; 5, moribund.
Real-time quantitative PCR for gene expression.
Total mRNA was extracted from tissue and cells by standard TRIzol-chloroform precipitation. For reverse transcription, the iScript cDNA synthesis kit (Bio-Rad) was used. Quantitative PCR (qPCR) reactions were prepared with LightCycler 480 DNA SYBR green master reaction mix (Roche Diagnostics). Primers were used at 0.2 μM, and sequences are available upon request. qPCRs were run in duplicates on a LightCycler 480 System (Roche diagnostics), and cDNA abundance was normalized to the reference gene Hprt. Results are expressed as the threshold cycle difference (ΔCT) (Hprt minus gene of interest).
Isolation of spleen, lymph node, and CNS leukocytes.
Mice were anesthetized with ketamine-xylazine and perfused intracardially with cold PBS. Spleens, lymph nodes, spinal cord, and brains were collected in complete RPMI (Gibco) supplemented with 10% (vol/vol) fetal calf serum (FCS; Gibco). Splenocytes were mashed through a 100-μm cell strainer (Falcon). Lymph node cells were mashed in a Potter and filtered through a 100-μm cell strainer (Falcon). Brain and spinal cord were collected separately, homogenized, and digested with collagenase D (2.5 mg/ml; Roche), DNase I (100 μg/ml; Sigma-Aldrich), and Nα-p-tosyl-l-lysine chloromethyl ketone (TLCK) (1 μg/ml, Roche) for 30 min at 37°C. Cells were then washed, suspended in 37% Percoll, and layered on 70% Percoll. After a 20-min centrifugation at 800 × g, the mononuclear cells were collected from the interface. In both cases, erythrocytes were lysed using ACK buffer (100 μM EDTA, 160 mM NH4Cl, and 10 mM NaHCO3).
Ex vivo T cell restimulation.
In the ECM model, 106 splenocytes and 3 × 105 brain leukocytes were incubated for 5 h at 37°C in the presence of brefeldin A (BfA) with MutuDC, a C57BL/6-derived DC line obtained from H. Acha-Orbea (82). MutuDC were loaded overnight with pRBC (ratio, 1 MutuDC to 10 P. berghei ANKA or P. berghei K173 pRBC) or just incubated with anti-CD3 or 5 μM of ETRAMP272–288 peptide (52) during the in vitro restimulation. Cells were stained with CD4 (APC-Cy7, GK1.5, 1/300; BD Pharmingen), CD8α (BV421, 53-6.7, 1/300; BD Biosciences), CD11a (PE, 2D7, 1/400; BD Biosciences), and CD49d (BV786, R1-2, 1/300; BD Biosciences). Intracellular IFN-γ (APC, XMG1.2, 1/200; eBioscience) and TNF-α (Alexa Fluor 700, MP6-XT22, 1/300; BD Pharmingen) were detected with an intracellular fixation and permeabilization buffer Set (eBioscience) according to the manufacturer’s instructions.
In the EAE model, 6 × 106 cells from spleen, 3 × 106 from dLN, 3 × 105 from brain, and 3 × 105 from spinal cord were incubated for 24 h at 37°C with MutuDC (ratio, 1 MutuDC to 10 cells) and 100 μg of MOG35–55. BfA was added for the last 5 h. Cells were stained with CD4 (BV510, RM4-5, 1/200; BD Horizon) and CD44 (BV605, IM7, 1/300; BD Horizon). Intracellular IFN-γ (AF700, XMG1.2, 1/300; BD Pharmingen), IL-17 (AF700, TC11-18H10, 1/200; BD Pharmingen), and GM-CSF (PE, MP1-22E9, 1/300; BioLegend) were detected with the same kit as described above.
Flow cytometry analysis of dendritic cells.
Spleens and dLN were treated with collagenase D (1 mg/ml; Roche) and DNase I (100 μg/ml; Sigma-Aldrich), minced into small pieces, and incubated at 37°C for 30 to 45 min. Cell preparations were filtered using 100-μm cell strainers. For DC identification, cells were stained for CD3 (PE, 145-2C11, 1/300; BD Biosciences), CD19 (PE, 1D3, 1/300; BD Biosciences), and NK1.1 (PE, PK136, 1/300; BD Biosciences) to exclude T, B, and NK cells. A mix containing CD11c (PE-Cy7, N418, 1/400; BioLegend), B220 (BV510, RA3-6B2, 1/900; BD Horizon), CD11b (PE-CF594, M1/70, 1/3,000; BD Horizon), MHC II (AF700, M5/114.15.2, 1/400; BD Pharmingen), CD8α (FITC, 53-6.7, 1/200; BD Biosciences), and CD172a (APC-Cy7, P84, 1/200; BioLegend) was used to characterize the different DC subsets. In some experiments, an anti-XCR1 antibody (PE, REA707, 1/200; Miltenyi Biotec) was used to confirm the identity of cDC1. All flow cytometry samples were run on a Fortessa (BD Biosciences) and analyzed using FlowJo software.
Sorting and transfer of splenic dendritic cells.
Spleens were injected with 1 ml of PBS supplemented with Liberase TL (0.125 mg/ml; Roche) and DNase I (100 μg/ml; Sigma-Aldrich), incubated for 30 min at 37°C, and mashed through a 100-μm cell strainer. RBC were lysed and cells washed in magnetically activated cell sorting (MACS) buffer. For DC sorting, cells were incubated 10 min with CD11c microbeads (Miltenyi Biotec), and CD11c+ cells were magnetically sorted according to the manufacturer’s instructions. Postsorting flow cytometry analysis showed that less than 0.5% of transferred CD11c+ cells were CD8+ CD3+ T cells. After sorting, 106 CD11c+ cells in 100 μl PBS were transferred to recipient mice by i.v. injection.
Measurements of soluble cytokines.
Enzyme-linked immunosorbent assay (ELISA) was used to measure cytokines in plasma (50 μl) and culture supernatants (72 h after restimulation). For IL-12p70, the ELISA MAX Deluxe Set (BioLegend) was used according to the manufacturer’s instructions. For the other cytokines, 96-well plates (Nunc Maxi Sorp; BioLegend) were coated for 2 h at 37°C with anti-IFN-γ (AN18), anti-IL-17 (TC11-18H10), or anti-GM-CSF (MP1-22E9) antibodies. Culture supernatants or standards (Peprotech) were incubated for 2 h at 37°C. The plates were then incubated for 2 h with a secondary biotinylated antibody, anti-IFN-γ (XMG1.2), anti-IL-17 (TC11-8H4), and anti-GM-CSF (MP1-31G6), followed by a 20-min incubation with streptavidin-phosphatase alkaline (Sigma-Aldrich) at 37°C. Plates were visualized by using phosphatase alkaline substrate (Sigma-Aldrich), and absorbance was measured at 450 nm to 540 nm. Luminex analyses were performed by Genotoul Anexplo platform using the kits EP0X10 and EPX020 (eBioscience), according to the manufacturer’s instructions.
Statistical analyses.
Statistical evaluation of differences between the experimental groups was done by using two-way analysis of variance followed by a Bonferroni’s posttest for clinical monitoring and parasitemia, Kaplan-Meier test for monitoring of ECM-free mice, Mann-Whitney U test for group versus group comparison, and Kruskal-Wallis test for multiple-group comparisons. All tests were performed with GraphPad Prism 5.00 (GraphPad Software Inc., San Diego, CA, USA). All data are presented as medians ± interquartile ranges (IQRs).
ACKNOWLEDGMENTS
We thank F. L’Faqihi-Olive, V. Duplan-Eche, A.-L. Iscache, and L. de la Fuente for technical assistance at the CPTP-Inserm U1043 flow cytometry facility, R. Balouzat and the zootechnicians at INSERM UMS006-CREFRE mouse facility, S. Ménard, M. Boos, and members of the Blanchard and Saoudi teams for technical help and discussions, I. Bernard for setting up the low-severity EAE model, H. Acha-Orbea for the MutuDC, D. Hudrisier and E. Meunier for the B6 Ifnar1 KO mice, S. Picot for the P. berghei ANKA stabilate, I. Landau for the P. berghei K173 stabilate, and BEI Resources, NIAID, NIH, for the Plasmodium yoelii subs p. yoelii strain YM stabilate.
This work was supported by Institut National de la Santé et de la Recherche Médicale, Association pour la Recherche sur la Sclérose en Plaques (to N.B. and A. Saoudi), Human Frontier Science Program Organization (CDA00047/2011 to N.B.), PIA PARAFRAP Consortium (ANR-11-LABX0024 to N.B.), PIA ANINFIMIP equipment (ANR-11-EQPX-0003 to N.B.), and the French Minister of Education, Research and Technology (PhD fellowship to A.H. and M.B.).
Conceived and designed the experiments: A.H., M.F.W., M.B., S.K., A. Saoudi, and N.B. Performed the experiments: A.H., M.F.W., M.B., E.B., A. Salvioni, and S.K. Analyzed the data: A.H., M.F.W., M.B., E.B., A. Salvioni, S.K., A.B., A. Saoudi, and N.B. Wrote the paper: A.H. and N.B. with help of coauthors.
We declare no competing interests.
Footnotes
Citation Hassan A, Wlodarczyk MF, Benamar M, Bassot E, Salvioni A, Kassem S, Berry A, Saoudi A, Blanchard N. 2020. A virus hosted in malaria-infected blood protects against T cell-mediated inflammatory diseases by impairing DC function in a type I IFN-dependent manner. mBio 11:e03394-19. https://doi.org/10.1128/mBio.03394-19.
Contributor Information
Noah Butler, University of Iowa.
Melissa Bruckner Lodoen, University of California, Irvine.
REFERENCES
- 1.Bach JF. 2018. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18:105–120. doi: 10.1038/nri.2017.111. [DOI] [PubMed] [Google Scholar]
- 2.Filyk HA, Osborne LC. 2016. The multibiome: the intestinal ecosystem’s influence on immune homeostasis, health, and disease. EBioMedicine 13:46–54. doi: 10.1016/j.ebiom.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nogueira AR, Shoenfeld Y. 2019. Microbiome and autoimmune diseases: cause and effect relationship. Curr Opin Rheumatol 31:471–474. doi: 10.1097/BOR.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 4.Tselis A. 2011. Evidence for viral etiology of multiple sclerosis. Semin Neurol 31:307–316. doi: 10.1055/s-0031-1287656. [DOI] [PubMed] [Google Scholar]
- 5.Correale J, Gaitan MI. 2015. Multiple sclerosis and environmental factors: the role of vitamin D, parasites, and Epstein-Barr virus infection. Acta Neurol Scand 132:46–55. doi: 10.1111/ane.12431. [DOI] [PubMed] [Google Scholar]
- 6.Geginat J, Paroni M, Pagani M, Galimberti D, De Francesco R, Scarpini E, Abrignani S. 2017. The enigmatic role of viruses in multiple sclerosis: molecular mimicry or disturbed immune surveillance? Trends Immunol 38:498–512. doi: 10.1016/j.it.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinbach K, Vincenti I, Egervari K, Kreutzfeldt M, van der Meer F, Page N, Klimek B, Rossitto-Borlat I, Di Liberto G, Muschaweckh A, Wagner I, Hammad K, Stadelmann C, Korn T, Hartley O, Pinschewer DD, Merkler D. 2019. Brain-resident memory T cells generated early in life predispose to autoimmune disease in mice. Sci Transl Med 11:eaav5519. doi: 10.1126/scitranslmed.aav5519. [DOI] [PubMed] [Google Scholar]
- 8.Maizels RM, McSorley HJ. 2016. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 138:666–675. doi: 10.1016/j.jaci.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap GS, Gause WC. 2018. Helminth infections induce tissue tolerance mitigating immunopathology but enhancing microbial pathogen susceptibility. Front Immunol 9:2135. doi: 10.3389/fimmu.2018.02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gieseck RL 3rd, Wilson MS, Wynn TA. 2018. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 11.Pinzon-Charry A, Woodberry T, Kienzle V, McPhun V, Minigo G, Lampah DA, Kenangalem E, Engwerda C, Lopez JA, Anstey NM, Good MF. 2013. Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med 210:1635–1646. doi: 10.1084/jem.20121972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodberry T, Minigo G, Piera KA, Amante FH, Pinzon-Charry A, Good MF, Lopez JA, Engwerda CR, McCarthy JS, Anstey NM. 2012. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J Infect Dis 206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]
- 13.Gotz A, Tang MS, Ty MC, Arama C, Ongoiba A, Doumtabe D, Traore B, Crompton PD, Loke P, Rodriguez A. 2017. Atypical activation of dendritic cells by Plasmodium falciparum. Proc Natl Acad Sci U S A 114:E10568–E10577. doi: 10.1073/pnas.1708383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ocana-Morgner C, Mota MM, Rodriguez A. 2003. Malaria blood stage suppression of liver stage immunity by dendritic cells. J Exp Med 197:143–151. doi: 10.1084/jem.20021072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundie RJ, Young LJ, Davey GM, Villadangos JA, Carbone FR, Heath WR, Crabb BS. 2010. Blood-stage Plasmodium berghei infection leads to short-lived parasite-associated antigen presentation by dendritic cells. Eur J Immunol 40:1674–1681. doi: 10.1002/eji.200939265. [DOI] [PubMed] [Google Scholar]
- 16.Bujila I, Schwarzer E, Skorokhod O, Weidner JM, Troye-Blomberg M, Östlund Farrants A-K. 2016. Malaria-derived hemozoin exerts early modulatory effects on the phenotype and maturation of human dendritic cells. Cell Microbiol 18:413–423. doi: 10.1111/cmi.12521. [DOI] [PubMed] [Google Scholar]
- 17.Wilson NS, Behrens GM, Lundie RJ, Smith CM, Waithman J, Young L, Forehan SP, Mount A, Steptoe RJ, Shortman KD, de Koning-Ward TF, Belz GT, Carbone FR, Crabb BS, Heath WR, Villadangos JA. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol 7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 18.Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, de Labastida Rivera F, Amante FH, Bunn PT, Sheel M, Sebina I, Koyama M, Varelias A, Hertzog PJ, Kalinke U, Gun SY, Renia L, Ruedl C, MacDonald KP, Hill GR, Engwerda CR. 2014. Type I IFN signaling in CD8− DCs impairs Th1-dependent malaria immunity. J Clin Invest 124:2483–2496. doi: 10.1172/JCI70698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2011. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Mazliah D, Nguyen MP, Hosking C, McLaughlin S, Lewis MD, Tumwine I, Levy P, Langhorne J. 2017. Follicular helper T cells are essential for the elimination of Plasmodium infection. EBioMedicine 24:216–230. doi: 10.1016/j.ebiom.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figueiredo MM, Costa PAC, Diniz SQ, Henriques PM, Kano FS, Tada MS, Pereira DB, Soares IS, Martins-Filho OA, Jankovic D, Gazzinelli RT, Antonelli L. 2017. T follicular helper cells regulate the activation of B lymphocytes and antibody production during Plasmodium vivax infection. PLoS Pathog 13:e1006484. doi: 10.1371/journal.ppat.1006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, Kallies A, Hansen DS. 2016. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep 14:68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. 2015. Circulating Th1-cell-type Tfh cells that exhibit impaired B cell help are preferentially activated during acute malaria in children. Cell Rep 13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly A, Hansen DS. 2019. Development of B cell memory in malaria. Front Immunol 10:559. doi: 10.3389/fimmu.2019.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwood BM. 1968. Autoimmune disease and parasitic infections in Nigerians. Lancet 2:380–382. doi: 10.1016/s0140-6736(68)90595-3. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood BM, Voller A. 1970. Suppression of autoimmune disease in NZB and NZB-NZW F1 hybrid mice by infection with malaria. Trans R Soc Trop Med Hyg 64:7. [PubMed] [Google Scholar]
- 27.Sotgiu S, Angius A, Embry A, Rosati G, Musumeci S. 2008. Hygiene hypothesis: innate immunity, malaria and multiple sclerosis. Med Hypotheses 70:819–825. doi: 10.1016/j.mehy.2006.10.069. [DOI] [PubMed] [Google Scholar]
- 28.Farias AS, Talaisys RL, Blanco YC, Lopes SC, Longhini AL, Pradella F, Santos LM, Costa FT. 2011. Regulatory T cell induction during Plasmodium chabaudi infection modifies the clinical course of experimental autoimmune encephalomyelitis. PLoS One 6:e17849. doi: 10.1371/journal.pone.0017849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thome R, Issayama LK, Costa TA, Gangi RD, Ferreira IT, Raposo C, Lopes SC, Hofling MA, Costa FT, Verinaud L. 2014. Dendritic cells treated with crude Plasmodium berghei extracts acquire immune-modulatory properties and suppress the development of autoimmune neuroinflammation. Immunology 143:164–173. doi: 10.1111/imm.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomé R, Bombeiro AL, Issayama LK, Rapôso C, Lopes SCP, da Costa TA, Di Gangi R, Ferreira IT, Longhini ALF, Oliveira ALR, da Cruz Höfling MA, Costa FTM, Verinaud L. 2014. Exacerbation of Autoimmune Neuro-Inflammation in Mice Cured from Blood-Stage Plasmodium berghei Infection. PLoS One 9:e110739. doi: 10.1371/journal.pone.0110739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black J, Hommel M, Snounou G, Pinder M. 1994. Mixed infections with Plasmodium falciparum and P. malariae and fever in malaria. Lancet 343:1095. doi: 10.1016/s0140-6736(94)90203-8. [DOI] [PubMed] [Google Scholar]
- 32.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. 1997. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg 91:256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 33.Villegas-Mendez A, Greig R, Shaw TN, de Souza JB, Gwyer Findlay E, Stumhofer JS, Hafalla JC, Blount DG, Hunter CA, Riley EM, Couper KN. 2012. IFN-gamma-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. J Immunol 189:968–979. doi: 10.4049/jimmunol.1200688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voza T, Vigário AM, Belnoue E, Grüner AC, Deschemin J-C, Kayibanda M, Delmas F, Janse CJ, Franke-Fayard B, Waters AP, Landau I, Snounou G, Rénia L. 2005. Species-specific inhibition of cerebral malaria in mice coinfected with Plasmodium spp. Infect Immun 73:4777–4786. doi: 10.1128/IAI.73.8.4777-4786.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC, Hunt NH. 2005. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun 73:5645–5653. doi: 10.1128/IAI.73.9.5645-5653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hassan A, Wlodarczyk MF, Benamar M, Bassot E, Salvioni A, Kassem S, Berry A, Saoudi A, Blanchard N. 2019. A virus hosted in malaria-infected blood protects against T cell-mediated inflammatory diseases by impairing DC function in a type I IFN-dependent manner. bioRxiv doi: 10.1101/830687v1. [DOI] [PMC free article] [PubMed]
- 37.Curfs JH, Schetters TP, Hermsen CC, Jerusalem CR, van Zon AA, Eling WM. 1989. Immunological aspects of cerebral lesions in murine malaria. Clin Exp Immunol 75:136–140. [PMC free article] [PubMed] [Google Scholar]
- 38.Hermsen CC, Mommers E, van de Wiel T, Sauerwein RW, Eling WM. 1998. Convulsions due to increased permeability of the blood-brain barrier in experimental cerebral malaria can be prevented by splenectomy or anti-T cell treatment. J Infect Dis 178:1225–1227. doi: 10.1086/515691. [DOI] [PubMed] [Google Scholar]
- 39.Mancio-Silva L, Slavic K, Grilo Ruivo MT, Grosso AR, Modrzynska KK, Vera IM, Sales-Dias J, Gomes AR, MacPherson CR, Crozet P, Adamo M, Baena-Gonzalez E, Tewari R, Llinás M, Billker O, Mota MM. 2017. Nutrient sensing modulates malaria parasite virulence. Nature 547:213–216. doi: 10.1038/nature23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanni LA, Rae C, Maitland A, Stocker R, Hunt NH. 2001. Is ischemia involved in the pathogenesis of murine cerebral malaria? Am J Pathol 159:1105–1112. doi: 10.1016/S0002-9440(10)61786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villegas-Mendez A, Strangward P, Shaw TN, Rajkovic I, Tosevski V, Forman R, Muller W, Couper KN. 2017. Gamma interferon mediates experimental cerebral malaria by signaling within both the hematopoietic and nonhematopoietic compartments. Infect Immun 85:e01035-16. doi: 10.1128/IAI.01035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claser C, Malleret B, Gun SY, Wong AY, Chang ZW, Teo P, See PC, Howland SW, Ginhoux F, Renia L. 2011. CD8+ T cells and IFN-gamma mediate the time-dependent accumulation of infected red blood cells in deep organs during experimental cerebral malaria. PLoS One 6:e18720. doi: 10.1371/journal.pone.0018720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. 2011. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res 17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Cai B, Wang M, Tan P, Ding X, Wu J, Li J, Li Q, Liu P, Xing C, Wang HY, Su XZ, Wang RF. 2016. Cross-regulation of two type i interferon signaling pathways in plasmacytoid dendritic cells controls anti-malaria immunity and host mortality. Immunity 45:1093–1107. doi: 10.1016/j.immuni.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spaulding E, Fooksman D, Moore JM, Saidi A, Feintuch CM, Reizis B, Chorro L, Daily J, Lauvau G. 2016. STING-licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog 12:e1005975. doi: 10.1371/journal.ppat.1005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notkins AL, Shochat SJ. 1963. Studies on the multiplication and the properties of the lactic dehydrogenase agent. J Exp Med 117:735–747. doi: 10.1084/jem.117.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inada T, Mims CA. 1986. Infection of mice with lactic dehydrogenase virus prevents development of experimental allergic encephalomyelitis. J Neuroimmunol 11:53–56. doi: 10.1016/0165-5728(86)90074-3. [DOI] [PubMed] [Google Scholar]
- 48.Gaignage M, Marillier RG, Uyttenhove C, Dauguet N, Saxena A, Ryffel B, Michiels T, Coutelier JP, Van Snick J. 2017. Mouse nidovirus LDV infection alleviates graft versus host disease and induces type I IFN-dependent inhibition of dendritic cells and allo-responsive T cells. Immun Inflamm Dis 5:200–213. doi: 10.1002/iid3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musaji A, Cormont F, Thirion G, Cambiaso CL, Coutelier JP. 2004. Exacerbation of autoantibody-mediated thrombocytopenic purpura by infection with mouse viruses. Blood 104:2102–2106. doi: 10.1182/blood-2004-01-0310. [DOI] [PubMed] [Google Scholar]
- 50.deWalick S, Amante FH, McSweeney KA, Randall LM, Stanley AC, Haque A, Kuns RD, MacDonald KP, Hill GR, Engwerda CR. 2007. Cutting edge: conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J Immunol 178:6033–6037. doi: 10.4049/jimmunol.178.10.6033. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Ruiz D, Lau LS, Ghazanfari N, Jones CM, Ng WY, Davey GM, Berthold D, Holz L, Kato Y, Enders MH, Bayarsaikhan G, Hendriks SH, Lansink LIM, Engel JA, Soon MSF, James KR, Cozijnsen A, Mollard V, Uboldi AD, Tonkin CJ, de Koning-Ward TF, Gilson PR, Kaisho T, Haque A, Crabb BS, Carbone FR, McFadden GI, Heath WR. 2017. Development of a novel CD4+ TCR transgenic line that reveals a dominant role for CD8+ dendritic cells and CD40 signaling in the generation of helper and CTL responses to blood-stage malaria. J Immunol 199:4165–4179. doi: 10.4049/jimmunol.1700186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Draheim M, Wlodarczyk MF, Crozat K, Saliou JM, Alayi TD, Tomavo S, Hassan A, Salvioni A, Demarta-Gatsi C, Sidney J, Sette A, Dalod M, Berry A, Silvie O, Blanchard N. 2017. Profiling MHC II immunopeptidome of blood-stage malaria reveals that cDC1 control the functionality of parasite-specific CD4 T cells. EMBO Mol Med 9:1605–1621. doi: 10.15252/emmm.201708123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 54.Riley V, Lilly F, Huerto E, Bardell D. 1960. Transmissible agent associated with 26 types of experimental mouse neoplasms. Science 132:545–547. doi: 10.1126/science.132.3426.545. [DOI] [PubMed] [Google Scholar]
- 55.Notkins AL. 1965. Lactic dehydrogenase virus. Bacteriol Rev 29:143–160. doi: 10.1128/MMBR.29.2.143-160.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mongini PK, Rosenberg LT. 1976. Inhibition of lymphocyte trapping by a passenger virus in murine ascitic tumors: characterization of lactic dehydrogenase virus (LDV) as the inhibitory component and analysis of the mechanism of inhibition. J Exp Med 143:100–113. doi: 10.1084/jem.143.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson DC, Tosta CE, Wedderburn N. 1978. Exacerbation of murine malaria by concurrent infection with lactic dehydrogenase-elevating virus. Clin Exp Immunol 33:357–359. [PMC free article] [PubMed] [Google Scholar]
- 58.Oldstone MB, Dixon FJ. 1972. Inhibition of antibodies to nuclear antigen and to DNA in New Zealand mice infected with lactate dehydrogenase virus. Science 175:784–786. doi: 10.1126/science.175.4023.784. [DOI] [PubMed] [Google Scholar]
- 59.Takei I, Asaba Y, Kasatani T, Maruyama T, Watanabe K, Yanagawa T, Saruta T, Ishii T. 1992. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J Autoimmun 5:665–673. doi: 10.1016/0896-8411(92)90184-r. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi T, Hasegawa K, Ohta A, Maeda K. 2001. Reduction of serum interferon (IFN)-gamma concentration and lupus development in NZBxNZWF1 mice by lactic dehydrogenase virus infection. J Comp Pathol 125:285–291. doi: 10.1053/jcpa.2001.0507. [DOI] [PubMed] [Google Scholar]
- 61.Ammann CG, Messer RJ, Peterson KE, Hasenkrug KJ. 2009. Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One 4:e6105. doi: 10.1371/journal.pone.0006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 63.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cousens LP, Orange JS, Su HC, Biron CA. 1997. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci U S A 94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biron CA. 2001. Interferons alpha and beta as immune regulators–a new look. Immunity 14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 66.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O'Reilly LA, Schneider P, Fasel N, Shortman K, Strasser A, Acha-Orbea H. 2011. Type I interferon drives dendritic cell apoptosis via multiple BH3-only proteins following activation by polyIC in vivo. PLoS One 6:e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, Kamoun M, Rostami A. 2002. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol 169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 68.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. 2011. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 69.Komuczki J, Tuzlak S, Friebel E, Hartwig T, Spath S, Rosenstiel P, Waisman A, Opitz L, Oukka M, Schreiner B, Pelczar P, Becher B. 2019. Fate-mapping of GM-CSF expression identifies a discrete subset of inflammation-driving T helper cells regulated by cytokines IL-23 and IL-1beta. Immunity 50:1289.e6–1304.e6. doi: 10.1016/j.immuni.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. 2012. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur J Immunol 42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 71.Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, Birnberg T, Blank T, Reizis B, Korn T, Wiendl H, Jung S, Prinz M, Kurschus FC, Waisman A. 2012. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor+ regulatory T cells. Immunity 37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 72.Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. 2009. IFN-beta inhibits human Th17 cell differentiation. J Immunol 183:5418–5427. doi: 10.4049/jimmunol.0803227. [DOI] [PubMed] [Google Scholar]
- 73.Kavrochorianou N, Evangelidou M, Markogiannaki M, Tovey M, Thyphronitis G, Haralambous S. 2016. IFNAR signaling directly modulates T lymphocyte activity, resulting in milder experimental autoimmune encephalomyelitis development. J Leukoc Biol 99:175–188. doi: 10.1189/jlb.3A1214-598R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zander RA, Guthmiller JJ, Graham AC, Pope RL, Burke BE, Carr DJ, Butler NS. 2016. Type I interferons induce T regulatory 1 responses and restrict humoral immunity during experimental malaria. PLoS Pathog 12:e1005945. doi: 10.1371/journal.ppat.1005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montes de Oca M, Kumar R, Rivera FL, Amante FH, Sheel M, Faleiro RJ, Bunn PT, Best SE, Beattie L, Ng SS, Edwards CL, Boyle GM, Price RN, Anstey NM, Loughland JR, Burel J, Doolan DL, Haque A, McCarthy JS, Engwerda CR. 2016. Type I interferons regulate immune responses in humans with blood-stage Plasmodium falciparum Infection. Cell Rep 17:399–412. doi: 10.1016/j.celrep.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teo TH, Howland SW, Claser C, Gun SY, Poh CM, Lee WW, Lum FM, Ng LF, Renia L. 2018. Co-infection with chikungunya virus alters trafficking of pathogenic CD8+ T cells into the brain and prevents Plasmodium-induced neuropathology. EMBO Mol Med 10:121–138. doi: 10.15252/emmm.201707885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ives A, Ronet C, Prevel F, Ruzzante G, Fuertes-Marraco S, Schutz F, Zangger H, Revaz-Breton M, Lye LF, Hickerson SM, Beverley SM, Acha-Orbea H, Launois P, Fasel N, Masina S. 2011. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331:775–778. doi: 10.1126/science.1199326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarr PI, Aline RF Jr, Smiley BL, Scholler J, Keithly J, Stuart K. 1988. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A 85:9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi M, Castiglioni P, Hartley MA, Eren RO, Prevel F, Desponds C, Utzschneider DT, Zehn D, Cusi MG, Kuhlmann FM, Beverley SM, Ronet C, Fasel N. 2017. Type I interferons induced by endogenous or exogenous viral infections promote metastasis and relapse of leishmaniasis. Proc Natl Acad Sci U S A 114:4987–4992. doi: 10.1073/pnas.1621447114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Charon J, Grigg MJ, Eden JS, Piera KA, Rana H, William T, Rose K, Davenport MP, Anstey NM, Holmes EC. 2019. Novel RNA viruses associated with Plasmodium vivax in human malaria and Leucocytozoon parasites in avian disease. PLoS Pathog 15:e1008216. doi: 10.1371/journal.ppat.1008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saravu K, Rishikesh K, Kamath A, Shastry AB. 2014. Severity in Plasmodium vivax malaria claiming global vigilance and exploration–a tertiary care centre-based cohort study. Malar J 13:304. doi: 10.1186/1475-2875-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuertes Marraco SA, Grosjean F, Duval A, Rosa M, Lavanchy C, Ashok D, Haller S, Otten LA, Steiner QG, Descombes P, Luber CA, Meissner F, Mann M, Szeles L, Reith W, Acha-Orbea H. 2012. Novel murine dendritic cell lines: a powerful auxiliary tool for dendritic cell research. Front Immunol 3:331. doi: 10.3389/fimmu.2012.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
P. berghei K173 coinfection dampens P. berghei ANKA-induced IFN-γ production by CD4+ T cells. (A) ECM development in C57BL/6 mice infected with 106 P. berghei (Pb) ANKA pRBC or 106 P. berghei ANKA pRBC plus 106 P. berghei K173 pRBC. (B) IFN-γ expression by CD4+ T cells from peripheral blood measured from day 3 to day 7 after infection. Data from 1 experiment with N = 5 mice per group. Dots show the medians ± IQRs. Download FIG S1, PDF file, 0.3 MB (344.6KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
P. berghei K173 protects against ECM independently from the presence of the parasite. (A) ECM development in C57BL/6 mice infected with 106 P. berghei (Pb) ANKA pRBC and injected with plasma prepared from mice infected either with P. berghei ANKA or P. berghei K173 pRBC. (B) Same as in panel A except that the plasma from P. berghei K173-infected mice was subjected to ultraviolet (UV) radiation before coinjection with P. berghei ANKA pRBC. (A and B) Data from 1 experiment with N = 5 to 7 mice per group. Download FIG S2, PDF file, 0.3 MB (342.1KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LDV dampens encephalitogenic CD4+ T cell differentiation in spleen and draining lymph node following MOG35–55/CFA immunization. C57BL/6 mice were injected with PBS or LDV one day before MOG35–55/CFA immunization. Mononuclear cells from spleen and dLN harvested 15 days after MOG35–55/CFA immunization were restimulated with 100 μg MOG peptide for 24 h. Proportions of activated CD44+ of CD4+ T cells in spleen (A) and dLN (C). Proportions of CD4+ T cells producing IFN-γ, IL-17, or GM-CSF, visualized by intracellular staining from spleen (B) and dLN (D). (E) Quantification of IFN-γ, IL-17, and GM-CSF cytokines measured by ELISA in the supernatant of MOG35–55-stimulated spleen cells. Data representative of 2 independent experiments with N = 7 mice/group. Download FIG S3, PDF file, 0.2 MB (218.4KB, pdf) .
Copyright © 2020 Hassan et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.