Abstract
Aim
We examined the use of a Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) catheter during cardiopulmonary resuscitation (CPR) after cardiac arrest (CA) to assess its effect on haemodynamics such as coronary perfusion pressure (CPP), common carotid artery blood flow (CCA-flow) and end-tidal CO2 (PetCO2) which are associated with increased return of spontaneous circulation (ROSC).
Methods
Six male swine were instrumented to measure CPP, CCA-Flow, and PetCO2. A 7Fr REBOA was advanced into zone-1 of the aorta through the femoral artery. Ventricular fibrillation was induced and untreated for 8 min. CPR (manual then mechanical) was initiated for 24 min. Continuous infusion of adrenaline (epinephrine) was started at minute-4 of CPR. The REBOA balloon was inflated at minute-16 for 3 min and then deflated/inflated every 3 min for 3 cycles. Animals were defibrillated up to 6 times after the final cycle. Animals achieving ROSC were monitored for 25 min.
Results
Data showed significant differences between balloon deflation and inflation periods for CPP, CCA-Flow, and PetCO2 (p < 0.0001) with an average difference (SD) of 13.7 (2.28) mmHg, 15.5 (14.12) mL min−1 and −4 (2.76) mmHg respectively. Three animals achieved ROSC and had significantly higher mean CPP (54 vs. 18 mmHg), CCA-Flow (262 vs. 135 mL min−1) and PetCO2 (16 vs. 8 mmHg) (p < 0.0001) throughout inflation periods than No-ROSC animals. Aortic histology did not reveal any significant changes produced by balloon inflation.
Conclusion
REBOA significantly increased CPP and CCA-Flow in this model of prolonged CA. These increases may contribute to the ability to achieve ROSC.
Keywords: REBOA, Cardiac arrest, Coronary perfusion pressure, Aorta, Return of spontaneous circulation
Introduction
Cardiac arrest is a major public health challenge. More than 400,000 people are treated in the United States annually with standard cardiopulmonary resuscitation (CPR) and advanced cardiac life support (ACLS).1 Despite intensive efforts over the past decades that include bystander CPR and hypothermic targeted temperature management, little progress has been made to improve the overall survival rate. Currently, only 10% of out-of-hospital cardiac arrest (OHCA) and 24% of in-hospital cardiac arrest (IHCA) patients survive to hospital discharge.1,2
Prior studies have demonstrated the association between optimal coronary perfusion pressure (CPP) and return of spontaneous circulation (ROSC).3–7 Paradise et al. concluded that a minimum CPP of 15 mmHg is critical for patients to achieve ROSC during CPR.3 Many factors affect the ability to achieve adequate CPP, including patient anatomy and quality and method of chest compression. The current major means to elevate CPP is with the use of vasopressors such as adrenaline (epinephrine) and vasopressin. However, their impact on patient outcomes have shown mixed results and debate continues concerning their proper use, benefits, and the risk of harm.4–12
Resuscitative endovascular balloon occlusion of the aorta (REBOA) has gained popularity as an effective bridge treatment for non-compressible torso haemorrhage until definitive surgical haemostasis is achieved.13–16 In addition to its ability to stop haemorrhage distal to aortic balloon occlusion, REBOA can also increase CPP, common carotid artery blood flow (CCA-flow), and cerebral perfusion pressure, and may thus have potential as an adjunct during CPR to increase ROSC. Sesma et al. have found that utilization of an aortic balloon occlusion catheter during CPR significantly increases both coronary and cerebral perfusion pressure in pigs.17 Rubertsson et al. as well as Gedeborg et al. found that balloon occlusion of the descending aorta contributed to an increased rate of ROSC in piglets and canines.18,19 However, these previous three studies utilized animals weighing less than 30 kg, and while exposing animals to untreated cardiac arrest times of 5–8 min, instituted aortic balloon inflation shortly after institution of CPR (1–5 min). Additionally, balloon inflation occurred at higher aortic levels and each did not simultaneously measure CPP, end-tidal CO2 (PetCO2) and markers of cerebral blood flow such as CCA-flow. Lastly, none used the current commercially available REBOA catheter.
Since institution of REBOA as an adjunct in cardiac arrest will likely occur in a longer time interval after cardiac arrest, this pilot investigation, used a larger swine model with prolonged arrest and CPR times to understand its primary effect on CPP, PetCO2, and CCA-Flow during CPR which are known to be associated with ROSC.3,4 We hypothesized that occlusion of the descending aorta during CPR using REBOA would significantly increase CPP and CCA-flow, and decrease PetCO2 even after prolonged cardiac arrest.
Methods and materials
All procedures outlined in this study adhered to the principles stated in the eighth edition of the Guide for the Care and Use of Laboratory Animals20 and were approved by the University of Michigan’s Institutional Animal Care and Use Committee.
Anesthesia
Six male Yorkshire mix swine with an average (SD) weight of 43 (2.9) kg were sedated using ketamine/xylazine (20/2 mg kg−1) to facilitate transport to the operating room. Core temperature was maintained at 37–38 °C using a warming blanket (Blanketrol1, Cincinnati Sub-Zero, Cincinnati, OH) during instrumentation then turned off after induction of cardiac arrest. Anesthesia induction was performed using 2.5% isoflurane in 100% oxygen through a face mask until animals lost their corneal and swallowing reflexes allowing intubation. Animals were intubated using a standard cuffed 7.5 mm endotracheal tube and anesthesia was maintained at 1.5–2.5% isoflurane. Fraction of inspired oxygen (FiO2) was maintained at 21–23% and mechanical ventilation was started using a volume control setting with initial tidal volume at 7–8 mL kg−1 (Draeger FabiusGS Premium. Draeger Inc. Telford, PA). Respiratory rate was adjusted to titrate PetCO2 to 35–45 mmHg (Biopac Data Acquisition System. Biopac Inc. Goleta, CA). A 22 ga catheter was placed in an ear vein for IV administration of crystalloids during surgical instrumentation.
Instrumentation
The left common femoral artery was cannulated for continuous arterial pressure measurements below the level of aortic balloon occlusion. The right common femoral artery was cannulated using a 9Fr introducer (Arrow International Inc., Reading, PA) for the placement of a 7Fr REBOA catheter (ER-REBOA™, PRYTIME MEDICAL, Lake-wood, CO). The right common carotid artery was cannulated with a catheter to the aortic arch for aortic blood pressure (AoBP) monitoring. An ultrasonic flow probe (3PS. Transonic, Ithaca, NY) was placed around the left common carotid artery to measure CCA-Flow.
A triple lumen central venous catheter (Arrow International Inc., Reading, PA) was placed in the right internal jugular vein to the right atrium for right atrial diastolic pressure measurement, fluid infusion, and blood sampling. The right external jugular vein was cannulated with a 9Fr introducer via ultrasound-guided Seldinger technique for the placement of a cardiac pacing catheter (Arrow International Inc., Reading, PA) into the right ventricle.
Procedure
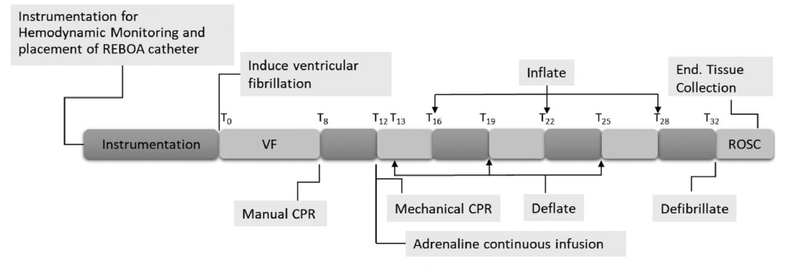
Baseline data and blood samples were collected 15 min following instrumentation. Prior to cardiac arrest, the REBOA catheter was placed through the right common femoral artery into zone 1 of the aorta, superior to the celiac artery, using a pre-measured approximated distance with placement verified post-mortem by direct visualization. Zone 1 was chosen to maximize the haemodynamic effect of REBOA on CPP and CCA-Flow.21 The balloon was briefly inflated with 7–9 mL of normal saline then deflated to ensure aortic occlusion, which was verified by the loss of femoral arterial pressure pulsatility. Ventricular fibrillation (VF) was induced with the pacing wire using a 9 V battery. VF was verified by ECG and loss of pulsatile AoBP and noted as time zero (T0). Isoflurane was discontinued, and animals were disconnected from the ventilator at the onset of VF. VF was left untreated for 8 min (T8). Manual CPR was started using American Heart Association Advanced Cardiac Life Support guidelines (100 compressions per minute) at T8 and continued for 4 min. The ventilator was resumed at the onset of CPR with a respiratory rate of 10 breaths per minute at the previous tidal volume (7–8 mL kg−1) and 100% FiO2. CPR provider was switched after 2 min (T10) to limit fatigue. At T12, manual CPR was discontinued in exchange for the mechanical CPR using LUCAS-II (Physio Control, Redmond, WA) which provided 100 compressions per minute at a depth of 5 cm and 50–50 duty cycle until the conclusion of the experiment. To avoid any compressions interruption during manual to mechanical transition, the LUCAS-II device was pre-fitted and the back plate was placed prior to the start of VF and CPR. Average (SD) time from manual to mechanical transition was 13(3) seconds. Simultaneous with mechanical CPR, a continuous IV infusion of adrenaline (0.0024 mg kg−1 min−1) into the central venous catheter began at T12 and continued until the conclusion of the CPR period (Fig. 1).
Fig. 1 -.
Timeline of the experimental design. (VF) Ventricular fibrillation. (ROSC) Return of spontaneous circulation. (T0–32) Time from start of VF to end of CPR.
Four minutes after the start of mechanical compressions (T16), the REBOA balloon was inflated. The balloon remained inflated for a period of 3 min, followed by deflation for 3 min. Using the 3 min prior to first inflation as first deflation, the deflation/inflation procedure was repeated 3 times. During each inflation period complete aortic occlusion was verified by loss of femoral arterial pressure pulsatility. At the conclusion of the third inflation period, the REBOA catheter was deflated and the animal received up to 6 repeated 200 J biphasic defibrillation attempts. Defibrillations were repetitively administered with no intervening chest compressions until ROSC was achieved or a total of 6 defibrillation attempts were administered. Animals achieving ROSC were immediately started on isoflurane anesthesia. Ventilation was adjusted to titrate PetCO2 to 35–45 mmHg and animals were monitored for an additional 25 min. Animals were then euthanized upon completion of the experiment using 1–2 mmol kg−1 KCl.
The descending aorta including segments above and below the balloon were immediately dissected and removed for histological analysis after euthanasia. Formalin fixed sections of the aorta around the area of the balloon placement were submitted for histopathology by a veterinary pathologist blinded to the study’s design. Standard H&E stain was used for histological analysis to evaluate any vascular injury caused by the balloon during CPR.
Data and sample collection
Continuous waveform data was collected of the following parameters: aortic and right atrial pressures, PetCO2, ECG, and CCA-flow using Biopac Data Acquisition System (Biopac Inc. Goleta, CA). During CPR, CPP was determined as the difference between aortic diastolic and right atrial diastolic blood pressure with diastole defined as the relaxation phase of chest compression.3 Intermittent blood samples were drawn for the following: CBC (Vetscan HM5: Abaxis, Union City, CA) at baseline, T30, and experiment completion, as well as arterial and venous blood gas analysis (ABL800 Radiometer America, Brea, CA) at baseline, T10, T20, T30, and completion of the experiment.
Statistical analysis
Physiologic data is presented as means, standard deviations and 95% confidence intervals. Repeated measures ANOVA (rmANOVA) as well as paired t-test were used to compare periods of REBOA inflation and deflation on pooled data and across all animals including comparing animals achieving ROSC versus No-ROSC. A level of significance was set at an a = 0.05. Prism7 (GraphPad, LaJolla, CA) and MATLAB R2017b (The Mathworks, Inc., Natick, MA) were used for data analysis.
Results
rmANOVA analysis on pooled data for all six animals demonstrated significant differences across all variables (CPP, CCA-flow, and PetCO2) between periods of REBOA inflation and deflation during CPR (p < 0.0001) (Table 1). Three of the six animals achieved ROSC. There were no significant differences in the above haemodynamic variables between ROSC and No-ROSC animals at baseline (table available in Supplemental material).
Table 1:
Difference between inflation and deflation periods for CPP, CCA-Flow and PetCO2
| Baseline | Deflation 1 | Inflation 1 | Deflation 2 | Inflation 2 | Deflation 3 | Inflation3 | ||
|---|---|---|---|---|---|---|---|---|
| CPP (mmHg) | Mean (SD) | 74 (0.59) | 23 (1.31) | 38 (1.92)* | 25 (1.03) | 37 (1.79)* | 20 (1.1) | 34 (1.28)* |
| 95% CI | 73.7, 74.2 | 22.7, 24 | 36.9, 38.7 | 24.1, 25.1 | 35.7, 37.4 | 18.5, 20.5 | 33.6, 34.8 | |
| CCA-Flow (mL/min) | Mean (SD) | 315 (3.6) | 260 (11) | 253 (19) | 165 (8) | 185 (14)* | 125 (5) | 158 (7)* |
| 95% CI | 313, 317 | 255, 265 | 244, 261 | 161, 169 | 179, 192 | 122, 127 | 155, 161 | |
| PetCO2 (mmHg) | Mean (SD) | 38.9 (0.11) | 30.2 (1.42) | 23.5 (2.57)* | 23.5 (1.52) | 19.4 (0.95)* | 19.5 (1.44) | 18.2 (0.46)* |
| 95% CI | 38.9, 39 | 29.5, 30.1 | 22.3, 24.7 | 22.8, 24.2 | 18.9, 19.8 | 18.9, 20.2 | 18, 18.5 |
Mean, standard deviation, and 95% confidence intervals for CPP, CCA-Flow, and PetCO2 during periods of deflation and inflation. (CPP) Coronary perfusion Pressure. (CCA-Flow) Carotid artery blood flow. (PetCO2) End-tidal CO2. (SD) Standard deviation. (95% CI) 95% Confidence intervals.
denotes significant difference between inflation and deflation periods.
Coronary perfusion pressure
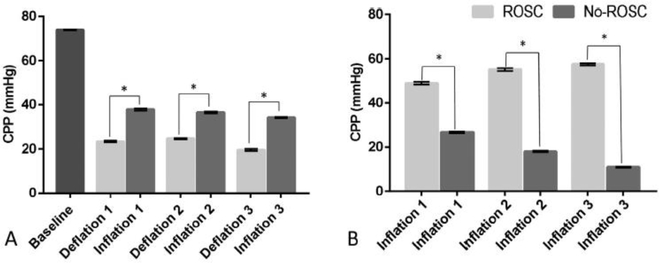
CPP on pooled data for 6 animals was significantly higher during periods of inflation than deflation. The mean (SD) of CPP was 36.2 (1.81) and 22.5 (2.63) mmHg during inflation and deflation respectively with mean difference (SD) of 14.5 (2.16), 12 (1.74), and 14.7 (2.94) mmHg (p < 0.001) for the first, second, and third cycle of deflation/inflation respectively (Fig. 2A). In addition, CPP was significantly higher during periods of inflation vs. periods of deflation in each individual animal (p < 0.001). The three animals that achieved ROSC also had significantly higher CPP during inflation periods compared to those that did not with mean (SD) CPP of 53.8 (4.55) and 18.5 (6.64) mmHg for ROSC and no-ROSC respectively (p < 0.001) (Fig. 2B).
Fig. 2 -.
Coronary perfusion pressure. (A) Differences between deflation and inflation. (B) Differences between ROSC and No-ROSC groups during inflation. (ROSC) Return of spontaneous circulation. Data is presented as means and standard errors (p < 0.001).
Common carotid artery flow
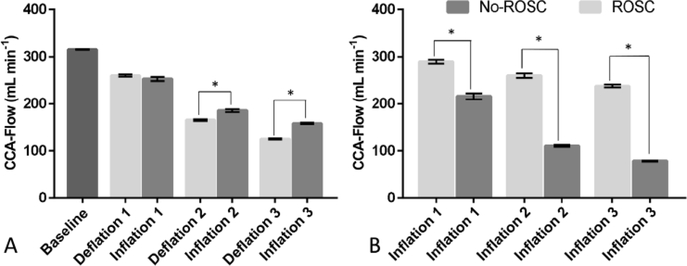
CCA-Flow on pooled data for 6 animals was significantly higher during the second and third periods of inflation than deflation. The mean (SD) of CCA-Flow was 198 (49) and 183 (69) mL/min during inflation and deflation respectively with mean difference (SD) of 20.1 (15.13) and 33.5 (6.91) mL min−1 (p < 0.001) (Fig. 3A). In addition, CCA-flow was significantly higher during periods of inflation vs. periods of deflation in each individual animal (p < 0.001) except for the first cycle in two of the No-ROSC animals. The three animals that achieved ROSC also had significantly higher CCA-flow during inflation compared to those that did not with mean(SD) CCA-Flow of 262 (28) and 135 (61) mL min−1 for ROSC and no-ROSC respectively (p < 0.001) (Fig. 3B).
Fig. 3 -.
Carotid artery blood flow. (A) Differences between deflation and inflation. (B) Differences between ROSC and No-ROSC groups during inflation. (ROSC) Return of spontaneous circulation. Data is presented as means and standard errors (p < 0.001).
End-tidal CO2
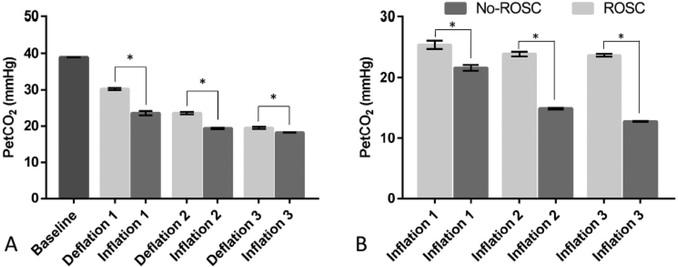
PetCO2 on pooled data for 6 animals was significantly decreased during the first, second, and third periods of inflation than deflation. The mean (SD) of PetCO2 was 20.4 (2.78) and 24.4 (5.38) mmHg during inflation and deflation respectively with mean difference (SD) of −6.6 (3.7), −4.1 (2.19), and −1.3 (1.79) mmHg (p = 0.005) (Fig. 4A). However, PetCO2 was significantly higher during inflation in animals achieving ROSC compared to no-ROSC with mean(SD) PetCO2 of 24.4 (2.23) and 16.4 (4.02) mmHg for ROSC and no- ROSC respectively (p < 0.001) (Fig. 4B).
Fig. 4 -.
End-tidal C02. (A) Differences between deflation and inflation. (B) Differences between ROSC and No-ROSC groups during inflation. (ROSC) Return of spontaneous circulation. Data is presented as means and standard errors (p < 0.001).
Aortic histology
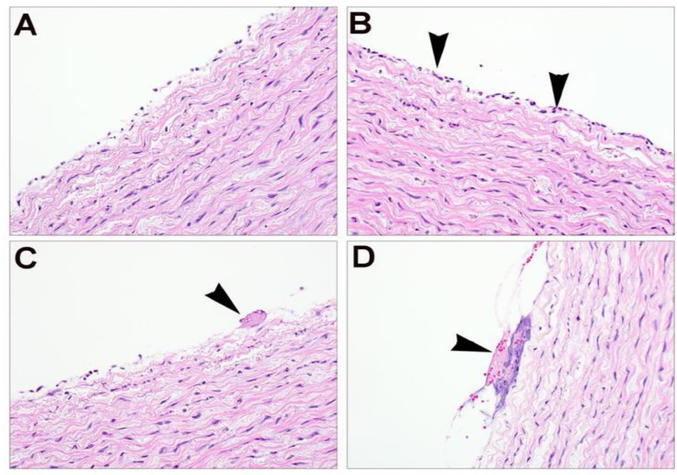
Zone 1 placement of the REBOA balloon was verified in all six animals by a post-mortem gross examination of the aorta. Pathology examination showed a few fibrin strands present at the center of the catheter balloon site that were not observed at the ends of aorta catheter balloon site or in adjacent aorta not in contact with the balloon. In one case, small fibrin strands were observed in adjacent control sites of the aorta, so this association is questionable in cases in which very small amounts of fibrin or few inflammatory cells are observed at the treatment site. Regardless, there were no significant alterations to the vessel wall or endothelial surface to raise concern for clinical implications due to the catheter exposure/treatment (Fig. 5).
Fig. 5 -.
Representative aortic histopathology from control region (A) and treatment area (B), showing discontinuous endothelial layer in both sections, but with few scattered neutrophils (B, arrowhead) in the treated area. (C-D) Treatment areas showing few neutrophils associated with minimal accumulation of fibrin (C, arrowhead) and cellular debris (D, arrowhead).
Discussion
Successful ROSC from cardiac arrest, especially in the setting of prolonged cardiac arrest, remains a challenge. Balloon occlusion of the aorta for control of truncal haemorrhage was first described in 1954.22 It is only within the last several years and with technical advances in catheter design that REBOA is being increasingly used as an adjunct in trauma centers as part of a strategy to obtain temporary control of truncal and pelvic haemorrhage until definitive surgical haemostasis.23 An immediate haemodynamic byproduct of REBOA inflation is an increase in cerebral and myocardial perfusion in patients with haemorrhagic shock.23,24 This effect makes the use of REBOA an attractive adjunct during CPR when CPP augmentation can be challenging and where patients can actually have reverse (negative) CPP values based on the mechanism of intrathoracic pressures produced during CPR.3 While adrenaline or vasopressin are often used to increase CPP, their immediate effectiveness on reaching a critical level of CPP during CPR is usually unknown. Instead, surrogates of CPP and CPR quality such as PetCO2 are used to guide CPR. In cases of prolonged cardiac arrest, increasing CPP becomes an even greater challenge. Some large animal studies have suggested that an even higher CPP (35–40 mmHg) may be needed in prolonged arrest, making resuscitative adjuncts such as REBOA more attractive.25 In a case report, Aslanger et al. described a refractory cardiac arrest during which an aortic occlusion catheter was used to occlude the descending aorta after more than 27 min of CPR including more than 5 min of asystole. The patient subsequently achieved ROSC and survived to hospital discharge.26
In this study, REBOA was effective in increasing aortic diastolic pressure without increasing right atrial diastolic pressure, thus achieving higher CPP and CCA-flow. The decrease in PetCO2 during balloon inflation was anticipated since the increase in afterload would reduce cardiac output generated by chest compressions. This same phenomenon is observed with high dose adrenaline during CPR.27 These findings suggest that REBOA favorably impacts haemodynamic parameters associated with improved chances of ROSC.
While CCA-flow may not indicate true oxygen delivery to the brain, it was used as a surrogate measure of brain blood flow, similar to middle cerebral artery Doppler for brain flow. Schultz et al. have shown that increased CCA-flow, together with CPP are associated with increased ROSC.28 In our investigation, animals demonstrated significantly higher CPP and CCA-flow values, and significantly lower PetCO2 values during periods of balloon inflation compared to deflation. Such parameters were also significantly higher during inflation in animals achieving ROSC vs. no-ROSC. Reasons for this significant disparity in response to REBOA are not clear but indicate the need for further exploration to better understand and differentiate responders from non-responders especially in the setting of prolonged cardiac arrest. Since all animals had complete loss of femoral artery pressure during balloon inflation, received the same mechanical CPR parameters and continuous epinephrine infusions, we can think of no technical issues which may have produced greater hemodynamic changes in “responders” versus “non-responders”.
Gross inspection of the REBOA catheter was performed and histological data were gathered from post mortem aortic tissues to assess safety of using the REBOA during CPR. The catheter and balloon maintained integrity after prolonged chest compressions while inflated, and after extraction the balloon functioned ex-vivo with no signs of damage on gross inspection. Histological analysis was performed and aortic tissues in contact with the balloon were compared to adjacent cranial-caudal segments and showed little difference. These findings suggest that cyclical pressure exerted on the inflated REBOA balloon during chest compressions did not significantly damage the aortic intima.
This study has several limitations. First, this was a pilot study in which only six animals were studied. The REBOA balloon was inflated periodically as opposed to continuous inflation or deflation during CPR. This was done in order to use each animal as its own internal control while also allowing for comparisons between all animals to better understand if REBOA could produce hemodynamic responses known to be favorable for ROSC in the setting of prolonged cardiac arrest. In addition, the differences in ROSC and survival outcome achieved by continuous versus cyclical balloon occlusion (producing any pre-conditioning effect) remain unknown. While the study was sufficiently powered to detect changes in CPP, PetCO2 and CCA-Flow, it was not powered to detect significant differences in ROSC. Finally, we did not monitor animals’ long term survival so cannot comment if ROSC would have resulted in favorable neurologic outcome.
Future studies are necessary to determine the effects of continuous aortic occlusion during CPR on haemodynamics, ROSC, long term survival and neurologic outcome. Furthermore, the optimal duration and adverse effects of intra-arrest REBOA implementation remain unknown. Additional studies should also examine the synergistic effect of REBOA with other CPR adjuncts such as the impedance threshold device and active compression/decompression CPR to further augment CPP. Lastly, the proximal port of the REBOA catheter may serve as an attractive way to deliver intra-arrest medications, hypothermic targeted temperature management, and neuroprotective agents.
Conclusion
In this model of prolonged cardiac arrest, REBOA significantly increased CPP and CCA-flow and decreased PetCO2. These changes may have contributed to the ability to achieve ROSC after prolonged CA. Additional investigation is warranted to understand the role of REBOA as an adjunct in the treatment of cardiac arrest.
Supplementary Material
Acknowledgement
The authors and MCIRCC are grateful for the donation of REBOA catheters by Prytime Inc. that were used in this study. Prytime Inc. had no involvement in the study design, data collection, analysis or writing of this manuscript. Dr. Hsu acknowledges her NIH K-12 training award (K12HL133304-01).
Footnotes
Conflict of interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.resuscitation.2019.05.010
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2010;3:63–81. [DOI] [PubMed] [Google Scholar]
- 3.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990;263:1106–13. [PubMed] [Google Scholar]
- 4.Callaham M, Madsen CD, Barton CW, Saunders CE, Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA 1992;268:2667–72. [PubMed] [Google Scholar]
- 5.Cantrell CL Jr, Hubble MW, Richards ME. Impact of delayed and infrequent administration of vasopressors on return of spontaneous circulation during out-of-hospital cardiac arrest. Prehosp Emerg Care 2013;17:15–22. [DOI] [PubMed] [Google Scholar]
- 6.Goto Y, Maeda T, Goto Y. Effects of prehospital epinephrine during out-of-hospital cardiac arrest with initial non-shockable rhythm: an observational cohort study. Crit Care 2013;17:R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA 2012;307:1161–8. [DOI] [PubMed] [Google Scholar]
- 8.Hansen M, Schmicker RH, Newgard CD, et al. Time to epinephrine administration and survival from nonshockable out-of-hospital cardiac arrest among children and adults. Circulation 2018;137:2032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koscik C, Pinawin A, McGovern H, et al. Rapid epinephrine administration improves early outcomes in out-of-hospital cardiac arrest. Resuscitation 2013;84:915–20. [DOI] [PubMed] [Google Scholar]
- 10.Machida M, Miura S, Matsuo K, Ishikura H, Saku K. Effect of intravenous adrenaline before arrival at the hospital in out-of-hospital cardiac arrest. J Cardiol 2012;60:503–7. [DOI] [PubMed] [Google Scholar]
- 11.Nakahara S, Tomio J, Nishida M, Morimura N, Ichikawa M, Sakamoto T. Association between timing of epinephrine administration and intact neurologic survival following out-of-hospital cardiac arrest in Japan: a population-based prospective observational study. Acad Emerg Med 2012;19:782–92. [DOI] [PubMed] [Google Scholar]
- 12.Perkins GD, Ji C, Deakin CD, et al. A randomized trial of epinephrine in out-of-hospital cardiac arrest. N Engl J Med 2018;379:711–21. [DOI] [PubMed] [Google Scholar]
- 13.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol 2008;22:999–1012. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 2016;80:324–34. [DOI] [PubMed] [Google Scholar]
- 15.Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am 2012;92:843–58 vii. [DOI] [PubMed] [Google Scholar]
- 16.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011;8:92–102. [DOI] [PubMed] [Google Scholar]
- 17.Sesma J, Sara MJ, Espila JL, Arteche A, Saez MJ, Labandeira J. Effect of intra-aortic occlusion balloon in external thoracic compressions during CPR in pigs. Am J Emerg Med 2002;20:453–62. [DOI] [PubMed] [Google Scholar]
- 18.Gedeborg R, Rubertsson S, Wiklund L. Improved haemodynamics and restoration of spontaneous circulation with constant aortic occlusion during experimental cardiopulmonary resuscitation. Resuscitation 1999;40:171–80. [DOI] [PubMed] [Google Scholar]
- 19.Rubertsson S, Bircher NG, Alexander H. Effects of intra-aortic balloon occlusion on hemodynamics during, and survival after cardiopulmonary resuscitation in dogs. Crit Care Med 1997;25:1003–9. [DOI] [PubMed] [Google Scholar]
- 20.Council NR. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 21.Tibbits EM, Hoareau GL, Simon MA, et al. Location is everything: the hemodynamic effects of REBOA in Zone 1 versus Zone 3 of the aorta. J Trauma Acute Care Surg 2018;85:101–7. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery 1954;36:65–8. [PubMed] [Google Scholar]
- 23.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery 2011;150:400–9. [DOI] [PubMed] [Google Scholar]
- 24.Spence PA, Lust RM, Chitwood WR, Iida H, Sun YS, Austin EH. Transfemoral balloon aortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res 1990;49:217–21. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care 2010;14:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslanger E, Golcuk E, Oflaz H, et al. Intraaortic balloon occlusion during refractory cardiac arrest. A case report. Resuscitation 2009;80:281–3. [DOI] [PubMed] [Google Scholar]
- 27.Chase PB, Kern KB, Sanders AB, Otto CW, Ewy GA. Effects of graded doses of epinephrine on both noninvasive and invasive measures of myocardial perfusion and blood flow during cardiopulmonary resuscitation. Crit Care Med 1993;21:413–9. [DOI] [PubMed] [Google Scholar]
- 28.Schultz J, Segal N, Kolbeck J, McKnite S, Caldwell E, Yannopoulos D. Sodium nitroprusside enhanced cardiopulmonary resuscitation (SNPeCPR) improves vital organ perfusion pressures and carotid blood flow in a porcine model of cardiac arrest. Resuscitation 2012;83:374–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.